Abstract
Research and therapeutic applications create a high demand for primary human hepatocytes. The limiting factor for their utilization is the availability of metabolically active hepatocytes in large quantities. Centrifugation through Percoll, which is commonly performed during hepatocyte isolation, has so far not been systematically evaluated in the scientific literature. 27 hepatocyte isolations were performed using a two-step perfusion technique on tissue obtained from partial liver resections. Cells were seeded with or without having undergone the centrifugation step through 25% Percoll. Cell yield, function, purity, viability and rate of bacterial contamination were assessed over a period of 6 days. Viable yield without Percoll purification was 42.4 × 106 (SEM ± 4.6 × 106) cells/g tissue. An average of 59% of cells were recovered after Percoll treatment. There were neither significant differences in the functional performance of cells, nor regarding presence of non-parenchymal liver cells. In five cases with initial viability of <80%, viability was significantly increased by Percoll purification (71.6 to 87.7%, p = 0.03). Considering our data and the massive cell loss due to Percoll purification, we suggest that this step can be omitted if the initial viability is high, whereas low viabilities can be improved by Percoll centrifugation.
Subject terms: Biomaterials - cells, Hepatocytes, Preclinical research, Translational research
Introduction
Primary human hepatocytes serve as the gold-standard model for in vitro testing of drugs that are metabolized in the liver1,2. In order to address the 3R-principle3, animal testing could be reduced with hepatocytes available on a grand scale. With highly metabolically active in vitro 3D-cultures of primary human hepatocytes, pharmacokinetic processes of drug metabolism can be investigated without the need for using a xenogenous animal model4,5. Primary human hepatocytes can be used to study the pathogenesis of human liver diseases more thoroughly, especially in regard to the increasing number of patients suffering from non-alcoholic fatty liver disease6. Moreover, in face of the growing organ scarcity for liver transplantation, hepatocytes can help to provide bridging strategies until a suitable donor organ is available, e.g. by hepatocyte transplantation7–9, or be used for bioengineering of neo-organs10,11. The wide application of primary human hepatocytes is, however, limited due to the lack of a safe, constant source of high-quality human liver cells.
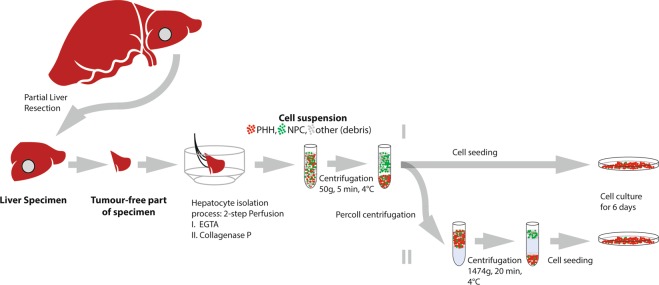
Collagenase perfusion protocols have been an established procedure since the 1980s for isolation of primary human hepatocytes12. The best available and most commonly used source for human liver cells is tissue obtained from partial liver resection for therapeutic purposes, for example after removing a tumor (Fig. 1). After cannulation, perfusion and enzymatic digestion of the tissue specimen, the resulting cell suspension contains liver cells that can be cultured for subsequent use. Optionally, hepatocyte suspensions are purified through density separation with Percoll, which is a low-density fluid containing colloidal silica particles coated with polyvinylpyrrolidone13,14. This additional purification step is included to the isolation protocol in almost 50% of published studies on primary human hepatocytes in the literature reviewed for this study (see supplementary data). Percoll purification can be used for splitting up different liver cell populations, for example Kupffer cells, stellate cells, hepatic progenitor cells, oval cells or liver sinusoidal endothelial cells by centrifuging the cell suspension through differently concentrated layers of Percoll15–18. Moreover, several publications19–21 report a Percoll purification step in their protocol to recover liver cells after cryopreservation. Nonetheless, density purification is also commonly performed to purify parenchymal human hepatocytes themselves22–26, adding about 45 minutes to the 3 hours isolation procedure. Systemic evaluation of this cell- and time-consuming step in hepatocyte isolation is not currently available in the literature, specifically not for human primary liver cells.
Figure 1.
Tissue retrieval and study design. Abbreviations: PHH: Primary human hepatocytes, NPC: non-parenchymal cells, EGTA: ethylene glycol tetraacetic acid.
With respect to the fact that the wider use of primary human hepatocytes is mainly limited by the lack of sufficient amount of hepatocytes11,27, it is crucial to know whether or not the Percoll purification step is necessary at all during hepatocyte isolation. The aim of this work is to investigate whether purification of primary human hepatocytes with density separation by Percoll has a beneficial effect on the performance of isolated human hepatocytes; e. g. yield, viability, plating efficiency, metabolic function, purity, bacterial contamination levels28 and purity of cell cultures.
Methods
Study design and tissue retrieval
From October 2015 to July 2016, a total of 27 hepatocyte isolations were performed at the Department of Surgery, Campus Charité Mitte | Campus Virchow-Klinikum of the Charité – Universitätsmedizin Berlin (Germany). Liver tissue specimens were retrieved from patients undergoing partial hepatectomies. Informed consent was obtained from all tissue donors, and all institutional and ethical guidelines were followed (approval of local ethics committee “Ethikkommission der Charité – Universitätsmedizin Berlin”: EA2/137/09). Patients with infectious diseases such as viral hepatitis, multi-resistant bacteria, or echinococcosis were excluded from the study. All donor-related information was collected from the patient’s hospital records.
Liver tissue was examined for macroscopically tumor-free areas immediately after resection in the operating room. Tissue specimens used for hepatocyte isolation were cut with a scalpel with only one dissection surface, the rest of the liver specimen being covered by Glisson’s capsule. Mass of these specimens was 18.21 g on average (SEM ± 2.64 g). Tissue specimens were stored in a container filled with 4 °C cold Williams E-medium and transferred to the laboratory. All steps were performed under sterile conditions and the isolation protocol began within 30 minutes after retrieval of the specimen (=cold ischemic time). To prevent interpersonal variability, all hepatocyte isolations were carried out by a single person (RH).
Hepatocyte isolation and culturing
Hepatocytes were isolated using an established two-step-perfusion protocol with Collagenase P29. For this technique, sectioned major vessels were cannulated with 3–5 cannulas (custum-made stainless-stell irrigation cannulas) that where connected to thin plastic tubes, merged in a larger tube, and then connected to a roller pump for perfusion. Cannulas and major leakages were sealed with a histoacryl-tissue-glue (3 M, St. Paul, MN, USA). First, the tissue was rinsed with pre-warmed (37 °C) perfusion solution (containing 1.42 M NaCl, 67 mM KCl, and 100 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid [all chemicals from Carl Roth GmbH, Karlsruhe, Germany]) supplemented with ethylene glycol tetraacetic acid (EGTA, Serva, Heidelberg, Germany) which is a chelator for Calcium ions that facilitates flushing the blood out of the specimen, hinder blood from clotting as well as disrupting the desmosomes between cells. This step was performed for 11.06 minutes (SEM ± 0.47) on average. Secondly, the liver specimen was perfused with recirculating perfusion solution containing 1 mg collagenase P/mL (Roche Diagnostics GmbH, Mannheim, Germany) at 37 °C. Digestion was stopped when the tissue was visually and tactically deemed digested with a mean digestion time of 8.07 minutes (SEM ± 0.24). Afterwards, the specimen was transferred into a petri dish containing perfusion solution without EGTA but supplemented with 4% human albumin to stop the enzymatic digestion. After removal of the cannulas, tissue was disrupted mechanically by shaking and using tweezers to disrupt cells from the remaining scaffold structures.
The resulting cell suspension was then centrifuged (5 minutes, 50 g, 4 °C) to eliminate cell debris. After resuspending in culture medium (phenol red free Williams E with supplements: 1 µM Insulin [Lilly, Indianapolis], 1 µM Fortecortin [Merck Serono GmbH, Darmstadt, Germany], 1 mM sodium pyruvate, 10 mM HEPES-Buffer and 10% fetal calf serum [all from Biochrom]), cells were counted, and viability was assessed using the trypan blue exclusion test. A predetermined part of this “crude” suspension, i.e. without having undergone the Percoll purification step, was then seeded at a density of 1041 cells/mm² (1 million cells/well of a 6-well-plate) onto collagen-coated culture plates (Biochrom GmbH, Berlin, Germany) for cultivation.
Remaining cell suspension underwent the Percoll density separation by centrifugation through a Percoll layer (25% Percoll [Biochrom GmbH, Berlin, Germany] in phosphate buffered saline, 1.037 g/ml, 300mosm) for 20 minutes at 1474 g (4 °C). Therefore, 5 mL of cell suspension was gently mounted over a cushion of 15 mL Percoll-suspension. After careful aspiration of the supernatant, cell pellets were re-suspended with ice-cold PBS and centrifuged (5 minutes, 50 g, 4 °C) to wash out Percoll particles. After resuspension in medium, cells were counted and seeded as described above (Fig. 1). The cells were washed with pre-warmed PBS 4 hours after seeding to discard non-attaching cells. Liver cells were cultured in modified Williams E (see above, additional 100 U/mL/100 µg penicillin/streptomycin [Biochrom GmbH, Berlin, Germany]) for 6 days at 37 °C, 21% O2, 5% CO2. Medium was changed every 24 hours during the cultivation period.
Measurement of cell culture parameters
To measure the aspartate aminotransferase (AST) activity, albumin production and urea content, the supernatant of 3 pooled wells was centrifuged (3000 g, 5 min, 4 °C) after culturing overnight and after 2, 4, and 6 days in culture. Measurement of AST and Urea was conducted by Labor Berlin – Charité Vivantes GmbH (Berlin, Germany) within 12 hours of cold storage of the samples using an enzymatic assay (Roche Hitachi cobas c 6000 system, Roche Diagnostics GmbH, Mannheim, Germany). Albumin production was assessed using the human albumin ELISA kit (Bethyl Laboratories, Montgomery, TX, USA) after supernatant sample storage at −80 °C. ELISA samples were measured as duplets and according to the manufacturer’s instructions. All data was set in relation to the protein-content of the respective wells at the end of culture (day 6). For protein content measurement, the cells from these wells were washed, scratched off the culture plates, pooled, suspended with RIPA-Buffer and stored at −80 °C until being measured. Finally, photometric analysis using BCA-Reagent (Pierce, Thermo Fisher Scientific, MA, USA) according to the manufacturers’ instructions was performed with all samples measured in triplets. Plating efficiency was evaluated using a protocol as described elsewhere29: The cells were washed 24 hours after seeding them. Subsequently, the adherent protein was set in relation to the sum of the adherent protein and the total protein contained in the supernatants at that time-point.
Purity assay
Purity of the composition of the cell culture was assessed using real-time quantitative (q-rt)-PCR. RNA samples were collected from 12 isolations 4 h after seeding, on days 2, 4 and 6 of culture. After washing with PBS, cells were pooled in RNAase-free Eppendorfer containers containing TRIzol® (Thermo Fisher Scientific, Waltham, MA, USA) and immediately shock frozen in liquid nitrogen. Isolation and RNA measurements were performed according to the established protocols using a NanoDrop 2000c UV-Vis spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) for calculating RNA-concentration. The RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA) in a PTC-100® Thermal Cycler (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for generating cDNA. Gene expression was assessed using a StepOne™ RT-PCR cycler (ThermoFisher Scientific, Venlo, Netherlands). The following established DNA-primers were used (Abcam, Cambridge, United Kingdom): KRT18 (hepatocytes, Vim (fibroblasts), GGT1 (cholangiocytes), Pecam1 (sinusoidal endothelial cells) and CD68 (Kupffer cells). B2M was chosen as an endogenous control because it had been established to be unregulated in random samples before. Results are shown using the R = 2(−ΔΔCT) method as described elsewhere30.
Additionally, these markers were stained in an immunofluorescence assay on culture plates that were fixed 24 h after seeding and at the end of the culture period. Cells were fixated with 4% formaldehyde and stored in methanol until staining.
Microbiological testing
In 16 isolations, cell suspension underwent microbiological testing directly before seeding. A fraction of 5 ml of the cell suspension were transferred into aerobic and anaerobic blood culture bottles before and after Percoll purification. During 5 days of incubation, bacterial growth was examined by the Labor Berlin – Charité Vivantes GmbH (Berlin, Germany).
Statistical analysis
Statistical analysis was performed using GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). After testing Gaussian distribution with the Shapiro-Wilk-test, either a paired t-test or a Wilcoxon matched-pairs-signed-rank-test was done to analyze paired quantitative parameters. A p-value less than 0.05 was considered significant. All data is expressed as the mean ± standard error of the mean (SEM).
Results
Isolation outcome
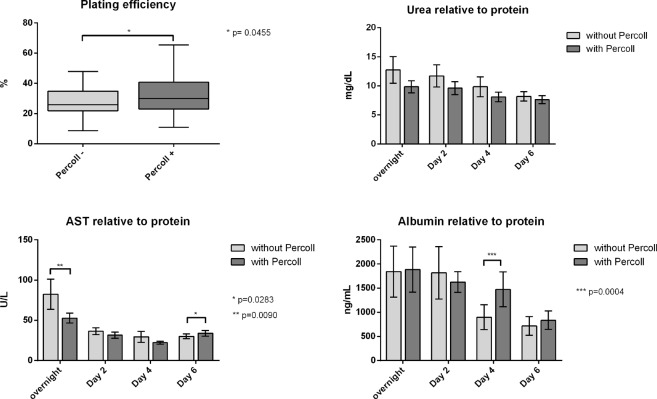
Isolation outcome parameters are expressed in relation to the respective mass of the liver specimen. The isolation outcome yielded 42.4 (±4.6) × 106 isolated cells per gram liver tissue on crude cells without Percoll purification and 26.6 (±4.0) × 106 cells per gram liver tissue after Percoll purification (Fig. 2). This resulted in an average Percoll survival rate of 59.03% (±3.9). Viability was significantly increased (p = 0.0299) from 84.47% (±1.60) to 88.18% (±1.13) by Percoll purification.
Figure 2.
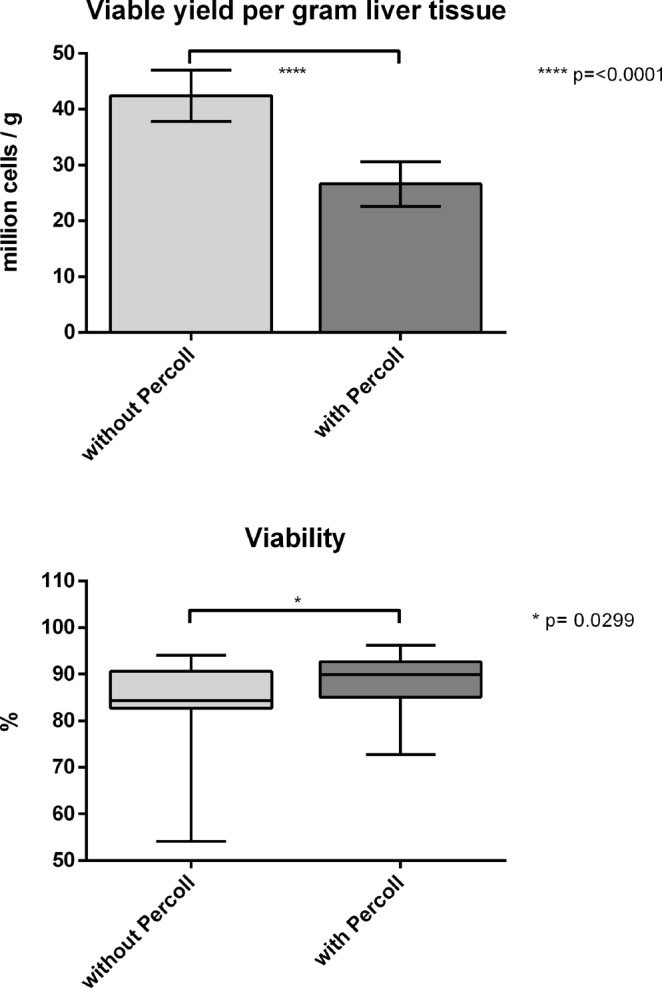
Yield and viability of the overall group. Comparison of liver cell isolation without vs. with Percoll purification, n = 27. Viability: Box plots show the 25th and 75th percentile and median value, whiskers display smallest and largest value. Yield: Columns display mean value, whiskers indicate SEM.
Functional parameters
Comparing functional parameters of isolated cells, plating efficiency was significantly (p = 0.046) increased in Percoll-purified cells (from 27.19 ± 2.19% to 31.77 ± 2.98%). Albumin, urea and AST levels are shown relative to the respective protein content at the end of culture (Table 1, Fig. 3).
Table 1.
In vitro hepatocyte function.
| Parameter | Overnight | Day 2 | Day 4 | Day 6 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | p-Value | Mean ± SEM | p-Value | Mean ± SEM | p-Value | Mean ± SEM | p-Value | |||||
| Percoll− | Percoll+ | Percoll− | Percoll+ | Percoll− | Percoll+ | Percoll− | Percoll+ | |||||
| Urea (mg/dL) | 12.74 ± 2.30 | 9.84 ± 1.03 | 0.0532 | 11.71 ± 1.89 | 9.61 ± 1.12 | 0.2722 | 9.83 ± 1.70 | 8.10 ± 0.83 | 0.3205 | 8.18 ± 0.81 | 7.63 ± 0.69 | 0.1111 |
| Albumin (ng/mL) | 1843.41 ± 529.30 | 1884.05 ± 466.00 | 0.8906 | 1817.28 ± 542.40 | 1625.02 ± 215.40 | 0.3683 | 897.36 ± 259.00 | 1474.38 ± 363.20 | 0.0004 | 716.08 ± 192.82 | 835.26 ± 191.45 | 0.3225 |
| AST (U/L) | 82.37 ± 18.67 | 52.71 ± 6.13 | 0.0090 | 36.56 ± 4.27 | 31.56 ± 3.83 | 0.0516 | 29.38 ± 6.67 | 22.14 ± 1.96 | 0.3525 | 29.98 ± 2.96 | 33.77 ± 3.62 | 0.0425 |
Figure 3.
Functional parameters of the overall group. Comparison of hepatocytes isolated without or with Percoll purification (n = 27). Plating efficiency: Box plots show the 25th and 75th percentile and median value, whiskers display smallest and largest value. Urea, AST, Albumin: Columns display mean value, whiskers indicate SEM. Abbreviations: AST: aspartate aminotransferase, SEM: standard error of mean.
Urea levels were similar in both groups throughout the cultivation period, being slightly higher in the untreated group, however without reaching statistical significance. Aspartate aminotransferase release was initially higher in the untreated group (82.37 ± 18.67 U/L vs. 52.71 ± 6.129 U/L, p = 0.009), whereas towards the end of culture Percoll-purified cells showed higher levels (29.98 ± 2.959 U/L vs 33.77 ± 3.622 U/L, p = 0.028). Albumin content decreases in both groups over the course of cell culture, however, additionally purified cells tended to decrease their production later and to a lesser degree than cells without Percoll purification. This reached statistical significance (p = 0.0004) on day 4: while Albumin content was 897.36 ng/mL (±259) without Percoll treatment, levels were higher on Percoll purified cells 1474.38 ng/mL (±363.2).
Microbiological analysis
Microbiological analysis revealed no difference in contamination rates between the two groups. Of the 16 isolations that underwent this investigation, 13 were sterile while three were contaminated. Identified pathogens were Enterococcus faecium, E. faecalis and E. coli as well as Klebsiella oxytoca and Staphylococcus haemolyticus. However, there was no difference between the two groups and we presume that this contamination most likely occurred in the operating room during retrieval of the tissue.
Cell culture purity
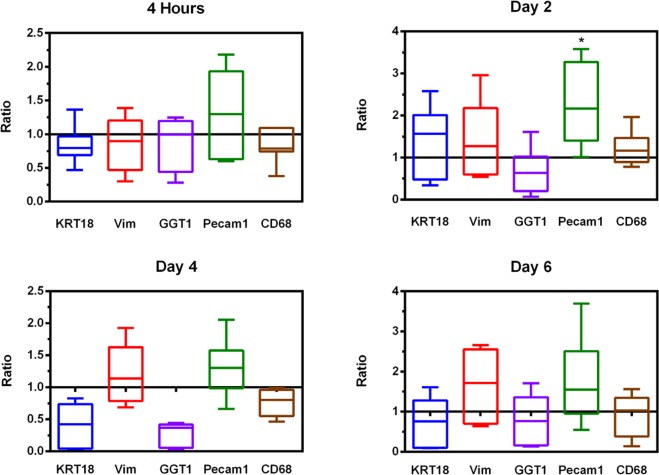
Purity assays showed no difference in the expression of liver-cell-specific DNA. KRT18 levels as well as CD68 on Kupffer cells and Vimentin on fibroblasts were the same in both the crude as well as the Percoll-purified liver cell cultures. However, there was a slight tendency in crude, i.e. non Percoll-purified, preparations to express more Pecam1, which is a marker for sinusoidal endothelial cells. This reached statistical significance on Day 2 of culturing (2.12-fold higher expression in crude cultures, p = 0.0220). Moreover, the presence of GGT1-expressing cells, namely cholangiocytes, tends to be higher in preparations that have undergone Percoll purification. This trend is visible throughout the culture period, and nearly reaches statistical significance on day 4, when expression is 5.66-fold higher in the untreated group (p = 0.0616, Fig. 4).
Figure 4.
q-rt-PCR. q-rt-PCR displayed by the 2−ΔΔCT-Method: values > 1 indicate higher expression of the gene in preparations without Percoll purification, while values < 1 mean higher expression in the Percoll-purified samples. Box plots show the 25th and 75th percentile and median value, whiskers display smallest and largest value.
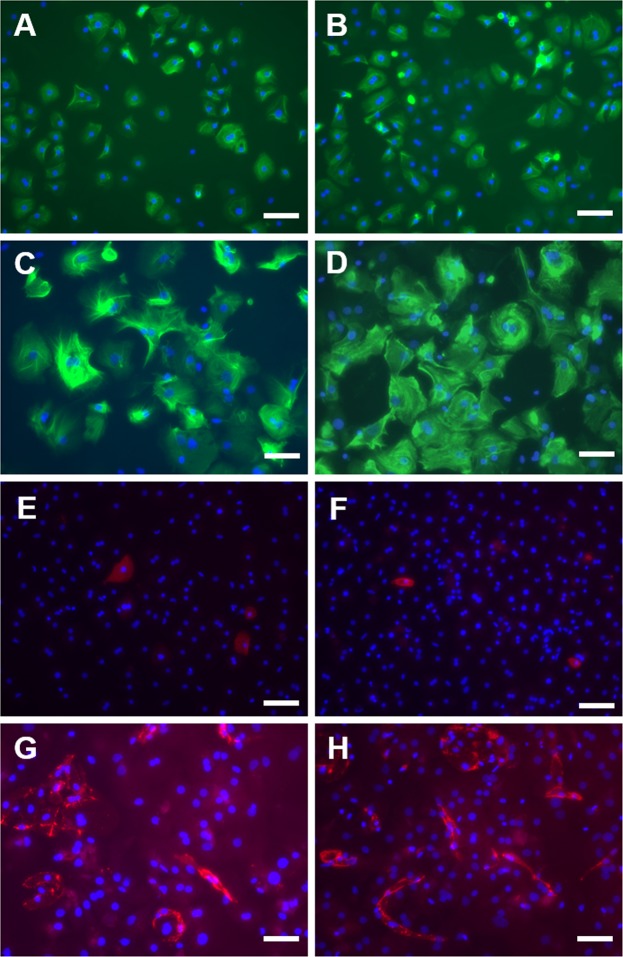
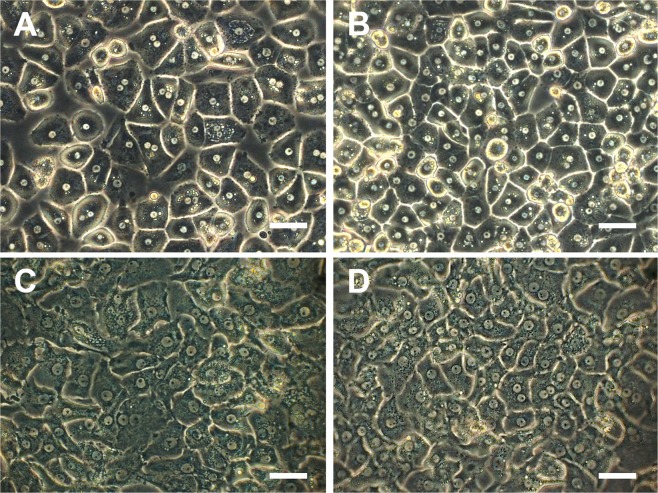
Immunofluorescence staining showed no differences between the two groups and generally supports the presence of all hepatic cell types in both groups, detected by mRNA-expression profiles. While hepatocytes in the KRT-18-staining look compact 24 hours after seeding, they appear protruded and not as dense as at the beginning of culture. This is true for both crude and Percoll-treated preparations. Interestingly, while Pecam-1-postive cells, i.e. endothelial cells, are also compact 24 hours after seeding, they form networks at the end of the culturing period (Fig. 5). There were again no differences detectable between the two treatment groups. Fractions of non-parenchymal cells were also stained, however due to difficult quantification not showed in this publication. Stellate cells were not found in either group. See supplementary information material for the immunofluorescence staining images of different non-parenchymal cell populations. Phase contrast microscopy of the cultures 24 hours after seeding and at the end of culture revealed no differences between the two treatment groups (Fig. 6).
Figure 5.
Immunofluorescence. Fluorescence microscopy of hepatocyte cultures without (A,C,E,G) of after (B,D,F,H) purification with Percoll. Time points: 24 hours after seeding (A,B,E,F) and on Day 6 (C,D,G,H). A-D display KRT18-staining (hepatocytes), E-H display Pecam1-staining (endothelial cells). Scale bar: 50 µm.
Figure 6.
Phase contrast images. Phase contrast microscopy of hepatocyte cultures without (A,C) of after (B,D) purification with Percoll. Time points: 24 hours after seeding (A,B) and on Day 6 (C,D). Scale bar: 50 µm.
Subgroup analysis
When analyzing the data more thoroughly, five cases presented an initial viability that was lower than 80%. Percoll purification increased the viability from 71.63 (±4.44) to 87.7% (±2.69) in these isolations, reaching statistical significance p = 0.0299. However, no benefit in functional parameters could be detected. An average of 56.4% of the cells could be recovered from Percoll-purification, whereas yield was significantly diminished from 25.90 (±9.72) × 106 to 15.3 (±8.66) × 106 cells per gram of liver tissue (Table 2). When analyzing the features of this subgroup, the three cases differed from the overall group in a few known factors24,31–33 that are known to affect the isolation outcome, including preoperative levels of ALT, AST, AP, GGT and in the operating technique (Table 3).
Table 2.
Subgroup analysis of isolation outcome parameters.
| Parameter | Overall | high initial viability > 80% | low initial viability <80% | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SEM | p-Value | Mean ± SEM | p-Value | Mean ± SEM | p-Value | ||||
| Percoll− | Percoll+ | Percoll− | Percoll+ | Percoll− | Percoll+ | ||||
| Plating efficiency (%) | 27.19 ± 2.19 | 31.77 ± 2.99 | 0.0455 | 29.92 ± 1.87 | 34.33 ± 3.05 | 0.0783 | 11.735 ± 2.47 | 16.46 ± 3.48 | 0.4312 |
| Viablility (%) | 84.47 ± 1.60 | 88.18 ± 1.13 | 0.0299 | 87.39 ± 0.91 | 88.29 ± 1.27 | 0.3209 | 71.63 ± 4.44 | 87.70 ± 2.79 | 0.0625 |
| Percoll survival (%) | 59.03 ± 3.90 | 59.63 ± 4.10 | 56.38 ± 12.02 | ||||||
| Relative yield in millions per gram liver tissue | 42.40 ± 4.60 | 26.60 ± 4.00 | <0.0001 | 46.15 ± 4.95 | 29.18 ± 4.40 | <0.0001 | 25.90 ± 9.72 | 15.30 ± 8.66 | 0.0299 |
| Total protein on culture plate on day 6 | 596.20 ± 66.79 | 662.70 ± 61.98 | 0.0102 | 643.76 ± 73.55 | 716.71 ± 66.50 | 0.0156 | 334.38 ± 79.79 | 365.58 ± 55.33 | 0.6250 |
Table 3.
Subgroup features.
| Parameter | High initial viability >80% | Low initial viability <80% |
|---|---|---|
| Mean ± SEM | Mean ± SEM | |
| Number of cases | 22 | 5 |
| Surgical Indication | ||
| HCC | 2 | 0 |
| Biliary tree carcinoma | 6 | 4 |
| CRLM | 12 | 0 |
| Benign disease | 2 | 1 |
| Preoperative bilirubin | 0.65 ± 0.08 | 1.21 ± 0.49 |
| Preoperative ALT | 35.32 ± 4.98 | 63.00 ± 19.99 |
| Preoperative AST | 41.24 ± 6.10 | 45.60 ± 4.41 |
| Preoperative AP | 152.50 ± 41.62 | 320.60 ± 108.62 |
| Preoperative GGT | 215.05 ± 59.80 | 356.80 ± 133.79 |
| Preoperative INR | 1.02 ± 0.01 | 1.04 ± 0.02 |
| Age of donor (years) | 61.50 ± 3.13 | 57.40 ± 7.69 |
| Fibrosis | ||
| No Fibrosis | 5 | 0 |
| Minimal (periportal) | 6 | 2 |
| Mild (septs) | 4 | 3 |
| Severe Fibrosis (portocentral septs, architecture changes) | 2 | 0 |
| Cirrhosis (definite architectural changes) | 1 | 0 |
| Data not available | 4 | 0 |
| Steatosis | ||
| No Steatosis | 2 | 0 |
| ≤30% Steatosis | 8 | 0 |
| >30% Steatosis | 3 | 0 |
| Data not available | 9 | 5 |
| Cold ischeamic time (min) | 23.27 ± 3.51 | 15.00 ± 2.24 |
| Time of first perfusion (min) | 11.18 ± 0.56 | 10.50 ± 0.61 |
| Time of second perfusion (min) | 8.09 ± 0.28 | 8.00 ± 0.57 |
| Mass of liver specimen (g) | 16.34 ± 1.39 | 26.42 ± 13.42 |
Abbreviations: GGT, Gamma-glutamyl transpeptidase; CIT, cold ischemic time; AST, aspartate aminotransferase; ALT, alanine aminotransferase; AP, alkaline phosphatase; INR, international normalized ratio; HCC, Hepatocellular carcinoma; CRLM, Colorectal liver metastasis.
Discussion
Isolation of primary human hepatocytes is an expensive and time-demanding process. It requires a high-level hepatobiliary surgical center, excellent communication between the personnel in the operation room to keep ischemic times as short as possible and experienced laboratory staff, as well as a well-equipped laboratory infrastructure. Tissue specimens retrieved from the operating room for hepatocyte isolation must therefore be utilized as effectively as possible.
Using Percoll density centrifugation is a double-edged sword: Is the tremendous loss of cells worth the better performance? In all publications that provide detailed information regarding the Percoll purification step during isolation of human liver cells22,23,33–36, the cell yield was diminished by this procedure, while the viability improved. According to Schröder et al.23 who performed purification through a 50% concentrated Percoll-suspension, a very pure cell culture was retrieved with few contaminating non-parenchymal liver cells (assessed using immunohistological staining) and a viability of 94%. However, in seven of 32 experiments no cells were recovered after the Percoll purification step, while a low-yield after Percoll purification was reported in the remaining 25 trials. Olinga et al.22 declared an average recovery threshold of 33% after Percoll purification, but viability improved from 69% to 92%. Information on the quality and purity of the resulting cells was not reported though. Laba et al.34 centrifuged their cell suspensions through a 29–31%-concentrated Percoll layer before and after cryopreservation. Mean viability of crude cells before Percoll purification ranged up to 72% and improved to 89% after Percoll purification. When comparing freshly isolated crude cell suspensions to cryopreserved cells that were purified using the Percoll method after thawing, a positive effect on the plating efficiency was found after usage of Percoll (35–40% improved to 65–85%) and lower levels of non-parenchymal cells were reported. However, this study focused on the comparison of fresh and cryopreserved and thawed hepatocytes, while there was no metabolic test and the study was limited due to the low number of experiments (n = 5). These authors also did not directly compare cells before and after Percoll purification regarding cell culture purity and plating efficiency, which are determining parameters for the subsequent application of hepatocytes:
Primary human hepatocytes are widely used to study pharmacodynamic processes as well as for investigating processes of the hepatocyte itself. Apart from in vitro research, human hepatocytes can also be applied in the clinic. Since the number of donated organs for whole organ transplantation is limited, but the number of patients with end-stage liver diseases is rising37 the demand for an alternative to whole orthotropic liver transplantation is high. Hepatocytes can be used to bridge the time until a suitable donor organ is available9. Hepatocyte transplantation has been successfully performed in children with inherited metabolic diseases such as Crigler-Najjar syndrome7,8 and in mice with hemophilia38. Primary hepatocytes can also be cultured in spheres or in multi-layered27 cell sheet technology. These three-dimensional constructs serve as a cell-based regenerative therapy by providing metabolic function when implanted in the kidney capsules of mice. Moreover, bioengineered organs are under investigation to overcome the shortage of donor organs for example by using human hepatocytes to engraft matrices derived from decellularized livers10,39,40. Tissue engineered organs still face problems of sufficient re-endothelialization, or reperfusion injury due to inflammatory mediators secreted by Kupffer cells contaminating the hepatocyte suspension41. Therefore, providing hepatocyte cultures in high purity is necessary. In contrary to ours and others expectations, our data show that Percoll purification at 25% concentration significantly contributes towards a more homogenously pure cell culture. Higher concentrated Percoll cushions (e.g. 50%) could achieve this. However, with increased silica particle density then fatty – but metabolically active – hepatocytes would be sorted out and lost. Hence, ideally, Percoll concentrations would be adjusted to the fat content of the donor’s liver. However, such an adjustment of the cell isolation procedure is not practical as often no suitable histopathological assessment is available prior to the isolation process. Although the overall metabolic performance of cells is similar in both treatment groups, AST levels differed significantly 24 hours and at the end of culture. We hypothesize that the difference on day 1 is due to the fact that there is more cell debris and dead cells in the non-Percoll-purified culture plates. This debris does not adhere for further culture, however, contributes to the high levels of AST in the non-purified group on day 1. The differences between albumin levels on day 4 and AST levels on day 6 might indicate a temporal higher metabolic activity of hepatocytes isolated with Percoll purification which is however not consistent throughout the culture period. Our study is limited by the fact that we analyzed hepatocytes isolations from surgically resected liver tissue, which would most likely not be applicable for therapeutic use. We do, however, expect that we would obtain similar results when comparing liver cell isolations from whole liver grafts with vs. without Percoll purification.
Primary liver cells isolated from human tissue still face the hurdle of availability. The limited preservation of metabolically capable primary hepatocytes in-vitro has created an urgent need for an alternative to primary human hepatocytes. Even though genetically engineered hepatic cells are under thorough investigation in stem cell research42–45, primary human hepatocytes are still the gold standard for the applications mentioned before9,27.
Given the fact that Percoll purification could not raise the function of the cells, we suggest that density separation is only useful to sort out initial dead cells and debris – if needed. If the cell suspension has a high initial viability, then there is no evidence to support the need for Percoll purification. Regarding functional effects of this step, there are no benefits, but also no contradictions to the centrifugation through the silica-particle-layer could be found. If donors do not meet the criteria for ensuring a good hepatocyte isolation outcome, as reported in the literature, then Percoll purification could still be considered to rescue a fraction of highly viably hepatocytes.
Supplementary information
Acknowledgements
The authors thank Prof. Dr. Robert Öllinger, Prof. Dr. Daniel Seehofer, Priv.-Doz. Dr. Moritz Schmelzle and Priv.-Doz. Dr. Robert Sucher for performing the surgical procedures and their good cooperation, Anja Schirmmeier for her excellent support in the tissue retrieval procedure and Bertram Linderkamp for his support in linguistic concerns. This work was supported by the Open Access Publication Fund of the Charité – Universitätsmedizin Berlin.
Author Contributions
M.K., K.H.H., R.H., I.M.S. and N.R. were responsible for planning the study. R.H. carried out all trials including the isolation process and analysis of isolation outcome parameters. R.H., J.G.M.V.G. and P.T. were involved in cell culturing and analysis of functional parameters throughout the culture period. S.L. carried out qRT-PCR. R.H., A.R.S. and S.M. performed statistical analysis. R.H. wrote the main manuscript text and prepared Figures 2, 3 and 5 and Tables 1–3; Figure 4 was provided by S.M., Figure 1 was created by I.M.S. N.R., I.M.S. and J.P. provided patient’s consent and gave genuine support in all steps of the study. All authors reviewed the manuscript.
Data Availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43042-8.
References
- 1.Gómez-Lechón MJ, Castell JV, Donato MT. The use of hepatocytes to investigate drug toxicity. Methods Mol Biol. 2010;640:389–415. doi: 10.1007/978-1-60761-688-7_21. [DOI] [PubMed] [Google Scholar]
- 2.Hengstler JG, et al. Cryopreserved primary hepatocytes as a constantly available in vitro model for the evaluation of human and animal drug metabolism and enzyme induction. Drug metabolism reviews. 2000;32(1):81–118. doi: 10.1081/DMR-100100564. [DOI] [PubMed] [Google Scholar]
- 3.Russell, W. M. S. & Burch, R. L. The principles of humane experimental technique. Methuen and Co ltd., United Kingdom (1959).
- 4.Godoy P, et al. Recent advances in 2D and 3D in vitro systems using primary hepatocytes, alternative hepatocyte sources and non-parenchymal liver cells and their use in investigating mechanisms of hepatotoxicity, cell signaling and ADME. Arch Toxicol. 2013;87(8):1315–530. doi: 10.1007/s00204-013-1078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vinken M, Vanhaecke T, Rogiers V. Primary hepatocyte cultures as in vitro tools for toxicity testing: quo vadis? Toxicol In Vitro. 2012;26(3):541–4. doi: 10.1016/j.tiv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Wong RJ, et al. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–55. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 7.Strom SC, et al. Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation. 1997;63(4):559–69. doi: 10.1097/00007890-199702270-00014. [DOI] [PubMed] [Google Scholar]
- 8.Fox IJ, et al. Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N. Engl. J. Med. 1998;338:1422–1426. doi: 10.1056/NEJM199805143382004. [DOI] [PubMed] [Google Scholar]
- 9.Squires JE, et al. A. Clinical Hepatocyte Transplantation: What Is Next? Curr Transplant Rep. 2017 Dec. 2017;4(4):280–289. doi: 10.1007/s40472-017-0165-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazza G, et al. Decellularized human liver as a natural 3D-scaffold for liver bioengineering and transplantation. Sci Rep. 2015;5:13079. doi: 10.1038/srep13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uygun BE, Yarmush ML, Uygun K. Application of whole-organ tissue engineering in hepatology. Nature reviews Gastroenterology & hepatology. 2012;9(12):738–744. doi: 10.1038/nrgastro.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strom SC, et al. Isolation, culture, and transplantation of human hepatocytes. J Natl Cancer Inst. 1982;68(5):771–8. [PubMed] [Google Scholar]
- 13.Smedsrød B, Pertoft H. Preparation of pure hepatocytes and reticuloendothelial cells in high yield from a single rat liver by means of Percoll centrifugation and selective adherence. J Leukoc Biol. 1985;38(2):213–30. doi: 10.1002/jlb.38.2.213. [DOI] [PubMed] [Google Scholar]
- 14.Pertoft H, Laurent TC, Låås T, Kågedal L. Density gradients prepared from colloidal silica particles coated by polyvinylpyrrolidone (Percoll) Anal Biochem. 1978;88(1):271–82. doi: 10.1016/0003-2697(78)90419-0. [DOI] [PubMed] [Google Scholar]
- 15.Kegel V, et al. Protocol for Isolation of Primary Human Hepatocytes and Corresponding Major Populations of Non-parenchymal Liver Cells. Journal of visualized experiments: JoVE. 2016;109:e53069. doi: 10.3791/53069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bourgognon M, Klippstein R, Al-Jamal KT. Kupffer Cell Isolation for Nanoparticle Toxicity Testing. Journal of visualized experiments: JoVE. 2015;102:e52989. doi: 10.3791/52989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SM, Schiergens TS, Demmel M, Thasler RM, Thasler WE. Isolation of Hepatocytes and Stellate Cells from a Single Piece of Human Liver. Methods in molecular biology. 2017;1506:247–58. doi: 10.1007/978-1-4939-6506-9_17. [DOI] [PubMed] [Google Scholar]
- 18.Pfeiffer E, et al. Featured Article: Isolation, characterization, and cultivation of human hepatocytes and non-parenchymal liver cells. Experimental biology and medicine. 2015;240(5):645–56. doi: 10.1177/1535370214558025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li AP. Human hepatocytes: Isolation, cryopreservation and applications in drug development. Chem Biol Interact. 2007;168(1):16–29. doi: 10.1016/j.cbi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Dou M, et al. Thawed human hepatocytes in primary culture. Cryobiology. 1992. 1992;29(4):454–69. doi: 10.1016/0011-2240(92)90048-7. [DOI] [PubMed] [Google Scholar]
- 21.Utesch D, Diener B, Molitor E, Oesch F, Platt KL. Characterization of cryopreserved rat liver parenchymal cells by metabolism of diagnostic substrates and activities of related enzymes. Biochem Pharmacol. 1992;44(2):309–15. doi: 10.1016/0006-2952(92)90014-A. [DOI] [PubMed] [Google Scholar]
- 22.Olinga P, et al. The capability of isolated hepatocytes and liver slices of donor livers to predict graft function after liver transplantation. Liver. 2000;20(5):374–80. doi: 10.1034/j.1600-0676.2000.020005374.x. [DOI] [PubMed] [Google Scholar]
- 23.Schröder AJ, Blaheta RA, Scholz M, Encke A, Markus BH. Isolation and separation of human adult hepatocytes from resected liver: cellular yield and purity using different methods. Zentralbl Chir. 1994;119(2):127–38. [PubMed] [Google Scholar]
- 24.Vondran FW, et al. Isolation of primary human hepatocytes after partial hepatectomy: criteria for identification of the most promising liver specimen. Artificial organs. 2008;32(3):205–13. doi: 10.1111/j.1525-1594.2007.00524.x. [DOI] [PubMed] [Google Scholar]
- 25.Richert L, et al. Tissue collection, transport and isolation procedures required to optimize human hepatocyte isolation from waste liver surgical resections. A multilaboratory study. Liver international. 2004;24(4):371–8. doi: 10.1111/j.1478-3231.2004.0930.x. [DOI] [PubMed] [Google Scholar]
- 26.Bhogal Ricky H., Hodson James, Bartlett David C., Weston Christopher J., Curbishley Stuart M., Haughton Emma, Williams Kevin T., Reynolds Gary M., Newsome Phillip N., Adams David H., Afford Simon C. Isolation of Primary Human Hepatocytes from Normal and Diseased Liver Tissue: A One Hundred Liver Experience. PLoS ONE. 2011;6(3):e18222. doi: 10.1371/journal.pone.0018222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatsumi K, Okano T. Hepatocyte Transplantation: Cell Sheet Technology for Liver. Cell Transplantation. Curr Transplant Rep. 2017;4(3):184–192. doi: 10.1007/s40472-017-0156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lehec SC, et al. Experience of microbiological screening of human hepatocytes for clinical transplantation. Cell transplantation. 2009;18(8):941–7. doi: 10.3727/096368909X471323. [DOI] [PubMed] [Google Scholar]
- 29.Horner R, et al. Hepatocyte Isolation After Laparoscopic Liver Resection. Tissue Eng Part C Methods. 2016;22(9):839–46. doi: 10.1089/ten.tec.2016.0187. [DOI] [PubMed] [Google Scholar]
- 30.Livak JK, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 31.Kluge M, et al. Human Hepatocyte Isolation: Does Portal Vein Embolization Affect the Outcome? Tissue Eng Part C Methods. 2016;22(1):38–48. doi: 10.1089/ten.tec.2015.0190. [DOI] [PubMed] [Google Scholar]
- 32.Alexandre E, et al. Influence of pre-, intra- and post-operative parameters of donor liver on the outcome of isolated human hepatocytes. Cell and tissue banking. 2002;3(4):223–33. doi: 10.1023/A:1024614000925. [DOI] [PubMed] [Google Scholar]
- 33.Lee SM, et al. An algorithm that predicts the Viability and yield of Human Hepatocytes Isolated from Remnant Liver Pieces Obtained from Liver Resections. PLoS one. 2014;9(10):e107567. doi: 10.1371/journal.pone.0107567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Laba A, Ostrowska A, Patrzalek D, Paradowski L, Lange A. Characterization of human hepatocytes isolated from non-transplantable livers. Archivum immunologiae et therapiae experimentalis. 2005;53(5):442–53. [PubMed] [Google Scholar]
- 35.Ostrowska A, et al. Investigation of functional and morphological integrity of freshly isolated and cryopreserved human hepatocytes. Cell and tissue banking. 2000;1(1):55–68. doi: 10.1023/A:1010175906791. [DOI] [PubMed] [Google Scholar]
- 36.Gramignoli R, et al. Rapid and sensitive assessment of human hepatocyte functions. Cell transplantation. 2014;23(12):1545–56. doi: 10.3727/096368914X680064. [DOI] [PubMed] [Google Scholar]
- 37.Pais R, et al. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016;65(6):1245–57. doi: 10.1016/j.jhep.2016.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tatsumi K, et al. Therapeutic effects of hepatocyte transplantation on hemophilia B. Transplantation. 2008;86(1):167–70. doi: 10.1097/TP.0b013e31817b9160. [DOI] [PubMed] [Google Scholar]
- 39.Uygun BE, et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat Med. 2010;16(7):814–20. doi: 10.1038/nm.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou P, et al. Decellularized liver matrix as a carrier for the transplantation of human fetal and primary hepatocytes in mice. Liver Transplantation. 2011;17(4):418–27. doi: 10.1002/lt.22270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clavien PA. Sinusoidal endothelial cell injury during hepatic preservation and reperfusion. Hepatology. 1998;28:281–5. doi: 10.1002/hep.510280201. [DOI] [PubMed] [Google Scholar]
- 42.Khan AA, et al. Human fetal liver-derived stem cell transplantation as supportive modality in the management of end-stage decompensated liver cirrhosis. Cell Transplant. 2010;19:409. doi: 10.3727/096368909X484707a. [DOI] [PubMed] [Google Scholar]
- 43.Marongiu F, et al. Hepatic differentiation of amniotic epithelial cells. Hepatology. 2011;53(5):1719–29. doi: 10.1002/hep.24255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsolaki E, Yannaki E. Stem cell-based regenerative opportunities for the liver: State of the art and beyond. World Journal of Gastroenterology. 2015;21(43):12334–12350. doi: 10.3748/wjg.v21.i43.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fanti M, et al. Differentiation of amniotic epithelial cells into various liver cell types and potential therapeutic applications. Placenta. 2017;59:139–145. doi: 10.1016/j.placenta.2017.03.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.