Abstract
This study evaluated the expression of genes involved in the concentration of Ca2+ in precursor osteoblast-like cell, MC3T3-E1 subjected to stretching stimuli. Transient receptor potential vanilloid 4 (Trpv4) gene expression, the factor that is activated by stretch stimulation and enables inflow of Ca2+ from the extracellular space, was not affected as a result of stretch stimulation; conversely, the expression of sodium-calcium exchanger 1 (Ncx1) gene involved in outflow of intracellular Ca2+ increased, depending on stimulation intensity. Localization of Ca2+ correlated with the positioning of the endoplasmic reticulum, and intracellular Ca2+ decreased in inverse proportion to the intensity of the stretching force. These results suggest that stretch stimulation activates intracellular Ca2+ elimination rather than Ca2+ uptake before osteoblast differentiation.
Keywords: Ncx1, Ncx3, osteoblast differentiation, stretch stimulus, Trpv4
Osteoblasts differentiate from mesenchymal stem cells, and they secrete bone matrix around themselves to form bone tissue [13]. Both physical and chemical signals have been reported to affect osteoblast differentiation in addition to various physiologically active substances. Examples of physical signals include shear and stretch stresses. These kinds of mechanical stimuli promote osteogenic activity [18, 21]. Examples of chemical signals include Ca2+ and Zn2+ ions, which have also been reported to promote osteoblast differentiation [2, 19].
Osteoblasts have a mechanism for regulating intracellular Ca2+ concentrations by controlling the uptake of Ca2+ into the endoplasmic reticulum (ER) and its outflow to the extracellular space [20]. The presence of multiple ion channels has been reported to be a factor regulating Ca2+ inflow [2], and the transient receptor potential vanilloid (TRPV) family of proteins is known as a channel responsive to changes in temperature, osmotic pressure, and mechanical stimuli [1]. Among these proteins, TRPV4 selectively infuses cations such as Ca2+ and Mg2+ into cells via a 6-transmembrane channel activated by cell stretching [9, 12]. Meanwhile, the sodium–calcium exchanger (NCX) protein acts as a Ca2+ excretion mechanism by utilizing molecules comprised of 3 Na+ ions to eject a single Ca2+ ion [17]. NCX can exist as subtype 1, 2, or 3, and osteoblasts have been confirmed to express NCX subtypes 1 and 3 [10, 14, 15]. Although osteoblast differentiation is induced in response to physical and chemical stresses, the extent of exposure to stretch stimulation and changes in the expression of factors related to the mechanism of Ca2+ regulation are not fully elucidated in an undifferentiated osteoblast. In this study, the precursor osteoblast-like cell, MC3T3-E1 cell line was used as the target of an analysis of the effect of stretch stimulation provided to an undifferentiated osteoblast with focus on Trpv4 and Ncxs gene expression.
Cells from the MC3T3-E1 mouse osteoblast-like cell line (DS Pharma Biomedical, Osaka, Japan) were cultured in an incubator using a minimum essential medium alpha (MEMα; Wako, Osaka, Japan) containing 5% fetal bovine serum (FBS; Atlas Biologicals, Fort Collins, CO, U.S.A.) and 1% penicillin-streptomycin (Wako) at 37°C and 5% CO2 concentration. An outline of this study is displayed in Fig. 1. Undifferentiated MC3T3-E1 cells (1 × 105 cells/ml) were seeded in a collagen-coated stretch chamber (Menicon Life Science, Nagoya, Japan) and were cultured under static conditions for 24 hr. After cells were adhered to the stretch chamber, they were exposed to 8% stretch stimuli at a stretch frequency of 0.1 Hz (1 stretch action every 10 sec) for 24 hr using the ShellPa mechanical cell stretching system (Menicon Life Science). Cells cultured for 24 hr without stretching (0% stretch stimulus) were used as controls.
Fig. 1.
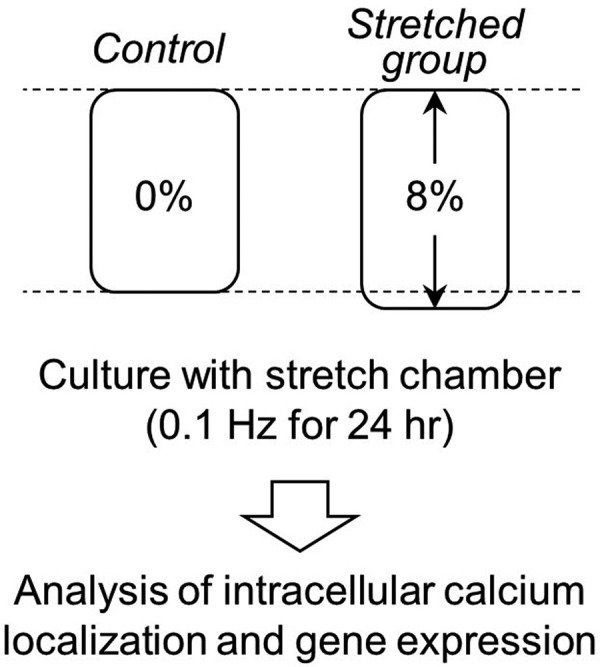
Overview of experimental procedures. After seeding of 1 × 105 cells/ml, the cells were exposed to 8% stretch stimuli at a stretching frequency of 0.1 Hz for 24 hr (1 stretch stimulation per 10-sec period). Cells cultured for 24 hr without stretching (0% stretch stimulus) were used as controls. After the stretch stimulation, the localization of intracellular Ca2+ and target gene expression in the stretched cells were measured.
The culture supernatant was removed from the stretch chamber, and the cells were fixed in 10% neutral buffered formalin (Wako) for 20 min. After rinsing with phosphate buffered saline (PBS, pH 7.4) for 5 min 3 times, the cells were exposed to a 10 µM calcium probe, CaTM-2AM (Goryo Chemical, Sapporo, Japan), for 60 min. After rinsing with PBS, the cells were exposed to 1 µM ER-Tracker Green (Thermo Fisher Scientific, Waltham, MA, U.S.A.), an ER-specific marker, for 15 min, and dyed with 5 µg/ml of Hoechst 33342 (Molecular Probe, Eugene, OR, U.S.A.). The stained cells were observed using an Olympus IX71 fluorescence microscope (Olympus, Tokyo, Japan) after staining.
Total RNA was extracted from cells using the RNeasy Mini Kit (QIAGEN, Hilden, Germany). Then, 30 ng/µl of RNA was reverse transcribed into cDNA using the ReverTra Ace qPCR RT with gDNA Remover Kit (Toyobo, Osaka, Japan). Quantitative PCR for comparing the rates of gene expression in each experimental group was performed using FastStart Essential DNA Green Master (Roche Diagnostics, Mannheim, Germany) and various primers (Table 1) for confirmation of gene expression of osteoblast differentiation markers (Runx2, runt related transcription factor 2; Alpl, alkaline phosphatase liver/bone/kidney; Bglap, bone gamma carboxyglutamate protein [osteocalcin]; and Col1a1, collagen type I alpha 1) and regulating factors involved in intracellular Ca2+ concentration (Trpv4, Ncx1, and Ncx3). Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) was used as an internal standard. Statistical analyses were performed using Student’s t-test or one-way analysis of variance with Bonferroni-Dunn’s post hoc tests. P values of <0.05 were considered statistically significant.
Table 1. Forward and reverse primer sequences for real time RT-PCR.
| Gene | Primer sequence | Product size (bp) | Gene accession number |
|---|---|---|---|
| Runx2 | 5′- AGC CTC TTC AGC GCA GTG AC-3′ | 59 | NM_001146038 |
| 5′- CTG GTG CTC GGA TCC CAA-3′ | |||
| Alpl | 5′- TCC TGG CTC TGC CTT TAT TCC-3′ | 62 | NM_007431 |
| 5′- TGC CCA AGA GAG AAA CCT GCT-3′ | |||
| Col1a1 | 5′- CCT GGC CTT GGA GGA AAC TTT-3′ | 73 | NM_007742 |
| 5′- GCA CGG AAA CTC CAG CTG ATT-3′ | |||
| Bglap | 5′- AAG CCT TCA TGT CCA AGC AGG-3′ | 170 | NM_007541 |
| 5′- TTT GTA GGC GGT CTT CAA GCC-3′ | |||
| Trpv4 | 5′- TCA CCT TCG TGC TCC TGT TG-3′ | 84 | NM_022017 |
| 5′- AGA TGT GCT TGC TCT CCT TG-3′ | |||
| Ncx1 | 5′- CTG GGG AAG ATG ACG ATG AT-3′ | 122 | NM_011406 |
| 5′- TTC TGT AGG TGG GAC GAA GG-3′ | |||
| Ncx3 | 5′- AGG AGG GGA TGA GGA TGA AG-3′ | 161 | NM_001167920 |
| 5′- TGA GGA TGG AGA CCA CGA AG-3′ | |||
| Gapdh | 5′- AGG TCG GTG TGA ACG GAT TTG-3′ | 123 | NM_008084 |
| 5′- TGT AGA CCA TGT AGT TGA GGT CA-3′ | |||
Fluorescence microscopic observation revealed that Ca2+ was distributed throughout the cytoplasm in MC3T3-E1 cells in the control group (0% stretch stimulus), and its localization was found to be consistent with the positioning of the ER (Fig. 2) on examining the localization of intracellular Ca2+ by CaTM-2AM. The presence of Ca2+ was also reported in the smooth ER in MC3T3-E1 cells [7], and the results of our intracellular Ca2+ localization analysis were supported by this previous study. Interestingly, the stainability of Ca2+ was markedly diminished in 8% of the experimental group cells compared with the control group. TRPV4, a Ca2+ ion-permeable channel, is known to be responsive to mechanical stimuli [4, 16]. At the start of the experiment, we predicted that Ca2+ entered cells as a result of stretch-related stress, and the cells would accumulate more Ca2+ for calcification. In contrast, however, the intracellular Ca2+ decreased proportionally to the intensity of the stretch stimulus.
Fig. 2.
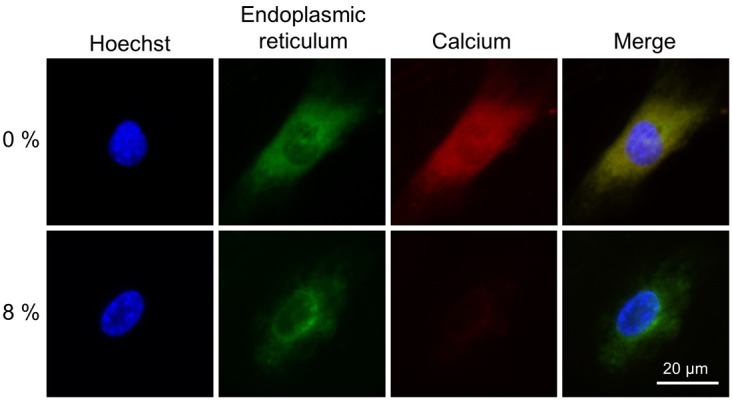
Fluorescent staining images of osteoblast endoplasmic reticula and intracellular Ca2+ in MC3T3-E1 cells following stretch stimulation. Endoplasmic reticulum was stained green, Ca2+ was stained red, and cell nuclei were stained blue using Hoechst. A marked decrease in intracellular Ca2+ staining was observed in the cells that underwent 8% stretch stimulation compared with the control cells (0% stretch stimulus).
Function of transcription factors is important for the initiation of osteoblast differentiation, and Runx2 is a representative factor [8]. To differentiate MC3T3-E1 cells into osteoblasts, osteogenic differentiation medium is usually used. In this study, we did not use such medium for culturing MC3T3-E1 cells, but interestingly, the stretch stimulation significantly increased Runx2 expression (Fig. 3). It was inferred that the stretch stimulation provided a clue to switch on osteogenic differentiation in the osteoblast precursor.
Fig. 3.
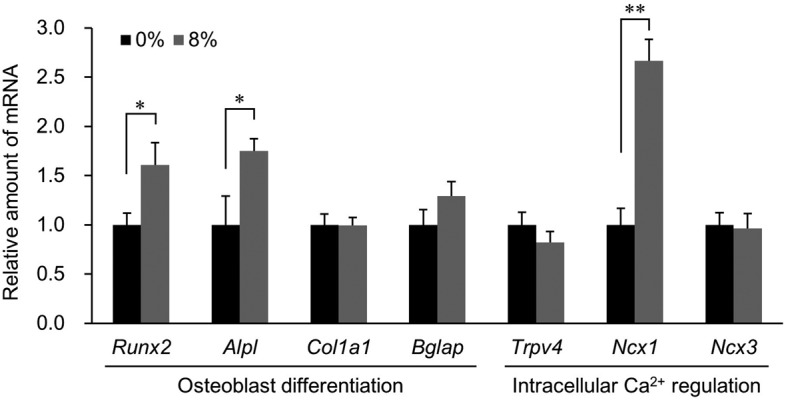
Gene expression of osteoblast differentiation markers (Runx2, Alpl, Col1a1, and Bglap) and regulating factors involved in intracellular Ca2+ concentration (Trpv4, Ncx1, and Ncx3) in the undifferentiated MC3T3-E1 cells exposed to 8% stretch stimulus. Each gene expression level was defined as the value obtained from realtime RT-PCR analysis divided by the Gapdh value. The data were analyzed using Student’s t-test. Mean ± SD, n=5, *P<0.05, **P<0.01.
In the differentiation of osteoblasts, the synthesis levels of proteins such as alkaline phosphatase (ALPL), type I collagen (COL1A1), and osteocalcin (BGLAP) change at each stage of differentiation. The expression levels of Alpl and Col1a1 mRNA increase in the early and middle stages of osteoblast differentiation, whereas Bglap expression is associated with terminal differentiation [8]. In this study, the stretch stimulation significantly increased the Alpl mRNA level; however, the expression of Col1a1 and Bglap showed no change (Fig. 3). Although Alpl and Col1a1 are known as markers expressed from the early stages of osteogenic differentiation, Alpl, whose protein serves to hydrolyze extracellular pyrophosphate, an inhibitor of calcium crystal formation in bone [11], is suggested to be more responsive to mechanical stimuli than Col1a1.
A comparison of gene expression of regulating factors involved in intracellular Ca2+ concentration, except for Ncx1, revealed no difference (Fig. 3). Although it is believed that TRPV4 is activated by mechanical stimuli [9, 12], no change was observed in mRNA expression after stretch stimulation. This result suggests that, in undifferentiated MC3T3-E1 cells, stretch stimulation does not affect Trpv4 gene expression. In contrast, expression of Ncx1 mRNA increased significantly (P<0.01) after exposure to 8% stretch stimulation (2.7 times compared to the control group; Fig. 3). To confirm whether the Ncx1 gene responds to lower stretch stimuli than 8%, qPCR analysis of Ncx1 expression in cells after 2, 5, and 8% stretch stimuli was performed. As a result, Ncx1 increased in proportion to the intensity of the stretch stimulus (Fig. 4). NCX typically plays a role in pumping Na+ into cells and Ca2+ out of cells along the Na+ concentration gradient via the cell membrane (forward mode). However, in pathological states, a Ca2+ flow reversal phenomenon (reverse mode) can occur [5]. The intracellular Ca2+ staining performed in this experiment was attenuated as stretch stimulation intensity increased, and accordingly, the stretch stimuli applied in this experiment caused stretch intensity-dependent forward-mode activation rather than nonphysiological reverse-mode activation, as described in Fig. 2. Sosnoski and Gay [14] have reported that NCX3 is a major contributor to Ca2+ translocation out of differentiated MC3T3-E1 osteoblasts into calcifying bone matrix and that NCX1 has little to no involvement. Considering our results of Ncx1 and Ncx3 gene expression patterns after stretch stimulation, the MC3T3-E1 cells may initially use NCX1 as the main NCX and gradually shift from NCX1 to NCX3 with progressive differentiation. In this experiment, whether increased Ncx1 expression was the direct result of the influence of stretch stimulation or whether the increase was due to uptake of Ca2+ via TRPV remains unclear. Analysis of restrictions on the flow of Ca2+ in and out of cells using TRPV4 antagonists such as HC-067047 [3] or Na+/Ca2+ exchanger inhibitors such as KB-R7943 [6] is believed to be necessary.
Fig. 4.
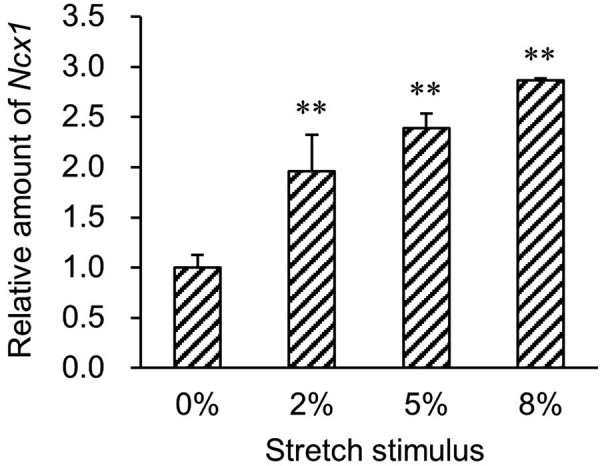
Quantitative PCR analysis of Ncx1 mRNA expression in cells after 2, 5, and 8% stretch stimuli. The data were normalized to the Gapdh mRNA level in each sample and are expressed as values relative to the internal control. The measurement values for each group were compared using Bonferroni-Dunn’s post hoc tests. Mean ± SD, n=5, **P<0.01.
Based on the above, a conclusion can be drawn that exposure to stretch stimulation facilitates greater Ca2+ excretion than inflow from the extracellular space in undifferentiated MC3T3-E1 cells due to NCX1 activity and promotes osteogenesis following osteoblast differentiation.
Acknowledgments
This work was supported in part by JSPS KAKENHI Grant Number 26450444 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
REFERENCES
- 1.Damann N., Voets T., Nilius B.2008. TRPs in our senses. Curr. Biol. 18: R880–R889. doi: 10.1016/j.cub.2008.07.063 [DOI] [PubMed] [Google Scholar]
- 2.Duncan R. L., Akanbi K. A., Farach-Carson M. C.1998. Calcium signals and calcium channels in osteoblastic cells. Semin. Nephrol. 18: 178–190. [PubMed] [Google Scholar]
- 3.Everaerts W., Zhen X., Ghosh D., Vriens J., Gevaert T., Gilbert J. P., Hayward N. J., McNamara C. R., Xue F., Moran M. M., Strassmaier T., Uykal E., Owsianik G., Vennekens R., De Ridder D., Nilius B., Fanger C. M., Voets T.2010. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc. Natl. Acad. Sci. U.S.A. 107: 19084–19089. doi: 10.1073/pnas.1005333107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamano K., Kawanishi T., Mizuno A., Suzuki M., Takagi Y.2016. Involvement of Transient Receptor Potential Vanilloid (TRPV) 4 in mouse sperm thermotaxis. J. Reprod. Dev. 62: 415–422. doi: 10.1262/jrd.2015-106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwamoto T., Kita S.2004. Development and application of Na+/Ca2+ exchange inhibitors. Mol. Cell. Biochem. 259: 157–161. doi: 10.1023/B:MCBI.0000021367.42752.54 [DOI] [PubMed] [Google Scholar]
- 6.Iwamoto T., Watano T., Shigekawa M.1996. A novel isothiourea derivative selectively inhibits the reverse mode of Na+/Ca2+ exchange in cells expressing NCX1. J. Biol. Chem. 271: 22391–22397. doi: 10.1074/jbc.271.37.22391 [DOI] [PubMed] [Google Scholar]
- 7.Koizumi T., Hikiji H., Shin W. S., Takato T., Fukuda S., Abe T., Koshikiya N., Iwasawa K., Toyo-oka T.2003. Cell density and growth-dependent down-regulation of both intracellular calcium responses to agonist stimuli and expression of smooth-surfaced endoplasmic reticulum in MC3T3-E1 osteoblast-like cells. J. Biol. Chem. 278: 6433–6439. doi: 10.1074/jbc.M210243200 [DOI] [PubMed] [Google Scholar]
- 8.Komori T.2008. Regulation of bone development and maintenance by Runx2. Front. Biosci. 13: 898–903. doi: 10.2741/2730 [DOI] [PubMed] [Google Scholar]
- 9.Mutai H., Heller S.2003. Vertebrate and invertebrate TRPV-like mechanoreceptors. Cell Calcium 33: 471–478. doi: 10.1016/S0143-4160(03)00062-9 [DOI] [PubMed] [Google Scholar]
- 10.Ousingsawat J., Wanitchakool P., Schreiber R., Wuelling M., Vortkamp A., Kunzelmann K.2015. Anoctamin-6 controls bone mineralization by activating the calcium transporter NCX1. J. Biol. Chem. 290: 6270–6280. doi: 10.1074/jbc.M114.602979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oyajobi B. O., Caswell A. M., Russell R. G.1994. Transforming growth factor β increases ecto-nucleoside triphosphate pyrophosphatase activity of human bone-derived cells. J. Bone Miner. Res. 9: 99–109. doi: 10.1002/jbmr.5650090114 [DOI] [PubMed] [Google Scholar]
- 12.Pedersen S. F., Nilius B.2007. Transient receptor potential channels in mechanosensing and cell volume regulation. Methods Enzymol. 428: 183–207. doi: 10.1016/S0076-6879(07)28010-3 [DOI] [PubMed] [Google Scholar]
- 13.Rutkovskiy A., Stensløkken K. O., Vaage I. J.2016. Osteoblast differentiation at a glance. Med. Sci. Monit. Basic Res. 22: 95–106. doi: 10.12659/MSMBR.901142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sosnoski D. M., Gay C. V.2008. NCX3 is a major functional isoform of the sodium-calcium exchanger in osteoblasts. J. Cell. Biochem. 103: 1101–1110. doi: 10.1002/jcb.21483 [DOI] [PubMed] [Google Scholar]
- 15.Stains J. P., Weber J. A., Gay C. V.2002. Expression of Na+/Ca2+ exchanger isoforms (NCX1 and NCX3) and plasma membrane Ca2+ ATPase during osteoblast differentiation. J. Cell. Biochem. 84: 625–635. doi: 10.1002/jcb.10050 [DOI] [PubMed] [Google Scholar]
- 16.Suzuki M., Mizuno A., Kodaira K., Imai M.2003. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 278: 22664–22668. doi: 10.1074/jbc.M302561200 [DOI] [PubMed] [Google Scholar]
- 17.Thurneysen T., Nicoll D. A., Philipson K. D., Porzig H.2002. Immunohistochemical detection of the sodium-calcium exchanger in rat hippocampus cultures using subtype-specific antibodies. Ann. N. Y. Acad. Sci. 976: 367–375. doi: 10.1111/j.1749-6632.2002.tb04763.x [DOI] [PubMed] [Google Scholar]
- 18.Yoo Y. M., Kwag J. H., Kim K. H., Kim C. H.2014. Effects of neuropeptides and mechanical loading on bone cell resorption in vitro. Int. J. Mol. Sci. 15: 5874–5883. doi: 10.3390/ijms15045874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yusa K., Yamamoto O., Iino M., Takano H., Fukuda M., Qiao Z., Sugiyama T.2016. Eluted zinc ions stimulate osteoblast differentiation and mineralization in human dental pulp stem cells for bone tissue engineering. Arch. Oral Biol. 71: 162–169. doi: 10.1016/j.archoralbio.2016.07.010 [DOI] [PubMed] [Google Scholar]
- 20.Zayzafoon M.2006. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J. Cell. Biochem. 97: 56–70. doi: 10.1002/jcb.20675 [DOI] [PubMed] [Google Scholar]
- 21.Zhang P., Wu Y., Jiang Z., Jiang L., Fang B.2012. Osteogenic response of mesenchymal stem cells to continuous mechanical strain is dependent on ERK1/2-Runx2 signaling. Int. J. Mol. Med. 29: 1083–1089. [DOI] [PubMed] [Google Scholar]


