Abstract
Background
Many adults with type 2 diabetes mellitus (T2DM) experience a psychosocial burden and mental health problems associated with the disease. Diabetes‐related distress (DRD) has distinct effects on self‐care behaviours and disease control. Improving DRD in adults with T2DM could enhance psychological well‐being, health‐related quality of life, self‐care abilities and disease control, also reducing depressive symptoms.
Objectives
To assess the effects of psychological interventions for diabetes‐related distress in adults with T2DM.
Search methods
We searched the Cochrane Library, MEDLINE, Embase, PsycINFO, CINAHL, BASE, WHO ICTRP Search Portal and ClinicalTrials.gov. The date of the last search was December 2014 for BASE and 21 September 2016 for all other databases.
Selection criteria
We included randomised controlled trials (RCTs) on the effects of psychological interventions for DRD in adults (18 years and older) with T2DM. We included trials if they compared different psychological interventions or compared a psychological intervention with usual care. Primary outcomes were DRD, health‐related quality of life (HRQoL) and adverse events. Secondary outcomes were self‐efficacy, glycosylated haemoglobin A1c (HbA1c), blood pressure, diabetes‐related complications, all‐cause mortality and socioeconomic effects.
Data collection and analysis
Two review authors independently identified publications for inclusion and extracted data. We classified interventions according to their focus on emotion, cognition or emotion‐cognition. We performed random‐effects meta‐analyses to compute overall estimates.
Main results
We identified 30 RCTs with 9177 participants. Sixteen trials were parallel two‐arm RCTs, and seven were three‐arm parallel trials. There were also seven cluster‐randomised trials: two had four arms, and the remaining five had two arms. The median duration of the intervention was six months (range 1 week to 24 months), and the median follow‐up period was 12 months (range 0 to 12 months). The trials included a wide spectrum of interventions and were both individual‐ and group‐based.
A meta‐analysis of all psychological interventions combined versus usual care showed no firm effect on DRD (standardised mean difference (SMD) ‐0.07; 95% CI ‐0.16 to 0.03; P = 0.17; 3315 participants; 12 trials; low‐quality evidence), HRQoL (SMD 0.01; 95% CI ‐0.09 to 0.11; P = 0.87; 1932 participants; 5 trials; low‐quality evidence), all‐cause mortality (11 per 1000 versus 11 per 1000; risk ratio (RR) 1.01; 95% CI 0.17 to 6.03; P = 0.99; 1376 participants; 3 trials; low‐quality evidence) or adverse events (17 per 1000 versus 41 per 1000; RR 2.40; 95% CI 0.78 to 7.39; P = 0.13; 438 participants; 3 trials; low‐quality evidence). We saw small beneficial effects on self‐efficacy and HbA1c at medium‐term follow‐up (6 to 12 months): on self‐efficacy the SMD was 0.15 (95% CI 0.00 to 0.30; P = 0.05; 2675 participants; 6 trials; low‐quality evidence) in favour of psychological interventions; on HbA1c there was a mean difference (MD) of ‐0.14% (95% CI ‐0.27 to 0.00; P = 0.05; 3165 participants; 11 trials; low‐quality evidence) in favour of psychological interventions. Our included trials did not report diabetes‐related complications or socioeconomic effects.
Many trials were small and were at high risk of bias for incomplete outcome data as well as possible performance and detection biases in the subjective questionnaire‐based outcomes assessment, and some appeared to be at risk of selective reporting. There are four trials awaiting further classification. These are parallel RCTs with cognition‐focused and emotion‐cognition focused interventions. There are another 18 ongoing trials, likely focusing on emotion‐cognition or cognition, assessing interventions such as diabetes self‐management support, telephone‐based cognitive behavioural therapy, stress management and a web application for problem solving in diabetes management. Most of these trials have a community setting and are based in the USA.
Authors' conclusions
Low‐quality evidence showed that none of the psychological interventions would improve DRD more than usual care. Low‐quality evidence is available for improved self‐efficacy and HbA1c after psychological interventions. This means that we are uncertain about the effects of psychological interventions on these outcomes. However, psychological interventions probably have no substantial adverse events compared to usual care. More high‐quality research with emotion‐focused programmes, in non‐US and non‐European settings and in low‐ and middle‐income countries, is needed.
Plain language summary
Psychological interventions for diabetes‐related distress in adults with type 2 diabetes mellitus
Review question
To investigate the effects of psychological interventions on diabetes‐related distress in adults aged 18 years and older with type 2 diabetes mellitus.
Background
Diabetes‐related distress has to do with the emotional experiences of people with diabetes mellitus, namely their concerns about disease management, support, emotional burden and access to health care. About half of people with type 2 diabetes mellitus experience this distress, which is associated with poor diabetes self‐care and disease control. Many psychological interventions have tried to reduce diabetes‐related distress, but it is uncertain which interventions are effective.
Study characteristics
We found 30 randomised controlled trials (clinical trials where people are randomly put into one of two or more treatment groups) with 9177 participants. The duration of the interventions ranged from 1 week to 12 months and follow‐up after treatment from 0 to 12 months. Most studies took place in community settings, almost all in high‐income countries and two each in Asia and Latin America. The studies included a wide spectrum of interventions and were both individual‐ and group‐based.
Key results
Psychological interventions have a small and positive effect on confidence for self‐care and glycosylated haemoglobin A1c (HbA1c ‐ a long‐term measure of glucose control) in adults with type 2 diabetes. Compared to usual care, psychological interventions showed no firm effect on diabetes‐related distress, health‐related quality of life, death from any cause, adverse events or blood pressure levels. No study reported on diabetes‐related complications (like stroke, heart attacks or kidney impairment) or socioeconomic effects (such as absence from work or costs for medication).
This evidence is up to date as of 21 September 2016.
Quality of the evidence
Overall, the quality of the evidence was low because of small studies, missing data, and limitations in the design and implementation of the included studies. Four studies are awaiting further assessment, and 18 studies are ongoing with results hopefully be published in the near future.
Summary of findings
Summary of findings for the main comparison. Psychological interventions versus usual care for diabetes‐related distress in adults with type 2 diabetes mellitus.
| Psychological interventions versus usual care for diabetes‐related distress in adults with type 2 diabetes mellitus | ||||||
|
Patient: type 2 diabetes participants with diabetes‐related distress
Settings: mostly community‐based primary care and general practicesa
Intervention: psychological interventions Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (trials) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | Psychological interventions | |||||
|
Diabetes‐related distress PAID and DDS scales Follow‐up: median 10 months |
No meaningful estimate for baseline score possible | The standardised mean difference for diabetes‐related distress in the intervention groups was 0.07 standard deviations lower (0.16 lower to 0.03 higher) | — | 3315 (12) | ⊕⊕⊝⊝ Lowb | A standard deviation of 0.07 represents a very small difference between groups |
| Health‐related quality of life Various questionnaires Follow‐up: median 11 months | No meaningful estimate for baseline score possible | The standardised mean difference for health‐related quality of life in the intervention groups was 0.01 standard deviations higher (0.09 lower to 0.11 higher) | — | 1932 (5) | ⊕⊕⊝⊝ Lowb | A standard deviation of 0.01 represents a very small difference between groups |
| Adverse events Self‐reported outcomes Follow‐up: median 9 months | 17 per 1000 | 41 per 1000 (13 to 125) | RR 2.40 (0.78 to 7.39) | 438 (3) | ⊕⊕⊝⊝ Lowc | — |
| Self‐efficacy Various questionnaires Follow‐up: median 10 months | No meaningful estimate for baseline score possible | The standardised mean difference for self‐efficacy in the intervention groups was 0.15 standard deviations higher (0.00 higher to 0.30 higher) | — | 2675 (6) | ⊕⊕⊝⊝ Lowb | A standard deviation of 0.15 represents a small difference between groups |
| HbA1c (%) Follow‐up: median 11 months | The mean HbA1c ranged across control groups from 6.8% to 9.4% | The mean Hba1c in the intervention groups was 0.14% lower (−0.27% lower to 0.0% lower) | — | 3165 (11) | ⊕⊕⊝⊝ Lowd | — |
| Diabetes‐related complications | Not reported | |||||
| All‐cause mortality Medical records or reported by family members Follow‐up: median 10 months | 11 per 1000 | 11 per 1000 (2 to 66) | RR 1.01 (0.17 to 6.03) | 1376 (3) | ⊕⊕⊝⊝ Lowc | Reported on data with mostly < 12 months follow‐up, only 1 trial had data > 12 months |
| *The basis for the assumed risk was the median control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; DDS: Diabetes Distress Scale; HbA1c: glycosylated haemoglobin A1c;PAID: Problem Areas In Diabetes; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aEight trials at general practices, outpatient clinics and community‐based setting; three trials at hospital‐based clinics. bDowngraded two levels for trial limitations (attrition and other biases). There was no blinding of participants and personnel, and no blinding of outcome assessment, but we judged the influence of these biases on this outcome as minimal (see Appendix 14). cDowngraded by two levels: one level for trial limitations (attrition bias) and one level for imprecision (low sample size and small trials) (see Appendix 14). dDowngraded by two levels: one level for trial limitations (attrition bias) and one level for imprecision (see Appendix 14).
Background
Description of the condition
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action or both. Insulin deficiency invariably leads to chronic hyperglycaemia (i.e. elevated levels of plasma glucose) causing disturbances in carbohydrate, fat and protein metabolism. There are various types of diabetes mellitus of differing aetiology. The most common are type 1 and type 2 diabetes mellitus (T2DM).
The prevalence of T2DM is increasing worldwide (Hu 2011; International Diabetes Federation 2015; Whiting 2011). People with T2DM suffer from complications such as cardiovascular disease, nephropathy, retinopathy and neuropathy as a result of suboptimal control of blood glucose, blood pressure and lipids. This poses a great challenge to many countries’ healthcare systems and budgets. There are about 415 million people living with diabetes mellitus today, and by 2040, there could be as many as 642 million (International Diabetes Federation 2015). It was estimated that diabetes mellitus has caused 5 million deaths and incurred healthcare costs of USD 673 to 1197 billion (International Diabetes Federation 2015). Furthermore, people with T2DM are at high risk of diminished psychological well‐being (Anderson 2002; Gask 2011; Rane 2011; Robertson 2012); Rane 2011 has reported this to be the case in about half the people with a new diagnosis (within three months). Sources of psychosocial problems could arise from strained coping with changed life routines (Rane 2011), worries about hypoglycaemia and complications of diabetes (Stuckey 2014), and non‐conducive living environments and social support (Hinder 2012). People with diabetes often show negative coping strategies (Rane 2011), and they frequently expect that diabetes will negatively affect their future, resulting in increased diabetes fatalism (perceptions of despair, hopelessness and powerlessness), decreased medication adherence, and decreased levels of self‐care behaviours (diet, exercise and blood sugar testing) (Walker 2012). Untreated psychological well‐being may lead to cardiovascular complications and depression (Ghiadoni 2000; Skinner 2010), and depression might be associated with cognitive decline, further impairing self‐care abilities (Sullivan 2013).
Diabetes‐related distress (DRD) is defined as a patient's concern about disease management, support, emotional burden and access to care (Polonsky 2005); it is an important condition distinct from depression (Fisher 2014), meant to capture the emotional experiences of people with diabetes mellitus. It is content‐ and context‐specific to living with diabetes mellitus. It differs from depressive symptoms and from major depressive disorder, which have an established symptomatology, in that DRD is viewed as part of the diabetes spectrum and not a separate clinical psychopathology (Fisher 2014). Past trials showed greater prevalence and incidence of DRD than major depressive disorder (Fisher 2007; Fisher 2008), ranging from 18% in Fisher 2008 to 63% in Browne 2013, with probably lower rates at the primary care setting compared to the hospital setting (Stoop 2014). Conversely, in Stoop 2014, DRD prevalence was much higher among non‐native Dutch patients (55% Turkish, 40% Suriname and 23% other ethnicities) compared to the native Dutch T2DM patients (primary care 4%, hospital 13%). Chew 2015a also noted similarly high prevalence rates (29.7% and 19.5% for moderate and high DRD, respectively) in Asian adults with T2DM of the Malay, Chinese and Indian ethnicities at public primary care clinics in Malaysia. In mainland China, the prevalence of DRD was 64% among the T2DM patients at two public hospitals (Zhang 2013). Therefore, it is possible that there are racial or regional differences in DRD. The definition of DRD was previously not clearly stated, since no appropriate measure was available to separate DRD from depression. DRD and stress are deemed to have similar psychological and physiological manifestations, except that DRD is specific to the diabetes context (Lloyd 2005). Validated scales such as the Problem Areas In Diabetes (PAID) instrument and the Diabetes Distress Scale (DDS) enable physicians to evaluate this construct separately from general stress and depressive disorders (Polonsky 1995; Polonsky 2005). More recently, trials have shown DRD and depression to have distinct effects on self‐care behaviours and disease control (Fisher 2007; Fisher 2010). In a recent review, Fisher 2014 suggested that in all trials of DRD, depression should also be measured to get insight into the association between DRD and depression. Indeed, what the literature widely reports as 'depression' among people with T2DM may really be a major depressive disorder, DRD or both, with only DRD showing an association with glycaemic control (Fisher 2010). At six months follow‐up, DRD was predictive for medication adherence and glycosylated haemoglobin A1c control (HbA1c), while depressive symptoms were predictive of behaviour‐oriented self‐management (Aikens 2012). In another study, DRD showed relationships with HbA1c at up to 18 months follow‐up (Fisher 2010). It is likely that in the spectrum of emotional disorders experienced by people with T2DM, DRD is at the milder end, and depression is at the more severe end (Das‐Munshi 2007; Fisher 2007).
Although DRD has a proven association with self‐management (Peyrot 2005), health‐related quality of life (Chew 2015c), and HbA1c (Aikens 2012; Fisher 2010), there is not necessarily a causal relationship between the two, especially because research has not found any significant prospective linkages between DRD and HbA1c over a period longer than 18 months. The relationship between DRD and glycaemic control does not assume the direct involvement of any physiological process, but instead, emphasises the ongoing negative subjective experience of emotional distress around the management of T2DM that has implications for ongoing disease‐related behaviours, motivation, self‐efficacy, problem solving and even depressive symptoms (Snoek 2015). For example, for some individuals, high disease distress can influence self‐management and medication adherence, with subsequent effects on glycaemic control, and for other people, poor control can lead to distress, which can influence disease management.
A Dutch study at community level in people with T2DM observed a significant relationship between DRD (measured by PAID) and microvascular (but not macrovascular) complications (Kasteleyn 2015). However, there are not many trials on the natural history of DRD or the relationship between DRD and diabetes‐related complications, morbidities and mortality. Much previous work on the relationship between depression and diabetes focused on major depressive disorder (Holt 2014; O'Connor 2009; Pan 2011; Park 2013), and some examined general stress (Lloyd 2005), but this research did not assess distress, which is likely far more prevalent than major depressive disorder, especially at primary and community care levels (Chew 2015a; Coyne 1994; Fechner‐Bates 1994). It is important to address the milder symptomatology of DRD, since it may progress to depression (Burns 2015; Ehrmann 2015; Skinner 2010), which is associated with increased disability, risk of health decline (Nakaya 2014), increased healthcare use (Callahan 1994), decreased quality of life (Egede 2013), and premature mortality (Kawamura 2007).
Description of the intervention
Existing self‐management and behavioural interventions for T2DM vary widely in their content, and their effectiveness is uncertain (Health Quality Ontario 2009a; Ismail 2004; Norris 2001; Van der Heijden 2013; Worswick 2013). These interventions include behavioural education (Sperl‐Hillen 2011), goal setting (Naik 2011), work on problem‐solving skills (Fitzpatrick 2013), and cognitive behavioural therapy (Safren 2014). In terms of delivery, interventions vary from being delivered by peer experts (Sinclair 2013), in groups versus individually (Quinones 2012; Sperl‐Hillen 2011), and in community‐ versus hospital‐based settings (D'Eramo 2010; Health Quality Ontario 2009b). These trials did not show consistent positive effects on psychological well‐being, self‐management skills or disease control (glycaemia, blood pressure and lipids). Previous trials and reviews suggested that behavioural interventions were more effective in people with a poorer baseline psychological state (Robertson 2013; Rosenbek 2011), while other studies have linked their effectiveness to people with poorer glycaemic control (HbA1c ≥ 9.0%) (Health Quality Ontario 2009a). However, these interventions showed different impacts on individuals with different personal traits and skills (Fisher 2013). On the other hand, many recent trials on psychological interventions that addressed DRD include effects of positive emotion as well (Robertson 2012). People with T2DM who experience distress and anxiety showed improved DRD, health‐related outcomes and self‐management with relaxation therapy (Mandel 2013), mindfulness‐based therapy (Van Son 2013), and Internet‐based programmes (Fonda 2009).
Considering the possible underlying fundamental mechanisms by which psychological interventions might exert their effects on an individual's behaviour (see below), and keeping in mind the need for a meaningful comparison between the interventions for this systematic review (Worswick 2013), we planned to categorise psychological interventions and programmes reported in the trials into either emotion‐focused or cognition‐focused interventions. We based categorisation on the description provided in the published reports and consensus among the authors (see below for further details).
Adverse effects of the intervention
In terms of the adverse effects of interventions, most reviews on psychological interventions in adults with T2DM have not reported this outcome (Baumeister 2012; Deakin 2005; Duke 2009; Health Quality Ontario 2009a; Ismail 2004; Norris 2001; Pal 2013). Investigators speculated that this omission was related to the relatively short duration of the trials and the physically non‐invasive nature of the interventions. However, one study reported that a participant withdrew from the study due to anxiety related to computer‐based learning on diabetes knowledge (Wise 1986).
Therefore, there is currently no good evidence documenting the adverse effects of psychological interventions. Possible adverse effects could include the following.
Increased psychological distress due to sensitisation from the intervention programmes.
Frustration about the absence of promised effects on clinical outcomes.
Sense of failure, loss of self‐esteem or self‐worth amongst individuals who cannot maintain newly learned skills from the interventions.
More hypoglycaemic events from increased self‐care activities.
Participants receiving incorrect advice or misinterpreting self‐management guidance.
Participants making decisions that clinicians would deem 'inappropriate'.
Strain on existing doctor‐patient relationships if there is a difference in advice from the intervention and the healthcare providers.
How the intervention might work
Emotion may interact with diabetes and patients' self‐care practices and influence health outcomes, although the pathways through which these processes occur are not yet fully understood (Chew 2014; Piette 2004). Positive feelings of well‐being and resilience may sustain long‐term coping efforts and protect people with T2DM from the negative consequences of prolonged distress and depression (Folkman 2000; Robertson 2012), thus facilitating diabetes self‐management behaviours, greater exercise and diet adherence, lower glycosylated haemoglobin A1c (HbA1c), fewer diabetic complications and lower risk of all‐cause mortality (Robertson 2012). The current perception is that emotion primarily regards motivation, while cognition primarily regards knowledge (Izard 2008). A recent meta‐analysis reported that several significant brain regions for emotion are situated in the bilateral amygdala, superior temporal gyrus, insula and medial anterior cingulate cortex (Cromheeke 2014). During emotional situations, neural activations emerged not only in the brain regions for emotions but also in brain regions commonly implicated in cognitive control, such as the lateral prefrontal cortex, the medial prefrontal cortex and the basal ganglia (Cromheeke 2014). The close interconnectedness of the neural circuits between emotion and cognition in the brain might underlie their mutual influences (Cromheeke 2014; Pessoa 2008), and many educational theories recognise the close relationship between the two.
Successful performance and maintenance of healthy behaviours are key elements in patient‐centred care and self‐management of chronic diseases. Appropriate application of underlying theories in this aspect would provide a good foundation for an effective health intervention or programme. Some of the most commonly cited models for health behavioural change invariably include cognition and emotional constructs within the personal attitudes, beliefs, perceptions and expectations. Examples of such models include the health belief model (Rosenstock 1966), the theory of reasoned action and planned behaviour (Ajzen 2011), the social cognitive theory (Bandura 1991; Bandura 2001), and the theory of self‐efficacy (Bandura 1997). Self‐efficacy is one's self‐ confidence in the ability to carry out or overcome difficulties inherent to specific tasks (Bandura 1977). This confidence is a learned capability, gained through past experiences. In the theory of self‐efficacy, differential experience and cognitive processing of information lead to different degrees of self‐efficacy attainment. Thus, having more self‐efficacy would lead to higher probability of acquiring a new and desired behaviour. Future‐oriented thinking or the proactive coping concept goes a step further in explaining how people could maintain an acquired behaviour (Aspinwall 2005; Thoolen 2009). In this model, a person has to continuously anticipate the potential barriers and threats to the desired behaviour to develop and realise strategies to offset these barriers and threats. In addition to the effective use of resources, people who are successful in maintaining their changed health behaviour would also use effective feedback to keep the goals viable.
All of these theories and constructs have cognitive and emotional components. An imbalance between emotional and cognitive support may explain faulty illness perceptions, inefficiency in coping, inefficacy in healthy behaviours, lower health status and quality of life (Petrie 2007). Since we will be evaluating complex interventions, we propose a conceptual framework (Figure 1) based on our research question, including elements of the above‐mentioned theories. Available evidence from the clinical trials and most of the present behavioural theories and concepts suggest that cognition has a stronger influence on self‐efficacy than emotion. Harkness 2010 reported in their meta‐regression that interventions that included psychological therapies had greater benefits on mental health; and interventions that included education and skills training components had greater effects on HbA1c. The model hypothesises that there is close interaction between emotion and cognition on the pathway to improved self‐efficacy (Bandura 2001; Pessoa 2008). Cognitive and/or emotional domains may generate some behavioural change and will be influenced by the new behaviour by means of a feedback system modifying illness perceptions (Petrie 2007), proactive coping (Aspinwall 2005), and self‐management. The extent to which psychological interventions address the emotional and cognitive needs might influence the effects of the interventions (Clark 2001). Robertson 2012 reported that healthy behaviours were associated with less distress, lower HbA1c, more positive emotions and better quality of life. Most current interventions are cognition‐focused (Worswick 2013), but we expect that emotion‐focused programmes could be more effective in addressing DRD.
1.
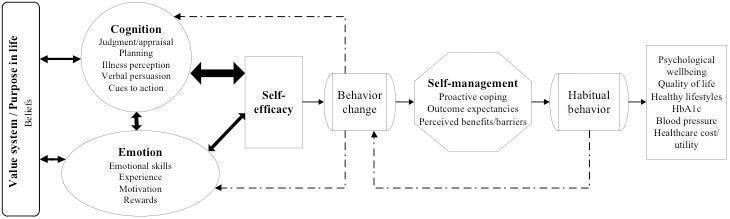
Conceptual framework of the influences of cognition and emotion on various aspects of diabetes management
Why it is important to do this review
To the best of our knowledge, there has not been any systematic review of interventions for DRD focussing on adults with T2DM. Sturt 2015 has conducted one in both adults with type 1 and type 2 diabetes mellitus. Other past reviews focused on diabetes self‐management and clinical outcomes (Deakin 2005; Duke 2009; Pal 2013; Vermeire 2005), or they looked at depression and health‐related quality of life in adults with diabetes (Baumeister 2012; Harkness 2010). There is consensus that DRD needs more attention (Nicolucci 2013; SIGN 2010; Snoek 2012). Improving DRD in adults with T2DM could improve psychological well‐being, health‐related quality of life, self‐care abilities and disease control (Fisher 2010; Fisher 2014), also reducing depression (Skinner 2010), which could in turn reduce diabetes‐related complications (Ghiadoni 2000; Kawamura 2007). However, the current evidence lacks strength and quality with regard to which cognition‐ and/or emotion‐focused interventions are most effective for managing DRD in adults with T2DM (Fisher 2013; Harkness 2010; Peyrot 2007).
Because DRD is at the mild end of the emotional spectrum, addressing it in primary care might be more suitable for future interventions since there are relatively more adults with T2DM and DRD in their early stages of disease. In particular, interventions delivered by nurses might be especially appropriate (Skelly 2009; Gabbay 2006), as these professionals are relatively more available and less expensive compared to physicians or mental health professionals such as psychologists. Thus, evidence on these interventions might encourage involvement of nurses in psychological interventions for adults with T2DM and DRD, potentially supporting the implementation of the minimal, most cost‐effective interventions to reduce DRD and improve self‐management.
Objectives
To assess the effects of psychological interventions for diabetes‐related distress in adults with type 2 diabetes mellitus.
Secondary objectives were to separately evaluate the effects of emotion‐focused and cognition‐focused psychological interventions for diabetes‐related distress in adults with type 2 diabetes mellitus (Chew 2015b).
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled clinical trials (RCTs).
Types of participants
We included trials evaluating participants (≥ 18 years old) with T2DM and DRD in different healthcare settings.
Diagnostic criteria for type 2 diabetes mellitus
To be consistent with changes in classification and diagnostic criteria of diabetes mellitus over the years, the diagnosis had to be established using the standard criteria valid at the time of the trial commencement (for example ADA 2003; ADA 2014; WHO 1998). Ideally, investigators should have described the diagnostic criteria. If necessary, we used the study authors' definition of diabetes mellitus. We planned to subject these diagnostic criteria to a sensitivity analysis.
Diagnostic criteria for diabetes‐related distress
This review includes trials that measure DRD with either the Problem Areas In Diabetes (PAID) questionnaire or the 17‐item Diabetes Distress Scale (DDS) (Polonsky 1995; Polonsky 2005). A mean item score of ≥ 3 for DDS indicates a substantial level of distress (Fisher 2012). Higher scores in all of these scales represent higher distress (Polonsky 1995; Polonsky 2005). For ,the PAID questionnaire, some trials interpreted an arbitrary cutoff score of one standard deviation above the mean or ≥ 40 (after the total score has been rescaled to 100) as a level of 'emotional burnout' that warranted special attention (Welch 2003). PAID and DDS are the most commonly used measures to assess diabetes distress, while. Other scales assess psychological distress or similar emotional distress in a way that is not diabetes‐specific to the same extent. We planned to subject these diagnostic criteria to a sensitivity analysis.
Types of interventions
Few trials in the past had a single domain or mode of psychological intervention but often some mixture of both emotional and cognitive domains (Soo 2009). Based on a systematic review (Harkness 2010), we classified the interventions as emotion‐focused (EF), cognition‐focused (CF) or a mixture of both components – an emotion‐cognition (EC) intervention.
We defined an intervention as an EF intervention if the content of the interventions described in the trials includes any one of the following aspects, but none of the CF interventions (further below).
Positive affects, e.g. hope, happiness, excitement, contentment.
Positive well‐being.
Resilience.
Managing negative affects such as anxiety, depression, distress, anger, hatred, fear, guilt, sadness or nervousness.
Integrating psychosocial adjustment to daily life.
Healthy coping. This is defined as coping skills that are taught mainly from the perspective of emotion management.
Motivation.
We defined an intervention as a CF intervention if the content of the interventions described in the trials include any one of the following aspects, but none of the EF interventions above.
Knowledge, comprehension or awareness about diabetes, complications and treatment options.
Taking medication.
Healthy eating.
Being active.
Goal setting to promote health.
Risk reduction.
Self‐efficacy and confidence in one's own ability to manage diabetes (categorised here because believed to manifest in 'know‐how' and thus more of cognition than emotion; this is consistent with a previous systematic review. Pal 2013).
We classified interventions with any mixture of emotion and cognition as an EC intervention.
The care providers or people involved in the delivery of the interventions needed training. We investigated different types of providers such as nurses, physicians and psychologists in subgroup analysis.
Therefore, we planned to investigate the following interventions versus each other or any control condition.
Intervention
Emotion‐focused (EF).
Cognition‐focused (CF).
Emotion‐cognition (EC).
All psychological interventions (EF, CF, EC).
Comparators
Usual care.
Waiting list.
Non‐interactive computer‐based programmes.
Paper educational material.
Concomitant treatments had to be the same in the intervention and comparator groups to establish fair comparisons.
Minimum duration of follow‐up after the intervention had to be six months.
Summary of specific exclusion criteria
Gestational diabetes mellitus.
Participants with life‐threatening illnesses, recent acute complications or hospitalisations.
Duration of follow‐up less than six months (with the exception of adverse events, see below).
We excluded trials if the independent effect of a psychosocial intervention could not be determined (e.g. antidepressant medication plus psychological intervention versus usual care).
Types of outcome measures
Primary outcomes
Diabetes‐related distress (DRD)
Health‐related quality of life
Adverse events
Secondary outcomes
Self‐efficacy
Glycosylated haemoglobin A1c (HbA1c)
Blood pressure
Diabetes‐related complications
All‐cause mortality
Socioeconomic effects
Method and timing of outcome measurement
DRD: evaluated with validated instruments (e.g. DDS (Polonsky 2005), PAID (Polonsky 1995)), measured at 6 to 12 months.
Health‐related quality of life: evaluated with validated instruments (e.g. the World Health Organization Quality of Life (WHOQOL) (WHOQOL Group 1998)) or diabetes‐specific measures (e.g. Audit of Diabetes Dependent Quality of Life (ADDQoL) (Bradley 1999; Wee 2006), Diabetes Quality of Life (DQOL) (DCCT Research Group 1988)), measured at 6 to 12 months.
Adverse events: such as increased psychological distress due to the interventions, hypoglycaemic events and others as mentioned above and measured at less than six months.
Self‐efficacy: defined as the individual's judgement of confidence to carry out tasks specific to diabetes management, measured with validated scales such as Diabetes Management Self Efficacy Scale (DMSES) (Bijl 1999), Diabetes Self‐Efficacy Scale (Rapley 2003), or Diabetes Empowerment Scale (DES) (Anderson 2000), and measured at 6 to 12 months.
HbA1c: measured at 6 to 12 months.
Systolic blood pressure: measured at 6 to 12 months.
Diabetes‐related complications: defined as ischaemic heart disease, cerebrovascular disease or stroke, retinopathy, nephropathy and diabetic foot problems, and measured at more than 12 months.
All‐cause mortality: defined as death from any cause reported during the study period and measured at more than 12 months.
Socioeconomic effects: defined as cost of treatments and visits to clinics or hospitals and measured at 6 to 12 months.
Summary of findings' table
We presented 'Summary of findings tables' reporting the following outcomes listed according to priority.
Diabetes‐related distress (DRD).
Health‐related quality of life.
Self efficacy.
Diabetes‐related complications.
All‐cause mortality.
Adverse events.
HbA1c.
Search methods for identification of studies
Electronic searches
We developed the search strategies based on text mining a set of 10 RCTs known to be relevant. We limited the search to studies published after 1 January 1995, as diabetes‐related distress is measured with two instruments developed in 1995 (PAID questionnaire) and 2005 (DDS). We placed no restrictions on the language of publication.
Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies Online (CRSO) (last searched 21 September 2016).
MEDLINE Ovid (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)) (1946 to 21 September 2016).
Embase Ovid (1974 to 20 September 2016).
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) (last searched 21 September 2016).
PsycINFO Ovid (1806 to December Week 4 2016).
LILACS (Latin American and Caribbean Health Science Information database) (last searched 21 September 2016).
BASE (Bielefeld Academic Search Engine) (last searched 16 December 2014).
ClinicalTrials.gov (www.clinicaltrials.gov) (last searched 21 September 2016).
World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/trialsearch/) (last searched 21 September 2016).
We continuously applied a MEDLINE (via Ovid SP) email alert service to identify newly published trials using the same search strategy as described for MEDLINE (for details on search strategies see Appendix 1) (Beller 2013).
Searching other resources
We tried to identify other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials, (systematic) reviews, meta‐analyses and health technology assessment reports. We contacted leading authors of each included trial and experts on this subject for additional data on published or unpublished trials.
Data collection and analysis
Selection of studies
Two review authors (BHC, RV) independently scanned the abstract, title or both, of every record retrieved, to determine which trials to assess further. One of these authors is knowledgeable in the area under review, and the other is not a content expert. We investigated all potentially relevant articles as full text. We resolved any discrepancies through consensus or recourse to a third review author (GR). If resolution of a disagreement was not possible, we planned to add the article to those 'awaiting assessment' and contact study authors for clarification.
We assessed eligibility criteria for each study in order of importance, so that the first 'no' response was the primary reason for exclusion of the study, and the remaining were not assessed. In other words, a single failed eligibility criterion was sufficient for excluding a study from the review. The order of importance was as follows: RCT, T2DM, age > 18 years, DRD is measured, psychological intervention and participants without life‐threatening illnesses. We then used this pilot test to refine and clarify the eligibility criteria before applying them to ensure that the review team applied the criteria consistently.
In the selection process, we did not mask information about the article, such as the journal that published it, the authors, the institution, or the magnitude and direction of the results. We presented an adapted Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) flow diagram showing the process of study selection (Liberati 2009).
Data extraction and management
For trials that fulfilled inclusion criteria, two review authors (BHC, MH) independently extracted data using standard data extraction templates as supplied by the CMED and modified for this review or if required, by consultation with a third review author (RV or GR) (for details see Table 2; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6; Appendix 7; Appendix 8; Appendix 9; Appendix 10; Appendix 11; Appendix 12).
1. Overview of trial populations.
| ID (trial design) | Main component of psychological intervention (type of intervention) | Sample sizea | Screened/eligible (N) | Randomised (N) | ITT (N) | Analysed (N) | Finishing trial (N) | Randomised finishing trial (%) | Follow‐up (extended follow‐up)b |
|
Beverly 2013 (parallel RCT) |
I: cognition focused (group education) |
— | 473/147 | 68 | 67 | 67 | 58 | 85.3 | 12 months |
| C: enhanced usual care (educational classes not focusing on diabetes care) |
67 | 67 | 67 | 63 | 94.0 | ||||
| total: | 135 | 134 | 134 | 121 | 90.3 | ||||
|
Davies 2008 (cluster‐RCT) |
I: cognition focused (group education) |
Assumption 1: SD HbA1c 2%, ICC 0.05, average 18 participants per practice, 315 per study arm to detect a clinically relevant difference in HbA1c of 1% (90% power at the 5% significance level). Assumption 2: failure to consent rate 20%, dropout rate 20%; 1000 participants (500 in each arm) needed to be referred |
1109/824 | 437 | 437 | 437 | 314 | 71.9 | 12 months |
| C: enhanced usual care (additional contact time with healthcare professionals) |
387 | 387 | 387 | 248 | 64.1 | ||||
| total: | 824 | 824 | 824 | 562 | 68.2 | ||||
|
Dennick 2015 (parallel RCT) |
I: emotion focused (writing about different aspects of life, thoughts and feelings) |
— | 1715/106 | 23 | 23 | 23 | 18 | 78.3 | 3 months |
| C: cognition focused (writing about previous days' activities) |
18 | 18 | 18 | 14 | 77.8 | ||||
| total: | 41 | 41 | 41 | 32 | 78.0 | ||||
|
D'Eramo Melkus 2010 (parallel RCT) |
I: emotion‐cognition components (cognitive behavioural self‐management training) |
Based on a power calculation of the estimated effect size for the primary outcome variable of HbA1c and a 20% attrition rate, recruitment was targeted to obtain a sample of 129 African American women with T2DM | 236/109 | 57 | 57 | 57 | 40 | 70.2 | 12 months |
| C: cognition focused (group education) |
52 | 52 | 52 | 37 | 71.2 | ||||
| total: | 109 | 109 | 109 | 77 | 70.6 | ||||
|
Fisher 2011 (cluster‐RCT) |
I: cognition focused (self‐monitoring of blood glucose) |
— | 770/483 | 256 | 256 | 256 | 188 | 73.4 | 12 months |
| C: enhanced usual care (additional quarterly diabetes‐focused physician visits) |
227 | 227 | 227 | 187 | 82.4 | ||||
| total: | 483 | 483 | 483 | 375 | 77.6 | ||||
|
Fisher 2013 (parallel RCT) |
I1: cognition focused (computer‐assisted self‐management) |
— | 2606/603 | 150 | 150 | 150 | 121 | 80.7 | 12 months |
| I2: emotion‐cognition components (computer‐assisted self‐management + problem solving) |
146 | 146 | 146 | 117 | 80.1 | ||||
| C: cognition focused (general diabetes support and education) |
96 | 96 | 96 | 81 | 84.4 | ||||
| total: | 392 | 392 | 392 | 319 | 81.4 | ||||
|
Gabbay 2013 (parallel RCT) |
I: cognition focused (motivational interviewing) |
— | 1178/545 | 232 | 232 | 232 | 188 | 81.0 | 24 months |
| C: usual care (standard diabetes care) |
313 | 313 | 313 | 233 | 74.4 | ||||
| total: | 545 | 545 | 545 | 421 | 77.2 | ||||
|
Glasgow 2005 (cluster‐RCT) |
I: cognition focused (computer‐assisted self‐management) |
— | 1187/886 | 469 | 469 | 469 | 379c | 80.8 | 6 months |
| C: enhanced usual care (computer information without self‐management) |
417 | 417 | 417 | 354c | 84.9 | 12 months | |||
| total: | 886 | 886 | 886 | 733 | 82.7 | — | |||
|
Grillo 2016 (parallel RCT) |
I: cognition focused (self‐management education) |
A sample of 136 participants (68 in each group) was required to detect a 0.5% difference in HbA1c, considering the repeat measurement design (baseline and 3 times during the follow‐up), 80% power and 5% alpha error | 1200/138 | 69 | 69 | 67 | 67 | 97.1 | 11 months |
| C: enhanced usual care (group meetings without education) |
68 | 68 | 60 | 60 | 88.2 | 12 months | |||
| total: | 137 | 137 | 127 | 127 | 92.7 | — | |||
|
Hermanns 2012 (parallel RCT) |
I: emotion‐cognition components (self‐management programme) |
Assumption of an equivalence region of 0.4% and an SD of 1.0% for the differences in HbA1c reduction between the 2 groups, 1‐sided therapeutic non‐inferiority can be shown with an error of alpha = 0.05 (1‐sided) and beta = 0.2 (power = 0.80) with 78 participants per group (total of 156 participants). Given an expected unevaluable rate of 15% (i.e. not suitable for per‐protocol analysis), a total of 184 individuals were needed with 92 participants in each group |
280/186 | 94 | 94 | 94 | 82 | 87.2 | 6 months |
| C: cognition focused (combination of 2 education programmes) |
92 | 92 | 92 | 85 | 92.4 | ||||
| total: | 186 | 186 | 186 | 167 | 89.8 | ||||
|
Hermanns 2015 (parallel RCT) |
I: emotion‐cognition components (cognitive behavioural treatment) |
An effect size of d = 0.5 was expected. Given this assumption, a 2‐sided therapeutic superiority could be shown with an error of alpha = 0.05 (2‐sided) and beta = 0.1 (power = 0.90) with 86 participants per group (total of 172 participants). Given an expected unevaluable rate of 20%, a total of 214 individuals were needed, with 107 participants in each group |
3156/214 | 106 | 106 | 93 | 93 | 87.7 | 12 months |
| C: cognition focused (group education) |
108 | 108 | 88 | 88 | 81.5 | ||||
| total: | 214 | 214 | 181 | 181 | 84.6 | ||||
|
Lamers 2011 (parallel RCT) |
I: emotion‐cognition components (cognitive behavioural therapy) |
Based on an alpha = 0.05 and beta = 0.9, 2 x 103 people were sufficient to detect a minimum clinically relevant difference of 0.72 on the DSC‐R total score, 9.03 on the PAID and 0.59% for HbA1c | 538/208 | 105 | 105 | 105 | 70 | 66.7 | 9 months |
| C: usual care (standard diabetes care) |
103 | 103 | 103 | 72 | 69.9 | ||||
| total: | 208 | 208 | 208 | 142 | 68.3 | ||||
|
Lerman 2009 (parallel RCT) |
I1: cognition focused (telephone contacts) |
— | — | 22 | — | 18 | 18 | 81.8 | 12 months |
| I2: cognition focused (group‐based education) | — | 26 | — | 24 | 24 | 92.3 | |||
| C: usual care (standard diabetes care) |
— | 22 | — | 17 | 17 | 77.3 | |||
| total: | 70 | — | 59 | 59 | 84.3 | ||||
|
Liu 2015 (parallel RCT) |
I: emotion‐cognition components (peer education) |
— | 127/536 | 63 | — | 63 | 63 | 100 | 6 months |
| C: cognition focused (diabetes health education) |
64 | — | 64 | 64 | 100 | ||||
| total: | 127 | — | 127 | 127 | 100 | ||||
|
Pibernik‐Okanovic 2015 (parallel RCT) |
I1: emotion‐cognition components (psycho‐educational intervention) | An improvement of 0.5 SDs in the absolute change in depressive symptoms as measured by the CES‐D questionnaire was considered clinically relevant with alpha = 0.05, samples of n = 59 per group were needed to have 80% power | 4858/365 | 74 | 74 | 64 | 65 | 87.8 | 12 months |
| I2: cognition focused (physical activity intervention) |
66 | 66 | 57 | 61 | 92.4 | ||||
| C1: emotion‐cognition components (enhanced usual diabetes care) |
69 | 69 | 57 | 62 | 89.9 | ||||
| total: | 209 | 209 | 178 | 188 | 90.0 | ||||
|
Quinn 2011 (cluster‐RCT) |
I1: cognition focused (coach + mobile diabetes management software) |
— | 2602/213 | 38 | 23 | 23 | 23 | 60.5 | 12 months |
| I2: cognition focused (coach + mobile diabetes management software + Internet portal) | 33 | 22 | 22 | 22 | 66.7 | ||||
| I3: cognition focused (coach + mobile diabetes management software + Internet portal + decision support) | 80 | 62 | 62 | 62 | 77.5 | ||||
| C: usual care (standard diabetes care) |
62 | 56 | 56 | 56 | 90.3 | ||||
| total: | 213 | 163 | 163 | 163 | 76.5 | ||||
|
Rosenbek 2011 (parallel RCT) |
I: emotion‐cognition components (motivational interviewing) |
With 352 patients, 176 in each group, the trial could detect a 0.4% difference in HbA1c. The power was set to 90%. This calculation was based on an SD of 1.15 in the HbA1c value and a 5% 2‐sided significance level | 469/464 | 173 | 173 | 145 | 145 | 83.8 | 12 months |
| C: usual care (standard diabetes care) |
176 | 176 | 153 | 153 | 86.9 | ||||
| total: | 349 | 349 | 298 | 298 | 85.4 | ||||
|
Shibayama 2007 (parallel RCT) |
I: emotion‐cognition components (behavioural counselling) |
With 64 participants in each group, there was an 80% power to detect 0.5% difference in the change in HbA1c assuming that the SD of the change was 1.0%, at an alpha (2‐sided) of 0.05. To allow for a 5% dropout rate, the sample was increased to 67 participants per group |
309/134 | 67 | 67 | 67 | 61 | 91.0 | 12 months |
| C: usual care (standard diabetes care) |
67 | 67 | 67 | 59 | 88.1 | ||||
| total: | 134 | 134 | 134 | 120 | 89.6 | ||||
|
Simmons 2015 (cluster‐RCT) |
I1: emotion‐cognition components (group peer support) | Predicted mean cluster size of 106 participants, ICC of 0.037 based upon an unpublished estimate from a previous study for HbA1c, a design effect of 1.36 was anticipated.
A sample size of 1250 participants from 106 clusters, after allowing 6 clusters to drop out and a further 10% participant loss to follow‐up, would leave 1060 participants in 100 clusters for primary outcome analysis. Based on an SD for HbA1c of 1.25, this provided (2‐sided tests, P < 0.05) 91% power to detect a difference of 0.3% (3 mmol/mol) in mean HbA1c for each factorial main effect, 88% power to detect a difference of 0.4% (4 mmol/mol) between any 2 arms in the case of an unexpected interaction between the factorial effects and 82% power to detect a 0.3% (3 mmol/mol) difference between combined intervention arms and the control arm. For questionnaire outcomes with the same ICC, based on 880 participants assuming a reduced 75% follow‐up rate, there was 90% power to detect effect size differences of 0.25 SD for factorial main effects, and 0.35 SD for pair‐wise comparisons |
3932/1366 | 330 | 330 | 272 | 272 | 82.4 | 12 months |
| I2: emotion‐cognition components (group&individual support) | 322 | 322 | 245 | 245 | 76.1 | ||||
| I3: emotion focused (individual peer support) |
325 | 325 | 264 | 264 | 81.2 | ||||
| C: usual care (standard diabetes care) |
322 | 322 | 283 | 283 | 87.9 | ||||
| total: | 1299 | 1299 | 1064 | 1064 | 81.9 | ||||
|
Skelly 2009 (parallel RCT) |
I1: cognition focused (symptom‐focused) | — | 308/180 | 60 | 60 | 60 | 54 | 90.0 | 9 months |
| I2: cognition focused (symptom‐focused with telephone booster) | 60 | 55 | 55 | 54 | 90.0 | 9 months | |||
| C: enhanced usual care (weight and diet programme) |
60 | 59 | 59 | 55 | 91.7 | 6 months | |||
| total: | 180 | 174 | 174 | 163 | 90.6 | — | |||
|
Spencer 2013 (parallel RCT) |
I: emotion‐cognition components (community health worker intervention) |
— | 1719/183 | 72 | 72 | 72 | 59d | 81.9 | 6 months |
| C: waiting list or usual care (information on community activities) |
92 | 92 | 92 | 71d | 77.2 | ||||
| total: | 164 | 164 | 164 | 130 | 82.9 | ||||
|
Sperl‐Hillen 2013 (parallel RCT) |
I1: cognition focused (individual education) |
— | 939/623 | 246 | 246 | 246 | 242 | 98.4 | 10 months |
| I2: cognition focused (group education) |
243 | 243 | 243 | 240 | 98.8 | 10 months | |||
| C: usual care (standard diabetes care) |
134 | 134 | 134 | 132 | 98.5 | 13 months | |||
| total: | 623 | 623 | 623 | 614 | 98.6 | — | |||
|
Sturt 2008 (cluster‐RCT) |
I: emotion‐cognition components (diabetes manual structured education) |
— | 2257/245 | 88 | 88 | 88 | 82 | 93.2 | 3 months |
| C: waiting list or usual care (standard diabetes care) |
114 | 114 | 114 | 112 | 98.2 | 6 months | |||
| total: | 202 | 202 | 202 | 194 | 96.0 | — | |||
|
Trief 2016 (parallel RCT) |
I1: emotion‐cognition components (behaviour change intervention, couples) |
The minimum sample size necessary, based on HbA1c data obtained from a 3‐month pilot study, showed that 80 participants/arm (N = 240) would exceed 80% power to detect significant differences between interventions | 280/350 | 104 | 97 | 97 | 97 | 93.3 | 12 months |
| I2: emotion‐cognition components (behaviour change intervention, individuals) |
94 | 93 | 93 | 93 | 98.9 | ||||
| C: cognition focused (individual diabetes education) |
82 | 78 | 78 | 78 | 95.1 | ||||
| Total: | 280 | 268 | 268 | 268 | 95.7 | ||||
|
Taylor 2006 (parallel RCT) |
I1: emotion‐cognition components (cognitive behavioural therapy) |
— | 126/96 | — | 26 | 26 | 26 | 89.7 | 5 weeks |
| I2: emotion‐cognition components (expressive writing) |
— | 23 | 23 | 23 | — | ||||
| C: waiting list or usual care (usual diabetes care) |
— | 18 | 18 | 18 | — | ||||
| total: | 96 | 67 | 67 | 67 | 69.8 | ||||
|
Van der Wulp 2012 (parallel RCT) |
I: cognition focused (peer‐led self‐management coaching programme) |
With an expected effect size (self‐efficacy) of 0.25, power set to 0.80 and alpha set to 0.05, a sample size of 40 participants per treatment group was needed | 332/133 | 68 | 59 | 59 | 59 | 86.8 | 6 months |
| C: usual care (standard diabetes care) |
65 | 60 | 60 | 60 | 92.3 | ||||
| total: | 133 | 119 | 119 | 119 | 89.5 | ||||
|
Van Dijk‐de Vries 2015 (cluster‐RCT) |
I: emotion‐cognition components (self‐management support in routine care) |
The power calculation was based on the dichotomous DFT. The basis was the group size of 46 practice nurses: a sample size of 232 participants (at least 5 participants per practice nurse) would have 90% power and an alpha of 0.05 to detect an improvement in perceived daily functioning (defined as DFT ≤ 4) at 12 months measurement occurring in 20% of participants in the intervention arm versus 5% of those in the control arm. An ICC of 0.04 was used. Assuming that not all positively screened participants would give informed consent for trial participation, and a 30% loss to follow‐up, 10 eligible participants were planned for each practice nurse |
3822/357 | 117 | 117 | 117 | 99 | 84.6 | 12 months |
| C: usual care (standard diabetes care) |
147 | 147 | 147 | 124 | 84.4 | ||||
| total: | 264 | 264 | 264 | 223 | 84.5 | ||||
|
Weinger 2011 (parallel RCT) |
I1: emotion‐cognition components (behavioural strategies) | For the primary endpoint of HbA1c level, 64 participants per arm were needed to detect a clinically significant 0.5% difference with 80% power (alpha = 0.05, 2‐tailed test). Based on prior experience with participants with poorly controlled diabetes, a 15% attrition rate was assumed and recruitment was targeted at approximately 74 participants per arm |
2027/464 | 74 | 74 | 74 | 70 | 94.6 | 12 months |
| C1: cognition focused (group attention) |
75 | 75 | 75 | 73 | 97.3 | ||||
| C2: cognition focused (individual attention) |
73 | 73 | 73 | 72 | 98.6 | ||||
| total: | 222 | 222 | 222 | 215 | 96.8 | ||||
|
Welch 2015 (parallel RCT) |
I: emotion‐cognition components (one‐to‐one diabetes education) |
— | 868/399 | 199 | 199 | 199 | 172 | 86.4 | 6 months |
| C: cognition focused (standard diabetes care) |
200 | 200 | 200 | 181 | 90.5 | ||||
| total: | 399 | 399 | 399 | 353 | 88.5 | ||||
|
Whittemore 2004 (parallel RCT) |
I: emotion‐cognition components (nurse coaching) |
— | 81/53 | 31 | 31 | 31 | 28 | 90.3 | 6 months |
| C: usual care (standard diabetes care) |
22 | 22 | 22 | 21 | 95.5 | ||||
| total: | 53 | 53 | 53 | 49 | 92.5 | ||||
| Grand total | All interventions | 5316e | 4458 | ||||||
| All comparators | 3794e | 3213 | |||||||
| All interventions and comparators | 9177e | 7671 | |||||||
aFollow‐up under randomised conditions until end of trial (= duration of intervention + follow‐up postintervention or identical to duration of intervention). bExtended follow‐up refers to follow‐up of participants once the original trial was terminated as specified in the power calculation. cData extracted from parallel publication (Williams 2007) on the same trial. d Data provided by trial author; the number of participants responded with the completed 'Problem Areas In Diabetes' questionnaire. eNumbers do not match exactly because only the total number of randomised participants was available in Taylor 2006.
—: denotes not reported
C: comparator; CES‐D: Center for Epidemiological Studies Depression scale; DFT: Daily Functioning Thermometer visual analogue scale (ranging from 0 = no burden to 10 = extreme burden); DSC‐R: Diabetes Symptom Checklist – Revised; I: intervention; ICC: intra‐cluster correlation;ITT: intention‐to‐treat; HbA1c: glycosylated haemoglobin A1c; PAID: Problem Areas in Diabetes; RCT: randomised controlled trial; SD: standard deviation; T2DM: type 2 diabetes mellitus.
We provided information (including trial identifier) about potentially relevant ongoing trials in the 'Characteristics of ongoing studies' table. We tried to find the protocol of each included study, either in trials registers or in publications of study designs, or both, and reported primary, secondary and other outcomes in comparison with data in publications in a joint 'Matrix of study endpoint (publications and trial documents)' (see Appendix 5).
We emailed all authors of included trials to enquire whether they were willing to answer questions regarding their trials. Appendix 13 shows the results of this survey. Thereafter, we sought relevant missing information on the trial from the primary author(s) of the article, if required.
For the inclusion of cross‐over trials in the meta‐analyses, we planned to use the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). For trials with several intervention groups, we planned to include only the intervention and control groups that met our eligibility criteria and report the trial only once in any one analysis. We coded in a standard way the following characteristics of the study sample: country of origin; number of participants at baseline and at follow‐up; age; baseline glycaemic and blood pressure control; type of diabetes treatment; duration of diabetes; presence of cardiovascular risk factors; presence of diabetes‐related complications; and basis of participant recruitment (poor diabetic control or identified psychological disorders). We categorised the different components of each intervention and extracted data on intervention intensity (number of sessions, duration), setting (e.g. primary care, hospital), the professionals involved, delivery method (e.g. individual or group, face‐to‐face or remote delivery), and quality control (training, supervision, written manuals, and assessments of adherence or competence). Where the trials reported two interventions versus a control group, we halved sample sizes to avoid double counting. Independent groups of two raters performed all coding and resolved disagreements by discussion.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents or multiple reports of a primary study, we maximised yield of information by collating all available data, and we used the most complete data set aggregated across all known publications. In case of doubt, we gave priority to the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Two review authors (BHC, MH) assessed the risk of bias of each included study independently. We resolved disagreements by consensus or by consultation with a third review author (RV or GR) in case of persisting disagreement. The review team tested the form for the assessments of risk of bias on a pilot sample of three to six papers that spanned a range from low to high risk of bias to ensure that we were consistently applying criteria and could reach a consensus. Review authors were not blinded to the names of the authors, institutions, journal or results of the study when assessing its methods for risk of bias.
We used the Cochrane 'Risk of bias' assessment tool and evaluated the following criteria (Higgins 2011a; Higgins 2011b).
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other potential sources of bias.
We evaluated individual bias items as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), assigning 'low', 'high' or 'unclear' risk of bias to each domain. We presented a 'Risk of bias' graph and a 'Risk of bias' summary figure. We assessed the impact of individual bias domains on study results at the endpoint and study levels. In case of high risk of selection bias, we marked all endpoints investigated in the associated study as being at high risk.
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessors), we evaluated risk of bias separately for each outcome (Hróbjartsson 2013). We noted whether trials measured outcomes subjectively or objectively, for example if blood pressure readings came from participants or study personnel.
We considered the implications of missing outcome data from individual participants per outcome such as high dropout rates (e.g. above 15%) or disparate attrition rates (e.g. difference of 10% or more between study arms).
We assessed outcome reporting bias by integrating the results of 'Examination of outcome reporting bias' (Appendix 6), 'Matrix of study endpoints (publications and trial documents)' (Appendix 5) and section 'Outcomes (outcomes reported in abstract of publication)' in the table Characteristics of included studies (Kirkham 2010). This analysis formed the basis of the judgement of selective reporting (reporting bias).
We defined the following endpoints as subjective outcomes.
Diabetes‐related distress (DRD).
Health‐related quality of life.
Self‐efficacy.
Adverse events, depending on measurement.
We defined the following endpoints as objective outcomes.
HbA1c.
Blood pressure.
Diabetes‐related complications.
All‐cause mortality.
Adverse events, depending on measurement.
Socioeconomic effects.
Measures of treatment effect
In general, we expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs). For continuous outcomes measured on the same scale, we extracted postintervention scores unless studies presented only change from baseline scores. We used the standardised mean difference (SMD) when trials assessed the same outcome measured on different scales (DRD, health‐related quality of life and self‐efficacy). The rule of thumb of how to interpret these measures is that an SMD less than 0.40 indicates a small effect, 0.40 to 0.70 a moderate effect, and more than 0.70 a large effect, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Unit of analysis issues
We planned to take into account the level at which randomisation occurred, such as cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome. In case of cross‐over trials or cluster‐randomised trials, we planned to extract effect estimates that took into account the correlation of the measurements.
Dealing with missing data
We obtained missing data from trial authors, if feasible, and carefully evaluated important numerical data such as screened, randomised participants as well as intention‐to‐treat, and as‐treated and per‐protocol populations. We investigated attrition rates, e.g. dropouts, losses to follow‐up and withdrawals, and we critically appraised issues of missing data and imputation methods (e.g. last observation carried forward).
Where standard deviations for outcomes were not reported and we did not receive information from study authors, we imputed these values by assuming the standard deviation of the missing outcome to be the average of the standard deviations from those trials where this information was reported. We planned to investigate the impact of imputation on meta‐analyses by means of sensitivity analysis.
Assessment of heterogeneity
In the event of substantial clinical or methodological heterogeneity, we did not report trial results as the pooled effect estimate in a meta‐analysis.
We identified heterogeneity (inconsistency) through visual inspection of the forest plots and by using a standard Chi² test with a significance level of α = 0.1. We also considered the I² statistic, which quantifies inconsistency across trials to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I² statistic of 75% or more indicates a considerable level of heterogeneity (Higgins 2011a).
In case of heterogeneity, we attempted to determine possible reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
If we included 10 trials or more investigating a particular outcome, we planned to use funnel plots to assess small study effects. There are several possible explanations for an asymmetrical funnel plot, including true heterogeneity of effect with respect to trial size, poor methodological design (and hence bias of small trials) and publication bias. We therefore interpreted results carefully (Sterne 2011).
Data synthesis
We calculated summary estimates of data that were primarily at low risk of bias by the use of the random‐effects model according to the statistical guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We interpreted random‐effects meta‐analyses with due consideration of the whole distribution of effects, ideally by presenting a prediction interval (Higgins 2009). A prediction interval specifies a predicted range for the true treatment effect in an individual study (Riley 2011). For rare events such as death, we used Peto odds ratio for meta‐analysis.
Quality of evidence
We presented the overall quality of the evidence for each outcome according to the GRADE approach, which takes into account issues not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity such as directness of results. Two review authors (BHC, MH) independently rated the quality for each outcome. We presented a summary of the evidence in a 'Summary of findings' table, which provides key information about the best estimate of the magnitude of the effect, in relative terms and absolute differences for each relevant comparison of alternative management strategies, numbers of participants and trials addressing each important outcome and the rating of the overall confidence in effect estimates for each outcome. We created the 'Summary of findings' table based on the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We presented the outcomes as described in the Types of outcome measures section. If meta‐analysis was not possible, we presented results in a narrative 'Summary of findings' table. We downgraded the evidence from 'high quality' by one level for serious (or by two for very serious) study limitations as specified in the following areas in the GRADEpro: risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias. We interpreted findings with the GRADE profiler (GRADEpro) which allowed us to import data from Review Manager 5 (RevMan 5) to create 'Summary of findings' tables (RevMan 2014). In addition, we established an appendix 'Checklist to aid consistency and reproducibility of GRADE assessments' (Meader 2014) which helped with standardisation of 'Summary of findings' tables (Appendix 14).
Subgroup analysis and investigation of heterogeneity
We expected the following characteristics to introduce clinical heterogeneity and planned to carry out subgroup analyses to investigate interactions.
Hospital versus community‐based trials.
Brief and simple versus longer and more advanced interventions.
Interventions delivered by nurses versus those delivered by physicians or psychologists.
Male versus female.
Age < 60 years versus age ≥ 60 years.
Hospital settings included the specialist outpatient clinics at the hospitals, and community‐based facilities are health clinics that provide general medical care. The two different healthcare settings entail differences in participants' sociodemographic and clinical profile, the healthcare professionals' qualification, and health systems (Chew 2013; Greenfield 2002).
We defined brief and simple interventions as those that involved fewer than four total sessions of less than three hours' duration each, completed within three months. This subgroup analysis was meant to ascertain the effectiveness of the minimal psychological interventions, which should be relatively more cost‐effective than advanced ones. Less costly interventions should be easier to implement at the primary care level, since this is usually characterised by a high patient load, relatively low use of technologies, staff without specialised training in psychological interventions, and budget constraints (Kamarudin 2012; Maeseneer 2008).
We performed a subgroup analysis for the difference between deliverers of the interventions (nurse versus doctor/psychologist) to determine whether interventions given by nurses who are generally more widely available and less expensive, are equally effective (Deakin 2005).
The rationale for the subgroup analysis between the sexes is based on previous reports that have alluded to gender differences in disease control, risk profile, health belief, behaviours and responses to health interventions (Cherrington 2010; Gouni‐Berthold 2008; Huxley 2006).
We used the age of 60 years as the cut‐off for another subgroup analysis based on similar age categorisation in past trials (Gouni‐Berthold 2008; Morley 1998; Soe 2011). People of 60 years or above are generally considered a high risk group (Chamnan 2009).
Sensitivity analysis
We planned to perform sensitivity analyses to assess the robustness of the following factors (when applicable) on effect sizes.
Restricting the analyses to published trials.
Restricting the analyses by only including trials that scored low overall risk of bias as specified in the Assessment of risk of bias in included studies section.
Restricting the analysis to very long or large trials to establish the extent to which they dominate the results. We defined long trials as having an active intervention beyond 12 months and large trials as involving more than 1000 participants.
Restricting the analysis to trials using the following filters: imputation, source of funding (industry versus other), country (Western versus Asian).
We also tested the robustness of the results for diabetes‐related distress (DRD), health‐related quality of life, health behaviours and physical outcomes by repeating the analysis using different measures of effect size (risk ratio and odds ratio) and different statistical models (fixed‐effect and random‐effects models). We compared the pooled effect size of psychological interventions against all control groups and against those control groups excluding trials evaluating another psychological therapy.
Results
Description of studies
For a detailed description of trials, see the Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies sections.
Results of the search
The database search and the continuous MEDLINE (via Ovid SP) updated search alerts yielded 1518 unique records (see Figure 2).
2.
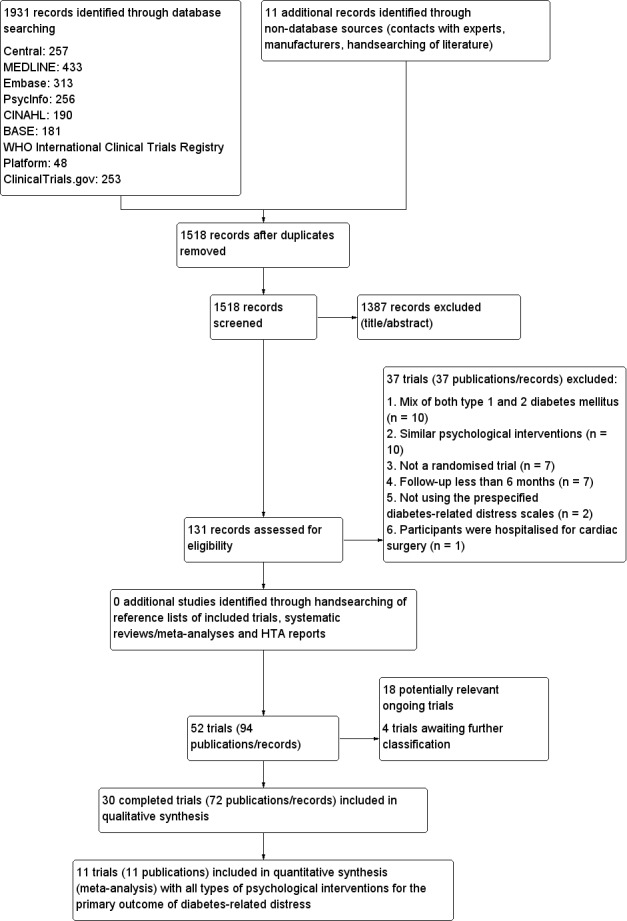
Study flow diagram.
Included studies
Thirty trials met the inclusion criteria, with two trials reported in two articles each (Glasgow 2005; Weinger 2011). Three of the 30 included trials included both type 1 and 2 diabetes mellitus participants, but we included them after the trial authors provided separate data on people with T2DM (Hermanns 2015; Rosenbek 2011; Weinger 2011). We present a detailed description of the characteristics of included trials elsewhere (see Characteristics of included studies and Appendices). The following is a succinct overview.
Source of data
All data presented in this review were from published literature. We contacted 32 trial authors for further information on the conduct of the trial such as the method of randomisation, allocation concealment, blinding and outcome measurement (Appendix 13). Fifteen trial authors replied with further clarification. Lerman 2009 took place in Mexico and was published in Spanish. It was a three‐arm RCT with one‐year follow‐up, assessing two different reinforcement strategies for diabetes self‐care management, psychological distress and glycaemic control. Taylor 2006 was a PhD dissertation, and no correspondence details were available to allow further clarification on details like the number of participants who were randomised to two of the three groups at the beginning of the trial. NCT01578096 had published baseline data but no reporting on the effects of the interventions on the outcome measures. Grillo 2016 did not provide data on DRD that were suitable for inclusion in the review.
Comparisons
Studies mainly used individual‐level interventions or system‐level interventions that might have an effect at the participant level. Rosenbek 2011 compared individual counselling sessions using motivational interviewing (MI) with usual care; Gabbay 2013 compared nurse case management plus MI versus usual care; Liu 2015, Simmons 2015 and Van der Wulp 2012 trained peer experts to provide necessary supports to the participants compared to usual care. Glasgow 2005 used computerised touch screen assessment and self‐management action planning procedures to assist doctor‐patient consultation, compared to similar computerised touch screen assessment but without self‐management action planning. The most common comparator in this review was usual care, waiting list, enhanced usual care or attention‐control. The most common comparison for the primary outcome of this review was cognition‐focused interventions versus usual care/enhanced usual care (11 trials), followed by emotion‐cognition focused interventions versus usual care (9 trials) and emotion‐cognition focused interventions versus cognition‐focused interventions (9 trials). There was only one trial contributing to the comparison between emotion‐focused versus cognition‐focused interventions, which was by Dennick 2015, and it contributed to the report of adverse events for this review. There was no included trial that compared an emotion‐focused intervention with usual care.
Overview of study participants
In total, 9177 participants were involved in the trials in this review (Table 2).
Trials explicitly reported randomising 5316 and 3794 participants to intervention and comparator groups, respectively.
A total of 83.9% (4458) and 84.7% (3213) of participants finished the trials in the intervention and comparator groups, respectively.
Individual sample size ranged from 41 to 1299.
Two trials had fewer than 30 participants per trial arm (Dennick 2015; Taylor 2006), whereas six trials had more than 200 participants per trial arm (Davies 2008; Fisher 2011; Gabbay 2013; Glasgow 2005; Simmons 2015; Sperl‐Hillen 2013).
Trial design
Twenty‐one RCTs had a two‐arm design, and seven had a three‐arm parallel design (Fisher 2013; Lerman 2009; Skelly 2009; Sperl‐Hillen 2013; Taylor 2006; Trief 2016; Weinger 2011). In addition, Simmons 2015 and Quinn 2011 were four‐arm cluster‐randomised trials.
Seven RCTs were cluster‐randomised (Davies 2008; Fisher 2011; Glasgow 2005; Quinn 2011; Simmons 2015; Sturt 2008; Van Dijk‐de Vries 2015). All the seven cluster‐RCTs included in this review used appropriate statistical analyses.
One trial had a non‐inferiority design (Hermanns 2012): all others were superiority trials.
Seventeen trials were multicentre trials (Davies 2008; D'Eramo Melkus 2010; Fisher 2011; Gabbay 2013; Glasgow 2005; Hermanns 2012; Lamers 2011; Quinn 2011; Simmons 2015; Skelly 2009; Spencer 2013; Sperl‐Hillen 2013; Sturt 2008; Taylor 2006; Trief 2016; Van der Wulp 2012; Welch 2015), with the number of centres ranging from 2 to 207.
No trial was double‐blinded or single‐blinded for participants because of the nature of the interventions under study. In 23 of the 30 trials, blinding of outcome assessors was absent or not clearly defined.
Six of 30 trials were blinded for outcome assessors with regard to outcomes such as manual blood pressure (Beverly 2013, D'Eramo Melkus 2010; Rosenbek 2011; Trief 2016; Weinger 2011; Welch 2015).
Trials were performed from the year 2000 to 2014.
Settings
See Appendix 3 for details on settings of all the included trials.
Fifteen of 30 trials were conducted in the USA, 4 in the UK (Davies 2008; Dennick 2015; Simmons 2015; Sturt 2008), 3 in the Netherlands (Lamers 2011; Van der Wulp 2012; Van Dijk‐de Vries 2015), 2 in Germany (Hermanns 2012; Hermanns 2015), and 1 each in China (Liu 2015), Brazil (Grillo 2016), Croatia (Pibernik‐Okanovic 2015), Denmark (Rosenbek 2011), Japan (Shibayama 2007), and Mexico (Lerman 2009).
Twenty‐one of 30 trials took place in a community‐based, primary care or general practice setting. Fisher 2013 took place mainly at community‐based health centres and also in diabetes education centres, whereas Whittemore 2004 reported undertaking the trial at an outpatient diabetes education centre. Taylor 2006 probably recruited participants from a mix of community‐based support groups and hospitals. The remaining nine trials took place in the hospital setting or specialist outpatient clinics (Beverly 2013; Hermanns 2012; Hermanns 2015; Lerman 2009; Liu 2015; Pibernik‐Okanovic 2015; Rosenbek 2011; Shibayama 2007; Weinger 2011). Liu 2015 also included participants from a mix of community‐based support groups and hospitals. Beverly 2013 and Weinger 2011 were conducted at the Joslin Clinic, which we consider to be a hospital setting.
Participants
All participants were from high‐income countries except those from China, Brazil, Croatia and Mexico.
Most trials included mainly white (non‐Hispanic, European white, British) participants except Quinn 2011, Skelly 2009 and Spencer 2013, where the main ethnic group was black or African American. Asian (in Liu 2015 and Shibayama 2007) and Latino (in Grillo 2016 and Lerman 2009) participants were only 2.8% (261/9177) and 2.3% (207/9177), respectively, of the total included trial participants in this review.
Twenty‐one trials reported the duration of diabetes; see Appendix 3 for the range of mean/median duration.
Fourteen trials had almost equal proportions of participants of both genders (Beverly 2013; Davies 2008; Fisher 2011; Fisher 2013; Glasgow 2005; Hermanns 2012; Hermanns 2015; Lamers 2011; Liu 2015; Quinn 2011; Rosenbek 2011; Van der Wulp 2012; Van Dijk‐de Vries 2015; Weinger 2011). Some trials recruited mostly women (Gabbay 2013; Grillo 2016; Lerman 2009; Spencer 2013; Taylor 2006; Welch 2015), while three trials recruited them exclusively (D'Eramo Melkus 2010; Skelly 2009 and Whittemore 2004).
Mean age of participants ranged from 43.2 to 70.7 years (see Appendix 4).
Mean HbA1c at baseline ranged from 6.9% to 9.3% (see Appendix 4).
Mean body mass index (BMI) at baseline ranged from 24.5 kg/m² to 36.9 kg/m² (see Appendix 4).
Twelve trials reported participants' comorbidities, and another 12, their co‐medications.
Eight trials used DDS to measure DRD (Fisher 2011; Fisher 2013; Glasgow 2005; Hermanns 2015; Liu 2015; Quinn 2011; Simmons 2015; Trief 2016). All of these trials except Glasgow 2005 reported mean baseline values, which ranged from 1.6 to 3.2. The rest of the trials used PAID to measure DRD; the mean total scores ranged from 14.5 to 59.9 at baseline (Davies 2008 did not report baseline PAID scores).
Major exclusion criteria were: being diagnosed with serious psychological, psychiatric or medical illness (severe depression, current schizophrenia, psychotic disorders, terminal renal disease, cancer, AIDS), cognitive impairment (such as dementia) or diabetes‐related complications or functional deficits (e.g. dialysis, blindness).
Diagnosis
In all the included trials, the diagnosis of T2DM was not defined according to any of the criteria (e.g. WHO, American Diabetes Association (ADA) criteria).
Interventions
See Appendix 2 and Characteristics of included studies for details on interventions of all the included trials.
Two of the 30 trials reported group education programmes before the start of the trial (Lerman 2009; Rosenbek 2011).
There were 49 psychological interventions in the 30 included trials. Only one intervention could be categorised as emotion‐focused (Dennick 2015). CF was the most common type of intervention (27 groups), followed by the EC (21 groups).
Eleven trials employed usual care as the control group (Gabbay 2013; Lamers 2011; Lerman 2009; Quinn 2011; Rosenbek 2011; Shibayama 2007; Simmons 2015; Sperl‐Hillen 2013; Van der Wulp 2012; Van Dijk‐de Vries 2015; Whittemore 2004), while three trials had control participants on a waiting list or delayed treatment (Spencer 2013; Sturt 2008; Taylor 2006), and six trials used enhanced usual care such as attention control as the comparator (Beverly 2013; Davies 2008; Fisher 2011; Glasgow 2005; Grillo 2016; Skelly 2009). There were another four 'enhanced usual care groups' that we classified as a psychological intervention: three as cognition‐focused (Hermanns 2015; Weinger 2011; Welch 2015), and one as emotion‐cognition (Pibernik‐Okanovic 2015). Six trials used active comparators that we also classified as a psychological intervention (Dennick 2015; D'Eramo Melkus 2010; Fisher 2013; Hermanns 2012; Liu 2015; Trief 2016).
Duration of interventions ranged from one week to 24 months; the mean was 7.8 months and the median was 6.0 months.
D'Eramo Melkus 2010 and Pibernik‐Okanovic 2015 involved psychologists and psychiatrists in one of their intervention programmes, and Lerman 2009 involved a doctor in one of its intervention programmes. Most included trials trained nurses and diabetes educators to deliver the interventions. Fisher 2013 trained non‐professional college graduates to deliver the interventions with supervision; Spencer 2013 trained community health workers to deliver the intervention. Fisher 2013, Glasgow 2005, Quinn 2011 and Welch 2015 used computer‐ and Internet‐based programmes in their interventions. Fisher 2011 used a collaborative self‐monitoring of blood glucose (SMBG) that involved one‐time training (classified as cognition‐focused) and participants' continuous involvement up to 12 months. Quinn 2011 used a mobile diabetes management software application and a web portal. Trief 2016 examined couples' interventions and diabetes education through telephone solely. Three trials involved practice‐embedded nurses' care, coaching and counselling throughout the trial period (Gabbay 2013; Shibayama 2007; Whittemore 2004). Simmons 2015 used peer support with interventional activities throughout the follow‐up period. In these six trials, the last assessments were taken as the postintervention (follow‐up) assessment because most parts of the intervention were conducted within the first six months, and only a few similar intervention parts were repeated afterwards. Many outcomes were medium‐term outcomes (6 to 12 months). Gabbay 2013 reported usable data for all‐cause mortality, Grillo 2016 had usable data for blood pressure and HbA1c, and Simmons 2015 had usable data for blood pressure outcome.
Duration of follow‐up ranged from immediate postintervention assessment (five weeks follow‐up in the control group of Taylor 2006) to 12 months after the end of the intervention (accumulated 24 months follow‐up in the control groups of D'Eramo Melkus 2010 and Gabbay 2013), with a mean and median follow‐up of 10.5 and 12 months, respectively.
No trial used a run‐in period.
No trial was terminated early.
All trials were using adequate interventions and comparators.
Outcomes
Seventeen trials explicitly stated a primary or secondary endpoint in the publication. See Appendix 7 and Appendix 8.
HbA1c was the most commonly defined primary outcome in publications. Some trials stated multiple primary outcomes, Fisher 2011 mentioned depressive symptoms and DRD, Rosenbek 2011 reported HbA1c and self‐efficacy, Welch 2015 included HbA1c, blood pressure and hypoglycaemia, and Gabbay 2013 included all its outcomes as primary outcomes and performance measure as the other outcome.
Thirteen of the 22 studies that were registered in a trials register or published protocols specified their primary outcomes in the study publications (Dennick 2015; Fisher 2013; Gabbay 2013; Hermanns 2015; Pibernik‐Okanovic 2015; Quinn 2011; Rosenbek 2011; Simmons 2015; Trief 2016; Van der Wulp 2012; Van Dijk‐de Vries 2015; Weinger 2011; Welch 2015). Four of these 13 trials specified multiple primary outcomes in their trials register records (Fisher 2013; Gabbay 2013; Van Dijk‐de Vries 2015; Weinger 2011). Eight trials did not have trials registers records or published design papers (see outcome reporting bias) (D'Eramo Melkus 2010; Glasgow 2005; Lerman 2009; Liu 2015; Shibayama 2007; Skelly 2009; Taylor 2006; Whittemore 2004).
The 30 included trials collected a median of three (range two to six) outcomes.
Eight of 30 trials reported adverse events, hypoglycaemic events or both (Dennick 2015; Fisher 2011; Lamers 2011; Pibernik‐Okanovic 2015; Quinn 2011; Taylor 2006; Weinger 2011; Welch 2015), with four of the eight trials reporting both adverse and hypoglycaemic events (Fisher 2011; Lamers 2011; Quinn 2011; Weinger 2011). Dennick 2015 assessed DRD only at three months' follow‐up instead of six months or later, so we did not include it in the meta‐analysis or in the 'Risk of bias' assessment for the DRD outcome measure. However, authors did report adverse events, so we still included the trial in the review for this outcome. The same holds true for Taylor 2006.
No trial was powered for investigating all‐cause mortality, but 7 of 30 trials reported death from any cause (Davies 2008; Gabbay 2013; Lamers 2011; Pibernik‐Okanovic 2015; Skelly 2009; Sperl‐Hillen 2013; Sturt 2008). Grillo 2016 excluded one death from analysis from each group. Quinn 2011 reported clinical measurements related to diabetes complications (blood pressure, lipid levels), but these did not constitute macro‐ or microvascular complications, and Spencer 2013 reported diabetes‐related complications as a self‐reported outcome measure at baseline and not as an outcome.
Sixteen trials reported health‐related quality of life (Beverly 2013; Davies 2008; D'Eramo Melkus 2010; Gabbay 2013; Hermanns 2012; Hermanns 2015; Lamers 2011; Liu 2015; Pibernik‐Okanovic 2015; Shibayama 2007; Simmons 2015; Skelly 2009; Taylor 2006; Van der Wulp 2012; Van Dijk‐de Vries 2015; Weinger 2011). D'Eramo Melkus 2010 did not provide suitable data to be included in the present review.
No trial investigated socioeconomic effects.
All trials provided a definition of endpoint measurement for the main outcomes of diabetes‐related distress, health‐related quality of life and self‐efficacy (see also Appendix 15; Appendix 16 and Appendix 17). Twenty of 28 trials provided a definition of the endpoint measurement for HbA1c. Seven of 12 trials provided a description on the process of blood pressure measurement (see Appendix 7 definition of endpoint measurement for blood pressure) (Davies 2008; D'Eramo Melkus 2010; Grillo 2016; Quinn 2011; Simmons 2015; Trief 2016; Welch 2015). D'Eramo Melkus 2010 had suitable data for diabetes‐related distress and HbA1c to be included in the present review.
Excluded studies
38 of 104 publications were excluded after evaluation of the full‐text.
The main reasons for exclusion were that the study population was a mix of type 1 and type 2 diabetes mellitus (10 trials), and the comparators were similar psychological interventions and differed only in their delivery methods (further details see Characteristics of excluded studies and Figure 2).
Risk of bias in included studies
For details on risk of bias of included trials see Characteristics of included studies.
For an overview of review authors' judgments about each risk of bias item for individual trials and across all trials see Figure 3 and Figure 4.
3.
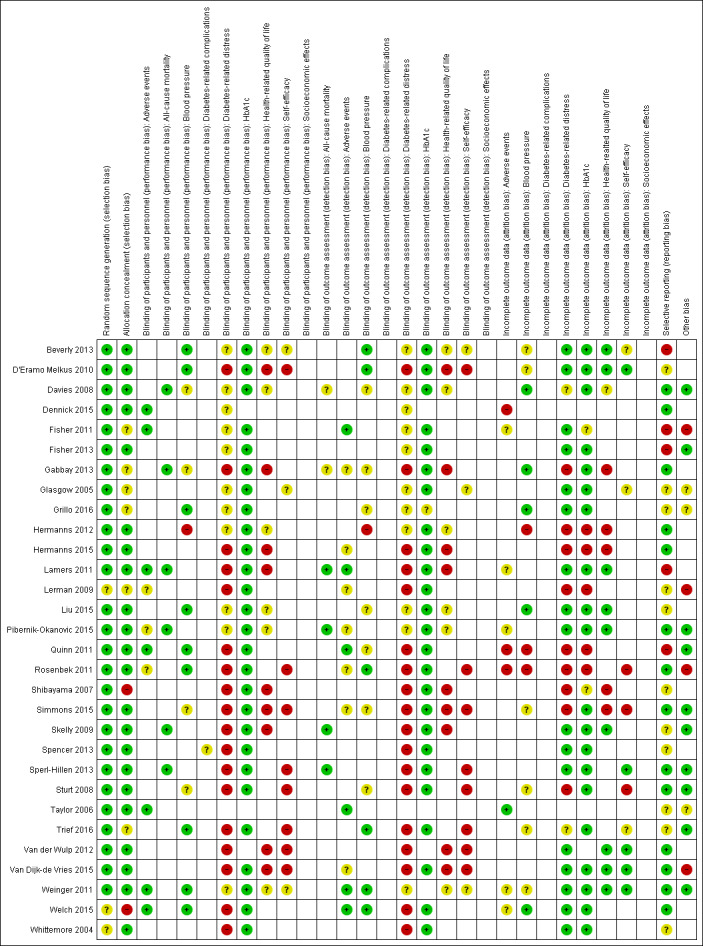
Risk of bias summary: review authors' judgements about each risk of bias item for each included study (blank cells indicate that the study did not report that particular outcome).
4.
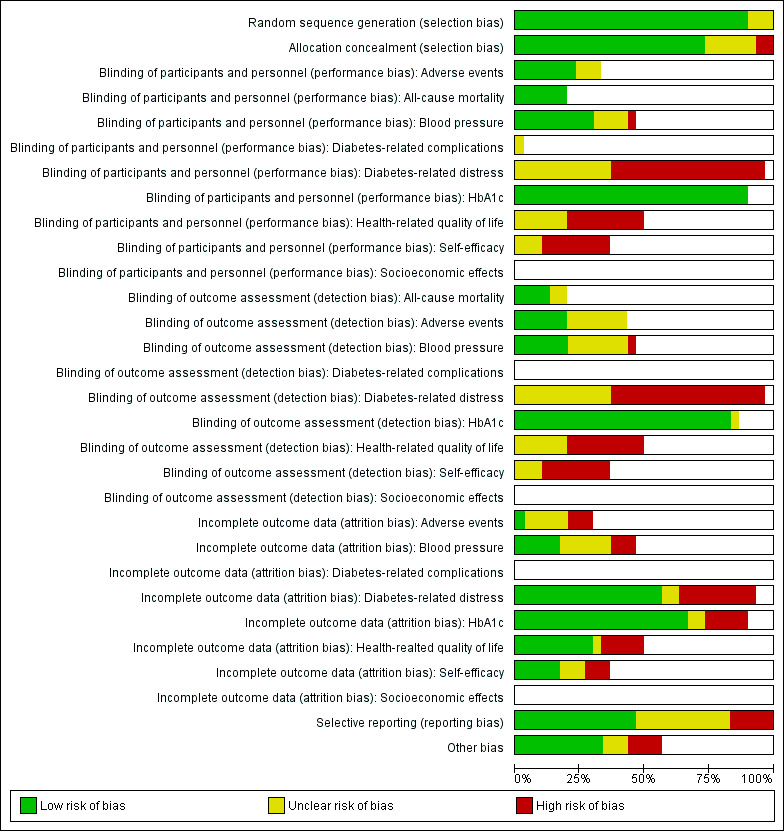
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included trials (blank cells indicate that the particular outcome was not investigated in some trials).
Allocation
Lerman 2009, Welch 2015 and Whittemore 2004 did not provide sufficient information on the random sequence generation procedure. Allocation concealment was probably inadequate in two trials: in Shibayama 2007, the investigators themselves were involved in allocating participants, and in Welch 2015, investigators obtained the healthcare providers' approval for participant participation. Allocation concealment was unclear in six trials (Fisher 2011; Gabbay 2013; Glasgow 2005; Grillo 2016; Lerman 2009; Trief 2016).
Blinding
As this review concerns psychological interventions, blinding of participants was not possible, so there was only blinding of healthcare providers or assessors. Below we report on blinding of the outcome assessor during blood pressure measurement, in particular using a sphygmomanometer, because blood pressure assessment might introduce a high risk of bias.
Five of the 12 trials reporting blood pressure measurements described performing single blinding. Trief 2016 reported so in the article, and four trial authors communicated through email correspondence (Beverly 2013; D'Eramo Melkus 2010; Rosenbek 2011; Weinger 2011).
Hermanns 2012 communicated that the blood pressure assessor was not blinded to the group assignment. This resulted in a high risk of bias.
Two trials did not provide a clear description of the blinding of the outcome assessor (Grillo 2016; Welch 2015), but blood pressure measurement was by means of an automatic digital blood pressure monitor, which resulted in low risk of bias.
Four of 12 trials had insufficient information about blinding procedures on blood pressure measurement (Davies 2008; Liu 2015; Quinn 2011; Simmons 2015).
Another two trials, apart from the above 12 trials, did not provide actual data that were needed for analysis in the review (Gabbay 2013; Sturt 2008).
Incomplete outcome data
Overall attrition rates ranged from 1.4% in Sperl‐Hillen 2013 to 31.8% in Davies 2008, and all included trials described them. Attrition rates within randomised groups in the included trials ranged from 0% in Liu 2015 and Skelly 2009 (in the symptom‐focused intervention group) to 35.9% in Davies 2008 (enhanced usual care). Taylor 2006 did not report complete attrition rates for all the intervention groups. Thus, all trials reported some losses to follow‐up, and one reported no attrition in two of the three intervention groups. Three of the 30 included trials had 10% or more difference in attrition rates between trial arms (Lerman 2009; Quinn 2011;Simmons 2015), with the largest differences reported by Quinn 2011 (coach‐only: attrition rate of 39.5% and usual care: attrition rate of 9.7%)
All included trials consistently used intention‐to‐treat analysis, except Hermanns 2015, which only used this for the primary outcome, and Lerman 2009, which analysed individuals who completed the study.
Fifteen of 29 trials reported losses to follow‐up and detailed descriptions of reasons for participants' withdrawals.
Gabbay 2013, Lerman 2009, Hermanns 2012, Hermanns 2015, Quinn 2011, Rosenbek 2011, Shibayama 2007, Simmons 2015 and Sturt 2008 had attrition rates with possible impact on the primary outcome of DRD.
Gabbay 2013, Hermanns 2012, Hermanns 2015, Shibayama 2007 and Simmons 2015 had attrition rates with possible impact on the outcome of health‐related quality of life.
Dennick 2015, Quinn 2011 and Rosenbek 2011 had attrition rates with possible impact on the outcome of adverse events.
Rosenbek 2011, Simmons 2015 and Sturt 2008 had attrition rates with possible impact on the self‐efficacy outcome.
Hermanns 2012, Hermanns 2015, Lerman 2009, Quinn 2011 and Rosenbek 2011 had attrition rates with possible impact on HbA1c.
Hermanns 2012, Quinn 2011 and Rosenbek 2011 had attrition rates with possible impact on blood pressure.
Selective reporting
We judged five trials to be at high risk of reporting bias (Beverly 2013; Fisher 2011; Fisher 2013; Lamers 2011; Quinn 2011), while we considered that 14 were at low risk based on the comparison of outcomes reported in published trials registers and results published in the respective papers (Davies 2008; Dennick 2015; Gabbay 2013; Hermanns 2012; Hermanns 2015; Pibernik‐Okanovic 2015; Rosenbek 2011; Simmons 2015; Sperl‐Hillen 2013; Sturt 2008; Van der Wulp 2012; Van Dijk‐de Vries 2015; Weinger 2011; Welch 2015). Nine trials had no published protocols or design papers to allow proper assessment of reporting bias (see also Appendix 5 and Appendix 6) (D'Eramo Melkus 2010; Glasgow 2005; Lerman 2009; Liu 2015; Shibayama 2007; Skelly 2009; Spencer 2013; Taylor 2006; Whittemore 2004).
Davies 2008 and Van der Wulp 2012 did not mention DRD as an outcome in the trials register records but reported it in the publications although DRD results were non‐significant. Weinger 2011 reported results on self‐efficacy, despite not pre‐specifying it as an outcome measure in the trials register record.
Funnel plots were possible for psychological interventions versus usual care for the outcome of diabetes‐related distress (11 trials, Analysis 8.1, Figure 5) and HbA1c (10 trials, Analysis 8.10, Figure 6). There was no clear evidence of reporting bias or small‐study effect in the former as the funnel plot is rather symmetrical. However, for the latter with HbA1c as the outcome, the funnel plot may indicate small‐study effect or true heterogeneity as discussed below.
Other potential sources of bias
Beverly 2013 and Whittemore 2004 recruited participants who had attended previous diabetes education programmes, and there was pre‐randomisation administration of a group education programme in the trials by Lerman 2009 and Rosenbek 2011. This could diminish the effect of the subsequent randomised experimental groups compared to the usual care control groups. There may have been no effect in the intervention in Van Dijk‐de Vries 2015 ('null bias' as described by Woods 1995). This trial used hybrid effectiveness‐implementation in its study design and saw low recruitment of eligible participants (only 16 of the 117 participants in the intervention arm) resulting in a low number of study participants (only 11) exposed to the complete intervention of self‐management support.
One trial on the effects of collaborative structured self‐measurement of blood glucose was sponsored by a pharmaceutical industry, so we judged it as having a potential conflict of interest (Fisher 2011). The trial on the Diabetes Priority Program by Glasgow 2005 did not provide clear funding sources except that it was a collaboration between the research team and the Copic Insurance Company, which provides malpractice insurance to 95% of the independent primary care physicians in Colorado, USA.
Effects of interventions
See: Table 1
None but two of all the included trials mentioned measuring diabetes‐related complications (Quinn 2011; Spencer 2013). Quinn 2011 defined diabetes‐related complications as blood pressure and lipid levels that are different from those stated for this review. Spencer 2013 included the number of diabetes‐related complications as a covariate in the analysis when making statistical adjustments for the effect of the intervention on DRD. Six trials reported on all‐cause mortality (Davies 2008; Gabbay 2013; Lamers 2011; Skelly 2009; Sperl‐Hillen 2013; Sturt 2008), which was not properly defined but mainly based on self‐report by the participant's family members or on mortality data in the electronic health record system (informed by the trial author Sperl‐Hillen 2013 through email correspondence). Grillo 2016 reported one death from each arm but did not define or report the source of the data. No trial examined the socioeconomic effects of psychological interventions in people with T2DM.
We combined outcomes for trials with more than two groups using similar interventions (Fisher 2013; Lerman 2009; Skelly 2009; Sperl‐Hillen 2013; Taylor 2006; Trief 2016; Weinger 2011). Hermanns 2015 used both DDS and PAID, and we included both outcomes in analyses but with the total sample halved. Quinn 2011 had three cognition‐focused groups combined for their outcome effects.
We describe the scale used by each included trial for DRD, HRQoL and self‐efficacy in Appendix 15, Appendix 16 and Appendix 17, respectively.
Baseline characteristics
For details of baseline characteristics, see Appendix 3 and Appendix 4. We describe notable differences in baseline characteristics in some of the included trials below. Van Dijk‐de Vries 2015 (EC versus usual care) recruited participants with emotional distress and impaired daily functioning, whereas Hermanns 2015 (EC versus CF), Lamers 2011 (EC versus usual care), Liu 2015 (EC versus CF) and Pibernik‐Okanovic 2015 (EC versus CF) recruited participants with depression. Conversely, Fisher 2013 (EC versus CF) included only participants who were clinically non‐depressed. Shibayama 2007 (EC versus usual care) excluded participants who were on insulin therapy. Gabbay 2013 (CF versus usual care) recruited participants who were considered to be at high risk for complications (HbA1c > 8.5%, blood pressure > 140/90 mmHg and/or low‐density lipoprotein (LDL) > 130 mg/dL). Regarding baseline HbA1c levels, Grillo 2016 (CF versus enhanced care) and Sperl‐Hillen 2013 (CF versus usual care) recruited participants with HbA1c > 7%; Trief 2016 (EC versus CF), Weinger 2011 (EC versus CF) and Welch 2015 (EC versus CF) included participants with HbA1c > 7.5%, and Sturt 2008 (EC versus usual care) recruited participants with baseline HbA1c > 8%.
Emotion‐focused (EF) interventions versus usual care
There was no trial comparing an EF intervention to usual care on any of the primary or secondary outcomes in this review.
Emotion‐focused interventions versus cognition‐focused (CF)
There was no study comparing EF to CF on diabetes‐related distress (DRD) or health‐related quality of life (HRQoL) at the pre‐determined timing of outcome measurement included in this review. However, Dennick 2015 examined the effect of writing thoughts and feelings about any stressful experience over the last month or current concern (known as the written emotional disclosure and classified as EF) and compared this intervention to neutral writing (classified as CF).
For this review, they only reported on adverse events. With only one participant‐reported adverse event of 'worried/stressed about what to write' reported in the intervention group, the relative risk was 2.38 (95% CI 0.10 to 55.06; P = 0.59; N = 41; Analysis 6.1). See also Appendix 9; Appendix 10; Appendix 11 and Appendix 12.
6.1. Analysis.
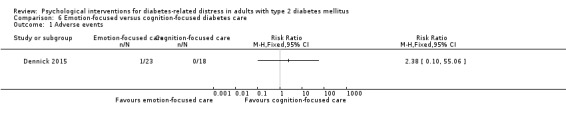
Comparison 6 Emotion‐focused versus cognition‐focused diabetes care, Outcome 1 Adverse events.
Cognition‐focused interventions versus usual care
Five trials compared a cognition‐focused intervention versus usual care (Gabbay 2013; Lerman 2009; Quinn 2011; Sperl‐Hillen 2013; Van der Wulp 2012). Six trials compared this type of programme to enhanced usual care (Beverly 2013; Davies 2008; Fisher 2011; Glasgow 2005; Grillo 2016; Skelly 2009). We performed separate analyses for comparisons of cognition‐focused psychological interventions: versus usual care (Analyses 1s), versus enhanced usual care (Analyses 2s) and versus combined usual and enhanced usual care (Analyses 3s). Gabbay 2013 and Skelly 2009 did not provide sufficient data for this outcome specifically between 6 to 12 months after intervention. Comparison between the cognition‐focused and usual care shows some significant beneficial effects for self‐efficacy. However, comparisons with enhanced usual care and combined usual and enhanced usual care do not result in substantial differences in effects. All three comparators (Analysis 1.2; Analysis 2.1 and Analysis 3.2) showed similar effects of cognition‐focused psychological interventions for DRD. Similar data of better effects on HbA1c in the longer and more advanced cognition‐focused psychological interventions were observed in all three comparators (Analysis 1.9; Analysis 2.9; Analysis 3.10).
1.2. Analysis.
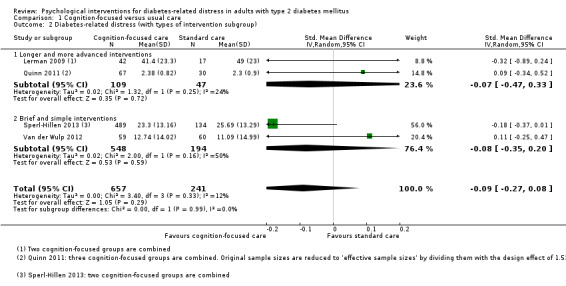
Comparison 1 Cognition‐focused versus usual care, Outcome 2 Diabetes‐related distress (with types of intervention subgroup).
2.1. Analysis.
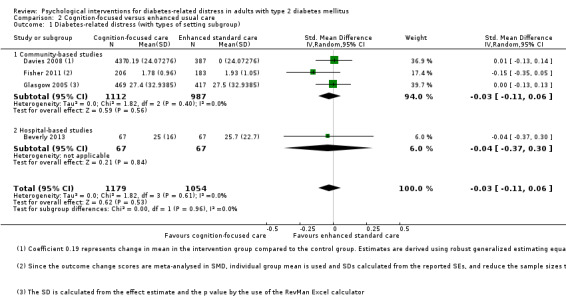
Comparison 2 Cognition‐focused versus enhanced usual care, Outcome 1 Diabetes‐related distress (with types of setting subgroup).
3.2. Analysis.
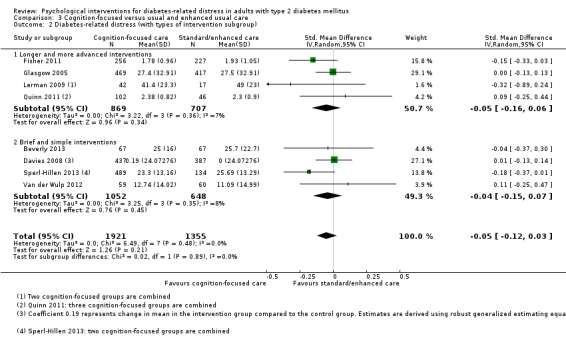
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 2 Diabetes‐related distress (with types of intervention subgroup).
1.9. Analysis.
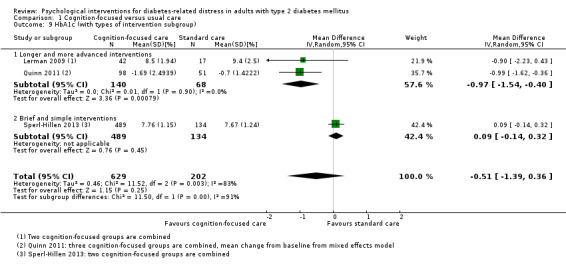
Comparison 1 Cognition‐focused versus usual care, Outcome 9 HbA1c (with types of intervention subgroup).
2.9. Analysis.
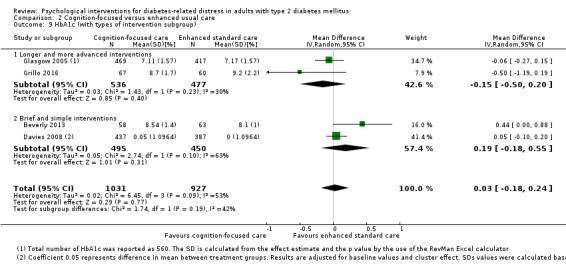
Comparison 2 Cognition‐focused versus enhanced usual care, Outcome 9 HbA1c (with types of intervention subgroup).
3.10. Analysis.
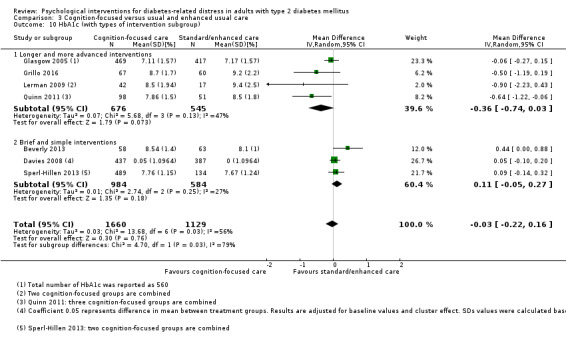
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 10 HbA1c (with types of intervention subgroup).
Primary outcomes
Diabetes‐related distress (DRD)
Four trials compared usual care versus cognition‐focused psychological interventions for DRD (measured with DDS and PAID) at 6 to 12 months (medium‐term) (Lerman 2009; Quinn 2011; Sperl‐Hillen 2013; Van der Wulp 2012). Interventions lasted from 3 to 12 months, and follow‐up periods ranged from 10 to 12 months. The meta‐analysis for DRD showed an SMD of −0.09 (95% CI −0.27 to 0.08; P = 0.29; 898 participants; 4 trials; Analysis 1.1, Analysis 1.2, Analysis 1.3).
1.1. Analysis.
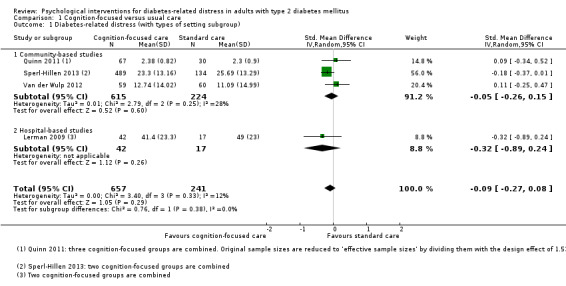
Comparison 1 Cognition‐focused versus usual care, Outcome 1 Diabetes‐related distress (with types of setting subgroup).
1.3. Analysis.
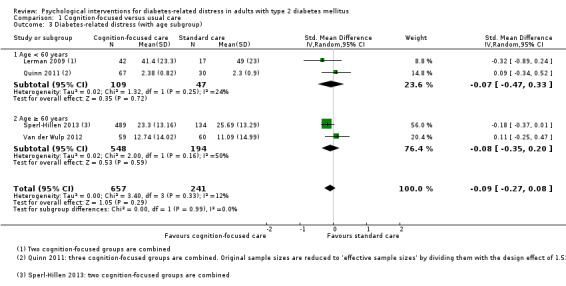
Comparison 1 Cognition‐focused versus usual care, Outcome 3 Diabetes‐related distress (with age subgroup).
Health‐related quality of life (HRQoL)
One trial assessed the effects of cognition‐focused psychological interventions versus usual care for HRQoL at 6 to 12 months after the intervention (Van der Wulp 2012). There was no substantial difference for HRQoL (MD 5 points; 95% CI −3 to 12; 119 participants; 1 trial; Analysis 1.4).
1.4. Analysis.
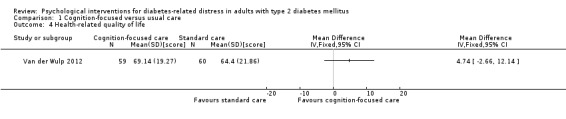
Comparison 1 Cognition‐focused versus usual care, Outcome 4 Health‐related quality of life.
Adverse events
One trial assessed the effects of cognition‐focused psychological interventions versus usual care on adverse events at less than 6 months (short‐term) postintervention (Quinn 2011), reporting 1/107 death in the intervention compared with 0/56 deaths in the control group (163 participants; 1 trial; very low‐quality evidence; Analysis 1.5). Quinn 2011 collated incidence of hypoglycaemia together with all the other adverse events, including hospitalisations and emergency‐room visits. With the enhanced usual care comparator (Analysis 2.5), Fisher 2011 reported the incidence of hypoglycaemia, based on downloaded meter data, to be 1.9% in the intervention group versus 1.8% in the usual care group. One participant in the second intervention group of symptom‐focused diabetes intervention with booster reported feeling depressed (Skelly 2009; see also Appendix 9; Appendix 10; Appendix 11; Appendix 12).
1.5. Analysis.
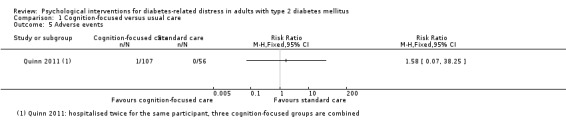
Comparison 1 Cognition‐focused versus usual care, Outcome 5 Adverse events.
2.5. Analysis.
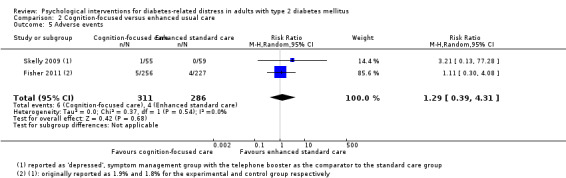
Comparison 2 Cognition‐focused versus enhanced usual care, Outcome 5 Adverse events.
Secondary outcomes
Self‐efficacy
Two trials assessed the effects of cognition‐focused psychological interventions compared to usual care on self‐efficacy at 6 to 12 months (medium‐term) postintervention (Sperl‐Hillen 2013; Van der Wulp 2012), and the meta‐analysis yielded an SMD of 0.21 (95% CI 0.04 to 0.38; P = 0.02; 742 participants; Analysis 1.6).
1.6. Analysis.
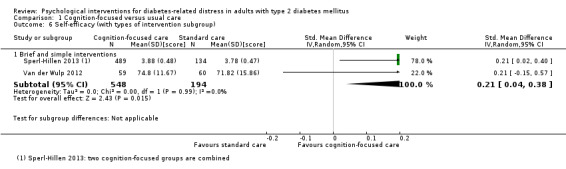
Comparison 1 Cognition‐focused versus usual care, Outcome 6 Self‐efficacy (with types of intervention subgroup).
HbA1c
Three trials assessed the effects of cognition‐focused psychological interventions on HbA1c at 6 to 12 months (medium‐term) postintervention (Lerman 2009; Quinn 2011; Sperl‐Hillen 2013). The meta‐analysis showed an MD for HbA1c of −0.51% (95% CI −1.39 to 0.36; P = 0.25; 831 participants; 3 trials; Analysis 1.9). Skelly 2009 did not provide sufficient data for this outcome.
Blood pressure
One trial compared usual care versus cognition‐focused psychological interventions for blood pressure (both systolic and diastolic) at 6 to 12 months (medium‐term) postintervention (Quinn 2011), and there were no substantial differences for systolic blood pressure (MD −1.8 mmHg (95% CI −9.3 to 5.7); 137 participants; Analysis 1.11) or diastolic blood pressure (MD −1.5 mmHg; 95% CI −6.0 to 3.0; Analysis 1.12).
1.11. Analysis.
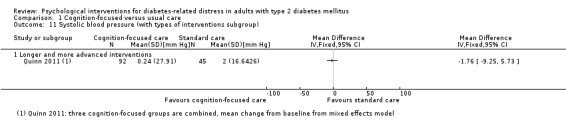
Comparison 1 Cognition‐focused versus usual care, Outcome 11 Systolic blood pressure (with types of interventions subgroup).
1.12. Analysis.
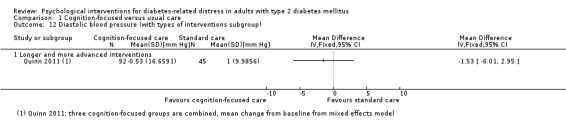
Comparison 1 Cognition‐focused versus usual care, Outcome 12 Diastolic blood pressure (with types of interventions subgroup).
Diabetes‐related complications
The included psychological intervention trials did not investigate diabetes‐related complications.
All‐cause mortality
Combining all the comparators for up to and more than 12 months (Davies 2008; Skelly 2009; Sperl‐Hillen 2013), the meta‐analysis showed no substantial differences (RR 0.79; 95% CI 0.31 to 2.02; P = 0.62; 1621 participants; 3 trials; moderate‐quality evidence; Analysis 3.14). The estimated effect on all‐cause mortality at all times was also not different between cognition‐focused versus usual care (10/721 deaths in the intervention groups versus 3/447 deaths in the comparator groups; RR 1.81, 95% CI 0.29 to 11.38; P = 0.17; 1168 participants; 2 trials; low‐quality evidence; Analysis 1.13).
3.14. Analysis.
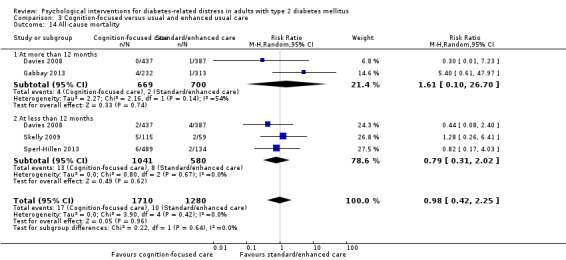
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 14 All‐cause mortality.
1.13. Analysis.
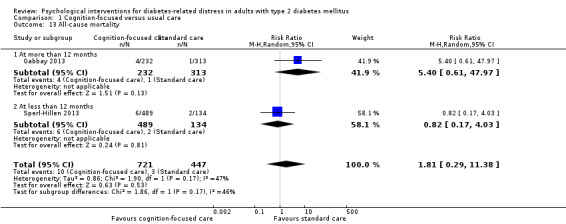
Comparison 1 Cognition‐focused versus usual care, Outcome 13 All‐cause mortality.
Emotion‐cognition (EC) focused interventions versus usual care
Trials included in this comparison used only usual care as a comparator.
Primary outcomes
Diabetes‐related distress
Nine trials assessed the effects of emotion‐cognition psychological interventions on DRD at 6 to 12 months (medium‐term) postintervention, but only eight reported sufficient information to pool effect sizes (Lamers 2011; Rosenbek 2011; Shibayama 2007; Simmons 2015; Spencer 2013; Sturt 2008; Van Dijk‐de Vries 2015; Whittemore 2004). Duration of interventions ranged from 6 weeks to 12 months, and follow‐up periods ranged from 6 months to 12 months. Skelly 2009 did not provide sufficient data for this outcome. The meta‐analysis for DRD showed an SMD of −0.07 (95% CI −0.19 to 0.06; P = 0.30; 2366 participants; 8 trials; Analysis 4.1).
4.1. Analysis.
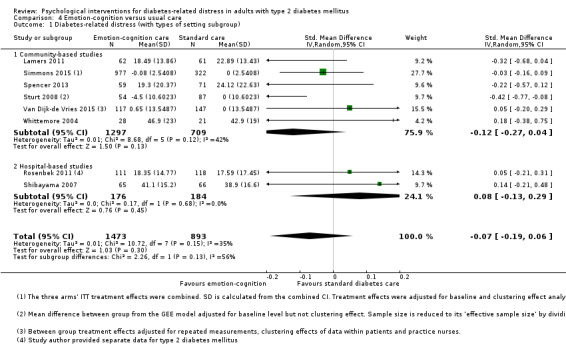
Comparison 4 Emotion‐cognition versus usual care, Outcome 1 Diabetes‐related distress (with types of setting subgroup).
Health‐related quality of life
Five trials assessed the effects of emotion‐cognition psychological interventions on HRQoL at 6 to 12 months (medium‐term) postintervention, but only four of these trials reported sufficient information to pool effect sizes (Lamers 2011; Shibayama 2007; Simmons 2015; Van Dijk‐de Vries 2015). Skelly 2009 did not provide sufficient data for this outcome. The meta‐analysis showed an SMD for HRQoL of −0.01 (95% CI −0.11 to 0.09; P = 0.85; 1813 participants; 4 trials; Analysis 4.5).
4.5. Analysis.
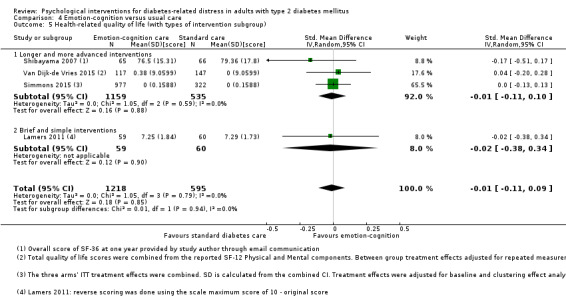
Comparison 4 Emotion‐cognition versus usual care, Outcome 5 Health‐related quality of life (with types of intervention subgroup).
Adverse events
Three trials examined adverse events of the emotion‐cognition psychological interventions compared to usual care (Lamers 2011; Taylor 2006; Rosenbek 2011), and two reported sufficient information for meta‐analysis. Fifteen events were reported: 12 in the intervention groups and 3 in the control groups. Lamers 2011 reported the most number of adverse events in the intervention group but did not specify hypoglycaemia; seven participants in the cognitive behavioural therapy with self‐management principles group reported that they perceived the questionnaire to be "burdensome", compared to three participants in the control group. Taylor 2006 reported only two adverse events in the intervention groups; one participant reported a "distinct dislike" of the emotion‐cognition therapy, and another participant was noted to be 'crying' during the expressive writing session. Rosenbek 2011 did not provide details on the reported events. The meta‐analysis showed an RR of 2.55 (95% CI 0.77 to 8.47; P = 0.13; 275 participants; 2 trials; low‐quality evidence; Analysis 4.4). See also Appendix 9; Appendix 10; Appendix 11 and Appendix 12.
4.4. Analysis.
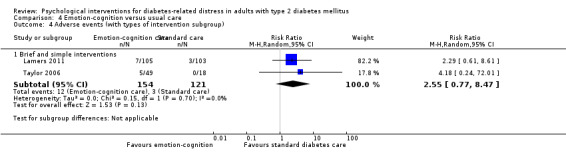
Comparison 4 Emotion‐cognition versus usual care, Outcome 4 Adverse events (with types of intervention subgroup).
Secondary outcomes
Self‐efficacy
Five trials assessed medium‐term effects of emotion‐cognition psychological interventions on self‐efficacy, with four reporting sufficient information for a pooled effect size estimation (Rosenbek 2011; Simmons 2015; Sturt 2008; Van Dijk‐de Vries 2015), which showed no substantial effect for the emotion‐cognition psychological intervention versus usual care (SMD 0.14; 95% CI −0.08 to 0.35; P = 0.22; 1933 participants; 4 trials; Analysis 4.7).
4.7. Analysis.
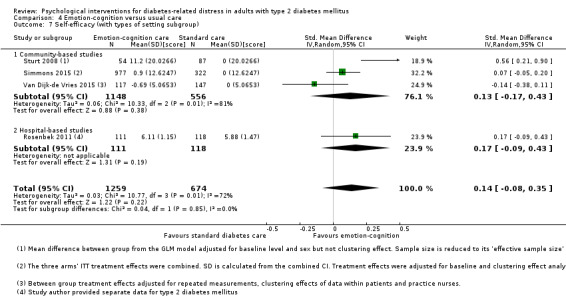
Comparison 4 Emotion‐cognition versus usual care, Outcome 7 Self‐efficacy (with types of setting subgroup).
HbA1c
Nine trials assessed the medium‐term effects of emotion‐cognition psychological interventions on HbA1c. Eight trials reported sufficient information for a pooled effect size estimation (Lamers 2011; Rosenbek 2011; Shibayama 2007; Simmons 2015; Spencer 2013; Sturt 2008; Van Dijk‐de Vries 2015; Whittemore 2004), which showed an MD for HbA1c of −0.09% (95% CI −0.19 to 0.0; P = 0.06; 2334 participants; 8 trials; Analysis 4.10).
4.10. Analysis.
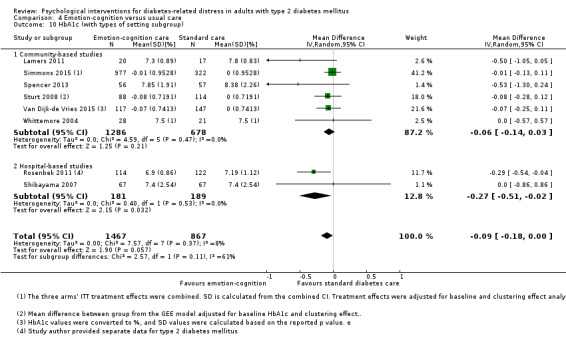
Comparison 4 Emotion‐cognition versus usual care, Outcome 10 HbA1c (with types of setting subgroup).
Blood pressure
Rosenbek 2011 and Simmons 2015 provided data on the effects of emotion‐cognition psychological interventions on both systolic and diastolic blood pressure at 6 to 12 months after the intervention. The meta‐analysis yielded no substantial differences for either systolic (MD −0.4 mmHg; 95% CI −2.1 to 1.2; P = 0.60; 1296 participants; Analysis 4.13) or diastolic blood pressure (MD −0.3 mmHg; 95% CI −1.4 to 0.7; P = 0.51; 1296 participants; Analysis 4.14).
4.13. Analysis.
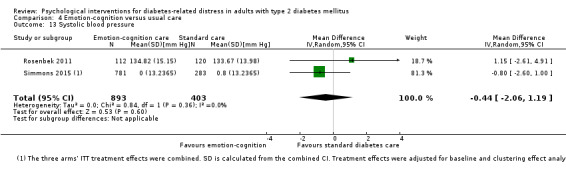
Comparison 4 Emotion‐cognition versus usual care, Outcome 13 Systolic blood pressure.
4.14. Analysis.
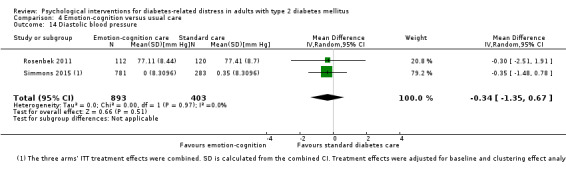
Comparison 4 Emotion‐cognition versus usual care, Outcome 14 Diastolic blood pressure.
Diabetes‐related complications
The included psychological intervention trials did not investigate diabetes‐related complications.
All‐cause mortality
Only Lamers 2011 reported on all‐cause mortality at less than 12 months, with one death reported at three months and two deaths at nine months following usual care (3/103 and 9/103 participants, respectively, versus 0/105 in the intervention group; Analysis 4.15).
4.15. Analysis.

Comparison 4 Emotion‐cognition versus usual care, Outcome 15 All‐cause mortality.
Emotion‐cognition focused interventions versus cognition‐focused interventions
Overall there were no substantial differences between these two types of psychological interventions on the outcomes in this review. Welch 2015 showed favourable effects for emotion‐cognition focused interventions in DRD and HbA1c, probably due to the high proportion and degree of distress and major depression among participants and their poor glycaemic control. Additionally, the emotion‐cognition intervention involved multiple team members of the healthcare professionals on top of continuous computer‐based support and reminders. Liu 2015 reported the results of a trial in China and showed favourable effects of the emotion‐cognition intervention for DRD and HRQoL. Liu 2015 recruited T2DM people with mild to moderate depression or anxiety and provided almost continuous personal contact with peers for exercises and discussion. Although Hermanns 2015 provided their emotion‐cognition intervention focused on managing DRD, it resulted in more beneficial effects in the subgroup of participants with T2DM. Hermanns 2015 included participants with depression and long duration of diabetes mellitus, and many participants already had diabetes‐related complications.
Primary outcomes
Diabetes‐related distress
Nine trials assessed the medium‐term effects of emotion‐cognition versus cognition‐focused interventions on DRD after the intervention (D'Eramo Melkus 2010; Fisher 2013; Hermanns 2012; Hermanns 2015; Liu 2015; Pibernik‐Okanovic 2015; Trief 2016; Weinger 2011; Welch 2015). Hermanns 2015 used both DDS and PAID, including both scores in the comparison and halving the study sample size. The meta‐analysis indicated a considerable between‐study heterogeneity, and the result was not pooled (Analysis 5.1). Besides differences in locations of the trial, there were differences in the participants' demographic and clinical characteristics at enrolment. D'Eramo Melkus 2010 included female participants only, employed a cognitive‐behavioural self‐management training up to 12 months and had a follow‐up of 24 months, whereas Weinger 2011 and Hermanns 2015 assessed a similar structured behavioural self‐managment training of five weeks with a follow‐up of 12 months. Fisher 2013, Hermanns 2012 and Welch 2015 used different computer‐based self‐management programmes with or without subsequent contacts with healthcare professionals. Fisher 2013 recruited clinically non‐depressed participants, while Liu 2015 recruited T2DM participants with mild to moderate depression or anxiety, and Welch 2015 included participants who were highly distressed (two‐thirds of the total participants) or had a major depression (one‐third of the total participants). Lastly, there was a large variation in the classification of control groups. For example, Weinger 2011 had both individual control and group attention control groups; although both were mainly cognition‐focused, there may also have been emotional components in the contacts with the diabetes nurses. Trief 2016 had as individual emotion‐cognition intervention and a cognitive‐focused diabetes education as two comparators.
5.1. Analysis.
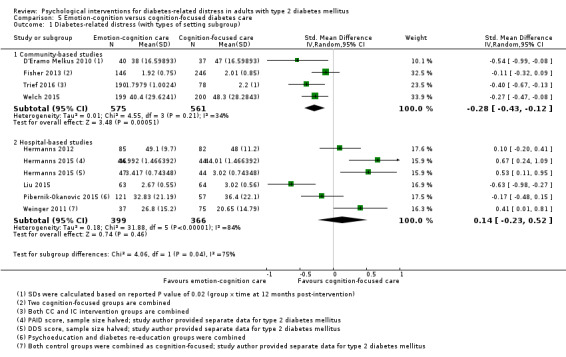
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 1 Diabetes‐related distress (with types of setting subgroup).
Health‐related quality of life
Five trials assessed the medium‐term effects (6 to 12 months) of emotion‐cognition versus cognition‐focused interventions on HRQoL (Hermanns 2012; Hermanns 2015; Liu 2015; Pibernik‐Okanovic 2015; Weinger 2011). Hermanns 2015 used both the EuroQol (EQ‐5D) and the World Health Organization five‐item (WHO‐5) Well‐Being Index, including both scores in the comparison and halving the study sample size. All these trials were hospital‐based or took place in specialist care settings. The meta‐analysis demonstrated an SMD for HRQoL of 0.01 (95% CI −0.27 to 0.29; P = 0.95; 765 participants; 5 trials; low‐quality evidence; Analysis 5.5).
5.5. Analysis.
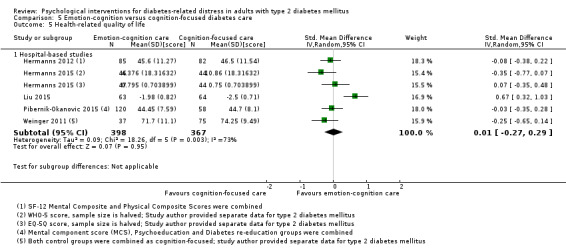
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 5 Health‐related quality of life.
Adverse events
Only one trial reported any adverse outcome (Welch 2015). Pibernik‐Okanovic 2015 reported unspecified 'other critical disease' as one of the dropout reasons in the study flow chart (Appendix 9), but we did not consider this as an adverse event. Welch 2015 examined the adverse effect of hypoglycaemic events. The reported rates were 22.1% (38/172 in the emotion‐cognition focused diabetes care group) and 20.4% (37/181 in the cognition‐focused diabetes care group) (Appendix 12). The RR was 1.08 (95% CI 0.72 to 1.62; low‐quality evidence; Analysis 5.6).
5.6. Analysis.
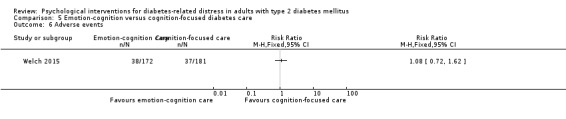
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 6 Adverse events.
Secondary outcomes
Self‐efficacy
Trief 2016 and Weinger 2011 reported on the effects of emotion‐cognition versus cognition‐focused psychological interventions on self‐efficacy at 6 to 12 months (medium‐term) postintervention. The estimated effect showed an SMD of −0.01 (95% CI −0.26 to 0.24; P = 0.91; 380 participants; 2 trials; low‐quality evidence; Analysis 5.7).
5.7. Analysis.
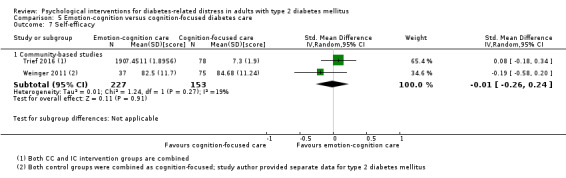
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 7 Self‐efficacy.
HbA1c
Nine trials investigated the effects of emotion‐cognition versus cognition focused psychological interventions on HbA1c at 6 to 12 months postintervention (D'Eramo Melkus 2010; Fisher 2013; Hermanns 2012; Hermanns 2015; Liu 2015; Pibernik‐Okanovic 2015; Trief 2016; Weinger 2011; Welch 2015). The meta‐analysis indicated a considerable between‐study heterogeneity (1934 participants; 9 trials; very low‐quality evidence; Analysis 5.8). This considerable heterogeneity might be due to characteristics that varied across the included trials as elaborated above, especially in D'Eramo Melkus 2010, where all participants were African American and in Welch 2015 with a high proportions of distressed and depressed participants. The outcomes of these two trials highly favoured the emotion‐cognition diabetes care arms.
5.8. Analysis.
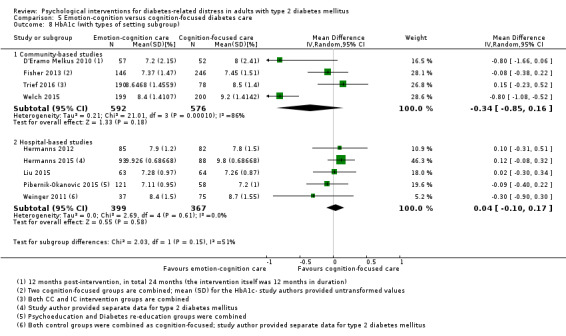
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 8 HbA1c (with types of setting subgroup).
Blood pressure
Five trials examined the effects of emotion‐cognition versus cognition‐focused psychological interventions on blood pressure (both systolic and diastolic) at 6 to 12 months postintervention (Hermanns 2012; Liu 2015; Trief 2016; Weinger 2011; Welch 2015). The meta‐analyses indicated that there were no substantial differences or inconsistencies in direction of effects for systolic and diastolic blood pressure (Analysis 5.12; Analysis 5.15).
5.12. Analysis.
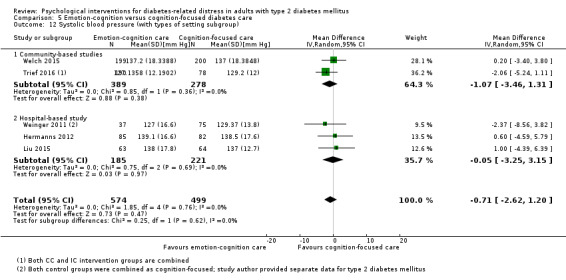
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 12 Systolic blood pressure (with types of setting subgroup).
5.15. Analysis.
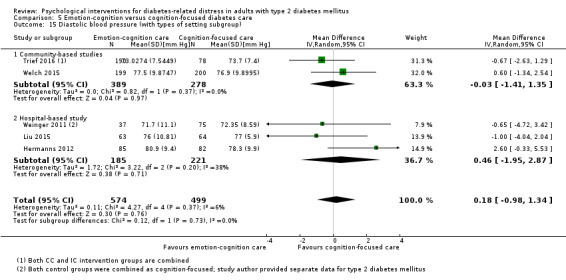
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 15 Diastolic blood pressure (with types of setting subgroup).
Diabetes‐related complications
The included psychological intervention trials did not investigate diabetes‐related complications.
All‐cause mortality
The emotion‐cognition focused interventions versus cognition‐focused interventions did not investigate all‐cause mortality.
All psychological interventions versus usual care
As specified in the review protocol, we examined the combined effects of any type of psychological intervention compared to usual care (Analysis 8.2; Analysis 8.3; Analysis 8.4; Analysis 8.5; Analysis 8.6; Analysis 8.7; Analysis 8.8; Analysis 8.9; Analysis 8.11; Analysis 8.12; Analysis 8.13; Analysis 8.14; Analysis 8.15; Analysis 8.16). There are 14 trials included in these analysis (Gabbay 2013; Lamers 2011; Lerman 2009; Quinn 2011; Rosenbek 2011; Shibayama 2007; Simmons 2015; Spencer 2013; Sperl‐Hillen 2013; Sturt 2008; Taylor 2006; Van der Wulp 2012; Van Dijk‐de Vries 2015; Whittemore 2004). We excluded control groups other than usual care (Beverly 2013; Davies 2008; Fisher 2011; Glasgow 2005; Grillo 2016; Skelly 2009). Enhanced usual care groups used attention control and provided some or similar numbers of contact with healthcare professionals or services but differed in the active element in the intervention groups. In these trials, the between‐group effects were lower and less consistent, as seen in Analysis 2.1, Analysis 2.6 and Analysis 2.8 (having the enhanced usual care as the comparator) and in Analysis 7.8, Analysis 7.12 and Analysis 7.13 (combining both usual and enhanced usual care as the comparators) when compared to trials employing usual care. Results of this comparison are tabulated in the Table 1; please see Appendix 14 for the quality of evidence assessment.
8.2. Analysis.
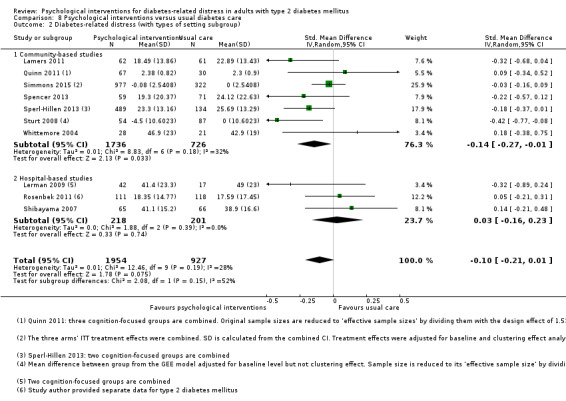
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 2 Diabetes‐related distress (with types of setting subgroup).
8.3. Analysis.
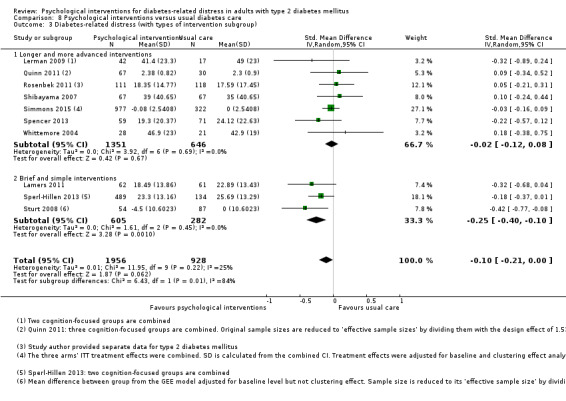
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 3 Diabetes‐related distress (with types of intervention subgroup).
8.4. Analysis.
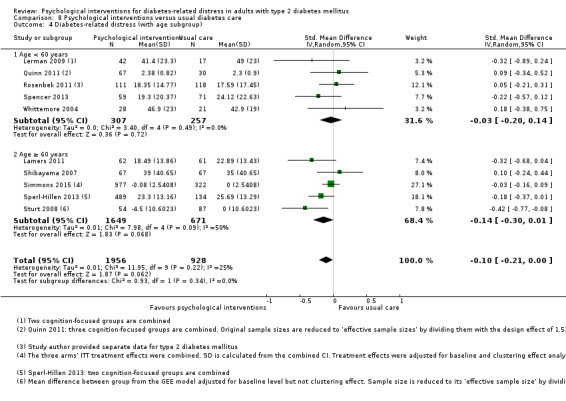
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 4 Diabetes‐related distress (with age subgroup).
8.5. Analysis.
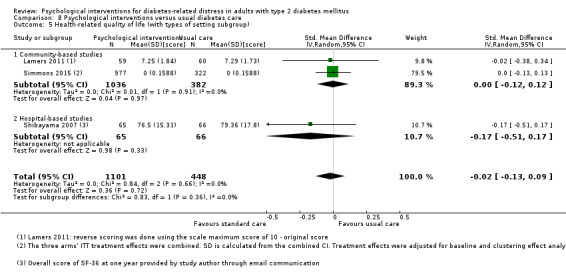
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 5 Health‐related quality of life (with types of setting subgroup).
8.6. Analysis.
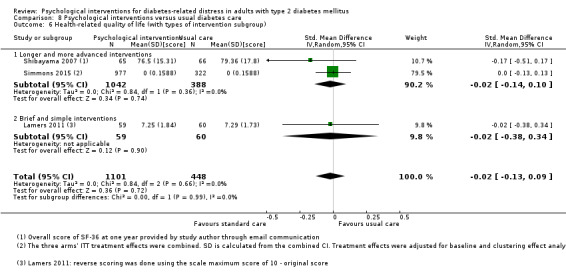
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 6 Health‐related quality of life (with types of intervention subgroup).
8.7. Analysis.
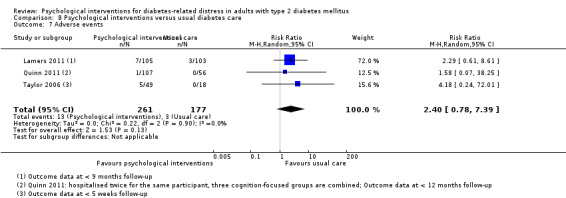
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 7 Adverse events.
8.8. Analysis.
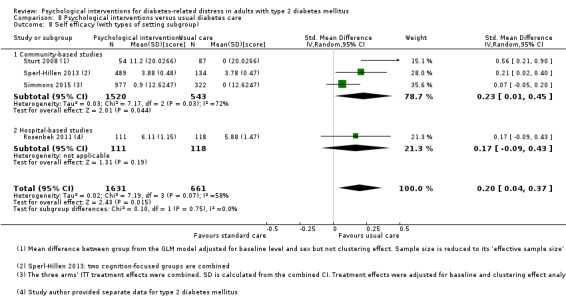
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 8 Self efficacy (with types of setting subgroup).
8.9. Analysis.
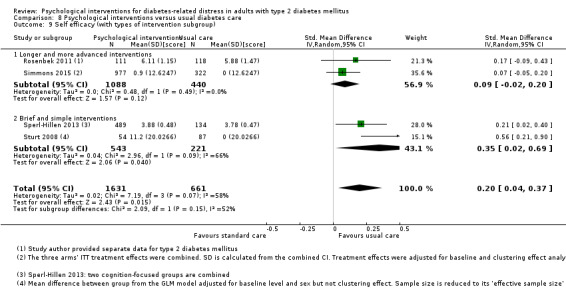
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 9 Self efficacy (with types of intervention subgroup).
8.11. Analysis.
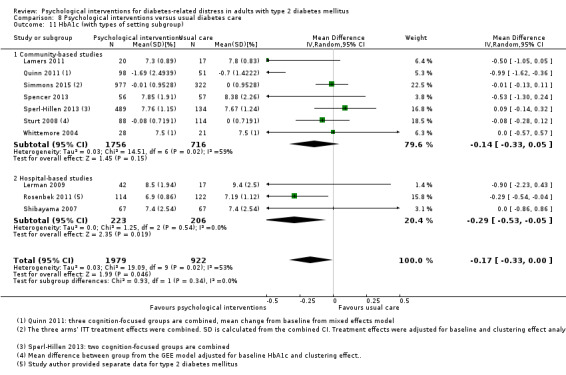
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 11 HbA1c (with types of setting subgroup).
8.12. Analysis.
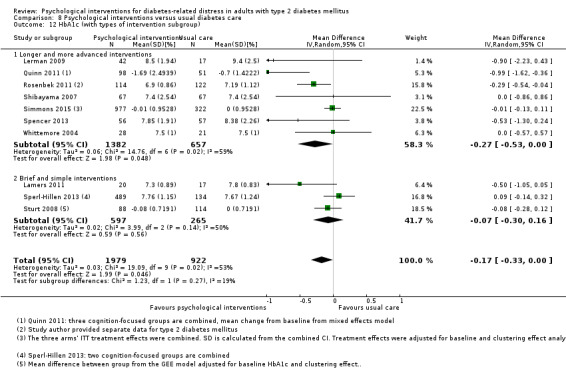
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 12 HbA1c (with types of intervention subgroup).
8.13. Analysis.
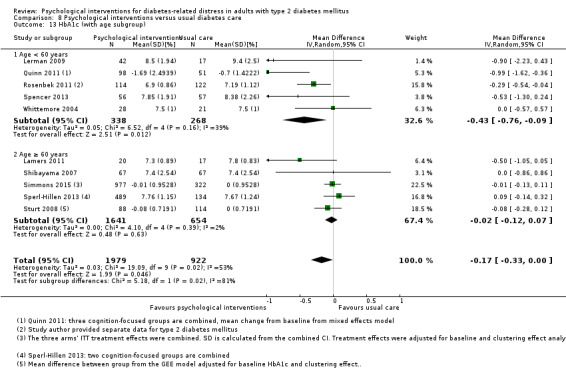
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 13 HbA1c (with age subgroup).
8.14. Analysis.
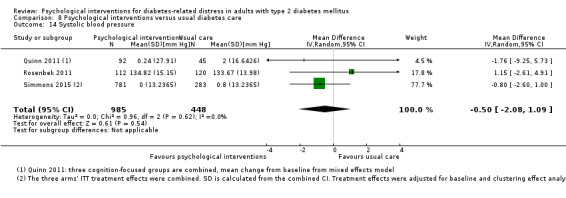
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 14 Systolic blood pressure.
8.15. Analysis.
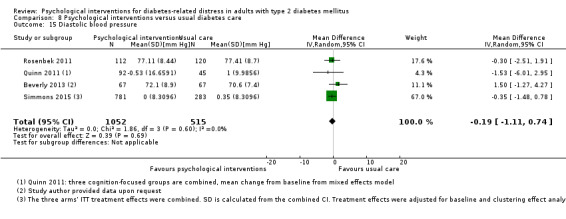
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 15 Diastolic blood pressure.
8.16. Analysis.
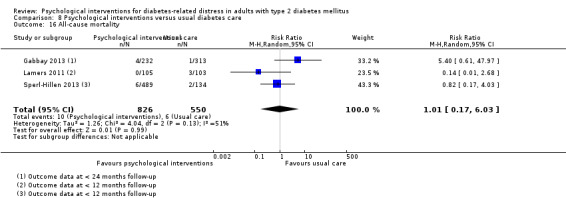
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 16 All‐cause mortality.
2.6. Analysis.
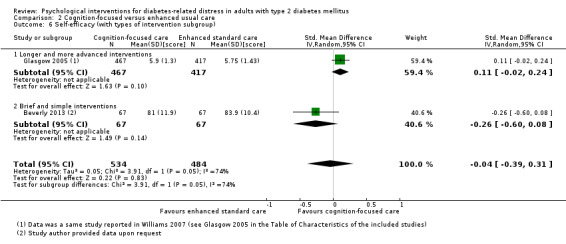
Comparison 2 Cognition‐focused versus enhanced usual care, Outcome 6 Self‐efficacy (with types of intervention subgroup).
2.8. Analysis.
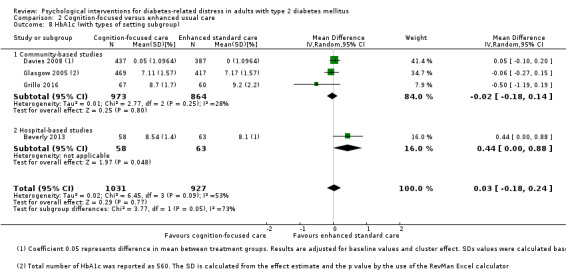
Comparison 2 Cognition‐focused versus enhanced usual care, Outcome 8 HbA1c (with types of setting subgroup).
7.8. Analysis.
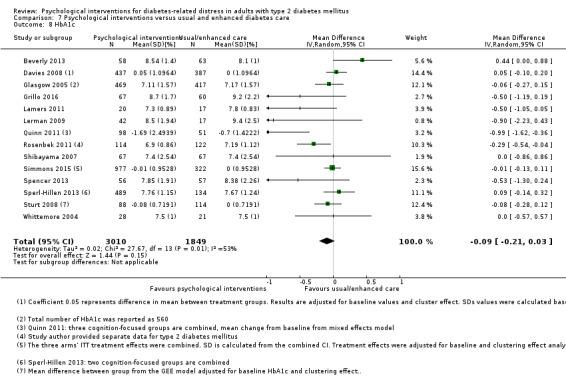
Comparison 7 Psychological interventions versus usual and enhanced diabetes care, Outcome 8 HbA1c.
7.12. Analysis.
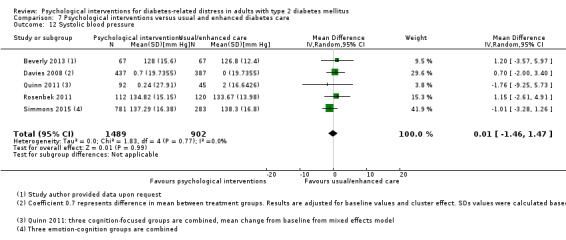
Comparison 7 Psychological interventions versus usual and enhanced diabetes care, Outcome 12 Systolic blood pressure.
7.13. Analysis.
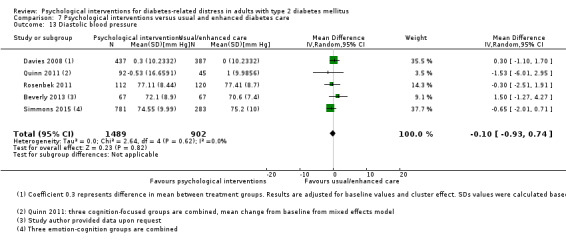
Comparison 7 Psychological interventions versus usual and enhanced diabetes care, Outcome 13 Diastolic blood pressure.
Primary outcomes
Diabetes‐related distress
All different types of psychological interventions taken together and compared to usual care for DRD showed an SMD of −0.07 (95% CI −0.16 to 0.03; P = 0.17; 3315 participants; 12 trials; low‐quality evidence; Analysis 8.1).
Health‐related quality of life
Similarly, psychological interventions compared to usual care for HRQoL showed an SMD of 0.01 (95% CI −0.09 to 0.11; P = 0.87; 1932 participants; 5 trials; low‐quality evidence; Analysis 8.5; Analysis 8.6).
Adverse events
From trials that reported adverse outcomes, the combined effect showed that participants in the psychological intervention groups experienced a higher risk of adverse events (RR 2.40; 95% CI 0.78 to 7.39; P = 0.90; 438 participants; 3 trials; low‐quality evidence; Analysis 8.7). See also Appendix 9, Appendix 10, Appendix 11 and Appendix 12.
Secondary outcomes
Self‐efficacy
The effect of psychological interventions versus usual care on self‐efficacy showed an SMD of 0.15 (95% CI 0.0 to 0.30; P = 0.005; 2675 participants; 6 trials; low‐quality evidence; Analysis 8.8; Analysis 8.9).
HbA1c
Psychological interventions compared to usual care for HbA1c showed an MD of −0.14% (95% CI −0.27 to 0.00; P = 0.050; 3165 participants; 11 trials; low‐quality evidence; Analysis 8.10).
Blood pressure
Psychological interventions compared to usual care for systolic blood pressure showed an MD of −0.5 mmHg (95% CI −2.1 to 1.1; P = 0.54; 1433 participants; 3 trials; Analysis 8.14); and for diastolic blood pressure an MD of −0.2 mmHg (95% CI −1.1 to 0.7; P = 0.69; 1567 participants; 3 trials; Analysis 8.15).
Diabetes‐related complications
The included psychological intervention trials did not investigate diabetes‐related complications.
All‐cause mortality
In the trials that reported all‐cause mortality, participants receiving psychological interventions did not experience more deaths from any cause compared to usual care (10/826 participants versus 6/550 participants, respectively; RR 1.01; 95% CI 0.17 to 6.03; P = 0.99; 1376 participants; 3 trials; low‐quality evidence; Analysis 8.16).
Subgroup analyses
It was possible to explore four of the five pre‐specified subgroup analyses in our protocol.
Setting (hospital versus community‐based trials): Analysis 1.1; Analysis 1.8; Analysis 2.1; Analysis 2.8; Analysis 3.1; Analysis 3.6; Analysis 3.9; Analysis 4.1; Analysis 4.7; Analysis 4.10; Analysis 5.1; Analysis 5.8; Analysis 5.12; Analysis 5.15; Analysis 7.2; Analysis 7.9; Analysis 8.2; Analysis 8.5; Analysis 8.8; Analysis 8.11; Analysis 10.1; Analysis 10.6; Analysis 10.8; Analysis 10.9.
1.8. Analysis.
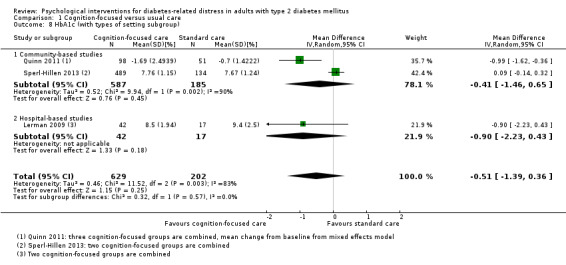
Comparison 1 Cognition‐focused versus usual care, Outcome 8 HbA1c (with types of setting subgroup).
3.1. Analysis.
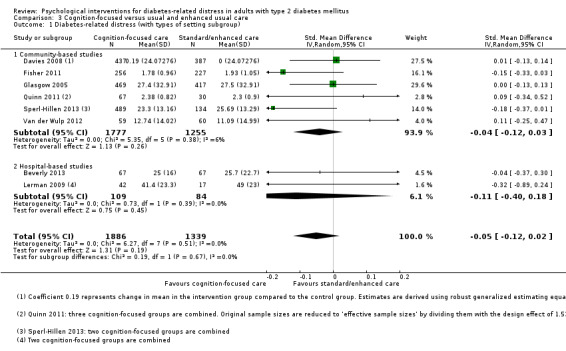
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 1 Diabetes‐related distress (with types of setting subgroup).
3.6. Analysis.
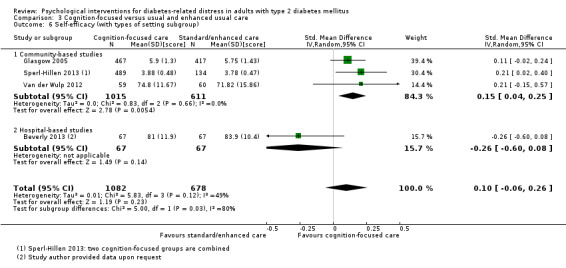
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 6 Self‐efficacy (with types of setting subgroup).
3.9. Analysis.
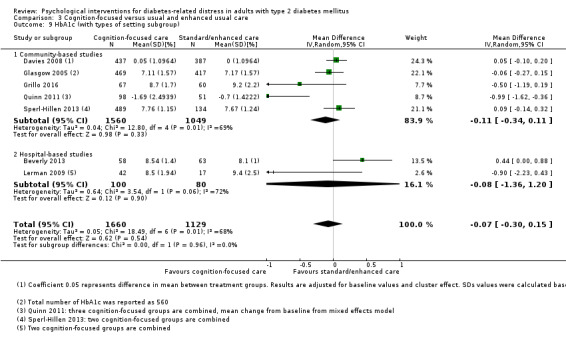
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 9 HbA1c (with types of setting subgroup).
7.2. Analysis.
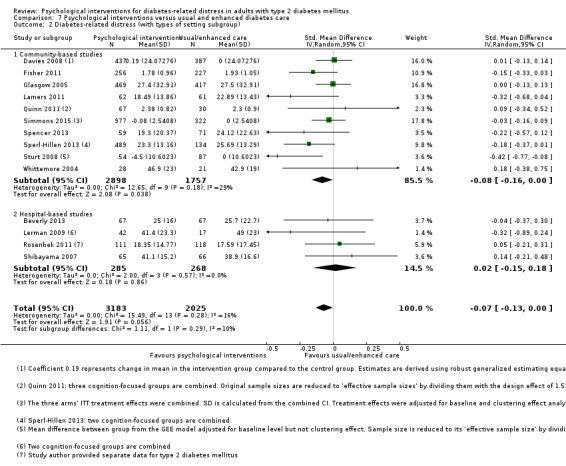
Comparison 7 Psychological interventions versus usual and enhanced diabetes care, Outcome 2 Diabetes‐related distress (with types of setting subgroup).
7.9. Analysis.
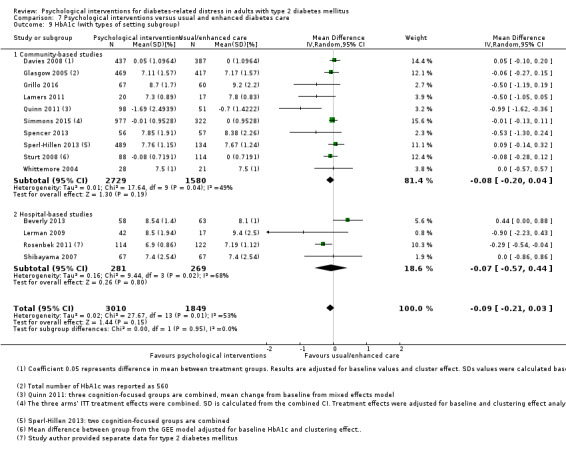
Comparison 7 Psychological interventions versus usual and enhanced diabetes care, Outcome 9 HbA1c (with types of setting subgroup).
10.1. Analysis.
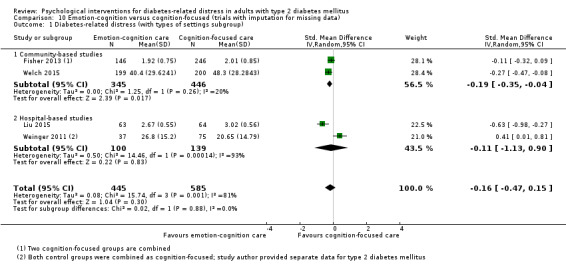
Comparison 10 Emotion‐cognition versus cognition‐focused (trials with imputation for missing data), Outcome 1 Diabetes‐related distress (with types of settings subgroup).
10.6. Analysis.
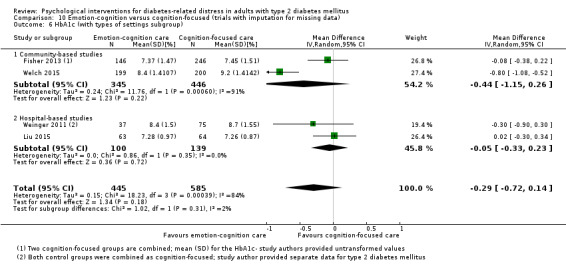
Comparison 10 Emotion‐cognition versus cognition‐focused (trials with imputation for missing data), Outcome 6 HbA1c (with types of settings subgroup).
10.8. Analysis.
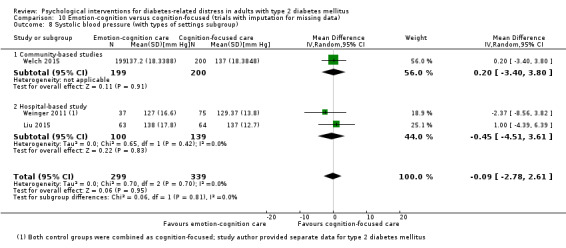
Comparison 10 Emotion‐cognition versus cognition‐focused (trials with imputation for missing data), Outcome 8 Systolic blood pressure (with types of settings subgroup).
10.9. Analysis.
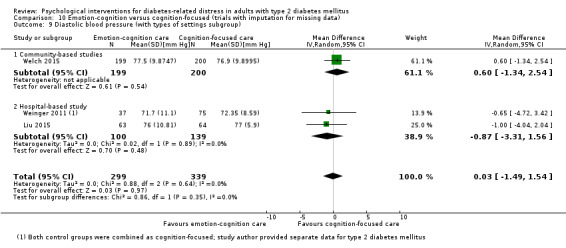
Comparison 10 Emotion‐cognition versus cognition‐focused (trials with imputation for missing data), Outcome 9 Diastolic blood pressure (with types of settings subgroup).
Type of intervention (brief and simple versus longer and more advanced): Analysis 1.2; Analysis 1.6; Analysis 1.9; Analysis 1.11; Analysis 1.12; Analysis 2.2; Analysis 2.6; Analysis 2.9; Analysis 2.11; Analysis 2.12; Analysis 3.2; Analysis 3.7; Analysis 3.10; Analysis 3.12; Analysis 3.13; Analysis 4.2; Analysis 4.4; Analysis 4.5; Analysis 4.11; Analysis 5.2; Analysis 5.9; Analysis 5.13; Analysis 5.16; Analysis 7.3; Analysis 7.10; Analysis 8.3; Analysis 8.6; Analysis 8.9; Analysis 8.12; Analysis 9.1; Analysis 9.7.
2.2. Analysis.
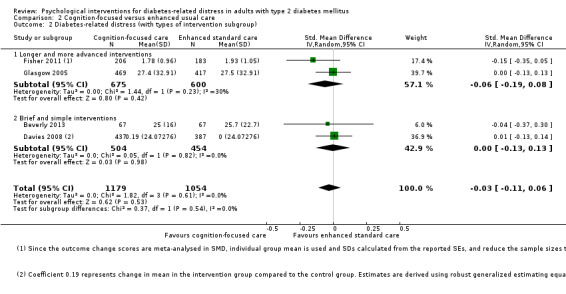
Comparison 2 Cognition‐focused versus enhanced usual care, Outcome 2 Diabetes‐related distress (with types of intervention subgroup).
2.11. Analysis.
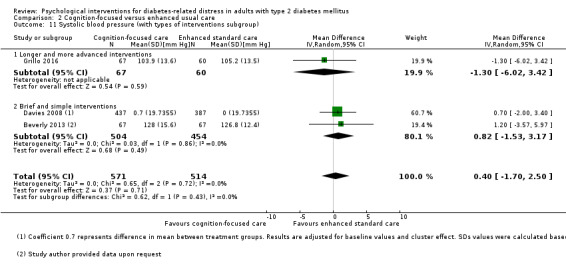
Comparison 2 Cognition‐focused versus enhanced usual care, Outcome 11 Systolic blood pressure (with types of interventions subgroup).
2.12. Analysis.
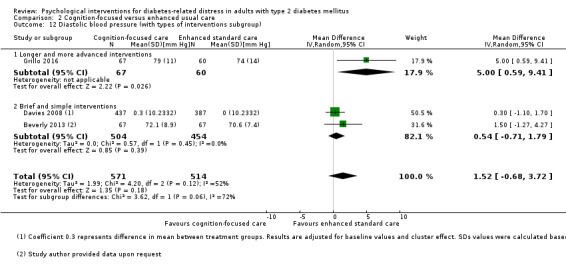
Comparison 2 Cognition‐focused versus enhanced usual care, Outcome 12 Diastolic blood pressure (with types of interventions subgroup).
3.7. Analysis.
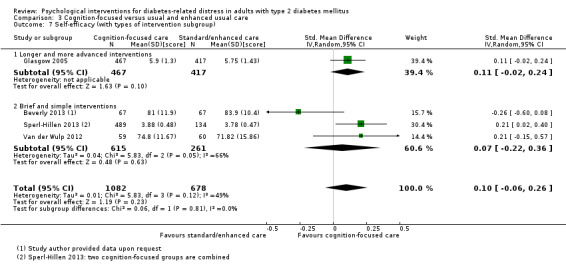
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 7 Self‐efficacy (with types of intervention subgroup).
3.12. Analysis.
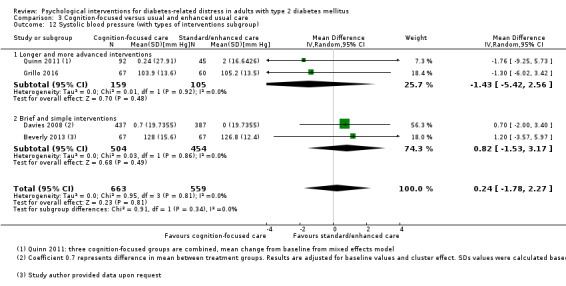
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 12 Systolic blood pressure (with types of interventions subgroup).
3.13. Analysis.
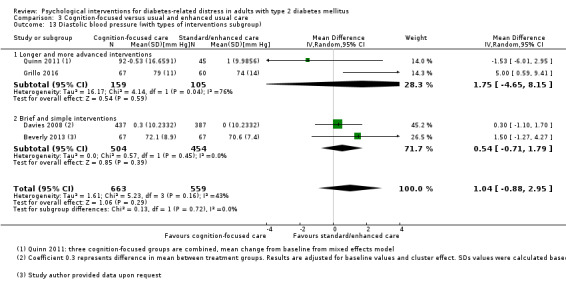
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 13 Diastolic blood pressure (with types of interventions subgroup).
4.2. Analysis.
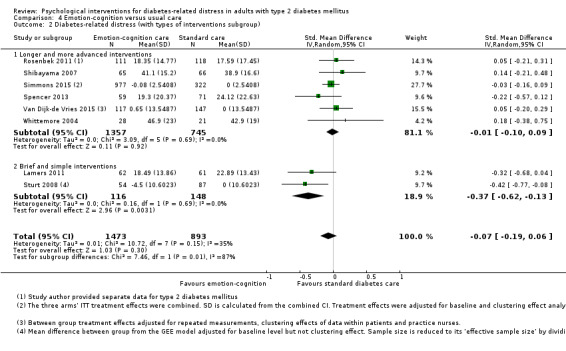
Comparison 4 Emotion‐cognition versus usual care, Outcome 2 Diabetes‐related distress (with types of interventions subgroup).
4.11. Analysis.
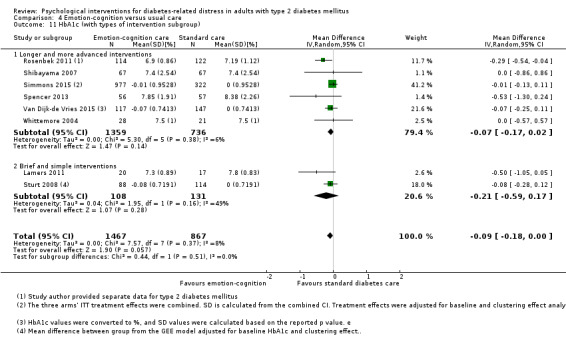
Comparison 4 Emotion‐cognition versus usual care, Outcome 11 HbA1c (with types of intervention subgroup).
5.2. Analysis.
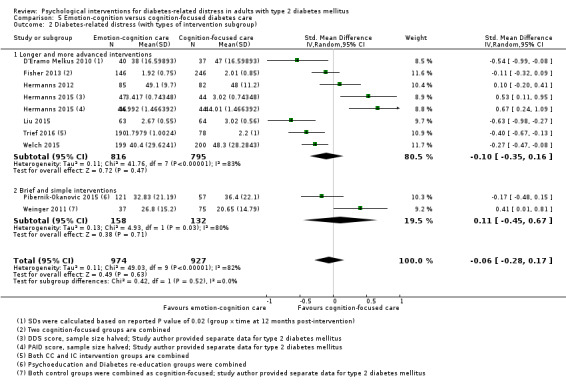
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 2 Diabetes‐related distress (with types of intervention subgroup).
5.9. Analysis.
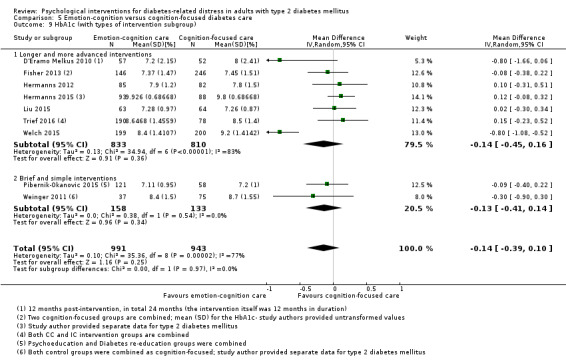
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 9 HbA1c (with types of intervention subgroup).
5.13. Analysis.
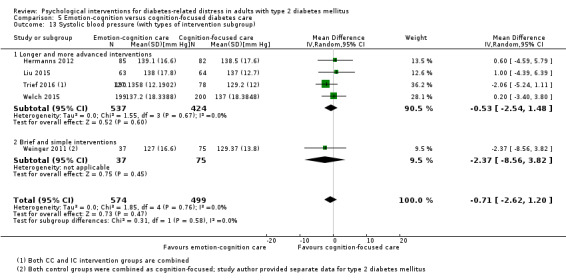
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 13 Systolic blood pressure (with types of intervention subgroup).
5.16. Analysis.
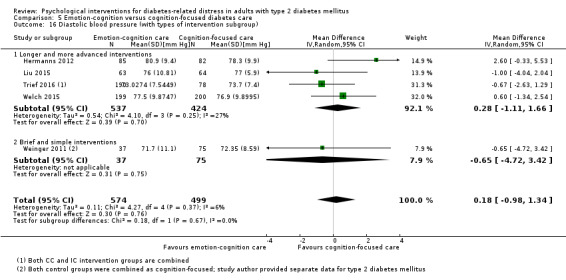
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 16 Diastolic blood pressure (with types of intervention subgroup).
7.3. Analysis.
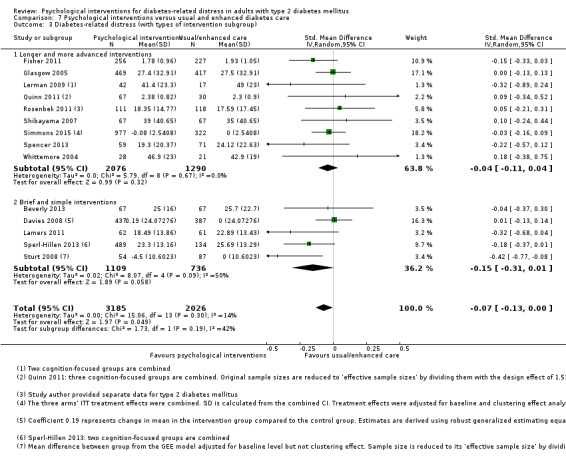
Comparison 7 Psychological interventions versus usual and enhanced diabetes care, Outcome 3 Diabetes‐related distress (with types of intervention subgroup).
7.10. Analysis.
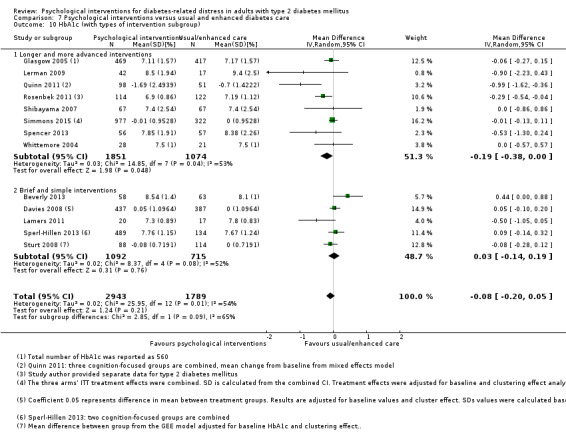
Comparison 7 Psychological interventions versus usual and enhanced diabetes care, Outcome 10 HbA1c (with types of intervention subgroup).
9.1. Analysis.
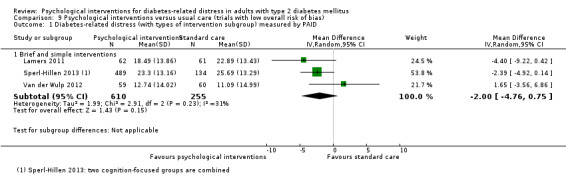
Comparison 9 Psychological interventions versus usual care (trials with low overall risk of bias), Outcome 1 Diabetes‐related distress (with types of intervention subgroup) measured by PAID.
9.7. Analysis.
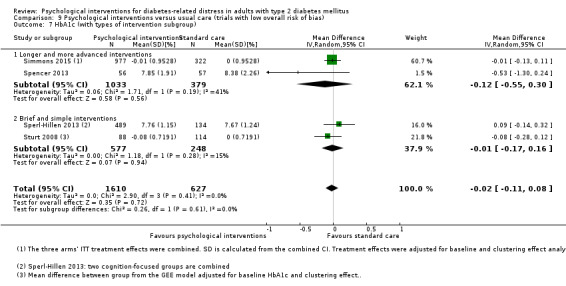
Comparison 9 Psychological interventions versus usual care (trials with low overall risk of bias), Outcome 7 HbA1c (with types of intervention subgroup).
Age, with the cut‐off at 60 years: Analysis 1.3; Analysis 1.7; Analysis 1.10; Analysis 1.14; Analysis 2.3; Analysis 2.7; Analysis 2.10; Analysis 2.14; Analysis 3.3; Analysis 3.8; Analysis 3.11; Analysis 3.15; Analysis 4.3; Analysis 4.6; Analysis 4.9; Analysis 4.12; Analysis 5.4; Analysis 5.11; Analysis 5.14; Analysis 5.17; Analysis 7.4; Analysis 7.11; Analysis 8.4; Analysis 8.13; Analysis 9.2; Analysis 9.8; Analysis 10.2; Analysis 10.7.
1.7. Analysis.
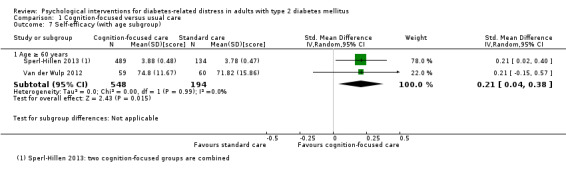
Comparison 1 Cognition‐focused versus usual care, Outcome 7 Self‐efficacy (with age subgroup).
1.10. Analysis.
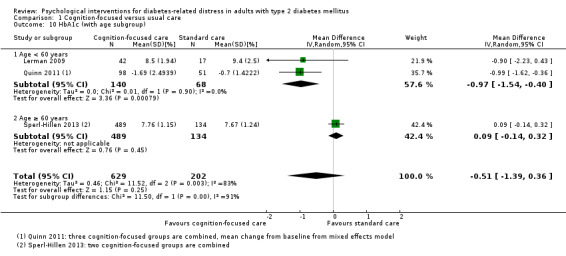
Comparison 1 Cognition‐focused versus usual care, Outcome 10 HbA1c (with age subgroup).
1.14. Analysis.
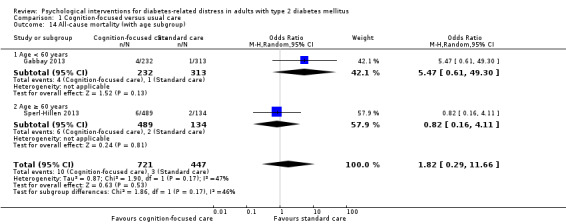
Comparison 1 Cognition‐focused versus usual care, Outcome 14 All‐cause mortality (with age subgroup).
2.3. Analysis.
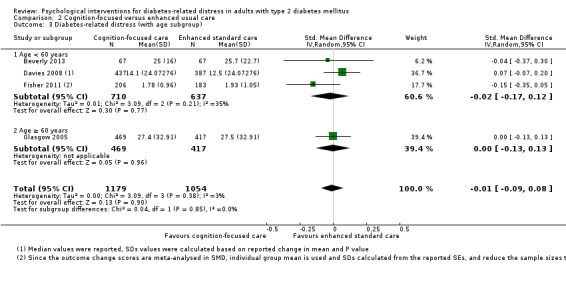
Comparison 2 Cognition‐focused versus enhanced usual care, Outcome 3 Diabetes‐related distress (with age subgroup).
2.7. Analysis.
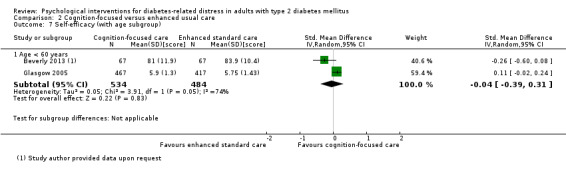
Comparison 2 Cognition‐focused versus enhanced usual care, Outcome 7 Self‐efficacy (with age subgroup).
2.10. Analysis.
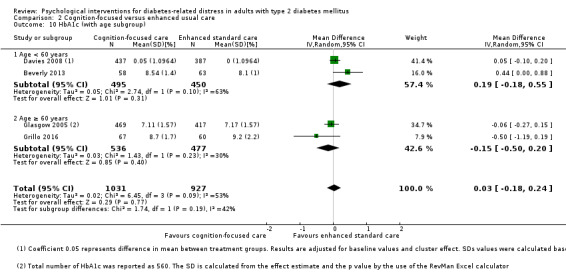
Comparison 2 Cognition‐focused versus enhanced usual care, Outcome 10 HbA1c (with age subgroup).
2.14. Analysis.
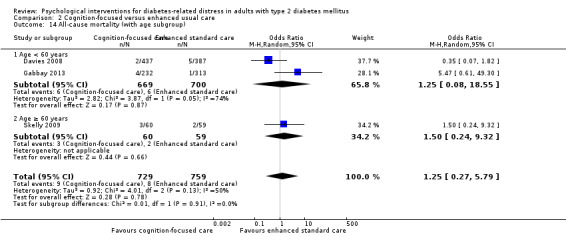
Comparison 2 Cognition‐focused versus enhanced usual care, Outcome 14 All‐cause mortality (with age subgroup).
3.3. Analysis.
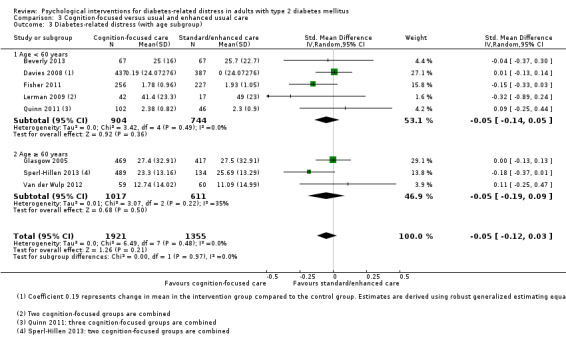
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 3 Diabetes‐related distress (with age subgroup).
3.8. Analysis.
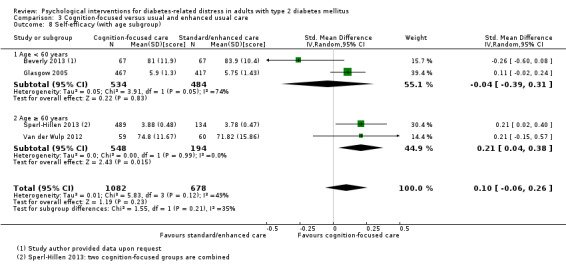
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 8 Self‐efficacy (with age subgroup).
3.11. Analysis.
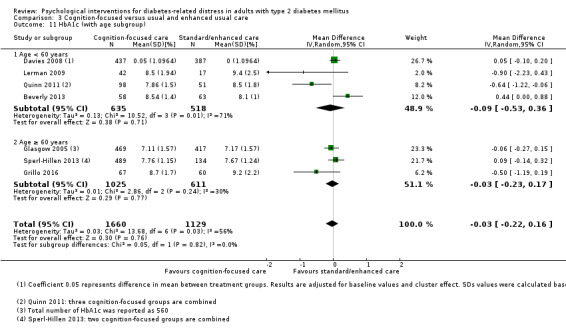
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 11 HbA1c (with age subgroup).
3.15. Analysis.
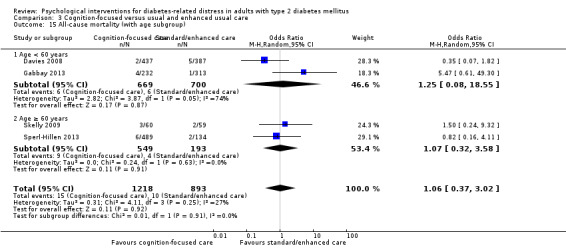
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 15 All‐cause mortality (with age subgroup).
4.3. Analysis.
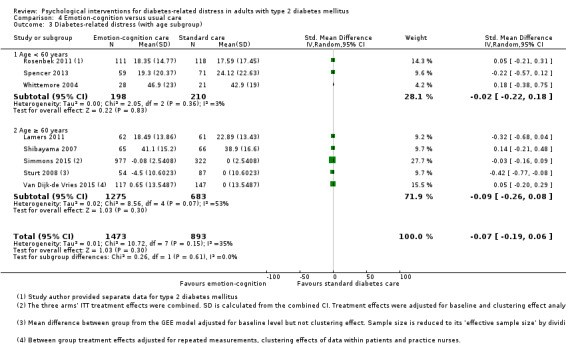
Comparison 4 Emotion‐cognition versus usual care, Outcome 3 Diabetes‐related distress (with age subgroup).
4.6. Analysis.
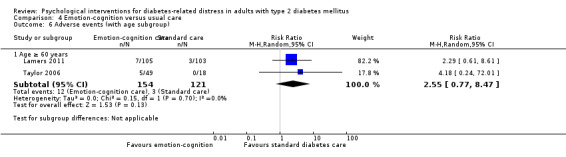
Comparison 4 Emotion‐cognition versus usual care, Outcome 6 Adverse events (with age subgroup).
4.9. Analysis.
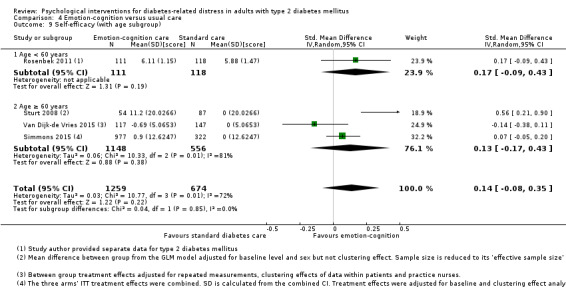
Comparison 4 Emotion‐cognition versus usual care, Outcome 9 Self‐efficacy (with age subgroup).
4.12. Analysis.
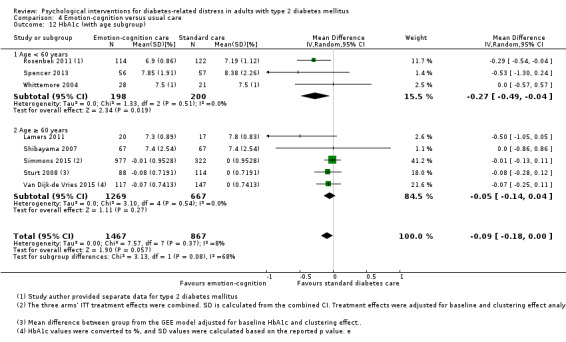
Comparison 4 Emotion‐cognition versus usual care, Outcome 12 HbA1c (with age subgroup).
5.4. Analysis.
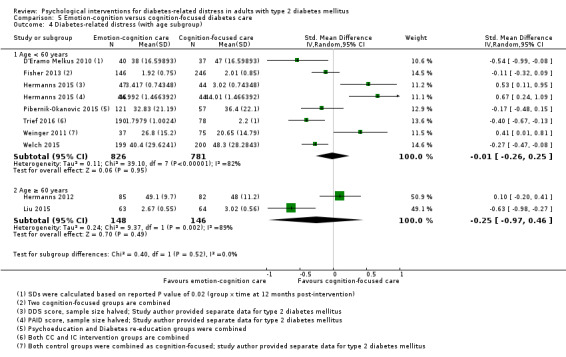
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 4 Diabetes‐related distress (with age subgroup).
5.11. Analysis.
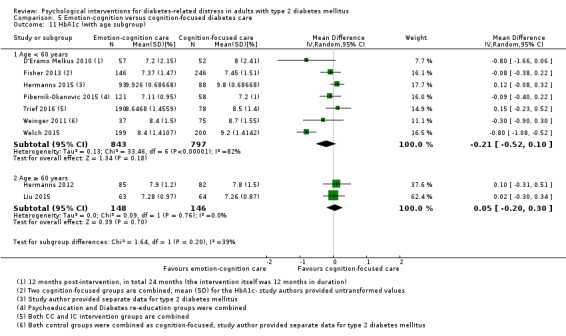
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 11 HbA1c (with age subgroup).
5.14. Analysis.
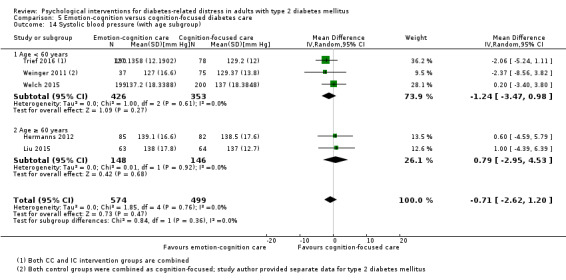
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 14 Systolic blood pressure (with age subgroup).
5.17. Analysis.
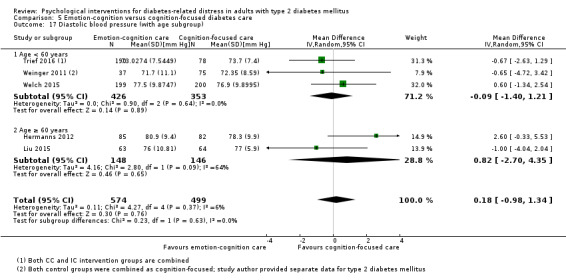
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 17 Diastolic blood pressure (with age subgroup).
7.4. Analysis.
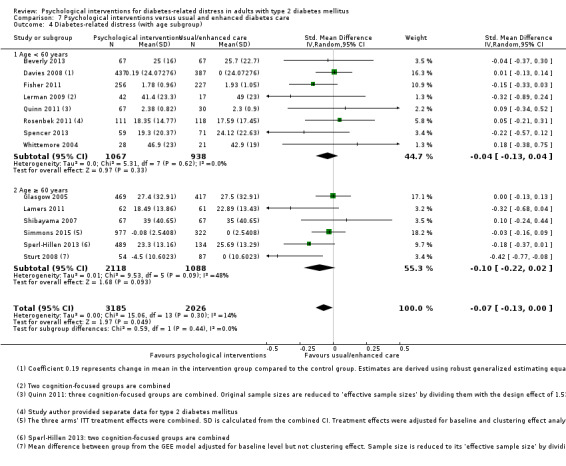
Comparison 7 Psychological interventions versus usual and enhanced diabetes care, Outcome 4 Diabetes‐related distress (with age subgroup).
7.11. Analysis.
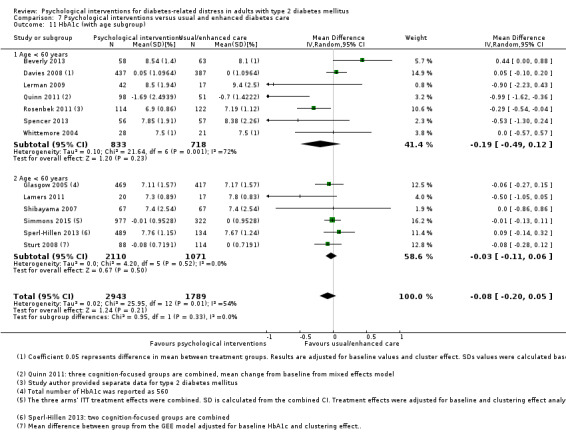
Comparison 7 Psychological interventions versus usual and enhanced diabetes care, Outcome 11 HbA1c (with age subgroup).
9.2. Analysis.
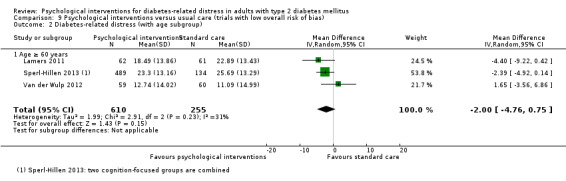
Comparison 9 Psychological interventions versus usual care (trials with low overall risk of bias), Outcome 2 Diabetes‐related distress (with age subgroup).
9.8. Analysis.
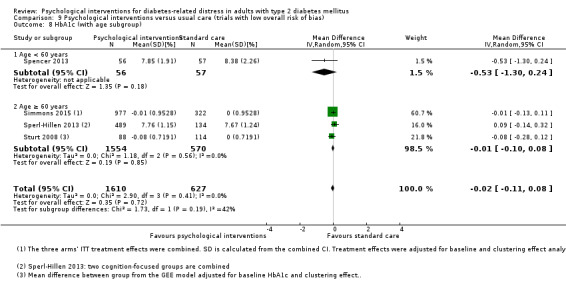
Comparison 9 Psychological interventions versus usual care (trials with low overall risk of bias), Outcome 8 HbA1c (with age subgroup).
10.2. Analysis.
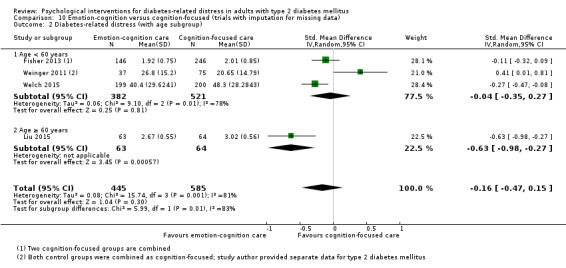
Comparison 10 Emotion‐cognition versus cognition‐focused (trials with imputation for missing data), Outcome 2 Diabetes‐related distress (with age subgroup).
10.7. Analysis.
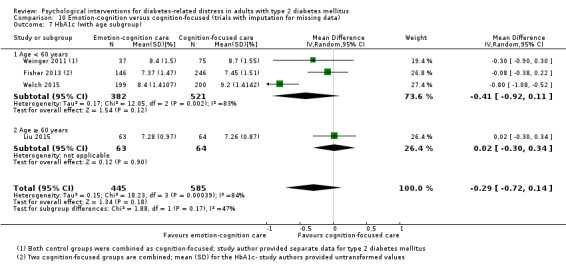
Comparison 10 Emotion‐cognition versus cognition‐focused (trials with imputation for missing data), Outcome 7 HbA1c (with age subgroup).
We also performed subgroup analyses (Analysis 5.3; Analysis 5.10) for the different intervention providers (nurses versus physician/psychologist), but only for the comparison between emotion‐cognition and cognition‐focused psychological interventions, because there were not enough trials to estimate effects in other comparisons (D'Eramo Melkus 2010 and Pibernik‐Okanovic 2015 used psychologists).
5.3. Analysis.
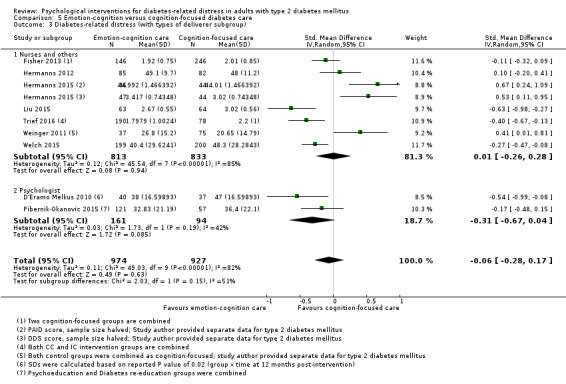
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 3 Diabetes‐related distress (with types of deliverer subgroup).
5.10. Analysis.
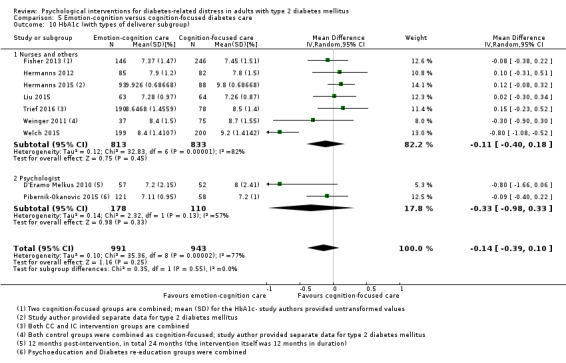
Comparison 5 Emotion‐cognition versus cognition‐focused diabetes care, Outcome 10 HbA1c (with types of deliverer subgroup).
We did not perform subgroup analyses for gender because very few included trials reported gender‐specific data. The three trials in only women compared different classes of psychological interventions (D'Eramo Melkus 2010; Skelly 2009; Whittemore 2004; see Appendix 2).
The summary estimates in the two groups of almost all significant subgroup comparisons had overlapping CIs (Analysis 2.8; Analysis 2.12; Analysis 3.6; Analysis 3.8; Analysis 4.10; Analysis 4.12; Analysis 5.1; Analysis 7.10; Analysis 8.3; Analysis 8.9; Analysis 8.11; Analysis 8.12; Analysis 8.13; Analysis 10.1; Analysis 10.2), thus making the observations hypothetical except in Analysis 1.9 and Analysis 1.10, where HbA1c was significantly lower in longer and more advanced cognition‐focused interventions and among those aged < 60 years (similar studies in both subgroup analyses) when compared to usual care; in Analysis 4.2, where DRD was significantly lower in brief and simple emotion‐cognition interventions compared to usual care; and in Analysis 4.8, where self‐efficacy was significantly higher in brief and simple emotion‐cognition interventions compared to usual care. Below we elaborate on subgroup comparisons that might be clinically relevant. We present results of the other subgroup analyses in the Data and analyses section.
4.8. Analysis.
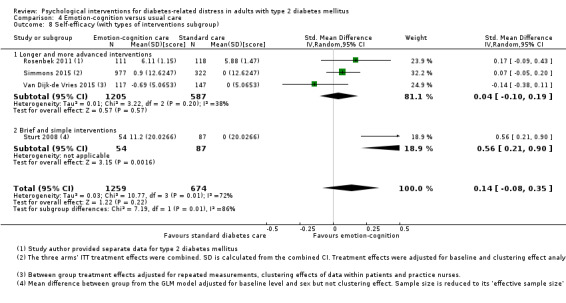
Comparison 4 Emotion‐cognition versus usual care, Outcome 8 Self‐efficacy (with types of interventions subgroup).
Setting
In the four community‐based trials (D'Eramo Melkus 2010; Fisher 2013; Trief 2016; Welch 2015) – but not in the five hospital‐based trials (Hermanns 2012; Hermanns 2015; Liu 2015; Pibernik‐Okanovic 2015; Weinger 2011) – emotion‐cognition programmes seemed to have more favourable results on DRD than the cognition‐focused interventions (SMD −0.28; 95% CI −0.43 to −0.12; P < 0.001; 1901 participants; 9 trials; Analysis 5.1; test for subgroup differences: P = 0.04). However, because the CIs of the summary estimates in the two groups overlap, this observation is hypothetical. Liu 2015 was organised by a hospital but conducted in the community; re‐categorising this study under the community‐based setting increased the above effect size to SMD −0.34 (95% CI −0.51 to −0.16; P < 0.001; test for subgroup differences: P < 0.001) without increased heterogeneity (51% vs 34%).
The overall effect size of hospital‐based psychological interventions compared to usual care showed an MD for HbA1c of −0.29% (95% CI −0.53 to −0.05; Analysis 8.11). This effect was mainly explained by the hospital‐based emotion‐cognition psychological interventions (HbA1c MD −0.27%; 95% CI −0.51 to −0.02; P = 0.03; 370 participants; 2 trials; Analysis 4.10; test for subgroup differences: P = 0.11).
Type of intervention
We classified eight interventions as brief and simple (Beverly 2013; Davies 2008; Dennick 2015; Lamers 2011; Sperl‐Hillen 2013; Sturt 2008; Taylor 2006; Van der Wulp 2012). In two of them (Lamers 2011; Sturt 2008), the effect of the emotion‐cognition intervention on DRD appeared better compared to usual care (SMD −0.37; 95% CI −0.62 to −0.13; P = 0.003; 264 participants; 2 trials; Analysis 4.2; test for subgroup differences: P = 0.006), with no overlapping CIs. In Analysis 8.3, four trials seem to show beneficial effects for all types of brief and simple psychological interventions on DRD (test for subgroup differences: P = 0.08) (Lamers 2011; Sperl‐Hillen 2013; Sturt 2008; Van der Wulp 2012). However, this is hypothetical because the CIs still overlap to a small degree.
Brief and simple emotion‐cognition focused interventions showed an RR of 2.55 (95% CI 0.77 to 8.47; P = 0.13; 275 participants; Lamers 2011; Taylor 2006; Analysis 4.4) for adverse events.
Sturt 2008 showed that brief and simple emotion‐cognition interventions improved self‐efficacy more than usual care (SMD 0.56; 95% CI 0.21 to 0.90; P = 0.002; 141 participants; Analysis 4.8; test for subgroup differences: P = 0.007). Although the beneficial effect of brief and simple psychological interventions on self‐efficacy persisted in subgroup analysis, the CIs overlap to a small degree (SMD 0.30; 95% CI 0.09 to 0.51; P = 0.005; 883 participants; 3 trials; Analysis 8.9; test for subgroup differences: P = 0.05).
Longer and more advanced cognition‐focused interventions compared to usual care seemed to reduce HbA1c slightly more (MD −0.97%; 95% CI −1.54 to −0.40; P < 0.001; 208 participants; 2 trials; Analysis 1.9; test for subgroup differences: P < 0.001). These effects did not hold in the comparison between cognition‐focused programmes versus enhanced usual care (Analysis 2.9), emotion‐cognition programmes versus usual care (Analysis 4.11), or all psychological interventions to usual care (MD in HbA1c of −0.19%; 95% CI −0.37 to 0.00; P = 0.04; 2303 participants; 8 trials; Analysis 8.12; test for subgroup differences: P = 0.43).
Effects on systolic blood pressure were inconsistent in the longer and more advanced intervention subgroups in cognition‐focused and usual care comparisons (Analysis 1.11; Analysis 1.12).
Age
Based on a cut‐off in mean or median age of 60 years, we included 12 trials in this subgroup analyses (Dennick 2015; Glasgow 2005; Hermanns 2012; Lamers 2011; Shibayama 2007; Simmons 2015; Skelly 2009; Sperl‐Hillen 2013; Sturt 2008; Taylor 2006; Van der Wulp 2012; Van Dijk‐de Vries 2015). Compared to usual care, the effects of cognition‐focused interventions on DRD did not substantially differ between subgroups (Analysis 1.3).
Overall, the age group of less than 60 years showed a better reduction in HbA1c compared with the age group of 60 years or older (test for subgroup differences: P = 0.002; Analysis 8.13). In the younger group, the cognition‐focused interventions seemed to improve HbA1c compared to usual care (MD −0.97%; 95% CI −1.54 to −0.40; P < 0.001; 208 participants; 2 trials; Analysis 1.10; test for subgroup differences: P < 0.001) but not when compared to enhanced usual care (Analysis 2.10). Emotion‐cognition interventions did not seem to be more beneficial for HbA1c in the younger age group (Analysis 4.12).
Subgroup analyses were not possible for HRQoL, adverse events, blood pressure or all‐cause mortality. Among people aged 60 years or older, the effect size of cognition‐focused interventions on self‐efficacy showed an SMD of 0.21 (95% CI 0.04 to 0.38; P = 0.02; 742 participants; 2 trials; Analysis 1.7).
People in the older age group attending emotion‐cognition psychological interventions showed an RR of 2.62 (95% CI 0.85 to 8.07; P = 0.09; 389 participants; 3 trials; Analysis 4.6) for adverse events.
Providers
We compared emotion‐cognition focused interventions delivered by psychologists or nurses and non‐physician/non‐health professionals on DRD (Analysis 5.3) and HbA1c (Analysis 5.10). Neither comparison indicated interaction effects (test for subgroup differences: P = 0.15 for DRD and P = 0.55 for HbA1c).
Sensitivity analyses
We performed sensitivity analyses for trials with low overall risk of bias and for trials with no missing data or that imputed missing data. Since all comparisons in this review used only published trials, we did not test the robustness of results by restricting the analyses to published trials.
We excluded three trials from the sensitivity analyses because they were either long (D'Eramo Melkus 2010 and Gabbay 2013 had an active intervention beyond 12 months) or large (Simmons 2015 included more than 1000 participants), but each of these studies had different psychological interventions and comparisons.
We likewise could not perform sensitivity analyses on source of funding (industry versus other) because only one trial used a commercial kit and was funded by the related industry (Fisher 2011). Many trials had non‐commercial funding, and six trials had a mix of non‐industry and industry funding sources (Beverly 2013; Davies 2008; Quinn 2011; Trief 2016; Weinger 2011; Whittemore 2004). Two trials that were purely industry‐funded did not use any related commercial goods that could pose a significant conflict of interest (Hermanns 2012; Sperl‐Hillen 2013). We also could not perform sensitivity analyses by world region (Western versus Asian) because only two studies took place in Asia (Liu 2015; Shibayama 2007), and they had different comparators (Appendix 2).
Trials with low overall risk of bias
We performed sensitivity analyses restricting the analyses to trials that scored low overall risk of bias as specified in the Assessment of risk of bias in included studies section. We judged trials with a low overall risk of bias further per outcome in the assessment of other biases. The trials that we considered as having low overall risk of bias were Beverly 2013, Pibernik‐Okanovic 2015, Sperl‐Hillen 2013, Taylor 2006, Van der Wulp 2012 and Weinger 2011. Included trials with low overall risk of bias in certain but not all outcomes were: Fisher 2011 (not for adverse events), Gabbay 2013 (not for DRD and HRQoL), Lamers 2011 (not for HbA1c or adverse events), Spencer 2013 (not for DRD), and Simmons 2015 (did not provide usable data for HbA1c).
Sensitivity analyses on the above‐mentioned low overall risk of bias trials comparing psychological interventions to usual care resulted in a similar interpretation of the findings (Gabbay 2013; Lamers 2011; Spencer 2013; Sperl‐Hillen 2013; Taylor 2006; Van der Wulp 2012; Weinger 2011; Analysis 9.1; Analysis 9.8).
Trials with no missing data or imputation for the missing data
One trial had no missing data (Liu 2015), and seven trials imputed missing data (Dennick 2015; Fisher 2013; Lamers 2011; Simmons 2015; Van der Wulp 2012; Weinger 2011; Welch 2015). In this sensitivity analyses, we included three trials for the comparison of combined psychological interventions versus usual care (Lamers 2011; Simmons 2015; Van der Wulp 2012), and four contributed to the comparison of emotion‐cognition versus cognition‐focused interventions (Fisher 2013; Liu 2015; Weinger 2011; Welch 2015).
Restricting the analysis to trials without missing data or which imputed data did not substantially change the results for the comparison between combined psychological interventions to usual care with respect to DRD (Analysis 11.1; Analysis 10.1), to HRQoL (Analysis 11.2) or to blood pressure (Analysis 10.8; Analysis 10.9).
11.1. Analysis.
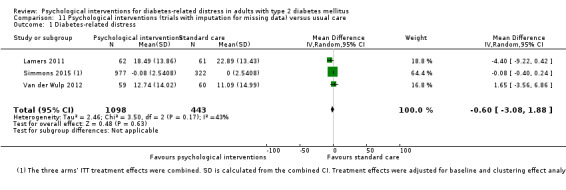
Comparison 11 Psychological interventions (trials with imputation for missing data) versus usual care, Outcome 1 Diabetes‐related distress.
11.2. Analysis.
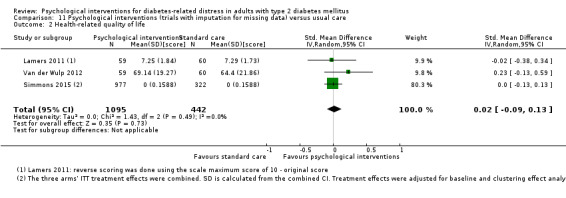
Comparison 11 Psychological interventions (trials with imputation for missing data) versus usual care, Outcome 2 Health‐related quality of life.
Effect of cluster trials on the results
Quinn 2011 did not change pooled effects of outcomes substantially in cognition‐focused vs usual care comparisons, except in subgroup Analysis 1.9 and Analysis 1.10, where adjustment for clustering was used and the effect size on HbA1c increased from −0.68 to −0.97. Davies 2008, Fisher 2011 and Glasgow 2005 also did not change the pooled effects of the outcomes substantially in cognition‐focused versus enhanced care comparisons (Analysis 2.1; Analysis 2.6; Analysis 2.8). Similarly, Simmons 2015, Sturt 2008 and Van Dijk‐de Vries 2015 also did not change the pooled effects of the outcomes substantially in the emotion‐cognition versus usual care comparisons.
Excluding Quinn 2011, Simmons 2015, Sturt 2008 and Van Dijk‐de Vries 2015 from the meta‐analyses on HbA1c outcome, the pooled effects size hardly changed, but the CIs narrowed. Only the results of HbA1c showed substantially lower estimates in the overall combined psychological intervention (Analysis 8.10) and in longer and more advanced interventions (CIs overlap) (Analysis 8.12) compared to usual care.
8.10. Analysis.
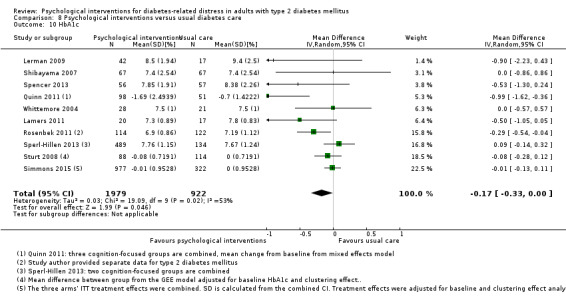
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 10 HbA1c.
Van Dijk‐de Vries 2015 contributed to the overall beneficial effect of psychological intervention on self‐efficacy (Analysis 8.9). The impact of data from Van Dijk‐de Vries 2015 was probably due to the implementation of the intervention itself rather than its cluster study design. This trial was at high risk of other bias, since it used a hybrid effectiveness‐implementation design, saw low recruitment rates of eligible participants (only 16 of the 117 participants in the intervention arm) and had low exposure (only 11 study participants) to the complete intervention of self‐management support. But exclusion of this study from the meta‐analysis hardly changed the resulted.
Assessment of reporting bias
We did not draw funnel plots due to the limited number of trials in many comparisons with specific psychological interventions. However, we did for the combined psychological interventions versus usual care comparison on DRD (12 trials; Figure 5) and HbA1c (11 trials; Figure 6). Trials on the effect of psychological interventions compared to usual care on DRD probably had no reporting bias or small study bias as shown by the funnel plot in Figure 5. However, trials with HbA1c as an outcome might have reporting bias or small study bias as indicated by an asymmetric funnel plot.
Trials awaiting classification and ongoing trials
There are four trials awaiting further classification (Dafoulas 2014; De Vries 2014; Ebert 2017; NCT01578096). Dafoulas 2014 has until now only been presented as a conference abstract. It was a parallel RCT conducted in Greece on the impact of a long‐term telemonitoring programme for people with T2DM on glycaemic control and health‐related quality of life compared to usual care. Ebert 2017 reported the results of a CF intervention (guided Internet‐based self‐help) compared to an EC intervention (treatment as usual plus online psychoeducation) for depression in a mixed cohort of people with type 1 and 2 diabetes mellitus in Germany. We contacted the trial authors, who promised to provide separate data for people with T2DM, but we had not received anything at the time of writing. The results of NCT01578096 were under review at a peer‐reviewed journal at the time of writing. This parallel RCT determined the effects of diabetes education combined with stress management versus diabetes education only among Latino participants with T2DM. De Vries 2014 was a community‐based study in 130 general practices in the Netherlands. It examined the effects of peer support in people with T2DM on quality of life, well‐being, diabetes‐related distress and self‐management behaviour.
We found 18 ongoing RCTs with 5 likely to be near completion (ACTRN12612000620820; ACTRN12616001010482; ISRCTN02123133; NCT01612520; NCT02748239), 11 recruiting (NCT01805245; NCT02021591; NCT02081586, NCT02137720, ACTRN12614001232628; ACTRN12615000931572; NCT02040038; NCT02370719; NCT02488785; NCT02675257; NCT02730078), and two for which recruitment was pending at the time of writing (NCT02066155; NCT02863523). NCT02066155 assesses ongoing diabetes self‐management support in church‐based settings for African Americans. NCT01805245 is about stress management and therefore likely to be an emotion‐focused psychological intervention.
Emotion‐cognition psychological interventions are likely implemented in NCT02081586 and NCT02137720 trials, with the former evaluating telephone‐based cognitive behavioural therapy and the latter, telephone‐based diabetes self‐managment support. NCT02675257 and NCT02863523 use two other possible emotion‐cognition focused psychological interventions with elements of cognitive behavioural therapy. NCT02675257 included both participants with type 1 and 2 diabetes mellitus. NCT02863523 incorporates problem‐solving therapy and behavioural counselling with strong community‐based support. Group‐based education and problem‐solving training feature in NCT02730078, NCT02748239 and ACTRN12616001010482. These are all likely to be emotion‐cognition focused psychological interventions, with NCT02730078 coming from a middle‐income Asian country in contrast to the others in high‐income Western countries.
Many ongoing trials are using Internet‐linked devices (such as smartphones, computers and tablets), applications and websites to deliver the interventions. NCT02021591 examines a cognition‐focused psychological intervention via a web application for problem solving in diabetes management. NCT01612520, NCT02370719 and NCT02488785 also investigate teleconsultation, telecoaching and application‐based cognition‐focused interventions, respectively. ACTRN12612000620820 investigates a self‐guided web‐based programme (likely to be an emotion‐cognition focused intervention) and aims to improve T2DM self‐management and dysphoria (depression, anxiety, and diabetes‐specific distress) by primarily targeting physical activity, nutrition, health routines and emotional well‐being. ACTRN12614001232628 includes both type 1 and 2 diabetes mellitus participants investigating an individually tailored package of text messages via mobile phone to increase the participant's diabetes self‐management (likely to be an emotion‐cognition based intervention). The text messages are informational and motivational in nature and cover a range of topics that include diabetes management tips, nutrition and diet, exercise, stress and mood management and foot care. ACTRN12615000931572 uses the active intervention 'myCompass', which is a fully automated, self‐help, public health intervention that is tailored to the user and has no therapist input. It provides real‐time self‐monitoring of symptoms (for example problem moods, thoughts and behaviours) via mobile phone, computer/tablet or both. ISRCTN02123133 compares two websites offering help and support for people with T2DM at primary care. The more complex website (HeLP‐Diabetes) has lots of online tips and tools to help diabetes self‐management, while the other more simple website focuses on the essential and general information on T2DM (likely to be an emotion‐cognition focused intervention versus cognition‐focused psychological intervention). NCT02040038 uses sophisticated information technology. It compares the effects of a virtual environment and traditional website on diet and physical activity in adults with T2DM. This diabetes self‐management training offers various virtual locations (such as a grocery store and a pharmacy) for participants to interact with peers or educators and learn to utilise informational resources, receive feedback on health behaviours and be awarded for achievements.
Discussion
The present systematic review investigated the effects of psychological interventions on diabetes‐related distress (DRD), health‐related quality of life (HRQoL), self‐efficacy, diabetes‐related complications, all‐cause mortality, adverse events and glycaemic control (HbA1c), and blood pressure in adults with T2DM. Our comprehensive search strategy yielded 30 RCTs fulfilling the inclusion criteria. Eleven trials compared cognition‐focused psychological interventions with usual care. Nine RCTs compared emotion‐cognition focused interventions with cognition‐focused interventions, and nine trials compared emotion‐cognition focused interventions with usual care. Only one trial compared an emotion‐focused with a cognition‐focused intervention. No trials compared an emotion‐focused intervention to usual care. Consequently, we can draw no conclusions on the differential effects of these treatment approaches for DRD. Other conclusions warrant caution due to the low number of trials per outcome in specific psychological interventions comparisons, the small sample sizes and – particularly in the emotion‐cognition psychological intervention trials – a wide variation in programmes.
Summary of main results
The results of the present review provide inconclusive evidence with regard to the effects of psychological interventions for DRD, HRQoL, all‐cause mortality and adverse events. The included psychological intervention trials did not investigate diabetes‐related complications or socioeconomic effects. Overall, psychological interventions improved self‐efficacy and glycaemic control (HbA1c) compared to usual care. Looking at the different types of psychological interventions, brief and simple emotion‐cognition focused interventions showed the best improvement in self‐efficacy when compared to usual care. This beneficial effect was sustained when we pooled only trials with a low overall risk of bias. The effect of emotion‐focused psychological interventions is uncertain due to the absence of such interventions. HbA1c improved significantly in the 6 to 12 month‐period with any type of psychological interventions compared to usual care. People with T2DM younger than 60 years old might benefit more from an emotion‐cognition or cognition‐focused interventions than older people with regard to the decrease of their HbA1c. Meta‐analyses further indicated that longer and more advanced cognition‐focused interventions might have stronger effects than emotion‐cognition focused interventions for reducing HbA1c in those under 60 years old. Enhanced usual care may be equally effective in reducing the HbA1c. For both the emotion‐cognition and the cognition‐focused interventions, delivery by nurses or physicians/psychologists seemed to have similar effects on HbA1c.
Compared to usual care, psychological interventions showed some beneficial effects on DRD 6 to 12 months after the end of the intervention; this effect occurred in four trials with brief and simple interventions, and there was an even larger effect in two trials of emotion‐cognition psychological interventions. Comparing emotion‐cognition versus cognition‐focused interventions, community‐based emotion‐cognition interventions showed a likely stronger effect than hospital‐based interventions, while delivery by nurses and physicians/psychologists seemed to yield a similar effect on DRD. Cognition‐focused interventions alone are probably not beneficial for reducing DRD in people with T2DM. It is reassuring to note that adverse events were not more likely to occur in people who underwent psychological interventions.
Overall completeness and applicability of evidence
This review synthesises the effects of psychological interventions that aimed to decrease DRD in adults with T2DM. In most included trials, trained nurses and healthcare professionals delivered the intervention, while three trials did not specify who delivered it. Physicians and clinical psychologists/psychiatrists were involved in only one of the groups in three trials. Therefore, we could not elucidate differential effects of the psychological interventions based on delivery by different healthcare professionals (nurses versus physician or psychologist). Four trials used non‐health professionals or peers, and four trials used computer or mobile applications. All included trials except four took place in the USA or Europe. Most included trials (18 of 30) had a community‐based or primary care setting. The variable preparedness of the healthcare providers and facilities may pose different challenges in providing the necessary psychological support and care for DRD in adults with T2DM.
Categorising the psychological interventions into emotion‐focused, cognition‐focused and emotion‐cognition focused could theoretically be helpful when applying psychological interventions in diabetes care. However, the variety of settings and interventions and the low scientific level of many may hamper the applicability of our findings.
To increase the robustness of our findings, we only included trials that defined and measured DRD with either a version of the Diabetes Distress Scale (DDS) or the Problem Areas in Diabetes (PAID) questionnaires. We had to exclude few trials that used other definitions of distress or other questionnaires. Sturt 2015 also observed this pattern, although that systematic review pooled the results of people with both T1DM and T2DM. We believe people with T2DM are distinct from those with T1DM in terms of the pathophysiology and aetiology of the disease, comorbidities, treatment complexity and psychosocial burden (Chiang 2014). It is obvious that the results of our review are mainly applicable to people with T2DM.
Because our primary outcome measure was DRD, we considered excluding trials with psychological interventions that did not measure DRD. However, other outcomes, namely HRQoL, self‐efficacy and glycaemic control are related to DRD (Fisher 2014). Therefore, we also examined trials of psychological interventions that could affect DRD for their effects on HRQoL, self‐efficacy and HbA1c. Readers should keep in mind that psychological interventions aiming to improve other psychological or emotional disorders such as depression may also have effects on DRD (Baumeister 2012; Ismail 2004). This means that the results of our review are indicative for the effects of psychological interventions on DRD, but that residual uncertainty remains.
This review framed the timing of outcome measurement to medium‐term, which means a 6‐ to 12‐month follow‐up period for most of the outcomes. Effects of psychological interventions at that time are considered sustainable and worthy of contemplation and implementation. We excluded some trials due to a follow‐up period of less than six months. This may lower the overall effect sizes of psychological interventions in this review. However, Cochrane Reviews are updated on a regular basis, and future versions of the review will incorporate the results from trials with longer follow‐up periods, thus increasing the knowledge regarding the long‐term effects of different psychological approaches.
A strength of this review is that we contacted authors for additional data if needed and received replies from 15 of 32 authors and investigators.
Quality of the evidence
We rated the overall quality of evidence for each outcome as low owing to the limitations in the design and implementation of the included trials, suggesting likelihood of bias, imprecision due to low sample size, and inability to exclude a clinically relevant benefit (see also Appendix 14). For the main comparisons between psychological interventions and usual care, the quality of evidence was low due to risk of bias and imprecision of results (wide confidence intervals). With regard to self‐efficacy and HbA1c, the quality of evidence was low because of additional attrition and other biases. The quality of evidence for other outcomes was also low. Including only trials that were at a low overall risk of bias, the overall quality of evidence for each outcome improved, but due to the small size and number of trials with similarly high clinical heterogeneity, we did not find substantial effects for psychological interventions in any outcome except self‐efficacy.
Many trials included participants with baseline imbalances, but few used statistical adjustment to correct it. Many trials did not describe blinding of the outcome assessors, which should have been possible. Even when blinding of participants is impossible in psychological interventions, we judged the influence of the performance and detection biases on the self‐reported outcomes to be minimal. Many trials reported incomplete outcome data (see Assessment of risk of bias in included studies and Table 2).
Many included trials showed discrepancies between the pre‐specified outcomes in trial register records and the published trials. This might have implications for the sample size calculation for the original primary outcome and suggests selective reporting of positive findings (see Appendix 5). For example, in Beverly 2013, the primary outcome was HbA1c, but in the trials register the primary outcome was the 'improved frequency of recommended self‐care behaviours'); while in Fisher 2011, the reported primary outcomes were 'depressive symptoms' and 'diabetes‐related distress', but it was HbA1c in the trials register.
We were not able to obtain published protocols for some of the included trials in this review and thus were not able to judge the risk of selective reporting for these trials (see Appendix 5). Despite our comprehensive search strategy, there may be unpublished trials with non‐significant results. All the cluster‐RCTs included in this review used appropriate statistical analyses, adjusting treatment effects and avoiding risk of bias in the effect estimation.
Potential biases in the review process
A potential bias in the review process may result from the classification of types of interventions. Many included trials described the essential content of the interventions but did not provide sufficient details of the delivery and possible interactions during the interventions. This might cause misclassification of the interventions. With two review authors reaching consensus on the type of an intervention, this potential bias was minimal. Another possible bias could arise from the different proportions of cognition‐ and emotion‐focused content in interventions of the emotion‐cognition category. This might cause differential impact on these two psychological domains. Separating these two domains in psychological intervention trials is almost impossible and at best arbitrary owing to the holistic approach. An intervention meant to be a cognition‐focused programme might have unintentionally used emotional strategies such as attentive listening and providing encouragement and consolation. Similarly, an intervention meant to be an emotion‐focused programme might draw on the participant's cognition in learning emotion skills and involving in expressive writing. This might explain the non‐differential effects of emotion‐cognition focused interventions and cognition‐focused interventions when compared to the usual care.
Psychological programmes that include emotion management are relatively new in diabetes care. Therefore, this category showed more variation than the established cognition‐focused interventions, resulting in lower heterogeneity among the latter category. Furthermore, we classified attention control or enhanced usual care into a cognition‐focused or emotion‐cognition intervention if the descriptions provided indications that participants in the group were indeed receiving input in these domains more than in usual care. We understood 'usual care' and waiting‐list controls to be real control conditions that included many sources of variance, which potentially might bias the results (Mohr 2009). Besides, waiting‐list control groups are viewed as more vulnerable to bias, which might lead to an overestimation of effect sizes (Mohr 2009). Nevertheless, to be in line with our conceptual framework (Chew 2014), we considered our classification of the interventions to be justified.
Decisions around the exclusion of trials that compared similar psychological interventions, but without a control group or with a different type of intervention not fitting in our classification, could have impacted the findings of the review (Characteristics of excluded studies). Trials tend to report larger within‐group changes (i.e. before‐and‐after interventions) than between‐group differences, as used in this review (Bland 2011). This decision on the use and analysis of data could have led to an underestimation of the effects of psychological interventions. Such an underestimation might also be the result of our choice to analyse only medium‐term outcomes rather than also including the short‐term outcomes, with usually larger effect sizes (Ricci‐Cabello 2014).
Adjusting treatment effects or sample sizes of the included cluster‐RCTs should be done to decrease bias in the estimates (Higgins 2011a). Since we used these adjusted treatment effects for the cluster RCTs, the pooled estimates were not biased. However, by doing this the results in the meta‐analyses and the subgroup analyses hardly changed.
Agreements and disagreements with other studies or reviews
An earlier systematic review also concluded that cognition‐based interventions were common (Worswick 2013). We noted that incorporating an emotional component into the cognition‐based intervention programmes has become common (Sturt 2015), compared to earlier reviews (Harkness 2010). We also noted that more trials were being conducted at the primary care level and delivered by nurses, diabetes educators and non‐medical specialists (Health Quality Ontario 2009a; Harkness 2010; Sturt 2015). The evidence did not suggest a preferred setting of care delivery, as suggested by Health Quality Ontario 2009b.
Sturt 2015 also reported small effects of psychological interventions or programmes for reducing DRD in both type 1 and type 2 diabetes mellitus. These generally small effects on DRD could be the consequence of multiple contributors to DRD, ranging from irreversible physical conditions, concurrent psychological disorders (such as depression), psychosocial circumstances in the family and society, healthcare professionals' support, health beliefs and personal perceptions of values in life (Berry 2015; Celano 2013; Chew 2014; Fisher 2014; Gary‐Webb 2013; Powers 2015), which the psychological interventions might not fully address. Although Sturt 2015 also reported that psycho‐education reduced DRD and that interventions delivered by generalists at the community setting were associated with reductions in DRD, Sturt 2015 reported in contrast to our review that more intense (6 sessions or longer) and longer (13 weeks or more) interventions reduced DRD more compared to those of lesser intensity and shorter duration. We hypothesise that brief and simple (fewer than 4 sessions in total and less than 3 hours per session or fewer than 10 session‐hours and completed within 3 months) psychological interventions of the emotion‐cognition category improve DRD more than usual care. Another systematic review by Ricci‐Cabello 2014 on characteristics and effectiveness of diabetes self‐management educational programmes targeted to ethnic minority groups suggested that simpler programmes in terms of teaching methods, contents and less involvement of different types of health professionals have more favourable effects on short‐term HbA1c. Another recent systematic review of cognitive‐behavioural therapy on glycaemic control and psychological outcomes in adults with type 1 and 2 diabetes mellitus found similar results (Uchendu 2017); the intervention seemed to improve short‐term (up to four months) DRD in mainly people with type 1 diabetes mellitus, but not in the longer term nor in trials that included a mixed group of participants of both diabetes types. Uchendu 2017 also reported improvement of short‐term quality of life, mainly in T2DM, in some individual trials, but investigators were not able to pool the results due to varying scales used to measure quality of life. Our subgroup analysis of medium‐term effects on HbA1c (−0.3%) in younger people indicates agreement with the results of Uchendu 2017 and Attridge 2014. There were also similar but larger positive effects on self‐efficacy at six months in Attridge 2014.
A review on web‐based emotion management in people with T2DM provides supplemental findings to our review on DRD, self‐efficacy and HRQoL because it included a number of trials that are also included in the current review (Hadjiconstantinou 2016). A meta‐analysis was only possible for DRD and showed no substantial differences between interventions. Narratively, four trials showed some improvement in self‐efficacy and little improvement in HRQoL (Hadjiconstantinou 2016). This confirms previous findings that Internet‐based interventions have little effect on DRD (Beatty 2013; Pal 2013). Thus, until further and better‐quality trials on web‐based emotion management interventions are available, emotion‐cognition interventional programmes with personal contact appear more likely to improve DRD in people with T2DM.
The overall lack of favourable effects on HRQoL may be due to the design of the psychological interventions in the included trials, which focused on negative emotions such as DRD, problems with treatments or poor behaviours. HRQoL consists of mainly positive perceptions of well‐being such as energy, vitality, optimism, life satisfaction and physical‐social‐spiritual functioning (Attridge 2014; Macaskill 2016; Robertson 2012). This review could not establish the relationship between psychological interventions and blood pressure based on good‐quality evidence. The inconsistent and small effects on blood pressure could be due to the uncertain direction of effects of negative emotions on blood pressure levels. Future trials in people with T2DM and uncontrolled hypertension will be needed to clarify the effects of psychological interventions on blood pressure.
Authors' conclusions
Implications for practice.
Compared to usual care, psychological interventions appear to have small and uncertain beneficial effects on self‐efficacy and HbA1c after 6 to 12 months. Not all psychological interventions have a substantial effect on DRD. DRD showed improvement following emotion‐cognition interventions that are brief and simple compared to usual care. There are no substantial adverse events or mortality in participants of psychological interventions. Existing psychological interventions have no different effect on HRQoL and blood pressure levels compared to usual care. Evidence is non‐existent on diabetes‐related complications and socioeconomic impacts.
The small difference of effects is a valid consideration when developing psychological interventions in resource‐challenged health facilities. Wise strategies include adoption of theory‐based and proven psychological interventions and need to be modified locally and in a culturally appropriate way.
Implications for research.
Careful consideration is needed when choosing the comparator in future trials examining psychological interventions for DRD. Higher sample sizes may be needed if the comparator is enhanced usual care or attention control group with equivalent number of contacts with healthcare professionals or health services.
There is a need for examination of socioeconomic effects of psychological interventions in adults with T2DM in order to better inform existing practices and policy‐makers considering development and implementation of such interventions. Trials of longer duration are required to provide evidence on the effects of psychological interventions for diabetes‐related complications.
More psychological interventions for adults with T2DM are needed in low‐ and middle‐income countries, particularly in Asia and other regions with high prevalence of T2DM and DRD (Chew 2016; Ikeda 2014; International Diabetes Federation 2015; Nicolucci 2013; Tan 2015; Zhang 2013). Disparate sources of data would also improve the quality of the evidence. There might also be a need for changing the current research agenda away from including all distressed people with T2DM regardless of their severity.
What's new
| Date | Event | Description |
|---|---|---|
| 23 October 2017 | Amended | Results for one trial (Simmons 2015) were missing in comparison 7 and 8 of the review published in issue 9, 2017. Inclusion of this trial did not substantially change the results. |
Notes
Portions of the Background and Methods sections, the Appendices, Additional tables and Figures 1 to 3 of this review are based on a standard template established by the Cochrane Metabolic and Endocrine Disorders Group.
Acknowledgements
We thank the Cochrane Metabolic and Endocrine Disorders Group for their kind and professional editorial assistance throughout the process of this review. A note of acknowledgement is recorded for Universiti Putra Malaysia and the Ministry of Higher Education, Malaysia for their support in sponsoring the PhD study and living allowances for Boon‐How Chew. We would like to thank Jackie Sturt and Kathryn Dennick from the Florence Nightingale Faculty of Nursing and Midwifery, King's College London for sharing full‐text articles with us.
Appendices
Appendix 1. Search strategies
| Cochrane Central Register of Controlled Trials (Cochrane Register of Studies Online) |
| 1. ((problem* next area*) near/4 "diabetes"):ti,ab,kw 2. (diabet* near/13 distress*):ti,ab,kw 3. (diabet* near/4 ("specific" or "related") near/4 "stress"):ti,ab,kw 4. (diabet* next "stress"):ti,ab,kw 5. or #1‐#4 6. Publication Year from 1995 to 2014 7. #5 and #6 |
| MEDLINE (Ovid SP) |
| 1. (problem? area? adj3 diabetes).tw. 2. (diabet* adj12 distress*).tw. 3. (diabet* adj3 (specific or related) adj3 stress).tw. 4. (diabet* stress).tw. 5. or/1‐4 6. limit 5 to yr="1995 ‐Current" (Cochrane Handbook 2008 RCT filter ‐ sensitivity maximizing version) 7. randomised controlled trial.pt. 8. controlled clinical trial.pt. 9. randomi?ed.ab. 10. placebo.ab. 11. drug therapy.fs. 12. randomly.ab. 13. trial.ab. 14. groups.ab. 15. or/7‐14 16. exp animals/ not humans/ 17. 15 not 16 18. 17 and 6 |
| Embase (Ovid SP) |
| 1. (problem? area? adj3 diabetes).tw. 2. (diabet* adj12 distress*).tw. 3. (diabet* adj3 (specific or related) adj3 stress).tw. 4. (diabet* stress).tw. 5. or/1‐4 6. limit 5 to yr="1995 ‐Current" (Wong et al. 2006 "sound treatment studies" filter – BS version) 7. random*.tw. or clinical trial*.mp. or exp health care quality/ 8. 6 and 7 9. limit 8 to embase |
| PsycINFO (Ovid SP) |
| 1. (problem? area? adj3 diabetes).tw. 2. (diabet* adj12 distress*).tw. 3. (diabet* adj3 (specific or related) adj3 stress).tw. 4. (diabet* stress).tw. 5. or/1‐4 6. limit 5 to yr="1995 ‐Current" (Eady et al. 2008 "PsycInfo Search Strategies" filter ‐ BS version) 7. control*.tw. OR random*.tw. OR exp Treatment/ 8. 6 and 7 |
| CINAHL (EBSCOhost) |
| S1. TI ("problem# area#" N3 diabetes) S2. AB ("problem# area#" N3 diabetes) S3. TI (diabet* N12 distress*) S4. AB (diabet* N12 distress*) S5. TI (diabet* N3 (specific OR related) N3 stress) S6. AB (diabet* N3 (specific OR related) N3 stress) S7. TI ("diabet* stress") S8. AB ("diabet* stress") S9. S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 S10. S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 and Limiters ‐ Published Date: 1995‐2014 (Wong et al. 2006 "therapy studies" filter ‐ BS version) S11. MH "prognosis+" OR MH "study design+" or random* S12. S10 AND S11 |
| Bielefeld Academic Search Engine |
| Advanced search: (diabetes OR diabetic) AND (distress OR "problem areas") year:(1995 TO 2015) doctype:(0003 0004) (0003 = Reports, Papers, Lectures, 0004 = Theses) |
| ClinicalTrials.gov |
| Advanced search: Search Terms: (diabetes OR diabetic) AND (distress OR problem areas) Age Group: Adult (18‐65) OR Senior (66+) |
| ICTRP Search Portal |
| Standard search: diabet* AND distress OR diabet* AND problem areas |
Appendix 2. Description of interventions
| Trial | Intervention | Intervention class | Comparator | Comparator class |
| Beverly 2013 |
Group education Deliverer: experienced diabetes nurses and dietitians, trained by certified trainers Hour(s) per session: 1 Number of sessions in total: 4 |
CF |
Educational classes not focusing on diabetes care Deliverer: registered nurse and dietitians, trained and certified Hour(s) per session: 2 Number of sessions in total: 2 |
Enhanced SC |
| Davies 2008 |
Group education Deliverer: registered healthcare professionals received formal training Hour(s) per session: 6 Number of sessions in total: 1 (one day or two half day equivalents) |
CF |
Additional contact time with healthcare professionals Deliverer: — Hour(s) per session: — Number of sessions in total: — |
Enhanced SC |
| Dennick 2015 |
Writing about different aspects of life, thoughts and feelings Deliverer: — Duration per session: 20 minutes Number of sessions in total: 3 |
EF |
Writing about previous days' activities Deliverer: — Duration per session: 20 minutes Number of sessions in total: 3 |
CF |
| D'Eramo Melkus 2010 |
Cognitive behavioural self‐management training Deliverer: clinical psychologist or psychiatric mental health nurse practitioner trained in coping skills training Hour(s) per session: 2 (first 6 sessions), 1 (the remaining 5 sessions) Number of sessions in total: 11 |
EC |
Group education Deliverer: nurse‐led (conventional care, uncertain of training received by nurses) Hour(s) per session: 1.5 (first 5 sessions), 1 (the last 5 sessions) Number of sessions in total: 10 |
CF |
| Fisher 2011 |
Self‐monitoring of blood glucose Deliverer: — Hour(s) per session: — Number of sessions in total: 1 |
CF |
Additional quarterly diabetes‐focused physician visits Deliverer: — Hour(s) per session: — Number of sessions in total: — |
Enhanced SC |
| Fisher 2013 |
Computer‐assisted self‐management Deliverer: non‐professional college graduate interventionists were trained and closely supervised by the investigators Hour(s) per session: 40 minutes Number of sessions in total: — |
CF |
General diabetes support and education Deliverer: non‐professional college graduate interventionists were trained and closely supervised by the investigators Hour(s) per session: 20 minutes Number of sessions in total: — |
CF |
|
Computer‐assisted self‐management + problem solving Deliverer: non‐professional college graduate interventionists were trained and closely supervised by the investigators Hour(s) per session: 60 minutes Number of sessions in total: — |
EC | |||
| Gabbay 2013 |
Motivational interviewing Deliverer: nurse case managers received intensive motivational interviewing training Hour(s) per session: 1 Number of sessions in total: > 6 |
CF |
Usual care Deliverer: — Hour(s) per session: — Number of sessions in total: — |
SC |
| Glasgow 2005 |
Computer‐assisted self‐management Deliverer: care managers Hour(s) per session: 30 minutes computerised touch screen assessment followed by 8‐10 minutes counselling session Number of sessions in total: 1 (probably 2, at the participant's visit 6‐monthly) |
CF |
Computer information without self‐management Deliverer: — Hour(s) per session: — Number of sessions in total: — |
Enhanced SC |
| Grillo 2016 |
Self‐management education Deliverer: generalist nurse trained in diabetes education Hour(s) per session: 2 Number of sessions in total: 5 + 2 reinforcement meetings |
CF |
Group meetings without education Deliverer: generalist nurse trained in diabetes education Hour(s) per session: — Number of sessions in total: 5 + 2 |
Enhanced SC |
| Hermanns 2012 |
Self‐management programme Deliverer: certified diabetes educators Hours per session: 90 minutes Number of sessions in total: 10 |
EC |
Combination of 2 education programmes Deliverer: certified diabetes educators Hours per session: — Number of sessions in total: 10 |
CF |
| Hermanns 2015 |
Cognitive behaviour treatment Deliverer: diabetes educators Hour(s) per session: 1.5 Number of sessions in total: 5 |
EC |
Group education Deliverer: diabetes educators Hour(s) per session: 1.5 Number of sessions in total: 5 |
CF |
| Lamers 2011 |
Cognitive behaviour therapy Deliverer: trained nurse Hour(s) per session: 1 Number of sessions in total: 4 |
EC |
Usual care Deliverer: — Hour(s) per session: — Number of sessions in total: — |
SC |
| Lerman 2009 |
Telephone contacts Deliverer: one of the doctors who participated in the study Hour(s) per session: — Number of sessions in total: 6 (monthly) |
CF |
Usual care Deliverer: trained nurse Hour(s) per session: 1 Number of sessions in total: 4 |
SC |
|
Group‐based education Deliverer: doctor, nurse educator in diabetes, nutrition and psychology graduate Hour(s) per session: 5 Number of sessions in total: 1 (at month 6) |
CF | |||
| Liu 2015 |
Peer education Deliverer: educators in diabetes and peer leaders, both were trained Hour(s) per session: 2 (diabetes health education), later much contact, not specified Number of sessions in total: many contacts in person, group, telephone and via social media |
EC |
Diabetes health education Deliverer: trained educators in diabetes Hour(s) per session: — Number of sessions in total: 4 |
CF |
| Pibernik‐Okanovic 2015 |
Psycho‐educational intervention Deliverer: psychologist Hour(s) per session: 1.5 Number of sessions in total: 6 |
EC |
One re‐educational intervention Deliverer: diabetologist Hour(s) per session: 1.5 Number of sessions in total: 1 |
EC |
|
Physical activity intervention Deliverer: physiotherapist Hour(s) per session: 1.5 Number of sessions in total: 6 |
CF | |||
| Quinn 2011 |
Coach+mobile diabetes management software Deliverer: — Hour(s) per session: — Number of sessions in total: — |
CF |
Usual care Deliverer: — Hour(s) per session: — Number of sessions in total: — |
SC |
|
Coach+mobile diabetes management software + Internet portal Deliverer: — Hour(s) per session: — Number of sessions in total: — |
CF | |||
|
Coach + mobile diabetes management software + Internet portal + decision support Deliverer: — Hour(s) per session: — Number of sessions in total: — |
CF | |||
| Rosenbek 2011 |
Motivational interviewing Deliverer: healthcare professional, trained in motivational interviewing Hour(s) per session: 45 minutes Number of sessions in total: 5 |
EC |
Usual care Deliverer: — Hour(s) per session: — Number of sessions in total: — |
SC |
| Shibayama 2007 |
Behavioural counselling Deliverer: certified expert nurse Hour(s) per session: 8‐76 minutes Number of sessions in total: 12 (monthly) |
EC |
Usual care Deliverer: — Hour(s) per session: — Number of sessions in total: — |
SC |
| Simmons 2015 |
Individual peer support Deliverer: — Hour(s) per session: — Number of sessions in total: — |
EC |
Usual care Deliverer: — Hour(s) per session: — Number of sessions in total: — |
SC |
|
Group peer support Deliverer: Hour(s) per session: — Number of sessions in total: — |
EC | |||
|
Combined group and individual support Deliverer: trained peer support facilitator Hour(s) per session: — Number of sessions in total: 6 (monthly) |
EC | |||
| Skelly 2009 | Symptom‐focused diabetes intervention Deliverer: registered nurse Hour(s) per session: 1 Number of sessions in total: 4 (bimonthly) | CF |
Weight and diet programme Deliverer: registered nurse Hour(s) per session: 1 Number of sessions in total: 4 (bimonthly) |
Enhanced SC |
|
Symptom‐focused diabetes intervention with telephone booster Deliverer: Hour(s) per session: — Number of sessions in total: — |
CF | |||
| Spencer 2013 |
Community health worker intervention Deliverer: trained community health worker Hour(s) per session: 2 Number of sessions in total: 11 (2‐weekly) |
EC |
Information on community activities Deliverer: — Hour(s) per session: — Number of sessions in total: — |
WL or SC |
| Sperl‐Hillen 2013 |
Individual education Deliverer: nurse or dietitian, certified diabetes educators Hour(s) per session: 1 Number of sessions in total: 3 |
CF |
Usual care Deliverer: — Hour(s) per session: — Number of sessions in total: — |
SC |
| Group education Deliverer: nurse or dietitian, certified diabetes educators Hour(s) per session: 2 Number of sessions in total: 4 (weekly) | CF | |||
| Sturt 2008 |
Diabetes manual structured education Deliverer: trained practice nurses Hour(s) per session: 15 minute introduction Number of sessions in total: 4 (1 introduction and 3 phone calls) |
EC |
Usual care Deliverer: — Hour(s) per session: — Number of sessions in total: — |
WL or SC |
| Taylor 2006 | Cognitive‐behavioural therapy Deliverer: — Hour(s) per session: 73 minutes Number of sessions in total: 5 weekly | EC |
Usual care Deliverer: — Hour(s) per session: — Number of sessions in total: — |
WL or SC |
| Expressive writing Deliverer: — Hour(s) per session: 75 minutes Number of sessions in total: 5 | EC | |||
| Trief 2016 |
Behaviour change intervention, couples Deliverer: trained dietitians (certified diabetes educators or with significant diabetes experience) Minutes per call: 57 Number of calls in total: 12 |
EC |
Individual diabetes education Deliverer: trained dietitians (certified diabetes educators or with significant diabetes experience) Minutes per call: 75 Number of calls in total: 2 |
CF |
|
Behaviour change intervention, individuals Deliverer: dietitians (certified diabetes educators or with significant diabetes experience), trained Minutes per call: 50 Number of calls in total: 12 |
EC | |||
| Van der Wulp 2012 | Peer‐led self‐management coaching programme Deliverer: trained peers (expert participant) Hour(s) per session: 1 home visit Number of sessions in total: 3 (monthly) | CF |
Usual care Deliverer: — Hour(s) per session: — Number of sessions in total: — |
SC |
| Van Dijk‐de Vries 2015 |
Self‐management support in routine care Deliverer: trained practice nurses Hour(s) per session: — Number of sessions in total: — |
EC |
Usual care Deliverer: practice nurses Hour(s) per session: — Number of sessions in total: — |
SC |
| Weinger 2011 |
Behavioural strategies Deliverer: certified and trained diabetes educators Hour(s) per session: 2 Number of sessions in total: 5 |
EC |
Group attention Deliverer: certified diabetes educators Hour(s) per session: — Number of sessions in total: 5 |
CF |
|
Individual attention Deliverer: certified diabetes educators (dietitian) Hour(s) per session: — Number of sessions in total: unlimited |
CF | |||
| Welch 2015 |
One‐to‐one diabetes education Deliverer: diabetes educators (two diabetes nurses and two diabetes dietitians) Hour(s) per session: 30 — 60 minutes Number of sessions in total: 5 |
EC |
Usual care Deliverer: four bilingual diabetes educators nurses and diabetes dietitians Hour(s) per session: — Number of sessions in total: — |
CF |
| Whittemore 2004 | Nurse coaching Deliverer: trained nurse Hour(s) per session: 1 Number of sessions in total: 6 | EC | Usual care Deliverer: — Hour(s) per session: — Number of sessions in total: — | SC |
| —: not reported; CF: cognition‐focused intervention; EC: intervention consists of a mixture of emotion and cognition components; EF: emotion‐focused intervention; SC: standard diabetes care; WL: waiting list | ||||
Appendix 3. Baseline characteristics (I)
| Study | Main component of psychological intervention (type of intervention) | Duration of intervention (duration of follow‐up) | Description of participants | Trial period (year to year) | Country | Setting | Ethnic groups (%) |
Duration of diabetes (mean/range years (SD) or as reported) |
| Beverly 2013 | I: cognition focused (group education) |
4 1‐hour sessions of unknown duration (12 months) | Adults with type 2 diabetes who had at least 3 hours of prior diabetes education | — | USA | Joslin Clinic | Non‐Hispanic white: 73 | 13.0 (6.1) |
| C: enhanced usual care (educational classes not focusing on diabetes care) |
Non‐Hispanic white: 70 | 13.6 (9.5) | ||||||
| Davies 2008 | I: cognition focused (group education) |
1‐2 days (12 months postintervention) | Adults with newly diagnosed type 2 diabetes | 2004‐2006 | UK | General practices | White European: 94 | — |
| C: enhanced usual care (additional contact time with healthcare professionals) |
White European: 94 | — | ||||||
| Dennick 2015 | I: emotion focused (writing about different aspects of life, thoughts and feelings) |
1 week (3 months postintervention) | Adults with type 2 diabetes | — | UK | General practices | White British: 96 | 76.9 (54.4) months |
| C: cognition focused (writing about previous days' activities) |
White British: 100 | 93.7 (95.9) months | ||||||
| D'Eramo Melkus 2010 | I: emotion‐cognition components (cognitive behavioural self‐management training) |
12 months (12 months postintervention) | Black women | — | USA | Primary care and community‐based | Black: 100 | — |
| C: cognition focused (group education) |
Black: 100 | — | ||||||
| Fisher 2011 | I: cognition focused (self‐monitoring of blood glucose) |
1 session (12 months) | Adults with type 2 diabetes who are able to read and write English | 2008‐2010 | USA | Primary care | White: 60 | 7.5 (6.1) |
| C: enhanced usual care (additional quarterly diabetes‐focused physician visits) |
— (12 months) |
White: 67 | 7.7 (6.1) | |||||
| Fisher 2013 | I1: cognition focused (computer‐assisted self‐management) |
48 weeks (12 months postintervention) | Non‐clinically depressed adults with type 2 diabetes mellitus | 2008‐2011 | USA | Community medical groups and diabetes education centres | White, non‐Hispanic: 41 | 6.9 (6.0) |
| I2: emotion‐cognition components (computer‐assisted self‐management + problem solving) |
White, non‐Hispanic: 42 | 6.5 (5.5) | ||||||
| C: cognition focused (general diabetes support and education) |
White, non‐Hispanic: 35 | 7.6 (6.4) | ||||||
| Gabbay 2013 | I: cognition focused (motivational interviewing) |
24 months (24 months) | High‐risk type 2 diabetes participants | 2006‐2008 | USA | Primary care clinics | White: 46 Hispanic: 38 |
— |
| C: usual care (standard diabetes care) |
White: 47 Hispanic: 39 |
— | ||||||
| Glasgow 2005 | I: cognition focused (computer‐assisted self‐management) |
6 months (6 months postintervention) | Adults with type 2 diabetes and able to read English | 2001‐2002 | USA | Primary care settings | Non‐Hispanic white: 84 Black: 2 |
— |
| C: enhanced usual care (computer information without self‐management) |
12 months (12 months) | Non‐Hispanic white: 78 Black: 3 |
— | |||||
| Grillo 2016 | I: cognition focused (self‐management education) |
1 + 8 months (11 months postintervention) | Uncontrolled type 2 diabetes mellitus participants | January 2009 to July 2010 | Brazil | Primary care unit | White: 87 | 10.1 (8.3) |
| C: enhanced usual care (group meetings without education) |
8 months (12 months) | White: 87 | 9.7 (7.3) | |||||
| Hermanns 2012 | I: emotion‐cognition components (self‐management programme) |
6 months (6 months postintervention) | Adult with type 2 diabetes mellitus on oral antidiabetic treatment, able to read and understand the German language | — | Germany | Outpatient medical practices run by a diabetologist and a diabetes educator or diabetes nurse | — | 13.8 (8.3) |
| C: cognition focused (combination of 2 education programmes) |
— | 13.6 (6.8) | ||||||
| Hermanns 2015 | I: emotion‐cognition components (cognitive behaviour treatment) |
2 weeks plus four intended phone visits of unknown duration (12 months) | Diabetes mellitus with depression | 2009‐2011 | Germany | Inpatient diabetes centre | — | 14.2 (10.3) |
| C: cognition focused (group education) |
12 months (12 months postintervention) |
— | 14.2 (10.7) | |||||
| Lamers 2011 | I: emotion‐cognition components (cognitive behaviour therapy) |
6 weeks (9 months postintervention) | Type 2 diabetes aged 60 years and over with co‐occurring depression | 2003‐2006 | Netherlands | Primary care practices | — | 8.2 (8.8) |
| C: usual care (standard diabetes care) |
9 months (9 months) | — | 9.8 (9.1) | |||||
| Lerman 2009 | I1: cognition focused (telephone contacts) |
6 months (12 months postintervention) | After finishing a course on diabetes education | — | Mexico | Internal medicine and diabetes clinic | — | 11.0 (8) |
| I2: cognition focused (group‐based education) | — | 12.0 (8) | ||||||
| C: usual care (standard diabetes care) |
6 weeks (12 months postintervention) | — | 14.0 (4) | |||||
| Liu 2015 | I: emotion‐cognition components (peer education) |
6 months (6 months) |
Type 2 diabetes ≥ 45 years with mental disorders (mild‐to‐moderate depression or anxiety) | — | China | Hospital‐based; diabetes education, community follow‐up by peer leaders | All Chinese | 9.8 (6.6) |
| C: cognition focused (diabetes health education) |
— (6 months postintervention) | 10.5 (6.4) | ||||||
| Pibernik‐Okanovic 2015 | I1: emotion‐cognition components (psycho‐educational intervention) | 6 weeks (12 months postintervention) | Type 2 diabetes participants who screened positively for depression and expressed a need for professional help with mood‐related issues | 2010‐2012 | Croatia | University hospital's clinic for diabetes | — | 11.4 (9.1) |
| I2: cognition focused (physical activity intervention) |
— | 12.9 (2.8) | ||||||
| C1: emotion‐cognition components (enhanced usual diabetes care) |
1 session (12 months postintervention) | — | 10.5 (6.9) | |||||
| Quinn 2011 | I1: cognition focused (coach + mobile diabetes management software) |
12 months (12 months) | Adults with type 2 diabetes mellitus who would benefit from an intensive diabetes intervention | — | USA | Primary care practices | Black: 44 Non‐Hispanic White: 52 |
7.7 (5.6) |
| I2: cognition focused (coach + mobile diabetes management software + Internet portal) | Black 46 Non‐Hispanic white: 41 |
6.8 (4.9) | ||||||
| I3: cognition focused (coach + mobile diabetes management software + Internet portal + decision support) | Black 27 Non‐Hispanic white: 63 |
8.2 (5.3) | ||||||
| C: usual care (standard diabetes care) |
Black 48 Non‐Hispanic white: 46 |
9.0 (7.0) | ||||||
| Rosenbek 2011 | I: emotion‐cognition components (motivational interviewing) |
12 months (12 months postintervention) | Adults with type 1 or type 2 diabetes mellitus who had participated in a group education programme before | 2005‐2009 | Denmark | University hospital | — | 57.1 (12.6) months |
| C: usual care (standard diabetes care) |
24 months (12 months postintervention) |
— | 55.8 (11.6) months | |||||
| Shibayama 2007 | I: emotion‐cognition components (behavioural counselling) |
12 months (12 months) | Participants with type 2 diabetes and HbA1c 6.5% to 8.5%, not using insulin | — | Japan | Outpatients of the University of Tokyo Hospital | — | 10 (6 to 14) |
| C: usual care (standard diabetes care) |
— | 13 (8 to 16) | ||||||
| Simmons 2015 | I1: emotion‐cognition components (group peer support) | 12 months (12 months) | Participants with type 2 diabetes for at least 12 months | 2011‐2013 | UK | Communities across Cambridgeshire and neighbouring areas of Essex and Hertfordshire, mainly general practices | Ethnic minority: 7 | 7.0 (3 to 12) |
| I2: emotion‐cognition components (group and individual support) | Ethnic minority: 7 | 6.0 (3 to 11) | ||||||
| I3: emotion focused (individual peer support) |
Ethnic minority: 8 | 7.0 (3 to 12) | ||||||
| C: usual care (standard diabetes care) |
Ethnic minority: 7 | 6.5 (3 to 12) | ||||||
| Skelly 2009 | I1: cognition focused (symptom‐focused) | 6 months (9 months postintervention) | Older African American women with type 2 diabetes | — | USA | Healthcare centres, health department clinics, and primary care practices | Black: 100 | 15 (7.3) |
| I2: cognition focused (symptom‐focused with telephone booster) | 6 months (9 months) | Black: 100 | 12 (6.2) | |||||
| C: enhanced usual care (weight and diet programme) |
3 months (6 months postintervention) | Black: 100 | 12 (5.2) | |||||
| Spencer 2013 | I: emotion‐cognition components (community health worker intervention) |
5.5 months (6 months postintervention) | African American and Latino participants with type 2 diabetes | — | USA | Community health centre, a major local health system | African American: 53 | — |
| C: waiting list or usual care (information on community activities) |
6 months (6 months postintervention) | African American: 61 | — | |||||
| Sperl‐Hillen 2013 | I1: cognition focused (individual education) |
3 months (10 months postintervention) | Type 2 diabetes participants with HbA1c > 7% | 2008‐2009 | USA | Health partners in Albuquerque, New Mexico, and Clinics in Minneapolis, Minnesota | White: 65 Black: 5 Hispanic: 22 | 11.7 |
| I2: cognition focused (group education) |
1 month (10 months postintervention) | |||||||
| C: usual care (standard diabetes care) |
13 months (13 months) | |||||||
| Sturt 2008 | I: emotion‐cognition components (diabetes manual structured education) |
3 months (3 months postintervention) | Adults with type 2 diabetes, not taking insulin and able to read and write English and a most recent HbA1c > 8.0%. | 2004‐2005 | UK | General practices | White: 81 | 1‐15 years: 76% |
| C: waiting list or usual care (standard diabetes care) |
6 months (6 months) | White: 79 | 1‐15 years: 80% | |||||
| Taylor 2006 | I1: emotion‐cognition components (cognitive‐behavioural therapy) |
5 weeks (5 weeks) | Adults with type 2 diabetes for at least 6 months | 2000 | USA | Diabetes support group, American Diabetes Association's referrals, and physician referrals from the Sutter Health Medical Group and Placer County's health agencies | White: 95 Hispanic: 3 African American: 1 Asian: 1 |
— |
| I2: emotion‐cognition components (expressive writing) |
— | |||||||
| C: waiting list or usual care (standard diabetes care) |
— | |||||||
| Trief 2016 | I1: emotion‐cognition components (behaviour change intervention, couples) |
4 months (12 months postintervention) | A willing partner able to speak and read English; in a self‐defined committed relationship for ≥ 1 year | 2009‐2014 | USA | Community | White: 74 Hispanic or Latino: 5 Asian: 4 Black or African American: 18 |
12.8 (8.5) |
| I2: emotion‐cognition components (behaviour change intervention, individuals) |
Type 2 diabetes for > 1 year; baseline HbA1c ≥ 7.5% (58 mmol/mol); ≥ 21 years of age; able to speak and read English | White: 64 Hispanic or Latino: 7 Asian: 12 Black or African American: 20 |
11.9 (6.9) | |||||
| C: cognition focused (individual diabetes education) |
2 weeks (12 months postintervention) | White: 70 Hispanic or Latino: 10 Asian: 12 Black or African American: 14 |
12.6 (8.3) | |||||
| Van der Wulp 2012 | I: cognition focused (peer‐led self‐management coaching programme) |
3 months (6 months postintervention) | Recently diagnosed participants with type 2 diabetes | 2008‐2010 | Netherlands | General practices | Dutch: 88 | — |
| C: usual care (standard diabetes care) |
9 months (6 months postintervention) | Dutch: 85 | — | |||||
| Van Dijk‐de Vries 2015 | I: emotion‐cognition components (self‐management support in routine care) |
12 months (12 months postintervention) | Type 2 diabetes participants with impaired daily functioning and emotional distress | 2011‐2012 | Netherlands | General practices | Non‐Western: 2 | 9 (8) |
| C: usual care (standard diabetes care) |
Non‐Western: 0 | 8 (6) | ||||||
| Weinger 2011 | I1: emotion‐cognition components (behavioural strategies) | 6 weeks (12 months postintervention) | Type 2 diabetes participants with diabetes ≥ 2 years taking insulin and/or oral medication ≥1 year, and HbA1c > 7.5% | 2003‐2008 | USA | Joslin Clinic | Non‐Hispanic white: 80 (subgroup with T2DM) | 10.7 (1.3 to 41.1) months (subgroup with T2DM) |
| C1: cognition focused (group attention) | ||||||||
| C2: cognition focused (individual attention) | ||||||||
| Welch 2015 | I: emotion‐cognition components (one‐to‐one diabetes education) |
6 months (6 months) | Self‐identified Hispanic ethnicity, HbA1c > 7.5% | 2010‐2012 | USA | Federally qualified health centres | White: 98 | — |
| C: usual care (standard diabetes care) |
White: 99 | — | ||||||
| Whittemore 2004 | I: emotion‐cognition components (nurse coaching) |
6 months (6 months) | Women with type 2 diabetes, who had previously participated in diabetes education | — | USA | Outpatient diabetes education centre | White: 89 Hispanic 11 | 2.7 (3.0) |
| C: usual care (standard diabetes care) | ||||||||
| —: not reported; C: comparator; HbA1c: glycosylated haemoglobin A1c; I: intervention; T2DM: type 2 diabetes mellitus | ||||||||
Appendix 4. Baseline characteristics (II)
| Study | Main component of psychological intervention (type of intervention) | Sex (female %) | Age (mean/range years (SD), or as reported) | HbA1c (%) | BMI (mean kg/m² (SD)) | Co‐medications/Co‐interventions (% of participants) | Comorbidities (% of participants) |
| Beverly 2013 | I: cognition focused (group education) |
48 | 59.9 (8.5) | 8.5 (1.4) | 34.6 (7.0) | — | — |
| C: enhanced usual care (educational classes not focusing on diabetes care) |
55 | 58.4 (9.0) | 8.3 (1.0) | 33.7 (7.1) | — | — | |
| Davies 2008 | I: cognition focused (group education) |
47 | 59.0 (28.0‐87.0) | 8.3 (2.2) | 32.3 (6.1) | Prescribed oral hypoglycaemic agents: 17% | Smokers: 14% |
| C: enhanced usual care (additional contact time with healthcare professionals) |
43 | 60.0 (29‐87) | 7.9 (2.0) | 32.4 (6.5) | Prescribed oral hypoglycaemic agents: 12% | Smokers: 16% | |
| Dennick 2015 | I: emotion focused (writing about different aspects of life, thoughts and feelings) |
39 | 63.9 (41–80 (9.2) | 7.0 | 30.6 (6.0) | Tablets and insulin: 4% | ≥ 1 complication: 52% |
| C: cognition focused (writing about previous days' activities) |
39 | 67.8 (52–84 (10.7) | 6.9 | 30.1 (7.1) | Tablets and insulin: 11% | ≥ 1 complication: 50% | |
| D'Eramo Melkus 2010 | I: emotion‐cognition components (cognitive behavioural self‐management training) |
100 | 47 (9) | 8.0 | — | — | Current smoker: 25% |
| C: cognition focused (group education) |
100 | 45 (10) | 8.3 | — | — | Current smoker: 25% | |
| Fisher 2011 | I: cognition focused (self‐monitoring of blood glucose) |
47 | 54.8 (10.1) | 8.9 (1.2) | 35.0 (7.8) | — | — |
| C: enhanced care (additional quarterly diabetes‐focused physician visits) |
46 | 57.0 (11.2) | 8.9 (1.2) | 35.1 (6.7) | — | — | |
| Fisher 2013 | I1: cognition focused (computer‐assisted self‐management) |
48 | 57.0 (8.8) | 7.45 (1.5)a | 32.1 (7.17) | Insulin use: 15.3% | No. of comorbidities/ complications: 3.4 |
| I2: emotion‐cognition components (computer‐assisted self‐management + problem solving) |
56 | 55.8 (9.4) | 7.34 (1.6)a | 33.9 (7.9) | Insulin use: 19.2% | No. of comorbidities/ complications: 3.2 | |
| C: cognition focused (general diabetes support and education) |
59 | 55.2 (10.9) | 7.45 (1.7)a | 33.3 (8.4) | Insulin use: 19.8% | No. of comorbidities/ complications: 3.6 | |
| Gabbay 2013 | I: cognition focused (motivational interviewing) |
62 | 58 (11) | 8.8 (2.4) | 34.0 (7.4) | — | — |
| C: usual care (standard diabetes care) |
55 | 58 (11) | 9.1 (2.3) | 34.8 (8.8) | — | — | |
| Glasgow 2005 | I: cognition focused (computer‐assisted self‐management) |
53 | 61.5 (12.6) | — | — | — | No. of chronic conditions: 1.9 (1.5) ≥ 5 comorbid illnesses: 6.1% |
| C: enhanced care (computer information without self‐management) |
51 | 64.6 (12.4) | — | — | — | No. of chronic conditions: 2.2 (1.4) ≥ 5 comorbid illnesses: 6.5% | |
| Grillo 2016 | I: cognition focused (self‐management education) |
71 | 61.7 (9.9) | 8.8 (1.9) | 30.7 (5.7) | Oral agents: 58% Oral agents and insulin: 36% Insulin alone: 6% | Hypertension: 91.3% Dyslipidaemia: 82.6% Smoking: 21.7% Sedentary: 84% |
| C: enhanced care (group meetings without education) |
56 | 63.2 (9.7) | 9.1 (2.0) | 29.9 (5.8) | Oral agents: 62% Oral agents and insulin: 34% Insulin alone: 4% | Hypertension: 91.2% Dyslipidaemia: 75.0% Smoking: 14.7% Sedentary: 88% | |
| Hermanns 2012 | I: emotion‐cognition components (self‐management programme) |
52 | 62.0 (8.7) | 8.4 (1.5) | 33.3 (5.6) | Oral antidiabetic: 46.2% Antihypertensive: 81.7% | No. of complications: 1.2 |
| C: cognition focused (combination of 2 education programmes) |
37 | 63.9 (7.8) | 8.3 (1.2) | 33.4 (6.2) | Oral antidiabetic: 60.4% Antihypertensive: 82.4% | No. of complications: 1.2 | |
| Hermanns 2015 | I: emotion‐cognition components (cognitive behaviour treatment) |
57 | 43.2 (14.9) | 8.9 (1.8) | 29.8 (7.7) | — | MicroCx: 53.8 MacroCx: 17.0 |
| C: cognition focused (group education) |
57 | 43.4 (13.8) | 8.9 (1.8) | 27.7 (6.3) | — | MicroCx: 45.4 MacroCx: 6.5 |
|
| Lamers 2011 | I: emotion‐cognition components (cognitive behaviour therapy) |
54 | 70.7 (6.6) | 7.5 (1.2) | — | Insulin and oral antidiabetic: 14.3% | — |
| C: usual care (standard diabetes care) |
52 | 69.7 (6.6) | 7.2 (1.4) | — | Insulin and oral antidiabetic: 20.8% | — | |
| Lerman 2009 | I1: cognition focused (telephone contacts) |
83 | 59.0 (9) | 8.5 (1.4) | 26.9 (4.5) | — | — |
| I2: cognition focused (group‐based education) | 63 | 58.0 (11) | 8.3 (1.7) | 27.8 (4.7) | — | — | |
| C: standard care (standard diabetes care) |
59 | 55.0 (10) | 9.3 (1.9) | 28.7 (6.2) | — | — | |
| Liu 2015 | I: emotion‐cognition components (peer education) |
73 | 62.6 (6.3) | 7.34 (1.2) | 24.5 (2.7) | — | Smoking 26% |
| C: cognition focused (diabetes health education) |
64 | 64.1 (4.7) | 7.39 (1.1) | 24.7 (2.7) | — | Smoking 23% | |
| Pibernik‐Okanovic 2015 | I1: emotion‐cognition components (psycho‐educational intervention) | 40 | 57.7 (6.2) | 7.4 (1.2) | 30.64 (4.5) | Insulin: 32 | — |
| I2: cognition focused (physical activity intervention) |
37 | 58.5 (4.8) | 7.2 (1.1) | 29.44 (4.7) | Insulin: 29 | — | |
| C1: emotion‐cognition components (enhanced diabetes care) |
36 | 58.2 (5.6) | 7.2 (1.1) | 29.96 (4.4) | Insulin: 32 | — | |
| Quinn 2011 | I1: cognition focused (coach + mobile diabetes management software) |
48 | 52.8 (8.0) | 9.3 | 36.9 (7.5) | — | Hypertension: 78.3% Hypercholesterolaemia: 47.8% Coronary artery disease: 8.7% Microvascular complications: 4.3% |
| I2: cognition focused (coach + mobile diabetes management software + Internet portal) | 55 | 53.7 (8.2) | 9.0 | 35.5 (10.3) | — | Hypertension: 59.1% Hypercholesterolaemia: 63.6% Coronary artery disease: 0 % Microvascular complications: 9.1% | |
| I3: cognition focused (coach + mobile diabetes management software + Internet portal + decision support) | 50 | 52 (8.0) | 9.9 | 35.8 (7.1) | — | Hypertension: 69.4% Hypercholesterolaemia: 58.1% Coronary artery disease: 8.1% Microvascular complications: 9.7% | |
| C: usual care (standard diabetes care) |
50 | 53.2 (8.4) | 9.2 | 34.3 (6.3) | — | Hypertension: 51.8% Hypercholesterolaemia: 60.7% Coronary artery disease: 8.9% Microvascular complications: 14.3% | |
| Rosenbek 2011 | I: emotion‐cognition components (motivational interviewing) |
48 | 57.1 (12.6) | 7.0 (1.2) | 30.8 (5.8) | Insulin: 27 OHA: 46 Antihypertensive treatment: 60 | — |
| C: usual care (standard diabetes care) |
51 | 55.8 (11.6) | 7.0 (1.2) | 31.1 (6.3) | Insulin: 30 OHA: 42 Antihypertensive treatment: 62 | — | |
| Shibayama 2007 | I: emotion‐cognition components (behavioural counselling) |
35 | 61 (8) | 7.3 (0.8) | 25 (6) | Oral antidiabetic: 89.6% | — |
| C: usual care (standard diabetes care) |
35 | 62 (7) | 7.4 (0.7) | 25 (5) | Oral antidiabetic: 82.1% | — | |
| Simmons 2015 | I1: emotion‐cognition components (group peer support) | 35 | 65.2 (10.2) | 7.5 (1.3) | 31.9 (5.8) | Insulin: 16.1 | Smoking: 8.8 |
| I2: emotion‐cognition components (group and individual support) | 41 | 65.3 (9.3) | 7.3 (1.3) | 32.1 (5.8) | Insulin: 17.4 | Smoking: 8.4 | |
| I3: emotion focused (individual peer support) |
42 | 65.2 (8.9) | 7.4 (1.3) | 32.7 (6.4) | Insulin: 19.1 | Smoking: 8.8 | |
| C: usual care (standard diabetes care) |
41 | 64.6 (10.3) | 7.3 (1.3) | 32.1 (6.1) | Insulin: 14.6 | Smoking: 11.8 | |
| Skelly 2009 | I1: cognition focused (symptom‐focused) | 100 | Median 68.5 | 8.4 (1.6) | — | Insulin and oral antidiabetic: 40% | Median no. of complication: 4 |
| I2: cognition focused (symptom‐focused with telephone booster) | 100 | Median 65 | 8.3 (1.6) | — | Insulin and oral antidiabetic: 33% | Median no. of complication: 4 | |
| C: enhanced usual care (weight and diet programme) |
100 | Median 68 | 8.1 (1.6) | — | Insulin and oral antidiabetic: 36% | Median no. of complication: 4 | |
| Spencer 2013 | I: emotion‐cognition components (community health worker intervention) |
75 | 50 | 8.6 | — | — | Diabetes complications: 2.4% |
| C: waiting list or usual care (information on community activities) |
67 | 55 | 8.5 | — | — | Diabetes complications: 2.9% | |
| Sperl‐Hillen 2013 | I1: cognition focused (individual education) |
49 | 62 | 8.1 | — | Insulin use: 32.5% | — |
| I2: cognition focused (group education) |
8.1 | — | Insulin use: 22.7% | — | |||
| C: usual care (standard diabetes care) |
8.1 | — | Insulin use: 36.7% | — | |||
| Sturt 2008 | I: emotion‐cognition components (diabetes manual structured education) |
39 | 62 (51‐71) | 8.9 (1.5) | 31.8 (6.7) | — | Other chronic conditions: 45% |
| C: waiting list or usual care (standard diabetes care) |
40 | 62 (53‐70) | 8.8 (1.5) | 31.6 (6.1) | — | Other chronic conditions: 50% | |
| Taylor 2006 | I1: emotion‐cognition components (cognitive‐behavioural therapy) |
72 | 69 | — | — | — | — |
| I2: emotion‐cognition components (expressive writing) |
66 | — | — | — | — | ||
| C: waiting list or usual care (standard diabetes care) |
68 | — | — | — | — | ||
| Trief 2016 | I1: emotion‐cognition components (behaviour change intervention, couples) |
37 | 57.8 (10.8) | 8.9 (1.3) | 35.7 (6.3) | — | — |
| I2: emotion‐cognition components (behaviour change intervention, individuals) |
38 | 55.6 (11.4) | 9.3 (1.7) | 36 (8.2) | — | — | |
| C: cognition focused (individual diabetes education) |
41 | 56.9 (10.4) | 9.1 (1.6) | 36 (8.1) | — | — | |
| Van der Wulp 2012 | I: cognition focused (peer‐led self‐management coaching programme) |
44 | 60.0 | — | — | Oral antidiabetic: 64.4% Insulin: 1.7% | — |
| C: usual care standard diabetes care) |
47 | 62.5 | — | — | Oral antidiabetic: 63.3% Insulin: 0% | — | |
| Van Dijk‐de Vries 2015 | I: emotion‐cognition components (self‐management support in routine care) |
47 | 64 (10) | 53.0 (11.2) mmol/mol | — | Oral antidiabetic: 61% Insulin: 9% | — |
| C: usual care (standard diabetes care) |
46 | 66 (9) | 51.8 (10.2) mmol/mol | — | Oral antidiabetic: 76% Insulin: 3% | — | |
| Weinger 2011 | I1: emotion‐cognition components (behavioural strategies) | 45 (subgroup with T2DM) |
58.4 (36.6‐75.1) (subgroup with T2DM) |
9.0 (7.6‐13.6) (subgroup with T2DM) |
32.4 (19.0‐57.8) (subgroup with T2DM) |
— | — |
| C1: cognition focused (group attention) | |||||||
| C2: cognition focused (individual attention) | |||||||
| Welch 2015 | I: emotion‐cognition components (one‐to‐one diabetes education) |
61 | 54.8 (10.3) | 8.9 (1.4) | 35.4 (7.7) | — | High distress: 62.9 Major depression: 32.7 |
| C: cognition focused (standard diabetes care) |
59 | 55.2 (11.9) | 9.0 (1.5) | 33.9 (7.5) | — | High distress: 50.5 Major depression: 41.2 | |
| Whittemore 2004 | I: emotion‐cognition components (nurse coaching) |
100 | 57.6 (10.9) | 7.7 (1) | 36.5 (7) | — | Overweight or obese: 96% |
| C: usual care (standard diabetes care) |
100 | 7.6 (1) | 34.8 (7) | — | |||
| —: not reported aTrial authors provided data that were not reported in the article BMI: body mass index; C: comparator; HbA1c: glycosylated haemoglobin A1c; I: intervention; OHA: oral hypoglycemic agents; SD: standard deviation | |||||||
Appendix 5. Matrix of study endpoints (publications and trial documents)
| Trial | Endpoints quoted in trial document(s) (ClinicalTrials.gov, FDA/EMA document, manufacturer's website, published design paper)a | Trial results or publications available in trials register Yes/No | Endpoints quoted in publication(s)b | Endpoints quoted in abstract of publication(s)b |
| Beverly 2013 |
Source: NCT00895986 Primary outcome measure(s): improved frequency of recommended self‐care behaviours (Self‐Care Inventory‐R) |
No (accessed 28 January 2016) | Primary outcome measure(s): HbA1c |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): HbA1c levels at 3 months, 6 and 12 months; frequency of self‐reported self‐care, diabetes quality of life, diabetes‐related distress and frustration with diabetes self‐care over time |
| Secondary outcome measure(s): HbA1c, QoL, DRD, psychological symptoms, coping styles, SE | Secondary outcome measure(s): BP, lipids, self‐care, psychological symptoms, coping styles, DRD, self‐management, QoL, SE, health literacy | |||
| Davies 2008 |
Source: ISRCTN17844016 Primary outcome measure(s): HbA1c |
Yes (Davies 2008) | Primary outcome measure(s): — |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): HbA1c levels at 12 months; weight loss at 12 months; the odds of not smoking at 12 months; changes in illness belief scores; depression score at 12 months; association between change in perceived personal responsibility and weight loss at 12 months |
| Secondary outcome measure(s): lipids, BP, QoL, self‐care, illness perception | Secondary outcome measure(s): — | |||
| Other outcome measure(s): | Other outcome measure(s): HbA1c, BP, lipids, weight, self‐care, physical activity, QoL, illness perception, DRD, depression | |||
| Dennick 2015 |
Source: ISRCTN18442976 Primary outcome measure(s): depression |
No (accessed 28 January 2016) | Primary outcome measure(s): depressive symptoms |
Primary outcome measure(s): Secondary outcome measure(s): Other outcome measure(s): Depressive symptoms; healthy dietary behaviour |
| Secondary outcome measure(s): DRD, health care use, diabetes self‐care behaviours, HbA1c, health status/QoL | Secondary outcome measure(s): DRD, health status/QoL and diabetes self‐care behaviours | |||
| D'Eramo Melkus 2010 | Source: none | Primary outcome measure(s): — |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): HbA1c from baseline to 3 months and at 12 and 24 months; systolic blood pressure and LDL cholesterol levels from baseline to 24 months. Baseline QoL and Medical Outcome Study Short Form‐36; social function, role‐emotional and mental health domains at 12 months and 24 months; general health, vitality, role physical and bodily pain domains over time. Perceived provider support for diet and exercise over time; diabetes‐related emotional distress |
|
| Secondary outcome measure(s): — | ||||
| Other outcome measure(s): HbA1c, BP, lipids, weight, anxiety, DRD, social support, SE, diabetes knowledge, QoL, health care provider support | ||||
| Fisher 2011 |
Source: NCT00674986 Primary outcome measure(s): HbA1c |
Yes (study results in trials register; Fisher 2011) | Primary outcome measure(s): depressive symptoms and DRD |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): depression and disease‐related distress from baseline to 12 months |
| Secondary outcome measure(s): number of visits with diabetic medication and/or lifestyle change, recommendations, depressive symptoms, DRD, well‐being/QoL, SE, mean number of subject‐monitored blood glucose (SMBG) tests per day, glycaemic variability | Secondary outcome measure(s): — | |||
| Other outcome measure(s): | Other outcome measure(s): HbA1c, adverse events such as hypoglycaemia; severe hypoglycaemia; extremely high blood glucose; severe hyperglycaemia with or without diabetic ketoacidosis; and any other any serious adverse effect on the health or safety or any life‐threatening problem or death | |||
| Fisher 2013 |
Source: NCT00714441 Primary outcome measure(s): diet, physical activity, medication adherence, DRD |
Yes (Fisher 2013) | Primary outcome measure(s): DRD |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): diabetes distress (DD) and regimen distress; reductions in DD were accompanied by significant improvements in healthy eating, physical activity, and medication adherence, although not by change in HbA1c |
| Secondary outcome measure(s): HbA1c, BP, fasting glucose, lipids | Secondary outcome measure(s): — | |||
| Other outcome measure(s): | Other outcome measure(s): physical activity, healthy eating, medication adherence, HbA1c | |||
| Gabbay 2013 |
Source: NCT00308386 Primary outcome measure(s): HbA1c, BP, lipids |
Yes (Gabbay 2013) | Primary outcome measure(s): HbA1c, LDL, BP, DRD, treatment satisfaction, depression, self‐care activities, QoL, BP, HbA1c |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): systolic blood pressure (SBP); HbA1c; low density lipoprotein (LDL); diastolic blood pressure; depression symptom scores; diabetes‐related distress |
|
Secondary outcome measure(s): percentages of participants with yearly ophthalmologic exam, with yearly foot exam, with assessment for nephropathy, with nephropathy on ACE inhibitor or ARB, participants on aspirin, depression, DRD, QoL, self‐care activities, participant satisfaction, cost‐effectiveness, physician satisfaction |
Secondary outcome measure(s): — | |||
| Glasgow 2005 | Source: none |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): participants' perceptions of provider autonomy support, SE, participant satisfaction, HbA1c, lipids, DRD, depression |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): participant perception of autonomy; perceived competence; change in lipids, diabetes distress and depressive symptoms |
|
| Grillo 2016 |
Source: NCT01473329 Primary outcome measure(s): HbA1c |
No (accessed 19 October 2016) | Primary outcome measure(s): HbA1c | Primary outcome measure(s): — |
Secondary outcome measure(s):
|
Secondary outcome measure(s): — | Secondary outcome measure(s): — | ||
| Other outcome measure(s): — | Other outcome measure(s): changes in diabetes mellitus literacy, blood pressure, BMI, and lipids | Other outcome measure(s): metabolic control, weight, blood pressure, distress scores, and knowledge on diabetes | ||
| Hermanns 2012 |
Source: NCT00901992 Primary outcome measure(s): HbA1c |
Yes (Hermanns 2012) | Primary outcome measure(s): — |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): Mean HbA1c at 6 months; diabetes‐related distress |
| Secondary outcome measure(s): QoL, diabetes knowledge, DRD, self‐care behaviour, lipids, weight | Secondary outcome measure(s): — | |||
| Other outcome measure(s): | Other outcome measure(s): HbA1c, lipids, DRD, knowledge, self‐care activities, QoL, weight | |||
| Hermanns 2015 |
Source:NCT01009138 Primary outcome measure(s): depressive symptoms (CES‐D‐Score) |
Yes (study results in trials register; Hermanns 2015) | Primary outcome measure(s): depression |
Primary outcome measure(s): depressive symptoms Secondary outcome measure(s): diabetes distress, well‐being, self‐care behaviour, diabetes acceptance, diabetes treatment satisfaction, HbA1c level, and subclinical inflammation Other outcome measure(s): — |
| Secondary outcome measure(s): QoL (EQ‐5D Score, WHO‐5 Score); diabetes distress (DDS‐Score, PAID‐Score); diabetes self‐care activity (SDSCA Score); diabetes acceptance (AADQ Score); inflammatory markers (CRP, IL‐6, IL‐1RA, IL‐18, TNF‐alpha, DHEA‐S, 5‐HIAA, cortisol); healthcare costs; glycaemic control (HbA1c); body weight (kg) | Secondary outcome measure(s): depressive symptoms (PHQ‐9), DRD, self‐care activities, QoL, diabetes acceptance and treatment satisfaction | |||
| Lamers 2011 |
Source: ISRCTN92331982 Primary outcome measure(s): depression, cost‐effectiveness, health status/QoL |
Yes (Lamers 2011) | Primary outcome measure(s): — |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): emotional distress and symptom distress (DSC‐R total score at 9 months; PAID at 9 months; HbA1c after 9 months) |
| Secondary outcome measure(s): QoL, daily functioning, SE, autonomy, participation | Secondary outcome measure(s): — | |||
| Other outcome measure(s): | Other outcome measure(s): QoL, DRD, HbA1c | |||
| Lerman 2009 | Source: none | Primary outcome measure(s): — |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): diabetes‐related knowledge, treatment compliance and adherence to the recommended meal plan, glycaemic control, prevalence of depression or diabetes‐related distress |
|
| Secondary outcome measure(s): — | ||||
| Other outcome measure(s): HbA1c, DRD, diabetes knowledge, depression | ||||
| Liu 2015 | Source: none | Primary outcome measure(s): — |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): metabolic index, diabetes‐related distress, emotional status and quality of life |
|
| Secondary outcome measure(s): — | ||||
| Other outcome measure(s): blood pressure, HbA1c levels, mentation and quality of life | ||||
| Pibernik‐Okanovic 2015 |
Source: ISRCTN05673017 Primary outcome measure(s): depressive symptoms, measured after the treatment (i.e. after 6 weeks for the 'diabetes treatment as usual' group), and after 6‐ and 12‐month follow‐up periods |
Yes (Pibernik‐Okanovic 2015) | Primary outcome measure(s): depressive symptoms |
Primary outcome measure(s): Depressive symptoms Secondary outcome measure(s): diabetes distress, diabetes self‐care, metabolic control and health‐related quality of life Other outcome measure(s): — |
|
Secondary outcome measure(s): 1. self‐management of diabetes, measured at 6 weeks for the "diabetes treatment as usual" group, and after 6‐ and 12‐month follow‐up periods 2. metabolic control, measured at 6 weeks for the "diabetes treatment as usual" group, and after 6‐ and 12‐month follow‐up periods 3. diabetes‐related distress, measured at 6 weeks for the "diabetes treatment as usual" group, and after 6‐ and 12‐month follow‐up periods 4. health‐related quality of life, measured at 6 weeks for the "diabetes treatment as usual" group, and after 6‐ and 12‐month follow‐up periods 5. treatment satisfaction, measured after the treatment |
Secondary outcome measure(s): diabetes distress, diabetes self‐care, metabolic control and health‐related quality of life | |||
| Quinn 2011 |
Source: NCT01107015 and design paper Quinn 2009 Primary outcome measure(s): HbA1c: mean change comparing Group 1 and Group 4 |
Yes (Quinn 2011) | Primary outcome measure(s): HbA1c |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): glycated haemoglobin over 12 months; differences between groups for patient‐reported diabetes distress, depression, diabetes symptoms, or blood pressure and lipid levels |
| Secondary outcome measure(s): change in HbA1c comparing all 4 groups, changes in measures related to BP and DRD, SE | Secondary outcome measure(s): depression, DRD, BP, lipids, hypoglycaemic events, hospitalisation, and emergency room visits | |||
| Rosenbek 2011 |
Source: NCT00555854 Primary outcome measure(s): HbA1c |
Yes (Rosenbek 2011) | Primary outcome measure(s): HbA1c, self‐efficacy, self‐care |
Primary outcome measure(s): HbA1c Secondary outcome measure(s): — Other outcome measure(s): competence of self‐management (using the PAID scale and PCDS |
| Secondary outcome measure(s): lipids profile, blood pressure, waist, BMI and medication questionnaire: PAID, PCDS, HCCQ, TSRQ and Health Care Behavioural | Secondary outcome measure(s): DRD | |||
| Shibayama 2007 | Source: none | Primary outcome measure(s): HbA1c |
Primary outcome measure(s): Secondary outcome measure(s): Other outcome measure(s): HbA1C, BMI, blood pressure, serum lipids and health‐related quality of life over 1 year between the 2 groups; modification of cognition and behaviour |
|
| Secondary outcome measure(s): — | ||||
| Other outcome measure(s): QoL, DRD, cognitive modification, behavioural modification and overall satisfaction | ||||
| Simmons 2015 |
Source: ISRCTN66963621 Primary outcome measure(s): HbA1c |
Yes (Simmons 2015) | Primary outcome measure(s): HbA1c |
Primary outcome measure(s): HbA1c Secondary outcome measure(s): quality of life, diabetes distress, blood pressure, waist, total cholesterol and weight Other outcome measure(s): — |
| Secondary outcome measure(s): BP and lipids; quality of life | Secondary outcome measure(s): total cholesterol | |||
| Other outcome measure(s): | Other outcome measure(s): depression, quality of life, diabetes self‐efficacy, the Revised Diabetes Knowledge Scale (RDKS), diabetes distress, and medication adherence | |||
| Skelly 2009 | Source: none | Primary outcome measure(s): — |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): HbA1c; symptom distress, perceived quality of life, impact of diabetes and self‐care activities |
|
| Secondary outcome measure(s): | ||||
| Other outcome measure(s): HbA1c, DRD, QoL, self‐care practices | ||||
| Spencer 2013 |
Source: NCT00800410 Primary outcome measure(s): Hemoglobin A1c |
Yes (Spencer 2013) | Primary outcome measure(s): — |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): PAID from pre‐intervention to post‐intervention; Patient Health Questionnaire (PHQ) score |
| Secondary outcome measure(s): — | ||||
| Secondary outcome measure(s): LDL cholesterol, blood pressure, Diabetes self‐management knowledge, Diabetes self‐management and self‐care activities (physical activity, healthy eating, glucose testing, medication taking, required screening tests/exams), diabetes‐specific emotional distress | Other outcome measure(s): HbA1c, health, health care, behaviours and attitudes toward diabetes, quality of diabetes care, relations with healthcare providers, and dietary and physical activity practices, self‐reported diabetes‐related complications, DRD, depressive symptoms | |||
| Sperl‐Hillen 2013 |
Source: NCT00652509 Primary outcome measure(s): programme satisfaction, behavioural and emotional outcomes |
Yes (Sperl‐Hillen 2013) | Primary outcome measure(s): — |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): HbA1c, PAID, Diabetes Self‐Efficacy (DES), Recommended Food Score (RFS) for the first 150 days post randomisation, and by 250 days |
| Secondary outcome measure(s): blood sugar level, BP, lipids, cost, comorbidities | Secondary outcome measure(s): — | |||
| Other outcome measure(s): | Other outcome measure(s): HbA1c, medication use, DRD, SE, recommended food score | |||
| Sturt 2008 |
Source: ISRCTN06315411 Primary outcome measure(s): HbA1c |
Yes (Sturt 2008) | Primary outcome measure(s): — |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): HbA1c; diabetes‐related distress scores; confidence to self‐care scores |
| Secondary outcome measure(s): lipids, BP, height, weight, DRD, QoL, SE | Secondary outcome measure(s): — | |||
| Other outcome measure(s): | Other outcome measure(s): HbA1c, BP, serum cholesterol, height, weight, DRD, SE | |||
| Taylor 2006 | Source: none | Primary outcome measure(s): — |
Primary outcome measure(s): Secondary outcome measure(s): Other outcome measure(s): well‐being; stress, increased energy, and an overall improvement in moods; awareness |
|
| Secondary outcome measure(s): — | ||||
| Other outcome measure(s): QoL, DRD, self‐care behavioural and social support, HbA1c | ||||
| Trief 2016 |
Source: NCT01017523 Primary outcome measure(s): HbA1c |
No (accessed 17 October 2016) | Primary outcome measure(s): HbA1c |
Primary outcome measure(s): HbA1c Secondary outcome measure(s): BMI, waist circumference, blood pressure, depressive symptoms, diabetes self‐efficacy, and diabetes distress Other outcome measure(s): — |
| Secondary outcome measure(s): BMI/waist circumference; measures of behaviour change (diet, physical activity); diabetes‐related quality of life outcome (distress) | Secondary outcome measure(s): BMI, waist circumference, blood pressure, depressive symptoms, diabetes self‐efficacy, and diabetes distress | |||
| Other outcome measure(s): | Other outcome measure(s): — | |||
| Van der Wulp 2012 |
Source: ISRCTN91626621 Primary outcome measure(s): SE |
No (accessed 28 January 2016) | Primary outcome measure(s): SE |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): self‐efficacy, coping and saturated fat intake over time; psychological well‐being |
| Secondary outcome measure(s): QoL, coping, self‐management behaviour, quality of care | Secondary outcome measure(s): — | |||
| Other outcome measure(s): | Other outcome measure(s): cognitive and behavioural coping, physical activity, dietary habits, QoL, depression, DRD | |||
| Van Dijk‐de Vries 2015 |
Source: NTR2764 Primary outcome measure(s): daily functioning as measured by means of the Daily Functioning Thermometer, a visual analogue scale; distress scale of the 4DSQ to assess changes in distress symptoms |
No (accessed 28 January 2016) | Primary outcome measure(s): daily functioning as measured by means of the Daily Functioning Thermometer |
Primary outcome measure(s): visual analogue scale of diabetes on daily functioning Secondary outcome measure(s): — Other outcome measure(s): diabetes‐related distress, quality of life, autonomy and participation, self‐efficacy, self‐management and glycaemic control |
| Secondary outcome measure(s): diabetes‐related emotional distress; the presence and severity of other mental health problems; participation and autonomy; quality of life; self‐efficacy; HbA1c; participant assessment of chronic illness Care; healthcare utilisation | Secondary outcome measure(s): DRD, participation and autonomy, self‐management knowledge and behaviours, QoL, self‐efficacy, HbA1c | |||
| Weinger 2011 |
Source: NCT00142922 Primary outcome measure(s): self‐care behaviours, glycaemic control (HbA1c), fitness |
No (accessed 28 January 2016) | Primary outcome measure(s): HbA1c |
Primary outcome measure(s): HbA1c Secondary outcome measure(s): frequency of diabetes self‐care, 3‐day pedometer readings, 24‐hour diet recalls, average number of glucose checks, physical fitness, depression, coping style, self‐efficacy, and quality of life Other outcome measure(s): — |
| Secondary outcome measure(s): QoL, diabetes‐related emotional distress | Secondary outcome measure(s): diabetes self‐care behaviours, physical fitness, DRD, depression and anxiety symptoms, controlled coping styles, diabetes‐specific self‐efficacy, frustration with self‐care, and diabetes QoL | |||
| Welch 2015 |
Source: NCT02156037 Primary outcome measure(s): HbA1c |
Yes (Welch 2015) | Primary outcome measure(s): percentage of participants achieving good blood glucose control (i.e. HbA1c < 7% (53 mmol/mol)), BP, BMI, hypoglycaemia |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): HbA1c, diabetes distress and social distress |
| Secondary outcome measure(s): diabetes distress, depression | Secondary outcome measure(s): diabetes distress, social distress, and depression | |||
| Whittemore 2004 | Source: none | Primary outcome measure(s): — |
Primary outcome measure(s): — Secondary outcome measure(s): — Other outcome measure(s): diet self‐management, diabetes‐related distress, integration and satisfaction with care, exercise self‐management and BMI; A1c levels |
|
| Secondary outcome measure(s): — | ||||
| Other outcome measure(s): HbA1C, BMI, self‐management (diet and exercise), DRD, integration and treatment satisfaction | ||||
|
aTrial document(s) refers to all available information from published design papers and sources other than regular publications (e.g. FDA/EMA documents, manufacturer's websites, trials register records).
bPublication(s) refers to trial information published in scientific journals (primary reference, duplicate publications, companion documents or multiple reports of a primary study). —: not reported 4DSQ: four dimensional symptom questionnaire; ACE: angiotensin‐converting enzyme; ARB: angiotensin II receptor blockers; BP: blood pressure; BMI: body mass index; DRD: diabetes‐related distress; EMA: European Medicines Agency; FDA: Food and Drug Administration (US); HbA1c: glycosylated haemoglobin; HCCQ: the Health Care Climate Questionnaire; LDL: low density lipoprotein; NA: not applicable; PAID: Problem Areas in Diabetes; PCDS: Perceived Competence for Diabetes Scale; QoL: quality of life; SBP: systolic blood pressure; SE: self‐efficacy; T2DM: type 2 diabetes mellitus; TSRQ: the Treatment Self‐Regulation Questionnaire. | ||||
Appendix 6. High risk of outcome reporting bias according to ORBIT classification
| Trial | Outcome | High risk of bias (category A)a | High risk of bias (category D)b | High risk of bias (category E)c | High risk of bias (category G)d |
| Beverly 2013 | NA | ||||
| Davies 2008 | NA | ||||
| Dennick 2015 | NA | ||||
| D'Eramo Melkus 2010 | BP, QoL, SE | BP, QoL, SE | — | — | — |
| Fisher 2011 | QoL, SE | — | — | QoL, SE | — |
| Fisher 2013 | BP | — | — | BP | — |
| Gabbay 2013 | NA | ||||
| Glasgow 2005 | NA | ||||
| Grillo 2016 | NA | ||||
| Hermanns 2012 | NA | ||||
| Hermanns 2015 | NA | ||||
| Lamers 2011 | SE | — | — | SE | — |
| Lerman 2009 | NA | ||||
| Liu 2015 | NA | ||||
| Pibernik‐Okanovic 2015 | NA | ||||
| Quinn 2011 | SE, DRD, BP | DRD, BP | — | SE | — |
| Rosenbek 2011 | NA | ||||
| Shibayama 2007 | NA | ||||
| Simmons 2015 | NA | ||||
| Skelly 2009 | NA | ||||
| Spencer 2013 | BP | — | — | BP | — |
| Sperl‐Hillen 2013 | NA | ||||
| Sturt 2008 | NA | ||||
| Taylor 2006 | NA | ||||
| Trief 2016 | NA | ||||
| Van der Wulp 2012 | NA | ||||
| Van Dijk‐de Vries 2015 | NA | ||||
| Weinger 2011 | NA | ||||
| Welch 2015 | NA | ||||
| Whittemore 2004 | NA | ||||
|
aClear that outcome was measured and analysed; trial report states that outcome was analysed but only reports that result was not significant (classification 'A', table 2, Kirkham 2010).
bClear that outcome was measured and analysed; trial report states that outcome was analysed but no results reported (classification 'D', table 2, Kirkham 2010).
cClear that outcome was measured; clear that outcome was measured but not necessarily analysed; judgement says likely to have been analysed but not reported because of non‐significant results (classification 'E', table 2, Kirkham 2010).
dUnclear whether the outcome was measured; not mentioned but clinical judgement says likely to have been measured and analysed but not reported on the basis of non‐significant results (classification 'G', table 2, Kirkham 2010). —: not reported BP: blood pressure; DRD: diabetes‐related distress; NA: not applicable; ORBIT: Outcome Reporting Bias In Trials; QoL: quality of life; SE: self‐efficacy | |||||
Appendix 7. Definition of endpoint measurement (I)a
| Trial | All‐cause mortality | Blood pressure (mmHg) | Diabetes‐related complications | Diabetes‐related distress | HbA1c | Health‐related quality of life | Self‐efficacy | Socioeconomic effects |
| Beverly 2013 | NI | NI | NI | SO (PAID) | IO | SO (DQOL) | SO (CIDS‐2) | NI |
| Davies 2008 | ND | IO | NI | SO (PAID) | IO | SO (WHOQOL‐BREF) | NI | NI |
| Dennick 2015 | NI | NI | NI | SO (PAID) | NI | NI (EQ‐5D) (at 3 months follow‐up) | NI | NI |
| D'Eramo Melkus 2010 | NI | IO (mercury manometer) | NI | SO (PAID) | IO | SO (SF‐36) | SO (DSEQ) | NI |
| Fisher 2011 | NI | NI | NI | SO (DDS) | IO | NI | NI | NI |
| Fisher 2013 | NI | NI | NI | SO (DDS) | IO | NI | NI | NI |
| Gabbay 2013 | ND | ND | NI | SO (PAID) | ND | SO (ADDQoL) | NI | NI |
| Glasgow 2005 | NI | NI | NI | SO (DDS) | IO | NI | SO (PCS) | NI |
| Grillo 2016 | ND | IO (digital sphygmomanometer) | NI | SO (PAID) | IO | NI | NI | NI |
| Hermanns 2012 | NI | ND | NI | SO (PAID) | IO | SO (SF‐12) | NI | NI |
| Hermanns 2015 | NI | NI | NI | SO (PAID and DDS) | IO | SO (EQ‐5D) | NI | NI |
| Lamers 2011 | ND | NI | NI | SO (PAID) | IO | SO (DSC‐R) | NI | NI |
| Lerman 2009 | NI | NI | NI | SO (PAID) | IO | NI | NI | NI |
| Liu 2015 | NI | IO (collected through clinical information systems) | NI | SO (DDS) | IO | SO (ADDQoL) | NI | NI |
| Pibernik‐Okanovic 2015 | ND | NI | NI | SO (PAID) | IO | SO (SF‐12) | NI | NI |
| Quinn 2011 | NI | IO (obtained from provider medical office records) | NI | SO (DDS) | IO | NI | NI | NI |
| Rosenbek 2011 | NI | ND | NI | SO (PAID) | IO | NI | SO (PCDS) | NI |
| Shibayama 2007 | NI | NI | NI | SO (PAID) | ND | SO (SF‐36) | NI | NI |
| Simmons 2015 | NI | IO (standardised methodology/ equipment) | NI | SO (DDS‐4) | IO | SO (EQ‐5D and WHO‐5 Well‐being Index) | SO (DSE‐8) | NI |
| Skelly 2009 | ND | NI | NI | SO (PAID) | IO | SO (Diabetes‐related Quality of life) | NI | NI |
| Spencer 2013 | NI | NI | NI | SO (PAID) | IO | NI | NI | NI |
| Sperl‐Hillen 2013 | ND | NI | NI | SO (PAID) | IO | NI | SO (DES‐SF) | NI |
| Sturt 2008 | ND | NI | NI | SO (PAID) | ND | NI | SO (DMSES) | NI |
| Taylor 2006 | NI | NI | NI | SO (PAID) | ND | SO (WBQ‐12) | NI | NI |
| Trief 2016 | NI | IO (automated) | NI | SO (DDS) | IO | NI | SO (DSE‐8) | NI |
| Van der Wulp 2012 | NI | NI | NI | SO (PAID) | NI | SO (WHO‐5 Well‐being Index) | SO (DMSES) | NI |
| Van Dijk‐de Vries 2015 | NI | NI | NI | SO (PAID) | IO | SO (SF‐12) | SO (GSES‐12) | NI |
| Weinger 2011 | NI | ND | NI | SO (PAID) | IO | SO (DQOL) | SO (CIDS‐2) | NI |
| Welch 2015 | NI | IO (automatic digital BP monitor (Omron model HEM‐705CP)) | NI | SO (PAID) | IO | NI | NI | NI |
| Whittemore 2004 | NI | NI | NI | SO (PAID) | IO | NI | NI | NI |
|
aIn addition to definition of endpoint measurement, description of who measured the outcome (AO: adjudicated outcome measurement; IO: investigator‐assessed outcome measurement;SO: self‐reported outcome measurement). ADDQoL: audit of diabetes dependent quality of life; CIDS‐2: confidence in diabetes self‐care scale; DDS: diabetes distress scale; DES‐SF: diabetes empowerment scale — short form; DMSES: diabetes management self‐efficacy scale; DQOL: diabetes quality of life scale; DSC‐R: diabetes symptom checklist — revised; DSEQ: diabetes self‐efficacy outcome expectancies questionnaire; GSES‐12: General Self‐Efficacy Scale; HbA1c: glycosylated haemoglobin A1c; ND: not defined; NI: not investigated; PAID: problem areas in diabetes; PCS: perceived competence scale; SF: short‐form health survey; WBQ‐12: well‐being questionnaire; WHO World Health Organization | ||||||||
Appendix 8. Definition of endpoint measurement (II)a
| Trial | All hypoglycaemic events | Severe/serious hypoglycaemia | Nocturnal hypoglycaemia | Severe/serious adverse events |
| Beverly 2013 | NI | NI | NI | NI |
| Davies 2008 | NI | NI | NI | NI |
| Dennick 2015 | NI | NI | NI | ND |
| D'Eramo Melkus 2010 | NI | NI | NI | NI |
| Fisher 2011 | IO (< 70 mg/dL or 3.9 mmol/L, based on downloaded meter data) | NI | NI | IO |
| Fisher 2013 | NI | NI | NI | NI |
| Gabbay 2013 | NI | NI | NI | NI |
| Glasgow 2005 | NI | NI | NI | NI |
| Grillo 2016 | NI | NI | NI | NI |
| Hermanns 2012 | NI | NI | NI | NI |
| Hermanns 2015 | NI | NI | NI | NI |
| Lamers 2011 | SO | NI | NI | ND |
| Lerman 2009 | NI | NI | NI | NI |
| Liu 2015 | NI | NI | NI | NI |
| Pibernik‐Okanovic 2015 | NI | NI | NI | ND |
| Quinn 2011 | SO (through quarterly telephone calls to patients) | NI | NI | SO |
| Rosenbek 2011 | NI | NI | NI | NI |
| Shibayama 2007 | NI | NI | NI | NI |
| Simmons 2015 | NI | NI | NI | NI |
| Skelly 2009 | NI | NI | NI | NI |
| Spencer 2013 | NI | NI | NI | NI |
| Sperl‐Hillen 2013 | NI | NI | NI | NI |
| Sturt 2008 | NI | NI | NI | NI |
| Taylor 2006 | NI | NI | NI | ND |
| Trief 2016 | NI | NI | NI | NI |
| Van der Wulp 2012 | NI | NI | NI | NI |
| Van Dijk‐de Vries 2015 | NI | NI | NI | NI |
| Weinger 2011 | ND | SO | ND | ND |
| Welch 2015 | SO (hypoglycaemia was defined in the Diabetes Self‐Care Profile as any "low blood sugars or sweating, nausea, heart pounding, trembling,cold and clammy skin, difficulty concentrating, and irritability" over the past month) | SO | NI | NI |
| Whittemore 2004 | NI | NI | NI | NI |
|
aIn addition to definition of endpoint measurement, description of who measured the outcome (AO: adjudicated outcome measurement; IO: investigator‐assessed outcome measurement;SO: self‐reported outcome measurement) ND: not defined; NI: not investigated | ||||
Appendix 9. Adverse events (I)
| Trial | Main component of psychological intervention (type of intervention) | Participants included in analysis (N) | Deaths (N) | Deaths (% of participants) | Participants with at least one adverse event (N) | Participants with at least one adverse event (%) | Participants with at least one severe/serious adverse event (N) | Participants with at least one severe/serious adverse event (%) |
| Beverly 2013 | I: cognition focused (group education) |
67 | — | — | — | — | — | — |
| C: enhanced usual care (educational classes not focusing on diabetes care) |
67 | — | — | — | — | — | — | |
| Davies 2008 | I: cognition focused (group education) |
437 | 2 | 0.005 | — | — | — | — |
| C: enhanced usual care (additional contact time with healthcare professionals) |
387 | 5 | 0.01 | — | — | — | — | |
| Dennick 2015 | I: emotion focused (writing about different aspects of life, thoughts and feelings) |
23 | — | — | 1 | 0.04 | — | — |
| C: cognition focused (writing about previous days' activities) |
18 | — | — | — | — | — | — | |
| D'Eramo Melkus 2010 | I: emotion‐cognition components (cognitive behavioural self‐management training) |
40 | — | — | — | — | — | — |
| C: cognition focused (group education) |
37 | — | — | — | — | — | — | |
| Fisher 2011 | I: cognition focused (self‐monitoring of blood glucose) |
256 | — | — | — | 1.8a | — | — |
| C: enhanced usual care (additional quarterly diabetes‐focused physician visits) |
227 | — | — | — | 1.9a | — | — | |
| Fisher 2013 | I1: cognition focused (computer‐assisted self‐management) |
150 | — | — | — | — | — | — |
| I2: emotion‐cognition components (computer‐assisted self‐management + problem solving) |
146 | — | — | — | — | — | — | |
| C: cognition focused (general diabetes support and education) |
96 | — | — | — | — | — | — | |
| Gabbay 2013 | I: cognition focused (motivational interviewing) |
232 | 4 | 1.7 | — | — | — | — |
| C: usual care (standard diabetes care) |
313 | 1 | 0.3 | — | — | — | — | |
| Glasgow 2005 | I: cognition focused (computer‐assisted self‐management) |
469 | — | — | — | — | — | — |
| C: enhanced usual care (computer information without self‐management) |
417 | — | — | — | — | — | — | |
| Grillo 2016 | I: cognition focused (self‐management education) |
67 | 1 | 1.5 | — | — | — | — |
| C: enhanced usual care (group meetings without education) |
60 | 1 | 1.6 | — | — | — | — | |
| Hermanns 2012 | I: emotion‐cognition components (self‐management programme) |
94 | — | — | — | — | — | — |
| C: cognition focused (combination of 2 education programmes) |
92 | — | — | — | — | — | — | |
| Hermanns 2015 | I: emotion‐cognition components (cognitive behaviour treatment) |
93 | — | — | — | — | — | — |
| C: cognition focused (group education) |
88 | — | — | — | — | — | — | |
| Lamers 2011 | I: emotion‐cognition components (cognitive behaviour therapy) |
105 | 0 | 0 | 14 | 13.3 | — | — |
| C: usual care (standard diabetes care) |
103 | 3 | 2.9 | 3 | 2.9 | — | — | |
| Lerman 2009 | I1: cognition focused (telephone contacts) |
18 | — | — | — | — | — | — |
| I2: cognition focused (group‐based education) | 24 | — | — | — | — | — | — | |
| C: usual care (standard diabetes care) |
17 | — | — | — | — | — | — | |
| Liu 2015 | I: emotion‐cognition components (peer education) |
63 | — | — | — | — | — | — |
| C: cognition focused (diabetes health education) |
64 | — | — | — | — | — | — | |
| Pibernik‐Okanovic 2015 | I1: emotion‐cognition components (psycho‐educational intervention) | 65 | 0 | 0 | 1 | 1.5 | — | — |
| I2: cognition focused (physical activity intervention) |
61 | 2 | 3.3 | 2 | 3.3 | — | — | |
| C1: emotion‐cognition components (enhanced usual diabetes care) |
62 | 1 | 1.6 | 1 | 1.6 | — | — | |
| Quinn 2011 | I1: cognition focused (coach + mobile diabetes management software) |
23 | 0 | 0 | 0 | 0 | 0 | 0 |
| I2: cognition focused (coach + mobile diabetes management software + Internet portal) | 22 | 0 | 0 | 0 | 0 | 0 | 0 | |
| I3: cognition focused (coach + mobile diabetes management software + Internet portal + decision support) | 62 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C: usual care (standard diabetes care) |
56 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Rosenbek 2011 | I: emotion‐cognition components (motivational interviewing) |
145 | — | — | 0 | 0 | 2 | 1.4 |
| C: usual care (standard diabetes care) |
153 | — | — | 0 | 0 | 4 | 2.6 | |
| Shibayama 2007 | I: emotion‐cognition components (behavioural counselling) |
67 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
67 | — | — | — | — | — | — | |
| Simmons 2015 | I1: emotion‐cognition components (group peer support) | 272 | — | — | — | — | — | — |
| I2: emotion‐cognition components (group and individual support) | 245 | — | — | — | — | — | — | |
| I3: emotion focused (individual peer support) |
264 | — | — | — | — | — | — | |
| C: usual care (standard diabetes care) |
283 | — | — | — | — | — | — | |
| Skelly 2009 | I1: cognition focused (symptom‐focused) | 60 | 3 | 5 | — | — | — | — |
| I2: cognition focused (symptom‐focused with telephone booster) | 55 | 2 | 3.6 | — | — | — | — | |
| C: enhanced usual care (weight and diet programme) |
59 | 2 | 3.4 | — | — | — | — | |
| Spencer 201 | I: emotion‐cognition components (community health worker intervention) |
72 | — | — | — | — | — | — |
| C: waiting list or usual care (information on community activities) |
92 | — | — | — | — | — | — | |
| Sperl‐Hillen 2013 | I1: cognition focused (individual education) |
246 | 4 | 1.6 | — | — | — | — |
| I2: cognition focused (group education) |
243 | 2 | 0.8 | — | — | — | — | |
| C: usual care (standard diabetes care) |
134 | 2 | 1.5 | — | — | — | — | |
| Sturt 2008 | I: emotion‐cognition components (diabetes manual structured education) |
88 | 1 | 1.1 | — | — | — | — |
| C: waiting list or usual care (standard diabetes care) |
114 | 1 | 0.9 | — | — | — | — | |
| Taylor 2006 | I1: emotion‐cognition components (cognitive‐behavioural therapy) |
26 | — | — | — | — | — | — |
| I2: emotion‐cognition components (expressive writing) |
23 | — | — | — | — | — | — | |
| C: waiting list or usual care (standard diabetes care) |
18 | — | — | — | — | — | — | |
| Trief 2016 | I1: emotion‐cognition components (behaviour change intervention, couples) |
97 | — | — | — | — | — | — |
| I2: emotion‐cognition components (behaviour change intervention, individuals) |
93 | — | — | — | — | — | — | |
| C: cognition focused (individual diabetes education) |
78 | — | — | — | — | — | — | |
| Van der Wulp 2012 | I: cognition focused (peer‐led self‐management coaching programme) |
59 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
60 | — | — | — | — | — | — | |
| Van Dijk‐de Vries 2015 | I: emotion‐cognition components (self‐management support in routine care) |
117 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
147 | — | — | — | — | — | — | |
| Weinger 2011 | I1: emotion‐cognition components (behavioural strategies) | 74 | — | — | 0 | 0 | 0 | 0 |
| C1: cognition focused (group attention) |
75 | — | — | 0 | 0 | 0 | 0 | |
| C2: cognition focused (individual attention) |
73 | — | — | 0 | 0 | 0 | 0 | |
| Welch 2015 | I: emotion‐cognition components (one‐to‐one diabetes education) |
199 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
200 | — | — | — | — | — | — | |
| Whittemore 2004 | I: emotion‐cognition components (nurse coaching) |
31 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
22 | — | — | — | — | — | — | |
| —: not reported aIncidence of hypoglycaemia (< 70 mg/dL or 3.9 mmol/L), based on downloaded meter data C: comparator; I: intervention | ||||||||
Appendix 10. Adverse events (II)
| Trial | Main component of psychological intervention (type of intervention) | Participants included in analysis (N) | Participants discontinuing trial due to an adverse event (N) | Participants discontinuing trial due to an adverse event (%) | Participants with at least one hospitalisation (N) | Participants with at least one hospitalisation (%) | Participants with at least one outpatient treatment (N) | Participants with at least one outpatient treatment (%) |
| Beverly 2013 | I: cognition focused (group education) |
67 | — | — | — | — | — | — |
| C: enhanced usual care (educational classes not focusing on diabetes care) |
67 | — | — | — | — | — | — | |
| Davies 2008 | I: cognition focused (group education) |
437 | — | — | — | — | — | — |
| C: enhanced usual care (additional contact time with healthcare professionals) |
387 | — | — | — | — | — | — | |
| Dennick 2015 | I: emotion focused (writing about different aspects of life, thoughts and feelings) |
23 | — | — | — | — | — | — |
| C: cognition focused (writing about previous days' activities) |
18 | — | — | — | — | — | — | |
| D'Eramo Melkus 2010 | I: emotion‐cognition components (cognitive behavioural self‐management training) |
40 | — | — | — | — | — | — |
| C: cognition focused (group education) |
37 | — | — | — | — | — | — | |
| Fisher 2011 | I: cognition focused (self‐monitoring of blood glucose) |
256 | — | — | — | — | — | — |
| C: enhanced usual care (additional quarterly diabetes‐focused physician visits) |
227 | — | — | — | — | — | — | |
| Fisher 2013 | I1: cognition focused (computer‐assisted self‐management) |
150 | — | — | — | — | — | — |
| I2: emotion‐cognition components (computer‐assisted self‐management + problem solving) |
146 | — | — | — | — | — | — | |
| C: cognition focused (general diabetes support and education) |
96 | — | — | — | — | — | — | |
| Gabbay 2013 | I: cognition focused (motivational interviewing) |
232 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
313 | — | — | — | — | — | — | |
| Glasgow 2005 | I: cognition focused (computer‐assisted self‐management) |
469 | — | — | — | — | — | — |
| C: enhanced care (computer information without self‐management) |
417 | — | — | — | — | — | — | |
| Grillo 2016 | I: cognition focused (self‐management education) |
67 | — | — | — | — | — | — |
| C: enhanced usual care (group meetings without education) |
60 | — | — | — | — | — | — | |
| Hermanns 2012 | I: emotion‐cognition components (self‐management programme) |
94 | — | — | — | — | — | — |
| C: cognition focused (combination of 2 education programmes) |
92 | — | — | — | — | — | — | |
| Hermanns 2015 | I: emotion‐cognition components (cognitive behaviour treatment) |
93 | — | — | — | — | — | — |
| C: cognition focused (group education) |
88 | — | — | — | — | — | — | |
| Lamers 2011 | I: emotion‐cognition components (cognitive behaviour therapy) |
105 | 7 | 6.7 | 2 | 1.9 | — | — |
| C: usual care (standard diabetes care) |
103 | 3 | 2.9 | 6 | 5.8 | — | — | |
| Lerman 2009 | I1: cognition focused (telephone contacts) |
18 | — | — | — | — | — | — |
| I2: cognition focused (group‐based education) | 24 | — | — | — | — | — | — | |
| C: usual care (standard diabetes care) |
17 | — | — | — | — | — | — | |
| Liu 2015 | I: emotion‐cognition components (peer education) |
63 | — | — | — | — | — | — |
| C: cognition focused (diabetes health education) |
64 | — | — | — | — | — | — | |
| Pibernik‐Okanovic 2015 | I1: emotion‐cognition components (psycho‐educational intervention) | 65 | — | — | — | — | — | — |
| I2: cognition focused (physical activity intervention) |
61 | — | — | — | — | — | — | |
| C1: emotion‐cognition components (enhanced diabetes care) |
62 | — | — | — | — | — | — | |
| Quinn 2011 | I1: cognition focused (coach + mobile diabetes management software) |
23 | — | — | — | — | — | — |
| I2: cognition focused (coach + mobile diabetes management software + Internet portal) | 22 | — | — | — | — | — | — | |
| I3: cognition focused (coach + mobile diabetes management software + Internet portal + decision support) | 62 | — | — | 1 (twice) | 1.6 | — | — | |
| C: usual care (standard diabetes care) |
56 | — | — | — | — | — | — | |
| Rosenbek 2011 | I: emotion‐cognition components (motivational interviewing) |
145 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
153 | — | — | — | — | — | — | |
| Shibayama 2007 | I: emotion‐cognition components (behavioural counselling) |
67 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
67 | — | — | — | — | — | — | |
| Simmons 2015 | I1: emotion‐cognition components (group peer support) | 272 | — | — | — | — | — | — |
| I2: emotion‐cognition components (group and individual support) | 245 | — | — | — | — | — | — | |
| I3: emotion focused (individual peer support) |
264 | — | — | — | — | — | — | |
| C: usual care (standard diabetes care) |
283 | — | — | — | — | — | — | |
| Skelly 2009 | I1: cognition focused (symptom‐focused) | 60 | — | — | — | — | — | — |
| I2: cognition focused (symptom‐focused with telephone booster) | 55 | — | — | — | — | — | — | |
| C: enhanced usual care (weight and diet programme) |
59 | — | — | — | — | — | — | |
| Spencer 2013 | I: emotion‐cognition components (community health worker intervention) |
72 | — | — | — | — | — | — |
| C: waiting list or usual care (information on community activities) |
92 | — | — | — | — | — | — | |
| Sperl‐Hillen 2013 | I1: cognition focused (individual education) |
246 | — | — | — | — | — | — |
| I2: cognition focused (group education) |
243 | — | — | — | — | — | — | |
| C: usual care (standard diabetes care) |
134 | — | — | — | — | — | — | |
| Sturt 2008 | I: emotion‐cognition components (diabetes manual structured education) |
88 | — | — | — | — | — | — |
| C: waiting list or usual care (standard diabetes care) |
114 | — | — | — | — | — | — | |
| Taylor 2006 | I1: emotion‐cognition components (cognitive‐behavioural therapy) |
26 | — | — | 3 | 11.5 | — | — |
| I2: emotion‐cognition components (expressive writing) |
23 | — | — | — | — | — | — | |
| C: waiting list or usual care (standard diabetes care) |
18 | — | — | — | — | — | — | |
| Trief 2016 | I1: emotion‐cognition components (behaviour change intervention, couples) |
97 | — | — | — | — | — | — |
| I2: emotion‐cognition components (behaviour change intervention, individuals) |
93 | — | — | — | — | — | — | |
| C: cognition focused (individual diabetes education) |
78 | — | — | — | — | — | — | |
| Van der Wulp 2012 | I: cognition focused (peer‐led self‐management coaching programme) |
59 | — | — | — | — | — | — |
| C: standard care standard diabetes care) |
60 | — | — | — | — | — | — | |
| Van Dijk‐de Vries 2015 | I: emotion‐cognition components (self‐management support in routine care) |
117 | — | — | — | — | — | — |
| C: standard care (standard diabetes care) |
147 | — | — | — | — | — | — | |
| Weinger 2011 | I1: emotion‐cognition components (behavioural strategies) | 74 | — | — | — | — | — | — |
| C1: cognition focused (group attention) |
75 | — | — | — | — | — | — | |
| C2: cognition focused (individual attention) |
73 | — | — | — | — | — | — | |
| Welch 2015 | I: emotion‐cognition components (one‐to‐one diabetes education) |
199 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
200 | — | — | — | — | — | — | |
| Whittemore 2004 | I: emotion‐cognition components (nurse coaching) |
31 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
22 | — | — | — | — | — | — | |
| —: not reported C: comparator; I: intervention | ||||||||
Appendix 11. Adverse events (III)
| Trial | Main component of psychological intervention (type of intervention) | Participants included in analysis (N) | Participants with a specific adverse event (description) | Participants with at least one specific adverse events (N) | Participants with at least one specific adverse event (%) |
| Beverly 2013 | I: cognition focused (group education) |
67 | — | — | — |
| C: enhanced usual care (educational classes not focusing on diabetes care) |
67 | — | — | — | |
| Davies 2008 | I: cognition focused (group education) |
437 | — | — | — |
| C: enhanced usual care (additional contact time with healthcare professionals) |
387 | — | — | — | |
| Dennick 2015 | I: emotion focused (writing about different aspects of life, thoughts and feelings) |
23 | ‘Worried/stressed about what to write' | 1 | 4.3 |
| C: cognition focused (writing about previous days' activities) |
18 | — | — | — | |
| D'Eramo Melkus 2010 | I: emotion‐cognition components (cognitive behavioural self‐management training) |
40 | — | — | — |
| C: cognition focused (group education) |
37 | — | — | — | |
| Fisher 2011 | I: cognition focused (self‐monitoring of blood glucose) |
256 | No intervention‐related adverse events | 0 | 0 |
| C: enhanced usual care (additional quarterly diabetes‐focused physician visits) |
227 | No intervention‐related adverse events | 0 | 0 | |
| Fisher 2013 | I1: cognition focused (computer‐assisted self‐management) |
150 | — | — | — |
| I2: emotion‐cognition components (computer‐assisted self‐management + problem solving) |
146 | — | — | — | |
| C: cognition focused (general diabetes support and education) |
96 | — | — | — | |
| Gabbay 2013 | I: cognition focused (motivational interviewing) |
232 | — | — | — |
| C: usual care (standard diabetes care) |
313 | — | — | — | |
| Glasgow 2005 | I: cognition focused (computer‐assisted self‐management) |
469 | — | — | — |
| C: enhanced usual care (computer information without self‐management) |
417 | — | — | — | |
| Grillo 2016 | I: cognition focused (self‐management education) |
67 | — | — | — |
| C: enhanced usual care (group meetings without education) |
60 | — | — | — | |
| Hermanns 2012 | I: emotion‐cognition components (self‐management programme) |
94 | — | — | — |
| C: cognition focused (combination of 2 education programmes) |
92 | — | — | — | |
| Hermanns 2015 | I: emotion‐cognition components (cognitive behaviour treatment) |
93 | — | — | — |
| C: cognition focused (group education) |
88 | — | — | — | |
| Lamers 2011 | I: emotion‐cognition components (cognitive behaviour therapy) |
105 | Perceived questionnaire to be burdensome | 7 | 6.7 |
| C: usual care (standard diabetes care) |
103 | Questionnaire burdensome | 3 | 2.9 | |
| Lerman 2009 | I1: cognition focused (telephone contacts) |
18 | — | — | — |
| I2: cognition focused (group‐based education) | 24 | — | — | — | |
| C: usual care (standard diabetes care) |
17 | — | — | — | |
| Liu 2015 | I: emotion‐cognition components (peer education) |
63 | — | — | — |
| C: cognition focused (diabetes health education) |
64 | — | — | — | |
| Pibernik‐Okanovic 2015 | I1: emotion‐cognition components (psycho‐educational intervention) | 65 | — | — | — |
| I2: cognition focused (physical activity intervention) |
61 | — | — | — | |
| C1: emotion‐cognition components (enhanced diabetes care) |
62 | — | — | — | |
| Quinn 2011 | I1: cognition focused (coach + mobile diabetes management software) |
23 | — | — | — |
| I2: cognition focused (coach + mobile diabetes management software + Internet portal) | 22 | — | — | — | |
| I3: cognition focused (coach + mobile diabetes management software + Internet portal + decision support) | 62 | — | — | — | |
| C: usual care (standard diabetes care) |
56 | — | — | — | |
| Rosenbek 2011 | I: emotion‐cognition components (motivational interviewing) |
145 | — | — | — |
| C: usual care (standard diabetes care) |
153 | — | — | — | |
| Shibayama 2007 | I: emotion‐cognition components (behavioural counselling) |
67 | — | — | — |
| C: usual care (standard diabetes care) |
67 | — | — | — | |
| Simmons 2015 | I1: emotion‐cognition components (group peer support) | 272 | — | — | — |
| I2: emotion‐cognition components (group and individual support) | 245 | — | — | — | |
| I3: emotion focused (individual peer support) |
264 | — | — | — | |
| C: usual care (standard diabetes care) |
283 | — | — | — | |
| Skelly 2009 | I1: cognition focused (symptom‐focused) | 60 | — | — | — |
| I2: cognition focused (symptom‐focused with telephone booster) | 55 | Depressed | 1 | 1.8 | |
| C: enhanced care (weight and diet programme) |
59 | — | — | — | |
| Spencer 2013 | I: emotion‐cognition components (community health worker intervention) |
72 | — | — | — |
| C: waiting list or usual care (information on community activities) |
92 | — | — | — | |
| Sperl‐Hillen 2013 | I1: cognition focused (individual education) |
246 | — | — | — |
| I2: cognition focused (group education) |
243 | — | — | — | |
| C: usual care (standard diabetes care) |
134 | — | — | — | |
| Sturt 2008 | I: emotion‐cognition components (diabetes manual structured education) |
88 | — | — | — |
| C: waiting list or standard care (standard diabetes care) |
114 | — | — | — | |
| Taylor 2006 | I1: emotion‐cognition components (cognitive‐behavioural therapy) |
26 | 'Distinct dislike' | 1 | 5.6 |
| I2: emotion‐cognition components (expressive writing) |
23 | Crying | 1 | 4.3 | |
| C: waiting list or usual care (standard diabetes care) |
18 | — | — | — | |
| Trief 2016 | I1: emotion‐cognition components (behaviour change intervention, couples) |
97 | — | — | — |
| I2: emotion‐cognition components (behaviour change intervention, individuals) |
93 | — | — | — | |
| C: cognition focused (individual diabetes education) |
78 | — | — | — | |
| Van der Wulp 2012 | I: cognition focused (peer‐led self‐management coaching programme) |
59 | — | — | — |
| C: usual care standard diabetes care) |
60 | — | — | — | |
| Van Dijk‐de Vries 2015 | I: emotion‐cognition components (self‐management support in routine care) |
117 | — | — | — |
| C: usual care (standard diabetes care) |
147 | — | — | — | |
| Weinger 2011 | I1: emotion‐cognition components (behavioural strategies) | 74 | — | — | — |
| C1: cognition focused (group attention) |
75 | — | — | — | |
| C2: cognition focused (individual attention) |
73 | — | — | — | |
| Welch 2015 | I: emotion‐cognition components (one‐to‐one diabetes education) |
199 | — | — | — |
| C: usual care (standard diabetes care) |
200 | — | — | — | |
| Whittemore 2004 | I: emotion‐cognition components (nurse coaching) |
31 | — | — | — |
| C: usual care (standard diabetes care) |
22 | — | — | — | |
| —: not reported C: comparator; I: intervention | |||||
Appendix 12. Adverse events (IV)
| Study | Main component of psychological intervention (type of intervention) | Participants included in analysis (N) | Participants with at least one hypoglycaemic episode (N) | Participants with at least one hypoglycaemic episode (%) | Participants with at least one nocturnal hypoglycaemic episode (N) | Participants with at least one nocturnal hypoglycaemic episode (% participants) | Participants with at least one severe/serious hypoglycaemic episode (N) | Participants with at least one severe/serious hypoglycaemic episode (%) |
| Beverly 2013 | I: cognition focused (group education) |
67 | — | — | — | — | — | — |
| C: enhanced usual care (educational classes not focusing on diabetes care) |
67 | — | — | — | — | — | — | |
| Davies 2008 | I: cognition focused (group education) |
437 | — | — | — | — | — | — |
| C: enhanced usual care (additional contact time with healthcare professionals) |
387 | — | — | — | — | — | — | |
| Dennick 2015 | I: emotion focused (writing about different aspects of life, thoughts and feelings) |
23 | — | — | — | — | — | — |
| C: cognition focused (writing about previous days' activities) |
18 | — | — | — | — | — | — | |
| D'Eramo Melkus 2010 | I: emotion‐cognition components (cognitive behavioural self‐management training) |
40 | — | — | — | — | — | — |
| C: cognition focused (group education) |
37 | — | — | — | — | — | — | |
| Fisher 2011 | I: cognition focused (self‐monitoring of blood glucose) |
256 | — | 1.8a | — | — | — | — |
| C: enhanced usual care (additional quarterly diabetes‐focused physician visits) |
227 | — | 1.9a | — | — | — | — | |
| Fisher 2013 | I1: cognition focused (computer‐assisted self‐management) |
150 | — | — | — | — | — | — |
| I2: emotion‐cognition components (computer‐assisted self‐management + problem solving) |
146 | — | — | — | — | — | — | |
| C: cognition focused (general diabetes support and education) |
96 | — | — | — | — | — | — | |
| Gabbay 2013 | I: cognition focused (motivational interviewing) |
232 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
313 | — | — | — | — | — | — | |
| Glasgow 2005 | I: cognition focused (computer‐assisted self‐management) |
469 | — | — | — | — | — | — |
| C: enhanced usual care (computer information without self‐management) |
417 | — | — | — | — | — | — | |
| Grillo 2016 | I: cognition focused (self‐management education) |
67 | — | — | — | — | — | — |
| C: enhanced usual care (group meetings without education) |
60 | — | — | — | — | — | — | |
| Hermanns 2012 | I: emotion‐cognition components (self‐management programme) |
94 | — | — | — | — | — | — |
| C: cognition focused (combination of 2 education programmes) |
92 | — | — | — | — | — | — | |
| Hermanns 2015 | I: emotion‐cognition components (cognitive behaviour treatment) |
93 | — | — | — | — | — | — |
| C: cognition focused (group education) |
88 | — | — | — | — | — | — | |
| Lamers 2011 | I: emotion‐cognition components (cognitive behaviour therapy) |
105 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
103 | — | — | — | — | — | — | |
| Lerman 2009 | I1: cognition focused (telephone contacts) |
18 | — | — | — | — | — | — |
| I2: cognition focused (group‐based education) | 24 | — | — | — | — | — | — | |
| C: usual care (standard diabetes care) |
17 | — | — | — | — | — | — | |
| Liu 2015 | I: emotion‐cognition components (peer education) |
63 | — | — | — | — | — | — |
| C: cognition focused (diabetes health education) |
64 | — | — | — | — | — | — | |
| Pibernik‐Okanovic 2015 | I1: emotion‐cognition components (psycho‐educational intervention) | 65 | — | — | — | — | — | — |
| I2: cognition focused (physical activity intervention) |
61 | — | — | — | — | — | — | |
| C1: emotion‐cognition components (enhanced usual diabetes care) |
62 | — | — | — | — | — | — | |
| Quinn 2011 | I1: cognition focused (coach + mobile diabetes management software) |
23 | 0 | 0 | — | — | — | — |
| I2: cognition focused (coach + mobile diabetes management software + Internet portal) | 22 | 0 | 0 | — | — | — | — | |
| I3: cognition focused (coach + mobile diabetes management software + Internet portal + decision support) | 62 | 0 | 0 | — | — | — | — | |
| C: usual care (standard diabetes care) |
56 | 0 | 0 | — | — | — | — | |
| Rosenbek 2011 | I: emotion‐cognition components (motivational interviewing) |
145 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
153 | — | — | — | — | — | — | |
| Shibayama 2007 | I: emotion‐cognition components (behavioural counselling) |
67 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
67 | — | — | — | — | — | — | |
| Simmons 2015 | I1: emotion‐cognition components (group peer support) | 272 | — | — | — | — | — | — |
| I2: emotion‐cognition components (group and individual support) | 245 | — | — | — | — | — | — | |
| I3: emotion focused (individual peer support) |
264 | — | — | — | — | — | — | |
| C: usual care (standard diabetes care) |
283 | — | — | — | — | — | — | |
| Skelly 2009 | I1: cognition focused (symptom‐focused) | 60 | — | — | — | — | — | — |
| I2: cognition focused (symptom‐focused with telephone booster) | 55 | — | — | — | — | — | — | |
| C: enhanced usual care (weight and diet programme) |
59 | — | — | — | — | — | — | |
| Spencer 2013 | I: emotion‐cognition components (community health worker intervention) |
72 | — | — | — | — | — | — |
| C: waiting list or usual care (information on community activities) |
92 | — | — | — | — | — | — | |
| Sperl‐Hillen 2013 | I1: cognition focused (individual education) |
246 | — | — | — | — | — | — |
| I2: cognition focused (group education) |
243 | — | — | — | — | — | — | |
| C: usual care (standard diabetes care) |
134 | — | — | — | — | — | — | |
| Sturt 2008 | I: emotion‐cognition components (diabetes manual structured education) |
88 | — | — | — | — | — | — |
| C: waiting list or usual care (standard diabetes care) |
114 | — | — | — | — | — | — | |
| Taylor 2006 | I1: emotion‐cognition components (cognitive‐behavioural therapy) |
26 | — | — | — | — | — | — |
| I2: emotion‐cognition components (expressive writing) |
23 | — | — | — | — | — | — | |
| C: waiting list or usual care (standard diabetes care) |
18 | — | — | — | — | — | — | |
| Trief 2016 | I1: emotion‐cognition components (behaviour change intervention, couples) |
97 | — | — | — | — | — | — |
| I2: emotion‐cognition components (behaviour change intervention, individuals) |
93 | — | — | — | — | — | — | |
| C: cognition focused (individual diabetes education) |
78 | — | — | — | — | — | — | |
| Van der Wulp 2012 | I: cognition focused (peer‐led self‐management coaching programme) |
59 | — | — | — | — | — | — |
| C: usual care standard diabetes care) |
60 | — | — | — | — | — | — | |
| Van Dijk‐de Vries 2015 | I: emotion‐cognition components (self‐management support in routine care) |
117 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
147 | — | — | — | — | — | — | |
| Weinger 2011 | I1: emotion‐cognition components (behavioural strategies) | 74 | 0 | 0 | 0 | 0 | 0 | 0 |
| C1: cognition focused (group attention) |
75 | 0 | 0 | 0 | 0 | 0 | 0 | |
| C2: cognition focused (individual attention) |
73 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Welch 2015 | I: emotion‐cognition components (one‐to‐one diabetes education) |
172 | 38 | 22b | — | — | — | — |
| C: usual care (standard diabetes care) |
181 | 37 | 20.6b | — | — | — | — | |
| Whittemore 2004 | I: emotion‐cognition components (nurse coaching) |
31 | — | — | — | — | — | — |
| C: usual care (standard diabetes care) |
22 | — | — | — | — | — | — | |
| —: not reported aIncidence of hypoglycaemia (< 70 mg/dL or 3.9 mmol/L), based on downloaded meter data. bOnly percentages were reported. C: comparator; I: intervention | ||||||||
Appendix 13. Survey of study investigators providing information on trials
| Trial | Date trial author contacted | Date trial author replied | Date trial author was asked for additional information (short summary) | Date trial author provided data (short summary) |
| Beverly 2013 | 22 June 2015 | 23 June 2015 | 22 June 2015 How was BP defined and measured? Blinding of the assessor? The actual effect sizes on the self‐efficacy (CIDS‐2) and BP, in mean (SD), at 12‐month postintervention, for both the treatment groups (reported only as no significant differences) |
23 June 2015 Blood pressure measurement was done using the CRC standard protocol (measured after 5 minutes sitting, using two measurements, with equipment calibrated yearly per state regulations) by CRC nurses who were blind to trial assignment and intervention details. Outcome data for CIDS‐2 and BP were provided as requested |
| Dafoulas 2014 | 18 February 2016 | No reply | Trial author was contacted with a request for full text when article with preliminary results was identified (Dafoulas 2014) | NA |
| Davies 2008 | 22 June 2015 | No reply | 22 June 2015 How was BP defined and measured? Blinding of the assessor? The actual effect sizes, in mean (SD), at 12‐month postintervention, on the DRD and HRQoL for the treatment group (reported only as no significant differences, given website www.leicestershirediabetes.org.uk but returned blank) Please provide the PAID score, in mean (SD), for treatment groups at baseline and 12‐month postintervention (reported as medians and IQR). |
NA |
| D'Eramo Melkus 2010 | 22 June 2015 | 24 June 2015 | 22 June 2015 Was BP measurement investigator‐assessed outcome measurement? Blinding of the assessor? Any published trials register record or trial design paper/protocol? PAID (total score) mean (SD) values for both treatment groups at 12 months postintervention, not reported but mentioned significant trend of changes and P value. SF‐36 (overall score) mean (SD) values for both treatment groups at 12 months postintervention, not reported but mentioned significant trend of changes and P value. Systolic and diastolic blood pressure, mean (SD) values for both treatment groups at 12 months postintervention, not reported but mentioned significant trend of changes and P value. |
24 June 2015 The blood pressure assessor was blinded to group assignment |
| Ebert 2017 | 14 October 2016 and 20 October 2016 | 14 October 2016 | 14 October 2016 We would like to have the following outcome data, in mean (SD), for the T2DM patients in the IG, intervention group and CG, control group at 6‐months follow‐up:1. PAID, Problem Areas in Diabetes scale; 2. SF‐12: Physical, Short Form Health Survey (Physical Health Summary Scale); 3. SF‐12: Mental, Short‐Form Health Survey (Mental Health Summary Scale) |
14 October 2016 The main study author relayed and requested the data from another author. |
| Fisher 2011 | 22 June 2015 | 22 June 2015 | 22 June 2015 Further publications on quality of life and self‐efficacy as the outcome measures? The actual number of participants with hypoglycaemia (reported in percentages, unclear of the denominator). |
22 June 2015 No data regarding the number of participants who experienced hypoglycaemia were available; only the incidence of values < 70 mg/dL from downloaded blood glucose data |
| Fisher 2013 | 22 June 2015 and 15 October 2015 | 24 June 2015 and 20 October 2015 | 22 June 2015 Further publication on BP as an outcome measure? HbA1c mean (SD) values for the 3 treatment groups at 12 months, reported in natural log transformed values. 15 October 2015 Separate mean (SD) values for HbA1c (untransformed in %) for the 3 treatment groups at 12 months |
24 June 2015 No further publication on BP as an outcome measure. HbA1c results were already reported as mean (SD) for the natural log transformed HbA1c. 24 June 2015 Provided untransformed HbA1c values 20 October 2015 Data provided as requested |
| Fonda 2009 | 22 June 2015 | No reply | 22 June 2015 Do you have any published trials register record or trial design paper/protocol? Separate outcome data for participants with type 2 diabetes mellitus (T2DM). Please provide mean (SD) for PAID total score and HbA1c in % at 12 months postintervention for all the treatment groups in T2DM only. |
NA |
| Gabbay 2006 | 22 June 2015 | No reply | 22 June 2015 Separate outcome data for participants with type 2 diabetes mellitus (T2DM). Please provide mean (SD) for blood pressure (systolic and diastolic), HbA1C in %, and PAID at 1‐year postintervention for all the treatment groups in T2DM only. Do you have any published trials register record or trial design paper/protocol for this trial? |
NA |
| Gabbay 2013 | 22 June 2015 | No reply | 22 June 2015 Was BP measurement investigator‐assessed outcome measurement, blinding of the assessor? PAID scores, in mean (SD) at year 1, for both treatment groups (only provided for the baseline and at year 2). Diabetes‐specific quality of life (ADDQoL) scores, in mean (SD) at year 1 (only provided for the baseline). All‐cause mortality reported in the CONSORT diagram ‐ what was the source of data; its definition of death? |
NA |
| Glasgow 2005 | 22 June 2015 | No reply | 22 June 2015 Number of participants with HbA1c results at 12 months, reported only the total for both groups of 560. |
NA |
| Grillo 2016 | 19 October 2016 | No reply | 19 October 2016 Final actual mean (SD) for the PAID scores at 12 months for the Educational Course and Control groups, respectively. |
NA |
| Hermanns 2012 | 22 June 2015 18 February 2016 |
22 June 2015 18 February 2016 |
22 June 2015 How was BP defined and measured, blinding of the assessor? Information on the SF‐12 questionnaire, its validation trial/publication, scoring, etc. What were the duration of interventions (in month or week) for the MEDIAS 2 ICT? |
22 June 2015 The blood pressure measurement was done according to the German hypertension guidelines. Auscultatory method of BP measurement was used. Participants were be seated quietly for 3‐5 minutes prior to the manual measurement. The cuff was inflated 20‐30 mmHg above the level of the auscultatory determinations; the cuff deflation rate for auscultatory readings should have been 2 mmHg per second. SBP was the point at which the first of two or more Korotkoff sounds was heard (onset of phase 1), and the disappearance of Korotkoff sound (onset of phase 5) is used to define DBP. There were no special measures undertaken to ensure that assessors were blinded against this outcome measurement. Validation trial of the German SF‐12 questionnaire (Bullinger 1995) and the normative values (Gandek 1998) were provided: Bullinger M. German translation and psychometric testing of the SF‐36 Health Survey: preliminary results from the IQOLA Project. International Quality of Life Assessment. Social Science & Medicine 1995;41:1359‐66. Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross‐validation of item selection and scoring for the SF‐12 Health Survey in nine countries: results from the IQOLA Project. International Quality of Life Assessment. Journal of Clinical Epidemiology 1998; 51:1171‐8. The intervention duration was 26 weeks or 6 months |
| Hermanns 2015 | 22 June 2015 and 22 October 2015 | 22 June 2015 | 22 June 2015 Separate outcome data for participants with type 2 diabetes mellitus (T2DM). Please provide mean (SD) for DDS total score, EQ‐5D overall score, HbA1c in % at 12 months postintervention for all the treatment groups in T2DM only. 22 October 2015 Separate baseline data for the T2DM for the DIAMOS and CG groups |
22 June 2015 Supplementary table 2 with diabetes type specific outcomes was provided by the trial author 23 October 2015 Data provided as requested |
| Lamers 2011 | 22 June 2015 | 23 June 2015 | 22 June 2015 Illness or hospital admittance as reported in the trial flow chart – source of data, definition? All‐cause mortality reported in the CONSORT diagram – source of data, definition? Is there a further publication on self‐efficacy as an outcome measure? |
23 June 2015 Illness or hospital admittance were based on self‐report by the participant. Mortality was not an outcome in this trial, no answer given to the query. A further publication on self‐efficacy as an outcome measure, but is not on participants with diabetes mellitus (Jonkers 2012 Int Psychogeriatrics) |
| Lerman 2009 | 22 June 2015 | No reply | 22 June 2015 Ask for a full‐text article as the original article is in Spanish and was not retrievable. |
NA |
| Munshi 2013 | 22 June 2015 | No reply | 22 June 2015 Separate outcome data for participants with type 2 diabetes mellitus (T2DM). Please provide mean (SD) for HbA1c in %, blood pressure (systolic and diastolic) and PAID at 12 months postintervention for both the treatment groups in T2DM only. |
NA |
| Quinn 2011 | 22 June 2015 | No reply | 22 June 2015 Was there blinding of outcome assessment? Was diabetes‐related distress questionnaire interviewed or self‐administered by the participants? Is there a further publication on self‐efficacy as an outcome measure? |
NA |
| Rosenbek 2011 | 22 June 2015 and 29 June 2015 | 26 June 2015 and 06 July 2015 | 22 June 2015 Separate outcome data for participants with type 2 diabetes mellitus (T2DM). Please provide mean (SD) for HbA1c in %, blood pressure (systolic and diastolic), PCDS and PAID at 12 months postintervention for both the treatment groups in T2DM only. |
26 June 2015 and 06 July 2015 The trial author replied and provided with the requested separate data for T2DM. Blood pressure was measured by the auscultatory method with use of a stethoscope and a sphygmomanometer. An inflatable cuff was placed around the upper left arm, at the same vertical height as the heart. Measurement was made in rest in a sitting position. Assessor was blinded. Both assessments tools were measured by self‐administered questionnaires. HCCQ (the Health Care Climate Questionnaire) evaluates the person's relationship with the health care practitioners when discussing health care issues. TSRQ (the Treatment Self‐Regulation Questionnaire) evaluates the people quality of motivation (i.e. psychological energy directed at a particular health outcome) along an autonomy continuum. |
| Shibayama 2007 | 22 June 2015 | 22 June 2015 and 15 October 2015 | 22 June 2015 1. Do you have any published trials register record or trial design paper/protocol? 2. Was there a random sequence generation? How was it done? 3. Was there an allocation concealment? How was it done? 4. Was there blinding of treating physicians? 5. Was there blinding of outcome assessment, such as were questionnaire/assessment on diabetes‐related distress and health‐related quality of life (DRD and HRQoL) interviewed or self‐administered? 6. SF‐36 (overall score) mean (SD) values for both treatment groups at one year (reported for each of the separate domain). |
22 June 2015 1. No trials register record or published protocol. 2. Yes. Every time a participant gave written consent to the participation of the trial, investigators generated a random number (from 0 to 1) with Microsoft Excel and allocated him/her to each group. For more detail, authors stratified participants by characteristics including age, sex, and glycaemic control at first. Secondly, they observed which treatment has the fewest participants in a subgroup of the participants so far: that treatment is then assigned with probability P > 2/3 to him/her. In order to get accurate probability, investigators used the random number above. 3. The random allocation was performed by two authors. Neither performed the intervention or directly measured outcomes. Allocation was not concealed to the participants or nurses who engaged the intervention because of the educational nature of the intervention. 4. Physicians were blinded to which treatments had been allocated to their participants. 5. The value of participants' HbA1c was measured by laboratory technicians who were not the members of our trial group and didn't know about the allocation. The questionnaires about DRD and HRQoL were self‐administered. 6. Overall score of SF‐36 at one year was shown below. Intervention (N = 65) mean 76.50, SD 15.31. Control (N = 66) mean 79.36, SD 17.80 (missing values were imputed with the last value carried forward method.) 15 October 2015 Trial author provided the means and SDs at one year for the PAIDS score. |
| Simmons 2015 | 22 June 2015 | No reply | 22 June 2015 HbA1c in unit % mean (SD) values for the 4 treatment groups at 8‐12 months (last) evaluation, reported in mmol/mol – unable to calculate SD in %. DDS‐4 scores in mean (SD) values for the 4 treatment groups at 8‐12 months (last) evaluation, reported as changes at follow‐up for some groups. EQ‐5D total score in mean (SD) values for the four treatment groups at 8‐12 months (last) evaluation, reported as changes at follow‐up for some groups. Self‐efficacy DSE‐8 score in mean (SD) values for the four treatment groups at 8‐12 months (last) evaluation, reported as changes at follow‐up for some groups. |
NA |
| Skelly 2009 | 22 June 2015 | No reply | 22 June 2015 Any published trials register record or trial design paper/protocol? 1. HbA1c in % mean (SD) values for the symptom management group and weight control group at 6‐month, reported significant changes and P values. 2. HbA1c in % mean (SD) values for the symptom management + booster group and weight control group at 9‐month, reported significant changes and P values. Also the PAID and QoL mean (SD) scores for the above no. 1 and 2 comparison and time points. |
NA |
| Van Son 2013 and Van Son 2014 | 22 June 2015 | No reply | 22 June 2015 Separate outcome data for participants with T2DM. If possible, please provide mean (SD) for PAID, SF‐12 and HbA1c at 6 months postintervention for both the treatment groups in T2DM only. |
NA |
| Spencer 2013 | 22 June 2015 | 26 June 2015 | 22 June 2015 Blinding of outcome assessment, interviewed or self‐administered (DRD)? PAID score in mean (SD) for both the immediate and delayed group at 6 month, reported in log transformation. HbA1c in % mean (SD) for both the immediate and delayed group at 6 month, not reported as an outcome measure. |
26 June 2015 Yes, there was blinding of the outcome assessment. The diabetes‐related distress questionnaire by interview‐administered. Data were provided in Excel file. |
| Sperl‐Hillen 2013 | 22 June 2015 | 22 June 2015 | 22 June 2015 All‐cause mortality reported in the CONSORT diagram — source of data, definition? |
22 June 2015 Deaths were either reported by family in return surveys, or the participant was listed as deceased in the EHR system. |
| Sturt 2008 | 22 June 2015 | No reply | 22 June 2015 BP mean (SD) for both intervention and delayed intervention group at 6 months, reported only no significant difference. |
NA |
| Taylor 2006 | 22 June 2015 No email could be found |
No | 22 June 2015 Any published trials register record or trial design paper/protocol? No email could be found |
NA |
| Van der Wulp 2012 | 22 June 2015 | No reply | 22 June 2015 Blinding of treating GP on the participating participants in their practices? |
NA |
| Weinger 2011 | 26 June 2015 | 30 June 2015 and 03 July 2015 | 26 June 2015 Subgroup of the type 2 diabetes for the outcomes measure (DRD, QoL, self‐efficacy, HbA1c, BP) between 6‐12 months How was blood pressure (BP) defined and measured, was there blinding of the assessor? One participant was reported to endorse suicidal idea when answering one of the questionnaire, which diabetes type and which intervention arm did this participant came from? Confirm the trial identifier as provided in the article because no trials with search of NCT000142922 were found |
30 June 2015 Trial author provided separate data on the T2DM. The blood pressure was measured with by nurses who were not involved in any other part of the trial (systolic and diastolic on calibrated equipment). They were blinded to the trial assignment. The participant endorsed a 2 ('moderate') on the Brief Symptoms inventory at 1 year postintervention. The participant was assessed and found not to be suicidal but was referred for psychological counselling‐ the participant had type 1 diabetes and was in the individual education arm. |
| Whittemore 2004 | 22 June 2015 | 26 June 2015 | 22 June 2015 Was there a random sequence generation? How was it done? Was there an allocation concealment? How was it done? Blinding of outcome assessment, interviewed or self‐administered (DRD), blinding of the nurse‐coach? Any published trials register record or trial design paper/protocol? |
26 June 2015 No register trial nor published trial protocol/design paper. Since this was a small trial, we had sealed opaque envelopes with the randomisation assignment. Participants selected an envelope after completion of baseline data collection. The diabetes distress was self‐administered. The nurse coach did not collect data. She only provided the intervention. |
| NCT01578096 | 18 February 2016 | 18 February 2016 | Trial authors were contacted to inquire on any published article, or when trial results will be published. | Manuscript reporting the diabetes distress outcomes of our intervention is currently under review. Investigators suggested checking back for a citation after a few months |
| ADDQoL: audit of diabetes dependent quality of life; BP: blood pressure;CIDS‐2: Confidence in Diabetes Self‐Care; DBP: diastolic blood pressure; DRD: diabetes‐related distress; HRQoL: health‐related quality of life;IG: intervention group; IQR: interquartile range; PAID: Problem Areas in Diabetes;PCDS: Perceived Competence for Diabetes Scale; NA: not applicable; SBP: systolic blood pressure; SD: standard deviation; SF‐36: Short Form Health Survey; T2DM: type 2 diabetes mellitus. | ||||
Appendix 14. Checklist to aid consistency and reproducibility of GRADE assessments
| Diabetes‐related distress | Health‐related quality of life | Self‐efficacy | Diabetes‐related complications | All‐cause mortality | Adverse events | HbA1c | ||
| Study limitations (risk of bias)a | 1. Was random sequence generation used (i.e. no potential for selection bias)? | Yes | Yes | Yes | NR | Yes | Yes | Yes |
| 2. Was allocation concealment used (i.e. no potential for selection bias)? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| 3. Was there blinding of participants and personnel (i.e. no potential for performance bias)? | No | No | No | Unclear | Unclear | Yes | ||
| 4. Was there blinding of outcome assessment (i.e. no potential for detection bias)? | No | No | No | Unclear | Unclear | Yes | ||
| 5. Was an objective outcome used? | No | No | No | Yes | No | Yes | ||
| 6. Were more than 80% of participants enrolled in trials included in the analysis (i.e. no potential reporting bias)?e | No (↓) | No (↓) | No (↓) | No (↓) | No (↓) | No (↓) | ||
| 7. Were data reported consistently for the outcome of interest (i.e. no potential selective reporting)? | Yes | Yes | Unclear | Yes | Yes | Unclear | ||
| 8. Were other biases reported (i.e. no potential of other bias)? | Unclear | Unclear | No (↓) | Yes | Yes | No | ||
| 9. Did the trials end as scheduled (i.e. not stopped early)? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Inconsistencyb | 1. Did point estimates vary widely? | Yes | Yes | Yes | Yes | Yes | Yes | |
| 2. To what extent did confidence intervals overlap (substantial: all confidence intervals overlap at least one of the included studies point estimate; some: confidence intervals overlap but not all overlap at least one point estimate; no: at least one outlier: where the confidence interval of some of the studies do not overlap with those of most included studies)? | Substantial | Substantial | Some | Substantial | Substantial | Some | ||
| 3. Was the direction of effect consistent? | Yes | Yes | No | Yes | Yes | Yes | ||
| 4. What was the magnitude of statistical heterogeneity (as measured by I²): low (I² < 40%), moderate (I² 40% to 60%) or high I² > 60%)? | Low | Low | Moderate | Moderate | Low | Moderate | ||
| 5. Was the test for heterogeneity statistically significant (P < 0.1)? | Not statistically significant | Not statistically significant | Not statistically significant | Not statistically significant | Not statistically significant | Statistically significant | ||
| Indirectnessa | 1. Were the populations in included studies applicable to the decision context? | Highly applicable | Highly applicable | Applicable | Applicable | Applicable | Highly applicable | |
| 2. Were the interventions in the included studies applicable to the decision context? | Highly applicable | Highly applicable | Applicable | Applicable | Applicable | Highly applicable | ||
| 3. Was the included outcome not a surrogate outcome? | Yes | No | No | Yes | Yes | No | ||
| 4. Was the outcome timeframe sufficient? | Sufficient | Sufficient | Sufficient | Insufficient | Sufficient | Sufficient | ||
| 5. Were the conclusions based on direct comparisons? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| Imprecisionc | 1. Was the confidence interval for the pooled estimate not consistent with benefit and harm? | Yes | Yes | Yes | No (↓) | No (↓) | No (↓) | |
| 2. What is the magnitude of the median sample size (high: 300 participants, intermediate: 100‐300 participants, low: < 100 participants)?e | Low | Low | Intermediate | Intermediate | Low (↓) | Low (↓) | ||
| 3. What was the magnitude of the number of included studies (large: > 10 studies, moderate: 5‐10 studies, small: < 5 studies)?e | Large | Moderate | Moderate | Small | Small | Large | ||
| 4. Was the outcome a common event (e.g. occurs more than 1/100)? | NA | NA | NA | Yes | Yes | NA | ||
| Publication biased | 1. Was a comprehensive search conducted? | Yes | Yes | Yes | Yes | Yes | Yes | |
| 2. Was grey literature searched? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| 3. Were any restrictions applied to study selection on the basis of language? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| 4. Was there an industry influence on studies included in the review? | Yes | Yes | Yes | Yes | Yes | Yes | ||
| 5. Was there evidence of funnel plot asymmetry? | Yes | Unclear | Unclear | Unclear | Unclear | No (↓) | ||
| 6. Was there any discrepancy in findings between published and unpublished trials? | Yes | Yes | Unclear | Yes | Yes | Unclear | ||
|
HbA1c: glycosylated haemoglobin A1c; NA: not applicable; NR: not reported.
aQuestions on risk of bias are answered in relation to most of the aggregated evidence in the meta‐analysis rather than to individual studies.
bQuestions on inconsistency are primarily based on visual assessment of forest plots and the statistical quantification of heterogeneity based on I². cWhen judging the width of the confidence interval it is recommended to use a clinical decision threshold to assess whether the imprecision is clinically meaningful. dQuestions address comprehensiveness of the search strategy, industry influence, funnel plot asymmetry and discrepancies between published and unpublished trials. eDepends on the context of the systematic review area. (↓): key item for possible downgrading the quality of the evidence (GRADE) as shown in the footnotes of the 'Summary of finding' table(s). | ||||||||
Appendix 15. Diabetes‐related distress: instruments
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| Diabetes Distress Scale (DDS) | 17‐items with four subscales: emotional burden (EB) subscale (5 items), physician‐related distress (PRD) subscale (4 items), regimen‐related distress (RRD) subscale (5 items), and diabetes‐related interpersonal distress (DRID) subscale (3 items) | Yes | 6‐point Likert‐scale from 'not a problem' to 'a serious problem'. | A total mean‐item score DRD (tDRD) scale score plus 4 subscale scores | Minimum score: 1 Maximum score: 6 A mean score of less than 2.0 indicates little to no distress, a score between 2.0 and 2.9 indicates moderate distress and 3.0 and greater is considered high distress worthy of clinical attention |
No | Higher values mean higher distress | |
| Fisher 2011 | Baseline mean tDDS (SD): active control: 2.25 (0.88); structured testing: 2.41 (0.98) 12‐months mean tDRD (SD): active control: 1.93 (0.07); structured testing: 1.78 (0.06) |
|||||||
| Fisher 2013 | Baseline mean tDDS (SD): Leap Ahead: 2.48 (0.95); computer‐assisted self‐management (CASM): 2.37 (0.86); CAPS: 2.38 (0.89) 12‐months mean tDRD (SD): Leap Ahead: 1.98 (0.88); CASM: 2.03 (0.83); CASM + problem solving therapy: 1.92 (0.75) |
|||||||
| Hermanns 2015 | Baseline tDRD (SD): intervention: 2.7 (0.9) (); control: 2.7 (0.8) 12‐months tDRD (SD): intervention:3.4; control: 3.0 |
|||||||
| Liu 2015 | Baseline mean tDDS (SD): peer education: 3.18 (0.2); usual education: 3.14 (0.9) 12‐months mean tDRD (SD): peer education: 2.67 (0.6); usual education: 3.02 (0.6) |
|||||||
| Quinn 2011 | Baseline mean tDDS (SD): usual care: 2.4 (0.9); group 2: 2.7 (0.9); group 3: 2.8 (0.7); group 4: 2.6 (0.9) 12‐month mean tDRD (SD): usual care: 2.3 (0.9); group 2: 2.6 (0.9); group 3: 2.4 (0.8)); group 4: 2.3 (0.8) |
|||||||
| Glasgow 2005 | No mean scores provided, just effect sizes | |||||||
| Trief 2016 | Baseline mean tDDS (SD): diabetes education: 2.2 (0.9); individual calls: 2.3 (1.1); change couples intervention: 2.4 (0.8) (CC) 12‐months mean tDRD (SD): diabetes education: 2.2 (1.0); individual calls: 1.9 (1.0); change couples intervention 1.7 (1.0) |
|||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| Diabetes Distress Scale (DDS‐4) | 4‐item with 2 items from the original 17‐item emotional burden (EB) subscale, and another 2 items from regimen‐related distress (RRD) subscale | Yes | 6‐point Likert‐scale from 'not a problem' to 'a serious problem'. | A total mean‐item score | Minimum score: 1 Maximum score: 6 |
No | Higher values mean higher distress | |
| Simmons 2015 | Baseline (SD): Control (SD): 6.61 (4.05) 1:1 : 6.53 (4.12) Group (SD): 6.27 (3.22) Combined (SD): 6.71 (4.27) |
|||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| Problem Areas in Diabetes (PAID) | None (20 items) | Yes | 5‐point Likert‐scale | Total score (TS) | Minimum score: 0 Maximum score: 100 |
No | Lower values mean better assessment | |
| Beverly 2013 | Baseline mean total score (SD): intervention: 33.3 (20.3); control: 34.8 (23.1) 12‐months mean total score (SD): intervention: 25.0 (16.0) (intervention)/ 25.7 (22.7) |
|||||||
| Davies 2008 | No baseline value was reported 12‐months mean total score: intervention: 14.1 (6.3‐28.1); control: 12.5 (4.7‐28.1) |
|||||||
| Dennick 2015 | Baseline mean total score (SD): intervention: 37.1 (2.5); control: 34.4 (2.3) Follow‐up mean total score (SD): intervention: 35.3 (1.4); control: 34.4 (1.6) |
|||||||
| D'Eramo Melkus 2010 | Baseline mean total score (SD): intervention: 54 (31); control: 60 (30) 24‐months mean total score (read from graph): intervention: about 38; control: 48 |
|||||||
| Gabbay 2013 | The baseline mean total score was 29 for both groups. PAID scores did not differ significantly at year 1, at Year 2 the scores were better in the intervention compared with the control group. | |||||||
| Glasgow 2005 | Mean total score (SD): baseline intervention (baseline control): 30.3 (28.5) Mean total score (SD): 12‐month intervention (12‐month control): 29.7 (26.8) |
|||||||
| Grillo 2016 | Baseline PAID score(SD): educational: 20 (14); control: 16 (13) 12‐month follow‐up (SD): decrease in the PAID score when compared to baseline (intervention: −34 (22) vs controls: —26 (18)) |
|||||||
| Hermanns 2015 | Mean total score (SD): baseline intervention (baseline control): 39.7 (37.5) Mean total score (SD): 12‐month intervention (12‐month control): 48.5 (40.1) |
|||||||
| Hermanns 2012 | Baseline mean score (SD): intervention: 52.5 (9.2); control: 47.6 (9.6) Endpoint mean score (SD): intervention: 49.1 (9.7); control: 48.0 (11.2) |
|||||||
| Lamers 2011 | Baseline mean total score (SD): intervention: 22.6 (20.5); control: 23.4 (19.5) 9‐months mean total score (SD): intervention: 18.49 (1.76); control: 22.89 (1.72) |
|||||||
| Lerman 2009 | Baseline mean total score (SD): intervention 1: 45 (23) (GRT); intervention 2: 49 (29) (GCR); control: 51 (19) 12‐month mean total score (SD): intervention 1: 46 (26) (GRT); intervention 2: 38 (21) (GCR); control: 49 (23) |
|||||||
| Pibernik‐Okanovic 2015 | Baseline mean total score (SD): psychoeducation: 37.9 (19.7); physical exercise: 42.6 (20.5) (physical exercise); re‐education: 39.1 (19.6) 12‐month mean total score (SD): psychoeducation: 32.5 (22.1); physical exercise: 36.4 (22.1); re‐education: 33.2 (20.3) |
|||||||
| Rosenbek 2011 | Baseline mean total score (SD): intervention: 20.0 (17.7); control: 19.6 (16.3) | |||||||
| Shibayama 2007 | Baseline mean total score (SD): intervention: 38 (28–52); control: 35 (26–51) | |||||||
| Skelly 2009 | Changed score (SD): Intervention: 2.05 (0.56) Intervention with booster: 2.28 (0.83) Weight and diet: 2.31 (0.75) |
|||||||
| Spencer 2013 | 12‐baseline (intervention): −12.1 (−16.3 to −6.0) 12‐baseline (delayed): −7.1 (−12.5 to 0.6) |
|||||||
| Sperl‐Hillen 2013a | Mean total score at baseline: usual care: 30.52; individual education: 29.81 group education: 29.62 () | |||||||
| Sturt 2008 | Baseline mean total score (SD): intervention: 21 (15); delayed: 21 (15) 6 months mean total score (SD): intervention: 17 (14); delayed: 22 (17) |
|||||||
| Taylor 2006 | Baseline mean total score: cognitive: 38.2; wait‐list: 30.72; intervention: 30.35 | |||||||
| Van der Wulp 2012 | Mean total score (SD): Intervention group: T0: 16.65 (18.95); T1: 13.19 (12.90); T2: 12.74 (14.02) Control group: T0: 14.48 (15.50); T1: 12.17 (11.90); T2: 11.09 (14.99) |
|||||||
| Van Dijk‐de Vries 2015 | Mean total score (SD): Intervention group: T0: 29.9 (16.9); T12: 27.8 (16.5) Control group: T0: 28.9 (19.4); T12: 27.0 (19.7) |
|||||||
| Weinger 2011 | Baseline: Type 2 diabetes: 32.5 (1.3 to 73.8) Structured behavioural: 34.4 (2.5 to 91.3) Attention control: 30.0 (3.8 to 85) Individual control: 32.5 (0.0 to 80.0) |
|||||||
| Welch 2015 | Baseline mean total score (SD): intervention: 59.0 (30.5); control: 51.9 (32.3) 6 months mean total score (SD): intervention: 40.4 (2.1); control: 48.3 (2.0) |
|||||||
| Whittemore 2004 | Baseline mean total score (SD): intervention: 59.9 (22); control: 42.3 (14) 6‐months mean total score (SD): intervention: 46.9 (23); control: 42.9 (19) |
|||||||
| aOne PAID question was inadvertently omitted on the survey resulting in a PAID score based on 19 instead of 20 questions. | ||||||||
Appendix 16. Health‐related quality of life: instruments
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| Diabetes Quality of Life (specific) | 4 subscales: satisfaction (SA) subscale (15 items), general health and impact of treatment (GT) subscale (20 items), future effects of diabetes (FE) subscale (4 items), and social effects (SE) subscale (7 items). | Yes | 5‐point Likert scale. A score of 1 represents no impact or worries and always satisfied. A score of 5 represents always affected, worried, or never satisfied. | Yields a total score (tDQOL) with plus 5 subscale scores. Scores are converted to a 100‐point scale | Minimum score: 0 Maximum score: 100 |
No | Higher values mean higher quality of life | |
| Beverly 2013 | tDQOL (SD): all: 67.4 (11.4); intervention: 67.9 (10.6); control: 66.9 (12.1) | |||||||
| Weinger 2011 | Baseline total score (SD): Type 2 diabetes: 69.6 (10.0) Structured behavioural: 67.1 (10.4) Attention control: 66.6 (10.4) Individual control: 67.8 (11.3) |
|||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| WHOQOL‐BREF (generic) | Two overall dimensions and four subscales for physical (7 items), psychological (6 items), social (3 items), and environmental (8 items) | Yes | 5‐point Likert scales | 2 overall scores and 4 subscale scores | Minimum score: 0 Maximum score: 100 The mean score of items within each domain is used to calculate the domain score. Mean scores are then transformed to a 0‐100 scale |
No | Higher scores denote higher quality of life | |
| Davies 2008 | The groups did not differ significantly in any of the scores for 6 dimensions of quality of life. The results of the analyses are available at www.leicestershirediabetes.org.uk. | |||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| EQ‐5D (generic) | Consists of a visual analogue scale (VAS) and a descriptive system covering 5 dimensions: mobility (3 items), self‐care (3 items), usual activity (3 items), pain/discomfort (3 items), anxiety and depression (3 items) (utility). | Yes | 3 levels (no problem, some problem, extreme problems) | Converted into a single summary index by applying a formula that essentially attaches values (also called weights) to each of the levels in each dimension. The index can be calculated by deducting the appropriate weights from 1, the value for full health (i.e. state 11111) |
VAS scores Minimum score: 0 Maximum score: 100 Utility scores Minimum score: 0 Maximum score: 1 |
Yes | Higher scores denote better state of health | |
| Dennick 2015 | VAS (SD): intervention‐baseline: 80.9 (4.0); control‐baseline: 79.1 (4.0); intervention‐follow‐up: 77.4 (2.8); control‐follow‐up: 82.1 (3.0) Utility (SD): intervention‐baseline: 0.86 (0.03); control‐baseline: 0.92 (0.03); intervention‐follow‐up: 0.86 (0.03); control‐follow‐up: 0.87 (0.03) |
|||||||
| Simmons 2015 | Baseline (SD): Control: 0.77 (0.27) 1:1 : 0.75 (0.30) Group: 0.76 (0.26) Combined: 0.76 (0.27) |
|||||||
| Hermanns 2015 | EQ‐5D (health‐related quality of life) intervention‐baseline (control‐baseline) (SD): 0.86 (0.88) intervention‐follow‐up (control‐follow up) (SD): 0.85 (0.86) |
|||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| 36‐item Short Form health survey (SF‐36) (generic) | Physical functioning (PF) (10 items) Role‐physical (RP) (4 items) Bodily pain (BP) (2 items) General health (GH) (5 items) Vitality (VT) (4 items) Social functioning (SF) (2 items) Role‐emotional (RE) (3 items) Mental health (MH) (5 items) Reported health transition (RHT) (1 item) | Yes | 3, 5 and 6‐point Likert‐scale | Scores for dimensions
Physical component summary (PCS‐36) Mental component summary (MCS‐36) |
Minimum scores: 0
scores for dimensions/PCS‐36/MCS‐36:
norm‐based scale Maximum scores: 100 scores for dimensions/PCS‐36/MCS‐36: norm‐based scale |
No | Higher score means better health‐related quality of life | |
| D'Eramo Melkus 2010 | PF (SD): control: 67 (29); intervention: 66 (28) RP (SD): control: 63 (41); intervention: 57 (45) BP (SD): control: 57 (29); intervention: 56 (26) GH (SD): control: 58 (20); intervention:56 (21) VT (SD): control: 50 (21); intervention: 49 (21) SF (SD): control: 66 (28); intervention: 72 (27) RE (SD): control: 60 (43); intervention: 61 (43) MH (SD): control: 64 (23); intervention: 65 (22) |
|||||||
| Shibayama 2007 | PF: control: 90 (85–95); intervention: 90 (80–95) RP: control: 100 (100–100); intervention: 100 (75–100) BP: control: 84 (62–100); intervention: 74 (52–100) GH: control: 57 (47–72); intervention: 57 (47–67) VT: control: 75 (60–90); intervention: 70 (50–85) SF: control: 100 (88–100); intervention: 100 (75–100) RE: control: 100 (100–100); intervention: 100 (67–100) MH: control: 88 (68–92); intervention: 76 (64–88) |
|||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| 12‐item Short Form health survey (SF‐12) (generic) | 2 dimensions: physical and mental health | Yes | 2, 3, 5 and 6‐point Likert‐scale | Scores for dimensions
Physical component summary (PCS‐12) Mental component summary (MCS‐12) |
Minimum score: 0 Maximum score: 100 |
Weighted and summed scales for physical and mental health | Higher score means higher level of health | |
| Hermanns 2012 | PCS‐12 (SD): baseline‐control: 40.9 (10.3); baseline‐intervention: 39.1 (10.4); endpoint‐control: 41.4 (10.3):; endpoint‐intervention: 41.2 (10.7) MCS‐12 (SD): baseline‐control: 52.0 (9.7); baseline‐intervention: 51.4 (9.0); endpoint‐control: 51.6 (10.5); endpoint‐intervention: 50.1 (10.1) |
|||||||
| Pibernik‐Okanovic 2015 | SF‐12v2 Baseline: PCS‐12 (SD): psychoeducation: 42.3 (8.7); physical exercise: 43.1 (8.8); re‐education: 42.7 (9.1) 0.871 MCS‐12 (SD): psychoeducation: 41.9 (7.4); physical exercise: 41.7 (8.3); re‐education: 41.2 (7.2) 0.872 |
|||||||
| Van Dijk‐de Vries 2015 | Baseline mean score: Intervention group (SD): SF‐12 physical component: 34.8 (9.6); SF‐12 mental component: 34.1 (11.3) Control group (SD): SF‐12 physical component: 35.0 (9.8); SF‐12 mental component: 35.2 (11.2) |
|||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| Audit of diabetes dependent quality of life (ADDQoL) (specific) | Two overview items that assess the global QOL and the impact of diabetes on quality of life and 13 domain‐specific items | Yes | 7‐point Likert‐scale of the two overview items and condition‐specific domains, and 4‐point Likert‐scale on the important of the item | Mean score for applicable domains are summed and divided by the number of applicable domains to give a final score | Minimum score: — 9 Maximum score: + 9 |
A weighted impact score is computed | More negative scores indicating poorer quality of life from diabetes | |
| Gabbay 2013 | Baseline (SD): control: –0.88 (3.32); intervention: –1.15 (3.33). Scores did not differ significantly between the 2 groups at the end of the study. | |||||||
| Liu 2015 | Baseline (SD): control: –2.52 (0.9); intervention: –2.53 (0.8) Follow‐up (SD): control: –2.50 (0.7); intervention: –1.98 (0.8) | |||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| Diabetes Symptom Checklist ‐ Revised (DSC‐R) (specific) | 8 dimensions (34 items): hyperglycaemia (HE), hypoglycaemia (HO), neuropathic pain (NP), sensibility (SS), fatigue (FG), cognitive distress (CD), cardiovascular symptoms (CS) and ophthalmological symptoms (OS) | Yes | 5‐point Likert scales. A score of 1 represents 'not at all'. A score of 5 represents 'extremely'. | A total score (TS) and subscores for the 8 dimensions | Minimum score: 0 Maximum score: 10 |
No | With 0 being most favourable outcome | |
| Lamers 2011 | Baseline TS (SD): usual care: 2.8 (1.6); intervention: 2.9 (1.6) Hyperglycaemic (SD): usual care: 3 (2.8); intervention: 3.4 (2.5) Hypoglycaemic (SD): usual care: 2.3 (2.1); intervention: 2.3 (2.1) Polyneuropathic pain (SD): usual care: 2.3 (2.5); intervention: 2.1 (2.5) Polyneuropathic sensory (SD): usual care: 2.4 (2.4); intervention: 2.4 (2.5) Psychological fatigue (SD): usual care: 5.3 (2.3); intervention: 5.1 (2.4) Cognitive stress (SD): usual care: 3.2 (2.4); intervention: 3.0 (2.3) Cardiovascular (SD): usual care: 2.4 (2.1); intervention: 2.6 (2.0) Ophthalmological symptoms (SD): usual care: 2.0 (2.3); intervention: 2.1 (1.9) | |||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| Diabetes‐related Quality of Life | 24‐item instrument has two subscales measuring quality of life in two domains: mental (MWB) (9 items) and social well‐being (SWB) (9 items); and a physical symptom index (6 items) | Yes | 4‐point Likert scale | Mean score for SWB and MWB | Minimum score: 1 Maximum score: 4 |
No | Higher scores mean better quality of life | |
| Skelly 2009 | Intervention (SD): SWB: 3.41 (0.57); MWB: 2.67 (0.60) Intervention with booster SWB (SD): 3.25 (0.66); MWB (SD): 2.55 (0.69) Weight and diet SWB (SD): 3.17 (0.71); MWB (SD): 2.56 (0.77) |
|||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| 12‐item Well‐Being Questionnaire (WBQ‐12) (generic) | 3 subscales to measure energy (4 items), positive well‐being (4 items), and negative well‐being (4 items) | Yes | 4‐point Likert scale. Score 0 represent 'not at all' and 3 means 'all the time'. | Total and sum subscales score | Total scores Minimum score: 0 Maximum score: 36 Subscale scores Minimum score: 0 Maximum score: 12 |
No | Higher scores mean better quality of life | |
| Taylor 2006 | Pre‐test/Post‐test Wait‐list: 22.88/23.08 CBT: 20.61/21.65 Expressive writing: 21.43/23.75 |
|||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| WHO (Five) Well‐being Index | 3 dimensions: positive mood (good spirits, relaxation), vitality (being active and waking up fresh and rested), and general interest (being interested in things) | Yes | 6‐point Likert scale | Total score. Total the 5 answers 0 to 25 and multiply by 4. | Minimum score: 0 Maximum score: 100 |
No | Higher scores mean better well‐being | |
| Hermanns 2015 | Intervention group: baseline: 8.5; follow‐up: 3.9 Control group: baseline: 9.6; follow‐up: 8.8 |
|||||||
| Van der Wulp 2012 | Intervention group (SD): T0: 62.58 (22.18); T1: 67.06 (18.82); T2: 69.14 (19.27) Control group (SD): T0: 60.13 (20.74); T1: 64.11 (18.10); T2: 64.40 (21.86) |
|||||||
| WHO: WHO World Health Organization | ||||||||
Appendix 17. Self efficacy: instruments
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| Confidence in Diabetes Self‐care Scale (CIDS‐2) | None (20 items) | Yes | 5‐point Likert scale ranging from 1 ("No, I am sure I cannot") to 5 ("Yes, I am sure I can") | A total score (TS) is calculated by summation of all item scores and then transformed to a 0–100 scale | Minimum score: 0 Maximum score: 100 |
No | Higher scores indicating higher self‐efficacy | |
| Beverly 2013 | TS (SD): all participants: 81.3 (11.8); intervention: 81.9 (11.6); control: 80.7 (12.1) | |||||||
| Weinger 2011 | Baseline total score (SD): Type 2 diabetes: 57.9 (15.7); structured behavioural: 56.3 (14.6); attention control: 57.1 (13.2); individual control: 57.9 (17.5) |
|||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| Diabetes Empowerment Scale ‐ Short Form (DES‐SF) | 8 conceptual dimensions: assessing the need for change (NC) (1 item), developing a plan (DP) (1 item), overcoming barriers (OB) (1 item), asking for support (AS) (1 item), supporting oneself (SO) (1 item), coping with emotion (CM) (1 item), motivating oneself (MO) (1 item), and making diabetes care choices appropriate for one's priorities and circumstances (CPC) (1 item) | Yes | 5‐point Likert scale ranging from 1 (strongly disagree) to 5 (strongly agree) | The average score of 8 items | Minimum score: 1 Maximum score: 5 |
No | Higher scores indicate higher levels of empowerment | |
| Sperl‐Hillen 2013 | Mean score at baseline: Usual care: 3.78; individual education: 3.8; group education; 3.79 |
|||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| Diabetes Self‐Efficacy Questionnaire (DSEQ) | 20‐items with 5 subscales: managing social, emotional and food‐related aspects of diabetes, communicating with health professionals and planning, managing low blood sugars, managing diabetes related to exercise, blood glucose and prevention and integrating knowledge and day to day care | Yes | 6‐point Likert scale ranging from 'never' to 'always', with 0 as 'Never' and 5 as 'Always' | Total score | Minimum score: 0 Maximum score: 100 |
No | Higher scores indicate higher levels of self‐efficacy | |
| D'Eramo Melkus 2010 | Baseline (SD): Control: 76 (12); intervention: 75 (11) |
|||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| Diabetes Self‐Efficacy Scale (DSE‐8) | 8‐item (none) | Yes | 10‐point Likert scale ranging from 1 as ' Not at all confident' to 10 as 'Totally confident' |
The score for the scale is the mean of the 8 items | Minimum score: 1 Maximum score: 10 |
No | Higher number indicates higher self‐efficacy | |
| Simmons 2015 | Baseline (SD): Control: 58.4 (17.2); one‐to‐one peer support: 56.3 (18.2); group: 57.6 (16.2); combined: 57.0 (17.1) |
|||||||
| Trief 2016 | Baseline mean (SD): diabetes education: 7.0 (1.8); individual calls: 6.9 (1.7); couples change: 7.0 (1.7) 12‐months mean (SD): diabetes education: 7.3 (1.9); individual calls: 7.4 (1.9); couples change: 7.5 (1.9) |
|||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| Diabetes Management Self‐efficacy Scale (DMSES) | 20‐items with 4 subscale: nutrition specific and weight, nutrition general and medical treatment, physical exercise and blood sugar | Yes | 5‐point Likert scale ranging from 1 as 'yes, surely' to 5 as 'no, surely not'. | Total score | Minimum score: 20 Maximum score: 100 |
No | Higher scores indicating more confidence in handling self‐management skills | |
| Sturt 2008 | Baseline (SD): intervention: 100 (27); delayed: 104 (28) 6 months (SD): intervention: 115 (23); delayed: 105 (29) |
|||||||
| Van der Wulp 2012 | Intervention group (SD): T0: 69.80 (13.90); T1: 73.14 (13.01); T2: 74.80 (11.67) Control group (SD): T0: 68.73 (14.17); T1: 71.37 (15.88); T2: 71.82 (15.86) |
|||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| Perceived Competence for Diabetes Scale (PCDS) | 5‐item (none) | Yes | 7‐point Likert scale ranging from 1 as 'not true at all' to 7 as 'very true'. | The score on the PCDS is calculated by averaging the responses on the 5 items. | Minimum score: 1 Maximum score: 7 |
No | Higher scores indicating higher perceived competence in dealing with diabetes | |
| Rosenbek 2011 | Mean (SD): intervention: 6.3 (1.0); usual care: 6.1 (1.1) | |||||||
| Perceived competence scale (PCS) | 4‐item (none) | Yes | 7‐point Likert scale with 1 as 'not at all true' to 7 as 'very true' | Mean score | Minimum score: 1 Maximum score: 7 |
No | Higher scores indicating more competency in self‐management skills | |
| Glasgow 2005 | Mean (SD): usual care: 5.75 (0.07); intervention: 5.90 (0.06) | |||||||
| Instrument | Dimensions (subscales, no. of items) | Validated instrument | Answer options | Scores |
Minimum score Maximum score |
Weighting of scores | Direction of scales | |
| General Self‐Efficacy Scale (GSES‐12) | 12‐item (none) | Yes | 5‐point Likert scale with 1 as 'strongly disagree' to 5 as 'strongly agree' |
Total score | Minimum score: 12 Maximum score: 60 |
No | Higher scores indicate higher levels of self‐efficacy | |
| Van Dijk‐de Vries 2015 | Mean (SD): Intervention group: T0: 38.6 (7.5); T12: 38.6 (7.6) Control group: T0: 39.2 (7.0);T12: 40.3 (6.9) |
|||||||
Data and analyses
Comparison 1. Cognition‐focused versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diabetes‐related distress (with types of setting subgroup) | 4 | 898 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.27, 0.08] |
| 1.1 Community‐based studies | 3 | 839 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.26, 0.15] |
| 1.2 Hospital‐based studies | 1 | 59 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.89, 0.24] |
| 2 Diabetes‐related distress (with types of intervention subgroup) | 4 | 898 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.27, 0.08] |
| 2.1 Longer and more advanced interventions | 2 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.47, 0.33] |
| 2.2 Brief and simple interventions | 2 | 742 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.35, 0.20] |
| 3 Diabetes‐related distress (with age subgroup) | 4 | 898 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.27, 0.08] |
| 3.1 Age < 60 years | 2 | 156 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.47, 0.33] |
| 3.2 Age ≥ 60 years | 2 | 742 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.35, 0.20] |
| 4 Health‐related quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Self‐efficacy (with types of intervention subgroup) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Brief and simple interventions | 2 | 742 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [0.04, 0.38] |
| 7 Self‐efficacy (with age subgroup) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Age ≥ 60 years | 2 | 742 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [0.04, 0.38] |
| 8 HbA1c (with types of setting subgroup) | 3 | 831 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.39, 0.36] |
| 8.1 Community‐based studies | 2 | 772 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐1.46, 0.65] |
| 8.2 Hospital‐based studies | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.23, 0.43] |
| 9 HbA1c (with types of intervention subgroup) | 3 | 831 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.39, 0.36] |
| 9.1 Longer and more advanced interventions | 2 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.54, ‐0.40] |
| 9.2 Brief and simple interventions | 1 | 623 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.14, 0.32] |
| 10 HbA1c (with age subgroup) | 3 | 831 | Mean Difference (IV, Random, 95% CI) | ‐0.51 [‐1.39, 0.36] |
| 10.1 Age < 60 years | 2 | 208 | Mean Difference (IV, Random, 95% CI) | ‐0.97 [‐1.54, ‐0.40] |
| 10.2 Age ≥ 60 years | 1 | 623 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.14, 0.32] |
| 11 Systolic blood pressure (with types of interventions subgroup) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Longer and more advanced interventions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Diastolic blood pressure (with types of interventions subgroup) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Longer and more advanced interventions | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 All‐cause mortality | 2 | 1168 | Risk Ratio (M‐H, Random, 95% CI) | 1.81 [0.29, 11.38] |
| 13.1 At more than 12 months | 1 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 5.40 [0.61, 47.97] |
| 13.2 At less than 12 months | 1 | 623 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.17, 4.03] |
| 14 All‐cause mortality (with age subgroup) | 2 | 1168 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [0.29, 11.66] |
| 14.1 Age < 60 years | 1 | 545 | Odds Ratio (M‐H, Random, 95% CI) | 5.47 [0.61, 49.30] |
| 14.2 Age ≥ 60 years | 1 | 623 | Odds Ratio (M‐H, Random, 95% CI) | 0.82 [0.16, 4.11] |
Comparison 2. Cognition‐focused versus enhanced usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diabetes‐related distress (with types of setting subgroup) | 4 | 2233 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.11, 0.06] |
| 1.1 Community‐based studies | 3 | 2099 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.11, 0.06] |
| 1.2 Hospital‐based studies | 1 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.37, 0.30] |
| 2 Diabetes‐related distress (with types of intervention subgroup) | 4 | 2233 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.11, 0.06] |
| 2.1 Longer and more advanced interventions | 2 | 1275 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.19, 0.08] |
| 2.2 Brief and simple interventions | 2 | 958 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.13, 0.13] |
| 3 Diabetes‐related distress (with age subgroup) | 4 | 2233 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.09, 0.08] |
| 3.1 Age < 60 years | 3 | 1347 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.17, 0.12] |
| 3.2 Age ≥ 60 years | 1 | 886 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.13, 0.13] |
| 4 Health‐related quality of life | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Adverse events | 2 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.39, 4.31] |
| 6 Self‐efficacy (with types of intervention subgroup) | 2 | 1018 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.39, 0.31] |
| 6.1 Longer and more advanced interventions | 1 | 884 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.02, 0.24] |
| 6.2 Brief and simple interventions | 1 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.60, 0.08] |
| 7 Self‐efficacy (with age subgroup) | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Age < 60 years | 2 | 1018 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.39, 0.31] |
| 8 HbA1c (with types of setting subgroup) | 4 | 1958 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.18, 0.24] |
| 8.1 Community‐based studies | 3 | 1837 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.18, 0.14] |
| 8.2 Hospital‐based studies | 1 | 121 | Mean Difference (IV, Random, 95% CI) | 0.44 [0.00, 0.88] |
| 9 HbA1c (with types of intervention subgroup) | 4 | 1958 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.18, 0.24] |
| 9.1 Longer and more advanced interventions | 2 | 1013 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.50, 0.20] |
| 9.2 Brief and simple interventions | 2 | 945 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.18, 0.55] |
| 10 HbA1c (with age subgroup) | 4 | 1958 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.18, 0.24] |
| 10.1 Age < 60 years | 2 | 945 | Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.18, 0.55] |
| 10.2 Age ≥ 60 years | 2 | 1013 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.50, 0.20] |
| 11 Systolic blood pressure (with types of interventions subgroup) | 3 | 1085 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐1.70, 2.50] |
| 11.1 Longer and more advanced interventions | 1 | 127 | Mean Difference (IV, Random, 95% CI) | ‐1.30 [‐6.02, 3.42] |
| 11.2 Brief and simple interventions | 2 | 958 | Mean Difference (IV, Random, 95% CI) | 0.82 [‐1.53, 3.17] |
| 12 Diastolic blood pressure (with types of interventions subgroup) | 3 | 1085 | Mean Difference (IV, Random, 95% CI) | 1.52 [‐0.68, 3.72] |
| 12.1 Longer and more advanced interventions | 1 | 127 | Mean Difference (IV, Random, 95% CI) | 5.0 [0.59, 9.41] |
| 12.2 Brief and simple interventions | 2 | 958 | Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.71, 1.79] |
| 13 All‐cause mortality | 2 | 1822 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.23, 2.07] |
| 13.1 At more than 12 months | 1 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.01, 7.23] |
| 13.2 At less than 12 months | 2 | 998 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.24, 2.48] |
| 14 All‐cause mortality (with age subgroup) | 3 | 1488 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.27, 5.79] |
| 14.1 Age < 60 years | 2 | 1369 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.08, 18.55] |
| 14.2 Age ≥ 60 years | 1 | 119 | Odds Ratio (M‐H, Random, 95% CI) | 1.5 [0.24, 9.32] |
2.4. Analysis.
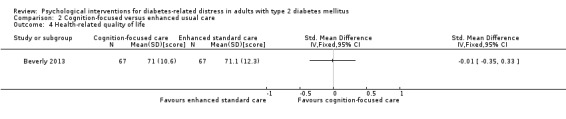
Comparison 2 Cognition‐focused versus enhanced usual care, Outcome 4 Health‐related quality of life.
2.13. Analysis.
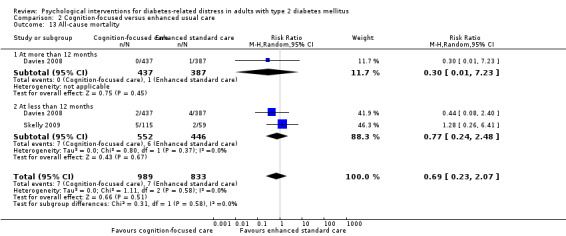
Comparison 2 Cognition‐focused versus enhanced usual care, Outcome 13 All‐cause mortality.
Comparison 3. Cognition‐focused versus usual and enhanced usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diabetes‐related distress (with types of setting subgroup) | 8 | 3225 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.12, 0.02] |
| 1.1 Community‐based studies | 6 | 3032 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.12, 0.03] |
| 1.2 Hospital‐based studies | 2 | 193 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.40, 0.18] |
| 2 Diabetes‐related distress (with types of intervention subgroup) | 8 | 3276 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.12, 0.03] |
| 2.1 Longer and more advanced interventions | 4 | 1576 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.16, 0.06] |
| 2.2 Brief and simple interventions | 4 | 1700 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.15, 0.07] |
| 3 Diabetes‐related distress (with age subgroup) | 8 | 3276 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.12, 0.03] |
| 3.1 Age < 60 years | 5 | 1648 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.14, 0.05] |
| 3.2 Age ≥ 60 years | 3 | 1628 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.19, 0.09] |
| 4 Health‐related quality of life | 2 | 253 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.14, 0.35] |
| 5 Adverse events | 3 | 760 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.43, 4.09] |
| 6 Self‐efficacy (with types of setting subgroup) | 4 | 1760 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.06, 0.26] |
| 6.1 Community‐based studies | 3 | 1626 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [0.04, 0.25] |
| 6.2 Hospital‐based studies | 1 | 134 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.26 [‐0.60, 0.08] |
| 7 Self‐efficacy (with types of intervention subgroup) | 4 | 1760 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.06, 0.26] |
| 7.1 Longer and more advanced interventions | 1 | 884 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.02, 0.24] |
| 7.2 Brief and simple interventions | 3 | 876 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.22, 0.36] |
| 8 Self‐efficacy (with age subgroup) | 4 | 1760 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.06, 0.26] |
| 8.1 Age < 60 years | 2 | 1018 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.39, 0.31] |
| 8.2 Age ≥ 60 years | 2 | 742 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [0.04, 0.38] |
| 9 HbA1c (with types of setting subgroup) | 7 | 2789 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.30, 0.15] |
| 9.1 Community‐based studies | 5 | 2609 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.34, 0.11] |
| 9.2 Hospital‐based studies | 2 | 180 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐1.36, 1.20] |
| 10 HbA1c (with types of intervention subgroup) | 7 | 2789 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.22, 0.16] |
| 10.1 Longer and more advanced interventions | 4 | 1221 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.74, 0.03] |
| 10.2 Brief and simple interventions | 3 | 1568 | Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.05, 0.27] |
| 11 HbA1c (with age subgroup) | 7 | 2789 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.22, 0.16] |
| 11.1 Age < 60 years | 4 | 1153 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.53, 0.36] |
| 11.2 Age ≥ 60 years | 3 | 1636 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.23, 0.17] |
| 12 Systolic blood pressure (with types of interventions subgroup) | 4 | 1222 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐1.78, 2.27] |
| 12.1 Longer and more advanced interventions | 2 | 264 | Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐5.42, 2.56] |
| 12.2 Brief and simple interventions | 2 | 958 | Mean Difference (IV, Random, 95% CI) | 0.82 [‐1.53, 3.17] |
| 13 Diastolic blood pressure (with types of interventions subgroup) | 4 | 1222 | Mean Difference (IV, Random, 95% CI) | 1.04 [‐0.88, 2.95] |
| 13.1 Longer and more advanced interventions | 2 | 264 | Mean Difference (IV, Random, 95% CI) | 1.75 [‐4.65, 8.15] |
| 13.2 Brief and simple interventions | 2 | 958 | Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.71, 1.79] |
| 14 All‐cause mortality | 4 | 2990 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.42, 2.25] |
| 14.1 At more than 12 months | 2 | 1369 | Risk Ratio (M‐H, Random, 95% CI) | 1.61 [0.10, 26.70] |
| 14.2 At less than 12 months | 3 | 1621 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.31, 2.02] |
| 15 All‐cause mortality (with age subgroup) | 4 | 2111 | Odds Ratio (M‐H, Random, 95% CI) | 1.06 [0.37, 3.02] |
| 15.1 Age < 60 years | 2 | 1369 | Odds Ratio (M‐H, Random, 95% CI) | 1.25 [0.08, 18.55] |
| 15.2 Age ≥ 60 years | 2 | 742 | Odds Ratio (M‐H, Random, 95% CI) | 1.07 [0.32, 3.58] |
3.4. Analysis.
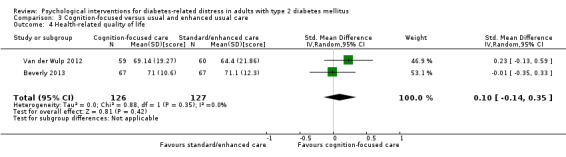
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 4 Health‐related quality of life.
3.5. Analysis.
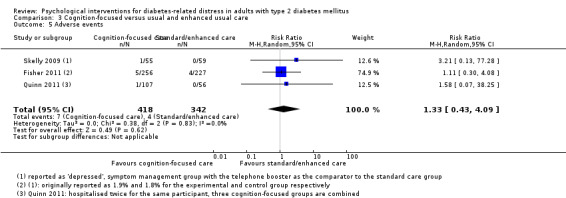
Comparison 3 Cognition‐focused versus usual and enhanced usual care, Outcome 5 Adverse events.
Comparison 4. Emotion‐cognition versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diabetes‐related distress (with types of setting subgroup) | 8 | 2366 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.19, 0.06] |
| 1.1 Community‐based studies | 6 | 2006 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.27, 0.04] |
| 1.2 Hospital‐based studies | 2 | 360 | Std. Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.13, 0.29] |
| 2 Diabetes‐related distress (with types of interventions subgroup) | 8 | 2366 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.19, 0.06] |
| 2.1 Longer and more advanced interventions | 6 | 2102 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 2.2 Brief and simple interventions | 2 | 264 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.62, ‐0.13] |
| 3 Diabetes‐related distress (with age subgroup) | 8 | 2366 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.19, 0.06] |
| 3.1 Age < 60 years | 3 | 408 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.22, 0.18] |
| 3.2 Age ≥ 60 years | 5 | 1958 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.26, 0.08] |
| 4 Adverse events (with types of intervention subgroup) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Brief and simple interventions | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 2.55 [0.77, 8.47] |
| 5 Health‐related quality of life (with types of intervention subgroup) | 4 | 1813 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.11, 0.09] |
| 5.1 Longer and more advanced interventions | 3 | 1694 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.11, 0.10] |
| 5.2 Brief and simple interventions | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.38, 0.34] |
| 6 Adverse events (with age subgroup) | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 Age ≥ 60 years | 2 | 275 | Risk Ratio (M‐H, Random, 95% CI) | 2.55 [0.77, 8.47] |
| 7 Self‐efficacy (with types of setting subgroup) | 4 | 1933 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.08, 0.35] |
| 7.1 Community‐based studies | 3 | 1704 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.17, 0.43] |
| 7.2 Hospital‐based studies | 1 | 229 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.09, 0.43] |
| 8 Self‐efficacy (with types of interventions subgroup) | 4 | 1933 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.08, 0.35] |
| 8.1 Longer and more advanced interventions | 3 | 1792 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.10, 0.19] |
| 8.2 Brief and simple interventions | 1 | 141 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.21, 0.90] |
| 9 Self‐efficacy (with age subgroup) | 4 | 1933 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.08, 0.35] |
| 9.1 Age < 60 years | 1 | 229 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.09, 0.43] |
| 9.2 Age ≥ 60 years | 3 | 1704 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.17, 0.43] |
| 10 HbA1c (with types of setting subgroup) | 8 | 2334 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.18, 0.00] |
| 10.1 Community‐based studies | 6 | 1964 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.14, 0.03] |
| 10.2 Hospital‐based studies | 2 | 370 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.51, ‐0.02] |
| 11 HbA1c (with types of intervention subgroup) | 8 | 2334 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.18, 0.00] |
| 11.1 Longer and more advanced interventions | 6 | 2095 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.17, 0.02] |
| 11.2 Brief and simple interventions | 2 | 239 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.59, 0.17] |
| 12 HbA1c (with age subgroup) | 8 | 2334 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.18, 0.00] |
| 12.1 Age < 60 years | 3 | 398 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.49, ‐0.04] |
| 12.2 Age ≥ 60 years | 5 | 1936 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.14, 0.04] |
| 13 Systolic blood pressure | 2 | 1296 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐2.06, 1.19] |
| 14 Diastolic blood pressure | 2 | 1296 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐1.35, 0.67] |
| 15 All‐cause mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 5. Emotion‐cognition versus cognition‐focused diabetes care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diabetes‐related distress (with types of setting subgroup) | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Community‐based studies | 4 | 1136 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.43, ‐0.12] |
| 1.2 Hospital‐based studies | 5 | 765 | Std. Mean Difference (IV, Random, 95% CI) | 0.14 [‐0.23, 0.52] |
| 2 Diabetes‐related distress (with types of intervention subgroup) | 9 | 1901 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.28, 0.17] |
| 2.1 Longer and more advanced interventions | 7 | 1611 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.35, 0.16] |
| 2.2 Brief and simple interventions | 2 | 290 | Std. Mean Difference (IV, Random, 95% CI) | 0.11 [‐0.45, 0.67] |
| 3 Diabetes‐related distress (with types of deliverer subgroup) | 9 | 1901 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.28, 0.17] |
| 3.1 Nurses and others | 7 | 1646 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.26, 0.28] |
| 3.2 Psychologist | 2 | 255 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.67, 0.04] |
| 4 Diabetes‐related distress (with age subgroup) | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Age < 60 years | 7 | 1607 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.26, 0.25] |
| 4.2 Age ≥ 60 years | 2 | 294 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.97, 0.46] |
| 5 Health‐related quality of life | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Hospital‐based studies | 5 | 765 | Std. Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.27, 0.29] |
| 6 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Self‐efficacy | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Community‐based studies | 2 | 380 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.26, 0.24] |
| 8 HbA1c (with types of setting subgroup) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Community‐based studies | 4 | 1168 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.85, 0.16] |
| 8.2 Hospital‐based studies | 5 | 766 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.10, 0.17] |
| 9 HbA1c (with types of intervention subgroup) | 9 | 1934 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.39, 0.10] |
| 9.1 Longer and more advanced interventions | 7 | 1643 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.45, 0.16] |
| 9.2 Brief and simple interventions | 2 | 291 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.14] |
| 10 HbA1c (with types of deliverer subgroup) | 9 | 1934 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.39, 0.10] |
| 10.1 Nurses and others | 7 | 1646 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.40, 0.18] |
| 10.2 Psychologist | 2 | 288 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.98, 0.33] |
| 11 HbA1c (with age subgroup) | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Age < 60 years | 7 | 1640 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.52, 0.10] |
| 11.2 Age ≥ 60 years | 2 | 294 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.20, 0.30] |
| 12 Systolic blood pressure (with types of setting subgroup) | 5 | 1073 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐2.62, 1.20] |
| 12.1 Community‐based studies | 2 | 667 | Mean Difference (IV, Random, 95% CI) | ‐1.07 [‐3.46, 1.31] |
| 12.2 Hospital‐based study | 3 | 406 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐3.25, 3.15] |
| 13 Systolic blood pressure (with types of intervention subgroup) | 5 | 1073 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐2.62, 1.20] |
| 13.1 Longer and more advanced interventions | 4 | 961 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐2.54, 1.48] |
| 13.2 Brief and simple interventions | 1 | 112 | Mean Difference (IV, Random, 95% CI) | ‐2.37 [‐8.56, 3.82] |
| 14 Systolic blood pressure (with age subgroup) | 5 | 1073 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐2.62, 1.20] |
| 14.1 Age < 60 years | 3 | 779 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐3.47, 0.98] |
| 14.2 Age ≥ 60 years | 2 | 294 | Mean Difference (IV, Random, 95% CI) | 0.79 [‐2.95, 4.53] |
| 15 Diastolic blood pressure (with types of setting subgroup) | 5 | 1073 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.98, 1.34] |
| 15.1 Community‐based studies | 2 | 667 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐1.41, 1.35] |
| 15.2 Hospital‐based study | 3 | 406 | Mean Difference (IV, Random, 95% CI) | 0.46 [‐1.95, 2.87] |
| 16 Diastolic blood pressure (with types of intervention subgroup) | 5 | 1073 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.98, 1.34] |
| 16.1 Longer and more advanced interventions | 4 | 961 | Mean Difference (IV, Random, 95% CI) | 0.28 [‐1.11, 1.66] |
| 16.2 Brief and simple interventions | 1 | 112 | Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐4.72, 3.42] |
| 17 Diastolic blood pressure (with age subgroup) | 5 | 1073 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.98, 1.34] |
| 17.1 Age < 60 years | 3 | 779 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐1.40, 1.21] |
| 17.2 Age ≥ 60 years | 2 | 294 | Mean Difference (IV, Random, 95% CI) | 0.82 [‐2.70, 4.35] |
Comparison 6. Emotion‐focused versus cognition‐focused diabetes care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 7. Psychological interventions versus usual and enhanced diabetes care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diabetes‐related distress | 14 | 5208 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.13, 0.00] |
| 2 Diabetes‐related distress (with types of setting subgroup) | 14 | 5208 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.13, 0.00] |
| 2.1 Community‐based studies | 10 | 4655 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.16, ‐0.00] |
| 2.2 Hospital‐based studies | 4 | 553 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.15, 0.18] |
| 3 Diabetes‐related distress (with types of intervention subgroup) | 14 | 5211 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.13, ‐0.00] |
| 3.1 Longer and more advanced interventions | 9 | 3366 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.11, 0.04] |
| 3.2 Brief and simple interventions | 5 | 1845 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.31, 0.01] |
| 4 Diabetes‐related distress (with age subgroup) | 14 | 5211 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.13, ‐0.00] |
| 4.1 Age < 60 years | 8 | 2005 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.13, 0.04] |
| 4.2 Age ≥ 60 years | 6 | 3206 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.22, 0.02] |
| 5 Health‐related quality of life | 4 | 1683 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 6 Adverse events | 5 | 1035 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.79, 4.09] |
| 7 Self efficacy | 6 | 3310 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [0.00, 0.27] |
| 8 HbA1c | 14 | 4859 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.21, 0.03] |
| 9 HbA1c (with types of setting subgroup) | 14 | 4859 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.21, 0.03] |
| 9.1 Community‐based studies | 10 | 4309 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.20, 0.04] |
| 9.2 Hospital‐based studies | 4 | 550 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.57, 0.44] |
| 10 HbA1c (with types of intervention subgroup) | 13 | 4732 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.20, 0.05] |
| 10.1 Longer and more advanced interventions | 8 | 2925 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.38, ‐0.00] |
| 10.2 Brief and simple interventions | 5 | 1807 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.14, 0.19] |
| 11 HbA1c (with age subgroup) | 13 | 4732 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.20, 0.05] |
| 11.1 Age < 60 years | 7 | 1551 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.49, 0.12] |
| 11.2 Age < 60 years | 6 | 3181 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.11, 0.06] |
| 12 Systolic blood pressure | 5 | 2391 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐1.46, 1.47] |
| 13 Diastolic blood pressure | 5 | 2391 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.93, 0.74] |
| 14 All‐cause mortality | 3 | 1376 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.17, 6.03] |
7.1. Analysis.
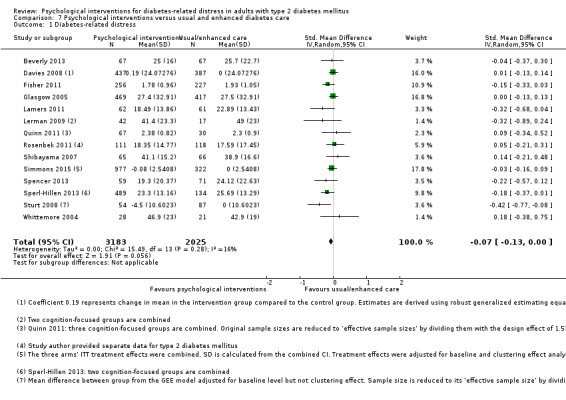
Comparison 7 Psychological interventions versus usual and enhanced diabetes care, Outcome 1 Diabetes‐related distress.
7.5. Analysis.
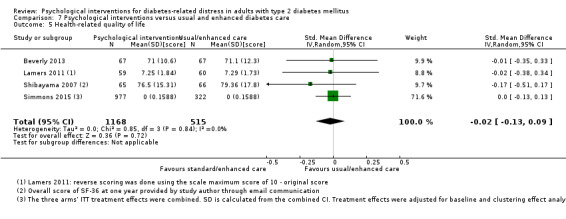
Comparison 7 Psychological interventions versus usual and enhanced diabetes care, Outcome 5 Health‐related quality of life.
7.6. Analysis.
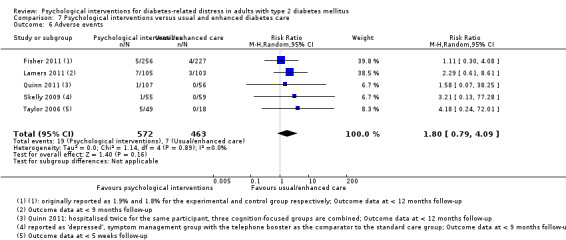
Comparison 7 Psychological interventions versus usual and enhanced diabetes care, Outcome 6 Adverse events.
7.7. Analysis.
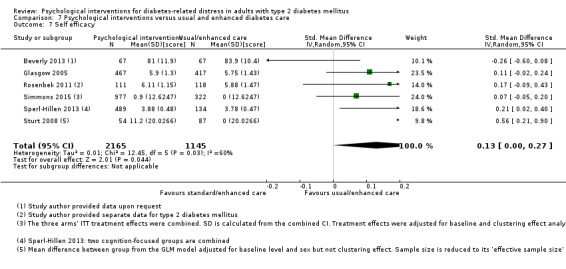
Comparison 7 Psychological interventions versus usual and enhanced diabetes care, Outcome 7 Self efficacy.
7.14. Analysis.
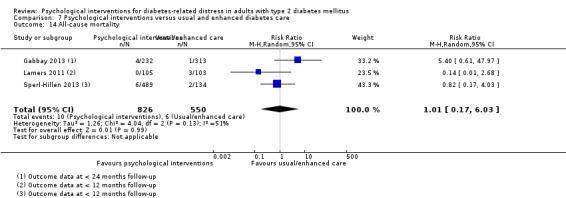
Comparison 7 Psychological interventions versus usual and enhanced diabetes care, Outcome 14 All‐cause mortality.
Comparison 8. Psychological interventions versus usual diabetes care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diabetes‐related distress | 10 | 2932 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.20, 0.01] |
| 2 Diabetes‐related distress (with types of setting subgroup) | 10 | 2881 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.21, 0.01] |
| 2.1 Community‐based studies | 7 | 2462 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.27, ‐0.01] |
| 2.2 Hospital‐based studies | 3 | 419 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.16, 0.23] |
| 3 Diabetes‐related distress (with types of intervention subgroup) | 10 | 2884 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.21, 0.00] |
| 3.1 Longer and more advanced interventions | 7 | 1997 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.12, 0.08] |
| 3.2 Brief and simple interventions | 3 | 887 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.40, ‐0.10] |
| 4 Diabetes‐related distress (with age subgroup) | 10 | 2884 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.21, 0.00] |
| 4.1 Age < 60 years | 5 | 564 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.20, 0.14] |
| 4.2 Age ≥ 60 years | 5 | 2320 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.30, 0.01] |
| 5 Health‐related quality of life (with types of setting subgroup) | 3 | 1549 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 5.1 Community‐based studies | 2 | 1418 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.12, 0.12] |
| 5.2 Hospital‐based studies | 1 | 131 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.51, 0.17] |
| 6 Health‐related quality of life (with types of intervention subgroup) | 3 | 1549 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.13, 0.09] |
| 6.1 Longer and more advanced interventions | 2 | 1430 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.14, 0.10] |
| 6.2 Brief and simple interventions | 1 | 119 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.38, 0.34] |
| 7 Adverse events | 3 | 438 | Risk Ratio (M‐H, Random, 95% CI) | 2.40 [0.78, 7.39] |
| 8 Self efficacy (with types of setting subgroup) | 4 | 2292 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.04, 0.37] |
| 8.1 Community‐based studies | 3 | 2063 | Std. Mean Difference (IV, Random, 95% CI) | 0.23 [0.01, 0.45] |
| 8.2 Hospital‐based studies | 1 | 229 | Std. Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.09, 0.43] |
| 9 Self efficacy (with types of intervention subgroup) | 4 | 2292 | Std. Mean Difference (IV, Random, 95% CI) | 0.20 [0.04, 0.37] |
| 9.1 Longer and more advanced interventions | 2 | 1528 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.02, 0.20] |
| 9.2 Brief and simple interventions | 2 | 764 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [0.02, 0.69] |
| 10 HbA1c | 10 | 2901 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.33, ‐0.00] |
| 11 HbA1c (with types of setting subgroup) | 10 | 2901 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.33, ‐0.00] |
| 11.1 Community‐based studies | 7 | 2472 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.33, 0.05] |
| 11.2 Hospital‐based studies | 3 | 429 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.53, ‐0.05] |
| 12 HbA1c (with types of intervention subgroup) | 10 | 2901 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.33, ‐0.00] |
| 12.1 Longer and more advanced interventions | 7 | 2039 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.53, ‐0.00] |
| 12.2 Brief and simple interventions | 3 | 862 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.30, 0.16] |
| 13 HbA1c (with age subgroup) | 10 | 2901 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.33, ‐0.00] |
| 13.1 Age < 60 years | 5 | 606 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.76, ‐0.09] |
| 13.2 Age ≥ 60 years | 5 | 2295 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.12, 0.07] |
| 14 Systolic blood pressure | 3 | 1433 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐2.08, 1.09] |
| 15 Diastolic blood pressure | 4 | 1567 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐1.11, 0.74] |
| 16 All‐cause mortality | 3 | 1376 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.17, 6.03] |
8.1. Analysis.
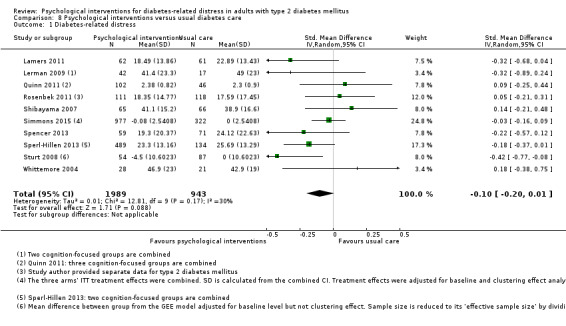
Comparison 8 Psychological interventions versus usual diabetes care, Outcome 1 Diabetes‐related distress.
Comparison 9. Psychological interventions versus usual care (trials with low overall risk of bias).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diabetes‐related distress (with types of intervention subgroup) measured by PAID | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Brief and simple interventions | 3 | 865 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐4.76, 0.75] |
| 2 Diabetes‐related distress (with age subgroup) | 3 | 865 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐4.76, 0.75] |
| 2.1 Age ≥ 60 years | 3 | 865 | Mean Difference (IV, Random, 95% CI) | ‐2.00 [‐4.76, 0.75] |
| 3 Health‐related quality of life | 2 | 238 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.15, 0.36] |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Self‐efficacy | 3 | 883 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [0.09, 0.51] |
| 6 HbA1c | 4 | 2237 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.11, 0.08] |
| 7 HbA1c (with types of intervention subgroup) | 4 | 2237 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.11, 0.08] |
| 7.1 Longer and more advanced interventions | 2 | 1412 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.55, 0.30] |
| 7.2 Brief and simple interventions | 2 | 825 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.17, 0.16] |
| 8 HbA1c (with age subgroup) | 4 | 2237 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.11, 0.08] |
| 8.1 Age < 60 years | 1 | 113 | Mean Difference (IV, Random, 95% CI) | ‐0.53 [‐1.30, 0.24] |
| 8.2 Age ≥ 60 years | 3 | 2124 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.10, 0.08] |
| 9 All‐cause mortality | 2 | 1168 | Odds Ratio (M‐H, Random, 95% CI) | 1.82 [0.29, 11.66] |
9.3. Analysis.
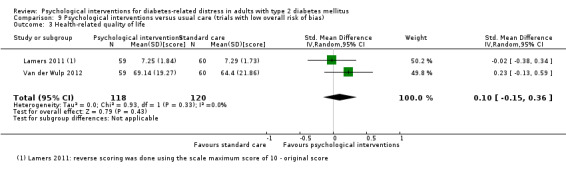
Comparison 9 Psychological interventions versus usual care (trials with low overall risk of bias), Outcome 3 Health‐related quality of life.
9.4. Analysis.
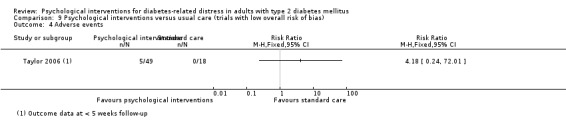
Comparison 9 Psychological interventions versus usual care (trials with low overall risk of bias), Outcome 4 Adverse events.
9.5. Analysis.
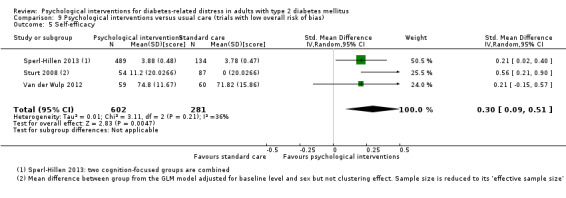
Comparison 9 Psychological interventions versus usual care (trials with low overall risk of bias), Outcome 5 Self‐efficacy.
9.6. Analysis.
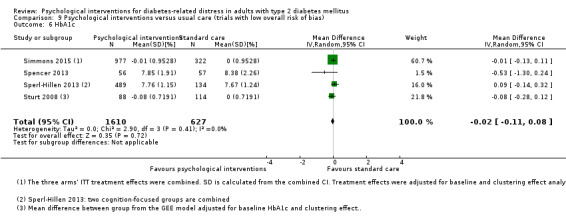
Comparison 9 Psychological interventions versus usual care (trials with low overall risk of bias), Outcome 6 HbA1c.
9.9. Analysis.
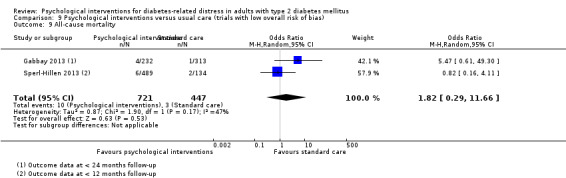
Comparison 9 Psychological interventions versus usual care (trials with low overall risk of bias), Outcome 9 All‐cause mortality.
Comparison 10. Emotion‐cognition versus cognition‐focused (trials with imputation for missing data).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diabetes‐related distress (with types of settings subgroup) | 4 | 1030 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.47, 0.15] |
| 1.1 Community‐based studies | 2 | 791 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.35, ‐0.04] |
| 1.2 Hospital‐based studies | 2 | 239 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐1.13, 0.90] |
| 2 Diabetes‐related distress (with age subgroup) | 4 | 1030 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.47, 0.15] |
| 2.1 Age < 60 years | 3 | 903 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.35, 0.27] |
| 2.2 Age ≥ 60 years | 1 | 127 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.98, ‐0.27] |
| 3 Health‐related quality of life | 2 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Hospital‐based studies | 2 | 239 | Std. Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.69, 1.12] |
| 4 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Self‐efficacy | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Community‐based studies | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 HbA1c (with types of settings subgroup) | 4 | 1030 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.72, 0.14] |
| 6.1 Community‐based studies | 2 | 791 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.15, 0.26] |
| 6.2 Hospital‐based studies | 2 | 239 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.33, 0.23] |
| 7 HbA1c (with age subgroup) | 4 | 1030 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.72, 0.14] |
| 7.1 Age < 60 years | 3 | 903 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.92, 0.11] |
| 7.2 Age ≥ 60 years | 1 | 127 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.30, 0.34] |
| 8 Systolic blood pressure (with types of settings subgroup) | 3 | 638 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐2.78, 2.61] |
| 8.1 Community‐based studies | 1 | 399 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐3.40, 3.80] |
| 8.2 Hospital‐based study | 2 | 239 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐4.51, 3.61] |
| 9 Diastolic blood pressure (with types of settings subgroup) | 3 | 638 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐1.49, 1.54] |
| 9.1 Community‐based studies | 1 | 399 | Mean Difference (IV, Random, 95% CI) | 0.60 [‐1.34, 2.54] |
| 9.2 Hospital‐based study | 2 | 239 | Mean Difference (IV, Random, 95% CI) | ‐0.87 [‐3.31, 1.56] |
10.3. Analysis.
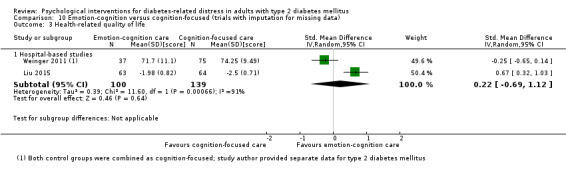
Comparison 10 Emotion‐cognition versus cognition‐focused (trials with imputation for missing data), Outcome 3 Health‐related quality of life.
10.4. Analysis.

Comparison 10 Emotion‐cognition versus cognition‐focused (trials with imputation for missing data), Outcome 4 Adverse events.
10.5. Analysis.
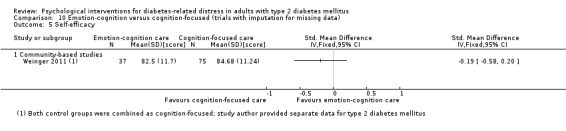
Comparison 10 Emotion‐cognition versus cognition‐focused (trials with imputation for missing data), Outcome 5 Self‐efficacy.
Comparison 11. Psychological interventions (trials with imputation for missing data) versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Diabetes‐related distress | 3 | 1541 | Mean Difference (IV, Random, 95% CI) | ‐0.60 [‐3.08, 1.88] |
| 2 Health‐related quality of life | 3 | 1537 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.09, 0.13] |
| 3 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Self‐efficacy | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 HbA1c | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Systolic blood pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Diastolic blood pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 All‐cause mortality | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
11.3. Analysis.
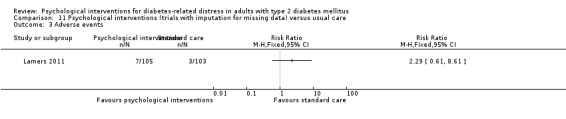
Comparison 11 Psychological interventions (trials with imputation for missing data) versus usual care, Outcome 3 Adverse events.
11.4. Analysis.
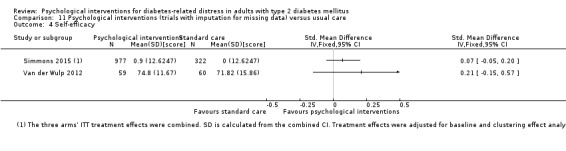
Comparison 11 Psychological interventions (trials with imputation for missing data) versus usual care, Outcome 4 Self‐efficacy.
11.5. Analysis.
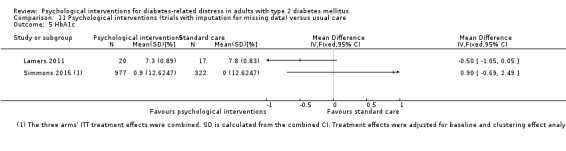
Comparison 11 Psychological interventions (trials with imputation for missing data) versus usual care, Outcome 5 HbA1c.
11.6. Analysis.
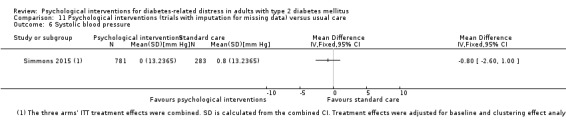
Comparison 11 Psychological interventions (trials with imputation for missing data) versus usual care, Outcome 6 Systolic blood pressure.
11.7. Analysis.
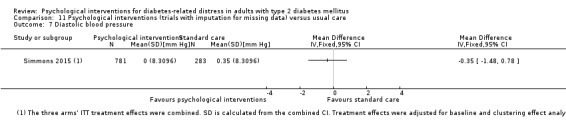
Comparison 11 Psychological interventions (trials with imputation for missing data) versus usual care, Outcome 7 Diastolic blood pressure.
11.8. Analysis.
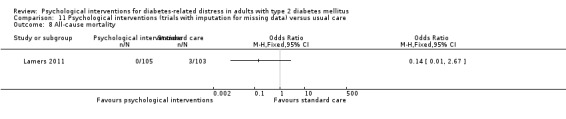
Comparison 11 Psychological interventions (trials with imputation for missing data) versus usual care, Outcome 8 All‐cause mortality.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beverly 2013.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: adults aged 25‐75 years diagnosed with type 2 diabetes for at least 2 years who were taking insulin and/or oral medication for at least 1 year, able to walk briskly, free of severe complications, had at least 3 hours of previous documented diabetes education, and who had a haemoglobin A1c level > 7.0% Exclusion criteria: inability to read and speak English, current or planned pregnancy, severe renal disease (microalbuminuria > 300 µg/mg), severe peripheral diabetic neuropathy and/or severe peripheral vascular disease, symptomatic severe autonomic neuropathy, proliferative diabetic retinopathy based on dilated eye examination within 1 year of study entry, A1c levels < 7.0% and A1c levels > 13.0%, a history of severe unstable myocardial infarction, congestive heart failure or other severe cardiac disease, and severe hypertension (systolic ≥ 160 mmHg or diastolic ≥ 90 mmHg); diagnosed with bipolar disorder, schizophrenia, mental retardation, organic mental disorder, and alcohol or drug abuse Diagnostic criteria: A1c measured via the Turbidimetric Inhibition Immunoassay using the Roche Integra 800 Analyzer (Roche Diagnostics Operations Inc, Indianapolis, Indiana; reference range is 4.0% ‐ 6.0%). Self‐Care Inventory‐R (SCI‐R); pedometer readings (Omron Healthcare, Inc, Lake Forest, Illinois); Brief Symptom Inventory (BSI); Coping Styles; Problem Areas in Diabetes (PAID); Problems With Diabetes Self‐Management Scale (PDSM); Diabetes Quality of Life Scale (DQOL); Confidence in Diabetes Self‐Care Scale (type 2; CIDS‐2); Test of Functional Health Literacy in Adults (TOFHLA) |
|
| Interventions |
Number of study centres: 1 Treatment before study: no Titration period: no Intervention: conversation maps. The 4 maps used for this study covered the following topics: diabetes overview, diabetes and healthy eating, blood glucose and monitoring, and the natural course of diabetes; each map had a programme manual for the group facilitator. At the end of each session, educators assisted participants in setting realistic health goals and developing a plan to achieve meaningful behaviour change in their lives Control: attention control ‐ heart healthy living. Educational classes focusing on dyslipidaemia and hypertension, but not specifically on diabetes self‐care |
|
| Outcomes | Outcomes reported in abstract of publication: A1c levels at 3 months, 6 and 12 months; frequency of self‐reported self‐care, diabetes quality of life, diabetes‐related distress and frustration with diabetes self‐care over time | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NCT00895986 |
|
| Publication details |
Language of publication: English Commercial and non‐commercial funding: American Diabetes Association (ADA) grant 7‐08‐CR‐62, the Diabetes and Endocrinology Research Core NIH P30 DK36836, and the NIH Training Grant No. T32 DK007260. Bayer Health Care LLC (Tarrytown, New York) contributed glucose meters and test strips Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "The purpose of the study was to assess the value of reinforcing diabetes self‐management for improving glycaemia and self‐care among adults with type 2 diabetes who had prior diabetes education." | |
| Notes | Multiple imputations with the Markov Chain Monte Carlo method (SAS Proc MI) were used to input missing data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "A block randomisation sequence based on a random number table was generated with randomization.com to ensure balance between the 2 groups at study end." Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Educators and study physicians had no role in randomisation." Comment: probably done |
| Blinding of participants and personnel (performance bias) Blood pressure | Low risk |
Quote from publication: "In addition to sociodemographic factors (age, sex, race/ethnicity, education level, marital status, occupation) and health factors (duration of diabetes, body mass index [BMI], waist circumference, blood pressure) ..." Comment: investigator‐assessed outcome measurement. Trial author communicated that standard measurement was undertaken, and the nurses were blinded to study assignment and intervention details |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "Finally, participants completed the following measures." No more direct quote is available in the publication Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "... A1c, measured via the Turbidimetric Inhibition Immunoassay using the Roche Integra 800 Analyzer." Comment: laboratory outcome measurement |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | Unclear risk |
Quote from publication: "Finally, participants completed the following measures." No more direct quote is available in the publication Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) Self‐efficacy | Unclear risk |
Quote from publication: "Finally, participants completed the following measures." No more direct quote is available in the publication Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) Blood pressure | Low risk |
Quote from publication: "In addition to sociodemographic factors (age, sex, race/ethnicity, education level, marital status, occupation) and health factors (duration of diabetes, body mass index [BMI], waist circumference, blood pressure),..." Comment: investigator‐assessed outcome measurement. Trial author communicated that standard measurement was undertaken, and the nurses were blinded to study assignment and intervention details |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "Finally, participants completed the following measures." No more direct quote is available in the publication Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "... A1c, measured via the Turbidimetric Inhibition Immunoassay using the Roche Integra 800 Analyzer." Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | Unclear risk |
Quote from publication: "Finally, participants completed the following measures." No more direct quote is available in the publication Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) Self‐efficacy | Unclear risk |
Quote from publication: "Finally, participants completed the following measures." No more direct quote is available in the publication Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Incomplete outcome data (attrition bias) Blood pressure | Unclear risk | Quote from publication: "None of the improvements in secondary outcomes differed by type of intervention. ... did not complete any surveys at follow‐up but provided physiological and laboratory data." Comment: not specifically reported |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "... diabetes‐related distress ... improved in both groups. None of the improvements in secondary outcomes differed by type of intervention ... An additional 6 participants (4 intervention, 2 control) did not complete any surveys at follow‐up ... As the pattern of our missing data was arbitrary, multiple imputations with the Markov Chain Monte Carlo method (SAS Proc MI) were used to input missing data.The results presented are based on combined inferences of the 15 complete data sets. The imputation model was built using demographic, psychosocial, and A1c values." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "Three other randomised participants did not return for follow‐up visits. All 4 (including dropped participant) were randomised to the intervention group ... An additional 6 participants (4 intervention, 2 control) did not complete any surveys at follow‐up but provided physiological and laboratory data." Comment: reported and reasons explained, more than 80% of the HbA1c measurements were available from every follow‐up time points |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | Low risk |
Quote from publication: "... diabetes quality of life ... improved in both groups. None of the improvements in secondary outcomes differed by type of intervention ... An additional 6 participants (4 intervention, 2 control) did not complete any surveys at follow‐up ... As the pattern of our missing data was arbitrary, multiple imputations with the Markov Chain Monte Carlo method (SAS Proc MI) were used to input missing data.The results presented are based on combined inferences of the 15 complete data sets. The imputation model was built using demographic, psychosocial, and A1c values." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) Self‐efficacy | Unclear risk |
Quote from publication: "None of the improvements in secondary outcomes differed by type of intervention..... An additional 6 participants (4 intervention, 2 control) did not complete any surveys at follow‐up... As the pattern of our missing data was arbitrary, multiple imputations with the Markov Chain Monte Carlo method (SAS Proc MI) were used to input missing data.The results presented are based on combined inferences of the 15 complete data sets. The imputation model was built using demographic, psychosocial, and A1c values." Comment: not specifically reported |
| Selective reporting (reporting bias) | High risk | Comment: self‐care behaviour was mentioned as the primary outcome in the trials register record but HbA1c was reported as the primary outcome in the publication, probably due to non‐significant results in the former and significant results in the HbA1c. All other outcomes including self‐care behaviour were reported as specified |
D'Eramo Melkus 2010.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: black women aged 21‐65 years, had a diagnosis of type 2 diabetes mellitus confirmed by C‐peptide assay, did not require insulin, had a body mass index (BMI) < 37 kg/m², were receiving diabetes treatment from a primary care provider, were not pregnant or lactating, and were able to read and speak English Exclusion criteria: diagnosed serious psychiatric or medical illness (cancer, AIDS) or diabetes‐related complication (renal disease), and subsequent treatment that would interfere with laboratory assays of the outcome variables as well as full study participation and completion Diagnostic criteria: anxiety was measured using the Crown‐Crisp Index; diabetes‐related emotional distress was measured using the 25‐item PAID; Diabetes‐specific social support was measured using a sub scale of the Diabetes Care Profile (DCP); Diabetes Self‐Efficacy Outcomes Expectancies Questionnaire (DSEQ); Diabetes Knowledge Test self‐developed by the investigators; The Medical Outcomes Study (MOS)‐SF‐36 was used to measure general quality of life; health care provider support was measured with the Modified Health Care Climate Questionnaires (MHCCQ) |
|
| Interventions |
Number of study centres: 2 Treatment before study: — Titration period: no Intervention: cognitive behavioural diabetes self‐management training (DSMT). The first 6 sessions: culturally relevant cognitive behavioural DSMT based on American Association of Diabetes Educators (AADE) standards. These sessions facilitate cognition and emotion used the transtheoretical model of behaviour change (TMBC) processes to move participants from the preparation to the action stage of behavioural change. The remaining 5 sessions address the following areas using the context of lifestyle behaviour for supporting diabetes self‐management: understanding stress (multiple life roles and the stress cycle); problem identification and explorations; problem‐solving strategies; managing your stress; and communication (active listening, assertiveness, and refusal techniques) Control: community hospital‐based group diabetes education classes. The first 5 sessions: standardised culturally neutral usual diabetes education. The last 5 sessions: providing diabetes discussion in addition to group sessions; both treatment arms also received nurse practitioner primary care diabetes medical management, based on American Diabetes Association standards, at 3‐month intervals |
|
| Outcomes | Outcomes reported in abstract of publication: haemoglobin A1c from baseline to 3 months and at 12 and 24 months; systolic blood pressure and low‐density lipoprotein cholesterol levels from baseline to 24 months. Baseline quality of life ((QOL) and Medical Outcome Study Short Form‐36); social function, role‐emotional and mental health domains at 12 months and 24 months; general health, vitality, role physical and bodily pain domains over time. Perceived provider support for diet and exercise over time; diabetes‐related emotional distress | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: — |
|
| Publication details |
Language of publication: English Non‐commercial funding: NIH Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "To test the effects of the intervention on glycaemic control, cardiac risk profile, diabetes self‐efficacy, diabetes‐related emotional distress, and QOL" | |
| Notes | No mention of missing data handling, probably no imputation of missing values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Enrolled participants were computer randomised to one of two interventions". Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Enrolled participants were computer randomised to one of two interventions". Comment: probably done |
| Blinding of participants and personnel (performance bias) Blood pressure | Low risk |
Quote from publication: "Physiological ... measures were obtained by trained study personnel ... Blood pressure, systolic (SBP) and diastolic (DBP), was measured by a mercury manometer meeting issued standards. Participants were instructed to refrain from smoking or caffeine intake 30 min prior to the readings. They were seated in a chair with arms and backs supported for a rest period of 5 min before the first blood pressure reading was taken with the appropriate size cuff. Two readings separated by 5 min were averaged to obtain the SBP and DBP". Comment: investigator‐assessed outcome measurement. Trial author communicated that assessor was blinded |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "Psychosocial measures were obtained by trained study personnel." Comment: self‐reported outcome measurement but modes of administration unclear, probably interviewed and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "... derived from a sample of venous blood using the Glyc‐affin Ghb (Isolab Inc., 1992) column method. " Comment: adjudicated outcome measurement |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | High risk |
Quote from publication: "Psychosocial measures were obtained by trained study personnel." Comment: self‐reported outcome measurement but modes of administration unclear, probably interviewed and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) Self‐efficacy | High risk |
Quote from publication: "Psychosocial measures were obtained by trained study personnel." Comment: self‐reported outcome measurement but modes of administration unclear, probably interviewed and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) Blood pressure | Low risk |
Quote from publication: "Physiological measures were obtained by trained study personnel ... Blood pressure, systolic (SBP) and diastolic (DBP), was measured by a mercury manometer meeting issued standards." Comment: investigator‐assessed outcome measurement. Trial author communicated that assessor was blinded |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "Psychosocial measures were obtained by trained study personnel. Procedures for data collection were routinely evaluated to ensure adherence to the measurement protocols and statistical conclusion validity." Comment: self‐reported outcome measurement but modes of administration unclear, probably interviewed and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "... derived from a sample of venous blood using the Glyc‐affin Ghb (Isolab Inc., 1992) column method. " Comment: adjudicated outcome measurement |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | High risk |
Quote from publication: "Psychosocial measures were obtained by trained study personnel. Procedures for data collection were routinely evaluated to ensure adherence to the measurement protocols and statistical conclusion validity." Comment: self‐reported outcome measurement but modes of administration unclear, probably interviewed and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) Self‐efficacy | High risk |
Quote from publication: "Psychosocial measures were obtained by trained study personnel. Procedures for data collection were routinely evaluated to ensure adherence to the measurement protocols and statistical conclusion validity." Comment: self‐reported outcome measurement but modes of administration unclear, probably interviewed and similarly done in intervention groups |
| Incomplete outcome data (attrition bias) Blood pressure | Unclear risk |
Quote from publication: "Discontinued (sporadic attendance) intervention (n = 6) due to time and travel, family‐/work‐related demands; Lost to follow‐up (n = 0)." Comment: not reported |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "Discontinued (sporadic attendance) intervention (n = 6) due to time and travel, family‐/work‐related demands; Lost to follow‐up (n = 0)." Comment: reported and reasons explained. Attrition rate was < 20% |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "Discontinued (sporadic attendance) intervention (n = 6) due to time and travel, family‐/work‐related demands; Lost to follow‐up (n = 0)." Comment: reported and reasons explained. Attrition rate was < 20% |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | Low risk |
Quote from publication: "Discontinued (sporadic attendance) intervention (n = 6) due to time and travel, family‐/work‐related demands; Lost to follow‐up (n = 0)." Comment: reported and reasons explained Comment: attrition rate was < 20% |
| Incomplete outcome data (attrition bias) Self‐efficacy | Low risk |
Quote from publication: "Discontinued (sporadic attendance) intervention (n = 6) due to time and travel, family‐/work‐related demands; Lost to follow‐up (n = 0)." Comment: reported and reasons explained. Attrition rate was < 20% |
| Selective reporting (reporting bias) | Unclear risk | Comment: BP, QoL and self‐efficacy (SE) outcomes were reported as non‐significant without details on the effect sizes. No trials register record or published study protocol available |
Davies 2008.
| Methods | Cluster‐randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: type 2 diabetes who were referred within 4 weeks of diagnosis, with those in the intervention arm attending a structured group education programme within 12 weeks of diagnosis Exclusion criteria: aged less than 18 years, had severe and enduring mental health problems, were not primarily responsible for their own care, were unable to participate in a group programme (for example, housebound or unable to communicate in English), or were participating in another research study Diagnostic criteria: WHOQOL‐BREF; illness perceptions questionnaire ‐ revised; PAID; HADS |
|
| Interventions |
Number of study centres: 207 Treatment before study: — Titration period: — Intervention: structured group education programme. Participant empowerment concepts and theories. Learning was elicited rather than taught, with the behaviour of the educators promoting a non‐didactic approach. Curriculum focused on lifestyle factors, such as food choices, physical activity, and cardiovascular risk factors. Participants to consider their own personal risk factors and to choose a specific, achievable goal of behaviour to change Control: enhanced standard care. Control practices were resourced to enable them to provide contact time with healthcare professionals equivalent to that provided by the structured group education programme. The practices were allowed to use the resources as they saw fit within their usual care routine |
|
| Outcomes | Outcomes reported in abstract of publication: haemoglobin A1c levels at 12 months; weight loss at 12 months; the odds of not smoking at 12 months; changes in illness belief scores; depression score at 12 months; association between change in perceived personal responsibility and weight loss at 12 months | |
| Study details |
Run‐in period: no Study terminated before regular end (for benefit /because of adverse events): no Trials register identifier: ISRCTN17844016 |
|
| Publication details |
Language of publication: English Commercial and non‐commercial funding: study was funded by Diabetes UK and the project office administration was funded by an unrestricted educational grant from Novo Nordisk Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "To evaluate the effectiveness of a structured group education programme on biomedical, psychosocial, and lifestyle measures in people with newly diagnosed type 2 diabetes." | |
| Notes | Missing outcomes were not replaced; adjustments were not made for multiple testing | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Randomisation was undertaken independently at the University of Sheffield using Random Log (D Machin, University of Southampton)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "The trial was carried out in 13 sites in primary care, involving 17 primary care organisations across England and Scotland. Randomisation was at practice level, with stratification by training status and type of contract with the primary care organisation (General Medical Services or Personal Medical Services). Randomisation was undertaken independently ... Participating practices represented the wide spectrum of routine care currently available in the UK ... " Comment: probably done |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: no direct quote is available; the CONSORT diagram reported death. Unclear of the method for this outcome measurement. Not defined but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Blood pressure | Unclear risk |
Quote from publication: "We measured ... blood pressure ... We collected data according to standard operating procedures." Comment: investigator‐assessed outcome measurement. Not clearly defined and described whether blinding was applied on the personnel who took the measurement |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "Questionnaire data were collected from participants at the beginning of the study and by postal questionnaire ..." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "Samples were drawn from a venous sample and assayed locally in an accredited laboratory that was part of the national external quality assurance programme, with haemoglobin A levels measured using an aligned method produced by the diabetes control and complications trial." Comment: laboratory outcome measurement |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | Unclear risk |
Quote from publication: "Questionnaire data were collected from participants at the beginning of the study and by postal questionnaire ..." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Unclear risk | Comment: no direct quote is available, the CONSORT diagram reported death. Unclear of the method for this outcome measurement. Not defined |
| Blinding of outcome assessment (detection bias) Blood pressure | Unclear risk |
Quote from publication: "We measured ... blood pressure ... We collected data according to standard operating procedures." Comment: investigator‐assessed outcome measurement. Not described whether blinding was applied on the personnel who took the measurement |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "Questionnaire data were collected from participants at the beginning of the study and by postal questionnaire ..." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "Samples were drawn from a venous sample and assayed locally in an accredited laboratory that was part of the national external quality assurance programme, with haemoglobin A levels measured using an aligned method produced by the diabetes control and complications trial." Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | Unclear risk |
Quote from publication: "Questionnaire data were collected from participants at the beginning of the study and by postal questionnaire ..." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Incomplete outcome data (attrition bias) Blood pressure | Low risk |
Quote from publication: "Statistical analysis was carried out on an intention to treat basis. Missing outcomes were not replaced and we derived an average over time of continuous outcomes. Biomedical data were collected at practice visits." Comment: dropouts reported but not explained. Attrition rates (not attended practices) were < 20% |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "The groups did not differ significantly for emotional impact of diabetes at eight and 12 months ..." Comment: dropouts reported but not explained. Attrition rates (non‐returning of questionnaire) were > 20% |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "Adjustment for baseline and cluster effect, however, indicated that the difference was not statistically significant (P = 0.52 at 12 months). Further analyses ... with an additional adjustment for oral hypoglycaemic agents showed no significant difference between the groups at all time points " "Statistical analysis was carried out on an intention to treat basis. Missing outcomes were not replaced and we derived an average over time of continuous outcomes. Biomedical data were collected at practice visits. " Comment: dropouts reported but not explained. Attrition rates (not attended practices) were < 20% |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | Unclear risk |
Quote from publication: "The groups did not differ significantly in any of the scores for six dimensions of quality of life ..." Comment: dropouts reported but not explained. Attrition rates (non‐returning of questionnaire) were > 20% |
| Selective reporting (reporting bias) | Low risk | Comment: DRD was not mentioned as an outcome in the trials register record ISRCTN17844016 but reported in the publication although DRD results were non‐significant |
| Other bias | Low risk |
Comment: right use of statistical analysis (generalised estimating equations) that adjust for a potential clustering effect Assessment of risk of bias in cluster‐randomised trials
|
Dennick 2015.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: adults with type 2 diabetes aged ≥ 18 years and diagnosed for at least 6 months Exclusion criteria: diagnosed psychiatric disorder, depression treatment/psychological therapy, history of self‐harm or general practitioner (GP) assessment as unsuitable; participants scoring ≥ 16 on the Centre for Epidemiological Studies Depression (CES‐D) scale Diagnostic criteria: depressive symptoms assessed with the CES‐D; PAID scale; perceived health status measured with the EQ‐5D; diabetes self‐care behaviours assessed with the Revised Summary of Diabetes Self‐care Activities questionnaire |
|
| Interventions |
Number of study centres: — Treatment before study: — Titration period: no Intervention: written emotional disclosure Control: neutral writing. Write at home in private. Wrote a description of the previous days' activities, without prompt to discuss thoughts or feelings in order to distinguish writing from content. To prevent inference of one's group assignment, the control exposure was identical except the writing foci |
|
| Outcomes | Outcomes reported in abstract of publication: depressive symptoms; healthy dietary behaviour | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: ISRCTN18442976 |
|
| Publication details |
Language of publication: English Non‐commercial funding: internally funded PhD studentship, with costs in excess of salary covered internally and by securing funds for unrelated consultation work. No specific grant from any funding agency, commercial or not‐for‐profit sectors was received Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "To test the feasibility of written emotional disclosure (WED) for UK primary care patients with Type 2 diabetes." | |
| Notes | Imputation by baseline observations carried forward (as available) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "A list of random numbers allocated sealed, opaque, serially numbered writing packs ..." Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "... which a researcher mailed blind and in sequence each time a primary care patient was enrolled ... Patients' group allocations were also withheld from GPs." Comment: probably done |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "Negative appraisals of WED (i.e., reasons for not completing/returning writing but also issues raised by those completing it) ..." Comment: self‐reported outcome measurement |
| Incomplete outcome data (attrition bias) Adverse events | High risk |
Quote from publication: "Thirty‐two participants (78%) were followed up at three months, of whom 12 (67%) WED and 13 (93%) control participants had returned their writing." Comment: reported and reasons explained |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes were reported as specified in the trials register record, DRD and QoL outcomes were reported although non‐significant |
Fisher 2011.
| Methods | Cluster‐randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: T2DM duration > 1 year; age 25 years; HbA1c level between 7.5% and 12.0%; currently treated by diet, exercise, oral diabetes medication and/or injectable incretin mimetic; able to read and write English; and had not participated in any other research protocol within the last 30 days Exclusion criteria: managed with insulin at the start of study; C‐peptide level > 0.50 ng/mL; used systemic oral or inhaled steroids < 14 days within last 3 months; treated with chemotherapy or radiation therapy; pregnant or breastfeeding; or had severe depression or other severe psychological condition Diagnostic criteria: depressive symptoms were assessed by the Patient Health Questionnaire, omitting the item on suicidality (PHQ‐8); the 17‐item Diabetes Distress Scale (DDS); HbA1c data were collected quarterly and analysed by a central laboratory (Covance, Indianapolis, IN, USA), using the Variant II and Variant II Turbo haemoglobin testing systems (Bio‐Rad Laboratories, Hercules, CA, USA) |
|
| Interventions |
Number of study centres: 34 Treatment before study: — Titration period: no Intervention: collaborative structured self‐monitoring of blood glucose (SMBG). Participants recorded a 7‐point SMBG profile during each of 3 consecutive days prior to each scheduled study visit (months 1, 3, 6, 9, 12), along with energy level and meal size. Participants received instruction on how to identify problematic glycaemic patterns and how best to address each through changes in physical activity, portion size and meal composition. Structured testing group (STG) participants and physicians reviewed the completed form at each visit and made lifestyle and medication changes accordingly. Physicians received training on interpreting the SMBG data and were provided with an algorithm that described various pharmacologic/lifestyle treatment strategies that could be utilised in response to specific SMBG patterns identified by the tool: low blood glucose, high fasting blood glucose, and excessive postprandial glucose excursions Control: active control. Participants did not receive blood glucose analysis system (Accu‐Chek 360 View) or any additional SMBG training. Physicians received no additional training or materials. Both groups received enhanced usual care that included quarterly diabetes‐focused physician visits and free blood glucose meters and strips |
|
| Outcomes | Outcomes reported in abstract of publication: depression and disease‐related distress from baseline to 12 months | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NCT00674986 |
|
| Publication details |
Language of publication: English Commercial funding: Roche Diagnostics, Indianapolis, IN, USA Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "To test whether a structured self‐monitoring of blood glucose (SMBG) protocol reduces depressive symptoms and diabetes distress." | |
| Notes | Missing data were estimated using maximum likelihood methods | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "[P]ractices were stratified by size and type, and then randomised to ..." Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "Patients were then randomly selected from the list, using an external, study‐defined protocol, until the pre‐determined sample size was reached." Comment: probably done |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "All reportable adverse events (AEs) and serious adverse events (SAEs) were documented." Comment: self‐reported outcome measurement; well‐defined |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "The primary outcomes were changes in two measures of diabetes‐related affective status over time." No more direct quote is available in the publication Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "HbA1c data were collected quarterly and analysed by a central laboratory (Covance, Indianapolis, IN, USA), using the Variant II and Variant II Turbo haemoglobin testing systems (Bio‐Rad Laboratories, Hercules, CA, USA)." Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Adverse events | Low risk |
Quote from publication: "All reportable adverse events (AEs) and serious adverse events (SAEs) were documented." Comment: self‐reported outcome measurement; well‐defined |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "The primary outcomes were changes in two measures of diabetes‐related affective status over time." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "HbA1c data were collected quarterly and analysed by a central laboratory (Covance, Indianapolis, IN, USA), using the Variant II and Variant II Turbo haemoglobin testing systems (Bio‐Rad Laboratories, Hercules, CA, USA). " Comment: laboratory outcome measurement |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk |
Quote from publication: "By 12 months, 40 (17.6%) ACG [active control group] patients and 68 (26.6%) STG patients had dropped out ... The incidence of hypoglycaemia (570 mg/dl or 3.9 mmol/l), based on downloaded meter data, was 1.9% in the ACG and 1.8% in the STG (P = ns)." Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "By 12 months, 40 (17.6%) ACG patients and 68 (26.6%) STG patients had dropped out, yielding a combined attrition of 108 (22.4%) patients. Dropouts in both groups were slightly younger (P < 0.02), more likely to be African American (P < 0.02), had a higher HbA1c at baseline (P < 0.01) and had fewer comorbid conditions at baseline (P < 0.02), but did not differ on PHQ‐8 or DDS scores." Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) HbA1c | Unclear risk |
Quote from publication: "By 12 months, 40 (17.6%) ACG patients and 68 (26.6%) STG patients had dropped out ... Dropouts in both groups were slightly younger (P < 0.02), more likely to be African American (P < 0.02), had a higher HbA1c at baseline (P < 0.01) and had fewer comorbid conditions at baseline (P < 0.02) ..." Comment: not reported |
| Selective reporting (reporting bias) | High risk | Comment: HbA1c was mentioned as the primary outcome in the trials register record but was treated as a covariate in the publication without details of its value or analyses as specified in the trials register. Results on HbA1c might have been reported in another publication by Polonsky 2011 that appeared in the in trials register record. DRD had been made a primary outcome from secondary outcome in the trials register record. Adverse event was not mentioned as an outcome in the trials register record. QoL and SE were not reported as specified in the trials register record |
| Other bias | High risk |
Comment: sponsored by a pharmaceutical industry and was thus judged as having a potential conflict of interest Assessment of risk of bias in cluster‐randomised trials
|
Fisher 2013.
| Methods | Randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: registry‐recorded diagnosis of type 2 diabetes ≥ 12 months, a mean score of ≥ 1.5 on the 2‐item Diabetes Distress Screener (confirmed later by the full scale) to indicate at least moderate diabetes distress, age ≥ 21 years, ability to read and speak English, at least moderate computer use facility, easy availability of a computer with Internet access, comfort with Internet use, and self‐reported problems with diabetes management (healthy eating or exercise plan not followed in 3 of 4 days during the previous week or medications not taken 2 or more days during the previous week, based on the Summary of Diabetes Self‐Care Activities Exclusion criteria: clinical depression (Patient Health Questionnaire 8 score ≥ 15) and severe diabetes complications or functional deficits (e.g. dialysis, blindness) Diagnostic criteria: diabetes distress was assessed by the 17‐item DDS; physical activity was assessed by the Community Health Activities Model Program For Seniors; Healthy eating was assessed by the NCI Percent Energy From Fat Screener; Medication adherence was assessed by the 8‐item Hill‐Bone Compliance Scale |
|
| Interventions |
Number of study centres: — Treatment before study: — Titration period: no Intervention 1: CASM ‐ Computer‐assisted self‐management diabetes support and education condition. A 40‐min, previously validated, web‐based diabetes self‐management improvement programme. Participants selected achievable goals for medication adherence, diet, or exercise and were shown how to monitor their daily progress on the site. They received immediate feedback on their success over the past 7 days. The predominately web‐based intervention also provided an ask‐the‐expert forum to enhance engagement. After 6 weeks, participants completed an "action plan" for each previously prioritised management problem. Also included was a list of personalised barriers and strategies to overcome barriers. Participants received 4 live phone calls from their interventionist at weeks 2, 4, 7, and 12 to check progress. At month 5, participants received an automated "behaviour chain" booster programme to reduce negative behavioural practices. This interactive component involved illustrative scenarios of prototypic participants experiencing 'chains of events,' e.g. negative thinking that triggered overeating, followed by an exercise to help 'break' the sequence. Finally, participants received 4 more live 15‐min phone calls at weeks 24, 28, 34, and 48 Intervention 2: CAPS ‐ CASM plus problem‐solving therapy (PST). Participants randomised to CAPS received a 60‐min in‐person intervention that included CASM plus PST. PST is an 8‐step process to identify and define diabetes distress, establish realistic goals, generate ways to meet these goals, weigh the pros and cons of each, choose and evaluate solutions, create a diabetes distress (DD) action plan, evaluate outcome, and engage in pleasant activities. As in CASM, CAPS participants received 4 live phone calls between baseline and month 4 and between month 4 and month 12 to check progress on CASM and PST, respond to problems, and provide encouragement and a live supplemental booster session at month 5 (a review of the PST steps) Control: leap ahead ‐ general. A 20‐min, computer‐delivered health risk appraisal (e.g. seat belt and sunscreen use) along with diabetes information regarding healthy living, diet, and physical activity. This was followed by 8 calls between baseline and month 12. The materials delivered diabetes information only, and participants were not directed to use the information to engage in a specific or structured programme of self‐management or diabetes distress change. Participants received a repeat of the risk appraisal at month 5, the same number and sequence of subsequent live phone calls to answer questions about provided diabetes management information, and assessments similar to those of CASM and CAPS |
|
| Outcomes | Outcomes reported in abstract of publication: DD and regimen distress; reductions in DD were accompanied by significant improvements in healthy eating, physical activity, and medication adherence, although not by change in HbA1c | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NCT00714441 |
|
| Publication details |
Language of publication: English Non‐commercial funding: National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "To compare three interventions to reduce diabetes distress (DD) and improve self‐management among non‐clinically depressed adults with type 2 diabetes mellitus (T2DM)." | |
| Notes | Missing data were imputed with multiple imputation procedures using NORM, version 2, software | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Patients were then randomised individually to one of the three study arms using a computer‐generated algorithm ..." Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Patients were then randomised individually to one of the three study arms using a computer‐generated algorithm ... Based on
telephone screening data, there were no significant differences between those contacted who participated and those who refused." Comment: probably done |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "A separate team of assistants undertook A0, A4, and A12 assessments." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "Glycemic control was assessed by HbA1c, which was analysed in a central laboratory". Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "A separate team of assistants undertook A0, A4, and A12 assessments." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "A separate team of assistants undertook A0, A4, and A12 assessments." Comment: laboratory outcome measurement |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "Attrition was 13.8% from A0 to A4, 5.7% from A4 to A12, and 18.7% from A0 to A12. Only 8.4% of patients missed both A4 and A12 follow‐up assessments. There were no significant between‐group differences in attrition across any time period on any key study variable." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "Attrition was 13.8% from A0 to A4, 5.7% from A4 to A12, and 18.7% from A0 to A12. Only 8.4% of patients missed both A4 and A12 follow‐up assessments. There were no significant between‐group differences in attrition across any time period on any key study variable." Comment: reported and reasons explained |
| Selective reporting (reporting bias) | High risk | Comment: BP was a secondary outcome measure in the trials register record but not reported in the publication; study author communicated and confirmed that no further publication on BP as an outcome measure. |
| Other bias | Low risk | Comment: all results were reported for the randomised groups |
Gabbay 2013.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: aged 18‐75 years with T2D with 1 or more of the following: (i) HbA1c > 8.5%; (ii) blood pressure > 140/90 mmHg; and /or (iii) low‐density lipoprotein (LDL) > 130 mg/dL. Exclusion criteria: could not communicate in either English or Spanish, or if they were residents of nursing homes Diagnostic criteria: PAID scale; the Diabetes Treatment Satisfaction Questionnaire (DTSQ); the CES‐D scale; the Summary of Diabetes Self‐Care Activities (SDSCA); the Audit of Diabetes Dependent Quality of Life (ADDQoL) |
|
| Interventions |
Number of study centres:12 Treatment before study: — Titration period: no Intervention: practice‐embedded nurse case managers (NCMs) care, including MI‐guided behaviour change counselling. Those assigned to the intervention group met individually within their primary care clinic with their NCM at baseline and then at 2 and 6 weeks, followed by 3, 6, and 12 months, and then at least every 6 months thereafter. Individual meeting within participants' primary care clinic with their NCM, and were usually not held on the same days as the participant's visit to his/her primary care provider (PCP). Participants could also contact their NCMs by phone and email between visits when appropriate. The frequency of these phone and email conversations varied based on participant need, as assessed by the NCM. The visits typically included a review of the participant's clinical laboratory test results, health‐related lifestyle behaviour relevant to managing diabetes, and medication adherence. The NCMs also checked whether the participant was due for complications screening and reminded them of follow‐up specialist visits when they were due. Referrals to a certified diabetes nurse educator or a dietitian were made when appropriate. Finally, NCMs prompted the PCPs for medication titrations when necessary. These were done via email, in person, or by telephone, depending on the PCP's preference. NCMs had standing orders for yearly ophthalmologic and foot exams and laboratory tests Control: usual care control. Routine care typically involved visits with a PCP every 3 months. The PCPs were not taught MI and control group participants had no contact with the NCMs |
|
| Outcomes | Outcomes reported in abstract of publication: systolic blood pressure (SBP); HbA1c; LDL; diastolic blood pressure; depression symptom scores; diabetes‐related distress | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NCT00308386 |
|
| Publication details |
Language of publication: English Non‐commercial funding: National Institutes of Health and National Institute of Diabetes and Digestive and Kidney Diseases Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "To determine whether the addition of NCMs trained in motivational interviewing (MI) to usual care would result in improved outcomes in high‐risk type 2 diabetes patients" | |
| Notes | No mention of missing data handling, probably no imputation of missing values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Participants were randomised to ..." Comment: probably done, since earlier reports from the same investigators clearly describe use of a stratified permuted block randomisation scheme (Stuckey 2009) |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "Participants were randomised to ..." Comment: probably done, since earlier reports from the same investigators clearly describe use of a stratified permuted block randomisation scheme (Stuckey 2009) |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: no direct quote is available, the CONSORT diagram reported death. Unclear of the method for this outcome measurement. Not defined but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Blood pressure | Unclear risk |
Quote from publication: "The participants' clinical data were still accessible through the registry as long as they continued to follow‐up with their PCPs." Comment: probably investigator‐assessed outcome measurement, since earlier reports from the same investigators describe use of patient registry system in retrieving the over time blood pressure levels (Stuckey 2009) |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "Surveys were mailed to the participants" Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "'The participants' clinical data were still accessible through the registry as long as they continued to follow‐up with their PCPs." Comment: probably adjudicated outcome measurement, since earlier reports from the same investigators describe use of patient registry system in retrieving the over time HbA1c levels (Stuckey 2009) |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | High risk |
Quote from publication: "Surveys were mailed to the participants" Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Unclear risk | Comment: no direct quote is available, the CONSORT diagram reported death. Unclear of the method for this outcome measurement; not defined |
| Blinding of outcome assessment (detection bias) Blood pressure | Unclear risk |
Quote from publication: "The participants' clinical data were still accessible through the registry as long as they continued to follow‐up with their PCPs." Comment: probably investigator‐assessed outcome measurement, since earlier reports from the same investigators describe use of patient registry system in retrieving the over time blood pressure levels (Stuckey 2009). Unclear of blinding of the assessor |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "Surveys were mailed to the participants" Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "The participants' clinical data were still accessible through the registry as long as they continued to follow‐up with their PCPs." Comment: probably adjudicated outcome measurement, since earlier reports from the same investigators describe use of patient registry system in retrieving the over time HbA1c levels (Stuckey 2009) |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | High risk |
Quote from publication: "Surveys were mailed to the participants" Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Incomplete outcome data (attrition bias) Blood pressure | Low risk |
Quote from publication: "At Year 1, the survey response rate was 56% for the control group and 68% for the intervention group ... Despite this, 81% of the intervention group still had clinical and laboratory data available for analysis. " Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | High risk |
Quote from publication: "At Year 1, the survey response rate was 56% for the control group and 68% for the intervention group. " Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "At Year 1, the survey response rate was 56% for the control group and 68% for the intervention group ... Despite this, 81% of the intervention group still had clinical and laboratory data available for analysis." Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | High risk |
Quote from publication: "At Year 1, the survey response rate was 56% for the control group and 68% for the intervention group." Comment: dropouts reported but not explained |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported, only within‐group improvements were significant and given in details whereas between‐groups results were largely non‐significant and no details reported |
Glasgow 2005.
| Methods | Cluster‐randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: over 25 years of age, ability to read English, and type 2 diabetes Exclusion criteria: — Diagnostic criteria: motivational variables included participants' perceptions of provider autonomy support, assessed by the 6‐item modified Health Care Climate Questionnaire (mHCCQ); perceptions of competence, assessed by the 4‐item Perceived Competence Scale (PCS); autonomy support; participant satisfaction was assessed by 5 items from the NCQA/ADA Provider Recognition Program; HbA1c assays, using a National Glycohemoglobin Standardization Program (NGSP) certified Bui‐Rad Variant 2 analyser (reference range: 4.1% to 6.5%); the DDS was administered to assess diabetes‐specific quality of life; the PHQ‐9 was administered to assess depressive symptoms |
|
| Interventions |
Number of study centres: 30 Treatment before study: — Titration period: no Intervention: Diabetes Priority Program. Participants were asked to come 30 minutes early to their scheduled primary care diabetes‐related visits to complete a computerised touch screen assessment and action planning procedure. The second part of the touch screen computerised program involved establishing a self‐management action plan related to dietary, physical activity, and/or smoking behaviours. The programme assessed current self‐management behaviours, provided tailored feedback, and guided users through selecting specific activities in the goal area, identifying barriers and selecting strategies to overcome the barriers. The computer generated for the participant an individualised action plan, including a summary of self‐management goals and assays for which the participant was due; a 1‐page summary of the participant's needed assessments and self‐management goals, highlighting issues the participant would like to discuss with the physician, and a detailed printout to be used by the office's designated care manager. This included review of participant self‐care goals and medical care needs and problem‐solving strategies to overcome barriers to their goals. The care manager also made brief follow‐up calls after visits. After 6 months, these procedures were repeated Control: enhanced standard care. Touch screen computer assessment procedures were completed by control participants who completed the ADA/NCQA Provider Recognition Program measures and general health risk issues (e.g. use of seatbelts, cancer screening) and were also matched for number of contacts and the novelty of using a diabetes care‐related, interactive touch screen computer programme. Control participants also received a printout on general health risks but did not set self‐management goals, meet with a care manager, or receive follow‐up phone calls |
|
| Outcomes | Outcomes reported in abstract of publication: significantly improved both the number of laboratory assays and patient‐centred aspects of diabetes care that participants received compared with those in the control condition. There was overall improvement on secondary outcomes of lipids, HbA1c, quality of life, and depression scores | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: — |
|
| Publication details |
Language of publication: English Commercial funding/non‐commercial funding/other funding: — Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "This report presents 12‐month follow‐up results from a computer‐assisted, patient‐centred intervention to improve the level of recommended services patients received from a variety of primary care settings." | |
| Notes | Same study as reported in Williams 2007 (see Glasgow 2005), which provided data on self‐efficacy, whereas Glasgow 2005 provided data on HbA1c and diabetes‐related distress. No mention of missing data handling, probably no imputation of missing values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "2‐group, cluster, randomised design. " Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "Randomization was conducted by the project statistician, who then notified research staff of condition assignment." Comment: probably done |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "... complete the computerized touch screen assessment ... The second part of the touch screen computerized program involved ... assessed current self‐management behaviours" Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "HbA1c assays were conducted at the University of Colorado Health Sciences Center using a National Glycohemoglobin Standardization Program certified Bio‐Rad Variant 2 analyser (Bio‐Rad, Richmond, CA) ..." Comment: laboratory outcome measurement |
| Blinding of participants and personnel (performance bias) Self‐efficacy | Unclear risk |
Quote from publication: "The second part of the touch screen computerized program involved ... assessed current self‐management behaviours" (Williams 2007) Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "... complete the computerized touch screen assessment ... The second part of the touch screen computerized program involved ... assessed current self‐management behaviours" Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "HbA1c assays were conducted at the University of Colorado Health Sciences Center using a National Glycohemoglobin Standardization Program certified Bio‐Rad Variant 2 analyser (Bio‐Rad, Richmond, CA) ..." Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Self‐efficacy | Unclear risk |
Quote from publication: "The second part of the touch screen computerized program involved ... assessed current self‐management behaviours" (Williams 2007) Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "Attrition rates were approximately equivalent (19% in intervention and 15% in control) ...There were no differences between the two conditions in the characteristics of patients who dropped out ... analyses were conducted on complete cases. Analyses using intent‐to‐treat procedures (and assuming those lost to follow‐up at 12 months were performing at their most recently collected levels) produced identical conclusions." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "Attrition rates were approximately equivalent (19% in intervention and 15% in control) ... There were no differences between the two conditions in the characteristics of patients who dropped out ... analyses were conducted on complete cases. Analyses using intent‐to‐treat procedures (and assuming those lost to follow‐up at 12 months were performing at their most recently collected levels) produced identical conclusions." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) Self‐efficacy | Unclear risk |
Quote from publication: no direct quote is available, no CONSORT diagram (Williams 2007) Comment: not reported |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome measures were reported as specified in the publication, no prior trials register record or study design paper was available |
| Other bias | Unclear risk |
Comment: did not provide clear funding sources except that it was a collaboration between the research team and the Copic Insurance Company, which provides malpractice insurance to 95% of the independent primary care physicians in Colorado, USA Assessment of risk of bias in cluster‐randomised trials
|
Grillo 2016.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: adult subjects (between 18 and 80 years old), with type 2 diabetes mellitus and HbA1c > 7%, attending the primary care unit at least once in the 6 months prior to the screening visit, and willing to attend the 5‐week course Exclusion criteria: history of active infection (e.g. osteomyelitis, pulmonary tuberculosis, AIDS), chronic corticosteroid use, unstable angina or myocardial infarction in the last 3 months, advanced renal disease requiring dialysis, heart failure (New York Heart Association classes III and IV), cirrhosis, alcohol abuse, illicit drug use, dementia, current pregnancy or breastfeeding, current cancer, or any disease that might affect survival in the subsequent 5 years Diagnostic criteria: psychological impact of diabetes mellitus was evaluated by the 20‐item PAID questionnaire; HbA1c measurements were performed by high‐performance liquid chromatography – HPLC (Merck‐Hitachi 9000, reference range: 4.7‐6.0%, Hercules, USA); Blood pressure was measured twice with a digital sphygmomanometer (ONROM, São Paulo, Brazil), with the patient in sitting position, after a 5‐min rest and with 1‐min interval between measurements |
|
| Interventions |
Number of study centres: 1 Treatment before study: no Titration period: no Intervention: Structured Diabetes Self‐management Education Course. Identification of modifiable risk factors for type 2 diabetes mellitus; nonpharmacological treatment, emphasising diet and exercise; pharmacological therapy, including mechanism of action and side effects of glucose‐lowering medications provided by the Brazilian public health system (metformin, glyburide, and NPH and regular insulin); an overview of chronic diabetes complications; and foot care. All patients received usual medical care at the discretion of their primary care physician. Control: attention‐control with same frequency of contact. The control group visited the centre at the same frequency as the intervention group, for a diabetic group meeting with the nurse, but no structured diabetes education was provided. During the control group meetings, participants discussed personal life issues or those related to other diseases. When control participants asked questions about diabetes, the nurse provided concise answers. Both groups were assisted by the same generalist nurse. All patients received usual medical care at the discretion of their primary care physician |
|
| Outcomes | Outcomes reported in abstract of publication: metabolic control, weight, blood pressure, distress scores, and knowledge on diabetes | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NCT01473329 |
|
| Publication details |
Language of publication: English Non‐commercial funding: Fundo de Incentivo à Pesquisa (FIPE) do HCPA (university's funding) Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication:"This study thus aimed to evaluate the effect of a group diabetes mellitus education program (a 5‐week course and reinforcement meetings every 4 months for one year applied by a generalist nurse) on HbA1c in uncontrolled type 2 diabetes mellitus patients attending a primary care unit." | |
| Notes | No mention of missing data handling, probably no imputation of missing values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Participants were randomly assigned to the intervention or control group following block randomization procedures." Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "Patients received a telephone invitation to participate, and a visit was scheduled to orient them on informed consent and protocol procedures." Comment: probably not done. However, the intervention and control groups were similar for all the clinical and laboratory variables at baseline except that there were 7 withdrawal in the control compared to 1 withdrawal in the intervention group. |
| Blinding of participants and personnel (performance bias) Blood pressure | Low risk |
Quote from publication: "While the course coordinator nurse and patients were aware of the allocated arm, outcome assessors and data analysts were blinded to the allocation." "Blood pressure was measured twice with a digital sphygmomanometer (ONROM, São Paulo, Brazil), with the patient in sitting position, after a 5‐min rest and with
1‐min interval between measurements." Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "While the course coordinator nurse and patients were aware of the allocated arm, outcome assessors and data analysts were blinded to the allocation." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "While the course coordinator nurse and patients were aware of the allocated arm, outcome assessors and data analysts were blinded to the allocation." Comment: investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) Blood pressure | Unclear risk |
Quote from publication: "Outcome assessors and data analysts were blinded to the allocation." Comment: investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "Outcome assessors and data analysts were blinded to the allocation." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) HbA1c | Unclear risk |
Quote from publication: "Outcome assessors and data analysts were blinded to the allocation." Comment: investigator‐assessed outcome measurement |
| Incomplete outcome data (attrition bias) Blood pressure | Low risk |
Quote from publication: "Reasons for loss to follow‐up (n = 10; 7%) were withdrawal of consent (n = 8) and death (n = 2). The drop‐out patients did not differ from those who completed the trial regarding age, diabetes mellitus duration, proportion of females, ethnicity, and baseline HbA1c (data not shown)." Comment: investigator‐assessed outcome measurement. There were 6 withdrawals in the control group compared to 1 withdrawal in the intervention group. Low dropout rates (< 15%) or minimal disparate attrition rates (e.g. difference of < 10% between study arms) |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "Reasons for loss to follow‐up (n = 10; 7%) were withdrawal of consent (n = 8) and death (n = 2). The drop‐out patients did not differ from those who completed the trial regarding age, diabetes mellitus duration, proportion of females, ethnicity, and baseline HbA1c (data not shown)." Comment: self‐reported outcome measurement. There were 6 withdrawals in the control group compared to 1 withdrawal in the intervention group. Low dropout rates (< 15%) or minimal disparate attrition rates (e.g. difference of < 10% between study arms) |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "At the end of the trial, 127 (93%) patients had at least one HbA1c value available" Comment: investigator‐assessed outcome measurement |
| Selective reporting (reporting bias) | Unclear risk |
Quote from publication: — Comment: all reported outcomes were mentioned in the publication. However, diabetes distress was not mentioned as an outcome in the trials register record. It is unclear whether there is any other selective or under‐reporting of other measurement |
| Other bias | Unclear risk | Intention‐to‐treat analysis. HbA1c values after the intervention were adjusted to baseline HbA1c and for possible changes in medication during the trial (doses of metformin, glyburide, and insulin/kg/day; when patients were not on one of these medications, they were not excluded from the analysis, but the dose was considered equal to zero) by multivariate analysis of covariance (MANCOVA) |
Hermanns 2012.
| Methods | Parallel randomised controlled trial (RCT); randomisation ratio 1:1 Non‐inferiority design: equivalence region of 0.4% and an SD of 1.0% for the differences in HbA1c reduction |
|
| Participants |
Inclusion criteria: type 2 diabetes mellitus, age 18‐75 years, at least 2 years diabetes duration with oral antidiabetic treatment, BMI 20.0‐40.0 kg/m², ability to read and understand the German language Exclusion criteria: current psychiatric disease, dementia or severe cognitive impairment, severe diabetes complications (e.g. terminal renal disease), gestational diabetes Diagnostic criteria: HbA1c was measured in a central laboratory using HPLC method (normal range 4.1% to 6.1%); PAID is used to assess the current level of diabetes‐related emotional distress; a knowledge test consisting of 14 items; self‐care activities were measured by the Summary of Self‐Care Activities Scale; health‐related quality of life was assessed by the short form (SF‐12) of the SF‐36 Health Survey |
|
| Interventions |
Number of study centres: 18 Treatment before study: — Titration period: no Intervention: MEDIAS 2 ICT: More Diabetes Self‐management for type 2 Diabetes – Intensive Conventional Insulin Therapy. To help participants perform multiple‐injection insulin therapy and adjust their insulin doses depending on carbohydrate consumption, physical exercise, and pre‐prandial glucose levels. In addition, MEDIAS 2 ICT focused on controlling metabolic risk factors such as elevated lipids and blood pressure. A key element of the empowerment/self‐management approach of MEDIAS 2 ICT is shared decision‐making between participants and diabetes educators concerning realistic treatment goals. During the lessons the participants discuss individual problems and barriers to achieving these treatment goals and methods to overcome the barriers. Based on these discussions, participants were enabled to establish realistic treatment goals. Also addressed during the MEDIAS 2 ICT lessons are attitudes and personal perceptions about certain aspects of diabetes treatment. Another key element of MEDIAS 2 ICT comprises participant materials, which are completed between the lessons (e.g. worksheets for assessing individual risk factors, nutrition diaries, calorie tables, and blood glucose logs). In a nutrition game, participants have to estimate the carbohydrate and calorie content of depicted meals. Social support for diabetes treatment is another important issue in MEDIAS 2 ICT. Family members, partners or friends of participants with diabetes are invited to attend the 7th lesson, during which social support issues are addressed Control: a combination of 2 previously established and evaluated education programmes. Didactic‐oriented, focusing primarily on the acquisition of knowledge, skills, and information about the correct treatment of diabetes and hypertension |
|
| Outcomes | Outcomes reported in abstract of publication: the mean HbA1c at 6 months; diabetes‐related distress | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NCT00901992 |
|
| Publication details |
Language of publication: English Commercial funding: unrestricted grant from Lilly, Germany Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "The primary objective of the study was to demonstrate the non‐inferiority of MEDIAS 2 ICT compared with the ACC [active comparator condition] control group regarding improvement of glycaemic control. A secondary objective was the analysis of the impact of this programme on diabetes‐related distress, diabetes knowledge, self‐care behavior, quality of life, and metabolic risk factors (lipids, blood pressure, and body mass index)." | |
| Notes | No mention of missing data handling, probably no imputation of missing values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "The study centre served as a stratification variable. For randomisation, statistical software (Systat 12.0) was used." Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Patients ... were individually randomised ... centrally by the coordinating centre." Comment: probably done |
| Blinding of participants and personnel (performance bias) Blood pressure | High risk |
Quote from publication: no direct quote is available Comment: unclear of the method for this outcome measurement. Not defined. Trial author communicated that manual auscultatory method was used in accordance to the German hypertension guideline in this outcome measurement; the assessor was not blinded |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | Unclear risk |
Quote from publication: no direct quote is available Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "HbA1c was measured in a central laboratory using HPLC method (normal range 4.1‐6.1%) ... in a central laboratory." Comment: adjudicated outcome measurement |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | Unclear risk |
Quote from publication: no direct quote is available Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) Blood pressure | High risk |
Quote from publication: no direct quote is available Comment: unclear of the method for this outcome measurement. Not defined. Trial author communicated that manual auscultatory method was used in accordance to the German hypertension guideline in this outcome measurement, the assessor was not blinded |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | Unclear risk |
Quote from publication: no direct quote is available Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "HbA1c was measured in a central laboratory using HPLC method (normal range 4.1‐6.1%) ... in a central laboratory." Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | Unclear risk |
Quote from publication: no direct quote is available Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Incomplete outcome data (attrition bias) Blood pressure | High risk |
Quote from publication: "A total of 19 patients (10.2%) were excluded from the per‐protocol analysis due to major protocol violations (attendance at fewer than 5 lessons or lost to follow‐up at the 6‐month follow‐up) ... A dropout analysis comparing the per‐protocol population and patients excluded from analysis showed no significant difference except for age." Comment: reported and reasons explained. Statistical adjustments were done for the baseline values and study centre, not for age |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | High risk |
Quote from publication: "A total of 19 patients (10.2%) were excluded from the per‐protocol analysis due to major protocol violations (attendance at fewer than 5 lessons or lost to follow‐up at the 6‐month follow‐up) ... A dropout analysis comparing the per‐protocol population and patients excluded from analysis showed no significant difference except for age." Comment: reported and reasons explained. Statistical adjustments were done for the baseline values and study centre, not for age |
| Incomplete outcome data (attrition bias) HbA1c | High risk |
Quote from publication: "A total of 19 patients (10.2%) were excluded from the per‐protocol analysis due to major protocol violations (attendance at fewer than 5 lessons or lost to follow‐up at the 6‐month follow‐up) ... A dropout analysis comparing the per‐protocol population and patients excluded from analysis showed no significant difference except for age." Comment: reported and reasons explained. Statistical adjustments were done for the baseline values and study centre, not for age |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | High risk |
Quote from publication: "A total of 19 patients (10.2%) were excluded from the per‐protocol analysis due to major protocol violations (attendance at fewer than 5 lessons or lost to follow‐up at the 6‐month follow‐up) ... A dropout analysis comparing the per‐protocol population and patients excluded from analysis showed no significant difference except for age." Comment: reported and reasons explained. Statistical adjustments were done for the baseline values and study centre, not for age |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported including BP, although not significant, that was not specified as an outcome measure |
Hermanns 2015.
| Methods | Parallel randomised clinical trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: diabetes mellitus; elevated depressive symptoms (CES‐D score ≥ 16); age 18‐70 years; sufficient German
language skills; and written informed consent Exclusion criteria: major depression; current schizophrenia/psychotic disorder, eating disorder, bipolar disorder, addictive disorder, or personality disorder; current use of antidepressant medication or ongoing psychotherapy; being bedridden; and under guardianship Diagnostic criteria: depressive symptoms were assessed using the German version of the CES‐D and the PHQ‐9; diabetes‐related distress was assessed by the German version of the DDS; self‐care activities were measured using the German version of the Summary of Diabetes Self‐Care Activities Measure (SDSCA); psychological well‐being was assessed using the WHO‐5 Well‐Being Index; health‐related quality of life was measured by the EuroQol (EQ‐5D); diabetes acceptance was assessed using the Acceptance and Action Diabetes Questionnaire (AADQ); diabetes treatment satisfaction was assessed by the Diabetes Treatment Satisfaction Questionnaire (DTSQ) |
|
| Interventions |
Number of study centres: 1 Treatment before study: — Titration period: no Intervention: the DIAMOS (Diabetes Motivation Strengthening) programme, based on a self‐management/empowerment approach. A key topic of DIAMOS is diabetes‐related distress originating from living with a chronic condition and the distress caused by treatment‐related factors. Another focus is the discrimination between diabetes‐related and unrelated problems and problem‐solving strategies addressing both issues. Another important aim is to prevent relapses in dysfunctional attitudes toward diabetes. A key element of this treatment approach is the exchange between group members about living with diabetes, and the use of master models for successfully coping with the challenges associated with diabetes and its treatment. After the lessons, the participants completed entries in a booklet in which they recorded personally important topics and individual problem solving strategies that emerged from the lesson (e.g. a personal distress model or development of personal coping strategies). At the beginning of each lesson, the entries recorded in this booklet were discussed Control: the participants in the CG participated in a standard group‐based diabetes education programme, including topics such as healthy diet in diabetes, diabetes and exercise, and diabetes and legal issues. |
|
| Outcomes | Outcomes reported in abstract of publication: the primary outcome was depressive symptoms. Secondary outcomes were diabetes distress, well‐being, self‐care behaviour, diabetes acceptance, diabetes treatment satisfaction, HbA1c level, and subclinical inflammation | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NCT01009138 |
|
| Publication details |
Language of publication: English Non‐commercial funding: Competence Network Diabetes Mellitus, which was funded by the Federal Ministry of Education and Research (BMBF) (grant FKZ 01GI0809); the Ministry of Science and Research of the State of North Rhine‐Westphalia; and the German Federal Ministry of Health; supported in part by a grant from the BMBF to the German Center for Diabetes Research (DZD e.V.). Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "In a randomised controlled trial, the efficacy of this newly developed program was evaluated after a 12‐month follow‐up period. The primary objective of this study was to test whether DIAMOS was superior in reducing depressive symptoms ... Since DIAMOS also focuses on coping with diabetes‐related distress, the impact of the program on diabetes distress was evaluated as a secondary outcome variable. " | |
| Notes | No mention of missing data handling, probably no imputation of missing values. For the main outcome, an intention‐to‐treat analysis was performed, using the last observation carried forward method. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "The randomisation occurred externally through the Coordination Centre for Clinical Trials" Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "A person independent from the recruitment process randomised the patients to the two treatment groups with a 1:1 allocation" Comment: probably done |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "The baseline and 12‐month measurements were performed at the study centre, and the other two measurements were performed by phone and mail ... All measurements were performed in a blinded fashion with respect to group assignment." Comment: self‐reported outcome measurement but involved interview |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "All measurements were performed in a blinded fashion with respect to group assignment." Comment: adjudicated outcome measurement |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | High risk |
Quote from publication: "The baseline and 12‐month measurements were performed at the study centre, and the other two measurements were performed by phone and mail ... All measurements were performed in a blinded fashion with respect to group assignment." Comment: self‐reported outcome measurement but involve interview |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "The baseline and 12‐month measurements were performed at the study centre, and the other two measurements were performed by phone and mail ... All measurements were performed in a blinded fashion with respect to group assignment." Comment: self‐reported outcome measurement but involved interview |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "All measurements were performed in a blinded fashion with respect to group assignment." Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | High risk |
Quote from publication: "The baseline and 12‐month measurements were performed at the study centre, and the other two measurements were performed by phone and mail ... All measurements were performed in a blinded fashion with respect to group assignment." Comment: self‐reported outcome measurement but involved interview |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | High risk |
Quote from publication: "Comparing the randomised and the analysed samples, no significant difference in dropout rates between the DIAMOS group and CG (13.9% vs. 22.7%, P = 0.205) was observed." Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) HbA1c | High risk |
Quote from publication: "Comparing the randomised and the analysed samples, no significant difference in dropout rates between the DIAMOS group and CG (13.9% vs. 22.7%, P = 0.205) was observed. A dropout analysis showed that patients who dropped out of the study were significantly ... younger years of age, P = 0.01) and had a lower BMI ... and poorer glycaemic control" Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | High risk |
Quote from publication: "Comparing the randomised and the analysed samples, no significant difference in dropout rates between the DIAMOS group and CG (13.9% vs. 22.7%, P = 0.205) was observed." Comment: dropouts reported but not explained |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcome measures were reported |
Lamers 2011.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: minor depression or mild to moderate major depression Exclusion criteria: severe major depression or with suicidal risk; treatment with antidepressants for depression, major psychiatric problems (bipolar depression, schizophrenia, alcohol or substance abuse), current psychosocial/psychiatric treatment, serious cognitive problems, on waiting list for nursing home, bedridden, loss of spouse in last 3 months and not being fluent in Dutch Diagnostic criteria: disease‐specific quality of life was operationalised as diabetes‐specific symptom distress assessed with the Diabetes Symptom Checklist – Revised (DSC‐R); emotional distress using the PAID questionnaire; haemoglobin A1c (HbA1c) retrieved from participants' records |
|
| Interventions |
Number of study centres: 89 Treatment before study: — Titration period: no Intervention: cognitive behavioural therapy (CBT) with self‐management principles. Its aim was to educate people to take responsibility for the daily management of their own illness and its consequences. The intervention consists of 5 steps. In the first step, the nurse explores the participant's feelings, cognitions and behaviours. In the second step, the participant keeps a diary, where he or she records symptoms, complaints, thoughts, worries, related feelings and behaviour. In the third step, the participants are challenged to link their mood to the consequent behaviour, using information from the diary, and then the self‐management approach is introduced in a fourth step. In this phase, the participant explores possibilities to alter his or her behaviour and draws up an action plan. By changing the behaviour that is linked to the depressed mood, mood itself can be altered. In the last step, the progress in achieving the goals of the action plan is evaluated. The intervention is tailor‐made: the number of visits depends upon progress Control: usual care. Regular treatment according to the practice guidelines of the Dutch College of General Practitioners (GP) for type 2 diabetes. These guidelines include regular follow‐up of somatic symptoms but do not involve the detection and treatment of depressive symptoms. Co‐interventions such as pharmacological depression treatments were allowed, and considered non‐differential between groups. Only after the follow‐up, GPs were informed about which participants had participated in the trial |
|
| Outcomes | Outcomes reported in abstract of publication: emotional distress and symptom distress (DSC‐R total score at 9 months P = 0.001; PAID, 9 months P = 0.03); haemoglobin A1c after 9 months | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: ISRCTN92331982 |
|
| Publication details |
Language of publication: English Non‐commercial funding: Netherlands Organisation for Health Research and Development (ZonMw) programme on Health Care Efficiency Research Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "The aim of this study was to examine whether a nurse‐administered minimal psychological intervention for depressive symptoms improves diabetes‐specific quality of life and glycaemic control in older persons with diabetes." | |
| Notes | Missing values on outcomes during follow‐up were imputed with the last available score of a given outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Randomization was then performed ... using a computerized random number generator with a block randomisation scheme stratified by general practice (block size of two)." Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "In total ... signed informed consent forms and completed a baseline questionnaire. Randomization was then performed, blinded for the researchers, by an external agency" Comment: probably done |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "The DSC‐R consists of ... hypoglycaemia ..." Comment: hypoglycaemic event was self‐reported outcome measurement, unclear of other adverse events such as illness or hospital admittance as reported in the study flow chart. Trial author communicated that these data were self‐reported |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: no direct quote is available; the study flow chart reported death. Unclear of the method for this outcome measurement. Not defined, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "Data were collected ... by mailed self‐administered questionnaires." Comment: self‐reported outcome measurement but actual modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "All general practices were contacted to retrieve participants' haemoglobin A1c (HbA1c) values that were determined between the inclusion phase and the end of the follow‐up" Comment: adjudicated outcome measurement |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | High risk |
Quote from publication: "Data were collected ... by mailed self‐administered questionnaires." Comment: self‐reported outcome measurement but actual modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Comment: no direct quote is available, the study flow chart reported death. Unclear of the method for this outcome measurement. Not defined, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Adverse events | Low risk |
Quote from publication: "The DSC‐R consists of ... hypoglycaemia ..." Comment: hypoglycaemic event was self‐reported outcome measurement, unclear of other adverse events such as illness or hospital admittance as reported in the study flow chart. Trial author communicated that these data were self‐reported |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "Data were collected ... by mailed self‐administered questionnaires." Comment: self‐reported outcome measurement but actual modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "All general practices were contacted to retrieve participants' haemoglobin A1c (HbA1c) values that were determined between the inclusion phase and the end of the follow‐up" Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | High risk |
Quote from publication: "Data were collected ... by mailed self‐administered questionnaires." Comment: self‐reported outcome measurement but actual modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk |
Quote from publication: "The dropout percentage throughout the follow‐up was comparable between the intervention and control groups (33% vs. 30%, P = 0.62). Dropout was associated only with higher age" Comment: reported and reasons explained, with many unknown reasons as reported in the study flow chart |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "The dropout percentage throughout the follow‐up was comparable between the intervention and control groups (33% vs. 30%, P = 0.62). Dropout was associated only with higher age ... Age, gender, educational level, treatment group, baseline value of outcome ... were standard inclusions in the model" Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "The dropout percentage throughout the follow‐up was comparable between the intervention and control groups (33% vs. 30%, P = 0.62). Dropout was associated only with higher age ... Age, gender, educational level, treatment group, baseline value of outcome ... were standard inclusions in the model" Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | Low risk |
Quote from publication: "The dropout percentage throughout the follow‐up was comparable between the intervention and control groups (33% vs. 30%, P = 0.62). Dropout was associated only with higher age ... Age, gender, educational level, treatment group, baseline value of outcome ... were standard inclusions in the model" Comment: reported and reasons explained |
| Selective reporting (reporting bias) | High risk | Comment: HbA1c was mentioned as a covariate in the study design paper (Lamers 2006) but reported as one of the outcome measure in the publication probably due to HbA1c being a significant result, SE was not reported in the publication although was specified as a secondary outcome measure |
Lerman 2009.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1:1 | |
| Participants |
Inclusion criteria: participants with type 2 diabetes aged 30‐75 years old, who regularly attended Internal Medicine and Diabetes Clinic of the National Institute of Medical Sciences and Nutrition Salvador Zubirán and could be contacted by telephone Exclusion criteria: participants with type 1 diabetes or secondary causes of diabetes, participants admitted to hospital in the previous 3 months or with chronic or disabling conditions that prevented them from attending regular appointments or affect their intellectual capacity Diagnostic criteria: diabetes self‐care by the Self Care lnventory; depression was assessed with 2 questions: "In recent weeks, how often it has happened that you feel 'low battery', depressed, hopeless?" and "During the past weeks, how often you had felt little interest or pleasure in doing things?"; emotional dysfunction associated with diabetes measured by the PAID questionnaire; knowledge of diabetes; HbA1c |
|
| Interventions |
Number of study centres: 1 Treatment before study: — Titration period: no Intervention 1: participants were contacted monthly by phone (GRT) to promote self‐management attitudes and address problems as they arose. During each call, several questions were asked to each participant in order to promote self‐care behaviours and to detect and to solve problems related to diabetes. A brief medical history, a set of questionnaires and laboratory tests were performed at the beginning and after a year of follow‐up Intervention 2: participants received a reinforcement group‐based education course at 6 months (RCG). The course consisted of group sessions for 6‐8 participants, lasting 5 hours, where again the basics of diabetes care and prevention of complications were taught. The sessions, conducted by a doctor, nurse educator in diabetes, nutrition and psychology graduate, were aimed at strengthening self‐care behaviours and solve problems encountered in daily life of participants. Finally, participants were encouraged to tell their personal experiences and to find ways to overcome their difficulties in achieving therapeutic goals and to improve their quality of life Control: participants in the control group (CG) continued with their normal treatment schedule. This involved regular appointments with the participants' doctor with a frequency of 3‐4 months, where the results of laboratory studies and glucose monitoring were discussed, a comprehensive clinical evaluation was made and the treatment was adjusted; optional consultation with a licensed nutritionist was allowed |
|
| Outcomes | Outcomes reported in abstract of publication: at 1‐year follow‐up, the three groups significantly increased their diabetes‐related knowledge. Both experimental groups displayed improved treatment compliance and had better adherence to the recommended meal plan. In addition, the PHCG significantly increased their adherence to pharmacological treatment. No significant differences were observed in glycaemic control, prevalence of depression or diabetes‐related distress | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: — |
|
| Publication details |
Language of publication: Spanish Commercial funding: unclear Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "The present study was performed in order to evaluate the impact of 2 strategies: monthly telephone and a biannual educational course reinforcement in glycaemic control calls, adherence to treatment, the presence of depression and emotional dysfunction associated with diabetes, after a year of follow‐up." | |
| Notes | Translated article, originally published in Spanish. No mention of missing data handling, probably no imputation of missing values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Consecutively 70 patients were randomly assigned to three study groups" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: no direct quote available Comment: probably not done |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "In the third group, patients were contacted monthly by telephone by one of the doctors who participated in the study (GRT)" Comment: self‐reported outcome measurement but modes of administration unclear, probably interviewed and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "In the third group, patients were contacted monthly by telephone by one of the doctors who participated in the study (GRT)" Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "For each call several questions were asked to each patient ... a set of questionnaires and laboratory tests were performed at the beginning and after a year of follow‐up. The variables included to assess ... emotional dysfunction associated with diabetes and glycaemic control (HbA1c)." Comment: investigator‐assessed outcome measurement but modes of administration unclear, probably interviewed and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "A set of questionnaires and laboratory tests were performed at the beginning and after a year of follow‐up. The variables included glycaemic control (HbA1c)." Comment: laboratory outcome measurement |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | High risk |
Quote from publication: "The study was completed by 59 patients, 11 were lost to follow up (five of GC , two from GCR and four GRT). The characteristics of these patients did not differ statistically from those who remained in the study. " Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) HbA1c | High risk |
Quote from publication: "The study was completed by 59 patients, 11 were lost to follow up (five of GC , two from GCR and four GRT). The characteristics of these patients did not differ statistically from those who remained in the study. " Comment: dropouts reported but not explained |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome measures were reported as specified in the publication, no prior trials register record or study design paper was available |
| Other bias | High risk | Comment: there is pre‐randomisation administration of a group education programme in the study that could diminish the effect of the subsequent educational intervention when compared to the control group |
Liu 2015.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: diagnosis of type 2 diabetes, mild‐to‐moderate depression or anxiety according to Self‐rating Depression Scale (SDS) and Self‐rating Anxiety Scale (SAS) criteria, respectively, and signed informed consent Exclusion criteria: diagnosed with a severe psychiatric disorder; treatment with an antipsychotic; undergoing current psychosocial treatment; experienced a recent negative life event (within < 3 months); known to have severe complications of diabetes; serious communication obstacles; and bedridden status Diagnostic criteria: laboratory measurements consisted of BMI, blood pressure, lipid profiles and HbA1c levels, and were collected through clinical information systems. Diabetes Distress Scale 17‐item is used to assess diabetes‐related distress, ADDQoL is a diabetes‐specific instrument comprised of 19 domain items to assess quality of life |
|
| Interventions |
Number of study centres: 1 Treatment before study: no Titration period: no Intervention: peer education group (PEG). The educators provided both groups with 4 diabetes health education lectures and relevant health knowledge materials. Peer leaders had to undergo 6 training sessions (2 h per training session) delivered by educators. Training methods included lectures and individual counselling. The training content focused on the relationship between blood glucose and diet, exercise, psychological status, emotions, and self‐management. Peer leaders were trained to grasp organisational skills, be active listeners, develop non‐judgmental communication skills, show expressive power and project charm. Peer leaders provided the patients in the PEG with diabetes self‐care skills, emotional support, encouragement for lifestyle changes, and medication understanding and adherence. In addition, peer leaders exercised with peer members at least 150 min per week. Arrangements were made to share experience sessions; that is, group discussions on diabetes diet, medications, psychological adjustment, regular life and homemade recipes at least once per month. Peer leaders used indefinite media (telephone, SMS, e‐mail and meetings) with the recipient once every 2 weeks to share experiences and lessons, focusing on providing psychological counselling and support, positive cues, communication with a pleasant interpersonal environment, and reminders of behavioural changes and regular healthy lifestyles. Peer leaders recorded the progress of each event, and could contact educators when problems occurred Control: usual education group (UEG). The educators provided both groups with 4 diabetes health education lectures and relevant health knowledge materials |
|
| Outcomes | Outcomes reported in abstract of publication: the metabolic index, diabetes knowledge, self‐management, diabetes‐related distress, emotional status and quality of life were compared at the end of the study | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: — |
|
| Publication details |
Language of publication: English Non‐commercial funding: National Natural Science Foundation of China (81170773 to Honglei Guo) Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication:"the aim of the present study was to develop a feasible and effective strategy to overcome these challenges and maintain behavioural health changes, and to implement and assess the effectiveness of PES (peer education support) compared to UDE (usual diabetes education) in patients with diabetes and mild affective disorders" | |
| Notes | There were no significant differences in metabolic indicators and self‐reported scales between the PEG and UEG at baseline. All participants completed the study. The PEG had higher attendance in group education (85%) than the UEG (74%), whereas the mean number of attendances did not differ between the 2 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Randomization was carried out by an external agency using a computerized random number generator." Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Randomization was carried out by an external agency using a computerized random number generator." Comment: probably done |
| Blinding of participants and personnel (performance bias) Blood pressure | Low risk |
Quote from publication: "Laboratory measurements consisted of ... blood pressure ... and HbA1c levels, and were collected through clinical information systems." Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "Participants in both groups completed the ... Diabetes‐related Distress Scale (DDS) and Audit of Diabetes Dependent Quality of Life (ADDQoL) before the intervention. At the end of the trial, participants provided the data, including the metabolic index, and questionnaire responses to the ... mentation and quality of life." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "Laboratory measurements consisted of ... blood pressure ... and HbA1c levels, and were collected through clinical information systems." Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | Unclear risk |
Quote from publication: "Participants in both groups completed the ... Diabetes‐related Distress Scale (DDS) and Audit of Diabetes Dependent Quality of Life (ADDQoL) before the intervention. At the end of the trial, participants provided the data, including the metabolic index, and questionnaire responses to the ... mentation and quality of life." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) Blood pressure | Unclear risk |
Quote from publication: "Laboratory measurements consisted of ... blood pressure ... and HbA1c levels, and were collected through clinical information systems." Comment: investigator‐assessed outcome measurement; unclear blinding |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "Participants in both groups completed the... Diabetes‐related Distress Scale (DDS) and Audit of Diabetes Dependent Quality of Life (ADDQoL) before the intervention. At the end of the trial, participants provided the data, including the metabolic index, and questionnaire responses to the ... mentation and quality of life." Comment: self‐reported outcome measurement, unclear whether self‐administered or by interview |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "Laboratory measurements consisted of ... blood pressure ... and HbA1c levels, and were collected through clinical information systems." Comment: laboratory |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | Unclear risk |
Quote from publication: "Participants in both groups completed the ... Diabetes‐related Distress Scale (DDS) and Audit of Diabetes Dependent Quality of Life (ADDQoL) before the intervention. At the end of the trial, participants provided the data, including the metabolic index, and questionnaire responses to the ... mentation and quality of life." Comment: self‐reported outcome measurement; unclear whether self‐administered or by interview |
| Incomplete outcome data (attrition bias) Blood pressure | Low risk |
Quote from publication: "All participants completed the study." Comment: investigator‐assessed outcome measurement |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "All participants completed the study." Comment: self‐reported outcome measurement |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "All participants completed the study." Comment: laboratory‐based outcome measurement |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | Low risk |
Quote from publication: "All participants completed the study." Comment: self‐reported outcome measurement |
| Selective reporting (reporting bias) | Unclear risk | Comment: no trials register record to compare with, within the publication probably no reporting bias |
Pibernik‐Okanovic 2015.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1:1 | |
| Participants |
Inclusion criteria: having had type 2 diabetes for at least 1 year, being aged 18‐65 years, and having had at least 1 medical check‐up during the previous year; reporting at least 1 depressive symptom over the past month, and a need for receiving professional help Exclusion criteria: major depression or dysthymia; current psychiatric treatment, advanced diabetes complications, and medical contraindications for physical exercise Diagnostic criteria: mood difficulties was done using the adapted PHQ‐2; clinical depression was determined by phone‐administered structured clinical interview; depressive symptoms were measured by the CES‐D; diabetes‐specific emotional distress was measured by the PAID; diabetes self‐care behaviours were measured by the Summary of Diabetes Self‐Care Activities (SDSCA); health‐related quality of life was measured by the version 2 of the 12‐Item Short Form Health Survey (SF‐ 12v2); HbA1c was measured by an automated immuno turbidimetric assay with dual reporting traceable to National Glycohaemoglobin Standardisation Programme (NGSP) (%) and International Federation of Clinical Chemistry (IFCC) (mmol/mol) reference systems (Integra 400 Tina‐quant, Roche, Mannheim, Germany) |
|
| Interventions |
Number of study centres: 1 Treatment before study: no Titration period: no Intervention 1: psycho‐educational intervention. The intervention comprised small‐group meetings (4‐6 members), with topics that included: recognising depressive symptoms; becoming aware of dysfunctional thinking patterns; alleviating the burden of depression through activities and problem solving; understanding cognitive processes that induced and maintained depression; gaining social support, and developing a personal plan for managing mood problems in the future. Meetings at the outpatient clinic were held at weekly intervals. The sessions consisted of a short standardised PowerPoint presentation aimed at acquainting participants with basic principles of cognitive behavioural approach to mood problems. The presentation provided a framework for group discussions and a basis for homework assignments. Each session alternated between presentations and discussions on personal experiences, based on the assumption that alternating giving and receiving information would stimulate participants' active participation. Whenever possible, participants' problems related to diabetes were used to explore a triad of feelings, thoughts and behaviour. Participants were provided with a self‐help manual. The manual's structure aimed to stimulate introducing personal examples and making notes. Participants also received a workbook containing exercises to recognise depressive symptoms, becoming aware of daily activities patterns, acquiring problem‐solving techniques, and to recognise and modify cognitive patterns that contributed to maintenance of depression Intervention 2: physical activity intervention. Small group sessions aimed at educating participants on the interaction between physical activity, mood and diabetes, practising warm‐up, flexibility, strengthening and stretching exercises, and at stimulating participants to increase daily physical activities. The sessions combined a short standardised PowerPoint presentation on the topic and practising exercise techniques considered suitable for the participants. Educational topics included: physical activity (PA) in treating diabetes; effects of exercise on glycaemic control and the cardiovascular system; PA and energy expenditure; effects of PA on mobility, muscles and peripheral nerves; effects of exercise on mood; acquiring strategies to maintain physical activities, and developing a personal plan for regular exercise. Educational topics were presented in the first 10‐15 minutes of each session including a possibility to exchange personal experiences. Exercise intensity was measured by a heart rate monitor and maintained in a light to medium intensity range. Blood glucose and blood pressure were measured before and after each session. Control: enhanced treatment as usual. 1 re‐educational intervention of 90 minutes duration was offered. It addressed: participants' understanding of their current HbA1c and lipid values; participants' goals in self‐managing diabetes; participants' concerns caused by diabetes in general and laboratory findings in particular. A method of delivery was small‐group patient‐centred counselling. In addition, participants were provided with written self‐help instructions to cope with mood difficulties. |
|
| Outcomes | Outcomes reported in abstract of publication: depressive symptoms (primary outcome) and diabetes distress, diabetes self‐care, metabolic control and health‐related quality of life (secondary outcomes) | |
| Study details |
Run‐in period: — Trial terminated early: no Trials register identifier: ISRCTN05673017 |
|
| Publication details | Language of publication: English Non‐commercial funding: European Foundation for the Study of Diabetes (EFSD) (Germany) Publication status: peer‐reviewed journal and full article | |
| Stated aim for study | Quote from publication: "This study explored the significance of treating sub‐syndromal depression in type 2 diabetes patients while examining the effects of three behavioural interventions – psycho education, physical exercise and enhanced treatment as usual – on depressive symptoms, diabetes distress, diabetes self‐management, health‐related quality of life and metabolic control at 1 year." | |
| Notes | Missing measurements were imputed using the baseline‐observation carried‐forward approach; missing questionnaires' scores were replaced by average individual scores | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "A computer‐generated algorithm ... provided two lists of random assignments to one of the three groups" Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "A computer‐generated algorithm ... provided two lists of random assignments to one of the three groups" Comment: probably done |
| Blinding of participants and personnel (performance bias) Adverse events | Unclear risk | Comment: no direct quotes. Unclear mode of outcome measurement, probably self‐reported; insufficient description |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: no direct quotes. Unclear mode of outcome measurement, probably self‐reported. Insufficient description but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "The outcome assessors were not blinded for the patients' group assignment, since the included measures (laboratory tests, standardised psychological questionnaires) were not considered likely to cause bias." Comment: no mention of blinding on the participants' usual healthcare providers. Self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "The outcome assessors were not blinded for the patients' group assignment, since the included measures (laboratory tests, standardised psychological questionnaires) were not considered likely to cause bias." Comment: no mention of blinding on the participants' usual healthcare providers. Laboratory‐based outcome measurement |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | Unclear risk |
Quote from publication: "The outcome assessors were not blinded for the patients' group assignment, since the included measures (laboratory tests, standardised psychological questionnaires) were not considered likely to cause bias." Comment: no mention of blinding on the participants' usual healthcare providers. Self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk |
Quote from publication: "The outcome assessors were not blinded for the patients' group assignment, since the included measures (laboratory tests, standardised psychological questionnaires) were not considered likely to cause bias." Comment: unclear mode of outcome measurement, probably self‐reported. Insufficient description but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk |
Quote from publication: "The outcome assessors were not blinded for the patients' group assignment, since the included measures (laboratory tests, standardised psychological questionnaires) were not considered likely to cause bias." Comment: unclear mode of outcome measurement, probably self‐reported. Insufficient description |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | Unclear risk |
Quote from publication: "The outcome assessors were not blinded for the patients' group assignment, since the included measures (laboratory tests, standardised psychological questionnaires) were not considered likely to cause bias." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "The outcome assessors were not blinded for the patients' group assignment, since the included measures (laboratory tests, standardised psychological questionnaires) were not considered likely to cause bias." Comment: laboratory‐based outcome measurement |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | Unclear risk |
Quote from publication: "The outcome assessors were not blinded for the patients' group assignment, since the included measures (laboratory tests, standardised psychological questionnaires) were not considered likely to cause bias. " Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk |
Quote from publication: "Fifty‐six patients withdrew their previous agreement to participate ... No differences between the participants and dropouts across the three study groups were observed." Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "Of the seven dropouts who completed the intervention but missed both follow‐up assessments, two missed the follow‐up appointments due to health problems, four were unwilling to come and one patient died. Four patients were excluded from per‐protocol analyses due to the initiation of pharmacological therapy or discovery of psychiatric co‐morbidities that were not reported during the recruitment period. No differences between the participants and dropouts across the three study groups were observed." Comment: reported and reasons explained. Attrition rate was < 10%, both per‐protocol and ITT analyses were carried to cross‐validate the results |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "Of the seven dropouts who completed the intervention but missed both follow‐up assessments, two missed the follow‐up appointments due to health problems, four were unwilling to come and one patient died. Four patients were excluded from per‐protocol analyses due to the initiation of pharmacological therapy or discovery of psychiatric co‐morbidities that were not reported during the recruitment period. No differences between the participants and dropouts across the three study groups were observed." Comment: reported and reasons explained. Attrition rate was < 10%, both per‐protocol and ITT analyses were carried to cross‐validate the results |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | Low risk |
Quote from publication: "Of the seven dropouts who completed the intervention but missed both follow‐up assessments, two missed the follow‐up appointments due to health problems, four were unwilling to come and one patient died. Four patients were excluded from per‐protocol analyses due to the initiation of pharmacological therapy or discovery of psychiatric co‐morbidities that were not reported during the recruitment period. No differences between the participants and dropouts across the three study groups were observed." Comment: reported and reasons explained. Attrition rate was < 10%, both per‐protocol and ITT analyses were carried to cross‐validate the results |
| Selective reporting (reporting bias) | Low risk | Comment: all the pre‐specified outcomes for this review were reported |
| Other bias | Low risk | Comment: all results were reported for the randomised groups |
Quinn 2011.
| Methods | Cluster‐randomised controlled trial; randomisation ratio 1.5:1:1:1.5 (group 1:group 2:group 3:group 4) | |
| Participants |
Inclusion criteria: eligible practices included groups of at least 3 physicians without academic affiliation who provided diabetes care to at least 10% of their participants and were identified. Participants eligible for recruitment met all inclusion criteria: physician diagnosis of type 2 diabetes for ≥ 6 months; glycated haemoglobin ≥ 7.5% within 3 months; age 18‐64 years Exclusion criteria: participants were excluded for any of the following: Medicare or Medicaid beneficiaries; uninsured; insulin pump users; not currently managed by study physicians; pregnant; active substance, alcohol, or drug abuser (sober < 1 year); psychotic or schizophrenic under active care; severe hearing or visual impairment; or no Internet or email access Diagnostic criteria: PHQ‐9 was administered to assess depressive symptoms; the 17‐item Diabetes Distress Scale; clinical measurement related to diabetes complications (blood pressure, lipid levels) was obtained from provider medical office records; hypoglycaemic events, hospitalisation, and emergency room visits were ascertained through quarterly telephone calls to participants |
|
| Interventions |
Number of study centres: 26 Treatment before study: — Titration period: no Intervention 1: coach‐only (CO).The participant‐coaching system included a mobile diabetes management software application and a web portal. The mobile software allowed participants to enter diabetes self‐care data (blood glucose values, carbohydrate intake, medications, other diabetes management information) on a mobile phone and receive automated, real‐time educational, behavioural, and motivational messaging specific to the entered data. The participant web portal augmented the mobile software application and consisted of a secure messaging centre (for participant provider communication), personal health record with additional diabetes information (e.g. laboratory values, eye examinations, foot screenings), learning library, and logbook to review historical data. Providers in the CO group received data from their participants if participants chose to share it. Participants in the 3 active treatment groups received identical study materials: mobile phones, 1‐year unlimited data and service plan, study mobile diabetes management software, and access to the web‐based participant portal. The mobile diabetes management software incorporated over 1000 automated self‐management messages into a feedback algorithm. The algorithm displayed educational and motivational messages to participants after participants self‐reported data into the mobile phone application. Diabetes educators were 'virtual' case managers that intermittently reviewed participant data. Educators could supplement automated messages with electronic messages sent to the participant portal. Educator messages were based on longitudinal data trends. Participants in all 3 treatment groups were allowed to make telephone calls to educators but were encouraged to communicate electronically Intervention 2: coach primary care providers portal (CPP). Coach primary care providers portal with decision support (CPDS). The participant‐coaching system as described in the CO group. The data‐only view allowed providers to access unanalysed participant data. Providers were trained on accessing the provider Internet portal on office compatible computers, allowing visual access to participants' unanalysed data Intervention 3: Coach primary care providers portal with decision support (CPDS). The participant‐coaching system as described in the CO group. The data‐only view allowed providers access to analysed participant data linked to standards of care and evidence‐based guidelines. Providers were trained on accessing the provider Internet portal on office‐compatible computers, allowing visual access to participants' unanalysed data, and also received quarterly reports (more often if needed) that summarised participants' glycaemic and metabolic control, adherence to medication, self‐management skills, and relevant evidence‐based guidelines Control: control‐usual care (UC). Providers assigned to UC were asked to care for participants as usual |
|
| Outcomes | Outcomes reported in abstract of publication: glycated haemoglobin over 12 months; differences between groups for patient‐reported diabetes distress, depression, diabetes symptoms, or blood pressure and lipid levels | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NCT01107015 |
|
| Publication details |
Language of publication: English Commercial and non‐commercial funding: a contract between the University of Maryland Baltimore and WellDoc in addition to contributions by WellDoc, CareFirst Blue Cross/Blue Shield of Maryland, LifeScan, and Sprint. Additional funding was provided by the Maryland Industrial Partnerships program through the University of Maryland Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "To test whether adding mobile application coaching and patient/provider web portals to community primary care compared with standard diabetes management would reduce glycated haemoglobin levels in patients with type 2 diabetes." | |
| Notes | No mention of missing data handling, probably no imputation of missing values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Primary care practices were randomised to ..." Comment: probably done, since earlier reports from the same investigators clearly describe use of a pseudo‐random number generator in the software package R (version 2.7.0) (Quinn 2009) |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Group assignment was concealed until a practice agreed to participate in the study. " Comment: probably done, earlier reports from the same investigators also clearly describe use of randomisation after the physician‐practice agreed to participate (Quinn 2009) |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "Hypoglycemic events, hospitalisation, and emergency room visits were ascertained through quarterly telephone calls to patients." Comment: self‐reported outcome measurement |
| Blinding of participants and personnel (performance bias) Blood pressure | Low risk |
Quote from publication: "Clinical measurement related to diabetes complications (blood pressure, lipid levels) was obtained from provider medical office records." Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "... administered at baseline and at follow‐up interviews ..." Comment: self‐reported outcome measurement. but modes of administration unclear, probably interviewed |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "Glycated haemoglobin was measured using one device, the Bayer DCA 2000, by trained staff blinded to patient group assignment ... Study data for primary and secondary outcomes were collected by research staff separately ..." Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Adverse events | Low risk |
Quote from publication: "Hypoglycemic events, hospitalisation, and emergency room visits were ascertained through quarterly telephone calls to patients." Comment: self‐reported outcome measurement |
| Blinding of outcome assessment (detection bias) Blood pressure | Unclear risk |
Quote from publication: "Clinical measurement related to diabetes complications (blood pressure, lipid levels) was obtained from provider medical office records." Comment: investigator‐assessed outcome measurement; unclear blinding |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "... administered at baseline and at follow‐up interviews ..." Comment: self‐reported outcome measurement. but modes of administration unclear, probably interviewed |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "Glycated haemoglobin was measured ... by trained staff blinded to patient group assignment" Comment: laboratory outcome measurement |
| Incomplete outcome data (attrition bias) Adverse events | High risk |
Quote from publication: "77% of those enrolled completed the study and were included in the analyses." Comment: dropouts reported but not explained. Groups differed in the dropout rates, from as low as 5% (in the control‐usual care) to as high as 21% (in the Coach‐PCP) |
| Incomplete outcome data (attrition bias) Blood pressure | High risk |
Quote from publication: "77% of those enrolled completed the study and were included in the analyses." Comment: dropouts reported but not explained. Groups differed in the drop out rates, from as low as 5% (in the control‐usual care) to as high as 21% (in the Coach‐PCP) |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | High risk |
Quote from publication: "77% of those enrolled completed the study and were included in the analyses." Comment: dropouts reported but not explained. Groups differed in the drop out rates, from as low as 5% (in the control‐usual care) to as high as 21% (in the Coach‐PCP) |
| Incomplete outcome data (attrition bias) HbA1c | High risk |
Quote from publication: "77% of those enrolled completed the study and were included in the analyses." Comment: dropouts reported but not explained. Groups differed in the drop out rates, from as low as 5% (in the control‐usual care) to as high as 21% (in the Coach‐PCP) |
| Selective reporting (reporting bias) | High risk | Comment: self‐efficacy was not reported in the publication although was mentioned as a secondary outcome measure using the Diabetes Stages of Change in the study design paper Quinn 2009. DRD and BP were reported as non‐significant without details |
| Other bias | Low risk |
Comment: right use of statistical analysis (linear mixed‐effect models) that adjust for a potential clustering effect Assessment of risk of bias in cluster‐randomised trials
|
Rosenbek 2011.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: type 1 or type 2 diabetes mellitus, were over 18 years of age and had participated in a group education programme offered at the diabetes clinic Exclusion criteria: pregnancy, severe debilitating disease and cognitive deficit Diagnostic criteria: PAID was used to measure diabetes‐related distress; the Perceived Competence for Diabetes Scale (PCDS) was used to measure competence at carrying out the diabetes treatment regime; glycaemic control was assessed using HbA1c, which was measured by a high‐performance liquid chromatography‐based ion exchanged procedure (Tosho Alc 2.2, Tokyo, Japan). The reference range was 4.3% to 6.3%. Total cholesterol, HDL cholesterol and triacylglycerol levels were measured in serum by enzymatic methods (Boehringer Mannheim Diagnostica, Mannheim, Germany). LDL cholesterol was calculated by Friedewald's equation |
|
| Interventions |
Number of study centres: 1 Treatment before study: had participated in a group education programme offered at the diabetes clinic Titration period: no Intervention: MI programme. The theoretical approach of the intervention was based on self‐efficacy theory and motivational interviewing (MI) spirit. Individual counselling sessions where the style of the interview was: seeking to understand the person's frame of reference; expressing acceptance and affirmation; eliciting and selectively reinforcing the client's own self‐motivational statements of problem recognition, concern, desire and intention to change, and ability to change; exploring the client's degree of readiness to change; and affirming the client's freedom of choice and self‐direction. Each session followed a semi‐structured interview format of MI, especially developed for this intervention programme. Participants brought up any problematic issues related to diabetes self‐care during sessions. The participants in the intervention group could be referred by the healthcare professional to individual counselling in changes of diet, a smoking cessation programme, counselling in alcohol abuse and an exercise programme, as they required Control: usual care. Participants underwent the same routine check‐up at their general practitioner or outpatient clinic in charge of their diabetes care. This usually involved 4 physician visits per year. Biochemical tests and examinations were usually performed during the visits in accordance with national diabetes guidelines. Individual counselling and recommendations based on the results of the examinations, biochemical tests and their self‐monitoring of blood glucose was given. Renewal of prescribed medication and test strips for blood glucose monitoring were also given at these check‐ups. Participants could be referred for individual counselling in change of diet, physical activity, smoking habits and alcohol abuse if required by their usual healthcare provider |
|
| Outcomes | Outcomes reported in abstract of publication: the primary outcome was glycated haemoglobin (HbA1c) and competence of self‐management (using the PAID and Perceived Competence for Diabetes Scale (PCDS)) | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NCT00555854 |
|
| Publication details |
Language of publication: English Non‐commercial funding: National Board of Health, Funen County, Danish Association of Diabetes, Odense University Hospital, University of Southern Denmark and TRYG Fonden Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "The aim was to study the effect of a 1‐year intervention programme based on MI following a group education programme on glycaemic control and competence of management in patients diagnosed with type 1 or type 2 diabetes mellitus. " | |
| Notes | No mention of missing data handling, probably no imputation of missing values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Randomisation was generated by random permuted blocks" Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "... with allocation concealment by sequentially numbered, sealed, opaque envelopes ... The person generating the allocation scheme did not administer the allocation of the patients to the two groups and was not part of the research team." Comment: probably done |
| Blinding of participants and personnel (performance bias) Adverse events | Unclear risk |
Quote from publication: no direct quote from the publication, data were reported in the study flow diagram Comment: unclear of the mode of this outcome measurement |
| Blinding of participants and personnel (performance bias) Blood pressure | Low risk |
Quote from publication: "All outcome measures were assessed at randomisation, 1 and 2 years after randomisation in both groups ... Blood pressure was measured by the auscultatory method with use of a stethoscope and a sphygmomanometer. An inflatable cuff was placed around the upper left arm, at the same vertical height as the heart. Measurement was made at rest in a sitting position." Comment: investigator‐assessed outcome measurement. Trial author communicated that the assessor was blinded to group assignment |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "All outcome measures were assessed at randomisation, 1 and 2 years after randomisation in both groups." No more direct quote is available in the publication Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "One laboratory analysed all the blood samples. Glycaemic control was assessed using HbA1c, which was measured by a high‐performance liquid chromatography‐based ion exchanged procedure (Tosho Alc 2.2, Tokyo, Japan)" Comment: laboratory outcome measurement |
| Blinding of participants and personnel (performance bias) Self‐efficacy | High risk |
Quote from publication: "All outcome measures were assessed at randomisation, 1 and 2 years after randomisation in both groups." No more direct quote is available in the publication Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) Adverse events | Unclear risk | Comment: no direct quote from the publication, data were reported in the study flow diagram. Unclear of the mode of this outcome measurement |
| Blinding of outcome assessment (detection bias) Blood pressure | Low risk |
Quote from publication: "All outcome measures were assessed at randomisation, 1 and 2 years after randomisation in both groups ... Blood pressure was measured by the auscultatory method with use of a stethoscope and a sphygmomanometer. An inflatable cuff was placed around the upper left arm, at the same vertical height as the heart. Measurement was made at rest in a sitting position." Comment: investigator‐assessed outcome measurement. Trial author communicated that the assessor was blinded to group assignment |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "All outcome measures were assessed at randomisation, 1 and 2 years after randomisation in both groups." No more direct quote is available in the publication Comment: self‐reported outcome measurement. but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "One laboratory analysed all the blood samples. Glycaemic control was assessed using HbA1c, which was measured by a high‐performance liquid chromatography‐based ion exchanged procedure (Tosho Alc 2.2, Tokyo, Japan)" Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Self‐efficacy | High risk |
Quote from publication: "All outcome measures were assessed at randomisation, 1 and 2 years after randomisation in both groups." No more direct quote is available in the publication Comment: self‐reported outcome measurement. but modes of administration unclear, probably self‐administered |
| Incomplete outcome data (attrition bias) Adverse events | High risk |
Quote from publication: "We found no difference in the characteristics of dropout participants compared with those who remained in the study, except for the mean age, where the dropouts were younger than the intervention group." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) Blood pressure | High risk |
Quote from publication: "We found no difference in the characteristics of dropout participants compared with those who remained in the study, except for the mean age, where the dropouts were younger than the intervention group." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | High risk |
Quote from publication: "We found no difference in the characteristics of dropout participants compared with those who remained in the study, except for the mean age, where the dropouts were younger than the intervention group." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) HbA1c | High risk |
Quote from publication: "We found no difference in the characteristics of dropout participants compared with those who remained in the study, except for the mean age, where the dropouts were younger than the intervention group." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) Self‐efficacy | High risk |
Quote from publication: "We found no difference in the characteristics of dropout participants compared with those who remained in the study, except for the mean age, where the dropouts were younger than the intervention group." Comment: reported and reasons explained |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes related to this review were reported as specified in the trials register record |
| Other bias | High risk | Comment: there is pre‐randomisation administration of a group education programme in the study that could diminish the effect of the subsequent randomised motivational interviewing that was to support problematic issues faced in self‐care |
Shibayama 2007.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: aged 20‐75 years; diagnosed with type 2 diabetes; had HbA1C values between 6.5% and 8.5% on an average in 3 tests assessed within recent 3 months; could not use insulin Exclusion criteria: serious ongoing illness or cognitive disorder Diagnostic criteria: health‐related quality of life was measured with SF‐36 Japanese version 1.2; PAID Japanese version; cognitive modification (3 items); behavioural modification (1 item) and overall satisfaction in Certified Expert Nurse counselling (1 item) |
|
| Interventions |
Number of study centres: 1 Treatment before study: no Titration period: no Intervention: one‐to‐one lifestyle counselling. The key features of the CEN counselling were assessment, participant participation in goal setting, selecting personalised strategies to overcome barriers and follow‐up including evaluation and problem solving. Also assessed were the participant's eating patterns, level of physical activity, adherence to medication, level of self‐care for diabetic complications and management of daily stress. Based on this information, the CEN established the participant current lifestyle, identified the most problematic areas and identified the participant's barriers to making lifestyle changes. A personalised programme was formulated in which realistic manageable goals for lifestyle change were negotiated, and specific intervention strategies to decrease barriers to change and empower the participant to change were developed. Relevant educational materials of the CEN's own making and printed laboratory results were also provided Control: usual care. Control participants were seen by the same physicians in charge of participants in the intervention group. Physicians did not know which participants served as control subjects for this study |
|
| Outcomes | Outcomes reported in abstract of publication: HbA1C, BMI, blood pressure, serum lipids and health‐related quality of life over 1 year between the 2 groups; modification of cognition and behaviour | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: — |
|
| Publication details |
Language of publication: English Non‐commercial funding: Ministry of Health, Labour, and Welfare Scientific Research Grants and Japanese Nursing Association Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "... to examine with randomised controlled design whether one‐to‐one lifestyle counselling by nurse for non‐insulin‐treated diabetic outpatients can improve their health outcomes ..." | |
| Notes | No mention of missing data handling, probably no imputation of missing values. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Subjects were randomly assigned to ..." Comment: not clear, probably done. Trial author clarified that random numbers were generated using Microsoft Excel |
| Allocation concealment (selection bias) | High risk |
Quote from publication: "Subjects were randomly assigned to ... " Comment: probably not done. Trial author clarified that they themselves did the allocation. Though they were not directly involved in the intervention but might not be blinded properly to the intervention |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "Physicians did not know which patients served as control subjects for this study." Comment: self‐reported outcome measurement. Trial author clarified that the questionnaire was self‐administered but modes of administration unclear, probably not similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk | Comment: no direct quote is available. Not defined, probably adjudicated outcome measurement. Trial author clarified that HbA1c was measured by laboratory technicians who were not the members of the study group and didn't know about the allocation. |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | High risk |
Quote from publication: "Physicians did not know which patients served as control subjects for this study." Comment: self‐reported outcome measurement. Trial author clarified that the questionnaire was self‐administered but modes of administration unclear, probably not similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "Physicians did not know which patients served as control subjects for this study." Comment: self‐reported outcome measurement. Trial author clarified that the questionnaire was self‐administered but modes of administration unclear, probably not similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk | Comment: no direct quote is available. Not defined, probably adjudicated outcome measurement. Trial author clarified that HbA1c was measured by laboratory technicians who were not the members of the study group and did not know about the allocation |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | High risk |
Quote from publication: "Physicians did not know which patients served as control subjects for this study." Comment: self‐reported outcome measurement. Trial author clarified that the questionnaire was self‐administered but modes of administration unclear, probably not similarly done in intervention groups |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | High risk |
Quote from publication: "During 1 year of follow‐up, 14 participants (10%) were dropped out, of whom 6 had been allocated to the intervention group. We found no differences in characteristics of dropout subjects between two groups." Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) HbA1c | Unclear risk |
Quote from publication: "During 1 year of follow‐up, 14 participants (10%) were dropped out, of whom 6 had been allocated to the intervention group. We found no differences in characteristics of dropout subjects between two groups." Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | High risk |
Quote from publication: "During 1 year of follow‐up, 14 participants (10%) were dropped out, of whom 6 had been allocated to the intervention group. We found no differences in characteristics of dropout subjects between two groups." Comment: dropouts reported but not explained |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcome measures in the publication were mentioned and reported although HbA1c and DRD were non‐significant; no prior design paper or trials register record |
Simmons 2015.
| Methods | Cluster‐factorial randomised controlled trial; randomisation ratio 1:1:1:1 | |
| Participants |
Inclusion criteria: participants had type 2 diabetes for at least 12 months Exclusion criteria: those with dementia or psychotic illness Diagnostic criteria: measures of depression (PHQ‐8), quality of life (EQ5D), diabetes self‐efficacy, the Revised Diabetes Knowledge Scale (RDKS), diabetes distress, and medication adherence. IFCC aligned HbA1c (high performance liquid chromatography, Tosoh G7, Tokyo, Japan) and lipid measurements (Dimension RxL Max Clinical Chemistry System, Siemens, Erlangen, Germany) were undertaken in 1 ?CPA' accredited laboratory to minimise variation in both the primary outcome, HbA1c and total cholesterol, a secondary outcome |
|
| Interventions |
Number of study centres: 130 Treatment before study: no Titration period: no Intervention 1: one‐to‐one (individual) peer support. Individual discussion of social and emotional aspects of living with diabetes Intervention 2: group peer support. Group discussion of social and emotional aspects of living with diabetes Intervention 3: combined group and individual. Within the combined individual and group support arm of the trial, participants will be encouraged to agree which topics should be covered individually, and which should be discussed in the group sessions. General content The intervention was delivered in 2 phases: an initial 4‐6 months discussing 3 core aspects: how to address barriers to care/practical issues arising from living with diabetes; social and emotional aspects of diabetes; and the health care received. Peer support facilitators (PSFs) were asked to be non‐directive and deploy the listening skills explored during the PSF training in order to support peers in their efforts to attain better control over their diabetes and its effects on everyday life. In the second phase, PSFs were invited to continue with the same themes, but to discuss other topics not yet covered and consider inviting speakers. A 'RAPSID nurse' met with groups of PSFs within each intervention arm, in each of 4 geographical areas on a monthly basis. These meetings enabled PSFs to share positive and challenging experiences, generate potential solutions, discuss clinical issues that arose and keep the delivered content of the interventions in a standardised form. A RAPSID nurse was also reachable by telephone during office hours if PSFs had pressing concerns. PSFs were asked to keep records of telephone contacts and meetings with their peers. They were also provided with diaries and encouraged to write reflections on their experiences of delivering the intervention. Even if a peer was unable to attend a meeting, PSFs were asked to attempt to make contact and discuss arrangements. Contact between peers within the same trial arm was not recorded. Throughout the trial, care was taken not to introduce those in different arms of the study to each other Controls: all participants received access to educational materials and normal care from their healthcare providers |
|
| Outcomes | Outcomes reported in abstract of publication: primary end point was HbA1c. Secondary outcomes included quality of life, diabetes distress, blood pressure, waist, total cholesterol and weight | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: ISRCTN66963621 |
|
| Publication details |
Language of publication: English Non‐commercial funding: peers for progress (peersforprogress.org ‐ no grant number) and National Institute for Health Research for Patient Benefit Programme (Ref PB‐PG‐0610‐22311) Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "We now describe the results of the RCT comparing different diabetes peer support strategies." | |
| Notes | Participants with missing outcome data were excluded. A sensitivity analysis including all participants was conducted by using multiple imputation (based on 50 imputed data sets), which did not change the conclusions of the primary outcome analysis. Any missing outcome values were assumed to be missing at random. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Clusters were then randomised electronically in blocks of four (one cluster in each arm)" Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "... by the statistician who had no trial involvement. Randomisation occurred once all clusters in the block were ready to proceed. All measurement staff were blind to the randomisation." Comment: probably done |
| Blinding of participants and personnel (performance bias) Blood pressure | Unclear risk |
Quote from publication: "A research nurse obtained consent, checked a self‐completed questionnaire, measured weight, height, waist circumference, BP and collected blood (HbA1c, lipids) using standardised methodology/equipment following training by the local Medical Research Council Epidemiology Unit." Comment: unclear of blinding and whether BP was an adjudicated (automated BP machine) or investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "A research nurse obtained consent, checked a self‐completed questionnaire ... measured using postal questionnaires at 4‐6 months and face‐to‐face measurements and questionnaires after 8‐12 months." Comment: self‐reported outcome measurement but modes of administration unclear, probably not similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "A research nurse obtained consent, checked a self‐completed questionnaire, measured weight, height, waist circumference, BP and collected blood (HbA1c, lipids) using standardised methodology/equipment following training by the local Medical Research Council Epidemiology Unit." Comment: laboratory outcome measurement |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | High risk |
Quote from publication: "A research nurse obtained consent, checked a self‐completed questionnaire ... measured using postal questionnaires at 4‐6 months and face‐to‐face measurements and questionnaires after 8‐12 months." Comment: self‐reported outcome measurement but modes of administration unclear, probably not similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) Self‐efficacy | High risk |
Quote from publication: "A research nurse obtained consent, checked a self‐completed questionnaire ... measured using postal questionnaires at 4‐6 months and face‐to‐face measurements and questionnaires after 8‐12 months." Comment: self‐reported outcome measurement but modes of administration unclear, probably not similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) Blood pressure | Unclear risk |
Quote from publication: "A research nurse obtained consent, checked a self‐completed questionnaire, measured weight, height, waist circumference, BP and collected blood (HbA1c, lipids) using standardised methodology/equipment following training by the local Medical Research Council Epidemiology Unit." Comment: unclear of blinding and whether BP was an adjudicated (automated BP machine) or investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "A research nurse obtained consent, checked a self‐completed questionnaire ... measured using postal questionnaires at 4‐6 months and face‐to‐face measurements and questionnaires after 8‐12 months." Comment: self‐reported outcome measurement but modes of administration unclear, probably not similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "A research nurse obtained consent, checked a self‐completed questionnaire, measured weight, height, waist circumference, BP and collected blood (HbA1c, lipids) using standardised methodology/equipment following training by the local Medical Research Council Epidemiology Unit." Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | High risk |
Quote from publication: "A research nurse obtained consent, checked a self‐completed questionnaire ... measured using postal questionnaires at 4‐6 months and face‐to‐face measurements and questionnaires after 8‐12 months." Comment: self‐reported outcome measurement but modes of administration unclear, probably not similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) Self‐efficacy | High risk |
Quote from publication: "A research nurse obtained consent, checked a self‐completed questionnaire ... measured using postal questionnaires at 4‐6 months and face‐to‐face measurements and questionnaires after 8‐12 months." Comment: self‐reported outcome measurement but modes of administration unclear, probably not similarly done in intervention groups |
| Incomplete outcome data (attrition bias) Blood pressure | Unclear risk |
Quote from publication: "Attenders were significantly older, more highly educated, with lower body mass index (BMI) and smoking prevalence. Analyses were on an intention to treatment (ITT) basis, two‐sided and assessed at P < 0.05. Each continuous outcome was analysed using linear mixed effects regression models ... with cluster as the random effect, and adjusting for the baseline of the outcome using the missing indicator method to include any participants for whom the baseline was missing." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | High risk |
Quote from publication: "Attenders were significantly older, more highly educated, with lower body mass index (BMI) and smoking prevalence." Comment: reported and reasons explained. Attrition rate for questionnaires was 26.4% in the group peer support compared to 18.3% in the control group |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "Compared with those without, those with an endpoint Hba1c were ... had longer diabetes duration ... lower BMI ... and were more likely to be treated with anti‐hyperglycaemic tablets ... hypertension treatment ... and dyslipidaemia treatment ... at baseline. A sensitivity analysis including all patients was conducted by using multiple imputation (based on 50 imputed data sets), which did not change the conclusions of the primary outcome analysis." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | High risk |
Quote from publication: "Attenders were significantly older, more highly educated, with lower body mass index (BMI) and smoking prevalence." Comment: reported and reasons explained. Attrition rate for questionnaires was 26.4% in the group peer support compared to 18.3% in the control group |
| Incomplete outcome data (attrition bias) Self‐efficacy | High risk |
Quote from publication: "Attenders were significantly older, more highly educated, with lower body mass index (BMI) and smoking prevalence." Comment: reported and reasons explained. Attrition rate for questionnaires was 26.4% in the group peer support compared to 18.3% in the control group |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported |
| Other bias | Low risk |
Comment: right use of statistical analysis (linear mixed‐effect models) that adjust for a potential clustering effect Assessment of risk of bias in cluster‐randomised trials
|
Skelly 2009.
| Methods | Parallel randomised controlled trial initially, later became 3‐group experimental design because at the end of the intervention, half of the symptom‐focused intervention participants were randomly assigned to receive the telephone booster. Randomisation ratio 2 (intervention):1 (control) | |
| Participants |
Inclusion criteria: female gender, age 50 years and older, African American ethnicity as defined by the participant, type 2 diabetes for greater than 1 year, and HbA1C greater than 7%; have access to a telephone and be English‐speaking Exclusion criteria: — Diagnostic criteria: HbA1c, using micro capillary samples were obtained in the home using the Accubase A1c Test Kit (FDA approved; K983172; MDE#903510) and submitted for analysis to Diabetes Technologies, Inc.; symptom distress was measured using the Diabetes Symptom Distress Scale; quality of life was measured using the Quality of Life in Diabetes Scale and the PAID; diabetes self‐care practices were measured using the Diabetes Self‐Care Practices questionnaire |
|
| Interventions |
Number of study centres: multicentre Treatment before study: — Titration period: no Intervention 1: symptom‐focused diabetes intervention. Teaching and counselling modules delivered by a nurse in the participant's home. Family members, if present, were invited to sit in during the intervention sessions, with the participant's approval. The intervention was guided by 4 modules addressing symptoms of hyperglycaemia, symptoms of hypoglycemia, numbness and tingling in the feet/foot pain, and prevention of cardiovascular symptoms Intervention 2: at the end of the intervention, half of the symptom‐focused intervention participants were randomly assigned to receive the telephone booster, provided between months 6 and 9 to symptom‐focused participants chosen randomly at month 6. 4 telephone calls at approximately 2‐3 week intervals with the spacing of the calls covering a 12‐week interval similar to that of the intervention. The purpose of the telephone booster was to reinforce the strategies developed during home visits, engage in problem‐solving, provide motivation and encouragement, and encourage reframing and adjustment as needed. Control: attention control. Weight and diet programme consisting of 4 modules addressed weight maintenance (2 modules), modifying fat, and modifying sodium in the diet |
|
| Outcomes | Outcomes reported in abstract of publication: HbA1c; symptom distress, perceived quality of life, impact of diabetes and self‐care activities | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: — |
|
| Publication details |
Language of publication: English Non‐commercial funding: National Institute of Nursing Research Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "to test of the effectiveness of a symptom‐focused approach to diabetes self‐care tailored for older African American women as compared to a more traditional skills‐based approach. Also assessed is the effect of a telephone booster follow‐up for the symptom‐focused approach." | |
| Notes | No mention of missing data handling, probably no imputation of missing values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Subjects randomly assigned to ... blocked by HbA1c (<10, >10), co morbidities (1, >1), and a factor to produce even accrual in the study arms over time." Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Arm assignments were kept in sealed, opaque envelopes that were opened using a verifiable system" Comment: probably done |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: no direct quote is available, the CONSORT diagram reported death. Probably adjudicated outcome measurement. Not defined but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "The home was chosen as the delivery site ... measures were read to participants rather than self‐administered." Comment: self‐reported outcome measurement but modes of administration unclear, probably not self‐administered |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "Microcapillary samples were obtained in the home using the Accubase A1c Test Kit (FDA approved; K983172; MDE#903510) and submitted for analysis to Diabetes Technologies, Inc." Comment: laboratory outcome measurement |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | High risk |
Quote from publication: "The home was chosen as the delivery site ... measures were read to participants rather than self‐administered." Comment: self‐reported outcome measurement but modes of administration unclear, probably not self‐administered |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Comment: no direct quote is available, the CONSORT diagram reported death. Probably adjudicated outcome measurement. Not defined but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "Data collection visits conducted by a research assistant, who was blind to the study arm assignment ..." Comment: self‐reported outcome measurement but modes of administration unclear, probably not self‐administered |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "Microcapillary samples were obtained in the home using the Accubase A1c Test Kit ..." Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | High risk |
Quote from publication: "Data collection visits conducted by a research assistant, who was blind to the study arm assignment ..." Comment: self‐reported outcome measurement but modes of administration unclear, probably not self‐administered |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "Retention rates for the four evaluation visits were 97% for time 1, 96% for time 2, 93% for time 3, and 91% for time 4 ... The likelihood of completing the study was not related to initial treatment assignment ... Completion of the study also was not related to the primary physiological outcome, glycaemic control." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "Retention rates for the four evaluation visits were 97% for time 1, 96% for time 2, 93% for time 3, and 91% for time 4 ... The likelihood of completing the study was not related to initial treatment assignment ... Completion of the study also was not related to the primary physiological outcome, glycaemic control." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | Low risk |
Quote from publication: "Retention rates for the four evaluation visits were 97% for time 1, 96% for time 2, 93% for time 3, and 91% for time 4 ... The likelihood of completing the study was not related to initial treatment assignment... Completion of the study also was not related to the primary physiological outcome, glycaemic control." Comment: reported and reasons explained |
| Selective reporting (reporting bias) | Unclear risk | Comment: no trials register record or study design paper available. DRD and QoL showed within‐group significant changes but did not show between‐group differences with some P values reported but no details on the effect sizes |
| Other bias | Low risk | Comment: all results were reported for the randomised groups |
Spencer 2013.
| Methods | Parallel randomised controlled trial; 45% of participants to immediate and 55% to the delayed group | |
| Participants |
Inclusion criteria: at least 18 years of age, had physician‐diagnosed type 2 diabetes, self‐identified as African American or Latino/Hispanic Exclusion criteria: had serious diabetes‐related complications, such as blindness, amputated limbs, or kidney failure Diagnostic criteria: haemoglobin A1c measurements were abstracted from medical records. The interview consisted of items from the Behavioral Risk Factor Surveillance System, a CDC‐administered survey used to track health risks in the USA (Center for Disease Control and Prevention 2004), and a battery of assessments about health, health care, behaviours and attitudes toward diabetes, quality of diabetes care, relations with healthcare providers, and dietary and physical activity practices; PAID is used to measure diabetes‐related emotional distress; depression severity is assessed with the PHQ‐9 |
|
| Interventions |
Number of study centres: multicentre Treatment before study: — Titration period: no Intervention: community health worker (CHW) intervention. Trained CHWs promoted healthy lifestyle and diabetes self‐management activities, including information on stress reduction, physical activity, and healthy eating. The diabetes education classes were culturally tailored group classes in both English and Spanish. CHWs helped participants improve their participant‐provider communication skills and facilitated necessary referrals to other service systems. CHWs also contacted participants by phone once every 2 weeks Control: 6‐month delayed group. Similar to the intervention group, participants received information on and had access to community activities that provided free, publicly available healthy eating demonstrations, physical fitness activity (e.g. dance and exercise classes, walking clubs), and a weekly community farmers' produce market. Participants also received health care at facilities in which healthcare providers were trained in culturally competent diabetes care. Participants in the delayed group were contacted once a month to update contact information until they were officially enrolled in the intervention |
|
| Outcomes | Outcomes reported in abstract of publication: PAID from pre‐intervention to postintervention; PHQ score | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NCT00800410 |
|
| Publication details |
Language of publication: English Non‐commercial funding: Centers for Disease Control and Prevention, the Michigan Diabetes Research and Training Center, and the Robert Wood Johnson Foundation Clinical Scholars Program Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "... investigated the influence of a community health worker (CHW) diabetes lifestyle intervention on mental health outcomes" | |
| Notes | No mention of missing data handling, probably no imputation of missing values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Participants were stratified by race/ethnicity and healthcare site during randomisation to assure that these variables were equally distributed". Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Community Health Worker (CHW) and interviewers were not blinded to the group assignment of the participants; however, data analysts were blinded." Comment: participants in the waiting list were informed of the delayed intervention |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "... survey was a comprehensive ... conducted in person, usually in the household of the participant, by trained staff ... The interview consisted of items ..." Comment: interview‐administered, self‐reported outcome measurement. Probably not blinded |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "Hemoglobin A1c measurements were abstracted from medical records." Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "... survey was a comprehensive ... conducted in person, usually in the household of the participant, by trained staff ... The interview consisted of items ..." Comment: interview‐administered, self‐reported outcome measurement. Probably not blinded |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "Hemoglobin A1c measurements were abstracted from medical records." Comment: laboratory outcome measurement |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "At the 6‐month follow‐up, 136 participants completed the study protocols and were analysed for the primary outcome (attrition rate = 17.1%). Withdrawal from the study was not independently associated with treatment arm, age, gender, education, diabetes duration, baseline HbA1c, low‐density lipoprotein cholesterol, or blood pressure. However, African Americans were more likely to withdraw from the study and to be missing HbA1c data." Comment: reported and reasons explained, statistical adjustments were done |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "At the 6‐month follow‐up, 136 participants completed the study protocols and were analysed for the primary outcome (attrition rate = 17.1 %). Withdrawal from the study was not independently associated with treatment arm, age, gender, education, diabetes duration, baseline HbA1c, low‐density lipoprotein cholesterol, or blood pressure. However, African Americans were more likely to withdraw from the study and to be missing HbA1c data." Comment: reported and reasons explained, statistical adjustments were done |
| Selective reporting (reporting bias) | Unclear risk | Comment: HbA1c measurements were reported that they would be abstracted from medical records but no details were actually reported in the paper. However, study authors communicated and provided the required data |
Sperl‐Hillen 2013.
| Methods | Parallel randomised controlled trial; randomisation ratio 2 (group education):2 (individual eduction):1 (usual care) | |
| Participants |
Inclusion criteria: type 2 diabetes and an A1c result of > 7% in the last 6 months Exclusion criteria: — Diagnostic criteria: depression measured by the PHQ‐9 depression module; understanding assessed by the diabetes care profile section; diabetes distress assessed by the 20‐item PAID; diabetes empowerment was measured by the diabetes empowerment scale‐short form (DES‐SF); nutrition was measured by the recommended food score (RFS); physical activity assessed by the behavioural risk factor surveillance system (BRFSS) method |
|
| Interventions |
Number of study centres: at least 2 Treatment before study: — Titration period: no Intervention 1: individual education (IE). The first session included an assessment of participant needs pertaining to American Association of Diabetes Educators (AADE) ‐ recommended content for 7 self‐care behaviours (healthy eating, monitoring blood sugars, taking medications, problem solving, risk reduction, healthy coping, and being active). Follow‐up sessions focused on the participant's individual concerns, reviewed self‐monitored blood sugars, and evaluated progress toward treatment targets. The sessions were intended to help the participant develop personalised behavioural modification goals needed to achieve care targets Intervention 2: group education (GE) using US Diabetes Conversation Maps. The programme was a non‐didactic group approach that promoted participant interaction and was intended to help participants overcome barriers to self‐management and to improve self‐efficacy Control: usual care (UC). The UC group was not assigned any educational intervention throughout the study. The study did not prohibit self‐management education recommended by usual providers or sought by the study participants |
|
| Outcomes | Outcomes reported in abstract of publication: A1c tests, PAID, Diabetes Self‐Efficacy (DES), Recommended Food Score (RFS) for the first 150 days post randomisation, and by 250 days | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NCT00652509 |
|
| Publication details |
Language of publication: English Commercial funding: Merck Sharp and Dohme Corp Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "To evaluate whether outcomes from diabetes self‐management education for patients with sub optimal control were sustained" | |
| Notes | Missing values for A1c and survey outcomes in the measurement period of interest were assigned the latest known result (e.g. the baseline value if no subsequent data were collected). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Consented subjects were randomly assigned ... using a random allocation sequence ..." Comment: probably done, since earlier reports from the same investigators describe use of computer‐generated random allocation (Sperl‐Hillen 2011) |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Consented subjects were randomly assigned ... using a random allocation sequence ..." Comment: Proabaly done, since earlier reports from the same investigators describe use of computer‐generated random allocation (Sperl‐Hillen 2011) |
| Blinding of participants and personnel (performance bias) All‐cause mortality | Low risk | Comment: no direct quote is available, the CONSORT diagram reported death. Unclear of the method for this outcome measurement. Not defined but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "Survey outcome variables for this analysis were obtained from validated instruments ... All study subjects received surveys at the baseline visit and by mail ..." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "HbA1C values ... were collected through passive surveillance of laboratory results contained in the electronic health record ..." Comment: laboratory outcome measurement |
| Blinding of participants and personnel (performance bias) Self‐efficacy | High risk |
Quote from publication: "Survey outcome variables for this analysis were obtained from validated instruments ... All study subjects received surveys at the baseline visit and by mail ..." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) All‐cause mortality | Low risk | Comment: no direct quote is available, the CONSORT diagram reported death. Unclear of the method for this outcome measurement. Not defined but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "All study subjects received surveys at the baseline visit and by mail at ..." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "HbA1C values ... were collected through passive surveillance of laboratory results contained in the electronic health record ... analysed at one of 2 accredited clinical laboratories using standard high‐pressure liquid chromatography assay methods ..." Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Self‐efficacy | High risk |
Quote from publication: "All study subjects received surveys at the baseline visit and by mail at ..." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "... responded to first survey ... second survey ... third survey ... fourth survey ..." Comment: dropouts reported but not explained. More than 80% responded to the survey across the treatment groups but no description on the non‐responders in each treatment group |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "... had an A1C result in the long‐term follow‐up period." Comment: dropouts reported but not explained. More than 90% had HbA1c data |
| Incomplete outcome data (attrition bias) Self‐efficacy | Low risk |
Quote from publication: "... responded to first survey ... second survey ... third survey ... fourth survey ..." Comment: dropouts reported but not explained. More than 80% responded to the survey across the treatment groups but no description on the non‐responders in each treatment group |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measures were reported |
| Other bias | Low risk | Comment: all results were reported for the randomised groups |
Sturt 2008.
| Methods | Cluster‐randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: adults with type 2 diabetes, not taking insulin and able to read and write English and, during the first 12 months of the study, a most recent HbA1c > 7.0% Exclusion criteria: — Diagnostic criteria: HbA1c, BP, serum cholesterol, BMI, diabetes‐related distress, measured with the PAID, and confidence to self‐care, measured with the Diabetes Management Self‐efficacy Scale (DMSES). Participants were assessed at baseline and 26 weeks |
|
| Interventions |
Number of study centres: 48 Treatment before study: — Titration period: no Intervention: the diabetes manual structured education. Practice nurses undertook a 15‐min face‐to‐face consultation with participants to introduce the 12‐week Diabetes Manual programme. Participants worked independently through the workbook. Workbook topics include diabetes facts/metabolism/goal setting and evaluation/exercise/nutrition/blood glucose monitoring/weight loss/smoking cessation/tests/complications/medication/stress, anxiety and depression/cholesterol/quizzes to self‐evaluate workbook topics/other peoples' stories/self‐assessment record sheets to encourage personal evaluation of current and new behaviours and activities. A relaxation audiotape was provided and the participant was encouraged within the workbook to use it and to explore alternative relaxation methods. An audiotape was provided mirroring a discussion between a general practitioner and a participant to be used as a brief introduction to diabetes and its management. Participants were encouraged to share it with family members. Nurse telephone support was provided in weeks 1, 5 and 11 Control: 6‐month delayed‐intervention control. The deferred intervention arm continued usual care, and following 26‐week data collection, nurses undertook training and delivered the Diabetes Manual to their participating participants |
|
| Outcomes | Outcomes reported in abstract of publication: HbA1c; diabetes‐related distress scores; confidence to self‐care scores | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: ISRCTN06315411 |
|
| Publication details |
Language of publication: English Non‐commercial funding: Diabetes UK Structured Education project grant and a Department of Health postdoctoral award Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "To determine the effects of the Diabetes Manual on glycaemic control, diabetes‐related distress and confidence to self‐care of patients with Type 2 diabetes" | |
| Notes | Analysis of complete data. Missing data set to equal baseline values for all primary and secondary outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "A two‐arm cluster randomised, controlled trial with participating practices randomised to ... Practices were allocated in blocks" Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Recruitment continued ... prior to planned and timed block randomisation and subsequent nurse training ... The practice nurse conducted pre‐randomization baseline clinical assessments ...Practices were allocated ... by a statistician blind to practice identity using computer‐aided minimization" Comment: probably done |
| Blinding of participants and personnel (performance bias) Blood pressure | Unclear risk |
Quote from publication: "Patients were assessed ... by the practice nurse" Comment: investigator‐assessed outcome measurement. Unclear of blinding on the practice nurse |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "Patients were assessed ... by the practice nurse ... administered by questionnaire mailed by the research team" Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "Patients were assessed ... by the practice nurse" Comment: laboratory outcome measurement. Earlier reports from the same investigators describe analysis was done outside the practice by the DCCT aligned laboratory blinded to practice or participant group allocation (Sturt 2006) |
| Blinding of participants and personnel (performance bias) Self‐efficacy | High risk |
Quote from publication: "Patients were assessed ... by the practice nurse... administered by questionnaire mailed by the research team" Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) Blood pressure | Unclear risk |
Quote from publication: "Patients were assessed ... by the practice nurse" Comment: investigator‐assessed outcome measurement. Unclear of blinding on the practice nurse |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "Patients were assessed ... by the practice nurse ... administered by questionnaire mailed by the research team" Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "Patients were assessed ... by the practice nurse" Comment: laboratory outcome measurement. Earlier reports from the same investigators describe analysis was done outside the practice by the DCCT aligned laboratory blinded to practice or participant group allocation (Sturt 2006) |
| Blinding of outcome assessment (detection bias) Self‐efficacy | High risk |
Quote from publication: "Patients were assessed ... by the practice nurse... administered by questionnaire mailed by the research team" Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Incomplete outcome data (attrition bias) Blood pressure | Unclear risk |
Quote from publication: "Follow‐up data for the primary outcome and clinical data were available for 202/245 participants" Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | High risk |
Quote from publication: "Questionnaire data were obtained for 148/245 participants. Completeness of PAID ... was only 50% for the intervention group and 69% for the delayed intervention group ... The characteristics of the participants according to their completeness of PAID ... data ... indicated that ... notable differences between the groups were observed ... related to demographic characteristics such as ethnicity, age and postcode ..." Comment: reported and reasons explained. Attrition rate was almost 40%, statistical adjustment only for baseline, intention‐to‐treat analysis maintained the statistical finding albeit with reduced effect size |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "Follow‐up data for the primary outcome and clinical data were available for 202/245 participants ... " Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) Self‐efficacy | High risk |
Quote from publication: "Questionnaire data were obtained for 148/245 participants. Completeness of ... DMSES data was only 50% for the intervention group and 69% for the delayed intervention group ... The characteristics of the participants according to their completeness of ... DMSES data ... indicated that ... notable differences between the groups were observed ... related to demographic characteristics such as ethnicity, age and postcode ..." Comment: reported and reasons explained. Attrition rate was almost 40%, statistical adjustment only for baseline and sex, intention‐to‐treat analysis maintained the statistical finding albeit with reduced effect size |
| Selective reporting (reporting bias) | Low risk | Comment: outcome measures were reported as specified |
| Other bias | Low risk |
Comment: right use of statistical analysis (generalised estimating equations) that adjust for a potential clustering effect Assessment of risk of bias in cluster‐randomised trials
|
Taylor 2006.
| Methods | Randomised controlled trial; randomisation ratio: 1:1:1 | |
| Participants |
Inclusion criteria: type 2 diabetes for at least 6 months Exclusion criteria: — Diagnostic criteria: psychological well‐being through the Well‐Being Questionnaire (WBQ‐12) and a diabetes‐specific well‐being measure through an administration of the PAID 1 scale; self‐care behavioural assessment of the 4 leading behaviours linked to successful diabetes management; and social support |
|
| Interventions |
Number of study centres: at least 3 Treatment before study: — Titration period: no Intervention 1: cognitive‐behavioural therapy (CBT). A total of 30 minutes was allocated to cognitive‐behavioural education and 20 minutes to small‐group interaction (teams) for practicing problem‐solving techniques on selected topics. In the final 25 minutes of the session, the team group reported to the class their thoughts on the topic and solutions to the dilemma situations. Topics presented over the course of 5 weeks included the following: week 1 ‐ mind‐behaviour connection: thoughts (cognitions) can raise your blood sugar; week 2 ‐ become an ANT (automatic negative thoughts) terminator!; week 3 ‐ transform one's stress into results and relaxation; week 4 ‐ coping, one's action plan for successful mood management; week 5 ‐ healthy habits for living well with diabetes. Participants were given a Diabetes Research and Wellness Diary and asked to document the self‐care behaviour that they chose on the questionnaire to monitor Intervention 2: expressive writing. This expressive writing programme followed a similar format of the CBT programme. The first 30 minutes focused on the health habit of the week, followed by 20 minutes of small group interaction (teams) for brainstorming ideas and problem‐solving situations related to the featured self‐management skill. The final 20 minutes followed the expressive writing protocol described below. Participants were instructed to follow the research assistant to an assigned quiet chair or bench located at different parts throughout the building and grounds. Once seated and comfortable, participants were instructed to write about a stressful event that had happened to them, noting details about the event, and describing their feelings or emotions at that time. They were asked to keep writing as thoughts came into their mind and to not worry about spelling or grammar. This group programme was designed to educate participants about the 5 behavioural skills required to manage their diabetes. A workbook was written and corresponded to the following weekly schedule, allowing participants to read the material and write down any information that they found helpful. The topics presented each week were: week 1 ‐ progress not perfection: healthy habits; week 2 ‐ focus on fitness and energising one's days; week 3 ‐ make nutrition come alive; week 4 ‐ the learning gap: balancing stress; week 5 ‐ healthy habits for life: communicating with your health professionals. Participants were given a Diabetes Research and Wellness Diary and asked to document the self‐care behaviour that they chose on the questionnaire to monitor Control: control group (wait‐list). Participants were given a Diabetes Research and Wellness Diary and asked to document the self‐care behaviour that they chose on the questionnaire to monitor |
|
| Outcomes | Outcomes reported in abstract of publication: well‐being; stress; energy levels; mood; awareness | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: — |
|
| Publication details |
Language of publication: English Non‐commercial funding: Diabetes Research and Wellness Foundation Publication status: dissertation submitted in partial fulfilment of the requirements for the degree of Doctor of Psychology |
|
| Stated aim for study | Quote from publication: "The goal of this research was to evaluate the effectiveness of both interventions at improving seniors' perceived psychological well‐being, increasing their self‐efficacy, and alleviating the severity of diabetes symptoms improving through self‐management skills." | |
| Notes | No mention of missing data handling, probably no imputation of missing values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "The diabetes educator coded all names on the list, and all participants were randomly assigned to ... In the interest of convenience... reassign[ed] 4 seniors to the group nearest their home." Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "The diabetes educator coded all names on the list, and all participants were randomly assigned to ..." Comment: probably done |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "If you are still upset we encourage you to call and talk to the researcher or the diabetes educator." Comment: self‐reported outcome measurement |
| Blinding of outcome assessment (detection bias) Adverse events | Low risk |
Quote from publication: "If you are still upset we encourage you to call and talk to the researcher or the diabetes educator." Comment: self‐reported outcome measurement |
| Incomplete outcome data (attrition bias) Adverse events | Low risk |
Expressive writing Quote from publication: "Four seniors dropped this program after the 2nd week because they did not want to write. " Comment: dropouts reported but not explained Cognitive behavioural therapy Quote from publication: "1 person stating a distinct dislike for the class. The 3 dropouts occurred because of hospitalisation for medical problems." Comment: reported and reasons explained |
| Selective reporting (reporting bias) | Unclear risk | Comment: adverse events were reported by participants in programme evaluation and during debriefing session |
| Other bias | Unclear risk | Comment: some of the results were incomplete for 2 of the 3 intervention groups |
Trief 2016.
| Methods | Parallel randomised controlled trial; randomisation ratio unequal "[A] smaller DE [diabetes education] sample was planned to provide more power to compare CC [couples change] to IC [individual calls]." |
|
| Participants |
Inclusion criteria: Couples were eligible if patients, with a willing partner able to speak and read English, met the following criteria: had a diagnosis of type 2 diabetes for > 1 year (diagnosis confirmed by medical record and/or A1c level); baseline A1c level of ≥ 7.5% (58 mmol/mol); ≥ 21 years of age; able to speak and read English; in a self‐defined committed relationship for ≥ 1 year; no severe medical or psychiatric conditions that might interfere with participation; and telephone access Exclusion criteria: — Diagnostic criteria: HbA1c was measured by the AccuBase A1c Test Kit (Diabetes Technologies, Inc); blood pressure was measured by an automated monitor with appropriate cuff sizes. 3 seated readings at 1‐min intervals; calculated mean of readings 2‐3; diabetes distress was assessed by the 17‐item Diabetes Distress Scale; diabetes self‐efficacy was assessed by the 8‐item scale developed for the Stanford English Diabetes Self‐Management Study |
|
| Interventions |
Number of study centres: multicentre Treatment before study: no Titration period: no Intervention 1: behaviour intervention change couples calls (CC) Interventions were delivered solely via telephone. All groups participated in 2 telephone sessions (mean length of calls 75 min) of comprehensive DE. CC interventions had 10 additional calls (mean length: 57 min/call). These behavioural interventions, based on social learning theory (which included knowledge development, goal setting, self‐monitoring, and behavioural contracting), promoted changes in diet, activity, medication adherence, and blood glucose testing. The CC intervention was also based on interdependence theory; partners were actively involved in calls and homework. Couples were encouraged to provide mutual support for change, using collaborative problem‐solving techniques and recognising their interdependence (i.e. reciprocal effects on one another). 2 sessions were relationship focused, as follows: couples practiced the "speaker‐listener technique" (partner shares concern, the other restates it until partner feels understood, then they switch roles), and communication/conflict management around a diabetes‐related issue. Both techniques are based on a research supported behavioural approach to relationship enhancement. Calls occurred weekly for 12 weeks. Workbooks included precall readings, content for discussion, goal‐setting forms, and diet/blood glucose/activity self‐monitoring logs Intervention 2: behaviour change intervention individual calls (IC). Interventions were delivered solely via telephone. All groups participated in 2 telephone sessions (mean length of calls 75 min) of comprehensive DE. IC interventions had 10 additional calls (mean length: 50 min/call). These behavioural interventions, based on social learning theory (which included knowledge development, goal setting, self‐monitoring, and behavioural contracting), promoted changes in diet, activity, medication adherence, and blood glucose testing. In the IC arm, the intervention was identical, except partners were not involved, and the 2 CC relationship‐focused calls addressed individual problem solving. Calls occurred weekly for 12 weeks. Workbooks included precall readings, content for discussion, goal‐setting forms, and diet/blood glucose/activity self‐monitoring logs Control: individual diabetes education (DE) calls. Interventions were delivered solely via telephone. All groups participated in 2 telephone sessions (mean length of calls 75 min) of comprehensive DE. In the DE arm, there was no further intervention |
|
| Outcomes | Outcomes reported in abstract of publication: the primary outcome was change in A1c; and secondary outcomes were BMI, waist circumference, blood pressure, depressive symptoms, diabetes self‐efficacy, and diabetes distress | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NCT01017523 |
|
| Publication details |
Language of publication: English Commercial funding: Roche, Inc, provided some material support Non‐commercial funding: National Institutes of Health (NIH) grant 1R18‐DK‐080867‐01A2. The first year of the study was funded by a NIH Diversity Fellowship Supplement Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "This is the first RCT we are aware of that tests the efficacy of a couples intervention for adults with type 2 diabetes." | |
| Notes | Randomisation produced treatment arms that differ in BP; statistically controlled for between‐arm differences when analysing BP, but no covariates were used for other outcomes. Longitudinal data were analysed with mixed linear model procedures. No mention of missing data handling, probably no imputation of missing values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Randomization was conducted using a computer‐generated random assignment scheme ..." Comment: probably done |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: "Participants were assigned to condition in the proper proportions." Comment: probably done |
| Blinding of participants and personnel (performance bias) Blood pressure | Low risk |
Quote from publication: assessors were blind to treatment group Comment: investigator‐assessed outcome measurement |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: assessors were blind to treatment group Comment: self‐reported outcome measurement but modes of administration unclear, probably interviewed |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: assessors were blind to treatment group. Comment: laboratory‐based outcome measurement |
| Blinding of participants and personnel (performance bias) Self‐efficacy | High risk |
Quote from publication: assessors were blind to treatment group. Comment: self‐reported outcome measurement but modes of administration unclear, probably interviewed |
| Blinding of outcome assessment (detection bias) Blood pressure | Low risk |
Quote from publication: assessors were blind to treatment group Comment: investigator‐assessed outcome measurement |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: assessors were blind to treatment group Comment: self‐reported outcome measurement but modes of administration unclear, probably interviewed |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: assessors were blind to treatment group Comment: laboratory‐based outcome measurement |
| Blinding of outcome assessment (detection bias) Self‐efficacy | High risk |
Quote from publication: assessors were blind to treatment group Comment: self‐reported outcome measurement but modes of administration unclear, probably interviewed |
| Incomplete outcome data (attrition bias) Blood pressure | Unclear risk |
Quote from publication: dropouts (n = 54, no follow‐up data) were less likely to be white (53% vs 74%) and retired (11% vs. 32%), and were more likely to be Asian (18% vs 7%) and single/widowed/separated/divorced (15% vs 4%) Comment: unclear of the significant of the differences in attrition between arms |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Unclear risk |
Quote from publication: dropouts (N = 54, no follow‐up data) were less likely to be white (53% vs 74%) and retired (11% vs 32%), and were more likely to be Asian (18% vs 7%) and single/widowed/separated/divorced (15% vs 4%) Comment: unclear of the significant of the differences in attrition between arms |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: Attrition (i.e. no follow‐up A1c level) was 17.9% (4 months), 19.8% (8 months), and 25.4% (12 months), with no significant differences in attrition between arms Comment: — |
| Incomplete outcome data (attrition bias) Self‐efficacy | Unclear risk |
Quote from publication: dropouts (N = 54, no follow‐up data) were less likely to be white (53% vs 74%) and retired (11% vs 32%), and were more likely to be Asian (18% vs. 7%) and single/widowed/separated/divorced (15% vs 4%) Comment: unclear of the significant of the differences in attrition between arms |
| Selective reporting (reporting bias) | Unclear risk |
Quote from publication: — Comment: all reported outcomes were mentioned in the publication. However, blood pressure and self‐efficacy were not mentioned as secondary outcomes in the trials register record. It is unclear whether there is any other selective or under‐reporting such as quality of life measure besides diabetes distress mentioned as the measure for this |
| Other bias | Low risk | Roche, Inc provided some material support. However, it is unlikely to bias the results of the study that is mainly on the behaviour intervention with and without couples involvement. Randomisation produced treatment arms that did not differ in any participant characteristics and intention‐to‐treat analyses were used |
Van der Wulp 2012.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: diagnosed with type 2 diabetes for less than 12 months Exclusion criteria: unable to complete a questionnaire because of an inability to read and understand the Dutch language or had cognitive impairments Diagnostic criteria: self‐efficacy was measured with the 20‐item Diabetes Management Self‐Efficacy Scale; the 21 item Diabetes Coping Measure was used to measure changes in cognitive and behavioural coping; physical activity was measured with the 12‐item Physical Activity Scale for the Elderly questionnaire; changes in dietary habits were measured with the 35‐item Fatlist; psychological well‐being was measured with the 5‐item WHO Well‐being Index; the 20‐item CES‐D was used to measure depressive symptoms; the Problem Areas In Diabetes questionnaire was used to measure psychological distress |
|
| Interventions |
Number of study centres: 54 Treatment before study: — Titration period: no Intervention: a peer‐led self‐management coaching programme. The primary objective of increasing self‐efficacy, with secondary objectives to improve physical activity and dietary habits. Expert participants conducted 3 monthly home visits to participating participants. During the first visit, areas for lifestyle change were explored. In the second visit, participants ranked the importance and feasibility of the proposed lifestyle change(s). In addition, goals were set to work on the upcoming month and possible obstacles for goal attainment were formulated. The expert participants made sure that their participants set feasible goals. These goals were evaluated in the third visit. The intervention focused on study participants solely (family, friends and others did not participate during the home visits). Within 2 weeks after each visit, the expert participants contacted their participants by telephone to evaluate the previous visit and to answer any questions. For medical advice, expert participants were instructed to refer the participants to their general practitioner, practice nurse or dietician as they kept receiving their usual care from these professionals, based on the Dutch guidelines on type 2 diabetes mellitus Control: usual care. Participants allocated to the control group received the same medical care as participants from the intervention group |
|
| Outcomes | Outcomes reported in abstract of publication: self‐efficacy, coping and saturated fat intake over time; psychological well‐being | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: ISRCTN91626621 |
|
| Publication details |
Language of publication: English Non‐commercial funding: Dutch Diabetes Research Foundation Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "To study the effectiveness of a peer‐led self‐management coaching intervention in recently diagnosed patients with Type 2 diabetes" | |
| Notes | Some imputations were done for missing data where possible | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "A computerized randomisation module allocated patients to ..." Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "Randomisation was conducted by a person who was not familiar with the study or the researchers." Comment: probably done |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "General practitioners selected eligible patients from their records ... Participants allocated to the control group received the same medical care as participants from the intervention group ... For medical advice, expert patients were instructed to refer the participants to their general practitioner, practice nurse or dietician as they kept receiving their usual care from." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered. Unsure of blinding on the general practitioners |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | High risk |
Quote from publication: "General practitioners selected eligible patients from their records... Participants allocated to the control group received the same medical care as participants from the intervention group ... For medical advice, expert patients were instructed to refer the participants to their general practitioner, practice nurse or dietician as they kept receiving their usual care from." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered. Unsure of blinding on the general practitioners |
| Blinding of participants and personnel (performance bias) Self‐efficacy | High risk |
Quote from publication: "General practitioners selected eligible patients from their records ... Participants allocated to the control group received the same medical care as participants from the intervention group ... For medical advice, expert patients were instructed to refer the participants to their general practitioner, practice nurse or dietician as they kept receiving their usual care from." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered. Unsure of blinding on the general practitioners |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "Participants filled in a questionnaire at ... patients were excluded from the analyses because they did not return the ... questionnaire ... " Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | High risk |
Quote from publication: "Participants filled in a questionnaire at ... patients were excluded from the analyses because they did not return the ... questionnaire ... " Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) Self‐efficacy | High risk |
Quote from publication: "Participants filled in a questionnaire at ... patients were excluded from the analyses because they did not return the ... questionnaire ... " Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "During the study, four participants met one of the exclusion criteria and were excluded from the analyses. In addition, 10 patients were excluded from the analyses because they did not return the T0 questionnaire and no sufficient data was available for imputation, leaving 119 patients for further analyses ... Thirteen participants dropped out during the study. Four of these provided a reason for dropping out ... one participant no longer received home visits. One participant became terminally ill and another could no longer participate because of a psychiatric illness. One participant indicated that he no longer needed the intervention because he knew enough about diabetes." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | Low risk |
Quote from publication: "During the study, four participants met one of the exclusion criteria and were excluded from the analyses. In addition, 10 patients were excluded from the analyses because they did not return the T0 questionnaire and no sufficient data was available for imputation, leaving 119 patients for further analyses ... Thirteen participants dropped out during the study. Four of these provided a reason for dropping out ... one participant no longer received home visits. One participant became terminally ill and another could no longer participate because of a psychiatric illness. One participant indicated that he no longer needed the intervention because he knew enough about diabetes." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) Self‐efficacy | Low risk |
Quote from publication: "During the study, four participants met one of the exclusion criteria and were excluded from the analyses. In addition, 10 patients were excluded from the analyses because they did not return the T0 questionnaire and no sufficient data was available for imputation, leaving 119 patients for further analyses ... Thirteen participants dropped out during the study. Four of these provided a reason for dropping out ... one participant no longer received home visits. One participant became terminally ill and another could no longer participate because of a psychiatric illness. One participant indicated that he no longer needed the intervention because he knew enough about diabetes." Comment: reported and reasons explained |
| Selective reporting (reporting bias) | Low risk | Comment: DRD was not mentioned as an outcome in the trials register record but was reported, although the result was not significant |
Van Dijk‐de Vries 2015.
| Methods | Cluster‐randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: participants with clinically established diagnosis of type 2 diabetes mellitus, scored Daily Functioning Thermometer (DFT) > 4 and Distress Screener (DS) > 3 Exclusion criteria: — Diagnostic criteria: daily functioning was measured by the DFT; diabetes‐related emotional distress was measured by the 20‐item PAID; participation and autonomy were measured by means of the Impact on Participation and Autonomy (IPA) questionnaire; self‐management knowledge and behaviours were measured using the Dutch version of the Partners in Health scale (PIH‐NL); the 12‐item Short‐Form Health Survey (SF‐12) measured the quality of life; the General Self‐Efficacy Scale (GSES‐12) assessed participants' belief in their ability to organise and engage in certain behaviours |
|
| Interventions |
Number of study centres: 40 Treatment before study: no Titration period: no Intervention: self‐management support (SMS) in routine care. Extra consultations delivered by practice nurses (PNs) were aimed at supporting participants in their day‐to‐day management of diabetes and its emotional and social consequences. The intervention strategy derived from the principles of learning theory. PNs supported participants in the processes of defining problems and finding solutions themselves, by applying problem‐solving and reattribution techniques. Problem‐solving consists of 7 stages that efficiently address problems and their possible solutions. The reattribution technique was applied to challenge participants to link feelings and cognition to consequent behaviour. Participants could use information from a diary in which they recorded symptoms, thoughts, worries, feelings, and behaviour. Both problem solving and reattribution techniques were intended to result in action plans indicating how participants would achieve their personal goals Control: usual care. PNs in the control arm provided usual diabetes care, conforming to the Dutch guidelines |
|
| Outcomes | Outcomes reported in abstract of publication: the primary outcome measure reported was the dichotomised score on a visual analogue scale of diabetes on daily functioning. Secondary measures included participants' diabetes‐related distress, quality of life, autonomy and participation, self‐efficacy, self‐management and glycaemic control. Outcomes were measured at baseline and at 4‐month and 12‐month follow‐ups. | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NTR2764 |
|
| Publication details |
Language of publication: English Non‐commercial funding/other funding: the Dutch Diabetes Research Foundation (Diabetes Fonds) with grant No. 2010.13.1366 (Voice of the Patient programme), and by the ‘Annadal Foundation' in Maastricht, an independent financial support fund in the field of healthcare. Both the training of practice nurses and operation of the system for registration of SMS were facilitated by the ‘HOZL' group of collaborating family practices in the eastern part of the Southern Limburg region. During the SMS project, CZ Health Insurance included a fee for SMS in the bundled payment arrangement for diabetes care Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "To evaluate the effectiveness of biopsychosocial Self‐Management Support (SMS) delivered by practice nurses in routine diabetes care." | |
| Notes | No mention of missing data handling, probably no imputation of missing values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "The randomisation was performed ... used a random number seed computer program ..." Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "The randomisation was performed by an independent research assistant ..." Comment: probably done |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "Patients who gave informed consent knew whether they would receive an addition to their usual care or not. No details were given about the content of the intervention ... PNs were blinded regarding the outcomes of the recruitment procedure and study participation of their patients. They applied SMS in all their consultations with patients with diabetes." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "PNs were blinded regarding the outcomes of the recruitment procedure and study participation of their patients ... The glycated haemoglobin in mmol/mol was measured during consultations." Comment: laboratory outcome measurement |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | High risk |
Quote from publication: "Patients who gave informed consent knew whether they would receive an addition to their usual care or not. No details were given about the content of the intervention ... PNs were blinded regarding the outcomes of the recruitment procedure and study participation of their patients. They applied SMS in all their consultations with patients with diabetes." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of participants and personnel (performance bias) Self‐efficacy | High risk |
Quote from publication: "Patients who gave informed consent knew whether they would receive an addition to their usual care or not. No details were given about the content of the intervention... PNs were blinded regarding the outcomes of the recruitment procedure and study participation of their patients. They applied SMS in all their consultations with patients with diabetes." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "PNs were blinded regarding the outcomes of the recruitment procedure and study participation of their patients. We used postal questionnaires for patient measurements." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "PNs were blinded regarding the outcomes of the recruitment procedure and study participation of their patients ... The glycated haemoglobin in mmol/mol was measured during consultations." Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | High risk |
Quote from publication: "PNs were blinded regarding the outcomes of the recruitment procedure and study participation of their patients. We used postal questionnaires for patient measurements." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) Self‐efficacy | High risk |
Quote from publication: "PNs were blinded regarding the outcomes of the recruitment procedure and study participation of their patients. We used postal questionnaires for patient measurements." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "One follow‐up measurement was missing. Three patients did not complete the baseline measurement and gave informed consent at the 4‐month follow‐up measurement. Another 23 patients completed only the baseline measurement. We found no baseline variables that were significantly related to incompleteness of measurements." Comment: dropouts reported but not explained. Imputation was done for missing value according to the scale recommendation. Analyses were performed on an intention‐to‐treat basis |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "One follow‐up measurement was missing. Three patients did not complete the baseline measurement and gave informed consent at the 4‐month follow‐up measurement. Another 23 patients completed only the baseline measurement. We found no baseline variables that were significantly related to incompleteness of measurements." Comment: dropouts reported but not explained. Analyses were performed on an intention‐to‐treat basis |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | Low risk |
Quote from publication: "One follow‐up measurement was missing. Three patients did not complete the baseline measurement and gave informed consent at the 4‐month follow‐up measurement. Another 23 patients completed only the baseline measurement. We found no baseline variables that were significantly related to incompleteness of measurements." Comment: dropouts reported but not explained. Imputation was done for missing value according to the scale recommendation. Analyses were performed on an intention‐to‐treat basis |
| Incomplete outcome data (attrition bias) Self‐efficacy | Low risk |
Quote from publication: "One follow‐up measurement was missing. Three patients did not complete the baseline measurement and gave informed consent at the 4‐month follow‐up measurement. Another 23 patients completed only the baseline measurement. We found no baseline variables that were significantly related to incompleteness of measurements." Comment: dropouts reported but not explained. Imputation was done for missing value according to the scale recommendation. Analyses were performed on an intention‐to‐treat basis |
| Selective reporting (reporting bias) | Low risk | Comment: all the outcomes for this review were reported as pre‐specified in the trials register record |
| Other bias | High risk |
Comment: this trial used a hybrid effectiveness‐implementation in its study design, experienced low recruitment of eligible participants (only 16 of the 117 participants in the intervention arm) and low exposure (only 11 study participants) to the complete intervention of self‐management support Assessment of risk of bias in cluster‐randomised trials
|
Weinger 2011.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1:1 | |
| Participants |
Inclusion criteria: adults aged 18‐70 years diagnosed as having type 1 or type 2 diabetes for at least 2 years who were taking insulin and/or oral medication for at least 1 year, were able to walk briskly, were free of severe complications, and whose HbA1c level was higher than 7.5% were eligible for enrolment Exclusion criteria: inability to read and speak English, current or planned pregnancy, severe psychopathologic condition, unstable depression, albumin to creatinine ratio higher than 300 µg/mg, untreated proliferative retinopathy, unstable heart disease, severe hypertension (within 1 year), participation in diabetes education within the previous 6 months, severe neuropathy, or any physical issue such as arthritis that prevented brisk walking Diagnostic criteria: HbA1c level was measured using the high‐performance liquid chromatography ion capture method (Tosoh Medics Inc, San Francisco, California) (reference range, 4.0% to 6.0%); diabetes‐related distress with PAID; diabetes‐specific self‐efficacy with the Confidence in Diabetes Self‐Care Scale; and diabetes quality of life with the Diabetes Quality of Life Questionnaire |
|
| Interventions |
Number of study centres: 1 Treatment before study: — Titration period: no Intervention: structured cognitive behavioural strategies. Highly structured behaviour based activities and information including group review of glucose logs to identify patterns and dietary, exercise, and medication factors that influence those patterns; educator‐facilitated self‐care goal setting to help participants achieve and evaluate progress toward self‐care goals; and instruction, modelling, and practice of problem‐solving skills to help participants identify and overcome barriers to implementing self‐care behaviours. Each session opened with a review of the prior week's homework including glucose logs, food choices, and physical activity Control 1. Group attention control: group education programme. Programme was designed with the same length of time and amount of contact with health professionals and of homework. The curriculum consisted of prepared slides, a detailed curriculum manual, and specific learning activities including homework and the importance of goal setting but not training in cognitive behaviour strategies or structured goal‐setting activities. Educators had access to all clinic teaching materials and assessment guides Control 2. Individual control: unlimited individual nurse and dietitian education sessions. Unlimited 1‐on‐1 appointments with diabetes nurse and dietitian educators. Participants were not required to attend any education appointments. The content was determined by the educator based on her assessment and not by study protocol. Participants were sent 2 reminders about the availability of these education services, and research assistants were available to help them schedule appointments. Educators had access to all clinic teaching materials and assessment guides |
|
| Outcomes | Outcomes reported in abstract of publication: outcomes were baseline and 3‐, 6‐, and 12‐month postintervention HbA1c levels (primary) and frequency of diabetes self‐care, 3‐day pedometer readings, 24‐hour diet recalls, average number of glucose checks, physical fitness, depression, coping style, self‐efficacy, and quality of life (secondary) | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NCT00142922 |
|
| Publication details |
Language of publication: English Commercial and non‐commercial funding: the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01 DK60115 (K.W.), the Diabetes and Endocrinology Research Core grant NIH P30 DK36836, and the Joslin Diabetes Center Clinical Research Center. Abbott Laboratories, Abbott Park, Illinois; LifeScan, Milpitas, California; and Roche Diagnostics, Indianapolis, Indiana, contributed glucose meters and test strips Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "The goal of this randomised controlled trial was to test the efficacy of a highly structured behavioral diabetes education program in helping patients with long duration, poorly controlled diabetes improve glycaemic control through comparisons with curriculum‐based standard group education and 1‐on‐1 education with nurse and dietitian educators. The secondary objective was to assess which factors (e.g. coping processes, affective issues, type of diabetes, adherence to recommendations) were associated with an improvement in glycaemic control." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: "Randomization consisted of a 2‐step process to ensure approximately equal groups ... using a computer‐generated block assignment scheme (performed by the principal investigator, K.W.) that ..." Comment: probably done |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "... research assistants unveiled during the randomisation visit." Comment: probably done |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "Participants reported no episodes of hypoglycaemia that required assistance of others" Comment: self‐reported outcome measurement |
| Blinding of participants and personnel (performance bias) Blood pressure | Low risk |
Quote from publication: "In addition to sociodemographic factors ... and health factors (... blood pressure), we also measured ..." Comment: investigator‐assessed outcome measurement. Trial author communicated that the nurses who measured blood pressure were blinded to the study assignment |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | Unclear risk |
Quote from publication: no relevant quote Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "... using the high‐performance liquid chromatography ..." Comment: laboratory outcome measurement |
| Blinding of participants and personnel (performance bias) Health‐related quality of life | Unclear risk |
Quote from publication: no relevant quote Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of participants and personnel (performance bias) Self‐efficacy | Unclear risk |
Quote from publication: no relevant quote Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) Adverse events | Low risk |
Quote from publication: "Participants reported no episodes of hypoglycaemia that required assistance of others" Comment: self‐reported outcome measurement |
| Blinding of outcome assessment (detection bias) Blood pressure | Low risk |
Quote from publication: "In addition to sociodemographic factors ... and health factors (... blood pressure), we also measured ..." Comment: investigator‐assessed outcome measurement. Trial author communicated that the nurses who measured blood pressure were blinded to the study assignment |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | Unclear risk | Comment: no relevant quote. Self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) Health‐related quality of life | Unclear risk | Comment: no relevant quote. Self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Blinding of outcome assessment (detection bias) Self‐efficacy | Unclear risk | Comment: no relevant quote. Self‐reported outcome measurement but modes of administration unclear, probably self‐administered and similarly done in intervention groups |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Comment: no direct quote. Not reported |
| Incomplete outcome data (attrition bias) Blood pressure | Unclear risk | Comment: no relevant quote. Not reported |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk | Comment: no direct quote; dropouts reported but not explained. Missing values were imputed in sensitivity analysis |
| Incomplete outcome data (attrition bias) HbA1c | Low risk | Comment: no direct quote, reported in the study flow diagram. Reported and reasons explained. Missing values were imputed in sensitivity analysis |
| Incomplete outcome data (attrition bias) Health‐realted quality of life | Low risk | Comment: no direct quote. Reported and reasons explained. Missing values were imputed in sensitivity analysis |
| Incomplete outcome data (attrition bias) Self‐efficacy | Low risk | Comment: no direct quote. Reported and reasons explained. Missing values were imputed in sensitivity analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all prespecified outcome measures were reported, including self‐efficacy that was not specifically stated in the trials register record |
| Other bias | Low risk | Comment: all results were reported for the randomised groups |
Welch 2015.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: age 18 years or older, self‐identified Latino ethnicity, diagnosis of T2DM, HbA1c > 7.5% (58 mmol/mol), and provider approval given for participant participation. Exclusion criteria: inability to consent, pregnant or planning to become pregnant in the next year, taking glucocorticoid therapy, or having serious psychiatric or medical complications (e.g. late‐stage diabetes complications, seizures, dementia or psychiatric hospitalisation) Diagnostic criteria: HbA1c was obtained using a validated finger stick blood test kit (Appraise Home HbA1c Kit; Heritage Labs International LLC). Heritage Labs is certified by the National Glycohemoglobin Standardization Program. The Appraise Home HbA1c Kit produces accurate and reliable test results equivalent to whole blood tests collected in physicians' offices. Other clinical variables assessed the percentage of participants at target BP (< 130/80 mmHg) and BMI. Systolic and diastolic BP measurements were obtained by research staff during baseline and follow‐up research visits based on a single seated assessment using an automatic digital BP monitor (Omron model HEM‐705CP). Hypoglycemia was defined in the Diabetes SelfCare Profile as any "low blood sugars or sweating, nausea, heart pounding, trembling, cold and clammy skin, difficulty concentrating, and irritability" over the past month. Assessment of diabetes distress involved the short (5‐item) version of the PAID questionnaire; social distress on a 0‐100 scale using the 20‐item Tool for Assessing Patients' Stress (TAPS) questionnaire; depression using the Patient Health Questionnaire |
|
| Interventions |
Number of study centres: 2 Treatment before study: no Titration period: no Intervention: diabetes dashboard intervention condition (IC). The IC involved a programme of 5, in‐person, one‐on‐one diabetes education visits with a diabetes nurse or diabetes dietitian, scheduled at baseline, 2 weeks, 1 month, 3 months, and 6 months post‐enrolment. The initial visit was an hour long, and the remaining visits were a half hour long each. The diabetes nurse and diabetes dietitian interventionists used an Internet‐based "diabetes dashboard" disease management tool to structure each education visit and to share information collected during each visit with each other and with clinic providers. This dashboard combines existing clinical data obtained from paper chart‐based and electronic health records (i.e. vital signs, laboratories, medications, admissions, procedures, and diagnoses) with additional participant data gathered using integrated surveys (described below) and during the course of ongoing care. The diabetes dashboard provides the following:
For the current study, each education visit with the diabetes nurse or diabetes dietitian interventionists began with a review based on a summary of participant‐reported self‐management behaviours and barriers (i.e. blood glucose testing, diet, physical activity, and medication adherence) and psychosocial challenges (i.e. diabetes distress, social distress, depression, hypoglycaemia, binge eating, alcohol abuse, and low social support) collected using an established survey integrated within the dashboard (i.e. the Diabetes Self‐Care Profile). Next, the interventionist reviewed the participant's vital signs and laboratory data, conducted a medication review and reconciliation process and updated the medication list, reviewed clinical alerts and reminders generated by the system, and updated the nursing or dietetic treatment plan using encounter forms. Following these steps, the interventionist delivered diabetes education tailored to the participant's individual clinical, behavioural, and psychosocial profile and referred the participant for psychosocial services (e.g. adjacent mental health clinic for depression) as needed and with notification to the primary care provider. Interventionists recorded clinical notes for each visit by free text using a "whiteboard" panel on the dashboard to facilitate internal team communication and participant handoff between sessions. The diabetes nurse and diabetes dietitian interventionists created clinical care recommendations for providers on pharmacological management of abnormal blood glucose, blood pressure, and lipid levels after several initial diabetes education evaluation and education sessions to develop rapport, assess current medication adherence, and provide individualised diabetes education and support Control: usual diabetes care (UDC). The UDC condition involved a series of individual participant visits with education content. Visit frequency was based on individual participant needs as determined by programme clinicians. Participants also had access to lifestyle and diabetes self‐management support groups run at the clinics by peer volunteers and clinical staff. Participants in the UDC condition completed the same assessment battery (i.e. Diabetes Self‐Care Profile) as that completed by participants in the IC. However, data from this assessment was used only for research purposes and was not used to guide clinical care delivered within the UDC condition |
|
| Outcomes | Outcomes reported in abstract of publication: HbA1c, diabetes distress and social distress | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: NCT02156037 |
|
| Publication details |
Language of publication: English Non‐commercial funding: National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "To compare usual diabetes care (UDC) to a comprehensive diabetes care intervention condition (IC) involving an Internet‐based "diabetes dashboard" management tool used by clinicians." | |
| Notes | Multiple imputation methods was used to address missing data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "Patients were randomised either to ..." Comment: insufficient description |
| Allocation concealment (selection bias) | High risk |
Quote from publication: "We used a parallel‐group randomised design ... inclusion criteria were as follows: ... provider approval given for patient participation." Comment: insufficient description, probably not done |
| Blinding of participants and personnel (performance bias) Adverse events | Low risk |
Quote from publication: "Hypoglycemia was defined in the Diabetes SelfCare Profile as any 'low blood sugars or sweating, nausea, heart pounding, trembling, cold and clammy skin, difficulty concentrating, and irritability' over the past month." Comment: self‐reported outcome measurement |
| Blinding of participants and personnel (performance bias) Blood pressure | Low risk |
Quote from publication: "... were obtained by research staff during baseline and follow‐up research visits based on a single seated assessment using an automatic digital BP monitor (Omron model HEM‐705CP). " Comment: investigator‐assessed outcome measurement. Unclear of blinding but was using an automatic digital BP monitor |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "Patients attended a 1‐h baseline research assessment and a 30‐min follow up assessment at 6 months." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "... obtained using a validated finger stick blood test kit (Appraise Home HbA1c Kit; Heritage Labs International LLC)." Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Adverse events | Low risk |
Quote from publication: "Hypoglycemia was defined in the Diabetes SelfCare Profile as any 'low blood sugars or sweating, nausea, heart pounding, trembling, cold and clammy skin, difficulty concentrating, and irritability' over the
past month." Comment: self‐reported outcome measurement |
| Blinding of outcome assessment (detection bias) Blood pressure | Low risk |
Quote from publication: "... were obtained by research staff during baseline and follow‐up research visits based on a single seated assessment using an automatic digital BP monitor (Omron model HEM‐705CP). " Comment: investigator‐assessed outcome measurement. Unclear of blinding but was using an automatic digital BP monitor |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "Patients attended a 1‐h baseline research assessment and a 30‐min follow up assessment at 6 months." Comment: self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "... obtained using a validated finger stick blood test kit (Appraise Home HbA1c Kit; Heritage Labs International LLC)." Comment: laboratory outcome measurement |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk |
Quote from publication: "Follow‐up research visits were completed by 86.4% of IC patients and 90.5% of UDC patients ... There were also no differences between the two conditions in new reports of hypoglycaemia at follow‐up (22 vs. 20.6%)" Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) Blood pressure | Low risk |
Quote from publication: "Follow‐up research visits were completed by 86.4% of IC patients and 90.5% of UDC patients ... Results were similar when multiple imputation methods were used to fill in missing data" Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "Follow‐up research visits were completed by 86.4% of IC patients and 90.5% of UDC patients" Comment: dropouts reported but not explained |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "Follow‐up research visits were completed by 86.4% of IC patients and 90.5% of UDC patients .. Results for mean HbA1c at follow‐up were similar in our sensitivity analysis based on imputed data" Comment: dropouts reported but not explained |
| Selective reporting (reporting bias) | Low risk | Comment: all pre‐specified outcome measures were reported, and more |
Whittemore 2004.
| Methods | Parallel randomised controlled trial; randomisation ratio 1:1 | |
| Participants |
Inclusion criteria: female, diagnosed with type 2 diabetes, between the ages of 30 and 70 years, cleared for exercise by a primary care provider, had no advanced complications of diabetes (e.g. amputation or renal failure), had an A1c level greater than 7%, were fluent in English, and had previously participated in diabetes education Exclusion criteria: — Diagnostic criteria: the A1c analysis was performed using a fingerstick blood sample and was analysed by the DCA 2000 Analyzer (normal range = 4.2% to 6.3%); dietary behaviour was measured by the Dietary Subscale of the Summary of Diabetes Self‐Care Activities Questionnaire; exercise behaviour was measured by a modified Paffenbarger Physical Activity Questionnaire; diabetes‐related distress was measured by the 20‐item PAID; how well diabetes is integrated into daily life was measured by The Diabetes Questionnaire (TDQ); satisfaction with care was measured by the Diabetes Treatment Satisfaction Questionnaire Change (DTSQc) |
|
| Interventions |
Number of study centres: 1 Treatment before study: — Titration period: no Intervention: nurse coaching. The nurse‐coaching sessions included educational, behavioural, and affective strategies. The nurse‐coaching protocol includes assessment of trajectory of diabetes diagnosis, treatment, and impact on life, patterns of daily living and important roles and values and the individual's diabetes self‐management programme. Education reinforcement, cognitive component clarify misconceptions, increase the personal relevance of diabetes knowledge, present diabetes information in greater depth and the ideal treatment recommendations and negotiate realistic goals. Problem solving and motivational guidance, the behavioural component identify personal barriers and facilitators to lifestyle change and brainstorm creative, concrete, and realistic strategies. The psychosocial support, affective component identify psychosocial issues related to living with diabetes, provide empathetic listening and an accepting environment, assist in identifying appropriate social support and mental health strategies, refer for psychological treatment as indicated and provide positive encouragement, praise, and support for efforts and relapses. 5 of the 6 sessions were provided in the first 3 months Control: standard care. Defined as regular appointments with a primary care provider at approximately 3‐ to 4‐month intervals. Providers included nurse practitioners, internists, family practice specialists, and endocrinologists. All women who were randomised to the control condition were invited to participate in the nurse‐coaching intervention at the end of the study |
|
| Outcomes | Outcomes reported in abstract of publication: diet self‐management, diabetes‐related distress, integration and satisfaction with care, exercise self‐management and BMI; A1c levels | |
| Study details |
Run‐in period: no Trial terminated early: no Trials register identifier: — |
|
| Publication details |
Language of publication: English Commercial and non‐commercial funding: National Institute of Nursing Research and the American Association of Diabetes Educators Roche Diagnostics Award Publication status: peer‐reviewed journal and full article |
|
| Stated aim for study | Quote from publication: "The purpose of this pilot study was to determine the efficacy of a 6‐month nurse‐coaching intervention that was provided after diabetes education for women with type 2 diabetes." | |
| Notes | No mention of missing data handling, probably no imputation of missing values | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: "... were randomised to ..." Comment: study author communicated that "Since this was a small study, we had sealed opaque envelopes with the randomisation assignment. Participants selected an envelope after completion of baseline data collection". Unclear of the generation of the random sequence |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: "... were randomised to ..." Comment: study author communicated that "[s]ince this was a small study, we had sealed opaque envelopes with the randomisation assignment. Participants selected an envelope after completion of baseline data collection." Probably done |
| Blinding of participants and personnel (performance bias) Diabetes‐related distress | High risk |
Quote from publication: "Data were collected on ... psychosocial (diabetes‐related distress and integration), and treatment satisfaction variables at baseline, 3 months, and 6 months." Comment: study author communicated that the nurse‐coach did not collect data. Self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of participants and personnel (performance bias) HbA1c | Low risk |
Quote from publication: "The A1c analysis was performed using a fingerstick blood sample and was analysed by the DCA 2000 Analyzer ..." Comment: laboratory outcome measurement |
| Blinding of outcome assessment (detection bias) Diabetes‐related distress | High risk |
Quote from publication: "Data were collected on ... psychosocial (diabetes‐related distress and integration), and treatment satisfaction variables at baseline, 3 months, and 6 months." Comment: study author communicated that the nurse‐coach did not collect data. Self‐reported outcome measurement but modes of administration unclear, probably self‐administered |
| Blinding of outcome assessment (detection bias) HbA1c | Low risk |
Quote from publication: "The A1C analysis was performed using a fingerstick blood sample and was analysed by the DCA 2000 Analyzer ..." Comment: laboratory outcome measurement |
| Incomplete outcome data (attrition bias) Diabetes‐related distress | Low risk |
Quote from publication: "The attrition rate was 8% (3 in the treatment group and 1 in the control group) ... Two women developed unrelated medical concerns and no longer had the time for the study, 1 woman became pregnant, and 1 woman developed a lack of interest in the study. There were no differences between the treatment (n=26) and control groups (n=23) on the variables of age, duration of diabetes, race, education, or income." Comment: reported and reasons explained |
| Incomplete outcome data (attrition bias) HbA1c | Low risk |
Quote from publication: "The attrition rate was 8% (3 in the treatment group and 1 in the control group) ... Two women developed unrelated medical concerns and no longer had the time for the study, 1 woman became pregnant, and 1 woman developed a lack of interest in the study. There were no differences between the treatment (n=26) and control groups (n=23) on the variables of age, duration of diabetes, race, education, or income." Comment: reported and reasons explained |
| Selective reporting (reporting bias) | Unclear risk | Comment: outcome measures were reported as specified in the publication, no prior trials register record or study design paper was available |
—: not reported
Note: where the judgement is 'Unclear' and the description is blank, the trial did not report that particular outcome.
ADDQoL: Audit of Diabetes Dependent Quality of Life; BMI: body mass index; BP: blood pressure; CBT: cognitive behavioural therapy; CES‐D: Center for Epidemiologic Studies Depression; DDS: Diabetes Distress Scale; DRD: diabetes‐related distress; HADS: hospital anxiety and depression scale; HbA1c: glycosylated haemoglobin A1c; HPLC: high‐performance liquid chromatography;LDL: low‐density lipoprotein; PAID: Problem Areas in Diabetes; PHQ: Patient Health Questionnaire; QoL: quality of life; SD: standard deviation; SE: self‐efficacy; T2DM: type 2 diabetes mellitus; WHO: World Health Organization; NIH: National Institutes of Health (USA); WHOQOL: WHO Quality of Life.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Carper 2014 | Not a randomised controlled trial |
| Chiu 2016 | Less than 6 months follow‐up for diabetes‐related distress. No specific adverse events are reported |
| Fisher 2014 | Not a randomised controlled trial |
| Fonda 2009 | Data of participants with both type 1 and 2 diabetes mellitus are included and no response was received on the request for separate data |
| Friis 2016 | Less than 6 months follow‐up. No specific adverse events are reported within the 3‐month post intervention |
| Gabbay 2006 | Data of participants with both type 1 and 2 diabetes mellitus are included; no response was received on the request for separate data |
| Heisler 2010 | Similar psychological interventions with only a difference in the methods of execution. The type of diabetes was not specified |
| Heisler 2014 | Similar psychological interventions with only a difference in the methods of execution. The type of diabetes was not specified |
| Imazu 2015 | Not a randomised controlled trial. No specific adverse events are reported |
| Izquierdo 2003 | Similar psychological interventions with only a difference in the methods of execution; participants with both type 1 and 2 diabetes mellitus are included; no response was received on the request for separate data |
| Jung 2015 | Less than 6 months follow‐up. No specific adverse events are reported |
| Lee 2014 | Hospitalisation for cardiac surgery |
| MacPhail 2014 | Less than 6 months follow‐up. No specific adverse events are reported |
| Mantwill 2015 | Less than 6 months follow‐up. No specific adverse events are reported |
| McMahon 2012 | Similar psychological interventions: all were cognition‐focused; participants with both type 1 and 2 diabetes mellitus are included; no response was received on the request for separate data |
| Munshi 2013 | Participants with both type 1 and 2 diabetes mellitus are included; no response was received on the request for separate data |
| Nobis 2015 | Less than 6 months follow‐up. No specific adverse events are reported |
| Safford 2015 | Similar psychological interventions: both were emotion‐cognition: peer coaches plus brief education compared with brief education alone |
| Samuel‐Hodge 2008 | Not a randomised controlled trial |
| Schoevers 2013 | Participants with both type 1 and 2 diabetes mellitus are included; no response was received on the request for separate data. Less than 6 months follow‐up; no reporting on adverse events. |
| Siminerio 2013 | Similar psychological interventions: both were cognition‐focused; participants with both type 1 and 2 diabetes mellitus are included; no response was received on the request for separate data. |
| Simson 2008 | Participants with both type 1 and 2 diabetes mellitus are included; no response was received on the request for separate data. Less than 1 month follow‐up |
| Sinclair 2013 | Less than 6 months follow‐up. No specific adverse events are reported |
| Skinner 2010 | Not a randomised controlled trial, descriptive statistics on prevalence and persistence of depressive symptoms |
| Surwit 2002 | Did not use the specified diabetes‐related distress scales |
| Tang 2014 | Similar psychological interventions: both were cognition‐focused |
| Tang 2015 | Similar emotion‐cognition interventions: 3‐month diabetes self‐management education programme versus ongoing diabetes self‐management support. The latter has extended peer‐support |
| Tovote 2014 | Participants with both type 1 and 2 diabetes mellitus are included; no response was received on the request for separate data. Less than 6 months follow‐up |
| Trief 2011 | Diabetes‐related distress was not reported as measured with the 2 instruments specified in inclusion criteria for this review |
| Van Bastelaar 2011 | Participants with both type 1 and 2 diabetes mellitus are included; no response was received on the request for separate data |
| Van Bastelaar 2012 | Not a randomised controlled trial |
| Van Son 2013 | Participants with both type 1 and 2 diabetes mellitus are included; no response was received on the request for separate data |
| Van Son 2014 | Participants with both type 1 and 2 diabetes mellitus are included; no response was received on the request for separate data |
| Welch 2011a | Similar psychological interventions: both were cognition‐focused |
| Welch 2011b | Similar psychological interventions: both were cognition‐focused |
| Whittemore 2005 | Not a randomised controlled trial |
| Zagarins 2012 | Similar psychological interventions: both were cognition‐focused |
Characteristics of studies awaiting assessment [ordered by study ID]
Dafoulas 2014.
| Methods | Trial design: parallel randomised control trial |
| Participants |
Inclusion criteria:
Exclusion criteria: pregnancy |
| Interventions | Number of centres: unknown In the tele monitoring (I) group participants' blood glucose profiles were collected weekly using a mobile phone health platform, for a period of 1 year. Allocated health professionals provided the appropriate counselling on lifestyle and medication changes by phone when required. Participants in control (C) group received usual care with face‐to‐face consultations. Country: Greece Setting: community and at home |
| Outcomes | Health‐related quality of life was assessed using a generic (SF36v2) questionnaire and a disease‐specific questionnaire, the Problem Areas in Diabetes (PAID) scale |
| Study details | Trials register identifier:NCT01498367 |
| Publication details | Language: English Funding: Regional Health Authority of Sterea & Thessaly Publication status: conference paper (peer reviewed journal) |
| Stated aim of study | To study the impact of a long‐term telemonitoring program for patients with type 2 diabetes mellitus on glycaemic control and health‐related quality of life compared to usual care |
| Notes | Currently classified as completed, but no study results posted nor full publication on the effects of the interventions on the outcomes identified (as of 17 October 2016) |
De Vries 2014.
| Methods | Trial design: parallel randomised control trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Number of centres: 130 general practices Intervention(s): usual care plus participation in a group‐based peer support programme consisting of 6 sessions Comparator(s): usual care plus attendance of 1 educational meeting on T2DM Country: northwestern, middle and southern parts of the Netherlands Setting: community |
| Outcomes |
Primary outcome(s):
Secondary outcome(s):
|
| Study details | Trials register identifier:NTR3474 |
| Publication details | Language: English Funding: Dutch Diabetes Research Foundation Publication status: conference abstract and oral presentation (peer reviewed journal), and published study protocol (De Vries 2014) |
| Stated aim of study | The aim of the study is to determine the effectiveness of a group‐based, peer support programme on diabetes‐related distress |
| Notes | Planned closing date is 1 September 2013, but no study results posted nor full publication identified (as of 18 October 2016). Discrepancies noted in the stated primary and secondary outcome between the trials register record and published study protocol. Trials register states that health‐related quality of life (both generic and diabetes‐specific (diabetes distress and well‐being)) and self‐management behaviour are primary outcomes, while self‐efficacy, self‐esteem and social support are secondary outcomes. Contact for public queries: MSc Lianne de Vries; contact for scientific queries: Dr Giel Nijpels |
Ebert 2017.
| Methods | Trial design: parallel randomised control trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Number of centres: unknown
Intervention(s): Internet guided self‐help intervention for depression Comparator(s): an online psychoeducation on depression Country: Germany Setting: community and at home |
| Outcomes | The primary outcome was the depressive symptom severity. Secondary outcomes are HbA1c, physical and mental functioning (Short Form Health Survey, SF‐12) and emotional distress related to living with diabetes (PAID‐5) |
| Study details | Trials register identifier: DRKS00004748 |
| Publication details | Language: English Funding: Regional Health Authority of Sterea & Thessaly Publication status: conference paper (peer reviewed journal) |
| Stated aim of study | The aim of this study is to test the 6‐month effectiveness of the GET.ON Mood Enhancer Diabetes intervention for comorbid depression and diabetes and examine the effects of these interventions on diabetes‐specific outcomes |
| Notes | Promised to provide separate data for participants with T2DM Trial website: http://www.geton‐training.de/Diabetes.php |
NCT01578096.
| Methods | Trial design: parallel randomised control trial |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Number of centres: unknown Intervention(s): diabetes education plus stress management Group‐based diabetes education plus stress management delivered to participants through community health workers Comparator(s): diabetes education Group‐based diabetes education delivered to participants through community health workers Country: New Haven, Connecticut, USA Setting: community |
| Outcomes |
Primary outcome(s):
Secondary outcome(s):
|
| Study details | Trials register identifier: NCT01578096 |
| Publication details | Language: English Funding: Yale University Publication status: conference paper (peer reviewed journal) |
| Stated aim of study | Quote: "The primary aims of this study are to: tailor a diabetes stress management intervention for delivery by community health workers (CHWs) serving an urban Latino population [and] investigate the efficacy of the stress management intervention on glycaemic control. Secondary aims of this study are to: investigate the efficacy of the stress management intervention on stress hormones, psychosocial functioning, and stress‐glucose reactivity. Study hypothesis: A CHW‐led group‐based diabetes education model enhanced with stress management education will improve glycaemic control more than CHW‐led group‐based diabetes education alone." |
| Notes | Currently classified as completed, but no study results posted nor full publication identified (as of 17 October 2016). However, a publication was noted based on the baseline data (Bermúdez‐Millán 2016). Contact: Rafael Pérez‐Escamilla, rafael.perez‐escamilla@yale.edu; Julie A Wagner, juwagner@uchc.edu |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12612000620820.
| Trial name or title | Evaluation of an online support program for type 2 diabetes self‐management and dysphoria (depression, anxiety, and diabetes‐specific distress) |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: blinded (masking used) Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes, dysphoria Enrollment: 300 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): An automated, web‐based programme aimed to improve T2DM self‐management and dysphoria (depression, anxiety, and diabetes‐specific distress) by primarily targeting physical activity, nutrition, health routines, and emotional well‐being. Being a self‐guided programme, participants use it at their own discretion. The intervention group are sent an email reminder if they have not logged on in ≥ 2 weeks. The programme has no set duration, as participants are free to access it indefinitely, although the main trial period is 12 months (or, for participants who choose to be followed up in the future, 5 years). Comparator(s): usual care and wait‐list control. Usual care receives access to limited components of the full programme throughout the trial (information resources, quizzes, and the health routines programme module). The wait‐list control arm receives access only to information resources and brief quizzes for the initial 3 months of participation, and then receives full programme access. |
| Outcomes |
Timepoint(s): baseline; 3, 6, and 12 months; and 5 years Primary outcome(s):
Secondary outcome(s):
Other outcome(s): — |
| Starting date |
Trial start date: 1 May 2012 Trial completion date: — |
| Contact information | Responsible party/principal investigator: Wesley Research Institute, Level 8, East Wing, The Wesley Hospital, 451 Coronation Drive, Auchenflower Brisbane/Mandy Cassimatis |
| Study identifier | Trials register identifier: ACTRN12612000620820 |
| Official title | Randomised controlled trial of OnTrack Diabetes: an online support program to improve type 2 diabetes self‐management and dysphoria |
| Stated purpose of study | Quote: "This study evaluates the efficacy of a novel, online support program that targets type 2 diabetes self‐management and dysphoria symptoms in aiming to improve glycaemic control and emotional well‐being. Secondary aims of the program are to improve behavioural outcomes (physical activity, dietary intake, and medication adherence), self‐efficacy for diabetes self‐care, and quality of life. Program evaluations include cost‐effectiveness and qualitative outcomes, for example implementation feasibility, user satisfaction, program usability and acceptability." |
| Notes | Retrospectively registered. Trial website: www.ontrack.org.au/diabetes |
ACTRN12614001232628.
| Trial name or title | Diabetes text message self management support Acronym: SMS4BG |
| Methods |
Type of study: interventional, efficacy Allocation: randomised Intervention model: parallel assignment Masking:open (masking not used) Primary purpose: treatment |
| Participants |
Condition: diabetes Enrollment: 1000 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): the intervention is an m‐health diabetes self‐management support program. Participants will receive an individually tailored package of text messages via their mobile phone to increase diabetes self‐management. The dose and duration of messaging is tailored to the patients' preferences ranging from 3 months to 9 months and from 2 messages per week to multiple messages per day. The messages are tailored based on participant demographics (e.g. ethnicity and age), preferences (e.g. timing of messages, module choice, frequency of reminders) personal characteristics (e.g. motivations) and clinical characteristics (e.g. foot risk category, treatment). Tailoring information is obtained from those participants randomised to the intervention group during the baseline phone interview with a research assistant (approximately 20‐30 min). Comparator(s): usual care that includes the standard diabetes care provided in primary care settings including (e.g. GP and nurse visits, HbA1c tests) and where needed the care provided by secondary care services. In addition usual care includes where appropriate access to current diabetes resources and services. |
| Outcomes |
Timepoint(s): baseline and 9 months Primary outcome(s):
Secondary outcome(s):
Other outcome(s): — |
| Starting date |
Trial start date: 16 June 2015 Trial completion date: — |
| Contact information | Responsible party/principal investigator: National Institute for Health Innovation, School of Population Health, The University of Auckland/Dr Robyn Whittaker |
| Study identifier | Trials register identifier: ACTRN12614001232628 |
| Official title | A randomised controlled trial to determine the efficacy of a text message based diabetes self management support program to improve glycaemic control, compared with usual care, in New Zealand adults with poorly controlled diabetes |
| Stated purpose of study | Quote: "This study will look at the benefits of a text message‐based program (SMS4BG) developed by the National Institute for Health Innovation and Waitemata DHB for people with poorly‐controlled diabetes." |
| Notes | Likely ongoing |
ACTRN12615000931572.
| Trial name or title | The springboarD trial: trial of a self‐help intervention to improve functioning and emotional well‐being for depression and diabetes‐related distress in people with type 2 diabetes |
| Methods |
Type of study: Interventional, efficacy endpoints Allocation: randomised Intervention model: parallel assignment Masking: open (masking not used) Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes, depression Enrollment: 600 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): the active intervention ('myCompass') is a fully automated, self‐help, public health intervention that is tailored to the user and has no therapist input. Real‐time self‐monitoring of symptoms (e.g. problem moods, thoughts and behaviours) via mobile phone and/or computer/tablet is a key therapeutic feature. Users can self‐monitor 3 symptoms of their choice at any one time, selected from a list of 20, or 3 that are recommended to them by the program (e.g. confidence, worry, irritability, motivation, diet, and medication use). Each symptom is rated on a 10‐point scale (e.g. "how confident do you feel right now?", "how worried do you feel right now?", "how satisfied are you that you have taken your prescribed medication today?"). At the time of rating, users also provide contextual information about where they are, what they are doing and who they are with, using a series of drop‐down menus. To improve adherence to the intervention users can schedule short message service (SMS) or email reminders to facilitate self‐monitoring (frequency of reminders determined by the user); receive and print graphical feedback about their monitoring, including contextual information, on their phone or computer (to monitor change and assist identification of triggers); and elect to receive helpful facts, mental healthcare tips or motivational statements by SMS or email. Comparator(s): the placebo control intervention ("Healthy Lifestyles") is an online and interactive health information program which provides information about a range of health topics including environmental and community health, stress and well being, sustainable living, healthy skin and eye health, safe road usage, and travelling. The program has no therapeutic content, and has been successfully used as a placebo in previous studies by members of the research team. Participants in the placebo control group will similarly have access to the intervention for 8 weeks with a tailing off of 4 weeks. |
| Outcomes |
Timepoint(s): baseline and at 3, 6, 12 and 24 months after commencement of intervention Primary outcome(s):
Secondary outcome(s):
Other outcome(s): — |
| Starting date |
Trial start date: 16 October 2015 Trial completion date: 30 December 2016 |
| Contact information | Responsible party/principal investigator: Black Dog Institute, School of Psychiatry, UNSW Australia/A/Prof Judy Proudfoot |
| Study identifier | Trials register identifier: ACTRN12615000931572 |
| Official title | The springboarD trial: Trial of a self‐help intervention to improve functioning and emotional well‐being for depression and diabetes‐related distress in people with type 2 diabetes |
| Stated purpose of study | Quote: "This project will test the hypothesis that functioning and mental well being will be improved in people with type 2 diabetes and comorbid depression following the use of a fully‐automated mobile phone and web‐based mental health intervention ('myCompass') for 12 weeks, compared with those who receive a placebo intervention." |
| Notes | Trial website: springboard.blackdoghealth.org.au |
ACTRN12616001010482.
| Trial name or title | Pilot randomised control trial of a problem‐solving intervention tailored to quality of life difficulties experienced by patients with diabetic retinopathy Acronym: DMP_INT |
| Methods |
Type of study: interventional Allocation: randomised Intervention model: parallel assignment Masking: single blind (outcomes assessor) of the people assessing the outcomes Primary purpose: treatment |
| Participants |
Condition: diabetic retinopathy, diabetes Enrollment: 40 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): participants randomised to the intervention arm will receive 6 (minimum) or 8 (maximum) complete weekly problem‐solving training (PST) sessions provided by trained eye care staff. The first PST session will be combined with the introductory session which will be delivered as an individual one‐on‐one session (face‐to‐face). The remaining PST sessions will be conducted over the telephone and the participant can decide whether they feel they need the 7th and 8th session, which are optional. Between sessions, participants will be expected to attempt to put problem‐solving techniques into practice and develop goals necessary to fulfil solutions to problems. Progress review will be conducted at the beginning of each session. All telephone calls are recorded and the frequency and duration of each session monitored. Comparator(s): participants randomised to this arm will be followed at the Royal Victorian Eye and Ear Hospital pragmatically and has the same face‐to‐face follow‐ups as the intervention group. They have access to the internal diabetes educator as deemed appropriate by their treating ophthalmologist (= usual care). |
| Outcomes |
Timepoint(s): baseline and 3 and 6 months postintervention Primary outcome(s):
Secondary outcome(s):
Other outcome(s): — |
| Starting date |
Trial start date: 13 August 2012 Trial completion date: 27 February 2014 |
| Contact information | Responsible party/principal investigator: Centre for Eye Research Australia, Department of Ophthalmology ‐ the University of Melbourne/Prof Ecosse Lamoureux |
| Study identifier | Trials register identifier: ACTRN12616001010482 |
| Official title | Pilot randomised control trial of a problem‐solving intervention tailored to quality of life difficulties experienced by patients with diabetic retinopathy |
| Stated purpose of study | Quote: "Aim 1: To develop a tailored, problem solving based program that targets individual quality of life difficulties. Aim 2: To assess, using a randomised control trial, the effectiveness of this program in improving participants' quality of life and psychological well‐being (reducing diabetes related distress and depressive symptoms) Investigation will also be undertaken to assess whether enhancing problem solving skills have a direct influence on a participant's ability to self‐manage their diabetes including improving overall glycaemic control and adopting recommended lifestyle practices." |
| Notes | Retrospectively registered trial |
ISRCTN02123133.
| Trial name or title | A web‐based self management programme (HeLP‐Diabetes) for people with type 2 diabetes in primary care Acronym: HeLP‐Diabetes |
| Methods |
Type of study: interventional study Allocation: randomised Intervention model: parallel assignment Masking: single blind (outcomes assessor) Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes Enrollment: 398 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): HeLP‐Diabetes is a web‐based self‐management programme we have developed for adults with T2DM Comparator(s): information‐only website created by the study team to compare with HeLP‐Diabetes |
| Outcomes |
Timepoint(s): baseline, 3 months, 12 months Primary outcome(s):
Secondary outcome(s):
Other outcome(s): — |
| Starting date |
Trial start date: 1 March 2013 Trial completion date: 1 September 2015 |
| Contact information | Responsible party/principal investigator: Department of Primary Care and Population Sciences, Hampstead Campus, Rowland Hill Street, London/Dr Charlotte Dack, c.dack@ucl.ac.uk |
| Study identifier | Trials register identifier: ISRCTN02123133 |
| Official title | Randomised controlled trial of a web‐based self management programme (HeLP‐Diabetes) for people with type 2 diabetes in primary care |
| Stated purpose of study | Quote: "We have developed two websites (one complex; one simple) offering help and support for people with type 2 diabetes. The aims of the study are to see if either website improves people's well being and clinical outcomes and if they are cost‐effective compared to usual care." |
| Notes | Trial website: public.ukcrn.org.uk/Search/StudyDetail.aspx?StudyID=13563 |
NCT01612520.
| Trial name or title | Telecoaching of people with type 2 diabetes in primary care |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: open Label Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes Enrollment: 574 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): The COACH program trains patients to 'drive' the process of achieving and maintaining the target levels for their risk factors while working in association with their GP. The telephone coaching is aimed at improving self‐efficacy by adhering to the prescribed therapy and making relevant behavior changes. The coaching model is a continuous 5‐stage coaching cycle:
The coach monitors and registers: the biomedical risk factors, the lifestyle/behavioral risk factors and use of the recommended medications. Coaching is focused on eliminating the knowledge gap and motivating the patient to apply the appropriate lifestyle and medical therapy. Comparator(s): the control group receives usual care alone. All study participants, including the control group, receive a DVD with educational material on type 2 diabetes, its complications and lifestyle recommendations. The laboratory results of the blood analysis are mailed to all study participants and their GPs after each assessment. |
| Outcomes |
Timepoint(s): baseline, 6 months and 18 months Primary outcome(s):
Secondary outcome(s):
Other outcome(s): — |
| Starting date |
Trial start date: April 2012 Trial completion date: January 2015 |
| Contact information | Responsible party/principal investigator: Katholieke Universiteit Leuven/Irina Odnoletkova |
| Study identifier | NCT number: NCT01612520 |
| Official title | Telecoaching of people with type 2 diabetes in primary care |
| Stated purpose of study | Quote: "The objective of the study is to analyse the effectiveness and the cost‐effectiveness of telecoaching in improving glycaemic control and other modifiable risk factors in patients with T2DM compared to usual care only." |
| Notes | This study has been completed |
NCT01805245.
| Trial name or title | Mindfulness: a novel approach for the management of diabetes‐related distress |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: single blind (outcomes assessor) Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes, emotional distress, stress Enrollment: estimated 90 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): mindfulness‐based stress reduction. Standard 8‐week programme; classes meet for 2.5 hours once weekly. Comparator(s): the health education control group meets at the same time and for the same amount of time. |
| Outcomes |
Timepoint(s): baseline, 8 weeks, 24 weeks Primary outcome(s):
Secondary outcome(s):
Other outcome(s): Mindfulness, assessed with the Five Facet Mindfulness Questionnaire |
| Starting date |
Trial start date: January 2012 Trial completion date: December 2015. |
| Contact information | Responsible party/principal investigator: University of North Carolina, Chapel Hill/Laura A Young |
| Study identifier | NCT number: NCT01805245 |
| Official title | Mindfulness: a novel approach for the management of diabetes‐related distress |
| Stated purpose of study | Quote: "The purpose of this study is to evaluate the impact of stress reduction on physiological and psychological variables in adults with Type 2 diabetes (T2DM) who have moderate to severe levels of diabetes‐related emotional distress. Subjects will be randomised to one of two interventions. We will evaluate the impact of the interventions on glucose metabolism, blood pressure, diabetes‐related distress and quality of life. Additionally, we will investigate the role of neuroendocrine dysfunction, systemic inflammation and diabetes self‐care practices as mediators in the relationship between increased stress, adverse glucose metabolism and elevated blood pressure in those subjects with T2DM." |
| Notes | Contact: Michelle Duclos, michelle_duclos@med.unc.edu |
NCT02021591.
| Trial name or title | Effectiveness study of interactive web application for problem solving in diabetes management Acronym: MoDD |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: cross‐over assignment Masking: open label Primary purpose: supportive care |
| Participants |
Condition: diabetes mellitus Enrollment: 240 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): early intervention (EI) Experimental arm: mobile diabetes detective (MoDD). Study participants attending 1 of the 4 EI sites will receive usual diabetes education provided by staff at the site and be given access to the MODD application and instructions for use for 4 weeks at the beginning of the study. After the initial 4 weeks of access to the MODD application, participants will be offered an option to continue using MODD for the duration of the study. Comparator(s): late intervention (LI) Control Arm: study participants attending 1 of the 4 LI centres will receive usual diabetes education provided by staff at the site; be provided with free test strips for their blood glucose meters during the 4‐week intervention period; given access to the MODD application at the end of the study. Instructions on how to use the MODD will be provided by site staff. |
| Outcomes |
Timepoint(s): baseline, postintervention 4 weeks, 3 months, 12 months Primary outcome(s):
Secondary outcome(s):
|
| Starting date |
Trial start date: December 2013 Trial completion date: August 2016 |
| Contact information | Responsible party/principal investigator: Columbia University/Olena Mamykina |
| Study identifier | NCT number: NCT02021591 |
| Official title | Randomized clinical trial of health information technology for problem solving in diabetes management |
| Stated purpose of study | Quote: "The main hypothesis of this research is that use of an informatics intervention for problem‐solving in diabetes management, Mobile Diabetes Detective (MoDD), by individuals with type 2 diabetes will lead to positive improvements on a number of primary and secondary outcomes related to their health and their management of diabetes. The primary outcomes are a reduction in individuals' glycolated haemoglobin (HbA1c), improvement in their problem‐solving abilities, and self‐care behaviours. Secondary outcomes include a reduction in individuals' fasting blood glucose (BG); improvement in individuals' self‐efficacy, and in emotional aspect of living with diabetes. We hypothesize that primary and secondary outcome effects will be sustained at three months and twelve months. Exploratory outcomes include a decrease in individuals' Cardiovascular Risk (Body Mass Index, Blood Pressure, Total, low‐density lipoprotein (LDL) and high‐density lipoprotein (HDL) Cholesterol levels, and Framingham Cardiovascular Risk Score). We also hypothesize that improvements in clinical outcomes (HbA1c, fasting BG and Cardiovascular Risk) will be mediated by the improvements in problem‐solving abilities and self‐efficacy." |
| Notes | Contact: Andrea Cassells, acass@cdnetwork.org |
NCT02040038.
| Trial name or title | Diabetes self‐management & support LIVE |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes, emotional distress, stress Enrollment: 300 Inclusion criteria:
Exclusion criteria: — |
| Interventions |
Intervention(s): participation in 3D virtual environment for DSMT/S for a period of 12 months. The intervention group have access to the LIVE site where they can find information, synchronous classes with diabetes educators, and peer support to enhance self‐management. Comparator(s): participation in 2D website for DSMT/S for a period of 12 months. The control group have access to the same informational and educational content in a traditional asynchronous Web format. |
| Outcomes |
Timepoint(s): baseline and at 3, 6, 12 and 18 months (for primary outcomes) and at baseline and 6, 12 and 18 months for secondary outcomes Primary outcome(s):
Secondary outcome(s):
Other outcome(s): — |
| Starting date |
Trial start date: July 2014 Trial completion date: January 2018 |
| Contact information | Responsible party/principal investigator: Duke University/Constance M Johnson, Allison Vorderstrasse and Gail Melkus |
| Study identifier | NCT number: NCT02040038 |
| Official title | Diabetes self‐management & support LIVE (learning in virtual environments) |
| Stated purpose of study | Quote: "The purpose of this study is to determine whether participation in virtual environment which incorporates real‐time diabetes self management and support (DSMT/S) is associated with positive changes in behavior and metabolic outcomes as compared to traditional web‐based DSMT/S." |
| Notes | This study is currently recruiting participants |
NCT02066155.
| Trial name or title | Ongoing diabetes self‐management support in church‐based settings |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: supportive care |
| Participants |
Condition: type 2 diabetes Enrollment: estimated 150 Inclusion criteria: IFor parish nurses
For peer leaders
For participants
Exclusion criteria: For parish nurses
For peer leaders and participants
|
| Interventions |
Intervention(s): behavioural: parish nurse Ongoing support following diabetes self‐management education provided by parish nurse Intervention(s): behavioural: peer support Ongoing support following diabetes self‐management education provided by a trained person with diabetes Comparator(s): control group No ongoing support provided |
| Outcomes |
Timepoint(s): baseline, 3, 9, 15, 27 months Primary outcome(s): HbA1c Secondary outcome(s): BMI Other outcome(s): Diabetes‐related distress |
| Starting date |
Trial start date: January 2015 Trial completion date: April 2017 |
| Contact information | Responsible party/principal investigator: University of Michigan/Gretchen Piatt |
| Study identifier | NCT number: NCT02066155 |
| Official title | Ongoing diabetes self‐management support in church‐based settings |
| Stated purpose of study | Quote: "African Americans are twice as likely to have diabetes compared to their White counterparts and experience higher rates of diabetes‐related complications. Diabetes‐related health disparities underscore the need for effective, culturally tailored approaches to promote and sustain diabetes self‐management over time. Diabetes self‐management education (DSME) is effective in improving diabetes outcomes in the short‐term. However, many adults with diabetes cannot sustain achieved improvements without continued follow‐up and support. The 2012 revisions of both the National Standards for Diabetes Care 6 and the National Standards for DSME and Support emphasize the importance of providing both initial DSME and on‐going diabetes self‐management support (DSMS) to assist people with diabetes in maintaining effective self‐management throughout a lifetime. While a great deal is understood about how to provide effective, initial DSME, less is known about who, where, when, and how to provide effective, sustained DSMS. One significant challenge is that DSME is a covered benefit in the healthcare system, while DSMS is not. This ultimately limits access and availability of DSMS programs, especially for low‐income African Americans. Accordingly, there is critical need to develop, evaluate, and understand effective DSMS models that are ongoing, patient‐driven, and embedded in the community." |
| Notes | Contact: Gretchen Piatt, piattg@umich.edu |
NCT02081586.
| Trial name or title | mHealth skill enhancement plus phone CBT for type 2 diabetes distress medication nonadherence: pilot study |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes Enrollment: estimated 12 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s):
Comparator(s): Treatment as usual |
| Outcomes |
Timepoint(s): baseline and 16 weeks Primary outcome(s): Acceptability questionnaire; feasibility and acceptability of the assessment protocol. Secondary outcome(s):
|
| Starting date |
Trial start date: May 2013 Trial completion date: August 2014 |
| Contact information | Responsible party/principal investigator: University of Pittsburgh/Judith A Callan |
| Study identifier | NCT number: NCT02081586 |
| Official title | mHealth skill enhancement plus phone CBT for type 2 diabetes distress medication nonadherence: pilot study |
| Stated purpose of study | Quote: "Primary aim: examine feasibility and acceptability of the assessment protocol, and the recruitment, and retention of study participants. Secondary aim: 1) collect preliminary data on the effect of the intervention on clinical outcomes, e.g., self‐reported adherence to medication and self‐management adherence, e.g., diet, exercise; levels of diabetes distress, diabetes medication beliefs, and distal T2DM outcomes (HbA1c level and body mass index)." |
| Notes | Contact: Judith A Callan, callanja@pitt.edu Contact: Lisa Tamres, ltamres@pitt.edu |
NCT02137720.
| Trial name or title | Translating telephonic diabetes self‐management support to primary care practice |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: single blind (outcomes assessor) Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes Enrollment: estimated 875 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): telephonic diabetes self‐management support This group receives all the educational print materials received by the comparison condition plus telephone calls from a health educator to provide tailored diabetes self‐management training and support. Participants with significant emotional distress at baseline also receive additional calls focused on distress management. Comparator(s): educational print materials Participants randomised to this arm will receive print materials on diabetes, glycaemic control, self‐management, and distress/depression. |
| Outcomes |
Timepoint(s): baseline and 12 months Primary outcome(s): HbA1c, obtained from electronic medical record Secondary outcome(s):
|
| Starting date |
Trial start date: June 2014 Trial completion date: June 2018 |
| Contact information | Responsible party/principal investigator: Albert Einstein College of Medicine of Yeshiva University/Jeffrey Gonzalez |
| Study identifier | NCT number: NCT02137720 |
| Official title | Translating telephonic diabetes self‐management support to primary care practice |
| Stated purpose of study | Quote: "The goal of this study is to evaluate the implementation and effectiveness of an intervention to improve diabetes self‐management, emotional distress and metabolic control among adults with type 2 diabetes receiving care in primary care practices throughout New York City. The program will be implemented by the New York City Department of Health, through their Primary Care Improvement Project." |
| Notes | Contact: Winfred Y Wu, wwu2@health.nyc.gov |
NCT02370719.
| Trial name or title | Evaluation of an mHealth behavioural intervention for the self‐management for type 2 diabetes |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes, emotional distress, stress Enrollment: 150 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): mobile application for diabetes self‐management Comparator(s): standard of care |
| Outcomes |
Timepoint(s): baseline, 3, 6, 9 and 12 months Primary outcome(s):
Secondary outcome(s):
Other outcome(s): — |
| Starting date |
Trial start date: June 2015 Trial completion date: July 2017 |
| Contact information | Responsible party/principal investigator: University Health Network, Toronto, Canada/Joseph A Cafazzo |
| Study identifier | NCT number: NCT02370719 |
| Official title | Evaluation of an mHealth behavioural intervention for the self‐management for type 2 diabetes mellitus |
| Stated purpose of study | Quote: "The purpose of this study is to evaluate a patient‐centered diabetes self‐management mobile application (app), which was developed with feedback from both patients and healthcare providers. During the 12 month participants in the intervention group will be provided with a mobile phone and commercial home medical devices, such as a weight scale, glucometer and activity monitor. The measurements taken from the medical devices will wirelessly transfer to the mobile phone, where the app will assess the data and provide patients with actionable self‐management knowledge." |
| Notes | This study is currently recruiting participants. Contact: Shivani Goyal, sgoyal@ehealhinnovation.org |
NCT02488785.
| Trial name or title | Impact of a virtual diabetes self‐care and education program on diabetes‐related outcomes in Latinos with type 2 diabetes mellitus |
| Methods |
Type of study: interventional study Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes Enrollment: — Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): participants will be able to share physical activity and glucose data with the diabetes educator using the smartphone they will receive. Participants will be given a Fitbit physical activity tracker, which they can use to record their activity and share the information with the diabetes educator using the device's smartphone application. In addition, participants will receive a Glooko MeterSync Blue cable which is able to connect to most glucose meters in order to download glucose data to the Glooko Population Management tool on their smartphones. Information downloaded to the Glooko Population Management tool can be shared with the diabetes educator. Device: Fitbit Device, Smartphone Comparator(s): patients in this group will attend regular clinical and education appointments as offered by the clinic for their diabetes care. |
| Outcomes |
Timepoint(s): baseline, 6 months (selected outcomes) and 9 months Primary outcome(s):
Secondary outcome(s):
Other outcome(s): — |
| Starting date |
Trial start date: June 2015 Trial completion date: March 2017 |
| Contact information | Responsible party/principal investigator: Joslin Diabetes Center/Enrique Caballero and Marcel Twahirwa |
| Study identifier | NCT number: NCT02488785 |
| Official title | The impact of a comprehensive virtual diabetes self‐care and education program on diabetes‐related outcomes in Latinos with type 2 diabetes |
| Stated purpose of study | Quote: "The goal of this study is to evaluate the impact of a comprehensive diabetes education and management program based on frequent communication with patients using teleconsultation, text messaging, and phone calls on diabetes related outcomes in Latino patients with type 2 diabetes. The investigators hypothesize that the decline in haemoglobin A1c value between the baseline and the six‐month visit will be at least 0.5 percent greater in the intervention group than in the control group." |
| Notes | This study was recruiting participants at the time of writing. Contact: Lana Yamba, yamba@dhr‐rgv.com |
NCT02675257.
| Trial name or title | Depression and diabetes control trial Acronym: DDCT |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: diabetes mellitus, affective disorders, depression, depressive symptoms, emotional distress, diabetes complications Enrollment: 212 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): cognitive‐behavioural group treatment. 5 group sessions of diabetes‐specific cognitive‐behavioural group treatment for diabetes patients with depressive symptoms and/or diabetes distress and suboptimal glycaemic control. Comparator(s): treatment as usual; standard diabetes education |
| Outcomes |
Timepoint(s): baseline and 12 months Primary outcome(s):
Secondary outcome(s):
Other outcome(s):
|
| Starting date |
Trial start date: July 2015 Trial completion date: June 2018 |
| Contact information | Responsible party/principal investigator: Forschungsinstitut der Diabetes Akademie Mergentheim, Bad Mergentheim, Baden‐Württemberg, Germany, 97980/Prof. Dr. Norbert Hermanns |
| Study identifier | NCT number: NCT02675257 |
| Official title | Depression and diabetes control trial (DDCT) |
| Stated purpose of study | Quote: "This randomised controlled trial evaluates a cognitive‐behavioural intervention for diabetes patients with suboptimal glycaemic control and comorbid depressive symptoms and/or diabetes distress. The main outcome is the improvement of suboptimal glycaemic control (HbA1c). Secondary outcomes are effects on depressive symptoms, diabetes distress, self‐care behaviour, diabetes acceptance and quality of life. The treatment group will be treated with a cognitive‐behavioural group treatment comprising specific interventions to improve glycaemic control and reduce diabetes distress as well as depressive symptoms. The control group will receive treatment‐as‐usual. A total of 212 study participants will be included. A secondary study objective is to analyse associations of suboptimal glycaemic control, depressive symptoms and diabetes distress with inflammatory markers." |
| Notes | This study is currently recruiting participants. Contact: Bernhard Kulzer, PhD (+49) 7931/594 ext 151 kulzer@diabetes‐zentrum.de Contact: Norbert Hermanns, Prof., PhD (+49) 7931/594 ext 553 hermanns@diabetes‐zentrum.de |
NCT02730078.
| Trial name or title | Value‐based emotion‐focused educational programme to reduce diabetes‐related distress Acronym: VEMOFIT |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes Enrollment: 200 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): VEMOFIT. The VEMOFIT intervention involves 4 biweekly 2‐hour sessions over a period of about 6 weeks, and a booster at 3 months follow‐up. It consists of a mixture of exploring illness perceptions and personal meanings of diabetes, cognition‐focused education on diabetes and practical skills in self‐management and emotion‐focused training on recognising emotions in the self and others. Each group will consist of 10 to 12 participants of equal representation by the patients and their significant others. Comparator(s): attention‐meetings (AG). Patients in the health clinics randomised to the AG, will receive the usual T2D care by the clinic doctors and education by the clinic paramedics based on the recommendations in the Malaysian clinical guidelines. At T1, T2 and T4, patients (not including their significant others) in AG will be gathered in groups of 10‐12 people for the primary and secondary outcomes evaluation. This session will include general discussion on feeling about and coping with diabetes, social support at home and satisfaction with treatment and care received at the respective clinics. |
| Outcomes |
Timepoint(s): baseline, 6 weeks, 6 months and 12 months Primary outcome(s):
Secondary outcome(s):
Other outcome(s):
|
| Starting date |
Trial start date: April 2016 Trial completion date: August 2018 |
| Contact information |
Responsible party/principal investigator: Universiti Putra Malaysia/Boon‐How Chew Sponsors: Ministry of Health, Malaysia; Collaborator: UMC Utrecht |
| Study identifier | NCT number: NCT02730078 |
| Official title | The effectiveness of a value‐based emotion‐focused educational programme to reduce diabetes‐related distress in Malay adults with type 2 diabetes (VEMOFIT): a cluster randomised controlled trial |
| Stated purpose of study | Quote: "The purpose of the clinical trial is to evaluate the effectiveness of a relatively simple and short value‐based emotion‐focused educational programme in adults with type 2 diabetes (VEMOFIT) on diabetes‐related distress, depressive symptoms, illness perception, medication adherence, quality of life, diabetes self‐efficacy, self‐care and clinical outcomes." |
| Notes | This study is enrolling participants by invitation only |
NCT02748239.
| Trial name or title | Evaluation of a diabetes self‐management education program for non‐intensified insulin therapy in type 2 diabetes Acronym: MEDIAS‐2‐CT |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes Enrollment: 182 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): the MEDIAS 2 CT is a education program for the initiation of a conventional insulin therapy in type 2 diabetic patients. The program consists of 6 lessons and is conducted in group settings (4‐8 participants) Comparator(s): the Current CT program is currently used for the initiation of conventional insulin therapy in type 2 diabetic patients. The program consists of 6 lessons and is conducted in group settings (4‐8 participants) |
| Outcomes |
Timepoint(s): baseline and 6 months Primary outcome(s):
Secondary outcome(s):
Other outcome(s): — |
| Starting date |
Trial start date: February 2013 Trial completion date: May 2016 |
| Contact information | Responsible party/principal investigator: Forschungsinstitut der Diabetes Akademie Mergentheim, Bad Mergentheim, Baden‐Württemberg, Germany, 97980/Prof Dr Norbert Hermanns |
| Study identifier | NCT number: NCT02748239 |
| Official title | Evaluation of a self‐management oriented diabetes education program for the initiation of non‐intensive insulin therapy in type 2 diabetic patients |
| Stated purpose of study | Quote: "A new diabetes education program for the initiation of non‐intensive insulin therapy in type 2 diabetic patients (MEDIAS 2 CT) was developed. In the evaluation, this new developed program is compared with an education programs which is currently used for diabetes education. It is expected that the new developed program (MEDIAS 2 CT) can demonstrate non‐inferiority with regard to the main outcome variable glycaemic control. If non‐inferiority can be demonstrated superiority of this program will be tested." |
| Notes | This study has been completed |
NCT02863523.
| Trial name or title | Collaborative care management for distress and depression in rural diabetes Acronym: COMRADE |
| Methods |
Type of study: efficacy study Allocation: randomised Intervention model: parallel assignment Masking: open label Primary purpose: treatment |
| Participants |
Condition: type 2 diabetes, diabetes‐related distress, depression Enrollment: 139 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention(s): integrated behavioural intervention. Patients receive intensive behavioural counselling that may include elements of cognitive behavioural therapy, problem solving therapy, and small changes lifestyle counselling in addition to medical care. Comparator(s): usual care |
| Outcomes |
Timepoint(s): baseline, 6 months and 12 months Primary outcome(s):
Secondary outcome(s):
Other outcome(s):
|
| Starting date |
Trial start date: September 2014 Trial completion date: February 2017 |
| Contact information | Responsible party/principal investigator: East Carolina University/Doyle M Cummings |
| Study identifier | NCT number: NCT02863523 |
| Official title | COMRADE: collaborative care management for distress and depression in rural diabetes |
| Stated purpose of study | Quote: "The study will implement and evaluate, using a pragmatic comparative effectiveness trial, a unique collaborative, stepped‐care intervention for patients with uncontrolled type 2 diabetes and co‐morbid distress and/or depression." |
| Notes | This study is ongoing, but not recruiting participants |
BMI; body mass index; CBT: cognitive behavioural therapy; CES‐D; Center for Epidemiological Studies Depression Scale; DDS; Diabetes Distress Scale; HbA1c: glycosylated haemoglobin; HDL: high‐density lipoprotein; LDL: low‐density lipoprotein; MEMS: medication event monitoring system; PAID; PHQ; T2DM;
Differences between protocol and review
The waiting list was combined with the usual care in order to increase the number of trials in comparisons. No comparison was made for non‐interactive computer‐based programmes and paper educational materials because there was no such stand‐alone intervention, and interventions that included similar features were classified accordingly.
We deleted the investigation of imbalances in baseline characteristics (chance bias) from risk of bias evaluations (newer reviews of the CMED Group investigate imbalances in baseline characteristics as part of selection bias).
We specified minimum duration of follow‐up as six months for all outcome measures except adverse events (as mentioned under 'Method and timing of outcome measurement') to better clarify duration of follow‐up as an exclusion criterion.
Contributions of authors
All review authors read and approved the final review draft.
Boon‐How Chew (BHC): acquiring trial reports, trial selection, data extraction, data analysis, data interpretation, review drafting, and future review updates.
Rimke Vos (RV): acquiring trial reports, trial selection, data analysis, data interpretation, review drafting, and future review updates.
Maria‐Inti Metzendorf (MIM): search strategy development, review drafting and future review updates.
Rob JPM Scholten (RS): acquiring trial reports, data analysis, data interpretation, review drafting, and future review updates.
Guy EHM Rutten (GR): acquiring trial reports, data interpretation, review drafting, and future review updates.
Sources of support
Internal sources
-
Universiti Putra Malaysia, Malaysia.
PhD study sponsorship‐ family living allowances
-
Ministry of Education, Malaysia, Malaysia.
PhD study sponsorship‐ tuition fees and living allowances
External sources
None, Other.
Declarations of interest
BHC: is receiving living allowances and tuition fees while doing his PhD and this systematic review from Ministry of Education Malaysia and Universiti Putra Malaysia.
RV: an unrestricted grant for a study in type 2 diabetes patients on insulin therapy (support of self‐managment by triggers) is provided by Sanofi.
MIM: none known.
RS: none known.
GR: received honoraria for consultancy (Novo Nordisk) and a grant for an investigator‐initiated study (Sanofi‐aventis).
Edited (no change to conclusions)
References
References to studies included in this review
Beverly 2013 {published data only}
- Beverly EA, Fitzgerald SM, Brooks KM, Hultgren BA, Ganda OP, Munshi M, et al. Impact of reinforcement of diabetes self‐care on poorly controlled diabetes: a randomized controlled trial. Diabetes Educator 2013;39(4):504‐14. [DOI: 10.1177/0145721713486837] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00895986. Using conversation maps to reinforce self‐care. www.clinicaltrials.gov/ct2/show/NCT00895986 (accessed 28 January 2016).
D'Eramo Melkus 2010 {published data only}
- D'Eramo Melkus G, Chyun D, Vorderstrasse A, Newlin K, Jefferson V, Langerman S. The effect of a diabetes education, coping skills training, and care intervention on physiological and psychosocial outcomes in black women with type 2 diabetes. Biological Research for Nursing 2010;12(1):7‐19. [DOI: 10.1177/1099800410369825] [DOI] [PubMed] [Google Scholar]
Davies 2008 {published data only}
- Davies MJ, Heller S, Skinner TC, Campbell MJ, Carey ME, Cradock S, et al. Effectiveness of the diabetes education and self management for ongoing and newly diagnosed (DESMOND) programme for people with newly diagnosed type 2 diabetes: cluster randomised controlled trial. BMJ 2008;336(7642):491‐5. [DOI: 10.1136/bmj.39474.922025] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN17844016. DESMOND (Diabetes Education and Self Management for Ongoing and Newly Diagnosed): a randomised controlled trial of a structured group education programme for people newly diagnosed with type two diabetes. www.isrctn.com/ISRCTN17844016 (accessed 28 January 2016).
- Khunti K, Gray LJ, Skinner T, Carey ME, Realf K, Dallosso H, et al. Effectiveness of a diabetes education and self management programme (DESMOND) for people with newly diagnosed type 2 diabetes mellitus: three year follow‐up of a cluster randomised controlled trial in primary care. BMJ 2012;344:e2333. [DOI: 10.1136/bmj.e2333] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dennick 2015 {published data only}
- Dennick K, Bridle C, Sturt J. Written emotional disclosure for adults with type 2 diabetes: a primary care feasibility study. Primary Health Care Research & Development 2015;16(2):179‐87. [DOI: 10.1017/S1463423614000188] [DOI] [PubMed] [Google Scholar]
- ISRCTN18442976. Writing for adults with type 2 diabetes. www.isrctn.com/ISRCTN18442976 (accessed 28 January 2016).
Fisher 2011 {published data only}
- Fisher L, Polonsky W, Parkin CG, Jelsovsky Z, Amstutz L, Wagner RS. The impact of blood glucose monitoring on depression and distress in insulin‐naive patients with type 2 diabetes. Current Medical Research and Opinion 2011;Suppl 3:39‐46. [DOI: 10.1185/03007995.2011.619176] [DOI] [PubMed] [Google Scholar]
- NCT00674986. A clinical study to evaluate the use of episodic, intensive blood glucose monitoring in persons with non‐insulin treated type 2 diabetes. www.clinicaltrials.gov/ct2/show/study/NCT00674986 (accessed 28 January 2016).
- Polonsky W, Fisher L, Schikman C, Hinnen D, Parkin C, Jelsovsky Z, et al. The value of episodic, intensive blood glucose monitoring in non‐insulin treated persons with Type 2 Diabetes: design of the Structured Testing Program (STeP) study, a cluster‐randomised, clinical trial [NCT00674986]. BMC Family Practice 2010;11:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polonsky WH, Fisher L, Schikman CH, Hinnen DA, Parkin CG, Jelsovsky Z, et al. Structured self‐monitoring of blood glucose significantly reduces A1C levels in poorly controlled, noninsulin‐treated type 2 diabetes: results from the Structured Testing Program study. Diabetes Care 2011;34(2):262‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2013 {published data only}
- Fisher L, Hessler D, Glasgow RE, Arean PA, Masharani U, Naranjo D, et al. REDEEM: a pragmatic trial to reduce diabetes distress. Diabetes Care 2013;36(9):2551‐8. [DOI: 10.2337/dc12-2493] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler D, Fisher L, Glasgow RE, Strycker LA, Dickinson LM, Arean PA, et al. Reductions in regimen distress are associated with improved management and glycemic control over time. Diabetes Care 2014;37(3):617‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00714441. Reducing distress and improving self‐care in diabetes (REDEEM). clinicaltrials.gov/ct2/show/NCT00714441 (accessed 28 January 2016).
Gabbay 2013 {published data only}
- Gabbay RA, Añel‐Tiangco RM, Dellasega C, Mauger DT, Adelman A, Horn DH. Diabetes nurse case management and motivational interviewing for change (DYNAMIC): results of a 2‐year randomized controlled pragmatic trial. Journal of Diabetes 2013;5(3):349‐57. [DOI: 10.1111/1753-0407] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00308386. DYNAMIC study: diabetes nurse case management and motivational interviewing for change. clinicaltrials.gov/ct2/show/NCT00308386 (accessed 28 January 2016).
- Stuckey HL, Dellasega C, Graber NJ, Mauger DT, Lendel I, Gabbay RA. Diabetes nurse case management and motivational interviewing for change (DYNAMIC):study design and baseline characteristics in the Chronic Care Model for type 2 diabetes. Contemporary Clinical Trials 2009;30(4):366‐74. [DOI: 10.1016/j.cct.2009.03.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Glasgow 2005 {published data only}
- Glasgow RE, Nutting PA, King DK, Nelson CC, Cutter G, Gaglio B, et al. A practical randomized trial to improve diabetes care. Journal of General Internal Medicine 2004;12(19):1167‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow RE, Nutting PA, King DK, Nelson CC, Cutter G, Gaglio B, et al. Randomized effectiveness trial of a computer‐assisted intervention to improve diabetes care. Diabetes Care 2005;28(1):33‐9. [PUBMED: 15616230] [DOI] [PubMed] [Google Scholar]
- Williams GC, Lynch M, Glasgow RE. Computer‐assisted intervention improves patient‐centered diabetes care by increasing autonomy support. Health Psychology 2007;26(6):728‐34. [PUBMED: 18020845] [DOI] [PubMed] [Google Scholar]
Grillo 2016 {published data only}
- Grillo Mde F, Neumann CR, Scain SF, Rozeno RF, Beloli L, Perinetto T, Gross JL, Leitão CB. Diabetes education in primary care: a randomized clinical trial. Cadernos De Saúde Pública 2016;32(5):pii: S0102‐311X2016000500502. [DOI: 10.1590/0102-311X00097115] [DOI] [PubMed] [Google Scholar]
- NCT01473329. Structured diabetes self‐management education in primary care and metabolic control. clinicaltrials.gov/ct2/show/NCT01473329 (accessed 19 October 2016).
Hermanns 2012 {published data only}
- Hermanns N, Kulzer B, Maier B, Mahr M, Haak T. The effect of an education programme (MEDIAS 2 ICT) involving intensive insulin treatment for people with type 2 diabetes. Patient Education and Counseling 2012;86(2):226‐32. [DOI: 10.1016/j.pec.2011.05.017] [DOI] [PubMed] [Google Scholar]
- NCT00901992. Evaluation of a self‐management oriented diabetes education program for intensified insulin therapy in type 2 diabetes (MEDIAS‐2‐ICT). clinicaltrials.gov/show/NCT00901992 (accessed 28 January 2016).
Hermanns 2015 {published data only}
- Chernyak N, Kulzer B, Hermanns N, Schmitt A, Gahr A, Haak T, et al. Within‐trial economic evaluation of diabetes‐specific cognitive behaviour therapy in patients with type 2 diabetes and subthreshold depression. BMC Public Health 2010;10:625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanns N, Schmitt A, Gahr A, Herder C, Nowotny B, Roden M, et al. The effect of a diabetes‐specific cognitive behavioral treatment program (DIAMOS) for patients with diabetes and subclinical depression: results of a randomized controlled trial. Diabetes Care 2015;38(4):551‐60. [PUBMED: 25605812] [DOI] [PubMed] [Google Scholar]
- NCT01009138. Evaluation of a diabetes‐specific cognitive behavioural treatment for subthreshold depression. clinicaltrials.gov/ct2/show/NCT01009138 (accessed 28 January 2016).
Lamers 2011 {published data only}
- ISRCTN92331982. Depression in chronically ill elderly. www.isrctn.com/ISRCTN92331982 (accessed 28 January 2016).
- Jonkers CC, Lamers F, Bosma H, Metsemakers JF, Eijk JT. The effectiveness of a minimal psychological intervention on self‐management beliefs and behaviours in depressed chronically ill elderly persons: a randomised trial. International Psychogeriatrics 2012;24(2):288‐97. [DOI] [PubMed] [Google Scholar]
- Jonkers CC, Lamers F, Evers SM, Bosma H, Metsemakers JF, Eijk JT. Economic evaluation of a minimal psychological intervention in chronically ill elderly patients with minor or mild to moderate depression: a randomised trial (the DELTA‐study). International Journal of Technol Assessment in Health Care 2009;25(4):497‐504. [DOI] [PubMed] [Google Scholar]
- Jonkers CC, Lamers F, Evers SM, Bosma H, Eijk JT. Cost‐utility estimates in depression: does the valuation method matter?. Journal of Mental Health Policy and Economics 2010;13(4):189‐97. [PubMed] [Google Scholar]
- Lamers F, Jonkers CC, Bosma H, Diederiks JP, Eijk JT. Effectiveness and cost‐effectiveness of a minimal psychological intervention to reduce non‐severe depression in chronically ill elderly patients: the design of a randomised controlled trial [ISRCTN92331982]. BMC Public Health 2006;6:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F, Jonkers CC, Bosma H, Knottnerus JA, Eijk JT. Treating depression in diabetes patients: does a nurse‐administered minimal psychological intervention affect diabetes‐specific quality of life and glycaemic control? A randomized controlled trial. Journal of Advanced Nursing 2011;67(4):788‐99. [DOI: 10.1111/j.1365-2648.2010.05540.x] [DOI] [PubMed] [Google Scholar]
Lerman 2009 {published data only}
- Lerman I, López‐Ponce A, Villa AR, Escobedo M, Caballero EA, Velasco ML, et al. Pilot study of two different strategies to reinforce self care behaviors and treatment compliance among type 2 diabetes patients from low income strata [Estudio piloto de dos diferentes estrategias para reforzar conductas de autocuidado y adherencia al tratamiento en pacientes de bajos recursos economicos con diabetes tipo 2]. Gaceta Medica de Mexico 2009;145(1):15‐9. [PUBMED: 19256406] [PubMed] [Google Scholar]
Liu 2015 {published data only}
- Liu Y, Han Y, Shi J, Li R, Li S, Jin N, Gu Y, Guo H. Effect of peer education on self‐management and psychological status in type 2 diabetes patients with emotional disorders. Journal of Diabetes Investigation 2015;6(4):479‐86. [DOI: 10.1111/jdi.12311] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pibernik‐Okanovic 2015 {published data only}
- ISRCTN66963621. Psycho‐education physical exercise effects: does treating subsyndromal depression improve depression‐ and diabetes‐related outcomes?. www.isrctn.com/ISRCTN05673017 (accessed 28 January 2016).
- Pibernik‐Okanović M, Ajduković D, Lovrenčić MV, Hermanns N. Does treatment of subsyndromal depression improve depression and diabetes related outcomes: protocol for a randomised controlled comparison of psycho‐education, physical exercise and treatment as usual. Trials 2011;12:17. [DOI: 10.1186/1745-6215-12-17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pibernik‐Okanović M, Hermanns N, Ajduković D, Kos J, Prašek M, Šekerija M, Lovrenčić MV. Does treatment of subsyndromal depression improve depression‐related and diabetes‐related outcomes? A randomised controlled comparison of psychoeducation, physical exercise and enhanced treatment as usual. Trials 2015;16:305. [DOI: 10.1186/s13063-015-0833-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vučić Lovrenčić M, Pibernik‐Okanović M, Šekerija M, Prašek M, Ajduković D, Kos J, et al. Improvement in depressive symptoms Is associated with reduced oxidative damage and inflammatory response in type 2 diabetic patients with subsyndromal depression: the results of a randomized controlled trial comparing psychoeducation, physical exercise, and enhanced treatment as usual. International Journal of Endocrinology 2015;2015:210406. [DOI: 10.1155/2015/210406] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quinn 2011 {published data only}
- NCT01107015. Mobile diabetes management. clinicaltrials.gov/show/NCT01107015 (accessed 28 January 2016).
- Quinn CC, Gruber‐Baldini AL, Shardell M, Weed K, Clough SS, Peeples M, et al. Mobile diabetes intervention study: testing a personalized treatment/behavioral communication intervention for blood glucose control. Contemp Clin Trials 2009;30(4):334‐46. [DOI] [PubMed] [Google Scholar]
- Quinn CC, Shardell MD, Terrin ML, Barr EA, Ballew SH, Gruber‐Baldini AL. Cluster‐randomized trial of a mobile phone personalized behavioral intervention for blood glucose control. Diabetes Care 2011;34(9):1934‐42. [DOI: 10.2337/dc11-0366] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenbek 2011 {published data only}
- NCT00555854. Self‐care behaviour treatment in patients with diabetes ‐ a randomised controlled trial. clinicaltrials.gov/show/NCT00555854 (accessed 28 January 2016).
- Rosenbek Minet LK, Wagner L, Lønvig EM, Hjelmborg J, Henriksen JE. The effect of motivational interviewing on glycaemic control and perceived competence of diabetes self‐management in patients with type 1 and type 2 diabetes mellitus after attending a group education programme: a randomised controlled trial. Diabetologia 2011;54(7):1620‐9. [DOI: 10.1007/s00125-011-2120-x] [DOI] [PubMed] [Google Scholar]
Shibayama 2007 {published data only}
- Shibayama T, Kobayashi K, Takano A, Kadowaki T, Kazuma K. Effectiveness of lifestyle counseling by certified expert nurse of Japan for non‐insulin‐treated diabetic outpatients: a 1‐year randomized controlled trial. Diabetes Research and Clinical Practice 2007;76(2):265‐8. [PUBMED: 17049662] [DOI] [PubMed] [Google Scholar]
Simmons 2015 {published data only}
- ISRCTN66963621. RAPSID: Can peer support, delivered as a group or individual intervention, enable people with diabetes and improve their health? A randomised controlled trial of peer support in type 2 diabetes. www.isrctn.com/ISRCTN66963621 (accessed 28 January 2016).
- Simmons D, Prevost AT, Bunn C, Holman D, Parker RA, Cohn S, et al. Impact of community based peer support in type 2 diabetes: a cluster randomised controlled trial of individual and/or group approaches. PLOS ONE 2015;10(3):e0120277. [DOI: 10.1371/journal.pone.0120277] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skelly 2009 {published data only}
- Skelly AH, Carlson J, Leeman J, Soward A, Burns D. Controlled trial of nursing interventions to improve health outcomes of older African American women with type 2 diabetes. Nursing Research 2009;58(6):410‐8. [DOI: 10.1097/NNR.0b013e3181bee597] [DOI] [PMC free article] [PubMed] [Google Scholar]
Spencer 2013 {published data only}
- NCT00800410. REACH Detroit partnership family intervention. www.clinicaltrials.gov/ct2/show/NCT00800410 (accessed 28 January 2016).
- Rosland AM, Kieffer E, Spencer M, Sinco B, Palmisano G, Valerio M, et al. Do pre‐existing diabetes social support or depressive symptoms influence the effectiveness of a diabetes management intervention?. Patient Education and Counseling 2015;98(11):1402‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer MS, Hawkins J, Espitia NR, Sinco B, Jennings T, Lewis C, et al. Influence of a community health worker intervention on mental health outcomes among low‐income Latino and African American adults with type 2 diabetes. Race and Social Problems 2013;5(2):137‐46. [DOI: 10.1007/s12552-013-9098-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sperl‐Hillen 2013 {published data only}
- NCT00652509. Journey for control of diabetes study (0431‐111). clinicaltrials.gov/show/NCT00652509 (accessed 28 January 2016).
- Sperl‐Hillen J, Beaton S, Fernandes O, Worley A, Vazquez‐Benitez G, Hanson A, et al. Are benefits from diabetes self‐management education sustained?. American Journal of Managed Care 2013;19(2):104‐12. [PUBMED: 23448107] [PubMed] [Google Scholar]
- Sperl‐Hillen J, Beaton S, Fernandes O, Worley A, Vazquez‐Benitez G, Parker E, et al. Comparative effectiveness of patient education methods for type 2 diabetes: a randomized controlled trial. Archives of Internal Medicine 2011;171(22):2001‐10. [DOI: 10.1001/archinternmed.2011.507] [DOI] [PubMed] [Google Scholar]
Sturt 2008 {published data only}
- ISRCTN06315411. Effectiveness of patient self‐managed structured education for type 2 diabetes (the diabetes manual): a cluster randomised‐controlled trial. www.isrctn.com/ISRCTN06315411 (accessed 28 January 2016).
- Sturt J, Hearnshaw H, Farmer A, Dale J, Eldridge S. The Diabetes Manual trial protocol ‐ a cluster randomized controlled trial of a self‐management intervention for type 2 diabetes [ISRCTN06315411]. BMC Family Practice 2006;7:45. [PUBMED: 16846517] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturt JA, Whitlock S, Fox C, Hearnshaw H, Farmer AJ, Wakelin M, et al. Effects of the Diabetes Manual 1:1 structured education in primary care. Diabetic Medicine 2008;25(6):722‐31. [DOI: 10.1111/j.1464-5491.2008.02451.x] [DOI] [PubMed] [Google Scholar]
Taylor 2006 {published data only}
- Taylor E. Improving the perceived psychological well‐being of seniors with type 2 diabetes through participation in two innovative programs. Dissertation Abstracts International: Section B: The Sciences and Engineering 2001:1‐99. [UMI Number: DP14197]
Trief 2016 {published data only}
- NCT01017523. Diabetes support project: couples intervention (DSP). clinicaltrials.gov/show/NCT01017523 (accessed 17 October 2016).
- Trief PM, Fisher L, Sandberg J, Cibula DA, Dimmock J, Hessler DM, et al. Health and psychosocial outcomes of a telephonic couples behavior change intervention in patients with poorly controlled type 2 diabetes: a randomized clinical trial. Diabetes Care 2016;39(12):2165‐73. [DOI: 10.2337/dc16-0035] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Wulp 2012 {published data only}
- ISRCTN91626621. Expert patients as a coach in a self‐management program for newly diagnosed patients with diabetes type two: a randomised controlled trial. www.isrctn.com/ISRCTN91626621 (accessed 28 January 2016).
- Wulp I, Leeuw JR, Gorter KJ, Rutten GE. Effectiveness of peer‐led self‐management coaching for patients recently diagnosed with Type 2 diabetes mellitus in primary care: a randomized controlled trial. Diabetic Medicine 2012;29(10):e390‐7. [DOI: 10.1111/j.1464-5491.2012.03629.x] [DOI] [PubMed] [Google Scholar]
Van Dijk‐de Vries 2015 {published data only}
- NTR2764. Implementation of a nurse‐led self‐management support in primary care for type 2 diabetes patients with emotional distress with problems with daily functioning. www.trialregister.nl/trialreg/admin/rctview.asp?TC=2764 (accessed 28 January 2016).
- Dijk‐de Vries A, Bokhoven MA, Winkens B, Terluin B, Knottnerus JA, Weijden T, et al. Lessons learnt from a cluster‐randomised trial evaluating the effectiveness of Self‐Management Support (SMS) delivered by practice nurses in routine diabetes care. BMJ Open 2015;5(6):e007014. [DOI: 10.1136/bmjopen-2014-007014] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinger 2011 {published data only}
- Beverly EA, Fitzgerald S, Sitnikov L, Ganda OP, Caballero AE, Weinger K. Do older adults aged 60‐75 years benefit from diabetes behavioral interventions?. Diabetes Care 2013;36(6):1501‐6. [DOI: 10.2337/dc12-2110] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00142922. Breaking down barriers to diabetes self‐care. clinicaltrials.gov/show/NCT00142922 (accessed 28 January 2016).
- Weinger K, Beverly EA, Lee Y, Sitnokov L, Ganda OP, Caballero AE. The effect of a structured behavioral intervention on poorly controlled diabetes: a randomized controlled trial. Archives of Internal Medicine 2011;171(22):1990‐9. [DOI: 10.1001/archinternmed.2011.502] [DOI] [PMC free article] [PubMed] [Google Scholar]
Welch 2015 {published data only}
- NCT02156037. An RCT evaluation of a diabetes dashboard team model in primary care. clinicaltrials.gov/show/NCT02156037 (accessed 28 January 2016).
- Welch G, Zagarins SE, Santiago‐Kelly P, Rodriguez Z, Bursell SE, Rosal MC, et al. An internet‐based diabetes management platform improves team care and outcomes in an urban Latino population. Diabetes Care 2015;38(4):561‐7. [DOI: 10.2337/dc14-1412] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whittemore 2004 {published data only}
- Whittemore R, Melkus GD, Sullivan A, Grey M. A nurse‐coaching intervention for women with type 2 diabetes. Diabetes Educator 2004;30(5):795‐804. [PUBMED: 15510531] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Carper 2014 {published data only}
- Carper MM, Traeger L, Gonzalez JS, Wexler DJ, Psaros C, Safren SA. The differential associations of depression and diabetes distress with quality of life domains in type 2 diabetes. Journal of Behavioral Medicine 2014;37(3):501‐10. [DOI: 10.1007/s10865-013-9505-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiu 2016 {published data only}
- Chiu CJ, Hu YH, Wray LA, Beverly EA, Yang YC, Wu JS, Lu FH. Dissemination of evidence‐base minimal psychological intervention for diabetes management in Taiwan adults with type 2 diabetes. International Journal of Clinical and Experimental Medicine 2016;9(7):14489‐98. [Google Scholar]
Fisher 2014 {published data only}
- Fisher L, Hessler D, Masharani U, Strycker L. Impact of baseline patient characteristics on interventions to reduce diabetes distress: the role of personal conscientiousness and diabetes self‐efficacy. Diabetic Medicine 2014;31(6):739‐46. [DOI: 10.1111/dme.12403] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fonda 2009 {published data only}
- Fonda SJ, McMahon GT, Gomes HE, Hickson S, Conlin PR. Changes in diabetes distress related to participation in an internet‐based diabetes care management program and glycemic control. Journal of Diabetes Science and Technology 2009;3(1):117‐24. [PUBMED: 20046656] [DOI] [PMC free article] [PubMed] [Google Scholar]
Friis 2016 {published data only}
- Friis AM, Johnson MH, Cutfield RG, Consedine NS. Kindness matters: a randomized controlled trial of a mindful self‐compassion intervention improves depression, distress, and HbA1c among patients with diabetes. Diabetes Care 2016;39(11):1963‐71. [DOI: 10.2337/dc16-0416] [DOI] [PubMed] [Google Scholar]
Gabbay 2006 {published data only}
- Gabbay RA, Lendel I, Saleem TM, Shaeffer G, Adelman AM, Mauger DT, et al. Nurse case management improves blood pressure, emotional distress and diabetes complication screening. Diabetes Research and Clinical Practice 2006;71(1):28‐35. [PUBMED: 16019102] [DOI] [PubMed] [Google Scholar]
Heisler 2010 {published data only}
- Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Annals of Internal Medicine 2010;153(8):507‐15. [DOI: 10.7326/0003-4819-153-8-201010190-00007] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heisler 2014 {published data only}
- Heisler M, Choi H, Palmisano G, Mase R, Richardson C, Fagerlin A, et al. Comparison of community health worker‐led diabetes medication decision‐making support for low‐income Latino and African American adults with diabetes using e‐health tools versus print materials: a randomized, controlled trial. Annals of Internal Medicine 2014;161(10 Suppl):S13‐22. [DOI: 10.7326/M13-3012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Imazu 2015 {published data only}
- Imazu MFM, Faria BN, Arruda GO, Sales CA, Marcon SS. Effectiveness of individual and group interventions for people with type 2 diabetes [Efetividade das intervenções individual e em grupo junto a pessoas com diabetes tipo 2]. Revista Latino‐Americana de Enfermagem 2015;23(2):200‐7. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Izquierdo 2003 {published data only}
- Izquierdo RE, Knudson PE, Meyer S, Kearns J, Ploutz‐Snyder R, Weinstock RS. A comparison of diabetes education administered through telemedicine versus in person. Diabetes Care 2003;26(4):1002‐7. [PUBMED: 12663564] [DOI] [PubMed] [Google Scholar]
Jung 2015 {published data only}
- Jung HY, Lee H, Park J. Comparison of the effects of Korean mindfulness‐based stress reduction, walking, and patient education in diabetes mellitus. Nursing & Health Sciences 2015;17(4):516‐25. [DOI: 10.1111/nhs.12229] [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee GA, Wyatt S, Topliss D, Walker KZ, Stoney R. A study of a pre‐operative intervention in patients with diabetes undergoing cardiac surgery. Collegian 2014;21(4):287‐93. [PUBMED: 25632725] [DOI] [PubMed] [Google Scholar]
MacPhail 2014 {published data only}
- MacPhail M, Mullan B, Sharpe L, MacCann C, Todd J. Using the health action process approach to predict and improve health outcomes in individuals with type 2 diabetes mellitus. Diabetes, Metabolic Syndrome and Obesity Targets and Therapy 2014;7:469‐79. [DOI: 10.2147/DMSO.S68428] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantwill 2015 {published data only}
- Mantwill S, Fiordelli M, Ludolph R, Schulz PJ. EMPOWER‐support of patient empowerment by an intelligent self‐management pathway for patients: study protocol. BMC Medical Informatics and Decision Making 2015;15:18. [DOI: 10.1186/s12911-015-0142-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
McMahon 2012 {published data only}
- McMahon GT, Fonda SJ, Gomes HE, Alexis G, Conlin PR. A randomized comparison of online‐ and telephone‐based care management with internet training alone in adult patients with poorly controlled type 2 diabetes. Diabetes Technology and Therapeutics 2012;14(11):1060‐7. [DOI: 10.1089/dia.2012.0137] [DOI] [PMC free article] [PubMed] [Google Scholar]
Munshi 2013 {published data only}
- Munshi MN, Segal AR, Suhl E, Ryan C, Sternthal A, Giusti J, et al. Assessment of barriers to improve diabetes management in older adults: a randomized controlled study. Diabetes Care 2013;36(3):543‐9. [DOI: 10.2337/dc12-1303] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nobis 2015 {published data only}
- Nobis S, Lehr D, Ebert DD, Baumeister H, Snoek F, Riper H, et al. Efficacy of a web‐based intervention with mobile phone support in treating depressive symptoms in adults with type 1 and type 2 diabetes: a randomized controlled trial. Diabetes Care 2015;38(5):776‐83. [DOI: 10.2337/dc14-1728] [DOI] [PubMed] [Google Scholar]
Safford 2015 {published data only}
- Safford MM, Andreae S, Cherrington AL, Martin MY, Halanych J, Lewis M, et al. Peer coaches to improve diabetes outcomes in rural Alabama: a cluster randomized trial. Annals of Family Medicine 2015;13(Suppl 1):S18‐26. [DOI: 10.1370/afm.1798] [DOI] [PMC free article] [PubMed] [Google Scholar]
Samuel‐Hodge 2008 {published data only}
- Samuel‐Hodge CD, Watkins DC, Rowell KL, Hooten EG. Coping styles, well‐being, and self‐care behaviors among African Americans with type 2 diabetes. Diabetes Educator 2008;34(3):501‐10. [DOI: 10.1177/0145721708316946] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schoevers 2013 {published data only}
- Schroevers MJ, Tovote KA, Keers JC, Links TP, Sanderman R, Fleer J. Individual mindfulness‐based cognitive therapy for people with diabetes: a pilot randomized controlled trial. Mindfulness 2013;6(1):99‐110. [DOI: 10.1007/s12671-013-0235-5] [DOI] [Google Scholar]
Siminerio 2013 {published data only}
- Siminerio L, Ruppert KM, Gabbay RA. Who can provide diabetes self‐management support in primary care? Findings from a randomized controlled trial. Diabetes Educator 2013;39(5):705‐13. [DOI: 10.1177/0145721713492570] [DOI] [PubMed] [Google Scholar]
Simson 2008 {published data only}
- Simson U, Nawarotzky U, Friese G, Porck W, Schottenfeld‐Naor Y, Hahn S, et al. Psychotherapy intervention to reduce depressive symptoms in patients with diabetic foot syndrome. Diabetic Medicine 2008;25(2):206‐12. [DOI: 10.1111/j.1464-5491.2007.02370.x] [DOI] [PubMed] [Google Scholar]
Sinclair 2013 {published data only}
- NCT02144909. Partners in care with semi‐structured support group. www.clinicaltrials.gov/ct2/show/NCT02144909 (accessed 1 January 2015).
- Sinclair KA, Makahi EK, Shea‐Solatorio C, Yoshimura SR, Townsend CK, Kaholokula JK. Outcomes from a diabetes self‐management intervention for Native Hawaiians and Pacific People: Partners in Care. Annals of Behavioral Medicine 2013;45(1):24‐32. [DOI: 10.1007/s12160-012-9422-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Skinner 2010 {published data only}
- Skinner TC, Carey ME, Cradock S, Dallosso HM, Daly H, Davies MJ, et al. Depressive symptoms in the first year from diagnosis of Type 2 diabetes: results from the DESMOND trial. Diabetic Medicine 2010;27(8):965‐7. [DOI: 10.1111/j.1464-5491.2010.03028.x] [DOI] [PubMed] [Google Scholar]
Surwit 2002 {published data only}
- Surwit RS, Tilburg MA, Zucker N, McCaskill CC, Parekh P, Feinglos MN, et al. Stress management improves long‐term glycemic control in type 2 diabetes. Diabetes Care 2002;25(1):30‐4. [PUBMED: 11772897] [DOI] [PubMed] [Google Scholar]
Tang 2014 {published data only}
- Tang TS, Funnell M, Sinco B, Piatt G, Palmisano G, Spencer MS, et al. Comparative effectiveness of peer leaders and community health workers in diabetes self‐management support: results of a randomized controlled trial. Diabetes Care 2014;37(6):1525‐34. [DOI: 10.2337/dc13-2161] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2015 {published data only}
- Tang TS, Funnell MM, Sinco B, Spencer MS, Heisler M. Peer‐Led, Empowerment‐based Approach to Self‐Management Efforts in Diabetes (PLEASED): a randomized controlled trial in an African American community. Annals of Family Medicine 2015;13(Suppl 1):S27‐35. [DOI: 10.1370/afm.1819] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tovote 2014 {published data only}
- Tovote KA, Fleer J, Snippe E, Peeters AC, Emmelkamp PM, Sanderman R, Links TP, et al. Individual mindfulness‐based cognitive therapy and cognitive behavior therapy for treating depressive symptoms in patients with diabetes: results of a randomized controlled trial. Diabetes Care 2014;37(9):2427‐34. [DOI: 10.2337/dc13-2918] [DOI] [PubMed] [Google Scholar]
Trief 2011 {published data only}
- Trief PM. Challenges and lessons learned in the development and implementation of a couples‐focused telephone intervention for adults with type 2 diabetes: the Diabetes Support Project. Translational Behavioral Medicine 2011;1(3):461‐7. [PUBMED: 22003374] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Bastelaar 2011 {published data only}
- Bastelaar KM, Pouwer F, Cuijpers P, Riper H, Snoek FJ. Web‐based depression treatment for type 1 and type 2 diabetic patients: a randomized, controlled trial. Diabetes Care 2011;34(2):320‐5. [DOI: 10.2337/dc10-1248] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Bastelaar 2012 {published data only}
- Bastelaar KM, Pouwer F, Cuijpers P, Riper H, Twisk JW, Snoek FJ. Is a severe clinical profile an effect modifier in a Web‐based depression treatment for adults with type 1 or type 2 diabetes? Secondary analyses from a randomized controlled trial. Journal of Medical Internet Research 2012;14(1):e2. [DOI: 10.2196/jmir.1657] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Son 2013 {published data only}
- Son J, Nyklícek I, Pop VJ, Blonk MC, Erdtsieck RJ, Spooren PF, et al. The effects of a mindfulness‐based intervention on emotional distress, quality of life, and HbA(1c) in outpatients with diabetes (DiaMind): a randomized controlled trial. Diabetes Care 2013;36(4):823‐30. [DOI: 10.2337/dc12-1477] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Son 2014 {published data only}
- Son J, Nyklíček I, Pop VJ, Blonk MC, Erdtsieck RJ, Pouwer F. Mindfulness‐based cognitive therapy for people with diabetes and emotional problems: long‐term follow‐up findings from the DiaMind randomized controlled trial. Journal of Psychosomatic Research 2014;77(1):81‐4. [DOI: 10.1016/j.jpsychores.2014.03.013] [DOI] [PubMed] [Google Scholar]
Welch 2011a {published data only}
- Welch G, Allen NA, Zagarins SE, Stamp KD, Bursell SE, Kedziora RJ. Comprehensive diabetes management program for poorly controlled Hispanic type 2 patients at a community health center. Diabetes Educator 2011;37(5):680‐8. [DOI: 10.1177/0145721711416257] [DOI] [PubMed] [Google Scholar]
Welch 2011b {published data only}
- Welch G, Zagarins SE, Feinberg RG, Garb JL. Motivational interviewing delivered by diabetes educators: does it improve blood glucose control among poorly controlled type 2 diabetes patients?. Diabetes Research and Clinical Practice 2011;91(1):54‐60. [DOI: 10.1016/j.diabres.2010.09.036] [DOI] [PMC free article] [PubMed] [Google Scholar]
Whittemore 2005 {published data only}
- Whittemore R, D'Eramo Melkus G, Grey M. Metabolic control, self‐management and psychosocial adjustment in women with type 2 diabetes. Journal of Clinical Nursing 2005;14(2):195‐203. [PUBMED: 15669928] [DOI] [PubMed] [Google Scholar]
Zagarins 2012 {published data only}
- Zagarins SE, Allen NA, Garb JL, Welch G. Improvement in glycemic control following a diabetes education intervention is associated with change in diabetes distress but not change in depressive symptoms. Journal of Behavioral Medicine 2012;35(3):299‐304. [DOI: 10.1007/s10865-011-9359-z] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Dafoulas 2014 {published data only}
- Dafoulas GE, Giata P, Giannakakos H, Stafylas P, Aletras V, Theodorou K, et al. Long‐term tele‐monitoring of patients with DMT2: preliminary results of the Greek pilot of the renewing health multicenter randomised trial. Diabetes Technology and Therapeutics 2014;16(Suppl 1):A26. [Google Scholar]
- NCT01498367. Life‐long tele‐monitoring of patients with type 2 diabetes mellitus in central Greece (RHCluster2GR). www.clinicaltrials.gov/ct2/show/NCT01498367 (accessed 17 October 2016).
De Vries 2014 {published and unpublished data}
- Vries L, Heijden AA, 't Riet E, Baan CA, Kostense PJ, Rijken M, et al. Peer support to decrease diabetes‐related distress in patients with type 2 diabetes mellitus: design of a randomised controlled trial [protocol]. BMC Endocrine Disorders 2014;14:21. [DOI: 10.1186/1472-6823-14-21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NTR3474. Peer support for patients with type 2 diabetes. www.trialregister.nl/trialreg/admin/rctview.asp?TC=3474 (accessed 17 October 2016).
Ebert 2017 {published data only}
- DRKS00004748. Internet‐based programme to treat depressive symptoms of patients with diabetes mellitus type 1 and type 2 [Online‐Programm zur Bewältigung von depressiven Beschwerden bei Personen mit Diabetes mellitus Typ 1 oder Typ 2]. www.drks.de/drks_web/navigate.do?navigationId=trial.HTML&TRIAL_ID=DRKS00004748 (accessed 1 December 2016).
- Ebert DD, Nobis S, Lehr D, Baumeister H, Riper H, Auerbach RP, et al. The 6‐month effectiveness of Internet‐based guided self‐help for depression in adults with type 1 and 2 diabetes mellitus. Diabetic Medicine 2017;34(1):99‐107. [DOI: 10.1111/dme.13173] [DOI] [PubMed] [Google Scholar]
NCT01578096 {published data only}
- Bermúdez‐Millán A, Pérez‐Escamilla R, Segura‐Pérez S, Damio G, Chhabra J, Osborn CY, et al. Psychological distress mediates the association between food insecurity and suboptimal sleep quality in Latinos with type 2 diabetes mellitus. Journal of Nutrition 2016;146(10):2051‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01578096. Stress management among Latinos with type 2 diabetes (CALMS‐D). www.clinicaltrials.gov/ct2/show/NCT01578096 (accessed 1 December 2015).
References to ongoing studies
ACTRN12612000620820 {published data only}
- ACTRN12612000620820. Evaluation of an online support program for type 2 diabetes self‐management and dysphoria (depression, anxiety, and diabetes‐specific distress). anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612000620820 (accessed 19 January 2017).
- Cassimatis M, Kavanagh DJ, Hills AP, Smith AC, Scuffham PA, Gericke C, et al. The OnTrack Diabetes web‐based program for type 2 diabetes and dysphoria self‐management: a randomized controlled trial protocol. JMIR Research Protocols 2015;4(3):e97. [DOI: 10.2196/resprot.2813; PUBMED: 26242916] [DOI] [PMC free article] [PubMed] [Google Scholar]
ACTRN12614001232628 {published data only}
- ACTRN12614001232628. Diabetes text message self management support. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367369 (accessed 19 January 2017).
- Dobson R, Whittaker R, Jiang Y, Shepherd M, Maddison R, Carter K, et al. Text message‐based diabetes self‐management support (SMS4BG): study protocol for a randomised controlled trial. Trials 2016;17:179. [DOI: 10.1186/s13063-016-1305-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
ACTRN12615000931572 {published data only}
- ACTRN12615000931572. The springboarD trial: trial of a self‐help intervention to improve functioning and emotional well‐being for people with type 2 diabetes. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=368109 (accessed 10 January 2017).
ACTRN12616001010482 {published data only}
- ACTRN12616001010482. Pilot randomised control trial of a problem‐solving intervention tailored to quality of life difficulties experienced by patients with diabetic retinopathy. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371127 (accessed 10 January 2017).
ISRCTN02123133 {published data only}
- ISRCTN02123133. A web‐based self management programme (HeLP‐Diabetes) for people with type 2 diabetes in primary care. www.isrctn.com/ISRCTN02123133 (accessed 10 January 2017).
- Murray E, Dack C, Barnard M, Farmer A, Li J, Michie S, et al. HeLP‐Diabetes: randomised controlled trial protocol. BMC health services research 2015;15:578. [PUBMED: 26715038] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01612520 {published data only}
- NCT01612520. Telecoaching of people with type 2 diabetes in primary care. clinicaltrials.gov/ct2/show/NCT01612520 (accessed 12 October 2016).
- Odnoletkova I, Goderis G, Nobels F, Aertgeerts B, Annemans L, Ramaekers D. Nurse‐led telecoaching of people with type 2 diabetes in primary care: rationale, design and baseline data of a randomised controlled trial. BMC Family Practice 2014;15:24. [DOI: 10.1186/1471-2296-15-24] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01805245 {published data only}
- NCT01805245. Mindfulness: a novel approach for the management of diabetes‐related distress. www.clinicaltrials.gov/ct2/show/NCT01805245 (accessed 1 December 2015).
NCT02021591 {published data only}
- NCT02021591. Effectiveness study of interactive web application for problem solving in diabetes management. www.clinicaltrials.gov/ct2/show/NCT02021591 (accessed 1 December 2015).
NCT02040038 {published data only}
- NCT02040038. Diabetes self‐management & support LIVE. www.clinicaltrials.gov/ct2/show/NCT02040038 (accessed 10 October 2016).
- Vorderstrasse AA, Melkus GD, Pan W, Lewinski AA, Johnson CM. Diabetes learning in virtual environments: testing the efficacy of self‐management training and support in virtual environments (randomized controlled trial protocol). Nursing Research 2015;64(6):485‐93. [DOI: 10.1097/NNR.0000000000000128] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02066155 {published data only}
- NCT02066155. Ongoing diabetes self‐management support in church‐based settings. www.clinicaltrials.gov/ct2/show/NCT02066155 (accessed 1 December 2015).
NCT02081586 {published data only}
- NCT02081586. mHealth skill enhancement plus phone CBT for type 2 diabetes distress medication nonadherence: pilot study. www.clinicaltrials.gov/ct2/show/NCT02081586 (accessed 1 December 2015).
NCT02137720 {published data only}
- NCT02137720. Translating telephonic diabetes self‐management support to primary care practice. www.clinicaltrials.gov/ct2/show/NCT02137720 (accessed 1 December 2015).
NCT02370719 {published data only}
- Goyal S, Lewis G, Yu C, Rotondi M, Seto E, Cafazzo JA. Evaluation of a behavioral mobile phone app intervention for the self‐management of type 2 diabetes: randomized controlled trial protocol. JMIR Research Protocols 2016;5(3):e174. [DOI: 10.2196/resprot.5959] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02370719. Evaluation of an mHealth behavioural intervention for the self‐management for type 2 diabetes. www.clinicaltrials.gov/ct2/show/NCT02370719 (accessed 1 December 2016).
NCT02488785 {published data only}
- NCT02488785. Impact of a virtual diabetes self‐care and education program on diabetes‐related outcomes in Latinos with type 2 diabetes mellitus. www.clinicaltrials.gov/ct2/show/NCT02488785 (accessed 1 December 2016).
NCT02675257 {published data only}
- NCT02675257. Depression and diabetes control trial (DDCT). www.clinicaltrials.gov/ct2/show/NCT02675257 (accessed 1 December 2016).
NCT02730078 {published data only}
- NCT02730078. Value‐based emotion‐focused educational programme to reduce diabetes‐related distress (VEMOFIT). www.clinicaltrials.gov/ct2/show/NCT02730078 (accessed 1 December 2016).
NCT02748239 {published data only}
- NCT02748239. Evaluation of a diabetes self‐management education program for non‐intensified insulin therapy in type 2 diabetes (MEDIAS‐2‐CT). www.clinicaltrials.gov/ct2/show/NCT02748239 (accessed 1 December 2016).
NCT02863523 {published data only}
- NCT02863523. Collaborative care management for distress and depression in rural diabetes (COMRADE). www.clinicaltrials.gov/ct2/show/NCT02863523 (accessed 1 December 2016).
Additional references
ADA 2003
- Expert Committee on the Diagnosis and Classification of Diabetes Mellitus, American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 1999;26(Suppl 1):S5‐19. [DOI: 10.2337/diacare.26.2007.S5] [DOI] [PubMed] [Google Scholar]
ADA 2014
- American Diabetes Association. Standards of medical care in diabetes ‐ 2014. Diabetes Care 2008;37(Suppl 1):S14‐80. [DOI: 10.2337/dc14-S014] [DOI] [PubMed] [Google Scholar]
Aikens 2012
- Aikens JE. Prospective associations between emotional distress and poor outcomes in type 2 diabetes. Diabetes Care 2012;35(12):2472‐8. [DOI: 10.2337/dc12-0181] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ajzen 2011
- Ajzen I. The theory of planned behaviour: Reactions and reflections. Psychology and Health 2011;26(9):1113‐27. [DOI: 10.1080/08870446.2011.613995] [DOI] [PubMed] [Google Scholar]
Anderson 2000
- Anderson RM, Funnell MM, Fitzgerald JT, Marrero DG. The Diabetes Empowerment Scale: a measure of psychosocial self‐efficacy. Diabetes Care 2000;23(6):739‐43. [DOI: 10.2337/diacare.23.6.739] [DOI] [PubMed] [Google Scholar]
Anderson 2002
- Anderson RJ, Grigsby AB, Freedland KE, Groot M, McGill JB, Clouse RE, et al. Anxiety and poor glycemic control: a meta‐analytic review of the literature. International Journal of Psychiatry in Medicine 2002;32(3):235‐47. [PUBMED: 12489699] [DOI] [PubMed] [Google Scholar]
Aspinwall 2005
- Aspinwall LG. The psychology of future‐oriented thinking: from achievement to proactive coping, adaptation, and aging. Motivation and Emotion 2005;29(4):203‐35. [DOI: 10.1007/s11031-006-9013-1] [DOI] [Google Scholar]
Attridge 2014
- Attridge M, Creamer J, Ramsden M, Cannings‐John R, Hawthorne K. Culturally appropriate health education for people in ethnic minority groups with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD006424.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bandura 1977
- Bandura A. Self‐efficacy: toward a unifying theory of behavioral change. Psychological Review 1977;84(2):191‐215. [PUBMED: 847061] [DOI] [PubMed] [Google Scholar]
Bandura 1991
- Bandura A. Social cognitive theory of self‐regulation. Organizational Behavior and Human Decision Processes 1991;50(2):248‐87. [DOI: 10.1016/0749-5978(91)90022-L] [DOI] [Google Scholar]
Bandura 1997
- Bandura A. Self‐efficacy: The Exercise of Control. New York: Worth Publishers, 1997. [Google Scholar]
Bandura 2001
- Bandura A. Social cognitive theory: an agentic perspective. Annual Review of Psychology 2001;52:1‐26. [PUBMED: 11148297] [DOI] [PubMed] [Google Scholar]
Baumeister 2012
- Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with diabetes mellitus and depression. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD008381.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beatty 2013
- Beatty L, Lambert S. A systematic review of internet‐based self‐help therapeutic interventions to improve distress and disease‐control among adults with chronic health conditions. Clinical Psychology Review 2013;33(4):609‐22. [DOI: 10.1016/j.cpr.2013.03.004] [DOI] [PubMed] [Google Scholar]
Beller 2013
- Beller EM, Chen JK, Wang UL, Glasziou PP. Are systematic reviews up‐to‐date at the time of publication?. Systematic Reviews 2013;2(1):36. [DOI: 10.1186/2046-4053-2-36] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berry 2015
- Berry E, Lockhart S, Davies M, Lindsay JR, Dempster M. Diabetes distress: understanding the hidden struggles of living with diabetes and exploring intervention strategies. Postgraduate Medical Journal 2015;91(1075):278‐83. [DOI: 10.1136/postgradmedj-2014-133017] [DOI] [PubMed] [Google Scholar]
Bijl 1999
- Bijl JV, Poelgeest‐Eeltink AV, Shortridge‐Baggett L. The psychometric properties of the diabetes management self‐efficacy scale for patients with type 2 diabetes mellitus. Journal of Advanced Nursing 1999;30(2):352‐9. [PUBMED: 10457237] [DOI] [PubMed] [Google Scholar]
Bland 2011
- Bland JM, Altman DG. Comparisons against baseline within randomised groups are often used and can be highly misleading. Trials 2011;12:264. [DOI: 10.1186/1745-6215-12-264] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 1999
- Bradley C, Todd C, Gorton T, Symonds E, Martin A, Plowright R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Quality of Life Research 1999;8(1‐2):79‐91. [DOI: 10.1023/A:1026485130100] [DOI] [PubMed] [Google Scholar]
Browne 2013
- Browne JL, Scibilia R, Speight J. The needs, concerns, and characteristics of younger Australian adults with Type 2 diabetes. Diabetic Medicine 2013;30(5):620‐6. [DOI: 10.1111/dme.12078] [DOI] [PubMed] [Google Scholar]
Burns 2015
- Burns RJ, Deschênes SS, Schmitz N. Cyclical relationship between depressive symptoms and diabetes distress in people with Type 2 diabetes mellitus: results from the Montreal Evaluation of Diabetes Treatment Cohort Study. Diabetic Medicine 2015;32(10):1272‐8. [DOI: 10.1111/dme.12860] [DOI] [PubMed] [Google Scholar]
Callahan 1994
- Callahan CM, Hui SL, Nienaber NA, Musick BS, Tierney WM. Longitudinal study of depression and health services use among elderly primary care patients. Journal of the American Geriatrics Society 1994;42(8):833‐8. [PUBMED: 8046192] [DOI] [PubMed] [Google Scholar]
Celano 2013
- Celano CM, Beale EE, Moore SV, Wexler DJ, Huffman JC. Positive psychological characteristics in diabetes: a review. Current Diabetes Reports 2013;13(6):917‐29. [DOI: 10.1007/s11892-013-0430-8] [DOI] [PubMed] [Google Scholar]
Chamnan 2009
- Chamnan P, Simmons RK, Sharp SJ, Griffin SJ, Wareham NJ. Cardiovascular risk assessment scores for people with diabetes: a systematic review. Diabetologia 2009;52(10):2001‐14. [DOI: 10.1007/s00125-009-1454-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cherrington 2010
- Cherrington A, Wallston KA, Rothman RL. Exploring the relationship between diabetes self‐efficacy, depressive symptoms, and glycemic control among men and women with type 2 diabetes. Journal of Behavioral Medicine 2010;33(1):81‐9. [DOI: 10.1007/s10865-009-9233-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chew 2013
- Chew BH, Shariff‐Ghazali S, Lee PY, Cheong AT, Mastura I, Haniff J, et al. Type 2 diabetes mellitus patient profiles, diseases control and complications at four public health facilities‐ a cross‐sectional study based on the Adult Diabetes Control and Management (ADCM) registry 2009. Medical Journal of Malaysia 2013;68(5):397‐404. [PUBMED: 24632869] [PubMed] [Google Scholar]
Chew 2014
- Chew BH, Shariff‐Ghazali S, Fernandez A. Psychological aspects of diabetes care: effecting behavioral change in patients. World Journal of Diabetes 2014;5(6):796‐808. [DOI: 10.4239/wjd.v5.i6.796] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chew 2015a
- Chew BH, Vos R, Mohd‐Sidik S, Rutten GE. Diabetes‐related distress, depression and distress‐depression among adults with type 2 diabetes mellitus in Malaysia. PLOS ONE 2016;11(3):e0152095. [DOI: 10.1371/journal.pone.0152095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chew 2015b
- Chew BH, Vos R, Heijmans M, Metzendorf M‐I, Scholten RJPM, Rutten GEHM. Psychological interventions for diabetes‐related distress in adults with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD011469] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chew 2015c
- Chew BH, Mohd‐Sidik S, Shariff‐Ghazali S. Negative effects of diabetes‐related distress on health‐related quality of life: an evaluation among the adult patients with type 2 diabetes mellitus in three primary healthcare clinics in Malaysia. Health and Quality of Life Outcomes 2015;13:187. [DOI: 10.1186/s12955-015-0384-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chew 2016
- Chew BH, Vos R, Mohd‐Sidik S, Rutten GE. Diabetes‐Related Distress, Depression and Distress‐Depression among Adults with Type 2 Diabetes Mellitus in Malaysia. PLoS One 2016;11(3):e0152095. [DOI: 10.1371/journal.pone.0152095] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chiang 2014
- Chiang JL, Kirkman MS, Laffel LM, Peters AL, Type 1 Diabetes Sourcebook Authors. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care 2014;37(7):2034‐54. [DOI: 10.2337/dc14-1140] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2001
- Clark NM, Gong M, Kaciroti N. A model of self‐regulation for control of chronic disease. Health Education and Behavior 2001;28(6):769‐82. [PUBMED: 11720277] [DOI] [PubMed] [Google Scholar]
Coyne 1994
- Coyne JC. Self‐reported distress: analog or Ersatz depression?. Psychological Bulletin 1994;116(1):29‐45. [PUBMED: 8078972] [DOI] [PubMed] [Google Scholar]
Cromheeke 2014
- Cromheeke S, Mueller SC. Probing emotional influences on cognitive control: an ALE meta‐analysis of cognition emotion interactions. Brain Structure and Function 2014;219(3):995‐1008. [DOI: 10.1007/s00429-013-0549-z] [DOI] [PubMed] [Google Scholar]
D'Eramo 2010
- D'Eramo Melkus G, Chyun D, Vorderstrasse A, Newlin K, Jefferson V, Langerman S. The effect of a diabetes education, coping skills training, and care intervention on physiological and psychosocial outcomes in black women with type 2 diabetes. Biological Research for Nursing 2010;12(1):7‐12. [DOI: 10.1177/1099800410369825] [DOI] [PubMed] [Google Scholar]
Das‐Munshi 2007
- Das‐Munshi J, Stewart R, Ismail K, Bebbington PE, Jenkins R, Prince MJ. Diabetes, common mental disorders, and disability: findings from the UK National Psychiatric Morbidity Survey. Psychosomatic Medicine 2007;69(6):543‐50. [PUBMED: 17636148] [DOI] [PubMed] [Google Scholar]
DCCT Research Group 1988
- DCCT Research Group. Reliability and validity of a diabetes quality‐of‐life measure for the diabetes control and complications trial (DCCT). Diabetes Care 1988;11(9):725‐32. [PUBMED: 3066604] [DOI] [PubMed] [Google Scholar]
Deakin 2005
- Deakin T, McShane CE, Cade JE, Williams RD. Group based training for self‐management strategies in people with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003417.pub2] [DOI] [PubMed] [Google Scholar]
Duke 2009
- Duke SA, Colagiuri S, Colagiuri R. Individual patient education for people with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD005268.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egede 2013
- Egede LE, Hernández‐Tejada MA. Effect of comorbid depression on quality of life in adults with type 2 diabetes. Expert Review of Pharmacoeconomics and Outcomes Research 2013;13(1):83‐91. [DOI: 10.1586/erp.12.86] [DOI] [PubMed] [Google Scholar]
Ehrmann 2015
- Ehrmann D, Kulzer B, Haak T, Hermanns N. Longitudinal relationship of diabetes‐related distress and depressive symptoms: analysing incidence and persistence. Diabetic Medicine 2015;32(10):1264‐71. [DOI: 10.1111/dme.12861] [DOI] [PubMed] [Google Scholar]
Fechner‐Bates 1994
- Fechner‐Bates S, Coyne JC, Schwenk TL. The relationship of self‐reported distress to depressive disorders and other psychopathology. Journal of Consulting and Clinical Psychology 1994;62(3):550‐9. [PUBMED: 8063981] [DOI] [PubMed] [Google Scholar]
Fisher 2007
- Fisher L, Skaff MM, Mullan JT, Arean P, Mohr D, Masharani U, et al. Clinical depression versus distress among patients with type 2 diabetes: not just a question of semantics. Diabetes Care 2007;30(3):542‐8. [PUBMED: 17327318] [DOI] [PubMed] [Google Scholar]
Fisher 2008
- Fisher L, Skaff MM, Mullan JT, Arean P, Glasgow R, Masharani U. A longitudinal study of affective and anxiety disorders, depressive affect and diabetes distress among adults with type 2 diabetes. Diabetic Medicine 2008;25(9):1096‐1101. [DOI: 10.1111/j.1464-5491.2008.02533.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2010
- Fisher L, Mullan JT, Arean P, Glasgow RE, Hessler D, Masharani U. Diabetes distress but not clinical depression or depressive symptoms is associated with glycemic control in both cross‐sectional and longitudinal analyses. Diabetes Care 2010;33(1):12‐8. [DOI: 10.2337/dc09-1238] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2012
- Fisher L, Hessler DM, Polonsky WH, Mullan J. When is diabetes distress clinically meaningful?: establishing cut points for the Diabetes Distress Scale. Diabetes Care 2012;35(2):259‐64. [PUBMED: 22228744] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzpatrick 2013
- Fitzpatrick SL, Schumann KP, Hill‐Briggs F. Problem solving interventions for diabetes self‐management and control: a systematic review of the literature. Diabetes Research and Clinical Practice 2013;100(2):145‐61. [DOI: 10.1016/j.diabres.2012.12.016] [DOI] [PMC free article] [PubMed] [Google Scholar]
Folkman 2000
- Folkman S, Moskowitz JT. Positive affect and the other side of coping. American Psychologist 2000;55(6):647‐54. [DOI] [PubMed] [Google Scholar]
Gary‐Webb 2013
- Gary‐Webb TL, Suglia SF, Tehranifar P. Social epidemiology of diabetes and associated conditions. Current Diabetes Reports 2013;13(6):850‐9. [DOI: 10.1007/s11892-013-0427-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gask 2011
- Gask L, Macdonald W, Bower P. What is the relationship between diabetes and depression? a qualitative meta‐synthesis of patient experience of co‐morbidity. Chronic Illness 2011;7(3):239‐52. [DOI: 10.1177/1742395311403636] [DOI] [PubMed] [Google Scholar]
Ghiadoni 2000
- Ghiadoni L, Donald AE, Cropley M, Mullen MJ, Oakley G, Taylor M, et al. Mental stress induces transient endothelial dysfunction in humans. Circulation 2000;102(20):2473‐8. [PUBMED: 11076819] [DOI] [PubMed] [Google Scholar]
Gouni‐Berthold 2008
- Gouni‐Berthold I, Berthold HK, Mantzoros CS, Böhm M, Krone W. Sex disparities in the treatment and control of cardiovascular risk factors in type 2 diabetes. Diabetes Care 2008;31(7):1389‐91. [DOI: 10.2337/dc08-0194] [DOI] [PMC free article] [PubMed] [Google Scholar]
Greenfield 2002
- Greenfield S, Kaplan SH, Kahn R, Ninomiya J, Griffith JL. Profiling care provided by different groups of physicians: effects of patient case‐mix (bias) and physician‐level clustering on quality assessment results. Annals of Internal Medicine 2002;136(2):111‐21. [PUBMED: 11790062] [DOI] [PubMed] [Google Scholar]
Hadjiconstantinou 2016
- Hadjiconstantinou M, Byrne J, Bodicoat DH, Robertson N, Eborall H, Khunti K, et al. Do web‐based interventions improve well‐being in type 2 diabetes? A systematic review and meta‐analysis. Journal of Medical Internet Research 2016;18(10):e270. [DOI: 10.2196/jmir.5991] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harkness 2010
- Harkness E, Macdonald W, Valderas J, Coventry P, Gask L, Bower P. Identifying psychosocial interventions that improve both physical and mental health in patients with diabetes: a systematic review and meta‐analysis. Diabetes Care 2010;33(4):926‐30. [DOI: 10.2337/dc09-1519] [DOI] [PMC free article] [PubMed] [Google Scholar]
Health Quality Ontario 2009a
- Health Quality Ontario. Behavioural interventions for type 2 diabetes: an evidence‐based analysis. Ontario Health Technology Assessment Series 2009;9(21):1‐45. [PUBMED: 23074526] [PMC free article] [PubMed] [Google Scholar]
Health Quality Ontario 2009b
- Health Quality Ontario. Community‐based care for the management of type 2 diabetes: an evidence‐based analysis. Ontario Health Technology Assessment Series 2009;9(23):1‐40. [PUBMED: 23074528] [PMC free article] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2009
- Higgins JPT, Thompson SG, Spiegelhalter DJ. A re‐evaluation of random‐effects meta‐analysis. Journal of the Royal Statistical Society: Series A (Statistics in Society) 2009;172(1):137‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hinder 2012
- Hinder S, Greenhalgh T. "This does my head in". Ethnographic study of self‐management by people with diabetes. BMC Health Services Research [electronic resource] 2012;12:83. [DOI: 10.1186/1472-6963-12-83] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holt 2014
- Holt RIG, Groot M, Lucki I, Hunter CM, Sortorius N, Golden SH. NIDDK international conference report on diabetes and depression: current understanding and future directions. Diabetes Care 2014;37(8):2067‐77. [DOI: 10.2337/dc13-2134] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hróbjartsson 2013
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomized clinical trials with measurement scale outcomes: a systematic review of trials with both blinded and nonblinded assessors. Canadian Medical Association Journal 2013;185(4):E201‐11. [DOI: 10.1503/cmaj.120744] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hu 2011
- Hu FB. Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 2011;34(6):1249‐57. [DOI: 10.2337/dc11-0442] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huxley 2006
- Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta‐analysis of 37 prospective cohort studies. BMJ 2006;332(7533):73‐8. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ikeda 2014
- Ikeda K, Fujimoto S, Morling B, Ayano‐Takahara S, Carroll AE, Harashima S, et al. Social orientation and diabetes‐related distress in Japanese and American patients with type 2 diabetes. PLOS ONE 2014;9(10):e109323. [DOI: 10.1371/journal.pone.0109323] [DOI] [PMC free article] [PubMed] [Google Scholar]
International Diabetes Federation 2015
- International Diabetes Federation. IDF Diabetes Atlas. 7th Edition. Brussels: International Diabetes Federation, 2015. [Google Scholar]
Ismail 2004
- Ismail K, Winkley K, Rabe‐Hesketh S. Systematic review and meta‐analysis of randomised controlled trials of psychological interventions to improve glycaemic control in patients with type 2 diabetes. Lancet 2004;363(9421):1589‐97. [PUBMED: 15145632] [DOI] [PubMed] [Google Scholar]
Izard 2008
- Izard CE. Emotion theory and research: highlights, unanswered questions, and emerging issues. Annual Review of Psychology 2008;60:1‐25. [DOI: 10.1146/annurev.psych.60.110707.163539] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kamarudin 2012
- Kamarudin MF, Noh KM, Jaafar S. Morbidity profiles at three primary care clinics in Perlis, Malaysia. The Medical Journal of Malaysia 2012;67(4):363‐8. [PUBMED: 23082442] [PubMed] [Google Scholar]
Kasteleyn 2015
- Kasteleyn MJ, Vries L, Puffelen AL, Schellevis FG, Rijken M, Vos RC, et al. Diabetes‐related distress over the course of illness: results from the Diacourse study. Diabetic Medicine 2015;32(12):1617‐24. [DOI: 10.1111/dme.12743] [DOI] [PubMed] [Google Scholar]
Kawamura 2007
- Kawamura T, Shioiri T, Takahashi K, Ozdemir V, Someya T. Survival rate and causes of mortality in the elderly with depression: a 15‐year prospective study of a Japanese community sample, the Matsunoyama‐Niigata suicide prevention project. Journal of Investigative Medicine 2007;55(3):106‐14. [PUBMED: 17481379] [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: 10.1136/bmj.c365] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLOS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lloyd 2005
- Lloyd C, Smith J, Weinger K. Stress and diabetes: a review of the links. Diabetes Spectrum 2005;18(2):121‐7. [10.2337/diaspect.18.2.121 1944‐7353] [Google Scholar]
Macaskill 2016
- Macaskill A. Review of positive psychology applications in clinical medical populations. Healthcare 2016;4(3):pii: E66. [DOI: 10.3390/healthcare4030066] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maeseneer 2008
- Maeseneer JD, Moosa S, Pongsupap Y, Kaufman A. Primary health care in a changing world. The British Journal of General Practice 2008;58(556):806‐6. [DOI: 10.3399/bjgp08X342697] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandel 2013
- Mandel SE, Davis BA, Secic M. Effects of music therapy and music‐assisted relaxation and imagery on health‐related outcomes in diabetes education: a feasibility study. Diabetes Educator 2013;39(4):568‐81. [DOI: 10.1177/0145721713492216] [DOI] [PubMed] [Google Scholar]
Meader 2014
- Meader N, King K, Llewellyn A, Norman G, Brown J, Rodgers M, et al. A checklist designed to aid consistency and reproducibility of GRADE assessments: development and pilot validation. Systematic Reviews 2014;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohr 2009
- Mohr DC, Spring B, Freedland KE, Beckner V, AreanP, Hollon SD, et al. The selection and design of control conditions for randomized controlled trials of psychological interventions. Psychotherapy and Psychosomatics 2009;78(5):275‐84. [DOI: 10.1159/000228248] [DOI] [PubMed] [Google Scholar]
Morley 1998
- Morley JE. The elderly Type 2 diabetic patient: special considerations. Diabetic Medicine 1998;15 Suppl 4:S41‐6. [PUBMED: 9868991] [DOI] [PubMed] [Google Scholar]
Naik 2011
- Naik AD, Palmer N, Petersen NJ, Street RL Jr, Rao R, Suarez‐Almazor M, et al. Comparative effectiveness of goal setting in diabetes mellitus group clinics: randomized clinical trial. Archives of Internal Medicine 2011;171(5):453‐9. [DOI: 10.1001/archinternmed.2011.70] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nakaya 2014
- Nakaya N, Kogure M, Saito‐Nakaya K, Tomata Y, Sone T, Kakizaki M, et al. The association between self‐reported history of physical diseases and psychological distress in a community‐dwelling Japanese population: the Ohsaki Cohort 2006 Study. European Journal of Public Health 2014;24(1):45‐9. [DOI: 10.1093/eurpub/ckt017] [DOI] [PubMed] [Google Scholar]
Nicolucci 2013
- Nicolucci A, Kovacs Burns K, Holt RI, Comaschi M, Hermanns N, Ishii H, et al. DAWN2 Study Group. Diabetes Attitudes, Wishes and Needs second study (DAWN2™): cross‐national benchmarking of diabetes‐related psychosocial outcomes for people with diabetes. Diabetic Medicine 2013;30(7):767‐77. [DOI: 10.1111/dme.12245] [DOI] [PubMed] [Google Scholar]
Norris 2001
- Norris SL, Engelgau MM, Narayan KM. Effectiveness of self‐management training in type 2 diabetes: a systematic review of randomized controlled trials. Diabetes Care 2001;24(3):561‐87. [PUBMED: 11289485] [DOI] [PubMed] [Google Scholar]
O'Connor 2009
- O'Connor PJ, Crain AL, Rush WA, Hanson AM, Fischer LR, Kluznik JC. Does diabetes double the risk of depression?. Annals of Family Medicine 2009;7(4):328‐35. [DOI: 10.1370/afm.964] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pal 2013
- Pal K, Eastwood SV, Michie S, Farmer AJ, Barnard ML, Peacock R, et al. Computer‐based diabetes self‐management interventions for adults with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD008776.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pan 2011
- Pan A, Sun Q, Okereke OI, Rexrode KM, Hu FB. Depression and risk of stroke morbidity and mortality: a meta‐analysis and systematic review. JAMA 2011;306(11):1241‐9. [DOI: 10.1001/jama.2011.1282] [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2013
- Park M, Katon WJ, Wolf FM. Depression and risk of mortality in individuals with diabetes: a meta‐analysis and systematic review. General Hospital Psychiatry 2013;35(3):217‐25. [DOI: 10.1016/j.genhosppsych.2013.01.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pessoa 2008
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews. Neuroscience 2008;9(2):148‐58. [DOI: 10.1038/nrn2317] [DOI] [PubMed] [Google Scholar]
Petrie 2007
- Petrie KJ, Jago LA, Devcich DA. The role of illness perceptions in patients with medical conditions. Current Opinion in Psychiatry 2007;20(2):163‐7. [PUBMED: 17278916] [DOI] [PubMed] [Google Scholar]
Peyrot 2005
- Peyrot M, Rubin RR, Lauritzen T, Snoek FJ, Matthews DR, Skovlund SE. Psychosocial problems and barriers to improved diabetes management: results of the Cross‐National Diabetes Attitudes, Wishes and Needs (DAWN) Study. Diabetic Medicine 2005;22(10):1379‐85. [PUBMED: PMID: 16176200] [DOI] [PubMed] [Google Scholar]
Peyrot 2007
- Peyrot M, Rubin RR. Behavioral and psychosocial interventions in diabetes: a conceptual review. Diabetes Care 2007;30(10):2433‐40. [PUBMED: 17666457] [DOI] [PubMed] [Google Scholar]
Piette 2004
- Piette JD, Richardson C, Valenstein M. Addressing the needs of patients with multiple chronic illnesses: the case of diabetes and depression. The American Journal of Managed Care 2004;10(2):152‐62. [PUBMED: 15005508] [PubMed] [Google Scholar]
Polonsky 1995
- Polonsky WH, Anderson BJ, Lohrer PA, Welch G, Jacobson AM, Aponte JE, et al. Assessment of diabetes‐related distress. Diabetes Care 1995;18(6):754‐60. [PUBMED: 7555499] [DOI] [PubMed] [Google Scholar]
Polonsky 2005
- Polonsky WH, Fisher L, Earles J, Dudl RJ, Lees J, Mullan J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care 2005;28(3):626‐31. [PUBMED: 15735199] [DOI] [PubMed] [Google Scholar]
Powers 2015
- Powers MA, Bardsley J, Cypress M, Duker P, Funnell MM, Hess Fischl A, et al. Diabetes self‐management education and support in type 2 diabetes: a joint position statement of the American Diabetes Association, the American Association of Diabetes Educators, and the Academy of Nutrition and Dietetics. Diabetes Care 2015;38(7):1372‐82. [DOI: 10.2337/dc15-0730] [DOI] [PubMed] [Google Scholar]
Quinones 2012
- Quinones AR, Richardson J, Freeman M, O'Neil ME, Kansagara D. Group Visits Focusing on Education for the Management of Chronic Conditions in Adults: A Systematic Review: VA Evidence‐based Synthesis Program Reports. Washington DC: Department of Veterans Affairs, 2012. [PUBMED: 24501785] [PubMed] [Google Scholar]
Rane 2011
- Rane K, Wajngot A, Wandell PE, Gafvels C. Psychosocial problems in patients with newly diagnosed diabetes: number and characteristics. Diabetes Research and Clinical Practice 2011;93(3):371‐8. [DOI: 10.1016/j.diabres.2011.05.009] [DOI] [PubMed] [Google Scholar]
Rapley 2003
- Rapley P, Passmore A, Phillips M. Review of the psychometric properties of the Diabetes Self‐Efficacy Scale: Australian longitudinal study. Nursing and Health Sciences 2003;5(4):289‐97. [PUBMED: 14622381] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ricci‐Cabello 2014
- Ricci‐Cabello I, Ruiz‐Pérez I, Rojas‐García A, Pastor G, Rodríguez‐Barranco M, Gonçalves DC. Characteristics and effectiveness of diabetes self‐management educational programs targeted to racial/ethnic minority groups: a systematic review, meta‐analysis and meta‐regression. BMC Endocrine Disorders 2014;14:60. [DOI: 10.1186/1472-6823-14-60] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Robertson 2012
- Robertson SM, Stanley MA, Cully JA, Naik AD. Positive emotional health and diabetes care: concepts, measurement, and clinical implications. Psychosomatics 2012;53(1):1‐12. [DOI: 10.1016/j.psym.2011.09.008] [DOI] [PubMed] [Google Scholar]
Robertson 2013
- Robertson SM, Amspoker AB, Cully JA, Ross EL, Naik AD. Affective symptoms and change in diabetes self‐efficacy and glycaemic control. Diabetic Medicine 2013;30:e189‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenstock 1966
- Rosenstock IM. Why people use health services. Milbank Memorial Fund Quarterly 1966;44(3):94‐127. [PUBMED: 5967464] [PubMed] [Google Scholar]
Safren 2014
- Safren SA, Gonzalez JS, Wexler DJ, Psaros C, Delahanty LM, Blashill AJ, et al. A randomized controlled trial of cognitive behavioral therapy for adherence and depression (CBT‐AD) in patients with uncontrolled type 2 diabetes. Diabetes Care 2014;37(3):625‐33. [DOI: 10.2337/dc13-0816] [DOI] [PMC free article] [PubMed] [Google Scholar]
SIGN 2010
- Health Improvement Scotland. Management of diabetes: a national clinical guideline. Edinburgh: Scottish Intercollegiate Guidelines Network; 2010 March (updated May 2014). Guideline No 116.
Snoek 2012
- Snoek FJ, Kersch NY, Eldrup E, Harman‐Boehm I, Hermanns N, Kokoszka A, et al. Monitoring of Individual Needs in Diabetes (MIND)‐2: follow‐up data from the cross‐national Diabetes Attitudes, Wishes, and Needs (DAWN) MIND study. Diabetes Care 2012;35(11):2128‐32. [DOI: 10.2337/dc11-1326] [DOI] [PMC free article] [PubMed] [Google Scholar]
Snoek 2015
- Snoek FJ, Bremmer MA, Hermanns N. Constructs of depression and distress in diabetes: time for an appraisal. Lancet Diabetes & Endocrinology 2015;3(6):450‐60. [DOI: 10.1016/S2213-8587(15)00135-7] [DOI] [PubMed] [Google Scholar]
Soe 2011
- Soe K, Sacerdote A, Karam J, Bahtiyar G. Management of type 2 diabetes mellitus in the elderly. Maturitas 2011;70(2):151‐9. [DOI: 10.1016/j.maturitas.2011.07.006] [DOI] [PubMed] [Google Scholar]
Soo 2009
- Soo H, Lam S. Stress management training in diabetes mellitus. Journal of Health Psychology 2009;14(7):933‐43. [DOI: 10.1177/1359105309341146] [DOI] [PubMed] [Google Scholar]
Sperl‐Hillen 2011
- Sperl‐Hillen J, Beaton S, Fernandes O, Worley A, Vazquez‐Benitez G, Parker E, et al. Comparative effectiveness of patient education methods for type 2 diabetes: a randomized controlled trial. Archives of Internal Medicine 2011;171(22):2001‐10. [DOI: 10.1001/archinternmed.2011.507] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
Stoop 2014
- Stoop CH, Nefs G, Pop VJ, Wijnands‐van Gent CJ, Tack CJ, Geelhoed‐Duijvestijn PH, et al. Diabetes‐specific emotional distress in people with Type 2 diabetes: a comparison between primary and secondary care. Diabetic Medicine 2014;31(10):1252‐9. [DOI: 10.1111/dme.12472] [DOI] [PubMed] [Google Scholar]
Stuckey 2014
- Stuckey HL, Mullan‐Jensen CB, Reach G, Kovacs Burns K, Piana N, Vallis M, et al. Personal accounts of the negative and adaptive psychosocial experiences of people with diabetes in the second Diabetes Attitudes, Wishes and Needs (DAWN2) study. Diabetes Care 2014;37(9):2466‐74. [DOI: 10.2337/dc13-2536] [DOI] [PubMed] [Google Scholar]
Sturt 2015
- Sturt J, Dennick K, Hessler D, Hunter BM, Oliver J, Fisher L. Effective interventions for reducing diabetes distress: systematic review and meta‐analysis. International Diabetes Nursing 2015;12(2):40‐55. [DOI: 10.1179/2057332415Y.0000000004] [DOI] [Google Scholar]
Sullivan 2013
- Sullivan MD, Katon WJ, Lovato LC, Miller ME, Murray AM, Horowitz KR, et al. Association of depression with accelerated cognitive decline among patients with type 2 diabetes in the ACCORD‐MIND trial. JAMA Psychiatry 2013;70(10):1041‐7. [DOI: 10.1001/jamapsychiatry.2013.1965] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tan 2015
- Tan LS, Khoo EY, Tan CS, Griva K, Mohamed A, New M, et al. Sensitivity of three widely used questionnaires for measuring psychological distress among patients with type 2 diabetes mellitus. Quality of Life Research 2015;24(1):153‐62. [DOI: 10.1007/s11136-014-0747-z] [DOI] [PubMed] [Google Scholar]
Thoolen 2009
- Thoolen BJ, Ridder D, Bensing J, Gorter K, Rutten G. Beyond good intentions: The role of proactive coping in achieving sustained behavioural change in the context of diabetes management. Psychology and Health 2009;24(3):237‐54. [DOI: 10.1080/08870440701864504] [DOI] [PubMed] [Google Scholar]
Uchendu 2017
- Uchendu C, Blake H. Effectiveness of cognitive‐behavioural therapy on glycaemic control and psychological outcomes in adults with diabetes mellitus: a systematic review and meta‐analysis of randomized controlled trials. Diabetic Medicine 2017;34(3):328‐39. [DOI: 10.1111/dme.13195] [DOI] [PubMed] [Google Scholar]
Van der Heijden 2013
- Heijden MM, Dooren FE, Pop VJ, Pouwer F. Effects of exercise training on quality of life, symptoms of depression, symptoms of anxiety and emotional well‐being in type 2 diabetes mellitus: a systematic review. Diabetologia 2013;56(6):1210‐25. [DOI: 10.1007/s00125-013-2871-7] [DOI] [PubMed] [Google Scholar]
Vermeire 2005
- Vermeire E, Wens J, Royen P, Biot Y, Hearnshaw H, Lindenmeyer A. Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003638.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2012
- Walker RJ, Smalls BL, Hernandez‐Tejada MA, Campbell JA, Davis KS, Egede LE. Effect of diabetes fatalism on medication adherence and self‐care behaviors in adults with diabetes. General Hospital Psychiatry 2012;34(6):598‐603. [DOI: 10.1016/j.genhosppsych.2012.07.005] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wee 2006
- Wee HL, Tan CE, Goh SY, Li SC. Usefulness of the Audit of Diabetes‐Dependent Quality‐of‐Life (ADDQoL) questionnaire in patients with diabetes in a multi‐ethnic Asian country. Pharmacoeconomics 2006;24(7):673‐82. [PUBMED: 16802843] [DOI] [PubMed] [Google Scholar]
Welch 2003
- Welch G, Weinger K, Anderson B, Polonsky WH. Responsiveness of the Problem Areas In Diabetes (PAID) questionnaire. Diabetic Medicine 2003;20(1):69‐72. [PUBMED: 12519323] [DOI] [PubMed] [Google Scholar]
Whiting 2011
- Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Research and Clinical Practice 2011;94(3):311‐21. [DOI: 10.1016/j.diabres.2011.10.029] [DOI] [PubMed] [Google Scholar]
WHO 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
WHOQOL Group 1998
- WHOQOL Group. Development of the World Health Organization WHOQOL‐BREF quality of life assessment. Psychological Medicine 1998;28(3):551‐8. [PUBMED: 9626712] [DOI] [PubMed] [Google Scholar]
Wise 1986
- Wise PH, Dowlatshahi DC, Farrant S, Fromson S, Meadows KA. Effect of computer‐based learning on diabetes knowledge and control. Diabetes Care 1986;9(5):504‐8. [PUBMED: 3533475] [DOI] [PubMed] [Google Scholar]
Woods 1995
- Wooks KL. Mega‐trials and management of acute myocardial infarction. Lancet 1995;346(8975):611‐4. [PUBMED: 7651008] [DOI] [PubMed] [Google Scholar]
Worswick 2013
- Worswick J, Wayne SC, Bennett R, Fiander M, Mayhew A, Weir MC, et al. Improving quality of care for persons with diabetes: an overview of systematic reviews ‐ what does the evidence tell us?. Systematic Reviews 2013;2:26. [DOI: 10.1186/2046-4053-2-26] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2013
- Zhang J, Xu CP, Wu HX, Xue XJ, Xu ZJ, Li Y, et al. Comparative study of the influence of diabetes distress and depression on treatment adherence in Chinese patients with type 2 diabetes: a cross‐sectional survey in the People's Republic of China. Neuropsychiatric Disease and Treatment 2013;9:1289‐94. [DOI: 10.2147/NDT.S49798] [DOI] [PMC free article] [PubMed] [Google Scholar]


