Abstract
Background
The prognosis and survival rate of women with breast cancer have significantly improved worldwide. Effective home‐based multidimensional programmes for breast cancer survivors have gained an ever greater emphasis in survivorship care to maximise women’s quality of life for their successful transition to rehabilitation and normal life. It is important to summarise the best available evidence to evaluate the effects of home‐based multidimensional survivorship programmes on quality of life in women within 10 years of the completion of surgery or adjuvant cancer therapy for breast cancer, or both.
Objectives
To assess the effects of home‐based, multidimensional survivorship (HBMS) programmes on maintaining or improving the quality of life in breast cancer survivors.
Search methods
In April 2016 we searched the Cochrane Breast Cancer Specialised Register, CENTRAL, PubMed, Embase, CINAHL Plus, PsycINFO, Web of Science, and the World Health Organization's International Clinical Trials Registry Platform (WHO ICTRP) and ClinicalTrials.gov. We also screened reference lists of all identified studies and contacted study authors.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs assessing the effects of HBMS programmes in maintaining or improving quality of life in women with stages 0 to 3 breast cancer who completed primary cancer treatment (surgery or adjuvant cancer therapy, or both) up to 10 years earlier. We considered studies where the interventions included more than one of the following listed components: educational (such as information provision and self‐management advice), physical (such as exercise training and resistance training) and psychological (such as counselling and cognitive therapies), to constitute a multidimensional programme. Interventions had to be allowed to be carried out at home.
Data collection and analysis
Two authors independently assessed eligible studies for inclusion, and performed quality assessment and extracted relevant data of the included studies. Quality of life was the primary outcome of the review.
Main results
We included 22 RCTs and four quasi‐RCTs on 2272 participants. We categorised the intervention components into four groups: educational and psychological; educational and physical; physical and psychological; and educational, physical and psychological. Most of the studies used usual care (routine medical follow‐up services) as the comparator. A few studies used a lower level or different type of intervention (e.g. stress management or exercise) or attention control as the comparator.
We used the Functional Assessment of Cancer Therapy‐Breast (FACT B), European Organisation for Research and Treatment of Cancer Quality of Life C30 (EORTC C30), Quality of Life (QoL) Breast Cancer, and SF36 questionnaires to assess quality of life. HBMS programmes may increase breast cancer‐specific quality of life and global quality of life immediately after the intervention, as measured by FACT‐B and EORTC C30 (FACT‐B: mean difference (MD) 4.55, 95% confidence interval (CI) 2.33 to 6.78, 7 studies, 764 participants; EORTC: MD 4.38, 95% CI 0.11 to 8.64, 6 studies; 299 participants; moderate‐quality evidence). There was no evidence of a difference in quality of life as measured by QoL‐Breast Cancer or SF‐36 (QoL‐Breast Cancer: MD 0.42, 95% CI ‐0.02 to 0.85, 2 studies, 111 participants, very low‐quality evidence; physical composite score SF36: MD 0.55, 95% CI ‐3.52 to 4.63, 2 studies, 308 participants, low‐quality evidence).
We observed a similar pattern at one to three months after the intervention: FACT‐B (MD 6.10, 95% CI 2.48 to 9.72, 2 studies, 426 participants), EORTC‐C30 (MD 6.32, 95% CI 0.61 to 12.04, 2 studies; 172 participants) and QoL‐Breast Cancer (MD 0.45, 95% CI ‐0.19 to 1.09, 1 study, 61 participants). At four to six months and 12 months, there was no evidence of a difference in quality of life between groups (four to six months: EORTC ‐ MD 0.08, 95% CI ‐7.28 to 7.44, 2 studies; 117 participants; SF‐36 ‐ MD ‐1.05, 95% CI ‐5.60 to 3.51, 2 studies, 308 participants; 12 months: EORTC ‐ MD 2.04, 95% CI ‐9.91 to 13.99, 1 study; 57 participants).
Functional status was incorporated into the quality of life subscale findings. HBMS programmes may decrease anxiety (MD of Hospital Anxiety and Depression Scale (HADS) ‐1.01, 95% CI ‐1.94 to ‐0.08, 5 studies, 253 participants, low‐quality evidence) compared to control immediately after the intervention but the effect did not persist at four to six months. There was no evidence of improvements in depression immediately after HBMS (MD of HADS ‐1.36, 95% CI ‐2.94 to 0.22, 4 studies, 213 participants, low‐quality evidence) or at follow‐up. HBMS programmes may also decrease fatigue (MD ‐1.11, 95% CI ‐1.78 to ‐0.45, 3 studies, 127 participants; low‐quality evidence) and insomnia (MD ‐1.81, 95% CI ‐3.34 to ‐0.27, 3 studies, 185 participants, low‐quality evidence).
None of the included studies reported service needs and utilisation and cost of care, and therefore the effect of HBMS programmes on healthcare utilisation and cost is unknown. Due to the variations in assessment methods of adherence among the eight studies, we could not combine the results for meta‐analysis. We synthesised the results narratively, with the reported adherence rates of 58% to 100%.
Authors' conclusions
The results of this systematic review and meta‐analysis revealed that HBMS programmes in breast cancer survivors appear to have a short‐term beneficial effect of improving breast cancer‐specific quality of life and global quality of life as measured by FACT‐B and EORTC‐C30, respectively. In addition, HBMS programmes are associated with a reduction in anxiety, fatigue and insomnia immediately after the intervention. We assessed the quality of evidence across studies as moderate for some outcomes, meaning that we are fairly confident about the results, while we assessed other outcomes as being low‐quality, meaning that we are uncertain about the result.
Plain language summary
Home‐based multidimensional survivorship programmes for breast cancer survivors
Background
The demands are growing for effective multidimensional survivorship programmes in women who have had breast cancer. This review was conducted to evaluate the effects of home‐based multidimensional survivorship programmes on the quality of life in women who had completed primary treatment (surgery and/or chemotherapy and/or radiotherapy) for breast cancer in the previous 10 years.
Study characteristics
We found 26 studies with 2272 participants receiving home‐based multidimensional survivorship programmes compared with control. The content and delivery approach of the home‐based multidimensional survivorship programmes were diverse among the included studies. The survivorship programme could incorporate any combination of at least two of the three identified components: educational (such as the provision of information and advice on how to self‐manage); physical (such as exercise or resistance training); and psychological (such as counselling and cognitive therapies). Most of the studies used usual care (routine medical follow‐up services) as a comparator. A few studies used a lower level or different type of intervention (e.g. stress management or exercise) or attention control as the comparator.
The results revealed that home‐based multidimensional survivorship programmes in breast cancer survivors appear to have a short‐term beneficial effect of improving quality of life. Several other studies examined the effects of home‐based multidimensional survivorship programmes on symptoms and psychosocial outcomes. Those breast cancer survivors who received home‐based multidimensional survivorship programmes showed a reduction in fatigue, insomnia and anxiety, but the effect was in the short term. There was no difference between groups with respect to symptoms of depression, flushes and night sweats. We found that a group‐based approach may be more effective than an individual‐based approach to deliver the home‐based multidimensional survivorship programmes. However, we found no evidence for a difference in quality of life with educational, psychological or physical components of the survivorship programmes.
Quality of evidence
The quality of evidence across studies for quality of life ranged from moderate to very low, meaning that in some cases we were fairly confident about the results (e.g. quality of life improvements) while in other cases we were uncertain about the results (e.g. reductions in fatigue, insomnia and anxiety).
Summary of findings
Summary of findings for the main comparison. Home‐based multidimensional survivorship programmes for breast cancer survivors.
| Home‐based multidimensional survivorship programmes compared to control for quality of life of breast cancer survivors | |||||
|
Patient or population: breast cancer survivors
Settings: home‐based
Intervention: home‐based multidimensional survivorship programmes Comparison: control (most of the studies used usual care (i.e. routine medical follow‐up services) as the comparator. A few studies used a lower level or different type of intervention (e.g. stress management or exercise), or attention control as the comparator) | |||||
| Outcomes | Anticipated absolute effects (95% CI)* | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Control | Home‐based multidimensional survivorship programmes | ||||
|
Quality of Life FACT‐B Functional Assessment of Cancer Therapy ‐ Breast Cancer Specific Scale from: 0‐144 Follow‐up: 0 to 3 months1 |
The mean quality of life in the control group was 109.4 | The mean quality of life ‐ FACT‐B in the intervention group was
4.55 higher
(2.33 to 6.78 higher) Higher score ==> Better Quality of Life |
764 (7 studies) | ⊕⊕⊕⊝ moderate2 | |
|
Quality of Life EORTC European Organisation for Research and Treatment of Cancer ‐ Global Scale from: 0‐100 Follow‐up: 0 to 10 months1 |
The mean quality of life in the control group was 67.95 | The mean quality of life ‐ EORTC in the intervention group was
4.38 higher
(0.11 to 8.64 higher) Higher score ==> Better Quality of Life |
299 (6 studies) | ⊕⊕⊕⊝ moderate3 | |
|
Quality of Life QoL Breast Cancer Quality of Life ‐ Breast Cancer Overall Follow‐up: 0 to 3 months1 |
The mean quality of life in the control group was 4.07 | The mean quality of life ‐ QOL Breast Cancer in the intervention group was
0.42 higher
(0.02 lower to 0.85 higher) Higher score ==> Better Quality of Life |
111 (2 studies) | ⊕⊝⊝⊝ very low4,5 | |
| Quality of Life ‐ SF‐36 (Physical) SF‐36 Physical Function Scale from: 0‐100 Follow‐up: 0 to 3 months1 | The mean quality of life (physical) in the control group was 79.7 | The mean quality of life ‐ SF‐36 (Physical) in the intervention group was
0.55 higher
(3.52 lower to 4.63 higher) Higher score ==> Better Quality of Life |
308 (2 studies) | ⊕⊕⊝⊝ low3,5 | |
| Anxiety Hospital Anxiety and Depression Scale Follow‐up: 0 to 12 months1 | The mean anxiety in the control group was 6.71 | The mean anxiety in the intervention group was
1.01 lower
(1.94 to 0.08 lower) Lower score ==> Less anxious |
253 (5 studies) | ⊕⊕⊝⊝ low3,5 | |
| Depression Hospital Anxiety and Depression Scale Follow‐up: median 0 to 12 months1 | The mean depression in the control group was 3.53 | The mean depression in the intervention group was
1.36 lower
(2.94 lower to 0.22 higher) Lower score ==> Less depression |
213 (4 studies) | ⊕⊕⊝⊝ low3,5 | |
| Fatigue Brief Fatigue Inventory Follow‐up: 0 months1 | The mean fatigue in the control group was 3.3 | The mean fatigue in the intervention group was
1.11 lower
(1.78 to 0.45 lower) Lower score ==> Less fatigue |
127 (3 studies) | ⊕⊕⊝⊝ low3,5 | |
| *CI: Confidence interval | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
1Length of follow‐up counted from end of intervention, 0 month refers to the measurements conducted at the end of the intervention. 2We downgraded the quality of evidence (risk of bias) 1 point for QoL FACT‐B because (i) blinding was not implemented in the studies, (ii) participants were recruited using non‐probability sampling and (iii) the participation rates were low. 3We downgraded the quality of evidence (risk of bias) 1 point for EOTRC ‐General because (i) allocation concealment was not mentioned in the studies, (ii) blinding was not implemented in the studies, (iii) selective reporting was unclear, (iv) participants were recruited using non‐probability sampling and (v) the participation rates were low. 4We downgraded the quality of evidence (risk of bias) 2 points for QoL Breast Cancer because (i) allocation concealment was not mentioned in the studies, (ii) blinding was not implemented in the studies, (iii) selective reporting was unclear, (iv) participants were recruited using non‐probability sampling and (v) the participation rates were low. Overall, around 50% of components were rated high risk of bias. 5We downgraded the quality of evidence (imprecision) 1 point for QoL Breast Cancer because the overall sample size < 400.
Background
Description of the condition
Breast cancer is the most common cancer in women worldwide (IARC 2014; WHO 2013). Early detection, advancements in adjuvant chemotherapy, radiological techniques, hormonal therapy and targeted therapies have significantly improved the survival rate of women with breast cancer. Overall five‐year survival rates are now approaching 100% for stage 0 and 1 breast cancer, and are about 93% and 72% for stage 2 and stage 3 breast cancers, respectively, in the USA (ACS 2016). Similarly, in the UK, five‐year survival rates are at 99.1%, 87.6% and 55.1% for stage 1, stage 2 and stage 3 breast cancer, respectively (Cancer Research UK 2017). Survival rates for breast cancer are expected to continue to increase, resulting in a growing population of patients with long‐term care needs: this poses a challenge both for patients themselves and their families, and for oncology services.
The concept of cancer survivorship has been discussed in the medical literature, but an exact definition is yet to be reached. Most medical and psycho‐oncology literature suggests that a cancer survivor is someone who has completed primary cancer treatment and moved towards a return to normal life, with a diminished risk of cancer recurrence, or who is living with cancer but is not in the terminal phase of illness (Gosain 2013; Hodgkinson 2007; Mullan 1985). Clinically, the first five to 10 years after breast cancer treatment is a vulnerable period, during which some women may face a multitude of short‐term and long‐term health and psychosocial problems, including persistent or late‐emerging symptoms (or both) following the cancer and its treatment, and psychosocial distress associated with the risk of cancer recurrence, chronic uncertainty and social disruption (Binkley 2012; Cheng 2014; Ewertz 2011; Gandhi 2010; Janz 2007; Kim 2012; Kuehn 2000; Levangie 2009; Mehnert 2009; Rosedale 2010).
For cancer survivors, the goal is to maximise their physical and psychosocial well‐being, and thus quality of life, for a successful transition to normal life patterns. Nevertheless, the healthcare services provided to cancer survivors post‐treatment may be insufficient when compared to those provided in the earlier phase of diagnosis and treatment. It has been indicated in the literature that the traditional medical follow‐up and surveillance functions of monitoring for recurrent cancer frequently fail to meet the supportive care needs of survivors, often resulting in feelings of abandonment during the transition from patient to survivor (Jefford 2008). Breast cancer survivors have said that off‐treatment symptoms were much worse and persisted longer than expected, mainly because they felt that they were not taught realistically what to expect and also not introduced to appropriate rehabilitation programmes after treatment (Binkley 2012; Cheville 2007; Collins 2004; Lee 2010a).
International studies on the supportive care needs of breast cancer survivors consistently indicate that about one in two survivors report one or more unmet needs, and these are mostly in the healthcare system/information and psychological domains (Armes 2009; Cheng 2014; Harrison 2011). A recent study has indicated that within six months of primary cancer treatment, more than 50% of women have some or strong care needs in physical functioning, psychological functioning, and self and body image (Pauwels 2013). Literature suggests that unmet needs are associated with poor quality of life (Akechi 2011; Cheng 2016; So 2014). The demands for evidence‐based, post‐treatment survivorship care programmes in the breast cancer population are enormous. The National Care Survivorship Initiative (NCSI) and the Institute of Medicine (IOM) recommend a shift towards best‐structured care for post‐treatment cancer survivors, with a greater emphasis on recovery, health and well‐being (NCSI 2010). In the USA, the National Coalition for Cancer Survivorship has highlighted the importance of survivorship care plans (NCCS 2009).
Description of the intervention
A home‐based programme is defined as interventions that an individual can carry out at home over any duration of follow‐up. The interventions should be easy to perform and facilitate long‐term adherence.
Home‐based, multidimensional survivorship programmes often take the form of multimodal interventions, including education, physical and psychological interventions, exercise training or dietary advice. An education intervention includes information about self‐management strategies for physical symptoms and recovery, including topics such as fatigue, arm pain, numbness/tingling, lymphoedema, problems with sleeping, and regaining and rebuilding lives. A key support for cancer survivors is the provision of information (Van de Poll‐Franse 2011). Beneficial effects of educational interventions on quality of life have been reported in breast cancer survivors (Fillion 2008; Meneses 2007). Dietary advice, including weight loss strategies, meal plans and caloric goal setting, is commonly present in breast cancer survivorship programmes. Positive effects of dietary advice on weight control, physical functioning and anxiety levels have been reported in breast cancer survivors (Kim 2011; Thompson 2012). Exercise programmes include muscle stretching, core stability exercises, physiotherapy and aquatic exercises. Physical activity and muscle strength have been shown to reduce fatigue and improve quality of life in breast cancer survivors (Cantereri‐Villanueva 2012; Cuesta‐Vargas 2014; Rogers 2009a). Psychological interventions, including psychotherapy and counselling, are important for assisting women in making the transition from treatment to recovery. Previous studies have shown that mindfulness‐based psychotherapy may reduce depression and anxiety, fatigue and disturbed sleep in breast cancer survivors (Lengacher 2011; Lengacher 2012).
A home‐based, multidimensional survivorship programme will contain training session(s) by a trained person or healthcare professional, with interventions delivered either individually or through group sessions in a healthcare or home‐based setting, with any mode of delivery (such as in person, over the telephone or internet, or through multimedia). Supplementary training materials (including videos, booklets, self‐help workbooks, internet‐based resources, etc.) may be provided to reinforce practice at home (Gautam 2011; Morey 2009; Pinto 2005; Spector 2014). This provides breast cancer survivors with the knowledge and self‐management skills necessary to manage their own care and to enhance their recovery, health and well‐being (Bodenheimer 2002; Gautam 2011; Morey 2009; Pinto 2005; Spector 2014). The feasibility of and level of adherence to programmes are often assessed through the use of logbooks, self‐monitoring diaries and motivational telephone calls (Cheville 2013; Gautam 2011; Jeffs 2013a; Lee 2013).
How the intervention might work
Given the multifaceted nature of the transition process of a cancer survivor from treatment to recovery, multimodal interventions are required to address its full impact. Since breast cancer survivors normally have infrequent clinical follow‐up (Grunfeld 2010), a home‐based programme may offer a viable option in providing a more feasible and sustainable post‐treatment survivorship programme. Previous studies have revealed that survivors need rehabilitation programmes that can assist them in the self‐management of their health and psychosocial problems, which can include persistent or late‐emerging symptoms (or both) following the cancer and its treatment, upper extremity dysfunction and psychosocial distress associated with the risk of cancer recurrence, chronic uncertainty and social disruption (Binkley 2012; Lattanzi 2010). Patient‐training to increase active participation and self‐care skills, which survivors can use for identifying and managing emerging symptoms and problems, and training to regain health, are essential elements for maximising quality of life and thus effective care transitions (Garrett 2013; Howell 2012). Positive correlations between levels of empowerment and self‐care, symptom control, psychological adjustment and quality of life in cancer survivors have been documented (Ganz 2004; Loh 2011).
Why it is important to do this review
Several studies encompassing multidimensional and home‐based programmes for breast cancer survivors have been conducted (Meneses 2007; Spector 2014). These programmes have varied in their intervention components and delivery structures. To date, a systematic review on the effectiveness of home‐based, multidimensional programmes in maintaining or improving the quality of life of breast cancer survivors is not available. The aim of this review was therefore to assess the effectiveness of home‐based, multidimensional survivorship programmes for breast cancer survivors, in order to guide evidence‐based healthcare decision‐making and policy in post‐treatment survivorship planning and services.
Objectives
To assess the effects of home‐based, multidimensional survivorship programmes (HBMS) on maintaining or improving quality of life in breast cancer survivors.
The review will evaluate the extent to which: • survivorship programmes exert a different impact on different domains of quality of life (physical, functional, psychological and social well‐being); • survivorship programmes exert a different impact on different patient‐reported and healthcare outcomes, including functional status, anxiety and depression, symptom severity and distress, and the need for and utilisation of services; • different components and delivery structures influence outcomes; • different survivorship periods influence outcomes; and • different levels of adherence influence outcomes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐RCTs with or without blinding, assessing the effects of home‐based, multidimensional survivorship programmes (HBMS) in maintaining or improving quality of life in women with breast cancer who had completed primary cancer treatment (surgery, adjuvant chemotherapy and radiotherapy) up to 10 years earlier.
Types of participants
Women (18 years and over) with a breast cancer diagnosis between stages 0 to 3 and within 10 years of the completion of primary cancer treatment (surgery, adjuvant chemotherapy and radiotherapy). Women with stage 4 or recurrent breast cancer were excluded.
Types of interventions
We considered studies where the interventions included more than one of the following listed components to constitute a multidimensional programme and included those that were allowed to be carried out at home over any duration of follow‐up. The components are:
educational (such as information provision, symptom management advice, dietary advice and self‐management advice);
physical (such as exercise training and resistance training); and
psychological (such as mindfulness‐based psychotherapy, counselling and cognitive therapies).
The interventions are often combined as multidimensional survivorship programmes for evaluation in RCTs and quasi‐RCTs. This review sets out the evidence for the full range of survivorship programmes in an effort to identify the best components and delivery structures for breast cancer survivors.
The interventions must contain training session(s) by a trained person or healthcare professional. The interventions may be delivered individually or through group sessions in a healthcare or home‐based setting, with any mode of delivery (such as in person, over the telephone or internet, or through multimedia). An intervention programme can be considered home‐based if the interventions are allowed to be carried out at home and participants are advised to practise the intervention at home over any duration of follow‐up after the delivery of the programme. Supplementary training materials (including videos, booklets, self‐help workbooks, internet‐based resources, etc.) must also be provided to reinforce practice at home. Examples of home‐based interventions include, but are not limited to:
exercise programmes that are taught to participants individually at the study site; participants are then asked to practise the programme at home and materials, such as videos and booklets, are provided to them (Cheville 2013);
booklets consisting of information and demonstrations of self‐management techniques are taught and provided to participants and they are instructed to practise the techniques at home (Jacobsen 2013; Morey 2009);
interventions implemented in a group‐based setting, with booklets to reinforce learning given to participants; participants are advised to practise the intervention at home (Cadmus‐Bertram 2013; Cho 2006).
The comparison group can include:
those who have received a lower level or different type of intervention, such as routine services available or standard care;
an active control, which provides the same amount of attention to the participants without the actual intervention;
waiting list controls or no treatment;
interventions given in different settings.
Types of outcome measures
Primary outcomes
Health‐related quality of life, measured using generic or disease‐specific, validated instruments such as the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (QLQ‐C30) (EORTC Quality of Life Department 2017), Functional Assessment of Cancer Therapy‐Breast Cancer (FACT‐B) (Cella 1993), Quality of Life Index ‐ Cancer Version (QOLI‐CV) (Ferrans 1990), Quality of Life – Cancer Survivor Tool (QOL‐CS) (Ferrell 1995) or Cancer Rehabilitation Evaluation System (CARES) (Schag 1989).
Secondary outcomes
Functional status (incorporated into measurements from functional subscales of quality of life instruments), symptom severity and distress (fatigue, insomnia, endocrine symptoms, symptom distress, and joint pain, stiffness and physical function), and psychosocial outcomes including anxiety and depression.
Service needs and utilisation, including psychosocial and supportive care needs, unplanned re‐admission and hospitalisation, and cost of care
Participants' adherence to the programme interventions.
Search methods for identification of studies
Electronic searches
The electronic search for literature focused on retrieving published manuscripts in academic journals through systematic searching of the following databases.
Cochrane Breast Cancer's Specialised Register (21 April 2016). Details of the search strategies used by Cochrane Breast Cancer for the identification of studies and the procedures used to code references are outlined in the Group's module (www.onlinelibrary.wiley.com/o/cochrane/clabout/articles/BREASTCA/frame.html). We planned to extract trials coded with the key words 'breast cancer survivor', 'survivorship', 'transitional care', 'home based', 'self care', 'self help', 'self management', 'self managed', 'survivorship surveillance', 'continuum of care', 'home‐based multidimensional survivorship program' and 'post treatment care' for consideration.
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 3; www.cochranelibrary.com/about/central‐landing‐page.html) (refer to Appendix 1)
Embase via OvidSP (1980 to 21 April 2016; www.ovid.com/site/catalog/databases/903.jsp) (refer to Appendix 2)
PubMed (1976 to 28 March 2016; www.ncbi.nlm.nih.gov/pubmed/) (refer to Appendix 3)
Cumulative Index to Nursing and Allied Health Literature (CINAHL) Plus via EBSCO (1966 to 28 March 2016; https://health.ebsco.com/products/the‐cinahl‐database) (refer to Appendix 4)
PsycINFO via OvidSP (1987 to 21 April 2016; www.ovid.com/site/catalog/databases/139.jsp) (refer to Appendix 5)
Web of Science via Web of Knowledge (1991 to 28 March 2016; www.webofknowledge.com/) (refer to Appendix 6).
We retrieved all peer‐reviewed journal articles published in English from all time periods. We performed a pilot search on CINAHL to identify relevant keywords contained in the title, abstract and subject descriptors. We used the following terms, with wildcards and modifications where necessary, when performing searches on electronic databases.
The basic search terms used were:
"Breast Neoplasm*" OR "Breast Cancer*" OR "Breast Carcinoma" OR "Breast Malignanc*" OR "Breast Tumor*" OR "Breast Lump*"
survivor*
survivorship OR therap* OR interven* OR program* OR evaluat* OR educat* OR rehabilit* OR effect* OR train* OR "post treatment care" OR "survivorship surveillance" OR "continuum of care" OR "transitional care" OR "home based" OR "home‐based" OR "self care" OR "self‐care" OR "self help" OR "self‐help" OR "self manag*" OR "self‐manag*" OR "multidimension*" OR "multi‐dimension*"
1 AND 2 AND 3
In addition, we used the search strategy recommended in the Cochrane Handbook for Systematic Reviews of Interventions to optimise the sensitivity of randomised controlled trial identification (Lefebvre 2011).
Searching other resources
We screened the bibliographies of all identified studies and reviews for any relevant publications.
We contacted the first authors of identified studies and experts in the area of interest to enquire whether they were aware of any other relevant unpublished literature in the area or any initial results.
We contacted the associations relevant to the field of oncology to identify any other relevant unpublished literature in the area. They included the American Cancer Society (ACS), Singapore Cancer Society (SCS), American Association for Cancer Research (AACR), Cancer Research UK (CRUK), Macmillan Cancer Support, the World Cancer Research Fund (WCRF), the Association for International Cancer Research (AICR) and the American Society of Clinical Oncology (ASCO).
We identified grey literature and unpublished studies through searching the OpenGrey database (www.opengrey.eu) (Appendix 7) and Web of Science.
We screened ongoing clinical trials for all prospectively registered trials. These included the World Health Organization's International Clinical Trials Registry Platform (WHO ICTRP) (21 April 2016) (Appendix 8) and www.clinicaltrials.gov (21 April 2016) (Appendix 9).
We screened databases for theses and dissertations, including Digital Dissertation Consortium, ProQuest Dissertation and Theses Global (Appendix 10).
Data collection and analysis
Selection of studies
Two review authors (EL and ZMK) independently searched the electronic databases and stored all articles obtained in EndNote version X6 (www.endnote.com). We screened articles obtained from keyword searching for duplicates electronically with EndNote X6 and then manually. After duplicate removal, we assessed the remaining articles for eligibility based on titles and abstracts. We included studies in the next round of full‐text screening if they met the following fundamental criteria:
RCTs including quasi‐RCTs;
female breast cancer survivors (aged 18 and over) with a breast cancer diagnosis between stages 0 to 3;
interventions were delivered to survivors who were within 10 years of the completion of primary cancer treatment (surgery, adjuvant chemotherapy and radiotherapy);
interventions included both home‐based and multidimensional components;
health‐related quality of life was reported as an outcome of the study, measured using a generic or disease‐specific validated instrument.
We retrieved full‐text articles if we considered the trials relevant and if there was insufficient information to determine inclusion or exclusion. We then examined each article against the established inclusion and exclusion criteria. We linked together multiple reports of the same study. We screened the bibliography of included trials and relevant reviews to identify additional relevant articles missed during the electronic search. We contacted the first authors of the eligible trials to seek further information about the methodology if necessary. The two review authors resolved any discrepancies in the inclusion or exclusion of the trials by discussion. Disagreements between review authors were resolved by consultation with a third review author (KC). We recorded a list of excluded trials and reasons for exclusion in the 'Characteristics of excluded studies' table.
Data extraction and management
Two review authors (EL and ZMK) independently reviewed and extracted the data from each eligible study. We extracted the following information and input this into a data extraction form.
Publication information: authors, year of publication, title of study, journal published, country of study, recruitment source, language of publication and aim of study.
Study characteristics: study design, total study duration, inclusion/exclusion criteria for participation in study, sample size and evidence of power calculation, sampling method, response rate, drop‐out and withdrawal rate, sequence generation, allocation sequence concealment, blinding and method, quality of delivery (e.g. training of implementers) and presence of safeguard checks against diffusion of treatments.
Participant information: age (range, mean, standard deviation), gender (only for trials performed on both men and women), tumour stage, cancer treatment modality, and distribution of participants to each group of the trial.
Intervention group characteristics: number of intervention arms in the study, types of intervention (e.g. symptom management, exercise training, dietary interventions, problem‐solving training, cognitive behavioural interventions, psycho‐educational interventions, complementary interventions, spiritually focused psychotherapy), delivery structure (e.g. individual, group‐based, telephone, internet, multimedia), delivery setting (e.g. hospital, home, community), frequency of intervention delivery (e.g. duration of intervention, number of sessions, duration of each session) and providers of the intervention (e.g. a trained person or healthcare professional).
Comparison group characteristics: description of comparison group, e.g. those who received lower levels or different types of intervention (e.g. routine available services or standard care, active control, waiting list control, no treatment and interventions given in different settings).
Outcome measures (quality of life, functional status, symptoms, psychosocial outcomes, services need and utilisation, cancer recurrence, participants' adherence and satisfaction): measurement tool used for each outcome, upper and lower limits and whether a high or low score is favourable, follow‐up timing, frequency and duration for each outcome, and any missing data.
The two review authors resolved disagreements in data extraction through discussion and consulted a third, independent review author (KC) if disagreements could not be resolved.
Assessment of risk of bias in included studies
Two review authors (EL and ZMK) independently assessed the risk of bias of the included studies using The Cochrane 'Risk of bias' tool (Higgins 2011).
The tool consists of seven domains:
sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessors;
incomplete outcome data;
selective outcome reporting;
other sources of bias.
A judgement of 'yes' indicated a low risk of bias, 'no' indicated a high risk of bias and 'unclear' indicated either unclear or unknown risk of bias. We recorded the assessment results in the 'Risk of bias' tables using Review Manager 5.3 (RevMan 5.3) software (RevMan 2014). We compared the judgements of the two independent review authors and resolved any disagreements through discussion. We consulted a third review author (KC) if disagreements could not be resolved.
Measures of treatment effect
Data reported in studies were dichotomous (e.g. satisfied or not satisfied), ordinal (e.g. different categories in quality of life scales) or continuous (e.g. changes in quality of life scores).
Dichotomous outcomes
We extracted the number of participants in each treatment arm who experienced the outcome of interest and the number of participants assessed at endpoint using a 2 x 2 table and computed risk ratio (RR) with 95% confidence interval (CI) for each study.
Ordinal outcomes
If odds ratios (OR), say from proportional odds models, were reported for the ordinal outcome, we extracted the OR and its 95% CI.
Continuous outcomes
We computed the mean difference (MD) with a 95% CI for each study when the outcome was measured with the same scale for all studies; otherwise we computed standardised mean difference (SMD) and its 95% CI. If standard deviations were not reported, we planned to compute them using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011), given sufficient information was available or authors of the included papers provided additional information (e.g. mean, standard deviation or sample size of their studies).
Unit of analysis issues
The unit of analysis was the individual study and therefore we did not anticipate any unit of analysis issues.
Dealing with missing data
We reported the number of participants included in the final analysis of each included study as a proportion of all participants in the study and we recorded the reasons for exclusion from analysis for each study. We contacted the primary authors of the study to request any missing data, if possible.
Assessment of heterogeneity
We assessed heterogeneity quantitatively using the Chi2 test (Deeks 2011) and I2 statistic (Higgins 2003). We used a P value of less than 0.10 and the I2 value (less than 30% as a low level, 30% to 50% as a moderate level and more than 50% as a substantial level of heterogeneity) to determine the heterogeneity (Deeks 2011).
Assessment of reporting biases
We searched grey literature to identify any unpublished studies that would be relevant. We used funnel plots to identify reporting bias where there were at least 10 studies included in the meta‐analysis, to ensure that the power was sufficient (Sterne 2011). Asymmetry in the plots could indicate reporting bias. We also used the trim and fill method to examine the bias and to compute the adjusted estimate due to the potential bias (Duval 2000).
Data synthesis
For the effect measure for continuous outcomes we used MD or SMD and for dichotomous outcomes we used RR (Borenstein 2009). We used a fixed‐effect model with an inverse variance approach when the level of heterogeneity was low, defined as I2 less than 30% (Normand 1999), and a DerSimonian and Laird (DH) random‐effects model when the heterogeneity was moderate, defined as I2 between 30% and 70% (DerSimonian 2000). We presented pooled and individual results using forest plots with the results presented according to the follow‐up period in chronological order (e.g. post intervention, one to three months after intervention, four to six months after intervention, etc.). Results from individual studies were not pooled when there was a substantial level of heterogeneity (i.e. I2 more than 70%). We planned to use meta‐regression to explore the source of heterogeneity only if there were enough studies (Borenstein 2009). Where there were insufficient studies for conducting meta‐analysis, we summarised the results in a narrative format.
Summary of Findings table:
The GRADE approach was used to assess the quality of evidence for the following main outcomes:
Fact‐B Breast Cancer Specific
EORTC ‐ Global
QoL Breast Cancer
QoL SF‐36 (Physical)
Anxiety
Depression
Fatigue
We used GRADEproGDT software (GRADEproGDT) to develop the Summary of Findings table and followed GRADE guidance (Schünemann 2011). Two authors (EL and WWST) graded the quality of the evidence. We selected to report the quality of evidence for Fact‐B Breast Cancer Specific because it is the overall score, computed by the sum of the general score and breast cancer sub‐scale, specifically designed for capturing the QoL for women with breast cancer.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses based on pre‐defined subgroups, namely the type of cancer treatment modality, survivorship period (less than five years versus five years or more) and components of the multidimensional interventions (physical plus psychological, physical plus educational, psychological plus educational, physical plus psychological plus educational) to attempt to explore heterogeneity across the subgroups. Since none of the included studies provided stratified analysis according to cancer treatment modality and survivorship period, we were unable to perform subgroup analyses as per the review protocol.
In future review updates, if sufficient data are available, potential covariates for meta‐regression will include the mean age of the participants and the mean time from first diagnosis. We may decide on additional covariates after data extraction from the included studies.
Sensitivity analysis
We performed sensitivity analysis for the primary outcome, quality of life, where meta‐analysis was possible, by repeating the analysis with the exclusion of the following studies:
studies with a high risk of bias, based on the Cochrane 'Risk of bias' tool;
studies that were unpublished (grey literature).
We used a fixed‐effect model for all outcomes regardless of the degree of heterogeneity so as to compare the sensitivity analyses with the results from the Data synthesis section. We identified further issues that threatened the robustness of the results in the meta‐analysis during the review process and performed sensitivity analysis accordingly, to ensure that the identified issues would not interfere with the quality of the meta‐analysis. We presented the results from the sensitivity analysis in a summary table. We computed the relative differences between the estimates from the main analysis and the sensitivity analysis for examination.
Results
Description of studies
Results of the search
The electronic search retrieved 29,198 studies, of which 25,907 were left after duplication removal. We retrieved another seven studies from other sources, including bibliography lists of included articles, and screened the titles and abstracts of 25,914 records in total, subsequently excluding 25,740 studies, leaving 174 articles for full‐text assessment. We retrieved each article and judged it against the inclusion and exclusion criteria. Following full‐text screening we excluded 140 articles for various reasons (see Excluded studies). We included 26 studies in this review, of which 25 were journal articles (Cho 2006; Dirksen 2008; Duijts 2012; Ergun 2013; Eyigor 2010; Fillion 2008; Galantino 2010; Heidrich 2009; Hoffman 2012; Kim 2011; Lahart 2016; Lengacher 2009; Loerzel 2008; Mann 2012; Matthews 2014; McClure 2010; Meneses 2007; Rogers 2009b; Rogers 2009c; Rogers 2015; Savard 2005; Sherman 2010; Spahn 2013; Swisher 2015; Wonghongkul 2008), and 1 was a dissertation (Fiorentino 2008). Eight abstracts or ongoing trials (Abrahams 2015; Befort 2014; Hummel 2015; Marcus 1998; Matthews 2002; McDonald 2014; Rock 2013; NCT01515124) were not included in the synthesis as no data were available. See Figure 1.
1.
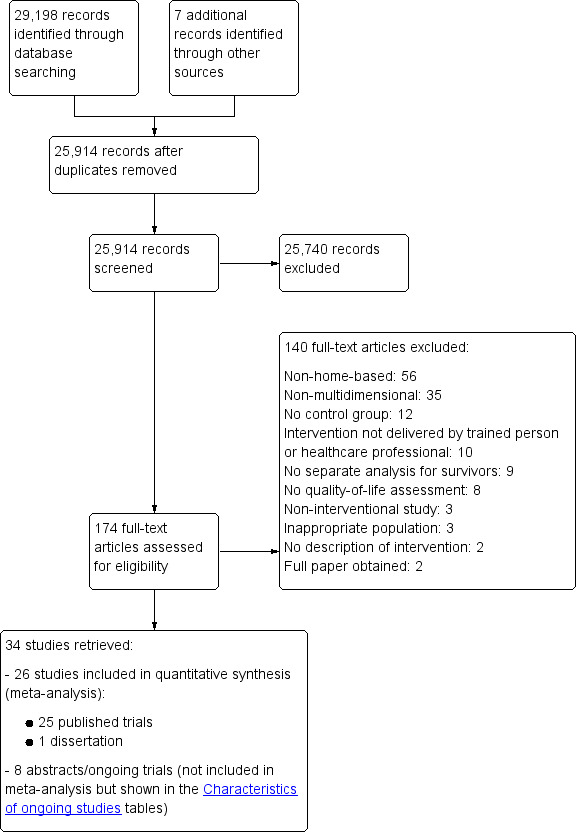
Study flow diagram
Included studies
We identified 26 included studies (25 journal articles and 1 dissertation) and 8 ongoing studies for this review. (See Characteristics of included studies and Characteristics of ongoing studies tables).
Type of studies
Of the 26 full studies, 21 were RCTs (Dirksen 2008; Duijts 2012; Ergun 2013; Eyigor 2010; Fillion 2008; Fiorentino 2008; Heidrich 2009; Hoffman 2012; Kim 2011; Lahart 2016; Lengacher 2009; Mann 2012; Matthews 2014; McClure 2010; Meneses 2007; Rogers 2009b; Rogers 2009c; Rogers 2015; Savard 2005; Spahn 2013; Swisher 2015) and four were quasi‐RCTs (Cho 2006; Galantino 2010; Sherman 2010; Wonghongkul 2008). The last study (Loerzel 2008) was a secondary analysis of a RCT by Meneses 2007, which was also included in this review. Rogers 2009b and Rogers 2009c were multiple reports of the same study using the same study sample. Rogers 2009c reported the study outcomes at baseline and immediately post intervention, while Rogers 2009b reported the study outcomes at baseline, immediately post intervention and at three months post intervention.
Type of participants
All participants were diagnosed with stages 0 to 3 breast cancer and had completed primary cancer treatment. Due to the unavailability of data in the included studies, we could not ascertain if local recurrence and metastatic disease occurred in participants. Sample sizes in the studies ranged from 14 to 422 participants. Fifteen studies (Cho 2006; Dirksen 2008; Fillion 2008; Fiorentino 2008; Hoffman 2012; Kim 2011; Lengacher 2009; Loerzel 2008; Matthews 2014; Rogers 2009b; Rogers 2009c; Rogers 2015; Savard 2005; Sherman 2010; Spahn 2013) provided information on the tumour stage at diagnosis while 11 studies did not (Duijts 2012; Ergun 2013; Eyigor 2010; Galantino 2010; Heidrich 2009; Lahart 2016; Mann 2012; McClure 2010; Meneses 2007; Swisher 2015; Wonghongkul 2008). Among the studies that reported the tumour stage at diagnosis, 36 women had stage 0, 231 women had stage 1, 215 women had stage 2 and 87 women had stage 3 tumours at diagnosis in the HBMS group. In the comparison group, 35 women had stage 0, 225 women had stage 1, 194 women had stage 2 and 71 women had stage 3 tumours at diagnosis. Forty‐one participants in the HBMS group and 15 participants from the comparison group did not report their tumour stage at diagnosis (Sherman 2010). Fiorentino 2008 and Loerzel 2008 did not provide information on the breakdown of the tumour stage at diagnosis by study group and overall 42 women had stage 1, 18 women had stage 2 and 4 women had stage 3 tumours at diagnosis.
Twenty‐three studies (Cho 2006; Dirksen 2008; Duijts 2012; Ergun 2013; Eyigor 2010; Fillion 2008; Fiorentino 2008; Heidrich 2009; Hoffman 2012; Kim 2011; Lengacher 2009; Loerzel 2008; Mann 2012; Matthews 2014; McClure 2010; Meneses 2007; Rogers 2009b; Rogers 2009c; Rogers 2015; Savard 2005; Spahn 2013; Swisher 2015; Wonghongkul 2008) provided information on the treatment modality, while three studies did not (Galantino 2010; Lahart 2016; Sherman 2010). In the studies that provided information on the treatment modalities, 634 participants had surgery, 640 participants had chemotherapy, 395 participants had radiation therapy and 546 participants had hormonal therapy in the HBMS group. In the comparison group, 423 participants had surgery, 430 participants had chemotherapy, 381 participants had radiation therapy and 369 participants had hormonal therapy. Dirksen 2008 and Eyigor 2010 did not indicate the type of adjuvant therapy given to the participants. Dirksen 2008 stated that 26 and 27 participants had surgery and adjuvant therapy in the HBMS and control group, respectively. Eyigor 2010 indicated that 11 and three participants underwent adjuvant therapy in the two intervention groups respectively. Heidrich 2009, Fiorentino 2008 and Loerzel 2008 did not report separately the treatment modality by study group. In these three studies, 124 participants underwent surgery, 29 participants underwent chemotherapy, 84 participants underwent radiation therapy and 79 participants underwent hormonal treatment. Lengacher 2009 and Meneses 2007 provided percentages reflecting the treatment modalities that participants underwent. In Lengacher 2009, 61% of participants in the HBMS group underwent radiotherapy only, while 39% of the participants in the HBMS group underwent chemotherapy and radiotherapy. In the comparison group, 60.5% of participants underwent radiotherapy only, while 39.5% of participants underwent both radiotherapy and chemotherapy Lengacher 2009. In Meneses 2007, more than 60% of participants underwent breast‐conserving surgery and 40% underwent single or bilateral mastectomy. In addition, more than 69% of participants underwent radiotherapy, 54% of participants underwent combination chemotherapy and more than 76% of participants were given tamoxifen or aromatase inhibitors.
Types of interventions
The interventions examined in the included studies were multi‐dimensional and encompassed a combination of at least two of the three identified components: educational, physical and psychological. A home‐based component was also present in all the interventions. Five studies (Duijts 2012; Rogers 2009b; Rogers 2009c; Rogers 2015; Swisher 2015) explored the effects of interventions comprising physical and psychological components, six studies (Ergun 2013; Eyigor 2010; Kim 2011; McClure 2010; Sherman 2010; Wonghongkul 2008) explored the effects of interventions comprising physical and educational components, while 12 studies (Dirksen 2008; Fiorentino 2008; Galantino 2010; Heidrich 2009; Hoffman 2012; Lahart 2016; Lengacher 2009; Loerzel 2008; Mann 2012; Matthews 2014; Meneses 2007; Savard 2005) explored the effects of interventions comprising psychological and educational components. The remaining three studies (Cho 2006; Fillion 2008; Spahn 2013) examined the effects of interventions comprising physical, psychological and educational components.
The duration of the delivered interventions ranged from four weeks to six months. Four studies had interventions that lasted between four to six weeks (Fillion 2008; Lengacher 2009; Mann 2012; Fiorentino 2008) while 13 studies had interventions that lasted between 7 to 12 weeks (Cho 2006; Dirksen 2008; Duijts 2012; Ergun 2013; Eyigor 2010; Hoffman 2012; Kim 2011; Rogers 2009b; Rogers 2009c; Rogers 2015; Sherman 2010; Spahn 2013; Swisher 2015). Amongst the remaining nine studies, the intervention lasted more than 12 weeks for five studies (Galantino 2010; Lahart 2016; Loerzel 2008; McClure 2010; Meneses 2007) and the duration was not reported in four studies (Heidrich 2009; Matthews 2014; Savard 2005; Wonghongkul 2008).
Thirteen studies delivered the HBMS programme using group‐based methods (Cho 2006; Dirksen 2008; Ergun 2013; Eyigor 2010; Hoffman 2012; Lengacher 2009; Mann 2012; McClure 2010; Rogers 2015; Savard 2005; Sherman 2010; Spahn 2013; Wonghongkul 2008), ten studies delivered the interventions on an individual basis (Fillion 2008; Galantino 2010; Heidrich 2009; Kim 2011; Lahart 2016; Loerzel 2008; Matthews 2014; Meneses 2007; Swisher 2015; Fiorentino 2008) while three studies used a combination of group‐ and individual‐based delivery methods (Duijts 2012; Rogers 2009b; Rogers 2009c).
Types of outcome
Baseline measurements were taken before the introduction of the study interventions in all studies. The period of follow‐up in the included studies ranged between immediately after the intervention to 12 months after the end of intervention delivery. Six studies had longer follow‐up periods of six months and above after the end of intervention delivery (Duijts 2012; Loerzel 2008; Mann 2012; Matthews 2014; Meneses 2007; Savard 2005).
Primary outcome
The included studies used a variety of instruments to assess quality of life, including the Functional Assessment of Cancer Therapy (FACT‐B, FACT‐G, FACT‐ES) instrument (Dirksen 2008; Hoffman 2012; Lahart 2016; Rogers 2009b; Rogers 2009c; Rogers 2015; Sherman 2010; Swisher 2015), European Organisation for Research and Treatment of Cancer Quality of Life C30 (EORTC QLQ‐C30, EORTC BR23) (Ergun 2013; Eyigor 2010; Kim 2011; Matthews 2014; Savard 2005; Spahn 2013), Short Form Health Survey (SF‐12) (Fillion 2008; Heidrich 2009), Short Form Health Survey (SF‐36) (Duijts 2012; Fiorentino 2008; Heidrich 2009; Lengacher 2009; Mann 2012; McClure 2010), the Quality of Life Patient/Cancer Survivor (QOLP/CS) scale (Galantino 2010), the Quality of Life‐Breast Cancer (QOL‐BC) scale (Loerzel 2008; Meneses 2007; Wonghongkul 2008), the WHO 5‐item well‐being questionnaire (WHO‐5) (Hoffman 2012) and a study‐specific instrument that was designed by the author (Cho 2006). Amongst the studies using the FACT instrument, four studies used only breast cancer‐specific versions including the FACT‐B and FACT‐ES, (Dirksen 2008; Hoffman 2012; Sherman 2010; Swisher 2015) and four studies used both generic and cancer‐specific versions (Lahart 2016; Rogers 2009b; Rogers 2009c; Rogers 2015). In the studies using the EORTC instrument, five (Ergun 2013; Kim 2011; Matthews 2014; Savard 2005; Spahn 2013) used only the generic EORTC QLQ‐C30 instrument while one study (Eyigor 2010) used both the generic (EORTC QLQ‐C30) and disease‐specific (EORTC BR23) versions of the instrument.
Secondary outcomes
Symptom Severity and Distress
Ten studies assessed anxiety using the State‐Trait Anxiety Inventory (STAI) (Dirksen 2008; Heidrich 2009), Profile of Mood States (POMS) (Fillion 2008), Brief Symptom Inventory (BSI‐18) (Fiorentino 2008) and Hospital Anxiety and Depression Scale (HADS) (Duijts 2012; Galantino 2010; Kim 2011; Matthews 2014; Savard 2005; Spahn 2013).
Fourteen studies assessed depression using the Center for Epidemiologic Studies‐Depression Scale (CES‐D) (Dirksen 2008; Fiorentino 2008; Heidrich 2009; Lengacher 2009), Beck Depression Inventory (BDI) (Ergun 2013; Eyigor 2010; McClure 2010), Profile of Mood States (Fillion 2008) and Hospital Anxiety and Depression Scale (HADS) (Duijts 2012; Galantino 2010; Kim 2011; Matthews 2014; Savard 2005; Spahn 2013).
Two studies (Dirksen 2008; Lengacher 2009) reported participants' depressive symptoms measured by Center for Epidemiological Studies Depression Scale (CES‐D)
Ten studies assessed fatigue using the Profile of Mood States Fatigue/Inertia Subscale (PONSF/I) (Dirksen 2008), the Brief Fatigue Inventory (BFI) (Ergun 2013; Eyigor 2010; Kim 2011), Multidimensional Fatigue Inventory (MFI) (Fiorentino 2008; Fillion 2008; Savard 2005; Spahn 2013), Piper Fatigue Scale (PFS) (Matthews 2014) and the German Fatigue Assessment Questionnaire (GEAQ) (Spahn 2013).
Seven studies assessed sleep‐related outcomes: four studies assessed insomnia, using the Insomnia Severity Index (ISI) (Dirksen 2008; Fiorentino 2008; Matthews 2014; Savard 2005) and three studies assessed sleep dysfunction using the Pittsburgh Sleep Quality Index (PSQI) (Fiorentino 2008; Rogers 2009b; Rogers 2009c).
Four studies assessed endocrine symptoms: two studies used the endocrine subscale of the Functional Assessment of Cancer Therapy (FACT‐ES) (Duijts 2012) and the Greene Climacteric Scale (GCS) (Fiorentino 2008). Two studies assessed hot flushes and night sweats using the Hot Flush Rating Scale (HFRS) (Duijts 2012; Mann 2012).
Two studies assessed symptom distress using the Symptom Bother‐Revised Scale (SB‐R) (Heidrich 2009) and M.D. Anderson Symptom Inventory (MDASI) (Lengacher 2009). However, Heidrich 2009 only reported the Symptom Distress score for the intervention group and not the control group, therefore we could not determine the combined MD.
Two studies assessed joint pain, stiffness and physical function using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) (Rogers 2009b; Rogers 2009c). As these two studies were multiple reports of the same study, we did not combine the results in a meta‐analysis.
Adherence
Eight studies assessed adherence (Duijts 2012; Kim 2011; Lengacher 2009; Mann 2012; McClure 2010; Rogers 2009c; Sherman 2010; Spahn 2013), which they measured using a variety of methods such as attendance records (Duijts 2012; Lengacher 2009; Mann 2012; Sherman 2010), number or intensity of training sessions (Duijts 2012; Lengacher 2009; Mann 2012; McClure 2010; Spahn 2013) and level of goal achievement (Kim 2011). Rogers 2009c did not describe the method of adherence assessment. Due to the variations in assessment methods of adherence across the various studies, we could not combine the results. Sherman 2010 and Spahn 2013 measured the level of participation in the intervention, while Kim 2011 measured the level of achievement of goals for the prescribed intervention at the end of the study. Duijts 2012, Lengacher 2009 and Mann 2012 measured attendance at intervention sessions and completion of homework assigned. McClure 2010 utilised an internally generated questionnaire, which asked the number of times each participant practised and used the intervention. Due to the limited data reporting and variations in data reporting, we did not perform meta‐analyses.
Excluded studies
During the screening of full‐text articles, we excluded 140 studies for various reasons. We excluded 56 studies as there was no home‐based component in the intervention and 35 studies because the intervention was not multi‐dimensional. Twelve studies did not have a control group; ten studies did not use a trained person or healthcare professional to deliver the intervention; nine studies did not perform a separate analysis for breast cancer survivors; eight studies did not perform quality‐of‐life assessments; three studies did not have an interventional component; three studies were not performed on breast cancer survivors; and two studies did not describe the intervention administered. We also excluded two abstracts as we were able to retrieve the full, published papers. See Characteristics of excluded studies table and Figure 1.
Risk of bias in included studies
Refer to Figure 2.
2.
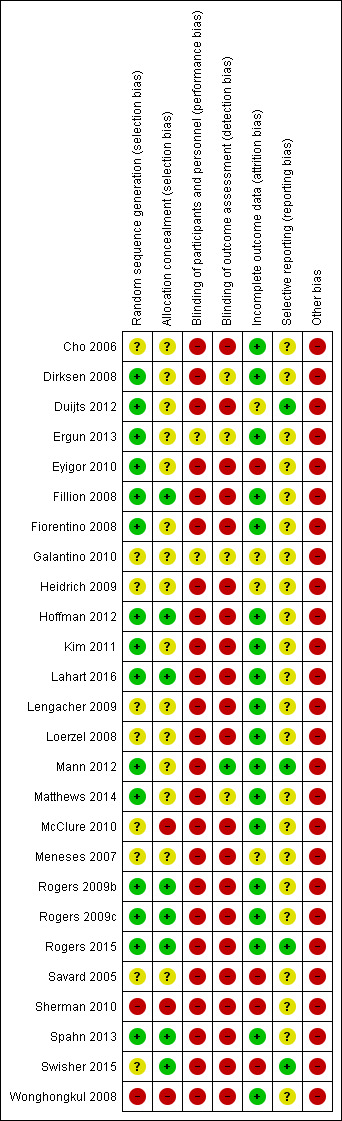
Risk of bias summary
Allocation
Random sequence generation
Fifteen studies stated clearly the methods used for random sequence generation (Dirksen 2008; Duijts 2012; Ergun 2013; Eyigor 2010; Fillion 2008; Fiorentino 2008; Hoffman 2012; Kim 2011; Lahart 2016; Mann 2012; Matthews 2014; Rogers 2009b; Rogers 2009c; Rogers 2015; Spahn 2013) therefore we judged the risk for selection bias as being low. We could not make a judgment of bias for nine studies due to the lack of information provided, and we gave them an unclear risk of bias rating (Cho 2006; Galantino 2010; Heidrich 2009; Lengacher 2009; Loerzel 2008; McClure 2010; Meneses 2007; Savard 2005; Swisher 2015). We allocated Sherman 2010 and Wonghongkul 2008 a high risk of selection bias due to the lack of random sequence generation of intervention and comparison groups.
Allocation concealment
We judged eight studies to have a low risk of selection bias resulting from allocation concealment (Fillion 2008; Hoffman 2012; Lahart 2016; Rogers 2009b; Rogers 2009c; Rogers 2015; Spahn 2013; Swisher 2015). However, this risk was elevated in Sherman 2010 and Wonghongkul 2008 due to the lack of randomisation. McClure 2010 performed randomisation in groups of eight with four participants allocated to the HBMS group and four to the comparison group. As a result, the same personnel who performed screening on the participants and informed them of group status could have guessed the group allocation for the next participants, so we judged the risk of selection bias to be high. We could not judge the presence of selection bias resulting from allocation concealment in 15 studies (Cho 2006; Dirksen 2008; Duijts 2012; Ergun 2013; Eyigor 2010; Fiorentino 2008; Galantino 2010; Heidrich 2009; Kim 2011; Lengacher 2009; Loerzel 2008; Mann 2012; Matthews 2014; Meneses 2007; Savard 2005) and so rated them 'unclear'.
Blinding
Blinding of participants and personnel
Blinding of participants and personnel was not achievable in 24 of the included studies due to the nature of the intervention, which made it difficult to blind the participants and personnel from the allocated groups (Cho 2006; Dirksen 2008; Duijts 2012; Eyigor 2010; Fillion 2008; Fiorentino 2008; Heidrich 2009; Hoffman 2012; Kim 2011; Lahart 2016; Lengacher 2009; Loerzel 2008; Mann 2012; Matthews 2014; McClure 2010; Meneses 2007; Rogers 2009b; Rogers 2009c; Rogers 2015; Savard 2005; Sherman 2010; Spahn 2013; Swisher 2015; Wonghongkul 2008). None of the included studies achieved a low risk of bias due to proper blinding of participants and personnel. The remaining two studies we judged as having an unclear risk of bias due to providing inadequate information (Ergun 2013; Galantino 2010).
Blinding of outcome assessors
One study blinded outcome assessors (Mann 2012) and so corresponded to a low risk of detection bias. Twenty‐one studies did not blind outcome assessors (Cho 2006; Duijts 2012; Eyigor 2010; Fillion 2008; Fiorentino 2008; Heidrich 2009; Hoffman 2012; Kim 2011; Lahart 2016; Lengacher 2009; Loerzel 2008; McClure 2010; Meneses 2007; Rogers 2009b; Rogers 2009c; Rogers 2015; Savard 2005; Sherman 2010; Spahn 2013; Swisher 2015; Wonghongkul 2008), and we could not make a judgment based on the information available in the remaining four studies (Dirksen 2008; Ergun 2013; Galantino 2010; Matthews 2014).
Incomplete outcome data
Incomplete outcome data contributed to a low risk of bias in 18 studies because withdrawals from the study groups appeared to be well‐balanced and unlikely to lead to attrition bias (Cho 2006; Dirksen 2008; Ergun 2013; Fillion 2008; Fiorentino 2008; Hoffman 2012; Kim 2011; Lahart 2016; Lengacher 2009; Loerzel 2008; Mann 2012; Matthews 2014; McClure 2010; Rogers 2009b; Rogers 2009c; Rogers 2015; Spahn 2013; Wonghongkul 2008). However, we judged four studies as having a high risk of attrition bias as a result of imbalanced attrition between the study groups (Eyigor 2010; Savard 2005; Sherman 2010; Swisher 2015). Four studies did not report enough information to allow a judgement (Duijts 2012; Galantino 2010; Heidrich 2009; ).
Selective reporting
The study protocols were available for four studies (Duijts 2012; Mann 2012; Rogers 2015; Swisher 2015) so we allocated low risk of reporting bias to these four studies that reported their outcomes as planned. Protocols were not available for the remaining 22 studies and so we could not make a judgment (Cho 2006; Dirksen 2008; Ergun 2013; Eyigor 2010; Fillion 2008; Fiorentino 2008; Galantino 2010; Heidrich 2009; Hoffman 2012; Kim 2011; Lahart 2016; Lengacher 2009; Loerzel 2008; Matthews 2014; McClure 2010; Meneses 2007; Rogers 2009b; Rogers 2009c; Savard 2005; Sherman 2010; Spahn 2013; Wonghongkul 2008).
Other potential sources of bias
We judged all the included studies as having a high risk of other biases due to the use of convenience sampling, where the study participants were recruited because of their convenient accessibility to the researcher. Bias might exist in the selection of the study sample (Cho 2006; Dirksen 2008; Duijts 2012; Ergun 2013; Eyigor 2010; Fillion 2008; Fiorentino 2008; Galantino 2010; Heidrich 2009; Hoffman 2012; Kim 2011; Lahart 2016; Lengacher 2009; Loerzel 2008; Mann 2012; Matthews 2014; McClure 2010; Meneses 2007; Rogers 2009b; Rogers 2009c; Rogers 2015; Savard 2005; Sherman 2010; Spahn 2013; Swisher 2015; Wonghongkul 2008).
Effects of interventions
See: Table 1
Primary outcome
Quality of life
Functional Assessment of Cancer Therapy ‐ Breast (FACT‐B)
Four studies (Dirksen 2008; Rogers 2009b; Rogers 2015; Lahart 2016) reported general scores post intervention. The pooled MD between the HBMS and control groups was 1.47 (95% CI ‐3.03 to 5.98; 399 participants; I2 = 51%) (Figure 3), indicating that there was no significant difference in general scores between the two groups. Rogers 2015 reported general scores at three months after the intervention but the difference was not significant (MD 3.30, 95% CI ‐0.77 to 7.37) (Analysis 1.1).
3.
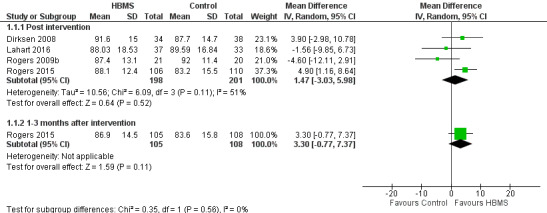
Forest plot of comparison 1, quality of life by Functional Assessment of Cancer Therapy‐Breast (FACT‐B), outcome: 1.1 General
1.1. Analysis.
Comparison 1 Quality of life by FACT‐B, Outcome 1 General.
Seven studies (Dirksen 2008; Hoffman 2012; Lahart 2016; Rogers 2009b; Rogers 2015; Sherman 2010; Swisher 2015) reported breast cancer scores post intervention. A significant pooled MD between HBMS and the control group was observed (MD 4.55, 95% CI 2.33 to 6.78; 764 participants; I2 = 17%; moderate‐quality evidence), demonstrating a significantly higher breast cancer score in the HBMS group (Analysis 1.2; Figure 4; Figure 5). Two studies (Hoffman 2012; Rogers 2015) reported the scores at one to three months post intervention and the pooled MD was also statistically significant (MD 6.10, 95% CI 2.48 to 9.72; 426 participants; I2 = 0%).
1.2. Analysis.
Comparison 1 Quality of life by FACT‐B, Outcome 2 Breast cancer.
4.
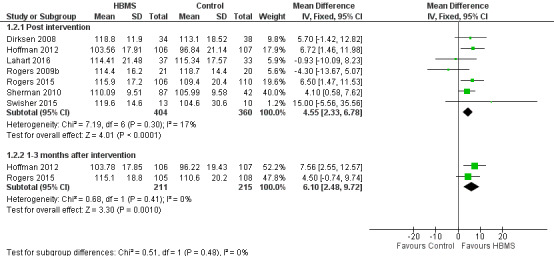
Forest plot of comparison 1, quality of life by Functional Assessment of Cancer Therapy‐Breast (FACT‐B), outcome: 1.2 Breast cancer
5.
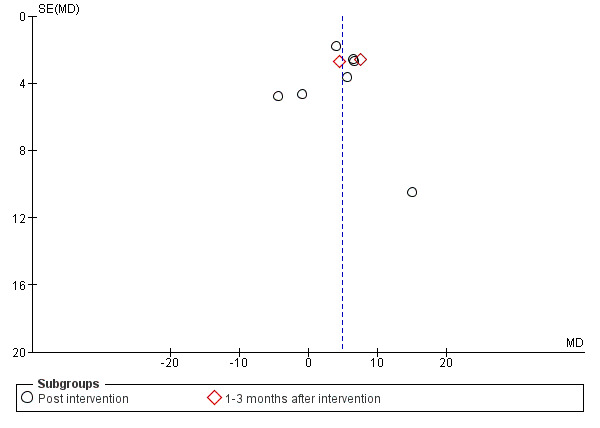
Funnel plot of comparison 1, quality of life by Functional Assessment of Cancer Therapy‐Breast (FACT‐B), outcome: 1.2 Breast cancer
The subscale results for physical well‐being, social well‐being, emotional well‐being, functional well‐being, endocrine subscale and Trial Outcome Index are provided in Table 2.
1. Quality‐of‐life subscale results.
| Questionnaires | HBMS group vs comparison group (MD, 95% CI) | |
| Assessed: immediately post intervention | Assessed: 1 to 3 months post intervention | |
| FACT‐B | ||
| Physical well‐being | MD 0.81a, 95% CI 0.04 to 1.58
764 participants 7 studies (Dirksen 2008; Hoffman 2012; Lahart 2016; Rogers 2009b; Rogers 2015; Sherman 2010; Swisher 2015) I2 = 39% Analysis 1.3 |
MD 1.25b, 95% CI 0.35 to 2.15 426 participants 2 studies (Hoffman 2012; Rogers 2015) I2 = 0% Analysis 1.3 |
| Social well‐being | MD 0.28, 95% CI ‐0.49 to 1.04 762 participants 7 studies (Dirksen 2008; Hoffman 2012; Lahart 2016; Rogers 2009b; Rogers 2015; Sherman 2010; Swisher 2015) I2 = 0% Analysis 1.4 |
MD 0.15, 95% CI ‐0.93 to 1.24 424 participants 2 studies (Hoffman 2012; Rogers 2015) I2 = 0% Analysis 1.4 |
| Emotional well‐being | MD 0.41, 95% CI ‐0.25 to 1.07 762 participants 7 studies (Dirksen 2008; Hoffman 2012; Lahart 2016; Rogers 2009b; Rogers 2015; Sherman 2010; Swisher 2015) I2 = 36% Analysis 1.5 |
MD 1.38, 95% CI ‐0.40 to 3.15 424 participants 2 studies (Hoffman 2012; Rogers 2015) I2 = 84% Analysis 1.5 |
| Functional well‐being | MD 0.97, 95% CI ‐0.33 to 2.28 740 participants 6 studies (Dirksen 2008; Hoffman 2012; Lahart 2016; Rogers 2009b; Rogers 2015; Sherman 2010) I2 = 72% Analysis 1.6 |
MD 1.46b, 95% CI 0.37 to 2.56 425 participants 2 studies (Hoffman 2012; Rogers 2015) I2 = 13% Analysis 1.6 |
| Endocrine symptoms | SMD 0.28b, 95% CI 0.09 to 0.47 421 participants 2 studies (Duijts 2012; Hoffman 2012) I2 = 0% Analysis 1.7 |
SMD 0.26b, 95% CI 0.07 to 0.46 421 participants 2 studies (Duijts 2012; Hoffman 2012) I2 = 34% Analysis 1.7 |
| Trial Outcome Index | MD 4.36b, 95% CI 2.20 to 6.53 345 participants 2 studies (Rogers 2015; Sherman 2010) I2 = 0% Analysis 1.8 |
MD 3.60a, 95% CI 0.01 to 7.19 213 participants 1 study (Rogers 2015) Analysis 1.8 |
| EORTC QLQ | ||
| Functional | MD 3.16a, 95% CI 0.09 to 6.22 127 participants 3 studies (Ergun 2013; Eyigor 2010; Kim 2011) I2 = 0% Analysis 2.2 |
Data were not available |
| Symptom | MD 2.53, 95% CI ‐5.56 to 10.61 82 participants 2 studies (Ergun 2013; Eyigor 2010) I2 = 56% Analysis 2.3 |
Data were not available |
| Role | MD 3.18, 95% CI ‐4.57 to 10.93 100 participants 2 studies (Kim 2011; Spahn 2013) I2 = 0% Analysis 2.4 |
MD 0.40, 95% CI ‐12.60 to 13.40 55 participants 1 study (Spahn 2013) Analysis 2.4 |
| Emotion | MD 12.22b, 95% CI 6.07 to 18.37 100 participants 2 studies (Kim 2011; Spahn 2013) I2 = 0% Analysis 2.5 |
MD 6.40, 95% CI ‐7.12 to 19.92 55 participants 1 study (Spahn 2013) Analysis 2.5 |
| Cognitive | MD 0.98, 95% CI ‐5.27 to 7.23 100 participants 2 studies (Kim 2011; Spahn 2013) I2 = 0% Analysis 2.6 |
MD ‐0.10, 95% CI ‐14.01 to 13.81 55 participants 1 study (Spahn 2013) Analysis 2.6 |
| Social | MD 1.57, 95% CI ‐6.98 to 10.13 100 participants 2 studies (Kim 2011; Spahn 2013) I2 = 0% Analysis 2.7 |
MD ‐3.90, 95% CI ‐19.01 to 11.21 55 participants 1 study (Spahn 2013) Analysis 2.7 |
| Physical Function | MD 3.29, 95% CI ‐2.80 to 9.38 115 participants 2 studies (Matthews 2014; Spahn 2013) I2 = 0% Analysis 2.8 |
MD 14.05b, 95% CI 4.00 to 24.10 107 participants 2 studies (Matthews 2014; Spahn 2013) I2 = 80% Analysis 2.8 |
| QOL ‐ BC | ||
| Physical | MD ‐0.06, 95% CI ‐0.65 to 0.53 50 participants 1 study (Loerzel 2008) Analysis 3.2 |
Data were not available |
CI: confidence interval; EORTC QLQ: European Organisation for Research and Treatment of Cancer ‐ General; FACT‐B Functional Assessment of Cancer Therapy ‐ Breast cancer; HBMS: home‐based multidimensional survivorship; MD: mean difference; QOL ‐ BC Quality of Life ‐ Breast Cancer Overall aSignificant results, P < 0.05 bSignificant results, P < 0.01
EORTC‐QLQ
Six studies (Ergun 2013; Eyigor 2010; Kim 2011; Matthews 2014; Savard 2005; Spahn 2013) reported the global scores post intervention. The pooled MD was statistically significant (MD 4.38, 95% CI 0.11 to 8.64; 299 participants; I2 = 30%; moderate‐quality evidence) implying that the EORTC‐QLQ global scores for women in the HBMS group were significantly higher than women in the control group by 4.38 units on average (Analysis 2.1; Figure 6). Three studies (Matthews 2014; Savard 2005; Spahn 2013) reported the global scores at one to three months post intervention and the pooled MD was significant (MD 6.32, 95% CI 0.61 to 12.04; 172 participants; I2 = 64%). Two studies (Matthews 2014; Savard 2005) reported scores at four to six months post intervention and the pooled MD was insignificant (MD 0.08, 95% CI ‐7.28 to 7.44; 117 participants; I2 = 0%).
2.1. Analysis.
Comparison 2 Quality of life measured by EORTC‐QLQ, Outcome 1 Global.
6.
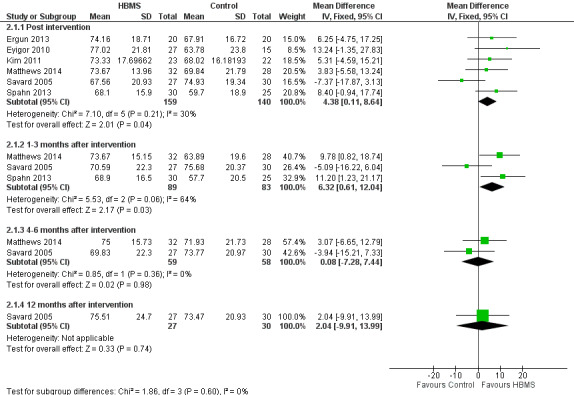
Forest plot of comparison 2, quality of life measured by European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC‐QLQ), outcome: 2.1 General
The subscale results for the functional, symptom, role, emotional, cognitive, social and physical function scores are provided in Table 2.
Quality of Life ‐ Breast Cancer
Three studies (Loerzel 2008; Meneses 2007; Wonghongkul 2008) reported the overall scores post intervention but the data were incomplete in one study (Meneses 2007). The pooled MD post intervention was 0.42 (MD 0.42, 95% CI ‐0.02 to 0.85; 111 participants; I2 = 74%; 2 studies; very low‐quality evidence) (Analysis 3.1). Two studies (Meneses 2007; Wonghongkul 2008) reported the overall scores at one to three months post intervention but the data for Meneses 2007 were incomplete; the MD of Wonghongkul 2008 was 0.45 (MD 0.45, 95% CI ‐0.19 to 1.09).
3.1. Analysis.
Comparison 3 Quality of life measured by Quality of Life‐Breast Cancer, Outcome 1 Overall.
The subscale results for the physical scores are provided in Table 2.
SF‐36
Three studies (Duijts 2012; Mann 2012; McClure 2010) reported SF‐36 physical component scores post intervention but complete data were not available for one study (McClure 2010). The pooled effect was statistically significant with a MD of 0.55 (95% CI ‐3.52 to 4.63; 308 participants; 2 studies; I2 = 7%; low‐quality evidence) (Analysis 4.1). Two studies reported the scores at four to six months post intervention and the pooled effect was MD ‐1.05 (95% CI ‐5.60 to 3.51; 308 participants; I2 = 0%).
4.1. Analysis.
Comparison 4 Quality of life measured by SF‐36, Outcome 1 Physical function.
Secondary outcomes
Anxiety
Seven studies (Dirksen 2008; Lengacher 2009; Galantino 2010; Kim 2011; Matthews 2014; Savard 2005; Spahn 2013) reported anxiety post intervention: two studies used the State‐Trait Anxiety Inventory (STAI) and five studies used Hospital Anxiety and Depression Scale (HADS). The pooled MD for STAI was ‐4.70 (95% CI ‐7.88 to ‐1.52; 154 participants; 2 studies) and HADS was ‐1.01 (95% CI ‐1.94 to ‐0.08; 253 participants; 5 studies; I2 = 53%; low‐quality evidence) (Analysis 5.1). The anxiety score in the HBMS group was significantly lower regardless of the instrument used. If we used the SMD to combine the results from the seven studies, the pooled SMD would be ‐0.34 (95% CI ‐0.55 to ‐0.14; 7 studies; I2 = 34%). Two studies (Matthews 2014; Savard 2005) reported scores on HADS at one to three months post intervention and the pooled MD was ‐0.68 (95% CI ‐2.04 to 0.68; 113 participants; I2 = 25%).
5.1. Analysis.
Comparison 5 Anxiety, Outcome 1 Anxiety.
Depression
Nine studies (Dirksen 2008; Ergun 2013; Eyigor 2010; Kim 2011; Lengacher 2009; Matthews 2014; McClure 2010; Savard 2005; Spahn 2013) reported participants' depression post intervention, where three of them used the Beck Depression Inventory (BDI) (Ergun 2013; Eyigor 2010; McClure 2010), two used the STAI (Dirksen 2008; Lengacher 2009), and four used the HADS (Kim 2011; Matthews 2014; Savard 2005; Spahn 2013). The pooled MD of BDI, STAI and HADS were ‐0.61 (95% CI ‐3.16 to 1.94), ‐3.29 (95% CI ‐5.82 to ‐0.77) and ‐1.36 (95% CI ‐2.94 to 0.22; 213 participants; low‐quality evidence). Matthews 2014, Savard 2005 and Spahn 2013 also reported the anxiety score at one to three months post intervention but the pooled MD was not statistically significant (MD ‐0.74, 95% CI ‐2.71 to 1.22) (Analysis 6.1).
6.1. Analysis.
Comparison 6 Depression, Outcome 1 Depression.
Depressive symptoms
Two studies (Dirksen 2008; Lengacher 2009) reported participants' depressive symptoms post intervention. Both studies showed lower symptom scores in the HBMS group but only one study (Lengacher 2009) was significant. The pooled MD was statistically significant (MD ‐2.61, 95% CI ‐4.93 to ‐0.29; 154 participants; I2 = 0%) (Analysis 10.1).
10.1. Analysis.
Comparison 10 Depressive symptoms measured by Center for Epidemiologic Studies‐Depression Scale (CES‐D), Outcome 1 Depressive symptoms.
Fatigue
Five studies (Ergun 2013; Eyigor 2010; Fillion 2008; Kim 2011; Savard 2005) reported fatigue post intervention: two studies used the Multidimensional Fatigue Inventory (MFI) (Fillion 2008; Savard 2005) and three studies used the used the Brief Fatigue Inventory (BFI) (Ergun 2013; Eyigor 2010; Kim 2011). The pooled MD of BFI was ‐1.11 (95% CI ‐1.78 to ‐0.45; 127 participants; I2 = 0%; low‐quality evidence) while the pooled MD of MFI was ‐0.04 (95% CI ‐0.69 to 0.62; 144 participants; I2 = 32%). Savard 2005 reported the MD of MFI scores at one to three and four to six months post intervention but none of them were statistically significant (Analysis 7.1).
7.1. Analysis.
Comparison 7 Fatigue, Outcome 1 Fatigue.
Insomnia
Three studies (Dirksen 2008; Matthews 2014; Savard 2005) reported the severity of insomnia measured by the Insomnia Severity Index (ISI) post intervention. The pooled MD was ‐1.18 (95% CI ‐3.34 to ‐0.27; 185 participants; I2 = 0%) (Analysis 8.1). Matthews 2014 and Savard 2005 reported the MD of ISI scores at one to three months post intervention and the pooled MD was significant (MD ‐2.27, 95% CI ‐4.22 to ‐0.33; 113 participants; I2 = 46%).
8.1. Analysis.
Comparison 8 Insomnia measured by Insomnia Severity Index (ISI) 3 months, Outcome 1 ISI.
Night Flushes and night sweats
Two studies (Duijts 2012; Mann 2012) reported flushes and night‐sweat symptoms post intervention. The pooled MD was ‐1.50 (95% CI ‐3.75 to 0.75; 216 participants; I2 = 0%) (Analysis 9.1). Both studies presented the long‐term follow‐up results but none was significant.
9.1. Analysis.
Comparison 9 Flushes and night sweats measured by Hot Flushes and Night Sweats Frequency Rating (HFRS), Outcome 1 HFRS.
Service needs and utilisation
None of the included studies reported data on these outcomes.
Participants' adherence to the programme interventions
Eight studies assessed participants’ adherence to the programme interventions (Duijts 2012; Kim 2011; Lengacher 2009; Mann 2012; McClure 2010; Rogers 2009c; Sherman 2010; Spahn 2013). Due to the variations in assessment methods of adherence among the eight studies, we could not combine the results for meta‐analysis. We report the results narratively here. Adherence rates varied across all eight studies and ranged from 58% to 100%. Six studies (Kim 2011; Lengacher 2009; Mann 2012; Rogers 2009c; Sherman 2010; Spahn 2013) reported adherence rates for programme interventions of more than 80%. Of these, four studies (Kim 2011; Mann 2012; Rogers 2009c; Spahn 2013) reported adherence rates of more than 90%. McClure 2010 reported adherence for programme interventions as good to excellent. Duijts 2012 reported adherence rates of 58% to 70% for the three programme intervention groups.
Subgroup analysis
We conducted two sets of subgroup analyses, the first one by the component of the intervention and the second by the mode (delivery structure) of the intervention. Since none of the included studies provided stratified analysis according to cancer treatment modality and survivorship period, we were unable to perform subgroup analyses as per the review protocol.
Components of the intervention
We divided the components of the intervention into four groups: educational and psychological components; educational and physical components; physical and psychological components; and educational, physical and psychological components. We performed subgroup analyses only for those meta‐analyses with four or more studies, and on quality of life outcomes.
FACT‐Breast Cancer
Educational and psychological components
There was a higher mean FACT‐B Breast Cancer Specific score in the HBMS group than in the control group (MD 5.08, 95% CI 1.24 to 8.92; 355 participants; 3 studies; I2 = 3%) (Analysis 11.1).
11.1. Analysis.
Comparison 11 Subgroup analyses: quality of life by FACT‐B ‐ components of intervention, Outcome 1 Breast cancer (post intervention).
Educational and physical components
There was a higher score in the HBMS group than in the control group (MD 4.10, 95% CI 0.58 to 7.62; 129 participants; 1 study) (Analysis 11.1).
Physical and psychological components
There was a higher score in the HBMS group than in the control group (MD 4.57, 95% CI 0.24 to 8.90; 280 participants; 3 studies; I2 = 60%) (Analysis 11.1).
We detected no significant difference in quality of life among the three groups (Chi2 = 0.14, P = 0.93) (Analysis 11.1). Refer to Table 3 for subgroup analysis results for physical, social, emotional and functional well‐being.
2. Subgroup analyses: quality‐of‐life subscale results by component.
| Questionnaires |
Educational and psychological group (group I) HBMS group vs comparison group |
Eductional and physical group (group 2) HBMS group vs comparison group |
Physical and psychological group (group 3) HBMS group vs comparison group |
Difference among subgroups |
| FACT‐B | ||||
| Physical well‐being | Insignificantly higher score in HBMS group MD 0.79, 95% CI ‐0.14 to 1.72 355 participants 3 studies (Dirksen 2008; Hoffman 2012; Lahart 2016) I2 = 0% Analysis 11.2 |
Significantly higher score in HBMS group MD 1.14, 95% CI 0.11 to 2.17b 129 participants 1 study (Sherman 2010) Analysis 11.2 |
Insignificantly lower score in HBMS group MD 0.45, 95% CI ‐1.85 to 2.74 280 participants 3 studies (Rogers 2009b; Rogers 2015; Swisher 2015) I2 = 78% Analysis 11.2 |
No significant difference (Chi2 = 0.41, P = 0.81) Analysis 11.2 |
| Social well‐being | MD 0.38, 95% CI ‐0.73 to 1.48 353 participants 3 studies (Dirksen 2008; Hoffman 2012; Lahart 2016) I2 = 15% Analysis 11.3 |
MD 0.00, 95% CI ‐2.04 to 2.04 129 participants 1 study (Sherman 2010) Analysis 11.3 |
MD 0.25, 95% CI ‐1.00 to 1.50 280 participants 3 studies (Rogers 2015; Rogers 2009b; Swisher 2015) I2 = 0% Analysis 11.3 |
No significant difference (Chi2 = 0.11, P = 0.95) Analysis 11.3 |
| Emotional well‐being | MD 0.51, 95% CI ‐0.77 to 1.80 353 participants 3 studies (Dirksen 2008; Hoffman 2012; Lahart 2016) I2 = 59% Analysis 11.4 |
MD 0.27, 95% CI ‐0.95 to 1.49 129 participants 1 study (Sherman 2010) Analysis 11.4 |
MD 0.07, 95% CI ‐1.37 to 1.51 280 participants 3 studies (Rogers 2015; Rogers 2009b; Swisher 2015) I2 = 52% Analysis 11.4 |
No significant difference (Chi2 = 0.21, P = 0.90) Analysis 11.4 |
| Functional well‐being | MD 0.89, 95% CI ‐0.83 to 2.61 354 participants 3 studies (Dirksen 2008; Hoffman 2012; Lahart 2016) I2 = 60% Analysis 11.5 |
MD 2.42, 95% CI 1.17 to 3.67b 129 participants 1 study (Sherman 2010) Analysis 11.5 |
MD ‐0.07, 95% CI ‐4.37 to 4.23 257 participants 2 studies (Rogers 2009b; Rogers 2015) I2 = 89% Analysis 11.5 |
No significant difference (Chi2 = 2.74, P = 0.25) Analysis 11.5 |
CI: confidence interval; FACT‐B Functional Assessment of Cancer Therapy ‐ Breast cancer; HBMS: home‐based multidimensional survivorship; MD: mean difference aSignificant results, P < 0.05 bSignificant results, P < 0.01
EORTC‐QLQ
Educational and psychological components
There was a lower score in the HBMS group than in the control group (MD ‐1.16, 95% CI ‐8.17 to 5.85; 117 participants; 2 studies; I2 = 59%) (Analysis 12.1).
12.1. Analysis.
Comparison 12 Sub‐group analysis: quality of life by EORTC‐QLQ ‐ components of intervention, Outcome 1 Global (post intervention).
Educational and physical components
There was a higher mean EORTC‐QLQ global score in the HBMS group than in the control group (MD 7.25, 95% CI 0.68 to 13.82; 127 participants; 3 studies; I2 = 0%) (Analysis 12.1).
Educational, physical and psychological components
There was an insignificantly higher score in the HBMS group than in the control group (MD 8.40, 95% CI ‐0.94 to 17.74; 55 participants; 1 study) (Analysis 12.1).
No significant difference in global quality of life was revealed among the three groups (Chi2 = 3.85, P = 0.15) (Analysis 12.1).
Mode of the intervention
We divided the mode (delivery structure) of intervention into three groups: group‐based interventions; individual‐based interventions; and both group‐ and individual‐based interventions. The mode of intervention was included as a subgroup analysis as it was one of the objectives of the review.
FACT‐Breast Cancer
Group‐based interventions
The pooled MD for group‐based interventions was 5.36 (95% CI 2.97 to 7.74; 630 participants; 4 studies) (Analysis 13.1).
13.1. Analysis.
Comparison 13 Subgroup analyses: quality of life by FACT‐B ‐ mode of intervention, Outcome 1 Breast cancer (post intervention).
Individual‐based interventions
The pooled MD for individual‐based interventions was 1.71 (95% CI ‐6.66 to 10.07; 93 participants; 2 studies; I2 = 48%) (Analysis 13.1).
Group‐ and individual‐based interventions
The MD for combined group‐ and individual‐based interventions was ‐4.30 (95% CI ‐13.67 to 5.07; 41 participants; 1 study) (Analysis 13.1).
No significant difference in quality of life was revealed among the three subgroups (Chi2 = 4.31, P = 0.12) (Analysis 13.1). Refer to Table 4 for subgroup analysis results for physical, social, emotional and functional well‐being.
3. Subgroup analyses: quality‐of‐life subscale results by mode.
| Questionnaires | Group‐based HBMS group vs comparison group | Individual‐based HBMS group vs comparison group |
Group‐ & individual‐based HBMS group vs comparison group |
Difference among subgroups |
| FACT‐B | ||||
| Physical well‐being | MD 1.15, 95% CI 0.55 to 1.75b 630 participants 4 studies (Dirksen 2008; Hoffman 2012;Rogers 2015; Sherman 2010) I2 = 0% Analysis 13.2 |
MD 1.17, 95% CI ‐0.90 to 3.24 93 participants 2 studies (Lahart 2016; Swisher 2015) I2 = 0% Analysis 13.2 |
MD ‐2.10, 95% CI ‐4.27 to 0.07 41 participants 1 study (Rogers 2009b) Analysis 13.2 |
Significant difference (Chi2 = 8.03, P = 0.02a) Analysis 13.2 |
| Social well‐being | MD 0.42, 95% CI ‐0.47 to 1.31 628 participants 4 studies (Dirksen 2008; Hoffman 2012;Rogers 2015; Sherman 2010) I2 = 0% Analysis 13.3 |
MD ‐0.45, 95% CI ‐2.20 to 1.30 93 participants 2 studies (Lahart 2016; Swisher 2015) I2 = 0% Analysis 13.3 |
MD 0.80, 95% CI ‐2.25 to 3.85 41 participants 1 study (Rogers 2009b) Analysis 13.3 |
No significant difference (Chi2 = 0.88, P = 0.64) Analysis 13.3 |
| Emotional well‐being | MD 0.84, 95% CI 0.28 to 1.39 628 participants 4 studies (Dirksen 2008; Hoffman 2012;Rogers 2015; Sherman 2010) I2 = 4% Analysis 13.4 |
MD ‐0.56, 95% CI ‐2.08 to 0.96 93 participants 2 studies (Lahart 2016; Swisher 2015) I2 = 0% Analysis 13.4 |
MD ‐1.10, 95% CI ‐2.94 to 0.74 41 participants 1 study (Rogers 2009b) Analysis 13.4 |
Significant difference (Chi2 = 6.16, P = 0.049a) Analysis 13.4 |
| Functional well‐being | MD 2.05, 95% CI 1.32 to 2.79b 629 participants 4 studies (Dirksen 2008; Hoffman 2012;Rogers 2015; Sherman 2010) I2 = 0% Analysis 13.5 |
MD ‐0.93, 95% CI ‐3.07 to 1.21 70 participants 1 study (Lahart 2016) Analysis 13.5 |
MD ‐2.40, 95% CI ‐4.89 to 0.09 41 participants 1 study (Rogers 2009b) Analysis 13.5 |
Significant difference (Chi2 = 16.56, P < 0.01b) Analysis 13.5 |
CI: confidence interval; FACT‐B Functional Assessment of Cancer Therapy ‐ Breast cancer; HBMS: home‐based multidimensional survivorship; MD: mean difference aSignificant results, P < 0.05 bSignificant results, P < 0.01
EORTC‐QLQ
Group‐based interventions
The pooled MD of the four studies with group‐based interventions was 4.28 (95% CI ‐1.19 to 9.74; 194 participants; 4 studies; I2 = 57%) (Analysis 14.1).
14.1. Analysis.
Comparison 14 Subgroup analysis: quality of life by EORTC QLQ ‐ mode of intervention, Outcome 1 Global (post intervention).
Individual‐based interventions
The pooled MD for the individual‐based interventions was 4.53 (95% CI ‐2.29 to 11.35; 105 participants; 2 studies; I2 = 0%) (Analysis 14.1).
No significant difference was revealed between the two groups (P = 0.95).
As Chapter 20 of Borenstein 2009 suggests at least 10 studies for each covariate in meta‐regression, and our meta‐analyses contained a smaller number than recommended, we did not conduct such analyses. We did not conduct sensitivity analyses because most studies had high risk of bias and none of the studies in the meta‐analyses were from grey literature.
Discussion
Summary of main results
This systematic review included 26 studies involving women who had been diagnosed with stage 0 to 3 breast cancer and had completed surgery, adjuvant chemotherapy or radiotherapy (or a combination or all of these) within the last 10 years. The aims of the home‐based multidimensional survivorship (HBMS) programmes in the included studies were mixed: some focused on reducing a multitude of long‐term or late symptoms associated with breast cancer and its treatment (e.g. fatigue, sleep problems, lymphoedema, hot flushes and night sweats, psychological distress) while some focused on improving health or psychosocial well‐being, or both. The HBMS programmes included symptom management, cognitive behavioural therapy, counselling, exercise, and/or wellness activities. All programmes were directed towards improving quality of life for women within their first 10 years after breast cancer treatment.
The results of this review showed beneficial effects of HBMS programmes for some measures of quality of life. For quality of life measured by FACT‐B, there was a significant improvement in breast cancer‐specific, physical and endocrine domains of quality of life post intervention and at one to three months post intervention. Similarly, for quality of life measured by the EORTC‐C30, there was a beneficial effect of HBMS programmes in global, functional and emotional domains of quality of life immediately and at one to three months post intervention. The quality of evidence across studies was moderate. However, no improvement in quality of life was shown either at four to six months nor at 12 months post intervention. The results suggested that HBMS programmes for women with breast cancer post treatment with surgery and/or adjuvant chemotherapy and/or radiotherapy are effective for improving quality of life and their effect persists for three months. As for the effects of HBMS programmes on quality of life subscales, physical and endocrine domains of quality of life (using FACT‐B), and functional and emotional domains of quality of life (using EORTC‐C30) showed an improvement compared to control.
QoL‐Breast Cancer and SF‐36 were relatively uncommon tools to measure quality of life in the included studies. The effect of HBMS programmes on overall quality of life or physical composite score was not demonstrated immediately post intervention or at follow‐up and we assessed the quality of evidence as very low. A possible explanation for the non‐significant finding could be due to the focus of the intervention targeted towards menopausal symptoms or lymphoedema in the studies of Duijts 2012, Mann 2012 and McClure 2010, and SF‐36 is a generic quality‐of‐life measure (Ware 2005) and thus it may lack sensitivity to detect differences in cancer populations.
Some of the included studies were designed to decrease psychological distress, fatigue and insomnia severity, that in turn lead to an increase in quality of life. Insomnia significantly reduced immediately and after one to three months after the intervention in women assigned to the HBMS programmes. We also observed a beneficial effect of HBMS programmes on anxiety immediately post intervention regardless of the measurement tools being used, and depression and fatigue immediately post intervention as measured by STAI and BFI, respectively. However, the effects could not be sustained at follow‐up.
Subgroup analysis on the different components of the survivorship programme did not show any difference in quality‐of‐life measures. In contrast, the mode of delivering HBMS programmes to women revealed a statistically significant effect on quality of life, in which group‐based HBMS interventions were effective in increasing the physical, emotional, and functional aspects of quality of life (using FACT‐B).
None of the included studies reported service needs and utilisation or cost of care, and therefore the effect of HBMS programmes on healthcare utilisation and cost is unknown. Due to the variations in assessment methods of adherence among the eight studies, we could not combine the results for meta‐analysis. The results were synthesised narratively, with the reported adherence rates of 58% to 100%.
Overall completeness and applicability of evidence
The evidence in this review is current to April 2016. Although the included studies were diverse in terms of the intervention components, delivery approach and duration of HBMS programmes and quality‐of‐life measures, it was possible to pool the results of seven studies that used FACT‐B, six studies that used EORTC‐C30, three studies that used QoL‐Breast Cancer, and three studies that used SF‐36. We included 17 out of 26 studies, involving 1482 participants, in the meta‐analyses but we were unable to include nine studies due to the unavailability of data and wide range of quality‐of‐life measures being used. Three of these studies measured quality of life with the SF‐36: Fiorentino 2008 reported mean change scores within an intervention group rather than mean score of each group; Lengacher 2009 presented the adjusted mean physical and mental composite scores of each group based on the population norm; and Heidrich 2009 did not present the mean score for quality of life. The authors of these studies concluded that there was no statistically significant difference in quality of life between groups, which was in line with the other three studies using SF‐36, whose data we were able to include in meta‐analysis. It was not possible to include Cho 2006 and Fillion 2008 in meta‐analysis since the former was the only study to use the authors’ own validated tool, while the latter was the only study to measure quality of life with SF‐12. We were unable to include Galantino 2010 in meta‐analysis since this was the only study to measure quality of life using QoL Patient/Cancer Survivors Scale and they did not report standard deviations for data pooling. In future, studies should standardise outcome measures and reporting parameters so as to reduce heterogeneity across studies and to permit robust meta‐analysis. Rogers 2009c was not used in the meta‐analyses because it used the same data as reported in Rogers 2009b but focused mainly on the change in physical activity–related outcomes. In addition, due to the unavailability of data, despite contacting the authors, Meneses 2007 and McClure 2010 were not included in the meta‐analysis.
Complex interventions have increasingly been recognised as an essential approach to address the complex needs of patients in oncology settings but they pose challenges in head‐to‐head comparisons and thus data synthesis across studies. In this review, the included studies were multi‐focused, targeting a multitude of common health problems and issues in women who were in transition from the primary cancer treatment phase into the rehabilitation and survivorship phase, which makes the survivorship programmes vary somewhat. In view of the diversity of individual survivorship programmes within the included studies, it was not possible to perform analysis of individual programmes (content and duration) that could contribute to the knowledge of exact components influencing or explaining quality of life. This limits our ability to provide evidence regarding the relative effects of different content and duration of survivorship programmes. Future studies addressing the specific content and duration of survivorship programmes in quality of life are needed to allow the possibility of data synthesis and conclusions.
The majority of the included studies had a short follow‐up, with only nine studies measuring quality of life at one to three months post intervention. The long‐term effects of HBMS programmes are unknown. In addition, the included studies did not provide data about service needs and utilisation or cost of care that could be used for data synthesis and meta‐analysis.
Quality of the evidence
We used the GRADE approach to assess the quality of evidence of the included studies (refer to Table 1). The quality of the evidence for the primary outcome of quality of life in 13 studies that used FACT‐B and EORTC‐C30 we judged as moderate, while quality of life in four studies that used QoL‐Breast Cancer and SF‐36 was low to very low. The quality of evidence for secondary outcomes of anxiety and depression, fatigue, and insomnia included in this review was low. The major contributors to the grading of moderate to very low quality of evidence include the high (92%) or unclear (8%) risk of performance bias and high (81%) or unclear (15%) risk of detection bias. Only one study (Mann 2012) used blinding of the trial statistician and researchers who collected outcome measures. Nevertheless, blinding is difficult to execute in such studies of survivorship programmes given the nature of interventions being delivered. There is also a possibility that the subjective nature of quality‐of‐life measurement would incur the 'Hawthorne' or social desirability effect in response to the participants’ own awareness of their intervention group assignments when blinding was impossible. Therefore, the limitation of this review might be the robustness of findings of included studies being compromised as a result of performance and detection bias, as well as the subjective nature of quality‐of‐life reporting.
Many of the included studies provided limited information pertaining to the criteria required to make a judgment of the risk of bias, which might also affect quality of evidence. Domains where the unclear risks of bias were common included selective outcome reporting (92%), allocation concealment (58%) and random sequence generation (35%). The majority of included studies were free of incomplete outcome data bias (69%). The withdrawals from the HBMS programme and control group seemed to be distributed evenly.
The variations in pooled estimates of the primary outcome of quality of life and secondary outcomes of fatigue and insomnia of the included studies were small (I2 = 30% or less). However, the studies that used QoL‐Breast Cancer (I2 = 74%) and HADS for anxiety (I2 = 53%) and depression (I2 = 73%) had high heterogeneity, which also contributed to the very low and low quality of evidence, respectively. In addition, data were not extractable for the planned subgroup analyses of type of cancer treatment modality and survivorship period (less than five years versus five years or more). As shown in the funnel plot, the publication bias affecting the overall quality of evidence was considered low.
There is a possibility of sampling bias associated with non‐probability sampling being employed by all of the included studies.
Taken together, this review resulted in our rating the evidence as moderate to very low quality for all primary and secondary outcomes.
Potential biases in the review process
We included 26 studies, of which 22 were RCTs and four were quasi‐RCTs (Cho 2006; Galantino 2010; Sherman 2010; Wonghongkul 2008). The selection bias associated with these four quasi‐RCTs could reduce the quality of the evidence, and increase heterogeneity in overall quality of life as measured by QoL‐Breast Cancer (Wonghongkul 2008). Nevertheless, the results of the review were robust when we excluded those quasi‐RCTs in a sensitivity analysis. We also identified eight abstracts/ongoing trials using the RCT design, however, at the time of writing, none of these studies had reported data.
The included studies used different quality‐of‐life measures and approaches to report data (e.g. group mean across each study time point versus mean change in certain time point within group), which hampered pooling of data of all included studies for a single meta‐analysis. Instead, we performed separate meta‐analyses by individual quality‐of‐life measures. This may have biased the estimation of the effect in this review.
Agreements and disagreements with other studies or reviews
We identified a systematic review of multidimensional rehabilitation programmes for adult cancer survivors (Scott 2013b). The review by Scott 2013b included 12 RCTs to examine the effectiveness of professional‐led multi‐ or uni‐dimensional rehabilitation interventions on physical and psychosocial well‐being of survivors with a broad range of cancer diagnosis (breast caner accounted for 41% of the total population of participants). The risk of bias ranged from moderate to high, and the level of heterogeneity across studies was high. The authors were only able to pool data on the SF‐36 in five studies. They found that SF‐36 physical composite score was significantly higher in participants who received multidimensional rehabilitation programmes compared to control participants (MD 2.2, 95% CI 0.12 to 4.31, P = 0.04). On the contrary, our meta‐analysis showed no effect of home‐based multidimensional survivorship programme on SF‐36 physical composite score of quality of life, neither immediately post intervention nor in follow‐up measurement (Duijts 2012;Mann 2012; McClure 2010). This discrepancy is likely to be related to the differences in the level of heterogeneity across studies between the two reviews, and mixed cancer populations in the review by Scott 2013b.
Authors' conclusions
Implications for practice.
Multimodal interventions have been recognised as an important approach in addressing the multifaceted nature of the transition process of a breast cancer survivor from treatment completion to long‐term survival. The results of this systematic review and meta‐analysis showed that home‐based, multidimensional survivorship (HBMS) programmes provide a short‐term beneficial effect by improving quality of life, and some domains of quality of life as measured by FACT‐B and EORTC‐C30 questionnaires. This review also suggested that home‐based multidimensional survivorship programmes may reduce anxiety, fatigue, and insomnia as measured by HADS, BFI and ISI, respectively. The result of this review revealed no difference in improving quality of life with educational, psychological or physical components of interventions in survivorship programmes. Group‐based intervention may be the most effective mode of intervention delivery in improving physical, emotional and functional quality of life. Nevertheless, there did not appear to be a beneficial effect of HBMS programmes three months after the intervention. The effect of HBMS programmes on healthcare service and utilisation, and cost of care is unknown. There were insufficient data to examine the variations in content or duration of the HBMS programmes.
Implications for research.
It is essential for future studies to improve the standards and rigours of conducting studies and reporting data in this field of research. Blinding is difficult to implement given the nature of interventions being delivered in HBMS programmes. Nevertheless, Mann 2012 involved blinding of the trial statistician and researchers who collected outcome measures, suggesting that at least single blinding is possible in such studies to reduce the risk of detection bias.
Because of the low number of included studies stratified by the type of cancer treatment modality and survivorship period (less than five years versus five years or more), it was not possible to perform subgroup analysis. There is a need for future studies to stratify and take account of these clinical parameters. Further high‐quality studies are also required to evaluate the long‐term effect (i.e. longer than three months) of HBMS programmes and compare different content and duration of the survivorship programmes. Additional studies are needed to evaluate the effects of HBMS programmes on unmet needs, healthcare cost, and clinical and patient outcomes. Future studies examining the mediating role of self‐efficacy, empowerment, self‐management skills and social support on behavioural change, and thus quality of life, would also help to shed some light on the path of HBMS programmes in improving quality of life of breast cancer survivors. Adverse effects or harms of HBMS programmes should also be addressed in future studies.
Acknowledgements
We would like to acknowledge the support of Cochrane Breast Cancer.
Appendices
Appendix 1. CENTRAL
1. MeSH descriptor: [Breast Neoplasms] explode all trees
2. breast near cancer*
3. breast near neoplasm*
4. breast near carcinoma*
5. breast near tumour*
6. breast near tumor*
7. #1 or #2 or #3 or #4 or #5 or #6
8. survivor*
9. #7 and #8
10. survivorship or therap* or interven* or program* or evaluat* or educat* or rehabilit* or effect* or train* or "post treatment care" or "survivorship surveillance" or "continuum of care" or "transitional care" or "home based" or "home‐based" or "self care" or "self‐care" or "self help" or "self‐help" or "self manag*" or "self‐manag*" or "multidimension*" or "multi‐dimension*"
11. #9 and #10
Appendix 2. Embase
Search strategy to 2014 (via Embase.com)
1. random* OR factorial* OR crossover* OR cross NEXT/1 over* OR placebo* OR (doubl* AND blind*) OR (singl* AND blind*) OR assign*orAND allocat* OR volunteer* OR 'crossover procedure'/exp OR 'double blind procedure'/exp OR 'randomized controlled trial'/exp OR'single blind procedure'/exp
2. 'breast cancer'/exp OR 'breast cancer'
3. 'breast neoplasm'
4. 'breast carcinoma'/exp OR 'breast carcinoma'
5. 'breast tumour'
6. 'breast tumor'/exp OR 'breast tumor'
7. 'mamma carcinoma'/exp OR 'mamma carcinoma'
8. 'mammary neoplasm'
9. 'mammary carcinoma'/exp OR 'mammary carcinoma'
10. 'mammary gland carcinoma'
11. #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
12. survivor*
13. #11 AND #12
14. survivorship OR therap* OR interven* OR program* OR evaluat* OR educat* OR rehabilit* OR effect* OR train* OR 'post treatment care' OR 'survivorship surveillance' OR 'continuum of care' OR 'transitional care'OR 'home based' OR 'home‐based' OR 'self care'/exp OR 'self care' OR 'self‐care'/exp OR 'self‐care' OR 'self help'/exp OR 'self help' OR'self‐help'/exp OR 'self‐help' OR 'self managed' OR 'self manage' OR 'self management'/exp OR 'self management' OR multidimension* OR 'multi dimension' OR 'multi dimensional' OR 'multi dimensions'
15. #1 AND #13 AND #14
16. #15 NOT ([animals]/lim NOT [humans]/lim)
17. #16 AND [embase]/lim
Search strategy for 2014‐2016 (via OvidSP)
| 1 | Randomized controlled trial/ |
| 2 | Controlled clinical study/ |
| 3 | Random$.ti,ab. |
| 4 | randomization/ |
| 5 | intermethod comparison/ |
| 6 | placebo.ti,ab. |
| 7 | (compare or compared or comparison).ti. |
| 8 | ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. |
| 9 | (open adj label).ti,ab. |
| 10 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. |
| 11 | double blind procedure/ |
| 12 | parallel group$1.ti,ab. |
| 13 | (crossover or cross over).ti,ab. |
| 14 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. |
| 15 | (assigned or allocated).ti,ab. |
| 16 | (controlled adj7 (study or design or trial)).ti,ab. |
| 17 | (volunteer or volunteers).ti,ab. |
| 18 | human experiment/ |
| 19 | trial.ti. |
| 20 | or/1‐19 |
| 21 | exp breast/ |
| 22 | exp breast disease/ |
| 23 | (21 or 22) and exp neoplasm/ |
| 24 | exp breast tumor/ |
| 25 | exp breast cancer/ |
| 26 | exp breast carcinoma/ |
| 27 | (breast$ adj5 (neoplas$ or cancer$ or carcin$ or tumo$ or metasta$ or malig$)).ti,ab. |
| 28 | 23 or 24 or 25 or 26 or 27 |
| 29 | survivor$.tw. |
| 30 | 28 and 29 |
| 31 | exp self help/ |
| 32 | exp self care/ |
| 33 | (survivorship or therap$ or intervene$ or program$ or evaluat$ or educat$ or rehabilit$ or effect$ or train$ or 'survivorship care plan' or 'post treatment care' or 'survivorship surveillance' or 'continuum of care' or 'individual care plan' or 'transitional care' or 'home based' or 'home‐based' or 'self care' or 'self‐care' or 'self help' or 'self‐help' or 'self managed' or 'self manage' or 'self management' or multidimension$ or 'multi dimension' or 'multi dimensional' or 'multi dimensions').tw. |
| 34 | 31 or 32 or 33 |
| 35 | 20 and 30 and 34 |
| 36 | limit 35 to (embase and yr="2014 ‐Current") |
| 37 | 36 not (1 or 2) |
Appendix 3. PubMed
STUDY DESIGN (i.e. RCTs and quasi‐RCTs):
1. (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tw] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab])
CONDITION (i.e. breast cancer survivors):
2. (((Breast neoplasms[mh] OR ((breast[mh] OR breast diseases[mh]) AND neoplasms[mh])) AND humans[mh]) OR DCIS[tiab] OR LCIS[tiab] OR ductal carcinoma in situ[tiab] OR lobular carcinoma in situ[tiab] OR (breast[tiab] AND (ductal carcinoma*[ti] OR lobular carcinoma*[ti])) OR ((Breast[ti] OR mammary[ti]) AND (cancer*[ti] OR neoplas*[ti] OR tumor*[ti] OR tumour*[ti] OR carcinoma*[ti] OR malignan*[ti] OR sarcoma[ti] OR lymphoma[ti])))
3. (survivor*)
4. (survivorship OR therap* OR interven* OR program* OR evaluat* OR educat* OR rehabilit* OR effect* OR train* OR "post treatment care" OR "survivorship surveillance" OR "continuum of care" OR "transitional care" OR "home based" OR "home‐based" OR "self care" OR "self‐care" OR "self help" OR "self‐help" OR "self manag*" OR "self‐manag*" OR "multidimension*" OR "multi‐dimension*")
5. #1 AND #2 AND #3 AND #4
6. Animals [MH] NOT Humans [MH]
7. #5 NOT #6
Appendix 4. Cumulative Index to Nursing and Allied Health Literature Plus (CINAHL Plus) via EBSCO host
S1. (MH "Clinical Trials+")
S2. PT Clinical trial
S3. TX clinic* n1 trial*
S4. TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) )
S5. TX randomi* control* trial*
S6. (MH "Random Assignment")
S7. TX random* allocat*
S8. TX placebo*
S9. (MH "Placebos")
S10. (MH "Quantitative Studies")
S11. TX allocat* random*
S12. S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11
S13. (MH "Breast Neoplasms+")
S14. TX breast cancer*
S15. TX breast neoplasm*
S16. TX breast carcinoma*
S17. TX breast tumour*
S18. TX breast tumor*
S19. S13 OR S14 OR S15 OR S16 OR S17 OR S18
S20. (MM "Cancer Survivors")
S21. TX survivor*
S22. S20 OR S21
S23. S19 AND S22
S24. TX survivorship OR TX therap* OR TX interven* OR TX program* OR TX evaluat* OR TX educat* OR TX rehabilit* OR TX effect* OR TX train* OR TX "post treatment care" OR TX "survivorship surveillance" OR TX "continuum of care" OR TX "transitional care" OR TX "home based" OR TX "home‐based" OR TX "self care" OR TX "self‐care" OR TX "self help" OR TX "self‐help" OR TX "self manag*" OR TX "self‐manag*" OR TX "multidimension*" OR TX "multi‐dimension*"
S25. S12 AND S23 AND S24
Appendix 5. PsycINFO via OvidSP
1. exp Treatment Effectiveness Evaluation/
2. exp Treatment Outcomes/
3. exp Placebo/
4. exp Followup Studies/
5. placebo*.mp.
6. random*.mp.
7. comparative stud*.mp.
8. (clinical adj3 trial*).mp.
9. (research adj3 design).mp.
10. (evaluat* adj3 stud*).mp
11. (clinical adj3 trial*).mp
12. (research adj3 design).mp.
13. (evaluat* adj3 stud*).mp.
14. (prospectiv* adj3 stud*).mp.
15. ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).mp.
16. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
17. exp Breast Neoplasms/
18. (breast adj6 cancer$).mp.
19. (breast adj6 neoplasm$).mp.
20. (breast adj6 carcinoma$).mp.
21. (breast adj6 tumour$).mp.
22. (breast adj6 tumor$).mp.
23. 17 or 18 or 19 or 20 or 21 or 22
24. survivor*.af.
25. 23 and 24
26. (survivorship or therap* or interven* or program* or evaluat* or educat* or rehabilit* or effect* or train* or "post treatment care" or "survivorship surveillance" or "continuum of care" or "transitional care" or "home based" or "home‐based" or "self care" or "self‐care" or "self help" or "self‐help" or "self manag*" or "self‐manag*" or "multidimension*" or "multi‐dimension*").af.
27. 16 and 25 and 26
Appendix 6. Web of Science via Web of Knowledge
1. TS= clinical trial* OR TS=research design OR TS=comparative stud* OR TS=evaluation stud* OR TS=controlled trial* OR TS=follow‐up stud* OR TS=prospective stud* OR TS=random* OR TS=placebo* OR TS=(single blind*) OR TS=(double blind*)
2. TS=("Breast Neoplasm*") OR TS=("Breast Cancer*") OR TS=("Breast Carcinoma*") OR TS=("Breast Tumour*") OR TS=("Breast Tumor*")
3. TS=(survivor*)
4. #3 AND #2
5. TS=(survivorship) OR TS=(therap*) OR TS=(interven*) OR TS=(program*) OR TS=(evaluat*) OR TS=(educat*) OR TS=(rehabilit*) OR TS=(effect*) OR TS=(train*) OR TS=("post treatment care") OR TS=("survivorship surveillance") OR TS=("continuum of care") OR TS=("transitional care") OR TS=("home based") OR TS=("home‐based") OR TS=("self care") OR TS=("self‐care") OR TS=("self help") OR TS=("self‐help") OR TS=("self manag*") OR TS=("self‐manag*") OR TS=("multidimension*") OR TS=("multi‐dimension*")
6. #5 AND #4 AND #1
Appendix 7. OpenGrey Database
1) breast AND survivor*
Appendix 8. WHO ICTRP
Basic search
1) Breast AND Survivor*
Appendix 9. ClinicalTrials.gov
To 2014 search
Basic search
1) Breast AND Survivor*
2014‐2016 search
Basic search:
1. breast cancer AND survivorship AND survivor
Advanced search:
1. Search terms: survivor
Recruitment: All studies
Study results: All studies
Study type: All studies
Gender: All studies
Conditions: breast cancer OR breast neoplasm
Interventions: survivorship OR mutlidimension OR self care OR self help OR self management
Appendix 10. ProQuest Dissertation and Theses Global
(SU.EXACT("Treatment Effectiveness Evaluation") OR SU.EXACT.EXPLODE("Treatment Outcomes") OR SU.EXACT("Placebo") OR SU.EXACT("Followup Studies") OR placebo* OR random* OR "comparative stud*" OR clinical NEAR/3 trial* OR research NEAR/3 design OR evaluat* NEAR/3 stud* OR prospectiv* NEAR/3 stud* OR (singl* OR doubl* OR trebl* OR tripl*) NEAR/3 (blind* OR mask*)) AND AB,FT,IF,TI("Breast Neoplasm*" OR "Breast Cancer*" OR "Breast Carcinoma" OR "Breast Malignanc*" OR "Breast Tumor*" ) AND AB,FT,IF,TI(survivor*) AND AB,FT,IF,TI(survivorship OR therap* OR interven* OR program* OR evaluat* OR educat* OR rehabilit* OR effect* OR train* OR "post treatment care" OR "survivorship surveillance" OR "continuum of care" OR "transitional care" OR "home based" OR "home‐based" OR "self care" OR "self‐care" OR "self help" OR "self‐help" OR "self manag*" OR "self‐manag*" OR "multidimension*" OR "multi‐dimension*")
Data and analyses
Comparison 1. Quality of life by FACT‐B.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 General | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Post intervention | 4 | 399 | Mean Difference (IV, Random, 95% CI) | 1.47 [‐3.03, 5.98] |
| 1.2 1‐3 months after intervention | 1 | 213 | Mean Difference (IV, Random, 95% CI) | 3.30 [‐0.77, 7.37] |
| 2 Breast cancer | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Post intervention | 7 | 764 | Mean Difference (IV, Fixed, 95% CI) | 4.55 [2.33, 6.78] |
| 2.2 1‐3 months after intervention | 2 | 426 | Mean Difference (IV, Fixed, 95% CI) | 6.10 [2.48, 9.72] |
| 3 Physical well‐being | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Post intervention | 7 | 764 | Mean Difference (IV, Random, 95% CI) | 0.81 [0.04, 1.58] |
| 3.2 1‐3 month after intervention | 2 | 426 | Mean Difference (IV, Random, 95% CI) | 1.25 [0.35, 2.15] |
| 4 Social well‐being | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Post intervention | 7 | 762 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.49, 1.04] |
| 4.2 1‐3 months after intervention | 2 | 424 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.93, 1.24] |
| 5 Emotional well‐being | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Post intervention | 7 | 762 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.25, 1.07] |
| 5.2 1‐3 months after intervention | 2 | 424 | Mean Difference (IV, Random, 95% CI) | 1.38 [‐0.40, 3.15] |
| 6 Functional well‐being | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Post intervention | 6 | 740 | Mean Difference (IV, Random, 95% CI) | 0.97 [‐0.33, 2.28] |
| 6.2 1‐3 months after intervention | 2 | 425 | Mean Difference (IV, Random, 95% CI) | 1.46 [0.37, 2.56] |
| 7 Endocrine subscale | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Post intervention | 2 | 421 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.09, 0.47] |
| 7.2 1‐3 months after intervention | 2 | 421 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.07, 0.46] |
| 8 Trial Outcome Index | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Post intervention | 2 | 345 | Mean Difference (IV, Fixed, 95% CI) | 4.36 [2.20, 6.53] |
| 8.2 1‐3 months after intervention | 1 | 213 | Mean Difference (IV, Fixed, 95% CI) | 3.60 [0.01, 7.19] |
1.3. Analysis.
Comparison 1 Quality of life by FACT‐B, Outcome 3 Physical well‐being.
1.4. Analysis.
Comparison 1 Quality of life by FACT‐B, Outcome 4 Social well‐being.
1.5. Analysis.
Comparison 1 Quality of life by FACT‐B, Outcome 5 Emotional well‐being.
1.6. Analysis.
Comparison 1 Quality of life by FACT‐B, Outcome 6 Functional well‐being.
1.7. Analysis.
Comparison 1 Quality of life by FACT‐B, Outcome 7 Endocrine subscale.
1.8. Analysis.
Comparison 1 Quality of life by FACT‐B, Outcome 8 Trial Outcome Index.
Comparison 2. Quality of life measured by EORTC‐QLQ.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Post intervention | 6 | 299 | Mean Difference (IV, Fixed, 95% CI) | 4.38 [0.11, 8.64] |
| 1.2 1‐3 months after intervention | 3 | 172 | Mean Difference (IV, Fixed, 95% CI) | 6.32 [0.61, 12.04] |
| 1.3 4‐6 months after intervention | 2 | 117 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐7.28, 7.44] |
| 1.4 12 months after intervention | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 2.04 [‐9.91, 13.99] |
| 2 Functional scale | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Post intervention | 3 | 127 | Mean Difference (IV, Fixed, 95% CI) | 3.16 [0.09, 6.22] |
| 3 Symptom scale | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Post intervention | 2 | 82 | Mean Difference (IV, Random, 95% CI) | 2.53 [‐5.56, 10.61] |
| 4 Role function | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Post intervention | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | 3.18 [‐4.57, 10.93] |
| 4.2 1‐3 months after intervention | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐12.60, 13.40] |
| 5 Emotion function | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Post intervention | 2 | 100 | Mean Difference (IV, Random, 95% CI) | 12.22 [6.07, 18.37] |
| 5.2 1‐3 months after intervention | 1 | 55 | Mean Difference (IV, Random, 95% CI) | 6.40 [‐7.12, 19.92] |
| 6 Cognitive function | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Post intervention | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | 0.98 [‐5.27, 7.23] |
| 6.2 1‐3 months after intervention | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐14.01, 13.81] |
| 7 Social function | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Post intervention | 2 | 100 | Mean Difference (IV, Fixed, 95% CI) | 1.57 [‐6.98, 10.13] |
| 7.2 1‐3 months after intervention | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐3.90 [‐19.01, 11.21] |
| 8 Physical function | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Post intervention | 2 | 115 | Mean Difference (IV, Fixed, 95% CI) | 3.29 [‐2.80, 9.38] |
| 8.2 1‐3 months after intervention | 2 | 107 | Mean Difference (IV, Fixed, 95% CI) | 14.05 [4.00, 24.10] |
| 8.3 4‐6 months after intervention | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 5.56 [‐3.71, 14.83] |
2.2. Analysis.
Comparison 2 Quality of life measured by EORTC‐QLQ, Outcome 2 Functional scale.
2.3. Analysis.
Comparison 2 Quality of life measured by EORTC‐QLQ, Outcome 3 Symptom scale.
2.4. Analysis.
Comparison 2 Quality of life measured by EORTC‐QLQ, Outcome 4 Role function.
2.5. Analysis.
Comparison 2 Quality of life measured by EORTC‐QLQ, Outcome 5 Emotion function.
2.6. Analysis.
Comparison 2 Quality of life measured by EORTC‐QLQ, Outcome 6 Cognitive function.
2.7. Analysis.
Comparison 2 Quality of life measured by EORTC‐QLQ, Outcome 7 Social function.
2.8. Analysis.
Comparison 2 Quality of life measured by EORTC‐QLQ, Outcome 8 Physical function.
Comparison 3. Quality of life measured by Quality of Life‐Breast Cancer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Post intervention | 2 | 111 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.02, 0.85] |
| 1.2 1‐3 months after intervention | 1 | 61 | Mean Difference (IV, Fixed, 95% CI) | 0.45 [‐0.19, 1.09] |
| 2 Physical | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Post intervention | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.65, 0.53] |
3.2. Analysis.
Comparison 3 Quality of life measured by Quality of Life‐Breast Cancer, Outcome 2 Physical.
Comparison 4. Quality of life measured by SF‐36.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Physical function | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Post‐intervention | 2 | 308 | Mean Difference (IV, Fixed, 95% CI) | 0.55 [‐3.52, 4.63] |
| 1.2 4‐6 months | 2 | 308 | Mean Difference (IV, Fixed, 95% CI) | ‐1.05 [‐5.60, 3.51] |
Comparison 5. Anxiety.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Anxiety | 7 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 State‐Trait Anxiety Inventory (STAI) ‐ post intervention | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐4.70 [‐7.88, ‐1.52] |
| 1.2 Hospital Anxiety and Depression Scale (HADS) ‐ post intervention | 5 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐1.01 [‐1.94, ‐0.08] |
| 1.3 Hospital Anxiety and Depression Scale (HADS) ‐ 1‐3 months after intervention | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐0.68 [‐2.04, 0.68] |
| 1.4 Hospital Anxiety and Depression Scale (HADS) ‐ 4‐6 months after intervention | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.37 [‐2.43, 1.69] |
Comparison 6. Depression.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Beck Depression Inventory (BDI) ‐ post intervention | 2 | 82 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐3.16, 1.94] |
| 1.2 State‐Trait Anxiety Inventory (STAI) ‐ post intervention | 2 | 154 | Mean Difference (IV, Random, 95% CI) | ‐3.29 [‐5.82, ‐0.77] |
| 1.3 Hospital Anxiety and Depression Scale (HADS) ‐ post intervention | 4 | 213 | Mean Difference (IV, Random, 95% CI) | ‐1.36 [‐2.94, 0.22] |
| 1.4 Hospital Anxiety and Depression Scale (HADS) 1‐3 months after intervention | 3 | 168 | Mean Difference (IV, Random, 95% CI) | ‐0.74 [‐2.71, 1.22] |
| 1.5 Hospital Anxiety and Depression Scale (HADS) 4‐6 months after intervention | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.77, 1.85] |
Comparison 7. Fatigue.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Fatigue | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Brief Fatigue Inventory (BFI) ‐ post intervention | 3 | 127 | Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.78, ‐0.45] |
| 1.2 Multidimensional Fatigue Inventory (MFI) ‐ post intervention | 2 | 144 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.69, 0.62] |
| 1.3 Multidimensional Fatigue Inventory (MFI) 1‐3 months after intervention | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.67 [‐0.63, 1.97] |
| 1.4 Multidimensional Fatigue Inventory (MFI) 4‐6 months after intervention | 1 | 57 | Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.77, 1.85] |
Comparison 8. Insomnia measured by Insomnia Severity Index (ISI) 3 months.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ISI | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Post intervention | 3 | 185 | Mean Difference (IV, Fixed, 95% CI) | ‐1.81 [‐3.34, ‐0.27] |
| 1.2 1‐3 months after intervention | 2 | 113 | Mean Difference (IV, Fixed, 95% CI) | ‐2.27 [‐4.22, ‐0.33] |
| 1.3 4‐6 months after intervention | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐3.08, 2.72] |
Comparison 9. Flushes and night sweats measured by Hot Flushes and Night Sweats Frequency Rating (HFRS).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 HFRS | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Post intervention | 2 | 216 | Mean Difference (IV, Fixed, 95% CI) | ‐1.50 [‐3.75, 0.75] |
| 1.2 1‐3 months after intervention | 1 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐0.51 [‐3.28, 2.26] |
| 1.3 4‐6 months after intervention | 1 | 96 | Mean Difference (IV, Fixed, 95% CI) | ‐2.19 [‐6.01, 1.63] |
Comparison 10. Depressive symptoms measured by Center for Epidemiologic Studies‐Depression Scale (CES‐D).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depressive symptoms | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Post intervention | 2 | 154 | Mean Difference (IV, Fixed, 95% CI) | ‐2.61 [‐4.93, ‐0.29] |
Comparison 11. Subgroup analyses: quality of life by FACT‐B ‐ components of intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breast cancer (post intervention) | 7 | 764 | Mean Difference (IV, Fixed, 95% CI) | 4.55 [2.33, 6.78] |
| 1.1 Intervention with education and psychological components | 3 | 355 | Mean Difference (IV, Fixed, 95% CI) | 5.08 [1.24, 8.92] |
| 1.2 Intervention with education and physical components | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 4.10 [0.58, 7.62] |
| 1.3 Intervention with physical and psychological components | 3 | 280 | Mean Difference (IV, Fixed, 95% CI) | 4.57 [0.24, 8.90] |
| 2 Physical well‐being (post intervention) | 7 | 764 | Mean Difference (IV, Random, 95% CI) | 0.81 [0.04, 1.58] |
| 2.1 Intervention with education and psychological components | 3 | 355 | Mean Difference (IV, Random, 95% CI) | 0.79 [‐0.14, 1.72] |
| 2.2 Intervention with education and physical components | 1 | 129 | Mean Difference (IV, Random, 95% CI) | 1.14 [0.11, 2.17] |
| 2.3 Intervention with physical and psychological components | 3 | 280 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐1.85, 2.74] |
| 3 Social well‐being (post intervention) | 7 | 762 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.49, 1.04] |
| 3.1 Intervention with education and psychological components | 3 | 353 | Mean Difference (IV, Fixed, 95% CI) | 0.38 [‐0.73, 1.48] |
| 3.2 Intervention with education and physical components | 1 | 129 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.04, 2.04] |
| 3.3 Intervention with physical and psychological components | 3 | 280 | Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐1.00, 1.50] |
| 4 Emotional well‐being (post intervention) | 7 | 762 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.25, 1.07] |
| 4.1 Intervention with education and psychological components | 3 | 353 | Mean Difference (IV, Random, 95% CI) | 0.51 [‐0.77, 1.80] |
| 4.2 Intervention with education and physical components | 1 | 129 | Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.95, 1.49] |
| 4.3 Intervention with physical and psychological components | 3 | 280 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐1.37, 1.51] |
| 5 Functional well‐being (post intervention) | 6 | 740 | Mean Difference (IV, Random, 95% CI) | 0.97 [‐0.33, 2.28] |
| 5.1 Intervention with education and psychological components | 3 | 354 | Mean Difference (IV, Random, 95% CI) | 0.89 [‐0.83, 2.61] |
| 5.2 Intervention with education and physical components | 1 | 129 | Mean Difference (IV, Random, 95% CI) | 2.42 [1.17, 3.67] |
| 5.3 Intervention with physical and psychological components | 2 | 257 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐4.37, 4.23] |
11.2. Analysis.
Comparison 11 Subgroup analyses: quality of life by FACT‐B ‐ components of intervention, Outcome 2 Physical well‐being (post intervention).
11.3. Analysis.
Comparison 11 Subgroup analyses: quality of life by FACT‐B ‐ components of intervention, Outcome 3 Social well‐being (post intervention).
11.4. Analysis.
Comparison 11 Subgroup analyses: quality of life by FACT‐B ‐ components of intervention, Outcome 4 Emotional well‐being (post intervention).
11.5. Analysis.
Comparison 11 Subgroup analyses: quality of life by FACT‐B ‐ components of intervention, Outcome 5 Functional well‐being (post intervention).
Comparison 12. Sub‐group analysis: quality of life by EORTC‐QLQ ‐ components of intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global (post intervention) | 6 | 299 | Mean Difference (IV, Fixed, 95% CI) | 4.38 [0.11, 8.64] |
| 1.1 Intervention with education and psychological components | 2 | 117 | Mean Difference (IV, Fixed, 95% CI) | ‐1.16 [‐8.17, 5.85] |
| 1.2 Intervention with education and physical components | 3 | 127 | Mean Difference (IV, Fixed, 95% CI) | 7.25 [0.68, 13.82] |
| 1.3 Intervention with education, physical and psychological components | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 8.40 [‐0.94, 17.74] |
Comparison 13. Subgroup analyses: quality of life by FACT‐B ‐ mode of intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Breast cancer (post intervention) | 7 | 764 | Mean Difference (IV, Fixed, 95% CI) | 4.55 [2.33, 6.78] |
| 1.1 Group‐based | 4 | 630 | Mean Difference (IV, Fixed, 95% CI) | 5.36 [2.97, 7.74] |
| 1.2 Individual‐based | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 1.71 [‐6.66, 10.07] |
| 1.3 Group‐ and individual‐based | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐13.67, 5.07] |
| 2 Physical well‐being (post intervention) | 7 | 764 | Mean Difference (IV, Random, 95% CI) | 0.81 [0.04, 1.58] |
| 2.1 Group‐based | 4 | 630 | Mean Difference (IV, Random, 95% CI) | 1.15 [0.55, 1.75] |
| 2.2 Individual‐based | 2 | 93 | Mean Difference (IV, Random, 95% CI) | 1.17 [‐0.90, 3.24] |
| 2.3 Group‐ and individual‐based | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐4.27, 0.07] |
| 3 Social well‐being (post intervention) | 7 | 762 | Mean Difference (IV, Fixed, 95% CI) | 0.28 [‐0.49, 1.04] |
| 3.1 Group‐based | 4 | 628 | Mean Difference (IV, Fixed, 95% CI) | 0.42 [‐0.47, 1.31] |
| 3.2 Individual‐based | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐2.20, 1.30] |
| 3.3 Group‐ and individual‐based | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [‐2.25, 3.85] |
| 4 Emotional well‐being (post intervention) | 7 | 762 | Mean Difference (IV, Random, 95% CI) | 0.41 [‐0.25, 1.07] |
| 4.1 Group‐based | 4 | 628 | Mean Difference (IV, Random, 95% CI) | 0.84 [0.28, 1.39] |
| 4.2 Individual‐based | 2 | 93 | Mean Difference (IV, Random, 95% CI) | ‐0.56 [‐2.08, 0.96] |
| 4.3 Group‐ and individual‐based | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐2.94, 0.74] |
| 5 Functional well‐being (post intervention) | 6 | 740 | Mean Difference (IV, Random, 95% CI) | 0.97 [‐0.33, 2.28] |
| 5.1 Group‐based | 4 | 629 | Mean Difference (IV, Random, 95% CI) | 2.05 [1.32, 2.79] |
| 5.2 Individual‐based | 1 | 70 | Mean Difference (IV, Random, 95% CI) | ‐0.93 [‐3.07, 1.21] |
| 5.3 Group‐ and individual‐based | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐2.40 [‐4.89, 0.09] |
13.2. Analysis.
Comparison 13 Subgroup analyses: quality of life by FACT‐B ‐ mode of intervention, Outcome 2 Physical well‐being (post intervention).
13.3. Analysis.
Comparison 13 Subgroup analyses: quality of life by FACT‐B ‐ mode of intervention, Outcome 3 Social well‐being (post intervention).
13.4. Analysis.
Comparison 13 Subgroup analyses: quality of life by FACT‐B ‐ mode of intervention, Outcome 4 Emotional well‐being (post intervention).
13.5. Analysis.
Comparison 13 Subgroup analyses: quality of life by FACT‐B ‐ mode of intervention, Outcome 5 Functional well‐being (post intervention).
Comparison 14. Subgroup analysis: quality of life by EORTC QLQ ‐ mode of intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global (post intervention) | 6 | 299 | Mean Difference (IV, Fixed, 95% CI) | 4.38 [0.11, 8.64] |
| 1.1 Group‐based | 4 | 194 | Mean Difference (IV, Fixed, 95% CI) | 4.28 [‐1.19, 9.74] |
| 1.2 Individual‐based | 2 | 105 | Mean Difference (IV, Fixed, 95% CI) | 4.53 [‐2.29, 11.35] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Cho 2006.
| Methods | Design: single centre, quasi‐RCT. Follow‐up: 10 weeks from baseline. | |
| Participants | Major inclusion criteria: stage 1‐2 breast cancer; within 2 years after mastectomy; completion of chemotherapy and/or radiotherapy with or without current hormone therapy use N = 55 (E: 28, C: 27) Mean age: E: 48.7 ± 9.1 years, C: 49.6 ± 6.2 years Stage of breast cancer: E: stage 1 (12, 42.9%), stage 2 (16, 57.1%); C: stage 1 (13, 48.2%), stage 2 (14, 51.8%) |
|
| Interventions | E: Psycho‐ educational (psychological‐based education) + physical (exercise) + psychological (peer support group activity) Psychological‐based education: group format, conducted by specialists (oncology, nurse, surgeon, dietician, and image consultant), 1 session per week of 90 minutes for ten weeks, topics included understanding breast cancer, treatment and complications of breast cancer, and advice about lymphedema prevention and management, nutrition and diet, sexual and daily life, and effective ways to manage relationships and communication. Exercise: group format, 2 sessions per week of 90 minutes for ten weeks, sessions included a warm up, main exercise and cool‐down; home‐based practice, at least twice per week for ten weeks, practiced basic stretching and stretching each part of the body. Peer support group activity: group format, 1 session per week of 60 minutes for ten weeks, involved participants sharing their feelings, conflicts and experiences. C: waiting‐list control. |
|
| Outcomes | QOL scale developed by the study authors | |
| Notes | E: experimental, C: comparison, RCT: randomised control trial. Dropouts or missing data where reported: Of the 65 participants entered into the study, 6 participants from experimental group and 4 from control were excluded from data analysis due to metastasis or did not complete the post‐test. Meta‐analysis was not possible as the QOL scale used was the study authors' own scale. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned into experimental and waiting‐list control group but method of randomisation was not described |
| Allocation concealment (selection bias) | Unclear risk | Study did not provide enough information to allow judgment as concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of allocated interventions since they had to go through the interventions. The researcher was involved in intervention administration |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were aware of allocated interventions since they had to go through the interventions. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition from intervention and control group described with reasons, and attrition balanced between groups (6 from intervention group, 4 from control group). Reasons for attrition: metastasis for 3 participants and refusal to be involved before completing post‐test for 7 participants |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Study design was quasi‐experimental with a non‐equivalent control group. Sampling method not described. Participation rate not provided |
Dirksen 2008.
| Methods | Design: single centre, RCT. Follow‐up: after experimental interventions. | |
| Participants | Major inclusion criteria: stage 1‐3 breast cancer; at least 3 months post‐completion of primary cancer treatment; has concerns about sleep N = 72 (E: 34, C: 38) Mean age: E: 57.2 ± 9.9 years, C: 59.2 ± 10.7 years Stage of breast cancer: E: stage 1 (19, 55.9%), stage 2 (9, 26.5%), stage 3 (4, 11.8%); C: stage 1 (17, 46.3%), stage 2 (12, 31.7%), stage 3 (5,12.2%) |
|
| Interventions | E: Psychological (cognitive behavioural therapy for insomnia) + Educational Cognitive behavioural therapy for insomnia: individual format, conducted by a master’s level registered nurse therapist, a face‐to‐face session of 2 hours at session 1 and of about 1 hour at sessions 2‐4, plus 2 phone sessions of about 15 minutes at sessions 5‐6, primary foci were stimulus control instructions, sleep restriction therapy, sleep education and hygiene, reviewing daily sleep diaries, discussing progress to data, encouraging adherence to treatment recommendation; home assignment, daily sleep diaries. Educational: integrated into cognitive behavioural therapy for insomnia; stimulus control instructions, sleep restriction therapy, sleep education and hygiene. C: received the same attention and number of contact hours as experimental group. |
|
| Outcomes | Quality of life: FACT‐B Others: STAI, CES‐D, POMSF/I, ISI |
|
| Notes | C: comparison, E: experimental, CES‐D: Center for Epidemiologic Studies‐Depression Scale, C: control, FACT‐B: Functional Assessment of Cancer Therapy‐Breast, ISI: Insomnia Severity Index, POMSF/I: Profile of Mood States Fatigue/Inertia Subscale, RCT: randomised control trial, STAI: State‐Trait Anxiety Inventory. Dropouts or missing data where reported: Of the 81 participants entered into the study, 6 from experimental group and 3 from control withdrew the study and were excluded from data analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups by the use of a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Study did not provide enough information to allow judgment as concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Research assistant was not blinded to group assignment. Participants were aware of allocated interventions since they had to go through the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants could be aware of allocated interventions, although control group received the same attention and number of contact hours as the intervention group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition from intervention and control group described with reasons, and attrition was quite balanced between groups (6 from intervention group, 3 from control group). Reasons for withdrawal from intervention group included treatment was not helping, did not like treatment, no longer interested, scheduling problems and a family death. Reasons for withdrawal from control group included not interested, scheduling problems and not able to contact |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Non‐probability sampling was performed and could lead to problems with external validity. Participation rate was about 26.2% |
Duijts 2012.
| Methods | Design: RCT. Follow‐up: 12 weeks and six months after study entry. | |
| Participants | Major inclusion criteria: stages 0‐3 breast cancer; within 4‐5 months of completion of chemotherapy; reported at least a minimal level of menopausal symptoms N = 422 (4 arms: E1: 109, E2: 104, E3: 106, C: 103) Mean age: E1: 48.2 ± 5.7 years, E2: 47.7 ± 5.6 years, E3: 49.0 ± 4.9 years, C: 47.8 ± 6.0 years Stage of breast cancer: not reported |
|
| Interventions | E1: psychological (cognitive behavioural therapy) E2: physical (exercise) E3: psychological (cognitive behavioural therapy) + physical (exercise) Cognitive behavioural therapy: group format, no information about who conducted the therapy, 1 session per week of 90 minutes for six weeks plus a booster session held 6 weeks after programme completion, primary foci were hot flushes and night sweats, and relaxation exercises. Exercise: individual format, one session per week of 2.5 to 3 hours for 12 weeks, physiotherapist‐guided exercise and level of physical activity. C: waiting‐list control. |
|
| Outcomes | Quality of life: SF 36, EORTC QLQ‐BR23 Others: FACT‐ES, HADS, HFRS, SAQ, BFLUTS | |
| Notes | BFLUTS: Incontinence scale of the Bristol Female Lower Urinary Tract Symptoms Questionnaire, C: comparison, E1: experimental 1, E2: experimental 2, E3: experimental 3, FACT‐ES: Endocrine subscale of the Functional Assessment of Cancer Therapy, HADS: Hospital Anxiety and Depression Scale, HFRS: Hot Flush Rating Scale, NS: not specified, EORTC QLQ‐BR23: European Organisation for Research and Treatment of Cancer‐Breast Cancer‐Specific Quality of Life Questionnaire, RCT: randomised controlled trial, SAQ: Sexual Activity Questionnaire, SF‐36: Short Form Health Survey. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned using computerised block randomisation |
| Allocation concealment (selection bias) | Unclear risk | Study did not provide enough information to allow judgment as concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Percentage available follow‐up data did not differ significantly between groups, but reason for missing outcome data not provided |
| Selective reporting (reporting bias) | Low risk | Study protocol was available and pre‐specified outcomes were reported in pre‐specified way |
| Other bias | High risk | Participation rate was about 23.4%. Sampling bias likely present as non‐probability sampling was used |
Ergun 2013.
| Methods | Design: single centre, RCT. Follow‐up: after experimental interventions. | |
| Participants | Major inclusion criteria: breast cancer with no recurrent or progressive breast cancer; completion of surgery, radiotherapy and chemotherapy, being post‐menopausal N = 60 (E1: 20, E2: 20, C: 20) Mean age: E1: 49.7 ± 8.3 years, E2: 55.1 ± 6.9 years, C: 50.3 ± 10.4 years Stage of breast cancer: not reported |
|
| Interventions | E1: educational + physical (supervised exercise) E2: educational + physical (home exercise) E3: educational Educational: no information about individual or group format, one session of 30 minutes, topics included adverse effects of breast cancer, prevention of lymphoedema and related activities, together with a booklet about lymphoedema‐specific exercises Physical (supervised exercise): no information about individual or group format, conducted by a specialist doctor of physical therapy and rehabilitation, supervised aerobic exercise and resistive exercise of 45 minutes per session with 3 sessions per week for 12 weeks, and brisk walking of 30 minutes per session with 3 sessions per week for 12 weeks. Physical (home exercise): home aerobic exercise for 12 weeks, and brisk walking of 30 minutes per session with 3 sessions per week for 12 weeks. C: educational only. |
|
| Outcomes | Quality of life: EORTC QLQ‐C30 Others: BDI, BFI |
|
| Notes | BDI: Beck Depression Inventory, BFI: Brief Fatigue Inventory, C: comparison, E1: experimental 1, E2: experimental 2, EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life C30, RCT: randomised control trial. Dropouts or missing data where reported: 2 participants from home exercise group dropped out due to development of metastasis and lack of time to do the exercises but were included in data analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables used for randomisation |
| Allocation concealment (selection bias) | Unclear risk | Study did not provide enough information to allow judgment as concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants could be aware of allocated interventions but as all three groups received programme, blinding could be possible too, but study did not provide enough information to allow judgment. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants could be aware of allocated interventions but as all three groups received programme, blinding could be possible too, but study did not provide enough information to allow judgment. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition described with reasons and quantity was small, 1 case for development of metastasis and 1 case for lack of time to do exercises. These participants were still included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Sampling bias was present as participants were volunteers |
Eyigor 2010.
| Methods | Design: single centre, RCT. Follow‐up: eight weeks after the experiment. | |
| Participants | Major inclusion criteria: breast cancer with no evidence of recurrent or progressive disease; completion of surgery, radiotherapy and/or chemotherapy with or without current hormone treatment N = 42 (E: 27, C: 15) Mean age: E: 48.5 ± 7.6 years, C: 49.7 ± 8.7 years Stage of breast cancer: not reported |
|
| Interventions | E: educational + physical (hospital pilates exercise + home exercise) Educational: no information about individual or group format, one session of 30 minutes, topics included the information of breast cancer, lymphoedema, and prevention of lymphoedema and related activities, together with a booklet about range‐of‐motion, stretching and respiratory exercises. Physical (hospital pilates exercise): no information about individual or group format, conducted by a specialist physiotherapist, supervised pilates exercise of 60 minutes per session with 3 sessions per week for 8 weeks. Physical (home exercise): home exercise in accordance with the booklet 1 session per day and walking exercise of 20 to 30 minutes per session with 3 sessions per week for 8 weeks. C: educational + physical (home exercise) |
|
| Outcomes | Quality of life: EORTC QLQ‐C30, EORTC BR23 Others: BDI, BFI |
|
| Notes | BDI: Beck Depression Inventory, BFI: Brief Fatigue Inventory, C: comparison, E: experimental, EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life C30, EORTC QLQ‐BR23: European Organisation for Research and Treatment of Cancer‐Breast Cancer‐Specific Quality of Life Questionnaire, RCT: randomised controlled trial. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised using a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Study did not provide enough information to allow judgment as concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether outcome assessors were blinded but the same physio made assessments before and after interventions |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition imbalanced across groups. 10 participants in group 2 failed to complete the programme due to loss of interest (2), difficulty in commuting to the hospital (6) and medical problems (2). No loss in group 1. Participants lost excluded from analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Sampling bias likely present due to non‐probability sampling. Participation rate was about 96.3% |
Fillion 2008.
| Methods | Design: single centre, RCT. Follow‐up: post‐experiment and at 3‐months post‐experiment. | |
| Participants | Major inclusion criteria non‐metastatic breast cancer; within 2 years after breast cancer treatment N = 87 (E: 44, C: 43) Mean age: E: 53.1 ± 9.7 years, C: 51.8 ± 10.3 years Stage of breast cancer: E: stage 0 (2, 4.5%), stage1 (21, 47.7%), stage 2 (18, 40.9%), stage 3 (3, 6.8%); C: stage 0 (4, 9.3%), stage 1 (17, 39.5%), stage 2 (12, 27.9%), stage 3 (10, 23.3%) |
|
| Interventions | E: psycho‐ educational + physical (exercise) Psycho‐educational + physical (exercise): group meeting format, one session per week of 2.5 hours for four weeks (1 hour was supervised walking training, 1.5 hours was psycho‐educative fatigue management session), with daily home‐based practice of relaxation and exercise, plus one booster telephone session of 5‐15 minutes between 7th and 8th week after completion of psycho‐educational and exercise interventions. C: usual care. |
|
| Outcomes | Quality of life: SF‐12 Others: POMS, MFI |
|
| Notes | C: comparison, E: experimental, MFI: Multidimensional Fatigue Inventory, POMS: Profile of Mood States, SF‐12: Short Form (12) Health Survey. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned to study groups through a sequence of randomisation which was computer‐generated, after a preliminary stratification, according to the adjuvant treatments received |
| Allocation concealment (selection bias) | Low risk | A kinesiologist randomly assigned each participant to groups using sealed envelopes, which were concealed to both kinesiologist and patient till then |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition from both groups balanced (4 from intervention, 3 from control) with reasons provided. Reasons for withdrawal in intervention group were disease recurrence (2), withdrawal (1) and metastatic disease (1) and withdrawal in control group was due to withdrawal (2) and disease recurrence (1). participants who did not receive allocated intervention were not included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported. |
| Other bias | High risk | Participation rate was 18.9%. Sampling bias likely present as non‐probability sampling was used |
Fiorentino 2008.
| Methods | Design: crossover‐experimental. Follow‐up: 6 and 12 weeks from baseline. | |
| Participants | Major inclusion criteria: Survivors of breast cancer who had finished their breast cancer treatment and met the DSM IV criteria for insomnia N = 14 (E: 11, C: 10) Mean age: 61 ± 11.6 years Stage of breast cancer: Stage 1 (7), Stage 2 (3), stage 3 (2) and advanced stage 3 (2) |
|
| Interventions | E: educational + psychology (behavioural) Education + Psychology (behavioural): 1‐hour session per week for 6 week; educational component covered sleep stages, processes regulating sleep , sleep throughout life, sleep in cancer patients, Spielman et al.’s (1987) insomnia 3‐P model and cognitive behavioral therapy basics; psychological (behavioural) component included entailed sleep restriction, stimulus control, adhering to the sleep hygiene rules, and training in progressive muscle relaxation techniques; conducted by a graduate level therapist trained in CBT‐I, and supervised by a licensed clinical psychologist C: usual care |
|
| Outcomes | Quality of life: SF‐36 Others: actigraphy, PSQI, ISI, Sleep Diaries, MFSI‐SF, FOSQ, CESD,BSI‐18, GCS |
|
| Notes | C: comparison, E: experimental, SF‐36: Short Form (36) Health Survey ISI: Insomnia, PSQI: Pittsburgh Sleep Quality Index, ISI: Insomnia Severity Index, Severity Index, MFSI‐SF: Multidimensional Fatigue Symptom Inventory, FOSQ: Functional Outcomes of Sleep Questionnaire, BSI‐18: Brief Symptom Inventory, GCS: Greene Climacteric Scale. Dropouts or missing data where reported: 5 in experimental and 2 in comparison lost to follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomly assigned through a computer‐generated random list |
| Allocation concealment (selection bias) | Unclear risk | Study did not provide enough information to allow judgment as concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 women dropped out, 1 reported study was too much and another realised in therapy that the root of her insomnia would be addressed with a more comprehensive psychotherapy addressing psychological events and traumas experienced in childhood |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Sampling bias likely present as non‐probability sampling method used. Participation rate was about 58.3% |
Galantino 2010.
| Methods | Design: single centre, quasi‐RCT. Follow‐up: 3 months from baseline. | |
| Participants | Major inclusion criteria: not reported N = 30 (E: 20, C: 10) Mean age: not reported Stage of breast cancer: not reported |
|
| Interventions | E: psychological (coaching) + educational Psychological (coaching): no information about individual or group format, conducted by a certified professional Health Fitness Instructor, an initial session of 90 minutes about a wellness vision and behavioural plan development, followed by five sessions of 30‐40 minutes each over a 3‐month period, topics included goal review and goal attainment, and discussion of obstacles and strategies. Educational: Web site information of health and fitness, and wellness vision and goals. (Web site information served as additional resources and was optional) C: long standing traditional social support |
|
| Outcomes | Quality of life: QOL P/CS Others: HADS | |
| Notes | C: comparison, E: experimental, HADS: HADS: Hospital Anxiety and Depression Scale, QOL P/CS: Quality of Life Patient/Cancer Survivor, CESD: Center of Epidemiological Studies‐Depression. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information about allocation to intervention and control groups was not stated clearly |
| Allocation concealment (selection bias) | Unclear risk | No information provided about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants could be aware of allocated interventions since they had to go through the intervention but it could also be possible that they were not aware as coaching and social support group appear similar. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants could be aware of allocated interventions since they had to go through the intervention but it could also be possible that they were not aware as coaching and social support group appear similar. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided about attrition |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Sample sizes in both groups seemed imbalanced (20 in coaching group, 10 in social support group). Sampling bias likely present as non‐probability sampling was used. No information provided about participation rate |
Heidrich 2009.
| Methods | This paper reports 3 pilot studies; 2 RCTs and 1 single group study. We included 2 RCTs in this review. P1: Design: single centre, RCT. Follow‐up: at 6 and 10 weeks post‐experiment. P2: Design: single centre, RCT. Follow‐up: at 2, 4, 6, 8 and 16 weeks post‐experiment. |
|
| Participants | The 2 pilot studies have the same major inclusion criteria: aged 65 years or older, at least 1 year post diagnosis of non‐metastatic breast cancer; at least 1 month post‐treatment for breast cancer except hormonal therapies
P1: N = 41 (E: not reported, C: not reported) Mean age: overall 72 years, range 65‐86 years (E: not reported, C: not reported) Stage of breast cancer: not reported P2: N = 20 (E: not reported, C: not reported) Mean age: overall 69.7 years, range 65‐82 years (E: not reported, C: not reported) Stage of breast cancer: not reported |
|
| Interventions | P1: Psychological (counselling interview) + Educational (symptom‐management strategies and information) Psychological (counselling interview): individual format, conducted by an advanced practice nurses, Individualised Representational Intervention to Improve Symptom Management (IRIS), 1 face‐to‐face session of 35‐70 minutes, involved participants describing their beliefs about most troublesome or serious symptoms and coping of symptoms, and developing a symptom management plan; and 1 phone session to review the progress of the symptom management plan. Educational: concurrent with the counselling interview, symptom‐management strategies and information. C: usual care P2: Psychological (counselling interview) + Educational (symptom‐management strategies and information) Psychological (counselling interview): same as P1 except for the addition of 4 bi‐weekly telephone reinforcement sessions beginning 2 weeks after the baseline interview, content included symptom distress assessment and symptom management review. Educational: same as PI C: waiting‐list control |
|
| Outcomes | PI*: Quality of life: SF‐36 Others: SB‐R, TSD, CES‐D, STAI P2*: Quality of life: SF‐12 Others: SB‐R, TSD, CES‐D, STAI, BPI |
|
| Notes | BPI: Brief Pain Inventory, CES‐D: Center for Epidemiologic Studies‐Depression Scale, SF‐36: Short Form (36) Health Survey, SF‐12: Short Form (12) Health Survey, P1: pilot study 1, P2: pilot study 2, RCT: randomised controlled trial SB‐R: Symptom Bother‐Revised Scale, STAI: State Anxiety Scale, TSD: Target Symptom Distress. Dropouts or missing where reported: P1*: 2 participants dropped out but were included in data analysis. P2*: 1 participant dropped out but were included in data analysis. *: Since Heidrich 2009 reported three pilot studies and two RCTs fulfilled our inclusion criteria; therefore, both pilot RCTs were included (labelled as P1 and P2 above) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study 1 & 2: randomisation performed but method not described |
| Allocation concealment (selection bias) | Unclear risk | Study 1 & 2: study did not provide enough information to allow judgment as concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study 1 & 2: participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study 1 & 2: participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study 1 & 2: reasons for attrition not described but drop out rate not substantial |
| Selective reporting (reporting bias) | Unclear risk | Study 1 & 2: study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Study 1 & 2: sampling bias likely present as participants were volunteers. Participation rate was 73% and 81% for study 1 and 2 respectively |
Hoffman 2012.
| Methods | Design: single centre, RCT. Follow‐up: 8‐12 weeks and 12‐14 weeks from baseline. | |
| Participants | Major inclusion criteria: stage 0‐3 breast cancer; within 2 months to 2 years after the completion of surgery, chemotherapy and/or radiotherapy
N = 229 (E: 114, C: 115) Mean age: E: 49.0 ± 9.3 years, C: 50.1 ± 9.14years Stage of breast cancer: E: stage 0 (11, 10%), stage 1 (34, 30%), stage 2 (47, 41%); stage 3 (22, 20%); C: stage 0 (6, 5%), stage 1 (45, 39%), stage 2 (47, 41%); stage 3 (17, 15%) |
|
| Interventions | E: Psychological (mindfulness‐based stress reduction) + Education (didactic teaching) Psychological (mindfulness‐based stress reduction): group format, conducted by a qualified mindfulness‐based stress reduction instructor, 1 session per week of 2 hours for 8 weeks (except the 1st and 8th weeks were 2.25 hours, and 6th week was 6 hours in length, topics of formal mindfulness practice included a body scan, gentle and appropriate lying and standing toga‐based stretches, sitting meditation, some group discussions, and home formal mindfulness practice for 40‐45 minutes for 6 or 7 days per week. Education (didactic teaching): integrated into the mindfulness‐based stress reduction programme C: waiting‐list control |
|
| Outcomes | Quality of life: FACT‐B Others: FACT‐ES, POMS, WHO‐5 |
|
| Notes | C: comparison, E: experimental, FACT‐B: Functional Assessment of Cancer Therapy‐Breast, FACT‐ES: Functional Assessment of Cancer Therapy‐Endocrine Symptoms scale, POMS: Profile of Mood States, RCT: randomised controlled trial, WHO‐5: WHO five item well‐being questionnaire. Dropouts or missing where reported: 7 participants from experimental group and 4 from control were excluded from analysis due to missing baseline questionnaire or with > 20% missing data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment was performed by using an externally computer‐generated randomisation programme in blocks of four |
| Allocation concealment (selection bias) | Low risk | Random assignment was performed by operations director of the organisation, who was independent from the study, which ensured allocation concealment because no clinician/researcher could anticipate or direct the allocation of the participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Clinician‐researcher conducting the study and delivering the intervention could not be blinded to allocation of participants to intervention or control group. Participants could be aware of allocated interventions since they had to go through the intervention |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Anonymised data were collected by a research assistant who was blinded to group assignment and independent from intervention delivery. Participants could be aware of allocated interventions since they had to go through the intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The proration method (standard mean imputation) was used to address missing data within questionnaires. When whole questionnaires were missing at T2 or T3, data were imputed by using previous values carried forward. There were 3 instances (2 from intervention, 1 from control) in which more than 20% of data was missing from participants at T1, and their data was excluded. Reasons for attrition described in CONSORT diagram and attrition does not differ too much (11 from intervention, 4 from control) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported. |
| Other bias | High risk | Participation rate was about 36.2%. Sampling bias likely present as non‐probability sampling was used. |
Kim 2011.
| Methods | Design: single centre, RCT. Follow‐up: 12 weeks post‐experiment. | |
| Participants | Major inclusion criteria: stage 0‐3 breast cancer; within 2 years of diagnosis, completion of primary cancer treatment, unmet behavioural goals (no moderate‐intensity exercise for a minimum of 30 min per day at least 5 day per week or had a poor diet)
N = 45 (E: 23, C: 22) Mean age: E: 44.6 ± 9.9 years, C: 47.1 ± 7.3 years Stage of breast cancer: E: stage 0 (3, 13%), stage 1 (9, 39%), stage 2 (8, 35%); stage 3 (3, 13%); C: stage 0 (2, 9%), stage 1 (10, 45%), stage 2 (7, 32%); stage 3 (3, 14%) |
|
| Interventions | Physical (exercise) + education (balanced diet) Exercise: individual format, telephone counselling, conducted by a specially trained nurse with Master degree in nursing, 1 session per week of 30 minutes for 2 weeks, topic included stage‐matched individualised prescription for regular exercise complemented with a workbook, with the goal to achieve a moderate‐intensity exercise for a minimum of 30 minutes per day at least 5 day per week. Education (balanced diet): integrated into telephone counselling for exercise, with the goal to achieve a balanced diet. C: control |
|
| Outcomes | Quality of life: EORTC QLQ‐C30 Others: HADS, BFI |
|
| Notes | BFI: Brief Fatigue Inventory, C: comparison, EORTC QLQ‐C30: European Organisation for the Research and Treatment of Cancer Quality‐of‐Life Questionnaire‐Core 30, E: experimental, HADS: Hospital Anxiety and Depression Scale , RCT: randomised controlled trial. Dropouts or missing where reported: 5 participants from experimental group and 4 participants from control dropped out but were included in data analysis as per intention‐to‐treat |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The authors used a random numbers table to assign participants to groups |
| Allocation concealment (selection bias) | Unclear risk | Study did not provide enough information to allow judgment as concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | For participants with missing data, the authors treated available data under the missing‐at‐random assumption of the generalised estimating equation analysis. 9 participants dropped out and reasons included car accident (1), aggravated health condition after colonoscopy (1), breast cancer recurrence (1), lack of interest (1) and loss to follow‐up (1). Reasons for drop out not related to intervention and no differences in attrition observed between two groups (5 from intervention, 4 from control). Analysis based on intention‐to‐treat |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Participation rate was about 5.3%. Sampling bias likely present as non‐probability sampling was used |
Lahart 2016.
| Methods | Design: single centre, RCT. Follow‐up: 6 months from baseline. | |
| Participants | Major inclusion criteria: stage 1‐3 breast cancer; post‐surgery and had no surgery planned for the next 6 months at least; completion of adjuvant radiotherapy and/or chemotherapy not including hormonal therapy N = 80 (E: 40, C: 40) Mean age: E: 52.4 ± 10.3 years, C: 54.7 ± 8.3 years Stage of breast cancer: not reported |
|
| Interventions | Psychological (physical activity counselling) + educational Physical activity counselling: face‐to‐face counselling, follow‐up phone calls and post‐card prompts. Face‐to‐face counselling; individual format, conducted by the primary researcher, 1 session of 30‐45 minutes for moderate‐intensity physical activity guided by motivational interviewing principles, topics included decision balance exercise, benefits of physical activity, seeking social support, goal setting, types and intensities of physical activity, safety advice, and basic lifestyle information. Follow‐up phone calls; individual format, conducted by the primary researcher, 3 session of 15‐20 minutes at the end of month for the first 3 months, topics are similar to the face‐to‐face counselling. Participants were required to do home‐based moderate‐intensity physical activity over each week for 6 months. Post‐card prompts: a reminder leaflets encouraging participants taking part in home‐based physical activity were mailed to participants at months 4 and 5. Educational: an information booklet and a DVD, topics included exercising safety, exercise intensity, dealing with fatigue and exercising with lymphedema, and local physical activity opportunities. C: usual care (standard information of physical activity) |
|
| Outcomes | Quality of life: FACT‐B | |
| Notes | C: comparison, E: experimental, FACT‐B: Functional Assessment of Cancer, RCT: randomised controlled trial. Dropouts or missing where reported: 3 participants from experimental group and 7 participants from control did not complete the post‐intervention assessment but were included in data analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐generated random numbers list |
| Allocation concealment (selection bias) | Low risk | Allocation performed at a different site |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to treatment group. Personnel blinding not possible |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants and scientist not blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants lost to follow‐up in intervention group as they discontinued intervention (1 due to sciatica, 1 due to recurrence, 1 due to personal reasons). 7 participants lost to follow‐up in control group (2 due to not wanting to come back to hospital to finish trial, 1 due to hip operation, 4 due to being unable to contact). Attrition somewhat balanced |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Participation rate was about 53.3%. The baseline characteristics of participants in the 2 groups were overall similar in most characteristics, with only a few dissimilarities (e.g. usual care group reported more comorbidities). Those in the usual care group were more physically active compared with the intervention group at baseline. For International Physical Activity Questionnaire Physical Activity categories at baseline, 15% (n = 6) more participants were categorised in the high activity category in the usual‐care group compared with the intervention group. Sampling bias likely present as non‐probability sampling used |
Lengacher 2009.
| Methods | Design: single centre, RCT. Follow‐up: 6 weeks after random assignment. | |
| Participants | Major inclusion criteria: stage 0‐3 breast cancer; within 18 months of treatment completion of surgery and adjuvant radiotherapy and/or chemotherapy N = 84 (E: 41, C: 43) Mean age: overall 57.5 ± 9.4 years, E: not reported, C: not reported Stage of breast cancer: E: stage 0 (12.2%), stage 1 (63.4%), stage 2 (17.1%); stage 3 (7.3%); C: stage 0 (20.9%), stage 1 (44.2%), stage 2 (27.9%); stage 3 (7%) |
|
| Interventions | E: Psychological (mindfulness‐based stress reduction) + educational Mindfulness‐based stress reduction: group format, conducted by a psychologist with Mindfulness‐based stress reduction certification, 1 session per week of 2 hours for 6 weeks, topics included meditation, body scan procedures and with response to stress, yoga, and modification of stress to help manage psychological and physical symptoms and thus cope better with the distress associated with cancer; group interaction, discussion and support; home‐based practice, formal meditation and yoga practice for at least 15‐45 minutes per day for 6 days per week for 6 weeks, informal daily meditation and yoga practice for at least 15‐45 minutes for 6 weeks. Educational: part of the components of mindfulness‐based stress reduction, a training manual and 4 audiotapes covering materials related to relaxation, mediation, and mind‐body connection so as to support participants’ home practice of meditation and yoga. C: usual care (standard post‐treatment clinic visits) + waitlisted control |
|
| Outcomes | Quality of life: SF‐36 Others: STAI, CES, PSS, MDASI, CRS, LOT |
|
| Notes | C: comparison, CES‐D: Center for Epidemiological Studies Depression Scale, CRS: fear of recurrence, E: experimental, LOT: Life Orientation Test, MBSR: Mindfulness‐based stress reduction, MDASI: M.D Anderson Symptom Inventory, PSS: Perceived Stress Scale; RCT: randomised controlled trial, SF‐36: Short Form (36) Health Survey, STAI: State‐Trait Anxiety Inventory. Dropouts or missing data where reported: 1 participant from experimental group and 1 from control lost to follow up and were excluded from analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed but method was not described |
| Allocation concealment (selection bias) | Unclear risk | Study did not provide enough information to allow judgment as concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to treatment group. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were aware of allocated interventions since they had to go through the intervention. Although data collectors were blinded to treatment assignment when baseline data were collected, no information was provided of whether they were blinded when data post‐intervention was collected |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition balanced between groups (1 from intervention for disease recurrence, 1 from control for family obligation) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Black people were more often assigned to usual‐care regimen. Sampling method not described. Participation rate was about 43.5% |
Loerzel 2008.
| Methods | Design: A secondary analysis of a RCT by Meneses 2007. Follow‐up: 3 and 6 months from baseline. | |
| Participants | Major inclusion criteria: aged 65 years and older who participated in the Breast Cancer Education Intervention research study of Meneses 2007, stage 1‐2 breast cancer; within the first year after cancer treatment completion N = 50 (E: not reported, C: not reported) Mean age: overall 72.1 ± 5.1 years, E: not reported, C: not reported Stage of breast cancer: overall: stage 1 (35, 70%), stage 2 (15, 30%) |
|
| Interventions | E: Psycho‐educational Psycho‐educational: individual format, conducted by an intervention nurse, 3 face‐to‐face education and support sessions of 60‐90 minutes followed by 5 support face‐to face or telephone sessions of 30 minutes over a 6‐month period, topics included fatigue, lymphedema, pain, menopausal symptoms, hot flushes, sleep problems, anxiety and depression, fear of cancer recurrence, uncertainty, sexual function, family and social relationships, work, financial, ways to maintain health, nutrition and healthy diet, adherence to cancer surveillance, and the unique problems and concerns that each individual participant facing, together with an education binder containing the corresponded materials with each education and support session; homework assignments, read about the topics, listen to audiotaped materials, and try new self‐management tips C: control (attention control) |
|
| Outcomes | QOL‐BC | |
| Notes | C: comparison, E: experimental, RCT: randomised controlled trial, QOL‐BC: Quality of Life‐Breast Cancer. Dropouts or missing data where reported 2 participants did not complete the study; leaving 49 at 3 months and 48 at 6 months for data analysis. The data used in Loerzel 2008 should be extracted from Meneses 2007 but only limited to subjects aged 65 or above. Since Meneses 2007 only reported the changes of the scores for different period, their data could not be used in the analysis. Therefore, we included Loerzel 2008 to facilitate meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was performed but method not described |
| Allocation concealment (selection bias) | Unclear risk | Study did not provide enough information to allow judgment as concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No unexpected missing data was noted. 2 participants did not complete study due to withdrawal before data collection at time 2 (1) and died prior to final data collection from causes unrelated to breast cancer or participation in the study. Attrition between groups insignificant |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Sampling bias likely present as non‐probability sampling used. Participation rate not described |
Mann 2012.
| Methods | Design: single centre, RCT. Follow‐up: 9 and 26 weeks after randomisation. | |
| Participants | Major inclusion criteria: diagnosed with breast cancer with no evidence of other cancers or metastases; had completed surgery, radiotherapy or chemotherapy; had had at least ten problematic HFNS per week for a duration of 2 months or more N = 88 (E: 43, C: 45) Mean age: E: 53.16 ± 8.1 years, C: 54.05 ± 7.8 years Stage of breast cancer: not reported |
|
| Interventions | E: Psycho‐educational (cognitive behavioural therapy) Cognitive behavioural therapy: group format, conducted by a clinical psychologist, 1 session per week of 90 minutes for 6 weeks, topics included physiological, cognitive, behavioural and emotional components of HFNS, sharing the experiences of HFNS in the context of breast cancer and group discussion, triggers of HFNS, cognitive behavioural therapy strategies to reduce stress and anxiety and to manage HFNS, relaxation and paced breathing, behavioural reactions to hot flushes and ways to manage hot flushes in social situations, cognitive component of sleep problems, behavioural strategies to reduce wakefulness after night sweats, action plans to maintain cognitive and behavioural change; home‐based practice, relaxation and paced breathing daily and during HFNS; educational material; a relaxation and paced breathing audio CD to support daily home practice. C: usual care |
|
| Outcomes | Quality of life: SF‐36 Others: HFRS, WHQ | |
| Notes | C: comparison, E: experimental, HFNS: hot flushes and night sweats, HFRS: Hot Flushes Rating Scale, RCT: randomised controlled trial, SF‐36: Short Form (36) Health Survey, WHQ: Women s Health Questionnaire. Dropouts where reported: 4 from experimental group and 4 from control did not complete data collection and were excluded from data analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done in cohort groups, whereby the trial clinical psychologist sent participants' identification details to a programmer for randomisation based on a computer‐generated randomisation sequence, allocating participants in a one‐to‐one ratio, stratified by age with randomly varying block size |
| Allocation concealment (selection bias) | Unclear risk | Study did not provide enough information to allow judgment as concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Neither participants nor the clinical psychologist could be masked to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Researchers collecting outcome data and analysing results were masked. Women were met by a separate researcher who collected questionnaires and who also asked the women not to disclose their treatment allocation to the researcher who did the outcome assessments |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal rates were much the same in both treatment groups and reasons for attrition were stated. Reasons included unable to attend (1), ill health (2), symptoms ceased (1), chose not to participate (2), died (1), could not be contacted (7) and family problems (1) |
| Selective reporting (reporting bias) | Low risk | Study protocol was available and pre‐specified outcomes were reported in pre‐specified way |
| Other bias | High risk | Sampling bias likely present as non‐probability sampling used. Participation rate was about 48.2% |
Matthews 2014.
| Methods | Design: single centre, RCT. Follow‐up: post‐experiment, and at 3‐and 6‐month post‐experiment. | |
| Participants | Major inclusion criteria: stage 1‐3 breast cancer; within 1‐36 months of completion of primary cancer treatment, having chronic insomnia N = 56 (E: 30, C: 26) Mean age: E: 52.17 ± 6.9 years, C: 52.85 ± 7.8 years Stage of breast cancer: E: stage 1 (9, 30%), stage 2 (11, 36.7%); stage 3 (10, 33.3%); C: stage 1 (11, 42.3%), stage 2 (9, 34.6%); stage 3 (6, 23.1%) |
|
| Interventions | E: Psychological (cognitive behavioural therapy for insomnia) + educational (sleep hygiene education) Cognitive behavioural therapy for insomnia: individual format, conducted by an advanced practice nurse with specialised training in cognitive behavioural therapy for insomnia, 4 face‐to‐face sessions of 30‐60 minutes at sessions 1‐3 and 6, plus 2 phone sessions of 15‐20 minutes at sessions 4‐5, primary foci were reviewing the participants, cognitive behavioural therapy for insomnia overview, conceptual model of insomnia, sleep restriction and stimulus control, sleep hygiene principles, cognitive therapy, cognitive behavioural therapy for insomnia principles reinforcement, sleep schedules adjustment based on participants’ sleep diary, and relapse prevention and skills to cope with setbacks; home assignment, adherence of the prescribed sleep schedule sleep diary at home. Educational: integrated into cognitive behavioural therapy for insomnia; sleep restriction, stimulus control, sleep hygiene education. C: behavioural placebo treatment |
|
| Outcomes | Quality of life: EORTC QLQ‐C30 Others: HADS, PFS, ISI, AFI |
|
| Notes | AFI: Attentional Function Index, C: comparison, E: experimental, EORTC QLQ‐C30: European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire‐Core 30, HADS: Hospital Anxiety and Depressions Scale, ISI: Insomnia Severity Index, PFS: Piper Fatigue Scale, RCT: randomised controlled trial. Dropouts or missing data where reported: Of the 60 participants enrolled in the study, 2 from experimental group and 2 from control withdrew from the study and were excluded in data analysis. 17 participants did not complete all the follow‐up measures but were included in data analysis as per intention‐to‐treat analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using an adaptive randomisation programme, controlling for age, insomnia severity, recruitment site, and breast cancer stage |
| Allocation concealment (selection bias) | Unclear risk | Study did not provide enough information to allow judgment as concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were blind to treatment condition, study therapist was not blind to treatment condition |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Participants were blind to treatment condition. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition from both groups equal (2 from each group, but reasons for attrition NS. Withdrawn participants included in analysis. No significant interactions were noted between missing data status and group, indicating that no systematic differences existed between those with or without data at follow‐up that might influence treatment effects |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Sampling bias likely to be present as non‐probability sampling and volunteers were used. Participation rate was 80.0% |
McClure 2010.
| Methods | Design: single centre, RCT. Follow‐up: 2.5 week, 5 week and 3 months from baseline. | |
| Participants | Major inclusion criteria: stage 1‐2 unilateral breast cancer related lymphoedema with ≥10% increased affected arm size compared with the unaffected arm, breast cancer surgery >3 months previously N = 21 (E: 10, C: 11) Mean age: E: 57.0 ± 2.9 years, C: 59.7 ± 2.1 years Stage of breast cancer: not reported |
|
| Interventions | E: physical (exercise) + education Exercise: group format, no information about intervention provider, 1 session per week of 60 minutes for 5 weeks, sessions included participation in the video of “From Lymphoedema Onto Wellness (exercise and relaxation programme)”, and the topics related to lymphoedema, coping and relaxation techniques, together with group discussion and hands‐on practice; self‐monitored home programme, daily practice of “From Lymphoedema Onto Wellness” and relaxation techniques for 3 months. Educational: written educational materials, topics included lymphedema, coping and relaxation techniques. C: usual care (to continue with the lymphoedema instructions from their medical team) |
|
| Outcomes | Quality of life: SF‐36 Others: BDI, Internally generated questionnaire for measuring adherence |
|
| Notes | BDI: Beck Depression Inventory, C: comparison, E: experimental, RCT: randomised controlled trial, SF‐36: Short Form (36) Health Survey. Dropouts or missing data where reported: Of the 32 participants entered into the study, 6 participants from experimental group and 5 subjects from control were excluded from analysis due to metastatic breast cancer, incomplete data collection or had lymphoedema < 10%. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation performed but method not described |
| Allocation concealment (selection bias) | High risk | Although each participant opened a randomised, sealed, sequentially numbered envelope informing him or her of group status, the next allocation may be guessed by the screener as randomisation was done in groups of 8 with 4 in intervention and 4 in control |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were aware of group allocation. Study did not provide enough information to allow judgment as to whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants were aware of group allocation. Therapist was not aware of participant group status |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 32 participants who entered the study, 11 were not included in analysis. Reasons for exclusion in intervention group are: metastatic breast cancer (1), did not continue past 2.5 weeks (3), L‐Dex scores and percentage swelling < 10 (2). Reasons for exclusion in control group are: triple bypass after baseline testing (1), L‐Dex scores and percentage swelling < 10 (4). No significant difference in participant characteristics for participants in primary analysis compared with those not included in primary analysis |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Participation rate not described. Sampling bias likely present as non‐probability sampling used |
Meneses 2007.
| Methods | Design: Single centre, RCT. Follow‐up: 3 and 6 months from baseline. | |
| Participants | Major inclusion criteria: stage 0‐2 breast cancer and no evidence of local recurrence and metastatic disease; within one year of diagnosis, had surgery at least 1 month before, received radiotherapy or chemotherapy, may have been on hormonal therapy N = 256 (E: 125, C: 131) Mean age: overall: 54.5 ± 11.6 years, E: not reported, C: not reportedStage of breast cancer: not reported |
|
| Interventions | E: Psycho‐educational Psycho‐educational: individual format, conducted by an intervention nurse, 3 face‐to‐face education and support sessions of 60‐90 minutes followed by 5 monthly support face‐to face or telephone sessions of 30 minutes over a 6‐month period, topics included fatigue, lymphedema, pain, menopausal symptoms, hot flushes, sleep problems, anxiety and depression, fear of cancer recurrence, uncertainty, sexual function, family and social relationships, work, financial, ways to maintain health, nutrition and healthy diet, adherence to cancer surveillance, and the unique problems and concerns that each individual participant facing, together with an education binder containing the corresponded materials with each education and support session; do homework assignments, read about the topics, listen to audiotaped materials, and try new self‐management tips C: control (attention control) |
|
| Outcomes | Quality of life: QOL‐BC | |
| Notes | C: comparison, E: experimental, RCT: randomised controlled trial, QOL‐BC: Quality of Life‐Breast Cancer. Dropouts or missing data where reported 4 participants from experimental group and 1 from control did not complete the study and were excluded from data analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation performed but method not described |
| Allocation concealment (selection bias) | Unclear risk | Study did not provide enough information to allow judgment as concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 women in intervention group withdrew during first month of participation but reasons NS. 1 participant in control group died from non‐cancer‐related cause during the study |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Sampling bias likely present as non‐probability sampling used. Participation rate was about 77.0% |
Rogers 2009b.
| Methods | Design: RCT. Follow‐up: 12 weeks from baseline. | |
| Participants | Major inclusion criteria: Stage 0‐3A breast cancer, currently taking aromatase inhibitors or selective oestrogen receptor modulators N = 41 (E: 21, C: 20) Mean age: E: 52 ± 15 years, C: 54 ± 8 years Stage of breast cancer: E: stage 1 (6, 29%), stage 2 (11, 52%), stage 3 (4, 19%); C: stage 1 (6, 30%), stage 2 (10, 50%), stage 3 (4, 20%) |
|
| Interventions | E: Physical (exercise) + Psychological (group discussion session) Exercise: individual format, 12 supervised sessions in the first 6 weeks and 3 face‐to‐face sessions at the 8th, 10th and 12th week; supervised and face‐to‐face sessions included walking where the intensity was derived from fitness test, discussion on flexibility exercises and exercise barriers, specific social cognitive theory constructs addressed by the individual sessions included self‐efficacy, outcome expectations, behavioral capability, perceived barriers, and goal setting with self‐monitoring; conducted by an exercise specialist. Group discussion session: group format, 6 sessions in the first 8 weeks, topics included social support, exercise role models, journaling, time management, stress management, exercise barriers, and behavior modification; specific social cognitive theory constructs included self‐efficacy, emotional coping, reciprocal determinism, perceived barriers, outcome expectations, behavioral capability, goal setting, environment, observational learning, and self‐control; conducted by a clinical psychologist. C: received written materials |
|
| Outcomes | Quality of life: FACT‐B Others: PSQI, WOMAC, Process and program evaluation checklist, activity monitoring by accelerometer, Godin Leisure‐Time Exercise Questionnaire, readiness for physical activity, fitness by submaximal treadmill test, muscle strength by a back/leg extensor dynamometer, anthropometric measures |
|
| Notes | RCT: Randomised controlled trial, C: Comparison, E: Experimental, FACT‐B: Functional Assessment of Cancer Therapy—Breast, PSQI: Pittsburgh Sleep Quality Index, WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index. Dropouts or missing data where reported: 1 in experimental group withdrew due to unrelated medical problems and 1 in comparison group withdrew due to distance. As the data of Rogers 2009b and 2009cwere from the same trial, only Rogers 2009b data were used in the meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated |
| Allocation concealment (selection bias) | Low risk | Allocation was kept in sealed envelopes until randomisation to prevent bias in group allocation by study personnel |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant refused to answer 4 of the endocrine symptom items and 2 of the cognitive function items and endocrine symptoms, cognitive function interference with functioning and total cognitive function could not be calculated for this participant. Attrition after randomisation was 7% (3 participants), with 1 not completing follow‐up in intervention group owing to unrelated illness, 1 not completing follow‐up in usual care group owing to distance and 1 providing incomplete follow‐up data in usual care group |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Sampling bias likely present as non‐probability sampling and volunteers used. Participation rate was about 46.1% |
Rogers 2009c.
| Methods | Design: RCT. Follow‐up: 12 weeks and 6 months from baseline. | |
| Participants | Major inclusion criteria: Stage 0‐3A breast cancer, currently taking aromatase inhibitors or selective oestrogen receptor modulators N = 41 (E: 21, C: 20) Mean age: E: 52 ± 15 years, C: 54 ± 8 years Stage of breast cancer: E: stage 1 (6, 29%), stage 2 (11, 52%), stage 3 (4, 19%); C: stage 1 (6, 30%), stage 2 (10, 50%), stage 3 (4, 20%) |
|
| Interventions | E: Physical (exercise) + Psychological (group discussion session) Exercise: individual format, 12 supervised sessions in the first 6 weeks and 3 face‐to‐face sessions at the 8th, 10th and 12th week; supervised and face‐to‐face sessions included walking where the intensity was derived from fitness test, discussion on flexibility exercises and exercise barriers, specific social cognitive theory constructs addressed by the individual sessions included self‐efficacy, outcome expectations, behavioral capability, perceived barriers, and goal setting with self‐monitoring; conducted by an exercise specialist. Group discussion session: group format, 6 sessions in the first 8 weeks, topics included social support, exercise role models, journaling, time management, stress management, exercise barriers, and behavior modification; specific social cognitive theory constructs included self‐efficacy, emotional coping, reciprocal determinism, perceived barriers, outcome expectations, behavioral capability, goal setting, environment, observational learning, and self‐control; conducted by a clinical psychologist. C: received written materials |
|
| Outcomes | Quality of life: FACT‐B Others: PSQI, WOMAC, activity monitoring by accelerometer, total daily activity counts, weekly minutes of moderate plus vigorous physical activity, readiness for physical activity |
|
| Notes | RCT: Randomised controlled trial, C: Comparison, E: Experimental, FACT‐B: Functional Assessment of Cancer Therapy—Breast, PSQI: Pittsburgh Sleep Quality Index, WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index. Dropouts or missing data where reported: 1 in experimental group withdrew due to unrelated medical problems and 1 in comparison group withdrew due to distance. As the data of Rogers 2009b and 2009cwere from the same trial, only Rogers 2009b data were used in the meta‐analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated |
| Allocation concealment (selection bias) | Low risk | Allocation was kept in sealed envelopes until randomisation to prevent bias in group allocation by study personnel. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. IStudy did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants withdrew at immediately post‐intervention follow‐up and another 2 withdrew at 6‐month follow‐up. 3 were in usual care and withdrew due to unrelated disease (1), distance (1) and ill spouse (1). 2 were from intervention group due to unrelated medical problems (1) and lost to follow‐up (1) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported. |
| Other bias | High risk | Sampling bias likely present as non‐probability sampling and volunteers used. Participation rate was about 46.1%. |
Rogers 2015.
| Methods | Design: RCT. Follow‐up: 12 weeks and 6 months from baseline. | |
| Participants | Major inclusion criteria: with history of ductal carcinoma in situ (DCIS) or Stage 1‐3A breast cancer, not currently receiving or planning to receive chemotherapy or radiation therapy, 8 weeks or more post‐surgical procedure. N = 222 (E: 110, C: 112) Mean age: E: 54.9 ± 9.3 years, C: 53.9 ± 7.7 years Stage of breast cancer: E: stage DCIS (13, 11.8%), stage 1 (47, 42.7%), stage 2 (37, 33.6%), stage 3 (13, 11.8%); C: DCIS (12, 10.7%), stage 1 (46, 41.1%), stage 2 (41, 36.6%), stage 3 (13, 11.6%) |
|
| Interventions | E: Physical (exercise) + Psychological (group discussion session) Exercise: individual format, 12 supervised sessions in the first 6 weeks and 3 bi‐weekly face‐to‐face sessions in the second 6 weeks, 1‐hour supervised session covered warm‐up, aerobic walking on the treadmill, cool‐down, and stretching, self‐selected exercise after week 6, 30‐minute face‐to‐face session covered progress discussion, activity log sheets review, adherence and barriers assessment, heart rate monitor use assessment, and goals setting for the next two weeks; conducted by an exercise specialist. Group discussion session: group format, 6 sessions in the first 8 weeks, topics included goal setting, journaling, exercise benefits, time and stress management, activity logs reviewed, barriers, benefits, safety, role model presentation, relapse and wrap‐up discussion; conducted by trained facilitators. C: received written materials |
|
| Outcomes | Quality of life: FACT‐B Others: activity monitoring by accelerometer, Godin Leisure‐Time Exercise Questionnaire, self‐reported exercise intensity, fitness by submaximal treadmill test |
|
| Notes | RCT: Randomised controlled trial, C: Comparison, E: Experimental, DCIS: Ductal carcinoma in situ, FACT‐B: Functional Assessment of Cancer Therapy—Breast. Dropouts or missing data where reported: 5 in experimental and 4 in comparison lost to follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation to one of the two study group conditions was completed using computer‐generated numbers in blocks of 4 within each recruiting site |
| Allocation concealment (selection bias) | Low risk | Random assignment was kept in sealed, opaque envelopes which were opened in the order in which participants completed baseline testing. Research staff members were unaware of the assignment until the envelope was opened |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel who delivered the intervention were not blinded to group allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition somewhat balanced between groups. After treatment allocation, 5 participants from intervention group dropped out (due to time, family obligation, knee injury and fatigue). At 3‐month follow‐up, 4 were lost to follow‐up in intervention group (due to time and family obligation) and 2 were lost to follow‐up in control group (due to time and family obligation). At 6‐month follow‐up, 1 was lost to follow‐up in intervention group (due to being unable to contact) and 2 were lost to follow‐up in control group (due to time and stress). The study groups were balanced with regard to all characteristics except that a greater percentage of participants in the usual‐care group had been on hormonal therapy for ≤ 1 year (P = 0.02) |
| Selective reporting (reporting bias) | Low risk | Protocol was available and outcomes are reported as stated in protocol |
| Other bias | High risk | Participation rate was about 68.9%. Sampling bias likely present as non‐probability sampling used |
Savard 2005.
| Methods | Design: RCT. Follow‐up: 8 weeks, 3, 6 and 12 months from baseline. | |
| Participants | Major inclusion criteria: Stage 1‐3 breast cancer, completed radiotherapy and chemotherapy at least 1 month prior to enrolment onto the study, met diagnostic criteria for a chronic insomnia syndrome N = 58 (E: 28, C: 30) Mean age: E: 54.81 ± 7.01 years, C: 53.37 ± 7.72 years Stage of breast cancer: E: stage 1 (16, 59.3%), stage 2 (10, 37%), stage 3 (1, 3.7%); C: stage 1 (17, 56.7%), stage 2 (11, 36.7%), stage 3 (2, 6.7%) |
|
| Interventions | E: Educational + Psychological Group format with 6 participants each, 90‐minute session for 8 week conducted by a master‐level psychologist. Psychological: Covered stimulus control therapy, sleep restriction, and cognitive restructuring Educational: Covered sleep hygiene, fatigue and stress management strategies through a treatment manual. C: waiting‐list control |
|
| Outcomes | Quality of life: QLQ‐C30+3 Others: IIS, SCID, Sleep diary, Polysomnography, ISI, HADS, MFI |
|
| Notes | RCT: Randomised controlled trial, C: comparison, E: experimental, QLQ‐C30+3: The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire, IIS: Insomnia Interview Schedule, SCID: Structured Clinical Interview for the DSM‐IV, ISI: Insomnia Severity Index, HADS: Hospital Anxiety and Depression Scale, MFI: Multidimensional Fatigue Inventory. Dropouts or missing data where reported: 12 in experimental and 5 in comparison group lost to follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation performed but method not described |
| Allocation concealment (selection bias) | Unclear risk | Study did not provide enough information to allow judgment as concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition at various time points described with reasons. For intervention group, 3 lost interest and 1 developed a myocardial infarction during post‐treatment follow‐up, 2 lost interest and 1 developed severe major depression during 3‐month follow‐up and 1 was not available and 4 not initially planned in protocol at 12 month follow‐up. In control group, 2 had metastatic evolution and 2 lost interest at 3‐month follow‐up and 1 lost interest at 6 month follow‐up. Attrition appeared quite imbalanced between groups. However, all missed treatment sessions were rescheduled and all participants received entire treatment programme, excluding the 4 participants who dropped out of study during course of interventions |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Group difference at pretreatment was for proportion of participants with a comorbid physical illness, which was greater in the treatment than in the control condition. Sampling bias likely present as non‐probability sampling and volunteers were used. Participation rate about 55.2% |
Sherman 2010.
| Methods | Design: quasi‐experimental. Follow‐up: 8 weeks from baseline. | |
| Participants | Major inclusion criteria: Diagnosed with primary breast cancer, at least eight weeks post‐breast cancer surgery and not actively in treatment N = 162 (E: 116, C: 46) Mean age: E: 57.75 ± 10.54 years, C: 54.30 ± 10.51 years Stage of breast cancer: E: stage 0 (1.8%), stage 1 (20.0%), stage 2 (20.9%), stage 3 (19.1%), stage 4 (2.7%); C: stage 0 (4.4%), stage 1 (26.7%), stage 2 (17.8%), stage 3 (17.8%), stage 4 (0.0%) |
|
| Interventions | E: Physical (exercise) + Educational Group format 2‐hour session per week for 8 weeks conducted by trained Encore exercise coordinators. Physical: included low intensity floor‐based mobility and stretching exercises (20 min), and slow and progressive hydrotherapy resistance exercises (30 min) with 5‐min warm‐up and cool‐down periods. Educational: covered information relating to aspects of breast cancer survivorship (e.g., managing lymphedema risk, nutrition, breast reconstruction), and provided opportunities for group discussion. C: waitlist control |
|
| Outcomes | Quality of life: FACT‐B Others: IES, STAI, SSQ‐6, Satisfaction, Health‐related Beliefs |
|
| Notes | C: comparison, E: experimental, FACT‐B: Functional Assessment of Cancer Therapy – Breast, IES: Impact of Event Scale, STAI: State‐Trait Anxiety Inventory, SSQ‐6: Social Support Questionnaire 6. Dropouts or missing data where reported: 4 in experimental and 29 in comparison groups lost to follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Non‐random methods of group allocation performed based on the availability of the intervention. Women immediately offered a place in Encore were invited to join the Intervention arm of the study and those registered to wait for a place in the Encore programme were invited to join the Waitlist control arm of the study |
| Allocation concealment (selection bias) | High risk | Non‐random methods of group allocation performed based on the availability of the intervention. Women immediately offered a place in Encore were invited to join the Intervention arm of the study and those registered to wait for a place in the Encore programme were invited to join the Waitlist control arm of the study |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In the intervention arm, 3 women had large amounts of missing data on the baseline questionnaire and 26 women either did not complete the follow‐up questionnaire or did not participate in a minimum of 6 out of the 8 sessions, without reasons specified. 4 women in control condition did not return follow‐up questionnaire. No difference between women who dropped out and women completing study in terms of demographic and baseline dependent variable values. 87 women completed intervention and 42 women completed control |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Groups found to differ on time since diagnosis, number of women who had developed lymphoedema symptoms, age and family history or breast cancer. These variables treated as covariates in further analysis. Study design was quasi‐experimental. Sampling bias likely present as non‐probability sampling was used. Participation rate was 90.0% |
Spahn 2013.
| Methods | Design: single centre RCT. Follow‐up: 10 and 22 weeks from baseline. | |
| Participants | Major inclusion criteria: Stage 1‐3 breast cancer patients, completed their tumour treatment at least 3 months before, felt unusual fatigue during the past month N = 64 (E: 32, C: 32) Mean age: E: 58.1 ± 8.5 years, C: 55.3 ± 11.4 years Stage of breast cancer: E: stage 1 (9, 30%), stage 2 (17, 56.7%), stage 3 (2, 6.7%); C: stage 1 (12, 48%), stage 2 (11, 44%), stage 3 (0, 0.0%) |
|
| Interventions | E: Educational+ Physical + Psychological Group format (10‐20 participants) 6‐hour session per week for 10 weeks conducted by experienced sports therapist (walking) and a multi‐professional team (other components). Education: Included whole‐food cooking, naturopathic and self‐help strategies Physical: physical component included supervised sessions on walking. Psychological: Covered meditation and mindfulness. C: received identical supervised sessions on walking |
|
| Outcomes | Quality of life: EORTC Others: German Fatigue Assessment Questionnaire, Unusual fatigue by VAS scale, MFI, HADS, MRS |
|
| Notes | RCT: randomised controlled trial, C: comparison, E: experimental, EORTC: European Organization for Research and Treatment of Cancer, MFI: Multidimensional Fatigue Inventory, HADS: Hospital Anxiety and Depression Scale, MRS: Menopausal Rating Scale. Dropouts or missing data where reported: 7 in experimental and 2 in comparison group lost to follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned to treatment groups by a non stratified block‐randomisation with fixed block length of 10. The biometrician drew random numbers from the "ranuni" random number generator of the SAS software |
| Allocation concealment (selection bias) | Low risk | Assignments were kept in sealed, sequentially‐numbered, opaque envelopes and when a patient fulfilled all enrolment criteria, the study physician opened the envelopes in ascending order to reveal that patient's assignment. The study physicians could not access the assignment sequence |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing data were multiply imputed by Markov chain Monte Carlo methods. 2 intervention participants withdrew consent due to time limitations or difficult access routes. 7 control participants dropped out because of dissatisfaction with result of randomisation (2), metastatic disease (1), worsening of hip and/or knee pain (2), unexpected lack of time (1) and unwilling to return questionnaire (1) |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Sampling bias likely present as participants were volunteers. Participation rate was about 42.1% |
Swisher 2015.
| Methods | Design: RCT. Follow‐up:12 weeks from baseline. | |
| Participants | Major inclusion criteria: Stage 1‐3 invasive breast cancer, > 3 months after completion of active treatment, BMI > 25, confirmed oestrogen/progesterone/HER2neu‐negative status N = 28 (E: 18, C: 10) Mean age: E: 53.8 years, C: 53.6 years Stage of breast cancer: Not mentioned |
|
| Interventions | E: Psychological + Physical Psychological: Two individual sessions for reviewing participants' 3‐day diet record, setting diet goals and monitoring the progress, Physical: Three individually supervised and two unsupervised sessions per week for 12 weeks, supervised sessions included 30‐mins moderate‐intensity aerobic exercise together with stretching and resistance training (optional), unsupervised session involved 30‐mins home exercise (typically walking), conducted by exercise physiologist and dietitian. C: received written materials on healthy eating and physical activity |
|
| Outcomes | Quality of life: FACT‐B Others: BMI, waist and hip circumferences, body fat percentage, HAES, assays for serum cytokines and adipokines |
|
| Notes | RCT: randomised controlled trial, C: comparison, E: experimental, BMI: Body Mass Index, FACT‐B: Functional Assessment of Cancer Therapy – Breast, HAES: Habitual Activity Estimation Scale. Dropouts or missing data where reported: 5 in experimental and 0 in comparison group lost to follow‐up |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was determined a priori by the study statistician, but method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Group assignments were placed in opaque envelopes and not revealed until the completion of all baseline testing. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 participants lost to follow‐up in intervention group but reasons not reported. Attrition somewhat imbalanced (0% in control group, 27.8% in intervention group). Baseline differences between groups not described |
| Selective reporting (reporting bias) | Low risk | Study previously registered as a randomised controlled clinical trial (NCT01498536). Outcomes were reported as described |
| Other bias | High risk | Sampling bias likely present as non‐probability sampling was used. Participation rate was about 42.4% |
Wonghongkul 2008.
| Methods | Design: quasi‐experimental. Follow‐up: 6 and 18 weeks from baseline. | |
| Participants | Major inclusion criteria: Breast cancer patients diagnosed for at least 5 years, no recurrence of disease during data collection. N = 61 (E: 30, C: 31) Mean age: E: 53.95 ± 5.59 years, C: 52.20 ± 7.37 years Stage of breast cancer: Not reported |
|
| Interventions | E: Educational+ Psychological Group format 4‐hour session every 2 weeks for a total of 8 weeks conducted by nurses, doctors, or breast cancer survivors. Educational: Included 1.5 hours lecture on the topics about self‐health management (e.g. living with cancer, maintaining wellness of mind and body, maintaining healthy relationships, and effectively managing family and daily living) and 1 hour video on cancer‐related information Psychological: Included 0.5 hour stress reduction and relaxation through cassette tape. C: received routine care from the hospital |
|
| Outcomes | Quality of life: Quality of Life: Breast Cancer Version Questionnaire Others: Health Status Questionnaire (self‐developed) |
|
| Notes | C: comparison, E: experimental. Dropouts or missing data where reported: Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomisation was performed |
| Allocation concealment (selection bias) | High risk | No randomisation was performed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether personnel were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Participants could be aware of allocated interventions since they had to go through the intervention. Study did not provide enough information to allow judgment whether outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants in intervention group dropped out due to study being inconvenient as a result of workload or travel. 2 participants in control group did not complete questionnaire |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was not available. Study did not provide enough information to allow conclusion if all expected outcome measures were reported |
| Other bias | High risk | Quasi‐experimental design due to inability to have full control over extraneous variables and conduct random assignment. Sampling bias likely present as non‐probability sampling was used. Participation rate was not described |
AFI: Attentional Function Index BDI: Beck Depression Inventory BFI: Brief Fatigue Inventory BFLUTS: Bristol Female Lower Urinary Tract Symptoms Questionnaire BMI: body mass index BPI: Brief Pain Inventory BPT: behavioural placebo treatment BSI‐18: Brief Symptom Inventory CBT: cognitive behavioural therapy CD: compact disc CES‐D: Center for Epidemiologic Studies‐Depression Scale CRS: Concerns about Recurrence Scale DCIS: ductal carcinoma in situ EORTC QLQ‐BR23: European Organisation for Research and Treatment of Cancer Quality of Life Breast Cancer Questionnaire EORTC QLQ‐C30: European Organisation for Research and Treatment of Cancer Quality of Life C30 FACT‐B: Functional Assessment of Cancer FACT‐Cog: FACT‐Cognitive FACT‐ES: Endocrine subscale of the Functional Assessment of Cancer Therapy FACT‐F: FACT‐Fatigue FACT‐G: Functional Assessment of Cancer Therapy‐General FOSQ: Functional Outcomes of Sleep Questionnaire GCS: Greene Climacteric Scale GEAQ: German Fatigue Assessment Questionnaire HADS: Hospital Anxiety and Depression Scale HFNS: Hot flushes and night sweats HFRS: Hot Flush Rating Scale IES: Impact of Event Scale ISI: Insomnia Severity Index LOT: Life Orientation Test MDASI: M.D Anderson Symptom Inventory MFI: Multidimensional Fatigue Inventory MFSI‐SF: Multidimensional Fatigue Symptom Inventory MBSR: mindfulness‐based stress reduction MRS: Menopausal Rating Scale NS: not stated PE: physical exercises PFS: Piper Fatigue Scale POMS: Profile of Mood States POMSF/I: Profile of Mood States Fatigue/Inertia Subscale PSQI: Pittsburgh Sleep Quality Index PSS: Perceived Stress Scale QOL‐BC: Quality of Life‐Breast Cancer QOL P/CS: Quality of Life Patient/Cancer Survivor SAQ: Sexual Activity Questionnaire SB‐R: Symptom Bother‐Revised Scale SD: standard deviation SE: standard error SF‐12: Medical Outcomes Study Short Form 12 SF‐36: Short Form Health Survey STAI: State‐Trait Anxiety Inventory TSD: Target Symptom Distress TOI: Trial Outcome Index VAS: visual analogue scale WHO‐5: WHO five item well‐being questionnaire WHQ: Women’s Health Questionnaire WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Appling 2012 | Non‐home‐based |
| Ashing 2016 | Non‐multidimensional |
| Basen‐Engquist 2006 | Non‐home‐based |
| Bjorneklett 2012 | Non‐home‐based |
| Brown 2002 | Non‐multidimensional |
| Cadmus‐Bertram 2013 | Non‐multidimensional |
| Cantarero‐Villanueva 2012 | Non‐home‐based |
| Carlson 2013 | Non‐home‐based |
| Casla 2015 | Non‐home‐based |
| Cheema 2006 | Non‐home‐based |
| Courneya 2003 | Non‐multidimensional |
| Coward 2003 | Non‐home‐based |
| Crane‐Okada 2012 | Non‐multidimensional |
| Cuesta‐Vargas 2014 | Non‐home‐based |
| Culos‐Reed 2006 | Non‐multidimensional |
| Daley 2004 | Non‐home‐based |
| Daley 2007 | Non‐home‐based |
| Darga 2007 | Non‐home‐based |
| Dieli‐Conwright 2014 | Non‐multidimensional |
| Djuric 2002 | Non‐home‐based |
| Dolbeault 2009 | Non‐home‐based |
| Dorai 2004 | Non‐multidimensional |
| Edelman 1999 | Non‐multidimensional |
| Fernandez‐Lao 2013 | Non‐multidimensional |
| Freeman 2015 | Non‐multidimensional |
| Ganz 2000 | Non‐home‐based |
| Ganz 2004 | Non‐home‐based |
| Garrett 1996 | Non‐multidimensional |
| Gellaitry 2010 | Non‐multidimensional |
| Graves 2002 | Non‐multidimensional |
| Grunfeld 2006 | Non‐home‐based |
| Heim 2007 | Non‐home‐based |
| Heiney 2012 | Non‐home‐based |
| Hershman 2013 | Non‐home‐based |
| Hockett 2005 | Non‐home‐based |
| Howell 2005 | Non‐multidimensional |
| Hughes 2008 | Non‐multidimensional |
| Jeffs 2013b | Non‐multidimensional |
| Johnston 2011 | Non‐home‐based |
| Jones 2010 | Non‐multidimensional |
| Juarez 2015 | Non‐home‐based |
| Jun 2011 | Non‐home‐based |
| Khan 2012 | Non‐home‐based |
| Korstjens 2006 | Non‐home‐based |
| Kwiatkowski 2013 | Non‐home‐based |
| Lechner 2014 | Non‐multidimensional |
| Lee 2010b | Non‐multidimensional |
| Lengacher 2014 | Non‐multidimensional |
| Levine 2012 | Non‐home‐based |
| Listing 2009 | Non‐multidimensional |
| Littman 2012 | Non‐multidimensional |
| Loprinzi 2011 | Non‐multidimensional |
| Lyons 2015 | Non‐multidimensional |
| Mandelblatt 2008 | Non‐home‐based |
| Manne 2005 | Non‐home‐based |
| McKenzie 2003 | Non‐home‐based |
| McKiernan 2010 | Non‐home‐based |
| Milbury 2013 | Non‐multidimensional |
| Milne 2008 | Non‐home‐based |
| Monti 2013 | Non‐multidimensional |
| Mustian 2004 | Non‐home‐based |
| Mustian 2008 | Non‐home‐based |
| Naumann 2012a | Non‐home‐based |
| Naumann 2012b | Non‐home‐based |
| Neil 2013 | Non‐home‐based |
| Park JH 2012 | Non‐home‐based |
| Piland 2011 | Non‐multidimensional |
| Poorkiani 2010 | Non‐home‐based |
| Rowland 2009 | Non‐home‐based |
| Schmitz 2009 | Non‐home‐based |
| Schover 2006 | Non‐home‐based |
| Schover 2011 | Non‐home‐based |
| Schultz 2011 | Non‐home‐based |
| Scott 2013a | Non‐home‐based |
| Shields 2004 | Non‐home‐based |
| Shields 2010 | Non‐home‐based |
| Simpson 2001 | Non‐home‐based |
| Speck 2010 | Non‐multidimensional |
| Speed Andrews 2010 | Non‐home‐based |
| Sprod 2010 | Non‐multidimensional |
| Sprod 2012 | Non‐home‐based |
| Sterba 2015 | Non‐multidimensional |
| Stolley 2009 | Non‐home‐based |
| Vallance 2007 | Non‐multidimensional |
| Vallance 2008 | Non‐multidimensional |
| Vallance 2010 | Non‐multidimensional |
| von Ah 2012 | Non‐home‐based |
| Winters‐Stone 2012 | Non‐multidimensional |
| Witek‐Janusek 2008 | Non‐home‐based |
Characteristics of ongoing studies [ordered by study ID]
Abrahams 2015.
| Trial name or title | A randomised controlled trial of web‐based cognitive behavioral therapy for severely fatigued breast cancer survivors (CHANGE‐study) |
| Methods | Design: Multicentre RCT. N (Target) = 120 Follow‐up: baseline, after 6 months |
| Participants | Major inclusion criteria: Breast cancer survivors finished treatment at least 3 months previously, severely fatigued, able to assess internet |
| Interventions | E: Individual web‐based CBT, consisting of three face‐to‐face sessions and maximally eight web‐based modules over a period of 6 months; conducted by licensed cognitive behavioral therapists C: Care as usual |
| Outcomes | Quality of life (EORTC‐QLQ‐C30) Others CIS, SIP, BSI‐18 |
| Starting date | NS |
| Contact information | Harriet.Abrahams@radboudumc.nl |
| Notes | E: experimental, C: comparison, RCT: randomised control trial, EORTC‐QLQ‐C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30; CIS: Fatigue severity; SIP: Functional impairments; BSI‐18: Psychological distress. The study is registered in the Dutch Trial Registry (reference no. NTR4309, date registered: December 6, 2013). |
Befort 2014.
| Trial name or title | Protocol and recruitment results from a randomised controlled trial comparing group phone‐based versus newsletter interventions for weight loss maintenance among rural breast cancer survivors |
| Methods | Design: Multicentre RCT N: 80 + 80 (target) Follow‐up: 6, 12, 18, and 24 months from baseline |
| Participants | Major inclusion criteria: Stage 0‐3c post‐menopausal breast cancer survivors, diagnosed within the past 10 years, BMI of 27–45 kg/m2 |
| Interventions | All received behavioral weight loss intervention delivered through group phone sessions
E: Group phone‐based treatment C: Newsletter by mail |
| Outcomes | Quality of life: SF‐12 Others; Severity of physical symptoms: Breast Cancer Prevention Trial Symptom Scales, Costs, Adherence |
| Starting date | Recruitment occurred between October 2011 and September 2013 |
| Contact information | Befort CA: cbefort@kumc.edu |
| Notes | E: experimental, C: comparison, RCT: randomised control trial; SF‐12: Short Form (12) Health Survey. |
Hummel 2015.
| Trial name or title | Internet‐based CBT for sexual dysfunctions in women treated for breast cancer: design of a multicenter, randomised controlled trial |
| Methods | Design: Multicentre RCT; N = 160 (target). Follop‐up: (E) 10 and 24 weeks from start of therapy, (C) 13 and 23 weeks from randomisation |
| Participants | Major inclusion criteria: Diagnosis of breast cancer 6 months‐5 years prior to study entry, completion of breast cancer treatment (with the exception of endocrine therapy and immunotherapy), formal diagnosis of sexual dysfunction |
| Interventions | E: a maximum of 20 therapy sessions that are completed within a period of 24 weeks, minimum of 5 lower limit sessions (90‐120 min/ week), weekly contact between therapist and client, internet‐based CBT program; conducted by therapist and sexologist. C: Waiting‐list control |
| Outcomes | Primary outcomes:
Quality of Life: SF‐36; FACT‐ES ESS‐18,
Others: SAQ, FSFI, FSDS‐R, PAIR Inventory, EORTC QLQ‐BR 23 Body image subscale, MMQ, HADS, IIEF Intimacy (PAIR Inventory) Secondary outcomes Body image (QLQ‐BR23 Body Image Subscale) Menopausal symptoms (FACT‐ES ESS‐18) Marital functioning (MMQ) Psychological distress (HADS) (SF‐36) Sexual function (male partners) (IIEF) |
| Starting date | September 2013 |
| Contact information | n.aaronson@nki.nl |
| Notes | E: experimental, C: comparison, RCT: randomised control trial; SF‐36: Short Form (36) Health Survey; FACT‐ES ESS‐18: Functional Assessment of Cancer Therapy‐Endocrine Subscale: SAQ: Sexual Activity Questionnaire; FFSI: Female Sexual Function Index; FSDS‐R: Female Sexual Distress Scale‐Revised; EORTC QLQ‐BR 23 Body image subscale: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Breast Cancer 23 items Body image subscale; MMQ: Maudsley Marital Questionnaire; HADS: Hospital Anxiety and Depression Scale; IIEF: International Index of Erectile Function The trial has been approved by the Institutional Review Board of The Netherlands Cancer Institute (under number NL44153.031.13). |
Marcus 1998.
| Trial name or title | Telephone counselling of breast cancer patients after treatment: a description of a randomised clinical trial |
| Methods | Design: Multicentre RCT Target: 400 breast cancer survivors with a good prognosis randomised into two groups Follow‐up: 3, 6, 12 and 18 months from baseline; |
| Participants | Major inclusion criteria: Stage 1‐3 cancer patients, completed treatment where treatment plan did not include bone marrow transplantation |
| Interventions | E: 16 telephone counselling over a 12‐month period; The intervention is structured as 6 discrete phases: (1) Orientation; (2) Assessment; (3) Coping skills training; (4) Educational counselling using thematic modules; (5) Reinforcement: contextual integration; and, (6) Summary; conducted by counsellors and supervisor C: standard care. Participants assigned to the control condition will receive standard care and a directory of breast cancer‐specific resources in their area |
| Outcomes | Quality of life: FACT‐B Others: Depressive symptoms: CES‐D, IES, ISEL, perceived social support, Rand Medical Outcomes Study Family Functioning Measure, Cancer Patient Adjustment Questionnaire, ECOG Self‐Description Questionnaire |
| Starting date | NS |
| Contact information | Marcus AC: Center for behavioural Studies, AMC Cancer Research Center, 1600 Pierce Street, Lakewood, CO 80214, USA. Tel:1 303 2393397; Fax:1 303 2331863 |
| Notes | E: experimental, C: comparison, RCT: randomised control trial; FACT‐B: Functional Assessment of Cancer Therapy Scale for Breast Cancer; CES‐D: Center for Epidemiological Studies Depression Scale; IES: Impact of Event Scale; ISEL: Interpersonal Support Evaluation List. |
Matthews 2002.
| Trial name or title | A home‐based walking intervention among breast cancer survivors |
| Methods | Design: RCT Follow‐up: 12 weeks from baseline |
| Participants | Major inclusion criteria: early stage breast cancer survivors |
| Interventions | E: Home‐based walking with in‐person counselling visit and follow‐up telephone‐calls comparison: Wait‐list control |
| Outcomes | Quality of life: SF‐36 Others: Adherence |
| Starting date | NS |
| Contact information | Matthews CE: cematthe@sph.sc.edu |
| Notes | E: experimental, C: comparison, RCT: randomised control trial; SF‐36: Short Form (36) Health Survey |
McDonald 2014.
| Trial name or title | The muscle mass, omega‐3, diet, exercise and lifestyle (MODEL) study – a randomised controlled trial for women who have completed breast cancer treatment |
| Methods | Design: Single‐centre RCT N = 144 (target) Follow‐up: 12 and 24 weeks from baseline |
| Participants | Major Inclusion criteria: Stage 0‐3a breast cancer survivor, completed treatment more than 6 week but within 1 year, BMI between 20 and 35. |
| Interventions | Intervention: arms 1 and 2 E1 (N‐3 group): daily consumption of LCn‐3 FAs for 24 weeks E2 (EX+N‐3 group): daily consumption of LCn‐3 FAs for 24 weeks plus a supervised 12‐week exercise and nutrition group education programme; conducted by dietitian and accredited exercise physiologist C: Placebo supplementation |
| Outcomes | Quality of life: Functional Assessment of Cancer Therapy‐Breast + 4 (FACT‐B + 4) Others: Body composition: Air displacement plethysmography, Adherence to exercise and dietary programme: Active Australia Survey, 7‐d Uniaxial accelerometry and exercise log (Exercise); Dietary Habits Questionnaire; Attendance at sessions; HAQ‐DI; Menopausal symptoms: Greene Climacteric Scale |
| Starting date | NS |
| Contact information | MacDonald C: c.mcdonald4@uq.edu.au |
| Notes | E1: Experimental group 1; E2: Experimental group 2, C: comparison, RCT: randomised control trial; HAQ‐DI: Body composition: Air displacement plethysmography. Funding for the study was put forward by the Wesley Research Institute, this funding covered blood analyses, purchase of necessary equipment and payment for research assistance when required. The authors acknowledge that all capsules were provided gratis by Blackmores Ltd, Australia. In addition, GymStick provided exercise equipment for all participants at cost price. Parties associated with the supply of capsules and exercise equipment have no part in the research design, administration, analyses and subsequent publications. |
NCT01515124.
| Trial name or title | The Women In Steady Exercise Research (WISER) Survivor Trial |
| Methods | Design: RCT N: 555 (target) Follow‐up: 6 and 12 months from baseline |
| Participants | Major inclusion Criteria: Breast cancer survivor, completed treatment for at least 2 months, overweight or obese |
| Interventions | E1: 60‐90 min twice‐weekly supervised weight‐lifting sessions with 180 min of weekly aerobic exercise over 6 weeks; conducted by certified fitness professionals. E2: 24‐week intensive phase that includes weekly meetings and provision of all meals and snacks from a commercial manufacturer, conducted by registered dietitians. E3: E1 + E2 C: No intervention |
| Outcomes | Quality of life: lymphoedema‐related quality of life Others: Clinical lymphoedema exacerbation rate: Incident events requiring medical care for lymphoedema (e.g. flare‐ups or cellulitic infections), arm swelling in the affected limb (Interlimb volume differences), and pain & lymphoedema symptoms (number and severity) |
| Starting date | January 2012 |
| Contact information | Schmitz KH: schmitz@mail.med.upenn.edu |
| Notes | E1: Experimental group 1; E2: Experimental group 2, E3: Experimental group 3, C: comparison, RCT: randomised control trial. ClinicalTrials.gov Identifier: NCT01515124 Sponsor of the trial: University of Pennsylvania. |
Rock 2013.
| Trial name or title | Reducing breast cancer recurrence with weight loss, a vanguard trial: The Exercise and Nutrition to Enhance Recovery and Good Health for You (ENERGY) Trial |
| Methods | Design: Multicentre RCT
N = 697 (E: 348, C:349) Follow‐up: 6, 12, 18 and 24 months from baseline |
| Participants | Major inclusion criteria: Stage 1‐3 breast cancer survivors, diagnosed within the previous 5 years, completed initial therapies, BMI 25‐45 |
| Interventions | E: group‐based cognitive‐behavioral weight loss program with telephone counselling and tailored newsletters to support initial weight loss and subsequent maintenance;four months of weekly one‐hour group sessions for closed‐group contingents of an average of 15 women, tapering to every other week for two months. from 6 months onward, the groups met monthly for the remainder of the year. C: standard weight loss and maintenance guidelines available for the general public |
| Outcomes | Quality of life: SF‐36 Other CES‐D, IOCv2, Breast Cancer Prevention Trial Symptom Scales Side effects of breast cancer treatment: Breast Cancer Prevention Trial Symptom Scales |
| Starting date | Fall of 2010 |
| Contact information | Rock CL: clrock@ucsd.edu |
| Notes | E: experimental, C: comparison, RCT: randomised control trial, SF‐36: Short Form (36) Health Survey, CES‐D: Center for Epidemiologic Studies Depression Scale; IOCv2: Impact of cancer: Impact of Cancer Scale. |
BMI: body mass index CBT: cognitive behavioural therapy HFNS: hot flushes and night sweats NS: not stated RCT: randomised controlled trial
Differences between protocol and review
We incorporated functional status into the functional subscales of the quality‐of‐life measures, and there is no separate reposting of the functional status as a secondary outcome in this review.
In relation to symptom severity and distress, the outcomes of anxiety, depression, fatigue, sleep‐related outcomes, endocrine symptoms, symptom distress and joint pain, stiffness and physical function are reported, as these outcomes were reported in the included studies. We performed meta‐analysis for anxiety, depression, fatigue, insomnia, hot flushes and night sweats, and depressive symptoms due to the availability of adequate studies.
The secondary outcomes of service needs and utilisation, including psychosocial and supportive care needs, unplanned readmission and hospitalisation, and cost of care are not reported due to the unavailability of these data in the included studies.
One of the criteria for considering types of participants of studies for this review was women with a breast cancer diagnosis between stages 0 to 3. It implied that we excluded studies with women diagnosed with stage 4 or recurrent breast cancer. In order to make the eligibility criteria more explicit and clearer in this review, we have further specified an exclusion criteria in the Methods sections as “women with stage 4 or recurrent breast cancer were excluded” in the Methods section.
Contributions of authors
Karis Cheng (KC) conceptualised the protocol, and contributed to the development and writing of the protocol and the review. She will also be responsible for the update.
Ethel Lim (EL) and Wilson Tam (WT) contributed to the development of the search strategies, and drafting of the protocol and the review.
EL and Zhi Min Koh (ZMK) were involved in screening studies and organizing data extraction.
KC and WT were involved in analysis and interpretation of the data.
All authors contributed to the final review for publication.
Sources of support
Internal sources
National University of Singapore, Singapore.
External sources
None, Other.
Declarations of interest
KC: nothing to declare EL: nothing to declare WT: nothing to declare ZMK: nothing to declare
New
References
References to studies included in this review
Cho 2006 {published data only}
- Cho OH, Yoo YS, Kim NC. Efficacy of comprehensive group rehabilitation for women with early breast cancer in South Korea. Nursing & Health Sciences 2006;8(3):140‐6. [DOI] [PubMed] [Google Scholar]
Dirksen 2008 {published data only}
- Dirksen SR, Epstein DR. Efficacy of an insomnia intervention on fatigue, mood and quality of life in breast cancer survivors. Journal of Advanced Nursing 2008;61(6):664‐75. [DOI] [PubMed] [Google Scholar]
Duijts 2012 {published data only}
- Duijts SFA, Beurden M, Oldenburg HSA, Hunter MS, Kieffer JM, Stuiver MM, et al. Efficacy of cognitive behavioral therapy and physical exercise in alleviating treatment‐induced menopausal symptoms in patients with breast cancer: results of a randomized, controlled, multicenter trial. Journal of Clinical Oncology 2012;30(33):4124‐33. [DOI] [PubMed] [Google Scholar]
Ergun 2013 {published data only}
- Ergun M, Eyigor S, Karaca B, Kisim A, Uslu R. Effects of exercise on angiogenesis and apoptosis‐related molecules, quality of life, fatigue and depression in breast cancer patients. European Journal of Cancer Care 2013;22(5):626‐37. [DOI] [PubMed] [Google Scholar]
Eyigor 2010 {published data only}
- Eyigor S, Karapolat H, Yesil H, Uslu R, Durmaz B. Effects of pilates exercises on functional capacity, flexibility, fatigue, depression and quality of life in female breast cancer patients: a randomized controlled study. European Journal of Physical and Rehabilitation Medicine 2010;46(4):481‐7. [PubMed] [Google Scholar]
Fillion 2008 {published data only}
- Fillion L, Gagnon P, Leblond F, Gelinas C, Savard J, Dupuis R, et al. A brief intervention for fatigue management in breast cancer survivors. Cancer Nursing 2008;31(2):145‐59. [DOI] [PubMed] [Google Scholar]
Fiorentino 2008 {published data only}
- Fiorentino L. Cognitive behavioral therapy for insomnia in breast cancer survivors: a randomized controlled crossover study [Dissertation]. San Diego: Universty of California, 2008. [3296705 Ph.D] [Google Scholar]
Galantino 2010 {published data only}
- Galantino ML, Schmid P, Botis S, Dagan C, Leonard SM, Milos A. Exploring wellness coaching and traditional group support for breast cancer survivors: a pilot study. Rehabilitation Oncology 2010;28(1):19‐25. [Google Scholar]
Heidrich 2009 {published data only}
- Heidrich SM, Brown RL, Egan JJ, Perez OA, Phelan CH, Yeom H, et al. An individualized representational intervention to improve symptom management (IRIS) in older breast cancer survivors: three pilot studies. Oncology Nursing Forum 2009;36(3):E133‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoffman 2012 {published data only}
- Hoffman CJ, Ersser SJ, Hopkinson JB, Nicholls PG, Harrington JE, Thomas PW. Effectiveness of mindfulness‐based stress reduction in mood, breast‐ and endocrine‐related quality of life, and well‐being in stage 0 to III breast cancer: a randomized, controlled trial. Journal of Clinical Oncology 2012;30(12):1335‐42. [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim SH, Shin MS, Lee HS, Lee ES, Ro JS, Kang HS. Randomized pilot test of a simultaneous stage‐matched exercise and diet intervention for breast cancer survivors. Oncology Nursing Forum 2011;38(2):E97‐106. [DOI] [PubMed] [Google Scholar]
Lahart 2016 {published data only}
- Lahart IM, Metsio GS, Nevill AM, Kitas GD, Carmichael AR. Randomised controlled trial of a home‐based physical activity intervention in breast cancer survivors. BMC Cancer 2016;16(1):234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lengacher 2009 {published data only}
- Lengacher CA, Johnson‐Mallard V, Post‐White J, Moscoso MS, Jacobsen PB, Klein TW. Randomized controlled trial of mindfulness‐based stress reduction (MBSR) for survivors of breast cancer. Psycho‐Oncology 2009;18(12):1261‐72. [DOI] [PubMed] [Google Scholar]
Loerzel 2008 {published data only}
- Loerzel VW, McNees P, Powel LL, Su X, Meneses K. Quality of life in older women with early‐stage breast cancer in the first year of survivorship. Oncology Nursing Forum 2008;35(6):924‐32. [DOI] [PubMed] [Google Scholar]
Mann 2012 {published data only}
- Mann E, Smith MJ, Hellier J, Balabanovic JA, Hamed H, Grunfeld EA, et al. Cognitive behavioural treatment for women who have menopausal symptoms after breast cancer treatment (MENOS 1): a randomised controlled trial. Lancet Oncology 2012;13(3):309‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthews 2014 {published data only}
- Matthews EE, Berger AM, Schmiege SJ, Cook PF, McCarthy MS, Moore CM, et al. Cognitive behavioral therapy for insomnia outcomes in women after primary breast cancer treatment: a randomized, controlled trial. Oncology Nursing Forum 2014;41(3):241‐53. [DOI] [PubMed] [Google Scholar]
McClure 2010 {published data only}
- McClure MK, McClure RJ, Day R, Brufsky AM. Randomized controlled trial of the breast cancer recovery program for women with breast cancer‐related lymphedema. American Journal of Occupational Therapy 2010;64(1):59‐72. [DOI] [PubMed] [Google Scholar]
Meneses 2007 {published data only}
- Meneses KD, McNees P, Loerzel VW, Su X, Zhang Y, Hassey LA. Transition From Treatment to Survivorship:Effects of a Psyclioeducationai Interventionon Quaiity ol Life in Breast Cancer Survivors. Oncology Nursing Forum 2007;34(5):1007‐1016. [DOI] [PubMed] [Google Scholar]
Rogers 2009b {published data only}
- Rogers LQ, Hopkins‐Price P, Vicari S, Markwell S, Pamenter R, Courneya KS, et al. Physical activity and health outcomes three months after completing a physical activity behavior change intervention: persistent and delayed effects. Cancer Epidemiology, Biomarkers & Prevention 2009;18(5):1410‐18. [DOI] [PubMed] [Google Scholar]
Rogers 2009c {published data only}
- Rogers LQ, Hopkins‐Price P, Vicari S, Pamenter R, Courneya KS, Markwell S, et al. A randomized trial to increase physical activity in breast cancer survivors. Medicine and Science in Sports and Exercise 2009;41(4):935‐46. [DOI] [PubMed] [Google Scholar]
Rogers 2015 {published data only}
- Rogers LQ, Courneya KS, Anton PM, Hopkins‐Price P, Verhulst S, Vicari SK, et al. Effects of the BEAT Cancer physical activity behavior change intervention on physical activity, aerobic fitness, and quality of life in breast cancer survivors: a multicenter randomized controlled trial. Breast Cancer Research and Treatment 2015;149(1):109‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Savard 2005 {published data only}
- Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive‐behavioural therapy for insomnia secondary to breast cancer, part I: sleep and psychological effects. Journal of Clinical Oncology 2005;23(25):6083‐96. [DOI] [PubMed] [Google Scholar]
Sherman 2010 {published data only}
- Sherman KA, Heard G, Cavanagh KL. Psychological effects and mediators of a group multi‐component program for breast cancer survivors. Journal of Behavioral Medicine 2010;33(5):378‐91. [DOI] [PubMed] [Google Scholar]
Spahn 2013 {published data only}
- Spahn G, Choi KE, Kennemann C, Ludtke R, Franken U, Langhorst J, et al. Can a multimodal mind‐body program enhance the treatment effects of physical activity in breast cancer survivors with chronic tumor‐associated fatigue? A randomized controlled trial. Integrative Cancer Therapies 2013;12(4):291‐300. [DOI] [PubMed] [Google Scholar]
Swisher 2015 {published data only}
- Swisher AK, Abraham J, Bonner D, Gilleland D, Hobbs G, Kurian S, et al. Exercise and dietary advice intervention for survivors of triple‐negative breast cancer: effects on body fat, physical function, quality of life, and adipokine profile. Supportive Care in Cancer 2015;23(10):2995‐3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wonghongkul 2008 {published data only}
- Wonghongkul T, Sawasdisingha P, Aree P, Thummathai K, Tungpunkom P, Muttarak M. Effect of educative ‐‐ supportive program on quality of life in breast cancer survivors. Thai Journal of Nursing Research 2008;12(3):179‐93. [Google Scholar]
References to studies excluded from this review
Appling 2012 {published data only}
- Appling SE, Scarvalone S, MacDonald R, McBeth M, Helzlsouer KJ. Fatigue in breast cancer survivors: the impact of a mind‐body medicine intervention. Oncology Nursing Forum 2012;39(3):278‐86. [DOI] [PubMed] [Google Scholar]
Ashing 2016 {published data only}
- Ashing LT, Miller AM. Assessing the utility of a telephonically delivered psychoeducational intervention to improve health‐related quality of life in African American breast cancer survivors: a pilot trial. Psycho‐Oncology 2016;25(2):236‐8. [DOI] [PubMed] [Google Scholar]
Basen‐Engquist 2006 {published data only}
- Basen‐Engquist K, Taylor CL, Rosenblum C, Smith MA, Shinn EH, Greisinger A, et al. Randomized pilot test of a lifestyle physical activity intervention for breast cancer survivors. Patient Education and Counseling 2006;64(1‐3):225‐34. [DOI] [PubMed] [Google Scholar]
Bjorneklett 2012 {published data only}
- Bjorneklett HG, Lindemalm C, Ojutkangas ML, Berglund A, Letocha H, Strang P, et al. A randomized controlled trial of a support group intervention on the quality of life and fatigue in women after primary treatment for early breast cancer. Supportive Care in Cancer 2012;20(12):3325‐34. [DOI] [PubMed] [Google Scholar]
Brown 2002 {published data only}
- Brown L, Payne S, Royle G. Patient‐initiated follow‐up of breast cancer. Psycho‐Oncology 2002;11(4):346‐55. [DOI] [PubMed] [Google Scholar]
Cadmus‐Bertram 2013 {published data only}
- Cadmus‐Bertram L, Littman AJ, Ulrich CM, Stovall R, Ceballos RM, McGregor BA, et al. Predictors of adherence to a 26‐week Viniyoga intervention among post‐treatment breast cancer survivors. Journal of Alternative and Complementary Medicine 2013;19(9):751‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cantarero‐Villanueva 2012 {published data only}
- Cantarero‐Villanueva I, Fernandez‐Lao C, Moral‐Avila R, Fernandez‐de‐las‐Penas C, Feriche‐Fernandez‐Castanys MB, Arroyo‐Morales M. Effectiveness of core stability exercises and recovery myofascial release massage on fatigue in breast cancer survivors: a randomized controlled clinical trial. Evidence‐based Complementary and Alternative Medicine 2012;2012:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Carlson 2013 {published data only}
- Carlson LE, Doll R, Stephen J, Faris P, Tamagawa R, Drysdale E, et al. Randomized controlled trial of mindfulness‐based cancer recovery versus supportive expressive group therapy for distressed survivors of breast cancer. Journal of Clinical Oncology 2013;31(25):3119‐26. [DOI] [PubMed] [Google Scholar]
Casla 2015 {published data only}
- Casla S, Lopez‐Tarruella S, Jerez Y, Marquez‐Rodas I, Galvao DA, Newton RU, et al. Supervised physical exercise improves VO2max, quality of life, and health in early stage breast cancer patients: a randomized controlled trial. Breast Cancer Research and Treatment 2015;153(2):371‐82. [DOI] [PubMed] [Google Scholar]
Cheema 2006 {published data only}
- Cheema BS, Gaul CA. Full‐body exercise training improves fitness and quality of life in survivors of breast cancer. Journal of Strength and Conditioning Research 2006;20(1):14‐21. [DOI] [PubMed] [Google Scholar]
Courneya 2003 {published data only}
- Courneya KS, Mackey JR, Bell GJ, Jones LW, Field CJ, Fairey AS. Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: cardiopulmonary and quality of life outcomes. Journal of Clinical Oncology 2003;21(9):1660‐8. [DOI] [PubMed] [Google Scholar]
Coward 2003 {published data only}
- Coward DD. Facilitation of self‐transcendence in a breast cancer support group: II. Oncology Nursing Forum 2003;30(2):291‐300. [DOI] [PubMed] [Google Scholar]
Crane‐Okada 2012 {published data only}
- Crane‐Okada R, Kiger H, Sugerman F, Uman GC, Shapiro SL, Wyman‐McGinty W, et al. Mindful movement program for older breast cancer survivors: a pilot study. Cancer Nursing 2012;35(4):E1‐E13. [DOI] [PubMed] [Google Scholar]
Cuesta‐Vargas 2014 {published data only}
- Cuesta‐Vargas A, Buchan J, Arroyo‐Morales M. A multimodal physiotherapy programme plus deep water running for improving cancer‐related fatigue and quality of life in breast cancer survivors. European Journal of Cancer Care 2014;23(1):15‐21. [DOI] [PubMed] [Google Scholar]
Culos‐Reed 2006 {published data only}
- Culos‐Reed SN, Carlson LE, Daroux LM, Hately‐Aldous S. A pilot study of yoga for breast cancer survivors: physical and psychological benefits. Psycho‐Oncology 2006;15(10):891‐7. [DOI] [PubMed] [Google Scholar]
Daley 2004 {published data only}
- Daley AJ, Mutrie N, Crank H, Coleman R, Saxton J. Exercise therapy in women who have had breast cancer: design of the Sheffield women's exercise and well‐being project. Health Education Research 2004;19(6):686‐97. [DOI] [PubMed] [Google Scholar]
Daley 2007 {published data only}
- Daley AJ, Crank H, Saxton JM, Mutrie N, Coleman R, Roalfe A. Randomized trial of exercise therapy in women treated for breast cancer. Journal of Clinical Oncology 2007;25(13):1713‐21. [DOI] [PubMed] [Google Scholar]
Darga 2007 {published data only}
- Darga LL, Magnan M, Mood D, Hryniuk WM, DiLaura NM, Djuric Z. Quality of life as a predictor of weight loss in obese, early‐stage breast cancer survivors. Oncology Nursing Forum 2007;34(1):86‐92. [DOI] [PubMed] [Google Scholar]
Dieli‐Conwright 2014 {published data only}
- Dieli‐Conwright CM, Mortimer JE, Schroeder ET, Courneya K, Demark‐Wahnefried W, Buchanan TA, et al. Randomized controlled trial to evaluate the effects of combined progressive exercise on metabolic syndrome in breast cancer survivors: rationale, design, and methods. BMC Cancer 2014;14:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
Djuric 2002 {published data only}
- Djuric Z, DiLaura NM, Jenkins I, Darga L, Jen CK, Mood D. Combining weight‐loss counseling with the Weight Watchers plan for obese breast cancer survivors. Obesity Research 2002;10(7):657‐65. [DOI] [PubMed] [Google Scholar]
Dolbeault 2009 {published data only}
- Dolbeault S, Cayrou S, Bredart A, Viala A, Desclaux B, Saltel P, et al. The effectiveness of a psycho‐educational group after early‐stage breast cancer treatment: results of a randomized French study. Psycho‐Oncology 2009;18(6):647‐56. [DOI] [PubMed] [Google Scholar]
Dorai 2004 {published data only}
- Dorai VK. Impact of a modified dietary plan on the health related quality of life of minority participants in the Women's Healthy Eating and Living (WHEL) Study [PhD thesis]. Houston: The University of Texas Health Sciences Center at Houston School of Public Health, 2004. [Google Scholar]
Edelman 1999 {published data only}
- Edelman S, Bell DR, Kidman AD. Group CBT versus supportive therapy with patients who have primary breast cancer. Journal of Cognitive Psychotherapy 1999;13(3):189‐202. [Google Scholar]
Fernandez‐Lao 2013 {published data only}
- Fernandez‐Lao C, Cantarero‐Villanueva I, Ariza‐Garcia A, Courtney C, Fernandez‐De‐Las‐Penas C, Arroyo‐Morales M. Water versus land‐based multimodal exercise program effects on body composition in breast cancer survivors: a controlled clinical trial. Supportive Care in Cancer 2013;21(2):521‐30. [DOI] [PubMed] [Google Scholar]
Freeman 2015 {published data only}
- Freeman LW, White R, Ratcliff CG, Sutton S, Stewart M, Palmer JL, et al. A randomized trial comparing live and telemedicine deliveries of an imagery‐based behavioral intervention for breast cancer survivors: reducing symptoms and barriers to care. Psycho‐Oncology 2015;24(8):910‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ganz 2000 {published data only}
- Ganz PA, Greendale GA, Petersen L, Zibecchi L, Kahn B, Belin TR. Managing menopausal symptoms in breast cancer survivors: results of a randomized controlled trial. Journal of the National Cancer Institute 2000;92(13):1054‐64. [DOI] [PubMed] [Google Scholar]
Ganz 2004 {published data only}
- Ganz PA, Kwan L, Stanton AL, Krupnick JL, Rowland JH, Meyerowitz BE, et al. Quality of life at the end of primary treatment of breast cancer: first results from the Moving Beyond Cancer randomized trial. Journal of the National Cancer Institute 2004;96(5):376‐87. [DOI] [PubMed] [Google Scholar]
Garrett 1996 {published data only}
- Garrett K. Counselling breast cancer survivors via a telephone intervention. Psycho‐Oncology 1996; Vol. 5, issue 3 Suppl:20.
Gellaitry 2010 {published data only}
- Gellaitry G, Peters K, Bloomfield D, Home R. Narrowing the gap: the effects of an expressive writing intervention on perceptions of actual and ideal emotional support in women who have completed treatment for early stage breast cancer. Psycho‐Oncology 2010;19(1):77‐84. [DOI] [PubMed] [Google Scholar]
Graves 2002 {published data only}
- Graves KD. Quality of life intervention for breast cancer survivors: application of social cognitive theory [Dissertation]. Blacksberg: Virginia Polytechnic Institute and State University, 2002. [3144015 Ph.D] [Google Scholar]
Grunfeld 2006 {published data only}
- Grunfeld E, Levine MN, Julian JA, Coyle D, Szechtman B, Mirsky D, et al. Randomized trial of long‐term follow‐up for early‐stage breast cancer: a comparison of family physician versus specialist care. Journal of Clinical Oncology 2006;24(6):848‐55. [DOI] [PubMed] [Google Scholar]
Heim 2007 {published data only}
- Heim ME, Malsburg MLE, Niklas A. Randomized controlled trial of a structured training program in breast cancer patients with tumor‐related chronic fatigue. Onkologie 2007;30(8‐9):429‐34. [DOI] [PubMed] [Google Scholar]
Heiney 2012 {published data only}
- Heiney SP, Underwood SM, Tavakoli A, Adams SA, Wells LM, Bryant LH. Randomized trial of therapeutic group by teleconference: African American women with breast cancer. Cancer 2012;118(15):3822‐32. [DOI] [PubMed] [Google Scholar]
Hershman 2013 {published data only}
- Hershman DL, Greenlee H, Awad D, Kalinsky K, Maurer M, Kranwinkel G, et al. Randomized controlled trial of a clinic‐based survivorship intervention following adjuvant therapy in breast cancer survivors. Breast Cancer Research and Treatment 2013;138(3):795‐806. [DOI] [PubMed] [Google Scholar]
Hockett 2005 {published data only}
- Hockett KA. The effects of a comprehensive post‐treatment recovery program for breast cancer survivors [Dissertation]. Tampa: University of South Florida, 2005. [3188412 Ph.D] [Google Scholar]
Howell 2005 {published data only}
- Howell D, Watson M. Evaluation of a pilot nurse‐led, community‐based treatment programme for lymphoedema. International Journal of Palliative Nursing 2005;11(2):62‐9. [DOI] [PubMed] [Google Scholar]
Hughes 2008 {published data only}
- Hughes DC, Leung P, Naus MJ. Using single‐system analyses to assess the effectiveness of an exercise intervention on quality of life for Hispanic breast cancer survivors: a pilot study. Social Work in Health Care 2008;47(1):73‐91. [DOI] [PubMed] [Google Scholar]
Jeffs 2013b {published data only}
- Jeffs E, Wiseman T. Randomised controlled trial to determine the benefit of daily home‐based exercise in addition to self‐care in the management of breast cancer‐related lymphoedema: a feasibility study. Supportive Care in Cancer 2013;21(4):1013‐23. [DOI] [PubMed] [Google Scholar]
Johnston 2011 {published data only}
- Johnston MF, Hays RD, Subramanian SK, Elashoff RM, Axe EK, Li JJ, et al. Patient education integrated with acupuncture for relief of cancer‐related fatigue randomized controlled feasibility study. BMC Complementary and Alternative Medicine 2011;11:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 2010 {published data only}
- Jones LW, Douglas PS, Eves ND, Marcom PK, Kraus WE, Herndon JE, et al. Rationale and design of the Exercise Intensity Trial (EXCITE): a randomized trial comparing the effects of moderate versus moderate to high‐intensity aerobic training in women with operable breast cancer. BMC Cancer 2010;10:531. [DOI] [PMC free article] [PubMed] [Google Scholar]
Juarez 2015 {published data only}
- Juarez G, Hurria A, Uman G, Ferrell B. Impact of a bilingual education intervention on the quality of life of Latina breast cancer survivors. Oncology Nursing Forum 2015;40(1):E50‐E60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jun 2011 {published data only}
- Jun EY, Kim S, Chang SB, Oh K, Kang HS, Kang SS. The effect of a sexual life reframing program on marital intimacy, body image, and sexual function among breast cancer survivors. Cancer Nursing 2011;34(2):142‐9. [DOI] [PubMed] [Google Scholar]
Khan 2012 {published data only}
- Khan F, Amatya B, Pallant JF, Rajapaksa I, Brand C. Multidisciplinary rehabilitation in women following breast cancer treatment: a randomized controlled trial. Journal of Rehabilitation Medicine 2012;44(9):788‐94. [DOI] [PubMed] [Google Scholar]
Korstjens 2006 {published data only}
- Korstjens I, Mesters I, Peet E, Gijsen B, Borne B. Quality of life of cancer survivors after physical and psychosocial rehabilitation. European Journal of Cancer Prevention 2006;15(6):541‐7. [DOI] [PubMed] [Google Scholar]
Kwiatkowski 2013 {published data only}
- Kwiatkowski F, Mouret‐Reynier MA, Duclos M, Leger‐Enreille A, Bridon F, Hahn T, et al. Long term improved quality of life by a 2‐week group physical and educational intervention shortly after breast cancer chemotherapy completion. Results of the 'Programme of Accompanying women after breast Cancer treatment completion in Thermal resorts' (PACThe) randomised clinical trial of 251 patients. European Journal of Cancer 2013;49(7):1530‐8. [DOI] [PubMed] [Google Scholar]
Lechner 2014 {published data only}
- Lechner SC, Whitehead NE, Vargas S, Annane DW, Robertson BR, Carver CS, et al. Does a community based stress management intervention affect psychological adaptation among underserved black breast cancer survivors?. Journal of the National Cancer Institute Monographs 2014;2014(50):315‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2010b {published data only}
- Lee SA, Kang J, Kim YD, An AR, Kim S, Kim Y, et al. Effects of a scapula‐oriented shoulder exercise programme on upper limb dysfunction in breast cancer survivors: a randomized controlled pilot trial. Clinical rehabilitation 2010;24(7):600‐13. [DOI] [PubMed] [Google Scholar]
Lengacher 2014 {published data only}
- Lengacher C, Shelton M, Reich R, Barta M, Johnson‐Mallard V, Moscoso M, et al. Mindfulness based stress reduction (MBSR(BC)) in breast cancer: evaluating fear of recurrence (FOR) as a mediator of psychological and physical symptoms in a randomized control trial (RCT). Journal of Behavioral Medicine 2014;37(2):185‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Levine 2012 {published data only}
- Levine AS, Balk JL. Pilot study of yoga for breast cancer survivors with poor quality of life. Complementary Therapies in Clinical Practice 2012;18(4):241‐5. [DOI] [PubMed] [Google Scholar]
Listing 2009 {published data only}
- Listing M, Reisshauer A, Krohn M, Voigt B, Tjahono G, Becker J, et al. Massage therapy reduces physical discomfort and improves mood disturbances in women with breast cancer. Psycho‐Oncology 2009;18(12):1290‐9. [DOI] [PubMed] [Google Scholar]
Littman 2012 {published data only}
- Littman AJ, Bertram LC, Ceballos R, Ulrich CM, Ramaprasad J, McGregor B, et al. Randomized controlled pilot trial of yoga in overweight and obese breast cancer survivors: effects on quality of life and anthropometric measures. Supportive Care in Cancer 2012;20(2):267‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Loprinzi 2011 {published data only}
- Loprinzi CE, Prasad K, Schroeder DR, Sood A. Stress Management and Resilience Training (SMART) program to decrease stress and enhance resilience among breast cancer survivors: a pilot randomized clinical trial. Clinical Breast Cancer 2011;11(6):364‐8. [DOI] [PubMed] [Google Scholar]
Lyons 2015 {published data only}
- Lyons KD, Hull JG, Kaufman PA, Li Z, Seville JL, Ahles TA. Development and initial evaluation of a telephone‐delivered, behavioral activation, and problem‐solving treatment program to address functional goals of breast cancer survivors. Journal of Psychosocial Oncology 2015;33(2):199‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandelblatt 2008 {published data only}
- Mandelblatt JS, Cullen J, Lawrence WF, Stanton AL, Yi B, Kwan L, et al. Economic evaluation alongside a clinical trial of psycho‐educational interventions to improve adjustment to survivorship among patients with breast cancer. Journal of Clinical Oncology 2008;26(10):1684‐90. [DOI] [PubMed] [Google Scholar]
Manne 2005 {published data only}
- Manne SL, Ostroff JS, Winkel G, Fox K, Grana G, Miller E, et al. Couple‐focused group intervention for women with early stage breast cancer. Journal of Consulting and Clinical Psychology 2005;73(4):634‐46. [DOI] [PubMed] [Google Scholar]
McKenzie 2003 {published data only}
- McKenzie DC, Kalda AL. Effect of upper extremity exercise on secondary lymphedema in breast cancer patients: a pilot study. Journal of Clinical Oncology 2003;21(3):463‐6. [DOI] [PubMed] [Google Scholar]
McKiernan 2010 {published data only}
- McKiernan A, Steggles S, Guerin S, Carr A. A controlled trial of group cognitive behavior therapy for Irish breast cancer patients. Journal of Psychosocial Oncology 2010;28(2):143‐56. [DOI] [PubMed] [Google Scholar]
Milbury 2013 {published data only}
- Milbury K, Chaoul A, Biegler K, Wangyal T, Spelman A, Meyers C, et al. Tibetan sound meditation for cognitive dysfunction: results of a randomized controlled pilot trial. Psycho‐Oncology 2013;22(10):2354‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Milne 2008 {published data only}
- Milne HM, Wallman KE, Gordon S, Courneya KS. Effects of a combined aerobic and resistance exercise program in breast cancer survivors: a randomized controlled trial. Breast Cancer Research and Treatment 2008;108(2):279‐88. [DOI] [PubMed] [Google Scholar]
Monti 2013 {published data only}
- Monti DA, Kash KM, Kunkel EJ, Moss A, Mathews M, Brainard G, et al. Psychosocial benefits of a novel mindfulness intervention versus standard support in distressed women with breast cancer. Psycho‐Oncology 2013;22(11):2565‐75. [DOI] [PubMed] [Google Scholar]
Mustian 2004 {published data only}
- Mustian KM, Katula JA, Gill DL, Roscoe JA, Lang D, Murphy K. Tai Chi Chuan, health‐related quality of life and self‐esteem: a randomized trial with breast cancer survivors. Supportive Care in Cancer 2004;12(12):871‐6. [DOI] [PubMed] [Google Scholar]
Mustian 2008 {published data only}
- Mustian KM, Palesh OG, Flecksteiner SA. Tai Chi Chuan for breast cancer survivors. Medicine and Sport Science 2008;52:209‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naumann 2012a {published data only}
- Naumann F, Martin E, Philpott M, Smith C, Groff D, Battaglini C. Can counseling add value to an exercise intervention for improving quality of life in breast cancer survivors? A feasibility study. Journal of Supportive Oncology 2012;10(5):188‐94. [DOI] [PubMed] [Google Scholar]
Naumann 2012b {published data only}
- Naumann F, Munro A, Martin E, Magrani P, Buchan J, Smith C, et al. An individual‐based versus group‐based exercise and counselling intervention for improving quality of life in breast cancer survivors. A feasibility and efficacy study. Psycho‐Oncology 2012;21(10):1136‐9. [DOI] [PubMed] [Google Scholar]
Neil 2013 {published data only}
- Neil SE, Klika RJ, Garland SJ, McKenzie DC, Campbell KL. Cardiorespiratory and neuromuscular deconditioning in fatigued and non‐fatigued breast cancer survivors. Supportive Care in Cancer 2013;21(3):873‐81. [DOI] [PubMed] [Google Scholar]
Park JH 2012 {published data only}
- Park JH, Bae SH, Jung YS, Kim KS. Quality of life and symptom experience in breast cancer survivors after participating in a psychoeducational support program: a pilot study. Cancer Nursing 2012;35(1):E34‐E41. [DOI] [PubMed] [Google Scholar]
Piland 2011 {published data only}
- Piland MN. Effectiveness of a cancer center based physical activity intervention in breast cancer survivors [Master's Thesis],. Greenville: East Carolina University. Retrieved from the Scholarship. (http://hdl.handle.net/10342/3740), 2011. [1505485] [Google Scholar]
Poorkiani 2010 {published data only}
- Poorkiani M, Abbaszadeh A, Hazrati M, Jafari P, Sadeghi M, Mohammadianpanah M. The effect of rehabilitation on quality of life in female breast cancer survivors in Iran. Indian Journal of Medical and Paediatric Oncology 2010;31(4):105‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rowland 2009 {published data only}
- Rowland JH, Meyerowitz BE, Crespi CM, Leedham B, Desmond K, Belin TR, et al. Addressing intimacy and partner communication after breast cancer: a randomized controlled group intervention. Breast Cancer Research and Treatment 2009;118(1):99‐111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmitz 2009 {published data only}
- Schmitz KH, Troxel AB, Cheville A, Grant LL, Bryan CJ, Gross CR, et al. Physical Activity and Lymphedema (the PAL trial): assessing the safety of progressive strength training in breast cancer survivors. Contemporary Clinical Trials 2009;30(3):233‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schover 2006 {published data only}
- Schover LR, Jenkins R, Sui D, Adams JH, Marion MS, Jackson KE. Randomized trial of peer counseling on reproductive health in African American breast cancer survivors. Journal of Clinical Oncology 2006;24(10):1620‐6. [DOI] [PubMed] [Google Scholar]
Schover 2011 {published data only}
- Schover LR, Rhodes MM, Baum G, Adams JH, Jenkins R, Lewis P, et al. Sisters Peer Counseling in Reproductive Issues After Treatment (SPIRIT): a peer counseling program to improve reproductive health among African American breast cancer survivors. Cancer 2011;117(21):4983‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schultz 2011 {published data only}
- Schultz SL, Dalton SO, Christensen J, Carlsen K, Ross L, Johansen C. Factors correlated with fatigue in breast cancer survivors undergoing a rehabilitation course, Denmark, 2002‐2005. Psycho‐Oncology 2011;20(4):352‐60. [DOI] [PubMed] [Google Scholar]
Scott 2013a {published data only}
- Scott E, Daley AJ, Doll H, Woodroofe N, Coleman RE, Mutrie N. Effects of an exercise and hypocaloric healthy eating program on biomarkers associated with long‐term prognosis after early‐stage breast cancer: a randomized controlled trial. Cancer Causes & Control 2013;24(1):181‐91. [DOI] [PubMed] [Google Scholar]
Shields 2004 {published data only}
- Shields CG, Rousseau SJ. A pilot study of an intervention for breast cancer survivors and their spouses. Family Process 2004;43(1):95‐107. [DOI] [PubMed] [Google Scholar]
Shields 2010 {published data only}
- Shields CG, Ziner KW, Bourff SA, Schilling K, Zhao Q, Monahan P, et al. An intervention to improve communication between breast cancer survivors and their physicians. Journal of Psychosocial Oncology 2010;28(6):610‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 2001 {published data only}
- Simpson JSA, Carlson LE, Trew ME. Effect of group therapy for breast cancer on healthcare utilization. Cancer Practice 2001;9(1):19‐26. [DOI] [PubMed] [Google Scholar]
Speck 2010 {published data only}
- Speck RM, Gross CR, Hormes JM, Ahmed RL, Lytle LA, Hwang WT, et al. Changes in the Body Image and Relationship Scale following a one‐year strength training trial for breast cancer survivors with or at risk for lymphedema. Breast Cancer Research and Treatment 2010;121(2):421‐30. [DOI] [PubMed] [Google Scholar]
Speed Andrews 2010 {published data only}
- Speed Andrews AE, Stevinson C, Belanger LJ, Mirus JJ, Courneya KS. Pilot evaluation of an Iyengar yoga program for breast cancer survivors. Cancer Nursing 2010;33(5):369‐81. [DOI] [PubMed] [Google Scholar]
Sprod 2010 {published data only}
- Sprod LK, Hsieh CC, Hayward R, Schneider CM. Three versus six months of exercise training in breast cancer survivors. Breast Cancer Research and Treatment 2010;121(2):413‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sprod 2012 {published data only}
- Sprod LK, Janelsins MC, Palesh OG, Carroll JK, Heckler CE, Peppone LJ, et al. Health‐related quality of life and biomarkers in breast cancer survivors participating in Tai Chi Chuan. Journal of Cancer Survivorship: Research and Practice 2012;6(2):146‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterba 2015 {published data only}
- Sterba KR, Armeson K, Franco R, Harper J, Patten R, Kindall S, et al. A pilot randomized controlled trial testing a minimal intervention to prepare breast cancer survivors for recovery. Cancer Nursing 2015;38(2):E48‐E56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stolley 2009 {published data only}
- Stolley MR, Sharp LK, Oh A, Schiffer L. A weight loss intervention for African American breast cancer survivors, 2006. Preventing Chronic Disease 2009;6(1):A22. [PMC free article] [PubMed] [Google Scholar]
Vallance 2007 {published data only}
- Vallance JK, Courneya KS, Plotnikoff RC, Yasui Y, Mackey JR. Randomized controlled trial of the effects of print materials and step pedometers on physical activity and quality of life in breast cancer survivors. Journal of Clinical Oncology 2007;25(17):2352‐9. [DOI] [PubMed] [Google Scholar]
Vallance 2008 {published data only}
- Vallance JK, Courneya KS, Plotnikoff RC, Dinu I, Mackey JR. Maintenance of physical activity in breast cancer survivors after a randomized trial. Medicine and Science in Sports and Exercise 2008;40(1):173‐80. [DOI] [PubMed] [Google Scholar]
Vallance 2010 {published data only}
- Vallance J, Plotnikoff RC, Karvinen KH, Mackey JR, Courneya KS. Understanding physical activity maintenance in breast cancer survivors. American Journal of Health Behavior 2010;34(2):225‐36. [DOI] [PubMed] [Google Scholar]
von Ah 2012 {published data only}
- Ah D, Carpenter JS, Saykin A, Monahan P, Wu J, Yu M, et al. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Research and Treatment 2012;135(3):799‐809. [DOI] [PMC free article] [PubMed] [Google Scholar]
Winters‐Stone 2012 {published data only}
- Winters‐Stone KM, Dobek J, Bennett JA, Nail LM, Leo MC, Schwartz A. The effect of resistance training on muscle strength and physical function in older, postmenopausal breast cancer survivors: a randomized controlled trial. Journal of Cancer Survivorship 2012;6(2):189‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witek‐Janusek 2008 {published data only}
- Witek‐Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo‐Arvizu R, Mathews HL. Effect of mindfulness based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behavior and Immunity 2008;22(6):969‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Abrahams 2015 {published data only}
- Abrahams HJG, Gielissen MFM, Goedendorp MM, Berends T, Peters MEWJ, Poort H, et al. A randomized controlled trial of web‐based cognitive behavioral therapy for severely fatigued breast cancer survivors (CHANGE‐study): study protocol. BMC Cancer 2015;15:765. [DOI] [PMC free article] [PubMed] [Google Scholar]
Befort 2014 {published data only}
- Befort CA, Klemp JR, Fabian C, Perri MG, Sullivan DK, Schmitz KH, et al. Protocol and recruitment results from a randomized controlled trial comparing group phone‐based versus newsletter interventions for weight loss maintenance among rural breast cancer survivors. Contemporary Clinical Trials 2014; Vol. 37, issue 2:261‐71. [DOI] [PMC free article] [PubMed]
Hummel 2015 {published data only}
- Hummel SB, Lankveld JJDM, Oldenburg HSA, Hahn DEE, Broomans E, Aaronson NK. Internet‐based cognitive behavioral therapy for sexual dysfunctions in women treated for breast cancer: design of a multicenter, randomized controlled trial. BMC Cancer 2015;15:321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marcus 1998 {published data only}
Matthews 2002 {published data only}
- Matthews CE, Wilcox S, Skiba A, Heiney S, Ananian CD. A home‐based walking intervention among breast cancer survivors. Era of Hope ‐ Department of Defense Breast Cancer Research program meeting 2002; Vol. 3, issue P58‐10.
McDonald 2014 {published data only}
- McDonald C, Bauer J, Capra S, Coll J. The muscle mass, omega‐3, diet, exercise and lifestyle (MODEL) study – a randomised controlled trial for women who have completed breast cancer treatment. BMC Cancer 2014; Vol. 14:264. [DOI] [PMC free article] [PubMed]
NCT01515124 {published data only}
- NCT01515124. The Women In Steady Exercise Research (WISER) Survivor Trial. clinicaltrials.gov/ct2/show/NCT01515124 first received 18 January 2012.
Rock 2013 {published data only}
- Rock CL, Byers TE, Colditz GA, Demark‐Wahnefried W, Ganz PA, Wolin KY, et al. Reducing breast cancer recurrence with weight loss, a vanguard trial: the Exercise and Nutrition to Enhance Recovery and Good health for You (ENERGY) Trial. Contemporary Clinical Trials 2013; Vol. 34, issue 2:282‐95. [DOI] [PMC free article] [PubMed]
Additional references
ACS 2016
- American Cancer Society. Breast Cancer Survival Rates. www.cancer.org/cancer/breast‐cancer/understanding‐a‐breast‐cancer‐diagnosis/breast‐cancer‐survival‐rates.html (accessed on 15 March 2017).
Akechi 2011
- Akechi T, Okuyama T, Endo C, Sagawa R, Uchida M, Nakaguchi T, et al. Patient’s perceived need and psychological distress and/or quality of life in ambulatory breast cancer patients in Japan. Psycho‐oncology 2011;20(5):497‐505. [DOI] [PubMed] [Google Scholar]
Armes 2009
- Armes J, Crowe M, Colbourne L, Morgan H, Murrells T, Oakley C, et al. Patients' supportive care needs beyond the end of cancer treatment: a prospective longitudinal survey. Journal of Clinical Oncology 2009;27(36):6172‐9. [DOI] [PubMed] [Google Scholar]
Binkley 2012
- Binkley JM, Harris SR, Levangie PK, Pearl M, Guglielmino J, Kraus V, et al. Patient perspectives on breast cancer treatment side effects and the prospective surveillance model for physical rehabilitation for women with breast cancer. Cancer 2012;118(8 Suppl):2207‐16. [DOI] [PubMed] [Google Scholar]
Bodenheimer 2002
- Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self‐management of chronic disease in primary care. JAMA 2002;288(19):2469‐75. [DOI] [PubMed] [Google Scholar]
Borenstein 2009
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to Meta‐analysis. Chichester, UK: John Wiley & Sons, 2009. [Google Scholar]
Cancer Research UK 2017
- Cancer Research UK. Breast cancer survival statistics. www.cancerresearchuk.org/health‐professional/cancer‐statistics/statistics‐by‐cancer‐type/breast‐cancer/survival#heading‐Three (accessed on 25 March 2017).
Cella 1993
- Cella D, Tulsky D, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy (FACT) scale: development and validation of the general measure. Journal of Clinical Oncology 1993;11(3):570‐79. [DOI] [PubMed] [Google Scholar]
Cheng 2014
- Cheng KKF, Darshini Devi R, Wong WH, Koh C. Perceived symptoms and the supportive care needs of breast cancer survivors six months to five years post‐treatment period. European Journal of Oncology Nursing 2014;18(1):3‐9. [DOI] [PubMed] [Google Scholar]
Cheng 2016
- Cheng KKF, Wong WH, Koh C. Unmet needs mediate the relationship between symptoms and quality of life in breast cancer survivors. Supportive Care in Cancer 2016;24(5):2055‐33. [DOI] [PubMed] [Google Scholar]
Cheville 2007
- Cheville AL, Tchou J. Barriers to rehabilitation following surgery for primary breast cancer. Journal of Surgical Oncology 2007;95(5):409‐18. [DOI] [PubMed] [Google Scholar]
Cheville 2013
- Cheville AL, Kollasch J, Vandenberg J, Shen T, Grothey A, Gamble G, et al. A home‐based exercise program to improve function, fatigue, and sleep quality in patients with stage IV lung and colorectal cancer: a randomized controlled trial. Journal of Pain and Symptom Management 2013;45(5):811‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collins 2004
- Collins LG, Nash R, Round T, Newman B. Perceptions of upper‐body problems during recovery from breast cancer treatment. Supportive Care in Cancer 2004;12(2):106‐13. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
DerSimonian 2000
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Duval 2000
- Duval S, Tweedie R. A simple funnel‐plot‐based method of testing and adjusting for publication bias in meta‐analysis. Biometrics 2000;56:455‐63. [DOI] [PubMed] [Google Scholar]
EORTC Quality of Life Department 2017
- EORTC Quality of Life Department. EORTC QLQ‐C30. groups.eortc.be/qol/eortc‐qlq‐c30 (accessed on 15 March 2017).
Ewertz 2011
- Ewertz M, Jensen AB. Late effects of breast cancer treatment and potentials for rehabilitation. Acta Oncologica 2011;50(2):187‐93. [DOI] [PubMed] [Google Scholar]
Ferrans 1990
- Ferrans CE. Development of a quality of life index for patients with cancer. Oncology Nursing Forum 1990;17(3 Suppl):15‐9. [PubMed] [Google Scholar]
Ferrell 1995
- Ferrell BR, Grant MM, Hassey‐Dow K. Quality of life breast cancer questionnaire. City of Hope National Medical Center. Duarte (CA) 1995.
Gandhi 2010
- Gandhi M, Oishi K, Zubal B, Lacouture ME. Unanticipated toxicities from anticancer therapies: survivors' perspectives. Supportive Care in Cancer 2010;18(11):1461‐8. [DOI] [PubMed] [Google Scholar]
Garrett 2013
- Garrett K, Okuyama S, Jones W, Barnes D, Tran Z, Spencer L, et al. Bridging the transition from cancer patient to survivor: pilot study results of the cancer survivor telephone education and personal support program. Patient Education & Counselling 2013;92(2):266‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gautam 2011
- Gautam AP, Maiya AG, Vidyasagar MS. Effect of home‐based exercise program on lymphedema and quality of life in female postmastectomy patients: pre‐post intervention study. Journal of Rehabilitation Research and Development 2011;48(10):1261‐8. [DOI] [PubMed] [Google Scholar]
Gosain 2013
- Gosain R, Miller K. Symptoms and symptom management in long‐term cancer survivors. Cancer Journal 2013;19(5):405‐9. [DOI] [PubMed] [Google Scholar]
GRADEproGDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 26 March 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Grunfeld 2010
- Grunfeld E, Hodgson DC, Giudice ME, Moineddin R. Population‐based longitudinal study of follow‐up care for breast cancer survivors. Journal of Oncology Practice 2010;6(4):174‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harrison 2011
- Harrison SE, Watson EK, Ward AM, Khan NF, Turner D, Adams E, et al. Primary health and supportive care needs of long‐term cancer survivors: a questionnaire survey. Journal of Clinical Oncology 2011;29(15):2091‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hodgkinson 2007
- Hodgkinson K, Butow P, Hunt GE, Pendlebury S, Hobbs KM, Wain G. Breast cancer survivors' supportive care needs 2‐10 years after diagnosis. Supportive Care in Cancer 2007;15(5):515‐23. [DOI] [PubMed] [Google Scholar]
Howell 2012
- Howell D, Hack TF, Oliver TK, Chulak T, Mayo S, Aubin M, et al. Models of care for post‐treatment follow‐up of adult cancer survivors: a systematic review and quality appraisal of the evidence. Journal of Cancer Survivorship 2012;6(4):359‐71. [DOI] [PubMed] [Google Scholar]
IARC 2014
- International Agency for Research on Cancer. Breast Cancer Estimated Incidence, Mortality and Prevalence Worldwide in 2012. globocan.iarc.fr/Pages/fact_sheets_cancer.aspx (accessed 10 June 2014).
Jacobsen 2013
- Jacobsen PB, Phillips KM, Jim HSL, Small BJ, Faul LA, Meade CD, et al. Effects of self‐directed stress management training and home‐based exercise on quality of life in cancer patients receiving chemotherapy: a randomized controlled trial. Psycho‐Oncology 2013;22(6):1229‐35. [DOI] [PubMed] [Google Scholar]
Janz 2007
- Janz NK, Mujahid M, Chung LK, Lantz PM, Hawley ST, Morrow M, et al. Symptom experience and quality of life of women following breast cancer treatment. Journal of Women's Health 2007;16(9):1348‐61. [DOI] [PubMed] [Google Scholar]
Jefford 2008
- Jefford M, Karahalios E, Pollard A, Baravelli C, Carey M, Franklin J, et al. Survivorship issues following treatment completion‐‐results from focus groups with Australian cancer survivors and health professionals. Journal of Cancer Survivorship 2008;2(1):20‐32. [DOI] [PubMed] [Google Scholar]
Jeffs 2013a
- Jeffs E, Wiseman T. Randomised controlled trial to determine the benefit of daily home‐based exercise in addition to self‐care in the management of breast cancer‐related lymphoedema: a feasibility study. Supportive Care in Cancer 2013;21(4):1013‐23. [DOI] [PubMed] [Google Scholar]
Kim 2012
- Kim SH, Lee R, Lee KS. Symptoms and uncertainty in breast cancer survivors in Korea: differences by treatment trajectory. Journal of Clinical Nursing 2012;21(7‐8):1014‐23. [DOI] [PubMed] [Google Scholar]
Kuehn 2000
- Kuehn T, Klauss W, Darsow M, Regele S, Flock F, Maiterth C, et al. Long‐term morbidity following axillary dissection in breast cancer patients – clinical assessment, significance for life quality and the impact of demographic, oncologic and therapeutic factors. Breast Cancer Research and Treatment 2000;64(3):275‐86. [DOI] [PubMed] [Google Scholar]
Lattanzi 2010
- Lattanzi JB, Giuliano S, Meehan C, Sander B, Wootten R, Zimmerman A. Recommendations for physical and occupational therapy practice from the perspective of clients undergoing therapy for breast cancer‐related impairments. Journal of Allied Health 2010;39(4):257‐64. [PubMed] [Google Scholar]
Lee 2010a
- Lee TS, Kilbreath SL, Sullivan G, Refshauge KM, Beith JM. Patient perceptions of arm care and exercise advice after breast cancer surgery. Oncology Nursing Forum 2010;37(1):85‐91. [DOI] [PubMed] [Google Scholar]
Lee 2013
- Lee DH, Kim JY, Lee MK, Lee C, Min JH, Jeong DH, et al. Effects of a 12‐week home‐based exercise program on the level of physical activity, insulin, and cytokines in colorectal cancer survivors: a pilot study. Supportive Care in Cancer 2013;21(9):2537‐45. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Lengacher 2011
- Lengacher CA, Johnson‐Mallard V, Barta M, Fitzgerald S, Moscoso MS, Post‐White J, et al. Feasibility of a mindfulness‐based stress reduction program for early‐stage breast cancer survivors. Journal of Holistic Nursing 2011;29(2):107‐17. [DOI] [PubMed] [Google Scholar]
Lengacher 2012
- Lengacher CA, Reich RR, Post‐White J, Moscoso M, Shelton MM, Barta M, et al. Mindfulness based stress reduction in post‐treatment breast cancer patients: an examination of symptoms and symptom clusters. Journal of Behavioral Medicine 2012;35(1):86‐94. [DOI] [PubMed] [Google Scholar]
Levangie 2009
- Levangie PK, Drouin J. Magnitude of late effects of breast cancer treatments on shoulder function: a systematic review. Breast Cancer Research and Treatment 2009;116(1):1‐15. [DOI] [PubMed] [Google Scholar]
Loh 2011
- Loh SY, Chew SL, Lee SY, Quek KF. Quality of life in breast cancer survivors: 2 years post self‐management intervention. Asian Pacific Journal of Cancer Prevention 2011;12(6):1497‐501. [PubMed] [Google Scholar]
Mehnert 2009
- Mehnert A, Berg P, Henrich G, Herschbach P. Fear of cancer progression and cancer‐related intrusive cognitions in breast cancer survivors. Psycho‐Oncology 2009;18(12):1273‐80. [DOI] [PubMed] [Google Scholar]
Morey 2009
- Morey MC, Snyder DC, Sloane R, Cohen HJ, Peterson B, Hartman TJ, et al. Effects of home‐based diet and exercise on functional outcomes among older, overweight long‐term cancer survivors: RENEW: A randomized controlled trial. JAMA 2009;301(18):1883‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mullan 1985
- Mullan F. Seasons of survival: reflections of a physician with cancer. New England Journal of Medicine 1985;313(4):270‐3. [DOI] [PubMed] [Google Scholar]
NCCS 2009
- National Coalition for Cancer Survivorship. www.canceradvocacy.org/ (accessed 30 December 2013).
NCSI 2010
- National Cancer Survivorship Initiative. The National Cancer Survivorship Initiative Vision. www.ncsi.org.uk/wp‐content/uploads/NCSI‐Vision‐Document.pdf (accessed 30 December 2013).
Normand 1999
- Normand SL. Meta‐analysis: formulating, evaluating, combining, and reporting. Statistics in Medicine 1999;18(3):321‐59. [DOI] [PubMed] [Google Scholar]
Pauwels 2013
- Pauwels E, Charlier C, Bourdeaudhuij l, Lechner L, Hoof E. Care needs after primary breast cancer treatment: survivors' associated sociodemographic and medical characteristics. Psycho‐Oncology 2013;22(1):125‐32. [DOI] [PubMed] [Google Scholar]
Pinto 2005
- Pinto BM, Frierson GM, Rabin C, Trunzo JJ, Marcus BH. Home‐based physical activity intervention for breast cancer patients. Journal of Clinical Oncology 2005;23(15):3577‐87. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rogers 2009a
- Rogers LQ, Hopkins‐Price P, Vicari S, Markwell S, Pamenter R, Courneya KS, et al. Physical activity and health outcomes three months after completing a physical activity behavior change intervention: persistent and delayed effects. Cancer Epidemiology, Biomarkers & Prevention 2009;18(5):1410‐8. [DOI] [PubMed] [Google Scholar]
Rosedale 2010
- Rosedale M, Fu MR. Confronting the unexpected: temporal, situational, and attributive dimensions of distressing symptom experience for breast cancer survivors. Oncology Nursing Forum 2010;37(1):E28‐33. [DOI] [PubMed] [Google Scholar]
Schag 1989
- Schag CAC, Heinrich RL. Cancer Rehabilitation Evaluation System (CARES) Manual. 1. Los Angeles: CARES Consultants, 1989. [Google Scholar]
Schünemann 2011
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glaziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook of Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Scott 2013b
- Scott DA, Mills M, Black A, Cantwell M, Campbell A, Cardwell CR, et al. Multidimensional rehabilitation programmes for adult cancer survivors. Cochrane Database of Systematic Reviews 2013;3:Art. No.: CD007730. [DOI] [PMC free article] [PubMed] [Google Scholar]
So 2014
- So WKW, Chow KM, Chan HYL, Choi KC, Wan RWM, Mak SSS, et al. Quality of life and most prevalent unmet needs of Chinese breast cancer survivors at one year after cancer treatment. European Journal of Oncology Nursing 2014;18(3):323‐8. [DOI] [PubMed] [Google Scholar]
Spector 2014
- Spector D, Deal AM, Amos KD, Yang H, Battaglini CL. A pilot study of a home‐based motivational exercise program for African American breast cancer survivors: clinical and quality‐of‐life outcomes. Integrative Cancer Therapies 2014;13(2):121‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Thompson 2012
- Thompson HJ, Sedlacek SM, Paul D, Wolfe P, McGinley JN, Playdon MC, et al. Effect of dietary patterns differing in carbohydrate and fat content on blood lipid and glucose profiles based on weight‐loss success of breast‐cancer survivors. Breast Cancer Research 2012;14(1):R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van de Poll‐Franse 2011
- Poll‐Franse LV, Nicolaije KA, Vos MC, Pijnenborg JM, Boll D, Husson O, et al. The impact of a cancer survivorship care plan on gynecological cancer patient and health care provider reported outcomes (ROGY Care): study protocol for a pragmatic cluster randomized controlled trial. Trials 2011;12:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 2005
- Ware JE, Kosinski M, Turner‐Bowker DM, Gandek, B. How to score version 2 of the SF‐12 health survey. Lincoln: QualityMetric Inc, 2005. [Google Scholar]
WHO 2013
- World Health Organization. Breast Cancer Awareness Month in October. www.who.int/cancer/events/breast_cancer_month/en/ (accessed 30 December 2013).


