Abstract
We aimed to evaluate patient factors including nonadherence and viral infection and de novo donor specific antibody (dnDSA) characteristics [Total IgG, C1q, IgG3, and IgG4] as predictors of renal allograft failure in a multicenter cohort with dnDSA. We performed a retrospective observational study of 113 kidney transplant recipients with dnDSA and stored sera for analysis. Predictors of death-censored allograft loss were assessed by Cox-proportional modeling Death censored allograft survival was 77.0%(87/113) during a median follow-up of 2.2(IQR 1.2–3.7) years after dnDSA detection. Predictors of allograft failure included: medication nonadherence [HR 6.5 (95% CI 2.6–15.9)], prior viral infection requiring immunosuppression reduction [HR 5.3 (95% CI 2.1–13.5)], IgG3 positivity [HR 3.8(95%CI 1.5, 9.3)], and time post-transplant (years) until DSA detection [HR 1.2 (95% CI 1.0,1.3)] . In the 67 patients who were biopsied at dnDSA detection; chronic antibody mediated rejection [HR 11.4(95% CI 2.3, 56.0)] and mixed rejection [HR 7.4(95% CI 2.2, 24.8)] were associated with allograft failure. We conclude that patient factors, including a history of viral infection requiring immunosuppression reduction or medication nonadherence, combined with DSA and histologic parameters must be considered to understand the risk of allograft failure in patients with dnDSA.
Introduction:
De novo donor specific antibody (dnDSA) is a major risk factor for chronic active antibody mediated rejection (ABMR) and subsequent renal allograft loss1–3, yet many patients with dnDSA have stable allograft function for years.4–7 A clear understanding of the patient characteristics and biomarkers at the time of dnDSA detection predictive of allograft loss is needed to inform management decisions. More importantly this information can be used to effectively design clinical trials and define inclusion criteria to enrich study populations with subjects most likely to reach meaningful clinical end-points.
Previous studies have shown that allograft dysfunction and histologic features of rejection help predict allograft loss 4,6, but this information is not always apparent at the time of initial dnDSA detection. The patients who develop dnDSA are also heterogeneous. The main precursors to de novo DSA include patient medication nonadherence and provider initiated immunosuppression reduction (ie. for infection)1,4,5,8, but it remains unclear whether these factors are important in predicting early allograft loss.
One important biomarker of allograft loss is DSA. The routine single antigen bead (SAB) assay for anti-HLA antibody detection provides valuable semi-quantitative information about immunoglobulin G (IgG) directed towards class I and/or class II HLA. However, other dnDSA information may also have prognostic value such as the specific IgG subclass profile or complement binding ability of the dnDSA. The different IgG subclasses have distinct effector functions, notably a differential ability to bind complement and the Fc receptor. These factors likely influence allograft histology and allograft loss 9,10,11. Previous studies have suggested that IgG3 positive DSA, C1q binding positivity, quantity of DSA as measured by mean fluorescence intensity (MFI) or titer, and the HLA class of DSA are predictive of allograft failure 4,12–20 in single center cohorts; but these factors have not been systematically studied in a diverse multicenter cohort in the context of other important predictors of allograft failure.
The objective of our project was two-fold. First, we aimed to determine the death-censored allograft survival and allograft histology following the identification of dnDSA in a well- characterized multicenter cohort of kidney transplant recipients. Second, we aimed to identify unique patient, histological, and dnDSA characteristics associated with early allograft failure. Patient factors studied included baseline demographics, nonadherence, or prior viral infection requiring immunosuppression reduction. De novo DSA characteristics included IgG subclasses and C1q binding positivity.
Methods:
Study Design
This was a retrospective observational multicenter study of solitary kidney transplant recipients transplanted from 1998 to 2015 [Mayo Clinic, Rochester, MN (Center A) ; New York Presbyterian Weill Cornell Medical College [NYP-WCM (Center B)], New York, NY; and University of Michigan, Ann Arbor, MI (Center C)] with dnDSA. A chart review was performed to identify patients meeting the following inclusion criteria: 1) no DSA at the time of transplant, 2) development of dnDSA with MFI > 1000 post-transplantation verified on two independent tests and 3) the availability of banked sera collected at the time of dnDSA detection to allow for additional DSA characterization at a central lab (Terasaki Research Institute, Los Angeles, CA). The overall aim of the study was to determine the factors identified at initial dnDSA detection that were associated with allograft loss. This study was approved by the Institutional Review Boards at Mayo Clinic, NYP-WCM, and University of Michigan. Clinical data were collected by chart review.
De novo Donor Specific Antibody Assessment
Donor specific antibody testing was performed using the SAB solid phase assay (LABscreen, One Lambda, Canoga Park, CA, USA)]. An MFI cut-off of 1000 was considered positive. All patients were negative for DSA pretransplant and had at least one SAB test negative for DSA post-transplant. Donor specific antibody testing and screening was performed for surveillance purposes at least yearly post-transplant and as indicated at the time of allograft dysfunction.
The stored sera obtained when dnDSA was initially detected was sent to the Terasaki Research Institute for repeat testing using a standard protocol to confirm the presence of dnDSA and perform IgG subclass and C1q testing. Pan IgG DSA testing was also done via the SAB assay. The dnDSA with the highest MFI at presentation was considered the Dominant DSA.
The methodology of IgG subclass typing with Luminex has been previously described in detail12. Briefly, the LABScreen® assay was performed according to the manufacturer’s instructions, except for the replacement of phycoerythrin (PE)-conjugated secondary mouse monoclonal anti-human IgG (One Lambda, Inc.) with different PE-mouse anti-human IgG specific to IgG subclass hinge regions (IgG3: HP6050, Southern Biotech Inc), and the Fc prime portion of the heavy chain (IgG4: HP6023, Southern Biotech Inc). The trimmed MFI values were normalized using the formula: ([sample #N beads-sample negative control beads]-[negative control #N beads-negative control beads]).
For the C1q assay, the test was performed using heat inactivated serum (56°C for 30 minutes) that was spiked with 150 mg/ml purified human C1q in HEPES buffer (One Lambda) to ensure equal functional amounts of C1q per sample. LABScreen® single antigen beads were added to the mixture and incubated for 20 minutes at room temperature, followed by addition of phycoerythrin conjugated anti-human C1q. Beads were washed twice and analyzed on a LABScan200 flow analyzer (i.e. Luminex). A cutoff of 1000 MFI was used to indicate positivity for all IgG and C1q testing unless otherwise indicated.
Assessment of Medication Adherence
We defined medical nonadherence as documented missing labs, unexplained low immunosuppressive drug levels, no-show to appointments, medications not refilled, documentation of non-adherence by treating physician in the medical record, or by the patient’s own admission. These events occurred prior to appearance of dnDSA, and were therefore considered a baseline variable.
Assessment of Viral Infection
Patients were monitored for viral infections based on center practices and clinical indications. The presence of a prior viral infection requiring immunosuppressive reduction was defined by both a positive blood PCR assay and physician initiated immunosuppressive reduction. The specific viruses considered for this study included BK viremia and/or nephropathy, Epstein Barr virus (EBV), cytomegalovirus (CMV), and parvovirus. BK viremia and/or nephropathy was also considered as a separate variable.
Biopsy Assessment
We analyzed the allograft biopsy findings from a subset of patients (N=67) who received a biopsy at the time of dnDSA detection. Biopsies were performed according to the individual transplant center’s surveillance protocol and provider discretion (i.e. dnDSA). Kidney biopsy tissue was processed for light microscopy and C4d if indicated. At Centers A and B, C4d was detected by immunofluorescence (AbD Serotec). At Center C, C4d was detected by immunohistochemistry. Biopsies were scored using the Banff 2017 classification system 21–23. Borderline acute cellular rejection was considered an acute cellular rejection (ACR) for our purposes. Specifically; active antibody mediated rejection (ABMR) was diagnosed if 2 features were present according to Banff 2017 classification system24: 1) Histologic evidence of acute tissue injury including g>0 and/or ptc >0, intimal or transmural arteritis (v>0), thrombotic microangiopathy, or acute tubular injury, in the absence of any other apparent cause and 2) Evidence of current/recent antibody interaction with vascular endothelium including at least one of the following: C4d ≥2 with immunofluorescence, C4d ≥ 1 with immunohistochemistry on frozen section, or g+ptc ≥2. The presence of cg score >0 signified in addition to active ABMR features signified chronic ABMR. Electron microscopy was not routinely done in all biopsies, and it was not used to determine the presence of chronic ABMR.
Patient treatment
Treatment for dnDSA and/or ABMR was based on the individual centers protocol and biopsy results. At Center A, only patients with ABMR and T cell mediated rejection received treatment with plasmapheresis, intravenous immunoglobulin (IVIG), and anti-thymocyte globulin. At Center B, patients received treatment based on biopsy findings and allograft function. Patients with dnDSA and ABMR with stable allograft function received steroid pulse with intravenous immunoglobulin (IVIG). Patients with dnDSA and ABMR who had allograft dysfunction received steroid pulse, plasmapheresis, IVIG, and bortezomib. Patients with dnDSA and ABMR with T cell mediated rejection received steroid pulse and anti-thymocyte globulin. At Center C, all patients with active ABMR received plasmapheresis and IVIG. If a combined T-cell mediated rejection was identified, the patient also received intravenous steroids and anti-thymocyte globulin. For chronic active ABMR, patients received intravenous immunoglobulin for 4 weeks. At all centers, no treatment was given to patients with dnDSA and no histologic evidence rejection (patients who did not receive a biopsy or patients who received a biopsy that was negative for ABMR).
Laboratory monitoring
All patients had serum creatinine levels and estimated glomerular filtration rate (GFR) reported at least every 3 months per center protocol.
Statistical Analysis
Statistical analysis was performed on JMPv10. (SAS, Cary, NC) and Rv3.4.1 (Austria). For numerical data, groups were compared with the t-test or the Wilcoxon rank sum test as indicated. Counts and percentages were compared using the chi-squared test . Matched pairs analysis was done to compare allograft function among individuals prospectively. Time-to-event data were summarized for each group using Kaplan-Meier estimates. Univariate and multivariate analysis for correlates with post-dnDSA allograft loss was done using Cox proportional hazards models using the date of dnDSA diagnosis as the index date. Variables were included in the multivariate analysis if the univariate p-value was less than 0.15 and variable selection was performed with backwards stepwise variable selection using the Schwarz’s Bayesian Criterion. Hazards ratios (HR) were described by their point estimate and corresponding 95% confidence intervals (CI). Statistical significance was defined by p<0.05 for two-sided p-values.
Assumptions of proportionality were tested through the Schoenfeld residuals using the cox.zph() routine in R. Non-linearity of variables entering models were tested using polynomial splines. An interaction term between C1q and IgG3 was included in the multivariable model in order to test for synergy between the two DSA subtypes.
Results:
Patient Characteristics
A total of 113 patients with dnDSA and banked serum collected at the time of initial DSA detection were included in the study (n=28 from Center A, n=35 from Center B, and n=50 from Center C) Table 2. The mean age ± SE was 41.4±1.5 years old, the majority of subjects were male [66.4% (75/113)], and the main cause of end stage renal disease was glomerulonephritis [35.4% (40/113)]. The racial composition of the cohort was diverse and varied among centers (p=0.02). Notably, 25.7% (29/113) of patients were African American and 12.4%(14/113) were Hispanic. The donor type and the proportion with prior transplant also varied by center (p<0.01 and p=0.02, respectively), and included 44.3% (50/113) deceased donor recipients and 16.8% (19/113) with prior failed kidney transplant. The proportion of patients with 0–2 HLA mismatches was 7.1% (8/113), 3–4 HLA mismatches was 39.8% (45/113), and 5–6 mismatches was 53.1% (27/113). The prevalence of documented medication nonadherence was 31.0% (35/113) overall and was similar among the participating centers, p=0.07. A viral infection requiring reduction in immunosuppression prior to the detection of DSA was present in 30.1% (34/113) of patients. Of note, 4.4% (5/113) patients had a documented history of medication nonadherence and also experienced a viral infection prior to the detection of dnDSA.
Table 2:
De novo Donor Specific Antibody Characteristics at the time of Detection
| All N=113 |
Center A N=28 |
Center B N=35 |
Center C N=50 |
P-value | |
|---|---|---|---|---|---|
| Time post-transplant to dnDSA detection median (IQR) year | 1.1(0.6–2.8) | 1.0(0.8–3.8) | 0.9(0.6–1.3) | 2.1(0.5–5.1) | P<0.01 |
| Class I only n(%) | 21(18.5) | 3(10.7) | 7(20.0) | 11(22.0) | P=0.34 |
| Class II only n(%) | 61(54.0) | 18(64.3) | 21(60.0) | 22(44.0) | |
| Class I + Class II n(%) | 31(27.4) | 7(25.0) | 7(20.0) | 17(34.0) | |
| Dominant DSA Median MFI (IQR) | 9592(3362–14923) | 7537(2652–12343) | 7707 (4052–11623) | 12751(3540–19233) | P=0.05 |
| Number of DSA Specificities Median (IQR) | 1(1–2) | 1(1–2) | 1(1–2) | 2(1–3) | P=0.27 |
| IgG 3* n(%) | 29(25.7) | 12(42.9) | 4(11.4) | 13(26.0) | P=0.02 |
| IgG 4* n(%) | 17(15.0) | 4(14.3) | 3(8.6) | 10(20.0) | P=0.35 |
| C1q* n(%) | 12(10.6) | 6(21.4) | 4(11.4) | 2(4.0) | P=0.06 |
based on MFI of > 1000.
Induction immunosuppression varied among centers (p<0.0001), but 73.5%(83/113) received anti-thymocyte globulin. The majority of recipients were treated with a combination maintenance immunosuppressive regimen including: tacrolimus [80.5% (91/113)], mycophenolate mofetil [94.7%(107/113)], and steroids [69.0%(78/113)]. A larger proportion of patients received maintenance immunosuppression with cyclosporine at Center C than other centers, and fewer patients were on a long term steroids at Center B (p<0.01). Other patient characteristics are included in Table 1.
Table 1:
Patient Characteristics
| Patient Characteristics | All N=113 |
Center A N=28 |
Center B N=35 |
Center C N=50 |
p-value |
|---|---|---|---|---|---|
| Age at transplantation mean +/− SE (years) | 41.4 +/− 1.5 | 41.0+/−3.0 | 43.6+/−2.7 | 40.2+/−2.2 | 0.61 |
| Gender Male n (%) | 75 (66.4) | 18(64.3) | 26(74.3) | 31(62.0) | 0.48 |
| Etiology of ESRD n (%) | 0.12 | ||||
| Race n (%) | 0.02 | ||||
| Donor type n (%) | <0.01 | ||||
| Re-transplant n(%) | 19(16.8) | 8(28.6) | 1(2.9) | 10(20.0) | P=0.02 |
| HLA mismatch | |||||
| History of nonadherence* n (%) | 35(31.0) | 12(42.9) | 6(17.1) | 17(34.0) | P=0.07 |
| Any viral infection requiring immunosuppression reduction*† | 34(30.1) | 6(21.4) | 17(48.6) | 11(22.0) | P=0.02 |
| Polyomavirus* n(%) | 26(23.0) | 6(21.4) | 11(31.4) | 9(18.0) | P=0.34 |
| Induction Immunosuppression | |||||
| Anti-thymocyte globulin | 83(73.5) | 17(60.7) | 32(91.4) | 34(68.0) | P<0.0001 |
| Immunosuppression at time of DSA n (%) | |||||
| Tacrolimus | 91(80.5) | 25(89.3) | 32(91.4) | 34(68.0) | P<0.01 |
| Mycophenolate mofetil | 107(94.7) | 28(100.0) | 33(94.3) | 46(92.0) | P=0.32 |
| Steroids | 78(69.0) | 22(78.6) | 9(25.7) | 47(94.0) | P<0.01 |
prior to the detection of dn DSA
Includes BK virus, Cytomegalovirus, Epstein Barr Virus, Parvovirus, or combination. At center A, all patients had BK virus (n=6). At center B, BK only (n=9) , BK and CMV (n=2), BK and EBV (n=1), CMV and EBV (n=1), CMV and parvovirus (n=1), CMV viremia (n=3). At center C, BK only (n=9) and CMV only (n=2).
DSA Characteristics at the Time of De novo DSA Detection
The median (IQR) time post-transplant until the detection of dnDSA was 1.1 (0.6–2.8) years, and this was different among centers, p<0.01. At Center A the median (IQR) time to detection was 1.0(0.8–3.8) years, at Center B it was 0.9 (0.6–1.3) years, and at Center C it was 2.1 (0.5–5.1) years post-transplant. Using the conventional LABScreen pan IgG assay, 18.5% (21/113) of the patients had dnDSA against class I only, 54.0%(61/113) had dnDSA against anti-class II only, and 27.4%(31/113) had dnDSA against both class I and class II Table 2 and Supplemental Table 1. The median (IQR) MFI of the dominant DSA was 9592 (IQR 3362–14923) and was similar among centers (p=0.05).
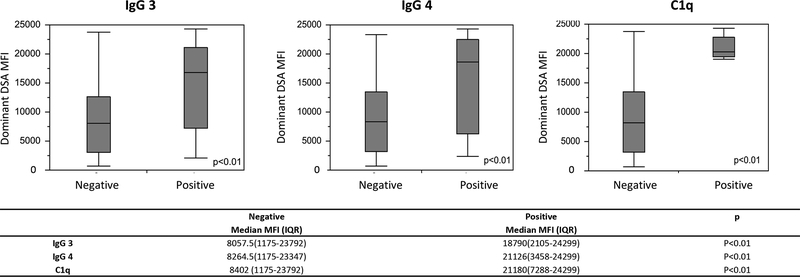
IgG3 (MFI ≥ 1000) at dnDSA onset was found in 25.7% (29/113) of patients and this was different among centers, p=0.02 [Center A = 42.9%(12/28), Center B = 11.4%(4/35), and Center C = 26.0% (13/50) Table 2. IgG4 positivity (MFI ≥ 1000) was found in only 15.0% (17/112) of patients and C1q binding was found in 10.6% (12/113). The prevalence of IgG4 and C1q binding was statistically similar among centers, p=0.35, and p=0.06 respectively Table 2. The presence of IgG3, IgG4, and C1q positivity at dnDSA initial detection was positively correlated with the MFI of the dominant DSA as shown in Figure 1.
Figure 1. IgG 3, IgG 4, and C1q positivity associated with high MFI of Dominant De novo DSA.
Patients with de novo DSA positive for IgG 3, IgG 4, and C1q were more likely to have an dominant de novo DSA with a high MFI. IgG 3, IgG 4, and C1q positivity was based on MFI of 1000.
The combinations of C1q and IgG subclass positivity and associated patient and pan IgG characteristics are detailed in Supplemental Figure 1. The majority of patients [66.4% (75/113) were negative for IgG3, IgG4, and C1q; while only 3.5% (4/113) were positive for all three of these characteristics (C1q, IgG 3, and IgG 4). C1q and IgG3 (+/− IgG4) was positive in 8.0%(9/113). Of the C1q positive patients, 75.0% (9/12) were also positive for IgG3. Conversely, of the IgG3 positive patients, 40.9% (9/22) were positive for C1q.
Allograft survival and Function
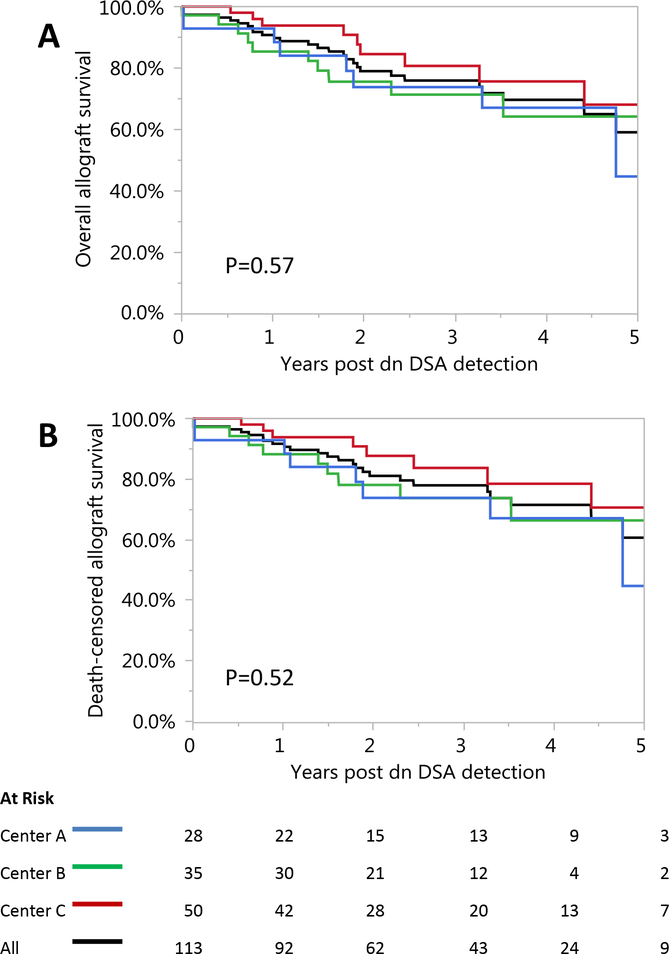
The incidence of death-censored allograft failure by one year post dnDSA was 8.0% (9/113); and by 3 years post-de novo DSA 32.2% (20/62) of the patients experienced allograft failure. Overall allograft survival was 75.2% (85/113) and death-censored allograft survival was 77.0% (87/113) during a median follow-up of 2.2 (IQR 1.2–3.7) years post-dnDSA detection Figure 2. Both were similar among centers (p=0.57 and p=0.52, respectively).
Figure 2. Overall and Death-censored Allograft Survival after De novo DSA Detection was Similar Among Centers.
The incidence of death-censored allograft failure by one year post dnDSA was 8.0% (9/113); and by 3 year post-de novo DSA 32.2% (20/62) of the patients lost their graft. Overall allograft survival was 75.2% (85/113) (A) and death-censored allograft survival was 77.0% (87/113) (B) during a median follow-up of 2.2 (IQR 1.2–3.7) years following dnDSA detection.
The median estimated GFR (IQR) at the time of dnDSA detection was 52.4 ml/min/1.73 m2 (IQR 37.8 – 67.2) mg/dl and was similar at 52.2 ml/min/1.73 m2 (IQR 33.85–70.3) within 6–12 month post-dnDSA detection, p=0.88. Within 24 months of dnDSA detection, the median estimated GFR decreased to 46.5 ml/min/1.73 m 2, p=0.02.
Factors Associated with Allograft Failure
Factors associated with death-censored allograft failure by univariate Cox-proportional hazard analysis and subsequently included in the multivariable analysis (p≤0.15) included history of nonadherence, viral infection requiring immunosuppression reduction prior to dnDSA detection, C1q (MFI >1000), IgG3 (MFI > 1000), and the time to dnDSA post-transplant in years Table 3. Factors not associated with death-censored allograft failure included the age of the recipient, race, deceased donor, steroid containing immunosuppression, history of prior kidney transplant, BK nephropathy prior to dnDSA, dominant DSA MFI, number of DSA specificities, class of DSA, and transplant center.
Table 3:
Univariate and Multivariate Predictors of Allograft Loss
| Univariate | Stepwise Model | |||
|---|---|---|---|---|
| Variable | HR | p | HR | p |
| Age of Recipient | 1.0 (1.0, 1.0) | 0.45 | ||
| Race | 0.9 (0.4, 2.0) | 0.86 | ||
| Deceased donor | 0.9 (0.4, 1.9) | 0.79 | ||
| Steroid containing immunosuppression | 1.8 (0.7, 4.9) | 0.22 | ||
| History of Nonadherence | 3.2 (1.5, 7.0) | 0.002 | 6.5 (2.6, 15.9) | <0.0001 |
| Prior Kidney Transplant | 0.8 (0.3, 2.1) | 0.60 | ||
| Viral Infection requiring immunosuppression reduction | 2.1 (0.9, 4.6) | 0.07 | 5.3 (2.1, 13.5) | 0.0004 |
| BK nephropathy prior to DSA | 1.2 (0.4, 4.1) | 0.75 | ||
| C1q (MFI >1000) | 5.9 (2.3, 15.6) | 0 | ||
| IgG 3 (MFI>1000) | 3.2 (1.5, 7.0) | 0.002 | 3.8 (1.5, 9.3) | 0.0039 |
| IgG 4 (MFI>1000) | 2.1 (0.8, 5.7) | 0.14 | ||
| Dominant MFI (Log) | 1.4 (0.46, 4) | 0.57 | ||
| Number of DSA specificities | 1.1 (0.9, 1.3) | 0.35 | ||
| Anti-class I DSA only | 0.7 (0.2, 2.1) | 0.52 | ||
| Anti-class II DSA only | 0.7 (0.3, 1.5) | 0.36 | ||
| Both Anti class I and II DSA | 2.0 (0.9, 4.3) | 0.10 | ||
| Center | ||||
| Time to dnDSA (years post-transplant) | 1.2 (1.1, 1.3) | 0.004 | 1.2 (1.0, 1.3) | 0.01 |
| C-stat | NA | NA | 0.80 | |
The interaction term between C1q and IgG subclasses was nonsignificant p> 0.05.
In a multivariate model using stepwise variable selection, predictors of allograft failure included history of medication nonadherence [HR 6.5 (95% CI 2.6–15.9)], viral infection prior to DSA detection [HR 5.3 (95% CI 2.1–13.5), IgG3 positivity [HR 3.8 (95% CI 1.5–9.3)], and the time post-transplant until detection of dnDSA [1.2 (1.0, 1.3) in years Table 3. The C-statistic was 0.80 for this model.
Allograft survival in the context of Medication Nonadherence and/or Viral Infection
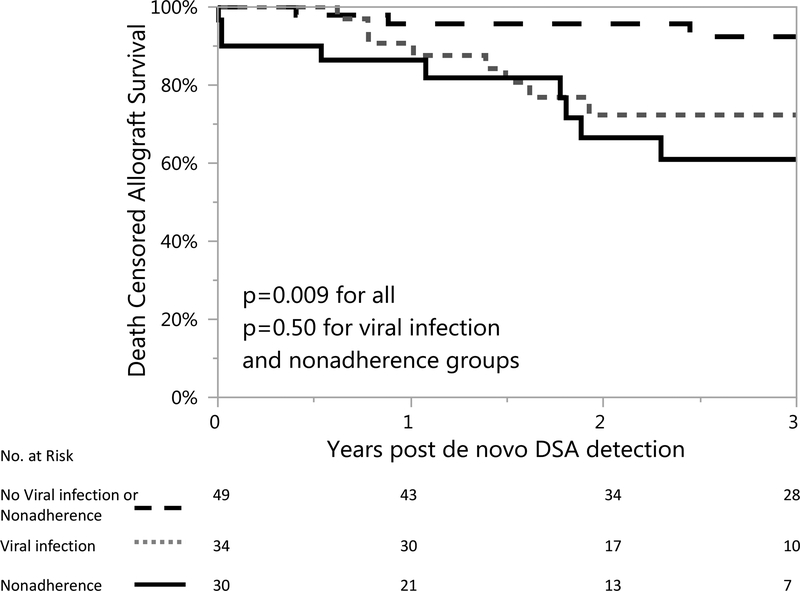
Given that both medication nonadherence and prior history of viral infection were associated with allograft failure, we examined allograft survival in the following subgroups: 1) those with documented history of medication nonadherence (n=30), 2) those with history of viral infection leading to immunosuppression reduction [n=34, five of which also had documented nonadherence], and 3) those with neither nonadherence or prior viral infection. Death-censored allograft survival following dnDSA was 70.0% (21/30) in the medication nonadherence group, 67.4% (23/34) in the prior viral infection group, and 87.8%(43/49) in the group with neither medication nonadherence nor prior viral infection during a mean follow-up of 2.2 (IQR 1.2–3.7) years post-dnDSA detection, p=0.009 Figure 3.
Figure 3. Death-censored allograft survival was decreased if there was prior history patient induced medication nonadherence or viral infection leading to immunosuppressive reduction.
Death-censored allograft survival following dnDSA was 70.0% (21/30) in the medication nonadherence group, 67.4% (23/34) in the prior viral infection group, and higher at 87.8%(43/49) in the group with neither medication nonadherence nor prior viral infection during a mean follow-up of 2.2 (IQR 1.2–3.7) years post-dnDSA detection, p=0.009.
There was numerical trend toward an increased frequency of IgG3+ DSA in the dnDSA-nonadherence group [33.3% (10/30) in the nonadherence group versus 17.7%(6/24) in dnDSA-viral infection group and 22.5% (11/49) in the neither group], but this did not reach statistical significance. The number of DSA, class of DSA, frequency of C1q+ DSA, and frequency of IgG4 DSA was similar among the three groups.
Allograft Histology at the time of De Novo DSA Detection
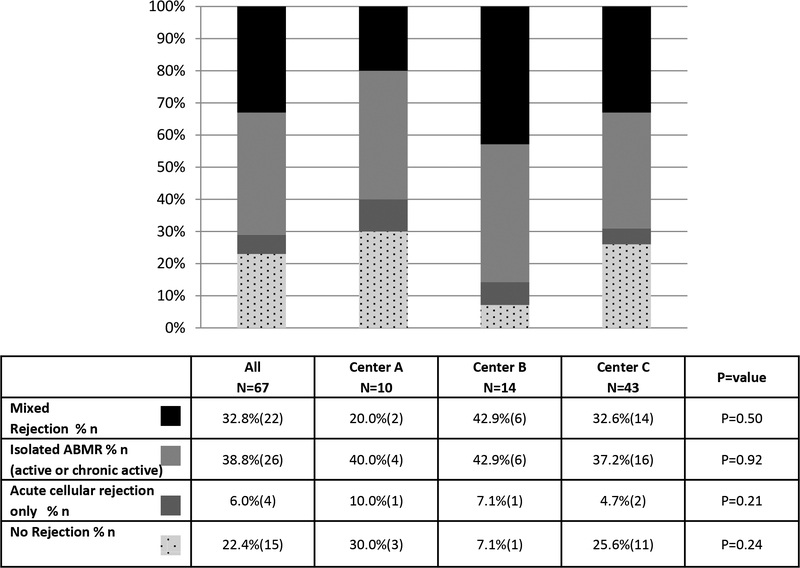
Sixty seven (59.3%) of patients received a kidney biopsy at the time of de novo DSA detection. The majority [71.6%(48/67)] showed evidence of ABMR. Of those cases, 33.3%(16/48) demonstrated chronic active ABMR. The specific biopsy findings stratified by center are shown in Figure 4. A mixed ABMR and T cell mediated rejection was present on 32.8%(22/67), while an isolated ABMR (active or chronic) was present on 38.8% (26/67) of biopsies. An isolated T-cell mediated rejection was found in only 6.0% (4/67) of biopsies and 22.4% (15/67) of biopsies were negative for rejection
Figure 4. Allograft Histology at the time of De novo DSA Detection.
Importantly, the biopsy findings at the time of de novo DSA detection were similar among centers (p=0.76) and no difference in death-censored graft loss was observed among those patients with or without biopsies performed at this time point [17.9% (12/67) allograft loss in patients with a biopsy versus 30.4% (14/46) allograft loss in patients without a biopsy, p=0.12].
The presence of chronic ABMR [HR 11.4 (95% CI 2.3–56.0)] or a mixed rejection [HR 7.4 (95% CI 2.2, 24.8)] were associated with allograft failure. When chronic ABMR was present; 43.8% (7/16) of patients had allograft loss. when mixed rejection was present 27.3% (6/22) had allograft loss. Isolated acute active ABMR, isolated ACR, or no rejection were not associated with early allograft loss after a mean follow-up of 2.2 (IQR 1.2–3.7) years.
Patients with chronic ABMR at the time of dnDSA detection were more likely to be nonadherent [56.3% (9/16) versus 25.5% (13/51), p<0.02] than patients with other biopsy findings. Additionally, patients with chronic ABMR presented with dnDSA later post-transplant than those without chronic ABMR (a mean ±SD of 7.1 ±4.0 versus 4.2 ± 2.2 ± SD years post-transplant respectively, p=0.01). Recipient age, history of BK infection, previous transplantation, and gender were similar among individuals with and without chronic ABMR.
Patients with dnDSA positive for IgG3 were more likely to have a mixed rejection, butwe did not detect a relationship among C1q binding, IgG3, or IgG4 subclasses and the histologic findings of no rejection, acute cellular rejection only, ABMR only, or chronic ABMR Supplemental Table 2.
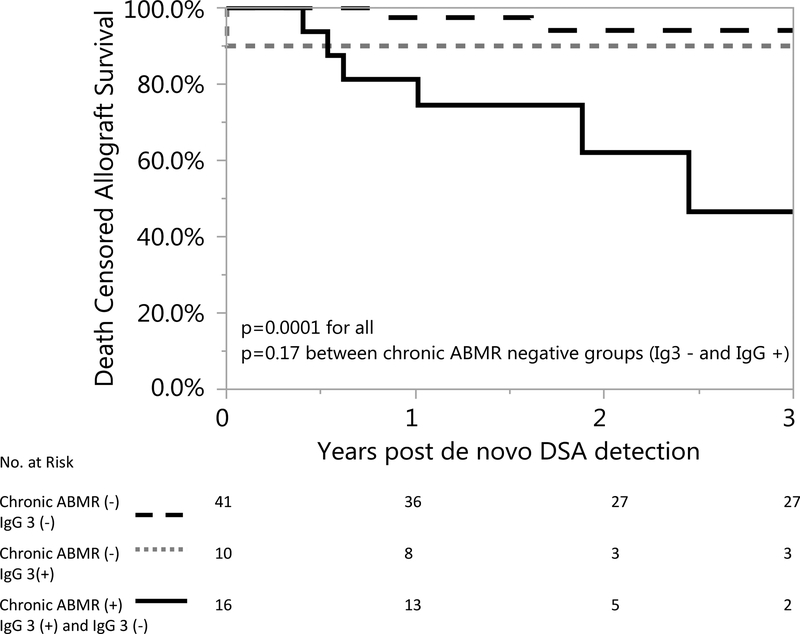
Given the relationship between IgG 3 and graft loss, we compared allograft survival among biopsied patients who were negative for chronic ABMR and IgG 3, patients who were IgG 3 positive but were negative for chronic ABMR, and those who had chronic ABMR (IgG 3 positive or negative). Allograft survival was decreased in patients with chronic ABMR (p=.0001), but allograft was similar among patients who did not have chronic ABMR regardless of IgG 3 status (p=.17) Figure 5.
Figure 5. Allograft similar in IgG 3+ and IgG – patients who were negative for chronic ABMR at De novo DSA detection.
Importantly, the biopsy findings at the time of de novo DSA detection were similar among centers (p=0.76) and no difference in death-censored allograft loss was observed among those patients with or without biopsies performed at this time point [17.9% (12/67) allograft loss in patients with a biopsy versus 30.4% (14/46) allograft loss in patients without a biopsy, p=0.12].
Discussion:
In our analysis of a large and diverse multicenter cohort with dnDSA; we confirmed that a history of nonadherence and IgG3 positivity are independently associated with death-censored allograft loss 1,2,4,6,25,26. In addition, we show that having a viral infection leading to immunosuppression reduction is an indicator of a poor prognosis, and that patients who develop dnDSA without a clear precipitant have the best prognosis. Lastly, we have added to the understanding of the histologic findings at the time of dnDSA and their prognostic value. The presence of chronic active ABMR or a mixed ABMR and T-cell mediated rejection are both associated with early allograft loss.
The association between prior viral infection and allograft loss in patients with dnDSA has not been previously well-described. Only recently has the link between BK nephropathy, DSA, and subsequent ABMR been well-recognized8,27–30; but the association between other viral infections (other than BK) and DSA has not been shown. Our findings are especially important because they suggest that dnDSA that develops following immunosuppression reduction for infection has a particularly poor prognosis. Our understanding of the complex interplay of infection, immunosuppression reduction, DSA, and ABMR remains limited because of the small number of cases in our cohort, and further study is needed. Nonetheless, our findings highlight the need for personalized immunosuppression reduction in the setting of infection and careful monitoring for dnDSA.
Our work is also distinctive because we studied many DSA characteristics simultaneously in a large diverse cohort tested at a centralized laboratory. We have confirmed that IgG3 positivity at the time of dnDSA detection is strongly associated with early allograft loss in patients with dnDSA31,32, but it is important to acknowledge that many IgG3 negative patients also had early allograft loss. IgG3 positivity at the time of dnDSA was present in only 43.2% (11/26) of the allograft loss cases. Other studies have indicated that patients develop IgG3 over time, thus it is possible that IgG3 DSA was present prior to dnDSA detection with screening or developed later.
We also found that when considering multiple factors, C1q positivity did not enter the final prediction model for early allograft loss in patients with dnDSA. This finding is likely because of the overlap between IgG3 and C1q positivity. Our results are consistent with other studies that have been mixed regarding the role of C1q positivity for risk stratification in patients with dnDSA33,34,25,35,36.
We acknowledge that evaluating DSA characteristics is complex. Alloantibody production is a dynamic process that can evolve. Additionally, IgG 3, IgG 4, C1q positivity, and the presence of class I and class II DSA were all correlated with pan IgG DSA MFI. However, the challenge of using MFI alone is that this result is semi-quantitative and issues such as prozone and assay interference need to be considered18. Obtaining DSA titer can be done to better quantify DSA, but this is impractical because it is labor intensive and expensive.
We have previously shown that histologic findings of ABMR (acute or chronic) were associated with allograft loss4, but it appears that the main factor leading to early allograft loss is chronic ABMR. Mixed ABMR is also associated with allograft loss, but to a lesser extent. These findings are supported by others 4,6. Although it is logical that patients with chronic ABMR will have earlier allograft loss, our findings are critical to consider when designing clinical trials. Patients with dnDSA who have isolated active ABMR on their initial biopsy are less likely to reach key end-points such as allograft loss in the short term. Likewise, patients with chronic ABMR should be cautiously selected in therapeutic clinical trials given the potential lack of response.
We acknowledge the significant heterogeneity in the centers who contributed patients for this study (varied baseline immunosuppression, follow-up, and treatment). However, “center” was not a univariate or multivariate predictor of allograft loss and death-censored allograft survival and allograft histology at the time of de novo DSA detection was similar among centers. It is possible that we were underpowered to detect center differences, but the allograft survival following dnDSA in our cohort was consistent with what has been previously published 13,17,25. Future multicenter prospective studies in which patients receive standardized treatment and long-term follow-up are needed to overcome the limitations of our retrospective study design. A standardized treatment approach and long term follow-up would also allow us to examine the effect of treatment on DSA characteristics and the evolution of DSA characteristics and histology. A prospective study would also allow us to determine the incidence of dnDSA, which was not the purpose of the present study. Further study is also needed to better understand the relationship between infection, dnDSA, and allograft loss.
In conclusion, a combination of patient historical factors, DSA characteristics, and histologic findings need to be considered to determine the risk of allograft failure in a patient with newly detected DSA after kidney transplant. DSA characteristics such as IgG 3 positivity are predictive of early graft loss, but other factors are also important. A prior history of viral infection leading to immunosuppressive reduction, nonadherence, and allograft histology must all be considered when designing therapeutic clinical trials to appropriately include patients most likely to reach meaningful clinical end-points such as allograft loss. Understanding of the risk factors associated with allograft loss can inform patient management decisions in clinical practice and improve outcomes.
Supplementary Material
Acknowledgments:
Carrie Schinstock received support from CTSA Grant Number KL2 TR000136 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Evan Farkash received support from NIAID 1K23AI108951. We acknowledge the work of Jennifer Mawby for her work as a research coordinator.
Abbreviations:
- DSA
De novo DSA
- SAB
Single antigen bead
- IgG
Immunoglobulin G
- MFI
Mean fluorescence intensity
- NYP-WCM
New York Presbyterian Weill Cornell Medical College
- EBV
Epstein Barr Virus
- CMV
Cytomegalovirus
- ABMR
Antibody mediated rejection
- IVIG
Intravenous immunoglobulin
- GFR
Glomerular filtration rate
- HR
Hazards ratio
- CI
Confidence intervals
- IQR
Interquartile range
Author Contributions:
All authors had substantial contributions to conception and design, or acquisition of data, or analysis and interpretation of data; 2) drafting the article or revising it critically for important intellectual content; 3) final approval of the version to be published; and 4) agree to be accountable for all aspects of the work.
C.A. Schinstock
study design and data acquisition
manuscript writing, preparation, and review
statistical analysis
D.M. Dadhania
study design and data acquisition
manuscript writing, preparation, and review
statistical analysis
M.J. Everly
study design
manuscript writing and critical review
centralized laboratory testing - DSA testing expertise
B. Smith
statistical analysis and interpretation
manuscript writing and critical review
M. Gandhi
study design and data acquisition
manuscript writing and critical review
DSA testing expertise
E. Farkash
data acquisition
review and DSA expertise.
V. Sharma
data acquisition
review and DSA expertise.
M Samaniego-Picota
study design and data acquisition
manuscript writing, preparation, and review
M.D. Stegall
study design and data acquisition
manuscript writing, preparation, and review
Footnotes
Disclosure:
The authors of this manuscript have no conflicts of interest to disclose as defined by the American Journal of Transplantation.
References:
- 1.Everly MJ, Rebellato LM, Haisch CE, et al. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 2013;95(3):410–417. [DOI] [PubMed] [Google Scholar]
- 2.Cooper JE, Gralla J, Chan L, Wiseman AC. Clinical significance of post kidney transplant de novo DSA in otherwise stable grafts. Clin Transpl. 2011:359–364. [PubMed] [Google Scholar]
- 3.Hourmant M, Cesbron-Gautier A, Terasaki PI, et al. Frequency and clinical implications of development of donor-specific and non-donor-specific HLA antibodies after kidney transplantation. J Am Soc Nephrol. 2005;16(9):2804–2812. [DOI] [PubMed] [Google Scholar]
- 4.Schinstock CA, Cosio F, Cheungpasitporn W, et al. The Value of Protocol Biopsies to Identify Patients With De Novo Donor-Specific Antibody at High Risk for Allograft Loss. Am J Transplant. 2017;17(6):1574–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12(5):1157–1167. [DOI] [PubMed] [Google Scholar]
- 6.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant. 2015;15(11):2921–2930. [DOI] [PubMed] [Google Scholar]
- 7.Parajuli S, Reville PK, Ellis TM, Djamali A, Mandelbrot DA. Utility of protocol kidney biopsies for de novo donor-specific antibodies. Am J Transplant. 2017;17(12):3210–3218. [DOI] [PubMed] [Google Scholar]
- 8.Cheungpasitporn W, Kremers WK, Lorenz E, et al. De novo Donor Specific Antibody following BK Nephropathy: The Incidence and Association with Antibody Mediated Rejection. Clin Transplant. 2018. [DOI] [PubMed] [Google Scholar]
- 9.Stegall MD, Diwan T, Raghavaiah S, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11(11):2405–2413. [DOI] [PubMed] [Google Scholar]
- 10.Feucht HE, Schneeberger H, Hillebrand G, et al. Capillary deposition of C4d complement fragment and early renal graft loss. Kidney Int. 1993;43(6):1333–1338. [DOI] [PubMed] [Google Scholar]
- 11.Mauiyyedi S, Pelle PD, Saidman S, et al. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001;12(3):574–582. [DOI] [PubMed] [Google Scholar]
- 12.Taniguchi M, Rebellato LM, Briley KP, et al. Risk Stratification of Human Leukocyte Antigen Class II Donor Specific Antibody Positive Patients by Immunoglobulin G Subclasses. Clin Transpl. 2015;31:293–301. [PubMed] [Google Scholar]
- 13.Freitas MC, Rebellato LM, Ozawa M, et al. The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation. 2013;95(9):1113–1119. [DOI] [PubMed] [Google Scholar]
- 14.Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369(13):1215–1226. [DOI] [PubMed] [Google Scholar]
- 15.Chen G, Sequeira F, Tyan DB. Novel C1q assay reveals a clinically relevant subset of human leukocyte antigen antibodies independent of immunoglobulin G strength on single antigen beads. Hum Immunol. 2011;72(10):849–858. [DOI] [PubMed] [Google Scholar]
- 16.Chin C, Chen G, Sequeria F, et al. Clinical usefulness of a novel C1q assay to detect immunoglobulin G antibodies capable of fixing complement in sensitized pediatric heart transplant patients. J Heart Lung Transplant. 2011;30(2):158–163. [DOI] [PubMed] [Google Scholar]
- 17.Zeevi A, Lunz J, Feingold B, et al. Persistent strong anti-HLA antibody at high titer is complement binding and associated with increased risk of antibody-mediated rejection in heart transplant recipients. J Heart Lung Transplant. 2013;32(1):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tambur AR, Herrera ND, Haarberg KM, et al. Assessing Antibody Strength: Comparison of MFI, C1q, and Titer Information. Am J Transplant. 2015. [DOI] [PubMed] [Google Scholar]
- 19.Kannabhiran D, Lee J, Schwartz JE, et al. Characteristics of Circulating Donor Human Leukocyte Antigen-specific Immunoglobulin G Antibodies Predictive of Acute Antibody-mediated Rejection and Kidney Allograft Failure. Transplantation. 2015;99(6):1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fidler SJ, Irish AB, Lim W, Ferrari P, Witt CS, Christiansen FT. Pre-transplant donor specific anti-HLA antibody is associated with antibody-mediated rejection, progressive graft dysfunction and patient death. Transpl Immunol. 2013;28(4):148–153. [DOI] [PubMed] [Google Scholar]
- 21.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2008;8(4):753–760. [DOI] [PubMed] [Google Scholar]
- 22.Solez K, Colvin RB, Racusen LC, et al. Banff ‘05 Meeting Report: differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2007;7(3):518–526. [DOI] [PubMed] [Google Scholar]
- 23.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int. 1999;55(2):713–723. [DOI] [PubMed] [Google Scholar]
- 24.Haas M, Sis B, Racusen LC, et al. Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 2014;14(2):272–283. [DOI] [PubMed] [Google Scholar]
- 25.Wiebe C, Gareau AJ, Pochinco D, et al. Evaluation of C1q Status and Titer of De Novo Donor-Specific Antibodies as Predictors of Allograft Survival. Am J Transplant. 2017;17(3):703–711. [DOI] [PubMed] [Google Scholar]
- 26.Schinstock C, Cheungpasitporn W, Cosio F, et al. Kidney Transplant with Low Level DSA and Low Positive B-Flow Crossmatch: An Underappreciated Option for Highly Sensitized Transplant Candidates. . Am J Transplant. 2016;16((suppl 3)). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sawinski D, Forde KA, Trofe-Clark J, et al. Persistent BK viremia does not increase intermediate-term graft loss but is associated with de novo donor-specific antibodies. J Am Soc Nephrol. 2015;26(4):966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nankivell BJ, Renthawa J, Sharma RN, Kable K, O’Connell PJ, Chapman JR. BK Virus Nephropathy: Histological Evolution by Sequential Pathology. Am J Transplant. 2017;17(8):2065–2077. [DOI] [PubMed] [Google Scholar]
- 29.Drachenberg CB, Papadimitriou JC, Chaudhry MR, et al. Histological Evolution of BK Virus-Associated Nephropathy: Importance of Integrating Clinical and Pathological Findings. Am J Transplant. 2017;17(8):2078–2091. [DOI] [PubMed] [Google Scholar]
- 30.Dieplinger G, Everly MJ, Briley KP, et al. Onset and progression of de novo donor-specific anti-human leukocyte antigen antibodies after BK polyomavirus and preemptive immunosuppression reduction. Transpl Infect Dis. 2015;17(6):848–858. [DOI] [PubMed] [Google Scholar]
- 31.Lefaucheur C, Viglietti D, Bentlejewski C, et al. IgG Donor-Specific Anti-Human HLA Antibody Subclasses and Kidney Allograft Antibody-Mediated Injury. J Am Soc Nephrol. 2016;27(1):293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viglietti D, Loupy A, Vernerey D, et al. Value of Donor-Specific Anti-HLA Antibody Monitoring and Characterization for Risk Stratification of Kidney Allograft Loss. J Am Soc Nephrol. 2017;28(2):702–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369(13):1215–1226. [DOI] [PubMed] [Google Scholar]
- 34.Yell M, Muth BL, Kaufman DB, Djamali A, Ellis TM. C1q Binding Activity of De Novo Donor-specific HLA Antibodies in Renal Transplant Recipients With and Without Antibody-mediated Rejection. Transplantation. 2015;99(6):1151–1155. [DOI] [PubMed] [Google Scholar]
- 35.Guidicelli G, Guerville F, Lepreux S, et al. Non-Complement-Binding De Novo Donor-Specific Anti-HLA Antibodies and Kidney Allograft Survival. J Am Soc Nephrol. 2016;27(2):615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calp-Inal S, Ajaimy M, Melamed ML, et al. The prevalence and clinical significance of C1q-binding donor-specific anti-HLA antibodies early and late after kidney transplantation. Kidney Int. 2016;89(1):209–216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.