Abstract
Evidence from several lines of research suggests decreased dopamine release in the prefrontal cortex as the neurochemical correlates of cognitive deficits in schizophrenia (SCZ). However, in vivo examination of cortical hypodopaminergia using positron emission tomography (PET) during cognitive task performance in SCZ remains to be investigated. We examined dopamine release in anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC), using PET while participants were performing a cognitive task. Thirteen drug-free patients with SCZ and 13 healthy volunteers (HV) matched for age and sex participated in the study. Data were acquired between 2011 and 2015. Two PET scans with [11C]FLB 457 were acquired while the participants were performing the Wisconsin Card Sorting Test (WCST) and a sensorimotor control task (SMCT). A magnetic resonance image was acquired for anatomical delineation. Differences in cortical dopamine release between SCZ and HV, indexed as percentage change in binding potential between WCST and SMCT (ΔBPND), were calculated in ACC and DLPFC. We observed significant differences in the ΔBPND in ACC (HV = 4.40 ± 6.00; SCZ = −11.48 ± 15.08; t = 3.52; P = .003) and a trend-level difference in ΔBPND in DLPFC (HV = −0.58 ± 8.45; SCZ = −7.79 ± 11.28; t = 1.84; P = .079), suggesting dopamine depletion in cortical brain regions in patients with SCZ while performing a cognitive task. These results provide the first in vivo evidence for reduced dopamine release or even dopamine depletion while performing cognitive task in ACC and DLPFC in patients with SCZ. The present results provide support for the frontal hypodopaminergia hypothesis of cognitive symptoms in SCZ.
Keywords: psychosis, cognition, PET, hypodopaminergia
Introduction
Cognitive deficits are a core feature of schizophrenia (SCZ), predict later functional status,1–6 and can be identified well before the onset of psychotic symptoms.7 Impaired executive functions are among the most widely observed and consistent findings across cognitive studies in patients with SCZ.8–11 Executive functions rely heavily on frontal lobes, importantly anterior cingulate cortex (ACC) and dorsolateral prefrontal cortex (DLPFC).12 Several functional neuroimaging studies indicate decreased frontal lobe activity in SCZ, which led to the hypofrontality hypothesis.13–16 Converging evidence from several lines of research in both animals and humans supported the concept of cortical hypodopaminergia as the underlying neurochemical abnormality in cognitive deficits.17–19
Although positron emission tomography (PET) can measure dopamine (DA) transmission in vivo, a few studies have directly examined cortical dopaminergic abnormalities in SCZ. Although earlier studies had examined DA D1 receptor availability in SCZ,20–23, a more recent study investigated DA release in frontal cortex of SCZ by using [11C]FLB 457, and the amphetamine challenge paradigm,24 reported blunted DA release in SCZ as compared to matched healthy volunteers (HV).
PET radioligand displacement of the D2 receptor antagonist [11C]raclopride by DA following acute administration of amphetamine has been widely examined and validated in the last 2 decades.25 However, studies with [11C]raclopride are restricted to the striatum. Cortical regions have a relatively low density of D2 receptors26 and, accordingly, a suitable PET radiotracer should have very high affinity. [11C]FLB 457 represents a very high affinity D2/D3 receptor ligand with desirable properties to study cortical D2 receptors.27 It has proven sensitive to competition from endogenous DA, and amphetamine-induced DA release results in a significant decrease in [11C]FLB 457 BPND (binding potential of the radiotracer with respect to the nondisplaceable compartment in the brain) in cortical regions.28 A linear relationship between magnitude of DA release, measured using microdialysis, and magnitude of reduction in [11C]FLB 457 BPND, measured using PET, has been established, further validating the use of [11C]FLB 457 to measure DA release in prefrontal cortex.29 A few studies have raised concerns about the quantitation methods in view of specific binding in cerebellum, change in cerebellum distribution volume with aripiprazole, and [11C]FLB 457 may not fit the 1-tissue compartment model.30,31 Despite these limitations, [11C]FLB 457 is superior to existing cortical D2 receptor radioligands such as [11C]fallypride, as it displays a higher signal-to-noise ratio in cortical areas such as ACC and DLPFC.28
It has also been shown that [11C]FLB 457 is sensitive to competition from endogenous DA release following a cognitive challenge in HV32,33 as well as patients with Parkinson’s disease.34,35 However, there are no in vivo studies examining DA release in cortical brain regions of SCZ during a cognitive challenge. This experimental design would provide direct support for the cortical hypodopaminergia hypothesis, which may explain the cognitive deficits seen in SCZ. Accordingly, in this study, we measured DA release in ACC and DLPFC in a unique sample of drug-free patients with SCZ and matched HV using [11C]FLB 457 while performing an executive function test—the Wisconsin Card Sorting Test (WCST)—that has been well documented to be impaired in SCZ.11 We hypothesized that cognitive task–induced cortical DA release in ACC and DLPFC will be decreased in SCZ compared with HV.
Methodology
Subjects
In this study, 14 patients with SCZ and 14 HV were initially enrolled and scanned. One patient and one control were excluded from the analysis because of excessive head motion that could not be corrected. Hence, the total number of individuals in the study was 26, with 13 participants in each group. All patients were antipsychotic free, whereas 5 of them were antipsychotic naïve. All patients had a diagnosis of SCZ, as determined using a structured clinical interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) Axis I disorders,36 and none had a concurrent axis I disorder. None of the HV had an axis I psychiatric disorder, as determined with structured clinical interview for DSM-IV Axis I disorders, or first-degree relatives with a major psychiatric disorder. Exclusion criteria included current substance dependence/abuse or a positive urine drug screen, pregnancy or current breast feeding, a clinically significant physical illness, the presence of metal implants in the body precluding a magnetic resonance imaging (MRI) scan, and claustrophobia.
Assessments
Clinical severity of psychotic symptoms was documented using the Positive and Negative Syndrome Scale,37 which is designed to measure severity of positive and negative symptoms, in addition to the Scale for the Assessment of Negative Symptoms,38 which is a sensitive instrument for the measurement of negative symptoms. All subjects were administered the Clinical Global Impression Scale,39 a standardized clinician-rated assessment of the subject’s current illness state, and the Global Assessment of Functioning scale,40 which rates level of functioning based on overall psychological, social, and occupational functioning. The Calgary Depression Scale for SCZ41 quantified depressive symptoms, the Fagerström Test for Nicotine Dependence determined severity of nicotine dependence,42 and the Snaith–Hamilton Pleasure Scale43 and Marin’s Apathy Evaluation Scale,44 determined apathy. All patients underwent neuropsychological assessment using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS).45 The study was approved by the Research Ethics Board of the Centre for Addiction and Mental Health (CAMH), University of Toronto, and all participants gave written informed consent after receiving a description of the study procedure.
Cognitive Challenge
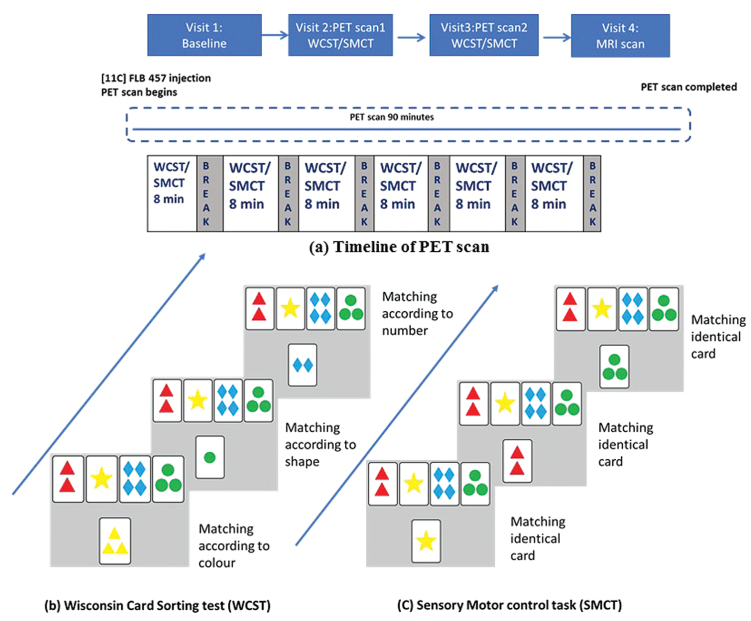
We used the WCST46 and a control task, the sensorimotor control task (SMCT), both of which have been validated in previous imaging studies.33,47,48 The tasks were displayed via video eyewear (VR920; Vuzix Corporation) while participants laid on the scanning table. Briefly, in the WCST 4 reference cards are displayed in a row at the top of a computer screen. On each classification trial, a new test card is presented in the middle of the screen below the reference cards and the subject must match the test card to 1 of 4 reference cards by pressing 1 of 4 buttons. The rule for classification is based on the shared single attribute (color, shape, or number). Each classification trial is followed by feedback (ie, correct or incorrect). The rule for classification changes after completion of a category and the subject is expected to learn the change in rule based on the feedback provided. In the SMCT, subjects are asked to match the test card with the identical reference card without any set shift.33 Hence, the SMCT served as control for motor movements and attention components of the WCST but isolating the executive function of set shifting. In both experiments, the task was started 8–10 min before the tracer injection and subjects performed six 8- to–10-min task segments while being scanned. A break of 2–3 min was given between the segments. Each segment consisted of 192 trials of the task, either SMCT or WCST. Both groups completed 6 such segments in each scan. The schematic representation of study design is given in figure 1. Over different days, all subjects underwent 2 PET scans, one while doing the WCST and the other while doing the SMCT (figure 1). The order of presentation of WCST and SMCT was counterbalanced.
Fig. 1.
Schematic representation of study design: (a) Representation of positron emission tomography (PET) scan timeline. Subjects underwent 2 PET scans, one while performing the Wisconsin Card Sorting Test (WCST) and the other while performing sensorimotor control task (SMCT) visit. (b) Representative trial of the WCST. (c) Representative trial of the SMCT.
PET Image Acquisition
PET scans were performed using a high-resolution PET–computed tomography (Siemens-BioGraph HiRez XVI; Siemens Molecular Imaging), which measures radioactivity in 81 brain sections with a thickness of 2.0 mm each. A custom-fitted thermoplastic mask was made for each subject and used with a head fixation system during PET measurements to minimize head movement. PET data were acquired for 90 min following an intravenous bolus injection of [11C]FLB 457. The images were reconstructed using 2-dimesional filter back-projection algorithms with a ramp filter at Nyquist cutoff frequency.
MRI Acquisition
All subjects underwent a structural MRI with the following parameters (imaging mode, 3-dimesional; sagittal plane; fast spoiled gradient-echo sequence; 8-channel head coil; repetition time = 6.7 s; echo time = 3 s; flip angle = 8°; frequency = 256; slice thickness = 0.9 mm). Magnetic resonance images were acquired using a 3-T MRI scanner at the CAMH. These images were used for delineation of individual region of interest (ROI) after coregistering with the PET image.
PET Data Analysis
PET data analysis was carried out using an in-house automated analysis software Region of Mental Interest (ROMI) for semiautomated generation of ROIs, thus completely overcoming manual ROI drawing bias.49 ROMI performs the following steps: (1) a standard brain template with a set of predefined ROIs is transformed to match with the individual high-resolution MRI scan; (2) the ROIs from the transformed template are refined based on the gray matter probability of voxels in the individual MRI; and (3) the individual MRI is registered to the PET images so that the individual refined ROIs are transformed to the PET image space to allow the time-activity curves (TACs) generation from each ROI. TACs from the ROIs, ACC, and DLPFC were obtained from the dynamic [11C]FLB images. ROMI uses validated, previously established methods to delineate the ROIs of our interest—DLPFC and ACC.49,50 Briefly, the prefrontal cortex was first sampled from the most rostral plane to the plane corresponding to the rostral boundary of the genu of the corpus callosum. Then, a line corresponding to anterior commissure (AC) and posterior commissure (PC) was drawn and regions dorsal to the AC–PC plane were divided into lateral region (DLPFC) and medial regions. The DLPFC consisted of dorsal part of the superior frontal gyrus, middle frontal gyrus, and triangular part of the inferior frontal gyrus. Medial regions included medial prefrontal cortex and ACC, dorsal and ventral to the cingulate gyrus, respectively. The ACC, hence, corresponds to perigenual ACC. We also calculated TACs for other extrastriatal brain regions, including occipital cortex, temporal cortex, inferior parietal cortex, and thalamus.
To obtain a quantitative estimate of binding, the TAC was analyzed using the simplified reference tissue model (SRTM)51 (accomplished under in-house software fMOD). The SRTM uses a within-brain reference region, cerebellum in this case, instead of the arterial input function. It provides an estimate of the BPND of the radiotracer, which is proportional to the more fundamental parameters of receptor number (Bmax) and affinity (Kd) [BPND ≈ Bmax/Kd]. This method has been validated and is commonly used with [11C]FLB 457.28,52,53 Though a few studies suggest small specific binding in cerebellum with [11C]FLB 457,30,54 there was no displaceable binding in cerebellum following amphetamine and methylphenidate challenge,28,55 and previous studies with [11C]FLB 457 have successfully used SRTM with cerebellum as the reference region.33,56,57 Further, a recent study showed that SRTM is a valid modeling approach to measure ΔBPND with [11C]FLB 457.58 Right and left ROIs were averaged together and used to derive binding potential of the radiotracer.
Statistical Analysis
We used independent t tests to examine our primary hypothesis of task-induced [11C]FLB 457 ΔBPND between SCZ and HV. Percentage change in BPND (NDΔBPND) is defined as follows:
Considering our main outcome measure ΔBPND in ROIs, DLPFC, and ACC, we considered a Bonferroni corrected α = .025 as significant. Findings of P value less than .1 were considered trend-level difference. Correlation analyses were used to test the association between ΔBPND and cognitive measures (performance measures on WCST and RBANS subscores) as well as psychopathology scores in those brain regions that were significantly different. Considering the exploratory nature of the correlation analysis, we did not control for the multiple comparisons and considered results less than 2-tailed conventional α = .05 as significant. To evaluate the potential influence of individual data points, we calculated the Cook’s distance to estimate influence of each observation on the fitted response values for those correlations that were significant.
Results
Demographics and PET Scan Parameters
There was no significant difference between patients with SCZ and HV in age and sex distribution. All subjects performed SMCT and WCST during the scans successfully. We acquired a total of 52 PET scans, with equal number of patients with SCZ (n = 13) and HV (n = 13). Details of demographic information, clinical scales, injected mass, injected activity, and specific activity of radioligand in both scans are given in table 1. There was no significant group difference in any of the PET scan parameters.
Table 1.
Participant’s Demographic and Clinical Characteristics
| SCZ | HV | t/χ2 | P | |
|---|---|---|---|---|
| Age | 26.61 ± 6.76 | 26.38 ± 4.78 | 0.10 | .92 |
| Sex | ||||
| Male | 9 | 6 | 1.41 | .42 |
| Female | 4 | 7 | ||
| Smoking status | ||||
| Nonsmoker | 8 | 11 | 1.75 | .37 |
| Smoker | 5 | 2 | ||
| Cigarettes/day | 3.38 ± 5.85 | 0.15 ± 0.37 | 2.01 | .07 |
| Years of education | 13.07 ± 1.65 | 15.53 ± 1.94 | 3.47 | .002 |
| Age at onset | 21.58 ± 6.77 | — | — | — |
| Duration of illness (months) | 59.91 ± 41.03 | — | — | — |
| Duration of untreated psychosis (months) | 31.00 ± 26.85 | — | — | — |
| Antipsychotic-free duration (months) | 26.00 ± 18.84 | — | — | — |
| Antipsychotic naïve | 5 (38.46%) | — | — | — |
| PANSS | ||||
| Positive | 18.76 ± 4.74 | — | — | — |
| Negative | 14.84 ± 7.05 | — | — | — |
| General psychopathology | 28.61 ± 11.55 | — | — | — |
| Total | 62.23 ± 19.40 | — | — | — |
| SANS | 27.84 ± 12.83 | — | — | — |
| GAF | 42.50 ± 12.63 | — | — | — |
| CGI | 4.00 ± 0.70 | — | — | — |
| Calgary Depression Scale | 4.46 ± 3.25 | — | — | — |
| Snaith–Hamilton Pleasure Scale | 13.53 ± 2.40 | — | — | — |
| Apathy Evaluation Scale | 21.46 ± 7.21 | — | — | — |
| RBANS total score | 83.30 ± 13.96 | 94.15 ± 13.85 | 1.98 | .05 |
| RBANS | ||||
| Immediate memory | 87.92 ± 23.23 | 93.53 ± 12.38 | 0.76 | .44 |
| Visuospatial ability | 82.61 ± 13.48 | 96.92 ± 19.92 | 2.14 | .04 |
| Language | 86.69 ± 15.67 | 96.30 ± 24.75 | 1.18 | .24 |
| Attention | 89.61 ± 20.41 | 95.30 ± 12.43 | 0.85 | .39 |
| Delayed memory | 89.38 ± 16.40 | 96.53 ± 7.90 | 1.41 | .17 |
| WCST | ||||
| Categories completed | 6.52 ± 2.44 | 7.16 ± 2.67 | 0.63 | .53 |
| Average correct responses | 149.75 ± 26.54 | 152.12 ± 10.98 | 0.29 | .76 |
| Errors | 29.27 ± 18.05 | 25.07 ± 15.01 | 0.64 | .52 |
| Perseverative responses | 48.91 ± 9.05 | 51.76 ± 9.83 | 0.76 | .45 |
| Perseverative errors | 14.53 ± 3.16 | 14.17 ± 6.97 | 0.17 | .86 |
| Mass injected | ||||
| SMCT | 1.26 ± 0.38 | 1.42 ± 0.42 | 1.00 | .32 |
| WCST | 1.28 ± 0.53 | 1.45 ± 0.52 | 0.83 | .43 |
| Specific activity | ||||
| SMCT | 3156.52 ± 706.03 | 2849.48 ± 939.75 | 0.94 | .35 |
| WCST | 3284.98 ± 999.45 | 2775.59 ± 815.62 | 1.42 | .16 |
| Amount injected | ||||
| SMCT | 9.83 ± 0.68 | 10.05 ± 0.77 | 0.74 | .46 |
| WCST | 10.14 ± 0.52 | 9.83 ± 0.52 | 1.47 | .15 |
Note: PANSS, Positive and Negative Symptoms Scale; SANS, Scale for Assessment of Negative Symptoms; GAF, Global Assessment of functioning; CGI, Clinical Global Impression; SCZ, schizophrenia patients; HV, healthy volunteers; WCST, Wisconsin Card Sorting Task; SMCT, sensorimotor control task; t, independent t test; P significant at <.05 (2-tailed).
PET Results
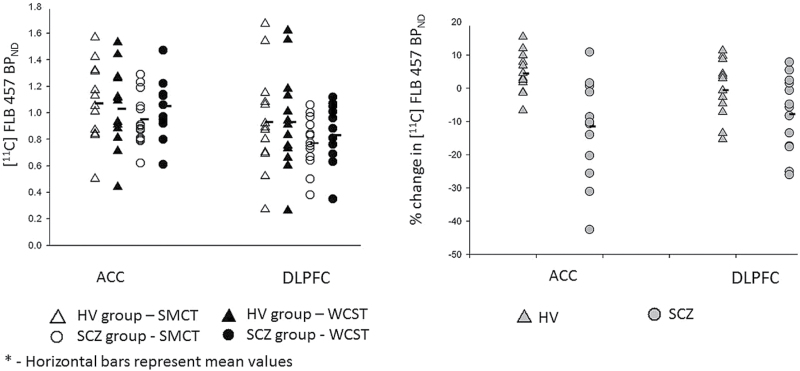
There was a significant difference between groups in ΔBPND of [11C]FLB 457, defined as percentage change in BPND, in ACC (t = 3.52; P = .003; df = 24), and a trend-level difference in DLPFC (t = 1.84; P = .079; df = 24). In both regions, HV (DLPFC = −0.58 ± 8.45; ACC = 4.40 ± 6.00) had higher [11C]FLB 457 ΔBPND than SCZ group (DLPFC = −7.79 ± 11.28; ACC = −11.48 ± 15.08), suggesting more DA release during the cognitive challenge as compared with the patients with SCZ, who instead showed reductions in DA release. There was no difference between the groups in other extrastriatal brain regions (table 2). There was no difference between SCZ and HV groups on SMCT BPND in any of the cortical ROIs. [11C]FLB 457 BPND in ROIs of SCZ and HV groups across tasks are reported in tables 2 and 3 and shown in figure 2.
Table 2.
[11C]FLB 457 BPNDs and ΔBPND in ROIs of SCZ and HV
| SCZ | HV | |||||||
|---|---|---|---|---|---|---|---|---|
| SMCT | WCST | ΔBPNDa | Paired t –test | SMCT | WCST | ΔBPNDa | Paired t–test | |
| ACC BPND | 0.95 ± 0.19 | 1.05 ± 0.21 | −11.48 ± 15.08 | t = 2.76; P = .01 | 1.07 ± 0.28 | 1.03 ± 0.30 | 4.40 ± 6.00 | t = 2.73; P = .01 |
| DLPFC BPND | 0.77 ± 0.19 | 0.83 ± 0.21 | −7.79 ± 11.28 | t = 2.86; P = .01 | 0.93 ± 0.38 | 0.93 ± 0.37 | −0.58 ± 8.45 | t = 0.04; P = .96 |
| Occipital cortex BPND | 0.82 ± 0.19 | 0.84 ± 0.23 | −1.83 ± 12.48 | t = 0.60; P = .55 | 0.92 ± 0.48 | 0.92 ± 0.52 | 3.17 ± 15.57 | t = 0.13; P = .89 |
| Inferior parietal cortex BPND | 0.87 ± 0.18 | 0.90 ± 0.21 | −3.35 ± 13.42 | t = 0.96; P = .35 | 1.58 ± 2.00 | 0.98 ± 0.44 | 6.06 ± 28.77 | t = 0.99; P = .34 |
| Temporal cortex BPND | 1.18 ± 0.28 | 1.14 ± 0.22 | 1.44 ± 15.58 | t = 0.63; P = .53 | 1.31 ± 0.57 | 1.30 ± 0.57 | 0.03 ± 9.01 | t = 0.54; P = .59 |
| Thalamus BPND | 3.40 ± 0.77 | 3.63 ± 0.71 | −8.73 ± 19.50 | t =1.42; P = .17 | 3.74 ± 1.28 | 3.94 ± 1.11 | −3.28 ± 18.36 | t = 1.01; P = .33 |
| Mass injected (µg) | 1.26 ± 0.38 | 1.28 ± 0.53 | t = 0.10; P=.92 | 1.42 ± 0.42 | 1.45 ± 0.52 | t = 0.14; P = .88 | ||
| Specific activity (Ci/mmol) |
3156.52 ± 706.03 | 3284.98 ± 999.45 | t =0.38; P = .70 | 2849.48 ± 939.75 | 2775.59 ± 815.62 | t = 0.23; P = .82 | ||
Note: Abbreviations are explained in the first footnote to table 1; DLPFC, dorsolateral prefrontal cortex; ACC, anterior cingulate cortex; P significant at <.05.
aPositive value in ΔBPND indicates decrease in the BPND during WCST compared with BPND during SMCT, which in turn suggests increased dopamine release during WCST compared with SMCT. On the other hand, negative value in ΔBPND indicates increase in the BPND during WCST compared with BPND during SMCT, which in turn suggests dopamine depletion.
Table 3.
Difference Between SCZ and HV [11C]FLB 457 ΔBPND in ROIs
| SCZ (ΔBPND) | HV (ΔBPND) | t | P | |
|---|---|---|---|---|
| DLPFC | −7.79 ± 11.28 | −0.58 ± 8.45 | 1.84 | .079 |
| ACC | −11.48 ± 15.08 | 4.40 ± 6.00 | 3.52 | .003a |
| Occipital cortex | −1.83 ± 12.48 | 3.17 ± 15.57 | 0.90 | .37 |
| Inferior Parietal cortex | −3.35 ± 13.42 | 6.06 ± 28.77 | 1.06 | .30 |
| Temporal cortex | 1.44 ± 15.58 | 0.03 ± 9.01 | 0.28 | .78 |
| Thalamus | −8.73 ± 19.50 | −3.28 ± 18.36 | 0.71 | .48 |
P significant at <.05; t, independent t test.
Note: Abbreviations are explained in the footnotes to tables 1 and 2.
aPositive value in ΔBPND indicates decrease in the BPND during WCST compared with BPND during SMCT which in turn suggests increased dopamine release during WCST compared with SMCT. On the other hand, negative value in ΔBPND indicate increase in the BPND during WCST compared with BPND during SMCT which in turn suggests dopamine depletion.
Fig. 2.
[11C]FLB 457 BPND in sensorimotor control task (SMCT) and Wisconsin Card Sorting Test (WCST) between groups (left); [11C]FLB 457 positron emission tomography (PET) ΔBPND with cognitive task in dorsolateral prefrontal cortex (DLPFC) and anterior cingulate cortex (ACC) (right).
Associations With PET Measures and Cognitive Functions and Clinical Symptoms
There was no difference in the number of trials completed between WCST and SMCT. Though patients performed more poorly compared to controls on WCST, the difference was not significant. Details of WCST performance are presented in table 1. There was also no difference between groups in the SMCT performance; patients performed with a mean accuracy of 96.95% ± 3.45% and controls performed with a mean accuracy of 97.88% ± 1.81% in the control task (t = 0.82; P = .40). We performed exploratory analyses between BPND of individual ROI and task performance, clinical assessments, and cognitive measurements. Although some correlations were significant, there was potential influence of individual data points and results were not significant after leave-one-out analysis. In addition, considering the exploratory nature of the correlational analysis, it was not corrected for multiple comparisons. Hence, these results need to be considered preliminary. The results of the correlation are given in Supplementary material.
Discussion
In the face of a cognitive challenge, our results suggest depletion of DA in the ACC of drug-free patients with SCZ as compared to HV, who had increased DA release. These findings provide further support for the cortical hypodopaminergia hypothesis in SCZ. To the best of our knowledge, no study has examined prefrontal cortical DA release during a cognitive challenge task in patients with SCZ before.
Though a large body of literature has documented striatal DA abnormalities in SCZ, very few PET studies have specifically evaluated cortical DA release abnormalities in SCZ, whereas no study investigated cortical DA release during a cognitive challenge performance. As cortical regions have a relatively low density of D2 receptors,26 a suitable PET radiotracer for cortical D2 receptors needs to demonstrate a very high affinity for D2 receptors. Thus, in this study, which used a 2-scan paradigm with a cognitive challenge, we chose [11C]FLB 457 to examine cortical D2 receptors. Our findings are consistent with the only available study, which examined DA release in patients with SCZ with an amphetamine challenge and reported blunted DA release in cortical regions.24
Previous neuroimaging studies have reported ACC as a key region associated with executive functions; lesions of the ACC are associated with impaired functioning.59,60 In addition, previous PET studies have also reported WCST performance to be associated with ACC DA33,61; however, this has not been directly investigated during a cognitive challenge. The absence of difference between the groups in displacement of [11C]FLB 457 in other brain regions (table 2) further supports the critical role of ACC. Moreover, the difference was seen in WCST but not in SMCT, suggesting the role of prefrontal cortex DA in executive functions. However, we did not find significant association between the performance scores and the DA release in prefrontal cortex. Although this may suggest the absence of a relation between cortical hypodopaminergia and cognitive deficits in our population, one cannot rule out the possibility of other compensatory mechanisms in the brain to compensate for the hypodopaminergia. Previous cognitive remediation treatment studies in SCZ have reported compensatory increase in activity of inferior parietal lobule, precuneus, and occipital cortex associated with improved performance in executive function.62–64 Strengthening the preserved cognitive reserve in compensatory structures and promoting new connections are proposed as possible mechanisms for these changes.65 As our study population did not have significant deficits compared to HV, it is possible that alternate strategies might have compensated for the DA reductions observed in prefrontal cortex.
Accurate quantification of [11C]FLB 457 has been challenging in view of recent findings. Some studies have reported challenges in using SRTM for quantification of [11C]FLB 457 in view of specific binding in cerebellum and change in cerebellum distribution volume by the D2 partial agonist, aripiprazole.30,31 However, other studies have supported the use of SRTM for extrastriatal regions with cerebellum as reference tissue.52,53,66 Concerns have also been raised as [11C]FLB 457 does not fit the 1-tissue compartment model that is ideal for SRTM. However, a recent study compared the 2-tissue compartment model (2TCM) and SRTM with cerebellum as reference tissue to estimate the ΔBPND of [11C]FLB 457 in extrastriatal regions. SRTM was more reliable than 2TCM for detecting ΔBPND after an amphetamine challenge and had lower relative standard error compared with 2TCM. In addition, there was no change in cerebellum distribution volume (VT) and VT/fp before and after amphetamine challenge, further supporting the use of cerebellum as reference tissue in this type of challenge experiment.58 Although SRTM may lead to underestimation of BPND compared with arterial input–based models,67 this underestimation applies to both SMCT and WCST such that the bias cancels out when computing radioligand ΔBPND, our main outcome measure (similar to amphetamine challenge studies58). Finally, as the study involved a 2-scan design, which were done on 2 separate days, within a short time frame, arterial blood sampling would have required 2 arterial lines to the nondominant hand (as the dominant hand was holding the response mouse), which would have been impossible to conduct. Considering these caveats and the additional benefit of using reference tissue models in patients, from a feasibility perspective, we chose to model our data with SRTM to also decrease variability30,68 and increase feasibility of a 2-scan paradigm with [11C]FLB 457 involving unmedicated patients with SCZ. Importantly, the absence of difference in other brain regions (table 2) not involved in executive functions suggests these methodological issues to have minimal influence on the present findings.
We used a well-validated cognitive challenge in the study. The WCST has been used in a number of neuroimaging studies and functional MRI studies have shown the involvement of cortical brain regions in particular ACC in the performance of WCST.59 Also, a previous study has shown the feasibility of using WCST and SMCT to measure DA release using [11C]FLB 457.33,34 Although one may object that the control task is not a neutral scan as it involved motor function, the use of SMCT cancels out the possible sustained attention and motor task–induced DA release,69 as participants performed similar motor activities in both tasks. This otherwise might have confounded the interpretation of results as motor involvement is known to cause DA release in striatum.69 As the tasks had similar motor components and subjects completed equal number of trials, the observed differences in DA release could not be the consequence of different motor performances. It is intriguing to note the differences in BPND in opposite direction in SCZ and HV in WCST and SMCT. At this stage, we do not have a definitive explanation for the DA depletion observed in patients. As mentioned above, control task also had cognitive demands on subjects, although comparatively less than the active task. It is possible that other compensatory mechanisms in the brain would have played an active role in the WCST to compensate for the deficits in strategy which might not be required in SMCT.62–64 This novel finding of DA depletion during a cognitive task needs to be examined and replicated in future studies.
One previous study used N-Back task, a working memory task to examine the frontal DA release in healthy individuals32 and reported DA release in DLPFC and ACC. The magnitude of DA release in the previous study32 was higher when compared to our study possibly due to the differences in the tasks used. Another study33 examined frontal DA release in healthy controls using similar 2-scan design and Montreal Card Sorting Task, a variation of WCST. The magnitude of DA release reported in this study33 is higher than the one reported in this study. It is important to note that Ko and colleagues used a voxel-wise analysis and the difference in BP reported by the authors was based on the mean BP extracted using a spherical region (3-mm diameter) around the statistical peak. On the other hand, we conducted an ROI analysis in anatomically defined regions. As not all voxels in a given ROI will have same level of DA release, this could explain the difference in findings between our study and the previous study.
Some limitations of the study need to be considered. First, except for the association between [11C]FLB 457 ΔBPND in ACC and WCST categories completed, the correlations we explored with clinical and cognitive measures and ΔBPND were not significant after correcting for multiple comparisons using the Bonferroni approach and accounting for the influence of individual data points. It is also possible that the study was not adequately powered to detect the correlations. Further, it is important to note that even in the only available previous study that examined amphetamine-induced cortical DA release using [11C]FLB 457,24 the correlational analyses were exploratory in both HV and SCZ. There were no significant correlations between working memory performance and DLPFC BPND, ΔBPND, VT, or ΔVT in SCZ, but there was a correlation between functional MRI blood-oxygen-level-dependent activity changes during the task and cortical DA release using [11C]FLB 457.24 Second, the mass of [11C]FLB 457 may not be at tracer dose.70 However, incorporation of the mass injected as a covariate into the analysis did not change the significance of percentage change in BPND in ACC (F = 11.63; P = .003) (supplementary table S1), and it was not significantly different between groups in the SMCT task (supplementary table S2). Third, to control for the potential effect of putative-specific binding of [11C]FLB 457 in cerebellum, we compared the cerebellar tracer uptake between SMCT and WCST tasks. There was a near-complete overlap between the tasks, and these results are shown in supplementary figure S1. Fourth, some patients had been on the treatment with antipsychotic earlier. Because none were on treatment with antipsychotics for at least 3 months at the time of examination, it is less likely that the previous antipsychotic treatment had a significant effect on the BPND. There was no difference between antipsychotic-naïve and antipsychotic-free patients in ΔBPND of [11C]FLB 457 in any cortical ROIs (see supplementary tables S3 and S4). Finally, the ΔBPND seen in our patient population is different compared to an earlier study71 using pharmacological depletion. However, it is important to note that the earlier study did not use [11C]FLB 457, but instead used [123I]IBZM and single-photon emission computed tomography. Hence, at this stage, it is not possible to comment whether the differences in findings are due to differences in the radiotracers, ROI (striatal vs extrastriatal), or due to design (behavioral depletion vs pharmacological). Future studies using pharmacological depletion and [11C]FLB 457 can give definitive answers.
Conclusion
In summary, our study indicates DA depletion in cortical brain regions in SCZ vs HV in the face of a cognitive challenge. In turn, these findings provide support to the cortical hypodopaminergia hypothesis in SCZ. This line of investigation has important clinical implications as it has the potential to advance our understanding of neurobiology of cognitive deficits in SCZ, which represent important determinants of functional outcome in this disorder.1–6 However, considering the absence of relation between cognitive performance and prefrontal cortex DA release in this study, further examination is required to investigate the nature of the relation between cortical hypodopaminergia and cognitive deficits in SCZ. It is also possible that the study was not adequately powered to detect the correlations with cognition and further studies with adequate power need to examine the correlational analyses.
Funding
This work was supported by the Canadian Institutes of Health Research (Canada Hope 116290 to NR and RM).
Supplementary Material
References
- 1. Bryson G, Bell MD. Initial and final work performance in schizophrenia: cognitive and symptom predictors. J Nerv Ment Dis. 2003;191:87–92. [DOI] [PubMed] [Google Scholar]
- 2. Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia?Am J Psychiatry. 1996;153:321–330. [DOI] [PubMed] [Google Scholar]
- 3. Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”?Schizophr Bull. 2000;26:119–136. [DOI] [PubMed] [Google Scholar]
- 4. Sánchez P, Ojeda N, Peña J, et al. Predictors of longitudinal changes in schizophrenia: the role of processing speed. J Clin Psychiatry. 2009;70:888–896. [DOI] [PubMed] [Google Scholar]
- 5. Nuechterlein KH, Subotnik KL, Green MF, et al. Neurocognitive predictors of work outcome in recent-onset schizophrenia. Schizophr Bull. 2011;37(suppl 2):S33–S40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lepage M, Bodnar M, Bowie CR. Neurocognition: clinical and functional outcomes in schizophrenia. Can J Psychiatry. 2014;59:5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erlenmeyer-Kimling L, Rock D, Roberts SA, et al. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: the New York High-Risk Project. Am J Psychiatry. 2000;157:1416–1422. [DOI] [PubMed] [Google Scholar]
- 8. Cohen AS, Saperstein AM, Gold JM, Kirkpatrick B, Carpenter WT Jr, Buchanan RW. Neuropsychology of the deficit syndrome: new data and meta-analysis of findings to date. Schizophr Bull. 2007;33:1201–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dibben CR, Rice C, Laws K, McKenna PJ. Is executive impairment associated with schizophrenic syndromes? A meta-analysis. Psychol Med. 2009;39:381–392. [DOI] [PubMed] [Google Scholar]
- 10. Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. [DOI] [PubMed] [Google Scholar]
- 11. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuropsychology. 1998;12:426–445. [DOI] [PubMed] [Google Scholar]
- 12. Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010;35:258–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carter CS, MacDonald AW III, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158:1423–1428. [DOI] [PubMed] [Google Scholar]
- 14. Laurens KR, Ngan ET, Bates AT, Kiehl KA, Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126:610–622. [DOI] [PubMed] [Google Scholar]
- 15. MacDonald AW III, Carter CS, Kerns JG, et al. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. Am J Psychiatry. 2005;162:475–484. [DOI] [PubMed] [Google Scholar]
- 16. Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sawaguchi T, Goldman-Rakic PS. D1 dopamine receptors in prefrontal cortex: involvement in working memory. Science. 1991;251:947–950. [DOI] [PubMed] [Google Scholar]
- 18. Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl). 1994;116:143–151. [DOI] [PubMed] [Google Scholar]
- 19.Alpert M, Friedhoff AJ. An un-dopamine hypothesis of schizophrenia. Schizophr Bull. 1980;6(3):387–389. [DOI] [PubMed] [Google Scholar]
- 20. Abi-Dargham A, Simpson N, Kegeles L, et al. PET studies of binding competition between endogenous dopamine and the D1 radiotracer [11C]NNC 756. Synapse. 1999;32:93–109. [DOI] [PubMed] [Google Scholar]
- 21. Abi-Dargham A, Xu X, Thompson JL, et al. Increased prefrontal cortical D₁ receptors in drug naive patients with schizophrenia: a PET study with [¹¹C]NNC112. J Psychopharmacol. 2012;26:794–805. [DOI] [PubMed] [Google Scholar]
- 22. Kosaka J, Takahashi H, Ito H, et al. Decreased binding of [11C]NNC112 and [11C]SCH23390 in patients with chronic schizophrenia. Life Sci. 2010;86:814–818. [DOI] [PubMed] [Google Scholar]
- 23. Okubo Y, Suhara T, Suzuki K, et al. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. [DOI] [PubMed] [Google Scholar]
- 24. Slifstein M, van de Giessen E, Van Snellenberg J, et al. Deficits in prefrontal cortical and extrastriatal dopamine release in schizophrenia: a positron emission tomographic functional magnetic resonance imaging study. JAMA Psychiatry. 2015;72:316–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. [DOI] [PubMed] [Google Scholar]
- 26. Hall H, Sedvall G, Magnusson O, Kopp J, Halldin C, Farde L. Distribution of D1- and D2-dopamine receptors, and dopamine and its metabolites in the human brain. Neuropsychopharmacology. 1994;11:245–256. [DOI] [PubMed] [Google Scholar]
- 27. Halldin C, Farde L, Högberg T, et al. Carbon-11-FLB 457: a radioligand for extrastriatal D2 dopamine receptors. J Nucl Med. 1995;36:1275–1281. [PubMed] [Google Scholar]
- 28. Narendran R, Frankle WG, Mason NS, et al. Positron emission tomography imaging of amphetamine-induced dopamine release in the human cortex: a comparative evaluation of the high affinity dopamine D2/3 radiotracers [11C]FLB 457 and [11C]fallypride. Synapse. 2009;63:447–461. [DOI] [PubMed] [Google Scholar]
- 29. Narendran R, Jedema H, Lopresti B, et al. Imaging dopamine transmission in the prefrontal cortex: a combined microdialysis and [11C]FLB 457 PET study. J Cereb Blood Flow Metab. 2012;32:s157. [Google Scholar]
- 30. Narendran R, Mason NS, Chen CM, et al. Evaluation of dopamine D₂/₃ specific binding in the cerebellum for the positron emission tomography radiotracer [¹¹C]FLB 457: implications for measuring cortical dopamine release. Synapse. 2011;65:991–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Asselin MC, Montgomery AJ, Grasby PM, Hume SP. Quantification of PET studies with the very high-affinity dopamine D2/D3 receptor ligand [11C]FLB 457: re-evaluation of the validity of using a cerebellar reference region. J Cereb Blood Flow Metab. 2007;27:378–392. [DOI] [PubMed] [Google Scholar]
- 32. Aalto S, Brück A, Laine M, Någren K, Rinne JO. Frontal and temporal dopamine release during working memory and attention tasks in healthy humans: a positron emission tomography study using the high-affinity dopamine D2 receptor ligand [11C]FLB 457. J Neurosci. 2005;25:2471–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ko JH, Ptito A, Monchi O, et al. Increased dopamine release in the right anterior cingulate cortex during the performance of a sorting task: a [11C]FLB 457 PET study. Neuroimage. 2009;46:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ko JH, Antonelli F, Monchi O, et al. Prefrontal dopaminergic receptor abnormalities and executive functions in Parkinson’s disease. Hum Brain Mapp. 2013;34:1591–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ray NJ, Miyasaki JM, Zurowski M, et al. Extrastriatal dopaminergic abnormalities of DA homeostasis in Parkinson’s patients with medication-induced pathological gambling: a [11C] FLB-457 and PET study. Neurobiol Dis. 2012;48:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. First M, Spitzer R, Gibbon M, Williams J.. Clinical Interview for DSM-IVTR (SCID-I): User’s Guide and Interview–Research Version. New York, NY: New York Psychiatric Institute Biometrics Research Department; 2001. [Google Scholar]
- 37. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 38. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City, IA: University of Iowa; 1981. [Google Scholar]
- 39. Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: Department of Heath, Education and Welfare Publication; 1976. [Google Scholar]
- 40. Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale. A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33:766–771. [DOI] [PubMed] [Google Scholar]
- 41. Addington D, Addington J, Maticka-Tyndale E, Joyce J. Reliability and validity of a depression rating scale for schizophrenics. Schizophr Res. 1992;6:201–208. [DOI] [PubMed] [Google Scholar]
- 42. Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the fagerström tolerance questionnaire. Br J Addict. 1991;86:1119–1127. [DOI] [PubMed] [Google Scholar]
- 43. Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith–Hamilton pleasure scale. Br J Psychiatry. 1995;167:99–103. [DOI] [PubMed] [Google Scholar]
- 44. Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the apathy evaluation scale. Psychiatry Res. 1991;38:143–162. [DOI] [PubMed] [Google Scholar]
- 45. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20:310–319. [DOI] [PubMed] [Google Scholar]
- 46. BERG EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychol. 1948;39:15–22. [DOI] [PubMed] [Google Scholar]
- 47. Monchi O, Ko JH, Strafella AP. Striatal dopamine release during performance of executive functions: a [(11)C] raclopride PET study. Neuroimage. 2006;33:907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Monchi O, Petrides M, Strafella AP, Worsley KJ, Doyon J. Functional role of the basal ganglia in the planning and execution of actions. Ann Neurol. 2006;59:257–264. [DOI] [PubMed] [Google Scholar]
- 49. Rusjan P, Mamo D, Ginovart N, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. [DOI] [PubMed] [Google Scholar]
- 50. Abi-Dargham A, Mawlawi O, Lombardo I, et al. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J Neurosci. 2002;22:3708–3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. Neuroimage. 1996;4:153–158. [DOI] [PubMed] [Google Scholar]
- 52. Ito H, Sudo Y, Suhara T, Okubo Y, Halldin C, Farde L. Error analysis for quantification of [(11)C]FLB 457 binding to extrastriatal D(2) dopamine receptors in the human brain. Neuroimage. 2001;13:531–539. [DOI] [PubMed] [Google Scholar]
- 53. Olsson H, Halldin C, Swahn CG, Farde L. Quantification of [11C]FLB 457 binding to extrastriatal dopamine receptors in the human brain. J Cereb Blood Flow Metab. 1999;19:1164–1173. [DOI] [PubMed] [Google Scholar]
- 54. Vandehey NT, Moirano JM, Converse AK, et al. High-affinity dopamine D2/D3 PET radioligands 18F-fallypride and 11C-FLB457: a comparison of kinetics in extrastriatal regions using a multiple-injection protocol. J Cereb Blood Flow Metab. 2010;30:994–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Montgomery AJ, Asselin MC, Farde L, Grasby PM. Measurement of methylphenidate-induced change in extrastriatal dopamine concentration using [11C]FLB 457 PET. J Cereb Blood Flow Metab. 2007;27:369–377. [DOI] [PubMed] [Google Scholar]
- 56. MacDonald SW, Cervenka S, Farde L, Nyberg L, Bäckman L. Extrastriatal dopamine D2 receptor binding modulates intraindividual variability in episodic recognition and executive functioning. Neuropsychologia. 2009;47:2299–2304. [DOI] [PubMed] [Google Scholar]
- 57. Mizrahi R, Rusjan P, Agid O, et al. Adverse subjective experience with antipsychotics and its relationship to striatal and extrastriatal D2 receptors: a PET study in schizophrenia. Am J Psychiatry. 2007;164:630–637. [DOI] [PubMed] [Google Scholar]
- 58. Sandiego CM, Gallezot JD, Lim K, et al. Reference region modeling approaches for amphetamine challenge studies with [11C]FLB 457 and PET. J Cereb Blood Flow Metab. 2015;35:623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum Brain Mapp. 2005;25:35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stuss DT, Alexander MP. Executive functions and the frontal lobes: a conceptual view. Psychol Res. 2000;63:289–298. [DOI] [PubMed] [Google Scholar]
- 61. Lumme V, Aalto S, Ilonen T, Någren K, Hietala J. Dopamine D2/D3 receptor binding in the anterior cingulate cortex and executive functioning. Psychiatry Res. 2007;156:69–74. [DOI] [PubMed] [Google Scholar]
- 62. Wykes T, Brammer M, Mellers J, et al. Effects on the brain of a psychological treatment: cognitive remediation therapy: functional magnetic resonance imaging in schizophrenia. Br J Psychiatry. 2002;181:144–152. [DOI] [PubMed] [Google Scholar]
- 63. Vianin P, Urben S, Magistretti P, Marquet P, Fornari E, Jaugey L. Increased activation in Broca’s area after cognitive remediation in schizophrenia. Psychiatry Res. 2014;221:204–209. [DOI] [PubMed] [Google Scholar]
- 64. Bor J, Brunelin J, d’Amato T, et al. How can cognitive remediation therapy modulate brain activations in schizophrenia? An fMRI study. Psychiatry Res. 2011;192:160–166. [DOI] [PubMed] [Google Scholar]
- 65. Thorsen AL, Johansson K, Løberg EM. Neurobiology of cognitive remediation therapy for schizophrenia: a systematic review. Front Psychiatry. 2014;5:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Olsson H, Farde L. Potentials and pitfalls using high affinity radioligands in PET and SPET determinations on regional drug induced D2 receptor occupancy–a simulation study based on experimental data. Neuroimage. 2001;14:936–945. [DOI] [PubMed] [Google Scholar]
- 67. Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. [DOI] [PubMed] [Google Scholar]
- 68. Narendran R, Himes M, Mason NS. Reproducibility of post-amphetamine [11C]FLB 457 binding to cortical D2/3 receptors. PLoS One. 2013;8:e76905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Goerendt IK, Messa C, Lawrence AD, Grasby PM, Piccini P, Brooks DJ; PET study Dopamine release during sequential finger movements in health and Parkinson’s disease: a PET study. Brain. 2003;126:312–325. [DOI] [PubMed] [Google Scholar]
- 70. Narendran R, Mason NS, May MA, et al. Positron emission tomography imaging of dopamine D₂/₃ receptors in the human cortex with [¹¹C]FLB 457: reproducibility studies. Synapse. 2011;65:35–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Abi-Dargham A, Rodenhiser J, Printz D, et al. Increased baseline occupancy of D2 receptors by dopamine in schizophrenia. Proc Natl Acad Sci USA. 2000;97:8104–8109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.