Abstract
C57BL/6J-XYPOS (B6J-XYPOS) mice, which have the Y chromosome derived from Mus musculus poschiavinus on a B6J genetic background, form ovotestes or ovaries. Previously, we replaced the genetic background of B6J-XYPOS mice with B6N and found that individuals with testes also appeared in addition to those with ovaries or ovotestes. To investigate the effect of the B6J genetic sequence on the testis differentiation, the genetic background of B6N-XYPOS mice was replaced with B6J again. The recovery of the B6J genetic background significantly decreased the incidence of testes; only ovaries developed. These results indicate that the testicular differentiation process tends to be perturbed especially in the B6J substrain. This shows the importance of substrain differences in mice usually treated as B6 collectively.
Keywords: C57BL/6, Mus musculus poschiavinus, sex reversal, substrain, testis differentiation
Mammalian undifferentiated bipotential gonads differentiate into testes in response to the appropriate expression of sex determination gene SRY/Sry (sex determining region of Y) [11, 14]. In mice, when the timing and level of Sry expression are inappropriate, the testis differentiation process is perturbed and ovotestes or ovaries are formed [4, 8]. The structure of Sry is not always the same among mouse strains. For example, Sry of Mus musculus domesticus has lost glutamic acid repeating regions in C terminal by nonsense mutation, so its stability is lower than that of M. m. musculus [1, 12]. Since C57BL/6 (B6) has a Sry allele derived from M. m. musculus, sex reversal may occur when the Domesticus-derived Sry gene is transferred into the B6 genetic background [1, 2]. The YPOS mouse, which has M. m. domesticus-derived Sry allele derived from M. m. poschiavinus, forms ovotestes or ovaries when it is maintained on the B6J genetic background [6, 7]. In our previous study, when the genetic background of B6J-XYPOS mice was replaced with B6N (B6N-XYPOS), individuals with testes also appeared in addition to those with ovotestes or ovaries [13]. This suggested that the difference between these phenotypes is attributable to the change in the genetic background from B6J to B6N [13].
The genomic sequence of the B6J mouse is regarded as a mouse standard (called reference sequence), and the B6 mice are widely used as both a genetic background for recombinant animals and a model of human pathology [9, 10, 16]. Various substrains exist in B6 animals, and genomic sequences and phenotypic differences occur between these substrains [9, 10]. Therefore, it is hypothesized that genomic differences among the B6 substrains affected the testis differentiation process in B6N-XYPOS. In this study, we investigated whether replacing the genetic background of B6N-XYPOS mice with the original B6J background would make testes appear.
B6N-XYPOS mice were generated and maintained as described elsewhere [13]. B6J-XYPOS mice were generated from B6N-XYPOS-Sry129 (N11) by backcrossing to B6JJmsSlc mice (purchased from Japan SLC, Hamamatsu, Japan). Their offspring, having only the Sry allele from M. m. poschiavinus, were selected and used after sexual maturation (9–24 weeks). Under isoflurane anesthesia, animals were euthanized by cervical dislocation. This study was approved by the Institutional Animal Care and Use Committee (Permission #25-06-03 and #29-05-02) and carried out according to the Kobe University Animal Experimental Regulations.
A gonad was classified as an ovary if uterine horn, oviduct and ovary like appearance of gonad were present. When a vas deferens, epididymis and testis like appearance of gonad were present, and when testicular descent was observed, the gonad was classified as a testis. In cases where the morphologies of a gonad and accessory gonads were ambiguous even on one side, the gonad was classified as an ovotestis.
Genital tissues were fixed in 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 24 hr at 4°C. The specimens were dehydrated with an ethanol series followed by xylene and then embedded in paraffin. Then, 5-µm-thick sections were cut by a sliding microtome and placed on slide glasses. For the general histological analyses, tissue sections were stained with hematoxylin and eosin (HE) after their deparaffinization and hydration.
Immunohistochemical staining was carried out as described previously [15]. For the primary antibody, we used anti-Sox9 (SRY-box 9) rabbit polyclonal antibody (1:1,000; #sc-20095; Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) for Sertoli cell maker and anti-Foxl2 (forkhead box L2) goat polyclonal antibody (1:12,800; #ab5096; Abcam, Cambridge, U.K.) for granulosa cell marker, and for the secondary antibody, we used Dako EnVision+ system-HRP (horseradish peroxidase)-labeled polymer (Dako Japan, Tokyo, Japan) or peroxidase-conjugated donkey anti-goat IgG (1:200; #705-035-147; Jackson ImmunoResearch, West Grove, PA, U.S.A.).
To examine the equivalence of incidence of each genotype or phenotype, the χ2 test and residual analysis were performed with Excel Statistics 2015 (version 1.00; SSRI, Tokyo, Japan). The results were considered significant when the P-value was <0.05.
In a comparison of the three latest generations that had a stable phenotype, recovery of the genetic background of B6J significantly increased the incidence of ovaries in YPOS mice (Fig. 1A). As the number of backcrosses increased, the incidence of individuals with ovaries increased while that of individuals with testes decreased (Fig. 1B). In particular, individuals with testis no longer appeared after four generations of backcrosses (Fig. 1B).
Fig. 1.
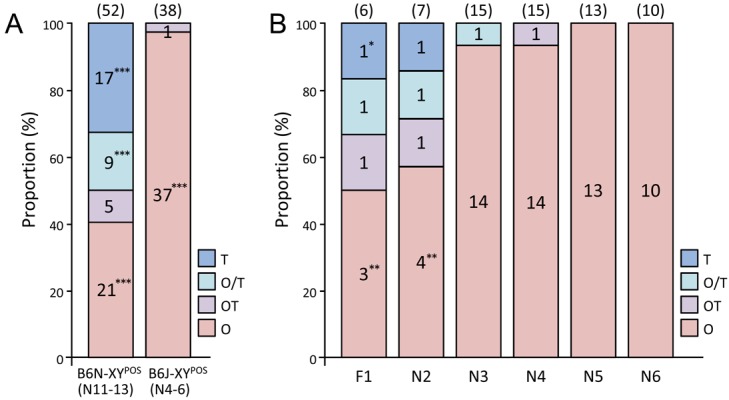
Phenotype frequency analysis in adult mice. (A) Evaluation of the equivalence of incidence of each phenotype by χ2 test showed that individuals with ovaries (O) were significantly higher in B6J-XYPOS than in B6N-XYPOS mice. On the other hand, individuals with testes (T) or testis and ovary (O/T) did not appear in B6J-XYPOS mice. (B) As the number of backcrosses by B6J increased, only individuals with ovaries appeared. Statistical evaluation with the hypothesis that the incidence of phenotypes in each generation is equal showed that the incidence of phenotypes with asterisks was significantly different. The numbers of animals examined are shown above the bars. OT, ovotestis. *P<0.05; **P<0.01; ***P<0.001.
Recovery of the B6J genetic background in B6N-derived YPOS mice increased the incidence of genotype XX (Fig. 2A). When autosomal composition ratio (presence of Sry129 or not) and sex chromosomal composition ratio (XX vs. XY) were reconsidered with the same data set using χ2 test, it was found that the appearance frequency of Sry129 was low in B6J background (data not shown). On the other hand, there was no statistically significant change in the appearance frequency of genotypes of each generation after the replacement to B6J (Fig. 2B). These results indicated that the genetic differences between the B6 substrains (B6JJmsSlc and B6NCrSlc) influence the appearance frequency of their litters’ genotypes.
Fig. 2.

Genotype frequency analysis in adult mice. (A) Evaluation of the equivalence of incidence of each genotype by χ2 test showed that replacement of the genetic background to B6J raised the incidence of XX. (B) There were no statistical differences in genotype appearance frequency even after increased backcrossing with B6J. The numbers of animals examined are shown above the bars.
The internal genitalia of B6J-XYPOS showed an anatomically ovarian morphology (Fig. 3A). The structure of ovaries including mature follicles and corpus luteum was observed histologically (Fig. 3B). Many granulosa cells with Foxl2 immunoreactivity were also observed (Fig. 3C), but primary follicle was not recognized. These results were same as ovaries of B6N-XYPOS after sexual maturation in our previous report [13]. On the other hand, seminiferous tubules containing Sox9-positive Sertoli cells were not observed (Fig. 3D). In gonadal supporting cells, Sox9 is expressed in Sertoli cells but not in granulosa cells. These results confirmed that the internal genitalia of B6J-XYPOS mice were ovaries, at least after the 5 times of backcrossing with B6J mice.
Fig. 3.
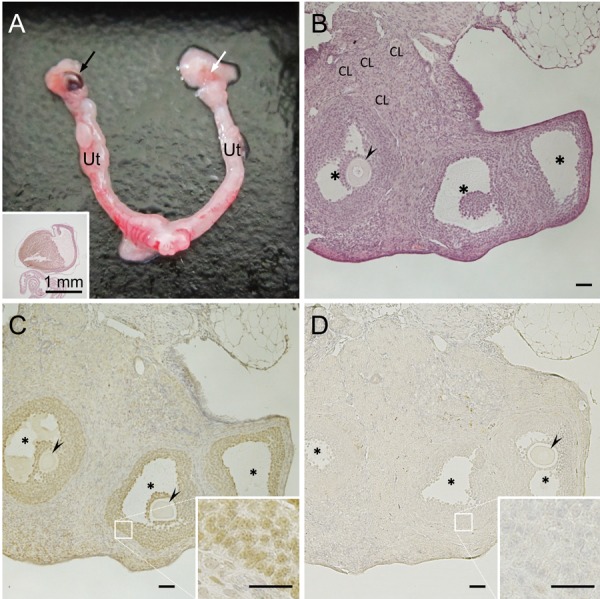
Isolated internal genitalia and histology of B6J-XYPOS(N6) mice. (A) Ovary (arrows), oviduct and uterus (Ut) were present on both sides. A part of the right ovary (black arrow) was brownish, and a follicle with blood leakage was observed in this region (inset). (B) Vesicular follicles (*) with germ cells (arrowhead) and corpus luteum (CL) were observed in the ovary. On the other hand, primary follicles were not observed. (C) Immunoreactivity of granulosa cell specific transcription factor Foxl2 was detected in the vesicular follicles. (D) Immunoreactivity of Sertoli cell specific transcription factor Sox9 was not detected in the ovary. Scale bars=50 µm.
Some Y chromosomes derived from M. m. domesticus on the B6 genetic background may cause sex reversal [1]. However, the phenotypic differences in YPOS mice in this study were not attributable to genomic sequence differences in the Y chromosome, since the origin of the Y chromosome was the same. Crossings of B6J-XYPOS male mice and female mice of other strains (DBA/2J, BLAB/cBy, C58/J) yield normal males; and half of the mice from crossings of B6J-XYPOS male mice and F1 female mice (B6 × DBA/2J) develop testes [5]. These findings indicate that the difference in autosomal chromosomes between mouse strains affects testis differentiation. Further, our results showed that the difference in autosomal sequences between B6 substrains also might affect testis differentiation.
Between substrains of B6 mice, some genomic sequence differences are related to the amount of time that has passed after the substrains first branched off [10]. B6N mice were branched from B6J mice in 1951, and SNP marker sets consisting of 100 SNPs that efficiently discriminate them were reported previously [10]. The B6NCrSlc mice used in this study branched off from B6N around 1972, and 30 SNPs in Mekada’s SNP marker sets of B6NCrSlc mice were different from those for B6J and B6JJmsSlc [10]. Eleven SNPs were also different between B6NCrSlc and B6J or B6JJmsSlc in an analysis using other SNP marker sets [9, 16]. Together, these results suggested that the differences in these regions are responsible for the differences in gonadal phenotype between B6N-XYPOS [13] and B6J-XYPOS with the B6JJmsSlc genetic background.
Sox9, an execution factor of the testis differentiation process, is upregulated after Sry expression [8, 11]. This suggested that a sufficient level of Sox9 expression was not induced during testis differentiation in this study, because the gonads of B6J-XYPOS differentiated into ovaries. To induce sufficient Sox9 expression, Sry should be expressed within the appropriate period [8]. The Sry expression of B6N-XYPOS was delayed and Sox9 expression was also insufficient, while Sry expression of B6J-XYPOS, which forms only ovotestes or ovaries, is further delayed [4, 13]. From these facts, it is presumed that the expression of Sry in B6J-XYPOS with the B6JJmsSlc genetic background, which forms only ovaries, was also delayed. The Chromosome 11 region has been reported as one of the candidate factors potentially involved in the delay of Sox9 expression in YPOS mice [3]. In addition, different SNP regions between B6N and B6J are also presumed to be involved in the regulation of Sry and Sox9 expression.
The results of this study revealed that the process of testis differentiation in YPOS mice is influenced by the differences between B6 substrains. In particular, it was revealed that testis differentiation tended to be perturbed in the B6J substrains. These results indicate the importance of substrain differences in these mice, which are usually treated as B6 collectively.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research (Nos. 24590401 and 16K08072 to N. Hoshi and Nos. 26460410 and 17K08686 to T. Yokoyama) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
REFERENCES
- 1.Albrecht K. H., Eicher E. M.1997. DNA sequence analysis of Sry alleles (subgenus Mus) implicates misregulation as the cause of C57BL/6J-YPOS sex reversal and defines the SRY functional unit. Genetics 147: 1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht K. H., Young M., Washburn L. L., Eicher E. M.2003. Sry expression level and protein isoform differences play a role in abnormal testis development in C57BL/6J mice carrying certain Sry alleles. Genetics 164: 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arboleda V. A., Fleming A., Barseghyan H., Délot E., Sinsheimer J. S., Vilain E.2014. Regulation of sex determination in mice by a non-coding genomic region. Genetics 197: 885–897. doi: 10.1534/genetics.113.160259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bullejos M., Koopman P.2005. Delayed Sry and Sox9 expression in developing mouse gonads underlies B6-YDOM sex reversal. Dev. Biol. 278: 473–481. doi: 10.1016/j.ydbio.2004.11.030 [DOI] [PubMed] [Google Scholar]
- 5.Eicher E. M., Washburn L. L.1986. Genetic control of primary sex determination in mice. Annu. Rev. Genet. 20: 327–360. doi: 10.1146/annurev.ge.20.120186.001551 [DOI] [PubMed] [Google Scholar]
- 6.Eicher E. M., Shown E. P., Washburn L. L.1995. Sex reversal in C57BL/6J-YPOS mice corrected by a Sry transgene. Philos. Trans. R. Soc. Lond. B Biol. Sci. 350: 263–268, discussion 268–269. doi: 10.1098/rstb.1995.0160 [DOI] [PubMed] [Google Scholar]
- 7.Eicher E. M., Washburn L. L., Whitney J. B., 3rd, Morrow K. E.1982. Mus poschiavinus Y chromosome in the C57BL/6J murine genome causes sex reversal. Science 217: 535–537. doi: 10.1126/science.7089579 [DOI] [PubMed] [Google Scholar]
- 8.Hiramatsu R., Matoba S., Kanai-Azuma M., Tsunekawa N., Katoh-Fukui Y., Kurohmaru M., Morohashi K., Wilhelm D., Koopman P., Kanai Y.2009. A critical time window of Sry action in gonadal sex determination in mice. Development 136: 129–138. doi: 10.1242/dev.029587 [DOI] [PubMed] [Google Scholar]
- 9.Mekada K., Abe K., Murakami A., Nakamura S., Nakata H., Moriwaki K., Obata Y., Yoshiki A.2009. Genetic differences among C57BL/6 substrains. Exp. Anim. 58: 141–149. doi: 10.1538/expanim.58.141 [DOI] [PubMed] [Google Scholar]
- 10.Mekada K., Hirose M., Murakami A., Yoshiki A.2015. Development of SNP markers for C57BL/6N-derived mouse inbred strains. Exp. Anim. 64: 91–100. doi: 10.1538/expanim.14-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Svingen T., Koopman P.2013. Building the mammalian testis: origins, differentiation, and assembly of the component cell populations. Genes Dev. 27: 2409–2426. doi: 10.1101/gad.228080.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sekido R.2010. SRY: A transcriptional activator of mammalian testis determination. Int. J. Biochem. Cell Biol. 42: 417–420. doi: 10.1016/j.biocel.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 13.Umemura Y., Miyamoto R., Hashimoto R., Kinoshita K., Omotehara T., Nagahara D., Hirano T., Kubota N., Minami K., Yanai S., Masuda N., Yuasa H., Mantani Y., Matsuo E., Yokoyama T., Kitagawa H., Hoshi N.2015. Ontogenic and morphological study of gonadal formation in genetically-modified sex reversal XYPOS mice. J. Vet. Med. Sci. 77: 1587–1598. doi: 10.1292/jvms.15-0292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Windley S. P., Wilhelm D.2015. Signaling pathways involved in mammalian sex determination and gonad development. Sex Dev. 9: 297–315. doi: 10.1159/000444065 [DOI] [PubMed] [Google Scholar]
- 15.Yamamoto A., Omotehara T., Miura Y., Takada T., Yoneda N., Hirano T., Mantani Y., Kitagawa H., Yokoyama T., Hoshi N.2018. The mechanisms underlying the effects of AMH on Müllerian duct regression in male mice. J. Vet. Med. Sci. 80: 557–567. doi: 10.1292/jvms.18-0023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zurita E., Chagoyen M., Cantero M., Alonso R., González-Neira A., López-Jiménez A., López-Moreno J. A., Landel C. P., Benítez J., Pazos F., Montoliu L.2011. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res. 20: 481–489. doi: 10.1007/s11248-010-9403-8 [DOI] [PubMed] [Google Scholar]


