Abstract
Background
Smoking cessation therapies are not effective for all smokers, and researchers are interested in identifying those subgroups of individuals (e.g. based on genotype) who respond best to specific treatments.
Objectives
To assess whether quit rates vary by genetically informed biomarkers within pharmacotherapy treatment arms and as compared with placebo. To assess the effects of pharmacotherapies for smoking cessation in subgroups of smokers defined by genotype for identified genome‐wide significant polymorphisms.
Search methods
We searched the Cochrane Tobacco Addiction Group specialised register, clinical trial registries, and genetics databases for trials of pharmacotherapies for smoking cessation from inception until 16 August 2016.
Selection criteria
We included randomised controlled trials (RCTs) that recruited adult smokers and reported pharmacogenomic analyses from trials of smoking cessation pharmacotherapies versus controls. Eligible trials included those with data on a priori genome‐wide significant (P < 5 × 10‐8) single‐nucleotide polymorphisms (SNPs), replicated non‐SNPs, and/or the nicotine metabolite ratio (NMR), hereafter collectively described as biomarkers.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. The primary outcome was smoking abstinence at six months after treatment. The secondary outcome was abstinence at end of treatment (EOT). We conducted two types of meta‐analyses‐ one in which we assessed smoking cessation of active treatment versus placebo within genotype groups, and another in which we compared smoking cessation across genotype groups within treatment arms. We carried out analyses separately in non‐Hispanic whites (NHWs) and non‐Hispanic blacks (NHBs). We assessed heterogeneity between genotype groups using T², I², and Cochrane Q statistics.
Main results
Analyses included 18 trials including 9017 participants, of whom 6924 were NHW and 2093 NHB participants. Data were available for the following biomarkers: nine SNPs (rs1051730 (CHRNA3); rs16969968, rs588765, and rs2036527 (CHRNA5); rs3733829 and rs7937 (in EGLN2, near CYP2A6); rs1329650 and rs1028936 (LOC100188947); and rs215605 (PDE1C)), two variable number tandem repeats (VNTRs; DRD4 and SLC6A4), and the NMR. Included data produced a total of 40 active versus placebo comparisons, 16 active versus active comparisons, and 64 between‐genotype comparisons within treatment arms.
For those meta‐analyses showing statistically significant heterogeneity between genotype groups, we found the quality of evidence (GRADE) to be generally moderate. We downgraded quality most often because of imprecision or risk of bias due to potential selection bias in genotyping trial participants.
Comparisons of relative treatment effects by genotype
For six‐month abstinence, we found statistically significant heterogeneity between genotypes (rs16969968) for nicotine replacement therapy (NRT) versus placebo at six months for NHB participants (P = 0.03; n = 2 trials), but not for other biomarkers or treatment comparisons. Six‐month abstinence was increased in the active NRT group as compared to placebo among participants with a GG genotype (risk ratio (RR) 1.47, 95% confidence interval (CI) 1.07 to 2.03), but not in the combined group of participants with a GA or AA genotype (RR 0.43, 95% CI 0.15 to 1.26; ratio of risk ratios (RRR) GG vs GA or AA of 3.51, 95% CI 1.19 to 10.3).
Comparisons of treatment effects between genotype groups within pharmacotherapy randomisation arms
For those receiving active NRT, treatment was more effective in achieving six‐month abstinence among individuals with a slow NMR than among those with a normal NMR among NHW and NHB combined participants (normal NMR vs slow NMR: RR 0.54, 95% CI 0.37 to 0.78; n = 2 trials). We found no such differences in treatment effects between genotypes at six months for any of the other biomarkers among individuals who received pharmacotherapy or placebo.
Authors' conclusions
We did not identify widespread differential treatment effects of pharmacotherapy based on genotype. Some genotype groups within certain ethnic groups may benefit more from NRT or may benefit less from the combination of bupropion with NRT. The reader should interpret these results with caution because none of the statistically significant meta‐analyses included more than two trials per genotype comparison, many confidence intervals were wide, and the quality of this evidence (GRADE) was generally moderate. Although we found evidence of superior NRT efficacy for NMR slow versus normal metabolisers, because of the lack of heterogeneity between NMR groups, we cannot conclude that NRT is more effective for slow metabolisers. Access to additional data from multiple trials is needed, particularly for comparisons of different pharmacotherapies.
Plain language summary
Do people's genes affect how effective medicines can be in helping people to quit smoking?
Background
Quitting smoking dramatically reduces risk of premature death, but rates of smoking cessation remain low, even with the help of smoking cessation treatments. Recent research has suggested that differences in parts of our genes, called 'genotypes', may help us to tell which smokers could be helped most by different treatments. However, more research is needed to confirm whether or not our genes affect how effective different treatments are at helping people to quit.
Study characteristics
We searched for studies of smokers treated with medicine to help them quit. We looked at people's genes and at how well their bodies could process nicotine, as this might help us to identify people more likely to quit successfully. We found 33 studies relevant to our review, and we were able to get enough information for 18 clinical trials, including over 9000 smokers, that looked at different medicines used to help people to stop smoking.
Key results
The results suggest that smokers with specific genotypes may be more likely to be successful quitting smoking with the use of nicotine replacement therapies compared with smokers who do not have those specific genotypes. Smokers whose bodies process nicotine more slowly may also benefit more from nicotine replacement therapy. We did not see effects of genes on the effectiveness of medicines other than nicotine replacement therapy.
Quality of evidence
These results should be interpreted with caution because the included studies did not assign treatments to smokers on the basis of genotype or the rate at which they process nicotine, and because a small number of clinical trials were included in some comparisons.
Summary of findings
Background
Table 8 presents a glossary of genetic terms.
1. Glossary of genetic terms.
| Genetic term | Explanation |
| Single‐nucleotide polymorphism (SNP) | A single base pair change in the DNA sequence at a particular point compared with the “common” or “wild‐type” sequence (Attia 2009) Most common form of genetic variation in the genome, in which a single‐base substitution has created 2 forms of a DNA sequence that differ by a single nucleotide (Pearson 2008) |
| Alleles | Alternate forms of a gene or chromosomal locus that differ in DNA sequence (Pearson 2008) |
| Genome‐wide association study (GWAS) | Any study of genetic variation across the entire human genome designed to identify genetic association with observable traits or the presence or absence of a disease, usually referring to studies with genetic marker density of 100,000 or more to represent a large proportion of variation in the human genome (Pearson 2008) |
| Genotype | The genetic constitution of an individual, either overall or at a specific gene (Attia 2009) |
| Hardy‐Weinberg equilibrium (HWE) | Population distribution of 2 alleles (with frequencies p and q) such that the distribution is stable from generation to generation and genotypes occur at frequencies of p2, 2pq, and q2 for the major allele homozygote, heterozygote, and minor allele homozygote, respectively (Pearson 2008) |
| Linkage disequilibrium | Measure of association between 2 alleles located near each other on a chromosome, such that they are inherited together more frequently than would be expected by chance (Pearson 2008) |
| Loci | The site(s) on a chromosome at which the gene for a particular trait is located on a gene at which a particular SNP is located (Attia 2009) |
| Minor allele | The allele of a biallelic polymorphism that is less frequent in the study population (Pearson 2008) |
| Major allele | The allele of a biallelic polymorphism that is more frequent in the study population (Pearson 2008) |
| Pharmacogenetics | The field of studying the genetic basis of drug response and applying this knowledge to clinical practice by guiding drug prescribing |
| Pharmacogenomics | Similar to pharmacogenetics but uses information across the whole genome |
| Population stratification | A form of confounding in genetic association studies caused by genetic differences between cases and controls unrelated to disease but due to sampling from populations of different ancestries (Pearson 2008) |
| Wild‐type allele | The allele at a particular single‐nucleotide polymorphism (SNP) that is most frequent in a population, also called “common” allele (Attia 2009) |
DNA = deoxyribonucleic acid; SNP = Single‐nucleotide polymorphism; GWAS = Genome‐wide association study; HWE = Hardy‐Weinberg equilibrium
Description of the condition
Tobacco smoking continues to be the leading cause of preventable death in the world, and yet the most efficacious behavioural and pharmacological treatments are ineffective for most treatment‐seeking smokers (Cahill 2012; Giovino 2012; Hughes 2014; Stead 2012a; Stead 2012b). Although social and other environmental determinants are major contributors to tobacco use, twin and family studies over decades have confirmed that genetic factors contribute substantially to smoking behavior and to disease that can be attributed to smoking (Bidwell 2016; Broms 2006; Carmelli 1992; Fisher 1958; Heath 2002; Li 2003; McCaffery 2008; True 1997; Xian 2008).
Given the low cessation success rate of the most efficacious smoking cessation treatments and the observation of substantial heritability for tobacco dependence, investigators have explored the association between polymorphisms among genes involved in tobacco dependence and genes for smoking cessation drug targets (many of which overlap), to better identify individuals who are more or less likely to successfully quit and abstain from smoking in response to specific medications (Mamoun 2015). It has been postulated that hundreds of genes contribute and may interact with each other and with the environment (e.g. via epigenetic processes) to affect the many dimensions of tobacco dependence through altered neuro‐adaptations, metabolism, and conditioned behaviour (Agrawal 2012; Sullivan 2004). Genomic analyses have the potential to improve the efficacy of smoking cessation treatment if meta‐analyses can identify polymorphisms associated with response to a given pharmacotherapy, and if this is followed by validation of the finding in independent clinical trials and treatment cohorts.
In this review, we used the term ’genomic’ to describe both single‐gene (traditionally described as ’genetic’) and whole‐genome (’genomic’) analyses. We did this because translational research will continue to test more genetic loci of interest; genome‐wide analyses of clinical trials will be performed eventually; and future reviews of more and more complex genetic data are better described as genomic or pharmacogenomic analyses. Our review assesses heterogeneity in treatment effects across genotype groups or nicotine metabolite ratio (NMR) status, and heterogeneity in treatment effects by genotype group or NMR status within treatment arms. These analyses are motivated by specific hypotheses that are pharmacogenomic (i.e. based on which genetic loci in the genome have the greatest effect on nicotine dependence) or pharmacometabolomic (i.e. based on the metabolite ratio of two stable nicotine metabolites that represent the nicotine metabolism rate of the enzyme with the greatest contribution to nicotine metabolism among all protein‐coding genes) in nature.
Description of the intervention
The US Food and Drug Administration (FDA) has approved pharmacotherapies as aids to smoking cessation including five forms of nicotine replacement therapy (NRT), bupropion SR (Zyban), and varenicline (Chantix). NRT forms include gum, lozenge, transdermal patch, nasal spray, and oral inhaler (numerous brands, over‐the‐counter) (Pharmacologic Product Guide). Different NRT therapies offer distinct benefits and limitations with regard to mode of administration and ease of titration; bupropion and varenicline offer alternative mechanisms of action to those offered by NRT, and varenicline offers greater efficacy than NRT monotherapy (Cahill 2013). Pharmacotherapies not approved by the FDA for use in smoking cessation as monotherapy or combination therapy include adrenergics, monoamine oxidase (MAO) inhibitors, selective serotonin reuptake inhibitors (SSRIs), and serotonin–norepinephrine reuptake inhibitors (SNRIs), as well as other drugs and preparations.
Meta‐analyses of the primary literature have identified significant effects of monotherapy and combination therapy versus control therapy on abstinence at six‐month follow‐up (Cochrane Tobacco Addiction Group). In a sample of 117 clinical trials of NRT versus placebo or non‐NRT control, data show a risk ratio (RR) of 1.60‐fold (95% confidence interval (CI) 1.53 to 1.68) (Stead 2012a); in a sample of trials of the antidepressant bupropion (44 trials) or nortriptyline (six trials) versus placebo, or of bupropion versus nortriptyline (three trials), results include significant RRs of 1.62 (95% CI 1.49 to 1.76) and 2.03 (95% CI 1.48 to 2.78) (Hughes 2014); and in a sample of trials comparing the cholinergic partial agonists varenicline (at standard dosage; 27 trials) and cytisine (two trials) versus placebo, investigators reported significant RRs of 2.24 (95% CI 2.06 to 2.43) and 3.98 (95% CI 2.01 to 7.87) (Cahill 2016). Multiple additional meta‐analytical comparisons provide evidence suggesting that varenicline is more effective than bupropion or NRT monotherapy (Mills 2012).
How the intervention might work
Research on the molecular genetics of smoking has pointed to at least two biologically plausible genetic loci contributing to nicotine dependence, cigarette consumption, and smoking cessation. The α5‐α3‐β4 nicotinic acetylcholine receptor gene cluster on chromosome 15q25.1 (CHRNA5‐CHRNA3‐CHRNB4) has been associated with cigarettes smoked per day in genome‐wide association studies; with lung cancer and chronic obstructive pulmonary disease; and with smoking cessation in retrospective pharmacogenetic analyses of clinical trials (Bergen 2013; Chen 2012; Chen 2014; David 2012; King 2012; Liu 2010; Munafò 2011; Saccone 2010; Thorgeirsson 2008; Thorgeirsson 2010; Tobacco and Genetics Consortium 2010; Zhu 2014). The cytochrome p450 2A6 enzyme inactivates approximately 80% of nicotine to cotinine and other metabolites through oxidative hepatic metabolism; extensive functional variation influencing the speed of nicotine metabolism is found at CYP2A6 (Benowitz 2006; Benowitz 2009; McDonagh 2012; Murphy 2014). The NMR 3‐hydroxycotinine/cotinine has been associated with cigarettes smoked per day, lung and other aerodigestive cancers, and smoking cessation (Bloom 2012; Canova 2009; Chen 2014; Dempsey 2004; Fujieda 2004; Gemignani 2007; Ho 2009; Lerman 2006; Lerman 2010; Liu 2011; Patterson 2008; Rotunno 2009; Schnoll 2009; Schnoll 2010; Schoedel 2004; Tamaki 2011; Thorgeirsson 2010; Tobacco and Genetics Consortium 2010; Topcu 2002). Variation in the CHRNA3‐A5‐B4 genetic locus is associated with functional changes in key nicotinic acetylcholine receptors in the brain, which in turn are related to behaviour (i.e. nicotine self‐administration and smoking quantity) (Fowler 2011; Scherf 2012; Ware 2012). Variation in the CYP2A6 genetic locus affects the rate of nicotine metabolism and is associated with smoking quantity (McDonagh 2012). Therefore, biologic plausibility underlies the relationship between these genetic loci and smoking‐related behaviour. Consistent with preclinical studies of nicotine reinforcement and conditioned place preference, genes in the dopamine pathway have been linked to smoking cessation (Balfour 2009; Herman 2014; Leventhal 2014; Tobacco and Genetics Consortium 2010). Many other candidate genes and pathways have been explored for association with smoking cessation in relation to the pharmacology of nicotine and tobacco dependence treatment. Bupropion and to a lesser degree nicotine are metabolised by cytochrome 2B6, which converts bupropion to the more pharmacologically active hydroxybupropion, which in turn has been associated with smoking cessation (Benowitz 2013; Zhu 2012). A low‐affinity organic cation transporter gene (SLC22A2) has been associated with varenicline adverse effects and with abstinence outcomes in individuals randomised to NRT or varenicline (Bergen 2014; King 2012). Both the endogenous opioid system and serotonin neuronal pathways are implicated in some dimensions of nicotine dependence, and polymorphisms in mu‐opioid receptor 1 (OPRM1) and serotonin 5‐HT3 receptor (HTR3B) genes, respectively, have been linked to response to smoking cessation drugs; however, results have been inconclusive and mixed or without consistent replication for a variety of polymorphisms across both neurotransmitter systems (Balfour 2009; David 2008; Hadjiconstantinou 2011; King 2012; Marteau 2012; Munafò 2007; Munafò 2013a; Quaak 2012; Ray 2007; Verhagen 2012; Wang 2010).
Current clinical practice guidelines recommend clinician assessment of every patient's smoking behaviour, which is followed by advice to quit, assessment of willingness to quit, assistance in quitting, and arranging follow‐up support (5 As) (European Smoking Cessation Guidelines 2012; Fiore 2008; West 2000). Clinical practice guidelines describe in detail aspects of patients, clinicians, clinician engagement, behavioural therapy, and pharmacotherapy that contribute towards abstinence (European Smoking Cessation Guidelines 2012; Fiore 2008; West 2000). Both behavioural therapies and pharmacotherapies are effective aids for patient smoking cessation ‐ therapies combining behavioural therapy and pharmacotherapy are recommended for greatest therapeutic efficacy (Patnode 2015). An intervention that addresses pharmacotherapeutic efficacy by genotype or by metabolic activity offers the prospect of improving therapeutic effectiveness beyond the minority of individuals who remain abstinent at 24 weeks with combination therapies delivered by a clinician (European Smoking Cessation Guidelines 2012; Fiore 2008; Kotz 2014; West 2000). A genotype‐based, or metabolism‐based, pharmacogenomic assay and associated intervention for smoking cessation might be developed if robust evidence for improved efficacy were to be developed through the established pathway of discovery and translation to clinical practice via retrospective and prospective tests of clinical data, analytical validity, clinical validity, and clinical utility. This review uses data from retrospective analyses of abstinence differences by genotype or metabolism subgroup in nearly all cases (one trial contributed data from a prospective stratification by metabolism); thus analyses of these data do not provide clinical guidance for pharmacogenomic testing and treatment selection. However, they do describe the scope of available data in terms of sample sizes, clinical treatments, and selected available genomic data. An intervention would be expected to be available as a pharmacogenomic test that the physician can order; results of the pharmacogenomic assay with clinical treatment recommendations based on prior clinical validity and utility analyses would be available to assist the physician in treating individuals for tobacco dependence.
Why it is important to do this review
Smoking cessation clinical trials have provided a growing body of genotype‐based subgroup analyses, but replication is rare, and there have been no comprehensive systematic reviews with meta‐analyses. This review evaluated the effectiveness of pharmacotherapy for smoking cessation in subgroups of treatment‐seeking smokers defined by genotype for genome‐wide significant single‐nucleotide polymorphisms (SNPs), replicated non‐SNP polymorphisms, and/or the NMR. Pharmacotherapies discussed in this review include medications approved to treat symptoms of withdrawal, craving, or other behavioural symptoms that affect the ability of the individual to stop smoking and to remain abstinent; these include all forms of NRT (e.g. patch, gum, lozenge, inhaler, spray) and non‐nicotine pharmacotherapies (e.g. bupropion, varenicline, cytisine, nortriptyline). Identifying whether clinically important differences in treatment response between genomically defined groups are likely is an essential first step in moving the field closer to personalised treatments guided by genomic testing.
Objectives
To assess whether quit rates vary by genetically informed biomarkers within pharmacotherapy treatment arms and as compared with placebo. To assess the effects of pharmacotherapies for smoking cessation in subgroups of smokers defined by genotype for identified genome‐wide significant polymorphisms.
Methods
Criteria for considering studies for this review
Types of studies
All published and unpublished primary and secondary analyses of randomised and quasi‐randomised controlled trials (RCTs) in which smoking cessation pharmacotherapies were initiated to enhance abstinence from smoking, and in which trial participants were genotyped for polymorphisms that are genome‐wide significant (alpha threshold < 5 × 10‐8) SNPs for cigarettes per day, nicotine dependence, or smoking cessation, as well as non‐SNP polymorphisms that were not included in genome‐wide association studies but have been associated with smoking cessation treatment response in at least two trials; or the NMR, which is highly influenced by the cytochrome p450 2A6 (CYP2A6) gene, hereafter collectively described as biomarkers (Chenoweth 2017; David 2012;Saccone 2010;Thorgeirsson 2008;Thorgeirsson 2010;Tobacco and Genetics Consortium 2010). Table 9 presents the polymorphisms of interest and NMR, which two review authors (AWB and SPD) identified via literature review. The comparator could be placebo, or usual/standard care, or a different pharmacotherapy, or a non‐pharmacotherapy intervention, or a no‐intervention control. We also considered trials comparing different doses or durations or regimens of pharmacotherapy, or comparing different formats of NRT, or comparing combinations of pharmacotherapy versus a single type, or comparing different types or intensities of behavioural support as adjuncts to pharmacotherapy. We applied no language or date restrictions. For inclusion of a study in the review, at least one other trial had to provide genetic or NMR data for the same type of smoking cessation medication (e.g. if we found only one genotyped venlafaxine or one genotyped nortriptyline trial, we did not perform meta‐analysis).
2. Polymorphisms of interest, together with their major/minor alleles, stratified by race.
| Polymorphism | Race group | ||||
| Gene/Region | SNP or VNTRa | Whiteb | Black or African Americanc | East Asiand | Mexicane |
| CHRNA3 | rs1051730 | G/A | G/A | G/A | G/A |
| CHRNA5 | rs16969968 | G/A | G/A | G/A | G/A |
| CHRNA5 | rs588765 | C/T | C/T | C/T | C/T |
| CHRNA5 | rs2036527 | G/A | G/A | G/A | G/A |
| CHRNB3 | rs13280604 | A/G | G/A | A/G | A/G |
| CYP2A6 | rs4105144 | T/C | T/C | T/C | T/C |
| CYP2B6 | rs6474412 | T/C | C/T | T/C | T/C |
| DBH | rs3025343 | G/A | G/A | G/A | G/A |
| DRD4 (exon 3 48 bp) | SI000224I | 4 (0.65), 7 (0.18), 3, 2 | 4 (0.75), 7 (0.14), 6, 5 | 4 (0.79), 2 (0.17), 5 | 7 (0.52), 4 (0.41), 2 |
| EGLN2 | rs3733829 | A/G | A/G | A/G | G/A |
| EGLN2 | rs7937 | T/C | C/T | T/C | T/C |
| LOC100188947 | rs1329650 | G/T | G/T | T/G | G/T |
| LOC100188947 | rs1028936 | A/C | A/C | C/A | A/C |
| PDE1C | rs215605 | T/G | G/T | G/T | T/G |
| SLC6A3 (3' 40 bp) | SI000156M | 10 (0.69), 9 (0.31) | 10 (0.73), 9 (0.21), 3, 8 | 10 (0.91), 9 | 10 (0.74), 9 (0.24) |
| SLC6A4 (Promoter) | SI664268Gf | L (0.62), S (0.38) | L (0.78), S (0.07), 19 (0.15) | L (0.27), S (0.71) | L (0.42), S (0.58) |
| aSNP rsID and frequencies from the 1000 Genomes Project database (http://analysistools.nci.nih.gov/LDlink/), VNTR UID and frequencies from ALFRED (http://alfred.med.yale.edu/alfred/index.asp). | |||||
| bGBR or European (PopID = 20, SampID = 20C). | |||||
| cASW or per VNTR (DRD4, Biaka (PopID = 5, SampID = 5F); SLC6A3, African American (PopID = 98R, SampID = 101C); SLC6A4, Biaka. | |||||
| dCHB + JPT, or mean of Han and Japanese, for SLC6A3 and SLC6A4, Han (SampID = 9J) and Japanese (SampID = 10B). | |||||
| eMXL or per VNTR (DRD4, see Table 2, Aguirre‐Samudio 2014; SLC6A3, Hispanic American from ALFRED; SLC6A4, see Table 2, Peralta‐Leal 2012). | |||||
| fFor Euro, African American and East Asian, extracted from Promoter VNTR + rs25531 Table in ALFRED. L = 16 repeats, S = 14 repeats. | |||||
SNP = Single‐nucleotide polymorphism; VNTR = variable number tandem repeat; G = nucleobase guanine; A = nucleobase adenine; C = nucleobase cytosine; T = nucleobase thymine; rsID=reference SNP cluster ID; UID=Unique Identifier, from ALFRED; GBR=British in England and Scotland population description code from 1000 Genomes Project; PopID=Population ID from ALFRED; SampID=Sample ID from ALFRED; ASW=Americans of African Ancestry in SW USA population description code, from 1000 Genomes Project; CHB=Han Chinese in Bejing, China population description code, from 1000 Genomes Project; JPT=Japanese in Tokyo, Japan population description code, from 1000 Genomes Project; Han=Han Chinese living in the San Francisco, California, from ALFRED; MXL=Mexican Ancestry from Los Angeles USA population description code, from 1000 Genomes Project; L=Long (16 repeats); S=Short (14 repeats)
Types of participants
Adult men and women who smoke, regardless of the setting from which they were recruited and/or their initial level of nicotine dependence. Participants could be of any ethnicity, but we analysed outcomes within different ethnic subgroups separately. We did not anticipate a sufficient number of genomic pharmacotherapy studies of minors (aged under 18 years) for inclusion in meta‐analyses.
Types of interventions
Smoking cessation pharmacotherapy, which included all forms of NRT (e.g. patch, gum, lozenge, inhaler, spray) and non‐nicotine pharmacotherapies (e.g. bupropion, varenicline, cytisine, nortriptyline).
Types of outcome measures
Primary outcomes
Smoking abstinence at six months from the start of treatment.
Secondary outcomes
Smoking abstinence at end of treatment defined as abstinence between 7 and 12 weeks after the start of treatment.
We did not collect information about adverse events reported in included trials, because we expected the occurrence of such events to be low, making power for analysis according to genotype insufficient.
Search methods for identification of studies
Electronic searches
On 16 August 2016, we searched the Cochrane Tobacco Addiction Group specialised register for trials of pharmacotherapies for smoking cessation, using the term 'genetic’, 'genomic’, 'pharmacogenetic’, or 'pharmacogenomic’ in the title, abstract, or keywords to identify relevant records. This register contains trials identified from:
MEDLINE.
Embase.
PsycINFO.
Reference lists of previous trials and overviews.
See details of search strategies applied for these databases in the Tobacco Addiction Group module.
Additionally, we searched international online clinical trial registers for ongoing and recently completed trials, including the WHO portal; the UK clinical trials gateway; the US clinical trials register; and the Australian and New Zealand clinical trials register.
In addition to the databases mentioned above, we searched genetics databases, including Pharmacogenomics Knowledgebase (PharmGKB) and Pharmacogenomics of Nicotine Addiction Treatment (PNAT). We considered additional searches of National Institutes of Health (NIH) databases, but at the time of this review, smoking cessation clinical trial data were not yet available on Genotypes and Phenotypes (dbGaP), a database developed to archive and distribute data and results from studies that have investigated the interaction of genotype and phenotype in humans.
Searching other resources
We checked the reference lists of published papers and consulted with experts in the field to identify any relevant forthcoming or unpublished research, or both. We contacted the authors of ongoing studies when necessary.
Data collection and analysis
Selection of studies
Two review authors (AWB and SPD) and Ms. Lindsey Stead (Cochrane Tobacco Addiction Group, Oxford) conducted database searches and performed initial screening of abstracts and manuscripts. Two review authors (ES and SPD) independently checked the abstracts and, if relevant, retrieved and reviewed the full texts of records for data. We observed that most of the studies eligible for inclusion were secondary analyses of pharmacotherapy trials. For each eligible study, we identified the primary trial report for long‐term cessation outcomes.
If a given genome‐wide significant SNP or non‐SNP polymorphism was not reported in an RCT that reported other genotyping data, we contacted study authors to request these data from unpublished genotyping data.
Data extraction and management
Four review authors (ES, OAP, AWB, and SPD) curated records for extraction of study characteristics of primary trials. We assessed each trial in duplicate. Review authors resolved disagreements by consensus, or by recourse to a third review author, who was not assigned to that particular trial. We recorded the following information in the Characteristics of included studies tables.
Methods: study design, study name (if applicable), study recruitment period.
Participants: number of participants in original trial, study recruitment procedure, country, number of study centres, study setting, inclusion and exclusion criteria.
Interventions: description of intervention(s) (treatment, dosage, regimen, behavioural support), description of control (treatment, dosage, regimen, behavioural support), number of participants in each treatment arm.
Outcomes: primary outcome and secondary outcomes of trials.
Funding source.
Declaration of interest.
Notes: information on genomic analyses: whether they were performed within the RCT or as secondary analysis, and the number of participants successfully genotyped.
Outcome data
We contacted trial authors and asked them to supply genotype counts by outcome, by pharmacotherapy arm, and by self‐identified race/ethnicity for participants in their study for (a) a group (N = 13) of single‐nucleotide polymorphisms (SNPs) with reported genome‐wide significance with smoking cessation, cigarettes per day, or nicotine dependence in genome‐wide association study (GWAS) investigations; (b) three variable numbers of tandem repeat polymorphisms (VNTRs); or (c) NMR data (David 2012; Thorgeirsson 2010; Tobacco and Genetics Consortium 2010). Table 9 provides an overview of all genetic polymorphisms and major and minor alleles stratified by race group. We asked trial authors to complete at least two predesigned data extraction forms ‐ one on the numbers of participants of different race/ethnicity groups included in the trial, and another on abstinence rates for each polymorphism, stratified by outcome, treatment arm, race/ethnicity, and genotype. Some identified papers presented data from multiple studies (e.g. from a meta‐analysis) (Bergen 2013). From the author of these combined analyses, we requested study level data so we could assess and account for differences across studies in the statistical analysis. We collected genotype information for a given polymorphism in a trichotomised fashion (homozygous major (M/M); heterozygous (M/m); homozygous minor (m/m) alleles), and we gathered NMR information in a dichotomised fashion (normal (NMR ≥ 0.31) vs slow (NMR < 0.31) metabolisers). For calculation of abstinence rates, we collected the number of participants who were abstinent at the time point of interest (i.e. at 6 months or at end of treatment) and the total number randomised in that subgroup.
We contacted trial authors twice. When they did not respond to our request, we tried to extract requested data from published reports. If trial authors supplied or if the paper reported genotype data in a dichotomised fashion (i.e. M/M vs M/m + m/m), we used this information to perform analysis in a similar fashion.
Assessment of risk of bias in included studies
Four review authors (ES, OAP, AWB, and SPD) independently assessed included studies for risks of selection bias (randomised sequence generation, allocation concealment), performance and detection bias (presence or absence of blinding), attrition bias (levels and reporting of loss to follow‐up), and any other threats to study quality, as recommended in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We assessed each trial in duplicate. We also assessed trials for other sources of bias specific to genotype studies: genotype frequencies for each study and polymorphism; potential deviation from Hardy‐Weinberg equilibrium; source of DNA (e.g. buccal swab, blood, saliva); genotyping protocol; and any quality control (QC) methods described. Review authors resolved disagreements by consensus, or by recourse to a third review author, who was not assigned to that particular trial.
Measures of treatment effect
We calculated the cessation rate as the number of people abstinent at six monthsand at end of treatment, based on point prevalence abstinence (preferably biochemically verified but otherwise self‐reported), divided by the total number of participants in that subgroup, separately in each genotype/treatment subgroup. We assessed treatment effects by meta‐analysing active versus placebo effects on abstinence separately in M/M, M/m. and m/m genotype strata; we then generated separate RRs and 95% CIs for active versus placebo for each genotype stratum for each biomarker. Afterwards, we assessed heterogeneity in treatment effect across genotype strata.
For analyses in which we found statistically significant heterogeneity in treatment effects across genotype subgroups, we estimated the difference in treatment effects in M/M versus m/m and in M/m versus m/m to capture the interaction effects of genotype x treatment. Because estimates of treatment effects were expressed in the log‐scale (i.e. logRR), we expressed the genotype x treatment interaction as the ratio of risk ratios (RRR) for treatment effect in one genotype group over treatment effect in the other genotype group. An RRR > 1 means that the treatment effect is greater in individuals with the M/M versus non‐M/M genotype.
Initially, we planned to address the potential effect of methodological heterogeneity in behavioural interventions across studies for those analyses in which we found statistically significant heterogeneity in treatment effects across genotype subgroups. However, because the number of included studies in these analyses was small, we deemed that such analysis was not reliable.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data when possible (e.g. when a study was reported as abstract only). In cases for which we received individual participant data, we treated participants lost to follow‐up as continuing smokers, which yields conservative absolute quit rates and makes little difference to the risk ratio unless dropout rates differ substantially between groups. For aggregate data, we relied on the decision of investigators of the primary trial, which conventionally reports smokers who are lost to follow‐up as smoking, in keeping with intention‐to‐treat analyses.
We noted in the ’Risk of bias’ tables whether results showed high or differential loss to follow‐up between treatment groups.
Assessment of heterogeneity
We evaluated levels of clinical heterogeneity between included studies to decide whether it would be appropriate to pool data in planned subgroups. We analysed different pharmacotherapies separately but pooled studies using different types of NRT.
We assessed statistical heterogeneity in each meta‐analysis using T², I², and Chi² statistics (Higgins 2003). We regarded statistical heterogeneity as substantial if I² was greater than 50% and either T² was greater than zero, or the P value was low (< 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
We decided that if 10 or more studies contributed data to the meta‐analysis for either outcome, we would investigate reporting biases (such as publication bias) using funnel plots, so as to assess possible asymmetry visually. If asymmetry was suggested by visual assessment, we planned to perform exploratory analyses to investigate this using more formal tests, such as Egger's and Begg's tests (Begg 1994; Egger 1997). This version of the review included too few studies in each of the meta‐analyses performed to allow assessment of reporting bias.
Data synthesis
For each polymorphism, we undertook two types of analysis.
-
Comparisons of relative treatment effect by genotype. We grouped studies based on the characteristics of intervention and control arms.
Each individual pharmacotherapy or combination compared with placebo/no pharmacotherapy control.
Comparisons between different pharmacotherapies.
Combinations of pharmacotherapy compared with a single pharmacotherapy.
We tested for heterogeneity in relative effects between genotype subgroups.
Comparisons of treatment effects between genotype groups, within pharmacotherapy randomisation arms. Some clinical trials included only one pharmacotherapy arm but more than one behavioural support arm, but we did not stratify analyses by behavioural support arm. Analyses of genotype effects within drug groups separately compared abstinence outcomes between 1 to 0 risk alleles and 2 to 0 risk alleles. If possible, we compared risk allele heterozygotes and homozygotes versus reference alleles in separate analyses (e.g. rs1051730 CT vs CC, or TT vs CC rather than CT/TT vs CC).
Differences in genetic architecture (allele frequencies and/or linkage disequilibrium) between ancestral groups may result in population structure, which may lead to confounding if smoking behaviour‐related outcomes are associated with both ancestry and genetic architecture. Conditions for population stratification are met for comparisons between African Americans and European Americans owing to differences in smoking behaviours (e.g. African Americans smoke fewer cigarettes per day than European Americans and in genetic architecture at relevant loci; we conducted separate analyses for white and black or African American study populations at genotype‐based biomarkers (Beirut 2008; Cardon 2003; Trinidad 2015; Wang 2013). We also considered other ancestries (e.g. Asian), but data were not sufficient to allow separate meta‐analyses in these populations. We did analyse the NMR across ancestry groups rather than separately by ancestry group because the NMR is a metabolic marker (not a genotype). The NMR is genetically informed by prior knowledge about the CYP2A6 genotype, but other genes and environmental factors affect NMR; because it is not a genotype, the same concerns about population stratification do not apply to the NMR.
We pooled risk ratios (RRs) using a Mantel‐Haenszel random‐effects model with a 95% confidence interval (CI) for our meta‐analysis, so as to account for statistical heterogeneity across included studies. When the event is defined as smoking cessation, an RR greater than one indicates that more people in the treatment group than in the control group successfully quit. We carried out statistical analysis using RevMan (Review Manager 5.3).
Subgroup analysis and investigation of heterogeneity
In the event of evidence of heterogeneity within planned subgroups based on genotype and type of pharmacotherapy, we planned to explore the impact of the following possible factors.
Different comparators.
Levels of behavioural support.
Dose or duration of treatment.
Gender differences.
Motivation to quit.
Level of nicotine dependence.
However, because heterogeneity within genotype groups was generally low and available data within genotype groups were generally sparse, we refrained from performing any such subgroup analyses.
Sensitivity analysis
We planned to conduct sensitivity analyses to determine whether inclusion of quasi‐RCTs and risk of bias made a difference in our findings. However, we did not identify any quasi‐RCTs, and we did not find enough studies at this time to undertake sensitivity analyses.
'Summary of findings' table
In keeping with standard Cochrane methods, we created a 'Summary of findings' table for comparisons between active NRT and placebo (because it is the most commonly administered intervention of those included in the review) for the genotypes most frequently studied. As part of this process, we used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for each outcome. This assessment of the quality of evidence within the review informs the confidence with which we view our conclusions.
Results
Description of studies
Results of the search
Through our search strategy, we identified a total of 479 unique papers, of which 94 were eligible for inclusion in our review (Figure 1). These corresponded to 35 primary randomised clinical trials of pharmacotherapies that compared two or more smoking cessation intervention arms that met the inclusion criteria stated (Ahluwalia 2006; Aveyard 2007; Bloch 2010; Brown 2007; Cinciripini 2005; Cox 2012; Gilbert 2009; Gonzales 2006; GPRG 1993; Hall 2008; Hall 2009; Jorenby 2006; Kalman 2011; Killen 2006; Killen 2008; Killen 2010; King 2012; Lerman 2002; Lerman 2004; Lerman 2015; Marteau 2012; McCarthy 2008; McClure 2013; Oncken 2006; Piper 2007; Piper 2009; Rose 2010; Schnoll 2010; Sun 2012; Swan 2003; Swan 2010; Verde 2014; Wagena 2005; Wilcox 2011; Winst 2006). We excluded three of these studies (Bloch 2010; King 2012; McClure 2013; see Excluded studies). Of the remaining 33 studies, we were able to obtain data from 14 studies by contacting the principal investigator and for four studies by extracting data from published reports (Ahluwalia 2006; Aveyard 2007; Brown 2007; Cox 2012; Gilbert 2009; GPRG 1993; Hall 2008; Hall 2009; Lerman 2002; Lerman 2004; Lerman 2015; Marteau 2012; McCarthy 2008; Piper 2007; Piper 2009; Swan 2010; Wagena 2005; Winst 2006). In total, we included 18 randomised clinical trials in the quantitative analysis (Ahluwalia 2006; Aveyard 2007; Brown 2007; Cox 2012; Gilbert 2009; GPRG 1993; Hall 2008; Hall 2009; Lerman 2002; Lerman 2004; Lerman 2015; Marteau 2012; McCarthy 2008; Piper 2007; Piper 2009; Swan 2010; Wagena 2005; Winst 2006). We did not consider 14 trials in the quantitative analysis because we obtained no relevant data via contact with the principal investigator or through review of published reports (Cinciripini 2005; Gonzales 2006; Jorenby 2006; Kalman 2011; Killen 2006; Killen 2008; Killen 2010; Oncken 2006; Rose 2010; Schnoll 2010; Sun 2012; Swan 2003; Verde 2014; Wilcox 2011). For the remainder of this report, we will focus only on those studies that contributed to the quantitative analysis.
1.
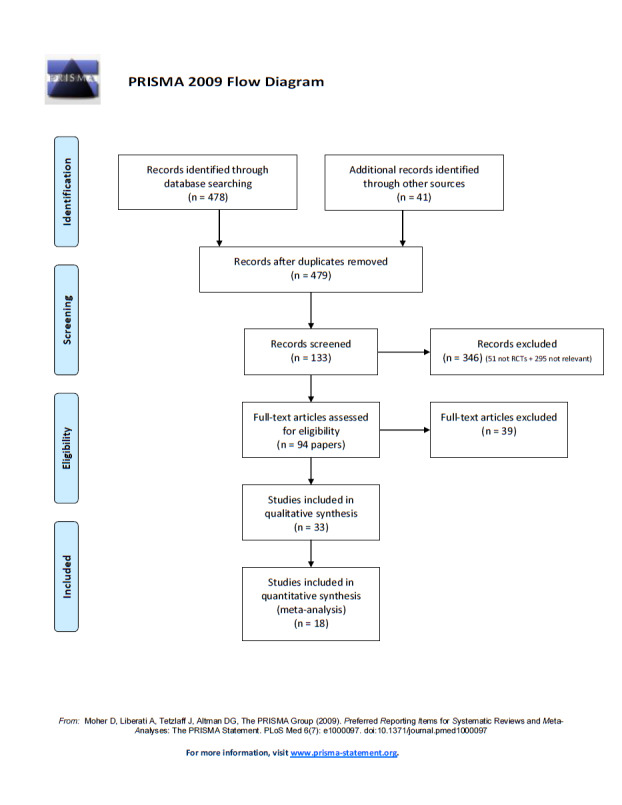
Flow diagram of the search and study selection.
Included studies
Refer to the Characteristics of included studies table for details.
We retrieved data (genotype counts by abstinence status, treatment arm, and race/ethnicity) from 18 randomised clinical trials including 9017 participants, of whom 6924 were non‐Hispanic whites (NHWs) and 2093 were non‐Hispanic black or African American (NHB) participants.
Data were available for 13 biomarkers: nine SNPs (rs1051730 (CHRNA3) (n = 13: Aveyard 2007; Becker 2008; Brown 2007; GPRG 1993; Hall 2008; Hall 2009; Lerman 2002; Lerman 2004; Marteau 2012; McCarthy 2008; Piper 2007; Piper 2009; Swan 2010; Winst 2006), rs16969968 (n = 7: Ahluwalia 2006; Cox 2012; Lerman 2015; McCarthy 2008; Piper 2007; Piper 2009), rs588765 (n = 10: Ahluwalia 2006; Brown 2007; Cox 2012; Hall 2008; Hall 2009; Lerman 2002; Lerman 2004; Lerman 2015; Piper 2009; Swan 2010), rs2036527 (CHRNA5) (n = 7: Ahluwalia 2006; Cox 2012; Lerman 2002; Lerman 2004; McCarthy 2008; Piper 2007; Piper 2009), rs3733829 (n = 4: Brown 2007; McCarthy 2008; Piper 2007; Piper 2009), rs7937 (EGLN2) (n = 3: McCarthy 2008; Piper 2007; Piper 2009), rs1329650 (n = 3: McCarthy 2008; Piper 2007; Piper 2009), rs1028936 (LOC100188947) (n = 3: McCarthy 2008; Piper 2007; Piper 2009), and rs215605 (PDE1C) (n = 3: McCarthy 2008; Piper 2007; Piper 2009)); two VNTRs (DRD4 (n = 4: Aveyard 2007; Brown 2007; Gilbert 2009; GPRG 1993) and SLC6A4 (n = 3: Aveyard 2007; Gilbert 2009; Wagena 2005)); and the NMR (n = 3: Ahluwalia 2006; GPRG 1993; Lerman 2015).
Data were insufficient to allow analysis for five additional biomarkers: four SNPs: rs13280604 (no trials), rs4105144 (no trials), rs6474412 (n = 1: Brown 2007), and rs3025343 (n = 1: Brown 2007); and one VNTR: SLC6A3 (3' 40 bp) (n = 1: Brown 2007), which were identified initially as relevant to investigate.
We identified placebo‐controlled trials of NRT (n = 4: Ahluwalia 2006; Gilbert 2009; GPRG 1993; Winst 2006); bupropion (n = 6: Brown 2007; Cox 2012; Hall 2008; Lerman 2002; McCarthy 2008; Wagena 2005); NRT, bupropion, and NRT + bupropion (n = 1: Piper 2009); NRT and varenicline (n = 1: Lerman 2015); and bupropion and NRT + bupropion (n = 1: Piper 2007). In the other trials, all participants received NRT (n = 4: Aveyard 2007; Hall 2009; Lerman 2004; Marteau 2012) or varenicline (n = 1: Swan 2010). NRT trials included gum (Ahluwalia 2006; Hall 2009; Piper 2007), lozenge (Piper 2009), patch (Aveyard 2007; Cinciripini 2005; Gilbert 2009; GPRG 1993; Hall 2008; Kalman 2011; Killen 2006; Killen 2008; Killen 2010; Lerman 2004; Lerman 2015; Marteau 2012; Piper 2009; Rose 2010; Schnoll 2010; Verde 2014; Winst 2006), and spray (Lerman 2004) NRT interventions. Piper et al included NRT lozenge and transdermal patch intervention arms in this five‐arm trial (Piper 2009).
Trials contained at least one arm of NRT (n = 10: Ahluwalia 2006; Aveyard 2007; Gilbert 2009; GPRG 1993; Hall 2009; Lerman 2004; Lerman 2015; Marteau 2012; Piper 2009; Winst 2006), bupropion (n = 7: Brown 2007; Cox 2012; Hall 2008; Lerman 2002; McCarthy 2008; Piper 2007; Piper 2009; Wagena 2005), varenicline (n = 2: Lerman 2015; Swan 2010), NRT + bupropion (n = 2: Piper 2007; Piper 2009), or placebo (n = 13: Ahluwalia 2006; Brown 2007; Cox 2012; Gilbert 2009; GPRG 1993; Hall 2008; Lerman 2002; Lerman 2015; McCarthy 2008; Piper 2007; Piper 2009; Wagena 2005; Winst 2006), resulting in a total of 40 active versus placebo, 16 active versus active, and 64 between‐genotype comparisons.
Data on the primary outcome ‐ abstinence at six months ‐ were available from 16 trials (Ahluwalia 2006; Aveyard 2007; Brown 2007; Cox 2012; Gilbert 2009; GPRG 1993; Hall 2008; Hall 2009; Lerman 2002; Lerman 2004; Lerman 2015; Marteau 2012; Piper 2009; Swan 2010; Wagena 2005; Winst 2006). Data on abstinence at end of treatment were available from 17 trials (Ahluwalia 2006; Aveyard 2007; Brown 2007; Cox 2012; Gilbert 2009; GPRG 1993; Hall 2008; Hall 2009; Lerman 2002; Lerman 2004; Lerman 2015; McCarthy 2008; Piper 2007; Piper 2009; Swan 2010; Wagena 2005; Winst 2006). Eleven trials included mostly NHW participants (Aveyard 2007; Brown 2007; Gilbert 2009; GPRG 1993; Hall 2008; Hall 2009; Marteau 2012; McCarthy 2008; Swan 2010; Wagena 2005; Winst 2006). Five trials included NHW and NHB participants (Lerman 2002; Lerman 2004; Lerman 2015; Piper 2007; Piper 2009). Two trials included NHB participants only (Ahluwalia 2006; Cox 2012). Available data were insufficient to allow meta‐analyses for Hispanic or Latino race or for non‐Hispanic or non‐Latino ethnic groups other than NHW or NHB.
Excluded studies
In total, we excluded three of the 36 identified randomised clinical trials ‐ one because researchers performed no individual genotyping, and two because investigators reported no genotypes of interest and/or made none available (Becker 2008; King 2012; McClure 2013). Additional information on these studies can be found in the Characteristics of excluded studies tables.
Risk of bias in included studies
Overall risk of bias of randomised clinical trials included in the quantitative analysis varied from low to high. Refer to the Characteristics of included studies table for details, and to Figure 2 and Figure 3 for a summary of ‘Risk of bias’ assessments. For one trial, we had only a protocol available, and this made it difficult to assess risk of bias of this study as actually performed (Winst 2006).
2.
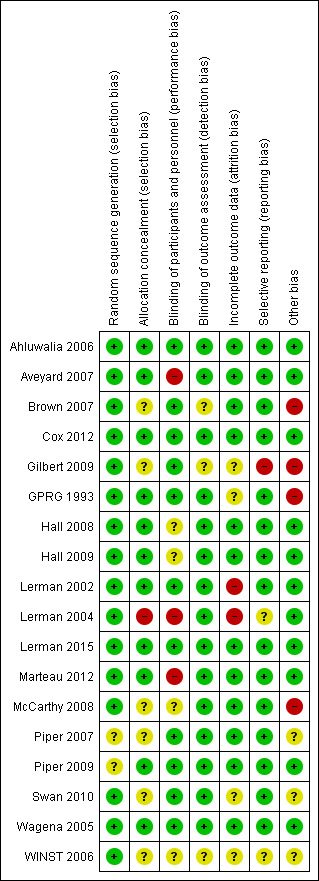
Risk of bias summary: review authors' judgements about each risk of bias item for each study.
3.
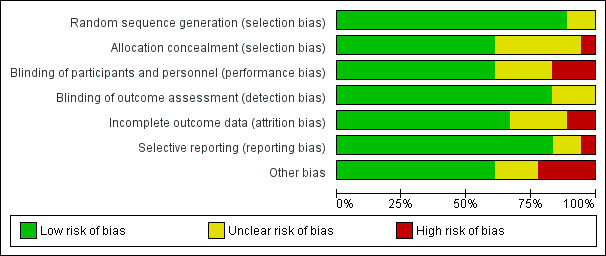
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
We assessed random sequence generation as having low risk of bias for 16 studies (Ahluwalia 2006; Aveyard 2007; Brown 2007; Cox 2012; Gilbert 2009; GPRG 1993; Hall 2008; Hall 2009; Lerman 2002; Lerman 2004; Lerman 2015; Marteau 2012; McCarthy 2008; Swan 2010; Wagena 2005; Winst 2006). We assessed two studies as having unclear risk (Piper 2007; Piper 2009). We considered allocation concealment as having low risk of bias for 10 studies (Ahluwalia 2006; Aveyard 2007; GPRG 1993; Hall 2008; Hall 2009; Lerman 2002; Lerman 2015; Marteau 2012; Piper 2009; Wagena 2005). We found unclear risk for seven studies (Brown 2007; Cox 2012; Gilbert 2009; McCarthy 2008; Piper 2007; Swan 2010; Winst 2006). We determined that one study was at high risk (Lerman 2004).
Blinding
We considered blinding of participants and personnel as having low risk of bias for 11 studies (Ahluwalia 2006; Brown 2007; Cox 2012; Gilbert 2009; GPRG 1993; Lerman 2002; Lerman 2015; Piper 2007; Piper 2009; Swan 2010; Wagena 2005). We judged four studies as having unclear risk (Hall 2008; Hall 2009; McCarthy 2008; Winst 2006). We found that three studies were at high risk (Aveyard 2007; Lerman 2004; Marteau 2012). We considered blinding of outcome assessment as having low risk of bias for 13 studies (Aveyard 2007; GPRG 1993; Hall 2008; Hall 2009; Lerman 2002; Lerman 2004; Lerman 2015; Marteau 2012; McCarthy 2008; Piper 2007; Piper 2009; Swan 2010; Wagena 2005). We determined that five studies were at unclear risk (Ahluwalia 2006; Brown 2007; Cox 2012; Gilbert 2009; Winst 2006).
Incomplete outcome data
We considered incomplete outcome data as having low risk of bias for 12 studies (Ahluwalia 2006; Aveyard 2007; Brown 2007; Cox 2012; Hall 2008; Hall 2009; Lerman 2015; Marteau 2012; McCarthy 2008; Piper 2007; Piper 2009; Wagena 2005). We deemed that four studies had unclear risk (Gilbert 2009; GPRG 1993; Swan 2010; Winst 2006). We found high risk for two studies (Lerman 2002; Lerman 2004).
Selective reporting
We considered selective reporting as having low risk of bias for 15 studies (Ahluwalia 2006; Aveyard 2007; Brown 2007; Cox 2012; GPRG 1993; Hall 2008; Hall 2009; Lerman 2002; Lerman 2015; Marteau 2012; McCarthy 2008; Piper 2007; Piper 2009; Swan 2010; Wagena 2005).We determined that two studies were at unclear risk (Lerman 2004; Winst 2006). We found that one study was at high risk (Gilbert 2009).
Other potential sources of bias
We assessed 11 studies as having low risk of bias for other potential sources of bias based on genotype frequencies (in study and polymorphism), potential deviation from Hardy‐Weinberg equilibrium, source of DNA (e.g. buccal swab, blood, saliva), genotyping protocol, and QC methods (Ahluwalia 2006; Aveyard 2007; Cox 2012; Hall 2008; Hall 2009; Lerman 2002; Lerman 2004; Lerman 2015; Marteau 2012; Piper 2009; Wagena 2005). For three studies, we were unable to assess the risk of other biases (Piper 2007; Swan 2010; Winst 2006). For four studies, we considered the risk of other bias to be high because a large proportion of original trial participants were not genotyped, thereby opening the possibility of selection bias, or because relapsers were excluded from analyses, which may have created study population imbalance for genetic predisposition to successfully quit smoking (Brown 2007; Gilbert 2009; GPRG 1993; McCarthy 2008).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7
Summary of findings for the main comparison. Active NRT compared with placebo ‐ rs1051730 ‐ non‐Hispanic white for smoking cessation.
| Active NRT compared with placebo ‐ rs1051730 ‐ non‐Hispanic white for smoking cessationa | ||||||
| Patient or population: people who smoke Setting: community and healthcare settings Intervention: active NRT Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with active NRT ‐ rs1051730 ‐ non‐Hispanic white | Risk with placebo | |||||
| Abstinence at end of treatment | Study population | RR 1.63 (1.14 to 2.32) | 1391 (2 RCTs) | ⊕⊕⊕⊝ MODERATEb,c | Pooled result across studies, including all genotypes. Between‐genotype group heterogeneity P value = 0.004 (see results for individual subgroups in below rows) | |
| 327 per 1000 (228 to 465) | 200 per 1000 | |||||
| Abstinence at end of treatment ‐ homozygous major | Study population | RR 1.09 (0.85 to 1.41) | 582 (2 RCTs) | ⊕⊕⊝⊝ LOWc,d | For participants with homozygous major genotype, low‐quality evidence suggests no effect. | |
| 286 per 1000 (223 to 370) | 263 per 1000 | |||||
| Abstinence at end of treatment ‐ heterozygous | Study population | RR 2.13 (1.52 to 2.97) | 631 (2 RCTs) | ⊕⊕⊕⊝ MODERATEc | For participants with heterozygous genotype, moderate‐quality evidence shows effect in favour intervention. | |
| 335 per 1000 (239 to 467) | 157 per 1000 | |||||
| Abstinence at end of treatment ‐ homozygous minor | Study population | RR 2.18 (1.04 to 4.58) | 178 (2 RCTs) | ⊕⊕⊝⊝ LOWc,e | For participants with homozygous minor genotype, low‐quality evidence shows effect in favour intervention. | |
| 338 per 1000 (161 to 710) | 155 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NRT: nicotine replacement therapy; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aThis is the first of seven 'Summary of findings' tables for the main comparison. For a complete list, see the 'Additional summary of findings' section.
bNot downgraded owing to inconsistency, as large statistical heterogeneity can be explained by differences between genotypes.
cDowngraded one level owing to risk of bias: high potential for selection bias in GRPG 1993 due to low fraction of genotyped participants.
dDowngraded one level owing to imprecision: optimal information size criterion is met, but 95% CIs include the null effect and fail to exclude important benefit or important harm.
eDowngraded one level owing to imprecision: optimal information size criterion is not met in this stratum. Counts in this stratum are smaller than in a single adequately powered trial.
Summary of findings 2. Active NRT compared with placebo ‐ rs16969968 ‐ non‐Hispanic white for smoking cessation.
| Active NRT compared with placebo ‐ rs16969968 ‐ non‐Hispanic white for smoking cessation | ||||||
| Patient or population: people who smoke Setting: community and healthcare settings Intervention: active NRT Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo ‐ rs16969968 ‐ non‐Hispanic white | Risk with active NRT | |||||
| End of treatment | Study population | RR 1.38 (0.97 to 1.98) | 1127 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa,b | Pooled result across studies, including all genotypes. Between‐genotype group heterogeneity P value = 0.03 (see results for individual subgroups in below rows) | |
| 251 per 1000 | 346 per 1000 (243 to 496) | |||||
| End of treatment ‐ homozygous major | Study population | RR 1.01 (0.77 to 1.33) | 449 (2 RCTs) | ⊕⊕⊝⊝ LOWa,c | For participants with homozygous major genotype, low‐quality evidence suggests no effect. | |
| 333 per 1000 | 337 per 1000 (257 to 443) | |||||
| End of treatment ‐ heterozygous | Study population | RR 1.85 (1.33 to 2.59) | 550 (2 RCTs) | ⊕⊕⊕⊕ HIGH | For participants with heterozygous genotype, high‐quality evidence shows effect in favour of intervention. | |
| 193 per 1000 | 358 per 1000 (257 to 501) | |||||
| End of treatment ‐ homozygous minor | Study population | RR 1.80 (0.45 to 7.23) | 128 (2 RCTs) | ⊕⊕⊝⊝ LOWd | For participants with homozygous minor genotype, low‐quality evidence shows that point estimate favours intervention, but 95% CI crosses null effect. | |
| 233 per 1000 | 420 per 1000 (105 to 1000) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NRT: nicotine replacement therapy; RCT, randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level owing to imprecision: Optimal information size criterion is met, but 95% CIs include the null effect and fail to exclude important benefit or important harm.
bNot downgraded owing to inconsistency, as large statistical heterogeneity can be explained by differences in genotypes.
cDowngraded one level owing to inconsistency: unexplained heterogeneity.
dDowngraded two levels owing to serious imprecision: optimal information size criterion not met, and 95% CIs include the null effect and fail to exclude important benefit or important harm.
Summary of findings 3. Active NRT compared with placebo ‐ rs16969968 ‐ non‐Hispanic black or African American for smoking cessation.
| Active NRT compared with placebo ‐ rs16969968 ‐ non‐Hispanic black or African American for smoking cessation | ||||||
| Patient or population: people who smoke Setting: community and healthcare settings Intervention: active NRT Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with active NRT ‐ rs16969968 ‐ non‐Hispanic black or African American | Risk with placebo | |||||
| Abstinence at 6 months | Study population | RR 1.11 (0.55 to 2.26) | 709 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa,b | Pooled result across studies, including all genotypes. Between‐genotype group heterogeneity P value = 0.03 (see results for individual subgroups in below rows) | |
| 203 per 1000 (101 to 414) | 183 per 1000 | |||||
| Abstinence at 6 months ‐ homozygous major | Study population | RR 1.47 (1.07 to 2.03) | 637 (2 RCTs) | ⊕⊕⊕⊕ HIGH | For participants with homozygous major genotype, high‐quality evidence shows effect in favour of intervention. | |
| 254 per 1000 (185 to 350) | 173 per 1000 | |||||
| Abstinence at 6 months ‐ heterozygous or homozygous minor | Study population | RR 0.43 (0.15 to 1.26) | 72 (2 RCTs) | ⊕⊕⊝⊝ LOWc | For participants with heterozygous or homozygous minor genotype, point estimate favours control, but 95% CI crosses null effect. | |
| 117 per 1000 (41 to 344) | 273 per 1000 | |||||
| Abstinence at end of treatment | Study population | RR 1.03 (0.36 to 2.94) | 709 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa,b | Pooled result across studies, including all genotypes. Between‐genotype group heterogeneity P value = 0.003 (see results for individual subgroups in below rows) | |
| 201 per 1000 (70 to 575) | 196 per 1000 | |||||
| Abstinence at end of treatment ‐ homozygous major | Study population | RR 1.57 (1.15 to 2.15) | 637 (2 RCTs) | ⊕⊕⊕⊕ HIGH | For participants with homozygous major genotype, high‐quality evidence shows effect in favour of intervention. | |
| 276 per 1000 (202 to 379) | 176 per 1000 | |||||
| Abstinence at end of treatment ‐ heterozygous or homozygous minor | Study population | RR 0.29 (0.10 to 0.86) | 72 (2 RCTs) | ⊕⊕⊕⊝ MODERATEd | For participants with heterozygous or homozygous minor genotype, moderate‐quality evidence shows effect in favour of control. | |
| 105 per 1000 (36 to 313) | 364 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NRT: nicotine replacement therapy; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aNot downgraded owing to inconsistency, as large statistical heterogeneity can be explained by differences in genotypes.
bDowngraded one level owing to imprecision: Optimal information size criterion is met, but 95% CIs include the null effect and fail to exclude important benefit or important harm.
cDowngraded two levels owing to serious imprecision: optimal information size criterion not met, but 95% CIs include the null effect and fail to exclude important benefit or important harm.
dDowngraded one level owing to imprecision: low number of events; optimal information size criterion not met.
Summary of findings 4. Active NRT compared with placebo ‐ rs 588765 ‐ non‐Hispanic white for smoking cessation.
| Active NRT compared with placebo ‐ rs 588765 ‐ non‐Hispanic white for smoking cessation | ||||||
| Patient or population: people who smoke Setting: community and healthcare settings Intervention: active NRT Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo ‐ rs 588765 ‐ non‐Hispanic white | Risk with active NRT | |||||
| End of treatment | Study population | RR 1.33 (1.04 to 1.71) | 923 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa | Pooled result across studies, including all genotypes. Between‐genotype group heterogeneity P value = 0.92 (see results for individual subgroups in below rows) | |
| 211 per 1000 | 281 per 1000 (220 to 361) | |||||
| End of treatment ‐ homozygous major | Study population | RR 1.39 (0.89 to 2.16) | 296 (2 RCTs) | ⊕⊕⊝⊝ LOWb | For participants with homozygous major genotype, low‐quality evidence suggests that point estimate favours intervention, but 95% CI crosses null effect. | |
| 208 per 1000 | 288 per 1000 (185 to 448) | |||||
| End of treatment ‐ heterozygous | Study population | RR 1.27 (0.89 to 1.79) | 469 (2 RCTs) | ⊕⊕⊝⊝ LOWb | For participants with heterozygous genotype, point estimate favours intervention, but 95% CI crosses null effect. | |
| 208 per 1000 | 265 per 1000 (185 to 373) | |||||
| End of treatment ‐ homozygous minor | Study population | RR 1.50 (0.74 to 3.06) | 158 (2 RCTs) | ⊕⊕⊝⊝ LOWb | For participants with homozygous minor genotype, low‐quality evidence suggests that point estimate favours intervention, but 95% CI crosses null effect. | |
| 226 per 1000 | 339 per 1000 (167 to 691) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NRT: nicotine replacement therapy; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level owing to imprecision: low number of events; optimal information size criterion not met.
bDowngraded two levels owing to serious imprecision: optimal information size criterion not met, but 95% CIs include the null effect and fail to exclude important benefit or important harm.
Summary of findings 5. Active NRT compared with placebo ‐ rs2036527 ‐ non‐Hispanic black or African American for smoking cessation.
| Active NRT compared with placebo ‐ rs2036527 ‐ non‐Hispanic black or African American for smoking cessation | ||||||
| Patient or population: people who smoke Setting: community and healthcare settings Intervention: active NRT Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo ‐ rs2036527 ‐ non‐Hispanic black or African American | Risk with active NRT | |||||
| 6‐Month abstinence | Study population | RR 1.18 (0.75 to 1.87) | 708 (2 RCTs) | ⊕⊕⊝⊝ LOWa | Pooled result across studies, including all genotypes. Between‐genotype group heterogeneity P value = 0.19 (see results for individual subgroups in below rows) | |
| 177 per 1000 | 208 per 1000 (132 to 330) | |||||
| 6‐Month abstinence ‐ homozygous major | Study population | RR 1.95 (0.46 to 8.26) | 425 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b | For participants with homozygous major genotype, very low‐quality evidence suggests that point estimate favours intervention, but 95% CI crosses null effect. | |
| 171 per 1000 | 333 per 1000 (79 to 1000) | |||||
| 6‐Month abstinence ‐ heterozygous or homozygous minor | Study population | RR 0.90 (0.40 to 2.03) | 283 (2 RCTs) | ⊕⊕⊕⊝ MODERATEc | For participants with heterozygous or homozygous minor genotype, moderate‐quality evidence shows no effect. | |
| 185 per 1000 | 167 per 1000 (74 to 377) | |||||
| End of treatment | Study population | RR 1.22 (0.77 to 1.93) | 708 (2 RCTs) | ⊕⊕⊝⊝ LOWa | Pooled results across studies, including all genotypes. Between‐genotype group heterogeneity P value = 0.19 (see results for individual subgroups in below rows) | |
| 199 per 1000 | 242 per 1000 (153 to 384) | |||||
| End of treatment ‐ homozygous major | Study population | RR 2.39 (0.43 to 13.35) | 425 (2 RCTs) | ⊕⊕⊝⊝ LOWb,c | For participants with homozygous major genotype, low‐quality evidence suggests that point estimate favours intervention, but 95% CI crosses null effect. | |
| 197 per 1000 | 471 per 1000 (85 to 1000) | |||||
| End of treatment ‐ heterozygous or homozygous minor | Study population | RR 0.89 (0.55 to 1.46) | 283 (2 RCTs) | ⊕⊕⊕⊝ MODERATEc | For participants with heterozygous or homozygous minor genotype, moderate‐quality evidence shows no effect. | |
| 202 per 1000 | 179 per 1000 (111 to 294) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NRT: nicotine replacement therapy; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
cDowngraded one level owing to imprecision: low number of events; optimal information size criterion not met.
Summary of findings 6. Active NRT compared with placebo ‐ DRD4 (exon 3 48 bp) ‐ non‐Hispanic white for smoking cessation.
| Active NRT compared with placebo ‐ DRD4 (exon 3 48 bp) ‐ non‐Hispanic white for smoking cessation | ||||||
| Patient or population: people who smoke Setting: community and healthcare settings Intervention: active NRT Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo ‐ DRD4 (exon 3 48 bp) ‐ non‐Hispanic white | Risk with active NRT | |||||
| 6‐Month abstinence | Study population | RR 1.44 (1.08 to 1.92) | 900 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | Pooled result across studies, including all genotypes. Between‐genotype group heterogeneity P value = 0.85 (see results for individual subgroups in below rows) | |
| 141 per 1000 | 203 per 1000 (152 to 271) | |||||
| 6‐month abstinence ‐ homozygous major | Study population | RR 1.34 (0.93 to 1.93) | 566 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | For participants with homozygous major genotype, very low‐quality evidence suggests that point estimate favours intervention, but 95% CI crosses null effect. | |
| 155 per 1000 | 207 per 1000 (144 to 299) | |||||
| 6‐Month abstinence ‐ heterozygous | Study population | RR 1.76 (1.00 to 3.10) | 290 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | For participants with heterozygous genotype, low‐quality evidence suggests effect in favour of intervention. | |
| 113 per 1000 | 198 per 1000 (113 to 349) | |||||
| 6‐Month abstinence ‐ homozygous minor | Study population | RR 1.28 (0.40 to 4.11) | 44 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | For participants with homozygous minor genotype, very low‐quality evidence suggests that point estimate favours intervention, but 95% CI crosses null effect. | |
| 167 per 1000 | 213 per 1000 (67 to 685) | |||||
| End of treatment | Study population | RR 1.20 (0.88 to 1.63) | 900 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | Pooled result across studies, including all genotypes. Between‐genotype group heterogeneity P value = 0.78 (see results for individual subgroups in below rows) | |
| 271 per 1000 | 325 per 1000 (238 to 441) | |||||
| End of treatment ‐ homozygous major | Study population | RR 1.22 (0.59 to 2.51) | 566 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | For participants with homozygous major genotype, very low‐quality evidence suggests that point estimate favours intervention, but 95% CI crosses null effect. | |
| 273 per 1000 | 334 per 1000 (161 to 686) | |||||
| End of treatment ‐ heterozygous | Study population | RR 1.40 (0.57 to 3.39) | 290 (2 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | For participants with heterozygous genotype, very low‐quality evidence suggests that point estimate favours intervention, but 95% CI crosses null effect. | |
| 258 per 1000 | 362 per 1000 (147 to 876) | |||||
| End of treatment ‐ homozygous minor | Study population | RR 1.02 (0.65 to 1.58) | 44 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | For participants with homozygous minor genotype, low‐quality evidence suggests no effect. | |
| 333 per 1000 | 340 per 1000 (217 to 527) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NRT: nicotine replacement therapy; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level owing to high risk of bias: high risk of selection bias in GRPG 1993 due to low fraction of genotyped participants.
bDowngraded one level owing to imprecision: low number of events; optimal information size criterion not met.
cDowngraded one level owing to imprecision: 95% CIs include the null effect and fail to exclude important benefit or important harm.
Summary of findings 7. Active NRT compared with placebo ‐ NMR ‐ non‐Hispanic black and white for smoking cessation.
| Active NRT compared with placebo ‐ NMR ‐ non‐Hispanic black and white for smoking cessation | ||||||
| Patient or population: people who smoke Setting: community and healthcare settings Intervention: active NRT Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo ‐ NMR ‐ non‐Hispanic black and white | Risk with active NRT | |||||
| 6‐Month abstinence | Study population | RR 1.51 (1.08 to 2.10) | 1417 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa | Pooled result across studies, including all genotypes. Between‐genotype group heterogeneity: P value = 0.29 (see results for individual subgroups in below rows) | |
| 99 per 1000 | 149 per 1000 (107 to 207) | |||||
| 6‐Month abstinence ‐ slow NMR | Study population | RR 1.82 (1.12 to 2.94) | 628 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa | For participants with slow NMR genotype, moderate‐quality evidence favours intervention. | |
| 116 per 1000 | 211 per 1000 (130 to 341) | |||||
| 6‐Month abstinence ‐ normal NMR | Study population | RR 1.21 (0.78 to 1.87) | 789 (2 RCTs) | ⊕⊕⊝⊝ LOWb | For participants with normal NMR genotype, point estimate favours intervention, but 95% CI crosses null effect. | |
| 85 per 1000 | 103 per 1000 (66 to 159) | |||||
| End of treatment | Study population | RR 1.51 (1.19 to 1.90) | 1417 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa | Pooled result across studies, including all genotypes. Between‐genotype group heterogeneity: P value = 0.52 (see results for individual subgroups in below rows) | |
| 136 per 1000 | 205 per 1000 (162 to 258) | |||||
| End of treatment ‐ slow NMR | Study population | RR 1.61 (1.18 to 2.20) | 847 (2 RCTs) | ⊕⊕⊕⊝ MODERATEa | For participants with slow NMR genotype, moderate‐quality evidence favours intervention. | |
| 127 per 1000 | 204 per 1000 (149 to 278) | |||||
| End of treatment ‐ normal NMR | Study population | RR 1.49 (0.85 to 2.60) | 570 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | For participants with normal NMR genotype, point estimate favours intervention, but 95% CI crosses null effect. | |
| 149 per 1000 | 222 per 1000 (127 to 388) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NMR: nicotine metabolite ratio; NRT: nicotine replacement therapy; RCT: randomised controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level owing to risk of bias: 755/1532 (49%) participants from Patch Trial (GPRG 1993) were genotyped, which could contribute to selection bias.
bDowngraded one level owing to imprecision: optimal information size criterion is met, but 95% CIs include the null effect and fail to exclude important benefit or important harm.
Results are presented according to type of analysis (comparisons of relative treatment effect by genotype, or comparisons of treatment effect between genotype groups, within pharmacotherapy randomisation arms) and type of treatment (comparison). Table 10, Table 11, Table 12, and Table 13 show the summary results for each type of analysis with separate tables for six‐month abstinence and abstinence at the end of treatment.
3. Data availability per treatment comparison for the outcome 6‐month abstinence.
| Treatment comparison | SNP or VNTRa | Ethnicity | Number of studies (individual treatment 1/treatment 2) included | Analysis number |
Significant treatment effect Letter indicates which treatment is favoured: a = active NRT b = bupropion ba = bupropion + active NRT p = placebo v = varenicline |
P value heterogeneity genotype groups | |||
| Active NRT vs placebo | O | HoMa | Het | HoMi | |||||
| rs1051730 | NHW | 3 (1023/574) | 1.1 | a | a | a | 0.40 | ||
| rs16969968 | NHB | 2 (392/317) | 3.1 | a | 0.03a | ||||
| rs2036527 | NHB | 2 (391/317) | 5.1 | 0.36a | |||||
| DRD4 (exon 3 48 bp) | NHW | 2 (453/447) | 6.1 | a | a | 0.72 | |||
| O | S | N | |||||||
| NMR | NHW or NHB | 2 (719/699) | 7.1 | a | a | 0.22 | |||
| Bupropion vs placebo | O | HoMa | Het | HoMi | |||||
| rs1051730 | NHW | 4 (797/532) | 8.1 | b | b | 0.77 | |||
| NHB | 3 (63/63) | 9.1 | 0.80 | ||||||
| rs16969968 | NHB | 2 (294/284) | 11.1 | 0.22a | |||||
| rs588765 | NHW | 4 (596/511) | 12.1 | b | b | 0.32 | |||
| rs2036527 | NHW | 2 (412/326) | 13.1 | b | b | 0.28 | |||
| NHB | 3 (331/329) | 14.1 | 0.65 | ||||||
| rs3733829 | NHW | 2 (307/264) | 15.1 | b | b | 0.25 | |||
Het: heterozygous; HoMa: homozygous major; HoMi: homozygous minor; N: normal NMR; NHB: non‐Hispanic black or African American; NHW: non‐Hispanic white; NMR: nicotine metabolite ratio; NRT: nicotine replacement therapy; O = overall; SNP: single‐nucleotide polymorphism; VNTR: variable number tandem repeat.
aHomozygous major vs heterozygous + homozygous minor.
4. Data availability per treatment comparison for the outcome abstinence at end of treatment.
| Treatment comparison | SNP or VNTRa | Ethnicity | Number of studies (individual treatment 1/treatment 2) included | Analysis number |
Significant treatment effect Letter indicates which treatment is favoured: a = active NRT b = bupropion ba = bupropion + active NRT p = placebo v = varenicline |
P value heterogeneity genotype groups | |||
| Active NRT vs placebo | O | HoMa | Het | HoMi | |||||
| rs1051730 | NHW | 2 (912/479) | 1.2 | a | a | a | 0.004 | ||
| rs16969968 | NHW | 2 (784/343) | 2.1 | a | 0.02 | ||||
| NHB | 2 (392/317) | 3.2 | a | pb | 0.003a | ||||
| rs588765 | NHW | 2 (587/336) | 4.1 | a | 0.89 | ||||
| rs2036527 | NHB | 2 (391/317) | 5.2 | 0.28a | |||||
| DRD4 (exon 3 48 bp) | NHW | 2 (453/447) | 6.2 | 0.79 | |||||
| O | S | N | |||||||
| NMR | NHW or NHB | 2 (718/699) | 7.2 | a | a | 0.80 | |||
| Bupropion vs placebo | O | HoMa | Het | HoMi | |||||
| rs1051730 | NHW | 6 (743/636) | 8.2 | b | b | b | b | 0.41 | |
| NHB | 3 (84/75) | 9.2 | 0.12 | ||||||
| rs16969968 | NHW | 3 (324/233) | 10.1 | b | b | 0.50 | |||
| NHB | 3 (315/296) | 11.2 | b | bb | 0.77a | ||||
| rs588765 | NHW | 4 (595/512) | 12.2 | b | b | 0.96 | |||
| rs2036527 | NHW | 4 (546/429) | 13.2 | b | b | b | b | 0.20 | |
| NHB | 4 (352/341) | 14.2 | b | b | 0.38 | ||||
| rs3733829 | NHW | 4 (440/367) | 15.2 | b | b | b | 0.71 | ||
| NHB | 2 (46/29) | 16.1 | 0.56a | ||||||
| rs7937 | NHW | 3 (324/233) | 17.1 | b | b | 0.54 | |||
| NHB | 2 (47/29) | 18.1 | 0.92a | ||||||
| rs1329650 | NHW | 3 (324/235) | 19.1 | b | b | 0.83 | |||
| NHB | 2 (47/29) | 20.1 | 0.59a | ||||||
| rs1028936 | NHW | 3 (324/235) | 21.1 | b | b | 0.90 | |||
| NHB | 2 (47/29) | 22.1 | 0.37a | ||||||
| rs215605 | NHW | 3 (324/234) | 23.1 | b | b | 0.43 | |||
| NHB | 2 (47/29) | 24.1 | 0.92a | ||||||
| Bupropion + any NRT vs placebo | O | HoMa | Het | HoMi | |||||
| rs1051730 | NHW | 2 (268/176) | 25.1 | ba | ba | 0.77 | |||
| NHB | 2 (40/29) | 26.1 | 0.07a | ||||||
| rs16969968 | NHW | 2 (266/175) | 27.1 | ba | ba | 0.77 | |||
| NHB | 2 (40/29) | 28.1 | 0.35a | ||||||
| rs2036527 | NHW | 2 (267/176) | 29.1 | ba | ba | 0.95 | |||
| NHB | 2 (40/29) | 30.1 | 0.59 | ||||||
| rs3733829 | NHW | 2 (266/175) | 31.1 | ba | ba | 0.83 | |||
| NHB | 2 (48/21[ES1] ) | 32.1 | 0.45a | ||||||
| rs7937 | NHW | 2 (268/174) | 33.1 | ba | 0.62 | ||||
| NHB | 2 (40/29) | 34.1 | 0.98a | ||||||
| rs1329650 | NHW | 2 (266/176) | 35.1 | ba | ba | 0.90 | |||
| NHB | 2 (40/29) | 36.1 | 0.78a | ||||||
| rs1028936 | NHW | 2 (268/176) | 37.1 | ba | ba | 0.88 | |||
| NHB | 2 (40/29) | 38.1 | 0.89a | ||||||
| rs215605 | NHW | 2 (267/175) | 39.1 | ba | ba | 0.79 | |||
| NHB | 2 (40/28) | 40.1 | 0.52a | ||||||
| Bupropion + any NRT vs bupropion | O | HoMa | Het | HoMi | |||||
| rs1051730 | NHW | 2 (268/265) | 41.1 | 0.97 | |||||
| NHB | 2 (40/47) | 42.1 | 0.41a | ||||||
| rs16969968 | NHW | 2 (266/265) | 43.1 | 0.98 | |||||
| NHB | 2 (40/47) | 44.1 | 0.57a | ||||||
| rs2036527 | NHW | 2 (267/265) | 45.1 | 0.99 | |||||
| NHB | 2 (40/47) | 46.1 | 0.61a | ||||||
| rs3733829 | NHW | 2 (267/264) | 47.1 | 0.82 | |||||
| NHB | 2 (48/46) | 48.1 | b | 0.58a | |||||
| rs7937 | NHW | 2 (268/265) | 49.1 | 0.69 | |||||
| NHB | 2 (40/47) | 50.1 | 0.85a | ||||||
| rs1329650 | NHW | 2 (266/265) | 51.1 | 0.66 | |||||
| NHB | 2 (40/47) | 52.1 | 0.49a | ||||||
| rs1028936 | NHW | 2 (268/265) | 53.1 | 0.60 | |||||
| NHB | 2 (40/47) | 54.1 | 0.49a | ||||||
| rs215605 | NHW | 2 (267/265) | 55.1 | 0.50 | |||||
| NHB | 2 (40/47) | 56.1 | 0.75a | ||||||
Het: heterozygous; HoMa: homozygous major; HoMi: homozygous minor; N: normal NMR; NHB: non‐Hispanic black or African American; NHW: non‐Hispanic white; NMR: nicotine metabolite ratio; NRT: nicotine replacement therapy; O: overall; SNP: single‐nucleotide polymorphism; VNTR: variable number tandem repeat.
aHomozygous major vs heterozygous + homozygous minor.
bHeterozygous + homozygous minor.
5. Data availability per treatment group for the outcome 6‐month abstinence.
| Treatment group | SNP or VNTRa | Ethnicity | Number of studies (individuals in homozygous major/heterozygous/homozygous minor) included | Analysis number |
Significant treatment effect Letter indicates which genotype is favoured: ma = genotype group with 1 or more major alleles mi = homozygous minor s = slow NMR n = normal NMR |
P value heterogeneity in genotype comparisons | |
| Active NRT | MaMi | HetMi | |||||
| rs1051730 | NHW | 7 (1352/1025/310) | 57.1 | 0.93 | |||
| NHB | 2 (181/50/6) | 58.1 | 0.98 | ||||
| rs16969968 | NHB | 2 (353/39a) | 59.1 | N/A | |||
| rs588765 | NHW | 3 (286/399/147) | 60.1 | 0.76 | |||
| rs2036527 | NHW | 2 (369/447/132) | 61.1 | 0.79 | |||
| NHB | 2 (232/159a) | 62.1 | N/A | ||||
| DRD4 (exon 3 48 bp) | NHW | 3 (798/253/68) | 63.1 | 0.74 | |||
| SLC6A4 (Promoter) | NHW | 2 (277/465/184) | 64.1 | 0.62 | |||
| Normal vs slow | |||||||
| NMR | NHW and NHB | 2 (400/318) | 65.1 | s | N/A | ||
| Bupropion | |||||||
| rs1051730 | NHW | 4 (220/294/96) | 66.1 | 0.82 | |||
| NHB | 2 (43/20a) | 67.1 | N/A | ||||
| rs16969968 | NHB | 2 (261/33a) | 69.1 | N/A | |||
| rs588765 | NHW | 4 (195/292/109) | 70.1 | 0.80 | |||
| rs2036527 | NHW | 2 (143/204/65) | 71.1 | 0.87 | |||
| NHB | 3 (187/144a) | 72.1 | N/A | ||||
| rs3733829 | NHW | 2 (146/116/45) | 73.1 | 0.65 | |||
| Varenicline | |||||||
| rs16969968 | NHW | 2 (269/349/89) | 83.1 | 0.81 | |||
| rs588765 | NHW | 2 (234/345/120) | 84.1 | 0.90 | |||
| Placebo | |||||||
| rs1051730 | NHW | 6 (377/441/156) | 101.1 | 0.54 | |||
| NHB | 2 (49/14a) | 102.1 | N/A | ||||
| rs16969968 | NHW | 2 (132/181/30) | 103.1 | 0.63 | |||
| NHB | 3 (519/65a) | 104.1 | N/A | ||||
| rs588765 | NHW | 5 (253/340/130) | 105.1 | 0.92 | |||
| NHB | 2 (275/291a) | 106.1 | N/A | ||||
| rs2036527 | NHW | 2 (117/166/43) | 107.1 | 0.81 | |||
| NHB | 4 (373/256a) | 108.1 | N/A | ||||
| DRD4 (exon 3 48 bp) | NHW | 3 (361/186/26) | 109.1 | 0.44 | |||
| rs3733829 | NHW | 2 (122/102/40) | 110.1 | 0.60 | |||
| SLC6A4 (Promoter) | NHW | 2 (128/23a) | 119.1 | N/A | |||
| Normal vs slow | |||||||
| NMR | NHW and NHB | 2 (389/310) | 120.1 | N/A | |||
HetMi: heterozygous vs homozygous minor; MaMi: homozygous major vs homozygous minor; NHB: non‐Hispanic black or African American; NHW: non‐Hispanic white; NMR: nicotine metabolite ratio; NRT: nicotine replacement therapy; NS: normal NMR vs slow NMR; SNP: single‐nucleotide polymorphism; VNTR: variable number tandem repeat.
aHeterozygous and homozygous minor combined.
6. Data availability per treatment group for the outcome abstinence at end of treatment.
| Treatment group | SNP or VNTRa | Ethnicity | Number of studies (individuals in homozygous major/heterozygous/homozygous minor) included | Analysis number |
Significant treatment effect Letter indicates which genotype is favored: ma = genotype group with 1 or more major alleles mi = homozygous minor s = slow NMR n = normal NMR |
P value heterogeneity in genotype comparisons | |
| Active NRT | MaMi | HetMi | |||||
| rs1051730 | NHW | 5 (917/1008/283) | 57.2 | 0.64 | |||
| rs16969968 | NHB | 2 (353/39a) | 59.2 | mab | N/A | ||
| rs588765 | NHW | 3 (286/399/147) | 60.2 | 0.97 | |||
| rs2036527 | NHW | 2 (369/447/132) | 61.2 | 0.74 | |||
| NHB | 2 (232/159a) | 62.2 | mab | N/A | |||
| DRD4 (exon 3 48 bp) | NHW | 3 (798/253/68) | 63.2 | 0.72 | |||
| SLC6A4 (Promoter) | NHW | 2 (277/465/184) | 64.2 | 0.83 | |||
| Normal vs slow | |||||||
| NMR | NHW and NHB | 2 (400/318) | 65.2 | N/A | |||
| Bupropion | |||||||
| rs1051730 | NHW | 6 (263/357/123) | 66.2 | 0.59 | |||
| NHB | 3 (58/26a) | 67.2 | N/A | ||||
| rs16969968 | NHW | 3 (108/164/52) | 68.1 | 0.51 | |||
| NHB | 3 (279/36a) | 69.2 | N/A | ||||
| rs588765 | NHW | 4 (195/292/109) | 70.2 | 0.65 | |||
| rs2036527 | NHW | 4 (185/269/92) | 71.2 | 0.53 | |||
| NHB | 4 (200/152a) | 72.2 | N/A | ||||
| rs3733829 | NHW | 4 (203/181/56) | 73.2 | 0.87 | |||
| NHB | 2 (35/11a) | 74.1 | N/A | ||||
| rs7937 | NHW | 3 (86/165/73) | 75.1 | 0.66 | |||
| NHB | 2 (20/24/3) | 76.1 | 0.62 | ||||
| rs1329650 | NHW | 3 (161/138/25) | 77.1 | 0.90 | |||
| NHB | 2 (42/5a) | 78.1 | N/A | ||||
| rs1028936 | NHW | 3 (212/103/9) | 79.1 | 1.00 | |||
| NHB | 2 (42/5a) | 80.1 | N/A | ||||
| rs215605 | NHW | 3 (125/154/45) | 81.1 | 0.94 | |||
| NHB | 2 (31/16a) | 82.1 | N/A | ||||
| Varenicline | |||||||
| rs16969968 | NHW | 2 (269/349/89) | 83.2 | 0.94 | |||
| rs588765 | NHW | 2 (234/345/120) | 84.2 | 0.84 | |||
| Bupropion + any NRT | |||||||
| rs1051730 | NHW | 2 (113/130/25) | 85.1 | 0.96 | |||
| NHB | 2 (33/7a) | 86.1 | N/A | ||||
| rs16969968 | NHW | 2 (113/130/23) | 87.1 | 0.98 | |||
| NHB | 2 (36/4a) | 88.1 | N/A | ||||
| rs2036527 | NHW | 2 (111/132/24) | 89.1 | 0.82 | |||
| NHB | 2 (23/12/5) | 90.1 | 0.87 | ||||
| rs3733829 | NHW | 2 (114/123/30) | 91.1 | 0.52 | |||
| NHB | 2 (30/10a) | 92.1 | mib | N/A | |||
| rs7937 | NHW | 2 (74/140/54) | 93.1 | 0.95 | |||
| NHB | 2 (13/27a) | 94.1 | N/A | ||||
| rs1329650 | NHW | 2 (149/96/21) | 95.1 | mi | 0.67 | ||
| NHB | 2 (34/6a) | 96.1 | N/A | ||||
| rs1028936 | NHW | 2 (183/75/10) | 97.1 | 0.67 | |||
| NHB | 2 (34/6a) | 98.1 | N/A | ||||
| rs215605 | NHW | 2 (105/119/43) | 99.1 | 0.25 | |||
| NHB | 2 (18/22a) | 100.1 | |||||
| Placebo | |||||||
| rs1051730 | NHW | 7 (378/441/164) | 101.2 | 0.71 | |||
| NHB | 3 (63/20a) | 102.2 | N/A | ||||
| rs16969968 | NHW | 4 (169/227/49) | 103.2 | 0.83 | |||
| NHB | 4 (530/66a) | 104.2 | mib | N/A | |||
| rs588765 | NHW | 5 (254/296/108) | 105.2 | 0.34 | |||
| NHB | 2 (277/289a) | 106.2 | N/A | ||||
| rs2036527 | NHW | 4 (154/213/62) | 107.2 | 0.90 | |||
| NHB | 5 (378/263a) | 108.2 | N/A | ||||
| DRD4 (exon 3 48 bp) | NHW | 3 (361/186/26) | 109.2 | 0.65 | |||
| rs3733829 | NHW | 4 (164/152/51) | 110.2 | 0.53 | |||
| rs7937 | NHW | 3 (73/120/40) | 111.1 | 0.67 | |||
| NHB | 2 (18/11a) | 112.1 | N/A | ||||
| rs1329650 | NHW | 3 (119/106/10) | 113.1 | 0.99 | |||
| NHB | 2 (21/8a) | 114.1 | N/A | ||||
| rs1028936 | NHW | 3 (158/72/5) | 115.1 | 0.79 | |||
| NHB | 2 (23/6a) | 116.1 | N/A | ||||
| rs215605 | NHW | 3 (94/105/35) | 117.1 | 0.70 | |||
| NHB | 2 (18/11a) | 118.1 | N/A | ||||
| SLC6A4 (Promoter) | NHW | 2 (128/23a) | 119.2 | N/A | |||
| Normal vs slow | |||||||
| NMR | NHW and NHB | 2 (400/318) | 120.2 | N/A | |||
HetMi: heterozygous vs homozygous minor; MaMi: homozygous major vs homozygous minor; NHB: non‐Hispanic black or African American; NHW: non‐Hispanic white; NS: normal NMR vs slow NMR; SNP: single‐nucleotide polymorphism; VNTR: variable number tandem repeat; NMR = nicotine metabolite ratio.
aHeterozygous and homozygous minor combined.
bHomozygous major vs heterozygous and homozygous minor.
Comparisons of relative treatment effects by genotype
Active NRT versus placebo
Six‐month abstinence
Data were available for the following SNPs: rs1051730 (n = 3, 1597 participants, NHW; Analysis 1.1), rs16969968 (n = 2, 709 participants, NHB; Analysis 3.1), rs2036527 (n = 2, 709 participants, NHB; Analysis 5.1), DRD4 (exon 3 48 bp) (n = 2, 900 participants, NHW; Analysis 6.1), and NMR (n = 2, 1418 participants, NHW or NHB; Analysis 7.1).
1.1. Analysis.
Comparison 1 Active NRT vs placebo ‐ rs1051730 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
3.1. Analysis.
Comparison 3 Active NRT vs placebo ‐ rs16969968 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
5.1. Analysis.
Comparison 5 Active NRT vs placebo ‐ rs2036527 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
6.1. Analysis.
Comparison 6 Active NRT vs placebo ‐ DRD4 (exon 3 48 bp) ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
7.1. Analysis.
Comparison 7 Active NRT vs placebo ‐ NMR ‐ non‐Hispanic black and white, Outcome 1 Six‐Month Abstinence.
Results showed statistically significant heterogeneity between genotype groups in the analysis of NHB for rs16969968 (P = 0.03; Analysis 3.1; Table 3). Six‐month abstinence was increased in the active NRT group as compared with the placebo group among participants with a GG genotype (RR 1.47, 95% CI 1.07 to 2.03), but not in the combined group of those with a GA or AA genotype (RR 0.43, 95% CI 0.15 to 1.26; Analysis 3.1; Table 3; RRR GG vs GA or AA 3.51, 95% CI 1.19 to 10.3). We found no such heterogeneity for any of the other biomarkers.
End‐of‐treatment abstinence
Data were available for the following SNPs: rs1051730 (n = 2, 1391 participants, NHW; Analysis 1.2), rs16969968 (n = 2, 1127 participants, NHW; Analysis 2.1; n = 2, 709 participants, NHB; Analysis 3.2), rs588765 (n = 2, 923 participants, NHW; Analysis 4.1), rs2036527 (n = 2, 708 participants, NHB; Analysis 5.2), DRD4 (exon 3 48 bp) (n = 2, 900 participants, NHW; Analysis 6.2), and NMR (n = 2, 1417 participants, NHW or NHB; Analysis 7.2).
1.2. Analysis.
Comparison 1 Active NRT vs placebo ‐ rs1051730 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
2.1. Analysis.
Comparison 2 Active NRT vs placebo ‐ rs16969968 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
3.2. Analysis.
Comparison 3 Active NRT vs placebo ‐ rs16969968 ‐ non‐Hispanic black or African American, Outcome 2 End of Treatment.
4.1. Analysis.
Comparison 4 Active NRT vs placebo ‐ rs 588765 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
5.2. Analysis.
Comparison 5 Active NRT vs placebo ‐ rs2036527 ‐ non‐Hispanic black or African American, Outcome 2 End of Treatment.
6.2. Analysis.
Comparison 6 Active NRT vs placebo ‐ DRD4 (exon 3 48 bp) ‐ non‐Hispanic white, Outcome 2 End of Treatment.
7.2. Analysis.
Comparison 7 Active NRT vs placebo ‐ NMR ‐ non‐Hispanic black and white, Outcome 2 End of Treatment.
We found statistically significant heterogeneity between genotype groups in the analysis of NHW for rs1051730 (heterogeneity P = 0.004; Analysis 1.2; Table 1) and rs16969968 (heterogeneity P = 0.02; Analysis 2.1; Table 1), and of NHB for rs16969968 (heterogeneity P = 0.003; Analysis 3.2; Table 3). For the rs1051730 analysis in NHW, abstinence at end of treatment was comparable between the active NRT group and the placebo group among participants with a GG genotype (RR 1.09, 95% CI 0.85 to 1.41), but was increased in those with GA or AA genotype (RR 2.13, 95% CI 1.52 to 2.97; and RR 2.18, 95% CI 1.04 to 4.58, respectively; Analysis 1.2; Table 1; RRR GG vs AA and GA vs AA genotype of 0.54, 95% CI 0.26 to 1.14; and 0.96, 95% CI 0.44 to 2.11, respectively). For the rs16969968 analysis in NHW, end‐of‐treatment abstinence increased with active NRT as compared with placebo among individuals with GA genotype (RR 1.85, 95% CI 1.33 to 2.59), but was comparable among those with GG or AA (RR 1.01, 95% CI 0.77 to 1.33; and RR 1.80, 95% CI 0.45 to 7.23, respectively; Analysis 2.1; Table 1; RRR GG vs AA and GA vs AA genotype of 0.78, 95% CI 0.33 to 1.47; and 1.04, 95% CI 0.45 to 2.41, respectively). For rs16969968 in NHB, active NRT increased end‐of‐treatment abstinence compared with placebo among those with GG genotype (RR 1.57, 95% CI 1.15 to 2.15), and it decreased end‐of‐treatment abstinence among those with GA or AA phenotype (RR 0.29, 95% CI 0.10 to 0.86; Analysis 3.2; Table 3; RRR GG vs GA or AA genotype of 5.84, 95% CI 1.89 to 18.1). We found no such heterogeneity for any of the other biomarkers.
Bupropion versus placebo
Six‐month abstinence
Data were available for the following SNPs: rs1051730 (n = 4, 1329 participants, NHW; Analysis 8.1; n = 3, 126 participants, NHB; Analysis 9.1), rs16969968 (n = 2, 578 participants, NHB; Analysis 11.1), rs588765 (n = 4, 1108 participants, NHW; Analysis 12.1), rs2036527 (n = 2, 738 participants, NHW; Analysis 13.1; n = 3, 660 participants, NHB; Analysis 14.1), and rs3733829 (n = 2, 571 participants, NHW; Analysis 15.1).
8.1. Analysis.
Comparison 8 Bupropion vs placebo ‐ rs1051730 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
9.1. Analysis.
Comparison 9 Bupropion vs placebo ‐ rs1051730 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
11.1. Analysis.
Comparison 11 Bupropion vs placebo ‐ rs16969968 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
12.1. Analysis.
Comparison 12 Bupropion vs placebo ‐ rs588765 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
13.1. Analysis.
Comparison 13 Bupropion vs placebo ‐ rs2036527 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
14.1. Analysis.
Comparison 14 Bupropion vs placebo ‐ rs2036527 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
15.1. Analysis.
Comparison 15 Bupropion vs placebo ‐ rs3733829 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
We found no heterogeneity between genotype groups when comparing bupropion versus placebo for any of the biomarkers.
End‐of‐treatment abstinence
Data were available for the following SNPs: rs1051730 (n = 6, 1379 participants, NHW; Analysis 8.2; n = 3, 159 participants, NHB; Analysis 9.2), rs16969968 (n = 3, 557 participants, NHW; Analysis 10.1; n = 3, 611 participants, NHB; Analysis 11.2), rs588765 (n = 4, 1107 participants, NHW; Analysis 12.2), rs2036527 (n = 4, 975 participants, NHW; Analysis 13.2; n = 4, 693 participants, NHB; Analysis 14.2), rs3733829 (n = 4, 807 participants, NHW; Analysis 15.2; n = 2, 75 participants, NHB; Analysis 16.1), rs7937 (n = 3, 557 participants, NHW; Analysis 17.1; n = 2, 76 participants, NHB; Analysis 18.1), rs1329650 (n = 3, 559 participants, NHW; Analysis 19.1; n = 2, 76 participants, NHB; Analysis 20.1), rs1028936 (n = 3, 559 participants, NHW; Analysis 21.1; n = 2, 76 participants, NHB; Analysis 22.1), and rs215605 (n = 3, 558 participants, NHW; Analysis 23.1; n = 2, 76 participants, NHB; Analysis 24.1).
8.2. Analysis.
Comparison 8 Bupropion vs placebo ‐ rs1051730 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
9.2. Analysis.
Comparison 9 Bupropion vs placebo ‐ rs1051730 ‐ non‐Hispanic black or African American, Outcome 2 End of Treatment.
10.1. Analysis.
Comparison 10 Bupropion vs placebo ‐ rs16969968 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
11.2. Analysis.
Comparison 11 Bupropion vs placebo ‐ rs16969968 ‐ non‐Hispanic black or African American, Outcome 2 End of Treatment.
12.2. Analysis.
Comparison 12 Bupropion vs placebo ‐ rs588765 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
13.2. Analysis.
Comparison 13 Bupropion vs placebo ‐ rs2036527 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
14.2. Analysis.
Comparison 14 Bupropion vs placebo ‐ rs2036527 ‐ non‐Hispanic black or African American, Outcome 2 End of Treatment.
15.2. Analysis.
Comparison 15 Bupropion vs placebo ‐ rs3733829 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
16.1. Analysis.
Comparison 16 Bupropion vs placebo ‐ rs3733829 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
17.1. Analysis.
Comparison 17 Bupropion vs placebo ‐ rs7937 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
18.1. Analysis.
Comparison 18 Bupropion vs placebo ‐ rs7937 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
19.1. Analysis.
Comparison 19 Bupropion vs placebo ‐ rs1329650 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
20.1. Analysis.
Comparison 20 Bupropion vs placebo ‐ rs1329650 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
21.1. Analysis.
Comparison 21 Bupropion vs placebo ‐ rs1028936 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
22.1. Analysis.
Comparison 22 Bupropion vs placebo ‐ rs1028936 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
23.1. Analysis.
Comparison 23 Bupropion vs placebo ‐ rs215605 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
24.1. Analysis.
Comparison 24 Bupropion vs placebo ‐ rs215605 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
We found no heterogeneity between genotype groups when comparing bupropion versus placebo for any of the biomarkers.
Bupropion + any NRT versus placebo
Six‐month abstinence
No data were available for this outcome.
End‐of‐treatment abstinence
Data were available for the following SNPs: rs1051730 (n = 2, 444 participants, NHW; Analysis 25.1; n = 2, 69 participants, NHB; Analysis 26.1), rs16969968 (n = 2, 441 participants, NHW; Analysis 27.1; n = 2, 69 participants, NHB; Analysis 28.1), rs2036527 (n = 2, 443 participants, NHW; Analysis 29.1; n = 2, 69 participants, NHB; Analysis 30.1), rs3733829 (n = 2, 441 participants, NHW; Analysis 31.1; n = 2, 69 participants, NHB; Analysis 32.1), rs7937 (n = 2, 442 participants, NHW; Analysis 33.1; n = 2, 69 participants, NHB; Analysis 34.1), rs1329650 (n = 2, 442 participants, NHW; Analysis 35.1; n = 2, 69 participants, NHB; Analysis 36.1), rs1028936 (n = 2, 444 participants, NHW; Analysis 37.1; n = 2, 69 participants, NHB; Analysis 38.1), and rs215605 (n = 2, 442 participants, NHW; Analysis 39.1; n = 2, 68 participants, NHB; Analysis 40.1).
25.1. Analysis.
Comparison 25 Bupropion + any NRT vs placebo ‐ rs1051730 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
26.1. Analysis.
Comparison 26 Bupropion + any NRT vs placebo ‐ rs1051730 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
27.1. Analysis.
Comparison 27 Bupropion + any NRT vs placebo ‐ rs16969968 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
28.1. Analysis.
Comparison 28 Bupropion + any NRT vs placebo ‐ rs16969968 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
29.1. Analysis.
Comparison 29 Bupropion + any NRT vs placebo ‐ rs2036527 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
30.1. Analysis.
Comparison 30 Bupropion + any NRT vs placebo ‐ rs2036527 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
31.1. Analysis.
Comparison 31 Bupropion + any NRT vs placebo ‐ rs3733829 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
32.1. Analysis.
Comparison 32 Bupropion + any NRT vs placebo ‐ rs3733829 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
33.1. Analysis.
Comparison 33 Bupropion + any NRT vs placebo ‐ rs7937 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
34.1. Analysis.
Comparison 34 Bupropion + any NRT vs placebo ‐ rs7937 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
35.1. Analysis.
Comparison 35 Bupropion + any NRT vs placebo ‐ rs1329650 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
36.1. Analysis.
Comparison 36 Bupropion + any NRT vs placebo ‐ rs1329650 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
37.1. Analysis.
Comparison 37 Bupropion + any NRT vs placebo ‐ rs1028936 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
38.1. Analysis.
Comparison 38 Bupropion + any NRT vs placebo ‐ rs1028936 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
39.1. Analysis.
Comparison 39 Bupropion + any NRT vs placebo ‐ rs215605 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
40.1. Analysis.
Comparison 40 Bupropion + any NRT vs placebo ‐ rs215605 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
We found no heterogeneity between genotype groups when comparing bupropion + any NRT versus placebo for any of the biomarkers.
Bupropion + any NRT versus bupropion alone
Six‐month abstinence
No data were available for this outcome.
End‐of‐treatment abstinence
Data were available for the following SNPs: rs1051730 (n = 2, 533 participants, NHW; Analysis 41.1; n = 2, 87 participants, NHB; Analysis 42.1), rs16969968 (n = 2, 531 participants, NHW; Analysis 43.1; n = 2, 87 participants, NHB; Analysis 44.1), rs2036527 (n = 2, 532 participants, NHW; Analysis 45.1; n = 2, 87 participants, NHB; Analysis 46.1), rs3733829 (n = 2, 531 participants, NHW; Analysis 47.1; n = 2, 94 participants, NHB; Analysis 48.1), rs7937 (n = 2, 533 participants, NHW; Analysis 49.1; n = 2, 87 participants, NHB; Analysis 50.1), rs1329650 (n = 2, 531 participants, NHW; Analysis 51.1; n = 2, 87 participants, NHB; Analysis 52.1), rs1028936 (n = 2, 533 participants, NHW; Analysis 53.1; n = 2, 87 participants, NHB; Analysis 54.1), and rs215605 (n = 2, 532 participants, NHW; Analysis 55.1; n = 2, 87 participants, NHB; Analysis 56.1).
41.1. Analysis.
Comparison 41 Bupropion + any NRT vs bupropion ‐ rs1051730 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
42.1. Analysis.
Comparison 42 Bupropion + any NRT vs bupropion ‐ rs1051730 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
43.1. Analysis.
Comparison 43 Bupropion + any NRT vs bupropion ‐ rs16969968 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
44.1. Analysis.
Comparison 44 Bupropion + any NRT vs bupropion ‐ rs16969968 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
45.1. Analysis.
Comparison 45 Bupropion + any NRT vs bupropion ‐ rs2036527 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
46.1. Analysis.
Comparison 46 Bupropion + any NRT vs bupropion ‐ rs2036527 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
47.1. Analysis.
Comparison 47 Bupropion + any NRT vs bupropion ‐ rs3733829 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
48.1. Analysis.
Comparison 48 Bupropion + any NRT vs bupropion ‐ rs3733829 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
49.1. Analysis.
Comparison 49 Bupropion + any NRT vs bupropion ‐ rs7937 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
50.1. Analysis.
Comparison 50 Bupropion + any NRT vs bupropion ‐ rs7937 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
51.1. Analysis.
Comparison 51 Bupropion + any NRT vs bupropion ‐ rs1329650 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
52.1. Analysis.
Comparison 52 Bupropion + any NRT vs bupropion ‐ rs1329650 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
53.1. Analysis.
Comparison 53 Bupropion + any NRT vs bupropion ‐ rs1028936 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
54.1. Analysis.
Comparison 54 Bupropion + any NRT vs bupropion ‐ rs1028936 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
55.1. Analysis.
Comparison 55 Bupropion + any NRT vs bupropion ‐ rs215605 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
56.1. Analysis.
Comparison 56 Bupropion + any NRT vs bupropion ‐ rs215605 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
We found no heterogeneity between genotype groups when comparing bupropion + any NRT versus bupropion for any of the biomarkers.
Comparisons of treatment effects between genotype groups within pharmacotherapy randomisation arms
Active NRT
Six‐month abstinence
Data were available for the following SNPs: rs1051730 (n = 7, 2687 participants, NHW; Analysis 57.1; n = 2, 237 participants, NHB; Analysis 58.1), rs16969968 (n = 2, 392 participants, NHB; Analysis 59.1), rs588765 (n = 3, 832 participants, NHW; Analysis 60.1), rs2036527 (n = 2, 948 participants, NHW; Analysis 61.1; n = 2, 391 participants, NHB; Analysis 62.1), DRD4 (exon 3 48 bp) (n = 3, 1119 participants, NHW; Analysis 63.1), SLC6A4 (Promoter) (n = 2, 926 participants, NHW; Analysis 64.1), and NMR (n = 2, 718 participants, NHW and NHB; Analysis 65.1).
57.1. Analysis.
Comparison 57 Active NRT ‐ rs1051730 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
58.1. Analysis.
Comparison 58 Active NRT ‐ rs1051730 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
59.1. Analysis.
Comparison 59 Active NRT ‐ rs16969968 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
60.1. Analysis.
Comparison 60 Active NRT ‐ rs588765 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
61.1. Analysis.
Comparison 61 Active NRT ‐ rs2036527 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
62.1. Analysis.
Comparison 62 Active NRT ‐ rs2036527 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
63.1. Analysis.
Comparison 63 Active NRT ‐ DRD‐4 (exon 3 48 bp) ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
64.1. Analysis.
Comparison 64 Active NRT ‐ SLC6A4 (Promoter) ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
65.1. Analysis.
Comparison 65 Active NRT‐NMR ‐ non‐Hispanic white or black or African American, Outcome 1 Six‐Month Abstinence.
Among those receiving active NRT, individuals with a slow NMR were more successful in achieving six‐month abstinence as compared with those with a normal NMR among NHW and NHB participants (normal NMR vs slow NMR: RR 0.54, 95% CI 0.37 to 0.78; Analysis 65.1).
We found no heterogeneity between genotype group comparisons (homozygous major vs homozygous minor compared with heterozygous vs homozygous minor, or slow vs normal) among individuals randomised to active NRT for any of the other biomarkers.
End‐of‐treatment abstinence
Data were available for the following SNPs: rs1051730 (n = 5, 2208 participants, NHW; Analysis 57.2), rs16969968 (n = 2, 392 participants, NHB; Analysis 59.2), rs588765 (n = 3, 832 participants, NHW; Analysis 60.2), rs2036527 (n = 2, 948 participants, NHW; Analysis 61.2; n = 2, 391 participants, NHB; Analysis 62.2), DRD4 (exon 3 48 bp) (n = 3, 1119 participants, NHW; Analysis 63.2), SLC6A4 (Promoter) (n = 2, 926 participants, NHW; Analysis 64.2), and NMR (n = 2, 718 participants, NHW and NHB; Analysis 65.2).
57.2. Analysis.
Comparison 57 Active NRT ‐ rs1051730 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
59.2. Analysis.
Comparison 59 Active NRT ‐ rs16969968 ‐ non‐Hispanic black or African American, Outcome 2 End of Treatment.
60.2. Analysis.
Comparison 60 Active NRT ‐ rs588765 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
61.2. Analysis.
Comparison 61 Active NRT ‐ rs2036527 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
62.2. Analysis.
Comparison 62 Active NRT ‐ rs2036527 ‐ non‐Hispanic black or African American, Outcome 2 End of Treatment.
63.2. Analysis.
Comparison 63 Active NRT ‐ DRD‐4 (exon 3 48 bp) ‐ non‐Hispanic white, Outcome 2 End of Treatment.
64.2. Analysis.
Comparison 64 Active NRT ‐ SLC6A4 (Promoter) ‐ non‐Hispanic white, Outcome 2 End of Treatment.
65.2. Analysis.
Comparison 65 Active NRT‐NMR ‐ non‐Hispanic white or black or African American, Outcome 2 End of Treatment.
Among those receiving active NRT, GG genotype individuals more often achieved end‐of‐treatment abstinence as compared with individuals with a combination of GA and AA genotypes for rs16969968 and rs2036527 in NHB (RR 2.75, 95% CI 1.08 to 7.02; Analysis 59.2; RR 1.73, 95% CI 1.20 to 2.49; Analysis 62.2; respectively).
We found no heterogeneity between genotype group comparisons (homozygous major vs homozygous minor compared with heterozygous vs homozygous minor, or slow vs normal) among individuals randomised to active NRT for any of the other biomarkers.
Bupropion
Six‐month abstinence
Data were available for the following SNPs: rs1051730 (n = 4, 610 participants, NHW; Analysis 66.1; n = 2, 63 participants, NHB; Analysis 67.1), rs16969968 (n = 2, 294 participants, NHB; Analysis 69.1), rs588765 (n = 4, 596 participants, NHW; Analysis 70.1), rs2036527 (n = 2, 412 participants, NHW; Analysis 71.1; n = 3, 331 participants, NHB; Analysis 72.1), and rs3733829 (n = 2, 307 participants, NHW; Analysis 73.1).
66.1. Analysis.
Comparison 66 Bupropion ‐ rs1051730 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
67.1. Analysis.
Comparison 67 Bupropion ‐ rs1051730 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
69.1. Analysis.
Comparison 69 Bupropion ‐ rs16969968 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
70.1. Analysis.
Comparison 70 Bupropion ‐ rs588765 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
71.1. Analysis.
Comparison 71 Bupropion ‐ rs2036527 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
72.1. Analysis.
Comparison 72 Bupropion ‐ rs2036527 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
73.1. Analysis.
Comparison 73 Bupropion ‐ rs3733829 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
Among those receiving bupropion, we identified no specific genotype groups that were more successful in achieving six‐month abstinence than other groups. Additionally, we found no heterogeneity between genotype group comparisons (homozygous major vs homozygous minor compared with heterozygous vs homozygous minor, or slow vs normal) among individuals randomised to bupropion for any of the biomarkers.
End‐of‐treatment abstinence
Data were available for the following SNPs: rs1051730 (n = 6, 743 participants, NHW; Analysis 66.2; n = 3, 84 participants, NHB; Analysis 67.2), rs16969968 (n = 3, 324 participants, NHW; Analysis 68.1; n = 3, 315 participants, NHB; Analysis 69.2), rs588765 (n = 4, 596 participants, NHW; Analysis 70.2), rs2036527 (n = 4, 546 participants, NHW; Analysis 71.2; n = 4, 352 participants, NHB; Analysis 72.2), rs3733829 (n = 4, 440 participants, NHW; Analysis 73.2; n = 2, 46 participants, NHB; Analysis 74.1), rs7937 (n = 3, 324 participants, NHW; Analysis 75.1; n = 2, 47 participants, NHB; Analysis 76.1), rs1329650 (n = 3, 324 participants, NHW; Analysis 77.1; n = 2, 47 participants, NHB; Analysis 78.1), rs1028936 (n = 3, 324 participants, NHW; Analysis 79.1; n = 2, 47 participants, NHB; Analysis 80.1), and rs215605 (n = 3, 324 participants, NHW; Analysis 81.1; n = 2, 47 participants, NHB; Analysis 82.1).
66.2. Analysis.
Comparison 66 Bupropion ‐ rs1051730 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
67.2. Analysis.
Comparison 67 Bupropion ‐ rs1051730 ‐ non‐Hispanic black or African American, Outcome 2 End of Treatment.
68.1. Analysis.
Comparison 68 Bupropion ‐ rs16969968 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
69.2. Analysis.
Comparison 69 Bupropion ‐ rs16969968 ‐ non‐Hispanic black or African American, Outcome 2 End of Treatment.
70.2. Analysis.
Comparison 70 Bupropion ‐ rs588765 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
71.2. Analysis.
Comparison 71 Bupropion ‐ rs2036527 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
72.2. Analysis.
Comparison 72 Bupropion ‐ rs2036527 ‐ non‐Hispanic black or African American, Outcome 2 End of Treatment.
73.2. Analysis.
Comparison 73 Bupropion ‐ rs3733829 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
74.1. Analysis.
Comparison 74 Bupropion ‐ rs3733829 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
75.1. Analysis.
Comparison 75 Bupropion ‐ rs7937 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
76.1. Analysis.
Comparison 76 Bupropion ‐ rs7937 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
77.1. Analysis.
Comparison 77 Bupropion ‐ rs1329650 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
78.1. Analysis.
Comparison 78 Bupropion ‐ rs1329650 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
79.1. Analysis.
Comparison 79 Bupropion ‐ rs1028936 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
80.1. Analysis.
Comparison 80 Bupropion ‐ rs1028936 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
81.1. Analysis.
Comparison 81 Bupropion ‐ rs215605 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
82.1. Analysis.
Comparison 82 Bupropion ‐ rs215605 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
Among those receiving bupropion, we identified no specific genotype groups that were more successful in achieving end‐of‐therapy abstinence than other groups. Additionally, we found no heterogeneity between genotype group comparisons (homozygous major vs homozygous minor compared with heterozygous vs homozygous minor, or slow vs normal) among individuals randomised to bupropion for any of the biomarkers.
Varenicline
Six‐month abstinence
Data were available for the following SNPs: rs16969968 (n = 2, 707 participants, NHW; Analysis 83.1) and rs588765 (n = 2, 699 participants; Analysis 84.1).
83.1. Analysis.
Comparison 83 Varenicline ‐ rs16969968 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
84.1. Analysis.
Comparison 84 Varenicline ‐ rs588765 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
Among those receiving varenicline, we identified no specific genotype groups that were more successful than other groups in achieving six‐month abstinence. Additionally, we found no heterogeneity between genotype group comparisons (homozygous major vs homozygous minor compared with heterozygous vs homozygous minor, or slow vs normal) among individuals randomised to varenicline for any of the biomarkers.
End‐of‐treatment abstinence
Data were available for the following SNPs: rs16969968 (n = 2, 707 participants, NHW; Analysis 83.2) and rs588765 (n = 2, 699 participants, NHW; Analysis 84.2).
83.2. Analysis.
Comparison 83 Varenicline ‐ rs16969968 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
84.2. Analysis.
Comparison 84 Varenicline ‐ rs588765 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
Among those receiving varenicline, we identified no specific genotype groups that were more successful than other groups in achieving end‐of‐therapy abstinence. Additionally, we found no heterogeneity between genotype group comparisons (homozygous major vs homozygous minor compared with heterozygous vs homozygous minor, or slow vs normal) among individuals randomised to varenicline for any of the biomarkers.
Bupropion + any NRT
Six‐month abstinence
No data were available for this outcome.
End‐of‐treatment abstinence
Data were available for the following SNPs: rs1051730 (n = 2, 268 participants, NHW; Analysis 85.1; n = 2, 40 participants, NHB; Analysis 86.1), rs16969968 (n = 2, 266 participants, NHW; Analysis 87.1; n = 2, 40 participants, NHB; Analysis 88.1), rs2036527 (n = 2, 267 participants, NHW; Analysis 89.1; n = 2, 40 participants, NHB; Analysis 90.1), rs3733829 (n = 2, 267 participants, NHW; Analysis 91.1; n = 2, 40 participants, NHB; Analysis 92.1), rs7937 (n = 2, 268 participants, NHW; Analysis 93.1; n = 2, 40 participants, NHB; Analysis 94.1), rs1329650 (n = 2, 266 participants, NHW; Analysis 95.1; n = 2, 40 participants, NHB; Analysis 96.1), rs1028936 (n = 2, 268 participants, NHW; Analysis 97.1; n = 2, 40 participants, NHB; Analysis 98.1), and rs215605 (n = 2, 267 participants, NHW; Analysis 99.1; n = 2, 40 participants, NHB; Analysis 100.1).
85.1. Analysis.
Comparison 85 Bupropion + any NRT ‐ rs1051730 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
86.1. Analysis.
Comparison 86 Bupropion + any NRT ‐ rs1051730 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
87.1. Analysis.
Comparison 87 Bupropion + any NRT ‐ rs16969968 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
88.1. Analysis.
Comparison 88 Bupropion + any NRT ‐ rs16969968 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
89.1. Analysis.
Comparison 89 Bupropion + any NRT ‐ rs2036527 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
90.1. Analysis.
Comparison 90 Bupropion + any NRT ‐ rs2036527 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
91.1. Analysis.
Comparison 91 Bupropion + any NRT ‐ rs3733829 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
92.1. Analysis.
Comparison 92 Bupropion + any NRT ‐ rs3733829 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
93.1. Analysis.
Comparison 93 Bupropion + any NRT ‐ rs7937 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
94.1. Analysis.
Comparison 94 Bupropion + any NRT ‐ rs7937 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
95.1. Analysis.
Comparison 95 Bupropion + any NRT ‐ rs1329650 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
96.1. Analysis.
Comparison 96 Bupropion + any NRT ‐ rs1329650 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
97.1. Analysis.
Comparison 97 Bupropion + any NRT ‐ rs1028936 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
98.1. Analysis.
Comparison 98 Bupropion + any NRT ‐ rs1028936 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
99.1. Analysis.
Comparison 99 Bupropion + any NRT ‐ rs215605 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
100.1. Analysis.
Comparison 100 Bupropion + any NRT ‐ rs215605 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
Among those receiving bupropion + any NRT, GG genotype individuals more often achieved six‐month abstinence as compared with AA genotype individuals for rs1329650 in NHW (AA vs GG, RR 0.64, 95% CI 0.43 to 0.94; Analysis 95.1), as did individuals with GG or AG genotype compared with AA genotype individuals for rs3733829 in NHB (AA vs AG or GG RR 0.36, 95% CI 0.13 to 0.99; Analysis 92.1).
We found no heterogeneity between genotype group comparisons (homozygous major vs homozygous minor compared with heterozygous vs homozygous minor, or slow vs normal) among individuals randomised to bupropion + any NRT for any of the other biomarkers.
Placebo
Six‐month abstinence
Data were available for the following SNPs: rs1051730 (n = 6, 974 participants, NHW; Analysis 101.1; n = 2, 63 participants, NHB; Analysis 102.1), rs16969968 (n = 2, 343 participants, NHW; Analysis 103.1; n = 3, 584 participants, NHB; Analysis 104.1), rs588765 (n = 5, 723 participants, NHW; Analysis 105.1; n = 2, 566 participants, NHB; Analysis 106.1), rs2036527 (n = 2, 326 participants, NHW; Analysis 107.1; n = 4, 629 participants, NHB; Analysis 108.1), DRD4 (exon 3 48 bp) (n = 3, 573 participants, NHW; Analysis 109.1), rs3733829 (n = 2, 264 participants, NHW; Analysis 110.1), SLC6A4 (Promoter) (n = 2, 151 participants, NHW; Analysis 119.1), and NMR (n = 2, 699 participants, NHW and NHB; Analysis 120.1).
101.1. Analysis.
Comparison 101 Placebo ‐ rs1051730 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
102.1. Analysis.
Comparison 102 Placebo ‐ rs1051730 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
103.1. Analysis.
Comparison 103 Placebo ‐ rs16969968 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
104.1. Analysis.
Comparison 104 Placebo ‐ rs16969968 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
105.1. Analysis.
Comparison 105 Placebo ‐ rs588765 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
106.1. Analysis.
Comparison 106 Placebo ‐ rs588765 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
107.1. Analysis.
Comparison 107 Placebo ‐ rs2036527 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
108.1. Analysis.
Comparison 108 Placebo ‐ rs2036527 ‐ non‐Hispanic black or African American, Outcome 1 Six‐Month Abstinence.
109.1. Analysis.
Comparison 109 Placebo ‐ DRD‐4 (exon 3 48 bp) ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
110.1. Analysis.
Comparison 110 Placebo ‐ rs3733829 ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
119.1. Analysis.
Comparison 119 Placebo ‐ SLC6A4 (Promoter) ‐ non‐Hispanic white, Outcome 1 Six‐Month Abstinence.
120.1. Analysis.
Comparison 120 Placebo ‐ NMR ‐ non‐Hispanic white or black or African American, Outcome 1 Six‐Month Abstinence.
Among those receiving placebo, abstinence rates were not statistically significantly different between specific genotype groups. Additionally, we found no heterogeneity between genotype group comparisons (homozygous major vs homozygous minor compared with heterozygous vs homozygous minor, or slow vs normal) among individuals randomised to placebo for any of the biomarkers.
End‐of‐treatment abstinence
Data were available for the following SNPs: rs1051730 (n = 7, 983 participants, NHW; Analysis 101.2; n = 3, 83 participants, NHB; Analysis 102.2), rs16969968 (n = 4, 445 participants, NHW; Analysis 103.2; n = 4, 596 participants, NHB; Analysis 104.2), rs588765 (n = 5, 658 participants, NHW; Analysis 105.2; n = 2, 566 participants, NHB; Analysis 106.2), rs2036527 (n = 4, 429 participants, NHW; Analysis 107.2; n = 5, 641 participants, NHB; Analysis 108.2), DRD4 (exon 3 48 bp) (n = 3, 573 participants, NHW; Analysis 109.2), rs3733829 (n = 4, 367 participants, NHW; Analysis 110.2), rs7937 (n = 3, 233 participants, NHW; Analysis 111.1; n = 2, 29 participants, NHB; Analysis 112.1), rs1329650 (n = 3, 235 participants, NHW; Analysis 113.1; n = 2, 29 participants, NHB; Analysis 114.1), rs1028936 (n = 3, 235 participants, NHW; Analysis 115.1; n = 2, 29 participants, NHB; Analysis 116.1), rs215605 (n = 3, 234 participants, NHW; Analysis 117.1; n = 2, 29 participants, NHB; Analysis 118.1), SLC6A4 (Promoter) (n = 2, 151 participants, NHW; Analysis 119.2), and NMR (n = 2, 718 participants, NHW and NHB; Analysis 120.2).
101.2. Analysis.
Comparison 101 Placebo ‐ rs1051730 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
102.2. Analysis.
Comparison 102 Placebo ‐ rs1051730 ‐ non‐Hispanic black or African American, Outcome 2 End of Treatment.
103.2. Analysis.
Comparison 103 Placebo ‐ rs16969968 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
104.2. Analysis.
Comparison 104 Placebo ‐ rs16969968 ‐ non‐Hispanic black or African American, Outcome 2 End of Treatment.
105.2. Analysis.
Comparison 105 Placebo ‐ rs588765 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
106.2. Analysis.
Comparison 106 Placebo ‐ rs588765 ‐ non‐Hispanic black or African American, Outcome 2 End of Treatment.
107.2. Analysis.
Comparison 107 Placebo ‐ rs2036527 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
108.2. Analysis.
Comparison 108 Placebo ‐ rs2036527 ‐ non‐Hispanic black or African American, Outcome 2 End of Treatment.
109.2. Analysis.
Comparison 109 Placebo ‐ DRD‐4 (exon 3 48 bp) ‐ non‐Hispanic white, Outcome 2 End of Treatment.
110.2. Analysis.
Comparison 110 Placebo ‐ rs3733829 ‐ non‐Hispanic white, Outcome 2 End of Treatment.
111.1. Analysis.
Comparison 111 Placebo ‐ rs7937 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
112.1. Analysis.
Comparison 112 Placebo ‐ rs7937 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
113.1. Analysis.
Comparison 113 Placebo ‐ rs1329650 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
114.1. Analysis.
Comparison 114 Placebo ‐ rs1329650 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
115.1. Analysis.
Comparison 115 Placebo ‐ rs1028936 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
116.1. Analysis.
Comparison 116 Placebo ‐ rs1028936 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
117.1. Analysis.
Comparison 117 Placebo ‐ rs215605 ‐ non‐Hispanic white, Outcome 1 End of Treatment.
118.1. Analysis.
Comparison 118 Placebo ‐ rs215605 ‐ non‐Hispanic black or African American, Outcome 1 End of Treatment.
119.2. Analysis.
Comparison 119 Placebo ‐ SLC6A4 (Promoter) ‐ non‐Hispanic white, Outcome 2 End of Treatment.
120.2. Analysis.
Comparison 120 Placebo ‐ NMR ‐ non‐Hispanic white or black or African American, Outcome 2 End of Treatment.
Abstinence at the end of treatment among those receiving placebo was greater among individuals with AA or GA genotype than among those with GG genotype for rs16969968 in NHB (GG vs GA or AA, RR 0.55, 95% CI 0.35 to 0.86; Analysis 104.2).
We found no heterogeneity between genotype group comparisons (homozygous major vs homozygous minor compared with heterozygous vs homozygous minor, or slow vs normal) among individuals randomised to placebo for any of the other biomarkers.
Discussion
Summary of main results
Using data from 18 randomised controlled trials (RCTs), we investigated whether a total of 13 biomarkers, including nine single‐nucleotide polymorphisms (SNPs), two variable number tandem repeats (VNTRs) (DRD4 and SLC6A4), and the nicotine metabolite ratio (NMR) modify the effects of different smoking cessation pharmacotherapies (nicotine replacement therapy (NRT), bupropion, varenicline, and combination therapy) on abstinence from smoking at six months and at end of treatment. We carried out analyses separately in non‐Hispanic whites (NHWs) and non‐Hispanic blacks (NHBs). Results showed statistical heterogeneity between genotypes for only NRT versus placebo in NHWs for rs1051730 and rs16969968 at end of treatment. In addition, rs16969968 showed evidence of heterogeneity in NHBs at six months, as well as at end of treatment, but sample sizes for some genotype subgroups were small, rendering these results unreliable. Results at rs16969968 for NRT versus placebo in NHWs and in NHBs reflect opposite directions. For the other seven SNPs evaluated in clinical trials, we found no evidence of SNP × treatment interaction. Within‐treatment arm analyses indicated that six‐month abstinence was less often achieved in NHW normal metabolisers and more often among NHB individuals with rs16969968 GG and and rs2036527 GG genotype who received NRT, and that end‐of‐treatment abstinence was less often achieved among NHWs with rs1329650 TT genotype and NHBs with rs3733829 GG or AG genotype who received bupropion + any NRT, and among NHBs with rs16969968 AA or GA genotype who received placebo. Trial results revealed no such differences for other treatments or biomarkers. In conclusion, although these findings suggest superior short‐term efficacy of NRT in specific rs1051730 and rs16969968 genotype subgroups among NHWs, lack of significant genotype differences within treatment arms limits confidence in the validity of these findings. The same holds true for results of within‐treatment analyses.
Overall completeness and applicability of evidence
For the vast majority of biomarkers for which we had data, we found no evidence that they may modify the effects of smoking cessation interventions, although evidence showed nominally significant treatment effects in specific genotype groups. Various reasons may explain this. First, identification of biomarker × treatment interactions may require large sample sizes (Cardon 2000), which were not available for the current RCTs. Traditionally, RCTs are designed to address comparative effectiveness questions regarding one or more active drugs. Genomic analyses are usually conducted retrospectively after trial completion and are rarely taken into account at design stages. Although we aimed to overcome sample size limitations of individual studies by combining data through meta‐analysis, for most comparisons we were not able to obtain summary level data that would allow us to quantitatively synthesise all available information. Second, RCTs that were included in our analyses used genotype information for a few preselected SNPs that were chosen on the basis of biological plausibility and prior genome‐wide significance with regards to smoking phenotypes. However, treatment response is likely to be under polygenic influences of biomarkers with small and large effects. Therefore, additional biomarkers across the genome may confer useful predictive information from which to identify individuals who will benefit from a particular smoking cessation intervention. To achieve this, RCTs should routinely collect and analyse DNA samples at the genome‐wide level. We refer readers to our recent reviews of progress of and recommendations for genomic studies of clinical trials of smoking cessation therapies (Chen 2017; Saccone 2017).
Quality of the evidence
Our analysis constitutes a synthesis of evidence from RCTs retrospectively evaluated for effects of smoking cessation treatments according to specific genotypes. Therefore, our results should be interpreted as evidence of heterogeneity of treatment effects (Varadhan 2013) according to specific biomarkers. Given the large number of analyses performed and assessment of the quality of evidence (GRADE) as moderate, one should interpret the results of this review with caution.
Although treatment effect heterogeneity is critical for making clinical and policy decisions, it provides only indirect evidence of the clinical utility of these genetic markers (i.e. whether measuring a particular biomarker will result in better or worse clinical outcomes).
The chr15q25.1 SNPs rs2036527, rs16969968, and rs1051730 are highly correlated (r2 = 0.90 for rs2036527 vs the latter two SNPs, and r2 = 1 vs the latter two SNPs) in individuals of European ancestry (Utah residents from North and West Europe), but are less highly correlated in African American individuals (r2 = 0.28, 0.38, and 0.46 for rs2036527 vs rs16969968, rs2036527 vs rs1051730, and rs16969968 vs rs1051730; Americans of African Ancestry in SW USA). In addition, the minor allele frequency of all three SNPs is lower among individuals of African American ancestry (from 0.40 to 0.22 for rs2036527, from 0.38 to 0.07 for rs16969968, and from 0.38 to 0.15 for rs1051730). In particular, the minor allele frequency of rs16969968 is much lower among those of African ancestry (means are < 0.01 in sub‐Saharan Africans, 0.027 in East Asians, 0.21 in Amerindians, 0.37 in Europeans, and 0.18 in South Asians; 1000 Genomes Project Consortium 2015). Therefore, one should use caution when comparing results at these chr15q25.1 SNPs between NHWs and NHBs owing to differences in linkage disequilibrium (LD) and power (due to differences in allele frequency). All three variants constitute susceptibility loci for lung cancer and are associated with measures of nicotine dependence (David 2012; Landi 2009; Liu 2010; McKay 2008; Thorgeirsson 2008;Thorgeirsson 2010;Tobacco and Genetics Consortium 2010; Zanetti 2016). rs16969968 (NM˙000745.3:c.1192G>A) is a missense variant (Asp398Asn) of the nicotinic acetylcholine receptor alpha 5 subunit gene (CHRNA5); African American individuals who are homozygous for the major allele have a higher probability of smoking abstinence with NRT than with placebo, but individuals of European ancestry who are heterozygous for rs16969968 (A/G genotype) have a higher probability of smoking abstinence with NRT than with placebo (see Results). Similarly, rs1051730 (NM˙000743.4:c.645C>T) is a synonymous substitution in the nicotinic acetylcholine receptor alpha 3 subunit gene (CHRNA3) that confers higher probability of abstinence among individuals of European ancestry who are heterozygous or homozygous for the minor allele (AA genotype) (see Results). rs2036527, found proximal to CHRNA5, is associated with nicotine dependence and with lung cancer in African Americans, but has much lower LD with the two other chr15q25.1 SNPs in African Americans than in European Americans. We did observe that NHB with GG genotype may benefit more from NRT than from placebo (see Results). Differences in SNP allele frequencies and in LD between NHW and NHB populations at chr15q25.1 (above) and differences in allele frequencies between NHW and NHB at chr19q13.2 (rs3733829) and chr10q23.32 (rs1329650), together with known differences in LD, suggest caution when results are compared between NHWs and NHBs. Given the small number of trials with available data for these and other comparisons, review findings should be interpreted with caution and should be revisited when results of future trials become available.
Potential biases in the review process
We should acknowledge that owing to the numbers of biomarkers, interventions, endpoints, and ancestry subgroups, our systematic review includes a large number of analyses. However, we did not correct for the multiplicity of comparisons because our aim was to synthesise existing evidence rather than focus on hypothesis testing. Therefore, we put greater emphasis on the estimation of effects of smoking cessation interventions than on testing for these effects (Higgins 2011). Moreover, in our review, we report all performed analyses regardless of whether they achieved statistical significance at the nominal P = 0.05 threshold; hence, we are not selectively reporting nominally significant results. Another issue of multiple comparisons relates to the selection of particular SNP × treatment interactions among many in the primary RCTs. Although most trials report only one or a few SNPs, we cannot exclude the possibility that these associations may have been selected among a number of analyses. Formally controlling for this type of multiplicity (e.g. using the approach proposed by Benjamini and Bogomolov) is difficult in the absence of detailed reporting in primary studies (Benjamini 2014). However, most RCTs had low risk of selective reporting and had specified a priori the SNPs of interest used in analyses of pharmacogenomics.
Agreements and disagreements with other studies or reviews
In European ancestry samples, minor alleles of both variants (rs1051730 and rs16969968) have been associated with increased abstinence (Bergen 2013; Chen 2012) and with decreased abstinence after NRT (Munafò 2011). Others have not been associated with abstinence (Tyndale 2015). In addition, a recent meta‐analysis revealed that neither of these SNPs was associated with smoking cessation among individuals randomised to NRT (Leung 2015). In contrast to our meta‐analysis, the studies of Leung et al evaluated only the per‐allele effect for each SNP among those randomised to NRT; these investigators did not examine effect modification, as we did in our analysis. In other words, they did not address the comparative effectiveness of NRT relative to placebo or some other smoking cessation intervention. On the other hand, in our meta‐analysis, we performed both within‐ and across‐treatment arms comparisons of effects of biomarkers to evaluate the efficacy of smoking cessation interventions in specific genotype groups. Hence, the fact that we did find evidence of treatment effect heterogeneity for both rs169969968 and rs1051730 suggests a likely gene × treatment interaction.
Authors' conclusions
Implications for practice.
These data suggest that response to nicotine replacement therapy (NRT) may be greater for non‐Hispanic black (NHB) individuals at six months with rs16969968 GG genotype than for those with GA or AA genotype; among individuals treated with active NRT, nicotine metabolite ratio (NMR) slow metaboliser status predicts higher six‐month abstinence outcomes than among those with normal metaboliser status. For NHWs and NHBs, rs1051730 GA and AA genotypes were associated with higher NRT response at end of treatment, and rs16969968 GG genotypes were associated with greater NRT response for abstinence at end of therapy.
Genotype comparisons within treatment arms were notable for higher abstinence rates on NRT among NHBs for rs2036527 GG (vs AA or AG) genotypes, and lower abstinence rates among NHWs treated with combination bupropion with NRT for rs1329650 TT (vs GG), and among NHBs for rs3733829 AG or GG (vs AA).
Although we did identify some genotype‐treatment interactions and genotype effects within treatment arms, the vast majority of analyses indicated no such results. Significant results should be considered preliminary and interpreted with caution because none of the statistically significant meta‐analyses included more than two trials per genotype comparison, many confidence intervals were wide, and the quality of evidence (GRADE) was generally moderate. In addition, our analyses could not adjust for ancestry informative markers; thus, these findings have the potential to be confounded by population stratification.
Implications for research.
We observed that smokers with slow NMR on NRT patch demonstrated higher abstinence rates than smokers with normal NMR on NRT patch. Our meta‐analyses did not detect heterogeneity in NRT patch efficacy (active vs placebo patch) between NMR groups, which could be a function of statistical power or lack of superior efficacy for slow metabolisers. Data provided by extant trials would increase the reliability of effect estimates and confidence in determining the potential clinical utility of NMR to guide selection of NRT versus an alternative treatment. In particular, access to individual participant data (IPD) would be desirable, as this may help to improve the quantity and quality of data, while standardising outcomes across included trials and enabling detailed data checking (Debray 2015; Tierney 2015). IPD offer greater flexibility in investigating effect modifiers and appear to be particularly useful for addressing genotype‐treatment interactions. Increases in data sharing by randomised controlled trials (RCTs) reporting response to treatment GWAS summary statistics (e.g. as exemplified by sharing of the response to treatment summary result from the Clinical Antipsychotic Trials of Intervention Effectiveness) may provide the catalyst to encourage future replications and meta‐analyses (McClay 2011).
Recent methodological advances may facilitate the design and analysis of such predictive trials (e.g. the adaptive signature design and the cross‐validated adaptive signature design are clinical trial designs used for development and validation of high‐dimensional biosignatures within single trials) (Freidlin 2005; Freidlin 2010). In a setting with SNP markers routinely available for patients, and with genomic architecture and genome‐wide significant single‐nucleotide polymorphisms (SNPs) known for nicotine dependence behavioural components, development and validation of predictive genomic signatures for response to smoking cessation therapies might begin (Sun 2015; Weiss 2016). Additionally, polygenic risk scores could be helpful, as even if many individual markers produce no detected effect on their own, combined scores could serve as a strong predictor of outcomes (Dudbridge 2013).
Prediction of response to smoking cessation pharmacotherapies based on genomic information is a complex issue that poses challenges for the design and analysis of clinical trials. As genotyping costs continue to fall, it is important that existing and future pharmacotherapy trials interrogate genome‐wide variation to identify biomarkers that predict treatment response for future assessment of clinical utility. Availability of genome‐wide data enables correction of population genetic variation in genome‐wide association studies, as exemplified in Pharmacogenomics of Nicotine Addiction Treatment (PNAT) Consortium Group RCT analyses, as well as examination of effects of genetic ancestry on treatment efficacy, as observed in analyses of response to treatment for tobacco dependence, major depression, and schizophrenia (Adkins 2013; Bress 2015; Murphy 2013). Genome‐wide genotype data will permit examination of multiple a priori SNPs in risk score format for treatment response, along with novel searches for SNPs associated with response to treatment at genome‐wide significance, as has recently been accomplished in relatively modest samples for lithium and bipolar disorder (Hou 2016; Uhl 2014).
What's new
| Date | Event | Description |
|---|---|---|
| 26 February 2018 | Amended | Significance of heterogeneity amended in Summary of Findings tables |
Acknowledgements
We acknowledge all prinicipal investigators who provided their study data to us. Furthermore, we acknowledge the assistance of Alexandros Christodoulou‐Rubalcava in drafting the protocol of this review, and the support of Lindsay Stead in identifying relevant literature.
Data and analyses
Comparison 1. Active NRT vs placebo ‐ rs1051730 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 3 | 1597 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.16, 1.75] |
| 1.1 Homozygous Major | 3 | 659 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.93, 1.72] |
| 1.2 Heterozygous | 3 | 731 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.08, 2.01] |
| 1.3 Homozygous Minor | 3 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 2.07 [1.06, 4.01] |
| 2 End of Treatment | 2 | 1391 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [1.14, 2.32] |
| 2.1 Homozygous Major | 2 | 582 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.85, 1.41] |
| 2.2 Heterozygous | 2 | 631 | Risk Ratio (M‐H, Random, 95% CI) | 2.13 [1.52, 2.97] |
| 2.3 Homozygous Minor | 2 | 178 | Risk Ratio (M‐H, Random, 95% CI) | 2.18 [1.04, 4.58] |
Comparison 2. Active NRT vs placebo ‐ rs16969968 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 1127 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.97, 1.98] |
| 1.1 Homozygous Major | 2 | 449 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.77, 1.33] |
| 1.2 Heterozygous | 2 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [1.33, 2.59] |
| 1.3 Homozygous Minor | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.45, 7.23] |
Comparison 3. Active NRT vs placebo ‐ rs16969968 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | 709 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.55, 2.26] |
| 1.1 Homozygous Major | 2 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.07, 2.03] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.15, 1.26] |
| 2 End of Treatment | 2 | 709 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.36, 2.94] |
| 2.1 Homozygous Major | 2 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [1.15, 2.15] |
| 2.2 Heterozygous or Homozygous Minor | 2 | 72 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.10, 0.86] |
Comparison 4. Active NRT vs placebo ‐ rs 588765 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 923 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [1.04, 1.71] |
| 1.1 Homozygous Major | 2 | 296 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.89, 2.16] |
| 1.2 Heterozygous | 2 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.89, 1.79] |
| 1.3 Homozygous Minor | 2 | 158 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.74, 3.06] |
Comparison 5. Active NRT vs placebo ‐ rs2036527 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | 708 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.75, 1.87] |
| 1.1 Homozygous Major | 2 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.46, 8.26] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.40, 2.03] |
| 2 End of Treatment | 2 | 708 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.77, 1.93] |
| 2.1 Homozygous Major | 2 | 425 | Risk Ratio (M‐H, Random, 95% CI) | 2.39 [0.43, 13.35] |
| 2.2 Heterozygous or Homozygous Minor | 2 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.55, 1.46] |
Comparison 6. Active NRT vs placebo ‐ DRD4 (exon 3 48 bp) ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | 900 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.08, 1.92] |
| 1.1 Homozygous Major | 2 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.93, 1.93] |
| 1.2 Heterozygous | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [1.00, 3.10] |
| 1.3 Homozygous Minor | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.40, 4.11] |
| 2 End of Treatment | 2 | 900 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.88, 1.63] |
| 2.1 Homozygous Major | 2 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.59, 2.51] |
| 2.2 Heterozygous | 2 | 290 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.57, 3.39] |
| 2.3 Homozygous Minor | 2 | 44 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.65, 1.58] |
Comparison 7. Active NRT vs placebo ‐ NMR ‐ non‐Hispanic black and white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | 1417 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.08, 2.10] |
| 1.1 Slow NMR | 2 | 628 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.12, 2.94] |
| 1.2 Normal NMR | 2 | 789 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.78, 1.87] |
| 2 End of Treatment | 2 | 1417 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.19, 1.90] |
| 2.1 Slow NMR | 2 | 847 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [1.18, 2.20] |
| 2.2 Normal NMR | 2 | 570 | Risk Ratio (M‐H, Random, 95% CI) | 1.49 [0.85, 2.60] |
Comparison 8. Bupropion vs placebo ‐ rs1051730 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 4 | 1329 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [1.11, 1.61] |
| 1.1 Homozygous Major | 4 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.94, 1.65] |
| 1.2 Heterozygous | 4 | 633 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.09, 1.91] |
| 1.3 Homozygous Minor | 4 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.57, 2.99] |
| 2 End of Treatment | 6 | 1379 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.19, 1.64] |
| 2.1 Homozygous Major | 6 | 496 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [1.05, 1.67] |
| 2.2 Heterozygous | 6 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.03, 1.79] |
| 2.3 Homozygous Minor | 6 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.20, 2.79] |
Comparison 9. Bupropion vs placebo ‐ rs1051730 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [0.75, 3.00] |
| 1.1 Homozygous Major | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.71, 3.64] |
| 1.2 Heterozygous | 2 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.32, 5.34] |
| 2 End of Treatment | 3 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.70, 2.06] |
| 2.1 Homozygous Major | 3 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [0.96, 3.23] |
| 2.2 Heterozygous | 3 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.32, 1.44] |
Comparison 10. Bupropion vs placebo ‐ rs16969968 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 3 | 557 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.13, 1.77] |
| 1.1 Homozygous Major | 3 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.92, 1.99] |
| 1.2 Heterozygous | 3 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [1.04, 2.04] |
| 1.3 Homozygous Minor | 3 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 2.55 [0.95, 6.85] |
Comparison 11. Bupropion vs placebo ‐ rs16969968 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | 578 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.84, 2.00] |
| 1.1 Homozygous Major | 2 | 512 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.75, 1.87] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 2.82 [0.76, 10.45] |
| 2 End of Treatment | 3 | 611 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.23, 3.08] |
| 2.1 Homozygous Major | 3 | 541 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [1.44, 3.10] |
| 2.2 Heterozygous or Homozygous Minor | 3 | 70 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.33, 8.36] |
Comparison 12. Bupropion vs placebo ‐ rs588765 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 4 | 1107 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.12, 1.67] |
| 1.1 Homozygous Major | 4 | 384 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.85, 2.08] |
| 1.2 Heterozygous | 4 | 526 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.95, 1.65] |
| 1.3 Homozygous Minor | 4 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.18, 3.22] |
| 2 End of Treatment | 4 | 1107 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.13, 1.65] |
| 2.1 Homozygous Major | 4 | 385 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.05, 1.79] |
| 2.2 Heterozygous | 4 | 525 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.86, 2.01] |
| 2.3 Homozygous Minor | 4 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.99, 2.06] |
Comparison 13. Bupropion vs placebo ‐ rs2036527 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | 738 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [1.05, 1.86] |
| 1.1 Homozygous Major | 2 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.73, 1.80] |
| 1.2 Heterozygous | 2 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.99, 2.06] |
| 1.3 Homozygous Minor | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 2.73 [1.02, 7.36] |
| 2 End of Treatment | 4 | 975 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.19, 1.73] |
| 2.1 Homozygous Major | 4 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.04, 1.80] |
| 2.2 Heterozygous | 4 | 482 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.04, 1.79] |
| 2.3 Homozygous Minor | 4 | 154 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.33, 5.25] |
Comparison 14. Bupropion vs placebo ‐ rs2036527 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 3 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.88, 1.92] |
| 1.1 Homozygous Major | 3 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.70, 1.98] |
| 1.2 Heterozygous or Homozygous Minor | 3 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.71, 2.91] |
| 2 End of Treatment | 4 | 693 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [1.36, 2.56] |
| 2.1 Homozygous Major | 4 | 395 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.39, 3.22] |
| 2.2 Heterozygous or Homozygous Minor | 4 | 298 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.93, 2.62] |
Comparison 15. Bupropion vs placebo ‐ rs3733829 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | 571 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.07, 1.87] |
| 1.1 Homozygous Major | 2 | 268 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.81, 1.82] |
| 1.2 Heterozygous | 2 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [1.22, 3.13] |
| 1.3 Homozygous Minor | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.58, 2.17] |
| 2 End of Treatment | 4 | 807 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.29, 1.87] |
| 2.1 Homozygous Major | 4 | 367 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [1.11, 1.86] |
| 2.2 Heterozygous | 4 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.24, 2.30] |
| 2.3 Homozygous Minor | 4 | 107 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.96, 2.95] |
Comparison 16. Bupropion vs placebo ‐ rs3733829 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.75, 3.04] |
| 1.1 Homozygous Major | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.75, 3.04] |
Comparison 17. Bupropion vs placebo ‐ rs7937 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 3 | 557 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.13, 1.78] |
| 1.1 Homozygous Major | 3 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.76 [1.13, 2.75] |
| 1.2 Heterozygous | 3 | 285 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.95, 1.76] |
| 1.3 Homozygous Minor | 3 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.82, 2.31] |
Comparison 18. Bupropion vs placebo ‐ rs7937 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.77, 3.18] |
| 1.1 Homozygous Major | 2 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.70 [0.57, 5.10] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.42, 5.84] |
Comparison 19. Bupropion vs placebo ‐ rs1329650 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 3 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [1.07, 1.74] |
| 1.1 Homozygous Major | 3 | 280 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.07, 2.22] |
| 1.2 Heterozygous | 3 | 244 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.88, 1.96] |
| 1.3 Homozygous Minor | 3 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.33, 4.74] |
Comparison 20. Bupropion vs placebo ‐ rs1329650 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.70, 2.99] |
| 1.1 Homozygous Major | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [0.67, 3.96] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 13 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.15, 5.70] |
Comparison 21. Bupropion vs placebo ‐ rs1028936 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 3 | 559 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.11, 1.74] |
| 1.1 Homozygous Major | 3 | 370 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [1.15, 2.06] |
| 1.2 Heterozygous | 3 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.75, 2.35] |
| 1.3 Homozygous Minor | 3 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.30, 8.49] |
Comparison 22. Bupropion vs placebo ‐ rs1028936 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.68, 3.21] |
| 1.1 Homozygous Major | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [0.74, 4.20] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 11 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.09, 4.77] |
Comparison 23. Bupropion vs placebo ‐ rs215605 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 3 | 558 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [1.18, 1.87] |
| 1.1 Homozygous Major | 3 | 219 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.23, 2.55] |
| 1.2 Heterozygous | 3 | 259 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.98, 1.87] |
| 1.3 Homozygous Minor | 3 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.49, 2.55] |
Comparison 24. Bupropion vs placebo ‐ rs215605 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.54, 3.69] |
| 1.1 Homozygous Major | 2 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.15, 24.95] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 1.67 [0.46, 6.13] |
Comparison 25. Bupropion + any NRT vs placebo ‐ rs1051730 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [1.07, 3.03] |
| 1.1 Homozygous Major | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [0.31, 14.60] |
| 1.2 Heterozygous | 2 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.23, 2.69] |
| 1.3 Homozygous Minor | 2 | 45 | Risk Ratio (M‐H, Random, 95% CI) | 2.84 [0.90, 8.89] |
Comparison 26. Bupropion + any NRT vs placebo ‐ rs1051730 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.27, 3.07] |
| 1.1 Homozygous Major | 2 | 58 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [0.56, 4.83] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 11 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.03, 1.43] |
Comparison 27. Bupropion + any NRT vs placebo ‐ rs16969968 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.07, 2.99] |
| 1.1 Homozygous Major | 2 | 183 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [0.31, 14.60] |
| 1.2 Heterozygous | 2 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [1.22, 2.66] |
| 1.3 Homozygous Minor | 2 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 2.81 [0.89, 8.88] |
Comparison 28. Bupropion + any NRT vs placebo ‐ rs16969968 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.46, 2.38] |
| 1.1 Homozygous Major | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.51, 2.85] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 6 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.03, 4.19] |
Comparison 29. Bupropion + any NRT vs placebo ‐ rs2036527 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 443 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [1.09, 2.77] |
| 1.1 Homozygous Major | 2 | 181 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [0.33, 14.75] |
| 1.2 Heterozygous | 2 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [1.23, 2.72] |
| 1.3 Homozygous Minor | 2 | 42 | Risk Ratio (M‐H, Random, 95% CI) | 2.12 [0.79, 5.65] |
Comparison 30. Bupropion + any NRT vs placebo ‐ rs2036527 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.52, 1.92] |
| 1.1 Homozygous Major | 2 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 2.03 [0.39, 10.56] |
| 1.2 Heterozygous | 2 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.38, 2.77] |
| 1.3 Homozygous Minor | 2 | 8 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.26, 2.05] |
Comparison 31. Bupropion + any NRT vs placebo ‐ rs3733829 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [1.20, 2.01] |
| 1.1 Homozygous Major | 2 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 1.48 [0.83, 2.65] |
| 1.2 Heterozygous | 2 | 201 | Risk Ratio (M‐H, Random, 95% CI) | 1.87 [1.04, 3.36] |
| 1.3 Homozygous Minor | 2 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.91, 3.74] |
Comparison 32. Bupropion + any NRT vs placebo ‐ rs3733829 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.14, 1.24] |
| 1.1 Homozygous Major | 2 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.14, 1.24] |
Comparison 33. Bupropion + any NRT vs placebo ‐ rs7937 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [1.16, 1.97] |
| 1.1 Homozygous Major | 2 | 131 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [0.84, 4.75] |
| 1.2 Heterozygous | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.99, 1.94] |
| 1.3 Homozygous Minor | 2 | 82 | Risk Ratio (M‐H, Random, 95% CI) | 2.34 [0.49, 11.08] |
Comparison 34. Bupropion + any NRT vs placebo ‐ rs7937 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.47, 2.38] |
| 1.1 Homozygous Major | 2 | 31 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.40, 2.73] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.23, 4.99] |
Comparison 35. Bupropion + any NRT vs placebo ‐ rs1329650 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 1.62 [1.10, 2.39] |
| 1.1 Homozygous Major | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.31, 3.00] |
| 1.2 Heterozygous | 2 | 172 | Risk Ratio (M‐H, Random, 95% CI) | 1.60 [0.70, 3.67] |
| 1.3 Homozygous Minor | 2 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.17, 25.25] |
Comparison 36. Bupropion + any NRT vs placebo ‐ rs1329650 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.48, 2.41] |
| 1.1 Homozygous Major | 2 | 55 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.40, 2.55] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.26, 6.80] |
Comparison 37. Bupropion + any NRT vs placebo ‐ rs1028936 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 444 | Risk Ratio (M‐H, Random, 95% CI) | 1.64 [1.08, 2.49] |
| 1.1 Homozygous Major | 2 | 305 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.34, 2.67] |
| 1.2 Heterozygous | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.48, 4.20] |
| 1.3 Homozygous Minor | 2 | 14 | Risk Ratio (M‐H, Random, 95% CI) | 1.93 [0.47, 8.02] |
Comparison 38. Bupropion + any NRT vs placebo ‐ rs1028936 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 69 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.48, 2.41] |
| 1.1 Homozygous Major | 2 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.43, 2.84] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 12 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.17, 5.39] |
Comparison 39. Bupropion + any NRT vs placebo ‐ rs215605 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 442 | Risk Ratio (M‐H, Random, 95% CI) | 1.72 [1.16, 2.53] |
| 1.1 Homozygous Major | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 2.00 [1.32, 3.03] |
| 1.2 Heterozygous | 2 | 200 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.60, 3.63] |
| 1.3 Homozygous Minor | 2 | 71 | Risk Ratio (M‐H, Random, 95% CI) | 2.29 [0.72, 7.27] |
Comparison 40. Bupropion + any NRT vs placebo ‐ rs215605 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 68 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.41, 1.77] |
| 1.1 Homozygous Major | 2 | 36 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.29, 1.76] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 32 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.34, 4.13] |
Comparison 41. Bupropion + any NRT vs bupropion ‐ rs1051730 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.90, 1.55] |
| 1.1 Homozygous Major | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.54, 2.42] |
| 1.2 Heterozygous | 2 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.93, 1.61] |
| 1.3 Homozygous Minor | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.68, 2.44] |
Comparison 42. Bupropion + any NRT vs bupropion ‐ rs1051730 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.37, 1.31] |
| 1.1 Homozygous Major | 2 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.40, 1.49] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 21 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.05, 2.19] |
Comparison 43. Bupropion + any NRT vs bupropion ‐ rs16969968 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.90, 1.55] |
| 1.1 Homozygous Major | 2 | 206 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.54, 2.42] |
| 1.2 Heterozygous | 2 | 262 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.93, 1.61] |
| 1.3 Homozygous Minor | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.66, 2.44] |
Comparison 44. Bupropion + any NRT vs bupropion ‐ rs16969968 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.37, 1.27] |
| 1.1 Homozygous Major | 2 | 74 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.38, 1.40] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 13 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.06, 2.76] |
Comparison 45. Bupropion + any NRT vs bupropion ‐ rs2036527 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.91, 1.52] |
| 1.1 Homozygous Major | 2 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.54, 2.52] |
| 1.2 Heterozygous | 2 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.92, 1.60] |
| 1.3 Homozygous Minor | 2 | 64 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.68, 2.25] |
Comparison 46. Bupropion + any NRT vs bupropion ‐ rs2036527 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.35, 1.23] |
| 1.1 Homozygous Major | 2 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.23, 1.35] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 37 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.32, 1.90] |
Comparison 47. Bupropion + any NRT vs bupropion ‐ rs3733829 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.96, 1.39] |
| 1.1 Homozygous Major | 2 | 236 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.77, 1.84] |
| 1.2 Heterozygous | 2 | 229 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.82, 1.51] |
| 1.3 Homozygous Minor | 2 | 66 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.80, 2.25] |
Comparison 48. Bupropion + any NRT vs bupropion ‐ rs3733829 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.25, 0.99] |
| 1.1 Homozygous Major | 2 | 65 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.18, 1.01] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.13, 4.20] |
Comparison 49. Bupropion + any NRT vs bupropion ‐ rs7937 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.95, 1.42] |
| 1.1 Homozygous Major | 2 | 145 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.65, 1.59] |
| 1.2 Heterozygous | 2 | 272 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.81, 2.00] |
| 1.3 Homozygous Minor | 2 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.90, 1.84] |
Comparison 50. Bupropion + any NRT vs bupropion ‐ rs7937 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.43] |
| 1.1 Homozygous Major | 2 | 33 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.13, 2.86] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.27, 1.93] |
Comparison 51. Bupropion + any NRT vs bupropion ‐ rs1329650 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 531 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.97, 1.49] |
| 1.1 Homozygous Major | 2 | 279 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.66, 2.25] |
| 1.2 Heterozygous | 2 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.92, 1.62] |
| 1.3 Homozygous Minor | 2 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 1.68 [0.89, 3.17] |
Comparison 52. Bupropion + any NRT vs bupropion ‐ rs1329650 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.33, 1.20] |
| 1.1 Homozygous Major | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.30, 1.15] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 11 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.17, 8.24] |
Comparison 53. Bupropion + any NRT vs bupropion ‐ rs1028936 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.92, 1.50] |
| 1.1 Homozygous Major | 2 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.86, 1.37] |
| 1.2 Heterozygous | 2 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.62, 2.75] |
| 1.3 Homozygous Minor | 2 | 15 | Risk Ratio (M‐H, Random, 95% CI) | 2.17 [0.51, 9.18] |
Comparison 54. Bupropion + any NRT vs bupropion ‐ rs1028936 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.33, 1.20] |
| 1.1 Homozygous Major | 2 | 76 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.30, 1.15] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 11 | Risk Ratio (M‐H, Random, 95% CI) | 1.2 [0.17, 8.24] |
Comparison 55. Bupropion + any NRT vs bupropion ‐ rs215605 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 532 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.93, 1.47] |
| 1.1 Homozygous Major | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 1.28 [0.91, 1.80] |
| 1.2 Heterozygous | 2 | 241 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.69, 1.45] |
| 1.3 Homozygous Minor | 2 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.64, 4.08] |
Comparison 56. Bupropion + any NRT vs bupropion ‐ rs215605 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | 87 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.37, 1.35] |
| 1.1 Homozygous Major | 2 | 49 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.09, 2.94] |
| 1.2 Heterozygous or Homozygous Minor | 2 | 38 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.26, 1.93] |
Comparison 57. Active NRT ‐ rs1051730 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 7 | 1559 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.71, 1.19] |
| 1.2 Heterozygous vs Homozygous Minor | 7 | 1650 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.75, 1.11] |
| 2 End of Treatment | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 5 | 1200 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.73, 1.06] |
| 2.2 Heterozygous vs Homozygous Minor | 5 | 1291 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.79, 1.10] |
Comparison 58. Active NRT ‐ rs1051730 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.26, 4.57] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.25, 4.99] |
Comparison 59. Active NRT ‐ rs16969968 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 2.24 [0.92, 5.45] |
| 2 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 392 | Risk Ratio (M‐H, Random, 95% CI) | 2.75 [1.08, 7.02] |
Comparison 60. Active NRT ‐ rs588765 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 3 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.80, 1.37] |
| 1.2 Heterozygous vs Homozygous Minor | 3 | 546 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.76, 1.29] |
| 2 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 3 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.76, 1.18] |
| 2.2 Heterozygous vs Homozygous Minor | 3 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.77, 1.17] |
Comparison 61. Active NRT ‐ rs2036527 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.60, 1.03] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 579 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 2 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 2 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.59, 1.35] |
| 2.2 Heterozygous vs Homozygous Minor | 2 | 579 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.61, 1.64] |
Comparison 62. Active NRT ‐ rs2036527 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.87, 1.81] |
| 2 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 391 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.20, 2.49] |
Comparison 63. Active NRT ‐ DRD‐4 (exon 3 48 bp) ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 3 | 866 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.51, 1.76] |
| 1.2 Heterozygous vs Homozygous Minor | 3 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.42, 1.60] |
| 2 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 3 | 866 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.23] |
| 2.2 Heterozygous vs Homozygous Minor | 3 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.58, 1.26] |
Comparison 64. Active NRT ‐ SLC6A4 (Promoter) ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 461 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.50, 1.67] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 649 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.66, 1.90] |
| 2 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 2 | 461 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.23] |
| 2.2 Heterozygous vs Homozygous Minor | 2 | 649 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.74, 1.18] |
Comparison 65. Active NRT‐NMR ‐ non‐Hispanic white or black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Normal NMR vs Slow NMR | 2 | 718 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.37, 0.78] |
| 2 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Normal NMR vs Slow NMR | 2 | 718 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.58, 1.03] |
Comparison 66. Bupropion ‐ rs1051730 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 4 | 316 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.67, 2.43] |
| 1.2 Heterozygous vs Homozygous Minor | 4 | 390 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.65, 2.03] |
| 2 End of Treatment | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 6 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.88, 1.50] |
| 2.2 Heterozygous vs Homozygous Minor | 6 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.81, 1.33] |
Comparison 67. Bupropion ‐ rs1051730 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.36, 1.95] |
| 2 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Heterozygous or Homozygous Minor | 3 | 84 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.51, 1.72] |
Comparison 68. Bupropion ‐ rs16969968 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 3 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.96, 2.25] |
| 1.2 Heterozygous vs Homozygous Minor | 3 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.79, 1.84] |
Comparison 69. Bupropion ‐ rs16969968 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.31, 1.21] |
| 2 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Heterozygous or Homozygous Minor | 3 | 315 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.48, 1.32] |
Comparison 70. Bupropion ‐ rs588765 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 4 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.34] |
| 1.2 Heterozygous vs Homozygous Minor | 4 | 401 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.66, 1.21] |
| 2 End of Treatment | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 4 | 304 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.69, 1.14] |
| 2.2 Heterozygous vs Homozygous Minor | 4 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.22] |
Comparison 71. Bupropion ‐ rs2036527 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 208 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.33, 1.81] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 269 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.51, 1.37] |
| 2 End of Treatment | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 4 | 277 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.79, 1.70] |
| 2.2 Heterozygous vs Homozygous Minor | 4 | 361 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.61, 1.51] |
Comparison 72. Bupropion ‐ rs2036527 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 3 | 331 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.55, 1.46] |
| 2 End of Treatment | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Heterozygous or Homozygous Minor | 4 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.87, 1.76] |
Comparison 73. Bupropion ‐ rs3733829 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 191 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.56, 1.46] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 161 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.65, 1.71] |
| 2 End of Treatment | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 4 | 259 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.73, 1.40] |
| 2.2 Heterozygous vs Homozygous Minor | 4 | 237 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.70, 1.36] |
Comparison 74. Bupropion ‐ rs3733829 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 46 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.37, 2.99] |
Comparison 75. Bupropion ‐ rs7937 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 3 | 159 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.71, 1.40] |
| 1.2 Heterozygous vs Homozygous Minor | 3 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.65, 1.23] |
Comparison 76. Bupropion ‐ rs7937 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.32, 4.26] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 27 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.18, 2.93] |
Comparison 77. Bupropion ‐ rs1329650 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 3 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.54, 1.35] |
| 1.2 Heterozygous vs Homozygous Minor | 3 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
Comparison 78. Bupropion ‐ rs1329650 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.35, 6.01] |
Comparison 79. Bupropion ‐ rs1028936 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 3 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.42, 1.55] |
| 1.2 Heterozygous vs Homozygous Minor | 3 | 112 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.40, 1.64] |
Comparison 80. Bupropion ‐ rs1028936 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.35, 6.01] |
Comparison 81. Bupropion ‐ rs215605 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 3 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.87, 3.00] |
| 1.2 Heterozygous vs Homozygous Minor | 3 | 199 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.79, 3.06] |
Comparison 82. Bupropion ‐ rs215605 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 47 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.54, 2.44] |
Comparison 83. Varenicline ‐ rs16969968 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.71, 1.25] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 438 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.30] |
| 2 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 2 | 358 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.78, 1.24] |
| 2.2 Heterozygous vs Homozygous Minor | 2 | 438 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
Comparison 84. Varenicline ‐ rs588765 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.91, 1.60] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.90, 1.54] |
| 2 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 2 | 354 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.91, 1.43] |
| 2.2 Heterozygous vs Homozygous Minor | 2 | 465 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.89, 1.37] |
Comparison 85. Bupropion + any NRT ‐ rs1051730 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.67, 1.78] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.69, 1.80] |
Comparison 86. Bupropion + any NRT ‐ rs1051730 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 2.26 [0.36, 14.38] |
Comparison 87. Bupropion + any NRT ‐ rs16969968 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 136 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.67, 1.85] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.68, 1.86] |
Comparison 88. Bupropion + any NRT ‐ rs16969968 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [0.27, 10.32] |
Comparison 89. Bupropion + any NRT ‐ rs2036527 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 135 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.70, 1.79] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 156 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.65, 1.66] |
Comparison 90. Bupropion + any NRT ‐ rs2036527 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 28 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.06, 4.51] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 17 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.03, 15.98] |
Comparison 91. Bupropion + any NRT ‐ rs3733829 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 144 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.66, 1.34] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.55, 1.14] |
Comparison 92. Bupropion + any NRT ‐ rs3733829 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.13, 0.99] |
Comparison 93. Bupropion + any NRT ‐ rs7937 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.57, 1.10] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.51, 1.28] |
Comparison 94. Bupropion + any NRT ‐ rs7937 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.12, 13.56] |
Comparison 95. Bupropion + any NRT ‐ rs1329650 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 170 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.94] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.50, 1.03] |
Comparison 96. Bupropion + any NRT ‐ rs1329650 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.15, 1.94] |
Comparison 97. Bupropion + any NRT ‐ rs1028936 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 193 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.37, 1.07] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 85 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.42, 1.32] |
Comparison 98. Bupropion + any NRT ‐ rs1028936 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.15, 1.94] |
Comparison 99. Bupropion + any NRT ‐ rs215605 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.84, 1.66] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.61, 1.26] |
Comparison 100. Bupropion + any NRT ‐ rs215605 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.04, 13.56] |
Comparison 101. Placebo ‐ rs1051730 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 6 | 533 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.86, 1.92] |
| 1.2 Heterozygous vs Homozygous Minor | 6 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.72, 1.62] |
| 2 End of Treatment | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 7 | 542 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.91, 2.13] |
| 2.2 Heterozygous vs Homozygous Minor | 7 | 605 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.81, 1.89] |
Comparison 102. Placebo ‐ rs1051730 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.08, 6.73] |
| 2 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Heterozygous or Homozygous Minor | 3 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.24, 2.45] |
Comparison 103. Placebo ‐ rs16969968 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.51, 2.14] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.32, 1.96] |
| 2 End of Treatment | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 4 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.68, 3.53] |
| 2.2 Heterozygous vs Homozygous Minor | 4 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.51, 3.53] |
Comparison 104. Placebo ‐ rs16969968 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 3 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.42, 1.33] |
| 2 End of Treatment | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Heterozygous or Homozygous Minor | 4 | 596 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.35, 0.86] |
Comparison 105. Placebo ‐ rs588765 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 5 | 383 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.69, 1.57] |
| 1.2 Heterozygous vs Homozygous Minor | 5 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.72, 1.58] |
| 2 End of Treatment | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 5 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.63, 1.21] |
| 2.2 Heterozygous vs Homozygous Minor | 5 | 404 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.80, 1.46] |
Comparison 106. Placebo ‐ rs588765 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.80, 1.79] |
| 2 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.71, 1.77] |
Comparison 107. Placebo ‐ rs2036527 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 1.85 [0.77, 4.44] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.66, 3.77] |
| 2 End of Treatment | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 4 | 216 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [0.91, 4.35] |
| 2.2 Heterozygous vs Homozygous Minor | 4 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 1.86 [0.94, 3.69] |
Comparison 108. Placebo ‐ rs2036527 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 4 | 629 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.71, 1.77] |
| 2 End of Treatment | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Heterozygous or Homozygous Minor | 5 | 641 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.71, 1.64] |
Comparison 109. Placebo ‐ DRD‐4 (exon 3 48 bp) ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 3 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.37, 3.16] |
| 1.2 Heterozygous vs Homozygous Minor | 3 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.22, 1.63] |
| 2 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 3 | 387 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.51, 2.29] |
| 2.2 Heterozygous vs Homozygous Minor | 3 | 212 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.61, 1.29] |
Comparison 110. Placebo ‐ rs3733829 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 2 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.46, 1.51] |
| 1.2 Heterozygous vs Homozygous Minor | 2 | 142 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.35, 1.26] |
| 2 End of Treatment | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major vs Homozygous Minor | 4 | 215 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.74, 2.05] |
| 2.2 Heterozygous vs Homozygous Minor | 4 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.57, 1.65] |
Comparison 111. Placebo ‐ rs7937 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 3 | 113 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.45, 1.45] |
| 1.2 Heterozygous vs Homozygous Minor | 3 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.57, 1.60] |
Comparison 112. Placebo ‐ rs7937 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.27, 5.04] |
Comparison 113. Placebo ‐ rs1329650 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 3 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.18, 3.62] |
| 1.2 Heterozygous vs Homozygous Minor | 3 | 116 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.39, 1.63] |
Comparison 114. Placebo ‐ rs1329650 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.19, 2.78] |
Comparison 115. Placebo ‐ rs1028936 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 3 | 163 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.29, 3.14] |
| 1.2 Heterozygous vs Homozygous Minor | 3 | 77 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.36, 4.03] |
Comparison 116. Placebo ‐ rs1028936 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.18, 1.32] |
Comparison 117. Placebo ‐ rs215605 ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Homozygous Minor | 3 | 129 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.52, 1.82] |
| 1.2 Heterozygous vs Homozygous Minor | 3 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.63, 2.12] |
Comparison 118. Placebo ‐ rs215605 ‐ non‐Hispanic black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major vs Heterozygous or Homozygous Minor | 2 | 29 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.03, 38.58] |
Comparison 119. Placebo ‐ SLC6A4 (Promoter) ‐ non‐Hispanic white.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Homozygous Major or Heterozygous vs Homozygous Minor | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.79] |
| 2 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Homozygous Major or Heterozygous vs Homozygous Minor | 2 | 151 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.83, 1.38] |
Comparison 120. Placebo ‐ NMR ‐ non‐Hispanic white or black or African American.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Six‐Month Abstinence | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Normal NMR vs Slow NMR | 2 | 699 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.70, 1.48] |
| 2 End of Treatment | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Normal NMR vs Slow NMR | 2 | 718 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.35, 1.14] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahluwalia 2006.
| Methods | 2 × 2 factorial randomised clinical trial conducted to evaluate the efficacy of nicotine gum and counseling for African American light smokers (the “Kick It at Swope II Trial” (KIS‐II)) Study period: March 2003 to June 2004 |
|
| Participants | N = 755 Participants were recruited using clinic, media, and community outreach efforts, including radio, television, gas pump, billboard advertising, community health fairs, signs posted in minority‐owned businesses, and referral letters mailed from physicians in the Kansas City, KS, USA, area Inclusion criteria: (a) self‐identified as ‘African American or black’, (b) ≥ 18 years, (c) smoked ≤ 10 cigarettes/d for ≥ 6 months, (d) before enrolment, smoked on ≥ 25 of the last 30 days, (e) was interested in quitting in the next 2 weeks, (f) spoke English, and (g) had a permanent home address and working telephone. Only 1 smoker per household was allowed to enrol Exclusion criteria: (a) contraindication for nicotine gum (jaw problems, irregular heartbeat, recent myocardial infarction, or stroke), (b) used other pharmacotherapy for smoking cessation in the last 30 days, (c) used other forms of tobacco within the last 30 days, (d) was pregnant or planning to become pregnant within the next 6 months, (e) was breastfeeding, or (f) was planning to move out of the local area within the next 6 months. Individuals demonstrating marked inappropriate affect or behavior were excluded from the study. |
|
| Interventions | Health education plus NRT gum (N = 189) Motivational interviewing plus NRT gum (N = 189) Health education plus placebo gum (N = 189) Motivational interviewing plus placebo gum (N = 188) Participants were assigned randomly to 1 of 4 study arms: 2 mg nicotine gum plus health education (HE); 2 mg nicotine gum plus motivational interviewing (MI); placebo gum plus HE; and placebo gum plus MI |
|
| Outcomes |
Primary outcomes: cotinine‐verified 7‐day abstinence at week 26, defined as having smoked no cigarettes ‐ not even a puff ‐ on the previous 7 days A salivary cotinine cutoff of ≤ 20 ng/mL was used to verify abstinence at 26 weeks; a cutoff of ≤ 10 ppm was used for CO. Secondary outcomes: Secondary outcome was 7‐day abstinence at week 8. Process measures included counseling attendance at randomisation and at weeks 1, 3, 6, and 8; and 16 counseling visits and self‐reported gum usage in the past 7 days at weeks 1, 3, and 8 (end of gum treatment). |
|
| Funding source | This project was supported by the National Cancer Institute at the National Institutes of Health (R01 CA091912). GlaxoSmithKline provided study medication but played no role in design or conduct of the study nor in interpretation and analysis of data. | |
| Declaration of interest | “NLB serves as a consultant to several pharmaceutical companies that market smoking cessation medications and has been a paid expert witness in litigation against tobacco companies. SPD is a scientific adviser to Genophen. RFT has participated in one‐day advisory meetings for Novartis and McNeil. RFT is an Associate Editor for Clinical Pharmacology & Therapeutics but was not involved in the review or decision process for this article. The other authors declared no conflict of interest.” |
|
| Notes | The present study did not report analyses of pharmacogenetics. However, 608/755 (80%) were successfully genotyped. These results were published in subsequent papers (Ho 2009; Zhu 2014). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation codes were generated in blocks and were linked to medication distribution and counseling assignment. |
| Allocation concealment (selection bias) | Low risk | “The Investigative Pharmacy at the University of Kansas Medical Center packaged the study medication using codes to maintain blinding. At the randomization visit, a sealed envelope with pre‐assigned randomization numbers was drawn to determine which form of counseling the participant would receive. The envelope and box of gum with matching randomization numbers were given to participants in the order in which they were randomized.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Study staff and participants were blinded to whether participants received active gum or placebo. However, assignment to MI counseling versus HE was not blinded.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers and participants were blinded to active or placebo medication. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomised participants were accounted for in the analyses. |
| Selective reporting (reporting bias) | Low risk | Primary endpoints were reported. |
| Other bias | Low risk | 80% of participants were genotyped, which was balanced across drug treatment groups. See ‘Notes’ above. |
Aveyard 2007.
| Methods | Randomised controlled clinical trial of behavioural support for smoking cessation (the “Patch in Practice Study”) Study period: July 2002 to March 2005 |
|
| Participants | N = 925 Recruited from 26 general practice clinics in Buckinghamshire and Oxfordshire, UK Inclusion criteria: current smokers, age ≥ 18, smoked ≥ 10 cigarettes/d Exclusion criteria: contraindications to nicotine replacement therapy (NRT) |
|
| Interventions | Basic support (N = 469) Weekly support (N = 456) Participants were randomised to behavioural support provided by practice nurse before quitting, telephoned around quit day, and seen 1 and 4 weeks after the initial appointment (basic support) vs basic support plus weekly support ‐ additional telephone call at 10 days and 3 weeks after the initial appointment, and an additional visit at 2 weeks to motivate adherence to nicotine replacement and to renew quit attempts. 15 mg/16 h nicotine patches were given to all participants. |
|
| Outcomes |
Primary outcomes: confirmed sustained abstinence at 1, 4, 12, and 26 weeks from quit day. Sustained abstinence was defined as self‐reported total abstinence after a 14‐day grace period from quit date confirmed by expired air carbon monoxide (CO) < 10 ppm. Secondary outcomes: not indicated |
|
| Funding source | “This study was funded by a programme grant from Cancer Research UK (trial registration ISRCTN 05689186). United Pharmaceuticals supplied the nicotine patches for the study free to be given without charge to the participants.” | |
| Declaration of interest | “PA has received free nicotine replacement products from Novartis and nortriptyline from King Pharmaceuticals for distribution to trial participants; personal income for advice to Xenova, a biotechnology company investigating a nicotine vaccine; small gifts and had numerous meals paid for by drug companies, including those producing medications for smoking cessation; and travel grants to attend conferences from the Society for Research in Nicotine and Tobacco. KB, CS, and AA have received small gifts and had meals paid for by drug companies, including those manufacturing medications for smoking cessation. M Munafó has received fees for invited lectures from the National Health Service, GlaxoSmithKline, Novartis, the Moffitt Cancer Research Center, and the Karolinska Institutet; benefits in kind (hospitality, etc.) from various pharmaceutical companies; research and travel support from the European Research Advisory Board, GlaxoSmithKline, Pfizer Consumer Healthcare and Novartis; and he has acted as a consultant to the European Commission, The American Institutes for Research, the National Audit Office, and G‐Nostics Ltd. EJ has received consultancy income from the European Network for Smoking Prevention. M Murphy has received consultancy income from the European Network for Smoking Prevention and has provided scientific consultancy services through the University of Oxford ISIS Innovation to the National Audit Office and G‐Nostics Ltd. The Childhood Cancer Research Group and the Cancer Research UK General Practice Research Group have received unrestricted educational grants, research project grants, and consultancy fees from Ciba Geigy/Novartis, GlaxoSmithKline, Pharmacia/Pfizer, Ares‐Serono, Sanofi‐Synthelabo, Third Wave Technologies, Astra Zeneca, and G‐Nostics.” | |
| Notes | This paper did not report analyses of pharmacogenetics. However, DNA was collected from all trial participants and analyses of pharmacogenetics were reported in subsequent papers (David 2008; David 2011; Munafò 2008;Munafò 2009; Munafò 2011; Munafò 2012;Spruell 2012; Uhl 2010). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number sequence generation specified |
| Allocation concealment (selection bias) | Low risk | Random number sequence sealed in numbered envelopes. Nurses opened sealed envelopes in sequence following eligibility and consent determination. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and nurses were necessarily not blinded to allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research staff was blinded to allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was balanced and transparently reported. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes were reported. |
| Other bias | Low risk | DNA samples were collected on all participants at the time of study entry. |
Bloch 2010.
| Methods | Double‐blind parallel‐group placebo‐controlled randomised clinical trial Study period: dates not reported |
|
| Participants | N = 61 Participants were schizophrenic patients on stable neuroleptic medication who were recruited by hospital doctors and clinicians from community mental health centres and ambulatory clinics. Inclusion criteria: age between 18 and 70 years, met DSM‐IV‐TR criteria for schizophrenia or schizoaffective disorder, clinically stable (based on psychotic and affective symptoms) as judged by treatment team and psychiatrists, had a stable dose of antipsychotic drugs for ≥ 1 month before the start date, in stable physical health, stable cigarette smoking habits (not defined), strong desire to quit smoking or at least to reduce significantly the number of cigarettes per day (score > 5 on motivational questionnaire) Exclusion criteria: not reported |
|
| Interventions | Sustained‐release bupropion + cognitive‐behavioural group therapy (n = 45) Placebo + cognitive‐behavioural group therapy (n = 16) All participants had 2‐week stabilisation period followed by 14 weeks of study medication. Initial dose was 150 mg/d for 3 days, then 300 mg/d. All participants participated in a 14‐week, 15‐session group cognitive‐behavioural therapy. Participants received no additional treatment. |
|
| Outcomes | Outcomes: self‐reports of daily cigarette consumption and the Fagerstrom Test of Nicotine Dependence (FTND), both measured at baseline, week 7, and week 14 | |
| Funding source | This research was supported by a Junior Investigator Award from the National Alliance for Research on Schizophrenia and Depression (NARSAD) (AR) and was partially supported by Phillip Morris USA and Phillip Morris International. | |
| Declaration of interest | Declaration of interest was not reported. | |
| Notes | This paper provided analysis of pharmacogenetics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Participants were randomly allocated according to order of arrival. |
| Allocation concealment (selection bias) | High risk | Allocation was based on order of arrival. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The study was double‐blind; however it is unclear who exactly was blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of participants and personnel was unclear; study outcome is objective and can be influenced by participants. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Incomplete outcome data were greater in the bupropion group, potentially influencing the overall treatment effect. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found. |
| Other bias | Low risk | Analysis of pharmacogenetics was performed on the total study population. |
Brown 2007.
| Methods | Randomised double‐blind placebo‐controlled trial of sustained‐release bupropion and behavioural support for smoking cessation (the “Zyban Collaborative Trial”) Study period: November 1997 to January 2001 |
|
| Participants | N = 524 Recruited from the general population and treated at 1 of 3 community‐based academic teaching hospitals in Pawtucket and Providence, RI, USA Inclusion criteria: current smokers, age ≥ 18, smoked ≥ 10 cigarettes/d Exclusion criteria: (a) current Axis I disorder according to the Diagnostic and Statistical Manual of Mental Disorders (4th edition; DSM‐IV; American Psychiatric Association, 1994), (b) DSM‐IV diagnosis of past‐year psychoactive substance abuse or dependence (other than nicotine), (c) current use of psychotropic medication or medication that may interact adversely with bupropion, (d) current weekly (or more frequent) psychotherapy, or (e) use of other tobacco products. Participants also were screened by a study physician to rule out the following: any unstable medical condition; hypertension; pregnancy, lactation, or refusal to use contraception while on study medication; history of seizure disorder or head injury with loss of consciousness; eating disorder; or panic disorder. Participants agreed to use only study‐supplied medication for smoking cessation for the duration of their study participation. |
|
| Interventions | Standard treatment + placebo (N = 157) Standard plus depression treatment + placebo (N = 112) Standard treatment + bupropion sustained‐release (SR) (N = 147) Standard plus depression treatment + bupropion SR (N = 108) Participants were randomised to 1 of 4 twelve‐week treatments: (a) standard, cognitive–behavioural smoking cessation treatment (ST) plus bupropion (BUP), (b) ST plus placebo (PLAC), (c) standard cessation treatment combined with cognitive–behavioural treatment for depression (CBTD) plus BUP, and (d) CBTD plus PLAC. Follow‐up assessments were conducted 2, 6, and 12 months after treatment, and self‐reported abstinence was verified biochemically. Bupropion was delivered according to the standard therapeutic dose (150 mg/d for the first 3 days, followed by 300 mg/d) for a total of 12 weeks. All participants and study staff were blind to medication condition. |
|
| Outcomes | Primary outcomes: biochemically verified point prevalence abstinence at end of treatment and at 2‐, 6‐ and 12‐month follow‐up. Abstinence was confirmed by a combination of CO ≤ 10 ppm and cotinine ≤ 15 ng/mL. Secondary outcomes: withdrawal and depression symptoms, craving | |
| Funding source | This study was funded in part by US Public Health Service grants HL32318, DA08511, CA84719, and DA14276, and by GlaxoSmithKline, Inc., which provided study medication. | |
| Declaration of interest | SPD is a scientific advisor with BaseHealth and participated in a 1‐day workshop with Pfizer. MRM has received research support from Pfizer and GlaxoSmithKline. CL has served as a consultant and has received research funding from Astra Zeneca, GlaxoSmithKline, and Pfizer. | |
| Notes | DNA was collected after start of trial, resulting in genotyping for 59% of trial participants. Participants contributing DNA were significantly more likely to be female (51% vs 40%) and older (45.4 vs 43.2 years), and had been smoking longer (27.1 vs 24.8 years). Analyses of pharmacogenetics were reported in subsequent papers from the original study sample (PMID: 17654295, PMID: 18058343). An additional 60 participants were recruited, randomised to bupropion vs placebo, and administered the same ST behavioural treatment following completion of the original trial. Analyses of the larger sample were reported in additional publications (David 2013a; Leventhal 2012; Uhl 2008). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to 1 of 2 treatment sites, where they were to receive 1 of 2 manualised group treatments, ST, or CBTD, and were randomly assigned to receive 1 of 2 medication conditions or 1 of 2 behavioural interventions, using the urn randomisation technique. |
| Allocation concealment (selection bias) | Unclear risk | Behavioural treatment allocation may not have been blinded but did appear to result in balanced pharmacological treatment arms. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All participants and study staff were blind to medication condition. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of outcome assessment was not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Handling of missing data was described. Analyses using intention‐to‐treat vs only complete data were conducted and demonstrated concordance. |
| Selective reporting (reporting bias) | Low risk | Primary endpoints were reported. |
| Other bias | High risk | 59% of original trial participants were genotyped, which may not be representative of the original study population. Analyses of pharmacogenetics were reported in multiple subsequent papers. See ‘Notes’ above. |
Cinciripini 2005.
| Methods | Double‐blind parallel‐group placebo‐controlled randomised clinical trial Study period: February 1996 to April 1997 |
|
| Participants | N = 147 Participants were smokers recruited from the Houston, Texas, metropolitan area via newspaper, radio, and TV advertisements and public service announcements. Inclusion criteria: smoking ≥ 10 cigarettes/d at baseline, between 18 and 75 years old Exclusion criteria: taking smoking cessation treatment; taking psychoactive medication; or having any uncontrolled systemic illness, contraindications for taking venlafaxine or the nicotine patch, current substance abuse, or other psychiatric disorders |
|
| Interventions | Venlafaxine (n = 71) Placebo (n = 76) 21 weeks of active venlafaxine or placebo. After a 1‐week no‐medication baseline (3 weeks before the quit date), participants began antidepressant therapy 2 weeks before quitting at an initial dose of 75 mg/d (37.5 mg/d twice daily). The dose was increased up to 150 mg/d during the week just before participants were to quit. During each subsequent week, the dose was raised in 37.5‐mg increments up to a maximum 225 mg/d. Two weeks before the end of treatment, the dose was decreased by 37.5 mg every 2 to 3 days. The medication cycle was completed 18 weeks after quitting. All participants also used the nicotine patch (Prostep, 22 mg) for 6 weeks, beginning on their quit date, and received smoking cessation counseling. |
|
| Outcomes | Outcomes: Abstinence was assessed on the quit date and at post quit weeks 1, 3, 6, 18 (end of treatment), 26, and 52. Abstinence was verified in person by expired air carbon monoxide ≤ 10 ppm or by a saliva cotinine sample of < 15 ng/µL at 26 or 52 weeks. | |
| Funding source | Support for this research was provided by grants from the MD Anderson Cancer Center (PRS), the National Cancer Institute (P50CA70907), and the National Institute on Drug Abuse (R01DA1182‐01) to Paul M. Cinciripini. Study medication was provided by Wyeth‐Ayerst Laboratories. | |
| Declaration of interest | Declaration of interest was not reported. | |
| Notes | Analysis of pharmacogenetics was reported in Cinciripini 2005. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed by the pharmacy, but details are absent. |
| Allocation concealment (selection bias) | Low risk | Randomisation was centralised and was performed by a third party (pharmacy). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, study staff, and study personnel with direct patient contact were blinded to group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was appropriate and the outcome of the study was objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data were similar between groups. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found. |
| Other bias | Low risk | Additional analyses of pharmacogenetics in Cinciripini 2004 were conducted in the large majority of the original randomised trial population, so selection bias seems unlikely. |
Cox 2012.
| Methods | Randomised double‐blind placebo‐controlled trial of sustained‐release bupropion for smoking cessation in African Americans (the “Kick It at Swope III Trial” (KIS‐III)) (ClinicalTrials.gov Identifier: NCT00666978) Study period: December 2007 to May 2010 |
|
| Participants | N = 540 Participants were recruited through clinic‐ and community‐based efforts. Clinic‐based efforts included use of fliers, posters, physician letters, pharmacy inserts, and lobby recruitment at the primary study site, Swope Health Services in Kansas City, 2 Swope affiliate clinics, and 2 regional hospitals (University of Kansas Medical Center and Truman Medical Center) in Kansas City, Kansas, USA. Inclusion criteria: (a) African American, (b) ≥ 18 years, (c) interested in stopping smoking, (d) smoked ≤ 10 cigarettes/d for ≥ 2 years, (e) smoked on ≥ 25 days in the past month, and (f) were willing to attend 4 clinic visits over the course of 6 months; (g) must have smoked ≥ 3 years, and (h) had a home address and (i) a functioning telephone number Exclusion criteria: (a) current use of bupropion; (b) use of psychoactive medications; (c) use of NRT, (d) fluoxetine, (e) clonidine, (f) buspirone, or (g) doxepin in the past 30 days; (h) history of alcohol or (i) substance abuse within the past year; (j) current drinking of 14 or more alcoholic drinks per week and/or binge drinking (≥ 5 drinks on 1 occasion) 2 or more times in the past month; (k) history of seizures or head trauma; (l) history of bulimia or anorexia nervosa; (m) pregnant (verified by over‐the‐counter pregnancy test kit for women of childbearing age only) or (n) contemplating pregnancy; (o) breastfeeding; (p) myocardial infarction in the past 30 days; (q) use of other forms of tobacco in the past 30 days; (r) reported use of opiates, (s) cocaine, (t) or stimulants; (u) diabetes treated with oral hypoglycaemics or insulin; (v) planning to move from the Kansas City metro area in the next 12 months; and (w) having another smoker in the household enrolled in the study |
|
| Interventions | Bupropion SR (N = 270) Placebo (N = 270) Participants were randomly assigned to receive 300 mg bupropion SR (150 mg once daily for 3 days, then 150 mg twice daily) (n = 270 participants) or placebo (n = 270 participants) for 7 weeks, and up to 6 sessions of health education counseling. |
|
| Outcomes |
Primary outcomes: salivary cotinine–verified 7‐day point prevalence smoking abstinence at week 26 (a cut point of 15 ng/mL differentiated smokers from non‐smokers) Secondary outcomes: Salivary cotinine–verified smoking abstinence at end of medication treatment at week 7 was also examined. |
|
| Funding source | “The Kick It at Swope III (KIS‐III) study is a federally funded registered clinical trial (ClinicalTrials.gov identifier: NCT00666978) from the grant “Enhancing Tobacco Use Treatment for African American Light Smokers”.” | |
| Declaration of interest | “JSA serves as a consultant to Pfizer Pharmaceuticals, Inc.; NLB serves as a consultant to Pfizer Pharmaceuticals, Inc., and has been a paid expert witness in litigation against tobacco companies; RFT holds shares in Nicogen Research, Inc., a company that is focused on novel smoking cessation treatment approaches; no Pfizer or Nicogen funds were used in this work.” “NLB serves as a consultant to several pharmaceutical companies that market smoking cessation medications and has been a paid expert witness in litigation against tobacco companies. SPD is a scientific adviser to Genophen. RFT has participated in one‐day advisory meetings for Novartis and McNeil. RFT is an Associate Editor for Clinical Pharmacology & Therapeutics but was not involved in the review or decision process for this article. The other authors declared no conflict of interest.” | |
| Notes | The present study did not report analyses of pharmacogenetics. However, 534/540 (˜ 90%) were successfully genotyped. These results were published in subsequent papers (Zhu 2013; Zhu 2014). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated table of random numbers was used to randomise. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed before group assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study staff and participants were blinded to treatment conditions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was appropriate, and the outcome of the study was objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was ˜ 30% in both groups, but all participants were accounted for in intention‐to‐treat follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes were reported. |
| Other bias | Low risk | 534/540 (˜ 90%) from the original trial were successfully genotyped, and distribution of participants across treatment groups was balanced. See ‘Notes’ above. |
Gilbert 2009.
| Methods | Randomised parallel‐group double‐blind placebo‐controlled trial of NRT vs placebo patch with non‐randomised continuing smoking control arm Study period: 1998 to 2004 (specific months not reported) |
|
| Participants | N = 171 Inclusion criteria: “Smokers wanting to quit” Exclusion criteria: (a) smoking < 7 cigarettes/d for the past 2 years, (b) habitual cigarette nicotine deliveries < 0.6 mg, (c) use of psychoactive drugs or medications other than alcohol and caffeine, (d) alcohol use in excess of 28 alcoholic drinks/week, (e) age < 18 or > 50 years, (f) non‐English speaking, (g) atypical sleep cycles, (h) pregnancy, and (i) serious medical or visual problems |
|
| Interventions | Smoke control (N = 38) Nicotine patch (N = 90) Placebo patch (N = 81) Participants were randomly assigned in an 80:20 ratio to a quit group or a group that continued to smoke; those in the quit group were randomised (50:50) to nicotine patch or placebo patch. Participants received an abbreviated form of the American Lung Association smoking cessation programme. Nicotine patches and placebo patches of corresponding size were 21 mg for the first 17 days of abstinence, 14 mg for days 18 to 26, and 7 mg for days 27 to 38. |
|
| Outcomes |
Primary outcomes: Implied primary outcomes are symptom trajectories of affect (anger, anxiety, depression). Secondary outcomes: Abstinence was biochemically verified but was not reported as an outcome. Abstinence failure was defined as smoking a total of more than 4 cigarettes after quitting. |
|
| Funding source | Research was supported by National Institute on Drug Abuse Grant R01 DA12289 awarded to David G. Gilbert and by nicotine and placebo patches from GlaxoSmithKline. | |
| Declaration of interest | None | |
| Notes | Participants were excluded for abstinence failure. DNA appears to have been collected for all participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence described using an urn technique |
| Allocation concealment (selection bias) | Unclear risk | Quit group allocation and pharmacological treatment allocation were randomised, but quit group allocation was not blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | 77% in the nicotine patch group correctly guessed treatment assignment, which may have been shared with data collectors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Treatment dropout high in both groups were reported as “all relapsed to smoking”. |
| Selective reporting (reporting bias) | High risk | Multiple gene x treatment interactions were reported for multiple behavioural phenotypes. |
| Other bias | High risk | Exclusion of relapsers from analyses may create study population imbalance for genetic predisposition to successfully quit smoking. |
Gonzales 2006.
| Methods | Double‐blind parallel‐group placebo and active treatment–controlled randomised clinical trial Study period: June 2003 to April 2005 |
|
| Participants | N = 1025 Participants generally were healthy smokers (≥ 10 cigarettes/d) with < 3 months of smoking abstinence in the past year, 18 to 75 years old, recruited via media advertising. Inclusion criteria: generally healthy, smoking ≥ 10 cigarettes/d, < 3 months of smoking abstinence in the past year, 18 to 75 years old, motivated to stop smoking Exclusion criteria: any serious or unstable disease within past 6 months; seizure risk; diabetes mellitus requiring insulin or oral hypoglycaemic medications; hepatic or renal impairment; clinically significant cardiovascular disease within past 6 months; uncontrolled hypertension; severe chronic obstructive pulmonary disease; history of cancer (except treated basal cell or squamous cell carcinoma of the skin); history of clinically significant allergic reactions; major depressive disorder within past year requiring treatment; history of panic disorder, psychosis, bipolar disorder, or eating disorders; alcohol or drug abuse/dependency within the past year; use of tobacco products other than cigarettes; use of nicotine replacement therapy, clonidine, or nortriptyline within the month before enrolment; body mass index < 15 or > 38 or weight < 45.5 kg; prior exposure to bupropion; and prior varenicline exposure |
|
| Interventions | Varenicline (n = 352) Bupropion SR (n = 329) Placebo (n = 344) Study medication was taken orally for 12 weeks. Active drugs were titrated as follows: varenicline 0.5 mg/d for days 1 to 3, 0.5 mg twice per day for days 4 to 7, then 1 mg twice per day through week 12; bupropion SR 150 mg/d for days 1 to 3, then 150 mg twice per day through week 12. All participants received brief (≤ 10‐minute), standardised, individual counseling to assist in problem solving and skills training for relapse prevention. |
|
| Outcomes |
Primary outcome: exhaled carbon monoxide–confirmed 4‐week continuous abstinence rate for weeks 9 through 12, defined as the proportion of participants who reported no smoking (not even a puff) or use of any nicotine‐containing products confirmed by an exhaled carbon monoxide measurement ≤ 10 ppm Other outcomes: continuous abstinence rates from week 9 through week 24, and from week 9 through week 52; 7‐day point prevalence abstinence rates at weeks 12, 24, and 52 |
|
| Funding source | This study was supported by Pfizer Inc., which provided funding, study drug and placebo, and monitoring. The database containing findings of the 19 individual investigator sites was maintained by Pfizer Inc., and statistical analyses were performed at Pfizer Inc. by Mr Billing and by Ann Pennington, MS. Independent analysis was performed to verify the findings of Pfizer Inc. | |
| Declaration of interest | Dr Gonzales reports having received research contracts from Pfizer, Sanofi‐Aventis, GlaxoSmithKline, and Nabi Biopharmaceuticals; and consulting fees and honoraria from Pfizer, Sanofi‐Aventis, and GlaxoSmithKline; and owning 5 shares of Pfizer stock. Dr Rennard reports having had or currently having a number of relationships with companies who provide products and/or services relevant to outpatient management of chronic obstructive pulmonary disease. These relationships include serving as a consultant for Adams, Almirall, Altana, Array Biopharma, AstraZeneca, Aventis, Biolipox, Centocor, Dey, Critical Therapeutics, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis,OnoPharma, Otsuka, RJ Reynolds, Roche, Sankyo, Schering‐Plough, Scios, and Wyeth; advising regarding clinical trials for Altana, AstraZeneca, Aventis, Centocor, GlaxoSmithKline, Novartis, Pfizer, and Philip Morris; and speaking at continuing medical education programs and performing funded research at both basic and clinical levels for Altana, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis. Dr Nides reports having received research grants, consulting fees, and honoraria from Pfizer, Sanofi‐Aventis, and GlaxoSmithKline. Dr Oncken reports having received research grants, consulting fees, and honoraria from Pfizer; receiving, at no cost, nicotine replacement and placebo products from GlaxoSmithKline for smoking cessation studies; and receiving honoraria from Pri‐Med. Drs Azoulay, Watsky, Gong, Williams, and Reeves and Mr Billing report owning Pfizer stock or having stock options in Pfizer. | |
| Notes | Analysis of pharmacogenetics was reported in King 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A predefined, central, computer‐generated randomisation sequence stratified by centre assigned participants to treatment groups using a block size of 6. |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blinded to drug treatment assignments. Participants were not encouraged to guess their treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was appropriate, and the outcome of the study was objective. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | More refusal to continue participation in the placebo group and more dropout in the bupropion SR group due to adverse effects. Dropouts were assumed to be smoking. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed on clinicaltrials.gov (NCT00141206) are reported. |
| Other bias | Low risk | Additional analyses of pharmacogenetics in King 2012 were conducted in a subset of the original randomised trial population, but baseline characteristics were comparable between treatment groups, so selection bias seems unlikely. |
GPRG 1993.
| Methods | Randomised double‐blind placebo‐controlled trial of smoking cessation with a 2 × 2 factorial design (the “Patch Trial”) Study period: June 1991 to March 1992 |
|
| Participants | N = 1686 Recruited from 19 general practice clinics in Oxfordshire, UK Inclusion criteria: current smokers, age ≥ 18 and ≤ 65 years, smoked ≥ 15 cigarettes/d Exclusion criteria: (a) known skin hypersensitivity to nicotine, (b) severe skin condition likely to make patch use impossible, (c) untreated peptic ulcer, (d) life‐threatening arrhythmia, (e) active cancer, (f) cerebrovascular or cardiovascular event within past 6 months, (g) lactation, and (h) existing or planned pregnancy. Patients were warned that they should not use other forms of nicotine, such as cigars, pipes, or nicotine chewing gum, during the trial, and that medication with centrally acting alpha activity (such as clonidine) was contraindicated. |
|
| Interventions | 16‐Page booklet plus nicotine patch (N = 422) 46‐Page booklet plus nicotine patch (N = 420) 16‐Page booklet plus placebo patch (N = 422) 46‐Page booklet plus placebo patch (N = 422) Participants were randomised to 1 of 4 treatment groups: (a) nicotine patch with a standard, 16‐page Health Education Authority pamphlet on smoking cessation; (b) nicotine patch with a 46‐page booklet giving specific and more detailed information on smoking cessation with the help of patches; (c) placebo patch with a standard, 16‐page Health Education Authority pamphlet on smoking cessation; or (d) placebo patch with a 46‐page booklet giving specific and more detailed information on smoking cessation with the help of patches. Patches were delivered 21 mg/d × 4 weeks, followed by 14 mg/d × 4 weeks, then 7 mg/d × 4 weeks. |
|
| Outcomes |
Primary outcomes: biochemically verified point prevalence abstinence at 1 and 4 weeks (CO ≤ 10 ppm), and at 12, 24, and 52 weeks by cotinine ≤ 20 ng/mL or CO ≤ 10 ppm. Non‐attenders were assumed to be smoking. Abstinence at follow‐up in 1999 to 2000 was confirmed by a plasma cotinine level ≤ 20 ng/mL. Secondary outcomes: withdrawal symptoms |
|
| Funding source | The Patch Trial was supported by Ciba‐Geigy Pharmaceuticals, which also supplied the nicotine and placebo patches. The Patch II Study was funded by the Imperial Cancer Research Fund and Cancer Research UK. “Personal funding to SPD provided by DA027331; National Institute for Health Research fellowship (to PA); and the UK Centre for Tobacco Control Studies (UKCTCS to P.A. and M.M.). The UKCTCS gratefully acknowledges funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and the Department of Health, under the auspices of the UK Clinical Research Collaboration.” | |
| Declaration of interest | No conflicts of interest were declared for the original Patch Trial. However, subsequent papers on pharmacogenetics report: “Paul Aveyard has done consultancy for McNeil, Pfizer, and Celtic Biotechnology and Sean David has done consultancy with Pfizer—both with regard to smoking cessation” (PMID: 21330274). | |
| Notes | The “Patch II Study”: 1532 of the original 1686 Patch Trial participants were contacted again between July 1999 and July 2000 and were invited to participate in 8‐year follow‐up of smoking status and to provide DNA samples, of whom 840 returned questionnaires and 755 were successfully genotyped. These results were published in subsequent papers (David 2007; David 2008; David 2008a; David 2011; Johnstone 2004; Johnstone 2006; Johnstone 2007; Munafò 2007; Munafò 2011; Munafò 2012; Uhl 2010; Yudkin 2004). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was carried out by prior random allocation of study numbers to each intervention group and by sequential allocation of a study number to patients on entry.” |
| Allocation concealment (selection bias) | Low risk | “Prepared precoded packages containing the patches were handed to the patients by the general practitioner.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “The packaging and appearance of the two types of patch were identical.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research staff were blinded to randomisation status throughout the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 755/1686 (45%) Patch Trial participants were genotyped and Patch II Study participants were more female, were slightly older, and were more likely to be abstinent than non‐genotyped Patch Trial participants. However, Patch II Study participants were balanced to treatment allocation. |
| Selective reporting (reporting bias) | Low risk | Primary outcome results were published for all candidate genes in comprehensive reviews and subsequent papers. |
| Other bias | High risk | 45% of original trial participants were genotyped, which may not be representative of the original study population. Analyses of pharmacogenetics were reported in multiple subsequent papers. See ‘Notes’ above. |
Hall 2008.
| Methods | Randomised parallel‐group open‐label efficacy clinical trial consisting of 2 phases: phase 1 lasting 12 weeks, during which all participants receive a standard treatment of NRT, bupropion, and 5 group counseling sessions; followed by phase 2, randomisation to 1 of 5 treatments for another 40 weeks. Outcomes are measured at multiple time points to 104 weeks. Study period: 2002 to 2004 |
|
| Participants | N = 407 Recruited through multi‐media advertising, public service announcements, flyers, and direct mailing Inclusion criteria: treatment‐seeking smokers 18 years of age or older who currently smoke 10 cigarettes/d, report a regular smoking history ≥ 5 years, and answer "yes" to the question, “Do you smoke within 30 minutes of arising?” Exclusion criteria: history of seizure or head injury resulting in unconsciousness; any condition that might predispose to seizures (brain tumour or stroke); current or history of anorexia nervosa or bulimia; any disease acutely life‐threatening or so severe that the patient is judged unable to comply with the protocol; use of a protease inhibitor or MAO inhibitor within the past 2 weeks; current use of psychiatric drugs that would interfere with interpretation of study results, including antidepressants; treatment for alcohol dependence during the past year, or evidence of alcohol abuse so severe that the patient is judged potentially unable to comply with the protocol; patients who know they are leaving the Bay Area within the study period and non‐English speakers; suicidal or homicidal ideation; current major depression; history of bipolar disorder; recent (within 12 months) myocardial infarction; any other medical condition that would contraindicate use of NRT or bupropion; physical limitation so severe that participation in a programme of moderate exercise is not possible; and pregnancy or lactation |
|
| Interventions | All participants receive 10 weeks of NRT (patch, tapering from 21 mg to 7 mg over 8 weeks, starting at week 3) and 12 weeks of bupropion treatment and 5 mandatory group counseling sessions. At week 11, subjects are randomly assigned to 1 of 5 treatment groups: (a) no further treatment, with assessments at weeks 12, 24, 36, 52, 64, and 104 weeks (standard assessments for all treatments); (b) extended active medication (bupropion) treatment through week 52 with low‐intensity (monthly) counseling with medical staff; (c) extended placebo medication treatment through week 52 with low‐intensity counseling with medical staff; (d) extended active medication treatment with high‐intensity (session 20 to 40 minutes in duration at weeks 12, 14, 16, 18, 20, 24, 28, 32, 36, 44, and 52, and telephone contact at weeks 13, 15, 18, 22, 26, 30, 34, 36, 40, and 48) counseling; and (e) extended placebo medication treatment with high‐intensity counseling. High‐intensity counseling sessions included additional information focusing on motivation, social support, mood management, weight gain, and dependence/withdrawal. | |
| Outcomes | Primary outcome: biochemically verified 7‐day point prevalence abstinence at weeks 12, 24, 52, 64, and 104 | |
| Funding source | The clinical trial was supported by a grant from the National Institute on Drug Abuse (R01DA015732, Maintaining Abstinence in Chronic Smokers, PI: Sharon M Hall). | |
| Declaration of interest | Declaration of interest was not reported. | |
| Notes | Analyses of pharmacogenetics were described in Bergen 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised allocation was performed by study statistician who did not have contact with participants. |
| Allocation concealment (selection bias) | Low risk | Assignment for individual participants was transmitted electronically to staff. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The trial was open‐label, but other aspects of the trial suggest rigorous management and lower probability of high performance bias to unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was done by self‐report and by 2 biochemical methods using exhaled breath and urine (i.e. very thorough outcome assessment). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were low for the companion trial. |
| Selective reporting (reporting bias) | Low risk | Outcome assessment was very thorough, with 3 pieces of information required to note an individual as abstinent. In the companion trial, only 17/905 urine determinations had data discordant with outcomes of self‐report and CO measurement (< 2%). |
| Other bias | Low risk | Analyses of pharmacogenetics were performed on ˜ 37% of total study, ˜ 50% of self‐identified “Caucasian”. Minimum arm N was 26. Data show no significant differences by arm with respect to proportion of the arm genotyped. Not generalisable to other ancestries |
Hall 2009.
| Methods | Randomised parallel‐group open‐label efficacy clinical trial consisting of 2 phases: phase 1, lasting 12 weeks during which all participants receive a standard treatment of NRT, bupropion, and 5 group counseling sessions; followed by phase 2, randomization to 1 of 4 treatments for another 40 weeks. Outcomes are measured at multiple time points to 104 weeks. Study period: 2002 to 2004 |
|
| Participants | N = 402 N = 403 were enrolled, but 1 individual died before randomisation. Recruited through multi‐media advertising, public service announcements, flyers, and direct mailing. Inclusion criteria: treatment‐seeking smokers 50 years of age or older who currently smoke 10 cigarettes/d Exclusion criteria: history of seizure or head injury resulting in unconsciousness; any condition that might predispose to seizures (brain tumour or stroke); current or history of anorexia nervosa or bulimia; any disease acutely life‐threatening or so severe that the patient is judged unable to comply with the protocol; use of a protease inhibitor or MAO inhibitor within the past 2 weeks; current use of psychiatric drugs that would interfere with interpretation of study results, including antidepressants; treatment for alcohol dependence during the past year, or evidence of alcohol abuse so severe that the patient is judged potentially unable to comply with the protocol; patients who know they are leaving the Bay Area within the study period and non‐English speakers; suicidal or homicidal ideation; current major depression; history of bipolar disorder; recent (within 12 months) myocardial infarction; any other medical condition that would contraindicate use of NRT or bupropion; physical limitation so severe that participation in a programme of moderate exercise is not possible; and pregnancy or lactation |
|
| Interventions | All participants receive 10 weeks of NRT (gum, 2 mg for those smoking < 25 cigarettes/d, up to 12 pieces a day, or 4 mg for heavier smokers or heavy users of 2‐mg gum still reporting withdrawal) and 12 weeks of bupropion treatment (150 mg for a week, then 300 mg the second week and thereafter, with no adverse effects) and 5 mandatory group counseling sessions (90 minutes each). At week 8 (to permit NRT tapering for those assigned to no further treatment), participants are randomly assigned to 1 of 4 treatment groups: (a) no further treatment, with assessments at weeks 12, 24, 36, 52, 64, and 104 (standard assessments for all treatments); (b) extended NRT (to week 52) with no further counseling; (c) extended NRT with extended cognitive‐behavioural therapy to prevent relapse and encourage abstinence in case of relapse before week 12, and in case of lapses after week 12, where extended individual (20 to 40 minutes) counseling occurred at week 10, every 2 weeks thereafter, then every 4 weeks, then at weeks 44 and 52, with telephone contact in between clinical visits; and (d) extended NRT and extended counseling. Extended counseling sessions included additional information focusing on motivation, social support, mood management, weight gain, and dependence/withdrawal. | |
| Outcomes | Primary outcome: biochemically verified 7‐day point prevalence abstinence at weeks 12, 24, 52, 64, and 104 | |
| Funding source | Clinical trial and publications (portion related to this trial) were supported by grants from the National Institute on Drug Abuse (R01 DA02538, K05 DA016752, K23 DA018691 and P50 DA 09253, and R01 DA15732). | |
| Declaration of interest | Declarations of interest were not reported. | |
| Notes | Neither this paper nor the clinicaltrials.gov record provided analyses of pharmacogenetics. These were provided in Bergen 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised allocation performed by study statistician who did not have contact with participants. |
| Allocation concealment (selection bias) | Low risk | Assignment for individual participants was transmitted electronically to staff. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Trial was open‐label, but other aspects of trial suggest rigorous management and lower probability of high performance bias to unclear. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was done by self‐report and by 2 biochemical methods using exhaled breath and urine (i.e. very thorough outcome assessment). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were low (ranging from 3.2% at week 12 to 13.4% at week 104). Per‐arm assessment rates averaged 93%, and abstinence assessments were available for 92% of participants. |
| Selective reporting (reporting bias) | Low risk | Outcome assessment was very thorough, with 3 pieces of information required to note an individual as abstinent. Only 17/905 urine determinations had data discordant with outcomes of self‐report and CO measurement (< 2%). |
| Other bias | Low risk | Analyses of pharmacogenetics performed on ˜ 42% of total study; ˜ 55% of self‐identified “Caucasian”; minimum arm N was 35; no significant differences by arm with respect to proportion of arm analysed. Not generalisable to other ancestries |
Jorenby 2006.
| Methods | Double‐blind parallel‐group placebo and active treatment–controlled randomised clinical trial Study period: June 2003 to March 2005 |
|
| Participants | N = 1027 Participants were generally healthy smokers. Inclusion criteria: smoking ≥ 10 cigarettes/d the previous year, < 3 months of smoking abstinence in the past year, and 18 to 75 years old Exclusion criteria: previous use of bupropion in any form; contraindications for use of bupropion (e.g. history of seizure, diagnosis of eating disorder, use of a monoamine oxidase inhibitor in the prior 14 days, hepatic or renal impairment, diabetes requiring insulin, oral hypoglycaemics); serious or unstable disease within previous 6 months; clinically significant cardiovascular disease in previous 6 months; uncontrolled hypertension; baseline systolic blood pressure higher than 150 mmHg or diastolic blood pressure higher than 95 mmHg; severe chronic obstructive pulmonary disease; history of cancer; clinically significant allergic reactions; body mass index < 15 or > 38; body weight < 45 kg; history of alcohol or other drug abuse or dependence in the previous 12 months (nicotine excepted); treatment for major depression in the previous 12 months; history of or current panic disorder, psychosis, or bipolar disorder; use of another investigational drug within the past 30 days; intention to donate blood or blood products during treatment phase of the study (12 weeks); previous participation in any varenicline study; use in the previous month or intention to use medications that might interfere with study medication evaluation (e.g. nicotine replacement, nortriptyline, clonidine); use of marijuana or other tobacco products during the study; clinically significant abnormalities in screening laboratory values |
|
| Interventions | Varenicline (n = 344) Bupropion SR (n = 342) Placebo (n = 341) Treatment phase doses were 1 mg of varenicline twice daily and 150 mg of bupropion SR twice daily for 12 weeks, with an initial dose titration to full strength during the first week for both drugs. All participants received a folder on the study medication without de‐blinding treatment allocation. |
|
| Outcomes |
Primary outcome: exhaled carbon monoxide–confirmed 4‐week continuous abstinence rate for weeks 9 through 12, defined as the proportion of participants who reported no smoking (not even a puff) and no use of nicotine‐containing products confirmed by an exhaled carbon monoxide measurement ≤ 10 ppm Other outcomes: continuous abstinence rates from week 9 through week 24, and from week 9 through week 52; 7‐day point prevalence abstinence rates at weeks 12, 24, and 52 |
|
| Funding source | The clinical trial was sponsored by Pfizer Inc., which provided funding, study drug and placebo, and monitoring. Drs Azoulay, Watsky, Williams, Gong, and Reeves, and Mr Billing, employees of Pfizer Inc., were involved in all elements of this study. In addition, the database containing findings of the 14 investigator sites was maintained by Pfizer Inc., and statistical analyses were performed at Pfizer Inc. by Mr Billing and Ann Pennington, MS. Independent analysis was performed to verify the findings of Pfizer Inc. | |
| Declaration of interest | Dr Jorenby reported receiving research support from Pfizer, Nabi Biopharmaceutical, and Sanofi‐Aventis, and consulting fees from Nabi Biopharmaceutical. Dr Hays reported receiving a research grant from Pfizer. Dr Rigotti reported receiving research grant funding and consulting fees from GlaxoSmithKline, which markets smoking cessation medications, and from Pfizer and Sanofi‐Aventis, which are developing smoking cessation medications. Dr Rigotti also reported receiving consulting fees from Merck, which is developing smoking cessation medications. | |
| Notes | Analysis of pharmacogenetics was reported in King 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was completed centrally with the use of a computer‐generated list; sites used an electronic system to assign participants to treatment. |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of personnel is not explicitly described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome of the study was objective. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | More refusal to continue participation in the placebo group. Dropouts were assumed to be smoking. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed on clinicaltrials.gov (NCT00143364) are reported. |
| Other bias | Low risk | Additional analyses of pharmacogenetics in King 2012 were conducted in a subset of the original randomised trial population, but baseline characteristics were comparable between treatment groups, so selection bias seems unlikely. |
Kalman 2011.
| Methods | Double‐blind parallel‐group placebo‐controlled randomised clinical trial Study period: June 2005 to April 2010 |
|
| Participants | N = 143 Participants were in recovery from alcohol problems and were recruited from a residential substance abuse treatment programme and from the community. Inclusion criteria: smoked ≥ 10 cigarettes/d, history of alcohol abuse or dependence, and between 2 and 12 months of abstinence from alcohol Exclusion criteria: older than age 70; diagnosis of schizophrenia; current psychotic episode; cardiac problems in the past 3 months; uncontrolled hypertension; history of seizure; history of head injury with neurological sequelae or prolonged loss of consciousness; and use of medications that lower the seizure threshold |
|
| Interventions | Bupropion + nicotine patch (n = 73) Placebo + nicotine patch (n = 70) Participants were taking study medication for 8 weeks. They began study medication (bupropion 150 mg SR tablets or placebo) 1 week before their quit day. Participants were instructed to take 1 tabletd for 3 days, then one 150‐mg tablet twice per day for the remainder of the treatment phase of the study. All participants received a nicotine patch for 7 weeks, starting 1 week after starting study medication on their quit day. They received a 21‐mg patch for 4 weeks, a 14‐mg patch for 2 weeks, and a 7‐mg patch for 1 week. |
|
| Outcomes | Outcomes: 7‐day point prevalence smoking abstinence at week 7 (end of treatment), week 11, and week 24. At week 7, smoking abstinence was defined via self‐report (complete abstinence during the 7 days before the time of assessment) and biochemical verification (CO reading < 8 ppm). At week 11 and week 24, smoking abstinence was defined via self‐report and biochemical verification (salivary cotinine levels ≤ 15 ng/mL) | |
| Funding source | Study was sponsored through personal funding by David Kalman: NIDA R01‐DA11713‐01; Peter Monti: NIAAA K05 Senior Scientist Award; and Marc Mooney: NIDA K01‐DA‐019446. NIDA and NIAAA had no further role in study design; in collection, analysis, and interpretation of data; in writing of the report; or in the decision to submit the paper for publication. | |
| Declaration of interest | No conflicts were declared. None of the review authors have any connection with the tobacco, alcohol, pharmaceutical, or gaming industries or with any body substantially funded by 1 of these organizations. | |
| Notes | Analysis of pharmacogenetics was reported in McGeary 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Urn randomisation with 4 variables was used to allocate participants. |
| Allocation concealment (selection bias) | Low risk | Randomisation was based on 4 variables. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Active and placebo medications were identical in appearance. No further details are presented. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome of the study was objective. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Excluded from analysis participants who did not receive the study medication; no ITT analysis was performed. |
| Selective reporting (reporting bias) | High risk | Weeks of abstinence assessment are different from those listed on clinicaltrials.gov (NCT00304707). |
| Other bias | Unclear risk | Additional analyses of pharmacogenetics were conducted in a subset of the original randomised trial population in McGeary 2012, but comparability of baseline characteristics between treatment groups is unclear. |
Killen 2006.
| Methods | Open‐label cessation and double‐blind extended treatment phase Setting: community cessation clinic, USA Recruitment: community volunteers Study period: December 2001 to March 2004 |
|
| Participants | N = 362 54% males; average age ˜ 45; average number of cigarettes/d: ˜ 20 Inclusion criteria: smokers; 18 to 65 years of age; smoking at least 10 cigarettes/d or 3.5 packs/week Exclusion criteria: pregnancy, current lactation, epilepsy, bipolar disorder, schizophrenia, receiving active treatment for or reporting current depression or substance abuse, current use of bupropion or NRT, use of medication that could interact with bupropion or NRT |
|
| Interventions | Open‐label phase: All participants received bupropion SR 300 mg/d (Zyban) for 11 weeks and nicotine patch therapy for 10 weeks. Double‐blind phase: bupropion SR (150 mg/d) (n = 181) vs placebo (n = 181) for 14 weeks; at week 12, those assigned to placebo had their bupropion SR dose tapered to 150 mg/d for 2 weeks, then were switched to placebo in week 14 |
|
| Outcomes |
Primary outcome: point prevalence abstinence rates at 25‐week and 52‐week follow‐up Secondary outcomes: repeated point prevalence abstinence; continuous abstinence; craving and withdrawal symptoms; physiological measurements; adverse events; medication compliance |
|
| Funding source | Support was provided solely by National Cancer Institute Grant CA 090300 awarded to Joel D. Killen. Nicotine patches and bupropion were kindly provided by GlaxoSmithKline. | |
| Declaration of interest | GlaxoSmithKline did not otherwise participate in the design, conduct, analysis, or reporting of this study. | |
| Notes | Analysis of pharmacogenetics was reported in Sarginson 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was conducted before study entry by the method of permuted block (block size = 2 to obtain balance between groups) and was stratified on gender in the order of participant ID numbers. |
| Allocation concealment (selection bias) | Low risk | When a participant was assigned to the next available ID number in the corresponding gender, he or she was associated with that treatment group. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and researchers were blinded to treatment at extended treatment phases. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Both participants and researchers were blinded to treatment at extended treatment phases. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost at 12 months' follow‐up: 8% bupropion; 13% placebo |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but detailed reporting of outcomes does not suggest selective reporting. |
| Other bias | Unclear risk | Of 304 trial participants, 270 provided samples for DNA extraction, but the selection process for those 270 is unclear. |
Killen 2008.
| Methods | Open‐label cessation and double‐blind extended treatment phase with follow‐up conducted at 20 and 52 weeks Setting: community cessation clinic, USA Recruitment: community volunteers Study period: February 2004 to March 2006 |
|
| Participants | N = 304; 3 were excluded because of wrong treatment 40% females; average age: ˜ 46; average cigarettes/d: ˜ 20 Inclusion criteria: smokers, 18 to 65 years of age; smoking ≥ 10 cigarettes/d or 3.5 packs/week Exclusion criteria: pregnancy, current lactation, epilepsy, bipolar disorder, schizophrenia, receiving active treatment for or reporting current depression or substance abuse, history of heart problems in the previous 6 months, head trauma leading to unconsciousness in the past year, history of severe head injury resulting in brain surgery or specific neurological problems, current use of bupropion or nicotine replacement therapy (NRT), or medication that could interact with bupropion or NRT |
|
| Interventions | Open‐label – pharmacotherapy: bupropion SR for 9 weeks and nicotine patch therapy for 8 weeks. During their first week, participants continued to smoke while taking bupropion SR (150 mg/d on days 1 to 3, then 300 mg/d on days 4 to 7). Nicotine patch (21 mg) was added to treatment with bupropion (300 mg) on the target quit date if the participant succeeded. After 1 month, participants were tapered to 14 mg nicotine patch for 2 weeks, then to 7 mg for 2 weeks. Open‐label – common behavioural therapy: 6 clinic sessions, 30 minutes each, at baseline, quit week, and weeks 1, 2, 4, and 6 Double‐blind – telephone support (control): n = 147 (excluded: n = 1); 5 minutes general support calls at weeks 8, 12, 16, and 20 Double‐blind – extended cognitive‐behavioural therapy: n = 154 (excluded: n = 2); 4 sessions, 30 minutes each, at weeks 8, 12, 16, and 20 and weekly check‐in calls to automated system; report of relapse or craving prompted proactive calls |
|
| Outcomes |
Primary outcomes: expired air CO‐confirmed 7‐day point prevalence abstinence evaluated at both 20 and 52 weeks Secondary outcomes: nicotine dependence (craving and withdrawal symptoms); major depressive disorder; medication compliance; heart rate and blood pressure; adverse events; medication compliance |
|
| Funding source | Support was provided solely by a grant from the National Institute on Drug Abuse. | |
| Declaration of interest | Dr Schatzberg serves as a consultant to GlaxoSmithKline. | |
| Notes | Analysis of pharmacogenetics is reported in Sarginson 2011. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation to extended treatment condition was conducted via a permuted block method (block size = 4 to obtain balance between groups) and was stratified by gender. |
| Allocation concealment (selection bias) | Low risk | Participants were assigned to the next available ID number in the corresponding gender. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Research team and participants were blinded to extended treatment assignment until the end of the open‐label phase. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research team and participants were blinded to extended treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: 89% in standard care; 90% in intervention group |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available but detailed reporting of outcomes does not suggest selective reporting. |
| Other bias | Unclear risk | Of 304 trial participants, 270 provided samples for DNA extraction, but the selection process for those 270 was unclear. |
Killen 2010.
| Methods | 8‐Week double‐blind randomized placebo‐controlled clinical trial Setting: community‐based, USA Recruitment: community recruitment through radio, newspapers, community website, and notices distributed via local organisations Study period: May 2006 to July 2008 |
|
| Participants | N = 243 Inclusion criteria: 18 to 65 years of age; smoked 10 or more cigarettes a day Males: 70%; average age: 45; average cigarettes/d: 19 |
|
| Interventions | Selegiline patch for 8 weeks, 6 mg/24 hours, starting on TQD vs identical placebo patch on same schedule Both groups received 9 sessions of individual cognitive‐behavioural therapy. |
|
| Outcomes |
Primary outcome: point prevalence abstinence (PPA) at week 25 and week 52 (i.e. report of non‐smoking (not even a puff) for 7 consecutive days before contact and an expired air carbon monoxide level < 10 ppm) Secondary outcomes: time to relapse; occurrence, duration, and severity of adverse events |
|
| Funding source | National Institute on Drug Abuse; medication and matching placebo were provided by Somerset Pharmaceuticals, Inc. | |
| Declaration of interest | Dr Schatzberg served as a consultant for Somerset Pharmaceuticals. | |
| Notes | Analyses of pharmacogenomics are reported in Sarginson 2015 (N = 231 with DNA samples; 77.1% Caucasian, 9.1% Hispanic, 3.9% Asian, 1.3% black, 0.4% other, and 8.2% mixed ancestry). All analyses of pharmacogenomics were performed in both the full cohort and the Caucasian‐only cohort. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of random number generator |
| Allocation concealment (selection bias) | Low risk | Participants were assigned sequential ID numbers corresponding to drugs used. Also: “The drug (active or placebo) associated with each ID was pre‐packaged and labelled by ID only at an off‐site location by an individual who had no association with the participants.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Treatment assignment was concealed from staff and both research staff and participants were blind to week 52.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Treatment assignment was concealed from staff and both research staff and participants were blind to week 52.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up at 12 months: 87% (same in both arms) |
| Selective reporting (reporting bias) | Low risk | Registered in clinicaltrials.gov under NCT01330030; no deviation from prespecified outcomes |
| Other bias | Low risk | 12 people did not provide DNA for analyses of pharmacogenomics; use of PCA was included in analyses of pharmacogenomics. |
Lerman 2002.
| Methods | Double‐blind randomised placebo‐controlled clinical trial of bupropion HCL (brand name Zyban) in adult male and female smokers Study period: June 1999 to March 2002 |
|
| Participants | N = 555 allocated and received intervention (Lerman 2006). Numbers included in published pharmacogenetic analyses include ˜ 414 (Lerman, 2006), ˜ 412 (Conti, 2008), and ˜ 416 (Bergen, 2013), which refer to European ancestry individuals only, but data made available for this review include non‐Hispanic white and non‐Hispanic black, total ˜ 494. Participants were smokers seeking treatment and were recruited through advertisements for a free smoking cessation programme at Georgetown University and at the State University of New York at Buffalo. Inclusion criteria: age ≥ 18 years, reported smoking ≥ 10 cigarettes/d for the previous 12 months, who provided informed consent for both genotyping and treatment Exclusion criteria: planning a pregnancy, pregnant, lactating; seizure disorder, history of head trauma or prior seizure, family history of a seizure disorder; brain (or CNS) tumour; history of or current diagnosis of bulimia or anorexia nervosa; diabetes treated with oral hypoglycaemics or insulin; excessive use of alcohol or alcoholism; current addiction to opiates, cocaine, or stimulants; use of other drugs containing bupropion (e.g. Wellbutrin, Wellbutrin SR); allergy to bupropion; currently taking particular medications (e.g. monoamine oxidase inhibitor, antipsychotics, antidepressants, theophylline, systemic steroids, over‐the‐counter stimulants and anorectics); recently taking an MAOI (< 14 days); or recent discontinuation of a benzodiazepine |
|
| Interventions | Bupropion and 7 sessions of in‐person behavioural group counseling (N = 285 allocated, 229 received allocation), and Placebo and 7 sessions of in‐person behavioural group counseling (N = 70 allocated, 211 received allocation) Participants received 10 weeks of pills (active or placebo) initiated on the first day of counseling. Bupropion treatment was standard (150 mg/d for 3 days, then 300 mg/d). The target quit day was day of the third counseling session. |
|
| Outcomes |
Primary outcomes: continuous abstinence measured at end of treatment and at 6 months after cessation Secondary outcomes: short‐term quit rates using 7‐day and 30‐day point prevalence 83% and 86% of participants reporting abstinence at end of treatment and at 6 months provided a CO sample for verification. |
|
| Funding source | Study medication for the bupropion trial was provided by GlaxoSmithKline. This research was supported by grants from the National Cancer Institute and the National Institutes of Drug Abuse, P50CA/DA84718 and RO1CA 63562. | |
| Declaration of interest | Dr Berrettini has consulted for GlaxoSmithKline. | |
| Notes | Lerman 2002 described an interim analysis of mediators on cessation and was the first published description of this trial. Lerman 2006 is the definitive description of the trial and the first published analysis of pharmacogenetics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer generated by a senior data manager. |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed from counselors and study assistants. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed via a timeline follow‐back method through phone interviews with study assistants. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | As described in Lerman 2006, 17% withdrew after allocation but before intervention (not included in ITT analysis), and 23% of the remainder were lost to follow‐up or discontinued treatment but were included in the ITT analysis. No significant differences in losses were reported at either stage by arm. However, as abstinence outcomes were 27% at EOT and 22% at 6 months, attrition proportion seems high. |
| Selective reporting (reporting bias) | Low risk | Conti 2008 reported on continuous abstinence. |
| Other bias | Low risk | Genotype completion rate was very high overall, so selection bias by treatment group is low. |
Lerman 2004.
| Methods | Open‐label randomised clinical trial of transdermal vs spray nicotine replacement therapy (brand names Nicoderm and Nicotrol), with behavioural group counseling provided to all participants Study period: February 2000 to August 2003 |
|
| Participants | Participants were smokers seeking treatment and were recruited through advertisements for a free smoking cessation programme at Georgetown University and at the University of Pennsylvania. Inclusion criteria: ≥ 18 years of age, reported smoking of ≥ 10 cigarettes/d for the previous 12 months, and provided informed consent for both genotyping and treatment Exclusion criteria: planning a pregnancy, pregnancy or lactation; seizure disorder, history of head trauma or prior seizure; unstable angina, heart attack, or stroke within past 6 months; current treatment for or recent diagnosis of cancer; drug or alcohol dependence; current diagnosis or history of a DSM‐IV axis I psychiatric disorder; current use of bupropion or nicotine‐containing products other than cigarettes; and skin allergies or chronic dermatitis |
|
| Interventions | N = 600 allocated and received intervention (Lerman 2006). Numbers included in published pharmacogenetic analyses include ˜ 368 (Lerman 2006) and ˜ 378 (Bergen 2013), which refer to European ancestry individuals only. Treatment allocation could not be concealed. Eight weeks of NRT (standard Nicoderm patch, N = 302, or Nicotrol spray, N = 298) was provided to participants on the target quit date after 2 weeks of counseling. A total of 7 sessions of behavioural group counselling was provided. |
|
| Outcomes |
Primary outcomes: continuous abstinence measured at end of treatment and at 6 months after cessation
Secondary outcomes: short‐term quit rates using 7‐day and 30‐day point prevalence 76% and 72% of participants reporting abstinence at end of treatment and at 6 months provided a CO sample for verification. |
|
| Funding source | This work was supported by a Transdisciplinary Tobacco Use Research Center Grant from the National Cancer Institute and the National Institute on Drug Abuse P5084718, and the Abramson Cancer Center and Annenberg Public Policy Center (CL), and PHS grants P60DA005186 (WB), DA02277, DA12393, CA078703, and the UCSF Comprehensive Cancer Center (NB), and Public Health Services Research Grant M01‐RR0040 from the National Institutes of Health. Nicotine nasal spray was provided by Pharmacia, Helsingborg, Sweden. | |
| Declaration of interest | Dr Berrettini acts as a consultant to GlaxoSmithKline. | |
| Notes | Lerman 2004 described an interim analysis of pharmacogenetics of abstinence and was the first published description of this trial. Lerman 2006 is the definitive description of the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer generated by a senior data manager. |
| Allocation concealment (selection bias) | High risk | Allocation could not be concealed from counselors and study assistants. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed via a timeline follow‐back method through phone interviews with study assistants. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | As described in Lerman 2006, 11% withdrew after allocation but before intervention (not included in ITT analysis), and 22% of the remainder were lost to follow‐up or discontinued treatment but were included in the ITT analysis. No significant differences in losses at either stage by arm. However, as abstinence outcomes were 33% at EOT and 20% at 6 months, attrition proportion seems high. |
| Selective reporting (reporting bias) | Unclear risk | Lerman et al reported on primary and secondary outcomes; Bergen et al on secondary outcomes. |
| Other bias | Low risk | Genotype completion rate was very high overall, so selection bias by treatment group is low. |
Lerman 2015.
| Methods | Double‐blind parallel‐group placebo and active treatment‐controlled randomised clinical trial Study period: November 2010 to September 2013 |
|
| Participants | N = 1246 Participants were smokers seeking treatment and were recruited through advertisements for a free smoking cessation programme. Inclusion criteria: age between 18 and 65 years, reported smoking ≥ 10 cigarettes/d for 6 months or longer (verified by carbon monoxide concentrations > 10 ppm) Exclusion criteria: use of non‐cigarette tobacco products, e‐cigarettes, or current smoking treatment; history of substance misuse treatment, current use of cocaine or methamphetamine, or more than 25 alcoholic drinks/week; medical contraindications; history of DSM‐IV Axis I psychiatric disorder or suicide risk score on the Mini‐International Neuropsychiatric Interview (MINI) > 1, or current major depression; current use of antipsychotics, stimulants, opiate medications, anticoagulants, rescue inhalers, antiarryhthmics, or medications altering CYP2A6 activity (e.g. monoamine oxidase inhibitors, tricyclic antidepressants); and inability to provide informed consent or any condition that could compromise safety |
|
| Interventions | Placebo pill + placebo patch (n = 408) Placebo pill + nicotine patch (n = 418) Varenicline + placebo patch (n = 420) Participants received 11 weeks of patches: 21 mg (6 weeks), 14 mg (2 weeks), and 7 mg (3 weeks). Varenicline (or placebo) was delivered for 12 weeks (1 week before the target quit date): days 1 to 3 (0·5 mg once daily); days 4 to 7 (0·5 mg twice daily); and days 8 to 84 (1·0 mg twice daily). |
|
| Outcomes |
Primary outcome: biochemically verified 7‐day point prevalence abstinence at end of treatment Secondary outcomes: side effects, withdrawal symptoms, 6‐month and 12‐month quit rates |
|
| Funding source | This work was supported by a grant from the National Institute on Drug Abuse, the National Cancer Institute, the National Human Genome Research Institute, and the National Institute on General Medical Sciences (U01‐DA20830) to CL and RFT; funding from the Abramson Cancer Center at the University of Pennsylvania (P30 CA16520) and a grant from the Commonwealth of Pennsylvania Department of Health was provided to CL; a grant from the Canadian Institutes of Health Research (CIHR TMH109787), an endowed Chair in Addiction for the Department of Psychiatry, CAMH Foundation, the Canada Foundation for Innovation (#20289 and #16014), and the Ontario Ministry of Research and Innovation were given to RFT. The Pennsylvania Department of Health disclaims responsibility for analyses, interpretations, or conclusions. Pfizer provided varenicline and placebo pills at no cost. | |
| Declaration of interest | CL received study medication and placebo, and support for medication packaging, from Pfizer; she has also consulted for Gilead, and has been a paid expert witness in litigation against tobacco companies. PC served on the scientific advisory board of Pfizer Pharmaceuticals, presented educational talks sponsored by Pfizer on smoking cessation from 2006 to 2008, and has received grant support from Pfizer. RAS received medication and placebo free of charge from Pfizer for a different project, and has consulted for Pfizer and GlaxoSmithKline. TPG has received both investigator‐initiated and industry‐sponsored grants from Pfizer in the past 12 months, and serves on a data monitoring committee for Novartis. NIB has served as a consultant to several pharmaceutical companies that market smoking cessation medications and has been a paid expert witness in litigation against tobacco companies. RFT has acted as a consultant to pharmaceutical companies, primarily on smoking cessation. The remaining authors declare no competing interests. | |
| Notes | This paper provided analysis of pharmacogenetics. Additional analyses are reported in Tyndale 2015. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A biostatistician, independent of study investigators, developed a randomisation procedure, which was integrated into a centralised data management system. |
| Allocation concealment (selection bias) | Low risk | Randomisation was centrally organised. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, study investigators, and personnel were masked to treatment group allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was appropriate, and the outcome of the study was objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Incomplete outcome data are similar in all treatment groups; different validation analyses were performed to deal with missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed on clinicaltrials.gov (NCT01314001) are reported. However, 12‐month quit rates were not listed in the protocol but are presented in the manuscript. |
| Other bias | Low risk | Additional analyses of pharmacogenetics in Tyndale 2015 were conducted in Caucasians only, but these seem to be distributed equally among treatment groups, so selection bias seems unlikely. |
Marteau 2012.
| Methods | Open‐label parallel‐group randomised controlled trial of genotype‐based vs dependence score‐based oral dose of NRT for smoking cessation (the “Personalised Extra Treatment (PET) Trial”) Study period: June 2007 to September 2009 |
|
| Participants | N = 633 Participants were recruited from 29 primary care practices in Birmingham and Bristol, UK. Inclusion criteria: (a) ≥ 18 years of age, (b) regular cigarette smoker of ≥ 10 cigarettes/d, (c) wants to stop smoking, (d) able to give informed consent to participate, (e) able to complete study questionnaires, alone or with assistance Exclusion criteria: (a) cigar, pipe, and oral tobacco users who do not also smoke ≥ 10 cigarettes/d, (b) contraindications to NRT use, (c) pregnant or lactating women and those who plan to become pregnant during the course of treatment, (d) previous severe adverse reactions to NRT patch or to oral NRT, (e) currently taking medication for smoking cessation that they are unwilling to stop, (f) taking medication with a known influence on smoking cessation that they should not stop (e.g. nortriptyline for depression), (g) non‐English speakers, (h) those deemed unsuitable for the study by their primary care physicians |
|
| Interventions | DNA‐tailored oral NRT dose (N = 315) Nicotine dependence‐tailored NRT dose (N = 318) Participants were randomly allocated on a 1:1 basis to 1 of 2 groups: (a) NRT oral dose tailored by DNA analysis (OPRM1 gene) (genotype), or (b) NRT oral dose tailored by nicotine dependence questionnaire score (phenotype). All participants were offered behavioural support and an NRT patch, tailored for all participants by phenotype (daily cigarettes smoked). Trial interventions comprised communication that the prescribed dose of oral NRT was based on either genotype (intervention) or phenotype (comparison). Support for behavioural change was based on withdrawal‐oriented therapy and was provided for all participants twice before quit day and weekly thereafter until 4 weeks after quitting, then once more 8 weeks after quitting. |
|
| Outcomes |
Primary outcomes: adherence to prescription of NRT over 28 days, motivation to make further quit attempts Secondary outcomes: adherence to prescription of NRT over 7 days, 6‐month abstinence |
|
| Funding source | “This study was funded as part of a grant from the Medical Research Council, UK (Risk communication in preventive medicine: Optimising the impact of DNA risk information; G0500274 PI: TMM). The trial protocol was peer reviewed by the Council. PA is funded by a personal award from the National Institute of Health Research (NIHR) and by the UK Centre for Tobacco Control Studies (UKCTCS). PA and MRM are members of UKCTCS, a UKCRC Public Health Research Centre of Excellence. Funding from British Heart Foundation, Cancer Research UK, Economic and Social Research Council, Medical Research Council, and National Institute for Health Research, under the auspices of the UK Clinical Research Collaboration, is gratefully acknowledged. The sponsors and funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.” | |
| Declaration of interest | “PA has done consultancy and research on smoking cessation for pharmaceutical companies. The remaining authors declare that they have no conflicts of interest.” | |
| Notes | DNA samples were collected at study entry. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The trial statistician generated the sequences and received the stratifier data and participant and family identifier required to randomise participants, and participant date of birth to confirm group allocation at trial closure.” |
| Allocation concealment (selection bias) | Low risk | Before group assignment, allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Randomisation sequence was revealed sequentially and was concealed from the study team, nurses, and participants. After assignment to groups, allocation was no longer blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was appropriate, and the outcome of the study was objective. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was modest and balanced, and all participants were analysed by intention‐to‐treat. |
| Selective reporting (reporting bias) | Low risk | Trial protocol was pre‐registered and was published before the results paper, which reported prespecified outcomes. |
| Other bias | Low risk | The trial and its outcomes were reported openly. |
McCarthy 2008.
| Methods | Open‐label behavioural intervention and placebo‐controlled pharmacotherapy intervention randomised clinical trial Study period: January 2001 to October 2003 |
|
| Participants | N = 463 Participants were smokers seeking treatment recruited through mass media and screened by 3 rounds (telephone, group orientation, office visit). Inclusion criteria: aged 18 years or older who reported smoking ≥ 10 cigarettes/d and whose expired carbon monoxide (CO) levels exceeded 9 ppm. Participants reported being motivated to quit smoking (3 or 4 on a 4‐point self‐report scale) and being willing to fulfil study requirements. Exclusion criteria: serious psychopathology (bipolar disorder or psychosis), current depression, contraindications to use of bupropion SR (e.g. uncontrolled hypertension, history of seizure disorder, history of eating disorders, current heavy drinking, risk of pregnancy, current breastfeeding). Participants were excluded if their score on the Center for Epidemiologic Studies Depression (CES‐D) scale was above 16, except when an interview with a licensed clinician suggested that symptoms were related to a cause other than clinical depression. |
|
| Interventions | Active (bupropion SR) pharmacotherapy and individual targeted counseling (n = 113) Active pharmacotherapy, general counseling (N = 116) Placebo pharmacotherapy, individual targeted counseling (N = 121) Placebo pharmacotherapy, general counseling (N = 113) Participants attended 5 office visits in the 3 weeks before the quit date. Participants received 9 weeks of study pills (active or placebo), to begin 1 week before and to end 8 weeks after the planned quit date. Participants attended another 8 office visits over the 8 weeks following the quit date (provided breath samples at each visit and a blood sample at baseline and at end of treatment), then completed 10 monthly follow‐up calls. All participants received education regarding medication use and adherence, quit day information, and general encouragement, and completed electronic diaries for 2 weeks before and 4 weeks after the quit date. Participants randomised to individual targeted counseling received eight 10‐minute individual counseling sessions (2 prequit, 1 quit day, 5 postquit over 4 weeks). |
|
| Outcomes |
Primary outcomes: electronic diary and retrospective report of lapse and of relapse (7 days) with 7‐day point prevalence abstinence confirmed via CO at each office visit and cotinine testing at end of treatment; 7‐day point prevalence abstinence at 6 and 12 months with CO testing Secondary outcomes: prolonged abstinence outcomes at end of treatment, at 6 and 12 months |
|
| Funding source | This work was supported by Transdisciplinary Tobacco Use Research Center grants CA084724 from the National Cancer Institute and DA19706 from the National Institute on Drug Abuse. GlaxoSmithKline provided complimentary active and placebo medication used in this study. GlaxoSmithKline was not involved in the design, data collection, analysis, or reporting of this study. | |
| Declaration of interest | DEJ has received research support from Nabi Biopharmaceutical and Pfizer, Inc., and consulting fees from Nabi Biopharmaceutical. SS serves as consultant to GlaxoSmithKline Consumer Healthcare on an exclusive basis regarding over‐the‐counter smoking cessation products and is a partner in a company that is developing a new nicotine medication. He is a cofounder of invivodata, Inc., which provides electronic diary services for clinical research. In 1998 the University of Wisconsin appointed MCF to a named Chair made possible by an unrestricted gift to the university from GlaxoWellcome. | |
| Notes | This work did not report analyses of pharmacogenetics, but these were reported in Bergen 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation via random number list |
| Allocation concealment (selection bias) | Unclear risk | Study pills did not differ in appearance between active and placebo but were packaged in containers before enrolment of participants. Randomisation was done via a random number list. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Research staff and participants were blind to pharmacotherapy randomisation but were not blind to counseling randomisation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was done by electronic diary through end of treatment, and by interview (self‐report), performed in office or by phone calls, with biochemical abstinence verified at each office visit (CO) and at 6 and 12 months (cotinine). Discordance between reports or verification methods analysed in multiple ways, but final prevalences highly similar |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was substantial and did not differ by treatment arm at quit, end of treatment, or any other follow‐up point. Attrition was related to various covariates included in abstinence models in the primary paper. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported. |
| Other bias | High risk | DNA samples were provided for 41% of self‐identified whites for analysis of pharmacogenetics. Most (˜ 99%) DNA samples were successfully genotyped. Analyses of pharmacogenetics in Bergen 2013 are distributed equally among treatment groups, so selection bias is unlikely in this sample. |
Oncken 2006.
| Methods | Double‐blind parallel‐group placebo and active treatment‐controlled randomised clinical trial Study period: study dates not reported |
|
| Participants | N = 647 Participants were healthy cigarette smokers. Recruitment methods not reported Inclusion criteria: 18 to 65 years of age, smoke ≥ 10 cigarettes/d Exclusion criteria: treatment with an investigational drug within the previous month; major depression within the prior year; panic disorder, psychosis, or bipolar disorder; use of nicotine replacement or bupropion within the previous 3 months; cardiovascular disease; clinically significant medical disease; drug or alcohol abuse or dependence within the past year; use of tobacco products other than cigarettes or marijuana within the previous month |
|
| Interventions | 0.5 mg twice‐daily non‐titrated varenicline (n = 129) 0.5 mg twice‐daily titrated varenicline (n = 130) 1.0 mg twice‐daily non‐titrated varenicline (n = 129) 1.0 mg twice‐daily titrated varenicline (n = 130) Placebo (n = 129) Participants received study medication for 12 weeks. Specifically, participants in each of these groups received the following medication: 0.5 mg twice daily non‐titrated (i.e. 0.5 mg twice daily for 12 weeks); 0.5 mg twice daily titrated (i.e. 0.5 mg once daily for 7 days, then 0.5 mg twice daily for 11 weeks); 1.0 mg twice daily non‐titrated (i.e. 1.0 mg twice daily for 12 weeks); 1.0 mg twice daily titrated (i.e. 0.5 mg once daily for 3 days, then 0.5 mg twice daily for 4 days, then 1.0 mg twice daily for 11 weeks); or placebo (i.e. 2 placebo tablets twice daily for 12 weeks). All participants received a smoking cessation booklet at the baseline visit and brief smoking cessation counseling (up to 10 minutes) at each visit. |
|
| Outcomes |
Primary outcomes: carbon monoxide–confirmed 4‐week continuous quit rate for weeks 4 through 7 and weeks 9 through 12 during treatment and continuous abstinence rates for weeks 9 through 52 for each dose relative to placebo. Continuous abstinence was defined as self‐report of no cigarette use during the specified time period confirmed by an exhaled carbon monoxide measurement ≤ 10 ppm Other outcomes: carbon monoxide–confirmed 7‐day point prevalence abstinence, changes in the Minnesota Nicotine Withdrawal Scale and the modified Cigarette Evaluation Questionnaire, carbon monoxide–confirmed 7‐day point prevalence abstinence at weeks 24 and 52 |
|
| Funding source | Pfizer Inc. provided funding for this study. Pfizer Inc. was involved in all elements of this study, including, but not limited to, study design and monitoring. | |
| Declaration of interest | Dr Oncken has received research grants, consulting fees, and honoraria from Pfizer; nicotine replacement and placebo products from GlaxoSmithKline at no cost for smoking cessation studies; and honoraria from Pri‐Med. Dr Gonzales has received research contracts, consulting fees, and honoraria from Pfizer, Sanofi‐Aventis, and GlaxoSmithKline, and owns 5 shares of Pfizer stock that he received as a gift from his parents. Dr Rennard has had or currently has a number of relationships with companies that provide products and/or services relevant to outpatient management of chronic obstructive pulmonary disease. These relationships include serving as a consultant (for Adams, Almirall, Altana, Array Biopharma, AstraZeneca, Aventis, Biolipox, Centocor, Dey, Critical Therapeutics, GlaxoSmithKline, Johnson & Johnson, Merck, Novartis, Ono Pharma, Otsuka, RJ Reynolds, Roche, Sankyo, Schering‐Plough, Scios, and Wyeth), advising regarding clinical trials (Altana, AstraZeneca, Aventis, Centocor, GlaxoSmithKline, Novartis, Pfizer, and Philip Morris), speaking at continuing medical education programs and performing funded research at both basic and clinical levels (Altana, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, and Novartis). He does not own any stock in any pharmaceutical companies. Dr Nides has received research grants, consulting fees, and honoraria from Pfizer, Sanofi‐Avenits, and GlaxoSmithKline. Drs Watsky and Reeves and Messrs Billing and Anziano are employees of Pfizer and own Pfizer stock or hold Pfizer stock options. | |
| Notes | Analysis of pharmacogenetics was reported in King 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and investigators were blinded to study drug treatment assignment. Participants were not encouraged to guess their treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding was appropriate, and the outcome of the study was objective. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | More dropout in the placebo group and specific dropout for lack of efficacy occurred more often in the 1.0 mg twice‐daily titrated varenicline group. Dropouts were assumed to be smoking. |
| Selective reporting (reporting bias) | Unclear risk | No protocol was found. |
| Other bias | Low risk | Additional analyses of pharmacogenetics were conducted in King 2012 in a subset of the original randomised trial population, but baseline characteristics were comparable between treatment groups, so selection bias seems unlikely. |
Piper 2007.
| Methods | Double‐blind placebo‐controlled combination pharmacotherapy intervention randomised clinical trial. Ten cohorts of participants were randomised in a block manner to 3 intervention arms. Study period: January 2001 to October 2002 |
|
| Participants | N = 608 Participants were smokers seeking treatment who were recruited through mass media. Inclusion criteria: 18 years of age or older, who reported smoking ≥ 10 cigarettes/d and being motivated to quit (3 or 4 on a 4‐point self‐report scale). Exclusion criteria: expired carbon monoxide (CO) levels < 10 ppm; CES‐D score > 16; suicidality; contraindications to use of bupropion SR (e.g. uncontrolled hypertension, history of seizure disorder, history of eating disorders, current heavy drinking, current breastfeeding). Female participants were not pregnant and agreed to prevent pregnancy during treatment. |
|
| Interventions | Active (bupropion SR) pharmacotherapy and active nicotine gum (4 mg as needed up to 12 mg/d) (n = 228) Active bupropion pharmacotherapy and placebo gum (N = 224) Placebo pharmacotherapy and placebo gum (N = 156) Participants attended a baseline screening (physical exam, questionnaires to assess medical/psychological exclusions, and nicotine dependence inventories), were randomised to 1 of 3 intervention arms for 9 weeks of treatment. After the baseline visit, participants attended office visits each week for 4 weeks, then every other week for 4 weeks (through the end of treatment). Participants received three 10‐minute counseling sessions (at baseline, quit day, and first post‐quit day weeks), providing medication instruction and Public Health Service Guideline elements. At remaining office visits, participants completed questionnaires and vital sign assessment and received medications. Participants completed a daily diary through treatment and used a cell phone for 2 weeks to collect real‐time data. Participants were followed monthly by telephone (smoking calendar and symptoms) to 6 months and 12 months. |
|
| Outcomes |
Primary outcome: 7‐day point prevalence abstinence at 6 months confirmed via CO (breath) or cotinine (blood) analysis Secondary outcomes: 7‐day point prevalence abstinence at 1 week post quit, at end of treatment, and at 12 months (12‐month abstinence confirmed via CO (breath) or cotinine (blood) analysis), continuous abstinence |
|
| Funding source | This work was supported by Transdisciplinary Tobacco Use Research Center grants CA084724 from the National Cancer Institute and DA019706 from the National Institute on Drug Abuse. | |
| Declaration of interest | Dr Fiore neither consults for nor accepts honoraria from the pharmaceutical industry, effective 1 January 2006. In 1998 the University of Wisconsin appointed Dr Fiore to a named chair, made possible by an unrestricted gift to the university from GlaxoWellcome. Dr Baker has received monies from pharmaceutical companies (Nabi, Glaxo, Pfizer, Sanofi) to conduct clinical trials; he has received no personal remuneration from these companies. | |
| Notes | This work did not report analyses of pharmacogenetics, but these were reported in Bergen 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description |
| Allocation concealment (selection bias) | Unclear risk | No description |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Research staff and participants were blind to pharmacotherapy randomisation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was done by daily diary and by self‐report through end of treatment, and by self‐report through the remaining 12 months, with biochemical abstinence verified at 6 and 12 months. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was moderate and did not differ by treatment arm at quit, at end of treatment, and at 6 and 12 months. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported. |
| Other bias | Unclear risk | DNA samples were provided for 60% of self‐identified white trial participants for analysis of pharmacogenetics. Most (˜ 99%) DNA samples were successfully genotyped. Analyses of pharmacogenetics are distributed equally among treatment groups in Bergen 2013, so selection bias is unlikely in this sample. |
Piper 2009.
| Methods | Double‐blind placebo‐controlled single and combination pharmacotherapy intervention randomised clinical trial Study period: September 2004 to August 2010 |
|
| Participants | N = 1504 Participants were smokers seeking treatment who were recruited through mass media. Inclusion criteria: 18 years of age or older who reported smoking ≥ 10 cigarettes/dfor the past 6 months, expired CO > 9 ppm, motivated to quit (3 or 4 on a 4‐point self‐report scale), able to read and write English, and willing to complete assessments Exclusion criteria: using any form of tobacco other than cigarettes, currently taking bupropion, or having a current psychosis or schizophrenia diagnosis. In addition, participants were excluded if they had medical contraindications for any of the study medications, including high alcohol consumption (6 drinks/d on 6 or 7 days of the week), a history of seizure, high blood pressure (> 160/100 mm Hg), bipolar disorder, an eating disorder, a recent cardiac event, or allergies to any of the medications. Only 1 person per household could participate. Finally, pregnant and breastfeeding women were not eligible for participation; eligible female participants had to agree to take steps to prevent pregnancy during the medication treatment phase of the study. |
|
| Interventions | Active (bupropion SR) pharmacotherapy for 9 weeks ‐ 1 week prequit, 8 weeks post quit (n = 264) Nicotine lozenge (2 or 4 mg for 12 weeks post quit) (n = 260) Nicotine patch (24‐hour patch 21, 14, and 7 mg over 8 weeks post quit) (n = 262) Nicotine patch (8 weeks post quit) plus nicotine lozenge (12 weeks post quit) (n = 267) Active bupropion pharmacotherapy plus nicotine lozenge (n = 262) Placebo pharmacotherapy (placebo bupropion, placebo lozenge, placebo patch, placebo patch plus lozenge, and placebo bupropion plus lozenge) (n = 189) Participants attended 5 baseline (prequit) screenings (physical exam, questionnaires to assess medical/psychological exclusions, and nicotine dependence inventories, and cardiovascular assessments). Participants were randomised to 1 of 6 intervention arms for 9 to 12 weeks of treatment at the fifth baseline visit. Participants attended study visits on their quit day and 1, 2, 4, and 8 weeks post quit, and received six 10‐ to 20‐minute counseling sessions at the third and fifth baseline visits, on their quit day, and at weeks 1, 2, and 4. |
|
| Outcomes |
Primary outcome: 7‐day point prevalence abstinence at 1 week post quit, at end of treatment, and at 6 months post quit, confirmed via CO (breath) analysis Secondary outcomes: initial cessation, days to lapse, days to relapse, latency to relapse after lapse |
|
| Funding source | This research was conducted at the University of Wisconsin–Madison and was supported by grant P50 DA019706 from the National Institute on Drug Abuse and by grant M01 RR03186 from the General Clinical Research Centers Program of the National Center for Research Resources. Dr Piper was supported by an Institutional Clinical and Translational Science Award, University of Wisconsin–Madison (KL2 grant 1KL2RR025012‐01). Medication was provided to participants at no cost under a research agreement with GlaxoSmithKline. | |
| Declaration of interest | Study authors report the following potential conflicts of interest for the past 5 years: Dr Smith has received research support from Elan Corporation. Dr Baker has served as an investigator on research projects sponsored by pharmaceutical companies, including Sanofi‐Synthelabo, Pfizer Inc., and Nabi Biopharmaceuticals. Dr Jorenby has received research support from the National Institute on Drug Abuse, the National Cancer Institute, Pfizer Inc., Sanofi‐Synthelabo, and Nabi Biopharmaceuticals. He has received support for educational activities from the National Institute on Drug Abuse and the Veterans Administration, and consulting fees from Nabi Biopharmaceuticals. Dr Fiore has received honoraria from Pfizer. He has served as an investigator on research studies at the University of Wisconsin that were funded by Pfizer, Sanofi‐ Synthelabo, GlaxoSmithKline, and Nabi Biopharmaceuticals. In 1998, the University of Wisconsin appointed Dr Fiore to a named chair funded by an unrestricted gift to University of Wisconsin from Glaxo Wellcome. | |
| Notes | This work did not report analyses of pharmacogenetics, but these were reported in Bergen 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Blocked on sex and self‐reported race but no other description |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed until the moment of randomisation (3 different types of pharmacotherapy). |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Research staff and participants were blind to active vs placebo pharmacotherapy randomisation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was done by self‐report, with biochemical (CO) verification at 6 and 12 months. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition was very modest and did not differ by treatment arm during treatment or follow‐up. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported. |
| Other bias | Low risk | DNA samples were provided for 83% of self‐identified white trial participants for analysis of pharmacogenetics. Most (˜ 99%) DNA samples were successfully genotyped. Pharmacogenetic analyses are distributed equally amongst treatment groups in Bergen 2013, so selection bias is unlikely in these arms and subsamples. |
Rose 2010.
| Methods | Randomised double‐blind parallel‐arm placebo‐controlled factorial trial with 2 levels of nicotine dependence and 2 nicotine doses Setting: academic centre, USA Recruitment: community volunteers Study period: not reported; August 2007 to May 2009 according to clinicaltrials.gov |
|
| Participants | N = 479 43.4% males; average age: ˜ 44; average cigarettes/d: 24 Inclusion criteria (as reported in clincatrials.gov): “18‐65 years old, smokers of at least 10 cigarettes per day for three cumulative or continuous years of a brand that delivers at least 0.5 mg nicotine, have an expired air carbon monoxide reading of at least 10 ppm, and express a desire to quit smoking. Additionally, subjects must express a willingness to switch to denicotinized cigarettes.” Exclusion criteria (as reported in clincatrials.gov): “Participants with hypertension or hypotension may, however, be allowed to participate in the study if the study physician or P.A. determines that the condition is stable, controlled by medication, and in no way jeopardizes the individual's safety. Subjects with no previous diagnosis of hypertension may have a screening blood pressure up to 160/100. Potential subjects who report coronary heart disease; heart attack; cardiac rhythm disorder (irregular heart rhythm); chest pains (unless history, exam, and EKG clearly indicate a non‐cardiac source); cardiac (heart) disorder (including but not limited to valvular heart disease, heart murmur, heart failure); history of skin allergy; active skin condition (psoriasis) within the last five years; skin disorder except minor skin conditions (including but not limited to facial acne, minor localized infections, and superficial minor wounds.); liver or kidney disorder (except kidney stones, gallstones); gastrointestinal problems or disease other than gastroesophageal reflux or heartburn; ulcers; lung disorder (including but not limited to COPD, emphysema, and asthma); brain abnormality (including but not limited to, stroke, brain tumour, seizure disorder); history of fainting; problems giving blood samples; difficulty passing urine; diabetes treated with insulin, non‐insulin treated diabetes (unless glucose is less than 180mg/dcl and HbA1c is less than 7%); current cancer or treatment for cancer in the past 6 months (except basal or squamous cell skin cancer); other major medical condition; current psychiatric disease (with the exception of depression, anxiety disorders, OCD and ADHD) will be excluded from the study. Potential subjects who do not have a self reported diagnosis of the above listed conditions may be excluded if the study physician or P.A. determines that the history, physical findings, EKG, or laboratory studies reveal information that may jeopardize the subject's safe study participation.” |
|
| Interventions | Nicotine patch 21 mg/24 h vs 42 mg/24 h in low vs high nicotine dependence groups Treatment groups: (a) less dependent/21 mg nicotine patch, (b) less dependent/42 mg nicotine patch, (c) more dependent/21 mg nicotine patch, and (d) more dependent/42 mg nicotine patch |
|
| Outcomes |
Primary outcome: continuous abstinence from target quit date through end of treatment (10 weeks) Secondary outcomes: 4‐week continuous abstinence during weeks 7 to 10 after target quit date; 7‐day point abstinence at 6 months |
|
| Funding source | National Institutes of Health (NIH), Intramural Research Program, National Institute on Drug Abuse, Department of Health and Social Services; grant to Duke University from Philip Morris USA, Richmond, VA, USA | |
| Declaration of interest | G.R. Uhl and J.E. Rose are listed as inventors for a patent application filed by Duke University based on genomic markers that distinguishes successful quitters from unsuccessful quitters in data from other clinical trials. Rose is the Principal Investigator for a grant from Philip Morris USA, Richmond, VA, USA, to Duke University; the company had no role in planning or execution of the study, data analysis, or publication of results. |
|
| Notes | This study provides analysis of pharmacogenetics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind design but no further details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind design but no further details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind design but no further details provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for withdrawal not reported |
| Selective reporting (reporting bias) | Low risk | Protocol available at clinicaltrials.gov (NCT00734617); no deviations from prespecified endpoints |
Schnoll 2010.
| Methods | Parallel randomised double‐blind placebo‐controlled trial Setting: academic centre, USA Recruitment: community volunteers Study period: October 2004 to March 2008 |
|
| Participants | N = 575 44.7% females; average age: 44.8; average cigarettes/d: 21.2 Inclusion criteria: age 18 to 65 years, smoked ≥ 10 cigarettes/d for at least the past year Exclusion criteria: pregnancy or lactation, uncontrolled hypertension, unstable angina, heart attack or stroke within previous 6 months, recent diagnosis of cancer or kidney or liver failure, history of organ transplantation, current diabetes, drug or alcohol dependence, history of Axis I psychiatric disorder, current use of a concomitant medication, or current treatment of nicotine addiction |
|
| Interventions | Transdermal nicotine (21 mg) for 8 weeks and placebo for 16 weeks (standard therapy) (n = 287) vs transdermal nicotine (21 mg) for 24 weeks (extended therapy) (n = 288) Behavioural counseling was provided to both groups at weeks ‐2, 0, 1, 4, 8, 12, 16, and 20. |
|
| Outcomes |
Primary outcome: self‐reported and biochemically confirmed 7‐day point prevalence abstinence at weeks 24 and 52 Secondary outcomes: self‐reported continuous abstinence; prolonged abstinence; time to relapse; incremental cost per additional quitter by treatment group at week 24 Also evaluated: side effects; adherence |
|
| Funding source | Transdisciplinary Tobacco Use Research Center Grant from the National Cancer Institute and the National Institute on Drug Abuse at the National Institutes of Health | |
| Declaration of interest | Dr Lerman has served as a consultant to GlaxoSmithKline ‐ one company that manufactures the nicotine patch. She has also served as a consultant for or has received research funding from AstraZeneca, Pfizer, and Novartis. Financial support for this study was not provided by an industry sponsor. Dr Lerman had full access to the data and had full responsibility for the decision to submit for publication. | |
| Notes | Analyses of pharmacogenetics are reported in Gold 2012 and Lerman 2010. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based randomisation; non‐stratified randomisation scheme was generated by sampling without replacement and by using small blocks of 20 participants. |
| Allocation concealment (selection bias) | Low risk | A computer programme linked randomisation to the patch supply, and only the database manager could link identification to treatment allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and all study personnel, except for the database manager, were blinded to randomisation. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and all study personnel, except for the database manager, were blinded to randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Completion rates at 52 weeks: 83% for extended therapy; 79% for standard therapy |
| Selective reporting (reporting bias) | Low risk | Protocol available at ClinicalTrials.gov (registration number: NCT00364156); no deviations from prespecified outcomes identified |
| Other bias | Low risk | Analyses of pharmacogenetics were conducted only in Caucasians. Distribution seems balanced between treatment groups because of randomisation. |
Sun 2012.
| Methods | Double‐blind randomised clinical trial Setting: community hospital, China Recruitment: community volunteers Study period: March to December 2004 |
|
| Participants | N = 249 93.5% males; average age: 41; average cigarettes/d: 23 Inclusion criteria: (a) Han Chinese 20 to 70 years of age who lived in the Haidian District of Beijing; (b) had to be motivated to stop smoking; (c) smoked C10 cigarettes/d and smoked for C3 years; (d) presented with carbon monoxide (CO) level C10 ppm in exhaled air; (e) provided written informed consent and able to take part in assessment Exclusion criteria: psychiatric disorder, alcohol abuse, and other drug abuse (per DSM‐IV); pathological changes in the floor of the mouth; cardiovascular disease; taking psychotropic medications; using other forms of tobacco or any other NRT products during the past 6 months; pregnant or breastfeeding |
|
| Interventions | Nicotine sublingual tablet (NST) vs placebo “Smokers were recommended to use one or two tablets (4 mg of nicotine) per hour, up to a maximum of 20 tablets per day. Subjects were advised to use the full treatment dose for 4 weeks (minimum of 15 tablets and maximum of 20 tablets per day). After this time‐point, treatment could be tapered off up to the 8‐week visit. During the next 4‐week follow‐up phase, no further medication was dispensed.” “In addition, all participants received six sessions of standardized behavioral group counseling focusing on self‐ monitoring and behavioral modification approaches.” |
|
| Outcomes | Continuous, self‐reported complete abstinence for ≥ 7 days, verified by exhaled CO level < 10 ppm | |
| Funding source | National Natural Science Foundation of China; Training Program Foundation for Excellent Talents by the Beijing Municipal Government, China; Stanley Medical Research Institute; Department of Veterans Affairs, Mental Illness Research, Education and Clinical Center (MIRECC); US National Institutes of Health | |
| Declaration of interest | Not reported | |
| Notes | This study provides analysis of pharmacogenetics. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computer‐generated randomisation scheme operated by a senior data manager |
| Allocation concealment (selection bias) | Low risk | “Allocation was concealed from the investigator who delivered the interventions and assessed the outcomes.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind design |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Interviewers were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers and reasons for missing participants are not given. |
| Selective reporting (reporting bias) | Unclear risk | No relevant details on protocol availability |
Swan 2003.
| Methods | Open‐label randomised trial Setting: HMO, USA Recruitment: volunteers from Group Health Cooperative (GHC) membership Study duration: April 1998 to May 1999 |
|
| Participants | N = 1524 57% females; average age: 45; average cigarettes/d: ˜ 23 Inclusion criteria: at least 18 years of age, smoked ≥ 10 cigarettes/d, were motivated to stop smoking, were otherwise in good general health, had sufficient verbal and written command of English to provide informed consent and study responses, and were enrolled and planned to stay enrolled in GHC for the next 12 months Exclusion criteria: (1) any predisposition to seizure, as defined by a personal or family history of a seizure disorder, such as epilepsy, or a personal history of febrile seizures; (2) history of stroke or transient ischaemic attack; (3) history of head injury resulting in loss of consciousness for longer than 1 hour; (4) current use of medications contraindicated to bupropion SR or known to lower the seizure threshold (complete list available on request); (5) history of or current diagnosis of anorexia nervosa or bulimia; (6) being of poor general health, as defined by the presence of severe and chronic cardiovascular disease (including myocardial infarction within the previous 3 months), severe and chronic pulmonary disease, renal or hepatic dysfunction, neurological disease, uncontrolled hypertension, or uncontrolled diabetes mellitus; (7) participation in GHC's Free & Clear (FC) smoking cessation programme in the previous 12 months (1 of the treatments included in the present study); (8) current depression; (9) current drinking of 14 or more alcoholic drinks/week and/or binge drinking 2 or more times in the past month; and (10) current pregnancy or plans to become pregnant or current nursing of a child |
|
| Interventions | Factorial design crossing 2 drug doses with 2 intensities of behavioural counseling: 150 mg bupropion SR with less intensive counseling (n = 382) or with more intensive counseling (n = 4381); or 300 mg bupropion SR with less intensive counseling (n = 4383) or with more intensive counseling (n = 4378) Free & Clear proactive telephone counseling (4 brief calls), access to quit‐line and S‐H materials vs Zyban Advantage Program (ZAP) tailored S‐H materials, single telephone call after TQD, access to Zyban support line Prescription was mailed. No face‐to‐face contact during enrolment or Rx |
|
| Outcomes |
Primary outcomes: self‐reported point prevalence 7‐day non‐smoking status at 3 and 12 months following target quit date Secondary outcomes: adverse and abstinence effects reported since beginning of treatment with bupropion SR |
|
| Funding source | US National Cancer Institute | |
| Declaration of interest | Study authors have no relevant financial interests and have received no financial support or medication from GlaxoSmithKline. | |
| Notes | Analyses of pharmacogenetics are reported in Swan 2005 and Swan 2007. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Open‐label randomized trial. The computer code for the procedure calculated probabilities of group assignment that were dynamically modified based on the number of members in each group so that final group sizes were equal. No restrictions such as stratification or blocking were used as part of the randomization process.” |
| Allocation concealment (selection bias) | Low risk | Procedure using a random number generator was built into study database. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up at 12 months: 83% intervention; 88% control |
| Selective reporting (reporting bias) | Unclear risk | No relevant information reported |
| Other bias | High risk | Participants who responded at 12‐month follow‐up (n = 1299) were invited to participate in the ‘genetic factors’ supplemental study; 496 (50%) actually provided samples. |
Swan 2010.
| Methods | Open‐label randomised behavioural intervention clinical trial Study period: October 2005 to June 2008 |
|
| Participants | N = 1202 Participants were smokers seeking treatment who were recruited through health plan advertisements, physician referrals, and a commercial smoking cessation plan’s advertisements. Inclusion criteria: age ≥ 18 years, reported smoking ≥ 10 cigarettes/d for 12 months and ≥ 5 cigarettes/d for the past week, with dependable telephone and Internet access; were financially eligible for smoking cessation services under their health plan and medically eligible for varenicline treatment Exclusion criteria: current pregnancy, plans to become pregnant during the medication treatment period, or breastfeeding; self‐report of poor health; severe or chronic heart disease; severe COPD; on dialysis with recent creatinine values > 3; current self‐reported diagnosis of or treatment for psychotic disorder; concurrent use of bupropion or nicotine replacement therapy; current use of recreational or street drugs; current drinking of ≥ 14 alcoholic drinks/week or binge drinking ≥ 2 times in the past month; current use of cimetidine, metformin, phenformin, pindolol, or procainamide; and recent participation in 2 specific smoking cessation programmes |
|
| Interventions | Varenicline and Proactive Telephone Counseling (PTC, n = 402) Varenicline and Web (N = 401) Varenicline, PTC and Web (N = 399) All randomised participants received a prescription for 12 weeks of varenicline, a 5‐ to 10‐minute orientation call, printed Quit Guides including recommended guidelines for varenicline, and access to a toll‐free telephone call in line for ad hoc calls. PTC arm participants received up to 5 phone calls. Web arm participants had online access to an interactive online programme modified from PTC content. PTC and Web arm participants received calls that encouraged them to use Web programme tools. |
|
| Outcomes |
Primary outcome: self‐reported 7‐day and 30‐day point prevalence abstinence at 3 months and at 6 months after the targeted quit day Secondary outcomes: probable side effects (6) and probable abstinence effects (9) at 3 months after the targeted quit day |
|
| Funding source | This work was supported by Grant R01CA071358 from the National Cancer Institute. Pfizer provided varenicline and nominal support for recruiting participants. | |
| Declaration of interest | SMZ owns stock in Free & Clear, Inc.; GES received financial support from Pfızer to attend a 1‐day advisory meeting in 2008, and a small grant from Pfızer to support recruitment and study intake. | |
| Notes | This paper did not provide analyses of pharmacogenetics, but these were reported in Bergen 2013. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Automated algorithm |
| Allocation concealment (selection bias) | Unclear risk | No data on which to make a judgement (method of concealment not described) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and staff were not blinded to treatment assignment. Differences between treatments were obvious to both participants and personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was done by self‐report, which was performed through follow‐up calls. Those who could not be contacted were assumed to be smoking. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Although missing outcome data were substantial (˜ 25% for 3‐month and 6‐month follow‐up), missing outcome data were balanced in numbers across intervention groups. In this trial, self‐report was not biochemically confirmed. |
| Selective reporting (reporting bias) | Low risk | Primary and secondary outcomes were reported. |
| Other bias | Unclear risk | Biospecimen collection was done post hoc; 47% of the trial population provided a biospecimen after a multiple‐step consent and exclusion process. Data show a highly significant difference in abstinence prevalence in the sample available for pharmacogenetic analysis vs the overall trial population (55% vs 44%, P = 1E‐5 at 3 months; 43% vs 33% at 6 months, P = 1E‐4). Most (˜ 94%) samples collected were genotyped. Pharmacogenetic analyses were conducted in self‐identified whites only in Bergen 2013, distributed equally among treatment groups. |
Verde 2014.
| Methods | Randomised open‐label trial of bupropion SR or nicotine patch in adult male and female smokers Setting: community hospital, Spain Recruitment period: 2007 to 2010 (months not indicated) |
|
| Participants | N = 76 "Heavy smokers" 100% were genotyped Inclusion criteria: (a) to be enrolled in a ‘standard’ (12‐week duration) smoking cessation programme with nicotine substitutive treatment (NST) or bupropion following medical criteria, (b) smoking history > 10 cigarettes/d and > 10 packs/y, and (c) > 3 scores in the Fagerstrom test for nicotine dependence (FTND) Exclusion criteria: not defined |
|
| Interventions | Bupropion (N = 34) NRT (N = 36) Doses of bupropion SR provided were 150 mg once per day for days 1 to 6, followed by 150 mg twice per day for days 7 to 84), for 12 weeks For NRT, doses were as follows: (1) for smokers ≥ 20 cigarettes/d, Nicotinell TTS 30 (4 weeks), Nicotinell TTS 20 (4 weeks), and Nicotinell TTS 10 (4 weeks); (2) for smokers < 20 cigarettes/d, Nicotinell TTS 20 (4 weeks), Nicotinell TTS 20 (4 weeks), and Nicotinell TTS 10 (4 weeks) |
|
| Outcomes | 12‐Month post‐treatment prolonged abstinence; abstinence rate was measured at 3, 6, 9, and 12 months during follow‐up visits. Biochemical verification was not mentioned. | |
| Funding source | Research was supported by the UEM 03‐2006 Internal Project of European University of Madrid and by the 035‐2006 Project of the Spanish Lung Foundation (SEPAR). | |
| Declaration of interest | No competing interests | |
| Notes | 6 candidate polymorphisms in CYP2A6, 5‐HTT, and HTR2A genes were genotyped. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random sequence generation was not mentioned in the paper. |
| Allocation concealment (selection bias) | High risk | Open‐label design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label design |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No mention of blinding of outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No frequencies of abstinence outcomes reported; thus no way to confirm reporting of outcomes for all participants |
| Selective reporting (reporting bias) | Unclear risk | Outcomes were not described transparently; thus one cannot confirm whether selective outcome reporting occurred. |
| Other bias | Unclear risk | Only 3 CYP2A6 alleles were genotyped. Therefore, *1 allele calls are likely overrepresented. SLC6A4 5‐HTTLPR "L" and "S" are not defined. |
Wagena 2005.
| Methods | Placebo‐controlled double‐dummy randomised trial of bupropion SR vs nortriptyline or placebo for smoking cessation in patients at risk for chronic obstructive pulmonary disease (COPD) or with COPD Study period: March 2002 to August 2003 |
|
| Participants | N = 255 Inclusion criteria: (a) current daily smokers at risk for COPD or with COPD, (b) 30 to 70 years of age, (c) smoking history ≥ 5 years, (d) smoked on average ≥ 10 cigarettes/d during the past year, and (e) were motivated to stop smoking Exclusion criteria: (a) having used or were still using bupropion SR or nortriptyline; (b) were using NRT or (c) psychoactive medication at the time of assessment; and (d) had any serious or unstable medical disorders that might affect lung function or for which bupropion SR or nortriptyline was contraindicated |
|
| Interventions | Bupropion SR (N = 86) Nortriptyline (N = 80) Placebo (N = 89) Eligible individuals were randomly assigned in a 1:1 ratio to receive the following: (1) bupropion SR, 150 mg once daily, for days 1 through 6, followed by 150 mg twice daily for days 7 through 84; (2) nortriptyline, 25 mg once daily, for days 1 through 3, followed by 50 mg once daily for days 3 through 7, then 75 mg once daily for days 8 through 84; or (3) placebo. At the baseline visit, the target quit date (TQD) was set for the second week, usually day 11 from the start of medication. |
|
| Outcomes |
Primary outcomes: prolonged abstinence from smoking from week 4 to week 26 after the target quit date. Prolonged abstinence was defined as a participant’s report of 0 cigarettes/d (not even a puff ) during weeks 4 through 26, confirmed by urinary cotinine values ≤ 60 ng/mL at weeks 4, 12, and 26 after TQD. Participants were allowed to miss 1 in‐person visit but not the last follow‐up visit. Secondary outcomes: prolonged abstinence during weeks 4 through 12 and 7‐day point prevalence abstinence (defined as having smoked 0 cigarettes, not even a puff, for the previous 7 days) at weeks 4, 12, and 26, confirmed by urinary cotinine levels ≤ 60 ng/mL |
|
| Funding source | This work has been supported by a grant from the Netherlands Organization for Health Research and Development (ZonMW, The Hague; Project no. 50–50101‐96–404). The original trial was funded by grants of the Dutch Asthma Foundation (NAF grant no. 3.2.00.21) and the Health Research and Development Council (ZorgOnderzoek Nederland, grant no. 2200.0111), the Netherlands. Lundbeck B.V. provided active nortriptyline free of charge. Lundbeck B.V. as well as GlaxoSmithKline B.V. did not play a role in the design and conduct of the study, nor in interpretation and analysis of the data and the decision to submit for publication. | |
| Declaration of interest | “CPVS has received financing (grants, consultancy and/or travel/accommodation costs) from AstraZeneca, Boehringer Ingelheim, and Pfizer, unrelated to this study. DSP has received financing (grants, consultancy, and/or travel/accommodation costs) from Chiesi, GlaxoSmithKline, AstraZeneca, Nycomed, and Boehringer Ingelheim, unrelated to this study. MQ, EJW, FJVS declare no conflict of interest.” | |
| Notes | The original study did not report pharmacogenetic outcomes. 214/255 (84%) participants in the original clinical trial were genotyped. Results were published in a subsequent pharmacogenetic paper (Quaak 2012). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer generated. |
| Allocation concealment (selection bias) | Low risk | Randomisation list by a pharmacist, stratified by COPD severity |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and research staff were blinded to treatment assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “No patient, research nurse, counselor, investigator, or any other staff member was aware of the treatment assignments for the duration of the study.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in analyses. |
| Selective reporting (reporting bias) | Low risk | Primary outcomes were reported. |
| Other bias | Low risk | 214/255 (84%) participants in the original clinical trial were genotyped, with results subsequently published. See ‘Notes’ above. |
Wilcox 2011.
| Methods | Double‐blind placebo‐controlled trial Pilot study Setting: (not specified), USA Recruitment: community volunteers Study period: not reported |
|
| Participants | N = 78 Inclusion criteria: male and female white smokers of European descent, at least 18 years of age, who were smoking ≥ 10 cigarettes/d for ≥ 5 years and were in generally good physical and mental health Exclusion criteria: not reported |
|
| Interventions | Rimonabant 20 mg/d (n = 48) vs placebo (n = 28) for 10 weeks | |
| Outcomes | Smoking status; exhaled CO level | |
| Funding source | This study reports analysis of pharmacogenetics. | |
| Declaration of interest | Pharmacology Research Institute and laboratory of one of the study authors at the University of California Los Angeles (UCLA) | |
| Notes | Not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No relevant information reported |
| Allocation concealment (selection bias) | Unclear risk | No relevant information reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Double‐blind design but no other information reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Double‐blind design but no other information reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | No information on protocol availability reported |
| Other bias | Unclear risk | Distribution of ethnicities between treatment groups unclear |
Winst 2006.
| Methods | Double‐blind parallel‐group placebo‐controlled randomised clinical trial Study period: June 2006 to February 2008 |
|
| Participants | N = 420
Participants included hospitalised smokers who needed to stop smoking at admission. Participants were approached by information presented in the general hospital admission leaflet and/or on the intranet and by active recruitment by smoking consults in specific departments of the hospital. Inclusion criteria: over 18 years of age; daily consumption of ≥ 15 cigarettes in the past 3 years and average daily consumption of 10 cigarettes the week before study inclusion; life expectancy of ≥ 1 year; conscious and approachable; able to read and sign an informed consent form; Dutch‐ or French‐speaking; expected duration of hospitalisation ≥ 72 hours; treating physician and anaesthetist agree on the patient’s study inclusion Exclusion criteria: already using NS within 14 days before study inclusion; use of tobacco products other than cigarettes; alcohol abuse of > 5 U/d; simultaneous use of psychoactive drugs or hallucinogens; referred from other hospitals; existing contraindications for NS use; predicted postoperative ICU stay longer than 48 hours; pregnant or lactating |
|
| Interventions | 15 minutes counseling + placebo patch (n = 210) 15 minutes counseling + nicotine transdermal patch (n = 210) Participants received a nicotine transdermal patch at a 15 mg/16 hours dose, daily, for maximum 7 days after hospital admission (study inclusion), or a placebo patch for the same study duration. |
|
| Outcomes |
Primary outcome: total addiction score, calculated from the Minnesota questionnaires, taken up at randomisation, after ≥ 72 hours of hospital admission and after a maximum of 7 treatment days Additional outcomes: point prevalence of quit rate at short (7 days) and long term (6 months) |
|
| Funding source | Study was conducted through a grant from the Foundation of Scientific Research (FWO number G.0604.06), Vlaamse Liga tegen Kanker, and an independent research grant from McNeil AB, Helsingborg, Sweden. | |
| Declaration of interest | Apart from the funding source, study authors report no other relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript, apart from those disclosed. | |
| Notes | All information from this study is extracted from the study protocol; thus information regarding sample size should be interpreted as the initial target rather than the actual number of recruited participants. Analysis of pharmacogenetics is reported in De Ruyck 2010. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed via an online‐available registration and randomisation website. |
| Allocation concealment (selection bias) | Unclear risk | Randomisation occurred online, but it is unclear whether allocation was properly concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Active and placebo medications were identical in appearance, and the study is labeled as double‐blind. No additional details are presented. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome is subjective and can be influenced by study participants. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Final study report not available |
| Selective reporting (reporting bias) | Unclear risk | Final study report not available |
| Other bias | Unclear risk | Final study report not available |
BUP: bupropion; CBTD: cognitive‐behavioural treatment for depression; CES‐D: Center for Epidemiologic Studies Depression Scale; CO: carbon monoxide; DSM‐IV: Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition; DSM‐IV‐TR, Diagnostic and Statistical Manual for Mental Disorders, Fourth Edition, Text Revision; EOT: end of therapy; FTND: Fagerstrom Test of Nicotine Dependence; GHC: Group Health Cooperative; HE: health education; ITT: intention‐to‐treat analysis; MAO: monoamine oxidase; MAOI: monoamine oxidase inhibitor; MI: myocardial infarction; MINI: Mini‐International Neuropsychiatric Interview; NARSAD: National Alliance for Research on Schizophrenia and Depression; NIAAA: National Institute on Alcohol Abuse and Alcoholism; NIDA: National Institute on Drug Abuse; NIHR: National Institute on Health Research; NRT: nicotine replacement therapy; NST: nicotine sublingual tablet; PCA: Principal Component Analysis; PLAC: placebo; PPA: point prevalence abstinence; ppm: parts per million; SR: sustained‐release; ST: standard treatment; TQD: target quit date; UKCRC: UK Clinical Research Collaboration; UKCTCS: UK Centre for Tobacco Control Studies.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baker 2009 | Primary paper was not a randomised controlled trial (RCT). |
| Becker 2008 | Pharmacogenetic analyses presented results from pooled (not individual) allelotyping (Drgon 2009). This investigation analysed results from Becker 2008 (no data available). |
| Uhl 2007 | Pharmacogenetic analyses presented results from pooled (not individual) allelotyping (Uhl 2008). This investigation analysed results from Lerman 2002, Lerman 2004, David 2007, and Uhl 2007 (no data available). |
Differences between protocol and review
We refrained from assessing reporting bias and performing subgroup analyses and sensitivity analyses because this version of the review includes too few studies and too few participants within genotype groups for these analyses to yield reliable results.
Contributions of authors
SPD and AWB conceived the study. ES, OAP, MRM, DAB, AWB, and SPD wrote the protocol and designed the study. LS, AWB, and SPD searched the literature and identified relevant studies. ES and SPD independently checked the abstracts and, if relevant, retrieved the full texts of records and reviewed them for data. SPD contacted study authors to ask them to share their study data and, in close collaboration with ES. ES, OAP, AWB, and SPD, extracted study characteristics and assessed risk of bias. ES extracted data and performed statistical analyses in RevMan 5.3 in close collaboration with OAP, AWB, and SPD. ES, OAP, AWB, and SPD drafted a first version of the manuscript. All review authors commented on consecutive versions of the manuscript, and read and agreed on submission of the final version of the manuscript.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Netherlands Organisation for Scientific Research, Netherlands.
ES acknowledges past support from the Netherlands Organisation for Scientific Research grant 825.14.001.
-
NIH, USA.
AWB acknowledges past support from NIH grants DA020830, DA028793, DA033813 and DA041211, and a Visiting Professorship at the University of Bristol.
Declarations of interest
ES: none known.
OAP: none known.
MRM: has received grant funding from Pfizer through its Investigator Initiated Research programme.
DAB: none known.
AWB: is an employee of BioRealm, LLC, which intends to commercialise an analysis platform for substance use disorder studies.
SPD: has received funding for study medication from Pfizer through its Investigator Initiated Research programme and is a scientific advisor for BaseHealth.
Edited (no change to conclusions)
References
References to studies included in this review
Ahluwalia 2006 {published data only}
- Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, et al. The effects of nicotine gum and counseling among African American light smokers: a 2 × 2 factorial design. Addiction 2006;101(6):883‐91. [DOI] [PubMed] [Google Scholar]
- Ho MK, Mwenifumbo JC, Al Koudsi N, Okuyemi KS, Ahluwalia JS, Benowitz NL, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African‐American light smokers. Clinical Pharmacology & Therapeutics 2009;85(6):635‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AZ, Zhou Q, Cox LS, David SP, Ahluwalia JS, Benowitz NL, et al. Association of CHRNA5‐A3‐B4 SNP rs2036527 with smoking cessation therapy response in African‐American smokers. Clinical Pharmacology & Therapy 2014;96(2):256‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aveyard 2007 {published and unpublished data}
- Aveyard P, Brown K, Saunders C, Alexander A, Johnstone E, Munafò MR, et al. Weekly versus basic smoking cessation support in primary care: a randomised controlled trial. Thorax 2007;62(10):898‐903. [PUBMED: 17483139] [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Johnstone EC, Churchman M, Aveyard P, Murphy MF, Munafò MR. Pharmacogenetics of smoking cessation in general practice: results from the Patch II and Patch in Practice trials. Nicotine & Tobacco Research 2011;13(3):157‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Johnstone EC, Murphy MF, Aveyard P, Guo B, Lerman C, et al. Genetic variation in the serotonin pathway and smoking cessation with nicotine replacement therapy: new data from the Patch in Practice trial and pooled analyses. Drug and Alcohol Dependence 2008;98(1‐2):77‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Johnstone EC, Guo B, Murphy MF, Aveyard P. Association of COMT Val108/158Met genotype with smoking cessation. Pharmacogenetics and Genomics 2008;18(2):121‐8. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Johnstone EC, Murphy MF, Aveyard P. Lack of association of DRD2 rs1800497 (Taq1A) polymorphism with smoking cessation in a nicotine replacement therapy randomized trial. Nicotine & Tobacco Research 2009;11(4):404‐7. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Johnstone EC, Walther D, Uhl GR, Murphy MF, Aveyard P. CHRNA3 rs1051730 genotype and short‐term smoking cessation. Nicotine & Tobacco Research 2011;13(10):982‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruell T, Colavita G, Donegan T, Egawhary M, Hurley M, Aveyard P, et al. Association between nicotinic acetylcholine receptor single nucleotide polymorphisms and smoking cessation. Nicotine & Tobacco Research 2012;14(8):993‐7. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Johnson C, Walther D, David SP, Aveyard P, et al. Genome‐wide association for smoking cessation success: participants in the Patch in Practice trial of nicotine replacement. Pharmacogenomics 2010;11(3):357‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloch 2010 {published data only}
- Bloch B, Reshef A, Cohen T, Tafla A, Gathas S, Israel S, et al. Preliminary effects of bupropion and the promoter region (HTTLPR) serotonin transporter (SLC6A4) polymorphism on smoking behavior in schizophrenia. Psychiatry Research 2010;175(1‐2):38‐42. [DOI] [PubMed] [Google Scholar]
Brown 2007 {published and unpublished data}
- Brown RA, Niaura R, Lloyd‐Richardson EE, Strong DR, Kahler CW, Abrantes AM, et al. Bupropion and cognitive‐behavioral treatment for depression in smoking cessation. Nicotine & Tobacco Research 2007;9(7):721‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Brown RA, Papandonatos GD, Kahler CW, Lloyd‐Richardson EE, Munafò MR, et al. Pharmacogenetic clinical trial of sustained‐release bupropion for smoking cessation. Nicotine & Tobacco Research 2007;9(8):821‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Strong DR, Leventhal AM, Lancaster MA, McGeary JE, Munafò MR, et al. Influence of a dopamine pathway additive genetic efficacy score on smoking cessation: results from two randomized clinical trials of bupropion. Addiction 2013;108(12):2202‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Strong DR, Munafò MR, Brown RA, Lloyd‐Richardson EE, Wileyto PE, et al. Bupropion efficacy for smoking cessation is influenced by the DRD2 Taq1A polymorphism: analysis of pooled data from two clinical trials. Nicotine & Tobacco Research 2007;9(12):1251‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, David SP, Brightman M, Strong D, McGeary JE, Brown RA, et al. Dopamine D4 receptor gene variation moderates the efficacy of bupropion for smoking cessation. Pharmacogenomics Journal 2012;12(1):86‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cinciripini 2005 {published data only}
- Cinciripini P, Wetter D, Tomlinson G, Tsoh J, Moor C, Cinciripini L, et al. The effects of the DRD2 polymorphism on smoking cessation and negative affect: evidence for a pharmacogenetic effect on mood. Nicotine & Tobacco Research 2004;6(2):229‐39. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Tsoh JY, Wetter DW, Lam C, Moor C, Cinciripini L, et al. Combined effects of venlafaxine, nicotine replacement, and brief counseling on smoking cessation. Experimenal & Clinical Psychopharmacology 2005;13(4):282‐92. [DOI] [PubMed] [Google Scholar]
Cox 2012 {published data only}
- Cox LS, Nollen NL, Mayo MS, Choi WS, Faseru B, Benowitz NL, et al. Bupropion for smoking cessation in African American light smokers: a randomized controlled trial. Journal of the National Cancer Institute 2012;104(4):290‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AZ, Cox LS, Nollen N, Faseru B, Okuyemi KS, Ahluwalia JS, et al. CYP2B6 and bupropion's smoking‐cessation pharmacology: the role of hydroxybupropion. Clinical Pharmacology & Therapeutics 2012;92(6):771‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AZ, Zhou Q, Cox LS, Ahluwalia JS, Benowitz NL, Tyndale RF. Gene variants in CYP2C19 are associated with altered in vivo bupropion pharmacokinetics but not bupropion‐assisted smoking cessation outcomes. Drug Metabolism & Disposition 2014;42(11):1971‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu AZ, Zhou Q, Cox LS, David SP, Ahluwalia JS, Benowitz NL, et al. Association of CHRNA5‐A3‐B4 SNP rs2036527 with smoking cessation therapy response in African‐American smokers. Clinical Pharmacology & Therapeutics 2014;96(2):256‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilbert 2009 {published and unpublished data}
- Gilbert DG, Zuo Y, Rabinovich NE, Riise H, Needham R, Huggenvik JI. Neurotransmission‐related genetic polymorphisms, negative affectivity traits, and gender predict tobacco abstinence symptoms across 44 days with and without nicotine patch. Journal of Abnormal Psychology 2009;118(2):322‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzales 2006 {published data only}
- Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline Phase 3 Study Group. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained‐release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006;296(1):47‐55. [DOI] [PubMed] [Google Scholar]
- King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo‐controlled clinical trials. Neuropsychopharmacology 2012;37(3):641‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
GPRG 1993 {published and unpublished data}
- David SP, Johnstone EC, Churchman M, Aveyard P, Murphy MF, Munafò MR. Pharmacogenetics of smoking cessation in general practice: results from the Patch II and Patch in Practice trials. Nicotine & Tobacco Research 2011;13(3):157‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafò MR, Murphy MF, Proctor M, Walton RT, Johnstone EC. Genetic variation in the dopamine D4 receptor (DRD4) gene and smoking cessation: follow‐up of a randomised clinical trial of transdermal nicotine patch. Pharmacogenomics Journal 2008;8(2):122‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Munafò MR, Murphy MF, Walton RT, Johnstone EC. The serotonin transporter 5‐HTTLPR polymorphism and treatment response to nicotine patch: follow‐up of a randomized controlled trial. Nicotine & Tobacco Research 2007;9(2):225‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imperial Cancer Research Fund General Practice Research Group. Effectiveness of a nicotine patch in helping people stop smoking: results of a randomised trial in general practice.. BMJ 1993;306(6888):1304‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Elliot KM, David SP, Murphy MF, Walton RT, Munafò MR. Association of COMT Val108/158Met genotype with smoking cessation in a nicotine replacement therapy randomized trial. Cancer Epidemiology Biomarkers & Prevention 2007;16(6):1065‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone EC, Yudkin PL, Hey K, Roberts SJ, Welch SJ, Murphy MF, et al. Genetic variation in dopaminergic pathways and short‐term effectiveness of the nicotine patch. Pharmacogenetics 2004;14(2):83‐90. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Elliot KM, Murphy MF, Walton RT, Johnstone EC. Association of the mu‐opioid receptor gene with smoking cessation. Pharmacogenomics Journal 2007;7(5):353‐61. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Johnstone EC, Walther D, Uhl GR, Murphy MF, Aveyard P. CHRNA3 rs1051730 genotype and short‐term smoking cessation. Nicotine & Tobacco Research 2011;13(10):982‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2008 {published and unpublished data}
- Bergen AW, Javitz HS, Krasnow R, Michel M, Nishita D, Conti DV, et al. Organic cation transporter variation and response to smoking cessation therapies. Nicotine & Tobacco Research 2014;16(12):1638‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Javitz HS, Krasnow R, Nishita D, Michel M, Conti DV, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenetics and Genomics 2013;23(2):94‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Humfleet GL, Gorecki JA, Muñoz RF, Reus VI, Prochaska JJ. Older versus younger treatment‐seeking smokers: differences in smoking behavior, drug and alcohol use, and psychosocial and physical functioning. Nicotine & Tobacco Research 2008;10(3):463‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2009 {published and unpublished data}
- Bergen AW, Javitz HS, Krasnow R, Michel M, Nishita D, Conti DV, et al. Organic cation transporter variation and response to smoking cessation therapies. Nicotine & Tobacco Research 2014;16(12):1638‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Javitz HS, Krasnow R, Nishita D, Michel M, Conti DV, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenetics and Genomics 2013;23(2):94‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall SM, Humfleet GL, Muñoz RF, Reus VI, Robbins JA, Prochaska JJ. Extended treatment of older cigarette smokers. Addiction 2009;104(6):1043‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jorenby 2006 {published data only}
- Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Varenicline Phase 3 Study Group. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained‐release bupropion for smoking cessation: a randomized controlled trial.. JAMA 2006;296(1):56‐63. [DOI] [PubMed] [Google Scholar]
- King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo‐controlled clinical trials. Neuropsychopharmacology 2012;37(3):641‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalman 2011 {published data only}
- Kalman D, Herz L, Monti P, Kahler CW, Mooney M, Rodrigues S, et al. Incremental efficacy of adding bupropion to the nicotine patch for smoking cessation in smokers with a recent history of alcohol dependence: results from a randomized, double‐blind, placebo‐controlled study. Drug and Alcohol Dependence 2011;118(2‐3):111‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeary JE, Knopik VS, Hayes JE, Palmer RH, Monti PM, Kalman D. Predictors of relapse in a bupropion trial for smoking cessation in recently‐abstinent alcoholics: preliminary results using an aggregate genetic risk score. Substance Abuse 2012;6:107‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Killen 2006 {published data only}
- Killen JD, Fortmann SP, Murphy GM Jr, Hayward C, Arredondo C, Cromp D, et al. Extended treatment with bupropion SR for cigarette smoking cessation. Journal of Consulting and Clinical Psychology 2006;74(2):286‐94. [DOI] [PubMed] [Google Scholar]
- Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Schatzberg AF, et al. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5‐A3‐B4) predict severity of nicotine addiction and response to smoking cessation therapy. American Journal of Medical Genetics B Neuropsychiatric Genetics 2011;156B(3):275‐84. [DOI] [PubMed] [Google Scholar]
Killen 2008 {published data only}
- Killen JD, Fortmann SP, Schatzberg AF, Arredondo C, Murphy G, Hayward C, et al. Extended cognitive behavior therapy for cigarette smoking cessation. Addiction 2008;103(8):1381‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Schatzberg AF, et al. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5‐A3‐B4) predict severity of nicotine addiction and response to smoking cessation therapy. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 2011;156B(3):275‐84. [DOI] [PubMed] [Google Scholar]
Killen 2010 {published data only}
- Killen JD, Fortmann SP, Murphy GM Jr, Hayward C, Fong D, Lowenthal K, et al. Failure to improve cigarette smoking abstinence with transdermal selegiline + cognitive behavior therapy. Addiction 2010;105(9):1660‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Ameli N, et al. Response to transdermal selegiline smoking cessation therapy and markers in the 15q24 chromosomal region. Nicotine & Tobacco Research 2015;17(9):1126‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lerman 2002 {published and unpublished data}
- Bergen AW, Javitz HS, Krasnow R, Michel M, Nishita D, Conti DV, et al. Organic cation transporter variation and response to smoking cessation therapies. Nicotine & Tobacco Research 2014;16(12):1638‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Javitz HS, Krasnow R, Nishita D, Michel M, Conti DV, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenetics and Genomics 2013;23(2):94‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Javitz HS, Su L, He Y, Conti DV, Benowitz NL, et al. The DRD4 exon III VNTR, bupropion, and associations with prospective abstinence. Nicotine & Tobacco Research 2013;15(7):1190‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini WH, Wileyto EP, Epstein L, Restine S, Hawk L, Shields P, et al. Catechol‐O‐methyltransferase (COMT) gene variants predict response to bupropion therapy for tobacco dependence. Biological Psychiatry 2007;61(1):111‐8. [DOI] [PubMed] [Google Scholar]
- Conti DV, Lee W, Li D, Liu J, Berg D, Thomas PD, et al. Pharmacogenetics of Nicotine Addiction and Treatment Consortium. Nicotinic acetylcholine receptor beta2 subunit gene implicated in a systems‐based candidate gene study of smoking cessation. Human Molecular Genetics 2008;17(18):2834‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David SP, Strong DR, Leventhal AM, Lancaster MA, McGeary JE, Munafò MR, et al. Influence of a dopamine pathway additive genetic efficacy score on smoking cessation: results from two randomized clinical trials of bupropion. Addiction 2013;108(12):2202‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AB, Wileyto EP, Lori A, Conti D, Cubells JF, Lerman C. Pharmacogenetic association of the galanin receptor (GALR1) SNP rs2717162 with smoking cessation. Neuropsychopharmacology 2012;37(7):1683‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitjan DF, Guo M, Ray R, Wileyto EP, Epstein LH, Lerman C. Identification of pharmacogenetic markers in smoking cessation therapy. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 2008;147B(6):712‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AM, Jepson C, Hoffmann E, Epstein L, Hawk LW, Lerman C, et al. CYP2B6 genotype alters abstinence rates in a bupropion smoking cessation trial. Biological Psychiatry 2007;62(6):635‐41. [DOI] [PubMed] [Google Scholar]
- Lee W, Bergen AW, Swan GE, Li D, Liu J, Thomas P, et al. Gender‐stratified gene and gene‐treatment interactions in smoking cessation. Pharmacogenomics Journal 2012;12(6):521‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Ray R, Bergen AW, Swan GE, Thomas P, Tyndale RF, et al. DRD1 associations with smoking abstinence across slow and normal nicotine metabolizers. Pharmacogenetics and Genomics 2012;22(7):551‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Roth D, Kaufmann V, Audrain J, Hawk L, Liu A, et al. Mediating mechanisms for the impact of bupropion in smoking cessation treatment. Drug and Alcohol Dependence 2002;67(2):219‐23. [DOI] [PubMed] [Google Scholar]
- Lerman C, Shields PG, Wileyto EP, Audrain J, Hawk LH Jr, Pinto A, et al. Effects of dopamine transporter and receptor polymorphisms on smoking cessation in a bupropion clinical trial. Health Psychology 2003;22(5):541‐8. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Lee W, Bergen AW, Swan GE, Tyndale RF, Lerman C, et al. Nicotine dependence as a moderator of genetic influences on smoking cessation treatment outcome. Drug and Alcohol Dependence 2014;138:109‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lerman 2004 {published and unpublished data}
- Bergen AW, Javitz HS, Krasnow R, Nishita D, Michel M, Conti DV, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenetics and Genomics 2013;23(2):94‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AB, Wileyto EP, Lori A, Conti D, Cubells JF, Lerman C. Pharmacogenetic association of the galanin receptor (GALR1) SNP rs2717162 with smoking cessation. Neuropsychopharmacology 2012;37(7):1683‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KE, Allen DL, Filbey FM, Jepson C, Lerman C, Benowitz NL, et al. CHRNA4 and tobacco dependence: from gene regulation to treatment outcome. Archives of General Psychiatry 2007;64(9):1078‐86. [DOI] [PubMed] [Google Scholar]
- Lee AM, Jepson C, Shields PG, Benowitz N, Lerman C, Tyndale RF. CYP2B6 genotype does not alter nicotine metabolism, plasma levels, or abstinence with nicotine replacement therapy. Cancer Epidemiology Biomarkers & Prevention 2007;16(6):1312‐4. [DOI] [PubMed] [Google Scholar]
- Lee W, Bergen AW, Swan GE, Li D, Liu J, Thomas P, et al. Gender‐stratified gene and gene‐treatment interactions in smoking cessation. Pharmacogenomics Journal 2012;12(6):521‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee W, Ray R, Bergen AW, Swan GE, Thomas P, Tyndale RF, et al. DRD1 associations with smoking abstinence across slow and normal nicotine metabolizers. Pharmacogenetics and Genomics 2012;22(7):551‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Kaufmann V, Rukstalis M, Patterson F, Perkins K, Audrain‐McGovern J, et al. Individualizing nicotine replacement therapy for the treatment of tobacco dependence: a randomized trial. Annals of Internal Medicine 2004;140(6):426‐33. [DOI] [PubMed] [Google Scholar]
- Lerman C, Wileyto EP, Patterson F, Rukstalis M, Audrain‐McGovern J, Restine S, et al. The functional mu opioid receptor (OPRM1) Asn40Asp variant predicts short‐term response to nicotine replacement therapy in a clinical trial. Pharmacogenomics Journal 2004;4(3):184‐92. [DOI] [PubMed] [Google Scholar]
- Leventhal AM, Lee W, Bergen AW, Swan GE, Tyndale RF, Lerman C, et al. Nicotine dependence as a moderator of genetic influences on smoking cessation treatment outcome. Drug and Alcohol Dependence 2014;138:109‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R, Jepson C, Wileyto EP, Dahl JP, Patterson F, Rukstalis M, et al. Genetic variation in mu‐opioid‐receptor‐interacting proteins and smoking cessation in a nicotine replacement therapy trial. Nicotine & Tobacco Research 2007;9(11):1237‐41. [DOI] [PubMed] [Google Scholar]
- Ray R, Mitra N, Baldwin D, Guo M, Patterson F, Heitjan DF, et al. Convergent evidence that choline acetyltransferase gene variation is associated with prospective smoking cessation and nicotine dependence. Neuropsychopharmacology 2010;35(6):1374‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lerman 2015 {published data only}
- Lerman C, Schnoll RA, Hawk LW Jr, Cinciripini P, George TP, Wileyto EP, et al. PGRN‐PNAT Research Group. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double‐blind placebo‐controlled trial. Lancet Respiratory Medicine 2015;3(2):131‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyndale RF, Zhu AZ, George TP, Cinciripini P, Hawk LW Jr, Schnoll RA, et al. PGRN‐PNAT Research Group. Lack of associations of CHRNA5‐A3‐B4 genetic variants with smoking cessation treatment outcomes in Caucasian smokers despite associations with baseline smoking. PLoS One 2015;10(5):e0128109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marteau 2012 {published and unpublished data}
- Marteau TM, Aveyard P, Munafò MR, Prevost AT, Hollands GJ, Armstrong D, et al. Effect on adherence to nicotine replacement therapy of informing smokers their dose is determined by their genotype: a randomised controlled trial. PLoS One 2014;7(4):e35249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Johnstone EC, Aveyard P, Marteau T. Lack of association of OPRM1 genotype and smoking cessation. Nicotine & Tobacco Research 2013;15(3):739‐44. [DOI] [PubMed] [Google Scholar]
McCarthy 2008 {published and unpublished data}
- Bergen AW, Javitz HS, Krasnow R, Michel M, Nishita D, Conti DV, et al. Organic cation transporter variation and response to smoking cessation therapies. Nicotine & Tobacco Research 2014;16(12):1638‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Javitz HS, Krasnow R, Nishita D, Michel M, Conti DV, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenetics and Genomics 2013;23(2):94‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy DE, Piasecki TM, Lawrence DL, Jorenby DE, Shiffman S, Fiore MC, et al. A randomized controlled clinical trial of bupropion SR and individual smoking cessation counseling. Nicotine & Tobacco Research 2008;10(4):717‐29. [DOI] [PubMed] [Google Scholar]
Oncken 2006 {published data only}
- Oncken C, Gonzales D, Nides M, Rennard S, Watsky E, Billing CB, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Archives of Internal Medicine 2006;166(15):151‐7. [DOI] [PubMed] [Google Scholar]
Piper 2007 {published and unpublished data}
- Bergen AW, Javitz HS, Krasnow R, Michel M, Nishita D, Conti DV, et al. Organic cation transporter variation and response to smoking cessation therapies. Nicotine & Tobacco Research 2014;16(12):1638‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Javitz HS, Krasnow R, Nishita D, Michel M, Conti DV, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenetics and Genomics 2013;23(2):94‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Federman EB, McCarthy DE, Bolt DM, Smith SS, Fiore MC, et al. Efficacy of bupropion alone and in combination with nicotine gum. Nicotine & Tobacco Research 2007;9(9):947‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piper 2009 {published and unpublished data}
- Bergen AW, Javitz HS, Krasnow R, Michel M, Nishita D, Conti DV, et al. Organic cation transporter variation and response to smoking cessation therapies. Nicotine & Tobacco Research 2014;16(12):1638‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Javitz HS, Krasnow R, Nishita D, Michel M, Conti DV, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenetics and Genomics 2013;23(2):94‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Baker TB, Piper ME, Breslau N, Cannon DS, Doheny KF, et al. Interplay of genetic risk factors (CHRNA5‐CHRNA3‐CHRNB4) and cessation treatments in smoking cessation success. American Journal of Psychiatry 2012;169(7):735‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LS, Bloom AJ, Baker TB, Smith SS, Piper ME, Martinez M, et al. Pharmacotherapy effects on smoking cessation vary with nicotine metabolism gene (CYP2A6). Addiction 2014;109(1):128‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper ME, Smith SS, Schlam TR, Fiore MC, Jorenby DE, Fraser D, et al. A randomized placebo‐controlled clinical trial of 5 smoking cessation pharmacotherapies. Archives of General Psychiatry 2009;66(11):1253‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 2010 {published data only}
- Rose JE, Behm FM, Drgon T, Johnson C, Uhl GR. Personalized smoking cessation: interactions between nicotine dose, dependence and quit‐success genotype score. Molecular Medicine 2010;16(7‐8):247‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnoll 2010 {published data only}
- Gold AB, Wileyto EP, Lori A, Conti D, Cubells JF, Lerman C. Pharmacogenetic association of the galanin receptor (GALR1) SNP rs2717162 with smoking cessation. Neuropsychopharmacology 2012;37(7):1683‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Jepson C, Wileyto EP, Patterson F, Schnoll R, Mroziewicz M, et al. Genetic variation in nicotine metabolism predicts the efficacy of extended‐duration transdermal nicotine therapy. Clinical Pharmacology & Therapeutics 2010;87(5):553‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnoll RA, Patterson F, Wileyto EP, Heitjan DF, Shields AE, Asch DA, et al. Effectiveness of extended‐duration transdermal nicotine therapy: a randomized trial. Annals of Internal Medicine 2010;152(3):144‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2012 {published data only}
- Sun H, Guo S, Chen D, Yang F, Zou Y, Di X, et al. Association of functional COMT Val108/Met polymorphism with smoking cessation in a nicotine replacement therapy. Journal of Neural Transmission (Vienna) 2012;119(12):1491‐8. [DOI] [PubMed] [Google Scholar]
Swan 2003 {published data only}
- Swan GE, Jack LM, Valdes AM, Ring HZ, Ton CC, Curry SJ, et al. Joint effect of dopaminergic genes on likelihood of smoking following treatment with bupropion SR. Health Psychology 2007;26(3):361‐8. [DOI] [PubMed] [Google Scholar]
- Swan GE, McAfee T, Curry SJ, Jack LM, Javitz H, Dacey S, et al. Effectiveness of bupropion sustained release for smoking cessation in a health care setting: a randomized trial. Archives of Internal Medicine 2003;163(19):2337‐44. [DOI] [PubMed] [Google Scholar]
- Swan GE, Valdes AM, Ring HZ, Khroyan TV, Jack LM, Ton CC, et al. Dopamine receptor DRD2 genotype and smoking cessation outcome following treatment with bupropion SR. Pharmacogenomics Journal 2005;5(1):21‐9. [DOI] [PubMed] [Google Scholar]
Swan 2010 {published and unpublished data}
- Bergen AW, Javitz HS, Krasnow R, Michel M, Nishita D, Conti DV, et al. Organic cation transporter variation and response to smoking cessation therapies. Nicotine & Tobacco Research 2014;16(12):1638‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen AW, Javitz HS, Krasnow R, Nishita D, Michel M, Conti DV, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenetics and Genomics 2013;23(2):94‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, McClure JB, Jack LM, Zbikowski SM, Javitz HS, Catz SL, et al. Behavioral counseling and varenicline treatment for smoking cessation. American Journal of Preventive Medicine 2010;38(5):482‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verde 2014 {published data only}
- Verde Z, Santiago C, Rodríguez González‐Moro JM, Lucas Ramos P, López Martín S, Bandrés F, et al. Are serotonergic system genes associated to smoking cessation therapy success in addition to CYP2A6?. Pharmacopsychiatry 2014;47(1):33‐6. [PUBMED: 24127329] [DOI] [PubMed] [Google Scholar]
Wagena 2005 {published data only}
- Quaak M, Schayck CP, Postma DS, Wagena EJ, Schooten FJ. Genetic variants in the serotonin transporter influence the efficacy of bupropion and nortriptyline in smoking cessation. Addiction 2012;107(1):178‐87. [DOI] [PubMed] [Google Scholar]
- Wagena EJ, Knipschild PG, Huibers MJ, Wouters EF, Schayck CP. Efficacy of bupropion and nortriptyline for smoking cessation among people at risk for or with chronic obstructive pulmonary disease. Archives of Internal Medicine 2005;165(19):2286‐92. [DOI] [PubMed] [Google Scholar]
Wilcox 2011 {published data only}
- Wilcox CS, Noble EP, Oskooilar N. ANKK1/DRD2 locus variants are associated with rimonabant efficacy in aiding smoking cessation: pilot data. Journal of Investigative Medicine 2011;59(8):1280‐3. [DOI] [PubMed] [Google Scholar]
Winst 2006 {published and unpublished data}
- Ruyck K, Nackaerts K, Beels L, Werbrouck J, Volder A, Meysman M, et al. Genetic variation in three candidate genes and nicotine dependence, withdrawal and smoking cessation in hospitalized patients. Pharmacogenomics 2010;11(8):1053‐63. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baker 2009 {published data only}
- Baker TB, Weiss RB, Bolt D, Niederhausern A, Fiore MC, Dunn DM, et al. Human neuronal acetylcholine receptor A5‐A3‐B4 haplotypes are associated with multiple nicotine dependence phenotypes. Nicotine & Tobacco Research 2009;11(7):785‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Becker 2008 {published data only}
- Becker KM, Rose JE, Albino AP. A randomized trial of nicotine replacement therapy in combination with reduced‐nicotine cigarettes for smoking cessation. Nicotine & Tobacco Research 2008;10(7):1139‐48. [DOI] [PubMed] [Google Scholar]
Uhl 2007 {published data only}
- Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE. Molecular genetics of nicotine dependence and abstinence: whole genome association using 520,000 SNPs. BMC Genetics 2007;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
1000 Genomes Project Consortium 2015
- 1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, Korbel JO, et al. A global reference for human genetic variation. Nature 2015;526(7571):68‐74. [PUBMED: 26432245] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adkins 2013
- Adkins DE, Souza RP, Aberg K, Clark SL, McClay JL, Sullivan PF, et al. Genotype‐based ancestral background consistently predicts efficacy and side effects across treatments in CATIE and STAR*D. PLoS One 2013;8(2):e55239. [PUBMED: 23405125] [DOI] [PMC free article] [PubMed] [Google Scholar]
Agrawal 2012
- Agrawal A, Verweij KJ, Gillespie NA, Heath AC, Lessov‐Schlaggar CN, Martin NG, et al. The genetics of addiction ‐ a translational perspective. Translational Psychiatry 2012;2(e140):1‐14. [PUBMED: 22806211] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aguirre‐Samudio 2014
- Aguirre‐Samudio AJ, Cruz‐Fuentes CS, González‐Sobrino BZ, Gutiérrez‐Pérez V, Medrano‐González L. Haplotype and nucleotide variation in the exon 3‐VNTR of the DRD4 gene from indigenous and urban populations of Mexico. Am J Hum Biol 2014;26(5):682‐9. [PUBMED: 24979719] [DOI] [PubMed] [Google Scholar]
Attia 2009
- Attia J, Ioannidis JP, Thakkinstian A, McEvoy M, Scott RJ, Minelli C, et al. How to use an article about genetic association: A: background concepts. JAMA 2009;301(1):74‐81. [PUBMED: 19126812] [DOI] [PubMed] [Google Scholar]
Balfour 2009
- Balfour DJ. The neuronal pathways mediating the behavioral and addictive properties of nicotine. Handbook of Experimental Pharmacology 2009;192:209‐33. [PUBMED: 19184651] [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PUBMED: 7786990] [PubMed] [Google Scholar]
Beirut 2008
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, et al. Variants in nicotinic receptors and risk for nicotine dependence. American Journal of Psychiatry 2008;165(9):1163‐71. [PUBMED: 18519524] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benjamini 2014
- Benjamini Y, Bogomolov M. Selective inference on multiple families of hypotheses. Journal of the Royal Statistical Society Series B 2014;76(1):297–318. [Google Scholar]
Benowitz 2006
- Benowitz NL, Swan GE, Jacob P 3rd, Lessov‐Schlaggar CN, Tyndale RF. CYP2A6 genotype and the metabolism and disposition kinetics of nicotine. Clinical Pharmacology and Therapeutics 2006;80(5):457‐67. [PUBMED: 17112802] [DOI] [PubMed] [Google Scholar]
Benowitz 2009
- Benowitz NL, Hukkanen J, Jacob P 3rd. Nicotine chemistry, metabolism, kinetics and biomarkers. Handbook of Experimental Pharmacology 2009;192:29‐60. [PUBMED: 19184645] [DOI] [PMC free article] [PubMed] [Google Scholar]
Benowitz 2013
- Benowitz NL, Zhu AZ, Tyndale RF, Dempsey D, Jacob P 3rd. Influence of CYP2B6 genetic variants on plasma and urine concentrations of bupropion and metabolites at steady state. Pharmacogenetics and Genomics 2013;23(3):135‐41. [PUBMED: 23344581] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergen 2013
- Bergen AW, Javitz HS, Krasnow R, Nishita D, Michel M, Conti DV, et al. Nicotinic acetylcholine receptor variation and response to smoking cessation therapies. Pharmacogenetics and Genomics 2013;23(2):94‐103. [PUBMED: 23249876] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bergen 2014
- Bergen AW, Javitz HS, Krasnow R, Michel M, Nishita D, Conti DV, et al. Organic cation transporter variation and response to smoking cessation therapies. Nicotine & Tobacco Research 2014;16(12):1638‐46. [PUBMED: 25143296] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bidwell 2016
- Bidwell LC, Palmer RH, Brick L, McGeary JE, Knopik VS. Genome‐wide single nucleotide polymorphism heritability of nicotine dependence as a multidimensional phenotype. Psychological Medicine 2016;46(10):2059‐69. [PUBMED: 27052577] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bloom 2012
- Bloom AJ, Harari O, Martinez M, Madden PA, Martin NG, Montgomery GW, et al. Use of a predictive model derived from in vivo endophenotype measurements to demonstrate associations with a complex locus, CYP2A6. Human Molecular Genetics 2012;21(13):3050‐62. [PUBMED: 22451501] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bress 2015
- Bress A, Kittles R, Wing C, Hooker SE Jr, King A. Genetic ancestry as an effect modifier of naltrexone in smoking cessation among African Americans: an analysis of a randomized controlled trial. Pharmacogenetics and Genomics 2015;25(6):305‐12. [PUBMED: 25918964] [DOI] [PMC free article] [PubMed] [Google Scholar]
Broms 2006
- Broms U, Silventoinen K, Madden PA, Heath AC, Kaprio J. Genetic architecture of smoking behavior: a study of Finnish adult twins. Twin Research and Human Genetics 2006;9(1):64‐72. [PUBMED: 16611469] [DOI] [PubMed] [Google Scholar]
Cahill 2012
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD006103.pub6; PUBMED: 22513936] [DOI] [PubMed] [Google Scholar]
Cahill 2013
- Cahill K, Stevens S, Perera R, Lancaster T. Pharmacological interventions for smoking cessation: an overview and network meta‐analysis. Cochrane Database of Systematic Reviews 2013;5:CD009329. [PUBMED: 23728690] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cahill 2016
- Cahill K, Lindson‐Hawley N, Thomas KH, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2016;9(5):CD006103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Canova 2009
- Canova C, Hashibe M, Simonato L, Nelis M, Metspalu A, Lagiou P, et al. Genetic associations of 115 polymorphisms with cancers of the upper aerodigestive tract across 10 European countries: the ARCAGE project. Cancer Research 2009;69(7):2956‐65. [PUBMED: 19339270] [DOI] [PubMed] [Google Scholar]
Cardon 2000
- Cardon LR, Idury RM, Harris TJ, Witte JS, Elston RC. Testing drug response in the presence of genetic information: sampling issues for clinical trials. Pharmacogenetics 2000;10(6):503‐10. [PUBMED: 10975604] [DOI] [PubMed] [Google Scholar]
Cardon 2003
- Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet 2003;361(9357):598‐604. [PUBMED: 12598158 ] [DOI] [PubMed] [Google Scholar]
Carmelli 1992
- Carmelli D, Swan GE. Genetic influence on smoking ‐ a study of male twins. New England Journal of Medicine 1992;327(12):829‐33. [PUBMED: 1508241] [DOI] [PubMed] [Google Scholar]
Chen 2012
- Chen LS, Baker TB, Piper ME, Breslau N, Cannon DS, Doheny KF, et al. Interplay of genetic risk factors (CHRNA5‐CHRNA3‐CHRNB4) and cessation treatments in smoking cessation success. American Journal of Psychiatry 2012;169(7):735‐42. [PUBMED: 22648373] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2014
- Chen LS, Bloom AJ, Baker TB, Smith SS, Piper ME, Martinez M, et al. Pharmacotherapy effects on smoking cessation vary with nicotine metabolism gene (CYP2A6). Addiction 2014;109(1):128‐37. [PUBMED: 24033696] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2017
- Chen LS, Zawertailo L, Piasecki T, Kaprio J, Foreman M, Elliott H, et al. Genetics and Treatment Workgroup of the Society for Research on Nicotine and Tobacco (SRNT). Leveraging genomic data in smoking cessation trials in the era of Precision Medicine: why and how. Nicotine & Tobacco Research 2017 May 12;(manuscript in press). [PUBMED: 28498934] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chenoweth 2017
- Chenoweth MJ, Tyndale RF. Pharmacogenetic optimization of smoking cessation treatment. Trends in Pharmacological Science 2017;38(1):55‐66. [PUBMED: 27712845] [DOI] [PMC free article] [PubMed] [Google Scholar]
David 2007
- David SP, Munafò MR, Murphy MF, Walton RT, Johnstone EC. The serotonin transporter 5‐HTTLPR polymorphism and treatment response to nicotine patch: follow‐up of a randomized controlled trial. Nicotine & Tobacco Research 2007;9(2):225‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
David 2008
- David SP, Johnstone EC, Murphy MF, Aveyard P, Guo B, Lerman C, et al. Genetic variation in the serotonin pathway and smoking cessation with nicotine replacement therapy: new data from the Patch in Practice trial and pooled analyses. Drug and Alcohol Dependence 2008;98(1‐2):77‐85. [PUBMED: 18562131] [DOI] [PMC free article] [PubMed] [Google Scholar]
David 2008a
- David SP, Munafò MR, Murphy MF, Proctor M, Walton RT, Johnstone EC. Genetic variation in the dopamine D4 receptor (DRD4) gene and smoking cessation: follow‐up of a randomised clinical trial of transdermal nicotine patch. Pharmacogenomics Journal 2008;8(2):122‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
David 2011
- David SP, Johnstone EC, Churchman M, Aveyard P, Murphy MF, Munafò MR. Pharmacogenetics of smoking cessation in general practice: results from the Patch II and Patch in Practice trials. Nicotine & Tobacco Research 2011;13(3):157‐67. [PUBMED: 21330274] [DOI] [PMC free article] [PubMed] [Google Scholar]
David 2012
- David SP, Hamidovic A, Chen GK, Bergen AW, Wessel J, Kasberger JL, et al. Genome‐wide meta‐analyses of smoking behaviors in African Americans. Translational Psychiatry 2012;2:e119. [PUBMED: 22832964 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
David 2013a
- David SP, Strong DR, Leventhal AM, Lancaster MA, McGeary JE, Munafò MR, et al. Influence of a dopamine pathway additive genetic efficacy score on smoking cessation: results from two randomized clinical trials of bupropion. Addiction 2013;108(12):2202‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Ruyck 2010
- Ruyck K, Nackaerts K, Beels L, Werbrouck J, Volder A, Meysman M, et al. Genetic variation in three candidate genes and nicotine dependence, withdrawal and smoking cessation in hospitalized patients. Pharmacogenomics 2010;11(8):1053‐63. [DOI] [PubMed] [Google Scholar]
Debray 2015
- Debray TP, Moons KG, Valkenhoef G, Efthimiou O, Hummel N, Groenwold RH, et al. GetReal Methods Review Group. Get real in individual participant data (IPD) meta‐analysis: a review of the methodology. Research Synthesis Methods 2015;6:293–309. [PUBMED: 26287812] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dempsey 2004
- Dempsey D, Tutka P, Jacob P 3rd, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clinical Pharmacology & Therapeutics 2004;76(1):64‐72. [PUBMED: 15229465] [DOI] [PubMed] [Google Scholar]
Drgon 2009
- Drgon T, Johnson C, Walther D, Albino AP, Rose JE, Uhl GR. Genome‐wide association for smoking cessation success: participants in a trial with adjunctive denicotinized cigarettes. Molecular Medicine 2009;15(7‐8):268‐74. [PUBMED: 19593411] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dudbridge 2013
- Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genetics 2013;9(3):e1003348. [PUBMED: 23555274] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
European Smoking Cessation Guidelines 2012
- Behrakis PK, Bilir N, Clancy L, Dautzenberg B, Demin AK, Gilljam H, et al. for the European Network for Smoking and Tobacco Prevention (ENSP). European Smoking Cessation Guidelines: The authoritative guide to a comprehensive understanding of the implications and implementation of treatments and strategies to treat tobacco dependence. http://ensp.org/wp‐content/uploads/2016/12/ENSP‐ESCG_FINAL.pdf 2012.
Fiore 2008
- Fiore MC, Jaen CR, Baker TB. Clinical practice guideline ‐ treating tobacco use and dependence: 2008 update. Rockville, MD: US Department of Health and Human Services, 2008; US Preventive Services Task Force. Behavioral and pharmacotherapy interventions for tobacco smoking cessation in adults, including pregnant women: recommendation statement. American Family Physician 2016;93:860A‐G. [PubMed] [Google Scholar]
Fisher 1958
- Fisher RA. Lung cancer and cigarettes?. Nature 1958;182(4628):108. [PUBMED: 13566198] [DOI] [PubMed] [Google Scholar]
Fowler 2011
- Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature 2011;471(7340):597‐601. [PUBMED: 21278726] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freathy 2009
- Freathy RM, Ring SM, Shields B, Galobardes B, Knight B, Weedon MN, et al. A common genetic variant in the 15q24 nicotinic acetylcholine receptor gene cluster (CHRNA5‐CHRNA3‐CHRNB4) is associated with a reduced ability of women to quit smoking in pregnancy. Human Molecular Genetics 2009;18(15):2922‐7. [PUBMED: 19429911] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freidlin 2005
- Freidlin B, Simon R. Adaptive signature design: an adaptive clinical trial design for generating and prospectively testing a gene expression signature for sensitive patients. Clinical Cancer Research 2005;11(21):7872‐8. [PUBMED: 16278411] [DOI] [PubMed] [Google Scholar]
Freidlin 2010
- Freidlin B, Jiang W, Simon R. The cross‐validated adaptive signature design. Clinical Cancer Research 2010;16(2):691‐8. [PUBMED: 20068112] [DOI] [PubMed] [Google Scholar]
Fujieda 2004
- Fujieda M, Yamazaki H, Saito T, Kiyotani K, Gyamfi MA, Sakurai M, et al. Evaluation of CYP2A6 genetic polymorphisms as determinants of smoking behavior and tobacco‐related lung cancer risk in male Japanese smokers. Carcinogenesis 2004;25(12):2451‐8. [PUBMED: 15308589] [DOI] [PubMed] [Google Scholar]
Gemignani 2007
- Gemignani F, Landi S, Szeszenia‐Dabrowska N, Zaridze D, Lissowska J, Rudnai P, et al. Development of lung cancer before the age of 50: the role of xenobiotic metabolizing genes. Carcinogenesis 2007;28(6):1287‐93. [PUBMED: 17259654] [DOI] [PubMed] [Google Scholar]
Giovino 2012
- Giovino GA, Mirza SA, Samet JM, Gupta PC, Jarvis MJ, Bhala N, et al. GATS Collaborative Group. Tobacco use in 3 billion individuals from 16 countries: an analysis of nationally representative cross‐sectional household surveys. Lancet 2012;380(9842):668‐79. [PUBMED: 22901888] [DOI] [PubMed] [Google Scholar]
Gold 2012
- Gold AB, Wileyto EP, Lori A, Conti D, Cubells JF, Lerman C. Pharmacogenetic association of the galanin receptor (GALR1) SNP rs2717162 with smoking cessation. Neuropsychopharmacology 2012;37(7):1683‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadjiconstantinou 2011
- Hadjiconstantinou M, Neff NH. Nicotine and endogenous opioids: neurochemical and pharmacological evidence. Neuropharmacology 2011;60(7‐8):1209‐20. [PUBMED: 21108953] [DOI] [PubMed] [Google Scholar]
Heath 2002
- Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PA. Estimating two‐stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Research 2002;5(2):113‐24. [PUBMED: 11931689] [DOI] [PubMed] [Google Scholar]
Herman 2014
- Herman AI, DeVito EE, Jensen KP, Sofuoglu M. Pharmacogenetics of nicotine addiction: role of dopamine. Pharmacogenomics 2014;15(2):221‐34. [PUBMED: 24444411] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Ho 2009
- Ho MK, Mwenifumbo JC, Al Koudsi N, Okuyemi KS, Ahluwalia JS, Benowitz NL, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African‐American light smokers. Clinical Pharmacology & Therapeutics 2009;85(6):635‐43. [PUBMED: 19279561] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hou 2016
- Hou L, Heilbronner U, Degenhardt F, Adli M, Akiyama K, Akula N, et al. Genetic variants associated with response to lithium treatment in bipolar disorder: a genome‐wide association study. Lancet 2016;387(10023):1085‐93. [PUBMED: 26806518] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2014
- Hughes JR, Stead LF, Hartmann‐Boyce J, Cahill K, Lancaster T. Antidepressants for smoking cessation. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD000031.pub4; PUBMED: 24402784] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnstone 2004
- Johnstone EC, Yudkin PL, Hey K, Roberts SJ, Welch SJ, Murphy MF, et al. Genetic variation in dopaminergic pathways and short‐term effectiveness of the nicotine patch. Pharmacogenetics 2004;14(2):83‐90. [DOI] [PubMed] [Google Scholar]
Johnstone 2006
- Johnstone E, Benowitz N, Cargill A, Jacob R, Hinks L, Day I, et al. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clinical Pharmacology & Therapeutics 2006;80(4):319‐30. [PUBMED: 17015050] [DOI] [PubMed] [Google Scholar]
Johnstone 2007
- Johnstone EC, Elliot KM, David SP, Murphy MF, Walton RT, Munafò MR. Association of COMT Val108/158Met genotype with smoking cessation in a nicotine replacement therapy randomized trial. Cancer Epidemiology Biomarkers & Prevention 2007;16(6):1065‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2012
- King DP, Paciga S, Pickering E, Benowitz NL, Bierut LJ, Conti DV, et al. Smoking cessation pharmacogenetics: analysis of varenicline and bupropion in placebo‐controlled clinical trials. Neuropsychopharmacology 2012;37(3):641‐50. [PUBMED: 22048466] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotz 2014
- Kotz D, Brown J, West R. Prospective cohort study of the effectiveness of smoking cessation treatments used in the "real world". Mayo Clinic Proceedings 2014;89(10):1360‐7. [PUBMED: 25282429] [DOI] [PMC free article] [PubMed] [Google Scholar]
Landi 2009
- Landi MT, Chatterjee N, Yu K, Goldin LR, Goldstein AM, Rotunno M, et al. A genome‐wide association study of lung cancer identifies a region of chromosome 5p15 associated with risk for adenocarcinoma. American Journal of Human Genetics 2009;85(5):679‐91. [PUBMED: 19836008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lerman 2006
- Lerman C, Tyndale R, Patterson F, Wileyto EP, Shields PG, Pinto A, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clinical Pharmacology & Therapeutics 2006;79(6):600‐8. [PUBMED: 16765148] [DOI] [PubMed] [Google Scholar]
Lerman 2010
- Lerman C, Jepson C, Wileyto EP, Patterson F, Schnoll R, Mroziewicz M, et al. Genetic variation in nicotine metabolism predicts the efficacy of extended‐duration transdermal nicotine therapy. Clinical Pharmacology and Therapeutics 2010;87(5):553‐7. [PUBMED: 20336063] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leung 2015
- Leung T, Bergen A, Munafò MR, Ruyck K, Selby P, Luca V. Effect of the rs1051730‐rs16969968 variant and smoking cessation treatment: a meta‐analysis. Pharmacogenomics 2015;16(7):713‐20. [PUBMED: 25950378] [DOI] [PubMed] [Google Scholar]
Leventhal 2012
- Leventhal AM, David SP, Brightman M, Strong D, McGeary JE, Brown RA, et al. Dopamine D4 receptor gene variation moderates the efficacy of bupropion for smoking cessation. Pharmacogenomics Journal 2012;12(1):86‐92. [PUBMED: 20661272] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leventhal 2014
- Leventhal AM, Lee W, Bergen AW, Swan GE, Tyndale RF, Lerman C, et al. Nicotine dependence as a moderator of genetic influences on smoking cessation treatment outcome. Drug and Alcohol Dependence 2014;1(138):109‐17. [PUBMED: 24667010] [DOI] [PMC free article] [PubMed] [Google Scholar]
Li 2003
- Li MD, Cheng R, Ma JZ, Swan GE. A meta‐analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 2003;98(1):23‐31. [PUBMED: 12492752] [DOI] [PubMed] [Google Scholar]
Liu 2010
- Liu JZ, Tozzi F, Waterworth DM, Pillai SG, Muglia P, Middleton L, et al. Meta‐analysis and imputation refines the association of 15q25 with smoking quantity. Nature Genetics 2010;42(5):436‐40. [PUBMED: 20418889] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2011
- Liu T, David SP, Tyndale RF, Wang H, Zhou Q, Ding P, et al. Associations of CYP2A6 genotype with smoking behaviors in southern China. Addiction 2011;106(5):985‐94. [PUBMED: 21205058] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mamoun 2015
- Mamoun M, Bergen AW, Shieh J, Wiggins A, Brody AL. Biomarkers of response to smoking cessation pharmacotherapies: progress to date. CNS Drugs 2015;29(5):359‐69. [PUBMED: 25895022] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsui 2015
- Matsui S, Buyse M, Simon R. Design and Analysis of Clinical Trials for Predictive Medicine. Boca Raton, FL: CRC Press, 2015. [Google Scholar]
McCaffery 2008
- McCaffery JM, Papandonatos GD, Lyons MJ, Koenen KC, Tsuang MT, Niaura R. Educational attainment, smoking initiation and lifetime nicotine dependence among male Vietnam‐era twins. Psychological Medicine 2008;38(9):1287‐97. [PUBMED: 17949517] [DOI] [PMC free article] [PubMed] [Google Scholar]
McClay 2011
- McClay JL, Adkins DE, Aberg K, Stroup S, Perkins DO, Vladimirov VI, et al. Genome‐wide pharmacogenomic analysis of response to treatment with antipsychotics. Molecular Psychiatry 2011;16(1):76‐85. [PUBMED: 19721433] [DOI] [PMC free article] [PubMed] [Google Scholar]
McClure 2013
- McClure JB, Swan GE, John J, Fauver R, Javitz HS, Bergen AW, et al. Pharmacogenetic smoking cessation intervention in a health care setting: a pilot feasibility study. Nicotine & Tobacco Research 2013;15(2):518‐26. [PUBMED: 22949583] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonagh 2012
- McDonagh EM, Wassenaar C, David SP, Tyndale RF, Altman RB, Whirl‐Carrillo M, et al. PharmGKB summary: very important pharmacogene information for cytochrome P‐450, family 2, subfamily A, polypeptide 6. Pharmacogenetics and Genomics 2012;22(9):695‐708. [PUBMED: 22547082] [DOI] [PMC free article] [PubMed] [Google Scholar]
McGeary 2012
- McGeary JE, Knopik VS, Hayes JE, Palmer RH, Monti PM, Kalman D. Predictors of relapse in a bupropion trial for smoking cessation in recently‐abstinent alcoholics: preliminary results using an aggregate genetic risk score. Substance Abuse 2012;6:107‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKay 2008
- McKay JD, Hung RJ, Gaborieau V, Boffetta P, Chabrier A, Byrnes G, et al. Lung cancer susceptibility locus at 5p15.33. Nature Genetics 2008;40(12):1404‐6. [PUBMED: 18978790] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2012
- Mills EJ, Wu P, Lockhart I, Thorlund K, Puhan M, Ebbert JO. Comparisons of high‐dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta‐analysis. Annals of Medicine 2012;44(6):588‐97. [PUBMED: 22860882] [DOI] [PubMed] [Google Scholar]
Munafò 2007
- Munafò MR, Elliot KM, Murphy MF, Walton RT, Johnstone EC. Association of the mu‐opioid receptor gene with smoking cessation [Association of the mu‐opioid receptor gene with smoking cessation]. Pharmacogenomics Journal 2007;7(5):353‐61. [17224915] [DOI] [PubMed] [Google Scholar]
Munafò 2008
- Munafò MR, Johnstone EC, Guo B, Murphy MF, Aveyard P. Association of COMT Val108/158Met genotype with smoking cessation. Pharmacogenetics and Genomics 2008;18(2):121‐8. [PUBMED: 18192898] [DOI] [PubMed] [Google Scholar]
Munafò 2009
- Munafò MR, Johnstone EC, Murphy MF, Aveyard P. Lack of association of DRD2 rs1800497 (Taq1A) polymorphism with smoking cessation in a nicotine replacement therapy randomized trial. Nicotine & Tobacco Research 2009;11(4):404‐7. [PUBMED: 19273465] [DOI] [PubMed] [Google Scholar]
Munafò 2011
- Munafò MR, Johnstone EC, Walther D, Uhl GR, Murphy MF, Aveyard P. CHRNA3 rs1051730 genotype and short‐term smoking cessation. Nicotine & Tobacco Research 2011;13(10):982‐8. [PUBMED: 21690317] [DOI] [PMC free article] [PubMed] [Google Scholar]
Munafò 2012
- Munafò MR, Timofeeva MN, Morris RW, Prieto‐Merino D, Sattar N, Brennan P, et al. EPIC Study Group, Davey Smith G. Association between genetic variants on chromosome 15q25 locus and objective measures of tobacco exposure. Journal of the National Cancer Institute 2012;104(10):740‐8. [PUBMED: 22534784] [DOI] [PMC free article] [PubMed] [Google Scholar]
Munafò 2013a
- Munafò MR, Johnstone EC, Aveyard P, Marteau T. Lack of association of OPRM1 genotype and smoking cessation. Nicotine & Tobacco Research 2013;15(3):739‐44. [DOI] [PubMed] [Google Scholar]
Murphy 2013
- Murphy E, Hou L, Maher BS, Woldehawariat G, Kassem L, Akula N, et al. Race, genetic ancestry and response to antidepressant treatment for major depression. Neuropsychopharmacology 2013;38(13):2598‐606. [PUBMED: 23827886] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murphy 2014
- Murphy SE, Park SS, Thompson EF, Wilkens LR, Patel Y, Stram DO, et al. Nicotine N‐glucuronidation relative to N‐oxidation and C‐oxidation and UGT2B10 genotype in five ethnic/racial groups. Carcinogenesis 2014;35(11):2526‐33. [PUBMED: 25233931] [DOI] [PMC free article] [PubMed] [Google Scholar]
Patnode 2015
- Patnode CD, Henderson JT, Thompson JH, Senger CA, Fortmann SP, Whitlock EP. Behavioral Counseling and Pharmacotherapy Interventions for Tobacco Cessation in Adults, Including Pregnant Women: A Review of Reviews for the US Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US) 2015 Sep; Vol. Report No.: 14‐05200‐EF‐1. [PUBMED: 26491759] [PubMed]
Patterson 2008
- Patterson F, Schnoll RA, Wileyto EP, Pinto A, Epstein LH, Shields PG, et al. Toward personalized therapy for smoking cessation: a randomized placebo‐controlled trial of bupropion. Clinical Pharmacology & Therapeutics 2008;84(3):320‐5. [PUBMED: 18388868] [DOI] [PubMed] [Google Scholar]
Pearson 2008
- Pearson TA, Manolio TA. How to interpret a genome‐wide association study. JAMA 2008;299(11):1335‐44. [PUBMED: 18349094] [DOI] [PubMed] [Google Scholar]
Peralta‐Leal 2012
- Peralta‐Leal V, Leal‐Ugarte E, Meza‐Espinoza JP, Dávalos‐Rodríguez IP, Bocanegra‐Alonso A, Acosta‐González RI, Gonzales E, Nair S, Durán‐González J. Association of a serotonin transporter gene (SLC6A4) 5‐HTTLPR polymorphism with body mass index categories but not type 2 diabetes mellitus in Mexicans. Genet Mol Biol 2012;35(3):589‐93. [PUBMED: 23055796] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quaak 2012
- Quaak M, Schayck CP, Postma DS, Wagena EJ, Schooten FJ. Genetic variants in the serotonin transporter influence the efficacy of bupropion and nortriptyline in smoking cessation. Addiction 2012;107(1):178‐87. [PUBMED: 21658141] [DOI] [PubMed] [Google Scholar]
Ray 2007
- Ray R, Jepson C, Wileyto EP, Dahl JP, Patterson F, Rukstalis M, et al. Genetic variation in mu‐opioid‐receptor‐interacting proteins and smoking cessation in a nicotine replacement therapy trial. Nicotine & Tobacco Research 2007;9(11):1237‐41. [PUBMED: 17978999] [DOI] [PubMed] [Google Scholar]
Review Manager 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rotunno 2009
- Rotunno M, Yu K, Lubin JH, Consonni D, Pesatori AC, Goldstein AM, et al. Phase I metabolic genes and risk of lung cancer: multiple polymorphisms and mRNA expression. PLoS One 2009;4(5):e5652. [PUBMED: 19479063] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saccone 2010
- Saccone NL, Culverhouse RC, Schwantes‐An TH, Cannon DS, Chen X, Cichon S, et al. Multiple independent loci at chromosome 15q25.1 affect smoking quantity: a meta‐analysis and comparison with lung cancer and COPD. PLoS Genetics 2010;6(8):e1001053. [PUBMED: 20700436] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saccone 2017
- Saccone N, Baurley JW, Bergen AW, David SP, Elliott H, Foreman M, et al. Genetics and Treatment Networks of the Society for Research on Nicotine and Tobacco (SRNT). The value of biosamples in smoking cessation trials: a review of genetic, metabolomic, and epigenetic findings. Nicotine & Tobacco Research 2017 May 3;(manuscript in press). [PUBMED: 28472521] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sarginson 2011
- Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Schatzberg AF, et al. Markers in the 15q24 nicotinic receptor subunit gene cluster (CHRNA5‐A3‐B4) predict severity of nicotine addiction and response to smoking cessation therapy. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics 2011;156B(3):275‐84. [DOI] [PubMed] [Google Scholar]
Sarginson 2015
- Sarginson JE, Killen JD, Lazzeroni LC, Fortmann SP, Ryan HS, Ameli N, et al. Response to transdermal selegiline smoking cessation therapy and markers in the 15q24 chromosomal region. Nicotine & Tobacco Research 2015;17(9):1126‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scherf 2012
- Scherf DB, Sarkisyan N, Jacobsson H, Claus R, Bermejo JL, Peil B, et al. Epigenetic screen identifies genotype‐specific promoter DNA methylation and oncogenic potential of CHRNB4. Oncogene 2012;32(28):3329‐38. [PUBMED: 22945651] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schnoll 2009
- Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacology, Biochemistry, and Behavior 2009;92(1):6‐11. [PUBMED: 19000709] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schoedel 2004
- Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult caucasians. Pharmacogenetics 2004;14(9):615‐26. [PUBMED: 15475735] [DOI] [PubMed] [Google Scholar]
Spruell 2012
- Spruell T, Colavita G, Donegan T, Egawhary M, Hurley M, Aveyard P, et al. Association between nicotinic acetylcholine receptor single nucleotide polymorphisms and smoking cessation. Nicotine & Tobacco Research 2012;14(8):993‐7. [PUBMED: 22180580] [DOI] [PubMed] [Google Scholar]
Stead 2012a
- Stead LF, Perera R, Bullen C, Mant D, Hartmann‐Boyce J, Cahill K, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD000146.pub4; PUBMED: 23152200] [DOI] [PubMed] [Google Scholar]
Stead 2012b
- Stead LF, Lancaster T. Behavioural interventions as adjuncts to pharmacotherapy for smoking cessation. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD009670.pub2; PUBMED: 23235680] [DOI] [PubMed] [Google Scholar]
Sullivan 2004
- Sullivan PF, Neale BM, Oord E, Miles MF, Neale MC, Bulik CM, et al. Candidate genes for nicotine dependence via linkage, epistasis, and bioinformatics. American Journal of Medical Genetics: Part B Neuropsychiatric Genetics 2004;126B(1):23‐36. [PUBMED: 15048644] [DOI] [PubMed] [Google Scholar]
Sun 2015
- Sun J, Kranzler HR, Bi J. An effective method to identify heritable components from multivariate phenotypes. PLoS One 2015;10(12):e0144418. [PUBMED: 26658140] [DOI] [PMC free article] [PubMed] [Google Scholar]
Swan 2005
- Swan GE, Valdes AM, Ring HZ, Khroyan TV, Jack LM, Ton CC, et al. Dopamine receptor DRD2 genotype and smoking cessation outcome following treatment with bupropion SR. Pharmacogenomics Journal 2005;5(1):21‐9. [DOI] [PubMed] [Google Scholar]
Swan 2007
- Swan GE, Jack LM, Valdes AM, Ring HZ, Ton CC, Curry SJ, et al. Joint effect of dopaminergic genes on likelihood of smoking following treatment with bupropion SR. Health Psychology 2007;26(3):361‐8. [DOI] [PubMed] [Google Scholar]
Tamaki 2011
- Tamaki Y, Arai T, Sugimura H, Sasaki T, Honda M, Muroi Y, et al. Association between cancer risk and drug‐metabolizing enzyme gene (CYP2A6, CYP2A13, CYP4B1, SULT1A1, GSTM1, and GSTT1) polymorphisms in cases of lung cancer in Japan. Drug Metabolism and Pharmacokinetics 2011;26(5):516‐22. [PUBMED: 21791872] [DOI] [PubMed] [Google Scholar]
Thorgeirsson 2008
- Thorgeirsson TE, Geller F, Sulem P, Rafnar T, Wiste A, Magnusson KP, et al. A variant associated with nicotine dependence, lung cancer and peripheral arterial disease. Nature 2008;452(7187):638‐42. [PUBMED: 18385739] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorgeirsson 2010
- Thorgeirsson TE, Gudbjartsson DF, Surakka I, Vink JM, Amin N, Geller F, et al. Sequence variants at CHRNB3‐CHRNA6 and CYP2A6 affect smoking behavior. Nature Genetics 2010;42(5):448‐53. [PUBMED: 20418888] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2015
- Tierney JF, Vale C, Riley R, Smith CT, Stewart L, Clarke M, et al. Individual participant data (IPD) meta‐analyses of randomised controlled trials: guidance on their use. PLoS Medicine 2015;12:e1001855. [PUBMED: 26196287] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tobacco and Genetics Consortium 2010
- Tobacco and Genetics Consortium. Genome‐wide meta‐analyses identify multiple loci associated with smoking behavior. Nature Genetics 2010;42(5):441‐7. [PUBMED: 20418890] [DOI] [PMC free article] [PubMed] [Google Scholar]
Topcu 2002
- Topcu Z, Chiba I, Fujieda M, Shibata T, Ariyoshi N, Yamazaki H, et al. CYP2A6 gene deletion reduces oral cancer risk in betel quid chewers in Sri Lanka. Carcinogenesis 2002;23(4):595‐8. [PUBMED: 11960911] [DOI] [PubMed] [Google Scholar]
Trinidad 2015
- Trinidad DR, Xie B, Fagan P, Pulvers K, Romero DR, Blanco L, et al. Disparities in the population distribution of African American and non‐Hispanic white smokers along the quitting continuum. Health Education & Behavior 2015;42(6):742‐51. [PUBMED: 25794519] [DOI] [PMC free article] [PubMed] [Google Scholar]
True 1997
- True WR, Heath AC, Scherrer JF, Waterman B, Goldberg J, Lin N, et al. Genetic and environmental contributions to smoking. Addiction 1997;92(10):1277‐87. [PUBMED: 9489045] [PubMed] [Google Scholar]
Tyndale 2015
- Tyndale RF, Zhu AZ, George TP, Cinciripini P, Hawk LW Jr, Schnoll RA, et al. PGRN‐PNAT Research Group. Lack of associations of CHRNA5‐A3‐B4 genetic variants with smoking cessation treatment outcomes in Caucasian smokers despite associations with baseline smoking. PLoS One 2015;10(5):e0128109. [PUBMED: 26010901] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uhl 2008
- Uhl GR, Liu QR, Drgon T, Johnson C, Walther D, Rose JE, et al. Molecular genetics of successful smoking cessation: convergent genome‐wide association study results. Archives of General Psychiatry 2008;65(6):683‐93. [PUBMED: 18519826] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uhl 2010
- Uhl GR, Drgon T, Johnson C, Walther D, David SP, Aveyard P, et al. Genome‐wide association for smoking cessation success: participants in the Patch in Practice trial of nicotine replacement. Pharmacogenomics 2011;11(3):357‐67. [PUBMED: 20235792] [DOI] [PMC free article] [PubMed] [Google Scholar]
Uhl 2014
- Uhl GR, Walther D, Musci R, Fisher C, Anthony JC, Storr CL, et al. Smoking quit success genotype score predicts quit success and distinct patterns of developmental involvement with common addictive substances. Molecular Psychiatry 2014;19(1):50‐4. [PUBMED: 23128154] [DOI] [PMC free article] [PubMed] [Google Scholar]
Varadhan 2013
- Varadhan R, Segal JB, Boyd CM, Wu AW, Weiss CO. A framework for the analysis of heterogeneity of treatment effect in patient‐centered outcomes research. Journal of Clinical Epidemiology 2013;66(8):818‐25. [PUBMED: 23651763] [DOI] [PMC free article] [PubMed] [Google Scholar]
Verhagen 2012
- Verhagen M, Kleinjan M, Engels RC. A systematic review of the A118G (Asn40Asp) variant of OPRM1 in relation to smoking initiation, nicotine dependence and smoking cessation. Pharmacogenomics 2013;13(8):917‐33. [PUBMED: 22676196] [DOI] [PubMed] [Google Scholar]
Wang 2010
- Wang J, Li MD. Common and unique biological pathways associated with smoking initiation/progression, nicotine dependence, and smoking cessation. Neuropsychopharmacology 2010;35(3):702‐19. [PUBMED: 19890259] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2013
- Wang JC, Spiegel N, Bertelsen S, Le N, McKenna N, Budde JP, et al. Cis‐regulatory variants affect CHRNA5 mRNA expression in populations of African and European ancestry. PLoS One 2013;8(11):e80204. [PUBMED: 24303001] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 2012
- Ware JJ, Bree M, Munafò MR. From men to mice: CHRNA5/CHRNA3, smoking behavior and disease. Nicotine & Tobacco Research 2012;14(11):1291‐9. [PUBMED: 22544838] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2016
- Weiss ST. Implementing personalized medicine in the academic health center. Journal of Personalize Medicine 2016;6(3):E18. [PUBMED: 27657137] [DOI] [PMC free article] [PubMed] [Google Scholar]
West 2000
- West R, McNeill A, Raw M. Smoking cessation guidelines for health professionals: an update. Health Education Authority. Thorax 2000;55(12):987‐99. [PUBMED: 11083883] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xian 2008
- Xian H, Scherrer JF, Grant JD, Eisen SA, True WR, Jacob T, et al. Genetic and environmental contributions to nicotine, alcohol and cannabis dependence in male twins. Addiction 2008;103(8):1391‐8. [PUBMED: 18855830] [DOI] [PubMed] [Google Scholar]
Yudkin 2004
- Yudkin P, Munafò M, Hey K, Roberts S, Welch S, Johnstone E, et al. Effectiveness of nicotine patches in relation to genotype in women versus men: randomised controlled trial. BMJ 2004;328(7446):989‐90. [PUBMED: 15033882] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zanetti 2016
- Zanetti KA, Wang Z, Aldrich M, Amos CI, Blot WJ, Bowman ED, et al. Genome‐wide association study confirms lung cancer susceptibility loci on chromosomes 5p15 and 15q25 in an African‐American population. Lung Cancer 2016;98:33‐42. [PUBMED: 27393504] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2012
- Zhu AZ, Cox LS, Nollen N, Faseru B, Okuyemi KS, Ahluwalia JS, et al. CYP2B6 and bupropion's smoking‐cessation pharmacology: the role of hydroxybupropion. Clinical Pharmacology & Therapeutics 2012;92(6):771‐7. [PUBMED: 23149928] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2013
- Zhu AZ, Zhou Q, Cox LS, Ahluwalia JS, Benowitz NL, Tyndale RF. Variation in trans‐3'‐hydroxycotinine glucuronidation does not alter the nicotine metabolite ratio or nicotine intake. PLoS One 2013;8(8):e70938. [PUBMED: 23936477] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhu 2014
- Zhu AZ, Zhou Q, Cox LS, David SP, Ahluwalia JS, Benowitz NL, et al. Association of CHRNA5‐A3‐B4 SNP rs2036527 with smoking cessation therapy response in African American smokers. Clinical Pharmacology & Therapeutics 2014;96(2):256‐65. [PUBMED: 24733007] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
David 2015
- Sean P David, Andrew W Bergen, Marcus R Munafò, Ewoud Schuit, Derrick A Bennett, Orestis A. Panagiotou. Genomic analysis to guide choice of treatment for smoking cessation. Cochrane Database of Systematic Reviews 3 August 2015. [DOI: 10.1002/14651858.CD011823] [DOI] [Google Scholar]


