Abstract
Background
Tartrazine is the best known and one of the most commonly used food additives. Food colorants are also used in many medications as well as foods. There has been conflicting evidence as to whether tartrazine causes exacerbations of asthma with some studies finding a positive association especially in individuals with cross‐sensitivity to aspirin.
Objectives
To assess the overall effect of tartrazine (exclusion or challenge) in the management of asthma.
Search methods
A search was carried out on the Cochrane Airways Group Specialised Register. Bibliographies of each RCT was searched for additional papers. Authors of identified RCTs were contacted for further information. Searches are updated annually and are current as of February 2006.
Selection criteria
RCTs of oral administration of tartrazine (as a challenge) versus placebo or dietary avoidance of tartrazine versus normal diet were considered. Studies which focused upon allergic asthma, were also included. Studies of tartrazine exclusion for other allergic conditions such as hay fever, allergic rhinitis and eczema were only considered if the results for subjects with asthma were separately identified. Trials could be in either adults or children with asthma or allergic asthma (e.g. sensitivity to aspirin or food items known to contain tartrazine).
Data collection and analysis
Study quality was assessed and data abstracted by two reviewers independently. Outcomes were analysed using RevMan 4.1.1.
Main results
Ninety four abstracts were found, of which 20 were potentially relevant. Six? met the inclusion criteria, but only three presented results in a format that permitted analysis and none could be combined in a meta‐analysis. In none of the studies did tartrazine challenge or avoidance in diet significantly alter asthma outcomes.
Authors' conclusions
Due to the paucity of available evidence, it is not possible to provide firm conclusions as to the effects of tartrazine on asthma control. However, the six RCTs that could be included in this review all arrived at the same conclusion. Routine tartrazine exclusion may not benefit most patients, except those very few individuals with proven sensitivity.
Plain language summary
Tartrazine exclusion for allergic asthma
Tartrazine is the best known and one of the most commonly used food additives. Food colorants are also used in many medications as well as foods. There is no evidence that tartrazine makes asthma worse or avoiding it makes asthma patients any better.
Background
In recent years, adverse reactions to food additives have been a subject of public debate and concern. There is considerable disagreement regarding its importance in the pathogenesis of asthma but ingested food additives can trigger episodes of wheezing. Clearly, it is important to determine the frequency and relevance of such hypersensitivity in individuals with asthma so that advice on dietary manipulation can be targeted more effectively.
Food additives are extensively used by the food industry to prevent spoilage, prolong shelf life, and enhance appearance or taste and in the processing of certain foodstuffs. Food colorants are used in the mass production of many foods, which are no longer obtained from their traditional sources or made with the original ingredients, to give them the appearance of the traditional product. Azo dyes, which possess a nitrogen‐nitrogen double bond, are commonly used because of their colourfastness. Tartrazine (FD&C yellow # 5) is the best known and one of the most commonly used azo dyes (Simon 1986). Food colorants are also used in many medications (Lockey 1977, MacCara 1982, Smith 1976) as well as foods (Freedman 1977). In a 1989 survey of colourings and preservatives in drugs in the UK, it was showed that in 2,204 drug formulations there were 419 different additives (Pollock 1989). Of these, 52 were dyes or preservatives, which had been implicated, in adverse reactions. Tartrazine was the fourth most commonly occurring colorant occurring in 124 drug formulations.
The observed association between food colorants and asthma is not a new one. Reports on children whose asthma appeared to be exacerbated by food colours date from 1958. There has been conflicting evidence as to whether tartrazine causes exacerbations of asthma with some studies (including double blind challenges) finding a positive association especially in individuals with cross‐sensitivity to aspirin (Genton 1985). Other researchers have not been able to demonstrate any link between tartrazine and asthma (again including double blind studies). However, it has been shown that withholding bronchodilator therapy on the day of the challenge led to false positives being recorded.
There is a need to resolve whether exclusion of a common food additive such as tartrazine from the diet of an individual with asthma confers any significant benefit in terms of improvement of symptoms and reduction in the need for medication. Advice to exclude such substances may involve considerable impact on the individual's lifestyle in terms of change of diet, cost of food and avoidance of certain drugs. It could also have implications for the food and pharmaceutical industries.
Objectives
To estimate the overall efficacy of tartrazine (exclusion or challenge) upon asthma symptoms, medication requirements, lung function, non‐specific bronchial hyper‐reactivity and allergen bronchial hyper‐reactivity (BHR).
Methods
Criteria for considering studies for this review
Types of studies
All trials were randomised controlled studies. Tartrazine challenge trials used placebo groups as controls and the dietary avoidance trials compared the intervention group with a group who had a normal diet. Double‐blinded trials were preferred, but single blind and open studies were also reviewed for possible inclusion. Studies, which focused upon allergic asthma, were also included but if there were a sufficient number of studies, we would have separated the two types of studies. Studies of tartrazine exclusion for other allergic conditions such as hay fever, allergic rhinitis and eczema were only considered if the results for subjects with asthma were separately identified.
Types of participants
Adults and children with asthma or allergic asthma (e.g. sensitivity to aspirin or food items known to contain tartrazine).
Types of interventions
Trial of oral administration of tartrazine (as a challenge) versus placebo or dietary avoidance of tartrazine versus normal diet.
Types of outcome measures
Primary outcome measures:
I. Lung function (e.g. FEV1, peak expiratory flow). ii. Medication requirements.
Secondary outcome measures:
I. Symptoms (including symptom scores). ii. Non‐specific bronchial hyper‐reactivity (to histamine or methacholine). iii. Allergen specific bronchial hyper‐reactivity.
Search methods for identification of studies
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and handsearching of respiratory journals and meeting abstracts. All records in the Specialised Register coded as 'asthma' were searched using the following terms:
"azo dye*" or "azo compound*" or tartrazine* or "sunset yellow" or azorubin or amarant or benzenesulfonate* or pyrazole* or sulfite* or (food* and (color* or colour*))
This search is updated annually. The most recent search was carried out in February 2006.
The reference list of each RCT was searched for additional papers. Authors of identified RCTs were contacted for further information for their trials and details of other published or unpublished studies.
Data collection and analysis
All trials that appeared potentially relevant were assessed by two reviewers independently who selected trials for inclusion. Agreement between the two reviewers was recorded. Disagreements were resolved by consensus.
All trials were graded using the Cochrane allocation concealment grades:
Grade A ‐ Adequate allocation concealment Grade B ‐ Unclear allocation concealment Grade C ‐ Inadequate allocation concealment Grade D ‐ Allocation concealment not used
Outcome data were extracted independently by two reviewers. All disagreements were resolved through discussion.
The planned comparisons for statistical consideration were as follows:
1. Tartrazine challenge vs placebo 2. Tartrazine exclusion or avoidance vs control (untreated controls or normal diet)
Categorical outcomes were analysed as odds ratios (OR) and 95% confidence intervals (95%CI) calculated for each outcome measure. Continuous outcomes were analysed as either weighted mean difference (WMD) or standardised mean difference (SMD) with effect sizes calculated using fixed or random effect model.
Tests for heterogeneity were performed for each outcome. If significant heterogeneity were demonstrated for individual comparisons, sensitivity analysis would have been undertaken to identify the source. A priori defined sub‐groups for this purpose were: dose of tartrazine, tartrazine and drug reactions, duration of intervention, adults vs children.
Results
Description of studies
Results of the search
Searches identified 90 titles and abstracts, that were screened to identify 18 potentially relevant studies involving tartrazine in patients with asthma. Full text versions of these 18 papers were obtained and assessed independently by two reviewers. They agreed to include six trials in the review. Twelve trials that were excluded have their reasons for exclusion noted in the table, "Characteristics of excluded studies". Details of the six included studies can be found in the table, "Characteristics of included studies". An attempt was made to contact all authors for verification of methodological quality, classification of the intervention(s) and outcomes of data. No replies have been received to date.
Update searches conducted in February 2005 and 2006 did not identify any relevant new studies. An updated search from 2011 identified four studies, two of which were potentially relevant and added to studies awaiting classification,
Included studies
Sample sizes:
Most of the trials had small sample sizes with the exception of one (Spector 1979) that had 277 subjects. The number of patients in each trial ranged between 10 and 277 with a mean value of 95.
Patient Characteristics:
All studies included patients with known well‐established asthma. Patients were recruited in trials if they had asthma (Tarlo 1982, Vedanthan 1977), perennial asthma (Weber 1979), aspirin sensitive asthma (Virchow 1988), sensitivity to food items containing tartrazine (Hariparsad 1984) and positive bronchial challenge with either histamine and or methacholine (Spector 1979). Patient age ranged from 7‐72 years.
Intervention Characteristics:
The type of intervention varied between studies. Hariparsad 1984 (challenge study): a two day study to test bronchial sensitivity to histamine before and after tartrazine administration, however this study also presented data after tartrazine administration but before histamine challenge. Spector 1979 (challenge study): patients were challenged with varying strengths of tartrazine ranging from 1 mg to 50 mg. Tarlo 1982 (avoidance study): this study employed dietary avoidance of tartrazine and benzoate and also excluded non‐steroidal anti‐inflammatory agents for one month of the study. Study measurements were made before and after patients were challenged with either tartrazine or lactose Vedanthan 1977 (challenge study): patients were challenge with 600 mg tartrazine or 25 mg aspirin on separate days and challenges were done on days when patients were not taking their corticosteroids medication Virchow 1988 (challenge study): this was a multicentre study and patients were challenged with tartrazine dissolved in water in increasing doses from 1 to 25 mg Weber 1979 (challenge study): patients underwent open challenges with tartrazine dose ranging from 2.5 to 20 mg. Trials that employed tartrazine challenges were separately entered from those that employed dietary avoidance of tartrazine.
None of the tartrazine challenge studies were preceded by a period of tartrazine avoidance.
Presentation of results:
Three of the studies (Hariparsad 1984, Spector 1979, Tarlo 1982) reported their results as means with a distribution statistic, but the remaining three (Vedanthan 1977, Virchow 1988, Weber 1979) did not present their data in a usable format. All authors have been contacted to provide further information for their studies.
Risk of bias in included studies
All studies were randomised and all had control arms (placebo or normal diet). Five were double blinded and one (Weber 1979) was open. Tarlo 1982 was the only trial that clearly and adequately described the method used for treatment allocation concealment. For all other studies, the method of allocation concealment was unclear. Using the Cochrane allocation concealment grading Tarlo 1982 was graded "A", Hariparsad 1984, Spector 1979, Vedanthan 1977, Virchow 1988 & Weber 1979 trials were graded "B". Description of dropouts and withdrawals was adequate in all studies.
Effects of interventions
The six studies examined the effects of tartrazine challenge or tartrazine avoidance versus placebo on the following outcomes: non‐specific bronchial hyper‐reactivity, lung function, asthma symptom scores, adverse events and asthma medication requirements. Only three of the trials (Hariparsad 1984, Spector 1979, Tarlo 1982) contributed any data fin a usable format, but none could be combined in a meta analysis.
None of the reported outcomes in this trials included in this review was statistically significant when compared to placebo or control.
Tartrazine challenge vs placebo:
Histamine PC20 (mg/ml) One trial (Hariparsad 1984) provided this data with 18 patients (WMD ‐0.87, 95%CI ‐6.30, 4.56, p=0.8).
PEFR (Litres/min) One trial (Hariparsad 1984) provided this data with 18 patients (WMD ‐7.0, 95%CI ‐103.4, 89.4, p=0.9).
FEV1 (Litres) One trial (Spector 1979) provided this data in this figure. The legend of this figure does not make it clear, but it appears to contain only data from the 11 patients (of 277 tested) who had a greater than 20% fall in FEV1 from baseline.
Adverse events (dichotomous data) One trial (Spector 1979) provided this data with 277 patients (RR 1.00, 95%CI 0.33, 3.06, p=1.0).
Tartrazine avoidance diet vs normal diet:
PEFR (Litres/min) One trial (Tarlo 1982) provided this data with 24 patients (WMD ‐3.0, 95%CI ‐66.9, 60.9, p=0.9).
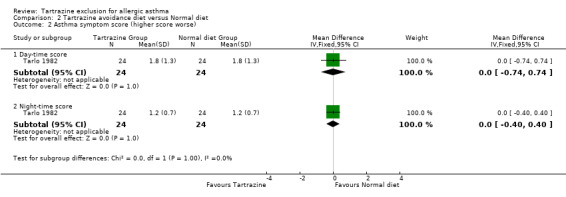
Asthma symptom scores (higher score worse) Day‐time score: One trial (Tarlo 1982) provided this data with 24 patients (WMD 0.00, 95%CI ‐0.74,0.74, p=1.0).
Night‐time score: One trial (Tarlo 1982) provided this data with 24 patients (WMD 0.00, 95%CI ‐0.40,0.40, p=1.0).
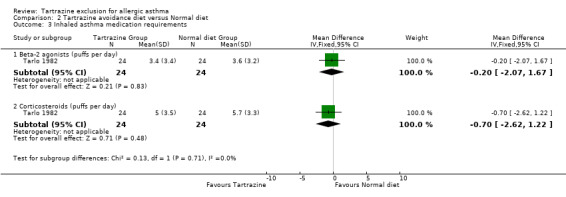
Asthma medication requirements (puffs per day) Inhaler beta‐2 agonists One trial (Tarlo 1982) provided this data with 24 patients (WMD ‐0.20, 95%CI ‐2.07,1.67, p=0.8).
Inhaled corticosteroids One trial (Tarlo 1982) provided this data with 24 patients (WMD ‐0.70, 95%CI ‐2.62,1.22, p=0.5).
None of the included trials provided data on allergen specific bronchial hyper‐reactivity.
Discussion
As data from the six included studies were limited, no firm conclusions can be drawn from this review regarding the effects of tartrazine exclusion in asthma. However, the six trials individually provided conclusions that were very similar. The five challenge trials (Hariparsad 1984; Spector 1979; Vedanthan 1977; Virchow 1988; Weber 1979) concluded that tartrazine did not change any clinical outcome, although it should be noted that none of these studies was preceded by a period of tartrazine avoidance. The one dietary avoidance trial (Tarlo 1982) also failed to show any benefit in the management of asthma.
There may be a need for further randomised controlled trials in order to confirm the absence of any effect of tartrazine on asthma control.
Authors' conclusions
Implications for practice.
There is no clear evidence that tartrazine challenge makes asthma worse or that tartrazine avoidance makes it better.
Implications for research.
To facilitate a greater understanding of the possible role of tartrazine and other food additives in exacerbating symptoms of asthma, it is recommended that a further larger scale RCT is conducted which considers the following methodological issues: blinded method to generate random sequence, state method used to conceal treatment allocation to intervention and control groups, blind assessors and state how they were blinded, include a larger sample size, include relevant outcome measures (e.g. lung function, asthma symptom scores, medication requirements) and report all relevant data completely.
What's new
| Date | Event | Description |
|---|---|---|
| 4 September 2017 | Amended | New literature search run to assess the need to update this review. No new studies identifed with search (5 July 2017). Two studies listed under Characteristics of studies awaiting classification from an earlier update search. |
History
Protocol first published: Issue 2, 1996 Review first published: Issue 3, 2001
| Date | Event | Description |
|---|---|---|
| 11 July 2012 | Review declared as stable | There are two eligible trials awaiting classification. The author team is no longer active. Expressions of interest in updating this review should be made to the managing editor of the Cochrane Airways Group. |
| 22 June 2012 | New search has been performed | Literature seacrh run. |
| 1 September 2008 | Amended | Converted to new review format. |
| 31 May 2001 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Kate Ardern wishes to acknowledge the support of the Nuffield Provincial Hospitals Trust through the award of a short‐term fellowship. The contribution of Felix Ram to previous versions of this review is acknowledged. Members of the Cochrane Airways Group (Steve Milan, Karen Blackhall, Bettina Reuben, Anna Bara) and Paul Jones the co‐ordinating editor of the Cochrane Airways Group are also acknowledged.
Data and analyses
Comparison 1. Tartrazine challenge versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PC20 ‐ Histamine (mg/ml) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.87 [‐6.30, 4.56] |
| 2 PEFR (litres/min) | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐7.0 [‐103.37, 89.37] |
| 4 Adverse Events | 1 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.33, 3.06] |
1.1. Analysis.
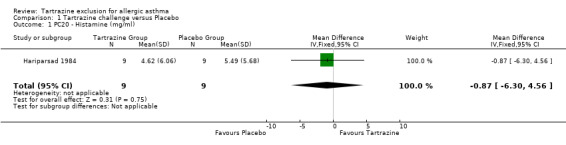
Comparison 1 Tartrazine challenge versus Placebo, Outcome 1 PC20 ‐ Histamine (mg/ml).
1.2. Analysis.
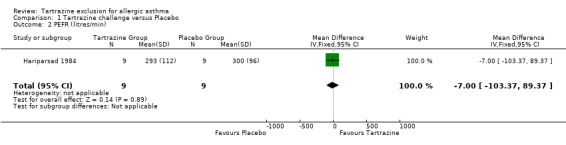
Comparison 1 Tartrazine challenge versus Placebo, Outcome 2 PEFR (litres/min).
1.4. Analysis.
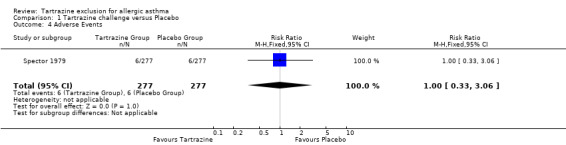
Comparison 1 Tartrazine challenge versus Placebo, Outcome 4 Adverse Events.
Comparison 2. Tartrazine avoidance diet versus Normal diet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PEFR (litres/min) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐66.94, 60.94] |
| 2 Asthma symptom score (higher score worse) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Day‐time score | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.74, 0.74] |
| 2.2 Night‐time score | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.40, 0.40] |
| 3 Inhaled asthma medication requirements | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Beta‐2 agonists (puffs per day) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.07, 1.67] |
| 3.2 Corticosteroids (puffs per day) | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐2.62, 1.22] |
2.1. Analysis.
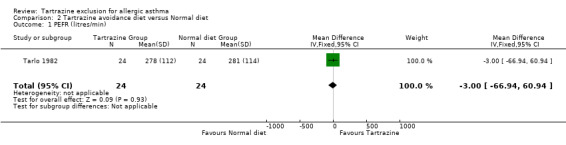
Comparison 2 Tartrazine avoidance diet versus Normal diet, Outcome 1 PEFR (litres/min).
2.2. Analysis.
Comparison 2 Tartrazine avoidance diet versus Normal diet, Outcome 2 Asthma symptom score (higher score worse).
2.3. Analysis.
Comparison 2 Tartrazine avoidance diet versus Normal diet, Outcome 3 Inhaled asthma medication requirements.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hariparsad 1984.
| Methods | Double blind randomised controlled crossover trial. | |
| Participants | 10 asthmatic children (6 boys, 4 girls) attending paediatric outpatient clinic with a history of cough or wheeze after orange drinks. Mean age 11.5 years (range 7‐16). Asthma medication was discontinued (aminophylline and cromoglycate 24 hours before and beta 2 agonists 8 hours before)before commencing each study. period. None of the children were taking anti‐histamine preparations. | |
| Interventions | A two day study to find out bronchial sensitivity to histamine before and after tartrazine administration. Baseline PEFR and histamine PC20 calculated (20% fall in PEF). Subjects were given either 1mg tartrazine capsule or placebo than challenged again with histamine (histamine challenge was repeated in those subjects that did not show a decrease in PEFR of at least 50%). 4 children who failed to respond to 1mg tartrazine after 2 days were given a single blind dose of 10mg followed by a histamine challenge. | |
| Outcomes | Non‐specific bronchial hyperreactivity (PC20), PEFR. 20% fall in PEFR defined a positive test result. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
Spector 1979.
| Methods | Double blind randomised controlled crossover trial. | |
| Participants | 304 patients (aged 14‐72 years) recruited of whom, underwent tartrazine (n = 277), aspirin, sodium salicylate and acetaminophen challenges. Selection criteria were that they had reversible obstructive airways disease (ATS criteria) and a positive bronchial challenge response to histamine and/or methacholine. Many were on oral corticosteroids. | |
| Interventions | After taking a detailed history about previous history of intolerance to foods containing tartrazine, subjects with no history of intolerance were given 50 mg tartrazine. Those with a positive history were given different doses ( 1, 5, 15, 25, 50 mg) in a double‐blind manner randomised with placebo (200mg lactose) on separate days. Challenges were monitored with half‐hourly lung function tests. All bronchodilators and anti‐histamines (but not corticosteroids) were withheld for 6 to 12 hours prior to the challenge. | |
| Outcomes | FEV1 (litres). 20% fall in FEV1 defined a positive test result. | |
| Notes | This study was primarily investigating aspirin intolerance and 74 patients were exclude from aspirin challenge tests because of recorded severe reactions to the drug. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
Tarlo 1982.
| Methods | Double blind randomised controlled crossover trial. | |
| Participants | 28 asthmatic patients (14 male 14 female) from several practices and an asthma clinic. Mean ages men 52 (sd +/‐ 14), women 53 (=/‐ 12). Selection criteria were that patients understood the requirements of the study, were able to attend the clinic and had severe enough asthma to motivate them to complete the protocol. All required daily asthma medication (82% took oral and /or inhaled corticosteroids). All were middle‐class and all were Caucasian except 1(Chinese male). | |
| Interventions | Dietary avoidance study: Double blind ingestion challenges were performed on separate days (following 24 hours on tartrazine and benzoate‐free diet) with lactose, tartrazine, benzoate and acetylsalicylic acid. 24 subjects then completed 1 month period of observation on firstly, normal diet and then on tartrazine and benzoate avoidance diets and eliminating non‐steriodal analgesics (e.g. aspirin). | |
| Outcomes | Lung function (FEV1), asthma symptom scores (day and night), asthma medication requirements (inhaled‐ puffs per day, oral ‐ tablets per day). 20% fall in FEV1 defined a positive test result. | |
| Notes | Used coded envelopes to conceal treatment allocation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment. |
Vedanthan 1977.
| Methods | Double blind randomised controlled crossover trial. | |
| Participants | 54 chronic asthmatic children 10 to 17 years of age who resided at the asthma centre. Only patients with airways obstruction demonstrated by spirometric and or plethysmographic pulmonary function testing were included, all required around‐the‐clock therapy with theophylline to prevent relapse of asthma. Approximately 60% required alternate‐day corticosteroid therapy and half of the patients were receiving cromolyn sodium. | |
| Interventions | All challenges were performed on days when corticosteroids were not administered. Bronchodilators were not administered for 6 hours but cromolyn sodium therapy was not suspended. All patients underwent double blind challenges to placebo, 600mg acetylsalicylic acid and 25mg tartrazine. on separate days. The study was discontinued ten months later when 32 of the original patients had completed the study. | |
| Outcomes | FEV1, FEF25‐75, blood pressure. 15% fall in FEV1 defined a positive test result. | |
| Notes | No data was presented for the tartrazine arm as the authors did not find and significant differences and therefore did not report results. No reply to correspondence received from author to date. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
Virchow 1988.
| Methods | Double blind randomised controlled crossover trial. | |
| Participants | 156 patients with aspirin‐induced asthma were studies. Oral or inhalation tests with aspirin were performed at least 10 days before the study and were positive in all patients. In the majority of patients oral or inhaled beta‐2 agonists were stopped at least 8 hours previously. In some patients medication was allowed 1‐2 hours before tartrazine challenge as these patients could not do with bronchodilators for longer periods. Age range 16‐63. | |
| Interventions | A multicenter study. Tartrazine was dissolved in water and administered orally at increasing doses of 1, 5, 10 and 25 mg. Placebo was white gelatine capsules. If FEV1 did not fall by >15% 1 hour post tartrazine challenge the next larger dose was given. | |
| Outcomes | FVC, FEV1, PEFR, specific airway resistance. 20% fall in FEV1 defined a positive test result. | |
| Notes | No data provided on placebo arm. Only positive tests to tartrazine provided. No reply to correspondence received from author to date. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
Weber 1979.
| Methods | Open randomised controlled crossover trial. | |
| Participants | 45 patients with severe perennial asthma. Patients were selected from asthmatics treated by the Allergy‐Immunology Service army medical centre. Age range was 15 to 62 years. All patients were taking a mixture of asthma medications including: prednisone, inhaled beclomethasone, methylxanthines and adrenergic bronchodilators. | |
| Interventions | The patients underwent open challenge over several days and in variable/random order with the following drugs and additives: acetylsalicylic acid, tartrazine, azo dye mix, non‐azo dye mix, sodium benzoate and butylated hydroxyanisole/hydroxytoluene. Placebo was clear gelatine capsules and could not be visually differentiated. Tartrazine challenge doses were 2.5, 5, 10, 15 and 20mg. A decrease in FEV1 of >25% was considered a positive challenge. Pulmonary function testing was repeated at 20‐min intervals following ingestion of the test does. If no significant change in FEV1 occurred by 1 hours the next higher dose was given. | |
| Outcomes | FVC, FEV1, PEFR. 25% fall in FEV1 defined a positive test result. | |
| Notes | No data provided on placebo arm. Only positive tests to tartrazine provided. No reply to correspondence received from author to date. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adler 1991 | No specific results on tartrazine and only uses skin tests and immunology to evaluate atopic response. |
| Collins‐W 1985 | Not a randomised controlled trial but a review of the literature. |
| Delaney 1976 | Not a randomised trial. Results were only given for the 2 patients found to be sensitive . Not able to calculate mean and standard deviations for group nor were these included in text. No replies to correspondence from author. |
| Freedman 1977 | Not a double blind randomised controlled trial ‐ no placebo used. |
| Fuglsang 1994 | Data for subjects with asthma not provided separately. No response to correspondence from author. |
| Grzelewska‐R 1986 | No placebo arm in study, all patients were challenge with tartrazine in order to develop tartrazine sensitivity. |
| Morales 1985 | Treatment not provided in a randomised manner but given sequentially. |
| Schneider 1996 | Review article not randomised controlled trial. |
| Settipane 1975 | This study was primarily designed to investigate cross‐reactivity in aspirin ‐intolerant patients. Not all the patients in the study were asthmatics and results for asthmatics were not given separately from those who were aspirin intolerant but not asthmatic. |
| Stenius 1976 | Not a double blind randomised controlled trial. Test where conducted whenever practicable and patients from one ward were included in the study. |
| Timberlake 1992 | Study also investigating precipitation of asthma by sodium metabisulphite, aspirin and betel nut. Only gave results for patients who were positive to any one of the substances and none were tartrazine sensitive. No details of placebo were provided. |
| Wilson 1989 | Study included respiratory, dermatological, behavioural and abdominal intolerance to food‐additives. Data on patients with asthma not provided separately. No response to correspondence from author. |
Contributions of authors
KA and Felix Ram (previous author no longer involved in this review) conducted all of the work for this review with help from various people as listed in the acknowledgement section.
Sources of support
Internal sources
No sources of support supplied
External sources
Nuffield Provincial Hospitals Trust, UK.
Felix Ram received funding from the Netherlands Astma Fonds, Netherlands.
Garfield Weston Foundation, UK.
Declarations of interest
There are no known conflicts of interest
Edited (no change to conclusions)
References
References to studies included in this review
Hariparsad 1984 {published data only}
- Hariparsad D, Wilson N, Dixon C, Silverman M. Oral tartrazine challenge in childhood asthma: effect on bronchial reactivity. Clinical Allergy 1984;4:81‐5. [DOI] [PubMed] [Google Scholar]
Spector 1979 {published data only}
- Spector SL, Wangaard C, Farr RS. Aspirin and concomitant idiosyncrasies in adult asthmatic patients. Journal of Allergy & Clinical Immunology 1979;64(6):500‐6. [DOI] [PubMed] [Google Scholar]
Tarlo 1982 {published data only}
- Tarlo SM, Broder I. Tartrazine and benzoate challenge and dietary avoidance in chronic asthma. Clinical Allergy 1982;12:303‐12. [DOI] [PubMed] [Google Scholar]
Vedanthan 1977 {published data only}
- Vedanthan PK, Menon MM, Bell TD, Bergin D. Aspirin and tartrazine oral challenge: incidence of adverse response in chronic childhood asthma. Journal of Allergy & Clinical Immunology 1977;60(1):8‐13. [DOI] [PubMed] [Google Scholar]
Virchow 1988 {published data only}
- Virchow C, Szczeklik A, Bianco S, Schmitz‐Schumann M, Juhl E, Robuschi M, et al. Intolerance to tartrazine in aspirin‐induced asthma: results of a multicenter study. Respiration 1988;53(1):20‐3. [DOI] [PubMed] [Google Scholar]
Weber 1979 {published data only}
- Weber RW, Hoffman M, Raine DA, Nelson HS. Incidence of bronchoconstriction due to aspirin, azo dyes, non‐azo dyes, and preservatives in a population of perennial asthmatics. Journal of Allergy & Clinical Immunology 1979;64(1):32‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adler 1991 {published data only}
- Adler BR, Assadullahi T, Warner JA, Warner JO. Evaluation of a multiple food specific IgE antibody test compared to parental perception, allergy skin tests and RAST. Clinical and Experimental Allergy 1991;21:683‐8. [DOI] [PubMed] [Google Scholar]
Collins‐W 1985 {published data only}
- Collins‐Williams C. Clinical spectrum of adverse reactions to tartrazine. Journal of Asthma 1985;22(3):139‐43. [DOI] [PubMed] [Google Scholar]
Delaney 1976 {published data only}
- Delaney JC. Response of patients with asthma and aspirin idosyncrasy to tartrazine (a drug commonly used in the food and drug industries). The Practitioner 1976;217:285‐7. [PubMed] [Google Scholar]
Freedman 1977 {published data only}
- Freedman BJ. Asthma induced by sulphur dioxide, benzoate and tartrazine contained in orange drinks. Clinical Allergy 1977;7:407‐15. [PMID: 412611] [DOI] [PubMed] [Google Scholar]
Fuglsang 1994 {published data only}
- Fulgsang GM, Halken C, Jorgensen M, Ostergaard PA, Osterballe O. Adverse reactions to food additives in children with atopic symptoms. Allergy 1994;49:31‐7. [DOI] [PubMed] [Google Scholar]
Grzelewska‐R 1986 {published data only}
- Grzelewska‐Rzymowska I, Szmidt M, Kowalski ML, Rozniecki J. Sensitivity and tolerance to tartrazine in aspirin‐sensitive asthmatics. Allergologia et Immunopathologia 1986;14(1):31‐6. [PMID: 3962814] [PubMed] [Google Scholar]
- Grzelewska‐Rzymowska I, Szmidt M, Kowalski ML, Rozniecki J. Tartrazine hypersensitivity in patients with asthma and aspirin hypersensitivity [Nadwrazliwosc na tartrazine u chorych na astme z nadwrazliwoscia na aspiryne]. Polskie Archiwum Medycyny Wewnetrznej 1984;72(5):237‐42. [PubMed] [Google Scholar]
Morales 1985 {published data only}
- Morales MC, Basomba A, Pelaez A, Garcia Villalmanzo I, Campos A. Challenge tests with tartrazine in patients with asthma associated intolerance to analgesics (ASA‐Triad). A comparative study with placebo. Clinical Allergy 1985;15(1):55‐9. [DOI] [PubMed] [Google Scholar]
Schneider 1996 {published data only}
- Schneider LC, Lester MR. Atopic disease, rhinitis and conjunctivitis and upper respiratory infections. Current Opinion in Pediatrics 1996;8:531‐40. [DOI] [PubMed] [Google Scholar]
Settipane 1975 {published data only}
- Settipane GA, Pudupakkam RK. Aspirin intolerance. iii. Subtypes, familial occurrence and cross‐reactivity with tartrazine. Journal of Allergy & Clinical Immunology 1975;56(3):215‐21. [DOI] [PubMed] [Google Scholar]
Stenius 1976 {published data only}
- Stenius BSM, Lemola M. Hypersensitivity to acetylsalicylic acid (ASA) and tartrazine in patients with asthma. Clinical Allergy 1976;6:119‐29. [DOI] [PubMed] [Google Scholar]
Timberlake 1992 {published data only}
- Timberlake CM, Toun AK, Hudson BJ. Precipitation of asthma attacks in Melanesian adults by sodium metabisulphite. Papua New Guinea Medical Journal 1992;35:186‐90. [PubMed] [Google Scholar]
Wilson 1989 {published data only}
- Wilson N, Scott A. A double‐blind assessment of additive intolerance in children using a 12 day challenge period at home. Clinical and Experimental Allergy 1989;19:267‐72. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Park 2008 {published data only}
- Park HW, Park CH, Park SH, Park JY, Park HS, Yang HJ, et al. Dermatologic adverse reactions to 7 common food additives in patients with allergic diseases: A double‐blind, placebo‐controlled study. Journal of Allergy and Clinical Immunology 2008;121(4):1059‐61. [DOI] [PubMed] [Google Scholar]
Pestana 2010 {published data only}
- Pestana S, Moreira M, Olej B. Safety of ingestion of yellow tartrazine by double‐blind placebo controlled challenge in 26 atopic adults. Allergologia Et Immunopathologia 2010;38(3):142‐6. [DOI] [PubMed] [Google Scholar]
Additional references
Genton 1985
- Genton C, Frei PC, Pecoud A. Value of oral provocation tests to aspirin and food additives in the routine investigation of asthma and chronic urticaria. Journal of Allergy & Clinical Immunology 1985;76(1):40‐5. [DOI] [PubMed] [Google Scholar]
Lockey 1977
- Lockey SD Sr. Hypersensitivity to tartrazine (FD&C Yellow No. 5) and other dyes and additives present in foods and pharmaceutical products. Annals of Allergy 1977;38(3):206‐10. [PubMed] [Google Scholar]
MacCara 1982
- MacCara ME. Tartrazine: a potentially hazardous dye in Canadian drugs. Canadian Medical Association Journal 1982;126(8):910‐4. [PMC free article] [PubMed] [Google Scholar]
Pollock 1989
- Pollock I, Young E, Stoneham M, Slater N, Wilkinson JD, Warner JO. Survey of colourings and preservatives in drugs. BMJ 1989;299(6700):649‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simon 1986
- Simon RA. Adverse reactions to food additives. New England & Regional Allergy Proceedings 1986;7(6):533‐42. [DOI] [PubMed] [Google Scholar]
Smith 1976
- Smith LJ, Slavin RG. Drugs containing tartrazine dye. Journal of Allergy & Clinical Immunology 1976;58(4):456‐70. [DOI] [PubMed] [Google Scholar]


