Abstract
Background
Primary postpartum haemorrhage (PPH) is one of the top five causes of maternal mortality in both developed and developing countries.
Objectives
To assess the effectiveness and safety of any intervention used for the treatment of primary PPH.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 August 2013).
Selection criteria
Randomised controlled trials comparing any interventions for the treatment of primary PPH.
Data collection and analysis
We assessed studies for eligibility and quality and extracted data independently. We contacted authors of the included studies to request more information.
Main results
Ten randomised clinical trials (RCTs) with a total of 4052 participants fulfilled our inclusion criteria and were included in this review.
Four RCTs (1881 participants) compared misoprostol with placebo given in addition to conventional uterotonics. Adjunctive use of misoprostol (in the dose of 600 to 1000 mcg) with simultaneous administration of additional uterotonics did not provide additional benefit for our primary outcomes including maternal mortality (risk ratio (RR) 6.16, 95% confidence interval (CI) 0.75 to 50.85), serious maternal morbidity (RR 0.34, 95% CI 0.01 to 8.31), admission to intensive care (RR 0.79, 95% CI 0.30 to 2.11) or hysterectomy (RR 0.93, 95% CI 0.16 to 5.41).
Two RCTs (1787 participants) compared 800 mcg sublingual misoprostol versus oxytocin infusion as primary PPH treatment; one trial included women who had received prophylactic uterotonics, and the other did not. Primary outcomes did not differ between the two groups, although women given sublingual misoprostol were more likely to have additional blood loss of at least 1000 mL (RR 2.65, 95% CI 1.04 to 6.75). Misoprostol was associated with a significant increase in vomiting and shivering.
Two trials attempted to test the effectiveness of estrogen and tranexamic acid, respectively, but were too small for any meaningful comparisons of pre‐specified outcomes.
One study compared lower segment compression but was too small to assess impact on primary outcomes.
We did not identify any trials evaluating surgical techniques or radiological interventions for women with primary PPH unresponsive to uterotonics and/or haemostatics.
Authors' conclusions
Clinical trials included in the current review were not adequately powered to assess impact on the primary outcome measures. Compared with misoprostol, oxytocin infusion is more effective and causes fewer side effects when used as first‐line therapy for the treatment of primary PPH. When used after prophylactic uterotonics, misoprostol and oxytocin infusion worked similarly. The review suggests that among women who received oxytocin for the treatment of primary PPH, adjunctive use of misoprostol confers no added benefit.
The role of tranexamic acid and compression methods requires further evaluation. Furthermore, future studies should focus on the best way to treat women who fail to respond to uterotonic therapy.
Plain language summary
Treatment for excessive bleeding after childbirth
After a woman gives birth, womb muscles contract, clamping down on the blood vessels and helping to limit bleeding when the placenta has detached. If the muscles do not contract strongly enough, very heavy bleeding (postpartum haemorrhage) can occur, which can be life threatening. These situations are common in resource‐poor countries, and maternal mortality is about 100 times higher than in resource‐rich countries. It is a very serious problem that requires effective treatments that might avoid the use of surgery to remove the womb (hysterectomy). This is often the last treatment option and leaves the woman unable to have more children. In most settings, women are given a drug at the time of birth (before excessive bleeding occurs) to reduce the likelihood of excessive blood loss. However, despite this intervention, some women bleed excessively, and this review looked to see what interventions might be used to reduce the amount of blood lost by these women. Treatment options include drugs to increase muscles contractions (such as oxytocin, ergometrine and prostaglandins like misoprostol), drugs to help with blood clotting (haemostatic drugs such as tranexamic acid and recombinant activated factor VII), surgical techniques (such as tying off or blocking of the uterine artery) and radiological interventions (to assist in blocking the main artery to the womb by using gel foams).
The review identified 10 randomised controlled trials involving 4052 women. Seven of these trials looked at a drug called misoprostol, which is a prostaglandin and so works by increasing muscle contractions. Overall, the trials suggest that misoprostol does not work as well as oxytocin infusion, and it has more side effects. However, oxytocin needs to be kept in a refrigerator, and so in settings where refrigeration and infusions are not readily available, misoprostol can be used.
Other clinical trials looked into using other types of drugs or squeezing the main artery that supplies blood to the woman. The number of women included in these studies was too small for any useful conclusions regarding their effectiveness and safety.
Background
Nearly half a million women die annually across the world from causes related to pregnancy and childbirth (Khan 2006; WHO 2010). Approximately one‐quarter of these deaths are caused by complications of the third stage of labour, that is, excessive bleeding within the first 24 hours after delivery, also known as primary postpartum haemorrhage (PPH) (Abou Zahr 1991). In the developing world, PPH remains the leading cause of maternal death, accounting for one‐third of maternal deaths in Asia and Africa (Khan 2006; WHO 2010). In the United Kingdom (UK), the risk of death from obstetrical haemorrhage is about one in 100,000 deliveries (Cantwell 2011).
Physiology
The uterus is composed of a unique interlacing network of muscle fibres known as 'myometrium'. The blood vessels that supply the placental bed pass through this latticework of uterine muscle (Baskett 2000). Myometrial contraction is the main driving force for both placental separation and haemostasis through constriction of these blood vessels. This blood‐saving mechanism is known as the 'physiological sutures' or 'living ligatures' (Baskett 2000). The physiological increase in clotting factors during labour helps to control blood loss after separation of the placenta.
Active management of the third stage of labour has been standard practice in many parts of the world for many years (Prendiville 1989). It is suggested that prophylactic administration of a uterotonic will help to reduce blood loss and blood transfusion after delivery (Begley 2011). The role of early cord clamping and controlled cord traction in the reduction of bleeding is less clear; although it was once thought important to deliver the placenta quickly after uterotonic drug administration, to prevent it from being retained (McDonald 2013), delayed cord clamping is now favoured.
Blood loss up to 500 mL at delivery is regarded as 'physiological’. It is part of the normal mechanism that brings the mother's blood parameters to their normal non‐pregnant levels, and a healthy pregnant woman can cope with it with no difficulty (Gyte 1992; Ripley 1999).
Definition
Traditionally, primary PPH is defined as bleeding from the genital tract of 500 mL or more in the first 24 hours following delivery of the baby (Cunningham 1993, Abou Zahr 1991). Alternative cutoff levels of 600 mL (Beischer 1986), 1000 mL (Burchell 1980), 1500 mL (Mousa 2002), with a substantial fall in haematocrit or the need for blood transfusion (ACOG 1998; Combs 1991), have also been used. Unfortunately, underestimation of blood loss following delivery is a common problem, as visually (clinically) assessed bleeding underestimates measured blood loss by an average of 100 to 150 mL (Pritchard 1962; Sloan 2010; Stafford 2008). Several methods have been proposed for measuring blood loss objectively, but they are used mainly for research purposes (Sloan 2010). In addition, women delivering by caesarean section lose more blood on average than women who have vaginal birth; therefore, 1000 mL is commonly used as a cutoff for significant blood loss after caesarean section. Overall, a trend towards increasing the rate of primary PPH has been seen in developed countries (Knight 2009).
Causes and risk factors
Several factors influence PPH rates, including whether blood loss is measured, how the third stage of labour is managed (e.g. the provision of uterotonic, uterine massage, controlled cord traction), obstetrical interventions carried out at the time of delivery (e.g. episiotomy, mode of delivery) and characteristics of the study population (Begley 2011; Carroli 2008). Lack of efficient uterine contraction (uterine atony) is the most common cause of primary PPH. Other aetiological factors include retained parts of the placenta and vaginal or cervical tears. Uterine rupture, clotting disorders and uterine inversion are extremely rare but often very dramatic causes of heavy bleeding. Several investigators have attempted to identify factors that may pre‐dispose women to excessive blood loss after delivery. Examples of risk factors include first pregnancy (Gilbert 1987; Hall 1985), maternal obesity (Aisaka 1988), a large baby (Stones 1993), twin pregnancy (Combs 1991; Suzuki 2012), prolonged or augmented labour (Gilbert 1987), chorioamnionitis, pre‐eclampsia, maternal anaemia and antepartum haemorrhage (Wetta 2013). High multiparity does not appear to be a risk factor in high‐ or low‐income countries, even after control for maternal age (Drife 1997; Stones 1993; Tsu 1993). Despite the identification of potential risk factors, primary PPH often occurs unpredictably in low‐risk women (Mousa 2008).
Complications
The most important consequences of severe PPH include death, hypovolaemic shock, disseminated intravascular coagulopathy, renal failure, hepatic failure and adult respiratory distress syndrome (Bonnar 2000). In low‐income countries, poor nutritional status, lack of easy access to treatment and inadequate intensive care and blood bank facilities are additional contributing factors that lead to high morbidity and mortality rates in these countries (Khan 2006; WHO 2010). As no definition of PPH has been universally accepted, the exact incidence of serious complications is difficult to ascertain (Knight 2009).
Management of primary PPH
Treatment for primary PPH requires a multidisciplinary approach. After exclusion of lower genital tract tears, in most cases, bleeding is due to uterine atony. Uterotonics that increase the efficiency of uterine contraction, including ergometrine and oxytocin, were introduced as first‐line therapy for atonic PPH in the 19th century. Women who continue to bleed require further assessment and interventions to control bleeding. These interventions may include additional uterotonics, haemostatic drugs, surgical interventions, radiological embolisation and/or compression devices (Abou El Senoun 2011).
A. Uterotonics
Ergometrine
John Stearns (Stearns 1822) was the first to emphasise the use of ergots for PPH. Earlier, he wrote describing ergot's action: "It expedites lingering parturition ... The pains induced by it are peculiarly forcing ... In most cases you will be surprised with the suddenness of its operation" (Stearns 1808). Moir 1932 noticed that administration of aqueous ergot extract by mouth is associated with dramatic and vigorous uterine contractions, which were described as the 'John Stearns effect'. In 1935, Dudley and Moir were able to isolate the pure crystallised substance from the water‐soluble extract of ergot that was responsible for the 'John Stearns effect', and they called it 'ergometrine' (Dudley 1935). The isolation of a new water‐soluble extract of ergot was announced almost simultaneously from three other centres: in America (Davis 1935), the UK (Thompson 1935) and Switzerland (Stoll 1935). It turned out to be the same substance. The Americans called their preparation ergonovine, and the Swiss used the name ergobasine. Although the use of oxytocin is usually free of adverse effects, the use of ergometrine may be associated with nausea, vomiting and hypertension (ACOG 1998).
Oxytocin
In 1953, Vincent Du Vigneaud (Du Vigneaud 1953) identified the structure of oxytocin and was able to synthesise the hormone. By the 1980s, several randomised controlled trials and their meta‐analyses confirmed the effectiveness of active management of the third stage in reducing PPH (Begley 2011). Oxytocin and ergometrine have traditionally formed essential components of first‐line therapy in the management of primary PPH. Ergometrine (and the mixed drug combination of oxytocin and ergometrine) is contraindicated in women with a history of hypertension, heart disease, pre‐eclampsia or eclampsia.
Carbetocin is a long‐acting synthetic oxytocin analogue that can be administered as a single dose either intravenously or intramuscularly; it produces a similar uterotonic effect as oxytocin. Intravenously administered carbetocin has a half‐life of 40 minutes (four to 10 times longer than oxytocin). Uterine activity persists for 120 minutes and 60 minutes following intramuscular and intravenous injection, respectively (Hunter 1992). In Europe, this drug is licenced only for prevention of uterine atony after caesarean section. Carbetocin is as effective, but more expensive, than oxytocin (Su 2007). It may have unpleasant side effects, including headaches, tremor, hypotension, flushing, nausea, abdominal pain, pruritus and a feeling of warmth (Rath 2009).
Prostaglandins
By the 1970s, the prostaglandin F2 alpha series was discovered by Sune Bergstrom, among others (Bergstrom 1962). The 15‐methyl analogue of prostaglandin F2 alpha has been reported to have a high success rate if used alone (88%) or in combination with other uterotonic agents (95%) (Oleen 1990). Prostaglandin administration could be associated with unpleasant side effects, including vomiting, diarrhoea, hypertension and fever (Oleen 1990).
Misoprostol, a methyl ester synthetic analogue of natural prostaglandin E1, is a thermo‐stable, inexpensive drug that can be used for prevention and treatment of PPH. It can be administered orally, sublingually, buccally, vaginally or rectally. A Cochrane systematic review of randomised trials of misoprostol versus injectable uterotonics in management of the third stage of labour suggests that the drug is less effective than injectable uterotonics in the prevention of severe PPH (blood loss ≥ 1000 mL) and has more adverse effects, including nausea, vomiting and diarrhoea (Hofmeyr 2008; Tunçalp 2012).
In most cases, uterotonic drugs will control postpartum bleeding, but if they do not, surgical intervention must be considered.
B. Haemostatic drugs
Haemostatic drugs, including tranexamic acid (As 1996) and recombinant activated factor VII (rFVIIa) (Moscardo 2001), have been used for the treatment of intractable haemorrhage unresponsive to first‐ and second‐line therapies. Tranexamic acid is a systemic antifibrinolytic agent that is widely used in surgery to prevent clot breakdown (fibrinolysis) and therefore to reduce blood loss. It is a simple, inexpensive drug that requires no training for administration and can be used for prevention and treatment of primary PPH (As 1996; Ferrer 2009; Novikova 2010). It has a short half‐life of two hours.The use of tranexamic acid may be associated with side effects, including nausea, vomiting and diarrhoea. Other rare complications include hypotension, thrombosis, blurred vision, renal cortical necrosis and retinal artery obstruction (Novikova 2010; Peitsidis 2011).
Recombinant activated factor VII (rFVIIa; Novo Nordisk A/S, Bagsvaerd, Denmark) has also been successfully used for controlling life‐threatening PPH. It reduces blood loss through enhancement of tissue factor–dependent coagulation. It is effective in up to 80% of cases (Alfirevic 2007) but is quite expensive. Adverse events were observed in 2.5% of treated cases (Franchini 2010). Of note, all adverse events were thrombotic, including deep venous thrombosis, pulmonary embolism, cerebral thrombosis and myocardial infarction.
C. Surgical interventions
Porro (Porro 1876) was the first to describe caesarean hysterectomy to prevent death from uterine haemorrhage. However, the technique is associated with major complications and sterility. Active attempts have been made to introduce other conservative measures to avoid hysterectomy.
Uterine tamponade
Uterine packing, using several yards of wide gauze placed inside the uterine cavity, was one of the earliest methods introduced to achieve a tamponade effect to control primary PPH (Eastman 1950). It fell out of favour in the 1950s, as it was thought to conceal haemorrhage and cause infection (Eastman 1950). However, this technique re‐emerged in the 1980s and 1990s after these concerns were not confirmed (Maier 1993).
Over the past decade, active attempts have been made to introduce better alternatives for uterine packing through the use of balloon tamponade, including Foley’s catheter (De Loor 1996), the Sengstaken‐Blakemore tube (Chan 1997), the Rusch catheter (Johanson 2001), the Bakri balloon (Bakri 1999) and the condom catheter (Akhter 2005). After exclusion of a genital tract laceration, these procedures can be considered for control of obstetrical haemorrhage secondary to uterine atony, placenta accreta and placenta praevia. Overall, the difference between them is related mainly to balloon volume and the presence or absence of a cavity for draining blood. The overall success rate is around 80% (Doumouchtsis 2007; Georgiou 2009). Close observation of uterine size and the general condition of the woman is mandatory, as significant bleeding may occur distal to the bulb (Alamia 1999).
Artery ligation and uterine compression sutures
Ligation of the uterine artery or its main supply (internal iliac artery) may be considered in selected cases (AbdRabbo 1994; Jouppila 1995). However, the latter may be technically difficult and is successful in less than 50% of cases (Clark 1985).
Uterine compression sutures have recently been described (B‐Lynch 1997; Cho 2000; Hayman 2002; Marasinghe 2011; Ouahba 2007; Pereira 2005; Zheng 2011). B‐Lynch was the first to describe a suture that runs through the full thickness of both uterine walls (anterior and posterior) (B‐Lynch 1997). When tied, the suture allows tight compression of the uterine walls and stops the bleeding (Mousa 2001). Single or multiple stitches may be inserted at the same time and, according to the shape, they may be called brace suture (B‐Lynch 1997), simple brace (Hayman 2002) or square sutures (Cho 2000). Although they are thought to be effective in selected cases, unexpected occlusion of the uterine cavity with subsequent development of intrauterine synechiae (Poujade 2011; Rathat 2011) or infection (pyometra) has been reported (Ochoa 2002). The choice of the type of surgical intervention depends on several factors, paramount of which is the experience of the surgeon. Other factors include parity and desire for future children, the extent of the haemorrhage and the general condition of the woman (Cantwell 2011).
D. Radiological embolisation
Selective radiological embolisation of the bleeding vessel may be a therapeutic option in centres where interventional radiologists are available and the bleeding is not life threatening (Arulkumaran 2007). In a systematic review, Doumouchtsis and colleagues evaluated the success rate of emergency embolisation for the control of major PPH. They reported a success rate of 91% (Doumouchtsis 2007). The procedure has many advantages including minimal morbidity and complication rates, shorter hospital stay and preservation of fertility; it can be carried out under local anaesthesia, and success can be verified. The procedure is not free of complications (Doumouchtsis 2007; Penninx 2010; Tseng 2011). Postprocedure fever is the most common complication and typically resolves within two to three days. Other complications include feet ischaemia, bladder and rectal wall necrosis and sciatic nerve injury (Doumouchtsis 2007). Late re‐bleeding is a rare but serious problem, and repeated embolisation or hysterectomy may be required. The use of interventional radiological techniques is limited by availability, and few centres have a 24‐hour trained, skilled team. Unlike with other procedures, an unstable patient has to be moved to the angiography suite (Mousa 2002).
E. Non‐pneumatic antishock garment (NASG) and aortic compression device
In the 1900s, an inflatable pressure suit was developed by George Crile (Vahedi 1995). After several modifications, it was used in the Vietnam War for resuscitation of soldiers with traumatic injuries (Cutler 1971). In the 1970s, the G‐suit was modified into a half‐suit, which became known as MAST (military antishock trousers) or PASG (pneumatic antishock garment). From the 1970s, the National Aeronautics and Space Administration (NASA) contributed to the development of a “non‐pneumatic version” of the antishock garment. This was originally used for children with haemophilia but has since been developed into the garment known as the non‐pneumatic antishock garment (NASG) (Haggerty 1996). The NASG is a low‐technology pressure device that decreases blood loss, restores vital signs and has the potential to reduce adverse outcomes by helping women survive delays in receiving adequate emergency obstetrical care. Use of this garment as a temporising measure to stabilise women awaiting transfer to higher levels of care began in 2002 (Hensleigh 2002). Use of NASG among women with primary PPH in low‐income countries was associated with significant reduction of measured blood loss, severe maternal morbidities and mortality and emergency hysterectomy (Miller 2009; Ojengbede 2011).
External aortic compression is an emergency manoeuvre proposed to reduce PPH and permit time for resuscitation and control of bleeding. This technique involves compression of the abdominal aorta using a strong metal spring that is cylindrical in shape and is fixed in place by a leather belt wrapped around the waist (Soltan 2009). It is a cost‐effective and easily applied manoeuvre that allows satisfactory management of PPH (Soltan 2009).
Rationale for the review
The quest for fast, effective and safe interventions in cases of major primary PPH is the focus of this review. Other relevant published Cochrane reviews are Begley 2011, which compares active with expectant third‐stage management; Tunçalp 2012, Cotter 2001, McDonald 2004, Su 2012, Liabsuetrakul 2007 and Oladapo 2012b, which consider the role of different prophylactic uterotonics in third‐stage management; Nardin 2011, which looks at the role of umbilical vein injection in the treatment of retained placenta; Oladapo 2012a, which evaluates advance community distribution of misoprostol for preventing or treating PPH; Novikova 2010, which evaluates the place of tranexamic acid for preventing PPH and Alexander 2002, which is examining drug treatment for secondary PPH. The current review focuses primarily on atonic primary PPH. Management of haemorrhage due to laceration of the genital tract is outside the scope of this review.
Objectives
To determine the effectiveness of any intervention used for the treatment of primary postpartum haemorrhage.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials of treatment for primary postpartum haemorrhage (PPH).
Types of participants
Women after delivery following a pregnancy of at least 24 weeks' gestation with a diagnosis of primary PPH, regardless of mode of delivery (vaginal or caesarean section) or other aspects of third‐stage management. Initially, our protocol stipulated that only studies in which primary PPH was defined as blood loss greater than 500 mL would be included. As it may be difficult to obtain an accurate measurement of blood loss before recruitment, we expanded our inclusion criteria to include trials in which PPH was defined in one of the following ways:
women with blood loss of 500 mL or more and/or
women with primary PPH requiring blood transfusion and/or blood products and/or
women with a clinical diagnosis of primary PPH (as defined by trialists).
Exclusion criteria
Women with PPH with gestational age less than 24 weeks.
Types of interventions
Eligible interventions included:
uterotonic agents that encourage uterine contractility (such as oxytocin, ergometrine, carbetocin and prostaglandins);
haemostatic agents that influence the clotting cascade (tranexamic acid and recombinant activated factor VII);
surgical interventions such as uterine packing or intrauterine catheter insertion, artery ligation, uterine compression sutures and/or hysterectomy;
interventional radiology (X‐ray–guided embolisation);
non‐pneumatic antishock garment (NASG) and aortic compression device; and
any other medical or surgical intervention.
Main comparisons included the following interventions.
Uterotonics versus control (no intervention) or placebo.
One uterotonic agent versus other single or multiple uterotonic drugs.
Haemostatic drugs versus other treatment, or versus control or placebo.
Uterine packing or balloon tamponade (e.g. Foley, hydrostatic catheter) versus other treatment, or versus control or placebo.
Uterine compression sutures (e.g. brace, square) versus other treatment, or versus control or placebo.
Vessel ligation versus other treatment, or versus control or placebo.
Hysterectomy versus other treatment, or versus control or placebo.
Radiological embolisation versus other treatment, or versus control or placebo.
Non‐pneumatic antishock garment (NASG) and aortic compression device versus other treatment, or versus control or placebo.
Any other medical or surgical intervention used for treatment of primary PPH versus other treatment or versus control or placebo.
Control group is defined as a group of participants randomly assigned to not receiving the active medication or factor under study and thereby serving as a comparison group for the intervention. Placebo group is defined as a group of women randomly assigned to receive a dummy treatment.
Treatment for primary PPH requires a multidisciplinary approach. Any measures and/or drug therapy taken as part of the initial treatment is considered first‐line therapy. In most cases, this includes resuscitation measures, exclusion of genital tract laceration, checking of the placenta and the use of uterotonics. Women who continue to bleed require further assessment and interventions to control the bleeding, commonly referred to as second‐line therapy. This may include additional uterotonics, haemostatic drugs, surgical interventions, radiological embolisation and/or compression devices (Abou El Senoun 2011).
Types of outcome measures
Primary outcomes
Maternal mortality.
Serious maternal morbidity (renal or respiratory failure, cardiac arrest or multiple‐organ failure).
Admission to intensive care.
Hysterectomy (provided it is not part of the intervention under investigation).
Secondary outcomes
Outcome measures related to blood loss
Number of women with total blood loss 500 mL or more after enrolment.
Number of women with total blood loss 1000 mL or more after enrolment.
Mean blood loss (mL).
Blood transfusion.
Duration from randomisation to cessation of bleeding or obtaining satisfactory response (as determined by the trialist).
Post‐randomisation additional uterotonic used to control bleeding.
Post‐randomisation surgical intervention used to control bleeding.
Side effects
Side effects of therapy or intervention (such as headache, vomiting, injuries). These will be related to the type of intervention under investigation.
Other
Days in hospital.
Iron therapy in the puerperium.
Secondary PPH (vaginal bleeding after 24 hours to 42 days following delivery).
Interventions to control secondary PPH (medical, surgical or both).
Hospital readmission and number of days in hospital.
Failure to continue breastfeeding at discharge from hospital and at 42 days of delivery.
Economic outcomes.
Maternal dissatisfaction with therapy.
Quality of life, including physiological activity and social and emotional changes.
Assessment of blood loss could vary between trials. It is expected that measurement of blood and blood clots in jars and weighing of linen are likely to be more precise than clinical judgement. The latter is known to underestimate blood loss (Pritchard 1962). The way of reporting the amount of loss as 'greater than' or 'greater than or equal to' a certain cutoff level (e.g. greater than 500 mL or greater than or equal to 500 mL) may affect the total reported amount of blood loss, especially when this amount is estimated. It is expected that trials evaluating uterotonic or haemostatic drugs may use other uterotonics to maintain contractions of the uterus after randomisation. Also, it should be taken into consideration that hysterectomy could be a method of intervention or co‐intervention, as well as an outcome measure.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 August 2013).
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of Embase;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and Embase, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.¬
We did not apply any language restrictions.
Data collection and analysis
For methods used in assessing the trials identified in the previous version of this review, see Mousa 2007.
For this update (2014), we used the following methods when assessing trials identified by the search.
Selection of studies
Two review authors (HAM and GAES or HAM and HS) independently assessed for inclusion all potential studies identified as a result of the search strategy. We resolved any disagreement through discussion and consultation with ZA.
Data extraction and management
HAM designed a special data extraction form. For eligible studies, at least two review authors (HAM and GAES or HAM and HS) extracted data using the agreed form. We resolved any discrepancies through discussion and consultation with ZA.
HAM and GAES entered data into Review Manager software (RevMan 2012) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
In addition to the main outcomes, we systematically extracted the following data for each study.
Trial entry criteria (specific inclusion and exclusion criteria).
Exclusions and missing data after randomisation.
Mode of delivery.
Management of the third stage of labour.
Duration and technique of assessment of blood loss.
Assessment of risk of bias in included studies
HAM and HS independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion with ZA.
(1) Random sequence generation (checking for possible selection bias)
We have described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We have assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number); or
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We have described for each included study in sufficient detail the method used to conceal the allocation sequence and have determined whether intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment.
We have assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth); or
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We have described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. Studies were judged at low risk of bias if they were blinded or if we judged that the lack of blinding could not have affected the results. Blinding was assessed separately for different outcomes or classes of outcomes.
We have assessed the methods as:
low, high or unclear risk of bias for participants; and
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We have described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We have assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
We have described for each included study, and for each outcome or class of outcomes, the completeness of data, including attrition and exclusions from the analysis. We have stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total of randomly assigned participants), reasons for attrition or exclusion where reported and whether missing data were balanced across groups or were related to outcomes. We have contacted authors regarding published data and to request any missing outcome data that was included in our analysis.
We have assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation); or
unclear risk of bias.
(5) Selective reporting bias
We have described for each included study how we investigated the possibility of selective outcome reporting bias and what we have found.
We have assessed the methods as:
low risk of bias (when it was clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (when not all of the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest were reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported); or
unclear risk of bias.
(6) Other sources of bias
We have described for each included study any important concerns that we have about other possible sources of bias.
We have assessed whether each study was free of other problems that could put it at risk of bias.
Low risk of other bias.
High risk of other bias.
Unclear whether there is risk of other bias.
(7) Overall risk of bias
We have made explicit judgements about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to the items listed above, we have assessed the likely magnitude and direction of the bias, and whether we consider it likely to impact the findings.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented the results as summary risk ratios with 95% confidence intervals.
Continuous data
For continuous data, we used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome but used different methods.
Unit of analysis issues
Cluster‐randomised trials
No cluster‐randomised trials were identified for inclusion. In the future, if eligible for inclusion, we will include cluster‐randomised trials in the analyses, along with individually randomised trials. We will adjust sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Section 16.3.4) based on an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and will conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if little heterogeneity is evident between the study designs and if interaction between the effect of the intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and will perform a (sensitivity or subgroup) analysis to investigate the effects of the randomisation unit.
Cross‐over trials
We considered cross‐over designs inappropriate for this review question.
Dealing with missing data
For included studies, levels of attrition were noted. The impact of including studies with high levels of missing data in the overall assessment of treatment effect was explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis (i.e. we have included in the analyses all participants randomly assigned to each group). The denominator for each outcome in each trial was the number randomly assigned minus any participants whose outcomes are known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero or a low P value (less than 0.10) was obtained in the Chi² test for heterogeneity.
Assessment of reporting biases
We planned to assess reporting biases if 10 or more studies were included in the meta‐analysis. In this update (2014), no meta‐analysis included 10 or more studies. In future updates, if more studies are included, we will investigate reporting biases (such as publication bias) using funnel plots. We will visually assess funnel plot asymmetry.
Data synthesis
We have carried out statistical analysis using the Review Manager software (RevMan 2012). We used fixed‐effect meta‐analysis for combining data in cases where it is reasonable to assume that studies are estimating the same underlying treatment effect, that is, when trials are examining the same intervention, and the trials’ populations and methods are judged sufficiently similar. If clinical heterogeneity is sufficient to expect that underlying treatment effects differ between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was to be treated as the average range of possible treatment effects, and we planned to discuss the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials.
If we used random‐effects analyses, results were presented as the average treatment effect with 95% confidence intervals and estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We have carried out subgroup analyses according to route of administration and dose of the drug used for misoprostol trials a priori, irrespective of heterogeneity.
In future updates, with the addition of new trials, if we identify substantial heterogeneity, we plan to investigate it further using the following subgroup analyses.
Mode of delivery (caesarean versus vaginal delivery).
Setting (hospital versus community).
All primary outcome measures will be used in subgroup analyses.
We plan to assess subgroup differences by using interaction tests available within RevMan (RevMan 2012). We will report the results of subgroup analyses by quoting the Chi² statistic and P value, as well as the interaction test I² value.
In the presence of significant heterogeneity (I² > 30%), we will use random‐effects.
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect on trial quality as assessed by concealment of allocation, high attrition rates or both, with poor‐quality studies excluded from the analyses to assess whether this made any difference to the overall result. Poor quality was defined as studies at high risk of bias for allocation concealment and/or incomplete outcome data.
Results
Description of studies
Included studies
Ten randomised clinical trials (RCTs) with a total of 4060 participants fulfilled our inclusion criteria and were included in this review
Uterotonic trials
Eight uterotonic studies were identified and considered for inclusion in this review. Of these, one was excluded (Takagi 1976) because the trial included women with blood loss less than 500 mL and the trial report did not allow analysis based on treatment allocation ('intention to treat'). Seven misoprostol trials were included in the review. Four placebo‐controlled trials compared misoprostol (at doses of 600 to 1000 mcg) versus placebo (1881 participants) among women receiving conventional uterotonics for primary postpartum haemorrhage (PPH) treatment (Hofmeyr 2004; Walraven 2004; Widmer 2010; Zuberi 2008). The main objective of these studies was to assess the effectiveness of the randomly selected drug to result in fewer women having additional blood loss of 500 mL of more. Lokugamage 2001 (64 participants) compared rectally administered misoprostol (800 mcg) versus oxytocics (combined syntometrine and oxytocin infusion) for the treatment of primary PPH, defined as blood loss greater than 500 mL. The main objective of the study was to assess the effectiveness of the randomly selected drug to stop PPH within 20 minutes. The Blum 2010 and Winikoff 2010 trials (1787 participants) compared sublingual misoprostol (800 mcg) versus oxytocin infusion (40 IU infusion) for the treatment of primary PPH among women who had a vaginal delivery with clinically diagnosed or measured blood loss of 700 mL or more within the first hour of delivery. The main objective of these studies was to assess the effectiveness of the randomly selected drug to stop PPH within 20 minutes and/or to result in additional blood loss of at least 300 mL. The latter was restricted to women who had received prophylactic oxytocin during the second or third stage of labour.
Haemostatic trials
Ducloy‐Bouthors 2011 (144 participants) evaluated the place of intravenous tranexamic acid (loading dose 4 g intravenously over one hour, then infusion of 1 g/hour over six hours) among women with primary PPH, defined as measured blood loss of more than 800 mL, following vaginal delivery. All participants with PPH > 500 mL were managed according to French practice guidelines: bladder catheter, manual removal of retained placenta, genital tract examination, uterine exploration and oxytocin (30 U/30 min), followed, and if these procedures were inefficacious, sulprostone was administered (500 μg in one hour) with no procoagulant treatment. Patients with PPH > 800 mL were included in the study. Immediately after inclusion, participants were randomly assigned to receive tranexamic acid (tranexamic acid group) or no antifibrinolytic treatment (control group). The main objective of the study was to assess the effect of randomly assigned tranexamic acid administration on blood loss at 30 minutes, two hours and six hours of administration.
Other drug therapy trials
Zhou 2006 (112 participants) assessed the additional benefit of estrogen adjuvant therapy (4 mg estradiol benzoate injected intramuscularly) for the amount of blood loss at two and 24 hours among women with primary PPH. 4 mg estradiol benzoate injected intramuscularly with routine management when bleeding exceeded 500 mL versus routine management only for the control group. Routine management of the control group was described as 'uterine massage and uterotonics administration' and included '20 U cervical muscle injection to contract the uterus; 20 U intravenous drip to contract the uterus'. In case of the cervical muscles not restoring, injection or intravenous drip did not exceed 80 U.
Surgical trials
Chantrapitak 2009 (64 participants) assessed the amount of blood loss at two hours after randomly assigning women with primary PPH (defined as blood loss 500 mL or more) to lower uterine segment compression in addition to conventional therapy for primary PPH versus conventional therapy alone.
We did not identify any trials related to uterine tamponade, uterine compression suturing techniques, artery ligations or radiological interventions.
For further details of included studies, see table of Characteristics of included studies.
Excluded studies
For details of excluded studies, see table of Characteristics of excluded studies.
Risk of bias in included studies
Please see Figure 1 and Figure 2 for summary of risk of bias assessments.
1.
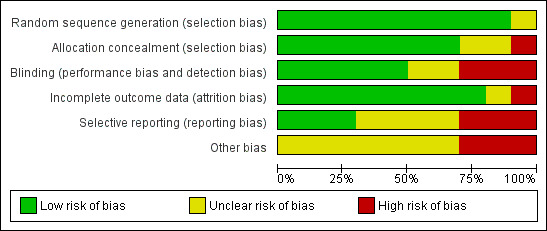
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
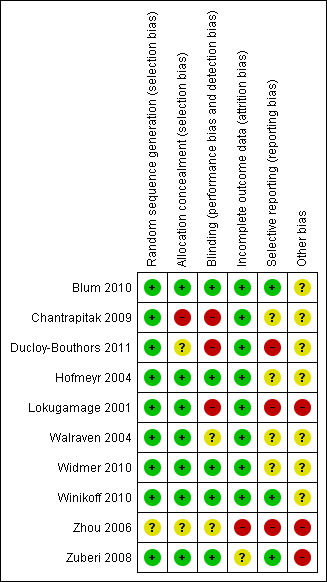
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
The Lokugamage 2001 trial compared misoprostol (800 mcg rectal) versus syntometrine combined with oxytocin infusion for treatment of PPH. The authors described clearly the random generation method and allocation concealment using consecutively numbered, sealed, opaque envelopes. It was a single‐blinded study, as obstetricians were aware of the type of drug given, although women and midwives were not. The trial authors indicated that single blinding was used mainly for safety "to prevent over‐dosage and to know what had been given in case of need of additional drugs". No description was provided of the method of measurement of blood loss or the management of the third stage of labour. The authors have been contacted to request more information. Post‐randomisation withdrawal of one woman (1/32) was reported in the misoprostol arm. The trial was prone to assessment bias, as physicians were aware of the treatment given. Generalisation of the results (external validity) is somewhat limited because effectiveness outcomes such as 'treatment failure' were susceptible to biased ascertainment. Furthermore, the authors performed an interim analysis after 12 months (30 recruited women), and it is unclear whether this information was shared with the clinicians participating in the trial. Therefore, one cannot rule out the possibility that postrandomisation management and outcome assessment were influenced by knowledge of interim results. The study was terminated after an interim analysis revealed an 80% difference between the two treatment arms for the pre‐specified outcome measure (effectiveness at stopping PPH within 20 minutes of trial drugs' administration). Only three outcome measures were adequately reported (hysterectomy, persistent vaginal bleeding following randomisation, medical and surgical co‐interventions). Maternal death was not reported as an outcome. Other reported outcome measures included blood transfusion, length of inpatient stay and drug side effects. However, they were reported as "P value of significance" with no numbers or percentages. No long‐term outcome data were presented.
The Walraven 2004 and Hofmeyr 2004 trials were double‐blinded studies that compared misoprostol (600 mcg in Walraven and 1000 mcg in Hofmeyr, delivered by multiple routes) versus placebo when used as an adjunct to standard uterotonics for the treatment of primary PPH. However, the authors of the former trial believed that blinding may have been compromised by differences in the size of the misoprostol tablets and the placebo. Both trials used active management of the third stage of labour and measured blood loss after administration of conventional oxytocics for primary PPH treatment and the trial drug. In Hofmeyr 2004, six of 244 data sheets did not include pack numbers and could not be included in the analysis. In the Walraven 2004 trial, no withdrawals after enrolment were reported. No long‐term outcome data were presented.
The Zuberi 2008 trial was a multi‐centre double‐blind randomised controlled study that compared sublingual misoprostol (600 mcg) versus placebo when used as an adjunct to standard uterotonics for the treatment of primary PPH. Blinding and allocation concealment were adequate, and participants were randomly assigned in blocks of 10, using a computer‐generated random sequence. Placebo tablets were identical in shape, colour, weight, feel and taste to misoprostol tablets. The study was powered to recruit 900 participants; however, investigators managed to recruit only 61 participants and reported results for 59 of them. The primary outcome measure was measured blood loss of 500 mL or more after treatment. Authors indicated that accurate use of the scales for assessment of blood loss proved difficult. Therefore, volume of blood was not analysed; instead measurement according to reading of the blood collection device was recorded and analysed. No long‐term outcome data were presented.
The Widmer 2010 trial was a multicentre double‐blind randomised controlled study that compared sublingual misoprostol (600 mcg) versus placebo when used as an adjunct to standard uterotonics for the treatment of primary PPH. Investigators used computer‐generated randomisation sequence in blocks of six and eight, stratified by country. Overall, blinding and allocation concealment were adequate. Placebo tablets were identical in shape, colour, weight, feel and taste to misoprostol tablets. A total of 1422 women were recruited to the study, three women did not receive interventions and five women were lost to follow‐up at 90 minutes, as blood loss was not recorded. The study was powered to measure impact on blood loss. Methods of blood collection and measurement varied between centres. However, trial authors indicated that some of the methods used had been previously evaluated in the World Health Organization trial of misoprostol for the prevention of PPH (Gülmezoglu 2001). Both groups received standard uterotonics for the treatment of primary PPH. No long‐term outcome data were presented.
The Blum 2010 and Winikoff 2010 trials were double‐blind randomised controlled trials that compared sublingual misoprostol (800 mcg) versus oxytocin infusion (40 IU in one 1000 mL of saline over 15 minutes). Both described clearly their methods of allocation concealment and blinding and used similar inclusion and exclusion criteria. Placebo tablets were identical in shape, colour, weight, feel and taste to misoprostol tablets. However, the latter trial included only participants for whom oxytocic drugs were not administered during the second and third stages of labour. They used cessation of active bleeding within 20 minutes after initial treatment and additional blood loss of 300 mL or more as primary end points and reported outcomes in 100% of cases. No long‐term outcome data were reported.
The Ducloy‐Bouthors 2011 trial was an open‐label randomised, controlled study. It was liable to selection and performance bias. Partial blinding was achieved, as obstetricians, midwives and participants were not aware of interventions used. However, anaesthetists were aware of the intervention and were responsible for randomisation and administration of the trial drug. It is unclear how the allocated intervention was concealed, as intravenous infusion would be visible to all. Investigators recruited 152 participants, but one was excluded, as it was found later that she did not fulfil the inclusion criteria. Protocol violations were reported for seven women (five in the tranexamic acid group and two in the control group), and the analysis reported on 144 participants (72 participants in each group). The study was not powered to measure any of our primary outcome measures. Investigators reported few long‐term outcome data.
The Zhou 2006 trial was a randomised controlled study in which women were randomly assigned to conventional therapy versus estrogen adjuvant therapy in addition to conventional therapy. No description of methods of randomisation and blinding was provided. The study was underpowered to measure any impact on primary outcome measures. Investigators reported impact of outcome on blood loss and hysterectomy. However, the method used for measurement of blood loss was not described.
The Chantrapitak 2009 trial was a randomised controlled study in which women were randomly assigned to lower uterine segment compression in addition to conventional therapy or conventional therapy only. Authors were contacted to clarify randomisation, and they have indicated that it occurred through random generation using opaque concealed envelopes. However, the study is prone to concealment bias, as clinicians were aware of interventions used. The trial was underpowered to measure impact on primary outcome measures.
Effects of interventions
Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics (four trials, comparison 1)
Sublingual misoprostol at a dose of 600 mcg was used by Zuberi 2008 and Widmer 2010 (total of 1483 women), in addition to conventional uterotonics, among women treated for primary PPH. A total dose of 600 mcg (200 mcg oral and 400 mcg sublingual) misoprostol was used simultaneously in Walraven 2004 (160 participants), and Hofmeyr 2004 (238 participants) used 1000 mcg misoprostol simultaneously (200 mcg oral, 400 mcg sublingual and 400 mcg rectal).
Primary outcomes
Compared with placebo, misoprostol conferred no additional benefit in terms of reduction in the rate of maternal mortality (risk ratio (RR) 6.16, 95% confidence interval (CI) 0.75 to 50.85; 5/930 versus 0/951; Analysis 1.1) and hysterectomy (average RR 0.93, 95% CI 0.16 to 5.41; random‐effects, Tau² = 0.83, I² = 33%; 5/930 versus 5/951; Analysis 1.4). Only Widmer 2010 and Zuberi 2008 reported serious maternal morbidity (RR 0.34, 95% CI 0.01 to 8.31; 0/734 versus 1/749; Analysis 1.2) and admission to the intensive care unit (RR 0.79, 95% CI 0.30 to 2.11; 7/734 versus 9/749; Analysis 1.3).
1.1. Analysis.
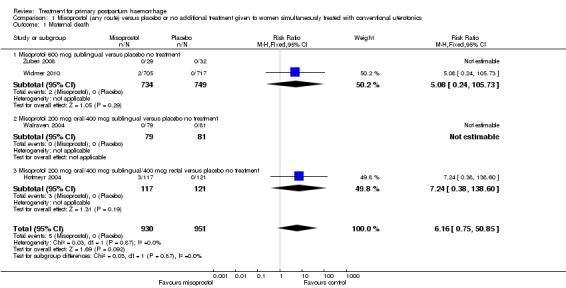
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 1 Maternal death.
1.4. Analysis.
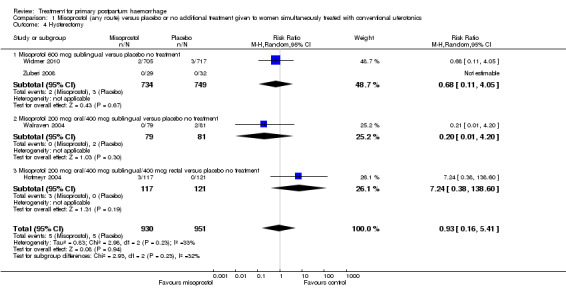
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 4 Hysterectomy.
1.2. Analysis.
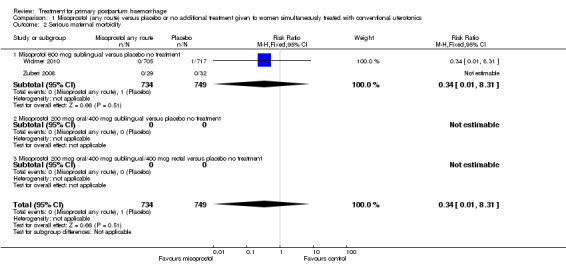
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 2 Serious maternal morbidity.
1.3. Analysis.
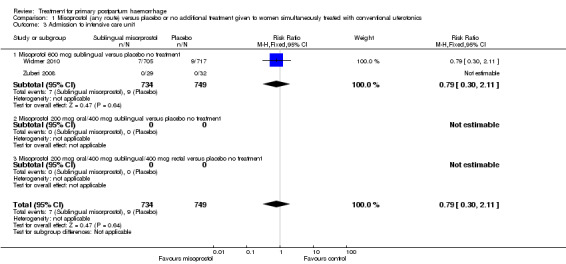
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 3 Admission to intensive care unit.
Secondary outcomes
Compared with placebo, misoprostol administered in addition to conventional uterotonics had no significant impact on blood loss of at least 500 mL (RR 0.89, 95% CI 0.71 to 1.12; 121/930 versus 138/950; Analysis 1.6), blood loss of at least 1000 mL (RR 0.88, 95% CI 0.42 to 1.86; 12/930 versus 14/950; Analysis 1.8) or blood transfusion (RR 0.95, 95% CI 0.77 to 1.17; 139/928 versus 150/949; Analysis 1.7).
1.6. Analysis.
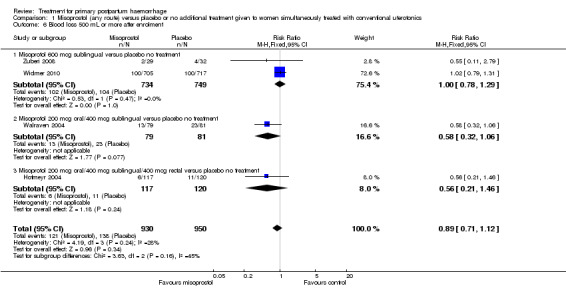
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 6 Blood loss 500 mL or more after enrolment.
1.8. Analysis.
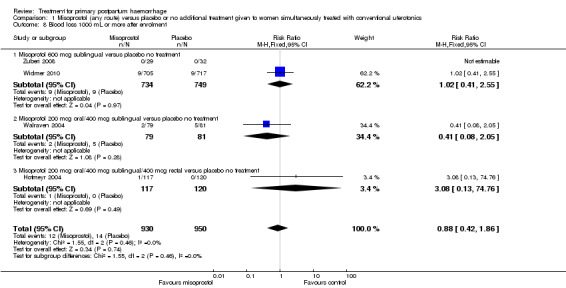
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 8 Blood loss 1000 mL or more after enrolment.
1.7. Analysis.
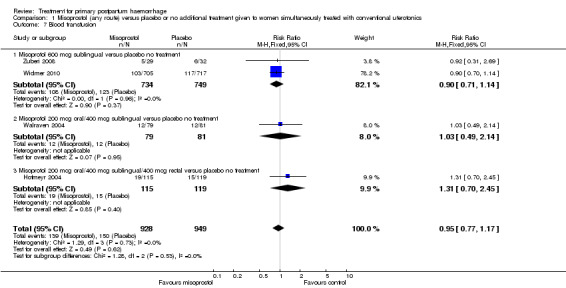
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 7 Blood transfusion.
Side effects
Compared with placebo, misoprostol intake by any route was associated with a significant increase in vomiting (RR 1.84, 95% CI 1.16 to 2.95; Analysis 1.18), shivering (average RR 2.25, 95% CI 1.76 to 2.88; heterogeneity: Tau² = 0.02; Chi² = 4.71, df = 3 (P = 0.19); I² = 36%; Analysis 1.23), maternal pyrexia of at least 38°C (RR 3.12, 95% CI 2.66 to 3.67; Analysis 1.20) and maternal pyrexia of 40°C or more (RR 13.58, 95% CI 4.93 to 37.44; Analysis 1.21).
1.18. Analysis.
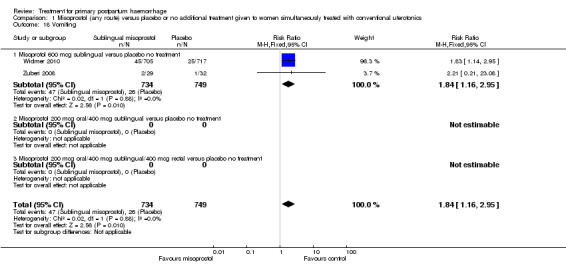
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 18 Vomiting.
1.23. Analysis.
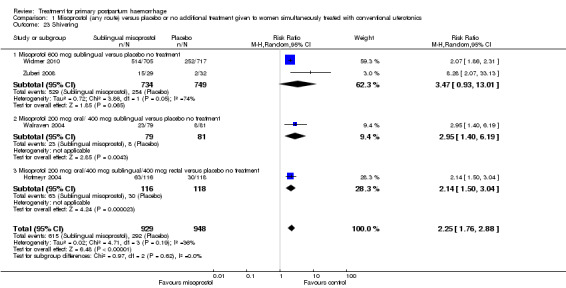
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 23 Shivering.
1.20. Analysis.
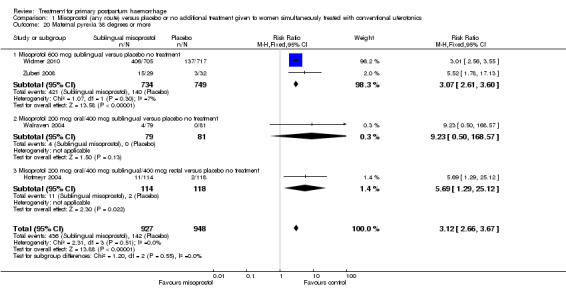
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 20 Maternal pyrexia 38 degrees or more.
1.21. Analysis.
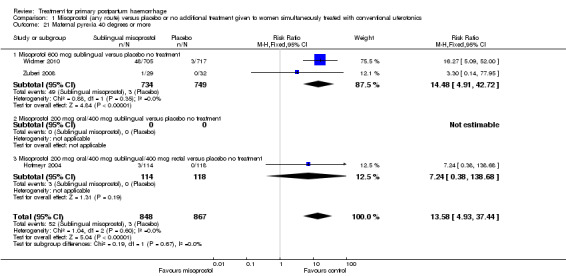
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 21 Maternal pyrexia 40 degrees or more.
Misoprostol versus other uterotonics given to women who have not received any conventional uterotonic therapy (three trials, comparisons 2 and 3)
Sublingual misoprostol (800 mcg) was compared with oxytocin infusion (40 IU) in two trials (Blum 2010; Winikoff 2010; 1787 women total). The latter was restricted to women who had received prophylactic oxytocin during the second or third stage of labour.
Lokugamage 2001 compared rectal misoprostol (800 mcg) with a combination of oxytocin infusion and syntometrine (64 women).
Primary outcomes
In the Blum 2010 and Winikoff 2010 trials, no significant differences were noted between the two groups for any of the primary outcomes: maternal mortality (RR 0.99, 95% CI 0.06 to 15.74; Analysis 2.1), hysterectomy (RR 1.98, 95% CI 0.36 to 10.72; 4/895 versus 2/892; Analysis 2.4), admission to intensive care unit (RR 0.33, 95% CI 0.01 to 8.06; 0/895 versus 1/892; Analysis 2.3) and serious maternal morbidity (RR 0.33, 95% CI 0.01 to 8.06; 0/895 versus 1/892; Analysis 2.2).
2.1. Analysis.
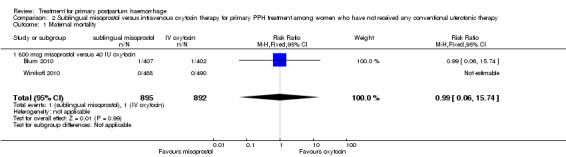
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 1 Maternal mortality.
2.4. Analysis.
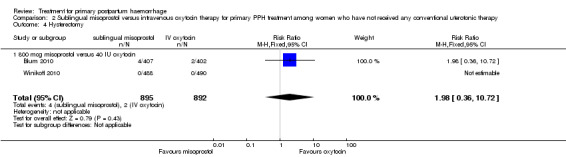
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 4 Hysterectomy.
2.3. Analysis.
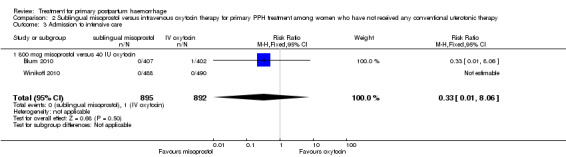
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 3 Admission to intensive care.
2.2. Analysis.
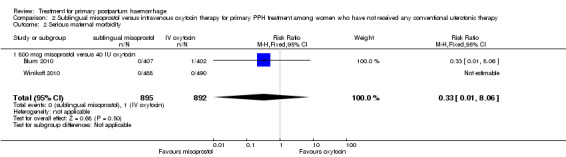
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 2 Serious maternal morbidity.
In Lokugamage 2001, the rate of hysterectomy did not differ between the two groups (RR 0.33, 95% CI 0.01, 7.89; 0/32 versus 1/32; Analysis 3.1). However, the authors did not report rates of maternal morbidity, mortality or admission to the intensive care unit.
3.1. Analysis.
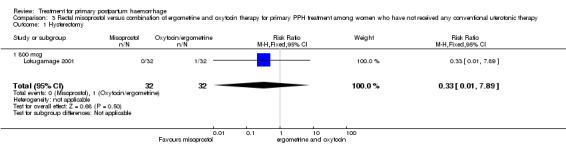
Comparison 3 Rectal misoprostol versus combination of ergometrine and oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 1 Hysterectomy.
Secondary outcome measures
Compared with oxytocin infusion, sublingual misoprostol use was associated with a significant increase in the number of women who had blood loss of at least 1000 mL (RR 2.65, 95% CI 1.04 to 6.75; Analysis 2.7) and blood transfusion (RR 1.47, 95% CI 1.02 to 2.14; Analysis 2.8). However, no significant differences were associated with blood loss of at least 500 mL (average RR 1.51, 95% CI 01.14 to 2.00; heterogeneity: Chi² = 8.54, df = 1 (P = 0.003); I² = 88%; Analysis 2.5) and postrandomisation use of additional uterotonics to control bleeding (average RR 1.30, 95% CI 0.57 to 2.94; random‐effects, Tau² = 0.30, I² = 88%; Analysis 2.10, analysed using a random‐effects model because of substantial heterogeneity). No significant differences were noted between the two groups regarding the number of women who required examination under anaesthesia, bimanual compression or surgical intervention to control bleeding.
2.7. Analysis.
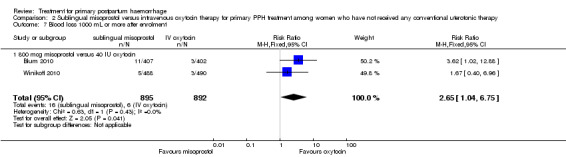
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 7 Blood loss 1000 mL or more after enrolment.
2.8. Analysis.
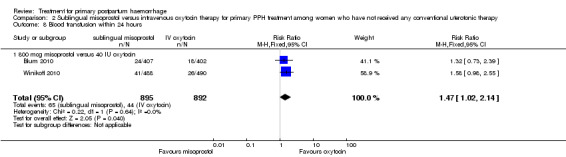
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 8 Blood transfusion within 24 hours.
2.5. Analysis.
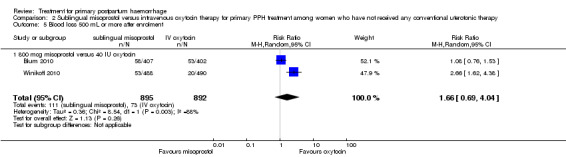
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 5 Blood loss 500 mL or more after enrolment.
2.10. Analysis.
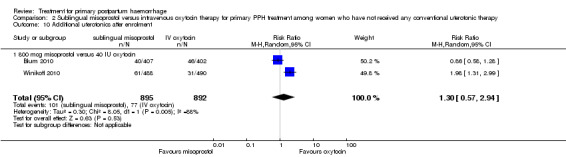
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 10 Additional uterotonics after enrolment.
The Lokugamage 2001 trial found that rectal misoprostol (800 mcg) was more effective than combined oxytocin and syntometrine in decreasing the need for additional uterotonics (RR 0.18, 95% CI 0.04, 0.76; Analysis 3.3). No significant differences in any other pre‐specified secondary outcomes were reported.
3.3. Analysis.
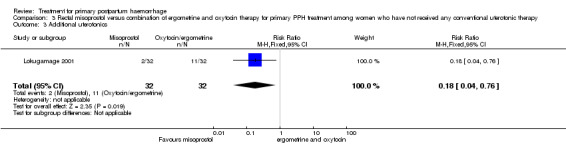
Comparison 3 Rectal misoprostol versus combination of ergometrine and oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 3 Additional uterotonics.
Side effects
Sublingual misoprostol use in 800 mcg was consistently associated with significantly higher rates of prostaglandin‐related side effects such as vomiting and shivering.
Estrogen adjuvant therapy trials (one trial, comparison 4)
Estrogen therapy (4 mg estradiol benzoate injected intramuscularly) in addition to conventional PPH treatment was evaluated in one single‐centre trial (Zhou 2006, 112 women). 4 mg estradiol benzoate was injected intramuscularly when routine management was ineffective and bleeding exceeded 500 mL versus routine management only. Management of the control group was described as 'uterine massage and uterotonics administration' and included '20 U cervical muscle injection to contract the uterus; 20 U intravenous drip to contract the uterus. In case of the cervical muscles not restoring, injection or intravenous drip did not exceed 80 U. Where rate of blood loss exceeded 2000 mL, hysterectomy was performed'.
Primary outcomes
Three women in the control group and no women in the estrogen group required hysterectomy (RR 0.16, 95% CI 0.01 to 3.11; Analysis 4.1).
4.1. Analysis.
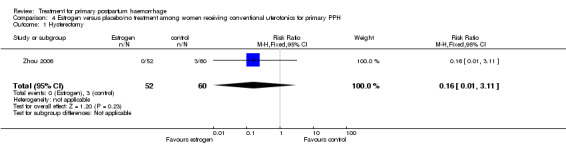
Comparison 4 Estrogen versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 1 Hysterectomy.
Secondary outcomes
We have included two additional measures to assess blood loss, as authors did not report any of our pre‐specified secondary outcome measures. The authors reported a significant reduction in blood loss within two hours (‐274.90 mL mean difference (MD), 95% CI ‐384.72 to ‐165.08 mL; Analysis 4.2) and between two and 24 hours from intervention (‐50.70 mL MD, 95% CI ‐83.07 to ‐18.33 mL; Analysis 4.3).
4.2. Analysis.

Comparison 4 Estrogen versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 2 Mean blood loss within two hours.
4.3. Analysis.
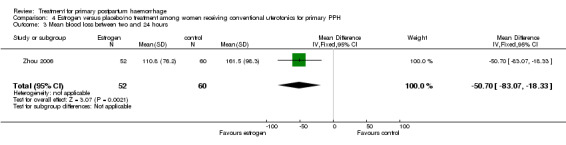
Comparison 4 Estrogen versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 3 Mean blood loss between two and 24 hours.
Tranexamic acid (one trial, comparison 5)
Ducloy‐Bouthors 2011 is the only placebo‐controlled trial of tranexamic acid (144 women).
Primary outcomes
No maternal deaths were reported in the study population. No significant difference was noted between the two groups regarding serious maternal morbidity (RR 0.33, 95% CI 0.01 to 8.05; 0/72 versus 1/72; Analysis 5.2), admission to the intensive care unit (RR 0.60, 95% CI 0.15 to 2.42; 3/72 versus 5/72; Analysis 5.3) and hysterectomy (RR 0.33, 95% CI 0.01 to 8.05; 0/72 versus 1/72; Analysis 5.4).
5.2. Analysis.
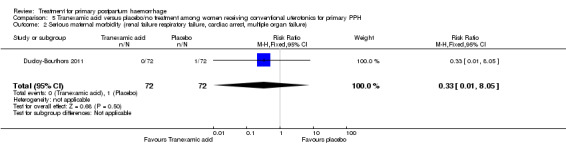
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 2 Serious maternal morbidity (renal failure respiratory failure, cardiac arrest, multiple organ failure).
5.3. Analysis.
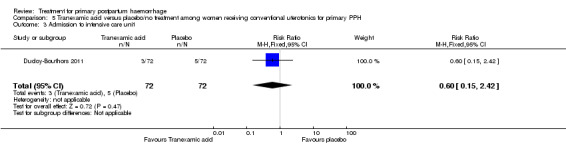
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 3 Admission to intensive care unit.
5.4. Analysis.
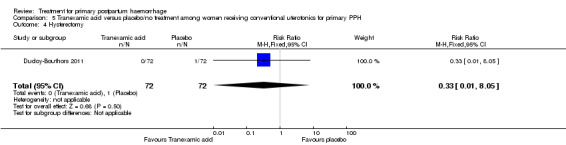
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 4 Hysterectomy.
Secondary outcomes
No significant differences were observed in any of the pre‐specified secondary outcome measures between women with primary PPH treated with tranexamic acid and those given placebo.
Side effects
Nausea was common with tranexamic acid administration (RR 11.00, 95% CI 1.46 to 82.99; Analysis 5.18). Three cases of deep vein thrombosis were reported in the study population (two in the tranexamic acid group and one in the control group; RR 2.00, 95% CI 0.19 to 21.57; Analysis 5.21).
5.18. Analysis.
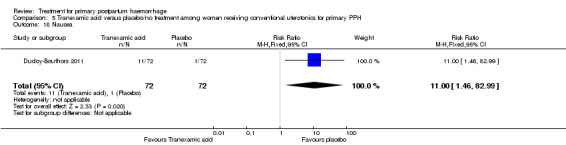
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 18 Nausea.
5.21. Analysis.
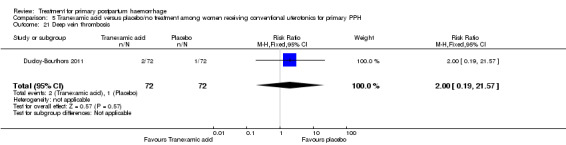
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 21 Deep vein thrombosis.
Lower uterine segment compression versus no compression (one trial, comparison 6)
Chantrapitak 2009 compared lower uterine segment compression versus no intervention (64 women).
Primary outcomes
No maternal death, admission to the intensive care unit, serious maternal morbidity or hysterectomy was reported in the two groups.
Secondary outcomes
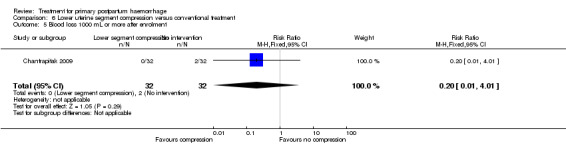
The number of women who had blood loss of at least 500 mL was significantly lower among women who had lower segment compression (RR 0.13, 95% CI 0.02 to 0.94; Analysis 6.4). However, no difference was observed between the two groups regarding mean blood loss (‐105.00 mL MD, 95% CI ‐262.00 to 52 mL; Analysis 6.6), rate of blood transfusion (RR 2.33, 95% CI 0.66 to 8.23; Analysis 6.7), number of women with blood loss of at least 1000 mL (RR 0.20, 95% CI 0.01 to 4.01; Analysis 6.5) and number who required surgical co‐intervention to control the bleeding (no events in either group, so not estimable) (Analysis 6.8).
6.4. Analysis.
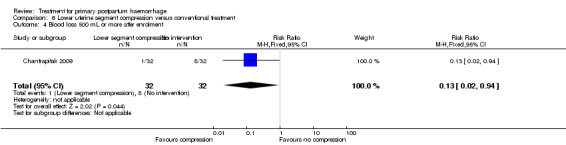
Comparison 6 Lower uterine segment compression versus conventional treatment, Outcome 4 Blood loss 500 mL or more after enrolment.
6.6. Analysis.
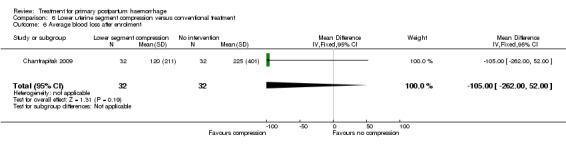
Comparison 6 Lower uterine segment compression versus conventional treatment, Outcome 6 Average blood loss after enrolment.
6.7. Analysis.
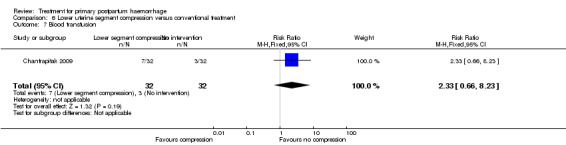
Comparison 6 Lower uterine segment compression versus conventional treatment, Outcome 7 Blood transfusion.
6.5. Analysis.
Comparison 6 Lower uterine segment compression versus conventional treatment, Outcome 5 Blood loss 1000 mL or more after enrolment.
6.8. Analysis.
Comparison 6 Lower uterine segment compression versus conventional treatment, Outcome 8 Other surgical interventions to control bleeding (other than hysterectomy).
Discussion
Summary of main results
The current review evaluated 10 clinical trials that fulfilled our inclusion criteria. None was adequately powered to address our primary outcome measures. Seven uterotonics trials evaluated the use of misoprostol for the treatment of primary PPH. Data from this review show that when misoprostol was compared with placebo (four clinical trials), intravenous oxytocin (two trials) or combined oxytocin and ergometrine (one trial), no statistically significant differences were seen in clinically important outcomes including maternal mortality, serious maternal morbidity, admission to intensive care and hysterectomy. Secondary outcomes, such as blood loss greater than 1000 mL and use of additional uterotonics, favoured intravenous oxytocin over misoprostol. The review suggests that conventional primary PPH treatment with intravenous oxytocin should be recommended as the more effective treatment.
The occurrence of five maternal deaths in the group of studies comparing misoprostol versus placebo is unexpected (5/930 versus 0/930; RR 6.16; 95% CI 0.75 to 50.85). This prompted Hofmeyr and colleagues to examine the frequency of maternal deaths among 40,000 participants in 46 clinical trials of misoprostol used for the prevention or treatment of PPH (Hofmeyr 2009). Of 11 maternal deaths reported in five clinical trials, eight occurred in women receiving misoprostol (odds ratio 2.49, 95% CI 0.67 to 8.13; Hofmeyr 2009). Subsequent trials comparing sublingual misoprostol versus intravenous oxytocin for treatment of primary PPH (Blum 2010; Winikoff 2010) have been more reassuring, with one maternal death reported in each group (1/895 versus 1/895; RR 0.99, 95% CI 0.06 to 15.74). Furthermore, another recent randomised trial comparing misoprostol versus placebo for PPH prevention reported no maternal deaths (Mobeen 2011).
Tranexamic acid has been used for the prevention of haemorrhage for quite some time (CRASH‐2 trial collaborators 2010; Ferrer 2009; Novikova 2010; Peitsidis 2011), but Ducloy‐Bouthors 2011 was the first published randomised trial that examined the use of tranexamic acid to treat primary PPH. The study is underpowered to assess any impact on pre‐specified primary outcome measures. The Zhou 2006 trial of estrogen adjuvant therapy was not big enough to evaluate the impact on primary and secondary outcomes. In the absence of any pharmacological studies to support this current approach, it is sensible to avoid any estrogen therapy for PPH, especially as risk of deep vein thrombosis is increased in the immediate postpartum period.
Chantrapitak 2009 described two new techniques to control blood loss through transabdominal compression of the lower uterine segment. These techniques appear simple and safe, do not require special skills and have no major side effects. Unfortunately, the authors did not specify which technique they used during the trial period. Lower uterine segment compression was associated with a modest reduction in mean blood loss and blood loss of at least 500 mL. The method warrants further evaluation, as it could be of considerable benefit in the management of women who develop primary PPH following home birth and require hospital transfer for further management.
We focused on four parameters to evaluate the impact of PPH treatments on postrandomisation blood loss: (1) blood loss of at least 500 mL; (2) blood loss of at least 1000 mL; (3) blood transfusion and (4) mean blood loss after enrolment. In this regard, misoprostol provided no additional benefit when compared with placebo when given to women simultaneously treated with conventional uterotonics. Misoprostol was evaluated as an effective and easy to administer alternative to intravenous oxytocin as first‐line therapy for the treatment of primary PPH in two other trials (Blum 2010; Winikoff 2010). Compared with 40 IU oxytocin infusion, 800 mcg sublingual misoprostol was associated with a significant increase in the number of women who had blood loss of at least 1000 mL (RR 2.65, 95% CI 1.04 to 6.75), blood transfusion (RR 1.47, 95% CI 1.02 to 2.14) and mean blood loss (mL) (MD 44.86, 95% CI 26.50, 63.22). Therefore, where available, oxytocin infusion should be recommended as first‐line treatment for primary PPH. Lack of significant differences in primary outcomes suggests that, when oxytocin infusion is not available, sublingual misoprostol may serve as a valid alternative for providers seeking a uterotonic therapy for their patients. No significant difference was reported between tranexamic acid and placebo in the Ducloy‐Bouthors 2011 study in terms of women who had blood loss of at least 500 mL or at least 1000 mL. This is consistent with the results of two systematic reviews (Ferrer 2009; Novikova 2010) that evaluated the use of tranexamic acid for the prevention of PPH.
Agreements and disagreements with other studies or reviews
Several potential reasons may explain why misoprostol randomised trials have not confirmed optimistic preliminary results from observational studies (Abdel‐Aleem 2001; Adekanmi 2001; O'Brien 1998; Oboro 2003). In previous reports, blood loss was subjectively assessed, but in the seven trials included in this review, blood loss was measured objectively. This is particularly important, as lack of blinding in previous studies may have affected the perception of effectiveness. The pharmacokinetics of misoprostol may also be a contributing factor: Variation in the route and dose of administration may result in significant variation in plasma therapeutic levels of the drug. Current evidence suggests that the oral route provides the advantage of rapid onset of action, although the vaginal and rectal routes confer the advantage of prolonged activity and greater bioavailability. The sublingual route possesses both of these advantages with a rapid onset of action, prolonged activity and greater bioavailability (Abdel‐Aleem 2003; Andolina 2003; Danielsson 1999; Hofmeyr 2005; Khan 2003; Tang 2002; Zieman 1997).
Misoprostol intake was associated with a significant increase in prostaglandin‐mediated side effects including maternal pyrexia (at least 38°C or at least 40°C), vomiting and shivering. Side effects appear to be dose dependent. Maternal pyrexia, in particular, is very rare when low‐dose misoprostol is used for induction of labour or termination of pregnancy (Alfirevic 2006; Dodd 2010). These side effects appear to be associated with high doses of misoprostol and may impact the management of patients with major obstetrical haemorrhage. For instance, blood transfusion forms an essential part of fluid resuscitation in women with major PPH, and a rise in body temperature following misoprostol use could be incorrectly labelled as a “transfusion reaction”, with subsequent stoppage of transfusion having a major impact on the general condition of the patient. Similarly, maternal pyrexia could mistakenly be labelled as “maternal sepsis”, which may result in the commencement of unnecessary intravenous antibiotic therapy.
Three cases of deep vein thrombosis were reported in the Ducloy‐Bouthors 2011 study: two in the tranexamic acid group and one in the control group (RR 2.00, 95% CI 0.19 to 21.57). It is difficult to draw any conclusion regarding safety and risk of thromboembolic complications after tranexamic acid administration. It is noteworthy that several large studies did not observe any significant increase in the risk of thromboembolism (CRASH‐2 trial collaborators 2010; Peitsidis 2011).
In this current update, we have not included postrandomisation haemoglobin level or disseminated intravascular coagulopathy (DIC) as an outcome of interest for several reasons. First, blood and clotting factor transfusions form an essential part of any primary PPH resuscitation protocol in women with massive obstetrical haemorrhage. Therefore, a corrected haemoglobin level and/or clotting factors may simply confirm adequate resuscitation, rather than effectiveness of the uterotonics or the intervention. Second, postdelivery haemoglobin level is directly related to pre‐delivery levels rather than the impact of intervention on blood loss. Ideally, one should measure the drop in haemoglobin and/or hematocrit levels before delivery and the first blood transfusion. However, as primary PPH occurs unexpectedly, often among low‐risk women, pre‐delivery haemoglobin and/or hematocrit levels usually are not available. The trials by Winikoff 2010, Blum 2010 and Zuberi 2008 have set an example by checking haemoglobin concentration for all labouring women before birth and after administration of the trial drug. However, this will be very difficult to replicate in other large pragmatic trials. Currently, lack of agreement has been noted between clinicians regarding the definition of DIC (Gando 2006).
Two excluded quasi‐randomised trials from the same centre (Soltan 2009; Soltan 2010) evaluated the use of external aortic compression devices in addition to conventional therapy in the treatment of primary PPH. Investigators observed less blood transfusion when this device was used (RR 0.55, 95% CI 0.45 to 0.66), but the studies were too small to show an impact on other substantive outcomes. Although the use of external aortic compression devices was associated with abdominal discomfort and numbness and a tingling sensation, the lack of any short‐ or long‐term Ischaemic manifestations was quite reassuring. Currently, interest in evaluating the non‐pneumatic antishock garment (NASG) is growing, especially in low‐resource areas. With brief training, it appears that individuals without a medical background can use this first‐aid device. Miller and colleagues examined the use of NASG in a pre‐intervention/intervention study involving 1442 participants with hypovolaemic shock secondary to obstetrical haemorrhage from any cause and an estimated blood loss of at least 750 mL (Miller 2010). The NASG intervention was associated with a significant reduction in measured blood loss, maternal mortality, severe morbidities and emergency hysterectomy.
The question related to the management of women with major primary PPH who remain unresponsive to medical management with uterotonics and/or tranexamic acid therapy remains largely unanswered. In the absence of randomised controlled trials, clinicians are left to make their own judgement on the best combination of surgical, radiological and/or pharmaceutical interventions to control bleeding. Large double‐blind, multi‐centre, randomised controlled trials are needed to evaluate the effects of surgical interventions and/or radiological interventions on the primary outcome measures; however, the inability to obtain informed consent from critically ill patients may make it difficult to recruit participants. Clinicians should be encouraged to conduct such trials provided that they are able to follow agreed procedures for getting consent from critically ill patients and to ensure that recruitment does not interfere with standard clinical management.
Authors' conclusions
Implications for practice.
Primary PPH is a life‐threatening condition and availability of first‐aid treatment (IV line, parenteral fluids and uterotonics) is crucial. Current evidence suggests that intravenous oxytocin should be used as first‐line therapy for the treatment of primary PPH due to uterine atony. Evidence suggests that misoprostol is less effective than oxytocin and provides no additional uterotonic effect when used simultaneously with conventional oxytocin treatment. Efforts should be made to make injectable oxytocin available for use at deliveries occurring outside of facilities. When injectable oxytocin is not available, misoprostol can be used.
Variation in dose regimens between the seven different misoprostol studies made it difficult for the review authors to draw clear conclusions regarding the most effective dose or route. As first‐line treatment, the largest body of evidence available supports the safety and effectiveness of an 800‐mcg sublingual dose. As an adjunct treatment to standard oxytocin infusion, various routes were examined, but none proved to be beneficial, so no regimen is suggested at this time. In general, the use of higher doses should be balanced against the likelihood of a greater incidence of maternal side effects associated with misoprostol. A system of "adverse event registration" might be helpful in identifying and tracking serious maternal morbidity and mortality associated with the use of all uterotonics in clinical practice.
Use of tranexamic acid in routine clinical practice is under investigation. The results of one ongoing trial, the WOMAN trial, should be large enough to provide information on the effectiveness and safety of this drug for women with primary PPH (Shakur 2010).
Lower segment compression is a simple and promising method, particularly when unwell patients with major haemorrhage are transferred between centres in low‐resource settings, where access to blood services is limited. Results from field studies of these methods will be included in a future review.
We are unable to provide any guidance regarding the management of women with primary PPH who fail to respond to uterotonics and/or haemostatic drug therapies. However, it is logical to consider conservative surgical techniques and/or radiological interventions in an effort to avoid hysterectomy. The main challenge is to ensure that adequate clinical expertise is available at all times to determine when the conservative approach should be abandoned in favour of hysterectomy in a timely fashion.
Implications for research.
High‐quality studies with adequate power are urgently needed to address our primary outcome measures. Future interventions addressing management of PPH at the community level would also be useful. Studies seeking to identify appropriate uterotonic management of primary PPH following home deliveries, particularly in developing countries, are of particular interest.
Currently, no randomised data are available on the effectiveness of carbetocin, a long‐acting synthetic oxytocin analogue, but recent reports suggest that it may be of benefit in the prevention of primary PPH (Su 2012) and, therefore, further research would be justified.
Three areas of research would be of particular interest for women with primary PPH unresponsive to uterotonics. First, further work is needed to identify the most effective tamponade procedures and uterine haemostatic suturing techniques in women with major primary PPH. Second, aortic compression devices and the non‐pneumatic antishock garment should be tested further in patients with major obstetrical haemorrhage. Finally, the benefits of interventional radiology for women at increased risk of bleeding during delivery and for those who bleed following childbirth should be critically evaluated in randomised trials. Ideally, first‐line uterotonics and second‐line surgical intervention trials should be conducted in both developed and developing countries.
What's new
| Date | Event | Description |
|---|---|---|
| 11 September 2017 | Amended | Added Published notes to explain that this review will no longer be updated in it's current form. |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 1, 2003
| Date | Event | Description |
|---|---|---|
| 31 August 2013 | New search has been performed | Search updated (31 August 2013). Seven new studies incorporated into review (Blum 2010; Chantrapitak 2009; Ducloy‐Bouthors 2011; Widmer 2010; Winikoff 2010; Zhou 2006; Zuberi 2008). One study awaiting classification (Lavigne‐Lissalde 2013), and five studies ongoing (Collins 2013; Miller 2008; Mirzazada 2011; Shakur 2010; Wikkelsoe 2012). Methods updated. |
| 31 August 2013 | New citation required and conclusions have changed | Additional data from new studies now suggest that in comparison with oxytocin, women given sublingual misoprostol are more likely to have greater blood loss. For other outcomes, the conclusions remain the same: misoprostol in comparison with placebo has no impact on maternal mortality, maternal morbidity, hysterectomy, and admission to intensive care; sublingual misoprostol in comparison with oxytocin increases the likelihood of adverse effects such as vomiting and shivering. |
| 8 May 2008 | Amended | Converted to new review format. |
| 14 November 2006 | New citation required and conclusions have changed | Substantive amendment |
Notes
This review will not be updated in it's current form. The review will be split into a number of reviews based on different types of treatment for postpartum haemorrhage. Once those reviews have been published we will add links to those reviews here.
Acknowledgements
We are very grateful for the corresponding authors and research teams of the following trials: Blum 2010; Chantrapitak 2009; Ducloy‐Bouthors 2011; Hofmeyr 2004; Widmer 2010; and Winikoff 2010, who completed our data collection forms.
As part of the pre‐publication editorial process, this review has been commented on by three peers (an editor and two referees external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the NIHR, the NHS or the Department of Health.
Data and analyses
Comparison 1. Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal death | 4 | 1881 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.16 [0.75, 50.85] |
| 1.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.08 [0.24, 105.73] |
| 1.2 Misoprotol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Misoprotol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.24 [0.38, 138.60] |
| 2 Serious maternal morbidity | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.31] |
| 2.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.31] |
| 2.2 Misoprostol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Misoprostol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Admission to intensive care unit | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.30, 2.11] |
| 3.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.30, 2.11] |
| 3.2 Misoprostol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Misoprostol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Hysterectomy | 4 | 1881 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.16, 5.41] |
| 4.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.11, 4.05] |
| 4.2 Misoprotol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 0.20 [0.01, 4.20] |
| 4.3 Misoprotol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 1 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 7.24 [0.38, 138.60] |
| 5 Average blood loss after enrolment in millilitres | 4 | 1880 | Mean Difference (IV, Fixed, 95% CI) | ‐3.87 [‐23.63, 15.88] |
| 5.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Mean Difference (IV, Fixed, 95% CI) | 1.18 [‐21.61, 23.98] |
| 5.2 Misoprotol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 1 | 160 | Mean Difference (IV, Fixed, 95% CI) | ‐85.0 [‐189.23, 19.23] |
| 5.3 Misoprotol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 1 | 237 | Mean Difference (IV, Fixed, 95% CI) | ‐8.0 [‐50.78, 34.78] |
| 6 Blood loss 500 mL or more after enrolment | 4 | 1880 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.71, 1.12] |
| 6.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.78, 1.29] |
| 6.2 Misoprotol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.32, 1.06] |
| 6.3 Misoprotol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 1 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.21, 1.46] |
| 7 Blood transfusion | 4 | 1877 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.77, 1.17] |
| 7.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.71, 1.14] |
| 7.2 Misoprotol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.49, 2.14] |
| 7.3 Misoprotol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 1 | 234 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.70, 2.45] |
| 8 Blood loss 1000 mL or more after enrolment | 4 | 1880 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.42, 1.86] |
| 8.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.41, 2.55] |
| 8.2 Misoprotol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.08, 2.05] |
| 8.3 Misoprotol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 1 | 237 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.08 [0.13, 74.76] |
| 9 Additional uterotonics | 4 | 1866 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.84, 1.08] |
| 9.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 9.2 Misoprotol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.15, 2.49] |
| 9.3 Misoprotol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 1 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.80, 1.27] |
| 10 Manual removal of the placenta after enrolment | 2 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.44, 1.08] |
| 10.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.44, 1.08] |
| 10.2 Misoprostol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Misoprostol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Uterine tamponade after enrolment | 2 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.07, 1.40] |
| 11.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 0.32 [0.07, 1.40] |
| 11.2 Misoprostol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Misoprostol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Artery ligation (uterine and/or hypogastric arteries) after enrolment | 2 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.14, 7.20] |
| 12.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.14, 7.20] |
| 12.2 Misoprostol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Misoprostol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Arterial embolisation after enrolment | 2 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Misoprostol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Misoprostol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Uterine compression stitch after enrolment | 2 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 Misoprostol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Misoprostol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Evacuation of retained product of conception | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.17 [0.25, 106.55] |
| 15.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 Misoprostol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Misoprotol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 1 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.17 [0.25, 106.55] |
| 16 Any surgical co‐interventions (uterine tamponade, artery ligations, arterial embolisation) excluding hysterectomy after enrolment | 2 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.15, 1.58] |
| 16.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.15, 1.58] |
| 16.2 Misoprostol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Misoprostol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Nausea | 3 | 1643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.84, 1.67] |
| 17.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.87, 1.77] |
| 17.2 Misoprotol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.15, 2.49] |
| 17.3 Misoprotol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Vomiting | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.16, 2.95] |
| 18.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [1.16, 2.95] |
| 18.2 Misoprostol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Misoprostol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Diarrhoea | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.37, 3.98] |
| 19.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.37, 3.98] |
| 19.2 Misoprostol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Misoprostol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Maternal pyrexia 38 degrees or more | 4 | 1875 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [2.66, 3.67] |
| 20.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.07 [2.61, 3.60] |
| 20.2 Misoprotol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.23 [0.50, 168.57] |
| 20.3 Misoprotol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.69 [1.29, 25.12] |
| 21 Maternal pyrexia 40 degrees or more | 3 | 1715 | Risk Ratio (M‐H, Fixed, 95% CI) | 13.58 [4.93, 37.44] |
| 21.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.48 [4.91, 42.72] |
| 21.2 Misoprostol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Misoprostol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 1 | 232 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.24 [0.38, 138.68] |
| 22 Headache | 3 | 1643 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.97, 1.53] |
| 22.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.01, 1.62] |
| 22.2 Misoprotol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 1 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.27, 1.60] |
| 22.3 Misoprostol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Shivering | 4 | 1877 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [1.76, 2.88] |
| 23.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 2 | 1483 | Risk Ratio (M‐H, Random, 95% CI) | 3.47 [0.93, 13.01] |
| 23.2 Misoprotol 200 mcg oral/ 400 mcg sublingual versus placebo no treatment | 1 | 160 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [1.40, 6.19] |
| 23.3 Misoprotol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 1 | 234 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [1.50, 3.04] |
| 24 Feeling faint or fainting | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.02, 8.66] |
| 24.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.02, 8.66] |
| 24.2 Misoprostol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Misoprostol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Allergy | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.1 Misoprotol 600 mcg sublingual versus placebo no treatment | 1 | 61 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Misoprostol 200 mcg oral/400 mcg sublingual versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Misoprostol 200 mcg oral/400 mcg sublingual/400 mcg rectal versus placebo no treatment | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
1.5. Analysis.
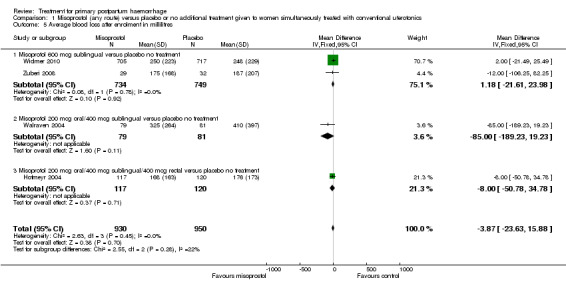
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 5 Average blood loss after enrolment in millilitres.
1.9. Analysis.
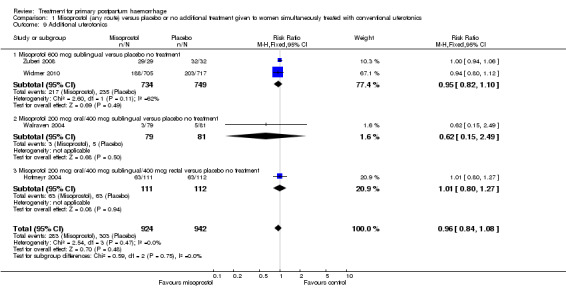
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 9 Additional uterotonics.
1.10. Analysis.
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 10 Manual removal of the placenta after enrolment.
1.11. Analysis.
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 11 Uterine tamponade after enrolment.
1.12. Analysis.
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 12 Artery ligation (uterine and/or hypogastric arteries) after enrolment.
1.13. Analysis.
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 13 Arterial embolisation after enrolment.
1.14. Analysis.
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 14 Uterine compression stitch after enrolment.
1.15. Analysis.
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 15 Evacuation of retained product of conception.
1.16. Analysis.
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 16 Any surgical co‐interventions (uterine tamponade, artery ligations, arterial embolisation) excluding hysterectomy after enrolment.
1.17. Analysis.
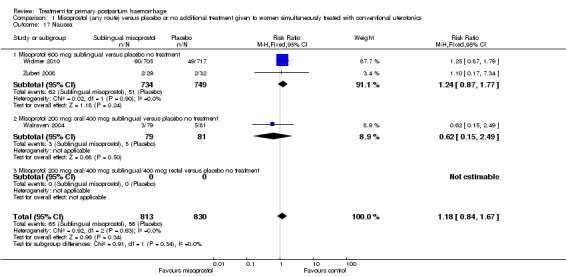
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 17 Nausea.
1.19. Analysis.
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 19 Diarrhoea.
1.22. Analysis.
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 22 Headache.
1.24. Analysis.
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 24 Feeling faint or fainting.
1.25. Analysis.
Comparison 1 Misoprostol (any route) versus placebo or no additional treatment given to women simultaneously treated with conventional uterotonics, Outcome 25 Allergy.
Comparison 2. Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal mortality | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.74] |
| 1.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.06, 15.74] |
| 2 Serious maternal morbidity | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.06] |
| 2.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.06] |
| 3 Admission to intensive care | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.06] |
| 3.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.06] |
| 4 Hysterectomy | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.36, 10.72] |
| 4.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.36, 10.72] |
| 5 Blood loss 500 mL or more after enrolment | 2 | 1787 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [0.69, 4.04] |
| 5.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Random, 95% CI) | 1.66 [0.69, 4.04] |
| 6 Mean blood loss after enrolment | 2 | 1787 | Mean Difference (IV, Fixed, 95% CI) | 44.86 [26.50, 63.22] |
| 6.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Mean Difference (IV, Fixed, 95% CI) | 44.86 [26.50, 63.22] |
| 7 Blood loss 1000 mL or more after enrolment | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.04, 6.75] |
| 7.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.04, 6.75] |
| 8 Blood transfusion within 24 hours | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.02, 2.14] |
| 8.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.02, 2.14] |
| 9 Duration from randomisation till cessation of bleeding or satisfactory response | 2 | 1787 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐1.02, 1.14] |
| 9.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐1.02, 1.14] |
| 10 Additional uterotonics after enrolment | 2 | 1787 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.57, 2.94] |
| 10.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.57, 2.94] |
| 11 Examination under anaesthesia | 2 | 1787 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.87, 1.87] |
| 11.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.87, 1.87] |
| 12 Uterine tamponade after enrolment | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.40] |
| 12.1 600 mcg | 2 | 1483 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.07, 1.40] |
| 12.2 800 mcg | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Bimanual compression | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.96, 1.18] |
| 13.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.96, 1.18] |
| 14 Artery ligation (uterine and/or hypogastric arteries) after enrolment | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Arterial embolisation after enrolment | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Uterine tamponade after enrolment | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Unsatisfactory response after enrolment after enrolment | 2 | 1787 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.02, 0.08] |
| 17.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.02, 0.08] |
| 18 Uterine compression stitch after enrolment | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Any surgical co‐interventions (uterine tamponade, artery ligations, arterial embolisation) excluding hysterectomy after enrolment | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.06] |
| 19.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.06] |
| 20 Nausea | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.25] |
| 20.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.76, 1.25] |
| 21 Vomiting | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.45, 4.38] |
| 21.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.52 [1.45, 4.38] |
| 22 Diarrhoea | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.44, 4.36] |
| 22.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.44, 4.36] |
| 23 Headache | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.23, 4.38] |
| 23.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.23, 4.38] |
| 24 Shivering | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [2.28, 3.19] |
| 24.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [2.28, 3.19] |
| 25 Feeling faint or fainting | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.39] |
| 25.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.39] |
| 26 Maternal pyrexia 38 degrees or more | 2 | 1787 | Risk Difference (M‐H, Random, 95% CI) | 0.23 [‐0.08, 0.54] |
| 26.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Difference (M‐H, Random, 95% CI) | 0.23 [‐0.08, 0.54] |
| 27 Maternal pyrexia 40 degrees or more | 2 | 1787 | Risk Ratio (M‐H, Random, 95% CI) | 23.54 [0.50, 1104.42] |
| 27.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Random, 95% CI) | 23.54 [0.50, 1104.42] |
| 28 Allergy | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.14, 7.09] |
| 28.1 800 mcg misoprostol versus 40 IU oxytocin | 2 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.14, 7.09] |
2.6. Analysis.
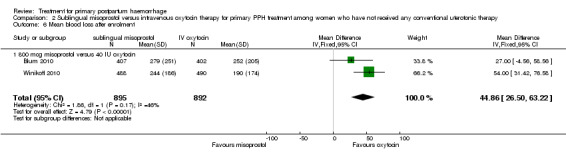
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 6 Mean blood loss after enrolment.
2.9. Analysis.
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 9 Duration from randomisation till cessation of bleeding or satisfactory response.
2.11. Analysis.
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 11 Examination under anaesthesia.
2.12. Analysis.
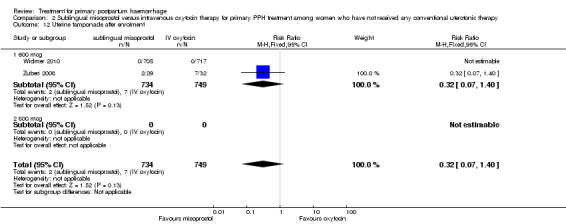
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 12 Uterine tamponade after enrolment.
2.13. Analysis.
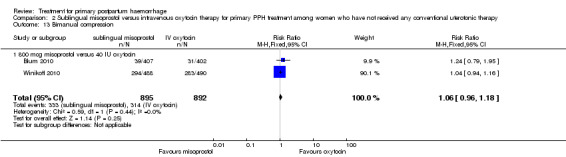
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 13 Bimanual compression.
2.14. Analysis.
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 14 Artery ligation (uterine and/or hypogastric arteries) after enrolment.
2.15. Analysis.
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 15 Arterial embolisation after enrolment.
2.16. Analysis.
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 16 Uterine tamponade after enrolment.
2.17. Analysis.
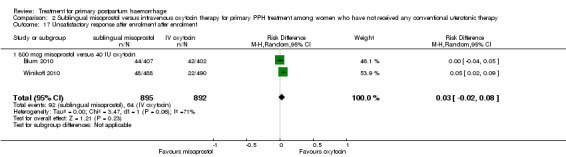
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 17 Unsatisfactory response after enrolment after enrolment.
2.18. Analysis.
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 18 Uterine compression stitch after enrolment.
2.19. Analysis.
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 19 Any surgical co‐interventions (uterine tamponade, artery ligations, arterial embolisation) excluding hysterectomy after enrolment.
2.20. Analysis.
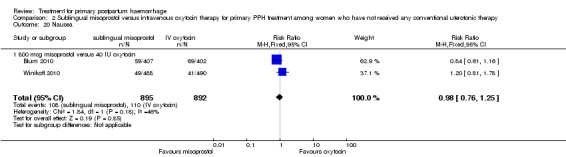
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 20 Nausea.
2.21. Analysis.
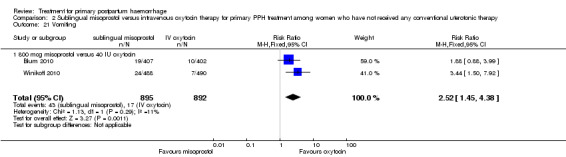
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 21 Vomiting.
2.22. Analysis.
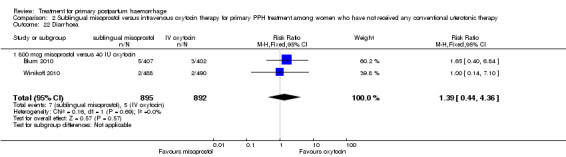
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 22 Diarrhoea.
2.23. Analysis.
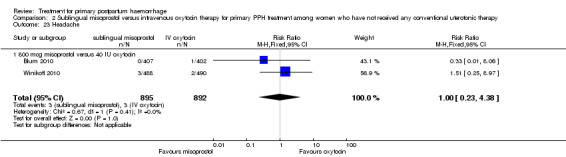
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 23 Headache.
2.24. Analysis.
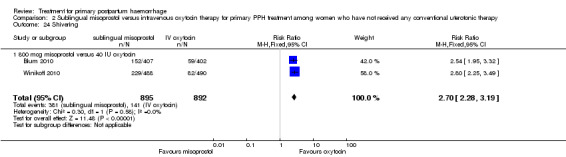
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 24 Shivering.
2.25. Analysis.
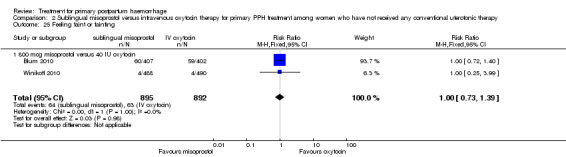
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 25 Feeling faint or fainting.
2.26. Analysis.
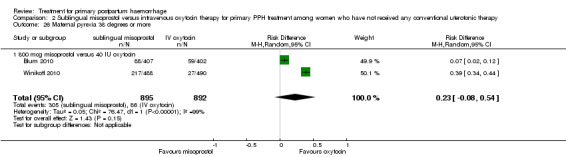
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 26 Maternal pyrexia 38 degrees or more.
2.27. Analysis.
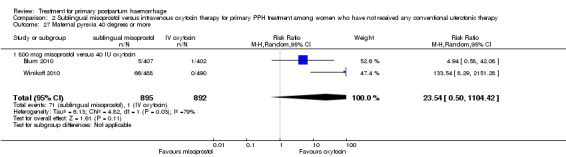
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 27 Maternal pyrexia 40 degrees or more.
2.28. Analysis.
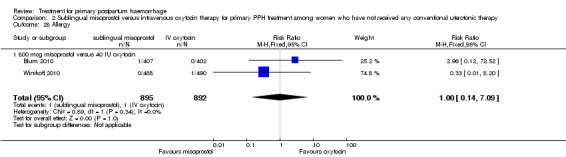
Comparison 2 Sublingual misoprostol versus intravenous oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 28 Allergy.
Comparison 3. Rectal misoprostol versus combination of ergometrine and oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hysterectomy | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.89] |
| 1.1 800 mcg | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.89] |
| 2 Persistent haemorrhage | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.76] |
| 3 Additional uterotonics | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.76] |
| 4 Surgical co‐interventions (excluding hysterectomy) | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.67] |
3.2. Analysis.
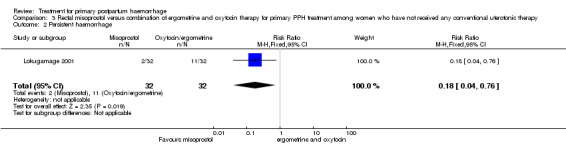
Comparison 3 Rectal misoprostol versus combination of ergometrine and oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 2 Persistent haemorrhage.
3.4. Analysis.
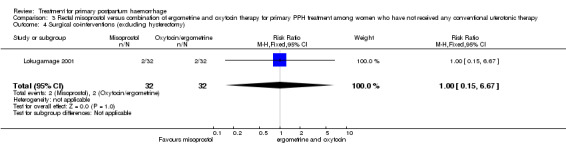
Comparison 3 Rectal misoprostol versus combination of ergometrine and oxytocin therapy for primary PPH treatment among women who have not received any conventional uterotonic therapy, Outcome 4 Surgical co‐interventions (excluding hysterectomy).
Comparison 4. Estrogen versus placebo/no treatment among women receiving conventional uterotonics for primary PPH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Hysterectomy | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 3.11] |
| 2 Mean blood loss within two hours | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐274.9 [‐384.72, ‐165.08] |
| 3 Mean blood loss between two and 24 hours | 1 | 112 | Mean Difference (IV, Fixed, 95% CI) | ‐50.7 [‐83.07, ‐18.33] |
Comparison 5. Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal mortality | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious maternal morbidity (renal failure respiratory failure, cardiac arrest, multiple organ failure) | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.05] |
| 3 Admission to intensive care unit | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.42] |
| 4 Hysterectomy | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.05] |
| 5 Blood loss 500 mL or more after enrolment | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.97, 1.03] |
| 6 Blood loss 1000 mL or more after enrolment | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.61, 2.09] |
| 7 Total mean blood loss after enrolment | 1 | 144 | Mean Difference (IV, Fixed, 95% CI) | ‐91.0 [‐242.00, 60.00] |
| 8 Blood transfusion within 24 hours | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.24, 1.40] |
| 9 Additional uterotonics after enrolment | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.76, 1.48] |
| 10 Unsatisfactory response after enrolment | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.03, 1.34] |
| 11 Uterine compression stitch after enrolment | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.09] |
| 12 Interventions to control bleeding for secondary postpartum haemorrhage | 1 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.93] |
| 12.1 Medical interventions to control bleeding (new subgroup) | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.68] |
| 12.2 Surgical evacuation | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.39] |
| 13 Examination under anaesthesia | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.97, 1.03] |
| 14 Uterine tamponade after enrolment | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Artery ligation (uterine and/or hypogastric arteries) after enrolment | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.05] |
| 16 Arterial embolisation after enrolment | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.22, 2.86] |
| 17 Headache | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Nausea | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.0 [1.46, 82.99] |
| 19 Maternal pyrexia 38 degrees or more | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.52, 2.31] |
| 20 Maternal pyrexia 40 degrees or more | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Deep vein thrombosis | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.57] |
| 22 Seizures | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Dizziness | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.75] |
| 24 Phosphenes | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.88, 18.19] |
| 25 Secondary postpartum haemorrhage | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.68] |
| 26 Surgical evacuation for secondary postpartum haemorrhage | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.39] |
| 27 Intravenous iron therapy in the puerperium | 1 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.89, 2.32] |
| 28 Hospital re‐admission for secondary postpartum haemorrhage | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.39] |
| 29 Postnatal depression at day 42 postpartum | 1 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.05] |
5.1. Analysis.
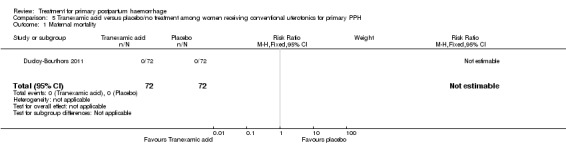
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 1 Maternal mortality.
5.5. Analysis.
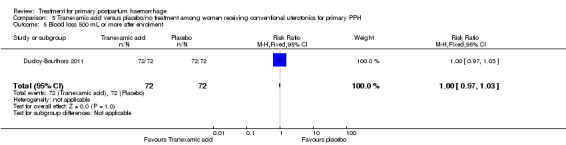
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 5 Blood loss 500 mL or more after enrolment.
5.6. Analysis.
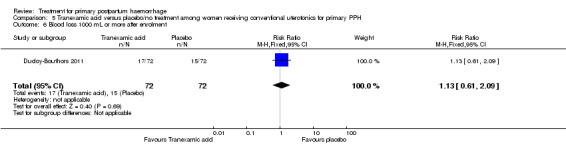
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 6 Blood loss 1000 mL or more after enrolment.
5.7. Analysis.
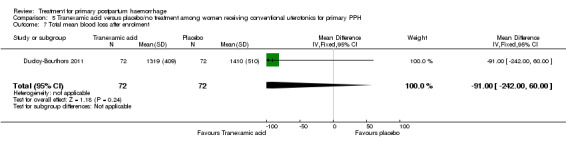
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 7 Total mean blood loss after enrolment.
5.8. Analysis.
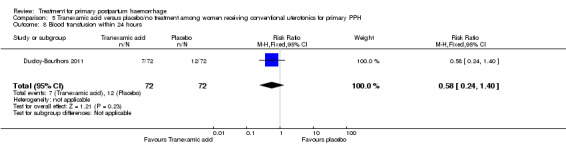
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 8 Blood transfusion within 24 hours.
5.9. Analysis.
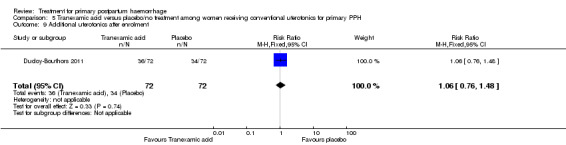
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 9 Additional uterotonics after enrolment.
5.10. Analysis.
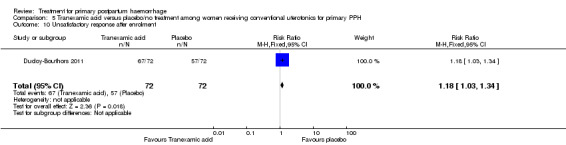
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 10 Unsatisfactory response after enrolment.
5.11. Analysis.
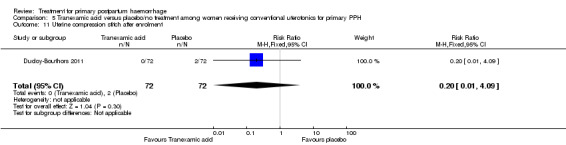
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 11 Uterine compression stitch after enrolment.
5.12. Analysis.
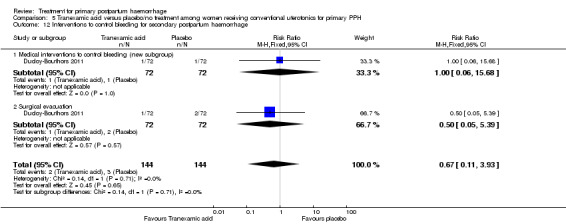
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 12 Interventions to control bleeding for secondary postpartum haemorrhage.
5.13. Analysis.
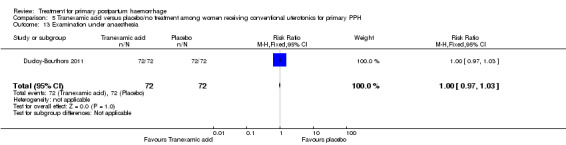
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 13 Examination under anaesthesia.
5.14. Analysis.
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 14 Uterine tamponade after enrolment.
5.15. Analysis.
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 15 Artery ligation (uterine and/or hypogastric arteries) after enrolment.
5.16. Analysis.
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 16 Arterial embolisation after enrolment.
5.17. Analysis.
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 17 Headache.
5.19. Analysis.
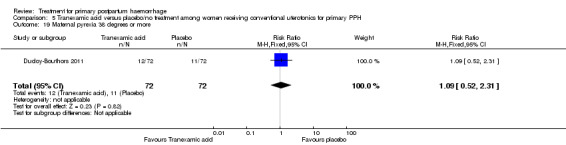
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 19 Maternal pyrexia 38 degrees or more.
5.20. Analysis.
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 20 Maternal pyrexia 40 degrees or more.
5.22. Analysis.
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 22 Seizures.
5.23. Analysis.
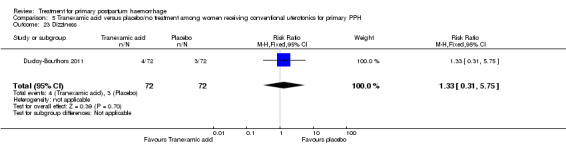
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 23 Dizziness.
5.24. Analysis.
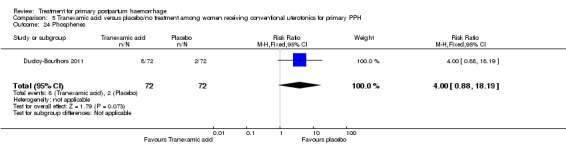
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 24 Phosphenes.
5.25. Analysis.
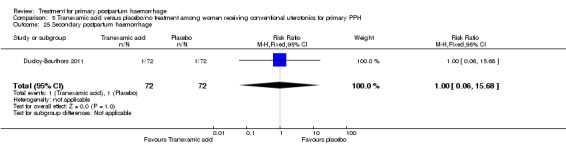
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 25 Secondary postpartum haemorrhage.
5.26. Analysis.
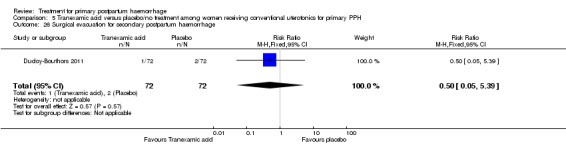
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 26 Surgical evacuation for secondary postpartum haemorrhage.
5.27. Analysis.
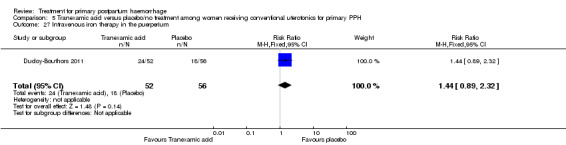
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 27 Intravenous iron therapy in the puerperium.
5.28. Analysis.
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 28 Hospital re‐admission for secondary postpartum haemorrhage.
5.29. Analysis.
Comparison 5 Tranexamic acid versus placebo/no treatment among women receiving conventional uterotonics for primary PPH, Outcome 29 Postnatal depression at day 42 postpartum.
Comparison 6. Lower uterine segment compression versus conventional treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Maternal mortality | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious maternal morbidity | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Hysterectomy | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Blood loss 500 mL or more after enrolment | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.94] |
| 5 Blood loss 1000 mL or more after enrolment | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.01] |
| 6 Average blood loss after enrolment | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐103.00 [‐260.00, 52.00] |
| 7 Blood transfusion | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [0.66, 8.23] |
| 8 Other surgical interventions to control bleeding (other than hysterectomy) | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Unsatisfactory response after enrolment | 1 | 64 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.67] |
6.1. Analysis.
Comparison 6 Lower uterine segment compression versus conventional treatment, Outcome 1 Maternal mortality.
6.2. Analysis.
Comparison 6 Lower uterine segment compression versus conventional treatment, Outcome 2 Serious maternal morbidity.
6.3. Analysis.
Comparison 6 Lower uterine segment compression versus conventional treatment, Outcome 3 Hysterectomy.
6.9. Analysis.
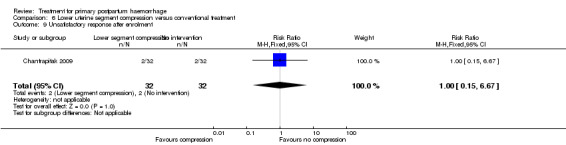
Comparison 6 Lower uterine segment compression versus conventional treatment, Outcome 9 Unsatisfactory response after enrolment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blum 2010.
| Methods | Computer‐generated random allocation sequence in blocks of 10. Sealed and numbered opaque boxes contained the treatment allocation and were opened in strict numerical sequence Participants received simultaneously either 40 IU oxytocin in a litre of intravenous solution over 15 minutes or 800 mcg (4 tablets of 200 mcg) misoprostol placed under the tongue for 20 minutes and a placebo for the other treatment (i.e. 4 placebo pills or an ampoule of saline). | |
| Participants | 809 women diagnosed with PPH due to uterine atony were randomly assigned to receive 800 mcg misoprostol or 40 IU intravenous oxytocin. Diagnosis of PPH was based on need for treatment, as determined by clinical judgement or measured blood loss of 700 mL in the first hour after delivery, whichever occurred first. Women were excluded if their PPH was suspected to have a cause other than uterine atony, if oxytocin was not received during the third stage of labour or if delivery was by caesarean section. |
|
| Interventions | Prophylactic oxytocin given during the third stage of labour plus 800 mcg misoprostol sublingually or 40 IU intravenous oxytocin after diagnosis of PPH due to uterine atony. | |
| Outcomes | Primary outcome: cessation of active bleeding within 20 minutes and additional blood loss of 300 mL or more after treatment. Secondary outcomes: total blood loss after treatment, change in haemoglobin after treatment, time to active bleeding cessation, provision of any additional interventions and side effects. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation sequence in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was not revealed until data collection and cleaning were completed. Periodic monitoring to ensure hospitals were following the numerical sequence of the boxes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants received simultaneously either 40 IU oxytocin in a litre of intravenous solution over 15 minutes or 800 mcg (4 tablets of 200 mcg) misoprostol placed under the tongue for 20 minutes and a placebo for the other treatment (i.e. 4 placebo pills or an ampoule of saline). Periodic monitoring of hospitals to ensure masking was successful. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% outcome data. |
| Selective reporting (reporting bias) | Low risk | Prior registration of protocol. |
| Other bias | Unclear risk | Outcome measure of additional blood loss of 300 mL or more after treatment may have included blood from episiotomy and other liquids collected during delivery. |
Chantrapitak 2009.
| Methods | Randomly assigned. | |
| Participants | Women, 28 to 42 weeks' gestational age pregnancy, with vaginal delivery with PPH defined as blood loss > 500 mL (assessed by weighing soaking drapes and blood in bucket). | |
| Interventions | Conventional treatment plus lower uterine compression (either compression of lower segment only or compression of lower segment with counteracting pressure from fundus) for 10 minutes versus conventional treatment alone for PPH. Conventional treatment is described as uterine massage, oxytocin (10 to 20 units in 1000 mL of intravenous solution, 200 mL/min), intravenous ergometrine), placing cold pack on the uterus and urinary catheterisation. | |
| Outcomes | Main outcome: amount of blood loss in conventionally treated group versus experimental group 2 hours after treatment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors indicated random generation using opaque concealed envelopes. |
| Allocation concealment (selection bias) | High risk | Clinicians were aware of intervention used. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Both participants and clinicians were aware of intervention used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data collected on all 64 randomly assigned women. |
| Selective reporting (reporting bias) | Unclear risk | No prior publication of protocol or statistical analysis plan against which to assess. |
| Other bias | Unclear risk | Outcome measure of blood loss may have included blood and other liquids collected during delivery. |
Ducloy‐Bouthors 2011.
| Methods | Randomised, open‐label, multi‐centre trial. Randomisation sequence was generated by a central computer, and randomisation was balanced by centre. | |
| Participants | Women with measured blood loss > 800 mL following vaginal delivery were included in the study. All participants with PPH > 500 mL were managed according to French practice guidelines: bladder catheter, manual removal of retained placenta, genital tract examination, uterine exploration and oxytocin (30 U/30 min), followed, and if these procedures were inefficacious, sulprostone was administered (500 μg in 1 hour) with no procoagulant treatment. Participants with PPH > 800 mL were included in the study. Immediately after inclusion, participants were randomly assigned to receive tranexamic acid (tranexamic acid group) or no antifibrinolytic treatment (control group). Exclusion criteria were age < 18 years, absence of informed consent, caesarean section, presence of known haemostatic abnormalities before pregnancy and history of thrombosis or epilepsy. All women were managed according to French practice guidelines: bladder catheter, manual removal of retained placenta, genital tract examination, uterine exploration and oxytocin (30 U/30 min), followed, and if these procedures were inefficacious, sulprostone (an analogue of prostaglandin E2 was administered 500 mcg in 1 hour) with no procoagulant treatment. |
|
| Interventions | Tranexamic acid of loading dose 4 grams intravenously over 1 hour, then infusion of 1 g/h over 6 hours versus no antifibrinolytics. | |
| Outcomes | Primary outcome was volume of blood loss between T1 and T4 (T1 = inclusion and T4 = T1 + 6 hours). Secondary outcomes were:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence was generated by a central computer, and randomisation was balanced by centre. |
| Allocation concealment (selection bias) | Unclear risk | Allocation is described as being concealed to outcome assessors. Obstetricians, midwives and participants were not aware of the intervention used. However, anaesthetists were aware of the intervention and were responsible for randomisation and administration of the trial drug. It is unclear how the allocated intervention was concealed, as the intravenous infusion would be visible to all. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open labelled. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 152 women randomly assigned, and data on 151 women reported in intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | High risk | Public registration of protocol done nearly 3 years after study completion. Report published several years after end of trial. |
| Other bias | Unclear risk | Outcome measure of blood loss may have included blood and other liquids collected during delivery. |
Hofmeyr 2004.
| Methods | Next in a series of treatment packs containing 5 tablets of independently prepared, ordered in computer‐generated random sequence and numbered consecutively. Packs contained either placebo or misoprostol 5 × 200 mcg. | |
| Participants | 244 women with bleeding more than expected at least 10 minutes after delivery thought to be due to uterine atony and requiring additional uterotonic therapy. | |
| Interventions | Routine active management of the third stage of labour with oxytocin 10 units or syntometrine 1 ampoule soon after birth. All participants were given all routine treatments for PPH (intravenous infusion, uterotonics, etc) from a special 'PPH Trolly'. Trial tablets (misoprostol 200 mcg or placebo) were administered: 1 orally, 2 sublingually and 2 rectally. | |
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | 6/244 data sheets did not have pack numbers completed and were excluded from the analysis. No abnormal outcomes were observed in any of the excluded group except 1 case of shivering and 1 of blood transfusion. No information given regarding allocation group. Authors were contacted to clarify amount of blood loss before recruitment, and they have provided the following information.
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | .Computer‐generated random sequence and numbered consecutively |
| Allocation concealment (selection bias) | Low risk | Adequate, as participants were allocated as next in a series of treatment packs containing 5 tablets of independently prepared trial drug (misoprostol or placebo). |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Treatment sequence was kept sealed, and the code was broken only after complete entry and checking of all trial data. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 244 women were randomly assigned. Pack numbers for 6 women were incompletely filled in on the data sheets. Group allocation of these women was therefore unknown, and they could not be included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No prior public registration of protocol. |
| Other bias | Unclear risk | Outcome measure of blood loss may have included blood and other liquids collected during delivery. |
Lokugamage 2001.
| Methods | Random allocation by sealed sequentially numbered envelopes. No blinding. | |
| Participants | 64 women with primary PPH > 500 mL in 2 centres. Women with hypertension at recruitment, cardiac abnormalities, ongoing severe asthma, connective tissue disorders, haemorrhage due to obvious genital tract trauma. Any contraindications to prostaglandin therapy were excluded. | |
| Interventions | Syntometrine + syntocinon intravenous infusion + 4 placebo tablets per rectum versus 800 mcg (4 tablets) misoprostol per rectum + a placebo normal saline 2 mL intramuscular injection + placebo crystalloid intravenous infusion. | |
| Outcomes | Effectiveness to control PPH within 20 minutes of administration. | |
| Notes | Single‐blinded study, as obstetricians were aware of the type of drug given, and women and midwives were blinded. No mention of (a) drugs used in the third stage; (b) measurement of blood loss. Outcome measures for the following factors were reported as P value only: (a) DIC; (b) blood transfusion; (c) length of hospital stay; (d) drug side effects. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed by generating random numbers via STATA, a statistical software package. |
| Allocation concealment (selection bias) | Low risk | Sealed, sequentially numbered opaque envelopes were used, and they were opened in succession once a participant had been recruited. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Midwives were blinded to treatment, although obstetricians and research doctor were aware of the randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 64 participants are presented in the final results, 32 participants having been allocated to each arm of the study. 1 participant was recruited to the misoprostol arm but was excluded from the analysis because the haemorrhage was due to uterine rupture. |
| Selective reporting (reporting bias) | High risk | Certain outcome data were reported as "P" value of significance. Therefore, data cannot be entered in a meta‐analysis. No attempt was made to measure blood loss, and it was not reported as an outcome measure. No prior public registration of protocol. |
| Other bias | High risk | Original sample size calculation: 142 participants needed to be recruited. However, the first year interim analysis, which included 15 participants per study arm, showed that misoprostol performed best. The decision was made to terminate the study after recruitment of 64 participants, as the difference between the 2 treatment regimens reached statistical significance with power in excess of 80%. As the study is a single‐blinded study, the interim results may have influenced clinicians' management. |
Walraven 2004.
| Methods | Next in a series of randomised treatment packs in opaque envelopes with 3 tablets of misoprostol 200 mcg or placebo. | |
| Participants | 160 women who delivered vaginally with measured postpartum blood loss of 500 mL or more within 1 hour of delivery and inadequate uterine contraction thought to be the possible factor. Exclusion criteria included women who delivered by caesarean section if blood loss was less than 500 mL in first hour following vaginal delivery, if gestational age was less than 28 weeks or if inadequate uterine contraction was not thought to be the causative factor for PPH. | |
| Interventions | Routine active management of third stage of labour with oxytocin 10 IU or syntometrine 1 ampoule (5 mL). All participants had standard management of PPH (rubbing the uterus, commencing intravenous infusion, administering oxytocics, delivering the placenta if undelivered and emptying the bladder). Trial tablets (misoprostol 200 mcg or placebo) were administered: 1 orally and 2 sublingually. |
|
| Outcomes | Primary outcome: additional blood loss after enrolment. Secondary outcomes: frequency and severity of side effects, additional blood loss of 500 mL or more after enrolment, clinical complications (blood transfusion, hysterectomy) and haemoglobin level at 12 to 24 hours after delivery. | |
| Notes | Blinding may have been compromised by non‐identical placebos. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Next in a series of randomised treatment packs in opaque envelopes containing misoprostol 3 200 Ag or placebo tablets. |
| Allocation concealment (selection bias) | Low risk | The randomisation code was broken only after entry and checking of data. An independent data monitor reviewed the data collected from the first 80 women and recommended that the study continue until complete recruitment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Although this is a double‐blind trial, the authors indicated that the tablets were similar in size and colour but not in shape. Efforts to obtain identical placebo tablets were unsuccessful. Although no account indicated that the midwife caught sight of the tablet, this is not a sufficient guarantee of adequate blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals after enrolment were reported, and all outcomes were analysed according to the allocated study group. |
| Selective reporting (reporting bias) | Unclear risk | No prior publication of protocol. |
| Other bias | Unclear risk | Outcome measure of blood loss may have included blood and other liquids collected during delivery. |
Widmer 2010.
| Methods | A computer‐generated randomisation sequence derived centrally in United States and stratified by country. Within the strata, women were individually allocated by block randomisation (varying blocks of 6 and 8). | |
| Participants | 1422 women with clinically diagnosed PPH that was suspected to be due to uterine atony, and they needed additional uterotonics. Participants were enrolled from hospitals in Argentina, Egypt, South Africa, Thailand and Vietnam. Women were not eligible for the trial if delivery was by caesarean section; misoprostol could not be given sublingually; any severe allergic or bleeding disorders (e.g. haemophilia) were recorded; temperature was higher than 38·5°C; delivery was defined as a miscarriage according to local gestational age limits; or the placenta was not delivered. | |
| Interventions | 600 μg misoprostol sublingually (3 tablets of 200 μg) or matching placebo in addition to standard care for PPH according to local protocol. | |
| Outcomes | Primary outcome: blood loss ≥ 500 mL within 60 minutes after randomisation. Secondary outcomes: need for blood transfusion; haemoglobin concentration of less than 80 g/L within 24 hours postpartum or need for blood transfusion; median blood loss at 60 minutes and 90 minutes after randomisation; blood loss of 500 mL or more within 90 minutes after randomisation; blood loss of 1000 mL or more within 60 minutes and 90 minutes after randomisation; need for any additional uterotonic; maternal death; severe morbidity (hysterectomy or admission to a maternal intensive care unit); side effects (shivering, pyrexia, diarrhoea, vomiting or nausea) within 60 minutes and 90 minutes after randomisation; and need for any other interventions. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation sequence derived centrally and stratified by country. Within the strata, women were individually allocated by block randomisation (varying blocks of 6 and 8). |
| Allocation concealment (selection bias) | Low risk | Randomisation code was not shown to any participating trial centre or member of the study team until the trial was closed. To conceal allocation, treatment boxes were sealed and numbered sequentially according to the randomisation sequence and were distributed in the order that women were judged to be eligible and were enrolled in the study. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Treatment boxes were identical in appearance for both groups, and placebo tablets were identical to misoprostol tablets in shape, colour, weight, feel and taste. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomly assigned participants included in analysis. |
| Selective reporting (reporting bias) | Unclear risk | No prior public registration of protocol. |
| Other bias | Unclear risk | Outcome measure of blood loss may have included blood and other liquids collected during delivery. |
Winikoff 2010.
| Methods | Computer‐generated random allocation sequence in blocks of 10 and concealed from study staff who enrolled and were allocated. Sealed and opaque packets were administered to participants in the order that they were diagnosed, and providers and women were masked to treatment assignment. | |
| Participants | 978 women with PPH where administration of oxytocic drugs during the second (e.g. induction or augmentation) and third stages of labour (active management) was not routine practice. Diagnosis of PPH could occur at any time and at any amount of blood loss; however, the protocol instructed providers to begin treatment immediately if measured blood loss exceeded 700 mL. Women who had a known allergy to prostaglandin, had received any uterotonic drug in labour or had a caesarean section or delivered outside the study site, or whose postpartum bleeding was not suspected to be due to atonic uterus, were excluded from the study. |
|
| Interventions | Either 1 ampoule of 40 IU oxytocin or 4 tablets of 200 mcg misoprostol and matching placebo (either 1 ampoule of saline solution or 4 placebo tablets resembling misoprostol), which were administered simultaneously. Oxytocin or saline solution was administered in a litre of intravenous solution over 15 minutes, and misoprostol or placebo tablets were placed under the tongue for 20 minutes. | |
| Outcomes | Primary outcomes were the proportion of women who ceased active bleeding within 20 minutes after study treatment alone and those who lost 300 mL or more of blood after treatment. Secondary outcomes were total blood loss after treatment, change in haemoglobin concentration after treatment, time to active bleeding cessation and any other additional interventions. All outcomes were assessed from the time of initial treatment. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation sequence in blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Randomisation codes were maintained centrally and were concealed from study staff who enrolled and allocated. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Sealed and opaque packets were administered to participants in the order in which they were diagnosed. Every packet contained 1 active treatment (either 1 ampoule of 40 IU oxytocin or 4 tablets of 200 μg misoprostol) and matching placebo (either 1 ampoule of saline solution or 4 placebo tablets resembling misoprostol). Note: Only visual matching of placebo tablets to active. Matching of taste not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcomes reported on all randomly assigned participants. |
| Selective reporting (reporting bias) | Low risk | Prior registration of protocol. |
| Other bias | Unclear risk | Outcome measure of blood loss may have included blood and other liquids collected during delivery. |
Zhou 2006.
| Methods | Randomised. | |
| Participants | 112 puerperants with PPH due to uterine atony who received routine management for uterine atony. Exclusions were as follows: younger than 18 years of age; any pre‐existing heart condition; high blood pressure for which they had received medication in the previous 2 years; any pre‐existing blood condition, whether from birth or contracted later in life, such as haemophilia; history of suffering from or exhibiting symptoms of progressive hepatitis or endocrinosis; having undergone traditional caesarean; having undergone general anaesthetic in case of placenta previa, or if the cervical muscles had undergone surgery. 52 assigned to test group, 60 to control group. |
|
| Interventions | 4 mg estradiol benzoate injected intramuscularly with routine management when bleeding exceeded 500 mL versus routine management only for the control group. Routine management of the control group was described as 'uterine massage and uterotonics administration' and included '20 U cervical muscle injection to contract the uterus; 20 U intravenous drip to contract the uterus. In case of the cervical muscles not restoring, injection or intravenous drip did not exceed 80 U. Where rate of blood loss exceeded 2000 ml, hysterectomy was performed'. | |
| Outcomes | Rate of blood loss at 2 hours and 2 to 24 hours and any reported instances of hysterectomy up to 24 hours. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Authors reported data only for blood loss and hysterectomy. |
| Selective reporting (reporting bias) | High risk | Authors reported data only for blood loss and hysterectomy. No prior public registration of protocol. |
| Other bias | High risk | Unclear how blood loss was measured. No sample size calculation. We were unable to identify management before randomisation. |
Zuberi 2008.
| Methods | Sample was randomly assigned in blocks of 10, stratified by site, using a computer‐generated random sequence. Eligible women were randomly assigned to next study envelope. Each study envelope contained 3 tablets of misoprostol (200 mcg × 3) or matching placebo. | |
| Participants | 61 participants from a planned sample of 900 women with PPH (defined as measured blood loss of 500 mL) had been reached. Women with cesarean section, gestational age less than 28 weeks at time of delivery or not consenting were excluded from the study. | |
| Interventions | 600 mcg of misoprostol or matching placebo taken sublingually, in addition to standard treatment for PPH. Standard treatment was management of the third stage of labour with standard uterotonics, controlled cord traction after delivery of baby and gentle uterine massage after delivery of the placenta. At delivery of the anterior shoulder of the baby, 1 of 2 uterotonic regimens was administered: intravenous 10 IU of oxytocin or 5 IU of oxytocin plus 0.4 mg of ergometrine given intramuscularly or intravenously. |
|
| Outcomes | Primary endpoint was measured blood loss ≥ 500 mL after PPH treatment. Secondary outcomes included change in haemoglobin, side effects, need for additional interventions including blood transfusion, additional uterotonics, balloon tamponade and hysterectomy and mean blood loss. |
|
| Notes | Only 61 participants of a planned sample of 900 recruited. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sample was randomly assigned in blocks of 10, stratified by site, using a computer‐generated random sequence. |
| Allocation concealment (selection bias) | Low risk | Randomisation code was concealed until all data were entered and cleaned. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Use of the next randomised study envelope; each contained 3 tablets of either misoprostol (200 mcg × 3) or matching placebo (matching not described). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Main outcome data presented for 59 of the 61 randomly assigned participants. |
| Selective reporting (reporting bias) | Low risk | Prior public registration of protocol. |
| Other bias | High risk | Failure to recruit sufficient participants to meet sample size and power requirements of the study. Bias in outcome measurement: Blood loss was collected on used gauze pieces and pads that were counted and placed in a plastic bag. The plastic bag was then weighed; however, accurate use of the scales proved difficult; these results could not be verified and were excluded. |
DIC: disseminated intravascular coagulopathies. EACD: external aortic compression device. IU: international units. min: minutes. PPH: postpartum haemorrhage.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Deneux‐Tharaux 2010 | A multifaceted intervention aimed at increasing the translation into practice of a protocol for early management of PPH. |
| Khalil 2011 | The current study was carried out to evaluate the effectiveness of a new technique for keeping the Bakri balloon in place among women with major primary PPH. All participants had a Bakri balloon inserted with the same technique, but they were randomly assigned to (a) Bakri balloon and a stitch to keep it in place or (b) Bakri balloon without a stitch. The current intervention of using "a stitch" was not direct for the treatment of primary PPH. |
| Khireddine 2012 | This study was excluded, as it was a non‐randomised population‐base case‐controlled study that examined the association between induction of labour and postpartum haemorrhage, according to its indications and methods, in low‐risk parturient women. |
| Magwali 2012 | This study was a non‐randomised study that compared blood loss and mortality in participants with severe obstetrical haemorrhage who received standard care in phase 1 (0ctober 2007 to October 2008) versus standard of care plus non‐pneumatic antishock garment (NASG) in phase 2 (October 2008 to October 2009) at 2 referral hospitals in Harare. |
| Soltan 2009 | This study was excluded, as it was a quasi‐randomisation trial. 300 participants, with blood loss of 500 mL from a non‐contracted uterus associated with signs of circulatory compromise (e.g. tachycardia and/or moderate to severe hypotension), were allocated in an alternative fashion to external aortic compression devices (EACDs) as a first intervention line simultaneously with conventional management versus conventional management alone. Main outcome measures were maternal mortality, surgical operation (e.g. hysterectomy) and quantity of uterotonic drugs and blood transfusion units used. Time in minutes required for cessation of uterine bleeding and side effects of EACD in relation to duration of use were recorded. Period of follow‐up was not defined. Authors presented data for only 240 women (120 participants in each arm). Reasons for exclusion of 60 participants were unclear. |
| Soltan 2010 | This study was excluded, as it was a quasi‐randomisation trial. 120 women with blood loss of 500 mL from a non‐contracted uterus associated with signs of circulatory compromise (e.g. tachycardia and/or moderate to severe hypotension) were allocated in an alternative fashion EACD as a first intervention line simultaneously with conventional management versus conventional management alone. Exclusion criteria included women undergoing a caesarean delivery, known lower limb ischaemia, deep venous thrombosis and peripheral neuritis or other neurological, respiratory, hepatic, renal or intestinal disorders. Aims of the study were to monitor femoral artery blood flow by Doppler velocimetry in women treated for PPH with and without the adjunct of the EACD and to assess possible adverse effects of the aortic compression device. Authors did not provide enough information regarding obstetrical outcome measures. |
| Takagi 1976 | The study consists of 2 parts. The first part was a retrospective analysis of data obtained before the clinical trial. The clinical trial compared the effects of prostaglandin F2 alpha and ergot derivatives on the amount of blood loss in women who suffered PPH as blood loss > 400 mL in primiparas and > 300 mL in multiparas. 13 women were randomly assigned to receive ergot derivatives, and 46 women received prostaglandin F2 alpha by 1 of the following routes: (1) gluteal intramuscular; (2) intravenous infusion; (3) transabdominal intramyometrial; or (4) transvaginal intramyometrial. Method of randomisation was not reported. We were unable to extract data according to allocated groups to perform an 'intention‐to‐treat' analysis. |
PPH: postpartum haemorrhage
Characteristics of studies awaiting assessment [ordered by study ID]
Lavigne‐Lissalde 2013.
| Methods | Block randomisation according to site. |
| Participants | Women with severe postpartum haemorrhage. |
| Interventions | Standard care plus recombinant activated factor VII (rhuVIIa) versus standard care only. |
| Outcomes | Impact on use of second‐line therapy. |
| Notes | Not enough information in abstract for appraisal. |
Characteristics of ongoing studies [ordered by study ID]
Collins 2013.
| Trial name or title | Fibrinogen concentrate to treat postpartum haemorrhage – OBS2 Study |
| Methods | A multicentre, prospective, double blind randomised control trial |
| Participants | Women experiencing major postpartum haemorrhage (PPH). About 1050 women will be recruited into the observational phase of the study so that 60 can be randomised to receive fibrinogen concentrate or placebo. |
| Interventions | Fibrinogen concentrate (RiaStap®) versus placebo. The woman will receive a bolus infusion of either fibrinogen concentrate or placebo plus standard treatment. The dose of fibrinogen concentrate or placebo to be infused will be calculated based on the woman's ideal body weight for height and the measured FIBTEM A5 with the aim of increasing the FIBTEM A5 to 23 mm. |
| Outcomes | Primary endpoints: The total number of allogeneic blood products transfused after study medication until discharge. The total number of allogeneic blood products transfused will be compared between the two arms. |
| Starting date | May 2013 |
| Contact information | Dr P Collins, Reader in Haematology, Dept of Haematology, School of Medicine, Cardiff University, Heath Park, Cardiff, CF14 4XN Tel : 02920744144 E‐mail :peter.collins@wales.nhs.uk |
| Notes | Estimated end: September 2014 |
Miller 2008.
| Trial name or title | Non‐pneumatic anti‐shock garment for obstetrical haemorrhage: Zambia and Zimbabwe (NASG). |
| Methods | Cluster‐randomised controlled trial. |
| Participants | Approximately 2340 women who are pregnant or postpartum and experiencing obstetrical haemorrhage with 2 of the following 3: blood loss > 500 mL (at SHF, 1000 mL at RH) SBP < 100 mm Hg, pulse > 100 bpm. |
| Interventions | Half of the study clinics will use the non‐pneumatic antishock garment on participants before transporting them to the referral hospital for intervention. |
| Outcomes | Frequency of mortalities and frequency of severe morbidities combined as extreme adverse outcomes. |
| Starting date | October 2007. |
| Contact information | suellenmiller@gmail.com |
| Notes | Estimated end: May 2012. |
Mirzazada 2011.
| Trial name or title | Misoprostol for the treatment of postpartum haemorrhage (PPH) following self‐administration of misoprostol prophylaxis in home deliveries. |
| Methods | Randomised, double‐blind, placebo‐controlled trial. |
| Participants | Women with clinical diagnosis of postpartum haemorrhage following home birth in 4 districts in Badakshan Province in Afghanistan. All women enrolled in the study will receive 600 mcg misoprostol to be self‐administered as prophylaxis for PPH after delivery of the baby and before delivery of the placenta. |
| Interventions | Misoprostol 800 mcg administered sublingually versus placebo. |
| Outcomes | Proportion of women who experience a drop in haemoglobin concentration greater than 2 g/dL from before delivery to after delivery. Outcomes will be compared between the 2 treatment arms. |
| Starting date | July 2012. |
| Contact information | dabbas@gynuity.org |
| Notes |
Shakur 2010.
| Trial name or title | Tranexamic acid for the treatment of postpartum haemorrhage: an international randomised, double blind placebo controlled trial (the WOMAN trial). |
| Methods | Randomised, double‐blind, placebo‐controlled trial. |
| Participants | 15,000 women with clinician‐diagnosed postpartum haemorrhage. |
| Interventions | Tranexamic acid versus placebo. |
| Outcomes | Proportion of women who die or undergo hysterectomy. The primary cause of death will be described. |
| Starting date | May 2009. |
| Contact information | thewomantrial@lshtm.ac.uk |
| Notes | Estimated end: February 2015. |
Wikkelsoe 2012.
| Trial name or title | FIB‐PPH trial: fibrinogen concentrate as initial treatment for postpartum haemorrhage: study protocol for a randomised controlled trial. |
| Methods | Randomised, double‐blind, placebo‐controlled trial. |
| Participants | 245 women with 1 of the following: (1) following vaginal delivery with estimated blood loss exceeding 500 mL and intended manual removal of the placenta, (2) estimated blood loss exceeding 1000 mL and intended manual exploration of the uterus due to continuous bleeding after the birth of the placenta, (3) following caesarean section with estimated perioperative blood loss exceeding 1000 mL. |
| Interventions | Fibrinogen versus placebo. |
| Outcomes | Need for blood transfusion. |
| Starting date | June 2011. |
| Contact information | wikkelsoe@gmail.com |
| Notes |
Differences between protocol and review
Methods updated.
Contributions of authors
Hatem Mousa assessed trial eligibility, extracted the data and co‐wrote the review.
Jennifer Blum provided data of published trials and co‐wrote the review.
Ghada Abou El Senoun assessed trial eligibility, entered data and co‐wrote the review.
Haleema Shakur assessed trial eligibility and co‐wrote the review.
Zarko Alfirevic verified trial eligibility, extracted data and co‐wrote the review.
Sources of support
Internal sources
The University of Liverpool, UK.
External sources
No sources of support supplied
Declarations of interest
Haleema Shakur and Zarko Alfirevic are investigators in the currently ongoing WOMAN trial. Jennifer Blum was a principal investigator in the Blum 2010, Winikoff 2010, Widmer 2010 and Zuberi 2008 trials. Hatem Mousa has received financial support from Novo Nordisk to investigate recombinant activated factor VII (rFVIIa) as a potential treatment for massive postpartum haemorrhage.
Edited (no change to conclusions)
References
References to studies included in this review
Blum 2010 {published data only}
- Blum J, Winikoff B, Raghavan S, Dabash R, Ramadan MC, Dilbaz B, et al. Treatment of post‐partum haemorrhage with sublingual misoprostol versus oxytocin in women receiving prophylactic oxytocin: a double‐blind, randomised, non‐inferiority trial. Lancet 2010;375(9710):217‐23. [DOI] [PubMed] [Google Scholar]
- Blum J, Winikoff B, Raghavan S, Dabash R, Ramadan MC, Dilbaz B, et al. Treatment of postpartum hemorrhage with sublingual misoprostol versus oxytocin: results from a randomized noninferiority trial among women receiving prophylactic oxytocin. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S22‐S23. [Google Scholar]
- Dao B, Blum J, Barrera G, Cherine Ramadan M, Dabash R, Darwish E, et al. Side effect profiles for misoprostol and oxytocin in the treatment of postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S150. [Google Scholar]
Chantrapitak 2009 {published data only}
- Chantrapitak W, Srijanteok K, Puangsa‐art S. Lower uterine segment compression for management of early postpartum hemorrhage after vaginal delivery at Charoenkrung Pracharak Hospital. Journal of the Medical Association of Thailand 2009;92(5):600‐5. [PubMed] [Google Scholar]
Ducloy‐Bouthors 2011 {published data only}
- Ducloy‐Bouthers A, Broisin F, Keita H, Fontaine S, Depret S, Legoeff F, et al. Tranexamic acid reduces blood loss in postpartum haemorrhage. Critical Care 2010;14()(Suppl 1):S124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducloy‐Bouthors AS, Duhamel A, Broisin F, Keita H, Fontaine S. Tranexamic acid reduces blood loss in post‐partum haemorrhage by reducing hyperfibrinolysis. British Journal of Anaesthesia 2012;108:ii191. [Google Scholar]
- Ducloy‐Bouthors AS, Duhamel A, Tournoys A, Prado‐Dupont A, Debize G, Peneau E, et al. Post‐partum haemorrhage induced hyperfibrinolysis. Thrombosis Research 2013;131(Suppl 1):S89‐90. [Google Scholar]
- Ducloy‐Bouthors AS, Jude B, Duhamel A, Broisin F, Huissoud C, Keita‐Meyer H, et al. High‐dose tranexamic acid reduces blood loss in postpartum haemorrhage. Critical Care (London, England) 2011;15(2):R117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmeyr 2004 {published data only}
- Hofmeyr GJ, Ferreira S, Nikodem VC, Mangesi L, Singata M, Jafta Z, et al. Misoprostol for treating postpartum haemorrhage: a randomized controlled trial [ISRCTN72263357]. BMC Pregnancy and Childbirth 2004;4(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maholwana B, Hofmeyr GJ, Nikodem C, Ferreira S, Singata M, Mangesi L, et al. Misoprostol for treating postpartum haemorrhage. Presented at: 21st Conference on Priorities in Perinatal Care in South Africa. 2002 March 5‐8; Eastern Cape, South Africa.
Lokugamage 2001 {published data only}
- Lokugamage AU, Moodley J, Sullivan K, Rodeck CH, Niculescu L, Tigere P. The Durban primary postpartum haemorrhage study. Women's Health—into the new millennium. Proceedings of the 4th International Scientific Meeting of the Royal College of Obstetricians and Gynaecologists; 1999 October 3‐6; Cape Town, South Africa. 1999:77‐8.
- Lokugamage AU, Sullivan KR, Niculescu I, Tigere P, Onyangunga F, Refaey H, et al. A randomized study comparing rectally administered misoprostol versus syntometrine combined with an oxytocin infusion for the cessation of primary post partum hemorrhage. Acta Obstetricia et Gynecologica Scandinavica 2001;80(9):835‐9. [DOI] [PubMed] [Google Scholar]
Walraven 2004 {published data only}
- Walraven G, Dampha Y, Bittaye B, Sowe M, Hofmeyr J. Misoprostol in the treatment of postpartum haemorrhage in addition to routine management: a placebo randomised control trial. BJOG: an international journal of obstetrics and gynaecology 2004;111:1014‐7. [DOI] [PubMed] [Google Scholar]
Widmer 2010 {published data only}
- Villar J. Misoprostol to treat postpartum hemorrhage (PPH): a randomized controlled trial (Argentina, Egypt, South Africa, Thailand and Viet Nam). Current Controlled Trials (www.controlled‐trials.com/mrct) (accessed 21 March 2006).
- Widmer M, Blum J, Hofmeyr GJ, Carroli G, Abdel‐Aleem H, Lumbiganon P, et al. Misoprostol as an adjunct to standard uterotonics for treatment of post‐partum haemorrhage: a multicentre, double‐blind randomised trial. Lancet 2010;375(9728):1808‐13. [DOI] [PubMed] [Google Scholar]
Winikoff 2010 {published data only}
- Winikoff B. Misoprostol for the treatment of postpartum hemorrhage. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
- Winikoff B, Dabash R, Durocher J, Darwish E, Ngoc NTN, Leon W, et al. Treatment of postpartum hemorrhage with sublingual misoprostol versus oxytocin: results from a randomized, non‐inferiority trial among women not exposed to oxytocin during labor. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S59. [Google Scholar]
- Winikoff B, Dabash R, Durocher J, Darwish E, Nguyen TN, Leon W, et al. Treatment of post‐partum haemorrhage with sublingual misoprostol versus oxytocin in women not exposed to oxytocin during labour: a double‐blind, randomised, non‐inferiority trial. Lancet 2010;375(9710):210‐6. [DOI] [PubMed] [Google Scholar]
Zhou 2006 {published data only}
- Zhou M, Yang CY, Zhao Y, Li P. Clinical value of adjuvant therapy with estrogen for postpartum hemorrhage. Journal of Southern Medical University 2006;26(6):865‐6. [PubMed] [Google Scholar]
Zuberi 2008 {published data only}
- Zuberi NF, Durocher J, Sikander R, Baber N, Blum J, Walraven G. Misoprostol in addition to routine treatment of postpartum hemorrhage: a hospital‐based randomized‐controlled trial in Karachi, Pakistan. BMC Pregnancy and Childbirth 2008;8:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Deneux‐Tharaux 2010 {published data only}
- Deneux‐Tharaux C, Dupont C, Colin C, Rabilloud M, Touzet S, Lansac J, et al. Multifaceted intervention to decrease the rate of severe postpartum haemorrhage: the PITHAGORE6 cluster‐randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2010;117(10):1278‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khalil 2011 {published data only}
- Khalil MI, Al‐Dohami H, Aldahish MM. A method to improve the effectiveness of the Bakri balloon for management of postpartum haemorrhage at caesarean. International Journal of Gynaecology and Obstetrics 2011;115(2):198‐200. [DOI] [PubMed] [Google Scholar]
Khireddine 2012 {published data only}
- Khireddine I, Le C, Dupont C, Rudigoz R, Bouvier‐Colle M, Deneux‐Tharaux C. Induction of labor and risk of postpartum hemorrhage in low risk parturient women. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(S2):101. [Google Scholar]
Magwali 2012 {published data only}
- Magwali TL, Butrick E, Mambo V, Ayadi A, Lippman S, Bergel E, et al. Non‐pneumatic anti‐shock garment (NASG) for obstetric hemorrhage:Harare, Zimbabwe. International Journal of Gynecology & Obstetrics 2012;119(Suppl 3):S410. [Google Scholar]
Soltan 2009 {published data only}
- Soltan MH, Faragallah MF, Mosabah MH, Al‐Adawy AR. External aortic compression device: the first aid for postpartum hemorrhage control. Journal of Obstetrics and Gynaecology Research 2009;35(3):453‐8. [DOI] [PubMed] [Google Scholar]
Soltan 2010 {published data only}
- Soltan MH, Imam HH, Zahran KA, Atallah SM. Assessing changes in flow velocimetry and clinical outcome following use of an external aortic compression device in women with postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2010;110(3):257‐61. [DOI] [PubMed] [Google Scholar]
Takagi 1976 {published data only}
- Takagi S, Yoshida T, Togo Y, Tochigi H, Abe M, Sakata H, et al. The effects of intramyometrial injection of prostaglandin F2alpha on severe post‐partum hemorrhage. Prostaglandins 1976;12(4):565‐79. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Lavigne‐Lissalde 2013 {published data only}
- Gris JC. rhuFVIIa in post‐partum hemorrhage. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
- Lavigne‐Lissalde G, Aya G, Mercier F, Roger‐Christophe S, Chauleur C, Morau E, et al. rhuFVIIa in women with a refractory primary postpartum haemorrhage: an international, multicenter, randomised, opened, controlled trial. Thrombosis Research 2013;131(Suppl 1):S74. [Google Scholar]
References to ongoing studies
Collins 2013 {published data only}
- Collins P. Fibrinogen concentrate versus placebo for treatment of postpartum haemorrhage: A multicentre, prospective, double blind randomised control trial. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 2013).
Miller 2008 {published data only}
- Miller S. Non‐pneumatic anti‐shock garment for obstetrical hemorrhage: Zambia and Zimbabwe. ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008).
Mirzazada 2011 {published data only}
- Mirzazada S. Misoprostol for the treatment of postpartum haemorrhage (PPH) following self‐administration of misoprostol prophylaxis in home deliveries. ClinicalTrials.gov (accessed 21 May 2013).
Shakur 2010 {published data only}
- Gulmezoglu M, Alfirevic Z, Elbourne D, Roberts I. Tranexamic acid for the treatment of postpartum haemorrhage: an international randomised, double blind, placebo controlled trial (woman trial—Protocol Number ISRCTN76912190). International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakur H, Elbourne D, Gulmezoglu M, Alfirevic Z, Ronsmans C, Allen E, et al. The WOMAN Trial (World Maternal Antifibrinolytic Trial): tranexamic acid for the treatment of postpartum haemorrhage: an international randomised, double blind placebo controlled trial. Trials 2010;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wikkelsoe 2012 {published data only}
- Wikkelsoe AJ, Afshari A, Stensballe J, Langhoff‐Roos J, Albrechtsen C, Ekelund K, et al. The FIB‐PPH trial: fibrinogen concentrate as initial treatment for postpartum haemorrhage: study protocol for a randomised controlled trial. Trials [Electronic Resource] 2012;13:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abdel‐Aleem 2001
- Abdel‐Aleem H, El‐Nashar I, Abdel‐Aleem A. Management of severe postpartum hemorrhage with misoprostol. International Journal of Gynecology & Obstetrics. 2001;72:75‐6. [DOI] [PubMed] [Google Scholar]
Abdel‐Aleem 2003
- Abdel‐Aleem H, Villar J, Gulmezoglu AM, Mostafa SA, Youssef AA, Shokry M, et al. The pharmacokinetics of the prostaglandin E1 analogue misoprostol in plasma and colostrum after postpartum oral administration. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;108:25‐8. [DOI] [PubMed] [Google Scholar]
AbdRabbo 1994
- AbdRabbo SA. Stepwise uterine devascularization: a novel technique for the management of uncontrollable postpartum hemorrhage with preservation of the uterus. American Journal of Obstetrics and Gynecology 1994;171:694‐700. [DOI] [PubMed] [Google Scholar]
Abou El Senoun 2011
- Abou El Senoun G, Singh M, Mousa HA, Alfirevic Z. Update on the new modalities on the prevention and management of postpartum haemorrhage. Fetal and Maternal Medicine Review 2011;22(4):247‐64. [Google Scholar]
Abou Zahr 1991
- Abou Zahr C, Royston E. Global Mortality: Global Factbook. Geneva: World Health Organization, 1991. [Google Scholar]
ACOG 1998
- ACOG Technical Bulletin. American College of Obstetricians and Gynecologists educational bulletin. Postpartum hemorrhage, Number 243. International Journal of Gynecology & Obstetrics 1998;61(1):79‐86. [PubMed] [Google Scholar]
Adekanmi 2001
- Adekanmi OA, Purmessur S, Edwards G, Barrington JW. Intrauterine misoprostol for the treatment of severe recurrent atonic secondary postpartum haemorrhage. BJOG: an international journal of obstetrics and gynaecology 2001;108:541‐2. [DOI] [PubMed] [Google Scholar]
Aisaka 1988
- Aisaka K, Ando S, Kokuho K, Tawada T, Kaneda S, Yoshimatsu J, et al. Effects of obesity and weight gain during pregnancy on obstetrical factors. Acta Obstetrica et Gynaecologica Japonica 1988;63:1851‐8. [PubMed] [Google Scholar]
Akhter 2005
- Akhter S, Begum MR, Kabir J. Condom hydrostatic tamponade for massive postpartum hemorrhage. International Journal of Gynecology & Obstetrics 2005;90:134–5. [DOI] [PubMed] [Google Scholar]
Alamia 1999
- Alamia V Jr, Meyer BA. Peripartum hemorrhage. Obstetrics and Gynecology Clinics of North America 1999;26(2):385‐98. [DOI] [PubMed] [Google Scholar]
Alexander 2002
- Alexander J, Thomas P, Sanghera J. Treatments for secondary postpartum haemorrhage. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD002867] [DOI] [PubMed] [Google Scholar]
Alfirevic 2006
- Alfirevic Z, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001338.pub2] [DOI] [PubMed] [Google Scholar]
Alfirevic 2007
- Alfirevic Z, Elbourne D, Pavord S, Bolte A, Geijn H, Mercier F, et al. Use of recombinant activated factorVII in primary postpartum hemorrhage: the Northern European registry 2000‐2004. Obstetrics and Gynecology 2007;110(6):1270‐8. [DOI] [PubMed] [Google Scholar]
Andolina 2003
- Andolina KL, Tolosa JE, Monzo JM, Roberts NS, Daly S. Oral misoprostol is rapidly absorbed in postpartum women at term. Journal of Maternal‐Fetal & Neonatal Medicine 2003;14:229‐32. [DOI] [PubMed] [Google Scholar]
Arulkumaran 2007
- Arulkumaran S, Walker JJ, Watkinson AF, Nicholson T, Kessel D, Patel J. The Role of Emergency and Elective Interventional Radiology in Postpartum Haemorrhage: Good Practice No 6. London: RCOG, 2007. [Google Scholar]
As 1996
- As AK, Hagen P, Webb JB. Tranexamic acid in the management of postpartum haemorrhage. British Journal of Obstetrics and Gynaecology 1996;103(12):1250‐1. [DOI] [PubMed] [Google Scholar]
B‐Lynch 1997
- B‐Lynch C, Coker A, Lawal AH, Abu J, Cowen MJ. The B‐Lynch surgical technique for the control of massive postpartum haemorrhage: an alternative to hysterectomy? Five cases reported. British Journal of Obstetrics and Gynaecology 1997;104(3):372‐5. [DOI] [PubMed] [Google Scholar]
Bakri 1999
- Bakri YN. Balloon device for control of obstetrical bleeding. European Journal of Obstetrics & Gynecology, and Reproductive Biology 1999;86:S84. [Google Scholar]
Baskett 2000
- Baskett TF. A flux of the reds: evolution of active management of the third stage of labour. Journal of the Royal Society of Medicine 2000;93:489‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begley 2011
- Begley CM, Gyte GML, Devane D, McGuire W, Weeks A. Active versus expectant management for women in the third stage of labour. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD007412] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beischer 1986
- Beischer NA, Mackay EV. Obstetrics and the Newborn. Eastbourne: Bailliere Tindall, 1986. [Google Scholar]
Bergstrom 1962
- Bergstrom S, Ryhag ER, Samuelson B, Sjovall J. The structure of prostaglandin E, F1, F2. Acta Chemica Scandinavica 1962;16:501‐2. [Google Scholar]
Bonnar 2000
- Bonnar J. Massive obstetric haemorrhage. Baillieres Best Practice and Research in Clinical Obstetrics and Gynaecology 2000;14(1):1‐18. [DOI] [PubMed] [Google Scholar]
Burchell 1980
- Burchell RC. Postpartum haemorrhage. In: Quilligan ES editor(s). Current Therapy in Obstetrics and Gynaecology. Philadelphia: WB Saunders, 1980. [Google Scholar]
Cantwell 2011
- Cantwell R, Clutton‐Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer: 2006–2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG: an international journal of obstetrics and gynaecology 2011;118(Suppl 1):1‐203. [DOI] [PubMed] [Google Scholar]
Carroli 2008
- Carroli G, Cuesta C, Abalos E, Gulmezoglu AM. Epidemiology of postpartum hemorrhage: a systematic review. Best Practice and Research. Clinical Obstetrics and Gynaecology 2008;22(6):999‐1020. [DOI] [PubMed] [Google Scholar]
Chan 1997
- Chan C, Razvi K, Tham KF, Arulkumaran S. The use of a Sengstaken‐Blakemore tube to control post‐partum hemorrhage. International Journal of Gynaecology and Obstetrics 1997;58:251–2. [DOI] [PubMed] [Google Scholar]
Cho 2000
- Cho JH, Jun HS, Lee CN. Hemostatic suturing technique for uterine bleeding during cesarean delivery. Obstetrics & Gynecology 2000;96(1):129‐31. [DOI] [PubMed] [Google Scholar]
Clark 1985
- Clark SL, Phelan JP, Yeh SY, Bruce SR, Paul RH. Hypogastric artery ligation for obstetric hemorrhage. Obstetrics & Gynecology 1985;66(3):353‐6. [PubMed] [Google Scholar]
Combs 1991
- Combs CA, Murphy EL, Laros RK. Factors associated with postpartum haemorrhage with vaginal birth. Obstetrics & Gynecology 1991;77:69‐76. [PubMed] [Google Scholar]
Cotter 2001
- Cotter AM, Ness A, Tolosa JE. Prophylactic oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001808] [DOI] [PubMed] [Google Scholar]
CRASH‐2 trial collaborators 2010
- CRASH‐2 trial collaborators, Shakur H, Roberts I, Bautista R, Caballero J, Coats T, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH‐2): a randomised, placebo‐controlled trial. Lancet 2010;376(9734):23‐32. [DOI] [PubMed] [Google Scholar]
Cunningham 1993
- Cunningham FG, MacDonald PC, Gant NF, Leveno KJ, Gilstrap LC. Abnormalities of the third stage of labor. Williams Obstetrics. 19th Edition. Norwalk, CT: Appleton & Lange, 1993. [Google Scholar]
Cutler 1971
- Cutler BS, Daggett WM. Application of the "G‐suit" to the control of hemorrhage in massive trauma. Annals of Surgery 1971;173(4):511‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Danielsson 1999
- Danielsson KG, Marions L, Rodriguez A, Spur BW, Wong PY, Bygdeman M. Comparison between oral and vaginal administration of misoprostol on uterine contractility. Obstetrics & Gynecology 1999;93:275‐80. [DOI] [PubMed] [Google Scholar]
Davis 1935
- Davis ME, Adher FL, Rogers G, Kharasch MS, Legault RR. A new active principle in ergot and its effects on uterine motility. American Journal of Obstetrics and Gynecology 1935;29:155‐67. [PubMed] [Google Scholar]
De Loor 1996
- Loor JA, Dam PA. Foley catheters for uncontrollable obstetric or gynecologic hemorrhage. Obstetrics & Gynecology 1996;88:737–8. [DOI] [PubMed] [Google Scholar]
Dodd 2010
- Dodd JM, Crowther CA. Misoprostol for induction of labour to terminate pregnancy in the second or third trimester for women with a fetal anomaly or after intrauterine fetal death. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD004901.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doumouchtsis 2007
- Doumouchtsis SK, Papageorghiou AT, Arulkumaran S. Systematic review of conservative management of postpartum hemorrhage: what to do when medical treatment fails. Obstetrical and Gynecological Survey 2007;62(8):540‐7. [DOI] [PubMed] [Google Scholar]
Drife 1997
- Drife J. Management of primary postpartum haemorrhage. British Journal of Obstetrics and Gynaecology 1997;104:275‐7. [DOI] [PubMed] [Google Scholar]
Du Vigneaud 1953
- Du Vigneaud V, Ressler C, Swan JM, Roberts CW, Katsoyannis PG, Gordon S. The synthesis of an octapeptide with the hormonal activity of oxytocin. Journal of the American Chemical Society 1953;75:4879‐80. [Google Scholar]
Dudley 1935
- Dudley HW, Moir C. The substance responsible for the traditional clinical effect of ergot. BMJ 1935;i:520‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eastman 1950
- Eastman NJ. Anomalies of the third stage of labor. William's Obstetrics. 10th Edition. New York: Appleton‐Century‐Crofts, 1950:917‐9. [Google Scholar]
Ferrer 2009
- Ferrer P, Roberts I, Sydenham E, Blackhall K, Shakur H. Anti‐fibrinolytic agents in post partum haemorrhage: a systematic review. BMC Pregnancy Childbirth 2009;15(9):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Franchini 2010
- Franchini M, Franchi M, Bergamini V, Montagnana M, Salvagno GL, Targher G, et al. The use of recombinant activated FVII in postpartum haemorrhage. Clinical Obstetrics and Gynecology 2010;53:219‐27. [DOI] [PubMed] [Google Scholar]
Gando 2006
- Gando S, Iba T, Eguchi Y, Ohtomo Y, Okamoto K, Koseki K, et al. A multicenter, prospective validation of disseminated intravascular coagulation diagnostic criteria for critically ill patients: comparing current criteria. Critical Care Medicine 2006;34(3):625‐31. [DOI] [PubMed] [Google Scholar]
Georgiou 2009
- Georgiou C. Balloon tamponade in the management of postpartum haemorrhage: a review. BJOG: an international journal of obstetrics and gynaecology 2009;116:748‐57. [DOI] [PubMed] [Google Scholar]
Gilbert 1987
- Gilbert L, Porter W, Brown VA. Postpartum haemorrhage: a continuing problem. British Journal of Obstetrics and Gynaecology 1987;94:67‐71. [DOI] [PubMed] [Google Scholar]
Gyte 1992
- Gyte G. The significance of blood loss at delivery. MIDIRS Midwifery Digest 1992;2(1):88‐92. [Google Scholar]
Gülmezoglu 2001
- Gülmezoglu AM, Villar J, Ngoc NT, Piaggio G, Carroli G, Adetoro L, et al. WHO multicentre randomised trial of misoprostol in the management of the third stage of labour. Lancet 2001;358(9283):689‐95. [DOI] [PubMed] [Google Scholar]
Haggerty 1996
- Haggerty J. Antishock garment. http://www.sti.nasa.gov/tto/spinoff1996/28. (accessed 20 February 2008).
Hall 1985
- Hall MH, Halliwell R, Carr‐Hill R. Concomitant and repeated happenings of complications of the third stage of labour. British Journal of Obstetrics and Gynaecology 1985;92:732‐8. [DOI] [PubMed] [Google Scholar]
Hayman 2002
- Hayman RG, Arulkumaran S, Steer PJ. Uterine compression sutures: surgical management of postpartum hemorrhage. Obstetrics & Gynecology 2002;99(3):502‐6. [DOI] [PubMed] [Google Scholar]
Hensleigh 2002
- Hensleigh PA. Anti‐shock garment provides resuscitation and haemostasis for obstetric haemorrhage. BJOG: an international journal of obstetrics and gynaecology 2002;109(12):1377‐84. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hofmeyr 2005
- Hofmeyr GJ, Walraven G, Gulmezoglu AM, Maholwana B, Alfirevic Z, Villar J. Misoprostol to treat postpartum haemorrhage: a systematic review. BJOG: an international journal of obstetrics and gynaecology 2005;112:547‐53. [DOI] [PubMed] [Google Scholar]
Hofmeyr 2008
- Hofmeyr GJ, Gülmezoglu AM. Misoprostol for the prevention and treatment of postpartum haemorrhage. Best Practice & Research Clinical Obstetrics & Gynaecology 2008;22(6):1025‐41. [DOI] [PubMed] [Google Scholar]
Hunter 1992
- Hunter DJ, Schulz P, Wassenaar W. Effect of carbetocin, a long acting oxytocin analogue in the postpartum uterus. Clinical Pharmacology and Therapeutics 1992;52:60‐7. [DOI] [PubMed] [Google Scholar]
Johanson 2001
- Johanson R, Kumar M, Obhrai M, Young P. Management of massive postpartum haemorrhage: use of a hydrostatic balloon catheter to avoid laparotomy. BJOG: an international journal of obstetrics and gynaecology 2001;108(4):420‐2. [DOI] [PubMed] [Google Scholar]
Jouppila 1995
- Jouppila P. Postpartum haemorrhage. Current Opinion in Obstetrics and Gynecology 1995;7:446‐50. [DOI] [PubMed] [Google Scholar]
Khan 2003
- Khan RU, El‐Refaey H. Pharmacokinetics and adverse‐effect profile of rectally administered misoprostol in the third stage of labour. Obstetrics & Gynecology 2003;101:968‐74. [DOI] [PubMed] [Google Scholar]
Khan 2006
- Khan KS, Wojdyla D, Say L, Gülmezoglu AM, Look PF. WHO analysis of causes of maternal death: a systematic review. Lancet 2006;367(9516):1066‐74. [DOI] [PubMed] [Google Scholar]
Knight 2009
- Knight M, Callaghan WM, Berg C, Alexander S, Bouvier‐Colle MH, Ford JB, et al. Trends in postpartum haemorrhage in high resource countries: a review and recommendations from the International Postpartum Haemorrhage Collaborative Group. BMC Pregnancy Childbirth 2009;27(9):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liabsuetrakul 2007
- Liabsuetrakul T, Choobun T, Peeyananjarassri K, Islam QM. Prophylactic use of ergot alkaloids in the third stage of labour. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD005456.pub2] [DOI] [PubMed] [Google Scholar]
Maier 1993
- Maier RC. Control of postpartum haemorrhage with uterine packing. American Journal of Obstetrics and Gynecology 1993;169:317‐23. [DOI] [PubMed] [Google Scholar]
Marasinghe 2011
- Marasinghe JP, Condous G, Seneviratne HR, Marasinghe U. Modified anchored B‐Lynch uterine compression suture for post partum bleeding with uterine atony. Acta Obstetricia et Gynecologica Scandinavica 2011;90:280–3. [DOI] [PubMed] [Google Scholar]
McDonald 2004
- McDonald SJ, Abbott JM, Higgins SP. Prophylactic ergometrine‐oxytocin versus oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD000201] [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2013
- McDonald SJ, Middleton P, Dowswell T, Morris PS. Effect of timing of umbilical cord clamping of term infants on maternal and neonatal outcomes. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD004074.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Miller 2009
- Miller S, Ojengbede O, Turan JM, Morhason‐Bello IO, Martin HB, Nsima D. A comparative study of the non‐pneumatic anti‐shock garment for the treatment of obstetric hemorrhage in Nigeria. International Journal of Gynecology & Obstetrics 2009;107(2):121‐5. [DOI] [PubMed] [Google Scholar]
Miller 2010
- Miller S, Fathalla MM, Ojengbede OA, Camlin C, Mourad‐Youssif M, Morhason‐Bello IO, et al. Obstetric hemorrhage and shock management: using the low technology non‐pneumatic anti‐shock garment in Nigerian and Egyptian tertiary care facilities. BMC Pregnancy Childbirth 2010;18:10‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mobeen 2011
- Mobeen N, Durocher J, Zuberi N, Jahan N, Blum J, Wasim S, et al. Administration of misoprostol by trained traditional birth attendants to prevent postpartum haemorrhage in homebirths in Pakistan: a randomised placebo‐controlled trial. BJOG: an international journal of obstetrics and gynaecology 2011;118:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moir 1932
- Moir JC. The action of ergot preparations on the puerperal uterus. BMJ 1935;i:520‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moscardo 2001
- Moscardo F, Perez F, Rubia J, Balerdi B, Lorenzo JI, Senent ML, et al. Successful treatment of severe intra‐abdominal bleeding associated with disseminated intravascular coagulation using recombinant activated factor VII. British Journal of Haematology 2001;114:174‐6. [DOI] [PubMed] [Google Scholar]
Mousa 2001
- Mousa HA, Walkinshaw S. Major postpartum haemorrhage. Current Opinion in Obstetrics and Gynecology 2001;13(6):595‐603. [DOI] [PubMed] [Google Scholar]
Mousa 2002
- Mousa H, Alfirevic Z. Major postpartum hemorrhage: survey of maternity units in the United Kingdom. Acta Obstetricia et Gynecologica Scandinavica 2002;81(8):727‐30. [DOI] [PubMed] [Google Scholar]
Mousa 2008
- Mousa HA, Cording V, Alfirevic Z. Risk factors and interventions associated with major primary postpartum hemorrhage unresponsive to first‐line conventional therapy. Acta Obstetricia et Gynecologica Scandinavica 2008;87(6):652‐61. [DOI] [PubMed] [Google Scholar]
Nardin 2011
- Nardin JM, Weeks A, Carroli G. Umbilical vein injection for management of retained placenta. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD001337] [DOI] [PubMed] [Google Scholar]
Novikova 2010
- Novikova N, Hofmeyr GJ. Tranexamic acid for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD007872] [DOI] [PubMed] [Google Scholar]
O'Brien 1998
- O'Brien P, El‐Refaey H, Gordon A, Geary M, Rodeck CH. Rectally administered misoprostol for the treatment of postpartum haemorrhage unresponsive to oxytocin and ergometrine: a descriptive study. Obstetrics & Gynecology 1998;92:212‐4. [DOI] [PubMed] [Google Scholar]
Oboro 2003
- Oboro VO, Tabowei TO, Bosah JO. Intrauterine misoprostol for refractory postpartum haemorrhage. International Journal of Gynecology & Obstetrics 2003;80:67‐8. [DOI] [PubMed] [Google Scholar]
Ochoa 2002
- Ochoa M, Allaire AD, Stitely ML. Pyometria after hemostatic square suture technique. Obstetrics & Gynecology 2002;99(3):506‐9. [DOI] [PubMed] [Google Scholar]
Ojengbede 2011
- Ojengbede OA, Morhason‐Bello IO, Galadanci H, Meyer C, Nsima D, Camlin C, et al. Assessing the role of the non‐pneumatic anti‐shock garment in reducing mortality from postpartum hemorrhage in Nigeria. Gynecologic and Obstetric Investigation 2011;71(1):66‐72. [DOI] [PubMed] [Google Scholar]
Oladapo 2012a
- Oladapo OT, Fawole B, Blum J, Abalos E. Advance misoprostol distribution for preventing and treating postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009336] [DOI] [PubMed] [Google Scholar]
Oladapo 2012b
- Oladapo OT, Okusanya BO, Abalos E. Intramuscular versus intravenous prophylactic oxytocin for the third stage of labour. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009332] [DOI] [PubMed] [Google Scholar]
Oleen 1990
- Oleen MA, Mariano JP. Controlling refractory atonic postpartum hemorrhage with hemabate sterile solution. American Journal of Obstetrics and Gynecology 1990;162(1):205‐8. [DOI] [PubMed] [Google Scholar]
Ouahba 2007
- Ouahba J, Piketty M, Huel C, Azarian M, Feraud O, Luton D, et al. Uterine compression sutures for postpartum bleeding with uterine atony. BJOG: an international journal of obstetrics and gynaecology 2007;114(5):619‐22. [DOI] [PubMed] [Google Scholar]
Peitsidis 2011
- Peitsidis P, Kadir RA. Antifibrinolytic therapy with tranexamic acid in pregnancy and postpartum. Expert Opinion on Pharmacotherapy 2011;12(4):503‐16. [DOI] [PubMed] [Google Scholar]
Penninx 2010
- Penninx JP, Pasmans HL, Oei SG. Arterial balloon occlusion of the internal iliac arteries for treatment of life‐threatening massive postpartum haemorrhage: a series of 15 consecutive cases. European Journal of Obstetrics & Gynecology, and Reproductive Biology 2010;148(2):131‐4. [DOI] [PubMed] [Google Scholar]
Pereira 2005
- Pereira A, Nunes F, Pedroso S, Saraiva J, Retto H, Meirinho M. Compressive uterine sutures to treat postpartum bleeding secondary to uterine atony. Obstetrics and Gynecology 2005;106(3):569‐72. [DOI] [PubMed] [Google Scholar]
Porro 1876
- Porro E. Della amputazione utero‐ovarica come complemento di taglio cesareo. Milan 1876.
Poujade 2011
- Poujade O, Grossetti A, Mougel L, Ceccaldi P, Ducarme G, Luton D. Risk of synechiae following uterine compression sutures in the management of major postpartum haemorrhage. BJOG: an international journal of obstetrics and gynaecology 2011;118:433–9. [DOI] [PubMed] [Google Scholar]
Prendiville 1989
- Prendiville WJ, Elbourne DR. Care during the third stage of labour.. In: Chalmers I, Enkin M, Keirse MJNC editor(s). Effective care in pregnancy and childbirth. Oxford: Oxford University Press,, 1989:1145–69. [Google Scholar]
Pritchard 1962
- Pritchard JA, Baldwin RM, Dickey JC, Wiggins KM. Blood volume changes in pregnancy and puerperium, II: red blood cell loss and changes in apparent blood volume during and following vaginal delivery, cesarean section, and cesarean section plus total hysterectomy. American Journal of Obstetrics and Gynecology 1962;84:1271‐82. [Google Scholar]
Rath 2009
- Rath W. Prevention of postpartum haemorrhage with the oxytocin analogue carbetocin. European Journal of Obstetrics & Gynecology, and Reproductive Biology 2009;147(1):15‐20. [DOI] [PubMed] [Google Scholar]
Rathat 2011
- Rathat G, Do Trinh P, Mercier G, Reyftmann L, Dechanet C, Boulot P, et al. Synechia after uterine compression sutures. Fertility and Sterility 2011;95(1):405‐9. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Ripley 1999
- Ripley DL. Uterine emergencies. Atony, inversion, and rupture. Obstetrics and Gynecology Clinics of North America 1999;26(3):419‐34. [DOI] [PubMed] [Google Scholar]
Sloan 2010
- Sloan NL, Durocher J, Aldrich T, Blum J, Winikoff B. What measured blood loss tells us about postpartum bleeding: a systematic review. BJOG: an international journal of obstetrics and gynaecology 2010;117(7):788‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stafford 2008
- Stafford I, Dildy GA, Clark SL, Belfort MA. Visually estimated and calculated blood loss in vaginal and cesarean delivery. American Journal of Obstetrics and Gynecology 2008;199(5):e511–e517. [DOI] [PubMed] [Google Scholar]
Stearns 1808
- Stearns J. Account of the pulvis parturiens, a remedy for quickening childbirth. Medical Repository of New York 1808;11:308‐9. [Google Scholar]
Stearns 1822
- Steans J. Observations on the secale cornutum or ergot, with directions for its use in parturition. Medical Research 1822;5:90. [Google Scholar]
Stoll 1935
- Stoll A, Burckhardt E. L'ergobasine, nouvel alkaloide de l'ergot de seigle, solubile dans l'eau. Bulletin of Pharmaceutical Sciences 1935;42:257‐66. [Google Scholar]
Stones 1993
- Stones RW, Paterson CM, Saunders NSTG. Risk factors for major obstetric haemorrhage. European Journal of Obstetrics & Gynecology and Reproductive Biology 1993;48:15‐8. [DOI] [PubMed] [Google Scholar]
Su 2007
- Su LL, Chong YS, Samuel M. Oxytocin agonists for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD005457] [DOI] [PubMed] [Google Scholar]
Su 2012
- Su LL, Chong YS, Samue lM. Carbetocin for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD005457.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suzuki 2012
- Suzuki S, Hiraizumi Y, Miyake H. Risk factors for postpartum hemorrhage requiring transfusion in cesarean deliveries for Japanese twins: comparison with those for singletons. Archives of Gynecology and Obstetrics 2012;286(6):1363‐7. [DOI] [PubMed] [Google Scholar]
Tang 2002
- Tang OS, Schweer H, Seyberth HW, Lee SW, Ho PC. Pharmacokinetics of different routes of administration of misoprostol. Human Reproduction 2002;17:332‐6. [DOI] [PubMed] [Google Scholar]
Thompson 1935
- Thompson MR. The active constituents of ergot. A pharmacological and chemical study. Journal of the American Pharmaceutical Association 1935;24:185‐96. [Google Scholar]
Tseng 2011
- Tseng JJ, Ho JY, Wen MC, Hwang JI. Uterine necrosis associated with acute suppurative myometritis after angiographic selective embolization for refractory postpartum hemorrhage. American Journal of Obstetrics and Gynecology 2011;204(6):e4‐e6. [DOI] [PubMed] [Google Scholar]
Tsu 1993
- Tsu VD. Postpartum haemorrhage in Zimbabwe: a risk factor analysis. British Journal of Obstetrics and Gynaecology 1993;100:327‐33. [DOI] [PubMed] [Google Scholar]
Tunçalp 2012
- Tunçalp Ö, Hofmeyr GJ, Gülmezoglu AM. Prostaglandins for preventing postpartum haemorrhage. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD000494.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vahedi 1995
- Vahedi M, Ayuyao A, Parsa M, Freeman H. Pneumatic antishock garment‐associated compartment syndrome in uninjured lower extremities. Journal of Trauma 1995;384(4):616‐8. [DOI] [PubMed] [Google Scholar]
Wetta 2013
- Wetta LA, Szychowski JM, Seals S, Mancuso MS, Biggio JR, Tita AT. Risk factors for uterine atony/postpartum hemorrhage requiring treatment after vaginal delivery. American Journal of Obstetrics and Gynecology 2013;209(1):51.e1‐51.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2010
- WHO, UNICEF, UNFPA. The World Bank World Health Organization (WHO) Press. Trends in Maternal Mortality: 1990 to 2008: Estimates Developed by WHO, UNICEF, UNFPA and The World Bank. Geneva: WHO, 2010. [Google Scholar]
Zheng 2011
- Zheng J, Xiong X, Ma Q, Zhang X, Li M. A new uterine compression suture for postpartum haemorrhage with atony. BJOG: an international journal of obstetrics and gynaecology 2011;118:370–4. [DOI] [PubMed] [Google Scholar]
Zieman 1997
- Zieman M, Fong SK, Benowitz NL, Banskter D, Darney PD. Absorption kinetics of misoprostol with oral or vaginal administration. Obstetrics & Gynecology 1997;90:88‐92. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hofmeyr 2009
- Hofmeyr GJ, Gülmezoglu AM, Novikova N, Linder V, Ferreira S, Piaggio G. Misoprostol to prevent and treat postpartum haemorrhage: a systematic review and meta‐analysis of maternal deaths and dose‐related effects. Bulletin of the World Health Organization 2009;87(9):666‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mousa 2003
- Mousa HA, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003249] [DOI] [PubMed] [Google Scholar]
Mousa 2007
- Mousa HA, Alfirevic Z. Treatment for primary postpartum haemorrhage. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD003249.pub2] [DOI] [PubMed] [Google Scholar]


