Abstract
Background
Health care‐associated infection is a major cause of morbidity and mortality. Hand hygiene is regarded as an effective preventive measure. This is an update of a previously published review.
Objectives
To assess the short‐ and long‐term success of strategies to improve compliance to recommendations for hand hygiene, and to determine whether an increase in hand hygiene compliance can reduce rates of health care‐associated infection.
Search methods
We conducted electronic searches of the Cochrane Register of Controlled Trials, PubMed, Embase, and CINAHL. We conducted the searches from November 2009 to October 2016.
Selection criteria
We included randomised trials, non‐randomised trials, controlled before‐after studies, and interrupted time series analyses (ITS) that evaluated any intervention to improve compliance with hand hygiene using soap and water or alcohol‐based hand rub (ABHR), or both.
Data collection and analysis
Two review authors independently screened citations for inclusion, extracted data, and assessed risks of bias for each included study. Meta‐analysis was not possible, as there was substantial heterogeneity across studies. We assessed the certainty of evidence using the GRADE approach and present the results narratively in a 'Summary of findings' table.
Main results
This review includes 26 studies: 14 randomised trials, two non‐randomised trials and 10 ITS studies. Most studies were conducted in hospitals or long‐term care facilities in different countries, and collected data from a variety of healthcare workers. Fourteen studies assessed the success of different combinations of strategies recommended by the World Health Organization (WHO) to improve hand hygiene compliance. Strategies consisted of the following: increasing the availability of ABHR, different types of education for staff, reminders (written and verbal), different types of performance feedback, administrative support, and staff involvement. Six studies assessed different types of performance feedback, two studies evaluated education, three studies evaluated cues such as signs or scent, and one study assessed placement of ABHR. Observed hand hygiene compliance was measured in all but three studies which reported product usage. Eight studies also reported either infection or colonisation rates. All studies had two or more sources of high or unclear risks of bias, most often associated with blinding or independence of the intervention.
Multimodal interventions that include some but not all strategies recommended in the WHO guidelines may slightly improve hand hygiene compliance (five studies; 56 centres) and may slightly reduce infection rates (three studies; 34 centres), low certainty of evidence for both outcomes.
Multimodal interventions that include all strategies recommended in the WHO guidelines may slightly reduce colonisation rates (one study; 167 centres; low certainty of evidence). It is unclear whether the intervention improves hand hygiene compliance (five studies; 184 centres) or reduces infection (two studies; 16 centres) because the certainty of this evidence is very low.
Multimodal interventions that contain all strategies recommended in the WHO guidelines plus additional strategies may slightly improve hand hygiene compliance (six studies; 15 centres; low certainty of evidence). It is unclear whether this intervention reduces infection rates (one study; one centre; very low certainty of evidence).
Performance feedback may improve hand hygiene compliance (six studies; 21 centres; low certainty of evidence). This intervention probably slightly reduces infection (one study; one centre) and colonisation rates (one study; one centre) based on moderate certainty of evidence.
Education may improve hand hygiene compliance (two studies; two centres), low certainty of evidence.
Cues such as signs or scent may slightly improve hand hygiene compliance (three studies; three centres), low certainty of evidence.
Placement of ABHR close to point of use probably slightly improves hand hygiene compliance (one study; one centre), moderate certainty of evidence.
Authors' conclusions
With the identified variability in certainty of evidence, interventions, and methods, there remains an urgent need to undertake methodologically robust research to explore the effectiveness of multimodal versus simpler interventions to increase hand hygiene compliance, and to identify which components of multimodal interventions or combinations of strategies are most effective in a particular context.
Plain language summary
Methods to improve healthcare worker hand hygiene to decrease infection in patient care
What is the aim of this review?
To find out what strategies can improve healthcare workers' compliance with recommendations for hand hygiene, either handwashing with soap and water or using alcohol‐based hand rub (ABHR), or both. This is an update of a previously published review.
Key messages
A variety of single intervention strategies and combinations of strategies, many based on current recommendations from the World Health Organization (WHO), led to increased hand hygiene compliance in most studies, regardless of setting. However, the certainty of the evidence varied from very low to moderate, depending on the strategy. What remains unclear is which strategy or combination of strategies is most effective in a given context.
What did we study in the review?
Traditionally hand hygiene has been considered the single most important way of reducing health care‐associated infections, many of which are spread by direct contact, especially by the hands of healthcare workers. Much time and effort is spent worldwide promoting hand hygiene. Many different strategies have been tried to improve hand hygiene compliance but the most effective methods remain unclear.
What are the main results of the review?
We included 26 studies in the review. Fourteen studies assessed the success of different combinations of strategies recommended by WHO to improve hand hygiene compliance. Strategies consisted of the following: increasing the availability of alcohol‐based hand hygiene products, different types of education for staff, reminders (written and verbal), different types of performance feedback, administrative support and staff involvement. Six studies assessed different types of performance feedback, two studies evaluated education, three studies evaluated cues such as signs or scent, and one study assessed placement of ABHR.
Multimodal (combinations of) strategies that include some but not all strategies recommended by WHO may slightly improve hand hygiene compliance and slightly reduce infection rates (low certainty of evidence). Multimodal interventions that include all strategies recommended by WHO may lead to little or no difference in methicillin‐resistant Staphylococcus aureus (MRSA) infection rates (low certainty of evidence), but it is uncertain whether such WHO‐based approaches improve hand hygiene compliance or reduce colonisation rates because the certainty of this evidence is very low. Multimodal interventions that contain all recommended strategies plus additional strategies may slightly improve hand hygiene compliance (low certainty of evidence). It is unclear whether such WHO‐enhanced interventions reduce infection rates because the certainty of this evidence is very low.
Performance feedback may improve hand hygiene compliance (low certainty of evidence) and probably slightly reduces infection and colonisation rates (moderate certainty of evidence). Education may improve hand hygiene compliance (low certainty of evidence). Cues, such as signs or scent, may slightly improve hand hygiene compliance (low certainty of evidence). Placement of ABHR close to the point of use probably slightly improves hand hygiene compliance (moderate certainty of evidence).
How up‐to‐date is this review?
The review authors searched for studies that had been published up to October 2016.
Summary of findings
Background
Description of the condition
The most recent prevalence study in England established that 6.4% of hospital inpatients develop health care‐associated infections (HCAIs) (Health Protection Agency 2011). In European acute care hospitals between 2011 and 2012, overall prevalence of HCAIs was 5.7% (ECDC 2013a). In a 2010 survey of 183 hospitals in the USA, prevalence of HCAIs was 4.0% (Magill 2014), while the prevalence was 8.7% in 30 Canadian paediatric hospitals in 2009 (Rutledge‐Taylor 2012). HCAI rates vary considerably by type of infection (e.g. surgical site infection or pneumonia), by hospital or long‐term care facility, and by causative micro‐organism. In the European Union (EU) study 12.3% of the HCAIs were caused by methicillin‐resistant Staphylococcus aureus (MRSA), while in the USA study 12.1% were caused by Clostridium difficile, 10.7% by Staphylococcus aureus (sensitive and resistant strains), and 9.9% by Klebsiella species. In general, rates of MRSA and Clostridium difficile infections have fallen but infections caused by Gram negative bacteria are increasing, especially those caused by antimicrobial‐resistant strains (ESPAUR 2015). However, not all facilities have been able to reduce overall or micro‐organism‐specific HCAI rates (CDC 2016). In Canada, for example, rates of vancomycin‐resistant enterococci (VRE) infections rose from 0.31 per 10,000 patient days in 2009 to 0.45 in 2014 (CNISP 2015).
Infections caused by antimicrobial‐resistant micro‐organisms place patients at risk of infection that cannot be treated easily and costs the EU 1.5 billion euros annually (UK Five Year Antimicrobial Resistance Strategy). It has been estimated that in general hospital populations in the USA the cost per case of HCAI ranges from USD 2027 to USD 12,197 (Etchells 2012). In acute care hospitals in the USA it has been estimated that the overall burden of HCAIs, including lost income and other direct and indirect health costs, result in an overall financial burden of USD 96 to USD 147 billion annually (Marchetti 2013 ). Collectively these figures demonstrate that strategies to prevent HCAIs have been more successful in some countries and healthcare facilities than others and for some pathogens more than others, and that HCAIs remain a major threat to patient safety globally and a drain on healthcare resources.
Most HCAIs are spread by direct contact, especially by the hands of healthcare workers. Hand hygiene has traditionally been considered the most important means of preventing HCAIs because it disrupts the chain of infection (Pittet 2004; Teare 1999). Transmission of micro‐organisms from the hands of healthcare workers to a patient or to the environment can be prevented either by mechanical removal by washing with soap and water or an aqueous antiseptic (e.g. chlorhexidine gluconate) and drying, or by use of alcohol‐based hand rubs (ABHRs). ABHRs kill many of the organisms that cause HCAIs, and are less time‐consuming and more convenient to use than traditional washing. However, they are effective only when used on physically clean hands. Furthermore, because they have low viscosity and evaporate rapidly, care must be taken to ensure that there is adequate contact with all hand surfaces. The availability of soap and water or of ABHR is insufficient, however, to ensure that healthcare workers perform hand hygiene when it is indicated.
In 2009 the World Health Organization (WHO) published guidelines for implementing and evaluating hand hygiene programmes in healthcare settings (WHO Guidelines 2009). The guidelines incorporate ‘My Five Moments for Hand Hygiene’, which sets out a framework for understanding, training, monitoring and reporting hand hygiene compliance (Sax 2007). The WHO guidelines also identify five components to be specifically implemented: ABHR at point of care or carried by the healthcare worker, training and education, observation and performance feedback, reminders (e.g. posters), and administrative support/institutional safety climate. The WHO guidelines have been widely disseminated internationally and are reported to be highly influential (Mathai 2011). Healthcare workers in many countries now spend considerable time and effort promoting hand hygiene, auditing hand hygiene compliance, and assessing the effectiveness of hand hygiene and other measures to reduce HCAIs. Multiple interventions have been implemented to improve hand hygiene compliance but the most effective method remains unclear.
Description of the intervention
Pittet 2000 published the results of a Swiss initiative that used an uncontrolled before‐after design to demonstrate that a hospital‐wide multimodal campaign led to a sustained improvement in hand hygiene compliance for nursing but not for medical staff, as well as a reduction in overall HCAIs and transmission of MRSA. The campaign consisted of visual cues (posters, signs), education to optimise use of ABHR, ABHR placed at every bedside, performance feedback and managerial support. Follow‐up data published independently revealed continuing success (Hugonnet 2002). These studies have been widely taken to indicate that multimodal campaigns are the most effective way of promoting hand hygiene compliance and reducing HCAIs. All of the interventions used in the Swiss initiative were incorporated into the recommendations for multimodal campaigns published in the WHO Guidelines 2009, which have since been implemented in many countries (Mathai 2011). Many different interventions have been tried over the years, both as individual interventions and as multimodal campaigns. The latter are usually based on the WHO recommendations, but multiple variations have been adopted. In the earliest studies, interventions were targeted mostly at nursing and sometimes at medical staff, but in recent years most have been targeted at inter‐professional audiences.
Interventions to promote hand hygiene compliance fall mainly under the heading of Implementation Strategies in the Evaluation of Practice and Organisation of Care (EPOC) Taxonomy of Topics (EPOC 2015a). Such strategies are designed to bring about changes in healthcare workers’ behaviour. Education is an important component of hand hygiene interventions. Information, usually based on the WHO Guidelines 2009, is displayed on posters and flyers. E‐learning materials and simulation have been used in wards in a few studies, while other studies have used lectures or workshops. Teaching is usually delivered by in‐house infection prevention teams or external consultants by outreach to clinical areas. Some studies have included reminders about hand hygiene. A common strategy is to use audit with performance feedback delivered to wards, units, organisations and sometimes to individuals. In some studies individual verbal as well as written feedback is given and in most studies there is graphical display of hand hygiene audit findings in clinical areas which may include infection rates.
Changes to the healthcare environment have also been incorporated into hand hygiene campaigns. These involve the introduction of ABHR, a new formulation of an alcohol‐based product (e.g. replacement of a liquid hand rub with gel), changes related to gloves, and in a few studies, rearranging the work environment to improve access to hand hygiene products in addition to increasing their availability. In a few studies consensus processes have been used to adapt guidelines for a local healthcare system, and a small number have employed administrators, opinion leaders or local champions to improve the practice culture.
Only a few studies have deployed incentives. These can take the form of individual rewards, financial incentives to healthcare workers in countries where money to pay for insurance claims arising from cases of HCAI is derived from hospital fines, or rewarding successful wards or healthcare workers by publicising their achievements throughout the organisation.
How the intervention might work
Education and training to use the different types of hand hygiene products are intended to increase compliance by increasing healthcare workers’ knowledge of when hand hygiene should be performed, and in some cases encouraging optimal technique. Audit and performance feedback are intended to increase awareness of behaviours, and, like incentives, may serve as a motivator to continue to perform well or to improve performance, depending on the level of compliance. Reminders serve as cues to action. Changes in the availability of products or the environment or both can facilitate performance of the behaviour; it is difficult to perform hand hygiene, for example, if sinks or ABHR are not readily available. Involvement of staff and leadership support help to create unit‐specific strategies to address local contributing factors to reduced compliance, and may reinforce behaviour through role‐modelling or creating expectations about hand hygiene. Performance feedback, reminders, and leadership support may serve to reinforce the need for hand hygiene in a continual Hawthorne effect (Roethlisberger 1939).
Multimodal interventions incorporate different components, including some of those advocated by WHO and, in some cases, different ones. The ideal components of multimodal campaigns remain to be established, and it is still unclear whether multimodal interventions are superior to single interventions, although a number of the most recent randomised trials are now exploring the impact of single interventions. Because few studies to improve hand hygiene compliance have incorporated any theoretical underpinning, the best way of encouraging compliance is unknown. A recent systematic review (Srigley 2015) has concluded that behavioural theories may help guide interventions.
Why it is important to do this review
An early systematic review by Naikoba 2001 of 21 studies published before 2000 suggested that multimodal campaigns held more promise of effectiveness than single interventions, and that education with written information, reminders and continuous performance feedback were more useful than single interventions such as automated sinks or provision of moisturised soaps. However, it was difficult to draw firm conclusions in this review. Naikoba 2001 noted numerous limitations associated with the studies they reviewed, including small sample sizes, short duration of follow‐up, lack of or inappropriate control groups, lack of generalisability from critical care units where most studies had been conducted to other clinical settings, and emphasis on frequency of hand hygiene as an outcome measure rather than microbiological data. One key limitation of the review was that it included studies that had weak designs for making causal inferences about the effects of interventions (mainly uncontrolled before‐after studies). Another disadvantage is the failure of the authors to consider variables that might influence rates of HCAIs. Seasonal variations are particularly likely to influence outcome measures in studies that examine hand hygiene and rates of HCAI. For example, bacterial counts are affected by seasonal factors such as humidity, while hand hygiene compliance is likely to be influenced by factors such as staffing levels and replacement of usual staff by temporary staff during national holidays or in the event of staff sickness.
Work published after Naikoba 2001 indicated that multimodal interventions to improve different aspects of healthcare delivery are not likely to change practice more effectively than single interventions (Grimshaw 2004), and that audit with performance feedback has only a modest effect on improving practice (Ivers 2012).
In 2007, we published a systematic review of interventions to improve hand hygiene compliance in patient care (Gould 2007), followed by an update in 2010 (Gould 2010). Only four studies met the inclusion criteria. Two examined education as a single intervention (Gould 1997; Huang 2002) while two evaluated multimodal campaigns (Vernaz 2008; Whitby 2008). Sample sizes were small and most studies lacked either a suitable comparison group or any control group at all. Consequently, we were unable to draw a conclusicn about the effectiveness of interventions to promote hand hygiene compliance due to the lack of high certainty of evidence (Gould 2010).
HCAIs remain a major threat to patient safety globally and a drain on healthcare resources (Badia 2017; PHAC 2016). The hospital microbial flora are constantly changing to present new infection prevention challenges, illustrated by a recent decline in MRSA and an upsurge in Gram negative bacteraemia in the UK (Health Foundation 2015). Some organisms are intrinsically resistant to antibiotics and for these, excellent non‐antibiotic approaches to prevention will always be essential. Hand hygiene has continued to be promoted as the foremost intervention that can be undertaken to prevent HCAIs and a large number of new studies have been published (Luangasanatip 2015). Since 2009 there has also been explicit guidance from WHO of what should be done to improve hand hygiene compliance, based on Pittet’s work in Geneva (Pittet 2000), but the components of multimodal campaigns vary considerably and do not always reflect the WHO recommendations.
Since evidence of the effectiveness of interventions to promote hand hygiene compliance and prevent HCAIs identified in Gould 2010 was limited and based on methodologically weak studies, it is important to review the large number of new studies and reassess the body of evidence. We undertook this review update to demonstrate the effectiveness or otherwise of new strategies, different approaches to performance feedback, the new combinations of approaches that have been adopted, and the impact of improved hand hygiene compliance on patient outcomes and healthcare expenditure.
Objectives
To assess the short‐ and long‐term success of strategies to improve hand hygiene compliance in patient care.
To determine whether an increase in hand hygiene compliance can reduce rates of health care‐associated infection.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised trials (RCTs), non‐randomised trials, controlled before‐after studies (CBAs) and interrupted time series (ITS) studies meeting the most recent explicit entry and quality criteria used by the Cochrane Effective Practice and Organisation of Care (EPOC) Group (EPOC 2013b). Studies reporting uncontrolled before‐after (UCBA) designs were not eligible for inclusion. To be eligible for review, ITS studies had to demonstrate a clearly‐defined point in time when the intervention occurred, and include at least three data collection points both before and after the intervention to take into account the influence of secular trends and the auto‐correlation among measurements repeatedly taken over time (Ramsay 2003). Data for CBAs had to be collected in at least two centres, with at least two intervention groups and two control groups.
Types of participants
We considered studies where the participants or target groups were nurses, doctors and other healthcare workers in any hospital, nursing home, long‐term care facility or community healthcare setting in any country. We excluded studies looking at surgical hand disinfection and surgical scrubbing, because their aims are not the same as hand hygiene for care in ward areas and clinics.
Types of interventions
We considered any intervention intended to improve compliance with hand hygiene using soap and water or alcohol‐based products, or both. For example, we considered education, audit with performance feedback, health promotion, and variations in availability and type of products used for hand hygiene. Studies of interventions to promote hand hygiene compliance were potentially eligible regardless of whether the intervention occurred in outbreak or non‐outbreak situations. We considered studies to promote compliance with universal or infection prevention and control precautions for inclusion, provided that data relating specifically to hand hygiene compliance were presented separately. Similarly, studies to promote hand hygiene compliance as part of a care bundle approach were eligible, provided that data relating specifically to hand hygiene compliance were presented separately. We excluded studies if hand hygiene compliance was assessed in simulations or artificial settings outside the clinical environment.
Types of outcome measures
Primary outcomes
Our primary outcome of interest was:
Hand hygiene compliance, measured through observation or a proxy indicator of hand hygiene compliance (e.g. increased use of hand hygiene products).
We considered studies reporting proxy indicators of hand hygiene compliance, for example use of soap or ABHRs or compliance with hand hygiene measured by an automated monitoring device. Automated devices vary in their degree of sophistication. The simplest are straightforward devices that deliver a measured amount (e.g. 5 mls) of product to the hands and record the number of times that the device has been used. The most sophisticated are body‐worn systems with sensors that indicate whether hands have been cleansed, linked to a computer that stores uptake. Healthcare workers’ self‐reports of their hand hygiene practices were not considered a valid measure of compliance because there is evidence that self‐reports are not accurate (Haas 2007).
Secondary outcomes
We also considered the following secondary outcomes of interest in our review, provided that hand hygiene compliance was also reported:
Reduction in health care‐associated infection.
Reduction in colonisation rates by clinically significant nosocomial pathogens. e.g. MRSA.
All studies had to demonstrate objective measurements of the outcome of interest, as well as relevant and interpretable data presented or obtainable.
Search methods for identification of studies
EPOC Information Specialists developed the search strategies according to EPOC recommendations.(Ballini 2010; EPOC 2014) and conducted the searches.
Electronic searches
We searched the following electronic databases up to 18 October 2016:
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 9) in the Cochrane Library
MEDLINE (including Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations) (1946 to 18 October 2016)
Embase Ovid (1974 to 17 October 2016)
Cumulative Index to Nursing and Allied Health Literature (CINAHL EBSCO); 1982 to 18 October 2016)
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 18 October 2016)
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 18 October 2016)
Full search strategies are available in Appendix 1.
Searching other resources
We handsearched the following high‐yield journals: BMJ; Journal of Hospital Infection; American Journal of Infection Control; Infection Control and Hospital Epidemiology; the Canadian Journal of Infection Control; and the Journal of Infection Prevention. Similarly, we handsearched the conference proceedings from the UK Hospital Infection Society; the Infection Prevention Society; the American Association for Professionals in Infection Control and Epidemiology (APIC); the Society for Health Care Epidemiology in America (SHEA); and Infection Prevention and Control Canada (IPAC Canada), formerly the Community and Hospital Infection Control Association (CHICA‐Canada).
We reviewed the reference lists of all papers and relevant reviews identified for additional references. Where relevant, we contacted authors of papers for any further published or unpublished work. We contacted colleagues from the professional organisations, WHO and pharmaceutical companies manufacturing hand hygiene products to ask if they were aware of any unpublished work within the field, as well as authors of other reviews in the field of effective professional practice for relevant studies of which they might be aware.
We also searched ISI Web of Science for relevant papers and the Database of Abstracts of Reviews of Effectiveness (DARE) for related reviews.
Data collection and analysis
We conducted the review using standard EPOC methods (Ballini 2010; EPOC 2013c).
Selection of studies
Three review authors (DJG, ND or DM) screened the results of searches to identify potentially relevant papers. Four review authors (DJG, JHC, ND or DM) independently selected the studies to be included in the review. ND or DM acted as arbiter for any unresolved difficulties.
Data extraction and management
Two review authors (DJG and ND or DM) abstracted data from each paper using the standard EPOC checklist (EPOC 2013c). The two review authors checked the abstracted data and resolved discrepancies through discussion, with MT, ND or DM acting as arbiter for any unresolved difficulties. Where key information was missing from the studies, we attempted to contact the authors for further information. None of our attempts to obtain additional information was successful . Authors either failed to respond or did not have the required information.
Assessment of risk of bias in included studies
Two review authors (DJG and DM) independently assessed the risks of bias using the standard EPOC 'Risk of bias' criteria (EPOC 2015b). All team members checked risk assessments and resolved discrepancies through discussion, with ND acting as arbiter for any unresolved difficulties.
We assessed randomised trials, non‐randomised trials and controlled before‐after studies according to nine standard criteria:
Adequate random sequence generation;
Concealment of allocation;
Blinding;
Adequately‐addressed incomplete outcome data;
Freedom from selective reporting;
Similar baseline outcome measures;
Similar baseline characteristics;
Adequate protection against contamination;
Freedom from other risks of bias.
We assessed ITS studies according to seven standard criteria, four of which were identical to criteria for the non‐ITS studies:
Blinding;
Adequately‐addressed incomplete outcome data;
Freedom from selective reporting;
Intervention independent of other changes;
Shape of the intervention prespecified;
Intervention unlikely to affect data collection;
Freedom from other risks of bias.
We divided blinding into two criteria to distinguish between blinding of participants and blinding of outcome assessment. The 'Risk of bias' tables therefore list 10 criteria for non‐ITS studies and eight criteria for ITS studies.
Measures of treatment effect
We described hand hygiene compliance using the measures reported by the authors: proportion of opportunities for hand hygiene in which hand hygiene was performed, or proportion of nurses who performed hand hygiene, hand hygiene events per hour, or volume of product. Measures of differences also varied across studies: adjusted odds ratios, risk ratios, mean difference (in percentage points), relative change in liquid soap procurement, or difference in events per hour.
Unit of analysis issues
We assessed whether appropriate analysis was conducted to adjust for clustering in estimating intervention effects in cluster RCTs and CBAs. Where clustering had not been accounted for, we planned to adjust the results using standard approaches incorporating measures of intra cluster correlation coefficients (ICCs) (Higgins 2011). This was not necessary to do however as we were not able to conduct a meta‐analysis because of heterogeneity. Unit of analysis errors were noted in our qualitative assessment of the studies’ results.
Data synthesis
Given the substantial heterogeneity of interventions and methods across studies, it was not sensible to undertake meta‐analysis to pool results; we therefore did not need to address clustering, matching, or inclusion of multi‐armed studies in a quantitative synthesis. Instead, we presented the results of studies in tabular form and made a qualitative assessment of the effects of studies, based on certainty of evidence. We reported the following data (where available): pre‐intervention and post‐intervention data, including absolute and percentage improvement. Where researchers did not report differences, the review authors calculated them based on available data and reported in Table 9 and Table 10. We noted inappropriate statistical analysis where relevant. We included studies with high or variable risks of bias in the qualitative summary, with the GRADE rating downgraded as appropriate.
1. Results from studies evaluating multimodal interventions.
| Study | Comparison | Estimate of compliance | Measure of difference or change |
| Intervention: Multimodal, not WHO | |||
| Ho 2012 | Cluster‐randomised trial Intervention: Multimodal not WHO · Also had study arms with powdered or powderless gloves Control: 2‐hour health talk |
Outcome: Hand hygiene compliance Inappropriate analysis: GEE but did not compare changes between arms Observed mean hand hygiene compliance: Intervention with powdered gloves: · Baseline: 27.0% · 1 month post: 59.2% · 4 months post: 60.6% Intervention with powderless gloves: · Baseline: 22.2% · 1 month post: 59.9% · 4 months post: 48.6% Control: · Baseline: 19.5% · 1 month post: 19.8% · 4 months post: 21.6% |
Not reported by researchers Calculated differences1 in percentage points between baseline and 1 month: · intervention with powdered gloves: 32.2 · intervention with powderless gloves: 37.7 · control: 0.3 Calculated differences1 in percentage points between baseline and 4 months: · intervention with powdered gloves: 33.6 · intervention with powderless gloves: 26.4 · control: 2.1 |
| Lee 2013 | ITS · 6 ‐ 7 month baseline · Intervention: Multimodal not WHO · 12 month intervention phase · 6‐month washout period · Control wards: no hand hygiene promotion |
Outcome: Hand hygiene compliance Intervention wards · Baseline: 49.3% (95% CI 47.2% to 51.4%) · Intervention phase: 63.8% (95% CI 62.3% to 64.4%) Control wards: · Baseline: 30.5% (95% CI 28.7% to 32.4%) · Washout period: 23.9% (95% CI 22.0% to 25.9%) |
Segmented regression analysis: · Increase after start of hand hygiene promotion: adjusted OR 1.19, 95% CI 1.01 to 1.42 · Decrease of 9% per month in washout period after campaign ended: adjusted OR 0.91, 95% CI 0.85 to 0.97 |
| Martin‐Madrazo 2012 | Cluster‐randomised trial Intervention: Multimodal not WHO Control: No intervention |
Outcome: Hand hygiene compliance Inappropriate analysis: Analysed at level of individual not cluster; inappropriate correction for missing data Mean observed hand hygiene compliance: Intervention group: Baseline: 7.98%, 95% CI 4.5 to 10.2 6 months post: 32.74 (no CI reported) Control group: Baseline: 8.26% (95% CI: 6.2‐11.6) 6 months post: 11.86 (no CI reported) |
Not reported by researchers Calculated differences1 in percentage points between baseline and 6 months post‐intervention: · intervention group: 24.76 · control group: 3.6 |
| Rodriguez 2015 | Stepped wedge RCT Intervention: Multimodal Not WHO Control: No intervention |
Outcome: Hand hygiene compliance Variation by site: · Pre: 47.2% to 79.8% · Post: 57.0% to 93.9% |
Absolute difference range: 1.9 to 26.7 Intervention effect: OR 1.17, 95% CI 1.13 to 1.22 Intervention effect adjusted by time: OR 1.08, 95% CI 1.03 to 1.14 |
| Yeung 2011 | Cluster‐randomised trial Intervention: Multimodal not WHO Control: Basic life support workshop |
Outcome: Hand hygiene compliance Inappropriate analysis: Analysed at level of individual not cluster Mean observed hand hygiene compliance (handwashing or ABHR use): Intervention group: Baseline: 25.8% Post‐intervention: 33.3% 7 months post: 36.7% Control group: Baseline: 25.8% Post‐intervention: 30.0% 7 months post: 37.7% |
Not reported by researchers Calculated differences1 in percentage points between baseline and post intervention: · intervention group: 7.5 · control group: 4.2 Calculated differences1 in percentage points between baseline and 7 months post‐intervention: · intervention group: 10.9 · control group: 11.9 |
| Intervention: Multimodal, WHO based | |||
| Derde 2014 | ITS Intervention: WHO based multimodal |
Outcome: Observed mean hand hygiene compliance: · Baseline: 52% · Optimised hand hygiene plus CHG bathing: 69% · Addition of MRSA screening and contact precautions: 77% |
Inappropriate analysis: No statistical analysis done Calculated difference1 in percentage points: · between baseline and optimised hand hygiene plus CHG bathing: 17 · between baseline and addition of MRSA screening and contact precautions: 25 |
| Mertz 2010 | Cluster‐randomised trial Intervention: WHO based multimodal Control: addition of ABHR |
Outcome: Hand hygiene compliance Intervention: · Pre: 15.8% · Post: 48.2% Control: · Pre: 15.9% · Post 42.6% |
Mean difference between groups at post‐test: · 6.3%, 95% CI 4.3% to 8.4% |
| Perlin 2013 | ITS Intervention: WHO‐based multimodal |
Outcome: Mean ounces of ABHR per adjusted pt‐day · Pre intervention: 21.3 · Post intervention: 48.75 |
Inappropriate analysis: No statistical analysis done Calculated difference1 between pre and post intervention: 27.45 ounces of ABHR per adjusted patient‐day |
| Interventions: Multimodal, WHO‐enhanced and WHO based | |||
| Vernaz 2008 | ITS VigiGerme campaign:WHO‐enhanced multimodal Clean Care is Safer Care campaign: WHO‐based multimodal |
Outcome: ABHR in litres per 100 patient‐days Did not report actual volume |
Increases in both VigiGerme and Clean Care campaigns via ARIMA modelling; no estimates of effect reported Overall increase in ABHR from 1.303 L/100 patient days to 2.016 L/patient days, but did not report by programme |
| Whitby 2008 | ITS Washington programme: WHO‐enhanced multimodal Geneva programme: WHO based multimodal |
Outcome: Electronic count of hand hygiene measured number of times ABHR dispensed from count Actual counts were not reported Noted that initial compliance was high in IDU |
GEE analysis: Washington program: increase in hand hygiene relative to baseline: RR 1.48 (95% CI: 1.2‐1.81) Geneva on medicine units: no increase in hand hygiene Geneva in IDU: increase in hand hygiene relative to baseline: RR 1.56, 95% CI 1.29 to 1.89 |
| Intervention: Multimodal, WHO‐enhanced | |||
| Huis 2013 | Cluster‐randomised trial Intervention: WHO‐enhanced multimodal Control: State of the art multimodal |
Outcome: Observed mean hygiene compliance Intervention: · Pre: 20% · Post: 53% · 6 months: 53% Control: · Pre: 23% · Post: 42% · 6 months: 46% |
OR of 1.64, 95% CI 1.33 to 2.02 in favour of team leader support |
| Midturi 2015 | ITS · 9‐month baseline Intervention: Multimodal WHO‐enhanced · 10‐month intervention period · 22‐month post‐intervention |
Outcome: Hand hygiene compliance · Baseline: 72.7% (range: 62.5% to 86.2%) · Intervention period: 79.7% (range not reported) · Post: 93.2% (range 7.9% to 97.7%) |
Inappropriate reporting of analysis for ITS · During intervention, average increase was 2% per month · Before‐after intervention, average increase was < 1% a month |
| Rosenbluth 2015 | ITS · 2‐year baseline Intervention: Multimodal WHO‐enhanced · 3‐year intervention period · 10‐month post‐intervention |
Outcome: Hand hygiene compliance Inappropriate analysis for ITS All healthcare workers: · During intervention: 85% to 92% · Pre‐intervention: variation (38% ‐ 100% but < 80% most months) · Post‐intervention: 83% ‐ 95% but most > 85% MDs: · During intervention: 75% ‐ 83% · Not reported for other time periods |
Not reported by researchers Because of the considerable variation by unit, it was not possible for the review authors to calculate a difference1 in percentage points between pre‐ and post‐intervention |
| Stevenson 2014 | Cluster‐randomised trial Intervention: WHO‐enhanced multimodal Control: Usual activities |
Outcome: Observed mean hand hygiene compliance Actual compliance rates were not reported |
Hand hygiene before and after patient contact, mean difference per group: Intervention: · 20.1% (range: 7.8% ‐ 35.5%) Control: · ‐3.1% (range: ‐6.3% ‐ +5.9%) Hand hygiene before or after patient contact, mean difference per group: Intervention: · 28.4% (range: 17.8% ‐ 38.2%) Control: · ‐0.7% (range: ‐16.7% ‐ +20.7%) |
1 Where researchers did not report differences, the review authors calculated the differences based on the data reported by the researchers and summarised in the column "estimate of compliance". ABHR: alcohol‐based hand rub; ARIMA: autoregressive integrated moving average; CHG: chlorhexidine gluconate; CI: confidence interval; GEE: generalised estimating equation; IDU: immunisation and diagnosis unit; ITS: interrupted time series; MDs: physicians; MRSA: methicillin‐resistant Staphylococcus aureus; OR: odds ratio; RCT: randomised (controlled) trial; RR: risk ratio; WHO: World Health Organizaiton
2. Results from studies evaluating interventions other than multimodal interventions.
| Study | Comparison | Estimate of compliance | Measure of difference or change |
| Intervention: Performance feedback | |||
| Armellino 2012 | ITS · 16‐week baseline · Intervention: video recording and feedback of hand hygiene rates · 16‐week post · 75‐week maintenance |
Outcome: Observed mean hand hygiene compliance: Baseline: 6.5% (weekly range: 3.5% to 9.8%) Post‐feedback period: 81.6% (weekly range: 30.8% to 91.2%) Maintenance phase: 87.9% (weekly range: 83.5% to 91.6%) |
Segmented regression analysis: · In week after start of intervention, estimated increase in compliance of 17.5% with additional 4% increase in each following week · In maintenance period, small weekly decrease of ‐0.04% |
| Fisher 2013a | Cluster‐randomised trial Intervention: wireless monitoring and feedback Control: No intervention |
Outcome: Mean hand hygiene compliance on entry as recorded by electronic monitor: Intervention group: · Baseline: 28% (21% ‐ 37%) · Phase 2: real time reminders: 33% (25% ‐ 41%) · Phase 3: feedback: 28% (16% ‐ 40%) Control group: · Baseline: 28% (21% ‐ 37%) · Phase 2: real time reminders: 26% (22% ‐ 32%) · Phase 3: feedback: 24% (19% ‐ 33%) Similar increases in compliance on exit Variation by study ward, professional category and opportunity load |
Unclear reporting of regression Not reported by researchers Calculated differences1 in percentage points between baseline and phase 2 real time reminders: · intervention group: 5 · control group: ‐2 Calculated differences1 in percentage points between baseline and phase 3 feedback: · intervention group: 0 · control group: ‐4 |
| Fuller 2012 | Stepped‐wedge RCT Intervention: feedback and personalised action planning Control: Clean Your Hands campaign |
Outcomes reported: · Estimated relative change in liquid soap procurement · Hand hygiene compliance Estimates of volume of soap use or observed hand hygiene compliance were not reported |
Estimated relative change in liquid soap: ACE: 1.133, 95% CI 0.987 to 1.3) ITU: 1.314, 95% CI 1.114 to 1.548 Absolute increase in compliance: ACE wards: · 13% if pre‐hand hygiene compliance was 50% · 10% if pre‐hand hygiene compliance was 70% ITU wards · 18% if pre‐hand hygiene compliance was 50% · 13% if pre‐hand hygiene compliance was 70% OR (compared to baseline) ACE wards: · 1.67, 95% CI 1.26 to 2.22 ITU wards: · 2.09, 95% CI 1.55 to 2.81 |
| Moghnieh 2016 | NRCT Intervention 1: Incentive Intervention 2: Audit and feedback Control: Usual hand hygiene campaign |
Outcome: Hand hygiene compliance Variation by week: · Baseline all groups: 16% ‐ 20% · During intervention 1: 60% at week 8 and 77% at week 14 · During intervention 2: 43% at week 8 and 51% at week 14 · Control group: unchanged from baseline Decreased post‐intervention at week 21: · Intervention 1: 34% · Intervention 2: 48% · Control: unchanged |
Not reported by researchers Calculated differences1 in percentage points between baseline and week 8: · intervention 1: 40 ‐ 44 · intervention 2: 23 ‐ 27 · control group: unchanged Calculated differences1 in percentage points between baseline and week 14: · intervention 1: 57 ‐ 61 · intervention 2: 31 ‐ 35 · control group: unchanged |
| Stewardson 2016 | Cluster‐randomised trial Intervention 1: Enhanced performance feedback Intervention 2: Enhanced performance feedback plus patient participation Control: Usual WHO‐based hand hygiene campaign |
Outcome: Hand hygiene compliance Performance feedback: · Baseline: 65% · Intervention period:75% · Follow‐up:72% Feedback plus patient participation: · Baseline: 66% · Intervention period: 77% · Follow‐up: 72% Control: · Baseline: 66% · Intervention period: 73% · Follow‐up: 70% |
Absolute change for performance feedback: · Intervention period: 10% with OR 1.61, 95% CI 1.41 to 1.84 · Follow‐up:7% with OR 1.38, 95% CI 1.19 to 1.60 Absolute change for feedback plus patient participation: · Intervention period: 11% with OR 1.73, 95% CI 1.51 to 1.98 · Follow‐up: 6% with OR 1.36, 95% CI 1.18 to 1.57 Absolute change for Control: · Intervention period: 7% with OR 1.41, 95% CI 1.21 to 1.63 · Follow‐up: 4% with OR 1.21, 95% CI 1.00 to 1.47 |
| Talbot 2013 | ITS · Baseline: 2004 ‐ 2009 · Intervention 2009 ‐ 10: feedback, leadership and incentives · Active accountability: 2010 ‐ 2012 |
Outcome: observed hand hygiene compliance Baseline: 52% Intervention: 75% Active accountability phase: 89% |
Segmented regression analysis done but no estimates of effect reported: · Increase in adherence in each phase · Changes in slope associated with each time period Calculated differences1 in percentage points between baseline and · intervention phase: 23 · active accountability phase 37 |
| Intervention: Education | |||
| Higgins 2013 | ITS Intervention: Education: E‐learning hand hygiene game |
Outcome: Observed mean hand hygiene compliance: · in 12 months pre‐e‐learning game: 42% · in 12 months post‐e‐learning game: 84% |
Appropriateness of analysis unclear: Did not specify statistical analysis done but only reported mean hand hygiene compliance Calculated differences1 in percentage points between pre and post: 42 |
| Huang 2002 | RCT Intervention: Education sessions on hand hygiene and UP Control: No intervention |
Outcome: % of nurses who performed hand hygiene Before patient contact: Intervention · Pre: 51.0% · Post: 85.7% Control · Pre: 53.1% · Post:53.1% After patient contact: Intervention · Pre: 75.5% · Post: 91.8% Control · Pre: 75.5% · Post: 71.4% |
Not reported by researchers Calculated differences1 in percentage points for before pt contact: · intervention: 24.5 · control group: no change Calculated differences1 in percentage points for after patient contact: · intervention: 16.3 · control group: 4.1 |
| Intervention: Cues | |||
| Diegel‐Vacek 2016 | NRCT Intervention: Light cue over sink Comparison: no light cue |
Outcome: Hand hygiene compliance Light cue: · Day 1: 23% · Day 2: 30% · Day 3: 23% No light cue: · Day 1: 7% · Day 2: 16% · Day 3: 23% |
Not reported by researchers Calculated differences1 in percentage points between day 1 and day 2: · light cue: 7 · no light cue: 9 Calculated differences1 in percentage points between day 1 and day 3: · light cue: 0 · no light cue: 16 |
| Grant 2011 | Pair‐matched cluster‐randomised trial Compared 2 signs: personal vs patient consequences as message |
Outcome: Observed mean hand hygiene compliance: Personal consequences sign: Pre‐test: 80.0% Post‐test: 79.71% Patient consequences sign: Pre‐test: 80.69% Post‐test: 89.2% Variation by type of practitioner but all had greater increase in hand hygiene in response to patient consequences sign |
Inappropriate analysis : Did not do a matched analysis Not reported by researchers Calculated differences1 in percentage points between pre and post test: · Personal consequences sign: ‐0.29 · Patient consequences sign: +8.51 |
| King 2016 | RCT Intervention: Olfactory cue (scent) or signs with male or female eyes Comparison: baseline without cues |
Outcome: Hand hygiene compliance · Baseline: 15.0% · Scent cue: 46.9% · Male eyes cue: 21.7% · Female eyes cue: 10.0% Some differences women vs men |
Not reported by researcher Calculated differences1 in percentage points between pre‐ and post‐test: · Scent cue: +31.9 · Stern male eyes: +6.7 · Female eyes: ‐5 |
| Intervention: Placement of ABHR | |||
| Munoz‐Price 2014 | RCT with cross‐over Intervention: placement of ABHR on cart Control: ABHR on wall |
Outcome: hand hygiene events per hour: Intervention: 0.84 Control: 0.54 |
Difference was an increase of 0.3 events per hour |
1 Where researchers did not report differences, the review authors calculated the differences based on the data reported by the researchers and summarized in the column "estimate of compliance". ABHR: alcohol‐based hand rub; ACE: acute care of the elderly; CI: confidence interval; ITS: interrupted time series; ITU: intensive care unit; NRCT: non‐randomised (controlled) trial; OR: odds ratio; RCT: randomised (controlled) trial
'Summary of findings' tables
We created 'Summary of findings' tables for each category of interventions (e.g. WHO‐based, WHO‐enhanced, performance feedback) as well as an overview Table 1. We included our primary outcome of hand hygiene compliance as well as our secondary outcomes of reduction in infection or colonisation rates. Since it was not possible to conduct a meta‐analysis, we summarise results narratively, using plain language statements (EPOC 2013c).
for the main comparison.
| Overview: interventions compared with different or no interventions for improving hand hygiene compliance in healthcare workers or reducing infection or colonisation rates | ||||
|
Patient or population: Healthcare workers Settings: Hospitals, nursing homes and long‐term care facilities Intervention: Strategies varied by study Comparison: Varied by study | ||||
| Types of Interventions1 | Impact | Outcomes and Certainty of the evidence (GRADE) 2 | ||
| Hand Hygiene Compliance3 | Change in infection rates4 | Change in colonisation rates4 | ||
| Multimodal, not WHO‐based5: contains some strategies recommended by WHO | Multimodal interventions that include some but not all strategies recommended in the WHO guidelines may slightly improve hand hygiene compliance and may slightly reduce infection rates (low certainty of evidence). | ⊕⊕⊝⊝
low (5 studies) |
⊕⊕⊝⊝
low (3 studies) |
‐‐‐ |
| Multimodal, WHO‐based: contains all strategies recommended by WHO | It is uncertain whether multimodal interventions that include all strategies recommended in the WHO guidelines improve hand hygiene compliance or reduces infection because the certainty of this evidence is very low. Such multimodal interventions may slightly reduce colonization rates (low certainty of evidence) | ⊕⊝⊝⊝
very low (5 studies) |
⊕⊝⊝⊝
very low (2 studies) |
⊕⊕⊝⊝
low (2 studies) |
| Multimodal, WHO‐enhanced: contains all strategies recommended by WHO and additional ones | Multimodal interventions that contain all strategies recommended in the WHO guidelines plus additional strategies may slightly improve hand hygiene compliance (low certainty of evidence). It is uncertain whether such multimodal interventions reduce infection rates because the certainty of this evidence is very low | ⊕⊕⊝⊝
low (6 studies) |
⊕⊝⊝⊝
very low (1 study) |
‐‐‐ |
| Performance feedback | Performance feedback may improve hand hygiene compliance (low certainty of evidence) and probably slightly reduces infection and colonisation rates | ⊕⊕⊝⊝
low (6 studies) |
⊕⊕⊕⊝
moderate (1 study) |
⊕⊕⊕⊝
moderate (1 study) |
| Education | Education may improve hand hygiene compliance (low certainty of evidence) | ⊕⊕⊝⊝
low (2 studies) |
‐‐‐ | ‐‐‐ |
| Cues | Cues such as signs or scent may slightly improve hand hygiene compliance (low certainty of evidence) | ⊕⊕⊝⊝
low (3 studies) |
‐‐‐ | ‐‐‐ |
| Placement of ABHR | Placement of ABHR close to point of use probably slightly improves hand hygiene compliance (moderate certainty of evidence). | ⊕⊕⊕⊝
moderate (1 study) |
‐‐‐ | ‐‐‐ |
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. Abbreviations: ABHR: alcohol‐based hand rub; WHO: World Health Organization | ||||
1Studies evaluated different strategies or combinations of strategies. 2See individual 'Summary of findings' tables (by intervention type) for specific impact and rationale for downgrading evidence. 3Hand hygiene compliance: measured through direct observation or a proxy indicator such as product use. 4Rates: infection or colonisation rates, or both, were reported for different micro‐organisms. 5Multiple strategies were used but were not consistent with WHO guidelines.
Two review authors (DM and DG) independently assessed the certainty of evidence (high, moderate, low or very low), using the five GRADE considerations (risk of bias, inconsistency, indirectness, imprecision, publication bias) and EPOC methods and recommendations (EPOC 2013d). We considered all measures of hand hygiene compliance together (e.g. observed hand hygiene or a proxy measure such as increased use of hand hygiene products) in assigning a GRADE rating. See Appendix 2 for the completed 'Calculation of GRADE ratings' worksheet. Justification for decisions to downgrade the ratings are placed in footnotes in each 'Summary of findings' table, and we have made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We did not perform any subgroup analysis or quantitative assessment of heterogeneity, since we did not perform a meta‐analysis.
Results
Description of studies
Results of the search
The searches yielded 4219 abstracts, excluding duplicates. We reviewed the full text of 534 potentially eligible articles and excluded 444. Of the remaining 90 full‐text articles, we excluded a further 67 studies with reasons primarily related to lack of adequate data points in an ITS design, or inadequacy of control groups (See Characteristics of excluded studies).
This review contains 26 studies. Figure 1 summarises the search and study selection results.
1.
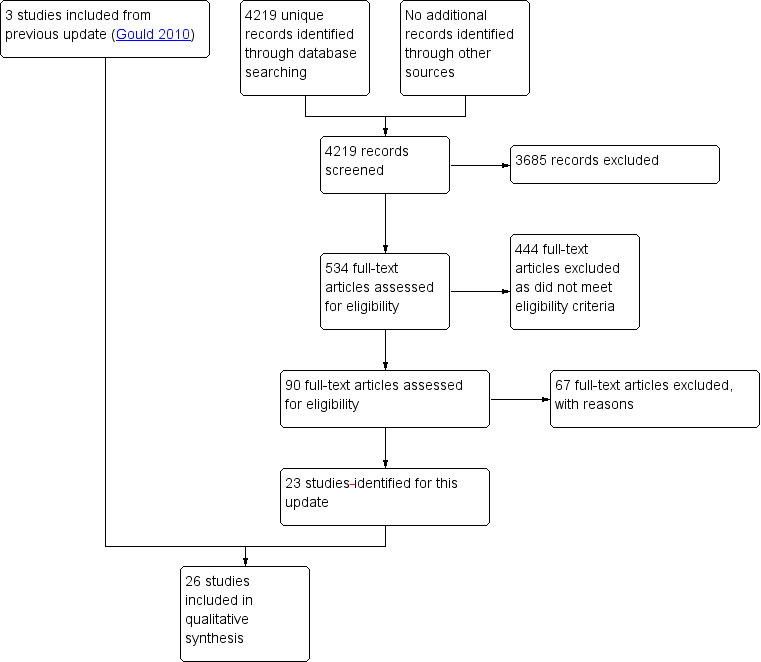
Study flow diagram.
Included studies
This review includes 26 studies; 23 from this update (Armellino 2012; Derde 2014; Diegel‐Vacek 2016; Fisher 2013a; Fuller 2012; Grant 2011; Higgins 2013; Ho 2012; Huis 2013; King 2016; Lee 2013; Martin‐Madrazo 2012; Mertz 2010; Midturi 2015; Moghnieh 2016; Munoz‐Price 2014; Perlin 2013; Rodriguez 2015; Rosenbluth 2015; Stevenson 2014; Stewardson 2016; Talbot 2013; Yeung 2011) and three from previous versions of the review (Huang 2002; Vernaz 2008; Whitby 2008). Details are provided in the Characteristics of included studies table and briefly summarised below.
Study design
Two studies were randomised trials (Huang 2002; King 2016). Nine studies were cluster‐randomised trials (Fisher 2013a; Grant 2011; Ho 2012; Huis 2013; Martin‐Madrazo 2012; Mertz 2010; Stevenson 2014; Stewardson 2016; Yeung 2011). Two studies were stepped‐wedge cluster‐randomised trials (Fuller 2012; Rodriguez 2015). One study was a randomised trial with cross‐over (Munoz‐Price 2014). Two were non‐randomised trials (Diegel‐Vacek 2016; Moghnieh 2016). Ten studies were ITS studies meeting the specific criteria stipulated by EPOC (Armellino 2012; Derde 2014Higgins 2013; Lee 2013; Midturi 2015; Perlin 2013; Rosenbluth 2015; Vernaz 2008; Whitby 2008). Some reports included different designs for assessing different interventions or outcomes, such as randomised trials to assess the effectiveness of bundles with an embedded ITS study for assessing hand hygiene compliance. We categorised the studies, and reviewed the appropriate methods related to the intervention(s) to assess hand hygiene.
Settings
Two studies were conducted in long‐term care facilities (Ho 2012; Yeung 2011) and one in a primary care setting (Martin‐Madrazo 2012). The remaining 23 studies were conducted in acute care hospitals on general wards and/or critical care units, except for Munoz‐Price 2014 which was conducted in an anaesthetic room.
In 15 studies data were collected in a single centre, although the size of the centre and number of units involved varied (Armellino 2012; Diegel‐Vacek 2016; Fisher 2013a; Grant 2011; Higgins 2013; Huang 2002; King 2016; Midturi 2015; Moghnieh 2016; Munoz‐Price 2014; Rosenbluth 2015; Stevenson 2014; Talbot 2013; Vernaz 2008; Whitby 2008). In two studies data were collected in three facilities (Huis 2013; Mertz 2010). In one study, data were collected in one multi‐state healthcare system with 166 hospitals and 116 outpatient surgery and endoscopy centres (Perlin 2013). In the remaining eight studies, data were collected from 7 to 18 centres.
Four studies took place in Southeast Asia (Fisher 2013a; Ho 2012; Midturi 2015; Yeung 2011), one took place in Spain (Martin‐Madrazo 2012), one in Canada (Mertz 2010), one in England and Wales (Fuller 2012), one in southern Ireland (Higgins 2013), two in Switzerland (Stewardson 2016; Vernaz 2008), one in Australia (Whitby 2008), one in Lebanon (Moghnieh 2016),one in the Netherlands (Huis 2013), one in Argentina (Rodriguez 2015), and 10 in the United States (Armellino 2012; Diegel‐Vacek 2016; Grant 2011; King 2016; Midturi 2015; Munoz‐Price 2014; Perlin 2013; Rosenbluth 2015; Stevenson 2014; Talbot 2013). Two studies were multinational (Derde 2014; Lee 2013), involving multiple European countries; Lee 2013 also included centres from Israel.
Staff participating
One study included staff in the anaesthetic room (Munoz‐Price 2014). In four studies data were collected from nurses (Huang 2002; Huis 2013; Moghnieh 2016; Yeung 2011); Huis 2013 also included student nurses, and Yeung 2011 also included nursing assistants and physiotherapists. In the remaining studies data were collected from all clinical staff present in the clinical areas during the period of data collection.
Interventions
Fourteen studies presented the results of multimodal campaigns featuring complex interventions that were similar to or based on the WHO Guidelines 2009 recommendations. Five studies reported multimodal campaigns that included some but not all of the elements recommended by WHO (Ho 2012; Lee 2013; Martin‐Madrazo 2012; Rodriguez 2015; Yeung 2011). They differed in the elements not included. We categorise them as non‐WHO multimodal interventions in this review. Three studies included all five types of strategies recommended by WHO; these campaigns are referred to as WHO‐based multimodal interventions in this review (Derde 2014; Mertz 2010; Perlin 2013). Derde 2014 did not describe their campaign. Four studies reported campaigns that included the interventions recommended by WHO in addition to other measures, such as social marketing or staff involvement in the development of the campaign (Huis 2013; Midturi 2015; Rosenbluth 2015; Stevenson 2014). These are called WHO‐enhanced multimodal interventions in this review. Midturi 2015, for example, evaluated the impact of rewards and alerts to the supervisor when hands were not cleansed, while Rosenbluth 2015 additionally evaluated role‐modelling, encouragement and incentives to cleanse hands.
Two studies reported on two separate multimodal interventions (Vernaz 2008; Whitby 2008). In Whitby 2008, one of the three interventions tested (Geneva) was categorised as WHO‐based, while the second (Washington) was categorised as WHO‐enhanced, as it included extensive staff involvement. Whitby 2008 also reported a third intervention that consisted of the addition of ABHR alone. Similarly, one of the two interventions in Vernaz 2008 (Clean Care is Safer Care) was categorised as WHO‐based, while the other (VigiGerme) was categorised as WHO‐enhanced, as it included social marketing. Vernaz 2008 did not describe their campaigns in any detail. As the researchers did not compare results between arms, we considered each intervention separately in this review (Vernaz 2008; Whitby 2008).
Table 11 summarises the individual components of the multimodal campaigns and illustrates the variation that existed.
3. Comparison of multimodal interventions.
|
Study/ Category* |
Education | Feedback |
Posters/ signs |
ABHR | Admin | Staff | Other |
| Intervention: Multimodal, not WHO | |||||||
| Ho 2012 | Yes (detailed) |
Individual and unit | Yes | Individual and point of care | No | No | Gloves with and without powder |
| Lee 2013 | Yes | No | Yes | Yes | Yes | No | ‐‐‐ |
| Martin‐Madrazo 2012 | Yes (details) | No | Yes | Yes | ‐‐‐ | No | ‐‐‐ |
| Rodriguez 2015 | Yes | Unit level | Yes | Yes | Yes | No | Role modelling Direct MD encouragement Incentives for MDs |
| Yeung 2011 | Yes (details) | 1 session to both groups at 3 months | Yes | Individual | No | No | Pens as reminder |
| Intervention: Multimodal, WHO based | |||||||
|
Mertz 2010 WHO‐based |
Yes | Unit level | Yes | Yes | Yes | Yes | ‐‐‐ |
|
Perlin 2013 WHO‐based |
Yes | Yes (at discretion) | Yes | Yes | Yes | No | ‐‐‐ |
|
Whitby 2008: Geneva Intervention WHO‐based |
Yes | Yes | Yes | Yes | Yes | No | ‐‐‐ |
| Intervention: Multimodal, WHO‐enhanced | |||||||
| Huis 2012 | Yes | Individual | Yes | Yes | Yes | Yes | Adequate supplies |
| Midturi 2015 | Yes | Individual and unit level | Yes | Yes | Yes | No | Rewards, alerts to immediate supervisor |
| Rosenbluth 2015 | No | Unit level | Yes | Yes | Yes | No | ‐‐‐ |
| Stevenson 2014 | Yes | Yes at unit level (variable) | Yes | Yes | Yes | Yes | Recognition and rewards programme (e.g. candy, buttons) |
|
Whitby 2008: Washington Intervention |
Yes | Informal | Yes | Yes | Yes (walk around by exec) | Yes | ‐‐‐ |
Note: Vernaz 2008 and Derde 2014 did not describe their multimodal campaigns and are not included in this table. Category: WHO‐based = included the 5 types of interventions recommended by WHO; WHO‐enhanced = included the 5 types of interventions recommended by WHO plus additional strategies; Not WHO = did not include at least the 5 types of interventions recommended by WHO. ABHR: alcohol‐based hand rub; MDs: physicians; WHO: World Health Organization
Of the remaining 12 studies, six reported a single intervention. Two of these studies focused on education; Huang 2002 evaluated education sessions while Higgins 2013 assessed the effect of an e‐learning hand hygiene game. Three studies evaluated the effectiveness of cues: signs with messages about personal consequences versus patient consequences of failing to cleanse hands (Grant 2011), lights above sinks switching on when staff entered the room (Diegel‐Vacek 2016), and signs portraying a stern pair of male eyes and clean scent to remind healthcare workers about hand hygiene (King 2016). Munoz‐Price 2014 evaluated the different placement of ABHR dispensers in the anaesthesia room.
The six remaining studies focused on performance feedback with additional components. Two of these evaluated feedback as their main intervention incorporating technology into the process; Armellino 2012 videotaped hand hygiene episodes and gave feedback to the units, while Fisher 2013a used a wireless monitoring system that had an audible beep as a real‐time reminder, and gave individual feedback. Fuller 2012 used two interventions, adding action planning to performance feedback; hand hygiene compliance results were reported to wards and staff were then supposed to develop action plans to address compliance issues. Stewardson 2016 evaluated performance feedback, enhanced performance feedback and patient participation. Moghnieh 2016 evaluated incentive‐based feedback and audit‐based feedback. The multimodal campaign by Talbot 2013 differed considerably from the others, focusing on feedback to individuals as well as leadership, goal setting, financial incentives to the centre, and institution‐wide marketing.
Three studies were complex and evaluated the effectiveness of interventions to address MRSA, such as screening, isolation precautions, and decolonisation, in addition to hand hygiene, but it was possible to extract hand hygiene data separately (Derde 2014; Lee 2013; Perlin 2013). One study adopted a two‐stage design in which the first stage refined the hand hygiene intervention which was then tested in the second stage (Grant 2011).
Six studies contained evidence of theoretical underpinning. In Vernaz 2008 the intervention was informed by Social Marketing Theory (Kotler 1971). A framework to support staff accountability for hand hygiene developed by the authors was used in Talbot 2013. Huis 2013 was based on a leadership and teamwork model developed for the study based on earlier descriptions of barriers and facilitators to hand hygiene. Diegel‐Vacek 2016 was based on an adaptation of the Theory of Planned Behaviour (Azjen 1991) which holds that behaviour is susceptible to change in environmental conditions and that such change can be manipulated to encourage the desired action (Shankar 2007). In Diegel‐Vacek 2016 the environmental cue was a light above a sink switching on when the room was entered. In King 2016, the intervention was based on psychological priming. This is the process through which exposure to particular cues (auditory, olfactory or visual) have the capacity to alter behaviour without the individual becoming aware that their behaviour is being manipulated (Bargh 1992). Fuller 2012 employed an intervention based on behavioural theory that applies psychological techniques to improve compliance through performance feedback, but no specific behavioural theory was named.
Exploring sustainability of the intervention
In 11 studies (Armellino 2012; Derde 2014; Fuller 2012; Higgins 2013; Ho 2012; Lee 2013; Midturi 2015; Perlin 2013; Stevenson 2014; Talbot 2013; Vernaz 2008) hand hygiene compliance measures continued longer‐term (12 months or longer). Four studies reported follow‐up data at six months post‐intervention (Huis 2013; Martin‐Madrazo 2012; Rodriguez 2015; Rosenbluth 2015), and one reported follow‐up data at seven months (Yeung 2011). In three studies follow‐up was less than three months (Diegel‐Vacek 2016; King 2016; Moghnieh 2016).
Outcomes
Data were collected by direct observation in 20 studies (Derde 2014; Diegel‐Vacek 2016; Grant 2011; Higgins 2013; Ho 2012; Huang 2002; Huis 2013; King 2016; Lee 2013; Martin‐Madrazo 2012; Mertz 2010; Midturi 2015; Moghnieh 2016; Munoz‐Price 2014; Rodriguez 2015; Rosenbluth 2015; Stevenson 2014; Stewardson 2016; Talbot 2013; Yeung 2011) and through videocamera observation in one study (Armellino 2012). One study measured hand hygiene compliance using an electronic monitoring device (Fisher 2013a). All but two of the studies using observation reported hand hygiene compliance in terms of opportunities for hand hygiene. The two exceptions were Huang 2002, who reported the proportion of nurses who performed hand hygiene, and Munoz‐Price 2014 who reported hand hygiene events per hour. Observation periods and time of day varied in all of the studies employing observation. One study measured both observed hand hygiene compliance and procurement of ABHR as a secondary measure (Fuller 2012). Three studies measured product usage alone (Perlin 2013; Vernaz 2008; Whitby 2008), but reported it differently.
Microbiological data were documented in nine studies (Derde 2014; Ho 2012; Lee 2013; Mertz 2010; Perlin 2013; Stevenson 2014; Stewardson 2016; Vernaz 2008; Yeung 2011). Stevenson 2014, however, did not report infection rates as an outcome measure, instead using the results as part of the hand hygiene campaign. Mertz 2010 measured MRSA colonisation, Derde 2014 measured colonisation with MRSA, VRE and highly‐resistant Enterobacteriaceae (HRE). Perlin 2013 and Lee 2013 documented rates of MRSA infection rather than colonisation, while Vernaz 2008 reported on the incidence of MRSA and C. difficile in clinical isolates as well as rates of antibiotic use. Derde 2014, in addition to reporting colonisation, also measured rates of intensive care unit (ICU)‐acquired bacteraemia. Both Ho 2012 and Yeung 2011 reported infections requiring hospitalisation, while Ho 2012 also reported the number of respiratory infection outbreaks, and Yeung 2011 reported on infection‐associated mortality. Stewardson 2016 documented clinical isolates of hospital pathogens, specifically MRSA, extended beta lactamase‐producing Enterobacteriaceae and C. difficile, at least 48 hours after admission in patients who were not known to be colonised.
Grant 2011 was the only study to estimate cost savings in terms of the number of infections prevented by cleansing hands.
Use of the World Health Organization Guidelines
Twenty‐three of the 26 included studies were published after 2009 when WHO released its guidelines for promoting hand hygiene compliance. However, the research was initiated prior to the publication of those guidelines in all but four of the included studies (Fisher 2013a; Grant 2011; Higgins 2013; Munoz‐Price 2014). Although refined for the WHO Guidelines 2009, WHO had made earlier recommendations regarding hand hygiene promotion. Their recommendations therefore would have been available to underpin 25 of the 26 studies, although not the much earlier study by Huang 2002. Use of the WHO definition for hand hygiene compliance was stated explicitly in three studies (Diegel‐Vacek 2016; Ho 2012; Yeung 2011). The WHO Guidelines 2009 recommend implementation of a multimodal hand hygiene campaign; 18 studies employed more than one intervention (Derde 2014; Higgins 2013; Ho 2012; Huis 2013; Lee 2013; Martin‐Madrazo 2012; Mertz 2010; Midturi 2015; Moghnieh 2016; Perlin 2013; Rodriguez 2015; Rosenbluth 2015; Stevenson 2014; Stewardson 2016; Talbot 2013; Vernaz 2008; Whitby 2008; Yeung 2011). The guidelines also identify five components to be specifically implemented: ABHR at point of care or carried by the healthcare worker; training and education; performance observation and feedback; reminders (e.g. posters); and administrative support/institutional safety climate. Leadership and staff involvement contribute to the latter. Three of the studies (Derde 2014; Mertz 2010; Perlin 2013), and one campaign in the studies by Vernaz 2008 and Whitby 2008, implemented all five recommendations. Derde 2014 and Vernaz 2008 may have adopted them but it is impossible to tell as details were not provided about their campaigns: they were simply described as based on the WHO guidelines, without further detail. Four studies we categorised as WHO‐enhanced (Huis 2013; Midturi 2015; Rosenbluth 2015; Stevenson 2014) and one campaign in the studies by Vernaz 2008 and Whitby 2008 implemented all the recommended interventions as well as additional ones. Five studies implemented many of the five recommended strategies, although not always the same ones (Ho 2012; Lee 2013; Martin‐Madrazo 2012; Rodriguez 2015; Yeung 2011). Table 11 shows the interventions implemented in the different multimodal campaigns.
The WHO Guidelines 2009 recommend that hand hygiene compliance should be assessed by direct observation because it is the only approach that can detect all hand hygiene opportunities, the number of times than an opportunity is acted on, and the appropriate timing of the hand hygiene episode in the sequence of care. In 20 studies data were collected solely by direct observation, with data collectors present on the units. In Armellino 2012, observation was recorded by video camera. Three studies employed product usage as the sole method of data collection (Perlin 2013; Vernaz 2008; Whitby 2008). Two studies documented direct observation and product usage (Fisher 2013a; Fuller 2012). Higgins 2013 also assessed hand‐washing technique using testing with adenosine triphosphase in addition to hand hygiene compliance. WHO acknowledges that the results of hand hygiene compliance derived through direct observation are open to bias. Thirteen studies using direct observation considered the possibility of bias in the discussion of their results (Derde 2014; Diegel‐Vacek 2016; Fisher 2013a; Fuller 2012; Higgins 2013; Ho 2012; Huis 2013; Martin‐Madrazo 2012; Mertz 2010; Munoz‐Price 2014; Stevenson 2014; Talbot 2013; Yeung 2011).
The WHO Guidelines 2009 also recommend use of their tools, including the Five Moments framework and their data collection checklist. Three studies reported that their checklists were based on the WHO audit tool (Higgins 2013; Ho 2012; Huis 2013). Mertz 2010 used a modified tool based on WHO tools that existed at the time, and Fuller 2012 used the Hand Hygiene Observation Tool which had been developed especially for their study. The other authors did not specify what observation tool was used, although five studies reported a link to the Five Moments indications for hand hygiene (Derde 2014; Martin‐Madrazo 2012; Moghnieh 2016; Rosenbluth 2015; Stewardson 2016). Only three studies clearly reported the use of the Five Moments as part of the promotional material to inform staff about hand hygiene (Derde 2014; Higgins 2013; Stewardson 2016).
Excluded studies
Forty‐five of the 67 excluded studies were ITS studies with inadequate numbers of pre‐ or post‐intervention data collection points, or unclear intervention periods, or both. Three of the excluded studies were non‐randomised trials with inadequate control groups, and 19 were controlled before‐after studies with only one intervention and one control group, rather than the required two groups of each.
We also excluded Gould 1997, which we had previously considered eligible and was included in the original 2007 review and the 2010 update (Gould 2007; Gould 2010). However, changes in the eligibility criteria of controlled before‐after studies meant this study no longer met the new criteria (EPOC 2013b). We have therefore removed it from the list of included studies and added it to the number and table of excluded studies (See Characteristics of excluded studies).
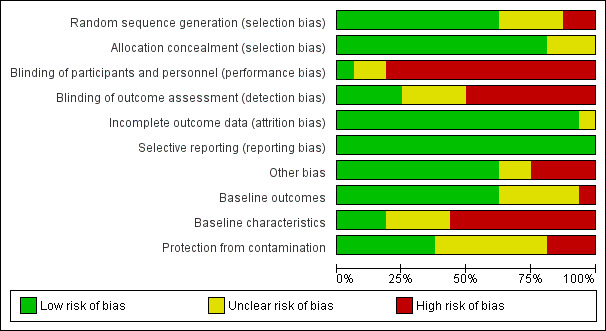
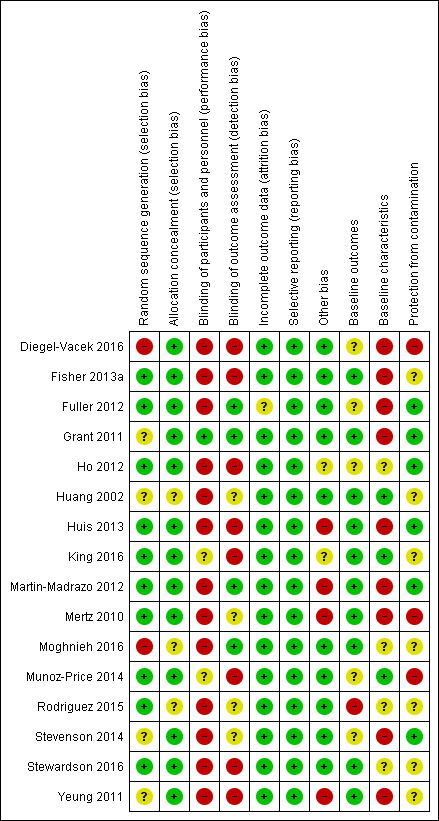
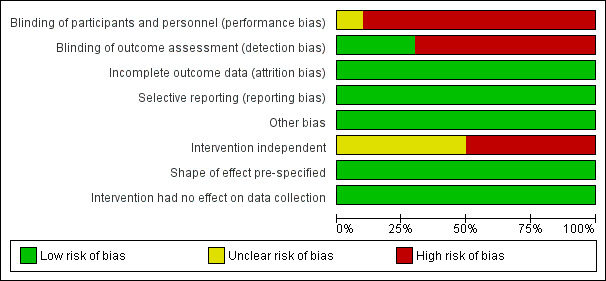
Risk of bias in included studies
We present details of the risks of bias for non‐ITS designs (randomised and non‐randomised trials and controlled before‐after studies) in Figure 2 and Figure 3, and details of the risk of bias for ITS studies in Figure 4 and Figure 5. Details are also provided in the Characteristics of included studies tables.
2.
Risk of bias graph for non‐ITS studies (RCTs, NRCTs, and CBAs)
3.
Risk of bias summary for non‐ITS studies (RCTs, NRCTs, and CBAs)
4.
Risk of bias graph for ITS studies
5.

Risk of bias summary for ITS studies
All the studies were at high risk of bias overall, with at least two items at high or unclear risk of bias. Of the 16 non‐ITS designs, Grant 2011 contained one source of high risk and one source of unclear risk of bias, while Huang 2002 and King 2016 each contained one source of high risk and four or three sources respectively, of unclear risks of bias. The remaining non‐ITS studies contained at least two sources of high risk of bias, with three having four such sources (Huis 2013; Mertz 2010; Yeung 2011) and Diegel‐Vacek 2016 having five such sources. Inadequate blinding and inadequate reporting of baseline characteristics were the most frequent sources of high risk of bias in non‐ITS studies, while most of the unclear risks stemmed from inadequacy of protection from contamination, inadequate reporting of baseline characteristics, and lack of clarity of random sequence generation.
Of the 10 ITS studies, Whitby 2008 contained one source of high risk of bias and one unclear risk of bias. All of the remaining studies contained two or three sources of high risk of bias. Inadequate blinding was the most frequent source of high risk of bias in ITS studies, while most of the unclear risks stemmed from lack of clarity related to whether the intervention was independent of other changes.
Allocation
Of the 16 studies reporting non‐ITS study designs, we considered the two non‐randomised trials, as in EPOC guidelines, to be at high risk of bias related to generation of allocation sequence (Diegel‐Vacek 2016; Moghnieh 2016). Four studies were at unclear risk of bias related to generation of allocation sequence because randomisation methods were not specified (Grant 2011; Huang 2002; Stevenson 2014; Yeung 2011). For the remainder, this risk of bias was low (Fisher 2013a; Fuller 2012; Ho 2012; Huis 2013; King 2016; Martin‐Madrazo 2012; Mertz 2010; Munoz‐Price 2014; Rodriguez 2015; Stewardson 2016). We rated three studies (Huang 2002, iMoghnieh 2016, Rodriguez 2015) at unclear risk of bias in terms of adequate concealment of allocation sequence; we judged the other 13 studies to be at low risk for this domain.
Blinding
We considered blinding of participants separately from blinding of outcome assessment.
Blinding of participants and personnel
Of the 16 studies reporting non‐ITS study designs, only one was at low risk of bias for blinding of participants to group allocation (Grant 2011). In this study staff were aware that signs to promote hand hygiene compliance had been positioned adjacent to sinks but were not aware that a research study was taking place. In the randomised trial with cross‐over reported by Munoz‐Price 2014, ABHR dispensers placed on the work surface were visible but staff did not know what was being assessed, so we considered risk of bias to be unclear. In King 2016, the participants would have noticed the signs and scent but the authors did not report whether the participants knew the purpose of the study.
In the remaining non‐ITS studies we rated risk of bias high through failure to blind staff and researchers to group allocation. In three studies (Ho 2012; Mertz 2010; Rodriguez 2015) posters and performance feedback were employed, so all staff knew about the intervention. In Martin‐Madrazo 2012 posters were displayed throughout all participating centres regardless of group allocation. In Yeung 2011 reminders to cleanse hands were given throughout all participating centres, regardless of group allocation. In the remaining studies blinding was impossible because campaigns were customised to the clinical setting (Stevenson 2014), the wireless technology used to promote hand hygiene was visible and audible (Fisher 2013a), the lights were visible and the participants aware of the purpose of the study (Diegel‐Vacek 2016), the intervention involved performance feedback and individualised action planning to improve hand hygiene (Fuller 2012; Stewardson 2016), the intervention was promoted by ward leaders (Huis 2013), the intervention involved an incentive or audit and feedback (Moghnieh 2016), or the education was very specific so participants were aware of the intervention (Huang 2002).
Of the 10 ITS studies we judged one to be at unclear risk of bias because insufficient information was provided in the study to draw conclusions (Derde 2014). Risks were high in the remaining ITS studies because of the changes introduced to collect the data or because the nature of the intervention indicated that hand hygiene compliance was being studied. In Armellino 2012, video cameras were installed to collect data. In the other ITS studies staff could have been alerted by extra AHBR stations being installed and the presence of the e‐learning hand hygiene game station (Higgins 2013), the appearance of posters and because it was apparent that managerial support was provided for the campaign (Lee 2013; Midturi 2015), use of incentives and feedback (Rosenbluth 2015), a care bundle to prevent MRSA was introduced and clinical leaders were involved in the delivery of the intervention (Perlin 2013), and pocket‐sized ABHR dispensers were introduced (Vernaz 2008). Leaders and staff were similarly involved in Talbot 2013 and by Whitby 2008.
Blinding of outcome assessment
Of the 16 studies reporting non‐ITS study designs, four were at low risk of bias because data collectors and staff were unaware of the outcome being assessed (Fuller 2012; Grant 2011; Martin‐Madrazo 2012; Moghnieh 2016). Eight were at high risk for different reasons, with three at high risk because no attempt was made to conceal the outcome assessment (Ho 2012; Munoz‐Price 2014; Yeung 2011). In one study with high risk of bias, researchers responsible for undertaking analysis were informed (Huis 2013), while in Fisher 2013a data collectors belonged to the research group and knew the outcome of interest. In three studies (King 2016; Stewardson 2016; Diegel‐Vacek 2016) scents and signs, posters, and lights, respectively would have been visible to the observers. In the other three studies risk was unclear, either because the authors did not discuss blinding (Huang 2002; Mertz 2010) or because observational data were collected by in‐house infection prevention staff (Stevenson 2014).
For three of the 10 ITS studies risk of bias was low because outcomes were assessed by AHBR usage (Perlin 2013; Vernaz 2008) or by an automated monitoring device (Whitby 2008). In the remaining ITS studies risks were high. In Armellino 2012 the video‐camera recordings were analysed by third‐party auditors who were not blind to the study outcomes, and in the ITS studies by Lee 2013, Midturi 2015, Rosenbluth 2015, and Talbot 2013, observers were not blinded. In Derde 2014, nurses from the study units were used as data collectors, while in Higgins 2013 the e‐learning hand hygiene game station was probably visible to data collectors.
Incomplete outcome data
Of the 16 studies reporting non‐ITS study designs, one study was at unclear risk of bias because of the difficulty of comparing loss to follow‐up given the different compositions of the groups (Fuller 2012). In the remaining non‐ITS studies (Diegel‐Vacek 2016; Fisher 2013a; Grant 2011; Ho 2012; Huang 2002; Huis 2013; King 2016; Martin‐Madrazo 2012; Mertz 2010; Moghnieh 2016; Munoz‐Price 2014; Rodriguez 2015; Stevenson 2014; Stewardson 2016; Yeung 2011) and in all 10 ITS studies (Armellino 2012; Derde 2014; Higgins 2013; Lee 2013; Midturi 2015; Perlin 2013; Rosenbluth 2015; Talbot 2013; Vernaz 2008; Whitby 2008) risk of bias was considered low, assuming that missed opportunities for hand hygiene were not different between each arm of the trial or study period, and because loss at follow‐up in a trial was minimal or addressed in the analysis.
Selective reporting
There was no evidence of selective reporting in any of the 16 studies reporting non‐ITS study designs or in any of the 10 ITS studies.
Other potential sources of bias
Similar baseline outcome measurements
Of the 16 studies reporting non‐ITS study designs, we rated five at unclear risk of bias. In Ho 2012 there were small differences in baseline hand hygiene compliance between groups. For four studies the risk was unclear because baseline outcome measures were not reported (Diegel‐Vacek 2016; Fuller 2012; Munoz‐Price 2014; Stevenson 2014). We considered risk of bias to be high in Rodriguez 2015, because there were differences between groups. For the remaining studies baseline measurements of outcomes were similar between groups, so we considered risk of bias to be low (Fisher 2013a; Grant 2011; Huang 2002; Huis 2013; King 2016; Martin‐Madrazo 2012; Mertz 2010; Moghnieh 2016; Stewardson 2016; Yeung 2011).
Similar baseline characteristics
Of the 16 studies reporting non‐ITS designs, we rated only three at low risk of bias because baseline characteristics were reported as similar (Huang 2002; Munoz‐Price 2014) or used a single unit (King 2016). We classified Ho 2012 and Rodriguez 2015 as having unclear risk of bias as there were minor differences in characteristics between groups, but the impact of such differences was unclear. Moghnieh 2016 and Stewardson 2016 reported baseline characteristics as similar but provided no supporting data. The remaining non‐ITS studies were all at high risk of bias. In Martin‐Madrazo 2012 types of healthcare workers were similar but the types of patients and baseline characteristics of the units in the healthcare centres participating in the different arms of the trial were not reported. In Mertz 2010, numbers of sinks and ABHR availability were similar but there was no comparison of characteristics between patients or staff. In the cluster‐randomised trial by Yeung 2011 there were more patients with severe disability in the test group and fewer sinks were available in the clinical areas where they received care. In six studies (Diegel‐Vacek 2016; Fisher 2013a; Fuller 2012; Grant 2011; Huis 2013; Stevenson 2014) no baseline characteristics were reported, so we judged the risk to be high in accordance with the EPOC criteria.
Adequate protection against contamination
Of the 16 studies reporting non‐ITS study designs there was adequate protection against contamination and we rated risk of bias as low in five studies because allocation was either by organisation (Ho 2012; Martin‐Madrazo 2012; Stevenson 2014) or by units in different hospitals (Fuller 2012; Huis 2013). We also judged risk of bias to be low in Grant 2011 because, although physicians and staff could have seen the different signs to promote hand hygiene displayed in different units, they were unaware that the research study was being conducted. We rated risk of bias as high in three studies (Diegel‐Vacek 2016; Mertz 2010; Munoz‐Price 2014). In the cross‐over randomised trial reported by Munoz‐Price 2014, there could have been a wash‐over effect among staff first allocated to the intervention group as they could have become primed to look for ABHR when it was no longer placed within reach. Similarly, staff may have become used to the light over the sink as a cue and changed behaviour when the light was not present (Diegel‐Vacek 2016). Mertz 2010 reported that contamination could have occurred in their study. In the remaining seven studies contamination was theoretically possible and we considered risks of bias to be unclear. In Fisher 2013a it was possible for information (audible bleep) to have been given to the test group, while in Huang 2002 nurses worked in the same hospital and could have communicated with one another. Yeung 2011 reported a high turnover of staff and as a result many in the intervention group did not in fact receive the intervention. Rodriguez 2015 and Stewardson 2016 reported that contamination could not be ruled out. It was unclear if staff in two studies moved within the unit or between units and may have been influenced by the intervention (King 2016; Moghnieh 2016).
Intervention independent of other changes (ITS)
In five ITS studies (Armellino 2012; Midturi 2015; Rosenbluth 2015; Talbot 2013; Whitby 2008) risk was unclear because there was no report concerning other events or activities (e.g. outbreaks, other campaigns, variations in staffing levels) that could have influenced findings while the research was in progress. The remaining ITS studies were at high risk for different reasons. In Higgins 2013 extra ABHR stations were added while the study was in progress and two separate interventions were ongoing at the same time: the multimodal intervention and the use of the e‐learning hand hygiene game station. Lee 2013 reported that mandatory MRSA screening was introduced while their study was in progress and although the intervention took place in 10 hospitals over a period of 25 months there were no reports of other events that could have influenced outcomes. Perlin 2013 reported that ABHR uptake varied in the different centres pre‐intervention and MRSA screening, isolation precautions and new policies for disinfection and cleaning were also introduced during that time. Two new infection prevention programmes were introduced throughout the seven years Vernaz 2008 was conducted. Multiple changes occurred related to MRSA screening and use of barrier and contact precautions during the course of Derde 2014.
Shape of intervention effect was prespecified (ITS)
Risk of bias was low in all 10 ITS studies because the point of analysis was the same as the point of intervention (Armellino 2012; Derde 2014; Higgins 2013; Lee 2013; Midturi 2015; Perlin 2013; Rosenbluth 2015; Talbot 2013; Vernaz 2008; Whitby 2008).
Intervention unlikely to affect data collection (ITS)
Risk of bias was low in all 10 ITS studies because the same methods of data collection were used pre‐ and post‐intervention (Armellino 2012; Derde 2014; Higgins 2013; Lee 2013; Midturi 2015; Perlin 2013; Rosenbluth 2015; Talbot 2013; Vernaz 2008; Whitby 2008).
Freedom from other risks of bias
Of the 16 studies reporting non‐ITS study designs, no evidence of further bias was apparent in 10 studies (Diegel‐Vacek 2016; Fisher 2013a; Fuller 2012; Grant 2011; Huang 2002; Moghnieh 2016; Munoz‐Price 2014; Rodriguez 2015; Stevenson 2014; Stewardson 2016). In Ho 2012 risk was unclear through possible selection bias: it was not clear which staff were invited to participate or refused. We rated risk of bias as unclear in King 2016 because it did not distinguish between healthcare workers and visitors, and it is unclear if their behaviour in response to the scent or signs would be different. The remaining studies contained high risk risks of bias. In two of these studies the research was reported to coincide with an outbreak of the respiratory virus H1N1 and the authors stated that additional measures were introduced to control spread (Huis 2013; Martin‐Madrazo 2012). In Mertz 2010, ABHR dispensers were installed throughout the hospital during the study period and there was an outbreak of MRSA that could have prompted efforts to improve hand hygiene. In Yeung 2011 a feedback session was delivered in the intervention and control groups three months after the intervention and at the conclusion of the study.
We judged the 10 ITS studies to be at low risk because we could identify no evidence of other risks of bias (Armellino 2012; Derde 2014; Higgins 2013; Lee 2013; Midturi 2015; Perlin 2013; Rosenbluth 2015; Talbot 2013; Vernaz 2008; Whitby 2008).
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
2.
| Multimodal interventions (not WHO‐based) compared with no intervention for promotion of hand hygiene or reduction of infection or colonisation rates | |||
|
Patient or population: Healthcare workers Settings: Long‐term care, primary care, hospital Intervention: Multimodal with some but not all of the strategies recommended by WHO; strategies varied by study Comparison: No hand hygiene promotion | |||
| Outcomes | Impact | Studies | Certainty of the evidence (GRADE) |
| Hand hygiene compliance | In the RCTs, the absolute differences in hand hygiene compliance compared to baseline ranged from 1.9 to 37.7 percentage points in intervention groups and from 0.3 to 11.9 in control groups. The ITS reported an adjusted OR of 1.19, 95% CI 1.01 to 1.42 favouring the intervention | 4 RCTs, 1 ITS 24 long‐term care facilities, 10 hospitals, 11 ICUs and 11 primary healthcare units |
⊕⊕⊝⊝ low1 |
| Infection rates | 1 RCT reported reduced respiratory outbreaks and MRSA infections requiring hospitalisation (IRR 0.12 to 0.61) favouring the intervention, while 1 ITS study reported no reduction in MRSA clinical isolates or infection. 1 RCT reported reductions of 0.27 to 0.77 cases per 1000 resident‐days in serious infections, pneumonia and death in the intervention group compared to no change or an increase of 0.57 cases per 1000 resident‐days in the control group | 2 RCT, 1 ITS 24 long‐term care facilities, 10 hospitals, |
⊕⊕⊝⊝ low2 |
| Colonisation rates | Not reported | ‐ | ‐ |
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: We are very uncertain about the estimate. Abbreviations: CI: confidence interval; ICU: intensive care unit; IRR: incidence rate ratio; ITS: interrupted time series; MRSA: methicillin‐resistant Staphylococcus aureus; OR: odds ratio; RCT: randomised (controlled) trial; WHO: World Health Organization | |||
1Evidence downgraded from high to low due to non‐randomised evidence (one of five studies); high risk of bias (all studies have two or more sources of bias), and inconsistency in effect sizes between studies and within multi‐unit studies. 2Evidence downgraded from high to low due to non‐randomised evidence (one of three studies), high risk of bias (all studies have two or more sources of high risk of bias), and (inconsistency in results with some studies reporting changes for some micro‐organisms but not others and 1 reporting no change.
3.
| WHO‐based multimodal interventions compared with some or no interventions for promotion of hand hygiene or reduction of infection or colonisation rates | |||
|
Patient or population: Healthcare workers Settings: Acute care hospitals Intervention: Multimodal with all five strategies recommended by WHO: ABHR at point of care, education, performance feedback, reminders, and administrative support. Comparison: Varied by study | |||
| Outcomes | Impact | Studies | Certainty of the evidence (GRADE) |
| Hand hygiene compliance | The absolute difference in hand hygiene compliance between intervention and control group was 6.3 percentage points in the RCT. One ITS reported a difference of 17 percentage points in hand hygiene compliance compared to baseline, while another ITS reported no change on medicine units and a RR of 1.56, 95% CI 1.29 to 1.89 in IDUs favouring intervention. One ITS in a multistate system reported an increase of 27.45 ounces of ABHR per adjusted bed‐day. One ITS did not report estimates of change | 1 RCT, 4 ITS 1 multistate system with 166 hospitals, 5 hospitals and 13 ICUs |
⊕⊝⊝⊝ very low1 |
| Infection rates | 1 ITS reported a decrease in blood stream infections of 0.191 cases per 1000 line‐days and a decrease in ventilator‐associated pneumonia of 0.538 cases per 1000 ventilator days. 1 ITS reported that MRSA decreased by 0.03 clinical isolates for each litre of ABHR per 100 patient‐days but there was no change in C. difficile | 2 ITS 3 hospitals and 13 ICUs |
⊕⊝⊝⊝ very low2 |
| Colonisation rates | 1 RCT reported no difference in MRSA colonisation. 1 ITS reported a slight decrease in MRSA acquisition (IRR 0.976 favouring intervention) but no change in VRE or HRE acquisition. | 1 RCT, 1 ITS 1 multistate system with 166 hospitals, 1 hospital |
⊕⊕⊝⊝ low3 |
| GRADE Working Group grades of evidence High certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very certainty: We are very uncertain about the estimate. Abbreviations: ABHR: alcohol‐based hand rub; C. difficile: Clostridium difficile; CI: confidence interval; HRE: highly‐resistant Enterobacteriaceae; ICU: intensive care unit; IDU: immunisation and diagnosis unit; ; ITS: interrupted time series; MRSA: methicillin‐resistant Staphylococcus aureus; RCT: randomised (controlled) trial; RR: risk ratio; VRE: vancomycin‐resistant enterococci; WHO: World Health Organization | |||
1Evidence downgraded from high to very low due to non‐randomised evidence (four of five studies); high risk of bias (four of five studies have two or more sources of high risk of bias), and inconsistency in effect sizes between studies and within multi‐unit studies. 2Evidence downgraded from high to very low due to non‐randomised evidence (two studies), high risk of bias (studies have two or more sources of high risk of bias), and inconsistency in effect sizes between studies and within multi‐unit studies. 3Evidence downgraded from high to low due to non‐randomised evidence (one of two studies), high risk of bias (both studies have two or more sources of high risk of bias), and inconsistency in results with one study reporting changes for some microorganisms but not others and the other reporting no change.
4.
| WHO‐enhanced multimodal interventions compared with some or no interventions for promoting hand hygiene | |||
|
Patient or population: Healthcare workers Settings: Acute care hospitals Intervention: Multimodal with all of the strategies recommended by WHO, plus additional interventions. Comparison: Varied by study | |||
| Outcomes | Impact | Studies | Certainty of the evidence (GRADE) |
| Hand hygiene compliance | 1 RCT and one ITS reported an increase in hand hygiene compliance with RR of 1.48 to 1.64 favouring intervention. 1 RCT reported increases in hand hygiene compliance of 20.1 to 28.4 percentage points in the intervention group compared to a decrease of 0.7 to 3.1 in the control. 1 ITS reported an increase in hand hygiene compliance of 2% per month during the intervention compared to < 1% a month before and after the intervention, while another ITS reported hand hygiene compliance of 83% ‐ 95% post‐intervention compared to 38% ‐ 100% at baseline, with variation by unit. 1 ITS did not report estimates of change | 2 RCTs, 4 ITS 15 hospitals |
⊕⊕⊝⊝ low1 |
| Infection rates | 1 ITS reported no change in MRSA clinical isolates or in C. difficile | 1 ITS 1 hospital |
⊕⊝⊝⊝ very low2 |
| Colonisation rates | Not reported | ‐ | ‐ |
| GRADE Working Group grades of evidence
High certainty: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: We are very uncertain about the estimate. Abbreviations:C. difficile: Clostridium difficile; ITS: interrupted time series; MRSA: methicillin‐resistant Staphylococcus aureus; RCT: randomised (controlled) trial; RR: risk ratio; WHO: World Health Organization | |||
1Evidence downgraded from high to low due to non‐randomised evidence (four of six studies; high risk of bias (five of six studies have two or more sources of high risk of bias), and inconsistency in effect sizes between studies and within multi‐unit studies.
2Evidence downgraded from high to very low due to non‐randomised evidence and high risk of bias (two sources of high risk of bias).
5.
| Performance feedback compared with some or no interventions for promoting hand hygiene | |||
|
Patient or population: Healthcare workers Settings: Acute care hospitals Intervention: Feedback with additional strategies such as focus on leadership; varied by study Comparison: Varied by study | |||
| Outcomes | Impact | Studies | Certainty of the evidence (GRADE) |
| Observed hand hygiene compliance | 1 RCT and 1 NRCT reported increases in hand hygiene compliance of 0 ‐ 61 percentage points in intervention groups compared to no changes or a slight decrease of 4 percentage points in control groups. 2 RCTs reported ORs of 1.61 to 2.09 favouring intervention. 1 ITS reported a weekly increase in hand hygiene compliance of 4% after an initial increase of 17.5%, while 1 ITS reported an increase of 37 percentage points during the active accountability phase of the study | 3 RCTs, 1 NRCT, 2 ITS 21 hospitals |
⊕⊕⊝⊝ low1 |
| Infection rates | 1 RCT reported reduced primary bloodstream infection in the enhanced feedback group (0.71, 95% CI 0.54 to 0.95) and control group (0.57, 95% CI 0.40 to 0.80) with little change in the enhanced feedback + patient participation group (1.02, 95% CI 0.78 to 1.34). Period prevalence of HCAIs was also reduced in the enhanced feedback group (0.91, 95% CI 0.68 to 1.23), with little change in the enhanced feedback + patient participation group (1.05, 95% CI 0.78 to 1.40) and an increase in the control group (1.33, 95% CI 0.94 to 1.88) | 1 RCT 1 hospital |
⊕⊕⊕⊝ moderate2 |
| Colonisation rates | 1 RCT reported reduced colonisation with MRSA in the enhanced feedback group (0.79, 95% CI 0.66 to 0.95) and the enhanced feedback + patient participation group (0.82, 95% CI 0.67 to 0.99), as well as in the control group (0.92, 95% CI 0.77 to 1.13) | 1 RCT 1 hospital |
⊕⊕⊕⊝ moderate2 |
| GRADE Working Group grades of evidence
High certainty: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: We are very uncertain about the estimate. Abbreviations: CI: confidence interval; HCAIs: healthcare‐associated infections; ITS: interrupted time series; MRSA: methicillin‐resistant Staphylococcus aureus; NRCT: non‐randomised (controlled) trial; OR: odds ratio; RCT: randomised (controlled) trial | |||
1Evidence downgraded from high to low due to non‐randomised evidence (three of six studies); high risk of bias (two or more sources in all studies), and inconsistency in effect sizes between studies and within multi‐unit studies. 2Evidence downgraded from high to moderate due to high risk of bias (two sources), and inconsistency in effect sizes within the study.
6.
| Education compared with no education for promotion of hand hygiene | |||
|
Patient or population: Healthcare workers Settings: Acute care hospitals Intervention: Education; content and delivery methods varied by study Comparison: No education | |||
| Outcomes | Impact | Studies | Certainty of the evidence (GRADE) |
| Observed hand hygiene compliance | 1 RCT reported increases of 16.3 to 24.5 percentage points in the proportion of nurses in the intervention group who complied with recommendations for hand hygiene, depending on moment of hand hygiene evaluated, compared to no changes or a decrease of 4.1 percentage points in the control group. 1 ITS reported an increase in hand hygiene compliance as a proportion of opportunities of 42 percentage points | 1RCT and 1 ITS 2 hospitals |
⊕⊕⊝⊝ low1 |
| Infection rates | Not reported. | ‐ | ‐ |
| Colonisation rates | Not reported. | ‐ | ‐ |
| GRADE Working Group grades of evidence
High certainty: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: We are very uncertain about the estimate. Abbreviations: ITS: interrupted time series; RCT: randomised (controlled) trial | |||
1Evidence downgraded from high to low due to non‐randomised evidence (one of two studies); and risk of bias (high and unclear).
7.
| Cues compared with no cue or different cue for promotion of hand hygiene | |||
|
Patient or population: Healthcare workers Settings: Acute care hospitals Intervention: Signs or scent as cue Comparison: No cue or different signs | |||
| Outcomes | Impact | No of Participants (studies) | Certainty of the evidence (GRADE) |
| Observed hand hygiene compliance | 1 RCT reported an increase in hand hygiene of 8.51 percentage points for the patient consequences sign compared to a slight decrease of 0.29 percentage points for the personal consequences sign. 1 RCT reported increases in hand hygiene compliance of 31.9 and 6.7 percentage points for the scent cue and sign of stern male eyes respectively, and a decrease of 5 percentage points for the sign with female eyes. One NRCT reported an increase of 7 percentage points in hand hygiene compliance with the light cue on day 2 compared to 9 percentage points with no light cue, whereas on day 3 compared to day 1 there was no difference with the light cue and an increase of 16 percentage points with no light cue | 2 RCTs, 1 NRCT 3 hospitals |
⊕⊕⊝⊝ low1 |
| Infection rates | Not reported | ‐ | ‐ |
| Colonisation rates | Not reported | ‐ | ‐ |
| GRADE Working Group grades of evidence
High certainty: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: We are very uncertain about the estimate. Abbreviations: NRCT: non‐randomised (controlled) trial; RCT: randomised (controlled) trial | |||
1Evidence downgraded from high to low due to non‐randomised evidence (one of three studies); risk of bias (all studies have two or more sources of high risk of bias), and inconsistency in effect sizes between studies.
8.
| Placement of ABHR on cart compared with placement of ABHR on wall for promotion of hand hygiene | |||
|
Patient or population: Anaesthesiologists and CRNAs Settings: Acute care surgical Intervention: Placement of ABHR on anaesthesia cart Comparison: Placement of ABHR on wall of anaesthesia room | |||
| Outcomes | Impact | No of Participants (studies) | Certainty of the evidence (GRADE) |
| Observed hand hygiene compliance | 1 RCT reported an increase of 0.3 hand hygiene events an hour in the intervention group compared to the control group | 1 RCT 1 hospital |
⊕⊕⊕⊝ moderate1 |
| Infection rates | Not reported | ‐ | ‐ |
| Colonisation rates | Not reported | ‐ | ‐ |
| GRADE Working Group grades of evidence
High certainty: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low certainty: We are very uncertain about the estimate. Abbreviations: ABHR: alcohol‐based hand rub; CRNA: certified registered nurse anaesthetist; RCT: randomised (controlled) trial | |||
1Evidence downgraded from high to moderate due to high risk for bias (two sources of high risk and two sources of unclear risk ).
We present an overview of the effects of the interventions in Table 1, while the evidence from specific types of interventions is summarised in Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; and Table 8. Table 9 and Table 10 contain details of key results for all interventions and hand hygiene outcome measures. Table 12 summarises the results related to infection or colonisation.
4. Results from studies reporting microbiological data.
| Study |
Design/ Intervention |
Results |
| Intervention: Multimodal, not WHO | ||
| Ho 2012 | RCT | · Reduced respiratory outbreaks: IRR 0.12, 95% CI 0.01 to 0.93 · Reduced MRSA infections requiring hospitalisation: IRR 0.61, 95% CI 0.38 to 0.97 |
| Lee 2013 | ITS | No reduction related to the hand hygiene promotion campaign alone in: · MRSA in clinical isolates: IRR 1.44, 95% CI 0.96 to 2.15 · MRSA infections: IRR 1.28, 95% CI 0.79 to 2.06 |
| Yeung 2011 | RCT | Reduced serious infections (cases per 1000 resident‐days): · Intervention group: pre: 1.42; post: 0.65 (difference: ‐0.77) · Control groups: pre: 0.49; post: 1.05 (difference: 0.56) Reduced pneumonia (cases per 1000 resident‐days) · Intervention group: pre: 0.91; post: 0.28 (difference: ‐0.63) · Control group: no change Reduced deaths per 1000 resident‐days: · Intervention group: pre: 0.37; post: 0.10 (difference: ‐0.27) · Control group: no change |
| Intervention: Multimodal, WHO based | ||
| Derde 2014 | ITS | · Trend in MRSA acquisition following hand hygiene campaign: IRR 0.976, 95% CI 0.954 to 0.999; · No changes in acquisition of VRE or HRE |
| Mertz 2010 | RCT | No difference in MRSA colonisation (cases per 1000 patient‐days): · Intervention group: 0.30 · Control group: 0.31 |
| Perlin 2013 | ITS | MRSA CLABSI per 1000 line days: · Pre: .497 (difference: ‐0.191) · Post: .306 MRSA VAP per 1000 ventilator days: · Pre: 1.088 (difference: ‐0.538) · Post: 0.550 |
| Vernaz 2008 | ITS | · MRSA decreased by 0.03 clinical isolates per 100 patient‐days for each litre of ABHR per 100 patient‐days · No change in C. difficile |
| Intervention: Multimodal, WHO‐enhanced | ||
| Vernaz 2008 | ITS | · No change in MRSA clinical isolates · No change in C. difficile |
| Intervention: Performance feedback | ||
| Stewardson 2016 | RCT | Primary bloodstream infection · Enhanced feedback: IRR 0.71, 95% CI 0.54 to 0.95 · Enhanced feedback + patient participation: IRR 1.02, 95% CI 0.78 to 1.34 · Control: IRR 0.57, 95% CI 0.40 to 0.80 Period prevalence of HCAIs · Enhanced feedback: IRR 0.91, 95% CI 0.68 to 1.23 · Enhanced feedback + patient participation: IRR 1.05, 95% CI 0.78 to 1.40 · Control: IRR 1.33, 95% CI 0.94 to 1.88 Colonisation with MRSA · Enhanced feedback: IRR 0.79, 95% CI 0.66 to 0.95 · Enhanced feedback + patient participation: IRR 0.82, 95% CI 0.67 to 0.99 · Control: IRR 0.92, 95% CI 0.77 to 1.13 |
AHBR: alcohol‐based hand rub; C. difficile: Clostridium difficile; CLABSI: central line‐associated blood stream infections; CI: confidence interval; HCAI: healthcare‐associated infection; HRE: highly‐resistant Enterobacteriaceae; IRR: incidence rate ratio; ITS: interrupted time series; MSRA: methicillin‐resistant Staphylococcus aureus; RCT: randomised (controlled) trial; VAP: ventilator‐associated pneumonia; VRE: vancomycin‐resistant enterococci; WHO: World Health Organization
As described in the following sections, overall, hand hygiene compliance increased in all studies, regardless of the intervention or the outcome measure employed. The level of increase varied, however, as did the level of hand hygiene compliance both at baseline and post‐intervention. Some studies reported reduction in infections (Ho 2012; Perlin 2013; Stewardson 2016; Yeung 2011) or mortality (Yeung 2011) while other studies reported no changes (Lee 2013) or a variation depending on the micro‐organism (Vernaz 2008). Three studies reported little or no changes in colonisation rates (Derde 2014; Mertz 2010; Stewardson 2016).
Appropriate statistical analysis was conducted in 11 of the 16 non‐ITS studies (Diegel‐Vacek 2016; Fuller 2012; Huang 2002; Huis 2013; King 2016; Mertz 2010; Moghnieh 2016; Munoz‐Price 2014; Rodriguez 2015; Stevenson 2014; Stewardson 2016) and in five of the 10 ITS studies (Armellino 2012; Lee 2013; Talbot 2013; Vernaz 2008; Whitby 2008). The nature of the inappropriate analysis is identified in the Characteristics of included studies table as well as in Table 9 and Table 10. Reasons varied for why we considered the analysis inappropriate. Grant 2011 did not conduct a matched analysis, while two cluster‐randomised trials analysed data at the level of individuals rather than clusters (Martin‐Madrazo 2012; Yeung 2011). Several studies did not conduct analyses appropriate for an ITS (Derde 2014; Higgins 2013; Perlin 2013; Rosenbluth 2015) while the reporting of results was unclear in other studies (Fisher 2013a; Midturi 2015). Ho 2012 used a generalised estimating equation (GEE) but did not compare changes between arms.
Re‐analysis of data from studies with inappropriate analysis was either not possible through lack of available data or not warranted, given the high risk of bias in the studies. However, where researchers did not report differences, the review authors calculated them based on available data and reported in Table 9 and Table 10.
Multimodal Campaigns
Fourteen studies evaluated multimodal campaigns, with two evaluating two different types of multimodal campaigns (Vernaz 2008; Whitby 2008). Overall, multimodal interventions led to an increase in hand hygiene compliance, with very low to low certainty of evidence, depending on the components of the multimodal intervention, i.e. whether they were not WHO‐based, WHO‐based, or WHO‐enhanced, as shown in Table 2; Table 3; Table 4. Table 9 summarises the key results. Outcomes for different measures of hand hygiene compliance are detailed, with both observed hand hygiene compliance or proxy measures considered together in determining the GRADE rating.
Multimodal campaigns not based on WHO recommendations
Five of the studies evaluated multimodal campaigns that did not contain all of the elements recommended by WHO (Ho 2012; Lee 2013; Martin‐Madrazo 2012; Rodriguez 2015; Yeung 2011). All showed improvements in hand hygiene compliance (low certainty of evidence) as shown in Table 2. All five measured observed hand hygiene compliance as the outcome; the four non‐ITS studies compared results of the intervention group to results from controls who had either received no intervention or education on another topic. Rodriguez 2015 reported on a stepped‐wedge randomised trial and used appropriate statistical analysis. They found an increase in observed hand hygiene compliance (odds ratio (OR) 1.17, 95% confidence interval (CI) 1.13 to 1.22), with absolute differences ranging from 1.9 to 26.7 percentage points. The other three studies were cluster‐randomised trials with none adopting appropriate statistical analysis: two analysed at the level of the individual rather than the cluster (Martin‐Madrazo 2012; Yeung 2011) and the other failed to compare changes between the arms of the trial (Ho 2012). However, all three studies showed increases in hand hygiene compliance in the intervention groups compared both to the control groups and to baseline levels. Baseline rates were low, ranging from 7.98% to 27%; post‐intervention rates in the intervention groups ranged from 32.74% to 48.6%. The absolute differences in hand hygiene compliance compared to baseline ranged from 1.9 to 37.7 percentage points in the intervention groups and from 0.3 to 11.9 percentage points in the control groups. Results varied by study and by time period, as none had the same post‐intervention assessment interval. The ITS study Lee 2013 reported appropriate statistical analysis. This team demonstrated increases after the start of hand hygiene promotion (OR 1.19, 95% CI 1.01 to 1.42) with monthly decreases after the campaign ended. The highest levels of hand hygiene compliance were observed during the intervention phase, with an average compliance of 63.8% in the intervention group compared to 49.3% at baseline.
Three of the studies also reported reduced infection (low certainty of evidence); none reported colonisation rates. Ho 2012 reported reduced respiratory outbreaks (incidence rate ratio (IRR) 0.12, 95% CI 0.01 to 0.93) and reduced MRSA infections requiring hospitalisation (IRR 0.61, 95% CI 0.38 to 0.97) favouring the intervention. Yeung 2011 reported reductions of 0.27 to 0.77 cases per 1000 resident‐days in serious infections, pneumonia and death in the intervention group compared to no change or an increase of 0.57 cases per 1000 resident‐days in the control group. In the ITS study Lee 2013, promotion of hand hygiene was incorporated into a bundle of strategies for the control of MRSA, so hand hygiene was a secondary outcome. Lee 2013 found that the bundle, rather than hand hygiene promotion alone or the other strategies without hand hygiene promotion, led to a decrease in MRSA isolated from clinical cultures.
Multimodal campaigns based on WHO recommendations
Table 3 summarises the five studies that evaluated WHO‐based campaigns that contained the five elements recommended by WHO (Derde 2014; Mertz 2010; Perlin 2013; Vernaz 2008; Whitby 2008). in the cluster‐randomised trial Mertz 2010, the multimodal intervention was compared to the addition of ABHR alone; each of the other studies was an ITS. All found an increase in hand hygiene compliance (very low certainty of evidence), regardless of whether hand hygiene compliance was assessed through observation or a proxy measure. Mertz 2010 and Derde 2014 both assessed observed hand hygiene compliance. Mertz 2010 reported an increase of 6.3 percentage points in the mean difference between groups at post‐test. Derde 2014 reported an increase in hand hygiene compliance from a baseline rate of 52% to 69%, a difference of 17 percentage points, after the campaign but did not conduct statistical analysis appropriate to an ITS study. The other three studies used different outcome measures. Whitby 2008 employed an electronic count of the number of times ABHR was dispensed, and reported an increase for the Geneva programme in hand hygiene compliance relative to baseline in immunisation and diagnosis units (IDUs) (risk ratio (RR) 1.56, 95% CI 1.29 to 1.89), but no increase on medical units. Vernaz 2008 measured ABHR in litres per 100 patient‐days and reported increases in use for the Clean Care is Safer Care programme but did not report volume changes. Perlin 2013 also measured volume of ABHR but used mean ounces of ABHR per adjusted patient‐day, so the results are not readily comparable to those reported by Vernaz 2008. Perlin 2013 reported an increase of 24.75 ounces of ABHR per adjusted bed‐day but did not conduct statistical analysis appropriate to an ITS study.
Two of the studies also reported effects of the intervention on infection rates (very low certainty of evidence). Both reported hand hygiene interventions as part of a bundle for control of specific infections. Perlin 2013 reported a decrease in central line‐associated blood stream infections (CLABSI) of 0.191 cases per 1000 line‐days, and a decrease of 0.538 cases of ventilator‐associated pneumonia (VAP) per 1000 ventilator‐days with the bundle, but did not conduct an appropriate statistical analysis for an ITS study, so results must be interpreted with caution. Vernaz 2008 reported that the WHO‐based campaign was associated with a decreased incidence of 0.03 clinical MRSA isolates for each litre of ABHR per 100 patient‐days but there was no effect on C. difficile rates.
Two studies reported colonisation rates (low certainty of evidence). Derde 2014 found that increasing hand hygiene compliance alone accounted for a trend towards reduction of MRSA acquisition (IRR 0.976, 95% CI 0.954 to 0.999) but neither hand hygiene alone nor the bundle of interventions had any effect on incidence of C. difficile or HRE. Mertz 2010 reported no difference in the incidence of MRSA colonisation, with 0.30 and 0.31 cases per 1000 patient days in the intervention and control groups respectively, but suggested that this might be because of the low hand hygiene compliance levels achieved post‐intervention (42% to 48%).
Multimodal campaigns with WHO enhanced recommendations
Six studies evaluated WHO‐enhanced campaigns, summarised in Table 4; all found an increase in hand hygiene compliance (low certainty of evidence). Two of the studies were randomised trials. Stevenson 2014 reported an increase in observed hand hygiene compliance with mean differences of 20.1 to 28.4 percentage points, depending on which of the moments for hand hygiene was assessed. In comparison, there was a very small average mean difference in the control group (0.7 to 3.1 percentage points) where no intervention was received. Huis 2013 also measured observed hand hygiene compliance and reported an OR of 1.64, 95% CI 1.33 to 2.02 when leadership support was enhanced compared to a state‐of‐the‐art health promotion campaign. Huis 2013 reported that hand hygiene compliance was the same immediately post‐intervention and six months later (53%) for the intervention group receiving leadership support, while there was a slight increase in the control group which had a state‐of‐the‐art multimodal campaign (42% post‐intervention and 46% at six months).
The other four studies used an ITS design. Vernaz 2008 and Whitby 2008, in addition to evaluating WHO‐based campaigns, also evaluated WHO‐enhanced campaigns that contained all of the elements recommended by WHO plus some additional elements. Whitby 2008 used an electronic count of the number of times ABHR was dispensed, and reported an increase for the Washington programme in hand hygiene relative to baseline (RR 1.48, 95% CI 1.2 to 1.81). In the Washington programme the intervention was customised to meet the requests of staff employed on the participating units. Unlike the results for the Geneva programme, which was WHO‐based, there was no difference between medical units and the IDUs. Vernaz 2008 reported an increase in ABHR use (litres per 100 patient‐days) for the VigiGerme programme which incorporated social marketing but did not report volume changes. Midturi 2015 reported an average increase in observed hand hygiene compliance of 2% per month during the intervention, with less than 1% per month before and after the intervention, while Rosenbluth 2015 reported that hand hygiene compliance ranged from 85% to 92% during the intervention, compared to 38% to 100% before the intervention. Neither Midturi 2015 nor Rosenbluth 2015 used appropriate statistical analysis for an ITS.
Only one study reported on infection rates; Vernaz 2008 reported that there was no change in MRSA clinical isolates or in C. difficile with the Vigigerme campaign (very low certainty of evidence). None of the studies reported on colonisation.
Performance feedback
Table 10 summarises key results from the 12 studies that evaluated interventions other than multimodal campaigns. Six studies evaluated interventions with a major emphasis on performance feedback. All reported increased hand hygiene compliance (low certainty of evidence) as shown in Table 5. Three of the studies were randomised trials. Fisher 2013a evaluated wireless monitoring with performance feedback of hand hygiene compliance and reported slight increases of 0 to 5 percentage points on both entry to and exit from patients’ rooms, compared to slight decreases of 2 to 4 percentage points in the control group who received no intervention. There was variation according to type of ward, occupational group, and opportunity for hand hygiene but the regression results were not clearly reported. Fuller 2012 focused on feedback and personalised action planning. This team reported increases in both the relative use of liquid soap and hand hygiene compliance documented by direct observation. They found an absolute increase in hand hygiene compliance from 10% to 18% (ORs 1.67 to 2.09) compared to baseline rates during the national Clean Your Hands campaign in England and Wales, with variation according to type of ward and baseline rates. Stewardson 2016 reported that observed hand hygiene was only slightly higher in the groups with performance feedback (75%) and with feedback and patient participation (77%) during the intervention compared to the control group (73%), although all groups improved over baseline (66%). Absolute changes in the intervention period compared to baseline were similar: 10% in the performance feedback group (OR 1.61, 95% CI 1.41 to 1.84) and 11% in the feedback and patient participation group (OR 1.73, 95% CI 1.51 to 1.98). Both changes were slightly higher than the absolute change of 7 percentage points in the control group (OR 1.41, 95% CI 1.21 to 1.63).
One study was a non‐randomised trial. Moghnieh 2016 reported observed hand hygiene compliance rates of 43% at week eight and 51% at week 14 for the audit and feedback group compared to 16% to 20% at baseline; rates were unchanged for the control group but higher in the group receiving an incentive at both week eight (60%) and week 14 (77%). Differences between baseline and week eight ranged from 23% to 44% in the intervention groups and between 31% and 61% between baseline and week 14. Each of the other studies was an ITS. Armellino 2012 reported increases in hand hygiene compliance with video‐recording and performance feedback to staff with an average increase of 4% weekly after an initial increase of 17%. Hand hygiene compliance ranged from 3.5% to 9.8% at baseline to an average of 81.6% (weekly range 30.8% to 91.2%) in the post‐feedback period. Talbot 2013 reported an increase in hand hygiene compliance following a programme focused on performance feedback, leadership goal setting, financial incentives to the centre and marketing, but did not report estimates of effect. Only Talbot 2013 reported a sustained and high level of hand hygiene compliance in the “active accountability phase” of their study, when strategies to increase staff accountability for hand hygiene had been incorporated in practice. Hand hygiene compliance was 89% in the "active accountability phase" and 75% during the intervention phase compared to 52% at baseline, with differences of 37 and 23 percentage points respectively.
Only one study reported on both infection rates and colonisation rates (moderate certainty of evidence). Stewardson 2016 reported reduced primary bloodstream infection in the enhanced feedback group (IRR 0.71, 95% CI 0.54 to 0.95) and control group (IRR 0.57, 95% CI 0.40 to 0.80) with little change in the enhanced feedback + patient participation group (IRR 1.02, 95% CI 0.78 to 1.34). Period prevalence of HCAIs was also reduced in the enhanced feedback group (IRR 0.91, 95% CI 0.68 to 1.23), with little change in the enhanced feedback + patient participation group (IRR 1.05, 95% CI 0.78 to 1.40) and an increase in the control group (IRR 1.33, 95% CI 0.94 to 0.1.88). They also reported reduced colonisation with MRSA in the enhanced feedback group (IRR 0.79, 95% CI 0.66 to 0.95) and the enhanced feedback + patient participation group (IRR 0.82, 95% CI 0.67 to 0.99), as well as in the control group (IRR 0.92, 95% CI, 0.77 to 1.13).
Education
Two studies evaluated educational interventions. Both reported increased hand hygiene compliance (low certainty of evidence) as summarised in Table 6. Huang 2002 reported increases of 16.3 to 24.5 percentage points in the proportion of nurses complying with hand hygiene recommendations following an educational intervention compared to no changes or a decrease of 4.1 percentage points in those without the intervention. Differences varied by moment of hand hygiene evaluated. They did not assess the appropriateness of the hand hygiene event or if hand hygiene opportunities were missed. Higgins 2013 reported that hand hygiene compliance doubled from 42% to 84%, after an intervention implementing an e‐learning hand hygiene game, compared to no intervention, but did not provide details of analysis. Neither study reported on infection or colonisation rates.
Cues
Three studies focused on cues. All reported increased hand hygiene compliance (low certainty of evidence) as shown in Table 7 none reported on infection or colonisation rates. Grant 2011 reported an increase of 8.51% in observed hand hygiene compliance on units where a sign with a message relating to patient consequences of poor hand hygiene was displayed, compared to a slight decrease of 0.29% on units where a sign with a message related to staff‐related consequences of poor hand hygiene was posted. They did not undertake the matched analysis that would have been most appropriate for their pair‐matched cluster‐randomised trial design. Compared to a baseline of 15%, King 2016 reported an increase in observed hand hygiene compliance of 31.9 percentage points (to 46%) with the scent cue, compared to an increase of 6.7 percentage points (to 21.7%) with a sign of stern male eyes, and a decrease of 5 percentage points (to 10%) with a sign of female eyes. Diegel‐Vacek 2016 found that observed hand hygiene compliance was higher on day 1 and day 2 with the light cue (23% and 30%) compared to no light cue (7% and 16%), but was the same on the third day with or without the light cue (23%).The differences from day 1 to day 2 were an increase of 7 percentage points with the light cue compared to 9 percentage points without the light cue,. On day 3, there was no difference compared to day 1 with the light cue, and an increase of 16 percentage points without the light cue.
None of the studies reported on infection or colonisation rates. However, Grant 2011 estimated potential savings of USD 300,000 a year in terms of infections prevented by improved hand hygiene, but did not report infection data.
Placement of ABHR
The remaining study focused on the single intervention of ABHR placement. As displayed in Table 8, Munoz‐Price 2014 reported an increase of 0.3 hand hygiene events an hour (moderate certainty of evidence) when ABHR dispensers were placed on anaesthesia carts compared to usual placement on wall‐mounted dispensers. They did not report on infection or colonisation rates.
Discussion
Summary of main results
Twenty‐six studies met the criteria for inclusion and reported on eight different types of interventions. All reported on hand hygiene compliance, although measures differed; some also reported either infection or colonisation rates. The main results are displayed in Table 1.
Multimodal interventions were evaluated by 14 studies. Multimodal interventions that include some but not all strategies recommended in the WHO guidelines, or that contain all recommended strategies plus additional strategies, may slightly improve hand hygiene compliance (low certainty of evidence; 11 studies). In contrast, it is uncertain whether multimodal interventions that include all strategies recommended in the WHO guidelines improve hand hygiene compliance, because the certainty of this evidence is very low (five studies). Multimodal interventions that include some but not all strategies recommended in the WHO guidelines may also slightly reduce infection rates (low certainty of evidence; three studies). It is uncertain whether multimodal interventions that include all strategies recommended in the WHO guidelines, or that contain all recommended strategies plus additional strategies, reduce infection rates because the certainty of this evidence is very low (three studies). Lack of impact of interventions on C. difficile is unsurprising, as alcohol does not destroy the spores produced by this bacterium. Multimodal interventions that include all strategies recommended in the WHO guidelines may lead to little or no difference in MRSA colonisation rates (low certainty of evidence; two studies).
We found considerable variation in hand hygiene compliance, regardless of type of intervention, the outcome measure used, the study design, or the setting. Most increases in hand hygiene compliance in intervention groups were small (less than 20% in observed compliance), although larger than the increases seen in the control groups. One ITS study reported a RR of 1.56 favouring the WHO‐based intervention, and one ITS and one randomised trial reported RRs of 1.48 to 1.64 favouring the WHO‐enhanced intervention. Similarly, there was variation in the types of infections evaluated. MRSA was the micro‐organism most commonly reported, with inconsistency in whether or not there were decreases, regardless of the type of intervention.
While the variation in results makes it difficult to draw conclusions about the effectiveness of the different types of multimodal interventions, the variation in the interventions was appropriate, as the WHO Guidelines 2009 are intended to be adapted to the local context.
Performance feedback was evaluated by six studies, with considerable variation in the nature and delivery of performance feedback. Performance feedback may improve hand hygiene compliance (low certainty of evidence; six studies). Some of the differences seen were as large as 37% to 61%, with ORs of 1.61 to 2.09 favouring the intervention. Performance feedback probably slightly reduces infection and colonisation rates (moderate certainty of evidence; one study).
Education may improve hand hygiene compliance (low certainty of evidence; two studies). Differences ranged from 16.3 to 42 percentage points, although different outcome measures were used. Cues, such as signs or scent, may slightly improve hand hygiene compliance (low certainty of evidence; three studies). There was considerable variation in results, with the scent cue resulting in greater increases in hand hygiene compliance than signs or light cues. Placement of ABHR close to the point of use probably slightly improves hand hygiene compliance (moderate certainty of evidence; one study).
In summary, because of the heterogeneity in the interventions, samples and outcome measures, inappropriate statistical analysis in a number of studies, and the limited number of studies evaluating a given intervention using similar measures, it is difficult to draw a clear conclusion about the effectiveness of different interventions, whether implemented as a single intervention or in combination. Given the variability of results between and within studies, we can draw no conclusion about which interventions or combination of interventions lead to clinically important improvements in hand hygiene compliance or reductions in infection or colonisation rates, and under what circumstances. In the studies that evaluated bundles for MRSA control, the role of hand hygiene could not be disentangled from the effects of other interventions. It does appear, however, that all interventions can potentially lead to some improvement in hand hygiene compliance. The main harm that may occur would be the use of resources that might have been directed elsewhere.
Overall completeness and applicability of evidence
The evidence is relevant to the primary and secondary review questions, namely to examine the effectiveness of different strategies to improve hand hygiene compliance in patient care and to reduce infection, respectively. Further research examining similar interventions in similar groups of participants, using robust methods and appropriate statistical analysis, will contribute to greater certainty of evidence (discussed in the next section). The results of this review, however, indicate that there is sufficient evidence to justify taking actions to improve hand hygiene, even though it is not yet clear which strategies would be the most useful for a specific context or whether a single intervention rather than a multimodal intervention would be sufficient for specific situations or clinical settings.
The review fits into the context of current practice because of the emphasis that hand hygiene continues to receive as an important component of infection prevention programmes in health care, international and national guidelines, and other regulations (e.g. accreditation standards in many countries). Considerable effort is put into promoting hand hygiene and monitoring hand hygiene performance and it is thus important to be able to identify effective practices. According to the WHO Guidelines 2009, it is appropriate for clinical settings to individualise strategies to their context. Leadership is crucial for infection prevention and control, but is not enough on its own; implementation of hand hygiene campaigns should also consider the opinions of healthcare workers who are required to implement infection prevention interventions and involve them in local decisions about strategies to promote hand hygiene (Freeth 2012; Health Foundation 2015). Some studies considered in this review involved staff in the design of the intervention, and involved leaders, but the contribution of these strategies was not assessed. The challenge is to encourage healthcare workers to accept personal responsibility for hand hygiene rather than imposing interventions ‘top down’ that are not tailored to the specific needs of each workplace. There is a risk that inflexible requirements for hand hygiene will be resented, as has been suggested for other aspects of infection prevention (Brewster 2016). Available evidence does not yet provide answers as to how best to address this challenge.
There are a number of additional problems affecting completeness and applicability of the evidence. A major problem is that there is currently a lack of agreement on what optimal hand hygiene compliance should be for a specific clinical setting or situation (Mahida 2016). It was not possible to draw conclusions about the ability of the interventions to achieve sustained high performance, because of the Hawthorne effect influencing performance and because of limited follow‐up evaluations. The evidence did not address the effects of limited evaluation of some of the moments of hand hygiene (Sax 2007); most studies documented the first and last moments only. Documentation of the Five Moments, and thus who performed hand hygiene and under what circumstance, is not possible in studies where hand hygiene compliance is assessed solely by product usage or electronic monitoring devices. The studies employing proxy measures of hand hygiene might not give valid or reliable indicators of compliance. Where product consumption is taken as the outcome measure apparent uptake could increase because more is being consumed each time the hands are cleansed or through wastage, spillage or improper use (e.g. cleaning equipment). Automated counters may break down or healthcare workers may ‘game’, resulting in under‐ or overestimation of hand hygiene compliance (Gould 2017). None of the included studies examined hand hygiene technique and its impact on infection/colonisation rates.
There is also very limited cost‐effectiveness data. This is a major omission, as healthcare workers require evidence not only of which hand hygiene interventions are effective, but also which are the most cost‐effective. A number of bundles incorporate hand hygiene promotion but do not measure it separately from other outcome measures. The effect of bundling is not clear; it might increase healthcare worker awareness of the risk of specific infections or place emphasis on specific technical skills that incorporate hand hygiene that are more effective than the components of typical multimodal campaigns incorporating performance feedback, reminders and education. Factors other than hand hygiene, such as environmental cleaning, antibiotic use or colonisation pressure, can have an impact on colonisation and infection rates but were not explored in any of the studies that evaluated bundles. Finally, most studies lack a clearly articulated theoretical underpinning. Linking interventions clearly to individual and organisational behaviour change theories would benefit our understanding of how interventions to promote hand hygiene work and how best to promote hand hygiene (Srigley 2015).
Certainty of the evidence
Our judgements of the certainty of evidence for the impact of multimodal interventions on hand hygiene compliance ranged from very low to low, depending on the combination of strategies recommended by WHO. The description of interventions was limited in three studies (Derde 2014, Vernaz 2008, Whitby 2008) so assumptions were made in categorizing the interventions as WHO‐based or WHO‐enhanced. The impact of incorrectly categorizing the studies on the GRADE rating would be minimal however as all three provided nonrandomized evidence and had at least two sources of high or unclear risk of bias. The overall conclusions about certainty of evidence for each of the three categories of multimodal interventions (not WHO, WHO based or WHO‐enhanced) would be unchanged if errors were made in our categorization of these studies. Certainty of evidence about performance feedback, education strategies and cues was low, while certainty about evidence for the impact of placement of ABHR was moderate. The evidence provides a clear indication of the direction of effect of interventions but not of the magnitude or consistency of effect of specific interventions in different contexts. The certainty of evidence was more variable for the outcomes of infection and colonisation rates, ranging from very low to moderate, with some studies reporting no effect.
We were unable to draw robust conclusions about which interventions to promote hand hygiene compliance are most effective either singly or in combination, because of the heterogeneity of interventions, participants, settings, and outcome measures. The evidence indicates that interventions can be effective, but there was inconsistency in the degree of improvement within and between studies.
Key methodological limitations of the studies relate to risk of bias. Most studies contained at least two or more sources of high risk of bias and many contained sources of unclear risk. Some of the sources of bias would be difficult to eliminate. These include risk of bias arising because healthcare workers were aware they were being observed and inability to blind them to the intervention. These sources of bias might not make a marked difference if the resultant overestimation of effect is taken into consideration and allowance is made during the interpretation of results.
Some sources of bias could have been readily addressed at the design stage by, for example, blinding observers. Although we found many more studies of eligible design in the searches for this update compared to the first update, a number did not meet the eligibility criteria because of inadequacies at the design stage. These included lack of sufficient data collection points pre‐ and post‐intervention and lack of documentation of a clear intervention period in the ITS studies. These omissions were particularly problematic when the studies introduced multiple different interventions at different stages in the study. Lack of an adequate control group was apparent in the randomised trials, non‐randomised trials and controlled before‐after studies. If more attention had been given at the design stage, more studies would have been eligible for inclusion. This in turn might have allowed the inclusion of a sufficient number of studies assessing specific interventions to enable us to draw conclusions about the effectiveness of those interventions. Furthermore, inappropriate statistical analysis was problematic in a number of studies and could also have been corrected at the design stage by identifying appropriate data to collect, and at the analysis phase.
Some biases could have been addressed at the reporting stage, for example, documenting baseline characteristics, blinding, and allocation methods. While it is difficult to control for other events such as outbreaks or changes in policy that could influence hand hygiene compliance, especially when studies continue over prolonged periods, reporting whether or not such changes occurred would allow for a more accurate assessment of risk of bias. Having fewer unclear risks of bias could lead to conclusions about interventions based on a stronger body of evidence.
Potential biases in the review process
The main source of potential bias arising from the conduct of the review was that we could not obtain relevant data for the studies with inadequate statistical analyses. Overall, the methods used to undertake the review were unlikely to have introduced bias: at least two review authors looked at all the outputs and there was extensive and detailed discussion amongst the team at all stages of the review.
Agreements and disagreements with other studies or reviews
Since the publication of Gould 2010, five systematic reviews have been published. One review by Huis 2012 considered only studies published until 2009 and so is not considered here.
Kingston 2016 reviewed intervention studies published between December 2009 (after the WHO guidelines were issued) and February 2014, and searched two databases (PubMed and CINAHL). They include UCBA and ITS studies which do not meet EPOC inclusion criteria. The review authors stated that heterogeneity precluded undertaking a meta‐analysis but nevertheless pooled the results and concluded that multimodal interventions are modestly effective, whether or not based on the WHO guidelines. While this review provides a useful descriptive account of the studies in terms of geographical locations and clinical settings where data were collected, it lacks critical appraisal and consideration of potential sources of bias is entirely absent.
Schweizer 2014 systematically reviewed all multimodal studies (referred to as bundles) to improve hand hygiene compliance. The searches were extensive; studies were published between 2000 and 2012. Many of 39 quasi‐experimental studies in their review did not meet EPOC criteria and were not included in this second Cochrane update. The authors concluded that the quality of the studies was poor but nevertheless proceeded to meta‐analysis of works that, in addition to a lack of rigour, demonstrated considerable heterogeneity. The authors concluded that multimodal interventions improve hand hygiene compliance and that the effectiveness of the different types of multimodal interventions should be evaluated employing better‐quality study designs. They did not address the question of whether multimodal interventions are more effective than simpler ones.
Srigley 2016 searched a number of databases and identified 10 studies but did not conduct a meta‐analysis because of heterogeneity. Eight of the 10 studies were UCBAs; all were considered to be at moderate to high risk of bias. Interventions varied from provision of ABHR with or without education, to multimodal strategies. They concluded that interventions may improve hand hygiene and reduce HCAI rates but that future research should be undertaken employing stronger designs.
The only systematic review published since 2009 to rival this second Cochrane update in terms of scope and the number of papers considered is Luangasanatip 2015. The aim of their review was to evaluate the effectiveness of WHO‐based and other interventions to promote hand hygiene in hospital settings. They undertook comprehensive searches covering the period up to 2014. Although they said they employed EPOC criteria, they included some studies that we did not consider eligible for inclusion in our review. They proceeded to meta‐analysis regardless of the marked heterogeneity between the included studies. Their conclusion differs from ours in that they considered that evidence of the effectiveness of multimodal interventions to promote hand hygiene, from a larger set of studies, is strong.
Our update is more rigorous than any of the four reviews discussed above and the conclusions drawn from it are correspondingly more cautious. However, our findings overall are broadly in line with those from the other reviews: there is evidence that interventions may be effective whether or not they are based on the WHO guidelines or are single strategies, and that more research studies are needed that employ stronger designs and address threats to internal validity.
Authors' conclusions
Implications for practice.
Since most HCAIs are likely spread by direct contact by the hands of staff, hand hygiene should logically provide an effective way of reducing risks of cross‐infection as well as being aesthetically desirable. Many organisations have provided guidelines to promote hand hygiene in healthcare settings but those issued by WHO are the most comprehensive. The multimodal package of interventions recommended (ABHR, education, reminders, performance feedback, and managerial support) are applicable to all settings and implementation should therefore be encouraged. However, the WHO intervention will need to be adapted to meet local needs and available resources; different strategies or combinations of interventions may be more effective for some groups or healthcare settings than others. Multimodal interventions that include some but not all strategies recommended in the WHO guidelines, and those that include all the recommended strategies plus additional strategies, may slightly improve hand hygiene compliance (low certainty of evidence), but it is uncertain whether multimodal interventions that contain all strategies recommended in the WHO guidelines improve hand hygiene compliance because the certainty of this evidence is very low.
There is sufficient evidence to justify taking actions to improve hand hygiene. However, it is unclear whether interventions need to be multimodal. When implemented as a single strategy, performance feedback and education may improve hand hygiene compliance (low certainty of evidence) and cues such as signs or scent may slightly improve hand hygiene compliance (low certainty of evidence). Placement of ABHR close to the point of use as a single strategy probably slightly improves hand hygiene compliance (moderate certainty of evidence). It will therefore be important for organisations to evaluate their own results and revise their interventions accordingly.
Organisations also need to make decisions about which approach to use for hand hygiene audit, as each has limitations. The presence of observers is very likely to increase hand hygiene frequency and overestimate compliance, but relying solely on product uptake or electronic counting devices results in loss of information because they provide no information about the hand hygiene event in the context of care delivery. Studies have not compared individual feedback to group feedback to identify which is more effective. Oganisations will need to interpret the results of audits in terms of the methods used, e.g. accounting for overestimation of effect.
Implications for research.
We have identified several implications for research. While study designs to evaluate the effectiveness of interventions intended to improve hand hygiene compliance and reduce HCAIs have improved since the last update of this review, there are still methodological inadequacies that should be addressed in future work. These include providing adequate controls, and blinding data collectors and those undertaking analysis to group allocation. There will always be some risk of bias in hand hygiene studies where data are collected by direct observation. Better reporting would allow risk of bias to be assessed more accurately and completely. ITS studies should include sufficient numbers of data collection points pre‐ and post‐intervention and report the period of time over which each phase of the study was conducted.
A key requirement of future studies is to frame the research question in terms of the value of the different components of the intervention: at present it is not clear if multimodal campaigns offer any advantage over single interventions, or which components add the most value. Similarly studies that investigate hand hygiene as part of a bundle to reduce infection also need to address the contribution of various components to the outcome. More sophistical research designs and analyses may be necessary to be able to determine the value of individual components.
Multimodal interventions and bundles have the potential to be more expensive than single strategies and it is important to demonstrate which component(s) of programmes are effective so that costly but ineffective elements are not recommended for practice. Many studies now continue over several years but only a few include economic outcomes, and this should be addressed.
We recommend that interventions should be considered in terms of underpinning theoretical frameworks, for example drawing on knowledge from the social sciences. Most studies continue to lack convincing theoretical underpinning and in some cases no rationale is given for including some of the components of multimodal interventions.
More studies are needed to address the same intervention employing consistent measures, so that results are comparable. Studies could consider hand hygiene techniques which still attract little attention, and data collection. A more thorough assessment of all of the Five Moments is also warranted.
Some potentially valuable studies that we reviewed were flawed because the data were not analysed appropriately. Two cluster‐randomised trials, for example, failed to analyse data at the level of the cluster, while multiple ITS studies did not use the correct statistical approach. Re‐analysis was not possible because too little information was provided. Research teams need to access high‐quality statistical support so that the appropriate analysis is conducted. Study results should also be reported as fully as possible, so that the effectiveness of the same approach can be evaluated in other hospitals and clinical settings.
What's new
| Date | Event | Description |
|---|---|---|
| 18 October 2016 | New citation required and conclusions have changed | Additional studies included with new conclusions reached: Multimodal interventions that include some but not all strategies recommended in the WHO guidelines, multimodal interventions that include all the recommended strategies plus additional strategies, and cues such as signs or scent may slightly improve hand hygiene compliance (low certainty evidence). It is uncertain whether multimodal interventions that contain all strategies recommended in the WHO guidelines improve hand hygiene compliance because the certainty of this evidence is very low. Performance feedback and education may improve hand hygiene compliance (low certainty evidence). Placement of ABHR close to point of use probably slightly improves hand hygiene compliance (moderate certainty evidence). |
| 18 October 2016 | New search has been performed | Updated searches performed to October 18 2016, with 23 new studies identified.This review now includes 26 studies. We have revised the searches to increase precision, and GRADE ratings are incorporated. One new author added (MT). |
History
Protocol first published: Issue 4, 2004 Review first published: Issue 2, 2007
| Date | Event | Description |
|---|---|---|
| 3 August 2010 | New citation required but conclusions have not changed | Two new studies added, no change in conclusions. Review now includes risk of bias table for all included studies and new searches up to November 2009. Review author order has been revised to reflect contribution for this update. |
| 3 August 2010 | New search has been performed | New search, screening, two new studies included |
| 24 June 2008 | Amended | Converted to new review format. |
| 7 February 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Julia Worswick and Alain Mayhew of EPOC for their helpful comments and assistance in preparing this review update.
Anna Selva for her assistance with preliminary screening of abstracts in the first search.
National Institute for Health Research, through Cochrane Infrastructure funding to the Effective Practice and Organisation of Care Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We would also like to thank the following for their helpful comments during the editorial and peer review stages of this update: Janet Squires (Contact Editor), Paul Miller (EPOC Information Specialist), Jane Marjoribanks and Toby Lasserson (Cochrane Central Editorial Unit), Kathryn Suh and Paul O'Connor (external referees), and Jemma Hudson and Villyen Nkengafac Motaze (EPOC Editors).
Appendices
Appendix 1. Search Strategies
Medline (OVID)
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) 1946 to Present
Search date: 18 October 2016
| No. | Search terms | Results |
| 1 | (doctor* or physician* or nurse* or clinician* or consultant* or healthcare assistant* or health care assistant* or health care professional* or healthcare professional* or team* or healthcare worker* or health care worker* or (health* adj2 personnel) or medical or nursing or staff).ti,ab. | 1811863 |
| 2 | exp health personnel/ | 430936 |
| 3 | exp health facilities/ | 682773 |
| 4 | (ward? or centre or centres or center or centers or department? or unit or units or hospital?).ti,ab. | 1960472 |
| 5 | long‐term care.ti,ab. | 16518 |
| 6 | (residential adj3 (care or healthcare or facilit*)).ti,ab. | 4658 |
| 7 | nursing home?.ti,ab. | 25235 |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 | 3711914 |
| 9 | hand disinfection/ | 4943 |
| 10 | (handwash* or hand wash* or hand hygiene or handrub* or hand rub*).ti,ab. | 6366 |
| 11 | (hand? adj2 (clean* or decontaminat* or disinfect* or hygiene or hygienic* or saniti* or sterili* or wash*)).ti,ab. | 6686 |
| 12 | (hand* adj3 (alcohol* or propanol* or ethanol*)).ti,ab. | 1322 |
| 13 | (hand* adj scrub*).ti,ab. | 101 |
| 14 | (antisepsis/ or sterilization/ or disinfection/) and hand/ | 432 |
| 15 | (hand? adj2 (aseps* or aseptic* or antisep*)).ti,ab. | 216 |
| 16 | or/9‐15 | 10437 |
| 17 | ((university adj student?) or school or preschool* or pre‐school* or daycare? or virolog* or parasitol* or home* or sanitat* or water).ti. | 344602 |
| 18 | 16 not 17 | 9861 |
| 19 | randomized controlled trial.pt. | 432907 |
| 20 | controlled clinical trial.pt. | 91818 |
| 21 | multicenter study.pt. | 212516 |
| 22 | pragmatic clinical trial.pt. | 427 |
| 23 | (randomis* or randomiz* or randomly).ti,ab. | 703141 |
| 24 | groups.ab. | 1648291 |
| 25 | (trial or multicenter or multi center or multicentre or multi centre).ti. | 192218 |
| 26 | (intervention? or effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. | 7780288 |
| 27 | non‐randomized controlled trials as topic/ | 83 |
| 28 | interrupted time series analysis/ | 226 |
| 29 | controlled before‐after studies/ | 185 |
| 30 | or/19‐29 | 8697386 |
| 31 | exp animals/ | 20710394 |
| 32 | humans/ | 16384441 |
| 33 | 31 not (31 and 32) | 4325953 |
| 34 | review.pt. | 2202629 |
| 35 | meta analysis.pt. | 74283 |
| 36 | news.pt. | 180656 |
| 37 | comment.pt. | 686913 |
| 38 | editorial.pt. | 420754 |
| 39 | cochrane database of systematic reviews.jn. | 16245 |
| 40 | comment on.cm. | 686917 |
| 41 | (systematic review or literature review).ti. | 85309 |
| 42 | or/33‐41 | 7512404 |
| 43 | 30 not 42 | 6043658 |
| 44 | 8 and 16 and 43 | 3925304 |
Embase (OVID)
Embase 1974 to 2016 October 17
Search date: 18 October 2016
| No. | Search terms | Results |
| 1 | (doctor* or physician* or nurse* or clinician* or consultant* or healthcare assistant* or health care assistant* or health care professional* or healthcare professional* or team* or healthcare worker* or health care worker* or (health* adj2 personnel) or medical or nursing or staff).ti,ab. | 2368813 |
| 2 | (ward? or centre or centres or center or centers or department? or unit or units or hospital?).ti,ab. | 2661525 |
| 3 | long‐term care.ti,ab. | 19913 |
| 4 | (residential adj3 (care or healthcare or facilit*)).ti,ab. | 5554 |
| 5 | nursing home?.ti,ab. | 31181 |
| 6 | exp *health care personnel/ | 506049 |
| 7 | exp *health care facility/ | 495390 |
| 8 | or/1‐7 | 4662077 |
| 9 | *hand washing/ | 3726 |
| 10 | (handwash* or hand wash* or hand hygiene or handrub* or hand rub*).ti,ab. | 8852 |
| 11 | (hand? adj2 (clean* or decontaminat* or disinfect* or hygiene or hygienic* or saniti* or sterili* or wash*)).ti,ab. | 9375 |
| 12 | (hand* adj3 (alcohol* or propanol* or ethanol*)).ti,ab. | 1902 |
| 13 | (hand* adj scrub*).ti,ab. | 125 |
| 14 | (hand? adj2 (aseps* or aseptic* or antisep*)).ti,ab. | 311 |
| 15 | (antisepsis/ or disinfection/) and Hand/ | 330 |
| 16 | or/9‐15 | 12308 |
| 17 | randomized controlled trial/ | 455978 |
| 18 | controlled clinical trial/ | 443330 |
| 19 | quasi experimental study/ | 4113 |
| 20 | pretest posttest control group design/ | 329 |
| 21 | time series analysis/ | 23372 |
| 22 | experimental design/ | 24295 |
| 23 | multicenter study/ | 153846 |
| 24 | (randomis* or randomiz* or randomly).ti,ab. | 931918 |
| 25 | groups.ab. | 2162571 |
| 26 | (trial or multicentre or multicenter or multi centre or multi center).ti. | 257431 |
| 27 | (intervention? or effect? or impact? or controlled or control group? or (before adj5 after) or (pre adj5 post) or ((pretest or pre test) and (posttest or post test)) or quasiexperiment* or quasi experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or time series or time point? or repeated measur*).ti,ab. | 9651646 |
| 28 | or/17‐27 | 10781079 |
| 29 | (systematic review or literature review).ti. | 100616 |
| 30 | "cochrane database of systematic reviews".jn. | 4951 |
| 31 | exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ | 23939845 |
| 32 | human/ or normal human/ or human cell/ | 18128043 |
| 33 | 31 not (31 and 32) | 5858620 |
| 34 | 29 or 30 or 33 | 5963444 |
| 35 | 28 not 34 | 8250546 |
| 36 | 8 and 16 and 35 | 4265 |
Cochrane Central Register of Controlled Trials (Wiley)
Search date: 18 October 2016
| No. | Search terms | Results |
| #1 | (doctor* or physician* or nurse* or clinician* or consultant* or healthcare assistant* or health care assistant* or health care professional* or healthcare professional* or team* or healthcare worker* or health care worker* or (health* near/2 personnel) or medical or nursing or staff):ti,ab | 93726 |
| #2 | (ward? or centre or centres or center or centers or department? or unit or units or hospital?):ti,ab | 89747 |
| #3 | long‐term care:ti,ab | 810 |
| #4 | (residential near/3 (care or healthcare or facilit*)):ti,ab | 395 |
| #5 | nursing home?:ti,ab | 1151 |
| #6 | [mh "health personnel"] | 7078 |
| #7 | [mh "health facilities"] | 13547 |
| #8 | {or #1‐#7} | 168722 |
| #9 | [mh handwashing] | 321 |
| #10 | (handwash* or (hand hygiene) or handrub* or hand rub*):ti,ab | 551 |
| #11 | (hand near/2 (clean* or decontaminat* or disinfect* or hygiene or hygienic* or saniti* or sterili* or wash*)):ti,ab | 457 |
| #12 | (hand* near/3 (alcohol* or propanol* or ethanol*)):ti,ab | 157 |
| #13 | (hand* near scrub*):ti,ab | 42 |
| #14 | [mh antisepsis] | 109 |
| #15 | [mh sterilization] | 490 |
| #16 | [mh disinfection] | 324 |
| #17 | [mh hand] | 2298 |
| #18 | (#14 or #15 or #16) and #17 | 18 |
| #19 | (hand near/2 (aseps* or aseptic* or antisep*)):ti,ab | 30 |
| #20 | #9 or #10 or #11 or #12 or #13 or #18 or #19 | 805 |
| #21 | #8 and #20 | 382 |
CINAHL (Ebsco)
Search date: 18 October 2016
| No. | Search terms | Results |
| S1 | TI (doctor* or physician* or nurse* or clinician* or consultant* or healthcare assistant* or health care assistant* or health care professional* or healthcare professional* or team* or healthcare worker* or health care worker* or (health* N2 personnel) or medical or nursing or staff) | 361,447 |
| S2 | AB (doctor* or physician* or nurse* or clinician* or consultant* or healthcare assistant* or health care assistant* or health care professional* or healthcare professional* or team* or healthcare worker* or health care worker* or (health* N2 personnel) or medical or nursing or staff) | 395,210 |
| S3 | TI (ward? or centre or centres or center or centers or department? or unit or units or hospital?) | 60,274 |
| S4 | AB (ward? or centre or centres or center or centers or department? or unit or units or hospital?) | 157,221 |
| S5 | TI (long‐term care) OR AB (long‐term care) | 12,209 |
| S6 | TI (residential N3 (care or healthcare or facilit*)) | 1,392 |
| S7 | AB (residential N3 (care or healthcare or facilit*)) | 1,948 |
| S8 | TI nursing home? OR AB nursing home? | 6,966 |
| S9 | (MH "Health Personnel+") | 342,921 |
| S10 | (MH "Health Facilities+") | 253,292 |
| S11 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 | 1,056,730 |
| S12 | (MH "Handwashing") | 5,414 |
| S13 | TI (handwash* or hand hygiene or handrub* or hand rub*) or AB (handwash* or hand hygiene or handrub* or hand rub*) | 2,942 |
| S14 | TI (hand* N2 (clean* or decontaminat* or disinfect* or hygiene or hygienic* or saniti* or sterili* or wash*) | 2,002 |
| S15 | AB (hand* N2 (clean* or decontaminat* or disinfect* or hygiene or hygienic* or saniti* or sterili* or wash*) | 2,152 |
| S16 | TI (hand* N3 (alcohol* or propanol* or ethanol*)) | 264 |
| S17 | AB (hand* N3 (alcohol* or propanol* or ethanol*)) | 427 |
| S18 | TI (hand* N1 scrub*) or AB (hand* N1 scrub*) | 67 |
| S19 | (MH "Hand") | 4,576 |
| S20 | (MH "Sterilization and Disinfection") | 6,710 |
| S21 | S19 AND S20 | 29 |
| S22 | S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S21 | 6,702 |
| S23 | PT randomized controlled trial | 30,693 |
| S24 | PT clinical trial | 52,806 |
| S25 | PT research | 986,950 |
| S26 | (MH "Randomized Controlled Trials") | 28,251 |
| S27 | (MH "Clinical Trials") | 85,144 |
| S28 | (MH "Intervention Trials") | 6,071 |
| S29 | (MH "Nonrandomized Trials") | 179 |
| S30 | (MH "Experimental Studies") | 15,081 |
| S31 | (MH "Pretest‐Posttest Design+") | 27,448 |
| S32 | (MH "Quasi‐Experimental Studies+") | 8,692 |
| S33 | (MH "Multicenter Studies") | 14,354 |
| S34 | (MH "Health Services Research") | 7,478 |
| S35 | TI ( randomis* or randomiz* or randomly) OR AB ( randomis* or randomiz* or randomly) | 114,306 |
| S36 | TI (trial or effect* or impact* or intervention* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) OR AB (trial or effect* or impact* or intervention* or before N5 after or pre N5 post or ((pretest or "pre test") and (posttest or "post test")) or quasiexperiment* or quasi W0 experiment* or pseudo experiment* or pseudoexperiment* or evaluat* or "time series" or time W0 point* or repeated W0 measur*) | 782,466 |
| S37 | S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 | 1,318,672 |
| S38 | S11 AND S22 AND S37 | 2,297 |
| S39 | S38 Limiters ‐ Exclude MEDLINE records | 576 |
ClinicalTrials.gov
Search date: 18 October 2016
| Search terms | Results |
| doctor OR physician OR nurse OR clinician OR consultant OR healthcare OR health care OR professional OR team OR worker OR personnel OR medical OR nursing OR staff OR ward OR centre OR center OR department OR unit OR hospital | Interventional Studies | “hand asepsis” OR “hand aseptic” OR “hand antiseptic” OR “hand sanitizer” OR “hand sterilization” | 63 |
| handwash OR “hand wash” OR “hand hygiene” OR handrub OR “hand rub” OR “hand clean” OR “hand disinfection” OR “hand sanitiser” OR “hand sterilisation” OR “hand alcohol” OR “hand doctor OR physician OR nurse OR clinician OR consultant OR healthcare OR health care OR professional OR team OR worker OR personnel OR medical OR nursing OR staff OR ward OR centre OR center OR department OR unit OR hospital | Interventional Studies | handwash OR “hand wash” OR “hand hygiene” OR handrub OR “hand rub” OR “hand clean” OR “hand disinfection” OR “hand sanitiser” OR “hand sterilisation” OR “hand alcohol” OR “hand propanol” OR “hand ethanol” OR “hand scrub” OR “hand ethanol” OR “hand scrub” | Interventional Studies | 159 |
WHO ICTRP
Search date: 18 October 2016
| Search terms | Results |
| “hand asepsis” OR “hand aseptic” OR “hand antiseptic” OR “hand sanitizer” OR “hand sterilization” | 14 |
| handwash OR “hand wash” OR “hand hygiene” OR handrub OR “hand rub” OR “hand clean” OR “hand disinfection” OR “hand sanitiser” OR “hand sterilisation” OR “hand alcohol” OR “hand propanol” OR “hand ethanol” OR “hand scrub” | 80 |
Appendix 2. Calculation of GRADE ratings
| No of studies | Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other | Certainty (overall score) |
|
Intervention: Multimodal not WHO‐based Outcome: Hand Hygiene Compliance | |||||||
| 5 | 4 RCT 1 ITS (3) |
Serious risk of bias (‐0.5) |
Important inconsistency in effect sizes (‐0.5) |
No serious indirectness | No serious imprecision | None | Low (2) |
|
Intervention: Multimodal not WHO‐based Outcome: Infection rates | |||||||
| 3 | 2 RCT 1 ITS (3) |
Serious risk of bias (‐0.5) |
Important inconsistency in effect sizes (‐0.5) |
No serious indirectness | No serious imprecision | None | Low (2) |
|
Intervention: Multimodal WHO‐based Outcome: Hand Hygiene Compliance | |||||||
| 5 | 1 RCT 4 ITS (2) |
Serious risk of bias (‐0.5) |
Important inconsistency in effect sizes (‐0.5) |
No serious indirectness | No serious imprecision | None | Very low (1) |
|
Intervention: Multimodal WHO‐based Outcome: Infection rates | |||||||
| 2 | 2 ITS (2) |
Serious risk of bias (‐0.5) |
Important inconsistency in effect sizes (‐0.5) |
No serious indirectness | No serious imprecision | None | Very low (1) |
|
Intervention: Multimodal WHO‐based Outcome: Colonisation rates | |||||||
| 2 | 1 RCT 1 ITS (3) |
Serious risk of bias (‐0.5) |
Important inconsistency in effect sizes (‐0.5) |
No serious indirectness | No serious imprecision | None | Low (2) |
|
Intervention: Multimodal WHO‐enhanced Outcome: Hand Hygiene Compliance | |||||||
| 6 | 2 RCT 4 ITS (3) |
Serious risk of bias (‐0.5) |
Important inconsistency in effect sizes (‐0.5) |
No serious indirectness | No serious imprecision | None | Low (2) |
|
Intervention: Multimodal WHO‐enhanced Outcome: Infection rates | |||||||
| 1 | 1 ITS (2) |
Serious risk of bias (‐.5) |
No inconsistency in effect sizes | No serious indirectness | No serious imprecision | None | Very low (1.5) |
|
Intervention: Performance feedback Outcome: Hand Hygiene Compliance | |||||||
| 6 | 3 RCT, 1 NRCT, 2 ITS (3) |
Serious risk of bias (‐0.5) |
Important inconsistency in effect sizes (‐0.5) |
No serious indirectness | No serious imprecision | None | Low (2) |
|
Intervention: Performance feedback Outcome: Infection rates | |||||||
| 1 | 1 RCT (4) |
Serious risk of bias (‐0.5) |
Important inconsistency in effect sizes (‐0.5) |
No serious indirectness | No serious imprecision | None | Moderate (3) |
|
Intervention: Performance feedback Outcome: Colonization rates | |||||||
| 1 | 1 RCT (4) |
Serious risk of bias (‐0.5) |
Important inconsistency in effect sizes (‐0.5) |
No serious indirectness | No serious imprecision | None | Moderate (3) |
|
Intervention: Education Outcome: Hand Hygiene Compliance | |||||||
| 2 | 1 RCT 1 ITS (3) |
Serious risk of bias (‐0.5) |
Important inconsistency in effect sizes (‐0.5) |
No serious indirectness | No serious imprecision | None | Low (2) |
|
Intervention: Cues Outcome: Hand Hygiene Compliance | |||||||
| 3 | 2 RCT, 1 NRCT (3) |
Serious risk of bias (‐0.5) |
Important inconsistency in effect sizes (‐0.5) |
No serious indirectness | No serious imprecision | None | Low (2) |
|
Intervention: Placement of ABHR Outcome: Hand Hygiene Compliance | |||||||
| 1 | 1 RCT (4) |
Serious risk of bias (‐0.5) |
No inconsistency in effect sizes | No serious indirectness | No serious imprecision | None | Moderate (3.5) |
Footnotes
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Armellino 2012.
| Methods | Design: ITS Study period: 16‐week baseline period (June to Sept 2008) followed by a 16‐week post‐intervention period (Oct 6 2008 to Jan 24 2009) then 75‐week maintenance period (Jan 25 2009 to July 4 2010) USA |
|
| Participants | All healthcare workers in a 17‐bed medical ICU | |
| Interventions | Video cameras recorded attempts at hand hygiene; feedback was given to staff in a variety of ways including continuous display of hand hygiene rates on electronic boards in hallways and detailed summaries sent to managers by email | |
| Outcomes | Hand hygiene compliance, defined as percentage of hand hygiene opportunities where hand hygiene was attempted within 10 seconds before or after access to a room | |
| Notes | Appropriate analysis for ITS Third‐party auditors remotely assessed video recordings Funding source: New York State Department of Health Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Presence of video cameras so staff aware of being monitored |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were not assessed blindly, although third‐party auditors were used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in various study periods |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | No evidence |
| Intervention independent | Unclear risk | No report of whether there were other campaigns, outbreaks, changes in staffing etc |
| Shape of effect pre‐specified | Low risk | Point of analysis is the point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection before and after intervention |
Derde 2014.
| Methods | Design: ITS Study period: May 2008‐April 2011 6 month baseline period, 7 month intervention period, 11 month follow up |
|
| Participants | Europe. 13 ICUs | |
| Interventions | Multimodal campaign based on WHO 5 Moments | |
| Outcomes | Direct observation of hand hygiene; not clear for how long or how often | |
| Notes | Inappropriate analysis for ITS (no segmented regression or equivalent) Funding source: European Commission Declaration of interest: None They also conducted a cluster‐randomised trial related to screening and barrier use which did not have hand hygiene as an outcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Data collectors were nurses from the study units trained in data collection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in the different study periods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | High risk | Other changes occurred in phase 3 of the study re screening for MRSA and other pathogens, plus concurrent use of barrier and contact precautions |
| Shape of effect pre‐specified | Low risk | Point of analysis is the point intervention |
| Intervention had no effect on data collection | Low risk | Same data collection method before and after |
Diegel‐Vacek 2016.
| Methods | Non‐randomised trial in 1 centre in the USA Study period: 3 observation days in a 3‐week period: day 1, day 14, day 21. Dates not stated. |
|
| Participants | All healthcare workers | |
| Interventions | Visual light as reminder | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Funding source: None Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐random allocation |
| Allocation concealment (selection bias) | Low risk | Room assigned to be intervention or control room prior to start of study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of observer and purpose of the light |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding was not possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Unclear risk | No baseline hand hygiene compliance assessed |
| Baseline characteristics | High risk | No report of characteristics of patients, staff or room set‐up |
| Protection from contamination | High risk | The same staff entered both rooms and were aware of the light cue in the intervention room |
Fisher 2013a.
| Methods | Design: cluster‐randomised trial Study period: Dates not stated Baseline period of 14 weeks, then phase 2 was 6 weeks (real‐time reminders) then phase 3 was 4 weeks (added individual feedback) Singapore |
|
| Participants | Healthcare workers in cardiology ward and SICU | |
| Interventions | Wireless monitoring system of hand hygiene with real‐time reminders and individual feedback Control: no intervention |
|
| Outcomes | Compliance with hand hygiene measured by system ABHR use (L per bed day) |
|
| Notes | Inappropriate analysis: Unclear reporting of regression Electronic monitoring so observer effect not a concern Funding source: Centre for Integration of Medicine and Innovative Technology which is licensed to HandGenix and by the Agency for Science, Technology and Research (Singapore). The equipment and its installation was paid for by HandGenix Declaration of interest: One of the co‐authors, S.Schiefen, holds shares in HandGenix |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated to arm using computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Allocation was by profession and performed at start of study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Visible and audible wireless technology so participants aware of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Observers were members of the study team and not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms Slightly more non‐participation in control group but this was unlikely to affect results |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Low risk | Similar hand hygiene compliance at baseline |
| Baseline characteristics | High risk | No baseline characteristics presented |
| Protection from contamination | Unclear risk | Those in control group could potentially hear reminder beep given to those in intervention group |
Fuller 2012.
| Methods | Design: stepped‐wedge cluster‐randomised trial Study period: Campagin rolled out in all centres between December 2004‐June 2005; data were collected from October 1, 2006‐December 31, 2009. 36 month trial overall, with units added to intervention at different periods in time UK |
|
| Participants | Healthcare workers in acute care and ICU: 60 wards in 16 hospitals | |
| Interventions | Feedback and personalised action planning plus National 'Clean Your Hands' campaign Control: 'Clean Your Hands' campaign only |
|
| Outcomes | Observation of hand hygiene compliance | |
| Notes | Appropriate analysis for stepped wedge Funding source: Patient Safety Research Programme and Trustees of the Royal Free Hospital Declaration of interest: Cookson and Stone have received consultancy fees from GoJo industries |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Hospitals were given a number, then the numbers were randomly allocated to arm using a research randomiser website |
| Allocation concealment (selection bias) | Low risk | Unit of allocation was the ward and was done at the start of the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Included feedback and personalised action planning so participants aware of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes were assessed blindly |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data (missed opportunities) unlikely to be very different in different arms Difficult to compare loss to follow‐up in both groups because of their different composition of types of units |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. Only 12 wards participated in MRSA swabbing but all participated in hand hygiene assessment |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Unclear risk | Baseline hand hygiene not reported; they reported relative changes from baseline with baseline as reference point |
| Baseline characteristics | High risk | No baseline characteristics presented |
| Protection from contamination | Low risk | Individualised unit‐based intervention so even if control units heard about it, they could not have the intervention |
Grant 2011.
| Methods | Design: pair‐matched cluster‐randomised trial Study period: Dates not stated Pre‐test: hand hygiene observations over a 2‐week period with no sign Post‐test: hand hygiene observations over a two 2‐week period with 1 of 2 signs displayed 4 matched pairs of units in one hospital in the USA |
|
| Participants | 3 categories of healthcare workers: MDs, nurses, and ancillary workers | |
| Interventions | 1 of 2 signs displayed. Signs had message related to personal consequences or to patient consequences | |
| Outcomes | Hand hygiene compliance | |
| Notes | Incorrect analysis: analysed by units rather than matched analysis Covert observation so observer effect unlikely to be a threat Funding source: None Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified how random allocation was done |
| Allocation concealment (selection bias) | Low risk | Unit of allocation was the ward and was done at the start of the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were aware of the signs but were not informed of the research underway |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observers were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be different in each arm. All units remained in study |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Low risk | Similar baseline hand hygiene rates for all 3 types of healthcare workers |
| Baseline characteristics | High risk | No baseline characteristics presented |
| Protection from contamination | Low risk | Participants were aware of the signs but were not informed of the research underway |
Higgins 2013.
| Methods | Design: ITS Study period: Baseline for 2 months in November ‐ December 2009 and multimodal campaign to end of 2010. Then in autumn 2011 an e‐learning hand hygiene game was added; it was moved from ward to ward on a mobile station. Data collected until end of first quarter of 2012 Ireland |
|
| Participants | Healthcare workers in 1 hospital | |
| Interventions | An e‐learning hand hygiene game: 1 week per unit, twice in1 year. Staff members had multiple opportunities to use it during that time on unit | |
| Outcomes | Hand hygiene compliance | |
| Notes | Inappropriate analysis for ITS (no segmented regression or equivalent) Funding source: None Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | E‐learning hand hygiene game stations used so participants aware of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | E‐learning hand hygiene game stations used and visible so observers aware of intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in various study periods |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | None noted |
| Intervention independent | High risk | Extra ABHR stations added during the study period and there were 2 interventions occurring at the same time: 1) multimodal and 2) e‐learning hand hygiene game |
| Shape of effect pre‐specified | Low risk | Point of analysis is point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection method before and after |
Ho 2012.
| Methods | Design: cluster‐randomised trial with 2 intervention groups and 1 control group Study period: Hand hygiene observations occurred at baseline, intervention, 1 month post‐intervention and 4 months post‐intervention Duration of observation periods were not reported but totaled 333 hours between November 2009 and July 2010 Hong Kong |
|
| Participants | Healthcare workers in 18 long‐term care facilities | |
| Interventions | WHO multimodal strategy including posters, reminders, education, pocket‐sized bottles of ABHR for personal use, and feedback. In addition, 1 test group received powdered disposable gloves and the other test group received powderless disposable gloves Control: 2‐hour health talk |
|
| Outcomes | Hand hygiene compliance, defined as proportion of hand hygiene opportunities resulting in compliant action. Number of respiratory outbreaks and MRSA infections requiring hospital admission |
|
| Notes | Logistic regression with GEE to account for clustering but did not compare changes between arms so inappropriate analysis Funding source: Centre for Health Protection, Hong Kong SAR, China Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 72 homes allocated to arm with a random‐number generator, then called in randomly selected order until 6 homes successfully recruited per group |
| Allocation concealment (selection bias) | Low risk | Unit of allocation was institution and performed at start of study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Included posters and feedback so participants aware of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were not assessed blindly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Possible selection bias as unclear who refused/did not have a chance to participate |
| Baseline outcomes | Unclear risk | Some difference in baseline hand hygiene compliance in the 3 groups (19.5, 27 and 22) |
| Baseline characteristics | Unclear risk | There were gender differences and a difference in the proportion of residents with dementia between arms |
| Protection from contamination | Low risk | Allocation was by institution |
Huang 2002.
| Methods | Design: RCT
Study period: September 2000‐January 2001 Questionnaires and observations done at baseline and at 4 months post‐intervention China |
|
| Participants | Nurses throughout a hospital | |
| Interventions | Education, mainly universal precautions | |
| Outcomes | % of nurses washing hands before and after patient contact Also evaluated knowledge scores, prevalence of Hepatitis B immunisation, self‐reported behaviours related to blood‐borne pathogens and universal precautions, self‐reported needlestick and sharps injury, and observed behaviours related to handling used needles. |
|
| Notes | Intervention successful after 4 months Appropriate analysis Funding source: No information given Declaration of Interest: No information given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified how were randomly selected to participate nor randomly allocated to group |
| Allocation concealment (selection bias) | Unclear risk | Allocation was done at the start of the study but method was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Education and questionnaire were very specific so participants aware of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Researchers did not specify if observers were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 98% follow‐up achieved in both groups Missing data (missed opportunities) unlikely to be different in both arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Low risk | Similar at baseline |
| Baseline characteristics | Low risk | Similar at baseline |
| Protection from contamination | Unclear risk | Participants worked in same institution so may have communicated with each other |
Huis 2013.
| Methods | Design: cluster‐randomised trial Study period:September 2008‐November 2009 Baseline (T1), then observations immediately after intervention (T2) then observations 6 months after end of intervention (T3) Netherlands |
|
| Participants | Nurses in patient wards | |
| Interventions | Multimodal: education, individual feedback, posters/signs, ABHR, admin support, staff involvement, adequate supplies Control: state of the art (no admin support or staff involvement) |
|
| Outcomes | Observation of hand hygiene compliance | |
| Notes | Appropriate analysis Observer effect not a concern as participants did not know what was being observed Funding source:Netherlands Organisation for Health Research and Development Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated to arm using computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Allocation was by unit at start of study after baseline assessment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Leaders directed strategy so participants aware of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Analysts were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms 10 intervention wards did not complete intervention; they did an ITT analysis so the loss to follow‐up may have resulted in underestimation of effect but not bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | H1N1 influenza publicity may have influenced hand hygiene |
| Baseline outcomes | Low risk | Similar hand hygiene compliance at baseline |
| Baseline characteristics | High risk | No baseline characteristics presented |
| Protection from contamination | Low risk | Individualised unit‐based intervention so even if control units heard about it, they could not have the intervention |
King 2016.
| Methods | RCT in an ICU Study period: November 2012‐January 2013 USA |
|
| Participants | All healthcare workers | |
| Interventions | Olfactory cue and visual cues | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Funding source: Not stated Declaration of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocks of observation periods (not individuals) were assigned to type of intervention using a random‐number generator |
| Allocation concealment (selection bias) | Low risk | Blocks assigned to intervention or control group prior to start of study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | They would have noticed signs and scent but authors did not specify whether they knew the purpose of the study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible to blind observers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | They did not collect data on number of visitors vs healthcare workers and unclear if their behaviour would be different |
| Baseline outcomes | Low risk | Single unit |
| Baseline characteristics | Low risk | Single unit |
| Protection from contamination | Unclear risk | Single unit, unclear if staff would have different behavior at end of 1 intervention period that could have affected performance when a different intervention occurred |
Lee 2013.
| Methods | Design: ITS Study period: March 2008‐July 2010 Baseline: 6 ‐ 7 months, Intervention 12 months, washout 6 months 9 countries in Europe, and Israel |
|
| Participants | 33 wards, 10 hospitals, all healthcare workers | |
| Interventions | WHO multimodal | |
| Outcomes | Hand hygiene compliance, no feedback Also studied MRSA screening and decolonisation, with MRSA rates as outcome of primary interest |
|
| Notes | Appropriate analysis for ITS ( segmented multilevel logistic regression) Funding source: European Commission 6th framework programme Declaration of interest: Harbarth is a member of the speakers' bureau for bioMerieux and Pfizer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Included posters and managerial support so participants aware of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Observers were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in various study periods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | High risk | Introduction of MRSA screening programme; 10 hospitals over 25 months with no report of whether there were other campaigns, outbreaks etc |
| Shape of effect pre‐specified | Low risk | Point of analysis is point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection before and after |
Martin‐Madrazo 2012.
| Methods | Design: Cluster‐randomised trial Study period: January 2009 to December 2009 3‐month baseline (first observation) then follow‐up (second observation) 6 months after intervention, although duration of data collection in the latter period was not specified Spain |
|
| Participants | Healthcare workers in 11 primary healthcare centres | |
| Interventions | Multimodal strategy based on WHO: posters, education sessions, and availability of ABHR Control: no intervention |
|
| Outcomes | Hand hygiene compliance, defined as number of hand hygiene opportunities taken by number of opportunities observed | |
| Notes | Unit of analysis error: analysed by healthcare worker type, not cluster, and inappropriate correction for missing data 10 opportunities were observed for each healthcare worker at each observation period Unlikely observer effect as participants did not know what outcome was being measured Funding source: Istituto de Salud Carlos III, Ministry of Health of Spain Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | EPIDAT3 program used to randomly select centres for each arm (reported in previous article listed in references) |
| Allocation concealment (selection bias) | Low risk | Unit of allocation was the centre and performed at the start of study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Included reminder posters so participants aware of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Observer was blinded (reported in discussion) and participants were unaware hand hygiene was being observed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms Similar loss to follow‐up in both groups |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | Additional measures taken for H1N1 |
| Baseline outcomes | Low risk | Similar hand hygiene compliance at baseline |
| Baseline characteristics | High risk | Similar types of healthcare workers but types of patients seen at the centres not reported and baseline characteristics of the units were not reported |
| Protection from contamination | Low risk | Intervention was by centre |
Mertz 2010.
| Methods | Design: Cluster‐randomised trial Study period: 3 month baseline assessment (October ‐ December 2006) then trial was conducted for 1 year (June 2007 ‐ May 2008) with assessments conducted weekly (5 randomly‐selected 15‐minute periods per week per unit) Canada |
|
| Participants | All healthcare workers on 30 adult hospital wards in 3 acute care hospitals | |
| Interventions | Performance feedback (pooled not individual), small‐group teaching seminars, posters and pamphlets, unit‐generated target adherence level and approaches to increase awareness of hand hygiene Control: ABHR dispensers installed |
|
| Outcomes | Adherence to hand hygiene: considered successful if hand hygiene occurred when it was deemed necessary (using WHO indications for hand hygiene) and if duration of hand hygiene met pre‐set criteria. Incidence of hospital‐acquired MRSA colonisation (cases per 1000 patient‐days) |
|
| Notes | Appropriate analysis: unit of analysis for hand hygiene was at the level of the clusters Funding source: Physicians’ Services Incorporated Foundation of Ontario, Canada and Swiss National Science Foundation Grant Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocated to arm using random numbers table; statistician was not part of study team |
| Allocation concealment (selection bias) | Low risk | Allocation was by unit and performed at start of study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Included posters and performance feedback so participants aware of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if outcomes were assessed blindly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | ABHR dispensers installed hospital wide during study; 1 MRSA outbreak |
| Baseline outcomes | Low risk | Similar hand hygiene compliance at baseline |
| Baseline characteristics | High risk | Only reported that sinks and ABHR availability were similar; no comparison of patients, staffing, etc |
| Protection from contamination | High risk | Authors suggested contamination of control group likely; control units were in same hospitals as intervention groups |
Midturi 2015.
| Methods | ITS in one hospital Study period: Pre‐intervention January‐September 2011; intervention October 2011‐July 2012; post‐intervention August 2012‐May 2014 USA |
|
| Participants | All healthcare workers | |
| Interventions | Multimodal: education and training; promotion; use of visual cues, covert direct observation of hand hygiene by peers; rewards; alerts to the immediate supervisor; and regular reports to leadership | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Unclear if analysis was appropriate for ITS but reported only compliance per period Funding source: Not stated Declaration of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants aware of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Observers not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in different time periods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | Unclear risk | No report of whether there were other campaigns, outbreaks, changes in staffing etc |
| Shape of effect pre‐specified | Low risk | Point of analysis is the point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection before and after the intervention |
Moghnieh 2016.
| Methods | Non‐randomised trial in 1 hospital Study period: November 2015‐March 2016 Lebanon |
|
| Participants | Nurses | |
| Interventions | Incentives in 1 intervention arm, and audit feedback in separate intervention arm vs education in control group | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Funding source: Not stated Declaration of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐random allocation |
| Allocation concealment (selection bias) | Unclear risk | Allocation was done at the start of the study but method was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Auditors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms Similar loss to follow‐up in both groups |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Low risk | Similar baseline hand hygiene compliance |
| Baseline characteristics | Unclear risk | Reported as similar but no supporting data provided |
| Protection from contamination | Unclear risk | Unclear if staff moved from unit to unit and would have been aware of feedback |
Munoz‐Price 2014.
| Methods | Design: RCT with cross‐over Study period: Dates not stated. Each participant was randomised to receive either the intervention or control first, was monitored for all activities with 1 patient (up to 120 minutes), then within a month was re‐monitored in the opposite arm USA |
|
| Participants | Anaesthesiologists and CRNAs | |
| Interventions | Placement of ABHR dispenser on cart + wall vs wall only | |
| Outcomes | Observation of hand hygiene compliance | |
| Notes | Appropriate analysis Observer effect not a concern since participants did not know what outcome was being measured Funding source: GoJo provided the alcohol product and dispensers Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number generator used to select OR, then group allocation determined by electronic files based on previous block randomisation |
| Allocation concealment (selection bias) | Low risk | Participants assigned to start as intervention or control prior to start of study, then evaluated within 30 days in opposite allocation; did not know what outcome was being assessed |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | ABHR dispenser was visible on cart but researchers said that participants were not aware of what was being assessed |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were not assessed blindly |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms Similar loss to follow‐up in both groups |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Unclear risk | Baseline hand hygiene not reported |
| Baseline characteristics | Low risk | Similar baseline characteristics |
| Protection from contamination | High risk | Participants were assessed once with intervention and once with control conditions but were blinded to outcome being assessed. They may have learned to look for ABHR on the cart when in the intervention arm first, affecting behaviour when they crossed over to the control arm |
Perlin 2013.
| Methods | Design: ITS Study period: Pre‐intervention: 3 quarters in 2006; intervention over 2 quarters in 2007; follow‐up over 10 quarters in 2007 ‐ 2009 USA |
|
| Participants | 1 multi‐state healthcare system with 166 hospitals and 116 outpatient surgery and endoscopy centres | |
| Interventions | Available ABHR, ongoing education, letters for awareness | |
| Outcomes | ABHR use in ounces per adjusted patient‐day | |
| Notes | Inappropriate analysis for ITS (no segmented regression or equivalent) Funding source: None Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Bundle for MRSA reinforced hand hygiene so participants aware of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective measure used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in various study periods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | High risk | Variable pre‐intervention ABHR use in different centres; introduction of MRSA screening, barrier precautions, cleaning and disinfection |
| Shape of effect pre‐specified | Low risk | Point of analysis is point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection method before and after |
Rodriguez 2015.
| Methods | Stepped‐wedge cluster‐randomised trial in 11 ICUs in hospitals
Study period: August 1, 2011 ‐May 1, 2012. A new intervention unit was added each month, and a new intervention component was added each month in each intervention unit. Argentina |
|
| Participants | All healthcare workers | |
| Interventions | Multimodal intervention with stepped introduction of leadership support, availability of ABHR, reminders, story boards, and unit feedback | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Funding source: Patient Safety Small Grant Program, WHO, Switzerland Declaration of interest: Not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Concealed table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Participants assigned to intervention or control group once a month as next units added to intervention |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms Similar loss to follow‐up in both groups |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | High risk | Some differences in baseline; authors identified sites as heterogeneous |
| Baseline characteristics | Unclear risk | Variation reported but impact unclear |
| Protection from contamination | Unclear risk | Authors identified that contamination could not be ruled out |
Rosenbluth 2015.
| Methods | ITS in 1 centre Study period; July 2008‐May 2014, with interventions introduced or altered between July 2010 and July 2013 USA |
|
| Participants | Physicians | |
| Interventions | Multimodal intervention with audit, role modelling, feedback, education, visual cues, direct physician engagement, incentives, and adequate resources | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Inappropriate analysis for an ITS (no segmented regression or equivalent) Funding source: None Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants aware of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Observers not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in time period |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | Unclear risk | No report of whether there other campaigns, outbreaks, changes in staffing etc; additional interventions added for physicians |
| Shape of effect pre‐specified | Low risk | Point of analysis is the point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection before and after the intervention |
Stevenson 2014.
| Methods | Design: cluster‐randomised trial Study period: March 2003‐February 2004 4‐month baseline, intervention period of 5 months USA |
|
| Participants | Healthcare workers in 10 community hospitals | |
| Interventions | Multimodal, customised to the unit: education, feedback at the unit level, posters/signs, ABHR, admin support, staff involvement, recognition and rewards programme (candy, buttons) Control: usual infection control practices |
|
| Outcomes | Observation of hand hygiene compliance | |
| Notes | Mixed effects logistic regression: appropriate analysis Funding source: None Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified how random allocation was done |
| Allocation concealment (selection bias) | Low risk | Unit of allocation was institution and performed at start of study after baseline assessment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Individualised campaigns so participants aware of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcomes were assessed blindly but local observers were used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms 1 withdrew early from the control group but this was unlikely to affect results |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Unclear risk | Baseline hand hygiene not reported; they compared absolute changes from baseline |
| Baseline characteristics | High risk | No baseline characteristics reported |
| Protection from contamination | Low risk | Allocation was by institution |
Stewardson 2016.
| Methods | Cluster‐randomised trial in 1 centre Study period: Baseline period April 1, 2009‐June 30, 2010; intervention period July 1, 2010 ‐June 30, 2012. Switzerland |
|
| Participants | All healthcare workers | |
| Interventions | Enhanced feedback or enhanced feedback with patient participation vs standard WHO‐based multimodal intervention | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Funding source: Swiss National Science Foundation Declaration of interest: None declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence with block randomisation of wards to groups |
| Allocation concealment (selection bias) | Low risk | Participants assigned to intervention or control group prior to start of study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not possible to blind as posters used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Baseline outcomes | Low risk | Similar baseline outcomes |
| Baseline characteristics | Unclear risk | Allocated by strata so patient characteristics likely similar but no data provided on healthcare workers or physical layout |
| Protection from contamination | Unclear risk | Unclear if staff moved from control to intervention wards; identified by authors in discussion as a possibility |
Talbot 2013.
| Methods | Design: ITS Study period: Baseline: 2004 ‐ 2009; Programme launch over 12‐month period (late 2009 ‐ late 2010); active accountability phase from late 2010 to fall 2012 USA |
|
| Participants | Healthcare workers in 1 centre | |
| Interventions | Leadership goal‐setting, financial incentives for centre, expanded hand hygiene observation programme including feedback to individuals, system‐wide marketing campaign | |
| Outcomes | Observed hand hygiene compliance | |
| Notes | Appropriate analysis for ITS Funding source: None Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Leaders were involved so participants were aware of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Observers were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in various study periods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | Unclear risk | They did not report whether or not there were other campaigns, outbreaks etc |
| Shape of effect pre‐specified | Low risk | Point of analysis is point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection before and after the intervention |
Vernaz 2008.
| Methods | Design: ITS Study period: February 2000 ‐ September 2006; VigiGerme® campaign occurred in spring 2003 and the Clean Care is Safer Care occurred in autumn 2005 University of Geneva Hospital Centre (2200 bed primary and tertiary care centre), Switzerland |
|
| Participants | Healthcare workers throughout hospital | |
| Interventions | Social marketing campaign (VigiGerme®) aimed at Standard Precautions in 2003 and Clean Care is Safer Care campaign in 2005. The campaigns were not described but were based on the Geneva campaign model which included the five components recommended in the WHO Guidelines 2009 | |
| Outcomes | Volume of hand hygiene products (litres per 100 patient‐days) Also measured new MRSA isolates per 100 patient‐days, newC. difficile isolates per 100 patient‐days, defined daily dose of antibiotics per 100 patient‐days |
|
| Notes | Analysis appropriate for ITS Funding source: None Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Pocket‐sized ABHR given so participants aware of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective measure used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be different in different time periods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | High risk | Multiple interventions occurred over the 7‐year period including 2 infection control programmes, so very likely there were confounding factors |
| Shape of effect pre‐specified | Low risk | Point of analysis same as point of intervention |
| Intervention had no effect on data collection | Low risk | Same data collection method before and after the intervention |
Whitby 2008.
| Methods | Design: ITS Duration: 2004‐2006, with 24 months of data collection following start of each campaign Geneva: pre‐intervention July‐October 2004; intervention October 2004‐May 2005 Washington: pre‐intervention July‐November 2004; intervention November 2004‐May 2005 Australia |
|
| Participants | All healthcare workers in multiple units | |
| Interventions | 3 separate interventions: 1) Simple substitutions: ABHR for soap, and 1 type of ABHR for another 2) Geneva campaign: based on the Geneva campaign (Pittet 2000) that existed at the time which consisted of all of the elements later included in the WHO Guidelines 2009 3) Washington campaign: based on a campaign that had taken place in Washington (Larson 2000) and consisted of the elements later included in the WHO Guidelines 2009 with informal feedback during the staff involvement in all aspects of design and implementation |
|
| Outcomes | Product use (electronic count of soap/AHBR dispensers) | |
| Notes | Appropriate analysis for ITS Funding source: None Declaration of interest: No information given |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Staff involved in developing campaign so participants aware of intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective measure used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be different in different time periods |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence |
| Intervention independent | Unclear risk | They did not comment on whether there were other changes, outbreaks etc. |
| Shape of effect pre‐specified | Low risk | Point of analysis same as point of intervention |
| Intervention had no effect on data collection | Low risk | Data collection method same before and after |
Yeung 2011.
| Methods | Design: cluster‐randomised trial Study period: intervention period April 1‐15, 2007; baseline assessment over 3 months; post intervention assessments over 36‐37 days starting April 16, 2007. Monthly monitoring for 3 months, then gave feedback to both intervention and control groups, then monitored for another 4 months |
|
| Participants | Hong Kong Healthcare workers in 6 long term care facilities |
|
| Interventions | Multimodal: education, feedback to group in one session, posters, individual ABHR, pens as reminder Control: basic life support workshop |
|
| Outcomes | Observed compliance to hand hygiene | |
| Notes | Unit of analysis error: analysed at level of individual not cluster Funding source: Grant to Support Academic Activities for Public Health and Social Medicine from the Chinese University of Hong Kong and by Vickmans Laboratories which supplied the pocket‐sized alcohol containers of hand rub Declaration of interest: None |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified how random allocation was done |
| Allocation concealment (selection bias) | Low risk | Unit of allocation was institution and performed at start of study after baseline assessment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Included reminders so participants aware of the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes were not assessed blindly (reported in discussion) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data (missed opportunities) unlikely to be very different in different arms Similar loss to follow up in both groups |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | High risk | A feedback session took place in both intervention and control units 3 months after intervention |
| Baseline outcomes | Low risk | Similar hand hygiene compliance at baseline |
| Baseline characteristics | High risk | Higher proportion with severe disabilities in treatment group and they had fewer handwashing sinks |
| Protection from contamination | Unclear risk | 43% of intervention group staff left by end of study and new staff may not have received education |
ABHR: alcohol‐based hand rub C.difficile: Clostridium difficile CRNA: certified registered nurse anaesthetist GEE: generalised estimating equation ICU: intensive care unit ITS: interrupted time series MRSA: methicillin‐resistant Staphylococcus aureus OR: operating room RCT: randomised (controlled) trial SICU: surgical intensive care unit WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aboumater 2012 | ITS design with insufficient data collection points |
| Adams 2013 | ITS design with unclear intervention period |
| Al Tawfiq 2013 | ITS design with inadeqate data collection points |
| Armellino 2013 | ITS design with inadeqate data collection points |
| Assanasen 2008 | ITS design with inadequate data collection points |
| Barnett 2013 | ITS design with inadeqate data collection points |
| Barrera 2011 | ITS design with inadequate data collection points |
| Barrow 2009 | ITS design with insufficient data collection points |
| Bellis 2006 | ITS design with inadequate data collection points |
| Bittner 2002 | CBA study design with 1 nonequivalent control group. |
| Chan 2013 | ITS design with inadeqate data collection points |
| Chen 2011 | ITS design with inadequate data collection points |
| Christiaens 2009 | ITS design with inadeqate data collection points |
| Colombo 2002 | CBA study design with 1 nonequivalent control group. |
| Conly 1989 | IITS design with inadeqate data collection points |
| Conrad 2010 | ITS design with unclear intervention period |
| Creel 2014 | ITS design with inadeqate data collection points |
| Crews 2013 | ITS design with inadeqate data collection points |
| Donnellan 2011 | ITS design with inadequate data collection points |
| Donowitz 1986 | ITS design with inadeqate data collection points |
| Dos Santos 2011 | ITS design with inadeqate data collection points and unclear intervention period |
| Duerink 2006 | CBA study inadequate control, no baseline |
| Eldridge 2006 | ITS design with inadeqate data collection points |
| Fisher 2013b | ITS design with inadeqate data collection points |
| Giannitsioti 2009 | Non‐randomised trial with inadequate control group |
| Golan 2006 | Cross‐over CBA design with only 1 intervention group and 1 control group |
| Gould 1997 | CBA design with only 1 intervention group and 1 control group |
| Grayson 2008 | ITS design with inadeqate data collection points |
| Grayson 2011 | ITS design with inadeqate data collection points |
| Harne‐Bittner 2011 | CBA design with 2 intervention groups but only 1 control group |
| Huang 2006 | ITS design with inadeqate data collection points |
| Huang 2008 | IITS design with inadeqate data collection points |
| Kohli 2009 | Non‐randomised clinical trial with inadequate control group |
| Larson 1991 | CBA study design with 1 nonequivalent control group |
| Larson 1997 | CBA study design with 1 nonequivalent control group |
| Larson 2000 | CBA study design with 1 nonequivalent control group |
| Linam 2011 | CBA design with only 1 intervention group and 1 control group |
| Lobo 2010 | ITS design with inadeqate data collection points |
| Madani 2006 | ITS design with inadequate data collection points |
| Marra 2008 | Non‐randomised trial, no baseline data, inadequate control group |
| Marra 2010 | CBA design with only 1 intervention group and 1 control group |
| Marra 2011 | CBA design with only 1 intervention group and 1 control group |
| Marra 2013a | CBA design with only 1 intervention group and 1 control group |
| Marra 2013b | ITS design with inadeqate data collection points |
| Marra 2014 | CBA design with only 1 intervention group and 1 control group |
| Mayer 1986 | CBA study design with 1 nonequivalent control group |
| McLaws 2009 | ITS design with inadequate data collection points |
| Miyachi 2007 | ITS design with inadequate data collection points |
| Molina‐Cabrillana 2010 | ITS design with inadequate data collection points |
| Peterson 2012 | ITS design with unclear intervention period |
| Picheansathian 2008 | ITS design with inadequate data collection points |
| Raju 1991 | ITS design with inadeqate data collection points |
| Rees 2013 | ITS design with inadeqate data collection points and unclear intervention period |
| Rupp 2008 | Cross‐over CBA design with only 1 intervention group and 1 control group |
| Sakamoto 2010 | ITS design with no clear intervention period |
| Schweon 2012 | ITS design with inadequate data collection points |
| Song 2013 | ITS design with inadeqate data collection points |
| Sopirala 2014 | ITS design with inadeqate data collection points |
| Stella 2013 | ITS design with inadeqate data collection points |
| Stoesser 2013 | ITS design with inadeqate data collection points |
| Stone 2007 | ITS design with inadeqate data collection points |
| Stone 2011 | ITS design with inadeqate data collection points |
| Trick 2007 | CBA study with only 1 control group |
| Van de Mortel 2006 | ITS design with inadequate data collection points |
| Vinci 2012 | ITS design with inadequate data collection points |
| Walker 2013 | CBA design with only 1 intervention group and 1 control group |
| Walker 2014 | CBA design with only 1 intervention group and 1 control group |
CBA: controlled before‐after ITS: interrupted time series RCT: randomised (controlled) trial
Differences between protocol and review
We refined the search strategy for this review to increase precision of the search results.
We include one new author (MT).
We added 'Summary of findings' tables and GRADE ratings.
We updated the eligibility criteria of study designs in line with changes to EPOC criteria for study designs eligible for inclusion.
We updated the objective "To determine whether a sustained increase in hand hygiene compliance can reduce rates of health care‐associated infection" and replaced it with "To determine whether an increase in hand hygiene compliance can reduce rates of health care‐associated infection", since studies examined the effect of changes in hand hygiene compliance, not a sustained increase in hand hygiene compliance.
The primary objective is: "To assess the short‐ and long‐term success of strategies to improve hand hygiene compliance in patient care". Because of inconsistency in duration of follow‐up, and no clear definition of 'long‐term success' in the literature or the studies, we focused only on short‐term success, with "short‐term" being the interval reported by the researchers.
In the original Gould 2007 review and the Gould 2010 update, the excluded studies lists summarised the reasons for lack of eligibility of the 129 excluded studies. The purpose was to highlight the types of studies being undertaken, most of which were uncontrolled before‐after designs, and explain why so few studies were eligible despite the large volume of publications. Since the Gould 2010 update, there has been a marked increase in the number of studies conducted using research designs that are potentially eligible for inclusion, so there is no longer a need to provide this level of detail. The excluded studies table now lists only randomised trials, non‐randomised trials, controlled before‐after studies and ITS studies failing to meet EPOC eligibility criteria. As previously discussed, one of the original studies (Gould 1997) is no longer eligible for inclusion and has therefore been removed from the list of included studies and added to the Characteristics of excluded studies table. Overall, while the types of studies differed between the original review and the first updated review, the reasons for exclusion were similar, primarily relating to insufficiency of control groups or inadequate data points in ITS studies.
Contributions of authors
Papers were reviewed by DJG, DM, ND and JC. ND or DM acted as arbiter in cases of disagreement. MT assisted in judgements related to eligibility, risk of bias, and/or statistical analyses for this update. DJG and DM compiled the final report.
Sources of support
Internal sources
City Hospital, London, UK.
Memorial University School of Nursing, St John's, Canada.
External sources
Department of Health Cochrane Review Incentive Scheme 2005 and 2010, UK.
Declarations of interest
DJG co‐authored one of the excluded studies (Gould 1997).
DM: none known
ND: none known
JC: none known
MT: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Armellino 2012 {published data only}
- Armellino D, Hussain E, Schilling ME, Senicola W, Eichorn A, Dlugacz Y, et al. Using high‐technology to enforce low‐technology safety measures: The use of third‐party remote video auditing and real‐time feedback in healthcare. Clinical Infectious Diseases 2012;54(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Derde 2014 {published data only}
- Derde LP, Cooper BS, Goossens H, Malhotra‐Kumar S, Willems RJL, Gniadkowski M, et al. Interventions to reduce colonisation and transmission of anti‐microbial resistant bacteria in intensive care units: an interrupted time series study and cluster randomized trial. Lancet 2014;14(1):31‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Diegel‐Vacek 2016 {published data only}
- Diegel‐Vacek L, Ryan C. Promoting hand hygiene with a lighting prompt. HERD 2016;10(1):65‐75. [DOI] [PubMed] [Google Scholar]
Fisher 2013a {published data only}
- Fisher DA, Seetoh T, Oh May‐Lin H, Viswanathan S, Toh Y, Yin WC, et al. Automated measures of hand hygiene compliance among healthcare workers using ultrasound: validation and a randomized controlled trial. Infection Control & Hospital Epidemiology 2013;34(9):919‐28. [DOI] [PubMed] [Google Scholar]
Fuller 2012 {published data only}
- Fuller C, Michie S, Savage J, McAteer J, Besser S, Charlett A, et al. The Feedback Intervention Trial (FIT)‐‐improving hand‐hygiene compliance in UK healthcare workers: a stepped wedge cluster randomised controlled trial. PLoS ONE [Electronic Resource] 2012;7(10):e41617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grant 2011 {published data only}
- Grant AM, Hofmann DA. It's not all about me: motivating hand hygiene among health care professionals by focusing on patients. Psychological Science 2011;22(12):1494‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2013 {published data only}
- Higgins A, Hannan MM. Improved hand hygiene technique and compliance in healthcare workers using gaming technology. Journal of Hospital Infection 2013;84(1):32‐7. [DOI] [PubMed] [Google Scholar]
Ho 2012 {published data only}
- Ho ML, Seto WH, Wong LC, Wong TY. Effectiveness of multifaceted hand hygiene interventions in long‐term care facilities in Hong Kong: a cluster‐randomized controlled trial. Infection Control & Hospital Epidemiology 2012;33(8):761‐7. [DOI] [PubMed] [Google Scholar]
Huang 2002 {published data only}
- Huang J, Jiang D, Wang X, Liu Y, Fennie K, Burgess J, et al. Changing knowledge, behavior and practice related to universal precautions among hospital nurses in China. Journal of Continuing Education in Nursing 2002;33(5):217‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Huis 2013 {published data only}
- Huis A, Schoonhoven L, Grol R, Donders R, Hulscher M, Achterberg T. Impact of a team and leaders‐directed strategy to improve nurses' adherence to hand hygiene guidelines: a cluster randomised trial. International Journal of Nursing Studies 2013;50(4):464‐74. [DOI] [PubMed] [Google Scholar]
King 2016 {published data only}
- King D, Vlaev I, Everett‐Thomas R, Fitzpatrick M, Darzi A, Birnbach DJ. "Priming" hand hygiene compliance in clinical environments. Health psychology 2016;35(1):96‐101. [DOI] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee AS, Cooper BS, Malhotra‐Kumar S, Chalfine A, Daikos GL, Fankhauser C, et al. Comparison of strategies to reduce meticillin‐resistant Staphylococcus aureus rates in surgical patients: a controlled multicentre intervention trial. BMJ open 2013;3(9):e003126, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin‐Madrazo 2012 {published data only}
- Martin‐Madrazo C, Soto‐Diaz S, Canada‐Dorado A, Salinero‐Fort MA, Medina‐Fernandez M, Carrillo de Santa Pau E, et al. Cluster randomized trial to evaluate the effect of a multimodal hand hygiene improvement strategy in primary care. Infection Control & Hospital Epidemiology 2012;33(7):681‐8. [DOI] [PubMed] [Google Scholar]
Mertz 2010 {published data only}
- Mertz D, Dafoe N, Walter SD, Brazil K, Loeb M. Effect of a multifaceted intervention on adherence to hand hygiene among healthcare workers: a cluster‐randomized trial. Infection Control & Hospital Epidemiology 2010;31(11):1170‐6. [DOI] [PubMed] [Google Scholar]
Midturi 2015 {published data only}
- Midturi JK, Narasimhan A, Barnett T, Sodek J, Schreier W, Barnett J, et al. A successful multifaceted strategy to improve hand hygiene compliance rates. American Journal of Infection Control 2015;43(5):533‐6. [DOI] [PubMed] [Google Scholar]
Moghnieh 2016 {published data only}
- Moghnieh R, Soboh R, Abdallah D, El‐Helou M, Al Hassan S, Ajjour L, et al. Health care workers' compliance to the My 5 Moments for Hand Hygiene: Comparison of 2 interventional methods. American Journal of Infection Control 2017;45(1):89‐91. [DOI: 10.1016/j.ajic.2016.08.012] [DOI] [PubMed] [Google Scholar]
Munoz‐Price 2014 {published data only}
- Munoz‐Price LS, Patel Z, Banks S, Arheart K, Eber S, Lubarsky DA, et al. Randomized crossover study evaluating the effect of a hand sanitizer dispenser on the frequency of hand hygiene among anesthesiology staff in the operating room. Infection Control & Hospital Epidemiology 2014;35(6):717‐20. [DOI] [PubMed] [Google Scholar]
Perlin 2013 {published data only}
- Perlin JB, Hickok JD, Septimus EJ, Moody JA, Englebright JD, Bracken RM. A bundled approach to reduce methicillin‐resistant Staphylococcus aureus infections in a system of community hospitals. Journal for Healthcare Quality 2013;35(3):57‐68. [DOI] [PubMed] [Google Scholar]
Rodriguez 2015 {published data only}
- Rodriguez V, Giuffre C, Villa S, Almada G, Prasopa‐Plaizier N, Gogna M, et al. A multimodal intervention to improve hand hygiene in ICUs in Buenos Aires, Argentina: a stepped wedge trial. International Journal for Quality in Health Care 2015;27(5):405‐11. [DOI] [PubMed] [Google Scholar]
Rosenbluth 2015 {published data only}
- Rosenbluth G, Garritson S, Green AL, Milev D, Vidyarthi AR, Auerbach AD, et al. Achieving hand hygiene success with a partnership between graduate medical education, hospital leadership, and physicians. American Journal of Medical Quality 2015;31(6):577‐83. [DOI] [PubMed] [Google Scholar]
Stevenson 2014 {published data only}
- Stevenson KB, Searle K, Curry G, Boyce JM, Harbarth S, Stoddard GJ, et al. Infection control interventions in small rural hospitals with limited resources: results of a cluster‐randomized feasibility trial. Antimicrobial Resistance & Infection Control 2014;3(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewardson 2016 {published data only}
- Stewardson AJ, Sax H, Gayet‐Ageron A, Touveneau S, Longtin Y, Zingg W, et al. Enhanced performance feedback and patient participation to improve hand hygiene compliance of health‐care workers in the setting of established multimodal promotion: a single‐centre, cluster randomised controlled trial. Lancet Infectious Diseases 2016;16(12):1345‐55. [PUBMED: 27599874] [DOI] [PubMed] [Google Scholar]
Talbot 2013 {published data only}
- Talbot TR, Johnson JG, Fergus C, Domenico JH, Schaffner W, Daniels TL, et al. Sustained improvement in hand hygiene adherence: utilizing shared accountability and financial incentives. Infection Control & Hospital Epidemiology 2013;34(11):1129‐36. [DOI] [PubMed] [Google Scholar]
Vernaz 2008 {published data only}
- Vernaz N, Sax H, Pittet D, Bonnabry P, Schrenzel J, Harbath S. Temporal effects of antibiotic use and hand rub consumption on the incidence of MRSA and Clostridium difficile. Journal of Antimicrobial Chemotherapy 2008;62(3):601‐7. [DOI] [PubMed] [Google Scholar]
Whitby 2008 {published data only}
- Whitby M, McLaws ML, Slater K, Tong E, Johnson Bl. Three successful interventions in health workers that improve compliance with hand hygiene: is sustained replication possible?. American Journal of Infection Control 2008;36(5):349‐55. [DOI] [PubMed] [Google Scholar]
Yeung 2011 {published data only}
- Yeung WK, Tam WS, Wong TW. Clustered randomized controlled trial of a hand hygiene intervention involving pocket‐sized containers of alcohol‐based hand rub for the control of infections in long‐term care facilities. Infection Control & Hospital Epidemiology 2011;32(1):67‐76. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aboumater 2012 {published data only}
- Aboumater H, Ristaino H, Davis RO, Thompson CB, Maragakis L, Cosgrove S, et al. Infection prevention promotion program based on the PRECEDE model: Improving hand hygiene behaviors among healthcare personnel. Infection Control & Hospital Epidemiology 2012;33(2):144‐51. [DOI] [PubMed] [Google Scholar]
Adams 2013 {published data only}
- Adams K, Heath D, Sood G, Grubb L, Bauernfiend J, Reuland C. Positive reinforcement to create and sustain a culture change in hand hygiene practices in a tertiary care academic facility. American Journal of Infection Control 2013;41(6 Suppl 1):S95. [Google Scholar]
Al Tawfiq 2013 {published data only}
- Al‐Tawfiq JA, Abed MS, Al‐Yami N, Birrer RB. Promoting and sustaining a hospital‐wide, multifaceted hand hygiene program resulted in significant reduction in health care‐associated infections. American Journal of Infection Control 2013;41(6):482‐6. [DOI] [PubMed] [Google Scholar]
Armellino 2013 {published data only}
- Armellino D, Trivedi M, Law I, Singh N, Schilling ME, Hussain E, et al. Replicating changes in hand hygiene in a surgical intensive care unit with remote video auditing and feedback. American Journal of Infection Control 2013;41(10):925‐7. [DOI] [PubMed] [Google Scholar]
Assanasen 2008 {published data only}
- Assanasen S, Edmond M, Bearman G. Impact of 2 different levels of performance feedback on compliance with infection control process measures in 2 intensive care units. American Journal of Infection Control 2008;36(6):407‐13. [DOI] [PubMed] [Google Scholar]
Barnett 2013 {published data only}
- Barnett T, Sodek J, Narasimhan A, Barnett J, Wheeler C, Schreier W, et al. A successful multifaceted strategy to improve hand hygiene compliance rates at a major teaching institution. American Journal of Infection Control 2013;41(6 Suppl 1):S11‐S12. [DOI] [PubMed] [Google Scholar]
Barrera 2011 {published data only}
- Barrera L, Zingg W, Mendez F, Pittet D. Effectiveness of a hand hygiene promotion strategy using alcohol‐based handrub in 6 intensive care units in Colombia. American Journal of Infection Control 2011;39(8):633‐9. [DOI] [PubMed] [Google Scholar]
Barrow 2009 {published data only}
- Barrow B, Mehler P, Price C. A communications campaign designed to improve hand hygiene compliance and reduce infection rates. Journal of Communication in Healthcare 2009;2(1):61‐77. [Google Scholar]
Bellis 2006 {published data only}
- Bellis K. Hand hygiene compliance in a Victorian multi‐centred study. Infection Control Association Fourth Biennial Conference 2006? The Key to Success 20‐22 September 2006, Sydney, Australia.. Australian Infection Control 2006;11(Part 4):141. [Google Scholar]
Bittner 2002 {published data only}
- Bittner MJ, Rich EC, Turner PD, Arnold WH Jr. Limited impact of sustained simple feedback based on soap and paper towel consumption on the frequency of hand washing in an adult intensive care unit. Infection Control & Hospital Epidemiology 2002;23(3):120‐6. [DOI] [PubMed] [Google Scholar]
Chan 2013 {published data only}
- Chan BP, Homa K, Kirkland KB. Effect of varying the number and location of alcohol‐based hand rub dispensers on usage in a general inpatient medical unit. Infection Control & Hospital Epidemiology 2013;34(9):987‐9. [DOI] [PubMed] [Google Scholar]
Chen 2011 {published data only}
- Chen YC, Sheng WH, Wang JT, S.Chang ST, Lin HC, Tien KL, et al. Effectiveness and limitations of hand hygiene promotion on decreasing healthcare‐associated infections. PLoS ONE 2011;6(11):e27163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christiaens 2009 {published data only}
- Christiaens G, Barbier C, Mitsers J, Warnotte J, Mol P, Bouffioux C. Hand hygiene: first measure to control nosocomial infections [Hygiène des mains: première mesure pour la maîtrise des infections nosocomiales]. Revue Medicale de Liege 2009;61(1):31‐6. [PubMed] [Google Scholar]
Colombo 2002 {published data only}
- Colombo C, Giger H, Grote J, Deplazes C, Pletscher W, Luthi R, et al. Impact of teaching interventions on nurse compliance with hand disinfection. Journal of Hospital Infection 2002;51(1):69‐72. [DOI] [PubMed] [Google Scholar]
Conly 1989 {published data only}
- Conly JM, Hill S, Ross J, Lertzman J, Louie TJ. Handwashing practices in an intensive care unit: the effects of an educational program and its relationship to infection rates. American Journal of Infection Control 1989;17(6):330‐9. [DOI] [PubMed] [Google Scholar]
Conrad 2010 {published data only}
- Conrad A, Kaier K, Frank U, Dettenkofer M. Are short training sessions on hand hygiene effective in preventing hospital‐acquired MRSA? A time‐series analysis. American Journal of Infection Control 2010;38(7):559‐61. [DOI] [PubMed] [Google Scholar]
Creel 2014 {published data only}
- Creel P. Deployment of an automated hand‐hygiene compliance monitoring system in an additional nursing unit yields faster success. American Journal Infection Control 2014;42(6 Suppl 1):S129‐S130. [Google Scholar]
Crews 2013 {published data only}
- Crews JD, Whaley E, Syblik D, Starke J. Sustained improvement in hand hygiene at a children's hospital. Infection Control & Hospital Epidemiology 2013;34(7):751‐3. [DOI] [PubMed] [Google Scholar]
Donnellan 2011 {published data only}
- Donnellan RA, Ludher J, Brydon M. A novel approach to auditing the compliance of hand hygiene and staff behaviour change. Healthcare Infection 2011;16(2):55‐60. [Google Scholar]
Donowitz 1986 {published data only}
- Donowitz LG. Failure of the overgown to prevent nosocomial infection in a pediatric intensive care unit. Pediatrics 1986;77(1):35‐8. [PubMed] [Google Scholar]
Dos Santos 2011 {published data only}
- Dos Santos RP, Jacoby T, Machado DP, Lisboa T, Gastal SL, Nagel FM, et al. Hand hygiene, and not ertapenem use, contributed to reduction of carbapenem‐resistant Pseudomonas aeruginosa rates. Infection Control & Hospital Epidemiology 2011;32(6):584‐90. [DOI] [PubMed] [Google Scholar]
Duerink 2006 {published data only}
- Duerink DO, Farida H, Nagelkirke NJ, Wahyono H, Keuter M, Lestari EM, et al. Preventing nosocomial infections: improving compliance with standard precautions in an Indonesian teaching hospital. Journal of Hospital Infection 2006;64(1):36‐43. [DOI] [PubMed] [Google Scholar]
Eldridge 2006 {published data only}
- Eldridge NE, Woods SS, Bonello RS, Clutter K, Ellingson L, Harris MA, et al. Using the six sigma process to implement the Centers for Disease Control and Prevention Guideline for Hand Hygiene in 4 intensive care units. Journal of General Internal Medicine 2006;21(Supplement 2):S35‐S42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2013b {published data only}
- Fisher D, Tambyah PA, Lin RT, Jureen R, Cook AR, Lim A, et al. Sustained meticillin‐resistant Staphylococcus aureus control in a hyper‐endemic tertiary acute care hospital with infrastructure challenges in Singapore. Journal of Hospital Infection 2013;85(2):141‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giannitsioti 2009 {published data only}
- Giannitsioti E, Athanasia S, Antoniadou A, Fytrou H, Athanassiou K, Bourvani P, et al. Does a bed rail system of alcohol‐based handrub antiseptic improve compliance of health workers with hand hygiene?. American Journal of Infection Control 2009;37(2):160‐3. [DOI] [PubMed] [Google Scholar]
Golan 2006 {published data only}
- Golan Y, Doron S, Griffin J, Gamal H, Tanios M, Blunt K, et al. The impact of gown‐use requirement on hand hygiene compliance. Clinics in Infectious Diseases 2006;42(3):370‐6. [DOI] [PubMed] [Google Scholar]
Gould 1997 {published data only}
- Gould DJ, Chamberlain A. The use of a ward‐based educational teaching package to enhance nurses’ compliance with infection control procedures. Journal of Clinical Nursing 1997;6(1):55–67. [DOI] [PubMed] [Google Scholar]
Grayson 2008 {published data only}
- Grayson ML, Jarvie LJ, Martin R, Johnson PD, Jodain ME, McMullan C, et al. Significant reductions in methicillin‐resistant Staphylococcus aureus bacteraemia and clinical isolates associated with a multisite, hand hygiene culture‐change program and subsequent successful statewide roll‐out. Medical Journal of Australia 2008;188(11):633‐40. [DOI] [PubMed] [Google Scholar]
Grayson 2011 {published data only}
- Grayson ML, Russo PL, Cruickshank M, Bear JL, Gee CA, Hughes CF, et al. Outcomes from the first 2 years of the Australian National Hand Hygiene Initiative. Medical Journal of Australia 2011;195(10):615‐9. [DOI] [PubMed] [Google Scholar]
Harne‐Bittner 2011 {published data only}
- Harne‐Bittner S, Allen M, Fowler KA. Improving hand hygiene adherence among nursing staff. Journal of Nursing Care Quality 2011;26(1):39‐48. [DOI] [PubMed] [Google Scholar]
Huang 2006 {published data only}
- Huang SS, Yokoe DS, Hinirichsen VL, Spurchise LS, Datta R, Miroshnik I, et al. Impact of routine intensive care unit surveillance cultures and resultant barrier precautions on hospital‐wide methicillin‐resistant Staphylococcus aureus bacteremia. Clinical Infectious Diseases 2006;43(8):971‐8. [DOI] [PubMed] [Google Scholar]
Huang 2008 {published data only}
- Huang TT, Wu SC. Evaluation of a training programme on knowledge and compliance of nurse assistants' hand hygiene in nursing homes. Journal of Hospital Infection 2008;68(2):164‐70. [DOI] [PubMed] [Google Scholar]
Kohli 2009 {published data only}
- Kohli E, Ptak J, Smith R, Taylor E, Talbot EA, Kirkland KB. Variability in the Hawthorne effect with regard to hand hygiene performance in high‐ and low‐performing inpatient care units. Infection Control & Hospital Epidemiology 2009;30(3):222‐5. [DOI] [PubMed] [Google Scholar]
Larson 1991 {published data only}
- Larson E, McGeer A, Quraishi ZA, Krenzischek D, Parsons BJ, Holdford J, et al. Effect of an automated sink on handwashing practices and attitudes in high‐risk units. Infection Control & Hospital Epidemiology 1991;12(7):422‐8. [DOI] [PubMed] [Google Scholar]
Larson 1997 {published data only}
- Larson EL, Bryan JL, Adler LM, Blane C. A multifactorial approach to changing handwashing behaviour. American Journal of Infection Control 1997;25(1):3‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Larson 2000 {published data only}
- Larson EL, Early E, Cloonan P, Sugrue S, Parides M. An organisational climate intervention associated with increased handwashing and decreased nosocomial infections. Behavioral Medicine 2000;26(1):14‐20. [DOI] [PubMed] [Google Scholar]
Linam 2011 {published data only}
- Linam WM. Improving patient safety by creating lasting behavior change: lessons learned from hand hygiene. Journal of the Arkansas Medical Society 2011;107(13):282‐3. [PubMed] [Google Scholar]
Lobo 2010 {published data only}
- Lobo RD, Levin AS, Oliveira MS, Gomes LMB, Gobara S, Park M, et al. Evaluation of interventions to reduce catheter‐associated bloodstream infection: Continuous tailored education versus one basic lecture. American Journal of Infection Control 2010;38(6):440‐448. [DOI] [PubMed] [Google Scholar]
Madani 2006 {published data only}
- Madani TA, Albarrak AM, Alhazmi MA, Alazraqi TA, Althaqafi AO, Ishaq AH. Steady improvement of infection control services in six community hospitals in Makkah following annual audits during Hajj for four consecutive years. BMC Infectious Diseases 2006;6:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marra 2008 {published data only}
- Marra AR, D'Arco C, Bravim BA, Bde A, Martino MD, Correa L, et al. Controlled trial measuring the effect of a feedback intervention on hand hygiene compliance in a step‐down unit. Infection Control & Hospital Epidemiology 2008;29(8):730‐5. [DOI] [PubMed] [Google Scholar]
Marra 2010 {published data only}
- Marra AR, Guastelli LR, Araújo CMP, Dos Santos JLS, Lamblet LC, Silva M, et al. Positive deviance: a new strategy for improving hand hygiene compliance. Infection Control & Hospital Epidemiology 2010;31(1):12‐20. [DOI] [PubMed] [Google Scholar]
Marra 2011 {published data only}
- Marra AR, Guastelli LR, Araujo CMP, Dos Santos JLS, Filho MAO, Silva CV, et al. Positive deviance: A program for sustained improvement in hand hygiene compliance. American Journal of Infection Control 2011;39(1):1‐5. [DOI] [PubMed] [Google Scholar]
Marra 2013a {published data only}
- Marra AR, Camargo TZS, Cardoso VJ, Moura DF Jr, Casemiro de Andrade E, Wentzcovitch J, et al. Hand hygiene compliance in the critical care setting: A comparative study of 2 different alcohol handrub formulations. American Journal of Infection Control 2013;41(2):136‐9. [DOI] [PubMed] [Google Scholar]
Marra 2013b {published data only}
- Marra AR, Noritomi DT, Westheimer Cavalcante AJ, Sampaio Camargo TZ, Bortoleto RP, Durao Junior MS, et al. A multicenter study using positive deviance for improving hand hygiene compliance. American Journal of Infection Control 2013;41(11):984‐8. [DOI] [PubMed] [Google Scholar]
Marra 2014 {published data only}
- Marra AR, Sampaio Camargo TZ, Magnus TP, Blaya RP, Dos Santos GB, Guastelli LR, et al. The use of real‐time feedback via wireless technology to improve hand hygiene compliance. American Journal of Infection Control 2014;42(6):608‐11. [DOI] [PubMed] [Google Scholar]
Mayer 1986 {published data only}
- Mayer JA, Dubbert PM, Miller M, Burkett PA, Chapman SW. Increasing handwashing in an intensive care unit. Infection Control 1986;7(5):259‐62. [DOI] [PubMed] [Google Scholar]
McLaws 2009 {published data only}
- McLaws ML, Pantle AC, Fitzpatrick KR, Hughes C. Improvements in hand hygiene across New South Wales public hospitals: clean hands save lives, Part III. Medical Journal of Australia 2009;191(Supplement):S18‐S25. [DOI] [PubMed] [Google Scholar]
Miyachi 2007 {published data only}
- Miyachi H, Furuya H, Umezawa K, Itoh Y, Ohshima T, Miyamoto M, et al. Controlling methicillin‐resistant Staphylococcus aureus by stepwise implementation of preventive strategies in a university hospital: impact of a link‐nurse system on the basis of multidisciplinary approaches. American Journal of Infection Control 2007;35(2):115‐21. [DOI] [PubMed] [Google Scholar]
Molina‐Cabrillana 2010 {published data only}
- Molina‐Cabrillana, Alvarez‐León JEE, Quori A, García‐De Carlos P, López‐Carrió I, Bolaños‐Rivero M, et al. Assessment of a hand hygiene program on healthcare‐associated infection control [Impacto de la mejora de la higiene de las manos sobre las infecciones hospitalarias]. Revista de Calidad Asistencial 2010;25(4):215‐22. [DOI] [PubMed] [Google Scholar]
Peterson 2012 {published data only}
- Peterson TH, Teman SF, Connors RH. A safety culture transformation: its effects at a children's hospital. Journal of Patient Safety 2012;8(3):125‐30. [DOI] [PubMed] [Google Scholar]
Picheansathian 2008 {published data only}
- Picheansathian W, Pearson A, Suchaxaya P. The effectiveness of a promotion programme on hand hygiene compliance and nosocomial infections in a neonatal intensive care unit. International Journal of Nursing Practice 2008;14(4):315‐21. [DOI] [PubMed] [Google Scholar]
Raju 1991 {published data only}
- Raju TK, Kobier C. Improving handwashing habits in the newborn nurseries. American Journal of the Medical Sciences 1991;302(6):355‐8. [DOI] [PubMed] [Google Scholar]
Rees 2013 {published data only}
- Rees S, Houlahan B, Safdar N, Sanford‐Ring S, Shore T, Schmitz M. Success of a multimodal program to improve hand hygiene compliance. Journal of Nursing Care Quality 2013;28(4):312‐8. [DOI] [PubMed] [Google Scholar]
Rupp 2008 {published data only}
- Rupp ME, Fitzgerald T, Puumala S, Anderson JR, Craig R, Iwen PC, et al. Prospective, controlled, cross‐over trial of alcohol‐based hand gel in critical care units. Infection Control & Hospital Epidemiology 2008;29(1):8‐15. [DOI] [PubMed] [Google Scholar]
Sakamoto 2010 {published data only}
- Sakamoto F, Yamada H, Suzuki C, Sugiura H, Tokuda Y. Increased use of alcohol‐based hand sanitizers and successful eradication of methicillin‐resistant Staphylococcus aureus from a neonatal intensive care unit: A multivariate time series analysis. American Journal of Infection Control 2010;38(7):529‐34. [DOI] [PubMed] [Google Scholar]
Schweon 2012 {published data only}
- Schweon SS, Edmonds S, Kirk JM. Effectiveness of a comprehensive hand hygiene program for reduction of infection rates in a long‐term care facility: Lessons learned. American Journal of Infection Control 2012;40(5):e149. [DOI] [PubMed] [Google Scholar]
Song 2013 {published data only}
- Song X, Stockwell DC, Floyd T, Short BL, Singh N. Improving hand hygiene compliance in health care workers: Strategies and impact on patient outcomes. American Journal of Infection Control 2013;41(10):e101‐5. [DOI] [PubMed] [Google Scholar]
Sopirala 2014 {published data only}
- Sopirala MM, Yahle‐Dunbar L, Smyer J, Wellington L, Dickman J, Zikri N, et al. Infection control link nurse program: An interdisciplinary approach in targeting health care‐acquired infection. American Journal of Infection Control 2014;42(4):353‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stella 2013 {published data only}
- Stella SA, Stace R, Miller A, Keniston A, Burden M, Price CS, et al. Eye images improve hand hygiene compliance in an emergency department. Journal of General Internal Medicine 2013;28:S76. [Google Scholar]
Stoesser 2013 {published data only}
- Stoesser N, Emary K, Soklin S, Peng An K, Sophal S, Chhomrath S, et al. The value of intermittent point‐prevalence surveys of healthcare‐associated infections for evaluating infection control interventions at Angkor Hospital for Children, Siem Reap, Cambodia. Transactions of the Royal Society of Tropical Medicine & Hygiene 2013;107:248‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2007 {published data only}
- Stone S, Slade R, Fuller C, Charlett A, Cookson B, Teare L, et al. Early communication: does a national campaign to improve hand hygiene in the NHS work? Initial English and Welsh experience from the NOSEC study (National Observational Study to Evaluate the CleanYourHands Campaign). Journal of Hospital Infection 2007;66:293–6. [DOI] [PubMed] [Google Scholar]
Stone 2011 {published data only}
- Stone S. The feedback intervention trial: A national stepped wedge cluster randomised controlled trial to improve hand hygiene. BMC Proceedings 2011;5 (Supp 6):066. [Google Scholar]
Trick 2007 {published data only}
- Trick WE, Vernon MO, Welbel SF, Demarais P, Hayden MK, Weinstein RA. Multicenter intervention program to increase adherence to hand hygiene recommendations and glove use and to reduce the incidence of antimicrobial resistance. Infection Control & Hospital Epidemiology 2007;28(1):42‐9. [DOI] [PubMed] [Google Scholar]
Van de Mortel 2006 {published data only}
- Mortel T, Murgo M. An examination of covert observation and solution audit as tools to measure the success of hand hygiene interventions. American Journal of Infection Control 2006;34(3):95‐9. [DOI] [PubMed] [Google Scholar]
Vinci 2012 {published data only}
- Vinci C, Bunson J, Govednik J, McGuckin M. Hand hygiene rates for rehabilitation and long term care facilities: One hospital's journey through the national goal and benchmarks. American Journal of Infection Control 2012;40(5):e88. [Google Scholar]
Walker 2013 {published data only}
- Walker JL, Sistrunk WW, Higginbotham MA, Burks K, Halford L, Goddard L, et al. Evaluation of a new hand hygiene monitoring program; feedback and salient observers critical to program success. American Journal of Infection Control 2013;41(6 Suppl 1):S14. [Google Scholar]
Walker 2014 {published data only}
- Walker J, Higginbotham MA, Sistrunk W, May T, Goddard L, Austin C, et al. Visible hand hygiene monitors are critical to improving and sustaining hand hygiene compliance. American Journal of Infection Control 2014;42(6 Suppl 1):S126. [DOI] [PubMed] [Google Scholar]
Additional references
Azjen 1991
- Azjen I. The theory of planned behavior. Organisational Behavior and Human Decision Processes 1991;50:179‐211. [Google Scholar]
Badia 2017
- Badia JM, Casey AL, Petrosillo N, Hudson PM, Mitchell SA, Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. Journal of Hospital Infection 2017;96(1):1‐15. [DOI] [PubMed] [Google Scholar]
Ballini 2010
- Ballini L, Bero L, Eccles MP, Grimshaw J, Gruen RL, Lewin S, et al. Cochrane Effective Practice and Organisation of Care Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2010; Vol. Issue 3, issue Art. No.: EPOC.
Bargh 1992
- Bargh JA. The ecology of automaticity: toward establishing the conditions needed to produce automatic processing effects. American Journal of Psychology 1992;105(2):181‐99. [PubMed] [Google Scholar]
Brewster 2016
- Brewster L, Tarrant C, Dixon‐Woods M. Qualitative study of views and experiences of performance management for healthcare‐associated infections. Journal of Hospital Infection 2016;94(1):41‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2016
- Centers for Disease Control. National and State Healthcare Associated Infections Progress Report. www.cdc.gov/HAI/pdfs/progress‐report/hai‐progress‐report.pdf 2016 (accessed 10th July 2017).
CNISP 2015
- Canadian Nosocomial Infection Surveillance Program. Antimicrobial Resistant Organisms Surveillance Summary Report from January 1, 2009 to December 31, 2014. Public Health Agency of Canada www.healthycanadians.gc.ca/publications/drugs‐products‐medicaments‐produits/antimicrobial‐summary‐sommaire‐antimicrobien/index‐eng.php#s3 2015 (accessed 10th July 2017).
ECDC 2013a
- European Centre for Disease Prevention and Control (ECDC). Point Prevalence Survey of Healthcare Associated Infections and Antimicrobial Use in European Acute Care Hospitals 2011‐2012. Stockholm: ECDC, 2013. [DOI] [PubMed] [Google Scholar]
EPOC 2013b
- Effective Practice, Organisation of Care (EPOC). What study designs should be included in an EPOC review?. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services. Available at: epoc.cochrane.org/epoc‐specific‐resources‐review‐authors 2013.
EPOC 2013c
- Effective Practice, Organisation of Care (EPOC). Data extraction and management. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services. Available at: epoc.cochrane.org/epoc‐specific‐resources‐review‐authors 2013.
EPOC 2013d
- Effective Practice, Organisation of Care (EPOC). EPOC worksheets for preparing a Summary of Findings (SoF) table using GRADE. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services. Available at: epoc.cochrane.org/epoc‐specific‐resources‐review‐authors 2013.
EPOC 2014
- Effective Practice, Organisation of Care (EPOC). How to develop a search strategy. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services. 2014.
EPOC 2015a
- EPOC. EPOC Taxonomy. epoc.cochrane.org/epoc‐taxonomy 2015.
EPOC 2015b
- Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC Resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services. Available at: epoc.cochrane.org/epoc‐specific‐resources‐review‐authors 2015.
ESPAUR 2015
- Public Health England. English Surveillance Programme for Antimicrobial Utilisation and Resistance Report. www.gov.uk/government/news/espaur‐report‐reveals‐continued‐rise‐in‐antibiotic‐resistant‐infections 2015 (accessed 10th July 2017).
Etchells 2012
- Etchells E, Koo M, Daneman N, McDonald A, Baker M, Matlow A, et al. Comparative economic analysis of patient safety improvement strategies in acute care: a systematic review. BMJ Quality and Safety 2012;21(6):448‐56. [DOI] [PubMed] [Google Scholar]
Freeth 2012
- Freeth D, Sandall J, Allan T, Warburton F, Berridge EJ, Mackintosh N, et al. A methodological study to compare survey‐based and observation‐based evaluations of organisational and safety culture and then compare both approaches with markers of the quality of health care. National Institute of Health Assessment Technology Programme 2012: www.ncbi.nlm.nih.gov/books/NBK97262/pdf/Bookshelf_NBK97262.pdf 2012 (accessed 10th July 2017). [DOI] [PubMed]
Gould 2017
- Gould DJ, Creedon S, Jeanes A, Drey NS, Chudleigh J, Moralejo D. The Hawthorne and avoidance effects in hand hygiene practice and research: a methodological reconsideration. Journal of Hospital Infection 2017;95(2):169‐74. [DOI] [PubMed] [Google Scholar]
Grimshaw 2004
- Grimshaw J, Eccles M, Tetroe J. Implementing clinical guidelines: current evidence and future implications. Journal of Continuing Education in the Health Professions 2004;24:S31‐7. [DOI] [PubMed] [Google Scholar]
Haas 2007
- Haas JP, Larson EL. Measurement of compliance with hand hygiene. Journal of Hospital Infection 2007;66(1):6‐14. [DOI] [PubMed] [Google Scholar]
Health Foundation 2015
- Health Foundation. Infection prevention and control: lessons from acute care in England. Towards a whole health economy approach. Health Foundation Learning Report. www.health.org.uk/publication/infection‐prevention‐and‐control‐lessons‐acute‐care‐england 2015 (accessed 10th July 2017).
Health Protection Agency 2011
- Health Protection Agency. English National Point Prevalence Survey of Health care‐Associated Infections and Antimicrobial Use 2011. webarchive.nationalarchives.gov.uk/20140714095446/http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317134304594 2011 (accessed 10th July 2017).
Higgins 2011
- Higgins JPT, Altman DG. Chapter 8: Assessing risk of bias in included studies.. In: Higgins JPT, Green S editor(s). ICochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org., March 2011. [Google Scholar]
Hugonnet 2002
- Hugonnet S, Perneger TV, Pittet D. Alcohol‐based handrub improves compliance with hand hygiene in intensive care units. Archives of Internal Medicine 2002;162(9):1037–43. [DOI] [PubMed] [Google Scholar]
Huis 2012
- Huis A, Achterberg T, Bruin M, Grol R, Schoonhoven L, Hulscher M. A systematic review of hand hygiene improvement strategies: a behavioural approach. Implementation Science 2012;7:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ivers 2012
- Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard‐Jensen J, French SD, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD000259.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kingston 2016
- Kingston L, O'Connell NH, Dunne CP. Hand hygiene‐related clinical trials reported since 2010: A systematic review. Journal of Hospital infection 2016;92(4):309‐20. [DOI] [PubMed] [Google Scholar]
Kotler 1971
- Kotler P, Zaltman G. Social marketing: an approach to planned social change. Journal of Marketing 1971;35(3):3‐12. [PubMed] [Google Scholar]
Luangasanatip 2015
- Luangasanatip N, Hongsuwan M, Limmathurotsakul D, Lubell Y, Lee AS, Harbath S, et al. Comparative efficacy of interventions to promote hand hygiene in hospital: systematic review and network meta‐analysis. BMJ 2015;351:h3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magill 2014
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point‐prevalence survey of healthcare‐associated infection. New England Journal of Medicine 2014;370(13):1198‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahida 2016
- Mahida N. Hand hygiene compliance: are we kidding ourselves?. Journal of Hospital Infection 2016;92(4):307‐8. [DOI] [PubMed] [Google Scholar]
Marchetti 2013
- Marchetti A, Rossiter R. Economic burden of healthcare‐associated infection in US acute care hospitals. Journal of Medical Economics 2013;16(12):1399‐404. [DOI] [PubMed] [Google Scholar]
Mathai 2011
- Mathai E, Allegranzi B, Kilpatrick C, Bagheri Nejad S, Graafmans W, Pittet D. Promoting hand hygiene in healthcare through national/subnational campaigns. Journal of Hospital Infections 2011;77(4):294‐8. [DOI] [PubMed] [Google Scholar]
Naikoba 2001
- Naikoba S, Hayward A. The effectiveness of interventions aimed at increasing handwashing in healthcare workers ‐ a systematic review. Journal of Hospital Infection 2001;47(3):173‐80. [DOI] [PubMed] [Google Scholar]
PHAC 2016
- Public Health Agency of Canada. Canadian Antimicrobial Resistance Surveillance System Report 2016. www.canada.ca/en/public‐health/services/publications/drugs‐health‐products/canadian‐antimicrobial‐resistance‐surveillance‐system‐report‐2016.html. Ottawa: Public Health Agency of Canada, 2016 (accessed 10th July 2017).
Pittet 2000
- Pittet D, Hugonnet S, Harbarth S, Mourouga P, SauvanV, Touveneau S, et al. Effectiveness of a hospital‐wide programme to improve compliance with hand hygiene Infection Control Programme. Lancet 2000;356(9238):1307–12. [DOI] [PubMed] [Google Scholar]
Pittet 2004
- Pittet D. The Lowbury lecture: behaviour in infection control. Journal of Hospital Infection 2004;58(1):1‐13. [DOI] [PubMed] [Google Scholar]
Ramsay 2003
- Ramsay CR, Matowe L, Griili R, Grimshaw JM, Thomas RE. Interrupted time series designs in health technology assessment: lessons from two systematic reviews of behavior change strategies. International Journal of Technology Assessment in Health Care 2003;19(4):613‐23. [DOI] [PubMed] [Google Scholar]
Roethlisberger 1939
- Roethlisberger FJ. Management and the Worker; an Account of a Research Program Conducted by the Western Electric Company, Hawthorne Works, Chicago. Cambridge, USA: Harvard University Press, 1939. [Google Scholar]
Rutledge‐Taylor 2012
- Rutledge‐Taylor K, Marlow A, Gravel D, EmbreeJ, Saux N, Johnston L, et al. A point prevalence survey of healthcare‐associated infection in Canadian pediatric inpatients. American Journal of Infection Control 2012;40(6):491‐6. [DOI] [PubMed] [Google Scholar]
Sax 2007
- Sax H, Allegranzi B, Uçkay I, Larson E, Boyce J, Pittet D. ‘My five moments for hand hygiene’: a user‐centred design approach to understand, train, monitor and report hand hygiene. Journal of Hospital Infection 2007;67(1):9‐21. [DOI] [PubMed] [Google Scholar]
Schweizer 2014
- Schweitzer ML, Schacht Reisinger H, Ohl M, Formanek MB, Blevins A, Ward MA, et al. Searching for an optimal hand hygiene bundle: A meta‐analysis. Clinical Infectious Diseases 2014;58(2):248‐59. [DOI] [PubMed] [Google Scholar]
Shankar 2007
- Shankar A, Conner M, Bodansky HJ. Can the theory of planned behavior predict maintenance of frequently repeated behavior?. Psychology, Health and Medicine 2007;12(2):213‐224. [DOI] [PubMed] [Google Scholar]
Srigley 2015
- Srigley JA, Corace K, Hargadon DP, Yu D, MacDonald T, Fabrigar L, et al. Applying psychological frameworks of behaviour change to improve healthworker hand hygiene: a systematic review. Journal of Hospital Infection 2015;91(3):202‐10. [DOI] [PubMed] [Google Scholar]
Srigley 2016
- Srigley JA, Furness CD, Gardam M. Interventions to improve patient hand hygiene: a systematic review. Journal of Hospital Infection 2016;94(1):23‐9. [DOI] [PubMed] [Google Scholar]
Teare 1999
- Teare EL, Cookson B, French GL, Jenner EA, Scott G, Pallett A, et al. UK handwashing initiative. Journal of Hospital Infection 1999;43(1):1‐3. [DOI] [PubMed] [Google Scholar]
UK Five Year Antimicrobial Resistance Strategy
- UK Five Year Antimicrobial Resistance Strategy 2013‐2018. The Five Year Antimicrobial Resistance Strategy 2013‐2018. www.gov.uk/government/publications/uk‐5‐year‐antimicrobial‐resistance‐strategy‐2013‐to‐2018 (accessed 10th July 2017).
WHO Guidelines 2009
- World Health Organization. World Health Organization Guidelines on Hand Hygiene in Health Care. www.who.int/gpsc/5may/tools/9789241597906/en/ 2009 (accessed 10th July 2017).
References to other published versions of this review
Chudleigh 2004
- Chudleigh JH, Gould DJ, Grol R, Moralejo D. Interventions to improve hand hygiene compliance in patient care. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD005186] [DOI] [PubMed] [Google Scholar]
Gould 2007
- Gould D, Chudleigh JH, Moralejo D, Drey N. Interventions to improve hand hygiene compliance in patient care. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005186.pub2] [DOI] [PubMed] [Google Scholar]
Gould 2010
- Gould DJ, Moralejo D, Drey N, Chudleigh JH. Interventions to improve hand hygiene compliance in patient care.. Cochrane Database of Systematic Reviews 2010, Issue 9 Art. No.: CD005186.. [DOI: 10.1002/14651858.CD005186.pub3] [DOI] [PubMed] [Google Scholar]


