Abstract
This is a protocol for a Cochrane Review (Diagnostic test accuracy). The objectives are as follows:
To determine the diagnostic accuracy of trans‐thoracic Doppler echocardiography for detecting pulmonary hypertension.
We will aim to study several possible source of heterogeneity as below.
Types of PH defined by WHO classification (group 3 or not). We expect that it will be more difficult to measure tricuspid regurgitation in patients with lung disease, especially COPD, than in patients without lung disease, which could decrease the sensitivity and specificity of echocardiography to detect PH.
Mechanical ventilation (including non‐invasive positive pressure ventilation) or not.
Estimation of right atrial pressure (whether estimated by using the diameter and collapse of the inferior vena cava during spontaneous respiration or by any other methods).
Background
Target condition being diagnosed
Pulmonary hypertension (PH) is an important cause of morbidity and mortality. Although the exact prevalence of PH is not known due to the various etiology, a previous study reported that PH affects up to 100 million people worldwide (Schermuly 2011). Pulmonary hypertension leads to a substantial loss of exercise capacity, as well as causing right ventricular overload, resulting in heart failure and early mortality. A recently published study reported that three‐year survival ranges from 58.2% to 73.3% (Ling 2012).
Pulmonary hypertension is diagnosed when resting mean pulmonary arterial pressure is 25 mmHg or more at right‐heart catheterization. It is a progressive disease that if left untreated can be fatal, although the rate of progression is highly variable.
Pulmonary hypertension is divided into five etiological categories according to World Health Organization (WHO) criteria (Simonneau 2013). Group 1 is pulmonary arterial hypertension (PAH). This group consists of sporadic idiopathic pulmonary hypertension (IPAH), heritable IPAH, and PAH due to diseases that localize to small pulmonary muscular arterioles. These diseases include connective tissue disease, HIV infection, portal hypertension, congenital heart disease, schistosomiasis, chronic hemolytic anemia, persistent pulmonary hypertension of the newborn, pulmonary veno‐occlusive disease, and pulmonary capillary hemangiomatosis. Drug‐induced PAH (including anorexigenic) and toxin‐induced PAH are also considered to belong to group 1 PAH.
Group 2 PH is pulmonary hypertension due to left heart disease, and is the most common form of PH worldwide. Pulmonary hypertension due to systolic dysfunction, diastolic dysfunction, or valvular heart disease is included in this group.
Group 3 PH is pulmonary hypertension due to lung disease or hypoxaemia. This includes PH caused by chronic obstructive pulmonary disease (COPD), interstitial lung disease, pulmonary disease with a mixed restrictive and obstructive pattern, sleep‐disordered breathing, and alveolar hypoventilation disorders.
Group 4 PH is chronic thromboembolic pulmonary hypertension due to chronic thromboembolic occlusion of the proximal or distal pulmonary vasculature.
Group 5 PH is pulmonary hypertension with unclear, multifactorial mechanisms.
Although the epidemiology of PH varies among the five groups, the majority of available evidence relates to group 1 PAH. The prevalence of group 1 PAH in the general population is estimated to be 5 to 15 cases per 1 million adults (Humbert 2006; Ling 2012). The prevalence of PH in groups 2 to 5 is unknown due to the broad classification and multiple etiologies. One cohort study reported that the proportion of each group among people with pulmonary hypertension is 0.18, 0.35, 0.17, 0.09, and 0.21 (Meredith 2014).
The prognosis of PH depends on various factors. The Registry to Evaluate Early and Long‐term PAH Disease Management (REVEAL) risk score is used to predict disease progression (Benza 2010). Poor prognostic indicators include age at initial presentation > 50 years (Peacock 2007), male sex and ≥ 60 years (Marcus 2008), persistent (WHO) functional class III or IV (Appendix 1), pericardial effusion and elevated right atrial pressure (Paulus 2007; Torbicki 2007). The natural history and prognosis of group 1 PAH is better studied than that of groups 2 through 5. In general, in the absence of therapy, those with group 1 PAH have worse survival than groups 2 through 5. Symptomatic patients with IPAH who do not receive treatment have a median survival of approximately three years. Data from the REVEAL registry reported that, from the time of diagnostic right‐heart catheterization, people with PAH had one‐, three‐, five‐, and seven‐year survival rates of 85%, 68%, 57%, and 49%, respectively (Benza 2010).
Early detection and treatment of PH is suggested because advanced disease may be less responsive to therapy (Galie 2013). Primary therapy of PH is directed at the underlying cause of the PH. People with persistent PH with WHO functional class II, III, or IV despite treatment of the underlying cause of the PH should be referred to a specialized center to be evaluated for advanced therapy. Advanced therapy is directed at the PH itself, rather than the underlying cause of the PH. It includes treatment with prostacyclin pathway agonists, endothelin receptor antagonists, phosphodiesterase 5 inhibitors, and soluble guanylate cyclase stimulant.
Index test(s)
Estimated systolic pulmonary artery pressure (PAP) by Doppler trans‐thoracic echocardiography will be an Index test. Systolic PAP can be estimated from the maximum tricuspid regurgitation (TR) jet velocity by using the modified Bernoulli equation and adding right atrial pressure (RAP) (systolic PAP = 4 (v)2 + RAP, where v is the peak velocity of the TR jet) (Berger 1985). The majority of people have some degree of TR, and the utilization of TR to estimate systolic PAP is the most common practice in echocardiography (Kaplan 2010). There are several methods to estimate RAP (e.g. clinical estimation from jugular venous pressure, using a fixed value from 5 mmHg to 10 mmHg, using the diameter and collapse of the inferior vena cava during spontaneous respiration); however, using the diameter and collapse of the inferior vena cava during spontaneous respiration is the most common method to estimate RAP (Rudski 2010). Several studies demonstrated adequate correlation between the estimated systolic PAP by Doppler trans‐thoracic echocardiography and the direct measurement of mean PAP with right‐heart catheterization (Zhang 2010), and if the estimated systolic PAP is higher than 35 to 40 mmHg, further evaluation is recommended to determine if PH is present (Rudski 2010). Although several studies demonstrated adequate correlation between the estimated systolic PAP and by echocardiography and the direct measurement of right‐heart catheterization, several studies reported that overinflated lung lowered the diagnostic accuracy of echocardiography (Fisher 2007).
Clinical pathway
Symptoms and signs of PH are non‐specific, which frequently results in a delay in diagnosis (Brown 2011). The initial symptoms of PH are exertional dyspnoea, fatigue, chest pain, syncope, palpitations, and peripheral edema. Many people with PH visit hospital complaining of these non‐specific symptoms.
Physicians suspect the presence of PH by these nonspecific symptoms or initial evaluation by chest radiograph or electrocardiogram. The classic chest radiograph of a person with PH shows enlargement of the central pulmonary arteries, right ventricular enlargement, and right atrial dilatation. Electrocardiogram of a person with PH may show signs of right ventricular disease, which include right axis deviation, an R wave/S wave ratio greater than 1 in lead V1, incomplete or complete right bundle branch block, or increased P wave amplitude in lead II.
When PH is suspected by these signs or initial screening tests, diagnostic evaluations are performed to confirm that PH exists and to determine its severity and identify its cause (Figure 1) (Rubin 2016).
Figure 1.
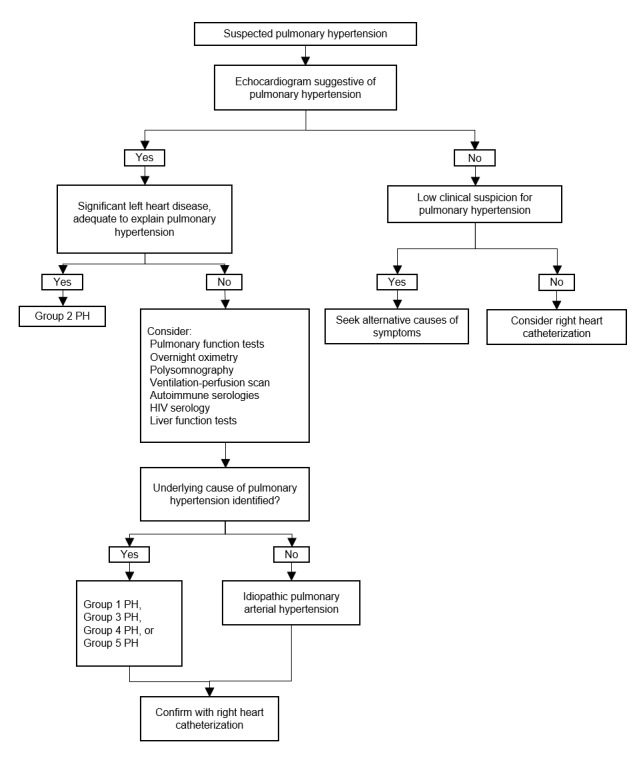
Clinical pathway of diagnostic evaluation for pulmonary hypertension among adults, adolescents, and children.
PH: pulmonary hypertension; PAH: pulmonary arterial hypertension (Rubin 2016)
The first step of diagnostic testing is evaluation with echocardiogram. Pulmonary artery pressure can be estimated by this non‐invasive method. When echocardiogram does not suggest PH, further evaluation depends on clinical suspicion. If clinical suspicion for PH is still high, right‐heart catheterization should be considered. When echocardiogram is suggestive of PH, clinicians should evaluate if left heart disease exists to adequately explain the degree of estimated PH. Patients with enough left heart disease on the echocardiogram to explain the degree of estimated PH do not require further evaluation to determine the etiology of PH.
Patients who have no left heart disease, which seems insufficient to explain the degree of estimated PH, should undergo additional diagnostic testing to determine the etiology of PH and appropriate treatment, which may include pulmonary function test, ventilation‐perfusion scanning, overnight oximetry, polysomnography, or laboratory testing (e.g. autoimmune serologies, HIV serology, and liver function tests) according to the history and physical examination.
Right‐heart catheterization is indicated to confirm the PH and its severity. Direct pressure measurement of pulmonary artery pressure with right‐heart catheterization is the clinical reference standard to confirm PH (Lewis 2016).
Alternative test(s)
Some additional tests (e.g. chest radiograph, magnetic resonance, computed tomography) are considered to confirm the etiology of PH, however Doppler echocardiography plays a central role in the diagnosis and management of PH. Doppler echocardiography or right‐heart catheterization is essential to confirm the elevation of PAP (Freed 2016).
Rationale
Many patients are diagnosed at a late stage of the disease because at the beginning of the disease symptoms and signs of PH are non‐specific (Brown 2011). Early and accurate detection of PH would therefore be of great benefit. While direct pressure measurement with right‐heart catheterization is the clinical reference standard for PH, it is not routinely used due to its invasiveness and complications (Connors 1996).
Trans‐thoracic Doppler echocardiography is less invasive, less expensive, and widely available compared with right‐heart catheterization; it is therefore recommended that echocardiography should be used as an initial screening method and as a method of monitoring disease progression (Rudski 2010).
However, several studies have questioned the accuracy of non‐invasively measured PAP (Arcasoy 2003; Fisher 2009; Rich 2011). To avoid late detection, accurate triage of PH is necessary. We will therefore evaluate the diagnostic accuracy of echocardiography to triage patients suspected PH.
We hypothesize that Doppler echocardiography could be a beneficial test to triage PH since ultrasound devices have become common in a variety of settings.
Objectives
To determine the diagnostic accuracy of trans‐thoracic Doppler echocardiography for detecting pulmonary hypertension.
Secondary objectives
We will aim to study several possible source of heterogeneity as below.
Types of PH defined by WHO classification (group 3 or not). We expect that it will be more difficult to measure tricuspid regurgitation in patients with lung disease, especially COPD, than in patients without lung disease, which could decrease the sensitivity and specificity of echocardiography to detect PH.
Mechanical ventilation (including non‐invasive positive pressure ventilation) or not.
Estimation of right atrial pressure (whether estimated by using the diameter and collapse of the inferior vena cava during spontaneous respiration or by any other methods).
Methods
Criteria for considering studies for this review
Types of studies
We will include reports on the diagnostic accuracy of trans‐thoracic Doppler echocardiography for detecting pulmonary hypertension. We will consider diagnostic test accuracy studies (consecutive series or random sample) of Doppler echocardiography against right‐heart catheterization (Bossuyt 2008). We will exclude diagnostic case‐control studies (two‐gate design). Case‐control designs are known to overestimate the sensitivity and specificity that a diagnostic test has in clinical practice (Rutjes 2005). We will exclude studies if right‐heart catheterization was not the reference standard, and the reference standard threshold is different to 25 mmHg. We will exclude case studies that did not provide sufficient diagnostic test accuracy data (true positive (TP), false positive (FP), true negative (TN), and false negative (FN) values, based on the reference standard). We will include studies that provided data from which we could extract TP, FP, TN, and FN values, based on the reference standard. We will contact study authors for missing data.
Participants
We will include all adults (16 years of age or older) with suspected PH. We will exclude any participant already diagnosed as having PH.
We will not exclude participants based on sex or cause of PH.
Index tests
Measurement by Doppler trans‐thoracic echocardiography will be an index test. Systolic PAP will be calculated from the maximum tricuspid regurgitation jet velocity by using the modified Bernoulli equation and adding RAP (Berger 1985). We will include all methods of measuring RAP (e.g. clinical estimation from jugular venous pressure, using a fixed value from 5 mmHg to 10 mmHg, using the diameter and collapse of the inferior vena cava during spontaneous respiration).
Target conditions
The target condition will be pulmonary hypertension (PH) regardless of WHO classification group 1 to 5 (Simonneau 2013).
Reference standards
We will define pulmonary hypertension as when mean of PAP assessed by right‐heart catheterization is ≥ 25 mmHg (Hoeper 2013).
Search methods for identification of studies
Electronic searches
We will search the following databases:
MEDLINE Ovid SP (1946 to date);
Embase Ovid SP (1974 to date);
Web of Science Core Collection (1970 to date).
We will search the following trials registries:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch/).
A proposed MEDLINE search strategy is detailed in Appendix 2, which we will adapt for use in the other databases. We have combined search terms describing the target condition and the index text. We have not used search terms to describe diagnostic study designs as this is not currently recommended in the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (De Vet 2008). We will not apply any restrictions on language or type of publication.
Searching other resources
To identify additional published, unpublished, and ongoing studies, we will enter relevant studies identified from the above sources into Web of Science and use the ‘Related Articles’ feature. We will check the reference lists of all primary studies and relevant systematic reviews and will contact authors of all included studies to identify other published, unpublished, or ongoing searches. We will search for conference proceedings through Embase and the Web of Science.
Data collection and analysis
Selection of studies
We will undertake the systematic review using the methods outlined in the Cochrane Handbook for Reviews of Diagnostic Test Accuracy (Deeks 2013). Two review authors (JK and SS) will independently review titles and abstracts identified by the search strategy. JK and SS will retrieve the full text of potentially relevant studies and will independently assess the full text against the eligibility criteria outlined in Criteria for considering studies for this review. Differences will be resolved by discussion between the review authors (JK and SS), with a further review author (YK) acting as arbiter. We will provide details of both included and excluded studies in the respective tables of the review.
Data extraction and management
Two review authors (JK and SS) will independently extract data on study characteristics, participant demographics, sample size, test methods, methodological quality, sensitivity, and specificity (Appendix 3; Appendix 4; Appendix 5). Both review authors will then extract data to construct a 2x2 contingency table. Disagreements will be resolved by consensus and with the further review author (YK) acting as arbiter.
Assessment of methodological quality
We will use the QUADAS‐2 tool to assess the quality of studies (Whiting 2011). We will record the assessment on a study quality assessment form (Appendix 6). The qualities to be assessed are described in detail in (Appendix 7). For each item in the quality assessment form, we will include a description of how the study addressed the issue and enter a judgement of 'low,' 'high,' or 'unclear' for overall risk of bias for each of the four domains. In addition, we will add a judgement of 'low,' 'high,' or 'unclear' for the overall concern of applicability to the review question for domains 1, 2, and 3. We will present an 'Assessment of methodological quality' table that will show all judgements made for all included studies. Two review authors (JK and SS) will independently assess methodological quality. Disagreements will be resolved by discussion between the review authors, with a further review author acting as an arbiter (YK).
Statistical analysis and data synthesis
We will perform data synthesis using the methods recommended by the working group of the Cochrane Collaboration on systematic reviews of diagnostic test accuracy (Deeks 2013). We will extract accuracy data for all thresholds used in the primary studies. We will represent individual studies' sensitivities and specificities in forest plots, sorted by sensitivity, in order to inspect between‐study variability. These individual studies' accuracy estimates will be also represented in a Receiver Operating Characteristic (ROC) plot of sensitivity versus 1‐specificity to visually assess the correlation between both indices. Given the lack of validated cut‐offs of the index tests (Galie 2009), we expect variability in cut‐off points chosen in the included studies. Therefore, if the included primary studies report accuracy data using different cut‐off values, we will meta‐analyze pairs of sensitivity and specificity using the hierarchical summary ROC (HSROC) model (Rutter 2001), which allows for the possibility of variation in threshold between studies, while also accounting for variation within and between studies and any potential correlation between sensitivity and specificity. If studies report data at multiple thresholds, we will use the threshold as primary analysis that was prespecified as primary endpoint in each study or was the nearest to 40 mmHg if not prespecified. If a sufficient number of primary studies report data using common cut‐off values, we will perform meta‐analyses using the bivariate model (Reitsma 2005). This model accounts for intrastudy accuracy variability and interstudy variations in test performance with the inclusion of random effects. We will analyze all studies sharing the same threshold at the same time and will obtain summary accuracy estimates. We will present these estimates with 95% confidence ellipses in the ROC space. We will use pooled estimates of sensitivity and specificity to calculate the positive and negative likelihood ratios and diagnostic odds ratio (Glas 2003). We will estimate explanatory variables for the bivariate model or HSROC model in order to analyze how these variables individually affect sensitivity and specificity. We will undertake all analyses using Review Manager 5 (Review Manager 2014), STATA software, version 13.0 (Stata 2013), or SAS software (SAS 2011).
Investigations of heterogeneity
To test whether either sensitivity or specificity, or both, differ in subgroups of studies we will use a bivariate model or HSROC model in which we will add explanatory variables representing the following subgroups.
Types of PH defined by WHO classification (group 3 or not). We expect that to measure tricuspid regurgitation will be more difficult in patients with lung disease, especially COPD, than in patients without lung disease, which could decrease the sensitivity and specificity of echocardiography to detect PH.
Mechanical ventilation (including non‐invasive positive pressure ventilation) or not.
Estimation of right atrial pressure (whether estimated by using the diameter and collapse of the inferior vena cava during spontaneous respiration or by any other methods).
Sensitivity analyses
We will examine the robustness of the meta‐analysis by conducting sensitivity analysis. We will check the impact of excluding from the meta‐analysis studies according to domains of the QUADAS‐2 assessment. We anticipate that studies at high risk of bias in domains 1 and 4 would have a great impact on meta‐analysis because high risk of bias in these domain would cause selection bias. Due to the invasiveness of the reference standard, less severe cases would not be verified by right‐heart catheterization, which would lead to partial verification bias. We will therefore perform additional sensitivity analysis by using prevalence as surrogate of partial verification, high prevalence or low prevalence, for which the median prevalence among included studies would be the cut‐off point.
Although uninterpretable results would be likely to occur among patients with overinflated lung, which is associated with the severity of pulmonary hypertension, it is not always considered test positive. We will therefore perform sensitivity analysis by including uninterpretable results as both test negative and positive.
Assessment of reporting bias
We do not plan to explore reporting bias due to a lack of suitable statistical methods (Deeks 2013).
Acknowledgements
We wish to thank Elizabeth Stovold, Information Specialist of the Cochrane Airway Group, for designing our search strategy. We based the methods section of this protocol on the Cochrane protocol Tsujimoto 2016 as a template.
The Cochrane Diagnostic Test Accuracy Editorial Team helped to edit this protocol and commented critically on the protocol.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. World Health Organization (WHO) functional classification for pulmonary hypertension
| Class | WHO functional classification for pulmonary hypertension |
| I | Patients with pulmonary hypertension but without resulting limitations of physical activity. Ordinary physical activity does not cause undue fatigue or dyspnoea, chest pain, or heart syncope. |
| II | Patients with pulmonary hypertension resulting in slight limitation of physical activity. They are comfortable at rest. Ordinary physical activity results in undue fatigue or dyspnoea, chest pain, or heart syncope. |
| III | Patients with pulmonary hypertension resulting in marked limitation of physical activity. They are comfortable at rest. Less‐than‐ordinary physical activity causes undue fatigue or dyspnoea, chest pain, or heart syncope. |
| IV | Patients with pulmonary hypertension resulting in inability to carry on any physical activity without symptoms. These patients manifest signs of right heart failure. Dyspnea or fatigue, or both may be present even at rest. Discomfort is increased by physical activity. |
Appendix 2. MEDLINE (Ovid) search strategy
1. exp Hypertension, Pulmonary/ 2. Pulmonary Heart Disease/ 3. (pulmonary adj3 hypertens$).tw. 4. pulmonary vascular disease.tw. 5. or/1‐4 6. exp Echocardiography/ 7. (echocardiogra$ or echo‐cardiogra$).tw. 8. doppler.tw. 9. or/6‐8 10. 5 and 9 11. Animals/ not (Animals/ and Humans/) 12. 10 not 11
Appendix 3. Data extraction sheet
| Study ID | ______________________________________ |
| Report ID | ______________________________________ |
| First author | ______________________________________ |
Study eligibility
|
Type of study Is the study a diagnostic test accuracy study? |
Yes | Unclear | No |
| Next question | Next question | Exclude | |
| Is the study a diagnostic case‐control study (two‐gate design)? | Yes | Unclear | No |
| Exclude | Next question | Next question | |
|
Participants Were the participants suspected as having pulmonary hypertension? |
Yes | Unclear | No |
| Next question | Next question | Exclude | |
| Were the participants already diagnosed with pulmonary hypertension? | Yes | Unclear | No |
| Exclude | Next question | Next question | |
|
Index tests Did the study evaluate pulmonary arterial pressure by Doppler echocardiography against the reference standard? |
Yes | Unclear | No |
| Next question | Next question | Exclude | |
|
Target conditions Was the aim of the diagnostic test to confirm pulmonary hypertension? |
Yes | Unclear | No |
| Next question | Next question | Exclude | |
|
Reference standard Was right‐heart catheterization performed as a reference standard of pulmonary hypertension? |
Yes | Unclear | No |
| Next question | Next question | Exclude | |
| Was the reference standard threshold different to 25 mmHg? | Yes | Unclear | No |
| Exclude | Next question | Next question | |
| Were the index test and reference standard performed within one week? | Yes | Unclear | No |
| Final decision | Include | Unclear | Exclude |
If the study is to be excluded, record the reason and details to add to 'Table of excluded studies.'
Appendix 4. Table of excluded studies
| Study ID | _________________________________________________________________ |
| Report ID | _________________________________________________________________ |
| Review author name | _________________________________________________________________ |
| Reason for exclusion (e.g. right‐heart catheterization was not reference standard) | _________________________________________________________________ |
Appendix 5. General information
| Study ID | __________________________________________________________________________ |
| Report ID | __________________________________________________________________________ |
| Review author name | __________________________________________________________________________ |
| Authors | __________________________________________________________________________ |
| Contact address | __________________________________________________________________________ |
| Country of the study | __________________________________________________________________________ |
| Language of publication | __________________________________________________________________________ |
| Participants | |
| Age (mean, median, range) | ______________________________________________________ |
| Sex (male numbers/%) | ______________________________________________________ |
| BMI | ______________________________________________________ |
| Study characteristics | |
| Design (prospective or retrospective) | ______________________________________________________ |
| Single or multicenter? | ______________________________________________________ |
| Inclusion criteria of participants | ______________________________________________________ |
| Classification of PH defined by WHO | ______________________________________________________ |
| Total number of participants | ______________________________________________________ |
| Total number of intubated patients | ______________________________________________________ |
| Index test (method to estimate right atrial pressure) | ______________________________________________________ |
| Index test (cut‐off to declare PH) | ______________________________________________________ |
| Index test (estimated mean systolic pulmonary arterial pressure) | ______________________________________________________ |
| Reference standard (threshold to define PH) | ______________________________________________________ |
| Reference standard (mean systolic pulmonary arterial pressure) | ______________________________________________________ |
| Time gap between index and reference standard | ______________________________________________________ |
| Study outcome (number of people) | |
| PH exists and correct detection by echocardiography (i.e. true positive of the test) | ______________________________________________________ |
| PH does not exist but interpreted as pulmonary hypertension (i.e. false positive of the test) | ______________________________________________________ |
| PH exists but failed to detect by echocardiography (i.e. false negative of the test) | ______________________________________________________ |
| PH does not exist and correctly detected by echocardiography (i.e. true negative of the test) | ______________________________________________________ |
| Total number of participants whose results of index test were uninterpretable | |
| Total number of participants whose results of reference standard were uninterpretable | |
| How uninterpretable results were handled within the analyses | |
| Additional information | |
| Conflict of interest | ______________________________________________________ |
BMI: body mass index: PH: pulmonary hypertension; WHO: World Health Organization
Appendix 6. Study quality assessment form (QUADAS‐2)
| Domain 1: Patient selection | |
| A. Risk of bias | |
| Describe methods of patient selection: | |
| ···· Was a consecutive or random sample of patients enrolled? | Yes/No/Unclear |
| ···· Did the study avoid inappropriate exclusions? | Yes/No/Unclear |
| Could the selection of patients have introduced bias? | RISK: LOW/HIGH/UNCLEAR |
| B. Concerns regarding applicability | |
| Describe included patients (prior testing, presentation, intended use of index test and setting): | |
| Is there concern that the included patients do not match the review question? | CONCERN: LOW/HIGH/UNCLEAR |
| Domain 2: Index test (echocardiography) | |
| A. Risk of bias | |
| Describe the index test and how it was conducted and interpreted: | |
| ···· Were the index test results interpreted without knowledge of the results of the reference standard? | Yes/No/Unclear |
| ···· If a threshold was used, was it prespecified? | Yes/No/Unclear |
| Could the conduct or interpretation of the index test have introduced bias? | RISK: LOW/HIGH/UNCLEAR |
| B. Concerns regarding applicability | |
| Is there concern that the index test, its conduct, or interpretation differ from the review question? | CONCERN: LOW/HIGH/UNCLEAR |
| Domain 3: Reference standard (right‐heart catheterization) | |
| A. Risk of bias | |
| Describe the reference standard and how it was conducted and interpreted: | |
| ···· Were the reference standard results interpreted without knowledge of the results of the index test? | Yes/No/Unclear |
| ···· Were the criteria of reference standard for target condition prespecified? | Yes/No/Unclear |
| Could the reference standard, its conduct, or its interpretation have introduced bias? | RISK: LOW/HIGH/UNCLEAR |
| B. Concerns regarding applicability | |
| Is there concern that the target condition as defined by the reference standard does not match the review question? | CONCERN: LOW/HIGH/UNCLEAR |
| Domain 4: Flow and timing | |
| A. Risk of bias | |
|
Describe any patients who did not receive the index test(s) and/or reference standard or who were excluded from the 2x2 table (refer to flow diagram): Describe the time interval and any interventions between index test(s) and reference standard: | |
| ···· Was the interval between the index test and reference standard less than one day? | Yes/No/Unclear |
| ···· Did all patients receive the same reference standard? | Yes/No/Unclear |
| ···· Were all patients included in the analysis? | Yes/No/Unclear |
| Could the patient flow have introduced bias? | RISK: LOW/HIGH/UNCLEAR |
Appendix 7. Study quality assessment details
Domain 1: Patient selection
Risk of bias: Could the selection of patients have introduced bias?
Signaling question 1: Was a consecutive or random sample of patients enrolled? Signaling question 2: Did the study avoid inappropriate exclusions?
Studies that make inappropriate exclusions, for example excluding 'difficult to diagnose' patients, whose image of echocardiography is difficult to detect due to obesity or overinflated lungs, may result in overoptimistic estimates of diagnostic accuracy. We will classify the studies as 'yes' if all patients were included in the study regardless of clinical suspicion following index test, 'no' if they conduct any inappropriate exclusions, for example excluding patients with low clinical suspicion based on index test, and 'unclear' if this information is not clear.
Applicability: Are there concerns that the included patients and the setting do not match the review question?
If studies include only a clinically relevant population that would have undergone index test in real practice and includes representative form of target condition, we will assess as 'low' concern. If the study population differed from the population defined in the review question in terms of demographic features (e.g. studies include mainly younger patients or specific group of WHO classification), we will assess as 'high' concern. If this information is unclear (e.g. WHO classification was not reported), we will assess as 'unclear'.
Domain 2: Index test
Risk of bias: Could the conduct or interpretation of the index test have introduced bias?
Signaling question 1: Were the index test results interpreted without knowledge of the results of the reference standard? Signaling question 2: If a threshold was used, was it prespecified?
We will classify the study as 'yes' if echocardiography results were interpreted without knowledge of the reference standard, 'no' if the echocardiography tests were interpreted with knowledge of the reference standard results, and 'unclear' if this information is not clear. We will classify the study as 'yes' if a threshold was prespecified, 'no' if authors selected a cut‐off value based on the analysis of data collected, and 'unclear' if insufficient information is provided.
Applicability: Are there concerns that the index test, its conduct, or its interpretation differ from the review question?
We will include studies where systolic pulmonary artery pressure calculated from the maximum tricuspid regurgitation jet velocity by using the modified Bernoulli equation and adding right atrial pressure (RAP) evaluation of pulmonary artery pressure by echocardiography is an index test regardless of threshold, and we will include index tests using various RAP estimation methods. However, the most common method is using the diameter and collapse of the inferior vena cava during spontaneous respiration. We will therefore judge studies where RAP was estimated by using the diameter and collapse of the inferior vena cava during spontaneous respiration as 'low' concern, those where RAP was estimated by using any other method as 'high' concern, and 'unclear' concern if this information is not clear.
Domain 3: Reference standard
Risk of bias: Could the reference standard, its conduct, or its interpretation have introduced bias?
Signaling question 1: Were the reference standard results interpreted without knowledge of the results of the index test? Signaling question 2: Were the criteria of reference standard for target condition prespecified?
We will classify the study as 'yes' if right‐heart catheterization results were interpreted without knowledge of the index test, 'no' if right‐heart catheterization was interpreted with knowledge of the index test results, and 'unclear' if this information is not clear. We will classify the study as 'yes' if the criteria of reference standard for target condition were prespecified, 'no' if the criteria of reference standard for target condition were not prespecified, and 'unclear' if this information is not clear.
Applicability: Are there concerns that the target condition as defined by the reference standard does not match the question?
The target condition is pulmonary hypertension defined as mean pulmonary artery pressure assessed by right‐heart catheterization of 25 mmHg or higher (Hoeper 2013). Use of right‐heart catheterization for reference standard and the threshold of 25 mmHg are inclusion criteria for this review, so we anticipate that all studies will be classified as 'low' concern.
Domain 4: Flow and timing
Risk of bias: Could the patient flow have introduced bias?
Signaling question 1: Was the interval between the index test and reference standard less than one day? Signaling question 2: Did all patients receive the same reference standard? Signaling question 3: Were all patients included in the analysis?
Both echocardiography and right‐heart catheterization are subject to disagreement when performed at different times (e.g. days or weeks apart), which is a major limitation of the studies that compare their accuracy in the diagnosis of pulmonary hypertension (Lewis 2016). We will classify the study as 'yes' if there is a delay of less than a day, 'no' if there is a delay of more than or equal to a day, and 'unclear' if the information is not clear. We will classify the study as 'yes' if all patients had the same reference standard, 'no' if some included patients did not undergo the same reference standard, and 'unclear' if this information is not clear. Uninterpretable results may be present (e.g. unclear echocardiography or failure to insert right‐heart catheterization). Additionally, withdrawals from the study may be present. We anticipate that uninterpretable results would occur associated with the severity of pulmonary hypertension (e.g. difficulty to detect Doppler echocardiography among severe chronic obstructive pulmonary disease patients due to overinflated lung) and not at random. If uninterpretable results are withdrawn from analysis, diagnostic accuracy will be overestimated. We will classify the study as 'yes' if uninterpretable results were reported and the study had no withdrawals or the withdrawals were unlikely to affect the results; 'no' if uninterpretable results were not reported or there were withdrawals that were likely to affect the results, or both; and 'unclear' if this information is not clear.
Contributions of authors
JK drafted the protocol with contributions from HT and YK. JK and YT devised the study selection criteria. JK, SS, YN, YK, HT, YT, and Elizabeth Stovold undertook the search strategy. JK, SS, YN, and HT developed the study design and research question. JK, HT, YK, and YT developed the statistical analysis/synthesis of data plan. All authors contributed to revising the protocol, reviewed all drafts, and agreed on the final version.
Sources of support
Internal sources
Sakai City Medical Center, Japan.
Kyoto University, Japan.
Hyogo Prefectural Amagasaki General Medical Center, Japan.
External sources
The authors declare that no such funding was received for this systematic review, Japan.
Declarations of interest
JK: none known.
SS: none known.
YN: none known.
YK: none known.
HT: none known.
YT: none known.
New
References
Additional references
- Arcasoy SM, Christie JD, Ferrari VA, Sutton MS, Zisman DA, Blumenthal NP, et al. Echocardiographic assessment of pulmonary hypertension in patients with advanced lung disease. American Journal of Resipratory and Critical Care Medicine 2003;167(5):735‐40. [DOI: 10.1164/rccm.200210-1130OC] [DOI] [PubMed] [Google Scholar]
- Benza RL, Miller DP, Gomberg‐Maitland M, Frantz RP, Foreman AJ, Coffey CS, et al. Predicting survival in pulmonary arterial hypertension: insights from the registry to evaluate early and long‐term pulmonary arterial hypertension disease management (REVEAL). Circulation 2010;122(2):164‐72. [DOI] [PubMed] [Google Scholar]
- Berger M, Haimowitz A, Van A, Berdoff RL, Goldberg E. Quantitative assessment of pulmonary hypertension in patients with tricuspid regurgitation using continuous wave Doppler ultrasound. Journal of the American College of Cardiology 1985;6(2):359‐65. [DOI: 10.1016/S0735-1097(85)80172-8] [DOI] [PubMed] [Google Scholar]
- Bossuyt PM, Leeflang MM, editor(s). Chapter 6: Developing criteria for including studies. In: Deeks JJ, Wisniewski S, Davenport C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
- Brown LM, Chen H, Halpern S, Taichman D, McGoon MD, Farber HW, et al. Delay in recognition of pulmonary arterial hypertension: factors identified from the REVEAL Registry. Chest 2011;140(1):19‐26. [DOI: 10.1378/chest.10-1166] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connors AF, Speroff T, Dawson NV, Thomas C, Harrell FE, Wagner D, et al. The effectiveness of right heart catheterization in the initial care of critically ill patients. SUPPORT Investigators. JAMA 1996;276(11):889‐97. [DOI: 10.1001/jama.1996.03540110043030] [DOI] [PubMed] [Google Scholar]
- Vet HCW, Eisinga A, Riphagen II, Aertgeerts B, Pewsner D, editor(s). Chapter 7: Searching for studies. In: Deeks JJ, Bossuyt PM, Gatsonis C, editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
- Deeks JJ, Bossuyt PM, Gatsonis C (editors). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Version 1.0.0. The Cochrane Collaboration, 2013. Available from srdta.cochrane.org.
- Fisher MR, Criner GJ, Fishman AP, Hassoun PM, Minai OA, Scharfe SM. Estimating pulmonary artery pressures by echocardiography in patients with emphysema. European Respiratory Journal 2007;30(5):914‐21. [DOI: 10.1183/09031936.00033007] [DOI] [PubMed] [Google Scholar]
- Fisher MR, Forfia PR, Chamera E, Housten‐Harris T, Champion HC, Girqis RE, et al. Accuracy of Doppler echocardiography in the hemodynamic assessment of pulmonary hypertension. American Journal of Respiratory and Critical Care Medicine 2009;179(7):615‐21. [DOI: 10.1164/rccm.200811-1691OC] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed BH, Collins JD, Francois CJ, Barker AJ, Cuttica MJ, Chesler NC, et al. MR and CT imaging for the evaluation of pulmonary hypertension. Journal of the American College of Cardiology: Cardiovascular Imaging 2016;9(6):715‐32. [DOI: 10.1016/j.jcmg.2015.12.015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galie N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. European Respiratory Journal 2009;34:1219‐63. [DOI: 10.1183/09031936.00139009] [DOI] [PubMed] [Google Scholar]
- Galiè N, Corris PA, Frost A, Girgis RE, Granton J, Jing ZC, et al. Updated treatment algorithm of pulmonary arterial hypertension. Journal of the American College of Cardiology 2013;62(25 Suppl):D60‐72. [DOI: 10.1016/j.jacc.2013.10.031] [DOI] [PubMed] [Google Scholar]
- Glas AS, Lijmer JG, Prins MH, Bonsel GJ, Bossuyt PM. The diagnostic odds ratio: a single indicator of test performance. Journal of Clinical Epidemiology 2003;56(11):1129‐35. [PUBMED: 14615004] [DOI] [PubMed] [Google Scholar]
- Hoeper MM, Bogaard HJ, Condliffe R, Frantz R, Khanna D, Kurzyna M, et al. Definitions and diagnosis of pulmonary hypertension. Journal of the American College of Cardiology 2013;62(25 Suppl):D42‐50. [DOI: 10.1016/j.jacc.2013.10.032] [DOI] [PubMed] [Google Scholar]
- Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. American Journal of Respiratory Care Medicine 2006;173(9):1023. [DOI] [PubMed] [Google Scholar]
- Kaplan A, Lean A, Vieillard‐Baron A. Hemodynamic Monitoring Using Echocardiography in the Critically Ill. Springer Berlin Heidelberg, 2010. [DOI: 10.1007/978-3-540-87956-5_13] [DOI] [Google Scholar]
- Lewis R, William H. Clinical features and diagnosis of pulmonary hypertension in adults. www.uptodate.com/contents/clinical‐features‐and‐diagnosis‐of‐pulmonary‐hypertension‐in‐adults (accessed 6 December 2016).
- Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine 2012;186(8):790‐6. [DOI] [PubMed] [Google Scholar]
- Marcus JT, Gan CT, Zwanenburg JJ, Boonstra A, Allaart CP, Gotte MJW. Interventricular mechanical asynchrony in pulmonary arterial hypertension: left‐to‐right delay in peak shortening is related to right ventricular overload and left ventricular underfilling. Journal of the American College of Cardiology 2008;51:750‐7. [DOI] [PubMed] [Google Scholar]
- Meredith EP, Lakshmi S, Li W, Ivan MR, John HN, Anna RH. Causes of pulmonary hypertension in the elderly. CHEST 2014;146(1):159‐66. [DOI: 10.1378/chest.13-1900] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the heart failure and echocardiography. European Heart Journal 2007;28:2539‐50. [DOI] [PubMed] [Google Scholar]
- Peacock AJ, Murphy NF, McMurray JJV, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. European Respiratory Journal 2007;30:104‐9. [DOI] [PubMed] [Google Scholar]
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [DOI] [PubMed] [Google Scholar]
- Nordic Cochrane Center, The Cochrane Collaboration. Review Manager. Version 5.3. Copenhagen: Nordic Cochrane Center, The Cochrane Collaboration, 2014.
- Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension: implications for clinical practice. CHEST 2011;139(5):988‐93. [DOI: 10.1378/chest.10-1269] [DOI] [PubMed] [Google Scholar]
- Rubin LJ, Hopkins W. Clinical features and diagnosis of pulmonary hypertension in adults. www.uptodate.com/contents/clinical‐features‐and‐diagnosis‐of‐pulmonary‐hypertension‐in‐adults (accessed 24 Jan 2017).
- Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. Journal of the American Society of Echocardiography 2010;23(7):685‐713. [DOI: 10.1016/j.echo.2010.05.010] [DOI] [PubMed] [Google Scholar]
- Rutjes AWS, Reitsma JB, Vandenbrouke JP, Glas AS, Bossuyt PMM. Case‐control and two‐gate designs in diagnostic accuracy studies. Clinical Chemistry 2005;51(8):1335‐41. [DOI: 10.1373/clinchem.2005.048595] [DOI] [PubMed] [Google Scholar]
- Rutter CM, Gatsonis CA. A hierarchical regression approach to meta‐analysis of diagnostic test accuracy evaluations. Statistics in Medicine 2001;20(19):2865‐84. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS. Version 9.3. Cary (NC): SAS Institute, 2011.
- Schermuly RT, Janssen W, Weissmann N, Stasch JP, Grimminger F, Ghofrani HA. Riociguat for the treatment of pulmonary hypertension. Expert Opinion on Investigational Drugs 2011;20(4):567‐76. [DOI] [PubMed] [Google Scholar]
- Simonneau G, Gatzoulis MA, Adatia I, Celermajer D, Denton C, Ghofrani A, et al. Updated clinical classification of pulmonary hypertension. Journal of the American College of Cardiology 2013;62(25 Suppl):D34‐41. [DOI: 10.1016/j.jacc.2013.10.029] [DOI] [PubMed] [Google Scholar]
- Stata Corporation. Stata. Version 13.0. College Station (TX): Stata Corporation, 2013.
- Torbicki A. Cardiac magnetic resonance in pulmonary arterial hypertension: a step in the right direction. European Heart Journal 2007;28:1187‐9. [DOI] [PubMed] [Google Scholar]
- Tsujimoto H, Tsujimoto Y, Nakata Y, Akazawa M, Kataoka Y. Ultrasonography for confirmation of gastric tube placement. Cochrane Database of Systematic Reviews 2016, Issue 2. [DOI: 10.1002/14651858.CD012083] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [PUBMED: 22007046] [DOI] [PubMed] [Google Scholar]
- Zhang RF, Zhou L, Ma GF, Shao FC, Wu XH, Ying KJ. Diagnostic value of transthoracic Doppler echocardiography in pulmonary hypertension: a meta‐analysis. American Journal of Hypertension 2010;23:1261‐4. [DOI: 10.1038/ajh.2010.188] [DOI] [PubMed] [Google Scholar]


