Structured Abstract
Objective:
To determine normative values for heart rate patterns in healthy fetuses.
Methods:
This research is from the Safe Passage Study conducted by the Prenatal Alcohol and SIDS and Stillbirth (PASS) Network. A standardized protocol assessed fetal heart rate (FHR), heart rate variability (HRV), and movement from 1,655 fetuses at three time points during gestation (20–24 weeks, 28–32 weeks, 34–38 weeks gestation).
Results:
FHR decreased while HRV increased over gestation. At the latter two ages, males had significantly lower FHR than females while there were no sex differences in FHR at 20–24 weeks. When accounting for fetal state during late gestation (34–28 weeks), we found that males had significantly lower FHR than females in the active fetal state only.
Conclusion:
Results demonstrate significant state, gestational age, and sex related changes in cardiac activity, somatic activity, and autonomic function as the fetus approaches birth.
Decreases in fetal heart rate (FHR) and increases in heart rate variability (HRV) during gestation have been well documented (1–7). HRV is the variation in the heart’s beat-to-beat intervals as a result of autonomic regulation of HR (8). In early gestation FHR is predominately under sympathetic control, whereas in later gestation the maturation of parasympathetic activity is responsible for the gradual decrease in FHR and increase in HRV (9, 10). The analysis of HR, HRV, and their coupling with movement allow for early assessment of perinatal development in physiological function (11, 12). Measures of change in fetal autonomic system (ANS) activity, which is modulated by fetal behavioral state, serve as a practical and non-invasive index for assessment of fetal brain functional development. These measures have also been implemented to study the impact of maternal clinical disorders, exposures, or other occurrences during gestation such as maternal depression (13), maternal stress (14), maternal smoking (15–19), maternal alcohol consumption (20–23), and the effect of aerobic exercise on the developing fetus (24). Measures of HR, HRV, and movement vary significantly by fetal behavioral state (25, 26), therefore accounting for state is imperative for the identification of potential developmental abnormalities.
The Prenatal Alcohol in SIDS and Stillbirth (PASS) Research Network was established to investigate the impact of prenatal exposure to alcohol, smoking, and other environmental factors on sudden infant death syndrome (SIDS), stillbirth, and fetal alcohol spectrum disorders (FASD). The Safe Passage Study conducted by the PASS Network enrolled approximately 12,000 maternal-fetal dyads in the Northern Plains of the US (North and South Dakota) and Cape Town, South Africa. The Safe Passage Study aimed to determine the role of exposure to alcohol, cigarettes, recreational drugs, and other environmental stressors on fetal autonomic functioning and subsequent developmental ability at 12 months in a subset of children. In addition to serving as potential markers for SIDS, stillbirth, or FASD, ANS measures of HR and HRV have been associated with cognitive and behavioral outcomes in early childhood (27, 28). The aims of this report are to: (a) present details of the standardized fetal assessment protocols used in the Safe Passage Study; (b) establish normative values for fetal heart rate and fetal body movement measures in medically healthy fetuses with maternal reports of no exposure to alcohol, smoking, or recreational drug use at any point during pregnancy; (c) determine fetal sleep state dependent differences on FHR, standard deviation of HR (SD-HR), and fetal body movements (FMOV); (d) identify sex dependent differences in FHR, SD-HR, and FMOVs. The fetal assessment measures and normative values presented in the current report provide meaningful information to researchers who lack normative control data for between-group comparisons and provide criteria for subsequent analyses focused on in-utero exposure or other research questions within and beyond the Safe Passage Study.
METHODS
Participants:
Institutional Review Board approvals for the Safe Passage Study were obtained from sponsoring organizations at the participating clinical sites in the Northern Plains (North and South Dakota) and South Africa, as well as for the Data Coordinating Center and the Physiologic Assessment Center. Consent was obtained from all participants in the Safe Passage Study. Participants in the current analyses include fetuses whose mothers self-reported on prenatal alcohol, smoking, and recreational drug exposures during in-person interviews. Additional exposure and medical history were gathered through maternal medical chart abstractions. This cohort is limited to mothers who reported no consumption of alcohol, smoking, and nor recreational drug use at any time during pregnancy. Additional exclusionary criteria consisted of preterm birth (< 37 weeks gestation), multiple births, NICU admission, or maternal illness during pregnancy. All fetuses included were singletons who delivered at term age (37 to 41 weeks gestation). A total of 1,655 participants met these criteria.
Data Acquisition Protocol:
Fetal assessments were attempted within three gestational age (GA) ranges: GA1: 20 – 24 weeks gestation, GA2: 28 – 32 weeks gestation, and GA3: 34 – 38 weeks gestation. All studies were completed between 9am and 4pm. Approximately 1 – 2 hours postprandial, mothers were seated in a reclining chair or were lying supine with a 15° lateral tilt and fitted with the recording equipment. The GA1 and GA2 assessments were of 30 minutes duration; the GA3 assessment was 50 minutes. Mothers were undisturbed for the first 20 minutes of data collection and were then asked to answer questions about alcohol and smoking habits, depression and anxiety symptoms, and recreational drug use through a clinical interview. FHR and movement (FMOV) data were collected using a single wide-array Doppler transducer placed on the maternal abdomen connected to a Toitu MT-320 or a MT-516 model Doppler actocardiograph (Toitu Company, Ltd., Toyko, Japan). FHR and FMOV signals were digitized at 20 Hz using a custom built physiological data acquisition hardware and software system (DATACQ, Medelex, Inc) interfaced to a laptop computer.
Quality Control (QC):
Oversight of data acquisition was accomplished through QC protocols implemented by the clinical sites and by site visits (2–3 visits per year) during which equipment was inspected and, when possible, test sessions were observed. These visits were supplemented by remote viewing of assessments and monthly calls with the site research staff. Although data were collected for research purposes, the protocol allowed research staff to refer mothers for clinical follow up in cases where the fetal monitor indicated fetal bradycardia (<110 bpm) for 10 minutes or fetal tachycardia (>160 bpm) for 20 minutes. Indications for the notification of the clinician or referral were also possible for ruptured membranes, abdominal pain, uterine contractions occurring over the course of one hour at < 36 weeks gestation, reduced fetal body movements, vaginal bleeding, severe headache, suicidal ideation, or ultrasound findings of poor fetal growth and/or congenital abnormalities. There were no such referrals in this normative cohort. Prior to processing, signals were displayed to ascertain study duration, data range, occurrence of severe artifact, and overall quality. This initial visual screening allowed a rapid assessment of acquisition systems performance and adherence to the study protocol.
Data Processing:
The digitized FHR signal for each DATACQ/fetal monitor system was transformed to beats per minute (bpm) using calibration values (intercepts and slopes) derived by activation of the transducer at known rates using an electronic vibrating metronome. For most systems, the accuracy of the calibrated FHR was confirmed and fine-tuned by comparison with FHR derived from a transabdominal ECG system. FMOV was calibrated for each collection system by determining the minimum and maximum values analog-to-digital units (AD units) across all studies collected on that system and then transforming the signal to an arbitrary 0 to 100 scale.
Data were analyzed using custom MATLAB programs. First, the FHR signal was processed to detect signal loss and artifact. The FHR signal can be lost for periods of time when the fetal heart is not physically within the field of the Doppler transducer. These periods can be in association with fetal and/or maternal movement or with repositioning of the transducer. To identify these potential cases, a FHR signal loss screening was conducted for all participants to identify FHR values < 100 or > 200 bpm. Gaps created by these exclusions were filled via linear interpolation. The resultant FHR series was then low–pass filtered at 3 Hz using a 16–point finite impulse response filter. Artifacts in the filtered FHR were further identified when the absolute sample–to–sample change in FHR exceeded 5 bpm. Values were excluded as artifact until the FHR returned to within 5 bpm of the previous valid value. Gaps created by this criterion were imputed by linear interpolation.
FHR was analyzed in 60 second epochs. The mean, median, and standard deviation of FHR were computed for each epoch using only the non–interpolated values. Epochs were excluded from analyses if 1) FHR signal loss for a gap was > 5 seconds, or 2) cumulative excluded data was > 20 seconds (not including gaps of 2 seconds or less), or 3) if the epoch had > 30% excluded data. The median FMOV was computed for each accepted FHR epoch except in cases where the FMOV signal exceeded the range of the Toitu FMOV amplifier or was not present. These cases comprised 2.5% of all records and were due to equipment failure or user error.
In addition, the cross-correlation of FHR/FMOV (heart rate/movement coupling) and the lag (seconds) between FMOV and FHR derived from the cross–correlation function were computed for each accepted 4 minute FHR epoch. The transformed FHR and FMOV signals were first low-pass filtered between 0.002 and 0.05 Hz using a 400–point FIR filter. The FMOV signal was z–transformed and the FHR was further processed by subtracting the mean from a local regression of 6 seconds and negative FHR values were set to zero (29). Finally, as a further control for artifact, each segment required a minimum FHR/FMOV covariance value of 0.5 and a lag at the maximum cross–correlation greater than –15 sec or less than 0 sec (i.e. changes in FMOV were required to precede changes in FHR).
Fetal State Coding:
For each GA3 record, medians for all of the above variables were computed for 30 second epochs each corresponding to a different FHR pattern for each fetal state. Fetal state was only coded for GA3 records (34 – 38 weeks gestation) since all four distinct states are not fully expressed and classifiable until late gestation (30). FHR patterns were based on those described previously and which were used in combination with fetal body and eye movements for coding fetal behavioral states (30). However, in the present report we did not have accompanying ultrasound to visualize fetal body or eye movements. Therefore, fetal state coding was accomplished by highly trained coders through visual inspection of the FHR tracings only. The four FHR patterns were defined as follows:
FHRP1 - Flat tracings (i.e. narrow oscillation bandwidth) with few and small accelerations (State 1F, also known as the quiet fetal behavioral state)
FHRP2 - Tracings with more frequent and larger accelerations than FHRP1 and with periods between accelerations displaying a greater oscillation band width than FHRP1 (State 2F, also known as the active fetal behavioral state)
FHRP3 - Tracings similar to FHRP1 in that there are few accelerations, but with bandwidths of oscillations broader than FHRP1 and more similar to the bandwidth between accelerations seen in FHRP2 (State 3F, also known as the quiet awake fetal behavioral state)
FHRP4 - Tracing with large and prolonged accelerations often fused into sustained tachycardia (State 4F, also known as the active awake fetal behavioral state)
Statistical Analyses:
For each reported variable, a standard algorithm was applied to determine values that were statistical outliers from the distributions (>1.5 x interquartile above the 75th percentile or <1.5 x interquartile range below the 25th percentile). To determine effects of gestational age and state, mixed models regression analyses (MMRA) were performed with gestational age (or state) as categorical fixed effects and random intercepts for individual participants to account for dependence of repeated measures. Effects of sex at each gestational age and within the 1F and 2F sleep/activity states were analyzed using t-tests. All statistical analyses were conducted in SYSTAT Version 13.
RESULTS
Summary statistics are presented in Table 1 for HR, SD- HR, FMOV, the cross-correlation of FHR/FMOV (heart rate/movement coupling), and the lag (seconds) between FMOV and FHR derived from the cross–correlation analyses. Table 2 shows these same variables and summary statistics by fetal behavioral state at GA3 (34–28 weeks) when states are measurable.
Table 1: Normative Fetal Heart Rate by Gestational Age:
Normative values (mean, SD, 5th and 95th percentiles), collapsing across all sleep/activity states, for fetal heart rate, heart rate variability, movement, heart rate/movement cross correlation, and heart rate movement lag from cross correlation analyses at each of three assessment gestational age ranges (GA1, 20–24 weeks gestation, n=994; GA2, 28–32 weeks gestation, n=507; GA3, 34–38, weeks gestation, n=1481). Outlier cutoffs were set at 1.5 x IQR above the 75th or below the 25th percentile. The 5th and 95th percentiles were determined after removing outliers.
| Variable GA Group | n with # epochs>4 (% of participants) | low cutoff | high cutoff | percentile | ||||
|---|---|---|---|---|---|---|---|---|
| final n | % outliers | mean ± SD | 5th | 95th | ||||
| Heart Rate (bpm) | ||||||||
| GA1 | 776 (78%) | 133.1 | 155.0 | 744 | 4% | 144.1 ±4.8 | 136.3 | 152.5 |
| GA2 | 470 (93%) | 126.3 | 153.1 | 443 | 6% | 139.5 ±5.7 | 130.2 | 148.9 |
| GA3 | 1422(96%) | 122.1 | 152.8 | 1334 | 4% | 136.4 ±6.7 | 124.7 | 147.8 |
| SD of Heart Rate (bpm) | ||||||||
| GA1 | 776 (78%) | 1.4 | 4.5 | 745 | 4% | 2.9 ±0.7 | 1.8 | 4.1 |
| GA2 | 470 (93%) | 1.8 | 5.9 | 444 | 6% | 3.7 ±0.9 | 2.3 | 5.2 |
| GA3 | 1422(96%) | 1.7 | 6.9 | 1353 | 5% | 4.3 ±1.1 | 2.5 | 6.3 |
| Fetal movement (arb units) | ||||||||
| GA1 | 750 (75%) | 0.7 | 3.3 | 713 | 5% | 1.9±0.6 | 1.1 | 2.9 |
| GA2 | 456 (90%) | 0.6 | 3.3 | 438 | 5% | 1.9±0.5 | 1.1 | 2.8 |
| GA3 | 1397(94%) | 0.6 | 3.6 | 1315 | 6% | 2.0 ±0.7 | 1.1 | 3.2 |
| Movement/HR Cross
correlation (no units) | ||||||||
| GA1 | 451 (45%) | 0.54 | 0.88 | 435 | 4% | 0.71 ±0.07 | 0.59 | 0.82 |
| GA2 | 355 (70%) | 0.59 | 0.89 | 339 | 5% | 0.75 ±0.06 | 0.63 | 0.85 |
| GA3 | 1230(83%) | 0.62 | 0.90 | 1178 | 4% | 0.77 ±0.06 | 0.66 | 0.86 |
| LAG of
Movement/HR (sec) | ||||||||
| GA1 | 451 (45%) | −11.4 | −3.3 | 432 | 4% | −7.4±1.8 | −10.5 | −4.5 |
| GA2 | 355 (70%) | −10.0 | −2.6 | 334 | 6% | −6.2 ±1.6 | −9.1 | −3.5 |
| GA3 | 1230(83%) | −9.1 | −2.9 | 1178 | 4% | −6.0 ±1.3 | −8.3 | −4.0 |
Table 2: Normative Fetal Heart Rate by Fetal Behavioral State:
Normative values (mean, SD, 5th and 95th percentiles) for three fetal sleep/activity states at the GA3 (34–38 weeks gestation) assessment for fetal heart rate, heart rate variability, movement, heart rate/movement cross correlation, and heart rate movement lag from cross correlation. Outlier cutoffs were set at 1.5 x IQR above the 75th or below the 25th percentile. The 5th and 95th percentiles were determined after removing outliers.
| Variable GA Age Group | n with # epochs>4 (% of participants) | low cutoff | high cutoff | percentile | ||||
|---|---|---|---|---|---|---|---|---|
| final n | % outliers | mean ± SD | 5tn | 95th | ||||
| Heart Rate (bpm) | ||||||||
| 1F | 543 (37%) | 119.6 | 149.8 | 515 | 5% | 134.8 ±6.8 | 122.7 | 145.6 |
| 2F | 1326(90%) | 122.4 | 152.9 | 1261 | 5% | 136.8 ±6.9 | 125.5 | 149.3 |
| 4F | 105(7%) | 133.7 | 172.0 | 90 | 14% | 152.0 ±8.5 | 136.7 | 166.8 |
| SD of Heart Rate (bpm) | ||||||||
| 1F | 543 (37%) | 1.1 | 3.4 | 507 | 7% | 2.1 ±0.5 | 1.3 | 3.0 |
| 2F | 1326(90%) | 2.3 | 7.2 | 1260 | 5% | 4.7 ±1.0 | 3.0 | 6.5 |
| 4F | 105(7%) | 2.0 | 8.6 | 99 | 6% | 5.3 ±1.6 | 2.6 | 7.9 |
| Fetal movement (arb units) | ||||||||
| 1F | 534 (36%) | 0.5 | 3.0 | 497 | 7% | 1.6±0.5 | 0.9 | 2.7 |
| 2F | 1304(88%) | 0.6 | 3.8 | 1232 | 5% | 2.1 ±0.7 | 1.1 | 3.4 |
| 4F | 103(7%) | 0.6 | 4.5 | 97 | 6% | 2.3 ±0.9 | 1.1 | 4.0 |
| Movement/HR Cross
correlation (no units) | ||||||||
| 1F | 57 (4%) | 0.60 | 0.93 | 54 | 5% | 0.78 ±0.07 | 0.67 | 0.89 |
| 2F | 1108(75%) | 0.62 | 0.90 | 1067 | 4% | 0.76 ±0.07 | 0.65 | 0.87 |
| 4F | 48 (3%) | 0.52 | 0.89 | 46 | 4% | 0.72 ±0.08 | 0.57 | 0.84 |
| LAG of
Movement/HR (sec) | ||||||||
| 1F | 57 (4%) | −10.6 | −0.8 | 57 | 0% | −5.6 ±2.0 | −8.6 | −3.2 |
| 2F | 1108 (75%) | −9.3 | −2.8 | 1063 | 4% | −6.0 ±1.3 | −8.3 | −3.9 |
| 4F | 48 (3%) | −10.6 | −2.95 | 45 | 6% | −6.8 ±1.8 | −10.2 | −3.7 |
Effects of Gestational Age on Heart Rate (Figure 1A):
Figure 1. Fetal Heart Rate (FHR) and the Standard Deviation of Fetal Heart Rate (SD-HR):
Panel A shows mean fetal heart rates (±SE) at each of the three assessment gestational age ranges (GA1, 20–24 weeks gestation; GA2, 28–32 weeks gestation; GA3, 34–38 weeks gestation). The three brackets depict gestational age group comparisons with p-values from mixed models regression above each bracket. Panel B shows mean fetal HRs (±SE) for each of three fetal sleep/activity states (1F, 2F, 4F) at the GA3 gestational age time point. The three brackets depict state comparisons with p-values from mixed models regression above each bracket. Panel C shows mean standard deviation of fetal heart rate (±SE) at each of the three assessment gestational age ranges. The three brackets depict gestational age group comparisons with p-values from mixed models regression above each bracket. Panel D shows the mean standard deviation of fetal heart rate (±SE) for each of three fetal sleep/activity states (1F, 2F, 4F) at the GA3 gestational age. The three brackets depict state comparisons with p-values from mixed models regression above each bracket.
FHR decreased throughout gestation: GA1 (144.1 bpm ± 4.8 bpm); GA2 (139.5 bpm ± 5.7 bpm); GA3 (136.4 bpm ± 6.7 bpm). The comparison between GA1 and GA3 revealed the HR was ~7 bpm lower at GA3 (p<0.000001, 2078 observations from 1495 participants; 583 participants had results at both time points). Similarly, the comparison between GA1 and GA2 revealed the HR was ~4 bpm lower at GA2 (p<0.000001, 1187 observations from 944 participants; 243 participants had results at both time points). HR was also ~3 bpm lower at GA3 than GA2 (p<0.000001, 1777 observations from 1436 participants; 347 participants had results at both time points).
Effects of Fetal State on Heart Rate (Figure 1D):
The mixed models comparison between 1F (quiet state) and 4F (active awake state) revealed HR was ~17 bpm higher in the 4F state (p<0.000001, 605 observations from 569 participants; 36 participants had results in both 1F and 4F). HR in 4F was ~14 bpm higher than in 2F (active state) (p<0.000001, 1351 observations from 1266 participants; 85 participants had results in both 2F and 4F). Finally, HR was ~3 bpm higher in state 2F than in state 1F (p<0.000001, 1776 observations from 1277 participants; 499 participants had results in both 1F and 2F).
Effects of Fetal State on Heart Rate Variability (Figure 1D):
The mixed models comparison between 1F and 4F revealed SD-HR was ~3.1 bpm higher in the 4F state (p<0.000001, 606 observations from 568 participants; 38 participants had results in both 1F and 4F). SD-HR in 4F was ~0.6 bpm higher than in state 2F (p<0.000001, 1359 observations from 1271 participants; 88 participants had results in both 2F and 4F). Finally, SD-HR was ~2.4 bpm higher in state 2F than in state 1F (p<0.000001, 1767 observations from 1285 participants; 482 participants had results in both 1F and 2F).
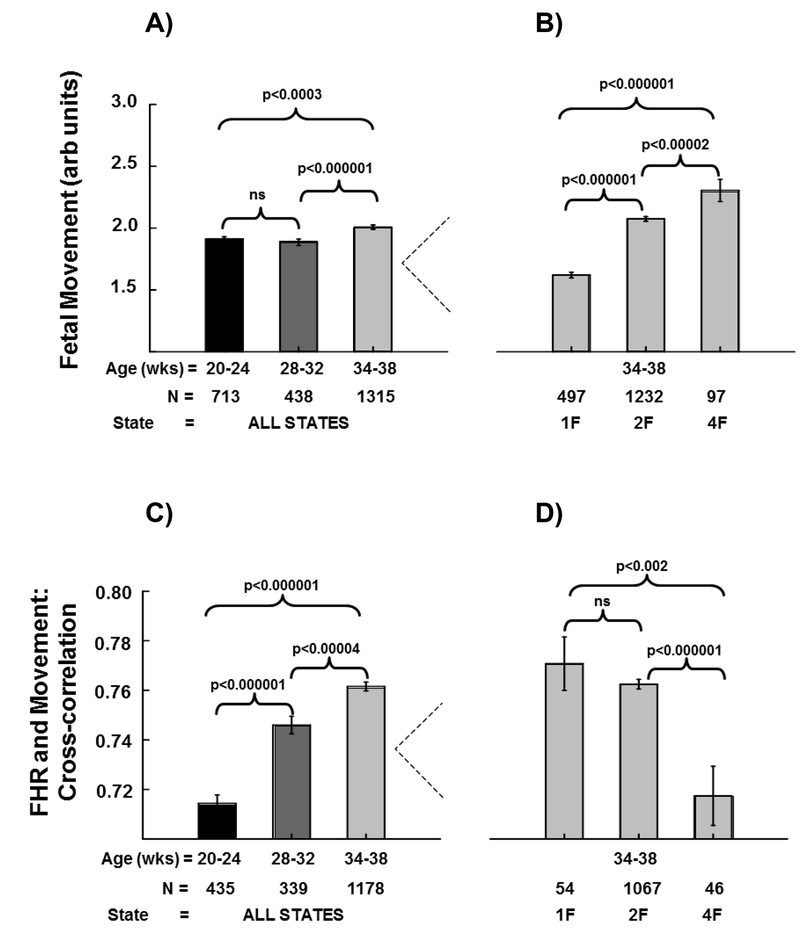
Effects of Gestational Age on Fetal Movement (FMOV) (Figure 2A):
Figure 2. Fetal Movement, Fetal Heart Rate (FHR)/Movement Cross-Correlation (CC):
Panel A shows mean of the fetal movement signal (±SE) at each of the three assessment age gestational ranges (GA1, 20–24 weeks gestation; GA2, 28–32 weeks gestation; GA3, 34–38 weeks gestation). The three brackets depict gestational age group comparisons with p-values from mixed models regression above each bracket. Panel B shows fetal movement signal (±SE) for each of three fetal sleep/activity states (1F, 2F, 4F) at the GA3 gestational age time point. The three brackets depict state comparisons with p-values from mixed models regression above each bracket. Panel C shows mean (±SE) cross-correlations between fetal heart rate and fetal movement at each of the three assessment gestational age ranges. The three brackets depict gestational age group comparisons with p-values from mixed models regression above each bracket. Panel D shows mean (±SE) cross-correlations between fetal heart rate and fetal movement for each of three fetal sleep/activity states (1F, 2F, 4F) at the GA3 gestational age time point. The three brackets depict state comparisons with p-values from mixed models regression above each bracket.
The comparison between GA1 and GA3 revealed FMOV was ~0.10 units higher at GA3 (p<0.0003, 2028 observations from 1473 participants; 545 participants had results at both time points). There was no significant difference in FMOV between GA1 and GA2 (1151 observations from 921 participants; 230 participants had results at both time points). FMOV was ~0.12 units higher at GA3 than at GA2 (p<0.0002, 1753 observations from 1425 participants; 328 participants had results at both time points).
Effects of Fetal State on Fetal Movement (Figure 2B):
The mixed models comparison between 1F and 4F revealed FMOV was ~0.70 units higher in the 4F state (p<0.000001, 594 observations from 557 participants; 37 participants had results in both 1F and 4F). FMOV in 4F was ~0.25 units higher than in state 2F (p<0.00002, 1329 observations from 1238 participants; 91 participants had results in both 2F and 4F). Finally, FMOV was ~0.40 units higher in state 2F than in state 1F (p<0.000001, 1729 observations from 1257 participants; 472 participants had results in both 1F and 2F).
Effects of Age on FHR/Movement Cross-correlation (CC) (Figure 2C):
The comparison between GA1 and GA3 revealed CC was ~0.05 correlation units higher at GA3 (p<0.000001, 1613 observations from 1290 participants; 323 participants had results at both time points). Similarly, the CC at GA2 was higher (~0.03 units) than at GA1 (774 observations from 639 participants; 135 participants had results at both time points), and CC was ~0.02 units higher at GA3 than at GA2 (p<0.00004, 1517 observations from 1266 participants; 251 participants had results at both time points).
Effect of Fetal Behavioral State on FHR/Movement Cross-correlation (CC) (Figure 4B):
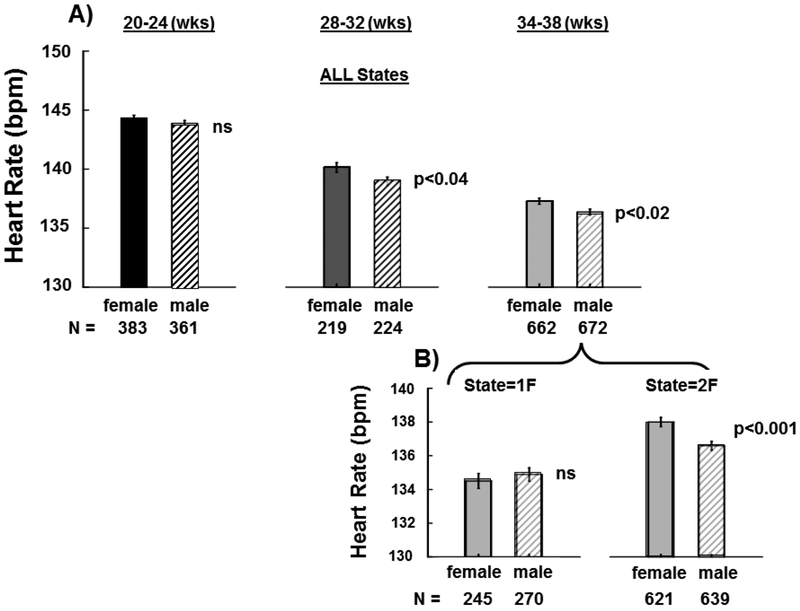
Figure 4. Fetal Heart Rate (FHR) by Sex:
Panel A shows mean fetal HRs (±SE) at each of the three assessment gestational age ranges (GA1, 20–24 weeks gestation; GA2, 28–32 weeks gestation; GA3, 34–38 weeks gestation) for females (solid black bars) and males (hatched bars). Panel B shows mean fetal HRs (±SE) at the GA3 (34–38 weeks) gestational age range for the two most common fetal sleep/activity states. ns = not significantly different.
The mixed models comparison between 1F and 4F revealed the CC between FHR and FMOV was ~0.05 units lower in the 4F state (p<0.002, 100 observations from 97 participants; 3 participants had results in both 1F and 4F). CC in 4F was ~0.05 units lower than in state 2F (p<0.000001, 1113 observations from 1074 participants; 39 participants had results in both 2F and 4F). Finally, CC was not significantly different in state 2F than in state 1F (1121 observations from 1076 participants; 45 participants had results in both 1F and 2F).
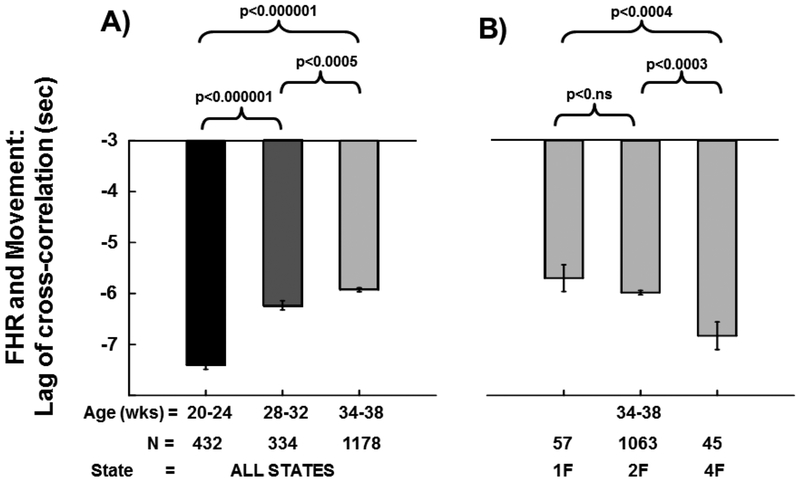
Effects of Gestational Age on FHR/Movement Lag of Cross-correlation (LAG) (Figure 3A):
Figure 3. Fetal Heart Rate (FHR)/Movement Lag of Cross-Correlation (sec):
Panel A shows mean (±SE) lag (in seconds) between fetal heart rate and fetal movement derived from the cross-correlation analyses at each of the three assessment gestational age ranges (GA1, 20–24 weeks gestation; GA2, 28–32 weeks gestation; GA3, 34–38 weeks gestation). The three brackets depict gestational age group comparisons with p-values from mixed models regression above each bracket. Panel B shows mean (±SE) lags between fetal heart rate and fetal movement for each of three fetal sleep/activity states (1F, 2F, 4F) at the GA3 gestational age time point. The three brackets depict state comparisons with p-values from mixed models regression above each bracket.
The comparison between GA1 and GA3 revealed LAG was ~1.5 sec shorter at the GA3 age (p<0.000001, 1610 observations from 1292 participants; 318 participants had results at both time points). Similarly, the LAG at GA2 was shorter (~1.2 sec) than at GA1 (766 observations from 630 participants; 136 participants had results at both time points), and the LAG of CC was ~0.3 sec shorter at GA3 than at GA2 (p<0.0005, 1512 observations from 1271 participants; 241 participants had results at both time points).
Effect of Fetal State on FHR/Movement Lag of Cross-correlation (LAG) (Figure 3B):
The mixed models comparison between 1F and 4F revealed the LAG of the CC between FHR and FMOV was ~1.1 sec shorter in the 1F state (p<0.004, 102 observations from 98 participants; 4 participants had results in both 1F and 4F). LAG in 4F was ~0.8 sec shorter than in state 2F (p<0.0003, 1108 observations from 1069 participants; 39 participants had results in both 2F and 4F). Finally, LAG was not significantly different in state 2F than in state 1F (1120 observations from 1072 participants; 48 participants had results in both 1F and 2F).
Effect of Sex on FHR (Figure 4):
Analyses (between group t-tests) indicated no significant sex difference at our earliest measured fetal time point (GA1, 20–24 weeks GA). However, at GA2 (28–32 weeks GA) and GA3 (34–38 weeks GA) females had significantly higher FHR than males (p<0.04 and p<0.02 respectively). Specifically, female FHR was ~1.2 beats above male FHR and at these time points. When examining the effects of fetal behavioral state in GA3, analyses revealed no significant difference between males and females in state 1F. But, females FHR was ~1.12 beats higher than males in state 2F (p<0.001). Similar analyses of SD, FMOV, CC and LAG revealed no significant differences between males and females at any of the three gestational ages or between sleep/activity states at the GA3 time point.
Discussion
There have been relatively few prior longitudinal studies that have measured changes in fetal heart rate and movement parameters at multiple time points during pregnancy. Defining normative fetal heart rate patterns longitudinally in diverse populations is imperative for optimally characterizing fetal autonomic system development and for laying the groundwork for future studies to identify potential developmental abnormalities. To the best of our knowledge, the present report is the largest longitudinal study to examine change in FHR, variability in FHR, and fetal body movements in healthy fetuses throughout gestation. Similar to prior reports [1–7], we found that FHR decreased over gestation: GA1 (144.1 bpm ± 4.8 bpm); GA2 (139.5 bpm ± 5.7 bpm); GA3 (136.4 bpm ± 6.7 bpm). In a report of multiple individual cohorts subsumed into a single analysis (n=~576), FHR was also found to decrease across gestation (~23 – 27 weeks GA: 147.6 bpm ± 5.6 bpm); (~30 – 34 weeks GA: 142.9 bpm ± 7.0 bpm); (~36 – 39 weeks GA: 141.7 bpm ± 8.1 bpm) (27). In the largest prospective longitudinal sample prior to the present report (n=~145), Amorin-Costa et al. (2016) (1) also reported a decrease in FHR throughout gestation: (24 weeks GA: 143.7 bpm); (28 – 32 weeks GA: 138.9 – 138.4 bpm); (34–38 weeks’ GA: 135.5 – 135.4 bpm). Neither of these prior studies reported on sex differences, however, similar to a previously published sex-specific review of cardiotocographic measures during gestation (2), we found sex differences in FHR emerge at ~28 weeks gestation. In the present report, females had significantly higher FHR than males at the GA2 and GA3 time points whereas there were no significant sex differences in FHR at 20 – 24 weeks gestation (GA1). When accounting for fetal state at the GA3 time point, we found that males had significantly lower FHR than females in the active fetal behavioral state (2F) and found no significant sex difference in FHR in the quiet fetal state (1F). Irrespective of sex, our mixed effect models revealed FHR was ~17 bpm higher in the awake fetal state (4F) state compared to the quiet sleep state (1F) and ~14 bpm higher compared to the active state (2F). FHR was also ~3 bpm higher in state 2F than in state 1F.
Although neither long-term variability (LTV) reported by Amorin-Costa (1) or short-term variability (STV) reported by DiPietro (27) cannot be directly compared to our measure of global HRV, similar to LTV and STV we found heart rate variability increased over gestation: GA1: (2.9 bpm ± 0.7 bpm); GA2: (3.7 bpm ± 0.9 bpm); GA3: (4.3 bpm ± 1.1 bpm). While these prior reports did not account for fetal state, we demonstrated that the standard deviation of FHR was highest in the 4F state. This measure of global FHR variability was also ~3 bpm higher in 4F than 1F and ~0.6 bpm higher than in 2F. Similar to Pillai and James (1990) (4), we also found that the standard deviation of FHR was higher in the active (2F) state than the quiet (1F) state. Some reports that have examined sex differences in fetal HRV have shown inconsistent results (31–33). However, at least three studies have demonstrated sexual dimorphism in fetal heart variability (2, 7, 34). DiPietro and colleagues found that males displayed higher heart rate variability throughout gestation (27) and more recently demonstrated males had significantly higher heart rate variability than females during late gestation (~ > 30 weeks GA) (34). In a large sample of CTG tracings, Amorim-Costa (2016) reported significantly lower short-term variability from 33 weeks gestation onwards whereas long-term variability was lower in female fetuses throughout gestation (2). In the current report, we also found that males had higher global heart rate variability, but this comparison did not reach statistical significance.
We additionally demonstrated that fetal movements increased with gestation. Specifically, we found that fetal movement significantly increased from GA1 (1.9 ± 0.6) to GA3 (2.0 ± 0.7), but found no statistically significant increase in fetal movement from GA1 to GA2 (1.9 ± 0.5). Although fetal breathing was not measured in the Safe Passage Study, work in non-human primates has identified respiratory sinus arrhythmia (RSA), the acceleration/deceleration cycle coordinated with inspiration, is present during fetal breathing movements (25). Specifically, FHR increases ~1–3 beats/minute with fetal inspiration and decreases during the expiratory phase (25). Prior work in human fetuses has demonstrated increased fetal breathing movements during maternal sleep and in association with increased maternal plasma glucose concentrations ~2–3 hours postprandial (35). Prior studies have reported a decrease in general movements and an increase in onset-onset interval successive bursts with advancing gestation (36, 37). When examining the effect of fetal behavioral state on fetal movement in the present report, some of our state specific observations were restricted by sample size (n=~100). However, we found movement was highest in the 4F state. Fetal movement was also higher in the 2F state than in the 1F state. Lastly, we found that the cross-correlation (CC) between FHR and movement increased throughout gestation while the FHR/movement lag of CC (seconds) decreased throughout gestation. The coupling between FHR and fetal movements (CC) was state dependent and was highest in the 1F state whereas the LAG was lowest in the 1F state.
The Safe Passage Study was successful in characterizing physiology in a large number of unexposed fetuses from a wide range of ethnic, racial, and geographic backgrounds, which will allow for generalizability to the population at-large. Restricting our analyses to unexposed fetuses allowed us to remove a confound that was not well controlled for in prior published studies. Our normative results demonstrate significant state and gestational age-related changes in cardiac activity, somatic activity, and autonomic function throughout gestation. Prenatal measurements of HR and HRV can provide reliable and sensitive markers of fetal brain developmental that could potentially identify those at-risk for stillbirth, SIDS, or neurodevelopmental disorders. In addition to providing criteria for subsequent analyses focused within the PASS network, the fetal assessment measures and normative values presented in the current report can also provide meaningful information to clinicians and researchers who lack normative reference data as a comparison group.
Limitations
In the present report, fetal behavioral state coding was based only on fetal HR patterns without accompanying ultrasound to visualize fetal body or eye movements. We only examined fetal behavioral state differences in variables of interest during 34 – 38 weeks gestation since all four distinct states are not fully expressed and classifiable until late gestation (30). In these analyses, we collapsed data from the Northern Plains and South African clinical sites in order to provide normative fetal physiology results that are representative of individuals from a wide range of racial, ethnic, geographic, and SES backgrounds. Due to the heterogeneity of subjects in the Safe Passage Study, our normative fetal physiology results may not be entirely representative of cardiac activity, somatic activity, or autonomic function in cohorts with restrictive inclusion/exclusion criteria or uniform population samples. The Safe Passage Study relied on maternal self-report to obtain estimates of maternal smoking and the Timeline Follow Back (TLFB) method in order to obtain quantitative estimates of alcohol usage (38). Although prior studies have reported high test-retest reliability on the TLFB interview (39), there may have been underreporting or misreporting of alcohol and/or cigarette usage during pregnancy in the present sample. Underreporting of drinking and smoking behaviors may have been greater in the Northern Plains US clinical sites than in the South African clinical site due to more stigma associated with drinking/smoking during pregnancy in the US.
Acknowledgements:
The authors gratefully acknowledge the cooperation of the study participants, PASS investigators, the PASS Steering Committee Chairman Gary D.V. Hankins, MD, and members of the NICHD advisory safety monitoring board: Elizabeth Thom, PhD (Chair); Reverend Phillip Cato, PhD; James W. Collins, Jr, MD, MPH; Terry Dwyer, MD, MPH; George Macones, MD; Philip A. May, PhD; Jeff Murray, MD; Richard M. Pauli, MD, PhD; Raymond W. Redline, MD; and Michael Varner, MD.
The PASS Research Network is supported by the National Institute on Alcohol Abuse and Alcoholism, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute on Deafness and Other Communication Disorders through the Cooperative Agreement Mechanism (U01HD055154, U01 HD045935, U01 HD055155, U01HD045991, and U01 AA016501).
Financial Support: This research was supported by grants T32MH016434-40 issued by the National Institute of Mental Health, U01HD055154, U01HD045935, U01HD055155, U01HD045991 and U01AA016501 issued by the National Institute on Alcohol Abuse and Alcoholism, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute on Deafness and Other Communication Disorders.
The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service (IHS) or the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), or the National Institute on Deafness and Other Communication Disorders (NIDCD).
Footnotes
Category of study: population study
Conflict of Interest:
The authors have no conflict of interests to declare.
References
- 1.Amorim-Costa C, Costa-Santos C, Ayres-de-Campos D, Bernardes J. Longitudinal evaluation of computerized cardiotocographic parameters throughout pregnancy in normal fetuses: a prospective cohort study. Acta Obstet Gynecol Scand. 2016;95(10):1143–52. [DOI] [PubMed] [Google Scholar]
- 2.Amorim-Costa C, Cruz J, Ayres-de-Campos D, Bernardes J. Gender-specific reference charts for cardiotocographic parameters throughout normal pregnancy: a retrospective cross-sectional study of 9701 fetuses. Eur J Obstet Gynecol Reprod Biol. 2016;199:102–7. [DOI] [PubMed] [Google Scholar]
- 3.Park MI, Hwang JH, Cha KJ, Park YS, Koh SK. Computerized analysis of fetal heart rate parameters by gestational age. Int J Gynaecol Obstet. 2001;74(2):157–64. [DOI] [PubMed] [Google Scholar]
- 4.Pillai M, James D. The development of fetal heart rate patterns during normal pregnancy. Obstet Gynecol. 1990;76(5 Pt 1):812–6. [DOI] [PubMed] [Google Scholar]
- 5.Snijders RJ, McLaren R, Nicolaides KH. Computer-assisted analysis of fetal heart rate patterns at 20–41 weeks’ gestation. Fetal Diagn Ther. 1990;5(2):79–83. [DOI] [PubMed] [Google Scholar]
- 6.Van Leeuwen P, Cysarz D, Edelhauser F, Gronemeyer D. Heart rate variability in the individual fetus. Auton Neurosci. 2013;178(1–2):24–8. [DOI] [PubMed] [Google Scholar]
- 7.DiPietro JA, Costigan KA, Shupe AK, Pressman EK, Johnson TR. Fetal neurobehavioral development: associations with socioeconomic class and fetal sex. Dev Psychobiol. 1998;33(1):79–91. [PubMed] [Google Scholar]
- 8.Rajendra Acharya U, Paul Joseph K, Kannathal N, Lim CM, Suri JS. Heart rate variability: a review. Med Biol Eng Comput. 2006;44(12):1031–51. [DOI] [PubMed] [Google Scholar]
- 9.Renou P, Newman W, Wood C. Autonomic control of fetal heart rate. Am J Obstet Gynecol. 1969;105(6):949–53. [DOI] [PubMed] [Google Scholar]
- 10.Spyridou K, Chouvarda I, Hadjileontiadis L, Maglaveras N. The effect of cigarette smoking on fetal heart rate tracing during pregnancy. J Perinat Med. 2017;45(4):403–11. [DOI] [PubMed] [Google Scholar]
- 11.Lucchini M, Pini N, Fifer WP, Burtchen N, Signorini MG. Entropy Information of Cardiorespiratory Dynamics in Neonates during Sleep. Entropy (Basel). 2017;19(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frasch MG, Zwiener U, Hoyer D, Eiselt M. Autonomic organization of respirocardial function in healthy human neonates in quiet and active sleep. Early Hum Dev. 2007;83(4):269–77. [DOI] [PubMed] [Google Scholar]
- 13.Allister L, Lester BM, Carr S, Liu J. The effects of maternal depression on fetal heart rate response to vibroacoustic stimulation. Dev Neuropsychol. 2001;20(3):639–51. [DOI] [PubMed] [Google Scholar]
- 14.Monk C, Fifer WP, Myers MM, Sloan RP, Trien L, Hurtado A. Maternal stress responses and anxiety during pregnancy: effects on fetal heart rate. Dev Psychobiol. 2000;36(1):67–77. [PubMed] [Google Scholar]
- 15.Zeskind PS, Gingras JL. Maternal cigarette-smoking during pregnancy disrupts rhythms in fetal heart rate. J Pediatr Psychol. 2006;31(1):5–14. [DOI] [PubMed] [Google Scholar]
- 16.Lehtovirta P, Forss M, Kariniemi V, Rauramo I. Acute effects of smoking on fetal heart-rate variability. Br J Obstet Gynaecol. 1983;90(1):3–6. [DOI] [PubMed] [Google Scholar]
- 17.Morrow RJ, Ritchie JW, Bull SB. Maternal cigarette smoking: the effects on umbilical and uterine blood flow velocity. Am J Obstet Gynecol. 1988;159(5):1069–71. [DOI] [PubMed] [Google Scholar]
- 18.Quigley ME, Sheehan KL, Wilkes MM, Yen SS. Effects of maternal smoking on circulating catecholamine levels and fetal heart rates. Am J Obstet Gynecol. 1979;133(6):685–90. [DOI] [PubMed] [Google Scholar]
- 19.Peterfi I, Kellenyi L, Peterfi L, Szilagyi A. The short-term effect of smoking on fetal ECG. J Matern Fetal Neonatal Med. 2017:1–10. [DOI] [PubMed] [Google Scholar]
- 20.McLeod W, Brien J, Loomis C, Carmichael L, Probert C, Patrick J. Effect of maternal ethanol ingestion on fetal breathing movements, gross body movements, and heart rate at 37 to 40 weeks’ gestational age. Am J Obstet Gynecol. 1983;145(2):251–7. [DOI] [PubMed] [Google Scholar]
- 21.Silva PD, Miller KD, Madden J, Keegan KA, Jr. Abnormal fetal heart rate pattern associated with severe intrapartum maternal ethanol intoxication. A case report. J Reprod Med. 1987;32(2):144–6. [PubMed] [Google Scholar]
- 22.Mulder EJ, Morssink LP, van der Schee T, Visser GH. Acute maternal alcohol consumption disrupts behavioral state organization in the near-term fetus. Pediatr Res. 1998;44(5):774–9. [DOI] [PubMed] [Google Scholar]
- 23.Pruett D, Waterman EH, Caughey AB. Fetal alcohol exposure: consequences, diagnosis, and treatment. Obstet Gynecol Surv. 2013;68(1):62–9. [DOI] [PubMed] [Google Scholar]
- 24.May LE, Glaros A, Yeh HW, Clapp JF 3rd, Gustafson KM. Aerobic exercise during pregnancy influences fetal cardiac autonomic control of heart rate and heart rate variability. Early Hum Dev. 2010;86(4):213–7. [DOI] [PubMed] [Google Scholar]
- 25.Myers MM, Fifer W, Haiken J, Stark RI. Relationships between breathing activity and heart rate in fetal baboons. Am J Physiol. 1990;258(6 Pt 2):R1479–85. [DOI] [PubMed] [Google Scholar]
- 26.Stark RI, Daniel SS, Kim YI, Leung K, Myers MM, Tropper PJ. Patterns of fetal breathing in the baboon vary with EEG sleep state. Early Hum Dev. 1994;38(1):11–26. [DOI] [PubMed] [Google Scholar]
- 27.DiPietro JA, Costigan KA, Voegtline KM. Studies in Fetal Behavior: Revisited, Renewed, and Reimagined. Monogr Soc Res Child Dev. 2015;80(3):vii; 1–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams IA, Tarullo AR, Grieve PG, Wilpers A, Vignola EF, Myers MM, et al. Fetal cerebrovascular resistance and neonatal EEG predict 18-month neurodevelopmental outcome in infants with congenital heart disease. Ultrasound Obstet Gynecol. 2012;40(3):304–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dipietro JA, Irizarry RA, Hawkins M, Costigan KA, Pressman EK. Cross-correlation of fetal cardiac and somatic activity as an indicator of antenatal neural development. Am J Obstet Gynecol. 2001;185(6):1421–1428. [DOI] [PubMed] [Google Scholar]
- 30.Nijhuis JG, Prechtl HF, Martin CB Jr., Bots RS. Are there behavioural states in the human fetus? Early Hum Dev. 1982;6(2):177–95. [DOI] [PubMed] [Google Scholar]
- 31.Fleisher LA, Dipietro JA, Johnson TR, Pincus S. Complementary and non-coincident increases in heart rate variability and irregularity during fetal development. Clin Sci (Lond). 1997;92(4):345–9. [DOI] [PubMed] [Google Scholar]
- 32.Lange S, Van Leeuwen P, Geue D, Hatzmann W, Gronemeyer D. Influence of gestational age, heart rate, gender and time of day on fetal heart rate variability. Med Biol Eng Comput. 2005;43(4):481–6. [DOI] [PubMed] [Google Scholar]
- 33.Nijhuis IJ, ten Hof J, Mulder EJ, Nijhuis JG, Narayan H, Taylor DJ, et al. Fetal heart rate in relation to its variation in normal and growth retarded fetuses. Eur J Obstet Gynecol Reprod Biol. 2000;89(1):27–33. [DOI] [PubMed] [Google Scholar]
- 34.DiPietro JA, Voegtline KM. The gestational foundation of sex differences in development and vulnerability. Neuroscience. 2017;342:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patrick J, Campbell K, Carmichael L, Natale R, Richardson B. Patterns of human fetal breathing during the last 10 weeks of pregnancy. Obstet Gynecol. 1980;56(1):24–30. [PubMed] [Google Scholar]
- 36.Roodenburg PJ, Wladimiroff JW, van Es A, Prechtl HF. Classification and quantitative aspects of fetal movements during the second half of normal pregnancy. Early Hum Dev. 1991;25(1):19–35. [DOI] [PubMed] [Google Scholar]
- 37.ten Hof J, Nijhuis IJ, Nijhuis JG, Narayan H, Taylor DJ, Visser GH, et al. Quantitative analysis of fetal general movements: methodological considerations. Early Hum Dev. 1999;56(1):57–73. [DOI] [PubMed] [Google Scholar]
- 38.Sobell LC SM. Timeline follow-back. New York: Springer Science; 1992. [Google Scholar]
- 39.Brown RA BE, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors 1998;12(2):101. [Google Scholar]