Abstract
Objective:
To compare the differential effects of the retinopathy of prematurity (ROP) examination on the physiology of premature infants with and without oxygen support.
Study Design:
We collected data from 42 premature infants (room air = 19, oxygen support = 23) and compared physiological metrics including heart rate (HR), systemic peripheral saturation (SpO2), mesenteric tissue oxygen saturation (StO2) and clinical events (oxygen desaturation episodes, bradycardia events, and gastric residuals).
Results:
We found significant differences between groups in HR during and briefly after the exam, and in mesenteric StO2, during eye drop administration, eye exam, and up to 8 minutes after the exam. SpO2 was significantly different between the groups at all time points. Gastric residuals were higher after the exam in infants on oxygen support, compared to baseline.
Conclusion:
Premature infants on oxygen support may be at a higher risk of adverse physiologic effects in response to the ROP exam.
Introduction
The retinopathy of prematurity (ROP) eye exam is a necessary and routine examination in the NICU that detects and identifies infants at risk for blindness due to retinal detachment (1). Although these eye examinations are the standard of care for the detection of ROP, the potential effect of mydriatic drugs absorbed systemically and the pain and stress associated with the physical manipulation of the eye during the exam (2–4) could result in adverse effects in neonates, despite the administration of oral sucrose and topical anesthetic. Documented adverse effects also include increased apnea events (5,6), and increased incidence of delayed gastric emptying (7). The characteristics of these negative after-effects are currently unknown and only an undetermined subset of the premature infant population present negative complications. The onset, extent, and duration of complications are unclear.
The mydriatic eye drop used at the study site before the ROP exam is composed of cyclopentolate and phenylephrine, both of which dilate the pupils to allow the ophthalmologist to clearly assess ROP progression. Adverse effects, including pain, have been linked to the use of mydriatic eye drops (8). Transient ileus (9), or a temporary arrest of intestinal peristalsis, and necrotizing enterocolitis (10) have also been observed in neonates that have received mydriatic eye drops.
The primary purpose of this prospective pilot study was to compare the physiological effects (heart rate, systemic peripheral oxygenation (SpO2), local mesenteric tissue oxygenation (StO2) and the calculated metric fractional tissue oxygen extraction, FTOE (11)) of the ROP eye exam on two groups of subjects: premature infants receiving oxygen support at least 24 hours before, after and at the time of the eye exam, and premature infants that were on spontaneous room air during the same time period.
Subjects and Methods
We conducted a prospective pilot study at Loma Linda University Children’s Hospital Neonatal Intensive Care Unit to measure the physiological effects of the ROP exam in premature neonates. The Loma Linda University Children’s Hospital Institutional Review Board (IRB) approved the study protocol and informed consent documents. Preterm infants less than 37 weeks estimated gestational age that met the following inclusion criteria from March 2015-September 2017 were considered for enrollment: (i) required the retinopathy of prematurity eye examination, (ii) met the screening guidelines published in the joint statement of the American Academy of Pediatrics and the American Academy Ophthalmology (12) and (iii) parents provided written consent. Exclusion criteria were infants (i) scheduled for laser eye surgery on the day of the examination, (ii) with intraventricular hemorrhage ≥ grade 3 (Papile classification) (13) diagnosed by head ultrasound, (iii) receiving the following medications: morphine, fentanyl, methadone, midazolam, lorazepam, muscle relaxants, phenobarbital, phenytoin, and levetiracetam, (iv) with renal injury defined as plasma creatinine > 1.5 mg/dL and (v) with severe cyanotic congenital heart disease (receiving inotropic support and/or prostaglandin E2 infusion), severe respiratory distress (unstable arterial blood gases) and gastrointestinal dysfunction (showing symptoms of necrotizing enterocolitis).
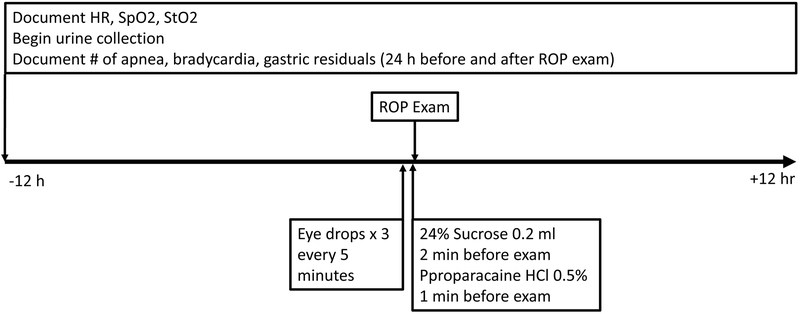
After consent was obtained, we collaborated with the ophthalmology team to schedule the ROP exam. The experimental procedure is described in Figure 1. Before the ROP exam can be performed, the pupils were dilated with mydriatic eye drops. Cyclomydril, the mydriatic drug used in this study, is a combination of cyclopentolate 0.2%, an anticholinergic drug that inhibits the sphincter pupillae muscle and blocks pupil constriction and phenylephrine 1%, an alpha-adrenergic drug that acts on the ciliary muscle to dilate the pupil. Two minutes before the exam, an analgesic, 0.2 mL dose of Sweet-Ease (24% sucrose) was orally administered to the neonate’s buccal mucosa via syringe. One minute before the eye exam, topical anesthetic eye drops (proparacaine HCl 0.5%) are administered into each eye. The eyes are examined one at a time using a binocular indirect ophthalmoscope. A speculum is used to keep the eyelids open while a depressor is used to manipulate the globe into various positions as required by the ophthalmologist. We examined longitudinal changes in heart rate, SpO2, StO2, and FTOE (i) 12 hours before the exam, (ii) during mydriatic eye drop administration, (iii) during the ROP exam, (iv) eight minutes after the ROP exam, and (v) 12 hours after the ROP exam. We also documented the number of apnea and bradycardia events 12 hours before and 12 hours after the ROP exam and gastric residuals 24 hours before and 24 hours after the exam.
Figure 1:
Experimental Protocol Flowchart
We obtained baseline heart rate and SpO2 using the Masimo Radical 7 Pulse Oximeter (Masimo, Irvine CA) and the CASMED FORE-SIGHT (Branfort CT) to collect StO2. FTOE (14) was calculated using the equation below:
where SpO2 is the peripheral oxygen saturation obtained from the Masimo Radical 7 and StO2 is the mesenteric tissue oxygen saturation obtained from the CASMED FORE-SIGHT.
Raw CSV files for StO2, HR and SpO2 were exported after the study period. The Casmed Foresight and the Masimo had a sampling rate of 0.5 Hz, which allowed for the consolidation of both CSV files into one clean file. The timestamps were matched with various phases of the ROP Exam: “Pre-Exam,” “Eyedrops 1,” “Eyedrops 2,” “Eyedrops 3,” “During Exam,” and “After Exam.” Each subject was labeled according to their study ID. The database was configured into a “long” format for proper analysis with these columns: ID, Group, Phase, HR, SpO2, StO2, and FTOE.
The physiological variables were grouped in different epochs as follows: the baseline value is the mean value of every recorded measurement 12 hours before the ROP exam. All other measurements were averaged into one-minute intervals. The “Eyedrop” epoch values represent the three doses of mydriatic eye drops given every five minutes, totaling a 15-minute period. The “Procedure” epoch values reflect the measurements during the ROP exam. The investigator worked with the ophthalmology team to standardize the ROP exam duration at four minutes; and the values were grouped in one-minute averages. The “Post” epoch values represent the eight-minute study period after the ROP exam. See statistical analysis for more information.
We prospectively reviewed the medical record 12 hours before and after the ROP exam for apnea, desaturation, and bradycardia events. Apnea was defined as a cessation of respiration for 20 seconds. Oxygen desaturations were defined as followed: mild (85–89% SpO2), moderate (81–84% SpO2), and severe (≤80% SpO2) (15). Bradycardia was defined as a heart rate less than 80 beats per minute.
Statistical Analysis
RStudio version 1.1.383 (https://www.rstudio.com/), a user-friendly interface to the R statistical language (https://www.r-project.org/) was used for all imputations and analyses. For the short-term statistical analysis (eye drops and up to eight minutes post-exam), the method is as follows. Missing values for this time series data were imputed using the Amelia package (https://cran.r-project.org/web/packages/Amelia/Amelia.pdf) to account for the correlation across time points with appropriate boundary restrictions for each outcome, ensuring that percentages stay within the range of 0 – 100 and pulse rate values remain above 0. Some subjects had no data available for certain outcomes—these were not used in the imputation step and subsequent analyses. Data was averaged into one-minute intervals. Each eye drop period consisted of 5 minutes, the eye exam procedure period consisted of 4 minutes, and up to 8 minutes of post-procedure time was used. Repeated measures analysis of variance (RMANOVA) approach with random intercepts was used to detect the timing of changes in SpO2, StO2, HR, and FTOE values relative to baseline and between the two groups. Time was modeled as a categorical variable to allow for post-hoc tests of individual time points with baseline time. A threshold alpha level of 0.05 was used throughout the analysis. All model assumptions were evaluated for each outcome.
Results
Subject Demographics and Baseline SpO2, StO2, HR, and FTOE
Subjects were grouped based on their oxygen requirement (FiO2). Subjects breathing spontaneously in room air had significantly higher weight and gestational age at the time of birth and at the time of the exam (Table 1). As expected, FiO2 requirement was significantly different 12 hours before, during and 12 hours after the examination (Table 1). There were no significant differences between the groups with regard to Apgar scores, number of ROP exams, ROP stage and zone, gender and ethnicity. Mean Score for Neonatal Acute Physiology with Perinatal Extension-II (SNAPPE-II) (16) scores of subjects receiving oxygen support was higher than those in room air (mean±SD: 22.9 ± 15.2 vs. 15.1 ± 10.9, respectively), but the difference was not statistically significant (P = 0.06).
Table 1:
Subject Demographics and Clinical Data
| On Room Air (n=19) |
Receiving Oxygen (n=23) |
P value# | |
|---|---|---|---|
| Birthweight (g) | 1220 ± 426 | 886 ± 279 | 0.004 |
| Weight at Exam time | 2352 ± 724 | 1804 ± 575 | 0.009 |
| Gestational Age (wks) | 29.5 ± 2.2 | 27.1 ± 2.1 | 0.001 |
| Postmenstrual age | 36.5 ± 2.6 | 34.6 ± 2.8 | 0.030 |
| Apgar-1 min, median (IQR) | 5 (4) | 4 (5) | 0.164++ |
| Apgar-5 min, median (IQR) | 7 (2) | 7 (3) | 0.732++ |
| Gender | Female: 9 Male: 10 |
Female: 8 Male: 15 |
0.408+ |
| SNAPPE II | 15.1 ± 10.9 | 22.9 ± 15.2 | 0.06 |
| Ethnicity | African-American: 6 Asian: 2 Hispanic/Latino: 1 Caucasian: 6 Other/More than one race: 2 Unknown: 2 |
African-American: 1 Asian: 1 Hispanic/Latino: 2 Caucasian: 14 Other/More than one race: 4 Unknown: 1 |
0.29+ |
| Number of ROP exam | 2.2 ± 1.7 | 2.3 ± 1.6 | 0.909 |
| Mode of oxygen support | Spontaneous room air: 19 | CPAP: 4 HFNC:11 Nasal cannula: 3 NAVA: 2 NCPAP: 1 NIMV: 1 NIPPV: 1 |
< 0.001+ |
| Time between the third eye drop and ROP exam (minutes) | 67 ± 48 | 70 ± 27 | 0.775 |
| FiO2 Min 24 hours before exam (%) | 21 ± 0 | 24.0 ± 3.7 | 0.001 |
| FiO2 Max 24 hours before exam (%) | 21 ± 0 | 30.4 ± 9.6 | < 0.001 |
| FiO2 During Exam | 21 ± 0 | 26.3 ± 6.0 | < 0.001 |
| FiO2 Min 24 hours after exam (%) | 21 ± 0 | 23.3. ± 3.9 | 0.017 |
| FiO2 Max 24 hours after exam (%) | 21 ± 0 | 33.6 ± 11.7 | < 0.001 |
Mean ± STD
CPAP: continuous positive airway pressure, HFNC: high flow nasal cannula, NAVA: neutrally adjusted ventilatory assist, NCPAP: nasal continuous positive airway pressure, NIMV: nasal intermittent mandatory ventilation, NIPPV: nasal intermittent positive pressure ventilation
Independent samples t-test
Mann-Whitney
Chi-square
Effect of eye drops and ROP exam on heart rate
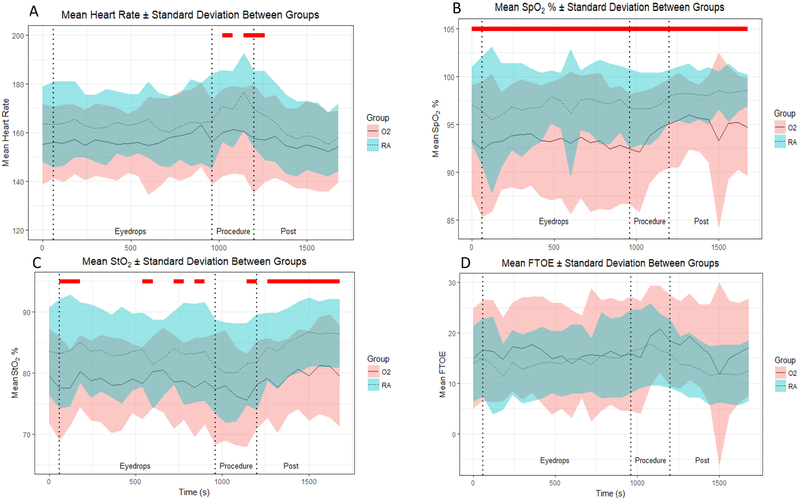
When comparing groups, we found no statistically significant difference in heart rate in response to the first two eyedrops (Figure 2A). However, we found that in neonates receiving oxygen, heart rate significantly increased above baseline one minute after the third eye drop administration, from baseline values of 155.3 ± 16 to 163.1 ± 17, a 5% increase (P < 0.01). This increase in heart rate was sustained throughout the last 4 minutes after the third eye drop. We did not find any sustained change in heart rate compared to baseline in neonates breathing room air.
Figure 2:
Mean and Standard Deviations for A) Heart Rate B) SpO2% C) mesenteric StO2% and D) FTOE. Red lines indicate time points with significant differences between groups. Blue lines indicate start of eye drop administration.
During the ROP exam, we found that heart rate significantly increased in neonates in room air, from baseline values of 163 ± 15 to 177 ± 16 bpm, an 8.5% increase (P < 0.01). In neonates requiring oxygen support, heart rate increased from 155 ± 16 to 161 ± 18 bpm, a 3.2% increase compared to baseline. When comparing groups, we observed significant differences in heart rate during the 2nd and 4th minute of the eye exam, with the difference persisting up to the first minute post eye exam. During the second minute of the exam, subjects on room air had a significantly higher mean heart rate of 171 ± 15 per minute compared to the group receiving oxygen support, whose mean heart rate was 160 ± 18. As the eye exam procedure continued to its fourth and final minute, mean heart rate continued to increase in neonates on room air, while heart rate plateaued in neonates receiving oxygen support. Heart rate returned to baseline within two minutes of completion of eye exam in both groups. There was no significant difference in heart rate between groups during the 12 hours post ROP exam.
Effect of eye drops and ROP exam on SpO2
We found a statistical but not a clinically significant difference in baseline SpO2 values between the two groups (Figure 2B). For neonates receiving oxygen, baseline SpO2 was 93% ± 6. For neonates in the room air group, baseline oxygen saturation was 97% ± 4.0. There were also statistically significant differences in SpO2 between the groups during all three eye drop applications, with the oxygen group having an average range of SpO2 of 92% ± 7 to 94% ± 5, and the room air group having an average range of SpO2 of 95% ± 8 to 98% ± 3. There was also a significant difference in SpO2 between groups during the eye exam. One subject in the oxygen group had a severe oxygen desaturation event (SpO2 below 80%) during the second minute of the ROP exam, which necessitated an increase in FiO2. This subject’s SpO2 normalized immediately and the FiO2 was adjusted back to its previous level within a few seconds of the change. The mean SpO2 of neonates in the oxygen group ranged from 92% ± 6 to 95% ± 5 while the room air group remained at an average SpO2 of 97% ± 4. The difference between groups persisted until the end of our analysis 12 hours after the exam (P < 0.005).
Effect of eye drops and ROP exam on mesenteric StO2
We observed no statistically significant group differences in baseline StO2 (Figure 2C). However, we noted that within two minutes after the third eye drop, StO2 began to decrease significantly in neonates in the oxygen group. This reduction in StO2 was sustained throughout the four minutes after the eye drop and throughout the entire eye exam procedure, with StO2 decreasing to 75% ± 8 in neonates in the oxygen group compared to 81% ± 7 in the room air group (P < 0.01). Group differences remained statistically different during the eight minutes after the eye exam, with mean values ranging from 78% ± 7 to 81% ± 8 in the oxygen group and 82% ± 8 to 86 ± 5 in the room air group. No significant differences in StO2 were observed during the 12-hour period post-exam.
Effect of eye drops and ROP exam on FTOE
As shown in Figure 2D, we found no significant group differences in FTOE at baseline. There were no significant differences in FTOE during all eye drop administrations. FTOE values ranged from 14 ± 11 to 18 ± 10 in the oxygen support group and 11 ± 7 to 16 ± 9 in the room air group. There were also no significant differences between groups during the eye exam. The values ranged from 15 ± 11 to 21 ± 9 in the oxygen group, and the room air group had values ranging from 16 ± 8 to 18 ± 8. There were no significant differences between groups after the eye exam, both in the short-term and the long-term analyses.
Effect of eye drops and ROP exam on apnea, bradycardia, oxygen desaturation, and gastric residuals
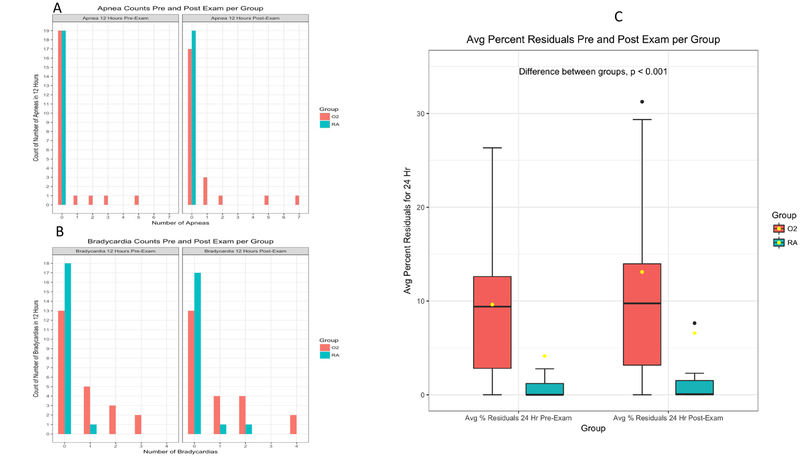
Prospective medical record review twelve hours before and after the ROP exam showed significant differences between groups in the number of bradycardia episodes (p < 0.005). Histograms of the increased apnea and bradycardia events in neonates in the oxygen group compared to neonates on spontaneous room air are shown in Figures 3A and 3B.
Figure 3:
Histogram of A) Apnea and B) Bradycardia Events 12 Hours Before and After the ROP Exam. C) Bar plots of average gastric residuals of both groups 24 Hours Before and After the ROP Exam
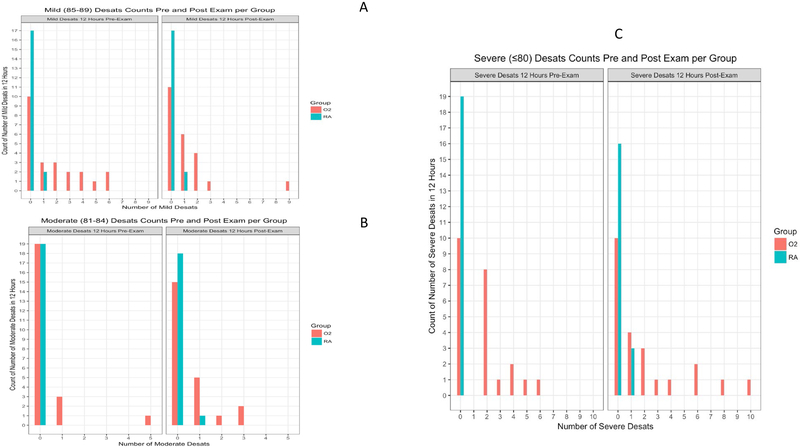
We also reviewed the medical record in the same time span for clinically relevant oxygen desaturations stratified into three different categories: mild (85–89% SpO2), moderate (81–84% SpO2), and severe (≤80% SpO2) (15). The differences between groups were statistically significant for all categories (mild: P < 0.001, moderate: P < 0.023, severe: P < 0.001). The ranges of clinically recorded desaturations in premature infants receiving oxygen support were as follows: before the ROP exam, mild (0 to 6), moderate (0 to 5), severe (0 to 6) and after the ROP exam, mild (0 to 9), moderate (0 to 3), and severe (0 to 10). For premature infants in the room air group: before the ROP exam, mild (0 to 1), moderate (0 to 0), severe (0 to 0) and after the ROP exam, mild (0 to 1), moderate (0 to 1), and severe (0 to 1). Histograms of oxygen desaturations are included in Figure 4A (mild), Figure 4B (moderate), and Figure 4C (severe).
Figure 4:
Histogram of A) Mild Desaturation Events (85-89%), B) Moderate Desaturation Events (81-84%), and C) Severe Desaturation Events (≤80%) 12 Hours Before and After the ROP Exam
The volume of gastric residuals was also prospectively reviewed for up to 24 hours before and after the ROP exam. The average percent residual for subjects before the ROP exam was 4.1± 3.6% for room air subjects and 9.2± 1.7% for oxygen support subjects. After the ROP exam, the average residual for room air subjects increased to 6.6± 5.5% and oxygen support subjects increased to 12.6± 2.9%. The difference between groups was statistically significant (P < 0.01); however, the clinical significance is unclear. See Figure 3C for a box plot of the gastric residual data.
Discussion
It is well documented that the ROP eye examination procedure that includes administration of topical mydriatics can result in signs of pain and stress (2–4) increased apnea events (5,6) and increased incidence of delayed gastric emptying (7). However, it is unclear which groups of infants will present with adverse effects and which component of the eye exam alters normal physiology. Our data suggest that the cardiovascular, pulmonary and gastrointestinal systems of neonates on oxygen are significantly altered by both the mydriatics and the exam. We found that neonates on oxygen responded with tachycardia to eye drop administration, which may be due to higher plasma levels of the muscarinic antagonist, cyclopentolate. This drug was shown by Mitchell et al. (5) to be higher in neonates in oxygen one hour after administration, suggesting increased systemic absorption of the drug in this population. Predictably, higher circulating levels of a drug that inhibits the vagus, administered with an adrenergic agonist, will cause an increase in heart rate. The relationship between oxygen support and higher plasma cyclopentolate requires further investigation, which may include a prolonged half-life and differences in drug metabolism and clearance. An investigation and/or standardization of the method by which eyedrops are administered is also needed to ensure that each neonate receives the full dose. Because neonates on oxygen were shown to have higher plasma levels of cyclopentolate, a re-evaluation of the dose of cyclopentolate given to this group is also needed.
Interestingly, we found that neonates on oxygen had a minimal response to the ROP exam itself, a 3.2% increase compared to neonates in room air, whose heart rate increased by 8.5% compared to baseline. The greater increase in heart rate in neonates on room air may be in response to scleral depression, especially if the ROP was in a zone that was difficult to visualize. Although we did not find any statistical difference in ROP stage or zone between the two groups, the subjects in room air had a higher gestational age and weight, with exams performed at an older age. The tachycardic response of these older neonates may be due to greater patient movement during the exam, a stiffer and thicker sclera, and the need for the ophthalmologist to use greater pressure during scleral depression. The larger increase in heart rate in neonates on room air may also be due to a nociceptive response to the exam, since the efficacy of proparacaine is not well-established in infants. Marsh et al. noted that although pain scores were lower in neonates who received proparacaine, 68% of these neonates still had a pain score of more than 10, which suggest moderate pain (17). Although the eye examination involves manipulation of the conjunctival fornix and touching the cornea is avoided, it is well-documented that the cornea is 300–600 times more sensitive to pain than the skin (18). It is unclear why the heart rate response was muted in the oxygen group, especially because of the well-documented correlation between chemoreceptors and autonomic activity (19). This diminished response may be indicative of this group’s limited ability to respond to an increase in allostatic load (20,21). It is also possible that the smaller, younger babies on oxygen required less scleral depression, and therefore had less pain. No infants experienced immediate bradycardia at the time of speculum insertion or scleral depression. This suggests that the oculocardiac reflex was not seen during the time of exam in either group. Heart rate returned to baseline soon after the eye exam in the oxygen group but dipped below baseline in the room air group.
In response to eye drop administration, neonates on oxygen had significant reductions in mesenteric StO2 compared to baseline. This may be also be an effect of the mydriatic drug cyclomydril, which inhibits the parasympathetic nervous system (cyclopentolate) and stimulates the sympathetic nervous system (phenylephrine), resulting in the reduction of gastric and duodenal motility (19) and a delay gastric emptying (7). Specifically, sympathetic nervous system activation hyperpolarizes enteric nervous system neurons (19). This decreases the frequency of phasic contractions of smooth muscle cells, thus decreasing gut motility. Akotia et al. has shown that mesenteric StO2 directly correlates with peristaltic motility as measured by transabdominal ultrasonography (22). Specifically, high StO2 values were associated with normal or hyperactive intestinal motility, and lower StO2 values were associated with slower intestinal motility.
Additionally, the reduction in StO2 in neonates on oxygen during the ROP exam may be due to sympathetic nervous system activation from the pain and stress of the exam, shown to still be present after proparacaine administration (17). Systematic reviews examining the analgesic effect of proparacaine report inconsistent results (23–25). Proparacaine only blocks mechano-nociception within the eye and not other types of nociceptors such as polymodal nociceptors (which respond to heat, irritation, and inflammation) or cold thermo-nociceptors (which respond to moderate temperature changes) (26). Furthermore, older neonates of the room air group that may have a more rigid sclera may require a greater magnitude of scleral depression, causing additional discomfort (27). Though the sources of nociception related to the eye exam are currently unknown, the presence of pain is reflected in the continuous stepwise increase in heart rate (sinoatrial beta-adrenergic activation) and a significant reduction in StO2 (alpha-adrenergic activation) during the exam.
Despite the significant effects of eye drops and ROP exam on heart rate and StO2, we found no statistical or clinically significant difference in SpO2 between the oxygen group and the room air group. We did not observe any statistical or clinically significant change in SpO2 during eye drop administration and ROP eye exam compared to baseline. Mean SpO2 remained above 90% in both groups before, during and after the procedure. This finding highlights the reduced utility of peripheral oxygen saturation monitoring in determining regional and systemic effects of the ROP eye exam.
We also found that neonates in oxygen had significantly higher bradycardia, oxygen desaturation and gastric residuals. It is unclear which specific component of the procedure resulted in these findings. The change in gastric residuals may be due to the mydriatic drugs and the ROP exam, which jointly enhanced sympathetic nervous system activity to enteric neurons. The higher number of oxygen desaturation events can alter adenosine triphosphate synthesis (28) which can increase the open probability of ATP-dependent potassium channels (KATP), hyperpolarizing sino-atrial nodal cells and reducing heart rate (19).
Limitations
The major limitation of this study is the small sample size. Future studies are needed, incorporating larger sample sizes that follow subjects through multiple eye examinations. Another limitation is the intermittent loss of signal in near infrared spectroscopy (NIRS) as well as some sporadic movement artifacts appearing during the ROP exam itself. This limitation was addressed through data imputation, as described under “Statistical Analysis.” In addition, it is clear that subject characteristics are different between the two groups. This highlights the relevance and specificity of our data, which suggest that neonates on oxygen (with its accompanying characteristics such as decreased weight and gestational age) are more vulnerable to the cardiovascular, pulmonary and gastrointestinal effects of a stressful procedure such as the ROP exam. Lastly, this study relied on the medical record for documentation of apnea, bradycardia and oxygen desaturations events, as well as gastric residuals volume. Although nurses were trained on how to clearly identify and document these variables, the number of nurses involved in the study may increase the variability of the data.
Conclusion
Our findings suggest that neonates on oxygen undergoing the ROP exam are at higher risk for alterations in heart rate and regional StO2 as well as increased apnea, bradycardia and gastric residuals compared to neonates in room air. This difference may be due to the systemic effects of cyclomydril as well as scleral manipulation. Future studies are needed to formulate an evidence-based plan of care for this group of infants. Specifically, the correct dose and type of mydriatics must be determined. In addition, eye drop administration should be standardized to ensure that the full dose is administered. The need for removal of residual eye drops should be explained and instituted, to prevent systemic absorption. The timing and reinstitution of oral feeding must be closely evaluated, especially in neonates on oxygen, and must be balanced with the neonate’s nutritional needs. Additionally, risk factors that can further increase cardiorespiratory events need to be examined in a study with a larger sample size. These factors include the effect of serial exams and the degree of difficulty of the ROP exam, repetitive and cumulative pain before and after the exam, fatigue/energy deficit and the neonate’s inability to self-regulate. In addition, interventions that may limit the occurrence of adverse effects during and after the ROP exam, such as analgesics, positioning, sensory stimulation (29), and kangaroo care (30–32) need to be investigated.
Acknowledgement:
We thank Dorothy Forde RN, Erin Hoch RN, Elena Kim-Saesim RN, and Sharon Lee RN for identifying and consenting subjects. We thank the LLU NICU physicians and nurses for their support. We thank the parents for allowing their babies to be part of this study.
Funding: This work was supported in part by NIH grant R01 NR011209 (Dr. Angeles)
Footnotes
Conflict of Interest: The authors declare no conflict of interest.
Works Cited
- 1.Hellström A, Smith LEH, Dammann O. Retinopathy of prematurity. Lancet. 2013. October 26;382(9902):1445–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Grabska J, Walden P, Lerer T, Kelly C, Hussain N, Donovan T, et al. Can oral sucrose reduce the pain and distress associated with screening for retinopathy of prematurity? J Perinatol Off J Calif Perinat Assoc. 2005. January;25(1):33–5. [DOI] [PubMed] [Google Scholar]
- 3.Belda S, Pallás CR, De la Cruz J, Tejada P. Screening for retinopathy of prematurity: is it painful? Biol Neonate. 2004;86(3):195–200. [DOI] [PubMed] [Google Scholar]
- 4.Şener Taplak A, Erdem E. A Comparison of Breast Milk and Sucrose in Reducing Neonatal Pain During Eye Exam for Retinopathy of Prematurity. Breastfeed Med Off J Acad Breastfeed Med. 2017. June;12:305–10. [DOI] [PubMed] [Google Scholar]
- 5.Mitchell A, Hall RW, Erickson SW, Yates C, Hendrickson H. Systemic Absorption of Cyclopentolate and Adverse Events After Retinopathy of Prematurity Exams. Curr Eye Res. 2016. December;41(12):1601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid B, Wang H, Guillet R. Apnea after Routine Eye Examinations in Premature Infants. Am J Perinatol. 2017. January;34(2):199–203. [DOI] [PubMed] [Google Scholar]
- 7.Bonthala S, Sparks JW, Musgrove KH, Berseth CL. Mydriatics slow gastric emptying in preterm infants. J Pediatr. 2000. September;137(3):327–30. [DOI] [PubMed] [Google Scholar]
- 8.Cohen AM, Cook N, Harris MC, Ying G-S, Binenbaum G. The pain response to mydriatic eyedrops in preterm infants. J Perinatol Off J Calif Perinat Assoc. 2013. June;33(6):462–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degirmencioglu H, Oncel MY, Calisici E, Say B, Uras N, Dilmen U. Transient ileus associated with the use of mydriatics after screening for retinopathy of prematurity in a very low birth weight infant. J Pediatr Ophthalmol Strabismus. 2014;51 Online:e44–47. [DOI] [PubMed] [Google Scholar]
- 10.Ozgun U, Demet T, Ozge KA, Zafer D, Murat S, Mehmet Y, et al. Fatal necrotising enterocolitis due to mydriatic eye drops. J Coll Physicians Surg--Pak JCPSP. 2014. May;24 Suppl 2:S147–149. [PubMed] [Google Scholar]
- 11.Cortez J, Gupta M, Amaram A, Pizzino J, Sawhney M, Sood BG. Noninvasive evaluation of splanchnic tissue oxygenation using near-infrared spectroscopy in preterm neonates. J Matern-Fetal Neonatal Med Off J Eur Assoc Perinat Med Fed Asia Ocean Perinat Soc Int Soc Perinat Obstet. 2011. April;24(4):574–82. [DOI] [PubMed] [Google Scholar]
- 12.Ophthalmology AAOPS on, Ophthalmology AAO, Strabismus AA for POA, Orthoptists AA of C. Screening Examination of Premature Infants for Retinopathy of Prematurity. Pediatrics. 2013. January 1;131(1):189–95. [DOI] [PubMed] [Google Scholar]
- 13.Papile L-A, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1,500 gm. J Pediatr. 1978. April 1;92(4):529–34. [DOI] [PubMed] [Google Scholar]
- 14.Naulaers G, Meyns B, Miserez M, Leunens V, Van Huffel S, Casaer P, et al. Use of tissue oxygenation index and fractional tissue oxygen extraction as non-invasive parameters for cerebral oxygenation. A validation study in piglets. Neonatology. 2007;92(2):120–6. [DOI] [PubMed] [Google Scholar]
- 15.Thoyre SM, Carlson J. Occurrence of oxygen desaturation events during preterm infant bottle feeding near discharge. Early Hum Dev. 2003. May;72(1):25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harsha SS, Archana BR. SNAPPE-II (Score for Neonatal Acute Physiology with Perinatal Extension-II) in Predicting Mortality and Morbidity in NICU. J Clin Diagn Res JCDR. 2015. October;9(10):SC10–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marsh VA, Young WO, Dunaway KK, Kissling GE, Carlos RQ, Jones SM, et al. Efficacy of topical anesthetics to reduce pain in premature infants during eye examinations for retinopathy of prematurity. Ann Pharmacother. 2005. May;39(5):829–33. [DOI] [PubMed] [Google Scholar]
- 18.Yang AY, Chow J, Liu J. Corneal Innervation and Sensation: The Eye and Beyond. Yale J Biol Med. 2018. March;91(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- 19.Boron WF, Boulpaep EL, editors. Medical physiology [Internet]. Third edition. Philadelphia, PA: Elsevier; 2017. [cited 2018 Apr 8]. 1297 p. Available from: https://www.clinicalkey.com/#!/browse/book/3-s2.0-C20110061677 [Google Scholar]
- 20.Tan JBC, Boskovic DS, Angeles DM. The Energy Costs of Prematurity and the Neonatal Intensive Care Unit (NICU) Experience. Antioxidants. 2018. March 2;7(3):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998. May 1;840:33–44. [DOI] [PubMed] [Google Scholar]
- 22.Akotia DH, Durham JT, Arnell KM, Petruzzelli DL, Katheria AC. Relationship Between Near-Infrared Spectroscopy and Transabdominal Ultrasonography: Noninvasive Monitoring of Intestinal Function in Neonates. Med Sci Monit Int Med J Exp Clin Res. 2016. January 6;22:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun X, Lemyre B, Barrowman N, O’Connor M. Pain management during eye examinations for retinopathy of prematurity in preterm infants: a systematic review. Acta Paediatr Oslo Nor 1992. 2010. March;99(3):329–34. [DOI] [PubMed] [Google Scholar]
- 24.Kandasamy Y, Smith R, Wright IMR, Hartley L. Pain relief for premature infants during ophthalmology assessment. J AAPOS Off Publ Am Assoc Pediatr Ophthalmol Strabismus. 2011. June;15(3):276–80. [DOI] [PubMed] [Google Scholar]
- 25.Samra HA, McGrath JM. Pain management during retinopathy of prematurity eye examinations: a systematic review. Adv Neonatal Care Off J Natl Assoc Neonatal Nurses. 2009. June;9(3):99–110. [DOI] [PubMed] [Google Scholar]
- 26.Belmonte C, Acosta MC, Merayo-Lloves J, Gallar J. What Causes Eye Pain? Curr Ophthalmol Rep. 2015;3(2):111–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trevino R, Stewart B. Change in intraocular pressure during scleral depression. J Optom. 2015. December;8(4):244–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Estévez-Herrera J, González-Santana A, Baz-Dávila R, Machado JD, Borges R. The intravesicular cocktail and its role in the regulation of exocytosis. J Neurochem. 2016;137(6):897–903. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Gonzalez F, Mu D. Apnea of prematurity: from cause to treatment. Eur J Pediatr. 2011. September;170(9):1097–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ludington-Hoe SM, Anderson GC, Swinth JY, Thompson C, Hadeed AJ. Randomized controlled trial of kangaroo care: cardiorespiratory and thermal effects on healthy preterm infants. Neonatal Netw NN. 2004. June;23(3):39–48. [DOI] [PubMed] [Google Scholar]
- 31.Hunt F The importance of kangaroo care on infant oxygen saturation levels and bonding. J Neonatal Nurs. 2008. April;14(2):47–51. [Google Scholar]
- 32.Bloch-Salisbury E, Zuzarte I, Indic P, Bednarek F, Paydarfar D. Kangaroo care: cardio-respiratory relationships between the infant and caregiver. Early Hum Dev. 2014. October 29;90(12):843–50. [DOI] [PubMed] [Google Scholar]