Abstract
Neuroinflammation is the coordinated response of the central nervous system (CNS) to threats to its integrity posed by a variety of conditions, including autoimmunity, pathogens and trauma. Activated astrocytes, in concert with other cellular elements of the CNS and immune system, are important players in the modulation of the neuroinflammatory response. During neurological disease, they produce and respond to cellular signals that often lead to dichotomous processes, which can promote further damage or contribute to repair. This occurs also in multiple sclerosis (MS), where astrocytes are now recognized as key components of its immunopathology. Evidence supporting this role has emerged not only from studies in MS patients, but also from animal models, among which the experimental autoimmune encephalomyelitis (EAE) model has proved especially instrumental. Based on this premise, the purpose of the present review is to summarize the current knowledge of astrocyte behavior in MS and EAE. Following a brief description of the pathological characteristics of the two diseases and the main functional roles of astrocytes in CNS physiology, we will delve into the specific responses of this cell population, analyzing MS and EAE in parallel. We will define the temporal and anatomical profile of astroglial activation, then focus on key processes they participate in. These include: 1) production and response to soluble mediators (e.g. cytokines and chemokines), 2) regulation of oxidative stress, and 3) maintenance of BBB integrity and function. Finally, we will review the state of the art on the available methods to measure astroglial activation in vivo in MS patients, and how this could be exploited to optimize diagnosis, prognosis and treatment decisions. Ultimately, we believe that integrating the knowledge obtained from studies in MS and EAE may help not only better understand the pathophysiology of MS, but also uncover new signals to be targeted for therapeutic intervention.
Keywords: Neuroinflammation, neuroimmune disease, astroglia, multiple sclerosis, experimental autoimmune encephalomyelitis, demyelinating disorder
Introduction
Astrocytes are key players in the complex cascade of cellular adaptations taking place in the central nervous system (CNS) in response to injury and disease. These adaptations, often referred to globally as neuroinflammation, occur in multiple sclerosis (MS) as well as its commonly used animal model experimental autoimmune encephalomyelitis (EAE), which has been successfully employed to investigate this and other aspects of MS immunopathology. Neuroinflammation is the coordinated response of the CNS to threats to its integrity posed by a variety of conditions, including pathogens and trauma. Activated astrocytes act in concert with other neural and non-neural cells to sustain the neuroinflammatory response by balancing cellular signals that often lead to opposing processes. As a result, the neurological outcome is dictated by the net combination of all effects, which tips the scale towards propagation or resolution of the damage at a specific time and place.
In recent years, many advances have been made in our understanding of astroglial biology, spearheaded by the development of new cutting edge tools, including gene targeting approaches and next generation transcriptomics. We now have an appreciation for the vast heterogeneity of the astroglial populations throughout the CNS and the timing and nature of their responses during disease. Because astrocyte-driven neuroinflammation is such a key feature of both MS and EAE, here we will review the astrocyte specific responses observed in both, focusing on how they contribute to the neuroinflammatory cascades at the basis of disease onset, evolution and resolution.
Multiple sclerosis immunopathology
MS is a chronic inflammatory demyelinating disease of the CNS, whose underlying cause remains uncertain [129]. It is the most common non-traumatic neurological disorder in young adults, affecting an estimated 1 million people in the US alone [192]. MS is believed to be initiated and sustained by the complex interplay of dysregulated innate and/or adaptive immunity, genetic susceptibility and environmental factors. MS manifests with distinct clinical phenotypes, the most frequent being relapsing remitting MS (RRMS), characterized by episodes of neurological dysfunction that spontaneously resolve [57]. Pathologically, relapses are associated with focal, inflammatory demyelination in white and gray matter, heavily infiltrated with immune cells. In over 75% of cases, RRMS evolves into secondary progressive MS (SPMS), where patients experience irreversible accumulation of disability associated with neurodegeneration [129]. In a small percentage of cases, a primary progressive phenotype is observed (PPMS), where irreversible and progressive neurodegeneration starts at onset. In progressive MS forms, chronic demyelinated lesions with axonal loss accumulate in the white matter, but diffuse changes also occur in the seemingly unaffected white matter, commonly referred to as normal appearing white matter. Diffuse and focal cortical gray matter demyelination and neurodegeneration are also seen [89, 150]. While major progress has been made in understanding disease mechanisms in RRMS, those driving progressive MS remain largely unresolved, which explains the lack of effective treatments for this disease forms. In this respect, a breakthrough came with the recent introduction of the B cell depleting biologic ocrelizumab, which became the first, and so far only, drug approved for PPMS, underscoring the importance of B cell function in MS pathogenesis [63].
In addition to the key inflammatory role of the different T and B cell subpopulations and other elements of the peripheral innate immune system (e.g. monocytes, dendritic cells), CNS glial cells, namely astrocytes and microglia, have been recognized as key components of MS immunopathology. This seems especially evident in progressive MS, where multiple lines of evidence suggest an association with chronic activation of the CNS innate immune system [91]. Furthermore, several studies point at the possibility that, at least in certain cases, MS may initially arise from a primary insult within the CNS, perhaps to oligodendrocytes. This may lead to glial activation and eventually immune-mediated inflammatory activation as a secondary phenomenon [105]
Experimental autoimmune encephalomyelitis: induction and pathological features
In its various forms, EAE has been the most widely utilized model of MS for decades [47]. It can be induced in multiple animal species, though rodents, particularly mice and rats, are usually preferred. This is due to their versatility, relatively low maintenance cost and potential for genetic manipulation that allows for investigations into specific cellular and molecular mechanisms of disease. Species, genetic strain, sex and age are factors that dictate the EAE phenotype that will manifest, whether with a chronic monophasic or a relapsing-remitting profile. EAE induction can be active or passive. Active EAE is obtained via direct immunization with CNS-specific antigens, namely highly immunogenic myelin peptides (e.g. MOG35–55, MBP84–104, PLP139–151) emulsified in complete Freund’s adjuvant (CFA) and usually accompanied by administration of pertussis toxin [47]. This leads to the priming of myelin-specific T cells in peripheral lymphoid organs, their differentiation into effector T cells (mostly Th1 and Th17) and entry into the CNS where they are reactivated via interaction with local antigen presenting cells (APCs). These events are followed by secretion of pro-inflammatory mediators from T cells, microglia and astrocytes, which further amplify the inflammatory response by sustaining the recruitment of more immune cells into the CNS [20]. Here, they accumulate predominantly in the white matter (especially in the spinal cord) where they cause demyelination and tissue damage. Passive EAE requires adoptive transfer of antigen-specific T cells obtained from actively immunized animals into recipient animals [47, 135]. Passive EAE is valuable to study the contribution of CD4+ T cells in the immunopathology of EAE/MS and to investigate the effector phase of disease in the absence of adjuvant. Amongst the various EAE models, active EAE induced with MOG35–55 peptide in C57BL/6 mice is by far the most common. Here, a monophasic response is observed, whereby, after a peak disease reached acutely, the clinical signs ameliorate to some degree to be then sustained chronically. Importantly, MOG immunization elicits not only an encephalitogenic T cell response, but also a demyelinating autoantibody response which enhances the severity of demyelination [95]. Anti-MOG autoantibodies are also found in MS patients and have been shown to induce demyelination when inoculated into rodents [203].
Both active and passive EAE models are not ideal to study immunological mechanisms at the basis of disease initiation and relapse. To overcome their limitations, transgenic models in which mice develop a spontaneous form of EAE have been generated. The first of these models was obtained via expression of a MBP-specific T cell receptor (TCR), where mice develop spontaneous EAE at various ages in about 50% of cases [62]. A number of similar models have since followed, in which other myelin-specific T cell receptors are expressed. These mouse lines can be crossed with RAG-deficient mice to obtain modified TCR expression in T cells in the absence of any other lymphocyte population [20]. Each line develops spontaneous EAE differently, and may require a triggering agent [20].
Regardless of the type of EAE, its pathological features include perivascular accumulation of immune cells in the CNS (especially at early disease stage) followed by their egress into the CNS parenchyma (maximal at disease peak) [29, 84], activation of microglia and astrocytes (throughout disease), demyelination (starting at peak disease, most evident chronically) and axonal loss (late chronic stage) [58]. The degree of immune cell entry and tissue damage directly correlates with functional impairment. Even though these immunopathological features are common to all EAE phenotypes, their timing, severity and distribution may vary significantly depending on the species and strain used, as well as the immunizing agent. For this reason, each individual EAE model is suited for investigating specific aspects of the human pathology. Overall, since EAE is primarily T cell driven and adaptive immune activation plays an integral role in its etiology, it is more amenable to study the induction and acute phases of RRMS, where accumulation of immune cells in active CNS lesions is the key driver of pathology and disability. The progressive MS forms where irreversible axonal injury and neurological damage are the predominant features, often observed in the absence of immune cell presence, are not modeled as accurately by EAE.
Similarly to MS, EAE features profound inflammatory activation of CNS glial cells, which largely contribute to EAE pathology at all stages of the disease. For this reason, EAE has been especially valuable in dissecting the processes and mechanisms driven by astrocytes, as well as other glia, in neuroimmune disease, thus helping to provide a more complete picture of MS pathophysiology.
Astrocyte function in health and disease
Over the last few decades, considerable efforts have been made to elucidate the complex functions of astrocytes in the healthy and diseased CNS. It is now established that astrocytes play essential roles that go far beyond the simplistic view of “supporting elements” to neurons. In fact, astrocytes are recognized to participate in functions once deemed to be exclusive prerogative of neurons, such as synaptic transmission and processing [171]. Astrocytes are found in a multitude of phenotypes (e.g. protoplasmic, fibrous, radial, velate, Bergmann glia, Muller glia) with diverse morphology, anatomical location and properties. They localize in all CNS districts, occupying discrete non-overlapping domains where they carry out homeostatic functions during development and adulthood [33, 187]. Their highly ramified processes make contacts with pre- and post- synaptic terminals forming the tripartite synapse [12]. Within this structure, astrocyte regulate synaptogenesis [43], synaptic plasticity and stability, and the efficiency of synaptic transmission by modulating the release of gliotransmitters (e.g. glutamate, ATP, D-serine) in the synaptic cleft [11], uptaking glutamate [132], and buffering extracellular K+ [70]. By extending and wrapping their end-feet around the cerebral vasculature, astrocytes constitute a key structural component of the blood brain barrier (BBB). Indeed, they participate in the formation of the glia limiting membrane, or glia limitans, a dense meshwork of processes covered by basal lamina that makes contact with the pia mater and regulates the movement of small molecules and cells into the CNS [169]. Key molecules expressed by the astrocytic end-feet at the glia limitans are the specialized channel-forming proteins aquaporin-4 (AQP4) and Kir4.1, and the gap junction-forming protein Connexin 43 (Cx43). They allow astrocytes a direct exchange with endothelial cells, conferring them the ability to regulate water, ion and soluble factor diffusion and redistribution across the BBB [81]. Via local production of vasoactive molecules (e.g. prostaglandins, arachidonic acid, nitric oxide (NO)), astrocytes regulate CNS blood flow [85]. They manage extracellular fluid composition and pH through the activity of specialized water and ion channels [165]. As they are positioned at the interface between blood vessels and neurons, astrocytes can uptake glucose from the circulation and provide it to neurons for energy supply [187]. Furthermore, they are the main storage sites of glycogen in the CNS and the highest accumulation of astrocytic glycogen occurs in areas of high synaptic density [142]. Lipid metabolism is also regulated by astrocytes, who are the primary source of CNS cholesterol needed for membrane homeostasis and myelin synthesis [35, 36]. Finally, astrocytes are recognized as important components of the innate immune response. Indeed, they participate in immunomodulatory functions through their ability to produce cytokines and chemokines, as well as to express MHC-II molecules. They have been attributed a role as APCs, although it is now recognized they act as weak APCs in vivo given their lack of expression of certain co-stimulatory molecules [40, 46].
Astrocytes respond to CNS disease and trauma with a complex process of activation that integrates cell proliferation, profound morphological changes (e.g. increased branching, augmented cellular size, elongation of cells processes) and functional modifications. This phenomenon, often defined as astrogliosis, bares both positive and negative repercussions on the neurological outcome [139]. A commonly used marker to identify astrogliosis is the intermediate filament glial fibrillary acidic protein (GFAP), whose upregulation can be reliably correlated with the acquisition of a reactive/astrogliotic phenotype by astrocytes during CNS stress and disease. Recently, it has been suggested that reactive astrocytes display two distinct phenotypes, A1 and A2. While A2 astrocytes have been attributed neuroprotective and anti-inflammatory properties, A1 astrocytes induced by neuroinflammatory microglia during CNS disease are highly neurotoxic [93].
Because of the vast diversity of astrocyte populations in the various areas of the brain and spinal cord, it is now recognized that the astroglial response to CNS damage is uniquely region- and disease-specific. For instance, in CNS autoimmunity, the processes astrocytes participate in are initiated by the multitude of molecules released by CNS damaged cells, neighboring glia and infiltrating immune cells, especially T cells. These affect the makeup of astrocytes resulting in gain or loss of functional properties. These include the production of soluble mediators such as chemokines (e.g. CCL2, CCL20, CXCL1), cytokines (e.g. TNF, IL1β, IL6), growth factors (e.g. NGF, BDNF, VEGF-A), and oxidants (NO), as well as the synthesis of insoluble matrix molecules (e.g. tenascin, chondroitin-sulphate proteoglycans), whose deposition in the extracellular space leads to the formation of the glial scar [170]. As the processes activated in reactive astrocytes during CNS autoimmunity are both detrimental and reparative, the roles these cells play in disease pathophysiology are complex. Fortunately, recent advances in cell-specific gene targeting and manipulations in vivo have enabled the in-depth investigation of the signals and mechanisms specifically induced in astrocytes in response to disease. This has uncovered that the contribution of astrocytes to MS and EAE etiopathology is broader than previously believed.
Temporal and anatomical profile of astrocyte reactivity in MS and EAE
In both MS and EAE, astrocyte reactivity is a widespread phenomenon that initiates at the early stage of lesion formation and persists into the chronic phases of lesion evolution, even after immune cell presence has receded. In MS, activated astrocytes with elevated GFAP expression are found throughout the CNS. Their morphology and distribution vary in relation to disease and lesion stage, as well as their positioning in the white or gray matter, suggesting they play different roles. In early pre-lesions, identified by focal mild perivascular lymphocyte infiltration and BBB breakdown, astrocytes are already hypertrophic [101]. In acute active lesions, both in the white and gray matter, astrocytes display a highly reactive phenotype with hypertrophic cell body and thick processes [32]. This phenotype extends into the adjacent normal appearing white and gray matter [32, 101], suggesting reactive astrocytes are early and active contributors to lesion development. A proportion of the hypertrophic astrocytes observed at the leading edge of actively demyelinating MS lesions show intracellular lipid inclusions. This is indicative of their participation in myelin debris phagocytosis that has been implicated in sustaining lesion pathology [144]. Within active lesions, it is also common to observe reactive astrocytes in close connection with the vasculature displaying signs of damage, with swollen cell bodies and disrupted astrocytic end-feet. This results in extensive gaps in the glia limitans and, consequently, in BBB dysfunction, which allows for increased CNS immune cell entry [32]. On this basis, there is growing support for the idea that the early pathogenic events of MS are dependent, at least in part, on the loss of astrocytic homeostatic function, rather than linked to excessive detrimental astrocytic activation [32]. In chronic lesions, astrocytes appear still reactive, however their GFAP expression is not as elevated as in acute lesions and their morphology is less rounded and with thinner processes [32]. They are concentrated at the lesion rim. The disruption of the glia limitans still remains, though astrocyte end-feet show reduced swelling and appear connected to the basal lamina [32]. As chronic lesions become inactive, reactive astrocytes also show accumulation of GFAP+ filaments, indicative of the progressive formation of an astroglial scar [32, 101]. Even though more subtle, changes occur also in the white and gray matter adjacent to MS lesions, defined “normal appearing” because of seemingly absent pathology. Here, astrocytes also show signs of activation, although to a lesser extent than within lesions. Overall, regardless of whether astroglial reactivity relates to a loss of homeostatic functions or acquisition of an inflammatory phenotype, the general consensus is that it correlates with disease pathology from the early stages and is a reliable indicator of disease evolution [32].
As far as the type of reactive astrocytes populating the lesion environment, a recent study reported them to be predominantly of the A1 type, which are considered highly pro-inflammatory and neurotoxic [93]. In demyelinating MS lesions, A1 astrocytes, identified by expression of complement component 3 (C3), are observed in close association with CD68+ activated microglia and/or macrophages, which are believed to be potent inducers of the A1 astroglial phenotype. Their number is significantly higher in acute active lesions compared to chronic active and inactive [93].
In EAE, astrocyte activation (assessed by increased GFAP immunoreactivity or gene expression, cellular hypertrophy, thickening of cell processes) is also observed from the early stages of disease, both in the white and gray matter, starting before the appearance of clinical symptoms (locomotor deficits), thus preceding CNS immune infiltration [27, 49, 102, 141, 168, 193]. Astroglial reactivity progressively increases from the pre-symptomatic stage, is maximal at peak/acute disease, particularly in the vicinity of immune cell infiltrates, and persists chronically, though to a lower extent [29, 66, 86, 168]. Astrogliosis, measured in the brain and spinal cord of EAE-induced GFAP-luc mice expressing luciferase under the control of the GFAP promoter, can be assessed as early as 3 days post induction (dpi) of EAE. Importantly, it correlates with and predicts the severity of the EAE clinical course [102]. In mice that develop spontaneous relapsing-remitting EAE (mutated to carry a TCR specific for MOG92–106 peptide), reactive perivascular astrocytes with elevated GFAP expression are found well before onset of symptoms in the absence of immune infiltrates [6]. Astrogliosis progresses as infiltration increases, manifesting more prominently on perivascular end-feet and parenchymal astrocytes [6].
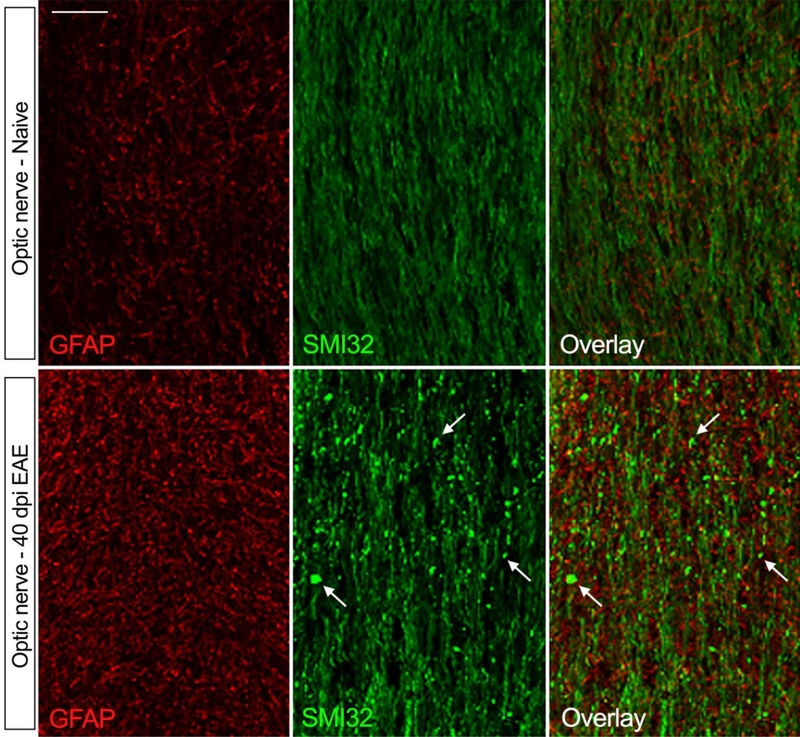
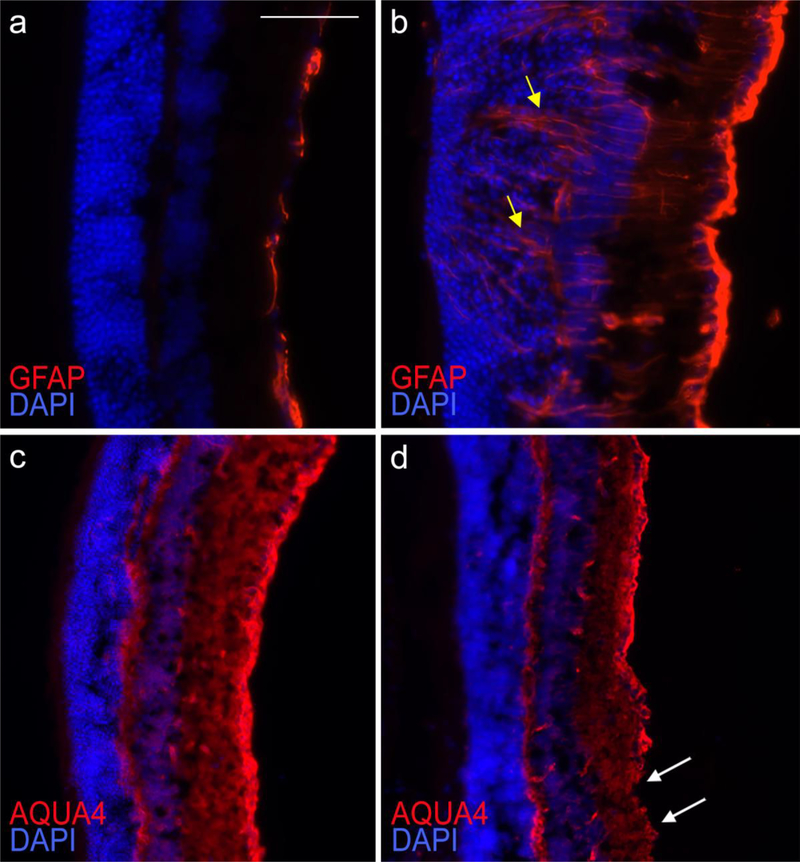
In addition to brain and spinal cord, astrocyte reactivity is evident in the optic nerve and retina of EAE affected mice that develop optic neuritis [27, 193]. In the optic nerve, astroglial activation occurs early on in disease, prior to immune cell infiltration, and has been associated with early signs of axonal injury [27, 193]. In our own observations, here astrogliosis remains elevated through the chronic stages of optic neuritis (Fig. 1) (40 dpi of MOG35–55 EAE), when axonal degeneration is severe and widespread, as shown by the accumulation of axonal retraction bulbs positive for dephosphorylated neurofilament (SMI32) (Fig. 1). At the same time point (40 dpi), we observe astroglial activation in the retina as well, associated with retinal swelling and disruption (Fig. 2b, yellow arrows) compared to naive mice (Fig. 2a). Here, astrocytes maintain elevated GFAP expression (Fig. 2b) and a concomitantly reduced AQP4 expression at the end-feet (Fig. 2d, white arrows) compared to naïve mice (Fig. 2c). We interpret this as a sign of astroglial damage, whereby astrocytes lacking AQP4 may have a compromised capacity to maintain water homeostasis and BBB functionality.
Figure 1. Astroglial reactivity and axonal damage in the mouse optic nerve at the chronic stage of EAE.
EAE was induced by MOG35–55 immunization in C57BL/6 mice. Optic nerves of mice sacrificed at chronic disease (40 dpi) were compared to naïve optic nerves. Astrocytes were immunostained for GFAP, and axons for non-phosphorylated neurofilament H (clone SMI32). In naïve conditions, GFAP expression was low, indicative of negligible astroglial activation. In parallel, SMI32 expression, which is elevated in correlation with axonal damage, was mild and diffuse to indicate axonal integrity. At chronic EAE, GFAP was highly upregulated demonstrating increased astroglial activation. This was accompanied by increased reactivity for SMI32, which concentrated in axon retraction bulbs (white arrows), demonstrating ongoing wallerian degeneration of axons. Scale bar: 20 μm.
Figure 2. Astroglial reactivity in the mouse retina at the chronic stage of EAE.
EAE was induced by MOG35–55 immunization in C57BL/6 mice. Retinas of mice sacrificed at chronic disease (40 dpi) (b, d) were compared to naïve retinas (a, c). Astrocytes were immunostained for GFAP (a, b), or AQP4 (c, d). Nuclei of retinal ganglion cells (RGCs) were identified by DAPI staining. In naïve conditions, GFAP expression was low (a), and AQP4 diffuse and uniformly distributed (c), indicative of negligible astroglial activation and intact glia limitans. The RGC layer was compact and uninterrupted (a, c). At chronic EAE, GFAP was highly upregulated indicating ongoing astroglial activation (b). Labelling was evident throughout the RGC layer (b, yellow arrows), which appeared disrupted. In parallel, AQP4 labeling was reduced and redistributed, absent in certain areas (d, white arrows), suggesting alterations of the glia limitans and potential BBB damage. Scale bar: 50 μm.
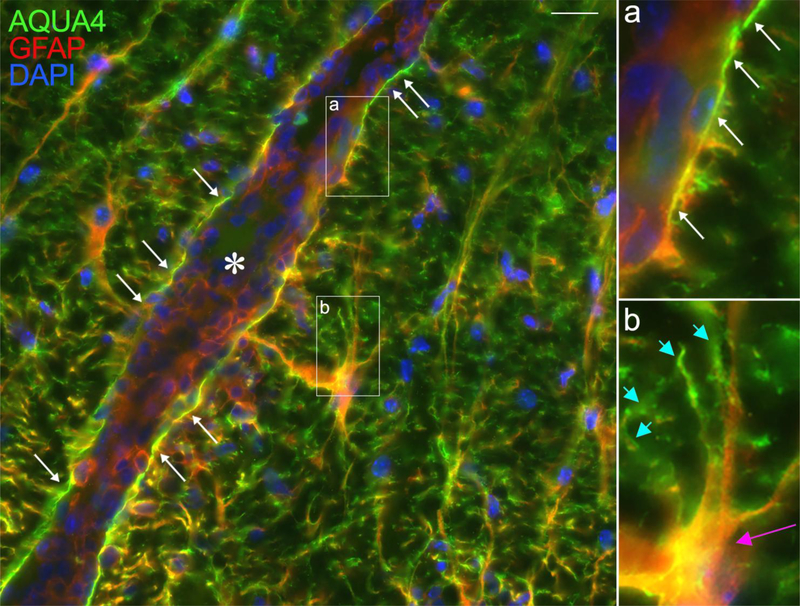
Similarly to MS, there is evidence that astrocytes suffer cell damage at the early stages of EAE as well. Electron microscopy studies in Lewis rats demonstrate the presence of swollen astrocytes with dispersed GFAP filaments in areas of the spinal cord yet to be infiltrated with immune cells [49]. These structural changes are correlated with increased permeability of the vascular walls, suggesting that astroglial alterations could in turn participate in the progressive disruption of the BBB at the basis of immune cell entry [49]. In our own observations at pre-symptomatic MOG35–55 EAE (10 dpi), just before onset, astrocytic activation is already elevated in the spinal cord with increased GFAP immunoreactivity and hypertrophy (Fig. 3). However, we do not find evident signs of structural damage. The astrocytic end-feed maintain AQ4 expression, and the glia limitans appear uninterrupted, suggesting no obvious disruption of the BBB (Fig. 3, white arrows). The discrepant findings may be attributed to various factors, including the different models used and the time and location where astroglial activation was assessed.
Figure 3. Astroglial reactivity in the mouse spinal cord at the pre-symptomatic stage of EAE.
EAE was induced by MOG35–55 immunization in C57BL/6 mice. In spinal cord sections of mice sacrificed at 10 dpi, before onset of symptoms, astrocytes were immunostained for GFAP and AQP4. Strong GFAP labeling was observed, indicative of astroglial activation already occurring. Intense labeling for AQP4 was also observed at the astrocyte end-feet (b, blue arrows) as well as on the cell body of hypertrophic astrocytes highly expressing GFAP (b, pink arrow). AQP4 and GFAP clearly colocalized within the glia limitans surrounding the blood vessels (a, white arrows), where no gaps in AQP4 labeling were observed, indicating that the structural integrity of the glia limitans was maintained at this early EAE stage. *Blood vessel. Scale bar: 10 μm.
Various studies have addressed the significance and main functional contribution of reactive astrocytes during the various phases of disease development and progression using ablation strategies. Selective depletion of proliferating reactive astrocytes achieved with administration of ganciclovir to GFAP-HSV-TK mice resulted in different clinical outcomes depending on the time of depletion. When depletion was initiated prior to EAE induction and protracted up to acute disease, the EAE clinical outcome was markedly exacerbated and associated with increased CNS immune cell infiltration [113, 191]. Similarly, reactive astrocyte depletion at onset with a mild 7-day ganciclovir cycle caused worsening of EAE associated with increased macrophage infiltration and upregulation of CNS inflammatory gene expression [180]. This may be attributed to the loss of astrocytic barrier function, which normally contains immune cells in the perivascular space preventing their entry into the CNS parenchyma [180, 191]. Earlier studies in EAE using GFAP knockout mice reached similar conclusions, with worsening of the EAE course dependent on uncontrolled immune cell ingress into the CNS [94]. Conversely, reactive astrocyte depletion with ganciclovir at the chronic disease phase (starting at 30 dpi) caused improvement of the EAE clinical outcome and was associated with decreased leukocyte infiltration into the CNS [113]. Collectively, these data suggest that, early on in disease, reactive astrocytes play a predominant role in maintaining barrier function against uncontrolled cell trafficking, thereby netting a protective effect, whereas chronically, their contribution to the detrimental neuroinflammatory response is preponderant, thereby netting a detrimental effect. This should be taken into consideration in devising possible therapeutic strategies targeting astroglial activation.
Signals regulating astrocyte reactivity in MS and EAE
Recent advances in cell-specific gene profiling methods have allowed to gain new insight into the signals driving or suppressing astroglial reactivity in neuroimmune disease. The glycolipid lactosylceramide (LacCer) has been identified as an autocrine signal synthesized in astrocytes by β−1,4-galactosyltransferase 6 (B4GALT6) that promotes pro-inflammatory astroglial activation in EAE [113]. LacCer and B4GALT6 are upregulated in astrocytes during EAE, particularly during the chronic phase. After release, LacCer directs astrocytes towards transcriptional programs that lead to neurodegeneration [113]. Specifically, astroglial LacCer signaling induces the recruitment and activation of microglia and macrophages via production of the chemokine CCL2 and the growth factor granulocyte-macrophage colony-stimulating factor (GM-CSF). Inhibition of B4GALT6 in vivo during EAE abrogates astroglial activation and suppresses the induction of their inflammatory program. This evidence, together with the finding that B4GALT6 is upregulated in hypertrophic reactive astrocytes in MS lesions, suggests that the astroglial LacCer-B4GALT6 axis may be a promising target for MS therapy [113]. Further analyses determined that LacCer operates in astrocytes through activation of the NF-κB and IRF-1 pathways [113]. A recent study showed that, during EAE, also TGFα and VEGF-B induce pro-inflammatory astroglial reactivity via NF-κB activation [152]. These reports are in line with previous studies that identified NF-κB as a primary driver of pro-inflammatory astroglial activation in EAE [28, 29] as well as other neurological disorders [26]. A more detailed account of astroglial responses dependent on NF-κB is provided in a following section (Pathophysiological roles of activated astrocytes in MS and EAE) where cytokine signaling in astrocytes is discussed.
Another key signaling pathway implicated in astroglial activation in CNS autoimmunity is the sphingosine 1-phosphate (S1P) receptor pathway [42, 153]. S1P receptors have been studied mostly in the immune system, as B and T cells have long been considered the target of the disease modifying drug FTY720 (fingolimod), which is recognized to function by sequestering lymphocytes inside lymph nodes, preventing them from entering the CNS. Nevertheless, S1P receptors are also expressed by astrocytes, and their contribution to FTY720 therapeutic effect is now starting to be elucidated. In MS, S1P1 and S1P3 receptors are strongly upregulated on hypertrophic astrocytes in active and inactive white matter lesions [184]. Similarly to MS, administration of FTY720 to EAE-induced mice improves the clinical outcome [42, 153]. This has been shown to be dependent, at least in part, on inhibition of S1P receptor signaling in astrocytes. Indeed, astroglial-specific ablation of S1P receptor drastically reduces the therapeutic effect of FTY720 in EAE [42]. Gene expression profiling of astrocytes isolated from EAE-induced mice untreated or treated with FTY720 showed that inhibition of S1P receptor signaling directed astrocytes towards a reparative program by suppressing proinflammatory astroglial activation (downregulation of TNF, IL6, CCL2, CCL20, CXCL10, etc.), while promoting the expression of anti-inflammatory molecules (e.g. IL33, CXCL12) [153].
Just as astrocytes are exposed to factors that stimulate their cellular reactivity during CNS autoimmune disease, they also respond to signals that suppress astrogliosis and inflammatory activation. Type I interferons (IFN-I) belong to this category of molecules. IFN-I, most notably IFNβ, have been used for decades in MS therapy, with their efficacy primarily attributed to suppression of leukocyte-mediated inflammation. However, it is now clear that glia can also respond to IFN-I that are endogenously produced in the CNS in both physiologic and disease conditions [21]. The cellular responses to IFN-I are mediated by the IFN-alpha receptor (IFNAR), composed of the IFNAR1 and IFNAR2 subunits [21], both of which are expressed by astrocytes. In a study by Rothhammer et al. [154], gene expression profiling of astrocytes isolated from EAE-induced and control mice showed that most genes upregulated in reactive astrocytes at acute EAE (28 dpi) are linked to IFN-I signaling [154]. Silencing of IFNAR1 specifically in astrocytes with a lentivirus-delivered shRNA caused worsening of EAE, uncovering an anti-inflammatory role for IFN-I signaling in astrocytes. This is due to IFN-I-dependent upregulation of the aryl hydrocarbon receptor (AhR) in astrocytes, which suppresses CNS inflammation through SOCS2-dependent inhibition of NF-κB in astrocytes [154]. Upregulation of AhR in astrocytes was also observed in active and chronic active white matter MS lesions. Importantly, this study showed that astroglial AhRs exert their suppressive function when stimulated by tryptophan metabolites specifically generated by the gut microbiota [154]. This highlights the importance of the cross-talk between CNS and microbiome in the maintenance of CNS homeostasis, and how dysregulation of this interaction may contribute to the pathogenesis of MS, establishing a link among environment, metabolism and inflammation.
Astroglial inflammatory activation in EAE is suppressed by estrogens. This occurs entirely through estrogen receptor α (ERα), whose conditional ablation specifically in astrocytes abolished the protective effect of estrogens [175, 176]. The mechanism is related to ERα-dependent silencing of NF-κB signaling [59]. ERα and aromatase, a key enzyme for estrogen synthesis, are highly upregulated in hypertrophic astrocytes found in chronic active and inactive lesions, suggesting that estrogen synthesis and signaling in reactive astrocytes may be part of an endogenous protective mechanism in CNS autoimmunity [100].
Pathophysiological roles of activated astrocytes in MS and EAE
- Synthesis and response to immunomodulatory factors
Cytokines.
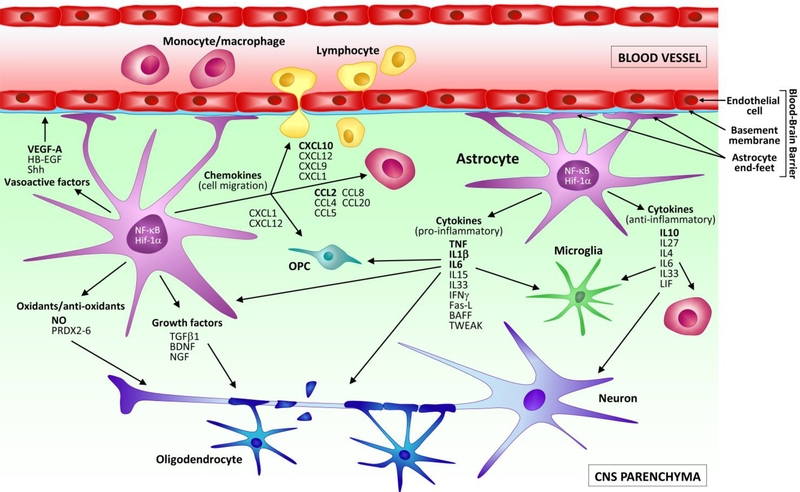
In the MS- and EAE-affected CNS, the ability of astrocytes to produce and respond to immunomodulatory cytokines with pro- and anti-inflammatory properties is well documented (Fig. 4, Table 1).
Figure 4. Schematic representation of the soluble mediators released by astrocytes in MS and EAE.
Astrocytes respond to neuroimmune disease with a process of cellular activation that leads to the production of soluble mediators. These signal in a cell autonomous and non-autonomous manner to promote both neurotoxic, and neuroprotective functions. Activated astrocytes release pro-inflammatory cytokines (e.g. TNF, BAFF, TWEAK, IL1β, IL6) that act on immune cells infiltrated in the CNS, as well as resident microglia to induce their activation. They also interact with neurons and oligodendrocytes to cause cellular damage. In parallel, astrocytes can also secrete anti-inflammatory cytokines and mediators (e.g. IL10, IL4, IL27, Shh) that behave as immuno-suppressants and contribute to turn off the pro-inflammatory activation of leukocytes and microglia, favouring repair and neuroprotection. Additionally, in MS and EAE astrocytes are a powerful source of chemokines (e.g. CCL2, CXCL10, CCL20, CXCL1), which serve as signals for the continued recruitment of immune cells into the CNS. Select chemokines (e.g. CXCL1 and CXCL12) have been shown to favour the migration of OPCs to the site of demyelination, thus exerting a reparative role. Some of the growth factors released by astrocytes (e.g. VEGF-A) can cause pathological alterations to the BBB, others can promote neuroprotective functions (e.g. BDNF, NGF). Finally, astrocytes participate in the oxidative stress response by producing neurotoxic radicals (e.g. NO), or neutralizing them, specifically via the production of peroxiredoxins (PRDX2–6).
Table 1.
Astrocyte-derived factors produced in MS and EAE
| Molecule | Expression in EAE: model type, time, location | Expression in MS: lesion type, time location | Function, mechanism |
|---|---|---|---|
| TNF | Various EAE models: acute and chronic disease, in and around lesions [113, 152, 154, 188] | WML: acute active; chronic active (edge); undetected in chronic inactive [72, 161] | Dual effect: pro-inflammatory (solTNF), anti-inflammatory (memTNF) |
| BAFF | n/a | WML: acute active; chronic active [88] | Pro-inflammatory: B cell survival and expansion (putative) |
| TWEAK | n/a | WML: chronic active (rim); NAGM: around subpial lesions [163] | Pro-inflammatory (putative) |
| Fas-L | MOG35–55 in C57BL/6 mice: acute disease [195] | n/a | Anti-inflammatory: immunosuppressant by causing T cell apoptosis (putative) |
| IFNγ | MOG35–55 in various C57BL/6 mouse lines [152–154] | n/a | Pro-inflammatory |
| IL1β | MOG35–55 in various C57BL/6 mouse lines [113, 152–154] | n/a | Pro-inflammatory |
| IL6 | MOG35–55 in various C57BL/6 mouse lines [54, 113, 152–154] | WML: acute active; chronic active(rim); adjacent parenchyma [13, 159] | Putative dual effect, pro- and anti-inflammatory |
| IL4 | n/a | WML: chronic active (rim), chronic inactive (rim) [76] | Anti-inflammatory |
| IL10 | MOG35_55 in various C57BL/6 mouse lines [113, 152–154] | WML: acute active (rim); chronic active (rim) [76] | Anti-inflammatory |
| IL15 | n/a | WML: acute active; chronic active (around vessels and in perivascular cuffs) [155] | Pro-inflammatory: CD8 T cell and NK cell survival and activation (putative) |
| IL27 | n/a | WML: chronic active [162] | Anti-inflammatory |
| IL33 | MOG35_55 in C57BL/6 mice: pre-onset, onset, peak disease [41] | WML: unspecified types; perilesional tissue; NAWM [44] | Putative dual effect, pro- and anti-inflammatory |
| LIF | MOG35_55 in various C57BL/6 mouse lines [113, 152–154] | n/a | Anti-inflammatory, pro-remyelination |
| CCL2 (MCP-1) | Various EAE models in rat and mouse: pre-onset, onset, acute disease [19, 60, 61, 82, 123, 138, 149] | WML: acute active (center); chronic active (rim) [147, 183]; chronic inactive (low) [116] | Pro-inflammatory: immune cell recruitment (macrophages) |
| CCL4 (MIP-1β) | MBP immunization in Lewis rats: acute disease only [122] | WML: acute active; chronic active [24] | Pro-inflammatory: immune cell recruitment |
| CCL5 (RANTES) | MBP immunization in Lewis rats: peak disease [122] | WML: acute active; chronic active [24] | Pro-inflammatory: immune cell recruitment |
| CCL8 (MCP-2) | n/a | WML: acute active; chronic active; chronic inactive (low) [116] | Pro-inflammatory: immune cell recruitment |
| CCL20 (MIP-3α) | PLP139–151 in SJL mice: during relapses [8]; MOG35–55 in C57BL/6 mice: high at acute EAE [65] | WML: acute active; chronic active [9] | Pro-inflammatory: immune cell recruitment (dendritic cells, macrophages) |
| CXCL1 (KC, Gro-1) | PLP139–151 in SJL mice: during relapses [60]; MOG35–55 in C57BL/6 mice: peak disease [79] | WML: acute active; chronic active [125, 133] | Pro-inflammatory: immune cell recruitment (neutrophils). Protective: OPC recruitment |
| CXCL9 (MIG) | MOG35–55 in C57BL/6 mice; not found in astrocytes, only microglia [37] | WML: acute active; chronic active [166] | Pro-inflammatory: immune cell recruitment (T cells) |
| CXCL10 (IP10) | Various EAE models in rat and mouse; high at peak [37, 60, 61, 121, 149] | WML: acute active; chronic active; NAWM [17, 166, 173, 174] | Pro-inflammatory: immune cell recruitment (T cells) |
| CXCL12 (SDF-1α) | MOG35–55 in C57BL/6 mice: in basolateral side of endothelium, not clear if in astrocytes [115] | WML: acute active; chronic active (high on glia limitans) [9, 34, 48, 87, 114 | Anti-inflammatory: confines immune cells in perivascular space; OPC recruitment, differentiation (putative) |
| BDNF | MOG35–55 in C57BL/6 mouse lines; acute disease [92, 96] | WML: acute active (high); chronic active (high); chronic inactive (low) [177] | Dual role: neuroprotective and neurotoxic (induces NO production) |
| HB-EGF | n/a | WML: acute active; chronic active; perilesional tissue [157] | Pro-inflammatory: promotes monocyte trafficking (putative) |
| NGF | Lewis rats immunized with guinea pig myelin: astrocytes throughout the CNS at acute disease [120] | n/a | Neuroprotective (putative) |
| TGFβ | MOG35–55 in C57BL/6 mice: before and at onset [90, 103] | WML: acute active; chronic active [51] | Pro-inflammatory |
| VEGF-A | MOG35–55 in C57BL/6 mouse lines; high at acute, peak disease in spinal cord lesions [13, 38] | WML: acute active; chronic active [14, 160] | Pro-inflammatory: BBB breakdown and CNS immune cell entry |
| Shh | MOG35–55 in C57BL/6 mouse lines: before onset, highest at acute disease [196] | WML: chronic active (rim); NAWM [5, 196] | Anti-inflammatory: induces endothelial quiescence and improves barrier function |
| PRDX2 | n/a | WML: lesion edge (unspecified stage) [189] | Antioxidant, neuroprotective |
| PRDX3 | n/a | WML: acute active (high); chronic active (low) [127] | Antioxidant, neuroprotective |
| PRDX5 | n/a | WML: acute active; chronic active; chronic inactive; NAWM [74] | Antioxidant, neuroprotective |
| PRDX6 | MOG35–55 in C57BL/6 mouse lines: acute disease, near lesions [201] | WML: unspecified stage [201] | Antioxidant, neuroprotective |
| NO (NOS2 expression) | Various EAE models in mice: acute disease, widespread in and around lesions [45, 154, 182] | WML: acute active (high); chronic active (rim); NAWM [23, 31, 97] | Oxidant, neurotoxic |
| ROS (activated NOX enzymes) | MOG35–55 in C57BL/6 mouse lines: acute disease, in/near lesions [124] | NOX activation not found in astrocytes, only microglia [56] | Oxidant, neurotoxic |
Soluble molecules produced by astrocytes during the course of MS and EAE are listed. For EAE, the type of model is reported, and for MS the type of tissue; n/a: not assessed. WML: white matter lesions; NAWM: normal appearing white matter; NAGM: normal appearing gray matter. In parenthesis: corresponding references.
In MS, tumor necrosis factor (TNF) is highly upregulated in astrocytes in active lesions, both acute and chronic, especially in astrocytes localized at the lesion edge [72, 161]. TNF is undetected in astrocytes in chronic silent lesions, where the few TNF+ cells display a macrophage morphology [161]. This establishes a correlation between astroglial TNF expression and lesion severity. It should be noted that, in addition to astrocytes, TNF is produced by a variety of other cell types during MS/EAE (neurons, microglia, immune cells) and its contribution to disease pathology is complex. Indeed, TNF has been attributed both protective and detrimental roles in the CNS affected by neurological disease [148]. The soluble form of TNF (solTNF) is mostly responsible for pro-inflammatory and pro-apoptotic processes via activation of TNFR1, whereas the membrane-bound form of TNF (memTNF) sustains anti-inflammatory and reparative processes via activation of TNFR2 [25, 148]. Given that astrocytes located within active MS lesions show upregulation of ADAM-17 (also known as TNF alpha converting enzyme, TACE), the metalloproteinase responsible for the shedding of pro-inflammatory solTNF [143], it is likely that astrocyte-derived TNF plays a neurotoxic role in MS. On the other hand, reactive astrocytes in MS lesions express TNFR2, the receptor primarily activated by protective memTNF, suggesting that by responding to TNF, astrocytes may participate in repair mechanisms [25]. Following EAE, astrocytes express TNF particularly in and around lesions [188]. Expression peaks at acute disease (12–15 dpi) and is still evident through the chronic disease stage [188]. Astrocytes also respond to TNF during EAE, as they express both TNFR1 and TNFR2 [10]. To date, only a few studies have addressed the function of astroglial TNF receptor-dependent signaling in EAE, as transgenic mice with cell-specific ablation of TNF receptors have only recently become available. Astroglial TNFR1 is responsible for the hippocampal synaptic alterations at the basis of learning/memory impairments observed in EAE. Indeed, Habbas and colleagues demonstrated that increase of TNF in the hippocampus of EAE mice triggers the activation of astrocytic TNFR1 and, in turn, a cascade of astrocyte-dependent neuronal alterations resulting in dysfunction of hippocampal synapses [67]. This could explain, at least in part, the cognitive disturbances associated with EAE and MS. Conversely, astroglial TNFR2 signaling has been attributed protective functions in demyelinating syndromes. Even though this was not investigated in the EAE model of MS, rather in the cuprizone model of demyelination, TNFR2 activation in astrocytes was shown to induce autocrine expression of the promyelinating factor CXCL12, which promotes oligodendrocyte precursor cell (OPC) proliferation and differentiation [137]. Collectively, the data available on TNF/TNFR signaling in MS and EAE indicate that astrocytes contribute to maintaining the balance between detrimental and beneficial TNF signaling in the CNS affected by neuroimmune disease.
Other cytokines of the TNF superfamily are produced by astrocytes in MS and EAE. BAFF, which is critical for B cell generation, development and survival, is exclusively expressed by astrocytes in the CNS and is upregulated in MS lesions [88]. In acute lesions, BAFF+ astrocytes are localized around inflammatory cuffs, and in chronic lesions are found in both the perivascular area and the parenchyma where they form a dense network. Interestingly, BAFF+ astrocytes are often in close proximity with immune cells expressing BAFF-receptor (BAFF-R), leading to the speculation that astroglial-derived BAFF promotes the survival of B cells, allowing their persistence and clonal expansion in the MS-affected CNS [88]. Recent studies showed high BAFF levels in the cerebrospinal fluid (CSF) of patients with high cortical lesion load at the time of diagnosis, as well as in the CNS of patients with rapidly progressive disease, suggesting BAFF could be a prognostic biomarker for a severe progressive clinical phenotype [104].
TWEAK, another TNF superfamily member, is found in astrocytes in MS tissues [163]. In chronic active white matter lesions, TWEAK+ astrocytes are localized at the lesion border. In the gray matter, TWEAK+ astrocytes are present in the non-demyelinated gray matter surrounding subpial lesions and in the transitional zone between gray and white matter. The TWEAK receptor Fn14 is also expressed by reactive astrocytes. In the white matter, it is found mainly along the borders of chronic active lesions and in the surrounding normal appearing white matter, and in the gray matter is found both within cortical lesions and in the surrounding normal appearing gray matter [163]. Furthermore, TWEAK and Fn14 are always observed in the areas of worse demyelination and cell damage. Given the pro-inflammatory role of TWEAK-Fn14 in CNS disease, this pattern of expression suggests a pathogenic involvement of astroglial TWEAK signaling in MS [163].
The TNF superfamily member Fas-ligand (Fas-L) is produced by astrocytes during EAE, and it has been attributed a protective role [195]. Studies in mice with astrocyte-specific Fas-L deletion show exacerbation of EAE, with increased immune cell infiltration and pro-inflammatory gene transcription [195]. The protective role of astroglial Fas-L has been associated with its role in inducing T cell apoptosis, thereby suppressing autoimmunity.
Astrocytes are the primary source of IL6 in the MS brain [106, 159]. In acute lesions, IL6+ astrocytes are distributed throughout, whereas in chronic active lesions they accumulate in areas of ongoing inflammation, such as the lesion edges [106]. IL6+ astrocytes are also seen in and around inactive lesions, particularly in the adjacent white matter with oligodendrocyte preservation [106, 159]. It is possible that, since IL6 is known to have a dual effect, pro- and anti-inflammatory, the association of IL6+ astrocytes with oligodendrocytes may signify a protective role for IL6 in myelin repair [159]. Nevertheless, based on current knowledge, the exact function of IL6 in lesion development is not defined. The same can be said for astrocyte-derived IL6 in EAE pathophysiology. Studies in conditional knockouts with astrocyte-specific ablation of IL6 paint a complex picture [54]. While male mice with astroglial IL6 ablation subjected to EAE do not show differences from WT male mice, female mice with astroglial IL6 ablation show a delayed EAE onset compared to female WT mice. This is accompanied by reduced demyelination and astrogliosis at chronic disease, suggesting a possible protective role for astroglial IL6 that appears to be sex-specific [54].
A common feature of TNF, IL6 and numerous other molecules (e.g. chemokines and growth factors discussed below) produced by activated astrocytes during neuroimmune disease, is that their synthesis is primarily controlled by the canonical NF-κB signaling pathway [119]. Because of its target gene signature, NF-κB has long been considered the master transcriptional regulator of inflammation [179]. The importance of NF-κB signaling in sustaining pro-inflammatory astroglial activation in EAE has been demonstrated using transgenic approaches. Mice with astrocyte-specific inhibition of NF-κB via GFAP-driven overexpression of the IκBα super-repressor (GFAP-IκBα-dn mice) show improved EAE with reduction of neuroinflammation assessed by suppression of inflammatory gene expression and immune cell infiltration in the CNS [27–29, 186]. Among the downregulated genes, many are themselves potent NF-κB activators, such as IL1β [29, 69]. This suggests that, via NF-κB, reactive astrocytes respond to EAE by producing inflammatory mediators, including cytokines, which in turn act in a cell autonomous manner and further stimulate their NF-κB-dependent production. It has been shown that this mechanism is kept in check by A20, a ubiquitin-modifying protein which physiologically inhibits NF-κB activation. Indeed, ablation of astrocytic A20 causes an increase in proinflammatory cytokine production (including TNF, IL6 and IFNγ) following EAE, resulting in disease exacerbation [194].
Astrocytic production of pro-inflammatory cytokines is also sustained by the T cell-derived cytokines IL17 and IFNγ, which are heavily released in the CNS during the acute phase of EAE. Astrocytes are responsive to IL17 as they physiologically express functional IL17 receptor A (IL17RA), which they upregulate following EAE [50]. Exposure of astrocytes in vitro to IL17 alone or in combination with other cytokines (e.g. TNF and IFNγ) stimulates the production of cytokines and chemokines, and increases the expression of the inducible nitric oxide synthase 2 (NOS2) [53, 117, 181]. Impairment of the IL17 signaling pathway in astrocytes via ablation of Act-1, an essential component of the IL17R complex, causes suppression of IL17-mediated chemokine expression and is associated with disease amelioration [78]. This indicates that via IL17 astrocytes play an important role in leukocyte recruitment during CNS autoimmunity. One of the mechanisms by which IL17 regulates the production of inflammatory mediators in astrocytes during EAE is the modulation of microRNA expression. With a combination of in vitro and in vivo studies, miR-873 induced by IL17 stimulation in astrocytes was shown to promote cytokine production via suppression of A20, thus lifting the inhibition of proinflammatory NF-κB activation [98]. Using a similar approach, miR-497 was also shown to participate in IL17-dependent pro-inflammatory gene expression in astrocytes following EAE. Indeed, activation of IL17 signaling in astrocytes causes a downregulation of miR-497 unleashing expression of the transcription factor Hif-1α and subsequent upregulation of pro-inflammatory genes. Restoring miR-497 to astrocytes of EAE-induced mice improved the clinical outcome, underscoring the relevance of the IL17-miR-497-HIF-1α axis to EAE pathophysiology [164].
Unlike IL17, the reports on the astrocytic response to IFNγ in EAE have been contradictory. Silencing of IFNγ signaling in astrocytes via lentiviral knockdown of IFNγR ameliorated EAE by suppressing the production of cytokines and chemokines, thus limiting CNS immune cell trafficking [52]. Yet, studies where astrocytic IFNγ signaling was suppressed via expression of a signaling-deficient dominant-negative IFNγ receptor 1 in astrocytes (GFAPγR1Δ mice) showed the opposite. Indeed, GFAPγR1Δ transgenic mice displayed remarkably increased EAE severity and progression, with enhanced astrocytic activation, demyelination and axonal damage [71]. These outcomes were linked to elevated expression of astrocytic IL6 and sustained microglial activation [156].
The cytokines IL33 and IL15 have also been detected in astrocytes in MS and EAE, and their function is yet to be understood. IL33, which has been attributed dual pro- and anti-inflammatory roles [44] is observed in astrocytes present both in lesional and perilesional tissue, as well as in the normal appearing white matter of MS brains. Its significance is unknown, although studies in EAE show that neutralization of IL33 actively secreted by astrocytes exacerbates disease [41], pointing at a protective role of astroglial derived IL33 in CNS autoimmunity. High expression of IL15 is detected in reactive astrocytes in MS tissues both in acute and subacute/chronic lesions [155]. IL15+ astrocytes are also found around blood vessels with or without perivascular cuffs [155]. Since IL15 is essential for development, activation, and survival of CD8 T lymphocytes and NK cells, it is plausible that production of IL15 is one of the ways by which astrocytes communicate with the adaptive immune system contributing to MS immunopathology [155].
Finally, various cytokines known for their anti-inflammatory and neuroprotective effects are expressed by reactive astrocytes in the MS brain. Strong IL10 immunoreactivity is observed in astrocytes within acute active lesions, as well as at the rim of chronic active lesions [76]. IL4 is detected in astrocytes within the hypocellular rim of chronic active and inactive lesions. [76]. Similarly, the immunosuppressive cytokine IL27 is upregulated in astrocytes within chronic lesions [162]. Importantly, their respective receptors IL10R, IL4R and IL27R are also expressed and upregulated in astrocytes in active and chronic active lesions [76, 162]. These patterns of expression suggest these cytokine systems may be implicated in MS lesion repair, potentially turning off lesion activity.
Chemokines.
In MS and EAE, astrocytes become a powerful source of chemokines, whose function is to guide cell migration and trafficking (Fig. 4, Table 1). While a handful of these chemokines display protective properties, most of them are chemoattractant for detrimental immune cells, thus their production is generally associated with sustaining and exacerbating the pathology.
In active demyelinating and chronic active MS lesions, reactive hypertrophic astrocytes highly express CCL2 (MCP-1). Immunoreactivity is especially elevated in the center of acute active lesions and within the rim of chronic active lesions, in close association with macrophages [183]. Low astroglial expression is also reported in chronic inactive lesions [116]. In a study looking at white matter versus gray matter lesions in the hippocampus, CCL2 expressing astrocytes were found only in active white matter lesions where macrophages were present and immunoreactive for the CCL2-specfic receptor CCR2. Astrocytes did not express CCL2 in gray matter lesions characterized by a paucity of immune cells [147]. Being CCL2 a powerful chemoattractant for monocytes/macrophages, this pattern of expression suggests that reactive astrocytes play a role in the recruitment of myelin-degrading macrophages, thereby specifically contributing to the evolution of white matter MS lesions [147, 183]. This may also explain the low immune cells presence in gray matter lesions where astrocytes do not express CCL2.
Multiple histological studies have localized CXCL10 (IP10) to reactive astrocytes in active demyelinating MS lesions and the surrounding parenchyma [17, 166, 173, 174]. Expression is observed especially around inflammatory infiltrates, both in the astrocyte cell body and end-feet [173]. A similar pattern was reported for CXCL9 (MIG), whose main localization is in astrocytes surrounding the lesions [166]. Importantly, T cells expressing CXCR3, the receptor responsive to both CXCL10 and CXCL9, are found within the lesions and accumulate as lesions develop [166, 174]. This indicates that astroglial CXCL10/CXCL9 signaling towards CXCR3 expressing T cells is an important mechanism driving T cell accumulation in the CNS perivascular space and parenchyma during MS lesion formation.
CCL2 and CXCL10 were the first two chemokines to be also identified in astrocytes during EAE [19, 37, 60, 61, 149]. For both, astroglial expression was only observed after the onset of clinical signs and was the highest at peak/acute disease, correlating with disease activity [19, 61]. Histological studies showed astrocytes in close proximity with infiltrating leukocytes had the most intense immunoreactivity [61]. This indicates that leukocyte-derived factors are likely the main stimuli triggering astroglial production of CCL2 and CXCL10 in EAE. Recent studies showed that transgenic mice with conditional ablation of CCL2 in astrocytes have less severe EAE, accompanied by reduced macrophage and T cell infiltration, reduced glial reactivity and improved neuroprotection [82, 123, 138]. Similarly, deletion of astroglial CXCL10 resulted in EAE improvement, with delayed onset and mild reduction of severity, despite not being effective in protecting from progressive axon loss [121]. Absence of CXCL10 reduced the ability of CD4+ T cells to enter the CNS parenchyma, accumulating in the perivascular cuffs instead [121]. Similar to the findings in MS, these reports underscore the crucial role astrocyte-derived CCL2 and CXCL10 play in the continued recruitment of immune cells that sustain disease progression.
In MS, astrocytes control CNS monocyte/macrophage trafficking also via CCL5 (RANTES) and CCL8 (MCP-2). These chemokines are upregulated in astrocytes within active and chronic active MS lesions [24, 116]. They both act through the receptor CCR5, which is highly expressed in monocytes (and few lymphocytes) concentrated in active lesions [17]. This profile has been reported in EAE as well, where astrocytes ramp up production of CCL5 as well as CCL4 (MIP-1β) after EAE onset [122]. Astroglial synthesis of CCL5 and CCL4 is initiated in response to T cell-derived IL17 and IL6 [122], indicating that, like CCL2 and CXCL10, leukocytes provide the initiating signals.
Astrocytes are the CNS cells expressing CXCL12 (SDF-1α) in the MS brain. A key mediator of B cell migration, CXCL12 is present in astrocytes and on blood vessels under normal conditions, and its expression is upregulated in MS, both in active and chronic inactive lesions [9, 87, 114]. The ability of astrocytes to produce CXCL12 has been amply demonstrated in vitro after stimulation with inflammatory cytokines [9, 22]. It has been observed that, in active MS lesions, CXCL12 on blood vessels changes distribution, rearranging toward the lumenal side of venules. At the same time, astrocytes increase CXCL12 expression within the glia limitans [114]. This change in CXCL12 polarity has been associated with impairment of the BBB and positively correlated with increased inflammation and demyelination in MS. This suggests that CXCL12 normally functions to localize infiltrating leukocytes to perivascular spaces, preventing their CNS parenchymal infiltration. When CXCL12 polarity is disrupted during MS, this leads to increased leukocyte adherence to vessels, which facilitates their migration into the CNS [114]. Similarly to MS, CXCL12 is upregulated in the CNS after EAE. At acute disease, it is found redistributed to the basolateral side of the CNS endothelium, and this is believed to be a mechanism by which leukocytes are allowed entry into the CNS during EAE [115]. Differently from MS, however, a clear expression in the astrocyte component of the glia limitans was not observed [115]. Beside these detrimental chemoattractant properties, astroglial CXCL12 has also been attributed protective functions. Indeed, it promotes the polarization of effector Th1 cells into IL10high antigen-specific regulatory T cells, thereby restraining the autoimmune process [118], and it may play a role in attracting CXCR4+ OPCs, contributing to remyelination and repair in CNS demyelination [136].
In MS, the chemokine CXCL1 (KC, Gro-1) is found on hypertrophic astrocytes in active lesions in close proximity to proliferating oligodendrocytes expressing CXCR2, the specific receptor for CXCL1. By acting through CXCR2, CXCL1 is a major chemoattractant for diverse cell populations, including neutrophils and OPCs [125]. This may indicate that, via release of CXCL1, reactive astrocytes recruit CXCR2+ OPCs to the sites of demyelination, thereby contributing to myelin repair [133]. Production of CXCL1 by astrocytes during EAE has also been reported [60]. Transgenic mice transiently overexpressing CXCL1 in astrocytes at the onset of EAE show a milder disease phenotype associated with reduced axonal pathology and improved remyelination at chronic stages, despite the acute increase in neutrophil infiltration driven by CXCL1. This indicates that the astroglial-oligodendroglial CXCL1-CXCR2 axis has a powerful neuroprotective function due to the CXCL1-dependent migration of OPCs to the lesion sites where they can initiate remyelination, which is sufficient to counteract the detrimental neutrophil effect [134]. Contrary to this study, a recent report showed that sustained overexpression of astroglial CXCL1 during EAE is detrimental and exacerbates the outcome by worsening inflammation and demyelination. This was dependent on overt and continuous neutrophil infiltration [64]. Interestingly, it has been shown that treatment of EAE-induced mice with ERβ ligands drastically reduced EAE severity. This correlated with increased astroglial production of CXCL1, leading to improved remyelination and neuroprotection [79].
Mild production of CCL20 (MIP3α) has been reported in astrocytes in MS lesions [9]. Expression is observed in EAE as well, where astrocytes are the principal source of CCL20 [8]. Here, astrocytic CCL20 production has been shown to be triggered by T cell-derived RANKL, a member of the TNF superfamily of cytokines [65]. Indeed, inhibition of RANKL leads to downregulation of CCL20 and results in suppression of EAE [65]. In addition to T cells, CCL20 is a powerful chemoattractant for dendritic cells, macrophages and, to a lesser extent, neutrophils. This may indicate a role for astroglial CCL20 in modulating the innate immune response in MS and EAE.
Growth factors.
Astrocytic production of a variety of growth factors has been demonstrated both in MS and EAE (Fig. 4, Table 1). Brain derived neurotrophic factor (BDNF) is found in reactive astrocytes within active and, to a lesser extent, inactive MS lesions [177]. It is also present in EAE lesions at the onset/acute stage of disease (14 dpi) [96]. Here, BDNF has been attributed neuroprotective functions. Indeed, genetic ablation of BDNF specifically in astrocytes exacerbated EAE and increased axonal damage [96], despite not altering CNS immune cell infiltration. An identical profile was observed when astroglial BDNF was inducibly ablated after EAE induction during the preclinical phase (up to 11 dpi) [92]. Studies with bone marrow chimeras showed this neuroprotective effect is solely attributed to CNS-derived and not immune cell-derived BDNF, uncovering an important reparative function for activated astrocytes in CNS autoimmunity [92]. Astrocytes not only produce BDNF, but also respond to it via activation of TrkB receptors. TrkB is markedly upregulated on astrocytes during EAE, as well as in MS chronic inactive lesions [45]. Surprisingly, astroglial TrkB activation is neurotoxic in EAE, as transgenic mice with astrocyte-specific TrkB ablation show reduced neuronal damage. This is due to NO released by astrocytes as a result of BDNF-dependent TrkB activation [45]. Together, these studies indicate that astroglial BDNF not only elicits neuroprotective effects in other cell types, but can also cell autonomously activate astrocytes to release toxic NO, thus causing neurodegeneration. This suggests a possible dual protective and degenerative role for astroglial BDNF in neuroimmune disease.
Astroglial expression of all the transforming growth factor beta (TGFβ) isoforms has been detected in MS lesions at various stages, from active demyelinating to chronic active and inactive [51], suggesting a role in lesion development and evolution. In EAE, TGFβ1 in particular was found to be upregulated in astrocytes before and during the onset phase of EAE [90, 103]. This has been shown to depend on astroglial production of angiotensin II, which can in turn stimulate astrocytes to produce TGFβ1 in a positive feed-back loop [90]. Both angiotensin II and its precursor angiotensinogen have been described in reactive perivascular astrocytes in MS lesions, thus indicating that a similar mechanism of TGFβ1 regulation may be relevant to MS pathogenesis as well. Blockade of TGFβ1 upregulation with the angiotensin II type 1 receptors (AT1Rs) inhibitor candesartan improved the clinical course of EAE and reduced lymphocyte infiltration, supporting a detrimental role for astroglial TGFβ1 in EAE [90]. This is further corroborated by studies with transgenic mice overexpressing astroglial TGFβ1, which, after EAE challenge, show earlier onset and increased disease severity [198]. Notably, the detrimental effects of astroglial-derived TGFβ1 are in contrast with peripherally produced or administered TGFβ1, which has been attributed protective functions in EAE due to its immunosuppressive and anti-inflammatory properties [68, 77].
Astrocytic expression of the angiogenic vascular endothelial growth factor A (VEGF-A) has been reported in active demyelinating and chronic MS lesions [14, 160], particularly in highly reactive astrocytes around blood vessels, and in EAE [13]. Astroglial VEGF-A is a key driver of BBB permeability in neuroimmune disease [13]. Indeed, transgenic ablation of astrocytic VEGF-A reduced BBB breakdown by preventing the disruption of endothelial claudin-5 (CLD5) and occludin (OCLN), thus preserving tight junctions. This limited lymphocyte infiltration, resulting in reduced paralysis and neuroprotection [13]. A primary signal directing the expression of VEGF-A during developmental angiogenesis is the transcription factor Hif-1α. Since Hif-1α and VEGF-A are highly expressed and colocalize to astrocytes in MS lesions, it has been suggested that reactivation of this developmental angiogenic pathway in astrocytes is responsible for BBB breakdown during CNS autoimmunity [14]. Furthermore, astroglial VEGF-A has been shown to synergize with astrocyte-produced thymidine phosphorylase (TYMP) to induce BBB disruption. Indeed, blockade of either one or both factors suppresses EAE and improves BBB function [38].
Reactive astrocytes in active and chronic active MS lesions, as well as perilesional tissue, highly express heparin-binding epidermal growth factor (HB-EGF) [158], a trophic and chemotactic factor induced by inflammatory stimuli. Expression is also observed on astrocyte end-feet wrapping blood vessels. Though its role in CNS autoimmunity is unclear, since blockade of HB-EGF reduced the migration of monocytes across brain endothelial cell monolayers, it is plausible to hypothesize it may be implicated in immune cell trafficking across the BBB, thus participating in lesion development and evolution [158].
Astroglial expression of nerve growth factor (NGF) has been observed throughout the CNS (spinal cord, corpus callosum, cortex, brain stem) during the acute stage of EAE in rats [120]. Whether astrocytes are a source of NGF also in the MS-affected CNS has not been reported. NGF function in neurodegenerative disorders is still a matter of debate, as this factor has been attributed both protective and detrimental effects on neuronal and OPC survival. One hypothesis is that enhanced NGF production may be an attempt at activating protective signals to counteract neuronal damage and promote oligodendrocyte survival [120].
- Modulation of oxidative stress
Oxidative stress is the complex cascade of deleterious events induced by reactive oxygen species (ROS) and reactive nitrogen species (RNS). It occurs when the antioxidative system is overwhelmed, particularly during pathological conditions. ROS are mostly byproducts of mitochondrial oxidative phosphorylation, generated via the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidases (or NOX enzymes). The main RNS is the free radical NO, generated by nitric oxide synthases. In the CNS, astrocytes are major players in the antioxidative system. Indeed, they have the capacity to produce ROS and RNS, but are also the main cell type responsible for their neutralization through glutathione and ascorbic acid, as well as detoxifying enzymes, such as superoxide dismutases (SODs) [187]. During MS and EAE, ROS and RNS are produced by astrocytes, as well as other CNS and immune cells, and they have been implicated in mechanisms underlying lesion pathogenesis (Fig. 4, Table 1).
In MS, extensive oxidative damage to lipids, proteins, and nucleic acids is observed throughout active demyelinating and chronic active lesions, especially within reactive astrocytes and myelin-laden macrophages [185]. Interestingly, at the same time astrocytes markedly upregulate the expression of the antioxidant enzymes superoxide dismutase 2 (SOD2) and NAD(P)H:quinone oxidoreductase 1 (NQO1), which may reflect an adaptive defense mechanism to reduce ROS-mediated cellular damage [185]. Astrocyte can counteract oxidative damage also via the activity of peroxiredoxins (PRDXs), which catalyze the reduction of peroxides to alcohols. In MS, PRDX2 is found upregulated in astrocytes in white matter lesions, particularly at the edges [189]. Expression levels positively correlate with the degree of inflammation and oxidative stress [189]. PRDX3 is detected strongly in acute active lesions and to a lesser extent in chronic active lesions [127]. PRDX5 is found in astrocytes in acute, chronic active and inactive MS lesions, as well as in the normal appearing white matter [74]. PRDX6 is also found in astrocytes within lesions, both in MS and EAE [201]. In EAE, transgenic mice overexpressing PRDX6 showed expression almost exclusively localized to astrocytes, mainly near demyelinating lesions. Increased levels of astroglial-derived PRDX6 correlated with a reduction in EAE clinical symptoms associated with diminished inflammation and CNS damage [201]. Collectively, these data indicate that, like SOD2 and NQO1 expression, PRDXs expression may function as an adaptive reaction of astrocytes to contain oxidative damage.
On the opposite side, astrocyte cause oxidative stress by producing NO. Intense upregulation of inducible nitric oxide synthase 2 (NOS2), as well as of its catalytic activity, is detected in hypertrophic astrocytes throughout active demyelinating lesions and in the adjacent normal appearing white matter [23, 31, 97]. In chronic active lesions, astroglial NOS2 expression is also evident, particularly at the lesion edge [23, 31, 97]. This indicates that astrocyte-derived NO produced via NOS2 may contribute to cytotoxicity mediated by oxidative damage. Astrocytes are also a major source of NO during EAE. Indeed NOS2 expressing astrocytes are found near inflammatory infiltrates in the spinal cord, as well as the adjacent white matter [182]. Astroglial expression of NOS2 has also been confirmed in recent transcriptomic studies [154]. BDNF has been identified as one of the signals driving astroglial NOS2 upregulation in EAE via TrkB stimulation and subsequent activation of the NF-κB pathway [154].
In EAE, astrocytes may contribute to oxidative stress also via the hyperactivation of NOX enzymes. NOX enzyme overactivation is detected in astrocytes in acute EAE lesions and is associated with accumulation of immune cells and damage [124]. This seem to contrast with MS lesions, where upregulation of NOX enzymes is observed only in microglia and macrophages [56].
- Maintenance of BBB integrity and function
Blood vessels, glia and neurons act as an integrated neurovascular unit that controls the functionality of the BBB [1]. Astrocytes, whose end-feet virtually wrap the entire vascular surface, are key structural elements of the BBB directing the bidirectional transit of molecules and cells between blood and CNS through multiple mechanisms. Their dysregulation results in BBB dysfunction and is a typical feature of neurological diseases including MS, where focal disturbances in the BBB have been associated with the formation of inflammatory lesions [7, 190].
In MS tissues, intense astrogliosis with increased numbers of GFAP+ end-feet is detected around blood vessels at the center of active lesions [4]. This is associated with degradation of endothelial junctional proteins (e.g. OCLN, VE-cadherin) which are necessary for proper BBB functionality [4]. This suggests that signals provided by activated astrocytes likely participate in BBB disruption and facilitate the entry of immune cells promoting lesion formation [4]. Furthermore, within active lesions, reactive astrocytes show severe disruption of the end-feet connecting them to the vasculature. This leads to gaps in the glia limitans and loss of BBB structural integrity, allowing for CNS immune cell entry [32]. Direct astrocyte-astrocyte and astrocyte-endothelial cell communication through gap junctions is also key to maintaining BBB function. Impairment of astrocytic gap junctions has been observed in MS lesions, both actively demyelinating and chronic active, with reduction in the expression of Cx43, their main structural component [111]. This has been linked to BBB disruption, as Cx43 loss leads to astrocyte end-feet edema causing detachment from the basal lamina and weakening of the BBB [55]. Severe loss of astrocytic Cx43 has been especially observed in actively demyelinating lesions of rapidly progressing MS cases who experienced higher relapse rates [112], whereas milder MS phenotypes did not show appreciable changes in astroglial Cx43. In the severe cases, the diffuse loss of Cx43 was also accompanied by a patchy loss of the water channel protein AQP4 at the astrocyte end-feet. Since this is also an integral component of the BBB structure, it is plausible that concomitant Cx43 and AQP4 astrocytopathy contributes to a higher degree of BBB damage that leads to unrestricted immune cell infiltration thus fulminant MS progression [112]. In other reports, reduced astrocytic Cx43 was observed in chronic inactive white matter lesions, whereas a modest increase was found in gray matter lesions and normal appearing gray matter [108, 109]. Rather than associated with BBB dysfunction, this increase has been interpreted as an indicator of astrogliosis causing aberrant astrocytic intercommunication that could be at the basis of cognitive impairments in MS patients [108, 109]. A systematic characterization of AQP4 expression and distribution in MS tissue was undertaken by Sinclair and colleagues, who found an increase in all categories of MS tissue, with the highest levels in active lesions [167]. Such increase was most pronounced at the astrocytic end-feet but was also found in the cell bodies of parenchymal astrocytes [167]. This suggests that aberrant AQP4 function at the BBB may be involved in the initiation of the diffuse vasogenic edema observed in MS [167].
In EAE, similarly to MS, BBB impairment due to altered astroglial AQP4 function has been associated with vasogenic edema and tissue damage [197]. Indeed, in the spinal cord and cerebellum of EAE-induced mice AQP4 was found to be disorganized and redistributed over the entire cell surface rather than confined to the end-feet. This loss of polarized AQP4 localization caused altered water redistribution across the BBB and edema formation [197]. A key protein in maintaining proper AQP4 organization, thus structural integrity of astrocyte end-feet and BBB, is dystroglycan [128, 197]. On one side, dystroglycan connects the astroglial end-feet to the endothelial basement membrane, on the other it is anchored to the astroglial cytoskeletal machinery. During EAE, loss of astrocytic dystroglycan due to cleavage by the metalloproteases MMP2 and MMP9 causes breakdown of the endothelial basement membrane and focal leakiness of the BBB, allowing unrestricted ingress of CD4+ T cells in the CNS parenchyma [3]. Recently, it has been reported that during CNS inflammation reactive astrocytes form de novo structural tight junction complexes within the end-feet in response to pro-inflammatory signals [75]. These contain claudin-1 (CLDN1), claudin-4 (CLDN4), and junctional adhesion molecule A (JAM-A). The newly formed tight junctions increase the impenetrability of the glia limitans component of the BBB, corralling T cells into segregated clusters and preventing their entry into the CNS [75]. Conditional ablation of CLDN4 specifically in astrocytes disrupts the formation of these protective tight junctions, leading to unchecked leukocyte infiltration and worsening of EAE [75]. Collectively, these studies underscore how the structural integrity of astrocytes is pivotal in maintaining the architecture and functionality of the BBB during neuroimmune disease. This is further supported by the observation that, in pre-symptomatic EAE, damaged swollen astrocytes with dispersed GFAP filaments are found in areas with increased vascular permeability, but yet to be infiltrated with immune cells [42]. This indicates that loss of astroglial cyto-integrity is a prerequisite for BBB disruption at the basis of immune cell entry [42].
Another important task operated by astrocytes at the BBB is the regulation of iron homeostasis. Toxic ferrous iron accumulates in disease and its detoxification requires conversion into ferric iron that can be dumped into circulation through the astrocyte end-feet in contact with the vasculature. In EAE, astrocytes near the site of lesions upregulate expression of iron importers, suggesting they possess mechanisms to actively uptake iron during disease progression [202]. Astrocytes also express iron exporters at all stages of EAE, indicating they are capable of effluxing iron and safely recycle it out of the CNS. These mechanisms likely explain why iron does not accumulate in astrocytes during EAE. The robust clearance of iron by astrocytes might also contribute to the lower level of iron buildup observed in the EAE spinal cord [202].
Astrocytes modulate BBB function also through the synthesis and release of soluble factors. In addition to those described in the paragraphs above (e.g. cytokines, VEGF-A, NO), Sonic Hedgehog (Shh) must be mentioned for its protective effect on barrier function. Shh is highly upregulated by reactive perivascular astrocytes in MS lesions [5, 196]. Using in vitro/in vivo approaches, astrocyte-derived Shh was shown to act on barrier-forming endothelial cells promoting their quiescence. Indeed, it downregulated their expression of intercellular adhesion molecule-1 (ICAM-1) as well as of the chemokine CCL2, thereby reducing the adhesion and migration potential of T cells across the BBB [5]. Furthermore, astroglial Shh reduced the activation state of Th1 and Th17 cells, dampening their production of pro-inflammatory molecules [5]. On this basis, it is no surprise that pharmacological inhibition of the Shh pathway caused significant EAE exacerbation, associated with increased immune cell presence in the CNS and worse demyelination. This underscores the crucial role of astroglial Shh in maintaining proper barrier function and sustaining the astroglial-specific anti-inflammatory response during CNS immune attack.
In vivo measurements of astroglial reactivity: diagnostic and/or prognostic tools for MS?
In MS, non-invasive imaging techniques are essential to aid diagnosis, monitor disease progression and treatment efficacy, as well as uncover underlying pathogenic mechanisms. Though magnetic resonance imaging (MRI) has been the gold standard in MS diagnosis and monitoring, its ability to assess functional processes and gain information on disease-associated molecular events is limited. To the contrary, this is possible with positron emission tomography (PET), which is now emerging as a powerful tool for in vivo functional imaging. It allows quantification of cellular targets that can help understand mechanisms underpinning MS even prior to the occurrence of structural changes detectable by MRI. Neuroinflammation can be effectively assessed by PET, and astroglial reactivity is one of its hallmarks that can be targeted for this purpose. Since a large body of evidence has now established a positive correlation between astroglial reactivity and disease progression in MS [32], PET imaging of this phenomenon is an appealing non-invasive method to potentially enhance early and accurate diagnosis for precision therapy. PET imaging of astroglial reactivity is still in its infancy and in need for the development of suitable imaging tracers [146]. Acetate is a metabolite known to accumulate in astrocytes through the activity of the monocarboxylate transporter (MCT). Since astroglial MCT expression is upregulated in MS lesions [126], astroglial accumulation of acetate is considered a feature of astrogliosis that can be measured by PET [199]. On this basis, 11C-acetate has been proposed as a tracer. 11C-acetate uptake significantly increases in the white and gray matter of MS patients, more predominantly in the white matter [178]. However, its overall low brain uptake renders 11C-acetate inadequate for quantitative assessments [200]. The derivatives 18F-fluoroacetate and benzyl 11C-acetate have been proposed as alternatives [131, 145]. However, more work needs to be done to develop the current tracers to a suitable standard, as well as to identify new ones before PET imaging of astroglial activation can become a reliable tool.
An alternate approach to MRI that can overcome some of its limitations proving useful in MS is MR spectroscopy (MRS) [15]. MRS allows to monitor pathological processes via changes in their characteristic metabolic markers [39]. These processes include astroglial activation, that can be indirectly assessed by measuring myo-inositol (Ins) content [30]. Because Ins plays an important role in the control of astrocyte volume, elevated levels of Ins are considered an indicator of astrogliosis, thus of neuroinflammation. The ratios between Ins and other metabolic markers such as N-acetylaspartate (tNAA), a surrogate for axonal integrity, or creatine (tCr), a surrogate for cell proliferation, are used to define astrocyte reactivity and disease evolution in MS. Increased Ins levels can be observed in the normal appearing white matter compared to control white matter [39, 83], indicating that alterations associated with astroglial reactivity may be assessed early in disease and may play a role in its development. Elevated Ins/tNAA in the normal appearing white matter is a reliable predictor of brain atrophy, thus of disease progression and neurological disability evolution [99, 130]. Elevation of Ins/tCr has been shown to correlate with the disruption of neuronal networks, thereby serving as a marker and predictor of cognitive dysfunction in MS [172]. Also, an increase in Ins, Ins/tCr and Ins/tNAA in chronic inactive white matter lesions, independently from the disease course, has been detected, indicating ongoing astrogliosis even during the late stages of disease [130].
In parallel to imaging modalities, quantification of GFAP levels in serum and CSF have been proposed as options for assessing astroglial activation in MS patients. The presence of elevated GFAP levels in the CSF of MS patients compared to healthy individuals has been described by several groups. GFAP levels are associated with disease severity [151], with the highest concentrations found in secondary progressive patients, particularly those exhibiting severe ambulatory deficits [107, 140]. In general, GFAP is considered a prognostic marker of disability progression [16, 107, 110, 151]. In patients with early RRMS and clinically isolated syndrome (CIS), GFAP levels correlate with the intensity of gadolinium-enhancement on MRI scans, indicating that GFAP may be a biomarker of highly active disease in these populations [80]. More recently, levels of GFAP in the serum have been proposed as disease biomarkers. Serum GFAP levels are elevated in all disease types [2, 73], and highest with increasing MRI-lesion count, especially in progressive patients [2]. This suggests that serum GFAP could be a suitable disease progression marker.
A few studies have looked at S100β, another astrocytic protein considered a marker of astrogliosis, in correlation with disease severity and progression. Levels of S100β are higher in the CSF of MS patients, especially in RRMS [18, 140], and seem to distinguish them from SPMS and PPMS patients [140]. Nevertheless, more evidence is needed to validate S100β as a disease biomarker.
Collectively, these studies show that, weather measured by imaging techniques or biological testing of CSF and serum samples, in vivo assessment of astroglial reactivity in MS patients is a reliable early diagnostic and prognostic tool for MS. The routine implementation of these assessments may in the future become the standard of practice, as it could be invaluable in guiding therapeutic decisions. More data need to be acquired in patients after treatment with disease modifying drugs to determine whether these in vivo assessments of astrocytic activation may also be indicative of therapeutic efficacy.
Concluding remarks
The contribution of astrocytes to MS and EAE immunopathology is now established. In both, astrocytes undergo a process of cellular activation that is evident from the initial stages of disease. This results in astrocytes gaining the ability of producing a host of soluble mediators, neurotoxic and neuroprotective, as well as losing the ability of maintaining homeostatic functions important for CNS integrity. In their neurotoxic capacity, astrocytes produce oxidants, cytokines and chemokines that exacerbate immune activation and recruitment, thus causing tissue damage. In their neuroprotective capacity, astrocyte produce anti-inflammatory and pro-remyelinating factors, and contain indiscriminate CNS immune cell invasion through the formation of a glial scar. Despite this dichotomy, numerous studies in both MS and EAE have established a positive correlation between astroglial activation and disease severity and evolution, suggesting that strategies aimed at an overall suppression of astroglial activation may prove beneficial. Non-invasive measurements of astroglial activation in patients may represent a way to monitor disease severity, predict disease progression, and possibly test the efficacy of MS therapeutics. Overall, continued efforts to better understand the nature of astroglial responses in neuroimmune disease are warranted, with the EAE model and current methodological and technical advances providing valuable tools to understand molecular signals and mechanisms that could be targeted for therapeutic purposes in MS.
Acknowledgments
We are grateful to Geoffrey Taghon for his contribution to the graphic design of Figure 4. R.B. was supported by NIH-NINDS (grant 1R01NS094522-01), the Italian Multiple Sclerosis Foundation (grant FISM 2015/R/7), the US National Multiple Sclerosis Society (grant NMSS PP-1804-30716), and The Miami Project To Cure Paralysis.
Abbreviations
- AhR
aryl hydrocarbon receptor
- APC
antigen presenting cell
- AQP4
aquaporin-4
- BBB
blood-brain barrier
- BDNF
brain derived neurotrophic factor
- CFA
complete Freund’s adjuvant
- CIS
clinically isolated syndrome
- CLD5
claudin-5
- CNS
central nervous system
- CSF
cerebro-spinal fluid
- Cx43
connexin 43
- EAE
experimental autoimmune encephalomyelitis
- ERα
estrogen receptor α
- GFAP
glial fibrillary acidic protein
- GM
gray matter
- HB-EGF
heparin-binding epidermal growth factor
- ICAM-1
intercellular adhesion molecule-1
- IFN-I
type I interferons
- Ins
myo-Inositol
- LacCer
lactosylceramide
- MOG
myelin oligodendrocyte glycoporotein
- MBP
myelin basic protein
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- MS
multiple sclerosis
- NADPH
nicotinamide adenine dinucleotide phosphate
- NAGM
normal appearing gray matter
- NAWM
normal appearing white matter
- NGF
nerve growth factor
- NO
nitric oxide
- NOS2
nitric oxide synthase 2
- OCLN
occludin
- OPC
oligodendrocyte precursor cell
- PET
positron emission tomography
- PLP
proteolipid protein
- PPMS
primary progressive multiple sclerosis
- ROS
reactive oxygen species
- RRMS
relapsing-remitting multiple sclerosis
- S1P
sphingosine 1-phosphate
- SPMS
secondary progressive multiple sclerosis
- tCr
creatine
- TGFβ
transforming growth factor β
- tNAA
N-acetylaspartate
- TNF
tumor necrosis factor
- memTNF
membrane-bound tumor necrosis factor
- solTNF
soluble tumor necrosis factor
- WM
white matter
- VEGF-A
vascular endothelial growth factor A
Footnotes
Conflict of interest: The author declares no conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Abbott NJ, Ronnback L, Hansson E (2006) Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci 7: 41–53 Doi 10.1038/nrn1824 [DOI] [PubMed] [Google Scholar]
- 2.Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M (2018) Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep 8: 14798 Doi 10.1038/s41598-018-33158-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrawal S, Anderson P, Durbeej M, van Rooijen N, Ivars F, Opdenakker G, Sorokin LM (2006) Dystroglycan is selectively cleaved at the parenchymal basement membrane at sites of leukocyte extravasation in experimental autoimmune encephalomyelitis. J Exp Med 203: 1007–1019 Doi 10.1084/jem.20051342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez JI, Cayrol R, Prat A (2011) Disruption of central nervous system barriers in multiple sclerosis. Biochim Biophys Acta 1812: 252–264 Doi 10.1016/j.bbadis.2010.06.017 [DOI] [PubMed] [Google Scholar]
- 5.Alvarez JI, Dodelet-Devillers A, Kebir H, Ifergan I, Fabre PJ, Terouz S, Sabbagh M, Wosik K, Bourbonniere L, Bernard M et al (2011) The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science 334: 1727–1731 Doi 10.1126/science.1206936 [DOI] [PubMed] [Google Scholar]
- 6.Alvarez JI, Katayama T, Prat A (2013) Glial influence on the blood brain barrier. Glia 61: 1939–1958 Doi 10.1002/glia.22575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alvarez JI, Saint-Laurent O, Godschalk A, Terouz S, Briels C, Larouche S, Bourbonniere L, Larochelle C, Prat A (2015) Focal disturbances in the blood-brain barrier are associated with formation of neuroinflammatory lesions. Neurobiol Dis 74: 14–24 Doi 10.1016/j.nbd.2014.09.016 [DOI] [PubMed] [Google Scholar]
- 8.Ambrosini E, Columba-Cabezas S, Serafini B, Muscella A, Aloisi F (2003) Astrocytes are the major intracerebral source of macrophage inflammatory protein-3alpha/CCL20 in relapsing experimental autoimmune encephalomyelitis and in vitro. Glia 41: 290–300 Doi 10.1002/glia.10193 [DOI] [PubMed] [Google Scholar]
- 9.Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, Lande R, Aloisi F, Coccia EM (2005) Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. J Neuropathol Exp Neurol 64: 706–715 [DOI] [PubMed] [Google Scholar]
- 10.Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV (2016) Astrocyte scar formation aids central nervous system axon regeneration. Nature 532: 195–200 Doi 10.1038/nature17623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A (2014) Gliotransmitters travel in time and space. Neuron 81: 728–739 Doi 10.1016/j.neuron.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Araque A, Parpura V, Sanzgiri RP, Haydon PG (1999) Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci 22: 208–215 [DOI] [PubMed] [Google Scholar]
- 13.Argaw AT, Asp L, Zhang J, Navrazhina K, Pham T, Mariani JN, Mahase S, Dutta DJ, Seto J, Kramer EG et al. (2012) Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 122: 2454–2468 Doi 10.1172/JCI60842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argaw AT, Zhang Y, Snyder BJ, Zhao ML, Kopp N, Lee SC, Raine CS, Brosnan CF, John GR (2006) IL-1beta regulates blood-brain barrier permeability via reactivation of the hypoxia-angiogenesis program. J Immunol 177: 5574–5584 [DOI] [PubMed] [Google Scholar]
- 15.Arnold DL, Wolinsky JS, Matthews PM, Falini A (1998) The use of magnetic resonance spectroscopy in the evaluation of the natural history of multiple sclerosis. J Neurol Neurosurg Psychiatry 64 Suppl 1: S94–101 [PubMed] [Google Scholar]
- 16.Axelsson M, Malmestrom C, Nilsson S, Haghighi S, Rosengren L, Lycke J (2011) Glial fibrillary acidic protein: a potential biomarker for progression in multiple sclerosis. J Neurol 258: 882–888 Doi 10.1007/s00415-010-5863-2 [DOI] [PubMed] [Google Scholar]
- 17.Balashov KE, Rottman JB, Weiner HL, Hancock WW (1999) CCR5(+) and CXCR3(+) T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc Natl Acad Sci U S A 96: 6873–6878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barateiro A, Afonso V, Santos G, Cerqueira JJ, Brites D, van Horssen J, Fernandes A (2016) S100B as a Potential Biomarker and Therapeutic Target in Multiple Sclerosis. Mol Neurobiol 53: 3976–3991 Doi 10.1007/s12035-015-9336-6 [DOI] [PubMed] [Google Scholar]
- 19.Berman JW, Guida MP, Warren J, Amat J, Brosnan CF (1996) Localization of monocyte chemoattractant peptide-1 expression in the central nervous system in experimental autoimmune encephalomyelitis and trauma in the rat. J Immunol 156: 3017–3023 [PubMed] [Google Scholar]
- 20.Bjelobaba I, Begovic-Kupresanin V, Pekovic S, Lavrnja I (2018) Animal models of multiple sclerosis: Focus on experimental autoimmune encephalomyelitis. J Neurosci Res 96: 1021–1042 Doi 10.1002/jnr.24224 [DOI] [PubMed] [Google Scholar]
- 21.Blank T, Prinz M (2017) Type I interferon pathway in CNS homeostasis and neurological disorders. Glia 65: 1397–1406 Doi 10.1002/glia.23154 [DOI] [PubMed] [Google Scholar]
- 22.Blazevski J, Petkovic F, Momcilovic M, Jevtic B, Mostarica Stojkovic M, Miljkovic D (2015) Tumor necrosis factor stimulates expression of CXCL12 in astrocytes. Immunobiology 220: 845–850 Doi 10.1016/j.imbio.2015.01.007 [DOI] [PubMed] [Google Scholar]
- 23.Bo L, Dawson TM, Wesselingh S, Mork S, Choi S, Kong PA, Hanley D, Trapp BD (1994) Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann Neurol 36: 778–786 Doi 10.1002/ana.410360515 [DOI] [PubMed] [Google Scholar]
- 24.Boven LA, Montagne L, Nottet HS, De Groot CJ (2000) Macrophage inflammatory protein-1alpha (MIP-1alpha), MIP-1beta, and RANTES mRNA semiquantification and protein expression in active demyelinating multiple sclerosis (MS) lesions. Clin Exp Immunol 122: 257–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brambilla R, Ashbaugh JJ, Magliozzi R, Dellarole A, Karmally S, Szymkowski DE, Bethea JR (2011) Inhibition of soluble tumour necrosis factor is therapeutic in experimental autoimmune encephalomyelitis and promotes axon preservation and remyelination. Brain 134: 2736–2754 Doi 10.1093/brain/awr199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A, Karmally S, Green EJ, Bethea JR (2005) Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med 202: 145–156 Doi 10.1084/jem.20041918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brambilla R, Dvoriantchikova G, Barakat D, Ivanov D, Bethea JR, Shestopalov VI (2012) Transgenic inhibition of astroglial NF-kappaB protects from optic nerve damage and retinal ganglion cell loss in experimental optic neuritis. J Neuroinflammation 9: 213 Doi 10.1186/1742-2094-9-213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brambilla R, Morton PD, Ashbaugh JJ, Karmally S, Lambertsen KL, Bethea JR (2014) Astrocytes play a key role in EAE pathophysiology by orchestrating in the CNS the inflammatory response of resident and peripheral immune cells and by suppressing remyelination. Glia 62: 452–467 Doi 10.1002/glia.22616 [DOI] [PubMed] [Google Scholar]
- 29.Brambilla R, Persaud T, Hu X, Karmally S, Shestopalov VI, Dvoriantchikova G, Ivanov D, Nathanson L, Barnum SR, Bethea JR (2009) Transgenic inhibition of astroglial NF-kappa B improves functional outcome in experimental autoimmune encephalomyelitis by suppressing chronic central nervous system inflammation. J Immunol 182: 2628–2640 Doi 10.4049/jimmunol.0802954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brand A, Richter-Landsberg C, Leibfritz D (1993) Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 15: 289–298 Doi 10.1159/000111347 [DOI] [PubMed] [Google Scholar]
- 31.Broholm H, Andersen B, Wanscher B, Frederiksen JL, Rubin I, Pakkenberg B, Larsson HB, Lauritzen M (2004) Nitric oxide synthase expression and enzymatic activity in multiple sclerosis. Acta Neurol Scand 109: 261–269 [DOI] [PubMed] [Google Scholar]
- 32.Brosnan CF, Raine CS (2013) The astrocyte in multiple sclerosis revisited. Glia 61: 453–465 Doi 10.1002/glia.22443 [DOI] [PubMed] [Google Scholar]
- 33.Bushong EA, Martone ME, Jones YZ, Ellisman MH (2002) Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22: 183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Calderon TM, Eugenin EA, Lopez L, Kumar SS, Hesselgesser J, Raine CS, Berman JW (2006) A role for CXCL12 (SDF-1alpha) in the pathogenesis of multiple sclerosis: regulation of CXCL12 expression in astrocytes by soluble myelin basic protein. J Neuroimmunol 177: 27–39 Doi 10.1016/j.jneuroim.2006.05.003 [DOI] [PubMed] [Google Scholar]
- 35.Camargo N, Brouwers JF, Loos M, Gutmann DH, Smit AB, Verheijen MH (2012) High-fat diet ameliorates neurological deficits caused by defective astrocyte lipid metabolism. FASEB J 26: 4302–4315 Doi 10.1096/fj.12-205807 [DOI] [PubMed] [Google Scholar]
- 36.Camargo N, Goudriaan A, van Deijk AF, Otte WM, Brouwers JF, Lodder H, Gutmann DH, Nave KA, Dijkhuizen RM, Mansvelder HD et al. (2017) Oligodendroglial myelination requires astrocyte-derived lipids. PLoS Biol 15: e1002605 Doi 10.1371/journal.pbio.1002605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carter SL, Muller M, Manders PM, Campbell IL (2007) Induction of the genes for Cxcl9 and Cxcl10 is dependent on IFN-gamma but shows differential cellular expression in experimental autoimmune encephalomyelitis and by astrocytes and microglia in vitro. Glia 55: 1728–1739 Doi 10.1002/glia.20587 [DOI] [PubMed] [Google Scholar]
- 38.Chapouly C, Tadesse Argaw A, Horng S, Castro K, Zhang J, Asp L, Loo H, Laitman BM, Mariani JN, Straus Farber R et al. (2015) Astrocytic TYMP and VEGFA drive blood-brain barrier opening in inflammatory central nervous system lesions. Brain 138: 1548–1567 Doi 10.1093/brain/awv077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chard DT, Griffin CM, McLean MA, Kapeller P, Kapoor R, Thompson AJ, Miller DH (2002) Brain metabolite changes in cortical grey and normal-appearing white matter in clinically early relapsingremitting multiple sclerosis. Brain 125: 2342–2352 [DOI] [PubMed] [Google Scholar]
- 40.Chastain EM, Duncan DS, Rodgers JM, Miller SD (2011) The role of antigen presenting cells in multiple sclerosis. Biochim Biophys Acta 1812: 265–274 Doi 10.1016/j.bbadis.2010.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen H, Sun Y, Lai L, Wu H, Xiao Y, Ming B, Gao M, Zou H, Xiong P, Xu Y et al. (2015) Interleukin-33 is released in spinal cord and suppresses experimental autoimmune encephalomyelitis in mice. Neuroscience 308: 157–168 Doi 10.1016/j.neuroscience.2015.09.019 [DOI] [PubMed] [Google Scholar]
- 42.Choi JW, Gardell SE, Herr DR, Rivera R, Lee CW, Noguchi K, Teo ST, Yung YC, Lu M, Kennedy G et al. (2011) FTY720 (fingolimod) efficacy in an animal model of multiple sclerosis requires astrocyte sphingosine 1-phosphate receptor 1 (S1P1) modulation. Proc Natl Acad Sci U S A 108: 751–756 Doi 10.1073/pnas.1014154108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P, Barres BA (2005) Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120: 421–433 Doi 10.1016/j.cell.2004.12.020 [DOI] [PubMed] [Google Scholar]
- 44.Christophi GP, Gruber RC, Panos M, Christophi RL, Jubelt B, Massa PT (2012) Interleukin-33 upregulation in peripheral leukocytes and CNS of multiple sclerosis patients. Clin Immunol 142: 308–319 Doi 10.1016/j.clim.2011.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Colombo E, Cordiglieri C, Melli G, Newcombe J, Krumbholz M, Parada LF, Medico E, Hohlfeld R, Meinl E, Farina C (2012) Stimulation of the neurotrophin receptor TrkB on astrocytes drives nitric oxide production and neurodegeneration. J Exp Med 209: 521–535 Doi 10.1084/jem.20110698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Colombo E, Farina C (2016) Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol 37: 608–620 Doi 10.1016/j.it.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 47.Constantinescu CS, Farooqi N, O’Brien K, Gran B (2011) Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br J Pharmacol 164: 1079–1106 Doi 10.1111/j.1476-5381.2011.01302.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Corcione A, Casazza S, Ferretti E, Giunti D, Zappia E, Pistorio A, Gambini C, Mancardi GL, Uccelli A, Pistoia V (2004) Recapitulation of B cell differentiation in the central nervous system of patients with multiple sclerosis. Proc Natl Acad Sci U S A 101: 11064–11069 Doi 10.1073/pnas.0402455101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.D’Amelio FE, Smith ME, Eng LF (1990) Sequence of tissue responses in the early stages of experimental allergic encephalomyelitis (EAE): immunohistochemical, light microscopic, and ultrastructural observations in the spinal cord. Glia 3: 229–240 Doi 10.1002/glia.440030402 [DOI] [PubMed] [Google Scholar]
- 50.Das Sarma J, Ciric B, Marek R, Sadhukhan S, Caruso ML, Shafagh J, Fitzgerald DC, Shindler KS, Rostami A (2009) Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis. J Neuroinflammation 6: 14 Doi 10.1186/1742-2094-6-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.De Groot CJ, Montagne L, Barten AD, Sminia P, Van Der Valk P (1999) Expression of transforming growth factor (TGF)-beta1, -beta2, and -beta3 isoforms and TGF-beta type I and type II receptors in multiple sclerosis lesions and human adult astrocyte cultures. J Neuropathol Exp Neurol 58: 174–187 [DOI] [PubMed] [Google Scholar]
- 52.Ding X, Yan Y, Li X, Li K, Ciric B, Yang J, Zhang Y, Wu S, Xu H, Chen W et al. (2015) Silencing IFN-gamma binding/signaling in astrocytes versus microglia leads to opposite effects on central nervous system autoimmunity. J Immunol 194: 4251–4264 Doi 10.4049/jimmunol.1303321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elain G, Jeanneau K, Rutkowska A, Mir AK, Dev KK (2014) The selective anti-IL17A monoclonal antibody secukinumab (AIN457) attenuates IL17A-induced levels of IL6 in human astrocytes. Glia 62: 725–735 Doi 10.1002/glia.22637 [DOI] [PubMed] [Google Scholar]
- 54.Erta M, Giralt M, Jimenez S, Molinero A, Comes G, Hidalgo J (2016) Astrocytic IL-6 Influences the Clinical Symptoms of EAE in Mice. Brain Sci 6: Doi 10.3390/brainsci6020015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ezan P, Andre P, Cisternino S, Saubamea B, Boulay AC, Doutremer S, Thomas MA, Quenech’du N, Giaume C, Cohen-Salmon M (2012) Deletion of astroglial connexins weakens the blood-brain barrier. J Cereb Blood Flow Metab 32: 1457–1467 Doi 10.1038/jcbfm.2012.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J, Mahad D, Bradl M, van Horssen J, Lassmann H (2012) NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury. Brain 135: 886–899 Doi 10.1093/brain/aws012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frohman EM, Racke MK, Raine CS (2006) Multiple sclerosis--the plaque and its pathogenesis. N Engl J Med 354: 942–955 Doi 10.1056/NEJMra052130 [DOI] [PubMed] [Google Scholar]
- 58.Gao H, Danzi MC, Choi CS, Taherian M, Dalby-Hansen C, Ellman DG, Madsen PM, Bixby JL, Lemmon VP, Lambertsen KL et al. (2017) Opposing Functions of Microglial and Macrophagic TNFR2 in the Pathogenesis of Experimental Autoimmune Encephalomyelitis. Cell Rep 18: 198–212 Doi 10.1016/j.celrep.2016.11.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Giraud SN, Caron CM, Pham-Dinh D, Kitabgi P, Nicot AB (2010) Estradiol inhibits ongoing autoimmune neuroinflammation and NFkappaB-dependent CCL2 expression in reactive astrocytes. Proc Natl Acad Sci U S A 107: 8416–8421 Doi 10.1073/pnas.0910627107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Glabinski AR, Tani M, Strieter RM, Tuohy VK, Ransohoff RM (1997) Synchronous synthesis of alpha- and beta-chemokines by cells of diverse lineage in the central nervous system of mice with relapses of chronic experimental autoimmune encephalomyelitis. Am J Pathol 150: 617–630 [PMC free article] [PubMed] [Google Scholar]
- 61.Glabinski AR, Tani M, Tuohy VK, Tuthill RJ, Ransohoff RM (1995) Central nervous system chemokine mRNA accumulation follows initial leukocyte entry at the onset of acute murine experimental autoimmune encephalomyelitis. Brain Behav Immun 9: 315–330 Doi 10.1006/brbi.1995.1030 [DOI] [PubMed] [Google Scholar]
- 62.Goverman J, Woods A, Larson L, Weiner LP, Hood L, Zaller DM (1993) Transgenic mice that express a myelin basic protein-specific T cell receptor develop spontaneous autoimmunity. Cell 72: 551–560 [DOI] [PubMed] [Google Scholar]
- 63.Greenfield AL, Hauser SL (2018) B-cell Therapy for Multiple Sclerosis: Entering an era. Ann Neurol 83: 13–26 Doi 10.1002/ana.25119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grist JJ, Marro BS, Skinner DD, Syage AR, Worne C, Doty DJ, Fujinami RS, Lane TE (2018) Induced CNS expression of CXCL1 augments neurologic disease in a murine model of multiple sclerosis via enhanced neutrophil recruitment. Eur J Immunol 48: 1199–1210 Doi 10.1002/eji.201747442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guerrini MM, Okamoto K, Komatsu N, Sawa S, Danks L, Penninger JM, Nakashima T, Takayanagi H (2015) Inhibition of the TNF Family Cytokine RANKL Prevents Autoimmune Inflammation in the Central Nervous System. Immunity 43: 1174–1185 Doi 10.1016/j.immuni.2015.10.017 [DOI] [PubMed] [Google Scholar]
- 66.Guo F, Maeda Y, Ma J, Delgado M, Sohn J, Miers L, Ko EM, Bannerman P, Xu J, Wang Y et al. (2011) Macroglial plasticity and the origins of reactive astroglia in experimental autoimmune encephalomyelitis. J Neurosci 31: 11914–11928 Doi 10.1523/JNEUROSCI.1759-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Habbas S, Santello M, Becker D, Stubbe H, Zappia G, Liaudet N, Klaus FR, Kollias G, Fontana A, Pryce CR et al. (2015) Neuroinflammatory TNFalpha Impairs Memory via Astrocyte Signaling. Cell 163: 1730–1741 Doi 10.1016/j.cell.2015.11.023 [DOI] [PubMed] [Google Scholar]
- 68.Harbige LS, Layward L, Morris-Downes MM, Dumonde DC, Amor S (2000) The protective effects of omega-6 fatty acids in experimental autoimmune encephalomyelitis (EAE) in relation to transforming growth factor-beta 1 (TGF-beta1) up-regulation and increased prostaglandin E2 (PGE2) production. Clin Exp Immunol 122: 445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hayden MS, Ghosh S (2014) Regulation of NF-kappaB by TNF family cytokines. Semin Immunol 26: 253–266 Doi 10.1016/j.smim.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Higashi K, Fujita A, Inanobe A, Tanemoto M, Doi K, Kubo T, Kurachi Y (2001) An inwardly rectifying K(+) channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am J Physiol Cell Physiol 281: C922–931 Doi 10.1152/ajpcell.2001.281.3.C922 [DOI] [PubMed] [Google Scholar]
- 71.Hindinger C, Bergmann CC, Hinton DR, Phares TW, Parra GI, Hussain S, Savarin C, Atkinson RD, Stohlman SA (2012) IFN-gamma signaling to astrocytes protects from autoimmune mediated neurological disability. PLoS One 7: e42088 Doi 10.1371/journal.pone.0042088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hofman FM, Hinton DR, Johnson K, Merrill JE (1989) Tumor necrosis factor identified in multiple sclerosis brain. J Exp Med 170: 607–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hogel H, Rissanen E, Barro C, Matilainen M, Nylund M, Kuhle J, Airas L (2018) Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Mult Scler: 1352458518819380 Doi 10.1177/1352458518819380 [DOI] [PubMed] [Google Scholar]
- 74.Holley JE, Newcombe J, Winyard PG, Gutowski NJ (2007) Peroxiredoxin V in multiple sclerosis lesions: predominant expression by astrocytes. Mult Scler 13: 955–961 Doi 10.1177/1352458507078064 [DOI] [PubMed] [Google Scholar]
- 75.Horng S, Therattil A, Moyon S, Gordon A, Kim K, Argaw AT, Hara Y, Mariani JN, Sawai S, Flodby P et al. (2017) Astrocytic tight junctions control inflammatory CNS lesion pathogenesis. J Clin Invest 127: 3136–3151 Doi 10.1172/JCI91301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hulshof S, Montagne L, De Groot CJ, Van Der Valk P (2002) Cellular localization and expression patterns of interleukin-10, interleukin-4, and their receptors in multiple sclerosis lesions. Glia 38: 24–35 [DOI] [PubMed] [Google Scholar]
- 77.Ishikawa M, Jin Y, Guo H, Link H, Xiao BG (1999) Nasal administration of transforming growth factor-beta1 induces dendritic cells and inhibits protracted-relapsing experimental allergic encephalomyelitis. Mult Scler 5: 184–191 Doi 10.1177/135245859900500308 [DOI] [PubMed] [Google Scholar]
- 78.Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C et al. (2010) Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity 32: 414–425 Doi 10.1016/j.immuni.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Karim H, Kim SH, Lapato AS, Yasui N, Katzenellenbogen JA, Tiwari-Woodruff SK (2018) Increase in chemokine CXCL1 by ERbeta ligand treatment is a key mediator in promoting axon myelination. Proc Natl Acad Sci U S A 115: 6291–6296 Doi 10.1073/pnas.1721732115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kassubek R, Gorges M, Schocke M, Hagenston VAM, Huss A, Ludolph AC, Kassubek J, Tumani H (2017) GFAP in early multiple sclerosis: A biomarker for inflammation. Neurosci Lett 657: 166–170 Doi 10.1016/j.neulet.2017.07.050 [DOI] [PubMed] [Google Scholar]
- 81.Keaney J, Campbell M (2015) The dynamic blood-brain barrier. FEBS J 282: 4067–4079 Doi 10.1111/febs.13412 [DOI] [PubMed] [Google Scholar]
- 82.Kim RY, Hoffman AS, Itoh N, Ao Y, Spence R, Sofroniew MV, Voskuhl RR (2014) Astrocyte CCL2 sustains immune cell infiltration in chronic experimental autoimmune encephalomyelitis. J Neuroimmunol 274: 53–61 Doi 10.1016/j.jneuroim.2014.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kirov II, Liu S, Tal A, Wu WE, Davitz MS, Babb JS, Rusinek H, Herbert J, Gonen O (2017) Proton MR spectroscopy of lesion evolution in multiple sclerosis: Steady-state metabolism and its relationship to conventional imaging. Hum Brain Mapp 38: 4047–4063 Doi 10.1002/hbm.23647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kivisakk P, Imitola J, Rasmussen S, Elyaman W, Zhu B, Ransohoff RM, Khoury SJ (2009) Localizing central nervous system immune surveillance: meningeal antigen-presenting cells activate T cells during experimental autoimmune encephalomyelitis. Ann Neurol 65: 457–469 Doi 10.1002/ana.21379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Koehler RC, Roman RJ, Harder DR (2009) Astrocytes and the regulation of cerebral blood flow. Trends Neurosci 32: 160–169 Doi 10.1016/j.tins.2008.11.005 [DOI] [PubMed] [Google Scholar]
- 86.Kothavale A, DiGregorio D, Smith ME (1995) Glial fibrillary acidic protein mRNA and the development of gliosis in mice with chronic relapsing experimental allergic encephalomyelitis. Prog Brain Res 105: 305–310 [DOI] [PubMed] [Google Scholar]
- 87.Krumbholz M, Theil D, Cepok S, Hemmer B, Kivisakk P, Ransohoff RM, Hofbauer M, Farina C, Derfuss T, Hartle C et al. (2006) Chemokines in multiple sclerosis: CXCL12 and CXCL13 up-regulation is differentially linked to CNS immune cell recruitment. Brain 129: 200–211 Doi 10.1093/brain/awh680 [DOI] [PubMed] [Google Scholar]
- 88.Krumbholz M, Theil D, Derfuss T, Rosenwald A, Schrader F, Monoranu CM, Kalled SL, Hess DM, Serafini B, Aloisi F et al. (2005) BAFF is produced by astrocytes and up-regulated in multiple sclerosis lesions and primary central nervous system lymphoma. J Exp Med 201: 195–200 Doi 10.1084/jem.20041674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kutzelnigg A, Lucchinetti CF, Stadelmann C, Bruck W, Rauschka H, Bergmann M, Schmidbauer M, Parisi JE, Lassmann H (2005) Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 128: 2705–2712 Doi 10.1093/brain/awh641 [DOI] [PubMed] [Google Scholar]
- 90.Lanz TV, Ding Z, Ho PP, Luo J, Agrawal AN, Srinagesh H, Axtell R, Zhang H, Platten M, Wyss-Coray T et al. (2010) Angiotensin II sustains brain inflammation in mice via TGF-beta. J Clin Invest 120: 2782–2794 Doi 10.1172/JCI41709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lassmann H, van Horssen J, Mahad D (2012) Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol 8: 647–656 Doi 10.1038/nrneurol.2012.168 [DOI] [PubMed] [Google Scholar]
- 92.Lee DH, Geyer E, Flach AC, Jung K, Gold R, Flugel A, Linker RA, Luhder F (2012) Central nervous system rather than immune cell-derived BDNF mediates axonal protective effects early in autoimmune demyelination. Acta Neuropathol 123: 247–258 Doi 10.1007/s00401-011-0890-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liddelow SA, Guttenplan KA, Clarke LE, Bennett FC, Bohlen CJ, Schirmer L, Bennett ML, Munch AE, Chung WS, Peterson TC et al. (2017) Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541: 481–487 Doi 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liedtke W, Edelmann W, Chiu FC, Kucherlapati R, Raine CS (1998) Experimental autoimmune encephalomyelitis in mice lacking glial fibrillary acidic protein is characterized by a more severe clinical course and an infiltrative central nervous system lesion. Am J Pathol 152: 251–259 [PMC free article] [PubMed] [Google Scholar]
- 95.Linington C, Lassmann H (1987) Antibody responses in chronic relapsing experimental allergic encephalomyelitis: correlation of serum demyelinating activity with antibody titre to the myelin/oligodendrocyte glycoprotein (MOG). J Neuroimmunol 17: 61–69 [DOI] [PubMed] [Google Scholar]
- 96.Linker RA, Lee DH, Demir S, Wiese S, Kruse N, Siglienti I, Gerhardt E, Neumann H, Sendtner M, Luhder F et al. (2010) Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis. Brain 133: 2248–2263 Doi 10.1093/brain/awq179 [DOI] [PubMed] [Google Scholar]
- 97.Liu JS, Zhao ML, Brosnan CF, Lee SC (2001) Expression of inducible nitric oxide synthase and nitrotyrosine in multiple sclerosis lesions. Am J Pathol 158: 2057–2066 Doi 10.1016/S0002-9440(10)64677-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu X, He F, Pang R, Zhao D, Qiu W, Shan K, Zhang J, Lu Y, Li Y, Wang Y (2014) Interleukin-17 (IL-17)-induced microRNA 873 (miR-873) contributes to the pathogenesis of experimental autoimmune encephalomyelitis by targeting A20 ubiquitin-editing enzyme. J Biol Chem 289: 28971–28986 Doi 10.1074/jbc.M114.577429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Llufriu S, Kornak J, Ratiney H, Oh J, Brenneman D, Cree BA, Sampat M, Hauser SL, Nelson SJ, Pelletier D (2014) Magnetic resonance spectroscopy markers of disease progression in multiple sclerosis. JAMA Neurol 71: 840–847 Doi 10.1001/jamaneurol.2014.895 [DOI] [PubMed] [Google Scholar]
- 100.Luchetti S, van Eden CG, Schuurman K, van Strien ME, Swaab DF, Huitinga I (2014) Gender differences in multiple sclerosis: induction of estrogen signaling in male and progesterone signaling in female lesions. J Neuropathol Exp Neurol 73: 123–135 Doi 10.1097/NEN.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 101.Ludwin SK, Rao V, Moore CS, Antel JP (2016) Astrocytes in multiple sclerosis. Mult Scler 22: 1114–1124 Doi 10.1177/1352458516643396 [DOI] [PubMed] [Google Scholar]
- 102.Luo J, Ho P, Steinman L, Wyss-Coray T (2008) Bioluminescence in vivo imaging of autoimmune encephalomyelitis predicts disease. J Neuroinflammation 5: 6 Doi 10.1186/1742-2094-5-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Luo J, Ho PP, Buckwalter MS, Hsu T, Lee LY, Zhang H, Kim DK, Kim SJ, Gambhir SS, Steinman L et al. (2007) Glia-dependent TGF-beta signaling, acting independently of the TH17 pathway, is critical for initiation of murine autoimmune encephalomyelitis. J Clin Invest 117: 3306–3315 Doi 10.1172/JCI31763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Magliozzi R, Howell OW, Nicholas R, Cruciani C, Castellaro M, Romualdi C, Rossi S, Pitteri M, Benedetti MD, Gajofatto A et al. (2018) Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann Neurol 83: 739–755 Doi 10.1002/ana.25197 [DOI] [PubMed] [Google Scholar]
- 105.Mahad DH, Trapp BD, Lassmann H (2015) Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol 14: 183–193 Doi 10.1016/S1474-4422(14)70256-X [DOI] [PubMed] [Google Scholar]
- 106.Maimone D, Guazzi GC, Annunziata P (1997) IL-6 detection in multiple sclerosis brain. J Neurol Sci 146: 59–65 [DOI] [PubMed] [Google Scholar]
- 107.Malmestrom C, Haghighi S, Rosengren L, Andersen O, Lycke J (2003) Neurofilament light protein and glial fibrillary acidic protein as biological markers in MS. Neurology 61: 1720–1725 [DOI] [PubMed] [Google Scholar]
- 108.Markoullis K, Sargiannidou I, Schiza N, Hadjisavvas A, Roncaroli F, Reynolds R, Kleopa KA (2012) Gap junction pathology in multiple sclerosis lesions and normal-appearing white matter. Acta Neuropathol 123: 873–886 Doi 10.1007/s00401-012-0978-4 [DOI] [PubMed] [Google Scholar]
- 109.Markoullis K, Sargiannidou I, Schiza N, Roncaroli F, Reynolds R, Kleopa KA (2014) Oligodendrocyte gap junction loss and disconnection from reactive astrocytes in multiple sclerosis gray matter. J Neuropathol Exp Neurol 73: 865–879 Doi 10.1097/NEN.0000000000000106 [DOI] [PubMed] [Google Scholar]
- 110.Martinez MA, Olsson B, Bau L, Matas E, Cobo Calvo A, Andreasson U, Blennow K, Romero-Pinel L, Martinez-Yelamos S, Zetterberg H (2015) Glial and neuronal markers in cerebrospinal fluid predict progression in multiple sclerosis. Mult Scler 21: 550–561 Doi 10.1177/1352458514549397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Masaki K (2015) Early disruption of glial communication via connexin gap junction in multiple sclerosis, Balo’s disease and neuromyelitis optica. Neuropathology 35: 469–480 Doi 10.1111/neup.12211 [DOI] [PubMed] [Google Scholar]
- 112.Masaki K, Suzuki SO, Matsushita T, Matsuoka T, Imamura S, Yamasaki R, Suzuki M, Suenaga T, Iwaki T, Kira J (2013) Connexin 43 astrocytopathy linked to rapidly progressive multiple sclerosis and neuromyelitis optica. PLoS One 8: e72919 Doi 10.1371/journal.pone.0072919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mayo L, Trauger SA, Blain M, Nadeau M, Patel B, Alvarez JI, Mascanfroni ID, Yeste A, Kivisakk P, Kallas K et al. (2014) Regulation of astrocyte activation by glycolipids drives chronic CNS inflammation. Nat Med 20: 1147–1156 Doi 10.1038/nm.3681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McCandless EE, Piccio L, Woerner BM, Schmidt RE, Rubin JB, Cross AH, Klein RS (2008) Pathological expression of CXCL12 at the blood-brain barrier correlates with severity of multiple sclerosis. Am J Pathol 172: 799–808 Doi 10.2353/ajpath.2008.070918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McCandless EE, Wang Q, Woerner BM, Harper JM, Klein RS (2006) CXCL12 limits inflammation by localizing mononuclear infiltrates to the perivascular space during experimental autoimmune encephalomyelitis. J Immunol 177: 8053–8064 [DOI] [PubMed] [Google Scholar]
- 116.McManus C, Berman JW, Brett FM, Staunton H, Farrell M, Brosnan CF (1998) MCP-1, MCP-2 and MCP-3 expression in multiple sclerosis lesions: an immunohistochemical and in situ hybridization study. J Neuroimmunol 86: 20–29 [DOI] [PubMed] [Google Scholar]
- 117.Meares GP, Ma X, Qin H, Benveniste EN (2012) Regulation of CCL20 expression in astrocytes by IL-6 and IL-17. Glia 60: 771–781 Doi 10.1002/glia.22307 [DOI] [PubMed] [Google Scholar]
- 118.Meiron M, Zohar Y, Anunu R, Wildbaum G, Karin N (2008) CXCL12 (SDF-1alpha) suppresses ongoing experimental autoimmune encephalomyelitis by selecting antigen-specific regulatory T cells. J Exp Med 205: 2643–2655 Doi 10.1084/jem.20080730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Memet S (2006) NF-kappaB functions in the nervous system: from development to disease. Biochem Pharmacol 72: 1180–1195 Doi 10.1016/j.bcp.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 120.Micera A, Vigneti E, Aloe L (1998) Changes of NGF presence in nonneuronal cells in response to experimental allergic encephalomyelitis in Lewis rats. Exp Neurol 154: 41–46 Doi 10.1006/exnr.1998.6864 [DOI] [PubMed] [Google Scholar]
- 121.Mills Ko E, Ma JH, Guo F, Miers L, Lee E, Bannerman P, Burns T, Ko D, Sohn J, Soulika AM et al. (2014) Deletion of astroglial CXCL10 delays clinical onset but does not affect progressive axon loss in a murine autoimmune multiple sclerosis model. J Neuroinflammation 11: 105 Doi 10.1186/1742-2094-11-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Miyagishi R, Kikuchi S, Takayama C, Inoue Y, Tashiro K (1997) Identification of cell types producing RANTES, MIP-1 alpha and MIP-1 beta in rat experimental autoimmune encephalomyelitis by in situ hybridization. J Neuroimmunol 77: 17–26 [DOI] [PubMed] [Google Scholar]
- 123.Moreno M, Bannerman P, Ma J, Guo F, Miers L, Soulika AM, Pleasure D (2014) Conditional ablation of astroglial CCL2 suppresses CNS accumulation of M1 macrophages and preserves axons in mice with MOG peptide EAE. J Neurosci 34: 8175–8185 Doi 10.1523/JNEUROSCI.1137-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mossakowski AA, Pohlan J, Bremer D, Lindquist R, Millward JM, Bock M, Pollok K, Mothes R, Viohl L, Radbruch M et al. (2015) Tracking CNS and systemic sources of oxidative stress during the course of chronic neuroinflammation. Acta Neuropathol 130: 799–814 Doi 10.1007/s00401-015-1497-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nguyen D, Stangel M (2001) Expression of the chemokine receptors CXCR1 and CXCR2 in rat oligodendroglial cells. Brain Res Dev Brain Res 128: 77–81 [DOI] [PubMed] [Google Scholar]
- 126.Nijland PG, Michailidou I, Witte ME, Mizee MR, van der Pol SM, van Het Hof B, Reijerkerk A, Pellerin L, van der Valk P, de Vries HE et al. (2014) Cellular distribution of glucose and monocarboxylate transporters in human brain white matter and multiple sclerosis lesions. Glia 62: 1125–1141 Doi 10.1002/glia.22667 [DOI] [PubMed] [Google Scholar]
- 127.Nijland PG, Witte ME, van het Hof B, van der Pol S, Bauer J, Lassmann H, van der Valk P, de Vries HE, van Horssen J (2014) Astroglial PGC-1alpha increases mitochondrial antioxidant capacity and suppresses inflammation: implications for multiple sclerosis. Acta Neuropathol Commun 2: 170 Doi 10.1186/s40478-014-0170-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Noell S, Wolburg-Buchholz K, Mack AF, Beedle AM, Satz JS, Campbell KP, Wolburg H, Fallier-Becker P (2011) Evidence for a role of dystroglycan regulating the membrane architecture of astroglial endfeet. Eur J Neurosci 33: 2179–2186 Doi 10.1111/j.1460-9568.2011.07688.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nylander A, Hafler DA (2012) Multiple sclerosis. J Clin Invest 122: 1180–1188 Doi 10.1172/JCI58649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Obert D, Helms G, Sattler MB, Jung K, Kretzschmar B, Bahr M, Dechent P, Diem R, Hein K (2016) Brain Metabolite Changes in Patients with Relapsing-Remitting and Secondary Progressive Multiple Sclerosis: A Two-Year Follow-Up Study. PLoS One 11: e0162583 Doi 10.1371/journal.pone.0162583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Okada M, Nakao R, Momosaki S, Yanamoto K, Kikuchi T, Okamura T, Wakizaka H, Hosoi R, Zhang MR, Inoue O (2013) Improvement of brain uptake for in vivo PET imaging of astrocytic oxidative metabolism using benzyl [1-(11)C]acetate. Appl Radiat Isot 78: 102–107 Doi 10.1016/j.apradiso.2013.04.025 [DOI] [PubMed] [Google Scholar]
- 132.Oliet SH, Piet R, Poulain DA (2001) Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science 292: 923–926 Doi 10.1126/science.1059162 [DOI] [PubMed] [Google Scholar]
- 133.Omari KM, John G, Lango R, Raine CS (2006) Role for CXCR2 and CXCL1 on glia in multiple sclerosis. Glia 53: 24–31 Doi 10.1002/glia.20246 [DOI] [PubMed] [Google Scholar]
- 134.Omari KM, Lutz SE, Santambrogio L, Lira SA, Raine CS (2009) Neuroprotection and remyelination after autoimmune demyelination in mice that inducibly overexpress CXCL1. Am J Pathol 174: 164–176 Doi 10.2353/ajpath.2009.080350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Panitch HS, McFarlin DE (1977) Experimental allergic encephalomyelitis: enhancement of cell-mediated transfer by concanavalin A. J Immunol 119: 1134–1137 [PubMed] [Google Scholar]
- 136.Patel JR, McCandless EE, Dorsey D, Klein RS (2010) CXCR4 promotes differentiation of oligodendrocyte progenitors and remyelination. Proc Natl Acad Sci U S A 107: 11062–11067 Doi 10.1073/pnas.1006301107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Patel JR, Williams JL, Muccigrosso MM, Liu L, Sun T, Rubin JB, Klein RS (2012) Astrocyte TNFR2 is required for CXCL12-mediated regulation of oligodendrocyte progenitor proliferation and differentiation within the adult CNS. Acta Neuropathol 124: 847–860 Doi 10.1007/s00401-012-1034-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Paul D, Ge S, Lemire Y, Jellison ER, Serwanski DR, Ruddle NH, Pachter JS (2014) Cell-selective knockout and 3D confocal image analysis reveals separate roles for astrocyte-and endothelial-derived CCL2 in neuroinflammation. J Neuroinflammation 11: 10 Doi 10.1186/1742-2094-11-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pekny M, Pekna M, Messing A, Steinhauser C, Lee JM, Parpura V, Hol EM, Sofroniew MV, Verkhratsky A (2016) Astrocytes: a central element in neurological diseases. Acta Neuropathol 131: 323–345 Doi 10.1007/s00401-015-1513-1 [DOI] [PubMed] [Google Scholar]
- 140.Petzold A, Eikelenboom MJ, Gveric D, Keir G, Chapman M, Lazeron RH, Cuzner ML, Polman CH, Uitdehaag BM, Thompson EJ et al. (2002) Markers for different glial cell responses in multiple sclerosis: clinical and pathological correlations. Brain 125: 1462–1473 [DOI] [PubMed] [Google Scholar]
- 141.Pham H, Ramp AA, Klonis N, Ng SW, Klopstein A, Ayers MM, Orian JM (2009) The astrocytic response in early experimental autoimmune encephalomyelitis occurs across both the grey and white matter compartments. J Neuroimmunol 208: 30–39 Doi 10.1016/j.jneuroim.2008.12.010 [DOI] [PubMed] [Google Scholar]
- 142.Phelps CH (1972) Barbiturate-induced glycogen accumulation in brain. An electron microscopic study. Brain Res 39: 225–234 [DOI] [PubMed] [Google Scholar]
- 143.Plumb J, McQuaid S, Cross AK, Surr J, Haddock G, Bunning RA, Woodroofe MN (2006) Upregulation of ADAM-17 expression in active lesions in multiple sclerosis. Mult Scler 12: 375–385 Doi 10.1191/135248506ms1276oa [DOI] [PubMed] [Google Scholar]
- 144.Ponath G, Ramanan S, Mubarak M, Housley W, Lee S, Sahinkaya FR, Vortmeyer A, Raine CS, Pitt D (2017) Myelin phagocytosis by astrocytes after myelin damage promotes lesion pathology. Brain 140: 399–413 Doi 10.1093/brain/aww298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ponde DE, Dence CS, Oyama N, Kim J, Tai YC, Laforest R, Siegel BA, Welch MJ (2007) 18F-fluoroacetate: a potential acetate analog for prostate tumor imaging--in vivo evaluation of 18F-fluoroacetate versus 11C-acetate. J Nucl Med 48: 420–428 [PubMed] [Google Scholar]
- 146.Poutiainen P, Jaronen M, Quintana FJ, Brownell AL (2016) Precision Medicine in Multiple Sclerosis: Future of PET Imaging of Inflammation and Reactive Astrocytes. Front Mol Neurosci 9: 85 Doi 10.3389/fnmol.2016.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Prins M, Dutta R, Baselmans B, Breve JJ, Bol JG, Deckard SA, van der Valk P, Amor S, Trapp BD, de Vries HE et al. (2014) Discrepancy in CCL2 and CCR2 expression in white versus grey matter hippocampal lesions of Multiple Sclerosis patients. Acta Neuropathol Commun 2: 98 Doi 10.1186/s40478-014-0098-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Probert L (2015) TNF and its receptors in the CNS: The essential, the desirable and the deleterious effects. Neuroscience 302: 2–22 Doi 10.1016/j.neuroscience.2015.06.038 [DOI] [PubMed] [Google Scholar]
- 149.Ransohoff RM, Hamilton TA, Tani M, Stoler MH, Shick HE, Major JA, Estes ML, Thomas DM, Tuohy VK (1993) Astrocyte expression of mRNA encoding cytokines IP-10 and JE/MCP-1 in experimental autoimmune encephalomyelitis. FASEB J 7: 592–600 [DOI] [PubMed] [Google Scholar]
- 150.Reynolds R, Roncaroli F, Nicholas R, Radotra B, Gveric D, Howell O (2011) The neuropathological basis of clinical progression in multiple sclerosis. Acta Neuropathol 122: 155–170 Doi 10.1007/s00401-011-0840-0 [DOI] [PubMed] [Google Scholar]
- 151.Rosengren LE, Lycke J, Andersen O (1995) Glial fibrillary acidic protein in CSF of multiple sclerosis patients: relation to neurological deficit. J Neurol Sci 133: 61–65 [DOI] [PubMed] [Google Scholar]
- 152.Rothhammer V, Borucki DM, Tjon EC, Takenaka MC, Chao CC, Ardura-Fabregat A, de Lima KA, Gutierrez-Vazquez C, Hewson P, Staszewski O et al (2018) Microglial control of astrocytes in response to microbial metabolites. Nature 557: 724–728 Doi 10.1038/s41586-018-0119-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rothhammer V, Kenison JE, Tjon E, Takenaka MC, de Lima KA, Borucki DM, Chao CC, Wilz A, Blain M, Healy L et al. (2017) Sphingosine 1-phosphate receptor modulation suppresses pathogenic astrocyte activation and chronic progressive CNS inflammation. Proc Natl Acad Sci U S A 114: 2012–2017 Doi 10.1073/pnas.1615413114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rothhammer V, Mascanfroni ID, Bunse L, Takenaka MC, Kenison JE, Mayo L, Chao CC, Patel B, Yan R, Blain M et al. (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat Med 22: 586–597 Doi 10.1038/nm.4106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Saikali P, Antel JP, Pittet CL, Newcombe J, Arbour N (2010) Contribution of astrocyte-derived IL-15 to CD8 T cell effector functions in multiple sclerosis. J Immunol 185: 5693–5703 Doi 10.4049/jimmunol.1002188 [DOI] [PubMed] [Google Scholar]
- 156.Savarin C, Hinton DR, Valentin-Torres A, Chen Z, Trapp BD, Bergmann CC, Stohlman SA (2015) Astrocyte response to IFN-gamma limits IL-6-mediated microglia activation and progressive autoimmune encephalomyelitis. J Neuroinflammation 12: 79 Doi 10.1186/s12974-015-0293-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S (2011) The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 1813: 878–888 Doi 10.1016/j.bbamcr.2011.01.034 [DOI] [PubMed] [Google Scholar]
- 158.Schenk GJ, Dijkstra S, van het Hof AJ, van der Pol SM, Drexhage JA, van der Valk P, Reijerkerk A, van Horssen J, de Vries HE (2013) Roles for HB-EGF and CD9 in multiple sclerosis. Glia 61: 1890–1905 Doi 10.1002/glia.22565 [DOI] [PubMed] [Google Scholar]
- 159.Schonrock LM, Gawlowski G, Bruck W (2000) Interleukin-6 expression in human multiple sclerosis lesions. Neurosci Lett 294: 45–48 [DOI] [PubMed] [Google Scholar]
- 160.Seabrook TJ, Littlewood-Evans A, Brinkmann V, Pollinger B, Schnell C, Hiestand PC (2010) Angiogenesis is present in experimental autoimmune encephalomyelitis and pro-angiogenic factors are increased in multiple sclerosis lesions. J Neuroinflammation 7: 95 Doi 10.1186/1742-2094-7-95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Selmaj K, Raine CS, Cannella B, Brosnan CF (1991) Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J Clin Invest 87: 949–954 Doi 10.1172/JCI115102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Senecal V, Deblois G, Beauseigle D, Schneider R, Brandenburg J, Newcombe J, Moore CS, Prat A, Antel J, Arbour N (2016) Production of IL-27 in multiple sclerosis lesions by astrocytes and myeloid cells: Modulation of local immune responses. Glia 64: 553–569 Doi 10.1002/glia.22948 [DOI] [PubMed] [Google Scholar]
- 163.Serafini B, Magliozzi R, Rosicarelli B, Reynolds R, Zheng TS, Aloisi F (2008) Expression of TWEAK and its receptor Fn14 in the multiple sclerosis brain: implications for inflammatory tissue injury. J Neuropathol Exp Neurol 67: 1137–1148 Doi 10.1097/NEN.0b013e31818dab90 [DOI] [PubMed] [Google Scholar]
- 164.Shan K, Pang R, Zhao C, Liu X, Gao W, Zhang J, Zhao D, Wang Y, Qiu W (2017) IL-17-triggered downregulation of miR-497 results in high HIF-1alpha expression and consequent IL-1beta and IL-6 production by astrocytes in EAE mice. Cell Mol Immunol: Doi 10.1038/cmi.2017.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Simard M, Nedergaard M (2004) The neurobiology of glia in the context of water and ion homeostasis. Neuroscience 129: 877–896 Doi 10.1016/j.neuroscience.2004.09.053 [DOI] [PubMed] [Google Scholar]
- 166.Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN (2000) Expression of the interferon-gamma-inducible chemokines IP-10 and Mig and their receptor, CXCR3, in multiple sclerosis lesions. Neuropathol Appl Neurobiol 26: 133–142 [DOI] [PubMed] [Google Scholar]
- 167.Sinclair C, Kirk J, Herron B, Fitzgerald U, McQuaid S (2007) Absence of aquaporin-4 expression in lesions of neuromyelitis optica but increased expression in multiple sclerosis lesions and normal-appearing white matter. Acta Neuropathol 113: 187–194 Doi 10.1007/s00401-006-0169-2 [DOI] [PubMed] [Google Scholar]
- 168.Smith ME, Somera FP, Eng LF (1983) Immunocytochemical staining for glial fibrillary acidic protein and the metabolism of cytoskeletal proteins in experimental allergic encephalomyelitis. Brain Res 264: 241–253 [DOI] [PubMed] [Google Scholar]
- 169.Sofroniew MV (2015) Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci 16: 249–263 Doi 10.1038/nrn3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32: 638–647 Doi 10.1016/j.tins.2009.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119: 7–35 Doi 10.1007/s00401-009-0619-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Solana E, Martinez-Heras E, Martinez-Lapiscina EH, Sepulveda M, Sola-Valls N, Bargallo N, Berenguer J, Blanco Y, Andorra M, Pulido-Valdeolivas I et al. (2018) Magnetic resonance markers of tissue damage related to connectivity disruption in multiple sclerosis. Neuroimage Clin 20: 161–168 Doi 10.1016/j.nicl.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sorensen TL, Tani M, Jensen J, Pierce V, Lucchinetti C, Folcik VA, Qin S, Rottman J, Sellebjerg F, Strieter RM et al. (1999) Expression of specific chemokines and chemokine receptors in the central nervous system of multiple sclerosis patients. J Clin Invest 103: 807–815 Doi 10.1172/JCI5150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sorensen TL, Trebst C, Kivisakk P, Klaege KL, Majmudar A, Ravid R, Lassmann H, Olsen DB, Strieter RM, Ransohoff RM et al. (2002) Multiple sclerosis: a study of CXCL10 and CXCR3 co-localization in the inflamed central nervous system. J Neuroimmunol 127: 59–68 [DOI] [PubMed] [Google Scholar]
- 175.Spence RD, Hamby ME, Umeda E, Itoh N, Du S, Wisdom AJ, Cao Y, Bondar G, Lam J, Ao Y et al. (2011) Neuroprotection mediated through estrogen receptor-alpha in astrocytes. Proc Natl Acad Sci U S A 108: 8867–8872 Doi 10.1073/pnas.1103833108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Spence RD, Wisdom AJ, Cao Y, Hill HM, Mongerson CR, Stapornkul B, Itoh N, Sofroniew MV, Voskuhl RR (2013) Estrogen mediates neuroprotection and anti-inflammatory effects during EAE through ERalpha signaling on astrocytes but not through ERbeta signaling on astrocytes or neurons. J Neurosci 33: 10924–10933 Doi 10.1523/JNEUROSCI.0886-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Stadelmann C, Kerschensteiner M, Misgeld T, Bruck W, Hohlfeld R, Lassmann H (2002) BDNF and gp145trkB in multiple sclerosis brain lesions: neuroprotective interactions between immune and neuronal cells? Brain 125: 75–85 [DOI] [PubMed] [Google Scholar]
- 178.Takata K, Kato H, Shimosegawa E, Okuno T, Koda T, Sugimoto T, Mochizuki H, Hatazawa J, Nakatsuji Y (2014) 11C-acetate PET imaging in patients with multiple sclerosis. PLoS One 9: e111598 Doi 10.1371/journal.pone.0111598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Taniguchi K, Karin M (2018) NF-kappaB, inflammation, immunity and cancer: coming of age. Nat Rev Immunol 18: 309–324 Doi 10.1038/nri.2017.142 [DOI] [PubMed] [Google Scholar]
- 180.Toft-Hansen H, Fuchtbauer L, Owens T (2011) Inhibition of reactive astrocytosis in established experimental autoimmune encephalomyelitis favors infiltration by myeloid cells over T cells and enhances severity of disease. Glia 59: 166–176 Doi 10.1002/glia.21088 [DOI] [PubMed] [Google Scholar]
- 181.Trajkovic V, Stosic-Grujicic S, Samardzic T, Markovic M, Miljkovic D, Ramic Z, Mostarica Stojkovic M (2001) Interleukin-17 stimulates inducible nitric oxide synthase activation in rodent astrocytes. J Neuroimmunol 119: 183–191 [DOI] [PubMed] [Google Scholar]
- 182.Tran EH, Hardin-Pouzet H, Verge G, Owens T (1997) Astrocytes and microglia express inducible nitric oxide synthase in mice with experimental allergic encephalomyelitis. J Neuroimmunol 74: 121–129 [DOI] [PubMed] [Google Scholar]
- 183.Van Der Voorn P, Tekstra J, Beelen RH, Tensen CP, Van Der Valk P, De Groot CJ (1999) Expression of MCP-1 by reactive astrocytes in demyelinating multiple sclerosis lesions. Am J Pathol 154: 45–51 Doi 10.1016/S0002-9440(10)65249-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Van Doorn R, Van Horssen J, Verzijl D, Witte M, Ronken E, Van Het Hof B, Lakeman K, Dijkstra CD, Van Der Valk P, Reijerkerk A et al. (2010) Sphingosine 1-phosphate receptor 1 and 3 are upregulated in multiple sclerosis lesions. Glia 58: 1465–1476 Doi 10.1002/glia.21021 [DOI] [PubMed] [Google Scholar]
- 185.van Horssen J, Schreibelt G, Drexhage J, Hazes T, Dijkstra CD, van der Valk P, de Vries HE (2008) Severe oxidative damage in multiple sclerosis lesions coincides with enhanced antioxidant enzyme expression. Free Radic Biol Med 45: 1729–1737 Doi 10.1016/j.freeradbiomed.2008.09.023 [DOI] [PubMed] [Google Scholar]
- 186.van Loo G, De Lorenzi R, Schmidt H, Huth M, Mildner A, Schmidt-Supprian M, Lassmann H, Prinz MR, Pasparakis M (2006) Inhibition of transcription factor NF-kappaB in the central nervous system ameliorates autoimmune encephalomyelitis in mice. Nat Immunol 7: 954–961 Doi 10.1038/ni1372 [DOI] [PubMed] [Google Scholar]
- 187.Verkhratsky A, Nedergaard M (2018) Physiology of Astroglia. Physiol Rev 98: 239–389 Doi 10.1152/physrev.00042.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Villarroya H, Violleau K, Ben Younes-Chennoufi A, Baumann N (1996) Myelin-induced experimental allergic encephalomyelitis in Lewis rats: tumor necrosis factor alpha levels in serum and cerebrospinal fluid immunohistochemical expression in glial cells and macrophages of optic nerve and spinal cord. J Neuroimmunol 64: 55–61 [DOI] [PubMed] [Google Scholar]
- 189.Voigt D, Scheidt U, Derfuss T, Bruck W, Junker A (2017) Expression of the Antioxidative Enzyme Peroxiredoxin 2 in Multiple Sclerosis Lesions in Relation to Inflammation. Int J Mol Sci 18: Doi 10.3390/ijms18040760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Vos CM, Geurts JJ, Montagne L, van Haastert ES, Bo L, van der Valk P, Barkhof F, de Vries HE (2005) Blood-brain barrier alterations in both focal and diffuse abnormalities on postmortem MRI in multiple sclerosis. Neurobiol Dis 20: 953–960 Doi 10.1016/j.nbd.2005.06.012 [DOI] [PubMed] [Google Scholar]
- 191.Voskuhl RR, Peterson RS, Song B, Ao Y, Morales LB, Tiwari-Woodruff S, Sofroniew MV (2009) Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J Neurosci 29: 11511–11522 Doi 10.1523/JNEUROSCI.1514-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wallin MT (2017) The prevalence of multiple sclerosis in the united states: a population-based healthcare database approach. In: ECTRIMS Online Library; Available at https://onlinelibrary.ectrims-congress.eu/ectrims/2017/ACTRIMS-ECTRIMS2017/199999/ [Google Scholar]
- 193.Wang D, Ayers MM, Catmull DV, Hazelwood LJ, Bernard CC, Orian JM (2005) Astrocyte-associated axonal damage in pre-onset stages of experimental autoimmune encephalomyelitis. Glia 51: 235–240 Doi 10.1002/glia.20199 [DOI] [PubMed] [Google Scholar]
- 194.Wang X, Deckert M, Xuan NT, Nishanth G, Just S, Waisman A, Naumann M, Schluter D (2013) Astrocytic A20 ameliorates experimental autoimmune encephalomyelitis by inhibiting NF-kappaB- and STAT1-dependent chemokine production in astrocytes. Acta Neuropathol 126: 711–724 Doi 10.1007/s00401-013-1183-9 [DOI] [PubMed] [Google Scholar]
- 195.Wang X, Haroon F, Karray S, Martina D, Schluter D (2013) Astrocytic Fas ligand expression is required to induce T-cell apoptosis and recovery from experimental autoimmune encephalomyelitis. Eur J Immunol 43: 115–124 Doi 10.1002/eji.201242679 [DOI] [PubMed] [Google Scholar]
- 196.Wang Y, Imitola J, Rasmussen S, O’Connor KC, Khoury SJ (2008) Paradoxical dysregulation of the neural stem cell pathway sonic hedgehog-Gli1 in autoimmune encephalomyelitis and multiple sclerosis. Ann Neurol 64: 417–427 Doi 10.1002/ana.21457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wolburg-Buchholz K, Mack AF, Steiner E, Pfeiffer F, Engelhardt B, Wolburg H (2009) Loss of astrocyte polarity marks blood-brain barrier impairment during experimental autoimmune encephalomyelitis. Acta Neuropathol 118: 219–233 Doi 10.1007/s00401-009-0558-4 [DOI] [PubMed] [Google Scholar]
- 198.Wyss-Coray T, Borrow P, Brooker MJ, Mucke L (1997) Astroglial overproduction of TGF-beta 1 enhances inflammatory central nervous system disease in transgenic mice. J Neuroimmunol 77: 45–50 [DOI] [PubMed] [Google Scholar]
- 199.Wyss MT, Magistretti PJ, Buck A, Weber B (2011) Labeled acetate as a marker of astrocytic metabolism. J Cereb Blood Flow Metab 31: 1668–1674 Doi 10.1038/jcbfm.2011.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Wyss MT, Weber B, Treyer V, Heer S, Pellerin L, Magistretti PJ, Buck A (2009) Stimulation-induced increases of astrocytic oxidative metabolism in rats and humans investigated with 1–11C-acetate. J Cereb Blood Flow Metab 29: 44–56 Doi 10.1038/jcbfm.2008.86 [DOI] [PubMed] [Google Scholar]
- 201.Yun HM, Park KR, Kim EC, Hong JT (2015) PRDX6 controls multiple sclerosis by suppressing inflammation and blood brain barrier disruption. Oncotarget 6: 20875–20884 Doi 10.18632/oncotarget.5205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zarruk JG, Berard JL, Passos dos Santos R, Kroner A, Lee J, Arosio P, David S (2015) Expression of iron homeostasis proteins in the spinal cord in experimental autoimmune encephalomyelitis and their implications for iron accumulation. Neurobiol Dis 81: 93–107 Doi 10.1016/j.nbd.2015.02.001 [DOI] [PubMed] [Google Scholar]
- 203.Zhou D, Srivastava R, Nessler S, Grummel V, Sommer N, Bruck W, Hartung HP, Stadelmann C, Hemmer B (2006) Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proc Natl Acad Sci U S A 103: 19057–19062 Doi 10.1073/pnas.0607242103 [DOI] [PMC free article] [PubMed] [Google Scholar]