Abstract
Background
Sickle cell disease comprises a group of genetic haemoglobin disorders. The predominant symptom associated with sickle cell disease is pain resulting from the occlusion of small blood vessels by abnormally 'sickle‐shaped' red blood cells. There are other complications, including chronic organ damage and prolonged painful erection of the penis, known as priapism. Severity of sickle cell disease is variable, and treatment is usually symptomatic. Priapism affects up to half of all men with sickle cell disease, however, there is no consistency in treatment. We therefore need to know the best way of treating this complication in order to offer an effective interventional approach to all affected individuals.
Objectives
To assess the benefits and risks of different treatments for stuttering (repeated short episodes) and fulminant (lasting for six hours or more) priapism in sickle cell disease.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Haemoglobinopathies Trials Register, which comprises references identified from comprehensive electronic database searches and handsearches of relevant journals and abstract books of conference proceedings. We also searched trial registries.
Date of the most recent search of the Group's Haemoglobinopathies Trials Register: 15 September 2017.
Date of most recent search of trial registries and of Embase: 12 December 2016.
Selection criteria
All randomised or quasi‐randomised controlled trials comparing non‐surgical or surgical treatment with placebo or no treatment, or with another intervention for stuttering or fulminant priapism.
Data collection and analysis
The authors independently extracted data and assessed the risk of bias of the trials.
Main results
Three trials with 102 participants were identified and met the criteria for inclusion in this review. These trials compared stilboestrol to placebo, sildenafil to placebo and ephedrine or etilefrine to placebo and ranged in duration from two weeks to six months. All of the trials were conducted in an outpatient setting in Jamaica, Nigeria and the UK. None of the trials measured our first primary outcome, detumescence but all three trials reported on the reduction in frequency of stuttering priapism, our second primary outcome. No significant effect of any of the treatments was seen compared to placebo. Immediate side effects were not found to be significantly different from placebo in the two trials where this information was reported. We considered the quality of evidence to be low to very low as all of the trials were at risk of bias and all had low participant numbers.
Authors' conclusions
There is a lack of evidence for the benefits or risks of the different treatments for both stuttering and fulminant priapism in sickle cell disease. This systematic review has clearly identified the need for well‐designed, adequately‐powered, multicentre randomised controlled trials assessing the effectiveness of specific interventions for priapism in sickle cell disease.
Keywords: Humans; Male; Anemia, Sickle Cell; Anemia, Sickle Cell/complications; Diethylstilbestrol; Diethylstilbestrol/therapeutic use; Estrogens, Non‐Steroidal; Estrogens, Non‐Steroidal/therapeutic use; Priapism; Priapism/drug therapy; Priapism/etiology
Treatments for long‐lasting and painful erection of the penis in boys and men with sickle cell disease
Review question
What are the benefits and risks of different treatments for stuttering (repeated short episodes) and fulminant (lasting for six hours or more) priapism in sickle cell disease?
Background
Priapism (the prolonged painful erection of the penis) is common in males with sickle cell disease. The length of time priapism lasts differs for different types and so does the medical treatment for it. Self‐management approaches may be helpful. We looked for randomised controlled trials of different treatments to find the best option.
Search date
We last looked for evidence on 15 September 2017.
Study characteristics
We found three trials set in Jamaica, Nigeria and the UK involving 102 people.
Key results
In the trials, four different drug treatments (stilboestrol, sildenafil, ephedrine and etilefrine) were compared to placebo. The trials all looked at whether the treatments reduced how often attacks of priapism occurred. There was no difference between any of the treatments compared to placebo. Due to lack of evidence, we are not able to conclude the best treatment of priapism in sickle cell disease. More research is needed.
Quality of the evidence
We considered the quality of evidence to be low to very low as all of the trials were at risk of bias and all had low participant numbers.
Summary of findings
Summary of findings for the main comparison.
Silboesterol versus placebo
| Silboesterol compared with placebo for stuttering priapism | ||||||
|
Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: stilboestrol 5 mg Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Stilboestrol 5 mg | |||||
| Detumescence Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in stuttering priapism Follow‐up: 2 weeks |
250 per 1000 | 730 per 1000 (183 to 1000) |
RR 2.92 (95% CI 0.73 to 11.70) |
7 (1) | ⊕⊝⊝⊝ very low1,2 | |
| Immediate side effects of treatment Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Effect on later sexual function Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. Downgraded once due to unclear methods of randomisation and allocation. 2. Downgraded twice for imprecision: confidence intervals around the relative effect are very wide as the trial has a small number of participants and a low event rate.
Summary of findings 2.
Sildenafil versus placebo
| Sildenafil 50 mg compared with placebo for stuttering priapism | ||||||
|
Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: sildenafil 50 mg daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Sildenafil 50 mg daily | |||||
| Detumescence Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 8 weeks |
286 per 1000 |
166 per 1000 (20 to 1000) |
RR 0.58 (95% CI 0.07 to 4.95) |
13 (1) |
⊕⊕⊝⊝ low1,2 | There was also no significant difference between treatments for the reduction in episodes of priapism by score tier: RR 1.17 (95% CI 0.36 to 3.76). |
| Immediate side effects of treatment Follow‐up: 16 weeks |
See comment | See comment | N/A | 13 (1) |
⊕⊕⊝⊝ low1,3 | Side effects of treatment were reported for the whole treatment phase including the open label phase. No significant differences were found between sildenafil and placebo. |
| Effect on later sexual function Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. Downgraded once due to unclear methods of randomisation and allocation. 2. Downgraded once due to imprecision. Confidence intervals around the relative effect are very wide as the trial has a low number of participants. 3. Downgraded once due to indirectness as we are only interested in the treatment phase but they have included the open label phase for reporting of adverse effects.
Summary of findings 3.
Etilefrine versus placebo
| Etilefrine 50 mg compared with placebo for stuttering priapism | ||||||
|
Patient or population: men and boys with SCD and stuttering priapism Settings: outpatient Intervention: etilefrine 50mg once daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Etilefrine 50 mg | |||||
| Detumescence Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 6 months |
See comment | See comment | N/A | 23 (1) |
⊕⊝⊝⊝ very low1,2 | No significant difference was found between etilefrine and placebo but there were concerns over the reliability of the published results. |
| Immediate side effects of treatment (palpitations) Follow‐up: 6 months |
333 per 1000 |
546 per 1000 (206 to 1000) |
RR 1.64 (95% CI 0.62 to 4.30) |
23 (1) |
⊕⊝⊝⊝ very low1,2 | No significant difference was seen between etilefrine and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Immediate side effects of treatment (tachycardia) Follow‐up: 6 months |
250 per 1000 |
545 per 1000 (178 to 1000) |
RR 2.18 (95% CI 0.71 to 6.68) | 23 (1) |
⊕⊝⊝⊝ very low1,2 | No significant difference was seen between etilefrine and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Effect on later sexual function Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. Downgraded twice due to high risk of bias from incomplete outcome data and unclear risk of bias from randomisation and allocation concealment. 2. Downgraded once due to imprecision:confidence intervals around the relative effect are very wide as the trial has a low number of participants.
Summary of findings 4.
Ephedrine versus placebo
| Ephedrine 15 mg compared with placebo for stuttering priapism | ||||||
|
Patient or population: men and boys with SCD and stuttering priapism Settings: outpatients Intervention: ephedrine 15 mg/30 mg once daily Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Ephedrine | |||||
| Detumescence Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Reduction in frequency of priapism Follow‐up: 6 months |
See comment | See comment | N/A | 24 (1) |
⊕⊝⊝⊝ very low1,2 | No significant difference was found between ephedrine 15mg and placebo or ephedrine 30mg and placebo but there were concerns over the reliability of the published results. |
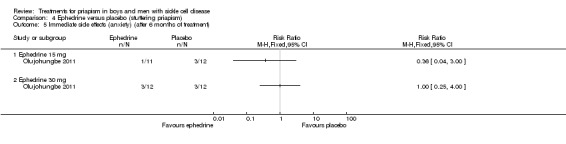
| Immediate side effects of treatment with 30 mg ephedrine (palpitations) Follow‐up: 6 months |
333 per 1000 |
333 per 1000 (107 to 1000) |
RR 1.00 (95% CI 0.32 to 3.10) |
24 (1) |
⊕⊝⊝⊝ very low1,2 | No significant difference was seen between ephedrine 15mg or 30mg and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
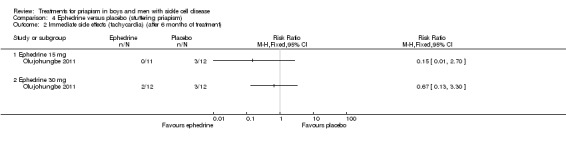
| Immediate side effects of treatment with 30 mg ephedrine (tachycardia) Follow‐up: 6 months |
250 per 1000 |
168 per 1000 (33 to 825) |
RR 0.67 (95% CI 0.13 to 3.30) |
24 (1) |
⊕⊝⊝⊝ very low1,2 | No significant difference was seen between ephedrine 15mg or 30mg and placebo for any of the other immediate side effects measured (lack of sleep, hand shaking, anxiety, dry mouth). |
| Effect on later sexual function Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Other untoward side effects of treatment Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| Efficacy of a prevention strategy Follow‐up: N/A |
See comment | See comment | N/A | N/A | N/A | This outcome was not measured. |
| *The basis for the assumed risk (e.g. the median control group risk across trials) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; N/A: not applicable; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
1. Downgraded twice due to high risk of bias from incomplete outcome data and unclear risk of bias from randomisation and allocation concealment. 2. Downgraded once due to imprecision: confidence intervals around the relative effect are very wide as the trial has a low number of participants.
Background
Description of the condition
Sickle cell disease (SCD) is a genetic haemoglobin disorder, caused by inheritance from both parents of an altered beta‐globin chain gene of which one at least is beta S. SCD affects people originating from Sub‐Saharan Africa, the African diaspora, Arab countries, the Mediterranean and South America (Davies 1997). Approximately 75,000 people in the USA (mostly African‐Americans) (NHLBI 2002), and about one in 60 West Africans (WHO 1994) are affected by SCD. There are on average 160 children born with SCD in England each year (Hickman 1999).
The pathophysiology of SCD is caused by the abnormal haemoglobin within red blood cells causing deformation or sickling of the cells when they give up oxygen. These abnormal cells block blood vessels causing vaso‐occlusion, resulting in symptoms and organ damage.
Priapism is the persistence of erection that does not result from sexual desire and fails to subside despite orgasm. There are two kinds of priapism: low‐flow ischaemic or veno‐occlusive priapism, which is the form seen in SCD and high‐flow priapism or non‐ischaemic, which is associated with external trauma causing damage to the cavernosal artery. Priapism is usually accompanied by pain and tenderness. It can occur in all age groups, with a reported peak incidence in SCD between the ages of 5 to 10 years and 20 to 50 years (Lue 1998). However, work from Jamaica suggests that the onset of priapism can be anytime between the ages of 5 and 45 years, with the peak incidence among young adults (aged 20 years to 25 years) (Emond 1980). Typically, priapism only affects the corpora cavernosa (very rarely the corpus spongiosum may be affected) (Lue 1998), thus surgical treatments to relieve priapism are designed to remove blood from the corpora cavernosa. Low‐flow priapism may result in impotence or erectile dysfunction secondary to corporeal fibrosis.
There is no clear demarcation between a prolonged erection and priapism. However, many would call an erection lasting over six hours fulminant or prolonged priapism. This is because in the low‐flow type (as occurs in the priapism of SCD) penile ischaemia and acidosis occur after six hours (Broderick 1994). Others, however, call an erection in males with SCD a priapic episode when it is in excess of one hour, or when it is painful.
Priapism is a common occurrence in SCD and may occur as acute episodes or the so‐called stuttering priapism, defined as recurrent episodes of prolonged erections. These can last from a few minutes to three hours. Stuttering priapism by repetitive vaso‐occlusion can lead to erectile dysfunction, and may develop into full‐blown priapism. Treatment is aimed at preventing such an occurrence. In one study of men with homozygous SCD (SS) in a Jamaican clinic, stuttering nocturnal attacks of priapism lasting two to six hours affected 42 per cent of participants (Serjeant 1985). A more recent questionnaire study from the USA reported an actuarial probability of experiencing priapism by the age of 20 years as 89% (+/‐ 9%) (Mantadakis 1999).
Red cell sickling and later sludging of blood occur in the corpora cavernosa, perhaps as a result of abnormal endothelial adherence, the relatively acidic state of the corpora during erection, mild acidosis accompanying hypoventilation during sleep, or mild trauma with masturbation and intercourse. When the venous channels are maximally compressed during nocturnal penile tumescence, the sludged red cells can block the microscopic subtunical venules and trigger diffuse veno‐occlusion (Lue 1998). More recent evidence has shown that priapism results from deficient erection control mechanisms at a molecular level (Bivalacqua 2006).
Description of the intervention
Interventions for priapism fall into two categories of preventing or reducing the frequency of priapic attacks, and treating fulminant priapic episodes. Several treatments have been described including: analgesia; hydration; blood alkalinisation; red cell transfusion; partial exchange transfusion to lower haemoglobin S; pharmaceutical agents and surgical procedures such as washouts. Surgical shunt procedures and even penile implantation have been described for intractable cases (Upadhyay 1998). Current treatments focus on resolving acute attacks and are often administered too late during an episode. With improved understanding of the true pathophysiology of priapism in SCD, newer treatments aim to target disease specific molecular abnormalities. Recent research suggests that there is abnormal signalling of the endothelium‐derived nitric oxide and phosphodiesterase type 5 (PDE5) signal transduction pathway in the penis (Bivalacqua 2012).
How the intervention might work
With a better understanding of the underlying pathophysiology of priapism in men with SCD, interventions can be better targeted at the underlying cause. Interventions to treat an acute attack aim to abort the attack and restore normal vascular homeostasis. It is thought that the nitric oxide imbalance leads to PDE5 dysregulation which then has a physiological impact on cyclic guanosine monophosphate (cGMP) and cyclic adenosine monophosphatecAMP activity. Treatments that interrupt abnormal mechanisms may be effective in reducing the frequency of attacks.
Why it is important to do this review
Priapism is associated with with long‐term erectile dysfunction and psychological consequences in often young men with SCD. It is important to review the evidence for interventions to treat acute episodes of priapism but also to prevent or reduce episodes in the longer term. Treatment of acute and stuttering priapism often depends on the type of clinician or institution to whom the male with SCD presents. With an increasing understanding of the underlying pathophysiology of the condition, new treatments such as etilefrine, an α adrenergic stimulator, and sildenafil, are showing encouraging results for preventing priapism (Bachir 1996; Okpala 2002; Virag 1996). This review seeks to determine safe, effective interventions for preventing or treating (or both) priapism.
Objectives
To assess the benefits and risks of the different treatments for both stuttering and fulminant priapism in SCD.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐randomised trials, whether published or unpublished.
Types of participants
Males with priapism, either fulminant or stuttering with any type of SCD including: homozygous SCD, i.e. sickle cell anaemia (SS); haemoglobin SC disease (SC); sickle beta thalassaemia (Sβº and Sβ⁺), irrespective of age, race, ethnic origin and setting.
Types of interventions
Any treatment for priapism in SCD, whether fulminant or stuttering, non‐surgical or surgical, compared with placebo or no treatment, or against another intervention.
Types of outcome measures
Primary outcomes
Detumescence or resolution of priapism and return of penis to non‐erect state (immediate or after one, two or four hours)
Reduction in frequency of stuttering priapism
Secondary outcomes
Return of priapism
Immediate side effects, e.g. pain, bleeding
Later sexual function
Other untoward systemic side effects, e.g. gynaecomastia, liver problems
Efficacy of a prevention strategy for stuttering priapism (and thus preventing fulminant priapism)
Search methods for identification of studies
There were no restrictions regarding language or publication status.
Electronic searches
Relevant trials were identified from the Group's Haemoglobinopathies Trials Register using the terms: (sickle cell OR (haemoglobinopathies AND general)) AND (priap* OR erection).
The Haemoglobinopathies Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library) and weekly searches of MEDLINE. Unpublished work is identified by searching the abstract books of five major conferences: the European Haematology Association conference; the American Society of Hematology conference; the British Society for Haematology Annual Scientific Meeting; the Caribbean Public Health Agency Annual Scientific Meeting (formerly the Caribbean Health Research Council Meeting); and the National Sickle Cell Disease Program Annual Meeting. For full details of all searching activities for the register, please see the relevant section of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of the most recent search of the Group's Haemoglobinopathies Trials Register: 15 September 2017.
We also searched Embase separately (1974 to 2003) (Appendix 1) and the Internet (December 2003) (Appendix 2) for the last update of this review. For this update Embase is included in the CENTRAL search but there may be a delay before references appear in CENTRAL. We therefore searched Embase for the six months prior to the main search being carried out (16 August 2016) to current (12 December 2016) (Appendix 3).
Date of most current Embase search: 12 December 2016.
We searched the clinical trial registries ClinicalTrials.gov (http://www.clinicaltrials.gov) and the World Health Organisation International Clinical Trials Registry Platform (http://www.who.int/ictrp/search/en/) for any ongoing and or unpublished trials using the terms " sickle cell" and "priapi*" (Appendix 4).
Date of most recent search 12 December 2016.
Searching other resources
Reference lists
We searched reference list of all included trials to identify any further relevant trials.
Date of the latest search: 12 December 2016.
Correspondence
Attempts were also made to identify any unpublished trials through contact with experts in the field and personal communication with known authors.
Data collection and analysis
Selection of studies
Each author independently identified potentially relevant trials from the search results. No disagreement occurred, so no other expert in the field was invited to make an independent assessment based on the selection criteria.
Data extraction and management
Two authors independently extracted data using standard data acquisition forms. Where there was disagreement or uncertainty, a third author arbitrated.
Outcome data were to be grouped into those measured at 1, 3, 6 and 12 months and annually thereafter. This was to gauge the long‐term benefit or risk from an intervention. Trials reporting on time periods not listed above, were considered as well and described within the review.
Assessment of risk of bias in included studies
Two review authors independently assessed trials following the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The assessments were compared and any inconsistencies between the review authors were discussed and resolved.
Assessment was made of the following domains, each being assessed as having either a low, unclear or high risk of bias:
generation of the allocation sequence
concealment of allocation
blinding (of participants, personnel and outcome assessors)
-
incomplete outcome data
record of participants lost to follow‐up or subsequently excluded from the trial
whether an intention‐to‐treat analyses has been performed
selective reporting
other bias
Measures of treatment effect
For binary outcome measures we calculated a pooled estimate of the treatment effect for each outcome across trials using the risk ratio (RR) with 95% confidence intervals (CIs), where appropriate.
For continuous outcomes, we planned to record either mean change from baseline for each group or mean post‐treatment or post‐intervention values and standard deviation for each group. We also intended to compute a pooled estimate of treatment effect by calculating the mean difference (MD) with 95% CIs; however; we were unable to do this because of insufficient data.
Unit of analysis issues
Elbourne discusses methods for meta‐analysing cross‐over trials (Elbourne 2002). The methods discussed rely on the data that is reported within the primary paper. In this review, we have adopted the method that uses the data from the first period only for the included cross‐over trial, as this is currently the only data available from the trial (Serjeant 1985). However, for future updates, we plan to use the most appropriate method that is available in RevMan to analyse cross‐over data (Review Manager 2014).
Dealing with missing data
We contacted authors, where further clarification was needed.
In order to allow an intention‐to‐treat analysis, we sought data on the number of participants with each outcome event, by allocated treated group, irrespective of compliance and whether or not the participant was later thought to be ineligible or otherwise excluded from treatment or follow‐up.
Assessment of heterogeneity
We could not test for heterogeneity between trial results using a standard Chi² test as intended as the included trials reported different outcomes and could not be combined. However, for future updates, when more trials are included we plan to test for heterogeneity using the Chi² test.
Assessment of reporting biases
We planned to generate a funnel plot to attempt to identify any publication bias in the included trials (Higgins 2011), however, there were less than 10 included trials and we were therefore unable to conduct this analysis. We attempted to identify any selective reporting in the included publications, by comparing the trial protocols or trial registry, where available, with the final papers and by examination of the trial publications. We considered reporting of both positive and negative effects of the intervention. Where trial protocols weren't available, we compared the outcomes reported in the results section against the methods section of the paper. We extracted information on the sponsors, sources of funding and competing interests of the authors to rule out external bias.
Data synthesis
We generated forest plots using the Review Manager software (Review Manager 2014) from the trial data but were unable to combine the results into a meta‐analysis due to the included trials reporting different interventions and outcomes. We carried out separate analyses for each of the interventions.
We have used the fixed‐effect approach. If further trials are included, in the absence of homogeneity of treatment effects, we will use a random‐effect approach, otherwise, we will use the fixed‐effect model.
Subgroup analysis and investigation of heterogeneity
We were unable to carry out subgroup analyses as there were insufficient trials with comparable outcomes. The management of stuttering priapism in people with SCD is about prevention, and therefore differs from the management of fulminant priapism. Thus, future analyses will not only be by group but also by acute management versus prevention.
Sensitivity analysis
We planned to carry out sensitivity analyses to look at the effect of the risk of bias findings and to look at the effect of adding in and taking out trials where there was high risk of bias. This was not possible due to there not being any trials included in a meta‐analysis.
Summary of findings tables and quality of the evidence (GRADE)
In a post hoc change in line with current Cochrane guidance, at the 2017 update we added a summary of findings table for each comparison presented in the review (Table 1; Table 2; Table 3; Table 4). We selected the following outcomes to report (chosen based on relevance to clinicians and consumers).
Detumescence
Reduction in stuttering priapism
Immediate side effects of treatment (up to two specific effects)
Effect on later sexual function
Other untoward side effects of treatment
Efficacy of a prevention strategy
We determined the quality of the evidence using the GRADE approach; and downgraded evidence in the presence of a high risk of bias in at least one trial, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, high probability of publication bias. We downgraded evidence by one level if they considered the limitation to be serious and by two levels if very serious.
Results
Description of studies
Results of the search
The previous version of the review included one trial (two references) (Serjeant 1985). The 2017 searches identified eight new references, one of which was not eligible for any section of the review. The remaining seven new references were to two new eligible trials (Burnett 2014; Olujohungbe 2011). One of these references was to an ongoing trial that appeared in the previous version of this review but the full paper has now been published and we can include it (Olujohungbe 2011). The results of the search are shown in a PRISMA flow diagram (Figure 1).
Figure 1.
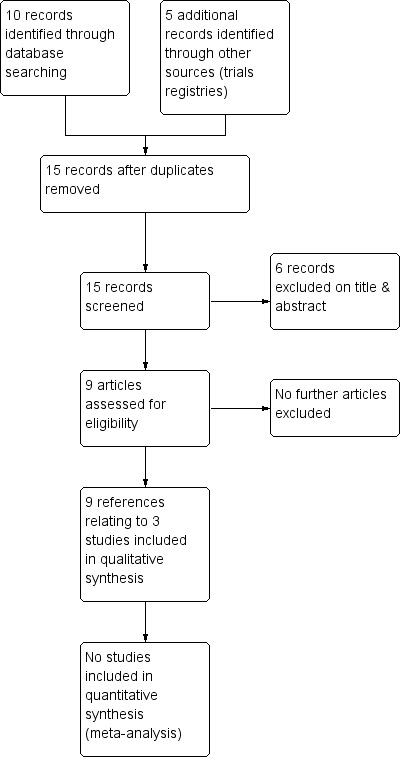
Study flow diagram.
Included studies
Nine references to three trials (with a total of 102 participants) on the treatment of stuttering priapism, were identified as eligible for inclusion in the review (Burnett 2014; Olujohungbe 2011; Serjeant 1985) (Characteristics of included studies). The two more recent trials are both double‐blind, placebo‐controlled parallel trials with treatment periods ranging from eight weeks (Burnett 2014) to 26 weeks (Olujohungbe 2011). The third trial is a double‐blind, placebo controlled cross‐over trial, in which participants who did not respond to the first arm of the trial were entered into the second arm of the trial with no washout period described (Serjeant 1985). There were no randomised trials of treatments for fulminant priapism. The literature reveals several case reports and case series of well‐established therapies and experimental therapies for stuttering and fulminant priapism.
Participants and setting
The 102 participants across the three trials were all outpatients with confirmed SCD and stuttering priapism from clinics in the USA, Nigeria, the UK and Jamaica. Olujohungbe recruited from clinics in the middle east for a short period due to poor recruitment in the UK (Olujohungbe 2011). The inclusion criteria for the Burnett and Serjeant trials stated that participants had to have at least two stuttering priapism episodes per week (Burnett 2014; Serjeant 1985). The Olujohungbe trial did not specify the number of episodes of stuttering priapism per week, but did exclude participants who had an attack which lasted longer than four hours or required hospitalisation (Olujohungbe 2011).
The mean (SD) age of participants ranged from 21.7 (5.3) years (Burnett 2014 Sildenafil group) to 24.3 (3.1) years (Serjeant 1985). Participant numbers were low in two of the trials with Burnett including 13 participants and Serjeant including only 11 (Burnett 2014; Serjeant 1985). The Olujohungbe trial required 320 participants to give 80% power to detect differences of 1.5 attacks per month between groups and did include larger numbers of participants but they only enrolled 131 of the required 320 and only 78 were eligible to be randomised to the intervention phase. Only 46 of these participants were included in the analysis and only 25 completed the treatment period (Olujohungbe 2011).
Type of intervention
All three trials report on different treatments from each other but they all compare to a placebo. The Serjeant trial looks at the preventive effects of 5 mg of stilboestrol on stuttering priapism (Serjeant 1985). Burnett looks at the effect of sildenafil 50 mg, a phosphodiesterase type 5 inhibitor, given once daily over eight weeks on preventing attacks of priapism (Burnett 2014). Olujohungbe looks at the effect of two oral alpha‐adrenergic agonists against placebo, etilefrine 50 mg, and two different doses of ephedrine (15 mg and 30 mg) (Olujohungbe 2011). In this trial the participants completed diaries in an observation phase for three to thirteen weeks before taking part in the randomised intervention phase. During the intervention phase participants were asked to take either 15 mg ephedrine, 30 mg ephedrine, 50 mg etilefrine or a placebo once daily, at bedtime for six months.
Type of outcomes
None of the included trials looked at our main primary outcome of detumescence or resolution of a priapism attack but all three trials measured the effect of treatment on the frequency of attacks. Burnett used a scoring system to record the frequency of attacks where 0 = no episodes, 1 = one to two episodes, 2 = three to four episodes, 3 = five to eight episodes and 4 = nine to sixteen episodes and also reported on the duration of episodes (Burnett 2014).The trial of stilboestrol also reported on the return of priapism after a period of no attacks (Serjeant 1985). The two more recent trials reported on immediate side effects of treatment but the specific side effects recorded were different in the two trials due to the actions of the medications being different. Burnett reported on headache, flushing, nasal congestion, abnormal vision, dyspepsia, myalgia whilst Olujohungbe reported on palpitations, tachycardia, lack of sleep, hand shaking, anxiety and dry mouth (Burnett 2014; Olujohungbe 2011). The ephedrine and etilefrine trial was the only trial to look at longer term effects by recording the effect on systolic and diastolic blood pressure but this was as a safety mechanism. Where participants were found to have an increase in systolic blood pressure of 20 mmHg and an increase in diastolic pressure of 10 mmHg the trial medication was discontinued (Olujohungbe 2011).
Excluded studies
There were no excluded studies.
Risk of bias in included studies
The risk of bias was assessed by both authors for all trials included in the review and the details are given in the table Characteristics of included studies. This has been summarised in Figure 2.
Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The method of randomisation was unclear and the concealment of allocation was not described in any of the three trials, therefore, we assessed each of these domains as having an unclear risk of bias for all three trials.
Blinding
All three trials were described as double‐blind (physician and participant) and we therefore assessed this domain as low risk. In the Olujohungbe trial, blinding of the trial drugs was done by an external clinical trial supplies company. In the Burnett trial, adverse events were reported for the whole of the treatment period (confirmed by the author team) including the open label phase and we therefore have not entered the data into our analysis as this would bias the results (Burnett 2014).
Incomplete outcome data
The Burnett trial included 13 participants and analysed the data both on an intention‐to‐treat basis and on a per protocol basis. All 13 participants' data were included in the analysis and the trial was deemed to be at low risk of bias for this domain (Burnett 2014).
Although the trial of ephedrine and etilefrine had higher numbers of participants, there was a high rate of attrition at each phase of the trial. The authors stated that 320 participants were needed to give 80% power to detect differences of 1.5 attacks per month between groups. The number enrolled was much less than this (131) and only a proportion completed the observation phase thus enabling them to be randomised to the treatment phase. Of the 78 participants that were randomised to the intervention phase, data was only available for 46 of them. The remainder were lost to follow up. Just under half of the participants analysed completed the full 26 weeks of treatment. We deemed this trial to be at a high risk of bias due to attrition (Olujohungbe 2011).
In the cross‐over trial one participant defaulted, and in two participants the attacks of stuttering priapism ceased spontaneously during the baseline observation period. There was a fourth participant who initially did not take the placebo because a painful crisis aborted the stuttering priapism prior to taking the assigned tablet. In this participant (identified as patient nine) attacks of priapism recurred two weeks later and data on a second baseline period were collected. The paper did not discuss an intention‐to‐treat analysis and we therefore assessed this paper as being at an unclear risk of bias for this domain (Serjeant 1985).
Selective reporting
We deemed two of the trials to be at low risk of bias for selective reporting (Olujohungbe 2011; Serjeant 1985) although we were unable to find a published protocol for any of the trials. The Burnett trial stated that there were no significant differences between groups for decreased median weekly change in priapism episode score but no data were shown (Burnett 2014). The same paper reported adverse effects in the published paper but did not include this as an outcome in either the methods section or in the trial registration document. We deemed this trial to be at an unclear risk of bias for this domain.
Other potential sources of bias
We did not find any other potential sources of bias.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Silboesterol versus placebo
One trial with 11 participants reported on the effect of 5 mg stilboestrol versus placebo (Serjeant 1985).
Primary outcomes
1. Detumescence (immediate or after one, two or four hours)
This outcome was not assessed in this trial.
2. Reduction in frequency of stuttering priapism
The trial of stilboestrol reported that in four out of the five participants randomised initially to placebo, the attacks of stuttering priapism continued unchanged. In the remaining one participant (participant seven) the attacks ceased immediately on placebo, but he developed a painful crisis six days later. Attacks of priapism recurred one month later and data for a second baseline period were collected before being started on stilboestrol. In all these five participants, switching over to stilboestrol led to the cessation of attacks, immediately in four and gradually in one.
Analysis of the first treatment period (in which no tablet was taken by four participants) showed no significant treatment effect, RR 2.92 (95% CI 0.73 to 11.70) (P = 0.13) (very low quality evidence) (Analysis 1.1). However, the fact that four participants did not take a tablet strongly biases the result (Serjeant 1985).
Analysis 1.1.
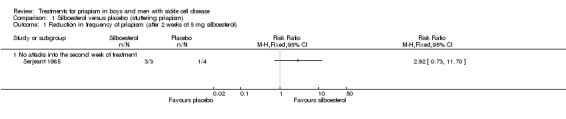
Comparison 1 Silboesterol versus placebo (stuttering priapism), Outcome 1 Reduction in frequency of priapism (after 2 weeks of 5 mg silboesterol).
Secondary outcomes
1. Return of priapism
Return of priapism was addressed in the Serjeant trial but not in a randomised manner (Serjeant 1985).
2. Immediate side effects, e.g. pain, bleeding
This outcome was not assessed (Serjeant 1985).
3. Later sexual function
This outcome was not assessed (Serjeant 1985).
4. Other untoward systemic side effects, e.g. gynaecomastia, liver problems
This outcome was not assessed (Serjeant 1985).
5. Efficacy of a prevention strategy for stuttering priapism (and thus preventing fulminant priapism)
This outcome was not assessed (Serjeant 1985).
Sildenafil versus placebo
One trial with 13 participants reported the effects of sildenafil 50 mg versus placebo (Burnett 2014).
Primary outcomes
1. Detumescence (immediate or after one, two or four hours)
This outcome was not assessed in this trial (Burnett 2014).
2. Reduction in frequency of stuttering priapism
The Burnett trial reported that there was no significant difference between sildenafil and placebo regarding decreases in episodes of priapism by score tier, RR 1.17 (95% CI 0.36 to 3.76) (low quality evidence) (Analysis 2.1), or decrease in frequency, RR 0.58 (95% CI 0.07 to 4.95) (low quality evidence) (Analysis 2.2) (Burnett 2014). One out of six participants taking sildenafil showed a reduction in frequency of episodes whilst two out of seven participants taking placebo showed a decrease in frequency (Burnett 2014).
Analysis 2.1.
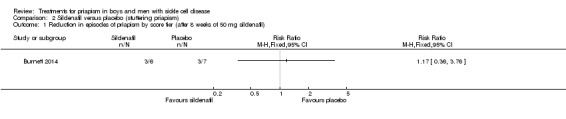
Comparison 2 Sildenafil versus placebo (stuttering priapism), Outcome 1 Reduction in episodes of priapism by score tier (after 8 weeks of 50 mg sildenafil).
Analysis 2.2.

Comparison 2 Sildenafil versus placebo (stuttering priapism), Outcome 2 Reduction in frequency of priapism after (8 weeks of 50 mg sildenafil).
Secondary outcomes
1. Return of priapism
This outcome was not assessed in this trial (Burnett 2014).
2. Immediate side effects, e.g. pain, bleeding
The Burnett trial reports adverse effects for the total treatment period including the open label phase (Burnett 2014). Burnett found no significant differences between sildenafil and placebo regarding headache (P = 1.0), flushing (P = 0.19), nasal congestion (P = 1), abnormal vision (P = 0.46), dyspepsia (P = 0.46), myalgia (P = 1.0) or hypotension (P = 1.0). Seven out of the 13 participants were hospitalised 14 times over the course of the trial (53.8%). Of these hospitalisations, 10 visits made by six participants were due to major priapism episodes. Two of these episodes occurred in participants who were taking sildenafil (20%) and the remaining eight occurred in participants who were taking placebo treatment (80%) (Burnett 2014).
3. Later sexual function
This outcome was not assessed in this trial (Burnett 2014).
4. Other untoward systemic side effects, e.g. gynaecomastia, liver problems
This outcome was not assessed in this trial (Burnett 2014).
5. Efficacy of a prevention strategy for stuttering priapism (and thus preventing fulminant priapism)
This outcome was not assessed in this trial (Burnett 2014).
Etilefrine or ephedrine versus placebo
One trial with 78 participants assessed the effect of etilefrine 50 mg versus placebo, ephedrine 15 mg versus placebo or ephedrine 30 mg within a four‐armed trial (Olujohungbe 2011).
Primary outcomes
1. Detumescence (immediate or after one, two or four hours)
This outcome was not assessed in this trial (Olujohungbe 2011).
2. Reduction in frequency of stuttering priapism
The results of the trial showed no significant differences between the four treatment groups in the weekly total number of attacks during the trial (Phase B) compared to a baseline pre‐randomisation period (Phase A). We do not quote any numerical results in this review due to concerns over the reliability of published results; the trial was substantially underpowered with only 46 out of a target 320 participants included in final analysis (14%) and the authors comment on poor compliance in the completion of symptom diaries during both Phase A and Phase B of the trial (Olujohungbe 2011).
Secondary outcomes
1. Return of priapism
This outcome was not assessed in this trial (Olujohungbe 2011).
2. Immediate side effects, e.g. pain, bleeding
Olujohungbe reported side effects of treatment in the blinded phase of the trial for etilefrine 50 mg, ephedrine 15 mg or ephedrine 30 mg (Olujohungbe 2011). Many of the participants experienced tachycardia but there was no significant difference between any of the treatments and placebo for any of the immediate side effects measured (Analysis 3.1; Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.6; Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6) (very low quality evidence). The three adverse events reported were three hospital admissions due to painful vaso‐occlusive crises in the same individual and were not thought to be related to the trial treatment.
Analysis 3.1.

Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 1 Immediate side effects (palpitations) (after 6 months of treatment).
Analysis 3.2.
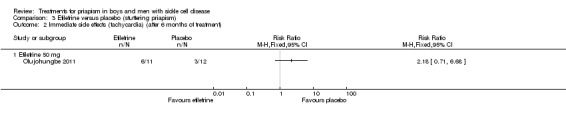
Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 2 Immediate side effects (tachycardia) (after 6 months of treatment).
Analysis 3.3.

Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 3 Immediate side effects (lack of sleep).
Analysis 3.4.
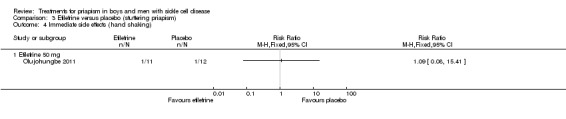
Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 4 Immediate side effects (hand shaking).
Analysis 3.5.
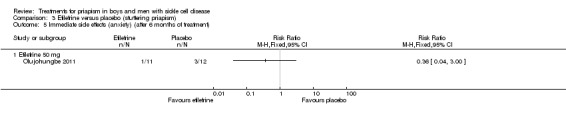
Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 5 Immediate side effects (anxiety) (after 6 months of treatment).
Analysis 3.6.
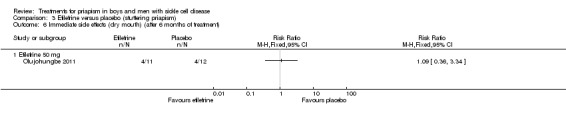
Comparison 3 Etilefrine versus placebo (stuttering priapism), Outcome 6 Immediate side effects (dry mouth) (after 6 months of treatment).
Analysis 4.1.
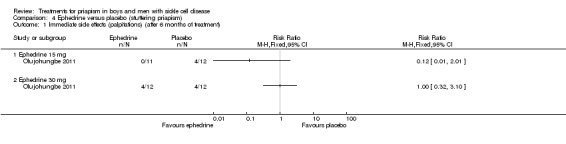
Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 1 Immediate side effects (palpitations) (after 6 months of treatment).
Analysis 4.2.
Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 2 Immediate side effects (tachycardia) (after 6 months of treatment).
Analysis 4.3.
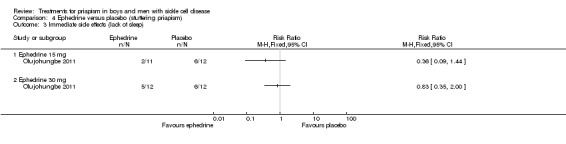
Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 3 Immediate side effects (lack of sleep).
Analysis 4.4.
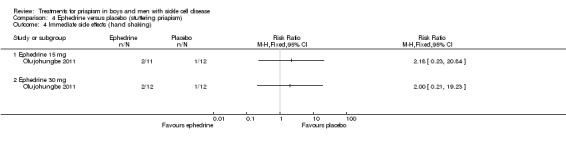
Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 4 Immediate side effects (hand shaking).
Analysis 4.5.
Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 5 Immediate side effects (anxiety) (after 6 months of treatment).
Analysis 4.6.
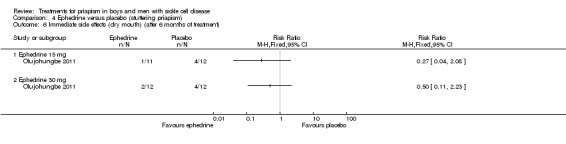
Comparison 4 Ephedrine versus placebo (stuttering priapism), Outcome 6 Immediate side effects (dry mouth) (after 6 months of treatment).
3. Later sexual function
This outcome was not assessed in this trial (Olujohungbe 2011).
4. Other untoward systemic side effects, e.g. gynaecomastia, liver problems
This outcome was not assessed in this trial (Olujohungbe 2011).
5. Efficacy of a prevention strategy for stuttering priapism (and thus preventing fulminant priapism)
This outcome was not assessed in this trial (Olujohungbe 2011).
Discussion
Summary of main results
Since the last update we have found two further trials of treatments for priapism in boys and men with sickle cell disease bringing the number of included trials to three (Burnett 2014; Olujohungbe 2011; Serjeant 1985). The three trials included small numbers of participants, had different trial designs and assessed four different drug treatments. None of the trials primarily looked at fulminant priapism and so no trials reported on detumescence, one of our primary outcomes. No significant effect of any of the treatments was seen in relation to our second primary outcome of reduction in frequency of stuttering priapism. The only secondary outcome reported was immediate side effects which were reported in two of the trials but in one of the trials, the reporting of side effects was done for the whole treatment period including the open label phase which may have biased the results. A summary of the results is presented in the summary of findings tables (Table 1; Table 2; Table 3; Table 4)
Overall completeness and applicability of evidence
We are confident that the searches were thorough and have identified all the available trials. The included trials were generally complete although we needed to clarify a couple of issues with the author team. However, the small number of participants and the high attrition rate limit the applicability of the results to the wider population of males with priapism related to sickle cell disease.
Quality of the evidence
The evidence provided by this review is low to very low quality, limited by the quantity and quality of the trials. All of the trials were randomised but the method of randomisation and allocation concealment were unclear. The risk of bias for blinding was deemed to be low as all three of the trials were double blind. The Burnett trial, however, reported on an open label phase for a period of time after the double‐blind phase of the trial. Immediate side effects of treatment were reported for the whole period, so there was the possibility that participants under or over reported any side effects due to the knowledge of the treatment they were on (Burnett 2014).
Poor recruitment and attrition bias were problems in the Olujohungbe trial where large numbers were required to show a significant effect. In stuttering priapism young males may be reluctant to report this complication of sickle cell disease and may also be reluctant to participate in research. Similarly, the nature of the disease may have been the reason for the number of men lost to follow‐up. Without information on why the participants failed to complete the trial it is not possible to comment on the evidence provided by those that did complete the trial (Olujohungbe 2011).
Potential biases in the review process
One of the co‐authors of this review, KA is also an author on one of the included trials (Olujohungbe 2011).
Agreements and disagreements with other studies or reviews
There are no similar reviews of treatment in this area with which we can compare our findings.
Authors' conclusions
The quality and quantity of the trials included in this review were not sufficient to allow firm conclusions about treatment for priapism in sickle cell disease to be made at the present time.
Well‐designed, adequately‐powered trials with multicentre collaboration are desperately required to provide evidence for the most effective treatment required to alleviate suffering in people with sickle cell disease. Large numbers of participants would be required in a randomised clinical trial to provide the evidence base for drug therapy of priapism and to demonstrate that one regimen is more efficacious than another. In acute or fulminant priapism, it would be unethical to have a placebo‐controlled (randomised) trial. However, trials could be conducted comparing one agent against another, e.g. different alpha agonists.
Acknowledgements
We are grateful to the following for comments:
Professor Dame Sally C Davies, Imperial College Faculty of Medicine, Department of Haematology, Central Middlesex Hospital, London, UK.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Original search strategy (Embase)
| OVID (online) |
| 1. sickle cell.mp. 2. priapism.mp. 3. priapic.mp. 4. erectile.mp. 5. 1 and 2 |
| 6. 1 and 3 7. 1 and 4 |
Appendix 2. Original search strategy (Google search)
| Internet |
| 1. sickle cell priapism 2. sickle cell priapic 3. sickle cell erectile |
Appendix 3. Search strategy Embase 2016 update
| 1. Clinical Trial/ |
| 2. Randomized controlled trial/ |
| 3. Randomization/ |
| 4. Single blind procedure/ |
| 5. Double blind procedure/ |
| 6. Crossover procedure/ |
| 7. Placebo/ |
| 8. Randomi?ed controlled trial$.tw. |
| 9. Rct.tw. |
| 10. Random allocation.tw. |
| 11. Randomly allocated.tw. |
| 12. Allocated randomly.tw. |
| 13. (allocated adj2 random).tw. |
| 14. Single blind$.tw. |
| 15. Double blind$.tw. |
| 16. ((treble or triple) adj blind$).tw. |
| 17. Placebo$.tw. |
| 18. Prospective study/ |
| 19. or/1‐18 |
| 20. Case study/ |
| 21. Case report.tw. |
| 22. Abstract report/ or letter/ |
| 23. or/20‐22 |
| 24. 19 not 23 |
| 25. sickle cell.mp. |
| 26. priapism.mp. |
| 27. priapic.mp. |
| 28. erectile.mp. |
| 29. 25 and 26 |
| 30. 25 and 27 |
| 31. 25 and 28 |
| 32. 29 or 30 or 31 |
| 33. 24 and 32 |
| 34. limit 33 to yr="2016" |
Appendix 4. Search terms clinical trials registries
Clinicaltrials.gov
Search terms: priapism
Study type: Interventional studies
Conditions: sickle cell
WHO International Clinical Trials Registry Platform
Priapi* AND sickle cell
Recruitment status set to ALL
Data and analyses
Comparison 1.
Silboesterol versus placebo (stuttering priapism)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in frequency of priapism (after 2 weeks of 5 mg silboesterol) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 No attacks into the second week of treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2.
Sildenafil versus placebo (stuttering priapism)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction in episodes of priapism by score tier (after 8 weeks of 50 mg sildenafil) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Reduction in frequency of priapism after (8 weeks of 50 mg sildenafil) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3.
Etilefrine versus placebo (stuttering priapism)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Immediate side effects (palpitations) (after 6 months of treatment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Immediate side effects (tachycardia) (after 6 months of treatment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Immediate side effects (lack of sleep) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Immediate side effects (hand shaking) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Immediate side effects (anxiety) (after 6 months of treatment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Immediate side effects (dry mouth) (after 6 months of treatment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Etilefrine 50 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4.
Ephedrine versus placebo (stuttering priapism)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Immediate side effects (palpitations) (after 6 months of treatment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Immediate side effects (tachycardia) (after 6 months of treatment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Immediate side effects (lack of sleep) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Immediate side effects (hand shaking) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Immediate side effects (anxiety) (after 6 months of treatment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Immediate side effects (dry mouth) (after 6 months of treatment) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Ephedrine 15 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Ephedrine 30 mg | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
What's new
Last assessed as up‐to‐date: 19 September 2017.
| Date | Event | Description |
|---|---|---|
| 19 September 2017 | New citation required but conclusions have not changed | Sherie Smith has been added to the author list. Changes have been made throughout most sections of the review, although the conclusions remain unchanged. |
| 19 September 2017 | New search has been performed | The trial previously listed as ongoing is now included (Olujohungbe 2011). A further trial has been included in the review (Burnett 2014). A total of three trials (102 participants) are included in the review. These trials compare stilboestrol to placebo, sildenafil to placebo and ephedrine or etilefrine to placebo. The review has been updated throughout and four new summary of findings tables have been added. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 27 August 2010 | New search has been performed | A search of the Group's Haemoglobinopathies Trials Register did not identify any trials potentially relevant for inclusion in the review. |
| 4 September 2008 | New search has been performed | A search of the Group's Haemoglobinopathies Trials Register identified no new trials eligible for inclusion in the review. |
| 4 September 2008 | Amended | Converted to new review format. |
| 1 February 2008 | New search has been performed | A search of the Group's Haemoglobinopathies Trials Register identified no new trials eligible for inclusion in the review. |
| 1 February 2008 | Amended | A new Plain Language Summary has replaced the previous Synopsis. |
| 1 February 2007 | New search has been performed | A search of the Group's Haemoglobinopathies Trials Register identified no new trials eligible for inclusion in the review. |
| 1 December 2005 | New search has been performed | A search of the Group's Haemoglobinopathies Trials Register identified no new trials eligible for inclusion in the review. |
Differences between protocol and review
In a post hoc change, we have added summary of findings tables to the review.
For the 2017, we also searched www.clinicaltrials.gov, Embase for 2016 and the WHO trial portal.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Double‐blind, placebo‐controlled parallel group trial followed by an open‐label extension for 8 weeks. Conducted prospectively from June 2008 to November 2012. | |
| Participants | 13 participants recruited from regional haematology and urology clinics randomised to sildenafil or placebo. Inclusion criteria were occurrences of at least two self‐reported priapism episodes per week and ability to provide written informed consent. Exclusion criteria were estimated GFR < 50 mL/min, clinical cirrhosis, pulmonary hypertension based on echocardiography, alcohol use exceeding 2 standard drinks daily, formal contraindications for using phosphodiesterase type 5 inhibitor therapy. Participant age: sildenafil (n = 6) mean (SD) age: 21.7 (5.3) years; placebo (n = 7) mean (SD) age: 23.0 (8.7) years. Disease status: SCD (confirmed SS or SC haemoglobinopathy) and recurrent ischaemic priapism. Hypertension no (%) placebo 1 (14.3%), sildenafil 2 (33.3%). Stroke no (%) placebo 3 (42.9%), sildenafil 1 (16.7%). Avascular necrosis no (%) placebo 1 (14.3%), sildenafil 0 (0). Acute chest syndrome no (%) placebo 1 (14.3%), sildenafil 2 (33.3%). Asthma no (%) placebo 2 (28.6%), sildenafil 2 (33.3%). Smoker no (%) placebo 1 (14.3%), sildenafil 2 (33.3%). Alcohol use no (%) placebo 3 (42.9%), sildenafil 3 (50%). |
|
| Interventions | Intervention: sildenafil 50 mg. Placebo: sugar pill. Either placebo or sildenafil was taken daily for 8 weeks. Participants were instructed to take the medication in the morning a few hours after awakening and without sexual stimulation. An 8‐week open‐label phase followed. |
|
| Outcomes | Primary efficacy outcome was 50% reduction in frequency in priapism episodes bi‐weekly from baseline at the end of the 8‐week double‐blind phase. Secondary outcomes included subjective improvements in episode frequency/duration and decrease in the number of of bi‐weekly episodes of priapism using a scoring system: 0 = no episodes; 1 = 1 to 2 episodes; 2 = 3 to 4 episodes; 3 = 5 to 8 episodes; 4 = 9 to 16 episodes; 5 = > 16 episodes. Adverse effects were also recorded for the whole trial period including the open label phase. Outcomes were measured via twice‐weekly nurse co‐ordinator phone calls to record progress, medication changes and adverse events. In‐clinic evaluations were carried out at baseline, week four and week eight which included repeat administration of trial instruments. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participats were randomised in a 1:1 allocation. Method of randomisation not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial where the participant, caregiver and investigator were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind trial where the participant, caregiver and investigator were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis. Both ITT and per protocol analyses were carried out. No data were shown for decreased median weekly change in priapism episode score, just that there were no significant differences between groups. Adverse effects were not listed in the methods section but were reported in the results. Adverse events were reported for the whole trial period including the open label phase (confirmed by author team). |
| Selective reporting (reporting bias) | Unclear risk | The publication states that there were no significant differences between groups for decreased median weekly change in priapism episode score but no data were shown. The same paper reported adverse effects in the published paper but did not include this as an outcome in either the methods section or in the trial registration document. |
| Other bias | Low risk | None identified. |
| Methods | Double‐blind, placebo controlled parallel group trial with four arms. The first phase of the trial was an observational phase lasting a minimum of three weeks and a maximum of 13 weeks to give a baseline before participants were randomised to intervention or placebo. The intervention phase ran for 26 weeks. | |
| Participants | 131 participants were enrolled onto the trial. 86 participants completed the observational phase of the trial. 78 participants were randomised to 1 of the 4 treatment arms. Inclusion criteria: males aged 12 or over with confirmed sickle cell anaemia or sickle cell beta‐thalassaemia on haemoglobin electrophoresis and stuttering priapism. Participants had to be in active attendance at a designated care centre (1 visit in last 6 months). Participant characteristics were only reported for the complete group of 86 not specifically for the 46 randomised participants. Age (participants who completed phase A n = 86): mean age was 23.6 (range 14.5 ‐ 46.1). Median weekly number of attacks in phase A ‐ 1.5 (range 0 – 7). Median weekly duration of attacks in phase A – 2.0h (range 0 – 42 h). Number (%) of weekly attacks > 4h duration in phase A – 22 (26%). Average attack pain score (mean (range)) in phase A – 3.4 (0 – 8.8). Participants were excluded if there was a history of an attack lasting longer than 4 hours or who needed acute hospital. admission and intervention or history of cerebrovascular accident or hypertension. |
|
| Interventions | Group 1: participants received 15 mg ephedrine per day. Group 2: participants received 30 mg ephedrine per day. Group 3: participants received 50 mg etilefrine per day. Group 4: participants received a placebo. All treatments were taken once a day at bedtime for 6 months. |
|
| Outcomes | Weekly total number of attacks expressed as a ratio of phase B to phase A. Mean difference in pain score from phase A to phase B. Number of participants who had an attack lasting more than four hours. Adverse events (palpitations, tachycardia, lack of sleep, hand shaking, anxiety, dry mouth) experienced during the trial period. Participants were reviewed on a 6‐weekly basis when diaries were monitored and BP was taken. |
|
| Notes | None of the participants were on a chronic hypertransfusion program. One participant had been on hydroxyurea for 18 months prior to the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding of the trial drug was carried out by Bilcare Ltd clinical trial supplies although the authors do not state who was blinded and what methods were used. It is a double‐blind trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of the trial drug was carried out by Bilcare Ltd clinical trial supplies although the authors do not state who was blinded and what methods were used. It is a double‐blind trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 86 participants completed the observational phase and of these, 59% entered the intervention phase. 78 participants were randomised to the intervention phase but data was only available for 46. The remainder were lost to follow‐up. 46 participants entered the intervention phase and 25 participants (54%) completed 26 weeks. |
| Selective reporting (reporting bias) | Low risk | No protocol available therefore outcomes reported in the results section against the methods section of the paper; no discrepancies found. |
| Other bias | Low risk | None identified. |
| Methods | Double‐blind, placebo‐controlled cross‐over trial, with two 14‐day treatment periods. Allocation concealment and method of randomisation was unclear. | |
| Participants | 11 males with stuttering priapism and homozygous sickle cell (SS) disease were randomised. 9 completed the trial, 1 participant defaulted after baseline and 1 participant had a painful crisis which aborted the priapism before any tablets were taken. Participants came from the sickle cell clinic of the University Hospital of the West Indies, Kingston, Jamaica. | |
| Interventions | Stilboestrol 5 mg daily versus placebo. | |
| Outcomes | Reduction in frequency of stuttering priapism. Return of priapism. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double blind. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described if outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 1 participant defaulted, and in two participants the attacks of stuttering priapism ceased spontaneously during the baseline observation period. There was a fourth participant who initially did not take the placebo because a painful crisis aborted the stuttering priapism prior to taking the assigned tablet. In this participant (identified as patient 9) attacks of priapism recurred 2 weeks later and data on a second baseline period were collected. The paper did not discuss an ITT analysis. |
| Selective reporting (reporting bias) | Low risk | No protocol available therefore outcomes reported in the results section against the methods section of the paper; no discrepancies found. |
| Other bias | Low risk | None identified. |
BP: blood pressure ITT: intention‐to‐treat SCD: sickle cell disease
Contributions of authors
The original review was conceived by the Cochrane Cystic Fibrosis and Genetic Disorders Group, who also conducted searches for relevant trials. Dr Chinegwundoh (FC) designed the original review with constructive comments from Dr Anie (KA).
FC and KA conducted further searches for relevant trials. FC took the lead in the write up of the review, with substantial revisions from KA.
FC is responsible for updating the review and acts as guarantor of the review.
For the 2017 version, Sherie Smith (SS) lead on this update with input from FC and KA.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Francis Chinegwundoh: none known.
Sherie Smith: none known.
Kofi Anie: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
- Burnett AL, Anele U, Trueheart IN, Dimitrakoff JD, Casella JF. Randomized, controlled study of the safety and efficacy of sildenafil for the treatment of recurrent ischemic priapism associated with sickle cell disease. Journal of Sexual Medicine 2014;11 Suppl 3:176. [Abstract no.: 124; CENTRAL: 1023232; CRS: 5500050000000245; EMBASE: 71682862] [Google Scholar]; Burnett AL, Anele UA, Trueheart IN, Strouse JJ, Casella JF. Randomized controlled trial of sildenafil for preventing recurrent ischemic priapism in sickle cell disease. American Journal of Medicine 2014;127(7):664‐8. [CENTRAL: 995412; CRS: 5500050000000244; EMBASE: 2014427964; PUBMED: 24680796] [DOI] [PMC free article] [PubMed] [Google Scholar]; NCT00940901. Sildenafil for treatment of priapism in men with sickle cell anaemia. https://clinicaltrials.gov/ct2/show/NCT00940901. https://www.clinicaltrials.gov/ct2/show/NCT00940901, (accessed 12 December 2016). ; Shakeri A, Asseldonk B, Elterman DS. Prevention of Ischemic Priapism in Sickle Cell Disease: Sildenafil: Commentary on: Randomized Controlled Trial of Sildenafil for Preventing Recurrent Ischemic Priapism in Sickle Cell Disease. Urology 2015;86(6):1055‐6. [DOI] [PubMed] [Google Scholar]
- ISRCTN54312363. A randomised double blind controlled trial of oral ephedrine/etilefrine in the prevention of recurrent (stuttering) attacks of priapism in sickle cell disease: a multicentre international study in older children and adults. http://isrctn.com/ISRCTN54312363 (accessed 12 December 2016). ; Olujohungbe AB, Adeyoju A, Yardumian A, Akinyanju O, Morris J, Westerdale N, et al. A prospective diary study of stuttering priapism in adolescents and young men with sickle cell anemia: report of an international randomized control trial‐the priapism in sickle cell study. Journal of Andrology 2011;32(4):375‐82. [CENTRAL: 802280; CRS: 5500050000000078; PUBMED: 21127308] [DOI] [PubMed] [Google Scholar]; Seftel AD. A prospective diary study of stuttering priapism in adolescents and young men with sickle cell anemia: Report of an international randomized control trial; The priapism in sickle cell study (PISCES study). Journal of Urology 2011;185(5):1837‐8. [CENTRAL: 1016434; CRS: 5500050000000464; EMBASE: 2011194426] [DOI] [PubMed] [Google Scholar]
- Serjeant GR, Ceulaer C, Maude GH. Stilboestrol and stuttering priapism in homozygous sickle‐cell disease. West Indian Medical Journal 1986;35(Suppl):39. [Google Scholar]; Serjeant GR, Ceulaer K, Maude GH. Stilboestrol and stuttering priapism in homozygous sickle‐cell disease. Lancet 1985;2(8467):1274‐6. [DOI] [PubMed] [Google Scholar]
Additional references
- Bachir D, Galacteros E, Lee K, Virag R. Two years experience in management of priapism and impotence in sickle cell disease. Blood 1996;88:14a. [Google Scholar]
- Bivalacqua TJ, Burnett AL. Priapism: new concepts in the pathophysiology and new treatment strategies. Current Urology Reports 2006;7(6):497‐502. [DOI] [PubMed] [Google Scholar]
- Bivalacqua TJ, Musicki B, Kutlu O, Burnett AL. New insights into the pathophysiology of sickle cell disease‐associated priapism. Journal of Sexual Medicine 2012;9(1):79‐87. [DOI] [PubMed] [Google Scholar]
- Broderick GA, Gordon D, Hypolite J, Levin RM. Anoxia and smooth muscle dysfunction: a model for ischaemic priapism. Journal of Urology 1994;151(1):259‐62. [DOI] [PubMed] [Google Scholar]
- Davies SC, Oni L. The management of patients with sickle cell disease. BMJ 1997;315(7109):656‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analysis involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
- Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Archives of Internal Medicine 1980;140(11):1434‐7. [PubMed] [Google Scholar]
- Hickman M, Modell B, Greengross P, Chapman C, Layton M, Falconer S, et al. Mapping the prevalence of sickle cell and beta thalassaemia in England: estimating and validating ethnic‐specific rates. British Journal of Haematology 1999;104(4):860‐7. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org2011.
- Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction and priapism. In: Walsh PC, Retik AB, Darracott Vaughan E Jr, Wein AJ editor(s). Campbell's Urology. 7th Edition. Vol. 2, Philadelphia: W.B. Saunders, 1998:1155‐80. [Google Scholar]
- Mantadakis E, Cavender JD, Rogers ZR, Ewalt DH, Buchanan GR. Prevalence of priapism in children and adolescents with sickle cell anaemia. Journal of Paediatric Hematology/Oncology 1999;21(6):518‐22. [PubMed] [Google Scholar]
- National Institutes of Health, USA. The Management of Sickle Cell Disease. Division of Blood Disease and Resources, National Heart, Lung and Blood Institute2002; Vol. NIH Publication No. 02‐2117.
- Okpala I, Westerdale N, Jegede T, Cheung B. Etilefrine for the prevention of priapism in adult sickle cell disease. British Journal of Haematology 2002;118(3):918‐21. [DOI] [PubMed] [Google Scholar]
- The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
- Upadhyay J, Shekarriz B, Dhabuwala CB. Penile implant for intractable priapism associated with sickle cell disease. Urology 1998;51(4):638‐9. [DOI] [PubMed] [Google Scholar]
- Virag R, Bachir D, Lee K, Galacteros F. Preventive treatment of priapism in sickle cell disease with oral and self‐administered intracavernous injection of etilefrine. Urology 1996;47(5):777‐81. [DOI] [PubMed] [Google Scholar]
- World Health Organisation. Joint WHO/TIF Meeting on the Control of Haemoglobinopathies. Report of the 7th Meeting of the WHO Working Group on the Control of Hereditary Anaemias; 1994 April 3‐4; Nicosia, Cyprus. Unpublished document WHO/HDP/TIF/HA/93.1. Geneva, 1994.


