Abstract
Introduction:
NRG protocols for glioblastoma allow for clinical target volume (CTV) reductions at natural barriers; however, literature examining CTV contouring and the relevant white matter pathways is lacking. This study proposes consensus CTV guidelines, with a focus on areas of controversy while highlighting common errors in glioblastoma target delineation.
Methods:
Ten academic radiation oncologists specializing in brain tumor treatment contoured CTVs on 4 glioblastoma cases. CTV expansions were based on NRG trial guidelines. Contour consensus was assessed and summarized by kappa statistics. A meeting was held to discuss the mathematically averaged contours and form consensus contours and recommendations.
Results:
Contours of the cavity plus enhancement (mean kappa 0.69) and T2-FLAIR signal (mean kappa 0.74) showed moderate to substantial agreement. Experts were asked to trim off anatomic barriers while respecting pathways of spread to develop their CTVs. Submitted CTV_4600 (mean kappa 0.80) and CTV_6000 (mean kappa 0.81) contours showed substantial to near perfect agreement. Simultaneous Truth and Performance Level Estimation (STAPLE) contours were then reviewed and modified by group consensus. Anatomic trimming reduced the amount of total brain tissue planned for radiation targeting by a 13.6% (range 8.7% - 17.9%) mean proportional reduction. Areas for close scrutiny of target delineation were described, with accompanying recommendations.
Conclusions:
Consensus contouring guidelines were established based on expert contours. Careful delineation of anatomic pathways and barriers to spread can spare radiation to uninvolved tissue without compromising target coverage. Further study is necessary to accurately define optimal target volumes beyond isometric expansion techniques for individual patients.
Keywords: Glioblastoma, clinical target volume, contouring, consensus
Introduction
Glioblastoma is the most common primary brain malignancy in the adult population. Although little variance exists regarding the recommendation of adjuvant chemoradiotherapy [1], there is significant heterogeneity in radiotherapy target delineation. Legacy trials utilized whole brain radiotherapy, based on the surgical observation that even a non-dominant hemispherectomy did not change the natural history of the disease, with recurrences occurring in the contralateral hemisphere, presumably through tumor cell migration across the corpus callosum. Current European practice is a single clinical target volume (CTV) based on the postoperative cavity and surrounding enhancement, with a volumetric 2 cm CTV expansion treated to 60 Gy, with consideration of the expansion to cover the T2 or FLAIR abnormality[2]. Recent Radiation Therapy Oncology Group (RTOG)/NRG volume recommendations have remain relatively unchanged over the past two decades, and are based upon autopsy series[3] as well as studies examining MRI-guided stereotactic biopsies[4] demonstrating that tumor cells can be found to the edge of the T2 hyperintensity and beyond. Therefore the initial phase (46 Gy) consists of including the T2 or FLAIR abnormalities on the post-operative MRI scan, plus a CTV margin of 2 cm, which may be reduced around natural barriers to tumor growth such as the skull, ventricles, falx, etc[5], with a subsequent boost to 60 Gy.
RTOG/NRG protocols are commonly referenced by practicing physicians as well as trainees in radiation oncology to guide their practice. While current RTOG/NRG protocols define the anatomic borders of organs at risk such as the brainstem, they do not address complex issues such as normal tissue exclusion near the brainstem or at the midline. Errors resulting in excess coverage of not-at-risk normal tissue, or inappropriate sparing of at-risk tissue, are commonly seen in contours submitted for central review on clinical trials. Furthermore, outside of mentor-mentee training guidance within individual institutions, no common reference exists to uniformly guide the crafting of glioblastoma CTVs; as a result significant variability exists amongst institutions regarding glioblastoma contours [6]. In the era of image-guided treatments, attempts at radiation dose escalation, and movement to inclusion of particle therapy in clinical practice, uniform delineation of CTVs is of paramount interest. Within this framework, a group of experts in central nervous system radiation oncology was formed to create a consensus document of glioblastoma contouring to provide practical guidance for routine practice.
Methods and Materials
A group of radiation oncologists specializing in CNS diseases was recruited during an NRG brain tumor meeting. Two radiation oncologists (TJK and KPM) identified cases submitted for central review on RTOG 0837 that provided a representative sample of complex target/normal tissue anatomy for contouring by the expert panel. The investigators were provided image sets of the selected cases with fused pre- and post-operative axial T1 post-contrast and axial T2-FLAIR MRI sequences co-registered to the planning CT image set for contouring. Each physician participant was then instructed to define CTVs using currently established NRG standard protocol guidelines of a customized 2 centimeter CTV margins for the initial and boost phases of treatment[5].
The investigators were asked to, as accurately as possible: (1) contour the initial and boost gross target volumes (2) expand the gross target volumes to an initial volume by 2 cm expansion of corresponding GTV for each phase of treatment (3) customize the CTV to exclude expansions into regions deemed not at-risk due to natural boundaries of tumor spread. No further instruction on the natural boundaries of spread was given, in line with the language in current RTOG protocols.
In order to have uniform names of the volumes, contours were crafted via the following protocol:
Contour “GTV2” (cavity + enhancement)
Contour “GTV1” (T2/FLAIR) ensuring that it encompassed all of GTV2 as well
Expand GTV1 to be “CTV1” as defined by (FLAIR) + 2 cm, limited to interior of brain tissue. “CTV1” is not a treatment volume.
Copy CTV1 to create a new “CTV_4600”
Manually trim CTV_4600 based on anatomical barriers, falx, ventricles, etc. This is a treatment volume.
Expand GTV2 by 2 cm to create “CTV2”, avoiding the exterior of newly defined CTV_4600. “CTV2” is not a treatment volume.
Copy CTV2 to create a new “CTV_6000”
Manually trim CTV_6000 based on anatomical barriers, falx, ventricles, etc. This is a treatment volume. (It is common that no further trimming of CTV_6000 is necessary as it has already been restricted to the anatomically limited CTV_4600)
Contours were submitted to the lead physicist (WRB), linked to the underlying CT scan via DICOM export to the IROC-St. Louis QA Center. Contours from each investigator were then compared for agreement using the open-source Computerized Environment for Radiation Research (CERR) software[7] implemented in MATLAB (MathWorks, Natick, MA). The group’s contours were then assessed using an expectation maximization algorithm called Simultaneous Truth and Performance Level Estimation (STAPLE) [8]. Kappa statistics with values between + 1 (perfect agreement) and −1 (complete disagreement) were generated for each of the CTV components. Kappa values between 0.41 and 0.60 correspond to a moderate level agreement, between 0.61 and 0.80 as substantial, and between 0.81 and 1.0 as almost perfect [9]. The investigators met to review the submitted and STAPLE contours. Areas of discrepant practice were identified, and discussion was held to form consensus recommendations for CTV delineation at these challenging areas. These recommendations were then applied to further modify the mathematically-generated STAPLE contours to result in consensus CTV contours more respective of anatomic considerations.
Results
CTV Variability
Ten physicians were asked to participate and returned contour sets; upon review one dataset was excluded for failure to comply with the instructions. The variability of the contours is quantitatively reported for all 4 cases in Table 1. Contours of the GTV2 (cavity plus enhancement; mean kappa 0.69) and GTV1 (T2-FLAIR signal; mean kappa 0.74) showed moderate to substantial agreement. Submitted CTV_4600 (mean kappa 0.80) and CTV_6000 (mean kappa 0.81) contours showed substantial to near perfect agreement. Case 1 (Figure 1) was a right temporal tumor with a STAPLE CTV1 of 487.1 cc (Fig. 1 h, green line); anatomic trimming and application of recommendations resulted in a STAPLE CTV_4600 of 441 cc (Fig. 1 h, blue line). Further modification of the STAPLE contours led to a consensus CTV_4600 of 432.8 cc (Fig. 1 h, orange line), representing an 11.1% volumetric reduction from the untrimmed STAPLE CTV1.
Table 1.
STAPLE estimates on interobserver agreement sensitivity, agreement specificity and kappa measures for each clinical case scenario
| Case 1 Right temporal |
Structure Measure |
GTV1 | GTV2 | CTV_4600 | CTV_6000 |
| Sensitivity | 88.8% | 84.2% | 92.4% | 92.1% | |
| Specificity | 97.4% | 97.0% | 98.5% | 97.1% | |
| Volume Min | 127.9 | 34.28 | 382.9 | 179.2 | |
| Volume Max | 285.8 | 67.30 | 874.8 | 226.4 | |
| Volume Mean | 173.3 | 47.10 | 471.0 | 205.0 | |
| Volume SD | 45.6 | 10.81 | 153.4 | 15.9 | |
| STAPLE Volume | 166.7 | 48.43 | 441.0 | 204.2 | |
| Kappa | 0.78 | 0.74 | 0.81 | 0.84 | |
| Case 2 Left frontal |
Structure Measure |
GTV1 | GTV2 | CTV_4600 | CTV_6000 |
| Sensitivity | 85.5% | 78.9% | 87.4% | 88.2% | |
| Specificity | 95.3% | 96.9% | 95.2% | 97.1% | |
| Volume Min | 72.1 | 25.2 | 324.8 | 202.1 | |
| Volume Max | 267.9 | 68.0 | 788.7 | 336.7 | |
| Volume Mean | 114.5 | 41.3 | 446.7 | 264.5 | |
| Volume SD | 62.6 | 13.3 | 144.4 | 46.2 | |
| STAPLE Volume | 95.2 | 42.1 | 418.8 | 270.1 | |
| Kappa | 0.63 | 0.66 | 0.72 | 0.79 | |
| Case 3 Right parietal |
Structure Measure |
GTV1 | GTV2 | CTV_4600 | CTV_6000 |
| Sensitivity | 88.3% | 70.9% | 91.7% | 85.4% | |
| Specificity | 98.0% | 97.6% | 96.3% | 96.2% | |
| Volume Min | 159.7 | 14.51 | 448.4 | 196.9 | |
| Volume Max | 233.2 | 42.21 | 654.5 | 312.8 | |
| Volume Mean | 189.7 | 29.72 | 538.6 | 245.7 | |
| Volume SD | 22.6 | 9.98 | 66.3 | 41.7 | |
| STAPLE Volume | 196.8 | 32.17 | 527.7 | 253.7 | |
| Kappa | 0.81 | 0.59 | 0.82 | 0.74 | |
| Case 4 L temporal |
Structure Measure |
GTV1 | GTV2 | CTV_4600 | CTV_6000 |
| Sensitivity | 86.2% | 87.5% | 94.7% | 93.6% | |
| Specificity | 96.4% | 97.5% | 95.8% | 96.7% | |
| Volume Min | 68.4 | 28.94 | 272.0 | 189.4 | |
| Volume Max | 122.7 | 46.16 | 372.3 | 218.2 | |
| Volume Mean | 81.5 | 38.70 | 299.4 | 204.9 | |
| Volume SD | 16.8 | 5.68 | 32.0 | 11.2 | |
| STAPLE Volume | 77.8 | 38.69 | 286.7 | 202.0 | |
| Kappa | 0.74 | 0.78 | 0.84 | 0.85 | |
Fig. 1.

Right temporal glioblastoma. (A, B) panels show the contrast-enhanced T1 MRI with GTV2 contours (cavity plus enhancement). (C) CTV_6000 contours demonstrate variability at the interface with the brainstem and optic structures. (D) the STAPLE GTV2 cavity contour in pink and the consensus CTV_6000 contour. (E, F) show T2 FLAIR MRI images with submitted GTV1 (FLAIR) contours and CTV_4600 contours. (G) CTV_4600 contours are demonstrated at level of brainstem and optic nerves with significant variation. (H) CTV1 expansion (without anatomic trimming) in green and mathematical STAPLE contours in blue. The space between the green and orange consensus CTV_4600 contour reflects anatomic trimming off the cerebellum (white arrow) due to cerebellar falx, while maintaining inclusion of optic and brainstem tissue in direct anatomic contiguity with the right temporal T2 FLAIR signal.
Case 2 (Figure 2) was a left frontal tumor with a STAPLE CTV1 of 444.5 cc (Fig. 2 g,h green line); anatomic trimming and application of recommendations resulted in a STAPLE CTV_4600 of 418.8 cc (not shown). Further modification of the STAPLE contours led to a consensus CTV_4600 of 405.9 cc (Fig. 2 g,h, orange line), representing an 8.7% volumetric reduction from the untrimmed STAPLE CTV1.
Fig. 2.
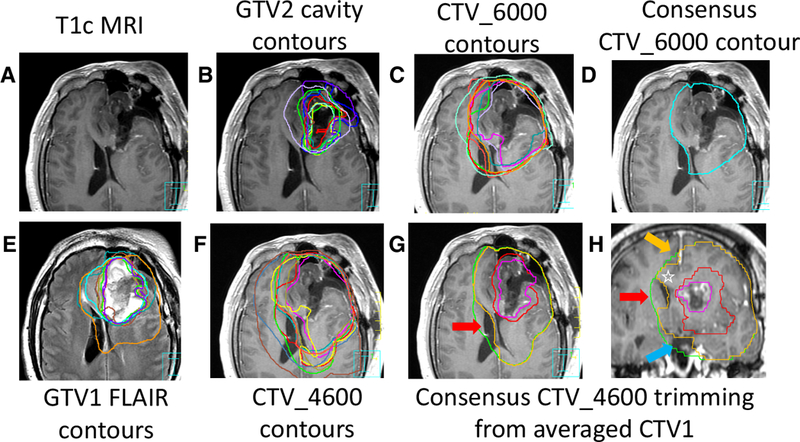
Left frontal glioblastoma. (A, B) panels show the contrast-enhanced T1 MRI with GTV2 contours (cavity plus enhancement). (C) CTV_6000 contours demonstrate variability at the interface with the ventricles. (D) the consensus CTV_6000 contour (E) shows T2 FLAIR MRI images with submitted GTV1 (FLAIR) contours (F) displays the CTV_4600 contours with significant variation at ventricle and corpus callosum. (G, H) shows average GTV2 (pink=cavity plus enhancement) and GTV1 (red=FLAIR) volumes in axial and coronal T1c scans. CTV1 expansion (without anatomic trimming) in green. The space between the green and orange consensus CTV_4600 contour reflecting anatomic trimming at the ventricular interface (red arrows), the interhemispheric falx (orange arrow) and pre-pontine cistern/pituitary fossa region (blue arrow). Note in panel (H) inclusion within the orange consensus CTV_4600 contour of the corpus callosum (white star) and the optic chiasm (superior to the blue arrow) given their anatomic contiguity with the red GTV1 (FLAIR) regions.
Case 3 (Figure 3) was a right parietal tumor with a STAPLE CTV1 of 637.5 cc (Fig. 3 g,h green line); anatomic trimming and application of recommendations resulted in a STAPLE CTV_46 of 527.7 cc (not shown). Further modification of the STAPLE contours led to a consensus CTV_4600 of 523.7 cc (Fig. 3 g,h, orange line), representing an 17.9% volumetric reduction from the untrimmed STAPLE CTV1.
Fig. 3.
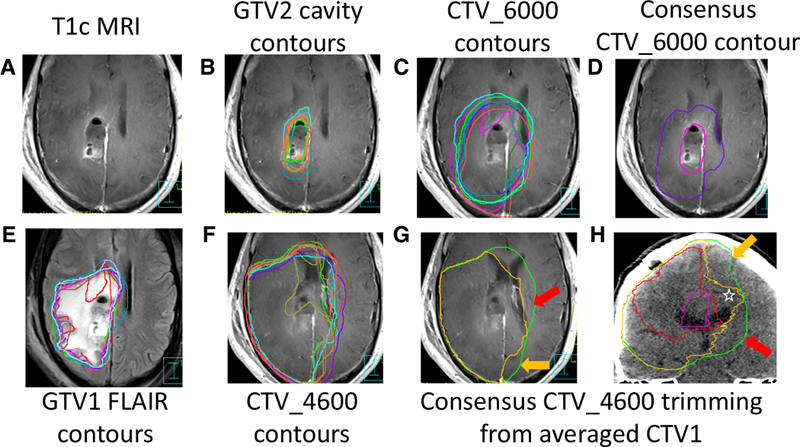
Right parietal glioblastoma. (A, B) panels show the contrast-enhanced T1 MRI with GTV2 contours (cavity plus enhancement). (C) CTV_6000 contours demonstrate variability at the at ventricle and corpus callosum (D) the STAPLE GTV2 cavity contour in pink and the consensus CTV_6000 contour (E) shows T2 FLAIR MRI images with submitted GTV1 (FLAIR) contours (F) displays the CTV_4600 contours with significant variation at ventricle and corpus callosum. (G, H) shows average GTV2 (pink=cavity plus enhancement) and GTV1 (red=FLAIR) volumes in axial and coronal T1c scans. CTV1 expansion (without anatomic trimming) in green. The space between the green and orange consensus CTV_4600 contour reflecting anatomic trimming at the ventricular interface (red arrows), the interhemispheric falx (orange arrow) and pre-pontine cistern/pituitary fossa region (blue arrow). Note in panel (H) inclusion within the orange consensus CTV_4600 contour of the corpus callosum overlying the right lateral ventricle (white star).
Case 4 (Figure 4) was a left temporal tumor with a STAPLE CTV1 of 358.0 cc (Fig. 4 d,g,h green line); anatomic trimming and application of recommendations resulted in a STAPLE CTV_4600 of 286.7 cc (Fig. 4d, blue line). Further modification of the STAPLE contours led to a consensus CTV_4600 of 297.5 cc (Fig 4 g,h, orange line), representing an 16.9% volumetric reduction from the untrimmed STAPLE CTV1. Therefore, anatomical trimming reduced the amount of total brain tissue planned for radiation targeting by a mean of 66.8 cc (range 38.6–113.9 cc) which represented a 13. 6% mean proportional reduction of the planned volume (range 8.7% - 17.9%).
Fig. 4.

Left temporal glioblastoma. (A) T1c MRI with submitted GTV2 (cavity plus enhancement) contours. (B) shows CTV_6000 contour variation at the interface of the brainstem and temporal lobe while (C) shows the averaged GTV2 volume (pink) with consensus CTV_6000 contour (purple) extending into contiguous brainstem. (D) T2-FLAIR MRI with GTV1 (FLAIR) contours. (E) CTV_4600 contours with significant variation at the interface of the brainstem and temporal lobe. The averaged GTV1 (FLAIR) contour is the smallest volume in red. (F) shows averaged GTV2 (pink=cavity plus enhancement) and GTV1 (red=FLAIR) volumes in axial T1c scans. The blue contour was the STAPLE CTV_4600 [averaged from contours in (E)], whereas the orange is the consensus CTV_4600, altered from the STAPLE CTV_4600 to include the contiguous brainstem (hash) but trimmed from the averaged CTV1 contour at the pre-pontine and quadrigeminal cisterns (asterisks). (G, H) shows average GTV2 (pink=cavity plus enhancement) and GTV1 (red=FLAIR) volumes (not present at midline on sagittal plane). CTV1 expansion (without anatomic trimming) in green. The space between the green and orange consensus CTV_4600 contour reflects anatomic trimming off the cerebellum (white arrow) due to cerebellar falx. Note the inclusion of brainstem in direct anatomic contiguity via the cerebral peduncle.
The contouring physicians then met to review the submitted contours and discuss areas of discrepancy. Areas of discrepant contouring practice were noted primarily at regions where clinical judgment varied between the practitioners, with observations of the contouring variation at the optic chiasm, brainstem, and interface with falxes prompting description from individual physicians as to their handling of these regions with respect to CTV delineation. These practices were then collated and distributed to the physician collaborative as a survey. The results were redistributed to the physicians and consensus was voiced to adopt the results as guideline recommendations (detailed below).
Furthermore during group review, discussion regarding common pitfalls in glioblastoma contouring were described. Recommendations to trainees included paying close attention to imaging registration between MRI and CT in the region of disease, as well as utilization of coronal and sagittal reconstructions to craft and review CTVs. Anatomic-specific areas of CTV contouring mistakes routinely noted are summarized in Table 2, and depicted where apparent in Figures 1–4. Of note, with acknowledgement that many institutions have adopted a simultaneous integrated boost approach utilizing 50 Gy and 60 Gy simultaneously delivered over 30 fractions [10] the group endorsed extension of the below principles to be applied to that schema. For consistency (with the submitted contour results) the below recommendations are written in terms of the sequential boost framework, and summarized in Table 3.
Table 2.
Common pitfalls in appropriate contouring of glioblastoma target volumes
| Issue | Appropriate contour example |
|---|---|
| Failure to trim the infratentorial component of post-expansion CTV for supratentorial (temporal, occipital) tumors. | Fig. 1h, and Fig 4h, white arrows |
| Failure to trim the contralateral hemisphere out of the post-expansion CTV, giving respect to the barrier that the interhemispheric falx represents to tumor spread. | Fig. 2g-h, and Fig. 3h, orange arrows show appropriate trimming |
| Over-aggressive trimming of the post-expansion CTV at the midline at the level of the corpus callosum—the interhemispheric falx does not continue caudally all the way to the top of the lateral ventricles, the corpus callosum drapes over the ventricles, best appreciated in coronal views. | Fig. 2h, and Fig. 3h, star bounded by orange contours |
| Over-aggressive trimming of the post-expansion CTV from the brainstem. While the ambient cistern separates the midbrain laterally from the temporal lobe, tumors or FLAIR signal that involve the thalamus have the potential for inferior spread down the cerebral peduncle into the mid-brain. Review volumes in the coronal plane to ensure adequate margin in this dimension | Figure 1h and Figure 4d. The difference between the blue averaged STAPLE CTV_4600 and orange consensus contours [(#) in Fig. 4d] show area to be properly included. Fig. 4G depicts appropriate brainstem inclusion in coronal plane. |
| Lack of awareness of anterior and posterior commissures and interthalamic adhesion and their potential role for contralateral tumor spread in the vicinity of the third ventricle and thalamus | Figure 5c, red arrow |
Table 3.
Guidelines for CTV delineation and planning goals in glioblastoma
| Contour | Definition/expansion | Brainstem | Optic chiasm/nerve |
|---|---|---|---|
| GTV1 | T2/FLAIR hyperintensity | Include to the extent of the MRI abnormality involvement | |
| GTV2 | Cavity plus T1c-enhancement | ||
| CTV_4600* | 2 cm beyond both GTV1 and GTV2, with anatomical trimming | •within 2 cm of T2-FLAIR should be included if in anatomic contiguity | •within 2cm of T2-FLAIR should be included if in anatomic contiguity |
| CTV_6000 | 2 cm beyond GTV2, with anatomical trimming | •within 2 cm of T1-contrast should be included if in anatomic contiguity; cover to ~54 Gy to keep brainstem PRV DMax <55 Gy •gross disease without margin should = CTV_6000. Recommend to prescribe 2 cm of surrounding brainstem as CTV54. Respect RTOG “variation acceptable” brainstem core/surface constraints (60, 64 Gy respectively) |
•within 2cm of T1-contrast should be included if in anatomic contiguity; cover to ~54 Gy, to keep PRV <55 Gy •gross disease abutting optic tissue should be treated to ~60 Gy, allowing Dmax on chiasm PRV up to 60 Gy |
These principles may also be applied to a simultaneous integrated boost approach of 50 Gy and 60 Gy all given in 30 fractions
Optic chiasm/nerves
In the perichiasmatic region, there was consensus that the pituitary gland could be routinely excluded from the CTV_4600. However significant variability was noted regarding inclusion of the optic chiasm in the CTV_4600. Upon discussion, survey, and review of survey results the following guidelines were adopted by consensus:
Optic chiasm/nerve within 2cm of T2-FLAIR signal abnormality should be included in the CTV_4600 if in anatomic contiguity with the FLAIR abnormality.
Optic chiasm/nerve within 2cm of T1-contrast signal abnormality should be included in the CTV_6000 if in anatomic contiguity with T1-contrast signal; cover this region to a dose of approximately 54 Gy, to keep PRV <55 Gy per current RTOG protocols given it may represent subclinical disease. This constraint should be prioritized over the 95% PTV coverage goal.
Optic chiasm/nerve abutting gross disease should be included in the CTV_6000; cover this region while allowing Dmax on PRV to ≤60 Gy. This constraint should be prioritized over the 95% PTV coverage goal.
Brainstem
Significant variability was noted regarding handling of the brainstem interface with the thalamus for tumors that demonstrated edema within <2 cm of this interface (see Fig. 1g). STAPLE contours (see Fig. 1b, red arrow). Utilization of coronal sequences (see Fig. 4g) was recommended to assist delineation of this complex target region, respecting the white matter tracts oriented in the craniocaudal dimension (as shown in Fig. 5a, white arrows). Upon discussion, survey, and review of survey results the following guidelines were adopted by consensus:
Brainstem within 2 cm of T2-FLAIR signal abnormality should be included in the CTV_4600 if in anatomic contiguity
Brainstem within 2 cm of T1-contrast signal abnormality via the cerebral peduncle/thalamus should be included in the CTV_6000; cover to ~54 Gy to keep brainstem PRV DMax <55 Gy given it may represent subclinical disease. This constraint should be prioritized over the 95% PTV coverage goal.
Brainstem gross disease without margin should be included in the CTV_6000. It is recommended to contour 2 cm of surrounding brainstem as a CTV_5400, and treat while respecting NRG “variation acceptable” brainstem core/surface constraints (60, 64 Gy respectively). These constraints should be prioritized over the 95% PTV coverage goal.
Fig. 5.
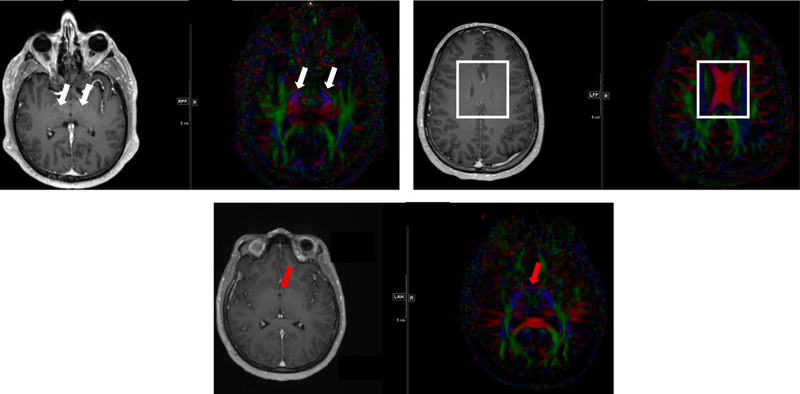
Representative MRIs and diffusion tensor imaging (DTI) showing white matter tracts for consideration in glioblastoma. Red indicates left-right pathways, blue indicates head-foot directionality, and green highlights anterior-posterior. In (A) the white arrows highlight blue ascending/descending white matter tracts at the level of the thalamus. These tracts are often not respected caudally, with inappropriate sparing of at-risk tissue below the level of the tentorial incisure. (B) The body of the corpus callosum is highlighted as indicated by the red pathways crossing midline. The falx cerebri is often inaccurately assumed to extend caudally to the top of the lateral ventricles. (C) Red arrows highlight the anterior commissure, with the DTI image demonstrating fibers in red that represent a white matter tract crossing midline. Similar fibers exist in the posterior commissure and variably in the interthalamic adhesion.
Falx
Significant variability was noted regarding handling the falx (see Fig. 3f posteriorly) Previous RTOG recommendations have stated that the CTV expansions may be reduced to 5 mm beyond the falx in the contralateral hemisphere. Upon discussion, survey, and review of survey results, the following guideline was adopted by consensus:
CTV expansions that cross the dura mater folds should be trimmed directly to the falx (not 5 mm); assume the falx is a hard barrier to tumor spread, as long as there is no FLAIR or T2 abnormality crossing the corpus callosum.
Discussion
Radiotherapy plays a central role in the management of glioblastoma, but significant controversy surrounds target delineation recommendations. In spite of the well-known propensity for disease spread up to several centimeters along white matter tracts, many investigators advocate for smaller margins than the expansions advocated by RTOG guidelines, attempting to minimize radiotherapy toxicity in the CNS in terms of neurocognitive, vascular, and endocrine impact[11–16]. Furthermore, significant publications have outlined organs at risk and have suggested dose-volume limitations to minimize normal tissue risk [17], including the brainstem [18], visual apparatus [19], cochlea[20], hippocampi, lenses, lacrimal glands, etc. While consensus efforts have described the elective at-risk areas in other anatomically complex sites such as the uninvolved neck in head-and-neck cancer [21], the relevant anatomy with relation to glioblastoma contouring is underreported in the literature. This effort demonstrates that through careful anatomic trimming a significant proportion of brain can be spared from high-dose radiation delivery.
This consensus effort furthermore highlighted discrepant areas of contour delineation between academic physicians who have a clinical focus in CNS tumors. Upon close review of individually submitted contours, discussions regarding contouring practices revealed a lack of uniform interpretation of current CTV delineation instructions at the optic chiasm/nerve, brainstem interface, and falx (cerebelli and cerebri). Through discussion, survey, and consensus endorsement of subsequent majority recommendations we have formulated guideline recommendations for CTV delineation at these areas across a number of commonly encountered clinical scenarios. These guidelines will help reduce clinical uncertainty that physicians face, and may provide more uniform CTV delineation for upcoming clinical trials.
An important limitation is that this study did not attempt to answer the question of how large of an isometric expansion should be utilized, an active area of debate. A previous South Korean examination of target delineation in glioblastoma demonstrated “substantial” agreement of submitted CTVs (mean kappa of 0.65) across 15 institutions [6], whereas our submitted CTVs showed “substantial to near perfect” agreement (mean kappas of ≥0.80), likely reflecting our usage of uniform 2 cm expansions before anatomic trimming, negating one source of disagreement. Nonetheless in off-protocol treatment this question of CTV expansions remains pertinent. Investigators from Emory[12] and Wake Forest[14] have suggested that glioblastoma CTVs may be reduced safely without compromising clinical outcomes. Furthermore Buglione et al demonstrated that larger volumes of the brain receiving 57 Gy were associated with higher rates of asthenia and leuko-encephalopathy, bolstering the rationale for smaller isometric expansions [11]. Additionally, a prospective randomized trial conducted in European and U.S. centers examining temozolomide dosing did not reveal outcome differences between those patients treated with RTOG volumes as compared to smaller EORTC volumes, further suggesting that more limited expansions may not negatively impact patient outcomes [22]. To the best of our knowledge, there is only one prospective randomized trial, as yet unpublished in manuscript form, that has asked this question and suggested similar recurrence patterns regardless of planning margin [23].
While these examples demonstrate the debate over the absolute magnitude of an isometric expansion in glioblastoma target delineation, our work highlights the importance of understanding the relevant neuroanatomy, with respect to white matter tracts and anatomic barriers to tumor spread. We demonstrated that significant volumes of brain tissue can be spared (mean of 66.8 cc; a mean proportional reduction of the target volume of 13.6%) with attention to anatomic barriers to spread such as the tentorium cerebelli, falx cerebri, and lateral ventricles. Furthermore, we have identified common areas of errant target delineation based on our combined experience. These errors are felt to be in part due to over-reliance on axial slices alone for glioblastoma CTV delineation, and utilization of coronal and sagittal slices was deemed essential for prudent target delineation.
While a “contouring atlas” was not felt to be feasible or generalizable given the vast differences between individual tumors in terms of involved anatomic regions, this work highlights areas of relevant neuroanatomy that warrant attention during isometric target expansion. The utilization of isometric expansions as a starting point is an inherent limitation of this work, as different tumor biology likely renders any rote isometric expansion beyond conventional MRI signals inaccurate for precise target risk delineation. While not yet widely utilized, novel PET tracers such as [11C] methionine [24] and FET-PET [25] hold the potential to more accurately delineate anatomic regions at high risk for harboring residual glioblastoma after resection. In fact, in a recent small Phase IIb multicenter trial from Japan[26], the synthetic amino acid, Fluciclovine, labeled with F-18 (known in the US as Axumin and used for imaging prostate cancer) was compared with MR imaging in 40 patients with clinically suspected high or low-grade gliomas undergoing resection. Multiple targeted biopsies were obtained during resection, using a neuro-navigation approach. The positive predictive value of Axumin in T1 contrast negative, but F-18 Fluciclovine positive regions was 26/26 (100%); in general, these T1 non-enhancing regions were either in the T2 abnormality or “normal” cortex, in the resection path. If validated, such an approach could potentially be utilized to determine which component of the T2/FLAIR abnormality should be considerd in future contouring recommendations. These methods, and other advanced radiologic techniques, warrant continued investigation and prospective validation as adjuncts for radiotherapy target delineations.
In conclusion, implementation of contouring guidelines for the unique infiltrative nature of malignant glioblastoma in the daily practice of radiation oncology is critical in the milieu of decreasing target volume expansions, smaller PTV due to daily image guidance and particle therapy; all of which carry the risk of inadequate target dosing. Attention to these consensus guidelines and knowledge of common pitfalls holds the potential to improve tumor control, minimize unneeded radiotherapy toxicity to tissue not at-risk, and facilitate the success of multi-institutional trials for infiltrative malignant glioblastoma. We welcome further radiologic advances to assist in more informed CTV delineation in this setting.
Acknowledgments
Funding: WRB’s work on this project was supported by a grant from National Institutes of Health grant U24 CA180803,”Imaging and Radiation Oncology (IROC) Group”, David Followill, PI.
Footnotes
Compliance with ethical standards:
Conflict of interest:
WRB’s reports travel expenses from AAPM, and honoraria from Augmenix Inc. These do not pertain to this work and WRB declares he has no conflict of interest with regard to this study.
TJK is on a speaker’s bureau for AstraZeneca, has served as a consultant to Varian Medical Systems and was on an advisory board for Abbvie Inc. These do not pertain to this work and TJK declares he has no conflict of interest with regard to this study.
CT is on a speaker’s bureau for Merck and Varian Medical Systems, advisory board for Novocure. These do not pertain to this work and CT declares she has no conflict of interest with regard to this study.
TJCW reports travel expenses from Abbvie, AstraZeneca, and Elekta, serves as a consultant for Abbvie, Merck, Doximity, and Elekta, is on advisory boards for American Cancer Society of New Jersey and AstraZeneca, and Honoria from Elekta and Wolthers Kluwer, and stock options from Doximity. These do not pertain to this work and TJCW declares he has no conflict of interest with regard to this study.
MPM has served as a consultant to Agenus, Insys, Remedy, IBA, Varian, Oncoceutics, Astra-Zeneca, Celgene, Tocagen, and is on the DSMB of Monteris, and the Board of Oncoceutics. These do not pertain to this work and MPM declares he has no conflict of interest with regard to this study.
KPM has served as a consultant for Via Oncology Pathways; this does not pertain to this work and KPM declares he has no conflict of interest with regard to this study.
MMK reports a research grant from EpicentRX, which does not pertain to this work and MMK declares she has no conflict of interest with regard to this study.
AAS has participated in an advisory committee, received travel expenses, received honoraria, and received research funding from Blue Earth Diagnostics, as well as travel expenses and honoraria from DAVA Oncology; this does not pertain to this work and AAS declares he has no conflict of interest with regard to this study.
SNB, JAB, AJG, SS do not have relevant financial relationships to disclose and declare they have no conflicts of interest with regard to this study.
Ethical approval: This article does not contain any studies with human participants performed by any of the authors
REFERENCES
- 1.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO, European Organisation for R, Treatment of Cancer Brain T, Radiotherapy G, National Cancer Institute of Canada Clinical Trials G (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352 (10):987–996. doi: 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 2.Niyazi M, Brada M, Chalmers AJ, Combs SE, Erridge SC, Fiorentino A, Grosu AL, Lagerwaard FJ, Minniti G, Mirimanoff RO, Ricardi U, Short SC, Weber DC, Belka C (2016) ESTRO-ACROP guideline “target delineation of glioblastomas”. Radiother Oncol 118 (1):35–42. doi: 10.1016/j.radonc.2015.12.003 [DOI] [PubMed] [Google Scholar]
- 3.Burger PC, Dubois PJ, Schold SC Jr., Smith KR Jr., Odom GL, Crafts DC, Giangaspero F (1983) Computerized tomographic and pathologic studies of the untreated, quiescent, and recurrent glioblastoma multiforme. J Neurosurg 58 (2):159–169. doi: 10.3171/jns.1983.58.2.0159 [DOI] [PubMed] [Google Scholar]
- 4.Earnest Ft, Kelly PJ, Scheithauer BW, Kall BA, Cascino TL, Ehman RL, Forbes GS, Axley PL (1988) Cerebral astrocytomas: histopathologic correlation of MR and CT contrast enhancement with stereotactic biopsy. Radiology 166 (3):823–827. doi: 10.1148/radiology.166.3.2829270 [DOI] [PubMed] [Google Scholar]
- 5.NRG-BN001 Randomized phase II tiral of hypofractionated dose-escalated photon IMRT or proton beam therapy versus conventional photon irradiation with concomitant and adjuvant temozolomide in patient with newly diagnosed glioblastoma. https://wwwnrgoncologyorg/Clinical-Trials/Protocol-Table
- 6.Wee CW, Sung W, Kang HC, Cho KH, Han TJ, Jeong BK, Jeong JU, Kim H, Kim IA, Kim JH, Kim SH, Kim S, Lee DS, Lee MY, Lim DH, Park HL, Suh CO, Yoon SM, Kim IH (2015) Evaluation of variability in target volume delineation for newly diagnosed glioblastoma: a multi-institutional study from the Korean Radiation Oncology Group. Radiat Oncol 10:137. doi: 10.1186/s13014-015-0439-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allozi R, Li XA, White J, Apte A, Tai A, Michalski JM, Bosch WR, El Naqa I (2010) Tools for consensus analysis of experts’ contours for radiotherapy structure definitions. Radiother Oncol 97 (3):572–578. doi: 10.1016/j.radonc.2010.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warfield SK, Zou KH, Wells WM (2004) Simultaneous truth and performance level estimation (STAPLE): an algorithm for the validation of image segmentation. IEEE transactions on medical imaging 23 (7):903–921. doi: 10.1109/TMI.2004.828354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Landis JR, Koch GG (1977) The measurement of observer agreement for categorical data. Biometrics 33 (1):159–174 [PubMed] [Google Scholar]
- 10.Baisden JM, Sheehan J, Reish AG, McIntosh AF, Sheng K, Read PW, Benedict SH, Larner JM (2011) Helical tomotherapy simultaneous integrated boost provides a dosimetric advantage in the treatment of primary intracranial tumors. Neurol Res 33 (8):820–824. doi: 10.1179/1743132811Y.0000000035 [DOI] [PubMed] [Google Scholar]
- 11.Buglione M, Pedretti S, Poliani PL, Liserre R, Gipponi S, Spena G, Borghetti P, Pegurri L, Saiani F, Spiazzi L, Tesini G, Uccelli C, Triggiani L, Magrini SM (2016) Pattern of relapse of glioblastoma multiforme treated with radical radio-chemotherapy: Could a margin reduction be proposed? J Neurooncol 128 (2):303–312. doi: 10.1007/s11060-016-2112-2 [DOI] [PubMed] [Google Scholar]
- 12.McDonald MW, Shu HK, Curran WJ Jr., Crocker IR (2011) Pattern of failure after limited margin radiotherapy and temozolomide for glioblastoma. Int J Radiat Oncol Biol Phys 79 (1):130–136. doi: 10.1016/j.ijrobp.2009.10.048 [DOI] [PubMed] [Google Scholar]
- 13.Minniti G, Amelio D, Amichetti M, Salvati M, Muni R, Bozzao A, Lanzetta G, Scarpino S, Arcella A, Enrici RM (2010) Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol 97 (3):377–381. doi: 10.1016/j.radonc.2010.08.020 [DOI] [PubMed] [Google Scholar]
- 14.Paulsson AK, McMullen KP, Peiffer AM, Hinson WH, Kearns WT, Johnson AJ, Lesser GJ, Ellis TL, Tatter SB, Debinski W, Shaw EG, Chan MD (2014) Limited margins using modern radiotherapy techniques does not increase marginal failure rate of glioblastoma. American journal of clinical oncology 37 (2):177–181. doi: 10.1097/COC.0b013e318271ae03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wernicke AG, Smith AW, Taube S, Mehta MP (2016) Glioblastoma: Radiation treatment margins, how small is large enough? Pract Radiat Oncol 6 (5):298–305. doi: 10.1016/j.prro.2015.12.002 [DOI] [PubMed] [Google Scholar]
- 16.Guram K, Smith M, Ginader T, Bodeker K, Pelland D, Pennington E, Buatti JM (2018) Using smaller-than-standard radiation treatment margins does not change survival outcomes in patients with high-grade gliomas. Pract Radiat Oncol doi: 10.1016/j.prro.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scoccianti S, Detti B, Gadda D, Greto D, Furfaro I, Meacci F, Simontacchi G, Di Brina L, Bonomo P, Giacomelli I, Meattini I, Mangoni M, Cappelli S, Cassani S, Talamonti C, Bordi L, Livi L (2015) Organs at risk in the brain and their dose-constraints in adults and in children: a radiation oncologist’s guide for delineation in everyday practice. Radiother Oncol 114 (2):230–238. doi: 10.1016/j.radonc.2015.01.016 [DOI] [PubMed] [Google Scholar]
- 18.Mayo C, Yorke E, Merchant TE (2010) Radiation associated brainstem injury. Int J Radiat Oncol Biol Phys 76 (3 Suppl):S36–41. doi: 10.1016/j.ijrobp.2009.08.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mayo C, Martel MK, Marks LB, Flickinger J, Nam J, Kirkpatrick J (2010) Radiation dose-volume effects of optic nerves and chiasm. Int J Radiat Oncol Biol Phys 76 (3 Suppl):S28–35. doi: 10.1016/j.ijrobp.2009.07.1753 [DOI] [PubMed] [Google Scholar]
- 20.Bhandare N, Jackson A, Eisbruch A, Pan CC, Flickinger JC, Antonelli P, Mendenhall WM (2010) Radiation therapy and hearing loss. Int J Radiat Oncol Biol Phys 76 (3 Suppl):S50–57. doi: 10.1016/j.ijrobp.2009.04.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gregoire V, Ang K, Budach W, Grau C, Hamoir M, Langendijk JA, Lee A, Le QT, Maingon P, Nutting C, O’Sullivan B, Porceddu SV, Lengele B (2014) Delineation of the neck node levels for head and neck tumors: a 2013 update. DAHANCA, EORTC, HKNPCSG, NCIC CTG, NCRI, RTOG, TROG consensus guidelines. Radiother Oncol 110 (1):172–181. doi: 10.1016/j.radonc.2013.10.010 [DOI] [PubMed] [Google Scholar]
- 22.Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT, Mahajan A, Schultz CJ, Erridge S, Baumert B, Hopkins KI, Tzuk-Shina T, Brown PD, Chakravarti A, Curran WJ Jr., Mehta MP (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31 (32):4085–4091. doi: 10.1200/JCO.2013.49.6968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar N, Kumar R, Sharma SC, Mukherjee KK, Khandelwal N, Kumar R, Gupta PK, Bansal A, Kapoor R, Ghosal S (2012) To compare the treatment outcomes of two different target volume delineation guidelines (RTOG vs MD Anderson) in glioblastoma multiforme patients: a prospective randomized study. Neuro-Oncology 14 ((Suppl 6)):vi133–vi141 [Google Scholar]
- 24.Matsuo M, Miwa K, Tanaka O, Shinoda J, Nishibori H, Tsuge Y, Yano H, Iwama T, Hayashi S, Hoshi H, Yamada J, Kanematsu M, Aoyama H (2012) Impact of [11C]methionine positron emission tomography for target definition of glioblastoma multiforme in radiation therapy planning. Int J Radiat Oncol Biol Phys 82 (1):83–89. doi: 10.1016/j.ijrobp.2010.09.020 [DOI] [PubMed] [Google Scholar]
- 25.Rieken S, Habermehl D, Giesel FL, Hoffmann C, Burger U, Rief H, Welzel T, Haberkorn U, Debus J, Combs SE (2013) Analysis of FET-PET imaging for target volume definition in patients with gliomas treated with conformal radiotherapy. Radiother Oncol 109 (3):487–492. doi: 10.1016/j.radonc.2013.06.043 [DOI] [PubMed] [Google Scholar]
- 26.Wakabayashi T, Iuchi T, Tsuyuguchi N, Nishikawa R, Arakawa Y, Sasayama T, Miyake K, Nariai T, Narita Y, Hashimoto N, Okuda O, Matsuda H, Kubota K, Ito K, Nakazato Y, Kubomura K (2017) Diagnostic Performance and Safety of Positron Emission Tomography Using (18)F-Fluciclovine in Patients with Clinically Suspected High- or Low-grade Gliomas: A Multicenter Phase IIb Trial. Asia Ocean J Nucl Med Biol 5 (1):10–21. doi: 10.22038/aojnmb.2016.7869 [DOI] [PMC free article] [PubMed] [Google Scholar]


