Abstract
Liver disease causing end organ failure is a growing cause of mortality. In most cases, the only therapy is liver transplantation. However liver transplantation is a complex undertaking and its success is dependent on a number of factors. In particular, liver transplantation is subject to the risks of ischemia-reperfusion injury (IRI). Liver IRI has significant effects on the function of a liver after transplantation. The cellular and molecular mechanisms governing IRI in liver transplantation are numerous. They involve multiple cells types such as liver sinusoidal endothelial cells, hepatocytes, Kupffer cells, neutrophils, and platelets acting via an interconnected network of molecular pathways such as activation of toll-like receptor signaling, alterations in micro-RNA expression, production of ROS, regulation of autophagy, and activation of hypoxia-inducible factors. Interestingly, the cellular and molecular events in liver IRI can be correlated with clinical risk factors for IRI in liver transplantation such as donor organ steatosis, ischemic times, donor age, and donor and recipient coagulopathy. Thus, understanding the relationship of the clinical risk factors for liver IRI to the cellular and molecular mechanisms that govern it are critical to higher levels of success after liver transplantation. This in turn will help in the discovery of therapeutics for IRI in liver transplantation--a process that will lead to improved outcomes for patients suffering from end stage liver disease.
Keywords: Ischemia-Reperfusion, Liver Transplantation, Therapeutics, Hypoxia Inducible Factors
Introduction:
Liver disease represents a growing cause of mortality worldwide. Up to 25% of the world’s population has risk factors for liver disease including viral infection, alcohol abuse, and non-alcoholic steatohepatitis (NASH). The high prevalence of risk factors has contributed to the rising incidence of primary liver cancer, which causes more than 800,000 deaths per year.1
In most instances, the only therapy for end-stage liver disease is liver transplantation. However, liver transplantation carries with it the risks of ischemia-reperfusion injury (IRI). IRI is defined as tissue injury that occurs when the blood supply to organs is interrupted and then returns.2 Liver IRI has significant effects on liver function and causes systemic injury.3 The liver-specific and systemic effects of IRI have important implications for the practice of liver transplantation.4
Patients awaiting liver transplant are at risk for IRI due to alterations in the balance between pro-coagulant and anti-coagulant processes. Patients with chronic liver injury are more susceptible to unregulated coagulation due to lack of matrix metalloproteinases, increased pro-thrombotic factors, and hypoalbuminemia.5
Organ procurement results in both cold and warm IRI. The severity of IRI in liver transplantation is affected by characteristics of the donor organ such as donor age, organ fat content, and brain death. Thus, organs at high risk for IRI, when put into recipients primed for ischemic injury, can lead to a potentially catastrophic scenario. Understanding IRI is, therefore, critical to success in liver transplantation.6
Liver IRI involves many cellular compartments. Liver sinusoidal endothelial cells and hepatocytes are targets of IRI-induced cell death. Early IRI-induced cell death is a result of metabolic disturbances and ATP depletion. Following reperfusion, neutrophils and macrophages are activated and accumulate in the liver. These cells exacerbate IRI through secretion of paracrine and autocrine signals such as reactive oxygen species (ROS) and inflammatory cytokines. Finally, hepatic stellate cells (HSC) become activated in IRI and promote long-term recovery from IRI, which can manifest as allograft fibrosis.7,8
The molecular pathways involved in IRI are similarly multifaceted. Release of inflammatory cytokines and chemokines results in activation of neutrophils and macrophages, promoting tissue destruction. Lipid peroxidation, ROS, and release of damage associated molecular patterns (DAMPS) during hepatocyte injury amplify the destructive activity of neutrophils and macrophages.9,10
Other molecular pathways regulate IRI after it is initiated. Autophagy can limit production of ROS. Hypoxia inducible factors (HIF) sense tissue hypoxia and help maintain neutrophil viability and promote macrophage activation. HIF are also critical in promoting tissue recovery through regulation of stem cell niches and matrix metalloproteinases (MMP).11,12
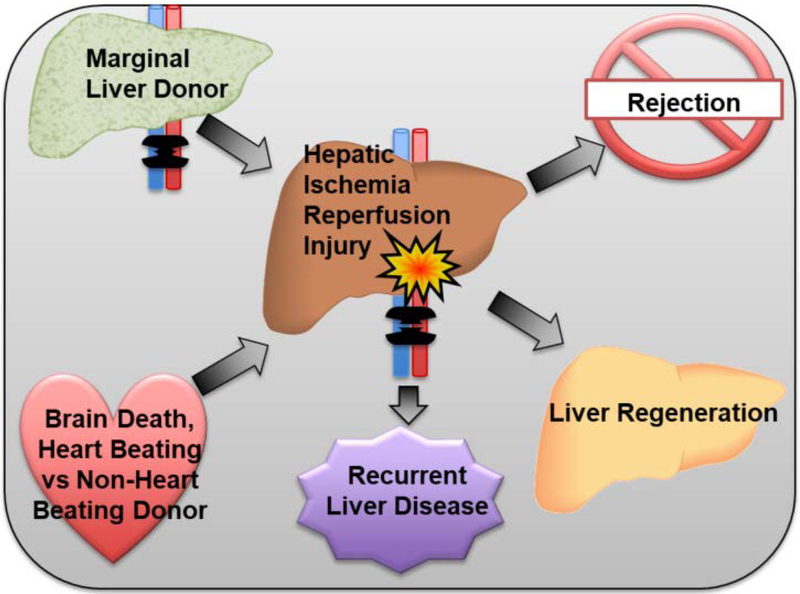
In short, hepatic IRI worsens the survival of patients needing liver transplants. It reduces the pool of organs available for transplant as some may suffer severe IRI if used. Those patients who experience severe IRI in their allografts have poor graft function and survival after liver transplantation. Thus, understanding the mechanisms of IRI in liver transplantation is critical to designing effective therapeutics. Effective therapeutics in turn will improve patient outcomes after liver transplantation and provide patients with liver disease a superior chance at survival (Figure 1).
Figure 1: Risk factors and sequelae of hepatic IRI in liver transplant patients.
Factors such as use of marginal donor organs due to donor scarcity and critical donor hemodynamics, including NHBBD donors, can increase the risk of hepatic IRI. Higher levels of hepatic IRI consequently contribute to poor outcomes, including rejection, recurrence of liver disease, and liver regeneration.
Clinical Context of IRI in Liver Transplantation
Recipient Challenges
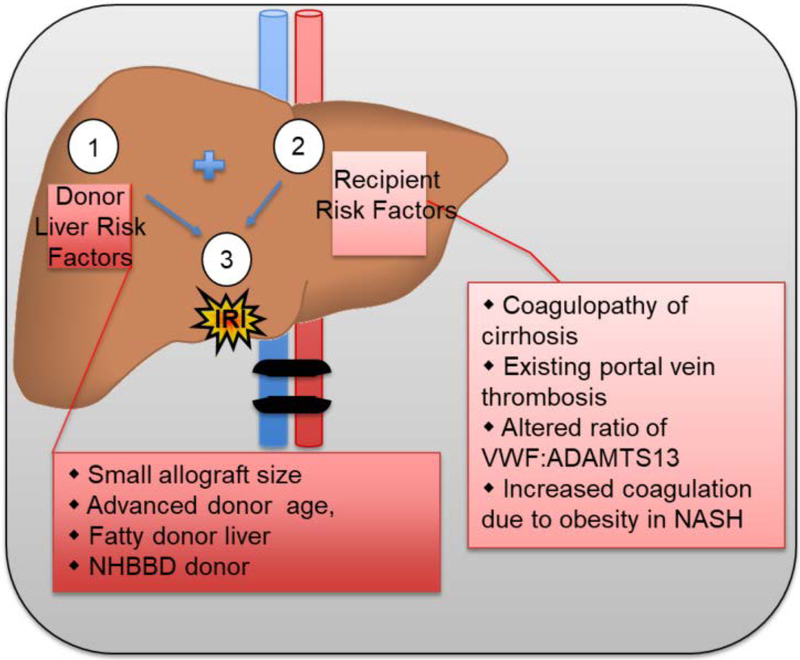
The risks of IRI in liver transplantation are present in the transplant recipient and the organ donor (Figure 2). Transplant recipients suffer from chronic liver injury, i.e. cirrhotics. Cirrhotics demonstrate altered coagulation profiles. Superficially, this appears to indicate impaired coagulation, but deeper investigation reveals a pro-thrombotic state.13 Clinically this manifests as increased rates of deep venous and portal vein thrombosis in cirrhotics as compared to patients without liver disease.14,15
Figure 2: Surgical, Donor, and Recipient factors that contribute to risk of IRI.
(1) Risks of IRI inherent to liver transplantation include: organwide ischemia during clamping and resection of donor organ, cold storage, re-anastomosis and circulation of widespread inflammatory factors, increased ischemic time. (2) Donor Liver risk factors can increase the severity of IRI: small allograft, advanced age, especially age >70, donor fatty liver, especially macrosteatosis > 30%, cause of donor death, NHBBD donor, use of marginal organs due to donor scarcity. (3) Recipient risk factors can further increase the severity of IRI: cirrhosis or hepatic fibrosis induced coagulopathy, nutritional coagulopathy, portal vein or hepatic artery thrombosis, altered VWF:ADAMTS13 ratio, history of NASH.
The pro-thrombotic profile of cirrhotics increases the risk of IRI before and after liver transplantation; up to 30% of cirrhotics may have thrombosis of the portal vein, the dominant blood supply to the liver. This may cause the patient awaiting a liver transplant to have IRI in their native liver, thus priming them for more severe IRI after liver transplantation.16 In the post-operative period, the pro-thrombotic state of the cirrhotic patient is a risk factor for thrombosis of vessels to the newly transplanted liver. This likely contributes to the risk of hepatic artery thrombosis that occurs in 5–7% of patients after liver transplant.17
The mechanisms of this pro-thrombotic state are multifactorial. Blood flow in cirrhotics is more turbulent due to increased resistance within the liver parenchyma.18 Liver cirrhosis leads to decreased production of the anti-coagulant proteins C and S.19 Cirrhotics have chronic nutritional deficiency leading to low albumin levels. Low serum albumin is associated with an increased risk of thrombosis.13
Of particular interest in cirrhotics is the imbalance between von Willebrand factor (VWF) and ADAMTS13. In homeostasis, VWF in vascular endothelium promotes platelet aggregation and clotting while ADAMTS13, produced by HSC, cleaves the multimeric form of VWF inhibiting its activity.20 Cirrhosis results in increased VWF in the hepatic sinusoids.21 This, coupled with decreased ADAMTS13, leads to platelet aggregation and microthrombi formation in cirrhotic livers. Microthrombotic injury increases the risk of IRI in cirrhotic livers, especially at times of physiologic stress. This correlates with the observation that the imbalance of VWF and ADAMTS13 increases as the severity of liver disease increases.22
The impaired ratio also affects recipients post liver transplant. Donor livers release large amounts of multimeric VWF further depleting already low levels of ADAMTS13. In turn, this increases generation of micothrombi in the liver, contributing to hepatic IRI and increasing the risk of acute rejection.23
Patients with NASH have additional risk factors for thrombosis. NASH is correlated with obesity.24 Obesity is associated with increased levels of plasminogen activator inhibitor-1.25 Thus, patients who are transplanted for NASH may not only be at risk for microthombotic complications in the liver due to an altered VWF/ADAMTS13 ratio, but could also be at increased risk for macrothrombotic complications due to altered regulation of the coagulation cascade.26
Donor Challenges
Donor related risk factors contribute to the severity of IRI in liver transplantation (Figure 2). Liver steatosis, older donor age, prolonged ischemic time, and nature of organ recovery are risk factors for increased hepatic IRI. Severe IRI is the cause of increased primary non-function of these categories of liver allografts. Liver donors with these risk factors have been labeled as “marginal.” However, due to the high risk of mortality while awaiting a liver transplant, there is increased demand for using these “marginal” organs.27
Steatosis of the donor liver is a strong predictor of graft function after liver transplant. Patients transplanted with livers with greater than 30% macrosteatosis have higher transaminases, decreased synthetic function, and higher rates of non-function of the liver after transplant. These complications are a result of more severe IRI in steatotic livers.28,29
Prolonged cold ischemic time is also a risk factor for poor allograft function. Currently, donor livers are preserved cold in solutions that slow down cellular respiration. However, as ischemic time accumulates, ATP stores are slowly depleted leading to large deficits of metabolic precursors once normal levels of cellular respiration are restored at reperfusion. This leads to cell death. Prolonged cold ischemic time is an additive threat for severe IRI in steatotic livers.30
Due to limited supply of available organs from heart-beating donors, there has been an increase in the number organs recovered from non-heart beating donors (NHBD). These represent up to 15% of all allografts used for liver transplantation in the United States.31 Livers from NHBD donors are subject to IRI from warm and cold ischemia. As opposed to heart-beating donors, NHBD have lack of blood flow prior to cold storage. This period of warm ischemia results in arrest of oxygen delivery, rapid depletion of ATP stores, blood stasis, and activation of the coagulation cascade.32 NHBD donor livers demonstrate increased levels of ceramide18, a pro-apoptotic activator correlating with increased cell death from IRI. VWF is also elevated in NHBD donor livers indicating higher risk of microthrombi formation and loss of sinusoidal blood flow contributing to ongoing IRI during organ recovery.33,34
Increasing donor age is a risk factor for IRI in liver transplantation. Livers from donors over the age of 70 experience more injury at prolonged cold ischemic times as compared to livers from younger donors. Older livers have a higher chance of severe IRI due to smaller volumes and less total blood flow. At the molecular level, older donors have fewer and less functional mitochondria making them more susceptible to depletion of ATP during cold storage and warm reperfusion. This results in increased production of ROS and activators of cell death. Homeostatic regulation of this process is altered as older livers have a muted stress response as manifest by diminished amounts of heat-shock protein 70.35–37
Brain Death and IRI
Donor organs are recovered from persons with brain death. Brain death is an inflammatory state that primes organs during the recovery phase for more severe IRI during reperfusion. End-organs in persons with brain death show increased accumulation of neutrophils, increased platelet deposition, and activation of Fas-FasL dependent apoptotic pathways. IRI is further primed in brain death by increased production of ROS and pro-inflammatory cytokines such as TNF-alpha. Finally, adhesion molecules such as ICAM-1 increase in expression along the vascular endothelium in response to brain death. This results in immune cell infiltration into tissues with concomitant end organ injury.38
The negative effects of brain death are amplified in marginal liver donors. Take, for example, steatotic livers. These livers suffer more severe IRI due to lipid peroxidation of the fat droplets in hepatocytes after reperfusion. This leads to increased production of ROS. Lipid accumulation also increases the amount of and metabolism of cholesterol in mitochondria in the liver leading to dysregulation of immune signaling pathways, such as those mediated by TLR4. This results in increased inflammatory cytokine production.39–41
Increased production of pro-inflammatory cytokines, such as IL-6 and TNF-alpha, activates the immune system. Activation of the immune system after IRI in steatotic livers leads to accumulation of neutrophils and macrophages at areas of fat deposition. Activation of these cell populations promotes production of ROS that exacerbate tissue damage.29,30
Thus, given that in brain death liver injury occurs via similar pathways as in IRI, it is no surprise steatotic livers have increased parenchymal injury in the setting of brain death as compared to non-steatotic livers. Mechanistically, as in IRI, this involves decrease in antioxidants, increase in ROS, alterations in TLR4 signaling, and reduction in heaptic microcirculation.42,43 Although data in other marginal liver donors regarding the effects of brain death are limited it is likely that brain death primes them for more severe IRI, thus, negatively affecting outcomes after liver transplantation.
Cellular Mechanisms of Liver IRI
Liver Sinusoidal Endothelial cells
Liver sinusoidal endothelial cells (LSEC) line the vascular endothelium of the liver. They control vascular tone and thereby blood flow and delivery of nutrients and oxygen to hepatocytes. Expression of cell adhesion molecules on LSEC is upregulated in hepatic injury allowing them to play a key role in accumulation, activation, and modulation of the cellular response to hepatic IRI. Thus, normal LSEC function is important to protecting the liver from the disruptions in homeostasis after IRI.44
LSEC are highly susceptible to injury during static cold preservation.45 Due to ATP depletion during cold storage, active transmembrane transport of ions is disrupted (Figure 3). This leads to cell swelling and mitochondrial dysfunction.46 Intensity of LSEC injury is increased by lack of active blood flow across the sinusoids during static cold storage.47
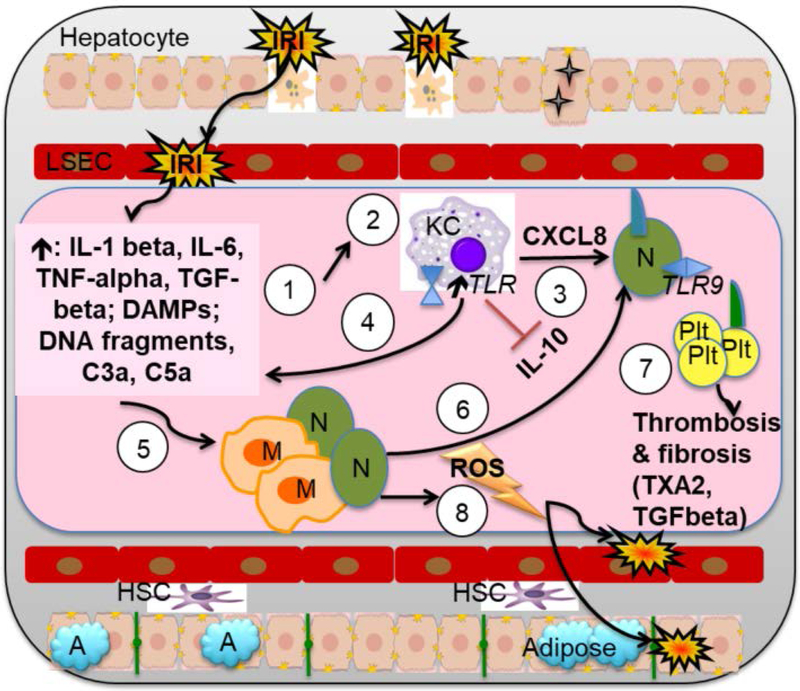
Figure 3: Cellular and molecular IRI pathway: A focus on the roles of Kupffer cells, macrophages, neutrophils, and platelets.
(1) IRI damage to LSECs and hepatocytes causes cell death and release of inflammatory cytokines IL-1 beta, IL-6, and TNF-alpha, TGF-beta, as well as DAMPS (HMGB1, FFA, and HSB), DNA fragments, and complement. (2) DAMPs cause KC to increase KC TLR activation, inhibit immunosuppressive IL-10 production, and increase KC cytokine production toward inflammatory phenotype. Inflammatory type KC release: (3) CXCL8, which amplifies recruitment and adhesion of neutrophils to LSEC; and (4) IL-1-beta, TNF-alpha, IFN-gamma, and IL-12. Together, the factors released from IRI damage to LSECs and hepatocytes, as well as those released by inflammatory KCs, (5) induce migration of neutrophils early in IRI and of macrophages late in IRI. (6) These factors also serve to promote further neutrophil adhesion and extravasation into the liver parenchyma via CD11b/CD18a on neutrophils and ICAM-1 on LSEC, while promoting platelet adhesion and activation via upregulation of P-selectin on LSEC. (7) Activated platelets release cytokines that affect vascular tone (TXA2 and serotonin), regulate local thrombosis (PAI-1), and induce fibrosis (TGF beta). (8) Simultaneously, neutrophils and macrophages cause further tissue injury and cellular destruction, through release of ROS and other destructive factors.
Upon reperfusion, damaged LSEC incur further injury. ROS derived from lack of energy sources for cellular respiration quickly reduce free radical scavengers. Lack of NO production from the injured LSEC, depletion of NO stores and the unopposed activity of vasoconstrictors such as thromboxane A2 cause an increase of vascular tone and reduction of blood flow to hepatocytes.48–50 Activated LSEC express vascular adhesion molecules such as P-selectin allowing for platelet adhesion and activation. Platelet adhesion to LSEC induces LSEC cell death as well as causing congestion and thrombus formation in the liver microcirculation.51,52 The cumulative effect of these processes worsens IRI in transplant allografts (Figure 3).
Kupffer Cells
Kupffer Cells (KC) are resident liver macrophages derived from erythro-myeloid progenitors in the fetal liver.53 During normal liver function, KC are central to a tolerogenic response as the liver is exposed to numerous circulating antigens. In homeostasis, KC scavenge circulating antigens passing through the liver, present them to T-cells, and induce expansion of tolerogenic T-cells. This leads to production of immunosuppressive cytokines such as IL-10.54
During liver injury, KC lose this tolerogenic profile and instead promote inflammation and injury.55 During IRI in liver transplantation, injury to LSEC and hepatocytes releases damage-associated molecular pattern molecules (DAMPS) such as high mobility group box-1 (HMGB1), free fatty acids (FFA), and heat shock proteins (HSP). KC recognize these injury signals through expression of toll-like receptors 3, 4, and 9. Activation of TLR’s, especially TLR4, drive cytokine production in KC toward an inflammatory phenotype.56
Pro-inflammatory cytokines secreted by KC during IRI include IL-1-beta, TNF-alpha, IFN-gamma, and IL-12. IL-1-beta and TNF-alpha induce upregulation of the adhesion molecules CD11b/CD18a (Mac-1) on neutrophils and intracellular adhesion molecule-1 (ICAM-1) on LSEC to promote neutrophil adhesion and extravasation into the liver parenchyma.57 These same cytokines drive release of ROS from neutrophils causing further tissue injury.58
TNF-alpha secretion from KC in IRI induces the upregulation of P-selectin on LSEC to promote platelet adhesion and activation. It also promotes chemokine secretion from KC during liver injury.59 Release of the chemokines CXCL1, −2, and −3, results in increased migration of neutrophils into the injured liver tissue (Figure 3). This amplifies recruitment and adhesion of neutrophils to LSEC and also promotes further activation of KC.60
Hepatocytes
Hepatocytes experience direct injury during IRI. (Figure 3). The respiratory chain in hepatocytes in IRI is disrupted due to lack of oxygen, impaired delivery of nutritional substrates, and depletion of residual ATP. Mitochondria deprived of O2 and glucose cannot replenish ATP supplies. This causes accumulation of toxic substances such as lactate.61 Further, active ionic transporters are disrupted leading to cell swelling and membrane damage.62
Although accumulation of ATP by-products such as AMP and activation of cAMP dependent protein kinase can protect against hepatic IRI, ultimately the disruption of cellular respiration results in production of ROS from mitochondria and tissue damage.41 During reperfusion, reintroduction of O2 does not restore normal respiratory chain function but rather exacerbates ROS production due to impaired mitochondrial function.39 Injured hepatocytes go on to release DAMPS that usher in a self-perpetuating inflammatory cycle of which LSEC and KC are the central regulators.63
Neutrophils
If LSEC and KC are central regulators of IRI in the liver, neutrophils represent the main actor in causing injury. Neutrophils are rapidly recruited to the liver after reperfusion. IRI causes activation of complement which leads to production of the chemotactic agents C3a and C5a. Neutrophils respond to C3a and C5a by infiltrating the liver after reperfusion.64
Once in the liver, chemokines secreted by activated KC, such as CXCL1 and CXCL2 (human CXCL8) create a chemoattractant gradient that is detected by neutrophils. Chemotaxis driven by chemokines brings neutrophils into liver sinusoids.65 Chemokines also bind to glycosoamino-glycans on the vascular surface of LSEC such that when neutrophils reach that location, chemokine-chemokine receptor interactions lead to activation of integrins and binding of neutrophils via the integrin Mac-1 to ICAM-1 on the sinusoidal endothelium.66
Once in the injured liver, neutrophils respond to the inflammatory signals therein. HMBG-1 and DNA released from damaged hepatocytes induces release of ROS from neutrophils.67 DNA from damaged hepatocytes activates TLR9 on neutrophils enhancing their production of ROS and secretion of chemokines such as CXCL1 and 2. This is mediated by TLR9 via activation of NF-κB.68,69
Activation of TLR9 in neutrophils results in their rapid degranulation during liver injury. Expression of TLR9 at the cell surface of neutrophils as opposed to endosomal expression leads to faster binding of DNA fragments to TLR9. This accelerates signaling via TLR9.70 Accelerated signaling via TLR9 amplifies tissue damage caused by neutrophils after hepatic IRI (Figure 3). Ongoing tissue damage creates a positive feedback loop in which neutrophil recruitment, migration, and tissue infiltration is promoted through further release of signals of tissue injury such as DAMPS, DNA, chemokines, and cytokines.71 In liver transplantation, modulating neutrophil activity is likely to improve allograft function.
Platelets
Platelet aggregation during hepatic IRI represents an important mechanism by which IRI is potentiated after liver transplantation.72 LSEC injury causes increased adhesion of platelets through upregulation of P-selectin. Increased platelet adhesion mechanically limits sinusoidal blood flow exacerbating ischemia; subsequent activation of other pathways amplifies this effect (Figure 3).51,52
LSEC express CD39 on their luminal surface. CD39 regulates the effect of ATP products on platelets. During homeostasis, CD39 cleaves ATP and ADP to AMP, limiting platelet activation. In IRI, as LSEC are injured, CD39 activity declines and ADP increases.73 ADP is a potent inducer of platelet activation and aggregation. Activated platelets release a variety of cytokines which 1. Affect vascular tone—thomboxane A2 (TXA2) and serotonin, 2. Regulate local thrombosis—plasminogen activator inhibitor-1 (PAI-1), and 3. Induce fibrosis—transforming growth factor-beta (TGF-beta).74–76
This process is termed extravasated platelet aggregation (EPA).72 LSEC injury induces vasoconstriction in the sinusoids.44 Platelet adhesion and aggregation increases leading to platelet activation and migration into the subendothelial space. With platelet activation comes sinusoidal vasoconstriction via TXA2 and serotonin.49,52 Thrombus degradation is inhibited by PAI-1; progression of platelet aggregation during EPA causes them to secrete TGF-beta.75,76 Secretion of TGF-beta induces a transition from the acute phase of IRI toward chronic remodeling of the liver allograft. TGF-beta activates HSC which results in collagen deposition and the initiation of graft fibrosis.77
Hepatic Stellate Cells
HSC reside in the perisinusoidal space and are central regulators of hepatic fibrosis.78 During homeostasis, HSC suppress excess inflammatory responses to hepatic injury. In this setting, HSC cause apoptosis of reactive T-cells, expand regulatory T-cell populations, and secrete antioxidants to scavenge ROS.79,80 However, severe injury leads to constitutive activation of HSC with negative consequences for parenchymal recovery.77
TNF-alpha, NO, and IL-6 released during IRI from KC activate HSC. Activated HSC undergo transformation to a myofibroblast phenotype. Immediate consequences of HSC activation are release of MMP, chemokines, and inflammatory cytokines which lead to ECM destruction, ingress of platelets and neutrophils, and further activation of HSC.8 HSC also contribute to endothelial vasoconstriction through secretion of endothelin-1 (ET-1).81
Long term consequences of HSC activation are deposition of ECM and fibrosis of the liver parenchyma.77 As noted above, this process is initiated through EPA with TGF-beta being the stimulator of persistent HSC activity after IRI.72 This affect is attenuated when the number of HSC in the liver are reduced in experimental models of IRI.8,82
Molecular Mechanisms of IRI
Autophagy
Autophagy is the process by which cells remove mis-folded, damaged, and over-age proteins and organelles through transport into lysosomes.83 There is some contradictory data regarding the effects of autophagy in liver IRI but in the context of liver transplantation, the balance favors regulated autophagy as a protective mechanism to limit injury.11,84
IRI in warm ischemia leads to rapid depletion of ATP and oxygen. Mitochondria in hepatocytes are overwhelmed by ROS. Loss of substrates for oxidative phosphorylation leads to increased mitochondrial permeability, especially in the reperfusion phase.85,86 This increases production of ROS and leads to further necrotic damage to hepatocytes.
Autophagy is protective in this setting as it leads to shuttling of damaged mitochondria into the autophagosome, limiting production of ROS.87 Evidence of this pathway is seen when expression of autophagosome localizing protein Beclin 1 is impaired leading to increased hepatocyte death.88,89 Overexpression of Beclin 1 or induction of autophagy through mTOR inhibitors such as rapamycin (in combination with delivery of ROS scavengers such as hydrogen sulfide) reduces IRI-induced hepatocyte death.11,90
The negative effects of older donor age and increased donor steatosis on hepatic IRI can be limited through upregulation of autophagy. Aging leads to decreased autophagy and increased hepatocyte injury after IRI. Activation of the PPAR-gamma nuclear receptor increases autophagy and limits hepatocyte damage after IRI. This effect is more pronounced in younger mice and diminished in older mice.91 Similarly, induction of autophagy through the HMG-CoA reductase inhibitor simvastatin reduces IRI in steatotic livers.45,92
The benefits of simvastatin are likely mediated through LSEC. In LSEC, injury from IRI is ameliorated by upregulating autophagy. Treating LSEC subject to static cold storage with simvastatin induces expression of the vasoprotective transcription factor KLF-2 (Kruppel-like factor 2). This prevents the arrest of autophagy that occurs during reperfusion and decreases LSEC death in IRI.45,93
As opposed to regulated autophagy, unregulated autophagy, may be detrimental to recovery from IRI. More severe hepatocyte necrosis is seen in correlation with increased autophagy as represented by higher levels of expression of the autophagosome related protein LC3-II.94 Further, HMGB1 released during IRI is a competitive binder of Beclin 1 and its binding to Beclin 1 induces autophagy. The protective effects of reduction of HMGB1 secretion after IRI may relate to decreased autophagy when lower levels of HMGB1 are present.95 Thus, although autophagy appears primarily protective in hepatic IRI and liver transplantation, these data suggest that manipulation of autophagy in liver transplantation should be undertaken with caution to avoid an unregulated response.
Hypoxia Inducible Factors
Hypoxia inducible factors (HIF) are heterodimeric transcription factors whose effects are directly tied to levels of oxygen present in tissue.96 HIF are stabilized in the presence of hypoxia.97 After hepatic IRI, stabilized HIF enter the nucleus and induce transcription of genes involved in cellular metabolism (Glut-1, PDK-1), angiogenesis (VEGF, NOS), and cytoprotection (Hmox-1, HSP) to ameliorate IRI induced hepatocyte injury and cell death.98–100
HIF have a constitutive beta subunit and a variant, oxygen sensing alpha subunit.96 Of the three known HIF alpha isoforms, HIF1-alpha is the best characterized in terms of its role in hepatic IRI. Inhibitors of prolyl hydroxylation of HIF, which targets it for destruction, appear to mitigate IR injury by increasing the amount of activated HIF1-alpha.98,101 Similarly, the positive effects of ischemic preconditioning on hepatic IRI appear to involve increased activation of HIF1-alpha.102
Data regarding the role of HIF2-alpha in hepatic IRI is limited. However, the growing amount of information regarding the role of HIF2-alpha in the development and homeostasis of the gastrointestinal tract may provide insight into its potential role in hepatic IRI.103 HIF2-alpha has selective expression in the endothelium of the GI tract.104 In macrophages, HIF2-alpha appears to be associated with an M2 phenotype. Reciprocally, Th2 type cytokines appear to increase HIF2-alpha expression.105 In neutrophils, HIF2-alpha promotes survival of neutrophils. In inflammatory bowel disease models, increased HIF2-alpha expression leads to increased tissue inflammation caused by neutrophils. Suppression of HIF2-alpha causes apoptosis of neutrophils and decreased inflammation.106
HIF2-alpha also regulates stem cell niches. HIF2-alpha knockout mice are genetically lethal with defects in vascular and mitochondrial development.107 In intestinal development, HIF2-alpha regulates stem cell niches through regulation of Oct4 and its downstream targets Sox2 and Nanog.108,109 Finally, evidence from hemopoietic stem cell development demonstrates HIF2-alpha is important for robust pluripotency of those stem cell populations.110
Further speculation regarding the role of HIF2-alpha in hepatic IRI comes from study of other diseases affecting the GI tract. In inflammatory bowel disease HIF2-alpha has a role in initiation and recovery from ischemic injury. Chronic activation of HIF2-alpha via NF-kappa B leads to colitis. Conversely, HIF2-alpha activation after ischemic injury from radiation leads to epithelial cell regeneration via VEGF pathways.111,112
Energy metabolism is an important part of initiation and recovery from IRI. Recent reports suggest HIF2-alpha regulates energy metabolism in the liver both in the context of insulin response and fatty acid metabolism.9 HIF2-alpha is a necessary component of the insulin response mediated by VEGF in the liver. Fat deposition and steatohepatitis in the liver are increased in the absence of HIF2-alpha.113,114
Lastly, HIF2-alpha may also regulate long term recovery from IRI through control of expression of MMP and collagen prolyl hydroxylases. HIF2-alpha is the major transcriptional regulator of these genes.115 As a corollary, activation of HIF2-alpha in the liver is sufficient to cause liver fibrosis.116
Based on these findings, one can speculate on the role of HIF2-alpha in hepatic IRI (Figure 4). Hypoxia in IRI would stabilize HIF2-alpha. HIF2-alpha expression would be increased in LSEC, KC, and neutrophils contributing to inflammation via increased vasoconstriction through decreased NO, enhanced production of cytokines, promotion of macrophage polarization, and enhanced survival of neutrophils. HIF2-alpha would also contribute to recovery from IRI by stabilizing the stem cell niche in hepatocytes. Finally, HIF2-alpha could affect the long-term architecture and function of the liver after IRI via remodeling of the ECM through regulation of MMP and collagen hydroxylases (Figure 4). Given the potential effects HIF2-alpha could have in heaptic IRI, further investigation in this area is warranted.
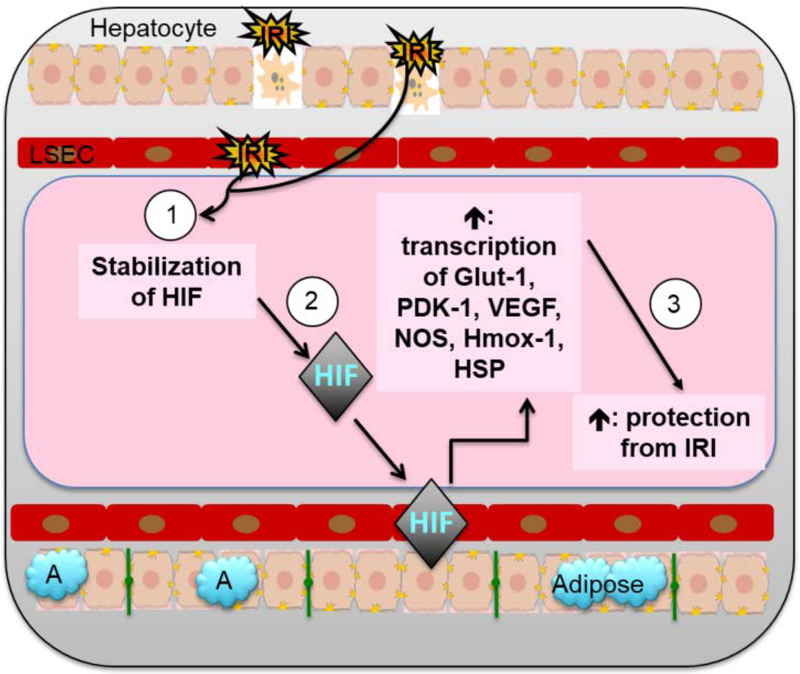
Figure 4: Cellular and molecular IRI pathway: A focus on the role of HIF.
(1) Hepatic IRI results in stabilization of HIF, which (2) enter the nucleus and induces transcription of genes in multiple pathways such as cellular metabolism (Glut-1, PDK-1), angiogenesis (VEGF, NOS), and cytoprotection (Hmox-1, HSP) to (3) ameliorate IRI induced hepatocyte injury and cell death.
MicroRNA
MicroRNA’s (miRNA) are single stranded, non-coding RNA’s which modulate gene expression via translational repression of target mRNAs.117 MiRNA expression has been linked to regulation of a number of cellular processes that affect hepatic IRI. These include promotion of inflammation, cellular regeneration, and autophagy.118
MiRNA 122 is the most abundant miRNA in the liver, representing 70% of the total.119 In miRNA-122 knock out mice, there are increased levels of monocytes and neutrophils. Further, loss of miRNA-122 leads to increased expression of the chemokine CCL2, which promotes migration of macrophage and neutrophil to the liver.120 After prolonged IRI, miRNA-122 levels are reduced in liver tissue but elevated in serum of patients with liver injury suggesting miRNA-122 is important in maintaining normal liver function and is released into the serum due to hepatocyte death after IRI.118
Other miRNAs upregulated in hepatic IRI include miRNA-34a, miRNA-17, and miRNA-155. MiRNA-34a appears to regulate the effect of ROS by decreasing expression of antioxidants after IRI and increasing apoptosis through upregulation of p53.121 Increased miRNA-34a decreases expression of Nrf2 a key molecule that promotes production of antioxidants. MiRNA-34a stabilizes and promotes increased expression of p53 resulting in increased liver injury after hepatic IRI, suggesting a multifaceted role for it in promotion and recovery from ischemic liver injury.122,123
MiRNA-17 is increased after hepatic IRI. It appears to regulate autophagy: increased miRNA-17 correlates with increased numbers of autophagosomes. Excess expression of miRNA-17 leads to unregulated autophagy and to decreased hepatocyte viability via increased release of caspases. MiRNA-17 controls autophagy through transcriptional regulation of STAT-3, a known inhibitor of autophagy. MiRNA-17 binds to the 3’ UTR of STAT-3 repressing its transcription.124
MiRNA-155 regulates the response of KC after IRI. MiRNA-155 deficient mice have less liver injury after IRI with decreased expression of CD80, CD86 and MHC class II on KC. Further, KC in these mice produced IL-10 which improved the viability of hepatocytes. Elimination of miRNA-155 negative KC led to increased injury after hepatic ischemia.125
Other miRNAs involved in apoptosis and regeneration show down- or up-regulation in expression respectively after hepatic IRI. Downregulated miRNAs involved in apoptosis include miRNA-200b and miRNA-183. Upregulated miRNAs involved in liver regeneration include miRNA-27a, −494, −1224, and −149.126 These findings indicate an important role for miRNA in the regulation of hepatic IRI.
Reactive oxygen species and inflammatory cytokines
In the ischemic phase of IRI, reducing substances are consumed and anaerobic metabolism dominates. In reperfusion, oxygen is reintroduced and due to the need for reducing substances, excess oxygen free radicals are produced. Oxygen free radicals cause direct cellular damage and induce an inflammatory response. In liver IRI, they induce release of HMGB1 and activate NF-kappa-B.127 This leads to production of a number of cytokines such as IL-1alpha and beta, IL-2, IL-3, IL-6, IL-8, and TNF-alpha and beta.128 Production of cytokines and release of ROS, HMBG1 and DAMP from damaged hepatocytes leads to neutrophil recruitment and KC activation.9 Thus ROS are critical initiators of the injury response (Figure 5).
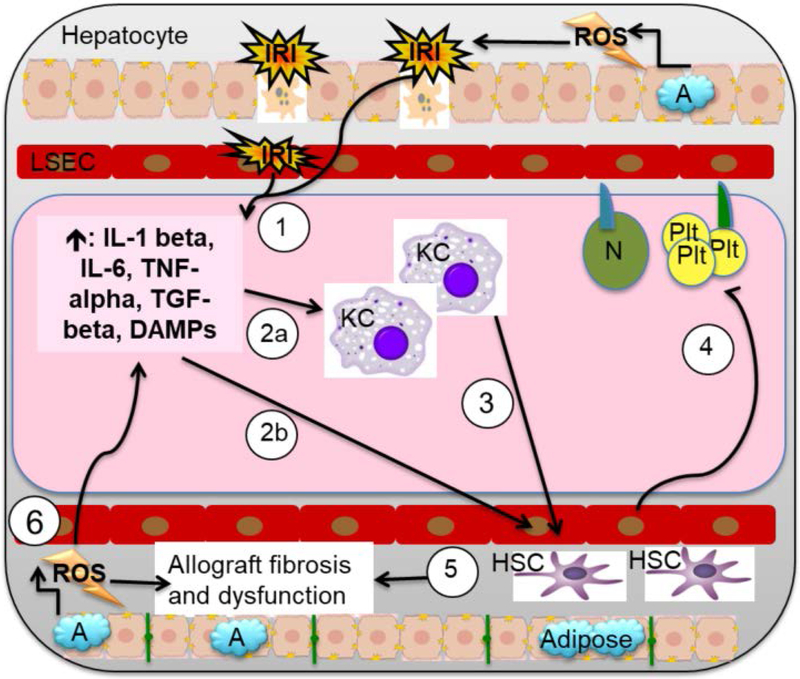
Figure 5: Cellular and molecular IRI pathway: A focus on the roles of HSC, adiposity, and ROS.
(1) IRI damage to hepatocytes and LSECs causes cell death and release of inflammatory cytokines IL-1 beta, IL-6, and TNF-alpha, DAMPS, HMGB1, TGF-beta, FFA, and HSB, as well as DNA fragments, which cause (2a) KC to lose tolerogenic profile and increasing cytokine production in KC toward inflammatory phenotype and (2b) hepatic stellate cell (HSC) activation. (3) KC activation is associated with increased KC cytokine production, which further recruits HSCs. The activation and recruitment of HSC to areas of IRI results in (4) ingress of platelets and neutrophils, (5) MMP release, ECM destruction, and endothelial vasoconstriction through ET-1. Together, this results in collagen deposition and graft fibrosis, increasing the risk of graft failure, morbidity, and mortality. (6) After organ reperfusion, lipid peroxidation due to high levels of steatosis causes release of ROS and DAMPs. This results in increased release of IL6 and TNF-alpha, resulting in further acute liver injury, ineffective autophagy, early graft dysfunction or non-function, and increased long-term fibrosis.
This is relevant in donors livers which are steatotic or are from NHBD. In steatotic livers, excess fat deposited in the hepatocytes is a target for peroxidation.129,130 Lipid peroxidation generates ROS and amplifies hepatic IRI.131 First, lipid peroxidation depletes NAD+ and increases the NADH/NAD+ ratio to promote production of superoxide free radicals.132 Second, to regulate this, mitochondria express mitochondrial uncoupling protein 2 (UCP2). UCP2 causes a leak in the proton gradient in cellular respiration to reduce ATP production. This has detrimental effects as rapid reduction of energy stores leads to further hepatocyte death due to loss of membrane integrity.133
In NHBD, the effects of ROS are more pronounced because of extended periods of warm ischemia. This leads to a rapid depletion of ATP and switch to anaerobic respiration. Subsequently, ROS production after reperfusion is greater and more pronounced leading to increased cellular injury.33 These mechanisms help explain the clinical observation as to why steatotic allografts and those from NHBD are at higher risk for early allograft dysfunction (Figure 5).
Recent data also indicates that a broad class of metabolic signaling molecules associated but not exclusive to adipose tissue, i.e. adipokines, affect liver regeneration after IRI. Some of the more well-known members of this family include IL-6, adiponectin, and leptin. In a comprehensive review, Peralta and colleagues examined the available data regarding adipokines and concluded that there was sufficient evidence to suggest that these molecules had a role in liver regeneration after IRI, particularly IL-6, but that more data in partial ischemia and steatotic liver injury models was required to clearly establish the positive or negative effects of the more than 600 adipokines known to date.134
Gut-Liver Axis of Injury
An emerging area of interest in liver IRI is the effect of the intestinal microbiome on liver injury. Clamping the portal circulation in murine models of liver IRI leads to impaired gut-barrier function and increased translocation of bacteria and bacterial debris into the portal circulation. This leads to activation of TLR4, promoting the inflammatory cascade after reperfusion and increasing liver injury. Removal of bacteria, abrogation of TLR4 signaling, and remote ischemic preconditioning all reduced the negative effects of the disruption of gut microbiome on liver IRI.135 In particular, remote ischemic preconditioning restores gut barrier function and normal composition of microbiota in these models.136 Thus, modulation of the gut-liver axis may provide new therapeutic avenues to treat IRI in liver transplantation.
Therapeutics for Hepatic IRI
Therapeutic strategies to limit IRI in liver transplantation generally fall into the following categories. 1. Reducing effects of ROS 2. Modulation of the cytokine response 3. Blocking activation of the immune system and 4. Improving organ preservation (Table 1).
Table 1.
Current Clinical Trials for Prevention of Ischemia Reperfusion Injury in Liver Transplantation
| Trial Name | Intervention Type | Description | Sponsor |
|---|---|---|---|
| Efficacy and Safety Pilot Study of Reparixin for Early Allograft Dysfunction Prevention in Orthotopic Liver Transplantation Patients | Pharmacotherapy | Use of Reparixin (CXCL8/IL-8) inhibitor to prevent IR injury in liver transplantation | Dompé Farmaceutici S.p.A (multicenter) |
| Safety and Efficacy of Treprostinil in Ischemia and Reperfusion Injury in Adult Orthotopic Liver Transplantation | Pharmacotherapy | Use of Treprostinil (prostacyclin analogue) to prevent IR injury in liver transplantation | University of Pittsburgh |
| Omega 3 Lipid Emulsions and Liver Transplantation | Pharmacotherapy | Perioperative Omega 3 rich lipid infusions to prevent IR injury in living donor liver transplantation | Mansoura University |
| YSPSL for Prevention of Ischemic Reperfusion Injury in Patients Undergoing Cadaveric Orthotopic Liver Transplantation | Pharmacotherapy | P-selectin Ig infusion during reperfusion of liver allograft to prevent IR injury during liver transplantation | UCLA Medical Center |
| Intermittent Portal and Graft Purge in Living Donor Liver Transplantation | Ischemic Preconditioning | Intermittent portal clamping during reperfusion of living donor liver transplantation | Mansoura University |
| Liver Protection of RIPC in Pediatric Living Donor Liver Transplantation | Ischemic Preconditioning | Donor and Recipient distant (limb) ischemic preconditioning to reduce IR injury in pediatric liver transplantation | Renji Hospital Shanghai |
| Efficacy Evaluation of Normothermic Perfusion Machine Preservation in Liver Transplant Using Very Old Donors | Ex Vivo Perfusion | Ex Vivo normothermic perfusion of older/marginal liver allografts to prevent IR injury in liver transplantation | Azienda Ospedaliero, Universitaria Pisana |
| Hypothermic Oxygenated Perfusion (HOPE) of human liver allografts | Ex Vivo Perfusion | Hypothermic perfusion with oxygenated perfusate after cold storage to prevent IR Injury in liver transplantation | Universisty of Zurich (multicenter) |
| Dual Hypothermic Oxygenated Perfusion of DCD Liver Grafts (HOPE-DCD) in Preventing Biliary Complications After Transplantation | Ex Vivo Perfusion | Hypothermic perfusion with oxygenated perfusate via portal vein and aorta of DCD livers to prevent IR Injury in liver transplantation | University Medical Center Groningen (multicenter) |
| HOPE for Human Extended Criteria and Donation After Brain Death Donor (ECD-DBD) Liver Allografts | Ex Vivo Perfusion | Hypothermic perfusion with oxygenated perfusate after cold storage of ECD livers to prevent IR Injury in liver transplantation | University of Aachen (multicenter) |
| Using Ex-vivo Normothermic Machine Perfusion With the Organox Metra™ Device to Store Human Livers for Transplantation | Ex Vivo Perfusion | Normothermic machine perfusion of liver allografts after procurement and prior to implantation to prevent IR injury in liver transplantation | OrganOx (multicenter, Europe, United States and Canada) |
Using free radical scavengers to limit IRI in liver transplantation is an elegant idea that unfortunately has not been clinically effective. Much effort has focused on the use of N-acetyl-cysteine (NAC), a glutathione precursor, as a free radical scavenger.137 NAC use in acetaminophen overdose has shown promise, but its effectiveness in liver transplantation is less clear.138 In NHBD, NAC was thought to be effective in reducing the injurious consequences of IRI on bile ducts, but this data has not been reproducible.139,140 Use of free radical scavengers and antioxidants such as S-adenosyl-methionine and Vitamin E has shown no clinical value in liver transplantation.141
A complex network of cytokine expression underlies the response to hepatic IRI. Modulating the cytokine response would be a logical way to blunt the effects of hepatic IRI in liver transplantation. To this end, both blocking cytokines produced during IRI and administration of cytokines to counter those released during IRI have been proposed as therapeutic strategies.
Prostaglandins E-1 and I2 experimentally promote vasodilation of the liver microcirculation. Unfortunately, neither has shown promise in clinical trials. PGE-2 administration in liver transplantation has no effect on the rates of allograft dysfunction or patient survival.142 Data for the effectiveness of PGI-2 is similarly lacking.143
TNF-alpha is a central cytokine in hepatic IRI. There are a number of anti-TNF-alpha biologic agents available for use. Unfortunately, none of these biologics has shown promise in liver transplantation. This may be due to the fact that TNF-alpha is essential for regeneration of the liver after IRI and blocking it eliminates its beneficial effects.144
Blockade of the immune system has been practiced in liver transplantation for some time with the use of steroids as part of an immunosuppressive regimen. Steroids suppress transcription of cytokines in immune cells.145 Steroids likely attenuate hepatic IRI, but the severity of IRI in steatotic, NHBD, and older livers, suggest steroids alone are not sufficient as a therapy.146
An intriguing direct strategy involves use of gadolinium chloride to inhibit KC activation. This drug has shown promise in animal models of liver transplantation where administration of gadolinium chloride reduced markers of liver injury and improved hepatic blood flow.60,147 Unfortunately at this time no correlative human data exists.
Finally, strategies to improve organ preservation have gained favor in recent years. Initial attempts to precondition liver allografts prior to cold preservation with brief periods of ischemia were found not be successful.148 However, the use of ex vivo perfusion has demonstrated reduction in liver injury.149 The concept underlying hypothermic ex vivo perfusion is that flow through the liver sinusoids limits LSEC injury. In normothermic ex vivo perfusion, the liver is preserved under normal physiologic conditions. Ongoing clinical trials are attempting to answer whether this reduction in liver injury has benefits in terms of early and late graft function and patient survival. Further, there is interest in extending these trials to preserve marginal liver allografts (Table 1). Early data suggests that ex vivo perfusion would have substantial benefit in reducing IRI in this setting although the mechanisms for this are yet to be fully elucidated.150
Conclusion
Hepatic IRI represents a barrier to the use of organs available for transplant. The risk of early and late graft dysfunction of donor livers is directly related to hepatic IRI. Therefore understanding the mechanisms of hepatic IRI and development of therapeutics are of enormous importance in reducing the mortality of patients awaiting liver transplant. This will allow more organs to be used and reduce the dysfunction of those organs once transplanted.
Key Points.
IRI negatively affects allograft function after liver transplant
The cellular and molecular mechanisms of IRI in liver transplantation can be correlated with clinical risk factors for IRI in donors and recipients
Developing therapies to ameliorate IRI in liver transplantation is critical to improving patient survival
Funding:
H. Eltzschig, NIH NHLBI (R01HL119837, R01HL133900); C. Ju NIH NIAAA (U01AA021723, R21AA024636), NIDDK (R01DK109574)
Abbreviations:
- IRI
Ischemia Reperfusion Injury
- DAMP
Damage associated molecular pattern
- HIF
hypoxia inducible factor
- MMP
matrix metalloproteinase
- VWF
von Willebrand factor
- NASH
non-alcoholic steatohepatitis
- NHBD
non heart beating donor
- LSEC
liver sinusoidal endothelial cells
- KC
Kupffer cells
- ROS
reactive oxygen species
- HSC
hepatic stellate cell
Footnotes
COI: Authors have no conflict of interests.
References
- 1.Marcellin P, Kutala BK. Liver diseases: A major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int 2018; 38 Suppl 1: 2–6. [DOI] [PubMed] [Google Scholar]
- 2.JENNINGS RB, SOMMERS HM, SMYTH GA, FLACK HA, LINN H. Myocardial necrosis induced by temporary occlusion of a coronary artery in the dog. Arch Pathol 1960; 70: 68–78. [PubMed] [Google Scholar]
- 3.Eltzschig HK, Eckle T. Ischemia and reperfusion--from mechanism to translation. Nat Med 2011; 17: 1391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siniscalchi A, Gamberini L, Laici C, et al. Post reperfusion syndrome during liver transplantation: From pathophysiology to therapy and preventive strategies. World J Gastroenterol 2016; 22: 1551–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stine JG, Northup PG. Coagulopathy Before and After Liver Transplantation: From the Hepatic to the Systemic Circulatory Systems. Clin Liver Dis 2017; 21: 253–74. [DOI] [PubMed] [Google Scholar]
- 6.Bolondi G, Mocchegiani F, Montalti R, Nicolini D, Vivarelli M, De Pietri L. Predictive factors of short term outcome after liver transplantation: A review. World J Gastroenterol 2016; 22: 5936–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peralta C, Jimenez-Castro MB, Gracia-Sancho J. Hepatic ischemia and reperfusion injury: effects on the liver sinusoidal milieu. J Hepatol 2013; 59: 1094–106. [DOI] [PubMed] [Google Scholar]
- 8.Stewart RK, Dangi A, Huang C, et al. A novel mouse model of depletion of stellate cells clarifies their role in ischemia/reperfusion- and endotoxin-induced acute liver injury. J Hepatol 2014; 60: 298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiniers MJ, van Golen RF, van Gulik TM, Heger M. Reactive oxygen and nitrogen species in steatotic hepatocytes: a molecular perspective on the pathophysiology of ischemia-reperfusion injury in the fatty liver. Antioxid Redox Signal 2014; 21: 1119–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu L, Zhou H, Ni M, et al. Innate Immune Regulations and Liver Ischemia-Reperfusion Injury. Transplantation 2016; 100: 2601–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cursio R, Colosetti P, Gugenheim J. Autophagy and liver ischemia-reperfusion injury. Biomed Res Int 2015; 2015: 417590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ju C, Colgan SP, Eltzschig HK. Hypoxia-inducible factors as molecular targets for liver diseases. J Mol Med (Berl) 2016; 94: 613–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tripodi A, Mannucci PM. The coagulopathy of chronic liver disease. N Engl J Med 2011; 365: 147–56. [DOI] [PubMed] [Google Scholar]
- 14.Aldawood A, Arabi Y, Aljumah A, et al. The incidence of venous thromboembolism and practice of deep venous thrombosis prophylaxis in hospitalized cirrhotic patients. Thromb J 2011; 9: 1,9560-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Violi F, Ferro D, Basili S, et al. Ongoing prothrombotic state in the portal circulation of cirrhotic patients. Thromb Haemost 1997; 77: 44–7. [PubMed] [Google Scholar]
- 16.Yerdel MA, Gunson B, Mirza D, et al. Portal vein thrombosis in adults undergoing liver transplantation: risk factors, screening, management, and outcome. Transplantation 2000; 69: 1873–81. [DOI] [PubMed] [Google Scholar]
- 17.Duffy JP, Hong JC, Farmer DG, et al. Vascular complications of orthotopic liver transplantation: experience in more than 4,200 patients. J Am Coll Surg 2009; 208: 896,903; discussion 903–5. [DOI] [PubMed] [Google Scholar]
- 18.Zoli M, Iervese T, Merkel C, et al. Prognostic significance of portal hemodynamics in patients with compensated cirrhosis. J Hepatol 1993; 17: 56–61. [DOI] [PubMed] [Google Scholar]
- 19.Qi X, Chen H, Han G. Effect of antithrombin, protein C and protein S on portal vein thrombosis in liver cirrhosis: a meta-analysis. Am J Med Sci 2013; 346: 38–44. [DOI] [PubMed] [Google Scholar]
- 20.Uemura M, Tatsumi K, Matsumoto M, et al. Localization of ADAMTS13 to the stellate cells of human liver. Blood 2005; 106: 922–4. [DOI] [PubMed] [Google Scholar]
- 21.Uemura M, Fujimura Y, Matsumoto M, et al. Comprehensive analysis of ADAMTS13 in patients with liver cirrhosis. Thromb Haemost 2008; 99: 1019–29. [DOI] [PubMed] [Google Scholar]
- 22.Matsuyama T, Uemura M, Ishikawa M, et al. Increased von Willebrand factor over decreased ADAMTS13 activity may contribute to the development of liver disturbance and multiorgan failure in patients with alcoholic hepatitis. Alcohol Clin Exp Res 2007; 31: S27–35. [DOI] [PubMed] [Google Scholar]
- 23.Ko S, Chisuwa H, Matsumoto M, Fujimura Y, Okano E, Nakajima Y. Relevance of ADAMTS13 to liver transplantation and surgery. World J Hepatol 2015; 7: 1772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Araujo AR, Rosso N, Bedogni G, Tiribelli C, Bellentani S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int 2018; 38 Suppl 1: 47–51. [DOI] [PubMed] [Google Scholar]
- 25.Verrijken A, Francque S, Mertens I, et al. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 2014; 59: 121–9. [DOI] [PubMed] [Google Scholar]
- 26.Stine JG, Argo CK, Pelletier SJ, Maluf DG, Caldwell SH, Northup PG. Advanced non-alcoholic steatohepatitis cirrhosis: A high-risk population for pre-liver transplant portal vein thrombosis. World J Hepatol 2017; 9: 139–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durand F, Renz JF, Alkofer B, et al. Report of the Paris consensus meeting on expanded criteria donors in liver transplantation. Liver Transpl 2008; 14: 1694–707. [DOI] [PubMed] [Google Scholar]
- 28.Spitzer AL, Lao OB, Dick AA, et al. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver Transpl 2010; 16: 874–84. [DOI] [PubMed] [Google Scholar]
- 29.Gehrau RC, Mas VR, Dumur CI, et al. Donor Hepatic Steatosis Induce Exacerbated Ischemia-Reperfusion Injury Through Activation of Innate Immune Response Molecular Pathways. Transplantation 2015; 99: 2523–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selzner M, Rudiger HA, Sindram D, Madden J, Clavien PA. Mechanisms of ischemic injury are different in the steatotic and normal rat liver. Hepatology 2000; 32: 1280–8. [DOI] [PubMed] [Google Scholar]
- 31.Fayek SA, Quintini C, Chavin KD, Marsh CL. The Current State of Liver Transplantation in the United States: Perspective From American Society of Transplant Surgeons (ASTS) Scientific Studies Committee and Endorsed by ASTS Council. Am J Transplant 2016; 16: 3093–104. [DOI] [PubMed] [Google Scholar]
- 32.Thuong M, Ruiz A, Evrard P, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int 2016; 29: 749–59. [DOI] [PubMed] [Google Scholar]
- 33.Xu J, Casas-Ferreira AM, Ma Y, et al. Lipidomics comparing DCD and DBD liver allografts uncovers lysophospholipids elevated in recipients undergoing early allograft dysfunction. Sci Rep 2015; 5: 17737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu J, Sayed BA, Casas-Ferreira AM, et al. The Impact of Ischemia/Reperfusion Injury on Liver Allografts from Deceased after Cardiac Death versus Deceased after Brain Death Donors. PLoS One 2016; 11: e0148815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lue A, Solanas E, Baptista P, et al. How important is donor age in liver transplantation? World J Gastroenterol 2016; 22: 4966–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wynne HA, Cope LH, Mutch E, Rawlins MD, Woodhouse KW, James OF. The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology 1989; 9: 297–301. [DOI] [PubMed] [Google Scholar]
- 37.Okaya T, Blanchard J, Schuster R, et al. Age-dependent responses to hepatic ischemia/reperfusion injury. Shock 2005; 24: 421–7. [DOI] [PubMed] [Google Scholar]
- 38.Watts RP, Thom O, Fraser JF. Inflammatory signalling associated with brain dead organ donation: from brain injury to brain stem death and posttransplant ischaemia reperfusion injury. J Transplant 2013; 2013: 521369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nardo B, Caraceni P, Pasini P, et al. Increased generation of reactive oxygen species in isolated rat fatty liver during postischemic reoxygenation. Transplantation 2001; 71: 1816–20. [DOI] [PubMed] [Google Scholar]
- 40.Ellett JD, Evans ZP, Atkinson C, Schmidt MG, Schnellmann RG, Chavin KD. Toll-like receptor 4 is a key mediator of murine steatotic liver warm ischemia/reperfusion injury. Liver Transpl 2009; 15: 1101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Caraceni P, Bianchi C, Domenicali M, et al. Impairment of mitochondrial oxidative phosphorylation in rat fatty liver exposed to preservation-reperfusion injury. J Hepatol 2004; 41: 82–8. [DOI] [PubMed] [Google Scholar]
- 42.Onumata O, Takahashi T, Sato K, Kakita A. Effects of ulinastatin on hypotension and hepatic circulation in brain dead rabbits. Transplant Proc 2000; 32: 2290–2. [DOI] [PubMed] [Google Scholar]
- 43.Jimenez-Castro MB, Merono N, Mendes-Braz M, et al. The effect of brain death in rat steatotic and non-steatotic liver transplantation with previous ischemic preconditioning. J Hepatol 2015; 62: 83–91. [DOI] [PubMed] [Google Scholar]
- 44.Poisson J, Lemoinne S, Boulanger C, et al. Liver sinusoidal endothelial cells: Physiology and role in liver diseases. J Hepatol 2017; 66: 212–27. [DOI] [PubMed] [Google Scholar]
- 45.Russo L, Gracia-Sancho J, Garcia-Caldero H, et al. Addition of simvastatin to cold storage solution prevents endothelial dysfunction in explanted rat livers. Hepatology 2012; 55: 921–30. [DOI] [PubMed] [Google Scholar]
- 46.Upadhya GA, Topp SA, Hotchkiss RS, Anagli J, Strasberg SM. Effect of cold preservation on intracellular calcium concentration and calpain activity in rat sinusoidal endothelial cells. Hepatology 2003; 37: 313–23. [DOI] [PubMed] [Google Scholar]
- 47.Schon MR, Kollmar O, Wolf S, et al. Liver transplantation after organ preservation with normothermic extracorporeal perfusion. Ann Surg 2001; 233: 114–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rauen U, Elling B, Gizewski ER, Korth HG, Sustmann R, de Groot H. Involvement of reactive oxygen species in the preservation injury to cultured liver endothelial cells. Free Radic Biol Med 1997; 22: 17–24. [DOI] [PubMed] [Google Scholar]
- 49.Ruan Z, Shibamoto T, Shimo T, et al. Effects of platelet-activating factor and thromboxane A2 on isolated perfused guinea pig liver. Prostaglandins Other Lipid Mediat 2004; 73: 73–85. [DOI] [PubMed] [Google Scholar]
- 50.Kageyama S, Yagi S, Tanaka H, et al. Graft reconditioning with nitric oxide gas in rat liver transplantation from cardiac death donors. Transplantation 2014; 97: 618–25. [DOI] [PubMed] [Google Scholar]
- 51.Massberg S, Enders G, Leiderer R, et al. Platelet-endothelial cell interactions during ischemia/reperfusion: the role of P-selectin. Blood 1998; 92: 507–15. [PubMed] [Google Scholar]
- 52.Sindram D, Porte RJ, Hoffman MR, Bentley RC, Clavien PA. Platelets induce sinusoidal endothelial cell apoptosis upon reperfusion of the cold ischemic rat liver. Gastroenterology 2000; 118: 183–91. [DOI] [PubMed] [Google Scholar]
- 53.Gomez Perdiguero E, Klapproth K, Schulz C, et al. Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 2015; 518: 547–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.You Q, Cheng L, Kedl RM, Ju C. Mechanism of T cell tolerance induction by murine hepatic Kupffer cells. Hepatology 2008; 48: 978–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heymann F, Peusquens J, Ludwig-Portugall I, et al. Liver inflammation abrogates immunological tolerance induced by Kupffer cells. Hepatology 2015; 62: 279–91. [DOI] [PubMed] [Google Scholar]
- 56.Toki Y, Takenouchi T, Harada H, et al. Extracellular ATP induces P2X7 receptor activation in mouse Kupffer cells, leading to release of IL-1beta, HMGB1, and PGE2, decreased MHC class I expression and necrotic cell death. Biochem Biophys Res Commun 2015; 458: 771–6. [DOI] [PubMed] [Google Scholar]
- 57.Witthaut R, Farhood A, Smith CW, Jaeschke H. Complement and tumor necrosis factor-alpha contribute to Mac-1 (CD11b/CD18) up-regulation and systemic neutrophil activation during endotoxemia in vivo. J Leukoc Biol 1994; 55: 105–11. [DOI] [PubMed] [Google Scholar]
- 58.Perry BC, Soltys D, Toledo AH, Toledo-Pereyra LH. Tumor necrosis factor-alpha in liver ischemia/reperfusion injury. J Invest Surg 2011; 24: 178–88. [DOI] [PubMed] [Google Scholar]
- 59.Peralta C, Fernandez L, Panes J, et al. Preconditioning protects against systemic disorders associated with hepatic ischemia-reperfusion through blockade of tumor necrosis factor-induced P-selectin up-regulation in the rat. Hepatology 2001; 33: 100–13. [DOI] [PubMed] [Google Scholar]
- 60.Mosher B, Dean R, Harkema J, Remick D, Palma J, Crockett E. Inhibition of Kupffer cells reduced CXC chemokine production and liver injury. J Surg Res 2001; 99: 201–10. [DOI] [PubMed] [Google Scholar]
- 61.Gonzalez-Flecha B, Cutrin JC, Boveris A. Time course and mechanism of oxidative stress and tissue damage in rat liver subjected to in vivo ischemia-reperfusion. J Clin Invest 1993; 91: 456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hasselgren PO, Jennische E, Fornander J, Hellman A. No beneficial effect of ATP-MgCl2 on impaired transmembrane potential and protein synthesis in liver ischemia. Acta Chir Scand 1982; 148: 601–7. [PubMed] [Google Scholar]
- 63.Tsung A, Klune JR, Zhang X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med 2007; 204: 2913–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ. Complement activates Kupffer cells and neutrophils during reperfusion after hepatic ischemia. Am J Physiol 1993; 264: G801–9. [DOI] [PubMed] [Google Scholar]
- 65.Sarris M, Masson JB, Maurin D, et al. Inflammatory chemokines direct and restrict leukocyte migration within live tissues as glycan-bound gradients. Curr Biol 2012; 22: 2375–82. [DOI] [PubMed] [Google Scholar]
- 66.Jaeschke H, Farhood A, Bautista AP, Spolarics Z, Spitzer JJ, Smith CW. Functional inactivation of neutrophils with a Mac-1 (CD11b/CD18) monoclonal antibody protects against ischemia-reperfusion injury in rat liver. Hepatology 1993; 17: 915–23. [PubMed] [Google Scholar]
- 67.Huang H, Tohme S, Al-Khafaji AB, et al. Damage-associated molecular pattern-activated neutrophil extracellular trap exacerbates sterile inflammatory liver injury. Hepatology 2015; 62: 600–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bamboat ZM, Balachandran VP, Ocuin LM, Obaid H, Plitas G, DeMatteo RP. Toll-like receptor 9 inhibition confers protection from liver ischemia-reperfusion injury. Hepatology 2010; 51: 621–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Roh YS, Zhang B, Loomba R, Seki E. TLR2 and TLR9 contribute to alcohol-mediated liver injury through induction of CXCL1 and neutrophil infiltration. Am J Physiol Gastrointest Liver Physiol 2015; 309: G30–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miyake K, Onji M. Endocytosis-free DNA sensing by cell surface TLR9 in neutrophils: rapid defense with autoimmune risks. Eur J Immunol 2013; 43: 2006–9. [DOI] [PubMed] [Google Scholar]
- 71.Oliveira THC, Marques PE, Proost P, Teixeira MMM. Neutrophils: a cornerstone of liver ischemia and reperfusion injury. Lab Invest 2018; 98: 51–62. [DOI] [PubMed] [Google Scholar]
- 72.Miyashita T, Nakanuma S, Ahmed AK, et al. Ischemia reperfusion-facilitated sinusoidal endothelial cell injury in liver transplantation and the resulting impact of extravasated platelet aggregation. Eur Surg 2016; 48: 92–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Enjyoji K, Sevigny J, Lin Y, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med 1999; 5: 1010–7. [DOI] [PubMed] [Google Scholar]
- 74.Katagiri H, Ito Y, Ito S, et al. TNF-alpha induces thromboxane receptor signaling-dependent microcirculatory dysfunction in mouse liver. Shock 2008; 30: 463–7. [DOI] [PubMed] [Google Scholar]
- 75.Watanabe K, Togo S, Takahashi T, et al. PAI-1 plays an important role in liver failure after excessive hepatectomy in the rat. J Surg Res 2007; 143: 13–9. [DOI] [PubMed] [Google Scholar]
- 76.Ueda S, Yamanoi A, Hishikawa Y, Dhar DK, Tachibana M, Nagasue N. Transforming growth factor-beta1 released from the spleen exerts a growth inhibitory effect on liver regeneration in rats. Lab Invest 2003; 83: 1595–603. [DOI] [PubMed] [Google Scholar]
- 77.Cheng F, Li Y, Feng L, Li S. Hepatic stellate cell activation and hepatic fibrosis induced by ischemia/reperfusion injury. Transplant Proc 2008; 40: 2167–70. [DOI] [PubMed] [Google Scholar]
- 78.Friedman SL Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem 2000; 275: 2247–50. [DOI] [PubMed] [Google Scholar]
- 79.Mehrfeld C, Zenner S, Kornek M, Lukacs-Kornek V. The Contribution of Non-Professional Antigen-Presenting Cells to Immunity and Tolerance in the Liver. Front Immunol 2018; 9: 635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng M, Wang Q, Wang H, Wang M, Guan W, Lu L. Adoptive transfer of hepatic stellate cells ameliorates liver ischemia reperfusion injury through enriching regulatory T cells. Int Immunopharmacol 2014; 19: 267–74. [DOI] [PubMed] [Google Scholar]
- 81.Rockey DC Hepatic blood flow regulation by stellate cells in normal and injured liver. Semin Liver Dis 2001; 21: 337–49. [DOI] [PubMed] [Google Scholar]
- 82.Reifart J, Rentsch M, Mende K, et al. Modulating CD4+ T cell migration in the postischemic liver: hepatic stellate cells as new therapeutic target? Transplantation 2015; 99: 41–7. [DOI] [PubMed] [Google Scholar]
- 83.Czaja MJ, Ding WX, Donohue TM Jr, et al. Functions of autophagy in normal and diseased liver. Autophagy 2013; 9: 1131–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Boteon YL, Laing R, Mergental H, et al. Mechanisms of autophagy activation in endothelial cell and their targeting during normothermic machine liver perfusion. World J Gastroenterol 2017; 23: 8443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lemasters JJ, Nieminen AL, Qian T, et al. The mitochondrial permeability transition in cell death: a common mechanism in necrosis, apoptosis and autophagy. Biochim Biophys Acta 1998; 1366: 177–96. [DOI] [PubMed] [Google Scholar]
- 86.Kim JS, He L, Qian T, Lemasters JJ. Role of the mitochondrial permeability transition in apoptotic and necrotic death after ischemia/reperfusion injury to hepatocytes. Curr Mol Med 2003; 3: 527–35. [DOI] [PubMed] [Google Scholar]
- 87.Go KL, Lee S, Zendejas I, Behrns KE, Kim JS. Mitochondrial Dysfunction and Autophagy in Hepatic Ischemia/Reperfusion Injury. Biomed Res Int 2015; 2015: 183469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 2011; 18: 571–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Evankovich J, Zhang R, Cardinal JS, et al. Calcium/calmodulin-dependent protein kinase IV limits organ damage in hepatic ischemia-reperfusion injury through induction of autophagy. Am J Physiol Gastrointest Liver Physiol 2012; 303: G189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang D, Ma Y, Li Z, et al. The role of AKT1 and autophagy in the protective effect of hydrogen sulphide against hepatic ischemia/reperfusion injury in mice. Autophagy 2012; 8: 954–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shin T, Kuboki S, Huber N, et al. Activation of peroxisome proliferator-activated receptor-gamma during hepatic ischemia is age-dependent. J Surg Res 2008; 147: 200–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature 2009; 458: 1131–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guixe-Muntet S, de Mesquita FC, Vila S, et al. Cross-talk between autophagy and KLF2 determines endothelial cell phenotype and microvascular function in acute liver injury. J Hepatol 2017; 66: 86–94. [DOI] [PubMed] [Google Scholar]
- 94.Cursio R, Colosetti P, Saint-Paul MC, et al. Induction of different types of cell death after normothermic liver ischemia-reperfusion. Transplant Proc 2010; 42: 3977–80. [DOI] [PubMed] [Google Scholar]
- 95.Shen M, Lu J, Dai W, et al. Ethyl pyruvate ameliorates hepatic ischemia-reperfusion injury by inhibiting intrinsic pathway of apoptosis and autophagy. Mediators Inflamm 2013; 2013: 461536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A 1995; 92: 5510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Iliopoulos O, Levy AP, Jiang C, Kaelin WG Jr, Goldberg MA. Negative regulation of hypoxia-inducible genes by the von Hippel-Lindau protein. Proc Natl Acad Sci U S A 1996; 93: 10595–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhong Z, Ramshesh VK, Rehman H, et al. Activation of the oxygen-sensing signal cascade prevents mitochondrial injury after mouse liver ischemia-reperfusion. Am J Physiol Gastrointest Liver Physiol 2008; 295: G823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yang YY, Lee PC, Huang YT, et al. Involvement of the HIF-1alpha and Wnt/beta-catenin pathways in the protective effects of losartan on fatty liver graft with ischaemia/reperfusion injury. Clin Sci (Lond) 2014; 126: 163–74. [DOI] [PubMed] [Google Scholar]
- 100.Xu Z, Chen X, Peng C, et al. Hypoxia-inducible factor-1alpha suppressed hepatocellular carcinoma cell apoptosis through influencing on Omi/HtrA2 expression and its releasing from the mitochondrion. Oncol Res 2012; 20: 213–20. [DOI] [PubMed] [Google Scholar]
- 101.Ben Mosbah I, Rosello-Catafau J, Alfany-Fernandez I, et al. Addition of carvedilol to University Wisconsin solution improves rat steatotic and nonsteatotic liver preservation. Liver Transpl 2010; 16: 163–71. [DOI] [PubMed] [Google Scholar]
- 102.Guo JY, Yang T, Sun XG, et al. Ischemic postconditioning attenuates liver warm ischemia-reperfusion injury through Akt-eNOS-NO-HIF pathway. J Biomed Sci 2011; 18: 79,0127-18-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ramakrishnan SK, Shah YM. Role of Intestinal HIF-2alpha in Health and Disease. Annu Rev Physiol 2016; 78: 301–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev 1997; 11: 72–82. [DOI] [PubMed] [Google Scholar]
- 105.Takeda N, O’Dea EL, Doedens A, et al. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev 2010; 24: 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Thompson AA, Elks PM, Marriott HM, et al. Hypoxia-inducible factor 2alpha regulates key neutrophil functions in humans, mice, and zebrafish. Blood 2014; 123: 366–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev 1998; 12: 3320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Covello KL, Kehler J, Yu H, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev 2006; 20: 557–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wicklow E, Blij S, Frum T, et al. HIPPO pathway members restrict SOX2 to the inner cell mass where it promotes ICM fates in the mouse blastocyst. PLoS Genet 2014; 10: e1004618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA. The HIF family member EPAS1/HIF-2alpha is required for normal hematopoiesis in mice. Blood 2003; 102: 1634–40. [DOI] [PubMed] [Google Scholar]
- 111.Xue X, Ramakrishnan S, Anderson E, et al. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology 2013; 145: 831–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Taniguchi CM, Miao YR, Diep AN, et al. PHD inhibition mitigates and protects against radiation-induced gastrointestinal toxicity via HIF2. Sci Transl Med 2014; 6: 236ra64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wei K, Piecewicz SM, McGinnis LM, et al. A liver Hif-2alpha-Irs2 pathway sensitizes hepatic insulin signaling and is modulated by Vegf inhibition. Nat Med 2013; 19: 1331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Xie C, Yagai T, Luo Y, et al. Activation of intestinal hypoxia-inducible factor 2alpha during obesity contributes to hepatic steatosis. Nat Med 2017; 23: 1298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang S, Kim J, Ryu JH, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med 2010; 16: 687–93. [DOI] [PubMed] [Google Scholar]
- 116.Qu A, Taylor M, Xue X, et al. Hypoxia-inducible transcription factor 2alpha promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology 2011; 54: 472–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ambros V The functions of animal microRNAs. Nature 2004; 431: 350–5. [DOI] [PubMed] [Google Scholar]
- 118.Farid WR, Pan Q, van der Meer AJ, et al. Hepatocyte-derived microRNAs as serum biomarkers of hepatic injury and rejection after liver transplantation. Liver Transpl 2012; 18: 290–7. [DOI] [PubMed] [Google Scholar]
- 119.Liang Y, Ridzon D, Wong L, Chen C. Characterization of microRNA expression profiles in normal human tissues. BMC Genomics 2007; 8: 166,2164-8-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hsu SH, Wang B, Kota J, et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest 2012; 122: 2871–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang G, Yao J, Li Z, et al. miR-34a-5p Inhibition Alleviates Intestinal Ischemia/Reperfusion-Induced Reactive Oxygen Species Accumulation and Apoptosis via Activation of SIRT1 Signaling. Antioxid Redox Signal 2016; 24: 961–73. [DOI] [PubMed] [Google Scholar]
- 122.Huang X, Gao Y, Qin J, Lu S. The mechanism of long non-coding RNA MEG3 for hepatic ischemia-reperfusion: Mediated by miR-34a/Nrf2 signaling pathway. J Cell Biochem 2018; 119: 1163–72. [DOI] [PubMed] [Google Scholar]
- 123.Huang X, Gao Y, Qin J, Lu S. The role of miR-34a in the hepatoprotective effect of hydrogen sulfide on ischemia/reperfusion injury in young and old rats. PLoS One 2014; 9: e113305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li S, Zhang J, Wang Z, et al. MicroRNA-17 regulates autophagy to promote hepatic ischemia/reperfusion injury via suppression of signal transductions and activation of transcription-3 expression. Liver Transpl 2016; 22: 1697–709. [DOI] [PubMed] [Google Scholar]
- 125.Li Y, Ma D, Wang Z, Yang J. MicroRNA-155 Deficiency in Kupffer Cells Ameliorates Liver Ischemia-Reperfusion Injury in Mice. Transplantation 2017; 101: 1600–8. [DOI] [PubMed] [Google Scholar]
- 126.Hashmi SK, Baranov E, Gonzalez A, Olthoff K, Shaked A. Genomics of liver transplant injury and regeneration. Transplant Rev (Orlando) 2015; 29: 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.van Golen RF, van Gulik TM, Heger M. The sterile immune response during hepatic ischemia/reperfusion. Cytokine Growth Factor Rev 2012; 23: 69–84. [DOI] [PubMed] [Google Scholar]
- 128.Csak T, Ganz M, Pespisa J, Kodys K, Dolganiuc A, Szabo G. Fatty acid and endotoxin activate inflammasomes in mouse hepatocytes that release danger signals to stimulate immune cells. Hepatology 2011; 54: 133–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mukhopadhyay P, Horvath B, Zsengeller Z, et al. Mitochondrial reactive oxygen species generation triggers inflammatory response and tissue injury associated with hepatic ischemia-reperfusion: therapeutic potential of mitochondrially targeted antioxidants. Free Radic Biol Med 2012; 53: 1123–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Murphy MP How mitochondria produce reactive oxygen species. Biochem J 2009; 417: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Murphy MP, Echtay KS, Blaikie FH, et al. Superoxide activates uncoupling proteins by generating carbon-centered radicals and initiating lipid peroxidation: studies using a mitochondria-targeted spin trap derived from alpha-phenyl-N-tert-butylnitrone. J Biol Chem 2003; 278: 48534–45. [DOI] [PubMed] [Google Scholar]
- 132.Llacuna L, Fernandez A, Montfort CV, et al. Targeting cholesterol at different levels in the mevalonate pathway protects fatty liver against ischemia-reperfusion injury. J Hepatol 2011; 54: 1002–10. [DOI] [PubMed] [Google Scholar]
- 133.Serviddio G, Bellanti F, Tamborra R, et al. Uncoupling protein-2 (UCP2) induces mitochondrial proton leak and increases susceptibility of non-alcoholic steatohepatitis (NASH) liver to ischaemia-reperfusion injury. Gut 2008; 57: 957–65. [DOI] [PubMed] [Google Scholar]
- 134.Alvarez-Mercado AI, Bujaldon E, Gracia-Sancho J, Peralta C. The Role of Adipokines in Surgical Procedures Requiring Both Liver Regeneration and Vascular Occlusion. Int J Mol Sci 2018; 19: 10.3390/ijms19113395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Orci LA, Lacotte S, Delaune V, et al. Effects of the gut-liver axis on ischaemia-mediated hepatocellular carcinoma recurrence in the mouse liver. J Hepatol 2018; 68: 978–85. [DOI] [PubMed] [Google Scholar]
- 136.Ren Z, Cui G, Lu H, et al. Liver ischemic preconditioning (IPC) improves intestinal microbiota following liver transplantation in rats through 16s rDNA-based analysis of microbial structure shift. PLoS One 2013; 8: e75950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cannistra M, Ruggiero M, Zullo A, et al. Hepatic ischemia reperfusion injury: A systematic review of literature and the role of current drugs and biomarkers. Int J Surg 2016; 33 Suppl 1: S57–70. [DOI] [PubMed] [Google Scholar]
- 138.Yoon E, Babar A, Choudhary M, Kutner M, Pyrsopoulos N. Acetaminophen-Induced Hepatotoxicity: a Comprehensive Update. J Clin Transl Hepatol 2016; 4: 131–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nguyen JH, Bonatti H, Dickson RC, et al. Long-term outcomes of donation after cardiac death liver allografts from a single center. Clin Transplant 2009; 23: 168–73. [DOI] [PubMed] [Google Scholar]
- 140.Aliakbarian M, Nikeghbalian S, Ghaffaripour S, et al. Effects of N-Acetylcysteine Addition to University of Wisconsin Solution on the Rate of Ischemia-Reperfusion Injury in Adult Orthotopic Liver Transplant. Exp Clin Transplant 2017; 15: 432–6. [DOI] [PubMed] [Google Scholar]
- 141.Augusto VS, Rodrigues AJ, Reis GS, et al. Evaluation of oxidative stress in the late postoperative stage of liver transplantation. Transplant Proc 2014; 46: 1453–7. [DOI] [PubMed] [Google Scholar]
- 142.Bharathan VK, Chandran B, Gopalakrishnan U, et al. Perioperative prostaglandin e1 infusion in living donor liver transplantation: A double-blind, placebo-controlled randomized trial. Liver Transpl 2016; 22: 1067–74. [DOI] [PubMed] [Google Scholar]
- 143.Cavalcanti AB, De Vasconcelos CP, Perroni de Oliveira M, Rother ET, Ferraz L Jr. Prostaglandins for adult liver transplanted patients. Cochrane Database Syst Rev 2011; (11):CD006006. doi: CD006006. [DOI] [PubMed] [Google Scholar]
- 144.Fingas CD, Beste M, Penndorf V, et al. Liver Regeneration-Related Cytokine Profiles in Donors and Recipients Before and After Living-Donor Liver Transplant. Exp Clin Transplant 2018. [DOI] [PubMed] [Google Scholar]
- 145.Barnes PJ Anti-inflammatory actions of glucocorticoids: molecular mechanisms. Clin Sci (Lond) 1998; 94: 557–72. [DOI] [PubMed] [Google Scholar]
- 146.Orci LA, Toso C, Mentha G, Morel P, Majno PE. Systematic review and meta-analysis of the effect of perioperative steroids on ischaemia-reperfusion injury and surgical stress response in patients undergoing liver resection. Br J Surg 2013; 100: 600–9. [DOI] [PubMed] [Google Scholar]
- 147.Wu Y, Wang Y, Li M, Yang X, Gong J, Zhang W. Gadolinium chloride suppresses acute rejection and induces tolerance following rat liver transplantation by inhibiting Kupffer-cell activation. Exp Ther Med 2014; 8: 1777–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Amador A, Grande L, Marti J, et al. Ischemic pre-conditioning in deceased donor liver transplantation: a prospective randomized clinical trial. Am J Transplant 2007; 7: 2180–9. [DOI] [PubMed] [Google Scholar]
- 149.Nasralla D, Coussios CC, Mergental H, et al. A randomized trial of normothermic preservation in liver transplantation. Nature 2018. [DOI] [PubMed] [Google Scholar]
- 150.op den Dries S, Karimian N, Sutton ME, et al. Ex vivo normothermic machine perfusion and viability testing of discarded human donor livers. Am J Transplant 2013; 13: 1327–35. [DOI] [PubMed] [Google Scholar]