Abstract
Background
Schizophrenia has been conceptualized as a brain network disorder rooted in dysregulated neurodevelopmental processes. Recent neuroimaging studies revealed disrupted brain connectomic organization in adult schizophrenia patients. However, altered developmental trajectories of the functional connectome during the adolescent maturational stage have not been examined.
Methods
The present study combined functional MRI with a graph theoretical approach to examine functional network topology and its age-related development in 39 medication naïve, first-episode patients with adolescent-onset schizophrenia and 31 matched controls (age range: 12–18 years).
Results
Patients demonstrated impaired large-scale integration as reflected by reduced global efficiency as well as decreased regional nodal efficiency in highly integrative network hubs, most consistently the hippocampal formation and the precuneus. Furthermore, the left hippocampus showed opposite age-efficiency associations in healthy controls and patients, indicating dysregulated maturational trajectories in adolescent schizophrenia and a particular vulnerability of this region during early pathological attack.
Conclusions
The findings allow an integrative perspective on network and neurodevelopmental perspectives on schizophrenia, suggesting that dysregulated maturation of the functional connectome during adolescence might reflect an early marker for the disorder.
Keywords: early-onset schizophrenia, functional connectome, graph theory, functional magnetic resonance imaging, adolescent maturation
Introduction
Schizophrenia is a devastating and chronically debilitating brain disorder with onset during early adulthood. Various pathogenic models of schizophrenia are under debate, but across these conceptualizations, neurodevelopmental and dysconnectivity perspectives remain central.1,2 Overarching conceptualizations propose that the underlying pathogenesis is rooted in abnormal processes that emerge from a dynamic interplay between genetic and environmental factors. These factors are thought to dysregulate neurodevelopmental synaptic plasticity,3,4 including myelogenesis5 and synaptic pruning,6 ultimately disrupting the integration of spatially distributed neural information on the network level.7 Efficient information processing in complex networks such as the human brain critically relies on topological organization principles reflecting a balanced interplay between integration and segregation.8 In line with the dysconnectivity hypothesis, neuroimaging studies assessing large-scale cortical organization in patients with schizophrenia consistently revealed disrupted brain connectomic organization on the level of white mater,9 structural covariant,10 and functional connectivity,7 suggesting that aberrant brain network topology might represent a promising imaging-based marker for the disorder.11
Despite the influential neurodevelopmental model of schizophrenia, research has only recently begun to map aberrant developmental trajectories at the level of the network organization of the brain. Recent findings suggest delayed development of brain connectivity,10 heterochronicity of adolescent maturation,12 as well as accelerated age-related decline in adult patients.13 However, these previous studies focused on patients in the chronic stage of disorder, and thus it cannot be excluded that the corresponding imaging based markers might partly reflect effects of antipsychotic medication, progressive neural changes with illness duration as well as accelerated aging-related decline.13 Indeed, previous research indicates a sensitivity of network level markers for acute and chronic administration of antipsychotic medication,14 and progressive alterations during the course of the disorder.15 Therefore, examining medication-naïve, first-episode patients with adolescent-onset schizophrenia (AOS) is essential to determine underlying core pathological dysregulations.16
Adolescence is a period of dramatic brain maturational changes, including progressive (ie, myelination) and regressive (ie, synaptic pruning) maturational processes, promoting functional specification as well as integration on the network level.17 Dysregulations in these complex maturational processes have been increasingly associated with schizophrenia.18 AOS, a rare form adolescent manifestation of the disorder is characterized by pronounced clinical symptomatology and neurocognitive impairments.19 Given that abnormal pruning is considered a key patho-mechanism underlying schizophrenia, examining aberrant age-related maturation of the functional connectome in AOS offers a unique opportunity to gain crucial insights into the neuropathophysiological developmental processes underlying the disorder. Despite the proposed importance of neurodevelopmental dysregulations in the dysconnectivity model and converging evidence for altered connectivity20–22 in AOS, altered developmental trajectories of functional brain topology have not been systematically examined.23
In the present study, we applied a graph theoretical approach to explore abnormal functional connectomics and maturation in antipsychotic-naïve first-episode AOS. In an initial step, aberrant brain functional network topological characteristics were mapped in comparison to matched controls. Next abnormal age-related maturational trajectories of the identified network metrics were explored comparing age-related changes between AOS and controls. Based on previous studies mapping brain structural connectivity alterations in early-onset schizophrenia across adolescence,10,24 we expected stronger functional connectomic alterations in younger patients, which may reflect early-phase biomarkers related to core pathophysiological mechanisms.
Methods
Participants and Protocols
A total of 39 antipsychotic-naïve patients with first-episode schizophrenia aged between 12 and 18 years were recruited at the Second Affiliated Hospital of Xinxiang Medical University. All participants were right-handed Han Chinese and had received more than 6 years of formal education. Exclusion criteria for all participants were: (1) any past or current neurological disorders or family history of hereditary neurological disorders; (2) history of head injury with loss of consciousness; (3) alcohol or substance abuse; (4) claustrophobia; (5) MRI contraindications. Patients had to fulfill the following inclusion criteria: (1) diagnosis of schizophrenia according to DSM criteria (DSM-VI-TR25); (2) no co-morbid Axis I diagnosis; (3) duration of illness less than 2 years; (4) no current or previous antipsychotic medication. Clinical symptoms were independently assessed by 2 experienced psychiatrists using DSM-VI based structured interviews (SCID-I/Patient version). To validate the initial diagnosis, all patients were re-assessed 6 months after the initial diagnostic interview. Clinical symptomatology was further evaluated using the Positive and Negative Syndrome Scale (PANSS). A total of 31 healthy adolescents without psychiatric or neurological symptoms, matched for age, gender, education, and general intelligence were included as healthy control group (HC). Clinical and demographical data are presented in table 1.
Table 1.
Demographic and Clinical Characteristics for AOS and HC Groups
| AOS (n = 35) | HC (n = 30) | Statistic | P | |
|---|---|---|---|---|
| Age, y, mean (SD) | 15.5 (1.7) | 15.4 (1.5) | t = 0.40 | .68a |
| Gender, male/female | 20/15 | 13/17 | χ2 = 0.74 | .39b |
| Education, y, mean (SD) | 8.7 (1.2) | 8.5 (1.4) | t = 0.52 | .61a |
| Duration of illness, months, mean (SD) | 6.6 (6.7) | NA | ||
| Handness, right/left | 35/0 | 30/0 | ||
| Motion translation (mm), mean (SD) | 0.61 (0.55) | 0.69 (0.37) | t = −0.71 | .48a |
| Motion rotation (degree), mean (SD) | 0.008 (0.008) | 0.013 (0.007) | t = −2.26 | .03a |
| Removed frames number, mean (SD) | 3.00 (6.00) | 2.00 (4.00) | t = 1.25 | .21 a |
| Frame-wise displacement, mean(SD) | 0.096 (0.04) | 0.093 (0.03) | t = 0.33 | .78 a |
| PANSS positive score, mean (SD) | 20.42 (5.72) | NA | ||
| PANSS negative score | 20.91 (8.41) | NA | ||
| PANSS general score | 33.28 (6.69) | NA | ||
| PANSS total score | 74.62 (10.61) | NA |
AOS, adolescent-onset schizophrenia; HC, healthy controls; PANSS, Positive and Negative Syndrome Scale; NA, not applicable.
aThe P values were obtained by 2 sample t-test.
bThe P value for gender distribution in the 3 groups was obtained by chi-squared test.
The study had full ethical approval by the Ethics Committee of the Departments of Psychiatry at the Second Affiliated Hospital of Xinxiang Medical University and the Second Xiangya Hospital of Central South University. All participants and their parents provided written informed consent after receiving a complete description of the study protocols.
Data Acquisition
MRI data were acquired on a 3 Tesla MRI system (Vision; Siemens MAGNETOM Verio) equipped with a high-speed gradient coil. Functional images were acquired using an echo-planar imaging sequence with the following parameters: TR/TE = 2000 ms/30 ms, 33 slices, 64 × 64 matrix, 90° flip angle, field of view = 220 × 220 mm2, inter-slice gap = 0.6 mm, voxel size = 3.44 × 3.44 × 4 mm3. For each participant 240 functional volumes were obtained.
Data Preprocessing
Functional image preprocessing was carried out using DPARSFA (http://www.restfmri.net).26 Specifically, the first 10 functional volumes were discarded and the remaining images were corrected for temporal differences and head motion. To minimize the effects of head movement, participants with translations >2 mm or rotations >2° were excluded. Moreover, mean frame-wise displacement (FD) was computed and frames with FD >0.5 were removed.27,28 All data retain more than 80% of the original time points after scrubbing. Third, functional images were warped into a standard stereotaxic space at a 2 × 2 × 2 mm3 resolution, using the Montreal Neurological Institute (MNI) echo-planar imaging template, and spatially smoothed with a 6-mm full-width half-maximum (FWHM) isotropic Gaussian kernel. Fourth, linear trends from the time courses were removed using temporal band-pass filtering (0.01–0.08 Hz). Finally, motion profiles including Friston’s 24-parameters, white matter and cerebrospinal fluid signals were regressed from the time courses. The residual signal were used to compute functional connectivity between regions.29
Functional Connectomic Graph Metrics
Functional brain network are composed of nodes which represent brain regions and edges mirroring the statistical interdependence in blood oxygen level–dependent signals between different regions. The Harvard–Oxford Atlas which includes a total of 110 (55 per hemisphere) cortical and subcortical brain regions was used in the present study.30 Functional connectivity between each pair of nodes in the brain network was computed as the correlation between their averaged regional time series. Details on construction of the functional connectomics are provided in the supplementary material.
Global efficiency and nodal efficiency measuring how efficient the information is transferred in the brain network or between nodes are outlined in detail in our study. These parameters correspond to the indices that have been examined and showed most consistently alterations in schizophrenia patients.31,32 More detailed description other network indices are provided in the supplementary material.
Statistical Analysis
Between-Group Differences in Network Metrics
To evaluate abnormal network topology in AOS, network topological characters were compared between AOS and HC using nonparametric permutation tests. In permutation test, we randomized assignment of the 2 group samples to yield an empirical null distribution of effects under the null hypothesis, 5000 permutations. P values were determined by the percentage of the computed null distribution that exceeded the measured difference value between groups. For global network metrics, we compared AOS and HC groups at each network density threshold and additionally calculated the areas under the curves (AUC) as a general comparison of global topological characters. Permutation results at P < .05 were considered significant. For each nodal character, multiple testing was controlled for by correcting P values using false discover rate (FDR correction for 110 comparisons, P < .05 was considered as significant).33
Associations Between Network Measures and Age and Clinical Variables
To determine age-related effect on network metrics in the experimental groups, analysis of covariance (ANCOVA) was performed in SPSS v20. The graph-theoretical indices were considered as dependent variables, fixed factor was “diagnosis” (HC, AOS) and age was included as covariate. Significant interaction effects (age × diagnosis) were further disentangled using post hoc analysis within each experimental group. Specifically, Shepherd’s pi correlation was calculated between age and topological metrics separately for both AOS and HC. Shepherd’s pi correlation was employed because it accounts for potential outliers and increases statistical power.34 Observations with bootstrapped mahalanobis distances equal to or greater than 6 are then removed from the sample. The 2 parameters Shepherd’s pi correlation returns are pi and p. The pi is Spearman’s r value estimated over the remaining data. However, because removing data points can inflate false positive rates, the resulting p value is then doubled to account for outlier removal.34
To further explore the aberrant age-related maturational processes in AOS, participants in both groups were divided into 3 age subgroups (age 12–14 years, n = 10 patients, n = 9 controls; age 15–16 years, n = 12 patients, n = 13 controls; age 17–18 years, n = 13 patients, n = 8 controls). Interaction effect between “diagnosis” (AOS, HC) and “age subgroup” (12–14 y, 15–16 y, 17–18 y) were assessed using analysis of variance (ANOVA). For significant interactions, permutation test (5000 times) was performed as post hoc analysis to determine network metric differences in each age subgroup between AOS and HC. Bonferroni corrected P < .05 was set as significance threshold.
Shepherd’s pi correlations between topological metrics and duration of illness, positive PANSS, negative PANSS scores were also estimated in AOS.
Results
Imaging data of 4 patients and 1 healthy participant were excluded due to high head motion (>2 mm translation or >2° rotation), leaving a total of n = 35 AOS and n = 30 HC for the final network analysis. Importantly, AOS and HC did not differ in the time points removed, the mean FD, motion rotation, and motion translation between the 2 groups (table 1).
Aberrant Overall Network Topology in AOS
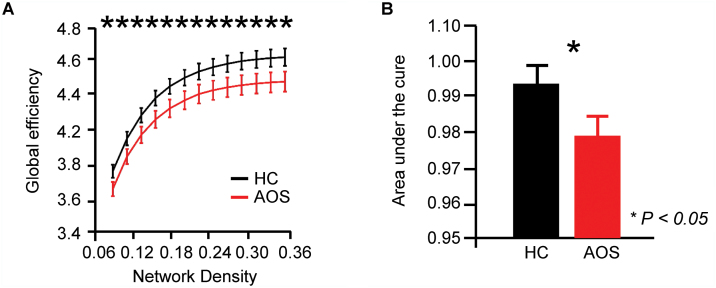
Compared to HC, AOS presented a significantly decreased global efficiency of the brain functional network at each network density threshold (figure 1). The overall network strength, local efficiency, and small-world character did not significantly differ between the groups (supplementary figure S1).
Fig. 1.
Global efficiency abnormality of brain network in AOS. (A) Global efficiency of brain functional network in AOS and HC groups were presented at each network sparse density; (B) The area under the curve (AUC) values were displayed and compared between groups. Error bars indicate standard deviations, permutation tests. AOS, adolescent-onset schizophrenia. HC, healthy controls.
Aberrant Regional Network Topology in AOS
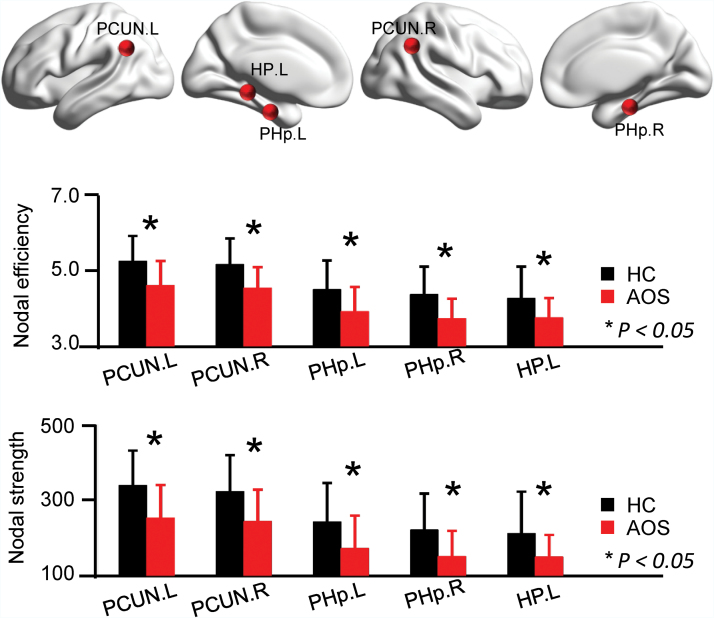
Compared to HC, AOS demonstrated reduced nodal efficiency and nodal strength in the bilateral posterior parahippocampus, bilateral precuneus, and left hippocampus (figure 2). Between-group comparisons in nodal path length, betweenness and clustering coefficient are additionally provided in supplementary figure S2. Regions with significantly altered efficiency and strength were used as topological targets to subsequently determine age- and disorder characteristic-associations.
Fig. 2.
Regional topological properties of brain network in AOS. (A) Nodal efficiency and nodal strength in 5 regions were significantly changed in AOS, permutation tests. Error bars indicate standard deviations. The locations of these 5 areas were presented on surface. PCUN, precuneus; PHp, parahippocampus posterior division; HP, hippocampus; L, left; R, right; AOS, adolescent-onset schizophrenia; HC, healthy controls.
Age Association of Network Metrics Alterations in AOS
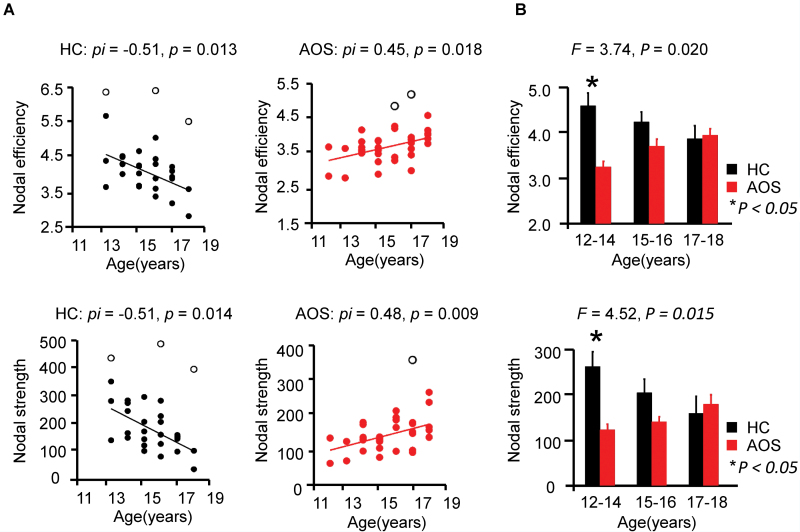
The ANCOVA did not reveal significant interaction effects between “diagnosis” and “age” on global efficiency (F = 0.47, P = .496). However, a significant interaction effect between “diagnosis” and “age” was found on nodal efficiency (F = 9.40, P = .003) and nodal strength (F = 9.67, P = .003) in the left hippocampus. Post hoc analysis demonstrated a significant, negative association between nodal efficiency and age in the left hippocampus of HC (pi = −.51, p = .013), whereas a significant positive association was observed in AOS (pi = .45, p = .018). Furthermore, nodal strength in the left hippocampus was significantly and negatively correlated with age in HC (pi = −.51, p = .014) and positively correlated with age in AOS (pi = .48, p = .009) (figure 3A).
Fig. 3.
Age related alterations of regional topological properties in AOS. (A) Age-related associations between nodal properties of the left hippocampus in AOS and HC subjects. Black hollow dots mark the outliers point in the linear estimation using Shepherd’s pi correlation. (B) Topological properties comparison in the age subgroups. ANOVA test was used to investigate the interactions between age (“12–14,” “15–16,” and “17–18”) and “diagnosis” (AOS, HC). Group comparison in each age subgroup was examined using permutation tests. Error bars indicate standard deviations. AOS, adolescent-onset schizophrenia; HC, healthy controls.
The ANOVA revealed a significant interaction effect between “diagnosis” and “age subgroup” on nodal efficiency (F = 3.74, P = .02) and nodal strength (F = 4.52, P = .01) in the left hippocampus. As shown in figure 3B, post hoc analysis in each age subgroup demonstrated significant and strongest between-group difference in the youngest AOS and HC group with respect to both, nodal efficiency (12–14 y: P = .004, Cohen’s d = 1.63) and nodal strength (12–14 y: P = .003, Cohen’s d = 1.88).
Associations Between Altered Regional Topological Characteristics and Clinical Characteristics
The reduced efficiency identified in the right posterior parahippocampus showed a negative correlation with illness duration within AOS (pi = −.50, p = .009). No significant association between illness duration and nodal efficiency (pi = −.28, p = .252) or nodal strength (pi = −.29, p = .244) in the left hippocampus was found (supplementary figure S3). No significant associations between network metrics in the left hippocampus and PANSS scores were observed in AOS (all P values > .05).
Validate and Additional Control Analysis
To validate results from the graph theoretical approach, network-based statistic (NBS) analysis35 aiming to identify aberrant functional network connectivity in AOS patients was additionally employed. The NBS analysis revealed 2 functional circuits with decreased functional connectivity in the AOS relative to controls, primarily involving frontal, temporal, and parietal regions (P < .05, supplementary figure S4 and supplementary table S2). Importantly, in concordance our findings on regional topological alterations in AOS, the NBS results point to the hippocampus, posterior parahippocampal gyrus, and the precuneus as main pathological hubs characterizing adolescent schizophrenia.
As shown in figure 3A, to further account for the potential impact of outliers, the association between age and nodal efficiency/strength in the left hippocampus, was computed using Shepherd’s pi correlation.34 Considering the potential impact of outlier exclusion in the small sample, we replicated the results in the entire sample using Spearman correlation (supplementary table S3). Second, the motion rotation between 2 groups is not matched very well (P = .03, table 1), though we have strictly controlled the head motion in data preprocessing and subject selection. Additionally, interaction between age and group may also be confounded by gender. We therefore replicated the ANCOVA including motion rotation and gender as confounders. Findings could be replicated (nodal efficiency: F = 9.01, P = .004; nodal strength: F = 10.50, P = .002), arguing against confounding effects of these variables on the present results.
Discussion
The present study combined resting state functional MRI and a graph theory approach to determine large scale brain functional network alterations in AOS. Focusing on medication-naïve first episode adolescent patients allowed for the first time to map aberrant adolescent brain maturation on the level of functional brain topology in schizophrenia. Compared with controls, patients demonstrated impaired large scale integration in highly integrative network hubs, most consistently the hippocampal formation and the precuneus. Notably, the left hippocampus showed opposite age-efficiency associations in the HCs and patients, indicating dysregulated maturational trajectories in adolescent schizophrenia. The present results extend previous findings on dysregulated adolescent brain-anatomical network maturation in schizophrenia10,24 and suggest a particular high vulnerability of the hippocampal functional networks for schizophrenia-associated maturational dysregulations during early adolescence. Together, the findings lend additional support for the neurodevelopmental hypothesis of schizophrenia and contribute to the growing evidence that network topological markers might represent early brain-based biomarkers for the disorder.
Global Measures
Deceased global efficiency in AOS suggests that low efficient communication capacity in brain functional networks critically contributes to the pathophysiology in schizophrenia. In support of putative disruptions in synaptic plasticity in schizophrenia, previous studies examining network topology on the level of white matter organization consistently reported decreased global network efficiency in schizophrenia.36,37 In contrast, previous research utilizing graph theory to determine schizophrenia-associated alterations in functional topology revealed inconsistent results regarding global efficiency in adult-onset schizophrenia, with both, decreased as well as increased efficiency being reported.28,38 Functional network characteristics have shown a high sensitivity to antipsychotic medication14 as well as progressive alterations during the course of the disorder.28 Notably, the only previous study in medication-naïve adult schizophrenia patients reported reduced global efficiency,32 which together with the current findings suggest that reduced efficiency might represent an early marker of the disorder.
In contrast to previous studies,22,23 the present study did not observe associations between brain network properties and symptom severity, possibly reflecting that network-level markers might not directly relate to the current symptom load at the onset of the disorder and might become more pronounced during the course of the disorder.39 However, a previous study in medication-naïve first-episode patients with childhood and adolescent schizophrenia patients23 reported that frontotemporal intrinsic network alterations were modulated by symptom severity, which together with the present results suggest that dysregulated brain network maturation during early adolescence represents a key pathophysiological substrate of the disorder.
Regional Measures
Decreased nodal strength and efficiency in the hippocampal formation and precuneus reflects impaired efficiency to assess information in AOS. Graph theoretical analyses in healthy subjects have consistently identified both, the hippocampal formation and the precuneus as densely connected regions with a central position in overall networks that subserve important roles in global communication.40 The precuneus has been considered a core hub of the default network and rich club organization, both of which have been consistently found to be disturbed in white matter anatomical networks of schizophrenia.37,41 Moreover, in line with their roles as integrative hubs across different networks,37 both the precuneus and the hippocampus critically contribute to highly integrative tasks, such as episodic memory,40 sensory gating,42 and self-processing,43 with marked impairments in these behavioral domains being reported in AOS.44,45 In line with the topological abnormalities, findings from the NBS-based analysis demonstrated that particularly long-range connections of the hippocampus and precuneus with frontal and temporal networks engaged in these behavioral domains were disrupted in patients with AOS. Together, convergent findings point to disconnections in hub regions critically engaged in integrative multimodal functions across brain systems which may lead to deficient communication capacity in the disorder and may contribute to the pronounced symptomatology commonly observed in AOS.46
Age-Maturation Associations
Most importantly, the present study mapped different maturation trajectories with regard to regional efficiency and nodal strength to the hippocampus in patients with adolescent schizophrenia. In adult-onset schizophrenia, alterations in the hippocampal morphology have been reported in first-episode47 and unmedicated patients48 suggesting that alterations in this region might represent an early marker of the disorder. Moreover, altered intrinsic hippocampal functioning has been consistently observed in adult-onset schizophrenia and high-risk populations, including reduced connectivity in unmedicated patients14 and schizotypal personality disorder49 as well as increased regional intrinsic activity in first-degree relatives,50 suggesting that medial temporal lobe pathology might be a core characteristic of schizophrenia spectrum disorders. Previous studies on network maturation during adolescence have reported age-related decline in both between as well as within local network integration,51 including decreasing hippocampal regional network metrics during the course of adolescent brain maturation.52 HCs in the present study displayed an age-related decline in local efficiency and strengths of the hippocampus, possibly reflecting fine-tuning of topological organization to promote adult-level cognitive processing.53 In contrast, patients displayed an age-related increase in these network metrics suggesting profound differences in the normal maturational time-course, which might mirror dysregulated maturational processes, ie, synaptic pruning and myelination as supported by previous studies in early-onset schizophrenia reporting gray matter loss,54 white matter integrity reduction,55 and structural dysconnectivity of the medial temporal lobe.
In line with our hypothesis and previous brain structural findings in patients with schizophrenia24 alterations were most pronounced during early adolescence. Aberrant maturational trajectories specifically affected the left hippocampus, suggesting either pronounced hippocampal functional deficits when the pathological attack occurred during early adolescence. Together with the prefrontal cortex, the medial temporal lobes undergo particularly strong changes during early adolescent brain maturation,56 with previous studies suggesting that an onset of schizophrenia during this period particularly affects temporal and frontal regions57 putatively reflecting a generally increased vulnerability of brain systems that undergo active adolescent maturation, particularly the hippocampus.58
Importantly, aberrant network metrics in the hippocampus were not associated with illness duration, suggesting that alterations in this region might reflect early markers of the disorder. In contrast, local efficiency of the parahippocampal gyrus further declined with illness duration, suggesting a particular high vulnerability of this region to disorder-related deteriorating during the early course of schizophrenia.
Conclusions
The present study illustrated that patients with adolescent-onset schizophrenia had a generally decreased efficiency in intrinsic functional brain networks. Patients additionally demonstrated aberrant age-related changes of hippocampal regional network topological features. Together the present findings support the dysconnectivity and the developmental pathology hypotheses of schizophrenia and suggest a particular high vulnerability of the hippocampal formation possibly reflecting an early marker for the disorder.
Limitations
There are 2 limitations related to the analyses employed in the present study. First, the sample size used in our study was sufficient, the sample size in the maturation analysis within each subgroup was relatively small, which limited the statistical power of our results. Although this type of data with antipsychotic-naïve, first-episode patients with adolescent-onset schizophrenia was collected extremely difficult, a larger sample size needs to be replicated. Second, to limit the number of comparisons for the determination of age-related changes in AOS a 2-step approach was employed and the examination of age-associations was limited to regions showing significant between group differences. Although this increased the sensitivity to detect different age-associations, this approach does not allow to draw conclusions regarding altered development of regions outside of this regionally restricted analyses.
Funding
The work was supported by 863 project (2015AA020505) and National Natural Science Foundation of China (61533006).
Supplementary Material
Acknowledgments
According to the informed consent subjects have provided, sharing of data and subject information is legally restricted. All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1. Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Mol Psychiatry. 2005;10:434–449. [DOI] [PubMed] [Google Scholar]
- 2. Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17:1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. [DOI] [PubMed] [Google Scholar]
- 4. Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. [DOI] [PubMed] [Google Scholar]
- 5. Lo CY, Su TW, Huang CC et al. Randomization and resilience of brain functional networks as systems-level endophenotypes of schizophrenia. Proc Natl Acad Sci U S A. 2015;112:9123–9128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Feinberg I. Schizophrenia: caused by a fault in programmed synaptic elimination during adolescence?J Psychiatr Res. 1982;17:319–334. [DOI] [PubMed] [Google Scholar]
- 7. Fornito A, Zalesky A, Pantelis C, Bullmore ET. Schizophrenia, neuroimaging and connectomics. Neuroimage. 2012;62:2296–2314. [DOI] [PubMed] [Google Scholar]
- 8. van den Heuvel MP, Sporns O. Network hubs in the human brain. Trends Cogn Sci. 2013;17:683–696. [DOI] [PubMed] [Google Scholar]
- 9. Zalesky A, Fornito A, Seal ML et al. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zalesky A, Pantelis C, Cropley V et al. Delayed development of brain connectivity in adolescents with schizophrenia and their unaffected siblings. JAMA Psychiatry. 2015;72:900–908. [DOI] [PubMed] [Google Scholar]
- 11. Kochunov P, Rowland LM, Fieremans E et al. Diffusion-weighted imaging uncovers likely sources of processing-speed deficits in schizophrenia. Proc Natl Acad Sci U S A. 2016;113:13504–13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kochunov P, Ganjgahi H, Winkler A et al. Heterochronicity of white matter development and aging explains regional patient control differences in schizophrenia. Hum Brain Mapp. 2016;37:4673–4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sheffield JM, Repovs G, Harms MP et al. Evidence for accelerated decline of functional brain network efficiency in schizophrenia. Schizophr Bull. 2016;42:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kraguljac NV, White DM, Hadley N et al. Aberrant hippocampal connectivity in unmedicated patients with schizophrenia and effects of antipsychotic medication: a longitudinal resting state functional MRI study. Schizophr Bull. 2016;42:1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Friedman JI, Tang C, Carpenter D et al. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. Am J Psychiatry. 2008;165:1024–1032. [DOI] [PubMed] [Google Scholar]
- 16. Douaud G, Smith S, Jenkinson M et al. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. [DOI] [PubMed] [Google Scholar]
- 17. Cao M, Huang H, Peng Y, Dong Q, He Y. Toward developmental connectomics of the human brain. Front Neuroanat. 2016;10:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence?Nat Rev Neurosci. 2008;9:947–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Frazier JA, McClellan J, Findling RL et al. Treatment of early-onset schizophrenia spectrum disorders (TEOSS): demographic and clinical characteristics. J Am Acad Child Adolesc Psychiatry. 2007;46:979–988. [DOI] [PubMed] [Google Scholar]
- 20. Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S. A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biol Psychiatry. 2008;63:519–523. [DOI] [PubMed] [Google Scholar]
- 21. Moran ME, Luscher ZI, McAdams H et al. Comparing fractional anisotropy in patients with childhood-onset schizophrenia, their healthy siblings, and normal volunteers through DTI. Schizophr Bull. 2015;41:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zheng J, Zhang Y, Guo X et al. Disrupted amplitude of low-frequency fluctuations in antipsychotic-naïve adolescents with early-onset schizophrenia. Psychiatry Res Neuroimaging. 2016;249:20–26. [DOI] [PubMed] [Google Scholar]
- 23. Yang Z, Xu Y, Xu T et al. Brain network informed subject community detection in early-onset schizophrenia. Sci Rep. 2014;4:5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Douaud G, Mackay C, Andersson J et al. Schizophrenia delays and alters maturation of the brain in adolescence. Brain. 2009;132:2437–2448. [DOI] [PubMed] [Google Scholar]
- 25. Kruskal JB. On the shortest spanning subtree of a graph and the traveling salesman problem. Proc Am Math Soc. 1956;7:48–50. [Google Scholar]
- 26. Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu Y, Liang M, Zhou Y et al. Disrupted small-world networks in schizophrenia. Brain. 2008;131:945–961. [DOI] [PubMed] [Google Scholar]
- 29. Liu F, Wang Y, Li M et al. Dynamic functional network connectivity in idiopathic generalized epilepsy with generalized tonic-clonic seizure. Hum Brain Mapp. 2017;38:957–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Di Martino A, Yan C, Li Q et al. The autism brain imaging data exchange: towards a large-scale evaluation of the intrinsic brain architecture in autism. Mol Psychiatry. 2013;19:659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. van den Heuvel MP, Fornito A. Brain networks in schizophrenia. Neuropsychol Rev. 2014;24:32–48. [DOI] [PubMed] [Google Scholar]
- 32. Hadley JA, Kraguljac NV, White DM, Ver Hoef L, Tabora J, Lahti AC. Change in brain network topology as a function of treatment response in schizophrenia: a longitudinal resting-state fMRI study using graph theory. NPJ Schizophr. 2016;2:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- 34. Schwarzkopf DS, De Haas B, Rees G. Better ways to improve standards in brain-behavior correlation analysis. Front Hum Neurosci. 2012;6:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zalesky A, Fornito A, Bullmore ET. Network-based statistic: identifying differences in brain networks. Neuroimage. 2010;53:1197–1207. [DOI] [PubMed] [Google Scholar]
- 36. van den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, Hulshoff Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci. 2010;30:15915–15926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. van den Heuvel MP, Sporns O, Collin G et al. Abnormal rich club organization and functional brain dynamics in schizophrenia rich club connectivity in schizophrenia. JAMA Psychiatry. 2013;70:783–792. [DOI] [PubMed] [Google Scholar]
- 38. Alexander-Bloch AF, Gogtay N, Meunier D et al. Disrupted modularity and local connectivity of brain functional networks in childhood-onset schizophrenia. Front Syst Neurosci. 2010;4:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gong Q, Hu X, Petterssonyeo W et al. Network-level dysconnectivity in drug-naïve first-episode psychosis: dissociating transdiagnostic and diagnosis-specific alterations. Neuropsychopharmacology. 2016;42:933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Geib BR, Stanley ML, Dennis NA, Woldorff MG, Cabeza R. From hippocampus to whole-brain: the role of integrative processing in episodic memory retrieval. Hum Brain Mapp. 2017;38:2242–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hagmann P, Cammoun L, Gigandet X et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Javitt DC, Freedman R. Sensory processing dysfunction in the personal experience and neuronal machinery of schizophrenia. Am J Psychiatry. 2015;172:17–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. [DOI] [PubMed] [Google Scholar]
- 44. Kumari V, Soni W, Mathew VM, Sharma T. Prepulse inhibition of the startle response in men with schizophrenia: effects of age of onset of illness, symptoms, and medication. Arch Gen Psychiatry. 2000;57:609–614. [DOI] [PubMed] [Google Scholar]
- 45. Frangou S. Cognitive function in early onset schizophrenia: a selective review. Front Hum Neurosci. 2010;3:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kumra S, Ashtari M, McMeniman M et al. Reduced frontal white matter integrity in early-onset schizophrenia: a preliminary study. Biol Psychiatry. 2004;55:1138–1145. [DOI] [PubMed] [Google Scholar]
- 47. Velakoulis D, Pantelis C, McGorry PD et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia: a high-resolution magnetic resonance imaging study. Arch Gen Psychiatry. 1999;56:133–141. [DOI] [PubMed] [Google Scholar]
- 48. Kraguljac NV, White DM, Reid MA, Lahti AC. Increased hippocampal glutamate and volumetric deficits in unmedicated patients with schizophrenia. JAMA Psychiatry. 2013;70:1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhu Y, Tang Y, Zhang T et al. Reduced functional connectivity between bilateral precuneus and contralateral parahippocampus in schizotypal personality disorder. BMC Psychiatry. 2017;17:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tang Y, Chen K, Zhou Y et al. Neural activity changes in unaffected children of patients with schizophrenia: a resting-state fMRI study. Schizophr Res. 2015;168:360–365. [DOI] [PubMed] [Google Scholar]
- 51. Gu S, Satterthwaite TD, Medaglia JD et al. Emergence of system roles in normative neurodevelopment. Proc Natl Acad Sci U S A. 2015;112:13681–13686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Supekar K, Musen M, Menon V. Development of large-scale functional brain networks in children. PLoS Biol. 2009;7:1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marek S, Hwang K, Foran W, Hallquist MN, Luna B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015;13:e1002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Thompson PM, Vidal C, Giedd JN et al. Mapping adolescent brain change reveals dynamic wave of accelerated gray matter loss in very early-onset schizophrenia. Proc Natl Acad Sci U S A. 2001;98:11650–11655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. White T, Kendi AT, Lehéricy S et al. Disruption of hippocampal connectivity in children and adolescents with schizophrenia—a voxel-based diffusion tensor imaging study. Schizophr Res. 2007;90:302–307. [DOI] [PubMed] [Google Scholar]
- 56. Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: what have we learned and where are we going?Neuron. 2010;67:728–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Pinacamacho L, ReyMejías ÁD, Janssen J et al. Age at first episode modulates diagnosis-related structural brain abnormalities in psychosis. Schizophr Bull. 2016;42:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hueston CM, Cryan JF, Nolan YM. Stress and adolescent hippocampal neurogenesis: diet and exercise as cognitive modulators. Transl Psychiatry. 2017;7:e1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.