Abstract
Objective:
To evaluate the variation in long-term opioid use in osteoarthritis patients by geography and healthcare access.
Methods:
We designed an observational cohort study among osteoarthritis patients undergoing total joint replacement (TJR) in the Medicare program (2010–2014). State of residence and healthcare access quantified at the primary care service area (PCSA) level as categories of number of practicing primary care providers (PCPs) and categories of rheumatologists per 1,000 Medicare beneficiaries were the independent variables of interest. The percentage of osteoarthritis patients using long-term opioids (≥ 90 days in the 360-day period immediately preceding TJR) within each PCSA was the outcome variable in a multi-level generalized linear regression model adjusting for case-mix at PCSA-level and policies including rigor of prescription drug monitoring programs and legalized medical marijuana at state-level.
Results:
A total of 358,121 advanced osteoarthritis patients with mean age of 74 years were included from 4,080 PCSAs. The unadjusted mean % of long-term opioid users varied widely across states, ranging from 8.9% (Minnesota) to 26.4% (Alabama), and this variation persisted in the adjusted models. Access to PCPs was only modestly associated with rates of long-term opioid use (adjusted mean difference (95% CI) between PCSAs with highest (>8.6) versus lowest (<3.6) concentration: 1.4% (0.8%−2.0%)); while access to rheumatologists was not associated with long-term opioid use.
Conclusion:
We noted substantial statewide variation in rates of treatment with long-term opioid therapy in osteoarthritis, which was not fully explained by differences in access to healthcare providers, varying case-mix, or state-level policies.
Keywords: osteoarthritis, prescription opioids, long-term opioid use, total joint replacement, geographic variation
INTRODUCTION
Long-term use of prescription opioids to treat chronic non-cancer pain has received intense scrutiny due to accumulating evidence of uncertain clinical benefit and well-recognized risks associated with their use (1). A comprehensive systematic review summarizing evidence from 40 studies concluded that evidence demonstrating the effectiveness of long-term opioid therapy for improving chronic pain and functioning was insufficient and available evidence supported potentially dose-dependent risk for serious harms including overdose, abuse, fractures, and cardiac events (2). Recently, a large randomized trial demonstrated equivalent outcomes between prescription opioids and non-opioid treatments at 12 months in patients with chronic back pain or hip or knee osteoarthritis pain (3). In light of all available evidence, the Centers for Disease Control (CDC) has recommended that in chronic pain patients, clinicians should assess risk benefit tradeoffs of long-term opioid therapy every three months or more frequently and gradually taper or discontinue when harms outweigh benefits (4).
Osteoarthritis of hip or knee is one of the most common reasons for chronic pain in the US, affecting nearly 30 million US adults and the prevalence is expected to rise with aging of the population (5). Moderate-to-severe pain in patients with osteoarthritis is often managed with non-steroidal anti-inflammatory drugs, steroids, and opioid analgesics (6), and in patients with severe pain that is inadequately controlled with medications, total joint replacement (TJR) is considered to improve quality of life (7, 8). Although two previous studies have investigated the cross-sectional prevalence of prescription opioid use in osteoarthritis patients, neither of them studied long-term use (9, 10). Studies conducted to describe patterns and predictors of opioid use on a societal level, without focusing on patients with chronic pain, have noted geographic variation in opioid prescribing practices and healthcare access to be important determinants of prescription opioid use (11, 12). However, patients with chronic pain is a population of special interest for studying prescription opioid use as the need for effective pain management in these patients can lead to long-term opioid use. Careful examination of long-term opioid use patterns in routine care patient populations affected by chronic pain is urgently needed to effectively disseminate opioid prescribing guidelines and target policy interventions minimizing harm. Therefore, we conducted an observational cohort study in a nationwide sample of Medicare enrollees with severe osteoarthritis to describe long-term opioid use and to evaluate the role of geography and healthcare access in determining long-term opioid use. We restricted the study sample to TJR recipients and evaluated opioid use in the year leading up to the TJR to include a homogenous patient population with advanced osteoarthritis and a clinical indication for pain control.
METHODS
Data sources
Medicare claims from Parts A (inpatient services), B (outpatient services), and D (pharmacy claims) between 2010 and 2014 were used in this study. These data sources contain longitudinally traceable information for their enrollees’ medical diagnoses recorded with International Classification of Disease, 9th Clinical Modification (ICD-CM) codes, medical procedures recorded as Current Procedure Terminology (CPT) or ICD-9 procedure codes, and medication dispensing recorded using National Drug Codes (NDC). In addition, we retrieved information regarding patient demographics including race, gender, age, and geography of their residence at ZIP code level from Medicare enrollment files.
Further, we used Primary Care Service Area (PCSA) data files (2010) available from the Health Resources & Services Administration (HRSA) Data Warehouse to quantify access to primary care providers (13). A PCSA is a discrete service area defined for Medicare beneficiaries based on receipt of primacy care services and contains one or more contiguous ZIP code tabulation areas (14). PCSA data files include information on the total number of Medicare enrollees in each PCSA along with a wide range of information related to healthcare services, for instance number of clinically active primary care providers, physician assistants, or federally qualified health centers, as well as socioeconomic status indicators derived from the American Community Survey conducted by the US Census Bureau (13). PCSA data files do not contain information on number of practitioners by specialty. Therefore, we obtained a comprehensive de-identified list of all practicing US-based rheumatologists as of 2010 and their corresponding business addresses from the American College of Rheumatology (15). Medicare files were linked with PCSA files and the list of rheumatologists using ZIP codes to assign a PCSA to each patient in this study. The institutional review board (IRB) of Brigham & Women’s Hospital approved the protocol for this study.
Study Population
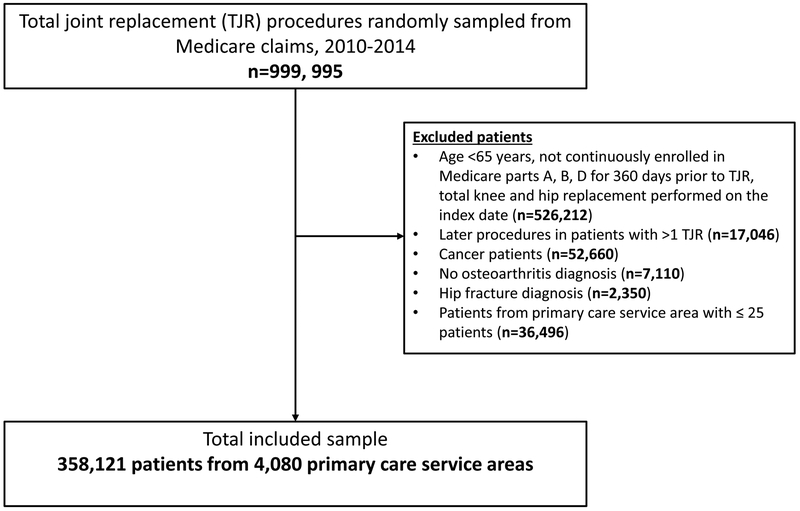
We randomly sampled 1 million Medicare beneficiaries undergoing a TJR (identified with ICD-9 procedure codes of 81.51 for total hip replacement or 81.54 for total knee replacement) between 2010 and 2014 with no record of a TJR in the year prior. Of these, we excluded patients who were younger than 65 years of age, who did not have continuous enrollment in Medicare Parts A, B, and D for a 12-month baseline period immediately preceding their TJR, who had both hip and knee replacement performed on the same date, or who had a diagnosis of cancer during the baseline period. We further restricted the study population to osteoarthritis patients by excluding patients with hip fracture (which could be the reason for THR) as well as patients without diagnosis codes for osteoarthritis during the baseline period. For patients undergoing multiple TJR procedures in the study period, we only included the first procedure. Patients meeting all our inclusion criteria were grouped by their PCSA, and the data were analyzed with PCSA as the unit of analysis. To reliably estimate the outcome, which is measured as percentage (see the next Section for details) in each PCSA, we excluded PCSAs with ≤ 25 patients from the analysis.
Outcome variable
We defined long-term opioid use for each patient in a 360 day period immediately preceding TJR based on prescription dispensing for any opioid with day supply totaling ≥ 90 days in accordance with the long-term opioid use definition outlined by the CDC (4). The opioids considered in our analysis included hydrocodone, dihydrocodeine, oxycodone, propoxyphene, tramadol, meperidine, hydromorphone, morphine, fentanyl, methadone, pentazocine, tapentadol, levorphanol, and oxymorphone. The percentage of patients on long-term opioid therapy within each PCSA was the main outcome variable of interest.
Independent variables of interest
Primary care provider (PCP) access: We quantified PCP access on PCSA level based on the total number of clinically active PCPs per 1,000 Medicare beneficiaries. This variable was categorized variable into 4 quartiles (Q1: 0–3.6, Q2: 3.6–5.5, Q3: 5.5–8.6, Q4:>8.6).
Rheumatologist access: The total number of rheumatologists in each PCSA was determined based on the ZIP code recorded on their business addresses listed in the list received from American College of Rheumatology. We quantified rheumatologist access on PCSA level based on the total number of practicing rheumatologists per 1,000 Medicare beneficiaries as a categorical variable. As >70% of the included PCSAs did not have any practicing rheumatologists, we created one category of no rheumatologist access and created three additional categories from PCSAs with at least 1 practicing rheumatologist based on tertiles (<0.15, 0.15–0.29, >0.29).
Geographic region: We identified states for each PCSA from the PCSA data files and used state indicator as an independent variable of interest. New York was selected as the reference state because of a large sample size and consistently low opioid use reported in previous investigations (12, 16).
Covariates
PCSA level case-mix adjustment:
To account for varying case-mix from one PCSA to another, we aggregated patient demographics at the PCSA-level, including age, race (white or non-white), and gender; dual enrollment in Medicare-Medicaid; type of joint replacement surgery (total knee or hip replacement); prevalence of other pain-related and co-morbid condition diagnoses that may influence prescription opioid use including back pain, neuropathic pain, migraine, rheumatoid arthritis, fractures, falls, depression, anxiety, bipolar disorder, drug abuse, and alcohol abuse. Further, as a marker for patients’ general health, we accounted for a comorbidity score that combines 20 chronic conditions from the Charlson and Elixhauser systems including metastatic cancer, congestive heart failure, dementia, renal failure, weight loss, hemiplegia, alcohol abuse, any tumor, cardiac arrythmias, chronic pulmonary disease, coagulopathy, complicated diabetes, deficiency anemias, fluid and electrolyte disorders, liver disease, peripheral vascular disorders, psychosis, pulmonary circulation disorders, HIV, and hypertension (17). Additionally, we included two indicators for socioeconomic status in each PCSA: percentage of population living under the federal poverty limit and percentage of population older than 25 years with less than high school education, to account for aggregate poverty and literacy levels in PCSAs.
State level policy interventions:
It is important to consider various state-level policies in the analysis to isolate the unexplained variation in long-term opioid use by state from the effect of these policies. Therefore, we accounted for the rigor of prescription drug monitoring programs (PDMP) and presence of medical marijuana policies in a state, both of which could have an impact on long-term prescription opioid use. Operationally, we identified the dates of implementation of these policies within each state. Additionally, for PDMPs, we identified program rigor and categorized state programs into three categories: 1) high rigor- included states that required prescribers to check PDMP database each time prior to prescribing opioids to all patients or to chronic pain patients, 2) low rigor- included states where an operational PDMP was available, but no requirement for checking PDMPs was implemented or checking PDMP was only required prior to prescribing opioids for the first time, 3) no operational PDMP. For medical marijuana, we only created a binary variable indicating presence or absence of state-laws for legally obtaining medical marijuana. To account for implementation of these policies over time during our study period (2010–2014), we first identified whether the concerned policy had been implemented by the time we started measuring each patients’ long-term opioid use to define policy-exposed patients. We then aggregated this information at the PCSA level by assigning each PCSA to a specific level of policy exposure when a majority of the patients (>50%) within that PCSA were exposed to that specific level of the policy. Policy implementation dates and details regarding the rigor of PDMPs were derived from the Prescription Drug Abuse Policy System (PDAPS) web portal (18), which is an National Institute on Drug Abuse supported initiative to track key state laws related to prescription drug abuse.
Statistical analysis
Patient characteristics were reported using descriptive statistics among opioid non-users, short-term users (<90 days), and long-term users (≥90 days) in the year leading up to TJR. Characteristics of opioid use, including total day supply, average daily dose in morphine milligram equivalents (MME), total number of different agents used, and frequency of the most frequently used agents, were described for short-term and long-term opioid users. The average daily dose was also reported in categories of <50, 50–90, and ≥90 MME. These categories were selected based on the CDC guidelines, which define average daily dose of 50–90 MME as the range where a careful assessment of risk-benefit is suggested and ≥90 MME as the range that should be avoided (4). We also described the case-mix across 4,080 PCSAs using descriptive statistics.
The proportion of long-term opioid users and density of clinically active PCPs and rheumatologists within each PCSA were plotted on the US map to visually demonstrate geographic variation in opioid use and healthcare access. To quantify the impact of access and geography on long-term opioid use rates, a generalized linear regression model with identity link was constructed with PCSA as the unit of analysis and percentage of long-term opioid users in each PCSA as the dependent variable. To account for the hierarchical structure of the data where PCSAs are clustered within states, we used a multi-level model. Level 1 variables included PCSA-level variables to adjust for case-mix and were modeled as fixed effects. Level 2 variables included state effects, which were modeled as random-effects, and policy interventions, which were modeled as fixed effects. The statistical analysis was conducted in SAS with PROC GLIMMIX in Version 9.4 (SAS Institute, Cary, NC).
Role of the Funding Source
The funding agency played no role in design or conduct of this study.
RESULTS
Study population
A total of 358,121 osteoarthritis patients with an average age of 74 years who underwent TJR met all our inclusion criteria and contributed to this analysis (Figure 1). A majority of the patients had white race (91.9%) and were females (67.8%). A total of 59,387 (16.5%) patients were identified as long-term opioid users (≥ 90 days) and 152,308 (42.3%) were identified as short-term users (<90 days). Over the study years from 2011 to 2014, the proportion of TJR patients using long-term opioids remained relatively stable (16.8% in 2011, 16.8% in 2012, 16.6% in 2013, and 16.3% in 2014). Table 1 summarizes patient characteristics across non-users, short-term users, and long-term users. The prevalence of pain-related conditions as well as the comorbidity burden were substantially higher among long-term opioid users compared to short-term and non-users.
Figure 1-.
Cohort selection flow-chart
Table 1-.
Patient-level descriptive statistics and opioid use characteristics in the year leading up to total joint replacement in a cohort of severe osteoarthritis patients among opioid non-users, short-term users (<90 days) and long-term users (≥90 days), Medicare data 2010–2014
| Non-users | Short-term users | Long-term users | |
|---|---|---|---|
| Total patients | 146426 | 152308 | 59387 |
| Patient characteristics | |||
| Average age ± SD | 74.3 (5.9) | 73.9 (5.9) | 73.5 (6.1) |
| Male gender (n (%)) | 52480 (35.8) | 48661 (31.9) | 14421 (24.3) |
| White race (n (%)) | 136213 (93.0) | 139730 (91.7) | 53119 (89.4) |
| Medicare-Medicaid dually eligible (n (%)) | 12089 (8.3) | 17929 (11.8) | 15017 (25.3) |
| Type of total joint replacement surgery | |||
| Total knee replacement (n (%)) | 105188 (71.8) | 101159 (66.4) | 37827 (63.7) |
| Total hip replacement (n (%)) | 41238 (28.2) | 51149 (33.6) | 21560 (36.3) |
| Pain related diagnoses and other comorbid conditions | |||
| Rheumatoid arthritis (n (%)) | 4603 (3.1) | 7374 (4.8) | 5862 (9.9) |
| Neuropathic pain (n (%)) | 29244 (20.0) | 49911 (32.8) | 27106 (45.6) |
| Back pain (n (%)) | 57840 (39.5) | 84146 (55.2) | 42393 (71.4) |
| Migraine (n (%)) | 8911 (6.1) | 14375 (9.4) | 8062 (13.6) |
| Falls (n (%)) | 4809 (3.3) | 9664 (6.3) | 6071 (10.2) |
| Fractures (n (%)) | 7209 (4.9) | 14549 (9.6) | 7799 (13.1) |
| Anxiety (n (%)) | 10980 (7.5) | 17579 (11.5) | 12027 (20.3) |
| Depression (n (%)) | 14165 (9.7) | 23163 (15.2) | 16212 (27.3) |
| Drug abuse (n (%)) | 67 (0) | 192 (0.1) | 506 (0.9) |
| Bipolar disorder (n (%)) | 988 (0.7) | 1505 (1) | 1268 (2.1) |
| Alcohol abuse (n (%)) | 919 (0.6) | 1468 (1) | 1003 (1.7) |
| Average omorbidity score ± SD | 0.6 (1.6) | 0.8 (1.8) | 1.3 (2.1) |
| Opioid use characteristics | |||
| Day supply of prescription opioids (Median, interquartile range) | - | 15 (6–34) | 218 (142–307) |
| Average daily dose of opioids in morphine mg equivalents (Median, interquartile range) | - | 30 (20–42.9) | 27.3 (17.8–41.7) |
| Average daily dose categories (n (%)) | - | ||
| <50 morphine mg equivalents | - | 128145 (84.1) | 48106 (81.0) |
| 50–90 morphine mg equivalents | - | 19911 (13.1) | 6836 (11.5) |
| >=90 morphine mg equivalents | - | 4252 (2.8) | 4445 (7.5) |
| Total number of different agents used (Median, interquartile range) | - | 1 (1–2) | 2 (1–2) |
| Five most commonly used agents | - | ||
| Tramadol | - | 55968 (36.8) | 27224 (45.8) |
| Hydrocodone | - | 58134 (38.2) | 17793 (30.0) |
| Oxycodone | - | 32998 (21.7) | 19154 (32.2) |
| Propoxyphene | - | 3018 (2.0) | 1953 (3.3) |
| Fentanyl | - | 731 (0.5) | 3671 (6.2) |
Table 1 further provides details regarding opioid use characteristics in long-term and short-term opioid users. The median (interquartile range) day supply for prescription opioids was 218 (142–307) among long-term users and 15 (6–34) among short-term users. A total of 19.0% of the long-term users and 15.9% of the short-term users consumed average daily dose of ≥ 50 MME. Compared to short-term opioid users, a notably higher use of tramadol (45.8% versus 36.8%), oxycodone (32.2% versus 21.7%), and fentanyl (6.2% versus 0.5%) was noted among long-term users.
A total of 4,080 PCSAs or 57.1% of the 7,144 total PCSAs defined in the US by HRSA were represented in our analysis. Average number of patients in each PCSA was 87.7 and ranged from 26 to 1,038. The case-mix across included PCSAs was heterogeneous with varying proportions of pain-related diagnoses, a wide range of socioeconomic status as well as access to healthcare providers (Table 2). Across the 4,080 included PCSAs, the average % of long-term opioid users was 17.2 (SD 7.8) and ranged from a low of 0% to a high of 60%. Appendix Figure 1 shows the distribution of the percentage of long-term opioid users across included PCSAs.
Table 2:
Primary care service area (PCSA) level descriptive statistics for the case-mix across 4,080 PCSAs in a cohort of severe osteoarthritis patients, Medicare data 2010–2014
| Population characteristic within each PCSA | Mean (SD) | Minimum | Maximum |
|---|---|---|---|
| Sample size | 87.7 (84.5) | 26.0 | 1038.0 |
| Demographics | |||
| Average age (years) | 74 (1.1) | 69.5 | 78.4 |
| Percent white | 92 (11.9) | 0.0 | 100.0 |
| Percent male | 32.4 (7.4) | 3.4 | 63.0 |
| Percent Medicare-Medicaid dually eligible | 13.6 (12.2) | 0.0 | 92.2 |
| Type of joint replacement surgery | |||
| Percent total knee replacement | 68.7 (8.1) | 38.5 | 96.3 |
| Percent total hip replacement | 31.3 (8.1) | 3.7 | 61.5 |
| Pain related diagnoses and other comorbid conditions | |||
| Percent rhematoid arthritis | 5 (3.4) | 0.0 | 36.8 |
| Percent neuropathic pain | 29.3 (7.5) | 7.1 | 68.5 |
| Percent back pain | 51.3 (8.1) | 20.5 | 85.6 |
| Percent migraine | 8.7 (4.2) | 0.0 | 30.2 |
| Percent falls | 5.8 (3.5) | 0.0 | 28.6 |
| Percent fracture | 8.2 (3.9) | 0.0 | 31.3 |
| Percent anxiety | 11.4 (5) | 0.0 | 38.5 |
| Percent depression | 14.9 (5.7) | 0.0 | 41.9 |
| Percent bipolar disorder | 1 (1.4) | 0.0 | 10.3 |
| Percent drug abuse | 0.2 (0.7) | 0.0 | 10.7 |
| Percent alcohol abuse | 0.9 (1.3) | 0.0 | 11.5 |
| Average combined comorbidity score | 0.8 (0.4) | −0.3 | 3.2 |
| Socioeconomic status variables | |||
| Percent below the poverty line | 9.5 (5.6) | 0.0 | 41.6 |
| Percent below highschool education | 5.6 (4.3) | 0.0 | 39.7 |
| Provider access | |||
| Number of primary care providers/1000 Medicare beneficiaries | 7 (6.1) | 0.0 | 116.6 |
| Number of rheumatologists/1000 Medicare beneficiaries | 0.1 (0.3) | 0.0 | 7.0 |
Association of geographic region and access to healthcare providers with long-term opioid use
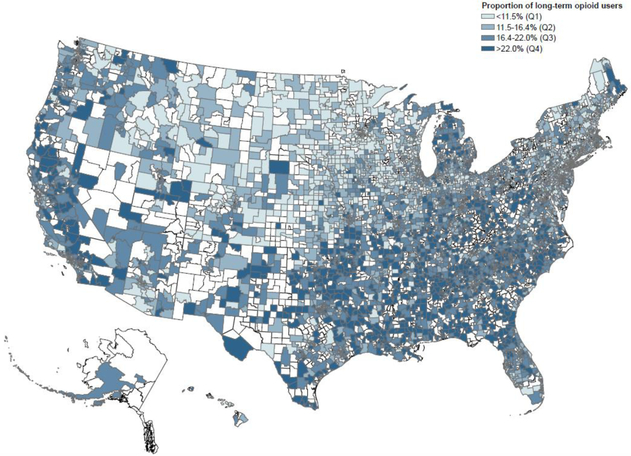
Figure 2 summarizes long-term opioid use by PCSAs in our study population across the US. PCSAs with a higher proportion of long-term opioid users were generally in the South, and PCSAs with a lower proportion of long-term opioid users were typically from the Northeast and the Midwest. Appendix Figure 2 summarizes healthcare provider access by PCSAs in our study population across the US. The distribution of PCPs and rheumatologists was noted to be more concentrated in the Northeast and the Midwest.
Figure 2-.
Long-term opioid use rates prior to total join replacement in primary care service areas across the United States, Medicare data 2010–2014
* Footnote- Primary care service areas in white did not contribute patients to this analysis.
The unadjusted mean % of long-term opioid users increased monotonically from the PCSA categories representing highest to lowest concentration of PCPs (16.0% to 18.3%) and rheumatologists (15.4% to 17.6%) (Table 3). Variation in the unadjusted mean % of long-term opioid users was substantial across states, ranging from a low of 8.9% in Minnesota to a high of 26.4% in Alabama (Appendix Table 1 provides this information for all states and DC).
Table 3-.
Association of healthcare access and geographic region with long-term pre-operative opioid use rates in in a cohort of severe osteoarthritis patients, Medicare data 2010–2014
| Independent variables of interest | N (%) of PCSAs (total N= 4,080) | Unadjusted mean (SD) of % long-term opioid users | Adjusted*mean difference in proportion (percentage points) of long term opioid users (95% confidence interval) |
|---|---|---|---|
| State† | |||
| New York | 181 (4.4) | 12.3 (6.1) | Reference |
| West Virginia | 26 (0.6) | 24.9 (8.6) | 10.3 (7.9–12.6) |
| Alabama | 69 (1.7) | 26.4 (7) | 10.2 (7.3–13.2) |
| Georgia | 111 (2.7) | 22.9 (8.2) | 9.4 (6.8–12) |
| Kentucky | 81 (2) | 24 (9.6) | 8.8 (7.2–10.4) |
| Lousiana | 53 (1.3) | 25 (8.6) | 8.3 (6.5–10.1) |
| Oklohama | 77 (1.9) | 22.3 (6.1) | 8.2 (6.6–9.8) |
| North Carolina | 135 (3.3) | 22.6 (8.1) | 7.9 (5.5–10.3) |
| Virginia | 123 (3) | 17 (6.3) | 7.2 (5.8–8.7) |
| Inidiana | 128 (3.1) | 19.1 (6.1) | 7.2 (5.8–8.6) |
| Mississipi | 67 (1.6) | 25 (7.7) | 7.1 (4.5–9.7) |
| Number of primary care providers/1000 Medicare beneficiaries in the primary care service area | |||
| >8.6 (Q4) | 1020 (25) | 16 (7.2) | Reference |
| 5.5–8.6 (Q3) | 1020 (25) | 16.4 (7.2) | 0.9 (0.4–1.4) |
| 3.6–5.5 (Q2) | 1020 (25) | 18.3 (8.0) | 1.7 (1.1–2.2) |
| <3.6 (Q1) | 1020 (25) | 18.3 (8.5) | 1.4 (0.8–2) |
| Number of rheumatologists/1000 Medicare beneficiaries in the primary care service area | |||
| >0.29 (T3) | 381 (9.4) | 15.4 (7.2) | Reference |
| 0.15–0.29 (T2) | 381 (9.4) | 16.3 (6.4) | 0.4 (−0.4–1.2) |
| <0.15 (T1) | 381 (9.3) | 17.5 (6.0) | 0.1 (−0.8–0.9) |
| None | 2938 (72) | 17.6 (8.2) | 0.6 (−0.1–1.3) |
Adjusted for case-mix including age, race, gender, dual Medicare-Medicaid enrollment, pain related diagnoses, co-morbid conditions, type of joint replacement surgery, and socioeconomic characteristics in each primary care service area as well as state level policies including rigor of prescription drug monitoring programs and legal availability of medical marijuana.
Based on the highest adjusted estimates, top 10 states presented in descending order of the magnitude of the effect. New York was selected as the reference based on low proportion of long term opioid users and a large sample size. Estimates for rest of the states are provided in Appendix Table 1.
In the regression model adjusted for case-mix, the variation across states in long-term opioid use rates persisted. Compared to the reference state (New York), the mean difference in long-term opioid users in percentage points was more than 10 for West Virginia and Alabama (Table 3 provides mean difference for 10 states with the highest mean differences). A total of 31 states had statistically significantly higher rates of long-term opioid users compared to New York, and in 19 states, the rates were similar (see Appendix Table 2 for results from the full regression model). The adjusted mean difference (95% CI) in long-term opioid users between PCSAs with highest (>8.6) versus lowest (<3.6) concentration of PCPs per 1,000 beneficiaries was 1.4% (0.8%−2.0%) and highest (>0.29) versus lowest (0) concentration of rheumatologists was 0.6% (−0.1%−1.3%) (Table 3).
DISCUSSION
In this large observational cohort study of Medicare enrollees with osteoarthritis undergoing TJR, we noted that one in six patients used long-term prescription opioids (≥ 90 days) for pain management in the year leading up to the TJR, with an average duration of approximately 7 months. Nearly 20% of the long-term users consumed an average daily dose of ≥50 MME, a range that is identified by the recent CDC guidelines as potentially imparting a high risk of opioid-related harms (4). Long-term opioid use varied substantially by state and had only a modest association with low access to primary care providers.
Although osteoarthritis is one of the most common reasons for chronic pain in the US, long-term prescription opioid use is not well studied in this population. One previous study using self-reported medication use data from the Medicare Current Beneficiary Surveys noted that 40% of osteoarthritis patients interviewed in 2009 used opioids at least once, but this study did not investigate the length of opioid use in these patients (9). Among all Medicare part D enrollees; the prevalence of long-term prescription opioid use was reported to be 7.3% in 2012 (19). The estimates for long-term opioid use observed in this study of Medicare patients with severe osteoarthritis from 2010–2014 are more than two-fold higher than this previously reported estimate. Thus, our study identifies patients with advanced OA as a patient population with substantially high rates of long-term prescription opioid use compared to an average Medicare enrollee. Further, we noted that one in five long-term users consumed an average daily dose of ≥50 MME and the average length of treatment with opioids was approximately 7 months in the year prior to TJR among long-term users. These observations are important to consider in light of results from a recent randomized controlled trial suggesting questionable effectiveness of long-term prescription opioids in treatment of chronic pain (3). In patients with severe OA, a special emphasis on periodically monitoring prescription opioid use is required to ensure benefits outweigh risks at prescribed doses.
We noted a substantial geographic variation in use of long-term prescription opioids in this study. This finding is in line with earlier studies, which also report wide geographic variation in prescribing of opioids with lower rates on average in the Northeast and Midwest and generally higher rates in the South (12, 16, 19–21). Using a comprehensive risk-adjustment approach, we further evaluated whether this geographic variation could be explained by differences in access to healthcare providers, differences in patient populations, differences in socioeconomic characteristics, or differences in policy interventions across states. We observed that state of residence had an independent association with rates of long-term opioid use after adjusting for these differences, suggesting that regional prescribing practices play a key role in determining rates of long-term opioid use in this population. This finding suggests that geographically targeted interventions to ensure widespread dissemination and implementation of safe opioid prescribing guidelines are necessary to make a meaningful impact on prescribing practices. While our study did not observe an association between state-level policies, including PDMPs and legal medical marijuana, and rates of long-term prescription opioid use (Appendix Table 2), this finding should not be interpreted as causal. Evidence regarding the impact of these policies on overall opioid prescribing is mixed with some studies indicating a modest reduction (22, 23), while some suggesting no consistent reduction in opioid prescribing rates as a result of implementation of these policies (24, 25). However, it must be noted that our study was not designed to evaluate the direct impact of these policies on rates of long-term prescription opioid use. Instead, our focus was on ruling out variation in these policies as a potential explanation for state to state variation in long-term prescription opioid rates. Future research employing more suitable methods for policy evaluations such as a controlled interrupted time-series design (26) should be considered to evaluate the impact of introduction of specific policies on long-term prescription opioid use in patient with severe osteoarthritis.
Further, our study also adds information to the literature regarding the complex association between healthcare access and use of prescription opioids in patients with chronic pain. Some previous studies have reported a positive correlation between number of clinically active practitioners in a geographic area and amount of opioids prescribed (11, 12), suggesting that higher access to multiple providers may make it easier for patients to find a provider readily willing to prescribe opioids or to seek opioid prescriptions from more than one providers. On the contrary, we noted a modestly negative association between the number of active PCPs in a PCSA and long-term opioid use rates and no association between number of rheumatologists and long-term opioid use rates in this patient population of elderly individuals suffering from severe osteoarthritis. Although, the magnitude of the association between higher PCP concentration and lower long-term opioid use was small, the contrast from previous studies suggests that factors driving long-term opioid use in chronic pain patients may be unique and easier access may not be a risk factor for higher opioid use in these patients.
There are some important strengths of this study. First, it describes rates of long-term opioid use in a nationally representative population of Medicare enrollees with severe osteoarthritis. We conducted comprehensive risk-adjustment based on patient demographics, co-morbid conditions, as well as variation in state-level policies. Next, since all patients included in this study had full pharmacy and medical benefits under fee for service Medicare during the study period, confounding by patient-level financial factors, such as differential health plan coverage of medications or differences in copays, is likely to be limited. However, there are some limitations that deserve mention. First, we did not have data on pain severity or pain related functioning for patients in this cohort, which makes residual confounding possible. However, restricting the study population to TJR recipients may have limited such confounding by ensuring inclusion of a somewhat homogenous population seeking pain relief for knee and hip osteoarthritis. Further, the data used in this study are not recent due to lag in release of Medicare claims by CMS. Therefore, our study may not have captured more recent shifts (after 2014) in prescription opioid use patterns in this population in response to the growing awareness about the opioid epidemic in the US. Another limitation of the current study is that we did not evaluate whether TJR changes opioid use in these patients. Future research should address the impact of pre-TJR opioid use on post-surgical functional outcomes as well as the impact of TJR on post-surgical opioid use. Next, we did not have complete information on access to other healthcare services such as physical therapy which precluded evaluation of the impact of access to these services on long-term opioid use in this population. An additional limitation is that we did not focus on variation in prescribing practices across individual providers, which may be important to consider while designing interventions. Finally, by including a population of TJR recipients and evaluating the opioid use prior to TJR, our study may underestimate the extent of prescription opioid use in this population due to exclusion of severe OA patients who may die without ever getting TJR. Further, there exists a substantial racial disparity in the use of TJR, with rates among blacks approximately 40% lower compared to whites (27); therefore, the restriction of study population to TJR recipients also limits the generalizability of our estimates.
In conclusion, we observed frequent use of long-term opioids in elderly patients with severe knee or hip osteoarthritis prior to TJR. Importantly, substantial statewide variation in rates of treatment with long-term opioid therapy was noted in this population, which was not fully explained by differences in access to PCPs or rheumatologists, variation in patient characteristics, or state-level policies including PDMPs and legalized medical marijuana. These findings suggest that geographically targeted dissemination strategies for safe opioid prescribing guidelines may be required to address the high use observed in certain states.
Supplementary Material
Acknowledgments
Funding:
This study was supported through funding from the National Institute for National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS- R01AR069557).
Conflict of interest/Financial disclosures:
SCK has received research grants to the Brigham and Women’s Hospital from Roche/Genentech, Pfizer, Bristol-Myers Squibb, Merck, and AstraZeneca for unrelated studies. RJD has received research grants to the Brigham and Women’s Hospital from Merck and Vertex for unrelated projects. DHS has received research grants to the Brigham and Women’s Hospital from Lilly, Pfizer, AstraZeneca, Genentech, Amgen and CORRONA. DHS serves in an unpaid role on a trial sponsored by Pfizer unrelated to the current study.
References
- 1.Volkow N, Benveniste H, McLellan AT. Use and Misuse of Opioids in Chronic Pain. Annu Rev Med. 2018;69:451–65. [DOI] [PubMed] [Google Scholar]
- 2.Chou R, Turner JA, Devine EB, Hansen RN, Sullivan SD, Blazina I, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(4):276–86. [DOI] [PubMed] [Google Scholar]
- 3.Krebs EE, Gravely A, Nugent S, Jensen AC, DeRonne B, Goldsmith ES, et al. Effect of Opioid vs Nonopioid Medications on Pain-Related Function in Patients With Chronic Back Pain or Hip or Knee Osteoarthritis Pain: The SPACE Randomized Clinical Trial. JAMA. 2018;319(9):872–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dowell D, Haegerich TM, Chou R. CDC Guideline for Prescribing Opioids for Chronic Pain--United States, 2016. JAMA. 2016;315(15):1624–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control (CDC). Osteoarthritis http://www.cdc.gov/arthritis/basics/osteoarthritis.htm. Accessed February 12, 2018.
- 6.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465–74. [DOI] [PubMed] [Google Scholar]
- 7.Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harris WH, Sledge CB. Total hip and total knee replacement (1). N Engl J Med. 1990;323(11):725–31. [DOI] [PubMed] [Google Scholar]
- 9.Wright EA, Katz JN, Abrams S, Solomon DH, Losina E. Trends in prescription of opioids from 2003–2009 in persons with knee osteoarthritis. Arthritis care & research. 2014;66(10):1489–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMik DE, Bedard NA, Dowdle SB, Burnett RA, McHugh MA, Callaghan JJ. Are We Still Prescribing Opioids for Osteoarthritis? The Journal of arthroplasty. 2017;32(12):3578–82. e1. [DOI] [PubMed] [Google Scholar]
- 11.Guy GP Jr., Zhang K, Bohm MK, Losby J, Lewis B, Young R, et al. Vital Signs: Changes in Opioid Prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. 2017;66(26):697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald DC, Carlson K, Izrael D. Geographic variation in opioid prescribing in the U.S. J Pain. 2012;13(10):988–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Primary Care Service Area Data Download – 2010 (Census Tract Basis). Health Resources & Services Administration, US Department of Health & Human Services; Available at https://datawarehouse.hrsa.gov/data/datadownload/pcsa2010download.aspx, accessed on 8-15-2017. . [Google Scholar]
- 14.Goodman DC, Mick SS, Bott D, Stukel T, Chang CH, Marth N, et al. Primary care service areas: a new tool for the evaluation of primary care services. Health Serv Res. 2003;38(1 Pt 1):287–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polinski JM, Brookhart MA, Ayanian JZ, Katz JN, Kim SC, Lii J, et al. Relationships between driving distance, rheumatoid arthritis diagnosis, and disease-modifying antirheumatic drug receipt. Arthritis Care Res (Hoboken). 2014;66(11):1634–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Desai RJ, Hernandez-Diaz S, Bateman BT, Huybrechts KF. Increase in prescription opioid use during pregnancy among Medicaid-enrolled women. Obstet Gynecol. 2014;123(5):997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gagne JJ, Glynn RJ, Avorn J, Levin R, Schneeweiss S. A combined comorbidity score predicted mortality in elderly patients better than existing scores. J Clin Epidemiol. 2011;64(7):749–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.9-January-2018. PDAPSPAahpoA. [Google Scholar]
- 19.Kuo YF, Raji MA, Chen NW, Hasan H, Goodwin JS. Trends in Opioid Prescriptions Among Part D Medicare Recipients From 2007 to 2012. Am J Med. 2016;129(2):221 e21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zerzan JT, Morden NE, Soumerai S, Ross-Degnan D, Roughead E, Zhang F, et al. Trends and geographic variation of opiate medication use in state Medicaid fee-for-service programs, 1996 to 2002. Med Care. 2006;44(11):1005–10. [DOI] [PubMed] [Google Scholar]
- 21.Paulozzi LJ, Mack KA, Hockenberry JM. Vital signs: variation among States in prescribing of opioid pain relievers and benzodiazepines - United States, 2012. MMWR Morb Mortal Wkly Rep. 2014;63(26):563–8. [PMC free article] [PubMed] [Google Scholar]
- 22.Bao Y, Pan Y, Taylor A, Radakrishnan S, Luo F, Pincus HA, et al. Prescription Drug Monitoring Programs Are Associated With Sustained Reductions In Opioid Prescribing By Physicians. Health affairs (Project Hope). 2016;35(6):1045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wen H, Hockenberry JM. Association of Medical and Adult-Use Marijuana Laws With Opioid Prescribing for Medicaid Enrollees. JAMA internal medicine. 2018;178(5):673–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brady JE, Wunsch H, DiMaggio C, Lang BH, Giglio J, Li G. Prescription drug monitoring and dispensing of prescription opioids. Public health reports (Washington, DC : 1974). 2014;129(2):139–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finley EP, Garcia A, Rosen K, McGeary D, Pugh MJ, Potter JS. Evaluating the impact of prescription drug monitoring program implementation: a scoping review. BMC health services research. 2017;17(1):420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez Bernal J, Cummins S, Gasparrini A. The use of controls in interrupted time series studies of public health interventions. International journal of epidemiology. 2018. [DOI] [PubMed] [Google Scholar]
- 27.Singh JA, Lu X, Rosenthal GE, Ibrahim S, Cram P. Racial disparities in knee and hip total joint arthroplasty: an 18-year analysis of national Medicare data. Ann Rheum Dis. 2013: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.