Abstract
Background
Genital Chlamydia trachomatis (C.trachomatis) infection may lead to pregnancy complications such as miscarriage, preterm labour, low birthweight, preterm rupture of membranes, increased perinatal mortality, postpartum endometritis, chlamydial conjunctivitis and C.trachomatis pneumonia.This review supersedes a previous review on this topic.
Objectives
To establish the most efficacious and best‐tolerated therapy for treatment of genital chlamydial infection in preventing maternal infection and adverse neonatal outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (26 June 2017) and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCTs) as well as studies published in abstract form assessing interventions for treating genital C.trachomatis infection in pregnancy. Cluster‐RCTs were also eligible for inclusion but none were identified. Quasi‐randomised trials and trials using cross‐over design are not eligible for inclusion in this review.
Data collection and analysis
Two review authors independently assessed studies for inclusion, assessed trial quality and extracted the data using the agreed form. Data were checked for accuracy. Evidence was assessed using the GRADE approach.
Main results
We included 15 trials (involving 1754 women) although our meta‐analyses were based on fewer numbers of studies/women. All of the included studies were undertaken in North America from 1982 to 2001. Two studies were low risk of bias in all domains, all other studies had varying risk of bias. Four other studies were excluded and one study is ongoing.
Eight comparisons were included in this review; three compared antibiotic (erythromycin, clindamycin, amoxicillin) versus placebo; five compared an antibiotic versus another antibiotic (erythromycin, clindamycin, amoxicillin, azithromycin). No study reported different antibiotic regimens.
Microbiological cure (primary outcome)
Antibiotics versus placebo: Erythromycin (average risk ratio (RR) 2.64, 95% confidence interval (CI) 1.60 to 4.38; two trials, 495 women; I2 = 68%; moderate‐certainty evidence), and clindamycin (RR 4.08, 95% CI 2.35 to 7.08; one trial, 85 women;low‐certainty evidence) were associated with improved microbiological cure compared to a placebo control. In one very small trial comparing amoxicillin and placebo, the results were unclear, but the evidence was graded very low (RR 2.00, 95% CI 0.59 to 6.79; 15 women).
One antibiotic versus another antibiotic: Amoxicillin made little or no difference in microbiological cure in comparison to erythromycin (RR 0.97, 95% CI 0.93 to 1.01; four trials, 466 women; high‐certainty evidence), probably no difference compared to clindamycin (RR 0.96, 95% CI 0.89 to 1.04; one trial, 101 women; moderate‐quality evidence), and evidence is very low certainty when compared to azithromycin so the effect is not certain (RR 0.89, 95% CI 0.71 to 1.12; two trials, 144 women; very low‐certainty evidence). Azithromycin versus erythromycin (average RR 1.11, 95% CI 1.00 to 1.23; six trials, 374 women; I2 = 53%; moderate‐certainty evidence) probably have similar efficacy though results appear to favour azithromycin. Clindamycin versus erythromycin (RR 1.06, 95% CI 0.97 to 1.15; two trials, 173 women; low‐certainty evidence) may have similar numbers of women with a microbiological cure between groups.
Evidence was downgraded for design limitations, inconsistency, and imprecision in effect estimates.
Side effects of the treatment (maternal) (secondary outcome)
Antibiotics versus placebo: side effects including nausea, vomiting, and abdominal pain, were reported in two studies (495 women) but there was no clear evidence whether erythromycin was associated with more side effects than placebo and a high level of heterogeneity (I2 = 78%) was observed (average RR 2.93, 95% CI 0.36 to 23.76). There was no clear difference in the number of women experiencing side effects when clindamycin was compared to placebo in one small study (5/41 versus 1/44) (RR 6.35, 95% CI 0.38 to 107.45, 62 women). The side effects reported were mostly gastrointestinal and also included resolving skin rashes.
One antibiotic versus another antibiotic: There was no clear difference in incidence of side effects (including nausea, vomiting, diarrhoea and abdominal pain) when amoxicillin was compared to azithromycin based on data from one small study (36 women) (RR 0.56, 95% CI 0.24 to 1.31).
However, amoxicillin was associated with fewer side effects compared to erythromycin with data from four trials (513 women) (RR 0.31, 95% CI 0.21 to 0.46; I2 = 27%). Side effects included nausea, vomiting, diarrhoea, abdominal cramping, rash, and allergic reaction.
Both azithromycin (RR 0.24, 95% CI 0.17 to 0.34; six trials, 374 women) and clindamycin (RR 0.44, 95% CI 0.22 to 0.87; two trials, 183 women) were associated with a lower incidence of side effects compared to erythromycin. These side effects included nausea, vomiting, diarrhoea and abdominal cramping.
One small study (101 women) reported there was no clear difference in the number of women with side effects when amoxicillin was compared with clindamycin (RR 0.57, 95% CI 0.14 to 2.26; 107 women). The side effects reported included rash and gastrointestinal complaints.
Other secondary outcomes
Single trials reported data on repeated infections, preterm birth, preterm rupture of membranes, perinatal mortality and low birthweight and found no clear differences between treatments.
Many of this review's secondary outcomes were not reported in the included studies.
Authors' conclusions
Treatment with antibacterial agents achieves microbiological cure from C.trachomatis infection during pregnancy. There was no apparent difference between assessed agents (amoxicillin, erythromycin, clindamycin, azithromycin) in terms of efficacy (microbiological cure and repeat infection) and pregnancy complications (preterm birth, preterm rupture of membranes, low birthweight). Azithromycin and clindamycin appear to result in fewer side effects than erythromycin.
All of the studies in this review were conducted in North America, which may limit the generalisability of the results. In addition, study populations may differ in low‐resource settings and these results are therefore only applicable to well‐resourced settings. Furthermore, the trials in this review mainly took place in the nineties and early 2000's and antibiotic resistance may have changed since then.
Further well‐designed studies, with appropriate sample sizes and set in a variety of settings, are required to further evaluate interventions for treating C.trachomatis infection in pregnancy and determine which agents achieve the best microbiological cure with the least side effects. Such studies could report on the outcomes listed in this review.
Plain language summary
Treatment of genital Chlamydia trachomatis infection in pregnancy
What is the issue?
This review aimed to assess whether the treatment of chlamydial infection during pregnancy cured the infection and prevented complications to the women and babies without causing side effects. This new review supersedes an earlier review on this topic.
Why is this important?
Chlamydia trachomatis is a bacterial infection which is sexually transmitted. It is more common in younger women. Women may have the infection without knowing it. In pregnant women, genital Chlamydia trachomatis can cause pregnancy complications such as preterm labour, preterm birth, premature rupture of the membranes, low birthweight of infants, and infection in the uterus after giving birth. Babies who acquire Chlamydia trachomatis during birth can develop infection of the lungs and the eyes.
Finding an effective treatment with minimal side effects is extremely important considering the complications that can occur with untreated Chlamydia trachomatis infection in pregnancy.
What evidence did we find?
We searched for evidence (June 2017) and included 15 studies in the review. The studies had a mixed risk of bias and were of limited quality, often with small numbers of participants. Three studies compared antibiotics (erythromycin, clindamycin, and amoxicillin) with placebo. The other studies compared different antibiotics with each other.
All of the studies reported on curing chlamydia, based on the elimination of the bacteria, with an antibiotic. Erythromycin (moderate‐quality evidence from two studies, 495 women) and clindamycin (low‐quality evidence from one study, 85 women) appeared to be more effective than placebo. The quality of the evidence for amoxicillin versus placebo (one study 15 women) was very low so we cannot be certain of the results.
When comparing different antibiotics with each other, no one antibiotic was substantially better than another at curing chlamydia in the studies that we examined: amoxicillin versus azithromycin (very low‐quality evidence from two studies, 144 women), amoxicillin versus erythromycin (high‐quality evidence from four studies, 466 women), azithromycin versus erythromycin (moderate‐quality evidence from six studies, 374 women), clindamycin versus erythromycin (low‐quality evidence from two studies, 173 women), amoxicillin versus clindamycin (moderate‐quality evidence from one study, 101 women). Only single trials assessed repeated infections, preterm birth, preterm rupture of membranes, perinatal mortality and low birthweight and found there were no clear differences between the different types of antibiotics examined.
Side effects were more common with erythromycin (two studies, 495 women) and clindamycin (one study, 85 women) than with placebo. Amoxicillin resulted in fewer side effects than azithromycin (one study, 36 women) or erythromycin (four studies, 513 women), and azithromycin caused fewer side effects than erythromycin (six studies, 374 women). Amoxicillin and clindamycin produced a similar number of side effects in one study (107 women).
What does this mean?
Treatment of chlamydia infection with antibiotics appears to be effective during pregnancy. There is no clear difference between amoxicillin, erythromycin, clindamycin, azithromycin in curing the infection or preterm birth, preterm rupture of membranes, and low birthweight. Azithromycin and clindamycin appear to result in fewer side effects than erythromycin.
The included studies were all carried out in North America. Chlamydia testing remains a problem in low‐resource settings because of its costs. We conclude that well‐designed studies of appropriate sample size, in different settings, are needed to further assess the effects of treatment of chlamydia infection in pregnancy. Resistance to the tested antibiotics could have changed since the studies included in this review were conducted. In particular, future research could report on the outcomes of focus in this review and target those antibiotics, such as amoxicillin and clindamycin, which may be effective in curing chlamydia with the least side effects.
Summary of findings
Background
The prevalence of chlamydial infection in pregnancy is between 2% to 30% depending on the patient's age and risk factors (Berggren 2011; Much 1991). It is particularly common in women younger than 25 years of age (Walker 2012). Genital Chlamydia trachomatis (C.trachomatis) infection has been shown to be associated with pregnancy complications such as miscarriage (Nigro 2011), preterm labour (Pararas 2006; Rours 2011), low birthweight (Attenburrow 1985) and increased perinatal mortality (Silva 2011). There may also be an association with preterm rupture of membranes (Blas 2007) and postpartum endometritis (Ismail 1987). If the mother is untreated, 20% to 50% of newborn babies may develop chlamydial conjunctivitis (Kakar 2010), and another 10% to 20% may develop C.trachomatis pneumonia (Rours 2009). Vaginal birth is associated with the highest risk of transmission of chlamydial infection, however, there is a small risk of acquiring the infection even in infants born by caesarean section with premature rupture of membranes and intact membranes (Pammi 2012; Yu 2009).
Genital C.trachomatis infection is detected by nucleic acid amplification test (NAAT) on the specimens of genital secretions or urine. This test has replaced tissue culture of C.trachomatis (Jespersen 2005).
Description of the condition
Genital C.trachomatis infection is a common bacterial sexually transmitted infection. The majority of women infected with this bacteria are asymptomatic and, therefore, may be more likely to transmit the infection because they do not seek treatment for the infection, which may result in a longer duration of the infection. The sequelae of C.trachomatis genital infection range from cervicitis to pelvic inflammatory disease, perihepatitis, ectopic pregnancy and infertility (Zenilman 2012). We have described complications of pregnancy and diseases of newborn related to genital Chlamydia infection in the Background section above.
C.trachomatis is a small gram‐negative intracellular bacterium with a two‐phased life‐cycle, which includes the form that infects new cells, (e.g. the small elementary body) and the active form (e.g. the reticulate body). The life‐cycle is about two to three days, and, therefore, sustained high serum minimum inhibitory concentration of antimicrobial agents is needed to achieve eradication of the infection, which can be achieved by long‐acting antimicrobials treatment or prolonged treatment. The incubation period of C.trachomatis infection varies between seven and 14 days (Zenilman 2012).
Description of the intervention
There are various treatment regimens for the management of chlamydial infection during pregnancy, however, there is no consensus on the most effective and safest option. In some, the hosts' immune system may even clear the infection.
According to the Centers for Disease Control and Prevention (CDC) guideline followed by many countries around the world, the recommended regimens for treatment of genital chlamydial infection in pregnancy are azithromycin (1 g orally given as a single dose) or amoxicillin (500 mg orally three times daily for seven days) (Workowski 2010). The alternative regimen according to the CDC guideline is erythromycin (500 mg or 250 mg orally four times daily for seven days), or erythromycin ethylsuccinate (800 mg orally four times daily for seven days, or 400 mg orally four times daily for 14 days) (Workowski 2010). Erythromycin is associated with a high degree of gastrointestinal side effects (primarily nausea) and the compliance may be an issue in such cases (Workowski 2010). Women who present in labour but were not treated for a prior positive chlamydial test are advised to be treated immediately with one of the above regimens. However, such late treatment is unlikely to substantially decrease the risk of transmission of Chlamydia to the newborn.
Clindamycin is another alternative drug for treatment of genital C.trachomatis infection. Despite it being safe in pregnancy, clindamycin is not used widely due to its cost (Miller 2000).
Other antibiotics such as doxycycline, levofloxacin, ofloxacin, and erythromycin estolate are used for the treatment of genital C.trachomatis outside of pregnancy. These drugs are contraindicated in pregnancy and lactation (Workowski 2010).
Azithromycin is believed to be the superior agent in comparison to other antibiotics for treatment of chlamydial infection but new research has emerged suggesting that there is a higher failure rate with azithromycin treatment of chlamydial infection than previously believed (Schwebke 2011). One of the explanations for this recent finding is a higher sensitivity of NAAT in comparison to that previously used in the tissue culture as a test of cure (Handsfield 2011), although it does not explain the similar cure rates reported after doxycycline treatment with both of these tests. Another explanation for treatment failure is heterotopic resistance with high Chlamydia loads which leads to treatment failures (Horner 2006). Re‐infection is also a cause of treatment failure (Horner 2006).
Cure rates of C.trachomatis in women who are pregnant are lower than in non‐pregnant women. The reasons behind this is a generally higher failure rate of treatment with amoxicillin, which has been traditionally used for treatment of C.trachomatis infection during pregnancy. A test of cure has always been recommended for all pregnant women and is performed no earlier than three weeks after treatment is initiated (Workowski 2010).
The previous Cochrane review on interventions for treating genital C.trachomatis infection in pregnancy found that amoxicillin was as effective as erythromycin (odds ratio (OR) 0.54, 95% confidence interval (CI) 0.28 to 1.02) (Brocklehurst 1998). Amoxycillin was found to be better tolerated than erythromycin (OR 0.16, 95% CI 0.09 to 0.30). Clindamycin and azithromycin were reported to be effective, however, the numbers of women included in trials were small (Brocklehurst 1998). New studies have been published in this area, therefore, it is important to update this review, which was done under new authorship.
How the intervention might work
Irradicating genital chlamydial infection during pregnancy with antibacterial drugs may lead to the following:
treatment of symptoms and sequelae of genital chlamydial infection such as discharge, cervicitis, pelvic inflammatory disease, tubal disease and infertility;
a decrease in perinatal complications such as preterm labour and early pregnancy loss, preterm rupture of membranes;
a decrease in transmission of the infection to the fetus or newborn and, therefore, prevention of intrauterine infection, neonatal conjunctivitis and pneumonia during pregnancy;
prevention of postpartum infection such as endometritis.
Why it is important to do this review
It is important to assess the different interventions for treating genital C.trachomatis in order to establish whether effective treatment of this infection improves perinatal outcomes and decreases maternal complications. This new review updates and replaces an earlier Cochrane review on this topic (Brocklehurst 1998).
Objectives
To establish the most efficacious and best‐tolerated therapy for treatment of genital chlamydial infection in preventing maternal infection and adverse neonatal outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials. Cluster‐randomised trials will be eligible for inclusion in this review in the future updates if identified. Quasi‐randomised trials and trials using cross‐over design were not eligible for inclusion. We included studies published in abstract form.
Types of participants
Pregnant women with a confirmed C.trachomatis infection.
Types of interventions
Any antibiotic versus no treatment or placebo for genital C.trachomatiss infection in pregnancy
One antibiotic versus another antibiotic
Different antibacterial regimens
Types of outcome measures
Primary outcomes
Microbiological cure ‐ negative Chlamydia test at least three weeks after treatment of the mother
Secondary outcomes
A. Maternal
Repeated infection
Preterm labour
Preterm birth
Preterm rupture of membranes
Chorioamnionitis
Postpartum endometritis
Sepsis
Prolonged hospital stay of the mother
Side effects of treatment
Maternal satisfaction with treatment
B. Fetal/neonatal
Perinatal mortality
Neonatal conjunctivitis
Neonatal pneumonia
Fetal anomalies
Low birthweight
Apgar score less than seven at five minutes
C. Cost
Cost of treatment
Search methods for identification of studies
The methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting their Information Specialist (26 June 2017).
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate the Pregnancy and Childbirth Group’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth Group in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, the Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies)
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) (26 June 2017) for unpublished, planned and ongoing trial reports. The search terms we used are given in Appendix 1.
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
The methods section of this review is based on a standard template used by the Cochrane Pregnancy and Childbirth Group.
Selection of studies
Two review authors independently assessed all the potential studies identified as a result of the search strategy for inclusion. Two review authors assessed the quality and extracted the data using the agreed form. Discrepancies were resolved through discussion with a third review author when needed. We entered data into Review Manager software and checked for accuracy. When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Studies published only in abstract form were included if they otherwise satisfied inclusion criteria. The authors of such studies were contacted if any additional information was required.
Data extraction and management
We designed a form to extract data. For eligible studies, review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted a third assessor. We entered data into Review Manager software (RevMan 2014), and checked for accuracy.
When information regarding any of the above is unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions(Higgins 2011). We resolved any disagreement by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding would be unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
A cut‐off point of 20% was used to assess the level of missing data as adequate for different outcomes.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update , we assessed the quality of the evidence for all comparisons using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the main outcome of microbiological cure.
We used GRADEpro GDT to import data from Review Manager 5.3 (RevMan 2014) to create 'Summary of findings' tables. A summary of the intervention effect and a measure of quality for each of the outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, we planned to use the mean difference if outcomes were measured in the same way between trials. We would have used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We did not identify any cluster‐randomised trials for inclusion in this version of the review. If we identify any cluster‐randomised trials for inclusion in future updates, we will include them in our analyses along with individually‐randomised trials. We will adjust their standard errors using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we will synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the studies and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will acknowledged heterogeneity in the randomisation unit and perform a subgroup analysis to investigate the effects of the randomisation unit.
Cross‐over trials
This study design is not eligible for inclusion in this review.
Other unit of analysis issues
We identified the trials with more than two treatment groups and included each pair‐wise comparison separately, but with shared intervention groups divided out approximately evenly among the comparisons. For dichotomous outcomes, both the number of events and the total number of patients were divided up. For continuous outcomes, only the total number of participants were divided up and the means and standard deviations left unchanged (Cochrane Handbook for Systematic Reviews of Interventions 16.5.4).
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis (see Sensitivity analysis).
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
We would have excluded studies with more than 20% missing data.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
In future updates of this review, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots and will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
Where we used random‐effects analyses, the results are presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
We did not carry out ant of the planned subgroup analyses as the outcomes only had a few included trials. In future updates if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will consider whether an overall summary is meaningful, and if it is, use random‐effects analysis to produce it.
We would consider carrying out the following subgroup analyses.
Women with a first episode versus women with recurrent (previously treated in pregnancy) genital C.trachomatis infection
Women in the first half (before 20 weeks) versus women in the second half (including 20 weeks and after 20 weeks) of pregnancy
The following outcome would be used in subgroup analysis.
Microbiological cure negative Chlamydia test after treatment for the mother
We would have assessed subgroup differences by interaction tests available within RevMan (RevMan 2014). We would have reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
Sensitivity analyses were not performed as there were no aspects of the review that may have affected the results, for example, the risk of bias associated with the quality of some of the included trials. We would have undertaken analysis of the primary outcome separately for trials with low risk of bias and high and unknown risk of bias (allocation concealment) if needed. Sensitivity analysis would have been carried out to explore the effects of random‐effects analyses for outcomes with statistical heterogeneity. We would also have carried out sensitivity analysis to investigate the effect of the randomisation unit if we had included cluster‐randomised controlled trials along with the individually‐randomised trials.
Results
Description of studies
Results of the search
The search of the Cochrane Pregnancy and Childbirth Group's Trials Register retrieved 23 reports of 20 trials and we retrieved no other studies from other sources (see: Figure 1). We included 15 studies, excluded four, and one is ongoing (Okunola 2013).
1.
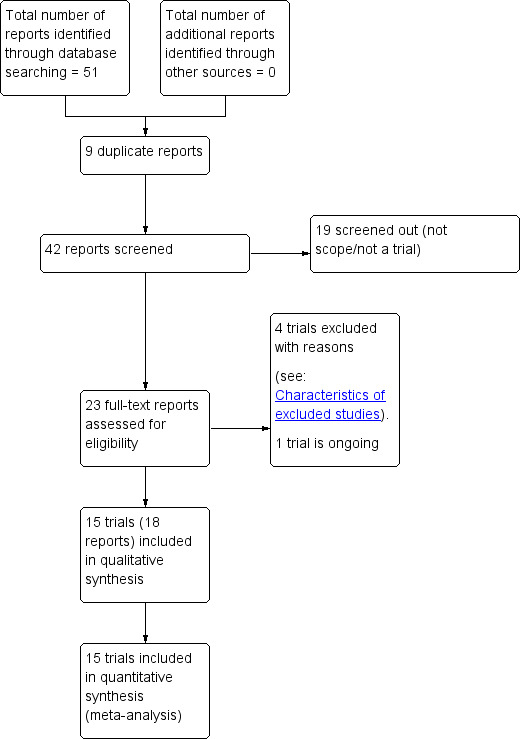
Study flow diagram.
Included studies
We included 15 studies into the meta‐analysis with a total of 1754 women. Meta‐analyses were mostly based on fewer numbers of studies.
Methods
All the trials were randomised control trials of pregnant women with confirmed Chlamydia trachomatis (C.trachomatis) infection.
Populations and settings
All of the included studies were undertaken in North America (14 in USA and one in Canada). One study took place in 1982, and the rest took place in the nineties and early 2000s.
Interventions and comparisons
Two studies compared erythromycin and placebo (Alger 1991; Martin 1997). One study compared clindamycin and placebo (Alger 1991). One study compared amoxicillin versus placebo (Bell 1982). Two studies compared azithromycin and amoxicillin (Jacobson 2001; Kacmar 2001). Four studies compared amoxicillin and erythromycin (Alary 1994; Magat 1993; Silverman 1994; Turrentine 1995). Six studies compared erythromycin and azithromycin (Adair 1998; Bush 1994; Edwards 1996; Gunter 1996; Rosenn 1995; Wehbeh 1998). Two studies compared clindamycin and erythromycin (Alger 1991; Turrentine 1995). One study compared amoxicillin and clindamycin (Turrentine 1995).
Funding sources
Adair 1998, Edwards 1996, and Turrentine 1995 had drugs donated by a pharmaceutical company at no cost. Alger 1991 was funded by a grant from the Upjohn company.
Alary 1994 was funded by a grant from the National Health Research and Development Program. Kacmar 2001 was funded by a NIH grant. Martin 1997 was funded by a National Institute of Child Health and Human Development grant. Bell 1982 was supported by a US Public Health Service grant.
Wehbeh 1998 was funded by local departmental funds.
Bush 1994, Gunter 1996, Jacobson 2001, Magat 1993, Rosenn 1995, and Silverman 1994 did not disclose any funding sources.
Trial authors' declarations of interest
Declarations of interest were not mentioned in any of the included studies.
Excluded studies
Reasons for exclusion are as follows.
El‐Shourbagy 2011 ‐ this study examines the rate of pre‐eclampsia in groups of treated and non‐treated Chlamydia pneumoniae infections in pregnancy.
McGregor 1990 ‐ this study included pregnant women with various genital tract infections and not only Chlamydia trachomatis. The data for Chlamydia trachomatis infection were not presented separately.
Nadafi 2005 ‐ this study included women with positive and negative Chlamydia test, it was a cohort study, sequence generation was not clear. The data for women with positive and negative Chlamydia test are presented together.
Zulkarneev 1998 ‐ this study was not a randomised controlled trial.
Risk of bias in included studies
2.
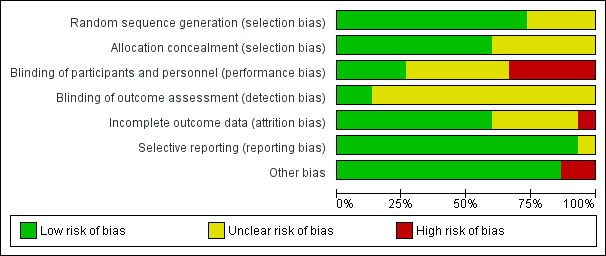
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
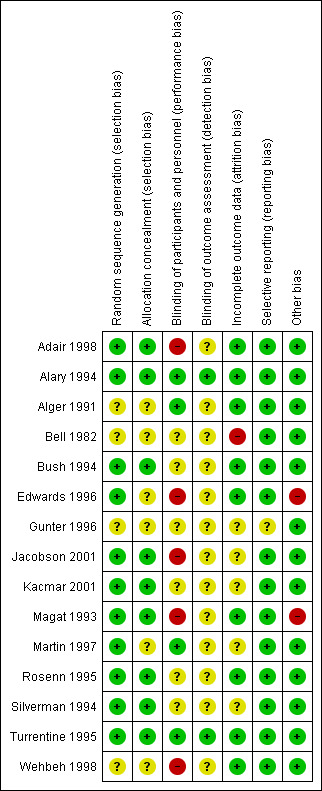
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Eleven studies had low risk of selection bias, e.g. three studies used number of blocks for allocation (Adair 1998; Alary 1994; Rosenn 1995), four studies used computer‐generated randomisation for allocation (Bush 1994; Jacobson 2001; Martin 1997; Turrentine 1995), four studies used random number tables (Edwards 1996; Kacmar 2001; Magat 1993; Silverman 1994). Four studies had unclear risk of selection bias, e.g. allocation method was not described (Alger 1991; Bell 1982; Gunter 1996; Wehbeh 1998).
Nine studies had a low risk of bias for allocation sequence. Six used sealed opaque envelopes (Adair 1998; Bush 1994; Jacobson 2001, Kacmar 2001; Rosenn 1995; Silverman 1994). One study used identical treatment packs (Alary 1994). In two trials the medications were dispensed by the pharmacy to prevent the healthcare practitioners knowing which medication and which dose were allocated (Magat 1993; Turrentine 1995). There was an unclear risk in six studies as allocation concealment was not described (Alger 1991; Bell 1982; Edwards 1996; Gunter 1996; Martin 1997; Wehbeh 1998).
Blinding
Performance bias
Blinding of participants and personnel was performed four studies (Alary 1994; Alger 1991; Martin 1997; Turrentine 1995).
Five studies did not implement blinding of participants or personnel and were assessed as high risk (Adair 1998; Edwards 1996; Jacobson 2001; Magat 1993; Wehbeh 1998).
Six studies did not describe performance blinding (Bell 1982; Bush 1994; Gunter 1996; Kacmar 2001; Rosenn 1995; Silverman 1994).
Assessment bias
Blinding of outcome assessment was unclear in 13 studies (Adair 1998; Alger 1991; Bell 1982; Bush 1994; Edwards 1996; Gunter 1996; Jacobson 2001; Kacmar 2001; Magat 1993; Martin 1997; Rosenn 1995; Silverman 1994; Wehbeh 1998).
Assessment bias was assessed as low risk in two studies were staff taking cultures were blinded to treatment group (Alary 1994; Turrentine 1995).
Incomplete outcome data
No studies had significant attrition bias. All losses to follow‐up were described. One study (Bell 1982) had high attrition for the final outcome reporting data for only 71% of participants. Five studies are at unclear risk of attrition bias due to insufficient information given in the study report (Gunter 1996), and some unexplained loss to follow‐up (Jacobson 2001; Kacmar 2001; Martin 1997; Silverman 1994).
Selective reporting
One study was published only in abstract form and states that it is an ongoing trial but no further information has been published (Gunter 1996). The remaining 14 studies were rated as being at low risk of reporting bias.
Other potential sources of bias
Two studies had unexplained different mean gestational ages in women in the two treatment arms (Edwards 1996; Magat 1993). The remaining 13 studies were assessed as being at a low risk of other bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
Summary of findings for the main comparison. Erythromycin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy.
| Erythromycin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection Setting: Obstetric Clinics, USA Intervention: Erythromycin Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Erythromycin | |||||
| Microbiological cure | Study population | Average RR 2.64 (1.60 to 4.38) | 495 (2 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 | ||
| 344 per 1000 | 908 per 1000 (550 to 1000) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Statistical Heterogeneity (I2 > 60%). (Inconsistency: ‐1)
2 One included study has design limitations but contributed < 40% weight. (Not downgraded)
Summary of findings 2. Clindamycin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy.
| Clindamycin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection Setting: Obstetric Clinic, USA Intervention: Clindamycin Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Clindamycin | |||||
| Microbiological cure | Study population | RR 4.08 (2.35 to 7.08) | 85 (1 RCT) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 227 per 1000 | 927 per 1000 (534 to 1000) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 The included study had design limitation (Design limitations: ‐1)
2 Wide confidence interval and small sample size (Imprecision: ‐1)
Summary of findings 3. Amoxicillin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy.
| Amoxicillin compared to placebo for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection Setting: USA Intervention: Amoxicillin Comparison: Placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 2.00 (0.59 to 6.79) | 15 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 333 per 1000 | 667 per 1000 (197 to 1000) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 The included study had design limitation (Design limitations: ‐1)
2 Wide confidence intervals crossing the line of no effect, few events, and small sample size (Imprecision: ‐2)
Summary of findings 4. Amoxicillin compared to azithromycin for treating genital Chlamydia trachomatis infection in pregnancy.
| Amoxicillin compared to azithromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection Setting: Prenatal clinics, USA Intervention: Amoxicillin Comparison: Azithromycin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with azithromycin | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 0.89 (0.71 to 1.12) | 144 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 716 per 1000 | 637 per 1000 (509 to 802) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 One study contributing to over 68% of weight to pooled analysis had some design limitations (Design limitations: ‐1)
2 Wide confidence intervals crossing the line of no effect and small size (Imprecision: ‐2)
Summary of findings 5. Amoxicillin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy.
| Amoxicillin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection Setting: Obstetric centre or prenatal clinics in Canada, USA Intervention: Amoxicillin Comparison: Erythromycin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with erythromycin | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 0.97 (0.93 to 1.01) | 466 (4 RCTs) | ⊕⊕⊕⊕ HIGH | One study contributing to 24% of weight had some design limitation. (not downgraded) | |
| 954 per 1000 | 925 per 1000 (887 to 963) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
Summary of findings 6. Azithromycin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy.
| Azithromycin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection Setting: Prenatal clinics, and university medical centres, USA Intervention: Azithromycin Comparison: erythromycin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with erythromycin | Risk with Azithromycin | |||||
| Microbiological cure | Study population | Average RR 1.11 (1.00 to 1.23) | 374 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 | ||
| 825 per 1000 | 916 per 1000 (825 to 1000) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Most studies have design limitations (Design limitations: ‐1)
2 Statistical heterogeneity at 53% (I2 < 60%) (not downgraded)
Summary of findings 7. Clindamycin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy.
| Clindamycin compared to erythromycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection Setting: Prenatal clinics, USA Intervention: Clindamycin Comparison: Erythromycin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with erythromycin | Risk with Clindamycin | |||||
| Microbiological cure | Study population | RR 1.06 (0.97 to 1.15) | 173 (2 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | ||
| 905 per 1000 | 959 per 1000 (878 to 1000) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 One study contributing to over 40% of weight to pooled analysis had some design limitations (Design limitations: ‐1)
2 Small sample size (Imprecision: ‐1)
Summary of findings 8. Amoxicillin compared to clindamycin for treating genital Chlamydia trachomatis infection in pregnancy.
| Amoxicillin compared to clindamycin for treating genital Chlamydia trachomatis infection in pregnancy | ||||||
| Patient or population: Pregnant women with a confirmed Chlamydia trachomatis infection Setting: Prenatal clinic, USA Intervention: Amoxicillin Comparison: Clindamycin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with clindamycin | Risk with Amoxicillin | |||||
| Microbiological cure | Study population | RR 0.96 (0.89 to 1.04) | 101 (1 RCT) | ⊕⊕⊕⊝ MODERATE 1 | ||
| 979 per 1000 | 940 per 1000 (871 to 1000) | |||||
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 The pooled effect was based on one study with a small sample size (Imprecision: ‐1)
Erythromycin versus placebo (comparison 1)
Primary outcome
Microbiological cure
Erythromycin appears to improve microbiological cure in comparison to placebo (moderate‐certainty evidence, Table 1; (average risk ratio (RR) 2.64, 95% confidence interval (CI) 1.60 to 4.38; 495 women; studies = two; I² = 68%; Analysis 1.1)). There was evidence of substantial heterogeneity between the studies (I² = 68%) in effect size; both studies found erythromycin improved microbiological cure.
1.1. Analysis.
Comparison 1 Erythromycin versus placebo, Outcome 1 Microbiological cure.
Secondary outcomes
Preterm birth
There was no clear difference in preterm births (RR 0.90, 95% CI 0.56 to 1.46; 405 women; studies = one; Analysis 1.2).
1.2. Analysis.
Comparison 1 Erythromycin versus placebo, Outcome 2 Preterm birth.
Preterm rupture of membranes
There was no clear difference in preterm rupture membranes between the treatment groups (RR 0.83, 95% CI 0.48 to 1.43; 389 women; studies = one; Analysis 1.3).
1.3. Analysis.
Comparison 1 Erythromycin versus placebo, Outcome 3 Preterm rupture of membranes.
Side effects of treatment
We are uncertain if erythromycin results in a higher incidence of side effects when compared to placebo (average RR 2.93, 95% CI 0.36 to 23.76; 495 women; studies = two; I² = 78%; Analysis 1.4). There was substantial heterogeneity between the studies, I² = 78%. The side effects reported included nausea, appetite loss (Martin 1997), vomiting, diarrhoea, and abdominal pain (Alger 1991).
1.4. Analysis.
Comparison 1 Erythromycin versus placebo, Outcome 4 Side effects of treatment.
Perinatal mortality
There was no clear difference in perinatal deaths between the groups (RR 3.01, 95% CI 0.32 to 28.74; 405 women; studies = one; Analysis 1.5).
1.5. Analysis.
Comparison 1 Erythromycin versus placebo, Outcome 5 Perinatal mortality.
Low birthweight
There was no clear difference in low birthweight between the groups (RR 0.77, 95% CI 0.42 to 1.40; 400 women; studies = one; Analysis 1.6).
1.6. Analysis.
Comparison 1 Erythromycin versus placebo, Outcome 6 Low birthweight.
Other secondary outcomes
No studies assessed the other secondary outcomes.
Clindamycin versus placebo (comparison 2)
Primary outcome
Microbiological cure
Clindamycin appears to improve microbiological cure in comparison to placebo (low‐certainty evidence, Table 2; (RR 4.08, 95% CI 2.35 to 7.08; 85 women; studies = one; Analysis 2.1)). One study (Alger 1991), which was funded by a pharmaceutical company contributed to this comparison.
2.1. Analysis.
Comparison 2 Clindamycin versus placebo, Outcome 1 Microbiological cure.
Secondary outcomes
Side effects of treatment
There was no clear difference in side effects between the two groups (RR 5.37, 95% CI 0.65 to 44.01; 85 women; studies = one; Analysis 2.2). The side effects included a rash and mild gastrointestinal complaints including nausea and vomiting, abdominal pain, cramps and diarrhoea.
2.2. Analysis.
Comparison 2 Clindamycin versus placebo, Outcome 2 Side effects of treatment.
Other secondary outcomes
No studies assessed the other secondary outcomes.
Amoxicillin versus placebo (comparison 3)
Primary outcome
Microbiological cure
It is uncertain whether amoxicillin improves microbiological cure in comparison to placebo but the certainty of this evidence is very low (Table 3; (RR 2.00, 95% CI 0.59 to 6.79; 15 women; studies = one; Analysis 3.1)).
3.1. Analysis.
Comparison 3 Amoxicillin versus placebo, Outcome 1 Microbiological cure.
Secondary outcomes
No secondary outcomes were reported for this outcome in the included studies.
Amoxicillin versus azithromycin (comparison 4)
Primary outcome
Microbiological cure
It is uncertain whether amoxicillin improves or reduces microbiological cure in comparison to azithromycin because the certainty of this evidence is very low (Table 4 ; (RR 0.89, 95% CI 0.71 to 1.12; 144 women; studies = two; Analysis 4.1)).
4.1. Analysis.
Comparison 4 Amoxicillin versus azithromycin, Outcome 1 Microbiological cure.
Secondary outcomes
Repeated infection
There was no clear difference for the outcome of repeated infections between amoxicillin and azithromycin in the single included study (RR 0.42, 95% CI 0.02 to 9.55; 34 women; studies = one; Analysis 4.2).
4.2. Analysis.
Comparison 4 Amoxicillin versus azithromycin, Outcome 2 Repeated infection.
Preterm birth
There was no clear difference in the incidence of preterm birth between amoxicillin and azithromycin (RR 1.17, 95% CI 0.43 to 3.20; 90 women; studies = one; Analysis 4.3).
4.3. Analysis.
Comparison 4 Amoxicillin versus azithromycin, Outcome 3 Preterm birth.
Side effects of treatment
There was no clear difference in side effects between the two groups (RR 0.56, 95% CI 0.24 to 1.31; 36 women; studies = one; Analysis 4.4). Side effects reported included nausea, vomiting, diarrhoea and abdominal pain.
4.4. Analysis.
Comparison 4 Amoxicillin versus azithromycin, Outcome 4 Side effects of treatment.
Other secondary outcomes
None were reported.
Amoxicillin versus erythromycin (comparison 5)
Primary outcome
Microbiological cure
Amoxicillin makes little or no difference to microbiological cure in comparison to erythromycin (high‐certainty evidence, Table 5; (RR 0.97, 95% CI 0.93 to 1.01; 466 women; studies = 4; Analysis 5.1)).
5.1. Analysis.
Comparison 5 Amoxicillin versus erythromycin, Outcome 1 Microbiological cure.
Secondary outcomes
Side effects of treatment
Amoxicillin was associated with reduced incidence of side effects in comparison to erythromycin (RR 0.31, 95% CI 0.21 to 0.46; 513 women; studies = four; I² = 27%; Analysis 5.2). Side effects associated with erythromycin use included nausea, vomiting, diarrhoea, abdominal cramping, rash, and an allergic reaction.
5.2. Analysis.
Comparison 5 Amoxicillin versus erythromycin, Outcome 2 Side effects of treatment.
Other secondary outcomes
None were reported.
Azithromycin versus erythromycin (comparison 6)
Primary outcome
Microbiological cure
It appears that azithromycin probably improves microbiological cure in comparison to erythromycin (moderate‐certainty evidence, Table 6; (average RR 1.11, 95% CI 1.00 to 1.23; participants = 374; studies = six; I² = 53%; Analysis 6.1)), however, there was substantial heterogeneity between the included studies (I² = 53%) and the lower confidence interval just touches the line of no effect.
6.1. Analysis.
Comparison 6 Azithromycin versus erythromycin, Outcome 1 Microbiological cure.
Secondary outcomes
Repeated infection
There was no clear difference between azithromycin and amoxicillin for the outcome of repeated infections (RR 1.37, 95% CI 0.32 to 5.73; 85 women; studies = one; Analysis 6.2).
6.2. Analysis.
Comparison 6 Azithromycin versus erythromycin, Outcome 2 Repeated infection.
Preterm birth
There was no clear difference in the rate of preterm birth between azithromycin and amoxicillin (RR 0.77, 95% CI 0.29 to 2.10; 126 women; studies = one; Analysis 6.3).
6.3. Analysis.
Comparison 6 Azithromycin versus erythromycin, Outcome 3 Preterm birth.
Preterm rupture of membranes
There was no clear difference for the outcome of preterm rupture of membranes between azithromycin and amoxicillin (RR 0.62, 95% CI 0.15 to 2.48; 126 women; studies = one; Analysis 6.4).
6.4. Analysis.
Comparison 6 Azithromycin versus erythromycin, Outcome 4 Preterm rupture of membranes.
Side effects of treatment
Fewer women in the azithromycin group experienced side effects in comparison to women receiving erythromycin (RR 0.24, 95% CI 0.17 to 0.34; 374 women; studies = six; Analysis 6.5). These side effects were mostly gastrointestinal in origin and included nausea, vomiting, diarrhoea and abdominal cramping.
6.5. Analysis.
Comparison 6 Azithromycin versus erythromycin, Outcome 5 Side effects of treatment.
Other secondary outcomes
None were reported.
Clindamycin versus erythromycin (comparison 7)
Primary outcome
Microbiological cure
Clindamycin may make little or no difference on microbiological cure in comparison to erythromycin (low‐certainty evidence,Table 7; (RR 1.06, 95% CI 0.97 to 1.15; 173 women; studies = two; Analysis 7.1)).
7.1. Analysis.
Comparison 7 Clindamycin versus erythromycin, Outcome 1 Microbiological cure.
Secondary outcomes
Side effects of treatment
Women in the clindamycin group experienced less side effects in comparison to erythromycin (RR 0.44, 95% CI 0.22 to 0.87; 183 women; studies = two; Analysis 7.2). These side effects were mostly gastrointestinal in origin and included nausea, vomiting, diarrhoea and abdominal cramping.
7.2. Analysis.
Comparison 7 Clindamycin versus erythromycin, Outcome 2 Side effects of treatment.
Other secondary outcomes
None were reported.
Amoxicillin versus clindamycin (comparison 8)
Primary outcome
Microbiological cure
Amoxicillin probably makes little or no difference on microbiological cure in comparison to clindamycin (moderate‐certainty evidence, Table 8; (RR 0.96, 95% CI 0.89 to 1.04; 101 women; studies = one; Analysis 8.1)).
8.1. Analysis.
Comparison 8 Amoxicillin versus clindamycin, Outcome 1 Microbiological cure.
Secondary outcomes
Side effects of treatment
There was no clear difference in number of side effects associated with amoxicillin and clindamycin (RR 0.57, 95% CI 0.14 to 2.26; 107 women; studies = one; Analysis 8.2). The side effects reported included rash and gastrointestinal complaints.
8.2. Analysis.
Comparison 8 Amoxicillin versus clindamycin, Outcome 2 Side effects of treatment.
Other secondary outcomes
None were reported.
Discussion
Summary of main results
Fifteen studies involving 1754 women were included in this review but our meta‐analyses are based on fewer numbers of studies/women. We excluded four studies and one study is ongoing.
Erythromycin (moderate‐certainty evidence) and clindamycin (low‐certainty evidence) were associated with a higher incidence of microbiological cure in comparison to placebo. Results were unclear in one very small study comparing amoxicillin placebo but the evidence was graded very‐low certainty.
There is no clear difference in microbiological cure between the assessed agents compared to each other: amoxicillin versus azithromycin (very low‐certainty evidence); amoxicillin versus erythromycin (high‐certainty evidence); azithromycin versus erythromycin (moderate‐certainty evidence); clindamycin versus erythromycin (low‐certainty evidence); amoxicillin versus clindamycin (moderate‐certainty evidence). There was no clear difference in repeat infections for amoxicillin versus azithromycin, or azithromycin versus erythromycin. Most secondary outcomes were not reported in any of the included studies.
Antibacterial treatment of genital Chlamydia trachomatis (C.trachomatis) infection was associated with side effects which were more common with the use of erythromycin and clindamycin than placebo as would be expected. Amoxicillin and clindamycin were associated with less side effects than azithromycin and erythromycin. Azithromycin caused less side effects than erythromycin. Side effects associated with erythromycin, azithromycin and clindamycin included nausea, vomiting, abdominal cramping and diarrhoea. Clindamycin use was occasionally associated with a non severe rash.
There were only a few studies that assessed the outcomes of preterm birth, preterm rupture of membranes and low birthweight. No studies assessed chorioamnionitis, postpartum endometritis, sepsis, prolonged hospital stay, maternal satisfaction, neonatal conjunctivitis, neonatal pneumonia, fetal anomalies, low birthweight and Apgar scores.
Overall completeness and applicability of evidence
All of the included studies were undertaken in North America (14 in USA and 1 in Canada) in 1982 and the mid to late nineties and early 2000s. Antibiotic resistance may have changed since these studies were performed. Study populations could differ in low‐resource settings and the results are therefore only applicable to well‐resourced settings. C.trachomatis testing remains a challenge in low‐resource settings because of the cost, and the treatment of genital infection is still based on a syndromic approach (South African STI guideline 2015). There was little or no information on the outcomes of preterm labour, preterm birth, preterm rupture of membranes, chorioamnionitis, postpartum endometritis, sepsis, prolonged hospital stay, maternal satisfaction with treatment, perinatal mortality, neonatal conjunctivitis, neonatal pneumonia, fetal anomalies, low birthweight, Apgar score less than seven at five minutes and cost of treatment.
Quality of the evidence
We assessed the included studies for risk of bias. Two studies (Alary 1994; Turrentine 1995) were assessed to be at low risk of bias in all domains. The remaining studies had varying risks of bias; blinding of participants and outcome assessors was unclear, not reported, or not attempted in most studies. We carried out formal assessments of quality of the evidence using GRADEpro for the review's primary outcome of microbiological cure. For this outcome, the evidence was graded from very low to high certainty for the different comparisons: amoxicillin versus placebo and versus azithromycin were graded very low quality; clindamycin versus placebo, and versus erythromycin were graded low quality; erythromycin versus placebo, azithromycin versus erythromycin, and amoxicillin versus clindamycin were graded moderate quality; amoxicillin versus erythromycin was graded high quality. Evidence was downgraded for limitations in study designs, inconsistency, and imprecision in effect estimates.
Potential biases in the review process
Evidence in this review was derived from studies identified in a detailed search process. Trials comparing interventions to treat C. trachomatis infection in pregnancy that have not been published may not have been identified. We attempted to minimise bias in the review process by having two review authors independently extract data.
Agreements and disagreements with other studies or reviews
We did not find any publications which included meta‐analysis of published studies, but we have identified two recent reviews/guidelines addressing the treatment of C.trachomatis during pregnancy.
CDC guidelines (CDC 2015) and the up‐to‐date review (Marrazzo 2016) recommends the treatment of C.trachomatis infection in pregnancy with azithromycin based on clinical practice as it is safe and effective. Recommended alternatives suggested by both documents are amoxicillin and erythromycin. A test of cure is recommended in pregnant women three to four weeks after treatment and again three months later. Resistance to amoxicillin is highlighted, however, it is referenced with respect to animal studies only. The review and guideline did not suggest clindamycin as an alternative, but according to limited data from this review it could be considered as a treatment option.
Authors' conclusions
Implications for practice.
The current evidence on individual antibiotic interventions for treating genital Chlamydia trachomatis (C.trachomatis) infection in pregnancy is limited ‐ the largest meta‐analysis in this review includes six studies involving 374 women, and most include only one or two studies. Clindamycin, erythromycin, and amoxicillin seem to be effective compared with placebo in achieving microbiological cure, however, the evidence related to amoxicillin is very low quality and we cannot be certain of this. There were no clear differences in microbiological cure between different antibiotics when compared against each other. Erythromycin was associated with more side effects than clindamycin, azithromycin, and amoxicillin, including nausea, vomiting, diarrhoea and abdominal cramps. The evidence related to effects of treatment on a number of maternal and most fetal outcomes is sparse.
Implications for research.
Further well‐designed studies of appropriate sample size are required to assess interventions for treating C.trachomatis infection in pregnancy with agents achieving the best microbiological cure and causing least side effects such as amoxicillin and clindamycin. The secondary outcomes in this review have been under‐reported. Future research could assess these outcomes: repeated infection, preterm labour, preterm birth, preterm rupture of membranes, chorioamnionitis, postpartum endometritis, sepsis, prolonged hospital stay of the mother, maternal satisfaction with treatment, perinatal mortality, neonatal conjunctivitis, neonatal pneumonia, fetal anomalies, low birthweight, Apgar score less than seven at five minutes and cost of treatment. A network meta‐analysis would be beneficial to compare agents which have not yet been compared directly. Future research is needed in low‐resource settings were population characteristics, cost, and treatment approach may differ from the studies included in this review.
Notes
This new review updates and supersedes an earlier review on this topic by Brocklehurst 1998.
Acknowledgements
The Cochrane Pregnancy and Childbirth Group and peer referees.
This project was supported by the National Institute for Health Research, via Cochrane infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who is external to the editorial team), a member of the Cochrane Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Appendices
Appendix 1. Search terms for ClinicalTrials.gov and ICTRP
ClinicalTrials.gov
chlamydia AND pregnancy
chlamydia AND pregnant
Data and analyses
Comparison 1. Erythromycin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Microbiological cure | 2 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 2.64 [1.60, 4.38] |
| 2 Preterm birth | 1 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.56, 1.46] |
| 3 Preterm rupture of membranes | 1 | 389 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.48, 1.43] |
| 4 Side effects of treatment | 2 | 495 | Risk Ratio (M‐H, Random, 95% CI) | 2.93 [0.36, 23.76] |
| 5 Perinatal mortality | 1 | 405 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [0.32, 28.74] |
| 6 Low birthweight | 1 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.42, 1.40] |
Comparison 2. Clindamycin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Microbiological cure | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.08 [2.35, 7.08] |
| 2 Side effects of treatment | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.37 [0.65, 44.01] |
Comparison 3. Amoxicillin versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Microbiological cure | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.59, 6.79] |
Comparison 4. Amoxicillin versus azithromycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Microbiological cure | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.12] |
| 2 Repeated infection | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.02, 9.55] |
| 3 Preterm birth | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.43, 3.20] |
| 4 Side effects of treatment | 1 | 36 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.24, 1.31] |
Comparison 5. Amoxicillin versus erythromycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Microbiological cure | 4 | 466 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.93, 1.01] |
| 2 Side effects of treatment | 4 | 513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.21, 0.46] |
Comparison 6. Azithromycin versus erythromycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Microbiological cure | 6 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [1.00, 1.23] |
| 2 Repeated infection | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.32, 5.73] |
| 3 Preterm birth | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.29, 2.10] |
| 4 Preterm rupture of membranes | 1 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.15, 2.48] |
| 5 Side effects of treatment | 6 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.17, 0.34] |
Comparison 7. Clindamycin versus erythromycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Microbiological cure | 2 | 173 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.97, 1.15] |
| 2 Side effects of treatment | 2 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.22, 0.87] |
Comparison 8. Amoxicillin versus clindamycin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Microbiological cure | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.89, 1.04] |
| 2 Side effects of treatment | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.14, 2.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adair 1998.
| Methods | Randomised controlled trial. | |
| Participants | 106 pregnant women screened positive by a direct DNA probe for Chlamydia trachomatis were enrolled. Exclusion criteria included hypersensitivity to erythromycin or azithromycin, lack of desire to participate in the study, or gestational age at most 14 weeks. 85 women entered and completed the entire trial protocol. 42 were assigned to azithromycin and 43 were assigned to erythromycin. | |
| Interventions | Azithromycin 1 g oral slurry in a single dose or erythromycin base 500 mg orally 4 times daily for 7 days. | |
| Outcomes | Cure rate, repeated infection, side effects (nausea and vomiting). | |
| Notes | In the azithromycin group, 9 were lost to follow‐up, 2 were not pregnant, 1 was treated off protocol.
In the erythromycin group, 7 were lost to follow‐up, 2 were not pregnant, 1 was treated off protocol.
74.4% in the erythromycin group and 50% in the azithromycin group completed the protocol as prescribed within the 3‐week period. This high rate of prolonged, unconfirmed test of cure could have resulted in higher positive tests of cure or possibly higher re‐infection rates in the azithromycin group.
Compliance in the azithromycin group was 97.6% and in the erythromycin group it was 53.5%.
Patients with positive Chlamydia assays at the test of cure were treated with the alternative agent to the originally assigned agent.
Sample size estimates suggested the need to enrol 120 patients. Performed at Bowman Gray School of Medicine of Wake Forest University, North Carolina, USA. Sources of trial funding: The drugs used in this study were supplied without charge by Pfizer, Incorporated, New York, New York. Declarations of interest: Not reported. Trial dates: 1995 ‐ 1997. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assignment to treatment was made by random numbers in blocks of 20 by the program Rancode‐Plus 3.1.1. |
| Allocation concealment (selection bias) | Low risk | Allocation cards were placed in a sealed opaque envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No attempt was made to blind the investigators to treatment allocations after enrolment. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 85 (80.2%) women completed the study. The number lost to follow‐up was similar in both the groups. |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Alary 1994.
| Methods | Randomised controlled trial. | |
| Participants | 210 women culture‐positive for Chlamydia trachomatis were included. Exclusion criteria: allergy to either drug, treatment with antibiotics after the chlamydial culture was done, abortion or miscarriage, moving away from the study area or more than 38 weeks of gestation at diagnosis. 11 were excluded from the final analysis (see below). Outcome data were available for 199 women. 100 were treated with amoxicillin. 99 were treated with erythromycin. |
|
| Interventions | Amoxycillin 500 mg 3 times daily for 7 days versus erythromycin 500 mg 4 times daily for 7 days. | |
| Outcomes | Cure rate. SIde effects (gastrointestinal). |
|
| Notes | Urethral samples from sexual partners of positive patients were cultured, and doxycycline treatment (100 mg twice a day for 10 days) was given free of charge. Eye, nose, pharyngeal, rectal and genital swabs were obtained in infants in the week after birth. Patients unable to tolerate their medication were offered the alternative treatment. In these cases, second cervical and urethral samples were cultured and a doctor independent of the study allocated the alternative treatment without informing the patients, investigators or the responsible physicians what the first therapy had been. 11 of the 210 enrolled women were excluded from the final analysis. 6 (4 amoxicillin, 2 erythromycin) did not attend to any follow‐up visits. 1 (amoxicillin) received another antibiotic during the early phase of the trial. 2 (erythromycin) delivered before any outcome measurements. In 2 women receiving erythromycin, treatment was interrupted by the physician because of side effects and the patients did not come for follow‐up tests. In the erythromycin group, 5 patients had a temporary treatment interruption due to severe side effects and 12 patients had to be stopped permanently. In the amoxicillin group, 1 had temporary treatment interruption and 1 permanent withdrawal due to side effects. Of the 13 patients who could not complete their treatment, 10 accepted an alternative treatment, and all but 1 were cured. Re‐infection cannot be ruled out for this patient, since her regular sexual partner did not attend follow‐up. Compliance was above 95% in both groups with exclusion of the 13 patients who could not complete their treatment because of side effects. Study performed in 9 obstetric centres in the province of Quebec, Canada. Sources of trial funding: Study was supported by a grant from the National Health Research and Development Program. Declarations of interest: Not reported. Trial dates: January 1990 ‐ April 1993. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Each woman was randomly assigned treatment in a predetermined order in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Identical treatment packs were used. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. "The drugs were issued in identical capsules in similar blister packs. In the amoxycillin packs, to maintain double‐blind nature of the project, the third daily dose was a placebo whereas other capsules contained 250 mg amoxycillin." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Staff taking sample cultures after treatment termination were unaware of the treatment group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After randomisation, only 5.6% women lost to follow ‐up, equally balanced across the two groups. |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Alger 1991.
| Methods | Randomised controlled trial. | |
| Participants | 135 pregnant women with culture‐positive endocervical Chlamydia trachomatis infection were enrolled. Data were available for 126. 40 women received erythromycin and a clindamycin placebo. 42 women received clindamycin and erythromycin placebo. 44 received a placebo for both clindamycin and erythromycin. |
|
| Interventions | Clindamycin (450 mg), erythromycin (331 mg), placebo orally 4 times per day for 14 days. | |
| Outcomes | Cure rate, side effects. | |
| Notes | Partners treated with doxycycline 100 mg orally twice a day for 7 days. 9 participants were delivered at another hospital and were lost to follow‐up. Study was performed at the University of Maryland Obstetric Clinic, Baltimore, USA. Sources of trial funding: Study was funded by a grant from the Upjohn company. Declarations of interest: Not reported. Trial dates: October 1985 ‐ April 1998. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. "Medications were dispensed in blister packs with each dose packaged sequentially in an individual cell, permitting accurate determination of the specific number and timing of missed doses." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | From table 3, it is clear that there was no missing data in the placebo group while the missing outcome data were balanced in numbers across the two interventions groups. |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Bell 1982.
| Methods | Randomised controlled trial. | |
| Participants | 21 gravidas beyond the 24th week of pregnancy with Chlamydial trachomatis infection who were not allergic to penicillin. Only had outcomes for 15 participants. 9 were in the amoxicillin group. 6 were in the placebo group. |
|
| Interventions | Amoxicillin 500 mg 3 times a day (11 participants) or placebo (10 participants). | |
| Outcomes | Postpartum culture: 3/9 had positive culture in amoxicillin group. 4/6 had a positive culture in the placebo group. | |
| Notes | Amoxicillin group: 2 were lost to follow‐up. Placebo: 1 was treated elsewhere with erythromycin, 2 were lost to follow‐up and 1 died due to a cerebrovascular incident. Site of the study was not stated but assumed to be performed at University of Washington, Seattle, USA. Sources of trial funding: Study was supported by a US Public Health Service grant. Declarations of interest: Not reported. Trial dates: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described, unlikely. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described, unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described, unlikely. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All participants accounted for but only 15/21 were assessed for final outcome (71%). |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Bush 1994.
| Methods | Randomised controlled trial. | |
| Participants | 30 pregnant women with positive cervical Chlamydia trachomatis screen analysed by direct DNA assay. 15 were randomised to azithromycin. 15 were randomised to erythromycin. |
|
| Interventions | Erythromycin 500 mg orally 4 times a day for 7 days versus azithromycin 1 g orally as a single dose. | |
| Outcomes | Cure rate, side effects. | |
| Notes | In those intolerant to erythromycin, 500 mg 4 times daily the dosage was lowered to 250 mg 4 times daily. This occurred in 5 of the 15 erythromycin cases. 1 of the patients treated with erythromycin who was intolerant got positive culture results after treatment, but was successfully treated with azithromycin. Sexual partners were treated in the standard fashion with doxycycline, 100 mg orally twice a day for 7 days. Site of study not directly described but assumed to be Willian Beaumont Army Medical Center, El Paso, Texas. Sources of trial funding: No funding source was declared. Declarations of interest: Not reported. Trial dates: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated number assignment. |
| Allocation concealment (selection bias) | Low risk | The women were assigned treatment in sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described, unlikely. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Edwards 1996.
| Methods | Randomised controlled trial. | |
| Participants | 140 pregnant women were enrolled. Patients were tested positive for C.trachomatis by DNA hybridisation from a cervical swab specimen. Exclusion criteria; allergy to or intolerance of either azithromycin or erythromycin. 65 were randomised to azithromycin. 65 were randomised to erythromycin. |
|
| Interventions | Azithromycin orally 1 g taken orally at enrolment versus erythromycin 500 mg orally 4 times a day for 7 days. | |
| Outcomes | Cure rate, preterm birth, preterm rupture of membranes, side effects. | |
| Notes | Compliance for the azithromycin group was 100%. Compliance for the erythromycin group was 59.4%. All sexual partners were referred to the appropriate county health department for treatment. Test of cure was repeated after 2 weeks. 62 of the 65 patients in the azithromycin group completed their post‐treatment questionnaires, while 64 of the 65 patients in the erythromycin group completed the same form. Study was undertaken at the Medical University of South Carolina, Charleston, USA. Sources of trial funding: Sponsored by Pfizer pharmaceuticals. Declarations of interest: Not reported. Trial dates: April 1993 ‐ July 1994. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using a pre‐established random number table. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No attempt was made to blind the study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups, 3 in the azithromycin group and 1 in the erythromycin group. |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted. |
| Other bias | High risk | There was an unexplained significant difference in mean gestational age of 8.2 weeks between the 2 treatment groups (erythromycin 28.6 weeks; azithromycin 20.4 weeks). |
Gunter 1996.
| Methods | Randomised controlled trial. | |
| Participants | 47 pregnant women with positive Gen‐probe test for C.trachomatis were enrolled. Outcome data were available for 22 who were assigned to the azithromycin group and18 were assigned to the erythromycin group. |
|
| Interventions | Erythromycin 500 mg 4 times a day for 7 days versus azithromycin powder 1 g orally once. | |
| Outcomes | Cure rate, side effects. | |
| Notes | Treatment compliance was 100% in the azithromycin group and 44.5% in the erythromycin group. Patients unable to tolerate original randomisation due to gastrointestinal side effects were allowed to cross‐over to the opposite study medication. 7 patients were excluded due to severe side effects from erythromycin and required cross‐over to azithromycin. Not directly stated but assumed that the study was undertaken at the Bowman Gray School of Medicine, Winston‐Salem, NC, USA. In published abstract it states it is an ongoing trial but no further publications were found. Sources of trial funding: No funding source was declared. Declarations of interest: Not reported. Trial dates: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of ‘Low risk’ or ‘High risk’. |
| Selective reporting (reporting bias) | Unclear risk | States it is an ongoing trial but no further results have been published or presented. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Jacobson 2001.
| Methods | Randomised controlled trial. | |
| Participants | 129 pregnant women with positive cervical C.trachomatis DNA test result were enrolled. Exclusion criteria included known allergy or hypersensitivity to amoxicillin, penicillin, or azithromycin; severe hyperemesis gravidarum at the time of entry; and concurrent use of an antibiotic with efficacy against Chlamydia trachomatis (fluoroquinolones, macrolides, clindamycin, tetracyclines, or sulphonamides). 110 completed the trial protocol. 55 were in the amoxicillin group and 55 were in the azithromycin group. |
|
| Interventions | Amoxicillin 500 mg orally 3 times a day for 7 days versus azithromycin 1 g once. | |
| Outcomes | Cure rate, preterm birth. | |
| Notes | The study was closed due to realisation that 3000 participants would be needed to complete it. The number of patients studied were too few. Data presented here include accrued cure rates for all patients studied. Among the 19 women excluded from the analysis, 14 were lost to follow‐up, (8 amoxicillin, 6 azithromycin) and 5 were involved in protocol violations (3 amoxicillin, 2 azithromycin). In the amoxicillin group, 3 patients were intolerant to treatment and in the azithromycin group, 6 patients were intolerant to treatment. Only 35% of the subjects were seen within 7 days of the scheduled appointment for test of cure. Study was undertaken in 2 university‐based inner‐city clinics in Milwaukee, USA. Sources of trial funding: No funding source was declared. Declarations of interest: Not reported. Trial dates: Ocotber 1988 ‐ February 2000. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was accomplished with a computer‐generated random‐number table in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Treatment assignments were placed in sequentially numbered, opaque, sealed envelopes by staff not involved in enrolment, treatment, or evaluation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Assignments were not blinded, and there was no attempt to directly visualise patients taking their first dose of medication. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Of the 19 women excluded from analysis, 14 were lost to follow‐up (8 amoxicillin, 6 azithromycin), but no reasons were provided. |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Kacmar 2001.
| Methods | Randomised controlled trial. | |
| Participants | 39 pregnant women. Routine Chlamydia screens using ligase chain reaction were performed on all patients attending. Exclusion criteria: other infections requiring antibiotic therapy, known allergy or sensitivity to either amoxicillin or azithromycin, or gestational age greater than 33 weeks. 19 received amoxicillin and 20 received azithromycin. |
|
| Interventions | Azithromycin 1 g orally as a single dose versus amoxicillin 500 mg orally 3 times a day for 7 days. | |
| Outcomes | Cure rate, repeated infection, side effects. | |
| Notes | A referral for treatment was given to all partners of patients testing positive for Chlamydia, and patients were instructed to abstain from sexual intercourse until treatment was completed. Compliance in the azithromycin group was 100%. Compliance in the amoxicillin group was 84%. A sample‐size calculation was performed, showing that 50 patients would be needed for each treatment group, but due to limitations and difficulties with recruitment only 39 were enrolled in this trial. The 1 positive test in the azithromycin group was in a patient who did not refer her partner for treatment, continued to have sexual intercourse and did not use a condom as recommended. Totals in table 2 vary due to missing data. Study was undertaken at the Women and Infants Hospital prenatal clinic, Providence, USA. Sources of trial funding: Funded by a NIH grant. Declarations of interest: Not reported. Trial dates: November 1998 ‐ May 2000. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a random number sequence. |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes were used for concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Four women in the amoxicillin group and one in the azithromycin group failed to return for follow‐up test of cure and the reasons are not provided. |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Magat 1993.
| Methods | Randomised controlled trial. | |
| Participants | 143 pregnant women with posItive culture for Chlamydia trachomatis enrolled before 36 weeks' gestation. Exlusion criteria: sensitivity to either study medication, persistent gastrointestinal symptoms or history of colitis or antibiotic therapy after screening and before enrolment. 72 were randomised to amoxicillin and 71 to erythromycin. |
|
| Interventions | Erythromycin (500 mg 4 times daily for 7 days) and amoxicillin (500 mg 3 times a day for 7 days). | |
| Outcomes | Cure rate, side effects of treatment. | |
| Notes | Partners of all women received doxycycline (100 mg twice a day for 7 days). 15 in the erythromycin group were intolerant to the therapy. 1 woman in the amoxicillin group was intolerant to the therapy. In both groups, this intolerance to therapy was developed the first or second day of treatment. Of the 15 who were intolerant to erythromycin, 11 were treated successfully with amoxicillin, 1 needed a further course of treatment, 2 refused further treatment and 1 was treated but not tested before delivery. 2 of the 9 failures reported partner non‐compliance with the doxycycline medication. Study was undertaken in by the University of Maryland School of Medicine, Baltimore, USA from October 1990 to August 1991. Sources of trial funding: No funding source was declared. Declarations of interest: Not reported. Trial dates: October 1990 ‐ August 1991. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by pseudo‐random number generator. |
| Allocation concealment (selection bias) | Low risk | The medications were dispensed by the hospital pharmacy to prevent the healthcare team from learning the assigned medication or dosage schedule. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 114 completed follow‐up (80% completed the study). Missing data in the amoxicillin group: out of 72 enrolled, 8 women were excluded. 7 had no follow‐up culture done before delivery. 5 of the 7 delivered before a test of cure was obtained; 1 woman was mistakenly entered into the study at 38.5 weeks; and the final woman was excluded because she was admitted with preterm labour and given erythromycin before the test of cure. 1 of the women had a allergic reaction and was also excluded. 64/72 finished treatment (88%). Missing data in the erythromycin group: out of 71 enrolled, 21 women were excluded. 6 women had no follow‐up culture for test of cure. 4 of these 6 had delivered before a test of cure culture was obtained, 1 had a therapeutic abortion, and 1 moved out of the state. 15 were intolerant to erythromycin and were excluded. In conclusion 50/71 finished treatment (70%). |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted. |
| Other bias | High risk | There was an unexplained significant difference in the mean gestational age at entry between the amoxicillin group (24.0 ± 8.4 weeks) and the erythromycin group (20.8 ± 8.0 weeks)(P = .05). |
Martin 1997.
| Methods | Randomised controlled trial. | |
| Participants | 414 pregnant women, between the 23rd and 26th week of pregnancy, who were diagnosed with positive reading of fluorescent isothiocyanate‐conjugated Chlamydia trachomatis‐specific monoclonal antibody were enrolled. Women were eligible if they were: more than 16 years old, were free of medical complications related to premature delivery, and were not taking selected medications. Women with positive screening cultures for Neisseria gonorrhoeae or > l0s micro‐organisms/mL of urine were treated and thus ineligible for the trial. 205 women were randomised to the erythromycin group. 209 women were randomised to the placebo group. |
|
| Interventions | Erythromycin 333 mg orally 3 times daily versus an identical placebo. | |
| Outcomes | Cure rate, preterm birth, preterm rupture of membranes, side effects, perinatal mortality, low birthweight. | |
| Notes | Trial participants with Chlamydia trachomatis were re‐treated with doxycycline, tetracycline, or erythromycin immediately postpartum, regardless of which trial medication they received. Infants were either treated empirically after delivery or were followed, cultured at their first postnatal visit, and treated with antibiotics if indicated.
Eligible women who agreed to participate in the clinical trial entered a week placebo run‐in. Those who took less than two‐thirds of the allotted placebo pills during the run‐in, who did not return to the clinic or refused further participation were not randomised.
Women identified as colonised with Ureaplasma urealyticum,group B streptococci, and/or Chlamydia trachomatis were considered for randomisation into the clinical trial. We included only the Chlamydia trachomatis results.
The study have different amounts of missing data on different outcomes. 25 erythromycin‐treated and 23 placebo‐treated women withdrew from the trial but were included in the intent‐to‐treat analysis. The primary outcome was measured mid‐treatment. The 20% failure rate of erythromycin in our study suggests the dose is less than optimal, possibly due to the 40% increase in blood and extracellular volume in pregnancy acting to reduce serum and tissue drug levels. Study was undertaken in 7 institutions utilising 6 antepartum clinics in Harlem Hospital, New York, Columbia University, New York, Louisiana State University and Tulane University, New Orleans, University of Oklahoma, Oklahoma City, University of Texas, San Antonio and University of Washington, Seattle, USA. Sources of trial funding: This work was supported by contracts from the National Institute of Child Health and Human Development ant the National Institute of Allergy and Infectious Diseases Declarations of interest: Not reported. Trial dates: November 1984 ‐ March 1989. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced randomisation scheme by Research Triangle Institute: computer randomised according to permuted‐block procedure with random block sizes. (Reference 13) |
| Allocation concealment (selection bias) | Unclear risk | Not described. Identical placebo. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 48 (11.6%) withdrew from the trial. Different amount of data missing in different aspects of outcomes. |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Rosenn 1995.
| Methods | Randomised controlled trial. | |
| Participants | 48 pregnant women with positive culture of Chlamydia trachomatis were enrolled. 24 were randomised to receive erythromycin and 24 were randomised to receive azithromycin. |
|
| Interventions | Azithromycin single dose 1 g orally versus erythromycin 500 mg 4 times daily for 7 days orally. | |
| Outcomes | Cure rate, side effects. | |
| Notes | All partners received doxycycline 100 mg twice a day orally. Follow‐up meeting 3 weeks after therapy. Compliance was then measured by pill count and the participants had filled out a questionnaire about: sexual activity, side effects and compliance. Azithromycin group compliance: 100%. Erythromycin group compliance: 61%. Study was undertaken at Thomas Jefferson University Hospital prenatal clinics, Philadelphia, USA, from August 1994 to April 1995. Sources of trial funding: no funding source reported. Declarations of interest: Not reported. Trial dates: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block of 6 randomisation generated from a random‐number table. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 were lost to follow‐up (94% completed the study). |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Silverman 1994.
| Methods | Randomised controlled trial. | |
| Participants | 74 pregnant women with culture positive Chlamydia trachomatis infection. Excluded if the first prenatal visit was after 36 weeks, recent antibiotic use for another indication (within 14 days), and known allergy or sensitivity to either of the study medications. 36 were treated with amoxicillin. 38 were treated with erythromycin. |
|
| Interventions | Amoxicillin 500 mg orally 3 times a day or erythromycin 500 mg orally 4 times daily for 7 days. | |
| Outcomes | Cure rate, side effects. | |
| Notes | The women who did not cure from the first dosage were crossed over to the alternative treatment. These results are not included in our analysis. Partners were treated with doxycycline 100 mg orally twice a day for 7 days. Study was undertaken at Thomas Jefferson University Hospital prenatal clinics, Philadelphia, USA. Sources of trial funding: no funding source reported. Declarations of interest: Not reported. Trial dates: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block of 6 randomisation generated from a random‐number table. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 women in each group were lost to follow‐up and no reasons were provided. |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Turrentine 1995.
| Methods | Randomised controlled trial. | |
| Participants | 168 pregnant women, < 36 weeks' gestation, with positive cervical Chlamydia trachomatis culture were enrolled. 56 received erythromycin, 57 received amoxicillin and 55 received clindamycin. |
|
| Interventions | Erythromycin‐base tablets 500 mg orally 4 times a day for 7 days, amoxicillin capsules 500 mg 2 times a day for 7 days or clindamycin tablets 600 mg orally 2 times a day for 10 days. | |
| Outcomes | Cure rate, side effects. | |
| Notes | All sexual partners in the study was offered treatment with doxycycline 100 mg twice a day for 7 days.
There were no statistically significant differences in age, racial distribution, gravidity, gestational age, or number of days to test‐of‐cure among the groups. 6 women elected not to participate. 8 patients were lost to follow‐up, 3 in the erythromycin, 2 in the amoxicillin, and 3 in the clindamycin group, and were excluded from the analysis. 5 women in the erythromycin group had severe side effects and discontinued the treatment. In the amoxicillin group, 2 women had severe side effects and discontinued the treatment. In the clindamycin group 2 women developed an allergic reaction to clindamycin and had to discontinue. 2 women had severe side effects to the treatment and discontinued. 1 developed an allergic reaction to erythromycin and had to discontinue. Study was undertaken by the University of Texas Health Science Center, Houston, USA. Sources of trial funding: Pharmaceutical companies Parke‐Davis, Lederle and Upjohn supplied the treatment medications. Declarations of interest: Not reported. Trial dates: July 1991 ‐ September 1993. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was accomplished by computer‐generated assignment. |
| Allocation concealment (selection bias) | Low risk | Unlabelled medications. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The unlabeled medications were dispensed by the hospital pharmacy to prevent the healthcare team from learning the assigned medication. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants had test of cure, assessors were still blinded to treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 148 (85%) completed the protocol. Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
Wehbeh 1998.
| Methods | Randomised controlled trial. | |
| Participants | 48 pregnant women screening positive for fluorescein‐conjugated monoclonal antibody toChlamydia trachomatis were enrolled. 17 received azithromycin and their partners received azithromycin, 10 received azithromycin and their partners received tetracycline and 21 received erythromycin and the partner received azithromycin. For this analysis the azithromycin group was considered as one group (27 women) and was compared to the erythromycin group (21 women). |
|
| Interventions | 3 groups. Single dose of azithromycin 1 g orally (partners got the same), erythromycin 500 mg orally 3 times a day for 7 days (partners got tetracycline) and single dose of azithromycin 1 g orally (partners got tetracycline). | |
| Outcomes | Cure rate, side effects. | |
| Notes | 7‐10 days after treatment started compliance was controlled by having a meeting with the couples. They were asked to bring their medication bottles and if it had at least 1 day of unused medications in it, the couple was considered non‐compliant.
Compliance rates: azithromycin: 92.6% (2 refused to participate after the randomisation), erythromycin: 71.4%, tetracycline: 75%.
There was no significant difference in age, gestational age at entry into the study or number of prior pregnancies between treatment groups.
Only 12% in group 1 reported having sexual intercourse during the study period whilst 42.9% and 30% in group 2 and 3 did.
Exact multiple logistic regression procedures was used to see if the treatment failure was due to reinfection from their sexual activity during the study. It showed no confounding of the observed treatment groups and is used as evidence that treatment failure is not due to reinfection of sexual partner. The third treatment group was included in order to assess the efficacy of multidose course of tetracycline versus single‐dose therapy with azithromycin, and to indirectly assess the possible reinfection of the pregnant women through sexual intercourse during the trial period. They considered that they did not need to make all the medications look the same and just placebo pills to make the azithromycin treatment identical to erythromycin because they thought compliance bias would be introduced. Study was undertaken in a prenatal clinic located within a large urban medical centre with New York, USA. Sources of trial funding: the trial was funded by local department funds. Declarations of interest: Not reported. Trial dates: Not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | States all participants were unaware of the exact nature of the antibiotic treatment given to them but that no placebo was used. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 in the first study group refused medications (96% completed the study). |
| Selective reporting (reporting bias) | Low risk | No selective reporting noted. |
| Other bias | Low risk | The study appears to be free of other sources of bias. |
g: gram mg: milligram mL: millilitre
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| El‐Shourbagy 2011 | This study examines the rate of pre‐eclampsia in groups of treated and non‐treated Chlamydia pneumoniae infections in pregnancy. |
| McGregor 1990 | This study included pregnant women with various genital tract infections and not only C.trachomatis. The data for C.trachomatis infection were not presented separately. |
| Nadafi 2005 | Not all participants of this trial had C.trachomatis infection, therefore, the study was excluded. |
| Zulkarneev 1998 | This study was not a randomised controlled trial. |
Characteristics of ongoing studies [ordered by study ID]
Okunola 2013.
| Trial name or title | Treatment of antenatal Chlamydia infection. |
| Methods | Randomised controlled trial. Open label. |
| Participants | Pregnant women at less than 36 weeks' gestation with positive C.trachomatis test on endocervical swab with rapid kit. Exlcuded with history of antibiotics in the last 2 weeks or low lying placenta, history of reaction to any of the drugs. Plan to recruit 200 participants. |
| Interventions | Group 1 ‐ amoxicillin 500 mg orally 3 times a day for a week. Group 2 ‐ erythromycin 500 mg 4 times a day for a week. Partners were treated with doxycycline for a week. |
| Outcomes | Completiion of course treatment. Microbiological cure. Side effects (nausea, diarrhoea, vomiting, loss of appetite). |
| Starting date | October 2013. |
| Contact information | Obafemi Awolowo University Teaching Hospital. |
| Notes | Clinical Trial identifier NCT01946256. |
Differences between protocol and review
There are some differences between our published protocol (Novikova 2013) and the full review; these are outlined below.
The contact person for the review has changed from Natalia Novikova to Cathy Cluver.
We have updated our methods text to include the use of GRADE and we have included eight 'Summary of findings' tables.
We have added the WHO International Clinical Trials Registry Platform (ICTRP) to sources searched.
Contributions of authors
Cathy Cluver and Natalia Novikova are the guarantors of the review. Natalia Novikova developed the protocol, provided clinical and methodological perspectives and drafted the review. Catherine Cluver provided general advice on the protocol, assisted with assessment of studies for inclusion into the meta‐analysis, checked the trials for inclusion criteria, checked data entry, checked assessment of bias, performed the data analysis and edited the final versions of the review. David OA Eriksson and Kevin Bengtsson assessed the studies for inclusion into the meta‐analysis, extracted the data and assisted with data analysis. Göran K Lingman checked the data and provided advice on the review.
Declarations of interest
Natalia Novikova: none known.
Catherine Cluver: none known.
David OA Eriksson: received a small travel scholarship from the international department of Lund University to finance some of the costs for travelling from Sweden to South Africa, to be a part of this review.
Kevin Bengtsson: received a small travel scholarship from the international department of Lund University to finance some of the costs for travelling from Sweden to South Africa, to be a part of this review.
Göran K Lingman: none known.
New
References
References to studies included in this review
Adair 1998 {published data only}
- Adair CD, Gunter M, Stovall TG, Mcelroy G, Veille J, Ernest JM. Chlamydia in pregnancy: a randomized trial of azithromycin and erythromycin. Obstetrics & Gynecology 1998;91(2):165‐8. [DOI] [PubMed] [Google Scholar]
Alary 1994 {published data only}
- Alary M, Joly JR, Moutquin JM, Mondor M, Boucher M, Fortier A, et al. Randomised comparison of amoxycillin and erythromycin in treatment of genital chlamydial infection in pregnancy. Lancet 1994;344:1461‐5. [DOI] [PubMed] [Google Scholar]
Alger 1991 {published data only}
- Alger LS, Lovchik JC. Comparative efficacy of clindamycin vs erythromycin in eradication of antenatal chlamydia trachomatis. American Journal of Obstetrics and Gynecology 1991;165:375‐81. [DOI] [PubMed] [Google Scholar]
Bell 1982 {published data only}
- Bell T, Sandstrom I, Eschenbach D, Hummel D, Kuo C, Wang S, et al. Treatment of Chlamydia trachomatis in pregnancy with amoxicillin. Fernstrom Foundation Series 1982;2:221‐4. [Google Scholar]
Bush 1994 {published data only}
- Bush MR, Rosa C. Azithromycin and erythromycin in the treatment of cervical chlamydial infection during pregnancy. Obstetrics & Gynecology 1994;84(1):61‐3. [PubMed] [Google Scholar]
Edwards 1996 {published data only}
- Edwards MS, Newman RB, Carter SG, LeBoeuf FW, Menard MK, Rainwater KP. Randomized clinical trial of azithromycin vs erythromycin for the treatment of chlamydia cervicitis in pregnancy. Infectious Diseases in Obstetrics & Gynecology 1996;4(6):333‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunter 1996 {published data only}
- Gunter ME, Adair CD, Ernest JM, McElroy G. Azithromycin powder versus erthromycin in the treatment of chlamydial cervicitis in pregnancy. Infectious Diseases in Obstetrics and Gynecology 1996;4:53. [Google Scholar]
Jacobson 2001 {published data only}
- Jacobson GF, Autry AM, Kirby RS, Liverman EM, Motley RU. A randomized controlled trial comparing amoxicillin and azithromycin for the treatment of chlamydia trachomatis in pregnancy. American Journal of Obstetrics and Gynecology 2001;184(7):1352‐4; discussion 1354‐6. [DOI] [PubMed] [Google Scholar]
Kacmar 2001 {published data only}
- Kacmar J, Cheh E, Montagno A, Peipert JF. A randomized trial of azithromycin versus amoxicillin for the treatment of chlamydia trachomatis in pregnancy. Infectious Diseases in Obstetrics & Gynecology 2001;9:197‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magat 1993 {published data only}
- Magat AH, Alger LS, Nagey DA, Hatch V, Lovchik JC. Double‐blind randomized study comparing amoxicillin and erythromycin for the treatment of chlamydia trachomatis in pregnancy. Obstetrics & Gynecology 1993;81(5):745‐9. [PubMed] [Google Scholar]
Martin 1997 {published data only}
- Martin DH, Eschenbach DA, Cotch MF, Nugent RP, Rao AV, Klebanoff MA, et al. Double‐blind placebo‐controlled treatment trial of Chlamydia trachomatis endocervical infections in pregnant women. Infectious Diseases in Obstetrics & Gynecology 1997;5:10‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosenn 1995 {published data only}
- Rosenn M, Macones GA, Silverman N. A randomized trial of erythromycin and azithromycin for the treatment of chlamydia infection in pregnancy. American Journal of Obstetrics and Gynecology 1996;174:410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenn M, Macones GA, Silverman N. Randomized trial of erythromycin and azithromycin for the treatment of chlamydial infection in pregnancy. Infectious Diseases in Obstetrics & Gynecology 1995;3:241‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silverman 1994 {published data only}
- Silverman N, Sullivan M, Hochman M, Womack M, Jungkind DL. A randomized, prospective trial of amoxicillin vs erythromycin for the treatment of chlamydia in pregnancy. American Journal of Obstetrics and Gynecology 1993;168:420. [DOI] [PubMed] [Google Scholar]
- Silverman NS, Sullivan M, Hochman M, Womack M, Jungkind DL. A randomized, prospective trial comparing amoxicillin and erythromycin for the treatment of chlamydia trachomatis in pregnancy. American Journal of Obstetrics and Gynecology 1994;170:829‐32. [DOI] [PubMed] [Google Scholar]
Turrentine 1995 {published data only}
- Turrentine MA, Troyer L, Gonik B. Randomized prospective study comparing erythromycin, amoxicillin, and clindamycin for the treatment of chlamydia trachomatis in pregnancy. Infectious Diseases in Obstetrics & Gynecology 1995;2:205‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wehbeh 1998 {published data only}
- Wehbeh H, Ruggiero R, Ali Y, Lopez G, Shahem S, Zarou D. A randomised clinical trial of a single dose of azithromycin in treatment of Chlamydia amongst pregnant women. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):361. [Google Scholar]
- Wehbeh HA, Ruggeirio RM, Shahem S, Lopez G, Ali Y. Single‐dose azithromycin for chlamydia in pregnant women. Journal of Reproductive Medicine 1998;43(6):509‐14. [PubMed] [Google Scholar]
References to studies excluded from this review
El‐Shourbagy 2011 {published data only}
- El‐Shourbagy MAA, El‐Refaie TA, Sayed KKA, Wahba KAH, El‐Din ASS, Fathy MM. Impact of seroconversion and antichlamydial treatment on the rate of pre‐eclampsia among Egyptian primigravidae. International Journal of Gynecology & Obstetrics 2011;113(2):137‐40. [DOI] [PubMed] [Google Scholar]
McGregor 1990 {published data only}
- McGregor JA, French JI, Richter R, Vuchetich M, Bachus V, Seo K, et al. Cervicovaginal microflora and pregnancy outcome: results of a double‐blind, placebo‐controlled trial of erythromycin treatment. American Journal of Obstetrics and Gynecology 1990;163:1580‐91. [DOI] [PubMed] [Google Scholar]
Nadafi 2005 {published data only}
- Nadafi M, Abdali KH, Parsanejad ME, Rajaee‐Fard AR, Kaviani M. A comparison of amoxicillin and erythromycin for asymptomatic chlamydia trachomatis infection in pregnancy. International Journal of Gynecology & Obstetrics 2005;90(2):142‐3. [DOI] [PubMed] [Google Scholar]
Zulkarneev 1998 {published data only}
- Zulkarneev RS, Kalinin IuT, Afanas'ev SS, Rubal'skii OV, Denisov LA, Vorob'ev AA, et al. Use of recombinant alpha2‐interferon and a complex immunoglobulin preparation for the treatment of chlamydiosis in pregnancy women [Primenenie rekombinantnogo alpha2‐interferona i kompleksnogo immunoglobulinovogo preparata pri lechenii khlamidioza u beremennykh.]. Zhurnal Mikrobiologii, Epidemiologii i Immunobiologii 1998;2:115‐8. [PubMed] [Google Scholar]
References to ongoing studies
Okunola 2013 {published data only}
- NCT01946256. Erythromycin versus amoxicillin for treatment of antenatal chlamydia trachomatis infection: a randomized controlled trial. clinicaltrials.gov/ct2/show/NCT01946256 (first received: 16 September 2013).
Additional references
Attenburrow 1985
- Attenburrow AA, Barker CM. Chlamydial pneumonia in the low birth weight neonate. Archives of Disease in Childhood 1985;60:1169‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Berggren 2011
- Berggren EK, Patchen L. Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae and repeat infection among pregnant urban adolescents. Sexually Transmitted Diseases 2011;38:172‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blas 2007
- Blas MM, Canchihuaman FA, Alva IE, Hawes SE. Pregnancy outcomes in women infected with Chlamydia trachomatis: a population‐based cohort study in Washington state. Sexually Transmitted Infections 2007;83(4):314‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2015
- Centers for Disease Control and Prevention. 2015 Sexually Transmitted Diseases Treatment Guidelines. Division of STD Prevention, National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, Centers for Disease Control and Prevention 2015.
Handsfield 2011
- Handsfield HH. Questioning azithromycin for chlamydial infection. Sexually Transmitted Diseases 2011;58:1028‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Horner 2006
- Horner P. The case for further treatment studies of uncomplicated genital Chlamydia trachomatis infection. Sexually Transmitted Infections 2006;82:340‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ismail 1987
- Ismail MA, Moawad AH, Poon E, Henderson C. Role of Chlamydia trachomatis in postpartum endometritis. Journal of Reproductive Medicine 1987;32(4):280‐4. [PubMed] [Google Scholar]
Jespersen 2005
- Jespersen DJ, Flatten KS, Jones MF, Smith TF. Prospective comparison of cell cultures and nucleic acid amplification tests for laboratory diagnosis of Chlamydia trachomatis infections. Journal of Clinical Microbiology 2005;43(10):5324‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kakar 2010
- Kakar S, Bhalla P, Maria A, Rana M, Chawla R, Mathur NB. Chlamydia trachomatis causing neonatal conjunctivitis in a tertiary care centre. Indian Journal of Medical Microbiology 2010;28(1):45‐7. [DOI] [PubMed] [Google Scholar]
Marrazzo 2016
- Marrazzo J. Treatment of Chlamydia trachomatis infection. http://www.uptodate.com/contents/treatment‐of‐chlamydia‐trachomatis‐infection (accessed Jan 19, 2016) 2016.
Miller 2000
- Miller JM, Martin DH. Treatment of Chlamydia trachomatis infections in pregnant women. Drugs 2000;60(3):597‐605. [DOI] [PubMed] [Google Scholar]
Much 1991
- Much DH, Yeh SY. Prevalence of Chlamydia trachomatis infection in pregnancy. Journal of Reproductive Medicine 1991;106(5):490. [PMC free article] [PubMed] [Google Scholar]
Nigro 2011
- Nigro G, Mazzocco M, Mattia E, Renzo GC, Carta G, Anceschi MM. Role of the infections in recurrent spontaneous abortion. Journal of Maternal‐Fetal and Neonatal Medicine 2011;24(8):983‐9. [DOI] [PubMed] [Google Scholar]
Pammi 2012
- Pammi M, Hammerschlag MR. Chlamydia trachomatis infections in the newborn. http://www.uptodate.com/contents/chlamydia‐trachomatis‐infections‐in‐the‐newborn (accessed 2012).
Pararas 2006
- Pararas MV, Skevaki CL, Kafetzis DA. Preterm birth due to maternal infection: causative pathogens and modes of prevention. European Journal of Clinical Microbiology and Infectious Diseases 2006;25(9):562‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rours 2009
- Rours GI, Hammerschlag MR, Doornum GJ, Hop WC, Groot R, Willemse HF, et al. Chlamydia trachomatis respiratory infection in Dutch infants. Archives of Disease in Childhood 2009;94(9):705‐7. [DOI] [PubMed] [Google Scholar]
Rours 2011
- Rours GI, Duijts L, Moll HA, Arends LR, Groot R, Jaddoe VW, et al. Chlamydia trachomatis infection during pregnancy associated with preterm delivery: a population‐based prospective cohort study. European Journal of Epidemiology 2011;26(6):493‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schwebke 2011
- Schwebke JR, Rompalo A, Taylor S. Re‐evaluating the treatment of nongonococcal urethritis: Emphasizing emerging pathogens—a randomized clinical trial. Clinical Infectious Diseases 2011;52:163‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Silva 2011
- Silva MJ, Florêncio GL, Gabiatti JR, Amaral RL, Eleutério Júnior J, Gonçalves AK. Perinatal morbidity and mortality associated with chlamydial infection: a meta‐analysis study. Brazilian Journal of Infectious Diseases 2011;15(6):533‐9. [DOI] [PubMed] [Google Scholar]
South African STI guideline 2015
- sahivsoc.org. Sexually Transmitted Infections Managment Guidelines 2015. http://www.sahivsoc.org/upload/documents/STIguidelines‐1‐28‐15(LC).pdf (accessed 16 May 2016) 2015.
Walker 2012
- Walker J, Tabrizi SN, Fairley CK, Chen MY, Bradshaw CS, Twin J, et al. Chlamydia trachomatis Incidence and re‐iInfection among young women – behavioural and microbiological characteristics. PLOS One 2012;7(5):37778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Workowski 2010
- Workowski KA, Berman S. Sexually Transmitted Diseases Treatment Guidelines. CDC, 2010. [DOI] [PubMed] [Google Scholar]
Yu 2009
- Yu J, Wu S, Li F, Hu L. Vertical transmission of Chlamydia trachomatis in Chongqing China. Current Microbiology 2009;58(4):315‐20. [DOI] [PubMed] [Google Scholar]
Zenilman 2012
- Zenilman JM. Genital Chlamydia trachomatis infections in women. http://www.uptodate.com/contents/genital‐chlamydia‐trachomatis‐infections‐in‐women (accessed 2012).
References to other published versions of this review
Brocklehurst 1998
- Brocklehurst P, Rooney G. Interventions for treating genital chlamydia trachomatis infection in pregnancy. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD000054] [DOI] [PMC free article] [PubMed] [Google Scholar]
Novikova 2013
- Novikova N, Cluver C. Interventions for treating genital Chlamydia trachomatis infection in pregnancy. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD010485] [DOI] [PMC free article] [PubMed] [Google Scholar]


