Abstract
Objective:
To evaluate temporal trends in medication-assisted treatment utilization among pregnant women with opioid use disorder.
Methods:
We conducted a retrospective cohort study using Pennsylvania Medicaid administrative data. Trends in medication-assisted treatment utilization, opioid pharmacotherapy (methadone and buprenorphine) and behavioral health counselling, were calculated using pharmacy records and procedure codes. Cochrane-Armitage tests evaluated linear trends in characteristics of pregnant women using methadone versus buprenorphine.
Results:
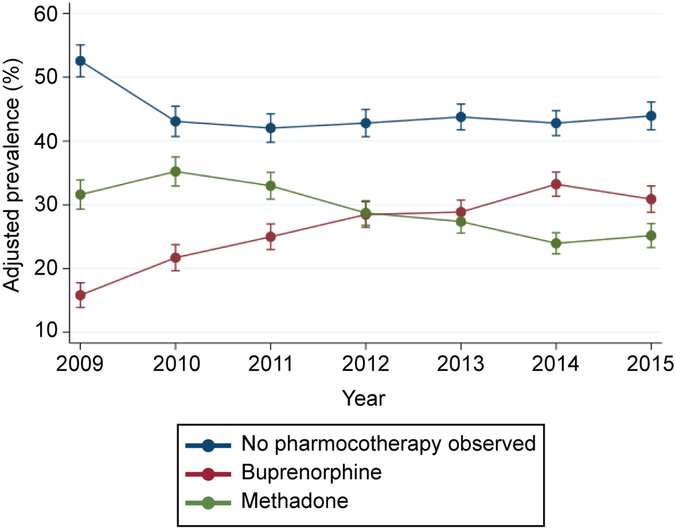
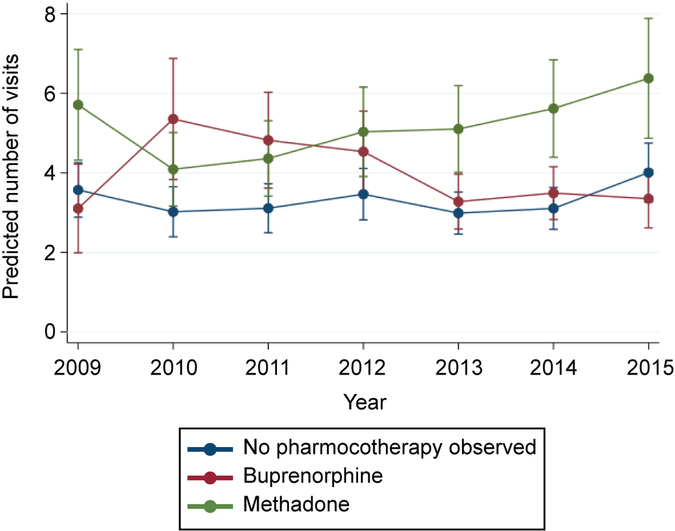
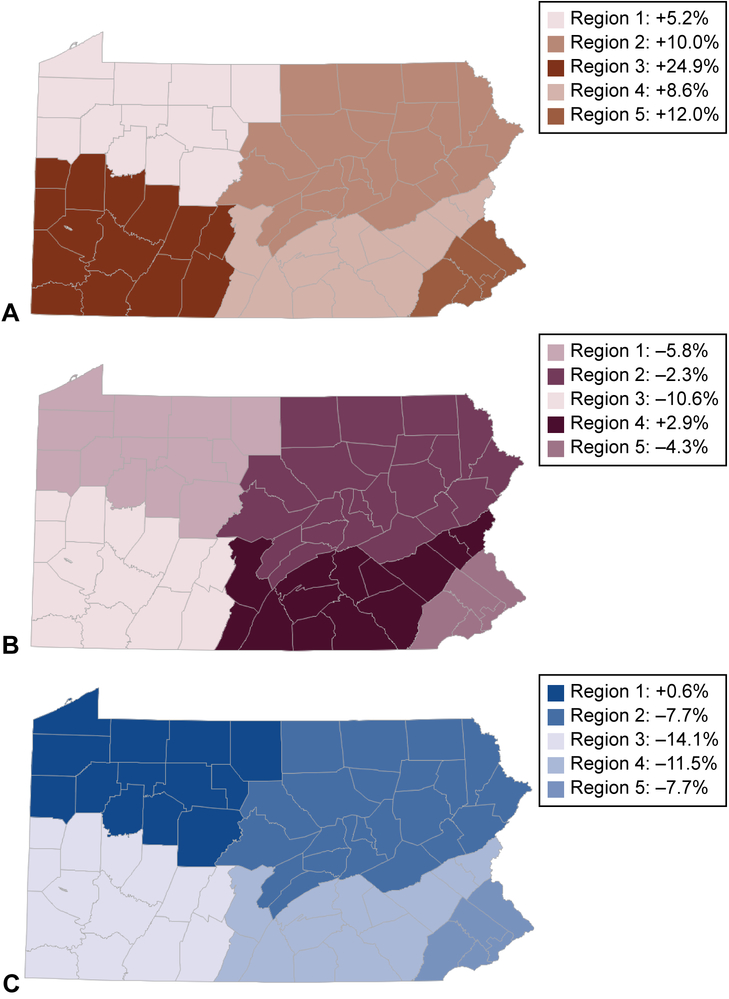
In total, we evaluated 12,587 pregnancies among 10,741 women with opioid use disorder who had a live birth between 2009 and 2015. Across all years, 44.1% of pregnant women received no opioid pharmacotherapy, 27.1% used buprenorphine, and 28.8% methadone. Fewer than half of women had any behavioral health counseling during pregnancy. The adjusted prevalence of methadone use declined from 31.6% (95% CI: 29.3%–33.9%) in 2009 to 25.2% (95% CI: 23.3%–27.1%) in 2015, while the adjusted prevalence of buprenorphine use increased from 15.8% (95% CI: 13.9%–17.8%) to 30.9% (95% CI: 28.8%–33.0%). Greater increases in buprenorphine use were found in geographic regions with large metropolitan centers, such as the Southwest (+24.9%) and the Southeast (+12.0%), compared to largely rural regions, such as the New West (+5.2%). In 2015, the adjusted number of behavioral health counseling visits during pregnancy was 3.4 (95% CI: 2.6–4.1) among women using buprenorphine, 4.0 (95% CI: 3.3–4.7) among women who did not use pharmacotherapy, and 6.4 (95% CI: 4.9–7.9) among women using methadone.
Conclusions:
Buprenorphine use among Medicaid-enrolled pregnant women with opioid use disorder increased significantly over time, with a small concurrent decline in methadone use. Behavioral health counseling utilization was low, but highest among women using methadone.
Precis
Although medication-assisted treatment use during pregnancy increased over the past decade, gaps between treatment need and receipt remain.
INTRODUCTION
Over the past 15 years, the prevalence of opioid use disorder among pregnant women has increased 333% resulting in increased treatment needs during pregnancy.1 Medication-assisted treatment, consisting of opioid pharmacotherapy and behavioral health counseling, is the recommended, evidence-based treatment approach for opioid use disorder during pregnancy.2–5 A consensus of government agencies and professional organizations, including the American College of Obstetrics and Gynecology (ACOG), endorse the effectiveness of medication-assisted treatment for pregnant women, which improves maternal health outcomes by providing a stable opioid dosing regimen, reducing illicit drug use and decreasing behaviors that increase the risk for HIV and HCV infection.2–5 Medication-assisted treatment use during pregnancy also improves neonatal health outcomes by minimizing the fetal stress response that results from illicit opioid use.6
Despite recommendations, medication-assisted treatment is underutilized during pregnancy. Evaluations of the Treatment Episodes Data Set (TEDS) indicate that only half of pregnant women with opioid use disorder admitted to substance use treatment facilities in the United States received opioid pharmacotherapy, which has remained relatively unchanged over the past 20 years.7,8 Although previous research highlights significant unmet treatment need, prior analyses are limited to publicly funded treatment programs, have not investigated differences in opioid pharmacotherapy type (methadone versus buprenorphine), and have not evaluated behavioral health counseling utilization, an important component of comprehensive substance use treatment.7,8 As such, a population-level assessment of temporal trends in medication-assisted treatment utilization patterns during pregnancy remain largely unknown.
State Medicaid programs comprise the single largest payer in the United States for pregnancy and births9 and are the primary insurer for women with opioid use disorder.10,11 Because of this, many women with opioid use disorder reengage or newly engage in healthcare services during pregnancy and obstetric providers often assume the critical role of linking patients to treatment.12 Thus, the purpose of this research is to describe temporal trends in medication-assisted treatment utilization among pregnant women with opioid use disorder enrolled in Pennsylvania Medicaid. Specifically, our objectives are to evaluate a) individual-level factors associated with medication-assisted treatment use during pregnancy, b) temporal trends in methadone versus buprenorphine use across rural and urban geographic regions, and c) corresponding temporal trends in behavioral health counseling use. Our evaluation can help focus ongoing efforts to expand treatment access and availability during pregnancy.
METHODS
For this retrospective cohort study, we utilized administrative healthcare claims data from the Pennsylvania Department of Health and Human Services Medicaid Program. This dataset is composed of claims, encounters and pharmacy data for all Medicaid enrollees in Pennsylvania (including Medicaid managed care and traditional fee-for-service plans) from January 1, 2008-September 30, 2015. In Pennsylvania, Medicaid provides reimbursement for all components of medication-assisted treatment including methadone, buprenorphine and behavioral health counseling services.13 Demographic information, including age, race-ethnicity, and county of residence identified by enrollee zip codes were obtained from patient enrollment files. Medication-assisted treatment utilization data, including buprenorphine prescription fills, methadone treatment services and behavioral health counseling visits were obtained from pharmacy, professional, outpatient and inpatient claims data. Pennsylvania Medicaid includes 5 regions that correspond to state population centers and are served by different managed care organizations.14 These Medicaid regional classifications were used to identify variation in medication-assisted treatment utilization across geographic regions within Pennsylvania. The study was approved by the University of Pittsburgh, Institutional Review Board (IRB).
We identified Medicaid-enrolled women ages 15–44 years who had a live birth from January 1, 2009-September 30, 2015 and who had an International Classification of Diseases, Ninth Revision (ICD-9) diagnosis of OUD (304.0X, 304.7X, 305.50, 305.51, 305.52) during their pregnancy. Because a woman could have more than one pregnancy in the dataset, the pregnancy was chosen as the unit of analysis and medication-assisted treatment use during each pregnancy was evaluated. Live births were identified using the date of delivery in inpatient data. The pregnancy period was approximated using an algorithm validated by the National Committee for Quality Assurance.15 Using this method, we calculated 280 days prior to the date of delivery to approximate the date of conception. Because we focused on pregnancies that resulted in a preterm or term live birth, if a woman had an interpregnancy interval of less than 24 weeks (<168 days), the later pregnancy was excluded from the analysis.
Demographic characteristics included continuous as well as categorical measures of patient age (15–19, 20–34, 35–45 years), race (African American, Caucasian, Asian, or other) and ethnicity (Hispanic or Latina). Urban versus rural county of patient residence was defined based on the Rural Urban Commuting Area (RUCA) codes developed by the United States Department of Agriculture.16 All metropolitan areas are defined as urban, and rural areas include micropolitan areas, small towns, and rural areas. Medical co-morbidities were identified by ICD-9 diagnostic codes that were associated with the pregnancy in the dataset. Medical co-morbidity diagnoses included in the analysis were pre-gestational diabetes mellitus, chronic hypertension, asthma, thyroid disorder, HIV, hepatitis C virus (HCV) and psychiatric disorders. Psychiatric disorders included major depressive disorder, bipolar disorder and schizophrenia. Pregnancy-associated co-morbidities included were gestational diabetes mellitus and gestational hypertensive disorders which included gestational hypertension, pre-eclampsia and eclampsia.
Substance use during pregnancy other than opioid use was identified by ICD-9 codes for tobacco, alcohol, cocaine, marijuana, amphetamine, hallucinogen, sedatives and other substance use or drug dependence during pregnancy. Opioid pharmacotherapy utilization with either methadone or buprenorphine was also identified. Buprenorphine use was identified according to National Drug Codes for buprenorphine formulations and was defined as having ≥ 1 outpatient prescription fill for buprenorphine during pregnancy. Methadone use in pregnancy was identified through outpatient and professional claims for methadone treatment services (procedure codes H0020 or J1230) during pregnancy. We created 3 mutually exclusive opioid pharmacotherapy utilization groups: methadone only, any buprenorphine, and no pharmacotherapy. In a small number of pregnancies (n=365, 2.9%) we observed claims for both buprenorphine and methadone. Because of the extremely small sample size of the combined group, we categorized these pregnancies in the “any buprenorphine group.” We chose to create an “any buprenorphine” and a “methadone only” group because women are clinically more likely to switch from buprenorphine to methadone than methadone to buprenorphine during pregnancy.
Behavioral health counseling visits for each patient during pregnancy were identified in professional claims using procedure codes. We also categorized behavioral health counseling professional claims by the ICD-9 diagnostic codes associated with those claims. Counseling for drug or alcohol abuse was defined as a behavioral health professional claim associated with a diagnostic code for drug or alcohol abuse. Counseling for a psychiatric disorder was defined as a behavioral health professional claim associated with a diagnostic code for a psychiatric disorder.
Geographic trends in opioid pharmacotherapy utilization over time were evaluated across five Medicaid regions designated by the Pennsylvania Department of Health and Human Services: New East, Southeast, Lehigh-Capital, New West and Southwest. Within each region, each county was defined as rural versus urban according to each county’s population density as defined by the Center for Rural Pennsylvania.17 The New West (Region 1) includes Erie and has 13 counties, 12 (92.3%) of which are rural. The New East (Region 2) includes Scranton and has 22 counties, 20 (90.9%) of which are rural. The Southwest (Region 3) includes the greater Pittsburgh area and has 14 counties, 11 (78.6%) of which are rural. The Lehigh-Capital (Region 4) includes Harrisburg and has 13 counties, 5 (38.5%) of which are rural. The Southeast (Region 5) includes the greater Philadelphia area and has 5 counties, none (0%) of which are rural.
We stratified the descriptive statistics of our study cohort by category of opioid pharmacotherapy to identify potential differences among the study population for each group using the chi-square test. Multivariable multinomial logistic regression was used to evaluate time trends in the prevalence of opioid pharmacotherapy utilization and negative binomial regression was used to evaluate the frequency of behavioral health visits during pregnancy. Both opioid pharmacotherapy and behavioral health visit models were adjusted for year of delivery, age, race, ethnicity, Medicaid region, medical co-morbidities including psychiatric disorder, HCV and HIV infection and substance use history including tobacco, alcohol and substance use other than opioid use. After running the regression analysis, predictive margins of the outcome for each year were obtained to generate adjusted prevalence estimates in each year. Adjusted prevalence estimates in each year were reported with 95% confidence intervals (CI).
We also calculated the changes over time in opioid pharmacotherapy utilization between geographic regions in Pennsylvania. Specifically, we calculated the percentage change from 2009–2015 in no opioid pharmacotherapy observed, buprenorphine, and methadone among pregnant women in each of the 5 geographic regions. All previously described variables were included as independent variables. P-values less than 0.05 were significant for all tests. All programming and statistical analyses were performed using the SAS software version 9.4.
RESULTS
From January 1, 2009-September 30, 2015, we identified 354,891 Medicaid enrolled pregnancies with a live birth. Within this sample, we identified a cohort of 12,587 pregnancies (3.5%) among 10,741 women who had a diagnosis of opioid used disorder (Table 1). Among 12,587 pregnancies, 5,553 (44.1%) did not use opioid pharmacotherapy during pregnancy. Of the 7,034 women who received opioid pharmacotherapy, 3,618 (51.4%) received methadone and 3,416 (48.6%) received buprenorphine. Overall, 4,930 (39.2%) women with opioid use disorder received behavioral health counseling during pregnancy. The majority of behavioral health claims (59.8%) were associated with drug and alcohol diagnoses with the remaining claims associated with psychiatric disorder diagnoses (7.9%), both drug and alcohol and psychiatric disorder diagnoses (13.2%), and a mix of other pregnancy-related diagnoses codes (19.1%).
Table 1.
Characteristics of pregnancies with opioid use disorder in Pennsylvania Medicaid, overall and stratified by opioid pharmacotherapy, 2009–2015*
| Characteristic | Overall n=12,587 |
No pharmacotherapy n=5,553 |
Methadone n=3,618 |
Buprenorphine n=3,416 |
p-value⸶ |
|---|---|---|---|---|---|
| Demographics | |||||
| Age [years; mean ±SD] | 27.7 ± 4.7 | 27.3 ± 4.8 | 28.0 ± 4.5 | 27.8 ± 4.6 | <0.01 |
| 15–19 | 331 (2.6) | 218 (3.9) | 59 (1.6) | 54 (1.6) | <0.01 |
| 20–34 | 11357 (90.2) | 4970 (89.5) | 3281 (90.7) | 3106 (90.9) | |
| ≥ 35 | 899 (7.1) | 365 (6.6) | 278 (7.7) | 256 (7.5) | |
| Race and ethnicity | |||||
| Non-Hispanic white | 11047 (87.8) | 4511 (81.2) | 3277 (90.6) | 3259 (95.4) | <0.01 |
| Non-Hispanic black | 961 (7.6) | 703 (12.7) | 174 (4.8) | 84 (2.5) | |
| Hispanic | 393 (3.1) | 253 (4.6) | 105 (2.9) | 35 (1.0) | |
| Other | 186 (1.5) | 86 (1.6) | 62 (1.7) | 38 (1.1) | |
| County of residence | |||||
| Urban | 10229 (81.3) | 4492 (80.9) | 3201 (88.5) | 2536 (74.2) | <0.01 |
| Rural | 2358 (18.7) | 1061 (19.1) | 417 (11.5) | 880 (25.8) | |
| Medical co-morbidities | |||||
| Psychiatric disorderǂ | 5370 (42.7) | 2288 (41.2) | 1617 (44.7) | 1465 (42.9) | <0.01 |
| Hepatitis C virus (HCV) | 3879 (30.8) | 1324 (23.8) | 1539 (42.5) | 1016 (29.7) | <0.01 |
| Asthma | 1812 (14.4) | 810 (14.6) | 528 (14.6) | 474 (13.9) | 0.60 |
| Chronic hypertension | 450 (3.6) | 209 (3.8) | 125 (3.5) | 116 (3.4) | 0.59 |
| Thyroid disorder | 390 (3.1) | 166 (3) | 107 (3.0) | 117 (3.4) | 0.43 |
| Diabetes mellitus | 188 (1.5) | 89 (1.6) | 62 (1.7) | 37 (1.1) | 0.06 |
| HIV | 78 (0.6) | 32 (0.6) | 31 (0.9) | 15 (0.4) | 0.07 |
| Pregnancy-associated co-morbidities | |||||
| Hypertensive disorder | 1335 (10.6) | 661 (11.9) | 370 (10.2) | 304 (8.9) | <0.01 |
| Gestational diabetes | 917 (7.3) | 447 (8.1) | 234 (6.5) | 236 (6.9) | 0.01 |
| Substance use in pregnancy | |||||
| Alcohol | 856 (6.8) | 467 (8.4) | 198 (5.5) | 191 (5.6) | <0.01 |
| Tobacco | 9009 (71.6) | 3747 (67.5) | 2767 (76.5) | 2495 (73.0) | <0.01 |
| Polysubstance use§ | 10338 (82.1) | 4132 (74.4) | 3377 (93.3) | 2829 (82.8) | <0.01 |
| Medication-assisted treatment (MAT) utilization | |||||
| Behavioral health counseling (≥ 1 visit) | 4930 (39.2) | 2007 (36.31) | 1466 (40.5) | 1457 (42.7) | <0.01 |
| Counseling classification | |||||
| Drug and alcohol | 2946 (59.8) | 1241 (61.8) | 817 (55.7) | 888 (60.9) | <0.01 |
| Psychiatric disorder | 391 (7.9) | 108 (5.4) | 124 (8.5) | 159 (10.9) | |
| Both | 649 (13.2) | 243 (12.1) | 204 (13.9) | 202 (13.9) | |
| Other | 944 (19.1) | 415 (20.7) | 321 (21.9) | 208 (14.3) | |
| Opioid pharmacotherapy | |||||
| None | 5553 (44.1) | 5553 (100) | |||
| Methadone | 3618 (28.8) | 3618 (100) | |||
| Buprenorphine | 3416 (27.1) | 3416 (100) | |||
n (%) unless otherwise specified;
difference comparing 2009 to 2015;
defined as a diagnosis of major depressive disorder, bipolar disorder, or schizophrenia;
defined as a diagnosis of cocaine, marijuana, amphetamine, hallucinogen and/or sedatives use during pregnancy
Between 2009 and 2015, the adjusted prevalence of no opioid pharmacotherapy observed during pregnancy decreased from 52.6% (95% CI: 50.1%–55.1%) to 43.9% (95% CI: 41.8%–46.1%) with the most significant decrease occurring between 2009 and 2010 (9.5%) (Figure 1). Increased utilization rates were largely due to buprenorphine use which increased from 15.8% (95% CI: 13.9%–17.8%) to 30.9% (95% CI: 28.8%–33.0%) between 2009 and 2015. In contrast, methadone use decreased from 31.6% (95% CI: 29.3%–33.9%) to 25.2% (95% CI: 23.3%–27.1%) during the study period.
Figure 1.
Opioid pharmacotherapy utilization among Medicaid-enrolled pregnant women with opioid use disorder, 2009–2015. Error bars represent 95% CIs. Prevalences are adjusted. Refer to the methods section for the list of all variables included in the multivariable model and Appendix 1 for crude and adjusted prevalences.
Between 2009 and 2015, an increase in the adjusted number of behavioral health visits occurred among women who received methadone during pregnancy, 5.7 (95% CI: 4.3–7.1) versus 6.4 (95% CI: 4.9–7.9) visits, although the increase was not statistically significant. (Figure 2). There were no significant changes over time in the adjusted number of behavioral health visits during pregnancy among women using buprenorphine (3.1 versus 3.4 visits) and among women with no observed opioid pharmacotherapy use (3.6 versus 4.0 visits). However, in 2014 and 2015, we did find significant differences in the number of counseling visits during pregnancy by opioid pharmacotherapy type. In 2015, the adjusted number of behavioral health counseling visits during pregnancy was 3.4 (95% CI: 2.6–4.1) among women using buprenorphine, 4.0 (95% CI: 3.3–4.7) among women who did not use pharmacotherapy, and 6.4 (95% CI: 4.9–7.9) among women using methadone (Figure 2). Crude and adjusted prevalences and counts for Figures 1 and 2 are described in Appendixes 1 and 2, available online at http://links.lww.com/xxx.
Figure 2.
Behavioral health counseling utilization by opioid pharmacotherapy type among Medicaid-enrolled pregnant women with opioid use disorder, 2009–2015. Error bars represent 95% CIs. Predicted number of visit counts are adjusted. Refer to the methods section for the list of all variables included in the multivariable model and Appendix 2 for crude and adjusted counts.
Between 2009 and 2015, we found a significant decrease in no opioid pharmacotherapy observed during pregnancy across all 5 Medicaid regions except for the New West (Region 1) which had a +0.6% increase. The New West region includes Erie and is the most rural Medicaid region with 12 of 13 counties (92.3%) designated as rural. Accordingly, we found an increase in buprenorphine use across all Medicaid regions with the most significant increases found in regions with large urban centers such as the Southwest (+24.9%) and the Southeast (+12.0%) compared to the largely rural New West region (+5.2%). The Southwest region includes the greater Pittsburgh metropolitan area and the Southeast region includes the greater Philadelphia area. Conversely, we found a decrease in methadone utilization during pregnancy across all Medicaid regions except for the Lehigh-Capital region which had a +2.9% increase in methadone utilization during pregnancy between 2009 and 2015.
DISCUSSION
Our evaluation of medication-assisted treatment utilization patterns among Medicaid-enrolled pregnant women with opioid use disorder demonstrates that opioid pharmacotherapy use increased by approximately 10% over the past decade with utilization rates largely driven by an increase in buprenorphine use in urban geographic areas. Despite these gains, a significant gap between treatment need and receipt remains. In 2015, only 56% of women with opioid use disorder received opioid pharmacotherapy during pregnancy and fewer than 40% had a claim for a behavioral health services. Behavioral health utilization varied by opioid pharmacotherapy type. In 2014 and 2015, women who received methadone had significantly more counseling visits during pregnancy than women who received buprenorphine and women with no pharmacotherapy observed. Therefore, despite current recommendations emphasizing the importance of medication-assisted treatment use during pregnancy, our findings demonstrate the need to further expand medication-assisted treatment access and availability for pregnant women with opioid use disorder.
Increases in pharmacotherapy utilization were largely driven by increases in buprenorphine use during pregnancy, which more than doubled over the study period and was accompanied by a corresponding decline in methadone use. Similar trends in pharmacotherapy utilization have been found in the general Medicaid population where buprenorphine has been the primary driver of increased pharmacotherapy use.18 Buprenorphine’s office-based availability, pharmacy-based dispensing, and decreased risk of overdose compared to methadone have resulted in a multitude of initiatives from federal, state and local agencies to rapidly expand the number of buprenorphine waivered physicians and outpatient programs to meet increased demands for substance use treatment.19,20 Further, randomized clinical trials have established buprenorphine’s safety in pregnancy and demonstrated superior neonatal outcomes including a shorter treatment duration for NAS and a shorter neonatal length of stay compared to methadone.21–23 As a result, buprenorphine has emerged a safe and effective, office-based treatment alternative to methadone and use during pregnancy has subsequently escalated.
While pharmacotherapy use increased overall, significant disparities in pharmacotherapy use were found among teens, African-American and Hispanic women. Approximately 66% of pregnant women aged 15–19 did not receive opioid pharmacotherapy, compared to pregnant women aged 20–34 (43.8%) and aged ≥ 35 (40.6%), which reflects additional barriers for adolescent populations. In the United States, buprenorphine-naltrexone is only approved for persons age 16 years or older, while persons younger than 18 years must fail two attempts at detoxification and provide a written consent from a parent prior to the initiation of methadone.24 While pregnant adolescents are emancipated to make their own decisions regarding their pregnancy, many providers may not extend this autonomy to medication-assisted treatment and provider willingness to provide medication-assisted treatment services to pregnant adolescents remains largely unknown. In 2016, a randomized clinical trial demonstrated that buprenorphine maintenance therapy was associated with significant improvements in treatment engagement and decreased rates of relapse among adolescents compared to medically-assisted withdrawal.25 As a result, the American Association of Pediatrics has advocated for increasing resources to improve medication-assisted treatment access for adolescent populations.26,27
Non-Hispanic black (73%) and Hispanic (64%) pregnant women were also significantly more likely to not use opioid pharmacotherapy than non-Hispanic white pregnant women (41%) suggesting racial and ethnic disparities in pharmacotherapy use. Further, among black and Hispanic pregnant women who received opioid pharmacotherapy, both groups were more likely to receive methadone compared to buprenorphine. Previous evaluations of racial and ethnic substance use treatment disparities in the adult, non-pregnant population have been mixed. In an analysis of the National Survey on Drug Use and Health, unadjusted differences in racial-ethnic substance use treatment receipt did not persist after adjustment for criminal history and socioeconomic factors.28 Likewise, racial-ethnic disparities in treatment completion using the TEDS-D data set were also largely explained by differences in socioeconomic status.29 Further evaluations are warranted to explain differential racial-ethnic opioid pharmacotherapy use rates among pregnant women.
Pharmacotherapy utilization during pregnancy increased across all Medicaid regions except for Region 1 (New West), the most rural region in Pennsylvania and was largely driven by increases in buprenorphine use in urban versus rural geographic areas. Rural-urban disparities in pharmacotherapy use is of significant concern as the prevalence of maternal opioid use and neonatal abstinence syndrome (NAS) have disproportionately increased in rural areas across the United States and over 80% of pregnant women living in rural areas experience barriers to substance use treatment services.30,31 In 2012, over 40% of counties in the United States did not have at least one outpatient substance use treatment facility that accepted Medicaid and only 3% of programs were located in rural areas.32 Further, addiction stigma is especially powerful in rural areas30,33 where fear of judgement, prosecution and child welfare involvement may prevent many women from self-disclosure during pregnancy and delay substance use treatment engagement.34,35
The majority of pregnant women in our cohort did not receive behavioral health counseling during pregnancy which is recommended as part of a comprehensive approach to treatment.5,36 Future research is necessary to determine the effect of counseling on substance use treatment outcomes among pregnant women and to identify subpopulations which may be most likely to benefit from behavioral health interventions.39,40
Our results must be interpreted with certain limitations. Our sample represents a cohort of women with opioid use disorder enrolled in Pennsylvania Medicaid which may limit the generalizability of our findings to privately-insured or uninsured women and to women in other states. However, this limitation is minimized as Pennsylvania’s Medicaid program is the fourth largest in the US, with expenditures of $27.6 billion in 2016,13 and has demographic and socioeconomic profiles that closely track national averages.41 While Pennsylvania Medicaid provides reimbursement for all components of medication-assisted treatment including methadone, buprenorphine and behavioral health counseling, our results may not be generalizable to states whose Medicaid programs do not cover the full range of treatment services. Methadone is not included on Medicaid preferred drug lists in 20 states while Medicaid covers buprenorphine in all 50 states.42 Further, women that were classified as not receiving pharmacotherapy in our data may have received pharmacotherapy from a non-Medicaid-billing provider or a provider that accepted cash payments for services.43 Finally, observational retrospective cohort analyses are vulnerable to bias and the possibility for confounding exists despite efforts to control for these factors.
Because many women with opioid use disorder newly engage or re-engage in healthcare services during pregnancy, obstetric providers play a critical role in linking pregnant women to treatment programs that provide opioid pharmacotherapy, behavioral health counseling and social services support.5,12 Future research efforts should focus on characterizing effective strategies to expand medication-assisted treatment access and availability for pregnant women such as identifying healthcare delivery models and clinical care pathways that increase medication-assisted treatment use and that can be implemented into a variety of obstetric settings.
Supplementary Material
Figure 3.
Change in opioid pharmacotherapy utilization during pregnancy by Medicaid geographic region in Pennsylvania, 2009–2015. Percent change in buprenorphine utilization (A), percent change in methadone utilization (B), and percent change in no pharmacotherapy observed (C).
Funding disclosure:
Research reported in this publication was supported by the National Institute on Drug Abuse under Award Number K23DA038789 (Krans) and R01DA045675 (Krans and Jarlenski). This research was also supported by an inter-governmental agreement between the University of Pittsburgh and the Pennsylvania Department of Human Services.
Financial Disclosure
Elizabeth E. Krans is an investigator on grants to Magee-Womens Research Institute from the National Institutes of Health, Gilead and Merck outside of the submitted work. The other authors did not report any potential conflicts of interest. Each author has confirmed compliance with the journal’s requirements for authorship.
Footnotes
Peer reviews and author correspondence are available at http://links.lww.com/xxx.
Contributor Information
Elizabeth E. Krans, Department of Obstetrics, Gynecology & Reproductive Sciences, Magee-Womens Research Institute, University of Pittsburgh, Pittsburgh, Pennsylvania.
Joo Yeon Kim, Department of Health Policy and Management, University of Pittsburgh, Pittsburgh, Pennsylvania.
Alton Everette James, III, Department of Health Policy and Management, University of Pittsburgh, Pittsburgh, Pennsylvania.
David Kelley, Pennsylvania Department of Human Services, Harrisburg, Pennsylvania.
Marian P. Jarlenski, Department of Health Policy and Management, University of Pittsburgh, Pittsburgh, Pennsylvania.
References
- 1.Haight SC, Ko JY, Tong VT, Bohm MK, Callaghan WM. Opioid Use Disorder Documented at Delivery Hospitalization - United States, 1999–2014. Morb Mortal Wkly Rep.. 2018;67(31):845–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Opioid use and opioid use disorder in pregnancy. Committee Opinion No. 711. American College of Obstetricians and Gynecologists. Obstet Gynecol 2017;130:e81–94. [DOI] [PubMed] [Google Scholar]
- 3.Amerian Society of Addiction Medicine (ASAM). (2015). National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. Available at https://www.asam.org/docs/default-source/practice-support/guidelines-and-consensus-docs/asam-national-practice-guideline-supplement.pdf. [DOI] [PMC free article] [PubMed]
- 4.Klaman SL, Isaacs K, Leopold A, Perpich J, Hayashi S, Vender J, Campopiano M, Jones HE. Treating Women Who Are Pregnant and Parenting for Opioid Use Disorder and the Concurrent Care of Their Infants and Children: Literature Review to Support National Guidance. J Addict Med. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Substance Abuse and Mental Health Services Administration. Clinical Guidance for Treating Pregnant and Parenting Women with Opioid Use Disorder and Their Infants. HHS Publication No. (SMA) 18–5054. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2018. [Google Scholar]
- 6.McCarthy JJ, Leamon MH, Finnegan LP, Fassbender C. Opioid dependence and pregnancy: minimizing stress on the fetal brain. Am J Obstet Gynecol. 2017;216(3):226–231. [DOI] [PubMed] [Google Scholar]
- 7.Bachhuber MA, Mehta PK, Faherty LJ, Saloner B. Medicaid Coverage of Methadone Maintenance and the Use of Opioid Agonist Therapy Among Pregnant Women in Specialty Treatment. Med Care. 2017;55(12):985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Short VL, Hand DJ, MacAfee L, Abatemarco DJ, Terplan M. Trends and disparities in receipt of pharmacotherapy among pregnant women in publically funded treatment programs for opioid use disorder in the United States. J Subst Abuse Treat. 2018;89:67–74. [DOI] [PubMed] [Google Scholar]
- 9.Markus AR, Andres E, West KD, Garro N, Pellegrini C. Medicaid covered births, 2008 through 2010, in the context of the implementation of health reform. Womens Health Issues. 2013;23(5):e273–280. [DOI] [PubMed] [Google Scholar]
- 10.Wen H, Druss BG, Cummings JR. Effect of Medicaid Expansions on Health Insurance Coverage and Access to Care among Low-Income Adults with Behavioral Health Conditions. Health Serv Res. 2015;50(6):1787–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, McAllister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA. 2012;307(18):1934–1940. [DOI] [PubMed] [Google Scholar]
- 12.Jones HE, Deppen K, Hudak ML, Leffert L, McClelland C, Sahin L, Starer J, Terplan M, Thorp JM Jr, Walsh J, Creanga AA. Clinical care for opioid-using pregnant and postpartum women: the role of obstetric providers Am J Obstet Gynecol. 2014;210(4):302–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaiser Family Foundation. State Health Facts: Total Medicaid Spending in FY 2015. 2016 ; http://kff.org/medicaid/state-indicator/total-medicaid-spending. Accessed April 12, 2017.
- 14.Pennsylvania HealthChoices Map. http://www.dhs.pa.gov/provider/healthcaremedicalassistance/managedcareinformation/statewidemanagedcaremap/index.htm. Accessed on December 7th, 2018.
- 15.National Committee for Quality Assurance. Prenatal and Postpartum Care Quality Measure. 2016; http://www.ncqa.org/portals/0/prenatal%20postpartum%20care.pdf. Accessed July 19, 2016.
- 16.United States Department of Agriculture: Economic Research Service. Rural-Urban Commuting Area Codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes/. Accessed on December 12, 2018.
- 17.The Center for Rural Pennsylvania. http://www.rural.palegislature.us/. Accessed October 15th, 2017.
- 18.Stein BD, Dick AW, Sorbero M, Gordon AJ, Burns RM, Leslie DL, Pacula RL. A population-based examination of trends and disparities in medication treatment for opioid use disorders among Medicaid enrollees. Subst Abuse. 2018:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dick AW, Pacula RL, Gordon AJ, Sorbero M, Burns RM, Leslie D, Stein BD. Growth In Buprenorphine Waivers For Physicians Increased Potential Access To Opioid Agonist Treatment, 2002–11. Health Aff. 2015;34(6):1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The President’s Commission on Combating Drug Addiction and the Opioid Crisis. Final Report. Washinton, DC: Executive Office of the President, The White House; 2017. [Google Scholar]
- 21.Jones HE, Heil SH, Baewert A, Arria AM, Kaltenbach K, Martin PR, Coyle MG, Selby P, Stine SM, Fischer G. Buprenorphine treatment of opioid-dependent pregnant women: a comprehensive review. Addiction. 2012;107 Suppl 1:5–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones HE, Kaltenbach K, Heil SH, Stine SM, Coyle MG, Arria AM, O’Grady KE, Selby P, Martin PR, Fischer G. Neonatal abstinence syndrome after methadone or buprenorphine exposure. N Engl J Med. 2010;363(24):2320–2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer G, Ortner R, Rohrmeister K, Jagsh R, Baewert A, Langer M, Aschauer H. Methadone versus buprenorphine in pregnant addicts: a double-blind, double-dummy comparison study. Addiction. 2006;101(2):275–281. [DOI] [PubMed] [Google Scholar]
- 24.Chang DC, Klimas J, Wood E, Fairbairn N. Medication-assisted treatment for youth with opioid use disorder: Current dilemmas and remaining questions. Am J Drug Alcohol Abuse. 2018;44(2):143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsch LA, Moore SK, Borodovsky JT, Solhkhah R, Badger GJ, Semino S, Jarrett K, Condon KD, Rossettie K, Vincent P, Hajizadeh N, Ducat E. A randomized controlled trial of buprenorphine taper duration among opioid-dependent adolescents and young adults. Addiction. 2016;111(8):1406–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ryan SA GP, Patrick SW, Quigley J, Siqueira L, Walker LR. Medication-assisted treatment of adolescents with opioid use disorders. Pediatrics. 2016;138(3). [DOI] [PubMed] [Google Scholar]
- 27.Hadland SE, Bagley SM, Rodean J, Silverstein M, Levy S, Larochelle MR, Samet JH, Zima BT. Receipt of Timely Addiction Treatment and Association of Early Medication Treatment With Retention in Care Among Youths With Opioid Use Disorder. JAMA Pediatr. 2018;172(11):1029–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cook BL, Alegria M. Racial-ethnic disparities in substance abuse treatment: the role of criminal history and socioeconomic status. Psychiatr Serv. 2011;62(11):1273–1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saloner B, Le Cook B. Blacks and Hispanics are less likely than whites to complete addiction treatment, largely due to socioeconomic factors. Health Aff. 2013;32(1):135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson A, Shannon L. Barriers to Receiving Substance Abuse Treatment Among Rural Pregnant Women in Kentucky. Matern Child Health J. 2012;16(9):1762–1770. [DOI] [PubMed] [Google Scholar]
- 31.Villapiano NL, Winkelman TN, Kozhimannil KB, Davis MM, Patrick SW. Rural and Urban Differences in Neonatal Abstinence Syndrome and Maternal Opioid Use, 2004 to 2013. JAMA Pediatr. 2017;171(2):194–196. [DOI] [PubMed] [Google Scholar]
- 32.Cummings JR, Wen HF, Ko M, Druss BG. Race/Ethnicity and Geographic Access to Medicaid Substance Use Disorder Treatment Facilities in the United States. JAMA Psychiat. 2014;71(2):190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keyes KM, Cerda M, Brady JE, Havens JR, Galea S. Understanding the rural-urban differences in nonmedical prescription opioid use and abuse in the United States. Am J Public Health. 2014;104(2):e52–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lander LR, Marshalek P, Yitayew M, Ford D, Sullivan CR, Gurka KK. Rural healthcare disparities: challenges and solutions for the pregnant opioid-dependent population. W Va Med J. 2013;109(4):22–27. [PubMed] [Google Scholar]
- 35.Kremer ME, Arora KS. Clinical, Ethical, and Legal Considerations in Pregnant Women With Opioid Abuse. Obstet Gynecol. 2015;126(3):474–478. [DOI] [PubMed] [Google Scholar]
- 36.Kampman K, Jarvis M. American Society of Addiction Medicine (ASAM) National Practice Guideline for the Use of Medications in the Treatment of Addiction Involving Opioid Use. J Addict Med. 2015;9(5):358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gossop M, Stewart D, Marsden J. Effectiveness of drug and alcohol counselling during methadone treatment: content, frequency, and duration of counselling and association with substance use outcomes. Addiction. 2006;101(3):404–412. [DOI] [PubMed] [Google Scholar]
- 38.Fiellin DA, Pantalon MV, Chawarski MC, Moore BA, Sullivan LE, O’Connor PG, Schottenfeld RS. Counseling plus buprenorphine-naloxone maintenance therapy for opioid dependence. N Engl J Med. 2006;355(4):365–374. [DOI] [PubMed] [Google Scholar]
- 39.Carroll KM, Weiss RD. The Role of Behavioral Interventions in Buprenorphine Maintenance Treatment: A Review. Am J Psychiatry. 2017;174(8):738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin SA, Chiodo LM, Bosse JD, Wilson A. The Next Stage of Buprenorphine Care for Opioid Use Disorder. Ann Intern Med. 2018;169(9):628–635. [DOI] [PubMed] [Google Scholar]
- 41.U.S. Census Bureau. Pennsvylania 2016. 2016; https://www.census.gov/quickfacts/table/PST045216/42. Accessed April 12, 2017.
- 42.Substance Abuse and Mental Health Services Administration, Medicaid Coverage and Financing of Medications to Treat Alcohol and Opioid Use Disorders. HHS Pblication No. SMA-14–4854. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2014. . [Google Scholar]
- 43.Patrick SW, Buntin MB, Martin PR, Scott TA, Dupont W, Richards M, Cooper WO. Barriers to Accessing Treatment for Pregnant Women with Opioid Use Disorder in Appalachian States. Subst Abuse. 2018:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz RP, Gryczynski J, O’Grady KE, Sharfstein JM, Warren G, Olsen Y, Mitchell SG, Jaffe JH. Opioid agonist treatments and heroin overdose deaths in Baltimore, Maryland, 1995–2009. Am J Public Health. 2013;103(5):917–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Terplan M, Laird HJ, Hand DJ, Wright TE, Premkumar A, Martin CE, Meyer MC, Jones HE, Krans EE. Opioid Detoxification During Pregnancy: A Systematic Review. Obstet Gynecol. 2018;131(5):803–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Terplan M, Longinaker N, Appel L. Women-Centered Drug Treatment Services and Need in the United States, 2002–2009. Am J Public Health. 2015;105(11):e50–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.