Abstract
Background and aims:
Detailed hepatitis C virus (HCV) kinetics modeling is scarce in patients with advanced liver disease receiving direct-acting antivirals (DAAs). Due to budget restrictions, patients and health systems would benefit from the shortest possible treatment course. We investigated whether modeling very early HCV kinetics in cirrhotic patients under DAAs therapy could be used to individualize care and reduce treatment duration to achieve cure.
Methods:
We included 74 patients with HCV-related liver cirrhosis who received interferon-free treatments for 12–24 weeks. HCV genotype, liver disease stage and treatment regimen were recorded. Viral load was determined prospectively at very frequent intervals until target-not-detected (TND, <15 IU/mL). A viral kinetic model was used to predict time to cure based on HCV clearance in extracellular-body fluid (CL-EF).
Results:
Sixty-eight patients (92%) achieved cure. Thirteen (18%) had MELD≥15, 35 (47%) were Child-Pugh (CTP)≥7. Median time to reach TND was 2 weeks (IQR:1–4 weeks). Modeling indicated an average DAAs efficacy in blocking viral production of ε=99.1%. HCV half-life (t1/2) was significantly shorter in patients with CTP<7, LSM<21 kPa or MELD<15 (1.5 vs 2.5 hours; p=0.0057). The overall median CL-EF was 5.6 weeks (4.1–7.8). A CTP>7 and a LSM≥21 kPa were significantly (p=0.016) associated with longer CL-EF.
Conclusions:
The study provides insights into HCV dynamics during DAAs therapy in patients with compensated and decompensated cirrhosis. Viral kinetics modeling suggests that treatment duration may be optimized in patients with compensated cirrhosis.
Keywords: modeling, hepatitis C, antivirals, liver disease, interferon-free
Introduction
Chronic hepatitis C virus (HCV) infection is a major global public health problem1. Chronic HCV infection may lead to hepatic fibrosis and cirrhosis, putting patients at risk for hepatic decompensation and hepatocellular carcinoma (HCC). This makes HCV-associated liver disease the most common indication for liver transplantation (LT) in the Western world2. The advent of highly effective direct-acting antivirals (DAAs) has revolutionized treatment for HCV infection with successful viral eradication (termed sustained virological response, SVR or cure) even in patients with advanced liver disease or significant portal hypertension3–9. However, in clinical trials as well as in clinical practice, patients with cirrhosis exhibited reduced SVR rates, less than 90% in different series10–14, mainly among decompensated liver disease groups. Therefore, increased treatment duration or addition of RBV is frequently used in decompensated cirrhotic patients or in genotype 3-HCV infected compensated cirrhotic patients, in an effort to improve response rates14.
Data on HCV kinetic analysis and modeling is scarce in patients with advanced liver disease during all-oral interferon (IFN)-free therapy. Understanding HCV viral kinetics in patients with cirrhosis, distinguishing between those with compensated and decompensated disease, could provide a means to optimize length of therapy and treatment outcomes in patients who would benefit from the shortest possible treatment course. Mathematical modeling has been applied to data from non-cirrhotic and compensated cirrhotic HCV-infected patients who were treated for 12 weeks with three different IFN-free sofosbuvir (SOF)-based regimens, and predicted that the majority of patients could have been cured with ≤8 weeks of SOF-based therapy15. Given the limited resources available for the costly DAA treatment, implementation of a response-guided treatment model to individualize length of DAA therapy could result in substantial cost saving on HCV drug expenditure (especially in resource-limited countries) in addition to improving patients’ compliance to treatment.
The aim of this study was to investigate in HCV-infected patients with compensated and decompensated cirrhosis whether modeling of very early HCV-RNA kinetics during DAAs-based antiviral therapy might give relevant insights regarding the minimal treatment duration needed to achieve cure.
Patients and Methods
Patient population and clinical data
Seventy-four consecutive patients with HCV-related cirrhosis who received IFN-free DAA-based treatments were prospectively enrolled at the Liver Unit, Hospital Clinic, Barcelona, Spain between January, 2015 and March, 2016. Cirrhosis was defined by at least one of the following criteria: biopsy-proven fibrosis stage METAVIR=4, hepatic venous pressure gradient (HVPG) ≥6 mmHg or the presence of esophageal varices, or liver stiffness measurement (LSM) ≥12 kPa. Decompensated cirrhosis was defined as Child-Turcotte-Pugh (CTP) score ≥7 points.
The following clinical and virological variables were recorded: demographics (age, gender), body mass index (BMI), treatment regimen and duration, baseline viral load, platelet count (Plt), albumin, bilirubin and International Normalized Ratio (INR), HCV RNA levels at specific time points during treatment (see below), HCV genotype, IL28B polymorphisms (rs12979860 and rs8099917), liver disease severity scores (MELD and CTP scores), HVPG and LSM. HBV and HIV co-infected patients were excluded.
The study was approved by the Ethics Committee of the Hospital Clínic of Barcelona and all patients provided written informed consent. The study was conducted in compliance with the Declaration of Helsinki, Good Clinical Practice guidelines, and local regulatory requirements.
Antiviral treatment regimens
Patients received different DAA-based regimens with or without RBV, for 12 or 24 weeks, according to EASL guidelines14 and compassionate use programs available at the time of enrollment: 1) sofosbuvir (SOF) plus ledipasvir (LDV) administered orally once daily at a fixed-dose combination (FDC) of 400/90 mg, 2) SOF 400 mg plus daclatasvir (DCV) 60 mg both administered once daily, or SOF 400 mg plus simeprevir (SMV) 150 mg both administered orally once daily, 3) DCV 60 mg plus SMV 150 mg, both administered orally once daily, 4) ombitasvir (OBV), ritonavir (r) and paritaprevir (PVT) administered orally once daily at FDC of 12,5 mg, 50 mg, 75 mg, respectively, taken in combination with dasabuvir (DSV) 250 mg administered orally twice daily.
Serum HCV-RNA quantification and HCV genotype determination
Blood samples were obtained to assess viral load at baseline, 4, 8 and 24 hours after treatment initiation, as well as days 2, 3, 4, 7 and weeks 2, 3 and 4 [or until target-not-detected (TND): HCV-RNA<15 IU/mL]. Viral load was determined by reverse transcription followed by real-time PCR using Versant HCV RNA 1.0 assay (kPCR) SIEMENS. Sustained virological response (SVR) was defined as HCV-RNA < 15 IU/mL that persisted 12 weeks after the end of antiviral treatment. The HCV genotype was determined by sequence analysis of the NS5B region of the HCV genome.
Serum HCV drug-resistant variants carrying resistant-associated substitutions (RASs) were explored, by population sequencing, in patients who did not respond to antiviral treatment (Supplementary Information).
Mathematical Modeling
HCV viral kinetics modeling was applied retrospectively using virological and clinical data collected prospectively. HCV viral kinetics under therapy was assumed to follow the standard biphasic model16 (Eq. S1, Supplementary Information) similar to previous modeling efforts15, 17. We used a mixed effect modeling approach. Parameters were estimated by maximum-likelihood using the stochastic approximation expectation-maximization algorithm implemented in MONOLIX 2016 R1 (Lixoft, Antony, France). Further details are given in the Supplementary Information.
Cure Boundaries
The time to cure was defined as the time to reach less than one HCV particle in the entire extracellular body fluid (CL-EF) volume, approximately 13.5L. A value of 7×10−5 IU/mL for viral load was used as the threshold for cure as the concentration of one virion per 13,500 mL. A secondary, more speculative analysis was done in which the time to cure was defined as <1 virus copy and <1 HCV-infected hepatocyte in the body (CL-C) as previously described15, 17.
Statistical analysis
Continuous variables were depicted using median and interquartile ranges (IQR) and categorical variables were expressed as absolute numbers and percentages. Univariate analysis was performed to define the differences in CL-EF and CL-C between different patient groups according to clinical parameters using Mann-Whitney tests. All differences and associations were considered significant at a 2-sided p-value of <0.05. Statistical analyses were performed with SPSS, version 18 (SPSS, Chicago, IL).
Results
Baseline characteristics
Seventy-four patients with HCV-related liver cirrhosis received the following IFN-free HCV treatment: 31 (42%) SOF+SMV±RBV, 14 (19%) SOF+LDV±RBV, 12 (16%) SOF+DCV±RBV, 6 (8%) DCV+SMV±RBV and 11 (15%) OBV+PTV/r+DSV±RBV (Table 1). Sixty-two patients (84%) received RBV dosed according to weight and tolerance. Forty-three patients (58%) were treated for 12 weeks and 31 (42%) for 24 weeks. Based on the treatment recommendations at the time of the study, patients treated for 24 weeks had more advanced liver disease compared to patients treated for 12 weeks including, lower baseline albumin level (p=0.027) and higher INR (p=0.012), creatinine (p=0.002), CTP score (p=0.019), and MELD score (p=0.007), (Table S1).
Table 1.
Baseline clinical and virological characteristics of patient population (n=74).
| All patients n=74 | n (%) or Median (IQR) |
|---|---|
| Age (years) | 61 (55–69) |
| Gender (male) | 50 (70%) |
| HCV genotype 1 | 65 (89%) |
| Baseline viral load (log10) | 6 (5–6) |
| Unfavorable IL28 polymorphism† | 46 (77%) |
| Albumin<35 g/L | 26 (35%) |
| Platelet count <100.000/mm3 | 53 (72%) |
| Bilirubin (mg/dL) | 1.4 (1–2) |
| Prothrombin time <70% | 49 (66%) |
| MELD MELD≥15 |
11 (8–13) 13 (18%) |
| Child-Turcotte-Pugh (CTP) A B C |
39 (53%) 25 (34%) 10 (13%) |
| Child-Turcotte-Pugh (CTP)> 7 points | 19 (26%) |
| LSM (kPa)* LSM>21kPa |
21.6 (17.3–35.8) 30 (50%) |
| HVPG (mmHg)** HVPG≥10 mmHg HVPG≥12 mmHg |
15.5 (11–18.8) 40 (83%) 35 (73%) |
| Previous HCC | 10 (13%) |
|
Antiviral Therapy Non-responders to previous IFN therapy SOF+SMV±RBV SOF+LDV±RBV SOF+DCV±RBV DCV+SMV±RBV OBV/PTV/r/DSV±RBV RBV use Treatment duration (12 weeks) |
41 (55%) 31 (42%) 14 (19%) 12 (16%) 6 (8%) 11 (15%) 62 (84%) 43 (58%) |
IL28 polymorphism (n=60)
Liver Stiffness Measurement (LSM) (n=60)
Hepatic Venous Pressure Gradient (HVPG) measurement was available in 48 out of 74 patients.
HCC: Hepatocellular Carcinoma. Clinical decompensation: ascites, hepatic encephalopathy or acute variceal bleeding. SOF: sofosbuvir, LDV: ledipasvir, RBV: ribavirin, DCV: daclatasvir, SMV: simeprevir, OBV: ombitasvir, PTV: paritaprevir, r: ritonavir, and DSV: dasabuvir.
Baseline clinical and virological features are summarized in Table 1. The median MELD score before antiviral therapy was 11, with 13 patients (18%) having a MELD score ≥15; 39 patients (53%) were CTP A (5–6 points), 25 (34%) were CTP B (7–9 points) and 10 patients (13%) were CTP C (>9 points). Ten patients (13%) had hepatocellular carcinoma (HCC) in complete response after tumor ablation or resection. Hepatic venous pressure gradient measurement was available in 48 patients (65%); of these, 40 (83%) had clinically significant portal hypertension (HVPG≥10 mmHg) and 35 (73%) had HVPG≥12 mmHg. LSM was available in 60 patients (81%) and 30 (50%) had LSM≥ 21kPa.
Virological response
Overall, the median time to reach target not detected (TND) was 2 weeks (IQR: 1–4 weeks). After 1, 2, 4 and 8 weeks of treatment 26% (n=19), 35% (n=26), 23% (n=17) and 16% (n=12) had serum HCV-RNA target not detected, respectively (Fig.1). However, comparing time to TND between patients revealed differences based on specific baseline characteristics. While we did not find significant differences in time to reach TND associated with any specific DAA regimen, use of RBV, patient age, history of HCC, IL28 polymorphism, or HCV genotype, patients who reached HCV RNA TND within 2 weeks had 1-log lower HCV RNA at baseline compared to those who reached HCV RNA TND after 2 weeks of treatment (p<0.001) (Table 2). Additionally, median time to HCV RNA TND was longer (2.5 vs 2 weeks) in patients with LSM≥21 kPa compared to those with LSM<21 kPa (p=0.009) (Table 2). We did not find significant differences when patients were classified according to HVPG (cut-off: ≥10 or ≥12 mmHg), platelet count (cut-off:≥100,000/mm3), MELD (cut-off:≥15) or CTP score (cut-off: ≥7).
Figure 1.
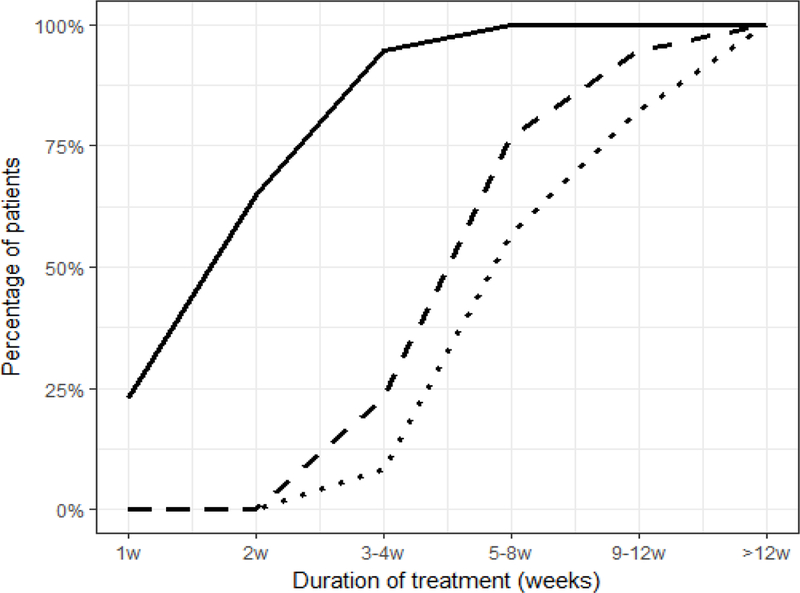
Percentage of patients who achieved target not detected (TND, solid line), and predicted to reach cure at specific time points during treatment based on extracellular body fluid (CL-EF, dashed line) and time to HCV-infected hepatocytes clearance (CL-C, dotted line) cure boundaries.
Table 2.
Differences in time to target not detected (TND), HCV clearance in extracellular body fluid (CL-EF) and HCV-infected hepatocytes clearance (CL-C) according to baseline clinical and virological characteristics.
| Variable | Time to TND (weeks)Ω |
p* | CL-EF (weeks) Ω |
p* | CL-C (weeks) Ω |
p* |
|---|---|---|---|---|---|---|
|
Gender Male vs female |
2 (1–4) vs 2 (1–4) |
0.924 |
6 (5–8) vs 6 (4–8) |
0.822 |
8 (6–10) vs 8 (5–10) |
0.782 |
|
Baseline VL ≤ 5
vs >5 (log) |
2 (1–2) vs 3 (2–5) |
<0.001 |
5 (3–6) vs 7 (5–8) |
<0.001 |
7 (5–9) vs 9 (8–12) |
<0.001 |
|
HCC Present vs absent |
2 (1–4) vs 2 (2–4) |
0.922 |
6 (4–8) vs 6 (3–8) |
0.752 |
8 (6–10) vs 8 (5–10) |
0.752 |
|
Child Pugh ≤7 vs > 7 |
2 (1–3) vs 3 (1–4) |
0.234 |
6 (4–7) vs 8 (5–10) |
0.016 |
7 (5–9) vs 9 (6–13) |
0.029 |
|
MELD <15 vs ≥15 |
2 (1.5–4) vs 2 (1–3) |
0.814 |
6 (4–8) vs 6 (4–8) |
0.904 |
8 (6–10) vs 8 (5–10) |
0.831 |
|
LSM LSM <21 kPa vs ≥21 kPa |
2 (1–3) vs 2.5 (2–5) |
0.009 |
5 (3–7) vs 6 (5–9) |
0.016 |
7 (5–9) vs 8 (7–11) |
0.047 |
|
HVPG <10 vs vs ≥ 10 mmHg <12 vs vs ≥ 12 mmHg |
2 (1–5) vs 2 (1–3) 2 (1–3) vs 2 (1–3) |
0.860 0.499 |
5 (3–7) vs 5 (4–7) 5 (3–7) vs 5 (4–7) |
0.596 0.417 |
7 (4–9) vs 7 (5–9) 7 (5–9) vs 7 (5–9) |
0.654 0.494 |
|
Plt count <100.000 vs ≥100.000/mm3 |
2 (1–3) vs 2 (2–4) |
0.126 |
5 (3–7) vs 6 (5–8) |
0.119 |
7 (5–9) vs 9 (6–11) |
0.215 |
|
Albumin
<35 vs ≥ 35 g/L |
2 (1–4) vs 2 (2–4) |
0.752 |
5 (4–7) vs 6 (5–8) |
0.191 |
7 (5–9) vs 8 (6–11) |
0.282 |
|
IL28 polymorphism
Non-favorable vs favorable |
2 (2–3) vs 2 (1–4) |
0.779 |
6 (6–8) vs 6 (4–7) |
0.882 |
7 (6–11) vs 8 (5–9) |
0.753 |
|
DAA regimen SOF+SMV±RBV DCV+SMV±RBV DCV+SOF±RBV OBV+PTV/r/DSV±RBV SOF+LDV±RBV |
2 (2–4) 3 (2–5) 2 (2–5) 2 (1–3) 2 (1–3) |
0.218 |
6 (2–12) 6 (3–7) 7 (5–10) 5 (4–6) 4 (3–8) |
0.362 |
8 (3–17) 7 (5–9) 9 (7–13) 8 (3–11) 6 (4–13) |
0.495 |
TND: target non detected, HVPG: hepatic venous pressure gradient, LSM: liver stiffness measurement, HCC: hepatocellular carcinoma, DAA: direct acting antivirals, Plt: platelet.
Univariate analysis
Data is expressed as median and IQR.
Overall, SVR rate was 92%. Only 6 patients were non-responders due to viral breakthrough (n=1) or relapse (n=5) after SMV-based treatment (n=4) or SOF+DCV (n=2) (Table S2). The patients experiencing relapse reached TND at week 1 (n=1), 3 (n=1) and 5 (n=3). Two patients had pre-existing RAS. Due to the small number of non-responders, we could not identify any virological or clinical variables related to treatment failure.
Viral kinetic parameter estimation
Data from all 74 patients were included in the viral kinetic modeling. The model fit the measured data well (Fig.2 and Fig.S1), the parameters were estimated (Tables S3 and S4) and the goodness-of-fit plots were satisfactory (Fig. S2). Overall, the initial HCV viral load was estimated at 5.7 log10 IU/mL (relative standard error, rse= 1%), with an inter-individual variability (IIV) of 11% (rse=9%). After a pharmacological delay of τ=3.6 h (rse=7%) with an IIV of 39% (rse=16%), DAA effectiveness in blocking viral production was estimated as ε=0.991 (rse~0) with an IIV of 0.5% (rse=15%). The virus clearance rate was estimated as c=7.68 d−1 (rse=10%) with an IIV of 80% (rse=9%), leading to a virus serum half-life of 2.17 h. Infected cells loss rate was estimated δ=0.49 d−1 (rse=6%) with an IIV of 46% (rse=10%), corresponding to an HCV-infected cell half-life of 1.42 days. Interestingly, in a model including covariates, patients with CTP<7 or MELD<15 or LSM<21 kPa had a significantly (p=0.0057) higher virus clearance rate c=11.2 d−1 (rse=14%) with an IIV of 49% (rse=25%), leading to a shorter virus serum half-life t1/2=1.5 h, compared to patients with more advanced liver disease (CTP≥7 or MELD≥15 or LSM≥21), where c=6.7 d−1 (rse=13%) with an IIV of 89% (rse=10%), leading to a longer t1/2=2.5 h (Table S3). Viral-kinetic parameters were not associated with previous IFN-based treatment status.
Figure 2:
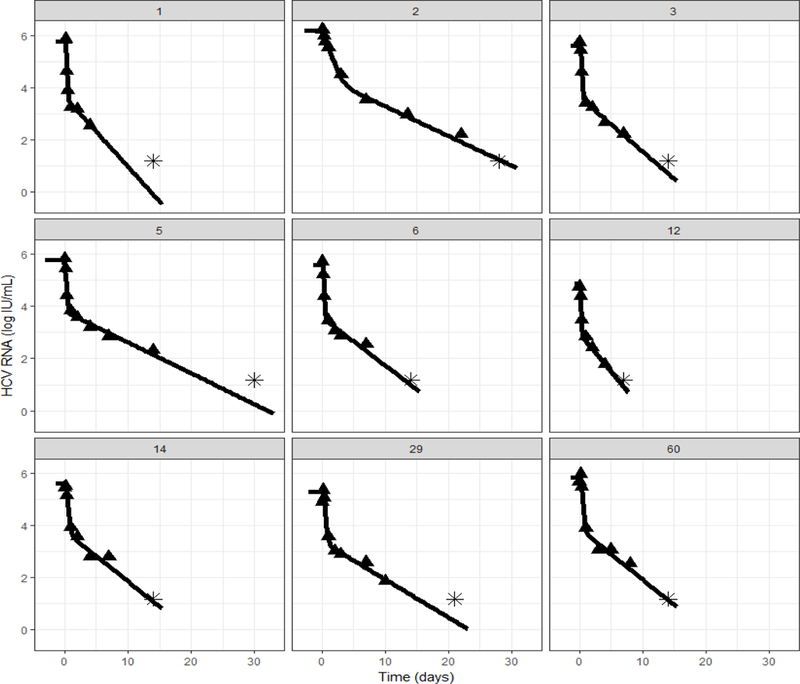
Representative model fit curves (black line) to the measured HCV data (triangle, stars represent data below the lower level of quantification). Model fits with HCV RNA measured data for all patients are shown in Fig. S1.
Predicting time to cure
Modeling predicted that after 4, 8, 12 and more than 12 weeks of therapy, 28% (n=21), 84% (n=62), 96% (n=71) and 100% (n=74) would achieve CL-EF, while 11% (n=8), 64% (n=47), 86% (n=64) and 100% (n=74) would achieve CL-C, respectively (Fig. 1).
Modeling predicted that, overall, the median CL-EF was 5.6 (4.1–7.8) weeks and the median CL-C was 7.7 (5.5–10.2) weeks. As expected, patients who reached HCV-RNA TND within the first week of treatment showed faster CL-EF (3 weeks) and CL-C (4.5 weeks), compared to those who reached HCV-RNA TND later (>1 week), who showed median CL-EF of 6 weeks and CL-C of 8 weeks (p<0.001). Similarly, patients with lower baseline viral load (<5 log IU/mL) had significantly faster CL-EF and CL-C compared to patients with higher baseline viral load (>5 log IU/mL) (p<0.001, Table 2).
No significant difference in CL-EF and CL-C was observed across gender, age, different treatment regimens, use of RBV or treatment duration (12 vs 24 weeks) (Table 2). However, both CL-EF and CL-C, did correlate with liver disease severity. Patients with CTP>7 had a significantly longer mean time to CL-EF and CL-C (8 and 9 weeks) than those patients with CTP≤7 (6 weeks and 7 weeks) (p=0.016 and p=0.029, respectively) (Table 2, Fig. 3). Similarly, patients with LSM≥21 kPa had longer CL-EF and CL-C (6 weeks and 8 weeks) compared to those with LSM<21 kPa (5 weeks and 7 weeks) (p=0.016 and p=0.047). No differences in CL-EF and CL-C were found grouping patients according pretreatment albumin, platelet levels, HVPG, or MELD score (Table 2).
Figure 3.
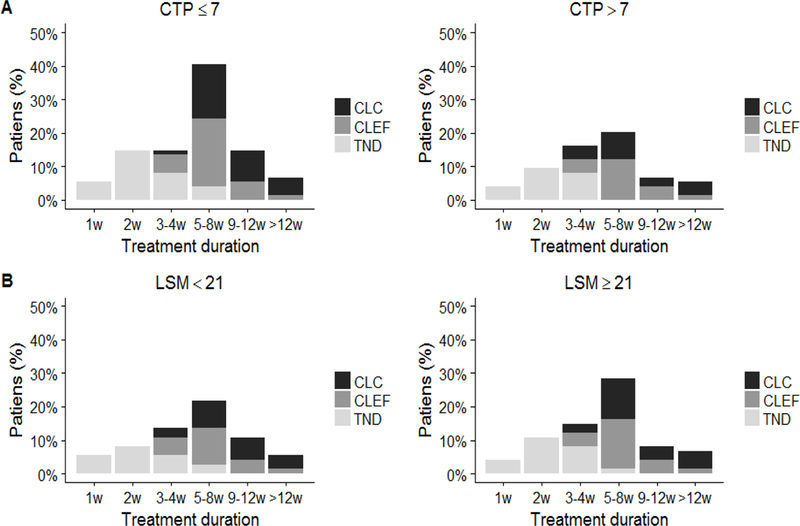
Time to HCV negativity and predicted time to cure based on Child-Turcotte-Pugh (CTP) or liver stiffness measurement (LSM). (A) Proportion of patients who achieved target not detected (TND), and predicted to achieve extracellular body fluid (CL-EF) and HCV-infected hepatocytes clearance (CL-C) at specific time points during the treatment, comparing those with CTP≤B7 and those with CTP>B7. (B) Proportion of patients who achieved TND, CL-EF and CL-C at specific time points during the treatment, comparing those with LSM>21 kPa and those with LSM≥21kPa.
Among patients achieving SVR after 12 weeks of DAA therapy, predicted time to cure based on CL-EF was ≤12 weeks in all but one patient (#42), who was predicted to require 3 additional weeks of therapy. On the other hand, modeling predicted that six patients, including one relapser, would need >12 weeks of treatment based on CL-C (Table S4). Interestingly, in the 24 week therapy group, the predicted time to cure based on CL-EF was ≤12 weeks in all but 2 patients. The CL-C boundary predicted that six patients would need between 12 and 24 weeks of therapy.
Discussion
Very early viral kinetics in patients with varying degrees of liver disease (47% with CPT B or C) receiving different DAAs combinations (with or without RBV) for 12 or 24 weeks was assessed. Using mathematical modeling, we estimated viral-host kinetic parameters and DAA efficacy in blocking viral production in order to predict the time to achieve HCV cure.
In the IFN era, mathematical models of HCV kinetics have provided a means to compare varied treatment regimens and outcomes in different patient populations18. The kinetics of early viral decline under interferon-based therapy was used as a stopping rule due to its high predictive value19. In addition, the detection of HCV-RNA at the end of treatment (EOT) with IFN-based therapies was an indicator of treatment failure20–22. In the era of DAA therapy, with exceptionally high SVR rates, it is far less important to predict outcome of therapy and early viral kinetics does not predict treatment failure23. Interestingly, viral kinetic analyses using the Abbott Real Time HCV quantification assay have indicated that some patients achieve SVR even if HCV-RNA is detectable at the EOT24–27. While new models were developed to explain this phenomenon28, 29, none of the patients in the current study, which used the SIEMENS assay to measure HCV RNA had detectable levels at EOT as in our previous modeling study15 in which Roche CobasTaqMan v2.0 was used to measure HCV RNA.
The median time to reach TND was overall rapid (within 2 weeks after initiation of DAA therapy), with a slightly shorter time to TND in patients with lower baseline viral load (≤5 log IU/mL) or LSM<21 kPa. This is in broad agreement with the viral kinetic study by Welzel et al30, which included patients with chronic HCV infection and advanced liver disease (SOLAR-1 trial). In the current study, all patients had a typical biphasic viral load decline with a first rapid phase viral decline followed by a second slower phase as previously described in non-cirrhotic and compensated cirrhotic patients who were treated with approved all-oral DAAs therapy15.
Modeling results reflect a pharmacological delay of 3.6 hours and a DAA effectiveness of 99.1% in blocking viral production. These results did not differ between patients with compensated cirrhosis and those with advanced cirrhosis. However, patients with CTP<7 or MELD<15 or LSM<21 kPa had a significantly shorter virus half-life (1.5 vs 2.5 hours, respectively), suggesting an inverse association between the rate of the first phase of viral decline under DAA therapy and the severity of liver disease. This is in agreement with a previous report using intravenous silibilin in the peri-transplant setting, which showed that the first phase of viral decline (and not the 2nd phase of viral decline, i.e., parameter δ) was inversely associated with the severity of liver disease31. Slower viral clearance rate from the blood in patients with CTP≥7, MELD≥15 or LSM≥21 kPa might reflect a decline in hepatic blood flow and portal systemic shunting, impairing drug delivery. Other potential explanations for reduced viral clearance in patients with decompensated cirrhosis include reduced drug metabolism with impaired liver function, or compromised immune responses needed to clear HCV from the liver32, 33.
In the current study, estimating the viral-host-drug parameters from each patient’s early viral kinetic profile allowed us to project the minimal treatment duration needed to achieve <1 virus copy in the entire extracellular body fluid (CL-EF) for each patient. Modeling results predicted that most patients achieved cure within 8 weeks of therapy, with a shorter required treatment duration in patients with low baseline viral load (<5 log IU/mL), LSM<21 kPa or CTP≤7. Interestingly, patients who reached HCV-RNA TND within the first week of treatment had a CL-EF of 3 weeks. This is in line with previous findings, documenting cure after 3 weeks of DAA therapy without ribavirin in non-cirrhotic patients who achieved HCV-RNA levels<500 IU/mL by day 2.34
The prediction of the time to the last virion in circulation (CL-EF) can be considered robust due to available measurements of the viral load in blood. We explored a second cure boundary, the time to eradication of the last infected cell (CL-C), which is more speculative due to lack of experimental data on the infected cell level. However, in the 12 week treatment group, the prediction of longer treatment duration to achieve <1 infected cell in 5 individuals who achieved SVR was clearly an overestimate as found in a previous DAA-inhibition modeling study in non-cirrhotic and compensated cirrhotic patients15. Therefore, the CL-EF provided a more accurate estimate of cure boundary.
Our study presents some limitations. First, viral kinetic parameters were estimated using a population modeling approach that borrows information from patients for whom sufficient data samples were quantifiable (see Supplementary Information). Additionally, the model did not include other important features such as RAS and unidentified host factors. All patients not achieving SVR (7%) had RASs (Table S2); since kinetic data of RASs were not available, it was not feasible to perform modeling in order to predict relapse due to RASs35. Finally, in patients who received antiviral regimens that include options that are currently considered sub-optimal; we cannot exclude different results when using next-generation DAAs. However, preliminary prospective data (NCT03603327) including cirrhotic subjects, shows that response-guided therapy can shorten treatment duration with newer DAAs to <12 weeks in up to 50% of patients, without compromising efficacy or safety36. Final results of ongoing clinical trials evaluating the possibility of shorter treatment duration regimens are awaited both in chronic and acute HCV infection (NCT03089944, NCT02625909, NCT02886624).
Real time modeling could provide a tool to individualize and optimize IFN-free antiviral therapy37. Shortening antiviral therapy is associated with DAA cost-saving36, and could possibly help improve patient compliance and reduce side effects of concurrent RBV treatment. In our study, patients were over-treated for a mean duration of 5.2±2.8 weeks (Table S4). If the observed viral kinetics are similar under the current approved DAAs and considering a standard treatment duration of 12 weeks, cost saving would be ~40% for patients with advanced liver disease.
In summary, very early viral kinetics modeling suggests that most patients with liver cirrhosis may achieve cure after less than 12 weeks of DAA treatment. Hence, understanding HCV infection dynamics and treatment response will help to optimize antiviral therapy (enhance drug synergy and increase the barrier to viral escape), predict the duration of therapy needed to achieve viral clearance, and ultimately allow for individualize therapy enabling a reduction in cost.
Supplementary Material
Lay Summary:
Data on HCV kinetic analysis and modeling is scarce in patients with advanced liver disease during all-oral interferon-free therapy with direct-acting antivirals (DAA).
In this study, modeling results predicted that most patients achieved cure within only 8 weeks of DAA therapy, with shorter required treatment duration in patients with lower baseline viral load and less advanced liver disease.
Real-time viral kinetics modeling has the potential to optimize duration of therapy in patients with compensated cirrhosis.
Considering a standard DAA treatment duration of 12 weeks for patients with advanced liver disease in Europe, modeling predicts DAA cost-saving of ~40% in patients with cirrhosis.
Acknowledgments
Financial Support: XF: received support in part by Instituto de Salud Carlos III (PI15/00151), Ministerio de Economía y Competitividad, co-funded by Fondo Europeo de Desarrollo Regional, Unión Europea, Una manera de hacer Europa. XF also received a grant from Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (grant 2017_SGR_1753). SL, ZM, MCL and XF also received support by the Spanish Health Ministry (Plan Estratégico Nacional contra la Hepatitis C). CIBERehd is funded by the Instituto de Salud Carlos III. LC: United Kingdom Biotechnology and Biological Sciences Research Council (grant reference 1698:BB/L001330/1. FG: Center for Modeling and Simulation in the Biosciences. SLU, SJC, and HD supported in part by the U.S. National Institute of Health (NIH) grants R01-AI078881 and R01GM121600.
Conflict of interest: XF received unrestricted grant support from Abbvie and has acted as advisor for Abbvie, and Gilead. SL has acted as advisor for Abbvie, Janssen, and Gilead. MCL has acted as advisor for MSD, Janssen, BMS and Gilead. ZM has acted as advisor for BMS. The remaining authors have nothing to declare.
Abbreviations:
- HCV:
hepatitis C virus
- HCC:
hepatocellular carcinoma
- DAAs:
direct-acting antivirals
- IFN:
interferon
- SOF:
sofosbuvir
- CTP:
Child Pugh score
- HVPG:
hepatic venous pressure gradient
- LSM:
liver stiffness measurement
- FDC:
fixed dose combination
- RBV:
ribavirin
- LDV:
ledipasvir
- DCV:
daclatasvir
- SMV:
simeprevir
- OBV:
ombitasvir
- r:
ritonavir
- PVT:
paritaprevir
- DSV:
dasabuvir
- SVR:
sustained virological response
- RAS:
resistant-associated substitutions
- CL-EF:
clearance in extracellular-body fluid
- CL-C:
clearance in hepatocytes
- EOT:
end of treatment
REFERENCES
- 1.Polaris Observatory HCVC. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol Hepatol 2017;2:161–176. [DOI] [PubMed] [Google Scholar]
- 2.Flemming JA, Kim WR, Brosgart CL, et al. Reduction in liver transplant wait-listing in the era of direct-acting antiviral therapy. Hepatology 2017;65:804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bourliere M, Bronowicki JP, de Ledinghen V, et al. Ledipasvir-sofosbuvir with or without ribavirin to treat patients with HCV genotype 1 infection and cirrhosis non-responsive to previous protease-inhibitor therapy: a randomised, double-blind, phase 2 trial (SIRIUS). Lancet Infect Dis 2015;15:397–404. [DOI] [PubMed] [Google Scholar]
- 4.Reddy KR, Bourliere M, Sulkowski M, et al. Ledipasvir and sofosbuvir in patients with genotype 1 hepatitis C virus infection and compensated cirrhosis: An integrated safety and efficacy analysis. Hepatology 2015;62:79–86. [DOI] [PubMed] [Google Scholar]
- 5.Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med 2014;370:1973–82. [DOI] [PubMed] [Google Scholar]
- 6.Bourliere M, Gordon SC, Flamm SL, et al. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N Engl J Med 2017;376:2134–2146. [DOI] [PubMed] [Google Scholar]
- 7.Forns X, Lee SS, Valdes J, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis 2017;17:1062–1068. [DOI] [PubMed] [Google Scholar]
- 8.Lens S, Alvarado-Tapias E, Marino Z, et al. Effects of All-Oral Anti-Viral Therapy on HVPG and Systemic Hemodynamics in Patients With Hepatitis C Virus-Associated Cirrhosis. Gastroenterology 2017;153:1273–1283 e1. [DOI] [PubMed] [Google Scholar]
- 9.Belli LS, Duvoux C, Berenguer M, et al. ELITA consensus statements on the use of DAAs in liver transplant candidates and recipients. J Hepatol 2017;67:585–602. [DOI] [PubMed] [Google Scholar]
- 10.Lawitz E, Poordad F, Gutierrez JA, et al. Simeprevir, daclatasvir and sofosbuvir for hepatitis C virus-infected patients with decompensated liver disease. J Viral Hepat 2017;24:287–294. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez Carrillo C, Lens S, Llop E, et al. Treatment of hepatitis C virus infection in patients with cirrhosis and predictive value of model for end-stage liver disease: Analysis of data from the Hepa-C registry. Hepatology 2017;65:1810–1822. [DOI] [PubMed] [Google Scholar]
- 12.Suraweera D, Saab S. Hepatitis C treatment threshold in patients with decompensated liver disease. Hepatology 2017;65:1789–1791. [DOI] [PubMed] [Google Scholar]
- 13.Curry MP, O’Leary JG, Bzowej N, et al. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N Engl J Med 2015;373:2618–28. [DOI] [PubMed] [Google Scholar]
- 14.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol 2018;69:461–511. [DOI] [PubMed] [Google Scholar]
- 15.Dahari H, Canini L, Graw F, et al. HCV kinetic and modeling analyses indicate similar time to cure among sofosbuvir combination regimens with daclatasvir, simeprevir or ledipasvir. J Hepatol 2016;64:1232–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neumann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-alpha therapy. Science 1998;282:103–7. [DOI] [PubMed] [Google Scholar]
- 17.Canini L, Imamura M, Kawakami Y, et al. HCV kinetic and modeling analyses project shorter durations to cure under combined therapy with daclatasvir and asunaprevir in chronic HCV-infected patients. PLoS One 2017;12:e0187409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dahari H, Guedj J, Perelson AS, et al. Hepatitis C Viral Kinetics in the Era of Direct Acting Antiviral Agents and IL28B. Curr Hepat Rep 2011;10:214–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried MW, Hadziyannis SJ, Shiffman ML, et al. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol 2011;55:69–75. [DOI] [PubMed] [Google Scholar]
- 20.Ferenci P, Laferl H, Scherzer TM, et al. Peginterferon alfa-2a and ribavirin for 24 weeks in hepatitis C type 1 and 4 patients with rapid virological response. Gastroenterology 2008;135:451–8. [DOI] [PubMed] [Google Scholar]
- 21.Poordad F, McCone J Jr., Bacon BR, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011;364:1195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobson IM, Dore GJ, Foster GR, et al. Simeprevir with pegylated interferon alfa 2a plus ribavirin in treatment-naive patients with chronic hepatitis C virus genotype 1 infection (QUEST-1): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet 2014;384:403–13. [DOI] [PubMed] [Google Scholar]
- 23.Zeuzem S, Dusheiko GM, Colombo M, et al. Early viral kinetics do not predict treatment outcome with sofosbuvir+ribavirin for 12 or 24 weeks in HCV genotype 2/3 patients in the Valence trial. J Hepatol 2014;60:S452. [Google Scholar]
- 24.Sidharthan S, Kohli A, Sims Z, et al. Utility of Hepatitis C Viral Load Monitoring on Direct-Acting Antiviral Therapy. Clin Infect Dis 2015. [DOI] [PMC free article] [PubMed]
- 25.Malespin M, Benyashvili T, Uprichard SL, et al. Prevalence of end of treatment RNA-positive/sustained viral response in HCV patients treated with sofosbuvir combination therapies. Therap Adv Gastroenterol 2017;10:68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shteyer E, Dahari H, Gafanovich I, et al. End of treatment RNA-positive/sustained viral response in an individual with acute hepatitis C virus infection treated with direct-acting antivirals. Therap Adv Gastroenterol 2017;10:429–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dahari H, Halfon P, Cotler SJ. Resurrection of response-guided therapy for sofosbuvir combination therapies. J Hepatol 2016;65:462–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goyal A, Lurie Y, Meissner EG, et al. Modeling HCV cure after an ultra-short duration of therapy with direct acting agents. Antiviral Res 2017;144:281–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen THT, Guedj J, Uprichard SL, et al. The paradox of highly effective sofosbuvir-based combination therapy despite slow viral decline: can we still rely on viral kinetics? Sci Rep 2017;7:10233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welzel TM, Reddy KR, Flamm SL, et al. On-treatment HCV RNA in patients with varying degrees of fibrosis and cirrhosis in the SOLAR-1 trial. Antivir Ther 2016;21:541–546. [DOI] [PubMed] [Google Scholar]
- 31.Canini L, DebRoy S, Marino Z, et al. Severity of liver disease affects HCV kinetics in patients treated with intravenous silibinin monotherapy. Antivir Ther 2015;20:149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serti E, Chepa-Lotrea X, Kim YJ, et al. Successful Interferon-Free Therapy of Chronic Hepatitis C Virus Infection Normalizes Natural Killer Cell Function. Gastroenterology 2015;149:190–200 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Al Marzooqi SH, Feld JJ. Sorting out cirrhosis: mechanisms of non-response to hepatitis C therapy. Liver Int 2015;35:1923–33. [DOI] [PubMed] [Google Scholar]
- 34.Lau G, Benhamou Y, Chen G, et al. Efficacy and safety of 3-week response-guided triple direct-acting antiviral therapy for chronic hepatitis C infection: a phase 2, open-label, proof-of-concept study. Lancet Gastroenterol Hepatol 2016;1:97–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rong L, Dahari H, Ribeiro RM, et al. Rapid emergence of protease inhibitor resistance in hepatitis C virus. Sci Transl Med 2010;2:30ra32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Etzion O, Dahari H, Yardeni D, et al. Response-guided therapy with DAA shortens treatment duration in 50% of HCV treated patients. Hepatology 2018;accepted.
- 37.Dahari H, Shteingart S, Gafanovich I, et al. Sustained virological response with intravenous silibinin: individualized IFN-free therapy via real-time modelling of HCV kinetics. Liver Int 2015;35:289–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


