Abstract
Background
This review is an update of a previously published review in Issue 2, 2012 (Derry 2012a). Migraine is a common, disabling condition and a burden for the individual, health services and society. Many sufferers choose not to, or are unable to, seek professional help and rely on over‐the‐counter (OTC) analgesics. Diclofenac is an established analgesic, and new formulations using the potassium or epolamine salts, which can be dissolved in water, have been developed for rapid absorption, which may be beneficial in acute migraine. Co‐therapy with an antiemetic should help to reduce the nausea and vomiting commonly associated with migraine.
Objectives
To determine the efficacy and tolerability of diclofenac, alone or in combination with an antiemetic, compared to placebo and other active interventions in the treatment of acute migraine headaches in adults.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the Oxford Pain Relief Database, ClinicalTrials.gov, and reference lists for studies through 27 September 2011 for the original review and 15 February 2013 for the update.
Selection criteria
We included randomised, double‐blind, placebo‐controlled or active‐controlled studies, or both, using self administered diclofenac to treat a migraine headache episode, with at least 10 participants per treatment arm.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. We used numbers of participants achieving each outcome to calculate relative risk (or 'risk ratio') and numbers needed to treat to benefit (NNT) or harm (NNH) compared to placebo or a different active treatment.
Main results
Five studies (1356 participants, 2711 attacks) compared oral diclofenac with placebo, and one also compared it with sumatriptan; none combined diclofenac with a self administered antiemetic. Four studies treated attacks with single doses of medication, and two allowed an optional second dose for inadequate response. Only two studies, with three active treatment arms, provided data for pooled analysis of primary outcomes. For single doses of diclofenac potassium 50 mg versus placebo (two studies), the NNTs were 8.9, 6.2, and 9.5 for pain‐free at two hours, headache relief at two hours, and pain‐free responses at 24 hours, respectively.
Similar numbers of participants experienced adverse events, which were mostly mild and transient, with diclofenac and placebo.
There were insufficient data to evaluate other doses of oral diclofenac, or to compare different formulations or different dosing regimens; only one study compared oral diclofenac with an active comparator (oral sumatriptan 100 mg).
Authors' conclusions
Oral diclofenac potassium 50 mg is an effective treatment for acute migraine, providing relief from pain and associated symptoms, although only a minority of patients experience pain‐free responses. Adverse events are mostly mild and transient and occur at the same rate as with placebo.
Plain language summary
Diclofenac with or without an antiemetic for acute migraine headaches in adults
This review found that oral diclofenac potassium 50 mg was an effective treatment for migraine headache, reducing moderate to severe pain to no more than mild pain within two hours in about half (55%) of those treated, to no pain at two hours in about one in five (22%), and to no pain sustained to 24 hours in about the same number (19%). Adverse events were mostly self limiting and of mild or moderate intensity, and were not significantly different from placebo over the short term. Although diclofenac provided good outcomes for some people, almost half did not experience adequate pain relief within two hours, and as few as one in five became pain‐free. It is not clear whether the 100 mg dose provides good outcomes for more people. For those who do not experience adequate responses, a different therapy will be needed.
There was no information about different formulations of diclofenac (e.g. suppositories) to treat acute migraine headaches.
Summary of findings
for the main comparison.
| Diclofenac compared with placebo for migraine headache | ||||||
|
Patient or population: adults with migraine headache ‐ moderate or severe pain Settings: community Intervention: diclofenac 50 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with intervention | Probable outcome with comparator | NNT, NNH or RR (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments |
| Pain‐free at 2 h | 220 in 1000 | 110 in 1000 | NNT 8.9 (6.7 to 13) | 2 studies, 1447 participants 262 events |
Moderate1 | Potassium salt; standard tablet and soluble formulations |
| Headache relief at 2 h | 550 in 1000 | 390 in 1000 | NNT 6.2 (4.7 to 9.1) | 2 studies, 1447 participants 718 events |
Moderate1 | Potassium salt; standard tablet and soluble formulations |
| Sustained pain‐free at 24 h | 190 in 1000 | 82 in 1000 | NNT 9.5 (7.2 to 14) | 2 studies, 1578 participants, 228 events2 | Moderate1 | Potassium salt; standard tablet and soluble formulations |
| Sustained headache relief at 24 h | No data | |||||
| At least one AE | 180 in 1000 | 160 in 1000 | RR 1.1 (0.86 to 1.6) | 3 studies, 1075 participants, 187 events | Low1 | Potassium salt; standard tablet and soluble formulations |
| Serious AE | No events | No events | ||||
| CI: Confidence interval; NNT: number needed to treat; NNH: number needed to harm; RR: relative risk | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 ‐ Quality of evidence downgraded from high because of threat from potential publication bias with modest effect size and numbers of events
2 ‐ includes a small proportion of participants with mild pain at baseline
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (Issue 2, 2012) on diclofenac with or without an antiemetic for acute migraine headaches in adults (Derry 2012a).
Description of the condition
Migraine is a common, disabling headache disorder, ranked seventh highest among specific causes of disability globally (Steiner 2013), and with considerable social and economic impact (Hazard 2009). Recent reviews found a one‐year prevalence of 15% globally (Vos 2012) and for adults in European countries (Stovner 2010), 13% for all ages in the United States (US) (Victor 2010), 21% in Russia (Ayzenberg 2012) and 9% for adults in China (Yu 2012). Migraine is more prevalent in women than in men (by a factor of two to three), and in the age range 30 to 50 years.
The International Headache Society (IHS) classifies two major subtypes. Migraine without aura is the most common subtype. It is characterised by attacks lasting 4 to 72 hours that are typically of moderate to severe pain intensity, unilateral, pulsating, aggravated by normal physical activity, and associated with nausea or photophobia and phonophobia. Migraine with aura is characterised by reversible focal neurological symptoms that develop over a period of 5 to 20 minutes and last for less than 60 minutes, followed by headache with the features of migraine without aura. In some cases the headache may lack migrainous features or be absent altogether (IHS 2004).
A recent large prevalence study in the US found that over half of migraineurs had severe impairment or required bed rest during attacks. Despite this high level of disability and a strong desire for successful treatment, only a proportion of migraine sufferers seek professional advice for the treatment of attacks. The majority were not taking any preventive medication, although one‐third met guideline criteria for offering or considering it. Nearly all (98%) migraineurs used acute treatments for attacks, with 49% using over‐the‐counter (OTC) medication only, 20% using prescription medication, and 29% using both. OTC medication included aspirin, other non‐steroidal anti‐inflammatory drugs (NSAIDs), paracetamol (acetaminophen), and paracetamol with caffeine (Bigal 2008; Diamond 2007; Lipton 2007). Similar findings have been reported from other large studies in France and Germany (Lucas 2006; Radtke 2009).
The significant impact of migraine with regard to pain, functional health and well‐being is well documented (Buse 2011; Leonardi 2005; Vos 2012) A cross‐sectional survey of eight European Union (EU) countries (representing 55% of the adult population) has estimated an annual direct and indirect cost of migraine per person of €1222, and a total annual cost for the EU of €111 billion for adults aged 18 to 65 years (Linde 2012). Costs are substantially greater for the minority with chronic migraine compared with episodic migraine; they also vary between countries, probably due to differences in available therapies and they way they are delivered, and structural differences in healthcare systems (Bloudek 2012). In the US, the average annual direct cost per person has been estimated at $1757 for episodic migraine and $7750 for chronic migraine (Munakata 2009). Whatever the exact direct and indirect costs are for each country, it is clear that migraine presents a significant economic burden. Successful treatment of acute migraine attacks not only benefits patients by reducing their disability and improving health‐related quality of life, but also has the potential to reduce the need for healthcare resources and increase economic productivity. Migraine is ranked in the top 10 disorders for global years lived with disability (Vos 2012).
Description of the intervention
Diclofenac is a NSAID with proven efficacy in treating mild to moderate pain and inflammation in acute and chronic musculoskeletal disorders, postoperative pain, gout, and dysmenorrhoea. It is available OTC in some countries, including parts of Europe, but remains prescription‐only in the US. In the United Kingdom (UK), diclofenac sodium is prescription‐only, while diclofenac potassium is available OTC as 12.5 mg tablets (with a maximum dose restriction of 25 mg) for headache relief (among other things). Diclofenac, like other NSAIDs, may cause irritation of the gastrointestinal tract and result in discomfort, ulcers, and bleeding, particularly with prolonged use.
Diclofenac is most often taken orally as a standard tablet or as a powder to be dissolved in water just before ingestion, but rectal (suppositories), intramuscular, and intravenous routes of administration are also used; generic formulations are widely available. As suggested above, diclofenac is widely available as the sodium or the potassium salt; in fewer places, it is available as the powdered epolamine salt (hydrosoluble diclofenac epolamine (DHEP)). In primary care in the UK, 6.4 million prescriptions for diclofenac sodium and 69,000 prescriptions for diclofenac potassium were issued in 2009, mostly as 50 mg tablets (PCA 2010). The potassium salt is more soluble and is thought to have a more rapid onset of action because it has been shown to be significantly more effective than the sodium salt in acute pain (Derry 2009). This rapid onset of action is exploited in the brand‐name formulation Voltarol Rapid, marketed by Novartis, with specific approval for treatment of acute migraine in the UK, and Cambia, marketed by Nautilus Neurosciences, with approval for acute migraine in the US. The epolamine salt (DHEP) is highly soluble in both hydrophilic and lipophilic tissues, and was developed to improve absorption of diclofenac. The complex is claimed to have both improved gastric absorption (leading to more rapid onset of action) and reduced mucosal irritation compared with diclofenac sodium, while bioavailability is maintained (IBSA 2011).
In order to establish whether diclofenac is an effective analgesic at a specified dose in acute migraine, it is necessary to study its effects in circumstances that permit detection of pain relief. Such studies are carried out in individuals with established pain of moderate to severe intensity, using single doses of the interventions. Participants who experience an inadequate response with either placebo or active treatment are permitted to use rescue medication, and the intervention is considered to have failed in those individuals. In clinical practice, however, individuals would not normally wait until pain is of at least moderate severity, and may take a second dose of medication if the first dose does not provide adequate relief. Once analgesic efficacy is established in studies using single doses in established pain, further studies may investigate different treatment strategies and patient preferences. These are likely to include treating the migraine early while pain is mild, and using a low dose initially, with a second dose if response is inadequate.
How the intervention might work
NSAIDs act by inhibiting the activity of cyclooxygenase (COX), now recognised to consist of two isoforms (COX‐1 and COX‐2), which catalyses the production of prostaglandins responsible for pain and inflammation. Diclofenac inhibits both COX isoforms, with low to moderate preference for COX‐2 (IC50 ratio of 29) (Patrono 2001; Patrono 2009). Suppression of prostaglandin synthesis is believed to underlie most of the analgesic effects of diclofenac, although other mechanisms probably contribute.
The efficacy of oral medications is reduced in many migraineurs because of impaired gastrointestinal motility, which is associated with nausea, and because of non‐absorption of the drug due to vomiting (Volans 1974). The addition of an antiemetic may improve outcomes by alleviating the often incapacitating symptoms of nausea and vomiting, and (at least potentially) by enhancing the bioavailability of the co‐administered analgesic. In particular, prokinetic antiemetics such as metoclopramide, which stimulate gastric emptying, may improve outcomes by increasing absorption of the analgesic. This has been investigated for metoclopramide and aspirin (Ross‐Lee 1983; Volans 1975). It has been claimed that treatment with intravenous metoclopramide alone can reduce pain in severe migraine (Friedman 2005; Salazar‐Tortolero 2008), but this claim requires further investigation, since metoclopramide has not been shown to be an analgesic in classical pain studies. This review seeks to determine whether treatment of acute migraine with diclofenac plus an antiemetic is in any way superior to treatment with diclofenac alone. In a recent review of aspirin with or without an antiemetic for acute migraine headaches, aspirin plus metoclopramide was significantly better than aspirin alone for headache relief and relief of nausea at two hours, but not for pain‐free at two or 24 hours (Kirthi 2013).
Why it is important to do this review
Diclofenac has proven efficacy in a variety of acute pain situations, and it is important to know where it fits in the range of therapeutic options for migraine therapy. Use of the more soluble potassium salt may provide the rapid relief that many migraineurs want. Where available OTC, it may offer a convenient and affordable alternative to migraine‐specific prescription medications. We could find no systematic review of the efficacy of diclofenac for acute migraine in adults.
This review is one of a series examining the efficacy of OTC treatments for migraine, including aspirin (Kirthi 2013), paracetamol (acetaminophen) (Derry 2013), and ibuprofen (Rabbie 2013), as well as oral sumatriptan (Derry 2012b [update in press]).
Objectives
The objective of this review is to determine the efficacy and tolerability of diclofenac, alone or in combination with an antiemetic, compared to placebo and other active interventions in the treatment of acute migraine headaches in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, double‐blind, placebo‐controlled or active‐controlled studies, or both, using diclofenac to treat a migraine headache episode. Studies had to have a minimum of 10 participants per treatment arm and report dichotomous data for at least one of the outcomes specified below. We accepted studies reporting treatment of consecutive headache episodes if outcomes for the first, or each, episode were reported separately; we used first‐attack data preferentially. We accepted cross‐over studies if there was adequate (at least 24 hours) washout between treatments.
Types of participants
Studies included adults (at least 18 years of age) with migraine. We used the definition of migraine specified by the International Headache Society (IHS 1988; IHS 2004). There were no restrictions on migraine frequency, duration, or type (with or without aura). We accepted studies including participants taking stable prophylactic therapy to reduce the frequency of migraine attacks. If reported, details on any prophylactic therapy prescribed or allowed are provided in the Characteristics of included studies table.
Types of interventions
We included studies in which self administered diclofenac was used to treat a migraine headache episode. There were no restrictions on dose, dosing regimen (e.g. single dose versus optional second dose), formulation, route of administration, or timing of the first dose in relation to headache intensity (e.g. taking the first dose when pain was of moderate or severe intensity versus when pain was only mild).
Included studies could use either diclofenac alone, or diclofenac plus an antiemetic. The antiemetic had to be taken either combined with diclofenac in a single formulation, or separately not more than 30 minutes before diclofenac, and be self administered.
A placebo comparator is essential to demonstrate that diclofenac is effective in this condition. We considered active‐controlled trials without a placebo as secondary evidence. We excluded studies designed to demonstrate prophylactic efficacy in reducing the number or frequency of migraine attacks.
Types of outcome measures
In selecting the main outcome measures for this review, we considered scientific rigour, availability of data and patient preferences. Patients with acute migraine headaches have rated complete pain relief, no headache recurrence, rapid onset of pain relief, and no side effects as the four most important outcomes (Lipton 1999).
In view of these patient preferences, and in line with the guidelines for controlled trials of drugs in migraine issued by the IHS (IHS 2000), the main outcomes to be considered were:
Primary outcomes
Pain‐free at two hours, without the use of rescue medication (PF2).
Reduction in headache pain ('headache relief') at two hours (HR2) ‐ (pain reduced from moderate or severe to none or mild without the use of rescue medication).
Data for pain‐free and headache relief outcomes at one hour would also be collected if reported and relevant, for example if a fast‐acting formulation of the intervention was tested.
Secondary outcomes
Sustained pain‐free during 24 hours (SPF24) ‐ pain‐free within two hours, with no use of rescue medication or recurrence of moderate to severe pain within 24 hours.
Sustained pain reduction over 24 hours (SHR24) ‐ headache relief at two hours, sustained for 24 hours, with no use of rescue medication or a second dose of study medication.
Adverse events: participants with any adverse event during 24 hours postdose; serious adverse events; adverse events leading to withdrawal.
Other outcomes
Data for a number of other outcomes were also collected, including:
use of rescue medication;
relief of headache‐associated symptoms;
relief of functional disability.
Pain intensity or pain relief had to be measured by the patient (not the investigator or care giver). Pain measures accepted for the main efficacy outcomes were:
Pain intensity (PI): 4‐point categorical scale, with wording equivalent to none, mild, moderate and severe; or 100 mm VAS), where < 30 mm was considered equivalent to mild or no pain and ≥ 30 mm equivalent to moderate or severe pain (Collins 1997);
Pain relief (PR): 5‐point categorical scale, with wording equivalent to none, a little, some, a lot, complete; or 100 mm VAS, where < 30 mm was considered equivalent to none or a little, and ≥ 30 mm equivalent to some, a lot or complete.
We considered only data obtained directly from the patient.
Definitions of important terms, including all measured outcomes, are provided in Appendix 1.
Search methods for identification of studies
Electronic searches
For the original review we searched the following databases to 27 September 2011:
the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 10).
MEDLINE (via Ovid).
EMBASE (via Ovid).
Oxford Pain Relief Database (Jadad 1996a).
For the update we searched:
the Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 1, 2013);
MEDLINE (via Ovid) from January 2011 to 15 February 2013;
EMBASE (via Ovid) from January 2011 to 15 February 2013.
See Appendix 2 for the MEDLINE search strategy, Appendix 3 for the EMBASE search strategy, and Appendix 4 for the CENTRAL search strategy. There were no language restrictions.
Searching other resources
For the original review we searched reference lists of retrieved studies and review articles for additional studies (we identified two unpublished studies). We also searched online databases of clinical trials (clinicaltrials.gov and novctrd.com). We made written requests to Novartis, who manufacture Voltarol Rapid tablets, and Nautilus Neurosciences, who manufacture Cambia, asking for details of any randomised controlled trials (RCTs) known to them involving diclofenac for acute treatment of migraine. Both manufacturers supplied citations for published papers that we had already identified, but no additional studies (published or otherwise) were identified. We did not search grey literature and short abstracts.
For the update we searched the online databases again (www.clinicaltrials.gov and www.novctrd.com).
Data collection and analysis
Selection of studies
Two review authors independently carried out the searches and selected studies for inclusion. We viewed titles and abstracts of all studies identified by electronic searches on screen and excluded any that clearly did not satisfy the inclusion criteria. We read full copies of the remaining studies to identify those suitable for inclusion. Disagreements were settled by discussion with a third review author.
Data extraction and management
Two review authors independently extracted data from included studies using a standard data extraction form. Disagreements were settled by discussion with a third review author. One author entered data into RevMan 5.1, and one author entered information for the update (RevMan 2012).
Assessment of risk of bias in included studies
We used the Oxford Quality Score (Jadad 1996b) as the basis for inclusion, limiting inclusion to studies that were randomised and double‐blind as a minimum. The scores for each study are reported in the 'Characteristics of included studies' table.
Two authors independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We assessed the following for each study:
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, e.g. random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). Studies using a non‐random process (e.g. odd or even date of birth; hospital or clinic record number) were excluded.
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions before assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). Studies that did not conceal allocation (e.g. open list) were excluded.
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study states that it was blinded and describes the method used to achieve blinding, e.g. identical tablets; matched in appearance and smell); unclear risk of bias (study states that it was blinded but does not provide an adequate description of how it was achieved). Studies that were not double‐blind were excluded.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (< 10% of participants provided no data without acceptable reason ‐ e.g. they were randomised but did not have a qualifying headache). Studies with high data loss were excluded.
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (≥ 200 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (< 50 participants per treatment arm).
Measures of treatment effect
We used relative risk (or 'risk ratio', RR) to establish statistical difference. We used numbers needed to treat (NNT) and pooled percentages as absolute measures of benefit or harm.
We planned to use the following terms to describe adverse outcomes in terms of harm or prevention of harm:
When significantly fewer adverse outcomes occur with diclofenac than with control (placebo or active) we would use the term the number needed to treat to prevent one event (NNTp).
When significantly more adverse outcomes occur with diclofenac compared with control (placebo or active) we would use the term the number needed to harm or cause one event (NNH).
In fact, the included studies did not provide the data needed to calculate these measures.
Unit of analysis issues
We accepted randomisation at the individual patient level only. For analysis of studies with more than one treatment arm contributing to any one analysis (e.g. two formulations of the same dose of diclofenac in the same study with a single placebo group), we split the placebo group equally between the two treatment arms so as not to double‐count placebo participants.
Dealing with missing data
The most likely source of missing data was in cross‐over studies; we planned to use only first‐period data where possible, but where that was not provided, we treated the results as if they were parallel group results. Where there were substantial missing data in any study, we would comment on this and perform sensitivity analyses to investigate their effect.
For all outcomes we carried out analyses, as far as possible, on a modified intention‐to‐treat (ITT) basis, i.e. we included all participants who were randomised and received an intervention. Where sufficient information was reported, we re‐included missing data in the analyses we undertook. We excluded data from outcomes where data from ≥ 10% of participants were missing with no acceptable reason provided or apparent.
Assessment of heterogeneity
We assessed heterogeneity of response rates using L'Abbé plots, a visual method for assessing differences in results of individual studies (L'Abbé 1987). Where data could be pooled, we report the I2 statistic.
Assessment of reporting biases
We assessed publication bias by examining the number of participants in trials with zero effect (relative risk of 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008). In this case, we specified a clinically useful level as a NNT ≥ 8 for pain‐free at two hours, and NNT ≥ 6 for headache relief at two hours.
Data synthesis
We analysed studies using a single dose of diclofenac in established pain of at least moderate intensity separately from studies in which medication was taken before pain became well established, or in which a second dose of medication was permitted.
We calculated effect sizes and combined data for analysis only for comparisons and outcomes where there were at least two studies and 200 participants (Moore 1998). We calculated relative risk of benefit or harm with 95% confidence intervals (CIs) using a fixed‐effect model (Morris 1995). We calculated NNT, NNTp, and NNH with 95% CIs, where possible, using the pooled number of events by the method of Cook and Sackett (Cook 1995). We assumed a statistically significant difference from control when the 95% CI of the relative risk of benefit or harm did not include the number one.
We determined significant differences between NNT, NNTp, and NNH for different groups in subgroup and sensitivity analyses, using the z test (Tramer 1997).
We describe data from comparisons and outcomes with only one study or fewer than 200 participants in the summary tables and text where appropriate for information and comparison, but they are not analysed quantitatively.
Subgroup analysis and investigation of heterogeneity
Issues for potential subgroup analysis were dose, monotherapy versus combination with an antiemetic, route of administration, and formulation. For combined treatment with an antiemetic, we planned to compare different antiemetics if there were sufficient data.
Sensitivity analysis
We planned sensitivity analysis for study quality (Oxford Quality Score of 2 versus 3 or more), and for migraine type (with aura versus without aura). A minimum of two studies and 200 participants were required for any sensitivity analysis.
Results
Description of studies
Included studies
New searches in February 2013 did not identify any additional studies.
We identified five studies, with 1356 participants, that satisfied our inclusion criteria (Dahlof 1993; Diener 2006; DKSMSG 1999; Lipton 2010; Vecsei 2007). We also identified two unpublished studies (Novartis 1995; Novartis 1998) from a narrative review (McNeely 1999), but we have been unable to obtain any further details. Judging from the information in the review, the studies are likely to have satisfied our inclusion criteria, but may not have provided dichotomous results for our primary outcomes (Characteristics of studies awaiting classification).
One of the included studies used a parallel‐group design (Lipton 2010) and the remainder a cross‐over design. A period of at least 48 hours was required between qualifying attacks in the cross‐over studies.
Studies recruited adults of both sexes who had a diagnosis of migraine according to IHS criteria (IHS 1988; IHS 2004) for at least one year. The median or mean ages reported in individual studies ranged from 33 to 44 years, and between 76% and 89% of participants were female. Four studies (Dahlof 1993; Diener 2006; DKSMSG 1999; Lipton 2010) included participants who experienced migraine with or without aura, while one (Vecsei 2007) included only participants who experienced migraine without aura. Participants were generally excluded for pregnancy or breast‐feeding, inadequate contraception, and known hypersensitivity or contraindication to diclofenac or other NSAIDs. Lipton 2010 excluded participants if they experienced vomiting in 20% of attacks or needed bed rest with most attacks, and Vecsei 2007 excluded participants if they usually experienced migraine of 'severe intensity'.
Included studies used oral diclofenac as the potassium salt taken either in a standard tablet formulation (Dahlof 1993; Diener 2006; DKSMSG 1999) or as a powder to be dissolved in water just before ingestion (Diener 2006; Lipton 2010), or as the powdered epolamine salt (DHEP) to be dissolved in water just before ingestion (Vecsei 2007). No study used an antiemetic in combination with diclofenac. In four studies (Dahlof 1993; Diener 2006; DKSMSG 1999; Lipton 2010) participants took a single dose of study medication for an attack, with rescue medication available after two hours if relief was inadequate. In the remaining study (Vecsei 2007) a second dose of study medication was available after one hour, and the majority of participants took the second dose (63% with diclofenac 50 mg, and 87% with placebo). We did not combine the different dosing regimens for analysis.
Three studies allowed participants to continue with migraine prophylaxis, provided it was stable and unchanged throughout the study (Diener 2006; DKSMSG 1999; Lipton 2010. Three studies specifically prohibited the use of various analgesics within a specific time of study medication: Dahlof 1993 prohibited NSAID therapy within one week; Diener 2006 prohibited long‐acting analgesics during the study, and acute headache medication within 48 hours; and Lipton 2010 prohibited any analgesics within 24 hours of study medication. Other medication was permitted if it did not interact with diclofenac and was unlikely to affect migraine outcomes.
All studies had a placebo control arm; one also compared diclofenac with sumatriptan 100 mg (DKSMSG 1999). There were 1356 participants who contributed data from 2711 attacks; 1111 attacks were treated with diclofenac potassium 50 mg, 422 with diclofenac potassium 100 mg, 238 with diclofenac DHEP, 115 with sumatriptan 100 mg and 825 with placebo.
In DKSMSG 1999, 144 participants were randomised to treat four consecutive attacks each with a single dose of the different study medications. There were no data suitable for analysis for the primary outcomes because the study reported only group mean data for these outcomes; in addition, only 115 patients had four attacks, giving an attrition rate of 20%. It was possible to use adverse event data from this study because these were reported according to participants receiving each treatment, irrespective of whether individuals completed all four treatments, and missing data were likely to be largely due to lack of qualifying headaches.
In one study (Lipton 2010) participants were instructed to wait until pain intensity was moderate or severe before taking study medication, while in four studies (Dahlof 1993; Diener 2006; DKSMSG 1999; Vecsei 2007) they were to take medication at the first sign of pain. Diener 2006 and Vecsei 2007 reported efficacy separately for participants with moderate or severe pain at baseline, and despite instructions to treat early, the vast majority (94% and 89% respectively) had at least moderate pain at baseline, so this subset was analysed together with Lipton 2010.
Full details of included studies are found in the Characteristics of included studies table.
Excluded studies
We excluded six studies after reading the full report (Bigal 2002; Comoglu 2011; Del Bene 1987; Karachalios 1992; Massiou 1991; Peroutka 2004). Reasons for exclusion are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
Included studies were all randomised and double‐blind. On the Oxford Quality Score three studies (Diener 2006; DKSMSG 1999; Lipton 2010) scored 4 of 5, and two (Dahlof 1993; Vecsei 2007) scored 3 of 5. Points were lost for failure to adequately describe the processes of randomisation or blinding, and failure to explicitly report withdrawals.
A risk of bias table was completed for randomisation, allocation concealment, blinding, incomplete outcome data, and study size. No study was considered at high risk of bias. The two studies that contributed to pooled analyses were both of good methodological quality and size (Figure 1.
1.
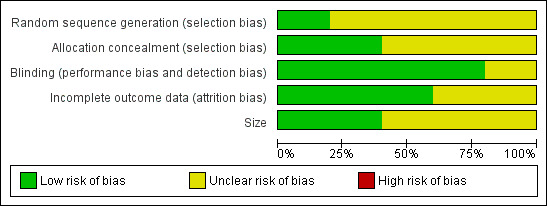
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
See: Table 1
Details of results in individual studies are available in Appendix 5 (efficacy) and Appendix 6 (adverse events and withdrawals).
Studies using a single dose of study medication
Two studies (three active treatment arms, 1477 participants) compared diclofenac potassium 50 mg with placebo in participants with moderate or severe baseline pain (Diener 2006; Lipton 2010). An oral solution was used in one active treatment arm of Diener 2006 and in Lipton 2010, and a tablet in the other active arm of Diener 2006. There were insufficient data for analysis of the 100 mg dose compared with placebo for any primary outcome, and no usable data for the 50 mg dose compared with placebo for outcomes at less than two hours, or for headache relief at 24 hours. Results for pain‐free and headache relief response for 50 mg are summarised in Summary of results A.
Pain‐free at two hours
Diclofenac potassium 50 mg versus placebo
The proportion of attacks pain‐free at two hours with diclofenac 50 mg was 22% (195/873; range 17% to 25%).
The proportion of attacks pain‐free at two hours with placebo was 11% (67/604; range 10% to 13%).
The relative benefit of treatment compared with placebo was 2.0 (95% CI 1.6 to 2.6; Analysis 1.1); the NNT was 8.9 (6.7 to 13).
1.1. Analysis.
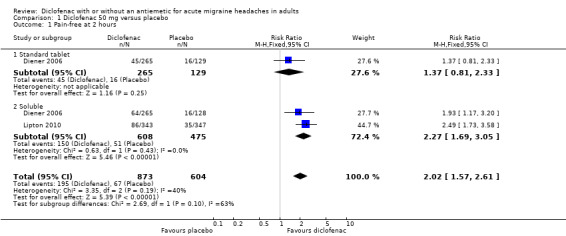
Comparison 1 Diclofenac 50 mg versus placebo, Outcome 1 Pain‐free at 2 hours.
For soluble formulations only the relative benefit was 2.3 (1.7 to 3.1; Figure 2); the NNT was 7.4 (5.6 to 11).
2.
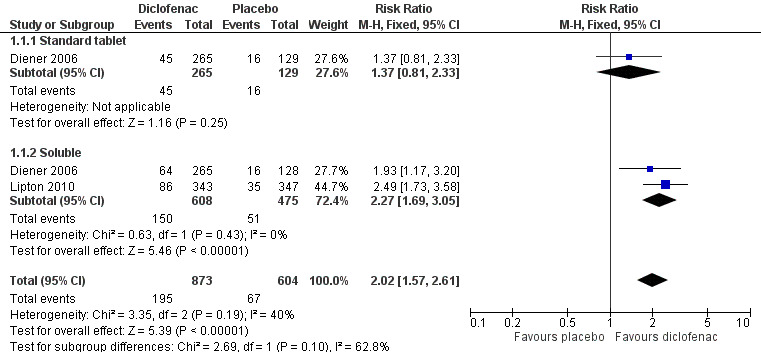
Forest plot of comparison: 1 Diclofenac 50 mg versus placebo, outcome: 1.1 Pain‐free at 2 hours.
Headache relief at two hours
Diclofenac potassium 50 mg versus placebo
The proportion of attacks with headache relief at two hours with diclofenac 50 mg was 55% (482/873; range 47% to 65%).
The proportion of attacks with headache relief at two hours with placebo was 39% (236/604; range 36% to 41%).
The relative benefit of treatment compared with placebo was 1.5 (1.3 to 1.7; Analysis 1.2); the NNT was 6.2 (4.7 to 9.1).
1.2. Analysis.
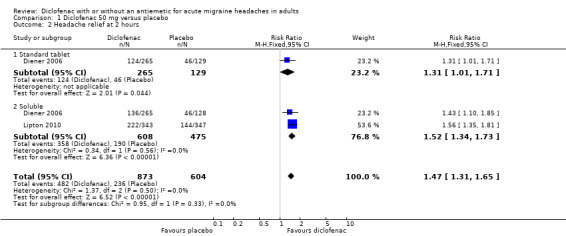
Comparison 1 Diclofenac 50 mg versus placebo, Outcome 2 Headache relief at 2 hours.
For soluble formulations only the relative benefit was 1.5 (1.3 to 1.7; Figure 3); the NNT was 5.1 (4.0 to 7.0).
3.
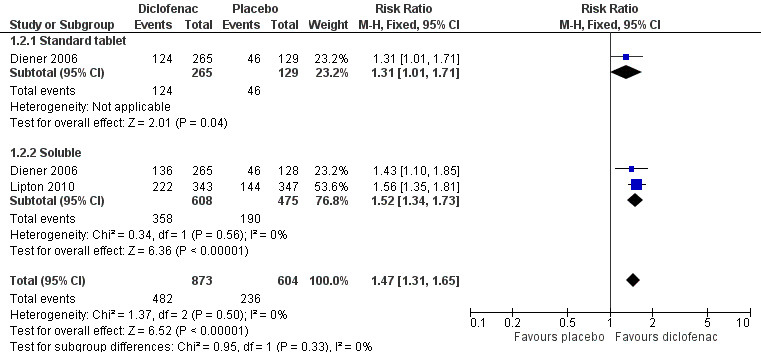
Forest plot of comparison: 1 Diclofenac 50 mg versus placebo, outcome: 1.2 Headache relief at 2 hours.
Sustained pain‐free at 24 hours
Diener 2006 reported this outcome for all included participants, a proportion (around 11%) of whom had mild baseline pain. All participants in Lipton 2010 had moderate or severe baseline pain. The total number of participants in this comparison was 1578.
Diclofenac potassium 50 mg versus placebo
The proportion of attacks with a 24‐hour sustained pain‐free response with diclofenac 50 mg was 19% (175/932; range 15% to 22%).
The proportion of attacks with a 24‐hour sustained pain‐free response with placebo was 8.2% (53/646; range 7.2% to 9.4%).
The relative benefit of treatment compared with placebo was 2.3 (1.7 to 3.0; Analysis 1.3); the NNT was 9.5 (7.2 to 14).
1.3. Analysis.
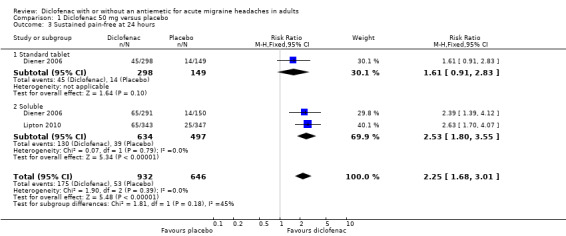
Comparison 1 Diclofenac 50 mg versus placebo, Outcome 3 Sustained pain‐free at 24 hours.
For soluble formulations only the relative benefit was 2.5 (1.8 to 3.6); the NNT was 8.1 (6.2 to 12).
| Summary of results A: Pain‐free and headache relief in single dose, placebo‐controlled studies | ||||||
| Studies |
Attacks treated |
Treatment (%) |
Placebo or comparator (%) |
Relative risk (95% CI) |
NNT (95% CI) |
|
| Pain‐free at 2 hours | ||||||
| Diclofenac potassium 50 mg (solution and tablet) | 2 | 1477 | 22 | 11 | 2.0 (1.6 to 2.6) | 8.9 (6.7 to 13) |
| Diclofenac potassium 50 mg (solution) | 2 | 1212 | 25 | 11 | 2.3 (1.7 to 3.1) | 7.4 (5.6 to 11) |
| Headache relief at 2 hours | ||||||
| Diclofenac potassium 50 mg (solution and tablet) | 2 | 1477 | 55 | 39 | 1.5 (1.3 to 1.7) | 6.2 (4.7 to 9.1) |
| Diclofenac potassium 50 mg (solution) | 2 | 1212 | 59 | 39 | 1.5 (1.3 to 1.7) | 5.1 (4.0 to 7.0) |
| 24‐hour sustained pain‐free | ||||||
| Diclofenac potassium 50 mg (solution and tablet) | 2 | 1578 | 19 | 8.2 | 2.3 (1.7 to 3.0) | 9.5 (7.2 to 14) |
| Diclofenac potassium 50 mg (solution) | 2 | 1280 | 21 | 8.2 | 2.5 (1.8 to 3.6) | 8.1 (6.2 to 12) |
Studies using an optional second dose of study medication
In one study (Vecsei 2007) participants were instructed to take hydrosoluble diclofenac epolamine (DHEP) 50 mg at the earliest sign of a migraine attack with an optional dose at one hour if needed, rather than waiting until pain was moderate or severe. The majority of attacks appear to have been of moderate or severe intensity at baseline.
The primary outcome was number of attacks reduced to less than 20 mm on a 100 mm VAS at two hours. 'Success', which the study authors regarded as equivalent to being 'pain‐free', was experienced in 109/238 attacks (46%) with diclofenac and 61/243 (25%) with placebo.
The authors also reported headache relief at two hours (defined, as in IHS 2000, as an improvement from severe or moderate pain to mild or no pain) for the subgroup of attacks that were treated when pain was moderate or severe. Relief was experienced in 123/226 attacks (54%) with diclofenac, and 77/228 (34%) with placebo.
Sensitivity analysis of primary outcomes
We had planned to conduct a sensitivity analysis for study quality, but all studies scored 3 or more on the Oxford Quality Scale, so no analysis was possible.
DKSMSG 1999 did not contribute any usable data for the primary outcomes, so no sensitivity analysis was carried out for the effect of missing data.
None of the studies that included both participants with and participants without aura analysed data according to migraine type, so no subgroup analysis for this criterion was possible in these studies. The study including only participants without aura (Vecsei 2007) could not be compared with the others because it also used a different dosing regimen.
Adverse events
Any adverse event
Three studies reported on the number of participants experiencing at least one adverse event with diclofenac or placebo. DKSMSG 1999 and Lipton 2010 used diclofenac 50 mg, and DKSMSG 1999 also used diclofenac 100 mg.
The proportion of attacks experiencing at least one adverse event with diclofenac was 18% (109/596; range 15% to 19%).
The proportion of attacks experiencing at least one adverse event with placebo was 16% (78/479; range 15% to 20%).
The relative benefit of treatment compared with placebo was 1.1 (0.86 to 1.5). There was no statistically significant difference between diclofenac and placebo (Analysis 1.5).
1.5. Analysis.
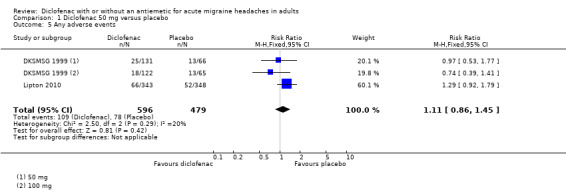
Comparison 1 Diclofenac 50 mg versus placebo, Outcome 5 Any adverse events.
For 50 mg alone, there was significant difference from placebo (relative benefit 1.2 (0.9 to 1.6)).
Serious adverse events
No serious adverse events were reported in any of the included studies.
Withdrawals
Four studies reported specifically on withdrawals due to adverse events (Dahlof 1993; Diener 2006; DKSMSG 1999; Lipton 2010). In total there were eight adverse event withdrawals amongst 1123 attacks (0.7%) treated with diclofenac (50 mg or 100 mg), and three amongst 587 attacks (0.5%) treated with placebo. There were too few events for statistical analysis.
Vecsei 2007 did not report any information about withdrawals, although 22 participants were excluded because they had missing data for "various reasons" (unspecified). In the remaining studies, with the exception of DKSMSG 1999, which has been discussed, relatively small numbers of participants withdrew or were excluded from analyses for reasons such as lost to follow‐up, withdrew consent or too few qualifying headaches. The treatment group of these participants was not always reported, but there was no evidence of any systematic bias.
Discussion
Summary of main results
For participants with moderate or severe baseline pain, diclofenac potassium 50 mg was statistically superior to placebo for the primary outcomes of pain‐free at two hours, headache relief at two hours, and sustained pain‐free at 24 hours, with NNTs of 8.9, 6.2 and 9.5, respectively. Efficacy appeared to be consistently better, but not significantly so, when diclofenac was taken as an oral solution rather than as a tablet. There was no significant difference between diclofenac and placebo for numbers of participants experiencing any adverse event, and adverse events were generally described as of mild or moderate severity. Withdrawals due to adverse events were too few to analyse, and no serious adverse events were reported.
These results are similar to those seen in comparisons with placebo for aspirin (Kirthi 2013) and ibuprofen 200 mg (Rabbie 2013).
Overall completeness and applicability of evidence
Although this review included five studies, with information from 1356 participants who received one or more treatments for one or more attacks, analyses were limited because the studies used two different doses of diclofenac (50 mg and 100 mg), different formulations of diclofenac (potassium salt as tablet or in solution, and epolamine salt in solution), different dosing regimens (single dose or with optional second dose), and different levels of baseline pain. Additionally, many of the outcomes of interest were either reported in a way that we could not use (e.g. group mean data for primary outcomes, unclear definitions for use of rescue medication) or were not reported at all by some studies (e.g. participants with any adverse events, relief of associated symptoms).
There were insufficient data for any analysis of the 100 mg dose, or to investigate the effects of treating early or mild pain compared to moderate or severe pain, different formulations of the potassium salt, or to compare diclofenac directly with other active comparators. The rationale for using the potassium salt or DHEP is that these salts are more soluble and therefore likely to be more rapidly absorbed than the sodium salt, giving a faster onset of action, which is important to migraine sufferers. Taking the medication as a solution rather than a tablet is thought to further speed up absorption and has led to development of powdered formulations.
Participants in the studies all had migraine diagnosed according to International Headache Society (IHS) criteria. It is likely that participants were recruited from patients attending migraine clinics, who may experience more severe, or more difficult to treat headaches than the general population; one study (Lipton 2010) excluded participants who frequently experienced vomiting, and another small study (Vecsei 2007) excluded individuals who normally experienced severe headaches. The overwhelming majority of participants were female, and most were white. The lack of participants from other ethnic backgrounds may limit generalisation to other populations.
Individual studies were underpowered to determine differences between treatments for adverse events, and even pooling studies may not provide adequate numbers of events to demonstrate differences or allow confidence in the size of the effect. Single dose studies are certainly unlikely to reveal rare, but potentially serious, adverse events. Furthermore, results may be confounded by recording of adverse events after taking rescue medication, which may disproportionately increase rates in the placebo group.
Quality of the evidence
Studies generally were of adequate or good methodological quality. One cross‐over study (DKSMSG 1999) was considered at risk of bias because it reported a four‐period 'completer analysis' for all efficacy outcomes which excluded 20% of randomised participants (12% for reasons other than lack of qualifying headache), but the study did not contribute to any primary outcomes.
Reporting of primary outcomes as group means is not considered valid (Moore 2010) because underlying distributions are frequently non‐Gaussian. Reporting the number or proportion of patients who achieve useful clinical outcomes is preferred, and IHS outcomes reflect this. The use of means to report efficacy in some studies limited the amount of data available for analyses.
Indirect comparisons of standard tablets and soluble salts (with placebo as the common comparator) were limited by numbers of studies and events, and were probably underpowered to show a statistical difference. Diclofenac formulations have very large differences in effect size in acute postoperative pain (Derry 2009).
While most studies reported some information about adverse events, the outcomes were not always our preferred ones, and the time over which data were collected was frequently not explicit. It is likely that data continued to be collected after intake of rescue medication or a second dose of study medication, so that total dose over the period assessed is uncertain.
We specified that a minimum of 200 participants in at least two studies were required before carrying out any pooled analysis, but ideally we would need at least 200 participants in each treatment arm where there is an event rate of 50% to be reasonably confident in the size of an effect (Moore 1998). The magnitude of effect for outcomes with fewer participants or lower event rates, or both, should be interpreted with caution.
Potential biases in the review process
Although we carried out a thorough search for relevant studies, we identified only a few. This may be largely because diclofenac is already an established analgesic in other conditions, and requirements by regulatory bodies for a new indication do not demand the same rigour as for a new drug. Nonetheless, the NNTs obtained for diclofenac potassium 50 mg compared with placebo are of borderline clinical utility (prespecified as ≥ 6 and ≥ 8 for headache relief and pain‐free at two hours, respectively), and it would take relatively few additional, unidentified studies or participants to increase them well beyond this cut‐off (Moore 2008). We identified two unpublished studies (Novartis 1995; Novartis 1998), but we have so far been unable to obtain any further information; the unpublished placebo‐controlled study alone would not significantly change the results of this review if it found no difference between diclofenac and placebo. On the other hand, randomised studies that we excluded for various reasons were consistent with the efficacy of oral diclofenac found in these analyses (Comoglu 2011; Massiou 1991).
The methods of the review were such as to minimise bias due to the review process itself, but use of data from both phases of cross‐over studies and from studies reporting combined data from several attacks may introduce unknown biases (Elbourne 2002). For cross‐over studies a 48‐hour period between qualifying attacks should limit the potential for carry‐over effects, and for multiple attacks there is some evidence of consistency of response (in terms of proportion of participants achieving the outcome) for aspirin in migraine (Kirthi 2013).
Agreements and disagreements with other studies or reviews
A narrative review considered the pharmacology, efficacy, tolerability, and dosage and administration of diclofenac for acute migraine (McNeely 1999). The review included four randomised controlled trials (RCTs), two of which are published and included in this review (Dahlof 1993; DKSMSG 1999), while two are unpublished, and we have been unable to obtain further information about them. It appears likely that these unpublished studies reported primary outcome data as group means, or mean differences (MDs), which we could not have used in our meta‐analyses. Secondary outcomes relating to associated symptoms, use of rescue medication, and adverse events may be dichotomous. The McNeely 1999 narrative review had no explicit search strategy and no meta‐analysis, and there were no disagreements with our review in the areas they have in common.
Authors' conclusions
Implications for practice.
Limited data indicate that diclofenac potassium is an effective treatment for the relief of headache pain in some patients with moderate or severe migraine headache. While the NNTs for headache relief at two hours, pain‐free at two hours and sustained pain‐free during the 24 hours postdose are of borderline clinical utility, the 50 mg dose achieves these three outcomes in 55%, 22%, and 19%, respectively, of patients who treat moderate or severe pain.
Implications for research.
Diclofenac is a well‐established analgesic in both acute and chronic pain conditions. It seems unlikely that more clinical trials will be conducted to show that any given formulation is more effective than placebo in migraine, though studies on faster‐acting formulations would be useful. Further head‐to‐head trials would establish its place relative to alternative treatments for migraine and should ideally also include a placebo. Studies investigating different dosing regimens, and combination with an antiemetic, could further establish the optimum dosing regimen for oral diclofenac. All future studies should use IHS‐preferred outcomes to facilitate comparison across studies.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 11 October 2017 | Review declared as stable | No new studies likely to change the conclusions are expected. |
History
Protocol first published: Issue 10, 2010 Review first published: Issue 2, 2012
| Date | Event | Description |
|---|---|---|
| 6 September 2017 | Review declared as stable | See Published notes. |
| 15 April 2016 | Amended | One of the review authors identified a transcription error in the Abstract for NNTs for PR2 and HR2. Now corrected. |
| 7 May 2013 | Review declared as stable | This review will be assessed for further updating in 2018. |
| 18 February 2013 | New citation required but conclusions have not changed | No new studies identified. |
| 18 February 2013 | New search has been performed | New searches carried out, Risk of bias tables expanded and updated, Summary of findings table added. |
| 27 June 2012 | Amended | Contact details updated. |
Notes
A restricted search in September 2017 did not identify any potentially relevant studies. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Acknowledgements
We would like to acknowledge the helpful comments and editorial expertise of Timothy Steiner, Douglas McCory, and Rebecca Gray, and those who contributed to the various stages of peer review.
The original review was carried out as part of a Cochrane Review Incentive Scheme 2010, and Lifting The Burden: the Global Campaign against Headache provided support for the editorial process.
The Oxford Pain Research Trust provided institutional support for this update, and editorial support was funded by the International Headache Society.
Appendices
Appendix 1. Definitions
All terms relating to primary efficacy outcomes are defined according to the effect of the treatment on headache pain, measured using a four‐point pain intensity scale (ranging from 0 to 3 or none, mild, moderate, and severe).
Baseline pain intensity ‐ level of pain participant must be experiencing in order to receive study medication, either 1 (mild pain) or 2/3 (moderate or severe pain).
Pain‐free at two hours ‐ number of participants with a pain intensity of 0 (none) at two hours after administration of study medication, expressed as a fraction of the treated participants with the appropriate baseline pain.
Pain‐free at one hour ‐ number of participants with a pain intensity of 0 (none) at one hour after administration of study medication, expressed as a fraction of the treated participants with the appropriate baseline pain.
Headache relief at two hours ‐ number of participants with a reduction in pain intensity from 2/3 (moderate/severe) to 0/1 (none/mild) at two hours after administration of study medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
Headache relief at one hour ‐ number of participants with a reduction in pain intensity from 2/3 (moderate/severe) to 0/1 (none/mild) at one hour after administration of study medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
24‐hour sustained headache relief ‐ number of participants with a reduction in pain intensity from 2/3 (moderate/severe) to 0/1 (none/mild) at two hours after administration of study medication which is then sustained between 2 and 24 hours without recurrence of headache or use of rescue medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
24‐hour sustained pain‐free ‐ number of participants with a pain intensity of 0 (none) at two hours after administration of study medication which is then sustained between 2 and 24 hours without recurrence of headache or use of rescue medication expressed as a fraction of the treated participants with the appropriate baseline pain.
Use of rescue medication ‐ number of participants requiring the use of additional medication to treat either recurrence of headache or an inadequate response to study medication, provided that the additional medication is not, or does not include, the study drug.
Relief of associated symptoms ‐ number of participants with an absence of a headache‐associated symptom (nausea, vomiting, photophobia, or phonophobia) at two hours after administration of study medication, expressed as a fraction of the treated participants for whom the symptom was present at baseline.
Complete relief of functional disability ‐ reduction in the level of functional disability, measured using a four‐point scale, from any degree of disability (grade 1/2/3) at baseline to grade 0 (able to work/function normally) at two hours after administration of study medication, expressed as a fraction of the treated participants with any functional disability at baseline.
Appendix 2. Search strategy for MEDLINE (via Ovid)
Diclofenac/ OR diclofenac.mp.
(Voltarol OR Voltaren OR Cataflam OR Cambia).mp
1 OR 2
Headache/ OR exp Headache Disorders/
exp Migraine Disorders/
(headach* OR migrain* OR cephalgi* OR cephalalgi*).mp.
4 OR 5 OR 6
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
OR/8‐15
3 AND 7 AND 16
Appendix 3. Search strategy for EMBASE (via Ovid)
Diclofenac/ OR diclofenac.mp.
(Voltarol OR Voltaren OR Cataflam OR Cambia).mp
1 OR 2
exp Headache and facial pain
exp Migraine
(headach* OR migrain* OR cephalgi* OR cephalalgi*).mp.
4 OR 5 OR 6
clinical trials.sh.
controlled clinical trials.sh.
randomized controlled trial.sh.
double‐blind procedure.sh.
(clin* adj25 trial*).ab.
((doubl* or trebl* or tripl*) adj25 (blind* or mask*)).ab.
placebo*.ab.
random*.ab.
OR/8‐15
3 AND 7 AND 16
Appendix 4. Search strategy for CENTRAL
MeSH descriptor Serotonin Agonists OR MeSH descriptor Tryptamines
(diclofenac OR Voltarol OR Voltaren OR Cataflam OR Cambia):ti,ab,kw
1 OR 2
MeSH descriptor Headache/ OR MeSH descriptor Headache Disorders explode all trees
MeSH descriptor Migraine Disorders explode all trees
(headach* OR migrain* OR cephalgi* OR cephalalgi*):ti,ab,kw
4 OR 5 OR 6
3 AND 7
Limit 8 to Clinical Trials (CENTRAL)
Appendix 5. Summary of outcomes: efficacy
| Study ID | Treatment | Headache relief 1 h | Headache relief 2 h | Pain‐free 2 h | Sustained headache relief 24 h | Sustained pain‐free 24 h | Use of rescue medication |
| Dahlof 1993 | (1) Diclofenac‐K 50 mg (2) Diclofenac‐K 100 mg (3) Placebo N = 72 (64 pts treated 3 attacks) |
No data | No usable dichotomous data (data reported only in graphical form and could not be used because of uncertainty about the denominator) Mean data: diclofenac significantly better than placebo, with no difference between doses |
No data | No data | No data | No usable data Within 2 h: (1) 46% (2) 37% (3) 58% Denominator unclear |
| Diener 2006 | (1) Diclofenac‐K sachet 50 mg, n = 291 (2) Diclofenac‐K tablet 50 mg, n = 298 (3) Placebo sachet and tablet, n = 299 N = 317 (888 attacks in total, 274 participants treated 3 attacks) Participants with moderate/severe pain at baseline: (1) 265/289 (2) 265/295 (3) 257/297 |
From moderate/severe (1) 146/265 (2) 169/265 (3) 192/257 | From moderate/severe baseline
(1) 136/265
(2) 124/265
(3) 92/257 This assumes no participants with < moderate at baseline become > moderate at 2 h |
From moderate/severe baseline (1) 64/265 (2) 45/265 (3) 32/257 | [Including some mild] (1) 107/291 (2) 92/298 (3) 55/299 | [Including some mild] (1) 65/291 (2) 45/298 (3) 28/299 | Within 3 h:
(1) 50/291
(2) 63/298
(3) 83/299 Within 8 h: (1) 102/291 (2) 108/298 (3) 150/299 Median time to use ˜3 h for all treatments in those who used it |
| DKSMSG 1999 | (1) Diclofenac‐K 50 mg (2) Diclofenac‐K 100 mg (3) Sumatriptan 100 mg (4) Placebo N = 144 (115 completed all 4 treatments) |
All active treatments significantly better than placebo for all time points 1.5 to 8 h. No significant differences between active treatments | All active treatments significantly better than placebo for all time points 1.5 to 8 h. No significant differences between active treatments | All active treatments significantly better than placebo for all time points 1.5 to 8 h. No significant differences between active treatments | All active treatments significantly better than placebo for all time points 1.5 to 8 h. No significant differences between active treatments | All active treatments significantly better than placebo for all time points 1.5 to 8 h. No significant differences between active treatments | No usable data. 36% of participants taking either dose of diclofenac required rescue medication during attacks, compared with 41% with sumatriptan and 60% with placebo. Unclear whether for failed response or recurrence Mean time to use reported as longer with active treatments (11 to 13 h) than placebo (8 h) ‐ probably includes participants with recurrence |
| Lipton 2010 | (1) Diclofenac‐K oral solution 50 mg, n = 343 (2) Placebo, n = 348 N = 691 |
No data | (1) 222/343 (2) 144/347 | (1) 86/343 (2) 35/347 | No data | (1) 65/343 (2) 25/347 | No data |
| Vecsei 2007 | (1) DHEP sachet 50 mg (equivalent) (2) Placebo N = 133 (481 attacks, 110 treated 4 attacks) |
No data | Majority of participants took 2nd dose any time after 1 h: (1) 63%, (2) 78% VAS < 20 mm at 2 h (1) 109/238 (2) 61/243 From moderate/severe baseline pain to mild/none Attacks: (1) 54.4% = 123/226 (2) 33.8% = 77/228 |
No data | No data | No data | No usable data. Majority of participants took 2nd dose for inadequate response at 1 h (63.6%, 78.4%) and many resorted to 'preferred rescue medication' within 48 h (30.5%, 56.7%). |
| DHEP ‐ hydrosoluble diclofenac epolamine; h ‐ hour(s); K ‐ potassium; N ‐ number of participants in study; n ‐ number of participants in treatment arm; Na ‐ sodium; VAS ‐ visual analogue scale | |||||||
Appendix 6. Summary of outcomes: adverse events and withdrawals
| Study ID | Treatment | Any adverse event | Specific adverse events | Serious adverse events | Adverse event withdrawal | Other withdrawals/exclusions |
| Dahlof 1993 | (1) Diclofenac‐K 50 mg (2) Diclofenac‐K 100 mg (3) Placebo |
Time not specified
(1) 27.7%
(2) 20.3%
(3) 20.9% Denominator unclear 23 pts in total reported any AE over 3 treatment periods, 8 had AEs in all periods Most mild to moderate intensity |
Fatigue/tiredness (1) 6 (2) 4 (3) 7 GI symptoms (1) 3 (2) 3 (3) 4 Denominator unclear |
None reported | (1 or 2) 1 (pulmonary embolism) (2) 2 (lack of efficacy, protocol violation) |
5 pts did not complete 3 treatments ‐ no reasons given, too few qualifying headaches |
| Diener 2006 | (1) Diclofenac‐K sachet 50 mg, n = 291 (2) Diclofenac‐K tablet 50 mg, n = 298 (3) Placebo sachet and tablet, n = 299 Pts with mod/sev pain at baseline: (1) 265/289 (2) 265/295 (3) 257/297 |
No usable data | Most common ‐ all < 2% Abdominal pain (1) 2/291 (2) 0/298 (3) 3/299 Dyspepsia (1) 2/291 (2) 1/298 (3) 1/299 Dizziness (1) 2/291 (2) 1/298 (3) 0/299 Diarrhoea (1) 1/291 (2) 1/298 (3) 4/299 Somnolence (1) 1/291 (2) 1/298 (3) 3/299 Nausea (1) 1/291 (2) 1/298 (3) 3/299 Vomiting (1) 3/291 (2) 1/298 (3) 1/299 |
None | (1) 2/291 (urticaria, vomiting) (2) 3/298 (urticaria, vomiting, haematuria) (3) 1/299 (eye swelling) |
< 3 qualifying attacks:
(1) 5 (2) 9 (3) 14 Withdrew consent: (1) 3 (2) 1 (3) 2 Lost to follow‐up: (1) 0 (2) 0 (3) 3 |
| DKSMSG 1999 | (1) Diclofenac‐K 50 mg (2) Diclofenac‐K 100 mg (3) Sumatriptan 100 mg (4) Placebo |
Time not specified (1) 25/131 (2) 18/122 (3) 43/130 (4) 26/131 Most mild to moderate intensity | Somnolence (1) 8/131 (2) 1/122 (3) 3/130 (4) 3/131 Paresthesia (1) 2/131 (2) 0/122 (3) 5/130 (4) 1/131 Fatigue (1) 5/131 (2) 1/122 (3) 7/130 (4) 4/131 Asthenia (1) 1/131 (2) 1/122 (3) 4/130 (4) 2/131 Dizziness (1) 1/131 (2) 0/122 (3) 7/130 (4) 3/131 Chest pain (1) (1) 0/131 (2) 0/122 (3) 4/130 (4) 1/131 Tachycardia (1) 2/131 (2) 0/122 (3) 7/130 (4) 2/131 Abdominal pain (1) 1/131 (2) 6/122 (3) 6/130 (4) 4/131 Dyspepsia (1) 3/131 (2) 3/122 (3) 1/130 (4) 1/131 Nausea (1) 3/131 (2) 1/122 (3) 3/130 (4) 5/131 |
None | Within 72 h:
(2) 2 (allergic‐type reaction, purpura of skin + pain + shortness of breath) (4) 2 (amentia + chills+ sweating, nausea + vomiting + gastritis) |
|
| Lipton 2010 | (1) Diclofenac‐K oral solution 50 mg, n = 343 (2) Placebo, n = 348 |
Within 24 h: (1) 66/343 (2) 52/348 Most mild to moderate intensity | Most common:
Nausea (1) 16/343 (2) 12/348 Dizziness (1) 5/343 (2) 3/348 Dyspepsia (1) 4/343 (2) 5/347 Vomiting (1) 4/343 (2) 1/348 |
None | None reported | 1 pt discontinued from placebo group after taking study medication ‐ no reason given |
| Vecsei 2007 | (1) DHEP sachet 50 mg (equivalent) (2) Placebo |
No data | No usable data | None possibly related to DHEP | None reported | Data regarding migraine attacks were missing for 22 pts (group not given), for different reasons (not specified) ‐ excluded from analysis |
| AE = adverse event; DHEP ‐ hydrosoluble diclofenac epolamine; h ‐ hour(s); K ‐ potassium; mod ‐ moderate; N ‐ number of participants in study; n ‐ number of participants in treatment arm; Na ‐ sodium; pt(s) ‐ participant(s); sev ‐ severe | ||||||
Appendix 7. Other outcomes
Use of rescue medication
Four studies reported some data on use of rescue medication (Dahlof 1993; Diener 2006; DKSMSG 1999; Vecsei 2007), but only in Dahlof 1993 and Diener 2006 was there a clear distinction between additional medication (second dose or alternative medication) taken because of inadequate response at two hours and additional medication taken because of recurrence of headache following initial response. There were no usable data from Dahlof 1993, in which the percentage of participants using rescue medication was reported, but the denominator was uncertain. Diener 2006 reported the percentage of participants using rescue medication at three hours and eight hours, with almost a doubling of numbers over that time; there were insufficient data for analysis.
Diener 2006 also reported median time to use of rescue medication, but only in those who took it, at about three hours for all treatments. DKSMSG 1999 reported a mean time to use of 'rescue medication' of eight hours with placebo and 11 to 13 hours with active treatments (diclofenac and sumatriptan). These somewhat implausible numbers almost certainly include participants who experienced recurrence. No analysis was possible.
Relief of headache‐associated symptoms
Only two studies reported sufficient data to calculate relief of headache‐associated symptoms (nausea, vomiting, photophobia, phonophobia) at two hours. In DKSMSG 1999, participants were asked to take study medication at the first sign of pain, while in Lipton 2010 they were to take it when pain was of moderate to severe intensity. Although the group mean pain score at baseline in DKSMSG 1999 is reported as ± 50 mm on a 100 mm VAS, which is equivalent to moderate pain (Collins 1997), the baseline incidences of nausea, photophobia, and vomiting were substantially lower than in Lipton 2010. For this reason, we thought it unwise to combine data for analysis. Where reported headache‐associated symptoms more participants experienced relief of symptoms with diclofenac than with placebo. Vomiting was infrequent (< 7%) in all studies reporting data.
Relief of functional disability
The same two studies reported data for relief of functional disability at two hours. At baseline about 80% of participants in DKSMSG 1999 reported some degree of functional disability, as did 97% in Lipton 2010. Participants in DKSMSG 1999 took medication at the first sign of pain (mean baseline pain reported as ˜50/100 mm), while those in Lipton 2010 were asked to wait until pain was at least moderate (˜70% moderate, 30% severe). The proportion of attacks experiencing relief of functional disability with diclofenac 50 mg was 33% (143/431) and with placebo was 14% (62/442). The relative benefit of treatment compared with placebo was 2.4 (1.8 to 3.1; Analysis 1.4); the NNT was 5.2 (4.1 to 7.3).
1.4. Analysis.
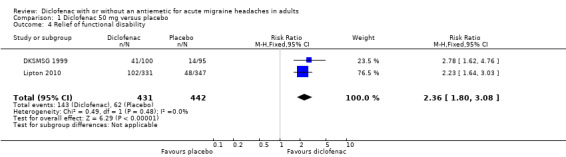
Comparison 1 Diclofenac 50 mg versus placebo, Outcome 4 Relief of functional disability.
Dahlof 1993 reported that functional disability was less frequent at two hours with diclofenac 100 mg than with 50 mg or placebo; Diener 2006 reported that more participants "improved" at two hours with diclofenac 50 mg than with placebo; and Vecsei 2007 reported that "working ability improved by > 1 point" in 54% and 40% of attacks treated with diclofenac 50 mg (with optional second dose) and placebo, respectively.
Data and analyses
Comparison 1. Diclofenac 50 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 hours | 2 | 1477 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.57, 2.61] |
| 1.1 Standard tablet | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.81, 2.33] |
| 1.2 Soluble | 2 | 1083 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.27 [1.69, 3.05] |
| 2 Headache relief at 2 hours | 2 | 1477 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.31, 1.65] |
| 2.1 Standard tablet | 1 | 394 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.01, 1.71] |
| 2.2 Soluble | 2 | 1083 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.34, 1.73] |
| 3 Sustained pain‐free at 24 hours | 2 | 1578 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.25 [1.68, 3.01] |
| 3.1 Standard tablet | 1 | 447 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.91, 2.83] |
| 3.2 Soluble | 2 | 1131 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.53 [1.80, 3.55] |
| 4 Relief of functional disability | 2 | 873 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.80, 3.08] |
| 5 Any adverse events | 2 | 1075 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.86, 1.45] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dahlof 1993.
| Methods | Multicentre, randomised, double‐dummy, placebo‐controlled, 3‐period, cross‐over study Single oral dose of each treatment for each of three consecutive attacks, with a minimum of 3 days between successively treated attacks. Medication to be taken at the earliest sign of a migraine attack (onset aura/headache) Assessments at 0.5, 1, 1.5, and 3 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication (of participant's choice) |
|
| Participants | Migraine with or without aura (IHS 1988). History: 2 to 8 migraine attacks/month Exclusions: participants with contraindication to study medication, concomitant NSAID therapy, ergotamine/analgesic addiction, pregnancy or inadequate contraception N = 72 M: 16, F: 56 Mean age 40 years, range 18 to 61 Migraine with aura 58% |
|
| Interventions | Diclofenac‐K 50 mg tablet, n = 64 Diclofenac‐K 100 mg tablet, n = 64 Placebo, n = 64 All prophylaxis stopped ≥ 2 weeks before start of study |
|
| Outcomes | Headache relief (100 mm VAS and 4‐point scale) at 2 hours Associated symptoms Working ability Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Medication supplied in packs with sequential patient number. Patient allocated lowest available number |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Tablets "of identical outward appearance and shape" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Denominator in graphs unclear |
| Size | Unclear risk | Group size 50 to 200 |
Diener 2006.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, cross‐over trial Single dose of each treatment for each of three separate migraine attacks, with at least 48 hours between attacks. Medication taken at the first sign of a migraine attack Assessments at 0, 15, 30, 45, 60, and 90 minutes and 2, 3, 4, 6, and 8 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication |
|
| Participants | Migraine with or without aura (IHS 1988). History: 2 to 6 migraine attacks/month in previous 3 months Exclusions: participants with interval headaches between attacks, other types of migraine, pregnancy or lactation or inadequate contraception, known hypersensitivity to study or related medications, significant systemic disease N = 317 M: 44, F: 273 Mean age: 39 years |
|
| Interventions | Diclofenac‐K sachet 50 mg, n = 291 Diclofenac‐K tablet 50 mg, n = 298 Placebo, n = 299 Prophylactic treatment allowed with a single agent if stable |
|
| Outcomes | Headache relief at 1, 2 hours Pain‐free at 2 hours Sustained headache relief at 24 hours Sustained pain‐free at 24 hours Use of rescue medication Relief from accompanying symptoms (combined outcome) Functional disability Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Low risk | Remote allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double dummy" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs described |
| Size | Low risk | Group size > 200 |
DKSMSG 1999.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐ and active‐controlled, cross‐over trial Single oral dose of each medication to treat each of 4 separate attacks; each patient was to receive all 4 treatments during the course of the trial. Medication taken at first sign of pain and attacks separated by ≥ 48 hours Assessments at 20 minutes, 40 minutes, and 1, 1.5, 2, 3, 4, 6 and 8 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication (paracetamol) |
|
| Participants | Migraine ± aura (IHS 1988). History: 2 to 6 attacks/month in previous 6 months Exclusions: participants experiencing non‐migrainous interval headaches or other types of migraine N = 156 M: 37, F: 119 Median age 33 years, range 19 to 70 years Median time since first diagnosis 15 years |
|
| Interventions | Diclofenac‐K 50 mg, n = 115 Diclofenac‐K 100 mg, n = 115 Sumatriptan 100 mg, n = 115 Placebo, n = 115 Beta‐blockers allowed if dose stable |
|
| Outcomes | Pain intensity (100 mm VAS) at 2 hours ‐ group mean data Associated symptoms Working ability Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double dummy" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs described. Completer analysis for efficacy, but did not contribute to efficacy analyses. Safety analysis on all participants receiving treatment. |
| Size | Unclear risk | Group size 50 to 200 |
Lipton 2010.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group, single‐attack trial Single dose of each medication to treat a single migraine attack, with at least 48 hours of treating previous migraine. Trial medication was to be taken at the earliest sign of a migraine attack, when migraine of moderate or severe intensity Assessments at 0, 15, 30 and 45 minutes and 1, 1.5, 2, 2.5, 3, 4, 8, 16 and 24 hours If pain not controlled, participants asked to wait 2 hours before taking rescue medication |
|
| Participants | Migraine with or without aura (IHS 2004). History: at least one migraine attack/month in previous year Exclusions: participants experiencing vomiting in 20% of attacks or needing bed rest with most attacks, pregnancy, lactation or inadequate contraception, hypersensitivity to study or related medication, traumatic injury to head or neck within 6 months, other significant medical history N = 690 (ITT population) M: 105, F: 585 Mean age: 40 years, range: 18 to 65 Migraine with aura 13% |
|
| Interventions | Diclofenac‐K oral solution 50mg, n = 343 Placebo, n = 347 Prophylactic treatment allowed if dose stable for ≥ 3 months |
|
| Outcomes | Headache relief at 2 hours Pain‐free at 2 hours Sustained pain‐free at 24 hours Associated symptoms Functional disability Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both treatments made up to clear solution |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs described |
| Size | Low risk | Group size > 200 |
Vecsei 2007.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, cross‐over trial Single oral dose of each treatment for four consecutive migraine attacks, with at least 48 hours between consecutive treatments Medication to be taken at the earliest sign of migraine attack, and a second tablet could be taken 1 hour later if relief was judged insufficient by the participant Assessments at 0, 2 and 24 hours "In the case of a migraine attack recurring within 48 hours, the patient was allowed to treat this attack with his 'usually used attack medicine' ('rescue medication')" |
|
| Participants | Migraine without aura. History: 1 to 6 migraine attacks/month in the 12 months prior to enrolment Exclusions: participants usually experiencing severe attacks, known hypersensitivity to study medication, concomitant treatment with drugs that interact with diclofenac, serious psychiatric disease, drug abuse headache N = 133 (ITT participants) M: 14, F: 119 Mean age 42 years |
|
| Interventions | Diclofenac epolamine (DHEP) 65 mg sachet, n = 133 Placebo, n = 133 |
|
| Outcomes | Headache relief at 2 hours "Pain‐free" at 2 hours Use of "rescue medication" (appears to refer to medication taken to treat recurrence ‐ see 'Methods', above) Working ability Associated symptoms Adverse events |
|
| Notes | Oxford Quality Score: R2, DB1, W0. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated using validated software" |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data missing for 22/155 participants without adequate reason |
| Size | Unclear risk | Group size 50 to 200 |
DB ‐ double blind; F ‐ female; IHS ‐ International Headache Society; ITT ‐ intention‐to‐treat; M ‐ male; N ‐ number of participants in study; n ‐ number of participants in treatment arm; R ‐ randomised; VAS ‐ visual analogue scale; W ‐ withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bigal 2002 | Intramuscular administration of diclofenac |
| Comoglu 2011 | Mixed baseline pain (some mild) with results not reported separately |
| Del Bene 1987 | Intramuscular administration of diclofenac |
| Karachalios 1992 | Intramuscular administration of diclofenac |
| Massiou 1991 | Diagnostic criteria not specified. Baseline pain not specified |
| Peroutka 2004 | 30% of participants unaccounted for in first period of cross‐over |
Characteristics of studies awaiting assessment [ordered by study ID]
Novartis 1995.
| Methods | Ransomised, double‐blind, active‐control, cross‐over Single dose, with additional tablets as needed after 2 hours (maximum 3 per attack for diclofenac or 6 per attack for Cafergot) |
| Participants | N = 63 (completed both phases?) |
| Interventions | Diclofenac potassium (Cataflam) 50 mg Cafergot (ergotamine 1 mg + caffeine 100 mg) |
| Outcomes | |
| Notes | Referenced in McNeely 1999 as follows: Data on file: GP 45 840 12, Cataflam tablets, diclofenac‐K. Novartis Pharma AG (Basel); 1995 |
Novartis 1998.
| Methods | Randomised, double‐dummy, placebo‐ and active‐control, parallel‐group Single dose, with additional tablets as needed after 2 hours (maximum 4 per attack for diclofenac or 5 per attack for Cafergot) |
| Participants | Migraine ± aura. Mean baseline pain 50/100 mm N = 423 |
| Interventions | Diclofenac potassium 50 mg, n = 140 (evaluable population) Cafergot (ergotamine 1 mg + caffeine 100 mg), n = 144 Placebo, n = 146 |
| Outcomes | |
| Notes | Referenced in McNeely 1999 as follows: Data on file: Novartis Pharma AG (Basel). 604man.doc/final draft; 1998 |
Differences between protocol and review
For the original review we considered data on one outcome not specified in the protocol. Use of rescue medication was reported by the majority of studies and provides a measure of efficacy from the point of view of the patient. In taking rescue medication the patient is saying that the efficacy of the medication is not adequate and that they need alternative analgesia. They are effectively withdrawing due to lack of efficacy, where efficacy is defined by their preparedness to carry on without additional analgesia, rather than a predefined outcome such as headache relief at two hours. We believe this is useful additional information relevant to clinical practice.
After discussion with headache specialists and editorial staff, and in line with Cochrane recommendations, for the update we decided to limit our outcomes for acute migraine headache reviews in order to focus attention on the most important outcomes and to make them more readable for both clinicians and patients. For the majority of interventions we will now include 2‐hour pain‐free and headache relief (PF2 and HR2) as primary outcomes, and 24‐hour sustained pain‐free and headache relief (SPF24 and SHR24) and adverse events as secondary outcomes. Pain‐free headache relief outcomes at earlier time points will be included if reported and relevant. We have moved results for use of rescue medication and relief of headache‐associated symptoms and functional disability to Appendix 7.
We have expanded the Risk of bias table; this review uses the new criteria for analysis.
Contributions of authors
RAM and SD wrote the protocol. For the original review SD and RR carried out searching, selection of studies, data extraction, analyses and writing. One author entered data into RevMan. All authors were involved with analyses and writing. RAM acted as arbitrator.
For the update SD carried out the searches. RAM and SD updated the review and RR checked it.
Sources of support
Internal sources
-
Oxford Pain Relief Trust, UK.
General institutional support
External sources
-
Cochrane Review Incentive Scheme 2010, UK.
For the original review
-
International Headache Society, UK.
Support for the editorial process
Declarations of interest
RAM has consulted for various pharmaceutical companies and has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. RAM and SD have received research support from charities, government and industry sources at various times. RR has no interests to declare. Support for the original review was from the Oxford Pain Research Trust and a Cochrane Incentive Scheme grant, and for the update from the Oxford Pain Research Trust. Neither funding body had any input into the review at any stage.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Dahlof 1993 {published data only}
- Dahlof C, Bjorkman R. Diclofenac‐K (50 and 100mg) and placebo in the acute treatment of migraine. Cephalagia 1993;13(2):117‐23. [DOI] [PubMed] [Google Scholar]
Diener 2006 {published data only}
- Diener H‐C, Montaga P, Gacs G, Lyczak P, Schumann G, Zoller B, et al on behalf of the Study Group. Efficacy and tolerability of diclofenac potassium sachets in migraine: a randomised, double‐blind, cross‐over study in comparison with diclofenac potassium tablets and placebo. Cephalagia 2006;26(5):537‐47. [DOI] [PubMed] [Google Scholar]
DKSMSG 1999 {published data only}
- The Diclofenac‐K/Sumatriptan Migraine Study Group. Acute treatment of migraine attacks: efficacy and safety of a nonsteroidal anti‐inflammatory drug, diclofenac‐potassium, in comparison to oral sumatriptan and placebo. Cephalagia 1999;19(4):232‐40. [DOI] [PubMed] [Google Scholar]
Lipton 2010 {published data only}
- Lipton RB, Grosberg B, Singer RP, Pearlman SH, Sorrentino JV, Quiring JN, et al. Efficacy and tolerability of a new powdered formulation of diclofenac potassium for oral solution for the acute treatment of migraine: results from the International Migraine Pain Assessment Clinical Trial (IMPACT). Cephalagia 2010;30(11):1136‐45. [DOI] [PubMed] [Google Scholar]
Vecsei 2007 {published data only}
- Vecsei L, Gallacchi G, Sagi I, Semjen J, Tajti J, Szok D, et al. Diclofenac epolamine is effective in the treatment of acute migraine attacks. A randomised, crossover, double blind, placebo‐controlled, clinical study. Cephalagia 2007;27(1):29‐34. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bigal 2002 {published data only}
- Bigal ME, Bordini CA, Speciali JG. Intramuscular diclofenac in the acute treatment of migraine: a double‐blind placebo controlled study. Arquivos de Neuro‐psiquiatria 2002;60(2‐B):410‐5. [PubMed] [Google Scholar]
Comoglu 2011 {published data only}
- Comoglu T, Dogan A, Comoglu S, Basci N. Formulation and evaluation of diclofenac potassium fast‐disintegrating tablets and their clinical application in migraine patients. Drug Development and Industrial Pharmacy 2011;37(3):260‐7. [DOI] [PubMed] [Google Scholar]
Del Bene 1987 {published data only}
- Bene E, Poggioni M, Garagiola U, Maresca V. Intra‐muscular treatment of migraine attacks using diclofenac sodium: a cross‐over clinical trial. Journal of International Medical Research 1987;15:44‐8. [DOI] [PubMed] [Google Scholar]
Karachalios 1992 {published data only}
- Karachalios GN, Fotiadou A, Chrisikos N, Karabetsos A, Kehagioglou K. Treatment of acute migraine attack with diclofenac sodium: a double‐blind study. Headache 1992;32(2):98‐100. [DOI] [PubMed] [Google Scholar]
Massiou 1991 {published data only}
- Massiou H, Serrurier D, Lasserre O, Bousser MG. Effectiveness of oral diclofenac in the acute treatment of common migraine attacks: a double‐blind study versus placebo. Cephalagia 1991;11(2):59‐63. [DOI] [PubMed] [Google Scholar]
Peroutka 2004 {published data only}
- Peroutka SJ, Lyon JA, Swarbrick J, Lipton RB, Kolodner K, Goldstein J. Efficacy of diclofenac sodium softgel 100mg with or without caffeine 100mg in migraine without aura: a randomised, double‐blind, crossover study. Headache 2004;44(2):136‐41. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Novartis 1995 {unpublished data only}
Novartis 1998 {unpublished data only}
Additional references
Ayzenberg 2012
- Ayzenberg I, Katsarava Z, Sborowski A, Chernysh M, Osipova V, Tabeeva G, et al. The prevalence of primary headache disorders in Russia: a countrywide survey. Cephalalgia 2012;32(5):373‐81. [DOI: 10.1177/0333102412438977] [DOI] [PubMed] [Google Scholar]
Bigal 2008
- Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology 2008;71(8):559‐66. [DOI: 10.1212/01.wnl.0000323925.29520.e7] [DOI] [PubMed] [Google Scholar]
Bloudek 2012
- Bloudek LM, Stokes M, Buse DC, Wilcox TK, Lipton RB, Goadsby PJ, et al. Cost of healthcare for patients with migraine in five European countries: results from the International Burden of Migraine Study (IBMS). Journal of Headache and Pain 2012;13(5):361‐78. [DOI: 10.1007/s10194-012-0460-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buse 2011
- Buse D, Manack A, Serrano D, Reed M, Varon S, Turkel C, et al. Headache impact of chronic and episodic migraine: results from the American Migraine Prevalence and Prevention study. Headache 2012;52(1):3‐17. [DOI: 10.1111/j.1526-4610.2011.02046.x] [DOI] [PubMed] [Google Scholar]
Collins 1997
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres?. Pain 1997;72(1‐2):95‐7. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2009
- Derry P, Derry S, Moore RA, McQuay HJ. Single dose oral diclofenac for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD004768.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012b
- Derry CJ, Derry S, Moore RA. Sumatriptan (oral route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008615] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2013
- Derry S, Moore RA, McQuay HJ. Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008040.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diamond 2007
- Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47(3):355‐63. [DOI: 10.1111/j.1526-4610.2006.00631.x] [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI: 10.1093/ije/31.1.140] [DOI] [PubMed] [Google Scholar]
Friedman 2005
- Friedman BW, Corbo J, Lipton RB, Bijur PE, Esses D, Solorzano C, et al. A trial of metoclopramide vs sumatriptan for the emergency department treatment of migraines. Neurology 2005;64(3):463‐8. [DOI: 10.1212/01.WNL.0000150904.28131.DD] [DOI] [PubMed] [Google Scholar]
Hazard 2009
- Hazard E, Munakata J, Bigal ME, Rupnow MF, Lipton RB. The burden of migraine in the United States: current and emerging perspectives on disease management and economic analysis. Value in Health 2009;12(1):55‐64. [DOI: 10.1111/j.1524-4733.2008.00404.x] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Altman DG, Sterne JAC editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. [Google Scholar]
IBSA 2011
- Institut Biochimque SA. Flector oral form. http://www.ibsa.ch/welcome‐intl/products‐nsaids‐flector_oral‐intl.htm?id=1240 (accessed 7 January 2012).
IHS 1988
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8 Suppl 7:1‐96. [PubMed] [Google Scholar]
IHS 2000
- International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 2000;20(9):765‐86. [DOI] [PubMed] [Google Scholar]
IHS 2004
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24 Suppl 1:1‐160. [DOI] [PubMed] [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2‐3):239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Kirthi 2013
- Kirthi V, Derry S, Moore RA, McQuay HJ. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008041.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. [DOI] [PubMed] [Google Scholar]
Leonardi 2005
- Leonardi M, Steiner TJ, Scher AT, Lipton RB. The global burden of migraine: measuring disability in headache disorders with WHO's Classification of Functioning, Disability and Health (ICF). Journal of Headache and Pain 2005;6(6):429‐40. [DOI: 10.1007/s10194-005-0252-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linde 2012
- Linde M, Gustavsson A, Stovner LJ, Steiner TJ, Barré J, Katsarava Z, et al. The cost of headache disorders in Europe: the Eurolight project. European Journal of Neurology 2012;19(5):703‐11. [DOI: 10.1111/j.1468-1331.2011.03612.x] [DOI] [PubMed] [Google Scholar]
Lipton 1999
- Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy?. Headache 1999;39 Suppl 2:S20‐6. [Google Scholar]
Lipton 2007
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF, AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68(5):343‐9. [DOI] [PubMed] [Google Scholar]
Lucas 2006
- Lucas C, Géraud G, Valade D, Chautard MH, Lantéri‐Minet M. Recognition and therapeutic management of migraine in 2004, in France: results of FRAMIG 3, a French nationwide population‐based survey. Headache 2006;46(5):715‐25. [DOI: 10.1111/j.1526-4610.2006.00430.x] [DOI] [PubMed] [Google Scholar]
McNeely 1999
- McNeely W, Goa KL. Diclofenac‐potassium in migraine. A review. Drugs 1999;57(6):991‐1003. [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [ISBN: 978‐0‐931092‐69‐5] [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ACTINPAIN Writing Group of the IASP Special Interest Group on Systematic Reviews in Pain Relief, Cochrane Pain, Palliative and Supportive Care Systematic Review Group Editors. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI: ] [DOI] [PubMed] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with confidence ‐ confidence intervals and statistical guidelines. London: British Medical Journal, 1995:50‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munakata 2009
- Munakata J, Hazard E, Serrano D, Klingman D, Rupnow MF, Tierce J, et al. Economic burden of transformed migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2009;49(4):498‐508. [DOI: 10.1111/j.1526-4610.2009.01369.x] [DOI] [PubMed] [Google Scholar]
Patrono 2001
- Patrono C, Patrignani P, García Rodríguez LA. Cyclooxygenase‐selective inhibition of prostanoid formation: transducing biochemical selectivity into clinical read‐outs. Journal of Clinical Investigation 2001;108(1):7‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patrono 2009
- Patrono C, Baigent C. Low‐dose aspirin, coxibs, and other NSAIDS: a clinical mosaic emerges. Molecular Interventions 2009;9:31‐9. [DOI] [PubMed] [Google Scholar]
PCA 2010
- The NHS Information Centre, Prescribing Support Unit. Prescription Cost Analysis for England 2009. The NHS Information Centre for health and social care, 2010. [ISBN: 978‐1‐84636‐408‐2] [Google Scholar]
Rabbie 2013
- Rabbie R, Derry S, Moore RA, McQuay HJ. Ibuprofen with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008039.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Radtke 2009
- Radtke A, Neuhauser H. Prevalence and burden of headache and migraine in Germany. Headache 2009;49(1):79‐89. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Ross‐Lee 1983
- Ross‐Lee LM, Eadie MJ, Heazlewood V, Bochner F, Tyrer JH. Aspirin pharmacokinetics in migraine. The effect of metoclopramide. European Journal of Clinical Pharmacology 1983;24(6):777‐85. [DOI] [PubMed] [Google Scholar]
Salazar‐Tortolero 2008
- Salazar‐Tortolero G, Huertas‐Campistol A, Vergez‐Pinto L, Ramos‐Brunet A, Lluch‐López J. Metoclopramide as a painkiller for intense migraine headache in emergency departments [Metoclopramida como analgésicoen la cefalea migrañosa intensa en urgencias]. Revista de Neurologia 2008;47(10):506‐8. [PubMed] [Google Scholar]
Steiner 2013
- Steiner TS, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. Journal of Headache and Pain 2013;14:1. [DOI: 10.1186/1129-2377-14-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stovner 2010
- Stovner LJ, Andree C. Prevalence of headache in Europe: a review for the Eurolight project. Journal of Headache and Pain 2010;11(4):289‐99. [DOI: 10.1007/s10194-010-0217-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tramer 1997
- Tramèr MR, Reynolds DJM, Moore RA, McQuay HJ. Impact of covert duplicate results on meta‐analysis: a case study. BMJ 1997;315(7109):635‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Victor 2010
- Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life‐span study. Cephalalgia 2010;30(9):1065‐72. [DOI: 10.1177/0333102409355601] [DOI] [PubMed] [Google Scholar]
Volans 1974
- Volans GN. Absorption of effervescent aspirin during migraine. BMJ 1994;4(5939):265‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Volans 1975
- Volans GN. The effect of metoclopramide on the absorption of effervescent aspirin in migraine. British Journal of Clinical Pharmacology 1975;2(1):57‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990‐2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163‐96. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2012
- Yu S, Liu R, Zhao G, Yang X, Qiao X, Feng J, et al. The prevalence and burden of primary headaches in China: a population‐based door‐to‐door survey. Headache 2012;52(4):582‐91. [DOI: 10.1111/j.1526-4610.2011.02061.x] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Derry 2012a
- Derry S, Rabbie R, Moore RA. Diclofenac with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008783.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


