Abstract
Background/Aims:
Enucleation for retinoblastoma is performed less often in the past decade due to increasingly widespread alternative therapies, but enucleation remains an important option. There is a paucity of reports on the current incidence of metastases and metastatic deaths in unilateral retinoblastoma from United States (US) centers.
Methods:
Retrospective chart review at 5 tertiary retinoblastoma centers in the US for unilateral retinoblastoma patients treated with primary enucleation, 2007–2017, with ≥1 year of follow-up or treatment failure.
Results:
Amongst 228 patients (228 eyes), there were 9 metastases (3.9%) and 4 deaths (1.7%). The Kaplan-Meier estimate at 5 years for metastasis-free survival was 96% (95% confidence interval (CI), 94%−99%), and for overall survival was 98% (95% CI 96%−100%). All metastases were evident within 12 months. Histopathology revealed higher risk pathology (post-laminar optic nerve and/or massive choroidal invasion) in 62 of 228 eyes (27%). Of these higher risk eyes, 39 received adjuvant chemotherapy. There were 4 subsequent metastases in this higher risk pathology with adjuvant chemotherapy group, with 3 deaths. Of the 9 overall with metastases, 7 (78%) showed higher risk pathology. All metastatic patients were classified as Reese-Ellsworth V and International Classification of Retinoblastoma Groups D or E. Initial metastases presented as orbital invasion in 7 of 9 cases.
Conclusions:
Primary enucleation for unilateral retinoblastoma results in a low rate of metastatic death, but is still associated with a 3.9% chance of metastases within a year of enucleation. Most but not all patients who developed metastases had higher risk histopathologic findings.
Synopsis /Precis:
Contemporary metastases and death rates in unilateral retinoblastoma after primary enucleation within the United States remain excellent. Five-year Kaplan-Meier estimates: metastasis-free survival 96% (95% confidence interval (CI), 94%−99%), and overall survival 98% (95% CI 96%−100%).
INTRODUCTION:
Enucleation has been performed for unilateral and bilateral retinoblastoma for more than 400 years.[1–2] Survival after enucleation for retinoblastoma was 5% in 1869,[3] 17% in 1897,[4] and 57% in 1916.[5] The mid-1900s saw further advances that allowed some unilateral retinoblastomas to be managed without primary enucleation.[2] In this period there were reported survival rates of 63%[6] by Reese and 70%[7] by Ellsworth. By the late-1900s 5-year retinoblastoma survival in the United States (US) reached 92%.[8] Eventually, the introduction of advanced globe sparing therapies allowed some unilateral eyes to be salvaged, often with useful vision and without compromising high patient survival rates.[9] Enucleation is still performed in every retinoblastoma center worldwide. Despite the ongoing importance of enucleation, there has been a lack of recent literature from the US on present day incidence of metastases and metastatic deaths following surgery.
Survival rates in the US have increased from the 1970s to 2010, with 5-year overall survival reported as 93.7% for 1975–1979, 93.7% for 1980–1989, 97.5% for 1990–1999, and 97% for 2000–2010.[10] Unfortunately, many such studies in the literature suffer from limited methodology, including drawing data from the Surveillance Epidemiology and End Results (SEER) datasets.[10–11] The SEER database has gaps, specifically from several regions of the country with high volume referral centers and does not allow for making detailed patient subsets.
A potentially more nuanced picture of modern enucleation outcomes can be patched together through reports from single institutions. For example, from 1995–2015 in Los Angeles, there was a subset of 206 primary enucleations (mix of unilateral and bilateral) with a 0.9% metastasis rate and 0.9% death rate.[12] While in New York from 2006–2014 the subset that underwent primary enucleation had a metastasis rate of 10% and death rate of 3% in 60 enucleations.[13]
Survival rates are lower for eyes with higher risk histopathology demonstrating invasive retinoblastoma beyond the lamina cribrosa of the optic nerve and/or massively into the choroid. Three studies on eyes with higher risk histopathology from Philadelphia include, first, a report on 80 enucleations from 1974–1999 with a higher metastatic rate for those who did not receive adjuvant chemotherapy (24%) compared to those who received chemotherapy (4%).[14] Second, a report on 519 enucleations between 1975–2011, with 117 showing higher risk histopathology. The overall metastasis rate was 8% and death rate 4%, with all metastasis/death events occurring only in those eyes with higher risk features.[15] And third, a study revealing post-enucleation adjuvant chemotherapy with vincristine etoposide, and carboplatin for high-risk retinoblastoma was effective in preventing metastasis in every case.[16]
Overall, these discussed reports and the broader literature show heterogeneity of research and clinical methodology. Additionally, unilateral enucleation outcomes are often merely incidental to recent studies’ primary purposes, and so they often lack Kaplan-Meier survival curves. Due to these limitations in outcome data, we designed this multi-center study within the United States as a retrospective cohort study of unilateral retinoblastoma treated with primary enucleation between 2007–2017. The results are intended to inform practitioners of the contemporary outcomes in the current patient population in which primary enucleation is performed.
MATERIALS AND METHODS:
Invitations to participate in this retrospective chart review study were sent to 17 centers in the US in 2017, and ultimately five centers agreed to participate: Memorial Sloan-Kettering Cancer Center (MSKCC), Children’s Hospital Los Angeles (CHLA), Wills Eye Hospital at Thomas Jefferson University, the Casey Eye Institute at Oregon Health & Science University (OHSU), and Vanderbilt University Medical Center. The institutional review boards (IRB) of each participating institution approved the study. Of the 12 invited centers that were not included, 3 responded that they did not have the time to take part in further projects, 2 others initially agreed to participate but were unable to have the data by collection deadline, and the rest did not reply. Final study size was based on all available patients that were submitted by each institution.
The patient data collected across centers were cases of unilateral retinoblastoma treated with primary enucleation at the reporting center July 2007 - July 2017. While the study design did not explicitly limit the reasons and indications for enucleation, all centers reported similar criteria. The available indications for primary enucleation across centers are: large Group D or E tumors without extraocular disease with patient/family informed preference for primary enucleation. Additionally it may be considered if there is limited view of fundus from media opacity including hemorrhage or corneal edema, advanced glaucoma, buphthalmos, or concern for optic nerve and/or uveal invasion on imaging.
Clinical characteristics including age at presentation, sex, laterality of disease, duration of follow-up, metastasis and survival status at follow-up, cause of death, pathology, and treatment details were collected via the medical record. This retrospective study included data collected before TNM was available. Centers used a variety of classification schemes: Memorial Sloan Kettering used the Children’s Oncology Group (COG) version,[17] CHLA used the Murphree version,[18] and Wills Eye Vanderbilt, and OHSU used the Philadelphia classification.[19]
The definition of higher risk pathology was retrolaminar optic nerve invasion and/or massive choroidal invasion. At initial data collection, disease at cut section was also defined as higher risk pathology, and was specifically noted if present.
The adjuvant chemotherapy regimen for high-risk enucleation pathology was based on the previously described Children’s Oncology Group protocol ARET0332.[20] Specifically: 6 cycles of vincristine, carboplatin, and etoposide. This was the standardized choice all participating institutions.
The systemic chemotherapy regimen for diagnosed metastases was based on the previously described Children’s Oncology Group protocol ARET0321.[21] Specifically: 4 cycles of vincristine, cisplatin, cyclophosphamide, and etoposide for all stages. Additionally there was external beam radiation for stage 2 and 3. There was also stem cell harvest, consolidation chemotherapy, and then possible external beam radiation for stage 4. This was the regimen of choice for any center in which retinoblastoma metastases were relevant, though one center noted dosing and other adjustments could be made at the discretion of the pediatric oncologist.
Eyes that received any prior treatments including systemic, intraarterial, or intravitreal chemotherapy or radiotherapy prior to enucleation were excluded from the analysis. Other exclusion criteria included follow-up time less than 1 year (unless metastasis occurred in under a year), metastasis present at the time of primary enucleation, and bilateral retinoblastoma.
A metastatic event was defined as the diagnosis of extraocular retinoblastoma following enucleation. Death events were defined as death directly from retinoblastoma; this definition excludes death from second cancers, treatment related toxicities, or any other causes. Deaths due to any other causes, if any, were also collected separately and noted in discussion.
Statistical Analysis:
Overall survival and metastases free survival odds were calculated with the Kaplan-Meier method. The subgroup analysis of proportion of metastases, with or without adjuvant chemotherapy, was calculated with the Chi-squared test.
If there was missing data, such as tumor classification due to different institutions using different classification systems in Table 1, the data was presented as absolute number of available but percentages were based on total patients.
Table 1:
Patient and Tumor Characteristics
| Total patients (n=228) | Metastasis (n=9) | Death (n=4) | |
|---|---|---|---|
| Age of Diagnosis (months) | |||
| Mean | 29 | 28 | 30 |
| Range | 1–127 | 19–40 | 20–40 |
| Gender: number (% of total) | |||
| Male | 104 (46%) | 4 (1.7%) | 1 (0.4%) |
| Female, number (% of total) | 124 (54%) | 5 (2.1%) | 3 (1.3%) |
| Laterality: number (% of total) | |||
| Right | 117 (51%) | 5 (2.1%) | 2 (0.8%) |
| Left | 111 (49%) | 4 (1.7%) | 2 (0.8%) |
| Follow-Up Time (months) | |||
| Mean | 60 | 35 | 25 |
| Range | 5–135 | 5–95 | 5–57 |
| Eye Classification at Diagnosis | |||
| Children’s Oncology Group: Number (% with classification) | |||
| D | 7 (3%) | 1 (0.4%) | 0 |
| E | 29 (13%) | 5 (2.1%) | 2 (0.8%) |
| Murphree: Number (% with classification) | |||
| D | 26 (11%) | 0 | 0 |
| E | 61 (27%) | 0 | 0 |
| Philadelphia: Number (% with classification) | |||
| C | 1 (0.4%) | 0 | 0 |
| D | 1 (0.4%) | 0 | 0 |
| E | 66 (29%) | 2 (0.8%) | 2 (0.8%) |
| Reese-Ellsworth: Number (% with classification) | |||
| V | 113 (49%) | 9 (3.9%) | 4 (1.7%) |
| Pathology: Number (% with pathology) | |||
| Anterior segment invasion | 9 (4%) | 1 (0.4%) | 1 (0.4%) |
| Ciliary body invasion | 0 | 0 | 0 |
| Massive Choroidal Invasion | 31 (14%) | 4 (1.7%) | 3 (1.3%) |
| Invasion into nerve, prelaminar | 69 (30%) | 1 (0.4%) | 0 |
| Invasion into nerve, postlaminar | 43 (19%) | 4 (1.7%) | 2 (0.8%) |
| Invasion into nerve, at cut section | 0 | 0 | 0 |
| Negative, none of the above | 95 (42%) | 1 (0.4%) | 0 |
| Unavailable | 1 (0.4%) | 0 | 0 |
All statistics were done with the software “R: The R Project for Statistical Computing” (v 3.3.1 GUI 1.68 Mavericks Build 7238) by R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. Graphing was also performed with the R Project software.
RESULTS:
Λ total, F228 patients (228 eyes) met the study ctitetia at the following centers: 48 patients from the Ocular Oncology Service at Memorial Sloan Kettering Cancer Center in New York, 77 patients from Wills Eye Hospital at Thomas Jefferson University in Philadelphia, 65 patients from Children’s Hospital Los Angeles, 26 patients from the Casey Eye Institute at OHSU, and 12 from Vanderbilt University Medical Center. None of the included patients had signs of metastases at diagnosis, as determined by treating ophthalmologist. All centers use magnetic resonance imaging (MRI) of brain and orbit as screening. Patients also receive thorough pediatric oncologist evaluation and standard blood work. Lumbar puncture and bone marrow biopsy are not regularly used at any of the participating centers, unless particularly high-risk. Baseline characteristics of patients and the tumors are presented in Table 1.
Metastasis developed in 9 patients (3.9%). The mean time from initial diagnosis to metastasis was 6.5 months (range 2–12 months) with metastatic events occurring at 2, 4, 4, 5, 5, 7, 7, 12, and 12 months after diagnosis. The mean age at diagnosis of retinoblastoma in this subgroup was 27.8 months (range 19.0–40.5 months), and mean age of metastasis in this subgroup was 34 months (range 24–48 months of age).
Pathology results In the overall group showed 62 of 228 eyes (27%) had a higher risk pathology (retrolaminar optic nerve invasion and/or massive choroidal invasion). Seven of 228 (3%) had both higher risk findings. Disease at cut section was also defined as higher risk pathology but there were no cases in this study.
Of the 62 higher risk pathology eyes, 39 received adjuvant chemotherapy (63%). Metastases developed in 4 of 39 (10.3%) higher risk eyes with adjuvant chemotherapy, with metastatic retinoblastoma deaths in 3 of 4 of these patients. Comparatively, metastases developed in 3 of 23 (13%) higher risk eyes that did not receive adjuvant chemotherapy (p=0.112, χ2). The reasoning for lack of adjuvant chemotherapy despite higher risk pathology in this group was collected for the 3 patients with metastases. The reason was family preference in all cases. Metastases developed in 0 of 10 eyes without higher risk features that still received adjuvant chemotherapy. Of the remaining 2 with metastasis, there were no higher risk features as the retinoblastoma had invaded prelaminar optic nerve in one case and the other case showed no invasion at all.
Seven of the nine cases of metastases involved the orbit; in 2 cases there was concurrent central nervous system (CNS) disease, one had concurrent marrow disease and one had concurrent CNS and marrow disease. In two cases the initial metastases were to CNS only. In these two cases, both had higher risk pathology (one with massive choroidal invasion, and one with both massive choroidal and retrolaminar invasion), and both had received adjuvant chemotherapy.
Amongst the 9 patients that developed metastases, 8 of them were treated with systemic chemotherapy for metastases. The 9th patient was not treated for metastases due to parental objection to administration of systemic chemotherapy. Additional therapy in the 8 patients treated for metastases with chemotherapy included: 2 patients with orbital radiation, 1 patient with orbital radiation plus stem cell transplant, 1 patient with additional intrathecal chemotherapy plus transplant, and 2 with stem cell transplant alone. Table 2 summarizes clinical outcomes and further detailed characteristics of patients that had metastases and/or death.
Table 2:
Metastatic Event Characteristics
| Metastasis Event | Follow Up (months) | Pathology: | Adjuvant Chemotherapy | Time from Diagnosis to Metastasis (months) | Location of Metastases: | Treatment of Metastases: |
|---|---|---|---|---|---|---|
| 1 | 44 | Into nerve - prelaminar | No | 5 | Orbit | Systemic Chemotherapy + Orbital Radiation |
| 2 | 66 | Massive Choroidal Invasion | No | 4 | Orbit, Marrow | Systemic Chemotherapy + Orbital Radiation + Transplant |
| 3 | 95 | Negative | No | 4 | Marrow | Systemic Chemotherapy + Transplant |
| 4 | 19 | Into nerve - postlaminar | No | 5 | Orbit, Marrow | Systemic Chemotherapy |
| 5 | 30 | Into nerve - postlaminar | Yes | 12 | Orbit, CNS | Systemic Chemotherapy + Orbital Radiation |
| 6* | 12 | Massive Choroidal Invasion | Yes | 7.2 | CNS | Systemic Chemotherapy |
| 7* | 5 | Massive Choroidal Invasion | No | 2 | Orbit, CNS, Marrow | None |
| 8* | 57 | Anterior Segment Invasion, Into nerve - postlaminar | Yes | 12 | Orbit, CNS | Systemic Chemotherapy + Transplant |
| 9* | 26 | Choroidal Invasion, Into nerve - postlaminar | Yes | 7 | CNS | Systemic Chemotherapy + Intrathecal Chemotherapy + Transplant |
Denoting patient death
Death was reported in 4 patients, all were from the subset that had metastatic disease, and all deaths were related to metastatic retinoblastoma. The mean time from metastasis to death was 18 months, with deaths occurring at 2.8 months, 4.9 months, 19 months, and 45 months after diagnosis of metastasis. The mean follow-up from initial diagnosis of retinoblastoma to death was 25 months (range 5–57 months). For the 5 of 9 patients with metastases that survived, the average follow-up was 45 months (range 14–91 months) following the development of metastases.
The causes of death were related to leptomeningeal metastases in one patient, central nervous system metastases in a second, and two other metastatic deaths without specification. No deaths were attributed to chemotherapy toxicity or secondary malignancy. Three of four patients who died of metastases had initially undergone chemotherapy for higher risk pathology after enucleation (adjuvant), as well as systemic chemotherapy later for metastases.. In the fourth case (metastatic event #9 in Table 2), the family refused both adjuvant chemotherapy for high-risk pathology and systemic chemotherapy for metastatic disease.
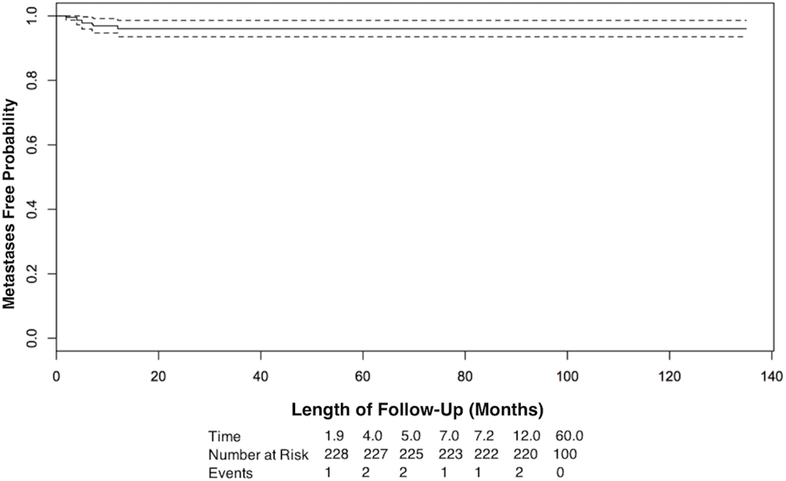
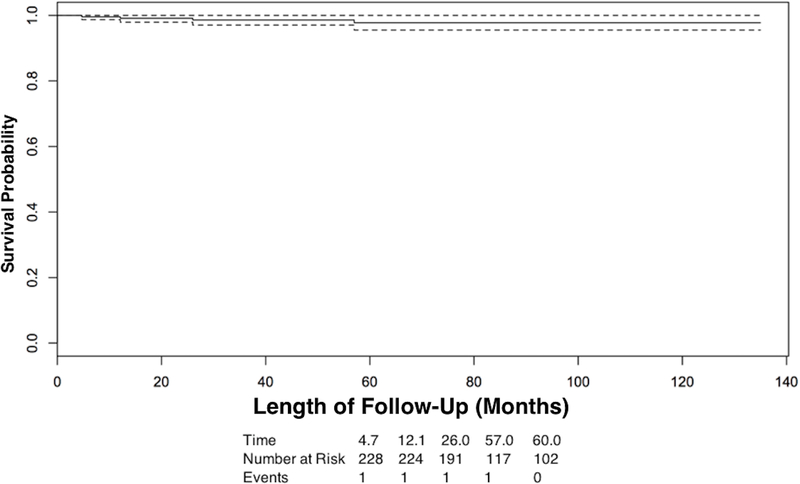
The Kaplan-Meier (KM) estimate of metastasis-free survival (Figure 1) was 96% at 5 years (95% confidence interval (CI), 94%−99%), and for overall survival (Figure 2) was 98% at 5 years (95% CI 96%−100%).
Figure 1:
Kaplan-Meier survival curve of metastasis-free survival over time of follow-up. The dotted line is the 95% confidence interval.
Figure 2:
Kaplan-Meier survival curve of metastasis-specific survival over time of follow-up. The dotted line is the 95% confidence interval.
DISCUSSION:
In this multi-center retrospective study of unilateral retinoblastoma treated primarily with enucleation, the primary outcomes of metastases and death rates continue to be favorable for patients, and consistent with previously published US and international literature. All of the metastases occurred within 12 months, and deaths occurred within 5 years of enucleation as previously described.[22] Seven of nine patients who developed metastases had orbital disease as the first site of metastasis, and the other two had CNS disease at metastasis.
Of the reported metastatic events, it is notable that there were significantly more metastases in the higher risk pathology group (7/62, 11%) versus the number of metastases in patients without higher risk pathology (2/166, 1%) (p value= 0.002). Four of four retinoblastoma deaths had 1 or more higher risk pathologic findings, and 3 of 4 died despite having received adjuvant chemotherapy for higher risk features. It is also notable that most of the patients who went on to have metastases developed orbital involvement. This reinforces a potential role for MRI screening for a period after enucleation. Participating centers do use repeat MRI screenings. Current imaging techniques can be helpful to distinguish between postsurgical contrast enhancement and orbital tumor recurrence.[23]
Among participating centers there were 3 different classification systems for retinoblastoma, the COG, and 2 versions of the International Classification of Retinoblastoma (Murphree and Philadelphia). This reinforces that there is still no widely accepted consensus regarding retinoblastoma classification.[24] While this ultimately did not affect our primary analysis of survival as it was independent of eye classification, the heterogeneity of systems may make other types of future studies more challenging as there is low but notable rate of inconsistencies between classification systems.[25] Interestingly all the patients with metastases were Reese Ellsworth V and all but one were Group E. There is also no consensus regarding indications for globe salvage therapy among these centers, though in each of these centers unilateral enucleation is being performed less often than in the past.[13]
The patients in this combined series include several of the largest retinoblastoma centers in the US, and international patients enucleated in these US centers were included. Evaluating the rate of metastases and deaths after secondary enucleation in the US is a worthwhile future endeavor. Additionally, studies such as ours for the US would be valuable to conduct internationally. A global epidemiological study of 20th and 21st century data showed the estimate of mortality varied as much as 3% in Japan and North America, to 70% in Africa.[26] There are other studies from the worldwide literature that separated their unilateral and bilateral data. This includes a 5-year survival of 97%[27] for English, Scottish, and Welsh Children with enucleated unilateral retinoblastoma from 19982002, and 94%[28] for Iranian children with enucleated unilateral retinoblastoma at a national referral center from 2001–2007. Global data for e nucleation outcomes is especially interesting in the context of differing classification systems, chemotherapy indications/regimens, and access to globe-salvaging therapies.
The results are not meant to compare enucleation against other treatment options or historic data. The proliferation of alternatives to enucleation, as previously discussed, may create selection bias for the type of patients who still require primary enucleation. Specifically, the primary enucleation patients may be the most advanced cases at each center. Another possible source of bias are patients that travel to a center of retinoblastoma excellence, then return to their home state or country for subsequent follow-up. To reduce this bias, we required 12 months of follow-up for inclusion. Overall, the changing trends in retinoblastoma care could make the study group different than historic populations. As shown in the results, the patients undergoing primary enucleation tend to have more advanced disease. The effect of this shift is part of the rationale of why our study would be informative to modern ocular oncologists.
In conclusion, this study provides a contemporary rate of metastasis and death outcomes for unilateral retinoblastoma treated with primary enucleation in the United States between 2007–2017. Although survival is excellent, metastases still develop and deaths from metastases occur. All metastases appeared within 12 months of retinoblastoma diagnosis. Seven of the nine patients with metastases had the orbit as the first site of metastases while the remaining 2 had CNS as the first site. All the patients with metastatic disease were classified as Reese-Ellsworth V or ICRB D or E. The results of this study have decision-making implications when counseling patients on options as well as in making treatment plans.
Transparency Statement:
The authors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported. No important aspects of the study have been omitted, any discrepancies from the as originally planned have been explained.
Acknowledgments
Funding Statement:
This work was supported by the Fund for Ophthalmic Knowledge, Inc and MSK Cancer Center Support Grant/Core Grant P30 CA008748 (JEL, JHF, IJD, and DHA). This work was also supported by the National Institutes of Health (NIH) grant P30 EY010572 and by unrestricted departmental funding from Research to Prevent Blindness (AHS and AKM). Additionally this work was supported by the NIH/NEI grant 5K08EY027464–02, a Research to Prevent Blindness Career Development Award, and by an unrestricted grant from Research to Prevent Blindness to the Vanderbilt Department of Ophthalmology and Visual Sciences (ABD). None of the funding sources were involved in the study design, execution, analysis, or interpretation.
Footnotes
Summary of Disclosure of Potential Conflicts of Interests
No authors have any interests relevant to this study to disclose.
Interests outside of the submitted work:
Dr. Lu has nothing to disclose.
Dr. Francis has nothing to disclose.
Dr. Dunkel reports other from Apexigen, personal fees from Bayer, grants and personal fees from Bristol-Myers Squibb, personal fees from Celgene, personal fees from Eisai, personal fees from Ipsen, personal fees from Pfizer, grants from Genentech, outside the submitted work.
Dr. Shields has nothing to disclose.
Mr. Yu has nothing to disclose Dr. Berry has nothing to disclose.
Ms. Kogachi has nothing to disclose
Dr. Skalet reports personal fees from Castle Biosciences, Inc., outside the submitted work
Dr. Miller has nothing to disclose
Mr. Santapuram has nothing to disclose
Dr. Daniels reports grants from Research to Prevent Blindness, grants from Knights Templar Eye Foundation, grants from Alcon Research Institute, grants from Spectrum Pharmaceuticals, outside the submitted work.
Dr. Abramson has nothing to disclose.
References
- 1.Albert DM. Historic Review of Retinoblastoma. Ophthalmology. 1987;94,(6):654–662. [DOI] [PubMed] [Google Scholar]
- 2.Dunphy EB. The Story of Retinoblastoma. Am.J. Ophthalmol 1964;58(4):539–552. [PubMed] [Google Scholar]
- 3.Hirschberg J Der Markschwamm der Netzhaut; eine monographic. Hirschwald, Berlin; 1869. [Google Scholar]
- 4.Wintersteiner H Das Neuroëpithelioma Retinae Eine anatomische and klinische Studie, Franz Deuticke, Vienna; 1897. [Google Scholar]
- 5.Leber T Beiträge zur Kenntnis der Struktur des Netzhautglioms. Albrecht von Graefes Arch Ophthalmol, 1911;78:381–411 [Google Scholar]
- 6.Reese AM. The treatment of retinoblastoma by x-rays and triethylene melamine. AMA Arch Ophthalmol. 1958;60:87. [DOI] [PubMed] [Google Scholar]
- 7.Ellsworth RM. The practical management of retinoblastoma. Trans Am Ophthalmol Soc 1960;67:462–534. [PMC free article] [PubMed] [Google Scholar]
- 8.Broaddus E, Topham A, Singh AD. Survival with retinoblastoma in the USA:1975–2004. Br J Ophthalmol. 2009;93:24–27. [DOI] [PubMed] [Google Scholar]
- 9.Abramson DH. Retinoblastoma: Saving Life with Vision. Review of Medicine. 2014;65(1):171–184 [DOI] [PubMed] [Google Scholar]
- 10.Tamboli D, Topham A, Singh N, Singh AD. Retinoblastoma: A SEER Dataset Evaluation for Treatment Patterns, Survival, and Second Malignant Neoplasms. Am. J. Ophthalmol 2015;160(5):953–958. [DOI] [PubMed] [Google Scholar]
- 11.Broaddus E, Topham A, Singh AD. Survival with retinoblastoma in the USA:1975–2004. Br J Ophthalmol. 2009;93:24–27. [DOI] [PubMed] [Google Scholar]
- 12.Berry JL, Kogachi K, Aziz HA, et al. Risk of Metastasis and Orbital Recurrence in Advanced Retinoblastoma Eyes Treated with Systemic Chemoreduction versus Primary Enucleation. Pediatr Blood Cancer. 2016;64:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abramson DH, Fabius AW, Issa R, et al. Advanced Unilateral Retinoblastoma: The Impact of Ophthalmic Artery Chemosurgery on Enucleation Rate and Patient Survival at MSKCC. Plos One. 2015;10(12):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honavar SG, Singh AD, Shields CL, Meadows AT, Demirci H, Cater J, Shields JA. Postenucleation adjuvant therapy in high-risk retinoblastoma. Arch Ophthalmol. 2002;120(7):923–31. [DOI] [PubMed] [Google Scholar]
- 15.Kaliki S, Shields CL, Rojanaporn D, Al-Dahmash S, McLaughlin JP, Shields JA, Eagle RC Jr. High-risk retinoblastoma based on international classification of retinoblastoma: analysis of 519 enucleated eyes. Ophthalmology. 2013;120(5):997–1003. [DOI] [PubMed] [Google Scholar]
- 16.Kaliki S, Shields CL, Shah SU, Eagle RC Jr, Shields JA, Leahey A. Postenucleation Adjuvant Chemotherapy with Vincristine, Etoposide, and Carboplatin for the Treatment of High-Risk Retinoblastoma. Arch Ophthalmol. 2011;129(11):1422–7. [DOI] [PubMed] [Google Scholar]
- 17.Just Diagnosed Staging. Children’s Oncology Group Website. [Accessed on March 23, 2016]. Available at https://www.childrensoncologygroup.org/index.php/newlydiagnosedwithretinoblastoma. September 2011.
- 18.Linn MA. Intraocular retinoblastoma: the case for a new group classification. Ophthalmol Clin North Am. 2005;18(1):41–53. [DOI] [PubMed] [Google Scholar]
- 19.Shields CL, Shields JA. Basic understanding of current classification and management of retinoblastoma. Curr Opin Ophthalmol. 2006;17:228–234. [DOI] [PubMed] [Google Scholar]
- 20.Children’s Oncology Group. Vincristine, Carboplatin, and Etoposide or Observation Only in Treating Patients Who Have Undergone Surgery for Newly Diagnosed Retinoblastoma. Available from: https://clinicaltrials.gov/ct2/show/NCT00335738.NLMIdentifierNCT00335738. Accessed August 15, 2018.
- 21.Children’s Oncology Group. Combination Chemotherapy, Autologous Stem Cell Transplant, and/or Radiation Therapy in Treating Young Patients With Extraocular Retinoblastoma. Available from: https://clinicaltrials.gov/ct2/show/NCT00554788?term=aret0321&rank=1.NLMIdentifierNCT00554788. Accessed August 15, 2018.
- 22.Kopelman JE, McLean IW, Rosenberg SH. Multivariate Analysis of Risk Factors for Metastasis in Retinoblastoma Treated by Enucleation. Ophthalmology. 1987;94(4):371–377 [DOI] [PubMed] [Google Scholar]
- 23.Sirin S, de Jong MC, de Graaf P, et al. High-Resolution Magnetic Resonance Imaging Can Reliably Detect Orbital Tumor Recurrence after Enucleation in Children with Retinoblastoma. Ophthalmology. 2016;123(3):635–45. [DOI] [PubMed] [Google Scholar]
- 24.Scelfo C, Francis JH, Ketan V, et al. An International Survey of Classification and Treatment Choices for Group D Retinoblastoma. Int J Ophthalmol. 2017;10(6):961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Novetsky DE, Abramson DH, Kim JW, et al. Published International Classification of Retinoblastoma (ICRB) Definitions Contain Inconsistencies An Analysis of Impact. Ophthalmic Genetics. 2009;30(1):40–44. [DOI] [PubMed] [Google Scholar]
- 26.Kivela T The epidemiological challenge of the most frequent eye cancer: retinoblastoma, an issue of birth and death. Br J Ophthalmol. 2009;93:1129–1131 [DOI] [PubMed] [Google Scholar]
- 27.MacCarthy A, Birch JM, Draper GJ, et al. Retinoblastoma: treatment and survival in Great Britain 1963–2002. Br J Ophthalmol. 2009;93:38–9 [DOI] [PubMed] [Google Scholar]
- 28.Masood N, Hossein N, Pejman B, et al. Retinoblastoma in Iran: outcomes in terms of patients survival and globe survival. Br J Ophthalmol. 2009;93:28–32. [DOI] [PubMed] [Google Scholar]