Abstract
Antibody-mediated lymphoablation is used in solid organ and stem cell transplantation and autoimmunity. Using murine anti-thymocyte globulin (mATG) in a mouse model of heart transplantation, we previously reported that the homeostatic recovery of CD8+ T cells requires help from depletion-resistant memory CD4+ T cells delivered through CD40-expressing B cells. This study investigated the mechanisms by which B cells mediate CD8+ T cell proliferation in lymphopenic hosts. While CD8+ T cell recovery required MHC class I expression in the host, the reconstitution occurred independently of MHC class I, MHC class II, or CD80/CD86 expression on B cells. mATG lymphoablation upregulated the B cell expression of several cytokine genes, including IL-15 and IL-27, in a CD4-dependent manner. Neither treatment with anti-CD122 mAb nor the use of IL-15Rα–/– recipients altered CD8+ T cell recovery after mATG treatment, indicating that IL-15 may be dispensable for T cell proliferation in our model. Instead, IL-27 neutralization or the use of IL-27Rα–/– CD8+ T cells inhibited CD8+ T cell proliferation and altered the phenotype and cytokine profile of reconstituted CD8+ T cells. Our findings uncover what we believe is a novel role of IL-27 in lymphopenia-induced CD8+ T cell proliferation and suggest that targeting B cell–derived cytokines may increase the efficacy of lymphoablation and improve transplant outcomes.
Keywords: Immunology, Transplantation
Keywords: B cells, Cytokines, T cells
interleukin-27 is induced by antibody-mediated lymphoablation and promotes reconstitution of CD8+ T cells in mouse heart allograft recipients.
Introduction
Antibody-mediated lymphoablation is commonly used in solid organ transplantation, stem cell transplantation, and autoimmunity (1). Rabbit anti-thymocyte globulin (ATG) is currently the most commonly used polyclonal antibody in clinical transplantation, with about half of renal transplant recipients in the United States receiving it as induction therapy (2). However, despite well-documented benefits of peritransplant lymphoablation for early graft function and prevention of acute rejection, the impact on long-term transplant survival remains controversial (3–7). Two major indications for the use of lymphoablative induction are recipient previous sensitization to donor alloantigens and cadaveric donor grafts subjected to prolonged cold ischemia storage. Even though recipient humoral and cellular immune memory is a primary reason for lymphoablation, memory T cells appear to be more resistant to depletion, having been observed in transplant recipients treated with ATG or anti-CD52 mAb (CAMPATH-1) in association with acute rejection episodes (8, 9). Consistent with these clinical findings, our previous studies in a mouse model of heart transplantation revealed that peritransplant lymphocyte depletion is followed by rapid memory T cell proliferation and only modestly prolongs allograft survival (10, 11). Understanding the mechanisms of T cell reconstitution and the resulting T cell repertoire composition is therefore critical for assessing and improving the long-term consequences of lymphoablative induction therapies.
Upon administration of lymphocyte-depleting antibody, remaining T cells undergo non–antigen-specific expansion known as homeostatic proliferation. Previous studies delineated 2 different types of lymphopenia-induced homeostatic proliferation (12). The first type is observed in experimental settings when small numbers of T cells are adoptively transferred into animals with acute lymphopenia (induced by irradiation or depleting antibody). This T cell expansion is dependent on IL-7 and cognate interactions with self MHC molecules but not on major costimulatory pathways (13–16). In the second type, rapid homeostatic proliferation of T cells injected into chronically lymphopenic animals is IL-7 independent but requires commensal microflora antigens and CD28 costimulation (17, 18). In addition, homeostatic cytokine-induced proliferation of naive T lymphocytes can be driven by 2 other γc cytokines, IL-2 and IL-15 (19, 20).
We previously reported that the mechanisms of endogenous T cell recovery after in situ antibody-mediated lymphoablation differ from those observed in the context of adoptive T cell transfer (10). We have used a mouse heart allograft model in which recipients were treated with a mouse analog of anti-thymocyte globulin (mATG) (11). Distinct from proliferation requirements observed in other models of lymphopenia, the reconstitution of the CD8+ T cell compartment is critically dependent on the presence of depletion-resistant memory CD4+ T cells, CD40/CD154 costimulation, and intact B lymphocytes. Furthermore, we demonstrated that CD40 signaling in B lymphocytes, but not in other cell types, is critical for homeostatic CD8+ T cell proliferation (10).
The goal of the current study was to investigate the mechanisms by which B cells mediate CD4+ T cell help for homeostatic reconstitution of CD8+ T cells. In particular, we tested the requirements for cognate MHC-peptide-TCR interactions between B cells and CD4+ or CD8+ T lymphocytes. While homeostatic CD8+ T cell recovery after mATG depletion was severely impaired in recipients with global deficiency in MHC class I expression, the reconstitution occurred independently of MHC class I, MHC class II, or CD80/CD86 expression on B cells. NanoString gene expression analysis revealed that several cytokine genes, including IL-15 and IL-27, are upregulated in B cells from recipients treated with mATG, and that this upregulation is abolished in the absence of CD4+ T cell help. Contrary to our expectations, neither treatment with anti-CD122 mAb nor the use of IL-15Rα–/– recipients altered the kinetics of CD8+ T cell homeostatic proliferation, indicating that IL-15 may be dispensable for T cell recovery. In contrast, IL-27 neutralization or the use of IL-27Rα–/– CD8+ T cells significantly decreased CD8+ T cell reconstitution, demonstrating the critical role of this cytokine in B cell–driven homeostasis of T cells following antibody-mediated lymphoablation.
Results
CD4+ T cell–B cell interactions following lymphoablation occur independently of MHCII expression on B cells and antigen recognition.
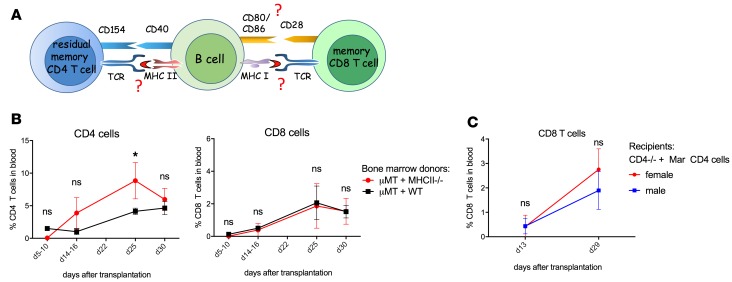
We previously reported that homeostatic recovery of CD8+ T lymphocytes following mATG depletion is critically dependent on depletion-resistant memory CD4+ T cells, B cells, and an intact CD40/CD154 pathway (10). Based on these findings, we proposed that depletion-resistant memory CD4+ T cells provide signals to B cells through CD40, thus enabling B cells to initiate CD8+ T cell proliferation (Figure 1A). To investigate whether cognate CD4+ T cell–B cell (hereafter referred to as CD4–B cell) interaction requires TCR recognition of antigens presented by B cells, we used mice deficient in all MHC class II genes to generate mixed bone marrow chimeras in which only B cells lack MHC class II expression. After hematopoietic cell reconstitution, the chimeric mice were transplanted with BALB/c heart allografts and treated with mATG. Unexpectedly, allograft recipient chimeras deficient in B cell MHC class II expression had no defects in T cell recovery compared with control recipient chimeras with MHC class II–sufficient B cells (Figure 1B). To further test the requirement for specific antigen recognition by residual helper T cells, we used Mar TCR-transgenic CD4+ T cells recognizing Y chromosome–encoded HYDby peptide presented in the context of I-Ab (21). Mar T cells were adoptively transferred into B6.CD4–/– male (contain HYDby antigen) or female (no specific antigen) mice followed by transplantation of BALB/c male heart allografts and mATG treatment. Consistent with our previous results, B6.CD4–/– mice had minimal recovery of CD8+ T cells after mATG injection (data not shown). In contrast, transferred Mar CD4+ T cells provided help for CD8+ T cell proliferation regardless of the antigen expression in the host (Figure 1C). Thus, while initial CD4–B cell interactions in lymphopenic recipients are CD40 dependent, they do not require recognition of antigen presented by B cell MHC class II by helper T cells.
Figure 1. CD4–B cell interactions following lymphoablation occur independently of MHCII expression on B cells and specific antigen recognition.
(A) Proposed model. (B) Lethally irradiated B cell–deficient μMT mice received a 1:1 mixture of μMT plus WT (control) or μMT plus MHCII−/− (B cells lacking MHC class II) bone marrow (BM). Resulting BM chimeras received BALB/c heart allografts and were treated with mATG (1 mg i.p. on days 0 and 4). Percentages of CD4+ and CD8+ among peripheral blood live cells were determined by flow cytometry. (C) Mar TCR-transgenic T cells (CD4+, HY-reactive) were transferred into male or female C57BL/6J mice followed by BALB/c heart transplantation and mATG treatment. Results are representative of 2 experiments with n = 3–5 animals/group/experiment; error bars represent SD. *P < 0.05; ns, P ≥ 0.05 by multiple t tests.
B cell MHC class I expression is dispensable for CD8+ T cell recovery.
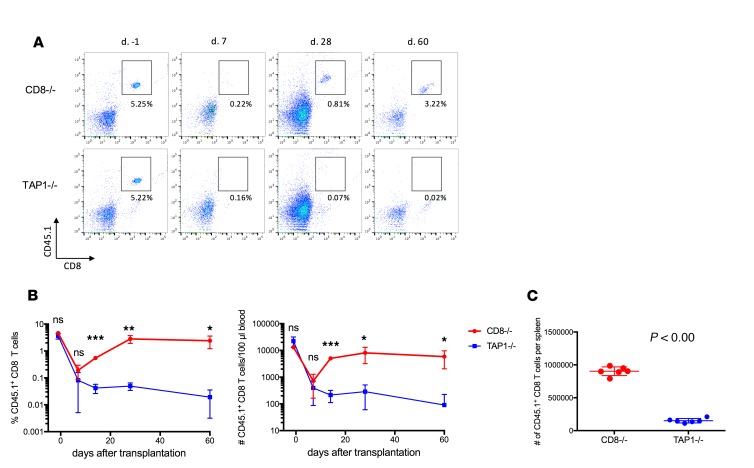
To investigate the requirement for TCR engagement during CD8+ T cell reconstitution, we adoptively transferred congenic B6.CD45.1 CD8+ T cells either into B6.TAP1–/– mice that have severely reduced cell surface expression of MHC class I or into B6.CD8–/– mice with normal MHC class I expression. After BALB/c heart transplantation and mATG treatment, transferred CD45.1+CD8+ T cells were similarly depleted in both groups (Figure 2, A and B). Despite prominent depletion, by day 35 after transplant CD8+ T cells injected into B6.CD8–/– recipients expanded to predepletion levels. In contrast, no CD8+ T cell reconstitution was observed in the absence of recipient MHC class I (Figure 2).
Figure 2. CD8+ T cell recovery is impaired in heart allograft recipients lacking MHC class I expression.
B6.CD45.1+ splenic CD8+ T cells were intravenously injected into CD8–/– or TAP1–/– B6 mice (10 × 106 per recipient) followed by BALB/c heart transplantation and mATG treatment. (A) Representative dot plots showing percentages of CD8+CD45.1+ T cells among peripheral blood live cells. (B) The kinetics of CD8+CD45.1+ T cell reconstitution. (C) Numbers of CD8+CD45.1+ T cells in spleen at day 60 after transplant. n = 6 animals per group; error bars represent SD. *P < 0.05, **P < 0.01, ***P < 0.001; ns, P ≥ 0.05 by multiple t tests (B) or Student’s t test (C).
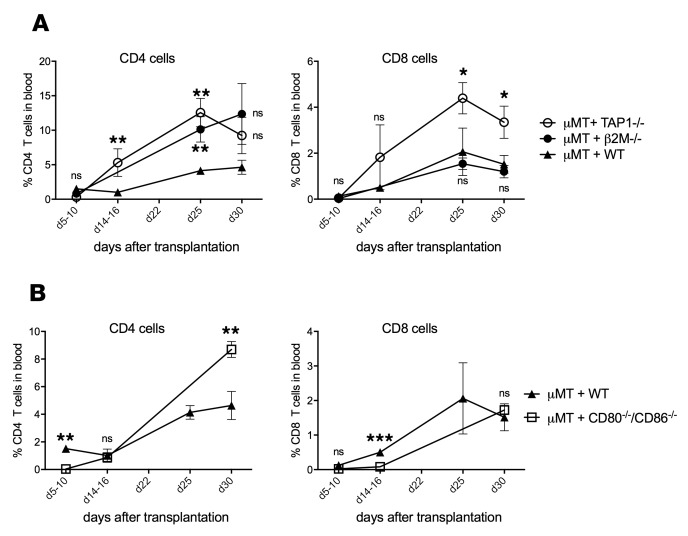
As class I MHC may support not only homeostatic proliferation but also the persistence of transferred CD8+ T cells (22, 23), our findings could be explained by poor survival of residual CD8+ T cells in the absence of MHC class I. To rule out inferior T cell survival in hosts with global MHC class I deficiency and to test whether CD8+ T cell proliferation is induced by recognition of MHC class I on B cells, we generated bone marrow chimeras with B cells deficient in TAP1 and MHC class I expression. We found that after heart transplantation and mATG treatment, such chimeras had normal or even modestly expedited CD8+ T cell reconstitution compared with control chimeric animals (Figure 3A). Analogous results were observed in mixed bone marrow chimeras in which B cells specifically lack β2 microglobulin (β2M) and therefore have minimal levels of class I MHC expression (Figure 3A) (24). Furthermore, CD8+ T cell reconstitution was not severely impaired in mixed bone marrow chimeras with B cells deficient in both CD80 and CD86 molecules (Figure 3B). These results indicate that although the CD8+ T cell TCR must interact with self MHC class I for homeostatic proliferation, MHC class I or costimulatory molecules on B cells are dispensable for optimal recovery following mATG lymphoablation.
Figure 3. CD8+ T cell recovery does not require expression of MHC class I or CD80/86 on B cells.
Lethally irradiated B cell–deficient μMT mice received bone marrow (BM) composed of a 1:1 mixture of μMT plus WT (control), μMT plus TAP1−/−, or μMT plus β2M−/− (in both, B cells lack MHC class I) (A); or μMT plus CD86−/− CD80−/− (B cells lack CD80 and CD86) (B). Resulting BM chimeras were transplanted with BALB/c heart allografts and treated with mATG (1 mg i.p. on days 0 and 4). Percentages of CD4+ and CD8+ among peripheral blood live cells were determined by flow cytometry. Results are cumulative of 2 experiments with n = 4–7 animals/group; error bars represent SD. *P < 0.05, **P < 0.01, ***P < 0.001; ns, P ≥ 0.05 compared with reconstitution in μMT plus WT chimeras by multiple t tests.
mATG lymphoablation induces B cell cytokine production in a CD4-dependent manner.
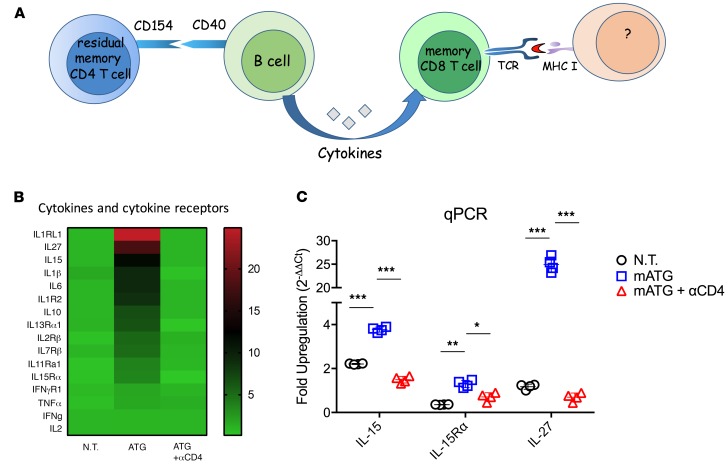
An alternative mechanism is that soluble factors produced by B cells after mATG treatment may play an important role in CD8+ T cell reconstitution (Figure 4A). In order to identify potential soluble mediators of B cell function, we isolated B cells from the spleens of heart allograft recipients on day 12 after transplant and performed NanoString Gene Expression analysis to evaluate the expression of 561 genes. Comparison was performed among untreated recipients and mATG-treated recipients with or without additional CD4+ T cell depletion. We found that mATG lymphoablation upregulated B cell expression of over 30 genes in a CD4-dependent manner. Among top candidates, there were several cytokines and cytokine receptors (Figure 4B and Supplemental Table 1; supplemental material available online with this article; https://doi.org/10.1172/jci.insight.125489DS1). We initially focused on IL-15, an important mediator of CD8+ T cell survival and proliferation (25, 26), and IL-27, a pleiotropic member of the IL-12 cytokine family that was shown to positively regulate CD8+ T cell expansion and functions (27). Real-time PCR analysis further confirmed the enhanced expression of IL-15 and IL-15Rα necessary for IL-15 trans presentation (28, 29), and IL-27 in B cells from recipients treated with mATG, whereas the absence of CD4+ T cell help completely abrogated lymphoablation-induced gene upregulation (Figure 4C). Of note, cytokines often associated with bystander B cell activation such as TNF-α, IFN-γ, and IL-2 (30–33) were not affected by mATG treatment (Figure 4B). These findings indicate the specific induction of IL-15 and IL-27 expression in B cells in response to lymphoablation.
Figure 4. mATG lymphoablation induces B cell cytokine production in a CD4-dependent manner.
(A) Revised model. (B and C) C57BL/6J mice were transplanted with BALB/c heart allografts and either left untreated (N.T.), treated with mATG alone (mATG), or with mATG and depleting anti-CD4 mAb (mATG + αCD4). RNA was isolated from flow-sorted splenic B cells and gene expression was analyzed by NanoString nCounter Gene Expression Assay (B) and real-time RT-PCR (qPCR) (C). Data represent fold increase in expression relative to B cells isolated from naive C57BL/6J mice. The results are representative of 2 experiments with n = 4 mice/group; error bars represent SD. *P < 0.05, **P < 0.01, ***P < 0.001 by ANOVA with Tukey’s multiple-comparisons post hoc test.
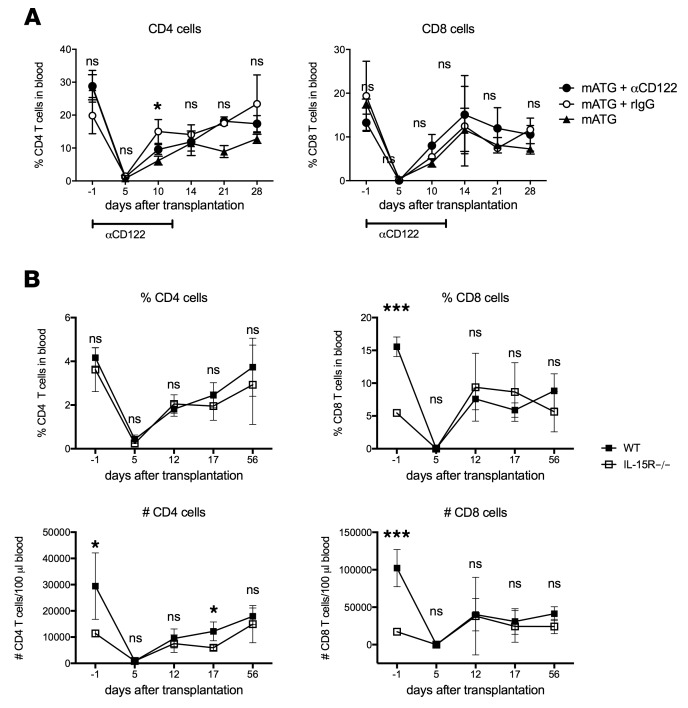
CD8+T cell reconstitution after mATG treatment may occur independently of IL-15 signaling. We proceeded to test the functional relevance of IL-15 to the CD8+ T cell recovery using 2 approaches. First, we injected mATG-treated heart allograft recipients with mAb blocking CD122, the common β chain of IL-15 and IL-2 receptors (34, 35). Unexpectedly, CD122 blockade for the first 12 days after transplant had no significant effect on CD8+ T cell proliferation and the kinetics of heart allograft rejection (Figure 5A and data not shown). The second approach used animals deficient in IL-15Rα chain required for trans presentation of IL-15 and IL-15–mediated signaling (36). As previously reported, the baseline numbers of CD4+ and CD8+ T cells in IL-15Rα–/– mice were significantly lower compared with WT animals (37). Nevertheless, after mATG depletion and heart transplantation, the rates of CD8+ and CD4+ T cell expansion in IL-15Rα–/– recipients were similar to those observed in WT recipients (Figure 5B). These results indicate that IL-15 is dispensable for CD4+ and CD8+ T cell recovery following lymphoablation.
Figure 5. IL-15 signaling is not required for CD8+ T cell reconstitution after mATG treatment.
(A) C57BL/6J mice were transplanted with BALB/c heart allografts and treated with mATG alone (1 mg i.p. on days 0 and 4), with mATG plus anti-CD122 mAb (0.2 mg i.p. on days –1, 3, 6, 9, and 12), or mATG plus control rat IgG (0.2 mg i.p. on days –1, 3, 6, 9, and 12). The results are representative of 2 experiments with n = 4–5 mice/group/experiment; error bars represent SD. *P < 0.05, ns, P ≥ 0.05 for the comparison of mATG plus anti-CD122 and mATG plus rIgG groups by multiple t tests. (B) B6.WT and B6.IL-15Rα–/– mice were transplanted with BALB/c heart allografts and treated with mATG (1 mg i.p. on days 0 and 4). n = 4–5 mice per group, error bars represent SD. *P < 0.05, ***P < 0.001; ns, P ≥ 0.05 by multiple t tests.
IL-27 facilitates CD8+ T cell reconstitution after mATG treatment.
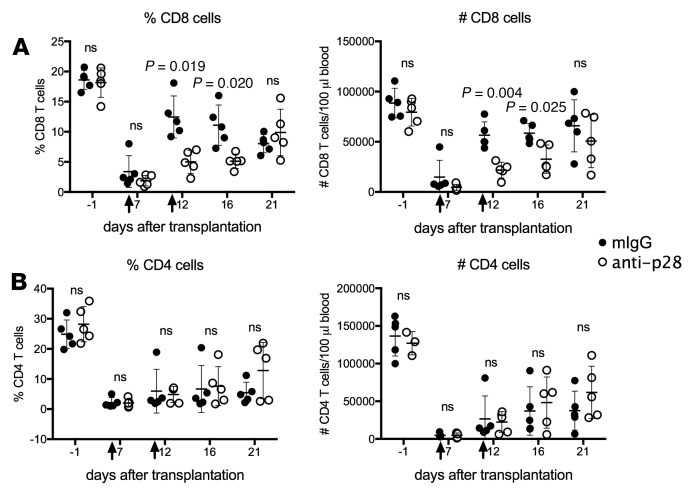
To test the role of IL-27 in T cell reconstitution, we used mAbs to specifically neutralize the IL-27 p28 subunit. B6 recipients of heart allografts were treated with mATG and injected with 0.5 mg anti-p28 mAb on days 6 and 11 after transplantation. IL-27 neutralization inhibited CD8+ T cell expansion measured on days 12 and 16, but by day 21 the effect of anti–IL-27 mAb treatment was not detectable, suggesting antibody clearance and restoration of IL-27 levels (Figure 6A). Notably, reconstitution of CD4+ T lymphocytes was not affected by IL-27 neutralization, consistent with our proposed model.
Figure 6. IL-27 neutralization delays CD8+ T cell reconstitution after mATG treatment.
C57BL/6J mice were transplanted with BALB/c heart allografts and treated with either mATG (1 mg i.p. on days 0 and 4) plus anti-p28 mAb (clone MM27-7B1, 0.25 mg i.p. on days 6 and 11 after transplant) or mATG plus control mouse IgG (0.25 mg i.p. on days 6 and 11). The percentages and numbers of CD8+ (A) and CD4+ (B) T cells in the peripheral blood were evaluated by flow cytometry. n = 5 mice per group; error bars represent SD. P values were determined by 2-tailed Student’s t test. ns, P ≥ 0.05.
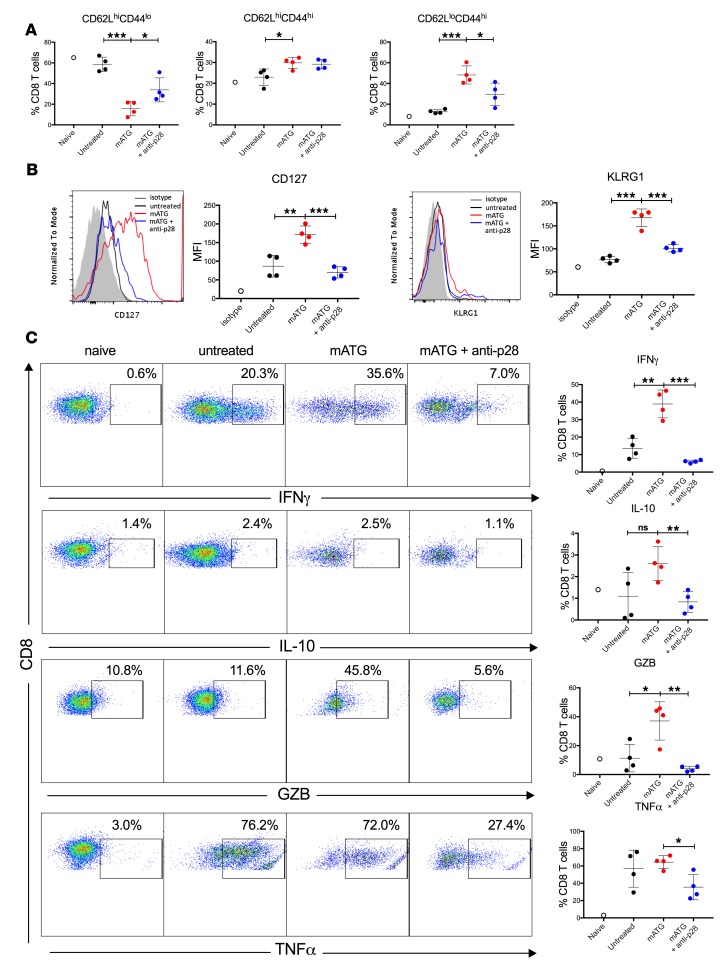
Evaluation of recipient spleen cells at day 12 after transplant demonstrated that mATG lymphoablation shifted the CD8+ T cell composition from naive (CD62LhiCD44lo) to Tcm (CD62LhiCD44hi) and Tem (CD62LloCD44hi) phenotypes and upregulated expression of the IL-7Rα chain, CD127 (Figure 7, A and B). Consistent with the increase in Tem cells, high proportions of reconstituted CD8+ T cells expressed proinflammatory cytokines IFN-γ and TNF-α and cytotoxicity mediator Granzyme B (Figure 7C). IL-27 neutralization shifted the resulting CD8+ T cell phenotype towards CD62LhiCD44lo naive cells (Figure 7A). The reduced frequency of the Tem cell population in recipients treated with anti–IL-27 mAb was associated with decreased numbers of CD8+ T cells producing effector cytokines (Figure 7C). Most importantly, IL-27 neutralization abrogated the upregulation of IL-7Rα induced by mATG lymphoablation (Figure 7B).
Figure 7. IL-27 neutralization alters the phenotype of reconstituted CD8+ T cells.
C57BL/6J mice were transplanted with BALB/c heart allografts and either left untreated or treated with either mATG (1 mg i.p. on days 0 and 4) plus anti-p28 mAb (clone MM27-7B1, 0.25 mg i.p. on days 6 and 11 after transplant) or mATG alone. Recipients were sacrificed on day 12 after transplant and splenic T cells were evaluated by flow cytometry. (A) Percentages of CD8+ T cells with naive (CD62LhiCD44lo), Tcm (CD62LhiCD44hi), or Tem (CD62LloCD44hi) phenotype. (B) Expression of CD127 (IL-7Rα, left) and KLRG1 (right) after gating on CD8+ cells. The results are shown as representative histograms (shaded, isotype control staining; black, untreated; red, mATG; blue, mATG + anti-p28) and MFI values. (C) Intracellular staining for IFN-γ, IL-10, Granzyme B (GZB), and TNF-α after gating on CD8+ cells. The results are shown as representative dot plots (left) and percentages of CD8+ T cells expressing respective cytokines (right). n = 4 mice per group, error bars represent SD. *P < 0.05, **P < 0.01, ***P < 0.001; ns, P ≥ 0.05 by multiple t tests.
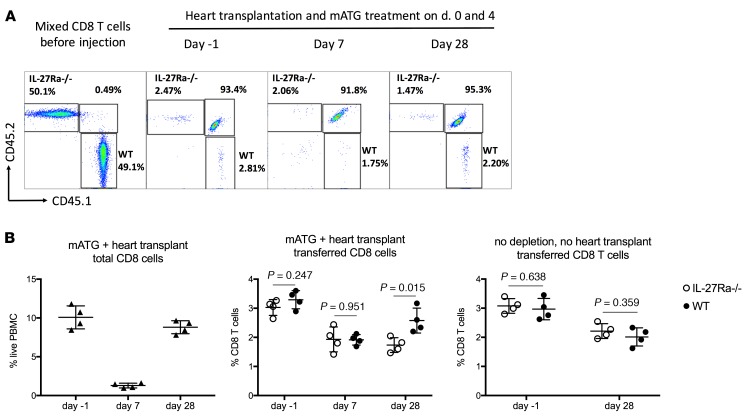
To test whether IL-27 signals directly to CD8+ T cells and thus induces their homeostatic recovery, we cotransferred congenic CD8+ T cells isolated from CD45.1 WT and CD45.2 IL-27Rα–/– mice into CD45.1/2 B6 recipients of BALB/c heart allografts treated with mATG so as to be able to separately track tracer WT, IL-27Rα–/–, and host CD8+ T cells within the same recipient. The absence of IL-27R on CD8+ T cells markedly impaired their reconstitution after mATG treatment (Figure 8). This was not due to higher attrition of IL-27Rα–/– T cells, as coinjected WT and IL-27Rα–/– CD8+ T cells survived equally well in animals not subjected to mATG lymphoablation (Figure 8B). Taken together, these results suggest IL-27 as a mediator of CD8+ T cell recovery in mATG-treated heart allograft recipients.
Figure 8. IL-27R expression is required for optimal CD8+ T cell reconstitution.
Splenic CD8+ T cells were isolated from B6.CD45.1+ WT and B6.IL-27Rα–/– CD45.2+ mice and coinjected into B6.CD45.1/2+ mice followed by BALB/c heart transplantation and mATG treatment. The control group of B6.CD45.1/2+ recipients was injected with CD8+ T cells but did not receive heart transplant or mATG treatment. (A) Representative dot plots analyzing transferred WT and IL-27Rα–/– CD8+ T cells in peripheral blood at indicated time points. Data are shown after gating on CD8+ cells. (B) The percentages of total and transferred CD8+ T cells in peripheral blood. The results are representative of 4 independent experiments with n = 4 mice/group; error bars represent SD. P values were determined by 2-tailed Student’s t test.
Discussion
Our study provides insights into the mechanisms of endogenous T cell reconstitution following antibody-mediated lymphoablation. We followed up on our initial observations that B cells are essential mediators of CD4+ T cell help required for CD8+ T cell homeostatic proliferation. Although these findings are distinct from the data gained during T cell adoptive transfers into lymphopenic hosts, the critical role of B cells in this context is not surprising. First, following ATG treatment in both humans and mice, the rates of B cell depletion are much lower compared with T lymphocytes. Second, the transient reduction in B cell numbers is compensated for by rapid reconstitution, so B cells become a majority of hematopoietic cells. Finally, in addition to antibody generation, B cells have been implicated as important regulators of various types of immune response via antigen presentation, delivery of costimulatory signals, cytokine production, and maintaining the structure of secondary lymphoid organs (38–40).
Given the importance of cognate TCR-MHC interactions for other types of homeostatic proliferation, we were surprised to find that optimal CD8+ T cell reconstitution does not require antigen presentation or costimulation by B cells themselves. Nevertheless, CD8+ T cell proliferation and/or survival were severely compromised in MHC class I–deficient hosts. This suggests that while important signals for T cell proliferation are delivered by B lymphocytes, other cell types may be responsible for MHC class I antigen presentation to CD8+ T cells. The exact nature of MHC class I–expressing cells and the nature of presented antigens (self peptides vs. microbial peptides) remain to be determined. The ongoing studies in our laboratory explore the requirement for class I expression by dendritic cells, radioresistant stromal or parenchymal cells, or even by T cells themselves.
The comparison of gene expression in “helped” versus “helpless” B cells from a lymphopenic environment yielded several groups of genes upregulated by mATG treatment including several proinflammatory cytokines and cytokine receptors (Figure 4 and Supplemental Table 1). The upregulation of these genes was not observed in recipients depleted of CD4+ T cells, ruling out the direct effect of mATG binding on B cells. B cell–derived cytokines can have potent effects on various aspects of immunity (38, 40). Studies in several models of infectious disease demonstrated that B cell–derived IL-2 promotes Th2 memory formation, whereas TNF-α– or IFN-γ–producing cells support Th1 type responses (30–33). Notably, neither IL-2 nor IFN-γ was induced by mATG treatment, suggesting that the cytokine production by B cells is context specific and is not merely the result of bystander B cell activation. We initially focused on the top hits among cytokines, IL-15 and IL-27. IL-15 facilitates CD8+ T cell survival and proliferation in many circumstances and appears the most plausible candidate for mediating homeostatic T cell recovery in our model (41–44). In fact, IL-2 and IL-15 can drive rapid expansion of naive T cells even in the absence of lymphopenia (20). However, from two complementary approaches using receptor-blocking antibody and a receptor-deficient animal model, we were unable to find any evidence supporting IL-15 involvement in CD8+ T cell recovery after mATG treatment. As CD122 is also a subunit of the IL-2 receptor, the inability of the anti-CD122 mAb to inhibit CD8+ T cell expansion argues against the critical role of IL-2 in our model.
IL-27 is a multifunctional cytokine with activities ranging from suppression to activation of various immune cell types (45). While immunosuppressive functions of IL-27 are well recognized, multiple studies demonstrated positive regulation of CD8+ T cell proliferation and functions particularly in the context of viral infections and antitumor responses (46–50). A recent study by Klarquist et al. demonstrated that both IL-27 and IL-15 are critical to vaccine-elicited T cell responses, as they drive clonal expansion through a mitochondrial metabolic program (51). Myeloid cells including dendritic cells and macrophages are generally thought to be the dominant IL-27 producers (52); therefore, finding more than 20-fold upregulation of IL-27 in B cells from mATG-treated hosts was unexpected. Heart allograft transplantation alone did not induce IL-27 expression in B cells, suggesting that production of this cytokine is a characteristic feature of B cells in a lymphopenic environment (Figure 4). Several groups reported that IL-27 directly enhances germinal center B cell activity and IgG production (53–55). However, little is known regarding the in vivo role of IL-27–producing B cells, although Hoyt et al. recently reported that B cell–derived IL-27 supports hematopoiesis in the context of pneumocystis infection (56). Despite prominent upregulation of IL-27 gene expression in B cells, our data do not rule out the possibility that IL-27 produced by myeloid cells is still important for supporting CD8+ T cell reconstitution. We initially attempted to specifically abolish IL-27 expression in B cells using a Cre/loxP approach (CD19cre × Il27p28fl/fl). However, it was unsuccessful because of the proximity of the Cd19, Mc2, and Il27 genes on mouse chromosome 7. Alternatively, our group is in the process of generating IL-27 reporter mice that will enable us to track IL-27–producing cells in lymphopenic conditions. Regardless of the source of IL-27, in vivo neutralization of this cytokine significantly delayed reconstitution of CD8+ but not CD4+ T cells in mATG-treated recipients. The delayed reconstitution was characterized by the increase in naive CD8+ T cells and respective decrease in cells with Tem and Tcm phenotypes (Figure 7A), possibly due to reduced contribution of homeostatic recovery and increased contribution of thymopoiesis. Notably, IL-27 neutralization abrogated the upregulated expression of IL-7Rα chain on CD8+ T cells caused by lymphoablation (Figure 7B). Given the critical importance of IL-7 signaling for T cell homeostatic proliferation in lymphopenic conditions (12, 15, 57, 58), our data strongly suggest that the reduced IL-7Rα expression may account for the observed reduction in CD8+ T cell reconstitution. These results are in consistence with previous reports that IL-7Rα blockade with mAb inhibited lymphocyte reconstitution following T cell depletion, thus prolonging skin allograft survival, and prevented graft-versus-host disease in a mouse model of allogeneic bone marrow transplantation (59, 60). The results of the WT and IL-27R–/– CD8+ T cell cotransfer experiment demonstrate the direct effect of IL-27 on this lymphocyte population (Figure 8). While IL-7Rα regulation by IL-7 and other prosurvival cytokines has been well documented (61, 62), to our knowledge the effects of IL-27 in this context have not been previously explored. In our future studies, we will further investigate how IL-27 signaling supports T cell IL-7Rα expression during depletion-induced lymphopenia.
It should be noted that IL-27 neutralization or receptor deficiency only partially inhibited CD8+ T cell recovery in our model, indicating the importance of other factors. Our pilot studies suggest that inflammation caused by transplantation and massive cell death (and reflected by enhanced production of IL-1β and IL-6 by B cells from lymphopenic mice) is a potent facilitator of T cell reconstitution. In addition to enhanced cytokine production, B cells from mATG-treated recipients had upregulated expression of genes responsible for tissue repair and remodeling (fibronectin 1), supporting lymphoid architecture (lymphotoxin β receptor and Tnfsf14, or LIGHT) and facilitating uptake of dead cells (Clec5a, Clec4e, and Card9). These results provide clues to other B cell functions that may facilitate T cell reconstitution following antibody-mediated depletion and will be pursued in our future studies.
Taken together, our results demonstrate that despite the importance of B cells for T cell reconstitution, their effects are mediated indirectly rather than via cognate TCR-MHC and costimulatory interactions. In particular, we identified what we believe is a novel function of IL-27 potentially produced by activated B cells in regulating homeostatic CD8+ T cell recovery following mATG treatment. These findings suggest a possibility of manipulating the resulting T cell repertoire by cytokine neutralization to achieve optimal benefits of lymphoablation in transplant recipients or patients with autoimmunity.
Methods
Animals.
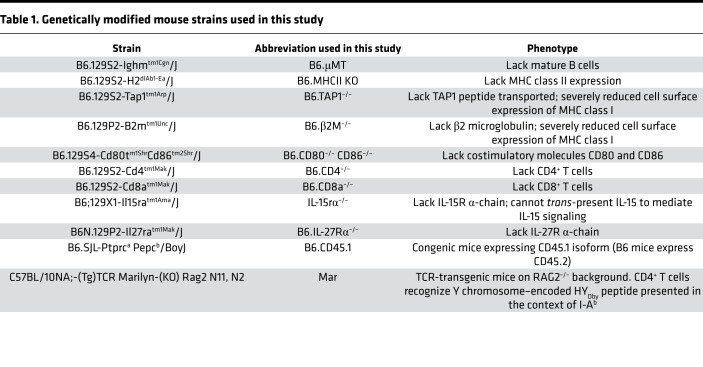
Male and female C57BL/6J (H-2b) [B6.WT], BALB/cJ (H-2d) [BALB/c], SJL/J-Pde6brd1 (H2s), C3H/HeJ (H-2k), DBA/1J (H-2q), B6.129S2-Ighmtm1Cgn/J (H-2b) [B6.μMT], B6.129S2-H2dlAb1-Ea/J (H-2b) [B6.MHCII-KO], B6.129S2-Tap1tm1Arp/J (H-2b) [B6.TAP1–/–], B6.129P2-B2mtm1Unc/J (H-2b) [B6.β2M–/–], B6.129S4-Cd80tm1ShrCd86tm2Shr/J (H-2b) [B6.CD80–/– CD86–/–], B6.129S2-Cd4tm1Mak/J (H-2b) [B6.CD4–/–], B6.129S2-Cd8atm1Mak/J (H-2b) [B6.CD8a–/–], B6;129X1-Il15ratm1Ama/J (H-2b) [IL-15rα–/–], B6N.129P2-Il27ratm1Mak/J (H-2b) [B6.IL-27Rα–/–], and B6.SJL-Ptprca Pepcb/BoyJ (H-2b) [B6.CD45.1] mice, aged 6–8 weeks, were purchased from The Jackson Laboratories. C57BL/10NA;-(Tg)TCR Marilyn-(KO) Rag2 N11, N2 mice (Mar, H-2b) were provided by Polly Matzinger (NIH). The specific characteristics of genetically modified mouse strains used in the study are listed in Table 1. All animals were maintained and bred in the pathogen-free facility at the Cleveland Clinic. All animal procedures were approved by the Institutional Animal Care and Use Committee at the Cleveland Clinic.
Table 1. Genetically modified mouse strains used in this study.
Heart transplantation.
Vascularized heterotopic cardiac transplants were performed as previously described (63). Rejection was defined as a loss of palpable heartbeat and confirmed by laparotomy.
mATG preparation and recipient treatment.
Rabbit anti–mouse thymocyte serum was generated by the Hybridoma Core at the Cleveland Clinic Research Institute by immunizing rabbits with C3H, DBA1, and SJL thymocytes. Total IgGs (mATG) were isolated by a sequential ammonium sulfate precipitation. Total protein concentration was measured using a BCA assay (Thermo Fisher Scientific), and the purity was confirmed by SDS-PAGE. Heart allograft recipients were treated with mATG (1 mg i.p.) on days 0 and 4 after transplant. For CD4+ T cell depletion, anti–mouse CD4+ mAbs (clones GK1.5 and YTS191, Bio X Cell) were injected i.p. on days –3, –2, and –1 relative to transplantation, 0.2 mg each per animal per day. To block CD122 (common β chain of IL-15R and IL-2R), recipients were injected i.p. with 0.2 mg anti–mouse CD122 mAb (clone 5H4, Bio X Cell) or control rat IgG (Bio X Cell) on days –1, 3, 6, 9, and 12 relative to transplantation. For IL-27 neutralization experiments, recipients were injected i.p. with 0.25 mg anti-p28 mAb (clone MM27-7B1, Thermo Fisher Scientific) or control mouse IgG (Bio X Cell) on days 6 and 11 after transplant.
Bone marrow chimeras.
Bone marrow was isolated from femurs of B6.WT, B6.MHCII-KO, B6.TAP1–/–, B6.β2M–/–, B6.CD80–/– CD86–/–, and μMT–/– mice. Female μMT–/– mice were irradiated (11 Gy) and injected i.v. with bone marrow cells so that each recipient received 5 × 106 to 7.5 × 106 μMT–/– cells plus equal numbers of either WT or respective KO cells. Four weeks later, the expression of MHC class I, MHC class II, CD80, and CD86 on peripheral blood B220+ and B220–cells was evaluated by flow cytometry to confirm the chimeric phenotype. The resulting chimeras were used as heart allograft recipients 5–6 weeks after bone marrow transplantation.
Adoptive cell transfers.
For adoptive transfer of Mar cells, CD4+ T cells were isolated from spleens and peripheral lymph nodes (inguinal, mesenteric, axial and brachial) of Mar mice using mouse negative-selection kits from STEMCELL Technologies according to the manufacturer’s instructions. Mar T cells (10 × 106) were i.v. injected into B6 male or female recipients 1 day prior to BALB/c heart transplantation. For cotransfer of IL-27R+/+ and IL-27Rα–/– cells, CD8+ T cells were isolated from spleens of B6.CD45.1 and B6.IL-27Rα–/– (CD45.2) mice using STEMCELL Technologies negative-selection kits. The resulting CD8+ T cells were mixed at a 1:1 ratio; 10 × 106 cells from this mixture were intravenously injected into B6.CD45.1/2 mice generated by crossing B6.WT and B6.CD45.1 mice. Two days after CD8+ T cell injection, animals were transplanted with BALB/c heart allografts and treated with mATG.
NanoString gene expression analyses.
Splenic B cells were purified on day 12 after transplant using a negative-selection kit from STEMCELL Technologies (>95% B220+ cells in all samples). Total RNA was isolated from individual samples using TRIzol reagent (Invitrogen). For measurements of gene expression by NanoString, RNA was hybridized to the Mouse Immunology Panel (561 genes including reference genes), and then processed on a GEN2 analysis system using the high-sensitivity protocol and high-resolution data capture (NanoString Technologies). Raw counts were normalized using nSolver version 3.0 software and controls incorporated into the codeset. Background counts were removed by subtracting the mean plus 2 standard deviations of the negative controls and lane-specific differences in hybridization and binding intensity were corrected using the geometric mean of the positive controls. Gene expression was normalized to the geometric mean of 12 reference genes that were expressed at stable levels in all samples. The resulting values were normalized to respective gene expression in B220+ cells from naive nontransplanted B6 mice.
Quantitative real-time PCR.
RNA samples were obtained from isolated B220+ cells as described for NanoString analyses. Reverse transcription was performed using the High-Capacity cDNA Reverse Transcription Kit, and quantitative real-time PCR was done on a 7500 Fast Real-Time PCR System instrument using Taqman Fast Universal PCR Master Mix (2×), No AmpEraseUNG (all from Applied Biosystems). Probes and primers were from Taqman gene expression assay reagents (Applied Biosystems): IL-27 (Mm004461162_m1), IL-15 (Mm0434210_m1), and IL-15rα (Mm01333387-m1). Data were normalized to Mrpl 32 (Mm00777741-sH) RNA amplification and calculated relative to the expression of the target gene in B220+ cells from naive B6 mice.
Flow cytometry.
The following conjugated antibodies were purchased from BD Biosciences or eBioscience: fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-anti-CD4 (GK1.5), allophycocyanin (APC)-anti-CD8α (53-6.7), PE-anti-CD44 (IM7), PE-Cy7- or FITC-anti-B220 (RA3-6B2), PE-Cy7-anti-CD45.1 (A20), FITC-anti-CD45.2 (104), PE-anti-H-2Kb (AF6-88.5.5.3), PE-Cy7-anti–I-A/I-E (M5/114.15.2), PE-Cy7-anti-CD80 (16-10A1), and PE-Cy7-anti-CD86 (GL1). Cells were isolated from peripheral blood and spleen and stained as previously described (64, 65). At least 200,000 events/sample were acquired on a BD Bioscience LSRII followed by data analysis using FlowJo software (Tree Star Inc.).
Statistics.
All data are expressed as the mean ± SD. For data comparisons between 2 groups, we used a 2-tailed Student’s t test. For data comparisons among 3 or more groups, we used 1-way analysis of variance (ANOVA) with Tukey’s multiple-comparisons post hoc test. Analyses were performed with GraphPad Prism 6. Differences were considered statistically significant at P < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001). Total numbers of animals in each experimental group and the number of experiments are indicated in respective figure legends.
Author contributions
KA, DBZ, RF, SH, VG, MN, and KSK performed the experiments. KA, DBZ, and KSK analyzed the data. BM, RLF, and AV designed the experiments. AV wrote and revised the manuscript.
Supplementary Material
Acknowledgments
The study was supported by NIH National Institute of Allergy and Infectious Diseases grant 1R01 AI113142-01A1 (to AV) and by the CTOT NanoString Core grant U01 AI063594 (to RLF).
Version 1. 04/04/2019
Electronic publication
Footnotes
Conflict of interest: The authors have declared that no conflict of interest exists.
Copyright: © 2019 American Society for Clinical Investigation
Reference information: JCI Insight. 2019;4(7):e125489. https://doi.org/10.1172/jci.insight.125489.
Contributor Information
Katayoun Ayasoufi, Email: k_ayasoufi@yahoo.com.
Ran Fan, Email: fanr@ccf.org.
Suheyla Hasgur, Email: hasgurs@ccf.org.
Michael Nicosia, Email: nicosim@ccf.org.
Victoria Gorbacheva, Email: gorbacv@ccf.org.
Booki Min, Email: minb@ccf.org.
Anna Valujskikh, Email: valujsa@ccf.org.
References
- 1.Mohty M, Bacigalupo A, Saliba F, Zuckermann A, Morelon E, Lebranchu Y. New directions for rabbit antithymocyte globulin (Thymoglobulin(®)) in solid organ transplants, stem cell transplants and autoimmunity. Drugs. 2014;74(14):1605–1634. doi: 10.1007/s40265-014-0277-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cai J, Terasaki PI. Induction immunosuppression improves long-term graft and patient outcome in organ transplantation: an analysis of United Network for Organ Sharing registry data. Transplantation. 2010;90(12):1511–1515. doi: 10.1097/TP.0b013e3181fecfcb. [DOI] [PubMed] [Google Scholar]
- 3.Bunnapradist S, Takemoto SK. Multivariate analysis of antibody induction therapy and their associated outcomes in deceased donor transplants. Transplant Proc. 2005;37(2):889–891. doi: 10.1016/j.transproceed.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 4.Hardinger KL, et al. Thymoglobulin induction is safe and effective in live-donor renal transplantation: a single center experience. Transplantation. 2006;81(9):1285–1289. doi: 10.1097/01.tp.0000209825.91632.ea. [DOI] [PubMed] [Google Scholar]
- 5.Libório AB, et al. Induction antibody therapy in renal transplantation using early steroid withdrawal: long-term results comparing anti-IL2 receptor and anti-thymocyte globulin. Int Immunopharmacol. 2011;11(11):1832–1836. doi: 10.1016/j.intimp.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Martins L, et al. Immunosuppression with antithymocyte globulin in renal transplantation: better long-term graft survival. Transplant Proc. 2005;37(6):2755–2758. doi: 10.1016/j.transproceed.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Opelz G, Naujokat C, Daniel V, Terness P, Döhler B. Disassociation between risk of graft loss and risk of non-Hodgkin lymphoma with induction agents in renal transplant recipients. Transplantation. 2006;81(9):1227–1233. doi: 10.1097/01.tp.0000219817.18049.36. [DOI] [PubMed] [Google Scholar]
- 8.Pearl JP, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5(3):465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 9.Zeevi A, et al. Recovery of functional memory T cells in lung transplant recipients following induction therapy with alemtuzumab. Am J Transplant. 2007;7(2):471–475. doi: 10.1111/j.1600-6143.2006.01641.x. [DOI] [PubMed] [Google Scholar]
- 10.Ayasoufi K, Fan R, Fairchild RL, Valujskikh A. CD4 T cell help via B cells is required for lymphopenia-induced CD8 T cell proliferation. J Immunol. 2016;196(7):3180–3190. doi: 10.4049/jimmunol.1501435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayasoufi K, Yu H, Fan R, Wang X, Williams J, Valujskikh A. Pretransplant antithymocyte globulin has increased efficacy in controlling donor-reactive memory T cells in mice. Am J Transplant. 2013;13(3):589–599. doi: 10.1111/ajt.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008;29(6):848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11(2):183–190. doi: 10.1016/S1074-7613(00)80093-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prlic M, Blazar BR, Khoruts A, Zell T, Jameson SC. Homeostatic expansion occurs independently of costimulatory signals. J Immunol. 2001;167(10):5664–5668. doi: 10.4049/jimmunol.167.10.5664. [DOI] [PubMed] [Google Scholar]
- 15.Schluns KS, Kieper WC, Jameson SC, Lefrançois L. Interleukin-7 mediates the homeostasis of naïve and memory CD8 T cells in vivo. Nat Immunol. 2000;1(5):426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 16.Tan JT, et al. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci USA. 2001;98(15):8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieper WC, et al. Recent immune status determines the source of antigens that drive homeostatic T cell expansion. J Immunol. 2005;174(6):3158–3163. doi: 10.4049/jimmunol.174.6.3158. [DOI] [PubMed] [Google Scholar]
- 18.Min B, Yamane H, Hu-Li J, Paul WE. Spontaneous and homeostatic proliferation of CD4 T cells are regulated by different mechanisms. J Immunol. 2005;174(10):6039–6044. doi: 10.4049/jimmunol.174.10.6039. [DOI] [PubMed] [Google Scholar]
- 19.Cho JH, et al. An intense form of homeostatic proliferation of naive CD8+ cells driven by IL-2. J Exp Med. 2007;204(8):1787–1801. doi: 10.1084/jem.20070740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramsey C, Rubinstein MP, Kim DM, Cho JH, Sprent J, Surh CD. The lymphopenic environment of CD132 (common gamma-chain)-deficient hosts elicits rapid homeostatic proliferation of naive T cells via IL-15. J Immunol. 2008;180(8):5320–5326. doi: 10.4049/jimmunol.180.8.5320. [DOI] [PubMed] [Google Scholar]
- 21.Braun MY, et al. Acute rejection in the absence of cognate recognition of allograft by T cells. J Immunol. 2001;166(8):4879–4883. doi: 10.4049/jimmunol.166.8.4879. [DOI] [PubMed] [Google Scholar]
- 22.Markiewicz MA, Brown I, Gajewski TF. Death of peripheral CD8+ T cells in the absence of MHC class I is Fas-dependent and not blocked by Bcl-xL. Eur J Immunol. 2003;33(10):2917–2926. doi: 10.1002/eji.200324273. [DOI] [PubMed] [Google Scholar]
- 23.Takada K, Jameson SC. Self-class I MHC molecules support survival of naive CD8 T cells, but depress their functional sensitivity through regulation of CD8 expression levels. J Exp Med. 2009;206(10):2253–2269. doi: 10.1084/jem.20082553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koller BH, Marrack P, Kappler JW, Smithies O. Normal development of mice deficient in beta 2M, MHC class I proteins, and CD8+ T cells. Science. 1990;248(4960):1227–1230. doi: 10.1126/science.2112266. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy MK, et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J Exp Med. 2000;191(5):771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schluns KS, Lefrançois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3(4):269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 27.Cox MA, Kahan SM, Zajac AJ. Anti-viral CD8 T cells and the cytokines that they love. Virology. 2013;435(1):157–169. doi: 10.1016/j.virol.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burkett PR, Koka R, Chien M, Chai S, Boone DL, Ma A. Coordinate expression and trans presentation of interleukin (IL)-15Ralpha and IL-15 supports natural killer cell and memory CD8+ T cell homeostasis. J Exp Med. 2004;200(7):825–834. doi: 10.1084/jem.20041389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandau MM, Schluns KS, Lefrancois L, Jameson SC. Cutting edge: transpresentation of IL-15 by bone marrow-derived cells necessitates expression of IL-15 and IL-15R alpha by the same cells. J Immunol. 2004;173(11):6537–6541. doi: 10.4049/jimmunol.173.11.6537. [DOI] [PubMed] [Google Scholar]
- 30.Barr TA, Brown S, Mastroeni P, Gray D. TLR and B cell receptor signals to B cells differentially program primary and memory Th1 responses to Salmonella enterica. J Immunol. 2010;185(5):2783–2789. doi: 10.4049/jimmunol.1001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris DP, et al. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1(6):475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- 32.Menard LC, et al. B cells amplify IFN-gamma production by T cells via a TNF-alpha-mediated mechanism. J Immunol. 2007;179(7):4857–4866. doi: 10.4049/jimmunol.179.7.4857. [DOI] [PubMed] [Google Scholar]
- 33.Wojciechowski W, et al. Cytokine-producing effector B cells regulate type 2 immunity to H. polygyrus. Immunity. 2009;30(3):421–433. doi: 10.1016/j.immuni.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J, et al. Insulin-dependent diabetes induced by pancreatic beta cell expression of IL-15 and IL-15Rα. Proc Natl Acad Sci USA. 2013;110(33):13534–13539. doi: 10.1073/pnas.1312911110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao W, Lin JX, Leonard WJ. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity. 2013;38(1):13–25. doi: 10.1016/j.immuni.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol Lett. 2010;127(2):85–92. doi: 10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lodolce JP, et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9(5):669–676. doi: 10.1016/S1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 38.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Curr Opin Immunol. 2008;20(3):332–338. doi: 10.1016/j.coi.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mauri C, Bosma A. Immune regulatory function of B cells. Annu Rev Immunol. 2012;30:221–241. doi: 10.1146/annurev-immunol-020711-074934. [DOI] [PubMed] [Google Scholar]
- 40.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15(7):441–451. doi: 10.1038/nri3857. [DOI] [PubMed] [Google Scholar]
- 41.Richer MJ, Pewe LL, Hancox LS, Hartwig SM, Varga SM, Harty JT. Inflammatory IL-15 is required for optimal memory T cell responses. J Clin Invest. 2015;125(9):3477–3490. doi: 10.1172/JCI81261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rubinstein MP, et al. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112(9):3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6(8):595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 44.Younes SA, et al. IL-15 promotes activation and expansion of CD8+ T cells in HIV-1 infection. J Clin Invest. 2016;126(7):2745–2756. doi: 10.1172/JCI85996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida H, Hunter CA. The immunobiology of interleukin-27. Annu Rev Immunol. 2015;33:417–443. doi: 10.1146/annurev-immunol-032414-112134. [DOI] [PubMed] [Google Scholar]
- 46.Brender C, et al. Suppressor of cytokine signaling 3 regulates CD8 T-cell proliferation by inhibition of interleukins 6 and 27. Blood. 2007;110(7):2528–2536. doi: 10.1182/blood-2006-08-041541. [DOI] [PubMed] [Google Scholar]
- 47.Hisada M, et al. Potent antitumor activity of interleukin-27. Cancer Res. 2004;64(3):1152–1156. doi: 10.1158/0008-5472.CAN-03-2084. [DOI] [PubMed] [Google Scholar]
- 48.Morishima N, Owaki T, Asakawa M, Kamiya S, Mizuguchi J, Yoshimoto T. Augmentation of effector CD8+ T cell generation with enhanced granzyme B expression by IL-27. J Immunol. 2005;175(3):1686–1693. doi: 10.4049/jimmunol.175.3.1686. [DOI] [PubMed] [Google Scholar]
- 49.Salcedo R, et al. IL-27 mediates complete regression of orthotopic primary and metastatic murine neuroblastoma tumors: role for CD8+ T cells. J Immunol. 2004;173(12):7170–7182. doi: 10.4049/jimmunol.173.12.7170. [DOI] [PubMed] [Google Scholar]
- 50.Schneider R, Yaneva T, Beauseigle D, El-Khoury L, Arbour N. IL-27 increases the proliferation and effector functions of human naïve CD8+ T lymphocytes and promotes their development into Tc1 cells. Eur J Immunol. 2011;41(1):47–59. doi: 10.1002/eji.201040804. [DOI] [PubMed] [Google Scholar]
- 51.Klarquist J, et al. Clonal expansion of vaccine-elicited T cells is independent of aerobic glycolysis. Sci Immunol. 2018;3(27):eaas9822. doi: 10.1126/sciimmunol.aas9822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hall AO, Silver JS, Hunter CA. The immunobiology of IL-27. Adv Immunol. 2012;115:1–44. doi: 10.1016/B978-0-12-394299-9.00001-1. [DOI] [PubMed] [Google Scholar]
- 53.Boumendjel A, Tawk L, Malefijt Rde W, Boulay V, Yssel H, Pène J. IL-27 induces the production of IgG1 by human B cells. Eur Cytokine Netw. 2006;17(4):281–289. [PubMed] [Google Scholar]
- 54.Larousserie F, Charlot P, Bardel E, Froger J, Kastelein RA, Devergne O. Differential effects of IL-27 on human B cell subsets. J Immunol. 2006;176(10):5890–5897. doi: 10.4049/jimmunol.176.10.5890. [DOI] [PubMed] [Google Scholar]
- 55.Yoshimoto T, et al. Induction of IgG2a class switching in B cells by IL-27. J Immunol. 2004;173(4):2479–2485. doi: 10.4049/jimmunol.173.4.2479. [DOI] [PubMed] [Google Scholar]
- 56.Hoyt TR, Dobrinen E, Kochetkova I, Meissner N. B cells modulate systemic responses to Pneumocystis murina lung infection and protect on-demand hematopoiesis via T cell-independent innate mechanisms when type I interferon signaling is absent. Infect Immun. 2015;83(2):743–758. doi: 10.1128/IAI.02639-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat Immunol. 2003;4(7):680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 58.Tchao NK, Turka LA. Lymphodepletion and homeostatic proliferation: implications for transplantation. Am J Transplant. 2012;12(5):1079–1090. doi: 10.1111/j.1600-6143.2012.04008.x. [DOI] [PubMed] [Google Scholar]
- 59.Chung B, Dudl EP, Min D, Barsky L, Smiley N, Weinberg KI. Prevention of graft-versus-host disease by anti IL-7Ralpha antibody. Blood. 2007;110(8):2803–2810. doi: 10.1182/blood-2006-11-055673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mai HL, et al. IL-7 receptor blockade following T cell depletion promotes long-term allograft survival. J Clin Invest. 2014;124(4):1723–1733. doi: 10.1172/JCI66287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mazzucchelli R, Durum SK. Interleukin-7 receptor expression: intelligent design. Nat Rev Immunol. 2007;7(2):144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 62.Park JH, et al. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21(2):289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 63.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol. 2004;172(9):5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 64.Ayasoufi K, Yu H, Fan R, Wang X, Williams J, Valujskikh A. Pretransplant antithymocyte globulin has increased efficacy in controlling donor-reactive memory T cells in mice. Am J Transplant. 2013;13(3):589–599. doi: 10.1111/ajt.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rabant M, Gorbacheva V, Fan R, Yu H, Valujskikh A. CD40-independent help by memory CD4 T cells induces pathogenic alloantibody but does not lead to long-lasting humoral immunity. Am J Transplant. 2013;13(11):2831–2841. doi: 10.1111/ajt.12432. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.