Abstract
Objective
Increasingly, studies have identified abnormalities in the functional connectivity (FC) of large-scale neural networks in early psychosis, but the findings thus far have been inconclusive. Therefore, the aim of this study was to identify robust alterations in FC of the default mode (DMN), salience (SN), and central executive networks (CEN), in patients with first-episode psychosis (FEP) using a meta-analytic approach.
Methods
Included studies were required to be resting-state, seed-to-whole brain, FC neuroimaging studies, comparing FEP patients to healthy controls (HC), with seeds within the boundaries of the region-of-interest networks. Peak effect coordinates and peak t, z, or p values were meta-analyzed using Seed-based d Mapping software.
Results
The DMN seeds primarily displayed within-network hypoconnectivity (largest clusters including the middle orbital gyrus; and ventral anterior cingulate gyrus). The SN seeds displayed hypoconnectivity with regions in the DMN and CEN (largest clusters located in the bilateral middle temporal gyri). Review of the limited CEN data revealed hypo- and hyperconnectivity across the networks. Negative symptoms were positively correlated with all DMN FC abnormalities in the FEP group. Antipsychotic-treated patients displayed greater hypoconnectivity than antipsychotic-naïve patients between both the DMN/SN seeds and prefrontal regions.
Conclusions
These findings provide substantial evidence of widespread resting-state FC abnormalities of the DMN, SN, and CEN in early psychosis; particularly implicating DMN and SN dysconnectivity as a core deficit underlying the psychopathology of psychosis. Additionally, we highlight the importance of disentangling connectivity abnormalities resulting from disease processes, from those that result from antipsychotic treatment.
Keywords: psychosis, first episode, dysconnectivity, connectivity, functional connectivity, networks
Introduction
Thus far, localized neurofunctional abnormalities have failed to fully account for the complex clinical presentation of psychosis.1 This has stimulated research into the integration of brain regions that have traditionally been considered functionally segregated.2–4 One approach to describe the extent of this integration is functional connectivity (FC). FC, the focal outcome measure in this meta-analysis, refers to the temporal relationship between functional activity in distinct brain regions or networks, either at rest or during a task.5
Neural networks of interest in psychosis include the default mode network (DMN), involved in internally directed thought processes, autobiographical memory, and conceiving others’ perspectives6; the central executive network (CEN), involved in higher level cognitive functions, attention, and external task performance7; and the salience network (SN), involved in the integration of sensory, emotional, and cognitive information, and the appropriate assignment of salience to these external and internal stimuli.8 The normal functioning of these networks is understood to depend on the appropriate “switching” between the anti-correlated CEN and DMN processes.9,10 In turn, this switching of engagement between the task positive (CEN) and task negative (DMN) states is thought to be mediated by the anterior insula (AI), a core node of the SN, when a salient internal or external event is detected.9,10
Abnormalities in the dynamic balance between these 3 networks have been proposed as a potential explanation for much of the core psychopathology of psychosis.11,12 All 3 networks have been found to display aberrant FC with the rest of the brain at different stages of psychotic illness, with varying consistency.1,3,13,14 One of the key findings is that such abnormalities differ over the course of the illness,15 and as a result of treatment.16 It follows that investigations in the early stages of psychosis are required to elucidate the abnormalities that underlie psychosis at onset, while limiting the confounding effects of disease progression and prolonged exposure to treatment.
Only one review thus far has explored FC abnormalities in first-episode psychosis (FEP), identifying a decrease in connectivity overall in FEP patients compared with healthy controls (HCs).3 However, as it was a systematic review, it did not formally assess the strength of the findings. Additionally, the inclusion of only a modest number (N = 7) of relevant studies, and methodological heterogeneity between the studies, limits confidence in their findings. Therefore, we aimed to conduct a meta-analysis of seed-based resting-state FC studies, to quantitatively summarize abnormalities in resting-state connectivity in FEP patients, and delineate the role of large-scale brain networks in early psychosis. An additional aim was to explore the effects of antipsychotic treatment on FC abnormalities in FEP, by comparing groups of antipsychotic-treated and antipsychotic-naïve FEP patients, an objective that is challenging within the context of an individual study. Furthermore, we also aimed to investigate any associations between network FC abnormalities and symptomology in the FEP groups.
Given the challenges in reconciling the results of the included studies into a rational paradigm for network FC, we decided to initially organize seed regions into groups of functional networks, using a previously described theoretically informed strategy for categorization based on coordinate locations.17 We then performed statistical analyses of the FC outcomes for each network and the rest of the whole brain based on this categorization. We hypothesized that for each network of interest, FEP patients would display distinct patterns of both hypo- and hyperconnectivity within- and between-networks, with an overall trend toward hypoconnectivity rather than hyperconnectivity.3,18 Additionally, we hypothesized that these patterns would differ between antipsychotic-treated and antipsychotic-naïve patients and that there would be a positive correlation between the severity of symptoms and FC abnormalities.
Materials and Methods
Search Strategy
Following PRISMA19 and MOOSE20 guidelines, 2 investigators (A.O’N., and S.B.) searched the online database PubMed on the 6th of June 2017, with no restrictions on time of publication previous to that date. The search terms used were: (“early” OR “first episode”) AND (“schizophrenia” OR “psychosis”) AND (“connectivity” OR “dynamic causal modelling”) AND (“fMRI” OR “functional magnetic resonance imaging” OR “functional neuroimaging”). We then manually searched the bibliographies of the included articles, and contacted the corresponding authors of the studies already identified, to identify any additional studies.
Selection Criteria
Articles were included if they met the following criteria:
Original peer-reviewed data-based manuscript.
Patients met standardized diagnostic criteria (eg, DSM,21 ICD,22 OPCRIT23) for FEP (schizophrenia spectrum psychoses [schizophrenia, schizoaffective, and schizophreniform] and affective psychoses [bipolar psychosis and psychotic depression]).
Included a HC group.
Employed seed-to-whole brain, resting-state, FC MRI methods.
Seeds were within DMN, SN, or CEN boundaries.
Either reported any Talairach (TAL) or Montreal Neurological Institute (MNI) peak effect coordinates and corresponding t, z, or P values that were significant at the whole-brain level, or reported no significant findings.
Articles were excluded if they did not meet the above criteria; peak coordinates and/or t, z, or P values were not available even after contacting the authors; or if they primarily investigated the effects of medication/drug use/therapeutic intervention on FC. Where multiple publications used the same data set and identical seed ROIs, only the original study was included.
Data Extraction and Coding
Information including the seed ROIs, peak coordinates of each significant between-group effect for each seed ROI, as well as additional study-specific information were extracted from each study (supplementary eMethods). Where only z or p values for the effect peaks were available, these were converted into t values.
Seed Network Parcellation
Based on the location of the seed coordinates, each seed ROI from each study was organized into 1 of 3 seed networks (DMN, CEN, or SN). Seed network definition described in the supplementary eMethods.
Seed-Based d Mapping
Meta-analysis was performed using Seed-Based d Mapping (SDM) software v5.14 (http://www.sdmproject.com/, accessed April 15, 2018),24 a well-validated method for meta-analyses.25–27 As the general SDM methods have been described in detail elsewhere,24 they are only briefly summarized here, along with the specific adaptations made here for meta-analysis of network FC (supplementary eMethods).
Once assigned to a seed network, each individual seed ROI was treated as a separate study, with its own corresponding sample, and reported peak effects/no reported peak effects (identified in the original studies). As SDM allows both positive and negative values in the same map, seeds for which there were no significant peak effects were also included. A separate SDM meta-analysis was then performed for each seed network, and Monte Carlo randomizations were used to create a null distribution of SDM-Z values at the whole-brain level.
Results were thresholded at a voxel-wise uncorrected value of P < .005. Previous studies have shown that such a threshold, given the context of the current method involving multiple randomizations, is equivalent to a corrected P value of 0.05.27
The resultant peak effects were identified and labeled using their MNI coordinates and the Atlas of the Human Brain.28
Sensitivity Analysis, Heterogeneity, and Publication Bias
Robustness of results was tested using jack-knife sensitivity analysis; between-study heterogeneity and publication bias were tested using visual inspection of the maps of between-study heterogeneity and Egger tests (supplementary eMethods).
Meta-regressions and Subgroup Analyses
Relationships between positive, negative, and general symptomology (indexed using the PANSS rating scale), and the peak effects identified in the DMN analysis were examined using meta-regressions. Effect of antipsychotic treatment was investigated using subgroup analyses and direct comparisons between antipsychotic-treated, and antipsychotic-naïve FEP groups. All results were thresholded at a voxel-wise uncorrected value of P < .005, as per the main analyses.27
Results
Results of Search and Network Parcellation
Although not screened on the basis of language, all studies included were in English. Of 124 articles initially identified, 11 were included in the final analyses (figure 1). Overall, there were 526 participants in the combined HC groups, and 420 participants in the combined patient groups. There were 406 FEP participants, and 383 HC in the DMN; 340 FEP participants, and 416 HC in the SN; and 34 FEP participants, and 34 HCs in the CEN analyses. Participant demographics and clinical characteristics are described in table 1.
Fig. 1.
PRISMA flow-chart depicting the study selection process, and the number of studies either included or excluded at each stage.
Table 1.
Demographic and Clinical Characteristics of the 11 Studies Included in the Meta-analysis
| Study | No. of Participants (% Female) | Mean Age (SD) | Diagnostic Group | Dx Tool | Mean Duration of Illness in Months (SD) | % Receiving Antipsychotic Meds. | Eyes Open/ Closed | Software | Tesla | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| HC | FEP | HC | FEP | ||||||||
| Alonso-Solis et al29 | 19 (36) | 19 (26) | 25.9 (4.7) | 24.9 (4.7) | FEP | SCID | 3.4 (1.9) | 84% | NR | AFNI–FSL | 3 T |
| Anticevic et al30 | 96 (55) | 28 (57) | 28.8 (10.5) | 25 (9.7) | ec-SCZ, brief PD, SFD | SCID | 4.27 (3.2) | 43% | Closed | FSL | 3 T |
| Duan et al31 | 62 (63) | 68 (47) | 35.2 (8.1) | 36.3 (7.3) | FE-SCZ | SCID | 40 (19) | 94% | NR | SPM8 | 1.5 T |
| Fornito et al32 | 26 (63) | 19 (26) | 26.4 (9.7) | 23.2 (4.3) | FEP-SCZ, BD, DD, PNOS | OPCRIT | <1 year | 53% | Closed | SPM8 | 3 T |
| Guo et al33 | 50 (54) | 49 (39) | 23.5 (2.5) | 22.7 (4.6) | FEP | SCID | 22.5 (6.71) | Antipsychotic naïve | Closed | SPM8, DPARSF, REST | 3 T |
| Li et al34 | 16 (56) | 20 (70) | 22.4 (4.4) | 22.9 (8.5) | FE-SCZ | SCID | 6.4 (13.6) | Antipsychotic naïve | Closed | SPM8 | 3 T |
| Lui et al35 | 68 (54) | 68 (56) | 24.7 (8.8) | 24.2 (8.6) | FE-SCZ | SCID | 8.6 (14.3) | Antipsychotic naïve | Closed | SPM2 | 3 T |
| Penner et al36 | 24 (50) | 24 (13) | 23.8 (4.3) | 23.2 (4.2) | ec-SCZ | SCID | 13.7 (10.9) | 96% | Closed | SPM8 | 3 T |
| Zhang et al37 | 30 (43) | 37 (54) | 15.3 (1.6) | 15.5 (1.8) | FE-SCZ | DSM-IV | 16 (14.4) | Antipsychotic naïve | closed | SPM8 | 3 T |
| Zheng et al38 | 30 (57) | 35 (43) | 15.43 (1.5) | 15.5 (1.8) | FE-SCZ | SCID | 6.6 (6.7) | Antipsychotic naïve | NR | SPM8 | 3 T |
| Zhou et al39 | 17 (29) | 17 (29) | 25.7 (5.6) | 22.9 (6) | FE-SCZ | DSM-IV | <2 years | 76% | Closed | SPM2 | 1.5 T |
Note: HC, healthy controls; FEP, first-episode psychosis; Dx Tool, diagnostic tool; ec-SCZ, early course schizophrenia; brief PD, brief psychotic disorder; SFD, schizophreniform disorder; SCZ, schizophrenia; BD, bipolar disorder; DD, delusional disorder; PNOS, psychotic disorder not otherwise specified; DSM-IV, diagnostic statistical manual; SCID, Structured Clinical Interview for DSM; OPCRIT, Operational Criteria Checklist for Psychotic Illness and Affective Illness; NR, not reported.
The network parcellation approach grouped the seed coordinates from each study into separate functional networks, resulting in 11 SN seed ROIs, 10 DMN seed ROIs, and 2 CEN seed ROIs (table 2). Only one study reported no significant connectivity differences between FEP and HC groups.35
Table 2.
Summary of Seed Networks and Anatomical Regions of Studies Included in Meta-analysis
| Study | Seed | DMN (FEP = 406; HC = 383) |
SN (FEP = 340; HC = 416) |
CEN (FEP = 34; HC = 34) |
|---|---|---|---|---|
| Alonso-Solis et al29 | dMPFC | X | ||
| PCC | X | |||
| TPJ | X | |||
| Anticevic et al30 | Amygdala | X | ||
| Duan et al31 | L. hippocampus | X | ||
| R. hippocampus | X | |||
| Fornito et al32 | Dorsal caudate | X | ||
| Sup. ventral caudate | X | |||
| Inf. ventral caudate | X | |||
| Guo et al33 | L. MPFC | X | ||
| R. dACC | X | |||
| Li et al34 | R. OFC | X | ||
| Lui et al35 | R. dACC | X | ||
| R. MTG | X | |||
| Penner et al36 | L. insula | X | ||
| R. insula | X | |||
| MPFC | X | |||
| Zhang et al37 | R. MTG | X | ||
| Zheng et al38 | L. OFC | X | ||
| R. OFC | X | |||
| Precuneus | X | |||
| Zhou et al39 | L. DLPFC | X | ||
| R. DLPFC | X | |||
| Total seed ROIs | 10 | 11 | 2 |
Note: DMN, default mode network; SN, salience network; CEN, central executive network; dMPFC, dorsomedial prefrontal cortex; PCC, posterior cingulate cortex; TPJ, temporoparietal junction; sup. ventral caudate, superior ventral caudate; inf. ventral caudate, inferior ventral caudate; MPFC, medial prefrontal cortex; dACC, dorsal anterior cingulate cortex; MTG, medial temporal gyrus; PFC thalamus, prefrontal thalamus; OFC, orbitofrontal cortex; DLPFC, dorsolateral prefrontal cortex; ROIs, regions of interest. X indicates regions that fall within the specified network. Note that the participant numbers for each network group are higher than the overall sample, as the participant sample of one study may be included in a network analysis multiple times, where multiple seed ROIs included in a single network analysis originate from a single original study.
Main Results
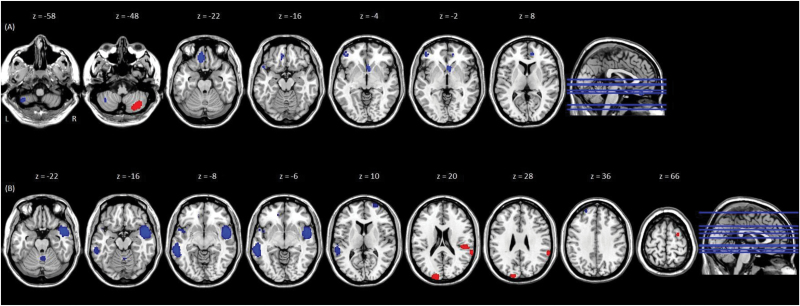
For the DMN meta-analysis, clusters displaying hypoconnectivity with DMN seeds in FEP relative to HC, surviving jack-knife sensitivity analyses, were identified in the medial orbital, inferior frontal and superior temporal gyri, and cerebellum on the left side, and right ventral and dorsal anterior cingulate gyri. One cluster, located in the right inferior semi-lunar cerebellar lobule, displayed significant hyperconnectivity with the DMN seeds in FEP relative to HC (table 3, figure 2).
Table 3.
Results of Meta-analysis of Resting-State Functional Connectivity in FEP, After Sensitivity Analysis
| Seed Network and Anatomy | Direction of Difference in FC | Effect Anatomy | Coordinates (MNI) | SDM-Z | P-value | SDM-Estimate | No. of Voxels | Jack-Knife Survival Rate | Egger Test, P-value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||||
| DMN: dMPFC, PCC, TPJ, hippocampus, MPFC, MTG, v. precuneus |
FEP > HC | Inf. semi-lunar cerebellar lobule | 36 | −68 | −48 | 1.304 | .00033 | 0.093 | 1118 | 10/11 | .394 |
| FEP < HC | Medial orbital gyrus | −12 | 36 | −22 | −1.59 | .00048 | −0.11 | 353 | 10/11 | .841 | |
| Cerebellum | −40 | −54 | −58 | −1.303 | .0026 | −0.093 | 145 | 10/11 | .348 | ||
| (Ventral anterior) cingulate gyrus | 6 | 14 | −4 | −1.526 | .00077 | −0.11 | 131 | 10/11 | .953 | ||
| Inferior frontal gyrus | −48 | 44 | −2 | −1.303 | .0027 | −0.093 | 99 | 10/11 | .377 | ||
| Superior temporal gyrus | −48 | 16 | −16 | −1.304 | .0026 | −0.093 | 64 | 10/11 | .377 | ||
| (Dorsal anterior) cingulate gyrus | 10 | 46 | 8 | −1.391 | .0018 | −0.099 | 40 | 10/11 | .451 | ||
| SN: amygdala, DC, sVC, iVC, dACC, insula, OFC |
FEP > HC | Occipital gyrus | −18 | −94 | 28 | 293 | .0004 | 1.205 | 293 | 10/12 | .448 |
| Superior temporal gyrus | 64 | −38 | 20 | 128 | .00038 | 1.205 | 128 | 11/12 | .449 | ||
| Post. transverse temporal gyrus | 54 | −30 | 20 | 126 | .00068 | 1.187 | 126 | 10/12 | .452 | ||
| Superior frontal gyrus | 26 | 0 | 66 | 38 | .00054 | 1.196 | 38 | 11/12 | .447 | ||
| FEP < HC | Middle temporal gyrus | 48 | 6 | −16 | −1.92 | .000015 | −0.223 | 1752 | 12/12 | .304 | |
| Middle temporal gyrus | −60 | −38 | −6 | −1.837 | .000034 | −0.174 | 1294 | 12/12 | .084 | ||
| Planum polare | −46 | 6 | −8 | −1.233 | .0029 | −0.095 | 85 | 10/12 | .499 | ||
| Cerebellum/culmen | 4 | −58 | −22 | −1.205 | .0033 | −0.093 | 85 | 11/12 | .449 | ||
| Middle frontal gyrus | 26 | 66 | 10 | −1.402 | .0011 | −0.151 | 82 | 10/12 | .141 | ||
| Superior frontal gyrus | −22 | 52 | 36 | −1.248 | .0027 | −0.107 | 23 | 10/12 | .167 | ||
Note: Results of comparison between individuals with first-episode psychosis (FEP) and healthy controls (HC), after jack-knife sensitivity analysis. Results were thresholded at P < .005 uncorrected (equivalent to P < .05 corrected), peak threshold of 1, extent threshold of 10. Seed networks included default mode network (DMN), and salience network (SN). dMPFC, dorsomedial prefrontal cortex; PCC, posterior cingulate cortex; TPJ, temporoparietal junction; MPFC, medial prefrontal cortex; dACC, dorsal anterior cingulate cortex; MTG, medial temporal gyrus; v. precuneus, ventral precuneus; DC, dorsal caudate; sVC, superior ventral caudate; iVC, inferior ventral caudate; OFC, orbitofrontal cortex; SDM-Z, SDM test statistic. SDM-estimate, effect size created by SDM. Coordinates are MNI standard stereotaxic space. Voxels = number of 1 mm × 1 mm × 1 mm voxels. Jack-knife survival rate refers to number of iterations the peak survived out of the total number of iterations, during the jack-knife sensitivity analyses.
Fig. 2.
Results of the meta-analysis of (A) DMN to whole-brain functional connectivity, and (B) SN to whole-brain functional connectivity, in FEP individuals, relative to controls. Brain regions that showed significant differences in functional connectivity with (A) the DMN seed network, and (B) the SN seed network, in individuals with first-episode psychosis (FEP), relative to healthy controls (HC). MNI z coordinates are displayed at the top of the figure. Peaks appear from left to right in the (A) left cerebellum (P = .0026); right inferior semi-lunar cerebellar lobule (P = .00033); medial orbital gyrus (P = .00048); left superior temporal gyrus (P = .0026); ventral anterior cingulate gyrus (P = .00077); the inferior frontal gyrus (P = .0027); and the dorsal anterior cingulate gyrus (P = .0018); (B) cerebellum/culmen (P = .0033); right middle temporal gyrus (P = .000015); left planum polare (P = .0029); left middle temporal gyrus (P = .000034); middle frontal gyrus (P = .0011); superior temporal gyrus (P = .00038) and posterior transverse temporal gyrus (P = .00068); occipital gyrus (P = .0004); left superior frontal gyrus (P = .0027); right superior frontal gyrus (P = .00054). For color, please see the figure online.
For the SN meta-analysis, clusters displaying hypoconnectivity with SN seeds in FEP relative to HC, surviving jack-knife sensitivity analyses, were identified in the middle temporal gyrus bilaterally, left planum polare, right middle frontal and left superior frontal gyri, and right cerebellum/culmen. Additionally, hyperconnectivity with SN seeds was identified in the occipital, superior and posterior transverse temporal, and the superior frontal gyri in FEP relative to HC (table 3, figure 2).
As the number of CEN seed ROIs was limited, we summarized these results qualitatively. Clusters displaying hypoconnectivity with the CEN seeds (located in the bilateral DLPFC) in FEP patients relative to HC were located in the parietal cortex, posterior cingulate cortex, striatum, and thalamus. Instances of hyperconnectivity were also observed between the left DLPFC CEN seed and the mid-posterior temporal lobe and paralimbic regions (orbitofrontal gyrus and insula), in FEP relative to HC.
Sensitivity, Heterogeneity, and Publication Bias Tests
No between-study heterogeneity was observed for the DMN analysis. For the SN analysis, between-study heterogeneity was present in the bilateral middle temporal and the right middle frontal gyri.
For the DMN analysis, 1 of the 8 identified peak effects did not survive jack-knife sensitivity analysis. For the SN analysis, all 10 identified peaks survived the sensitivity analyses (for all peak effects before sensitivity testing, see supplementary eTables 1 and 2).
Significant publication bias was not observed for either the DMN or the SN analyses (p > 0.05 for all significant peak effects, table 3).
Meta-regressions and Subgroup Analyses
The DMN subgroup analysis of studies that included patients currently being treated with antipsychotic medication (medFEP, N = 6), compared with HC, revealed between-group differences comparable to the main analysis (supplementary eTable 3). In contrast, the DMN subgroup analysis of studies including only antipsychotic naïve patients (nomedFEP, N = 4), relative to HC, did not identify any of the peaks identified in the main analysis (supplementary eTable 4). Results from the direct comparison of medFEP and nomedFEP studies supported these findings, revealing hypo- and hyperconnectivity, in the medFEP group, between the DMN seeds and peaks corresponding with all those identified in the main DMN analysis (supplementary eTable 5, supplementary eFigure 1). Investigation of the differences in duration of illness and age between the medFEP and nomedFEP groups (DMN seed analysis) revealed the former to be significantly older and to have a numerically longer duration of illness than the latter. After controlling for these potentially confounding variables in the direct comparison of the subgroups (medFEP vs nomedFEP), only the medial orbital gyrus (MOG) (SDM-Z = −1.4, P = .0009), and dorsal anterior cingulate gyrus peaks survived (SDM-Z = −1.347, P = .0015). Meta-regression of the main DMN analysis (FEP vs HC) identified a positive correlation between patient age and the hypo- and hyperconnectivity for all the significant peaks. In the medFEP vs HC contrast, age was positively correlated with the FC abnormalities of all the prior significant peaks, excluding the MOG and ventral anterior cingulate peaks. In contrast, no significant correlation was observed between age and the FC abnormalities of the prior significant peaks in the nomedFEP group.
The SN subgroup analysis of the medFEP studies (N = 6) revealed hypoconnectivity between the SN and the right middle frontal, left superior frontal, and left middle temporal gyri, relative to HC, overlapping with the same regions identified in the main analysis (supplementary eTable 3). The SN subgroup analysis of the nomedFEP studies (N = 5) revealed hyperconnectivity, relative to HC, between the SN and 2 clusters overlapping with those identified in the main analysis: the right superior temporal and superior frontal gyri (supplementary eTable 4). For the same nomedFEP analysis, 2 clusters displaying hypoconnectivity with the SN corresponding to those seen in the main analysis were identified in the right middle temporal gyrus, and the cerebellum, in the nomedFEP groups (supplementary eTable 4). Direct comparison of medFEP and nomedFEP studies supported these findings, demonstrating hypoconnectivity between the SN and the middle and superior frontal, occipital, and left middle temporal gyri in the medFEP group, and hypoconnectivity between the SN and the cerebellum and right middle temporal gyrus clusters in the nomedFEP group (supplementary eTable 6, supplementary eFigure 2). Illness duration and age were observed to be greater in the medFEP group than the nomedFEP group (SN seed analysis), but not significantly so. Controlling for both potential confounders in the direct comparison of the subgroups (medFEP vs nomedFEP), only the middle frontal gyrus (SDM-Z = −2.292, P = .000018), and superior frontal gyrus peaks survived (SDM-Z = −2.197, P = .000066). Meta-regression of the main SN analysis (FEP vs HC) identified a negative correlation for patient age and the hypoconnectivity between the SN seeds and the bilateral middle temporal gyrus, and planum polare clusters. Similar correlations were observed in both the subgroup analyses (medFEP/nomedFEP compared with HC), such that younger age was associated with greater hypoconnectivity between the SN seeds and the left middle temporal gyrus in the medFEP group, and also with the right middle temporal gyrus, and planum polare in the nomedFEP group.
The DMN meta-regression analyses revealed a significant correlation between higher negative symptoms ratings and greater hypoconnectivity, for all the clusters that displayed DMN hypoconnectivity in the main analysis, except the ventral anterior cingulate gyrus (vACG), ie, the medial orbital, inferior frontal, superior temporal, and dorsal anterior cingulate gyri clusters, and the cerebellum cluster (supplementary eFigure 1). Positive and negative symptom ratings in FEP were both found to be positively associated with the hyperconnectivity between the DMN and the right inferior semi-lunar cerebellar lobule, identified in the main analysis in FEP relative to HC (supplementary eFigure 1). There were no significant associations between general psychopathology and any of the DMN group differences.
Due to a lack of consistency across the SN studies regarding the symptom rating scales used, exploration of the relationship between symptoms and SN connectivity was not feasible.
Discussion
This meta-analysis presents robust evidence of widespread functional dysconnectivity of the DMN and SN in patients with FEP, compared with HC, implicating aberrant functional integration of these networks in the core pathophysiology underlying psychosis, from the early illness stages. To our knowledge, this is the first meta-analysis investigating abnormalities in resting-state FC of the DMN, SN, and CEN in FEP.
Key Findings
An overall trend toward hypoconnectivity with the rest of the brain was identified for the DMN, while the SN and CEN displayed more mixed results. Specifically, the DMN primarily displayed decreased connectivity with other regions within the DMN, but also with regions in the SN and CEN. The SN also displayed reduced connectivity with regions in the DMN and CEN, while demonstrating additional hyperconnectivity particularly with regions involved in visual and auditory processing. Review of the limited CEN data revealed hypo and hyperconnectivity across the DMN, CEN, and SN.
The most significant finding relating to the DMN was of decreased within-network connectivity in the FEP group, relative to HCs, the largest cluster of which was located in the MOG. Additionally, the extent of all the DMN abnormalities was positively associated with the severity of negative symptoms in the FEP group.
The MOG is involved in a range of reward processing functions,40,41 the learning of associations between emotions and salient situations,42 and processes requiring the individual to assess the perspective of others.43 Impairments in these functions are often linked to the negative symptoms observed in psychosis—with aberrant reward processing and maintenance of reward value representations, in particular, being associated with amotivation.44
The vACG, also within the DMN, contained the second largest cluster of DMN hypoconnectivity and is involved in the modulation of emotional behavior and social cognition.45,46 Consistent with this finding, Anticevic et al47 observed a decrease in connectivity between the ventral anterior cingulate cortex and the dorsal medial prefrontal cortex in patients with chronic schizophrenia, and also in patients with bipolar disorder who had a history of psychosis. The same decrease was not observed in patients with bipolar disorder that did not have a history of psychosis. This is somewhat consistent with the findings of Gong et al,1 who reported decreased within-network DMN connectivity across 3 patient groups including FEP patients, patients with major depressive disorder (MDD), and patients with post-traumatic stress disorder (PTSD), relative to controls. Both MDD and PTSD may also present with psychotic symptoms. However, whether decreased FC within the DMN may specifically link the mechanisms underlying psychotic symptoms across disorders remains to be investigated.
The most significant finding of the SN meta-analysis was that of hypoconnectivity with 2 large DMN clusters, located in the bilateral middle temporal gyri (MTG). The MTG play central roles in language and semantic memory processing, and multimodal sensory integration.48 In keeping with the functionality of both the MTG and the SN; aberrant MTG activity, abnormal SN connectivity, and reduced gray matter volumes in both the SN and the MTG have all been associated with severity of hallucinations across schizophrenia spectrum disorders.48–52 These findings suggest a role for abnormal SN–MTG FC underlying the manifestation of hallucinations in psychosis, and the potential for the distinction between subtypes of psychosis based on the same. Though it was impossible to include symptom ratings in the current SN analysis, such an approach should prove useful in the future study of SN–MTG FC.
Collectively, the results from both the SN and DMN analyses are consistent with the model proposing a pivotal role for the SN in the appropriate switching between DMN and CEN engagement.9,10 In schizophrenia, abnormal activity of the SN is thought to result in aberrant salience attribution to everyday experiences and stimuli, and subsequently, inappropriate switching between attentional, higher-order cognitive processes, and internal thought processes.11,12,18,53 Results of the present meta-analysis suggest that this inappropriate switching between the CEN and DMN may be the result of reduced SN–DMN connectivity in FEP leading to the aberrant allocation of neural resources and the characteristic presentation of psychosis. However, whether this reflects a loss of influence of the SN over the switching between task positive and task negative states remains to be tested. This was not possible in the present meta-analysis as the studies included herein did not report on the directional influence of these neural systems on each other, also described as effective connectivity.
These findings are also broadly in keeping with studies of patients with chronic schizophrenia, which describe widespread hypoconnectivity in comparison to HCs.3,14,18 However, instances of hyperconnectivity appear to be more consistent in chronic illness than in FEP, particularly in relation to the FC of the DMN,54,55 and frontal regions generally.3,56
The identification of widespread hypoconnectivity in FEP patients, relative to controls, is also largely in keeping with both Pettersson-Yeo et al.’s review of FC in FEP,3 and with seed-based FC studies in schizophrenia, overall.57–62 However, studies that employed independent component analysis (ICA, another commonly used method of connectivity analysis) reported more mixed findings.63–66 In line with the goals of this article, seed-based analysis enables the investigation of the FC of a specific region with the rest of the whole brain, while ICA models data by identifying sets of voxels whose activity varies together over time and is maximally distinguishable from other sets.67 Though neither method is without limitations, in terms of the current goal, the data reduction involved in the initial stages of ICA—requiring the researcher to determine a priori the number of components in the data—may introduce some methodological bias (supplementary eDiscussion).68 Nonetheless, future meta-analysis of ICA data would prove useful in exploring these differences in outcomes further.
Another finding of interest was the presence of FC abnormalities between both the DMN and SN, and language, auditory, and visual regions. Specifically, the DMN displayed hypoconnectivity with regions involved in the comprehension, processing, and production of language (inferior frontal gyrus),69 and also with the primary auditory cortex (superior temporal gyrus),70,71 while the SN displayed hypoconnectivity with a subregion of the primary auditory cortex (planum polare),70 and hyperconnectivity with other regions within the primary auditory cortex (right posterior transverse temporal gyrus, and superior temporal gyrus).71 The largest cluster to display hyperconnectivity with the SN lay within the occipital lobe, another sensory region, containing most of the visual cortex. Whether hyperconnectivity between the SN and regions involved in auditory and visual processing reflects greater allocation of attentional resources and aberrant attribution of salience, and in turn underlies perceptual disturbances typically present in FEP, remains to be investigated. In contrast, DMN hypoconnectivity with the regions involved in the comprehension, processing, and production of language may underlie deficits in social role function and negative symptoms in psychosis, and warrant further investigation.
Finally, the CEN data—though limited—illustrated increases and decreases in FC between the CEN and both the DMN and the SN for the FEP group. While novel in itself, further studies are required to draw any decisive conclusions about CEN connectivity in FEP.
The most prominent finding from the additional analyses was of the disparity between FC abnormalities identified in the antipsychotic-naïve FEP groups, and those identified in the antipsychotic-treated FEP groups as well as in the main analyses. When age and length of illness were controlled for in the direct comparison of the 2 groups, the deficits in prefrontal regions were more severe in the medFEP group, compared to the nomedFEP group. This was also reflected in both the DMN and SN subgroup analyses, where the medFEP or nomedFEP groups were compared with HC, when controlling for age. These findings may suggest an effect of antipsychotic medication on the FC between the DMN/SN and prefrontal regions that are rich in dopaminergic inputs,72 and hence susceptible to the dopamine modulating mechanism of action of antipsychotic drugs.73 Another possibility is that patients in the medFEP groups were more unwell than patients in the nomedFEP group, and that after controlling for the effects of age and illness duration, these prefrontal regions were the most severely affected by psychosis. It is important to note, however, that for both main analyses, the medFEP studies outnumbered the nomedFEP studies. This should be taken into consideration when interpreting these findings, particularly considering the disparity between the results of the 2 subgroups in the DMN analysis. Additionally, as these data were cross-sectional, they do not address the effects of antipsychotic treatment on FC over time. Previous research supports the longitudinal effects of antipsychotic treatment on FC in psychosis, however, the direction and localization of these effects are still unclear.16,74–76
Collectively, these results suggest that the effect of antipsychotic treatment on FC of DMN/SN seeds with prefrontal regions may have driven some, but not all of the differences in FC between FEP patients and HC. Nonetheless, these results highlight a need for future appropriately designed studies to address these issues definitively. Although we were unable to do this here due to lack of available data, future meta-analytic endeavours may consider taking into account the potential confounding effects of between-study differences in antipsychotic dosage, duration of treatment, duration of untreated illness, and ethnic differences while trying to disentangle abnormalities that are intrinsic to the pathophysiology of psychosis from those that may be an effect of antipsychotic exposure.
Considerations for Future Research
A potential limitation of our investigation is that some of the studies had used multiple seeds within the same network and therefore were included more than once within the same meta-analysis. While this raises the possibility of dependence among some of the entries within a meta-analysis, at present there are no established methods for estimating and correcting for this in the context of seed-based resting-state FC studies.
It may also be questioned whether connectivity patterns of individual seeds truly reflect the connectivity pattern of the larger network that the seeds may be a part of. It is worth noting that the large-scale networks examined here have been defined on the basis of empirical data that has been consistently replicated by independent research groups,7,77–81 with the broad consensus about the regions included in each network, corresponding to the seeds used in the current analyses.7,8,82 Given that brain regions included as part of these large-scale networks have been identified on the basis of temporal correlation between the time-series of these regions, it is reasonable to assume that the FC observed for a region which is part of a certain network can be used as a proxy for the behavior of that network as whole. However, in the absence of empirical data, we cannot be certain that this is the case.
Additional discussion of the advantages and limitations of this study can be found in the supplementary eDiscussion.
Conclusions
The results of this meta-analysis provide robust evidence of resting-state FC abnormalities in early psychosis, specifically implicating the DMN and SN in early psychosis psychopathology. The discovery of functional dysconnectivity of large-scale networks even in early psychosis is consistent with the view that this represents a core neural deficit of the illness. The DMN, SN, and CEN were all found to display a combination of hyper- and hypoconnectivity, to different degrees, though the most noteworthy disturbances were hypoconnectivity within the DMN, and between the SN and the DMN. These findings in particular support a contributory role, previously proposed in schizophrenia, for abnormalities of the SN in the DMN dysfunction observed in early psychosis.11,12,53 What effect antipsychotic treatment has on such abnormalities, however, requires further clarification.
Funding
This work was supported by grants from the Medical Research Council (MRC), UK (MR/J012149/1 and MC_PC_14105 v.2 to S.B.). S.B. has also received support from the National Institute for Health Research (NIHR) (NIHR Clinician Scientist Award; NIHR CS-11-001), and from the NIHR Mental Health Biomedical Research Centre at South London and Maudsley National Health Service (NHS) Foundation Trust and King’s College London. A.O’N. was supported by the NIHR Collaboration for Leadership in Applied Health Research and Care South London, at King’s College Hospital NHS Foundation Trust.
Supplementary Material
Acknowledgments
We certify that this article is not being considered for publication elsewhere, and has not been published previously. The views expressed are those of the author(s), and not necessarily those of the NHS, the NIHR, or the Department of Health. All the authors contributed in a substantial way to the study and approved the manuscript content. A.O’N. and S.B. were involved in the design and analysis, and all authors contributed to the interpretation of findings. A.O’N. wrote the first draft of the manuscript, and all authors contributed to its critical revision and gave final approval of the version for publication. The authors have no other biomedical financial interests or potential conflicts of interest related to this publication.
References
- 1. Gong Q, Hu X, Pettersson-Yeo W, et al. . Network-level dysconnectivity in drug-naive first-episode psychosis: dissociating transdiagnostic and diagnosis-specific alterations. Neuropsychopharmacology. 2016; 42: 933–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friston KJ. Dysfunctional connectivity in schizophrenia. World Psychiatry. 2002;1:66–71. [PMC free article] [PubMed] [Google Scholar]
- 3. Pettersson-Yeo W, Allen P, Benetti S, McGuire P, Mechelli A. Dysconnectivity in schizophrenia: where are we now?Neurosci Biobehav Rev. 2011;35:1110–1124. [DOI] [PubMed] [Google Scholar]
- 4. Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Friston KJ. Functional and effective connectivity: a review. Brain Connect. 2011;1:13–36. [DOI] [PubMed] [Google Scholar]
- 6. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 7. Seeley WW, Menon V, Schatzberg AF, et al. . Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Menon V. Salience network. In: Toga AW, ed. Brain Mapping: An Encyclopedic Reference. Vol 2 Cambridge: Academic Press; 2015:597–611. [Google Scholar]
- 9. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA. 2008;105:12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. [DOI] [PubMed] [Google Scholar]
- 12. Nekovarova T, Fajnerova I, Horacek J, Spaniel F. Bridging disparate symptoms of schizophrenia: a triple network dysfunction theory. Front Behav Neurosci. 2014;8:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alderson-Day B, Diederen K, Fernyhough C, et al. . Auditory hallucinations and the brain’s resting-state networks: findings and methodological observations. Schizophr Bull. 2016;42:1110–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mamah D, Barch DM, Repovš G. Resting state functional connectivity of five neural networks in bipolar disorder and schizophrenia. J Affect Disord. 2013;150:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li T, Wang Q, Zhang J, et al. . Brain-wide analysis of functional connectivity in first-episode and chronic stages of schizophrenia. Schizophr Bull. 2016;43: 436–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bolding MS, White DM, Hadley JA, Weiler M, Holcomb HH, Lahti AC. Antipsychotic drugs alter functional connectivity between the medial frontal cortex, hippocampus, and nucleus accumbens as measured by H215O PET. Front Psychiatry. 2012;3:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry. 2015;72:603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dong D, Wang Y, Chang X, Luo C, Yao D. Dysfunction of large-scale brain networks in schizophrenia: a meta-analysis of resting-state functional connectivity. Schizophr Bull. 2017;44:168–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moher D, Shamseer L, Clarke M, et al. ; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stroup DF, Berlin JA, Morton SC, et al. . Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 21. American Psychiatric Association. DSM-5 task force. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. 5th ed. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- 22. World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization; 1992. [Google Scholar]
- 23. Rucker J, Newman S, Gray J, et al. . OPCRIT+: an electronic system for psychiatric diagnosis and data collection in clinical and research settings. Br J Psychiatry. 2011;199:151–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Radua J, Mataix-Cols D. Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry. 2009;195:393–402. [DOI] [PubMed] [Google Scholar]
- 25. Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–198. [DOI] [PubMed] [Google Scholar]
- 26. Fusar-Poli P. Voxel-wise meta-analysis of fMRI studies in patients at clinical high risk for psychosis. J Psychiatry Neurosci. 2012;37:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Luijten M, Schellekens AF, Kühn S, Machielse MW, Sescousse G. Disruption of reward processing in addiction: an image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry. 2017;74:387–398. [DOI] [PubMed] [Google Scholar]
- 28. Mai JK, Majtanik M, Paxinos G.. Atlas of the Human Brain. 4th ed. Amsterdam: Elsevier Ltd; 2015. http://www. thehumanbrain.info/. [Google Scholar]
- 29. Alonso-Solís A, Corripio I, de Castro-Manglano P, et al. . Altered default network resting state functional connectivity in patients with a first episode of psychosis. Schizophr Res. 2012;139:13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anticevic A, Tang Y, Cho YT, et al. . Amygdala connectivity differs among chronic, early course, and individuals at risk for developing schizophrenia. Schizophr Bull. 2014;40:1105–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Duan HF, Gan JL, Yang JM, et al. . A longitudinal study on intrinsic connectivity of hippocampus associated with positive symptom in first-episode schizophrenia. Behav Brain Res. 2015;283:78–86. [DOI] [PubMed] [Google Scholar]
- 32. Fornito A, Harrison BJ, Goodby E, et al. . Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry. 2013;70:1143–1151. [DOI] [PubMed] [Google Scholar]
- 33. Guo W, Liu F, Liu J, et al. . Abnormal causal connectivity by structural deficits in first-episode, drug-naive schizophrenia at rest. Schizophr Bull. 2015;41:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li F, Lui S, Yao L, et al. . Longitudinal changes in resting-state cerebral activity in patients with first-episode schizophrenia: a 1-year follow-up functional MR imaging study. Radiology. 2016;279:867–875. [DOI] [PubMed] [Google Scholar]
- 35. Lui S, Deng W, Huang X, et al. . Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166:196–205. [DOI] [PubMed] [Google Scholar]
- 36. Penner J, Ford KA, Taylor R, et al. . Medial prefrontal and anterior insular connectivity in early schizophrenia and major depressive disorder: a resting functional MRI evaluation of large-scale brain network models. Front Hum Neurosci. 2016;10:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang Y, Zheng J, Fan X, et al. . Dysfunctional resting-state connectivities of brain regions with structural deficits in drug-naive first-episode schizophrenia adolescents. Schizophr Res. 2015;168:353–359. [DOI] [PubMed] [Google Scholar]
- 38. Zheng J, Zhang Y, Guo X, et al. . Disrupted amplitude of low-frequency fluctuations in antipsychotic-naïve adolescents with early-onset schizophrenia. Psychiatry Res Neuroimaging. 2016;249:20–26. [DOI] [PubMed] [Google Scholar]
- 39. Zhou Y, Liang M, Jiang T, et al. . Functional dysconnectivity of the dorsolateral prefrontal cortex in first-episode schizophrenia using resting-state fMRI. Neurosci Lett. 2007;417:297–302. [DOI] [PubMed] [Google Scholar]
- 40. Rolls ET. Prefrontal contributions to reward encoding. In: Squire LR, ed. Encyclopedia of Neuroscience. Cambridge: Academic Press; 2009. [Google Scholar]
- 41. Nakamura M, Nestor PG, Levitt JJ, et al. . Orbitofrontal volume deficit in schizophrenia and thought disorder. Brain. 2008;131:180–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. [DOI] [PubMed] [Google Scholar]
- 43. Hynes CA, Baird AA, Grafton ST. Differential role of the orbital frontal lobe in emotional versus cognitive perspective-taking. Neuropsychologia. 2006;44:374–383. [DOI] [PubMed] [Google Scholar]
- 44. Strauss GP, Waltz JA, Gold JM. A review of reward processing and motivational impairment in schizophrenia. Schizophr Bull. 2014;40(suppl 2):S107–S116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mars RB, Neubert FX, Noonan MP, Sallet J, Toni I, Rushworth MF. On the relationship between the “default mode network” and the “social brain”. Front Hum Neurosci. 2012;6:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anticevic A, Savic A, Repovs G, et al. . Ventral anterior cingulate connectivity distinguished nonpsychotic bipolar illness from psychotic bipolar disorder and schizophrenia. Schizophr Bull. 2015;41:133–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Onitsuka T, Shenton ME, Salisbury DF, et al. . Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am J Psychiatry. 2004;161:1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Cui Y, Liu B, Song M, et al. . Auditory verbal hallucinations are related to cortical thinning in the left middle temporal gyrus of patients with schizophrenia. Psychol Med. 2017;48: 115–112. [DOI] [PubMed] [Google Scholar]
- 50. Thoma RJ, Chaze C, Lewine JD, et al. . Functional MRI evaluation of multiple neural networks underlying auditory verbal hallucinations in schizophrenia spectrum disorders. Front Psychiatry. 2016;7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73–81. [DOI] [PubMed] [Google Scholar]
- 52. Pu W, Li L, Zhang H, et al. . Morphological and functional abnormalities of salience network in the early-stage of paranoid schizophrenia. Schizophr Res. 2012;141:15–21. [DOI] [PubMed] [Google Scholar]
- 53. Manoliu A, Riedl V, Zherdin A, et al. . Aberrant dependence of default mode/central executive network interactions on anterior insular salience network activity in schizophrenia. Schizophr Bull. 2014;40:428–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Alonso-Solís A, Vives-Gilabert Y, Grasa E, et al. . Resting-state functional connectivity alterations in the default network of schizophrenia patients with persistent auditory verbal hallucinations. Schizophr Res. 2015;161:261–268. [DOI] [PubMed] [Google Scholar]
- 55. Whitfield-Gabrieli S, Thermenos HW, Milanovic S, et al. . Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Baker JT, Holmes AJ, Masters GA, et al. . Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry. 2014;71:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Zhou Y, Shu N, Liu Y, et al. . Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr Res. 2008;100:120–132. [DOI] [PubMed] [Google Scholar]
- 58. Bluhm RL, Miller J, Lanius RA, et al. . Spontaneous low-frequency fluctuations in the BOLD signal in schizophrenic patients: anomalies in the default network. Schizophr Bull. 2007;33:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bluhm RL, Miller J, Lanius RA, et al. . Retrosplenial cortex connectivity in schizophrenia. Psychiatry Res. 2009;174:17–23. [DOI] [PubMed] [Google Scholar]
- 60. Liang M, Zhou Y, Jiang T, et al. . Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. [DOI] [PubMed] [Google Scholar]
- 61. Moran LV, Tagamets MA, Sampath H, et al. . Disruption of anterior insula modulation of large-scale brain networks in schizophrenia. Biol Psychiatry. 2013;74:467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Palaniyappan L, Simmonite M, White TP, Liddle EB, Liddle PF. Neural primacy of the salience processing system in schizophrenia. Neuron. 2013;79:814–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Khadka S, Meda SA, Stevens MC, et al. . Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry. 2013;74:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Orliac F, Naveau M, Joliot M, et al. . Links among resting-state default-mode network, salience network, and symptomatology in schizophrenia. Schizophr Res. 2013;148:74–80. [DOI] [PubMed] [Google Scholar]
- 65. Ongür D, Lundy M, Greenhouse I, et al. . Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res. 2010;183:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Camchong J, MacDonald AW III, Bell C, Mueller BA, Lim KO. Altered functional and anatomical connectivity in schizophrenia. Schizophr Bull. 2011;37:640–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Huettel SA, Song AW, McCarthy G.. Functional Magnetic Resonance Imaging. Sunderland, MA: Sinauer Associates; 2004. [Google Scholar]
- 68. Sheffield JM, Barch DM. Cognition and resting-state functional connectivity in schizophrenia. Neurosci Biobehav Rev. 2016;61:108–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Iwashiro N, Koike S, Satomura Y, et al. . Association between impaired brain activity and volume at the sub-region of Broca’s area in ultra-high risk and first-episode schizophrenia: a multi-modal neuroimaging study. Schizophr Res. 2016;172:9–15. [DOI] [PubMed] [Google Scholar]
- 70. Ahveninen J, Jaaskelainen IP, Raij T, et al. . Task-modulated “what” and “where” pathways in human auditory cortex. Proc Natl Acad Sci USA. 2006;103:14608–14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ritsner MS. The Handbook of Neuropsychiatric Biomarkers, Endophenotypes, and Genes. Dordrecht: Springer; 2009. [Google Scholar]
- 72. Artigas F. The prefrontal cortex: a target for antipsychotic drugs. Acta Psychiatr Scand. 2010;121:11–21. [DOI] [PubMed] [Google Scholar]
- 73. Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol. 2015;29:97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kraguljac NV, White DM, Hadley JA, et al. . Abnormalities in large scale functional networks in unmedicated patients with schizophrenia and effects of risperidone. Neuroimage Clin. 2016;10:146–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lui S, Li T, Deng W, et al. . Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792. [DOI] [PubMed] [Google Scholar]
- 76. Sambataro F, Blasi G, Fazio L, et al. . Treatment with olanzapine is associated with modulation of the default mode network in patients with Schizophrenia. Neuropsychopharmacology. 2010;35:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–430. [DOI] [PubMed] [Google Scholar]
- 78. Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci USA. 2006;103:10046–10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BT. The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:2322–2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Choi EY, Yeo BT, Buckner RL. The organization of the human striatum estimated by intrinsic functional connectivity. J Neurophysiol. 2012;108:2242–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yeo BT, Krienen FM, Sepulcre J, et al. . The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann N Y Acad Sci. 2014;1316:29–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.