Abstract
Background
Invasive aspergillosis is the most common life‐threatening opportunistic invasive mycosis in immunocompromised patients. A test for invasive aspergillosis should neither be too invasive nor too great a burden for the already weakened patient. The serum galactomannan enzyme‐linked immunosorbent assay (ELISA) seems to have the potential to meet both requirements.
Objectives
To obtain summary estimates of the diagnostic accuracy of galactomannan detection in serum for the diagnosis of invasive aspergillosis.
Search methods
We searched MEDLINE, EMBASE and Web of Science with both MeSH terms and text words for both aspergillosis and the sandwich ELISA. We checked the reference lists of included studies and review articles for additional studies. We conducted the searches in February 2014.
Selection criteria
We included cross‐sectional studies, case‐control designs and consecutive series of patients assessing the diagnostic accuracy of galactomannan detection for the diagnosis of invasive aspergillosis in patients with neutropenia or patients whose neutrophils are functionally compromised. The reference standard was composed of the criteria given by the European Organization for Research and Treatment of Cancer (EORTC) and the Mycoses Study Group (MSG).
Data collection and analysis
Two review authors independently assessed quality and extracted data. We carried out meta‐analysis using the bivariate method. We investigated sources of heterogeneity by adding potential sources of heterogeneity to the model as covariates.
Main results
We included 54 studies in the review (50 in the meta‐analyses), containing 5660 patients, of whom 586 had proven or probable invasive aspergillosis. When using an optical density index (ODI) of 0.5 as a cut‐off value, the sensitivity of the test was 78% (70% to 85%) and the specificity was 85% (78% to 91%). At a cut‐off value of 1.0 ODI, the sensitivity was 71% (63% to 78%) and the specificity was 90% (86% to 93%). At a cut‐off value of 1.5 ODI, the sensitivity was 63% (49% to 78%) and the specificity was 93% (89% to 97%). None of the potential sources of heterogeneity had a statistically significant effect on either sensitivity or specificity.
Authors' conclusions
If we used the test at a cut‐off value of 0.5 ODI in a population of 100 patients with a disease prevalence of 11% (overall median prevalence), two patients who have invasive aspergillosis would be missed (sensitivity 78%, 22% false negatives), and 13 patients would be treated unnecessarily or referred unnecessarily for further testing (specificity 85%, 15% false negatives). If we used the test at a cut‐off value of 1.5 in the same population, that would mean that four invasive aspergillosis patients would be missed (sensitivity 61%, 39% false negatives), and six patients would be treated or referred for further testing unnecessarily (specificity 93%, 7% false negatives). These numbers should, however, be interpreted with caution because the results were very heterogeneous.
Plain language summary
Measurement of serum galactomannan to detect invasive aspergillosis in immunocompromised patients
When the immune system of a patient is unable to fight infections (for example because of prolonged corticosteroid therapy, immunosuppressive drugs, haematological malignancies or HIV/AIDS) invasive or systemic aspergillosis can be a life‐threatening mycotic (fungal) infection. Establishing a diagnosis of invasive aspergillosis at an early stage of infection allows early antifungal treatment, but a definitive diagnosis can only be established after death. To enable early diagnosis in a way that is not burdensome for the already weakened patient, galactomannan testing may be promising. Galactomannan is a cell wall component of Aspergillus that is excreted by the fungus.
Study design
The authors of this systematic review found 54 studies that looked at the error rates of this galactomannan test. These studies compared the results of the galactomannan test with the results of a more elaborate diagnostic workup, so that the percentages of false positive results (patients without invasive aspergillosis, according to the elaborate testing, but with a positive galactomannan test) and false negative results (patients with invasive aspergillosis, according to the elaborate testing, but with a negative galactomannan test) could be calculated. The galactomannan test does not result in a yes/no answer, but in a so‐called 'optical density index' (ODI). The authors of the different studies defined the galactomannan test as positive when the ODI was above 0.5, 1.0 or 1.5. Four studies used a different ODI and these were not included in the meta‐analysis.
Studies and results
When an ODI of 0.5 or higher was said to be positive, the galactomannan test missed 22 out of every 100 patients with invasive aspergillosis and it resulted in a false positive test in 15 out of every 100 patients without invasive aspergillosis.
When an ODI of 1.0 or higher was said to be positive, the galactomannan test missed 29 out of every 100 patients with invasive aspergillosis and it resulted in a false positive test in 10 out of every 100 patients without invasive aspergillosis.
When an ODI of 1.5 or higher was said to be positive, the galactomannan test missed 37 out of every 100 patients with invasive aspergillosis and it resulted in a false positive test in only 7 out of every 100 patients without invasive aspergillosis.
Limitations
The studies showed variable results and had small numbers of patients with invasive aspergillosis.
Summary of findings
Summary of findings'. 'Summary of results table: different cut‐offs.
|
What is the diagnostic accuracy of the galactomannan ELISA for invasive aspergillosis for different cut‐off values? Patients/population: immunocompromised patients, mostly haematology patients Prior testing: varied, mostly underlying disease or symptoms (fever, neutropenia) Setting: mainly haematology or cancer departments, mainly inpatients Index test: a sandwich ELISA for galactomannan, an Aspergillus antigen Importance: depends on the time‐gain the test may give Reference standard: gold standard is autopsy, but that is almost never done; in most studies therefore the reference standard is composed of clinical and microbiological criteria Studies: patient series or case‐control studies, not using an in‐house test and not excluding possibly infected patients. Studies had to report cut‐off values that were used (n = 29). Each study can be present in more than one subgroup | |||||
| Subgroup |
Effect (95% CI) |
No. of participants (studies) |
Prevalence (median, range) |
Comments | What do these results mean? |
| Cut‐off 0.5 |
Sensitivity 0.78 (0.70 to 0.85) Specificity 0.85 (0.78 to 0.91) |
394 proven or probable 3549 possible or no IA (27) |
Median 11% (IQR 6.5% to 16%) |
— | With a prevalence of 11%*, 11 out of 100 patients will develop IA Of these, 2 will be missed by the Platelia test (22% of 11), but will be tested again Of the 89 patients without IA, 13 will be unnecessarily referred for CT scanning |
| Cut‐off 1.0 |
Sensitivity 0.71 (0.63 to 0.78) Specificity 0.90 (0.86 to 0.93) |
145 proven or probable 1246 possible or no IA (8) |
Median 13% (IQR 4.2% to 31%) |
— | With a prevalence of 11%*, 11 out of 100 patients will develop IA Of these, 3 will be missed by the Platelia test (29% of 11), but will be tested again Of the 89 patients without IA, 9 will be unnecessarily referred for CT scanning |
| Cut‐off 1.5 |
Sensitivity 0.63 (0.49 to 0.77) Specificity 0.93 (0.89 to 0.97) |
209 proven or probable 2412 possible or no IA (15) |
Median 7.4% (IQR 4.3% to 16%) |
— | With a prevalence of 11%*, 11 out of 100 patients will develop IA Of these, 4 will be missed by the Platelia test (36% of 11), but will be tested again Of the 89 patients without IA, only 6 will be unnecessarily referred for CT scanning |
| Children |
Sensitivity 0.84 (0.66 to 0.93) Specificity 0.88 (0.60 to 0.97) |
47 proven or probable 308 possible or no IA (in 6 studies) |
Median 16% (IQR 10% to 16%) |
5 studies had a cut‐off of 0.5 and one had a cut‐off of 1.5 | Of the 100 children, 16 had IA Of these, 2 or 3 (2.5) will be missed; while 10 out of the 84 children without IA will test positive and be referred unnecessarily for CT scanning |
* Median prevalence over all studies was 11% (range 0.8% to 56%).
CI: confidence interval; CT: computerised tomography; ELISA: enzyme‐linked immunosorbent assay; IA: invasive aspergillosis; IQR: interquartile range
Background
Target condition being diagnosed
Invasive aspergillosis is the most common life‐threatening opportunistic invasive mycosis in immunocompromised patients (Kontoyiannis 2002). Mortality in patients diagnosed with this condition ranges from 70% to 90% at one year (Upton 2007). Invasive aspergillosis is caused by ubiquitous Aspergillus species that invade (most often) from the lungs into the adjacent organs if the immune system is not able to fight the infection. Its incidence is still increasing, mainly because of the increasing number of patients undergoing intensified chemotherapy or receiving prolonged corticosteroid therapy, and due to the increasing number of transplant recipients (Denning 1998; Marr 2002).
Establishing a diagnosis of invasive aspergillosis at an early stage of infection and subsequent early treatment improves the chances of survival (Upton 2007). However, clinical signs and symptoms are non‐specific and characteristic lesions on chest radiographs are frequently absent. The only definite reference standard to confirm invasive aspergillosis is autopsy, combined with culture from autopsy specimens. As a clinical reference standard, the demonstration of hyphen invasion in tissue specimens obtained by invasive procedures, in combination with a positive culture for Aspergillus species from the same specimens, establishes a diagnosis of invasive aspergillosis (Hope 2005; Singh 2005). The problem is that the patient's status often prohibits the use of invasive techniques. In addition, culturing of the causative agent can result in false negative or false positive results.
In 2001, a committee consisting of the Invasive Fungal Infections Cooperative Group of the European Organization for Research and Treatment of Cancer (EORTC), the Mycoses Study Group (MSG) and the National Institute of Allergy and Infectious Diseases proposed to grade the diagnosis of invasive aspergillosis at three levels of probability (Ascioglu 2002): proven, probable and possible invasive aspergillosis (seeTable 2). Unfortunately, these levels are only useful in research settings, because in clinical practice a large number of patients will be classified as 'possible', which may lead to overexposure to antifungal therapy if all 'possible' patients are treated (Subira 2003).
1. EORTC/MSG criteria.
| Proven IA | Histopathologic or cytopathologic examination showing hyphae from needle aspiration or biopsy specimen with evidence of associated tissue damage; or positive culture result for a sample obtained by sterile procedure from a normally sterile and clinically or radiologically abnormal site consistent with infection |
| Probable IA | At least 1 host factor criterion; and 1 microbiological criterion; and 1 major (or 2 minor) clinical criteria from abnormal site consistent with infection |
| Possible IA | At least 1 host factor criterion; and 1 microbiological or 1 major (or 2 minor) clinical criteria from abnormal site consistent with infection. This category is not recommended for use in clinical trials of antifungal agents |
Host factor criteria are, for example, neutropenia, persistent fever, predisposing conditions, prolonged use of corticosteroids.
Microbiological criteria are positive culture from sputum, bronchoalveolar lavage fluid (BAL) samples or from sinus aspirate specimen; positive result for Aspergillus antigen in specimens of BAL, cerebrospinal fluid or two or more blood samples.
Major clinical criteria are, for example, new infiltrates on CT imaging (e.g. halo sign), suggestive radiological findings.
Minor clinical criteria are suggestive symptoms and signs.
The exact definitions of the EORTC/MSG criteria and their host factor, microbiological or clinical criteria can be found here (Ascioglu 2002).
CT: computerised tomography; EORTC: European Organization for Research and Treatment of Cancer; MSG: Mycoses Study Group
The main issues in the diagnosis of invasive aspergillosis are the following: a test needs to be sensitive in the early phase of the infection in order to start treatment early, but should not pose a large burden on the already weakened patient. Screening immunocompromised patients for invasive aspergillosis weekly or twice a week with such a test may lead to earlier treatment and better outcomes. When screening results are positive, patients may be referred for further confirmation of the diagnosis or they may be treated immediately.
Imaging techniques are neither invasive nor too great a burden for most patients. The presence of the so‐called 'halo sign' or the 'air crescent sign' on radiographs or computed tomography is indicative of invasive aspergillosis. These signs are, however, not long‐lasting: approximately a week after infection these signs disappear. The costs and the rapid accumulation of radiation associated with computed tomography (CT) scanning prevent its use as a screening tool for invasive aspergillosis. Furthermore, imaging techniques only give a clinical diagnosis, not a microbiological diagnosis. Microbiological diagnosis can be achieved through culturing of the fungus from normally sterile tissues or through histology of those tissues. These techniques, however, are time‐consuming and often too invasive for the patient.
An alternative is the use of laboratory tests. These include the detection of antigens (beta‐glucan or galactomannan), measurement of antibodies or nucleic acid detection techniques. Of these tests, the detection of galactomannan is currently the one that is most often used in practice. Galactomannan is a cell wall component of Aspergillus spp. and Penicillium spp. (Latgé 1994). It is excreted by the fungus during the growth phase and it has been suggested that the level of galactomannan is proportional to the fungal load in tissue and that the level of galactomannan has a prognostic value.
Index test(s)
There are currently two commercially available assays for the detection of galactomannan: the Pastorex® latex agglutination test and the Platelia® sandwich enzyme‐linked immunosorbent assay (ELISA) test. Of these two, the Pastorex® kit is only rarely used nowadays. The ELISA is mostly used for the detection of antigen in serum and in fluid that is obtained via bronchoalveolar lavage (BAL). Other specimens in which the test can also be used are cerebrospinal fluid (CSF) or urine (Ascioglu 2001; Hope 2005). We focused on the ELISA test in serum, because obtaining serum is less of a burden for the patient than collecting BAL fluid. Results of the ELISA are given as an optical density index (ODI), which is the ratio of the optical density of (usually) 1 ng/mL galactomannan versus the optical density of the sample. In order to enhance the sensitivity of the test, in the USA the manufacturer changed the recommended cut‐off for positivity in from 1.5 to 0.5 ODI.
Clinical pathway
There is substantial variation in the way the galactomannan ELISA is currently used in the clinic. Some clinicians do not use it at all, while others use the galactomannan ELISA as a screening tool, to monitor whether patients at risk develop invasive aspergillosis or not. In those cases, serum is tested for invasive aspergillosis once or twice every week. Sometimes the galactomannan ELISA is used to test for invasive aspergillosis in BAL fluid when it is already suspected and in those situations the test is only used in serum when there is no BAL fluid. In most situations, the galactomannan ELISA is used as a triage test: if the ELISA is positive, patients will be referred for further diagnostic testing or they will be referred for antifungal therapy (Segal 2006). Further diagnostic testing may involve either laboratory testing of BAL fluid, CT scanning or radiography, or a combination of tests. Patients may also be referred for further diagnostic work‐up on the basis of clinical signs and symptoms.
Objectives
Our primary objective was to assess the diagnostic accuracy of galactomannan detection in serum for the diagnosis of invasive aspergillosis in immunocompromised patients, at different cut‐off values for test positivity.
Secondary objectives
We aimed to study several possible sources of heterogeneity: subgroups of patients, different interpretations of the EORTC/MSG criteria as the reference standard and study design features.
Methods
Criteria for considering studies for this review
Types of studies
Studies that assessed the diagnostic accuracy of galactomannan detection by the Platelia© sandwich ELISA test, with either prospective or retrospective data collection, were eligible. The galactomannan ELISA could be assessed alone or in comparison to other tests.
Participants
Studies had to include patients with neutropenia or patients whose neutrophils are functionally compromised. We included studies with the following patient groups:
patients with haematological malignancies, receiving haematopoietic stem cell transplants, chemotherapeutics or immunosuppressive drugs;
solid organ transplant recipients and other patients who are receiving immunosuppressive drugs for a prolonged time;
patients with cancer who are receiving chemotherapeutics;
patients with a medical condition compromising the immune system, such as HIV/AIDS and chronic granulomatous disease (CGD, an inherited abnormality of the neutrophils).
Index tests
A commercially available galactomannan sandwich ELISA (Platelia©) was the test under evaluation. We only included studies concerning galactomannan detection in serum. We excluded studies addressing detection in BAL fluid, a number of other body fluids, such as CSF or peritoneal fluid, and tissue. We also excluded studies evaluating in‐house serum galactomannan tests.
Target conditions
The target condition of this review was invasive aspergillosis, also called invasive pulmonary aspergillosis or systemic aspergillosis.
Reference standards
The following reference standards can be used to define the target condition:
autopsy;
the criteria of the EORTC/MSG (Ascioglu 2002; De Pauw 2008); or
the demonstration of hyphal invasion in biopsies, combined with a positive culture for Aspergillus species from the same specimens.
The gold standard for this diagnosis is autopsy, combined with a positive culture of Aspergillus species from the autopsy specimens, or with histopathological evidence of Aspergillus. Autopsy is rarely reported, therefore we decided to take the criteria of the EORTC/MSG as the reference standard. These criteria divide the patient population into four categories: patients with proven invasive aspergillosis, patients who probably have invasive aspergillosis, patients who possibly have invasive aspergillosis and patients without invasive aspergillosis (seeTable 2). This division is based on host factor criteria, microbiological criteria and clinical criteria. Clinical studies have shown that these criteria do not match autopsy results perfectly. This especially true for the possible category. For clinical trials investigating the effect of treatment, for example, it is recommended that only the proven and probable categories are used (Borlenghi 2007; Subira 2003).
The exclusion of patients with 'possible' invasive aspergillosis, which can be regarded as group of 'difficult or atypical' patients, is likely to affect the observed diagnostic accuracy of a test. Also, the exclusion of any other of the reference standard groups may affect the accuracy of the index test. We therefore excluded studies explicitly excluding one of the four categories of patients from the review, as well as studies in which it is not clear how many patients with proven, probable, possible or no invasive aspergillosis had positive or negative index test results.
Search methods for identification of studies
Electronic searches
We searched MEDLINE (through PubMed), EMBASE (through Ovid) and ISI Web of Knowledge for relevant articles. We updated the search on 24 June 2011 and again on 17 February 2014 by searching the complete databases again with revised search terms. We compared the results of the updated search with the results of the previous search and removed duplicates. The revised search strategies can be found in Appendix 1.
Searching other resources
To identify additional published, unpublished and ongoing studies, we:
entered relevant studies identified from the above sources into PubMed and then use the 'Related Articles' feature;
searched the Science Citation Index to identify articles that cite the relevant articles;
checked the reference lists of all relevant studies.
In the protocol, we stated that we would also contact authors and industry, but due to time constraints we were not able to do this.
Data collection and analysis
Selection of studies
After we removed all articles on animal studies, plant studies, mycotoxin studies and studies of allergic aspergillosis from the set of retrieved articles, two authors (ML, YD) selected potentially relevant articles based on title and abstract. Afterwards, we obtained the full paper of each potentially eligible article located in the search. Three review authors (ML, CV, YD) independently assessed eligible articles for inclusion. We resolved disagreements by discussion. We included all articles on which disagreement could not be resolved.
Data extraction and management
We extracted the following:
author, year of publication and journal;
study design;
study population;
reference standard and performance of the reference standard;
performance of the index test;
QUADAS‐2 items;
data for two‐by‐two tables.
The data extraction form was accompanied by a background document that stated how each item on the form should be interpreted. We standardised the form and piloted it on two primary diagnostic studies, including the quality assessment. Six review authors in total extracted data and assessed quality. Two review authors independently assessed each article. One author had a methodological background and the other a microbiological background. The articles were randomly allocated to a pair of assessors. We resolved disagreements by discussion.
Assessment of methodological quality
Study quality was assessed using QUADAS‐2 (Whiting 2003; Whiting 2011). The items of the QUADAS‐2 tool and their interpretation are described in Appendix 2. Results are presented in the text and in a graph. We did not calculate a summary score estimating the overall quality of a article since the interpretation of such summary scores is problematic and potentially misleading (Juni 1999; Whiting 2005).
Statistical analysis and data synthesis
Our reference standard was the set of EORTC/MSG criteria that can be used to classify patients to one of four groups: proven, probable, possible and no invasive aspergillosis. This resulted in a two‐by‐four table: a positive or negative galactomannan test result in each one of the four reference groups. To calculate test accuracy and to reflect the categories that are used in clinical practice to guide further management, we made the post hoc decision to define the proven and probable patients as having invasive aspergillosis and we defined the possible and no invasive aspergillosis patients as not having invasive aspergillosis, in order to construct two‐by‐two tables. We assessed other divisions between patients having and not having invasive aspergillosis in a subgroup analysis, but because of the limited clinical value and statistical limitations (not enough proven invasive aspergillosis patients, for example) our focus was on the ability of the galactomannan ELISA to discriminate between patients that were either classified as proven or probable and patients who were classified as possible or no invasive aspergillosis. We excluded studies reporting insufficient data for the construction of a two‐by‐two table from the final analyses.
We used the data from the two‐by‐two tables to calculate sensitivity and specificity for each study. We present individual study results graphically by plotting the estimates of sensitivity and specificity (and their 95% confidence intervals) in both forest plots and the receiver operating characteristic (ROC) space. We used a bivariate random‐effects approach for the meta‐analysis of the pairs of sensitivity and specificity and for the construction of a summary ROC curve (Reitsma 2005). We incorporated covariates in the bivariate model to examine the effect of potential sources of bias and variation across subgroups of studies. Due to the bivariate nature of the model, effects of covariates on sensitivity and specificity can be modelled separately.
If more than one threshold was reported in a study, we selected one of those thresholds to incorporate in the meta‐analysis. In that case, we chose the threshold of 0.5, if reported, because this is the positivity threshold currently recommended by the manufacturer. Meta‐analyses were restricted to those studies that reported one of the most often used cut‐off values (0.5, 1.0 or 1.5).
Investigations of heterogeneity
We investigated heterogeneity in the first instance through visual examination of forest plots of sensitivities and specificities and through visual examination of the ROC plot of the raw data. We addressed the following three sources of heterogeneity: effect of cut‐off value, effect of the reference standard and existence of clinical subgroups.
a. Effect of cut‐off value
A main source of heterogeneity in diagnostic test accuracy reviews is differences in the applied cut‐off value between studies. We expected studies to report three different cut‐off values: 1.5 ODI (the value previously prescribed by the manufacturer), 0.5 ODI (the value prescribed by the manufacturer nowadays) and 1.0 ODI (an intermediate value). We therefore first investigated what was the influence of these cut‐off values on sensitivity and specificity by including the cut‐off value as a covariate in the meta‐regression model.
Some studies defined a positive test result as one single sample that exceeded the cut‐off value, while others defined a test result as positive when at least two subsequent samples (taken within a week) exceeded the cut‐off value. The latter was only reported in studies that used the galactomannan ELISA to monitor whether the patients developed invasive aspergillosis. The single sample definition was both used in these screening studies and in studies that only tested for galactomannan when there was suspicion of invasive aspergillosis (e.g. fever not responsive to antibacterial medication). We examined the impact of single sample versus subsequent sample by adding subsequent testing as a covariate to the previous analysis.
b. Effect of the definition of invasive aspergillosis
Our reference standard consists of the criteria of the EORTC/MSG, as published by Ascioglu 2002 or De Pauw 2008. This reference standard classified patients into four groups. We studied what the effect was of our definition of 'diseased' patients (i.e. either proven or probable invasive aspergillosis) and 'non‐diseased' patients (either possible or no invasive aspergillosis) versus other definitions of diseased (only proven patients or all patients except no invasive aspergillosis) and non‐diseased (all patients except the proven invasive aspergillosis patients or only no invasive aspergillosis patients).
c. Clinical subgroups
We explored the possible influence of clinical subgroups by stratified analyses and by including additional covariates in the regression analyses. We carried out these additional analyses by adding these variables as covariates to the analyses. We used the following variables as covariates in the meta‐analyses:
children versus adults;
distinctive groups of patients (e.g. patients at high risk versus patients at low risk of invasive aspergillosis; solid organ transplants versus haematological patients);
use of antifungal prophylaxis (yes versus no);
use of antifungal therapy (yes versus no).
Sensitivity analyses
To assess whether methodological quality influenced the results we found, we compared the results of only studies fulfilling certain quality criteria with the overall results. We also did a sensitivity analysis for Chinese studies only and for our choice to regard proven and probable as being diseased versus possible and no invasive aspergillosis as being non‐diseased.
Results
Results of the search
Our updated search in 2011 resulted in 4377 unique titles (Figure 1). After removing totally irrelevant titles (allergic aspergillosis, plants, animals, studies published before 1995), we checked 972 titles and abstracts in duplicate for eligibility. After assessment of the selected 158 full‐text articles, 87 articles seemed to be eligible for inclusion. From these 87 articles, we excluded 49 from the review for various reasons. We found no extra studies through additional searches or reference checking. The updated search in 2011 revealed 6 extra studies compared to the original version of the review. The updated search in 2014 resulted in 1605 additional unique titles; of these, we checked the full texts of 168 papers and included 38 studies. During data extraction, we excluded a further 22 studies and thus included another 16 studies in the review. In total, this review includes 54 relevant articles (of which 6 plus 16 were not in the original version). As four studies did not report the results of a cut‐off value of 0.5 ODI, 1.0 ODI or 1.5 ODI, we used 50 studies in the meta‐analyses.
1.
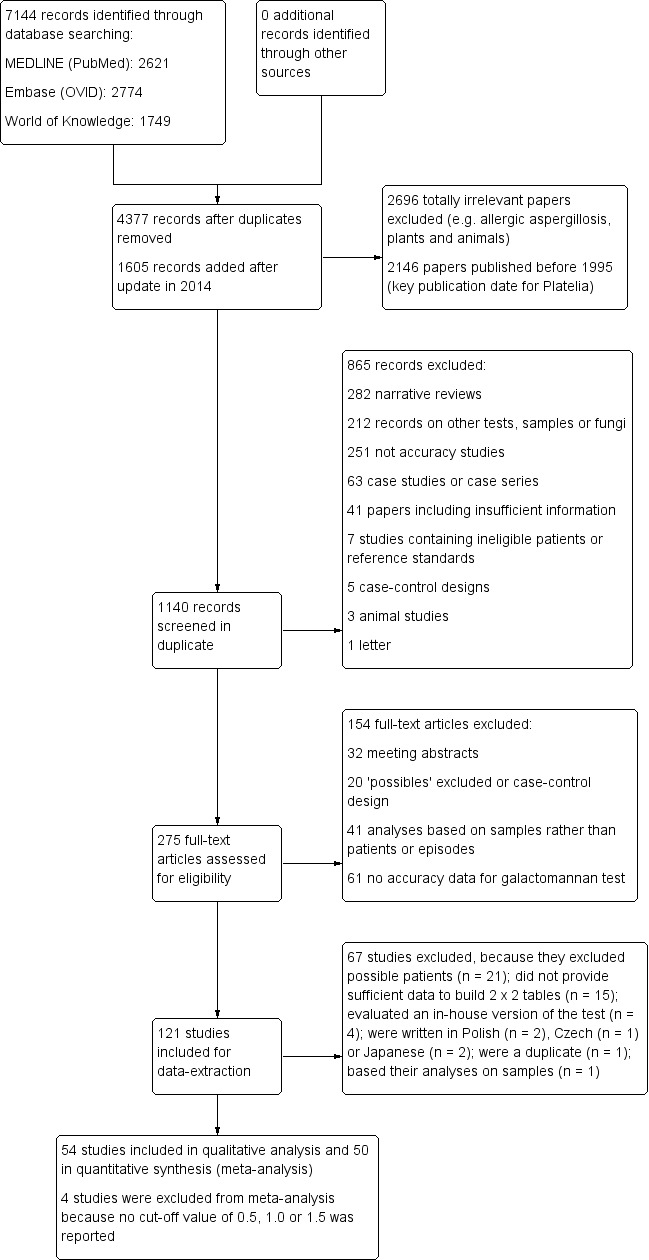
Study flow diagram.
Included studies
The Characteristics of included studies table lists the characteristics of the 54 included studies, containing a total of 197 participants with proven invasive aspergillosis, 573 participants with probable invasive aspergillosis, 43 participants that were classified in one group as proven or probable invasive aspergillosis, 980 participants with possible invasive aspergillosis, 5284 participants with no invasive aspergillosis and 1219 participants that were classified in one group as possible or no invasive aspergillosis. One study included a group of nine so‐called 'suspected' patients, in between proven and no invasive aspergillosis (Williamson 2000). We considered this group as possibles.
Most studies reported diagnostic accuracy based on the results in individual patients, whereas seven studies reported test results for treatment, neutropenic or disease episodes, without exactly stating how many episodes there were per patient. As most patients will have only one or two episodes, we did not expect diagnostic test accuracy to change by the inclusion of these studies and therefore we have included them in the analyses.
The reference standard was formed by the EORTC/MSG criteria that defined the proven, probable, possible or no invasive aspergillosis categories. We also included studies that used criteria that were similar to the EORTC criteria (thus, defining groups of patients with ordinal certainty of invasive aspergillosis). Eleven studies were done in China and used the criteria from the Editorial Board of the Chinese Journal of Internal Medicine. As these criteria also divide the patients into proven, probable, possible and no invasive aspergillosis categories, we assumed that they were sufficiently similar to include these studies. However, we did carry out a sensitivity analysis to assess their effect on the results.
Twenty studies presented the results for a cut‐off value of a single test above 0.5 ODI; 17 presented the results for a cut‐off value of two subsequent tests above 0.5. Ten studies reported the results for a cut‐off value of a single test above 1.0, while seven presented the results for a cut‐off value of two subsequent tests above 1.0. Fifteen studies reported the results for a cut‐off value of a single test above 1.5, while 10 presented the results for a cut‐off value of two subsequent tests above 1.5. A few of these studies also reported the results for other cut‐off values, such as 0.38 (one study) or 0.80 (two studies). One study did not report a cut‐off value at all and three studies reported a mixed cut‐off value of either subsequently above 0.5 or a single value above 0.8.
Excluded studies
We excluded 67 articles (seeCharacteristics of excluded studies table). Possible invasive aspergillosis patients were excluded by 24 studies, which we excluded from the review for that reason. Four assessed another test than the commercially available galactomannan ELISA or an obsolete version, and 14 studies did not provide sufficient details to make two‐by‐two tables. One study was published in duplicate, one was a letter to the editor and one provided two‐by‐two tables based on samples rather than individual patients. Five studies needed to be translated, but we could not find a translator.
Methodological quality of included studies
Figure 2 shows the overall quality of the 54 included studies.
2.
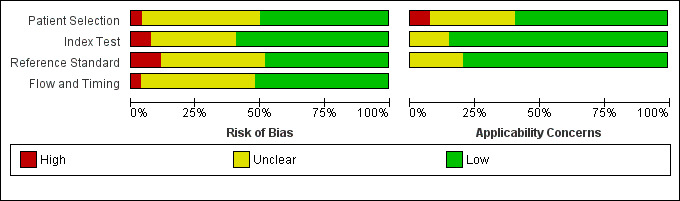
Methodological quality graph: Review authors' judgements about each methodological quality item presented as percentages across all included studies.
Slightly more than half of the studies had included a representative patient spectrum. Two studies reported the results of a case‐control study, but in these studies the controls were randomly selected from the previously tested population. In eight studies it was not clear whether they used a case‐control design or not. Eleven studies were not clear about how they interpreted the EORTC/MSG criteria or whether they used other criteria as reference standard, five of which were of Chinese origin. The time between the galactomannan ELISA and the actual diagnosis was reported in 10 studies and eight of these reported an acceptable time gap. Partial and differential verification was not a problem. Most studies (n = 29) reported explicitly that they did not include the galactomannan ELISA in the EORTC/MSG criteria, but there were 12 studies that explicitly reported that galactomannan testing was part of the reference standard. Blinding of both the results of the reference standard and the results of the index test was reported variably. Most studies reported no details at all about any uninterpretable or indeterminate index test results.
The quality assessment results for the individual studies can be found in Figure 3.
3.
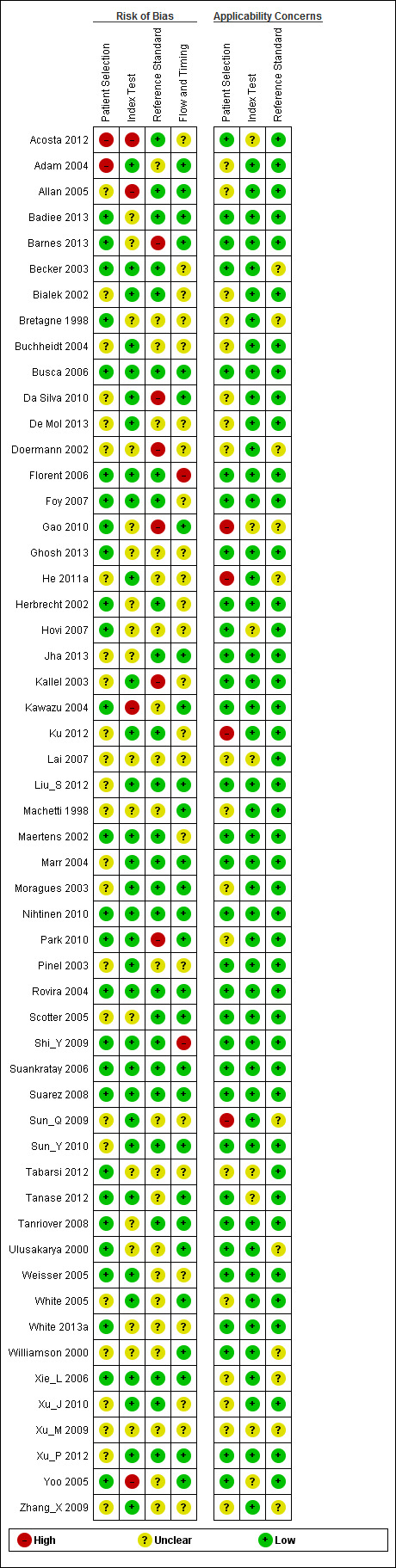
Methodological quality summary: Review authors' judgements about each methodological quality item for each included study.
Findings
The sensitivity of the 54 studies varied from 0% to 100% and the specificity from 21% to 100% (see Figure 4 and Figure 5). The wide range of the sensitivity was largely due to chance variation, because of small numbers of patients with the target condition (proven or probable) in the various studies, ranging from 1 to 98 (median 12). For instance, if there is only one patient with proven or probable invasive aspergillosis in a study and this patient had a positive test, the sensitivity would be 100%, but if he or she had a negative test result, the sensitivity would be 0%. Small numbers of patients were not an issue in the possible or no invasive aspergillosis groups (median 92, range 8 to 773).
4.
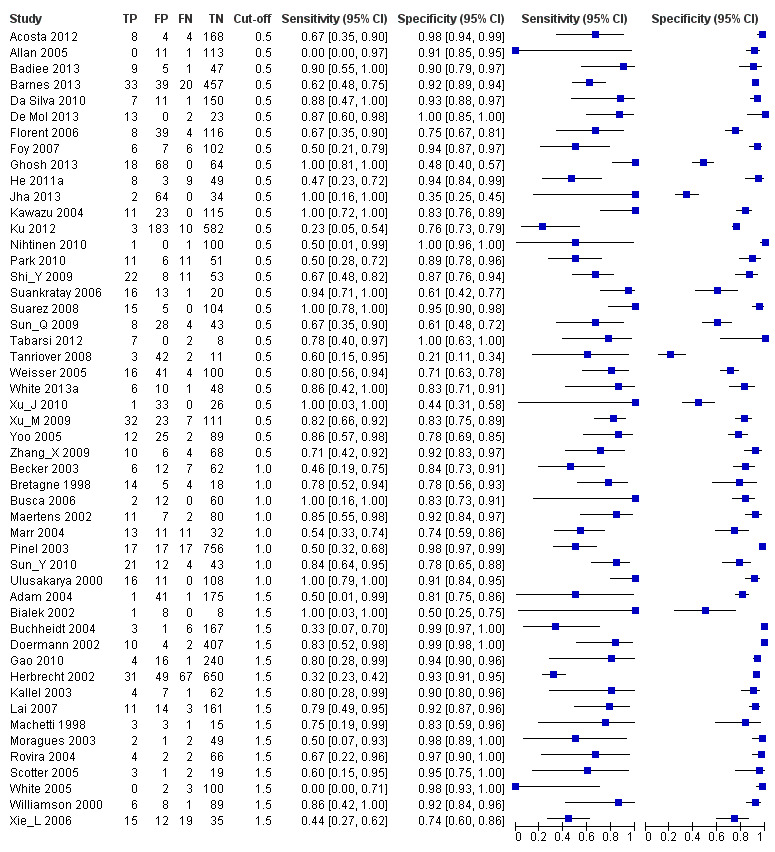
Forest plots of sensitivity and specificity. The squares represent the sensitivity and specificity of one study, the black line its confidence interval. Studies are grouped by reported cut‐off value. If a study reported accuracy data for more than one cut‐off, its results are included in more than one subgroup. TP = True Positive; FP = False Positive; FN = False Negative; TN = True Negative.
Forest plot of the included studies. TP = True Positive; FP = False Positive; FN = False Negative; TN = True Negative. Between brackets the 95% confidence intervals (CI) of sensitivity and specificity. The figure shows the estimated sensitivity and specificity of the study (blue square) and its 95% confidence interval (black horizontal line).
5.
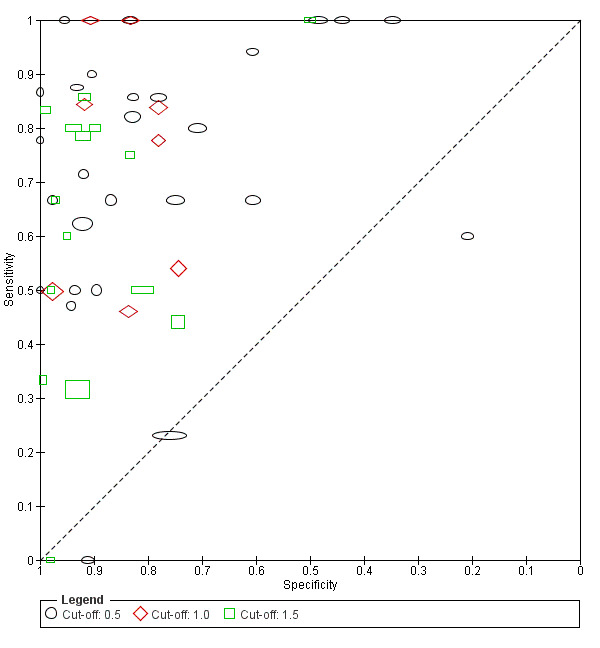
Plot of sensitivity versus specificity for all 54 studies, with different symbols for the different cut‐off values. The width of the symbols is proportional to the inverse standard error of the specificity in every study and the height of the symbols is proportional to the inverse standard error of the sensitivity.
The median prevalence of invasive aspergillosis patients was 11% (range 0.8% to 56%; interquartile range 4.5% to 21%). This prevalence is based on the proportion of proven and probable patients in the studies that included consecutive series of patients with a comparable risk of developing invasive aspergillosis (in contrast to case‐control studies, where the numbers of cases and controls, and thus the prevalence, is determined by the researchers).
We carried out meta‐analyses on the 50 studies that reported a cut‐off value of 0.5, 1.0 or 1.5, either as a single test result or subsequently.
After selecting one two‐by‐two table from each study, we had 27 two‐by‐two tables for a cut‐off value of 0.5, eight two‐by‐two tables for 1.0 and 15 two‐by‐two tables for 1.5. As expected, applying a higher cut‐off value led to lower sensitivities and higher specificities; these effects of cut‐off value were statistically significant for specificity only (P value = 0.07 for sensitivity and P value = 0.03 for specificity) (Figure 6).
6.
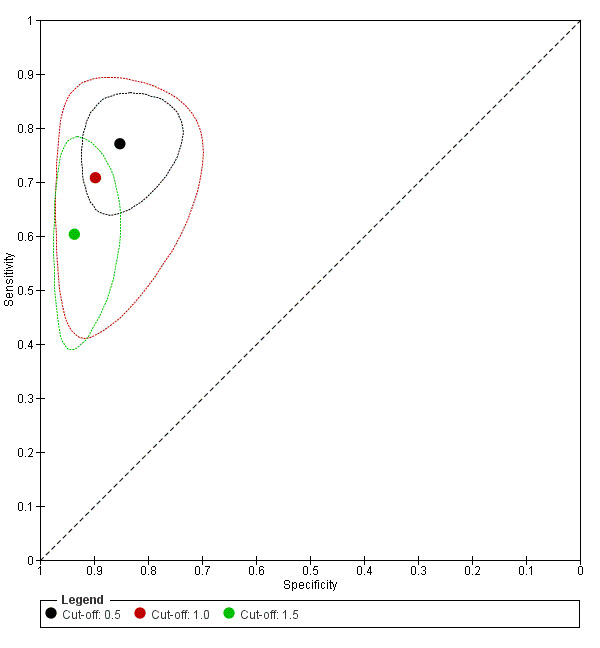
Summary ROC plots for galactomannan test at three different cut‐off values. The graph shows the point estimates of sensitivity and specificity (solid dots) and the 95% confidence regions (dotted lines) around it. Data for individual studies are not shown in this plot.
A participant could be defined as test‐positive in two ways: a single sample above the cut‐off value; or two subsequent samples above the cut‐off value. In those situations, a participant was only defined as having a positive enzyme‐linked immunosorbent assay (ELISA) when two subsequent test results were both above the cut‐off value. This increases specificity, as the number of false positive results will decrease, and it decreases sensitivity. This effect was not statistically significant (P value = 0.73 for sensitivity and P value = 0.12 for specificity).
The results for both analyses are presented in Table 3.
2. Effect of definition of test positivity.
| Cut‐off | Analysis | Studies (n) | Sensitivity (95% CI) | Specificity (95% CI) |
| 0.5 | 27 | 0.78 (0.70 to 0.85) | 0.85 (0.78 to 0.91) | |
| Single sample | 13 | 0.79 (0.69 to 0.88) | 0.80 (0.71 to 0.90) | |
| Subsequent samples | 14 | 0.77 (0.67 to 0.87) | 0.88 (0.81 to 0.94) | |
| 1.0 | 8 | 0.71 (0.63 to 0.78) | 0.90 (0.86 to 0.93) | |
| Single sample | 4 | 0.72 (0.62 to 0.82) | 0.87 (0.81 to 0.93) | |
| Subsequent samples | 4 | 0.70 (0.59 to 0.80) | 0.92 (0.88 to 0.96) | |
| 1.5 | 15 | 0.63 (0.49 to 0.77) | 0.93 (0.89 to 0.97) | |
| Single sample | 8 | 0.64 (0.48 to 0.80) | 0.92 (0.86 to 0.97) | |
| Subsequent samples | 7 | 0.61 (0.45 to 0.78) | 0.95 (0.91 to 0.98) |
CI: confidence interval
Effect of different definitions of 'diseased' patients
Table 4 shows the results of the analyses based on different definitions of 'diseased' patients. In the analyses above, sensitivity was calculated based on both the proven and the probable patients. If we calculate sensitivity with the results of only the proven patients, sensitivity improves from 78% (70% to 85%) to 89% (79% to 99%) for subgroups with a cut‐off value of 0.5 optical density index (ODI). In these analyses, the probable patients were considered to have no invasive aspergillosis and are used (with the possibles and the no invasive aspergillosis group) to calculate specificity, which decreased from 85% (78% to 91%) to 72% (62% to 82%). These effects, increasing sensitivity and decreasing specificity, are the same for all cut‐off values.
3. Effect definition of 'diseased' patients.
|
Cut‐off value and analysis |
Proven and probable versus possible and no IA | Proven versus probable, possible and no IA | Proven, probable and possible versus no IA | ||||||
| n |
Sensitivity (95% CI) |
Specificity (95% CI) |
n |
Sensitivity (95% CI) |
Specificity (95% CI) |
n |
Sensitivity (95% CI) |
Specificity (95% CI) |
|
| 0.5 ODI | 27 | 0.78 (0.70 to 0.85) | 0.85 (0.78 to 0.91) | 18 | 0.89 (0.79 to 0.99) | 0.72 (0.62 to 0.82) | 19 | 0.55 (0.41 to 0.69) | 0.87 (0.80 to 0.94) |
| 1.0 ODI | 8 | 0.71 (0.63 to 0.78) | 0.90 (0.86 to 0.93) | 8 | 0.79 (0.70 to 0.89) | 0.83 (0.78 to 0.88) | 8 | 0.54 (0.44 to 0.65) | 0.93 (0.90 to 0.96) |
| 1.5 ODI | 15 | 0.63 (0.49 to 0.77) | 0.93 (0.89 to 0.97) | 14 | 0.65 (0.48 to 0.83) | 0.91 (0.86 to 0.96) | 14 | 0.54 (0.36 to 0.71) | 0.97 (0.94 to 0.99) |
CI: confidence interval; IA: invasive aspergillosis; ODI: optical density index
When we calculated sensitivity based on all patients except the no invasive aspergillosis patients, then sensitivity decreased (from 78% to 55%). This is due to the addition of the possible patients, who will be more often falsely negative if we classify them as having invasive aspergillosis. Specificity in these analyses was only based on no invasive aspergillosis patients and increased from 85% (78% to 91%) to 87% (80% to 94%) for subgroups with a cut‐off value of 0.5 ODI. Again this effect is present for all cut‐off values.
Clinical subgroups
It is possible that the accuracy of the following clinical subgroups could differ and therefore they are a potential source of heterogeneity:
children versus adults;
distinctive groups of patients;
use of antifungal prophylaxis;
use of antifungal therapy.
Children versus adults
Within the set of 54 studies there were seven studies that reported data on children. Foy 2007 reported separate results for both adults and children. Badiee 2013, Bialek 2002, De Mol 2013, Hovi 2007, Jha 2013 and Zhang_X 2009 only reported results for children. Five studies reported a cut‐off of 0.5; one a cut‐off of 1.5 and one study did not report a cut‐off (Hovi 2007).
Whether the analyses were based only on children or not had no significant effect on either sensitivity (P value = 0.09) or specificity (P value = 0.69).
Effect of distinctive groups of patients
We were not able to investigate the effect of distinctive groups of patients either due to the absence of such patient groups in the included studies or because this information was not presented in the articles. Some studies reported the inclusion of high‐risk patients, but the definition of high‐risk was not always clear or the definition of high‐risk matched the inclusion criteria of studies that did not report that they included high‐risk patients. Also the type of underlying disease was not always clearly reported.
Therefore, we decided post hoc to analyse the effect of prevalence of invasive aspergillosis on the accuracy of the galactomannan test and the effect of the way the patients were selected for the study, as a proxy for disease severity. High prevalence of invasive aspergillosis may reflect a population that is at high risk of developing invasive aspergillosis. The effect of prevalence on sensitivity and specificity was not significant when it was in addition to cut‐off value as a covariate in the regression analysis (sensitivity, P value = 0.99; specificity, P value = 0.96).
Another post hoc analysis to investigate the effect of distinctive patient groups was the assessment of the effect of the selection of patients on the accuracy of the galactomannan test. We divided the studies into three groups: (1) studies that did not restrict the patients that would be included in the study and that used the galactomannan ELISA as a screening test in all patients (n = 15; median prevalence 7.6%, interquartile range 2.0% to 12%); (2) studies that included only patients who had fever for a certain number of days and whose fever was not responsive to antibiotic treatment (n = 17; median prevalence 12%, interquartile range 8.6% to 23.0%); (3) studies that used other selection methods, mostly based on underlying diseases, or that did not report clearly how they selected their patients, or that did use a combination of selection methods (n = 14; median prevalence 14%, interquartile range 6.7% to 34%). The difference in selection methods had no significant effect on either sensitivity (P value = 0.21 for fever; P value = 0.21 for unselected) or specificity (P value = 0.088 for fever; P value = 0.89 for unselected).
Use of antifungal prophylaxis or antifungal therapy
Eighteen studies used antifungal prophylaxis, 22 studies did not and 11 studies provided no details on the use of prophylaxis. The use of prophylaxis was not significantly associated with either sensitivity (P value = 0.16) or specificity (P value = 0.63). Thirty‐eight studies used a therapeutic antifungal intervention (mostly amphotericin B), three studies did not and 10 studies were not clear on whether they used therapy or not. Most studies that did use antifungal therapy kept monitoring galactomannan levels during therapy. Use of antifungal therapy had a significant effect on sensitivity (P value = 0.04), but not on specificity (P value = 0.32).
Sensitivity analysis
We explored the impact of risk of bias by doing a sensitivity analysis only for studies that had a low risk of bias for each of the QUADAS‐2 domains (Figure 7). We also analysed the effect of different reference standards, studies including haematology patients only and Chinese articles. For all the sensitivity analyses the confidence intervals overlap with the overall meta‐analysis.
7.
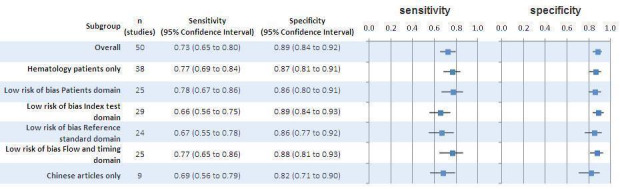
Sensitivity analyses
Discussion
Summary of main results
We included 54 studies in the review, but the results of the meta‐analyses are based on the 50 studies that explicitly reported the use of the commercially available galactomannan enzyme‐linked immunosorbent assay (ELISA) and the cut‐off value(s), and that included results for all four categories of invasive aspergillosis patients: proven, probable, possible and no invasive aspergillosis. Quality features that were poorly reported were: the time between the galactomannan ELISA and the actual diagnosis, whether reference and index tests were performed in a blinded fashion and the source of funding.
The mean sensitivity of the galactomannan ELISA at a cut‐off of 0.5 optical density index (ODI) was 78% (70% to 85%) and the specificity was 85% (78% to 91%). At a cut‐off value of 1.0 ODI, sensitivity was 71% (63% to 78%) and specificity was 90% (86% to 93%). At a cut‐off value of 1.5 ODI, sensitivity was 63% (49% to 77%) and specificity was 93% (89% to 97%). Sensitivity in particular was very heterogeneous. Part of this heterogeneity can be explained by the inclusion of small studies and by the inclusion of studies with low prevalence. SeeTable 1. When two subsequent positive test results were needed to indicate a patient as being 'positive', sensitivity decreased slightly and specificity increased considerably. There were no potential sources of heterogeneity that had a significant effect on either sensitivity or specificity, except that in studies in which the patients were curatively treated, the sensitivity was slightly higher than in studies that did not report curative treatment during the study period.
Our results compared with other reports
Several reviews have been published in recent years about the (lack of) usefulness of the galactomannan ELISA for the diagnosis of invasive aspergillosis (Mennink‐Kersten 2004; Pfeiffer 2006; Segal 2006; Verdaguer 2007). Most of these reviews, however, are based on non‐systematic methods. Pfeiffer and colleagues undertook a systematic approach to summarise all available studies until 2005 (Pfeiffer 2006). Although this meta‐analysis has methodological limitations (sensitivity and specificity were summarised separately, for example), their results for the different cut‐off value subgroups did not differ much from ours (Leeflang 2006). As a change in cut‐off value will always lead to an opposite change in sensitivity and specificity across studies, we studied the effect of other potential factors by including them as covariates additional to the cut‐off value. This gives a more realistic estimation of the sensitivity and specificity belonging to a certain group of studies. Pfeiffer 2006 also recommended that a higher rather than a lower cut‐off value improves diagnostic test accuracy. They only looked, however, at the diagnostic odds ratio (DOR) for this conclusion. Using the DOR to guide clinical decisions regarding the use of a diagnostic test has some serious limitations. It does not take into account the relative importance of false negative or false positive results. A test with a sensitivity of 70% and a specificity of 90% has the same DOR as a test with a sensitivity of 90% and a specificity of 70%, but the clinical consequences of missing a diseased patient (false negative) are not identical to those of given unnecessary treatment to a non‐diseased patient (false positive).
Strengths and weaknesses of the review
We reviewed the diagnostic accuracy of a commercially available galactomannan ELISA to diagnose invasive aspergillosis according to the most recent insights and methods for diagnostic meta‐analyses. The results can, however, be biased by the use and implementation of the reference standard in a way that we have not been able to detect. We only included studies that used the European Organization for Research and Treatment of Cancer (EORTC)/Mycoses Study Group (MSG) criteria or a similar reference standard, but we can imagine that these criteria may still be interpreted subjectively, especially regarding the host factor criteria. Differences in interpretation of the reference standard may have been the reason for the large differences we found in the distribution of patients with proven, probable, possible and no invasive aspergillosis. A relatively large proportion of proven and probable patients may suggest that the reference standard is interpreted in a liberal way, which would then lead to more patients with proven/probable invasive aspergillosis that in reality might not have it. In that case, the estimated sensitivity will be lower than the true sensitivity.
Another indication that the reference standard may not be interpreted in the same way for each study is the variation in prevalence that we found among the studies. Prevalence of proven and probable invasive aspergillosis combined varied from 0.8% to 44%, with an overall median of 9.3%. Variation in prevalence can be caused by several different mechanisms, among which are differences in reference standard interpretation, differences in underlying population, differences in selection of participants and differences in referral pattern (the way through which the participants have been referred to the study location). These mechanisms may also cause differences in diagnostic test accuracy, but their effect on sensitivity and specificity may vary. For example, patient groups with a higher prevalence may include more severe cases of disease (Mulherin 2002). In that situation, one would expect that patient groups with a higher prevalence would also show a higher sensitivity, because more severe cases will result in more true positives and fewer false negatives. We found, however, no significant effect of prevalence on diagnostic accuracy, either when we used prevalence as a continuous covariate (results not shown), or when we used prevalence as dichotomous covariate. This may be because the patients were selected for being 'at high risk' before they developed invasive aspergillosis, so that this only changes the proportion of patients with proven or probable invasive aspergillosis in this group, but not the severity of this disease in the high‐risk group. Another explanation may be that there is no relationship between the severity of invasive aspergillosis and the serum galactomannan titre.
Another factor that we could not control was the time between the index test and the reference standard. Our reference standard was a composite reference standard, therefore the final diagnosis could have been made at several time points and at different time intervals from the index test. If the time between the index test and the reference standard is too long, the true disease status of the patient may have changed by the time the reference standard is assessed.
We defined the proven and probable patients as having invasive aspergillosis and we defined the possible and no invasive aspergillosis patients as not having invasive aspergillosis, in order to construct two‐by‐two tables. Whether this would have influenced our results depends on the association between the galactomannan test results and the true underlying invasive aspergillosis status in the 'probables' and in the 'possibles'.
Applicability of findings to the review question
We reviewed the diagnostic accuracy of only one test, but it would have been worthwhile to investigate the relative value of the galactomannan ELISA in addition to all other tests that can be performed. However, the galactomannan test has the advantage that it is not an invasive test and hence can be assessed in very ill patients. In some patients, it may therefore be the only available test. In that case, this review gives a valuable overview of the possibilities and weaknesses of the test. Furthermore, the current use of the galactomannan ELISA and its place in the clinic differs from place to place. It would therefore have been very difficult to make comparisons that would have been relevant for a broader public.
In some clinics, the galactomannan test is used in addition to the clinical presentation of the patient and chest radiographs, as a tool to monitor whether the immunocompromised patient develops invasive aspergillosis. If a patient has fever and pulmonary symptoms that do not respond to antimicrobial therapy, he or she will be referred for high‐resolution computed tomography (HRCT). If the galactomannan test is positive, the patient will also be referred for HRCT; it is generally believed that the galactomannan test becomes positive before clinical signs of aspergillosis develop. Hence, the use of this test will lead to earlier referral for HRCT, before clear symptoms develop, and to earlier treatment, if the test is positive. This, in turn, may lead to a higher treatment success rate.
This supposed advantage of the galactomannan test, however, leans on three assumptions: (1) the Platelia test is indeed positive before the patient shows signs and symptoms; (2) the HRCT also shows signs of invasive aspergillosis at that moment; and (3) earlier treatment results in a higher success rate. Of the 42 studies that we included in our review, 24 did not report any useful information about point in time at which the galactomannan test was positive. Five studies reported that the test was never positive before either CT, diagnosis or clinical signs. The other studies that reported the time between a positive galactomannan test and other tests or clinical signs reported time periods varying from around 60 days before to around 50 days after any other evidence (either CT, radiology, clinical signs, fever, diagnosis) for aspergillosis. It was not possible to calculate a mean or median time span, or even a probability of the galactomannan test being positive earlier than other diagnostic evidence. So we could not evaluate the probability that the first two assumptions are true.
Authors' conclusions
Implications for practice.
The value of the galactomannan test will depend on the role that the results of this test will play in clinical decisions about starting therapy for aspergillosis. We can compare the cut‐off value of 0.5 optical density index (ODI) with that of 1.5 ODI in a group of 200 potential invasive aspergillosis patients with a disease prevalence of 11% (the overall median prevalence). In such a population, 22 patients will have proven or probable invasive aspergillosis and 178 will not. If we used the test at a cut‐off value 0.5, then we would miss five patients with invasive aspergillosis (sensitivity 78%, 22% false negative rate). Although these patients would still be monitored for clinical signs in most clinical situations, the expectation is that invasive aspergillosis would be detected later. Twenty‐seven patients would be treated unnecessarily with antimycotics or would be unnecessarily referred for further diagnostic testing (e.g. HRCT) (specificity of 85%, 15% false positive rate). If we used the test at a cut‐off value of 1.5, then we would miss seven patients with invasive aspergillosis (sensitivity 63%, 37% false negative rate) and 13 others would be treated or referred unnecessarily (specificity 93%, 7% false positive rate). Clinicians should decide whether the numbers that follow from the use of the test at 0.5 ODI are more or less acceptable than the numbers that follow from the use of the test at 1.5 ODI.
Whether the galactomannan test may be preferred over or replaced by polymerase chain reaction (PCR) for invasive aspergillosis can be debated. In a recent Cochrane review PCR had a mean sensitivity of 80.5% (95% confidence interval (CI) 73.0 to 86.3) and a mean specificity of 78.5% (95% CI 67.8 to 86.4) for a single positive test result (Cruciani 2015). This is a higher sensitivity than the galactomannan test at any of the investigated cut‐off values, but also a lower specificity. Using the PCR on two consecutive positive test results would lead to a higher specificity than the galactomannan test (96.2%, 95% CI 89.6 to 98.6), but also a lower sensitivity (58.0%, 95% CI 36.5 to 76.8). Besides, PCR may require more resources and may be more expensive than the Platelia test.
Implications for research.
This review showed that although we do have a good estimate of the test accuracy of the galactomannan ELISA for the diagnosis of invasive aspergillosis, we do not have enough data to estimate its value in clinical practice. Future studies should report the spectrum of patients in which the test is used unambiguously, as well as the time between the index test result and actual diagnosis, or between the index test result and the results of other tests. It would also be helpful if researchers reported more clearly the individual results of the components of the reference standard.
The diagnostic accuracy of the Platelia assay has been evaluated in several studies. It is time now for studies that evaluate this test as monitoring tool, taking into account the time to diagnosis. It would also be useful to investigate the additional value of the Platelia on top of the other tests used to diagnose invasive aspergillosis.
What's new
| Date | Event | Description |
|---|---|---|
| 27 September 2017 | Amended | Typos in the abstract changed to match data in Table 1. |
History
Review first published: Issue 4, 2008
| Date | Event | Description |
|---|---|---|
| 26 June 2015 | New citation required but conclusions have not changed | The original version of the review included 42 studies, but ten of those were not in the meta‐analyses because they excluded patients with possible aspergillosis. These ten studies have been excluded from this version of the review completely, leaving 32 studies in the review that were also in the original version. On top of that, we added 22 newly retrieved studies to the review, leading to a total of 54 studies. We added a plain language summary. |
| 17 November 2014 | New search has been performed | QUADAS‐2 implemented. |
| 17 February 2014 | New search has been performed | New literature search run. |
| 22 July 2008 | Amended | First published version of the review. |
Notes
No published notes.
Acknowledgements
The Cochrane Diagnostic Test Accuracy Editorial Team helped to edit this review and commented critically on the review.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Electronic searches
|
A. MEDLINE (through PubMed) 1. "Aspergillus"[MeSH] 2. "Aspergillosis"[MeSH] 3. "Pulmonary Aspergillosis"[MeSH] 4. aspergill*[tiab] 5. fungal infection[tw] 6. (invasive[tiab] AND fungal[tiab]) 7. #1 OR #2 OR #3 OR #4 OR #5 OR 6 43462 hits 8. "Serology"[MeSH] 9. Serology"[MeSH] 10. (serology[tiab] OR serodiagnosis[tiab] OR serologic[tiab]) 11. #8 OR #9 OR #10 190568 hits 12. "Immunoassay"[MeSH] 13. (immunoassay[tiab] OR immunoassays[tiab]) 14. (immuno assay[tiab] OR immuno assays[tiab]) 15. (ELISA[tiab] OR ELISAs[tiab] OR EIA[tiab] OR EIAs[tiab]) 16. immunosorbent[tiab] 17. #12 OR #13 OR #14 OR #15 OR #16 452423 hits 18. Platelia[tw] 19. "Mannans"[MeSH] 20. galactomannan[tw] 21. #18 OR #19 OR #20 3860 hits 22. #11 OR #17 OR #21 609778 hits 23. #7 AND #22 2621 hits |
|
B. EMBASE (through OVID) 1. exp aspergillosis/ 2. aspergill*.ti,ab. 3. exp Aspergillus/ 4. (fungal adj2 infection).mp. 5. (fungal adj2 invasive).mp. 6. 1 or 2 or 3 or 4 or 5 52624 hits 7. exp serology/ 8. exp serodiagnosis/ 9. (serology or serodiagnosis).ti,ab. 10. exp immunoassay/ 11. immunoassay*.mp. 12. immuno assay*.mp. 13. immunosorbent.mp. 14. ELISA.ti,ab. 15. (EIA or EIAs).ti,ab. 16. Platelia.mp. 17. galactomannan.ti,ab. 18. exp mannan/ 19. or/ 7‐18 480456 hits 20. 6 and 19 2773 hits |
|
C. ISI Web of Knowledge 1. Topic=(Aspergillosis OR aspergillus) OR Title=(Aspergillosis OR aspergillus) 2. TS=(aspergill*) OR TI=(aspergill*) 3. TS=(fungal SAME infection*) OR TI=(fungal SAME infection*) 4. TS=(invasive SAME fungal) OR TI=(invasive SAME fungal) 5. #1 OR #2 OR #3 OR #4 (59479 hits) 6. TS=(immunosorbent) OR TI=(immunosorbent) 7. TS=(ELISA) OR TI=(ELISA) OR TS=(ELISAs) OR TI=(ELISAs) 8. TS=(EIA) OR TI=(EIA) OR TS=(EIAs) OR TI=(EIsA) 9. TS=(Platelia) OR TI=(Platelia) OR FT=(Platelia) 10. TS=(galactomannan) OR TI=(galactomannan) 11. TS=(serology) OR TS=(serodiagnosis) 12. #6 OR #7 OR #8 OR #9 OR #10 OR #11 (>100000 hits) 13. #5 AND #12 (1749 hits) |
Appendix 2. QUADAS‐2 checklist
1. Patient selection domain ‐ risk of bias
1a. Was a consecutive or random sample of patients enrolled?
YES ‐ if this was clearly stated in the methods section or if the study stated that 'all' eligible patients were enrolled. NO ‐ if it was clear that the clinician made the selection. UNCLEAR ‐ if we could not decide between YES or NO.
1b. Was a case‐control design avoided?
This was scored YES for all included studies, as case‐control designs were excluded.
1c. Did the study avoid inappropriate exclusions?
YES ‐ if, for example, only solid tumour transplants were excluded or if no one was excluded. NO ‐ if, for example, exclusion was done based on EORTC criteria or index test results. Studies excluding 'possible IA' patients were excluded. UNCLEAR ‐ if it was not clear whether excluded patients were inappropriately excluded.
RISK OF BIAS: HIGH ‐ when at least one question was answered with 'NO'. LOW ‐ when at least two questions were answered with 'YES'. UNCLEAR: all other situations.
CONCERNS RE. APPLICABILITY: We made an inventory of whether participants were inpatients or outpatients, the age groups of the participants and the cause of their increased risk for IA (neutropenia, corticosteroids etc). HIGH CONCERN ‐ xxx LOW CONCERN ‐ xxx UNCLEAR CONCERN ‐ xxx
2. Index tests domain
2a. Were the index test results interpreted without knowledge of the results of the reference standard?
YES ‐ if the index test was done before the reference standard was assessed, or when the authors clearly stated that the assessment of the index test was blinded. NO ‐ if the authors stated that the assessment of the index test was not blinded. UNCLEAR ‐ all other situations.
2b. If a threshold was used, was it pre‐specified?
YES ‐ if the threshold was mentioned in the methods section, or if the authors stated that they followed the manufacturer's directions (which include guidance on the threshold). NO ‐ if the authors drew a ROC plot, or if multiple thresholds (outside the commonly reported ones of 0.5, 1.0 and 1.5) were reported, or if the authors stated that the threshold they used was the optimal threshold in their study. UNCLEAR ‐ all other situations.
RISK OF BIAS: HIGH ‐ when at least one question was answered with 'NO'. LOW ‐ when only 2b was answered 'YES' and 2a with 'UNCLEAR' (as the galactomannan test is a laboratory test) or when both questions were answered with 'YES'. UNCLEAR: if both were answered 'UNCLEAR'.
CONCERNS RE. APPLICABILITY: HIGH CONCERN ‐ if a threshold was used that was not one of the more commonly used thresholds (i.e. 0.5 or 1.0 or 1.5). LOW CONCERN ‐ if a threshold of 0.5, 1.0 or 1.5 was used; all studies used a commercially available and thus relatively standard and commonly available test. UNCLEAR CONCERN ‐ if the threshold used was not clear, or if it was not entirely clear whether the authors evaluated the Platelia® kit.
3. Reference standard domain
3a. Is the reference standards likely to correctly classify the target condition?
As we regarded the EORTC/MSG criteria as being acceptable, this item was always fulfilled by all included studies. We did, however, register whether the authors of the primary study used the exact criteria of the EORTC/MSG and (if reported) how they were interpreted. If they only mentioned that they did divide their patients into categories, but did not explain on what basis, we scored this item as 'unclear'.
3b. Were the reference standard results interpreted without knowledge of the results of the index tests?
YES ‐ when the authors clearly stated that the assessment of the index test was blinded. NO ‐ if the authors stated that the assessment of the index test was not blinded, or if the index test formed part of the reference standard (incorporation bias). UNCLEAR ‐ all other situations.
RISK OF BIAS: HIGH ‐ when at least one question was answered with 'NO'. LOW ‐ when both questions were answered 'YES'. UNCLEAR ‐ if one was answered 'UNCLEAR' and the other 'YES' or if both were answered 'UNCLEAR'.
CONCERNS RE. APPLICABILITY: HIGH CONCERN ‐ if the EORTC criteria were used in a different way than described in the reference papers from 2002 and 2008 (e.g. if the authors used five or three categories instead of four). LOW CONCERN ‐ if the EORTC criteria were used in the same way as described in the reference papers from 2002 and 2008. UNCLEAR CONCERN ‐ if it was not clear how the EORTC criteria were implemented.
4. Flow and timing domain
4a. Was there an appropriate interval between index test and reference standard?
The calculation of the diagnostic accuracy of a test is more reliable when the time between the Platelia test and the final diagnosis is not too long. If the galactomannan test is negative on day 1 and the patient is diagnosed as having IA on day 20, this test result will be regarded as a false negative result. The patient's true status on day 1, however, was not known in this case and the false negative result may have been a true negative result at that moment. We judged a time interval of less than 15 days as appropriate (YES).
4b. Did all patients receive the same reference standard?
The reference test was in most studies a composite reference while the index test was often used as screening tool to monitor whether patients developed IA. So some patients fulfilled more criteria than others. However, we considered the EORTC criteria as one reference standard. Partial verification would have been a problem in studies were only autopsy is used as reference standard, because it is only done when a patient dies and his or her family gives permission.
4c. Were all patients included in the analysis?
YES ‐ if all included patients also ended up in the 2 x 2 table. NO ‐ if there was a discrepancy between these two numbers. UNCLEAR ‐ if it was unclear whether patients were missing or not.
RISK OF BIAS: HIGH ‐ when at least one question was answered with 'NO'. LOW ‐ when at least two questions were answered 'YES' and the remaining one with 'UNCLEAR'. UNCLEAR ‐ all other situations.
Data
Presented below are all the data for all of the tests entered into the review.
Tests. Data tables by test.
| Test | No. of studies | No. of participants |
|---|---|---|
| 1 Platelia ‐ all cut‐offs | 50 | 7955 |
| 2 Platelia in children | 7 | 472 |
1. Test.
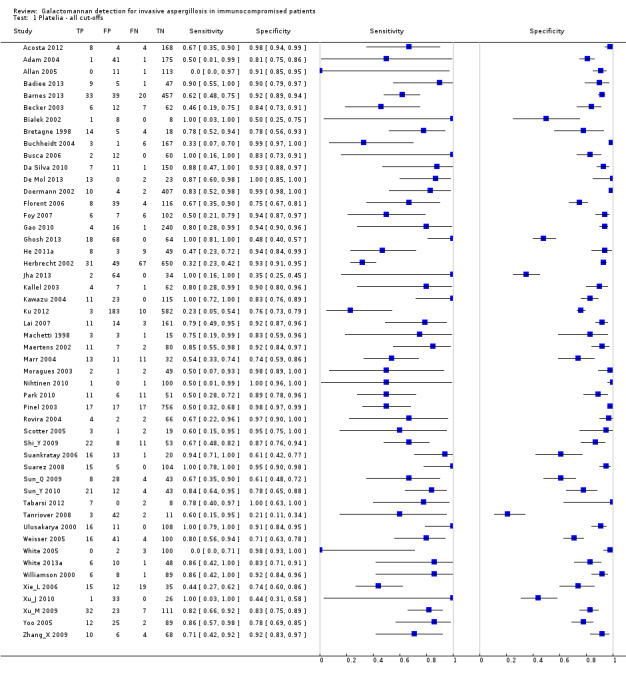
Platelia ‐ all cut‐offs.
2. Test.
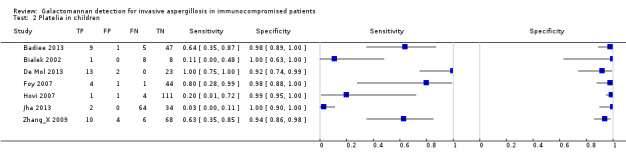
Platelia in children.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Acosta 2012.
| Study characteristics | |||
| Patient sampling | Testing of serum samples was requested when judged clinically relevant | ||
| Patient characteristics and setting | Over a period of 24 months (between June 2008 and May 2010) 965 patients were admitted to the ICU. Of these, 149 (15.4%) had a compatible clinical syndrome and host factors of IFD, and 98 (10.2%) met the criteria for inclusion (23 patients in the IFD group and 75 patients in the control group without evidence of IFD; Tables 1, 2 and 3). The overall prevalence of IFD in this cohort was 23.4% | ||
| Index tests | Platelia was used; a ROC curve was drawn. Multiple cut‐offs reported, not all the 'standard' ones | ||
| Target condition and reference standard(s) | Proven and probable IA diagnoses were based on the modified definitions of De Pauw 2008, excluding the detection of GM in BAL and in serum samples. | ||
| Flow and timing | Time interval not reported; all patients were classified using the EORTC criteria; no withdrawals reported | ||
| Comparative | |||
| No patients per category | 4 proven, 7 probable, no possibles reported, 75 no‐IFD + 12 other fungal diseases | ||
| Notes | M. Finkelman (second last author) is an employee of Associates of Cape Cod, Inc., the manufacturer of the Fungitell kit | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | No | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| High | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Adam 2004.
| Study characteristics | |||
| Patient sampling | Prospective; consecutive enrollment of patients not reported Platelia ODI between 1.0 and 1.5 were excluded |
||
| Patient characteristics and setting | 225 participants
Age ranged from 16 to 74 years
No information about gender
France Adults with haematological malignancies who were likely to be severely neutropenic. Inpatients, monitoring clinical course. Representative spectrum? Unclear: although galactomannan antigenaemia is monitored weekly in those patients with haematological malignancies who are likely to experience severe neutropenia, it is not clear whether the patients were enrolled consecutively |
||
| Index tests | Platelia: galactomannan antigenaemia is monitored weekly; first positive result is regarded positive. Cut‐off 1.5 ODI | ||
| Target condition and reference standard(s) | Invasive aspergillosis, defined according to EORTC criteria, reference Ascioglu 2002 Incorporation avoided? Unclear: not reported Acceptable reference standard? Yes: a diagnosis of invasive aspergillosis was classified as proven, probable or possible, according to criteria established by the EORTC/MSG (Ascioglu 2002) |
||
| Flow and timing | Not reported Partial verification avoided? Yes: all patients were classified according to the reference criteria Withdrawals explained? Yes: see above Uninterpretable results reported? No: Platelia ODI between 1.0 and 1.5 were excluded |
||
| Comparative | |||
| No patients per category | 0 proven; 2 probable; 5 possible; 218 non‐IA | ||
| Notes | Sponsoring? Nothing reported on financial resources | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | No | ||
| High | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Allan 2005.
| Study characteristics | |||
| Patient sampling | Prospective; series of patients with same risk profile; consecutive enrollment not reported. Episode‐based analysis | ||
| Patient characteristics and setting | 125 participants
Age ranged from 16 to 76 years
No information about gender
Scotland Adults undergoing allogeneic or autologous stem cell transplantation or intensive chemotherapy; no other details reported Representative spectrum? Unclear: adult haemato‐oncology patients |
||
| Index tests | Platelia: twice‐weekly screening; different ways of defining positive result reported and different cut‐off values | ||
| Target condition and reference standard(s) | Invasive aspergillosis, according to EORTC criteria (Ascioglu 2002) Incorporation avoided? Yes: as the Platelia Aspergillus was being evaluated it was excluded from the EORTC/MSG definitions Acceptable reference standard? Yes: IFI was classified as proven, probable or possible according to the EORTC/MSG definitions (Ascioglu 2002) |
||
| Flow and timing | Timing not reported Partial verification avoided? Unclear: it was unclear if really all patients were classified using the reference criteria Withdrawals explained? No: not reported Uninterpretable results reported? No: there were no uninterpretable results |
||
| Comparative | |||
| No patients per category | 0 proven, 1 probable, 11 possible, 113 non‐IA | ||
| Notes | Sponsoring: grants from Chief Scientists Office, Scotland, Wyeth Healthcare and Gilead Sciences | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Badiee 2013.
| Study characteristics | |||
| Patient sampling | The study included all the paediatric patients (1 to 14 years old) with haematology disorders who were treated at the haematology/oncology unit of Faghihi Hospital, Shiraz University of Medical Sciences, Iran. All study participants were identified as being at increased risk of developing IA | ||
| Patient characteristics and setting | 1 to 14 years old; mean age 9.3; all had haematological malignancies Study done at a haematology/oncology unit in Iran |
||
| Index tests | Detection of Aspergillus GM was performed using the Platelia Aspergillus EIA (immunoenzymatic sandwich microplate assay, Bio‐Rad, Platelia, Marnes La Coquette, France), according to the manufacturer's protocol. GM assay index ≥ 0.5 was considered positive | ||
| Target condition and reference standard(s) | Classification of patients was performed according to the protocols of the European Organization for Research and Treatment of Cancer‐Mycosis Study Group (De Pauw 2008). Accordingly, the reference gold standards (positive culture from clinical samples) used to calculate the specificity and sensitivity were the mycological criteria (without indirect tests including GM) along with host factors and clinical criteria | ||
| Flow and timing | No information on time to diagnosis; all included patients were classified according to the same criteria; no withdrawals mentioned | ||
| Comparative | |||
| No patients per category | 1 proven, 9 probable, 26 possible, 26 no IA | ||
| Notes | No COI declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Barnes 2013.
| Study characteristics | |||
| Patient sampling | Between October 2005 and June 2009 all adult patients entered into the pathway were audited. 612 patients were entered into the care pathway, 27 children < 18 years were excluded. A further 36 patients did not have a minimum of at least 1 specimen each for EIA and PCR sent and were excluded from the analysis, leaving 549 patients for full analysis | ||
| Patient characteristics and setting | Not much information on patient characteristics, but underlying diseases (Table 1) suggests a representative sample | ||
| Index tests | Serum was collected twice weekly. Platelia kits (Bio‐Rad, UK) were used for the detection of galactomannan; any value above 0.5 was considered significant although 0.5 to 0.7 was considered borderline and a repeat was requested | ||
| Target condition and reference standard(s) | IFD was defined according to revised EORTC/MSG criteria (De Pauw 2008). GM is included within the EORTC/MSG consensus criteria to define probable infection | ||
| Flow and timing | Timing not reported; all patients classified in the same way; all patients included in analyses | ||
| Comparative | |||
| No patients per category | There were 6 cases of proven IA, 47 cases of probable IA and 23 cases of possible IA | ||
| Notes | Work was supported by a grant from Gilead Sciences | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Becker 2003.
| Study characteristics | |||
| Patient sampling | Prospective; consecutive series of patients | ||
| Patient characteristics and setting | 160 participants
Age ranged from 18 to 79 years
No information about gender
Netherlands Adult haematological patients with neutropenia Inpatients, monitoring of clinical course Representative spectrum? Yes: haematology patients that had an expected neutropenia for at least 10 days and had an age of at least 18 years. Serum samples were taken from all patients twice weekly (= consecutive) Prospective; consecutive patient series |
||
| Index tests | Platelia: serum was sampled twice weekly during neutropenia. 2 subsequent positive samples were considered positive. Cut‐off 1.0 ODI | ||
| Target condition and reference standard(s) | Invasive aspergillosis, modified EORTC criteria (they added 2 extra categories) (Ascioglu 2002) Incorporation avoided? Yes: results of galactomannan detection were excluded from the criteria Acceptable reference standard? Yes: invasive fungal infections were classified according to the EORTC case definitions, with some modifications (1 extra category). Ascioglu 2002 cited |
||
| Flow and timing | Time interval not reported Partial verification avoided? Yes: all patients were classified according to reference criteria Withdrawals explained? Yes Uninterpretable results reported? Yes: suspected and possible patients |
||
| Comparative | |||
| No patients per category | 2 proven, 11 probable, 22 possible (18 suspected plus 4 possible), 125 non‐IA | ||
| Notes | Sponsoring not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Bialek 2002.
| Study characteristics | |||
| Patient sampling | Study design not clear | ||
| Patient characteristics and setting | 17 participants
Age ranged from 1 month to 9 years; 59% males France or Germany Children undergoing bone marrow transplantation (BMT), no other details reported Paediatric bone marrow recipients; other details not reported |
||
| Index tests | Platelia: screening (but not reported how often), single sample positive is positive; < 1.0 is negative and > 1.5 is positive | ||
| Target condition and reference standard(s) | Invasive aspergillosis, according to EORTC criteria, EORTC website cited They excluded the antigen detection as a microbiological criterion in the EORTC criteria |
||
| Flow and timing | None very clearly reported: not clearly reported whether all patients who underwent the Platelia test also underwent the reference standard No withdrawals reported Uninterpretable results not clearly reported: below 1.0 ODI a sample is negative, above 1.5 ODI a sample is positive, but they do not describe how samples between 1.0 and 1.5 ODI are handled |
||
| Comparative | |||
| No patients per category | 0 proven, 1 probable, 2 possible, 14 non‐IA | ||
| Notes | Research fund and university fund reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Bretagne 1998.
| Study characteristics | |||
| Patient sampling | Retrospective study Patients considered to have a high risk (criteria mentioned) of IA, with sufficient samples stored and reliable clinical data available were selected; n = 41 |
||
| Patient characteristics and setting | 41 participants; no information about age or gender Haematology patients with neutropenia or receiving steroid therapy following allogeneic bone marrow transplantation (BMT) France Inpatients; monitoring clinical course |
||
| Index tests | Platelia: serum was collected on admission and then once weekly Quantitative results were used instead of index Cut‐off value was single sample of 1 ng/ml |
||
| Target condition and reference standard(s) | Invasive aspergillosis, according to EORTC‐like criteria. "those considered to have clinical invasive aspergillosis were categorized according to the following criteria: (1) confirmed: histologically proven disease and an Aspergillus‐positive culture of a specimen obtained by percutaneous aspiration; (2) probable: development of a new opacity in lung and isolation of an Aspergillus species or of septate branched hyphae on a wet mount examination of BAL fluid, transtracheal aspirate, or sputum (or histologically proven disease without any positive culture to confirm the species of filamentous fungus involved); and (3) suspected: temperature of >38◦ C for >5 days that was unresponsive to antibacterial agents, in a patient at risk for invasive aspergillosis who started receiving empirical antifungal treatment and had a new opacity on a chest radiograph (no evident etiology). On the basis of these criteria, 22 patients were considered to have invasive aspergillosis (table 1), of whom 6 had confirmed aspergillosis (patients 1–6), 12 (patients 7–18) had probable invasive aspergillosis, and 4 (patients 19–22) had suspected invasive aspergillosis. Nineteen patients did not develop invasive aspergillosis." Galactomannan test not mentioned in definitions categories |
||
| Flow and timing | Not reported; retrospective study Partial verification avoided? Yes: all patients were classified according to the same reference criteria Withdrawals explained? No Uninterpretable results reported? No |
||
| Comparative | |||
| No patients per category | 6 proven, 12 probable, 4 possible, 19 non‐IA | ||
| Notes | Sponsoring precluded? ELISA was performed as previously described with plates provided by Sanofi Diagnostics Pasteur | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Unclear | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Buchheidt 2004.
| Study characteristics | |||
| Patient sampling | Study design not clear. Episode‐based analysis | ||
| Patient characteristics and setting | 177 participants
Age ranged from 17 to 81 years
Germany
No further info Adults with haematological malignancies undergoing chemotherapy or bone marrow transplantation and fulfilled host factor criteria (Ascioglu 2002) Inpatients Representative spectrum? Unclear: selection process not clearly reported |
||
| Index tests | Platelia: positivity was defined as 2 or more serial samples with ODI > 1.5 times the cut‐off index and with > 0.7 times the cut‐off index. On average every 3 days samples were measured | ||
| Target condition and reference standard(s) | Invasive aspergillosis, according to EORTC criteria (Ascioglu 2002) Incorporation avoided? Not reported Acceptable reference standard? Yes: all assays were evaluated for sensitivity and specificity in the detection of IA after classification of patient episodes according to the 2002 guidelines established by the EORTC/MSG (Ascioglu 2002) |
||
| Flow and timing | Time interval: not reported Partial verification avoided? Yes: all assays were evaluated for sensitivity and specificity in the detection of IA after classification of patient episodes according to the 2002 guidelines established by the EORTC/MSG (Ascioglu 2002) Withdrawals explained? No Uninterpretable results reported? No |
||
| Comparative | |||
| No patients per category | 6 proven, 3 probable, 75 possible, 93 non‐IA | ||
| Notes | Sponsoring? Funded by the German Jose Carreras Leukemia Fund | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Busca 2006.
| Study characteristics | |||
| Patient sampling | Prospective; consecutive series of patients "Between February 2002 and October 2004, all adult patients transplanted at our institution were analyzed" |
||
| Patient characteristics and setting | 74 participants
Age ranged from 19 to 70 years
No information about gender
Italy All adult patients undergoing allogeneic haematopoietic stem cell transplantation Inpatients; monitoring clinical course Representative spectrum: all adult patients undergoing allogeneic haematopoietic stem cell transplantation were analysed with the commercially available galactomannan sandwich‐ELISA assay Both inpatients and outpatients (outpatients only where possible) |
||
| Index tests | Platelia. Serum samples were taken twice weekly; galactomannan positivity was defined as an ODI of 1.0 or higher in 2 subsequent sera | ||
| Target condition and reference standard(s) | Invasive aspergillosis, according to the EORTC criteria (Ascioglu 2002) Incorporation avoided? Yes: invasive fungal infections were classified according to the EORTC/MSG case definitions Galactomannan results were excluded as microbiologic criteria Acceptable reference standard? Yes: invasive fungal infections were classified according to the EORTC/MSG case definitions (Ascioglu 2002). Galactomannan results were excluded as microbiologic criteria |
||
| Flow and timing | Timing not reported Partial verification avoided: all patients were classified according to the reference criteria No withdrawals: the study included all patients = 74; the results for all 74 patients were given No uninterpretable results: the study included all patients = 74; the results for all 74 patients were given in terms of negative or positive result, so no uninterpretable results |
||
| Comparative | |||
| No patients per category | 2 proven, 0 probable, 7 possible, 65 no IA | ||
| Notes | Sponsoring: not reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Da Silva 2010.
| Study characteristics | |||
| Patient sampling | "Between April 2005 and April 2008, 169 patients (median age 55 years) with AML received intensive chemotherapy (107 patients) as induction or consolidation therapy and 62 patients received high dose chemotherapy for auto‐HSCT. All patients were screened twice a week for GM analysis." This however says nothing about the enrollment and whether it was consecutive or not |
||
| Patient characteristics and setting | See above Study done in Portugal; not sure where patients came from |
||
| Index tests | Platelia test; both serum and BAL GM samples were considered positive when the index value was > 0.5 ng/mL All patients were screened twice a week for GM analysis Nothing reported on blinding |
||
| Target condition and reference standard(s) | Fungal infections were classified according to EORTC/MSG revised consensus (De Pauw 2008) They did not exclude Platelia from criteria |
||
| Flow and timing | Timing not reported; blinding not reported | ||
| Comparative | |||
| No patients per category | 2 proven, 6 probable, ? possible, 161 no IA (or 161 possible + no?) | ||
| Notes | No conflicts reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
De Mol 2013.
| Study characteristics | |||
| Patient sampling | Retrospectively, BAL fluid obtained from 456 bronchoscopies between July 2002 and June 2008 were evaluated. Paediatric patients fulfilling the host factor criteria as defined by the EORTC/MSG were included. Patients suffering from cystic fibrosis, allergic bronchopulmonary aspergillosis and other primary lung diseases were excluded. If more than one bronchoscopy was performed within a patient, the bronchoscopy done for diagnosing IPA was taken; if bronchoscopy was related in time with a CT scan, then the patient was excluded | ||
| Patient characteristics and setting | Setting: children's hospital in The Netherlands The median age of the 47 children was 9.8 years (range 1.1 to 18.2). Most were diagnosed with a haematologic disease (n = 31) of whom 2 received a HSCT |
||
| Index tests | The Platelia ELISA (Bio‐Rad Laboratories, France) was used to measure the levels of GM in serum and BAL according to the instructions of the manufacturer. An optical density index of 0.5 was considered positive All tests were performed by technicians who were unaware of the clinical condition of the patient Serum GM samples were taken twice weekly in paediatric patients considered to be at high risk of IA |
||
| Target condition and reference standard(s) | Children were classified as proven, probable or possible IPA according to the EORTC/MSG criteria (De Pauw 2008). Children with a CT scan not indicative for IPA were regarded as having no IPA | ||
| Flow and timing | No information on timing; all patients were classified by EORTC criteria; not sure if all patients were included in analyses | ||
| Comparative | |||
| No patients per category | 2 proven, 17 probable, 12 possible and 16 no IA | ||
| Notes | No conflicts of interest reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Doermann 2002.
| Study characteristics | |||
| Patient sampling | Study design not clear | ||
| Patient characteristics and setting | 423 adult participants Age of cases ranged from 16 to 70 years; no information about gender Haematology department France Inpatients, no further information provided |
||
| Index tests | Platelia: no further details reported Sera were tested twice a week in patients at risk 2 consecutive samples is positive, the rest are negative Cut‐off = 1.5 ng/ml |
||
| Target condition and reference standard(s) | Invasive aspergillosis, according to EORTC‐like criteria Antigenaemia reported as criterion in one of the definitions for disease classifications Acceptable reference standard? Yes EORTC‐like criteria; definitions of separate groups reported completely |
||
| Flow and timing | All patients were evaluated according to the same criteria, but not clear whether there were withdrawals or uninterpretable results | ||
| Comparative | |||
| No patients per category | 3 proven, 9 probable, 6 possible, 405 non‐IA | ||
| Notes | Language: French Sponsoring precluded? Unclear: not reported |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| High | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Florent 2006.
| Study characteristics | |||
| Patient sampling | Consecutive series of patients. From April 2001 through November 2002, all patients with haematological malignancies who were routinely screened for GM detection and 15 years old were included in the study | ||
| Patient characteristics and setting | 167 participants who were at least 15 years old and who had samples collected within 1 week from diagnosis
No information about gender
France Patients with haematological malignancies Inpatients, monitoring of clinical course Representative spectrum? Patients with haematological malignancies who had samples collected within 1 week from diagnosis Consecutive patient series |
||
| Index tests | Platelia: patients were tested twice weekly; a single positive sample was required to be test positive. Cut‐off = 0.5 ODI | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by the EORTC criteria (Ascioglu 2002) Incorporation avoided? Yes: to evaluate the performance of the GM assay either alone or in combination with the PCR‐ELISA, the results of the GM assay were not included in the microbiological criteria for the diagnosis of probable IA Acceptable reference standard? Yes: EORTC criteria (Ascioglu 2002) |
||
| Flow and timing | Timing not reported Partial verification avoided? All patients were classified according to the reference criteria Withdrawals explained? Not reported Uninterpretable results reported? Yes: 34 patients did not have consecutive serum samples that were collected within 1 week and therefore they were excluded from the final analysis |
||
| Comparative | |||
| No patients per category | 4 proven, 8 probable, 39 possible, 116 no‐IA | ||
| Notes | Also results for PCR assay Potential conflicts of interest: none reported. |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Foy 2007.
| Study characteristics | |||
| Patient sampling | Consecutive series of patients. Retrospective study In 2004, all patients undergoing HSCT at the University of Minnesota Medical Center were screened with biweekly serum GM ELISAs while hospitalised |
||
| Patient characteristics and setting | 121 participants
Age ranged from 4 months to 68 years
57% male USA. Inpatients undergoing haematopoietic stem cell transplantation were screened biweekly; and outpatients when possible Representative spectrum? Yes: patients undergoing HSCT Retrospective study, but consecutive series of patients |
||
| Index tests | Platelia; biweekly serum samples; single sample was enough. Cut‐off 0.5, mentioned in methods section | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by the EORTC criteria (Ascioglu 2002) Incorporation avoided? Yes, galactomannan excluded from criteria Acceptable reference standard? Yes: EORTC criteria (Ascioglu 2002). Galactomannan excluded from criteria |
||
| Flow and timing | Maximum of 18 months No partial verification: all patients were classified according to the reference criteria Withdrawals explained? No: some strange results, not clear if patients are missing but no explanation provided either Uninterpretable results reported? No intermediates; so N/A |
||
| Comparative | |||
| No patients per category | 12 proven or probable, 81 possible, 28 no IA | ||
| Notes | No financial support reported at all | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Gao 2010.
| Study characteristics | |||
| Patient sampling | All patients diagnosed with an acute exacerbation of COPD during the study period were included. No exclusion criteria recorded. Prospective study | ||
| Patient characteristics and setting | October 2006 to November 2008; No. 150 Hospital of the Chinese People's Liberation Army (PLA), China. There are 1100 beds in this hospital, with 60 beds in the respiratory diseases department No haematological or transplant patients; COPD patients |
||
| Index tests | The detection of GM antigen by the Platelia Aspergillus EIA test (Bio‐Rad Laboratories, Marnes, France) was carried out according to the manufacturer's instructions. A sample was considered positive if the index was ≥ 1.5 | ||
| Target condition and reference standard(s) | The EORTC/MSG (European Organization for the Treatment of Cancer/Mycoses Study Group) guidelines for IPA were not designed for patient categories other than cancer patients and bone marrow transplant recipients. One important at‐risk group, patients with COPD, was not included in this definition. So we used modified IPA definitions. Reference to De Pauw 2008. Platelia was included in the criteria |
||
| Flow and timing | No information about time intervals; all patients were classified according to the EORTC criteria; all patients were included in the analyses | ||
| Comparative | |||
| No patients per category | 2 had proven IPA, 3 had probable IPA and 254 patients did not have IPA; the remaining 2 patients had possible IPA | ||
| Notes | No conflicts of interest reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| High | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ghosh 2013.
| Study characteristics | |||
| Patient sampling | This was a prospective, single institutional, cohort study conducted during the period October 2008 to February 2010. A convenient sample of 150 consecutive episodes of high‐risk neutropenia was chosen. Patients who were planned for discharge soon after completion of chemotherapy and those not willing to participate were excluded | ||
| Patient characteristics and setting | We included patients aged ≥ 15 years, with a diagnosis of leukemia or recipients of auto‐ and allo‐HSCT. Episodes in patients diagnosed with possible or probable IA were also eligible for inclusion Median age of the patients in the episodes was 33 years (range 15.65 years). Male sex predominated with M:F ratio of 2.6:1. Acute myeloid leukaemia induction constituted 50 episodes (33.3%), consolidation with high‐dose cytarabine 30 episodes (20%) and recipients of autologous haematopoietic stem cell transplantation (auto‐HSCT) 37 episodes (24.7%), respectively. 6 episodes had a prior history of IA (3 possible and 3 probable) |
||
| Index tests | A double‐sandwich ELISA GM assay (Platelia Aspergillus, Bio‐Rad laboratories) capable of detecting GM at concentrations as low as 0.5 ng/mL was used. The assay was carried out at the Medical Oncology Laboratory of the hospital as per manufacturer's guidelines. A cut‐off of optical density index (ODI) > 0.5 was taken as positive | ||
| Target condition and reference standard(s) | Each episode was categorised as no IA, possible IA, probable IA or proven IA according to European Organization for Research and Treatment of Cancer (EORTC) 2008 criteria (De Pauw 2008). Unclear if GM results were also in criteria | ||
| Flow and timing | Timing not reported | ||
| Comparative | |||
| No patients per category | 25 possible, 17 probable, 1 proven, 107 no IA | ||
| Notes | No COI declared | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Unclear | |||
He 2011a.
| Study characteristics | |||
| Patient sampling | The study was conducted in the respiratory intensive care unit (RICU) of Beijing Chao‐Yang Hospital, a teaching facility of the Capital Medical University in Beijing, China. Critically ill stage III or IV COPD patients were included. Samples of all patients included in this study were taken once a day for the first 3 days of their ICU stays and again once a week if the patient remained in the ICU for more than 7 days | ||
| Patient characteristics and setting | COPD patients, thus high concern China |
||
| Index tests | Consecutive serum samples for GM studies were collected on the first and the fourth days of the patient's admission to the ICU. A sandwich ELISA assay for GM detection (Platelia Aspergillus; Sanofi Diagnostics Pasteur, Marnes‐La‐Coquette, France) was used according to the manufacturer's instructions. An optical density (OD) ratio of 0.5 or greater was considered positive. Diagnosis was not based on a serum GM test |
||
| Target condition and reference standard(s) | Based on case definitions of the EORTC/MSG, reference to De Pauw 2008. Thus, cases were interpreted as 'proven', 'probable' IPA or non‐IPA Patients from whom Aspergillus was recovered from non‐sterile sites, but who had no other evidence of fungal infections, were considered to be colonised. (ML: 'colonised' is not a classification that is mentioned in the original (2008) EORTC criteria) Diagnosis was not based on a serum GM test |
||
| Flow and timing | Timing is unclear/not reported. All patients were classified according to EORTC criteria. Unclear whether all patients were included in analyses. No flow chart reported | ||
| Comparative | |||
| No patients per category | 90 patients; 1 proven and 18 probable | ||
| Notes | No conflicts of interest declared or found | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Herbrecht 2002.
| Study characteristics | |||
| Patient sampling | Consecutive ("all patients presenting with ... had a serum sample collected") and prospective series of patients Episode‐based analysis | ||
| Patient characteristics and setting | 177 participants
Age ranged from 4 months to 88 years France Neutropenic children and adults (neutrophil count < 500/ųl) with a persistent fever despite antibiotics, but without any other signs of infection Daily monitoring of clinical course |
||
| Index tests | Platelia: sera with an index > 1.5 ODI were considered positive | ||
| Target condition and reference standard(s) | Invasive aspergillosis; EORTC‐criteria, citation Ascioglu 2002 The results of the antigen testing were not included in our classification |
||
| Flow and timing | All patients fulfilling the inclusion criteria were diagnosed with the EORTC criteria Withdrawals explained? No. Not clear how they came from 797 episodes to 640 episodes Uninterpretable results reported? Not reported |
||
| Comparative | |||
| No patients per category | 6 proven, 3 probable, 75 possible, 93 non‐IA | ||
| Notes | Nothing reported on sponsoring | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Hovi 2007.
| Study characteristics | |||
| Patient sampling | Prospective; consecutive series of patients. Episode‐based analysis "The study comprised all consecutive pediatric patients who were treated at the hematology/oncology unit of the Hospital for Children and Adolescents, University of Helsinki, from January 2000 to June 2002 and at increased risk for developing IFI. Eligible patients were those receiving therapy for remission induction of acute leukemia or myeloablative high‐dose chemo‐radiotherapy followed by SCT." No exclusion criteria or exclusions reported |
||
| Patient characteristics and setting | 117 paediatric patients
Age ranged from 1 to 16 years
57% male
Finland Inpatients at the haematology/oncology department, who had an increased risk for developing IA (receiving therapy for remission induction of acute leukaemia or myeloablative high‐dose chemotherapy followed by stem cell transplantation); monitoring clinical course Representative spectrum: consecutive patients, increased risk for developing IA |
||
| Index tests | Platelia. Sera were tested once a week; antigen levels were recorded as positive, borderline or negative. Single and subsequent samples analysed. Cut‐off not reported | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by the EORTC criteria (Ascioglu 2002) Incorporation avoided? Not reported Acceptable reference standard? Yes, EORTC criteria (Ascioglu 2002) |
||
| Flow and timing | Time interval not reported Partial verification avoided: all patients were classified according to the reference criteria Withdrawals not reported Uninterpretable results not reported |
||
| Comparative | |||
| No patients per category | 1 proven, 1 probable, 27 possible, 88 no IA | ||
| Notes | Sponsoring precluded? Nothing reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Jha 2013.
| Study characteristics | |||
| Patient sampling | The study was conducted prospectively from July 2010 to December 2011 in a paediatric oncology unit
Children, up to 14 years, on treatment for haematological malignancies and admitted with fever were enrolled
Patients who received piperacillin‐tazobactam and/or amoxicillin‐clavulanic acid were excluded as their administration has been associated with a false‐positive GM assay. Stem cell transplant (SCT) recipients were excluded as well A febrile episode was considered as an independent episode in a patient when it was > 4 weeks apart from the previous one, with the patient being clinically well in between |
||
| Patient characteristics and setting | Children, until 14 years, on treatment for haematological malignancies and admitted with fever were enrolled Setting: haematology‐oncology unit in India Mean age: 6.1 years (1 to 13 years) 3.5 males:1 female |
||
| Index tests | Blood for GM assay was drawn on the day of admission along with the sample for blood counts and bacterial culture. Serial estimation of GM was performed once a week, until discharge or death in limited patients. The GM assay obtained at admission was considered for analysis Serum GM levels were measured using the Platelia Aspergillus enzyme immunoassay test (Bio‐Rad, Hercules, CA, USA) as per the manufacturer's instructions. Results were recorded as the ratio of optical density of the sample to that of threshold control samples |
||
| Target condition and reference standard(s) | Diagnosis of fungal infections was classified as proven, probable, possible or no aspergillosis, based on criteria adapted from the 2002 EORTC/MSG definitions (Ascioglu 2002). For analysis, episodes with a proven, probable or possible disease were considered to have IA unless otherwise stated. EORTC /MSG definitions permit the GM assay results to be used to meet microbiological criteria for IA. However, the GM values were not included in the criteria for classification of diagnosis of IA, as the assay was itself being validated |
||
| Flow and timing | They do report the time span of the episodes and they state that the GM test evaluated was the one done at admission. So then the maximum amount of time between index test and diagnosis could have been the time span of an episode, which was 14 days on average, with a maximum of 60 days. Also, they define episodes clearly | ||
| Comparative | |||
| No patients per category | Proven 1; probable 1; possible 23; no IA 70; other fungal infections n = 5 | ||
| Notes | Analyses based on episodes; 100 episodes in 78 patients; no clear distinction No conflicts of interest stated |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Kallel 2003.
| Study characteristics | |||
| Patient sampling | Prospective; series of patients with same risk profile Not reported whether enrollment was consecutive |
||
| Patient characteristics and setting | 74 participants
Age ranged from 8 to 47 years
No information about gender
Tunisia Children and adults who were neutropenic; predominantly allograft patients Inpatients, monitoring clinical course Representative spectrum? Yes: children and adults that were neutropenic; predominantly allograft patients Prospective, consecutive patient series |
||
| Index tests | Platelia. Sera were monitored weekly on Mondays and Tuesdays. Both days positive = positive. All other results = negative. Cut‐off 1.5 ODI | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria (Ascioglu 2002) Incorporation avoided? No: a positive galactomannan test was one of the requirements for probable IA |
||
| Flow and timing | Not reported Partial verification avoided? Yes: all patients were classified according to reference criteria Withdrawals explained? No Uninterpretable results reported? No |
||
| Comparative | |||
| No patients per category | 1 proven, 4 probable, 2 possible, 67 no | ||
| Notes | Sponsoring precluded? Unclear: nothing reported about financial support French |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Kawazu 2004.
| Study characteristics | |||
| Patient sampling | Prospective; consecutive series of patients. Episode‐based analysis | ||
| Patient characteristics and setting | 149 participants
Age ranged from 17 to 74 years
70% males
Japan Adults with haematological disorders that were neutropenic, underwent chemotherapy, had persistent fever despite antibiotics, acute graft versus host disease or received corticosteroids. Weekly screening of inpatients Representative spectrum? Yes: adults with haematological disorders that were neutropenic, underwent chemotherapy, had persistent fever despite antibiotics, acute graft versus host disease or received corticosteroids |
||
| Index tests | Platelia. Serum was monitored weekly. Treatment episodes with only 1 or 2 measurements were excluded. Positive is either 1 positive sample or 2 consecutive positive samples. All the rest are negative. Cut‐off 0.6 ODI | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by the EORTC criteria (Ascioglu 2002) Incorporation avoided? Yes: plasma GM level was not included in the microbiological criteria Acceptable reference standard? Yes: EORTC criteria (Ascioglu 2002) |
||
| Flow and timing | Not reported Partial verification avoided? All patients were classified according to the reference criteria Withdrawals explained? N/A Uninterpretable results reported? N/A |
||
| Comparative | |||
| No patients per category | 9 proven, 2 probable, 13 possible, 125 no IA | ||
| Notes | ROC curves and timelines reported Sponsoring precluded? Unclear: nothing reported about support |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | No | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ku 2012.
| Study characteristics | |||
| Patient sampling | Non‐haematological patients who underwent GM testing from January 2007 to December 2009 were evaluated retrospectively. Non‐haematological patients were defined as patients without haematological disease, including malignancies, or those who have not undergone HSCT | ||
| Patient characteristics and setting | Non‐haematological patients, so high concern | ||
| Index tests | Platelia test; an OD index of 0.5 was considered positive. All positive samples were retested and considered positive only if the repeat test was also positive | ||
| Target condition and reference standard(s) | Each of the patients was classified according to the criteria of the European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC) revised in 2008 (De Pauw 2008). Thus, the patients were categorised as proven, probable, possible and non‐IA. Proven and probable IA cases were defined as IA in this study. The definition of IA was not based on the GM test | ||
| Flow and timing | Nothing reported on time intervals; all patients were classified by the same criteria; not clear if all patients were included in analyses | ||
| Comparative | |||
| No patients per category | 778 patients in total; 9 proven and 4 probable | ||
| Notes | No conflicts of interest reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Lai 2007.
| Study characteristics | |||
| Patient sampling | Study design not clear No inclusion or exclusion criteria reported |
||
| Patient characteristics and setting | 189 patients
Age ranged from 12 to 76 years
No information about gender
Taiwan Inpatients from intensive care units and haematology/oncology departments Representative spectrum? Unclear: selection criteria and patient population not clearly described |
||
| Index tests | Platelia. No further information, except about definition of positive test result and cut‐off values. Positive sample = subsequent samples of ≥ 1.5 ODI | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria (Ascioglu 2002) Incorporation avoided? Yes: galactomannan excluded from criteria Acceptable reference standard? Yes: EORTC criteria (Ascioglu 2002) |
||
| Flow and timing | Time interval not reported Partial verification avoided? Yes: all patients were classified according to the reference criteria Withdrawals explained? No Uninterpretable results reported? Yes |
||
| Comparative | |||
| No patients per category | 5 proven, 9 probable, 26 possible, 149 no IA | ||
| Notes | Nothing reported about financial support | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Liu_S 2012.
| Study characteristics | |||
| Patient sampling | 40 patients with haematologic malignancies at high risk of IFI | ||
| Patient characteristics and setting | Patients with high risk of IFI | ||
| Index tests | Platelia Aspergillus GM; threshold: two OD > 0.5 or one OD > 0.7 | ||
| Target condition and reference standard(s) | Invasive fungal infection; EORTC/MSG | ||
| Flow and timing | Difficult to extract due to Chinese language; time interval unclear. As reference standard was EORTC criteria and study used a one‐gate inclusion process, we assumed that all patients received the same reference standard. As there were no withdrawals or dropouts reported and the numbers in the 2x2 were the same as the numbers included, we assumed that all patients were included in the analyses. |
||
| Comparative | |||
| No patients per category | 5 proven, 13 probable, 4 possible, 10 no IA | ||
| Notes | Chinese language; no information on conflicts of interest provided. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Machetti 1998.
| Study characteristics | |||
| Patient sampling | 22 allogeneic BMT patients were followed from transplant to 90 days onwards; 5 developed IA. No information on how the 22 were selected | ||
| Patient characteristics and setting | 22 participants undergoing allogeneic bone marrow transplantation (BMT) No information about age or gender Setting: haematology department Country: Italy Inpatients; monitoring clinical course No further details |
||
| Index tests | Platelia Serum samples were collected 3 times a week during the first month and once a week during the second and third month Positivity was defined as at least 2 consecutive positive samples. One or less positive was considered negative Cut‐off: positive if > 1.5 ODI and negative if < 1.0 ODI |
||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC‐like criteria Galactomannan test was not mentioned as part of the reference criteria |
||
| Flow and timing | Until day 90 after transplantation All patients were classified according to the same reference criteria No withdrawals reported and no intermediate results reported |
||
| Comparative | |||
| No patients per category | 1 proven, 3 probable, 1 possible, 17 no IA | ||
| Notes | Sponsoring precluded? No: ELISA was kindly provided by Sanofi Pasteur and they received financial support from Pfizer | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Maertens 2002.
| Study characteristics | |||
| Patient sampling | Prospective; consecutive series of patients | ||
| Patient characteristics and setting | 100 participants
Age ranged from 17 to 58 years
67% males Belgium Adults with haematological disorders who underwent myeloablative allogeneic stem cell transplantation (ASCT) Exclusion of autologous transplants and patients undergoing non‐myeloablative conditioning |
||
| Index tests | Platelia Serum samples were collected twice weekly, and more often if patients were proven or probable 2 consecutives was positive. Results were reported back to clinicians once a week Cut‐off value 1.0 ODI |
||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria (Ascioglu 2002) Galactomannan ELISA was not mentioned as part of the reference criteria |
||
| Flow and timing | All patients were classified according to the same reference criteria Withdrawals explained? Yes Uninterpretable results reported? No |
||
| Comparative | |||
| No patients per category | 5 proven, 8 probable, 34 possible, 53 no IA | ||
| Notes | Also postmortem details given Nothing reported about financial support |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Marr 2004.
| Study characteristics | |||
| Patient sampling | Patients were enrolled prospectively; blood samples were analysed after storage | ||
| Patient characteristics and setting | 67 participants
Age ranged from 5 to 66 years
No information about gender
USA Children and adults undergoing bone marrow transplantation. Monitoring clinical course, no further information provided. Representative spectrum? Unclear: children and adults undergoing bone marrow transplantation; although it seems not to be a case‐control design, this can not completely be ruled out |
||
| Index tests | Platelia. Blood samples were obtained weekly. Samples were frozen and relabeled randomly; samples were analysed blinded to both the source of the samples and clinical data. Samples that had an ODI above 0.5 were tested again to verify positive result. At least 1 sample had to be obtained within 1 week before or after diagnosis | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria (Ascioglu 2002) Incorporation avoided? Yes: ELISA was explicitly excluded from EORTC criteria Acceptable reference standard? Yes: EORTC criteria (Ascioglu 2002) |
||
| Flow and timing | Time interval not reported Partial verification avoided? Unclear: all cases were classified according to the reference criteria. What they did to find controls is not entirely clear Withdrawals explained? Yes Uninterpretable results reported? Not reported |
||
| Comparative | |||
| No patients per category | 13 proven, 11 probable, 8 possible, 35 no IA | ||
| Notes | Sponsoring precluded? No: financed by National Institute of Health and Bio‐Rad Labs. 3 authors have also worked as consultants for Bio‐Rad Labs | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Moragues 2003.
| Study characteristics | |||
| Patient sampling | Retrospective study, not much reported | ||
| Patient characteristics and setting | 54 participants
No information about age or gender
Spain Severe neutropenic patients in the haematological department Monitoring clinical course, inpatients Representative spectrum? Unclear: severe neutropenic patients in the haematological department, without any further description |
||
| Index tests | Platelia. Sampled twice a week. 2 consecutive positive samples was considered as a positive result. No explanation of negative result. Cut‐off 1.5 ODI | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria (Ascioglu 2002) Incorporation avoided? Yes: the results of the ELISA were not used for the reference criteria Acceptable reference standard? Yes: EORTC criteria (Ascioglu 2002) |
||
| Flow and timing | Time interval not reported Partial verification avoided? Yes: all patients were classified according to reference criteria Withdrawals explained? Yes Uninterpretable results reported? No |
||
| Comparative | |||
| No patients per category | 3 proven, 1 probable, 17 possible, 33 no IA | ||
| Notes | Sponsoring? Financed by university Article in Spanish |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Nihtinen 2010.
| Study characteristics | |||
| Patient sampling | "All adult allo‐SCT recipients transplanted between January 2001 and December 2002 in Helsinki University Central Hospital were eligible for this study" Exclusion of patients receiving reduced‐intensity conditioning that does not lead to severe neutropenia Single group; prospective study; patients consecutively enrolled |
||
| Patient characteristics and setting | 138 patients were transplanted; 102 patients were left for the final analysis
Mean age 44 years
Finland "All adult allo‐SCT recipients transplanted [...] were eligible for this study" Representative spectrum? Yes: single group of allo‐SCT recipients; prospective study; patients consecutively enrolled |
||
| Index tests | Analyses were performed using the GM ELISA test (Platelia Aspergillus, Bio‐Rad, Hercules, CA, USA) according to the manufacturer's instructions. An optical density index of 0.5 was used as the criterion for test positivity | ||
| Target condition and reference standard(s) | The revised EORTC/MSG criteria were used to define the cases GM ELISA results were excluded from the criteria for IA Acceptable reference standard? Yes |
||
| Flow and timing | 36 patients were excluded because of reduced‐intensity conditioning or patient refusal; at 1 year after transplantation, 75 patients (73.5%) were alive Partial verification avoided? Yes: all patients were classified according to the reference criteria Withdrawals explained? No: some patients refused, but no reason is given as to why Uninterpretable results reported? No: there must have been indeterminate results; but they were not explained |
||
| Comparative | |||
| No patients per category | Unclear reporting: 1 proven?; 1 probable?; 0 possible?; 100 no IA | ||
| Notes | Sponsoring precluded? M Richardson is the founder and a shareholder of MoBiAir Diagnostics Ltd. but this seems to have nothing to do with Platelia; so there was no sponsoring by Platelia | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Park 2010.
| Study characteristics | |||
| Patient sampling | A prospective cohort study was performed at the Asan Medical Center, a 2700‐bed tertiary hospital in Seoul, South Korea, between May 2008 and January 2009. They included all adult patients (16 years of age) who underwent bronchoscopy with BAL to evaluate new pulmonary infiltrates and for whom GM assays from BAL were submitted were included. Informed consent was obtained from each patient. The study protocol was approved by the Institutional Review Board of the hospital. | ||
| Patient characteristics and setting | Unclear; patients requiring BAL or undergoing BAL may be less severely ill than patients who can undergo serum GM | ||
| Index tests | Transplant recipients and neutropenic cancer patients were measured weekly by ELISA (Platelia Aspergillus, Bio‐Rad, Hercules, CA). Serum GM was not regularly measured in other patients, although the assay was performed whenever IPA was suspected. An optical density (OD) cut‐off value of 0.5 or greater was considered positive for GM in serum or BAL samples, as previously recommended | ||
| Target condition and reference standard(s) | Patients were categorised with proven, probable or possible IPA according to revised consensus definitions of the EORTC/MSG (De Pauw 2008); Platelia was part of that | ||
| Flow and timing | Time between index test and diagnosis not reported; all patients were classified by the same reference standard; they provide a clear flow chart of patient flow | ||
| Comparative | |||
| No patients per category | 1 proven, 17 probable, 4 possible, 337 no IA | ||
| Notes | No financial disclosure reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | No | ||
| High | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Pinel 2003.
| Study characteristics | |||
| Patient sampling | Prospective; series of patients with same risk profile Not reported whether selection was done consecutively, but no reason to assume it was not |
||
| Patient characteristics and setting | 3327 serum samples from 807 participants
No information about age
62% of the cases were male
France Patients from haematological and intensive care units that were at risk for invasive fungal infections Inpatients, monitoring clinical course Representative spectrum? Yes: patients from haematological and intensive care units that were at risk of invasive fungal infections |
||
| Index tests | Platelia. 2 consecutive positive patient samples were necessary to suspect IA. In analyses also looked at single sample results. Cut‐offs analysed: > 1.0 ODI subsequently; 0.5, 1.0, 1.5 as single sample | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria (Ascioglu 2002) Incorporation avoided? The authors did not explicitly report the exclusion of the ELISA results from the EORTC criteria, so blinding also unclear Acceptable reference standard? Yes: EORTC criteria (Ascioglu 2002) |
||
| Flow and timing | Time interval was not reported. Partial verification avoided? Yes: all patients were classified according to reference criteria Withdrawals explained? No Uninterpretable results reported? No |
||
| Comparative | |||
| No patients per category | 3 proven, 31 probable, 22 possible, 751 no IA | ||
| Notes | Also clinical and radiological signs reported Nothing reported about financial support |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Rovira 2004.
| Study characteristics | |||
| Patient sampling | Prospective; consecutive series of patients | ||
| Patient characteristics and setting | 74 participants
Age ranged from 15 to 60 years
61% males
Spain Adults undergoing allogeneic haematopoietic stem cell transplantation in institution and adult outpatients receiving immunosuppressive therapy. Inpatients were screened twice weekly; outpatients weekly, if possible Representative spectrum? Yes: adults undergoing allogeneic haematopoietic stem cell transplantation |
||
| Index tests | Platelia. Serum was monitored twice a week until discharge or death. Outpatients were monitored weekly where possible. Positive was above 1.5 and negative was below 1.0. In between was undetermined. Total of 832 samples from 74 patients. Positive was one or more positive; negative was all negative | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria (Ascioglu 2002) Incorporation avoided? Yes: Aspergillus galactomannan antigen test results were excluded as microbiologic criteria Acceptable reference standard? Yes: EORTC criteria (Ascioglu 2002) |
||
| Flow and timing | Time interval not reported Partial verification avoided? Yes: all patients were classified according to reference criteria Withdrawals explained? Unclear Uninterpretable results reported? Yes: when a sample was undetermined, an additional sample was immediately tested |
||
| Comparative | |||
| No patients per category | 1 proven, 5 probable, 2 possible, 66 no IA | ||
| Notes | Clinical course and timelines for 8 patients Sponsoring? Governance funds and a leukaemia foundation |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Scotter 2005.
| Study characteristics | |||
| Patient sampling | Prospective; series of patients with same risk profile | ||
| Patient characteristics and setting | 25 participants
Age of cases ranged from 3 to 79 years
60% males
New Zealand Children and adults undergoing stem cell transplantation or chemotherapy for haematological malignancy and had fever for > 96 hours Inpatients Representative spectrum? Yes: patients undergoing stem cell transplantation or chemotherapy for haematological malignancy and had fever for > 96 hours |
||
| Index tests | Platelia. If the patient was febrile at least once per day for 4 days or if there was a high suspicion of invasive fungal infection, samples were assayed for galactomannan testing and PCR. Number of samples per patient varied from 2 to 32. At each time point of sample/assay, the patients were classified according to their status at that moment. Negative = all samples negative; positive = at least 1 sample positive. Many different cut‐off values analysed (0.5, 1.0, 1.5, 2.0 ODI) | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria (Ascioglu 2002) Incorporation avoided? Yes: galactomannan results were excluded from EORTC criteria Acceptable reference standard? EORTC criteria (Ascioglu 2002) |
||
| Flow and timing | Time interval not reported Partial verification avoided? Yes: all patients were classified according to the reference criteria Withdrawals explained? Yes Uninterpretable results reported? No |
||
| Comparative | |||
| No patients per category | 4 proven, 1 probable, 7 possible, 13 no IA | ||
| Notes | Sponsoring: work was supported by Gilead Sciences, a Bone Marrow Transplantation trust and the Canterbury District Health Board. No connections with Platelia | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Shi_Y 2009.
| Study characteristics | |||
| Patient sampling | Seems to be a single‐arm study; consecutive enrolment of patients | ||
| Patient characteristics and setting | China; only adults; 94 participants Inpatients admitted to ICU and suffering from suspected IPA Representative spectrum? Yes: seems to be eligible (consecutive enrolment; all suspected of IPA) |
||
| Index tests | Platelia; cut‐off is 0.5 ODI (single sample is sufficient); test was done routinely, twice a week | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria (Ascioglu 2002). They also used Chinese criteria, but not for these two‐by‐two tables Incorporation avoided? Not explicitly reported, so 'unclear' Acceptable reference standard? Yes: EORTC criteria |
||
| Flow and timing | Time interval NR Partial verification avoided? Yes Withdrawals explained? No Uninterpretable results reported? No |
||
| Comparative | |||
| No patients per category | 4 proven, 29 probable, 34 possible, 27 no IA | ||
| Notes | No information on financial support reported Chinese language |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | No | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | No | ||
| High | |||
Suankratay 2006.
| Study characteristics | |||
| Patient sampling | From June 2002 to January 2004 a consecutive series of adult patients with haematological disorders who were at risk of developing IA were included Eligible patients were 1) receiving chemotherapy with an expected duration of neutropenia of less than 500 cells/μL of at least 7 days or 2) undergoing allogeneic bone marrow or peripheral blood stem cell transplantation Those patients who were undergoing autologous bone marrow transplantation or were less than 16 years old were excluded from the present study (not sure if autologous BMT would fall under inappropriate exclusion) |
||
| Patient characteristics and setting | All 50 patients older than 16
46% male
At KCMH, Bangkok, Thailand Patients receiving chemotherapy or allogeneic haematopoietic stem cell transplantation. Patients under 16 and patients undergoing autologous stem cell transplantation were excluded Inpatients; monitoring of clinical course Representative spectrum? Patients receiving chemotherapy or allogeneic haematopoietic stem cell transplantation |
||
| Index tests | Platelia Aspergillus. Blood samples were obtained once or twice weekly until death or discharge. 2 subsequent positive samples were needed to get a positive test result. Cut‐off 0.5, 0.75, 1.0, 1.25, 1.5 ODI | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria (Ascioglu 2002) Incorporation avoided? Galactomannan not included in description of reference criteria Acceptable reference standard? Yes: EORTC (Ascioglu 2002) |
||
| Flow and timing | Timing not reported Partial verification avoided? Yes, all patients were classified according to the reference criteria Withdrawals explained? No Uninterpretable results reported? Yes |
||
| Comparative | |||
| No patients per category | 5 proven, 12 probable, 33 possible or no IA | ||
| Notes | Timelines GM for 17 patients Sponsoring: nothing reported about financial support |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Suarez 2008.
| Study characteristics | |||
| Patient sampling | Prospective study; consecutive enrolment; singe group | ||
| Patient characteristics and setting | All adult patients receiving allogeneic or autologous HSCT, or intensive chemotherapy for haematological malignancies, and who were routinely monitored for biweekly GM detection were included in the study "This study was conducted prospectively […] in the adult hematology and bone marrow transplant unit at […] a tertiary‐care university hospital (Paris, France)" Representative spectrum? Yes: seems to be eligible (consecutive enrollment; all suspected of IPA) |
||
| Index tests | "The GM assay was performed […] twice weekly […] using the Platelia Aspergillus enzyme immunoassay (Bio‐Rad Laboratories, Marnes‐la‐Coquette, France). Serum samples with an index of >0.5 were retested the following day and were considered positive if the GM index was again >0.5" | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria (Ascioglu 2002) Incorporation avoided? Yes: we used the EORTC/MSG criteria (except the GM results) for diagnosis of IA Acceptable reference standard? Yes: EORTC criteria |
||
| Flow and timing | Study lasted 13 months Partial verification avoided? Yes Withdrawals explained? Seems as if there were no withdrawals (N/A) Uninterpretable results reported? Yes: seems that there were no indeterminates etc. (so N/A) |
||
| Comparative | |||
| No patients per category | 1 proven, 14 probable, 2 possible, 107 no IA | ||
| Notes | Support has been reported; no Platelia support mentioned | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Sun_Q 2009.
| Study characteristics | |||
| Patient sampling | Enrolment not reported | ||
| Patient characteristics and setting | 83 adult; inpatients; China participants Haematology department; inclusion criteria were: low WBC for more than 5 days and: (1) non‐responding fever; (2) CT signs; (3) culture/microscope positive No representative spectrum: spectrum seems to be highly selective (halo signs etc.) |
||
| Index tests | Platelia test; cut‐off single sample 0.5 | ||
| Target condition and reference standard(s) | Criteria of the Editorial Board of the Chinese Journal of Medicine Not explicitly reported if Platelia formed part of the reference standard Acceptable reference standard? Unclear: Chinese criteria |
||
| Flow and timing | Time interval not reported. Partial verification avoided? Yes Withdrawals explained? Unclear, not reported Uninterpretable results reported? Unclear, not reported |
||
| Comparative | |||
| No patients per category | 4 proven; 8 probable; 45 possible; 26 no IA | ||
| Notes | Not supported by Platelia Chinese language |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | High | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Sun_Y 2010.
| Study characteristics | |||
| Patient sampling | Enrolment not reported | ||
| Patient characteristics and setting | 80 patients
China Haematology department; patients with host factor of IFI and symptoms and signs of infection, plus: (1) unresponsive fever; or (2) imaging shows focus of infection in lung, head and paranasal sinus; or (3) other evidence of suspicious IPA infection Representative spectrum? No: seems to be highly selective |
||
| Index tests | Platelia test; cut‐off single sample 1.0 or consecutive samples 0.8 | ||
| Target condition and reference standard(s) | Criteria of the Editorial Board of the Chinese Journal of Medicine Incorporation avoided? Unclear: not explicitly reported Acceptable reference standard? Unclear: Chinese criteria |
||
| Flow and timing | NR Partial verification avoided? Yes Withdrawals explained? Unclear: nothing reported on withdrawals Uninterpretable results reported? Yes: there were no uninterpretable results: N/A |
||
| Comparative | |||
| No patients per category | 5 proven, 20 probable, 34 possible, 21 no IA | ||
| Notes | Not supported by Platelia | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Tabarsi 2012.
| Study characteristics | |||
| Patient sampling | This study took place from January 2009 to January 2010 at the lung transplant centre of Massih Daneshvari Hospital, which is the primary hospital for heart and lung transplants in Iran. All patients who had clinical and radiological manifestations suggestive of pulmonary infection were prospectively included | ||
| Patient characteristics and setting | 15 lung transplants, 1 heart transplant patient and 1 heart‐lung transplant patient (17 in total); not haematological patients Iran Mean age was 34.6 years (range, 12 to 50 years) |
||
| Index tests | The Aspergillus galactomannan antigen was detected in serum or bronchoalveolar lavage by direct double‐sandwich enzyme‐linked immunosorbent assay (Platelia Aspergillus; Bio‐Rad, Marnes, La Coquette, France). No further information | ||
| Target condition and reference standard(s) | Classification of invasive aspergillosis was done based on the Infectious Diseases Society of America guidelines for aspergillosis. 3 categories of 'definite', 'probable', and 'possible' were defined. Reference to Ascioglu 2002 | ||
| Flow and timing | Nothing on timing; all patients were classified according to EORTC criteria; not sure if all patients were included in analyses | ||
| Comparative | |||
| No patients per category | Probable or definite invasive aspergillosis was diagnosed in 9 patients. In 8 patients, invasive aspergillosis was not confirmed and alternative pathogens were isolated. Unclear what happened to possibles | ||
| Notes | No conflicts of interest reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Tanase 2012.
| Study characteristics | |||
| Patient sampling | In 2011, all patients with HSCT at the Fundeni Clinical Institute, Bucharest, were screened with serum GM ELISAs while hospitalised. Patients were retrospectively evaluated from 1 January to 31 December 2011 | ||
| Patient characteristics and setting | A total of 148 adult and 14 paediatric patients had at least 2 GM ELISA tested while undergoing HSCT in 2011. These patients ranged in age from 1 to 68.1 years (mean 32.4 years). 96 of the recipients were male (59.9%). With 1 exception, all the tested patients received peripheral haematopoietic stem cells | ||
| Index tests | A commercially available sandwich ELISA (Platelia Aspergillus, Bio‐Rad, France) was used. The optical density (OD) of the test specimen is divided by the mean OD of the cut‐off control, and results with an index value of 0.5 or higher are considered positive. A positive GM test result was defined as 2 consecutive tests with an optical density index of ≥ 0.5 or a single test with an optical density index of ≥ 0.8 (this is different from other studies ‐> impact on applicability?) | ||
| Target condition and reference standard(s) | The probability of having an invasive fungal infection at any time after HSCT was determined by EORTC/MSG criteria. Reference to Ascioglu 2002. Not clear if the EIA results were included | ||
| Flow and timing | The date of the first positive GM test result was considered the date of diagnosis of IA, in high‐risk patients with radiological signs of IA | ||
| Comparative | |||
| No patients per category | 102 of the 162 patients had no clinical, radiographic or microbiological criteria for IA. 1 had proven IA by biopsy; 6 patients had a probable diagnosis of IA and 53 had a possible diagnosis of IA | ||
| Notes | No conflicts of interest reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Low | |||
Tanriover 2008.
| Study characteristics | |||
| Patient sampling | Reported as a prospective cohort study All patients (> 16 years of age) with high‐risk haematological malignancies admitted to the Internal Medicine wards during the 2‐year study period were interviewed and those who consented were recruited for follow‐up Patients who gave informed consent were included in the study starting from the day they were admitted to the wards and followed up until death, discharge or withdrawal of consent, whichever occurred earlier. Death or discharge within 10 days of hospitalisation, less than 10 days of neutropenia or major difficulty in obtaining blood samples were the exclusion criteria. |
||
| Patient characteristics and setting | 58 treatment episodes in 45 participants
Age between 15 and 74 years
Turkey
Analyses are based on episodes All patients (> 16 years of age) with high‐risk haematological malignancies admitted to the Internal Medicine wards during the 2‐year study period were interviewed and those who consented were recruited for follow‐up Representative spectrum? Yes: all patients (> 16 years of age) with high‐risk haematological malignancies; prospective study |
||
| Index tests | Platelia Aspergillus; Bio‐Rad Laboratories; according to manufacturer's instructions. GM index was expressed as the ratio of the optical density of the sample relative to the optical density of the threshold control (ODI). Cut‐points tested: 0.5, 0.7, 1.0, 1.5. Calculations made separately for single positive values and at least 2 consecutive positive results (within 1 week) as well as classifying the data as proven plus probable or proven plus probable plus possible | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria (Ascioglu 2002) Incorporation avoided? Yes: "GM positivity was not used as a microbiological criterion for classifying IA." Acceptable reference standard? Yes: EORTC criteria |
||
| Flow and timing | The mean time between the first febrile day and the first CT scan was reported: 15.7 +/‐ 12 days Partial verification avoided; withdrawals were explained; there were no uninterpretable results |
||
| Comparative | |||
| No patients per category | 1 proven, 4 probable, 20 possible, 33 no IA | ||
| Notes | Sponsoring reported and no Platelia support mentioned This study is registered as being published in 2010, which is correct, but it was already published online in 2008 |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Ulusakarya 2000.
| Study characteristics | |||
| Patient sampling | Consecutive series of patients; retrospectively selected | ||
| Patient characteristics and setting | 507 samples from 135 patients were analysed during 193 neutropenic periods Age ranged from 6 to 78 years; 47% males Haematology unit France Children and adults undergoing bone marrow transplantation (BMT); all consecutive patients with haematological malignancies and treated with high‐dose chemotherapy Monitoring clinical course |
||
| Index tests | Platelia Antigenaemia was monitored weekly Positive = one or more positives; negative = all negatives Cut‐off: 1.0 and 1.5 reported |
||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC‐like criteria, citation Machetti 1998. Proven + probable + possible versus no IA Galactomannan ELISA was not mentioned as part of the reference criteria |
||
| Flow and timing | Not clear if all patients were categorised according to the same criteria Withdrawals were explained Uninterpretable results reported: these were ignored | ||
| Comparative | |||
| No patients per category | 10 proven, 6 probable, 2 possible, 117 no IA | ||
| Notes | Nothing reported about financing | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Weisser 2005.
| Study characteristics | |||
| Patient sampling | Prospective; consecutive series of patients. Episode‐based analysis | ||
| Patient characteristics and setting | 161 participants Age ranged from 16 to 78 years 51% males Switzerland Adults undergoing autologous or allogeneic haematopoietic stem cell transplantation (HSCT) or receiving chemotherapy Inpatients; monitoring clinical course Representative spectrum? Yes: adults undergoing autologous or allogeneic HSCT or receiving chemotherapy |
||
| Index tests | Platelia Aspergillus. Sera were tested twice weekly. 2 consecutives positive was considered positive. Cut‐off = 0.5 ODI | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria Incorporation avoided? Not explicitly reported whether galactomannan results were excluded from EORTC criteria Acceptable reference standard? Yes: EORTC criteria |
||
| Flow and timing | Timing not reported Partial verification avoided? Not clear Withdrawals explained? Not reported Uninterpretable results reported? Not reported |
||
| Comparative | |||
| No patients per category | 20 proven or probable, 32 possible, 109 no IA | ||
| Notes | Sponsoring: Science Grant reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Yes | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
White 2005.
| Study characteristics | |||
| Patient sampling | Study design not clear | ||
| Patient characteristics and setting | 105 participants
No information about age or gender
UK Patients considered to be at high risk for invasive fungal infection, no further details provided Representative spectrum? Patients considered to be at high risk, without further information |
||
| Index tests | Platelia Aspergillus. One positive is positive; no positive is negative; not reported how often samples were taken. Positive result: single sample above 1.5 ODI | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria (no citation) Incorporation avoided? Not explicitly reported whether galactomannan results were excluded from EORTC criteria Acceptable reference standard? Yes: EORTC criteria (no citation) |
||
| Flow and timing | Timing not reported Uninterpretable results reported? No | ||
| Comparative | |||
| No patients per category | 1 proven, 2 probable, 4 possible, 98 no IA | ||
| Notes | Sponsoring unclear; nothing reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
White 2013a.
| Study characteristics | |||
| Patient sampling | As part of the local neutropenic fever care pathway, twice weekly samples were routinely taken. Serum was prospectively tested by GM EIA. Although they speak of controls, it seems that the inclusion was consecutively done, as they have 1 proven, 6 probables, 10 possibles and 48 'controls'. This is very much in agreement with what one would expect | ||
| Patient characteristics and setting | Haematology population; not much further information. No reasons to have high concern regarding applicability of this population. | ||
| Index tests | Platelia test; cut‐off 0.5 Serum‐positive EIA results were confirmed by retesting if the results from plasma and serum were incongruent or if the result represented a single positive among the samples tested per patient and was not confirmed by plasma testing. Otherwise, agreement between samples or multiple positive results were considered confirmation |
||
| Target condition and reference standard(s) | Over a 6‐month period, cases (proven, probable and possible IA) were selected according to disease status as defined, at the time of testing, by the revised EORTC/MSG criteria (De Pauw 2008). (They also took plasma samples; the results of these were not included in EORTC criteria, but it is not clear what they did with the serum samples) | ||
| Flow and timing | No information on timing | ||
| Comparative | |||
| No patients per category | 1 proven, 6 probables, 10 possibles and 48 'controls' | ||
| Notes | Authors are involved with and paid by (for conferences and talks) Giliad Sciences and Pfizer, 2 therapeutic companies | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Williamson 2000.
| Study characteristics | |||
| Patient sampling | Prospective; consecutive series of patients; blood samples re‐analysed later for ELISA | ||
| Patient characteristics and setting | 104 participants Age ranged from 3 months to 56 years No information about gender UK Children and adults undergoing bone marrow transplantation (BMT) or chemotherapy for haematological malignancy with severe neutropenia Inpatients Monitoring clinical course |
||
| Index tests | Platelia Aspergillus Serum samples were collected and tested twice weekly One positive is positive; no positive at all is negative Cut‐off value not reported |
||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC‐like criteria, no reference; 3 groups Galactomannan ELISA was not mentioned as part of the reference criteria |
||
| Flow and timing | All patients were classified according to the same reference criteria Withdrawals explained? Yes Uninterpretable results reported? No |
||
| Comparative | |||
| No patients per category | 7 proven, no probables, 9 possible, 88 no IA | ||
| Notes | Nothing reported on conflicts of interest or sponsoring | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Yes | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Yes | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Low | |||
Xie_L 2006.
| Study characteristics | |||
| Patient sampling | Prospective; consecutive series of patients | ||
| Patient characteristics and setting | 81 participants
Both children and adults
Inpatients
China Haematology department; included when fever (T > 38.5 ℃); ineffective after 4 days therapy of broad spectrum antibiotic; neutrophile granulocyte < 1.0 × 109/L Representative spectrum? Yes: all patients in the haematology department who fulfilled the inclusion criteria |
||
| Index tests | Platelia Aspergillus; index test was done every 3 to 4 days; cut‐off was 1.5 ODI and 2 subsequent positive samples were needed for a positive result | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by the Editorial Board of the Chinese Journal of Internal Medicine Incorporation avoided? Unclear: this was not clearly reported Acceptable reference standard? Unclear: we are not sure that the Chinese criteria are exactly the same as the 'Western' criteria |
||
| Flow and timing | Not reported Partial verification avoided? Yes: all patients were classified according to the reference criteria Withdrawals explained? Unclear: not reported Uninterpretable results reported? Unclear: not reported |
||
| Comparative | |||
| No patients per category | 11 proven, 23 probable, 33 possible and 14 no IA | ||
| Notes | Sponsoring precluded? No financial support reported | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Xu_J 2010.
| Study characteristics | |||
| Patient sampling | Patients enrolled if at least 1 sample was collected before treatment and if consecutive testing was possible for at least 2 weeks | ||
| Patient characteristics and setting | 60 patients
Both children and adults
China Haematology department; non‐responsive fever; no antifungal therapy Representative spectrum? Unclear: haematology department; non‐responsive fever; no antifungal therapy No information about enrollment and whether the study was prospective |
||
| Index tests | Platelia, at 3 different thresholds | ||
| Target condition and reference standard(s) | Criteria of the Editorial Board of the Chinese Journal of Medicine Incorporation avoided? Yes: Platelia explicitly excluded from criteria Acceptable reference standard? Unclear: Chinese criteria |
||
| Flow and timing | Time interval not reported Partial verification avoided? Yes Withdrawals explained? Not reported Uninterpretable results reported? Not reported |
||
| Comparative | |||
| No patients per category | 0 proven; 1 probable; 24 possible; 35 no IA | ||
| Notes | Sponsoring precluded? Not supported by Platelia | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Xu_M 2009.
| Study characteristics | |||
| Patient sampling | Enrolment not reported | ||
| Patient characteristics and setting | 172 inpatients; China Haematology department; non‐responsive fever Representative spectrum? Unclear: haematology department; non‐responsive fever. No information about enrollment and whether the study was prospective |
||
| Index tests | Platelia test; cut‐off single sample 0.7; or twice 0.5 | ||
| Target condition and reference standard(s) | Criteria of the Editorial Board of the Chinese Journal of Medicine Incorporation avoided? Yes: Platelia explicitly excluded from criteria Acceptable reference standard? Unclear: Chinese criteria. The patients were divided into 3 groups: proven/probable; possible; no IA |
||
| Flow and timing | Timing not reported Partial verification avoided? Yes Withdrawals explained? No: not reported Uninterpretable results reported? No: not reported |
||
| Comparative | |||
| No patients per category | 2 proven; 37 probable; 58 possible; 76 no IA | ||
| Notes | Not supported by Platelia Chinese language |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
Xu_P 2012.
| Study characteristics | |||
| Patient sampling | 113 patients with suspected lPA admitted in 'Department of Respiratory Medicine, Infectious Diseased, Kidney Disease Centre and ICU' were included in the study | ||
| Patient characteristics and setting | ICU patients | ||
| Index tests | Galactomannan antigen; threshold: two OD > 0.5 or one OD > 0.8. | ||
| Target condition and reference standard(s) | Invasive pulmonary aspergillosis, EORTC/MSG | ||
| Flow and timing | Difficult to extract due to Chinese language; time interval unclear. As reference standard was EORTC criteria and study used a one‐gate inclusion process, we assumed that all patients received the same reference standard. As there were no withdrawals or dropouts reported and the numbers in the 2x2 were the same as the numbers included, we assumed that all patients were included in the analyses. |
||
| Comparative | |||
| No patients per category | 4 proven, 36 probable, 16 possible, 57 no IA | ||
| Notes | No information on conflicts of interest; Chinese language. | ||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Low | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Yes | ||
| Low | |||
Yoo 2005.
| Study characteristics | |||
| Patient sampling | Prospective; consecutive series of patients. Possible cases were regarded as non‐IA | ||
| Patient characteristics and setting | 14 participants
No information about age or gender
Korea Neutropenic adults with fever that did not respond to antibiotic therapy Inpatients, monitoring of clinical course Representative spectrum? Yes: neutropenic adults with fever that did not respond to antibiotic therapy |
||
| Index tests | PlateliaAspergillus. Blood samples were usually obtained twice a week until the patient recovered from neutropenia. 2 consecutive positive samples was considered as a positive result. No explanation of negative result. Many different cut‐off values analysed | ||
| Target condition and reference standard(s) | Invasive aspergillosis, as defined by EORTC criteria, Ascioglu 2002 cited. In analyses proven and probable versus possible and no IA Incorporation avoided? Not explicitly stated that galactomannan results were excluded from EORTC criteria Acceptable reference standard? Yes: EORTC criteria (Ascioglu 2002) |
||
| Flow and timing | Time: until recovering from neutropenia Partial verification avoided? Unclear: not entirely clear whether all patients were classified according to the reference criteria Withdrawals explained? Yes Uninterpretable results reported? No: not reported |
||
| Comparative | |||
| No patients per category | 2 proven or probable, 12 possible or no IA | ||
| Notes | ROC curve provided Sponsoring precluded? Yes: financial support by the Korean Research Foundation |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Yes | ||
| Was a case‐control design avoided? | Yes | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Low | Low | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | No | ||
| High | Unclear | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Yes | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Low | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Yes | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Low | |||
Zhang_X 2009.
| Study characteristics | |||
| Patient sampling | No information provided | ||
| Patient characteristics and setting | 88 children; China; no further info Little information provided; no inclusion criteria given; department not reported Representative spectrum? Unclear: no information provided except that they were children |
||
| Index tests | Platelia test, but not used as a standard care procedure. Cut‐off single sample 0.5 | ||
| Target condition and reference standard(s) | Criteria of the Editorial Board of the Chinese Journal of Medicine Incorporation avoided? Yes, Platelia explicitly excluded from criteria Acceptable reference standard? Unclear: Chinese criteria and only 3 categories |
||
| Flow and timing | Time interval not reported Partial verification avoided? Yes Withdrawals explained? Not reported Uninterpretable results reported? Not reported |
||
| Comparative | |||
| No patients per category | 14 proven/probable; 16 possible; 58 no IA | ||
| Notes | Sponsored by Platelia Chinese language |
||
| Methodological quality | |||
| Item | Authors' judgement | Risk of bias | Applicability concerns |
| DOMAIN 1: Patient Selection | |||
| Was a consecutive or random sample of patients enrolled? | Unclear | ||
| Was a case‐control design avoided? | Unclear | ||
| Did the study avoid inappropriate exclusions? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 2: Index Test All tests | |||
| Were the index test results interpreted without knowledge of the results of the reference standard? | Unclear | ||
| If a threshold was used, was it pre‐specified? | Yes | ||
| Low | Low | ||
| DOMAIN 3: Reference Standard | |||
| Is the reference standards likely to correctly classify the target condition? | Unclear | ||
| Were the reference standard results interpreted without knowledge of the results of the index tests? | Unclear | ||
| Unclear | Unclear | ||
| DOMAIN 4: Flow and Timing | |||
| Was there an appropriate interval between index test and reference standard? | Unclear | ||
| Did all patients receive the same reference standard? | Yes | ||
| Were all patients included in the analysis? | Unclear | ||
| Unclear | |||
IFD: Invasive Fungal Disease; IFI: Invasive Fungal Infection; IPA: Invasive Pulmonary Aspergillosis; BAL: bronchoalveolar lavage; BMT: bone marrow transplantation; COI: conflict of interest; COPD: chronic obstructive pulmonary disease; CT: computerised tomography; ELISA: enzyme‐linked immunosorbent assay; EIA: enzyme immuno‐assay (equals ELISA); EORTC: European Organization for Research and Treatment of Cancer; GM: galactomannan; HSCT: haematopoietic stem cell transplantation; IA: invasive aspergillosis; ICU: intensive care unit; MSG: Mycoses Study Group; N/A: not applicable; NR: not reported; ODI: optical density index; PCR: polymerase chain reaction; ROC: receiver operating characteristic; SCT: stem cell transplantation; WBC: white blood cells.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Acosta 2010 | Possibles excluded |
| Acosta 2011 | Duplicate of Acosta 2012 |
| Asano‐Mori 2008 | Possibles excluded |
| Bellanger 2011 | Possibles excluded |
| Bergeron 2010 | No control group |
| Bergeron 2012 | No control group |
| Bochenek 2007 | Polish; no translation available |
| Bretagne 1997 | In‐house test |
| Castagnola 2010 | Possibles excluded |
| Cesaro 2008 | Possibles excluded |
| Challier 2004 | Case‐control design; included healthy controls |
| Cuenca‐Estrella 2009 | Possibles excluded |
| Desai 2009 | Insufficient information provided |
| Fortun 2001 | Possibles excluded |
| Fujiuchi 2009 | Japanese; no translation available |
| Guinea 2008 | Possibles excluded |
| Hachem 2009 | Possibles excluded |
| Hadrich 2011 | Possibles excluded |
| Hayden 2008 | Possibles excluded |
| He 2011 | Possibles excluded |
| Hu_H 2012 | Possibles excluded |
| Husain 2004 | Possibles excluded |
| Jarque 2003 | Possibles excluded |
| Jathavedam 2009 | Insufficient information to derive 2 x 2 tables |
| Ji 2007 | Full text unavailable |
| Ji 2011 | Not a diagnostic accuracy study |
| Jin_J 2010 | Case‐control study with healthy controls |
| Khanna 2013 | Case‐control design including healthy blood donors as controls |
| Kimura 2009 | Possibles excluded |
| Kitasato 2009 | Insufficient information to derive 2 x 2 tables |
| Leng_Y 2010 | No EORTC criteria (or something similar) used |
| Li_L 2011 | No EORTC criteria (or something similar) used |
| Lim 2004 | Insufficient information to derive 2 x 2 tables |
| Lopes 2010 | Sample‐based analysis |
| Maertens 2004 | Possibles excluded |
| Maertens 2007 | Possibles excluded |
| Maertens 2007a | Possibles excluded |
| Marr 2005 | Possibles excluded |
| Meersseman 2008 | Possibles excluded |
| Pazos 2005 | Possibles excluded |
| Penack 2008 | How specificity was estimated and whether possibles were included or excluded is not reported |
| Pereira 2005 | Cases were patients with invasive fungal infections, including candida infections and other fungal infections than aspergillosis; cases were from different department |
| Perkins 2007 | Letter |
| Racil 2008 | Czech; no translation available |
| Rogers 2013 | Possibles excluded |
| Sarrafzadeh 2010 | Control group was made up from probables and possibles; no patients without IA included |
| Steinbach 2007 | Possibles excluded |
| Sulahian 1996 | In‐house test |
| Sulahian 2001 | In‐house test |
| Tabone 1997 | In‐house test |
| Uryu 2006 | Japanese; full text not available |
| Verweij 1995 | In‐house test |
| Wang_Y 2013 | 2 x 2 tables could not be derived |
| Xiang_J 2010 | 2 x 2 tables could not be derived in a correct way (the numbers in the tables and the sensitivity/specificity estimates did not match) |
| Yao 2009 | 2 x 2 tables could not be derived |
| Yu 2010 | Possibles and no IA excluded |
| Zedek 2006 | Insufficient data |
| Zeng 2011 | Case‐control design with healthy volunteers |
EORTC: European Organization for Research and Treatment of Cancer; IA: invasive aspergillosis
Differences between protocol and review
We stated that we would contact authors and industry and this has not been done. This is now stated in the 'Methods' section.
We limited the accepted reference standard to EORTC(‐like) criteria. We originally stated that it would be either autopsy, combined with a positive culture or with histopathological evidence, or the EORTC/MSG criteria, or the demonstration of hyphal invasion in biopsies, combined with a positive culture. The rationale for this is that autopsy is almost never done and that biopsy and culture are included in the EORTC/MSG criteria.
QUADAS‐2 did not exist when the protocol was written; we updated the review to incorporate QUADAS‐2.
We did not calculate likelihood ratios and odds ratios, as described in the protocol. The reason for this is that we think that the value of this test is better described by explaining the consequences of false positive (1‐specificity) and false negative (1‐sensitivity) results.
We added some extra explanation about the independence of index and reference tests to the 'Methods' section (under 'Assessment of methodological quality').
In the protocol we stated that we would investigate the effect of: cut‐off values, reference standard, distinctive groups of patients, children versus adults and the use of antifungal therapy. In the review we did investigate the effects of: cut‐off values, reference standard and clinical subgroups (children versus adults; distinctive groups of patients (high‐risk versus low‐risk); use of antifungal prophylaxis; use of antifungal therapy).
In the protocol we stated that the main purpose for a test for invasive aspergillosis would be to guide therapy. During the review process, we discovered that the test is used in many different ways and in most studies it is not used to guide therapy (although a test that could guide therapy would still be ideal). We have therefore changed the text in such a way that there is less focus on guidance of therapy.
In the protocol we did not mention that we would divide the four reference categories into diseased versus non‐diseased, because at the time the protocol was written we were not aware that this could be an issue.
Contributions of authors
ML: drafted protocol; searches; study selection and data extraction; analyses; drafted review.
YD: study selection and data extraction; commented on protocol and review.
JW: study selection and data extraction in second round of update (2014); translation of Chinese articles.
CV: study selection and data extraction; commented on protocol and review.
HB: data extraction; commented on protocol and review.
LH: data extraction; commented on protocol and review.
RS: data extraction; commented on protocol and review.
JBR: data analysis; commented on review.
MZ: data extraction and translation of Chinese articles in first‐round (2011) update.
PMB: commented on protocol and review.
CVG: initiator; commented on protocol and review.
Sources of support
Internal sources
-
None, Other.
The authors declare that no funding was received for this systematic review.
External sources
-
None, Other.
The authors declare that no funding was received for this systematic review.
Declarations of interest
Authors ML, YD, JW, CV, HB, LH, RS, JBR, MZ, PMB, CVG state no conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Acosta 2012 {published data only}
- Acosta J, Catalan M, Palacio‐Perez‐Medel A, Montejo J‐C, Cruz‐Bertolo J, Moragues MD, et al. Prospective study in critically ill non‐neutropenic patients: diagnostic potential of (1,3)‐beta‐D‐glucan assay and circulating galactomannan for the diagnosis of invasive fungal disease. European Journal of Clinical Microbiology and Infectious Diseases 2012;31(5):721‐31. [DOI] [PubMed] [Google Scholar]
Adam 2004 {published data only}
- Adam O, Auperin A, Wilquin F, Bourhis JH, Gachot B, Chachaty E. Treatment with piperacillin‐tazobactam and false‐positive Aspergillus galactomannan antigen test results for patients with hematological malignancies. Clinical Infectious Diseases 2004;38(6):917‐20. [DOI] [PubMed] [Google Scholar]
Allan 2005 {published data only}
- Allan EK, Jordanides NE, McLintock LA, Copland M, Devaney M, Stewart K, et al. Poor performance of galactomannan and mannan sandwich enzyme‐linked immunosorbent assays in the diagnosis of invasive fungal infection. British Journal of Haematology 2005;128(4):578‐9. [DOI] [PubMed] [Google Scholar]
Badiee 2013 {published data only}
- Badiee P, Alborzi A, Karimi M, Pourabbas B, Haddadi P, Mardaneh J, et al. Diagnostic potential of nested PCR, galactomannan EIA, and beta‐D‐glucan for invasive aspergillosis in pediatric patients. Journal of Infection in Developing Countries 2012;6(4):352‐7. [PUBMED: 22505446] [DOI] [PubMed] [Google Scholar]
Barnes 2013 {published data only}
- Barnes RA, Stocking K, Bowden S, Poynton MH, White PL. Prevention and diagnosis of invasive fungal disease in high‐risk patients within an integrative care pathway. Journal of Infection 2013;67(3):206‐14. [DOI] [PubMed] [Google Scholar]
Becker 2003 {published data only}
- Becker MJ, Lugtenburg EJ, Cornelissen JJ, Schee C, Hoogsteden HC, Marie S. Galactomannan detection in computerized tomography‐based broncho‐alveolar lavage fluid and serum in haematological patients at risk for invasive pulmonary aspergillosis. British Journal of Haematology 2003;121(3):448‐57. [DOI] [PubMed] [Google Scholar]
Bialek 2002 {published data only}
- Bialek R, Moshous D, Casanova JL, Blanche S, Hennequin C. Aspergillus antigen and PCR assays in bone marrow transplanted children. European Journal of Medical Research 2002;7(4):177‐80. [PubMed] [Google Scholar]
Bretagne 1998 {published data only}
- Bretagne S, Costa JM, Bart‐Delabesse E, Dhedin N, Rieux C, Cordonnier C. Comparison of serum galactomannan antigen detection and competitive polymerase chain reaction for diagnosing invasive aspergillosis. Clinical Infectious Diseases 1998;26(6):1407‐12. [DOI] [PubMed] [Google Scholar]
Buchheidt 2004 {published data only}
- Buchheidt D, Hummel M, Schleiermacher D, Spiess B, Schwerdtfeger R, Cornely OA, et al. Prospective clinical evaluation of a LightCycler‐mediated polymerase chain reaction assay, a nested‐PCR assay and a galactomannan enzyme‐linked immunosorbent assay for detection of invasive aspergillosis in neutropenic cancer patients and haematological stem cell transplant recipients. British Journal Haematology 2004;125(2):196‐202. [DOI] [PubMed] [Google Scholar]
Busca 2006 {published data only}
- Busca A, Locatelli F, Barbui A, Limerutti G, Serra R, Libertucci D, et al. Usefulness of sequential Aspergillus galactomannan antigen detection combined with early radiologic evaluation for diagnosis of invasive pulmonary aspergillosis in patients undergoing allogeneic stem cell transplantation. Transplantation Proceedings 2006;38(5):1610‐3. [DOI] [PubMed] [Google Scholar]
Da Silva 2010 {published data only}
- Silva RL, Ribeiro P, Abreu N, Ferreira T, Fernandes T, Monteiro A, et al. Early diagnosis of invasive aspergillosis in neutropenic patients. comparison between serum galactomannan and polymerase chain reaction. Clinical Medicine Insights: Oncology 2010;4:81‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Mol 2013 {published data only}
- Mol M, Jongste JC, Westreenen M, Merkus PJFM, Vries AHC, Hop WCJ, et al. Diagnosis of invasive pulmonary aspergillosis in children with bronchoalveolar lavage galactomannan. Pediatric Pulmonology 2013;48(8):789‐96. [DOI] [PubMed] [Google Scholar]
Doermann 2002 {published data only}
- Doermann F, Accoceberry I, Weill FX, Agape P, Boiron JM, Marit G, et al. Evaluation of Aspergillus antigen detection in sera by ELISA for diagnosis of invasive aspergillosis in a hematological unit. Journal de Mycologie Medicale 2002;12(3):131‐5. [Google Scholar]
Florent 2006 {published data only}
- Florent M, Katsahian S, Vekhoff A, Levy V, Rio B, Marie JP, et al. Prospective evaluation of a polymerase chain reaction‐ELISA targeted to Aspergillus fumigatus and Aspergillus flavus for the early diagnosis of invasive aspergillosis in patients with hematological malignancies. Journal of Infectious Diseases 2006;193(5):741‐7. [DOI] [PubMed] [Google Scholar]
Foy 2007 {published data only}
- Foy PC, Burik JA, Weisdorf DJ. Galactomannan antigen enzyme‐linked immunosorbent assay for diagnosis of invasive aspergillosis after hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation 2007;13(4):440‐3. [DOI] [PubMed] [Google Scholar]
Gao 2010 {published data only}
- Gao X, Chen L, Hu G, Mei H. Invasive pulmonary aspergillosis in acute exacerbation of chronic obstructive pulmonary disease and the diagnostic value of combined serological tests. Annals of Saudi Medicine 2010;30(3):193‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghosh 2013 {published data only}
- Ghosh I, Raina V, Kumar L, Sharma A, Bakhshi S, Iqbal S. Serum galactomannan assay for diagnosis of probable invasive Aspergillosis in acute leukemia and hematopoietic stem cell transplantation. Indian Journal of Medical and Paediatric Oncology 2013;34(2):74‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
He 2011a {published data only}
- He H, Ding L, Chang S, Li F, Zhan Q. Value of consecutive galactomannan determinations for the diagnosis and prognosis of invasive pulmonary aspergillosis in critically ill chronic obstructive pulmonary disease. Medical Mycology 2011;49(4):345‐51. [DOI] [PubMed] [Google Scholar]
Herbrecht 2002 {published data only}
- Herbrecht R, Letscher‐Bru V, Oprea C, Lioure B, Waller J, Campos F, et al. Aspergillus galactomannan detection in the diagnosis of invasive aspergillosis in cancer patients. Journal of Clinical Oncology 2002;20(7):1898‐906. [DOI] [PubMed] [Google Scholar]
Hovi 2007 {published data only}
- Hovi L, Saxen H, Saarinen‐Pihkala UM, Vettenranta K, Meri T, Richardson M. Prevention and monitoring of invasive fungal infections in pediatric patients with cancer and hematologic disorders. Pediatric Blood and Cancer 2007;48(1):28‐34. [DOI] [PubMed] [Google Scholar]
Jha 2013 {published data only}
- Jha AK, Bansal D, Chakrabarti A, Shivaprakash MR, Trehan A, Marwaha RK. Serum galactomannan assay for the diagnosis of invasive aspergillosis in children with haematological malignancies. Mycoses 2013;56(4):442‐8. [DOI] [PubMed] [Google Scholar]
Kallel 2003 {published data only}
- Kallel K, Ladeb S, Belhadj S, Jerbi A, Ben Othman T, Abdelkefi A, et al. Evaluation of aspergillary antigenemia for monitoring neutropenic patients. Journal de Mycologie Medicale 2003;13(4):199‐202. [Google Scholar]
Kawazu 2004 {published data only}
- Kawazu M, Kanda Y, Nannya Y, Aoki K, Kurokawa M, Chiba S, et al. Prospective comparison of the diagnostic potential of real‐time PCR, double‐sandwich enzyme‐linked immunosorbent assay for galactomannan, and a (1‐‐>3)‐beta‐D‐glucan test in weekly screening for invasive aspergillosis in patients with hematological disorders. Journal of Clinical Microbiology 2004;42(6):2733‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ku 2012 {published data only}
- Ku NS, Han SH, Choi JY, Kim SB, Kim H‐W, Jeong SJ, et al. Diagnostic value of the serum galactomannan assay for invasive aspergillosis: it is less useful in non‐haematological patients. Scandinavian Journal of Infectious Diseases 2012;44(8):600‐4. [DOI] [PubMed] [Google Scholar]
Lai 2007 {published data only}
- Lai CC, Hsu HL, Lee LN, Hsueh PR. Assessment of Platelia Aspergillus enzyme immunoassay for the diagnosis of invasive aspergillosis. Journal of Microbiology, Immunology, and Infection 2007;40(2):148‐53. [PubMed] [Google Scholar]
Liu_S 2012 {published data only}
- Liu SJ, Wang X, Wang XH, Zhou H, Yuan D, Jiang H. Quantitative PCR for early diagnosis of invasive fungal infections in patients with hematologic malignancies. Journal of Experimental Hematology/Chinese Association of Pathophysiology 2012;20(5):1225‐30. [PubMed] [Google Scholar]
Machetti 1998 {published data only}
- Machetti M, Feasi M, Mordini N, Lint MT, Bacigalupo A, Latge JP, et al. Comparison of an enzyme immunoassay and a latex agglutination system for the diagnosis of invasive aspergillosis in bone marrow transplant recipients. Bone Marrow Transplantation 1998;21(9):917‐21. [DOI] [PubMed] [Google Scholar]
Maertens 2002 {published data only}
- Maertens J, Eldere J, Verhaegen J, Verbeken E, Verschakelen J, Boogaerts M. Use of circulating galactomannan screening for early diagnosis of invasive aspergillosis in allogeneic stem cell transplant recipients. Journal of Infectious Diseases 2002;186(9):1297‐306. [DOI] [PubMed] [Google Scholar]
Marr 2004 {published data only}
- Marr KA, Balajee SA, McLaughlin L, Tabouret M, Bentsen C, Walsh TJ. Detection of galactomannan antigenaemia by enzyme immunoassay for the diagnosis of invasive aspergillosis: variables that affect performance. Journal of Infectious Diseases 2004;190(3):641‐9. [DOI] [PubMed] [Google Scholar]
Moragues 2003 {published data only}
- Moragues MD, Amutio E, Garcia‐Ruiz JC, Ponton J. Usefulness of galactomannan detection in the diagnosis and follow‐up of hematological patients with invasive aspergillosis [Utilidad de la deteccion de galactomanano en el diagnostico y seguimiento de la aspergilosis invasora en pacientes hematologicos]. Revista Iberoamericana de Micologia 2003;20(3):103‐10. [PubMed] [Google Scholar]
Nihtinen 2010 {published data only}
- Nihtinen A, Anttila VJ, Richardson M, Ruutu T, Juvonen E, Meri T, et al. Invasive aspergillus infections in allo‐SCT recipients: environmental sampling, nasal and oral colonization and galactomannan testing. Bone Marrow Transplant 2010;45(2):333‐8. [DOI] [PubMed] [Google Scholar]
Park 2010 {published data only}
- Park SY, Lee S‐O, Choi S‐H, Sung H, Kim M‐N, Choi C‐M, et al. Aspergillus galactomannan antigen assay in bronchoalveolar lavage fluid for diagnosis of invasive pulmonary aspergillosis. Journal of Infection 2010;61(6):492‐8. [DOI] [PubMed] [Google Scholar]
Pinel 2003 {published data only}
- Pinel C, Fricker‐Hidalgo H, Lebeau B, Garban F, Hamidfar R, Ambroise‐Thomas P, et al. Detection of circulating Aspergillus fumigatus galactomannan: value and limits of the Platelia test for diagnosing invasive aspergillosis. Journal of Clinical Microbiology 2003;41(5):2184‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rovira 2004 {published data only}
- Rovira M, Jimenez M, Bellacasa JP, Mensa J, Rafel M, Ortega M, et al. Detection of Aspergillus galactomannan by enzyme immunoabsorbent assay in recipients of allogeneic hematopoietic stem cell transplantation: a prospective study. Transplantation 2004;77(8):1260‐4. [DOI] [PubMed] [Google Scholar]
Scotter 2005 {published data only}
- Scotter JM, Campbell P, Anderson TP, Murdoch DR, Chambers ST, Patton WN. Comparison of PCR‐ELISA and galactomannan detection for the diagnosis of invasive aspergillosis. Pathology 2005;37(3):246‐53. [DOI] [PubMed] [Google Scholar]
Shi_Y 2009 {published data only}
- Shi Y, Liu DW, Long Y, Liu Y, Rui X, Zhou X, et al. The role of galactomannan detection in the diagnosis of invasive pulmonary aspergillosis in critically ill patients. Zhonghua Nei Ke Za Zhi 2009;48:225‐30. [PubMed] [Google Scholar]
Suankratay 2006 {published data only}
- Suankratay C, Kanitcharaskul P, Arunyingmongkol K. Galactomannan antigenemia for the diagnosis of invasive aspergillosis in neutropenic patients with hematological disorders. Journal of the Medical Association of Thailand 2006;89(11):1851‐8. [PubMed] [Google Scholar]
Suarez 2008 {published data only}
- Suarez F, Lortholary O, Buland S, Rubio MT, Ghez D, Mahe V, et al. Detection of circulating Aspergillus fumigatus DNA by real‐time PCR assay of large serum volumes improves early diagnosis of invasive aspergillosis in high‐risk adult patients under hematologic surveillance. Journal of Clinical Microbiology 2008;46:3772‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun_Q 2009 {published data only}
- Sun Q‐X, Meng F‐Y, Tan X‐J, Li J‐T, Cai Y‐X, Zhu Z‐G. Utility of serum galactomannan in diagnosis of invasive fungal infection in hematologic malignancy [Chinese]. Chinese Journal of Infection and Chemotherapy 2009;9(2):140‐2. [Google Scholar]
Sun_Y 2010 {published data only}
- Sun YQ, Ji Y, Xu LP, Liu DH, Liu KY, Huang XJ. Combination of real‐time polymerase chain reaction assay and serum galactomannan in the diagnosis of invasive aspergillosis in patients with hematological malignancies and recipients of hematopoietic stem cell transplantation. Zhonghua Yi Xue Za Zhi 2010;90(6):375‐8. [PubMed] [Google Scholar]
Tabarsi 2012 {published data only}
- Tabarsi P, Soraghi A, Marjani M, Zandian P, Baghaei P, Najafizadeh K, et al. Comparison of serum and bronchoalveolar lavage galactomannan in diagnosing invasive aspergillosis in solid‐organ transplant recipients. Experimental and Clinical Transplantation 2012;10(3):278‐81. [DOI] [PubMed] [Google Scholar]
Tanase 2012 {published data only}
- Tanase AD, Colita A, Marculescu A, Berteanu C, Streinu Cercel A, Stoica M, et al. Using the galactomannan antigen assay in the diagnosis of invasive aspergillosis after hematopoietic stem cell transplantation. Romanian Journal of Morphology and Embryology 2012;53(2):379‐82. [PubMed] [Google Scholar]
Tanriover 2008 {published data only}
- Tanriover MD, Ascioglu S, Altun B, Uzun O. Galactomannan on the stage: prospective evaluation of the applicability in routine practice and surveillance. Mycoses 2010;53(1):16‐25. [DOI] [PubMed] [Google Scholar]
Ulusakarya 2000 {published data only}
- Ulusakarya A, Chachaty E, Vantelon JM, Youssef A, Tancrede C, Pico JL, et al. Surveillance of Aspergillus galactomannan antigenemia for invasive aspergillosis by enzyme‐linked immunosorbent assay in neutropenic patients treated for hematological malignancies. Hematology Journal 2000;1(2):111‐6. [DOI] [PubMed] [Google Scholar]
Weisser 2005 {published data only}
- Weisser M, Rausch C, Droll A, Simcock M, Sendi P, Steffen I, et al. Galactomannan does not precede major signs on a pulmonary computerized tomographic scan suggestive of invasive aspergillosis in patients with hematological malignancies. Clinical Infectious Diseases 2005;41(8):1143‐9. [DOI] [PubMed] [Google Scholar]
White 2005 {published data only}
- White PL, Archer AE, Barnes RA. Comparison of non‐culture‐based methods for detection of systemic fungal infections, with an emphasis on invasive Candida infections. Journal of Clinical Microbiology 2005;43(5):2181‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2013a {published data only}
- White PL, Jones T, Whittle K, Watkins J, Barnes RA. Comparison of galactomannan enzyme immunoassay performance levels when testing serum and plasma samples. Clinical and Vaccine Immunology 2013;20(4):636‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williamson 2000 {published data only}
- Williamson EC, Oliver DA, Johnson EM, Foot AB, Marks DI, Warnock DW. Aspergillus antigen testing in bone marrow transplant recipients. Journal of Clinical Pathology 2000;53(5):362‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xie_L 2006 {published data only}
- Xie LP, Liu T, Meng WT, Zhou J. The value of serum galactomannan antigen for detection of invasive aspergillosis in hematological patients. Zhonghua Nei Ke Za Zhi 2006;45(12):992‐5. [PubMed] [Google Scholar]
Xu_J 2010 {published data only}
- Xu J, Sun ZM, Wang XB, Zhang XH. Early antigen diagnosis of invasive fungal infection in patients with hematological malignancies and patients receiving hematopoietic stem cell transplantation. Zhongguo Shi Yan.Xue Ye Xue Za Zhi 2010;18(5):1302‐5. [PubMed] [Google Scholar]
Xu_M 2009 {published data only}
- Xu M‐Z, Sun A‐N, Wu D‐P, Qiu H‐Y, Tang X‐W, Fu Z‐Z, et al. Utility of serum galactomannan antigen for diagnosis and follow‐up of invasive aspergillosis in hematological patients [Chinese]. Chinese Journal of Infection and Chemotherapy 2009;9(3):233‐6. [Google Scholar]
Xu_P 2012 {published data only}
- Xu PF, Zhou JY, Zhou H, Shen P. Serum antigens assay combined with chest CT scan in diagnosis of invasive pulmonary aspergillosis [血清抗原检测联合肺部CT诊断侵袭性肺曲霉病的探讨]. Journal of Zhejiang University Medical Sciences 2012;41(3):332‐8. [DOI] [PubMed] [Google Scholar]
Yoo 2005 {published data only}
- Yoo JH, Choi JH, Choi SM, Lee DG, Shin WS, Min WS, et al. Application of nucleic acid sequence‐based amplification for diagnosis of and monitoring the clinical course of invasive aspergillosis in patients with hematologic diseases. Clinical Infectious Diseases 2005;40(3):392‐8. [DOI] [PubMed] [Google Scholar]
Zhang_X 2009 {published data only}
- Zhang XY, Zhao SY, Jiang ZF. Value of serum galactomannan assay in the diagnosis of pediatric invasive pulmonary aspergillosis. Zhonghua Er Ke Za Zhi 2009;47(2):83‐6. [PubMed] [Google Scholar]
References to studies excluded from this review
Acosta 2010 {published data only}
- Acosta J, Catalan M, Palacio‐Perez‐Medel A, Lora D, Montejo JC, Cuetara MS, et al. A prospective comparison of galactomannan in bronchoalveolar lavage fluid for the diagnosis of pulmonary invasive aspergillosis in medical patients under intensive care: comparison with the diagnostic performance of galactomannan and of (1‐‐> 3) ‐beta‐d‐glucan chromogenic assay in serum samples. Clinical Microbiology and Infection 2011;17(7):1053‐60. [DOI] [PubMed] [Google Scholar]
Acosta 2011 {published data only}
- Acosta J, Catalan M, Palacio‐Perez‐Medel A, Lora D, Montejo J‐C, Cuetara M‐S, et al. A prospective comparison of galactomannan in bronchoalveolar lavage fluid for the diagnosis of pulmonary invasive aspergillosis in medical patients under intensive care: Comparison with the diagnostic performance of galactomannan and of (1‐> 3) ‐beta‐d‐glucan chromogenic assay in serum samples. Clinical Microbiology and Infection 2011;17(7):1053‐60. [DOI] [PubMed] [Google Scholar]
Asano‐Mori 2008 {published data only}
- Asano‐Mori Y, Kanda Y, Oshima K, Kako S, Shinohara A, Nakasone H, et al. False‐positive Aspergillus galactomannan antigenaemia after haematopoietic stem cell transplantation. Journal of Antimicrobial Chemotherapy 2008;61(2):411‐6. [DOI] [PubMed] [Google Scholar]
Bellanger 2011 {published data only}
- Bellanger AP, Grenouillet F, Henon T, Skana F, Legrand F, Deconinck E, et al. Retrospective assessment of beta‐D‐(1,3)‐glucan for presumptive diagnosis of fungal infections. APMIS 2011;119(4‐5):280‐6. [DOI] [PubMed] [Google Scholar]
Bergeron 2010 {published data only}
- Bergeron A, Belle A, Sulahian A, Lacroix C, Chevret S, Raffoux E, et al. Contribution of galactomannan antigen detection in BAL to the diagnosis of invasive pulmonary aspergillosis in patients with hematologic malignancies. Chest 2010;137(2):410‐5. [DOI] [PubMed] [Google Scholar]
Bergeron 2012 {published data only}
- Bergeron A, Porcher R, Menotti J, Poirot JL, Chagnon K, Vekhoff A, et al. Prospective evaluation of clinical and biological markers to predict the outcome of invasive pulmonary aspergillosis in hematological patients. Journal of Clinical Microbiology 2012;50(3):823‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bochenek 2007 {published data only}
- Bochenek M, Rup E, Macura AB. Mycoserological tests in the diagnosis of invasive aspergillosis [Polish]. Mikologia Lekarska 2007;14(3):174‐8. [Google Scholar]
Bretagne 1997 {published data only}
- Bretagne S, Marmorat‐Khuong A, Kuentz M, Latge JP, Bart‐Delabesse E, Cordonnier C. Serum Aspergillus galactomannan antigen testing by sandwich ELISA: practical use in neutropenic patients. Journal of Infection 1997;35(1):7‐15. [DOI] [PubMed] [Google Scholar]
Castagnola 2010 {published data only}
- Castagnola E, Furfaro E, Caviglia I, Licciardello M, Faraci M, Fioredda F, et al. Performance of the galactomannan antigen detection test in the diagnosis of invasive aspergillosis in children with cancer or undergoing haemopoietic stem cell transplantation. Clinical Microbiology and Infection 2010;16(8):1197‐203. [DOI] [PubMed] [Google Scholar]
Cesaro 2008 {published data only}
- Cesaro S, Stenghele C, Calore E, Franchin E, Cerbaro I, Cusinato R, et al. Assessment of the lightcycler PCR assay for diagnosis of invasive aspergillosis in paediatric patients with onco‐haematological diseases. Mycoses 2008;51(6):497‐504. [DOI] [PubMed] [Google Scholar]
Challier 2004 {published data only}
- Challier S, Boyer S, Abachin E, Berche P. Development of a serum‐based Taqman real‐time PCR assay for diagnosis of invasive aspergillosis. Journal of Clinical Microbiology 2004;42(2):844‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cuenca‐Estrella 2009 {published data only}
- Cuenca‐Estrella M, Meije Y, Diaz‐Pedroche C, Gomez‐Lopez A, Buitrago MJ, Bernal‐Martinez L, et al. Value of serial quantification of fungal DNA by a real‐time PCR‐based technique for early diagnosis of invasive Aspergillosis in patients with febrile neutropenia. Journal of Clinical Microbiology 2009;47(2):379‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Desai 2009 {published data only}
- Desai R, Ross LA, Hoffman JA. The role of bronchoalveolar lavage galactomannan in the diagnosis of pediatric invasive aspergillosis. Pediatric Infectious Disease Journal 2009;28(4):283‐6. [DOI] [PubMed] [Google Scholar]
Fortun 2001 {published data only}
- Fortun J, Martin‐Davila P, Alvarez ME, Sanchez‐Sousa A, Quereda C, Navas E, et al. Aspergillus antigenemia sandwich‐enzyme immunoassay test as a serodiagnostic method for invasive aspergillosis in liver transplant recipients. Transplantation 2001;71(1):145‐9. [DOI] [PubMed] [Google Scholar]
Fujiuchi 2009 {published data only}
- Fujiuchi S, Yamamoto Y, Takeda A, Nishigaki Y, Fujit Y, Yamazaki Y, et al. Evaluation of galactomannan antigen and beta‐D‐glucan value for diagnosis of chronic necrotizing pulmonary aspergillosis. Nihon Kokyuki Gakkai Zasshi 2009;47(1):7‐11. [PubMed] [Google Scholar]
Guinea 2008 {published data only}
- Guinea J, Jensen J, Pelaez T, Gijon P, Alonso R, Rivera M, et al. Value of a single galactomannan determination (Platelia) for the diagnosis of invasive aspergillosis in non‐hematological patients with clinical isolation of Aspergillus spp. Medical Mycology 2008;46(6):575‐9. [DOI] [PubMed] [Google Scholar]
Hachem 2009 {published data only}
- Hachem RY, Kontoyiannis DP, Chemaly RF, Jiang Y, Reitzel R, Raad I. Utility of galactomannan enzyme immunoassay and (1,3) beta‐D‐glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients. Journal of Clinical Microbiology 2009;47(1):129‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hadrich 2011 {published data only}
- Hadrich I, Mary C, Makni F, Elloumi M, Dumon H, Ayadi A, et al. Comparison of PCR‐ELISA and Real‐Time PCR for invasive aspergillosis diagnosis in patients with hematological malignancies. Medical Mycology 2011;49(5):489‐94. [DOI] [PubMed] [Google Scholar]
Hayden 2008 {published data only}
- Hayden R, Pounds S, Knapp K, Petraitiene R, Schaufele RL, Sein T, et al. Galactomannan antigenemia in pediatric oncology patients with invasive aspergillosis. Pediatric Infectious Disease Journal 2008;27(9):815‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
He 2011 {published data only}
- He H, Ding L, Chang S, Li F, Zhan Q. Value of consecutive galactomannan determinations for the diagnosis and prognosis of invasive pulmonary aspergillosis in critically ill chronic obstructive pulmonary disease. Medical Mycology 2011;49(4):345‐51. [DOI] [PubMed] [Google Scholar]
Hu_H 2012 {published data only}
- Hu H, Yang Y, Teng GL, Ju YF, Zhang LL, Wei M. Application of detecting bronchoalveolar lavage fluid Aspergillus galactomannan antigen in the diagnosis of pulmonary aspergillosis [支气管肺泡灌洗液半乳甘露聚糖抗原检测在肺曲霉病诊断中的价值]. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2012;92(32):2268‐70. [PubMed] [Google Scholar]
Husain 2004 {published data only}
- Husain S, Kwak EJ, Obman A, Wagener MM, Kusne S, Stout JE, et al. Prospective assessment of Platelia Aspergillus galactomannan antigen for the diagnosis of invasive aspergillosis in lung transplant recipients. American Journal of Transplantation 2004;4(5):796‐802. [DOI] [PubMed] [Google Scholar]
Jarque 2003 {published data only}
- Jarque I, Andreu R, Salavert M, Gomez D, Peman J, Gobernado M, et al. Value of Aspergillus galactomannan antigen detection in the diagnosis and follow‐up of invasive aspergillosis in hematological patients. Revista Iberoamericana de Micología 2003;20(3):116‐8. [PubMed] [Google Scholar]
Jathavedam 2009 {published data only}
- Jathavedam A, Dure DC, Taur Y, Weinstock DM. Limited utility of serum galactomannan assay after auto‐SCT. Bone Marrow Transplant 2009;44(1):59‐61. [DOI] [PubMed] [Google Scholar]
Ji 2007 {published data only}
- Ji Y, Liu DH, Xu LP, Liu KY, Huang XJ. The value of serum galactomannan detection for diagnosis of invasive aspergillosis in hematopoietic stem cell transplant recipients. Zhonghua Xue Ye Xue Za Zhi 2007;28(2):83‐6. [PubMed] [Google Scholar]
Ji 2011 {published data only}
- Ji Y, Xu LP, Liu DH, Chen YH, Han W, Zhang XH, et al. Positive results of serum galactomannan assays and pulmonary computed tomography predict the higher response rate of empirical antifungal therapy in patients undergoing allogeneic hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation 2011;17(5):759‐64. [DOI] [PubMed] [Google Scholar]
Jin_J 2010 {published data only}
- Jin, J.Sun, T.Y.Hu, Y.J.Li, Y.M. Multiple serum antigenic assays for diagnosis of invasive fungal infections in non‐neutropenic adult patients. Chinese journal of tuberculosis and respiratory diseases 2010;33(9):not reported. [PubMed] [Google Scholar]
Khanna 2013 {published data only}
- Khanna S, Oberoi JK, Datta S, Aggarwal S, Wattal C. Variables affecting the performance of galactomannan assay in high‐risk patients at a tertiary care centre in India. Indian Journal of Medical Microbiology 2013;31(1):34‐9. [DOI] [PubMed] [Google Scholar]
Kimura 2009 {published data only}
- Kimura S, Odawara J, Aoki T, Yamakura M, Takeuchi M, Matsue K. Detection of sputum Aspergillus galactomannan for diagnosis of invasive pulmonary aspergillosis in haematological patients. International Journal of Hematology 2009;90(4):463‐70. [DOI] [PubMed] [Google Scholar]
Kitasato 2009 {published data only}
- Kitasato Y, Tao Y, Hoshino T, Tachibana K, Inoshima N, Yoshida M, et al. Comparison of Aspergillus galactomannan antigen testing with a new cut‐off index and Aspergillus precipitating antibody testing for the diagnosis of chronic pulmonary aspergillosis. Respirology 2009;14(5):701‐8. [DOI] [PubMed] [Google Scholar]
Leng_Y 2010 {published data only}
- Leng Y, Li LH. Feasibility of serum galactomannan assay in hematologic malignancy patients with invasive fungal infections. Journal of Leukemia and Lymphoma 2010;19(7):551‐2. [PubMed] [Google Scholar]
Li_L 2011 {published data only}
- Li LL, Wang JM, Zhang WP, Hou J, Cheng H, Yang JM, et al. Value of circulating galactomannan screening for early diagnosis of invasive aspergillosis in hematological patients. Journal of Leukemia and Lymphoma 2011;20(7):418‐21. [Google Scholar]
Lim 2004 {published data only}
- Lim ZY, Pagliuca A, Wade J, Ho A, Devereux S, Mufti GJ. Use of the galactomannan ELISA in the detection of invasive aspergillosis: a retrospective review and potential pitfalls in application. British Journal of Haematology 2004;125:59‐60. [Google Scholar]
Lopes 2010 {published data only}
- Lopes da Silva R, Ribeiro P, Abreu N, Ferreira T, Fernandes T, Monteiro A, et al. Early diagnosis of invasive aspergillosis in neutropenic patients. Comparison between serum galactomannan and polymerase chain reaction. Clinical Medicine Insights. Oncology 2010;4:81‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maertens 2004 {published data only}
- Maertens J, Theunissen K, Verbeken E, Lagrou K, Verhaegen J, Boogaerts M, et al. Prospective clinical evaluation of lower cut‐offs for galactomannan detection in adult neutropenic cancer patients and haematological stem cell transplant recipients. British Journal of Haematology 2004;126(6):852‐60. [DOI] [PubMed] [Google Scholar]
Maertens 2007 {published data only}
- Maertens JA, Klont R, Masson C, Theunissen K, Meersseman W, Lagrou K, et al. Optimization of the cutoff value for the Aspergillus double‐sandwich enzyme immunoassay. Clinical Infectious Diseases 2007;44(10):1329‐36. [DOI] [PubMed] [Google Scholar]
Maertens 2007a {published data only}
- Maertens J, Theunissen K, Lodewyck T, Lagrou K, Eldere J. Advances in the serological diagnosis of invasive Aspergillus infections in patients with haematological disorders. Mycoses 2007;50 Suppl 1:2‐17. [DOI] [PubMed] [Google Scholar]
Marr 2005 {published data only}
- Marr KA, Laverdiere M, Gugel A, Leisenring W. Antifungal therapy decreases sensitivity of the Aspergillus galactomannan enzyme immunoassay. Clinical Infectious Diseases 2005;40(12):15. [DOI] [PubMed] [Google Scholar]
Meersseman 2008 {published data only}
- Meersseman W, Lagrou K, Maertens J, Wilmer A, Hermans G, Vanderschueren S, et al. Galactomannan in bronchoalveolar lavage fluid: a tool for diagnosing aspergillosis in intensive care unit patients. American Journal of Respiratory and Critical Care Medicine 2008;177(1):27‐34. [DOI] [PubMed] [Google Scholar]
Pazos 2005 {published data only}
- Pazos C, Ponton J, Palacio A. Contribution of (1 ‐> 3)‐beta‐D‐glucan chromogenic assay to diagnosis and therapeutic monitoring of invasive aspergillosis in neutropenic adult patients: a comparison with serial screening for circulating galactomannan. Journal of Clinical Microbiology 2005;43(1):299‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penack 2008 {published data only}
- Penack O, Rempf P, Graf B, Blau IW, Thiel E. Aspergillus galactomannan testing in patients with long‐term neutropenia: implications for clinical management. Annals of Oncology 2008;19(5):984‐9. [DOI] [PubMed] [Google Scholar]
Pereira 2005 {published data only}
- Pereira CN, Nero G, Lacaz CS, Machado CM. The contribution of galactomannan detection in the diagnosis of invasive aspergillosis in bone marrow transplant recipients. Mycopathologia 2005;159(4):487‐93. [DOI] [PubMed] [Google Scholar]
Perkins 2007 {published data only}
- Perkins JD. Use of Aspergillus galactomannan enzyme‐linked immunosorbent assay (ELISA) in liver transplant patients. Liver Transplantation 2007;13(2):304‐5. [PubMed] [Google Scholar]
Racil 2008 {published data only}
- Racil Z, Kocmanova I, Wagnerova B, Winterova J, Lengerova M, Moulis M, et al. The use of galactomannan detection in diagnosing invasive aspergillosis in hemato‐oncological patients. Vnitrni Lekarstvi 2008;54(1):45‐52. [PubMed] [Google Scholar]
Rogers 2013 {published data only}
- Rogers TR, Morton CO, Springer J, Conneally E, Heinz W, Kenny C, et al. Combined real‐time PCR and galactomannan surveillance improves diagnosis of invasive aspergillosis in high risk patients with haematological malignancies. British Journal of Haematology 2013;161(4):517‐24. [DOI] [PubMed] [Google Scholar]
Sarrafzadeh 2010 {published data only}
- Sarrafzadeh SA, Hoseinpoor Rafati A, Ardalan M, Mansouri D, Tabarsi P, Pourpak Z. The accuracy of serum galactomannan assay in diagnosing invasive pulmonary aspergillosis. Iranian Journal of Allergy, Asthma, and Immunology 2010;9(3):149‐55. [PubMed] [Google Scholar]
Steinbach 2007 {published data only}
- Steinbach WJ, Addison RM, McLaughlin L, Gerrald Q, Martin PL, Driscoll T, et al. Prospective Aspergillus galactomannan antigen testing in pediatric hematopoietic stem cell transplant recipients. Pediatric Infectious Disease Journal 2007;26(7):558‐64. [DOI] [PubMed] [Google Scholar]
Sulahian 1996 {published data only}
- Sulahian A, Tabouret M, Ribaud P, Sarfati J, Gluckman E, Latge JP, et al. Comparison of an enzyme immunoassay and latex agglutination test for detection of galactomannan in the diagnosis of invasive aspergillosis. European Journal of Clinical Microbiology and Infectious Diseases 1996;15(2):139‐45. [DOI] [PubMed] [Google Scholar]
Sulahian 2001 {published data only}
- Sulahian A, Boutboul F, Ribaud P, Leblanc T, Lacroix C, Derouin F. Value of antigen detection using an enzyme immunoassay in the diagnosis and prediction of invasive aspergillosis in two adult and pediatric hematology units during a 4‐year prospective study. Cancer 2001;91(2):311‐8. [DOI] [PubMed] [Google Scholar]
Tabone 1997 {published data only}
- Tabone MD, Vu‐Thien H, Latge JP, Landman‐Parker J, Perez‐Castiglioni P, Moissenet D, et al. Value of galactomannan detection by sandwich enzyme‐linked immunosorbent assay in the early diagnosis and follow‐up of invasive aspergillosis. Opportunistic Pathogens 1997;9(1):5‐15. [Google Scholar]
Uryu 2006 {published data only}
- Uryu H, Kozeki M, Asada N, Takeuchi M, Suenaga T. Analysis of serum Aspergillus galactomannan contents conducted at our hospital during the past 2 years. Japanese Journal of Antibiotics 2006;59(5):413‐4. [PubMed] [Google Scholar]
Verweij 1995 {published data only}
- Verweij PE, Latge JP, Rijs AJ, Melchers WJ, Pauw BE, Hoogkamp‐Korstanje JA, et al. Comparison of antigen detection and PCR assay using bronchoalveolar lavage fluid for diagnosing invasive pulmonary aspergillosis in patients receiving treatment for hematological malignancies. Journal of Clinical Microbiology 1995;33(12):3150‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang_Y 2013 {published data only}
- Wang Y Y, Xiao CL, Li JM, Zhao WL, Mi JQ, Hu J, Ni YX, Shen ZX. The retrospective study of serum aspergillus galactomannan (GM) antigen assay in invasive aspergillosis on hematological diseases. Chung‐Hua Hsueh Yeh Hsueh Tsa Chih [Chinese Journal of Hematology] 2013;34(6):498‐501. [DOI] [PubMed] [Google Scholar]
Xiang_J 2010 {published data only}
- Xiang J, Sun Z, Xu Z, Xia J, Huan J. Contribution of galactomannan antigen assay to early diagnosis of aspergillosis in patients with extensive burns [血清半乳甘露聚糖抗原动态检测对烧伤伴曲霉感染早期诊断的意义]. Chinese Journal of Infection and Chemotherapy 2010;10(4):304‐7. [Google Scholar]
Yao 2009 {published data only}
- Yao JF, Su D, Huang Y, Zhang P, Lin QS, Wang ZY, et al. A preliminary investigation on early diagnosis of invasive aspergillusis in patients with blood diseases by using circulating galactomannan test. Zhongguo Shi Yan.Xue Ye Xue Za Zhi 2009;17(3):765‐9. [PubMed] [Google Scholar]
Yu 2010 {published data only}
- Yu J, Li RY, Gao LJ, Lu QY, Wang XH. Utility of galactomannan enzyme immunoassay and (1,3)beta‐D‐glucan assay in invasive fungal infection. Zhonghua Yi Xue Za Zhi 2010;90(6):371‐4. [PubMed] [Google Scholar]
Zedek 2006 {published data only}
- Zedek DC, Miller MB. Use of galactomannan enzyme immunoassay for diagnosis of invasive aspergillosis in a tertiary‐care center over a 12‐month period. Journal of Clinical Microbiology 2006;44(4):1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zeng 2011 {published data only}
- Zeng SY, Liu T, Meng WT, Chen YN. The significance of serum GM and BG antigens assay for invasive fungal infections in hematological malignancies patients. Zhonghua Xue Ye Xue Za Zhi 2011;32(1):43‐6. [PubMed] [Google Scholar]
Additional references
Ascioglu 2001
- Ascioglu S, Pauw BE, Donnelly JP, Collette L. Reliability of clinical research on invasive fungal infections: a systematic review of the literature. Medical Mycology 2001;39(1):35‐40. [DOI] [PubMed] [Google Scholar]
Ascioglu 2002
- Ascioglu S, Rex JH, Pauw B, Bennett JE, Bille J, Crokaert F, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clinical Infectious Diseases 2002;34(1):7‐14. [DOI] [PubMed] [Google Scholar]
Borlenghi 2007
- Borlenghi E, Cattaneo C, Capucci MA, Pan A, Quaresmini G, Franco F, et al. Usefulness of the MSG/IFICG/EORTC diagnostic criteria of invasive pulmonary aspergillosis in the clinical management of patients with acute leukaemia developing pulmonary infiltrates. Annals of Hematology 2007;86(3):205‐10. [DOI] [PubMed] [Google Scholar]
Cruciani 2015
- Cruciani M, Mengoli C, Loeffler J, Donnelly P, Barnes R, Jones BL, et al. Polymerase chain reaction blood tests for the diagnosis of invasive aspergillosis in immunocompromised people. Cochrane Database of Systematic Reviews 2015, Issue 10. [DOI: 10.1002/14651858.CD009551.pub3] [DOI] [PubMed] [Google Scholar]
De Pauw 2008
- Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clinical infectious Diseases 2008;46(12):1813‐21. [DOI: 10.1086/588660; PUBMED: 18462102] [DOI] [PMC free article] [PubMed] [Google Scholar]
Denning 1998
- Denning DW, Marinus A, Cohen J, Spence D, Herbrecht R, Pagano L, et al. An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. EORTC Invasive Fungal Infections Cooperative Group. Journal of Infection 1998;37(2):173‐80. [DOI] [PubMed] [Google Scholar]
Hope 2005
- Hope WW, Walsh TJ, Denning DW. Laboratory diagnosis of invasive aspergillosis. Lancet Infectious Diseases 2005;5(2):609‐22. [DOI] [PubMed] [Google Scholar]
Juni 1999
- Juni P, Witschi A, Bloch R, Egger M. The hazards of scoring the quality of clinical trials for meta‐analysis. JAMA 1999;282(11):1054‐60. [DOI] [PubMed] [Google Scholar]
Kontoyiannis 2002
- Kontoyiannis DP, Bodey GP. Invasive aspergillosis in 2002: an update. European Journal of Clinical Microbiology and Infectious Diseases 2002;21(3):161‐72. [DOI] [PubMed] [Google Scholar]
Latgé 1994
- Latgé JP, Kobayashi H, Debeaupuis JP, Diaquin M, Sarfati J, Wieruszeski JM, et al. Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus. Infection and Immunity 1994;62(6):5424‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leeflang 2006
- Leeflang MM, Debets‐Ossenkopp YJ, Visser CE, Bossuyt PM. Meta‐analysis of diagnostic test accuracy. Clinical Infectious Diseases 2006;43(9):1220. [DOI] [PubMed] [Google Scholar]
Marr 2002
- Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clinical Infectious Diseases 2002;34(7):909‐17. [DOI] [PubMed] [Google Scholar]
Mennink‐Kersten 2004
- Mennink‐Kersten MASH, Donnelly JP, Verweij PE. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infectious Diseases 2004;4(6):349‐57. [DOI] [PubMed] [Google Scholar]
Mulherin 2002
- Mulherin SA, Miller WC. Spectrum bias or spectrum effect? Subgroup variation in diagnostic test evaluation. Annals of Internal Medicine 2002;137:598‐602. [DOI] [PubMed] [Google Scholar]
Pfeiffer 2006
- Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta‐analysis. Clinical Infectious Diseases 2006;42(10):1417‐27. [DOI] [PubMed] [Google Scholar]
Reitsma 2005
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. Journal of Clinical Epidemiology 2005;58(10):982‐90. [DOI] [PubMed] [Google Scholar]
Segal 2006
- Segal BH, Walsh TJ. Current approaches to diagnosis and treatment of invasive aspergillosis. American Journal of Respiratory and Critical Care Medicine 2006;173(7):707‐17. [DOI] [PubMed] [Google Scholar]
Singh 2005
- Singh N. Invasive aspergillosis in organ transplant recipients: new issues in epidemiologic characteristics, diagnosis, and management. Medical Mycology 2005;43 Suppl 1:S267‐70. [DOI] [PubMed] [Google Scholar]
Subira 2003
- Subira M, Martino R, Rovira M, Vazquez L, Serrano D, Camara R. Clinical applicability of the new EORTC/MSG classification for invasive pulmonary aspergillosis in patients with hematological malignancies and autopsy‐confirmed invasive aspergillosis. Annals of Hematology 2003;82(2):80‐2. [DOI] [PubMed] [Google Scholar]
Upton 2007
- Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clinical Infectious Diseases 2007;44(4):531‐40. [DOI] [PubMed] [Google Scholar]
Verdaguer 2007
- Verdaguer V, Walsh TJ, Hope W, Cortez KJ. Galactomannan antigen detection in the diagnosis of invasive aspergillosis. Expert Review of Molecular Diagnostics 2007;7(1):21‐32. [DOI] [PubMed] [Google Scholar]
Whiting 2003
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Medical Research Methodology 2003;3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2005
- Whiting P, Harbord R, Kleijnen J. No role for quality scores in systematic reviews of diagnostic accuracy studies. BMC Medical Research Methodology 2005;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiting 2011
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS‐2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of Internal Medicine 2011;155(8):529‐36. [PUBMED: 22007046] [DOI] [PubMed] [Google Scholar]


