Abstract
Background
Hepatorenal syndrome is defined as severe renal failure occurring in people with cirrhosis and ascites. Systematic reviews of randomised clinical trials found that, compared with placebo, terlipressin may reduce mortality and improve renal function in people with hepatorenal syndrome, but we need current evidence from systematic reviews on the benefits and harms of terlipressin versus other vasoactive drugs.
Objectives
To evaluate the beneficial and harmful effects of terlipressin versus other vasoactive drugs for people with hepatorenal syndrome.
Search methods
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register, CENTRAL, MEDLINE, Embase, and Science Citation Index Expanded; conducted manual searches of references in relevant literature; and wrote to experts and pharmaceutical companies (date of last search November 2016).
Selection criteria
Randomised clinical trials comparing terlipressin versus any other type of vasoactive drugs for hepatorenal syndrome. We allowed albumin and other cointerventions if provided equally in the comparison groups.
Data collection and analysis
Three authors independently extracted data. The primary outcomes were mortality, hepatorenal syndrome (persistent hepatorenal syndrome despite treatment), and serious adverse events. We conducted meta‐analyses and present the results as risk ratios (RR) with 95% confidence intervals (CI). We performed sensitivity, subgroup, and Trial Sequential Analyses and evaluated bias control based on the Cochrane Hepato‐Biliary Group domains.
Main results
We included 10 randomised clinical trials with 474 participants. The trials compared terlipressin versus noradrenaline (seven trials), octreotide (one trial), midodrine and octreotide (one trial), or dopamine (one trial). All participants in both groups received albumin as cointervention. We classified two trials at low risk of bias and eight trials at high risk of bias in the assessment of mortality and all trials at high risk of bias for remaining outcomes. In five trials, investigators specifically stated that they did not receive funding from for‐profit organisations. We had no information about the funding source from the remaining five trials.
Terlipressin was not superior or inferior compared with other vasoactive drugs in regard to mortality when including the two trials with a low risk of bias (RR 0.92, 95% CI 0.63 to 1.36; 94 participants, very low quality evidence) or when including all 10 trials (RR 0.96, 95% CI 0.88 to 1.06; 474 participants; I² = 0%; very low quality evidence). One meta‐analysis including nine trials suggested a beneficial effect of terlipressin on hepatorenal syndrome (RR 0.79, 95% CI 0.63 to 0.99; 394 participants; I² = 26%; very low quality evidence). Due to the high mortality of hepatorenal syndrome, the registration of other serious adverse events is uncertain, but comparing terlipressin and other vasoactive drugs we found no significant difference (RR 0.96, 95% CI 0.88 to 1.06; 474 participants; I² = 0%; very low quality evidence). Several trials did not report systematically of adverse events, but terlipressin seemed to increase the risks of diarrhoea or abdominal pain, or both (RR 3.50, 95% CI 1.19 to 10.27; 221 participants; 5 trials, I² = 0%). However, Trial Sequential Analyses found insufficient evidence to support or refute any differences between interventions for all outcomes. Considering reversal of hepatorenal syndrome, subgroup analyses on the type of other vasoactive drugs found that terlipressin was superior compared with midodrine and octreotide (RR 0.47, 95% CI 0.30 to 0.72) or octreotide alone (RR 0.56, 95% CI 0.33 to 0.96), but each subgroup only included one small trial. None of the remaining subgroup or sensitivity analyses found differences between terlipressin and other vasoactive drugs. We downgraded the evidence to very low quality because of the high risk of bias, imprecision, and the results of the Trial Sequential Analyses.
Authors' conclusions
This review found insufficient evidence to support or refute beneficial or harmful effects of terlipressin and albumin versus other vasoactive drugs and albumin. Additional research is needed to evaluate if clinically meaningful differences exist between interventions.
Plain language summary
Terlipressin versus other vasoactive drugs for hepatorenal syndrome
Background
Hepatorenal syndrome is a type of renal (relating to the kidneys) failure occurring in people with severe liver disease and fluid in the abdomen (ascites). We do not fully understand why some people with liver disease develop hepatorenal syndrome, but it is generally believed that low blood pressure and reduced blood supply to the kidneys is one of the main reasons. Theoretically, drugs that increase the blood pressure may be beneficial. The drug terlipressin combined with albumin (a protein) infusion is the recommended treatment for people with hepatorenal syndrome according to guidelines. Some countries (e.g. the USA) have not approved the use of terlipressin and researchers have suggested that other vasoactive drugs may be used instead.
Review question
Is terlipressin more beneficial or safe than other vasoactive drugs for the treatment of hepatorenal syndrome?
Search date
November 2016.
Study characteristics
We included 10 clinical trials with 474 participants. Seven trials compared terlipressin and albumin versus noradrenaline and albumin. The remaining three trials compared terlipressin and albumin versus midodrine and octreotide, or octreotide alone, or dopamine. In total, 241 participants received terlipressin and 233 participants received other vasoactive drugs (drugs that change blood pressure; noradrenaline, octreotide, midodrine, or dopamine).
Study funding sources
In five trials, investigators specifically stated that they did not receive funding from organisations that could profit from the trial results. We did not have information about the funding source from the remaining five trials.
Key results
Our analyses found uncertain evidence to support or refute terlipressin versus other vasoactive drugs in the treatment of hepatorenal syndrome when evaluating mortality or serious side effects. Our analyses suggested that treatment with terlipressin may have a beneficial effect on hepatorenal syndrome by reducing the number of participants with persistent hepatorenal syndrome. Additional analyses showed that the number of participants in the trials was too small for us to be sure about this. Accordingly, we found that important differences between terlipressin and other vasoactive drugs may be overlooked.
Quality of the evidence
We found that the evidence was of very low quality due to the high risk of bias and the small number of participants.
Authors' conclusions
We need additional large trials of a high quality to evaluate if terlipressin is more beneficial or safer than other vasoactive drugs.
Summary of findings
Summary of findings for the main comparison. Terlipressin compared to other vasoactive drugs for hepatorenal syndrome.
| Terlipressin compared to other vasoactive drugs for hepatorenal syndrome | ||||||
| Patient or population: people with cirrhosis and hepatorenal syndrome Setting: hospital Intervention: terlipressin Comparison: other vasoactive drugs | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with other vasoactive drugs | Risk with terlipressin | |||||
| Mortality (All‐cause) | Study population | RR 0.96 (0.88 to 1.06) | 474 (10 randomised clinical trials*) | ⊕⊝⊝⊝ Very lowa,b,c | Downgraded because of clinical heterogeneity, 8/10 randomised clinical trials were at high risk of bias and, the results of Trial Sequential Analysis. | |
| 601 per 1000 | 577 per 1000 (529 to 637) | |||||
|
Hepatorenal syndrome (Number of participants who did not achieve reversal of hepatorenal syndrome) |
Study population | RR 0.79 (0.63 to 0.99) | 394 (9 randomised clinical trials) | ⊕⊝⊝⊝ Very lowb,c,d | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of Trial Sequential Analysis. | |
| 560 per 1000 | 442 per 1000 (353 to 554) | |||||
| Serious adverse events | Study population | RR 0.96 (0.88 to 1.06) | 474 (10 randomised clinical trials) | ⊕⊝⊝⊝ Very lowb,c,d | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of Trial Sequential Analysis. | |
| 609 per 1000 | 585 per 1000 (536 to 646) | |||||
| Non‐serious adverse events: diarrhoea or abdominal pain, or both | Study population | RR 3.50 (1.19 to 10.27) | 221 (5 randomised clinical trials) | ⊕⊝⊝⊝ Very lowb,c,d | Downgraded because of clinical heterogeneity, all trials were judged as high risk of bias, and results of the Trial Sequential Analysis. | |
| 19 per 1000 | 65 per 1000 (22 to 190) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
aIn the assessment of mortality, we classified two randomised clinical trials at low risk of bias and eight at high risk of bias.
bThe randomised clinical trials were not designed for equivalence or inferiority analysis. The Trial Sequential Analysis showed that sample size did not reach the required information size for equivalence/inferiority meta‐analysis.
cClinical heterogeneity.
dWe classified all randomised clinical trials at high risk of bias in all non‐mortality outcomes.
Background
Description of the condition
Hepatorenal syndrome is a potentially reversible acute kidney injury associated with severe liver disease (Arroyo 1996). The diagnosis hepatorenal syndrome includes cirrhosis, ascites, and impaired renal function as well as exclusion of parenchymal renal disease and factors that may precipitate renal insufficiency (Salerno 2007). Among people hospitalised with cirrhosis and ascites, 25% develop acute renal insufficiency (Fede 2012). Among people with cirrhosis and acute renal insufficiency, 25% have hepatorenal syndrome (Fede 2012). In one cohort study of 234 non‐azotaemic participants with cirrhosis and ascites, 18% developed hepatorenal syndrome after one year (Gines 1993).
Hepatorenal syndrome is divided into two types. Type 1 has the most rapid course. Factors such as infection, alcoholic hepatitis, and bleeding may precipitate type 1 hepatorenal syndrome (Israelsen 2015). Without treatment, the median survival is about two weeks. Type 2 hepatorenal syndrome is often associated with refractory ascites and has a more protracted course with a median survival of about six months (Arroyo 1996; Gines 2003; Salerno 2007).
Description of the intervention
Main factors in the pathophysiology of hepatorenal syndrome are splanchnic vasodilation and increased cardiac output. Hepatorenal syndrome develops when the arterial pressure drops as the underlying condition progresses or due to factors leading to low blood pressure. Therefore, administration of vasoconstrictors may be beneficial. Terlipressin, an analogue of vasopressin, induces vasoconstriction mediated by type 1 vasopressin receptors in the smooth muscle cells of the blood vessel wall (Krag 2008). Other vasoactive drugs such as noradrenaline, octreotide, and midodrine may be equally effective. Noradrenaline is a catecholamine with high affinity for the alpha‐adrenergic receptors that mediate vasoconstriction in the venous and arterial system (Duvoux 2002). Octreotide is an octapeptide that mimics somatostatin. Octreotide is a potent vasoconstrictor and is used in bleeding oesophageal varices. The active metabolite of midodrine is an alpha‐1‐receptor agonist which increases the vascular tone (Angeli 1999).
Albumin infusions are recommended in combination with vasoconstrictors for type 1 hepatorenal syndrome (EASL 2010; Runyon 2013). By increasing the cardiac preload and cardiac output, albumin improves the effective arterial blood volume (Sort 1999). Furthermore, albumin infusions decrease the risk of developing hepatorenal syndrome, and, since 2007, plasma expansion with albumin for 48 hours is mandatory prior to diagnose hepatorenal syndrome (Salerno 2007; Angeli 2015). Consequently, almost all randomised clinical trials assessing vasoactive drugs for hepatorenal syndrome used standardised doses of cotreatment with albumin.
How the intervention might work
Hepatorenal syndrome is associated with the circulatory changes seen in cirrhosis including portal hypertension leading to splanchnic vasodilation; effective underfilling of the renal arteries; and activation of the endogenous vasoconstrictors; renin‐angiotensin‐aldosterone, the arginine‐vasopressin, and the sympathetic nervous systems (Pasqualetti 1998; Cardenas 2003; Moller 2004; Ruiz‐del‐Arbol 2005). Activation of the the endogenous vasoconstrictors may result in severe vasoconstriction of the renal arteries leading to hepatorenal syndrome (Cardenas 2003). Vasoactive drugs that increase splanchnic arterial tone may reverse the process.
Why it is important to do this review
Six randomised clinical trials compared terlipressin versus placebo for hepatorenal syndrome (Hadengue 1998; Solanki 2003; Martín‐Llahí 2008; Neri 2008; Sanyal 2008; Boyer 2016). One meta‐analysis including participants with type 1 hepatorenal syndrome found a beneficial effect of terlipressin versus placebo on reversal of hepatorenal syndrome (Fabrizi 2009). Our previous Cochrane systematic review including participants with type 1 or type 2 hepatorenal syndrome also found that terlipressin versus placebo or no intervention may reduce mortality and increase the proportion of participants with improved renal function (Gluud 2010; Gluud 2012). At present, guidelines recommend terlipressin as the treatment of choice for people with hepatorenal syndrome (EASL 2010). The drug is not available in the USA and other countries (Runyon 2013). Randomised clinical trials have compared terlipressin versus noradrenaline (Duvoux 2002; Alessandria 2007; Sharma 2008). The results suggested that the interventions were equally effective. However, due to the size of the trials, the results may have been inconclusive. Our previous Cochrane Review and one subsequent meta‐analysis found no significant differences between terlipressin and other vasoactive drugs (Gluud 2010; Gluud 2012; Nassar 2014). The Cochrane Review included two randomised clinical trials comparing terlipressin versus noradrenaline and the meta‐analysis included four randomised clinical trials. Both reviews found inconclusive evidence. Therefore, we conducted this updated review.
Objectives
To evaluate the beneficial and harmful effects of terlipressin versus other vasoactive drugs for people with hepatorenal syndrome.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised clinical trials regardless of blinding, publication status, or language in analyses of benefits and harms. We planned to include quasi‐randomised and observational studies in the assessment of harms identified in the searches. If, during the selection of trials, we had identified observational studies (i.e. quasi‐randomised studies, cohort studies, or patient reports) reporting adverse events caused by, or associated with, the interventions in our review, we planned to include these studies for a review of the adverse events only. We did not specifically search for observational studies for inclusion, which is a known limitation of our systematic review.
Types of participants
People with cirrhosis and type 1 or type 2 hepatorenal syndrome according to current or earlier diagnostic criteria (Arroyo 1996; Salerno 2007; Angeli 2015).
Types of interventions
Comparisons of terlipressin versus other vasoactive drugs (including noradrenaline, octreotide, midodrine, or dopamine) regardless of dose or duration of interventions. We accepted cointerventions including albumin.
Types of outcome measures
We assessed all outcomes at the maximum duration of follow‐up (Gluud 2017).
Primary outcomes
Mortality (all‐cause).
Hepatorenal syndrome (persistent hepatorenal syndrome despite treatment).
Serious adverse events: defined as any untoward medical occurrence that led to death, was life‐threatening, or required hospitalisation or prolongation of hospitalisation (ICH‐GCP 1997). We assessed serious adverse events as a composite outcome and conducted analyses of individual serious adverse events.
Secondary outcomes
Health‐related quality of life.
Non‐serious adverse events defined as adverse events that did not fulfil the criteria for serious adverse events.
Search methods for identification of studies
Electronic searches
We searched The Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2017; November 2016), Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2016, Issue 11), MEDLINE Ovid (1946 to November 2016), Embase Ovid (1974 to November 2016), and Science Citation Index Expanded (Web of Science; 1900 to November 2016) (Royle 2003), using the search strategies described in Appendix 1.
Searching other resources
We scanned the reference lists of relevant articles, and proceedings from meetings of the British Society for Gastroenterology, the British Association for the Study of the Liver, the European Association for the Study of the Liver, the United European Gastroenterology Week, the American Gastroenterological Association, and the American Association for the Study of Liver Diseases. We wrote to the principal authors of randomised clinical trials and the pharmaceutical companies involved in the production of vasoactive drugs for additional information about completed randomised clinical trials and for information about any ongoing randomised clinical trials, and searched the database ClinicalTrials.gov (clinicaltrials.gov) and the World Health Organization (WHO) online trial meta‐register (apps.who.int/trialsearch/).
Data collection and analysis
We performed the review following the recommendations of the Cochrane Hepato‐Biliary Group (Gluud 2017).
Selection of studies
All authors participated independently in the literature searches, the identification of potentially eligible trials and studies, and in the decision regarding inclusion or exclusion of trials. We reached the final selection through consensus and resolved disagreements through discussion. We listed details of all included randomised clinical trials in summary tables and listed all excluded studies with the reasons for their exclusion.
Data extraction and management
Three authors (MI, AA, and LG) independently collected data using pilot‐tested data extraction sheets and resolved contrary opinions through discussion. We requested missing data and other information from authors of included randomised clinical trials.
We collected the following data:
-
general study information:
year, country, and language of publication (if published);
funding;
design;
-
intervention:
type, dose and duration;
cointerventions;
-
participants:
characteristics (age, proportion with cirrhosis, proportion of men/women, aetiology of liver disease);
criteria used to diagnose hepatorenal syndrome;
withdrawals and losses to follow‐up;
-
outcomes:
outcomes assessed and duration of follow‐up.
Assessment of risk of bias in included studies
We assessed bias control using the domains described in the Cochrane Hepato‐Biliary Group Module (Gluud 2017), and classified the risk of bias for separate domains as high, unclear, or low (Higgins 2011). We also combined the bias domains in an overall assessment as described below.
Allocation sequence generation
Low risk of bias: the study authors performed sequence generation using computer random number generation or a random number table. We considered drawing lots, tossing a coin, shuffling cards, and throwing dice as adequate if an independent person not otherwise involved in the study performed them.
Unclear risk of bias: the study authors did not specify the method of sequence generation.
High risk of bias: the sequence generation method was not random. We only considered such studies for assessment of harms.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. A central and independent randomisation unit controlled allocation. The investigators were unaware of the allocation sequence (e.g. if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Unclear risk of bias: the study authors did not describe the method used to conceal the allocation so the intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: it was likely that the investigators who assigned the participants knew the allocation sequence. We only considered such studies for assessment of harms.
Blinding of participants and personnel
Low risk of bias: any of the following: no blinding or incomplete blinding, but the outcome was not likely to be influenced by lack of blinding (e.g. mortality) (Wood 2008; Savović 2012); or blinding of participants and key study personnel ensured, and it was unlikely that the blinding could have been broken.
Unclear risk of bias: insufficient information to permit judgement of 'low risk' or 'high risk.'
High risk of bias: any of the following: no blinding or incomplete blinding, and the outcome was likely to be influenced by lack of blinding (non‐mortality outcomes).
Blinded outcome assessment
Low risk of bias: any of the following: no blinding of outcome assessment, but outcome measurement was not likely to be influenced by lack of blinding (mortality) (Wood 2008; Savović 2012); or blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
Unclear risk of bias: insufficient information to permit judgement of 'low risk' or 'high risk.'
High risk of bias: any of the following: no blinding of outcome assessment, and the outcome measurement was likely to be influenced by lack of blinding (non‐mortality outcomes); or blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement was likely to be influenced by lack of blinding (non‐mortality outcomes).
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. The study used sufficient methods, such as multiple imputations, to handle missing data.
Unclear risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to induce bias on the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: the trial reported the following predefined outcomes: all‐cause mortality, hepatorenal syndrome, and serious adverse events. If the original trial protocol was available, the outcomes should have been those called for in that protocol. We only considered information from trial registries (e.g. www.clinicaltrials.gov) if the protocol was registered at the time that the trial was begun.
Unclear risk of bias: the study authors did not report all predefined outcomes fully, or it was unclear whether the study authors recorded data on these outcomes or not.
High risk of bias: the study authors did not report one or more predefined outcomes.
For‐profit bias
Low risk of bias: the trial appeared free of industry sponsorship or other type of for‐profit support.
Unclear risk of bias: the trial did not provide any information on clinical trial support or sponsorship.
High risk of bias: the trial was sponsored by industry or received other type of for‐profit support.
Other bias
Low risk of bias: the trial appeared free of other factors that could put it at risk of bias (e.g. different follow‐up or administration of an inappropriate doses).
Unclear risk of bias: the trial may or may not have been free of other factors that could put it at risk of bias.
High risk of bias: there were other factors in the trial that could put it at risk of bias.
Overall bias assessment
Low risk of bias: all domains were low risk of bias using the definitions described above.
High risk of bias: one or more of the bias domains were of unclear or high risk of bias.
Measures of treatment effect
We used risk ratios (RR) with 95% confidence intervals (CI).
Unit of analysis issues
We planned to include the first period from cross‐over trials due to the severe prognosis associated with the condition. None of the identified randomised clinical trials used a cross‐over design.
Dealing with missing data
We extracted data on all participants randomised to allow intention‐to‐treat analyses, the number of participants with missing outcome, and reasons for missing data. To evaluate the importance of missing data, we planned to conduct a worst‐case scenario analysis and a best‐worst case scenario analysis (Gluud 2017). We did not conduct the analyses because we were unable to identify the number of participants who had missing data.
Assessment of heterogeneity
We visually inspected forest plots and expressed heterogeneity as I² values using the following thresholds: 0% to 40% (unimportant), 41% to 60% (moderate), 61% to 80% (substantial), and greater than 80% (considerable).
Assessment of reporting biases
For meta‐analyses with at least 10 randomised clinical trials, we planned to assess reporting biases through regression analyses using the Harbord test (Harbord 2006).
Data synthesis
We performed the analyses in Review Manager 5 (RevMan 2014), STATA version 14 (Stata 2014), and Trial Sequential Analysis (TSA 2011), and used the GRADEpro software (GRADEpro) to prepare a 'Summary of findings' table.
Meta‐analysis
In our primary analyses, we stratified randomised clinical trials based on the type of control intervention. We compared the fixed‐effect and random‐effects estimates of the intervention effect. The estimates were similar. Accordingly, we assumed that any small‐study effects had little influence on the intervention effect estimate. If the random‐effects estimate had been more beneficial, we planned to re‐evaluate whether it was reasonable to conclude that the intervention was more effective in the smaller studies. If the larger studies tended to be those conducted with greater methodological rigour, or conducted in circumstances more typical of the use of the intervention in practice, then we planned to report the results of meta‐analyses restricted to the larger, more rigorous studies. Based on the expected clinical heterogeneity, we expected that a number of analyses would display statistical between‐trial heterogeneity (I² greater than 0%). For random‐effects models, precision decreased with increasing heterogeneity and CIs widened correspondingly. Therefore, we expected that the random‐effects model would give the most conservative (and a more correct) estimate of the intervention effect. Accordingly, we reported the results of our analyses based on random‐effects meta‐analyses.
Trial Sequential Analysis
We performed Trial Sequential Analyses for our primary outcomes (Higgins 2008; Wetterslev 2008; Thorlund 2011; Wetterslev 2017). We defined the required information size (also known as the heterogeneity‐adjusted required information size) as the number of participants needed to detect or reject an intervention effect based on the relative risk reduction (RRR) and the control group risk (CGR). The analyses show firm evidence if the Z‐curve crosses the monitoring boundary (also known as the trial sequential monitoring boundary) before reaching the required information size. We constructed futility boundaries to evaluate the uncertainty of obtaining a chance negative finding and performed the analyses with alpha set to 3% and power to 90% model‐based diversity. We originally planned to limit the analyses to randomised clinical trials with a low risk of bias, but we only identified two such trials. Therefore, we included all randomised clinical trials in our analyses. We reduced the RRR based on the recommendations from the Hepato‐Biliary Group and based the CGR on the proportions of events in the terlipressin group in the meta‐analysis in Allegretti 2017.
Mortality: CGR 52%, RRR 20%, heterogeneity correction 30%.
Hepatorenal syndrome (persistent hepatorenal syndrome despite treatment): CGR 63%, RRR 25%, heterogeneity correction 50%.
Serious adverse events: CGR 11%, RRR 25%, heterogeneity correction 20%.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses comparing types of other vasoactive drug and participants with type 1 or type 2 hepatorenal syndrome. We conducted subgroup analyses on mortality based on our assessment of bias control.
Sensitivity analysis
We performed a sensitivity analysis excluding randomised clinical trials published in abstract form. We planned to conduct a worse‐case‐scenario analysis as described above, but we did not identify randomised clinical trials describing the number of participants with missing outcome data in the two groups.
'Summary of findings' table
We used the GRADE system to evaluate the quality of the evidence for outcomes reported in the review considering the within‐study risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimate, and risk of publication bias (GRADEpro). Two authors (MI and LG) created the 'Summary of findings' table, which included the primary outcomes and the most common adverse events; that is, diarrhoea and abdominal pain.
Results
Description of studies
We included 10 randomised clinical trials (see Characteristics of included studies table). Our searches did not identify eligible non‐randomised studies. We excluded 11 randomised clinical trials, one quasi‐randomised trial, and one observational study (see Characteristics of excluded studies table).
Results of the search
We identified 619 potentially relevant references in electronic databases and four additional records through manual searches (Figure 1). After removing duplicates and clearly irrelevant references, 427 references remained. After screening these 427 references, we retrieved 26 references for further assessment. In total, we excluded 14 references referring to 13 randomised clinical trials because they did not compare terlipressin with other vasoactive drugs. The remaining 12 references referred to 10 randomised clinical trials fulfilling all of our inclusion criteria and none of our exclusion criteria. Eight of these were published in full‐paper articles (Alessandria 2007; Sharma 2008; Singh 2012; Badawy 2013; Ghosh 2013; Srivastava 2015; Cavallin 2016; Goyal 2016), and two in abstracts (Copaci 2013; Indrabi 2013).
1.
Study flow diagram.
We wrote to the authors of included randomised clinical trials to ask for additional information about included participants. However, we received no additional data.
Included studies
Participants
The countries of origin were India (Sharma 2008; Singh 2012; Ghosh 2013; Indrabi 2013; Srivastava 2015; Goyal 2016), Italy (Alessandria 2007; Cavallin 2016), Egypt (Badawy 2013), and Romania (Copaci 2013). The trials included 474 participants. The mean age ranged from 39 to 65 years and the proportion of men from 52% to 93%. The proportion of participants with alcoholic liver disease ranged from 20% to 94%.
Four randomised clinical trials (Alessandria 2007; Sharma 2008; Badawy 2013; Ghosh 2013) used the 1996 criteria (Arroyo 1996; Appendix 2) to diagnose hepatorenal syndrome and four randomised clinical trials (Singh 2012; Srivastava 2015; Cavallin 2016; Goyal 2016) used the 2007 criteria (Salerno 2007; Appendix 2). The remaining two randomised clinical trials did not specify the criteria used to diagnose hepatorenal syndrome (Copaci 2013; Indrabi 2013). Seventy‐seven per cent of the included participants had type 1 hepatorenal syndrome and the remaining 23% had type 2 hepatorenal syndrome. Two papers did not provide separate outcome data for participants with type 1 and type 2 hepatorenal syndrome (Copaci 2013; Cavallin 2016). The vast majority (80/88 participants) in these two randomised clinical trials had type 1 hepatorenal syndrome, and consequently due to the lack of separate outcome data, we included all 88 participants in our subgroup analyses of type 1 hepatorenal syndrome.
Interventions
All randomised clinical trials compared terlipressin versus other vasoactive drugs. All participants received cointervention with albumin. Seven randomised clinical trials used a treatment duration protocol running until reversal of hepatorenal syndrome, death, liver transplantation, or a maximum of two weeks (Alessandria 2007; Sharma 2008; Singh 2012; Badawy 2013; Ghosh 2013; Cavallin 2016; Goyal 2016). One randomised clinical trial used five days of treatment (Srivastava 2015), and two randomised clinical trials did not describe the treatment duration (Copaci 2013; Indrabi 2013).
Terlipressin
Six randomised clinical trials used an intravenous bolus injection (Alessandria 2007; Sharma 2008; Singh 2012; Ghosh 2013; Srivastava 2015; Goyal 2016), and two randomised clinical trials used continuous infusions (Badawy 2013; Cavallin 2016). We were unable to gather information of the administration form (bolus or continuous infusion) from two trials (Copaci 2013; Indrabi 2013). Eight randomised clinical trials used a dose titration regimen for terlipressin (Alessandria 2007; Sharma 2008; Singh 2012; Badawy 2013; Copaci 2013; Ghosh 2013; Cavallin 2016; Goyal 2016). The initial daily dose ranged from 2 mg to 6 mg and was increased in a stepwise manner to a maximum dose of 6 mg to 12 mg until reaching an absolute reduction in serum creatinine of less than 1 mg/dL or less than 25% from baseline after 48 to 72 hours. One randomised clinical trial used a fixed dose of 0.5 mg per six hours (Srivastava 2015). One randomised clinical trial did not provide information about the dose (Indrabi 2013).
Other vasoactive drugs
Seven randomised clinical trials compared terlipressin versus noradrenaline (Alessandria 2007; Sharma 2008; Singh 2012; Badawy 2013; Ghosh 2013; Indrabi 2013; Goyal 2016), one randomised clinical trial compared terlipressin versus midodrine and octreotide (Cavallin 2016), one randomised clinical trial compared terlipressin versus octreotide (Copaci 2013), and one randomised clinical trial compared terlipressin versus dopamine (Srivastava 2015). Noradrenaline and dopamine were administrated as continuous intravenous infusions. Octreotide was given as subcutaneous bolus injections and midodrine as oral tablets. The dose depended on the drug.
Noradrenaline: in six randomised clinical trials, the initial dose of 0.5 mg/hour was increased in a stepwise manner to a maximum dose of 3 mg/hour (Sharma 2008; Singh 2012; Badawy 2013; Ghosh 2013; Indrabi 2013; Goyal 2016). One randomised clinical trial used an initial dose of 0.1 μg/kg/minute increased stepwise to a maximum of 0.7 μg/kg/minute in lack of response (Alessandria 2007). All randomised clinical trials adjusted the dose based on the mean arterial pressure and urine output.
Dopamine: 2 μg/kg/minute (Srivastava 2015).
Midodrine: the initial dose of 7.5 mg was increased to 12.5 mg if the change in serum creatinine was less than 25% within 48 hours (Cavallin 2016).
Octreotide: the initial dose of 100 μg was increased to 200 μg if the change in serum creatinine was less than 25% within 48 hours (Copaci 2013; Cavallin 2016).
Cointerventions
All included randomised clinical trials treated both intervention groups using equal doses of intravenous albumin infusion in combination with the vasoactive drugs. Overall, the mean dose of albumin ranged from 20 g/day to 56 g/day. Four trials used 20 g/day to 40 g/day (Sharma 2008; Singh 2012; Ghosh 2013; Srivastava 2015). Two randomised clinical trials used 1 g/kg bodyweight at the inclusion day, followed by 20 g/day to 40 g/day (Copaci 2013; Cavallin 2016). Two randomised clinical trials titrated the dose to maintain a central venous pressure between 10 cmH2O and 15 cmH2O (Alessandria 2007; Badawy 2013). One randomised clinical trial did not report the albumin dose (Indrabi 2013).
Outcome measures
All 10 randomised clinical trials described mortality and serious adverse events using follow‐up between 15 and 90 days. Nine randomised clinical trials reported reversal of hepatorenal syndrome (Alessandria 2007; Sharma 2008; Singh 2012; Badawy 2013; Copaci 2013; Ghosh 2013; Indrabi 2013; Cavallin 2016; Goyal 2016) and six randomised clinical trials reported non‐serious adverse events (Sharma 2008; Singh 2012; Ghosh 2013; Srivastava 2015; Goyal 2016; Cavallin 2016). None reported health‐related quality of life.
Excluded studies
In total, we excluded 13 trials evaluating vasoactive drugs for hepatorenal syndrome (see Characteristics of excluded studies table). We excluded eight randomised clinical trials evaluating terlipressin versus placebo (Hadengue 1998; Yang 2001; Solanki 2003; Martín‐Llahí 2008; Neri 2008; Pulvirenti 2008; Sanyal 2008; Boyer 2016), and one randomised clinical trial comparing noradrenaline versus midodrine and octreotide (Tavakkoli 2012). In addition, we excluded two randomised clinical trials comparing the administration form (Cavallin 2015) or dose of terlipressin (Wan 2014). Finally, we excluded two non‐randomised studies without information about harms (Silawat 2011 (quasi‐randomised trial); Nguyen‐Tat 2015 (observational study)).
Risk of bias in included studies
For 'Risk of bias' summary, see Figure 2 and Figure 3.
2.
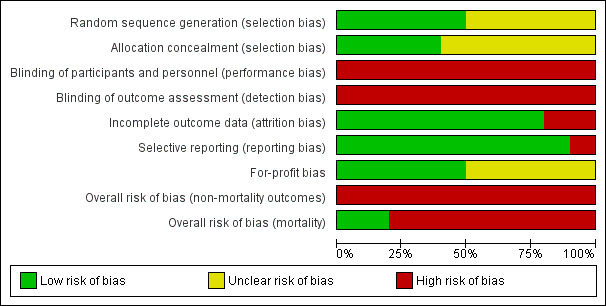
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
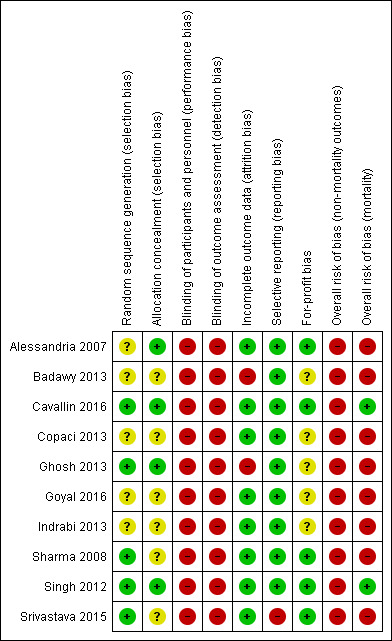
"Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
'+' = low risk of bias;
'‐' = high risk of bias;
'?' = unclear risk of bias.
Allocation
Three randomised clinical trials generated the allocation sequence using a computer‐generated list of random numbers and concealed the allocation by using serially numbered opaque sealed envelopes (Singh 2012; Ghosh 2013; Cavallin 2016). We classified these three randomised clinical trials as low risk of selection bias. The remaining seven randomised clinical trials did not provide adequate descriptions of both randomisation and concealment and were classified at unclear risk of bias in at least one of the domains (Alessandria 2007; Sharma 2008; Badawy 2013; Copaci 2013; Indrabi 2013; Srivastava 2015; Goyal 2016).
Blinding
All randomised clinical trials were open and none used blinded outcome assessment. We classified all randomised clinical trials as high risk of bias for these two domains in the evaluation of non‐mortality outcomes (Alessandria 2007; Sharma 2008; Singh 2012; Badawy 2013; Copaci 2013; Ghosh 2013; Indrabi 2013; Cavallin 2016; Srivastava 2015; Goyal 2016).
Incomplete outcome data
Eight randomised clinical trials had no losses to follow‐up or withdrawals, and included all participants in their analyses (Alessandria 2007; Sharma 2008; Singh 2012; Copaci 2013; Indrabi 2013; Srivastava 2015; Goyal 2016; Cavallin 2016). Two randomised clinical trials excluded participants after randomisation in their analyses, but they did not describe the number of participants with missing outcomes (Badawy 2013; Ghosh 2013). Consequently, we classified eight randomised clinical trials at low risk and two at high risk of attrition bias.
Selective reporting
Nine randomised clinical trials defined and described clinically relevant outcomes (Alessandria 2007; Sharma 2008; Singh 2012; Copaci 2013; Badawy 2013; Ghosh 2013; Srivastava 2015; Goyal 2016; Cavallin 2016). One randomised clinical trial reported mortality and serious adverse events but not hepatorenal syndrome (Srivastava 2015). We classified nine randomised clinical trials at low risk and one at high risk of reporting bias.
For‐profit bias
Five randomised clinical trials described competing interests (Alessandria 2007; Sharma 2008; Singh 2012; Srivastava 2015; Cavallin 2016). None received funding or other support from for‐profit companies. The remaining five randomised clinical trials did not describe funding (Badawy 2013; Copaci 2013; Ghosh 2013; Indrabi 2013; Goyal 2016).
Overall bias assessment
In the assessment of mortality, we classified two randomised clinical trials at low risk of bias (Singh 2012; Cavallin 2016), and eight at high risk of bias (Alessandria 2007; Sharma 2008; Badawy 2013; Copaci 2013; Ghosh 2013; Indrabi 2013; Srivastava 2015; Goyal 2016).
All randomised clinical trials were high risk of bias in all remaining outcomes (Alessandria 2007; Sharma 2008; Singh 2012; Badawy 2013; Copaci 2013; Ghosh 2013; Indrabi 2013; Srivastava 2015; Goyal 2016; Cavallin 2016).
Effects of interventions
See: Table 1
We evaluated the effects of interventions on the primary outcomes; mortality, hepatorenal syndrome (persistent despite treatment), and serious adverse events.
Mortality (all‐cause)
We retrieved mortality data from all 10 randomised clinical trials (Analysis 1.1). The analysis showed no significant difference between terlipressin and other vasoactive drugs when including all trials (RR 0.96, 95% CI 0.88 to 1.06; 474 participants; I² = 0%) or when including the two trials with a low risk of bias in the overall assessment (RR 0.92, 95% CI 0.63 to 1.36; 94 participants). In Trial Sequential Analysis including all trials regardless of bias control (Figure 4), the cumulative Z‐curve did not cross the monitoring boundaries for benefit, harm, or futility. Subgroup analyses found lack of evidence supporting different effects between terlipressin and noradrenaline (RR 0.98, 95% CI 0.88 to 1.08; 306 participants; 7 trials; I² = 0%); midodrine and octreotide (RR 0.71, 95% CI 0.40 to 1.28; 48 participants; 1 trial); octreotide alone (RR 0.75, 95% CI 0.32 to 1.77; 40 participants; 1 trial); or dopamine and furosemide (RR 0.97, 95% CI 0.77 to 1.22; 80 participants; 1 trial) (Analysis 1.2), in participants with type 1 or type 2 hepatorenal syndrome (Analysis 1.3), or trials published as full paper articles or abstracts (Analysis 1.4).
1.1. Analysis.
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 1 Mortality: bias control.
4.
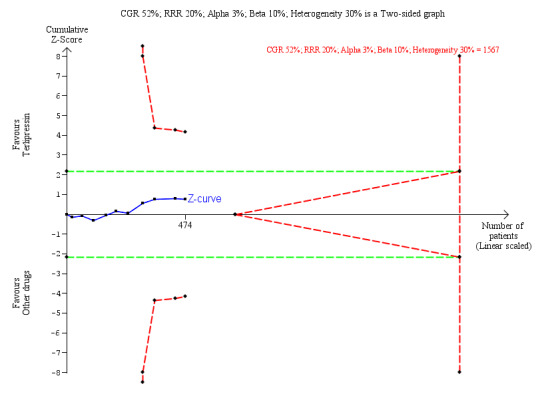
Trial Sequential Analysis of 10 randomised clinical trials (474 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on mortality. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 20%, a control group risk (CGR) of mortality of 52%, and a model variance ‐ based heterogeneity correction of 30%. The risk ratio was 0.96 (97% confidence interval 0.79 to 1.18). The cumulative Z‐curve (blue line) did not cross the diversity‐adjusted trial monitoring boundary for benefit.
1.2. Analysis.
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 2 Mortality: type of vasoactive drug.
1.3. Analysis.
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 3 Mortality: type of hepatorenal syndrome.
1.4. Analysis.
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 4 Mortality: publication status.
Hepatorenal syndrome
One trial did not report the number of participants with persistent hepatorenal syndrome despite treatment (Srivastava 2015). The meta‐analysis of remaining nine trials found a significant beneficial effect of terlipressin versus other vasoactive drugs (RR 0.79, 95% CI 0.63 to 0.99; 394 participants; I² = 26%; Analysis 1.5). The analysis did not include trials with a low risk of bias. In Trial Sequential Analysis, including the nine trials (Figure 5), the cumulative Z‐curve did not cross the monitoring boundaries for benefit, harm, or futility. Subgroup analyses showed that terlipressin was superior to octreotide alone (RR 0.56, 95 % CI 0.33 to 0.96) and midodrine combined with octreotide (RR 0.47, 95 % CI 0.30 to 0.72), but each analysis was only based on one trial (Analysis 1.5). There were no differences between trials comparing terlipressin and noradrenaline (Analysis 1.5), participants with type 1 or type 2 hepatorenal syndrome (Analysis 1.6), or trials published as full‐paper articles or abstracts (Analysis 1.7).
1.5. Analysis.
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 5 Hepatorenal syndrome: type of vasoactive drug.
5.
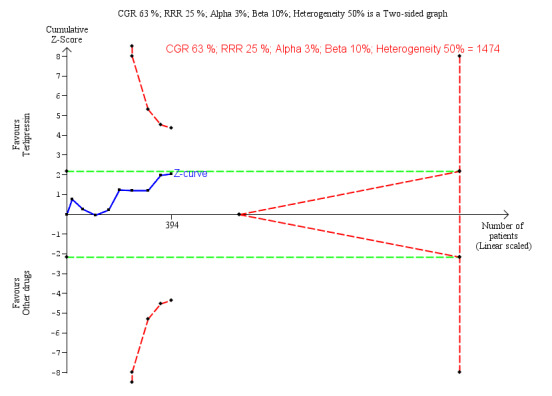
Trial Sequential Analysis of nine randomised clinical trials (394 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on lack of reversal of hepatorenal syndrome. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of lack of reversal of hepatorenal syndrome of 63%, and a heterogeneity correction of 50%. The risk ratio was 0.79 (97% confidence interval 0.48 to 1.31). The cumulative Z‐curve (blue line) does not cross the diversity‐adjusted trial monitoring boundary for benefit.
1.6. Analysis.
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 6 Hepatorenal syndrome: type hepatorenal syndrome.
1.7. Analysis.
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 7 Hepatorenal syndrome: publication status.
Serious adverse events
We extracted data of serious adverse events as a composite outcome from all trials (Analysis 1.8). None had a low risk of bias in the overall assessment. Overall, there was no significant difference between terlipressin and other vasoactive drugs (RR 0.96, 95% CI 0.88 to 1.06; I² = 0%). In subgroup analysis, terlipressin did not increase the risk of major cardiovascular events (RR 0.88, 95% CI 0.13 to 5.98; Analysis 1.9). Trial Sequential Analysis showed insufficient evidence to support or refute a beneficial or detrimental effect of terlipressin versus other vasoactive drugs (Figure 6).
1.8. Analysis.
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 8 Serious adverse events, type of vasoactive drug.
1.9. Analysis.
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 9 Serious adverse events, type of event.
6.
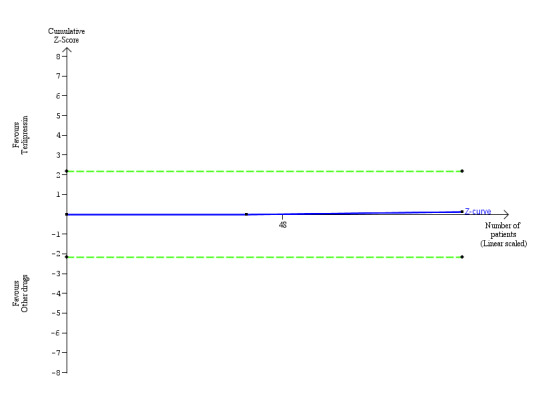
Trial Sequential Analysis of two randomised clinical trials (88 participants) evaluating terlipressin versus other vasoactive drugs for people with hepatorenal syndrome on cardiovascular adverse events. The analysis was made with power 90%, alpha 3%, a relative risk reduction (RRR) of 25%, a control group risk (CGR) of cardiovascular adverse events of 15%, and a heterogeneity correction of 20%. The diversity‐adjusted trial monitoring boundary for harm was not included in the figure due to insufficient information. The estimated required information size was 4831 participants. Accordingly, with an accrued number of participants of 88, the required number of participants was not achieved.
Secondary outcomes
Health‐related quality of life
None of the included trials evaluated the effect of the interventions on health‐related quality of life.
Non‐serious adverse events
We were able to gather data on non‐serious adverse events from six trials (Analysis 1.10). Overall, we found no evidence supporting differences between terlipressin and other vasoactive drugs regarding the overall risk of non‐serious adverse events (RR 1.82, 95 % CI 1.00 to 3.31; 301 participants; I² = 0%), but that terlipressin increased the risk of diarrhoea or abdominal pain, or both (RR 3.50, 95% CI 1.19 to 10.27; 221 participants; 5 trials; I² = 0%; Analysis 1.11). We were unable to evaluate these two non‐serious adverse events (diarrhoea or abdominal pain) separately.
1.10. Analysis.
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 10 Non‐serious adverse events.
1.11. Analysis.
Comparison 1 Terlipressin versus other vasoactive drugs, Outcome 11 Non‐serious adverse event: types.
'Summary of findings' tables
We downgraded the evidence to very low quality (Table 1). The main reasons for downgrading the evidence were risk of bias, imprecision, and the results from the Trial Sequential Analyses.
Discussion
Summary of main results
This systematic review included a small number of randomised clinical trials comparing terlipressin versus other vasoactive drugs. The statistical strength of the evidence was weak and the risk of bias considerable. The analyses found no evidence supporting differences between terlipressin and other vasoactive drugs regarding mortality and serious adverse events, but we found a beneficial effect of terlipressin on hepatorenal syndrome based on the proportion of participants without reversal. Subgroup analysis of the outcome hepatorenal syndrome showed a potential benefit of terlipressin compared to octreotide alone or octreotide combined with midodrine, but that analysis only included two small trials. Terlipressin is associated with an increased risk of abdominal pain or diarrhoea, or both. Trial Sequential Analyses confirmed that our review found insufficient evidence to support or refute a beneficial or harmful effect of terlipressin and that additional randomised clinical trials are needed. In this review, we both conducted overall analyses (to increase power and precision) and subgroup analyses comparing terlipressin versus the individual different comparators. The overall analysis was difficult to interpret, but apparently we found no heterogeneity.
Overall completeness and applicability of evidence
We included randomised clinical trials with participants diagnosed with hepatorenal syndrome. The initial diagnostic recommendations from 1996 included several major and minor criteria (Arroyo 1996). The subsequent revised diagnostic criteria now focus on evidence of severe liver disease, ascites, and exclusion of other causes of renal failure (Salerno 2007). We originally assumed that using the different diagnostic criteria would result in some degree of clinical heterogeneity. The potential influence of the diagnostic criteria did not lead to statistical heterogeneity. However, the lack of heterogeneity may also reflect the small number of trials and participants. Recommendations have suggested a revision based on the accepted criteria for diagnosing acute renal failure (Angeli 2015; Appendix 4). This suggestion was based on the criteria of acute kidney injury defined by the Acute Kidney Injury Network (Mehta 2007). In the previous criteria, a fixed value of serum creatinine greater than 133 mmol/L was used in diagnostic assessment. The revised criteria suggest that we should use an increase of serum creatinine of 0.3 mg/dL or greater (26.5 μmol/L or greater) or greater than 50% from baseline within 48 hours. The reasoning is that changes in serum creatinine are more sensitive to an acute reduction of renal function. The aim of these criteria is to allow earlier detection and intervention to improve the overall outcome for people diagnosed with cirrhosis and acute kidney injury or hepatorenal syndrome. The consensus recommendation suggests that people meeting the new criteria should be treated with vasoconstrictors and albumin. Additional studies are needed to evaluate if this is correct. In addition, the potential importance of albumin should be addressed in future randomised clinical trials. One meta‐analysis comprising 19 studies found a dose‐response association between survival and increased cumulative doses of albumin (Salerno 2015). We were unable to address the question in our review and were, therefore, unable to evaluate if the results reflect bias.
One study used three months of follow‐up and had one of the highest response rates (83%) and the lowest mortality (32%) (Alessandria 2007). During the follow‐up, all survivors except one had reversal of hepatorenal syndrome and underwent liver transplantations after the reversal of hepatorenal syndrome. This may suggest that some populations, such as candidates for liver transplantation, are more likely to benefit from treatment with vasoactive drugs.
Quality of the evidence
The lack of large, high‐quality randomised clinical trials is the main limitation of this review. We only identified two randomised clinical trials with a low risk of bias in the assessment of mortality. For the remaining outcomes, we classified all trials at high risk of bias. Lack of blinding was a concern as was an unclear control of selection bias. In addition, two trials had a high risk of attrition bias (Badawy 2013; Ghosh 2013). These trials excluded more than 15% of the participants after randomisation. Unfortunately, we were unable to gather data that allowed a worst‐case or an extreme worst‐case scenario analysis and we were, therefore, unable to evaluate the influence of losses to follow‐up. We contacted the authors, but we were unable to gather additional data. Another major concern is that several trials did not report systematically adverse events. Consequently, our results may underestimate the actual risk of adverse events. Similarly to this present meta‐analysis none of the included trials were powered for equivalence or inferiority analysis. We increased the risk of clinical heterogeneity by pooling type 1 and type 2 hepatorenal syndrome, two diagnostic criteria of hepatorenal syndrome and all types of other vasoactive drugs than terlipressin. The trials were not designed for equivalence or inferiority analysis. The Trial Sequential Analysis showed that the sample size did not reach the required information size for equivalence/inferiority meta‐analysis. Due to the lack of power, increased risk of clinical heterogeneity combined with the large proportion of trials classified at high risk of bias, we must classify the overall quality of evidence as very low in this present review.
Potential biases in the review process
One of the main limitations in this present review was the small sample size. Another limitation was the predominance of randomised clinical trials at high risk of bias.
According to the results of our Trial Sequential Analyses, none of our findings reached the required information size for superiority, equivalence, or inferiority meta‐analyses. This means that our significant findings of terlipressin versus other vasoactive drugs were inconclusive. In the same way, our non‐significant findings were inconclusive and may cover efficacy differences between the type of other vasoactive drugs.
Methodological concerns are highlighted in the Risk of bias in included studies section. However, it must be emphasised in particular, that the majority of trials in this present review had unsystematic reporting of adverse events, which may compromise the validity of our results on safety. Treatment with terlipressin is associated with an increased risk of ischaemia including cardiovascular events and with less serious events such as abdominal pain or diarrhoea, or both (Krag 2008). However, the risk of ischaemia is not unique to terlipressin as a vasoactive drug. One large‐scale randomised clinical trial including 778 participants tested noradrenaline versus vasopressin in septic shock and found equal risk of ischaemia, of which the majority of adverse events included cardiovascular and intestinal ischaemia (Russell 2008). The profile of adverse events of terlipressin and noradrenaline are closely linked to the mode of action of vasoconstrictors. From our results, we cannot suggest which vasoactive drug is safest. However, we must emphasise that treatment with all types of vasoactive drugs require close monitoring to balance between improving renal perfusion and prevent ischaemia.
We were unable to assess long‐term benefits and harms of terlipressin versus other vasoactive drugs because the majority of trials had 30 days' follow‐up and the longest follow‐up was three months.
We contacted the pharmaceutical companies producing vasoactive drugs, but we did not search for files of regulatory authorities.
Agreements and disagreements with other studies or reviews
Two meta‐analyses comparing terlipressin versus noradrenaline for hepatorenal syndrome included four randomised clinical trials (Nassar 2014; Mattos 2016). In agreement with our findings, the meta‐analyses found no clear difference between terlipressin and noradrenaline. Two additional meta‐analyses evaluated vasoactive drugs for type 1 hepatorenal syndrome and found results similar to ours (Facciorusso 2017; Gilford 2017). One of the meta‐analyses had a positive evaluation of the quality of the evidence (Facciorusso 2017). In agreement with the second meta‐analysis, we found no convincing evidence and that the majority of randomised clinical trials were too small and entailed a high risk of bias (Gilford 2017). The guidelines of management of hepatorenal type 1 by the European Association of Studying the Liver suggest that the first‐line treatment should be terlipressin combined with albumin (EASL 2010). The guidelines of management of hepatorenal syndrome type 1 by the American Association for Study of Liver Diseases recommends vasoactive drugs in combination with albumin (Runyon 2013). Terlipressin is not available in the USA and other countries; consequently the recommended vasoactive drugs include midodrine and octreotide, whereas noradrenaline should be considered in intensive care units.
Authors' conclusions
Implications for practice.
This review found insufficient evidence to support or refute terlipressin versus other vasoactive drugs for people with cirrhosis and hepatorenal syndrome. The main body of evidence described the management of people with type 1 hepatorenal syndrome. The review only includes a small number of participants with type 2 hepatorenal syndrome.
Implications for research.
Large, high‐quality randomised clinical trials are needed to evaluate if terlipressin is more beneficial or safer than other vasoactive drugs that have been shown to be superior when compared with placebo.
Acknowledgements
We would like to thank Dimitrinka Nikolova and Sarah Klingenberg from the Cochrane Hepato‐Biliary Group for their assistance with the preparation of our review protocol and search strategy.
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in The Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark. Disclaimer: the views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or The Copenhagen Trial Unit.
Peer reviewers: Fiona J Gifford, UK; Jonel Trebicka, Germany. Contact editor: Janus Christian Jakobsen, Denmark. Sign‐off editor: Christian Gluud, Denmark.
Appendices
Appendix 1. Search strategies
| Database | Time span | Search strategy | No of hits |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | November 2016. | (terlipressin* OR glypressin* OR vasoconstric*) AND hepatorenal syndrom* | 31 |
| The Cochrane Central Register of Controlled Trials (CENTRAL) | 2016, Issue 11. | #1 MeSH descriptor: [Vasoconstrictor Agents] explode all trees #2 terlipressin* or glypressin* or vasoconstric* #3 #1 or #2 #4 MeSH descriptor: [Hepatorenal Syndrome] explode all trees #5 hepatorenal syndrom* #6 #4 or #5 #7 #3 and #6 |
35 |
| MEDLINE Ovid | 1946 to November 2016. | 1. exp Vasoconstrictor Agents/ 2. (terlipressin* or glypressin* or vasoconstric*).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 3. 1 or 2 4. exp Hepatorenal Syndrome/ 5. hepatorenal syndrom*.mp. [mp=title, original title, abstract, name of substance word, subject heading word] 6. 4 or 5 7. 6 and 3 8. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, original title, abstract, name of substance word, subject heading word] 9. 8 and 7 |
59 |
| Embase Ovid | 1974 to November 2016. | 1. exp Terlipressin/ 2. exp Vasoconstrictor Agent/ 3. (terlipressin* or glypressin* or vasoconstric*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 4. 1 or 3 or 2 5. exp Hepatorenal Syndrome/ 6. hepatorenal syndrom*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 7. 6 or 5 8. 4 and 7 9. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 10. 8 and 9 |
204 |
| Science Citation Index Expanded (Web of Science) | 1900 to November 2016. | #5 #4 AND #3 #4 TS=(random* or blind* or placebo* or meta‐analysis) #3 #1 AND #2 #2 TS=(hepatorenal syndrom*) #1 TS=(terlipressin* or glypressin* or vasoconstric*) |
118 |
Appendix 2. Diagnostic criteria of hepatorenal syndrome (Arroyo 1996)
Diagnostic criteria described in Arroyo 1996.
Major criteria
Chronic or acute liver disease with advanced hepatic failure and portal hypertension.
Low glomerular filtration rate, as indicated by serum creatinine of greater than 133 mmol/L (1.5 mg/dL) or 24‐hour creatinine clearance less than 40 mL/minute.
Absence of shock, ongoing bacterial infection, and current or recent treatment with nephrotoxic drugs. Absence of gastrointestinal fluid losses (repeated vomiting or intense diarrhoea) or renal fluid losses (weight loss greater than 500 g/day for several days in people with ascites without peripheral oedema or 1000 g/day in people with peripheral oedema).
No sustained improvement in renal function (decrease in serum creatinine to 1.5 mg/dL or less or increase in creatinine clearance to 40 mL/minute or more) following diuretic withdrawal and expansion of plasma volume with 1.5 L of isotonic saline.
Proteinuria less than 500 mg/dL and no ultrasonographic evidence of obstructive uropathy or parenchymal renal disease.
Additional criteria
Urine volume less than 500 mL/day.
Urine sodium less than 10 mEq/L.
Urine osmolality greater than plasma osmolality.
Urine red blood cells less than 50 per high power field.
Serum sodium concentration less than 130 mEq/L.
Appendix 3. Diagnostic criteria of hepatorenal syndrome (Salerno 2007)
Diagnostic criteria described in Salerno 2007.
Cirrhosis with ascites.
Serum creatinine greater than 133 mmol/L (1.5 mg/dL).
No improvement of serum creatinine (decrease to a level of 133 mmol/L or less after at least two days with diuretic withdrawal and volume expansion with albumin. The recommended dose of albumin 1 g/kg of bodyweight per day up to a maximum of 100 g/day.
Absence of shock.
No current or recent treatment with nephrotoxic drugs.
Absence of parenchymal kidney disease as indicated by proteinuria greater than 500 mg/day, microhaematuria (more than 50 red blood cells per high power field) and abnormal renal ultrasonography.
Appendix 4. Diagnostic criteria of hepatorenal syndrome type of acute kidney injury in people with cirrhosis
Diagnostic criteria described in Angeli 2015.
Diagnosis of cirrhosis and ascites.
Diagnosis of acute kidney injury according to the International Ascites Club‐Acute Kidney Injury criteria (see below).
No response after two consecutive days of diuretic withdrawal and plasma volume expansion with albumin 1 g/kg of bodyweight.
Absence of shock.
No current or recent use of nephrotoxic drugs (non‐steroidal anti‐inflammatory drugs, aminoglycosides, iodinated contrast media, etc.).
No macroscopic signs of structural kidney injury, defined as: absence of proteinuria (greater than 500 mg/day), absence of microhaematuria (more than 50 red blood cells per high power field), normal findings on renal ultrasonography.
Definition of acute kidney injury according to the International Ascites Club‐Acute Kidney Injury criteria
Increase in serum creatinine of 0.3 mg/dL or greater (26.5 μmol/L or greater) within 48 hours; or a percentage increase serum creatinine of 50% or greater from baseline which is known, or presumed, to have occurred within the prior seven days.
| Staging of acute kidney injury | Stage 1 | Stage 2 | Stage 3 |
| ‐ | Increase in serum creatinine ≥ 0.3 mg/dL (≥ 26.5 μmol/L) or an increase in serum creatinine ≥ 1.5‐fold to 2‐fold from baseline. | Increase in serum creatinine > 2‐fold to 3‐fold from baseline. | Increase of serum creatinine > 3‐fold from baseline or serum creatinine ≥ 4.0 mg/dL (≥ 353.6 μmol/L) with an acute increase ≥ 0.3 mg/dL (≥ 26.5 μmol/L) or initiation of renal replacement therapy. |
| Progression of acute kidney injury | Progression | ‐ | Regression |
| ‐ | Progression of acute kidney injury to a higher stage or need for renal replacement therapy, or both. | ‐ | Regression of acute kidney injury to a lower stage. |
| Response to treatment | No response | Partial response | Full response |
| ‐ | No regression of acute kidney injury. | Regression of acute kidney injury stage with a reduction of serum creatinine to ≥ 0.3 mg/dL (≥ 26.5 μmol/L) above baseline value. | Return of serum creatinine to within 0.3 mg/dL (26.5 μmol/L) of baseline value. |
Baseline serum creatinine: a value of serum creatinine obtained in the previous three months, when available, can be used as baseline serum creatinine. In people with more than one value within the previous three months, the value closest to the admission time to the hospital should be used In people without a previous serum creatinine value, the serum creatinine on admission should be used as baseline.
Data and analyses
Comparison 1. Terlipressin versus other vasoactive drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality: bias control | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 1.1 Low risk of bias | 2 | 94 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.63, 1.36] |
| 1.2 High risk of bias | 8 | 380 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.07] |
| 2 Mortality: type of vasoactive drug | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 2.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 2.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.40, 1.28] |
| 2.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.77] |
| 2.4 Dopamine/furosemide | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 3 Mortality: type of hepatorenal syndrome | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 3.1 Type 1 hepatorenal syndrome | 9 | 375 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.87, 1.06] |
| 3.2 Type 2 hepatorenal syndrome | 3 | 99 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.68, 1.33] |
| 4 Mortality: publication status | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Full paper | 8 | 374 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.83, 1.14] |
| 4.2 Abstract | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.86, 1.08] |
| 5 Hepatorenal syndrome: type of vasoactive drug | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.99] |
| 5.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.76, 1.21] |
| 5.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.30, 0.72] |
| 5.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.33, 0.96] |
| 6 Hepatorenal syndrome: type hepatorenal syndrome | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| 6.1 Type 1 hepatorenal syndrome | 8 | 335 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.62, 1.01] |
| 6.2 Type 2 hepatorenal syndrome | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.36, 2.10] |
| 7 Hepatorenal syndrome: publication status | 9 | 394 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.63, 0.98] |
| 7.1 Full paper articles | 7 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.63, 1.06] |
| 7.2 Abstracts | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.44, 1.17] |
| 8 Serious adverse events, type of vasoactive drug | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 8.1 Noradrenaline | 7 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.88, 1.08] |
| 8.2 Midodrine/octreotide | 1 | 48 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.42, 1.23] |
| 8.3 Octreotide | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.32, 1.77] |
| 8.4 Dopamine/furosemide | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.77, 1.22] |
| 9 Serious adverse events, type of event | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Death | 10 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 9.2 Major cardiovascular events | 7 | 323 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.13, 5.98] |
| 10 Non‐serious adverse events | 6 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [1.00, 3.31] |
| 11 Non‐serious adverse event: types | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11.1 Diarrhoea or abdominal pain, or both | 5 | 221 | Risk Ratio (M‐H, Random, 95% CI) | 3.50 [1.19, 10.27] |
| 11.2 Peripheral cyanosis | 2 | 92 | Risk Ratio (M‐H, Random, 95% CI) | 3.0 [0.32, 27.83] |
| 11.3 Minor cardiovascular events | 6 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.37, 1.93] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alessandria 2007.
| Methods | Open‐label, single‐centre randomised clinical trial. | |
| Participants |
Criteria used to define hepatorenal syndrome:Arroyo 1996 (Appendix 2). Type 1 hepatorenal syndrome = 9 participants included. Type 2 hepatorenal syndrome = 13 participants included. Demographics: Terlipressin group: mean age 55 years, 75% men, alcoholic cirrhosis 33%. Other vasoactive drug group: mean age 56 years, 70% men, alcohol‐related cirrhosis 20%. |
|
| Interventions |
Terlipressin: Administration form: intravenous bolus injection. Dose: dose titration regimen. Initial dose 1 mg/4 hours. With no response, dose increased to 2 mg/4 hours. Response defined as reduction in serum creatinine ≥ 25% from baseline after 3 days of treatment. Other vasoactive drug:noradrenaline. Administration form: continuous intravenous infusion. Dose: dose titration regimen. Initial dose 0.1 μg/kg/minute. Dose increased in steps of 0.05 μg/kg/minute every 4 hours until the mean arterial pressure was increased to at least 10 mmHg compared to baseline. Maximum dose 0.7 μg/kg/minute. Cointervention: Both arms treated with albumin to maintain a central venous pressure between 10 cmH2O and 15 cmH2O. Mean dose of albumin in terlipressin group. 46 g/day (range 35 to 65). Mean dose of albumin in noradrenaline group 56 g/day (range 40 to 75). During follow‐up, participants with ascites were treated with diuretics and large volume paracentesis followed by albumin infusions as needed. |
|
| Outcomes | No predefined outcome (pilot study). Survival and reversal of hepatorenal syndrome reported. | |
| Treatment duration |
Treatment duration: until reversal of hepatorenal syndrome, death, or a maximum 2 weeks. Follow‐up: 90 days. |
|
| Country of origin | Italy. | |
| Inclusion period | Data not available. | |
| Notes | Full paper. All survivors underwent liver transplantation at end of follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Low risk | Serially numbered sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. All participants included in analyses. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes defined and reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Low risk | No funding or other support from for‐profit organisations. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
Badawy 2013.
| Methods | Open‐label multicentre randomised clinical trial. | |
| Participants |
Criteria used to define hepatorenal syndrome:Arroyo 1996 (Appendix 2). Type 1 hepatorenal syndrome = 51 participants included. Demographics: Terlipressin group: mean age 43 years, 67% men, aetiology mostly viral hepatitis. Other vasoactive drug group: mean age 46 years, 71% men, aetiology mostly viral hepatitis. |
|
| Interventions |
Terlipressin: Administration form: continuous intravenous infusion. Dose: dose titration regimen. Initial dose 3 mg/24 hours. With no response, dose primarily increased to 6 mg/24 hours and secondarily to 12 mg/24 hours. Response defined as a reduction of serum creatinine ≥ 25% compared to baseline after every 48 hours of treatment. Other vasoactive drug:noradrenaline. Administration form: continuous intravenous infusion Dose: dose titration regimen. Initial dose 0.5 mg/hour. Dose increased in steps of 0.5 mg/hour every 4 hours guided by a mean arterial pressure around 85 mmHg to 90 mmHg. Maximum dose 3 mg/hour. Cointervention: Both arms treated with albumin to maintain a central venous pressure between 10 cmH2O and 15 cmH2O. Dose not reported. |
|
| Outcomes |
Primary outcome: reversal of hepatorenal syndrome. Secondary outcomes: 30 days survival and treatment costs. |
|
| Treatment duration |
Treatment duration: until reversal of hepatorenal syndrome, death, or a maximum of 15 days. Follow‐up: 30 days. |
|
| Country of origin | Egypt. | |
| Inclusion period | January 2009 to April 2012. | |
| Notes | Full paper. Participants who died within 72 hours after randomisation excluded from study. We contacted the authors, but were unable gather any further information. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | Sealed opaque envelopes (text did not explain if envelopes were serially numbered). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No missing outcome data described. Participants who died within 72 hours excluded from analyses, but number allocated to 2 intervention groups not given. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcome reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Unclear risk | No description. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
Cavallin 2016.
| Methods | Open label, multicentre randomised clinical trial. | |
| Participants |
Criteria used to define hepatorenal syndrome:Salerno 2007 (Appendix 2). Type 1 hepatorenal syndrome = 44 participants included. Type 2 hepatorenal syndrome = 4 participants included. Demographics: Terlipressin group: mean age 60 years, men 78%, viral aetiology 37%. Other vasoactive drug group: mean age 65 years, men 52%, viral aetiology 38%. |
|
| Interventions |
Terlipressin: Administration form: continuous intravenous infusion. Dose: dose titration regimen. Initial dose 3 mg/24 hours. With no response, dose primarily increased to 6 mg/24 hours and then to 12 mg/24 hours. Response defined as a reduction of serum creatinine of ≥ 25% compared to baseline after every 48 hours of treatment. Other vasoactive drugs:midodrine and octreotide Midodrine Administration form: oral tablet. Dose: dose titration regimen. Initial dose 7.5 mg/8 hours. With no response, dose increased to 12.5 mg/8 hours. Response defined as a reduction in serum creatinine of ≥ 25% from baseline after 3 days of treatment. Octreotide Administration form: subcutaneous bolus injection. Dose: dose titration regimen. Initial dose 100 mg/8 hours. With no response, dose increased to 200 mg/8 hours. Response defined as a reduction in serum creatinine of ≥ 25% from baseline after 3 days of treatment. Cointervention: both arms treated with albumin; 1 g/kg bodyweight at day 1, followed by 20 g/day to 40 g/day. |
|
| Outcomes |
Primary: reversal of hepatorenal syndrome. Secondary: 3 months survival. |
|
| Treatment duration |
Treatment duration: until reversal of hepatorenal syndrome, death, or a maximum of 14 days. Follow‐up: 3 months. |
|
| Country of origin | Italy. | |
| Inclusion period | 2008 to 2012. | |
| Notes | Full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Serially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data and all participants were accounted for. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Low risk | Authors declared no conflict of interests and the trial did not receive funding from for‐profit organisations. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | Low risk | |
Copaci 2013.
| Methods | Open‐label, single‐centre randomised clinical trial. | |
| Participants |
Criteria used to define hepatorenal syndrome: not reported. Type 1 hepatorenal syndrome = 36 participants included. Type 2 hepatorenal syndrome = 4 participants included. Demographics: Terlipressin group: not available. Other vasoactive drug group: not available. |
|
| Interventions |
Terlipressin: Administration form: continuous intravenous infusion. Dose: dose titration regimen. Initial dose 4 mg/24 hours. With no response, dose increased stepwise to 12 mg/24 hours. Response defined as a reduction in serum creatinine of ≥ 50% from baseline or reversal of hepatorenal syndrome. Other vasoactive drug:octreotide. Administration form: subcutaneously bolus injection. Dose: dose titration regimen. Initial dose 100 mg/8 hours. With no response, dose increased to 200 mg/8 hours. Response defined as a reduction in serum creatinine of ≥ 50% from baseline or reversal of hepatorenal syndrome. Cointervention: both arms treated with albumin; 1 g/kg bodyweight at day 1, followed by 20 g/day to 40 g/day. |
|
| Outcomes | No description. Data on reversal of hepatorenal syndrome and mortality available. | |
| Treatment duration |
Treatment duration: data not available. Follow‐up: 30 days. |
|
| Country of origin | Romania. | |
| Inclusion period | Data not available. | |
| Notes | Abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data and all participants accounted for. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes defined and reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Unclear risk | No description. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
Ghosh 2013.
| Methods | Open‐label, single‐centre randomised clinical trial. | |
| Participants |
Criteria used to define hepatorenal syndrome:Salerno 2007 (Appendix 2). Type 2 hepatorenal syndrome = 46 participants included. Demographics: Terlipressin group: mean age 46 years, 87% men, alcohol‐related cirrhosis 65%. Other vasoactive drug group: mean age 48 years, 70% men, alcohol‐related cirrhosis 70%. |
|
| Interventions |
Terlipressin: Administration form: intravenous bolus injection. Dose: dose titration regimen. Initial dose 0.5 mg/6 hours. With no response, dose increased primarily to 1 mg/6 hours and then to 2 mg/6 hours. Response defined as a reduction in serum creatinine of 1 mg/dL after 3 days of treatment. Other vasoactive drug:noradrenaline. Administration form: continuous intravenous infusion. Dose: dose titration regimen. Initial dose 0.5 mg/hour. Dose increased in steps of 0.5 mg/hour every 4 hours until mean arterial pressure increased to ≥ 10 mmHg compared to baseline or an increase in urine output to > 200 mL/4 hours. Maximum dose 3 mg/hour. Cointervention: both arms treated with albumin 20 g/day to 40 g/day. Treatment temporarily stop if central venous pressure exceeded 18 cmH2O. |
|
| Outcomes |
Primary: reversal of hepatorenal syndrome. Secondary: 3 months mortality. |
|
| Treatment duration |
Treatment duration: until reversal of hepatorenal syndrome, death, or a maximum of 15 days. Follow‐up: 3 months. |
|
| Country of origin | India. | |
| Inclusion period | January 2009 to December 2011. | |
| Notes | Full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Serially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Investigators excluded 12 participants from analyses after randomisation. Reasons for exclusions/withdrawals included sepsis (7), severe coronary artery disease (1), hepatocellular carcinoma (1), diabetic nephropathy (1), and refusal to participate (2). Authors did not provide information about allocation group. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes defined and reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Unclear risk | No description. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
Goyal 2016.
| Methods | Open‐label, single‐centre randomised clinical trial. | |
| Participants |
Criteria used to define hepatorenal syndrome:Salerno 2007 (Appendix 2). Type 1 hepatorenal syndrome = 41 participants included. Demographics: Terlipressin group: mean age 56.9 years, 85% men, alcohol‐related cirrhosis 75%. Other vasoactive drug group: mean age 54.7 years, 95.2% men, alcohol‐related cirrhosis 61.9%. |
|
| Interventions |
Terlipressin: Administration form: intravenous bolus injection. Dose: dose titration regimen. Initial dose 0.5 mg/6 hours. With no response, dose increased stepwise to maximum of 2 mg/6 hours. Response defined as a reduction in serum creatinine of 1 mg/dL after 3 days of treatment. Other vasoactive drug:noradrenaline (+furosemide). Noradrenaline Administration form: continuous intravenous infusion Dose: dose titration regimen. Initial dose 0.5 mg/hour. Dose increased in steps of 0.5 mg/hour every 4 hours until mean arterial pressure increased to ≥ 10 mmHg compared to baseline or an increase in urine output to > 200 mL/4 hours. Maximum dose 3 mg/hour. Furosemide Administration form: continuous intravenous infusion. Dose: dose titration regimen. Furosemides added, if urine output < 200 mL/4 hours despite reaching an increase in mean arterial pressure of ≥ 10 mmHg. Initial dose 0.001 mg/kg/minute and adjusted to maintain a urine output of > 40 mL/hour. Cointervention: both arms treated with intravenous third‐generation cephalosporins and albumin 20 g/day to 40 g/day. Administration stopped temporarily if central venous pressure increased > 12 cm/H2O or if serum albumin > 4 g/L. |
|
| Outcomes |
Primary: reversal of hepatorenal syndrome. Secondary: 14 days mortality. |
|
| Treatment duration |
Treatment duration: until reversal of hepatorenal syndrome, death, or a maximum of 14 days. Follow‐up: 14 days. |
|
| Country of origin | India. | |
| Inclusion period | 3 years. | |
| Notes | Full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data and all participants included in analyses. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes defined and reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Unclear risk | No description. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
Indrabi 2013.
| Methods | Open‐label randomised clinical trial. | |
| Participants |
Criteria used to define hepatorenal syndrome: no description. Type 1 hepatorenal syndrome = 60 participants included. Demographics: no description. |
|
| Interventions |
Terlipressin: Administration form: intravenous. Other vasoactive drug:noradrenaline. Administration form: intravenous. Cointervention: both arms treated with albumin. Dose not reported. |
|
| Outcomes | No description. Data on reversal of hepatorenal syndrome and mortality available. | |
| Treatment duration |
Treatment duration: no description. Follow‐up: 90 days or death. |
|
| Country of origin | India. | |
| Inclusion period | No description. | |
| Notes | Full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No description. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes described and reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Unclear risk | No description. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
Sharma 2008.
| Methods | Open‐label, single‐centre randomised clinical trial. | |
| Participants |
Criteria used to define hepatorenal syndrome:Arroyo 1996 (Appendix 2). Type 1 hepatorenal syndrome = 40 participants included. Demographics: Terlipressin group: mean age 48 years, 85% men, alcohol‐related cirrhosis 60%. Other vasoactive drug group: mean age 48 years, 85% men, alcohol‐related cirrhosis 70%. |
|
| Interventions |
Terlipressin: Administration form: intravenous bolus injection. Dose: dose titration regimen. Initial dose 0.5 mg/6 hours. With no response, dose increased stepwise to a maximum 2 mg/6 hours. Response defined as a reduction in serum creatinine of 1 mg/dL after 3 days of treatment. Other vasoactive drug:noradrenaline. Administration form: continuous intravenous infusion. Dose: dose titration regimen. Initial dose 0.5 mg/hour. Dose increased in steps of 0.5 mg/hour every 4 hours until mean arterial pressure increased to ≥ 10 mmHg compared to baseline or an increase in urine output to > 200 mL/4 hours. Maximum dose 3 mg/hour. Cointervention: both arms treated with albumin 20 g/day to 40 g/day. Treatment temporarily stop if central venous pressure exceeded 18 cmH2O. Participants with tense ascites had 3 L to 5 L paracentesis combined with infusions of 8 g of albumin for each litre of ascitic fluid removed. |
|
| Outcomes |
Primary outcome: reversal of hepatorenal syndrome. Secondary outcomes: 30 days survival. |
|
| Treatment duration |
Treatment duration: until reversal of hepatorenal syndrome, death, or a maximum of 15 days. Follow‐up: 30 days. |
|
| Country of origin | India. | |
| Inclusion period | August 2005 to December 2006. | |
| Notes | Full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation sequence. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data. All participants included in analyses. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Low risk | Authors declared no conflict of interests and trial did not receive funding from for‐profit organisations. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
Singh 2012.
| Methods | Open‐label, single‐centre randomised clinical trial. | |
| Participants |
Criteria used to define hepatorenal syndrome:Salerno 2007 (Appendix 2). Type 1 hepatorenal syndrome = 46 participants included. Demographics: Terlipressin group: mean age 51 years, 83% men, alcohol‐related cirrhosis 43%. Other vasoactive drug group: mean age 48 years, 83% men, alcohol‐related cirrhosis 52%. |
|
| Interventions |
Terlipressin: Administration form: intravenous bolus injection. Dose: dose titration regimen. Initial dose 0.5 mg/6 hours. With no response, dose increased stepwise to maximum 2 mg/6 hours. Response defined as a reduction in serum creatinine of 1 mg/dL after 3 days of treatment. Other vasoactive drug:noradrenaline. Administration form: continuous intravenous infusion. Dose: dose titration regimen. Initial dose 0.5 mg/hour. Dose increased in steps of 0.5 mg/hour every 4 hours until mean arterial pressure increased to > 10 mmHg compared to baseline or an increase in urine output to > 200 mL/4 hours. Maximum dose 3 mg/hour. Cointervention: both arms treated with albumin 20 g/day to 40 g/day. Treatment temporarily stopped if central venous pressure exceeded 18 cmH2O. Participants with tense ascites had 3 L to 5 L paracentesis combined with infusions of 8 g of albumin for each litre of ascitic fluid removed. |
|
| Outcomes |
Primary outcome: reversal of hepatorenal syndrome. Secondary outcomes: 30 days survival. |
|
| Treatment duration |
Treatment duration: until reversal of hepatorenal syndrome, death, or a maximum of 15 days. Follow‐up: 30 days. |
|
| Country of origin | India. | |
| Inclusion period | January 2009 to 2011 October. | |
| Notes | Full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation list. |
| Allocation concealment (selection bias) | Low risk | Serially numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data and all participants included in analyses. |
| Selective reporting (reporting bias) | Low risk | Clinically relevant outcomes reported. No differences between trial registration/protocol and published paper identified. |
| For‐profit bias | Low risk | Authors declared no conflict of interests and trial did not receive funding from for‐profit organisations. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | Low risk | |
Srivastava 2015.
| Methods | Open‐label, single‐centre randomised clinical trial. | |
| Participants |
Criteria used to define hepatorenal syndrome:Salerno 2007 (Appendix 2). Type 1 hepatorenal syndrome = 40 participants included. Type 2 hepatorenal syndrome = 40 participants included. Demographics: type 1 hepatorenal syndrome. Terlipressin group: mean age 46 years, 83% men, alcohol‐related cirrhosis 50%. Other vasoactive drug group: mean age 39 years, 83% men, alcohol‐related cirrhosis 50%. Demographics: type 2 hepatorenal syndrome. Terlipressin group: mean age 45 years, 83% men, alcohol‐related cirrhosis 53%. Other vasoactive drug group: mean age 43 years, 83% men, alcohol‐related cirrhosis 55%. |
|
| Interventions |
Terlipressin: Administration form: intravenous bolus injection. Dose: fixed dose 0.5 mg/6 hours. Other vasoactive drug:dopamine and furosemide. Administration form: continuous intravenous infusion. Dose: fixed doses of dopamine 2 μg/kg/minute and furosemide 0.01 mg/kg/hour. Cointervention: both arms treated with albumin 20 g/day. |
|
| Outcomes |
Primary outcomes: reversal of hepatorenal syndrome, 15 and 30 days' survival. Secondary outcomes: cost of treatment. |
|
| Treatment duration |
Treatment duration: 5 days. Follow‐up: 30 days. |
|
| Country of origin | India. | |
| Inclusion period | February 2005 to June 2010. | |
| Notes | Full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | No description. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants or personnel. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data and all participants included in analyses. |
| Selective reporting (reporting bias) | High risk | Number of participants with (or without) reversal of hepatorenal syndrome not reported. |
| For‐profit bias | Low risk | Authors declared no conflict of interests. The randomised clinical trial received financial support from the Indian Council of Medical Research and did not receive funding from for‐profit organisations. |
| Overall risk of bias (non‐mortality outcomes) | High risk | |
| Overall risk of bias (mortality) | High risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Boyer 2016 | Comparing terlipressin with placebo. |
| Cavallin 2015 | Compared bolus injections of terlipressin with continuous infusions of terlipressin. |
| Hadengue 1998 | Compared terlipressin with placebo. |
| Martín‐Llahí 2008 | Compared terlipressin with placebo. |
| Neri 2008 | Compared terlipressin with placebo. |
| Nguyen‐Tat 2015 | Observational study. No information about harms. |
| Pulvirenti 2008 | Compared terlipressin with placebo. |
| Sanyal 2008 | Compared terlipressin with placebo. |
| Silawat 2011 | Quasi‐randomised trial. No information about harms. |
| Solanki 2003 | Compared terlipressin with placebo. |
| Tavakkoli 2012 | Compared noradrenaline with midodrine and octreotide. |
| Wan 2014 | Compared high dose of terlipressin with low dose of terlipressin. |
| Yang 2001 | Compared terlipressin with placebo. |
Differences between protocol and review
The review author's team expanded with four more people.
We changed the inclusion criteria from including "participants with hepatorenal syndrome" to including "people with cirrhosis and type 1 or type 2 hepatorenal syndrome." Cirrhosis has been mandatory in the diagnostic criteria of hepatorenal syndrome since Salerno 2007. All randomised clinical trials included in this review that used the former diagnostic criteria by Arroyo 1996 included participants only diagnosed with cirrhosis.
Regarding all outcome assessments, we changed the follow‐up from "end of treatment and at maximum follow‐up" to "maximum duration of follow‐up". Due to the severe prognosis of hepatorenal syndrome, the treatment is usually ended at reversal of hepatorenal syndrome or death. We choose to leave out the assessments at end of treatment because our primary outcomes included reversal of hepatorenal syndrome and all‐cause mortality.
We did not assess "death from renal failure," a secondary outcome in the protocol. Hepatorenal syndrome occurs in people with end‐stage liver disease and it is usually not possible to point to a single cause that leads to death. This is reflected in randomised clinical trials on hepatorenal syndrome that do not usually report the cause of death.
We updated the parameters for the Trial Sequential Analyses according to latest findings (see Data synthesis).
Contributions of authors
MI and LG collaborated on the initial draft. All authors participated in the revision of the review and approved of the final version.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
MI, MJ, AHG, RWW: no conflicts of interest.
ASA: served on a Scientific Advisory Board for Ferring Pharmaceuticals (makers of terlipressin in Europe) after the submission of this manuscript. AK: served on a Scientific Advisory Board for Norgine, planned scientific meetings for Norgine and Intercept, and received funding for research from Norgine. LG: acted as investigator in studies funded by AbbVie, Intercept, Merck and Norgine, received funding for travel expenses and consultancy from Novo Nordisk, and for scientific presentations at meetings funded by Norgine and Eli Lily.
New
References
References to studies included in this review
Alessandria 2007 {published data only}
- Alessandria C, Marzano A, Ottobrelli A, Debernardi‐Venon W, Todros L, Torrni M, et al. Noradrenaline vs terlipressin in hepatorenal syndrome: a prospective, randomized study. Journal of Hepatology 2006;44:S83. [DOI] [PubMed] [Google Scholar]
- Alessandria C, Ottobrelli A, Debernardi‐Venon W, Todros L, Cerenzia MT, Martini S, et al. Noradrenalin vs terlipressin in patients with hepatorenal syndrome: a prospective, randomized, unblinded, pilot study. Journal of Hepatology 2007;47:499‐505. [DOI: 10.1016/j.jhep.2007.04.010] [DOI] [PubMed] [Google Scholar]
Badawy 2013 {published data only}
- Badawy S, Meckawy N, Ahmed A. Norepinephrine versus terlipressin in patients with type 1 hepatorenal syndrome refractory to treatment with octreotide, midodrine, and albumin: a prospective randomized comparative study. Egyptian Journal of Cardiothoracic Anesthesia 2013;7:13‐8. [DOI: 10.7123/01.EJCA.0000431078.82595.1f] [DOI] [Google Scholar]
Cavallin 2016 {published data only}
- Cavallin M, Kamath PS, Merli M, Fasolato S, Toniutto P, Salerno F, et al. Terlipressin plus albumin versus midodrine and octreotide plus albumin in the treatment of hepatorenal syndrome: a randomized trial. Hepatology (Baltimore, Md.) 2016;63:983‐92. [DOI: 10.1002/hep.27709] [DOI] [PubMed] [Google Scholar]
Copaci 2013 {published data only}
- Copaci I, Chiriac G, Micu L, Mindrut E. Alternative treatments for hepato‐renal syndrome in patients with cirrhosis. Falk Symposium 191. Abstract book: Liver Diseases in 2013: Advances in Pathogenesis and Treatment 2013:87.
- Copaci I, Micu L, Chiriac G. Reversal of type 1 hepato‐renal syndrome with terlipressin and octreotide. Journal of Hepatology 2016;64:S660. [Google Scholar]
Ghosh 2013 {published data only}
- Ghosh S, Choudhary NS, Sharma AK, Singh B, Kumar P, Agarwal R, et al. Noradrenaline vs terlipressin in the treatment of type 2 hepatorenal syndrome: a randomized pilot study. Liver International 2013;33:1187‐93. [DOI: 10.1111/liv.12179] [DOI] [PubMed] [Google Scholar]
Goyal 2016 {published data only}
- Goyal I. Terlipressin versus noradrenalin in hepatorenal syndrome: a prospective, randomised, unblinded study. Journal of Gastroenterology and Hepatology 2008;23:A76. [Google Scholar]
- Goyal O, Sidhu S, Sehgal N, Puri S. Noradrenaline is as effective as terlipressin in hepatorenal syndrome type 1: a prospective, randomized trial. Journal of the Association of Physicians of India 2016;64:30‐5. [PUBMED: 27762512] [PubMed] [Google Scholar]
Indrabi 2013 {published data only}
- Indrabi RA, Javid G, Zargar SA, Khan BA, Yattoo GN, Shah SH, et al. Noradrenaline is equally effective as terlipressin in reversal of type 1 hepatorenal syndrome: a randomized prospective study. Journal of Clinical and Experimental Hepatology 2013;3:S97. [Google Scholar]
Sharma 2008 {published data only}
- Sharma P, Kumar A, Shrama BC, Sarin SK. An open label, pilot, randomized controlled trial of noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome and predictors of response. American journal of Gastroenterology 2008;103:1689‐97. [DOI: 10.1111/j.1572-0241.2008.01828.x] [DOI] [PubMed] [Google Scholar]
- Sharma P, Kumar A, Shrama BC, Sarin SK. Noradrenaline versus terlipressin in the treatment of type 1 hepatorenal syndrome: a randomized controlled trial. Hepatology (Baltimore, Md.) 2006;44:449A. [DOI] [PubMed] [Google Scholar]
Singh 2012 {published data only}
- Singh V, Ghosh S, Singh B, Kumar P, Sharma N, Bhalla A, et al. Noradrenaline vs. terlipressin in the treatment of hepatorenal syndrome: a randomized study. Journal of Hepatology 2012;56:1293‐8. [DOI: 10.1016/j.jhep.2012.01.012] [DOI] [PubMed] [Google Scholar]
Srivastava 2015 {published data only}
- Srivastava S, Shalimar, Vishnubhatla S, Prakash S, Sharma H, Thakur B, et al. Randomized controlled trial comparing the efficacy of terlipressin and albumin with a combination of concurrent dopamine, furosemide, and albumin in hepatorenal syndrome. Journal of Clinical and Experimental Hepatology 2015;5:276‐85. [DOI: 10.1016/j.jceh.2015.08.003; PUBMED: 26900268] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Boyer 2016 {published data only}
- Boyer TD, Medicis JJ, Pappas SC, Potenziano J, Jamil K. A randomized, placebo‐controlled, double‐blind study to confirm the reversal of hepatorenal syndrome type 1 with terlipressin: the REVERSE trial design. Open Access Journal of Clinical Trials 2012;4:39‐49. [Google Scholar]
- Boyer TD, Sanyal AJ, Wong F, Frederick RT, Lake JR, O'Leary JG, et al. Terlipressin plus albumin is more effective than albumin alone in improving renal function in patients with cirrhosis and hepatorenal syndrome type 1. Gastroenterology 2016;150:1579‐89. [PUBMED: 26896734] [DOI] [PubMed] [Google Scholar]
Cavallin 2015 {published data only}
- Angeli P, Fasolato S, Cavallin M, Maresio G, Callegaro A, Sticca A, et al. Terlipressin given as continuous intravenous infusion is the more suitable schedule for the treatment of type 1 hepatorenal syndrome (HRS) in patients with cirrhosis: results of a controlled clinical study. Hepatology (Baltimore, Md.) 2008;48:378A. [Google Scholar]
- Cavallin M, Piano S, Romano A, Fasolato S, Frigo AC, Benetti G, et al. Terlipressin given by continuous intravenous infusion versus intravenous boluses in the treatment of hepatorenal syndrome: a randomized controlled study. Hepatology (Baltimore, Md.) 2015;63:983‐92. [PUBMED: 25644760] [DOI] [PubMed] [Google Scholar]
Hadengue 1998 {published data only}
- Hadengue A, Gadano A, Giostra E, Negro F, Moreau R, Valla D, et al. Terlipressin in the treatment of hepatorenal syndrome (HRS): a double blind cross‐over study. Hepatology (Baltimore, Md.) 1995;22:165A. [Google Scholar]
- Hadengue A, Gadano A, Giostra E, Negro F, Moreau R, Valla D, et al. Terlipressin in the treatment of hepatorenal syndrome (HRS): a double blind cross‐over study. Journal of Hepatology 1998;29:565‐70. [PUBMED: 9824265] [DOI] [PubMed] [Google Scholar]
Martín‐Llahí 2008 {published data only}
- Martin‐Llahi M, Pepin MN, Guevara M, Torre A, Monescillo A, Soriano G, et al. Randomized, comparative study of terlipressin and albumin vs albumin alone in patients with cirrhosis and hepatorenal syndrome. Journal of Hepatology 2007;46:S36. [Google Scholar]
- Martín‐Llahí M, Pépin MN, Guevara M, Díaz F, Torre A, Monescillo A, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology 2008;134:1352‐9. [PUBMED: 7939047] [DOI] [PubMed] [Google Scholar]
Neri 2008 {published data only}
- Neri S, Pulvirenti D, Malaguarnera M, Cosimo BM, Bertino G, Ignaccolo L, et al. Terlipressin and albumin in patients with cirrhosis and type I hepatorenal syndrome. Digestive Diseases and Sciences 2008;53:830‐5. [PUBMED: 17939047] [DOI] [PubMed] [Google Scholar]
Nguyen‐Tat 2015 {published data only}
- Nguyen‐Tat M, Gotz E, Scholz‐Kreisel P, Ahrens J, Sivanathan V, Schattenberg J, et al. Response to Terlipressin and albumin is associated with improved outcome in patients with cirrhosis and hepatorenal syndrome [Leberzirrhose und hepatorenales Syndrom: Das Ansprechen auf Terlipressin und Albumin ist mit besserem Überleben assoziiert]. Deutsche Medizinische Wochenschrift 2015;140:21‐6. [PUBMED: 25612289] [DOI] [PubMed] [Google Scholar]
Pulvirenti 2008 {published data only}
- Pulvirenti D, Tsami A. Low doses of terlipressin and albumin in the type I hepatorenal syndrome [Terlipressina a basso dosaggio e albumina nella sindrome epatorenale di tipo I]. Italian Journal of Medicine 2008;2:34‐8. [Google Scholar]
Sanyal 2008 {published data only}
- Sanyal A, Boyer T, Garcia‐Tsao G, Regenstein F, Rossaro L, Teuber P. A prospective, randomized, double blind, placebo‐controlled trial of terlipressin for type 1 hepatorenal syndrome (HRS). Hepatology (Baltimore, Md.) 2006;44:694A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal AJ, Boyer T, Garcia‐Tsao G, Regenstein F, Rossaro L, Appenrodt B, et al. A randomized, prospective, double‐blind, placebo‐controlled trial of terlipressin for type 1 hepatorenal syndrome. Gastroenterology 2008;134:1360‐8. [MEDLINE: ; clinicaltrials.gov NCT00089570] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal AJ, Boyer TD, Garcia‐Tsao G, Blei AT, Teuber P, Terlipressin Study Group. Effect of terlipressin on mean arterial pressure (MAP) and its relation to serum creatinine concentration in type 1 hepatorenal syndrome (HRS): analysis of data from the pivotal phase 3 trial. Hepatology (Baltimore, Md.) 2008;48:1071A. [Google Scholar]
- Sanyal AJ, Boyer TD, Teuber P. Prognostic factors for hepatorenal syndrome (HRS) in patients with type 1 HRS enrolled in a randomized double‐blind, placebo‐controlled trial. Hepatology (Baltimore, Md.) 2007;46:565A. [Google Scholar]
Silawat 2011 {published data only}
- Silawat FN, Shaikh MK, Lohana RK, Devrajani BR, Shah SZA, Ansari A. Efficacy of terlipressin and albumin in the treatment of hepatorenal syndrome. World Applied Sciences Journal 2011;12:1946‐50. [Google Scholar]
Solanki 2003 {published data only}
- Solanki P, Chawla A, Garg R, Gupta R, Jain M, Sarin SK. Beneficial effects of terlipressin in hepatorenal syndrome: a prospective, randomized placebo‐controlled clinical trial. Journal of Gastroenterology and Hepatology 2003;18:152‐6. [DOI] [PubMed] [Google Scholar]
Tavakkoli 2012 {published data only}
- Tavakkoli H, Yazdanpanah K, Mansourian M. Noradrenalin versus the combination of midodrine and octreotide in patients with hepatorenal syndrome: randomized clinical trial. International Journal of Preventive Medicine 2012;3:764‐69. [PMC free article] [PubMed] [Google Scholar]
Wan 2014 {published data only}
- Wan S, Wan X, Zhu Q, Peng J. A comparative study of high‐or low‐dose terlipressin therapy in patients with cirrhosis and type 1 hepatorenal syndrome. Chinese Journal of Hepatology 2014;22:349‐53. [PUBMED: 25180869] [DOI] [PubMed] [Google Scholar]
Yang 2001 {published data only}
- Yang YZ, Dan ZL, Liu NZ, Liu M. Efficacy of terlipressin in treatment of liver cirrhosis with hepatorenal syndrome. Journal of Internal Intensive Medicine 2001;7:123‐5. [Google Scholar]
Additional references
Allegretti 2017
- Allegretti AS, Israelsen M, Krag A, Jovani M, Goldin AH, Schulman AR, et al. Terlipressin versus placebo or no intervention for people with cirrhosis and hepatorenal syndrome. Cochrane Database of Systematic Reviews 2017, Issue 6. [DOI: 10.1002/14651858.CD005162.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Angeli 1999
- Angeli P. Reversal of type 1 hepatorenal syndrome with the administration of midodrine and octreotide. Hepatology (Baltimore, Md.) 1999;29:1690‐7. [DOI] [PubMed] [Google Scholar]
Angeli 2015
- Angeli P, Gines P, Wong F, Bernardi M, Boyer TD, Gerbes A, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut 2015;64:531‐7. [DOI] [PubMed] [Google Scholar]
Arroyo 1996
- Arroyo V, Gines P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, et al. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology (Baltimore, Md.) 1996;23:164‐76. [DOI] [PubMed] [Google Scholar]
Cardenas 2003
- Cardenas A, Arroyo V. Hepatorenal syndrome. Annals of Hepatology 2003;2:23‐9. [PubMed] [Google Scholar]
Duvoux 2002
- Duvoux C, Zanditenas D, Hezode C, Chauvat A, Monin JL, Roudot‐Thoraval F, et al. Effects of noradrenalin and albumin in patients with type I hepatorenal syndrome: a pilot study. Hepatology (Baltimore, Md.) 2002;36:374‐80. [DOI] [PubMed] [Google Scholar]
EASL 2010
- European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. Journal of Hepatology 2010;53:397‐417. [DOI] [PubMed] [Google Scholar]
Fabrizi 2009
- Fabrizi F, Dixit V, Messa P, Martin P. Terlipressin for hepatorenal syndrome: a meta‐analysis of randomized trials. International Journal of Artificial Organs 2009;32:133‐40. [PubMed] [Google Scholar]
Facciorusso 2017
- Facciorusso A, Chandar A, Murad M, Prokop L, Muscatiello N, Kamath P, et al. Comparative efficacy of pharmacological strategies for management of type 1 hepatorenal syndrome: a systematic review and network meta‐analysis. Lancet Gastroenterology and Hepatology 2017;2(2):94‐102. [DOI] [PubMed] [Google Scholar]
Fede 2012
- Fede G, D'Amico G, Arvaniti V, Tsochatzis E, Germani G, Georgiadis D, et al. Renal failure and cirrhosis: a systematic review of mortality and prognosis. Journal of Hepatology 2012;56:810‐8. [DOI] [PubMed] [Google Scholar]
Gilford 2017
- Gifford F, Morling J, Fallowfield J. Systematic review with meta‐analysis: vasoactive drugs for the treatment of hepatorenal syndrome type 1. Alimentary Pharmacology & Therapeutics 2017;45(5):593‐603. [DOI] [PubMed] [Google Scholar]
Gines 1993
- Gines A, Escorsell A, Gines P, Salo J, Jimenez W, Inglada L, et al. Incidence, predictive factors, and prognosis of hepatorenal syndrome in cirrhosis. Gastroenterology 1993;105:229‐36. [DOI] [PubMed] [Google Scholar]
Gines 2003
- Gines P, Guevara M, Arroyo V, Rodes J. Hepatorenal syndrome. Lancet 2003;362:1819‐27. [DOI] [PubMed] [Google Scholar]
Gluud 2017
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2017, Issue 6. Art. No.: LIVER.
GRADEpro [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. Grade Working Group, 2008.
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25:3443‐57. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, White IR, Wood AM. Imputation methods for missing outcome data in meta‐analysis of clinical trials. Clinical Trials 2008;5:225‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Philadelphia (PA): Barnett International/PAREXEL, 1997. [Google Scholar]
Israelsen 2015
- Israelsen ME, Gluud LL, Krag A. Acute kidney injury and hepatorenal syndrome in cirrhosis. Journal of Gastroenterology and Hepatology 2015;30:236‐43. [DOI: 10.1111/jgh.12709] [DOI] [PubMed] [Google Scholar]
Krag 2008
- Krag A, Borup T, Moller S, Bendtsen F. Efficacy and safety of terlipressin in cirrhotic patients with variceal bleeding or hepatorenal syndrome. Advances in Therapy 2008;25:1105‐40. [DOI] [PubMed] [Google Scholar]
Mattos 2016
- Mattos ÂZ, Mattos AA, Ribeiro RA. Terlipressin versus noradrenaline in the treatment of hepatorenal syndrome: systematic review with meta‐analysis and full economic evaluation. European Journal of Gastroenterology & Hepatology March 2016;28(3):345‐51. [DOI] [PubMed] [Google Scholar]
Mehta 2007
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care 2007;11:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moller 2004
- Moller S, Henriksen JH. Review article: pathogenesis and pathophysiology of hepatorenal syndrome ‐ is there scope for prevention?. Alimentary Pharmacology & Therapeutics 2004;20:31‐41. [DOI] [PubMed] [Google Scholar]
Nassar 2014
- Nassar AP, Farias AQ, D'Albuquerque LA, Carrilho FJ, Malbouisson LM. Terlipressin versus norepinephrine in the treatment of hepatorenal syndrome: a systematic review and meta‐analysis. PloS One 2014;9:e107466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pasqualetti 1998
- Pasqualetti P, Festuccia V, Collacciani A, Acitelli P, Casale R. Circadian rhythm of arginine vasopressin in hepatorenal syndrome. Nephron 1998;78:33‐7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Ruiz‐del‐Arbol 2005
- Ruiz‐del‐Arbol L, Monescillo A, Arocena C, Valer P, Gines P, Moreira V, et al. Circulatory function and hepatorenal syndrome in cirrhosis. Hepatology (Baltimore, Md.) 2005;42:439‐47. [DOI] [PubMed] [Google Scholar]
Runyon 2013
- Runyon BA, AASLD. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology (Baltimore, Md.) 2013;57(4):1651‐3. [DOI] [PubMed] [Google Scholar]
Russell 2008
- Russell JA, Walley KR, Singer J, Gordon AC, Hebert PC, Cooper DJ, et al. Vasopressin versus norepinephrine infusion in patients with septic shock. New England Journal of Medicine 2008;358:877‐87. [PUBMED: 18305265] [DOI] [PubMed] [Google Scholar]
Salerno 2007
- Salerno F, Gerbes A, Ginès P, Wong F, Arroyo V. Diagnosis, prevention and treatment of hepatorenal syndrome in cirrhosis. Gut 2007;56:1310‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salerno 2015
- Salerno F, Navickis R, Wilkes M. Albumin treatment regimen for type 1 hepatorenal syndrome: a dose‐response meta‐analysis. BMC Gastroenterology 2015;15(67):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Savović 2012
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157:428‐38. [DOI] [PubMed] [Google Scholar]
Sort 1999
- Sort P, Navasa M, Arroyo V, Aldeguer X, Planas R, Ruiz‐del‐Arbol L, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. New England Journal of Medicine 1999;341(6):403‐9. [DOI] [PubMed] [Google Scholar]
Stata 2014 [Computer program]
- StataCorp LP. Stata. Version 14. Texas: StataCorp LP, 2014.
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 27 July 2017).
TSA 2011 [Computer program]
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61:64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2017
- Wetterslev J, Jakobsen JC, Gluud C. Trial Sequential Analysis in systematic reviews with meta‐analysis. BMC Medical Research Methodology 2017;17(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Gluud 2010
- Gluud LL, Christensen K, Christensen E, Krag A. Systematic review of randomized trials on vasoconstrictor drugs for hepatorenal syndrome. Hepatology (Baltimore, Md.) 2010;51:576‐84. [PUBMED: 19885875] [DOI] [PubMed] [Google Scholar]
Gluud 2012
- Gluud LL, Christensen K, Christensen E, Krag A. Terlipressin for hepatorenal syndrome. Cochrane Database of Systematic Reviews 2012, Issue 9. [DOI: 10.1002/14651858.CD005162.pub3] [DOI] [PubMed] [Google Scholar]


