Abstract
Background
Heated, humidified air has long been used by people with the common cold. The theoretical basis is that steam may help congested mucus drain better and that heat may destroy the cold virus as it does in vitro. This is an update of a review last published in 2013.
Objectives
To assess the effects of inhaling heated water vapour (steam) in the treatment of the common cold by comparing symptoms, viral shedding, and nasal resistance.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (to February 2017), MEDLINE (1966 to 24 February 2017), Embase (1990 to 24 February 2017), and Current Contents (1998 to 24 February 2017). We also searched World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (8 March 2017) and ClinicalTrials.gov (8 March 2017) as well as reference lists of included studies.
Selection criteria
Randomised controlled trials using heated water vapour in participants with the common cold or experimentally induced common cold were eligible for inclusion.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Three review authors independently screened titles and abstracts for inclusion of potential studies identified from the search. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram. We used a data collection form for study characteristics and outcome data that was developed and used for previous versions of this review. Two review authors independently extracted data, and a third review author resolved any disagreements. We used Review Manager 5 software to analyse data.
Main results
We included six trials from five publications involving a total of 387 participants. We included no new studies in this 2017 update. The 'Risk of bias' assessment suggested an unclear risk of bias in the domain of randomisation and a low risk of bias in performance, detection, attrition, and reporting.
It was uncertain whether heated, humidified air provides symptomatic relief for the common cold, as the fixed‐effect analysis showed evidence of an effect (odds ratio (OR) 0.30, 95% confidence interval (CI) 0.16 to 0.56; 2 studies, 149 participants), but the random‐effects analysis showed no significant difference in the results (OR 0.22, 95% CI 0.03 to 1.95). There is an argument for using either form of analysis. No studies demonstrated an exacerbation of clinical symptom scores. One study conducted in the USA demonstrated worsened nasal resistance, but an earlier Israeli study showed improvement. One study examined viral shedding in nasal washings, finding no significant difference between treatment and placebo groups (OR 0.47, 95% CI 0.04 to 5.19). As judged by the subjective response to therapy (i.e. therapy did not help), the number of participants reporting resolution of symptoms was not significantly higher in the heated humidified group (OR 0.58, 95% CI 0.28 to 1.18; 2 studies, 124 participants). There was significant heterogeneity in the effects of heated, humidified air on different outcomes, therefore we graded the quality of the evidence as low. Some studies reported minor adverse events (including discomfort or irritation of the nose).
Authors' conclusions
The current evidence does not show any benefits or harms from the use of heated, humidified air delivered via the RhinoTherm device for the treatment of the common cold. There is a need for more double‐blind, randomised trials that include standardised treatment modalities.
Plain language summary
Heated, humidified air for the common cold
Review question
We examined the effects of inhaling heated, humidified air delivered by a device (RhinoTherm) for people with common cold.
Background
Common cold is the most common infection in humans. It does not usually cause complications, but can lead to days off work or school due to discomfort caused by symptoms. The diagnosis is based on symptoms, and the treatments are mainly symptomatic. Symptoms include fever, loss of appetite, feeling unwell, feeling chilled, with headache, muscle aches, and pains. Many signs and symptoms are caused by congestion from swelling of membranes and thickened mucus inside the nose. The common cold has been treated for decades with inhaled steam to help the mucus drain more easily. There is laboratory evidence that the cold virus may be sensitive to heat, but no large scale clinical trials have tested its effectiveness. Steam inhalation continues to be used because it provides subjective relief of common cold symptoms.
Search date
The search is current to 24 February 2017.
Study characteristics
We included six randomised, double‐blind trials from five publications involving a total of 387 participants published between 1987 and 1995 in the English language. All included trials used the RhinoTherm device, which delivered heated, humidified air for different lengths of time and at different flow rates to treat common cold symptoms. Three trials were conducted in the USA, two in the UK, and one in Israel. Most studies recruited people with naturally occurring colds, but one study induced colds by infecting participants.
Study funding sources
The RhinoTherm devices were provided by Netzer Sereni in four studies and A Beacham in two studies. One study was funded by Cleveland Clinic internal funding, and another was supported by authors' discretionary funds. The remaining studies did not mention funding sources.
Key results
None of the included studies reported any worsening in clinical symptom scores after inhaling heated, humidified air. Participants in two trials showed a lack of persistent symptoms, however the results were inconsistent. Two studies reported minor adverse events. There was no effect of treatment on rhinovirus shedding.
Quality of evidence
Using GRADE criteria, we assessed the quality of the evidence as low for the outcomes reduction in the clinical severity of the common cold (measured by decrease in the symptom score index); number of participants with the subjective response: therapy did not help; and number of participants with a positive viral culture in the nasal washings, due to risk of bias and inconsistency of the study results.
Summary of findings
Summary of findings for the main comparison. Heated, humidified air compared to control for treating the common cold.
| Heated, humidified air compared to control for treating the common cold | ||||||
| Patient or population: People with the common cold Setting: Clinics, university communities, general practice Intervention: Heated, humidified air administered using a RhinoTherm device Comparison: Ambient air heated to 20 °C to 30 °C at various flow rates | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with rhinothermy | |||||
| Reduction in the clinical severity of the common cold (measured by decrease in the symptom score index) | Study population |
Fixed‐effect model OR 0.30 (0.16 to 0.56) Random‐effects model OR 0.22 (0.03 to 1.95) |
149 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | The significance of the effect is uncertain because use of the fixed‐effect model produces a different result than use of the random‐effects model. | |
| 681 per 1000 |
Fixed‐effect model 390 per 1000 (254 to 544) Random‐effects model 319 per 1000 (60 to 806) |
|||||
| Number of participants with the subjective response: therapy did not help | Study population | OR 0.58 (0.28 to 1.18) | 124 (2 RCTs) | ⊕⊕⊝⊝ LOW 3 4 | We downgraded the evidence for risk of bias and imprecision. | |
| 524 per 1000 | 389 per 1000 (235 to 565) | |||||
| Number of participants with positive nasal wash cultures | Study population | OR 0.47 (0.04 to 5.19) | 20 (1 RCT) | ⊕⊕⊝⊝ LOW 5 6 | We downgraded the evidence for risk of bias and imprecision. | |
| 900 per 1000 | 809 per 1000 (265 to 979) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Ophir 1987 had high attrition rates; Tyrrell 1989b did not perform allocation concealment. 2Downgraded for inconsistency. 3Forstall 1994 and Macknin 1990 did not clearly describe randomisation and allocation concealment methods. 4Downgraded one point for imprecision. 5Hendley 1994 did not clearly state randomisation and allocation methods. Downgraded one point. 6Downgraded one point for imprecision.
Background
Description of the condition
The common cold is an acute, self limiting viral infection of the upper respiratory tract involving, sneezing, nasal congestion and discharge (rhinorrhoea), sore throat, cough, low‐grade fever, headache, and malaise. Different viruses can cause the common cold, the most common of which are the more than 100 serotypes of rhinoviruses (Sahin 2015).
"Life is made up of sobs, sniffles and smiles, with sniffles predominating" (Adams 1967). Sniffles, the common cold, and other acute respiratory infections account for about 40% of employee absenteeism and about 30% of absenteeism from school (Predy 2005). Separate studies of families have shown that the average preschool child has six to 10 colds per year, and the average adult has two to four colds per year (Monto 1974). Care for people with the common cold imposes significant economic burden.
Description of the intervention
Many remedial measures such as antihistamines, decongestants, intranasal ipratropium bromide, vitamin C, interferon, and traditional remedies such as Chinese herbs, garlic, and ginseng are used in the treatment of the common cold (AlBalawi 2013; De Sutter 2012; Karsch‐Volk 2014; Smith 1993; Zhang 2009). Inhaling warm, damp air is thought to offer relief from symptoms of the common cold. Hot water, hot soup, and tea have been used for centuries for this purpose and have been subject to scientific investigation (Saketkhoo 1978; Sanu 2008).
How the intervention might work
Lwoff 1969 suggested that raising the mucosal temperature to 43 °C for three 30‐minute periods can block rhinoviral replication and stop the common cold. Studies of the effect of heated, humidified air suggest that raising nasal mucosal temperature may inhibit rhinoviral replication (Forstall 1994). A device to raise nasal mucosal temperature (RhinoTherm) has been developed. It has been claimed that 80% of participants who used RhinoTherm in the early stages of common cold felt better the next day (Ophir 1987; Yerushalmi 1980).
Why it is important to do this review
A multimillion dollar industry thrives on treatments for alleviating symptoms of the common cold (Fendrick 2003). Treatments range from antihistamines, decongestants, antibiotics, vitamins, and minerals from the conventional medical system to several physical therapies ranging from inhaling steam with herbs, 'neti' treatment, and 'Pranayaam' from the complementary systems of medicine. The common cold and allergic rhinitis constitute a global health problem that affects social life, sleep, school and work performance, and imposes a substantial economic burden on society due to absence from work and reduced working capacity (Hellgren 2010). Two studies estimated the productivity lost to the common cold by using a telephone‐administered survey that measured three sources of loss: absenteeism, on‐the‐job productivity, and caregiver absenteeism (Bramley 2002; Fendrick 2003). Each cold experienced by a working adult caused an average of 8.7 work hours lost (2.8 absenteeism hours and 5.9 hours of on‐the‐job loss) and 1.2 work hours lost due to caring for children aged under 13 years who had colds. The economic cost of lost productivity due to the common cold approaches nearly USD 25,000 million, of which USD 16,600 million is attributed to on‐the‐job productivity loss, USD 8000 million is attributed to absenteeism, and USD 230 million is attributed to caregiver absenteeism (Bramley 2002).
It was therefore important to review the evidence to provide a scientific foundation for the safety and efficacy of heated, humidified air to treat common cold symptoms.
Objectives
To assess the effects of inhaling heated water vapour (steam) in the treatment of the common cold by comparing symptoms, viral shedding, and nasal resistance.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials investigating rhinothermy. "Rhinothermy involves the application of heated and humidified air to the nasal passages" to treat common cold symptoms (Goodall 2016).
Types of participants
The treatment group consisted of people of all ages with natural or experimentally induced common cold or acute viral rhinopharyngitis, receiving warm vapour inhalation via a RhinoTherm device. The control group included people with natural or experimentally induced cold who received room temperature air or room temperature humidified air.
We excluded people who did not meet the predefined inclusion criteria.
Types of interventions
We included trials that compared inhalation from a device delivering warm, humidified air (40 °C to 47 °C) that raised intranasal temperature with breathing from a similar device that delivered humidified or ambient temperature air at up to 30 °C.
Types of outcome measures
Primary outcomes
Reduction in the clinical severity of the common cold (i.e. a decrease in the symptom score index). This was measured based on symptoms of nasal blockage, sneezing, and nasal drainage, which were scored on a four‐point scale (0 = no symptoms, 1 = mild, 2 = moderate, 3 = severe).
Number of participants with the subjective response: therapy did not help.
Secondary outcomes
Decrease in the weight of nasal secretions.
Decrease in nasal resistance measured using a rhinomanograph (ICS Medical Corporation, Schaumburgh, IL).
Number of participants with a positive viral culture in the nasal washings.
Adverse events.
Search methods for identification of studies
Electronic searches
We searched the following databases:
Cochrane Central Register of Controlled Trials, which contains the Cochrane Acute Respiratory Infection Group's Specialised Register, in the Cochrane Library searched on 24 February 2017 using the strategy in Appendix 1;
MEDLINE (Ovid) (from 1966 to 24 February 2017) using the strategy in Appendix 2;
Embase (Elsevier) (from 1990 to 24 February 2017) using the strategy in Appendix 3; and
Current Contents (Thomson Reuters) (from 1998 to 24 February 2017) (Appendix 4).
We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision); Ovid format (Lefebvre 2011). Where applicable, it was modified appropriately for other databases.
We searched the following clinical trials registries:
the World Health Organization (WHO) ICTRP (8 March 2017); and
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov, 8 March 2017).
We did not restrict results by language or publication status (published, unpublished, in press, or in progress). Details of search strategies used for previous versions of this review are presented in Appendix 5.
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We planned to contact experts in the field to identify any additional unpublished materials.
Data collection and analysis
Selection of studies
Three review authors (MaS, NJ, AC) independently screened titles and abstracts of the studies identified by the search for potential inclusion in the review. We retrieved the full‐text reports of the studies deemed potentially eligible, and the same two review authors independently screened the full texts to identify studies for inclusion, and identified and recorded reasons for exclusion of ineligible studies. Any disagreements were resolved through discussion, or by consulting a third review author (MeS) when necessary. We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and 'Characteristics of excluded studies' table (Moher 2009). We did not impose any language restrictions.
Data extraction and management
We used a data collection form for study characteristics and outcome data that was developed for previous versions of this review. One review author (MaS or MeS) extracted study characteristics from included studies. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (MaS, MeS) independently extracted outcome data from included studies. We noted in the 'Characteristics of included studies' table if outcome data were not reported in a usable way. We resolved disagreements by consensus. Two review authors (NJ, AC) transferred data into the Review Manager 5 file (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with the study reports. A third review author (MeS) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
All review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). We assessed the risk of bias according to the following domains.
Random sequence generation
Allocation concealment
Blinding of participants and personnel
Blinding of outcome assessment
Incomplete outcome data
Selective outcome reporting
Other bias
We graded each potential source of bias as high, low, or unclear and provided quotes from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the 'Risk of bias' judgements across different studies for each domain listed. We considered blinding separately for different key outcomes where necessary. Where information on risk of bias related to unpublished data or correspondence with an author, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for the studies that contributed data to that outcome.
Measures of treatment effect
We entered outcome data for each study into data tables in Review Manager 5 to calculate the treatment effects (RevMan 2014). We used odds ratio for dichotomous outcomes, and planned to use mean differences or standardised mean differences for continuous outcomes.
Dealing with missing data
All of the included studies provided dropout rates; none conducted intention‐to‐treat analyses (Higgins 2011a). We contacted trial authors of two included studies to verify key study characteristics.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis (Deeks 2011).
Assessment of reporting biases
If we were able to pool more than 10 trials, we planned to create and examine a funnel plot to explore possible small‐study and publication biases.
Data synthesis
We planned to pool data from studies judged to be clinically homogeneous using Review Manager 5 (RevMan 2014). We planned to perform meta‐analysis where more than one study provided usable data in any single comparison using fixed‐effect and random‐effects models.
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: reduction in the clinical severity of the common cold (measured by decrease in the symptom score index); number of participants with the subjective response: therapy did not help; and number of participants with a positive viral culture in the nasal washings. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the evidence quality as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We employed methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions,Higgins 2011b, using GRADEpro GDT software (GRADEpro GDT 2014). We justified all decisions to down‐ or upgrade the quality of studies using footnotes, and made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
No subgroup analyses were planned for this review.
Sensitivity analysis
We conducted sensitivity analysis for the number of participants with persistent symptoms using the Mantel‐Haenszel random‐effects model.
Results
Description of studies
See Characteristics of included studies.
Results of the search
Of the 499 records identified in the searches for the 2017 update, we excluded 496 after title and abstract screening. We obtained full‐text copies of three studies (Murdoch 2014; Varricchio 2013; Yu 2013), which we excluded following assessment. We included no new studies in this 2017 update. The previous version of this review included five randomised controlled trials, which we retained in this update (Figure 1).
1.
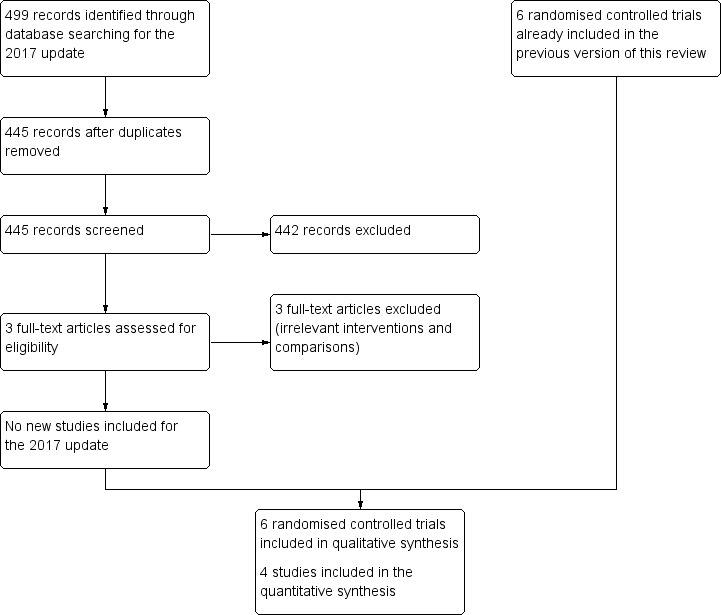
Study flow diagram for 2017 update.
Included studies
We included six trials reported in five publications involving a total of 387 participants (Forstall 1994; Hendley 1994; Macknin 1990; Ophir 1987; Tyrrell 1989a; Tyrrell 1989b). Tyrrell 1989 included the general population and volunteer arms, which were analysed separately as Tyrrell 1989a and Tyrrell 1989b, respectively. All included studies were randomised and double‐blinded. All trials were conducted in the 1980s to 1990s, with small sample sizes.
Tyrrell 1989a and Tyrrell 1989b used a RhinoTherm invented by A. Beacham, and four trials used RhinoTherm devices manufactured by Netzer Sereni (Forstall 1994; Hendley 1994; Macknin 1990; Ophir 1987). RhinoTherm is a microprocessor‐controlled device that delivers warm, humidified air at a controlled temperature.
Forstall 1994 was conducted in the USA in 1992 and included 75 participants, of whom seven were excluded from the final analysis. Of the included participants, 81% and 61% were females in the intervention and control groups, respectively. Participants were aged over 18 years and had symptoms of moderate to severe cold, nasal congestion, discharge or sneezing at the time of enrolment. The intervention was administered to 32 participants via RhinoTherm delivering 40 L per minute of saturated constant air flow at 47 °C for one hour. The control group (N = 36) received 2 L per minute of ambient air at 20 °C to 24 °C for one hour. This study was funded by Cleveland Clinic internal funding.
Hendley 1994 was conducted in the USA with 20 healthy participants whose colds were an experimentally induced rhino viral infection. The average age of participants was 20 years, and 80% were female. Participants with fever, respiratory illness, or taking antihistamines, decongestants, steroids, or nasal spray were excluded. The intervention was delivered to 10 participants via RhinoTherm to provide 38 L to 40 L per minute humidified air at 42 °C to 44 °C after 24 hours and 48 hours. The control group (N = 10) received 2 L per minute of ambient air at 22 °C to 23 °C. The study was supported by authors' discretionary funds.
Ophir 1987 was conducted in Israel and included 62 participants with a mean age of 34 years. The trial randomised 70 participants, of whom eight dropped out (3 and 5 from the intervention and control groups, respectively). The intervention arm included 32 participants with common cold who were treated with heated vapour at 42 °C to 44 °C at a flow rate of 40 L per minute delivered via RhinoTherm in two 20‐minute sessions at 60‐ to 90‐minute intervals. The control group (N = 30) were treated with 22 °C to 24 °C at 2 L per minute of ambient air.
Macknin 1990 was conducted in the USA in 1989 and included a total of 66 participants. The mean ages of participants were 36 years for the intervention group and 32 years for the control group. The percentage of female participants was 75% in the intervention group and 79% in the control group. The intervention group (N = 32) received heated, distilled vapour at 42 °C to 45 °C at 40 L per minute in two 20‐minute sessions at 60‐ to 90‐minute intervals. The control group (N = 34) received 2 L per minute at 20 °C to 24 °C.
The trial by Tyrell 1989 included two study arms.
Tyrrell 1989a was conducted in a UK general practice in 1989 and included 96 participants, of which data records for 87 participants were obtained. Of these 87 participants, 45 received humidified air at 43 °C, and 42 received humidified air at 30 °C at a rate of 40 L for 20 minutes. The ratio of male to female was 48% to 52% in the intervention group and 60% to 40% in the control group. The participants in this study arm did not cross‐over from Tyrrell 1989b.
Tyrrell 1989b was a separate study arm. After two days of quarantine, 75 participants aged 18 to 50 years were experimentally induced with intranasal drops of human rhinovirus type 14. Volunteers were randomised to receive water vapour at 43 °C or 30 °C for 30 minutes. Participants received three 30‐minute sessions with 90‐minute intervals.
Excluded studies
See Characteristics of excluded studies.
We excluded six studies that did not meet the inclusion criteria; three were previously excluded (Baroody 2000; Grübber 2003; Yerushalmi 1980), and three were excluded from the 2017 search (Murdoch 2014; Varricchio 2013; Yu 2013).
Three studies assessed populations that were not relevant to this review (Baroody 2000; Yerushalmi 1980; Yu 2013); two investigated interventions that this review did not assess (Grübber 2003; Varricchio 2013); and one reported on an intervention and population not relevant to this review (Murdoch 2014).
Risk of bias in included studies
The overall risk of bias is presented graphically in Figure 2 and summarised in Figure 3.
2.
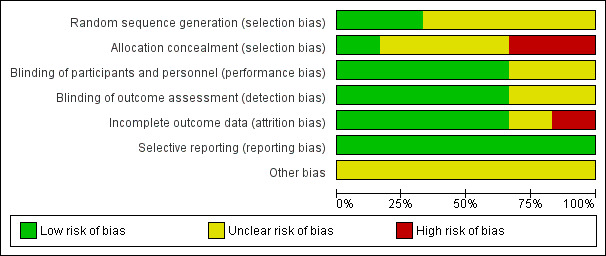
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Four of six included studies randomised participants. However, we assessed these studies as at unclear risk of bias for random sequence generation because they did not describe randomisation methods (Forstall 1994; Hendley 1994; Macknin 1990; Ophir 1987). We assessed two studies as at low risk of bias (Tyrrell 1989a; Tyrrell 1989b).
Allocation concealment was not stated clearly in three trials, which were assessed as at unclear risk of bias for this domain (Forstall 1994; Macknin 1990; Ophir 1987). The other three studies did not mention allocation concealment and were assessed as at high risk of bias (Hendley 1994; Tyrrell 1989a; Tyrrell 1989b).
Blinding
We assessed four studies as at low risk for performance bias (Forstall 1994; Hendley 1994; Macknin 1990; Ophir 1987). We assessed two studies as at unclear risk of bias because it was not clear who was blinded or how blinding was done (Tyrrell 1989a; Tyrrell 1989b).
We assessed four studies as at low risk of detection bias (Forstall 1994; Hendley 1994; Macknin 1990; Ophir 1987). We judged two studies to be at unclear risk of detection bias (Tyrrell 1989a; Tyrrell 1989b).
Incomplete outcome data
We assessed one study as at high risk of bias because the attrition rate in the placebo group was more than the event rate in the placebo group (Ophir 1987). In Tyrrell 1989a, data were presented for analysis for 87 of 96 enrolled participants with no reason given, hence it was classified as at unclear risk of bias. The remaining four included studies were at low risk of bias for this domain (Forstall 1994; Hendley 1994; Macknin 1990; Tyrrell 1989b).
Selective reporting
The protocols for the published studies were not available for review. We compared the outcomes listed in the methods section to reported outcomes for all included studies. We found that all outcomes listed in the methods sections were reported in the results of the studies. We therefore assessed all studies as at low risk of bias for this domain (Forstall 1994; Hendley 1994; Macknin 1990; Ophir 1987; Tyrrell 1989a; Tyrrell 1989b).
Other potential sources of bias
None known.
Effects of interventions
See: Table 1
Primary outcomes
1. Reduction in the clinical severity of the common cold (measured by decrease in the symptom score index)
The included studies did not provide unequivocal evidence supporting the use of warm vapour inhalations for treatment of the common cold. No studies demonstrated a worsening of clinical symptom scores, but two studies reported the persistence of symptoms (odds ratio (OR) 0.30, 95% confidence interval (CI) 0.16 to 0.56; 2 studies, 149 participants, fixed‐effect model) (Analysis 1.1). There was significant heterogeneity (I² = 87%).
1.1. Analysis.
Comparison 1 Rhinothermy versus control, Outcome 1 Number of participants with persistent symptoms.
Three studies used similar symptom index scores (Forstall 1994; Macknin 1990; Ophir 1987). Ophir 1987 showed a significant improvement in symptom index score; however, Forstall 1994 and Macknin 1990 did not show any improvements. Macknin 1990 showed a greater change towards symptom improvement from baseline in the placebo group. We could not pool these studies because standard deviations were unavailable. Tyrrell 1989a in their general practice study and Tyrrell 1989b in the volunteer study, showed more improvements in the participants given the hot humidified air at 43 C by the rhinotherm device. A different symptom score used by Hendley 1994 showed no significant difference between study and control interventions.
2. Number of participants with subjective response: therapy did not help
As judged by subjective response to therapy (therapy did not help), there was a statistically non‐significant resolution of symptoms in the heated, humidified group (OR 0.58, 95% CI 0.28 to 1.18; 2 studies, 124 participants, I² = 22%) (Analysis 1.2).
1.2. Analysis.
Comparison 1 Rhinothermy versus control, Outcome 2 Number of participants with subjective response to therapy: therapy did not help.
Secondary outcomes
1. Decrease in the weight of nasal secretions
None of the included studies commented on weight of nasal secretions of participants.
2. Decrease in nasal resistance as measured by a rhinomanograph
Three studies showed improvement in nasal resistance (Ophir 1987; Tyrrell 1989a; Tyrrell 1989b). Forstall 1994 demonstrated increased nasal resistance one week after steam inhalation (data skewed at entry). This contrasted with an earlier study that showed improvement in nasal resistance (Macknin 1990). We could not pool data for analysis.
3. Number of participants with a positive viral culture in the nasal washings
There was no statistically significant difference in number of participants with positive nasal wash cultures (OR 0.47, 95% CI 0.04 to 5.19; 1 study, 20 participants) (Analysis 1.3).
1.3. Analysis.
Comparison 1 Rhinothermy versus control, Outcome 3 Number of participants with positive nasal wash culture.
4. Adverse events
Macknin 1990 reported that adverse events were statistically significant in the rhinothermy group (OR 4.73, 95% CI 1.46 to 15.30; 1 study, 65 participants, P = 0.010) (Analysis 1.4). Minor adverse events included nasal and lip irritation, lightheadedness, increased congestion, and discomfort from the mask delivering heated, humidified air (Macknin 1990). Forstall 1994 reported episodes of nasal congestion, minor mucosal burns, and discomfort from condensation in the mask. One participant was reported to have experienced temporary dizziness for two to three minutes following treatment with saturated, hot air (Ophir 1987). However, the studies reporting adverse events related to thermal discomfort used the treatment for longer durations.
Sensitivity analysis
We conducted a sensitivity analysis for the number of participants with persistent symptoms using the Mantel‐Haenszel random‐effects model, which showed no significant difference in results (OR 0.22, 95% CI 0.03 to 1.95) (Analysis 2.1).
2.1. Analysis.
Comparison 2 Rhinothermy versus control (random‐effects model), Outcome 1 Number of participants with persistent symptoms.
Discussion
Summary of main results
We included six trials from five publications (one publication included two trials that we assessed separately) that investigated heated, humidified air for the treatment of people with the common cold. The RhinoTherm devices were provided by Netzer Sereni in four studies (Forstall 1994; Hendley 1994; Macknin 1990; Ophir 1987), and by A Beacham in two studies (Tyrrell 1989a; Tyrrell 1989b). One study was funded by Cleveland Clinic internal funding (Forstall 1994), while another study was supported by authors' discretionary funds (Hendley 1994). The remaining studies did not mention funding sources (Macknin 1990; Ophir 1987; Tyrrell 1989a; Tyrrell 1989b).
Three trials reported benefits of heated, humidified air for symptom relief in people with the common cold (Ophir 1987; Tyrrell 1989a; Tyrrell 1989b). However, sample sizes were small. Results on symptom indices were equivocal. No studies demonstrated an exacerbation of clinical symptom scores (Analysis 1.1; Analysis 1.2). Forstall 1994 demonstrated worsened nasal resistance, but Ophir 1987 showed improvements. Hendley 1994 examined viral shedding in nasal washings through cultures, finding no difference between treatment and placebo groups. Two studies reported minor adverse events (including discomfort or nasal irritation) (Forstall 1994; Macknin 1990).
Overall completeness and applicability of evidence
We aimed to examine the evidence for heated, humidified air delivered using a RhinoTherm device for treating the common cold. The trials assessed participants that included healthy volunteers with experimentally‐induced cold. This added an element of indirectness. The small sample sizes and the fact that all outcomes were not addressed made the effect estimates susceptible to change. The included trials are now more than two decades old, and we identified no new studies (completed or ongoing) for this update. Hence, cautious interpretation of the findings is suggested.
Quality of the evidence
We used GRADE (GRADEpro GDT 2014), as described in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011), to assess the quality and certainty of the evidence (Table 1). The existing evidence was of low quality, which implies that further research is very likely to change our effect estimates.
We downgraded the evidence for potential risk of bias, inconsistency among the trials, imprecision and indirectness of the trials. We attributed imprecision and inconsistency to the small sample sizes of the trials.
Potential biases in the review process
We were unable to pool the data for most outcomes due to a lack of included studies reporting the outcome. We could not estimate publication bias due to the small number of included studies. We aimed to reduce bias in the review process by using a standardised search strategy and including studies up to the most recent search date. We performed searching and data extraction according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). The strength of this review is in the inclusion of studies using similar methodologies and instrument for the intervention. There was a difference in the duration of warm vapour inhalation, with a longer period (30 minutes) associated with no benefit and increased resistance of the nasal passages. We found potential biases in most included studies (unclear risk of bias for randomisation and allocation concealment).
Agreements and disagreements with other studies or reviews
A review of non‐antibiotic treatments for upper respiratory tract infections (the common cold), based on seven Cochrane Reviews and presenting risk ratios for outcomes, drew similar conclusions (Arroll 2005).
Authors' conclusions
Implications for practice.
The current evidence does not show any benefits or harms from the use of heated, humidified air delivered via a RhinoTherm device for the treatment of the common cold. Cautious interpretation of the evidence is suggested.
Implications for research.
There is a need for large, multicentre studies using rhinothermy to treat the symptoms of the common cold, including a detailed cost‐benefit analysis of using this device. The outcome measures should be dependent upon the frequency of common cold symptoms and a definitive diagnosis based on viral cultures, using a uniform symptom score index and nasal resistance measurements.
Feedback
Heated, humidified air for the common cold
Summary
1. I am delighted to see this review: a common and important but long neglected problem ‐ and a marvellous introductory quote! I hope my comments may help you clarify some aspects.
2. Objectives: it would be good to specify the comparisons to be made in the review.
3. Types of participants: the treatment group includes people with "acute viral rhinopharyngitis" [avr], but the control group does not. Is avr synonymous with "common cold"? Healthy volunteers inoculated with virus are not patients, nor are people with a spontaneous or 'natural' cold who have not consulted a health professional.
4. Types of intervention: "... delivering hot humidified air ... at 40‐44 deg C." This should exclude the Forstall 1994 study, in which the air was delivered at 47 deg, or if it is included it must at least be analysed separately. It would be useful to add an illustration of the Rhinotherm apparatuses ‐ say line drawings.
5. Types of outcome measures: detailed reporting and discussion of the symptom scores used in the trials is necessary. They are unlikely to have used the same scoring methods, and cannot be combined without explicit justification.
6. Methods of the review: the validity score used assumes that all the points are equally important, and is therefore misleading. As the Cochrane Reviewers' Handbook 4.0 [end of section 6.7.2] says " ... it is preferable to ... report how each trial scored on each criterion".
7. Results: the results should be reported separately for each of the different comparisons to be made, and also separately for each of the 5 outcome measures. Only then will it be possible to decide whether it is appropriate to lump together any of the comparisons or the outcome measures. In the absence of such an analysis the MetaView summary cannot be interpreted.
8. Discussion: it is not clear where each trial was done. This should be noted in the table of Characteristics of included trials. Geographical differences may not only imply different epidemiology of rhinovirus infection, but also climatic and seasonal differences which could affect outcomes. The two experimental studies by Tyrrell should not be lumped with the others.
9. Characteristics of included trials: At what stage in the infection, for how long, and how many times were the inhalations used in each trial? For some trials details are incomplete, e.g. Ophir: source of patients, length of follow up. Tyrrell‐1 and ‐2: length of follow up.
10. Conclusions: I think the implications for practice need to be rethought in the light of a more detailed analysis.
Reply
The review has been revised after these comments.
Contributors
Andrew Herxheimer Feedback and reply added 27 July 2001
What's new
| Date | Event | Description |
|---|---|---|
| 24 February 2017 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 24 February 2017 | New search has been performed | Searches updated and we identified three new trials for exclusion (Murdoch 2014; Varricchio 2013; Yu 2013). New authors joined to update the review. |
History
Review first published: Issue 3, 1999
| Date | Event | Description |
|---|---|---|
| 8 March 2015 | New search has been performed | Searches updated. We excluded three new trials (Murdoch 2014; Varricchio 2013; Yu 2013). |
| 8 March 2015 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 12 March 2013 | New search has been performed | We conducted new searches. We did not identify any new trials for inclusion or exclusion in this updated review. |
| 12 March 2013 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 21 July 2010 | New search has been performed | We conducted an updated search but found no new trials for inclusion in the review. Although the main conclusions remain unchanged, due to the increasing prevalence of common cold‐like symptoms due to H1N1, it has become essential to conduct well‐designed clinical trials to test the effect of hot, humid air on respiratory virus inactivation. |
| 20 December 2009 | New citation required but conclusions have not changed | A new coauthor, Dr Meenu Singh, joined to update this review. |
| 8 May 2009 | Amended | Contact details updated. |
| 3 May 2008 | Amended | Converted to new review format. |
| 19 December 2005 | New search has been performed | Searches conducted. |
| 14 December 2003 | New search has been performed | Searches conducted. |
| 29 July 2001 | New search has been performed | Searches conducted. |
| 26 July 2001 | Feedback has been incorporated | Feedback comment and reply added to review. |
| 3 December 1998 | New search has been performed | Searches conducted. |
Notes
The original version of this review, Singh 1999, was submitted to the Cochrane Library as a review; no Cochrane Review protocol was published. The initial protocol and review were conducted for presentation at an acute respiratory infection conference held in Canberra, Australia.
Acknowledgements
The Library Service, Post Graduate Institute of Medical Education and Research (PGIMER), Chandigarh, India. We thank Cochrane Acute Respiratory Infections Group Information Specialist David Honeyman for database searching and Managing Editor Liz Dooley and Assistant Managing Editor Ann Jones for editorial assistance. We would also like to acknowledge Michael Macknin for sharing study funding details.
Appendices
Appendix 1. CENTRAL search strategy
#1 [mh ^"Common Cold"] #2 "common cold*":ti,ab #3 coryza:ti,ab #4 [mh ^Nasopharyngitis] #5 (nasopharyngit* or rhinopharyngit*):ti,ab #6 (acute near/2 rhinit*):ti,ab #7 (upper near/2 ("respiratory infection*" or "respiratory tract infection*")):ti,ab #8 ((nasal or nose*) near/1 (blocked or blockage* or obstruct* or congest* or stuffiness or stuffy or discharge or runny or running)):ti,ab #9 ("nasal mucus" or "nasal mucous"):ti,ab #10 [mh ^Rhinovirus] #11 [mh ^coronavirus] or [mh ^"coronavirus 229e, human"] or [mh ^"coronavirus nl63, human"] or [mh ^"coronavirus oc43, human"] #12 [mh ^"Coronavirus Infections"] #13 [mh ^"Adenoviruses, Human"] #14 [mh ^"Adenovirus Infections, Human"] #15 ((rhinovir* or coronavir* or adenovir*) near/2 infect*):ti,ab #16 {or #1‐#15} #17 [mh ^Steam] #18 steam*:ti,ab #19 [mh ^Humidity] #20 ((heat* or hot or warm* or humid*) near/3 air):ti,ab #21 [mh "Nebulizers and Vaporizers"] #22 [mh ^"Administration, Inhalation"] #23 (inhal* or atomi* or vapor* or vapour* or nebuli*):ti,ab #24 {or #17‐#23} #25 #16 and #24
Appendix 2. MEDLINE (Ovid) search strategy
1 Common Cold/ 2 common cold*.tw. 3 coryza.tw. 4 Nasopharyngitis/ 5 (nasopharyngit* or rhinopharyngit*).tw. 6 (acute adj2 rhinit*).tw. 7 (upper adj2 (respiratory infection* or respiratory tract infection*)).tw. 8 ((nasal or nose*) adj1 (blocked or blockage* or obstruct* or congest* or stuffiness or stuffy or discharge or runny or running)).tw. 9 (nasal mucus or nasal mucous).tw. 10 Rhinovirus/ 11 coronavirus/ or coronavirus 229e, human/ or coronavirus nl63, human/ or coronavirus oc43, human/ 12 Coronavirus Infections/ 13 Adenoviruses, Human/ 14 Adenovirus Infections, Human/ 15 ((rhinovir* or coronavir* or adenovir*) adj2 infect*).tw. 16 or/1‐15 17 Steam/ 18 steam*.tw. 19 Humidity/ 20 ((heat* or hot or warm* or humid*) adj3 air).tw. 21 exp "Nebulizers and Vaporizers"/ 22 Administration, Inhalation/ 23 (inhal* or atomi* or vapor* or vapour* or nebuli*).tw. 24 or/17‐23 25 16 and 24
Appendix 3. Embase.com search strategy
#18 #12 AND #17 #17 #13 OR #14 OR #15 #16 inhal*:ab,ti OR atomi*:ab,ti OR vapor*:ab,ti OR vapour*:ab,ti OR nebuli*:ab,ti #15 'nebulizer'/exp OR 'inhalation'/de #14 steam*:ab,ti OR ((heat* OR warm* OR hot OR humid*) NEAR/3 air):ab,ti #13 'water vapor'/de OR 'humidity'/de #12 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 #11 ((rhinovir* OR coronavir* OR adenovir*) NEAR/2 infect*):ab,ti #10 'human adenovirus'/exp OR 'human adenovirus infection'/de #9 'coronavirus'/de OR 'human coronavirus nl63'/de #8 'rhinovirus infection'/de OR 'human rhinovirus'/de #7 'nasal mucus':ab,ti OR 'nasal mucous':ab,ti #6 ((nasal OR nose*) NEAR/1 (blocked OR blockage* OR obstruct* OR congest* OR stuffiness OR stuffy OR discharge OR runny OR running)):ab,ti #5 'upper respiratory tract infection'/de OR 'viral upper respiratory tract infection'/de #4 nasopharyngit*:ab,ti OR rhinopharyngit*:ab,ti #3 'rhinopharyngitis'/de #2 'common cold':ab,ti OR 'common colds':ab,ti OR coryza:ab,ti OR (acute NEXT/2 rhinitis):ab,ti #1 'common cold'/de OR 'common cold symptom'/de
Appendix 4. Current Contents (Thomson Reuters) search strategy
| # 6 | 15 | #4 AND #3 Refined by: Publication Years=(2011 OR 2010 OR 2012) Databases=CM, LS Timespan=All Years Lemmatization=On |
| # 5 | 105 | #4 AND #3 Databases=CM, LS Timespan=All Years Lemmatization=On |
| # 4 | 528,340 | Topic=(random* or placebo* or allocat* or crossover* or "cross over" or ((singl* or doubl*) NEAR/1 blind*)) OR Title=(trial) Databases=CM, LS Timespan=All Years Lemmatization=On |
| # 3 | 321 | #2 AND #1 Databases=CM, LS Timespan=All Years Lemmatization=On |
| # 2 | 80,936 | Topic=(steam* or ((heat* or hot or warm* or humid*) NEAR/3 air) or inhal* or atomi* or vapor* or vapour* or nebuli*) Databases=CM, LS Timespan=All Years Lemmatization=On |
| # 1 | 7,377 | Topic=("common cold" or "common colds" or coryza or (acute NEAR/2 rhinitis) or rhinopharyngit* or nasopharyngit* or "upper respiratory tract infection" or "upper respiratory tract infections" or "upper respiratory infection" or "upper respiratory infections") OR Topic=((nasal or nose*) NEAR/1 (blocked or blockage* or obstruct* or congest* or stuffiness or stuffy or discharge or runny or running)) OR Topic=("nasal mucus" or "nasal mucous") Databases=CM, LS Timespan=All Years Lemmatization=On |
Appendix 5. Previous search strategies
In 1999 when we published the first review we searched MEDLINE using the following MeSH headings: common cold; rhinopharyngitis; inhalation; steam and heated vapour. We used the highly sensitive search strategy for identifying RCTs as given in the Cochrane Handbook for Systematic Reviews of Interventions. We used different combinations of terms to retrieve the maximum number of studies. We searched EMBASE, Current Contents, review articles and cross‐references. We wrote letters to the manufacturers of the rhinotherm equipment for any unpublished trials. We did not receive any replies.
In 2003, we updated this review to identify all recent RCTs in any language. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2003, Issue 4), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 1966 to November Week 2, 2003), EMBASE (January 1990 to November 2003) and Current Contents (current five years).
In 2005, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2005, Issue 4), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (2003 to December Week 2, 2005); EMBASE (July 2003 to September 2005) and Current Contents (current five years). No new trials were identified.
In the 2010 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 3), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (December 2005 to July week 1, 2010), EMBASE.com (September 2005 to July 2010) and Current Contents (2005 to July 2010). We used the following search terms to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE.
MEDLINE
1 Common Cold/ 2 common cold*.tw. 3 coryza.tw. 4 Rhinovirus/ 5 coronavirus/ or coronavirus 229e, human/ or coronavirus oc43, human/ 6 Picornaviridae/ 7 Adenoviridae/ 8 ((rhinovir* or coronavir* or picornavir* or adenovir*) adj2 infect*).tw. 9 or/1‐8 10 Steam/ 11 steam*.tw. 12 Humidity/ 13 humid*.tw. 14 ((heat* or hot or warm*) adj5 air*).tw. 15 Inhalation/ 16 Administration, Intranasal/ 17 ((nose* or nasal or intranasal) adj5 inhal*).tw. 18 exp "Nebulizers and Vaporizers"/ 19 ((heat* or hot or warm*) adj3 (vapour* or vapour* or nebul* or atomi*)).tw. 20 or/10‐19 21 9 and 20
Data and analyses
Comparison 1. Rhinothermy versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with persistent symptoms | 2 | 149 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.16, 0.56] |
| 1.1 Number of participants with persistent symptoms at the end of therapy | 2 | 149 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.16, 0.56] |
| 2 Number of participants with subjective response to therapy: therapy did not help | 2 | 124 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.28, 1.18] |
| 3 Number of participants with positive nasal wash culture | 1 | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.04, 5.19] |
| 4 Subjective response: side effects were present | 1 | 65 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.73 [1.46, 15.30] |
1.4. Analysis.
Comparison 1 Rhinothermy versus control, Outcome 4 Subjective response: side effects were present.
Comparison 2. Rhinothermy versus control (random‐effects model).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with persistent symptoms | 2 | 149 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.03, 1.95] |
| 1.1 Number of participants with persistent symptoms at the end of the therapy | 2 | 149 | Odds Ratio (M‐H, Random, 95% CI) | 0.22 [0.03, 1.95] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Forstall 1994.
| Methods | Study design: double‐blind, randomised, placebo‐controlled, in vivo study Study duration: 14 January 1992 to 8 April 1992 | |
| Participants |
Inclusion criteria
A total of 75 participants were randomised in a double‐blind fashion to 2 groups; 68 participants aged at least 18 years had symptoms of common cold such as nasal congestion, nasal discharge, or sneezing. The study population was 75% female. The average age was 35 years for both groups. Exclusion criteria
|
|
| Interventions |
Treatment group
Control group
After the initial nasal airway resistance measurement, each participant underwent a single 1‐hour treatment with either the steam or the placebo treatment |
|
| Outcomes |
Symptom score index: Symptom score indexes were completed by participants on days 0 to 7. A symptom score card was also maintained by a masked study investigator. Percentage change in score from baseline was calculated for each participant. Both groups had identical symptom experiences, as measured by the participant and investigator symptom index cards. Placebo participants had a higher initial nasal resistance than the treatment participants (6.0 versus 3.9, P = 0.04). The unit of measurement of nasal resistance is percentage of change Nasal resistance: Showed an increase in participants who received rhinothermy. Data were skewed at entry. The difference was attributed to an imbalance between the groups rather than due to the treatment effect The results of this study show that warm, humidified air at 47 °C is no better than non‐humidified air at 20 °C to 24 °C for treatment of the common cold. Heated, humidified air may be detrimental to nasal resistance 7 days after treatment. |
|
| Notes | Funding source was not mentioned in the published report; we contacted the authors for this information. The manufacturer (Netzer Sereni) provided the RhinoTherm, and other costs were borne by Cleveland Clinic internal funding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were allocated randomly to the 2 groups in a blinded manner. The method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both devices looked similar, and the investigator did not know which delivered heated air |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessment by symptom index assessed by participants and investigator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7 participants were excluded from the final analysis (5 lost to follow‐up and 2 withdrew from the study) |
| Selective reporting (reporting bias) | Low risk | No published protocol was available. All outcomes listed in the methods section of the published trial were reported in the results |
| Other bias | Unclear risk | None known. |
Hendley 1994.
| Methods |
Study design: double‐blind, randomised controlled trial on people with experimental induction of rhinoviral infection Study duration: 2 days, 1994 |
|
| Participants |
Inclusion criteria:
20 healthy participants susceptible to rhino viral infection as determined by their neutralising antibody titre were inoculated intranasally with coarse drops containing untyped human rhinovirus culture. Exclusion criteria:
|
|
| Interventions |
Treatment group
"Instrument: rhinotherm, Netzer‐Sereni, Beer Yacov, Israel. The digital temperature readout showed 45 to 50 °C on the active machine and was preset at 55 °C on the placebo machine. The airstream from both the machines was humidified and visible as steam. The vapour from the active machine was delivered at a temperature of 42 °C to 44 °C at the flow rate of 38 to 40 L/min. Vapour from the placebo machine was delivered at 22 °C to 23 °C at a flow rate of 2 L/min. These differences were apparent if the machines were compared side by side" (p.1112). The air stream from both the machines was humidified, hence temperature difference was the only active intervention. Control group
"Two treatments were given; the first 24 hours after inoculation when the symptoms started appearing and the second after 48 hours of inoculation when the symptoms were at their peak" (p.1112) |
|
| Outcomes |
Symptom score index: "Viral shedding was assessed daily for 5 days after viral inoculation. The symptom severity during each period was assessed by using a scale 0 to 4 of sneezing, rhinorrhoea, nasal obstruction, sore throat, cough, headache, malaise and chills" (p.1113) |
|
| Notes | "The titre of rhinovirus were the same in the active and the placebo group on day 1, prior to treatment, and on all four days after treatment. The proportion of participants who shed the virus was also the same in the 2 groups. Symptom scores in the 2 treatment groups were not statistically different" (p.1113) Funding source: equipment used was provided by RhinoTherm, Netzer Sereni, Beer Yacov, Israel. Study was supported by investigators from their discretionary funds. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not described |
| Allocation concealment (selection bias) | High risk | Allocation concealment not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both devices looked similar, and the investigator did not know which delivered heated air |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessed using scale grading different levels of severity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details of all participants' outcomes were described |
| Selective reporting (reporting bias) | Low risk | No published protocol was available. All outcomes listed in the methods section of the published trial were reported in the results |
| Other bias | Unclear risk | None known |
Macknin 1990.
| Methods |
Study design: randomised, double‐blind, parallel‐group comparison Study duration: study conducted in 1990 |
|
| Participants |
Inclusion criteria
There were 4 dropouts (3 from the treatment group and 1 from the placebo group). Gender and racial differences were non‐significant between groups. Exclusion criteria
|
|
| Interventions |
Treatment
Control
In the active group the intervention was operated through "rhinotherm (Netzer Sereni, Beer Yacov, Israel), which is a microprocessor‐controlled ultrasonic heater, from the vibrated heated, distilled water (42 to 44 °C)" (p.989). These droplets were delivered through 2 exhaust nozzles with a constant flow of 40 L/min saturated, warm air. Comparatively, the placebo group received ambient air at room temperature (20 °C to 24 °C at a flow rate of only 2 L/min) with a machine identical to the active device. Clinical nurse instructed participants to inhale through the nozzle, which was adjusted at 2.5 cm from the nares, and exhale through the mouth, which maintained a "temperature of 40 to 42 °C at the distal nostrils. Treatment were given in front of a mirror to ensure self monitoring of the nozzle position throughout the treatment" (p.990). "The active intervention was constituted by raised temperature as well as saturation with water vapour" (p.990) |
|
| Outcomes |
Symptom score index: Outcomes were assessed days 1 to 7 by participants on daily score card. Outcomes assessed were "severity of 3 symptoms (nasal drainage, nasal congestion and sneezing) on a 4 point scale each day" (p.990). Any change in the severity index symptom index score was taken for evaluation. Nasal resistance: A single blinded research associate measured the total nasal resistance using rhinomanographer, before the treatment and on day 1 and 7 after the treatment. The calculation formula used to measure resistance in each nostril was "R = (RNxLB/RN+LN), where R indicates the total nasal resistance, right nostril (RN) resistance, left nostril (LN) resistance" (p.990). "The two groups were similar for symptom index score measurements at the time of entry but significantly favoured the placebo on day 3, 6 and 7. On day 1, the measurement of nasal resistance showed a median 2% worse in the placebo group on day 1 (P > 0.05)" (p.990). "But on day 7, nasal resistance got better by 11% in the placebo group and 6% worse in the active group (P < 0.05)" (p.990) |
|
| Notes | "The results of this study do not support the use of rhinothermy in the common cold. The authors attribute the difference to a difference in the epidemiology of the disease in the USA. This study also raises the possibility of hot, humid air damaging the nasal epithelium as improvement in the nasal resistance was noted more in the placebo group." (p.991) Funding agency: the brand name of the equipment is mentioned. However, no clear information about who funded the study was available |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was mentioned that the participants were randomly allocated to 1 of 2 groups in a blinded manner. The method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Methods not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | An attempt was made to keep the intervention blinded by using identical‐ looking and ‐sounding equipment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessed using similar scale for placebo and intervention group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details of all participants' outcomes were described |
| Selective reporting (reporting bias) | Low risk | No published protocol was available. All outcomes listed in the methods section of the published trial were reported in the results |
| Other bias | Unclear risk | None known |
Ophir 1987.
| Methods |
Study design: 2 groups of participants were randomised in a double‐blind design Study duration: 1987 |
|
| Participants |
Inclusion criteria
Participants with a naturally acquired common cold Exclusion criteria
|
|
| Interventions |
Treatment group
Warm vapour inhalation via RhinoTherm device delivering heated vapour at 42 °C to 44 °C (study group) at 40 L/min. An identical‐looking and ‐sounding instrument delivered air at room temperature (22 °C to 24 °C) to the placebo group at the flow rate of 2 L/min. A full course consisted of 2 treatments lasting 20 minutes, with 60‐ to 90‐minute intervals Control group
|
|
| Outcomes |
Symptom score index: Symptom score index was calculated for each day by dividing the sum of recorded on that day by 3. Symptomatic improvement was noticed in 26/32 steam‐treated participants and 7/30 placebo‐treated participants Nasal resistance: Objective measure of nasal potency the morning after treatment improved in 61% to 74% of participants in the steam‐treated group and only 6% to 8% of participants in the placebo‐treated group. There was a significant improvement in the nasal blockade index in the active group (P < 0.01) |
|
| Notes | The results of this study demonstrate a clear‐cut improvement in the symptom index in the treatment group. Symptomatic improvement at the end of the follow‐up period was reported by 92.9% of participants in the active treatment group and 84.6% of participants in the placebo group. Nasal patency was increased significantly in the active treatment group Funding source: RhinoTherm device used in study manufactured by Netzer Serani, Israel |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not clearly described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | An attempt was made to keep the intervention blinded by using identical‐looking and ‐sounding equipment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessed using similar scale for placebo and intervention group |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The attrition rate of the placebo group was more than the event rate of the placebo group |
| Selective reporting (reporting bias) | Low risk | No published protocol was available. All outcomes listed in the methods section of the published trial were reported in the results |
| Other bias | Unclear risk | None known. |
Tyrrell 1989a.
| Methods |
Study design: randomised, double‐blind trial in a general practice setting Study duration: study conducted in 1989 |
|
| Participants |
Inclusion criteria
96 participants were enrolled, of whom complete data were available for 87. The study involved participants with typical acute nasal and upper respiratory symptoms (general practice study). 45/87 participants received humidified air at 43 °C and 42/87 received humidified air at 30 °C. Male‐to‐female ratio was 23:22 in the intervention group and 17:25 in the placebo group. History of other conditions such as hay fever or sinusitis was present in 11 participants in the intervention group and 18 participants in the control group. There were more participants with symptoms in the control group than the intervention group Exclusion criteria Not described |
|
| Interventions |
Treatment:
Control:
|
|
| Outcomes |
Symptom score index: Immediately after treatment, 22/45 participants given air at 43 °C and 16/42 given air at 30 °C reported improvement. There were small differences in the response of other symptoms During the subsequent days, the mean symptom scores were substantially less in the group given humidified air at 43 °C than in the group given humidified air at 30 °C, the mean total scores being 9.3 and 25.9, respectively. Since the groups were imbalanced, at the time of entry, rank sum analysis was performed that gave highly significant results between the 2 groups. On the fourth day of observation, 21/45 of the participants given air at 43 °C and only 1/42 given air at 30 °C were absolutely free of symptoms |
|
| Notes | Some imbalances were present in the study groups, which were overcome by the authors by using a rank analysis of variance. The groups were also blocked for scores before treatment. The data from this study strongly support the beneficial effect of inhaling warm, humidified air through a RhinoTherm for the treatment of common cold The RhinoTherm device was supplied by A. Beacham |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated to groups based on random numbers from a list |
| Allocation concealment (selection bias) | Low risk | Used a random numbers list |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details of who were blinded and how this was done were not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of who were blinded and how this was done were not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The study presents data for 87 out of 96 enrolled participants without providing a reason |
| Selective reporting (reporting bias) | Low risk | No published protocol was available. All outcomes listed in the methods section of the published trial were reported in the results |
| Other bias | Unclear risk | None known. |
Tyrrell 1989b.
| Methods |
Study design: experimental study on human participants with experimental induction Study duration: conducted in 1989 |
|
| Participants |
Inclusion criteria
After 2 days of quarantine, 75 participants were inoculated with intranasal drops containing about 100 times the median tissue culture dose of human rhinovirus type 14. 27 participants who developed early signs of cold, used 4 tissue papers more than their baseline, or developed at least 1 more symptom of the common cold were entered into the trial and allocated at random to receive humidified air through the RhinoTherm at 43 °C or 30 °C. Participants received 3 treatments of 30 minutes each at 90‐minute intervals. The proportion of participants showing improvement was reported to be significantly greater in the group receiving steam at 43 °C Exclusion criteria Not described |
|
| Interventions |
Treatment
Control
The machines delivering humidified air were placed on a table, and the participants sat on a chair to breathe through a vented anaesthetic mask. Some machines were set at 43 °C and some at 30 °C. Both machines gave the sensation of breathing warm, moist air |
|
| Outcomes |
Symptom score index: The number of participants rating symptoms as 'better' immediately after treatment was 14 in the 30 °C group compared with 39 in the 43 °C group. The difference in the mean total score was significant (P = 0.02) The difference in the weight of the nasal secretions was also significant (26 versus 33, P = 0.027) Nasal washings for viral titre: There was no difference in the proportion of participants shedding virus between the 2 groups. On the day of treatment, the group given air at 43 °C had insignificantly lower viral titre. The frequency of antibody response was not significantly different (5/14 in the 43 °C group and 7/13 in the 30 °C group). The mean titres at convalescence were also not significantly different |
|
| Notes | The evidence in this study was derived from participants who had experimental induction of the common cold. There was a significant difference between the mean total scores and in the total weight of the secretions, the reductions being 43% and 21%, respectively Funding agency: the RhinoTherm used in this study was manufactured by Netzer Serani |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated into groups based on random numbers list |
| Allocation concealment (selection bias) | High risk | There was no mention of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Details of who were blinded and how this was done were not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Details of who were blinded and how this was done were not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Details of all participants' outcomes were described |
| Selective reporting (reporting bias) | Low risk | No published protocol was available. All outcomes listed in the methods section of the published trial were reported in the results |
| Other bias | Unclear risk | None known. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baroody 2000 | RCT involving participants with allergic rhinitis undergoing a nasal allergen challenge with hot, humid air delivered in a chamber or by a mask |
| Grübber 2003 | Intervention was physical application of hot and cold water. The main outcome assessed was incidence of the common cold. |
| Murdoch 2014 | RCT evaluating the role of TRPV1 antagonists on cold, dry air‐induced symptoms in people with non‐allergic rhinitis |
| Varricchio 2013 | RCT comparing inhaled crenotherapy (vapour therapy) with salso‐sulphide thermal water and isotonic saline for the prevention of recurrent respiratory infections in children |
| Yerushalmi 1980 | Participants with persistent allergic rhinitis |
| Yu 2013 | RCT examining the role of heated humidifiers during CPAP titration in people with obstructive sleep apnoea‐hypopnoea syndrome |
CPAP: continuous positive airway pressure RCT: randomised controlled trial TRPV1: transient receptor potential vanilloid 1
Differences between protocol and review
In this 2017 update, we moved the secondary outcome "Number of participants with no symptoms" to the primary outcomes and rephrased it as "Number of participants with subjective response: therapy did not help" because we believed it addressed an important question about the efficacy of heated, humidified air. We also rephrased the secondary outcome "Decrease in viral culture titre in the nasal secretions" to " Number of participants with a positive viral culture in the nasal washings", as it added clarity for the readers about what exactly is reported in the review.
Contributions of authors
Meenu Singh was the sole author of this review and subsequent updates until 2005. The 2011 and 2013 updates were conducted by Meenu Singh and Manvi Singh.
The current update (2017) was conducted by Meenu Singh (MeS), Manvi Singh (MaS), Nishant Jaiswal (NJ), and Anil Chauhan (AC).
| Roles and responsibilities | |
| Task | Undertaken by |
| Review stage: select which trials to include | NJ, AC, MeS |
| Review stage: extract data from trials | MaS, MeS |
| Review stage: enter data into Review Manager 5 | NJ, AC |
| Review stage: carry out the analysis | MaS, MeS |
| Review stage: interpret the analysis | MaS, MeS |
| Review stage: draft the final review | MeS, AC, NJ, MaS |
| Update stage: update the review | MeS, AC, NJ, MaS |
Sources of support
Internal sources
Post Graduate Institute of Medical Education and Research, Chandigarh, India.
External sources
-
eHealth Project, Ministry of Health and Family Welfare, Government of India, India.
Financial Support to Poonam Chaudhary, B. Lib. Information specialist.
Declarations of interest
Meenu Singh: none known. Manvi Singh: none known. Nishant Jaiswal: none known. Anil Chauhan: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Forstall 1994 {published data only}
- Forstall GJ, Macknin LM, Yen‐Lieberman BR, Medendorp SV. Effect of inhaling heated vapor on symptoms of the common cold. JAMA 1994;271(14):1109‐11. [PubMed] [Google Scholar]
Hendley 1994 {published data only}
- Hendley OJ, Abbot RD, Beasley PP, Gwaltney JM Jr. Effect of inhalation of hot humidified air on experimental rhinovirus infection. JAMA 1994;271(14):1112‐3. [PubMed] [Google Scholar]
Macknin 1990 {published data only}
- Macknin ML, Mathew S, Medendorp SV. Effect of inhaling heated vapor on symptoms of the common cold. JAMA 1990;264(8):989‐91. [PubMed] [Google Scholar]
Ophir 1987 {published data only}
- Ophir D, Elad Y. Effects of steam inhalation on nasal patency and nasal symptoms in patients with the common cold. American Journal of Otolaryngology 1987;8(3):149‐53. [DOI] [PubMed] [Google Scholar]
Tyrrell 1989a {published data only}
- Tyrrell D, Barrow I, Arthur J. Local hyperthermia benefits natural and experimental common colds. BMJ 1989;298(6683):1280‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tyrrell 1989b {published data only}
- Tyrrell D, Barrow I, Arthur J. Local hyperthermia benefits natural and experimental common colds. BMJ 1989;298(6683):1280‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Baroody 2000 {published data only}
- Baroody FM, Assanasen P, Chung J, Naclerio RM. Hot, humid air partially inhibits the nasal response to allergic provocation. Archives of Otolaryngology ‐ Head & Neck Surgery 2000;126(6):749‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grübber 2003 {published data only}
- Grübber C, Riesberg A, Mansmann U, Knipschild P, Wahn U, Bühring M. The effect of hydrotherapy on the incidence of common cold episodes in children: a randomized controlled trial. European Journal of Pediatrics 2003;162(3):168‐76. [DOI] [PubMed] [Google Scholar]
Murdoch 2014 {published data only}
- Murdoch RD, Bareille P, Denyer J, Newlands A, Bentley J, Smart K, et al. TRPV1 inhibition does not prevent cold dry air‐elicited symptoms in non‐allergic rhinitis. International Journal of Clinical Pharmacology and Therapeutics 2014;52(4):267‐76. [DOI] [PubMed] [Google Scholar]
Varricchio 2013 {published data only}
- Varricchio AM, Giuliano M, Capasso M, Gaizo D, Ascione E, Lucia A, et al. Salso‐sulphide thermal water in the prevention of recurrent respiratory infections in children. International Journal of Immunopathology and Pharmacology 2013;26(4):941‐52. [DOI] [PubMed] [Google Scholar]
Yerushalmi 1980 {published data only}
- Yerushalmi A, Lwoff A. Treatment of infectious coryza and persistent allergic rhinitis by thermotherapy [Traitement du coryza infectieux et des rhinites persistantes allergiques par la thermotherapie]. Comptes Rendus de l'Academie des Sciences de Paris 1980;291(12):957‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Yu 2013 {published data only}
- Yu CC, Luo CM, Liu YC, Wu HP. The effects of heated humidifier in continuous positive airway pressure titration. Sleep & Breathing 2013;17(1):133‐8. [DOI: 10.1007/s11325-012-0661-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Adams 1967
- Adams JM. Viruses and Colds: the Modern Plague. New York: Elsevier Science Publishing Co. Inc, 1967. [Google Scholar]
AlBalawi 2013
- AlBalawi ZH, Othman SS, AlFaleh K. Intranasal ipratropium bromide for the common cold. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD008231.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arroll 2005
- Arroll B. Non‐antibiotic treatments for upper‐respiratory tract infections (common cold). Respiratory Medicine 2005;99(12):1477‐84. [PUBMED: 16291073] [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2014;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bramley 2002
- Bramley TJ, Lerner D, Sames M. Productivity losses related to the common cold. Journal of Occupational and Environmental Medicine 2002;44(9):822‐9. [DOI] [PubMed] [Google Scholar]
De Sutter 2012
- Sutter AIM, Driel ML, Kumar AA, Lesslar O, Skrt A. Oral antihistamine‐decongestant‐analgesic combinations for the common cold. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD004976.pub3] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analysis. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Fendrick 2003
- Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non‐influenza‐related viral respiratory tract infection in the United States. Archives of Internal Medicine 2003;163(4):487‐94. [DOI] [PubMed] [Google Scholar]
Goodall 2016
- Goodall S, Molloy P. Treatment and prevention of the common cold using povidone‐iodine. Google Patents 2016.
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version (accessed 3 May 2017). Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Hellgren 2010
- Hellgren J, Cervin A, Nordling S, Bergman A, Cardell LO. Allergic rhinitis and the common cold ‐ high cost to society. Allergy 2010;65(6):776‐83. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Wiley‐Blackwell.
Higgins 2011b
- Higgins JP, Green S, editor(s). Cochrane Handbook of Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Karsch‐Volk 2014
- Karsch‐Völk M, Barrett B, Kiefer D, Bauer R, Ardjomand‐Woelkart K, Linde K. Echinacea for preventing and treating the common cold. Cochrane Database of Systematic Reviews 2014, Issue 2. [DOI: 10.1002/14651858.CD000530.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). Available from www.cochrane‐handbook.org. Wiley‐Blackwell.
Lwoff 1969
- Lwoff A. Death and transfiguration of a problem. Bacteriological Reviews 1969;33(3):390‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Monto 1974
- Monto AS, Ulman BM. Acute respiratory illness in an American community. The Tecumseh study. JAMA 1974;227(2):164‐9. [PubMed] [Google Scholar]
Predy 2005
- Predy GN, Goel V, Lovlin R, Donner A, Stitt L, Basu TK. Efficacy of an extract of North American ginseng containing polyfuranosyl‐pyranosyl‐saccharides for preventing upper respiratory tract infections: a randomized controlled trial. Canadian Medical Association Journal 2005;175(9):1043‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5.3). Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sahin 2015
- Sahin ÖNA, Gülen F. Approach to common cold in children. Journal of Pediatric Research 2015;2(1):1‐6. [Google Scholar]
Saketkhoo 1978
- Saketkhoo K, Januszkiewicz A, Sackner MA. Effects of drinking hot water, cold water, and chicken soup on nasal mucus velocity and nasal airflow resistance. Chest 1978;74(4):408‐10. [DOI] [PubMed] [Google Scholar]
Sanu 2008
- Sanu A, Eccles R. The effects of a hot drink on nasal airflow and symptoms of common cold and flu. Rhinology 2008;46(4):271. [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. ..
Smith 1993
- Smith MBH, Feldman W. Over the counter cold medications. A critical review of trials between 1950‐1991. JAMA 1993;269(17):2258‐63. [DOI] [PubMed] [Google Scholar]
Zhang 2009
- Zhang X, Wu T, Zhang J, Yan Q, Xie L, Liu GJ. Chinese medicinal herbs for the common cold. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004782.pub2] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Singh 1999
- Singh M. Heated, humidified air for the common cold. Cochrane Database of Systematic Reviews 1999, Issue 3. [DOI: 10.1002/14651858.CD001728] [DOI] [PubMed] [Google Scholar]
Singh 2001
- Singh M. Heated, humidified air for the common cold. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001728.pub3] [DOI] [PubMed] [Google Scholar]
Singh 2004
- Singh M. Heated, humidified air for the common cold. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD001728.pub3] [DOI] [PubMed] [Google Scholar]
Singh 2006
- Singh M. Heated, humidified air for the common cold. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001728.pub3] [DOI] [PubMed] [Google Scholar]
Singh 2011
- Singh M, Singh M. Heated, humidified air for the common cold. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD001728.pub4] [DOI] [PubMed] [Google Scholar]
Singh 2013
- Singh M, Singh M. Heated, humidified air for the common cold. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD001728.pub5] [DOI] [PubMed] [Google Scholar]


