Abstract
Background
Perioperative fluid strategies influence clinical outcomes following major surgery. Many intravenous fluid preparations are based on simple solutions, such as normal saline, that feature an electrolyte composition that differs from that of physiological plasma. Buffered fluids have a theoretical advantage of containing a substrate that acts to maintain the body’s acid‐base status ‐ typically a bicarbonate or a bicarbonate precursor such as maleate, gluconate, lactate, or acetate. Buffered fluids also provide additional electrolytes, including potassium, magnesium, and calcium, more closely matching the electrolyte balance of plasma. The putative benefits of buffered fluids have been compared with those of non‐buffered fluids in the context of clinical studies conducted during the perioperative period. This review was published in 2012, and was updated in 2017.
Objectives
To review effects of perioperative intravenous administration of buffered versus non‐buffered fluids for plasma volume expansion or maintenance, or both, on clinical outcomes in adults undergoing all types of surgery.
Search methods
We electronically searched the Clinicaltrials.gov major trials registry, the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 6) in the Cochrane Library, MEDLINE (1966 to June 2016), Embase (1980 to June 2016), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to June 2016). We handsearched conference abstracts and, when possible, contacted leaders in the field. We reran the search in May 2017. We added one potential new study of interest to the list of ‘Studies awaiting classification' and will incorporate this trial into formal review findings when we prepare the review update.
Selection criteria
Only randomized controlled trials that compared buffered versus non‐buffered intravenous fluids for surgical patients were eligible for inclusion. We excluded other forms of comparison such as crystalloids versus colloids and colloids versus different colloids.
Data collection and analysis
Two review authors screened references for eligibility, extracted data, and assessed risks of bias. We resolved disagreements by discussion and consensus, in collaboration with a third review author. We contacted trial authors to request additional information when appropriate. We presented pooled estimates for dichotomous outcomes as odds ratios (ORs) and for continuous outcomes as mean differences (MDs), with 95% confidence intervals (CIs). We analysed data via Review Manager 5.3 using fixed‐effect models, and when heterogeneity was high (I² > 40%), we used random‐effects models.
Main results
This review includes, in total, 19 publications of 18 randomized controlled trials with a total of 1096 participants. We incorporated five of those 19 studies (330 participants) after the June 2016 update. Outcome measures in the included studies were thematically similar, covering perioperative electrolyte status, renal function, and acid‐base status; however, we found significant clinical and statistical heterogeneity among the included studies. We identified variable protocols for fluid administration and total volumes of fluid administered to patients intraoperatively. Trial authors variably reported outcome data at disparate time points and with heterogeneous patient groups. Consequently, many outcome measures are reported in small group sizes, reducing overall confidence in effect size, despite relatively low inherent bias in the included studies. Several studies reported orphan outcome measures. We did not include in the results of this review one large, ongoing study of saline versus Ringer's solution.
We found insufficient evidence on effects of fluid therapies on mortality and postoperative organ dysfunction (defined as renal insufficiency leading to renal replacement therapy); confidence intervals were wide and included both clinically relevant benefit and harm: mortality (Peto OR 1.85, 95% CI 0.37 to 9.33; I² = 0%; 3 trials, 6 deaths, 276 participants; low‐quality evidence); renal insufficiency (OR 0.82, 95% CI 0.34 to 1.98; I² = 0%; 4 trials, 22 events, 276 participants; low‐quality evidence).
We noted several metabolic differences, including a difference in postoperative pH measured at end of surgery of 0.05 units ‐ lower in the non‐buffered fluid group (12 studies with a total of 720 participants; 95% CI 0.04 to 0.07; I² = 61%). However, this difference was not maintained on postoperative day one. We rated the quality of evidence for this outcome as moderate. We observed a higher postoperative serum chloride level immediately after operation, with use of non‐buffered fluids reported in 10 studies with a total of 530 participants (MD 6.77 mmol/L, 95% CI 3.38 to 10.17), and this difference persisted until day one postoperatively (five studies with a total of 258 participants; MD 8.48 mmol/L, 95% CI 1.08 to 15.88). We rated the quality of evidence for this outcome as moderate.
Authors' conclusions
Current evidence is insufficient to show effects of perioperative administration of buffered versus non‐buffered crystalloid fluids on mortality and organ system function in adult patients following surgery. Benefits of buffered fluid were measurable in biochemical terms, particularly a significant reduction in postoperative hyperchloraemia and metabolic acidosis. Small effect sizes for biochemical outcomes and lack of correlated clinical follow‐up data mean that robust conclusions on major morbidity and mortality associated with buffered versus non‐buffered perioperative fluid choices are still lacking. Larger studies are needed to assess these relevant clinical outcomes.
Plain language summary
Buffered versus non‐buffered fluids given to adults during surgery
Review question
To review evidence from randomized controlled trials on safety and effects of administration of buffered versus non‐buffered fluids into the veins of adult patients undergoing surgery.
Background
During surgery, adults are given fluids into their veins to prevent or treat excessive loss of body water and salts (dehydration) and to compensate for loss of blood. Some fluids consist of a simple salt solution in the same salt concentration as cells and blood, such as isotonic saline; others are buffered solutions that resist changes in pH when small quantities of an acid or a base are added to them. Buffered fluids include additional electrolytes, including potassium, magnesium, and calcium, so they are matched more closely to fluid in the blood.
Study characteristics
We searched the literature up to June 2016 and found 19 studies, with a total of 1096 adults randomly assigned to receive buffered or non‐buffered fluids. Some included trials involved minor surgery in otherwise fit and healthy patients. Other trials analysed outcomes after major surgery in high‐risk patients, and five trials included patients undergoing renal transplant surgery. We reran the search in May 2017 and decided that we will deal with one new study of interest when we update the review.
Key results
Overall results show that the number of deaths was low and provide no evidence that choice of fluids ‐ buffered or non‐buffered ‐ influenced the number of deaths that occurred around the time of surgery in the three trials that looked at this outcome (involving 267 participants). We found no differences between groups in the numbers of participants whose kidney function was adversely affected. Analysis of clinical outcomes suggests that buffered fluids are an equally safe and effective alternative to non‐buffered fluids for adult patients undergoing surgery. The pH of the blood after surgery was reduced among patients receiving saline (pH 7.32 vs 7.38), suggesting that buffered fluids are associated with less metabolic acidosis. The saline group had higher serum chloride and sodium levels than the buffered fluid group. This might be expected, as members of the saline group were receiving saline and no other electrolytes. Higher serum chloride is a cause of metabolic acidosis.
Quality of the evidence
We assessed the quality of evidence as generally moderate, although quality of evidence showing effects of fluid choice on kidney function was low because of the presence of other factors that could affect kidney function in these participants. Evidence shows wide variation in the types of surgery performed and in drivers for and volumes of fluid administered across trials. Reported outcomes varied a great deal between included trials, and some results were expressed in ways that did not allow their inclusion in our findings.
Summary of findings
Summary of findings for the main comparison. Buffered versus non‐buffered crystalloid intravenous fluid for adults undergoing any form of surgery.
| Buffered versus non‐buffered crystalloid intravenous fluid for adults undergoing any form of surgery | ||||||
| Patient or population: adults receiving intravenous fluids whilst undergoing any form of surgery Setting: elective, major surgery in hospitals in Europe, USA, Asia, and the Middle East Intervention: buffered crystalloid intravenous fluid Comparison: non‐buffered crystalloid intravenous fluid | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with non‐buffered fluid | Risk with buffered fluid | |||||
| Mortality (in‐hospital death and 30‐day mortality) | Study population | OR 1.85 (0.37 to 9.33) | 267 (3 RCTs) | ⊕⊕⊕⊝ LOWa | ||
| 15 per 1000 | 28 per 1000 (6 to 126) | |||||
| Organ system failure ‐ renal insufficiency requiring support | Study population | OR 0.82 (0.34 to 1.98) | 267 (4 RCTs) | ⊕⊕⊝⊝ LOWb | ||
| 92 per 1000 | 77 per 1000 (33 to 168) | |||||
| Plasma pH ‐ postoperative pH | Mean postoperative pH was 7.32. | Mean postoperative pH in intervention group was 0.05 higher (0.04 to 0.07). | ‐ | 720 (12 RCTs) | ⊕⊕⊕⊝ MODERATEc | |
| Serum chloride (mmol/L) ‐ postoperative chloride | Mean postoperative chloride was 114.3 mmol/L. | Mean postoperative chloride in intervention group was 6.77 mmol/L lower (3.38 to 10.17). | ‐ | 530 (10 RCTs) | ⊕⊕⊕⊝ MODERATEd | |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded ‐2. Data from 3 studies only with few events. All 3 studies have unclear risk of bias for outcome assessment. Waters et al. also has unclear risk of reporting bias and attrition bias. Overall confidence in the effect estimate is low.
bDowngraded ‐2. Half of studies included patients with the confounding effect of existing organ failure, i.e. participants undergoing renal transplant for renal failure. All studies had unclear risk of detection bias. 2 studies had unclear risk of reporting bias. 2 studies had unclear risk of attrition bias.
cDowngraded ‐1. Significant heterogeneity in methods, included participant characteristics, and outcomes between studies. Small numbers of participants in each trial.
dDowngraded ‐1. Significant heterogeneity in methods, included participant characteristics, and outcomes between studies.
Background
Description of the condition
Administration of intravenous fluids is a nearly universal practice for patients undergoing major surgery who need to maintain intravascular volume at a time when that volume may be depleted owing to preoperative dehydration, intraoperative haemorrhage, or movement of fluid into a different physiological space. Intravenous fluids in these circumstances offer the advantages of being relatively cheap and easily available and causing few side effects as compared with blood transfusion. The ideal intravenous fluid would allow splinting of the circulation for an adequate time to replace missing plasma volume whilst not leading to adverse effects.
Intravenous fluids are manufactured with the addition of a mixture of electrolytes to water (making a crystalloid solution), and sometimes with the addition of a suspension of particles to water (making a colloid solution). A wide variety of available fluid formulations differ in two basic ways: first, by the different component electrolytes that are in solution with water, which can interact with the body's internal equilibrium once infused; and second, by the addition of a suspended non‐soluble colloid material to exert oncotic pressure. The colloid versus crystalloid debate has been extensively explored, but the electrolyte formulation itself has been less often examined (O'Connor 2001). In 2011, a series of guidelines on administration of intravenous fluids were released (GIFTASUP 2011). One key recommendation was that balanced solutions should be used for fluid resuscitation and replacement to avoid metabolic derangement. A recent consensus statement from the International Fluid Optimization Group also recommended the use of balanced crystalloids for low‐risk patients undergoing surgery of short duration (Navarro 2015).
Description of the intervention
Different intravenous solutions have been available for human use for many years (Cosnett 1989). For the past half‐century, the most widely used fluids have been based on a 0.9% sodium chloride solution (normal saline). Similarly, most colloids have been available only suspended in normal saline. This reliance on normal saline has been due in large part to its ease of manufacture and its ability to counteract effects of fluid loss ‐ capabilities that have been shown historically. Thus, saline‐based fluids have remained the standard of care when intravenous volume is required but administration of blood or blood products is not needed.
The electrolyte composition of normal saline is significantly different from that of plasma given to replace it. This may cause electrolyte imbalance, in particular, hyperchloraemic metabolic acidosis (Prough 1996), as plasma is diluted with saline. An electrolyte imbalance such as this alters the body's internal milieu and has a wide range of effects (Bellomo 2001; Kellum 2004).
How the intervention might work
Intravenous fluid formulations that closely match the constituents of human plasma have been available for some years (Hartmann 1934). In particular, these fluids contain a physiological buffer that helps to maintain the body's acid‐base balance. Other notable differences in the composition of these buffered fluids include variable quantities of other electrolytes, such as potassium, magnesium, and calcium, which closely reflect the composition of plasma. Several types of crystalloid and colloid solutions contain this physiological buffer (Table 5). Over the past few years, researchers have investigated and compared the effects of buffered and non‐buffered fluids by conducting in vitro (Roche 2006), animal (Wilcox 1983), and healthy volunteer studies (Reid 2003; Williams 1999).
1. Components of individual fluids (mmol/L when appropriate).
| Normal saline |
Plasmalyte 148 (buffered) |
Ringer's solution (buffered) |
Hextend (buffered) |
Hespan |
'Crystalloid A' (buffered) |
HES 130/0.42 (buffered) |
Vitafusal (buffered) |
VitaHES | |
| Sodium | 154 | 140 | 130 | 143 | 154 | 140 | 140 | 130 | 154 |
| Chloride | 154 | 98 | 112 | 124 | 154 | 127 | 118 | 112.5 | 154 |
| Potassium | 0 | 5 | 5.4 | 3 | 0 | 4 | 4 | 5.5 | 0 |
| Calcium | 0 | 0 | 1.8 | 2.5 | 0 | 2.5 | 2.5 | 1 | 0 |
| Magnesium | 0 | 1.5 | 0 | 0.5 | 0 | 1 | 1 | 1 | 0 |
| Lactate (buffer) |
0 | 0 | 27 (if lactated) | 28 | 0 | 0 | 0 | 0 | 0 |
| Acetate (buffer) | 0 | 27 | 27 (if acetated) | 0 | 0 | 24 | 24 | 27 | 0 |
| Maleate (buffer) |
0 | 0 | 0 | 0 | 0 | 5 | 5 | 0 | 0 |
| Gluconate (buffer) |
0 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Colloid | 0 | 0 | 0 | HES 670/0.8 | HES 450/0.8 | 0 | HES 130/0.42 | HES 130/0.42 | HES 130/0.42 |
| Dextrose (g/L) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
Manufacturers (when appropriate):
Plasmalyte 148: Baxter Healthcare Corporation, Deerfield, IL 60015, USA.
Ringer's solution: non‐proprietary.
Hextend: BioTime Inc., Berkeley, CA, USA.
Hespan: B Braun Medical Inc., Irvine, CA, USA.
Vitafusal: Serumwerk Bernburg AG, Bernberg, Germany.
VitaHES: Serumwerk Bernburg AG, Bernberg, Germany.
We planned to perform several subgroup analyses to identify patients for whom optimal selection of fluids may be important. Planned analyses involved (1) examining the effects of different colloid fluid types among elderly versus younger patients on the basis that younger patients may have greater physiological reserve; and (2) assessing the ability of the body to compensate for effects of different fluids among patients undergoing elective versus emergency procedures on the basis that patients treated in an emergency situation may have less capacity to deal with non‐buffered fluids, and among patients undergoing cardiac versus non‐cardiac surgery on the basis that cardiopulmonary bypass may have profound effects on acid‐base and electrolyte status.
Why it is important to do this review
Over the past decade, several published clinical trials have examined outcomes of surgery among adult patients, but these trials differed in outcomes measured, case mix included, size of study samples enrolled, and quality of evidence provided. Therefore, the clinical effects of buffered versus non‐buffered fluids among adult surgical patients remain uncertain. This systematic review is the first conducted to examine this topic.
Objectives
To review effects of perioperative intravenous administration of buffered versus non‐buffered fluids for plasma volume expansion or maintenance, or both, on clinical outcomes in adults undergoing all types of surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included only published randomized controlled trials (RCTs). We considered unpublished studies and studies published only in abstract format for inclusion if adequate information regarding study methods and results could be obtained.
Types of participants
We included studies on adults (aged 16 years and over) receiving intravenous fluids whilst undergoing any form of surgery.
Types of interventions
We included administration of intravenous fluids with and without a buffer (bicarbonate or bicarbonate precursor buffer, such as maleate, gluconate, lactate, or acetate) for the purpose of plasma volume expansion or maintenance during the perioperative period. To minimize confounding factors, we considered only trials in which the sole difference between experimental and control arms involved the presence or absence of a buffer in the fluid.
We excluded studies that compared crystalloids with colloids and those that compared fluids with different colloid components. However, we included trials with three or more arms that satisfied the other inclusion criteria.
We included as much as possible data reported by trials with three or more arms: If two groups could be combined, we attempted to do so by using the statistical methods presented in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We defined the perioperative period as extending from two hours before the start of surgery up to six hours after surgery or until arrival to a postanaesthetic care unit. We included only studies that used isotonic fluids (osmolarity 250 to 350 mmol/L) and a broadly physiological concentration of sodium (120 to 160 mmol/L).
Types of outcome measures
Primary outcomes
Mortality (all time frames reported)
Secondary outcomes
Clinically significant organ system dysfunction as defined in individual papers, including renal, pulmonary, hepatic, gastrointestinal, coagulation, and central nervous system
Surrogate measures of organ system dysfunction including urine output, serum creatinine, partial pressure of arterial carbon dioxide (PaCO2), nausea, and vomiting
Blood loss or transfusion requirement
Serum measures of coagulation such as prothrombin time, activated partial thromboplastin time, von Willebrand factor, antithrombin 3 activity, fibrinogen, and thromboelastography
Biochemical or electrolyte disturbances including pH, base excess, and serum bicarbonate, sodium, potassium, calcium, and chloride
Postoperative hospital length of stay
Functional health status and quality of life measures as described by identified papers
Cost
Search methods for identification of studies
Electronic searches
For this updated review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 6) in the Cochrane Library (see Appendix 1); MEDLINE via OvidSP (1966 to June 2016) (see Appendix 2); Embase via OvidSP (1980 to June 2016) (see Appendix 3); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) via EBSCOhost (1982 to June 2016) (see Appendix 4).
We reran the search in May 2017 and will deal with the one study of interest when we update the review.
We did not impose language restrictions for the search criteria.
(In the original review, we searched to May 2011 (Burdett 2012). We preserved topic search terms but updated filters for identifying RCTs in accordance with theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The initial search strategy ‐ used up to 2003 ‐ is available in Appendix 5.)
Searching other resources
We handsearched relevant journals and conference abstracts not previously handsearched by Cochrane Review groups and therefore not included in CENTRAL. For this update, we searched the following conference abstracts for relevant studies published to June 2016. (For the original review (Burdett 2012), we searched from 1998 to 2010.)
American Society of Anesthesiologists (ASA).
International Anesthesia Research Society (IARS).
Society of Cardiovascular Anesthesiologists (SCA).
Society of Critical Care Medicine (SCCM).
European Society of Intensive Care Medicine (ESICM).
American Thoracic Society (ATS).
European Association of Cardiothoracic Anesthesiologists (EACTA).
International Symposium on Intensive Care and Emergency Medicine (ISICEM).
American College of Surgeons (ACS).
Network for the Advancement of Transfusion Alternatives (NATA).
Association of University Anesthesiologists (AUA).
Society of Thoracic Surgeons (STS).
European Society of Anesthesiologists (ESA).
American Society of Critical Care Medicine (ASCCM).
We checked the reference lists of all identified trials and reviews and, when possible, contacted trial authors to ask if any studies had been missed.
Data collection and analysis
Selection of studies
Five review authors (TG, EB, AR, SB, and PO) independently identified appropriate studies after screening conference abstracts and abstracts identified via electronic searches.
Data extraction and management
Two review authors (SB and PO) independently extracted study characteristics and outcomes for each trial using a standardized data extraction form. We resolved disagreements by consensus or by consultation with a third review author (EB). We entered data into Review Manager software (RevMan 5.3) and checked them for accuracy. We attempted to contact study authors to obtain further information, when necessary.
Assessment of risk of bias in included studies
Two independent review authors (SB and PO) assessed risk of bias of included studies. When details on published manuscripts were not available, we attempted to contact study authors directly for clarification. When data were published in graphical form, we converted results to numerical form by enlarging and measuring the diagrams. We assessed potential risk of bias for each study using the Cochrane 'Risk of bias' tool (Higgins 2011).
For full details of included studies, see the Characteristics of included studies table. We discussed in the Results section the results of our 'Risk of bias' assessment. We performed sensitivity analyses to determine whether treatment effects on primary and secondary outcomes were the same when we assessed only studies that used adequate methods of randomization, allocation concealment, and study blinding, and provided a description of withdrawals.
Measures of treatment effect
When appropriate, we pooled trial data. We calculated the treatment effect across all trials using the Cochrane statistical package Review Manager 5.3 (RevMan 5.3). We calculated mean differences (MDs) with 95% confidence intervals (CIs) using an inverse variance method for continuous variables. When outcome data were skewed to the extent that the mean divided by the standard deviation (SD) was less than 1, indicating strong evidence of a skewed distribution (Altman 1996), and when only means and SDs on the un‐logged scale were available, we performed statistical manipulation as described in Higgins 2008 and Jones 2011 by transforming raw means and SDs to the log scale. We then analysed data on the log scale using a generic inverse variance method available in RevMan 5.3. We exponentiated pooled MDs between buffered and non‐buffered groups on the log scale to determine the ratio of geometric means of the variable on the un‐logged scale, which quantifies the relative difference in the original untransformed outcome variable between buffered and non‐buffered groups as a percentage difference, to aid interpretation (Bland 1996). For dichotomous variables, we used the Mantel‐Haenszel method with odds ratios (ORs) for common outcomes (> 5%) and Peto OR for rare outcomes (< 5%).
Unit of analysis issues
When studies included more than two groups, we merged data into groups when the intervention was equivalent. Some studies included groups of participants who did not receive the interventions of interest and excluded these groups from analyses.
Dealing with missing data
We contacted the authors of included trials to obtain required data that were missing from manuscripts and to discover missing information about methodological properties (randomization, allocation concealment, blinding) of these trials.
Assessment of heterogeneity
We quantified the degree of heterogeneity in trial results using the I² statistic, which expresses the percentage of total variation observed between studies due to differences between studies rather than to sampling error (Higgins 2011). We assumed significant heterogeneity when I² was 40% or greater. When heterogeneity was significant, we used random‐effects models. When I² was less than 40%, we used a fixed‐effect model.
Assessment of reporting biases
We planned to assess the presence of possible publication bias and heterogeneity for the primary outcome by using funnel plot analysis (Egger 1997; Sterne 2001). In the case of suspected publication bias, we intended to use the trim and fill method to assess the impact of potential publication bias and the robustness of the estimate (Gilbody 2000; Sutton 2000).
Data synthesis
We used Review Manager software (RevMan 5.3) to conduct our meta‐analyses when it was reasonable to assume that studies were estimating the same underlying treatment effect. When clinical heterogeneity was sufficient to suggest that the underlying treatment effect was not clinically meaningful, we did not combine trials, for example, when trials were examining insufficiently similar populations. We tested dichotomous outcomes using ORs and 95% CIs, and continuous outcomes using MDs between groups and 95% CIs. We assumed P < 0.05 to be of statistical significance.
Subgroup analysis and investigation of heterogeneity
To explore sources of heterogeneity between studies on those occasions when it was possible to do so, we planned to perform subgroup analyses for the primary outcome by colloid categories, age groups (≤ 65 years, > 65 years), elective and emergency surgery, cardiac and non‐cardiac surgery, fluids with and without calcium, fluids with and without magnesium, fluids with and without glucose, and fluids containing bicarbonate and fluids containing a bicarbonate precursor buffer. However, this was not possible because we found insufficient studies reporting our anticipated primary outcome of mortality at all time frames.
Sensitivity analysis
We planned to perform sensitivity analysis for the primary outcome to explore robustness of results by study quality, in particular in the presence or absence of adequate randomization, allocation concealment, study blinding, and other bias, as shown in the risk of bias assessment, when numbers of trials were adequate for us to do so. Second, when applicable, we planned to examine the results of skewed data using un‐logged data for comparisons.
'Summary of findings' table and GRADE
We judged the quality of evidence and generated a 'Summary of findings' table using GRADEproGDT (GRADEproGDT 2015;Guyatt 2011a). We based our assessment of the quality of evidence on assessments of imprecision, inconsistency, risk of bias, and indirectness for all studies reporting specific outcome measures. We considered the starting point to be 'high quality' because of the randomized design of all included studies. We downgraded quality by one or two levels on the basis of assessment of GRADE criteria and assessment of the methodological quality and design of included studies. The 'Summary of findings' table in this review presents mortality, organ system failure, postoperative pH, and postoperative chloride. Mortality was the primary outcome in this review; we therefore have presented these data in the table. We have reported organ system failure, postoperative pH, and postoperative chloride as secondary outcomes of interest.
Results
Description of studies
Results of the search
See Figure 1.
1.
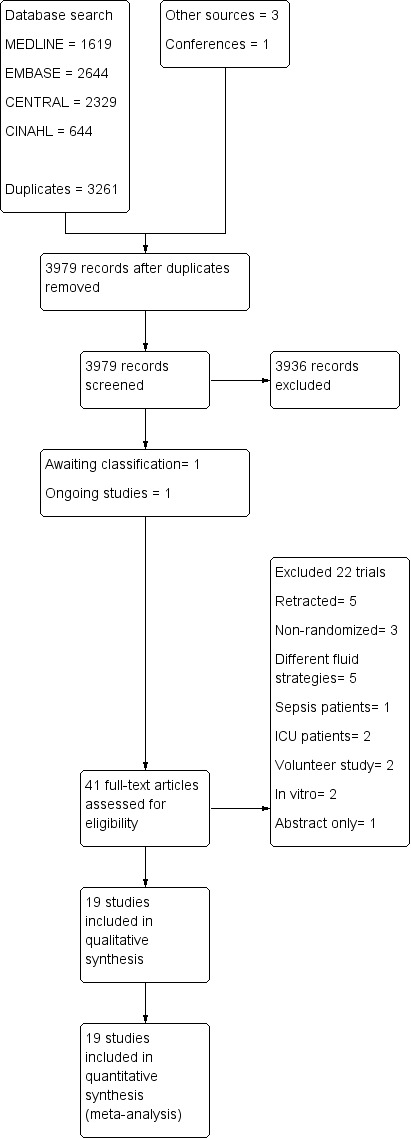
Prisma study flow diagram.
We reran the search in May 2017. We found one study of interest. We added this study to a list of ‘Studies awaiting classification' and will incorporate it into formal review findings during the review update.
We identified 3546 citations through database searches, manual searches, citation reviews, and contact with experts. After screening by title, then by abstract, we obtained full‐paper copies of 41 citations that were potentially eligible for inclusion in the review. We analysed each citation by hand and included 19 publications of 18 trials or comparisons.
We reran the search in May 2017, which revealed 432 new citations and one potential new study of interest. We added this potential new study of interest to a list of ‘Studies awaiting classification' and will incorporated it into formal review findings when we prepare the review update.
Included studies
Populations
Nineteen studies met our inclusion criteria for study design, participants, and interventions. We identified a total of 1096 participants, of whom 563 received buffered fluids and 533 received non‐buffered fluids. Two papers reported one trial, but reported outcomes were different in the two papers and showed no overlap, so we considered these publications separately (Martin 2002; Moretti 2003). We took care to ensure that participants were not counted twice. All included trials were fully published in peer‐reviewed journals. We were unable to identify any unpublished studies suitable for inclusion in our review. We have listed additional study characteristics in Table 2 and have described these studies under Characteristics of included studies.
2. Summary of trial interventions.
| Trial | Buffered arm intervention | Non‐buffered arm intervention | Participants given buffered fluids | Participants given non‐buffered fluids | Fluids given to both arms | Notes |
| Base 2011 | 6% HES 130/0.4 in balanced solution (Volulyte) | 6% HES 130/0.4 in saline (Voluven) |
43 | 38 | Lactated Ringer's | Cardiac surgery patients |
| Chin 2006 | Lactated Ringer's | Normal saline | 16 | 16 | None ‐ completely buffered vs completely non‐buffered | Minor surgery, low fluid volumes given |
| Gan 1999 | Hextend and lactated Ringer's | Hespan and lactated Ringer's | 60 | 60 | Lactated Ringer's (buffered) | |
| Hadimioglu 2008 | Lactated Ringer's or Plasmalyte 148 | Normal saline | 60 | 30 | None | We combined both buffered fluid arms for analysis |
| Heidari 2011 | Lactated Ringer's | Normal saline | 30 | 30 | None | |
| Khajavi 2008 | Lactated Ringer's | Normal saline | 26 | 26 | Blood as needed | Administered during renal transplantation surgery |
| Kim 2013 | Plasmalyte 148 | Normal saline | 30 | 30 | 750 mL 5% albumin | Administered during renal transplantation surgery |
| Kulla 2008 | 130/0.42 buffered HES and buffered crystalloid | 130/0.42 non‐buffered HES and normal saline | 29 | 32 | None | Acetate buffer in the buffered group |
| Martin 2002 | Hextend and lactated Ringer's | Hespan and lactated Ringer's | 30 | 30 | None | Same participant group as in Moretti 2003 but different outcomes described |
| McFarlane 1994 | Plasmalyte 148 | Normal saline | 15 | 15 | None | |
| Moretti 2003 | Hextend and lactated Ringer's | Hespan and lactated Ringer's | 30 | 30 | Lactated Ringer's | Same participant group as in Martin 2002 but different outcomes described |
| Nuraei 2010 | Lactated Ringer's | Normal saline | 54 | 54 | None | Administered during renal transplantation surgery |
| O'Malley 2005 | Lactated Ringer's | Normal saline | 25 | 26 | Normal saline with 20 mmol/L bicarbonate given to each group postoperatively | |
| Scheingraber 1999 | Lactated Ringer's | Normal saline | 12 | 12 | None | |
| Song 2015 | Plasmalyte 148 | Normal saline | 25 | 25 | 6% hydroxyethyl starch 130/0.4 in 0.9% saline administered to replace blood loss > 500 mL | |
| Takil 2002 | Lactated Ringer's | Normal saline | 15 | 15 | 500 mL gelofusine given to each participant | |
| Walsh 1983 | Lactated Ringer's | Normal saline | 7 | 7 | None | |
| Waters 2001 | Lactated Ringer's | Normal saline | 33 | 33 | Human albumin given to both arms | |
| Wilkes 2001 | Hextend and lactated Ringer's | Hespan and normal saline | 23 | 24 | None |
Manufacturer:
Gelofusine: B Braun Melsungen AG, Melsungen, Germany/
HES: hydroxyethyl starch.
Five studies included patients with renal transplants (Hadimioglu 2008; Khajavi 2008; Kim 2013; Nuraei 2010; O'Malley 2005). As this population was different from the population undergoing other perioperative procedures, we performed sensitivity analysis, when possible, for renal outcomes such as intraoperative urine output. Further, data described in Analysis 1.11.1 and Analysis 1.11.2 were significantly skewed to the extent that SDs divided by means were less than 1 for all included studies and SDs were comparable with mean values. We transformed data according to the third method described by Higgins, which is a suitable method for rendering skewed data appropriate for meta‐analysis, and we inserted the transformed data into RevMan for analysis (Higgins 2008; RevMan 5.3).
1.11. Analysis.
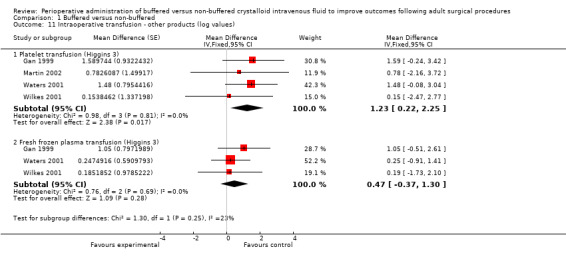
Comparison 1 Buffered versus non‐buffered, Outcome 11 Intraoperative transfusion ‐ other products (log values).
Interventions
Interventions varied between studies. Of 19 included publications, 13 used only crystalloids in their experimental and control arms (Chin 2006; Hadimioglu 2008; Heidari 2011; Khajavi 2008; Kim 2013; McFarlane 1994; Nuraei 2010; O'Malley 2005; Scheingraber 1999; Song 2015; Takil 2002; Walsh 1983; Waters 2001). Of these studies, nine compared lactated Ringer's solution versus normal saline (Chin 2006; Heidari 2011; Khajavi 2008; Nuraei 2010; O'Malley 2005; Scheingraber 1999; Takil 2002; Walsh 1983; Waters 2001), and four compared Plasmalyte 148 versus normal saline (Hadimioglu 2008; Kim 2013; McFarlane 1994; Song 2015). One trial included two buffered crystalloid arms, each consisting of 30 participants (Plasmalyte 148 and lactated Ringer's solution), and one normal saline arm of 30 participants (Hadimioglu 2008). We combined the two buffered arms numerically using the statistical methods described in Chapter 7 of the Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1; Higgins 2011), so in effect we compared one buffered arm of 60 participants consisting of combined Plasmalyte and lactated Ringer's arms versus the non‐buffered arm of 30 participants.
Six publications described five trials that used colloid solutions in their experimental and control arms (Base 2011; Gan 1999; Kulla 2008; Martin 2002; Moretti 2003; Wilkes 2001). All compared a buffered hydroxyethyl starch (HES) solution versus a non‐buffered HES solution. Of these, four trials used high molecular weight (MW) HES (Gan 1999; Martin 2002; Moretti 2003; Wilkes 2001), and two used low MW HES (Base 2011; Kulla 2008).
All trials used lactate as the buffering agent in the group given buffering fluids except one (Kulla 2008), which used a fluid containing acetate.
Of 19 identified publications, only seven had protocols that compared completely buffered versus completely non‐buffered fluids (Chin 2006; Heidari 2011; McFarlane 1994; Nuraei 2010; Scheingraber 1999; Walsh 1983; Wilkes 2001). The other reports described trials that administered a combination of buffered and non‐buffered fluids in one arm of the study (Base 2011; Gan 1999; Hadimioglu 2008; Khajavi 2008; Kim 2013; Kulla 2008; Martin 2002; Moretti 2003; O'Malley 2005; Song 2015; Takil 2002; Waters 2001). Hence these trials compared a partially buffered fluid regimen versus a totally buffered fluid regimen, although only one trial reported this after collecting study data (Hadimioglu 2008). More details appear in Table 2.
Outcomes
Outcomes obtained from these trials were similar in theme but were heterogeneous in units, statistical reporting methods, and time scales; as such, not all were suitable for meta‐analysis. When practical, we sought unpublished data in an attempt to rectify this. Our attempts were successful in some cases, as some trial authors provided outcome information beyond published details (Base 2011; Gan 1999; Martin 2002; Moretti 2003; Waters 2001; Wilkes 2001).
Data reported were heterogeneous. Data potentially of interest to this review but reported only in single studies and therefore not suitable for numerical analysis are listed as orphan outcomes in Table 3. In particular, the timing of reported endpoints of these trials was heterogeneous for the first 48 hours postoperatively. Only three studies described biochemical data beyond this time point (Khajavi 2008; Nuraei 2010; O'Malley 2005). We synthesized these data for analysis by dividing them into the following time categories.
3. Orphan outcomes.
| Study | Outcome | Participants | Results |
| Base 2011 | Respiratory failure | 81 | Four participants (9.3%) in buffered group and 1 (2.6%) in non‐buffered group developed respiratory failure. |
| Gan 1999 | Need for intraoperative calcium | 120 | Mean intraoperative calcium given was 4.2 mg in the buffered group and 220 mg in the non‐buffered group (P < 0.05). |
| Gan 1999 | Prothrombin time | 120 | Prothrombin time for buffered group was 16 seconds (± 4) and for non‐buffered group was 17 seconds (± 7). No important differences between groups |
| Khajavi 2008 | Renal artery thrombosis | 52 | 2 participants in buffered fluids group (lactated Ringer's) developed renal artery thrombosis and subsequent graft failure. No similar incidences were recorded in the non‐buffered fluids group (normal saline). No important differences between groups |
| Kulla 2008 | Blood loss on first postop day | 61 | Blood loss on first postoperative day in the buffered group was 289 ± 325 mL and in the non‐buffered group was 309 ± 250 mL. No important differences between groups |
| Kulla 2008 | Factor VIII at 6 hours postop and on first postop day | 61 | Factor VIII levels at 6 hours postop was 141% ± 49 and 142% ± 49 for buffered and non‐buffered groups, respectively. No statistically significant differences Factor VIII levels first postoperative day was 123% ± 47 and 132% ± 43 for buffered and non‐buffered groups, respectively. No important differences between groups |
| Kulla 2008 | Ristocetin cofactor at 6 hours postop and on first postop day | 61 | Ristocetin cofactor levels at 6 hours postop were 143% ± 15 and 148% ± 10 for buffered and non‐buffered groups, respectively. No statistically significant differences Ristocetin cofactor levels on first postop day were 145% ± 14 and 150% ± 0 for buffered and non‐buffered groups, respectively. No important differences between groups |
| Moretti 2003 | Postop nausea | 60 | Postoperative nausea was reported in 22 (73%) participants in the buffered group and in 14 (47%) participants in the non‐buffered group. |
| O'Malley 2005 | Serum creatinine 6 months postop | 51 | Serum creatinine at 6 months was 133 ± 54 μmol/L and 133 ± 35 μmol/L for buffered and non‐buffered groups, respectively. |
| Song 2015 | Rotational Thromboelastography | 50 | ROTEM analyses revealed that values of MCF in FibTEM, CFT, a angle and MCF in INTEM, CT, CFT, a angle and MCF in EXTEM at end of surgery were changed towards a hypocoagulable state compared with their corresponding baseline values in both groups (P < 0.05). However, no important differences in FibTEM, INTEM, and EXTEM analyses were noted between Plasmalyte and NS groups. |
| Takil 2002 | Serum bicarbonate at 5 to 10 hours postop | 30 | Serum bicarbonate at 6 hours was 23.6 ± 2.2 mmol/L and 19.3 ± 2.2 mmol/L for buffered and non‐buffered groups, respectively (P < 0.01). |
| Wilkes 2001 | Gastric tonometry | 47 | Gastric tonometry showed P(g‐a)CO2 of 0.9 ± 1.1 kPa in the balanced group and 1.7 ± 0.5 kPa in the unbalanced group (P = 0.04). |
| Wilkes 2001 | Serum total calcium | 47 | Postoperative total serum calcium was 2.0 ± 0.2 mmol/L for the balanced group and 1.6 ± 0.2 mmol/L for the non‐balanced group (P = 0.0001). |
Data potentially of interest to this review but reported only in single studies and therefore not suitable for numerical analysis.
CFT: clot formation time; CT: clotting time; EXTEM, FibTEM, INTEM: Trade names for types of ROTEM assay; kPa: kilopascals; MCF: maximum clot firmness; mL: millilitres; mmol/L: millimols per litre; NS: normal saline; P(g‐a)CO2: partial pressure of gastric minus arterial carbon dioxide; postop: postoperative; ROTEM: manufacturer's name of device ‐ Rotational Thromboelastometry; s: seconds;:μmol/L: micromols/litre.
Immediately postoperatively: This category consisted of the first reported postoperative data unless stated otherwise in the trial.
Five to 10 hours postoperatively: All studies that included data within these times are included here.
First postoperative day (POD1): This category included all data described as reported 12 to 24 hours postoperatively or on postoperative day one.
We included additional time categories as appropriate.
Funding sources
Five of the included trials received funding from pharmaceutical companies that manufactured an intervention examined in the trial (Base 2011; Gan 1999; Martin 2002; Moretti 2003; Wilkes 2001). Each included study appropriately disclosed all funding.
Excluded studies
We excluded 22 clinical studies for reasons described in the Characteristics of excluded studies table (Bennett‐Guerrero 2001; Bick 1995; Boldt 1993; Boldt 2002a; Boldt 2002b; Boldt 2007; Boldt 2009; Boldt 2010; Campbell 1990; Choi 2010; Evans 2003; Javnrin 1980; Kaplan 2001; Krebbel 2014; Lowery 1967; Protsenko 2009; Reid 2003; Roche 2006; Ruttman 1996; Walker 2001; Williams 1999; Young 2015).
Studies awaiting classification
We identified one study awaiting classification for the updated review in the May 2017 search (Pfortmueller 2017). Please refer to the Characteristics of studies awaiting classification table for details.
Of two studies awaiting classification from the previous review (Burdett 2012), we included one in this updated review following translation into English (Nuraei 2010) and we excluded the other (Choi 2010).
Ongoing studies
We identified one ongoing study on the ClinicalTrials.gov trials registry (NCT02565420). This study is currently recruiting participants for a trial of saline versus Ringer's lactate. The primary outcome measure is major postoperative complications, and the study has an estimated completion date of 2022.
Risk of bias in included studies
Allocation
All trials were randomized. Fifteen trials provided details about allocation sequence generation and were considered to be at low risk of bias (Base 2011; Chin 2006; Gan 1999; Hadimioglu 2008; Heidari 2011; Khajavi 2008; Kim 2013; Martin 2002; Moretti 2003; Nuraei 2010; O'Malley 2005; Song 2015; Takil 2002; Waters 2001; Wilkes 2001). Generally, participant numbers in these trials were low, with four trials enrolling fewer than 20 participants in each arm (McFarlane 1994; Scheingraber 1999; Takil 2002; Walsh 1983).
Twelve studies mentioned the method of allocation concealment used and we considered them to be at low risk of selection bias (Base 2011; Chin 2006; Gan 1999; Khajavi 2008; Kim 2013; Martin 2002; Moretti 2003; O'Malley 2005; Song 2015; Takil 2002; Waters 2001; Wilkes 2001). Two studies showed no evidence of allocation concealment, and we considered them to be at high risk of bias for this criterion (McFarlane 1994; Walsh 1983).
We have summarized this information in Figure 2 and Figure 3.
2.
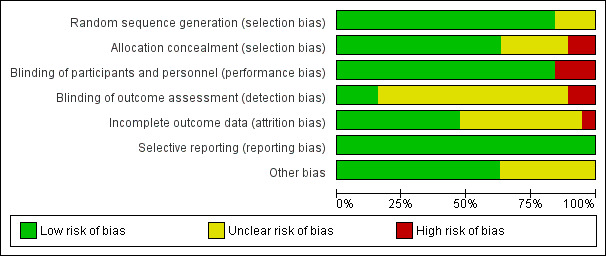
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
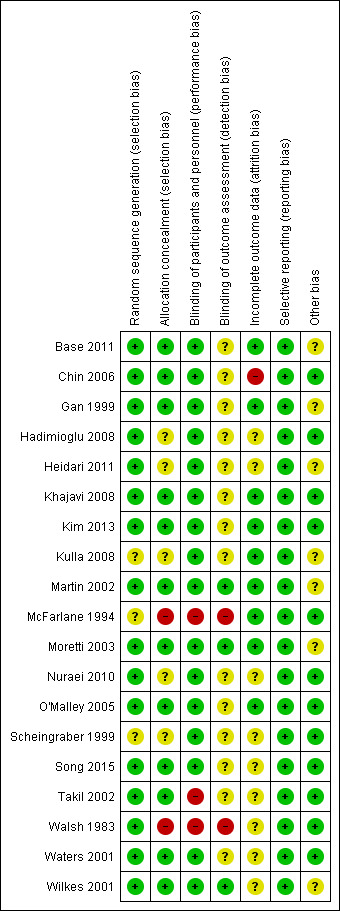
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Blinding
All included studies were randomized trials. A total of 16 studies referred to blinding or double‐blinding in their design (Base 2011; Chin 2006; Gan 1999; Hadimioglu 2008; Heidari 2011; Khajavi 2008; Kim 2013; Kulla 2008; Martin 2002; Moretti 2003; Nuraei 2010; O'Malley 2005; Scheingraber 1999; Song 2015; Waters 2001; Wilkes 2001); hence, we considered these studies to be at low risk of performance and detection bias. Only three studies made no reference to blinding, and we considered them to be at high risk for potential bias (McFarlane 1994; Takil 2002; Walsh 1983). We did not consider the included studies to be at risk for any other potential performance or detection bias.
Incomplete outcome data
Investigators lost few participants to follow‐up and gave reasons for all lost participants. Researchers performed intention‐to‐treat analysis, when possible, except in one case, when the trial excluded three participants after randomization because they met an exclusion criterion preoperatively (O'Malley 2005). The manuscript does not detail which group each participant was excluded from, and investigators analysed the data after these participants were removed. We judged only one trial to be at high risk of attrition bias because a high proportion of participants dropped out of the trial owing to administration of non‐protocol intravenous fluids (Chin 2006).
Selective reporting
We did not detect reporting bias and therefore categorized all studies as low risk.
Other potential sources of bias
Of note, pharmaceutical companies that manufactured an intervention of interest funded five of the included studies (Base 2011; Gan 1999; Martin 2002; Moretti 2003; Wilkes 2001). Although each study clearly disclosed these funding sources, we considered these studies to be at unclear risk of bias. Two other studies did not report sufficient detail about outcomes of interest, and we therefore considered them to be at unclear risk of bias (Heidari 2011; Kulla 2008).
Effects of interventions
See: Table 1
All included studies reported at least one outcome of interest, as described above in Types of outcome measures. Reported outcomes varied a great deal between included trials, and some study authors expressed data in ways that made them unsuitable for statistical synthesis.
Primary outcome
Mortality
Three clinical trials with a total of 267 participants reported mortality (Base 2011: Gan 1999; Waters 2001). Mortality was low in both groups: 2.9% (4/136) in the buffered group and 1.5% (2/131) in the non‐buffered group. Pooling of these limited data suggests no important mortality differences between groups (Peto OR 1.85, 95% CI 0.37 to 9.33; I² = 0%; Analysis 1.1). We downgraded the quality of evidence from high to low owing to imprecision of trial results due to small sample sizes, wide confidence intervals, and methodological variability between studies. Studies reporting mortality presented few events, and we consider all three studies to be at unclear risk of bias for outcome assessment. One of these studies is also at unclear risk of reporting bias and attrition bias (Waters 2001). Overall confidence in the effect estimate is low.
1.1. Analysis.
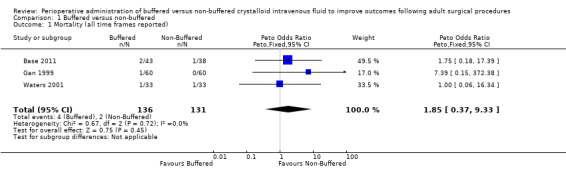
Comparison 1 Buffered versus non‐buffered, Outcome 1 Mortality (all time frames reported).
Secondary outcomes
Organ system failure
Four trials with a total of 267 participants reported on renal insufficiency leading to the requirement for renal replacement therapy (Hadimioglu 2008; Kim 2013; O'Malley 2005; Waters 2001). Data show lower risk of renal insufficieny requiring renal support with the use of buffered fluids (4.7% (11/148) vs 9.2% (11/119)), but we have little confidence that this difference is real (OR 0.82, 95% CI 0.34 to 1.98; I² = 0%; Analysis 1.2). We rated the quality of evidence as low because of limitations in study design and implementation. Three of these studies included participants with the confounding effect of pre‐existing organ failure (i.e. participants undergoing renal transplant for renal failure) (Hadimioglu 2008; Kim 2013; O'Malley 2005).
1.2. Analysis.
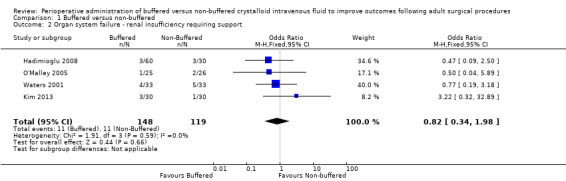
Comparison 1 Buffered versus non‐buffered, Outcome 2 Organ system failure ‐ renal insufficiency requiring support.
The single study that examined respiratory failure enrolled 81 participants and reported four cases (9.3%) of postoperative respiratory failure in the buffered group and one case (2.6%) in the non‐buffered group (Base 2011). Authors of the primary study offered no comment on reported differences between groups. We did not subject these data to further analysis, and we listed this outcome as an orphan outcome in Table 3.
No trials specified any outcomes regarding failure of other organ systems (cardiac, gastrointestinal, or neurological).
Surrogate measures of organ system dysfunction (urine output, serum creatinine, PaCO2, nausea and vomiting)
Urine output
Eight trials with a total of 459 participants reported intraoperative urine output during the intraoperative period and on the first postoperative day(Gan 1999; Kulla 2008; Kim 2013; O'Malley 2005; Scheingraber 1999; Takil 2002; Waters 2001; Wilkes 2001). Mean urine output reported intraoperatively was 872 mL for the buffered fluid group and 799 mL for the non‐buffered fluid group. Data show no important differences between groups. The mean difference was 6.1 mL higher in the buffered group (95% CI ‐128.41 to 140.61; I² = 49%). We encountered significant heterogeneity for this analysis (I² = 49%); therefore we calculated this comparison using a random‐effects model (Analysis 1.3).
1.3. Analysis.
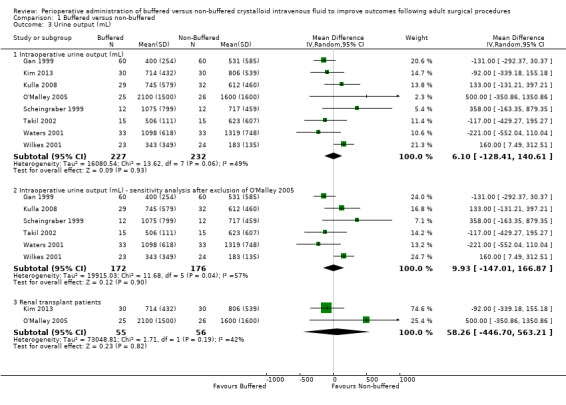
Comparison 1 Buffered versus non‐buffered, Outcome 3 Urine output (mL).
Four studies included renal transplant patients, and we believe that this group represented a different population with abnormal renal function and may not be comparable with the standard perioperative participants included in other studies (Khajavi 2008; Kim 2013; Nuraei 2010; O'Malley 2005). We performed sensitivity analysis while excluding these studies, which confirmed no important differences between groups for intraoperative urine output, with a mean difference of 10 mL (95% CI ‐147 to 167; I² = 57%; Analysis 1.3). We were unable to perform a subgroup analysis of intraoperative urine output for these renal transplant participants, as only one study reported this outcome (O'Malley 2005), and another study reported on urine output on the first operative day (Hadimioglu 2008).
Two trials with a total of 151 participants reported urine output by the first postoperative day (Hadimioglu 2008; Kulla 2008). We were unable to pool these data owing to clinical heterogeneity between studies (Analysis 1.3). One study enrolled renal transplant patients, and these participants had a large amount of urine output on the first postoperative day (Hadimioglu 2008). Once again, this patient group may not reflect perioperative participants in the other study (Kulla 2008). We performed a subgroup analysis of data from the only two studies that reported intraoperative urine output in participants undergoing renal transplant surgery (Kim 2013; O'Malley 2005). Pooled outcomes for both studies had wide 95% confidence intervals and did not indicate an important difference between groups;MD was 58.26 mL greater with buffered solutions (95% CI ‐446.7 to 563.21; P = 0.82; I² = 42%).
One study reported 24‐hour urine output in mL/kg body weight, and we were unable to get further information from trial authors (Base 2011). A second study reported intraoperative urine output in mL/kg/h, and we were unable to obtain further information from these authors as well (Song 2015).
Postoperative serum creatinine change
Two trials with a total of 113 participants reported postoperative serum creatinine change (Waters 2001; Wilkes 2001). Data show no important differences in the postoperative creatine change between buffered and non‐buffered groups; the mean difference was 6.96 µmol/L lower in the buffered group (95% CI ‐27.42 to 13.50; I² = 89%). Owing to significant heterogeneity (I² = 89%), we used random‐effects models for this comparison (Analysis 1.4).
1.4. Analysis.
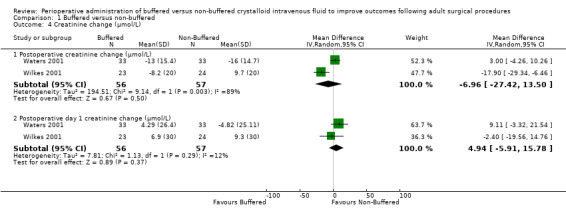
Comparison 1 Buffered versus non‐buffered, Outcome 4 Creatinine change (µmol/L).
Postoperative day one creatinine change
Two trials with a total of 113 participants reported postoperative day one creatinine change (Waters 2001; Wilkes 2001). Data show no important differences in postoperative day one creatinine change between groups. The mean difference was 4.94 µmol/L lower in the non‐buffered group (95% CI ‐5.91 to 15.78; I² = 12%; Analysis 1.4).
Postoperative absolute creatinine values
Three trials with a total of 235 participants reported postoperative creatinine (Kulla 2008; Nuraei 2010; Waters 2001). One trial reported postoperative creatinine in participants undergoing renal transplant surgery (Nuraei 2010). In this study, investigators reported that mean creatinine was 530 µmol/L in the buffered fluid group and 460 µmol/L in the non‐buffered group. Data show no important differences between groups. Trials conducted in non‐renal transplant participants reporting absolute creatinine reported that mean creatinine was 76.72 µmol/L in the buffered fluid group and 79.53 µmol/L in the non‐buffered group. Overall, for all three trials together, the MD was ‐1.31 µmol/L lower in the non‐buffered group (95% CI ‐9.30 to 6.68; I² = 71%; Analysis 1.5).
1.5. Analysis.
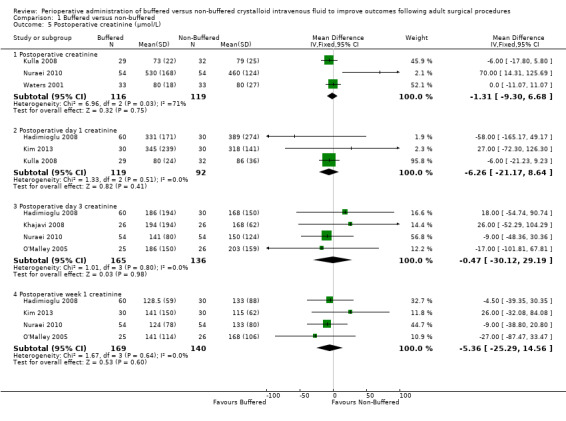
Comparison 1 Buffered versus non‐buffered, Outcome 5 Postoperative creatinine (µmol/L).
Three trials with a total of 211 participants reported postoperative day one creatinine (Hadimioglu 2008; Kim 2013; Kulla 2008). Two studies enrolled renal transplant patients (Hadimioglu 2008; Kim 2013). Data show a mean difference 6.26 µmol/L lower in the buffered group (95% CI ‐21.17 to 8.64; I² = 0%; Analysis 1.5).
Four trials with a total of 301 participants reported postoperative day three creatinine (Hadimioglu 2008; Khajavi 2008; Nuraei 2010; O'Malley 2005). All four studies enrolled renal transplant patients. Investigators reported mean postoperative day three creatinine of 172.5 µmol/L in the buffered group and 167.5 µmol/L in the non‐buffered group. Data show no important differences between groups; the MD was 0.47 µmol/L lower in the buffered group (95% CI ‐30.12 to 29.19; I² = 0%; Analysis 1.5).
Four trials with a total of 309 participants reported postoperative week one serum creatinine (Hadimioglu 2008; Kim 2013; Nuraei 2010; O'Malley 2005). All four studies enrolled renal transplant patients. Mean postoperative week one serum creatinine was 131.1 µmol/L in the buffered group and 114.2 in the non‐buffered group. Data show no important differences between groups; MD was 5.36 µmol/L lower in the buffered group (95% CI ‐25.29 to 14.56; I² = 0%; Analysis 1.5).
One trial with a total of 51 participants reported six‐month serum creatinine of 132.64 (± 53.84) µmol/L in the buffered group and 132.6 (± 35.3) µmol/L in the non‐buffered group (O'Malley 2005). Trial authors reported no clear differences between groups. We did not attempt to perform analysis for this comparison (Table 3).
Postoperative creatinine clearance
Three trials with a total of 222 participants reported postoperative creatinine clearance (Base 2011; Hadimioglu 2008; O'Malley 2005). Results show no important differences in this outcome between groups; MD was 12.61 mL/min higher in the non‐buffered group (95% CI ‐2.31 to 27.54; I² = 0%; Analysis 1.6).
1.6. Analysis.
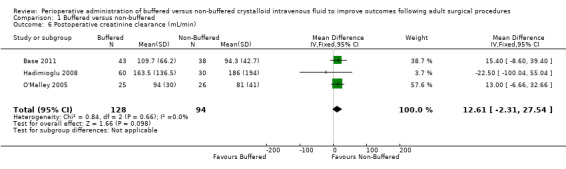
Comparison 1 Buffered versus non‐buffered, Outcome 6 Postoperative creatinine clearance (mL/min).
Partial pressure of arterial carbon dioxide (PaCO²)
Seven trials with a total of 446 participants reported postoperative PaCO² at two time points (Hadimioglu 2008; Kim 2013; Kulla 2008; Nuraei 2010; Song 2015; Takil 2002; Wilkes 2001). Results show mean PaCO² of 34.9 mmHg in the buffered fluid group and 35.0 mmHg in the non‐buffered fluid group. PaCO₂ was higher in the buffered group (MD 1.05 mmHg, 95% CI 0.15 to 1.94; I² = 0%; Analysis 1.7).
1.7. Analysis.
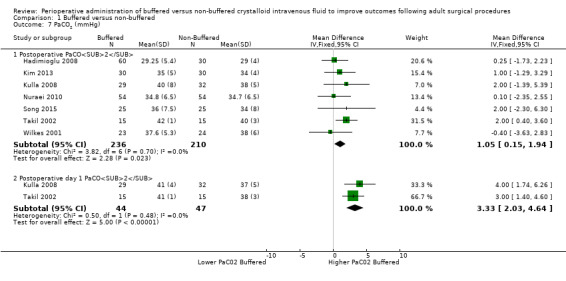
Comparison 1 Buffered versus non‐buffered, Outcome 7 PaCO2 (mmHg).
Two trials with a total of 91 participants reported postoperative day one PaCO² of 41 mmHg in the buffered fluid group and 37.7 mmHg in the non‐buffered fluid group (Kulla 2008; Takil 2002). PaCO₂ was higher in the buffered group (MD 3.3 mmHg, 95% CI 2.03 to 4.64; I² = 0%; Analysis 1.7).
Postoperative vomiting
Three trials reported 21 episodes of postoperative vomiting in 84 participants (25%) in the buffered fluid group and 28 episodes of postoperative vomiting in 84 participants (33%) in the non‐buffered fluid group (Heidari 2011; Moretti 2003; Wilkes 2001). Data show no clear differences between groups (OR 0.66, 95% CI 0.34 to 1.30; I² = 20%; Analysis 1.8).
1.8. Analysis.
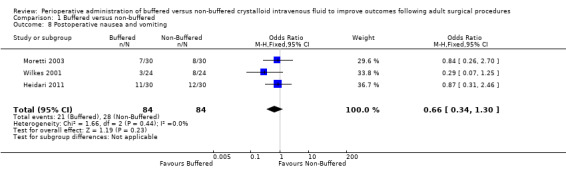
Comparison 1 Buffered versus non‐buffered, Outcome 8 Postoperative nausea and vomiting.
One trial with a total of 60 participants reported that 22 participants (73%) in the buffered group and 14 participants (47%) in the non‐buffered group had experienced nausea postoperatively (Moretti 2003). The authors of this study did not perform statistical analysis of differences between these groups. We did not attempt numerical analysis for this orphan outcome (Table 3).
One trial with a total of 47 participants reported differences between PaCO₂ outcomes measured by gastric tonometry and arterial blood gas analysis (Pg‐aCO₂) of 0.9 ± 1.1 kPa for the buffered group and 1.7 ± 0.5 kPa for the non‐buffered group (P = 0.04) (Wilkes 2001). We did not attempt numerical analysis for this orphan outcome (Table 3).
Blood loss and transfusion requirement
Blood loss
Thirteen trials reported on intraoperative blood loss (mL) (Base 2011; Gan 1999; Khajavi 2008Kulla 2008; Martin 2002; McFarlane 1994; O'Malley 2005; Scheingraber 1999; Song 2015; Takil 2002; Walsh 1983; Waters 2001; Wilkes 2001). Two studies reported estimated blood loss in mL/kg and could not be included in the analysis because they did not report patient weight (Base 2011; McFarlane 1994). Analysis of data from the other nine studies with a total of 576 participants revealed 287 in the buffered fluid groups and 287 in the non‐buffered fluid groups. Clinical heterogeneity between these trials was great, with two trials reporting less than 400 mL of estimated blood loss (O'Malley 2005; Walsh 1983) and two trials reporting estimated blood loss of 2 L or more (Takil 2002; Waters 2001). These findings reflect the type of surgery conducted, showing relatively large amounts of blood loss for abdominal aortic aneurysm repair (Waters 2001) and during major spinal surgery (Takil 2002). Results made it unlikely that any analysis would yield a clinically significant result if the group was analysed as a whole.
We performed subgroup analysis to attempt to reduce clinical heterogeneity by arbitrarily grouping trials with less than 1000 mL of blood loss and those with blood loss of 1000 mL or more (Analysis 1.9). Trials reporting blood loss less than 1000 mL (five studies with 202 participants) reported no important differences between group sand showed mean difference in intraoperative blood loss that was 5.90 mL higher in the buffered group (95% CI ‐45.18 to 56.99; I² = 0%) (Khajavi 2008; Kulla 2008; O'Malley 2005; Scheingraber 1999; Walsh 1983). Trials reporting blood loss was of 1000 mL or more (six studies with 374 participants) also reported no important differences in blood loss between groups and showed mean difference in intraoperative blood loss that was 173 mL lower in the buffered group (95% CI ‐438.8 to 92.7; I² = 13%; Analysis 1.9) (Gan 1999; Martin 2002; Song 2015; Takil 2002; Waters 2001; Wilkes 2001).
1.9. Analysis.
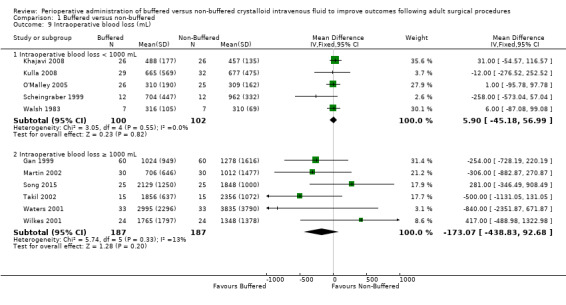
Comparison 1 Buffered versus non‐buffered, Outcome 9 Intraoperative blood loss (mL).
One trial with a total of 61 participants reported estimated blood loss during the first postoperative day (Kulla 2008). Trial authors reported no important differences in blood loss between buffered (289 ± 325 mL) and non‐buffered groups (309 ± 250 mL). We did not include these data in the analysis and listed this as an orphan clinical outcome in Table 3.
Intraoperative red cell transfusion
Four trials with a total of 152 participants reported on the quantity of intraoperative red cell transfusion (O'Malley 2005; Scheingraber 1999; Takil 2002; Wilkes 2001). Data show no important differences in the quantity of red cells transfused between individuals given buffered fluids and those given non‐buffered solutions. The mean difference was 29 mL less in the buffered fluid group (95% CI ‐117 to 59; I² = 28%; Analysis 1.10).
1.10. Analysis.
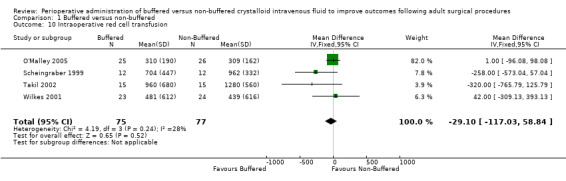
Comparison 1 Buffered versus non‐buffered, Outcome 10 Intraoperative red cell transfusion.
Other blood products given
Outcomes for this comparison include data that were significantly skewed. Therefore, we first transformed data according to the third method described in an article written by Higgins (Higgins 2008) and subsequently analysed them using the inverse variance method. Estimated effect sizes and associated 95% CIs consequently quantified the relative difference in the original un‐transformed outcome variable between groups (ratio of geometric means), expressed as a percentage.
Platelet transfusion
Data from four studies with a total of 293 participants that detailed the volume of platelets transfused in each arm were suitable for analysis (Gan 1999; Martin 2002; Waters 2001; Wilkes 2001). Analysis revealed an important difference between treatment groups in the volume of platelets transfused. The pooled estimate showed that 242% (log ratio 1.23) more platelets were transfused (mL) in the non‐buffered group than in the buffered group (95% CI 24.61% to 848.77%; I² = 0%; Analysis 1.11).
Fresh frozen plasma given
Three studies with a total of 233 participants reported the volume of fresh frozen plasma given (Gan 1999; Waters 2001; Wilkes 2001). Results revealed no important differences between groups. The pooled estimate showed that 60% (log ratio 0.47) more fresh frozen plasma (mL) was given in the non‐buffered group, but this finding was not statistically significant (95% CI ‐30.93% to 266.93%; I² = 0%; Analysis 1.11).
None of these trials reported administration of any other blood product, such as cryoprecipitate or blood factor concentrate.
Serum measures of coagulation
Measures included activated partial thromboplastin time, prothrombin time, Factor VIII, von Willebrand factor, ristocetin cofactor, and thromboelastography, antithrombin 3 activity, fibrinogen and thromboelastogram.
Two studies with a total of 181 participants reported serum activated partial thromboplastin time (APTT) at a series of time intervals (Gan 1999; Kulla 2008). APTT at end of surgery was 35.5 seconds in the buffered fluid group and 34.6 seconds in the non‐buffered fluid group, showing no important differences between groups. The mean difference was 1 second higher in the buffered group (95% CI ‐1.82 to 3.58; I² = 0%; Analysis 1.12). One trial involving 62 participants reported that APTT on postoperative day one was 35 ± 4 seconds in the buffered group and 34 ± 4 seconds in the non‐buffered group (Kulla 2008). This difference was not statistically significant, and we did not subject these data to numerical analysis (Table 3).
1.12. Analysis.
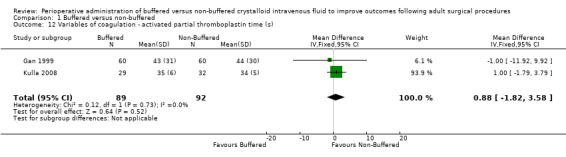
Comparison 1 Buffered versus non‐buffered, Outcome 12 Variables of coagulation ‐ activated partial thromboplastin time (s).
Only one trial reported prothrombin time (PT) (Gan 1999). At end of surgery, PT was 16 ± 4 seconds in the buffered group and 17 ± 7 seconds in the non‐buffered group, showing no important differences between groups. We did not subject these data to numerical analysis (Table 3).
Two trials with a total of 181 participants reported postoperative Factor VIII (Gan 1999; Kulla 2008) levels of 92.8 IU/L in the buffered group and 122.4 IU/L in the non‐buffered group. Data show an important difference between groups, with a mean difference 29.6 IU/L lower in the buffered group (95% CI ‐46.2 to ‐12.9; I² = 0%; Analysis 1.13). One trial reported postoperative Factor VIII levels 5 to 10 hours postoperatively and on the first postoperative day (Kulla 2008). Data show no important differences between groups. We did not analyse these data and listed this as an orphan outcome in Table 3.
1.13. Analysis.
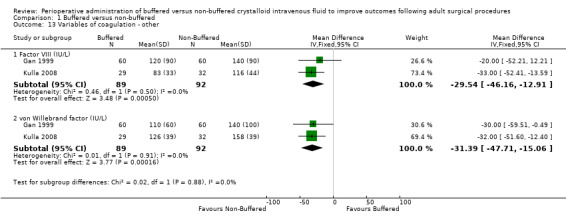
Comparison 1 Buffered versus non‐buffered, Outcome 13 Variables of coagulation ‐ other.
Two trials with a total of 181 participants reported serum levels of von Willebrand factor (vWF) of 121.1 IU/L in the buffered fluid group and 152.5 IU/L in the non‐buffered fluid group (Gan 1999; Kulla 2008). Results show an important difference between groups, with a mean difference 31.4 IU/L lower in the buffered fluid group than in the non‐buffered fluid group (95% CI ‐47.7 to ‐15.1; I² = 0%; Analysis 1.13).
Only one trial with a total of 62 participants reported ristocetin cofactor (Kulla 2008). Five to 10 hours postoperative and postoperative day one ristocetin cofactor levels showed no important differences between groups. We did not analyse these data and listed this as an orphan outcome in Table 3.
Three trials reported thromboelastographic (TEG) data (Gan 1999; Martin 2002; Song 2015). Two studies reported postoperative TEG data graphically (Gan 1999; Martin 2002). Therefore we did not subject this measure to meta‐analysis.
Serum biochemical or electrolyte disturbances
Measures included pH, base excess, serum bicarbonate, glucose, chloride,sodium, potassium, lactate, and calcium.
pH
Twelve studies with a total of 720 participants reported postoperative pH (Base 2011; Hadimioglu 2008; Khajavi 2008; Kim 2013; Kulla 2008; Nuraei 2010; O'Malley 2005; Scheingraber 1999; Song 2015; Takil 2002; Waters 2001; Wilkes 2001). Reporting was heterogeneous with different time intervals. Mean postoperative pH was 7.38 in the buffered fluid group and 7.32 in the non‐buffered fluid group. Data show that postoperative pH was 0.05 units lower (95% CI ‐0.04 to ‐0.07; I² = 61%) in the non‐buffered fluid group than in the buffered group ‐ an important difference between groups. However, we downgraded the quality of this evidence by one level to moderate because we noted a significant degree of heterogeneity. Two trials with a total of 91 participants reported pH on postoperative day one (Kulla 2008; Takil 2002). Results show no important differences between groups; MD was 0.01 units lower in the non‐buffered fluid group (95% CI ‐0.00 to 0.03; I² = 0%; Analysis 1.14).
1.14. Analysis.
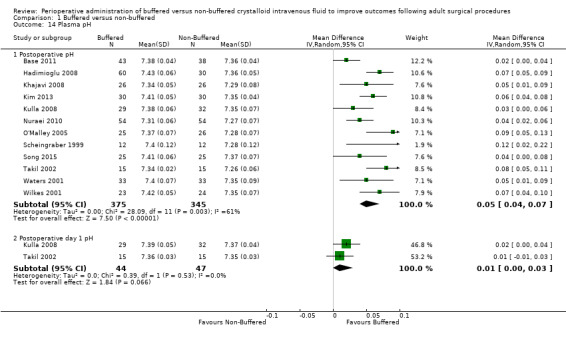
Comparison 1 Buffered versus non‐buffered, Outcome 14 Plasma pH.
Base excess
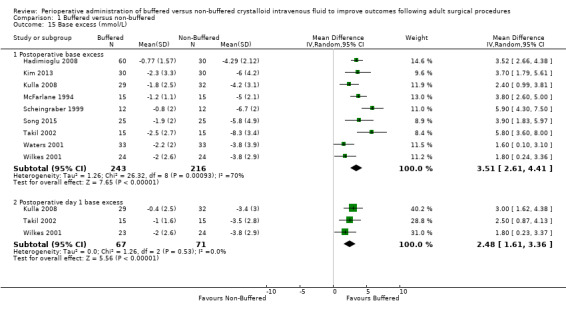
Investigators reported this outcome at various time intervals. Nine studies with a total of 459 participants reported postoperative base excess of ‐1.65 mmol/L in the buffered fluid group and ‐5.02 mmol/L in the non‐buffered fluid group (Hadimioglu 2008; Kim 2013; Kulla 2008; McFarlane 1994; Scheingraber 1999; Song 2015; Takil 2002; Waters 2001; Wilkes 2001). Data show an important difference between groups, with MD 3.51 mmol/L lower in the non‐buffered fluid group than in the buffered fluid group (95% CI 2.61 to 4.41). We noted statistical heterogeneity between trials (I² = 70%); therefore, we calculated this comparison using a random‐effects model (Analysis 1.15).
1.15. Analysis.
Comparison 1 Buffered versus non‐buffered, Outcome 15 Base excess (mmol/L).
Three studies with a total of 138 participants reported base excess on postoperative day one of ‐1.07 mmol/L in the buffered fluid group and ‐3.55 mmol/L in the non‐buffered fluid group (Kulla 2008; Takil 2002; Wilkes 2001). Data show an important difference between groups, with MD 2.48 mmol/L lower in the non‐buffered fluid group (95% CI 1.61 to 3.36; I² = 0%; Analysis 1.15).
Serum bicarbonate
Seven studies with a total of 478 participants reported postoperative serum bicarbonate of 21.6 mmol/L in the buffered fluid group and 18.6 mmol/L in the non‐buffered fluid group (Hadimioglu 2008; Kim 2013; O'Malley 2005; Scheingraber 1999; Song 2015; Takil 2002; Waters 2001). Results show an important difference between groups, with MD 3.14 mmol/L lower in the non‐buffered group (95% CI 2.30 to 3.98). We noted significant statistical heterogeneity between trials (I² = 59%); therefore, we calculated this comparison using a random‐effects model (Analysis 1.16).
1.16. Analysis.
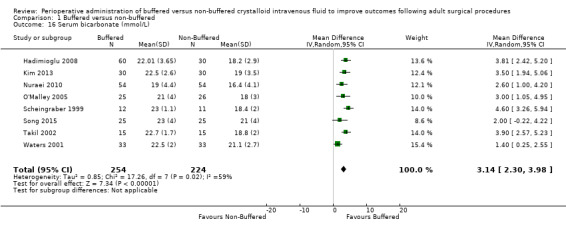
Comparison 1 Buffered versus non‐buffered, Outcome 16 Serum bicarbonate (mmol/L).
Serum glucose
Three trials reported postoperative serum glucose of 6.0 mmol/L for both buffered and non‐buffered groups, showing no mean differences between groups (95% CI ‐0.29 to 0.29; I² = 0%; Analysis 1.17) (Chin 2006; Waters 2001; Wilkes 2001).
1.17. Analysis.
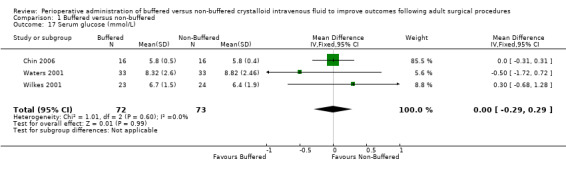
Comparison 1 Buffered versus non‐buffered, Outcome 17 Serum glucose (mmol/L).
Serum chloride
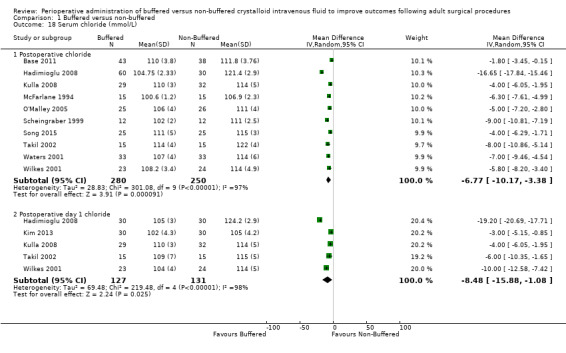
Ten studies with a total of 530 participants reported postoperative serum chloride of 107.5 mmol/L in the buffered fluid group and 114.3 mmol/L in the non‐buffered fluid group at this time point (Base 2011; Hadimioglu 2008; Kulla 2008; McFarlane 1994; O'Malley 2005; Scheingraber 1999; Song 2015; Takil 2002; Waters 2001; Wilkes 2001). Data show an important difference between groups, with MD ‐6.77 mmol/L higher in the non‐buffered fluid group (95% CI ‐10.17 to ‐3.38). We noted statistical heterogeneity between trials (I² = 97%); therefore, we calculated this comparison using a random‐effects model (Analysis 1.18). In light of this heterogeneity, we downgraded the quality of evidence to moderate because of significant inconsistency.
1.18. Analysis.
Comparison 1 Buffered versus non‐buffered, Outcome 18 Serum chloride (mmol/L).
Five studies with a total of 258 participants reported mean serum chloride on the first postoperative day of 105.7 mmol/L in the buffered fluid group and 114.4 mmol/L in the non‐buffered fluid group (Hadimioglu 2008; Kim 2013; Kulla 2008; Takil 2002; Wilkes 2001). Data show an important differences between groups, with MD ‐8.48 mmol/L higher in the non‐buffered fluid group (95% CI ‐15.88 to ‐1.08). We noted statistical heterogeneity between trials (I² = 98%); therefore, we calculated this comparison using a random‐effects model (Analysis 1.18).
Serum potassium
Seven trials with a total of 459 participants reported postoperative serum potassium of 4.13 mmol/L in the buffered group and 4.22 mmol/L in the non‐buffered group (Hadimioglu 2008; Khajavi 2008; Kulla 2008; Nuraei 2010; O'Malley 2005; Song 2015; Wilkes 2001). Data show no important differences between groups, with MD ‐0.04 mmol/L lower in the buffered group (95% CI ‐0.14 to 0.06; I² = 65%; Analysis 1.19).
1.19. Analysis.
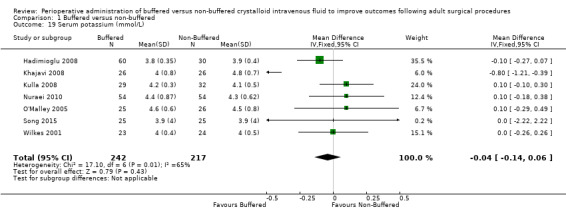
Comparison 1 Buffered versus non‐buffered, Outcome 19 Serum potassium (mmol/L).
Serum sodium
Investigators reported this outcome at two time points. Eight trials with a total of 447 participants reported a serum sodium level of 137.3 mmol/L in the buffered fluid group and 139.4 mmol/L in the non‐buffered fluid group (Khajavi 2008; Kim 2013; Kulla 2008; Nuraei 2010; Song 2015; Takil 2002; Waters 2001; Wilkes 2001). Data show an important difference between groups, with MD ‐2.26 mmol/L higher in the non‐buffered group (95% CI ‐2.84 to ‐1.68; I² = 56%; Analysis 1.20).
1.20. Analysis.
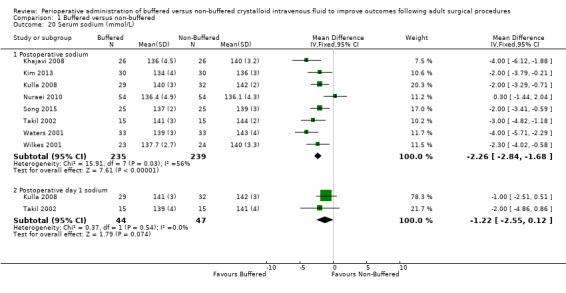
Comparison 1 Buffered versus non‐buffered, Outcome 20 Serum sodium (mmol/L).
Two trials with a total of 91 participants reported postoperative day one serum sodium of 140.6 mmol/L in the buffered fluid group and 141.8 mmol/L in the non‐buffered fluid group (Kulla 2008; Takil 2002). Data show no important differences between groups, with MD 1.2 mmol/L higher in the non‐buffered fluid group (95% CI ‐2.55 to 0.12; I² = 0; Analysis 1.20).
Serum lactate
Four trials with a total of 199 participants reported serum lactate of 2.27 mmol/L in the buffered fluid group and 1.62 mmol/L in the non‐buffered fluid group (Kulla 2008; Song 2015; Waters 2001; Wilkes 2001). Data show no important differences between groups, with MD 0.52 mmol/L higher in the buffered group (95% CI ‐0.04 to 1.08). Analysis suggested statistical heterogeneity between trials (I² = 87%); therefore, we calculated this comparison using a random‐effects model (Analysis 1.21).
1.21. Analysis.
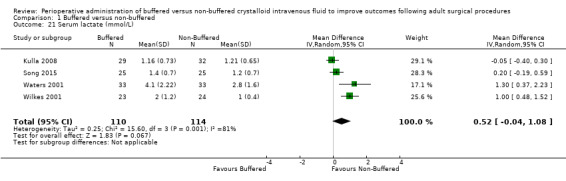
Comparison 1 Buffered versus non‐buffered, Outcome 21 Serum lactate (mmol/L).
Serum calcium
One trial reported postoperative serum calcium of 2.0 ± 0.2 mmol/L in the buffered fluid group and 1.6 ± 0.2 mmol/L in the non‐buffered fluid group (Wilkes 2001). Data show an important difference between groups. We did not analyse these data and listed this as an orphan outcome in Table 3.
Hospital length of stay (days)
Five trials with a total of 348 participants reported hospital length of stay (Base 2011; Gan 1999; O'Malley 2005; Takil 2002; Waters 2001). Reporting was heterogeneous, with data presented as the median (range) by O'Malley 2005 and as the mean (range) by Base 2011. We applied the formula used by Hozo to numerically convert these data to mean (± SD) (Hozo 2005). Data show no important differences between groups, with MD in hospital stay of 0.37 (95% CI ‐0.72 to 1.47; I² = 16%; favouring the non‐buffered groupAnalysis 1.22).
1.22. Analysis.
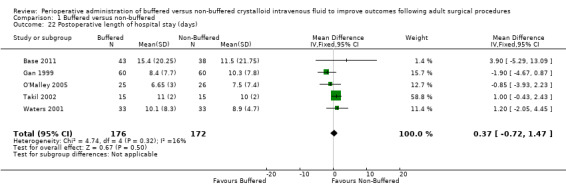
Comparison 1 Buffered versus non‐buffered, Outcome 22 Postoperative length of hospital stay (days).
Functional health status and quality of life measures
None of the included trials addressed this outcome.
Cost
None of the included trials addressed this outcome.
Discussion
Summary of main results
Three studies of 267 participants contributing data to the primary outcome of this review provided evidence suggesting that overall mortality was low and provided no evidence indicating that choice of fluids ‐ buffered or non‐buffered ‐ influenced mortality (Peto odds ratio (OR) 1.88, 95% confidence interval (CI) 0.37 to 9.33). We graded the quality of this evidence as moderate. Analysis of all secondary outcomes measured by 18 different randomized controlled trials of 1096 participants suggests that intravenous fluids containing a physiological buffer are a safe alternative to saline‐based fluids for adult patients undergoing surgery. Data show no differences between groups in terms of renal dysfunction or surrogate markers of renal dysfunction (urine output and serum creatinine). We rated this evidence as low quality. Results for some of the other secondary outcomes revealed differences between groups, including reduced postoperative pH (pH 7.32 vs 7.38, with mean difference (MD) 0.05 lower in the non‐buffered group (95% CI ‐0.04 to ‐0.07)), which suggests that buffered fluids given perioperatively are associated with a lesser degree of metabolic acidosis. We rated this evidence as moderate quality. Four trials with a total of 267 participants provided evidence on renal insufficiency leading to the requirement for renal replacement therapy suggesting that risk of renal insufficiency requiring renal support is lowered by the use of buffered fluids (4.7% (11/148) vs 9.2% (11/119)), but we have little confidence that this difference is real (OR 0.82, 95% CI 0.34 to 1.98; I² = 0%). We downgraded the quality of this evidence to low because of limitations in the design of three studies that included participants with the confounding effect of pre‐existing organ failure (i.e. participants undergoing renal transplant for renal failure) (Hadimioglu 2008; Kim 2013; O'Malley 2005).
Data show higher postoperative serum chloride levels in the non‐buffered group than in the buffered group (chloride 114.3 mmol/L vs 107.5 mmol/L, for MD of 6.7 with 95% CI ‐10.17 to ‐3.38). We rated the quality of this evidence as moderate. Higher chloride concentrations in these fluids might suggest this outcome. Higher serum chloride is a cause of metabolic acidosis and may explain our findings of both lower pH and lower partial pressure of arterial carbon dioxide (PaCO2) (secondary to respiratory compensation for metabolic acidosis) when non‐buffered fluids were used (Stewart 1978).
Overall completeness and applicability of evidence
This systematic review includes published trials comparing buffered and non‐buffered fluid administration during major elective surgery in adults only. Such fluids are used in a variety of clinical contexts including trauma resuscitation, burns, and sepsis, and are given to paediatric patients. Our review did not assess trials conducted outside the adult perioperative setting, hence we cannot draw conclusions beyond those applicable to elective surgery in adults.
Whilst these trials reported many outcomes, very few addressed our primary outcome of death or major organ system failure ‐ most studies reported metabolic or symptomatic differences between groups of patients. Therefore, the numbers reported for our primary outcome are low, and our analyses may not have detected differences between patient groups for these outcomes.
Trials within our review included a total of 1096 participants. We cannot rule out rare adverse effects of buffered or non‐buffered fluid administration for adult surgical patients, but we can conclude that both fluids appear equally safe. Some evidence indicates that selection of buffered or non‐buffered fluids may have measurable effects on the composition of plasma in surgical patients. None of these effects were primary outcomes, and none has been shown to directly affect patient outcomes. However, these metabolic differences may have clinically significant effects that were not detected by our study. Meta‐analysis of high versus low chloride content of fluid administered to surgical and critical care patients has indicated a weak but significant association with risk of acute kidney injury among surgical and critical care patients (Krajewski 2015). Likewise, a systematic review of near‐isotonic or isotonic crystalloids found evidence to support varying acid–base status and other physiological outcomes between even relatively similar fluid types (Orbegozo 2014). Results of our review are compatible with the conclusion that different crystalloid fluids have different profiles of effects on acid–base status and plasma electrolyte concentration. However, current available evidence is insufficient to clearly resolve the question of whether such differences have clinical applicability by beneficially altering prognosis for postoperative patients.
Given the lack of evidence on harm and putative benefit for biochemical status, recent guidelines related to perioperative fluid management have begun to recommend use of balanced salt solutions, such as Hartmann’s, in routine clinical practice (GIFTASUP 2011). However, different countries, and indeed different clinicians, continue to report conflicting views and practices. The phenomenon of hyperchloraemic metabolic acidosis and its relationship to saline‐based fluid administration are well understood among perioperative physicians, and many favour a buffered fluid preparation. Clinicians who work with other acutely unwell patient groups may still not be aware of this phenomenon.
Quality of the evidence
A total of 19 publications reported results from 18 randomized controlled trials enrolling a total of 1096 participants. We judged the evidence from these trials to be of moderate to low quality by using GRADEproGDT software (GRADEproGDT 2015). Data for several outcomes show considerable heterogeneity between study populations. Differences in the characteristics of patients undergoing surgery ranged from American Society of Anesthesiologists (ASA) I and II status in patients who were relatively well, to ASA III or greater status among those with end‐stage organ failure. Such a broad range of patients would suggest a similarly broad range of expected outcomes, and thus reduced generalizability of observed outcomes to the surgical population as a whole. For example, the observation that choice of fluid may be associated with differences in the numbers of patients with end‐stage renal insufficiency who progress to postoperative organ failure may be true of that specific population of patients but may not be true for a population with normal renal function. Therefore, one must exercise caution when interpreting these results. For this reason, we downgraded the quality of evidence for this specific outcome by two levels to low. Similarly, trials show major differences in the types of surgery under investigation. Some included trials examined very minor surgery in otherwise fit and healthy patients (Chin 2006). Others analysed outcomes after major surgery in high‐risk patient groups (Waters 2001; Wilkes 2001), and some focused only on renal transplant surgery. This clinical heterogeneity among surgical procedures exacerbates the difficulty encountered in interpreting trial results and in ascertaining their applicability to the general population.
Most included trials were of high methodological quality (see Figure 3), although some did not specify blinding methods used and one was explicitly unblinded. Figure 2 illustrates that few studies demonstrated high risk of bias. This same figure reveals that many studies had unclear risk of bias. It remains unclear to the authors of this review whether these findings were simply a result of poor reporting of methods, or were reflective of poor trial design.
Data for some outcomes were highly skewed. Whilst we analysed these data using the most appropriate method that we could identify, one must regard review conclusions with caution. For example, patients receiving non‐buffered fluids were given an increased volume of platelets compared with those in the buffered fluid group. These data were highly skewed (standard deviation divided by the mean was < 1). Confidence intervals for this pooled effect were large for logged data, indicating that the pooled estimate is not very precise. More data are required to determine whether differences between groups in the quantity of platelets transfused are indeed important.
We also found differences in various estimates of coagulation function, such as end of surgery levels of von Willebrand factor and Factor VIII. Two studies reported these outcomes upon comparing buffered colloids versus non‐buffered colloids (Gan 1999; Kulla 2008). These results should be interpreted with caution, as we analysed only two studies with few participants.
This review identified small numbers of patients and low numbers of events across outcomes of interest, including the primary outcome of mortality. Awareness of this, along with unclear risk of bias, perhaps reflective of poor reporting, should lead us to interpret review results with caution. Larger randomized controlled trials are needed to assess the clinical implications of our findings.
Potential biases in the review process
We took measures to reduce bias throughout this systematic review process, and we adhered to guidance provided in the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2011). Multiple review authors worked independently to assess risk of bias and study eligibility, and to extract data from studies included in this review.
Two review authors (PO and SB) worked independently to assess eligibility of studies against inclusion criteria and to extract data from the 19 primary publications. We consulted a third review author (EB) when we sought to resolve disagreements. Two review authors independently assessed risk of bias and ranked the quality of studies.
Trials identified for inclusion in this review were heterogeneous with regards to groups studied, reported outcomes, and time points of assessment. This led to us report many outcomes based on small patient groups at different time points. Many studies included co‐interventions that were not ubiquitous across all studies. For example, some studies administered different types of fluids such as colloids and blood alongside trial fluids. In addition, heterogeneity within some analyses was pronounced (this is particularly true of Analysis 1.19), and this may have weakened the robustness of review results.
Some trial data did not contribute to our analyses because they were reported in weight‐based units rather than in absolute amounts. We attempted to contact trial authors to obtain individual participant data, but we were not always successful in these attempts.
Although we made all efforts possible to retrieve relevant trials, we included only data that were published in peer‐reviewed journals. Other high‐quality data may remain unpublished, or may be published in the grey literature. Therefore, our analysis is at risk of publication bias. Additionally, we discovered only two trials that were published in a language other than English ‐ one bi‐lingually in German and English, the other in Farsi (Kulla 2008, Nuraei 2010). We might have failed to identify other trials. We reran the search in May 2017 and found one study of interest (Pfortmueller 2017). We added this study to a list of Studies awaiting classification and will incorporate it into formal review findings during the review update.
Agreements and disagreements with other studies or reviews
To the best of our knowledge, no other systematic reviews have compared effects of buffered versus non‐buffered fluid administration in any patient group.
The UK clinical guideline on intravenous fluid therapy (GIFTASUP) states that owing to the relationship between non‐buffered fluids and hyperchloraemic metabolic acidosis, these fluids should be avoided in favour of buffered fluids, except in special circumstances. The data from our review support this statement.
Authors' conclusions
Implications for practice.
Perioperative fluid administration includes an array of different fluid types, including buffered and non‐buffered fluids. Our systematic review identified moderate‐quality evidence to support the safety of buffered fluids in terms of their low risk of precipitating electrolyte disturbance. In particular, evidence in our review shows that perioperative buffered fluid resuscitation is associated with hyperchloraemic metabolic acidosis in a reduced proportion of patients when compared with non‐buffered fluid resuscitation. Some patients, including those with hypernatraemic acidaemia, are at increased risk of postoperative metabolic derangement. Buffered fluids are appropriate for fluid replacement during surgery and should be considered especially for patients with, or at risk of, metabolic derangement. However, our review has not presented evidence to support a difference in our primary outcome (i.e. mortality) between buffered and non‐buffered fluids. Likewise, although buffered fluids are demonstrably safe when compared with non‐buffered fluids, the ultimate choice regarding administration of fluid to meet individual patient requirements remains at the discretion of the clinician.
Implications for research.
These data were derived from studies of variable quality and remain underpowered to detect any morbidity or mortality arising from selection of buffered or non‐buffered fluids for administration during the perioperative period. One of the key limitations of these studies, which has consequences for the generalizability of the findings of this review, was the heterogeneity of protocols for fluid administration, including wide variation in volumes of fluid administered across studies and in targets used to drive fluid administration. Additional studies are needed, including specifically a large, adequately powered and appropriately blinded randomized controlled trial of sufficient power to detect differences in clinical outcomes arising from the physician's choice of fluid.
Future trials should seek to identify trends in meaningful patient‐centred outcomes such as mortality, quality of recovery, length of hospital stay, and organ dysfunction, and in quality of life measures such as postoperative pain. One large study of saline versus Ringer's lactate that assessed major postoperative complications as its primary outcome measure is currently ongoing and is expected to be completed in 2022.
Our review examined effects of buffered and non‐buffered fluids on adult surgical patients. Several other patient groups, for example, surgical and medically unstable paediatric patients and critically ill adult patients in the intensive care unit, also are treated with large volumes of intravenous fluid. Clinical trials are comparing use of buffered and non‐buffered fluids in these patient populations. A systematic review of these data may reveal findings consistent with the findings presented in our review.
Feedback
Errors noted in Table 1, 5 February 2018
Summary
Table 1 incorrectly states that Plasmalye 148 contains calcium when it contains none.
Reply
Thank you for informing us of the error in table one. There were in fact a number of errors in table one that were also present in the 2012 review (Burdett 2012). I have corrected all of these.
Components of individual fluids (mmol/L when appropriate)
|
Plasmalyte 148 (buffered) Previously stated |
Plasmalyte 148 (buffered) Corrected to read |
|
| Potassium | 0 | 5 |
| Calcium | 2.5 | 0 |
| Lactate (buffer) | 23 | 0 |
| Acetate (buffer) | 0 | 27 |
Contributors
Summary
I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback
Peter Alston
Consultant in Anaesthesia, Critical care and Pain Medicine
Royal Infirmary of Edinburgh
UK
Reply
Sohail Bampoe
Centre for Anaesthesia and Perioperative Medicine, University College London, London, UK
What's new
| Date | Event | Description |
|---|---|---|
| 8 March 2018 | Feedback has been incorporated | Errors corrected in Table 5 |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 12, 2012
| Date | Event | Description |
|---|---|---|
| 3 June 2016 | New search has been performed | We performed a new search in June 2016 and identified 6 new publications. We reviewed 2 trials that previously were awaiting classification, and included 1 (Nuraei 2010) and excluded 1 (Choi 2010). In total, we extracted data from 5 new trials included in this updated review (Heidari 2011; Khajavi 2008; Kim 2013; Nuraei 2010; Song 2015). We excluded 3 trials (Choi 2010; Krebbel 2014; Young 2015). No further trials are awaiting classification. To ensure that this updated review complied with MECIR Reporting Standards, we re‐extracted and analysed data from all 19 primary publications. In this review, we updated the following sections: Abstract, Plain language summary, Summary of main findings, Methods, Results, PRISMA flow chart, Discussion, References, Characteristics of studies, Data and analysis (1.2, 1.3, 1.4, 1.5, 1.7, 1.8, 1.9, 1.14, 1.15, 1.16, 1.18, 1.20, 1.21), and Additional tables. |
| 3 June 2016 | New citation required but conclusions have not changed | Conclusions of this updated review have not been changed by the inclusion of new studies. We have added 2 new review authors (Bampoe S and Odor P) to the team. We have updated the methods used to comply with current MECIR Reporting Standards and have updated quality assessments to incorporate the GRADE method. |
| 11 February 2013 | Amended | We updated contact details. |
| 4 January 2008 | Amended | We converted this review to new review format. |
Notes
8 March 2018
Error corrected in table 1 (Feedback 1)
Acknowledgements
We thank the following for statistical advice.
Tony Brady, Statistician/Data Manager, Intensive Care National Audit and Research Council.
Rafael Perera, Statistician, Cochrane Collaboration, Oxford, UK.
We would like to thank Bita Mesgarpour and Sorahi Bampoe for extracting data from Nuraei 2010.
We would like to thank Mike Bennett (Content Editor); Nathan Pace (Statistical Editor); and Daniel Chappell and Giovanni FM Strippoli (Peer Reviewers) for help and editorial advice provided during preparation of the review (Burdett 2012).
We would like to thank Michael Bennett (Content Editor); Jing Xie (Statistical Editor); Janne Vendt (Information Specialist); and Janet Wale (Consumer Editor) for help and editorial advice provided during preparation of this updated systematic review.
We thank Karen Hovhannisyan (former Trials Search Co‐ordinator, Cochrane Anaesthesia, Critical and Emergency Care Review Group (ACE)) for help with search strategies.
Finally, we thank Jane Cracknell (Managing Editor, ACE) for her advice, support, and patience.
Appendices
Appendix 1. Search strategy for CENTRAL, the Cochrane Library
#1 MeSH descriptor Colloids, this term only #2 colloid*:ti,ab #3 crystalloid* #4 MeSH descriptor Plasma Substitutes, this term only #5 (lactated or colloid* or hyperchlor?emi* or crystalloid* or ringer or hartmann or "Fluid Therapy" or buffered):ti,ab #6 (fluid* near (intravenous or replacement or resuscitation or balanced or non‐balanced)):ti,ab #7 saline:ti #8 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) #9 MeSH descriptor Surgery explode all trees #10 (surgery or surgical):ab #11 (#9 OR #10) #12 (#8 AND #11)
Appendix 2. Search strategy for MEDLINE (OvidSP)
1. colloid*.ti,ab. or Colloids/ 2. crystalloid*.mp. 3. lactated.ti,ab. or Plasma Substitutes/ 4. (hyperchlor?emi* or crystalloid* or ringer or hartmann).ti,ab. 5. Fluid Therapy.ti,ab. or Fluid Therapy/ 6. (intravenous adj3 fluid*).ti,ab. 7. (fluid adj3 replacement).mp. 8. (fluid and resuscitation).ti,ab. 9. buffered.mp. or exp Bicarbonates/ 10. ((balanced or non‐balanced) adj3 fluid*).mp. 11. saline.ti. 12. 7 or 5 or8 or 1 or 6 or 2 or 10 or 4 or 3 or 11 or 9 13. Surgery/ or (surgery or surgical*).ti,ab. 14. 13 and 12 15. ((randomised controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) and humans.sh. 16. 15 and 14
Appendix 3. Search strategy for Embase (OvidSP)
1. colloid*.ti,ab. or Colloids/ 2. crystalloid*.mp. 3. actated.ti,ab. or Plasma Substitutes/ 4. (hyperchlor?emi* or crystalloid* or ringer or hartmann).ti,ab. 5. Fluid Therapy.ti,ab. or Fluid Therapy/ 6. (intravenous adj3 fluid*).ti,ab. 7. (fluid adj3 replacement).mp. 8. (fluid and resuscitation).ti,ab. 9. buffered.mp. or exp Bicarbonates/ 10. ((balanced or non‐balanced) adj3 fluid*).mp. 11. saline.ti. 12. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 13. Surgery/ or (surgery or surgical*).ti,ab. 14. 13 and 12 15. (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab.) and human*.ec,hw,fs. 16. 15 and 14
Appendix 4. Search strategy for CINAHL (EBSCOhost)
#1. TX (Fluid Therapy)
#2. (resuscitation or replacement or intravenous or balanced or non balanced) and fluid
#3. TX buffered or colloid* or crystalloid* or acetated or hyperchloremi* or hyperchloraemi* or crystalloid* or ringer or hartmann
#4. TI saline
#5. MW Bicarbonates or Fluid Therapy or Plasma Substitutes or Colloids
#6. #1 or #2 or #3 or #4 or #5
#7. TX surgery or surgical
#8. #6 and #7
#9. (MH "Clinical Trials+")
#10. ("randomised") or (MM "Random Assignment")
#11. #9 or #10
#12. #8 and #11
Appendix 5. Search strategy in 2003
In 2003, the following search strategy, or variations of this strategy were performed: #1 colloid.mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #2 crystalloid.mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #3 ringer$.mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #4 hartmann$.mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #5 lactated.mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #6 hyperchloremic.mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #7 hyperchloremia.mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #8 hyperchloraemic.mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #9 hyperchloraemia.mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #10 fluidtherapy.mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #11 fluid‐therapy.mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #12 (intravenous and fluid).mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #13 (intravascular and fluid).mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #14 (fluid adj2 therapy).mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #15 (fluid and replacement).mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #16 (fluid and resuscitation).mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #17 saline.mp. [mp=ti, ab, rw, sh, tn, ot, dm, mf, dv] #18 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 #19 RANDOMIZED CONTROLLED TRIAL.pt. #20 CONTROLLED CLINICAL TRIAL.pt. #21 RANDOMIZED CONTROLLED TRIALS.sh. #22 RANDOM ALLOCATION.sh. #23 DOUBLE BLIND METHOD.sh. #24 SINGLE‐BLIND METHOD.sh. #25 or/19‐24 26 (ANIMAL not HUMAN).sh. #27 #25 not #26 #28 CLINICAL TRIAL.pt. #29 exp CLINICAL TRIALS/MINOR #30 (clin$ adj25 trial$).ti,ab. #31 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. #32 PLACEBOS.sh. #33 placebo$.ti,ab. #34 random$.ti,ab. #35 RESEARCH DESIGN.sh. #36 or/28‐35 #37 #36 not #26 #38 #37 not #27 #39 COMPARATIVE STUDY.sh. #40 exp EVALUATION STUDIES/ #41 FOLLOW UP STUDIES.sh. #42 PROSPECTIVE STUDIES.sh. #43 (control$ or prospectiv$ or volunteer$).ti,ab. #44 or/39‐43 #45 #44 not #26 #46 #45 not (#27 or #38) #47 #27 or #38 or #46 #48 ((CHILD or PAEDIATRIC or PEDIATRIC or INFANT) not ADULT).sh. #49 #47 not #48 #50 #18 and #49
Data and analyses
Comparison 1. Buffered versus non‐buffered.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all time frames reported) | 3 | 267 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.85 [0.37, 9.33] |
| 2 Organ system failure ‐ renal insufficiency requiring support | 4 | 267 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.34, 1.98] |
| 3 Urine output (mL) | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Intraoperative urine output (mL) | 8 | 459 | Mean Difference (IV, Random, 95% CI) | 6.10 [‐128.41, 140.61] |
| 3.2 Intraoperative urine output (mL) ‐ sensitivity analysis after exclusion of O'Malley 2005 | 6 | 348 | Mean Difference (IV, Random, 95% CI) | 9.93 [‐147.01, 166.87] |
| 3.3 Renal transplant patients | 2 | 111 | Mean Difference (IV, Random, 95% CI) | 58.26 [‐446.70, 563.21] |
| 4 Creatinine change (µmol/L) | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Postoperative creatinine change (µmol/L) | 2 | 113 | Mean Difference (IV, Random, 95% CI) | ‐6.96 [‐27.42, 13.50] |
| 4.2 Postoperative day 1 creatinine change (µmol/L) | 2 | 113 | Mean Difference (IV, Random, 95% CI) | 4.94 [‐5.91, 15.78] |
| 5 Postoperative creatinine (µmol/L) | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Postoperative creatinine | 3 | 235 | Mean Difference (IV, Fixed, 95% CI) | ‐1.31 [‐9.30, 6.68] |
| 5.2 Postoperative day 1 creatinine | 3 | 211 | Mean Difference (IV, Fixed, 95% CI) | ‐6.26 [‐21.17, 8.64] |
| 5.3 Postoperative day 3 creatinine | 4 | 301 | Mean Difference (IV, Fixed, 95% CI) | ‐0.47 [‐30.12, 29.19] |
| 5.4 Postoperative week 1 creatinine | 4 | 309 | Mean Difference (IV, Fixed, 95% CI) | ‐5.36 [‐25.29, 14.56] |
| 6 Postoperative creatinine clearance (mL/min) | 3 | 222 | Mean Difference (IV, Fixed, 95% CI) | 12.61 [‐2.31, 27.54] |
| 7 PaCO2 (mmHg) | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Postoperative PaCO2 | 7 | 446 | Mean Difference (IV, Fixed, 95% CI) | 1.05 [0.15, 1.94] |
| 7.2 Postoperative day 1 PaCO2 | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | 3.33 [2.03, 4.64] |
| 8 Postoperative nausea and vomiting | 3 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.34, 1.30] |
| 9 Intraoperative blood loss (mL) | 11 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Intraoperative blood loss < 1000 mL | 5 | 202 | Mean Difference (IV, Fixed, 95% CI) | 5.90 [‐45.18, 56.99] |
| 9.2 Intraoperative blood loss ≥ 1000 mL | 6 | 374 | Mean Difference (IV, Fixed, 95% CI) | ‐173.07 [‐438.83, 92.68] |
| 10 Intraoperative red cell transfusion | 4 | 152 | Mean Difference (IV, Fixed, 95% CI) | ‐29.10 [‐117.03, 58.84] |
| 11 Intraoperative transfusion ‐ other products (log values) | 4 | Mean Difference (Fixed, 95% CI) | Subtotals only | |
| 11.1 Platelet transfusion (Higgins 3) | 4 | Mean Difference (Fixed, 95% CI) | 1.23 [0.22, 2.25] | |
| 11.2 Fresh frozen plasma transfusion (Higgins 3) | 3 | Mean Difference (Fixed, 95% CI) | 0.47 [‐0.37, 1.30] | |
| 12 Variables of coagulation ‐ activated partial thromboplastin time (s) | 2 | 181 | Mean Difference (IV, Fixed, 95% CI) | 0.88 [‐1.82, 3.58] |
| 13 Variables of coagulation ‐ other | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13.1 Factor VIII (IU/L) | 2 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐29.54 [‐46.16, ‐12.91] |
| 13.2 von Willebrand factor (IU/L) | 2 | 181 | Mean Difference (IV, Fixed, 95% CI) | ‐31.39 [‐47.71, ‐15.06] |
| 14 Plasma pH | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 14.1 Postoperative pH | 12 | 720 | Mean Difference (IV, Random, 95% CI) | 0.05 [0.04, 0.07] |
| 14.2 Postoperative day 1 pH | 2 | 91 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.00, 0.03] |
| 15 Base excess (mmol/L) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 Postoperative base excess | 9 | 459 | Mean Difference (IV, Random, 95% CI) | 3.51 [2.61, 4.41] |
| 15.2 Postoperative day 1 base excess | 3 | 138 | Mean Difference (IV, Random, 95% CI) | 2.48 [1.61, 3.36] |
| 16 Serum bicarbonate (mmol/L) | 8 | 478 | Mean Difference (IV, Random, 95% CI) | 3.14 [2.30, 3.98] |
| 17 Serum glucose (mmol/L) | 3 | 145 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.29, 0.29] |
| 18 Serum chloride (mmol/L) | 11 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18.1 Postoperative chloride | 10 | 530 | Mean Difference (IV, Random, 95% CI) | ‐6.77 [‐10.17, ‐3.38] |
| 18.2 Postoperative day 1 chloride | 5 | 258 | Mean Difference (IV, Random, 95% CI) | ‐8.48 [‐15.88, ‐1.08] |
| 19 Serum potassium (mmol/L) | 7 | 459 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.14, 0.06] |
| 20 Serum sodium (mmol/L) | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20.1 Postoperative sodium | 8 | 474 | Mean Difference (IV, Fixed, 95% CI) | ‐2.26 [‐2.84, ‐1.68] |
| 20.2 Postoperative day 1 sodium | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | ‐1.22 [‐2.55, 0.12] |
| 21 Serum lactate (mmol/L) | 4 | 224 | Mean Difference (IV, Random, 95% CI) | 0.52 [‐0.04, 1.08] |
| 22 Postoperative length of hospital stay (days) | 5 | 348 | Mean Difference (IV, Fixed, 95% CI) | 0.37 [‐0.72, 1.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Base 2011.
| Methods | Design: randomized controlled study Withdrawals: no withdrawals Setting: 2 European cardiac surgery hospitals Sample size: 81 cardiac surgical patients |
|
| Participants | Age (mean): 64.6/67.9 years Gender (M/F): 62/19 ASA grade: not reported Surgery type: CABG/valve surgery/CABG + valve surgery Surgery duration (mean): 4.1/3.8 hours Anaesthesia type: general |
|
| Interventions |
Interventionn = 43 Buffered arm‐ Balanced 6% HES 130/0.4 (Volulyte) for intraoperative and postoperative fluid administration Control n = 38 Non‐Buffered arm ‐ 6% HES 130/0.4 in saline for intraoperative and postoperative fluid administration Co‐interventions: none |
|
| Outcomes | Acid‐base status, serum biochemistry up to 24 hours postoperatively. Duration of ICU stay, duration of hospital stay, and mortality all measured up to 30 days postoperatively | |
| Notes | Funding source: not disclosed Declarations of interest: 1 study author was employed by Fresenius Kabi, which manufactured the intervention being studied (Voluven). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using SAS" and "patients were randomized to treatment groups per study centre in blocks of 6" |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial was blinded to participants and investigators. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not specified whether outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis ‐ no withdrawals |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Unclear risk | One study author is employed by the manufacturer of the fluids compared in the study. |
Chin 2006.
| Methods | Design: randomized controlled study Withdrawals: 10 participants excluded for protocol violations (from total 60 participants recruited) Setting: single hospital in Singapore Sample size: 50; 3 arms to the trial One arm consisted of participants who received a fluid formulation that was not relevant to this review (dextrose 5% in 0.9% saline). Details for this arm of the study are not extracted here. |
|
| Participants | Age (mean): 50/35 years Gender (M/F): 21/11 ASA grade: I or II Surgery type: elective surgery that was not expected to enter into a major body cavity, or to require intravenous fluid volume in excess of 500 mL in the first 2 hours of perioperative care. Covered orthopaedic, ENT, breast, minor general surgery Surgery duration (mean): 1.5/1.2 hours Anaesthesia type: general |
|
| Interventions |
Intervention n = 16 Buffered arm ‐ 500 mL lactated Ringer's solution administered over 45 to 60 minutes Control n = 16 Non‐buffered arm ‐ 500 mL 0.9% normal saline administered over 45 to 60 minutes Co‐interventions: none |
|
| Outcomes | Serum glucose and electrolytes, measured up to 1 hour postoperatively | |
| Notes | This trial was performed on participants undergoing only minor surgery, who received only a small amount of intravenous fluid. Study was designed to investigate effects on serum glucose of infusion fluids containing or not containing a small amount of glucose. It was not designed as a trial to compare effects of buffered vs non‐buffered fluids. Data were reported as means (95% CIs) and were converted into means (SDs) for analysis. Funding source: not disclosed Declarations of interest: not disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomized via a computer‐generated random number table and sealed opaque envelopes. |
| Allocation concealment (selection bias) | Low risk | See above. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Trial was blinded to participants and investigators (personal communication). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not disclosed |
| Incomplete outcome data (attrition bias) All outcomes | High risk | A high proportion of participants (20%) dropped out owing to administration of non‐protocol intravenous fluids. |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Low risk | No evidence of other potential bias |
Gan 1999.
| Methods | Design: randomized controlled study Withdrawals: 3 participants owing to protocol violations Setting: 2 centres (USA) Sample size: 120 |
|
| Participants | Age (mean): 58/57 Gender (M/F): 69/61 ASA grade: I to III Surgery type: major elective surgery, which covered orthopaedic, general, gynaecological, and urological needs Surgery duration (mean): 5.3/5.2 hours Anaesthesia type: general |
|
| Interventions |
Intervention n = 60 Buffered arm ‐ Hextend administered via a hypovolaemia algorithm to ensure adequate volume during the operation Control n = 60 Non‐buffered arm ‐ Hespan administered via a hypovolaemia algorithm to ensure adequate volume during the operation Co‐interventions: Each arm was given a maintenance dose of lactated Ringer's solution (a buffered fluid). |
|
| Outcomes | Urine output, EBL, intraoperative transfusion, death, length of postoperative stay, requirement for calcium measured and recorded during hospital stay | |
| Notes | Funding source: BioTime biotechnology company Declarations of interest: disclosure of funding source |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequences were generated via a computer programme. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinding of participants, trial conductors and assessors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not disclosed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants did not receive study solution. |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported. All exclusions for protocol violations. No cross‐overs |
| Other bias | Unclear risk | Analysis was done on an intention‐to‐treat basis. Study was funded in part by BioTime Inc., which manufactures Hextend. |
Hadimioglu 2008.
| Methods | Design: randomized double‐blinded controlled trial Withdrawals: Not reported Setting: not specified whether this is a single‐centre or multi‐centre study Sample size: 90 3 arms to the study, each with 30 participants |
|
| Participants | Age (mean): normal saline 44.4, lactated Ringer's 48.3, Plasmalyte 46.3 years Gender (M/F): not specified ASA grade: III to IV with end‐stage renal failure Surgery type: living‐related kidney transplants Surgery duration (mean): normal saline 2 hours, lactated Ringer's 2 hours, Plasmalyte 2 hours Anaesthesia type: general |
|
| Interventions |
Intervention 1 n = 30 Buffered arm ‐ Lactated Ringer's Intervention 2 n = 30 Buffered arm ‐ Plasmalyte 148 Control n = 30 Non‐buffered arm‐ Normal saline Co‐interventions: all fluids administered at 20 to 30 mL/kg/h to maintain CVP at 12 to 15 mmHg The 2 buffered arms (lactated Ringer's solution and Plasmalyte 148) were numerically combined, so that the buffered fluid arm included 60 participants and the non‐buffered fluid arm included 30 participants. |
|
| Outcomes | Urine output, pH, base excess, lactate, bicarbonate, potassium, CO2, chloride, creatinine, creatinine clearance, chloride, requirement for dialysis measured daily until the third postoperative day and then once more on the seventh postoperative day | |
| Notes | Funding source: not disclosed Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer randomization programme was used for participant group assignments. |
| Allocation concealment (selection bias) | Unclear risk | No information forthcoming |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded ‐ study solutions prepared in unlabeled bags by the hospital pharmacy. Participants and clinicians blinded to group assignments |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not disclosed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported. No participants lost to follow‐up |
| Other bias | Low risk | No further data available |
Heidari 2011.
| Methods | Design: randomized double‐blinded controlled trial Withdrawals: none Setting: single centre in the Middle East Sample size: 90; 3 groups within the trial |
|
| Participants | Age (mean): 43.6/40.9 years Gender (M/F): 28/32 ASA grade: I to II Surgery type: elective lower abdominal surgery Surgery duration (mean): 1.3/1.5 hours Anaesthesia type: general One arm consisted of participants who received a fluid formulation that was not relevant to this review. Details for this arm of the study are not extracted here. |
|
| Interventions |
Intervention n = 30 Buffered arm ‐ Ringer's lactate 15 mL/kg administered 30 minutes preoperatively Control n = 30 Non‐buffered arm ‐ normal saline 15 mL/kg administered 30 minutes preoperatively Co‐interventions: none |
|
| Outcomes | Postoperative nausea using VAS and incidence of postoperative vomiting at 6, 12, and 24 hours postoperatively | |
| Notes | Funding source: not disclosed Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated randomly according to random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Reported as 'double‐blinded' but blinding methods not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported as 'double‐blinded' but blinding methods not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Unclear risk | Type of surgery not described in detail |
Khajavi 2008.
| Methods | Design: randomized double‐blinded controlled trial Withdrawals: none Setting: single centre in the Middle East Sample size: 52 |
|
| Participants | Age (mean): 40/37 years Gender (M/F): not described in detail, but groups described as sex‐matched ASA grade: not described Surgery type: elective renal transplantation surgery Surgery duration (mean): not described Anaesthesia type: general |
|
| Interventions |
Intervention n = 26 Buffered arm ‐ Ringer's lactate 60 mL/kg titrated to a CVP of 10 to 15 mmHg Control n = 26 Non‐buffered arm ‐ normal saline 60 mL/kg titrated to a CVP of 10 to 15 mmHg Co‐interventions: none |
|
| Outcomes | Electrolytes, pH, blood loss, graft failure, urine output Blood samples measured at the start of surgery, at 1 hour, and at end of surgery. Urine output measured first 4 hours postoperatively |
|
| Notes | Funding source: not disclosed Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Fluids covered in opaque tape |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and personnel blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not disclosed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Low risk | No other obvious bias |
Kim 2013.
| Methods | Design: randomized double‐blinded controlled trial Withdrawals: none Setting: single centre in Korea Sample size: 60 |
|
| Participants | Age (mean): 48/44 years Gender (M/F): 38/22 ASA grade: not described Surgery type: elective renal transplantation surgery Surgery duration (mean): 4.4/4.3 hours Anaesthesia type: general |
|
| Interventions |
Intervention n = 30 Buffered arm ‐ Ringer's lactate infusion to maintain CVP of 12 to 15 mmHg Control n = 30 Non‐buffered arm ‐ normal saline infusion to maintain CVP of 12 to 15 mmHg Co‐interventions: none |
|
| Outcomes | pH, base excess, strong ion difference, urine output, postoperative creatinine, graft failure requiring dialysis up to day 7 postoperatively | |
| Notes | Funding source: not disclosed Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized according to random number sequence |
| Allocation concealment (selection bias) | Low risk | Solutions in unlabelled bags |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Low risk | All participant data reported |
Kulla 2008.
| Methods | Design: randomized double‐blinded controlled trial Withdrawals: 1 Setting: single centre in Germany Sample size: 62 |
|
| Participants | Age (mean): adult Gender (M/F): not described ASA grade: 2 to 3 Surgery type: elective major abdominal surgery Surgery duration: Not reported Anaesthesia type: general Anticipated surgery longer than 90 minutes with more than 1.5 L fluid requirement. Postoperative intensive care required |
|
| Interventions |
Intervention n = 29 Buffered arm ‐ buffered HES as colloid and acetated Ringer's solution Control n = 33 Non‐buffered arm ‐ HES in saline‐based solution as colloid plus a non‐balanced crystalloid Co‐interventions: none |
|
| Outcomes | Endpoints taken preoperatively, during surgery, at end of surgery, 6 hours postoperatively, and at POD1 blood loss, urine output, creatinine, lactate, sodium, chloride, PaO2, PaCO2, pH, base excess, thromboplastin time "quick", partial thromboplastin time, antithrombin III, Factor VIII, von Willebrand factor, ristocetin cofactor |
|
| Notes | Very heterogeneous group of surgical procedures, including 1 each of oesophagectomy, gastrectomy, prostatectomy, nephrectomy, and colonic surgery Funding source: not disclosed Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized ‐ method unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Unclear risk | Of 62 participants enrolled, 1 was lost to follow‐up, as the operation was not performed as expected. No further information is available. One author of this study was employed by Serumwerk Bernburg AG, which manufactures hydroxyethyl starch (HES). |
Martin 2002.
| Methods | Design: randomized controlled trial Withdrawals: none Setting: single centre (USA) Sample size: 90 |
|
| Participants | Age (mean): 6% Hetastarch in normal saline 58, 6% Hetastarch in balanced saline 59, lactated Ringer’s solution 58 Gender (M/F): not described ASA grade: I to III Surgery type: major elective non‐cardiac surgery with anticipated blood loss of 500 mL Surgery duration (mean): not described Anaesthesia type: general |
|
| Interventions | Three arms, only 2 of which are included (60 participants) 30 patients were enrolled into the buffered (Hextend) arm; and 30 into the non‐buffered arm (Hespan). Trial fluids were given according to a protocol, to ensure adequate volume during the operation. In addition, each arm was given a maintenance dose of lactated Ringer's solution (a buffered fluid). |
|
| Outcomes | TEG data measured before induction, at end of surgical procedure, and 24 hours postoperatively | |
| Notes | These are the same participant data as in Moretti 2003, but different outcomes are reported. Funding source: supported in part by a grant from BioTime, Inc. Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded to participants, trial conductors and assessors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded to participants, trial conductors and assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant in each group fell out ‐ 1 did not need study fluid, the other had surgery rescheduled. |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported. Analysis completed on an intention‐to‐treat basis |
| Other bias | Unclear risk | Study was funded in part by BioTime Inc., which manufactures Hextend. |
McFarlane 1994.
| Methods | Design: randomized controlled trial Withdrawals: none Setting: single centre (UK) Sample size: 30 |
|
| Participants | Age (mean): 57/54 years Gender (M/F): not described ASA grade: I or II Surgery type: major elective hepatobiliary or pancreatic surgery Surgery duration (mean): 3.3/3.7 hours Anaesthesia type: general |
|
| Interventions |
Intervention n = 15 Buffered arm ‐ Plasmalyte 148 infusion 15 mL/kg/h Control n = 30 Non‐buffered arm ‐ normal saline infusion 15 mL/kg/h Rate adjusted in response to clinical state, but not according to a clinical protocol Co‐interventions: all fluids warmed |
|
| Outcomes | Blood loss (mL/kg) mean and SD, chloride, bicarbonate, base excess, PaCO2, lactate up to 24 hours postoperatively | |
| Notes | Funding source: not disclosed Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization method not clear |
| Allocation concealment (selection bias) | High risk | No evidence of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No evidence of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No evidence of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All outcome data complete |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Low risk | No other information forthcoming |
Moretti 2003.
| Methods | Design: randomized controlled trial Withdrawals: 2 Setting: single centre USA Sample size: 90 |
|
| Participants | Age (mean): buffered (Hextend) arm 59, non‐buffered arm (Hespan) 58 Gender (M/F): not described ASA grade: I to III Surgery type: major elective general, gynaecological, orthopaedic, or urological with anticipated blood loss > 500 mL Surgery duration (mean): 4.6/4.8 hours Anaesthesia type: general |
|
| Interventions | Three arms, only 2 of which are included 30 participants were enrolled into the buffered (Hextend) arm, and 30 into the non‐buffered arm (Hespan). Trial fluids were given according to a protocol, to ensure adequate volume during the operation. In addition, each arm was given a maintenance dose of lactated Ringer's solution (a buffered fluid). |
|
| Outcomes | Postoperative nausea and vomiting, oedema up to 24 hours after surgery | |
| Notes | These are the same participant data as in Martin 2002, but different outcomes are reported. Funding source: supported in part by a grant from BioTime, Inc. Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Blinded to participants, trial conductors and assessors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent research personnel unaware of participant's collected randomization data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant from each group did not proceed with fluid allocation. |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Unclear risk | One participant in each group fell out ‐ 1 did not need study fluid, and the other had surgery rescheduled. Analysis was completed on an intention‐to‐treat basis. Study was funded in part by BioTime Inc., which manufactures Hextend. |
Nuraei 2010.
| Methods | Design: randomized controlled trial Withdrawals: none Setting: single centre, Iran Sample size: 108 |
|
| Participants | Age (mean): 38/39 years Gender (M/F): 68/40 ASA grade: "I and II" ‐ NB: All participants had end‐stage renal disease and were awaiting transplant. Surgery type: renal transplant surgery Surgery duration (mean): 2.1/2.0 hours Anaesthesia type: general |
|
| Interventions |
Intervention n = 54 Buffered arm ‐ Ringer's lactate Control n = 54 Non‐buffered arm ‐ normal saline Protocol for fluid administration not reported Co‐interventions: none |
|
| Outcomes | Renal function, acid‐base status up to 24 hours after surgery | |
| Notes | Funding source: not disclosed Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized double‐blinded trial |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All outcomes adequately reported |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Low risk | No other obvious bias |
O'Malley 2005.
| Methods | Design: prospective randomized double‐blind controlled study Withdrawals: 3 Setting: single centre, USA Sample size: 54 |
|
| Participants | Age (mean): 44/44 years Gender (M/F): 32/19 ASA grade: > III Surgery type: renal transplantation Surgery duration (mean): 5.6/5.6 hours Anaesthesia type: general A total of 54 participants with end‐stage renal failure undergoing kidney transplantation were recruited. Of these, 3 were excluded owing to high preoperative potassium (total analysed 51). |
|
| Interventions |
Intervention n = 25 Buffered arm ‐ Ringer's lactate Control n = 26 Non‐buffered arm ‐ normal saline Fluids titrated to routine clinical endpoints Co‐interventions: dopamine infusion 2 mcg/kg/min |
|
| Outcomes | Serum potassium, pH, serum creatinine up to 1 week, postoperative urine output, creatinine clearance, requirement for dialysis, blood loss, transfusion requirements, length of stay in hospital | |
| Notes | Trial was stopped early when severe hyperkalaemia was noted in some participants in the control arm. Funding source: not disclosed Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Low risk | Closed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind" ‐ method unclear |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not disclosed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants excluded preoperatively on the basis of predetermined exclusion criteria |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Low risk | No evidence of other potential bias |
Scheingraber 1999.
| Methods | Design: prospective randomized double‐blind controlled study Withdrawals: none Setting: single centre, USA Sample size: 24 |
|
| Participants | Age (mean): 53/46 years Gender (M/F): all female ASA grade: I to II Surgery type: elective gynaecological surgery Surgery duration (mean): 2.3/2.3 hours Anaesthesia type: general |
|
| Interventions |
Intervention n = 12 Buffered arm ‐ Ringer's lactate Control n = 12 Non‐buffered arm ‐ normal saline 30 mL/kg/h Co‐interventions: supplemental intravenous potassium administered according to intraoperative serum potassium levels During the study, no participants received colloids, plasma products, or blood transfusions. |
|
| Outcomes | Estimated intraoperative blood loss, urine output, pH, carbon dioxide, base excess, lactate, chloride, sodium | |
| Notes | Funding source: not disclosed Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomization details not available |
| Allocation concealment (selection bias) | Unclear risk | No details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Published data state that double‐blinding took place. No other details are available. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not disclosed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No participants lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Low risk | No other information forthcoming |
Song 2015.
| Methods | Design: randomized double‐blinded study Withdrawals: 0 Setting: single centre, Korea Sample size: 50 |
|
| Participants | Age (mean): 60/63 Gender (M/F): 17/33 ASA grade: I to II Surgery type: elective lumbar spinal surgery Surgery duration (mean): 4.9/4.85 hours Anaesthesia type: general |
|
| Interventions |
Intervention n = 25 Buffered arm ‐ Plasmalyte Control n = 25 Non‐buffered arm ‐ normal saline 6 mL/kg/h increased at discretion of anaesthetist Co‐interventions: If blood loss > 500 mL, participants were given a colloid (6% HES in saline). If haematocrit decreased to < 24%, blood was given. No significant differences between groups in volumes of blood or colloid administered |
|
| Outcomes | Rotational thromboelastometry (ROTEM) at end of surgery. Intraoperative blood loss, pH, BE, bicarbonate. Postoperative electrolytes at 12 hours Urea and creatinine at 24 hours |
|
| Notes | Funding source: supported by a university grant Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number sequence |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Anaesthetists performing ROTEM were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No participants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Low risk | No other apparent bias |
Takil 2002.
| Methods | Design: randomized controlled study Withdrawals: 0 Setting: single centre, Turkey Sample size: 30 |
|
| Participants | Age (mean): 37/45 years Gender (M/F): not reported ASA grade: I to II Surgery type: elective major spinal surgery Surgery duration (mean): 4.9/4.9 hours Anaesthesia type: general |
|
| Interventions |
Intervention n = 15 Buffered arm ‐ Ringer's lactate Control n = 15 Non‐buffered arm ‐ normal saline 20 mL/kg/h Co‐interventions: If blood loss > 500 mL, participants were given a colloid (Gelofusine). If blood loss > 20%, blood was given. |
|
| Outcomes | Sodium, chloride, bicarbonate, base deficit, pH, blood transfusion, blood loss, urine output, PaCO2 up to 12 hours after surgery. Length of intensive care unit and hospital stay recorded Time points were first, second, fourth, sixth, and twelfth hours postoperatively. |
|
| Notes | Funding source: not disclosed Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial was randomized, but no details about randomization are available. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes were used. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not mentioned at all in the manuscript. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not disclosed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Low risk | No other information available |
Walsh 1983.
| Methods | Design: randomized controlled study Withdrawals: 0 Setting: single centre, UK Sample size: 21 |
|
| Participants | 3 arms included in the trial, with 7 participants in each. Arms were given lactated Ringer's solution, 5% dextrose, and normal saline. Only 2 arms were included for analysis (N = 14). Age (mean): Hartmann's 55/normal saline 50 years Gender (M/F): not reported ASA grade: not reported Surgery type: elective cholecystectomy Surgery duration (mean): not reported Anaesthesia type: general |
|
| Interventions |
Intervention n = 7 Buffered arm ‐ Hartmann's solution Control n = 7 Non‐buffered arm ‐ normal saline 6 mL/kg/h Co‐interventions: none |
|
| Outcomes | Estimated blood loss measured intraoperatively | |
| Notes | Funding source: not disclosed Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods of randomization unclear |
| Allocation concealment (selection bias) | High risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Low risk | No other information given |
Waters 2001.
| Methods | Design: randomized controlled study Withdrawals: 0 Setting: single centre, USA Sample size: 66 |
|
| Participants | Age (mean): 70/70 years Gender (M/F): not reported ASA grade: average III.1 Surgery type: open abdominal aortic aneurysm surgery Surgery duration (mean): not reported Anaesthesia type: general + thoracic epidural |
|
| Interventions |
Intervention n = 33 Buffered arm ‐ lactated Ringer's Control n = 33 Non‐buffered arm ‐ normal saline Fluids administered to maintain CVP to within 10% of baseline. Colloid administration restricted to period of rapid blood loss Co‐interventions: Protocol allowed sodium bicarbonate to be given to participants if their metabolic acidosis was significant. Participants were given human albumin solution in addition to study fluid at the discretion of the anaesthetic team. All cell‐salvaged blood was washed in normal saline. Non‐buffered arm received an average of 1500 mL more fluid intraoperatively. |
|
| Outcomes | Urine output, creatinine, need for renal replacement therapy, EBL, transfusion requirements, base deficit, chloride, death, length of postoperative stay Variables were measured at start of surgery, on admission to ICU, and every 24 hours until normalization of measured variable. |
|
| Notes | Funding source: supported in part by a grant from Centre for Health Outcomes Research Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by a computerized random number generator |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded: labels of solutions covered from participants, trial conductors and assessors |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No withdrawals |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Low risk | No other information available |
Wilkes 2001.
| Methods | Design: prospective randomized double‐blinded Withdrawals: 5 Setting: 2 centres, UK Sample size: 47 |
|
| Participants | Age (mean): 71.6/73.1 years Gender (M/F): 23/24 ASA grade: average I to III Surgery type: major non‐cardiac surgery Surgery duration (mean): 3.3/3.1 hours Anaesthesia type: general |
|
| Interventions |
Intervention n = 23 Buffered arm ‐ Hartmann's and 6% Hetastarch Control n = 24 Non‐buffered arm ‐ normal saline and 6% Hetastarch 500 mL of colloid at induction as a bolus followed by 7 mL/kg/h of crystalloid as an infusion according to a predefined algorithmic protocol Co‐interventions: 6% Hetastarch |
|
| Outcomes | Chloride, sodium, RBCs transfused, platelets transfused, FFP transfused, urine output, base excess, pH, PaCO2, bicarbonate measured postoperatively | |
| Notes | Trial was stopped early after 1 participant experienced adverse effects that may have been caused by the study fluid. Funding source: supported in part by a grant from Abbott Laboratories and from BioTime Inc. Declarations of interest: none disclosed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization and stratification were instituted with the use of permuted blocks with a size of 4. |
| Allocation concealment (selection bias) | Low risk | All clinicians involved in the care of participants were blinded to group allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded ‐ participants, trial conductors and assessors |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blinded ‐ participants, trial conductors and assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 withdrawals. Study was stopped early after adverse effects possibly linked to study fluid. |
| Selective reporting (reporting bias) | Low risk | No evidence of selection bias. All proposed outcome measures adequately reported |
| Other bias | Unclear risk | Study was funded in part by BioTime Inc., which manufactures Hextend. |
ASA: American Society of Anesthesiologists; BE:base excess; CABG: coronary artery bypass graft; CI:chloride; CO2: carbon dioxide; CVP: central venous pressure; EBL: estimated blood loss; ENT: ear, nose, and throat; FFP: fresh frozen plasma; HES: hydroxyethyl starch; Hg: mercury; ICU: intensive care unit; kg: kilogram; L: litre; min: minutes; mL: millilitres; mm: millimetres; NS: normal saline; PaCO2: partial pressure of arterial carbon dioxide; PaO2: partial pressure of arterial oxygen; POD1: postoperative day 1; RBCs: red blood cells; ROTEM: Rotational Thromboelastometry (trade name); SAS: Statistical Analysis Software (trade name); SD: standard deviation; TEG: thromboelastography; VAS:visual analogue scale; vs: versus.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bennett‐Guerrero 2001 | Information available in abstract form only. No further information available |
| Bick 1995 | Not a randomized controlled trial |
| Boldt 1993 | No buffered fluid group |
| Boldt 2002a | Study retracted |
| Boldt 2002b | Study retracted |
| Boldt 2007 | Study retracted |
| Boldt 2009 | Study retracted |
| Boldt 2010 | Study retracted |
| Campbell 1990 | Non‐balanced group received a non‐isotonic solution. |
| Choi 2010 | Difference between 3 groups was not just buffered vs non‐buffered (1 group received high molecular weight HES, whilst the other received low molecular weight HES). |
| Evans 2003 | Different fluid categories. No buffered group |
| Javnrin 1980 | No buffered fluid group |
| Kaplan 2001 | ICU patients ‐ not perioperative. Retrospective, not controlled |
| Krebbel 2014 | Balanced group received crystalloid and colloid. |
| Lowery 1967 | Non‐randomized study |
| Protsenko 2009 | Sepsis patients |
| Reid 2003 | Volunteer study |
| Roche 2006 | In vitro study |
| Ruttman 1996 | In vitro study. No buffered group |
| Walker 2001 | Retrospective, case control study |
| Williams 1999 | Volunteer study |
| Young 2015 | ICU patients ‐ not perioperative |
HES: hydroxyethyl starch; ICU: intensive care unit; vs: versus.
Characteristics of studies awaiting assessment [ordered by study ID]
Pfortmueller 2017.
| Methods | Randomized controlled trial of acetate‐buffered crystalloid infusion vs infusion of 0.9% saline and haemodynamic stability in patients undergoing renal transplantation |
| Participants | 150 participants |
| Interventions | 76 participants received 0.9% saline perioperatively. 74 participants received an acetate‐buffered balanced crystalloid (Elomel Isoton). |
| Outcomes | Cumulative vasopressor dose (μg/kg/min) Use of catecholamines Mean minimum arterial blood pressure Peak chloride levels Serum sodium levels |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
NCT02565420.
| Trial name or title | Saline versus lactated Ringer's solution: the SOLAR fluid trial |
| Methods | Randomized controlled trial of lactated Ringer's solution vs normal saline for intraoperative fluid management |
| Participants | Estimated enrolment: 8548 participants |
| Interventions | Active comparator: lactated Ringer's solution
During perioperative period of colorectal, orthopaedic, or similar surgery, participant will receive an intervention of lactated Ringer's solution fluids Placebo comparator: normal saline During perioperative period of colorectal, orthopaedic, or similar surgery, participant will receive an intervention of normal saline solution |
| Outcomes | Primary: major postoperative complications Secondary: economic evaluation |
| Starting date | September 2015 |
| Contact information | Roberta Johnson, 216‐444‐9950; johnsor13@ccf.org |
| Notes | ClinicalTrials.gov identifier: NCT02565420 |
Differences between protocol and review
When appropriate, we performed subgroup analysis for patients undergoing renal transplant surgery. We did not perform any of the other planned subgroup analyses owing to lack of sufficient data.
Contributions of authors
Edward Burdett (EB), Sohail Bampoe (SB), Peter M Odor (PO), Ahilanandan Dushianthan (AD), Elliott Bennett‐Guerrero (EBG), Suzie Cro (SC), Tong J Gan (TJG), Michael PW Grocott (MPWG), Michael FM James (MFMJ), Michael G Mythen (MGM), Anthony M Roche (AR), Kathy Rowan (KR), and Catherine MN O'Malley (COM).
Conceiving the review: MGM, AR, MPWG, KR, EB, MFMJ, EBG, COM.
Co‐ordinating the review: EB, PO, SB.
Undertaking manual searches: PO, SB.
Screening search results: PO, SB.
Organizing retrieval of papers: PO, SB.
Screening retrieved papers against inclusion criteria: PO, SB.
Appraising quality of papers: PO, SB.
Abstracting data from papers: PO, SB.
Writing to authors of papers for additional information: EB, AD, PO, SB.
Providing additional data about papers: PO, SB.
Obtaining and screening data on unpublished studies: EB, PO, SB.
Managing data for the review: PO, SB.
Entering data into Review Manager (RevMan 5.3): PO, SB.
Analysing RevMan statistical data: EB, SC, AD, PO, SB.
Performing other statistical analysis not using RevMan: SC.
Performing double entry of data (person one: PO; person two: SB).
Interpreting data: EB, PO, SB.
Making statistical inferences: MGM, EB, PO, SB.
Writing the review: EB, PO, SB.
Securing funding for the review: MGM, AR.
Performing previous work that was the foundation of the present study: MGM, EBG, TJG, COM.
Serving as guarantor for the review (one review author): MGM.
Taking responsibility for reading and checking the review before submission: EB, PO, SB, COM.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Cochrane Collaboration, UK.
Review completion course.
-
University Hospital Southampton NHS Foundation Trust ‐ University of Southampton Respiratiry Biomedical Research Unit, UK.
Some of the work for this review was conducted in this institution, which receives a proportion of funding from the UK Department of Health's National Institute for Healthcare Research Biomedical Research Units funding scheme.
Declarations of interest
For this updated review, new review authors (SB and PO) performed searches, extracted data, analysed data, and prepared the manuscript. Conflicts of interest include the following.
Edward Burdett ‐ none known.
Sohail Bampoe ‐ none known.
Peter M Odor ‐ none known.
Ahilanandan Dushianthan ‐ none known.
Elliott Bennett‐Guerrerois ‐ author of the following included studies: Gan 1999, Martin 2002, and O'Malley 2005.
Suzie Cro ‐ none known.
Tong J Gan is an author of the following included studies: Gan 1999, Martin 2002, and Moretti 2003.
Michael PW Grocott ‐ none known.
Michael FM James continues to receive ongoing lecture support and honoraria from Fresenius Kabi, which manufactures Voluven ‐ an intervention provided in one of the primary studies included in this review (Base 2011). Professor James did not conduct searches or extract or analyse data for this updated review.
Michael G Mythen is an author of the following included studies: Gan 1999, Martin 2002, and Wilkes 2001.
Catherine O'Malley is an author of the following included study: O'Malley 2005.
Anthony M Roche ‐ none known.
Kathy Rowan ‐ none known.
Edward Burdett, Sohail Bampoe, Peter M Odor, Ahilanandan Dushianthan, Elliott Bennett‐Guerrero, Tong J Gan, Michael PW Grocott, Michael FM James, Michael G Mythen, Anthony M Roche, Kathy Rowan, and Catherine O'Malley all work within the specialities of anaesthesia or critical care medicine, in which both buffered and non‐buffered fluids are used.
Authors of this review authored five of the primary studies included in this Cochrane review (Gan 1999; Martin 2002; Moretti 2003; O'Malley 2005; Wilkes 2001). PO and SB, who were not authors of these primary studies, extracted data from all studies in this updated review.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Base 2011 {published data only}
- Base EM, Standl T, Lassnigg A, Skhirtladze K, Jungheinrich C, Gayko D, et al. Efficacy and safety of hydroxyethyl starch 6% in a balanced electrolyte solution (Volulyte) during cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 2011;25(3):407‐14. [PUBMED: 21345699] [DOI] [PubMed] [Google Scholar]
Chin 2006 {published and unpublished data}
- Chin J, Macachor J, Ong KC, Ong BC. A comparison of 5% dextrose in 0.9% normal saline versus non‐dextrose‐containing crystalloids as the initial intravenous replacement fluid in elective surgery. Anaesthesia and Intensive Care 2006;34(5):613‐7. [PUBMED: 17061636] [DOI] [PubMed] [Google Scholar]
Gan 1999 {published and unpublished data}
- Gan TJ, Bennett‐Guerrero E, Phillips‐Bute B, Wakeling H, Moskowitz DM, Olufolabi Y, et al. Hextend, a physiologically balanced plasma expander for large volume use in major surgery: a randomized phase III clinical trial. Anesthesia and Analgesia 1999;88(5):992‐8. [PUBMED: 10320157 ] [DOI] [PubMed] [Google Scholar]
Hadimioglu 2008 {published data only (unpublished sought but not used)}
- Hadimioglu N, Saadawy I, Saglam T, Ertug Z, Dinckan A. The effect of different crystalloid solutions on acid‐base balance and early kidney function after kidney transplantation. Anesthesia and Analgesia 2008;107(1):264‐9. [PUBMED: 18635497] [DOI] [PubMed] [Google Scholar]
Heidari 2011 {published data only}
- Heidari SM, Saryazdi H, Shafa A, Arefpour R. Comparison of the effect of preoperative administration of Ringer’s solution, normal saline and hypertonic saline 5% on postoperative nausea and vomiting: a randomized, double blinded clinical study. Pakistan Journal of Medical Sciences 2011;27(4):771‐4. [Google Scholar]
Khajavi 2008 {published data only}
- Khajavi MR, Etezadi F, Moharari RS, Imani F, Meysamie AP, Khashayar P, et al. Effects of normal saline vs. lactated Ringer's during renal transplantation. Renal Failure 2008;30(5):535‐9. [PUBMED: 18569935] [DOI] [PubMed] [Google Scholar]
Kim 2013 {published data only}
- Kim SY, Huh KH, Lee JR, Kim SH, Jeong SH, Choi YS. Comparison of the effects of normal saline versus Plasmalyte on acid‐base balance during living donor kidney transplantation using the Stewart and base excess methods. Transplantation Proceedings 2013;45:2191‐6. [PUBMED: 23953528] [DOI] [PubMed] [Google Scholar]
Kulla 2008 {published data only (unpublished sought but not used)}
- Kulla M, Weidhase R, Lampl L. Hydroxyethyl starch 6% 130/0.42 In acetate‐buffered Ringer's solution as a part of a balanced‐volume resuscitation in abdominal surgery. Anasthesiologie und Intensivmedizin 2008;49:7‐18. [Google Scholar]
Martin 2002 {published and unpublished data}
- Martin G, Bennett‐Guerrero E, Wakeling H, Mythen MG, el‐Moalem H, Robertson K, et al. A prospective randomised comparison of thromboelastographic coagulation profile in patients receiving lactated Ringer's solution, 6% Hetastarch in a balanced‐salt vehicle, or 6% Hetastarch in saline during major surgery. Journal of Cardiothoracic and Vascular Anesthesia 2002;16(4):441‐6. [PUBMED: 12154422] [DOI] [PubMed] [Google Scholar]
McFarlane 1994 {published data only (unpublished sought but not used)}
- McFarlane C, Lee A. A comparison of Plasmalyte 148 and 0.9% saline for intra‐operative fluid replacement. Anaesthesia 1994;49(9):779‐81. [PUBMED: 7978133] [DOI] [PubMed] [Google Scholar]
Moretti 2003 {published data only (unpublished sought but not used)}
- Moretti EW, Robertson KM, El‐Moalem H, Gan TJ. Intraoperative colloid administration reduces postoperative nausea and vomiting and improves postoperative outcomes compared with crystalloid administration. Anesthesia and Analgesia 2003;96(2):611‐7. [PUBMED: 12538221] [DOI] [PubMed] [Google Scholar]
Nuraei 2010 {published data only}
- Nuraei N, Khajenouri R, Soleimani M, Dabbagh A. The effects of intraoperative normal saline versus lactated Ringer solution on clinical outcomes and laboratory findings in renal transplant patients. Tehran University Medical Journal 2010;68(4):243‐9. [Google Scholar]
O'Malley 2005 {published data only (unpublished sought but not used)}
- O'Malley CM, Frumento RJ, Hardy MA, Benvenisty AI, Brentjens TE, Mercer JS, et al. A randomized, double‐blind comparison of lactated Ringer's solution and 0.9% NaCl during renal transplantation. Anesthesia and Analgesia 2005;100(5):1518‐24. [DOI] [PubMed] [Google Scholar]
Scheingraber 1999 {published data only (unpublished sought but not used)}
- Scheingraber S, Rehm M, Sehmisch C, Finsterer U. Rapid saline infusion produces hyperchloremic acidosis in patients undergoing gynecologic surgery. Anesthesiology 1999;90(5):1265‐70. [PUBMED: 10319771 ] [DOI] [PubMed] [Google Scholar]
Song 2015 {published data only}
- Song JW, Shim JK, Kim NY, Jang J, Kwak YL. The effect of 0.9% saline versus Plasmalyte on coagulation in patients undergoing lumbar spinal surgery: a randomized controlled trial. International Journal of Surgery 2015;20:128‐34. [DOI] [PubMed] [Google Scholar]
Takil 2002 {published data only (unpublished sought but not used)}
- Takil A, Eti Z, Irmak P, Yilmaz Gogus F. Early postoperative respiratory acidosis after large intravascular volume infusion of lactated Ringer's solution during major spine surgery. Anesthesia and Analgesia 2002;95(2):294‐8. [PUBMED: 12145036 ] [DOI] [PubMed] [Google Scholar]
Walsh 1983 {published data only (unpublished sought but not used)}
- Walsh ES, Traynor C, Paterson JL, Hall GM. Effect of different intraoperative fluid regimens on circulating metabolites and insulin during abdominal surgery. British Journal of Anaesthesia 1983;55(2):135‐40. [PUBMED: 6338893] [DOI] [PubMed] [Google Scholar]
Waters 2001 {published and unpublished data}
- Waters JH, Gottlieb A, Schoenwald P, Popovich MJ, Sprung J, Nelson DR. Normal saline versus lactated Ringer's solution for intraoperative fluid management in patients undergoing abdominal aortic aneurysm repair: an outcome study. Anesthesia and Analgesia 2001;93(4):817‐22. [PUBMED: 11574339 ] [DOI] [PubMed] [Google Scholar]
Wilkes 2001 {published and unpublished data}
- Wilkes NJ, Woolf R, Mutch M, Mallett SV, Peachey T, Stephens R, et al. The effects of balanced versus saline‐based Hetastarch and crystalloid solutions on acid‐base and electrolyte status and gastric mucosal perfusion in elderly surgical patients. Anesthesia and Analgesia 2001;93(4):811‐6. [PUBMED: 11574338 ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bennett‐Guerrero 2001 {published data only (unpublished sought but not used)}
- Bennett‐Guerrero E, Frumento RJ, Berend Mets MPH, Manspeizer HE, Hirsh AL. Impact of normal saline based versus balanced‐salt intravenous fluid replacement on clinical outcomes: a randomized blinded clinical trial. Anesthesiology 2001;95:A147. [Google Scholar]
Bick 1995 {published data only}
- Bick RL. Evaluation of a new hydroxyethyl starch preparation on selected coagulation variables. Clinical and Applied Thrombosis/Hemostasis 1995;1:215‐9. [Google Scholar]
Boldt 1993 {published data only}
- Boldt J, Knothe C, Zickmann B, Andres P, Dapper F, Hempelmann G. Influence of different intravascular volume therapies on platelet function in patients undergoing cardiopulmonary bypass. Anesthesia and Analgesia 1993;76(6):1185‐90. [PUBMED: 7684579 ] [DOI] [PubMed] [Google Scholar]
Boldt 2002a {published data only (unpublished sought but not used)}
- Boldt J, Haisch G, Suttner S, Kumle B, Schellhase F. Are lactated Ringer's solution and normal saline equal with regard to coagulation? Retraction in: Are lactated Ringer's solution and normal saline solution equal with regard to coagulation?: Retraction. [Anesth Analg 2011]. Anesthesia and Analgesia 2002;94(2):378‐84. [PUBMED: 11812703] [DOI] [PubMed] [Google Scholar]
Boldt 2002b {published data only}
- Boldt J, Haisch G, Suttner S, Kumle B, Schellhaass A. Effects of a new modified, balanced hydroxyethyl starch preparation (Hextend) on measures of coagulation. Retraction. Notice of formal retraction of articles by Dr. Joachim Boldt. [Br J Anaesth 2011]. British Journal of Anaesthesia 2002;89(5):722‐8. [PUBMED: 12393770] [DOI] [PubMed] [Google Scholar]
Boldt 2007 {published data only (unpublished sought but not used)}
- Boldt J, Schollhorn T, Munchbach J, Pabsdorf M. A total balanced volume replacement strategy using a new balanced hydroxyethyl starch preparation (6% HES 130/0.42) in patients undergoing major abdominal surgery. European Journal of Anaesthesiology 2007;24(3):267‐75. [PUBMED: 17054812 ] [DOI] [PubMed] [Google Scholar]
Boldt 2009 {published data only (unpublished sought but not used)}
- Boldt J, Suttner S, Brosch C, Lehmann A, Rohm K, Mengistu A. The influence of a balanced volume replacement concept on inflammation, endothelial activation, and kidney integrity in elderly cardiac surgery patients. Retraction Note: The influence of a balanced volume replacement concept on inflammation, endothelial activation, and kidney integrity in elderly cardiac surgery patients. [Intensive Care Med 2011]. Intensive Care Medicine 2009;35(3):462‐70. [PUBMED: 18807007] [DOI] [PubMed] [Google Scholar]
Boldt 2010 {published data only}
- Boldt J, Mayer J, Brosch C, Lehmann A, Mengistu A. Volume replacement with a balanced hydroxyethyl starch (HES) preparation in cardiac surgery patients. Retractions. [J Cardiothorac Vasc Anesth 2011]. Journal of Cardiothoracic and Vascular Anesthesia 2010;24(3):399‐407. [DOI] [PubMed] [Google Scholar]
Campbell 1990 {published data only}
- Campbell IT, Baxter JN, Tweedie IE, Taylor GT, Keens SJ. IV fluids during surgery. British Journal of Anaesthesia 1990;65:726‐9. [PUBMED: 2248854 ] [DOI] [PubMed] [Google Scholar]
Choi 2010 {published data only}
- Choi SJ, Ahn HJ, Chung SS, Kim MH, Choi DH, Lee SM, et al. Hemostatic and electrolyte effects of hydroxyethyl starches in patients undergoing posterior lumbar interbody fusion using pedicle screws and cages. Spine 2010;35(7):829‐34. [PUBMED: 20072091] [DOI] [PubMed] [Google Scholar]
Evans 2003 {published data only}
- Evans PA, Heptinstall S, Crowhurst EC, Davies T, Glenn JR, Madira W, et al. Prospective double‐blind randomized study of the effects of four intravenous fluids on platelet function and hemostasis in elective hip surgery. Journal of Thrombosis and Haemostasis 2003;1(10):2140‐8. [PUBMED: 14521596] [DOI] [PubMed] [Google Scholar]
Javnrin 1980 {published data only}
- Janvrin SB, Davies G, Greenhalgh RM. Postoperative deep vein thrombosis caused by intravenous fluids during surgery. British Journal of Surgery 1980;67(10):690‐3. [PUBMED: 7000224] [DOI] [PubMed] [Google Scholar]
Kaplan 2001 {published data only}
- Kaplan LJ, Bailey H. Large volume resuscitation with hydroxyethyl starch (HES) in lactated Ringer's (LR) solution restores perfusion, minimally induces hyperchloremia or impairs coagulation. Critical Care 2001;5 Suppl 1:113. [Google Scholar]
Krebbel 2014 {published data only}
- Krebbel H, Feldheiser A, Müller O, Boemke W, Sander M, Perka C, et al. Influence of goal‐directed therapy with balanced crystalloid‐colloid or unbalanced crystalloid solution on base excess. Journal of International Medical Research 2014;42(2):468‐86. [PUBMED: 24514432] [DOI] [PubMed] [Google Scholar]
Lowery 1967 {published data only}
- Lowery BD, Cloutier CT, Carey LC. Electrolye solutions in resuscitation in human hemorrhagic shock. Surgery, Gynecology & Obstetrics 1971;133(2):273‐84. [PubMed] [Google Scholar]
Protsenko 2009 {published data only}
- Protsenko DN, Leiderman IN, Grigor'ev EV, Kokarev EA, Levit AL, Gel'fand BR. Evaluation of the effectiveness and safety of synthetic colloid solutions in the treatment of severe abdominal sepsis: a randomized comparative study. Anesteziologiia i Reanimatologiia 2009;5:9‐13. [PUBMED: 19938709] [PubMed] [Google Scholar]
Reid 2003 {published data only}
- Reid F, Lobo DN, Williams RN, Rowlands BJ, Allison SP. (Ab)normal saline and physiological Hartmann's solution: a randomized double‐blind crossover study. Clinical Science (London) 2003;104(1):17‐24. [PUBMED: 12519083] [DOI] [PubMed] [Google Scholar]
Roche 2006 {published data only}
- Roche A, James MF, Bennett‐Guerrero E, Mythen MG. A head‐to‐head comparison of the in vitro coagulation effects of saline‐based and balanced electrolyte crystalloid and colloid intravenous fluids. Anesthesia and Analgesia 2006;102(4):1274‐9. [PUBMED: 16551936] [DOI] [PubMed] [Google Scholar]
Ruttman 1996 {published data only}
- Ruttmann TG, James MF, Viljoen JF. Haemodilution induces a hypercoagulable state. British Journal of Anaesthesia 1996;76(3):412‐4. [PUBMED: 8785143] [DOI] [PubMed] [Google Scholar]
Walker 2001 {published data only}
- Walker SC, Hoover LR, Shepherd JM, Cancio L, Goodwin C. Balanced electrolyte solution reduces acidosis in the resuscitation of perioperative burn patients. Anesthesiology 2001;95(3A):A375. [Google Scholar]
Williams 1999 {published data only}
- Williams EL, Hildebrand KL, McCormick SA, Bedel MJ. The effect of intravenous lactated Ringer's solution versus 0.9% sodium chloride solution on serum osmolality in human volunteers. Anesthesia and Analgesia 1999;88(5):999‐1003. [PUBMED: 10320158] [DOI] [PubMed] [Google Scholar]
Young 2015 {published data only}
- Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. SPLIT Investigators, ANZICS CTG. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA 2015;314(16):1701‐10. [PUBMED: 26444692] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Pfortmueller 2017 {published data only}
- Pfortmueller C, Funk G, Potura E, Reiterer C, Luf F, Kabon B, et al. Acetate‐buffered crystalloid infusate versus infusion of 0.9% saline and hemodynamic stability in patients undergoing renal transplantation. The Wiener Klinische Wochenschrift ‐ The Central European Journal of Medicine 2017;e‐pub. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
NCT02565420 {published data only}
- NCT02565420. Saline versus Lactated Ringer's Solution: The SOLAR Fluid Trial. https://clinicaltrials.gov/ct2/show/NCT02565420. First received 28 September 2015.
Additional references
Altman 1996
- Altman DG, Bland JM. Detecting skewness from summary information. BMJ 1996;313(7066):1200. [PUBMED: 8916759] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bellomo 2001
- Bellomo R, Liskaser F. What is the clinical relevance of dilutional acidosis?. Anesthesiology 2001;95(3):810‐11. [PUBMED: 11575565] [DOI] [PubMed] [Google Scholar]
Bland 1996
- Bland JM, Altman DG. The use of transformation when comparing the two means. BMJ 1996;312(7039):1153. [PUBMED: 8620137] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cosnett 1989
- Cosnett JE. The origins of intravenous fluid therapy. Lancet 1989;1(8641):768‐71. [PUBMED: 2564573 ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GIFTASUP
GIFTASUP 2011
- Powell‐Tuck J, Gosling P, Lobo DN, Allison SP, Carlson GL, Gore M, et al. British consensus guidelines on intravenous fluid therapy for adult surgical patients. GIFTASUP 2011.
Gilbody 2000
- Gilbody SM, Song F, Eastwood AJ, Sutton A. The causes, consequences and detection of publication bias in psychiatry. Acta Psychiatrica Scandinavica 2000;102(4):241‐9. [PUBMED: 11089723] [DOI] [PubMed] [Google Scholar]
GRADEproGDT 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version Accessed July 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Guyatt 2011a
- Guyatt G, Oxman AD, Akl EA, Kunz ZR, Vist G, Brozek J, et al. GRADE guideline: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):389‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Hartmann 1934
- Hartmann AF. Theory and practice of parenteral fluid administration. JAMA 1934;103:1349‐54. [Google Scholar]
Higgins 2008
- Higgins J, White IR, Anzures‐Cabera J. Meta‐analysis of skewed data: combining results reported on log‐transformed or raw scales. Statistics in Medicine 2008;27(29):6072‐92. [PUBMED: 18800342] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman D, Sterne J (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated 2011). The Cochrane Collaboration. www.cochrane‐handbook.org. The Cochrane Collaboration.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of the sample. BMC Medical Research Methodology 2005;5:13. [PUBMED: 15840177] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2011
- Jones RM, Arlidge J, Gillham R, Reagu S, Bree M, Taylor PJ. Efficacy of mood stabilisers in the treatment of impulsive or repetitive aggression: systematic review and meta‐analysis. British Journal of Psychiatry 2011;198(2):93‐8. [PUBMED: 21282779] [DOI] [PubMed] [Google Scholar]
Kellum 2004
- Kellum JA, Song M, Alasri E. Hyperchloremic acidosis increases circulating inflammatory molecules in experimental sepsis. Chest 2004;130(4):962‐7. [PUBMED: 17035425 ] [DOI] [PubMed] [Google Scholar]
Krajewski 2015
- Krajewski ML, Raghunathan K, Paluszkiewicz SM, Schermer CR, Shaw AD. Meta‐analysis of high‐ versus low‐chloride content in perioperative and critical care fluid resuscitation. British Journal of Surgery 2015;102(1):24‐36. [PUBMED: 25357011] [DOI] [PMC free article] [PubMed] [Google Scholar]
Navarro 2015
- Navarro L, Bloomstone J, Auler J, Cannesson M, Rocca G, Gan TJ, et al. Perioperative fluid therapy: a statement from the international Fluid Optimization Group. Perioperative Medicine 2015; Vol. 4, issue 3:published online 2015. [DOI: 10.1186/s13741-015-0014-z; PUBMED: 25897397] [DOI] [PMC free article] [PubMed]
O'Connor 2001
- O'Connor MF, Roizen MF. Lactate versus chloride: which is better?. Anesthesia and Analgesia 2001;4(93):809‐10. [PUBMED: 11574337 ] [DOI] [PubMed] [Google Scholar]
Orbegozo 2014
- Orbegozo Cortes D, Rayo Bonor A, Vincent JL. Isotonic crystalloid solutions: a structured review of the literature. British Journal of Anaesthesia 2014;112(6):968–81. [PUBMED: 24736393] [DOI] [PubMed] [Google Scholar]
Prough 1996
- Prough DS. Crystalloids versus colloids in the perioperative period. Anesthesiology Clinics of North America 1996;14(2):341‐68. [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sterne 2001
- Sterne J, Egger M. Funnel plots for detecting bias in meta‐analysis: guidelines on choice of axis. Journal of Clinical Epidemiology 2001;54(10):1046‐55. [PUBMED: 11576817] [DOI] [PubMed] [Google Scholar]
Stewart 1978
- Stewart PA. Independent and dependent variables of acid‐base control. Respiration Physiology 1978;33(1):9‐26. [PUBMED: 27857] [DOI] [PubMed] [Google Scholar]
Sutton 2000
- Sutton AJ, Duval SJ, Tweedie, RL, Abrams KR, Jones DR. Empirical assessment of effect of publication bias on meta‐analyses. BMJ 2000;320(7249):1574‐7. [PUBMED: 10845965] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilcox 1983
- Wilcox CS. Regulation of renal blood flow by plasma chloride. Journal of Clincal Investigation 1983;71(3):726‐35. [PUBMED: 6826732 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Burdett 2003
- Burdett E, Bennett‐Guerrero E, Frumento R, James M, Mythen MG, Roche T, et al. Perioperative buffered versus non‐buffered fluid administration for surgery in adults. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD004089] [DOI] [PubMed] [Google Scholar]
Burdett 2012
- Burdett E, Dushianthan A, Bennett‐Guerrero E, Cro S, Gan TJ, Grocott MPW, et al. Perioperative buffered versus non‐buffered fluid administration for surgery in adults. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD004089.pub2] [DOI] [PubMed] [Google Scholar]


