Abstract
MKRN3 mutations represent the most common genetic cause of central precocious puberty (CPP) but associations between genotype and clinical features have not been extensively explored. This systematic review and meta-analysis investigated genotype-phenotype associations and prevalence of MKRN3 mutations in CPP. The search was conducted in seven electronic databases (Cochrane, EMBASE, LILACS, LIVIVO, PubMed, Scopus, and Web of Science) for articles published until 4 September 2018. Studies evaluating MKRN3 mutations in patients with CPP were considered eligible. A total of 22 studies, studying 880 subjects with CPP, fulfilled the inclusion criteria. Eighty-nine subjects (76 girls) were identified as harboring MKRN3 mutations. Girls, compared with boys, exhibited earlier age at pubertal onset (median, 6.0 years; range, 3.0 to 7.0 vs 8.5 years; range, 5.9 to 9.0; P < 0.001), and higher basal FSH levels (median, 4.3 IU/L; range, 0.7 to 13.94 IU/L vs 2.45 IU/L; range, 0.8 to 13.70 IU/L; P = 0.003), and bone age advancement (ΔBA; median, 2.3 years; range, −0.9 to 5.2 vs 1.2 years; range, 0.0 to 2.3; P = 0.01). Additional dysmorphisms were uncommon. A total of 14 studies evaluating 857 patients were included for quantitative analysis, with a pooled overall mutation prevalence of 9.0% (95% CI, 0.04 to 0.15). Subgroup analysis showed that prevalence estimates were higher in males, familial cases, and in non-Asian countries. In conclusion, MKRN3 mutations are associated with nonsyndromic CPP and manifest in a sex-dimorphic manner, with girls being affected earlier. They represent a common cause of CPP in western countries, especially in boys and familial cases.
Keywords: central precocious puberty, MKRN3, systematic review, meta-analysis
Precocious puberty is traditionally defined by the onset of progressive pubertal development before the age of 8 years in girls and 9 years in boys. Central precocious puberty (CPP), also known as gonadotropin-dependent precocious puberty, is a condition resulting from the early reactivation of the hypothalamic-pituitary-gonadal axis [1, 2]. In the absence of central nervous system abnormalities, CPP is classified as idiopathic, and it predominantly affects girls. Idiopathic CPP is familial in as many as 27.5% of the cases, emphasizing the genetic origin of this disorder [3].
The involvement of the makorin ring finger protein 3 (MKRN3) gene in the pathogenesis of CPP was first described by Abreu et al. in 2013 after evaluating patients with familial CPP using whole-exome sequencing [4]. Subsequently, mutations in MKRN3 have been described in multiple patients with CPP from different countries [5–16].
MKRN3, previously known as ZNF127, is an intronless and imprinted gene located on chromosome 15q11-q13, in the Prader-Willi syndrome (PWS) region [17]. Because of maternal imprinting, only the paternal allele is expressed. Therefore, CPP can be expected only if the mutated allele is paternally inherited [2, 4, 18]. It encodes a protein belonging to the Makorin family of zinc finger proteins with proposed ubiquitin-protein ligase (E3) activity, based on its structure and on activity of the other members of the makorin family. The findings of loss of function mutations in MKRN3 associated with the pattern of the hypothalamic Mkrn3 mRNA expression in mice suggest that MKRN3 acts to inhibit puberty initiation [4, 18], though its exact mechanism of action remains to be elucidated.
Currently, MKRN3 mutations are recognized as the main genetic cause of CPP and appear to be particularly common in familial cases (up to 33% to 46%) [7, 18], although no mutations were described in the subgroup of Korean girls with familial CPP [12]. In the sporadic form of CPP, the prevalence was 3.9% in a large cohort of Brazilian girls [5], however it was variable in other reports, ranging from 0% to 20% in small cohorts of Italian and Bulgarian girls, respectively [19, 20]. Thus, the exact prevalence of mutations in patients with CPP and possible geographical differences worldwide has not been explored.
Patients carrying MKRN3 mutations exhibit typical clinical and biochemical features of premature reactivation of the reproductive axis. Few studies compared data of patients with and without mutations, and higher levels of basal FSH and an earlier age at pubertal onset had been described among girls with mutations [5, 7], whereas affected boys exhibited later pubertal onset in comparison with those with CPP and no mutations [15]. Thus, it is still not known whether their phenotype differs from those without mutations. Therefore, we performed a systematic review and meta-analysis aiming to identify and synthesize data on MKRN3 mutations in CPP, to investigate genotype-phenotype associations, and to estimate the worldwide prevalence of MKRN3 mutations in patients with idiopathic CPP.
1. Methods
The methodology of this systematic review was based on the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [21]. The protocol was registered at the international prospective register of systematic reviews with the number CRD42018095144 (http://www.crd.york.ac.uk/PROSPERO/).
A. Eligibility Criteria
Observational human studies and clinical trials evaluating MKRN3 mutations in patients with idiopathic CPP were considered eligible. Idiopathic CPP was defined by progressive pubertal development before the age of 8 years in girls and 9 years in boys, pubertal basal and/or GnRH-stimulated LH levels, advancement of BA as assessed by the Greulich and Pyle method, and absence of central nervous system abnormalities on MRI.
Exclusion criteria were defined as follows: (i) review articles, letters, conference abstracts, personal opinions, and book chapters; (ii) articles that were not in the Latin (Roman) alphabet; (iii) studies that had duplicate data; and (iv) studies investigating single nucleotide polymorphisms in patients without a confirmed diagnosis of CPP.
B. Search Strategy and Study Selection
A systematic electronic search was conducted in seven databases with neither language nor year restriction (Cochrane, EMBASE, LILACS, LIVIVO PubMed, Scopus, and Web of Science). The grey literature [22] search was performed using Google Scholar and ProQuest. At the end of the search phases, the bibliographies of selected articles were manually screened for the identification of additional eligible studies. The details of search strategy are important to the comprehension of this study [23].
Two independent authors (L.P.V. and C.G.M.) conducted the study selection. First, titles and abstracts were evaluated and publications that did not fulfill the inclusion criteria were discarded. In the second phase, full texts of selected articles were reviewed independently by the same authors (L.P.V. and C.G.M.) to confirm their eligibility. Any disagreement between reviewers was resolved by consensus. A third author (I.P.D.T) was consulted if disagreements between the two initial evaluators were not resolved by consensus.
C. Data Extraction
The following data were extracted from each study: first author, year and country of publication, study design, sample size, number of patients with MKRN3 mutations, family history of CPP, and sequencing technique. In addition, clinical variables of interest including sex, age at pubertal onset, Tanner stage, BA, hormonal parameters at diagnosis, type of MKRN3 mutation, and information regarding treatment were extracted for individual patients. Data and genetic analyses from adult parents with a diagnosis of CPP based on past medical history were not included.
D. Risk of Bias in Individual Studies
Critical appraisal of included studies was performed independently by two authors (L.P.V. and C.G.M.) according to a checklist developed by Joanna Briggs Institute for Studies Reporting Prevalence Data [24]. Disagreements in the assessment of the risk of bias were resolved by discussion, with the involvement of a third reviewer author if necessary.
E. Statistical Analysis
We pooled the predefined clinical and biochemical features that were more frequently reported in the selected studies (age at pubertal onset and at diagnosis, Tanner stage, BA, basal and poststimulated LH, basal FSH, estradiol, testosterone, and type of MKRN3 mutation).
Possible differences between data of girls and boys with MKRN3 mutations, and of Asian girls and girls from western countries, were analyzed using the nonparametric Mann-Whitney test because data did not follow a normal distribution. Statistical significance was defined as P < 0.05. Analyses were performed using SPSS, version 20.
In studies eligible for analysis of prevalence, the number of patients screened positive for MKRN3 mutations (numerator) and the total number of CPP patients screened (denominator) were used to estimate prevalence. Meta-analysis was performed using StataCorp Software, version 13 (College Station, TX) with the “metaprop” command; a random effect model was used to estimate the pooled prevalence of MKRN3 mutations and the 95% CIs. Potential sources of heterogeneity were examined by performing a series of prespecified subgroup analyses for the following variables: sex, family history of CPP, and geographical distribution (Asian and non-Asian countries). Heterogeneity was assessed using the I2 statistic, where ≤25%, 50%, and ≥75% indicated low, moderate, and high heterogeneity, respectively.
2. Results
A. Study Selection
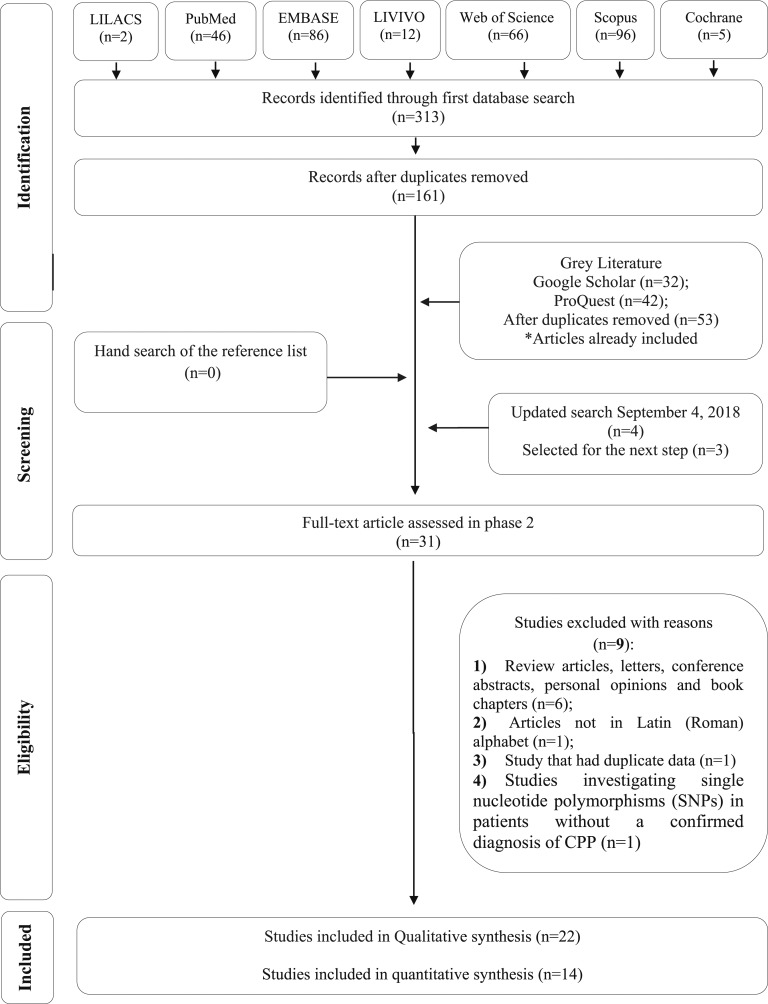
The initial search through the electronic databases was conducted on 6 February 2018 and resulted in a total of 161 studies after removing duplicates. Four other articles were identified through updated search on 4 September 2018. No additional study was included from the references of the retrieved articles or from grey literature. After a comprehensive evaluation of titles and abstracts, 31 articles were considered for the second phase, in which the full texts were reviewed. Finally, 22 studies complied with the inclusion criteria. A flow chart detailing the selection process is shown in Fig. 1.
Figure 1.
Flow chart for identifying eligible studies.
B. Study Characteristics
The main study characteristics are summarized elsewere [23]. All studies were published between 2013 and 2018 in the English language. Of them, 7 were case reports, 2 were case series, 11 were cross-sectional, and 2 studies were classified as cross-sectional and translational. The 14 studies included in the quantitative synthesis (meta-analysis) of prevalence comprised a total of 857 patients with idiopathic CPP screened for MKRN3 mutations. In the study of Bessa et al. [15], data of some patients had been previously published [4] and therefore were counted only once for the analysis of prevalence.
Regarding risk of bias by Joanna Briggs Institute Critical Appraisal Checklist for Studies Reporting Prevalence Data, 2 studies were classified as carrying moderate risk of bias [25, 26] and 20 were classified as with low risk of bias [4–16, 19, 20, 23, 27–31].
C. Demographic and Phenotypic Characteristics Associated With MKRN3 Mutations
MKRN3 defects were described in 89 patients (76 girls and 13 boys) with CPP from 17 countries: Argentina [15], Belgium [4], Brazil [4, 5, 15, 16, 31], Bulgaria [20], Cyprus [25, 26], Denmark [28], France [7], Germany [9], Greece [6], Israel [8], Italy [7, 10, 19, 27], Japan [14], Korea [12], Spain [13], Taiwan [29], Turkey [11, 30], and United States of America [4]. Eighty-eight patients harbored mutations in the coding sequence and one exhibited a deletion in the promoter region of the gene.
Patients with MKRN3 mutations presented with signs and symptoms of early reactivation of the hypothalamic-pituitary-gonadal axis, represented by precocious development of sexual characteristics, BA advancement, and pubertal levels of basal or poststimulated LH. The detailed clinical and hormonal profiles of patients with MKRN3 mutations identified are shown elsewhere [23].
In the pooled analysis, the median age at pubertal onset of girls with MKRN3 mutations (n = 76) was 6.0 years (range, 3.0 to 7.8). Median basal and poststimulated LH levels were 1.27 IU/L (range, 0.1 to 6.1) and 22.0 IU/L (range, 4.0 to 95.9), respectively. Asian girls were significantly older at diagnosis (8.0 years; range, 6.8 to 10.3 years) in comparison with girls from western countries (6.7 years; range, 3.6 to 8.4; P < 0.001) and tended to be older at pubertal onset (6.8 years; range, 5.0 to 7.8 years vs 6.0 years; range, 3.0 to 7.5 years; P = 0.072). In addition, Asian girls presented with lower basal FSH levels than western girls (3.0 IU/L; range, 0.8 to 7.64 IU/L vs 4.5 IU/L; range, 0.7 to 13.94 IU/L, respectively; P = 0.042), as shown in Table 1.
Table 1.
Pooled Comparative Analysis of Clinical and Biochemical Data of Girls From Western Countries (n = 65) and Girls From Asian Countries (n = 11)
| Clinical and Biochemical Data | Western Girls, Median (Range) | Asian Girls, Median (Range) | P Value |
|---|---|---|---|
| Age at pubertal onset, y (64/6) | 6.0 (3.0–7.5) | 6.8 (5.0–7.80) | 0.072 |
| Age at diagnosis, y (61/11) | 6.7 (3.6–8.4) | 8.0 (6.8–10.3) | <0.001a |
| ΔBA-CA, y (58/11) | 2.3 (−0.9 to 5.2) | 2.1 (1.2–3.0) | 0.928 |
| Basal FSH, IU/L (58/11) | 4.5 (0.7–13.94) | 3.0 (0.8–7.64) | 0.042a |
| Basal LH, IU/L (55/11) | 1.1 (0.1–6.1) | 1.6 (0.36–3.74) | 0.454 |
| Poststimulated LH, IU/L (54/9) | 22.1 (4.9–95.9) | 11.6 (5.6–52.5) | 0.321 |
| Estradiol, pg/mL (52/2) | 25.5 (2.0–80.0) | 38.5 (29.0–48.0) | 0.507 |
In the first column, numbers in parentheses refer to the number of patients from each group in which the information was available for comparative analysis.
Abbreviation: ΔBA-CA, difference between bone age and chronological age.
P < 0.05, using the Mann-Whitney U test for comparison between the groups.
The median age at pubertal onset of boys (n = 13) was 8.5 years (range, 5.9 to 9.0). In comparison with boys, girls exhibited a significantly earlier age at pubertal onset (2.0 years below the sex-specific lower age limit vs 0.5 years below this limit in boys, P = 0.001), higher basal FSH levels (median, 4.3 IU/L; range, 0.7 to 13.94 IU/L vs 2.45 IU/L; range, 0.8 to 13.70 IU/L in boys; P = 0.003), and greater ΔBA (median, 2.3 years; range, −0.9 to 5.2 years vs 1.2 years; range, 0.0 to 2.3 years, respectively; P = 0.01) (Table 2).
Table 2.
Pooled Comparative Analysis of Clinical and Biochemical Data of Girls (n = 76) and Boys (n = 13) With MKRN3 Mutations
| Clinical and Biochemical Data | Girls, Median (Range) | Boys, Median (Range) | P Value |
|---|---|---|---|
| Age at pubertal onset, y (70/8) | 6.0 (3.0 7.8) | 8.5 (5.9–9.00) | <0.001a |
| Δ Age at pubertal onset (70/8) | 2.0 (0.2–5.0) | 0.5 (0.0–3.9) | 0.001a |
| Age at diagnosis, y (72/12) | 6.8 (3.6–10.3) | 9.45 (8.1–13.2) | <0.001a |
| ΔBA-CA, y (69/11) | 2.3 (−0.9-5.2) | 1.2 (0.0–2.3) | 0.01a |
| Basal FSH, UI/L (69/12) | 4.3 (0.7–13.94) | 2.45 (0.8–13.70) | 0.003a |
| Basal LH, UI/L (66/12) | 1.27 (0.1–6.1) | 1.35 (0.7–5.41) | 0.239 |
| Poststimulated LH, UI/L (63/7) | 22.0 (4.9–95.9) | 13.9 (6.7–20.00) | 0.089 |
In the first column, numbers between parentheses refer to the number of patients from each group in which the information was available for comparative analysis; Δ age at pubertal onset express the difference between the sex-specific lower age limit for pubertal development and the age at onset of puberty.
P < 0.05, using the Mann-Whitney U test for comparison between the groups.
There was no difference among the age at pubertal onset, ΔBA, and hormonal data when comparing patients harboring different subtypes of MKRN3 mutations, according to their predicted severity. However, patients with missense mutations were older at diagnosis than patients with mutations that encode premature stop codons (nonsense and frameshift) and a promoter deletion (7.72 years; range, 3.6-10.3 vs 6.75 years; range, 3.8 to 13.2, respectively). This finding may suggest a slower rate of puberty progression associated with mutation subtype (Table 3). Notably, the majority of mutations found in Asian girls were missense [9 of 11 (82%)] whereas in girls from western countries missense mutations were found only in 21 of 65 (32%) of the patients (P = 0.002).
Table 3.
Pooled Comparative Analysis of Clinical and Biochemical Data of Patients With MKRN3 Mutations According to Subtype of Mutation
| Clinical and Biochemical Data | Severe Mutationsa (n = 52) Median (Range) | Missense Mutations (n = 37), Median (Range) | P Value |
|---|---|---|---|
| Age at pubertal onset, y (51/27) | 6.0 (3.0–9.0) | 6.0 (3.5–8.5) | 0.645 |
| Age at diagnosis, y (48/36) | 6.75 (3.8–13.2) | 7.72 (3.6–10.3) | 0.028 |
| ΔBA-CA (44/36) | 2.0 (−0.9 to 5.2) | 2.5 (0.2–3.7) | 0.166 |
| Basal FSH, UI/L (47/34) | 4.0 (0.7–13.94) | 3.85 (0.8–8.9) | 0.497 |
| Basal LH, UI/L (44/34) | 1.3 (0.1–6.1) | 1.29 (0.2–3.74) | 0.341 |
| Poststimulated LH, UI/L (40/30) | 20.0 (4.9–62.5) | 19.75 (5.6– 95.9) | 0.506 |
| Estradiol, pg/mL (34/20)b | 27.85 (5.0–80.0) | 25.5 (2.0–61.0) | 0.502 |
| T, ng/mL (5/5)c | 216.0 (78.0–548.0) | 198.8 (41.0–466.0) | 0.347 |
In the first column, numbers in parentheses refer to the number of patients from each group in which the information was available for comparative analysis.
Abbreviation: T, testosterone.
Severe mutations encompassed frameshift mutations (n = 43), nonsense mutations (n = 8) and promoter deletions (n = 1).
Estradiol comparative analysis was performed between girls.
T comparative analysis was performed between boys; P < 0.05, using the Mann-Whitney U test for comparison between the groups.
Additional dysmorphisms were rare and include esotropia detected in two affected siblings [18], and clinodactyly and lumbar hyperlordosis in two unrelated girls, one of whom also exhibited a high-arched palate and dental abnormalities [5, 10]. Premature ovarian failure was reported by Grandone et al. [10] in the mutated grandmother of the index case, although it could be an incidental finding.
There were few studies comparing data of patients with and without MKRN3 mutations. Macedo et al. [5] described higher levels of basal FSH in girls with MKRN3 mutations compared with nonmutated girls (median, 4.9 IU/L; ranging from 4.4 to 10 UI/L vs 3.6 IU/L; range, 1.0 to 9.8 IU/L; P = 0.016). Simon et al. [7] found that girls with MKRN3 mutations were younger at puberty onset than nonmutated ones [median age, 6.0 years (range, 5.4 to 6.0) vs 7.0 years (range, 6.0 to 7.0), P = 0.01], but no additional differences regarding hormonal levels, uterus length, Tanner Stage, and ΔBA were observed between the groups. Data of male patients are limited because of the small number of reported boys with MKRN3 mutations (n = 13). Data from Bessa et al. [15] suggested a later pubertal onset in mutated boys (median age, 8.2 years vs 7.0 years in boys with CPP without MKRN3 mutations; P = 0.033), although no differences in hormonal status were identified between the groups.
Younger carriers of MKRN3 mutations identified by family screening before the development of pubertal signs have also been described [7, 11], and their clinical follow-up are still not known. Stecchini et al. reported a case of a girl identified as a carrier of MKRN3 mutation at the age of 4 because of a positive family history, who was diagnosed with CPP at the age of 6.7 years [16].
Ultimately, information regarding treatment was available only for 54 patients and all were treated with GnRH analog. A satisfactory therapeutic response was described in all but two girls who were noncompliant with treatment. One Turkish girl whose parents refused treatment had menarche at the age of 8.9 years [11]. However, menarche seemed to occur in an appropriate age after an adequate treatment adherence with GnRH analog. In addition, the predicted final height appeared to be within the target height in the few cases in which this information was available [5].
D. MKRN3 Mutations
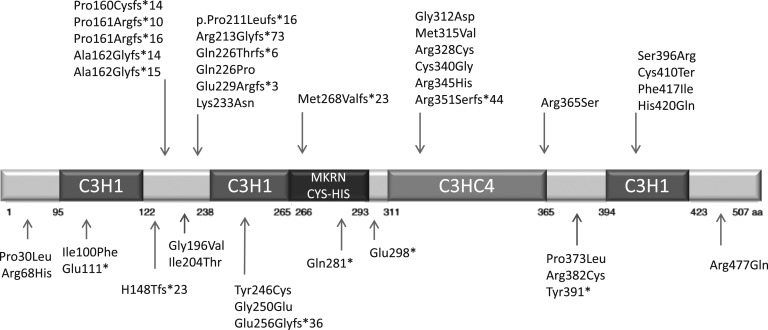
To date, 39 inactivating mutations in the coding sequence of MKRN3 have been described, including 4 nonsense, 13 frameshift, and 22 missense mutations (Fig. 2).
Figure 2.
Structure of the MKRN3 human protein showing the location of the mutations in coding sequence identified to date. Notably, 36% (14 of 39) of mutations are located in the loop structure between 2 C3H1 zinc finger motifs in the aminoterminal region of the protein and these are almost all frameshift mutations, thereby affecting protein structure. The C3HC4 is the second domain where mutations tend to cluster; this area has mostly missense mutations.
Frameshift variants are predicted to result in the translation of truncated proteins by creation of premature stop codons. Notably, 5 frameshift mutations, identified in almost one-third of the patients (n = 27), are located in a cytosine-rich region between nucleotides 476 and 482, confirming this site as a hotspot.
Most of the missense mutations were predicted to be pathogenic by in silico analysis. Of these, six are located within the C3HC4 ring zinc finger domain of the protein (p.Gly312Asp, p.Met315Val, p.Arg328Cys, p.Cys340Gly, p.Arg345His, p.Arg356Ser) and five within a C3H zinc finger motif (p.Tyr246Cys, p.Gly250Glu, p.Cys410Ter, p.Phe417Ile, p.His420Gln). These domains are related to the E3 ubiquitin-ligase activity and RNA-binding, respectively, and seem to be essential for protein function. Six missense variants were predicted to be benign or neutral by in silico analysis (p.Ile100Phe, p.Ile204Thr, p.Gln226Pro, p.Lys233Asn, and p.Ser396Arg), which were detected in Korean girls, and p.Arg477Gln, detected in a single Taiwanese girl [12, 29]. However, the lack of in vitro analysis of the missense mutations may be a possible limitation of interpretation.
In addition to defects in the coding sequence, a heterozygous 4-nucleotide deletion (c.-150_147delTCAG) in the promoter region of MKRN3 was recently described in 1 of 110 patients with CPP without mutations in the coding sequence. This deletion is predicted to cause the loss of a binding site to a putative transcriptional factor for MKRN3 expression. Using an in vitro luciferase reporter assay, the authors showed a substantial reduction of MKRN3 promoter activity in cells transfected with a plasmid encoding the deletion [32].
Other genetic or epigenetic mechanisms that could disrupt the expression of an imprinted gene, such as copy number variations or methylation abnormalities, of the 15q11locus were not detected in 52 patients evaluated by methylation-specific multiplex ligation-dependent probe amplification [5]. In addition, partial or complete MKRN3 deletions were not found in 32 CPP patients without MKRN3 mutations investigated through a quantitative PCR gene dosage assay [7].
E. Prevalence of MKRN3 Mutations in Idiopathic CPP
MKRN3 mutations have been described in patients with familial and sporadic CPP from multiple countries and may account for a substantial proportion of familial cases (33% to 46%) [4, 7]. However, MKRN3 mutations were not identified in a subgroup of Korean girls with familial CPP [12], and a lower frequency of mutations in familial cases was also described in other series (8.7% and 5.3%, respectively) [27, 30]. In addition, the frequency of MKRN3 mutations appears to be higher among males (40% in one report) [15].
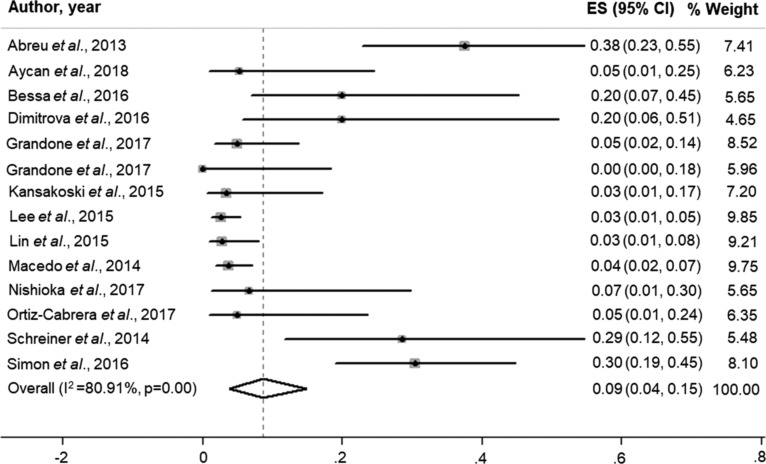
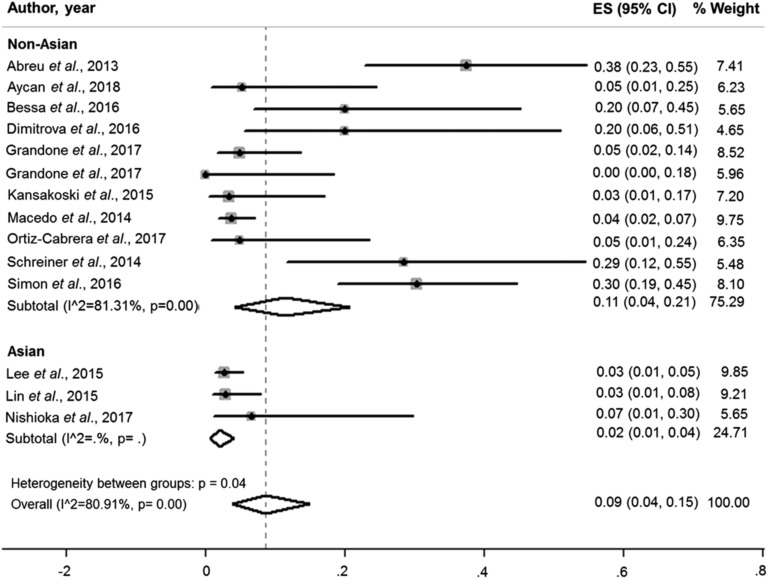
The pooled overall prevalence of MKRN3 mutations in patients with idiopathic CPP was 9.0% (95% CI, 0.04 to 0.15; I2, 80.91%; P = 0.00; Fig. 3). Interestingly, the pooled prevalence of MKRN3 mutations was much lower in patients from Asian countries (2.0%; 95% CI, 0.01 to 0.04; I2, 0.0%) than in those from non-Asian countries (11.0%; 95% CI, 0.04 to 0.21; I2, 81.31%) (Fig. 4).
Figure 3.
Pooled overall prevalence of MKRN3 mutations in patients with central precocious puberty (9.0%; 95% CI, 0.04 to 0.15; I2, 80.91%; P = 0.00). ES, prevalence estimate.
Figure 4.
Pooled prevalence of MKRN3 mutations among central precocious puberty patients from Asian (2.0%; 95% CI, 0.01 to 0.04; I2, 0.0%) and non-Asian countries (11.0%; 95% CI, 0.04 to 0.21; I2, 81.31%).
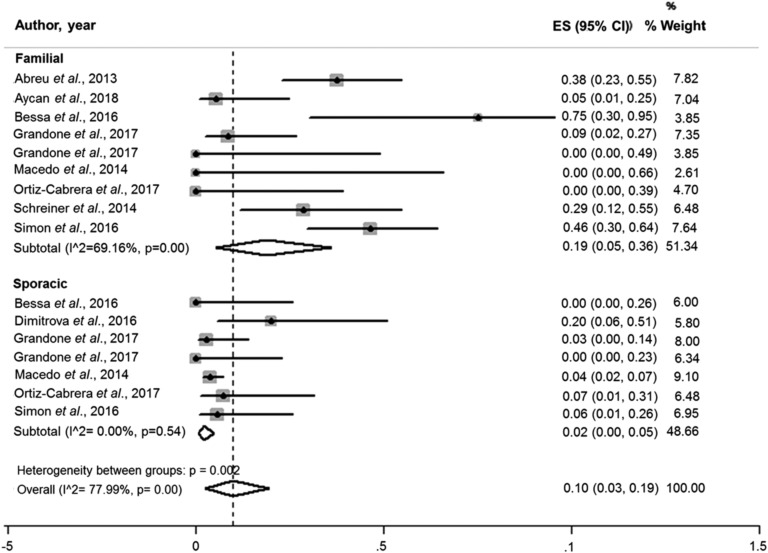
Considering the variation in the prevalence of MKRN3 mutations according to the geographical distribution and the lack of information regarding family history in some reports, studies from Asian countries were not included in the subanalysis by family history. The pooled prevalence of MKRN3 mutations in patients with familial CPP was 19.0% (95% CI, 0.05 to 0.36; I2, 69.16%). Considering the sporadic cases, the estimated pooled prevalence was 2.0% (95% CI, 0.01 to 0.04; I2, 0%) (Fig. 5).
Figure 5.
Pooled prevalence of MKRN3 mutations according to family history. The pooled prevalence of MKRN3 mutations in patients with familial CPP was 19.0% (95% CI, 0.05 to 0.36; I2, 69.16%). Considering the sporadic cases, the pooled estimates for prevalence was 2.0% (95% CI, 0.01 to 0.04; I2, 0%).
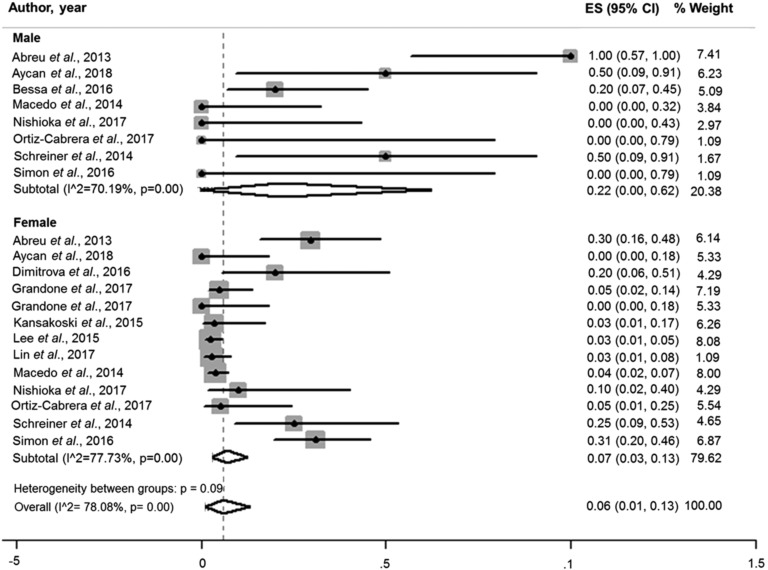
Afterward, in sex-based subanalysis, the pooled prevalence of MKRN3 mutations in males (22.0%; 95% CI 0.0 to 0.62; I2, 70.19) was higher than in females (7.0%; 95% CI, 0.03 to 0.13; I2, 77.73%) (Fig. 6).
Figure 6.
Pooled prevalence of MKRN3 mutations according sex. The prevalence of MKRN3 mutations in males was 22.0% (95% CI, 0.0 to 0.62; I2, 70.19) and in females 7.0% (95% CI, 0.03 to 0.13; I2, 77.73%).
3. Discussion
This systematic review and meta-analysis investigated data of patients with CPP caused by MKRN3 mutations, and, to our knowledge, it represents the most comprehensive review of the reported data published to date. In addition, it provides qualitative and quantitative analysis of published data, expanding the knowledge on the epidemiology of MKRN3 mutations.
There was no specific clinical or laboratory finding that identified a patient with CPP as a carrier of a MKRN3 mutation. In one report, higher basal FSH levels were the only parameter found to differ between girls with and without MKRN3 mutations [5]. In another study, lower age at puberty onset was recorded in the subgroup of girls with mutations in MKRN3 compared with girls without mutations [7]. On the other hand, later age at pubertal onset is described in mutated boys compared with those without mutations [15].
Additional dysmorphisms were rare (described in only four patients worldwide) and major criteria for PWS were not described in any patient, reinforcing the hypothesis that MKRN3 mutations are associated with the phenotype of nonsyndromic CPP. Curiously, premature ovarian failure was described in the grandmother of an Italian girl identified as a carrier of MKRN3 gene mutation. Although it could be an incidental finding, gonadal dysfunction has been described in patients with PWS [33, 34], and long-term follow-up of mutated girls is necessary to evaluate a possible relationship between MKRN3 mutations and premature ovarian failure.
The onset of physiologic puberty in girls occurs 1 to 2 years earlier than in boys, which is consistent with sexual dimorphism of the major drivers of puberty [35]. The pooled analysis of available data showed that mutations in MKRN3 appear to affect pubertal onset in a sex-dimorphic manner, with girls entering puberty at a younger age than boys (median age, 2 years vs 0.5 years, below their sex-specific lower age limit, respectively; P = 0.001). In addition, girls with CPP exhibited higher basal FSH levels and greater ΔBA in comparison with boys. These differences do not reflect a more advanced stage of puberty at the time of diagnosis in girls than in boys; rather, the proportion of subjects with Tanner stages 3 and 4 was higher in boys than in girls (76% vs 58%, respectively). Moreover, FSH predominance is routinely seen during minipuberty [36] and female puberty [37], whether it is precocious or normally timed. A marked sex difference in circulating MKRN3 levels was also reported, with prepubertal boys displaying a 50% lower mean serum MKRN3 level compared with prepubertal girls [38]. Of note, serum levels of MKRN3 negatively correlated with circulating FSH and LH in prepubertal girls [39], albeit no substantial correlation were observed between MKRN3 and gonadotropin levels in boys. The mechanisms underlying this sex difference are not yet understood, and a sex-specific action of MKRN3 on signaling pathways with a role on GnRH secretion cannot be ruled out. However, more studies are needed to clarify a putative sexually dimorphic action of MKRN3.
Girls from Asian countries with MKRN3 mutations exhibited a tendency of older age at pubertal onset than girls from western countries (median, 6.8 years vs 6.0 years), older age at diagnosis (median, 8.0 years vs 6.7 years), and lower FSH levels (median, 3.0 IU/L vs 4.5 IU/L). It is well known that pubertal timing is influenced by genetic, metabolic, nutritional, and ethnic factors. In previous reports, menarche in Asian girls occurred later than in girls from western countries (at an average age of 14.5 years) [40]. However, two recent Asian studies reported that the mean age at menarche of Chinese and Korean girls declined to 12.6 and 12.7 years, respectively, throughout the past decade [41, 42]. These recent findings are similar to European and American studies, in which the reported mean age at menarche was near 12.5 years [43, 44]. The differences in clinical presentation of Asian girls with MKRN3 mutations from that of girls from western countries may have been influenced by the difference observed in mutation subtypes, ethnicity, and environmental factors, such as diet and exposure to endocrine disruptors. In particular, higher body mass index and body adiposity are associated with earlier onset of puberty in girls [35, 45]. Unfortunately, it was not possible to analyze differences in body mass index between the groups because this information was absent in most of the reports. Additionally, interactions of metabolic and environmental factors that could potentially coregulate MKRN3 signaling pathways are still under investigation. Other possibility may be attributed to selection bias. For example, selection criteria for MKRN3 testing may have been more comprehensive for Asian studies compared with western studies.
Regarding treatment-related outcomes, patients with CPP caused by MKRN3 mutations seem to be satisfactorily treated with GnRH analogs, although information regarding response to treatment was absent in almost one-third of the cases [4, 6, 9, 10, 12, 13, 15, 29]. The predicted final height appears to be preserved; however, the limited number of patients in whom this information was available precludes a definite conclusion about the potential of achieving target height [5, 12].
Early pubertal timing and menarche have been associated with an increased risk of obesity, type 2 diabetes, cardiovascular disease, and estrogen-dependent cancers [2, 46, 47]. It is still not known if MKRN3 mutations are associated with a higher risk for adverse health outcomes in later life in comparison with patients without mutations. Long-term clinical follow-up of these patients is necessary to clarify this issue.
MKRN3 is expressed ubiquitously in human tissues. The human protein has 507 amino acids and contains a typical C3HC4 RING zinc finger motif and several C3H zinc finger motifs with predicted E3 ubiquitin-ligase activity and RNA-binding activity, respectively [18]. To date, 39 inactivating mutations in the coding sequence (including nonsense, missense, and frameshift mutations) and 1 promotor deletion have been described in 89 patients worldwide. The fact that more girls (n = 76) than boys (n = 13) have been identified to harbor such mutations may reflect the well-known higher prevalence of precocious puberty in girls than in boys [2]. Recent familial CPP studies have demonstrated that affected males were retrospectively identified based on medical history and genetic analysis and perhaps this prevalence might reflect an under diagnosis of CPP in the male group [7, 11]. Another explanation could be the relative deficit of boys among the offspring of men with MKRN3 mutations, as observed by Simon et al., who speculated that Y-bearing sperm cells carrying MKRN3 mutations may be defective [7]. Accordingly, in this systematic review, the offspring of 41 MKRN3 mutated men consists of 68 (71%) girls and 28 (29%) boys, supporting this hypothesis. However, confirmation in a larger group of men with MKRN3 mutations is required for a definitive conclusion.
Mutations are distributed over the entire coding region, but tend to cluster in the loop structure between two C3H zinc finger motifs in the aminoterminal region of the protein. Notably, the poly-C region between nucleotides 476 and 482 seems to be a mutation hotspot, comprising 5 of the common frameshift mutations identified in almost one-third of the patients. In particular, the p.Ala162Glyfs15* mutation was identified in 8 families from France and Italy [7], suggesting a possible founder effect among individuals from those countries.
The C3HC4 RING zinc finger motif, which is responsible for the ubiquitin-ligase activity, was recognized as another region where mutations, especially missense mutations, tend to cluster. This may reflect the location of functionally important amino acid residues within the protein and suggests that variants in this domain are more likely to cause deleterious effects on protein function. It is notable that six of the missense mutations were predicted to be benign according to in silico analysis [12, 29]. However, considering the typical phenotype of the girls harboring these missense variants, it is reasonable to assume their role in the pathogenesis of CPP, but complementary functional studies would strengthen this association.
Abnormalities in the regulatory region of MKRN3 may be rarely implicated in the pathogenesis of CPP because a deletion in the promotor region has been recently identified in one girl [31]. Other genetic or epigenetic mechanisms that could disrupt the expression of imprinted genes, such as deletions, uniparental disomy or alterations in DNA methylation markers within the imprinted cluster have not been detected so far, indicating these abnormalities may represent very rare mechanisms in this disorder, although they have not been widely investigated to date [5, 7].
Currently, MKRN3 mutations represent the main genetic cause of CPP. The prevalence is quite variable, ranging from 0 in a small sample from Italy [19] to 46% considering the subgroup of patients with familial CPP reported by Simon et al. [7]. In the current meta-analysis of 14 studies including 857 patients with CPP, the pooled overall prevalence of MKRN3 mutations was 9.0% (95% CI, 0.04 to 0.15). Interestingly, our data set demonstrates geographic predominance of MKRN3 mutations in patients from western countries (prevalence estimates of 11%; 95% CI, 0.04 to 0.21) and a lower frequency of mutations in patients from Asian countries (2%; 95% CI, 0.01 to 0.04), suggesting genetic variation according to ethnic background. In addition, it is noteworthy that specific single nucleotide polymorphisms near the paternal allele of MKRN3 have also been associated with earlier age at menarche in large populational dataset studies, although those articles were not included in our meta-analysis because they did not fulfill the inclusion criteria [48, 49].
Our meta-analysis also supports the previous observation that the frequency of MKRN3 mutations is higher among boys with CPP than in girls (pooled prevalence of 22%; 95% CI, 0.00 to 0.62 vs 7%; 95% CI, 0.03 to 0.13, respectively). Considering that MKRN3 is located on an autosomal chromosome, the reasons underlying the predominance of mutations in males are still not understood. Of note, all mutated boys exhibited a positive family history of precocious puberty; these data highlight the importance of evaluating boys, especially those with familial CPP, for MKRN3 mutations.
The prevalence of mutations in familial cases was variable among different studies. Our meta-analysis showed a pooled prevalence of 19.0% (95% CI, 0.05 to 0.36) in patients with familial CPP from western countries, with a moderate level of heterogeneity (I2, 69.16%). Potential sources of variation could be due to different criteria of selection of familial cases and small sample sizes in some reports. In addition, considering the imprinted pattern of inheritance in which a generation can be skipped, the familial nature may be underrecognized if not carefully investigated, whereas the prevalence of MKRN3 mutations in familial cases might have been underestimated in some series.
Our study also had some potential limitations. First, a meta-analysis of specific association between genotype and phenotype was not possible because of the limited number of studies comparing clinical data of patients with and without MKRN3 mutations. Second, the number of affected boys identified so far is still small and information about pubertal onset was absent in some of them. Further limitations relate to the type of studies, with exclusively observational data, including case reports and case series, to the small sample size of some reports and to inconsistency of study designs. Thus, larger studies evaluating patients with idiopathic CPP and with comparative phenotypic analysis of individuals with and without mutations are needed to establish a more precise genotype-phenotype relationship.
In summary, our meta-analysis shows that MKRN3 mutations are associated with the phenotype of nonsyndromic CPP. Girls are more severely affected than boys and exhibit an early age at pubertal onset, higher basal FSH levels, and greater ΔBA. Overall, the pooled estimated prevalence of MKRN3 mutations in CPP was 9.0% (95% CI, 0.04 to 0.15), with variations according to sex, family history, and geographical distribution. Additional studies are needed to establish a precise genotype-phenotype relationship and long-term follow-up of patients harboring MKRN3 mutations is necessary to clarify whether they exhibit higher risks of adverse health outcomes later in life.
Acknowledgments
The authors thank Luiz Guilherme Grossi Porto, Professor of Applied Physiology to Physical Education at the Faculty of Physical Education, University of Brasilia, Brazil, for his contribution in statistical analysis.
Financial Support: This work was supported by National Institutes of Health Grant R01 HD082314, the Brigham and Women’s Hospital Women’s Brain Initiative (to U.B.K.), and Conselho Nacional de Desenvolvimento Cientifico e Tecnológico Grant 462346/2014-5 (to A.L.-P.).
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- BA
bone age
- CPP
central precocious puberty
- E3
ubiquitin-protein ligase
- MKRN3
makorin ring finger protein 3
- PWS
Prader-Willi syndrome
References and Notes
- 1. Carel JC, Léger J. Clinical practice. Precocious puberty. N Engl J Med. 2008;358(22):2366–2377. [DOI] [PubMed] [Google Scholar]
- 2. Latronico AC, Brito VN, Carel JC. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. 2016;4(3):265–274. [DOI] [PubMed] [Google Scholar]
- 3. de Vries L, Kauschansky A, Shohat M, Phillip M. Familial central precocious puberty suggests autosomal dominant inheritance. J Clin Endocrinol Metab. 2004;89(4):1794–1800. [DOI] [PubMed] [Google Scholar]
- 4. Abreu AP, Dauber A, Macedo DB, Noel SD, Brito VN, Gill JC, Cukier P, Thompson IR, Navarro VM, Gagliardi PC, Rodrigues T, Kochi C, Longui CA, Beckers D, de Zegher F, Montenegro LR, Mendonca BB, Carroll RS, Hirschhorn JN, Latronico AC, Kaiser UB. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368(26):2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Macedo DB, Abreu AP, Reis ACS, Montenegro LR, Dauber A, Beneduzzi D, Cukier P, Silveira LF, Teles MG, Carroll RS, Junior GG, Filho GG, Gucev Z, Arnhold IJ, de Castro M, Moreira AC, Martinelli CE Jr, Hirschhorn JN, Mendonca BB, Brito VN, Antonini SR, Kaiser UB, Latronico AC. Central precocious puberty that appears to be sporadic caused by paternally inherited mutations in the imprinted gene makorin ring finger 3. J Clin Endocrinol Metab. 2014;99(6):E1097–E1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Settas N, Dacou-Voutetakis C, Karantza M, Kanaka-Gantenbein C, Chrousos GP, Voutetakis A. Central precocious puberty in a girl and early puberty in her brother caused by a novel mutation in the MKRN3 gene. J Clin Endocrinol Metab. 2014;99(4):E647–E651. [DOI] [PubMed] [Google Scholar]
- 7. Simon D, Ba I, Mekhail N, Ecosse E, Paulsen A, Zenaty D, Houang M, Jesuran Perelroizen M, de Filippo GP, Salerno M, Simonin G, Reynaud R, Carel JC, Léger J, de Roux N. Mutations in the maternally imprinted gene MKRN3 are common in familial central precocious puberty. Eur J Endocrinol. 2016;174(1):1–8. [DOI] [PubMed] [Google Scholar]
- 8. de Vries L, Gat-Yablonski G, Dror N, Singer A, Phillip M. A novel MKRN3 missense mutation causing familial precocious puberty. Hum Reprod. 2014;29(12):2838–2843. [DOI] [PubMed] [Google Scholar]
- 9. Schreiner F, Gohlke B, Hamm M, Korsch E, Woelfle J. MKRN3 mutations in familial central precocious puberty. Horm Res Paediatr. 2014;82(2):122–126. [DOI] [PubMed] [Google Scholar]
- 10. Grandone A, Cantelmi G, Cirillo G, Marzuillo P, Luongo C, Miraglia del Giudice E, Perrone L. A case of familial central precocious puberty caused by a novel mutation in the makorin RING finger protein 3 gene. BMC Endocr Disord. 2015;15(60):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Simsek E, Demiral M, Ceylaner S, Kırel B. Two frameshift mutations in MKRN3 in Turkish patients with familial central precocious puberty. Horm Res Paediatr. 2017;87(6):405–411. [DOI] [PubMed] [Google Scholar]
- 12. Lee HS, Jin HS, Shim YS, Jeong HR, Kwon E, Choi V, Kim MC, Chung IS, Jeong SY, Hwang JS. Low frequency of MKRN3 mutations in central precocious puberty among Korean girls. Horm Metab Res. 2016;48(2):118–122. [DOI] [PubMed] [Google Scholar]
- 13. Ortiz-Cabrera NV, Riveiro-Álvarez R, López-Martínez MÁ, Pérez-Segura P, Aragón-Gómez I, Trujillo-Tiebas MJ, Soriano-Guillén L. Clinical exome sequencing reveals MKRN3 pathogenic variants in familial and nonfamilial idiopathic central precocious puberty. Horm Res Paediatr. 2017;87(2):88–94. [DOI] [PubMed] [Google Scholar]
- 14. Nishioka J, Shima H, Fukami M, Yatsuga S, Matsumoto T, Ushijima K, Kitamura M, Koga Y. The first Japanese case of central precocious puberty with a novel MKRN3 mutation. Hum Genome Var. 2017;4(4):17017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bessa DS, Macedo DB, Brito VN, França MM, Montenegro LR, Cunha-Silva M, Silveira LG, Hummel T, Bergadá I, Braslavsky D, Abreu AP, Dauber A, Mendonca BB, Kaiser UB, Latronico AC. High frequency of MKRN3 mutations in male central precocious puberty previously classified as idiopathic. Neuroendocrinology. 2017;105(1):17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stecchini MF, Macedo DB, Reis ACS, Abreu AP, Moreira AC, Castro M, Kaiser UB, Latronico AC, Antonini SR. Time course of central precocious puberty development caused by an MKRN3 gene mutation: a prismatic case. Horm Res Paediatr. 2016;86(2):126–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jong MTC, Gray TA, Ji Y, Glenn CC, Saitoh S, Driscoll DJ, Nicholls RD. A novel imprinted gene, encoding a RING zinc-finger protein, and overlapping antisense transcript in the Prader-Willi syndrome critical region. Hum Mol Genet. 1999;8(5):783–793. [DOI] [PubMed] [Google Scholar]
- 18. Abreu AP, Macedo DB, Brito VN, Kaiser UB, Latronico AC. A new pathway in the control of the initiation of puberty: the MKRN3 gene. J Mol Endocrinol. 2015;54(3):R131–R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grandone A, Cirillo G, Sasso M, Capristo C, Tornese G, Marzuillo P, Luongo C, Rosaria Umano G, Festa A, Coppola R, Miraglia Del Giudice E, Perrone L. MKRN3 levels in girls with central precocious puberty and correlation with sexual hormone levels: a pilot study. Endocrine. 2018;59(1):203–208. [DOI] [PubMed] [Google Scholar]
- 20. Dimitrova-Mladenova MS, Stefanova EM, Glushkova M, Todorova AP, Todorov T, Konstantinova MM, Kazakova K, Tincheva RS. Males with paternally inherited MKRN3 mutations may be asymptomatic. J Pediatr. 2016;179:263–265. [DOI] [PubMed] [Google Scholar]
- 21. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341. [DOI] [PubMed] [Google Scholar]
- 22.The Twelfth International Conference on Grey Literature, Prague. 2010. Available from: https://guides.mclibrary.duke.edu/sysreview/greylit.
- 23. Valadares LP, Meireles CG, Toledo IP, Oliveira RS, Castro LCG, Abreu AP, Carrol RS, Latronico AC, Kaiser UB, Guerra ENS, Lofrano-Porto A. Supplemental material of MKRN3 mutations in central precocious puberty: a systematic review and meta-analysis. figshare 2018. figshare 2018. Deposited 19 November 2018. 10.6084/m9.figshare.7339700.v1. [Google Scholar]
- 24. Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manag. 2014;3(3):123–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Neocleous V, Shammas C, Phelan MM, Nicolaou S, Phylactou LA. In silico analysis of a novel MKRN3 missense mutation in familial central precocious puberty. Clin Endocrinol (Oxf). 2016;84(1):80–84. [DOI] [PubMed] [Google Scholar]
- 26. Christoforidis A, Skordis N, Fanis P, Dimitriadou M, Sevastidou M, Phelan MM, Neocleous V, Phylactou LA. A novel MKRN3 nonsense mutation causing familial central precocious puberty. Endocrine. 2017;56(2):446–449. [DOI] [PubMed] [Google Scholar]
- 27. Grandone A, Capristo C, Cirillo G, Sasso M, Umano GR, Mariani M, Miraglia Del Giudice E, Perrone L. Molecular screening of MKRN3, DLK1, and KCNK9 genes in girls with idiopathic central precocious puberty. Horm Res Paediatr. 2017;88(3-4):194–200. [DOI] [PubMed] [Google Scholar]
- 28. Känsäkoski J, Raivio T, Juul A, Tommiska J. A missense mutation in MKRN3 in a Danish girl with central precocious puberty and her brother with early puberty. Pediatr Res. 2015;78(6):709–711. [DOI] [PubMed] [Google Scholar]
- 29. Lin WD, Wang CH, Tsai FJ. Genetic screening of the makorin ring finger 3 gene in girls with idiopathic central precocious puberty. Clin Chem Lab Med. 2016;54(3):e93–e96. [DOI] [PubMed] [Google Scholar]
- 30. Aycan Z, Savaş-Erdeve Ş, Çetinkaya S, Kurnaz E, Keskin M, Muratoğlu Şahin N, Bayramoğlu E, Ceylaner G. Investigation of MKRN3 mutation in patients with familial central precocious puberty. J Clin Res Pediatr Endocrinol. 2018;10(3):223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Macedo DB, França MM, Montenegro LR, Cunha-Silva M, Best DS, Abreu AP, Kaiser UB, Mendonca BB, Jorge AAL, Brito VN, Latronico AC. Central precocious puberty caused by a heterozygous deletion in the MKRN3 promoter region [published correction appears in Neuroendocrinology. 2018;107:313–314]. Neuroendocrinology. 2018;107(2):127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bulcao Macedo D, Nahime Brito V, Latronico AC. New causes of central precocious puberty: the role of genetic factors. Neuroendocrinology. 2014;100(1):1–8. [DOI] [PubMed] [Google Scholar]
- 33. Gross-Tsur V, Hirsch HJ, Benarroch F, Eldar-Geva T. The FSH-inhibin axis in prader-willi syndrome: heterogeneity of gonadal dysfunction. Reprod Biol Endocrinol. 2012;10(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Radicioni AF, Di Giorgio G, Grugni G, Cuttini M, Losacco V, Anzuini A, Spera S, Marzano C, Lenzi A, Cappa M, Crinò A. Multiple forms of hypogonadism of central, peripheral or combined origin in males with Prader-Willi syndrome. Clin Endocrinol (Oxf). 2012;76(1):72–77. [DOI] [PubMed] [Google Scholar]
- 35. Bianco SD. A potential mechanism for the sexual dimorphism in the onset of puberty and incidence of idiopathic central precocious puberty in children: sex-specific kisspeptin as an integrator of puberty signals. Front Endocrinol (Lausanne). 2012;3(149):149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Johannsen TH, Main KM, Ljubicic ML, Jensen TK, Andersen HR, Andersen MS, Petersen JH, Andersson A-M, Juul A. Sex differences in reproductive hormones during mini-puberty in infants with normal and disordered sex development. J Clin Endocrinol Metab. 2018;103(8):3028–3037. [DOI] [PubMed] [Google Scholar]
- 37. Styne DM, Grumbach MM. Puberty: Ontogeny, Neuroendocrinology, Physiology, and Disorders. In: Melmed S, Polonsky KS, Larsen PR, and Kronenberg HM, eds. Willians Textbook of Endocrinology. 12th edPhiladelphia: Elsevier/Saunders; 2011:1054–1201. [Google Scholar]
- 38. Busch AS, Hagen CP, Almstrup K, Juul A. Circulating MKRN3 levels decline during puberty in healthy boys. J Clin Endocrinol Metab. 2016;101(6):2588–2593. [DOI] [PubMed] [Google Scholar]
- 39. Hagen CP, Sørensen K, Mieritz MG, Johannsen TH, Almstrup K, Juul A. Circulating MKRN3 levels decline prior to pubertal onset and through puberty: a longitudinal study of healthy girls. J Clin Endocrinol Metab. 2015;100(5):1920–1926. [DOI] [PubMed] [Google Scholar]
- 40. Ahn JH, Lim SW, Song BS, Seo J, Lee JA, Kim DH, Lim JS. Age at menarche in the Korean female: secular trends and relationship to adulthood body mass index. Ann Pediatr Endocrinol Metab. 2013;18(2):60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee MH, Kim SH, Oh M, Lee KW, Park MJ. Age at menarche in Korean adolescents: trends and influencing factors. Reprod Health. 2016;13(1):121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Meng X, Li S, Duan W, Sun Y, Jia C. Secular trend of age at menarche in Chinese adolescents born from 1973 to 2004. Pediatrics. 2017;140(2):e20170085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morris DH, Jones ME, Schoemaker MJ, Ashworth A, Swerdlow AJ. Secular trends in age at menarche in women in the UK born 1908-93: results from the Breakthrough Generations Study. Paediatr Perinat Epidemiol. 2011;25(4):394–400. [DOI] [PubMed] [Google Scholar]
- 44. Papadimitriou A, Fytanidis G, Douros K, Bakoula C, Nicolaidou P, Fretzayas A. Age at menarche in contemporary Greek girls: evidence for levelling-off of the secular trend. Acta Paediatr. 2008;97(6):812–815. [DOI] [PubMed] [Google Scholar]
- 45. Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016;4(3):254–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gong T-T, Wang Y-L, Ma X-X. Age at menarche and endometrial cancer risk: a dose-response meta-analysis of prospective studies. Sci Rep. 2015;5(1):14051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, Wareham NJ, Ong KK. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab. 2009;94(12):4953–4960. [DOI] [PubMed] [Google Scholar]
- 48. Day FR, Thompson DJ, Helgason H, Chasman DI, Finucane H, Sulem P, Ruth KS, Whalen S, Sarkar AK, Albrecht E, Altmaier E, Amini M, Barbieri CM, Boutin T, Campbell A, Demerath E, Giri A, He C, Hottenga JJ, Karlsson R, Kolcic I, Loh PR, Lunetta KL, Mangino M, Marco B, McMahon G, Medland SE, Nolte IM, Noordam R, Nutile T, Paternoster L, Perjakova N, Porcu E, Rose LM, Schraut KE, Segrè AV, Smith AV, Stolk L, Teumer A, Andrulis IL, Bandinelli S, Beckmann MW, Benitez J, Bergmann S, Bochud M, Boerwinkle E, Bojesen SE, Bolla MK, Brand JS, Brauch H, Brenner H, Broer L, Brüning T, Buring JE, Campbell H, Catamo E, Chanock S, Chenevix-Trench G, Corre T, Couch FJ, Cousminer DL, Cox A, Crisponi L, Czene K, Davey Smith G, de Geus EJCN, de Mutsert R, De Vivo I, Dennis J, Devilee P, Dos-Santos-Silva I, Dunning AM, Eriksson JG, Fasching PA, Fernández-Rhodes L, Ferrucci L, Flesch-Janys D, Franke L, Gabrielson M, Gandin I, Giles GG, Grallert H, Gudbjartsson DF, Guénel P, Hall P, Hallberg E, Hamann U, Harris TB, Hartman CA, Heiss G, Hooning MJ, Hopper JL, Hu F, Hunter DJ, Ikram MA, Im HK, Järvelin MR, Joshi PK, Karasik D, Kellis M, Kutalik Z, LaChance G, Lambrechts D, Langenberg C, Launer LJ, Laven JSE, Lenarduzzi S, Li J, Lind PA, Lindstrom S, Liu Y, Luan J, Mägi R, Mannermaa A, Mbarek H, McCarthy MI, Meisinger C, Meitinger T, Menni C, Metspalu A, Michailidou K, Milani L, Milne RL, Montgomery GW, Mulligan AM, Nalls MA, Navarro P, Nevanlinna H, Nyholt DR, Oldehinkel AJ, O’Mara TA, Padmanabhan S, Palotie A, Pedersen N, Peters A, Peto J, Pharoah PDP, Pouta A, Radice P, Rahman I, Ring SM, Robino A, Rosendaal FR, Rudan I, Rueedi R, Ruggiero D, Sala CF, Schmidt MK, Scott RA, Shah M, Sorice R, Southey MC, Sovio U, Stampfer M, Steri M, Strauch K, Tanaka T, Tikkanen E, Timpson NJ, Traglia M, Truong T, Tyrer JP, Uitterlinden AG, Edwards DRV, Vitart V, Völker U, Vollenweider P, Wang Q, Widen E, van Dijk KW, Willemsen G, Winqvist R, Wolffenbuttel BHR, Zhao JH, Zoledziewska M, Zygmunt M, Alizadeh BZ, Boomsma DI, Ciullo M, Cucca F, Esko T, Franceschini N, Gieger C, Gudnason V, Hayward C, Kraft P, Lawlor DA, Magnusson PKE, Martin NG, Mook-Kanamori DO, Nohr EA, Polasek O, Porteous D, Price AL, Ridker PM, Snieder H, Spector TD, Stöckl D, Toniolo D, Ulivi S, Visser JA, Völzke H, Wareham NJ, Wilson JF, Spurdle AB, Thorsteindottir U, Pollard KS, Easton DF, Tung JY, Chang-Claude J, Hinds D, Murray A, Murabito JM, Stefansson K, Ong KK, Perry JRB; LifeLines Cohort Study; InterAct Consortium; kConFab/AOCS Investigators; Endometrial Cancer Association Consortium; Ovarian Cancer Association Consortium; PRACTICAL consortium. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet. 2017;49(6):834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Perry JRB, Day F, Elks CE, Sulem P, Thompson DJ, Ferreira T, He C, Chasman DI, Esko T, Thorleifsson G, Albrecht E, Ang WQ, Corre T, Cousminer DL, Feenstra B, Franceschini N, Ganna A, Johnson AD, Kjellqvist S, Lunetta KL, McMahon G, Nolte IM, Paternoster L, Porcu E, Smith AV, Stolk L, Teumer A, Tšernikova N, Tikkanen E, Ulivi S, Wagner EK, Amin N, Bierut LJ, Byrne EM, Hottenga JJ, Koller DL, Mangino M, Pers TH, Yerges-Armstrong LM, Zhao JH, Andrulis IL, Anton-Culver H, Atsma F, Bandinelli S, Beckmann MW, Benitez J, Blomqvist C, Bojesen SE, Bolla MK, Bonanni B, Brauch H, Brenner H, Buring JE, Chang-Claude J, Chanock S, Chen J, Chenevix-Trench G, Collée JM, Couch FJ, Couper D, Coveillo AD, Cox A, Czene K, D’adamo AP, Smith GD, De Vivo I, Demerath EW, Dennis J, Devilee P, Dieffenbach AK, Dunning AM, Eiriksdottir G, Eriksson JG, Fasching PA, Ferrucci L, Flesch-Janys D, Flyger H, Foroud T, Franke L, Garcia ME, García-Closas M, Geller F, de Geus EEJ, Giles GG, Gudbjartsson DF, Gudnason V, Guénel P, Guo S, Hall P, Hamann U, Haring R, Hartman CA, Heath AC, Hofman A, Hooning MJ, Hopper JL, Hu FB, Hunter DJ, Karasik D, Kiel DP, Knight JA, Kosma VM, Kutalik Z, Lai S, Lambrechts D, Lindblom A, Mägi R, Magnusson PK, Mannermaa A, Martin NG, Masson G, McArdle PF, McArdle WL, Melbye M, Michailidou K, Mihailov E, Milani L, Milne RL, Nevanlinna H, Neven P, Nohr EA, Oldehinkel AJ, Oostra BA, Palotie A, Peacock M, Pedersen NL, Peterlongo P, Peto J, Pharoah PD, Postma DS, Pouta A, Pylkäs K, Radice P, Ring S, Rivadeneira F, Robino A, Rose LM, Rudolph A, Salomaa V, Sanna S, Schlessinger D, Schmidt MK, Southey MC, Sovio U, Stampfer MJ, Stöckl D, Storniolo AM, Timpson NJ, Tyrer J, Visser JA, Vollenweider P, Völzke H, Waeber G, Waldenberger M, Wallaschofski H, Wang Q, Willemsen G, Winqvist R, Wolffenbuttel BH, Wright MJ, Boomsma DI, Econs MJ, Khaw KT, Loos RJ, McCarthy MI, Montgomery GW, Rice JP, Streeten EA, Thorsteinsdottir U, van Duijn CM, Alizadeh BZ, Bergmann S, Boerwinkle E, Boyd HA, Crisponi L, Gasparini P, Gieger C, Harris TB, Ingelsson E, Järvelin MR, Kraft P, Lawlor D, Metspalu A, Pennell CE, Ridker PM, Snieder H, Sørensen TI, Spector TD, Strachan DP, Uitterlinden AG, Wareham NJ, Widen E, Zygmunt M, Murray A, Easton DF, Stefansson K, Murabito JM, Ong KK; Australian Ovarian Cancer Study; GENICA Network; kConFab; LifeLines Cohort Study; InterAct Consortium; Early Growth Genetics (EGG) Consortium. Parent-of-origin-specific allelic associations among 106 genomic loci for age at menarche. Nature. 2014;514(7520):92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]