Abstract
Background
Sustained oral corticosteroid use can lead to complications, so there is interest in identifying agents that can reduce oral steroid use in people with asthma. Methotrexate has attracted attention as a possible steroid sparing agent in patients with chronic oral steroid dependent asthma.
Objectives
The objective of this review was to assess the effects of adding methotrexate to oral corticosteroids in adults with stable asthma who are dependent on oral corticosteroids.
Search methods
The Cochrane Airways Group Specialised Register and reference lists of identified articles were searched. Searches are current as of February 2006.
Selection criteria
Randomised trials of the addition of methotrexate compared with placebo in adult steroid dependent asthmatics. Duration of therapy needed to be at least 12 weeks.
Data collection and analysis
Trial quality was assessed and data extraction was carried out by two reviewers independently. Study authors were contacted for missing information.
Main results
Ten trials involving a total of 185 people were included. Study design and quality, corticosteroid dosages and outcomes varied widely. There was a reduction in oral corticosteroid dose favouring methotrexate in parallel trials (weighted mean difference ‐4.1 mg per day, 95% confidence interval ‐6.8 to ‐1.3) and also in cross‐over trials (weighted mean difference ‐2.9 mg per day, 95% confidence interval ‐5.9 to ‐0.2). There was no difference between methotrexate and placebo for forced expiratory volume in one minute (weighted mean difference 0.12 litre, 95% confidence interval ‐0.21 to 0.45). Hepatotoxicity was a common adverse effect with methotrexate compared to placebo (odds ratio 6.9, 95% confidence interval 3.1 to 15.5).
Authors' conclusions
Methotrexate may have a small steroid sparing effect in adults with asthma who are dependent on oral corticosteroids. However, the overall reduction in daily steroid use is probably not large enough to reduce steroid‐induced adverse effects. This small potential to reduce the impact of steroid side‐effects is probably insufficient to offset the adverse effects of methotrexate.
Plain language summary
Methotrexate as a steroid sparing agent for asthma in adults
Some people with asthma need to rely on corticosteroid drugs to control their asthma. Corticosteroids reduce the inflammation (swelling) of the airways (passages to the lungs). Long‐term use has serious adverse effects, so ways to try and cut down on the need for corticosteroids are sometimes tried. Methotrexate may also be able to reduce inflammation, and is sometimes used for arthritis. Its adverse effects include headaches, dizziness, fatigue and altered moods, and stomach, lung and liver complications. The review of trials found methotrexate provides little relief for asthma, and adds its own adverse effects.
Background
Most asthmatics require some form of prophylactic therapy. Inhaled corticosteroids are used most commonly for this purpose, but some patients have symptoms sufficiently severe to require prolonged or chronic use of oral corticosteroids. There are a large number of well recognised complications of chronic corticosteroid administration and concern regarding these complications has spurred the development of steroid sparing strategies. Following work done using steroid sparing agents in patients with rheumatoid arthritis, it was proposed that these agents would similarly be of benefit in reducing steroid dosage in asthmatics. A number of agents have been tried, including gold, cyclosporin & methotrexate.
Methotrexate (MTX) has been the most studied agent in asthma and its use in this role was first suggested in 1986 (Mullarkey 1986). MTX is a folic acid antagonist, which has anti‐neoplastic, immuno‐suppressive & anti‐ inflammatory effects. Its mechanism of action in producing a steroid‐sparing effect in asthma is uncertain (Cott 1994a, Delaney 1992, Moss 1995, Markham 1994, Weinblatt 1995). Glynn‐Barnhart 1991 have demonstrated that MTX does not affect prednisolone pharmacokinetics although it does appear to decrease theophylline clearance. MTX may work through inhibition of neutrophil chemotaxis to C5a, LTB4, and formyl‐methionyl‐leucyl‐phenylalanine & of monocyte chemotaxis to C5a. There may also be anti‐mitotic effects on mononuclear cells & MTX may directly affect granulocyte locomotion. Additionally there may be interference with IL‐1 production (Tsai 1994, Suarex 1987, Ternowitz 1987, Lammers 1987). A fuller discussion is provided by Cott 1994b; Lynch 1997 and Calderon 1991.
The toxicity profile differs from that found when MTX is used in cancer chemotherapy, but side effects occur in up to 60% of patients. Those most frequently seen are gastrointestinal, but headaches, dizziness, fatigue & altered moods also occur. Haematological toxicity is uncommon at low doses, but pulmonary toxicity may occur at any dose. Hepatotoxicity is uncommon and opportunistic infections are rare.
MTX is generally given as a once weekly dose in chronic disease, with dosage between 7.5 & 25 mg. Parenteral dosage is recommended when there is no response to oral therapy, if there is gastrointestinal toxicity, if the dose exceeds 20 mg per week or if there is doubt about oral bioavailability due to the disease process (Weinblatt 1995). MTX is used usually in combination with corticosteroid therapy. A literature search failed to identify any studies that investigated the use of MTX alone, so it is not possible to test whether there may be an effect of MTX in asthma in the absence of concurrent treatment with corticosteroids.
The use of methotrexate as an adjunct to oral corticosteroids has been reported in both open & randomised controlled studies in asthma. Most studies have only involved small numbers of subjects and the methodology has varied. To date, results have been conflicting. Previous narrative reviews give the overall impression that it is of benefit, but this has not been established. A systematic review with meta‐analysis may help to synthesise the data. One systematic review of methotrexate therapy for asthma has been carried out recently (Marin 1997), but this did not separate cross‐over and parallel group studies.
Objectives
To conduct a systematic review of the literature concerning the benefit of adding MTX to oral corticosteroids in stable adult asthmatics who are dependent on oral corticosteroids.
Methods
Criteria for considering studies for this review
Types of studies
All studies that were randomised double blind placebo controlled trials of methotrexate in stable steroid dependent adult asthmatics were included. Studies in languages other than English were to be included.
Types of participants
(a) Inclusion criteria
All trial patients were required to have "asthma", defined in operational terms.
Adults, arbitrarily defined as greater than 16 years old.
All trial patients to be "oral" corticosteroid dependent, defined in operational terms as at least three months continuous oral corticosteroid (OCS) use for control of asthma.
(b) Exclusion Criteria
Subjects not on chronic oral corticosteroids prior to trial.
Types of interventions
The addition of MTX or placebo in a blinded randomised fashion, to existing asthma medications, including chronic OCS use. The duration of therapy should have been at least 12 weeks. The route of administration could be oral or parenteral.
Types of outcome measures
Study outcomes comprised a wide range of measurements, including at least one of the following:
Alterations in maintenance oral corticosteroid (OCS) dosage.
Pulmonary function testing: peak expiratory flow rate (PEFR), forced expiratory volume in one second (FEV1) & any others
Symptoms.
Use of rescue medications (e.g. bronchodilators)
Frequency of asthma exacerbations.
Quality of Life Scores.
Frequency of use and variation in dosages of other drugs.
Side effects & adverse events.
Health economics.
Deaths.
Hospital admissions.
Search methods for identification of studies
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and handsearching of respiratory journals and meeting abstracts. All records in the Specialised Register coded as 'asthma' were searched using the following terms:
Methotrexate* or MTX or "steroid dependan*"
Reference lists of all primary studies and review articles identified from this process were reviewed for additional references. Trial authors were contacted for more information.
Searches were current as of February 2006 and will be updated annually.
Data collection and analysis
The titles and abstracts were reviewed to identify all potential RCTs. Full text versions of these articles were obtained. In addition full text versions of review articles were obtained. Bibliographic searches of all retrieved articles were performed to identify any further RCTs.
Inclusion of studies was decided by the reviewers who independently read the methods section of all identified papers and applied the stated criteria. Trials were scored according to the Cochrane assessment of allocation concealment.
A number of comparisons were carried for this analysis. They are grouped as follows:
A. Comparisons of the effect of MTX vs placebo on the reduction in oral steroid dose by the end of the trial ‐ the primary outcome variable. Comparison 1 includes all evaluable trials. Comparisons 2 ‐ 5 are sub‐group analyses that address issues of: trial duration, initial dose of OCS, mode of MTX delivery, inhaled corticosteroid (ICS) dose.
B. A test of the influence of an attempt to taper the dose of OCS to a minimum prior to MTX treatment, with the aim to have the patients enter the trial at the lowest steroid dose compatible with adequate asthma control (Comparison 6).
C. A comparison of the effect of MTX vs placebo on the FEV1 by the end of the trial ‐ the secondary outcome variable (Comparison 7).
D. A comparison of the withdrawal rate between MTX vs placebo treated patients (Comparison 8).
E. A comparison of the side‐effects between MTX vs placebo treated patients (Comparison 9).
Details of these comparisons are as follows:
Comparison 1.0: Effect of MTX vs placebo on reduction in OCS
1.1 Parallel studies
1.2 Cross‐over studies
Comparison 2.0: MTX vs placebo ‐ effect of trial duration on reduction in OCS
2.1 Parallel trials ‐ 16 weeks or less
2.2 Parallel trials ‐ greater than 16 weeks
2.3 Cross‐over trials ‐ 16 weeks or less
2.4 Cross‐over trials ‐greater than 16 weeks
Comparison 3.0: MTX vs placebo ‐ effect of initial steroid dose on reduction in OCS
3.1 Initial OCS dose less than 22.5 mg ‐ parallel trials
3.2 Initial OCS dose less than 22.5 mg ‐ cross‐over trials
3.3 Initial OCS dose greater than 22.5 mg ‐ parallel trials
3.4 Initial OCS dose greater than 22.5 mg ‐ crossover trials
Comparison 4.0: Intramuscular MTX vs placebo ‐ effect on reduction in OCS
4.1 Intramuscular MTX vs placebo
Comparison 5.0: MTX vs placebo ‐ effect of ICS on reduction in OCS
5.1 Optimal inhaled corticosteroids
5.2 Sub‐optimal inhaled corticosteroids
5.3 Unclear dose of inhaled corticosteroids
Comparison 6.0: MTX vs placebo ‐ effect of steroid reduction during run‐in on reduction in OCS
6.1 Trial of steroid reduction
6.2 No trial of steroid reduction
Comparison 7.0: MTX vs placebo ‐ effect on FEV1
7.1 FEV1 at end of trial
Comparison 8.0: MTX vs placebo ‐ withdrawals from studies
8.1 Withdrawals ‐ all causes
8.2 Withdrawals ‐ hepatic
Comparison 9.0: MTX vs placebo ‐ side Effects
9.1 Hepatic
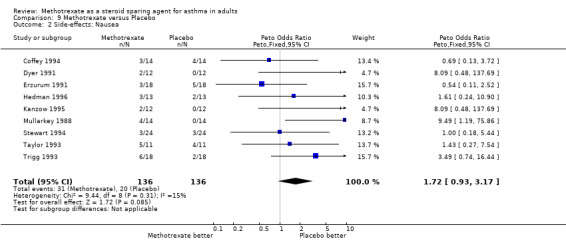
9.2 Nausea
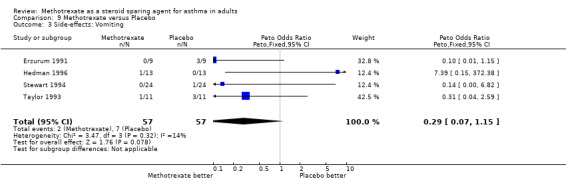
8.3 Vomiting
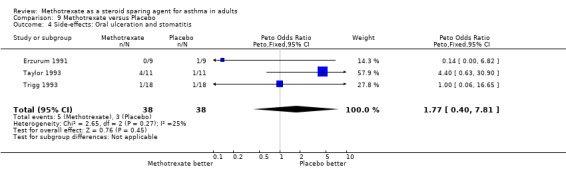
9.4 Oral ulceration and stomatitis
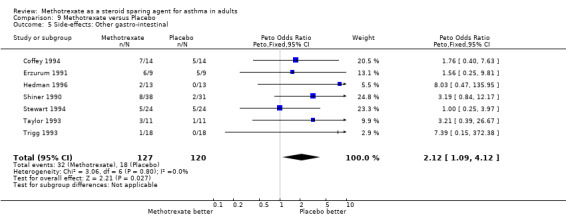
9.5 Other GIT
9.6 Rash
9.7 Alopecia
9.8 Headache
9.9 Pneumonia
Inter‐rater reliability was assessed by simple agreement, Kappa & weighted Kappa statistics. Outcome data was entered into RevMan for statistical analysis. Categorical outcomes were assessed as odds ratios (OR) and 95% confidence intervals. Continuous outcomes were analysed as effect sizes. Fixed effects models were used to obtain summary statistics for the overall efficacy of MTX upon specific outcomes.
FEV1 data were entered as negative values to conform to the Cochrane convention whereby effects that favour the treatment under review move to the left.
Results
Description of studies
A total of 13 RCTs were identified, 11 published as full articles and a further two reported in abstract form. All were published since 1988. All studies were published in English language publications. One excluded study was published in Spanish as a dissertation (Caldwell 1992). They have mostly been small scale, short term cross‐over studies, with somewhat varying methodologies. There was a total of 186 patients completing the studies considered suitable for meta‐analysis. All patients included in the studies had demonstrated reversible airflow limitation (15‐20%, depending upon study). All trials excluded patients with significant renal or hepatic disease, diabetes mellitus, alcohol abuse, pregnant women or where non‐compliance was thought to be a potential problem. There were additional exclusion criteria in some studies. An update search in February 2006 identified two studies of potential relevance. Both were excluded as no tapering of OCS was attempted in one (Comet Monte 2002), and although the second was a placebo‐controlled crossover study, the allocation of these treatments was not randomised (Mathur 1992). The first published RCT using MTX as a steroid sparing agent was that of Mullarkey 1988. This was a randomised, double‐blinded, placebo‐controlled cross‐over trial of 24 weeks duration with 2x12 week treatment periods and analysis performed over the last 8 weeks of each treatment period. Initially 22 patients were enrolled, with 13 completing the study and one withdrawal before the trial was unblinded. The mean age was 49 years, with 5 male and 9 female subjects. All patients had been on OCS for at least 12 months and all were taking an unspecified dose of ICS. The mean initial dose of OCS was 24.8 mg/day. The trial used 15 mg oral MTX, given as three 5 mg doses, 12 hours apart once per week, or identical placebo medication. Assessment was performed on a three weekly basis, with patient initiated reduction in OCS dose by 5 mg every five days if symptoms had improved.
The authors found that they were able to reduce the dose of OCS to 16.6 mg/day with MTX (P=0.01), compared to 26.2 mg/day with placebo. There was one withdrawal. MTX associated adverse effects included four episodes of nausea, one of rash and one of abnormally elevated LFTs. Complications of OCS use during the trial included the development of diabetes in one patient and a pathological cervical spinal fracture in another. They concluded that MTX had a significant steroid sparing effect in steroid‐dependent asthmatics. However the trial was only a small, short term study, with only 12 weeks of active treatment and no attempt at tapering of OCS prior to treatment. There was no indication that there was optimal use of inhaled corticosteroids (ICS) by the trial subjects.
The next trial was that of Shiner 1990 et al. This was a randomised, double‐blinded, placebo‐controlled parallel trial with a total duration of 38 weeks. It had a four week run‐in, 24 week treatment and 10 week run‐out periods. Originally there were 69 subjects, but only 60 completed the study, 32 in the MTX arm and 28 in the placebo arm. The mean age was 49 years for MTX and 48 years for placebo treated patients. Subjects with a significant smoking history were included in the trial, in contrast to some other studies. All patients were on OCS at a minimum dose of 7.5 mg/day for at least 12 months before the trial and all were taking at least 800 mcg/day of ICS. Mean initial doses of OCS were 13.2 mg/day for the MTX group and 15.0 mg/day for placebo. There were no significant differences in pulmonary function or symptom scores between the groups. Oral MTX 15 mg weekly or identical placebo tablets was added to each patient's usual asthma management. Monitoring was by daily diary card entries and clinical assessment every four weeks. Physician initiated reduction in OCS by 2.5 mg occurred if the diary card criteria and spirometry were unchanged or improved.
These authors observed a 50% reduction in OCS dose with MTX. The final dose fell to 6.6 mg/day compared to a 14% fall in OCS dose in the placebo group whose final dose was 12.9 mg/day (P < 0.005). There were six withdrawals from the MTX arm and three from the placebo arm. Twelve of the 38 patients enrolled originally in the MTX arm had some degree of disturbed LFTs and eight had other GI symptoms. Only two of the 31 subjects in the placebo arm suffered GI symptoms.
The authors concluded that MTX had a significant steroid sparing effect, but this did not become apparent until after the twelve week of treatment. There was no formal attempt to taper steroids prior to commencement of the trial, but there were previously documented attempts at steroid weaning in the trial subjects.
The next two trials were published in 1991. Erzurum 1991 conducted a three stage randomised, double‐blind, placebo‐controlled, parallel study of 13 weeks duration. It had a one week run‐in (Stage I), and 12 week treatment period (Stages II‐III), with steroid tapering in last 6 weeks (Stage III). There were 19 patients, with 17 completing the study, eight in the MTX arm and nine in the placebo arm. The mean age was 54 years in the MTX group, and 42 in the control group. All patients had been on long term OCS, for a mean of 5.6 years and 9.8 years in the MTX and placebo arms respectively. Initial OCS doses were 20.2 mg/day for the MTX arm and 19.1 mg/day for placebo. There were no statistically significant differences in any of the measured variables between the two arms at randomisation. All patients had been given a "previous adequate trial" on ICS and, in each arm, seven out of nine patients were on ICS during the trial. The trial was structured as three stages. Stage I involved baseline studies. Stage II involved randomisation to intramuscular MTX or colour matched placebo, given once weekly in clinic for 6 weeks without any tapering of steroids. The dose given was 5 mg in the first week, 10 mg in the second and 15 mg thereafter. In Stage III, steroid taper was attempted in weeks 7‐12. This was individualised, but the trial guidelines suggested a 5 mg reduction in OCS dose per week. There was a subsequent open phase that was not analysed for this review.
The authors found that the final OCS dose was 12.2 mg/day in the MTX arm and 11.9 mg/day in the placebo arm. These represented falls of 39.6% and 40.2% respectively (P < 0.003). There were two withdrawals, one due to non‐compliance and one from the MTX arm with severe asthma. These data removed from the analysis by the authors. There was also considerable minor toxicity, particularly gastrointestinal. There was one death due to Pneumocystis carinii pneumonia in the open portion of the trial.
Although they were able to demonstrate a significant overall reduction in mean OCS dose by careful monitoring and cautious dose reduction in both arms of the study, they were unable to find any significant benefit from MTX. There was no initial OCS tapering period. It was not possible to determine if the trial subjects were on adequate ICS therapy.
Dyer 1991, reported a randomised, double‐blinded, placebo‐controlled cross‐over study of 31 weeks duration, involving a three week taper (or longer if further reduction was possible) and 2x12 week treatment periods with a 4 week washout between treatments. Twelve patients were initially enrolled, with 10 completing the trial. The mean age was 54 years. All patients had been on OCS in a dose equivalent to 7.5 mg/day or more for at least 24 months and all were taking at least 16 puffs per day of beclomethasone or triamcinolone. The initial dose of OCS was 13.1 mg/day. Baseline FEV1 was 1.97 L. During the run‐in, OCS dose was reduced by 1‐2 mg per week until either symptoms worsened or peak flow measurements declined. This taper was extended up to a maximum of 10 weeks, if further reduction was possible. Patients were then randomised to oral MTX 15 mg once a week or identical placebo. OCS dose was tapered by 2.5 mg every two weeks if reduction was tolerated. Clinical assessment was performed every three weeks.
There was a reduction in mean OCS dose of 1.4 mg/day following the initial taper period, but no SD was calculable. The final OCS dose achieved with MTX was 8.4 mg/day and 12.0 mg/day with placebo (P < 0.01). This corresponded to a 30% reduction in OCS dosage from the initial dose with the addition of MTX to therapy, compared to an 8.8% fall with placebo. There were two withdrawals on placebo. All adverse effects occurred in patients in the MTX arm.
The study was only short‐term and the absolute decrease in OCS dose was small (a mean fall of 3.6 mg/day). The authors considered that MTX associated complications were minimal.
Trigg 1993 conducted a randomised, double‐blinded, placebo‐controlled cross‐over trial over 24 weeks, with 2 x 12 week treatment periods. They used a higher dose of oral MTX than in other studies, 30 mg once a week or identical placebo tablets. The dose was increased progressively 7.5 mg for first week, 15 mg for second week, 30 mg if tolerated for remainder of trial. There were daily symptom diary cards and PEFR monitoring, with clinical assessment and testing every three weeks. Patients initiated OCS reduction by 5 mg every five days when well to 10 mg/day, then by 2.5 mg every five days. Statistical analysis was performed on the last 8 weeks of each period, to allow for a washout period between arms of the trial.
18 patients were included, with 6 withdrawals. Mean age was 50 years. All had been on at least 10 mg/day of OCS for at least 6 months. All patients were taking at least 1,500 mcg of ICS daily. Initial OCS dose was 17.5 mg/day. Initial FEV1 was 1.77 L.
They found no significant difference in final OCS doses between the two treatment periods, 10 mg/day with MTX, compared to 12.5 mg/day with placebo.
There were six withdrawals, three from the MTX period and two from the placebo period. In addition, one patient was removed from analysis after completion of the trial because they were found to have been taking additional OCS. Five patients on MTX required dose reduction due to adverse effects, as did two patients on placebo. Adverse effects were common.
They found no evidence that MTX was a steroid sparing agent. It had a high incidence of side effects at the dose used, often requiring dose reduction. However, patients only had 11 weeks on 15 mg or more of MTX as the trial was only of short duration.
Taylor 1993 conducted a randomised, double‐blinded, placebo‐controlled cross‐over trial over 48 weeks, with 2 x 24 week treatment periods. There were 11 patients, aged between 32 and 62 years. Nine completed the study. The duration of prior OCS use varied between 2 and 28 years, with a mean initial dose of 16.1 mg/day. All patients were on at least 2,000 mcg of ICS daily. Initial FEV1 was 47.2% of predicted normal. Patients were randomised to oral MTX 15 mg once a week or identical placebo tablets (7.5 mg in first week of each treatment period). OCS dosage was adjusted after the first four weeks of each treatment period by 1.0 ‐ 2.5 mg according to clinical assessment. Daily symptom diaries, peak flows, OCS dose and bronchodilator use were monitored, with four weekly clinical assessment. Data from the first four weeks of each period was excluded from analysis to reduce any carry over effect.
They found a final OCS dose of 14.4 mg/day in the MTX arm (a fall of 15.6%); with a final dose of 12.9 mg/day in the placebo arm (a 29.4% fall). This was not statistically significant. There were two withdrawals during the MTX period. Side effects were frequent.
Overall, Taylor 1993 and co‐investigators found no evidence of any significant steroid‐sparing effect with MTX. Only small numbers of subjects were enrolled in the trial, which was however, a long‐term study.
Stewart 1994 and co‐workers described a 33 week randomised, double‐blinded, placebo‐controlled cross‐over trial. It had a three week run‐in, two 12 week treatment periods, with an intervening three week washout period, then a three week run‐out. They initially had 24 subjects, with 21 completing the study. The OCS dose for one patient was not provided. Patients ages varied between 19 and 69 years, with a mean of 48 years. Mean duration of OCS use was 78 months (range 5 ‐ 360 months). The initial dose of OCS for patients completing the study was 21.5 mg/day. There was no indication as to whether patients were receiving ICS or not. After an initial three week screening period, patients were randomised to oral MTX 15 mg once a week or identical placebo tablets for 12 weeks (7.5 mg only for first three weeks). All subjects were seen at three weekly intervals and OCS reduction attempted at each visit if clinically indicated.
There was no significant change in OCS dose during the placebo period, but the dose fell by 14.2% in the MTX period (P = 0.045). There were three patient withdrawals, all from the placebo arm. Adverse effects were wide‐ranging and frequent.
The authors concluded that short term, low dose, pulse therapy with MTX had a steroid‐sparing effect and did not have an unacceptable level of side‐effects even though there were a large number of minor adverse effects documented. The study was only short‐term. It is not clear whether patients were on adequate and appropriate ICS and OCS therapy prior to commencing the trial. There was no attempt at weaning of OCS in the running‐in phase of the trial.
Coffey 1994 produced the results of a randomised, double‐blinded, placebo‐controlled cross‐over study lasting between 28 and 34 weeks in duration. It had a four to 10 week run‐in period for optimisation of OCS dose and 2x12 week treatment periods. They used oral MTX or placebo, initially 7.5 mg weekly, rising by 2.5 mg every two weeks to a maximum of 15 mg. OCS dose was adjusted at weekly or bi‐weekly assessments. Analysis was performed over the last four weeks of each period only. There were 14 enrolments and 11 completed the study. The mean age was 36 years. All had been on OCS for at least 12 months, in large doses. After the run‐in phase, the mean OCS dose was 30.8 mg/day. All patients took ICS in a dose of at least 800 mcg /day. At initialisation, the FEV1 was 2.3 L.
They found a significant fall (P < 0.01) in final OCS dose for both MTX and placebo, to 20.1 mg/day and 24.5 mg/day respectively. There were three withdrawals. In addition four patients had one dose of MTX withheld due to toxicity. Reported toxicities included nausea, abdominal pain, diarrhoea, alopecia, rash and headache.
Overall, the authors found no additional benefit from the addition of MTX.
Kanzow 1995 et al. performed a randomised, double‐blinded, placebo‐controlled parallel study using 15 mg of oral MTX or placebo over a total of 27 weeks, with a three week run‐in period without steroid taper and a 16 week treatment period, followed by an eight week run‐out. OCS were tapered during the treatment and run‐out periods at a maximum of 5 mg/week, There were initially 24 patients of whom 21 completed the study, 12 in the MTX arm and 9 in the placebo arm. All patients were on at least 15 mg of OCS daily. The two arms were matched for age, sex, duration of asthma, duration of OCS therapy; ICS dose (MTX 1350 mcg/day, placebo 1656 mg/day) and initial OCS dose (MTX 29.8 mg/day, placebo 25.1 mg/day).
They found a significant fall of 25 % in OCS dose with MTX (22.7 mg/day) but not with placebo, which fell 5 % to 22.8 mg/day. A further reduction in OCS dose occurred in the placebo group during the run‐out phase, which reached significance (P < 0.05). There were three withdrawals, all from the placebo arm, one due to non‐compliance, the other two due to worsening of asthma. The only reported toxicities were two episodes of nausea with MTX.
The authors concluded that the trial demonstrated a significant reduction in OCS dosage in the MTX arm, but that overall there was only a marginal steroid‐sparing effect and that there was no difference in absolute OCS dose between the two arms of the trial.
The most recent trial, was from Hedman 1996 et al. It was a randomised, double‐blinded, placebo‐controlled cross‐over trial lasting 26 weeks, with a 2 week run‐in and 2x12 week treatment periods. MTX 15 mg orally or placebo were used. Thirteen subjects were included, with one withdrawal. Their mean age was 47 years. All were on high dose ICS (2250 mcg/day) and had been on OCS for at least 12 months at a mean dose of 10.9 mg per day. Assessment occurred four‐weekly, with reduction in OCS by 2.5 mg/week if stable or improved. The final OCS doses were 7.9 mg/day for MTX and 12.8 mg/day for placebo (P < 0.05). The steroid sparing effect of MTX became significant in the last 2 weeks of the trial only. The one withdrawal was due to excessive nose bleeds whilst on MTX. There were five reports of nausea (three on MTX) and two reports of abdominal pain in patients on MTX.
The authors concluded that the trial demonstrated a significant reduction in OCS dosage with MTX.
Risk of bias in included studies
Each study was scored according to the 0‐5 point scale of Jadad.
Other characteristics of trial validity were also reviewed.
(I) "Chronic" & "Stable" were assessed operationally in terms of duration of prior oral corticosteroid therapy & variation in dose during that period.
(ii) Use of inhaled corticosteroids This was graded as either: A. Optimal. B. Sub‐optimal. C. Not stated
(iii) Prior attempts at reduction in oral corticosteroid dose should have been unsuccessful in eliminating chronic use. A "run in" period on a steady dose of oral corticosteroid or with an attempt at reduction of corticosteroid dose was considered to be an important component of the trial design. Studies lacking this were graded accordingly: A. Steroid dose reduction attempted B. No steroid dose reduction attempted C. Not stated
Many studies lacked sufficient detail regarding methods of randomisation & this was the major reason why trials failed to obtain a Cochrane "A" rating. We chose to allocate a "C" rating to these trials, rather than a "B", as all papers claimed to have used randomisation procedures. Jadad scoring varied between 2 & 4.
The ratings for the trials were:‐ Coffey 1994 C3, Dyer 1991 A3, Erzurum 1991 C3, Hedman 1996 A3, Mullarkey 1988 A4, Kanzow 1995 A4, Shiner 1990 A3, Stewart 1994 C3, Taylor 1993 A3 & Trigg 1993 C3.
Effects of interventions
The literature searches identified a total of 13 possible studies. Data was in a form adequate for analysis in 10 of the 11 published trials, but in neither of the two studies reported in abstract. Attempts to contact the authors of these trials for more detailed information were unsuccessful. Study design, initial OCS doses, ICS use and reported outcomes varied widely. These are described in the Table of Included Studies.
The 10 trials that were analysed were comprised of seven cross‐over and three parallel randomised placebo‐controlled trials. Nine out of 10 studies used a dose of 15 mg of MTX per week and one used 30 mg per week. One trial used intramuscular MTX, the remainder using oral administration.
There were a total of 218 patients enrolled in the 10 trials reviewed. Of these 186 completed the study, with 32 withdrawals. Sixty nine of these patients came from one large parallel trial (Shiner 1990). Data was lacking for one patient.
Most trials were of relatively short duration, with 16 weeks or less on active treatment. The long‐term trials (Shiner 1990; Taylor 1993) showed variable results.
Only a few trials incorporated an attempt at tapering the dose of OCS to a minimum before commencing treatment with MTX (Dyer 1991, Coffey 1994). Several studies (Kanzow 1995; Hedman 1996; Stewart 1994; Shiner 1990; Erzurum 1991) had a brief run‐in period without any attempt at steroid tapering.
The duration of prior OCS use by individual patients varied widely, from a minimum of 5 months in one study, to a maximum of 28 years in another. All but two studies specified a minimum of 12 months on chronic OCS therapy for inclusion. The other two (Trigg 1993; Stewart 1994) had a minimum duration less than one year.
The specified minimum dose also varied, but was at least 7.5 mg/day (or its equivalent alternate‐day dose) in all trials. Some trials included patients on far higher mean daily doses than others. Mean daily doses varied between 10.9 mg/day (Hedman 1996) and 30.8 mg/day (Coffey 1994).
The final OCS dose was the primary outcome measure in all of the trials reviewed. Five studies found that the introduction of MTX to the treatment of chronic steroid‐dependent asthmatics produced a significant decrease in dose of oral corticosteroids, while five found no such change. Two studies (Coffey 1994; Erzurum 1991) also reported a significant fall in OCS dose on placebo, and the fall with placebo reached significance in the run‐out phase of the Kanzow 1995 study.
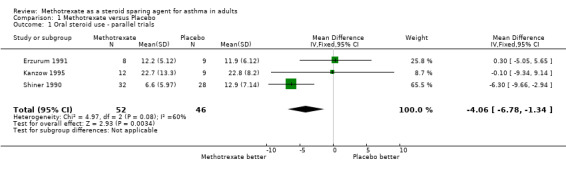
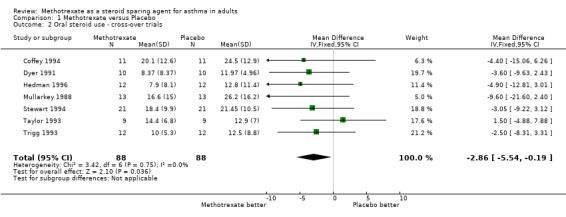
Meta‐analysis of the data for final the OCS dose was performed separately on the parallel and cross‐over studies. These found a WMD in daily steroid dose of ‐4.1 mg/day favouring MTX in parallel studies, (95% CI ‐6.8, ‐1.3) and a WMD of ‐2.9 mg/day favouring MTX in the cross‐over studies. (95% CI ‐5.5, ‐0.2). No significant heterogeneity was detected amongst these studies.
Not all studies involved optimal use of ICS. Shiner 1990, Dyer 1991, Trigg 1993, Taylor 1993 and Hedman 1996 all examined groups receiving 1500 mcg/day or more of ICS, whilst the patients in the Kanzow 1995 study straddled this level (MTX 1400 mcg/day, placebo 1656 mcg/day). The patients in the Coffey 1994 paper were on 800 mcg/day and on an undetermined amount in the Mullarkey 1988 study. It is unclear whether they, along with those in the Erzurum 1991 and Stewart 1994 trials were receiving adequate ICS. Overall, in three out of five positive studies it is unclear as to whether ICS therapy was optimal. Meta‐analysis showed that, in trials in which there was optimal use of ICS, the WMD that favoured MTX more than those in whom ICS use was sub‐optimal or unclear (Optimal ICS: WMD ‐4.0 mg/day, 95% CI ‐6.1, ‐2.0; Suboptimal ICS: WMD ‐2.0 mg/day, 95% CI ‐9.0, 5.0; Unclear ICS: WMD ‐2.0 mg/day, 95% CI ‐5.8, 1.9).
Most studies failed to find significant differences in any other outcome. In those that did, these were minor, even where statistical significance was reached. These outcomes are described in the Table of Included Studies. The FEV1 was reported in a form suitable for analysis in four trials. These showed no significant difference between MTX and placebo, with a WMD of 0.12 L (95% CI ‐0.18, 0.42) Other pulmonary function tests were either reported without sufficient data to calculate an SD or differed between studies, for example reporting either minimum PEFR, evening PEFR or PEFR variability. The method of collecting symptom scores varied between trials. None of the studies included an examination of the bronchial tree with bronchial biopsy or broncho‐alveolar lavage to determine whether there was any reduction in airway inflammation.
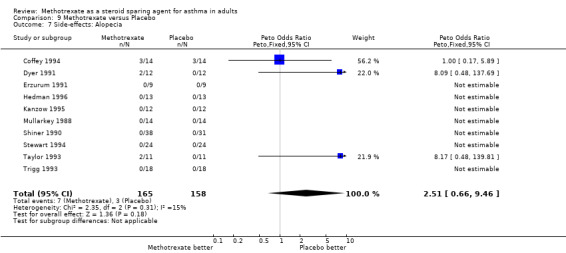
Side‐effects were frequent in most studies, the exception being the Kanzow 1995 study. The side‐effects were reported in a number of different ways, making direct comparison difficult. Where possible, this data was extracted and analysed. Hepatotoxicity with MTX was a frequent problem, abnormal LFTs or more significant toxicity being reported in 26 of 141 individuals, compared with 2 of 134 in the placebo arm (one of which was due to a paracetamol overdose). The Peto OR for the frequency of side‐effects was 6.9 favouring placebo (95% CI 3.1, 15.5). Overall, there were five withdrawals due to hepatotoxicity from MTX. Other frequent side effects due to MTX included nausea (OR 1.72; 95% CI 0.93, 3.17), oral ulceration and stomatitis (OR 1.77; 95% CI 0.40, 7.81), alopecia (OR 2.51; 95% CI 0.66, 9.46) and other GI symptoms (OR 2.12; 95% CI 1.09, 4.12) There were no reported episodes of cytopenia or pulmonary toxicity in any of the studies. No deaths were reported during the blinded phase of the trials, although two patients died subsequently during the open phase of two trials. One died of Pneumocystis cariniae pneumonia (an opportunistic infection associated with impaired immunity ‐ and therefore possibly treatment related) and one of a cardiac complaint.
Withdrawals were relatively frequent, 32 in total. Of these, six occurred at an unspecified phase in the trial, but also included one non‐steroid dependent patient who was enrolled in error. These patients were not included in the analysis of withdrawals. Of the remaining 26, there were a variety of reasons for withdrawal. The commonest medical reason was hepatotoxicity and worsening of asthma (4 patients). These withdrawals are detailed in the description of each trial in the Table of Included Trials.
Discussion
We have reviewed 10 RCTs which added MTX or placebo in a double‐blinded, randomised fashion to conventional asthma management. These trials varied in methodology and design. Most were small in scale, with the Shiner 1990 trial standing out by virtue of its size. Results were presented in conflicting and sometimes contradictory ways and often data had to be estimated from small graphs.
Meta‐analysis confirmed the benefits of MTX in reducing chronic OCS use. Due to the methodological differences between parallel and cross‐over trials, they were analysed separately. The beneficial effect of MTX was greater in the parallel trial group, with a WMD of ‐4.1 mg/ml than in the cross‐over trial (WMD ‐2.9 mg/day). This was due largely to the size effect of one large study (Shiner 1990). The other two parallel trials both reaching negative conclusions. The mean reduction in OCS dose was modest, although some positive trials showed a 50 % fall. It is unclear whether the decreases in OCS dose that were obtained were sufficient to ameliorate the morbidity that accrues from the chronic use of such agents.
It is noteworthy that the cross‐over studies produced a smaller reduction in OCS use. This may have been due to a carry‐over effect of MTX when given first. However, this conclusion must be qualified because two of the three parallel studies found no effect at all, the overall result being dominated by the larger trial of Shiner 1990.
The only previously published meta‐analysis performed in the area was that of Marin 1997. Our results vary slightly from his due to different interpretations of graphical result tables and methodology, but the differences are minor. He found a significant steroid‐sparing effect, with an average reduction in OCS dosage of 4.4 mg/day, or 23.7% of the initial dosage. In his review, which did not differentiate between parallel and cross‐over studies, the effect was most marked in those studies in which MTX was used for twenty‐four weeks. However, this was due to the sample size in the Shiner 1990 trial. Eleven studies were identified in that review, one of which we elected not to use in view of the differing nature of that particular study (Ogriala 1995) and our inability to extract the appropriate data from the published material concerning this trial.
There were a considerable number of variations between trials which may have affected the results of each trial. A number of these variations may be of some importance in trying to analyse the effect of MTX.
Few trials formally attempted to taper the steroid dose prior to initiation of the treatment phase. In the two that did, Coffey 1994 et al. found a mean increase of 3.2 mg/day in the daily OCS dose needed to obtain optimal control from 27.6 mg/day to 30.8 mg/day in contrast. In contrast, Dyer 1991 et al. found a mean decrease of 1.4 mg/day from 13.1 mg/day to 11.7 mg/day. These changes reflect the difficulty in establishing a stable dose of OCS, in addition to the variation in initial OCS dose between the studies. Other authors (Klaustermeyer 1991) have attempted to demonstrate that prolonged observation of PEFR and FEV1 is necessary to establish a true baseline for investigation as trends in pulmonary function may only become apparent over several months. They argue that a period of one to two months observation is inadequate to establish stability. None of the studies included in this review had more than a ten week run‐in period and some had no run‐in at all. Coupled with the significant falls in OCS dose seen in the placebo arms of some of the studies (Erzurum 1991, Coffey 1994) it becomes clear that a determined and sustained attempt at reducing OCS dose prior to introducing MTX may in fact produce the same desired effect ‐ i.e. a reduction in daily steroid dose.
The duration of trials may have been inadequate to allow for the full benefit of MTX to appear. It is known from work with MTX in rheumatoid arthritis and psoriasis that no steroid sparing effect can be seen for at least 6 weeks and maximal benefits are not seen for at least 3 months (Markham 1994; Weinblatt 1995; Lynch 1997 & McCune). This is may also be the case in asthma. All of the studies reviewed here were terminated after 12 weeks or more, so there should have been sufficient time for some benefit to become apparent, although this may not have been optimal. Of the two trials which continued past 16 weeks, one (Shiner 1990) showed the greatest proportional fall in OCS of all (50%). The other failed to show any difference between treatment arms (Taylor 1993). Another study, (Kanzow 1995) found that a significant fall in OCS dose occurred only between weeks 12 and 16 of their study. Thus it is possible that some of the short‐term negative trials may have had greater effect if they had been designed to run for a longer period of time.
Most studies employed a cross‐over design, with either short or no washout periods between arms. Whilst many designs incorporated a period in which no analysis was carried out, in an attempt to minimise carry‐over effects, it is not clear how long this period should be. The three studies which incorporated a run‐out period, (Shiner 1990, Stewart 1994, Kanzow 1995) showed that the effects of MTX did not persist.
The patients in each trial may not have been directly comparable across trials. Although most factors were well matched across studies, there was a marked discrepancy in mean initial doses of OCS, from 10.9 mg/day (Hedman 1996) to 30.78 mg/day (Coffey 1994). Division of the studies into higher and lower dose groups and subsequent analysis showed that the benefits of MTX were seen at both dose ranges, with the exception of one negative parallel trial at higher dosage (Kanzow 1995).
There may be a subset of what Ledford 1996 has called "MTX responders", those patients who show a much greater response to MTX than the overall group of steroid‐dependent patients. Coffey 1994 et al. and Stewart 1994 et al. both felt that they were able to identify these patients retrospectively, but other groups did not find any such subsets. If these so‐called responders exist, they may explain the occasional patients who were able to dramatically reduce or even stop OCS altogether. It may also explain the observation, made in some open studies, of sustained "remission" from chronic OCS use. However it has to be recognised that these "responders" were identified retrospectively when the identity of the treatment had been identified. Similar spontaneous improvements can also been seen in other asthmatic patients when followed over a long period.
Not all studies involved the optimal usage of ICS as recommended by various national consensus statements. The Shiner 1990, Dyer 1991, Trigg 1993, Taylor 1993 and Hedman 1996 trials all included patients who were receiving 1500 mcg/day or more of ICS, while the patients in the Kanzow 1995 study were at the borderline of this level. The patients in the Coffey 1994 paper were on 800 mcg/day. In Mullarkey's study they were on an undetermined amount. It is unclear whether they, along with those in the Erzurum 1991 and Stewart 1994 trials, were receiving adequate ICS. Of the five positive trials, three involved patients on an uncertain and possibly inadequate dose of ICS. Meta‐analysis showed that those trials in which there was optimal use of ICS had a WMD favouring MTX greater than those in whom ICS use was sub‐optimal or unclear. However, this result is weighted heavily by the results of the Shiner 1990 trial. It is possible that more aggressive use of ICS could produce falls in OCS use to similar those seen with MTX.
Few of the trials demonstrated any significant improvement in airway function (as measured by FEV1 and other PFTs). In those that did (Mullarkey 1988, Erzurum 1991, Davies, Trigg 1993), the differences were small and their clinical effect likely to be minor. This may have been due to methodological factors in the trial design. The primary endpoint of the trial was a reduction in steroid dose in patients who were judged to be treated optimally already. In such circumstances, an improvement in airways function may not have been expected. Perhaps, more importantly, the dose of steroid was reduced without a reduction in airways function.
There was no attempt in any study to systematically follow inflammatory markers to see whether there was any reduction in the underlying airway inflammation. One study (Erzurum 1991) did perform serial methacholine challenges which showed no significant differences. In the same study, peripheral blood eosinophils showed significant reductions in both placebo and MTX arms, probably due to overall better control. These studies shed little light on the mechanisms by which MTX may act in chronic steroid‐dependent asthmatics.
Side effects were frequent in the MTX treated group. Hepatotoxicity and other GI symptoms were particularly prominent. OCS can cause abnormal liver function tests in isolation, (Wald 1991] but the results of our analysis confirmed the potential hepatotoxicity of MTX. Others have reviewed the literature and found MTX‐associated side effects in a third of patients (Bardin 1993), although a recent review found that MTX was well tolerated at the low dosages used in asthma (Shulimzon 1996 & Shiner 1990). Pulmonary side effects were limited to respiratory infections in the studies reviewed, but the possibility of MTX inducing asthma has previously been raised (Fertel 1991, Jones 1991) and other serious pulmonary and haematological effects are well described (Weinblatt 1995, Shulimzon 1996 & Shiner 1990, Lynch 1997). Regular monitoring of LFTs and blood indices is mandatory with MTX, but the utilisation of other forms of monitoring such as liver biopsy and pulmonary gas diffusion studies remains unclear. Current recommendations are that liver biopsy should be performed when the cumulative dose of MTX reaches 1.5 to 2.0 gm and measurement of DLCO when there is a prior problem or when clinically indicated (Szefler 1992, Cott 1994a, Shulimzon 1996 & Shiner 1990, Lynch 1997).
Close supervision of patients on MTX is necessary. One possible explanation for the improvements seen is that there was more intensive and closer supervision of patients and more incentive for patient compliance in these trials than in routine practice. This may well explain the beneficial decrease in OCS seen in both arms of two trials, as well as the changing dose seen in the steroid tapering phases.
It is unclear as to when MTX should be commenced. It has been argued that it may be more efficacious to start MTX early, for its potent anti‐inflammatory effects, rather than wait until other treatments have been unsuccessful (Mullarkey 1988, Mullarkey 1991, Mullarkey 1997, Szefler 1997). There are no clinical trial data to support this suggestion, however. The trials reviewed here do not help, since they were all performed in patients on long‐term OCS therapy.
Another issue that remains unaddressed is that of therapy duration. Those studies which had a run‐out period demonstrated that the effects of MTX disappeared once it was ceased. Case reports and open trials suggest that relapse is not inevitable (Mullarkey 1988), however such observations are open to bias through lack of blinding. None of the studies reviewed here involved MTX therapy for greater than six months in duration and most were considerably shorter. We do not know the long‐term outcome in MTX treated steroid‐dependent asthmatics. The only studies which have examined the long‐term sequelae of MTX have been open trials (Shiner 1990, Shiner 1994, Stanziola 1995 and Sorkness 1991).
The results of the ten studies reviewed here are varied. Most have a number of methodological flaws, including small numbers and inadequately described randomisation methods. In addition, the duration of many of these trials may have been inadequate to allow the full benefit of MTX to become apparent. Inadequate or unstated inhaled corticosteroid usage, the lack of a stable baseline oral corticosteroid dose during the run‐in period, or of a formal attempt to reduce this dose prior to the trial would suggest that other steroid‐sparing stratagems may not have been optimally employed. In addition, supervision of the patients was closer than would be practicable in non‐trial conditions. It is difficult to make any firm conclusions as to how these factors may have influenced the size of the MTX effect on OCS use. The size of the reductions in OCS dose during MTX therapy was very modest. The trial data suggest that it is reasonable to assume that a determined effort to maximise inhaled steroid use and minimise oral steroid use could produce reductions of OCS dose of comparable magnitude. Whether such a process would magnify or reduce an apparent MTX effect is open to conjecture.
It is clear that a MTX produced a significantly greater side‐effect rate than steroids alone. It is difficult to weight the relative importance of different side‐effects. A risk‐benefit analysis would have to weigh a significant increase in specific side‐effects due to MTX against a reduction in side‐effects due to a 4 mg/day reduction in oral steroids, bearing in mind that the latter would probably be quite small. The studies reviewed here showed no evidence of benefit in terms of improved lung function with MTX to offset the side‐effects and the trials are too small and too short to assess the rate of acute exacerbations or hospital admissions. It should be noted, however, that of 186 patients included in this review, one died from a complication that may well have been MTX related.
Authors' conclusions
Implications for practice.
There appears to be a modest steroid‐sparing effect with MTX. This is accompanied by a significant incidence of side‐effects, predominantly hepatic & gastrointestinal but including potentially serious haematological events. There does not appear to be any significant improvement in airways function or reduction in airway hyper‐responsiveness. Anti‐inflammatory effects of MTX are currently unclear.
At this stage it is not possible to recognise in advance which patients will benefit most from the addition of MTX to their asthma regimen.
The maximal benefit from MTX may not be seen for at least three months and the reduction in OCS dose achieved is generally modest. It is unclear whether this reduction in OCS dose is sufficient to produce any reduction in the incidence of corticosteroid‐associated side‐effects, or how long it will take for this benefit to be seen. The benefit gained from MTX may not persist following its cessation. Close monitoring for the development of side‐effects is mandatory in any patient commenced upon MTX.
Currently the decision to commence MTX in any patient must be based upon an individual assessment of the risks & benefits that may accrue to that patient. At this stage of our knowledge we cannot conclude that MTX will be of benefit in all steroid‐dependent asthmatics. Maximal conventional asthma therapy should be employed & other stratagems for reducing OCS dose may also need to be pursued in this group.
Implications for research.
There is a need for further research into the role of MTX in asthma. The reasons for this are: first, to establish whether the apparent reduction in OCS dose is of any clinical significance. It may be that the reduction, whilst statistically significant, does not produce any appreciable clinical benefit. Second, it may prove possible to identify a group of patients in whom MTX has a greater benefit than others. The dramatic reductions in OCS dose seen in some individual patients may be due to an increased response to MTX in a susceptible group. Third, MTX is known to have a direct anti‐inflammatory effect although it is unknown whether this plays a role in the improvement in asthma control seen in some individuals. Fourth, the long‐term sequelae of MTX use in asthma are not yet known. Observational studies report an acceptable level of toxicity, but data are lacking.
A long‐term prospective randomised trial of adequate size may answer some of these questions. It will, however, require a run‐in time of several months with meticulous attention to the employment of other steroid sparing strategies to enable patients to be on a truly stable dose of OCS. Measurement of inflammatory markers, possibly including biopsy specimens will be needed. Follow‐up should be over the course of several years, both for long term side effects from MTX and to determine if there is benefit from the reduced consumption of OCS. There is also a need for further research into the mechanisms by which MTX works.
What's new
| Date | Event | Description |
|---|---|---|
| 4 September 2017 | Amended | New literature search run (1 February 2017) to assess the need to update this review. One potentially eligible ongoing study identified and added to Ongoing studies and three potentially eligible studies added to Studies awaiting classification. |
History
Protocol first published: Issue 1, 1998 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 30 September 2008 | Amended | Converted to new review format. |
| 4 March 1998 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank the Cochrane Airways Group editorial base staff for their continued support in the update of this review.
Data and analyses
Comparison 1. Methotrexate versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral steroid use ‐ parallel trials | 3 | 98 | Mean Difference (IV, Fixed, 95% CI) | ‐4.06 [‐6.78, ‐1.34] |
| 2 Oral steroid use ‐ cross‐over trials | 7 | 176 | Mean Difference (IV, Fixed, 95% CI) | ‐2.86 [‐5.54, ‐0.19] |
1.1. Analysis.
Comparison 1 Methotrexate versus Placebo, Outcome 1 Oral steroid use ‐ parallel trials.
1.2. Analysis.
Comparison 1 Methotrexate versus Placebo, Outcome 2 Oral steroid use ‐ cross‐over trials.
Comparison 2. Methotrexate versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral steroid use ‐ parallel trials: 16 weeks or less | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐4.43, 4.83] |
| 2 Oral steroid use ‐ parallel trials: 16 weeks or more | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐6.3 [‐9.66, ‐2.94] |
| 3 Oral steroid use ‐ crossover trials: 16 weeks or less | 6 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐3.73 [‐6.21, ‐1.25] |
| 4 Oral steroid use ‐ crossover trials: 16 weeks or more | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | 1.50 [‐4.88, 7.88] |
2.1. Analysis.
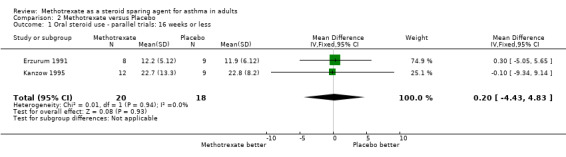
Comparison 2 Methotrexate versus Placebo, Outcome 1 Oral steroid use ‐ parallel trials: 16 weeks or less.
2.2. Analysis.
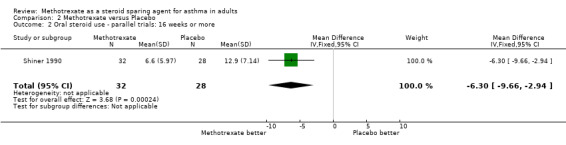
Comparison 2 Methotrexate versus Placebo, Outcome 2 Oral steroid use ‐ parallel trials: 16 weeks or more.
2.3. Analysis.
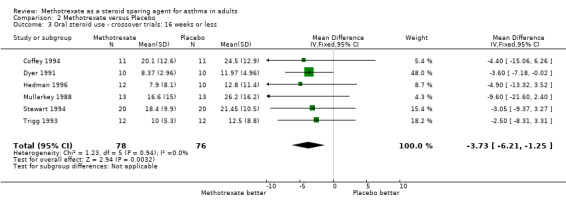
Comparison 2 Methotrexate versus Placebo, Outcome 3 Oral steroid use ‐ crossover trials: 16 weeks or less.
2.4. Analysis.
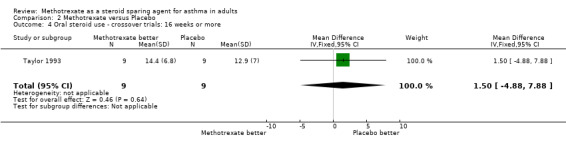
Comparison 2 Methotrexate versus Placebo, Outcome 4 Oral steroid use ‐ crossover trials: 16 weeks or more.
Comparison 3. Methotrexate versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral steroid use ‐ parallel trials: initial dose <22.5 mg/day | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | ‐4.43 [‐7.28, ‐1.59] |
| 2 Oral steroid use ‐ crossover trials: initial dose <22.5 mg/day | 5 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐2.73 [‐5.13, ‐0.33] |
| 3 Oral steroid use ‐ parallel trials: initial dose >22.5 mg | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐9.34, 9.14] |
| 4 Oral steroid use ‐ crossover trials: initial dose >22.5 mg/day | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐6.69 [‐14.66, 1.28] |
3.1. Analysis.
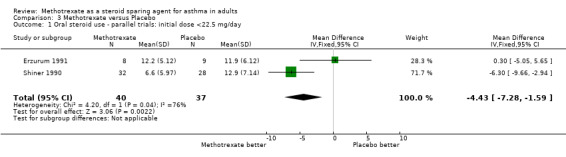
Comparison 3 Methotrexate versus Placebo, Outcome 1 Oral steroid use ‐ parallel trials: initial dose <22.5 mg/day.
3.2. Analysis.
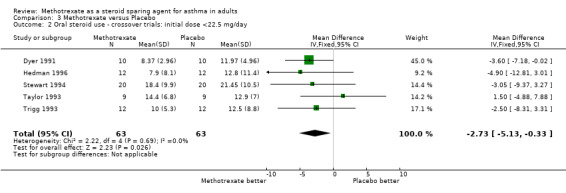
Comparison 3 Methotrexate versus Placebo, Outcome 2 Oral steroid use ‐ crossover trials: initial dose <22.5 mg/day.
3.3. Analysis.
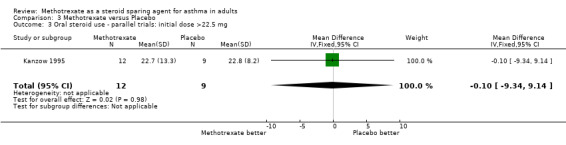
Comparison 3 Methotrexate versus Placebo, Outcome 3 Oral steroid use ‐ parallel trials: initial dose >22.5 mg.
3.4. Analysis.
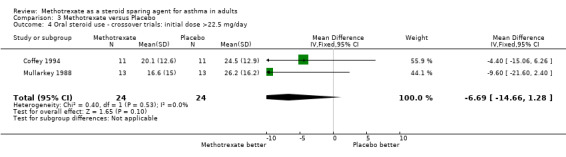
Comparison 3 Methotrexate versus Placebo, Outcome 4 Oral steroid use ‐ crossover trials: initial dose >22.5 mg/day.
Comparison 4. Intramuscular Methotrexate versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral steroid use | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐5.05, 5.65] |
4.1. Analysis.
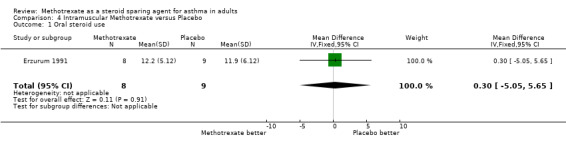
Comparison 4 Intramuscular Methotrexate versus Placebo, Outcome 1 Oral steroid use.
Comparison 5. Methotrexate versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral steroid use ‐ inhaled steroid use optimal | 5 | 146 | Mean Difference (IV, Fixed, 95% CI) | ‐4.03 [‐6.09, ‐1.98] |
| 2 Oral steroid use ‐ inhaled steroid use non‐optimal | 2 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐1.94 [‐8.92, 5.04] |
| 3 Oral steroid use ‐ inhaled steroid use unclear | 3 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐1.98 [‐5.84, 1.89] |
5.1. Analysis.
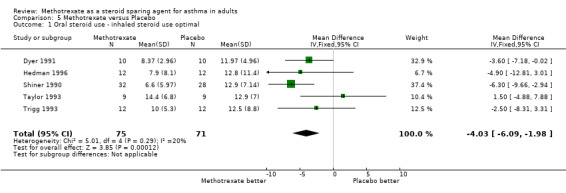
Comparison 5 Methotrexate versus Placebo, Outcome 1 Oral steroid use ‐ inhaled steroid use optimal.
5.2. Analysis.
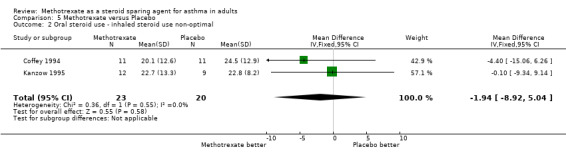
Comparison 5 Methotrexate versus Placebo, Outcome 2 Oral steroid use ‐ inhaled steroid use non‐optimal.
5.3. Analysis.
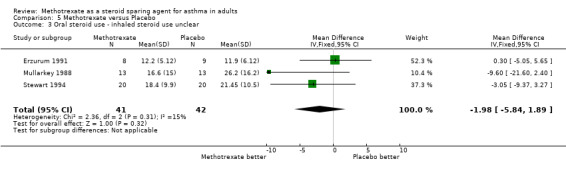
Comparison 5 Methotrexate versus Placebo, Outcome 3 Oral steroid use ‐ inhaled steroid use unclear.
Comparison 6. Methotrexate versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oral steroid use ‐ trial of steroid reduction in run in | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐3.68 [‐7.07, ‐0.29] |
| 2 Oral steroid use ‐ no trial of steroid reduction in run in | 8 | 233 | Mean Difference (IV, Fixed, 95% CI) | ‐1.95 [‐3.99, 0.09] |
6.1. Analysis.
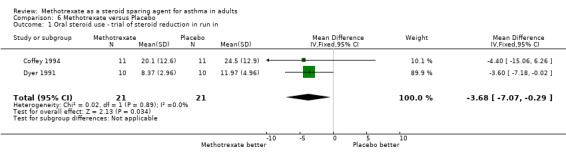
Comparison 6 Methotrexate versus Placebo, Outcome 1 Oral steroid use ‐ trial of steroid reduction in run in.
6.2. Analysis.
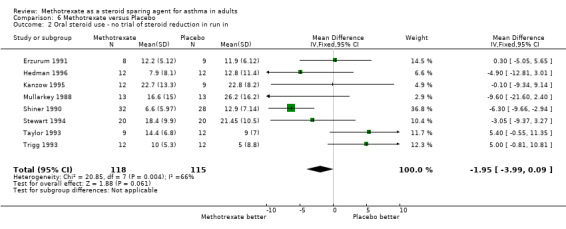
Comparison 6 Methotrexate versus Placebo, Outcome 2 Oral steroid use ‐ no trial of steroid reduction in run in.
Comparison 7. Methotrexate versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 at end of trial | 4 | 85 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.21, 0.45] |
7.1. Analysis.
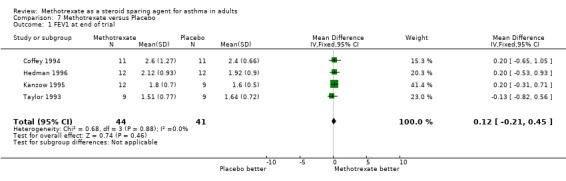
Comparison 7 Methotrexate versus Placebo, Outcome 1 FEV1 at end of trial.
Comparison 8. Methotrexate versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Withdrawals ‐ all causes | 10 | 337 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.42, 2.14] |
| 2 Withdrawals ‐ hepatic | 10 | 347 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.11 [1.19, 42.68] |
8.1. Analysis.
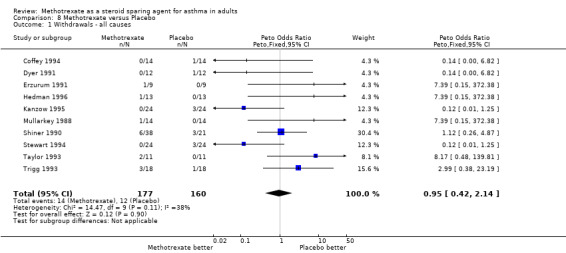
Comparison 8 Methotrexate versus Placebo, Outcome 1 Withdrawals ‐ all causes.
8.2. Analysis.
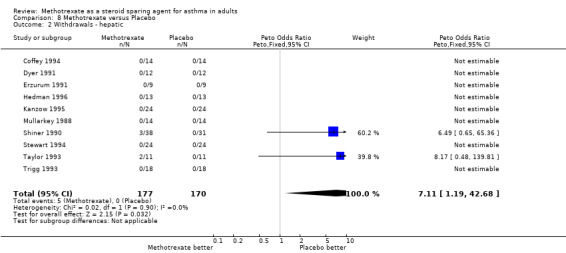
Comparison 8 Methotrexate versus Placebo, Outcome 2 Withdrawals ‐ hepatic.
Comparison 9. Methotrexate versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Side‐effects: Hepatic | 9 | 275 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.90 [3.08, 15.46] |
| 2 Side‐effects: Nausea | 9 | 272 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [0.93, 3.17] |
| 3 Side‐effects: Vomiting | 4 | 114 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.07, 1.15] |
| 4 Side‐effects: Oral ulceration and stomatitis | 3 | 76 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.77 [0.40, 7.81] |
| 5 Side‐effects: Other gastro‐intestinal | 7 | 247 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.12 [1.09, 4.12] |
| 6 Side‐effects: Rash | 3 | 78 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.61 [0.54, 12.50] |
| 7 Side‐effects: Alopecia | 10 | 323 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.51 [0.66, 9.46] |
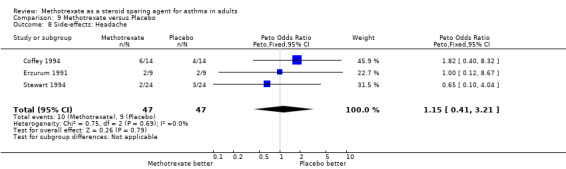
| 8 Side‐effects: Headache | 3 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.15 [0.41, 3.21] |
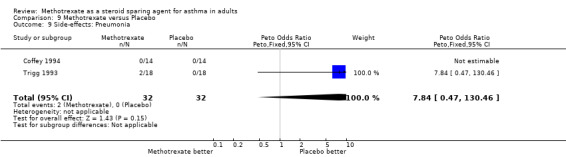
| 9 Side‐effects: Pneumonia | 2 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.84 [0.47, 130.46] |
9.1. Analysis.
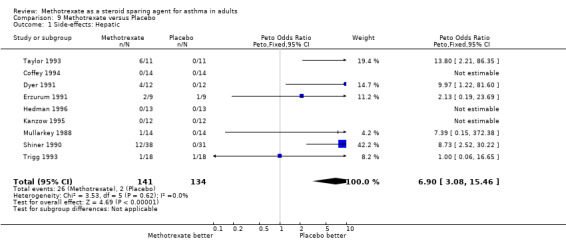
Comparison 9 Methotrexate versus Placebo, Outcome 1 Side‐effects: Hepatic.
9.2. Analysis.
Comparison 9 Methotrexate versus Placebo, Outcome 2 Side‐effects: Nausea.
9.3. Analysis.
Comparison 9 Methotrexate versus Placebo, Outcome 3 Side‐effects: Vomiting.
9.4. Analysis.
Comparison 9 Methotrexate versus Placebo, Outcome 4 Side‐effects: Oral ulceration and stomatitis.
9.5. Analysis.
Comparison 9 Methotrexate versus Placebo, Outcome 5 Side‐effects: Other gastro‐intestinal.
9.6. Analysis.
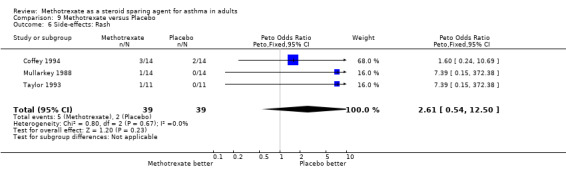
Comparison 9 Methotrexate versus Placebo, Outcome 6 Side‐effects: Rash.
9.7. Analysis.
Comparison 9 Methotrexate versus Placebo, Outcome 7 Side‐effects: Alopecia.
9.8. Analysis.
Comparison 9 Methotrexate versus Placebo, Outcome 8 Side‐effects: Headache.
9.9. Analysis.
Comparison 9 Methotrexate versus Placebo, Outcome 9 Side‐effects: Pneumonia.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Coffey 1994.
| Methods | Randomised, double‐blinded, placebo‐controlled crossover study. 28 ‐ 34 weeks duration. 4 ‐ 10 week run‐in, 2 X 12 week treatment periods. Analysis over last 4 weeks of each period only. | |
| Participants | Patient Numbers 12 ‐ 11 completed study. Age Range 35.5 (21 ‐ 46) Sex ‐ M / F 3/8 No. Smokers None Duration OCS At least 12 months Dose ICS At least 0.8 mg /day Initial dose OCS 30.78 mg/day (SD 16.25) *After run‐in phase (Mean 27.6 mg at enrolment) FEV1 2.3 L (SD 0.66) FVC 3.8 L (SD 1.32) FEV1/FVC 61.2 (SD 11.61) PFM 310 L/min (SD 122.7) Symptom Score (0 ‐ 4) 2.01 (SD 0.06) | |
| Interventions | Initial optimisation of OCS dose over 4 ‐ 10 week run‐in period. Randomisation into 2 treatment arms of 12 weeks duration; of oral MTX, initially at 7.5 mg once a week, increasing by 2.5 mg per 2 weeks to a dose of 15 mg weekly ; or identical placebo. Cross‐over occurred after 12 weeks. Adjustment of OCS dose based upon PFTs & clinical assessment weekly or biweekly. | |
| Outcomes | Final OCS dose ‐ MTX 20.1mg/day (SD 12.6)
‐ placebo 24.5mg/day (SD 12.9)
p < 0.01 for both placebo & MTX vs initial OCS dose. FEV1 ‐ MTX 2.6 L (SD 1.27) ‐ placebo 2.4 L (SD 0.66) NS FVC ‐ MTX 3.9 L (SD 1.66) ‐ placebo 3.8 L (SD 1.32) NS FEV1/FVC ‐ MTX 62.7 (SD 10.28) ‐ placebo 64.0 (SD 11.61) NS PFM ‐ MTX 308 (SD 122.71) ‐ placebo 319 (SD 126.03) NS Symptom Score ‐ MTX 1.89 (SD 0.60) ‐ placebo 2.03 (SD 0.60) 8 patients only Toxicity Withdrawals 1 placebo Nausea ‐ MTX 4, placebo 3. Abdominal pain ‐ MTX 4, placebo 5 Diaorrhoea ‐ MTX 5, placebo 7 Stomatitis ‐ MTX 2, placebo 1 Alopecia ‐ MTX 3, placebo 3 Rash ‐ MTX 2, placebo 3 Headache ‐ MTX 4, placebo 6 No haematologic, hepatic or pneumonic complications. |
|
| Notes | Paper analysed results for last 4 weeks of each treatment period only. Inadequate information provided regarding allocation concealment. Authors did not respond to correspondence. Initial OCS dose used for analysis is that at time of randomisation. (After run‐in phase) Results given in paper as mean dose & SEM, converted to SD for analysis. The trial failed to show any significant reduction in OCS dose with MTX. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Investigators aware as to order of treatment group assignment (Cochrane Grade C) |
Dyer 1991.
| Methods | Randomised, double‐blinded, placebo‐controlled crossover study. Duration 31 weeks. 3 week taper,(longer if further reduction possible) 2 X 12 week treatment periods with 4 week washout between treatment periods. | |
| Participants | Patient Numbers 12 ‐ 10 completed study. Age ‐ Range 54 (38 ‐ 64) Sex ‐ M / F 5/5 Duration OCS At least 24 months Dose ICS 16 puffs per day of beclomethasone or triamcinolone. Initial dose OCS 13.12 (SD 5.31) (Mean dose 11.77 mg after taper. ‐ SD not calculable) FEV1 1.97 L (SD 0.82) | |
| Interventions | Reduction of OCS by 1 ‐ 2 mg per week in run‐in until symptoms worsened or PFs declined. Oral MTX 15 mg once a week or identical placebo. OCS tapered by 2.5 mg every 2 weeks if tolerated. | |
| Outcomes | Final OCS dose ‐ MTX 8.37 mg/day (SD 2.96)
‐ placebo 11.97 mg/day (SD 4.96)
p = 0.01 FEV1 ‐ MTX 1.89L ‐ placebo 1.91 L NS FVC ‐ MTX 2.85 L ‐ placebo 2.85 L NS PF (am) ‐ MTX 289 ‐ placebo 283 NS PF (pm) ‐ MTX 323 ‐ placebo 323 NS Symptoms (am) ‐ MTX 2.1 ‐ placebo 2.2 NS Symptoms (pm) ‐ MTX 2.1 ‐ placebo 2.3 NS ß agonist ‐ MTX 6.0 ‐ placebo 6.2 NS Residual volume ‐ MTX 2.74 L ‐ placebo 2.65 L NS Se Theophylline ‐ MTX 11.11 µg/ml ‐ placebo 13.66 µg/ml NS AST ‐ MTX 48.6 IU/ml ‐ placebo 24.2 IU/ml p = 0.06 Toxicity Withdrawals 2: 1 non‐compliance, 1 pancreatic pseudocyst ‐ placebo. Nausea 2 MTX Alopecia 2 MTX Herpes 1 MTX High AST 4 MTX |
|
| Notes | Mean reduction in OCS dose of 1.4mg per day during initial taper. No information available to calculate SD. Individual patient values given for average daily dose in graphical format. SD values calculated from these. Inadequate data provided to allow calculation of other SD values. The trial demonstrated a significant reduction in OCS dosage with MTX. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Erzurum 1991.
| Methods | Randomised, double‐blind, placebo‐controlled, parallel study. Duration 13 weeks. 1 week run‐in, (Stage I) 12 week treatment period, (Stages II ‐ III) with steroid tapering in last 6 weeks. (Stage III) | |
| Participants | Patient Numbers 19 ‐ 17 completed study. MTX n = 8, placebo n = 9. Age ‐ Range ‐ MTX 53.8, (SD 11.3) ‐ Placebo 42.4. (SD16.7) p = 0.11 Sex ‐ M / F ‐ MTX 7 M, 2 F. ‐ Placebo 5 M, 4 F. p > 0.2 Race ‐ MTX 9 white. ‐ Placebo 2 black, 9 white. p > 0.2 Weight ‐ MTX 80.3 (SD 10.9) ‐ Placebo 75.0 (SD 14.5) p > 0.2 Duration asthma ‐ MTX 23.4 years. (SD 21.6) ‐ Placebo 24.1 years. (SD 15.0) p > 0.2 Duration OCS ‐ MTX 5.6 years. (SD 5.3) ‐ Placebo 9.8 years. (SD 9.1) p > 0.2 Dose ICS "Previous adequate trial". 7/9 in both groups actually on ICS. Initial dose OCS ‐ MTX 20.2 mg (SD 5.0) ‐ Placebo 19.1 mg (SD 4.2) p > 0.2 FEV1 ‐ MTX 75.7 % (SD 23.5) ‐ Placebo 72.6 % (SD 11.6) p > 0.2 Past smoker ‐ MTX 6 ‐ Placebo 3 p = 0.15 Eosinophil count ‐ MTX 62 per mm3 (CI 9 ‐ 229) ‐ placebo 18 per mm3 (CI 18 ‐ 35) |
|
| Interventions | Stage I ‐ baseline studies. (Week 0) Stage II ‐ Randomisation to intramuscular MTX or colour matched placebo, given once weekly in clinic for 6 weeks without any attempt to taper steroids. Dose of 5 mg in first week, 10 mg in second, 15 mg thereafter. Blood tests every 2 weeks, PFTs after week 6. Stage III ‐ Steroid taper attempted in weeks 7 ‐ 12. Individualised, but guidelines suggested 5 mg reduction in OCS dose per week. Repeated blood tests, PFTs, methacholine challenge tests at completion. Subsequent open phase. (Not analysed) |
|
| Outcomes | Final OCS dose ‐ MTX 12.2 mg/day (SD 5.12) (Mean fall of 8.0 mg or 39.6%)
p =0.001
‐ placebo 11.9 mg/day (SD 6.12) (Mean fall of 7.2 mg or 40.2%)
p = 0.003 TLC NS FEV1 pre ‐ MTX 74.3 % (Mean fall of 1.4 %) NS ‐ Placebo 61.5 % (Mean fall of 6.1 %) p = 0.03 FEV1 post ‐ MTX 71.0 % (Mean fall of 4.7 %) NS ‐ Placebo 68.4 % (Mean fall of 4.2 %) p = 0.02 PEFR pre ‐ MTX 31.5 L/min fall p = 0.04 ‐ placebo 4.5 L/min rise NS PEFR post ‐ MTX 25.6 L/min fall p = 0.03 ‐ placebo 1.5 L/min rise NS DLCO NS Methacholine challenge NSD in PC 20. Eosinophil count ‐ MTX 246 per mm3 (CI 97 ‐ 326) ‐ placebo 405 per mm3 (CI 229 ‐ 704) Both groups p < 0.001 Symptom scores (0 ‐ 4) ‐ MTX increased 0.9 (95 % CI ‐ 0.4 ‐ 2.3) ‐ Placebo increased 0.9 (95 % CI 0.0 ‐ 1.8) No significant increase from baseline. ß agonist use ‐ MTX increased 4.6% (95%CI ‐7.7 ‐ 16.9%) ‐ placebo increased 8.0 % (CI ‐ 2.8 ‐ 18.8) NS Exacerbations ‐ MTX 4 patients, 6 episodes. ‐ placebo 5 patients, 8 episodes. p > 0.2 Toxicity 2 withdrawals, 1 non‐compliance, 1 severe asthma (MTX ‐ not analysed) GI ‐ 6 MTX, 5 placebo Nausea ‐ 3 MTX, 5 placebo Vomiting ‐ 3 placebo Diarrhoea ‐ 6 MTX, 5 placebo Oral ulcers ‐ 1 placebo High LFTs ‐ 2 MTX, 1 placebo Headache ‐ 2 MTX, 2 placebo Pain at injection ‐ 1 placebo High CK ‐ 1 MTX |
|
| Notes | No formal run‐in period. Only study to use im MTX. Analysis performed between mean steroid dose in stage II & last 4 weeks in stage III. Values actually given as mean reduction in OCS dose & 95 % CI. It was assumed that 95 % CI for fall can be translated into 95 % CI for final dose. 95 % CI then converted to SD. Baseline values for PFTs & other parameters not given in sufficient detail to calculate SD values. 1 death in open phase of study ‐ PCP. The trial failed to show any reduction in OCS dose attributable to MTX. Inadequate detail provided regarding details of randomisation. Authors did not respond to correspondence. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Investigators aware as to order of treatment group assignment (Cochrane Grade C) |
Hedman 1996.
| Methods | Randomised, double‐blinded, placebo‐controlled crossover trial. 26 weeks duration, with 2 week run‐in period & 2 X 12 week treatment periods. | |
| Participants | Patient Numbers 13 ‐ 12 completed study. Age ‐ Range 46.9 (27 ‐ 64) Sex ‐ M / F 4/8 BMI 25.1 (18 ‐ 30) (>30 excluded from trial) Duration of OCS 12 months + Dose of ICS 2.25 mg (Range 1.6 ‐ 3.2) Initial dose OCS 10.9 mg (SD 8.4) FEV1 2.11L ( SD 0.98) FVC 3.22 L (SD 1.28) DLCO 94.5 % (SD 16.6) Evening PEFR 372 L/min (SD 120) PEF variability 10.7 % (SD 11.8) | |
| Interventions | 2 week run‐in period. Randomisation to MTX 15 mg orally once a week or identical placebo tablets. 4 weekly assessment with reduction in OCS by 2.5 mg per week if stable or improved. Blood tests & spirometry performed every 4 weeks, DLCO at 0, 12, 24 weeks, CXR at 0, 24 weeks. | |
| Outcomes | Final OCS dose ‐ MTX 7.9 mg/day (SD 8.1)
‐ placebo 12.8 mg/day (SD 11.4)
p < 0.05 FEV1 ‐ MTX 2.12 L (SD 0.93) ‐ placebo 1.91L (SD 0.90) NS FVC ‐ MTX 3.19 L (SD 1.26) ‐ Placebo 2.94 L (SD 1.19) NS DLCO ‐ MTX 90.8 % (SD 14.1) ‐ placebo 88.8 % (SD 12.1) NS Evening PEFR ‐ MTX 376 L/min (SD 123) ‐ placebo 344 L/min (SD 135) NS PEFR variability ‐ MTX 9.4 % (SD 7.0) ‐ placebo 7.0 % (SD8.5) NS ß agonist use ‐ MTX decreased by 22 % p < 0.05 Wheeze MTX favoured p < 0.001 Dyspnoea MTX favoured p < 0.001 Cough MTX favoured p < 0.01 Serum cortisol ‐ MTX 252 nmol per L ‐ Placebo 272 nmol per L NS Toxicity ‐ 1 withdrawal ‐ MTX nosebleeds. ‐ Nausea ‐ 3 MTX, 2 placebo ‐ Abdominal pain ‐ 2 MTX. |
|
| Notes | Results given with SD values. Steroid sparing effect of MTX became significant in last 2 weeks of trial only. The trial demonstrated a significant reduction in OCS dosage with MTX. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Kanzow 1995.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel study. Duration 27 weeks, with 3 week run‐in period & 16 week treatment period, followed by 8 week run‐out period. | |
| Participants | Patient Numbers 24 ‐ 21 completed study. MTX n = 12, placebo n = 9. Age ‐ Range ‐ MTX 52 (SD 12) All > 30 yo ‐ Placebo 59 (SD 6) NS Sex ‐ M / F ‐ MTX 7/5 ‐ Placebo 6/3 NS Duration asthma ‐ MTX 12 years (SD 14) ‐ Placebo 19 years (SD 17) NS Duration OCS ‐ MTX 5.2 years (SD 2.6) ‐ Placebo 7.8 years (SD 6.8) NS Dose ICS ‐ MTX 1.350 mg (SD 0.639) ‐ placebo 1.656 mg (SD 0.321) NS Initial dose OCS ‐ MTX 29.8 mg (SD 13.9) Minimum 15 mg/day ‐ Placebo 25.1 mg (SD 9.2) NS FEV1 ‐ MTX 1.5 L (SD 0.4), 53 % predicted (SD 10) ‐ Placebo 1.7 L (SD 0.5), 58 % predicted (SD 24) NS FVC ‐ MTX 3.4 L (SD 0.6), 91 % predicted (SD 12) ‐ Placebo 3.5 L (SD 0.7), 95 % predicted (SD 20) NS PEFR‐minimum ‐ MTX 240 L/min (SD 80) ‐ placebo 210 L/min (SD 90) NS Eosinophil count ‐ MTX 107 /µL (SD 141) ‐ placebo 168 /µL (SD 87) NS Serum theophylline ‐ MTX 11.0 µg/ml (SD 4.0) ‐ placebo 12.0 µg/ml (SD 4.8) NS Symptom score ‐ MTX 1.7 (SD 0.7) ‐ placebo 2.0 (SD 0.67) NS Nocturnal awakenings ‐ MTX 1.15 (SD 0.8) ‐ placebo 0.9 (SD 0.6) NS |
|
| Interventions | 3 week run‐in period. No steroid taper. Randomisation to MTX 15 mg orally once a week or identical placebo. Steroid taper during treatment & run‐out phases with maximal reduction of 5 mg per week. Patients allowed to increase OCS after consulting study if asthma worsened 2 weekly clinical review,with spirometry, blood gas analysis, FBC & LFTs performed. Diary of night awakenings & symptom score, OCS dose & PEFR. At run‐in, week 8 & at week 21, CXR, spirometry, blood gas analysis, DLCO, FBC & eosinophil count, LFTs & creatinine, serum theophylline & US of liver were obtained. | |
| Outcomes | Final OCS dose ‐ MTX 22.7 mg/day (SD 13.3) 25 % fall. (p < 0.01)
‐ placebo 22.8 mg/day (SD 8.2) 5 % fall. (NS) FEV1 ‐ MTX 1.8 L (SD 0.7) ‐ placebo 1.6 L (SD 0.5) NS FVC NS PEFR‐minimum ‐ MTX 220 L/min (SD 80) ‐ placebo 250 L/min (SD 80) NS Symptom score ‐ MTX 1.7 (SD 0.8) ‐ placebo 1.9 (SD 0.7) NS Nocturnal awakening ‐ MTX 1.2 (SD 06) ‐ placebo 1.0 (SD 0.8) NS Toxicity ‐ 3 withdrawals ‐ placebo, 1 noncompliant, 2 worsening of asthma. ‐ Nausea ‐ 2 MTX |
|
| Notes | Values given as means & SD, some calculated from graphs. Comparisons given between baseline & week 16 of the treatment period. The steroid sparing effect of MTX was not sustained during the run‐out period. A further reduction in OCS dose occurred in the placebo group during the run‐out phase, which reached significance. (p < 0.05) The trial demonstrated a significant reduction in OCS dosage with MTX, but no difference in OCS dose between MTX & placebo arms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Mullarkey 1988.
| Methods | Randomised, double‐blinded, placebo‐controlled crossover trial. 24 week duration with 2 X 12 week treatment periods. Analysis over last 8 weeks of each period only. | |
| Participants | Patient Numbers 14 ‐ 13 completed study. Age ‐ Range 49 (30 ‐69) Sex ‐ M / F 5/9 Duration OCS at least 12 months Dose ICS yes but not stated Initial dose OCS 24.8 mg/day (SD 15.3) | |
| Interventions | Oral methotrexate 15 mg once a week or identical placebo. 3 weekly assessment. Patient initiated reduction in OCS by 5 mg every 5 days if symptoms improved. | |
| Outcomes | Final OCS dose ‐ MTX 16.6 mg/day (SD 15.0)
‐ placebo 26.2 mg/day (SD 16.2)
p = 0.01 FVC ‐ MTX 2.92 L ‐placebo 2.74 L p = 0.16 FEV1 ‐ MTX 1.84 L ‐placebo 1.74 L p = 0.17 Breathing ‐ MTX 0.70 ‐placebo 1.03 p = 0.01 Wheeze ‐ MTX 0.70 ‐placebo 0.90 p = 0.02 Nocturnal symptoms ‐ MTX 5.44 ‐ placebo 7.38 p = 0.08 Coughing ‐ MTX 0.49 ‐ placebo 0.57 p = 0.46 WCC ‐ MTX 10,023 mm3 ‐ placebo 11,218 mm3 p = 0.07 SGOT(AST) ‐ MTX 20.0 IU/L ‐ placebo 17.4 IU/L p = 0.01 Serum theophylline ‐ MTX 11.9 µg/ml ‐ placebo 14.2 µg/ml p = 0.26 DLCO ‐ MTX 86.15 % predicted ‐ placebo 87.15 % predicted p = 0.54 Toxicity 1 withdrawal ‐ surgery on MTX Nausea ‐ 4 MTX Rash ‐ 1 MTX High AST ‐ 1 MTX Diabetes ‐ 1 OCS Cervical # ‐ 1 OCS Total of 6 episodes of toxicity due to MTX & 2 attributed to OCS. |
|
| Notes | Trial unblinded after 14 completions/withdrawals. Originally 22 enrolled. Results originally given as mg per week, converted to mg per day for assessment. Standard deviation per week divided by 7 to give SD per day. The trial showed a significant difference in final doses of OCS, subjective breathing ability & frequency of wheezing; Other differences failed to reach significance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Shiner 1990.
| Methods | Randomised, double‐blinded, placebo‐controlled parallel trial. Duration 38 weeks, with 4 week run‐in, 24 week treatment & 10 week run‐out periods. | |
| Participants | Patient Numbers 69, 60 completed study. n MTX = 32, n Placebo = 28. Age ‐ Range MTX 49 (22 ‐ 73) Placebo 48 (22 ‐ 75) NS Sex ‐ M / F MTX 9/23 Placebo 10/18 NS No. Smokers MTX 7 Placebo 4 NS Duration OCS MTX at least 12 months Placebo at least 12 months NS Dose ICS MTX 1.655 (SD 0.548) (All > 0.8 mg/day) Placebo 1.793 (SD 0.745) NS Initial dose OCS MTX 13.2 mg (SD 5.0) (minimum 7.5 mg) Placebo 15.0 mg (SD 6.0) NS PEFR‐morning ‐ MTX 208 L/min (SD 14) ‐ placebo 229 L/min (SD 18) NS PEFR‐evening ‐ MTX 226 L/min (SD 13) ‐ placebo 249 L/min (SD 14) NS FEV1 ‐ MTX 56.3 % (SD 19) ‐ placebo 55.2 % (SD 19) NS FVC ‐ MTX 73.2 % (SD 21) ‐ placebo 73.7 % (SD 16) NS TLC ‐ MTX 89.2 % (SD 18) ‐ placebo 95.3 % (SD 14) NS DLCO ‐ MTX 91.6 % (SD 17) ‐ placebo 97.6 % (SD 18) NS PEFR variability ‐ MTX 13 % (SD 4) ‐ placebo 10 % (SD 3) NS Symptom score ‐ MTX 2.05 (SD 0.2) ‐ placebo 1.95 (SD 0.2) NS Night waking ‐ MTX 0.7 (SD 0.2) ‐ placebo 1.4 (SD 0.3) NS |
|
| Interventions | Oral methotrexate 15 mg once a week or identical placebo tablets. Daily diary card entries. Assessment every 4 weeks. Physician initiated reduction in OCS by 2.5 mg if diary card criteria & spirometry unchanged or improved. FBC & LFTs at 4 weekly intervals, PFTs & CXR every 3 months. | |
| Outcomes | Final OCS dose ‐ MTX 6.6 mg/day (SD 6.0) 50 % fall.
‐ placebo 12.9 mg/day (SD 7.1) 14 % fall.
p<0.005 Symptom score ‐ MTX 1.95 (SD 0.2) ‐ placebo 1.75 (SD 0.3) NS Night waking ‐ MTX 0.9 (SD 0.2) ‐ placebo 1.1 (SD 0.3) NS Mean PEFR ‐ MTX 230 L/min (SD 15) ‐ placebo 255 L/min (SD 15) NS PEFR variation ‐ MTX 12 % (SD 2) ‐ placebo 15 % (SD 3) NS Exacerbations ‐ MTX 1.4 (SD 1.1) ‐ placebo 2.6 (SD 1.8) p < 0.01 Toxicity ‐ Withdrawals ‐ 6 MTX (3 LFTs, 2 GIT, 1 compliance) ‐ 3 placebo ( 2 GIT, 1 non compliance) ‐ LFTs ‐ 12 MTX. ‐ GI ‐ 8 MTX, 2 placebo. No haematological or respiratory complications. |
|
| Notes | Analysis based on conversion of percentage fall & SEM to mean & SEM dosage in mg & calculation of SD from SEM. Other values calculated from graphs. ( All ?SD values) Both placebo & MTX groups showed a 16 % fall in OCS dose from baseline at 12 weeks. After this, only the MTX group continued to show a fall in OCS. Fall not sustained in run‐out period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Stewart 1994.
| Methods | Randomised, double‐blinded, placebo‐controlled crossover trial. Duration 33 weeks, with 3 week run‐in period, first 12 week treatment period, 3 week washout period, second 12 week treatment & 3 week run‐out periods. | |
| Participants | Patient Numbers 24 ‐ 21 completed study. (Data for 20 patients provided) Age ‐ Range 48 (19 ‐ 69) Sex ‐ M / F 11/13 Duration OCS 78 months (5‐360 months) Dose ICS Not stated. Initial dose OCS 23.8 mg (12.5 ‐ 85 mg) 21.5 mg for patients completing study. (SD 6.0) | |
| Interventions | Initial 3 week screening period. Randomisation to either oral methotrexate 15 mg once a week or identical placebo tablets for 12 weeks. 7.5 mg only for first 3 weeks of each treatment period. 3 week washout period followed by second 12 week treatment period. All subjects seen at 3 weekly intervals. OCS reduction attempted at each visit if clinically indicated. Monitoring by diary & symptom score & FEV1, serum theophylline, FBC & LFTs 3 weekly. | |
| Outcomes | Final OCS dose ‐ MTX 18.4 mg/day (SD 9.9 ) p = 0.0447
‐ placebo 21.45 mg/day (SD 10.5) p = 0.9190 FEV1 ‐ MTX 2.05 L (5.1%) ‐placebo 1.95 L p = 0.1053 Subjective symptom scores ‐ MTX 1.84 ‐placebo 2.34 p < 0.05 Physician symptom score ‐ MTX 2.17 ‐placebo 2.53 p < 0.05 AST, ALT NS Serum theophylline NS Toxicity ‐ Withdrawals‐ 3:‐ patient concern, severe asthma, non‐compliance. all from placebo arm. ‐ Dizziness ‐ MTX 3 ‐ Musculoskeletal ‐ MTX 7 ‐ placebo 12 ‐ Respiratory infection ‐ MTX 9 ‐ placebo 16 ‐ Nausea ‐ MTX 6 ‐ placebo 3 ‐ Dyspnoea ‐ MTX 3 ‐ placebo 2 ‐ Headache ‐ MTX 5 ‐ placebo 3 ‐ Rhinitis ‐ MTX 3 ‐ placebo 2 ‐Skin infection/cellulitis ‐ MTX 3 ‐ Syncope ‐ MTX 1 ‐ Abdominal pain ‐ MTX 3 ‐ placebo 5 ‐ Facial flushing ‐ placebo 1 ‐ Chest pain ‐ placebo 4 ‐ Constipation ‐ placebo 1 ‐ Urinary incontinence ‐ MTX 4 ‐ placebo 2 ‐ DVT ‐ placebo 1 ‐ Fever ‐ MTX 2 ‐ placebo 2 ‐ Diarrhoea ‐ MTX 3 ‐ placebo 5 ‐ Depression ‐ MTX 3 ‐ Ear infection ‐ MTX 1 ‐ placebo 2 ‐ Ear pain ‐ placebo 1 ‐ Vaginal bleeding ‐ MTX 1 ‐ placebo 1 ‐ Alopecia ‐ placebo 1 ‐ Abdominal bloating ‐ MTX 1 ‐ Sweating ‐ MTX 1 ‐ Polydipsia ‐ MTX 1 ‐ Polyphagia ‐ MTX 1 ‐ Polyuria ‐ MTX 1 ‐ Blurred vision ‐ placebo 1 ‐ Vomiting ‐ placebo 1 ‐ Hoarseness ‐ placebo 1 ‐ Arm pain ‐ placebo 1 ‐ Irritable bowel ‐ placebo 1 ‐ Oedema ‐ placebo 1 ‐ Numbness ‐ placebo 1 ‐ Weakness ‐ placebo 1 ‐ Pruritis ‐ placebo 1 26 episodes of abnormal LFTs in 8 patients reported, however not broken down between MTX & placebo. |
|
| Notes | Inadequate details of randomisation allocation & concealment. Authors did not respond to correspondence. Dosages for 20 patients only provided on graphs. Values for SDs & means calculated from graphs. 1 death 1 week after completion of trial. (Cardiac arrest) Some patients had more than one episode of each side effect ‐ Only entered once for each patient in outcomes. Tabulated results for side effects differ from those in text of study. The trial demonstrated a significant reduction in OCS dosage with MTX. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Investigators aware as to order of treatment group assignment (Cochrane Grade C) |
Taylor 1993.
| Methods | Randomised, double‐blinded, placebo‐controlled crossover trial. Duration 48 weeks, with 2 X 24 week treatment periods. | |
| Participants | Patient Numbers 11 ‐ 9 completed study Age ‐ Range 32 ‐ 62 Sex ‐ M / F 7/4 Duration OCS 2‐28 years. Dose ICS At least 2 mg per day. FEV1 47.2 % (95 % CI 37.2, 57.1) (SD 16.84) Initial dose OCS 16.1 mg per day. (SD 7.5) | |
| Interventions | Randomisation to either oral methotrexate 15 mg once a week or identical placebo tablets for 24 weeks. (7.5 mg in first week of each treatment period.) OCS dosage adjusted after first 4 weeks of each treatment period by 1 ‐ 2.5 mg according to clinical assessment. Daily symptom diaries, PFs, OCS dose & bronchodilator use monitored, with 4 weekly clinical assessment. CXR performed at entry & 24 & 48 weeks, PFTs, FBC & biochemical investigations performed monthly, DLCO second monthly. | |
| Outcomes | Final OCS dose ‐ MTX 14.4 mg/day (SD 6.8) (15.6% fall)
‐ placebo 12.9 mg/day (SD 7.0) (29.4% fall)
NS FVC ‐ MTX 2.82 L (SD 0.78) ‐placebo 2.99 L (SD 0.77) NS FEV1 ‐ MTX 1.51 L (SD 0.77) ‐placebo 1.64 L (SD 0.72) NS PFs (am/ pm) ‐ MTX 289 L/min (SD 13.01) 298 L/min (SD 13.77) ‐ placebo 298 L/min (SD 13.77) 308 L/min (SD 13.77) NS Daytime symptoms ‐ MTX 2.7 (SD 1.76) ‐placebo 3.2 (SD 1.76) NS Nocturnal symptoms ‐ MTX 2.2 (SD 1.84) ‐ placebo 2.5 (SD 1.84) NS No. admissions ‐ MTX 5 ‐ placebo 4 NS Exacerbations ‐ MTX 3.2 (SD 3.60) ‐ placebo 4.6 (SD 4.51) NS Infections ‐ MTX 1.8 (SD 2.45) ‐ placebo 2.6 (SD 2.52) NS Toxicity 2 Withdrawals ‐ 2 MTX ‐ 1 intractable nausea, alopecia, 1 abnormal LFTs Nausea ‐ 5 MTX, 4 placebo Rash ‐ 1 MTX Abnormal LFTs ‐ 6 MTX Vomiting ‐ 1 MTX, 3 placebo Diarrhoea ‐ 3 MTX, 1 placebo Alopecia ‐ 2 MTX Oral ulcers ‐ 4 MTX, 1 placebo |
|
| Notes | Results given as 95% confidence intervals & SEMs. Converted to SD for analysis. 3 patients able to cease OCS completely. The trial failed to show any significant reduction in OCS dose with MTX. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Trigg 1993.
| Methods | Randomised, double‐blinded, placebo‐controlled crossover trial. Duration 24 weeks, with 2 X 12 week treatment periods. | |
| Participants | Patient Numbers 18 ‐ 12 completed study. Age ‐ Range 49.7 (23‐68) (SD 12.0) Sex ‐ M / F 9/9 Duration OCS At least 6 months Dose ICS 10 ‐ 1.5mg/day, 3 ‐ 1.6mg/day, 5 ‐ 2mg/day Initial dose OCS 17.5 mg/day (Range 10‐50) (SD 11.55) FEV1 1.77 L (SD 0.86) | |
| Interventions | Oral methotrexate 30 mg once a week or identical placebo tablets. (7.5 mg for first week, 15 mg for second week, 30 mg if tolerated for remainder of trial) FBC, biochemistry, spirometry performed at screening & 3 weekly; skin prick tests performed at screening, CXR, PFTs & DLCO performed at screening, 12 & 24 weeks. Daily symptom diary cards & PEFRs monitored. Patient initiated OCS reduction by 5 mg every 5 days when well to 10 mg/day, then 2.5mg every 5 days. | |
| Outcomes | Final OCS dose ‐ MTX 10mg/day (SD 5.3)
‐ placebo 12.5mg/day (SD 8.8)
NS FEV1 ‐ Mean difference 0.24 L (SD 0.35) favouring MTX NS MMEFR ‐ Mean difference 0.35 L/min (SD 0.53) favouring MTX Significant PEFRs (am/pm) ‐ MTX 339 L/min (SD 150.2) / 361 L/min (SD 144.9) ‐ placebo 331 L/min (SD 185.6) / 357 L/min (SD 171.4) NS Wheeze ‐ MTX 0.59 (SD 0.87) (0 ‐ 3) ‐ placebo 0.67 (SD 0.92) NS Night waking ‐ MTX 0.56 (SD 0.97) (0 ‐ 3) ‐ placebo 0.61 (SD 0.99) NS Dyspnoea ‐ MTX 0.62 (SD 0.72) (0 ‐ 3) ‐ placebo 0.79 (SD 0.94) NS Cough ‐ MTX 0.50 (SD 0.64) (0 ‐ 3) ‐ placebo 0.64 (SD 0.64) NS Phlegm ‐ MTX 0.63 (SD 0.58) (0 ‐ 3) ‐ placebo 0.58 (SD 0.51) NS Toxicity 6 Withdrawals ‐ 3 MTX ‐ 1 headache & nausea, 2 pneumonia ‐ 2 placebo ‐ 1 depression, 1 non‐compliant ‐ 1 at completion (On additional OCS, data discarded) ‐ Dose reduction ‐ MTX ‐ 5 patients ‐ placebo ‐ 2 patients ‐ Nausea ‐ MTX 6, placebo 2 ‐ Stomatitis ‐ MTX 1, placebo 1 ‐Elevated LFTs ‐ MTX 1, placebo 1(paracetamol OD?) ‐ Pneumonia ‐ MTX 2 ‐ Zoster ‐ placebo 1 ‐ Skin infections ‐ MTX 3 ‐ Gastroenteritis ‐ MTX 1 ‐ Bronchitis ‐ MTX 2, placebo 2 ‐ URTI ‐ MTX 2, placebo 4 Exacerbations ‐ MTX 5, placebo 7 Depression, ‐ MTX 9, placebo 6 lethargy, headache |
|
| Notes | Only trial to use 30 mg /week of MTX. Statistical analysis over last 8 weeks of each treatment period to allow for washout period. Values calculated from graph, or given as SD or 95% CI limits. Auhtors failed to respond to correspondence, | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Investigators aware as to order of treatment group assignment (Cochrane Grade C) |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Caldwell 1992 | Small study published in abstract form only. Data unsuitable for analysis. 19 week cross‐over study, 17 patients with 5 withdrawals. No significant differences between arms. 20% reduction (SD 0.59) in OCS dose with MTX & 19.8% reduction (SD 0.59) with placebo. Unable to contact authors for further information. |
| Comet Monte 2002 | Study recruited participants treated with inhaled steroids; no chronic OCS use in the study. |
| Fennerty 1991 | A small study published in abstract form only. Data unsuitable for analysis. 24 week cross‐over study with 2X12 week treatment periods. 12 patients. 28% fall in OCS dose with MTX & 26% fall with placebo. Unable to contact authors for more information. |
| Mathur 1992 | This was a placebo‐controlled crossover study of 10 months duration (2 x 20 week treatment periods). Participants were allocated to either placebo or MTX at a dose of 15mg once per week. This study was excluded because there was no randomised process of allocation to either treatment. |
| Ogriala 1995 | A three armed study which compared the effects of a single dose of intramuscular triamcinolone Vs methotrexate Vs placebo on peak flow, PEFRs, airway reactivity, ER visits & hospitalisations over 6 months. The study did not examine fall in daily OCS dosage but only compared cumulative dosage after 6 months. Triamcinolone improved FEV1, peak flow rates & airway hyper‐responsiveness & reduced hospitalisation & emergency visits. Not possible to calculate effects of MTX upon OCS use with available data. Unable to contact authors. |
Contributions of authors
Davies H ‐ Inclusion / Exclusion, quality assessment, data extraction, preparation of text, data entry and analysis. Gibson P ‐ Intellectual direction and supervision, Inclusion / Exclusion, quality assessment, data extraction, interpretation and conclusions. Olson L ‐ Inclusion / Exclusion, quality assessment, data extraction, quality assessment editing of text.
Sources of support
Internal sources
NHS Research and Development, UK.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Coffey 1994 {published data only}
- Coffey M, Sanders G, Eschenbacher WL, Tsien A, Ramesh S, Weber RW, et al. The role of methotrexate in the management of steroid‐dependent asthma. Chest 1994;105:117‐21. [DOI] [PubMed] [Google Scholar]
Dyer 1991 {published data only}
- Dyer PD, Vaughan TR, Weber RW. Methotrexate in the treatment of steroid dependent asthma. Journal of Allegy & Clinical Immunology 1991;88:208‐12. [DOI] [PubMed] [Google Scholar]
Erzurum 1991 {published data only}
- Erzurum SC, Leff JA, Cochran JE, Ackerson LM, Szefler SJ, Martin RJ, et al. Lack of benefit of methotrexate in severe steroid‐dependent asthma. A double‐blind, placebo‐controlled study. Annals of Internal Medicine 1991;114:353‐60. [DOI] [PubMed] [Google Scholar]
Hedman 1996 {published data only}
- Hedman J, Seideman P, Albertioni F, Stenius‐Aarniala B. Controlled trial of methotrexate in patients with chronic severe asthma. European Journal of Clinical Pharmacology 1996;49:347‐9. [DOI] [PubMed] [Google Scholar]
Kanzow 1995 {published data only}
- Kanzow G, Nowak D, Magnussen H. Short term effect of methotrexate in severe steroid‐dependent asthma. Lung 1995;173:223‐31. [DOI] [PubMed] [Google Scholar]
Mullarkey 1988 {published data only}
- Mullarkey MF, Blumenstein BA, Andrade WP, Bailey GA, Olasen I, Wetzel CE. Methotrexate in the treatment of corticosteroid‐dependent asthma. A double‐blind crossover study. New England Journal of Medicine 1988;318:603‐7. [DOI] [PubMed] [Google Scholar]
- Shiner RJ, Nunn AJ, Chung KF, Geddes DM. Randomised, double‐blind, placebo‐controlled trial of methotrexate in steroid‐dependent asthma. Lancet 1990;336:137‐40. [DOI] [PubMed] [Google Scholar]
Shiner 1990 {published data only}
- Shiner RJ, Nunn A.J, Chung KF, Geddes DM. Randomised, double‐blind, placebo‐controlled trial of methotrexate in steroid‐dependent asthma. Lancet 1990;336:137‐40. [DOI] [PubMed] [Google Scholar]
Stewart 1994 {published data only}
- Stewart GE, Diaz JD, Lockey RF, Seleznick MJ, Trudeau WL, Ledford DK. Comparison of oral pulse methotrexate with placebo in the treatment of severe glucocorticosteroid‐dependent asthma. Journal of Allery & Clinical Immunology 1994;94:482‐9. [DOI] [PubMed] [Google Scholar]
Taylor 1993 {published data only}
- Taylor DR, Flannery EM, Herbison GP. Methotrexate in the management of severe steroid dependent asthma. New Zealand Journal of Medicine 1993;106:409‐11. [PubMed] [Google Scholar]
Trigg 1993 {published data only}
- Trigg CJ, Davies RJ. Comparison of methotrexate 30 mg per week with placebo in chronic steroid‐dependent asthma: a 12‐week double‐blind, cross‐over study. Respiratory Medicine 1993;87:211‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Caldwell 1992 {published data only}
- Caldwell EJ, Vogel BM, Dziodzio JT, Bagwell S. The effects of methotrexate on prednisone dosing in steroid‐dependent asthma. American Review of Respiratory Medicine 1992;145:A420. [Google Scholar]
Comet Monte 2002 {published data only}
- Comet Monte R. Efficacy and tolerance of methotrexate to decrease steroid tolerance in steroid‐dependend asthmatics [Dissertation] [Eficàcia i tolerància del metotrexate com a tractament a llarg termini a pacients amb asma corticodepenent]. Universitat Autònoma de Barcelona 2002.
Fennerty 1991 {published data only}
- Fennerty AG, Crossland P, Chappell AG. Methotrexate in steroid dependent asthmatics. Scottish Medical Journal 1991;36:124‐5. [Google Scholar]
Mathur 1992 {published data only}
- Mathur R, Bhasin RC. Methotrexate as steroid sparing drug in bronchial asthma. Lung India 1992;10(2):57‐60. [Google Scholar]
Ogriala 1995 {published data only}
- Ogirala RG, Sturm TM, Aldrich TK, Meller FF, Pacia EB, Keane AM, et al. Single, high‐dose intramuscular triamcinolone acetonide versus weekly oral methotrexate in life‐threatening asthma: a double‐blind study. American Journal of Respiratory and Critical Care Medicine 1995;152:1461‐6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Comet 2006 {published data only}
- Comet Monte R. Efficacy and tolerance of methotrexate to decrease steroid tolerance in steroid‐dependend asthmatics. [Dissertation]. 2002. [Google Scholar]
- Comet R, Domingo C, Larrosa M, Morón A, Rué M, Amengual MJ, et al. Benefits of low weekly doses of methotrexate in steroid‐dependent asthmatic patients. A double‐blind, randomized, placebo‐controlled study. Respiratory medicine 2006 Mar;100(3):411‐9. [DOI] [PubMed] [Google Scholar]
Gazdik 1997 {published data only}
- Gazdik F, Jahnova E, Keleova H, et al. The influence of methotrexate on the immune system of patients with corticoiddependent (sic) bronchial asthma (randomised double‐blind study) [Vplyv Metotrexatu na imunity system pacientov s kortikodependentnou bronchialnou astmou (randomizovana dvojite slepa, paralelne prebiehajuca studia slepa)]. Klinicka Imunologia a Alergologia 1997;7(2):26‐30. [Google Scholar]
Kumar 2013 {published data only}
- Kumar S, Kumar P. Asthma diagnosis and treatment‐1026. Long term twice weekly. World Allergy Organization journal 2013;6(Suppl 1):P25. [Google Scholar]
Obojski 2007 {published data only}
- Obojski A, Chmielowicz B, Dobek R, Bielous‐Wilk A, Krzysztof W, Barg W, et al. Methotrexate in treatment of severe difficult to treat asthma: randomized, double blind, placebo controlled study [Abstract]. 2007; Vol. 30:248s [1584].
References to ongoing studies
Polosa 2014 {published data only}
- Polosa R, Bellinvia S, Caruso M, Emma R, Alamo A, Kowalski ML, et al. Weekly low‐dose methotrexate for reduction of Global. Trials 2014;15(1):492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Bardin 1993
- Bardin PG, Fraenkel DJ, Beasley RW. Methotrexate in asthma A safety perspective. Drug Safety 1993;9:151‐5. [DOI] [PubMed] [Google Scholar]
Calderon 1991
- Calderon E, Coffey RG, Lockey RF. Methotrexate in bronchial asthma. Journal of Allergy & Clinical Immunology 1991;88:274‐6. [DOI] [PubMed] [Google Scholar]
Cott 1994a
- Cott GR. Current status of alternative anti‐inflammatory therapies in the treatment of asthma: critical appraisal. Seminars in Respiratory and Critical Care Medicine 1994;15:136‐46. [Google Scholar]
Cott 1994b
- Cott GR. Perspectives on methotrexate in the treatment of asthma. Chest 1994;105:649‐50. [DOI] [PubMed] [Google Scholar]
Delaney 1992
- Delaney SG, Taylor DR. Steroid‐sparing agents in the treatment of asthma. Medical Journal of Australia 1992;157:437‐9. [DOI] [PubMed] [Google Scholar]
Fertel 1991
- Fertel D, Wanner A. Methotrexate: does it treat or induce asthma?. American Review of Respiratory Disease 1991;143:1‐2. [DOI] [PubMed] [Google Scholar]
Glynn‐Barnhart 1991
- Glynn‐Barnhart AM, Erzurum SC, Leff JA, Martin RJ, Cochran JE, Cott GR, et al. Effect of low‐dose methotrexate on the disposition of glucocorticoids and theophylline. Journal of Allergy & Clinical Immunology 1991;88:180‐6. [DOI] [PubMed] [Google Scholar]
Jones 1991
- Jones G, Mierins E, Karsh J. Methotrexate induced asthma. American Review of Respiratory Disease 1991;143:179‐81. [DOI] [PubMed] [Google Scholar]
Klaustermeyer 1991
- Klaustermeyer WB, Garb KS, Santiago SM, Kinney JL. Variability of pulmonary function tests in stable corticosteroid dependent asthma patients. Allergy Proceedings 1991;12:255‐9. [DOI] [PubMed] [Google Scholar]
Lammers 1987
- Lammers AM, Kerkhof PCM, Mier PD. Reduction of leukotriene B4‐induced intraepidermal accumulation of polymorphonuclear leukocytes by methotrexate in psoriasis. British Journal Dermatology 1987;116:667‐71. [DOI] [PubMed] [Google Scholar]
Ledford 1996
- Ledford DK. Treatment of steroid‐resistant asthma. Immunology Allergy Clinics of North America 1996;16:777‐96. [Google Scholar]
Lynch 1997
- Lynch JP, McCune WJ. Immunosuppressive and cytotoxic pharmacotherapy for pulmonary disorders. American Journal Respiratory Critical Care Medicine 1997;155:395‐420. [DOI] [PubMed] [Google Scholar]
Marin 1997
- Marin MG. Low‐dose methotrexate spares steroid usage in steroid‐dependent asthmatic patients ‐ a meta‐analysis. Chest 1997;112:29‐33. [DOI] [PubMed] [Google Scholar]
Markham 1994
- Markham A, Faulds D. Methotrexate: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in rheumatoid arthritis and other immunoregulatory disorders. Clinical Immunotherapy 1994;1:217‐44. [Google Scholar]
Moss 1995
- Moss RB. Alternative pharmacotherapies for steroid‐dependent asthma. Chest 1995;107:817‐25. [DOI] [PubMed] [Google Scholar]
Mullarkey 1986
- Mullarkey MF, Webb R, Pardee NE. Methotrexate in the treatment of steroid dependent asthma. Annals of Allergy 1986;56:347‐50. [PubMed] [Google Scholar]
Mullarkey 1991
- Mullarkey MF. Methotrexate and asthma. Journal of Allergy & Clinical Immunology 1991;88:272‐3. [DOI] [PubMed] [Google Scholar]
Mullarkey 1997
- Mullarkey MF. Methotrexate revisited. Chest 1997;112:1‐2. [DOI] [PubMed] [Google Scholar]
Shiner 1994
- Shiner RJ, Katz I, Shulimzon T, Silkoff P, Benzaray S. Methotrexate in steroid‐dependent asthma: long‐term results. Allergy 1994;49:565‐8. [DOI] [PubMed] [Google Scholar]
Shulimzon 1996
- Shulimzon TR, Shiner RJ. A risk‐benefit assessment of methotrexate in corticosteroid‐dependent asthma. Drug Safety 1996;15:283‐90. [DOI] [PubMed] [Google Scholar]
Sorkness 1991
- Sorkness CA, Busse WW, Bush RK. Use of oral methotrexate in corticosteroid‐dependent adult asthma: a twenty‐four month analysis. Journal of Allergy and Clinical Immunology 1991;87:298. [Google Scholar]
Stanziola 1995
- Stanziola A, Sofia M, Mormile M, Molino A, Carratu L. Long‐term treatment with methotrexate in patients with corticosteroid‐dependent bronchial asthma. Monaldi Archives of Chest Disease 1995;50:109‐13. [PubMed] [Google Scholar]
Suarex 1987
- Suarex CR, Pickett WC, Bell DH, McClintock, Oronosky AL, Kerwar SS. Effect of low dose methotrexate on neutrophil chemotaxis induced by leukotriene B4 and complement C5a. Journal of Rheumatology 1987;14:9‐11. [PubMed] [Google Scholar]
Szefler 1992
- Szefler SJ. Anti‐inflammatory drugs in the treatment of allergic disease. Medical Clinics of North America 1992;76:953‐75. [DOI] [PubMed] [Google Scholar]
Szefler 1997
- Szefler SJ, Leung DYM. Glucocorticoid‐resistant asthma: pathogenesis and clinical implications for management. European Respiratory Journal 1997;10:1640‐7. [DOI] [PubMed] [Google Scholar]
Ternowitz 1987
- Ternowitz T, Bjerring P, Anderson PH, et al. Methotrexate inhibits the human C5a‐induced skin response in patients with psoriasis. Journal of Investigative Dermatology 1987;89:192‐6. [DOI] [PubMed] [Google Scholar]
Tsai 1994
- Tsai JJ, Wang TF, Wang SR. The inhibitory effect of methotrexate on PAF‐induced neutrophil and eosinophil locomotion in asthmatic patients. Asian Pacific Journal of Allergy & Immunology 1994;12:65‐71. [PubMed] [Google Scholar]
Wald 1991
- Wald JA, Farr RS. Abnormal liver‐function tests associated with long‐term systematic corticosteroid use in subjects with asthma. Journal of Allergy & Clinical Immunology 1991;88(2):277‐8. [DOI] [PubMed] [Google Scholar]
Weinblatt 1995
- Weinblatt ME. Methotrexate for chronic diseases in adults. New England Journal of Medicine 1995;332:330‐1. [DOI] [PubMed] [Google Scholar]


