Abstract
In canine electrocardiography, the reference interval of the ventricular mean electrical axis (MEA) is between +40° and +100°. MEA values in dogs can be influenced by the patient position as well as by the shape of the thorax. The aim of this study was to evaluate the MEA in healthy Doberman Pinschers, hypothesizing that some present a left shift of the MEA. In this retrospective study, 41 healthy, client-owned Doberman Pinschers were included. Echocardiography and standard six-lead ECG examination were performed in all dogs. The MEA was calculated using the isoelectric method. The morphology of the QRS complex and the Q/R ratio in lead II were also evaluated. The median MEA was +45° and ranged from −45° to +90°. MEA was deviated in 16/41 dogs (39%), all presenting a left axis deviation (range, −45° to + 30°). Age was significantly associated with the MEA (P=0.008), showing a negative linear correlation. A deep Q wave in lead II was present in 26/41 (63%) dogs. The Q/R ratio was higher in dogs presenting left shift of the MEA (0.66; range, 0.28–1.35) in comparison to dogs with a MEA within reference range (0.44; range, 0.04–0.73; P<0.001). In conclusion, a significant number of healthy Doberman Pinschers present a left shift of the MEA compare to the reference range, and dogs with MEA deviation show a higher Q/R ratio in lead II. This data should be considered when electrocardiographic evaluation is performed in Doberman Pinschers.
Keywords: canine, deep Q wave, dilated cardiomyopathy, electrocardiography, left axis deviation
In electrocardiography (ECG), the ventricular mean electrical axis (MEA) is defined as the average direction of the ventricular myocardial activation vectors during the cardiac cycle [20]. In the general canine population, normal MEA values on the frontal plane range between +40° and + 100° [5, 9, 20]. In clinical practice, an abnormal MEA may indicate variation of the cardiac axis, cardiac chamber enlargement or intraventricular conduction disturbances [20]. Previous studies stated that the MEA can be influenced by the shape of the thorax, with narrow-chested dogs having the anatomical orientation of the cardiac axis inside the thorax more vertical in comparison to broad-chested dogs, in which the cardiac axis is more horizontal [4, 7, 13, 15, 20].
In Doberman Pinschers, standard ECG and Holter ECG are commonly used for the screening and the diagnosis of dilated cardiomyopathy [2, 3, 14, 19, 21, 23]. A previous study showed that in a population of apparently healthy Doberman Pinschers the MEA range from −42° to +101°, with a left shift deviation occurring in 25% of dogs which was ascribed to a possible preclinical stage of dilated cardiomyopathy [11]. Nevertheless, no thoracic x-Ray and/or echocardiography was performed in that study, and the left shift of the MEA was considered a possible indication of left ventricular enlargement and/or left bundle branch block [20].
The aim of this study was to evaluate the MEA in a sample population of healthy Doberman Pinschers in order to describe a reference range in this specific breed. Since Doberman Pinschers is a narrow-chested breed, we hypothesized that some healthy Doberman Pinchers may present a left axis deviation of the MEA.
MATERIALS AND METHODS
This was a retrospective, multicentric, observational study. Case inclusion was performed at the Department of Cardiology of the Istituto Veterinario di Novara and at the Department of Veterinary Sciences of the University of Pisa. Due to the retrospective study design, no institutional animal care and use approval or client consent was sought.
Case selection
Medical records from June 2013 to May 2017 were reviewed for healthy, client-owned Doberman Pinschers. Dogs were defined healthy base on history, physical examination, ECG and echocardiography. All cases had to have undergone a standard 6-lead ECG and a complete echocardiographic examination on the same day. Exclusion criteria consisted of any finding indicating cardiac or systemic disease. All echocardiographic exams were reviewed by a board-certified cardiologist (O.D.), who verified that all dogs included in the study were not affected by dilated cardiomyopathy according to echocardiographic criteria of the more recent published screening guidelines for dilated cardiomyopathy [21]. In addition, the clinical follow-up of each dog was ascertained by telephone interview with the owner at the time of inclusion. The owner was asked if the dog was dead or alive. If the dog was dead, the owner was asked if the dog had been euthanized or died spontaneously, reasons for euthanasia, and, in case of spontaneous death, the possible causes. Cardiac-related death was defined as death occurring because of occurrence and progression of heart failure. Sudden death was regarded as cardiac-related if no other cause of death was obvious. Only dogs that were still alive at the time of the study or that died from non-cardiac related death were included in the study.
Echocardiography
All echocardiographic examinations were performed as previously described with the dogs awake in right and left lateral recumbency [22], using ultrasonographic units (Vivid I, General Electric Healthcare, Boston, MA, U.S.A.; Xario XG, Toshiba, Tokyo, Japan) equipped with phased-array transducers. Linear and volume measurements of the left ventricle were made with standard techniques, and dilated cardiomyopathy was excluded using the left ventricular end-diastolic and end-systolic diameters, the left ventricular end-diastolic and end-systolic volume indexed for body surface area, and the left ventricular ejection fraction as previously described [14, 17, 21, 23]. Continuous ECG monitoring was performed during the echocardiographic examination.
Electrocardiography
All 6-lead ECGs were acquired with the dogs in right lateral recumbence and without the use of sedation. The electrocardiographic leads were positioned as previously described [20]. The electrocardiographic units (Elan 1100 ECG system, Cardioline, Trento, Italy; MAC 800 ECG system, General Electric Healthcare) were set with a sampling frequency of 1,000 Hz for acquisition, a 100 Hz low-pass filter and a 0.3–0.5 Hz high-pass filter to decrease respiratory artefact [10].
Mean HR (beats per min, bpm) was calculated as the number of QRS complexes recorded in 30 sec and multiplied by two. Measurements of waves, intervals and segments were made in lead II according to the standard recommendations [20]. Amplitude of the P, Q, R, and S waves (mV), together with duration of the PQ interval (msec), QRS complex (msec) and QT interval (msec) were measured. Morphology of the QRS complex in lead II was evaluated. Each deflection composing the QRS complex was described based on its amplitude, with capital letters if the amplitude was major or equal than 0.5 mV or with tiny letters if it was lower than 0.5 mV [15, 18, 20]. Presence of a Q wave greater than 0.5 mV in lead II was defined as “deep” Q wave. The ratio between the amplitude of Q and R waves in lead II (Q/R ratio) was calculated.
The MEA was calculated using the isoelectric method. With this method all six leads are inspected, and the lead with the algebraic sum of the QRS deflection closer or equal to zero is defined as the isoelectric lead. The lead perpendicular to the isoelectric lead represents the direction of the MEA. If the dominant deflection of the QRS complex is positive the MEA is directed to the positive pole of the perpendicular lead, while if the lead is negative the MEA is directed towards the negative pole of the same perpendicular lead [20]. This method approximates the value of MEA of ± 30°.
To reduce the degree of error to ± 15° is necessary to evaluate the isoelectric lead and verify if the sum of the QRS complex is zero, slightly positive or slightly negative.
If the sum is zero, the MEA contains yet an error of ± 15°. If the sum is slightly positive the MEA is directed an additional 15° towards the positive pole of the isoelectric lead, while if the sum is slightly negative the MEA is directed an additional 15° towards the negative pole of the isoelectric lead [18]. A MEA less than +40° was defined as left shift, and a MEA greater than +100° as right shift [20].
Statistical analysis
Statistical analysis was performed using commercially available statistical software (GraphPad Prism version 5.0 for Windows, GraphPad Software, San Diego, CA, U.S.A.; Minitab version 18 for Windows, Minitab Inc., Pennsylvania, PA, U.S.A.).
Descriptive statistics were generated. The normality of data distribution was tested using the Shapiro-Wilk test, and parametric or non-parametric tests were used according to the Gaussian distribution. The chi-squared or Fisher’s exact tests were used to compare categorical variables. Continuous variables were compared using the unpaired t-test or the Mann-Whitney U-test, based on data distribution. Data are reported as median and range (minimum-maximum), unless otherwise stated. Multiple linear regression analysis was used to identify significant independent predictors of the MEA among selected clinical variables (age, sex, body weight). A value of P<0.05 was considered statistically significant.
RESULTS
The sample population included 41 healthy Doberman Pinschers. Twenty-six were females and 15 males, with a mean age of 5.2 ± 2.7 years (range, 1 to 12 years), and a mean body weight of 35.1 ± 6.3 kg (range, 28 to 50 kg). Electrocardiographic findings in our population are reported in Table 1. All dogs had sinus rhythm, with no arrhythmias observed during the standard ECG and on the continuous ECG during the echocardiographic exam. Electrocardiographic measurements were within the reference range, apart from 3 dogs (7%) that had a R wave amplitude greater than 3 mV in lead II. The median MEA was +45°, with a range from −45° to +90°. Deviation of the MEA was found in 16 over 41 dogs (39%). All dogs with a MEA deviation presented a left shift of the MEA, with median MEA of 0° and a range from −45° to + 30° (Figs. 1 and 2). According to multivariate analyses, sex (P=0.205) and body weight (P=0.091) were not associated with MEA values. Only age was significantly associated with MEA (P=0.008), showing a negative linear correlation (r= −0.41).
Table 1. Electrocardiographic findings of the all study population.
| Variable | Study population (n=41) | Reference range a) |
|---|---|---|
| HR (bpm) | 100 (60–160) | 70–160 |
| P (msec) | 38 (20–50) | <40–50 |
| P (mV) | 0.2 (0.1–0.4) | ≤0.4 |
| PQ (msec) | 108 (60–130) | 60–130 |
| QRS (msec) | 45 (40–60) | ≤60 |
| QRS MEA (°) | +45 (−45–+90) | +40–+100 |
| Q (mV) | 0.7 (0.1–1.9) | - |
| R (mV) | 1.6 (0.5–3.4) | ≤3 |
| S (mV) | 0.2 (0.1–0.7) | - |
| QT (msec) | 200 (160–245) | 150–250 |
Data from study population are reported as median and range. a) From Tilley, 1992.
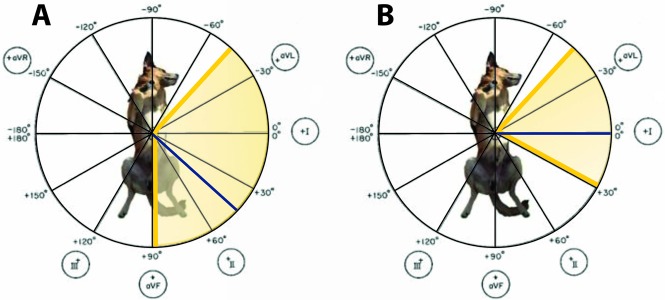
Fig. 1.
Mean electrical axis in all study population (A) and in dogs with left-shift (B). Median MEA value is depicted with the blue line and yellow area represents the MEA range.
Fig. 2.
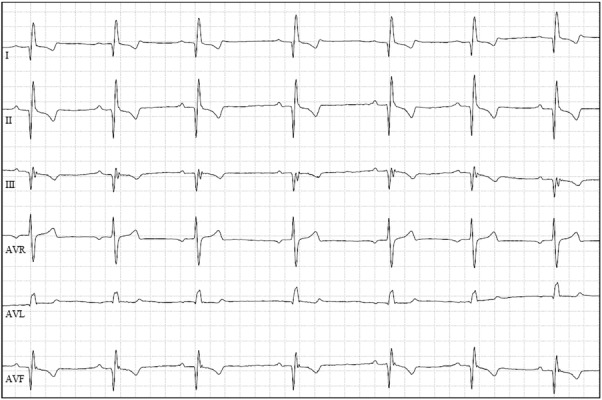
Example of left-shift of the MEA in a Doberman Pinscher of the study. Note the deep Q wave in lead II. Paper speed=50 mm/sec; 5 mm/mV.
Regarding QRS complex morphology in lead II, 15 (36%) dogs had a QR morphology, 13 (32%) had a qRs morphology, 9 (22%) had a QRs morphology, 2 (5%) had a QRS morphology, 1 (2.5%) had a qR morphology and 1 (2.5%) had a qrs morphology.
In dogs with left shift of the MEA (n=16), QRS complex showed a QRs morphology in 5 dogs (31%), QR morphology in 4 dogs (25%), qRs morphology in 4 dogs (25%), QRS morphology in 2 dogs (13%) and qrs morphology in 1 (6%).
A deep Q wave in lead II was present in 26/41 (63%) dogs. Prevalence of deep Q wave was not different between dogs with left shift of the MEA (11/16, 69%) and dogs having a MEA within reference range (15/25, 60%; P=0.742).
The median Q/R ratio in all study population was 0.52, with a range from 0.04 to 1.35. The Q/R ratio was significantly higher in dogs presenting left shift of the MEA (0.66; range, 0.28–1.35) in comparison to dogs with a MEA within reference range (0.44; range, 0.04–0.73; P<0.001; Fig. 3). Among dogs with left axis deviation, 5/11 (45%) showed a Q/R ratio >1; differently, none of the dogs with normal MEA had Q/R>1 (P=0.006).
Fig. 3.
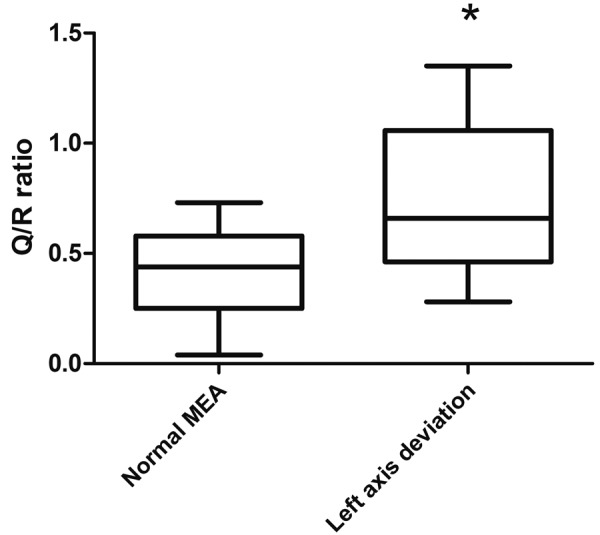
Box and whiskers plot for the Q/R ratio in lead II. Dogs presenting left axis deviation showed a higher Q/R ratio in comparison to dogs having a MEA within reference range (*P<0.001).
DISCUSSION
In our population of healthy Doberman Pinschers, the median MEA was +45° with a range from −45° to +90°. According to the normal reference range in dogs [20], in our study 39% of healthy Doberman Pinschers showed a left axis deviation of the MEA, ranging from −45° to +30°. Similar findings were reported in a previous study in Doberman Pinschers, where the mean MEA was +51° with a range from −42° to +101° and with a left shift deviation occurring in 25% of dogs [11]. However, in that study echocardiography was not performed in all dogs, and the authors concluded that the left shift of the MEA found in some dogs could have been a consequence of occult dilated cardiomyopathy. In our study we performed the echocardiographic examination in all dogs to exclude structural heart diseases and we similarly found a left shift of the MEA in healthy Doberman Pinchers. Similar to our findings, in a previous study, 17% of healthy dogs from 25 different breeds presented a left shift of the MEA in comparison to the standard reference range [9]. Left shift of the MEA can be induced by the standing up position, causing a more vertical anatomical cardiac axis into the thoracic cavity compared to the right lateral recumbence [6, 8, 10, 16]. This change of the position of the heart into the thoracic cavity during the standing up recording could explain the MEA variation [16]. Thus, anatomical cardiac axis has a definitive influence on MEA. Anatomical cardiac axis is different among dogs according to thoracic conformation, with narrow-chested dogs having the anatomical orientation of the cardiac axis inside the thorax more vertical in comparison to broad-chested dogs in which the cardiac axis is more horizontal [4, 7, 13, 15, 20]. Similarly, significant differences in MEA values have been reported between narrow and broad chested dogs [4, 9, 13]. Considering the dolicomorphic conformation of Doberman pinchers, the different MEA range of our study in comparison to standard reference range could be due to a different anatomical cardiac axis in this narrow-chested breed in comparison to general canine population. However further studies are needed to verify our results in a large population of narrow-chested dogs.
Left axis deviation without an increase in QRS complex duration can be caused by left anterior fascicular block. In this type of fascicular block, the pattern of ventricular depolarization is characterized by a “rS” morphology in lead II (i.e. small r waves and deep S waves), and Q wave is not present in lead II [18, 20]. In our study all dogs showed a Q wave in lead II, and none of the dogs with left axis deviation had a rS morphology in inferior leads. Thus, a left ventricular conduction disturbance as a cause of left axis deviation was considered unlikely in this population.
In our study, age was significantly associated with MEA, showing a negative linear correlation. Thus, older dogs tended to present a lower value of the MEA. A possible explanation is a progressive variation of cardiac anatomical axis with age. According to several authors the variability of the canine ECG is largely due to variations in the degree with which the canine heart is suspended in the mediastinum compare to that of the human. The heart depending on its weight, becomes considerably displaced especially in the lateral recumbent position [6]. This possibility of the heart to move into the thorax can influence the values of the MEA, especially in older dogs where an increase of the ligament laxity could lead to heart displacement.
In our study, heart rate, duration and amplitude of the electrocardiographic waves and intervals were found to be within the normal range for dogs, except the R wave amplitude which was over the upper limit in 3 dogs [20]. In previous studies it has been found that in healthy Whippet and Alaskan sled dogs, the R wave normal amplitude values were up to 4.3 mV and 4.4 mV, respectively [1, 10]. It has been shown that R wave amplitude can be influenced by several factors such as, left ventricular enlargement, physical exercise or thoracic conformation [1, 20].
Regarding the morphology of the QRS complex, the majority of dogs in our study presented a deep Q wave in lead II observed in 63% of dogs. In a previous study in Dobermans [11], most dogs showed a deep Q wave in lead II, with a mean amplitude of the Q wave equal to 0.65 ± 0.4 mV. This is in line with our study, where we found a median amplitude of the Q wave of 0.7 mV (range, 0.1–1.9 mV). A high voltage of the Q wave has been described as a physiologic finding in dogs with a deep chest [7, 20]. A previous study reported a median Q/R ratio in lead II of 0.18 (range, 0.02–0.33) in healthy Golden retrievers [12]. In our study the median Q/R was 0.52, with a range from 0.04 to 1.35. This could confirm that Doberman pinschers probably present deeper Q waves in lead II in comparison to other breeds.
The study has some limitations. First, the retrospective nature of the study and the relatively small sample size. Second, Holter monitoring were not performed to exclude occult dilated cardiomyopathy, although standard echocardiography to exclude structural heart disease was performed in all dogs.
In conclusion, the reference normal range of the MEA in the Doberman Pinschers is different from the standard reference values used for the general canine population, with a significant number of healthy Doberman Pinchers presenting a left axis deviation of the MEA. In lead II, most healthy Dobermans have a deep Q wave and the Q/R ratio is higher in dogs presenting left shift of the MEA. We suggest that these results should be taken into consideration when electrocardiographic interpretation is done in Doberman Pinschers, and breed-related reference range for ECG parameters should be used in clinical practice.
Acknowledgments
The study was not supported by a grant. Part of the study was presented as a poster at the 28th ECVIM-CA Congress, September 2018, Rotterdam, The Netherlands. The authors are grateful to Valentina Meucci for the statistical analysis and to Jan Polverini for technical assistance.
REFERENCES
- 1.Bavegems V., Duchateau L., Ham L. V., Rick A. D., Sys S. U.2009. Electrocardiographic reference values in whippets. Vet. J. 182: 59–66. doi: 10.1016/j.tvjl.2008.05.003 [DOI] [PubMed] [Google Scholar]
- 2.Calvert C. A.1995. Diagnosis and management of ventricular tachyarrhythmias in Doberman Pinschers with cardiomyopathy. pp. 799–806. In: Kirk’s Current Veterinary Therapy. Small Animal Practice XII (Bonagura, J. D. ed.), W.B. Saunders, Philadelphia. [Google Scholar]
- 3.Calvert C. A., Wall T. M.2001. Correlations among time and frequency measures of heart rate variability recorded by use of a Holter monitor in overtly healthy Doberman pinschers with and without echocardiographic evidence of dilated cardiomyopathy. Am. J. Vet. Res. 62: 1787–1792. doi: 10.2460/ajvr.2001.62.1787 [DOI] [PubMed] [Google Scholar]
- 4.Chastain C. B., Riedesel D. H., Pearson P. T.1974. McFee and Parungao orthogonal lead vectocardiography in normal dogs. Am. J. Vet. Res. 35: 275–280. [PubMed] [Google Scholar]
- 5.Dubin S., Beard R., Staib J., Hunt P.1977. Variation of canine and feline frontal-plane QRS axes with lead choice and augmentation ratio. Am. J. Vet. Res. 38: 1957–1962. [PubMed] [Google Scholar]
- 6.Eckenfels A., Trieb G.1979. The normal electrocardiogram of the conscious beagle dog. Toxicol. Appl. Pharmacol. 47: 567–584. doi: 10.1016/0041-008X(79)90527-1 [DOI] [PubMed] [Google Scholar]
- 7.Gugjoo M. B., Hoque M., Saxena A. C., Zama M. M.2014. Reference values of six-limb-lead electrocardiogram in conscious Labrador retriever dogs. Pak. J. Biol. Sci. 17: 689–695. doi: 10.3923/pjbs.2014.689.695 [DOI] [PubMed] [Google Scholar]
- 8.Hanton G., Rabemampianina Y.2006. The electrocardiogram of the Beagle dog: reference values and effect of sex, genetic strain, body position and heart rate. Lab. Anim. 40: 123–136. doi: 10.1258/002367706776319088 [DOI] [PubMed] [Google Scholar]
- 9.Hill J. D.1968. The electrocardiogram in dogs with standardized body and limb positions. J. Electrocardiol. 1: 175–182. doi: 10.1016/S0022-0736(68)80025-1 [DOI] [PubMed] [Google Scholar]
- 10.Hinchcliff K. W., Constable P. D., Farris J. W., Schmidt K. E., Hamlin R. L.1997. Electrocardiographic characteristics of endurance-trained Alaskan sled dogs. J. Am. Vet. Med. Assoc. 211: 1138–1141. [PubMed] [Google Scholar]
- 11.Kovacevic A., Duras M., Gomercic T.1999. Contribution to standardization of heart rate and electrocardiographic values in Doberman Pinschers. Vet. Arh. 69: 211–219. [Google Scholar]
- 12.Moise N. S., Valentine B. A., Brown C. A., Erb H. N., Beck K. A., Cooper B. J., Gilmour R. F.1991. Duchenne’s cardiomyopathy in a canine model: electrocardiographic and echocardiographic studies. J. Am. Coll. Cardiol. 17: 812–820. doi: 10.1016/S0735-1097(10)80202-5 [DOI] [PubMed] [Google Scholar]
- 13.Mukherjee J., Das P. K., Ghosh P. R., Banerjee D., Sharma T., Basak D., Sanyal S.2015. Electrocardiogram pattern of some exotic breeds of trained dogs: A variation study. Vet. World 8: 1317–1320. doi: 10.14202/vetworld.2015.1317-1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Grady M. R., O’Sullivan M. L., Minors S. L., Horne R.2009. Efficacy of benazepril hydrochloride to delay the progression of occult dilated cardiomyopathy in Doberman Pinschers. J. Vet. Intern. Med. 23: 977–983. doi: 10.1111/j.1939-1676.2009.0346.x [DOI] [PubMed] [Google Scholar]
- 15.Rezakhani A., Atwell R. B., Webster J.1990. Electrocardiographic values of German shepherd dogs. Aust. Vet. J. 67: 307–309. doi: 10.1111/j.1751-0813.1990.tb07806.x [DOI] [PubMed] [Google Scholar]
- 16.Rishniw M., Porciello F., Erb H. N., Fruganti G.2002. Effect of body position on the 6-lead ECG of dogs. J. Vet. Intern. Med. 16: 69–73. doi: 10.1111/j.1939-1676.2002.tb01608.x [DOI] [PubMed] [Google Scholar]
- 17.Sahn D. J., DeMaria A., Kisslo J., Weyman A.1978. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 58: 1072–1083. doi: 10.1161/01.CIR.58.6.1072 [DOI] [PubMed] [Google Scholar]
- 18.Santilli R., Moise N. S., Pariaut R., Perego M.2018. Electrocardiography of the Dog and Cat: Diagnosis of Arrhythmias. pp. 53, 60, 277–279. Edra, Milano. [Google Scholar]
- 19.Tidholm A., Jönsson L.1997. A retrospective study of canine dilated cardiomyopathy (189 cases). J. Am. Anim. Hosp. Assoc. 33: 544–550. doi: 10.5326/15473317-33-6-544 [DOI] [PubMed] [Google Scholar]
- 20.Tilley L. P.1992. The normal canine and feline electrocardiogram. In: Essentials of Canine and Feline Electrocardiography: Interpretation and Treatment. pp. 48, 50–52, 68, 116, 438, 3rd ed., Lea & Febiger, Philadelphia. [Google Scholar]
- 21.Wess G., Domenech O., Dukes-McEwan J., Häggström J., Gordon S.2017. European Society of Veterinary Cardiology screening guidelines for dilated cardiomyopathy in Doberman Pinschers. J. Vet. Cardiol. 19: 405–415. doi: 10.1016/j.jvc.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 22.Thomas W. P., Gaber C. E., Jacobs G. J., Kaplan P. M., Lombard C. W., Moise N. S., Moses B. L.1993. Recommendations for standards in transthoracic two-dimensional echocardiography in the dog and cat. Echocardiography Committee of the Specialty of Cardiology, American College of Veterinary Internal Medicine. J. Vet. Intern. Med. 7: 247–252. doi: 10.1111/j.1939-1676.1993.tb01015.x [DOI] [PubMed] [Google Scholar]
- 23.Wess G., Mäurer J., Simak J., Hartmann K.2010. Use of Simpson’s method of disc to detect early echocardiographic changes in Doberman Pinschers with dilated cardiomyopathy. J. Vet. Intern. Med. 24: 1069–1076. doi: 10.1111/j.1939-1676.2010.0575.x [DOI] [PubMed] [Google Scholar]