Abstract
The haematopoietic stem cell (HSC) microenvironment in the bone marrow, termed the niche, ensures haematopoietic homeostasis by controlling the proliferation, self-renewal, differentiation and migration of HSCs and progenitor cells at steady state and in response to emergencies and injury. Improved methods for HSC isolation, driven by advances in single-cell and molecular technologies, have led to a better understanding of their behaviour, heterogeneity and lineage fate, and of the niche cells and signals that regulate their function. Niche regulatory signals can be in the form of cell-bound or secreted factors and other local physical cues. A combination of technological advances in bone marrow imaging and genetic manipulation of crucial regulatory factors has enabled the identification of several candidate cell types regulating the niche, including both non-haematopoietic (e.g. perivascular mesenchymal stem and endothelial cells) and HSC-derived (e.g. megakaryocytes, macrophages and regulatory T cells), with better topographical understanding of HSC localization in the bone marrow. Here, we review advances in our understanding of HSC regulation by niches during homeostasis, ageing and malignancy, and discuss their implications for the development of therapies to rejuvenate aged HSCs or niches or to disrupt self-reinforcing malignant niches.
Introduction
Haematopoiesis is the process by which the cellular constituents of blood are continually replenished throughout the lifetime of an organism. The haematopoietic system consists of various populations of highly specialized cells that have unique functions, such as oxygen transport and immune defence1. It is estimated that an adult human generates approximately 4–5×1011 haematopoietic cells per day2. The continuous production of many blood cell types requires a highly regulated, yet highly responsive, system. Within the mammalian haematopoietic organization, rare haematopoietic stem cells (HSCs) sit at the top of the hierarchy. In adults, HSCs are found primarily in the bone marrow (BM) and are characterized by their ability to self-renew and produce various progenitors that proliferate and differentiate into mature blood cells3. HSCs are essential to replenish the haematopoietic system after transplantation into marrow-ablated recipients4,5 or after injury or infection6–9. By contrast, committed progenitors have limited self-renewal ability and exhibit restricted lineage differentiation potential, and exhaust within a few weeks after transplantation3. At steady state, most HSCs are quiescent, which protects them from genotoxic insults 10–13, whereas the bulk of haematopoiesis is ensured by downstream progenitors14–16. Several studies using single-cell transplantation and in vitro differentiation have challenged the classical hierarchical differentiation tree of haematopoietic progenitors, instead revealing lineage-restricted progenitors (restricted to one or two lineages) that may bypass multipotent progenitors and are generated directly from HSCs17–19.
To ensure haematopoietic homeostasis throughout life, the balance between differentiation and self-renewal needs to be tightly regulated: excessive differentiation or insufficient self-renewal depletes the HSC pool, whereas insufficient differentiation or unrestrained self-renewal can lead to myeloproliferative diseases or leukaemia. HSC activity is regulated by an intricate interplay of cell-intrinsic factors, such as transcriptional and epigenetic regulators and metabolic pathways, and cell-extrinsic cues, including long-range humoral and neural signals or local cues from the BM microenvironment, which is referred to as the stem cell niche.
The concept of the ‘niche’ was proposed by R. Schofield in 1978 (REF20) and refers to the regulatory unit that maintains and directs HSC self-renewal and differentiation. Building on this and other early observations, as well as new functional genetic tools and advancements in imaging methods and the discovery of new markers for HSCs and niche cells, have enabled a better understanding of the HSC microenvironment. The HSC niche is now viewed as a complex multicellular network that provides molecular cues and physical interactions that are essential for HSC localization, maintenance and differentiation. The field has grown rapidly in recent years; a search of PubMed using ‘haematopoietic stem cell niche’ as a search term retrieves >2,000 articles since Schofield’s seminal 1978 article20; >85% of these articles were published in the past 10 years. Although distinct BM niche constituents have been identified, predominantly in mice, and include both HSC progeny and non-haematopoietic cell types21, the exponential growth of knowledge has led to a paradoxical situation in which almost all cellular constituents of the BM have been proposed to contribute to the niche, many of which act in redundant ways. The situation is further complicated by the fact that the HSC pool itself is functionally and molecularly heterogeneous6,19,22–29, raising the possibility that distinct ‘specialized’ niches exist for distinct subpopulations of HSCs30–32.
HSCs are the basis of bone marrow transplantation — a curative therapy for haematological diseases involving the replacement of an individual’s haematopoietic and immune systems with transplanted donor bone marrow33,34. The number of available donor HSCs is often insufficient for marrow reconstitution, necessitating the ex vivo expansion of HSCs, which remains a major challenge. As the HSC population can expand substantially in its native niche, an understanding of the mechanisms of HSC maintenance is a prerequisite for the development of protocols to successfully expand HSC populations ex vivo for transplantation. Here, we review the progress of the past several years in the understanding of the HSC niche, emphasizing the cellular composition of the BM HSC niche and the molecular mechanisms and signals that underlie HSC–niche communication during homeostasis, ageing and malignancy. Finally, we discuss some unanswered questions in the field and their implications for regenerative medicine and the treatment of haematological and other cancers.
Bone marrow architecture
Understanding how HSCs and niche regulators interact requires knowledge of the microanatomical organization and properties of adult BM (FIG. 1). The BM is an intricate organ that encompasses several haematopoietic and non-haematopoietic cell types that are interconnected by a vascular and innervated network within the cavities of long bones and axial bones35. Most current knowledge about the HSC niche comes from studies of the long bones in mice (FIG. 1a). However, whereas all bones support haematopoiesis in mice, in humans the axial skeleton (which includes the cranium, sternum (FIG. 1b–d), ribs, vertebrae and ilium) is the major haematopoietic site. The haematopoietic (red) marrow of human long bones is progressively replaced in adolescence by ‘fatty’ yellow marrow that has negligible haematopoietic activity, except for in the proximal regions of the long bones36.
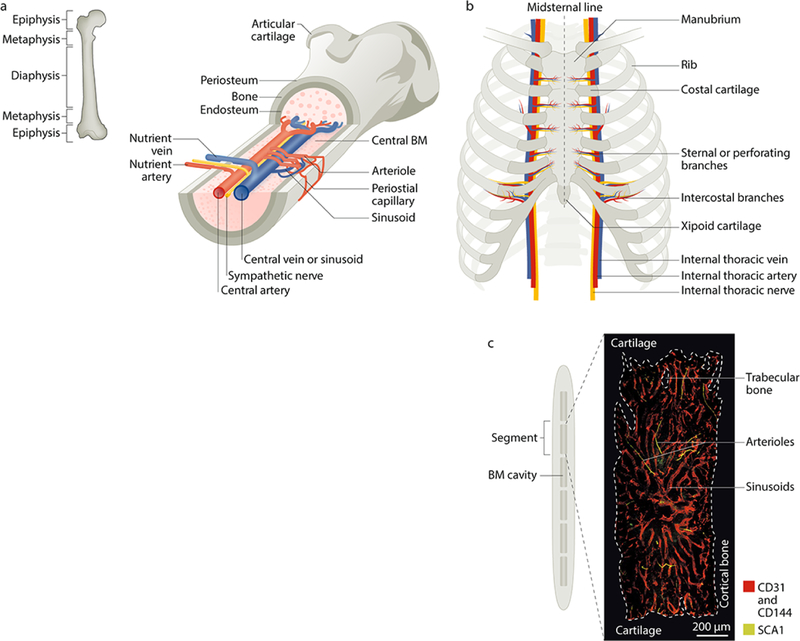
Figure 1. Microarchitecture of the adult mouse femur and sternum bone marrow.
Postnatally, the bone marrow (BM) is the primary site of haematopoietic stem cell (HSC) maintenance and haematopoiesis. a | Longitudinal view of a femur illustrating the arrangement of blood vessels and nerves (marked in yellow) that accompany the arteries within the BM cavity. The periosteal layer covers the outer surface of the bone and the endosteal layer is at the interface of bone and BM. Branching arteries (red) run parallel to the long axis of the marrow cavity, often close to the endosteum. These vessels feed into the sinusoidal network, which is distributed evenly throughout the marrow cavity and then coalesces to form the venous circulation (blue). b | Anterior view of the adult human sternum with attached ribs. Unlike the long bones, which mostly contain adipocytes in adult humans, the sternum has rich haematopoietic activity in both mice and humans, which makes it a suitable site to study haematopoiesis. The blood supply to the sternum originates from sternal/perforating branches located in the intersegmental spaces (between the ribs) from internal thoracic arteries that extend along the inside of the ribcage parallel to the sternum. c | Mid-sagittal section of the mouse sternum illustrating the six BM compartments (the human sternum comprises two bones, the manubrium and the body) with a representative image of a sternal segment showing the vasculature, which is labelled using intravenously injected antibodies against CD31/CD144 (red) and SCA1 (green). Arterioles can be distinguished from the sinusoidal network by their SCA1high expression.
Despite the differences in morphology, trabecular content and the ratio of endosteal to central marrow of various bones (such as the femur, pelvis and sternum), the HSCs isolated from them have similar gene expression profiles and long-term multilineage reconstitution abilities37. Analyses of the distribution patterns of ex vivo-purified and transplanted HSCs revealed that they tend to home to near the endosteal bone surfaces after bone marrow ablation by irradiation38,39 as well as in non-ablated recipients40. Haematopoietic stem and progenitor cells (HSPCs) also home preferentially to the trabecular-rich metaphysis of femurs in non-ablated mice, a process that depends in part on hyaluronic acid41. Other imaging studies have reported that endogenous HSCs localize eccentrically toward the bone surface rather than in the central medullary regions12,30,42–44. HSCs isolated from the endosteal region show greater in vivo homing, lodgement and reconstitution potential than their central marrow counterparts45,46. However, a recent study using optically cleared BM suggested that HSCs identified by α-catulin and KIT expression (TABLE 1) are uniformly distributed in the marrow space47 (see BOX 1).
TABLE 1:
Transgenic mice for labelling of adult bone marrow HSPCs.
| Mouse strain | Genetic modification | Specificity within the adult haematopoietic compartment | Analysis | Refs |
|---|---|---|---|---|
| Hoxb5-Tri-mCherry | Knock in | Specific to long-term HSCs | • Modified CUBIC clearing and light-sheet microscopy • Flow cytometry • Transplantation |
243 |
| Ctnnal1–GFP | Knock in | Restricted to HSCs and haematopoietic progenitors; requires KIT staining to enrich for HSCs | • Modified Murray’s clearing, immunostaining and confocal and multi-photon microscopy • Flow cytometry • Transplantation |
47 |
| Fgd5–mCherry | Knock in | Restricted to HSCs; low expression in haematopoietic progenitors | • Flow cytometry • Transplantation |
246 |
| Vwf–GFP | Transgenic | Labels platelet/myeloid-biased HSCs, megakaryocyte progenitors, megakaryocytes and platelets | • Flow cytometry • Transplantation • Immunostaining and whole-mount confocal microscopy |
29,32 |
| Msi2–GFP | Knock in | Also labels haematopoietic progenitors | • Confocal microscopy • Flow cytometry |
247 |
| Pdzk1ip1–GFP | Transgenic | Enriches for highly purified HSCs but also labels a small subpopulation of haematopoietic progenitors and mature granulocytes | • Doxycycline chase • Transplantation • Flow cytometry |
16 |
| Evi1–GFP | Knock in | Also labels haematopoietic progenitors | • Flow cytometry • Transplantation |
248 |
| Scl-tTA-H2B–GFP | Transgenic | H2B-GFPhigh label-retaining cells are enriched in quiescent long-term HSCs; also labels haematopoietic progenitors | • Doxycycline chase • Transplantation • Flow cytometry • Immunostaining and confocal microscopy |
6,249 |
| Tie2–GFP | Transgenic | Enriches for highly purified HSCs but also labels haematopoietic progenitors | • Flow cytometry • Transplantation • Immunostaining and whole-mount confocal and multi-photon microscopy |
245 |
| Gprc5c–GFP | Transgenic | Enriches for dormant HSCs but also labels haematopoietic progenitors | • Flow cytometry • Transplantation |
250 |
| Hdc–GFP | Transgenic | Enriches for myeloid-biased HSCs but also labels haematopoietic progenitors and mature myeloid cells | • Flow cytometry • Transplantation • Immunostaining and confocal microscopy |
251 |
| Krt7–GFP | Knock in | Specific to HSCs | • Flow cytometry | 252 |
| Gata2–GFP | Knock in | Enriches for HSCs and haematopoietic progenitors; SCA1 or Lineage staining are required for HSC selectivity | • Flow cytometry • Transplantation • Immunostaining and microscopy |
44 |
| Hoxb4–YFP | Knock in | Also labels haematopoietic progenitors | • Flow cytometry • Transplantation |
253 |
Ctnnal1, α-catulin; CUBIC, clear, unobstructed brain imaging cocktails and computational analysis; Evi1, ecotropic virus integration site 1 homologue; Fgd5, FYVE, RHOGEF and PH domain-containing 5; Gata2, GATA-binding 2; Gprc5c, G-protein coupled receptor family C group 5 member C; H2B, histone H2B; Hdc, histidine decarboxylase; HSCs, haematopoietic stem cells; HSPCs, haematopoietic stem and progenitor cells; Hoxb5, homeobox b5; Krt7, keratin 7; Msi2, Musashi 2; Pdzk1ip1, PDZK1-interacting 1; Scl, stem cell leukaemia; Tie2, tyrosine kinase with immunoglobulin and EGF homology domains 2; tTA, tetracycline-controlled transactivator protein; Vwf, von Willebrand factor.
Box 1 – The localization of HSCs in the niche.
Assessment of the anatomical location of haematopoietic stem cells (HSCs) and their proximity to candidate niche cells are major criteria for defining bona fide niches240 and are major topics of debate in the field. Direct visualization of the bone marrow (BM) is therefore essential to fully understand how HSCs are maintained and regulated in situ. However, this is a major challenge owing to a lack of exclusive markers for the identification and tracking of endogenous HSCs and niche cells and to the calcified nature of bones. Initial studies traced the homing of exogenously labelled or GFP-marked haematopoietic stem and progenitor cells (HSPCs) transplanted into the BM of recipient mice using classical histological or intravital microscopy imaging techniques. These studies indicated that HSPCs have a close relationship with the endosteum38–40. However, the extensive manipulation required to purify HSCs, the possible effect of anaesthesia or surgical stress during transplantation and the limited depth of imaging are all major drawbacks of this approach.
The discovery of signalling lymphocytic activation molecule (SLAM) family receptors (such as CD150, CD48 and CD244) as HSPC markers, studies using thinly sliced BM sections revealed that CD150+CD48–CD41–Lineage– HSCs are distributed broadly in close proximity to endothelial cells and Nes–GFP+ perivascular cells54,65. In mice expressing GFP from the haematopoietic promoter of Gata2, GFP+SCA1+ cells enriched in HSC activity localize in close proximity to the endosteum44. Endogenous HSCs have also been shown to localize in perivascular niches that are enriched near the endosteum43,241,242. However, the use of histological sections is limited by the restricted z-depths. Further technological improvements enabling 3D visualization of endogenous HSCs in situ uncovered a substantial association between a subset of quiescent HSCs and small arterioles, which themselves show a higher density in the endosteum region30. Upon activation and proliferation, this HSC subset distributes away from arterioles. 3D whole-mount imaging analyses revealed that a subset of quiescent HSCs is located adjacent to megakaryocytes in a niche that seems to be distinct from the arteriolar niche156. Although the ‘non-arteriolar niche’ is often referred to as the ‘sinusoidal niche’, it is important to emphasize that sinusoids occur throughout the BM and that most, if not all, haematopoietic cells are located in close proximity to sinusoids. However, HSC distribution in perivascular niches may not be random and may be influenced by specific microenvironments.
Of note, other studies using different tissue processing to improve translucence and transgenic mice with selective expression of HSC reporters (TABLE 1) suggested that HSCs are uniformly distributed in perisinusoidal niches. Studies using KIT immunostaining of optically cleared BM from Ctnnal1–GFP (Ctnnal1 encodes α-catulin) reporter mice found that GFP+ HSCs are not associated with arterial vessels but instead are broadly and randomly distributed in the BM (in a manner indistinguishable from randomly placed dots)47. Furthermore, there was no enrichment of quiescent HSCs in arteriolar areas. Another study found that Hoxb5–mCherry+ HSCs (imaged after chemical clearing) are homogeneously associated with VE-cadherin+ endothelial cells243. In the future, it will be interesting to evaluate in greater detail the localization of HOXB5+ HSCs in the context of vascular compartments. Although clearing techniques enable deep imaging of the marrow, they also require tissue decalcification or marrow plug extraction and additional processing steps, that may compromise the marrow architecture and the expression of surface markers244. By contrast, and in support of the existence of multiple niche structures, other studies detected arteriolar and/or megakaryocyte niches containing quiescent HSCs with low levels of reactive oxygen species (ROS) 31, quiescent HSCs in close proximity to arteriolar-associated Schwann cells136 and HSCs associated with transitional vessel endothelial cells connecting arterioles and sinusoids58. Tie2-GFP+ HSCs that are highly enriched for HSC activity also show a strong association with arterioles245 as well as the CD82+ HSC subset that is enriched for long-term HSC potential169. Imaging of myeloid-biased HSCs using Vwf–GFP shows separate BM niches for Vwf–GFP+ HSCs and Vwf–GFP– HSCs, which are regulated by megakaryocyte and arteriole-associated niche cells, respectively32. Thus, these studies using different HSC markers have reported differing results about HSC localization in the BM; whether these discrepancies result from the use of different methodologies or reflect the existence of distinct HSC subpopulations will require further investigation.
The periosteum is a highly vascularized and innervated tissue, allowing the entry and exit of blood vessels48 and nerve fibres to the bone and marrow cavity49. Of the autonomic nerves that reach the bone, the sympathetic nerves have been shown to penetrate the BM space50,51. Conversely, parasympathetic fibres may innervate the distal femoral metaphysis52. In the adult mouse BM, arterioles are wrapped by sympathetic nerves and perivascular stromal cells that express both the pericyte marker neural–glial antigen 2 (NG2) and the type VI intermediate filament protein nestin (NES), forming structural networks termed the neuro-reticular complex30,53,54.
A unique dense vascular network enables the efficient removal of waste products and the optimal delivery of nutrients, oxygen, hormones, neurotransmitters and growth factors to various locations in the bone and BM. Independently of the bone type, the oxygenated arterial blood connects with an extended network of sinusoids and exits through collecting veins55. Typical long bones, such as the femur, contain a central artery that enters the cortical bone through a nutrient canal and branches to form ascending and descending smaller arteries and thin-walled arterioles that mostly run parallel to the long axis of the marrow cavity, close to the endosteum 30,56. The connection between arterioles and the sinusoidal network preferentially occurs adjacent to endosteal and trabecular bone, mostly at the metaphysis but also in the diaphysis, and is mediated by type H vessels (also called transitional vessels). The endothelium of type H vessels expresses both arteriolar and sinusoidal markers. The number of type H vessels declines when bones have reached their adult size and is markedly reduced in old mice57–59. Sinusoidal endothelial cells represent the majority of BM endothelium and seem to be less affected by ageing than transitional vessel endothelial cells. Sinusoidal vessels connect to the central venous sinus, which is found at the centre of the marrow cavity in cross-section and runs the entire length of the diaphysis and metaphysis31,57,60. In turn, the central venous sinus empties into nutrient veins that pass through the nutrient canal along with the central artery, carrying deoxygenated blood and nutrient waste out of the marrow into the general circulation61. The vasculature of the skeletal system has been extensively studied (reviewed elsewhere55,62).
Blood vessels are crucial for haematopoiesis. Definitive HSCs emerge from endothelial cells of the largest artery and HSCs continue to be closely associated with blood vessels in embryonic and extra-embryonic sites63. In the adult mouse BM, early studies suggested the existence of a vascular niche that regulates haematopoiesis64. However, the idea of a vascular HSC niche was supported by direct imaging of HSC distribution in their native microenvironment close to blood vessels54,65. As discussed below, the niche has grown in complexity with several stromal and haematopoietic cells contributing to its activity66.
Constituents of the HSC niche
Transgenic mouse models have been developed that enable the ablation of various endogenous cell types or depletion of niche factors or the tracing of lineages. Coupled with the use of advanced microscopy techniques (BOX 2), these models have led to the identification of several non-haematopoietic and haematopoietic cell types, as well as niche factors and their receptors as indispensable regulators of HSC activity; these cell types and factors (FIG. 2 and FIG. 3) are discussed below.
Box 2. The identification of HSC niche cells.
A “stem cell niche” refers to the local microenvironment within a tissue in which stem cells are maintained in an undifferentiated and self-renewable state and receive stimuli that determine their fate. Therefore, the location of stem cells in a tissue is not enough to define a niche, as the niche must have both anatomical and functional consequences. In the haematopoietic system, niche inputs are experimentally evaluated by determining their effects on haematopoietic stem cell (HSC) numbers, proliferation, localization, in vivo repopulating capacity and trafficking. Stem cell niches share several key characteristics, such as physical association between niche and stem cells or selective synthesis of stem cell maintenance factors, that make their in vivo identification possible using different methodologies.
Targeted cell ablation studies.
Genetic manipulation can be used to deplete candidate niche cells in vivo and evaluate its effect on HSCs and haematopoiesis. Ablation can be achieved by the expression of a suicide gene, such as thymidine kinase (TK)72 or the diphtheria toxin receptor (DTR), under the control of the promoter of a gene specifically expressed in the target cell type. Use of the DTR approach led to the identification of different perivascular constituents of the bone marrow niche30,54,102,156–158. Conversely, genetic manipulations can also be used to expand or activate putative niche candidates; for example, a correlation between the number of osteoblasts and haematopoietic stem and progenitor cells (HSPCs) was demonstrated using this approach70,71. However, these approaches rely on the specificity of the genetic manipulation in the candidate niche cell type. Additionally, in vivo genetic cell depletion cannot discriminate between direct niche-dependent and indirect niche-independent effects that result from the systemic depletion of the cell population. The experimental dissection of direct and indirect effects should always be an important consideration in studies manipulating candidate niche cells.
Identification of niche cells by the expression of niche factors.
An alternative approach to identify niche cells is determining which cells synthesize HSC regulators, which include CXC chemokine ligand 12 (CXCL12), stem cell factor (SCF), thrombopoietin (THPO), osteopontin (OPN), transforming growth factor-β (TGFβ), CXCL4, vascular cell adhesion molecule 1 (VCAM1), GP130, Notch ligands, fibroblast growth factor 1 (FGF1) and pleiotrophin (FIG 2).
Identification of niche cells by conditional deletion of niche factors.
Putative niche cells can also be functionally identified by conditionally deleting a gene encoding a known HSC niche factor (such as Cxcl1285,86,109 or Scf84,109) by Cre-mediated recombination in candidate niche cells. This approach minimizes the off-target effects associated with the disruption of the BM cellular architecture that occurs in cell ablation studies. Furthermore, it enables a much more detailed evaluation of the individual contributions of candidate cells to overall niche activity. The main drawbacks of this approach are that it also relies on the availability of genetic models and the final readout will depend on the specificity of Cre expression and recombination efficiency. In addition, the niche capacity of some cells might also be overlooked owing to compensation by other cell types.
Other approaches.
The physical proximity of HSCs and candidate niche structures may offer further insight into the HSC niche. Imaging techniques can reliably identify HSCs in their native microenvironment, and the distance between HSCs and the nearest putative niche can be easily measured. To determine whether potential associations are functionally relevant, the distribution of HSCs relative to candidate structures can be statistically compared to computer-generated models of random spots distribution in the marrow space30,47,156,157,243. The HSC niche capacity of stromal cells can also be tested using heterotopic transplantation assays. In these assays, candidate niche cells are embedded in a matrix or ossicle and transplanted into recipient mice to test their ability to generate haematopoietic niches with active haematopoiesis of host origin supported by a stroma of donor origin54,95,97,101.
Figure 2. Cellular and molecular constituents of the HSC niche.
Various cell types have been implicated in regulating haematopoietic stem cell (HSC) activity, including perivascular mesenchymal stem cells (MSCs), endothelial cells, osteoblasts, adipolineage cells, sympathetic nervous system (SNS) nerves, nonmyelinating Schwann cells, macrophages, megakaryocytes and regulatory T (Treg) cells. The target plot illustrates how bone marrow (BM) niche cells contribute to HSC regulation indirectly or directly by synthesizing niche factors in the form of cell-bound or secreted molecules. The colour of the radial spokes indicates the HSC activity that is affected. *Molecules involved in bone marrow regeneration after ablation. Bold type indicates those molecules for which there is functional data using cell-specific genetic evidence. SCF, stem cell factor; CXCL12, CXC-chemokine ligand 12; CXCL4, CXC chemokine ligand 4; FGF1, fibroblast growth factor 1; TGFβ, transforming growth factor-β; DARC, duffy antigen receptor for chemokines; GP130, glycoprotein 130; VCAM1, vascular cell adhesion molecule 1; IL-10, interleukin-10.
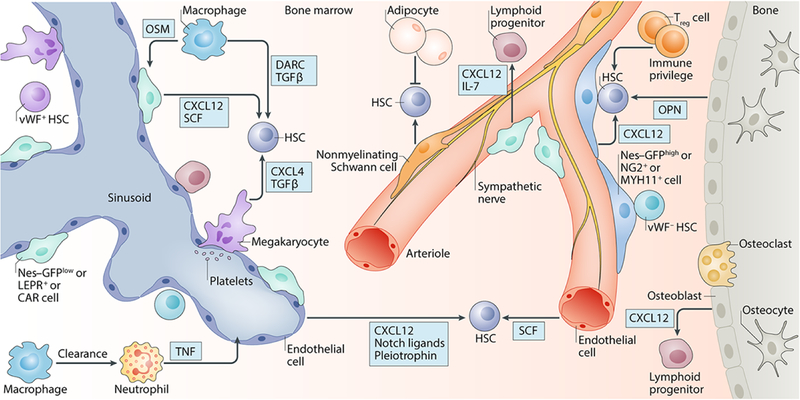
Figure 3. The adult bone marrow HSC niche in homeostasis.
Schematic representation of the adult bone marrow haematopoietic stem cell (HSC) niche in homeostasis, showing various cell types and niche factors that directly or indirectly regulate HSC activity. Emerging evidence highlights the vasculature and associated stromal cells, such as periarteriolar Nes–GFPhigh cells, NG2+ cells and MYH11+ cells and perisinusoidal Nes–GFPlow cells, CAR cells and LEPR+ cells, as key regulators of HSC maintenance. Sympathetic nervous system (SNS) nerves regulate HSC mobilization and non-myelinating Schwann cells may contribute to HSC quiescence. Osteoblasts have been implicated in HSC regulation but the precise molecular signals have not been clearly elucidated; however, they may have a role in regulating lymphoid progenitors. Adipocytes may negatively affect HSC maintenance. Haematopoietic cells, such as macrophages, neutrophils, regulatory T (Treg) cells and megakaryocytes, are examples of HSC-derived progeny that can feedback and contribute to HSC maintenance or mobilization. Regional localization of HSC subsets has shown that platelet-biased or myeloid-biased Vwf–GFP+ HSCs and Vwf–GFP– HSCs are located in, and regulated by, separate BM niches containing megakaryocytes and arterioles, respectively. CAR cells, CXCL12-abundant reticular cells; SCF, stem cell factor; CXCL4, C-X-C-chemokine ligand 4; CXCL12, CXC chemokine ligand 12; DARC, duffy antigen receptor for chemokines; IL-7, interleukin-7; LEPR, leptin receptor; MYH11, myosin heavy chain 11; NG2, neuron/glial antigen 2; OPN, osteopontin; OSM, oncostatin M; SCF, stem cell factor; TGFβ, transforming growth factor-b; TNF, tumour necrosis factor; vWF, von Willebrand factor.
Regulation by non-haematopoietic cells
Various bone marrow stromal cells and other cell types have been implicated in the regulation of HSC activity, including mesenchymal stem cells (MSCs), adipocytes and glial cells.
Osteolineage cells.
Osteolineage cells were the first population of BM cells to be linked to the regulation of HSPCs. Early studies preceding Schofield’s niche concept suggested that HSPCs are enriched in the endosteal region of the bone67,68. In addition, studies in which HSC-enriched cell populations were transplanted into non-myeloablated recipients suggested that HSPCs are preferentially located near the bone surface40. The presence of osteoblasts in the endosteal region, together with their ability to support expansion of haematopoietic progenitor cells in vitro69 led to the hypothesis that osteoblasts are a candidate niche cell. Later, in vivo studies using early cell-surface markers (which define Lineage–SCA1+KIT+ (LSK) cells) showed a correlation between the numbers of osteoblasts and HSPCs70–72. Conditional ablation of osteoblasts, by ganciclovir treatment of mice expressing a truncated form of the herpes virus thymidine kinase (TK) gene under the control of a 2.3 kb fragment of the rat α1 type I collagen promoter, induces acute alterations in haematopoiesis, including reduced numbers of lymphoid, erythroid, and myeloid progenitors72. Enforced signalling through parathyroid hormone, a potent regulator of bone turnover, increases the number of osteoblastic cells and leads to HSC expansion mediated, at least in part, by the Notch ligand Jagged 1 (REF70). Similarly, the conditional inactivation of bone morphogenetic protein receptor type IA (BMPRIA) using Cre recombinase (Cre) expressed from the Mx1 promoter (Mx1-Cre) increases the number of N-cadherin+ osteoblasts, which also leads to HSC expansion71. This effect was thought to be the result of interactions between HSCs and osteoblasts mediated by the adhesion molecule N-cadherin, although functional evidence implicating N-cadherin in this interaction is still lacking71. Indeed, subsequent studies using new markers that are highly specific for HSCs dispute these findings73–75.
Two of the best studied cytokines that are known to be non-cell-autonomously required for HSC maintenance — CXC-chemokine ligand 12 (CXCL12, also known as SDF1)76–79 and stem cell factor (SCF, also known as KIT ligand)80,81—bind to CXC-chemokine receptor 4 (CXCR4) and KIT on HSCs, respectively82,83. Deletion of Cxcl12 or Scf in mature osteoblasts or osteoblastic progenitors using Col.2.3–Cre, Bglap–Cre (Bglap encodes osteocalcin) or Osx–Cre (Osx encodes osterix) mice has little effect on HSCs, suggesting that these osteolineage cells do not directly maintain HSCs, at least not via CXCL12 or SCF84–86. Osteoblasts can also produce other molecules that are implicated in the maintenance of HSCs, including osteopontin (OPN), a negative regulator of HSC pool size87,88, and thrombopoietin (THPO)89,90 and angiopoietin 1 (ANGPT1)12, which bind to their receptors myeloproliferative leukaemia protein (MPL) and tyrosine kinase with Ig and EGF homology domains-2 (TIE2), respectively, and regulate HSC quiescence. However, the physiological contribution of osteoblast-derived THPO and ANGPT1 in HSC regulation has been questioned91,92. Hepatocytes, not local marrow niche cells, seem to be the major functional source of THPO required for HSC maintenance92. HSPCs, megakaryocytes and leptin receptor-positive (LEPR+) stromal cells seem to be the main sources of ANGPT1 in the mouse BM91. This study also suggested that ANGPT1 does not directly affect HSC function but rather regulates niche regeneration after irradiation.
Although osteoblasts were the first cell population in the BM to be linked to HSC regulation, 3D imaging studies have shown that endogenous HSCs are not significantly associated with osteoblasts30,43. Taken together, these studies suggest that mature osteoblasts do not have a direct role in regulating HSC activity. However, osteolineage cells seem to support the maintenance of more committed haematopoietic progenitors, in particular, the lymphoid lineage72,85,86,93,94. The regulation of lineage-committed progenitors by BM niches has been reviewed elsewhere66.
Perivascular cells.
The discovery that adult mouse BM HSCs and haematopoietic progenitor cells differentially express receptors of the signalling lymphocytic activation molecule (SLAM) family (which include CD150, CD48 and CD244) has greatly facilitated imaging of highly purified HSCs in their native microenvironment. Bone marrow CD150+CD48–CD41–Lineage– HSCs are localized in relatively close proximity to sinusoids rather than being confined to the endosteum, suggesting the existence of a vascular niche65. Subsequent studies revealed that perivascular cells in human and mouse BM are highly enriched in niche factor expression and mesenchymal stem cell (MSC) activity54,95.
Bone marrow-derived MSCs are rare haematopoiesis-supporting stromal cells that have self-renewal potential and the capacity to differentiate into bone, fat and cartilage. They wrap tightly around arterioles and more loosely around sinusoidal blood vessels. MSCs form colony-forming unit-fibroblasts (CFU-Fs) or mesenspheres (non-adherent mesenchymal spheres) in vitro and, when transplanted, they contribute to ectopic formation of an organized haematopoietic niche in which BM stromal cells and active haematopoiesis can be detected96. Furthermore, human BM CD146+ mesenchymal stem and progenitor cells (MSPCs) subcutaneously transplanted into mice can generate a heterotopic haematopoietic niche with host-derived HSCs and donor-derived perivascular CD146+ MSPCs95. CD105+CD51+ MSPCs isolated from fetal mouse bones and transplanted into the renal capsule of adult mice are capable of inducing ectopic haematopoietic marrow97. These studies indicate that MSPCs may organize the HSC niche.
HSCs are released into the circulation in a circadian manner in response to adrenergic signals from the sympathetic nervous system (SNS) that regulate the synthesis of CXCL1298–100. Tracking the stromal cells that are targeted by SNS nerves has led to their identification as perivascular cells that are marked by GFP expression from regulatory elements of the Nes promoter. Nes–GFP+ cells associate tightly with nerves and HSCs and are enriched for MSC activity, suggesting that the BM niche may consist of the two stem cell populations, MSCs and HSCs54. Nes–GFP+ perivascular cells express high levels of key factors involved in HSC maintenance, such as CXCL12 and SCF, as well as other regulatory factors, such as ANGPT1, OPN, interleukin-7 (IL-7) and vascular cell adhesion molecule 1 (VCAM1). A similar cell population can also be isolated from the BM of non-transgenic mice and from human fetal BM using the surface markers platelet-derived growth factor receptor-α (PDGFRα) and CD51 (REF. 101). Knock-in of a GFP reporter at the Cxcl12 locus has revealed the existence of CXCL12-abundant reticular (CAR) stromal cells, which are adipo-osteogenic progenitors that are mainly distributed around sinusoids79,102. CAR cells are also major producers of SCF102, IL-7, which is required for the maintenance of lymphoid progenitors and mature B cells103, and the transcription factors forkhead box C1 (FOXC1) and early B cell factor 3 (EBF3), which are both required for CAR cell self-maintenance104,105.
Subsequent studies in mice with a GFP knock-in at the Scf locus showed GFP expression by endothelial cells and perivascular cells marked by the adipo-osteogenic regulator Lepr84,106. Approximately 90% of Lepr–Cre+ cells overlap with CAR cells107 and represent a large subset (~80%) of Nes–GFP+ cells30,108. Deletion of Scf or pleiotrophin (Ptn) using Lepr–Cre results in depletion of HSCs in the BM84,109,110. However, conditional deletion of Cxcl12 using Lepr–Cre mobilizes HSCs from the BM to the circulation and the spleen but has no effect on their abundance or localization in the marrow85,109. In Nes–CreER transgenic mice, which express a tamoxifen-inducible Cre–oestrogen receptor (ER) fusion protein, deletion of Cxcl12 or Scf does not markedly alter HSC numbers84,85, even though Nes–GFP+ cells overlap substantially with LEPR+ cells in the BM30,101,108. As Nes transgenic reporters were generated and screened to label neural stem cells, their expression in the BM is variable and depends on the transgenic strain. Accordingly, Nes–CreER recombination efficiency in Nes–GFP+ cells is very low and a Nes transgene may not necessarily reflect endogenous Nes expression, possibly owing to the incomplete Nes promoter regulatory elements in the Nes–GFP construct and the slow turnover of GFP. Nes–CreER may be more appropriate for early development studies, as the deletion of Cxcl12, when tamoxifen is administered within the first 3 weeks after birth, depletes HSCs in the early postnatal BM111.
Whole-mount 3D imaging of the BM has uncovered heterogeneity among perivascular cells — rare Nes–GFPhigh cells that express the pericyte marker NG2 (REF30) or the smooth-muscle marker MYH11 (REF109) are associated with arterioles and a subset of quiescent HSCs, whereas Nes–GFPlow stromal cells overlap with LEPR+ cells and CAR cells and are ubiquitously distributed near sinusoids30. In contrast to Lepr–Cre, Cxcl12 deletion using Ng2–Cre, which has high Cre-mediated recombination efficiency in almost all Nes–GFP+ cells and in >80% in LEPR+ cells and CAR cells, leads to a severe reduction in the number of HSCs in the BM, their exit from quiescence and redistribution of a subset away from arterioles109. The conditional deletion of Cxcl12 using paired-related homeobox 1 (Prx1)–Cre also depletes HSCs from the BM, likely because PRX1 is expressed in limb bud mesoderm during development and broadly by adult BM mesenchymal progenitor cells85,86.
Using Nes–GFP and inducible Ng2–CreER together with a reporter line, ~30% of Nes–GFPhigh cells are labelled with the reporter but no labelling of Nes–GFPlow cells is detected. Although the recombination efficiency of Ng2–CreER inducible diphtheria toxin receptor (iDTR) is sufficient to reduce Nes–GFPbright cells and the marrow HSC population after tamoxifen and diphtheria toxin administration30, conditional deletion of Cxcl12 or Scf using Ng2–CreER has no effect in BM HSCs using this tamoxifen administration protocol is used47. However, with an extended tamoxifen administration period, starting at 2–3 weeks of age to maximize the efficiency of depletion, the selective deletion of Cxcl12, but not of Scf, in Ng2–CreER cells reduced HSC numbers in the BM and altered HSC localization109. These studies suggest that distinct inputs of cytokines required for HSC maintenance are derived from distinct perivascular niches (LEPR+ cells versus NG2+ cells), although it is still unclear if this differential contribution is a simple reflection of a difference in niche cell numbers (to explain the lack of an effect of Scf deletion) or a reflection of the crucial role of cytokines in HSC localization (to explain strong effect of Cxcl12 deletion). Because bone marrow arterial endothelial cells secrete SCF112, it is also possible that the difference in phenotype may be due to the contributions of other stromal cell types. Recent studies further support the notion of niche heterogeneity — the quiescence and localization of von Willebrand factor (Vwf)–GFP– HSCs are regulated by NG2+ perivascular cells, whereas those of myeloid-biased Vwf–GFP+ HSCs are selectively regulated by megakaryocytes32 (see below).
It is now clear that there is substantial overlap between the adult BM MSPC populations that have been identified using Nes–GFP, Ng2–Cre, CAR, Scf–GFP, Lepr–Cre and Prx1–Cre101,106,108,113,114. In addition, two populations of bone MSCs have been identified based on the expression of PDGFRα and SCA1(REF115) or Cd73–GFP(REF116), and two populations of skeletal stem cells isolated from whole bones have been identified based on the expression of CD51 and CD200 (REF117) or gremlin 1 (REF118). The contributions of these populations of cells to steady-state regulation of HSCs, as well as their precise lineage relationship with the previously described MSPC populations, have yet to be resolved.
When dissecting the relationship between different MSPC populations identified using cell surface markers or genetic reporters or when comparing studies, the tissue processing technique utilized for cell isolation is an important consideration. Different enzymatic treatments or bone-processing methods, such as crushing or marrow flushing, can elicit phenotypically and functionally different cellular subsets, preventing direct comparison of studies. For example, SCA1 and GREMLIN1 seem to be expressed mostly by bone-associated MSPCs (for example, in the periosteum), which are not in direct contact with haematopoietic cells. Cells isolated from the bone exhibit distinct gene expression profiles and greater clonogenicity, growth and differentiation capacity compared to those in the BM96,119,120. Bulk-cell analysis shows that niche activity measured by the expression of HSC niche factors correlates with MSC activity in the mouse and human fetal BM101. However, only a fraction of Nes–GFP+ cells, CAR cells, LEPR+ cells and Prx1–Cre cells contain CFU-F and multilineage differentiation capacity, suggesting that heterogeneity exists in the stromal cell population. However, the homogeneity of the stromal cell population can be substantially improved by the routine inclusion of CD51 as a marker in the analysis121. Interestingly, the majority of BM CD45–Ter119–CD31– stromal cells are HSC-derived and contain previously unappreciated populations of CD45– erythroid and B lymphoid progenitors121. Although the hierarchical organization of bona fide stromal cell types may be as complex as the haematopoietic lineage tree, there is currently no evidence supporting this idea; the on-going characterization of stromal cell types using single-cell techniques will undoubtedly provide further insight.
Adipocytes.
In humans, ageing accelerates BM adiposity, which is especially apparent in long bones where haematopoietic sites are progressively replaced by ‘fatty’ yellow marrow with reduced haematopoietic activity36. Adiponectin, an adipocyte-secreted protein, impairs the proliferation of haematopoietic progenitors in vitro122. Furthermore, adipocyte-rich marrow (such as in the vertebrae of the mouse tail) contains fewer HSPCs than adipocyte-poor marrow (such as in the thoracic vertebrae). In the A-ZIP/F1 ‘fatless’ mouse, BM engraftment by HSCs is accelerated123. In addition, the BM recovers more quickly after transplantation or chemotherapy when mice are treated with a peroxisome proliferator-activated receptor γ (PPARγ) antagonist, which inhibits adipogenesis123,124. These results suggest that adipocytes are negative regulators of HSC function, although alterations in MSPCs may also indirectly contribute to this result. A novel MSC population (CD45–CD31–SCA1+CD24+) that is regulated by diet and ageing and can give rise to osteochondrogenic and adipogenic precursors, has been identified125. Consistent with the negative influence of adipocytes, co-transplantation of adipogenic precursors in an intratibial injection model inhibits HSPC engraftment and bone fracture healing, in a manner mediated by the negative regulator dipeptidyl peptidase 4125. By contrast, adipocyte progenitors derived from Adipoq–CreER-labelled cells transiently support the regeneration of HSCs by producing SCF126. Further studies are needed to clarify the mechanisms of adipolineage cell regulation of the HSC niche, particularly at steady state.
Neural regulation.
Innervation in the skeletal system has been suggested to regulate haematopoiesis in the bone and BM49,98,127. Whereas parasympathetic nerves only innervate the bone and do not fully penetrate the marrow52,128, sympathetic nerves50,51 and sensory nerves51,129 innervate both the bone and BM. Sympathetic fibres release the neurotransmitter noradrenaline, which signals through adrenergic receptors. HSPC mobilization out of the marrow to peripheral organs depends on an intact gradient of CXCL12. Granulocyte colony-stimulating factor (G-CSF) decreases the steepness of the gradient by selectively reducing BM CXCL12 levels, leading to rapid HSPC egress from the BM77,130. The SNS has a role in HSC mobilization in response to G-CSF treatment98, as pharmacological or genetic ablation of adrenergic neurotransmission suppresses the HSC migratory response to G-CSF stimulation, which indicates that adrenergic signalling controls G-CSF-induced osteoblast suppression and CXCL12 downregulation98. Furthermore, an intact osteocyte network, which is regulated by SNS fibres, is also required for G-CSF-induced HSPC mobilization131.
Bone marrow Nes-GFP+ MSCs are targeted by the SNS, as at steady state, adrenergic signals transduced by the β3 adrenergic receptor (ADRβ3), which is expressed by Nes-GFP+ stromal cells mediate circadian oscillations of CXCL12 expression and HSC egress in mice54,99 and probably in humans100. Importantly, G-CSF-induced mobilization is associated with suppression of MSC and osteolineage cell function98,99,131–133.
SNS signals are also important for haematopoietic regeneration following genotoxic stress134,135. Chemotherapy and/or irradiation-mediated toxicity can promote sympathetic neuropathy in the BM and excessive proliferation of MSCs and endothelial cells, which leads to a reduction in their niche support capacity. The administration of neuroprotective135 or neuroregenerative134 drugs rescues sympathetic nerve fibres and promotes the survival of niche cells and haematopoietic recovery after genotoxic insults, highlighting the potential benefit of using these drugs to protect haematopoietic niches from therapeutic injury.
Nonmyelinating Schwann cells, which are glial cells that express the marker glial fibrillary acidic protein (GFAP), insulate sympathetic and sensory nerves along arteries and promote HSC quiescence through activation of transforming growth factorβ (TGFβ) and SMAD signalling136. Surgical sympathectomy by transection of the postganglionic sympathetic nerve results in the loss of BM GFAP+ cells and HSCs by increased proliferation. However, as less invasive methods of surgical sympathectomy do not result in reduced HSC numbers98,99,134, it is unclear how SNS nerves signal to Schwann cells and to what extent marrow denervation contributes to the effects on HSCs.
Whether nerves can directly influence HSC function remains poorly understood. Adrenergic signals can act directly on human haematopoietic progenitors that express the β2-adrenergic receptor, promoting their migration and engraftment137. Furthermore, the BM is also innervated by sensory nerves51,129, although very few studies have implicated them in haematopoiesis138,139. Similarly, very little is known about the role of parasympathetic signalling in regulating HSC activity or their microenvironment. However, the type 1 muscarinic receptor (CHRM1), which is one of the receptors for the parasympathetic neurotransmitter acetylcholine in the hypothalamus, has been shown to regulate G-CSF-induced HSC mobilization from the BM via glucocorticoid hormone release140.
Endothelial cells.
The BM is a well-vascularized tissue, enabling several blood cell types, including HSCs, to enter or leave the bloodstream. Bone marrow endothelial cells lining the interior of all blood vessels produce various factors, such as Notch ligands, CXCL12, SCF and pleiotrophin, which regulate HSCs and progenitors’ activity58,84,86,110,141–143. Endothelial cells also play a crucial role after myelosuppressive injury, by producing factors that promote haematopoietic regeneration110,141,142,144–147. In vivo studies have shown that deletion of Gp130 REF148, Scf84, Cxcl1285,86 or Jag1REF145 using endothelial cell-specific Cre strains (Tie2–Cre or Cdh5–Cre (Cdh5 encodes VE-cadherin)), impairs HSC maintenance at steady state, and confirms the role of endothelial cells as niche regulators. Arteriolar endothelial cells (AECs) and sinusoid endothelial cells (SECs) can be distinguished by their differential expression of SCA1 and podoplanin (PDPN); AECs are CD45–Ter119–SCA1highPDPN–, whereas SECs are uniformly CD45–Ter119–SCA1+PDPN+. Interestingly, AECs secrete nearly all detectable endothelial-derived SCF in the BM, as genetic deletion of Scf in AECs, but not in SECs, substantially reduced the number of functional HSCs112. Intriguingly, although the overall abundance of BM endothelial cells is comparable to that of MSPCs149, the levels of CXCL12 and SCF in BM endothelial cells are much lower than in MSPCs109. Thus, it remains possible that extra-medullary Tie2–Cre+ cells may influence the BM niche. Additionally, as the structural integrity and function of endothelial cells and perivascular cells are intimately connected, it is conceivable that deletion of a specific niche or loss of specific factors could have deleterious effects on other niches. In fact, depletion of LEPR+ cells using an inducible diphtheria toxin system produced alterations in VEGFR3+ sinusoids, which became more tortuous, dilated and leaky150. Similarly, the conditional deletion of Angpt1 in perivascular cells and haematopoietic cells resulted in increased vascular leakiness91.
Although hypoxia has been suggested to be an inherent feature of HSPCs that is independent of their spatial localization in the BM architecture43, a recent study indicated that the permeability of different vessels (arterioles versus sinusoids) to blood plasma affects the levels of reactive oxygen species (ROS) in neighbouring HSCs and consequently their localization in the marrow. HSPCs in the vicinity of the less permeable AECs contain low levels of ROS and are thus quiescent, whereas those close to the leakier SECs have increased ROS levels, leading to their activation and augmenting their differentiation and migration31. It is unclear whether these differences arise from their specific metabolic profiles. Future studies should focus on imaging more refined HSC populations, as the higher ROS tolerance of contaminating progenitors may confound the analysis. The large number of studies that investigated the metabolic requirements for HSC activity have been reviewed elsewhere151.
In-depth analyses of the BM vasculature in mice of different ages identified distinct subtypes of blood vessels that have unique molecular identifiers57,59,152 and are associated with distinct types of perivascular cells31,58,60. Notch signalling in BM endothelial cells promotes osteogenesis and the expansion of type-H and arterial vessels as well as their coverage by perivascular PDGFRβ+ mesenchymal cells58,152, which is accompanied by increased SCF levels and HSC abundance in the BM58. Together, these studies further emphasize the complexity of BM niches by revealing the existence of molecular pathways that couple the behaviour of endothelial cells and perivascular mesenchymal cells.
Regulation by HSC descendants
In addition to the stromal niche components, HSCs’ own progeny can also regulate HSC activity in a feedback loop.
Megakaryocytes.
Emerging models of haematopoiesis propose that there is a close relationship between the megakaryocyte lineage and HSCs. Megakaryocyte lineage may even bypass differentiation from multipotent progenitors and instead differentiate directly from HSCs16,18,19,29,153–155. In fact, a subpopulation of HSCs exists that is biased toward megakaryocyte potential and expresses platelet markers, such as CD41 and von Willebrand factor (vWF)19,29, and megakaryocytes directly regulate HSC quiescence156–159. Whole-mount 3D imaging studies of HSC localization combined with computational randomization analyses showed that a subset of HSCs specifically associate with megakaryocytes and that megakaryocyte depletion leads to HSC proliferation156. Megakaryocytes might regulate HSC quiescence by several mechanisms, including secretion of the chemokine CXCL4 (also known as PF4)156, TGFβ (signalling in HSCs requires SHIP1 (also known as PTPN6))157,160 and THPO (mediated by C-type lectin-like receptor 2)158,159. However, selective deletion of Thpo in haematopoietic cells (including megakaryocytes) using Vav1–Cre mice does not alter HSC abundance in the BM92. In contrast to steady-state conditions, megakaryocytes promote HSC niche remodelling following a lethal dose of radiation, through osteolineage cell expansion161,162 and the secretion of fibroblast growth factor 1 (FGF1)157. During emergency myelopoiesis, the production of TGFβ1 and CXCL4 by megakaryocytes surrounding clusters of granulocyte/macrophage progenitor cells (cGMPs) may be an important mechanism to re-establish HSC quiescence and limit the duration of the regenerative response163. Thus, these studies suggest that separate HSC niches exist that promote quiescence — megakaryocytes and arterioles with NG2+ cells and GFAP+ nerves30,136. Vwf–GFP+ HSCs, which are biased towards both platelet formation and the myeloid lineage, closely associate with and are regulated by megakaryocytes, indicating that HSCs are regulated in a feedback loop by their progeny32. Furthermore, megakaryocyte depletion selectively expands Vwf–GFP+ HSCs, whereas depletion of NG2+ arteriolar niche cells selectively depletes Vwf–GFP– HSCs. Thus, these studies demonstrate the existence of two spatially and functionally distinct niches for HSC subpopulations that have distinct developmental potential.
Phagocytic cells.
Investigation of the mechanism of G-CSF-induced HSC mobilization revealed that phagocytic cells in the BM regulate HSC migration. BM macrophages promote HSC retention by regulating osteolineage cells and MSCs, thus antagonising the SNS-mediated inhibition of HSC retention in the BM133,164,165. Splenic red pulp macrophages have an important role in HSC retention in the splenic niche by providing adhesion via VCAM1166. In vivo depletion of macrophages using genetic models or clodronate-loaded liposomes promotes the mobilization of HSPCs into the blood164. Furthermore, BM CD169+ macrophages promote the retention of HSCs in the BM, by inducing the expression of CXCL12 and other HSC retention factors by Nes–GFP+ MSCs165. Additionally, G-CSF-receptor signalling in BM monocytic cells has an important role in eliciting HSPC mobilization133. The crosstalk between macrophages and Nes–GFP+ MSCs may be mediated by macrophage-derived oncostatin M167. Macrophages may also directly regulate HSCs168,169. A rare population of α-smooth muscle actin+ macrophages localized adjacent to HSCs in the BM may protect HSPCs from exhaustion by limiting the production of ROS under stress168. A subset of macrophages expressing duffy antigen receptor for chemokines (DARC) regulates HSC quiescence via TGFβ–SMAD3 signalling169. However, whether the macrophages that directly influence HSCs and those that exert their effects indirectly via the niche are the same, is unknown.
The homeostatic clearance of aged CD62LlowCXCR4high neutrophils from the circulation also regulates the HSC niche170. The daily release and clearance of neutrophils is regulated in a circadian manner, altering CAR cell function and promoting the circadian egress of HSPCs into the circulation. Modulation of the niche is ultimately mediated by BM phagocytic macrophages — which are responsible for neutrophil clearance — through liver X receptor signalling170. While studying the mechanism of fever in models of mobilization, another group showed that G-CSF-induced sympathetic tone stimulates the production of prostaglandin E2 by neutrophils, which can target osteolineage cells to promote HSPC retention171. Tumour necrosis factor (TNF) produced by neutrophils improves vascular recovery following irradiation via TNF receptors on host endothelial cells, which results in enhanced haematopoietic engraftment172. This study highlights a potential novel approach to improve BM transplantation by enhancing the recovery of the niche compartment. Together, these studies provide a formal demonstration that macrophages and neutrophils directly and indirectly regulate HSC activity.
Regulatory T cells.
Initial studies suggested that subsets of T lymphocytes may be beneficial in allogeneic HSC (allo-HSC) engraftment after BM transplantation173,174. Improvements in intravital microscopy techniques enabled researchers to show that allo-HSCs co-localized with FOXP3+ regulatory T (Treg) cells in the endosteal area after transplantation175. Treg cells promote the survival of donor allo-HSCs by secreting the immunoregulatory cytokine IL-10. Moreover, transplanted donor-derived HSCs can persist in non-irradiated recipient mice for 30 days without immunosuppression. However, depletion of FOXP3+ Treg cells leads to rapid loss of allo-HSCs, establishing that Treg cells are thus capable of endowing the HSC niche with immune privilege. Bone marrow CD150high Treg cells seem to localize close to HSCs and promote their quiescence at steady state (potentially by the CD39-catalysed production of adenosine), thereby facilitating allo-HSC engraftment176. The direct and indirect roles of Treg cells in the HSC niche may establish an immune-privileged ‘sanctuary’ from immune attack, not only for healthy HSPCs but also for malignant cells; thus, further investigation of these cells in cancer therapy is warranted.
The ageing niche
Mammalian ageing is characterized by progressive tissue attrition, which is associated with degenerative diseases and an increased incidence of many types of cancers. In the haematopoietic system, age-associated alterations in immune responses that have been attributed to a decline in HSC function contribute to increased susceptibility to infections, autoimmunity, anaemia and myeloproliferative diseases177. Although the absolute number of phenotypically identified HSCs increases with age in mice, the regenerative potential of aged HSCs is reduced178–181. However, these changes in HSC number are not observed in all mouse strains; in fact, the abundance of functional HSCs in BALB/c mice decreases with age182. Furthermore, aged HSCs show myeloid-biased differentiation upon transplantation180,181,183–185, enhanced mobilization into the circulation and reduced homing back to the BM178,186,187. Aged HSCs also localize to sites further away from the endosteum in competitive short-term homing assays in conditioned mice188 and are redistributed away from arteriolar and megakaryocytic niches at steady state189 compared with young HSCs, suggesting that altered HSC distribution is an additional hallmark of ageing.
The functional impairment of aged HSCs compared with their young counterparts when transplanted into young recipients178,180,181 suggests that HSC ageing is a consequence of cell-intrinsic alterations. Indeed, many cell-intrinsic mechanisms involved in HSC ageing have been described, including defects in cell polarity, altered transcriptional and epigenetic profiles, and altered metabolism and DNA damage (reviewed elsewhere190,191). However, transplanted young HSCs engraft at a lower efficiency in aged recipients than in young mice180,192, indicating a role for cell-extrinsic mechanisms in HSC ageing (FIG. 4a). Interestingly, a subpopulation of aged, myeloid-restricted HSCs behaved as ‘latent’ multipotent HSCs, as they had a myeloid-restricted potential in primary transplantation but not in secondary transplantation193. This study suggests that the rejuvenation of HSCs and restoration of their multilineage potential are complex but attainable and highlights a possible role of the HSC microenvironment in this process194 (TABLE 2). For example, increased secretion of the pro-inflammatory chemokine CC-chemokine ligand 5 (CCL5; also known as RANTES) may contribute to the myeloid-biased differentiation of aged HSCs192. Ex vivo treatment of HSCs with CCL5 results in fewer T cells and deletion of Ccl5 in mice rescues the myeloid-biased differentiation. OPN expression by BM stromal cells decreases with age and the injection of thrombin-cleaved OPN attenuates the functional deficits of aged HSCs after transplantation195. MSCs also show skewed differentiation during ageing, which leads to decreased bone formation and increased adipogenesis196,197. The accumulation of adipocytes in the BM may contribute to haematopoietic alterations in aged mice, owing to their negative effects on haematopoiesis123,125 and the concomitant expansion of the myeloid lineage198,199. Age-related bone loss in mice has also been associated with changes in the BM vasculature58,189,200. The number of transitional vessels, arterioles and Osterix+ cells is substantially reduced in aged mice and can be restored by the activation of endothelial Notch signalling, although HSC function is not rejuvenated58. Furthermore, the BM vasculature exhibits increased leakiness, elevated ROS levels and decreased expression of CXCL12, SCF and Jagged 1 with age200. Co-culture of young HSPCs with aged endothelial cells, as well as injection of endothelial cells into mice following myelosuppressive injury, demonstrated that aged endothelial cells impair the function and increase the myeloid differentiation bias of young HSCs. Conversely, young endothelial cells can restore the repopulation capacity of aged HSCs but are unable to reverse their myeloid differentiation bias200.
Figure 4. The adult bone marrow HSC niche in ageing and malignancy.
a | Schematic representation of the aged haematopoietic stem cell (HSC) niche. Aged HSCs are highly proliferative and exhibit increased myeloid-biased differentiation and reduced regenerative capacity. Ageing-related alterations of the bone marrow (BM) niche that influence HSC ageing include alterations in the vasculature and mesenchymal stem and progenitor cells (MSPCs), increased adipogenesis and reduced osteogenesis, altered secretion of niche factors and a reduced number of adrenergic nerves. b | HSC niche alterations that promote cancer. Epigenetic or genetic lesions (lightning bolt) in stromal niche regulators can lead to the loss of inhibitory signals that control the growth of premalignant clones and ultimately promote myeloid malignancies. These lesions include alterations in the expression of RBPJ in endothelial cells, β-catenin in osteoblasts and Dicer 1, SBDS and PTPN11 in MSPCs; and deletion of Rarg (which encodes RARγ), Rb or Mib1 in undefined stromal cells. c | Cancer promotes niche remodelling. The majority of myeloid malignancies are caused by epigenetic and/or genetic mutations in haematopoietic stem and progenitor cells (HSPCs), which lead to BM niche remodelling that supports cancer cell growth at the expense of normal haematopoiesis. Alterations produced by different malignancies can lead to a proinflammatory environment characterized by impaired MSPC differentiation, fibrosis, vascular remodelling, neuropathy and reduced production of HSC-niche factors by stromal cells. Leukemic stem cells (LSCs) can also upregulate the expression of CXCR4, VLA4 and CD44, to hijack the mechanisms of adhesion used by healthy HSPCs. ADRβ3, adrenergic β3 receptor; AML, acute myeloid leukaemia; CCL3, CC chemokine ligand 3; CML, chronic myeloid leukaemia; CXCL12, CXC chemokine ligand 12; CXCR4, CXC chemokine receptor type 4; IL-1 β, interleukin-1β; MDS, myelodysplastic syndrome; MIB1, mind bomb 1; MPN, myeloproliferative neoplasm; NG2, neuron/glial antigen 2; OPN, osteopontin; PTPN11, protein tyrosine phosphatase, non-receptor type 11; RARγ, retinoic acid receptor-γ; RB, retinoblastoma protein; RBPJ, recombination signal binding protein for immunoglobulin-κ J region; ROS, reactive oxygen species; SBDS, Shwachman–Bodian–Diamond syndrome protein; SCF, stem cell factor; SNS, sympathetic nervous system; T-ALL, T cell acute lymphoblastic leukaemia; TGFβ, transforming growth factor-b; THPO, thrombopoietin; VLA-4, very late antigen 4.
TABLE 2:
Niche alterations associated with ageing in the mouse bone marrow.
| Cell type | Alterations associated with ageing |
|---|---|
| Endothelial cells | • Overall increase in vascular density189 but reduced number and length of arterioles58,189 and type H capillaries58 • Increased vascular leakiness and elevated intracellular ROS levels200 • Decreased expression of CXCL12, Jagged 1200 and SCF58,200 |
| Bone marrow MSPCs |
• Decreased osteogenesis and increased adipogenesis189,196,197 • Reduced number of osteoprogenitors58 • Reduced number of perivascular αSMA+ cells58,189, PDGFRβ+ cells and NG2+ cells58 • Expansion of bone marrow MSCs with reduced CFU-F capacity and reduced expression of HSC niche factors189 |
| Compact bone MSPCs | • Reduced number and CFU-F capacity of bone-associated Nes–GFP+ cells189 |
| Osteolineage cells | • Increased expression of CCL5192 • Decreased expression of OPN195 • Reduced number of CD45–Ter119–CD31–SCA1–CD51+ osteoblast-enriched cells195 |
| Adipolineage cells | • Increased number of adipolineage cells125 |
| Nerves | • Reduced number of TH+ adrenergic and β-III tubulin+ nerve fibres189 • Reduced number of synaptophysin+ synapses189 |
| Megakaryocytes | • Increased number of megakaryocytes and megakaryocyte progenitors189 |
αSMA, α-smooth muscle actin; CCL5, CC-chemokine ligand 5; CFU-F, colony-forming unit-fibroblast; CXCL12, CXC-chemokine ligand 12; HSC, haematopoietic stem cell; MSCs, mesenchymal stem cells; MSPCs, mesenchymal stem and progenitor cells; Nes, nestin; NG2, neural/glial antigen 2; OPN, osteopontin; PDGFRβ, platelet-derived growth factor receptor-β; ROS, reactive oxygen species; SCA1, stem cell antigen 1; SCF, stem cell factor; TH, tyrosine 3-hydroxylase.
Ageing also leads to marked remodelling of the BM vasculature, including shortening of arteriolar segments and loss of innervation of arterioles189. Surgical denervation of the bone in young mice results in all the major features of HSC ageing, such as the expansion of myeloid-biased HSCs that have reduced repopulating activity, the acquisition of cell polarity defects, and an increased number of γH2AX foci (a marker of DNA damage) in HSCs. Denervation also induced remodelling of the BM microenvironment that is reminiscent of that in aged mice, including shortening of arteriolar vessels and expansion of MSCs that have reduced clonogenic capacity and expression of HSC niche factors, such as CXCL12 and SCF. Importantly, administration of an ADRβ3-selective agonist in old mice substantially rejuvenated the in vivo function of aged HSCs by acting on BM stromal cells189. Future studies to investigate the interplay between HSC-intrinsic and HSC-extrinsic mechanisms that regulate HSC ageing should offer therapeutic opportunities to mitigate the ageing-related decline in the haematopoietic system.
The haematopoietic stem cell niche in cancer
Consistent with the regulation of haematopoiesis by the niche (FIG. 3), emerging evidence suggests that niche constituents can also drive neoplasia (FIG. 4b) or be remodelled to support malignant cells (FIG. 4c).
Niche-driven malignancies
One of the first indications that an altered BM niche may contribute to malignant transformation comes from two studies201,202 that found that retinoic acid receptor-γ (RARγ) deficiency triggers a myeloproliferative neoplasm (MPN)-like phenotype. This phenotype is observed even when wild-type cells are transplanted into RARγ knockout mice201, showing that RARγ expression in the stromal cells rather than in haematopoietic cells causes neoplasia. In addition, deletion of the gene encoding retinoblastoma-associated protein (Rb1) in both the stromal compartment and myeloid cells results in MPN-like disease202. Similarly, deletion of mind bomb-1 (Mib1), an essential component for Notch ligand endocytosis, in non-haematopoietic cells causes MPN-like disease that can be reverted by activation of Notch in the microenvironment203. These studies show that signals from the microenvironment can influence malignant transformation.
Mesenchymal-derived stromal cells have also been implicated in driving malignancies. Deletion of the gene encoding the RNA-processing enzyme Dicer 1 in MSPCs using Osx–Cre causes myelodysplastic syndrome (MDS) that can sporadically transform to acute myeloid leukaemia (AML). However, deletion of Dicer1 in mature osteoblasts using Bglap–Cre does not result in malignancy204. Loss of Dicer 1 in MSPCs also leads to the reduced expression of Sbds, the gene mutated in human Shwachman–Bodian–Diamond syndrome, which is associated with BM failure and leukaemic predisposition. Sbds deletion in MSPCs stimulates p53 signalling and secretion of the inflammatory molecules S100A8 and S100A9, which impairs haematopoiesis and favours leukaemogenesis by inducing genotoxic stress in HSPCs205. Conditional expression of a mutant protein tyrosine phosphatase non-receptor type 11 (Ptpn11), a positive regulator of RAS signalling, in MSCs and osteoprogenitors can also drive MPN development and progression206. Additional support for the role of osteolineage cells in leukaemogenesis comes from studies of mice with osteoblast-specific expression of constitutively active β-catenin, which results in MDS and from increased Notch signalling in HSCs207. Interestingly, activation of the parathyroid hormone receptor in mouse osteoblasts augments MLL–AF9 oncogene-driven AML, whereas it reduces BCR–ABL chronic myelogenous leukaemia (CML)-like disease, suggesting a disease-specific role for osteolineage cells in leukaemogenesis208. Other cells of the microenvironment may also influence malignant transformation, as genetic disruption of Notch signalling in endothelial cells alters miR-155 expression, activates NF-kB signalling, and increases proinflammatory cytokine production, which can trigger MPN-like disease209. Overall, these studies provide evidence that the microenvironment can cause and/or support haematological malignancy in mice (FIG. 4b). However, it is unclear whether similar alterations in human in the niche only can cause human malignancies, although there are anecdotal reports of donor cell-derived neoplasias after allogeneic stem cell transplantation from a healthy donor210. Whether this results from undetected malignant clones in the donor sample or from an altered microenvironment in the recipient has not been resolved.
Niche remodelling by malignancies
Just as healthy HSPCs can modulate the niche91, so too can leukaemia stem cells (LSCs) in order to compete for the BM niche211 and further remodel it to create a cancer-supportive environment. Leukaemic cells upregulate the expression of several molecules, including CXCR4 REF212–215, VLA4 REF216 (also known as integrin α4β1) and the glycoprotein CD44REF217,218; these molecules mediate leukaemia cell adhesion and survival that confer chemoresistance and are thus being explored as therapeutic targets. BCR–ABL CML cells can induce the differentiation of MSCs into altered osteolineage progenitors through direct cell–cell contact and the secretion of THPO and CCL3, which may impair their capacity to support healthy HSCs and instead promote the growth of leukaemia cells219. Similar observations were reported for various mouse models of acute leukaemia, such as an MLL–AF9-driven model of AML220,221, a BCR–ABL-driven model of blast-crisis CML222 and a model of T cell acute lymphoblastic leukaemia (T-ALL)223,224. Furthermore, studies of primary AML samples from patients also showed that leukaemia cells suppressed BM adipogenesis in favour of AML-supportive osteolineage differentiation from MSCs225,226. Other HSC niche regulators, such as vascular endothelial cells, are also altered in leukaemia. In mouse and human AML, an overall increase in the BM vasculature occurs220,227, although a selective reduction in the number of endosteal blood vessels is observed by intravital microscopy221. In the latter study, vascular remodelling results from the accumulation of local proinflammatory and anti-angiogenic cytokines produced by endosteal AML cells. Interestingly, chemical or genetic rescue of endosteal vessel defects prevented the loss of healthy HSCs and increased the efficacy of chemotherapy221. Similarly, inhibition of endothelial-derived nitric oxide normalizes the BM vascular leakiness observed in AML xenografts and improves the response to the chemotherapeutic agent cytarabine228, suggesting that vascular regulation by malignant cells is a key component of chemoresistance.
Emerging data suggest that nerves — which are predominantly associated with blood vessels in tissues — can regulate solid cancers229–231 and haematologic malignancies220,232. In the MLL–AF9 mouse model of AML, infiltration of leukaemia cells into the BM leads to the degeneration of sympathetic nerve fibres around arterioles, which further promotes AML220. Consistent with this observation, treatment of these mice with the neurotoxin 6-hydroxydopamine, which specifically damages catecholaminergic neurons98,99, increases the number of phenotypic LSCs. Neuropathy is accompanied by the proliferation of NES+ MSPCs that are primed for osteoblastic differentiation and a reduction in the abundance of HSC-maintaining NG2+ periarteriolar niche cells. Blockade of the β2-adrenergic receptor enhances AML infiltration in the BM, whereas a β2-adrenergic agonist delays disease progression220. In a mouse model of MPN induced by the expression of a mutant form of Janus kinase 2 (JAK2V617F), the number of sympathetic fibres and Schwann cells is also reduced, which is triggered by the production of the proinflammatory cytokine IL-1β by mutant HSPCs232. In contrast to its effect in the MLL–AF9 mouse model of AML, neuropathy in this MPN model induces apoptosis of NES+ MSCs. MPN progression is attenuated by the administration of a β3-adrenergic agonist, which restores the sympathetic regulation of MSCs and indirectly reduces the number of LSCs232. Other studies of MPN myelofibrosis found that abnormal megakaryocytes secrete proinflammatory paracrine signals, such as TGFβ233, whereas the aberrant differentiation of LEPR+ MSPCs234 and/or Gli1+ MSPCs235 gives rise to excess myofibroblasts that promote BM fibrosis. Collectively, these studies indicate that although different haematological malignancies share common oncogenesis pathways, it is likely that their interaction with the BM microenvironment may be specific to the leukaemia subtype. Solid tumours of non-haematopoietic origin, such as prostate and breast cancer, can metastasize to the BM and modulate the HSC niche to support tumour growth236. Human metastatic prostate cancer cells can bind to niche-derived CXCL12 and compete with donor HSCs to occupy the niche237,238. Manipulating the neoplastic niche in combination with chemotherapy may thus be a beneficial therapeutic strategy, particularly in malignancies for which conventional therapies have been unsuccessful.
Conclusions and future perspectives
The past 10 years have seen extraordinary growth in the understanding of BM niches and the specific cellular and molecular components that regulate HSC activity. A range of genetically modified mouse strains, combined with reporters and sophisticated imaging techniques associated with computational analyses have enabled the study of the dynamics of endogenous HSCs in their native environment. These studies support the idea that the niches for HSCs and their progeny consist of complex arrangements of cellular constituents whose contributions remain to be fully elucidated. Accumulating data have identified a major role for vascular and perivascular cells in HSC niche regulation. However, the most purified candidate niche cells (for example, CAR cells, Nes–GFP+ cells or LEPR+ cells) far outnumber HSCs, which suggests that these mesenchymal-derived populations require further fractionation or that establishment of a bona fide niche requires the participation of other cellular constituents66. Ongoing studies using single-cell approaches should provide new insights about the heterogeneity of stromal cells in the HSC niche. Although conditional gene deletions or cellular depletion are powerful tools to investigate the factors and cellular participants that are involved in establishment and/or regulation of the HSC niche, the specificity of promoter and Cre recombinase excision activity is an important consideration for the interpretation of experiments in these models. Studies in which single factors are deleted in niche cells must be cautiously interpreted, as there may be compensatory production of the factor by other cells or long-range signals from outside the bone marrow (BOX 2).
The fact that ‘space’ must be made in the BM niche, using preparative myeloablative regimens, to enable HSCs to engraft has led to the notion that niches are saturated at steady state. However, a recent study suggested that the BM may contain numerous unoccupied niches at steady state239, which raises a number of important questions, such as how vacant niches can be unlocked, whether they form reservoirs for haematopoietic emergencies and whether leukaemia cells take advantage of these reservoirs to promote disease progression. These important questions also raise the basic yet important issue of how HSC numbers are regulated in the BM. As current imaging approaches to localize endogenous HSCs in fixed tissues may underestimate niche availability, improvements in intravital microscopy methods and genetic reporters to image HSC–niche interactions in real-time may bypass this limitation.
Studies suggest that haematopoietic progenitors have a major role in the regulation of steady-state haematopoiesis; therefore, further studies are needed to carefully evaluate the structure of putative niches established by haematopoietic progenitors and to identify novel factors that regulate stemness and the maintenance of specific types of progenitors. RNA sequencing and gene-targeting approaches may help with the discovery of these factors and their specific role in HSC and progenitor function, but proteomics-based and lipidomics-based methods ultimately be necessary to investigate more thoroughly the various secreted factors that are presumably required to sustain haematopoiesis. These advances would enable the development of improved chemically defined culture systems and the design of ex vivo HSC expansion protocols to improve gene editing and transplantation efficiency. The development of novel mouse models with a humanized microenvironment that is more permissive for human HSC niche studies would be beneficial, as currently it is difficult to recapitulate certain haematological malignancies in mice.
The underlying changes in the BM niches that promote the development of leukaemia suggest that different leukaemia subtypes can induce specific niche abnormalities and may differ in their niche dependence. Other important questions about niche involvement in malignancy include whether LSCs reside in specific niches and if so, whether LSCs of different classes (for example, myeloid versus lymphoid) localize in different niches; and what the role of these niches is in transformation from pre-leukaemia to acute leukaemia. As the incidence of haematological malignancies increases with age, understanding the mechanisms of HSC and niche ageing and their contribution to malignant transformation may help in the design of therapeutic approaches to rejuvenate an aged haematopoietic system or prevent or treat haematological malignancies.
Acknowledgements
The authors apologize to investigators whose work could not be cited owing to space limitations. The authors thank the members of the Frenette laboratory for helpful discussions and recognize funding support for their laboratory from the US National Institutes of Health (R01DK056638, R01HL069438, U01DK116312, R01DK112976), the Leukemia and Lymphoma Society (LLS-TRP 6475-15) and the New York State Department of Health (NYSTEM IIRP C029570 and C029154).
Glossary terms
- Transplantation
An in vivo procedure to replace the haematopoietic system of a recipient by delivering a sufficient number of donor total bone marrow cells or enriched populations of haematopoietic stem and progenitor cells.
- Self-renewal
The capacity of a cell to divide and give rise to identical cells with equivalent developmental potential, resulting from either an asymmetric cell division that yields a daughter cell and a cell committed to differentiation, or from a symmetric cell division that yields two identical daughter cells.
- Multipotent
The capacity of a single cell to give rise to progeny of multiple lineages.
- Quiescence
The state of being inactive or dormant in the G0 phase of the cell cycle, which is important for long-term function.
- Exhaustion
A state in which the numbers and/or function of HSPCs are compromised owing to high turnover as a consequence of high demand for reconstitution in stress or serial transplantation.
- Homing
The return of haematopoietic stem cells to the bone marrow by trans-endothelial migration.
- Lodgement
The process by which, following homing, a haematopoietic stem cells anchor in a specific region.
- Engraftment
The process by which donor haematopoietic stem cells persist in the host, generate mature progeny as well as re-populate the haematopoietic stem cell pool.
- Trabecular bone
A type of bone tissue, also known as cancellous or spongy bone, which actively remodels and is mostly present in flat and irregular bones, as well as in the epiphysis and metaphysis of long bones.
- Cortical bone
A type of bone tissue, also known as compact bone, which has a mechanical function, forming a hard shell for the long bone diaphysis and accounting for the majority of total bone mass in the human skeleton.
- Endosteum
Inner surface of the bone cavities and the outer surface of the trabeculae bone spicules within the cavities covered by bone-forming osteoblasts and bone-resorbing osteoclasts.
- Periosteum
Tissue that covers the outer surface of the bone and consists of two layers: an outer fibrous layer that contains fibroblasts and collagen fibres and an inner cambium layer that consists of osteoblasts and skeletal stem cells with bone regeneration capacity.
- Sinusoids
Specialized thin-walled capillaries with a wide lumen. Sinusoids distribute evenly throughout the bone marrow and form a web of fenestrated vessels that facilitate trafficking of haematopoietic cells and factors in and out of the circulation.
- Perivascular niche
A specialized microenvironment located adjacent to the bone marrow vasculature, which regulates the maintenance of haematopoietic stem cells and/or progenitor cells.
- Bone marrow stromal cells
Cells of non-haematopoietic origin (classically defined as CD45–Ter119–) in the bone marrow. Stromal cells may or may not include endothelial cells, depending on whether cells that express CD31 or TIE2 are included in the definition.
- Mesenchymal stem cell
(MSC). A self-renewing precursor cell that can differentiate into bone, fat, cartilage and forms stromal cells of the bone marrow.
- Mesenchymal stem and progenitor cells
(MSPCs). A population of stromal cells that remains undefined but is expected to contain self-renewing mesenchymal stem cells and colony-forming mesenchymal progenitor cells.
- Sympathetic nervous system
(SNS). A branch of the autonomic nervous system that emerges from the thoracolumbar spinal cord and prepares the body for situations requiring alertness by releasing noradrenaline, which binds to adrenergic receptors.
- Genotoxic insults.
Radiation or chemical agents that induce damage to the genetic material in cells.
- Allogeneic.
In the context of transplantation, cells or tissue that are from the same species but are genetically different from the recipient.
Footnotes
Competing interests
The authors declare no competing interests.
REFERENCES
- 1.Jagannathan-Bogdan M & Zon LI Hematopoiesis. Development 140, 2463–2467, doi: 10.1242/dev.083147 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaushansky K Lineage-specific hematopoietic growth factors. N Engl J Med 354, 2034–2045, doi: 10.1056/NEJMra052706 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Laurenti E & Gottgens B From haematopoietic stem cells to complex differentiation landscapes. Nature 553, 418–426, doi: 10.1038/nature25022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobson LO, Simmons EL, Marks EK & Eldredge JH Recovery from radiation injury. Science 113, 510–511 (1951). [DOI] [PubMed] [Google Scholar]
- 5.Till JE & Mc CE A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res 14, 213–222 (1961). [PubMed] [Google Scholar]
- 6.Wilson A et al. Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129, doi: 10.1016/j.cell.2008.10.048 (2008). [DOI] [PubMed] [Google Scholar]
- 7.Essers MA et al. IFNalpha activates dormant haematopoietic stem cells in vivo. Nature 458, 904–908, doi: 10.1038/nature07815 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Baldridge MT, King KY, Boles NC, Weksberg DC & Goodell MA Quiescent haematopoietic stem cells are activated by IFN-gamma in response to chronic infection. Nature 465, 793–797, doi: 10.1038/nature09135 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walter D et al. Exit from dormancy provokes DNA-damage-induced attrition in haematopoietic stem cells. Nature 520, 549–552, doi: 10.1038/nature14131 (2015). [DOI] [PubMed] [Google Scholar]
- 10.Cheshier SH, Morrison SJ, Liao X & Weissman IL In vivo proliferation and cell cycle kinetics of long-term self-renewing hematopoietic stem cells. Proc Natl Acad Sci U S A 96, 3120–3125 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng T et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287, 1804–1808 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Arai F et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell 118, 149–161, doi: 10.1016/j.cell.2004.07.004 (2004). [DOI] [PubMed] [Google Scholar]
- 13.Passegue E, Wagers AJ, Giuriato S, Anderson WC & Weissman IL Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. The Journal of experimental medicine 202, 1599–1611, doi: 10.1084/jem.20050967 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun J et al. Clonal dynamics of native haematopoiesis. Nature 514, 322–327, doi: 10.1038/nature13824 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Busch K et al. Fundamental properties of unperturbed haematopoiesis from stem cells in vivo. Nature 518, 542–546, doi: 10.1038/nature14242 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Sawai CM et al. Hematopoietic Stem Cells Are the Major Source of Multilineage Hematopoiesis in Adult Animals. Immunity 45, 597–609, doi: 10.1016/j.immuni.2016.08.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas S, Trumpp A & Milsom MD Causes and Consequences of Hematopoietic Stem Cell Heterogeneity. Cell Stem Cell 22, 627–638, doi: 10.1016/j.stem.2018.04.003 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Notta F et al. Distinct routes of lineage development reshape the human blood hierarchy across ontogeny. Science 351, aab2116, doi: 10.1126/science.aab2116 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto R et al. Clonal analysis unveils self-renewing lineage-restricted progenitors generated directly from hematopoietic stem cells. Cell 154, 1112–1126, doi: 10.1016/j.cell.2013.08.007 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Schofield R The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25 (1978). [PubMed] [Google Scholar]
- 21.Asada N, Takeishi S & Frenette PS Complexity of bone marrow hematopoietic stem cell niche. Int J Hematol 106, 45–54, doi: 10.1007/s12185-017-2262-9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller-Sieburg CE, Cho RH, Thoman M, Adkins B & Sieburg HB Deterministic regulation of hematopoietic stem cell self-renewal and differentiation. Blood 100, 1302–1309 (2002). [PubMed] [Google Scholar]
- 23.Dykstra B et al. Long-term propagation of distinct hematopoietic differentiation programs in vivo. Cell Stem Cell 1, 218–229, doi: 10.1016/j.stem.2007.05.015 (2007). [DOI] [PubMed] [Google Scholar]
- 24.Foudi A et al. Analysis of histone 2B-GFP retention reveals slowly cycling hematopoietic stem cells. Nat Biotechnol 27, 84–90, doi: 10.1038/nbt.1517 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benveniste P et al. Intermediate-term hematopoietic stem cells with extended but time-limited reconstitution potential. Cell Stem Cell 6, 48–58, doi: 10.1016/j.stem.2009.11.014 (2010). [DOI] [PubMed] [Google Scholar]
- 26.Challen GA, Boles NC, Chambers SM & Goodell MA Distinct hematopoietic stem cell subtypes are differentially regulated by TGF-beta1. Cell Stem Cell 6, 265–278, doi: 10.1016/j.stem.2010.02.002 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morita Y, Ema H & Nakauchi H Heterogeneity and hierarchy within the most primitive hematopoietic stem cell compartment. The Journal of experimental medicine 207, 1173–1182, doi: 10.1084/jem.20091318 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Benz C et al. Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell 10, 273–283, doi: 10.1016/j.stem.2012.02.007 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Sanjuan-Pla A et al. Platelet-biased stem cells reside at the apex of the haematopoietic stem-cell hierarchy. Nature, doi: 10.1038/nature12495 (2013). [DOI] [PubMed]
- 30.Kunisaki Y et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 502, 637–643, doi: 10.1038/nature12612 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itkin T et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature 532, 323–328, doi: 10.1038/nature17624 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinho S et al. Lineage-Biased Hematopoietic Stem Cells Are Regulated by Distinct Niches. Dev Cell, doi: 10.1016/j.devcel.2018.01.016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Notta F et al. Isolation of single human hematopoietic stem cells capable of long-term multilineage engraftment. Science 333, 218–221, doi: 10.1126/science.1201219 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Osawa M, Hanada K, Hamada H & Nakauchi H Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science 273, 242–245 (1996). [DOI] [PubMed] [Google Scholar]
- 35.Birbrair A & Frenette PS Niche heterogeneity in the bone marrow. Ann N Y Acad Sci 1370, 82–96, doi: 10.1111/nyas.13016 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kricun ME Red-yellow marrow conversion: its effect on the location of some solitary bone lesions. Skeletal Radiol 14, 10–19 (1985). [DOI] [PubMed] [Google Scholar]
- 37.Kiel MJ, Iwashita T, Yilmaz OH & Morrison SJ Spatial differences in hematopoiesis but not in stem cells indicate a lack of regional patterning in definitive hematopoietic stem cells. Dev Biol 283, 29–39, doi: 10.1016/j.ydbio.2005.03.037 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Lo Celso C et al. Live-animal tracking of individual haematopoietic stem/progenitor cells in their niche. Nature 457, 92–96, doi: 10.1038/nature07434 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Y et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature 457, 97–101, doi: 10.1038/nature07639 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Nilsson SK, Johnston HM & Coverdale JA Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood 97, 2293–2299 (2001). [DOI] [PubMed] [Google Scholar]
- 41.Ellis SL et al. The relationship between bone, hemopoietic stem cells, and vasculature. Blood 118, 1516–1524, doi: 10.1182/blood-2010-08-303800 (2011). [DOI] [PubMed] [Google Scholar]
- 42.Guezguez B et al. Regional localization within the bone marrow influences the functional capacity of human HSCs. Cell Stem Cell 13, 175–189, doi: 10.1016/j.stem.2013.06.015 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Nombela-Arrieta C et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol 15, 533–543, doi: 10.1038/ncb2730 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Suzuki N et al. Combinatorial Gata2 and Sca1 expression defines hematopoietic stem cells in the bone marrow niche. Proc Natl Acad Sci U S A 103, 2202–2207, doi: 10.1073/pnas.0508928103 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haylock DN et al. Hemopoietic stem cells with higher hemopoietic potential reside at the bone marrow endosteum. Stem Cells 25, 1062–1069, doi: 10.1634/stemcells.2006-0528 (2007). [DOI] [PubMed] [Google Scholar]
- 46.Grassinger J, Haylock DN, Williams B, Olsen GH & Nilsson SK Phenotypically identical hemopoietic stem cells isolated from different regions of bone marrow have different biologic potential. Blood 116, 3185–3196, doi: 10.1182/blood-2009-12-260703 (2010). [DOI] [PubMed] [Google Scholar]
- 47.Acar M et al. Deep imaging of bone marrow shows non-dividing stem cells are mainly perisinusoidal. Nature 526, 126–130, doi: 10.1038/nature15250 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chanavaz M Anatomy and histophysiology of the periosteum: quantification of the periosteal blood supply to the adjacent bone with 85Sr and gamma spectrometry. J Oral Implantol 21, 214–219 (1995). [PubMed] [Google Scholar]
- 49.Maryanovich M, Takeishi S & Frenette PS Neural Regulation of Bone and Bone Marrow. Cold Spring Harb Perspect Med, doi: 10.1101/cshperspect.a031344 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bellinger DL, Lorton D, Felten SY & Felten DL Innervation of lymphoid organs and implications in development, aging, and autoimmunity. Int J Immunopharmacol 14, 329–344 (1992). [DOI] [PubMed] [Google Scholar]
- 51.Mach DB et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 113, 155–166 (2002). [DOI] [PubMed] [Google Scholar]
- 52.Bajayo A et al. Skeletal parasympathetic innervation communicates central IL-1 signals regulating bone mass accrual. Proc Natl Acad Sci U S A 109, 15455–15460, doi: 10.1073/pnas.1206061109 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamazaki K & Allen TD Ultrastructural morphometric study of efferent nerve terminals on murine bone marrow stromal cells, and the recognition of a novel anatomical unit: the “neuro-reticular complex”. Am J Anat 187, 261–276, doi: 10.1002/aja.1001870306 (1990). [DOI] [PubMed] [Google Scholar]
- 54.Mendez-Ferrer S et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466, 829–834, doi: 10.1038/nature09262 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Watson EC & Adams RH Biology of Bone: The Vasculature of the Skeletal System. Cold Spring Harb Perspect Med, doi: 10.1101/cshperspect.a031559 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mysorekar VR Diaphysial nutrient foramina in human long bones. J Anat 101, 813–822 (1967). [PMC free article] [PubMed] [Google Scholar]
- 57.Kusumbe AP, Ramasamy SK & Adams RH Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature 507, 323–328, doi: 10.1038/nature13145 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kusumbe AP et al. Age-dependent modulation of vascular niches for haematopoietic stem cells. Nature 532, 380–384, doi: 10.1038/nature17638 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langen UH et al. Cell-matrix signals specify bone endothelial cells during developmental osteogenesis. Nat Cell Biol 19, 189–201, doi: 10.1038/ncb3476 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ramasamy SK et al. Blood flow controls bone vascular function and osteogenesis. Nat Commun 7, 13601, doi: 10.1038/ncomms13601 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trueta J & Morgan JD The vascular contribution to osteogenesis. I. Studies by the injection method. J Bone Joint Surg Br 42-B, 97–109 (1960). [DOI] [PubMed] [Google Scholar]
- 62.Ramasamy SK et al. Regulation of Hematopoiesis and Osteogenesis by Blood Vessel-Derived Signals. Annu Rev Cell Dev Biol 32, 649–675, doi: 10.1146/annurev-cellbio-111315-124936 (2016). [DOI] [PubMed] [Google Scholar]
- 63.Gao X, Xu C, Asada N & Frenette PS The hematopoietic stem cell niche: from embryo to adult. Development 145, doi: 10.1242/dev.139691 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Avecilla ST et al. Chemokine-mediated interaction of hematopoietic progenitors with the bone marrow vascular niche is required for thrombopoiesis. Nat Med 10, 64–71, doi: 10.1038/nm973 (2004). [DOI] [PubMed] [Google Scholar]
- 65.Kiel MJ, Yilmaz OH, Iwashita T, Terhorst C & Morrison SJ SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121, doi: 10.1016/j.cell.2005.05.026 (2005). [DOI] [PubMed] [Google Scholar]
- 66.Wei Q & Frenette PS Niches for Hematopoietic Stem Cells and Their Progeny. Immunity 48, 632–648, doi: 10.1016/j.immuni.2018.03.024 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lord BI, Testa NG & Hendry JH The relative spatial distributions of CFUs and CFUc in the normal mouse femur. Blood 46, 65–72 (1975). [PubMed] [Google Scholar]
- 68.Gong JK Endosteal marrow: a rich source of hematopoietic stem cells. Science 199, 1443–1445 (1978). [DOI] [PubMed] [Google Scholar]
- 69.Taichman RS, Reilly MJ & Emerson SG Human osteoblasts support human hematopoietic progenitor cells in vitro bone marrow cultures. Blood 87, 518–524 (1996). [PubMed] [Google Scholar]
- 70.Calvi LM et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature 425, 841–846, doi: 10.1038/nature02040 (2003). [DOI] [PubMed] [Google Scholar]
- 71.Zhang J et al. Identification of the haematopoietic stem cell niche and control of the niche size. Nature 425, 836–841, doi: 10.1038/nature02041 (2003). [DOI] [PubMed] [Google Scholar]
- 72.Visnjic D et al. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood 103, 3258–3264, doi: 10.1182/blood-2003-11-4011 (2004). [DOI] [PubMed] [Google Scholar]
- 73.Bromberg O et al. Osteoblastic N-cadherin is not required for microenvironmental support and regulation of hematopoietic stem and progenitor cells. Blood 120, 303–313, doi: 10.1182/blood-2011-09-377853 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Greenbaum AM, Revollo LD, Woloszynek JR, Civitelli R & Link DC N-cadherin in osteolineage cells is not required for maintenance of hematopoietic stem cells. Blood 120, 295–302, doi: 10.1182/blood-2011-09-377457 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kiel MJ, Acar M, Radice GL & Morrison SJ Hematopoietic stem cells do not depend on N-cadherin to regulate their maintenance. Cell Stem Cell 4, 170–179, doi: 10.1016/j.stem.2008.10.005 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagasawa T et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 382, 635–638, doi: 10.1038/382635a0 (1996). [DOI] [PubMed] [Google Scholar]
- 77.Petit I et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol 3, 687–694, doi: 10.1038/ni813 (2002). [DOI] [PubMed] [Google Scholar]
- 78.Ara T et al. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 19, 257–267 (2003). [DOI] [PubMed] [Google Scholar]
- 79.Sugiyama T, Kohara H, Noda M & Nagasawa T Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 25, 977–988, doi: 10.1016/j.immuni.2006.10.016 (2006). [DOI] [PubMed] [Google Scholar]
- 80.Barker JE Sl/Sld hematopoietic progenitors are deficient in situ. Exp Hematol 22, 174–177 (1994). [PubMed] [Google Scholar]
- 81.Ogawa M et al. Expression and function of c-kit in hemopoietic progenitor cells. The Journal of experimental medicine 174, 63–71 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ikuta K & Weissman IL Evidence that hematopoietic stem cells express mouse c-kit but do not depend on steel factor for their generation. Proc Natl Acad Sci U S A 89, 1502–1506 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zou YR, Kottmann AH, Kuroda M, Taniuchi I & Littman DR Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 393, 595–599, doi: 10.1038/31269 (1998). [DOI] [PubMed] [Google Scholar]
- 84.Ding L, Saunders TL, Enikolopov G & Morrison SJ Endothelial and perivascular cells maintain haematopoietic stem cells. Nature 481, 457–462, doi: 10.1038/nature10783 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ding L & Morrison SJ Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 495, 231–235, doi: 10.1038/nature11885 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Greenbaum A et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature 495, 227–230, doi: 10.1038/nature11926 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stier S et al. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. The Journal of experimental medicine 201, 1781–1791, doi: 10.1084/jem.20041992 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nilsson SK et al. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood 106, 1232–1239, doi: 10.1182/blood-2004-11-4422 (2005). [DOI] [PubMed] [Google Scholar]
- 89.Qian H et al. Critical role of thrombopoietin in maintaining adult quiescent hematopoietic stem cells. Cell Stem Cell 1, 671–684, doi: 10.1016/j.stem.2007.10.008 (2007). [DOI] [PubMed] [Google Scholar]
- 90.Yoshihara H et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell 1, 685–697, doi: 10.1016/j.stem.2007.10.020 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Zhou BO, Ding L & Morrison SJ Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting Angiopoietin-1. Elife 4, e05521, doi: 10.7554/eLife.05521 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Decker M, Leslie J, Liu Q & Ding L Hepatic thrombopoietin is required for bone marrow hematopoietic stem cell maintenance. Science 360, 106–110, doi: 10.1126/science.aap8861 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhu J et al. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood 109, 3706–3712, doi: 10.1182/blood-2006-08-041384 (2007). [DOI] [PubMed] [Google Scholar]
- 94.Yu VW et al. Specific bone cells produce DLL4 to generate thymus-seeding progenitors from bone marrow. The Journal of experimental medicine 212, 759–774, doi: 10.1084/jem.20141843 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sacchetti B et al. Self-renewing osteoprogenitors in bone marrow sinusoids can organize a hematopoietic microenvironment. Cell 131, 324–336, doi: 10.1016/j.cell.2007.08.025 (2007). [DOI] [PubMed] [Google Scholar]
- 96.Frenette PS, Pinho S, Lucas D & Scheiermann C Mesenchymal stem cell: keystone of the hematopoietic stem cell niche and a stepping-stone for regenerative medicine. Annual review of immunology 31, 285–316, doi: 10.1146/annurev-immunol-032712-095919 (2013). [DOI] [PubMed] [Google Scholar]
- 97.Chan CK et al. Endochondral ossification is required for haematopoietic stem-cell niche formation. Nature 457, 490–494, doi: 10.1038/nature07547 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Katayama Y et al. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124, 407–421, doi: 10.1016/j.cell.2005.10.041 (2006). [DOI] [PubMed] [Google Scholar]
- 99.Mendez-Ferrer S, Lucas D, Battista M & Frenette PS Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–447, doi: 10.1038/nature06685 (2008). [DOI] [PubMed] [Google Scholar]
- 100.Lucas D, Battista M, Shi PA, Isola L & Frenette PS Mobilized hematopoietic stem cell yield depends on species-specific circadian timing. Cell Stem Cell 3, 364–366, doi: 10.1016/j.stem.2008.09.004 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pinho S et al. PDGFRalpha and CD51 mark human nestin+ sphere-forming mesenchymal stem cells capable of hematopoietic progenitor cell expansion. The Journal of experimental medicine 210, 1351–1367, doi: 10.1084/jem.20122252 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Omatsu Y et al. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity 33, 387–399, doi: 10.1016/j.immuni.2010.08.017 (2010). [DOI] [PubMed] [Google Scholar]
- 103.Cordeiro Gomes A et al. Hematopoietic Stem Cell Niches Produce Lineage-Instructive Signals to Control Multipotent Progenitor Differentiation. Immunity 45, 1219–1231, doi: 10.1016/j.immuni.2016.11.004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Omatsu Y, Seike M, Sugiyama T, Kume T & Nagasawa T Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature 508, 536–540, doi: 10.1038/nature13071 (2014). [DOI] [PubMed] [Google Scholar]
- 105.Seike M, Omatsu Y, Watanabe H, Kondoh G & Nagasawa T Stem cell niche-specific Ebf3 maintains the bone marrow cavity. Genes Dev 32, 359–372, doi: 10.1101/gad.311068.117 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yue R, Zhou BO, Shimada IS, Zhao Z & Morrison SJ Leptin Receptor Promotes Adipogenesis and Reduces Osteogenesis by Regulating Mesenchymal Stromal Cells in Adult Bone Marrow. Cell Stem Cell 18, 782–796, doi: 10.1016/j.stem.2016.02.015 (2016). [DOI] [PubMed] [Google Scholar]
- 107.Zhou BO, Yue R, Murphy MM, Peyer JG & Morrison SJ Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell 15, 154–168, doi: 10.1016/j.stem.2014.06.008 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mizoguchi T et al. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell 29, 340–349, doi: 10.1016/j.devcel.2014.03.013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Asada N et al. Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol 19, 214–223, doi: 10.1038/ncb3475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Himburg HA et al. Distinct Bone Marrow Sources of Pleiotrophin Control Hematopoietic Stem Cell Maintenance and Regeneration. Cell Stem Cell 23, 370–381 e375, doi: 10.1016/j.stem.2018.07.003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Isern J et al. The neural crest is a source of mesenchymal stem cells with specialized hematopoietic stem cell niche function. Elife 3, e03696, doi: 10.7554/eLife.03696 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xu CL et al. Stem cell factor is selectively secreted by arterial endothelial cells in bone marrow. Nature Communications 9, doi:ARTN 244910.1038/s41467–018-04726–3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Joseph C et al. Deciphering hematopoietic stem cells in their niches: a critical appraisal of genetic models, lineage tracing, and imaging strategies. Cell Stem Cell 13, 520–533, doi: 10.1016/j.stem.2013.10.010 (2013). [DOI] [PubMed] [Google Scholar]
- 114.Ono N et al. Vasculature-associated cells expressing nestin in developing bones encompass early cells in the osteoblast and endothelial lineage. Dev Cell 29, 330–339, doi: 10.1016/j.devcel.2014.03.014 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Morikawa S et al. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. The Journal of experimental medicine 206, 2483–2496, doi: 10.1084/jem.20091046 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Breitbach M et al. In Vivo Labeling by CD73 Marks Multipotent Stromal Cells and Highlights Endothelial Heterogeneity in the Bone Marrow Niche. Cell Stem Cell 22, 262–2760 e267, doi: 10.1016/j.stem.2018.01.008 (2018). [DOI] [PubMed] [Google Scholar]
- 117.Chan CK et al. Identification and specification of the mouse skeletal stem cell. Cell 160, 285–298, doi: 10.1016/j.cell.2014.12.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Worthley DL et al. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell 160, 269–284, doi: 10.1016/j.cell.2014.11.042 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Short BJ, Brouard N & Simmons PJ Prospective isolation of mesenchymal stem cells from mouse compact bone. Methods Mol Biol 482, 259–268, doi: 10.1007/978-1-59745-060-7_16 (2009). [DOI] [PubMed] [Google Scholar]
- 120.Duchamp de Lageneste O et al. Periosteum contains skeletal stem cells with high bone regenerative potential controlled by Periostin. Nat Commun 9, 773, doi: 10.1038/s41467-018-03124-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Boulais PE et al. The Majority of CD45– Ter119– CD31– Bone Marrow Cell Fraction Is of Hematopoietic Origin and Contains Erythroid and Lymphoid Progenitors. Immunity (2018). [DOI] [PMC free article] [PubMed]
- 122.Yokota T et al. Adiponectin, a new member of the family of soluble defense collagens, negatively regulates the growth of myelomonocytic progenitors and the functions of macrophages. Blood 96, 1723–1732 (2000). [PubMed] [Google Scholar]
- 123.Naveiras O et al. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 460, 259–263, doi: 10.1038/nature08099 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhu RJ, Wu MQ, Li ZJ, Zhang Y & Liu KY Hematopoietic recovery following chemotherapy is improved by BADGE-induced inhibition of adipogenesis. Int J Hematol 97, 58–72, doi: 10.1007/s12185-012-1233-4 (2013). [DOI] [PubMed] [Google Scholar]
- 125.Ambrosi TH et al. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 20, 771–784 e776, doi: 10.1016/j.stem.2017.02.009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhou BO et al. Bone marrow adipocytes promote the regeneration of stem cells and haematopoiesis by secreting SCF. Nat Cell Biol, doi: 10.1038/ncb3570 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Calvo W The innervation of the bone marrow in laboratory animals. Am J Anat 123, 315–328, doi: 10.1002/aja.1001230206 (1968). [DOI] [PubMed] [Google Scholar]
- 128.Artico M et al. Noradrenergic and cholinergic innervation of the bone marrow. Int J Mol Med 10, 77–80 (2002). [PubMed] [Google Scholar]
- 129.Bjurholm A, Kreicbergs A, Brodin E & Schultzberg M Substance P- and CGRP-immunoreactive nerves in bone. Peptides 9, 165–171 (1988). [DOI] [PubMed] [Google Scholar]
- 130.Hattori K et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood 97, 3354–3360 (2001). [DOI] [PubMed] [Google Scholar]
- 131.Asada N et al. Matrix-embedded osteocytes regulate mobilization of hematopoietic stem/progenitor cells. Cell Stem Cell 12, 737–747, doi: 10.1016/j.stem.2013.05.001 (2013). [DOI] [PubMed] [Google Scholar]
- 132.Semerad CL et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood 106, 3020–3027, doi: 10.1182/blood-2004-01-0272 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Christopher MJ, Rao M, Liu F, Woloszynek JR & Link DC Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. The Journal of experimental medicine 208, 251–260, doi: 10.1084/jem.20101700 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lucas D et al. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat Med 19, 695–703, doi: 10.1038/nm.3155 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Park MH et al. Neuropeptide Y regulates the hematopoietic stem cell microenvironment and prevents nerve injury in the bone marrow. EMBO J 34, 1648–1660, doi: 10.15252/embj.201490174 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Yamazaki S et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell 147, 1146–1158, doi: 10.1016/j.cell.2011.09.053 (2011). [DOI] [PubMed] [Google Scholar]
- 137.Spiegel A et al. Catecholaminergic neurotransmitters regulate migration and repopulation of immature human CD34+ cells through Wnt signaling. Nat Immunol 8, 1123–1131, doi: 10.1038/ni1509 (2007). [DOI] [PubMed] [Google Scholar]
- 138.Rameshwar P & Gascon P Substance P (SP) mediates production of stem cell factor and interleukin-1 in bone marrow stroma: potential autoregulatory role for these cytokines in SP receptor expression and induction. Blood 86, 482–490 (1995). [PubMed] [Google Scholar]
- 139.Broome CS & Miyan JA Neuropeptide control of bone marrow neutrophil production. A key axis for neuroimmunomodulation. Ann N Y Acad Sci 917, 424–434 (2000). [DOI] [PubMed] [Google Scholar]
- 140.Pierce H et al. Cholinergic Signals from the CNS Regulate G-CSF-Mediated HSC Mobilization from Bone Marrow via a Glucocorticoid Signaling Relay. Cell Stem Cell 20, 648–658 e644, doi: 10.1016/j.stem.2017.01.002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Butler JM et al. Endothelial cells are essential for the self-renewal and repopulation of Notch-dependent hematopoietic stem cells. Cell Stem Cell 6, 251–264, doi: 10.1016/j.stem.2010.02.001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Himburg HA et al. Pleiotrophin regulates the expansion and regeneration of hematopoietic stem cells. Nat Med 16, 475–482, doi: 10.1038/nm.2119 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Winkler IG et al. Vascular niche E-selectin regulates hematopoietic stem cell dormancy, self renewal and chemoresistance. Nat Med 18, 1651–1657, doi: 10.1038/nm.2969 (2012). [DOI] [PubMed] [Google Scholar]
- 144.Kobayashi H et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol 12, 1046–1056, doi: 10.1038/ncb2108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Poulos MG et al. Endothelial Jagged-1 is necessary for homeostatic and regenerative hematopoiesis. Cell Rep 4, 1022–1034, doi: 10.1016/j.celrep.2013.07.048 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Doan PL et al. Tie2(+) bone marrow endothelial cells regulate hematopoietic stem cell regeneration following radiation injury. Stem Cells 31, 327–337, doi: 10.1002/stem.1275 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guo P et al. Endothelial jagged-2 sustains hematopoietic stem and progenitor reconstitution after myelosuppression. J Clin Invest 127, 4242–4256, doi: 10.1172/JCI92309 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yao L, Yokota T, Xia L, Kincade PW & McEver RP Bone marrow dysfunction in mice lacking the cytokine receptor gp130 in endothelial cells. Blood 106, 4093–4101 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nombela-Arrieta C & Manz MG Quantification and three-dimensional microanatomical organization of the bone marrow. Blood Adv 1, 407–416, doi: 10.1182/bloodadvances.2016003194 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ramalingam P, Poulos MG & Butler JM Regulation of the hematopoietic stem cell lifecycle by the endothelial niche. Curr Opin Hematol 24, 289–299, doi: 10.1097/MOH.0000000000000350 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ito K & Ito K Hematopoietic stem cell fate through metabolic control. Exp Hematol 64, 1–11, doi: 10.1016/j.exphem.2018.05.005 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ramasamy SK, Kusumbe AP, Wang L & Adams RH Endothelial Notch activity promotes angiogenesis and osteogenesis in bone. Nature 507, 376–380, doi: 10.1038/nature13146 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Grover A et al. Single-cell RNA sequencing reveals molecular and functional platelet bias of aged haematopoietic stem cells. Nat Commun 7, 11075, doi: 10.1038/ncomms11075 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rodriguez-Fraticelli AE et al. Clonal analysis of lineage fate in native haematopoiesis. Nature 553, 212–216, doi: 10.1038/nature25168 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Carrelha J et al. Hierarchically related lineage-restricted fates of multipotent haematopoietic stem cells. Nature 554, 106–111, doi: 10.1038/nature25455 (2018). [DOI] [PubMed] [Google Scholar]
- 156.Bruns I et al. Megakaryocytes regulate hematopoietic stem cell quiescence through CXCL4 secretion. Nat Med 20, 1315–1320, doi: 10.1038/nm.3707 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhao M et al. Megakaryocytes maintain homeostatic quiescence and promote post-injury regeneration of hematopoietic stem cells. Nat Med 20, 1321–1326, doi: 10.1038/nm.3706 (2014). [DOI] [PubMed] [Google Scholar]
- 158.Nakamura-Ishizu A, Takubo K, Fujioka M & Suda T Megakaryocytes are essential for HSC quiescence through the production of thrombopoietin. Biochem Biophys Res Commun 454, 353–357, doi: 10.1016/j.bbrc.2014.10.095 (2014). [DOI] [PubMed] [Google Scholar]
- 159.Nakamura-Ishizu A, Takubo K, Kobayashi H, Suzuki-Inoue K & Suda T CLEC-2 in megakaryocytes is critical for maintenance of hematopoietic stem cells in the bone marrow. The Journal of experimental medicine 212, 2133–2146, doi: 10.1084/jem.20150057 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Jiang L et al. SHP-1 regulates hematopoietic stem cell quiescence by coordinating TGF-beta signaling. The Journal of experimental medicine, doi: 10.1084/jem.20171477 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dominici M et al. Restoration and reversible expansion of the osteoblastic hematopoietic stem cell niche after marrow radioablation. Blood 114, 2333–2343, doi: 10.1182/blood-2008-10-183459 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Olson TS et al. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood 121, 5238–5249, doi: 10.1182/blood-2012-10-463414 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Herault A et al. Myeloid progenitor cluster formation drives emergency and leukaemic myelopoiesis. Nature 544, 53–58, doi: 10.1038/nature21693 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Winkler IG et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood 116, 4815–4828, doi: 10.1182/blood-2009-11-253534 (2010). [DOI] [PubMed] [Google Scholar]
- 165.Chow A et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. The Journal of experimental medicine 208, 261–271, doi: 10.1084/jem.20101688 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dutta P et al. Macrophages retain hematopoietic stem cells in the spleen via VCAM-1. The Journal of experimental medicine 212, 497–512, doi: 10.1084/jem.20141642 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Albiero M et al. Bone Marrow Macrophages Contribute to Diabetic Stem Cell Mobilopathy by Producing Oncostatin M. Diabetes 64, 2957–2968, doi: 10.2337/db14-1473 (2015). [DOI] [PubMed] [Google Scholar]
- 168.Ludin A et al. Monocytes-macrophages that express alpha-smooth muscle actin preserve primitive hematopoietic cells in the bone marrow. Nat Immunol 13, 1072–1082, doi: 10.1038/ni.2408 (2012). [DOI] [PubMed] [Google Scholar]
- 169.Hur J et al. CD82/KAI1 Maintains the Dormancy of Long-Term Hematopoietic Stem Cells through Interaction with DARC-Expressing Macrophages. Cell Stem Cell 18, 508–521, doi: 10.1016/j.stem.2016.01.013 (2016). [DOI] [PubMed] [Google Scholar]
- 170.Casanova-Acebes M et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 153, 1025–1035, doi: 10.1016/j.cell.2013.04.040 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kawano Y et al. G-CSF-induced sympathetic tone provokes fever and primes antimobilizing functions of neutrophils via PGE2. Blood 129, 587–597, doi: 10.1182/blood-2016-07-725754 (2017). [DOI] [PubMed] [Google Scholar]
- 172.Bowers E et al. Granulocyte-derived TNFalpha promotes vascular and hematopoietic regeneration in the bone marrow. Nat Med 24, 95–102, doi: 10.1038/nm.4448 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gandy KL, Domen J, Aguila H & Weissman IL CD8+TCR+ and CD8+TCR− cells in whole bone marrow facilitate the engraftment of hematopoietic stem cells across allogeneic barriers. Immunity 11, 579–590 (1999). [DOI] [PubMed] [Google Scholar]
- 174.Kaufman CL et al. Phenotypic characterization of a novel bone marrow-derived cell that facilitates engraftment of allogeneic bone marrow stem cells. Blood 84, 2436–2446 (1994). [PubMed] [Google Scholar]
- 175.Fujisaki J et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature 474, 216–219, doi: 10.1038/nature10160 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hirata Y et al. CD150(high) Bone Marrow Tregs Maintain Hematopoietic Stem Cell Quiescence and Immune Privilege via Adenosine. Cell Stem Cell 22, 445–453 e445, doi: 10.1016/j.stem.2018.01.017 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Geiger H, de Haan G & Florian MC The ageing haematopoietic stem cell compartment. Nat Rev Immunol 13, 376–389, doi: 10.1038/nri3433 (2013). [DOI] [PubMed] [Google Scholar]
- 178.Morrison SJ, Wandycz AM, Akashi K, Globerson A & Weissman IL The aging of hematopoietic stem cells. Nat Med 2, 1011–1016 (1996). [DOI] [PubMed] [Google Scholar]
- 179.de Haan G, Nijhof W & Van Zant G Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood 89, 1543–1550 (1997). [PubMed] [Google Scholar]
- 180.Rossi DJ et al. Cell intrinsic alterations underlie hematopoietic stem cell aging. Proc Natl Acad Sci U S A 102, 9194–9199, doi: 10.1073/pnas.0503280102 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Dykstra B, Olthof S, Schreuder J, Ritsema M & de Haan G Clonal analysis reveals multiple functional defects of aged murine hematopoietic stem cells. The Journal of experimental medicine 208, 2691–2703, doi: 10.1084/jem.20111490 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chen J, Astle CM & Harrison DE Development and aging of primitive hematopoietic stem cells in BALB/cBy mice. Exp Hematol 27, 928–935 (1999). [DOI] [PubMed] [Google Scholar]
- 183.Sudo K, Ema H, Morita Y & Nakauchi H Age-associated characteristics of murine hematopoietic stem cells. The Journal of experimental medicine 192, 1273–1280 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Cho RH, Sieburg HB & Muller-Sieburg CE A new mechanism for the aging of hematopoietic stem cells: aging changes the clonal composition of the stem cell compartment but not individual stem cells. Blood 111, 5553–5561, doi: 10.1182/blood-2007-11-123547 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Beerman I et al. Functionally distinct hematopoietic stem cells modulate hematopoietic lineage potential during aging by a mechanism of clonal expansion. Proc Natl Acad Sci U S A 107, 5465–5470, doi: 10.1073/pnas.1000834107 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Liang Y, Van Zant G & Szilvassy SJ Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood 106, 1479–1487, doi: 10.1182/blood-2004-11-4282 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Xing Z et al. Increased hematopoietic stem cell mobilization in aged mice. Blood 108, 2190–2197, doi: 10.1182/blood-2005-12-010272 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Florian MC et al. Cdc42 activity regulates hematopoietic stem cell aging and rejuvenation. Cell Stem Cell 10, 520–530, doi: 10.1016/j.stem.2012.04.007 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Maryanovich M et al. Adrenergic nerve degeneration in bone marrow drives aging of the hematopoietic stem cell niche. Nat Med, doi: 10.1038/s41591-018-0030-x (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Beerman I & Rossi DJ Epigenetic regulation of hematopoietic stem cell aging. Exp Cell Res 329, 192–199, doi: 10.1016/j.yexcr.2014.09.013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.de Haan G & Lazare SS Aging of hematopoietic stem cells. Blood 131, 479–487, doi: 10.1182/blood-2017-06-746412 (2018). [DOI] [PubMed] [Google Scholar]
- 192.Ergen AV, Boles NC & Goodell MA Rantes/Ccl5 influences hematopoietic stem cell subtypes and causes myeloid skewing. Blood 119, 2500–2509, doi: 10.1182/blood-2011-11-391730 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Yamamoto R et al. Large-Scale Clonal Analysis Resolves Aging of the Mouse Hematopoietic Stem Cell Compartment. Cell Stem Cell 22, 600–607 e604, doi: 10.1016/j.stem.2018.03.013 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nakamura-Ishizu A & Suda T Aging of the hematopoietic stem cells niche. Int J Hematol 100, 317–325, doi: 10.1007/s12185-014-1641-8 (2014). [DOI] [PubMed] [Google Scholar]
- 195.Guidi N et al. Osteopontin attenuates aging-associated phenotypes of hematopoietic stem cells. EMBO J 36, 1463, doi: 10.15252/embj.201796968 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Nishikawa K et al. Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J Clin Invest 120, 3455–3465, doi: 10.1172/JCI42528 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Singh L et al. Aging alters bone-fat reciprocity by shifting in vivo mesenchymal precursor cell fate towards an adipogenic lineage. Bone 85, 29–36, doi: 10.1016/j.bone.2016.01.014 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Adler BJ, Green DE, Pagnotti GM, Chan ME & Rubin CT High fat diet rapidly suppresses B lymphopoiesis by disrupting the supportive capacity of the bone marrow niche. PLoS One 9, e90639, doi: 10.1371/journal.pone.0090639 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Doucette CR et al. A High Fat Diet Increases Bone Marrow Adipose Tissue (MAT) But Does Not Alter Trabecular or Cortical Bone Mass in C57BL/6J Mice. J Cell Physiol 230, 2032–2037, doi: 10.1002/jcp.24954 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Poulos MG et al. Endothelial transplantation rejuvenates aged hematopoietic stem cell function. J Clin Invest 127, 4163–4178, doi: 10.1172/JCI93940 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Walkley CR et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor gamma deficiency. Cell 129, 1097–1110, doi: 10.1016/j.cell.2007.05.014 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Walkley CR, Shea JM, Sims NA, Purton LE & Orkin SH Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell 129, 1081–1095, doi: 10.1016/j.cell.2007.03.055 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kim YW et al. Defective Notch activation in microenvironment leads to myeloproliferative disease. Blood 112, 4628–4638, doi: 10.1182/blood-2008-03-148999 (2008). [DOI] [PubMed] [Google Scholar]
- 204.Raaijmakers MH et al. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature 464, 852–857, doi: 10.1038/nature08851 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zambetti NA et al. Mesenchymal Inflammation Drives Genotoxic Stress in Hematopoietic Stem Cells and Predicts Disease Evolution in Human Pre-leukemia. Cell Stem Cell 19, 613–627, doi: 10.1016/j.stem.2016.08.021 (2016). [DOI] [PubMed] [Google Scholar]
- 206.Dong L et al. Leukaemogenic effects of Ptpn11 activating mutations in the stem cell microenvironment. Nature 539, 304–308, doi: 10.1038/nature20131 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kode A et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature 506, 240–244, doi: 10.1038/nature12883 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Krause DS et al. Differential regulation of myeloid leukemias by the bone marrow microenvironment. Nat Med 19, 1513–1517, doi: 10.1038/nm.3364 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang L et al. Notch-dependent repression of miR-155 in the bone marrow niche regulates hematopoiesis in an NF-kappaB-dependent manner. Cell Stem Cell 15, 51–65, doi: 10.1016/j.stem.2014.04.021 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Wiseman DH Donor cell leukemia: a review. Biol Blood Marrow Transplant 17, 771–789, doi: 10.1016/j.bbmt.2010.10.010 (2011). [DOI] [PubMed] [Google Scholar]
- 211.Boyd AL et al. Niche displacement of human leukemic stem cells uniquely allows their competitive replacement with healthy HSPCs. The Journal of experimental medicine 211, 1925–1935, doi: 10.1084/jem.20140131 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Nervi B et al. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood 113, 6206–6214, doi: 10.1182/blood-2008-06-162123 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zeng Z et al. Targeting the leukemia microenvironment by CXCR4 inhibition overcomes resistance to kinase inhibitors and chemotherapy in AML. Blood 113, 6215–6224, doi: 10.1182/blood-2008-05-158311 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Uy GL et al. A phase ½ study of chemosensitization with the CXCR4 antagonist plerixafor in relapsed or refractory acute myeloid leukemia. Blood 119, 3917–3924, doi: 10.1182/blood-2011-10-383406 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Pitt LA et al. CXCL12-Producing Vascular Endothelial Niches Control Acute T Cell Leukemia Maintenance. Cancer Cell 27, 755–768, doi: 10.1016/j.ccell.2015.05.002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Matsunaga T et al. Interaction between leukemic-cell VLA-4 and stromal fibronectin is a decisive factor for minimal residual disease of acute myelogenous leukemia. Nat Med 9, 1158–1165, doi: 10.1038/nm909 (2003). [DOI] [PubMed] [Google Scholar]
- 217.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F & Dick JE Targeting of CD44 eradicates human acute myeloid leukemic stem cells. Nat Med 12, 1167–1174, doi: 10.1038/nm1483 (2006). [DOI] [PubMed] [Google Scholar]
- 218.Krause DS, Lazarides K, von Andrian UH & Van Etten RA Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med 12, 1175–1180, doi: 10.1038/nm1489 (2006). [DOI] [PubMed] [Google Scholar]
- 219.Schepers K et al. Myeloproliferative neoplasia remodels the endosteal bone marrow niche into a self-reinforcing leukemic niche. Cell Stem Cell 13, 285–299, doi: 10.1016/j.stem.2013.06.009 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Hanoun M et al. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell 15, 365–375, doi: 10.1016/j.stem.2014.06.020 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Duarte D et al. Inhibition of Endosteal Vascular Niche Remodeling Rescues Hematopoietic Stem Cell Loss in AML. Cell Stem Cell 22, 64–77 e66, doi: 10.1016/j.stem.2017.11.006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Frisch BJ et al. Functional inhibition of osteoblastic cells in an in vivo mouse model of myeloid leukemia. Blood 119, 540–550, doi: 10.1182/blood-2011-04-348151 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Hawkins ED et al. T-cell acute leukaemia exhibits dynamic interactions with bone marrow microenvironments. Nature 538, 518–522, doi: 10.1038/nature19801 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Wang W et al. Aberrant Notch Signaling in the Bone Marrow Microenvironment of Acute Lymphoid Leukemia Suppresses Osteoblast-Mediated Support of Hematopoietic Niche Function. Cancer Res 76, 1641–1652, doi: 10.1158/0008-5472.CAN-15-2092 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Battula VL et al. AML-induced osteogenic differentiation in mesenchymal stromal cells supports leukemia growth. JCI Insight 2, doi: 10.1172/jci.insight.90036 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Boyd AL et al. Acute myeloid leukaemia disrupts endogenous myelo-erythropoiesis by compromising the adipocyte bone marrow niche. Nat Cell Biol 19, 1336–1347, doi: 10.1038/ncb3625 (2017). [DOI] [PubMed] [Google Scholar]
- 227.Hussong JW, Rodgers GM & Shami PJ Evidence of increased angiogenesis in patients with acute myeloid leukemia. Blood 95, 309–313 (2000). [PubMed] [Google Scholar]
- 228.Passaro D et al. Increased Vascular Permeability in the Bone Marrow Microenvironment Contributes to Disease Progression and Drug Response in Acute Myeloid Leukemia. Cancer Cell 32, 324–341 e326, doi: 10.1016/j.ccell.2017.08.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Magnon C et al. Autonomic nerve development contributes to prostate cancer progression. Science 341, 1236361, doi: 10.1126/science.1236361 (2013). [DOI] [PubMed] [Google Scholar]
- 230.Zahalka AH et al. Adrenergic nerves activate an angio-metabolic switch in prostate cancer. Science 358, 321–326, doi: 10.1126/science.aah5072 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hayakawa Y et al. Nerve Growth Factor Promotes Gastric Tumorigenesis through Aberrant Cholinergic Signaling. Cancer Cell 31, 21–34, doi: 10.1016/j.ccell.2016.11.005 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Arranz L et al. Neuropathy of haematopoietic stem cell niche is essential for myeloproliferative neoplasms. Nature 512, 78–81, doi: 10.1038/nature13383 (2014). [DOI] [PubMed] [Google Scholar]
- 233.Zingariello M et al. Characterization of the TGF-beta1 signaling abnormalities in the Gata1low mouse model of myelofibrosis. Blood 121, 3345–3363, doi: 10.1182/blood-2012-06-439661 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Decker M et al. Leptin-receptor-expressing bone marrow stromal cells are myofibroblasts in primary myelofibrosis. Nat Cell Biol 19, 677–688, doi: 10.1038/ncb3530 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Schneider RK et al. Gli1(+) Mesenchymal Stromal Cells Are a Key Driver of Bone Marrow Fibrosis and an Important Cellular Therapeutic Target. Cell Stem Cell 20, 785–800 e788, doi: 10.1016/j.stem.2017.03.008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Croucher PI, McDonald MM & Martin TJ Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer 16, 373–386, doi: 10.1038/nrc.2016.44 (2016). [DOI] [PubMed] [Google Scholar]
- 237.Wang J et al. The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem 283, 4283–4294, doi: 10.1074/jbc.M707465200 (2008). [DOI] [PubMed] [Google Scholar]
- 238.Shiozawa Y et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest 121, 1298–1312, doi: 10.1172/JCI43414 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Shimoto M, Sugiyama T & Nagasawa T Numerous niches for hematopoietic stem cells remain empty during homeostasis. Blood 129, 2124–2131, doi: 10.1182/blood-2016-09-740563 (2017). [DOI] [PubMed] [Google Scholar]
- 240.Silberstein L et al. Proximity-Based Differential Single-Cell Analysis of the Niche to Identify Stem/Progenitor Cell Regulators. Cell Stem Cell 19, 530–543, doi: 10.1016/j.stem.2016.07.004 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Kubota Y, Takubo K & Suda T Bone marrow long label-retaining cells reside in the sinusoidal hypoxic niche. Biochem Biophys Res Commun 366, 335–339, doi: 10.1016/j.bbrc.2007.11.086 (2008). [DOI] [PubMed] [Google Scholar]
- 242.Takaku T et al. Hematopoiesis in 3 dimensions: human and murine bone marrow architecture visualized by confocal microscopy. Blood 116, e41–55, doi: 10.1182/blood-2010-02-268466 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Chen JY et al. Hoxb5 marks long-term haematopoietic stem cells and reveals a homogenous perivascular niche. Nature 530, 223–227, doi: 10.1038/nature16943 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Richardson DS & Lichtman JW Clarifying Tissue Clearing. Cell 162, 246–257, doi: 10.1016/j.cell.2015.06.067 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Ito K et al. Self-renewal of a purified Tie2+ hematopoietic stem cell population relies on mitochondrial clearance. Science 354, 1156–1160, doi: 10.1126/science.aaf5530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Gazit R et al. Fgd5 identifies hematopoietic stem cells in the murine bone marrow. The Journal of experimental medicine 211, 1315–1331, doi: 10.1084/jem.20130428 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Koechlein CS et al. High-resolution imaging and computational analysis of haematopoietic cell dynamics in vivo. Nat Commun 7, 12169, doi: 10.1038/ncomms12169 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Kataoka K et al. Evi1 is essential for hematopoietic stem cell self-renewal, and its expression marks hematopoietic cells with long-term multilineage repopulating activity. The Journal of experimental medicine 208, 2403–2416, doi: 10.1084/jem.20110447 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sugimura R et al. Noncanonical Wnt signaling maintains hematopoietic stem cells in the niche. Cell 150, 351–365, doi: 10.1016/j.cell.2012.05.041 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Cabezas-Wallscheid N et al. Vitamin A-Retinoic Acid Signaling Regulates Hematopoietic Stem Cell Dormancy. Cell 169, 807–823 e819, doi: 10.1016/j.cell.2017.04.018 (2017). [DOI] [PubMed] [Google Scholar]
- 251.Chen X et al. Bone Marrow Myeloid Cells Regulate Myeloid-Biased Hematopoietic Stem Cells via a Histamine-Dependent Feedback Loop. Cell Stem Cell 21, 747–760 e747, doi: 10.1016/j.stem.2017.11.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Tajima Y et al. Continuous cell supply from Krt7-expressing hematopoietic stem cells during native hematopoiesis revealed by targeted in vivo gene transfer method. Sci Rep 7, 40684, doi: 10.1038/srep40684 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Hills D et al. Hoxb4-YFP reporter mouse model: a novel tool for tracking HSC development and studying the role of Hoxb4 in hematopoiesis. Blood 117, 3521–3528, doi: 10.1182/blood-2009-12-253989 (2011). [DOI] [PubMed] [Google Scholar]