Abstract
Background
Invasive ventilation is used to assist or replace breathing when a person is unable to breathe adequately on their own. Because the upper airway is bypassed during mechanical ventilation, the respiratory system is no longer able to warm and moisten inhaled gases, potentially causing additional breathing problems in people who already require assisted breathing. To prevent these problems, gases are artificially warmed and humidified. There are two main forms of humidification, heat and moisture exchangers (HME) or heated humidifiers (HH). Both are associated with potential benefits and advantages but it is unclear whether HME or HH are more effective in preventing some of the negative outcomes associated with mechanical ventilation. This review was originally published in 2010 and updated in 2017.
Objectives
To assess whether heat and moisture exchangers or heated humidifiers are more effective in preventing complications in people receiving invasive mechanical ventilation and to identify whether the age group of participants, length of humidification, type of HME, and ventilation delivered through a tracheostomy had an effect on these findings.
Search methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, Embase and CINAHL up to May 2017 to identify randomized controlled trials (RCTs) and reference lists of included studies and relevant reviews. There were no language limitations.
Selection criteria
We included RCTs comparing HMEs to HHs in adults and children receiving invasive ventilation. We included randomized cross‐over studies.
Data collection and analysis
We assessed the quality of each study and extracted the relevant data. Where possible, we analysed data through meta‐analysis. For dichotomous outcomes, we calculated the risk ratio (RR) and 95% confidence interval (95% CI). For continuous outcomes, we calculated the mean difference (MD) and 95% CI or standardized mean difference (SMD) and 95% CI for parallel studies. For cross‐over trials, we calculated the MD and 95% CI using correlation estimates to correct for paired analyses. We aimed to conduct subgroup analyses based on the age group of participants, how long they received humidification, type of HME and whether ventilation was delivered through a tracheostomy. We also conducted sensitivity analysis to identify whether the quality of trials had an effect on meta‐analytic findings.
Main results
We included 34 trials with 2848 participants; 26 studies were parallel‐group design (2725 participants) and eight used a cross‐over design (123 participants). Only three included studies reported data for infants or children. Two further studies (76 participants) are awaiting classification.
There was no overall statistical difference in artificial airway occlusion (RR 1.59, 95% CI 0.60 to 4.19; participants = 2171; studies = 15; I2 = 54%), mortality (RR 1.03, 95% CI 0.89 to 1.20; participants = 1951; studies = 12; I2 = 0%) or pneumonia (RR 0.93, 95% CI 0.73 to 1.19; participants = 2251; studies = 13; I2 = 27%). There was some evidence that hydrophobic HMEs may reduce the risk of pneumonia compared to HHs (RR 0.48, 95% CI 0.28 to 0.82; participants = 469; studies = 3; I2 = 0%)..
The overall GRADE quality of evidence was low. Although the overall methodological risk of bias was generally unclear for selection and detection bias and low risk for follow‐up, the selection of study participants who were considered suitable for HME and in some studies removing participants from the HME group made the findings of this review difficult to generalize.
Authors' conclusions
The available evidence suggests no difference between HMEs and HHs on the primary outcomes of airway blockages, pneumonia and mortality. However, the overall low quality of this evidence makes it difficult to be confident about these findings. Further research is needed to compare HMEs to HHs, particularly in paediatric and neonatal populations, but research is also needed to more effectively compare different types of HME to each other as well as different types of HH.
Keywords: Adolescent; Adult; Child; Child, Preschool; Humans; Infant; Infant, Newborn; Young Adult; Humidity; Respiration, Artificial; Respiration, Artificial/adverse effects; Steam; Cross‐Over Studies; Heating; Heating/instrumentation; Pneumonia; Pneumonia/etiology; Randomized Controlled Trials as Topic
Plain language summary
Heat and moisture exchangers compared to heated humidifiers for ventilated adults and children
Review question
Are heat and moisture exchangers or heated humidifiers more effective in preventing complications such as airway blockages and pneumonia in adults, children or infants who receive invasive mechanical ventilation.
Background
When mechanical ventilation is used to keep critically ill people breathing effectively, the upper airway must be humidified by artificial means. Heat and moisture exchangers and heated humidifiers are the most commonly used methods of artificial humidification. Both have been associated with specific advantages and disadvantages; for example, heat and moisture exchangers are thought to be more likely to cause airway obstruction while heated humidifiers have been associated with an increased risk of pneumonia (swelling (inflammation) of the tissue in one or both lungs).
Study characteristics
We searched for studies up to May 2017. We included 34 trials in the review, with 2848 participants from 12 countries. The majority of trials (27) were set in an intensive care unit with one in a neonatal intensive care unit. The remaining seven studies were done in an operating department. Participants were infants in three studies with adults (average age of 40 to 69 years) in the remainder.
Key results
There was no overall difference in the rates of airway blockage, pneumonia or death in adults who were ventilated through heat and moisture exchangers compared to adults ventilated through a heated humidifier. There was some evidence that the occurrence of pneumonia may be lowered by using heat and moisture exchangers that capture less moisture. There was not enough information to make any conclusions about either of these methods in children or infants.
Quality of the evidence
The overall low quality of this evidence was low, making it difficult to be confident about these findings.
Summary of findings
Summary of findings for the main comparison. Heat and moisture exchangers (HME) compared to heated humidifiers (HH) for ventilated adults and children.
| Heat and moisture exchangers (HME) compared to heated humidifiers (HH) for ventilated adults and children | ||||||
|
Patient or population: ventilated adults (18 trials) and children (1 trial) Settings: ICUs (17), NICU (1), and hospitals (1) in France (7), USA (3), Australia (2), Brazil (2), Denmark (1), Italy (1), Saudi Arabia (1), Spain (1), Switzerland (1) Intervention: HME Comparison: HH | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| HH | HME | |||||
|
Artificial airway occlusion (measured over 3‐15 days (median 4 days)) |
23 per 1000 | 37 per 1000 | RR 1.59 (0.6 to 4.19) | 2171 (15 studies) | ⊕⊕⊝⊝ 1 L ow |
Allocation and blinding unclear in 13 studies; moderate heterogeneity. |
|
Mortality ‐ all cause (Measured over 3‐15 days (median 8 days)) |
247 per 1000 | 257 per 1000 | RR 1.03 (0.89 to 1.20) | 1951 (12 studies) | ⊕⊕⊝⊝ 2 L ow |
Allocation and blinding unclear in 9 and 11 studies; low heterogeneity. |
|
Pneumonia (Measured over 4‐21 days (median 4 days)) |
32 per 1000 | 30 per 1000 | RR 0.93 (0.73 to 1.19) | 2251 (13 studies) | ⊕⊕⊝⊝ 3 L ow |
Allocation and blinding unclear in more than half of studies; moderate heterogeneity though this was due to only 1 study. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: 95% confidence interval; HH: heated humidification; HME: heat and moisture exchanger; ICU: intensive care unit; NICU: neonatal intensive care unit; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
The assumed and corresponding risks were calculated from data in included trials.
1 Quality downgraded two levels for serious indirectness because participants may not have been considered suitable for HME in three studies and could be taken out of the HME group in three studies.
2 Quality downgraded two levels for serious indirectness because participants may not have been considered suitable for HME in two studies and could be taken out of the HME group in three studies.
3 Quality downgraded two levels for serious indirectness because participants may not have been considered suitable for HME in two studies and could be taken out of the HME group in three studies.
Background
Description of the condition
Mechanical ventilation is used to assist or replace breathing when a person is unable to breathe adequately on their own (AARC 2012). Mechanical ventilation is provided through an endotracheal tube (ETT) inserted into the trachea through the mouth or nose, or directly into the trachea through a surgical incision known as a tracheostomy and now more commonly through a technique known as percutaneous dilatational tracheostomy which can be conducted at the bedside using a guidewire and tracheal inflation (Mehta 2017).
Because the upper airway is bypassed during mechanical ventilation, the respiratory system is no longer able to warm and moisten inhaled gases (Al Ashry 2014; Schiffmann 2006). The consequent delivery of cooler and dryer gases can cause a range of problems including decreased body temperature, increased difficulty in breathing and airway obstruction in people who already require assisted breathing (AARC 2012). To prevent these problems, humidification is routinely provided for people who receive invasive mechanical ventilation (Branson 2007).
Description of the intervention
The two main forms of humidification used during mechanical ventilation are active or passive.
Active humidification is provided by a heated humidifier (HH), which warms and moistens gases as they pass over the surface of a heated water reservoir attached to the ventilator. The system may also have a heated wire in the inspiratory limb of the ventilator circuit to prevent the warmed air cooling and condensing as it moves from the reservoir to the person (Al Ashry 2014).
Inhaled gases may also be passively humidified with a heat and moisture exchanger (HME). This device contains a condenser, which retains heat and moisture from every exhaled breath and returns it back to the person in the inspired breath (Al Ashry 2014). Because there are concerns that the performance of HMEs decrease with prolonged use, most manufacturers recommend changing HMEs every 24 hours (AARC 2012).
The type of condenser used in an HME varies. They can be hydrophobic, hygroscopic or combined condensers (AARC 2012; Al Ashry 2014). In hydrophobic HMEs, the condenser is made of a water repelling element. Hygroscopic condensers are based on salts (such as calcium or lithium chloride) which absorb water vapour during expiration and release it during inspiration. In combination HMEs, a hygroscopic salt is added inside the hydrophobic HME.
HMEs may have filters which prevent viruses or bacteria from inspired air reaching the person's airway (Al Ashry 2014).
An active heated water source can be added to HMEs converting them from passive to active, increasing their humidification capacity. Another active HME model releases heat through chemical reactions with exhaled carbon dioxide (AARC 2012).
Why it is important to do this review
Ventilator‐associated pneumonia (VAP) is a major source of morbidity and mortality in people who receive invasive mechanical ventilation (Lorente 2010). It increases hospital stay by a mean of seven to nine days per person and is associated with an "attributable mortality" of 33% to 50% (ATS 2005; Koenig 2006). VAP accounts for up to 25% of all intensive care unit (ICU) infections (ATS 2005; Koenig 2006; Kola 2005). The high level of bacterial colonization associated with HHs means they may be considered a greater risk for VAP than HME (Al Ashry 2014; Koenig 2006; Ricard 2006).
HMEs may be more cost effective than HHs in people without contraindications for their use (Dodek 2004; Lorente 2010; Ricard 2006), but are also associated with complications. Because HMEs are thought to provide lower levels of humidification than HHs and increase inspiratory and expiratory airway resistance (Al Ashry 2014; Rathgeber 2006), they are associated with increased rates of airway occlusion (AARC 2012; Hess 2003; Koenig 2006). Thus, they are not recommended in people with thick, copious secretions, pulmonary trauma or inflammation (AARC 2012; Branson 2007; Rathgeber 2006). HMEs may also be inappropriate for children and babies as their smaller airways may mean they are at a greater risk of airway occlusion (Rathgeber 2006; Schiffmann 2006). In addition, because HMEs require heat retention to provide effective warming of inspired gases, they can be contraindicated for people with hypothermia particularly children and babies who are more susceptible to heat loss (AARC 2012). There may, however, be differences in risk and benefit between different types of HME. Hydrophobic HMEs may be more likely to cause ETT occlusion compared to HMEs with a hygroscopic element (AARC 2012; Al Ashry 2014).
Although humidification for mechanically ventilated people is widely accepted as an essential practice, there is a lack of consensus over which method of humidification is preferable. In their review of VAP, Lorente and colleagues commented that while new evidence‐based guidelines for prevention of VAP had been published in the previous two years by American, Canadian and European scientific societies, recommendations on whether HMEs or HHs should be used were not consistent (Lorente 2010).
Our original systematic review of 33 trials, with 2833 participants of all randomized controlled trials (RCTs) comparing the use of HH to HME in people undergoing invasive mechanical ventilation found no clear evidence that either method was less likely to be associated with adverse events (Kelly 2010a; Kelly 2010b). This review update included the relevant literature from 2010 to 2015.
Objectives
To assess whether heat and moisture exchangers or heated humidifiers are more effective in preventing complications in people receiving invasive mechanical ventilation and to identify whether the age group of participants, length of humidification, type of HME, and ventilation delivered through a tracheostomy had an effect on these findings.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs comparing HMEs to HH in adults and children undergoing invasive mechanical ventilation. We included cross‐over studies if the order of the device was randomized.
Types of participants
We included children (aged 0 to 16 years) and adults (aged over 16 years) who were receiving invasive mechanical ventilation in any setting.
We imposed no any exclusion criteria.
Types of interventions
Comparison of HME and HH:
any type of HME (e.g. hygroscopic, hydrophobic or combination);
any model of HH.
Types of outcome measures
Primary outcomes
Artificial airway occlusion.
Mortality (all‐cause and related to respiratory events).
Pneumonia (all‐cause, nosocomial and ventilator‐associated).
Secondary outcomes
Respiratory complications, as defined by study authors and including: hypoxaemia, hypercapnia, aspiration from any cause, aspiration due to condensate in ventilator circuit.
Respiratory measures including: partial pressure of arterial oxygen (PaO2), partial pressure of arterial carbon dioxide (PaCO2), breathing rate, work of breathing, tidal volume or minute ventilation.
Secretion clearance or inspissated (thickened or congealed) mucous.
Change in body temperature.
Length of stay: ICU, hospital.
Supplemental humidification with nebulized or directly instilled saline.
Cost of devices.
Quality of life measures.
Search methods for identification of studies
Electronic searches
Before beginning this review, we searched the Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews of Effectiveness (DARE) to identify whether any relevant systematic reviews already existed.
We searched the current issue of the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 5); MEDLINE, OvidSP (1966 to 30 May 2017); Embase, OvidSP (1980 to 30 May 2017); and CINAHL, EBSCO host (1982 to 30 May 2017) for trials.
The CENTRAL, MEDLINE, Embase and CINAHL search strategies used are detailed in Appendix 1.
Methodology filter
A modified version of the first two phases of the Cochrane RCT search strategy was used to identify RCTs and controlled clinical trials. We combined the population and intervention terms, as outlined above, with the methodology terms. Where duplicate publication occurred, we used the publication with the most data as the primary publication for the review.
Searching other resources
We checked reference lists of included studies and relevant reviews for other potentially relevant studies which may not have been identified by the electronic search.
Non‐English language articles were eligible for selection to reduce the risk of publication bias. We had the papers translated or had data extraction performed by translators identified within Cochrane (see Acknowledgements).
Data collection and analysis
Selection of studies
Two authors (of DG, DT, JF, BB) independently examined the citations retrieved by the search strategy and retrieved those reports thought to fulfil the selection criteria in full. Where a judgement could not be made, based on the citation alone, we obtained the full article. We resolved differences by consensus.
We excluded studies if there was no report of randomization in the study design. The definition of 'mechanically ventilated' was taken to be invasive ventilation via an ETT or tracheostomy. We excluded studies involving participants undergoing non‐invasive ventilation, such as mask continuous positive airway pressure or participants self‐ventilating via a tracheostomy or a T‐tube.
Data extraction and management
We devised a standard data extraction form and piloted the form on a mixed set of articles. Two authors (DG, DT, JF, BB) independently extracted the data from each study and each pair met to compare the data. We resolved differences by consensus. In the case of papers written in a language other than English, data were extracted by Cochrane identified translators. Where data was missing or further information was required, we made all reasonable attempts to contact the authors to obtain the required information. We extracted the following information:
country and setting where study was performed;
inclusion and exclusion criteria;
participant characteristics: age group, gender, diagnosis, severity of illness;
details of interventions;
outcomes measured;
duration of study;
numbers enrolled and completing in each group;
baseline characteristics of each group;
results per group.
Assessment of risk of bias in included studies
We independently assessed trials as 'low,' 'high' or 'unclear' risk of bias according to the following quality criteria (Higgins 2011).
Random sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other identified or potential bias.
If there was any disagreement about whether or not a trial fulfilled a particular quality criterion, we resolved the differences by consensus or referral to a third member of the review team.
Measures of treatment effect
Dichotomous data
For meta‐analyses of dichotomous data, we calculated the risk ratio (RR) and 95% confidence interval (95% CI) using a random‐effects model. If data were only reported by a single study, these data were also calculated and reported but a forest plot was not generated.
Continuous data
For continuous outcomes, we calculated the mean difference (MD) and corresponding 95% CI using a random‐effects model.
As a meta‐analysis is based on assumptions of normality, all continuous data were checked for skew before inclusion. Data were considered substantially skewed if the standard deviation was greater than the mean (Higgins 2011). If data were substantially skewed, they were not included in meta‐analyses and were reported separately.
We calculated the standardized mean difference (SMD) and 95% CI rather than MD for saline instillations as two different units of measurement (i.e. volume or number) were used.
Unit of analysis issues
Cross‐over trials
Since the results in a cross‐over trial are based on paired data, the meta‐analysis of cross‐over trials used the MD and standard error (SE) of the mean difference (SE (MD)) from each trial. Only one study provided adequate information to derive the SE (MD) for the outcome PaCO2 (MacIntyre 1983). Therefore, we calculated the results of the cross‐over studies based on low (0.3), moderate (0.5) and high (0.7) estimates of correlation. Meta‐analyses using each of the different correlation values are reported for each outcome.
Dealing with missing data
If information about study methods or adequate data to be used in meta‐analysis were not reported, we attempted to contact study authors to obtain this information.
Assessment of heterogeneity
We used the I2 statistic to assess heterogeneity (Higgins 2011). The I2 statistic lies between 0% and 100%, with larger values being indicative of increasing heterogeneity. Higgins 2011 suggested assigning low, moderate and high heterogeneity to I2 statistical values of 25%, 50% and 75%. Based on this, we considered that there was evidence of substantial heterogeneity when the I2 statistic was greater than 50%.
Assessment of reporting biases
We entered the primary outcome data from all included studies into a funnel plot (trial effect against trial size) to investigate the possibility of publication bias (Higgins 2011).
The quality of the included studies was assessed independently by two authors (DG, DT, JF, BB) without blinding to authorship or journal of publication. We resolved differences in the authors' allocation of studies into quality categories by consensus.
Data synthesis
Where cluster randomized trials were identified, we planned to make appropriate corrections for the cluster design if appropriate data had been reported and these data incorporated into the meta‐analyses. However, no data which could be used to make these corrections were reported in included studies.
If parallel studies reported data for more than one group for each intervention (e.g. two ventilation pressures for both HME and HH), we calculated pooled means and SDs and used them in the meta‐analysis. In three of the four studies that used more than one type of HME or HH, data were given separately for each type of intervention. Therefore, we pooled data for HME or HH in these studies (Luchetti 1998; Ricard 1999; Villafane 1996).
As there is no method for pooling mean and variance data from cross‐over studies, data were used from only one of the two groups of participants receiving each intervention in Campbell 2000 and Girault 2003. In the study by Campbell 2000, we used the data reported for people who were chemically paralysed and sedated rather than those who were breathing spontaneously as this was more consistent with the other included studies. Girault 2003 reported data for people receiving two different levels of pressure support ventilation so we used data from the group receiving ventilation at a pressure of 15 cm H2O as this was similar to what was used in the other included studies.
Subgroup analysis and investigation of heterogeneity
As adults and children have different respiratory anatomy and physiology, we decided a priori to perform subgroup analysis by age group if there were adequate data. Since the anatomical and physiological differences are even more pronounced in preterm and term neonates, the age group categories were:
neonates: term and preterm infants up to 28 days of age (or corrected age for preterm infants). Preterm infants have a gestational age of less than 37 weeks while term infants have a gestational age of 37 weeks or greater;
infants and children, aged 28 days to 16 years; and
adults, aged over 16 years.
As respiratory complications and damage are linked to the length of time a person is intubated and the duration of mechanical ventilation, subgroup analysis by the duration of the experimental humidification was conducted. These were:
ultra‐short humidification: up to 12 hours;
short‐term humidification: from 12 to 48 hours;
medium‐term humidification: from 48 hours to seven days;
long‐term humidification: more than seven days.
We conducted subgroup analyses based on whether the HME used was:
hydrophobic;
hygroscopic;
combined hydrophobic/hygroscopic.
Finally, a subgroup analysis was planned by artificial airway:
ETT;
tracheostomy.
conditional on whether sufficient studies were available.
Sensitivity analysis
We conducted sensitivity analysis based on whether studies were rated as a low, unclear or high risk of selection and detection bias.
Therefore, the sensitivity analyses were as follows.
Selection bias: low versus unclear versus high risk.
Detection bias: low versus unclear versus high risk.
'Summary of findings' table and GRADE
We produced a 'Summary of findings' table for the primary outcomes of artificial airway occlusion, all‐cause mortality and pneumonia when HME was compared to HH using GRADEpro software (GRADEpro). Baseline risk was based on the risk rates calculated by GRADEpro from included studies. We used the GRADE system to assess the quality of studies and data contributing to these analyses (Guyatt 2008). The GRADE approach gives an assessment of within‐study risk of bias (methodological quality), that may affect the directness of the evidence, heterogeneity of the data, precision of the effect estimates and potential for publication bias. For assessments of the overall quality of evidence for each outcome, we downgraded the evidence from 'high quality' by one level for single or two for multiple risks of bias.
Results
Description of studies
Results of the search
(Figure 1)
1.
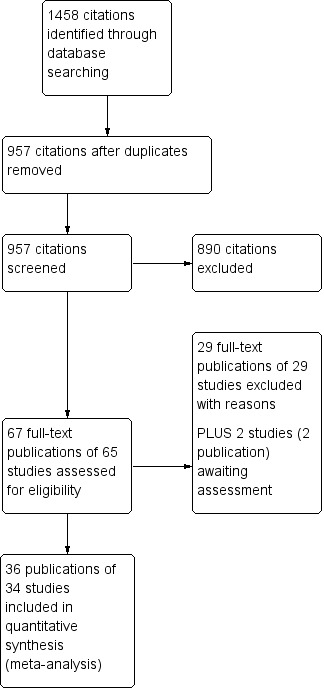
Study flow diagram.
In total, we identified 1458 citations using the search strategies detailed in Appendix 1. After the removal of duplicates, 957 citations remained. After title and abstract review, 65 of these studies (67 citations) were considered potentially relevant. Following data extraction, we included 34 studies (36 citations) and excluded 29 (29 citations). Two studies (76 participants) are awaiting classification, one by Nadir Oziş 2009 until data can be obtained from authors and one by Oguz 2013 identified from a recently published review (Vargas 2017).
Included studies
We included 34 trials in the review, with 2848 participants completing the trials (see Characteristics of included studies table). Thirteen of the trials were conducted in France (Daoud 1991; Deriaz 1992; Dreyfuss 1995; Girault 2003; Lacherade 2005; Le Bourdelles 1996; Martin 1990; Martin 1994; Misset 1991; Ricard 1999; Roustan 1992; Thomachot 2001; Villafane 1996); six in the USA (Branson 1996; Campbell 2000; Goldberg 1992; Kirton 1997; Kollef 1998; MacIntyre 1983); three in Italy (Iotti 1997; Luchetti 1998; Pelosi 1996); two each in Australia (Boots 1997; Boots 2006), Canada (Bissonnette 1989a; Bissonnette 1989b); and Brazil (Alcoforado 2012; Diaz 2002); and one each in Saudi Arabia (Memish 2001), Denmark (Kirkegaard 1987), Finland (Linko 1984), Spain (Lorente 2006), Switzerland (Hurni 1997), and the UK (Yam 1990). Seven studies were performed in the operating department (Bissonnette 1989a; Bissonnette 1989b; Deriaz 1992; Goldberg 1992; Kirkegaard 1987; Le Bourdelles 1996; Yam 1990); the remaining 27 studies were set in an ICU, one of these in a neonatal ICU (Daoud 1991). Three studies were conducted on children and infants (Bissonnette 1989a; Bissonnette 1989b; Daoud 1991). Bissonnette 1989a reported data from infants weighing between 5 kg and 10 kg; Bissonnette 1989b reported data from infants and children weighing between 5 kg and 30 kg; and Daoud 1991 reported data from a neonatal population. The remaining studies were conducted in adults; although participants in these trials were aged 15 to 95 years, the mean age was 40 to 69 years).
Twenty‐six studies used a parallel‐group design in which at least two independent groups were studied (Alcoforado 2012; Bissonnette 1989a; Bissonnette 1989b; Boots 1997; Boots 2006; Branson 1996; Daoud 1991; Deriaz 1992; Diaz 2002; Dreyfuss 1995; Goldberg 1992; Hurni 1997; Kirkegaard 1987; Kirton 1997; Kollef 1998; Lacherade 2005; Linko 1984; Lorente 2006; Luchetti 1998; Martin 1990; Memish 2001; Misset 1991; Ricard 1999; Roustan 1992; Villafane 1996; Yam 1990). Eight studies used a cross‐over design whereby two interventions were studied in the same group of participants (Campbell 2000; Girault 2003; Iotti 1997; Le Bourdelles 1996; MacIntyre 1983; Martin 1994; Pelosi 1996; Thomachot 2001). In each of the cross‐over studies, the order of the intervention (HH or HME) was randomized. Twenty‐nine of the included studies compared one type of HME to one type of HH (Alcoforado 2012; Bissonnette 1989a; Bissonnette 1989b; Boots 1997; Branson 1996; Campbell 2000; Daoud 1991; Deriaz 1992; Diaz 2002; Dreyfuss 1995; Girault 2003; Goldberg 1992; Hurni 1997; Iotti 1997; Kirkegaard 1987; Kirton 1997; Kollef 1998;Lacherade 2005; Le Bourdelles 1996; Linko 1984; Lorente 2006; MacIntyre 1983; Martin 1990; Martin 1994; Memish 2001; Pelosi 1996; Roustan 1992; Thomachot 2001; Yam 1990). Four parallel studies used more than one type of HH or HME. Two of these studies compared one HME to two HHs (Luchetti 1998; Misset 1991); one compared two HMEs to one HH (Villafane 1996); and one compared four HMEs to one HH (Ricard 1999).
Across the 34 included studies, the outcomes reported were: artificial airway occlusion, mortality, pneumonia, respiratory complications, ventilation, change in body temperature, length of stay, secretion clearance, supplemental humidification and cost. None of the included studies reported quality of life.
Excluded studies
We excluded 29 studies because participants were not undergoing invasive mechanical ventilation, there was no randomization, no relevant clinical outcomes, HME was used in conjunction with an HH, or data could not be obtained from study authors (see Characteristics of excluded studies table).
Studies awaiting classification
Two studies (76 participants) are awaiting classification. One study by Nadir Oziş 2009 is awaiting classification as we have not yet been able to obtain additional information including outcome data from the study authors. The second study (Oguz 2013) was identified from a recently published review (Vargas 2017). See Characteristics of studies awaiting classification table for further information.
Ongoing studies
We identified no ongoing studies.
Risk of bias in included studies
We rated all of the studies for risk of bias in six domains: random sequence generation and allocation concealment (both selection bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias) and other bias (see Figure 2, Figure 3; Characteristics of included studies table). Seven studies had a low risk of bias for one domain (Bissonnette 1989b; Hurni 1997; Kirkegaard 1987; Linko 1984; Luchetti 1998; Martin 1994; Villafane 1996), 13 studies for two domains (Alcoforado 2012; Bissonnette 1989a; Boots 2006; Branson 1996; Daoud 1991; Dreyfuss 1995; Lorente 2006; MacIntyre 1983; Martin 1990; Ricard 1999; Roustan 1992; Thomachot 2001; Yam 1990), nine studies for three domains (Boots 1997; Campbell 2000; Girault 2003; Iotti 1997; Kirton 1997; Lacherade 2005; Le Bourdelles 1996; Memish 2001; Pelosi 1996), and two studies for four domains (Diaz 2002; Kollef 1998). Two studies were not at low risk of bas for any domain (Deriaz 1992; Misset 1991). Fourteen studies were at high risk of bias for one domain (Alcoforado 2012; Boots 2006; Deriaz 1992; Dreyfuss 1995; Hurni 1997; Kirkegaard 1987; Lacherade 2005; Luchetti 1998; Martin 1990; Martin 1994; Memish 2001; Roustan 1992; Thomachot 2001; Villafane 1996) and three studies in two domains (Branson 1996; Lorente 2006; Misset 1991).
2.
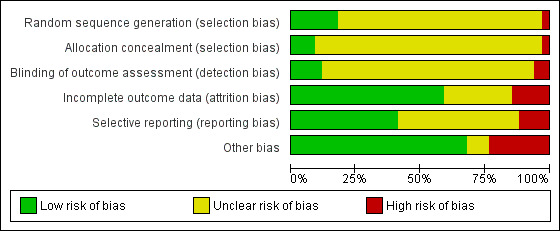
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
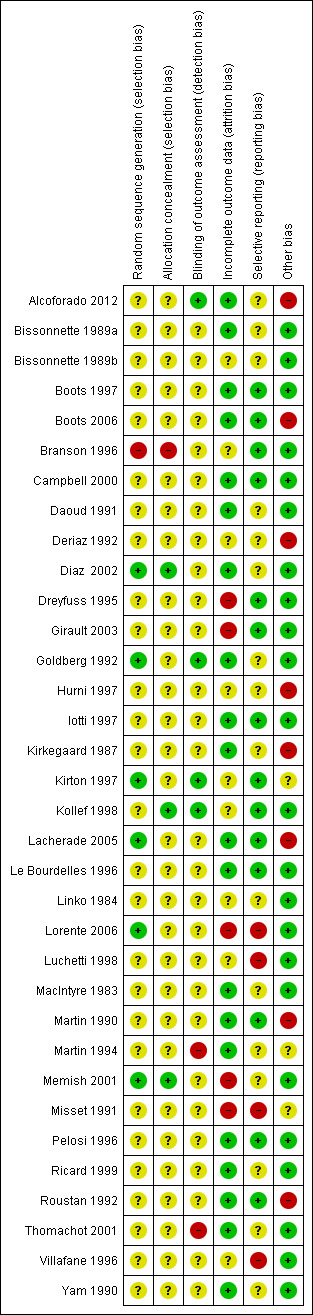
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Six studies described adequate generation of randomization sequences (Diaz 2002; Goldberg 1992; Kirton 1997; Lacherade 2005; Lorente 2006; Memish 2001), while it was inadequate in one study (Branson 1996). The remainder did not describe sequence generation. Three trials had adequate allocation concealment (Diaz 2002; Kollef 1998; Memish 2001), and one had inadequate allocation concealment (Branson 1996); 30 trials did not explain the allocation process.
Blinding
Four trials reported that outcome assessment was blinded (Alcoforado 2012; Goldberg 1992; Kirton 1997; Kollef 1998), while two stated that it was not blinded (Martin 1994; Thomachot 2001). Most studies contained no information about blinding.
Incomplete outcome data
Most studies were at low risk of attrition bias because they appeared to have had 100% follow‐up (Alcoforado 2012; Bissonnette 1989a; Boots 1997; Boots 2006; Campbell 2000; Diaz 2002; Goldberg 1992; Iotti 1997; Kirkegaard 1987; Lacherade 2005; Le Bourdelles 1996; MacIntyre 1983; Martin 1990; Martin 1994; Pelosi 1996; Ricard 1999; Roustan 1992; Thomachot 2001; Yam 1990). Five trials were at high risk of attrition bias (Dreyfuss 1995; Girault 2003; Lorente 2006; Memish 2001; Misset 1991). The risk due to attrition was unclear in the remaining studies.
Selective reporting
The majority of studies were at low risk of selective reporting because all expected primary outcomes were reported (Boots 1997; Boots 2006; Branson 1996; Campbell 2000; Dreyfuss 1995; Girault 2003; Iotti 1997; Kirton 1997; Kollef 1998; Lacherade 2005; Le Bourdelles 1996; Martin 1990; Pelosi 1996; Roustan 1992). Four studies were at high risk of reporting bias because they were all long‐term studies that did not report at least one of the expected outcome of pneumonia or mortality (Lorente 2006; Luchetti 1998; Misset 1991; Villafane 1996). The remainder were at unclear risk of bias.
Other potential sources of bias
Six studies were at high risk of other bias. In Alcoforado 2012, the mean time in hospital was much longer in the HH group (29 days versus 9 days) although the median duration of ventilation was shorter (161 hours versus 201 hours), and 100% of the HH group received antibiotics compared to 87.5% in the HME group. Participants in the HME group were ventilated for a significantly shorter period (six days versus eight days) in Boots 2006; the HME group were anaesthetized for significantly longer than the HH group (155 minutes versus 116 minutes) in Deriaz 1992; circuits were changed every 48 hours in the HH group and only every seven days in the HME group in Hurni 1997; in Lacherade 2005, there were five times as many participants in the HH group who had HIV and this group also had higher partial pressure arterial oxygen/fraction of inspired oxygen (PaO2/FiO2) values; and in Martin 1990 the HH group remained in the study for a mean of 14 days compared to 10 days in the HME group.
Four studies were funded by the makers of the HME (Kollef 1998) or HH (Branson 1996; Lacherade 2005), or both HME and HH (Boots 2006). The maker supplied the HME in Yam 1990.
Effects of interventions
See: Table 1
Data from parallel studies
(See Analysis 1.1; Analysis 1.2; Table 2; Table 3.)
1.1. Analysis.
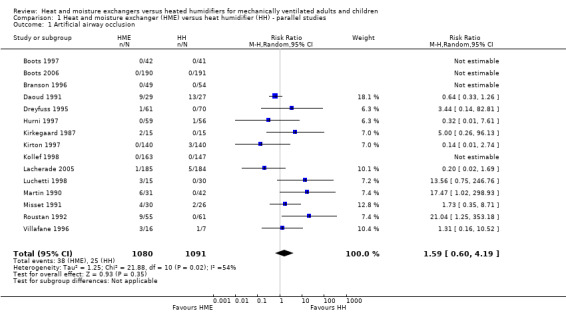
Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 1 Artificial airway occlusion.
1.2. Analysis.
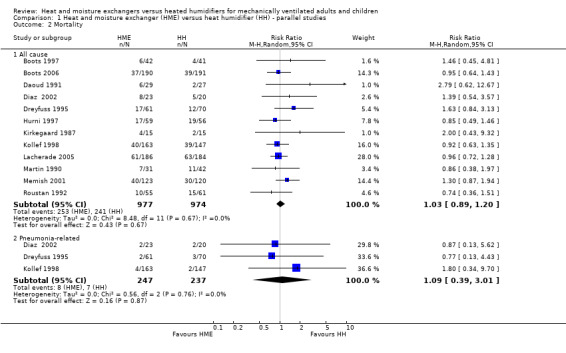
Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 2 Mortality.
1. Length of stay ‐ intensive care unit (mean days).
| Study | HH | HME | No of participants |
| Boots 2006 | 9 | 7 | 381 |
| Diaz 2002 | 4 | 4 | 43 |
| Hurni 1997 | 13.80 | 11.10 | 104 |
| Kollef 1998 | 5.30 | 5.70 | 310 |
| Lacherade 2005 | 25.3 | 21.4 | 369 |
| Roustan 1992 | 9.30 | 13.90 | 116 |
HH: heated humidification; HME: heat and moisture exchangers.
2. Length of stay ‐ hospital (mean days).
| Study | HH | HME | No of participants |
| Diaz 2002 | 11 | 10 | 43 |
| Kollef 1998 | 16.50 | 16.50 | 310 |
HH: heated humidification; HME: heat and moisture exchangers.
Data for the binary outcomes of artificial airway occlusion, mortality, pneumonia, atelectasis and pneumothorax came from the 26 parallel‐group studies (Alcoforado 2012; Bissonnette 1989a; Bissonnette 1989b; Boots 1997; Boots 2006; Branson 1996; Daoud 1991; Deriaz 1992; Diaz 2002; Dreyfuss 1995; Goldberg 1992; Hurni 1997; Kirkegaard 1987; Kirton 1997; Kollef 1998; Lacherade 2005; Linko 1984; Lorente 2006; Luchetti 1998; Martin 1990; Memish 2001; Misset 1991; Ricard 1999; Roustan 1992; Villafane 1996; Yam 1990). Data for the continuous outcomes of PaO2, PaCO2, tidal volume, minute ventilation, tracheal aspirations, number and volume of saline instillations, absolute and change in body temperature, length of stay (ICU and hospital) and cost also came from the parallel trials.
Primary outcomes
1. Artificial airway occlusion
Fifteen trials involving 2171 participants measured artificial airway occlusion (Boots 1997; Boots 2006; Branson 1996; Daoud 1991; Dreyfuss 1995; Hurni 1997; Kirkegaard 1987; Kirton 1997; Kollef 1998; Lacherade 2005; Luchetti 1998; Martin 1990; Misset 1991; Roustan 1992; Villafane 1996). There was no statistical difference between groups in the occurrence of artificial airway occlusion although there was substantial heterogeneity between studies (RR 1.59, 95% CI 0.60 to 4.19; I2 = 54%; Analysis 1.1).
2. Mortality
There was no statistically significant difference in all‐cause mortality (RR 1.03, 95% CI 0.89 to 1.20; 1951 participants; 12 studies; Boots 1997; Boots 2006; Daoud 1991; Diaz 2002; Dreyfuss 1995; Hurni 1997; Kirkegaard 1987; Kollef 1998; Lacherade 2005; Martin 1990; Memish 2001; Roustan 1992; I2 = 0%; Analysis 1.2) or pneumonia‐related mortality (RR 1.09, 95% CI 0.39 to 3.01; 484 participants; 3 studies; Diaz 2002; Dreyfuss 1995; Kollef 1998; I2 = 0%; Analysis 1.2).
3. Pneumonia
There was no statistically significant difference in the prevalence of pneumonia overall (RR 0.93, 95% CI 0.73 to 1.19; 2251 participants; 13 studies; Alcoforado 2012; Boots 1997; Boots 2006; Branson 1996; Diaz 2002; Dreyfuss 1995; Kirton 1997; Kollef 1998; Lacherade 2005; Lorente 2006; Martin 1990; Memish 2001; Roustan 1992; I2 = 27%; Analysis 1.3). Neither was there any difference when pneumonia was diagnosed at any time within the ventilation period (RR 0.94, 95% CI 0.69 to 1.27; 1090 participants; 7 studies; I2 = 0%; Analysis 1.3) or if the diagnosis was made at least 48 hours after ventilation was begun (RR 0.96, 95% CI 0.64 to 1.46; 1161 participants; 6 studies; I2 = 57%; Analysis 1.3), although there was substantial heterogeneity in this latter subgroup.
1.3. Analysis.
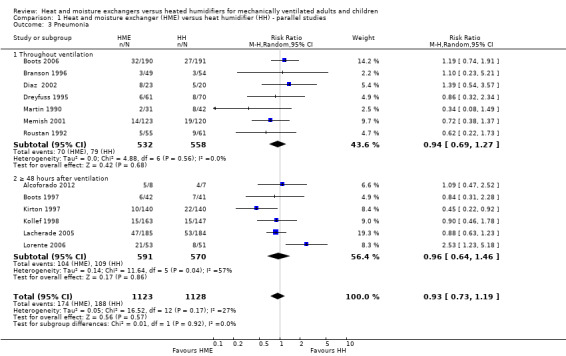
Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 3 Pneumonia.
Secondary outcomes
1. Respiratory complications
Atelectasis
There was no statistical difference between the two forms of humidification for atelectasis (RR 0.85, 95% CI 0.52 to 1.40; 303 participants; 3 studies; Daoud 1991; Dreyfuss 1995; Roustan 1992; I2 = 0%; Analysis 1.4).
1.4. Analysis.
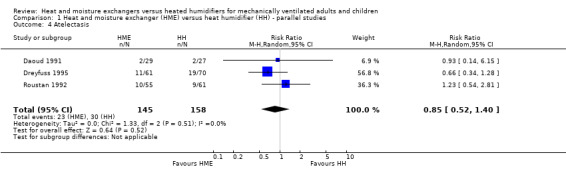
Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 4 Atelectasis.
Pneumothorax
There was no statistical difference between the HME and HH groups in the prevalence of pneumothorax in the one study that reported this outcome (RR 2.79, 95% CI 0.62 to 12.67; 56 participants; 1 study; Daoud 1991; P = 0.18).
2. Respiratory measures
Arterial pressure of oxygen
The PaO2 was significantly higher in the HME group compared with the HH group (MD 2.80 kPa, 95% CI 0.13 to 5.47; 30 participants; 1 study; Kirkegaard 1987; P = 0.04 ).
Arterial pressure of carbon dioxide
There was no statistical difference between groups in PaCO2 levels (MD ‐0.20 kPa, 95% CI ‐0.67 to 0.27; 30 participants; 1 study; Kirkegaard 1987; P = 0.40).
Tidal volume
There was no difference between the HME and HH groups in tidal volume (MD ‐0.03 L, 95% CI ‐38.81 to 38.75; 85 participants; 1 study; Ricard 1999; P = 1.00).
Minute ventilation
There was no difference between groups in minute ventilation (MD ‐0.70 L/minute, 95% CI ‐1.97 to 0.57; 85 participants; 1 study; Ricard 1999; P = 0.28).
3. Secretion clearance
Tracheal aspirations
There was no significant difference between groups in tracheal aspirations (MD ‐0.47 aspirations per day, 95% CI ‐1.41 to 0.47; 290 participants; 3 studies; Branson 1996; Dreyfuss 1995; Misset 1991; I2 = 64%; Analysis 1.5).
1.5. Analysis.
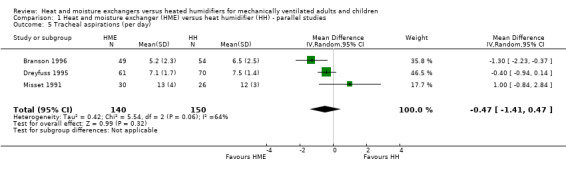
Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 5 Tracheal aspirations (per day).
Data from the study by Boots 1997, which measured the number of suctions per hour, could not be included in this meta‐analysis as there was no measure of variance reported.
Saline instillations
The number or volume of saline instillations per day was significantly lower in the HME group (SMD ‐0.40 instillations per day, 95% CI ‐0.64 to ‐0.17; 276 participants; 3 studies; Branson 1996; Dreyfuss 1995; Martin 1990; I2 = 0%; Analysis 1.6).
1.6. Analysis.
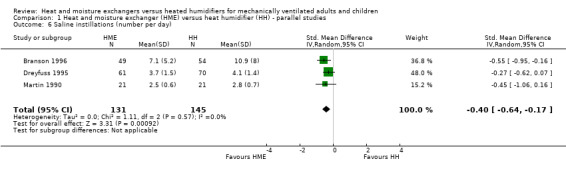
Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 6 Saline instillations (number per day).
4. Change in body temperature
Six parallel‐group studies reported absolute body temperature (Bissonnette 1989a; Branson 1996; Deriaz 1992; Goldberg 1992; Martin 1990; Yam 1990), and three studies reported change in body temperature (Branson 1996; Dreyfuss 1995; Martin 1990). Body temperature was significantly lower in the HME group when analysing absolute data (MD ‐0.49 ºC, 95% CI ‐0.96 to ‐0.02; 321 participants; I2 = 88% Analysis 1.7) and mean change data (MD ‐0.59 ºC, 95% CI ‐0.82 to ‐0.36; 78 participants; I2 = 8%; Analysis 1.8).
1.7. Analysis.
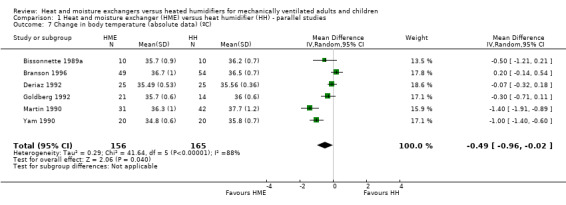
Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 7 Change in body temperature (absolute data) (ºC).
1.8. Analysis.
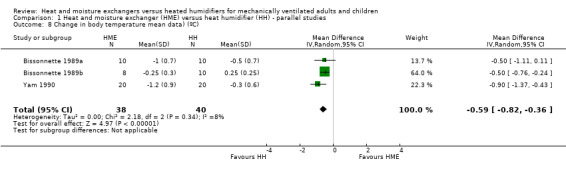
Comparison 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, Outcome 8 Change in body temperature mean data) (ºC).
5. Length of stay
Six studies reported ICU length of stay (Boots 2006; Diaz 2002; Hurni 1997; Kollef 1998; Lacherade 2005; Roustan 1992; 1323 participants) and two studies reported length of stay in hospital (Diaz 2002; Kollef 1998; 353 participants); however, all these data were skewed or the SDs were not reported and the data could not be added to a meta‐analysis. There was no consistent pattern in length of stay in either group (Table 2; Table 3).
6. Supplemental humidification
None of the studies reported supplemental humidification.
7. Cost of devices
Six studies reported cost data (Boots 1997; Boots 2006; Branson 1996; Dreyfuss 1995; Kirton 1997; Kollef 1998; 1284 participants). As a measure of variance was not available for these studies, these data could not be meta‐analysed. However, all studies reported lower costs for HMEs compared to HHs (Table 4).
3. Cost.
| Study | HME | HH | Units | Participants |
| Boots 1997 | 6.72 | 8.20 | AUD/day | 83 |
| Boots 2006 | 8.62 | 9.27 | AUD/day | 381 |
| Branson 1996 | 4.70 | 8.97 | USD/day | 99 |
| Dreyfuss 1995 | 5.00 | 11.00 | USD/day (France) | 131 |
| Kirton 1997 | 17.46 | 27.80 | USD/participant | 280 |
| Kollef 1998 | 15.98 | 38.26 | USD/participant | 310 |
HH: heated humidification; HME: heat and moisture exchangers.
8. Quality of life
None of the studies reported quality of life.
Data from cross‐over studies
Data for the continuous outcomes: SaO2, PaO2, PaCO2, breathing rate, tidal volume and minute ventilation came from the included cross‐over trials.
1. Respiratory complications
Arterial oxygen saturation
Only one cross‐over trial involving 11 participants reported SaO2 data (Campbell 2000). There was no significant difference between the HME and HH groups in the SaO2 at the low (MD ‐2.00, 95% CI ‐4.84 to 0.84; P = 0.17), moderate (MD ‐2.00, 95% CI ‐4.70 to 0.70; P = 0.15) or high correlation estimates (MD ‐2.00, 95% CI ‐4.57 to 0.57; P = 0.13).
2. Respiratory measures
Arterial pressure of oxygen
Four cross‐over trials involving 65 participants reported PaO2 (Campbell 2000; Girault 2003; Le Bourdelles 1996; MacIntyre 1983). There was no significant difference between groups at low (MD ‐3.24 mmHg, 95% CI ‐16.08 to 9.60; Analysis 2.1), moderate (MD ‐3.87 mmHg, 95% CI ‐16.73 to 9.00; Analysis 2.1) and high correlation estimates (MD ‐4.41 mmHg, 95% CI ‐17.09 to 8.27; P = 0.50; Analysis 2.1).
2.1. Analysis.
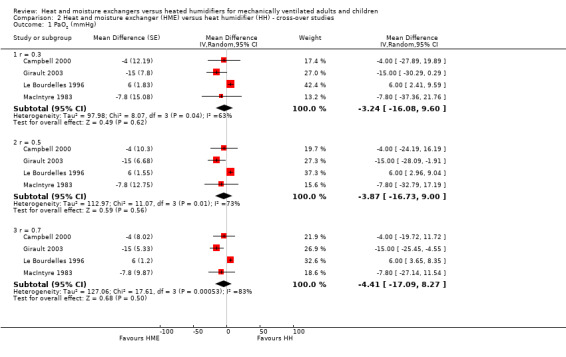
Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 1 PaO2 (mmHg).
Arterial pressure of carbon dioxide
Five cross‐over trials involving 88 participants reported PaCO2 (Campbell 2000; Girault 2003; Iotti 1997; Le Bourdelles 1996; MacIntyre 1983). The PaCO2 was significantly higher in the HME group at all correlation estimates: low (MD 1.93 mmHg, 95% CI 0.27 to 3.59; Analysis 2.2), moderate (MD 2.02 mmHg, 95% CI 0.19 to 3.85; Analysis 2.2) and high (MD 2.21 mmHg, 95% CI 0.33 to 4.09; Analysis 2.2).
2.2. Analysis.
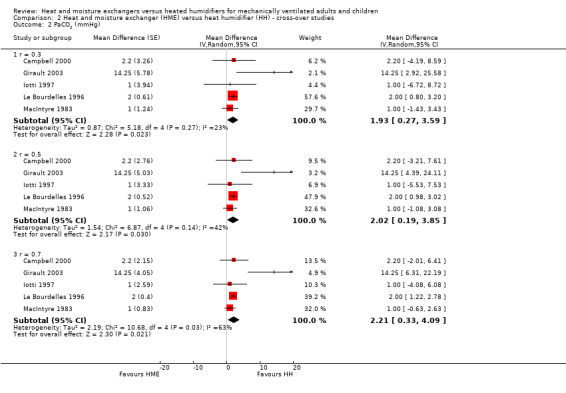
Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 2 PaCO2 (mmHg).
Breathing rate
Four cross‐over trials involving 65 participants reported breathing rate (Campbell 2000; Iotti 1997; Le Bourdelles 1996; Pelosi 1996). The breathing rate was significantly higher in the HME group at the low correlation estimate (MD 1.40 breaths per minute, 95% CI 0.33 to 2.46; Analysis 2.3), but there was no significant difference between groups at moderate (MD 1.15 breaths per minute, 95% CI ‐0.13 to 2.44; Analysis 2.3) or high (MD 1.02 breaths per minute, 95% CI ‐0.38 to 2.41; Analysis 2.3) correlation estimates.
2.3. Analysis.
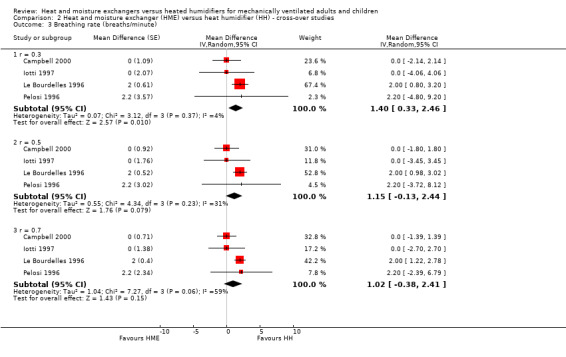
Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 3 Breathing rate (breaths/minute).
Work of breathing
Two studies involving 21 participants measured work of breathing (Girault 2003; Iotti 1997). However, as these data were skewed, they could not be meta‐analysed. Work of breathing was higher in the HME group in both studies (Table 5).
4. Work of breathing (joules/minute ‐ mean).
| Study | HH | HME | Participants |
| Girault 2003 | 9.86 | 16.50 | 11 |
| Iotti 1997 | 13.6 | 20.8 | 10 |
HH: heated humidification; HME: heat and moisture exchangers.
Tidal volume
Five studies involving 76 participants measured tidal volume (Campbell 2000; Girault 2003; Iotti 1997; Le Bourdelles 1996; Pelosi 1996). There was no difference between groups at the low (MD 0.02 L, 95% CI ‐0.00 to 0.03; Analysis 2.4) or moderate (MD 0.02 L, 95% CI 0.00 to 0.04; Analysis 2.4) correlation estimates, but tidal volume was significantly higher at the high correlation estimate (MD 0.03 L, 95% CI 0.01 to 0.06; Analysis 2.4).
2.4. Analysis.
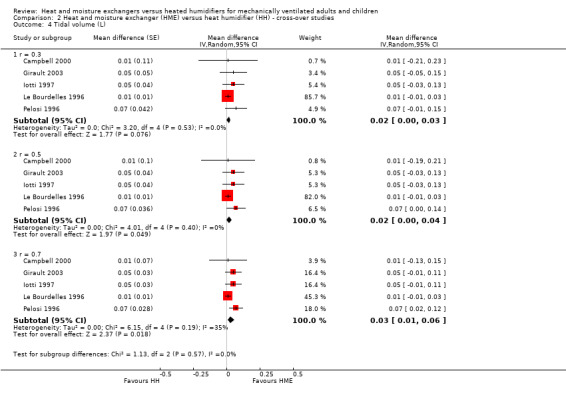
Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 4 Tidal volume (L).
Minute ventilation
The five cross‐over studies involving 76 participants measuring minute ventilation were the same studies as those measuring tidal volume (Campbell 2000; Girault 2003; Iotti 1997; Le Bourdelles 1996; Pelosi 1996). There was a significantly higher minute ventilation in the HME group at the low (MD 1.20 L/minute, 95% CI 0.78 to 1.61; Analysis 2.5), moderate (MD 1.19 L/minute, 95% CI 0.63 to 1.75; Analysis 2.5) and high (MD 1.18 L/minute, 95% CI 0.55 to 1.80 Analysis 2.5) correlation estimates.
2.5. Analysis.
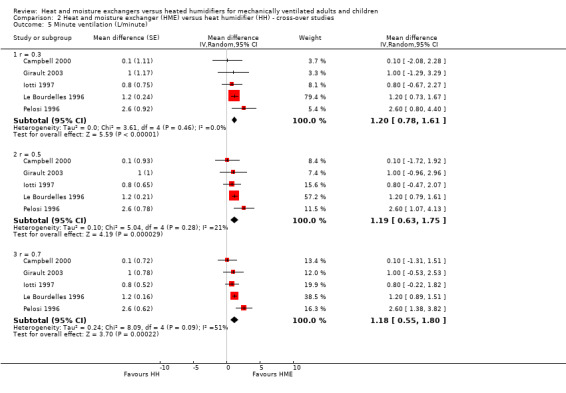
Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 5 Minute ventilation (L/minute).
3. Change in body temperature
Two cross‐over studies involving 21 participants reported body temperature (Martin 1994; Thomachot 2001). There was no difference between groups at low (MD ‐1.12 ºC, 95% CI ‐3.77 to 1.52; Analysis 2.6), moderate (MD ‐1.13 ºC, 95% CI ‐3.77 to 1.52; Analysis 2.6) or high (MD ‐1.13 ºC, 95% CI ‐3.78 to 1.51; Analysis 2.6) correlation estimates, but heterogeneity was very high, ranging from an I2 statistic of 96% to 98%.
2.6. Analysis.
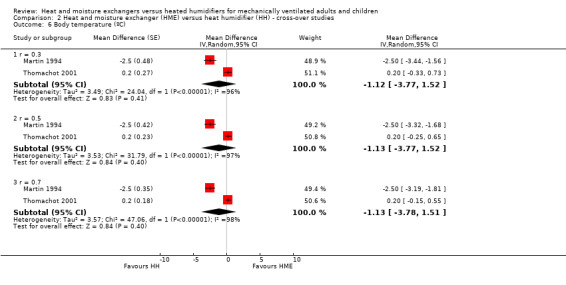
Comparison 2 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies, Outcome 6 Body temperature (ºC).
Subgroup analysis
Age group
Only two of the 34 included studies were carried out in children (Bissonnette 1989a; Bissonnette 1989b), and one was performed in the neonatal population (Daoud 1991). Daoud 1991 was the only study in infants or children which reported primary outcomes, namely: artificial airway occlusion and mortality. The remaining studies were all conducted in adults.
There was no difference between age groups for the outcomes of artificial airway occlusion (Analysis 3.1) or mortality (Analysis 3.2).
3.1. Analysis.
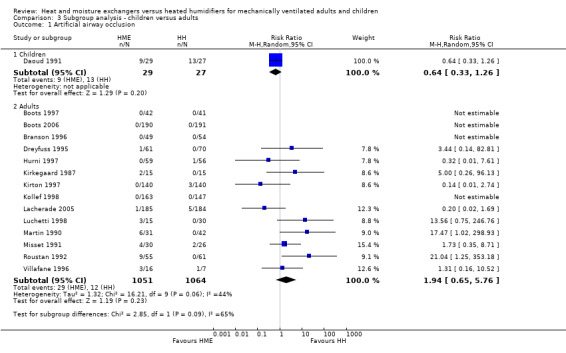
Comparison 3 Subgroup analysis ‐ children versus adults, Outcome 1 Artificial airway occlusion.
3.2. Analysis.
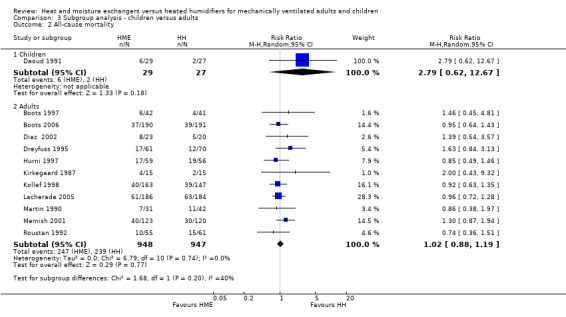
Comparison 3 Subgroup analysis ‐ children versus adults, Outcome 2 All‐cause mortality.
Length of ventilation
Twelve of the included studies compared methods of humidification that we categorized as ultra‐short term (up to 12 hours) (Bissonnette 1989a; Bissonnette 1989b; Campbell 2000; Deriaz 1992; Girault 2003; Goldberg 1992; Iotti 1997; Le Bourdelles 1996; Linko 1984; Pelosi 1996; Ricard 1999; Yam 1990). Three were short‐term humidification studies (12 to up to 48 hours) (MacIntyre 1983; Martin 1994; Thomachot 2001); nine were medium‐term (from 48 hours to seven days) (Alcoforado 2012; Boots 1997; Boots 2006; Branson 1996; Daoud 1991; Kirkegaard 1987; Kollef 1998; Luchetti 1998; Villafane 1996); and eight were long‐term (more than seven days) (Dreyfuss 1995; Hurni 1997; Lacherade 2005; Lorente 2006; Martin 1990; Memish 2001; Misset 1991; Roustan 1992). Two studies did not state the length of humidification (Diaz 2002; Kirton 1997).
Data were only available to compare the primary outcomes for medium and long‐term trials.
There was a no difference between medium‐term and long‐term studies for the outcome of artificial airway (Analysis 4.1).
4.1. Analysis.
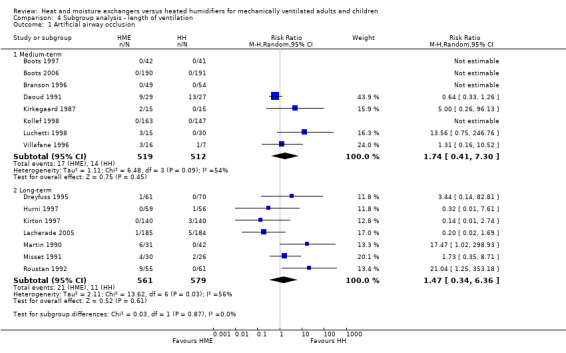
Comparison 4 Subgroup analysis ‐ length of ventilation, Outcome 1 Artificial airway occlusion.
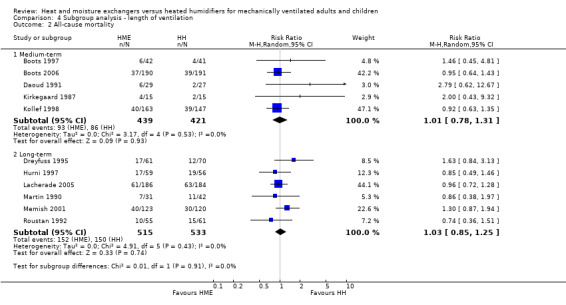
There was no apparent subgroup difference between medium‐term and long‐term studies in all‐cause mortality (Analysis 4.2) or pneumonia (Analysis 4.3).
4.2. Analysis.
Comparison 4 Subgroup analysis ‐ length of ventilation, Outcome 2 All‐cause mortality.
4.3. Analysis.
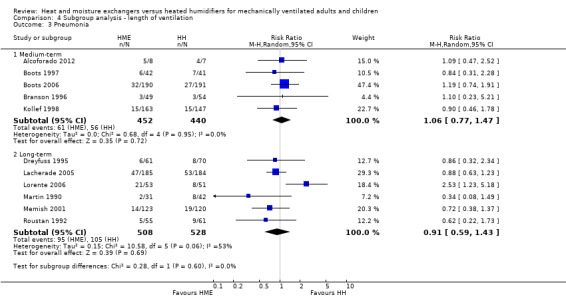
Comparison 4 Subgroup analysis ‐ length of ventilation, Outcome 3 Pneumonia.
Hygroscopic versus hydrophobic heat and moisture exchangers
Eight studies used hydrophobic HMEs (Goldberg 1992; Kirton 1997; Martin 1990; Misset 1991; Ricard 1999; Roustan 1992; Thomachot 2001; Villafane 1996). In two studies, it was unclear whether an HME with hygroscopic properties was used (Linko 1984; Lorente 2006). The remaining studies appeared to use hygroscopic HMEs.
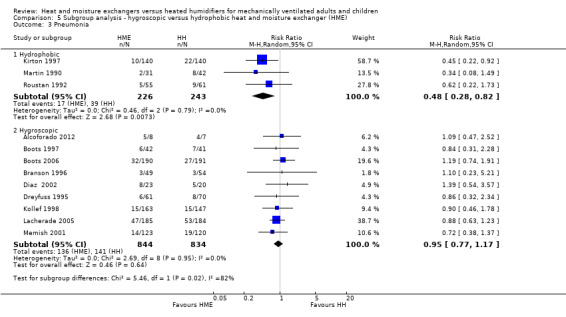
There was no difference between subgroups for the outcomes of artificial airway occlusion (Analysis 5.1) or mortality (Analysis 5.2). However, there was a significant difference between those studies using a hydrophobic HME compared to those using a hygroscopic HME for the outcome of pneumonia (Analysis 5.3). The likelihood of pneumonia appeared to be halved in studies using a hydrophobic HME (RR 0.48, 95% CI 0.28 to 0.82; 469 participants; 3 studies) compared to those using a hygroscopic HME where the risk was not significantly different from the HH group (RR 0.95, 95% CI 0.77 to 1.17; 1678 participants; 9 studies; Analysis 5.3).
5.1. Analysis.
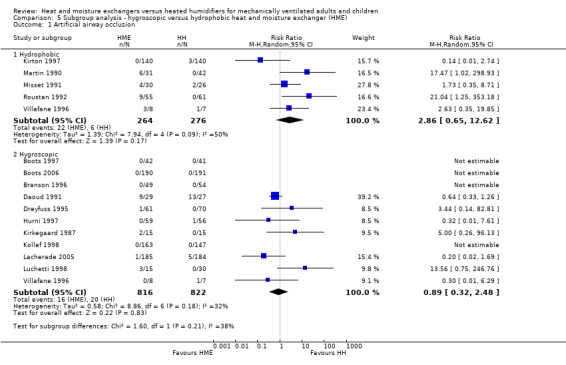
Comparison 5 Subgroup analysis ‐ hygroscopic versus hydrophobic heat and moisture exchanger (HME), Outcome 1 Artificial airway occlusion.
5.2. Analysis.
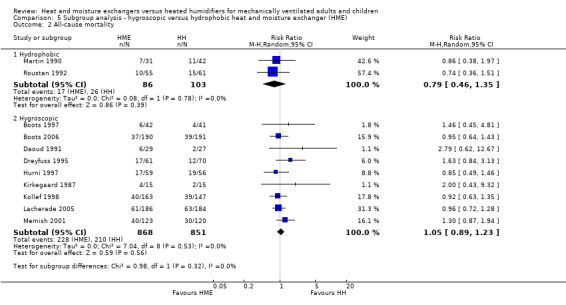
Comparison 5 Subgroup analysis ‐ hygroscopic versus hydrophobic heat and moisture exchanger (HME), Outcome 2 All‐cause mortality.
5.3. Analysis.
Comparison 5 Subgroup analysis ‐ hygroscopic versus hydrophobic heat and moisture exchanger (HME), Outcome 3 Pneumonia.
Endotracheal tube versus tracheostomy
Only one study stated that ventilation was given via a tracheostomy (Martin 1990), and data for participants with a tracheostomy were not given separately from participants ventilated through an ETT. Therefore, there were insufficient data to do a subgroup analysis of people who were ventilated via tracheostomy.
Sensitivity analyses
Sensitivity analyses based on the adequacy of allocation concealment were done for the outcomes of mortality (Analysis 7.1) and pneumonia (Analysis 7.2). There was no difference between the studies rated as having either adequate or unknown allocation concealment for either outcome. A sensitivity analysis was not done for artificial airway occlusion as only one study which reported this outcome was rated as having adequate allocation concealment (Kirton 1997) and one as inadequate (Branson 1996). There was also no difference between studies that we rated as low and unclear risk of detection bias for the outcomes of artificial airway occlusion (Analysis 8.1) and pneumonia (Analysis 8.2). We only rated one study which reported mortality at low risk of detection bias and none rated as high risk reported mortality.
7.1. Analysis.
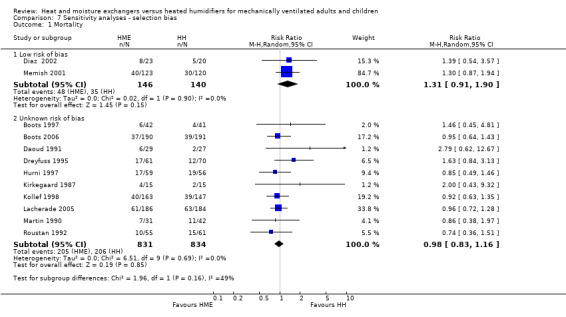
Comparison 7 Sensitivity analyses ‐ selection bias, Outcome 1 Mortality.
7.2. Analysis.
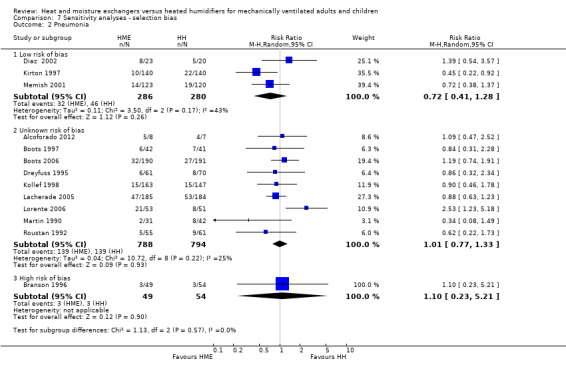
Comparison 7 Sensitivity analyses ‐ selection bias, Outcome 2 Pneumonia.
8.1. Analysis.
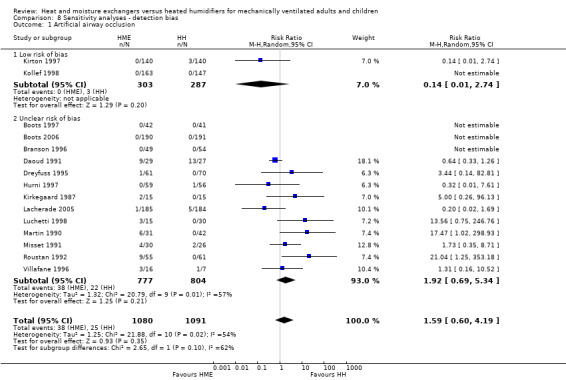
Comparison 8 Sensitivity analyses ‐ detection bias, Outcome 1 Artificial airway occlusion.
8.2. Analysis.
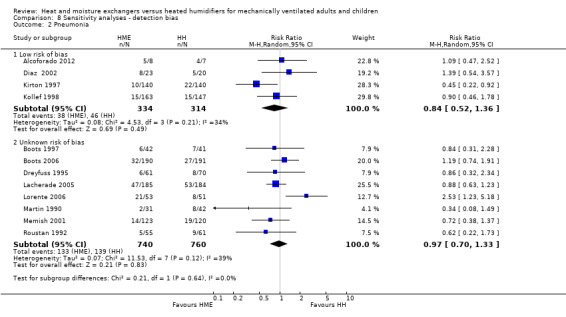
Comparison 8 Sensitivity analyses ‐ detection bias, Outcome 2 Pneumonia.
Funnel plot analyses
There was no evidence of funnel plot asymmetry for the outcomes of artificial airway occlusion (Figure 4), mortality (Figure 5), and pneumonia (Figure 6).
4.
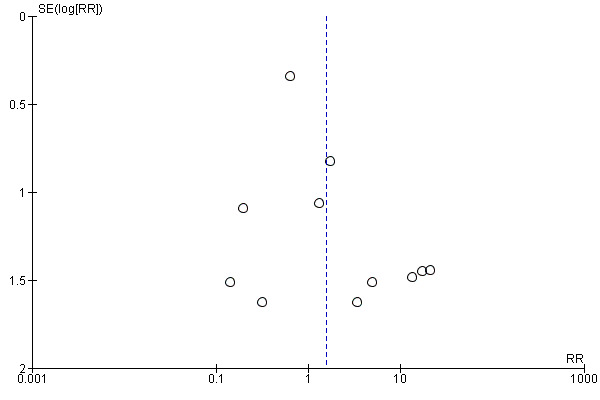
Funnel plot of comparison: 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, outcome: 1.1 Artificial airway occlusion.
5.
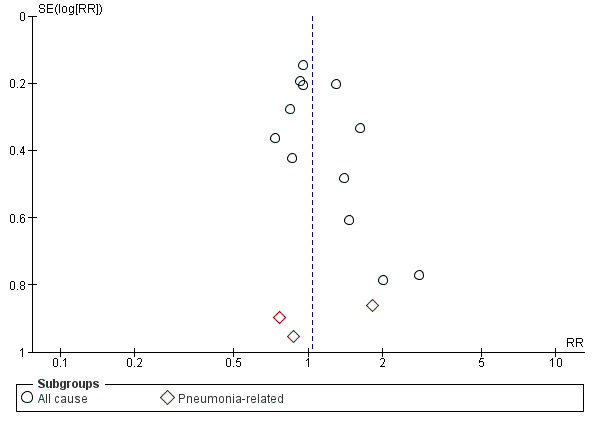
Funnel plot of comparison: 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, outcome: 1.2 Mortality.
6.
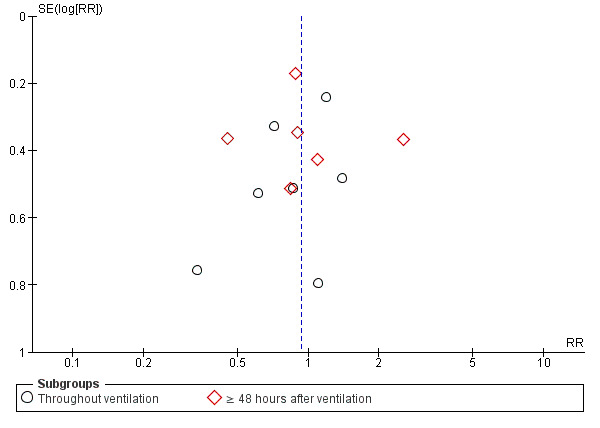
Funnel plot of comparison: 1 Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies, outcome: 1.3 Pneumonia.
Discussion
Summary of main results
Overall, our results showed no evidence that either HMEs or HHs reduce the risk of artificial airway occlusion, mortality or pneumonia. However, there was substantial heterogeneity between studies for the outcomes of airway occlusion and pneumonia. HMEs may increase the PaCO2 and minute ventilation and decrease body temperature. Hydrophobic HMEs appear to reduce the prevalence of pneumonia compared to HHs but this requires further investigation.
Artificial airway occlusion
Blockage of the endotracheal or tracheostomy tube is a life‐threatening adverse event that can occur in a ventilated person. Over all studies, we found that there was no difference in the risk of artificial airway occlusion with either method of humidification. This was also the finding in the review by Bench 2003, although these data came from only one study.
Mortality
We found no effect of the type of artificial humidification on mortality. It would be difficult to attribute a person's death directly to the type of humidification used since the studies were performed in ventilated people who were critically ill. However, death could result from complications associated with the type of humidification used, for example, occlusion of the artificial airway or pneumonia.
Pneumonia
Overall, we found that the prevalence of pneumonia was no different when an HME was used. However, the use of a hydrophobic HME without hygroscopic properties may reduce the risk of pneumonia compared to an HH.
This apparent difference between HMEs may be due to the higher moisture‐retaining properties of hygroscopic HMEs, although both Dreyfuss 1995 and Boots 1997 reported that contamination was still higher with HH compared to hygroscopic HMEs. As HHs without a heated wire have been associated with a higher risk of VAP (Siempos 2007), we also considered whether the apparent difference between HME may have been explained by the HH they were compared to. All hydrophobic HME were compared to HH without a heated wire while six of the eight studies using a hygroscopic HME used an HH with a heated wire (Boots 1997; Boots 2006; Branson 1996; Dreyfuss 1995; Kollef 1998; Lacherade 2005). However, this does not appear to be the case as the prevalence of pneumonia was 16% in the HH groups with and without a heated wire, as well as the hygroscopic HME group, but only 7.5% in the hydrophobic HME group. Therefore, the prevalence of pneumonia does appear to be lower in the group with a hydrophobic HME.
Respiratory complications
There was no difference between groups for the outcomes of atelectasis, pneumothorax or tracheal aspirations. The number or volume of saline instillations was significantly lower in the HME group but given the subjectivity of these outcomes, these results may not be valid measures of potential airway blockage.
Respiratory measures
There was no difference in PaO2 over the three cross‐over studies that reported this outcome although these data were highly heterogenous (I2 statistic between 63% and 83%). The PaCO2 was higher in the HME group at all correlation estimates over the five studies that reported PaCO2, which may be because of carbon dioxide retained within the HME. This higher PaCO2 may also be related to the higher measures of work of breathing reported for the HME group in two studies although these data could not be meta‐analysed.
In the cross‐over studies, minute ventilation was significantly higher for HMEs across the five studies reporting this outcome. However, the findings for tidal volume were more equivocal, only being statistically higher in the HME group at the high correlation estimate.
While HMEs appeared to increase minute ventilation relative to HHs, as with many of the respiratory variables this may be affected by ventilatory settings and should, therefore, be interpreted with caution.
Change in body temperature
There was good evidence across parallel studies that body temperature was significantly decreased by approximately 0.5 ºC when an HME was used. Although the results of cross‐over studies were equivocal, these data came from two small contradictory studies. This decrease in body temperature in many cases may not be considered clinically relevant when the participants are adults. However, temperature control is vitally important and very finely balanced in neonates, and a drop of 0.5 ºC could have adverse effects. While to a lesser extent, a temperature drop of 0.5 ºC may also have adverse effects on older children and elderly people, particularly if they were initially hypothermic.
Cost of devises
There was evidence of cost savings with HMEs in our review but the variation in the way that the information was reported made it difficult to be definitive about how great the cost saving could be. The apparent cost advantages associated with an HME need to be weighed against the potential disadvantages.
Frequency of heat and moisture exchanger changes
Less‐frequent HME changes may increase the risk of adverse outcomes such as artificial airway occlusion (Karpadia 2001), although a number of studies have advocated that HMEs can be used safely for 48 hours (Boyer 2003; Markowicz 2000). We were unable though to make any conclusions about the frequency of HME changes from data in this review. Although HMEs were used for at least 24 hours in 16 parallel studies, six studies did not report the frequency of HME changes (Branson 1996; Diaz 2002; Dreyfuss 1995; Kollef 1998; Martin 1990; Memish 2001). In the 10 studies that did report the frequency, eight reported changing the HME at least every 24 hours (Boots 1997; Boots 2006; Daoud 1991; Kirton 1997; Luchetti 1998; Misset 1991; Roustan 1992; Villafane 1996), and only two reported 48‐hour changes (Lacherade 2005; Lorente 2006).
Overall completeness and applicability of evidence
Since the original version of this review in 2010, we identified only one new study. Therefore, there is little information which has been added to our original findings. Thus, evidence to date remains inadequate to make any conclusions about the relative risk associated with either method of humidification. We endeavoured to compare patient‐related factors (age group and type of ventilation) and humidification factors (length of ventilation and type of HME) that may have important implications on findings; however, there were inadequate data to make any conclusions about the effects of these factors. Data were particularly poor for infants and children, and people who were ventilated through a tracheostomy.
Quality of the evidence
The majority of included studies did not describe most quality criteria. We only considered six and three studies at low risk of selection bias due to sequence generation and allocation, respectively. Only four were at low risk of detection bias. We deemed the majority (22 studies) at low risk of attrition bias and of other biases (23 studies).
Potential biases in the review process
The findings of this review need to be considered with caution as a number of the studies excluded participants who were not considered suitable for an HME (Alcoforado 2012; Boots 2006; Branson 1996; Daoud 1991; Lacherade 2005; Memish 2001). In addition, participants could be swapped to the HH group because of thick and bloody secretions in the study by Branson 1996; because of hypothermia and to minimize deadspace in Hurni 1997; and due to "specific indications" in Kollef 1998. Memish 2001 excluded participants who discontinued HME although it was not clear why they were discontinued. The study by Martin 1990 was interrupted after a participant in the HME group died following total occlusion of the artificial airway. Two studies changed HMEs more frequently, if considered necessary (Boots 1997; Boots 2006).
Agreements and disagreements with other studies or reviews
Two earlier narrative reviews found that VAP was reduced when an HME was used compared to an HH but these conclusions were not strongly supported by the data (Bench 2003; Cook 1998). Only one of the five studies in the review by Cook 1998, and neither of the two studies in Bench 2003, showed a significant difference in VAP. One meta‐analysis of eight trials with 1368 participants by Kola 2005 also found pneumonia was reduced when an HME was used in people who had mechanical ventilation for seven days or more.
However, as with this review update and our original Cochrane Review (Kelly 2010a; Kelly 2010b), we found no difference in the risk of VAP when HMEs or HHs were used. These findings were also reported by Siempos 2007 in their meta‐analysis of 2580 participants in 13 trials and by Niël‐Weise 2007 in their meta‐analysis of 2014 participants in 10 trials.
As with our reviews, other reviews also reported no difference between HH and HME in the risk of artificial airway occlusion (Bench 2003; Siempos 2007), mortality (Siempos 2007), or length of ICU stay (Siempos 2007).
As with our findings, Siempos and colleagues found that HME reduced humidification costs (Siempos 2007).
An additional meta‐analysis and meta‐regression of HME versus HH in critically ill adults was published during this update (Vargas 2017). We had previously included 17 of the 18 studies included by Vargas 2017 who found no overall difference between HME and HH in the rates of airway occlusion, pneumonia or mortality.
Authors' conclusions
Implications for practice.
Overall, the type of humidification device (either passive or active) does not appear to affect the prevalence of artificial airway occlusion, mortality or pneumonia in people undergoing invasive mechanical ventilation. Heat and moisture exchangers (HMEs) were consistently cheaper to use than heated humidification (HH). Hydrophobic HMEs may reduce the risk of pneumonia although HMEs may also increase the risk of artificial airway occlusion in some groups of patients. There are insufficient data to make any conclusions about the use of HMEs or HHs in children or infants.
Implications for research.
There has been little new work in this area since 2012. Further research is needed comparing HH and HME, particularly relating to the use of in the paediatric and neonatal populations. Much more research comparing different types of HME and different types of HH is needed. Clear information on the types of devices used, if filters are used, the types of condensers, the use of heated wires in HH, the length of humidification and change frequency of humidification devices should be reported. Information that can help us make better judgements about the quality of trials must also be clearly reported. In particular, information about the number of people not deemed suitable for HMEs or taken off HMEs during the study must be reported.
Trials are needed to evaluate the effectiveness of the newer HMEs, and their combination with new devices such as boosters (Kola 2005). Trials investigating the use of HMEs beyond 24 hours are also needed (AARC 2012).
What's new
| Date | Event | Description |
|---|---|---|
| 13 December 2018 | Amended | Editorial team changed to Cochrane Emergency and Critical Care |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 4, 2010
| Date | Event | Description |
|---|---|---|
| 14 September 2017 | Amended | Typos corrected in "Why it is important to do this review" and the "Implications for practice" section |
| 30 May 2017 | New search has been performed | Search rerun to May 2017 |
| 30 May 2017 | New citation required but conclusions have not changed | We included one new study which did not change the conclusions We added a 'Summary of Findings' table to the review Change in authorship: two authors of the published review (Kelly 2010a), Kelly M and Lockwood C did not contribute to the updated review. Two new authors contributed to the updated review: Foster JP, Batuwitage BT |
| 11 July 2012 | Amended | Contact details updated |
| 17 April 2012 | Amended | Contact details updated. |
| 2 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank Harald Herkner (content editor), Vibeke E Horstmann (statistical editor), Antonio M Esquinas (peer reviewer), Davide Chiumello (peer reviewer), Odie Geiger (consumer referee) for their help and editorial advice during the preparation of this updated systematic review.
We would like to thank and acknowledge the important role of Margaret Kelly and Catherine Lockwood as authors of the original review (Kelly 2010a). We would also like to thank and acknowledge Mariana Correia and Raul Carvalho for their assistance in translating and extracting data from Alcoforado 2012.
Appendices
Appendix 1. Search strategies
Search strategy for CENTRAL, the Cochrane Library
#1 MeSH descriptor Respiration, Artificial explode all trees #2 ventilat* #3 artificia* near (respir* or airway*) #4 MeSH descriptor Tracheostomy explode all trees #5 tracheostom* #6 #1 or #2 or #3 or #4 or #5 #7 MeSH descriptor Humidity explode all trees #8 MeSH descriptor Hot Temperature explode all trees #9 humidif* #10 heat near (moisture and exchang*) #11 hme #12 (artificial or swedish) near nose #13 (#7 OR #8 OR #9 OR #10 OR #11 OR #12) #14 (#6 AND #13)
Search strategy for MEDLINE (OvidSP)
1 . Respiration‐Artificial/ or exp TRACHEOSTOMY/ or ventilat*.mp. or (artificia* adj3 (respir* or airway*)).mp. or tracheostom*.mp. 2. exp HUMIDITY/ or exp Heat/ or humidifi*.mp. or ((heat adj5 (moisture and exchanger*)).mp. or hme.mp. or ((artificial or Swedish) adj3 nose).mp. 3. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 4. 1 and 2 and 3
Search strategy for Embase (OvidSP)
1 exp artificial‐ventilation/ or exp TRACHEOSTOMY/ or ventilat*.mp. or (artificia* adj5 (respir* or airway*)).mp. or tracheostom*.mp. 2 exp humidity/ or exp heat/ or humidifi*.mp. or (heat adj5 (moisture and exchanger*)).mp. or hme.mp. or ((artificial or swedish) adj5 nose).mp. 3 (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or factorial* or placebo* or volunteer* or (singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*))).ti,ab.) not (animals not (humans and animals)).sh. 4 1 and 2 and 3
Search strategy for CINAHL (EBSCO host)
S1 (MH "Respiration, Artificial+") S2 (MM "Tracheostomy") S3 TX ventilat* or (artificia* and (respir* or airway*)) or tracheostom* S4 S1 or S2 or S3 S5 (MM "Humidity") S6 (MM "Heat") S7 TX humidif* or (heat and (moisture and exchang*)) or hme or ((artificial or Swedish) and nose) S8 S5 or S6 or S7 S9 S4 and S8 S10 (MM "Random Assignment") S11 (MH "Clinical Trials+") S12 (MM "Double‐Blind Studies") or (MM "Single‐Blind Studies") or (MM "Triple‐Blind Studies") S13 (MM "Placebos") S14 (MH "Prospective Studies+") S15 (MM "Multicenter Studies") S16 TX random* or trial* or placebo* or multicenter* or prospective or ((single or double or triple or treble) and (mask* or blind*)) S17 S10 or S11 or S12 or S13 or S14 or S15 or S16 S18 S9 and S17
Data and analyses
Comparison 1. Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ parallel studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Artificial airway occlusion | 15 | 2171 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.60, 4.19] |
| 2 Mortality | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 All cause | 12 | 1951 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.89, 1.20] |
| 2.2 Pneumonia‐related | 3 | 484 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.39, 3.01] |
| 3 Pneumonia | 13 | 2251 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.73, 1.19] |
| 3.1 Throughout ventilation | 7 | 1090 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.69, 1.27] |
| 3.2 ≥ 48 hours after ventilation | 6 | 1161 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.64, 1.46] |
| 4 Atelectasis | 3 | 303 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.40] |
| 5 Tracheal aspirations (per day) | 3 | 290 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐1.41, 0.47] |
| 6 Saline instillations (number per day) | 3 | 276 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.64, ‐0.17] |
| 7 Change in body temperature (absolute data) (ºC) | 6 | 321 | Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.96, ‐0.02] |
| 8 Change in body temperature mean data) (ºC) | 3 | 78 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.82, ‐0.36] |
Comparison 2. Heat and moisture exchanger (HME) versus heat humidifier (HH) ‐ cross‐over studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PaO2 (mmHg) | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 r = 0.3 | 4 | Mean Difference (Random, 95% CI) | ‐3.24 [‐16.08, 9.60] | |
| 1.2 r = 0.5 | 4 | Mean Difference (Random, 95% CI) | ‐3.87 [‐16.73, 9.00] | |
| 1.3 r = 0.7 | 4 | Mean Difference (Random, 95% CI) | ‐4.41 [‐17.09, 8.27] | |
| 2 PaCO2 (mmHg) | 5 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 2.1 r = 0.3 | 5 | Mean Difference (Random, 95% CI) | 1.93 [0.27, 3.59] | |
| 2.2 r = 0.5 | 5 | Mean Difference (Random, 95% CI) | 2.02 [0.19, 3.85] | |
| 2.3 r = 0.7 | 5 | Mean Difference (Random, 95% CI) | 2.21 [0.33, 4.09] | |
| 3 Breathing rate (breaths/minute) | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 3.1 r = 0.3 | 4 | Mean Difference (Random, 95% CI) | 1.40 [0.33, 2.46] | |
| 3.2 r = 0.5 | 4 | Mean Difference (Random, 95% CI) | 1.15 [‐0.13, 2.44] | |
| 3.3 r = 0.7 | 4 | Mean Difference (Random, 95% CI) | 1.02 [‐0.38, 2.41] | |
| 4 Tidal volume (L) | 5 | Mean difference (Random, 95% CI) | Subtotals only | |
| 4.1 r = 0.3 | 5 | Mean difference (Random, 95% CI) | 0.02 [‐0.00, 0.03] | |
| 4.2 r = 0.5 | 5 | Mean difference (Random, 95% CI) | 0.02 [0.00, 0.04] | |
| 4.3 r = 0.7 | 5 | Mean difference (Random, 95% CI) | 0.03 [0.01, 0.06] | |
| 5 Minute ventilation (L/minute) | 5 | Mean difference (Random, 95% CI) | Subtotals only | |
| 5.1 r = 0.3 | 5 | Mean difference (Random, 95% CI) | 1.20 [0.78, 1.61] | |
| 5.2 r = 0.5 | 5 | Mean difference (Random, 95% CI) | 1.19 [0.63, 1.75] | |
| 5.3 r = 0.7 | 5 | Mean difference (Random, 95% CI) | 1.18 [0.55, 1.80] | |
| 6 Body temperature (ºC) | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 6.1 r = 0.3 | 2 | Mean Difference (Random, 95% CI) | ‐1.12 [‐3.77, 1.52] | |
| 6.2 r = 0.5 | 2 | Mean Difference (Random, 95% CI) | ‐1.13 [‐3.77, 1.52] | |
| 6.3 r = 0.7 | 2 | Mean Difference (Random, 95% CI) | ‐1.13 [‐3.78, 1.51] |
Comparison 3. Subgroup analysis ‐ children versus adults.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Artificial airway occlusion | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Children | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.33, 1.26] |
| 1.2 Adults | 14 | 2115 | Risk Ratio (M‐H, Random, 95% CI) | 1.94 [0.65, 5.76] |
| 2 All‐cause mortality | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Children | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 2.79 [0.62, 12.67] |
| 2.2 Adults | 11 | 1895 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.88, 1.19] |
Comparison 4. Subgroup analysis ‐ length of ventilation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Artificial airway occlusion | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Medium‐term | 8 | 1031 | Risk Ratio (M‐H, Random, 95% CI) | 1.74 [0.41, 7.30] |
| 1.2 Long‐term | 7 | 1140 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.34, 6.36] |
| 2 All‐cause mortality | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Medium‐term | 5 | 860 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.78, 1.31] |
| 2.2 Long‐term | 6 | 1048 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.85, 1.25] |
| 3 Pneumonia | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Medium‐term | 5 | 892 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.77, 1.47] |
| 3.2 Long‐term | 6 | 1036 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.59, 1.43] |
Comparison 5. Subgroup analysis ‐ hygroscopic versus hydrophobic heat and moisture exchanger (HME).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Artificial airway occlusion | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Hydrophobic | 5 | 540 | Risk Ratio (M‐H, Random, 95% CI) | 2.86 [0.65, 12.62] |
| 1.2 Hygroscopic | 11 | 1638 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.32, 2.48] |
| 2 All‐cause mortality | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Hydrophobic | 2 | 189 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.46, 1.35] |
| 2.2 Hygroscopic | 9 | 1719 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.89, 1.23] |
| 3 Pneumonia | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Hydrophobic | 3 | 469 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.28, 0.82] |
| 3.2 Hygroscopic | 9 | 1678 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.77, 1.17] |
Comparison 6. Heat and moisture exchanger (HME) with and without filters.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Artificial airway occlusion | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 With filter | 8 | 1203 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.41, 3.80] |
| 1.2 No filter | 7 | 968 | Risk Ratio (M‐H, Random, 95% CI) | 2.46 [0.33, 18.47] |
| 2 All‐cause mortality | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 With filter | 5 | 1080 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.82, 1.22] |
| 2.2 No filter | 7 | 871 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.85, 1.36] |
| 3 Pneumonia | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 With filter | 5 | 979 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.76, 1.24] |
| 3.2 no filter | 8 | 1272 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.55, 1.38] |
6.1. Analysis.
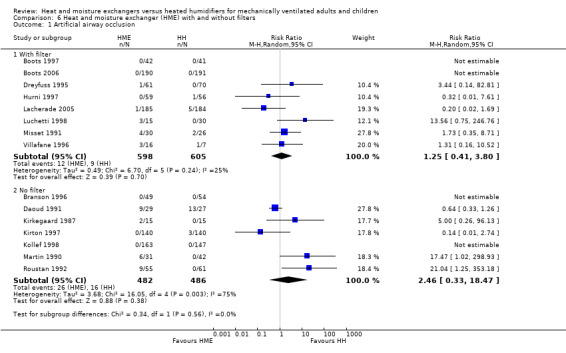
Comparison 6 Heat and moisture exchanger (HME) with and without filters, Outcome 1 Artificial airway occlusion.
6.2. Analysis.
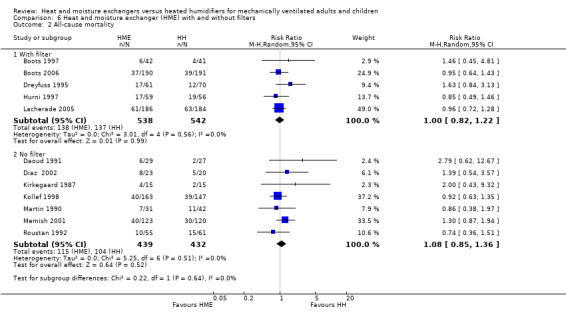
Comparison 6 Heat and moisture exchanger (HME) with and without filters, Outcome 2 All‐cause mortality.
6.3. Analysis.
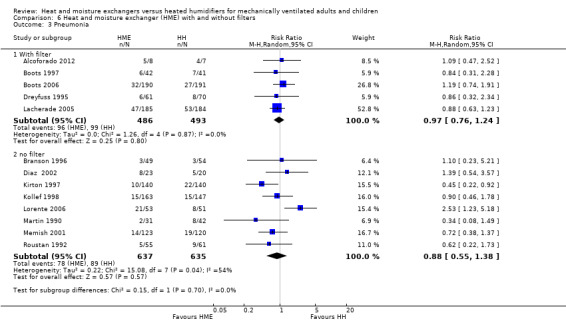
Comparison 6 Heat and moisture exchanger (HME) with and without filters, Outcome 3 Pneumonia.
Comparison 7. Sensitivity analyses ‐ selection bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Low risk of bias | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.91, 1.90] |
| 1.2 Unknown risk of bias | 10 | 1665 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.83, 1.16] |
| 2 Pneumonia | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Low risk of bias | 3 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.41, 1.28] |
| 2.2 Unknown risk of bias | 9 | 1582 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.77, 1.33] |
| 2.3 High risk of bias | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.23, 5.21] |
Comparison 8. Sensitivity analyses ‐ detection bias.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Artificial airway occlusion | 15 | 2171 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.60, 4.19] |
| 1.1 Low risk of bias | 2 | 590 | Risk Ratio (M‐H, Random, 95% CI) | 0.14 [0.01, 2.74] |
| 1.2 Unclear risk of bias | 13 | 1581 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.69, 5.34] |
| 2 Pneumonia | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Low risk of bias | 4 | 648 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.52, 1.36] |
| 2.2 Unknown risk of bias | 8 | 1500 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.70, 1.33] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alcoforado 2012.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: men and women aged > 18 years admitted to an ICU and requiring mechanical ventilation for ≥ 72 hr. Mean (± SD) age: 62.66 ± 14.48 years. Respiratory diagnosis: HME 37.5%, HH 42.8%. Exclusion criteria: contraindication to HME such as a large haemoptysis. hypothermia or excessive tracheal secretions. Severity: mean APACHE II score: HME 27.50, HH 24.27. Setting: ICU, Brazil. |
|
| Interventions | HME (hygroscopic): DAR filter Hygrobac S (Mallinckrodt Tyco Healthcare) changed every 24 hr. n = 8. HH: Misty 3 Intermed. n = 7. Time in study (median): HME 7 days, HH 7 days. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized by 'simple lottery.' |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes measured by 2 different researchers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, only VAP reported. |
| Other bias | High risk | Mean time in hospital: HME 9 days, HH 29 days ; median duration of ventilation: HME 201 hr, HH 161 hr; antibiotics used: HME 87.5%, HH 100%. |
Bissonnette 1989a.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: infants weighing 5‐10 kg, ASA status I or II having peripheral surgery lasting 2‐3 hr. Mean age: HME 12 months, HH 11 months. Exclusion criteria: not stated. Respiratory diagnosis: not stated. Severity: ASA status I or II. Setting: paediatric hospital, Canada. |
|
| Interventions | HME (hygroscopic): Humid‐Vent (Gibeck). n = 10. HH: MR450 (Fisher & Paykel) set at 37 ºC. n = 10. Time in study: 120 min. |
|
| Outcomes |
|
|
| Notes | Funding: National Institutes of Health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None identified. |
Bissonnette 1989b.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: infants and children weighing 5‐30 kg, ASA status I or II, having peripheral surgery lasting 1‐3 hr. Mean age: HME 4 years, HH 5 years. Exclusion criteria: without history of tympanic or middle ear problems. Respiratory diagnosis: not stated. Severity: ASA status I or II. Setting: paediatric hospital, Canada. |
|
| Interventions | HME (hygroscopic): Humid‐Vent (Gibeck). n = 8. HH: MR450 (Fisher & Paykel) set at 37 ºC. n = 10. Time in study: 120 min. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number enrolled not stated. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None identified. |
Boots 1997.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: all admissions to general ICU requiring mechanical ventilation for > 48 hr. Mean age: 51 years. Exclusion criteria: people with asthma, airway burns or pulmonary haemorrhage. Respiratory diagnosis: respiratory failure: HME 38/42, HH 37/41. Mean APACHE II score: HME 19, HH 18. Setting: adult ICU, Australia. |
|
| Interventions | HME (hygroscopic): Humid‐Vent (Gibeck) changed every 24 hr. n = 42. HH (heated wire): MR730 (Fisher & Paykel) set at 37 ºC. n = 41. Change of circuit every 48 hr in both groups. Time in study (median): HME 6 days, HH 6 days. |
|
| Outcomes |
|
|
| Notes | Funding: Teleflex, Wayne, PA. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all primary outcomes reported. |
| Other bias | Low risk | None identified. |
Boots 2006.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people requiring mechanical ventilation for ≥ 48 hr. Mean age: HME 59 years, HH 60 years. Exclusion criteria: presenting history which suggested need for hot water humidification, e.g. airway haemorrhage, asthma or airway burns. Mean APACHE II score: HME 20, HH 20. Setting: ICU, Australia. |
|
| Interventions | HME (hygroscopic): Humid‐Vent (Gibeck) changed every 24 hr or more frequently if required. n = 190. HH (heated wire): MR730 (Fisher & Paykel, single heated wire) set at 37 ºC or MR290 (Fisher & Paykel, double heated wire) set at 40 ºC. n = 191. Circuit unchanged for duration of ventilation. Time in study (median): HME 6 days, HH 8 days. |
|
| Outcomes |
|
|
| Notes | Funding: Teleflex, Wayne, PA. and Fisher & Paykel. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all primary outcomes reported. |
| Other bias | High risk | Participants in HME group ventilated for a significantly shorter period (i.e. HME 6 days, HH 8 days). |
Branson 1996.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people in ICU requiring mechanical ventilation deemed suitable for HME. Mean age: HME 44 years, HH 41 years. Exclusion criteria: people deemed unsuitable for HME. Respiratory diagnosis: not stated. Mean SAPS score: HME 9, HH 8. Setting: surgical and medical ICUs, USA. |
|
| Interventions | HME (hygroscopic): Baxter. n = 49. HH (heated wire): MR730 (Fisher & Paykel) set at 36 ºC. n = 54. Time in study (mean): HME 5 days, HH 4 days. |
|
| Outcomes |
|
|
| Notes | Funding: Fisher & Paykel. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Randomization was accomplished using the last digit in the patient's medical record number ‐‐‐patients with an odd medical record number received an HCH [HME] and those with an even number received a heated humidifier." |
| Allocation concealment (selection bias) | High risk | "Randomization was accomplished at the bedside." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number enrolled not stated. |
| Selective reporting (reporting bias) | Low risk | No protocol available but the 2 primary outcomes of airway occlusion and pneumonia reported. |
| Other bias | Low risk | None identified. |
Campbell 2000.
| Methods | Randomized cross‐over study comparing 2 types of HME to HH. | |
| Participants | Inclusion criteria: people following surgery, 15/26 breathing spontaneously and 11/26 ventilated. Mean age: 44 years. Exclusion criteria: not stated. Respiratory diagnosis: 58%. Severity: not stated. Setting: surgical ICU, USA. |
|
| Interventions | HME (hygroscopic): Humid‐Vent 2 (Gibeck). n = 26. HME: Extended use (Mallinckrodt). n = 26. HH (heated wire): MR730 (Fischer & Paykel) set at 34 ºC. n = 26. Time in study: 1 hr for each type of humidification. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but a range of respiratory variables reported for this short‐term trial. |
| Other bias | Low risk | None identified. |
Daoud 1991.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: neonates requiring ventilation via ETT. Ventilated for > 8 hr before admission, obstruction in tracheobronchial tree, ventilated at a frequency > 60 cycles/min. Mean age: not stated. Exclusion criteria: not stated. Respiratory diagnosis: HME 55%, HH 44%. Severity: not stated. Setting: NICU, France. |
|
| Interventions | HME (hygroscopic): Hygroflux (Vygon) changed daily. n = 29. HH: (Fisher & Paykel) set at 32‐34 ºC. n = 27. Time in study (mean): HME 4 days, HH 5 days. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, 2 of the 3 primary outcomes, i.e. airway occlusion and mortality, reported but pneumonia not reported. |
| Other bias | Low risk | None identified. |
Deriaz 1992.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people with ASA status I or II scheduled for gynaecological surgery. Mean age: 40 years. Exclusion criteria: not stated. Respiratory diagnosis: not stated. Severity: ASA status I or II. Setting: hospital, France. |
|
| Interventions | HME (hydrophobic): Ultipor hydrophobic (Pall). n = 25. HH: Aquapor (Dräger) set at 42 ºC. n = 25. Time in study (mean): HME 155 min, HH 116 min. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number enrolled not stated. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | High risk | Participants in HME group anaesthetized for significantly longer than those in HH group (HME 155 min, HH 116 min). |
Diaz 2002.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people receiving intubation. Median age: HME 61 years, HH 66 years. Exclusion criteria: previous pulmonary disease, hypothermia, pulmonary secretion or low expiratory volume. Respiratory diagnosis: not stated. Severity: not stated. Setting: ICU, Brazil. |
|
| Interventions | HME (hygroscopic): not stated. n = 23. HH: not stated. n = 20. Time in study: not stated. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers list used to determine treatment sequence. |
| Allocation concealment (selection bias) | Low risk | Allocations made available in sealed and consecutively numbered envelopes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, 2 of the 3 primary outcomes, i.e. pneumonia and mortality, reported but airway occlusion not reported. |
| Other bias | Low risk | None identified. |
Dreyfuss 1995.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people receiving mechanical ventilation for > 48 hr. Mean age: HME 58 years, HH 62 years. Exclusion criteria: not stated. Respiratory diagnosis: HME 64%, HH 63%. Mean SAPS score: HME 16, HH 16. Setting: ICU, France. |
|
| Interventions | HME (hygroscopic/hydrophobic): Hygrobac II (DAR) changed daily. n = 61. HH: Bennett cascade (Puritan‐Bennett) or MR460 (Fischer‐Paykel). n = 70. Time in study (median): HME 10 days, HH 12 days. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data from participants available at follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all 3 primary outcomes reported. |
| Other bias | Low risk | None identified. |
Girault 2003.
| Methods | Randomized cross‐over study comparing HME to HH. | |
| Participants | Inclusion criteria: people with acute or chronic respiratory failure difficult to wean from mechanical ventilation. Mean age: 69 years. Exclusion criteria: non‐co‐operative people, unable to have an oesophageal tube. Respiratory diagnosis: 100%. Mean SAPS score: 44. Setting: ICU, France. |
|
| Interventions | HME (hygroscopic): Hygrobac (DAR). n = 11. HH (heated wire): MR730 (Fischer & Paykel). n = 11. Time in study: 2 × 20 min for HME (7 cm H2O/min and 18 cm H2O/min ventilation pressure), 2 × 20 min for HH (7 cm H2O/min and 18 cm H2O/min ventilation pressure). |
|
| Outcomes |
* Data not used as this was unlikely to be a valid outcome in a cross‐over study. |
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3/15 participants withdrawn from study early because they could not "tolerate" HME and 1 participant refused to continue study. |
| Selective reporting (reporting bias) | Low risk | No protocol available but a range of respiratory variables reported for this short‐term study. |
| Other bias | Low risk | None identified. |
Goldberg 1992.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: adults with ASA status I, II and III, scheduled lower abdominal surgery under endotracheal anaesthesia for 1‐4 hr. Mean age: HME: 43 years, HH: 45 years. Exclusion criteria: not stated. Respiratory diagnosis: not stated. Severity: ASA status I, II and III. Setting: operating theatre, USA. |
|
| Interventions | HME (hydrophobic): Ultipor (Pall). n = 21. HH: Fisher & Paykel set at 37 ºC. n = 14. Time in study (mean): HME 123 min, HH 141 min. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned using a computer‐generated random table." |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Nursing personnel ‐‐‐ blinded to the patient group ‐‐‐ recorded temperatures." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None identified. |
Hurni 1997.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people receiving mechanical ventilation for ≥ 48 hr. Mean age: HME 53 years, HH 59 years. Exclusion criteria: hypothermic; intubated for 12 hr prior to ICU admission. Respiratory diagnosis: 40%. Mean SAPS score: HME 13, HH 13. Setting: ICU, Switzerland. |
|
| Interventions | HME (hygroscopic): Hygroster (DAR). n = 59. HH: Fisher & Paykel set at 32 ºC. n = 56. Time in study (mean): HME 8 days, HH 8 days. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT analysis used but there was a very high rate, i.e. 51%, that did not receive ventilation for ≥ 48 hr. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, 2 of the 3 primary outcomes, i.e. airway occlusion and mortality, reported but pneumonia not reported. |
| Other bias | High risk | Circuits changed every 48 hr in HH group and every 7 days in HME group. |
Iotti 1997.
| Methods | Randomized cross‐over study comparing 2 types of HME to HH | |
| Participants | Inclusion criteria: people receiving mechanical ventilation for acute respiratory failure. Mean age: 58 years. Exclusion criteria: COPD. Respiratory diagnosis: 100%. Severity: not stated. Setting: ICU, Italy. |
|
| Interventions | HME (hygroscopic): Umid‐Vent 2S (Gibeck). n = 10. HME: Hygroster (DAR). n = 10. HH: MR 450 (Fisher & Paykel) set at 32‐34 ºC. n = 10. Time in study: 1‐2 hr. |
|
| Outcomes |
|
|
| Notes | Funding: Polytechnico S. Matteo and support from Hamilton Bonaduzz AG. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but range of respiratory variables reported for this short‐term study. |
| Other bias | Low risk | None identified. |
Kirkegaard 1987.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people undergoing neurosurgery. Median age: HME 52 years, HH 36 years. Exclusion criteria: not stated. Respiratory diagnosis: not stated. Severity: not stated. Setting: hospital, Denmark. |
|
| Interventions | HME (hygroscopic): Engström Edith (Gambro). n = 15. HH: Hygrotherm. n = 15. Time in study: 72 hr. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, 2 of the 3 primary outcomes, i.e. airway occlusion and mortality, reported but pneumonia not reported. |
| Other bias | High risk | Participants in HME group were older. |
Kirton 1997.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people requiring mechanical ventilation. Mean age: 47 years. Exclusion criteria: ventilated elsewhere. Respiratory diagnosis: not stated. Severity: not stated. Setting: trauma ICU, USA. |
|
| Interventions | HME (hydrophobic): BB100‐F (Pall). n = 140. HH (heated wire). n = 140. Time in study: until removal of ETT, time not stated. |
|
| Outcomes |
|
|
| Notes | All 6 lost to follow‐up appeared to have been in HME group. Funding: University of Miami and Jackson Memorial Hospital. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was by a random number generated from a personal computer." |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Laboratory and chest radiograph interpretation were blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether the 6 lost to follow‐up included in analysis. |
| Selective reporting (reporting bias) | Low risk | No protocol available, 2 of the 3 primary outcomes, i.e. airway occlusion and pneumonia, reported but mortality not reported. |
| Other bias | Unclear risk | None identified but potential differences between groups not reported. |
Kollef 1998.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: adults requiring mechanical ventilation. Mean age: HME 58 years, HH 59 years. Exclusion criteria: ventilated elsewhere, previous heart or liver transplant, massive haemoptysis. Respiratory diagnosis: HME 16%, HH 18%. Mean APACHE II score: HME 17, HH 18. Setting: medical and surgical ICUs, USA. |
|
| Interventions | HME (hygroscopic): duration extended use (Nellcor Puritan‐Bennett). n = 163. HH (heated wire): MR730 (Fisher & Paykel) set at 35‐36 ºC. = 147. Time in study (mean): both groups 4 days. |
|
| Outcomes |
|
|
| Notes | Funding: Nellcor Puritan‐Bennett. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Low risk | "Randomization was done using opaque, sealed envelopes;" opened when person enrolled in study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants suspected of having VAP were reviewed by an investigator blinded to treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number enrolled not stated. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all 3 primary outcomes reported. |
| Other bias | Low risk | None identified. |
Lacherade 2005.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people expected to require mechanical ventilation for > 48 hr. Mean age: HME 55 years, HH 55 years. Exclusion criteria: people already ventilated, contraindications to an HME or HH, admitted after cardiac arrest, enrolled in clinical trial or early decision to withdraw treatment. Respiratory diagnosis: HME 38%, HH 28%. Mean SAPS II score: HME 45.4, HH 49.3. Setting: 2 ICUs, France. |
|
| Interventions | HME (hygroscopic): DAR Hygrobac (Tyco Healthcare/Nellcor) changed at 48‐hr intervals. n = 185. HH (heated wire): MR730 (Fisher & Paykel) and Aerodyne 2000 (Tyco Healthcare/Nellcor). n = 184. Time in study (mean): HME 14 days, HH 15 days. |
|
| Outcomes |
|
|
| Notes | Funding: Fisher & Paykel. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised according to a computer‐generated randomization list, stratified by participating ICU." |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all 3 primary outcomes reported. |
| Other bias | High risk | 5 times as many participants with HIV in HH group. Also higher PaO2/FiO2 in HH group. |
Le Bourdelles 1996.
| Methods | Randomized cross‐over study comparing HME to HH. | |
| Participants | Inclusion criteria: people receiving inspiratory pressure support during weaning trials from mechanical ventilation. Mean age: 63 years. Exclusion criteria: not stated. Respiratory diagnosis: 53%. Mean SAPS score: 16. Setting: hospital, France. |
|
| Interventions | HME (hygroscopic): Hygrobac (DAR).
n = 15. HH: MR450 (Fisher & Paykel). n = 15. Time in study: 20 min. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but a range of respiratory variables reported for this short‐term study. |
| Other bias | Low risk | None identified. |
Linko 1984.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: adults ASA status I or II undergoing laparotomies > 3 hr. Mean age: HME 43 years, HH 40 years. Exclusion criteria: serious circulatory, pulmonary or metabolic disease, heavy intraoperative bleeding or shock. Respiratory diagnosis: not stated. Severity: ASA status I or II. Setting: hospital, Finland. |
|
| Interventions | HME: Servo 150 (Siemens‐Elema AB). n = 10. HH: MR418 (Fisher & Paykel) set at 37‐40 ºC. n = 10. Time in study: 4 hr. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number enrolled not stated. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None identified. |
Lorente 2006.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: adults expected to require ventilation for ≥ 5 days. Mean age: HME 56 years, HH 55 years. Exclusion criteria: aged < 18 years, HIV, blood leukocytes < 1000/mm3, solid or haematological tumour, immunosuppressive therapy. Respiratory diagnosis: HME 24%, HH 31%. Mean APACHE II score: HME 18.11, HH 18.72. Setting: ICU, Spain. |
|
| Interventions | HME: Edith Flex (Datex‐Ohmeda) changed at 48‐hr intervals. n = 53. HH (heated wire): MR 850 (Fisher & Paykel) and Aerodyne 2000 (Tyco Healthcare/Nellcor) set at 37 ºC. n = 51. Time in study (mean): HME 20 days, HH 21 days. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were assigned ‐‐‐ by a random number list generated using Excel software." |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data from participants available at follow‐up. |
| Selective reporting (reporting bias) | High risk | No protocol available and only 1 primary outcome reported. |
| Other bias | Low risk | None identified. |
Luchetti 1998.
| Methods | Randomized parallel study comparing an HME to 2 types of HH. | |
| Participants | Inclusion criteria: critically ill people undergoing mechanical ventilation. Age range: 18‐34 years. Exclusion criteria: not stated. Respiratory diagnosis: not stated. Severity: not stated. Setting: ICU, Italy. |
|
| Interventions | HME (hygroscopic): Hygrobac (DAR) changed every 24 hr. n = 15. HH: Cascade II set at 8 (Puritan‐Bennett). n = 15. HH: MR600 (Fisher & Paykel) set at 37 ºC. n = 15. Time in study: 3‐7 days. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number enrolled not stated. |
| Selective reporting (reporting bias) | High risk | No protocol available and only 1 primary outcome reported. |
| Other bias | Low risk | None identified. |
MacIntyre 1983.
| Methods | Randomized cross‐over study comparing HME to HH. | |
| Participants | Inclusion criteria: clinically stable people requiring mechanical ventilation. Mean age: 60 years. Exclusion criteria: not stated. Respiratory diagnosis: 35%. Severity: not stated. Setting: hospital, USA. |
|
| Interventions | HME (hygroscopic): Servo humidifier. n = 26. HH: Puritan Bennett. n = 26. Time in study: 24 hr for each type of humidification. |
|
| Outcomes |
* Not considered valid because of the cross‐over design and, therefore, not used. |
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None identified. |
Martin 1990.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people receiving mechanical ventilation for > 24 hr. Mean age: HME 61 years, HH 54 years. Exclusion criteria: not stated. Respiratory diagnosis: HME 48%, HH 51%. Severity: not stated. Setting: ICU, France. |
|
| Interventions | HME (hydrophobic): Ultipor (Pall) replaced at least daily. n = 31. HH: set at 31 ºC. n = 42. Time in study (mean): HME 10 days, HH 14 days. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all 3 primary outcomes reported. |
| Other bias | High risk | Mean number of days: HH 14, HME 10. |
Martin 1994.
| Methods | Randomized cross‐over study comparing HME to HH. | |
| Participants | Inclusion criteria: sedated, paralysed people requiring mechanical ventilation for > 3 days. Mean age: 64 years. Exclusion criteria: not stated. Respiratory diagnosis: 100%. Severity: not stated. Setting: ICU, France. |
|
| Interventions | HME (hygroscopic): Humid‐Vent (Gibeck). n = 11. HH: Cascade II (Bennett). n = 11. Time in study: 24 hr for each method of humidification. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "This study was ‐ unblinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Unclear risk | None identified. |
Memish 2001.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: intubated people requiring mechanical ventilation with evidence of respiratory or systemic infection. Mean age: HME 48 years, HH 46 years. Exclusion criteria: ventilated < 48 hr. Respiratory diagnosis: 36%. Mean APACHE score: HME 32, HH 31. Setting: medical/surgical ICU, Saudi Arabia. |
|
| Interventions | HME (hygroscopic): Hudson RCI. n = 120. HH: not stated. n = 123. Time in study (mean): HME 9 days, HH 9 days. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Group balance was maintained within each block of 20." |
| Allocation concealment (selection bias) | Low risk | "The randomization record was kept with the hospital biostatistician." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data from participants available at follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, 2 of the 3 primary outcomes, i.e. mortality and pneumonia, reported but airway occlusion not reported. |
| Other bias | Low risk | None identified. |
Misset 1991.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people expected to be mechanically ventilated for > 5 days. Mean age: HME 53 years, HH 49 years. Exclusion criteria: ventilated < 5 days. Respiratory diagnosis: not stated. Mean SAPS score: HME 14, HH 13. Setting: ICU, France. |
|
| Interventions | HME (hydrophobic): Ultipor BB2215 (Pall) changed daily. n = 30. HH: Cascade II (Puritan‐Bennett) or MR450 (Fisher & Paykel) set at 32‐34 ºC. n = 26. Time in study (mean): HME 12 days, HH 11 days. |
|
| Outcomes |
|
|
| Notes | Variance reported as SEMs but based on P values appear to have been SDs. Funding: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data from participants available at follow‐up. |
| Selective reporting (reporting bias) | High risk | No protocol available and only 1 primary outcome, airway occlusion, was reported for this long‐term study. |
| Other bias | Unclear risk | None identified. |
Pelosi 1996.
| Methods | Randomized cross‐over study comparing 2 types of HME to HH. | |
| Participants | Inclusion criteria: people with moderate acute respiratory failure, receiving pressure‐support ventilation. Mean age: 54 years. Exclusion criteria: COPD. Respiratory diagnosis: 100%. Mean SAPS score: 12. Setting: ICU, Italy. |
|
| Interventions | HME (hygroscopic): Hygroster. n = 7. HME: Hygrobac‐S (DAR). n = 7. HH: MR450 (Fisher & Paykel). n = 14. Time in study: 90 min. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but 3 respiratory variables reported for this short‐term study. |
| Other bias | Low risk | None identified. |
Ricard 1999.
| Methods | Randomized parallel/cross‐over* study comparing 4 types of HME to HH. * Participants randomized to 2/5 interventions. |
|
| Participants | Inclusion criteria: people requiring mechanical ventilation. Mean age: 54 years. Exclusion criteria: not stated. Respiratory diagnosis: 58%. Mean SAPS score: 14. Setting: ICU, France. |
|
| Interventions | HME (hydrophobic): Ultipor Filter BB2215 (Pall). n = 20. HME (hydrophobic): Biomedical BB50 (Pall). n = 20. HME (hydrophobic/hygroscopic): Ultipor Filter BB100 (Pall). n = 20. HME (hydrophobic/hygroscopic): Hygrobac (DAR). n = 10. HH: Fischer‐Paykel. n = 15. Time in study: 3 hr. |
|
| Outcomes |
|
|
| Notes | Funding: authors declared they did not have any funding support and had no conflict of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available but 2 respiratory variables reported for this short‐term study. |
| Other bias | Low risk | None identified. |
Roustan 1992.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: people requiring mechanical ventilation through an ETT. Mean age: HME 53 years, HH 49 years. Exclusion criteria: requiring high‐frequency jet ventilation. Respiratory diagnosis: HME 53%, HH 38%. Severity: HME 12, HH 12. Setting: ICU, France. |
|
| Interventions | HME (hydrophobic): BB2215 (Pall), changed daily. n = 55. HH: Aquapor (Dräger) set at 31 ºC. n = 61. Time in study (mean): HME 11 days, HH 8 days. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Low risk | No protocol available but all 3 primary outcomes reported. |
| Other bias | High risk | HME group in study for mean of 11 days compared to 8 days in HH group. |
Thomachot 2001.
| Methods | Randomized cross‐over study comparing 2 types of HME to HH. | |
| Participants | Inclusion criteria: people requiring mechanical ventilation through an ETT for acute respiratory failure. Mean age: 45 years. Exclusion criteria: not stated. Respiratory diagnosis: 100%. Mean SAPS score: 15. Setting: ICU, France. |
|
| Interventions | HME (hydrophobic): BB100 (Pall). n = 10. HME: BactHME (Pharma system AB). n = 10. HH: Cascade II (Puritan‐Bennett). n = 10. Time in study: 24 hr. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "A prospective controlled unblinded study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None identified. |
Villafane 1996.
| Methods | Randomized parallel study comparing 2 types of HME to HH. | |
| Participants | Inclusion criteria: people in ICU requiring mechanical ventilation. Mean age: HME 63 years, HH 60 years. Exclusion criteria: haemorrhagic disorders, intubated for > 24 hr before admission. Respiratory diagnosis: not stated. Mean SAPS score: HME 17, HH 17. Setting: ICU, France. |
|
| Interventions | HME (hydrophobic): BB2215 (Pall). n = 8. HME: Hygrobac (DAR) changed daily. n = 8. HH: MR310 (Fisher & Paykel) set at 32 ºC. n = 7. Time in study (mean): HME 6 days, HH 6 days. |
|
| Outcomes |
|
|
| Notes | Funding: not stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number enrolled not stated. |
| Selective reporting (reporting bias) | High risk | No protocol available and only 1 primary outcome reported. |
| Other bias | Low risk | None identified. |
Yam 1990.
| Methods | Randomized parallel study comparing HME to HH. | |
| Participants | Inclusion criteria: elderly people having total hip arthroplasty for osteoarthritis. Mean age: HME 70 years, HH 68 years. Exclusion: grossly obese, endocrine diseases, people with pyrexia. Respiratory diagnosis: not stated. Severity: not stated. Setting: operating theatre, UK. |
|
| Interventions | HME: Thermovent 1200 (Portex) n = 20. HH: Cascade (Puritan Bennett) n = 20. Time in study (mean): HME 128 min, HH 133 min. |
|
| Outcomes |
|
|
| Notes | Funding: HME supplied by Portex. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | None identified. |
APACHE: Acute Physiology & Chronic Health Score; ASA: American Society of Anesthesiologists; COPD: chronic obstructive pulmonary disease; ETT: endotracheal tube; HH: heated humidifier; HME: heat and moisture exchanger; hr: hour; ICU: intensive care unit; ITT: intention‐to‐treat analysis; LOS: length of stay; min: minute; n: number of participants; NICU: neonatal intensive care unit; PaCO2: arterial pressure of carbon dioxide; PaO2: arterial pressure of oxygen; SaO2: arterial oxygen saturation; SAPS: Simplified Acute Physiologic Score; SD: standard deviation; SEM: standard error; VAP: ventilator‐associated pneumonia.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alagar 2000 | Data unavailable. |
| Branson 1993 | Not randomized. Allocation based on clinical algorithm. |
| Branson 1999 | HME connected to HH. |
| Christiansen 1998 | Did not measure any relevant clinical outcomes ‐ measured inspired humidity. |
| Cohen 1988 | Studied HME. Only switched to HH if there were complications. |
| Conti 1990 | Only studied HMEs. |
| Dias 1993 | Not a randomized controlled trial. |
| Dias 1997 | Not a randomized controlled trial. |
| Fujita 2006 | Not a randomized controlled trial. |
| Jaber 2004 | Not randomized ‐ all participants received HH first. |
| Johnson 1995 | HME in ventilator circuit with HH. |
| Kranabetter 2004 | Not randomized. |
| Lellouche 2006 | Did not measure any relevant clinical outcomes ‐ measured core temperature and inspired and expired humidity. |
| Luchetti 1999 | Non‐randomized study of HMEs use in children during anaesthesia. |
| MacLeod 2006 | Not a trial. Commentary on trial by Lacherade 2005. |
| Martin 1992 | All participants received HH first. |
| Martin 1995 | HME used in conjunction with HH. |
| McEvoy 1995 | Participants were cold to start with ‐ study compared no heat via HME with HH. |
| McNamara 2014 | Invasive ventilation was not used. |
| Nakagawa 2000 | Data unavailable. Results presented only in graphical form. |
| Nava 2008 | Used non‐invasive ventilation. |
| Prat 2003 | Not randomized. |
| Prin 2002 | Not randomized. HME removed when person was hypercapnic and HH used. |
| Rathgeber 1996 | Did not measure any relevant clinical outcomes ‐ measured inspired humidity. |
| Rathgeber 2001 | Did not measure any relevant clinical outcomes ‐ measured water vapour pressure. |
| Schiffmann 1997 | Not randomized. |
| Takumi 1970 | Home‐made humidifier compared to no humidification. |
| Thomachot 1998 | Participants spontaneously breathing via tracheostomy. |
| Wilmshurst 1999 | Not randomized ‐ alternate allocation. |
HH: heated humidifier; HME: heat and moisture exchanger.
Characteristics of studies awaiting assessment [ordered by study ID]
Nadir Oziş 2009.
| Methods | Randomized trial of HME‐Booster and HH. |
| Participants | 41 mechanically ventilated people in an ICU in Turkey. |
| Interventions | 21 participants randomized to HME‐Booster and 20 participants to HH with conventional microbiological filter. |
| Outcomes | Endotracheal tube occlusion due to secretions, VAP, PaCO2. |
| Notes | Study awaiting classification, attempted to contact authors for additional information including outcome data. |
Oguz 2013.
| Methods | Randomized trial of HME and HH. |
| Participants | 35 mechanically ventilated participants from the first day of intubation, did not have preintubation pneumonia, no infections or antibiotics for pulmonary infections or evidence of infiltration with chest radiography in a private hospital ICU in Turkey. |
| Interventions | 18 participants randomized to HME and 17 participants to HH. |
| Outcomes | Pneumonia ‐ presence of infiltrate over 7 days. |
| Notes | Infiltrate identified in 6 participants in HME group and 5 participants in HH group. |
HH: heated humidifier; HME: heat and moisture exchange; ICU: intensive care unit; PaCO2: arterial pressure of carbon dioxide; VAP: ventilator‐associated pneumonia.
Differences between protocol and review
We made the following changes to the published protocol (Kelly 2004)in the earlier version of the review (Kelly 2010a).
Moved mortality and pneumonia from secondary to primary outcomes.
In line with the new 'Risk of bias' table, added sequence generation to the assessed quality criteria.
There was significant heterogeneity between studies of people who had been ventilated for at least 48 hours compared to people ventilated for less than 48 hours. Therefore, a further analysis was conducted based on the types of HMEs, that is, whether they were hydrophobic or hygroscopic.
-
In the earlier version of the review (Kelly 2010a), we conducted the following subgroup analysis:
short‐term ventilation, defined as less than six hours;
medium‐term ventilation, defined as between six and 48 hours;
long‐term ventilation, defined as greater than 48 hours.
However, in this updated version of the review, the following additional changes were made:
The title was changed from "Heated humidification versus heat and moisture exchangers for mechanically ventilated adults and children" to "Heat and moisture exchangers versus heated humidifiers for mechanically ventilated adults and children." This was done because it was not clear in the original title that we were only including people who were undergoing invasive ventilation (though it was stated in the inclusion criteria). In addition, the order of heated humidification and heat and moisture exchangers was reversed because heat and moisture exchangers are treated as the intervention group in all analyses and therefore throughout the text.
We made the following changes to the published protocol (Kelly 2004), in the 2017 update:
Margaret Kelly and Catherine Lockwood, authors of the protocol and original review were no longer authors on this review update and there are two new authors, Jann P Foster and Bisanth T Batuwitage.
Incorporated a 'Summary of findings' table.
Modified the subgroup analysis for length of ventilation to the length of humidification, which was often, but not always, the same as the length of ventilation.
Contributions of authors
2017 Update
Co‐ordinating the review: Gillies D.
Screening search results: Gillies D, Todd D, Foster J, Batuwitage B.
Appraising quality of papers: Kelly MT, Gillies D, Todd D, Lockwood C.
Data extraction: Gillies D.
Writing to authors of papers for additional information: Gillies D.
Entering data into Review Manager: Gillies D.
Writing the review: Gillies D, Todd D, Foster J, Batuwitage B, Correia M, Carvalho R.
Guarantor for the review (one author): Gillies D.
People responsible for reading and checking review before submission: Gillies D, Todd D, Foster J, Batuwitage B.
Contributions of authors in original review (Kelly 2010a)
Developing protocol: Kelly MT, Gillies D, Todd D, Lockwood C.
Co‐ordinating the review: Kelly MT, Gillies D.
Screening search results: Kelly MT, Todd D, Lockwood C.
Organizing retrieval of papers: Kelly MT, Gillies D.
Screening retrieved papers against inclusion criteria: Kelly MT, Gillies D, Todd D, Lockwood C.
Data extraction: Kelly MT, Gillies D, Todd D, Lockwood C.
Writing to authors of papers for additional information: Kelly MT, Gillies D.
Entering data into Review Manager: Kelly MT, Gillies D.
Other statistical analysis not using RevMan: Kelly MT, Gillies D.
Writing the review: Kelly MT, Gillies D, Todd D.
Guarantor for the review (one author): Gillies D.
People responsible for reading and checking review before submission: Kelly MT, Gillies D.
Sources of support
Internal sources
The Children's Hospital at Westmead, Australia.
Sydney West Area Health Service, Australia.
External sources
No sources of support supplied
Declarations of interest
Donna Gillies: no conflict of interest.
David A Todd: no conflict of interest.
Jann P Foster: no conflict of interest.
Bisanth T Batuwitage: no conflict of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Alcoforado 2012 {published and unpublished data}
- Alcoforado L, Paiva D, Souza da Silva F, Martins Glavúo A, Galindo Filho V, Cunha Brandúo D, et al. Heat and moisture exchanger: protection against lung infections? Pilot study [Trocador de calor e humidade: proteção contra infeções pulmonares? Estudo piloto]. Fisioterapia e Pesquisa 2012;19(1):57‐62. [Google Scholar]
Bissonnette 1989a {published data only}
- Bissonnette B. Passive or active inspired gas humidification increases thermal steady‐state temperatures in anesthetized infants. Anesthesia and Analgesia 1989;69(6):783‐7. [PUBMED: 2589661] [PubMed] [Google Scholar]
Bissonnette 1989b {published data only}
- Bissonnette B, Sessler DI, LaFlamme P. Passive and active inspired gas humidification in infants and children. Anesthesiology 1989;71(3):350‐4. [PUBMED: 2774261] [DOI] [PubMed] [Google Scholar]
Boots 1997 {published data only}
- Boots R, Howe S, George N, Harris F, Faoagali J. Clinical utility of hygroscopic heat and moisture exchangers in intensive care patients. Critical Care Medicine 1997;25(10):1707‐12. [PUBMED: 9377886] [DOI] [PubMed] [Google Scholar]
Boots 2006 {published data only}
- Boots RJ, George N, Faoagali JL, Druery J, Dean K, Heller RF. Double‐heater‐wire circuits and heat‐and‐moisture exchangers and the risk of ventilator‐associated pneumonia. Critical Care Medicine 2006;34(3):687‐93. [PUBMED: 16505654] [DOI] [PubMed] [Google Scholar]
Branson 1996 {published data only}
- Branson RD, Davis K Jr, Brown R, Rashkin M. Comparison of three humidification techniques during mechanical ventilation: patient selection, cost and infections considerations. Respiratory Care 1996;41(9):809‐16. [EMBASE: 1996281309 ] [Google Scholar]
Campbell 2000 {published data only}
- Campbell RS, Davis K Jr, Johannigman JA, Branson RD. The effects of passive humidifier dead space on respiratory variables in paralyzed and spontaneously breathing patients. Respiratory Care 2000;45(3):306‐12. [PUBMED: 10771799] [PubMed] [Google Scholar]
Daoud 1991 {published data only}
- Daoud P, Casadevall I, Hartmann JF, Mercier JC, Beaufils F. Artificial noses versus cascade humidifiers: compared clinical effects in ventilated neonates [Condenseurs et rechauffers: effets cliniques compares chez le nouveau‐ne en ventilation mechanique]. Reanimation Soins Intensifs Medecine D'Urgence 1991;7(1):5‐8. [EMBASE: 1991117245 ] [Google Scholar]
Deriaz 1992 {published data only}
- Deriaz H, Fiez N, Lienhart A. Comparative effects of a hygrophobic filter and a heated humidifier on intraoperative hypothermia [Influence d'un filtre hygrophobe ou d'un humidificateur‐rechauffeur sur l'hypothermie peroperatoire]. Annales Francaises d'Anesthesie et de Reanimation 1992;11(2):145‐9. [PUBMED: 1503286] [DOI] [PubMed] [Google Scholar]
Diaz 2002 {published data only}
- Diaz RB, Barbosa DA, Bettencourt AR, Vianna LAC, Gir E, Guimaraes T. Evalution [sic] the use of hygroscopic humidifier filters to prevent nosocomial pneumonia [Avaliacao do uso de filtros umidificadores higroscopicos para prevencao de pneumonia hospitalar]. Acta Paulista de Enfermagem 2002;15(4):32‐44. [Google Scholar]
Dreyfuss 1995 {published data only}
- Dreyfuss D, Djedaini K, Gros I, Mier L, LeBourdelles G, Cohen Y, et al. Mechanical ventilation with heated humidifiers or heat and moisture exchangers: effects on patient colonization and incidence of nosocomial pneumonia. American Journal of Respiratory and Critical Care Medicine 1995;151(4):986‐92. [PUBMED: 7697277] [DOI] [PubMed] [Google Scholar]
Girault 2003 {published data only}
- Girault C, Breton L, Richard J, Tamion F, Vandelet P, Aboab J, et al. Mechanical effects of airway humidification devices in difficult to wean patients. Critical Care Medicine 2003;31(5):1306‐11. [PUBMED: 12771595] [DOI] [PubMed] [Google Scholar]
Goldberg 1992 {published data only}
- Goldberg ME, Epstein R, Rosenblum F, Larijani GE, Marr A, Lessin J, et al. Do heated humidifiers and heat and moisture exchangers prevent temperature drop during lower abdominal surgery?. Journal of Clinical Anesthesia 1992;4(1):16‐20. [PUBMED: 1540363] [DOI] [PubMed] [Google Scholar]
Hurni 1997 {published data only}
- Hurni J, Feihl F, Lazor R, Leuenberger P, Perret C. Safety of combined heat and moisture exchanger filters in long‐term mechanical ventilation. Chest 1997;111(3):686‐91. [PUBMED: 9118709] [DOI] [PubMed] [Google Scholar]
Iotti 1997 {published data only}
- Iotti GA, Olivei MC, Palo A, Galbusera C, Veronesi R, Comelli A, et al. Unfavorable mechanical effects of heat and moisture exchangers in ventilated patients. Intensive Care Medicine 1997;23(4):399‐405. [PUBMED: 9142578] [DOI] [PubMed] [Google Scholar]
Kirkegaard 1987 {published data only}
- Kirkegaard L, Andersen BN, Jensen S. Moistening of inspired air during respirator treatment. Comparison between the water‐bath evaporator and hygroscopic moisture heat exchanger [Fugtning af inspirationsluft under respiratorbehandling. En sammenligning imellem vandbadsfordamper og hygroskopisk fugt‐og varmeveksler]. Ugeskrift for Laeger 1987;149(3):152‐5. [PUBMED: 3547970] [PubMed] [Google Scholar]
Kirton 1997 {published data only}
- Kirton O, Haven B, Morgan J, Morejon O, Civetta J. A prospective, randomised comparison of an in‐line heat moisture exchange filter and heated wire humidifiers: rates of ventilator‐associated early‐onset (community‐acquired) or late‐onset (hospital‐acquired) pneumonia and incidence of endotracheal tube occlusion. Chest 1997;112(4):1055‐9. [PUBMED: 9377917] [DOI] [PubMed] [Google Scholar]
- Kirton O, Haven B, Morgan J, Morejon O, Civetta J. Rates of nosocomial pneumonia associated with HME/bacterial filter and heated wire humidifiers: a prospective, randomised trial. International Journal of Intensive Care 1997;4(1):6‐13. [EMBASE: 1997140409 ] [Google Scholar]
Kollef 1998 {published data only}
- Kollef MH, Shapiro SD, Boyd V, Silver P, Harz B, Trovillion E, et al. A randomized clinical trial comparing an extended‐use hygroscopic condenser humidifier with heated‐water humidification in mechanically ventilated patients. Chest 1998;113(3):759‐67. [PUBMED: 9515854] [DOI] [PubMed] [Google Scholar]
Lacherade 2005 {published data only}
- Lacherade JC, Auburtin M, Cerf C, Louw A, Soufir L, Rebufat Y, et al. Impact of humidification systems on ventilator‐associated pneumonia: a randomized multicenter trial. American Journal of Respiratory and Critical Care Medicine 2005;172(10):1276‐82. [PUBMED: 16126933] [DOI] [PubMed] [Google Scholar]
Le Bourdelles 1996 {published data only}
- Bourdelles G, Mier L, Fiquet B, Djedaini K, Saumon G, Coste F, et al. Comparison of the effects of heat and moisture exchangers and heated humidifiers on ventilation and gas exchange during weaning trials from mechanical ventilation. Chest 1996;110(5):1294‐8. [PUBMED: 8915237] [DOI] [PubMed] [Google Scholar]
Linko 1984 {published data only}
- Linko K, Honkavaara P, Nieminen MT. Heated humidification in major abdominal surgery. European Journal of Anaesthesiology 1984;1(3):285‐91. [PUBMED: 6536517] [PubMed] [Google Scholar]
Lorente 2006 {published data only}
- Lorente L, Lecuona M, Jimenez A, Mora ML, Sierra A. Ventilator‐associated pneumonia using a heated humidifier or a heat and moisture exchanger: a randomized controlled trial. Critical Care 2006;10(4):R116. [PUBMED: 16884530] [DOI] [PMC free article] [PubMed] [Google Scholar]
Luchetti 1998 {published data only}
- Luchetti M, Stuani A, Castelli G, Marraro G. Comparison of three different humidification systems during prolonged mechanical ventilation. Minerva Anestesiologica 1998;64(3):75‐81. [PUBMED: 9677791] [PubMed] [Google Scholar]
MacIntyre 1983 {published data only}
- MacIntyre NR, Anderson HR, Silver RM, Schuler FR, Coleman RE. Pulmonary function in mechanically‐ventilated patients during 24‐hour use of a hygroscopic condensor humidifier. Chest 1983;84(5):560‐4. [PUBMED: 6628007] [DOI] [PubMed] [Google Scholar]
Martin 1990 {published data only}
- Martin C, Perrin G, Gevaudan MJ, Saux P, Gouin F. Heat and moisture exchangers and vaporizing humidifiers in the intensive care unit. Chest 1990;97(1):144‐9. [PUBMED: 2295234] [DOI] [PubMed] [Google Scholar]
Martin 1994 {published data only}
- Martin C, Papazian L, Perrin G, Saux P, Gouin F. Preservation of humidity and heat of respiratory gases in patients with a minute ventilation greater than 10 L/min. Critical Care Medicine 1994;22(11):1871‐6. [PUBMED: 7956294] [PubMed] [Google Scholar]
Memish 2001 {published data only}
- Memish ZA, Oni GA, Djazmati W, Cunningham G, Mah MW. A randomized clinical trial to compare the effects of a heat and moisture exchanger with a heated humidifying system on the occurrence rate of ventilator‐associated pneumonia. American Journal of Infection Control 2001;29(5):301‐5. [PUBMED: 11584255] [DOI] [PubMed] [Google Scholar]
Misset 1991 {published data only}
- Misset B, Escudier B, Rivara D, Leclercq B, Nitenberg G. Heat and moisture exchanger vs heated humidifier during long‐term mechanical ventilation. A prospective randomized study. Chest 1991;100(1):160‐3. [PUBMED: 2060336] [DOI] [PubMed] [Google Scholar]
Pelosi 1996 {published data only}
- Pelosi P, Solca M, Ravagnan I, Tubiolo D, Ferrario L, Gattinoni L. Effects of heat and moisture exchangers on minute ventilation, ventilatory drive, and work of breathing during pressure‐support ventilation in acute respiratory failure. Critical Care Medicine 1996;24(7):1184‐8. [PUBMED: 8674333] [DOI] [PubMed] [Google Scholar]
Ricard 1999 {published data only}
- Ricard JD, Markowicz P, Djedaini K, Mier L, Coste F, Dreyfuss D. Bedside evaluation of efficient airway humidification during mechanical ventilation of the critically ill. Chest 1999;115(6):1646‐52. [PUBMED: 10378563] [DOI] [PubMed] [Google Scholar]
Roustan 1992 {published data only}
- Roustan JP, Kienlen J, Aubas P, Aubas S, du Cailar J. Comparison of hydrophobic heat and moisture exchangers with heated humidifier during prolonged mechanical ventilation. Intensive Care Medicine 1992;18(2):97‐100. [PUBMED: 1613206] [DOI] [PubMed] [Google Scholar]
- Roustan JP, Kienlen J, Aubas S, du Cailar J. Evaluation of an exchange filter on heat and humidity in long‐duration mechanical ventilation. Comparison with heated humidification. Annales Francaises d Anesthesie et de Reanimation 1989;8 Suppl:R275. [PUBMED: 2604223] [PubMed] [Google Scholar]
Thomachot 2001 {published data only}
- Thomachot L, Viviand X, Lagier P, Dejode JM, Albanese J, Martin C. Measurement of tracheal temperature is not a reliable index of total respiratory heat loss in mechanically ventilated patients. Critical Care (London, England) 2001;5(1):24‐30 EP ‐ 30. [PUBMED: 11178222] [DOI] [PMC free article] [PubMed] [Google Scholar]
Villafane 1996 {published data only}
- Villafane MC, Cinnella G, Lofaso F, Isabey D, Harf A, Lemaire F, et al. Gradual reduction of endotracheal tube diameter during mechanical ventilation via different humidification devices. Anesthesiology 1996;85(6):1341‐9. [PUBMED: 8968181] [DOI] [PubMed] [Google Scholar]
Yam 1990 {published data only}
- Yam PC, Carli F. Maintenance of body temperature in elderly patients who have joint replacement surgery. A comparison between the heat and moisture exchanger and heated humidifier. Anaesthesia 1990;45(7):563‐5. [PUBMED: 2386281] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alagar 2000 {published data only (unpublished sought but not used)}
- Alagar R. Heated humidification reduces saline instillations, nebulized therapy, and cost in long term ventilated patients. American Journal of Respiratory and Critical Care Medicine 2000;161; 3 Suppl:A552. [Google Scholar]
Branson 1993 {published data only}
- Branson RD, Davis K Jr, Campbell RS, Johnson DJ, Porembka DT. Humidification in the intensive care unit: prospective study of a new protocol utilizing heated humidification and a hydroscopic condenser humidifier. Chest 1993;104(6):1800‐5. [PUBMED: 8252968] [DOI] [PubMed] [Google Scholar]
Branson 1999 {published data only}
- Branson RD, Campbell RS, Johannigman JA, Ottaway M, Davis K Jr, Luchette FA, et al. Comparison of conventional heated humidification with a new active hygroscopic heat and moisture exchanger in mechanically ventilated patients. Respiratory Care 1999;44(8):912‐7. [EMBASE: 1999297233 ] [Google Scholar]
Christiansen 1998 {published data only}
- Christiansen S, Renzing K, Hirche H, Reidemeister JC. Measurements of inspired air humidity as provided by different humidifiers. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 1998;33(5):300‐5. [EMBASE: 1998188789 ] [DOI] [PubMed] [Google Scholar]
Cohen 1988 {published data only}
- Cohen IL, Weinberg PF, Fein IA, Rowinski GS. Endotracheal tube occlusion associated with the use of heat and moisture exchangers in the intensive care unit. Critical Care Medicine 1988;16(3):277‐9. [PUBMED: 3422625] [DOI] [PubMed] [Google Scholar]
Conti 1990 {published data only}
- Conti G, Blasi RA, Rocco M, Pelaia P, Antonelli M, Bufi M, et al. Effects of the heat‐moisture exchangers on dynamic hyperinflation of mechanically ventilated COPD patients. Intensive Care Medicine 1990;16(7):441‐3. [PUBMED: 2269712] [DOI] [PubMed] [Google Scholar]
Dias 1993 {published data only}
- Dias MD, Pellacine EN, Zechineli CA. The new system of gaseous mixture humidification and heating of respirators: comparative study [Novo sistema de umidificação e aquecimento de mistura gasosa dos respiradores: estudo comparativo]. Revista da Associacao Medica Brasileira 1993;39(4):207‐12. [PUBMED: 8162083] [PubMed] [Google Scholar]
Dias 1997 {published data only}
- Dias MD, Pellacine EN, Zechineli CA. Bacterial aerosol generated by mechanical ventilators: comparative study [Aerossol bacteriano gerado por respiradores mecanicos: estudo comparativo]. Revista da Associacao Medica Brasileira 1997;43(1):15‐20. [PUBMED: 9224986] [PubMed] [Google Scholar]
Fujita 2006 {published data only}
- Fujita Y, Imanaka H, Fujino Y, Takeuchi M, Tomita T, Mashimo T, et al. Effect of humidifying devices on the measurement of tidal volume by mechanical ventilators. Journal of Anesthesia 2006;20(3):166‐72. [PUBMED: 16897234] [DOI] [PubMed] [Google Scholar]
Jaber 2004 {published data only}
- Jaber S, Pigeot J, Fodil R, Maggiore S, Harf A, Isabey D, et al. Long‐term effects of different humidification systems on endotracheal tube patency: evaluation by the acoustic reflection method. Anesthesiology 2004;100(4):782‐8. [PUBMED: 15087611] [DOI] [PubMed] [Google Scholar]
Johnson 1995 {published data only}
- Johnson PA, Raper RF, Fisher McDM. The impact of heat and moisture exchanging humidifiers on work of breathing. Anaesthesia and Intensive Care 1995;23(6):697‐701. [PUBMED: 8669603] [DOI] [PubMed] [Google Scholar]
Kranabetter 2004 {published data only}
- Kranabetter R, Leier M, Kammermeier D, Just HM, Heuser D. The effects of active and passive humidification on ventilation‐associated nosocomial pneumonia [Einfluss von aktiver und passiver Befeuchtung auf die beatmungsassoziierte nosokomiale Pneumonie]. Anaesthesist 2004;53(1):29‐35. [PUBMED: 14749873] [DOI] [PubMed] [Google Scholar]
Lellouche 2006 {published data only}
- Lellouche F, Qader S, Taille S, Lyazidi A, Brochard L. Under‐humidification and over‐humidification during moderate induced hypothermia with usual devices. Intensive Care Medicine 2006;32(7):1014‐21. [PUBMED: 16791663] [DOI] [PubMed] [Google Scholar]
Luchetti 1999 {published data only}
- Luchetti M, Pigna A, Gentili A, Marraro G. Evaluation of the efficiency of heat and moisture exchangers during paediatric anaesthesia. Paediatric Anaesthesia 1999;9(1):39‐45. [PUBMED: 10712714] [PubMed] [Google Scholar]
MacLeod 2006 {published data only}
- MacLeod R, Bucknall T. Mechanical ventilation with heated humidifiers or with heat and moisture exchangers with microbiological filters did not reduce ventilator associated pneumonia in adults. Evidence‐Based Nursing 2006;9(3):82. [PUBMED: 16865833] [DOI] [PubMed] [Google Scholar]
Martin 1992 {published data only}
- Martin C, Papazian L, Perrin G, Bantz P, Gouin F. Performance evaluation of three vaporizing humidifiers and two heat and moisture exchangers in patients with minute ventilation > 10 L/min. Chest 1992;102(5):1347‐50. [PUBMED: 1424849] [DOI] [PubMed] [Google Scholar]
Martin 1995 {published data only}
- Martin C, Thomachot L, Quinio B, Viviand X, Albanese J. Comparing two heat and moisture exchangers with one vaporizing humidifier in patients with minute ventilation greater than 10 L/min. Chest 1995;107(5):1411‐5. [PUBMED: 7750340] [DOI] [PubMed] [Google Scholar]
McEvoy 1995 {published data only}
- McEvoy MT, Carey TJ. Shivering and rewarming after cardiac surgery: comparison of ventilator circuits with humidifier and heated wires to heat and moisture exchangers. American Journal of Critical Care 1995;4(4):293‐9. [PUBMED: 7663593] [PubMed] [Google Scholar]
McNamara 2014 {published data only}
- McNamara DG, Asher MI, Rubin BK, Stewart A, Byrnes CA. Heated humidification improves clinical outcomes, compared to a heat and moisture exchanger in children with tracheostomies. Respiratory Care 2014;59(1):46‐53. [PUBMED: 23764867] [DOI] [PubMed] [Google Scholar]
Nakagawa 2000 {published data only}
- Nakagawa NK, Macchione M, Petrolino HM, Guimaraes ET, King M, Saldiva PH, et al. Effects of a heat and moisture exchanger and a heated humidifier on respiratory mucus in patients undergoing mechanical ventilation. Critical Care Medicine 2000;28(2):312‐7. [PUBMED: 10708159] [DOI] [PubMed] [Google Scholar]
Nava 2008 {published data only}
- Nava S, Cirio S, Fanfulla F, Carlucci A, Navarra A, Negri A, et al. Comparison of two humidification systems for long‐term noninvasive mechanical ventilation. European Respiratory Journal 2008;32(2):460‐4. [PUBMED: 18669787] [DOI] [PubMed] [Google Scholar]
Prat 2003 {published data only}
- Prat G, Renault A, Tonnelier JM, Goetghebeur D, Oger E, Boles JM, et al. Influence of the humidification device during acute respiratory distress syndrome. Intensive Care Medicine 2003;29(12):2211‐5. [PUBMED: 12904858] [DOI] [PubMed] [Google Scholar]
Prin 2002 {published data only}
- Prin S, Chergui K, Augarde R, Page B, Jardin F, Vieillard‐Baron A. Ability and safety of a heated humidifier to control hypercapnic acidosis in severe ARDS. Intensive Care Medicine 2002;28(12):1756‐60. [PUBMED: 12447519] [DOI] [PubMed] [Google Scholar]
Rathgeber 1996 {published data only}
- Rathgeber J, Henze D, Zuchner K. Air conditioning with a high‐performance HME (heat and moisture exchanger) ‐ an effective and economical alternative to active humidifiers in ventilated patients. A prospective and randomized clinical study [Atemgasklimatisierung mit leistungsfahigen HME (Heat and Moisture Exchanger) ‐ eine effektive und kostengunstige Alternative zu aktiven Befeuchtern bei beatmeten Patienten. Eine prospektive und randomisierte klinische Studie]. Der Anaesthesist 1996;45(6):518‐25. [PUBMED: 8767565] [DOI] [PubMed] [Google Scholar]
Rathgeber 2001 {published data only}
- Rathgeber J, Betker T, Zuchner K. Measurement of water vapour pressure in the airways of mechanically ventilated patient using different types of humidifiers [Wassergehaltsmessungen im Tracheobronchialsystem beatmeter Patienten bei Verwendung unterschiedlicher Befeuchtersysteme]. Anasthesiologie, Intensivmedizin, Notfallmedizin, Schmerztherapie 2001;36(9):560‐5. [PUBMED: 11577355] [DOI] [PubMed] [Google Scholar]
Schiffmann 1997 {published data only}
- Schiffmann H, Rethgeber J, Singer D, Harms K, Bolli A, Zuchner K. Airway humidification in mechanically ventilated neonates and infants: a comparative study of a heat and moisture exchanger vs. a heated humidifier using a new fast‐response capacitive humidity sensor. Critical Care Medicine 1997;25(10):1755‐60. [PUBMED: 9377894] [DOI] [PubMed] [Google Scholar]
Takumi 1970 {published data only}
- Takumi Y, Aochi O. Effect of emphysematous expansion and humidification on the intrapulmonary gas exchange [Der Effekt der Uberblahung und Anfeuchtung auf den intrapulmonalen Gasaustrausch]. Der Anaesthesist 1970;19(10):373‐83. [PUBMED: 5274102] [PubMed] [Google Scholar]
Thomachot 1998 {published data only}
- Thomachot L, Viviand X, Arnaud S, Vialet R, Albanese J, Martin C. Preservation of humidity and heat of respiratory gases in spontaneously breathing, tracheostomized patients. Acta Anaesthesiologica Scandinavica 1998;42(7):841‐4. [PUBMED: 9698962] [DOI] [PubMed] [Google Scholar]
Wilmshurst 1999 {published data only}
- Wilmshurst JM, Rahman MA, Shah V, Elton P, Long D, Martin N. The heat moisture exchange device (HME) in neonatal ventilation. American Journal of Perinatology 1999;16(1):13‐6. [PUBMED: 10362076] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Nadir Oziş 2009 {published data only}
- Nadir Oziş T, Ozcan Kanat D, Oguzulgen IK, Aydogdu M, Hizel K, Gursel G. The clinical and microbiological comparison of the use of heated humidifiers and heat and moisture exchanger filters with Booster in mechanically ventilated patients. Tuberkuloz ve Toraks 2009;57(3):259‐67. [PubMed] [Google Scholar]
Oguz 2013 {published data only}
- Oguz S, Deger I. Ventilator‐associated pneumonia in patients using HME filters and heated humidifiers. Irish Journal of Medical Science 2013;182(4):651–5. [DOI] [PubMed] [Google Scholar]
Additional references
AARC 2012
- Restrepo RD, Walsh BK. AARC (American Association for Respiratory Care) clinical practice guideline: humidification during invasive and noninvasive mechanical ventilation 2012. Respiratory Care 2012;57(5):782‐8. [DOI: 10.4187/respcare.01766] [DOI] [PubMed] [Google Scholar]
Al Ashry 2014
- Al Ashry HS, Modrykamien AM. Humidification during mechanical ventilation in the adult patient. BioMed Research International 2014;2014:Article ID 715434. [PUBMED: 25089275] [DOI] [PMC free article] [PubMed] [Google Scholar]
ATS 2005
- American Thoracic Society and Infectious Diseases Society of America. Guidelines for the management of adults with hospital‐acquired, ventilator‐associated, and healthcare associated pneumonia. American Journal of Respiratory and Critical Care Medicine 2005;171(4):388‐416. [PUBMED: 15699079] [DOI] [PubMed] [Google Scholar]
Bench 2003
- Bench S. Humidification in the long‐term ventilated patient; a systematic review. Intensive and Critical Care Nursing 2003;19:75‐84. [PUBMED: 12706733] [DOI] [PubMed] [Google Scholar]
Boyer 2003
- Boyer A, Thiery G, Lasry S, Pigne E, Salah A, Lassence A, et al. Long‐term mechanical ventilation with hygroscopic heat and moisture exchangers used for 48 hours: a prospective clinical, hygrometric, and bacteriologic study. Critical Care Medicine 2003;31(3):823‐9. [PUBMED: 12626991] [DOI] [PubMed] [Google Scholar]
Branson 2007
- Branson RD. Secretion management in the mechanically ventilated patient. Respiratory Care 2007;52(10):1328‐47. [PUBMED: 17894902] [PubMed] [Google Scholar]
Cook 1998
- Cook D, Jonghe B, Brochard L, Brun‐Buisson C. Influence of airway management on ventilator‐associated pneumonia: evidence from randomized trials. JAMA 1998;279(10):781‐7. [PUBMED: 9508156] [DOI] [PubMed] [Google Scholar]
Dodek 2004
- Dodek P, Keenan S, Cook D, Heyland D, Jacka M, Hand L, et al. Evidence‐based clinical practice guideline for the prevention of ventilator‐associated pneumonia. Annals of Internal Medicine 2004;141(4):305‐13. [PUBMED: 15313747] [DOI] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- GRADE Working Group, McMaster University. GRADEpro. Version 3.6 for Windows. Hamilton (ON): GRADE Working Group, McMaster University, 2011.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hess 2003
- Hess DR, Kallstrom TJ, Mottram CD, Myers TR, Sorenson HM, Vines DL, et al. Care of the ventilator circuit and its relation to ventilator‐associated pneumonia. Respiratory Care 2003;48(9):869‐79. [PUBMED: 14513820] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Karpadia 2001
- Karpadia, FN, Bajan KB, Singh S, Mathew B, Nath A, Wadkar S. Changing patterns of airway accidents in intubated ICU patients. Intensive Care Medicine 2001;27(1):296‐300. [PUBMED: 11280652] [DOI] [PubMed] [Google Scholar]
Koenig 2006
- Koenig SM, Truwit JD. Ventilator‐associated pneumonia: diagnosis, treatment, and prevention. Clinical Microbiology Reviews 2006;19(4):637‐57. [PUBMED: 17041138] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kola 2005
- Kola A, Eckmanns T, Gastmeier P. Efficacy of heat and moisture exchangers in preventing ventilator‐associated pneumonia: meta‐analysis of randomized controlled trials. Intensive Care Medicine 2005;31:5‐11. [PUBMED: 15368038] [DOI] [PubMed] [Google Scholar]
Lorente 2010
- Lorente L, Blot S, Rello J. New issues and controversies in the prevention of ventilator‐associated pneumonia. American Journal of Respiratory & Critical Care Medicine 2010;182(7):870‐6. [PUBMED: 20448095] [DOI] [PubMed] [Google Scholar]
Markowicz 2000
- Markowicz P, Ricard JD, Dreyfuss D, Mier L, Brun P, Coste F, et al. Safety, efficacy, and cost‐effectiveness of mechanical ventilation with humidifying filters changed every 48 hours: a prospective, randomized study. Critical Care Medicine 2000;28(3):665‐71. [PUBMED: 10752812] [DOI] [PubMed] [Google Scholar]
Mehta 2017
- Mehta C, Mehta Y. Percutaneous tracheostomy. Annals of Cardiac Anaesthesia 2017;20(Suppl 1):S19‐25. [DOI: 10.4103/0971-9784.197793] [DOI] [PMC free article] [PubMed] [Google Scholar]
Niël‐Weise 2007
- Niël‐Weise BS, Wille JC, Broek PJ. Humidification policies for mechanically ventilated intensive care patients and prevention of ventilator associated pneumonia: a systematic review of randomized controlled trials. Journal of Hospital Infection 2007;65(4):285‐91. [PUBMED: 17320243] [DOI] [PubMed] [Google Scholar]
Rathgeber 2006
- Rathgeber J. Devices used to humidify respired gases. Respiratory Care Clinics of North America 2006;12(2):165‐82. [PUBMED: 16828689] [DOI] [PubMed] [Google Scholar]
Ricard 2006
- Ricard JD, Boyer A, Dreyfuss D. The effect of humidification on the incidence of ventilator‐associated pneumonia. Respiratory Care Clinics of North America 2006;12(2):263‐73. [PUBMED: 16828694] [DOI] [PubMed] [Google Scholar]
Schiffmann 2006
- Schiffmann H. Humidification of respired gases in neonates and infants. Respiratory Care Clinics of North America 2006;12(2):321‐36. [PUBMED: 16828698] [DOI] [PubMed] [Google Scholar]
Siempos 2007
- Siempos II, Vardakas KZ, Kopterides P, Falagas ME. Impact of passive humidification on clinical outcomes of mechanically ventilated patients: a meta‐analysis of randomized controlled trials. Critical Care Medicine 2007;35(12):2843‐51. [PUBMED: 18074484] [DOI] [PubMed] [Google Scholar]
Vargas 2017
- Vargas M, Chiumello D, Sutherasan Y, Ball L, Esquinas AM, Pelosi P, et al. Heat and moisture exchangers (HMEs) and heated humidifiers (HHs) in adult critically ill patients: a systematic review, meta‐analysis and meta‐regression of randomized controlled trials. Critical Care 2017;21:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Kelly 2004
- Kelly M, Gillies D, Todd DA, Lockwood C. Heated humidification versus heat and moisture exchangers for ventilated adults and children. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004711] [DOI] [PubMed] [Google Scholar]
Kelly 2010a
- Kelly M, Gillies D, Todd DA, Lockwood C. Heated humidification versus heat and moisture exchangers for ventilated adults and children. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD004711.pub2] [DOI] [PubMed] [Google Scholar]
Kelly 2010b
- Kelly M, Gillies D, Todd DA, Lockwood C. Heated humidification versus heat and moisture exchangers for ventilated adults and children. Anesthesia and Analgesia 2010;111(4):1072. [DOI] [PubMed] [Google Scholar]


