Abstract
Succinic acid is widely applied to chemical, pharmaceutical, food, and agricultural industries. With the rapid development of these industries, a great demand of succinic acid is required. The acid-tolerance and succinic acid production of Actinobacillus succinogenes strain were improved by using genome shuffling. Results showed that one modified strain AS-F32, with the best acid resistance and the highest succinic acid production, was obtained after 3 cycles of genome shuffling. The minimum growth pH of AS-F32 was 3.5, and the acid production and cell dry weight were 5.1 and 4.8 g/L in flask, improved 2.6 and 1.85 times over the start strain As-R2. Furthermore, the succinic acid yield of As-32 was 31.2 g/L and the dry cell weight was increased 44.4% by maintaining pH 4.8 with 7.0 M NH4OH in 5 L bioreactor, increased 1.1 times than the original strain As-R2.
Keywords: Actinobacillus succinogenes, Succinic acid, Genome shuffling, Acid resistance, High production
Introduction
Succinic acid, a kind of organic acids, can be acquired from plants, animals or microorganisms. It belongs to C4-dicarboxylic acid family and plays an essential part in biological metabolism of many organisms (Wittmann et al., 2017). It is also an important material that is widely applied in many industries, including food, pharmaceuticals, chemicals, and agricultural products (Carlson et al., 2016; Oh et al., 2009). Furthermore, its derivatives succinic acid imide can be used as biofuels and water purifying agents (Jiang et al., 2014). Nowadays, the primary methods for producing succinic acid are chemical synthesis and microbial fermentation (Alonso et al., 2015). For the former, it not only occupies unrenewable resource but also seriously pollutes the environment. In contrast, microbial fermentation is accepted because the advantages of low cost, non-pollution and high conversion rate. It also can fix carbon dioxide to drive down the emission load (Pinazo et al., 2015). Many microorganisms, including Actinobacillus succinogenes, Mannheimia succiniciproducens, recombinant Escherichia coli, and Anaerobiospirillum succiniciproducens can be used to produce succinic acid (Ahn et al., 2016; Lee et al., 2003; Liang et al., 2011; Wu et al., 2016). Owing to the better acid tolerance and higher succinic acid production, A. succinogenes is considered as the most promising strain in industry (Liu et al., 2013; Song and Sang, 2006; Thuy et al., 2017). However, for industries, the better acid tolerance of strains is a critical factor of production. Succinic acid is produced with the reproduction of cells, the accumulation of succinic acid will inhibit cell growth and limit to produce succinic acid substantially. Normally, typical commercial fermentation of A. succinogenes runs at a minimum pH of 6.8, the succinic acid solution contained 93.5% succinate2−, 6.4% succinate1−, and 0.1% free acid. While at pH 2.0, succinic acid solution composed of 0.6% succinate1− and 99.4% free acid (Li et al., 2010). Thus, when higher pH values, it requires a more expensive and wasteful purification to obtain succinic acid. But at lower pH values, succinic acid exists in the free-acid form, which may be purified by extracting the fermentation broth directly and used in continuous biofilm reactor production, and decrease the requirement for neutralizing agents and lower the cost of downstream processing. In addition, it also reduces potential contamination. The improving of growth and succinic acid production of microorganisms at low pH is a desired commercial goal.
It is difficult to improve the acid tolerance phenotype of industrial strains and increase the production of succinic acid controlled by multi-genes and un-cloned genes. Genome shuffling is a potential method, by which the target characters, especially ones controlled by multi-genes or un-cloned genes (Dai and Copley, 2004). It is a typical technique of combinatorial engineering and an efficient way to improve the phenotype of industrial strains as well as suited well in industrial application compared with traditional breeding methods, which is difficult and impractical to reform specific genes (Biot-Pelletier and Martin, 2014). The traditional mutation breeding techniques and microbial protoplast fusion were successfully reorganized by gene technology for combination to the reorganization of objects from a single gene to the entire genome, while many parent strains were excellent phenotype with a reorganization of the strains, which could be efficiently and quickly filter out the fermentation of mutant strains for positive good character, instead of the mutants that is difficult to improve the performance of the defects after a long tradition of mutagen glazing. In a word, this technique combines the merits of conventional breeding methods. Recently, it has been successfully used for enhancing the glucose and acid resistance in Lactobacillus (Wang et al., 2007; Yu et al., 2008). Zhang et al. has used it to increase the yield of Streptomyces (Zhang et al., 2002). Moreover, the antifungal activity of L. plantarum has been improved by using genome shuffling (Wang et al., 2013). Up to now, the developed strains by genome shuffling have been reported to be used for producing many biotechnological products, including L-lactic acid, ethanol, antibiotics, bio-insecticide, ribo-flavin, ayamycin, avilamycin, alkaliphilic lipase, and so on (Luna-Flores et al., 2016; Zhang et al., 2014; Zhao et al., 2015).
The aim of our research is to improve the acid tolerance and succinic acid yield of A. Succinogenes simultaneously. By using NTG and UV treatments, the mutant strains with slight improvements compared to wild type strain A. succinogenes ATCC 55618 (AS-R2) were obtained, then they were subjected to recursive protoplast fusion and the performance of shuffled strains was analyzed in shake flask and bioreactor.
Materials and methods
Parental strains and mediums
AS-R2 was obtained from the American Type Culture Collection (ATCC 55618, Manassas, VA, USA) and grown in liquid medium. Inoculum medium contains 5.0 g yeast extract, tryptone 10.0 g, 5.0 g soy peptone, 5.0 g NaCl per liter at pH 7.0–7.2 (20 g agar for agar plates). Screening plate medium contains 5.0 g yeast extract, tryptone 10.0 g, 5.0 g soy peptone, 20 g glucose, 20 g agar, 5.0 g NaCl per liter. Regeneration medium (RM) includes 30 g glucose, 10 g yeast extract, 0.3 g Na2HPO4, 9.6 g NaH2PO4, 3.0 g K2HPO4, 0.4 g MgCl2, 171.13 g sucrose and 2% agar for one liter at pH 7.0–7.2. Fermentation medium including 10 g yeast extract, 0.3 g Na2HPO4, 9.6 g NaH2PO4, 30 g glucose, 3 g K2HPO4, and 0.4 g MgCl2 per liter and the pH was adjusted. A. succinogenes protoplast formation buffer (APB) contained 2.33 g maleic acid, 4.07 g MgCl2·6H2O, 171.13 g sucrose per liter, and the initial pH was regulated to 7.0. All medias were sterilized at 121 °C for 20 min and the pH value was adjusted by 1.0 M HCl. All other chemicals were of analytical grade.
Strain mutagenesis and screening
The mutagenesis were carried out as described (Yu et al., 2008). In brief, for NTG treatment, the AS-R2 cell suspension was handled with 500 µg/mL NTG at 37 °C for 90 min, and for UV treatment, the AS-R2 cell suspension was exposed directly under the 30 W UV lamp at a distance of 30 cm for 50 s. After 10 times of gradient dilution to remove the NTG, the dilutions were spread on screening plates and cultured at 37 °C for 5 days in Anaerobic System (Thermo Fisher Scientific, Waltham, MA, USA). The colonies, grew on pH 4.5 screening plates were selected and incubated on screening plates with 1% CaCO3. Colonies were selected with the larger transparent circles and then used for shake-flasks analysis. The more productive strains were screened and named AS-UV1, AS-UV2, AS-NTG1, AS-NTG2 as starter strains for genome shuffling.
Genome shuffling
Genome shuffling was conducted as described (Wang et al., 2007). First, different mutant strains were centrifuged, washed, suspended, and diluted in APB buffer and harvested when OD600 reached 0.8, then lysozyme (Sigma Chemical, Co. St. Louis, MO, USA) was added until the final concentration to 0.08 mg/mL. Protoplasts were obtained by identifying the formation of protoplast through light microscopy and inactivated by UV and heating treatment. Before genome shuffling, both inactivated protoplast populations were mixed equivalently, and then spread on RM plates in an Anaerobic System at 37 °C for 5 days. After that, the strains were selected on screening plates at pH 4.3 under 37 °C for 5 days. Then the shuffled strains were spread on screening plates containing 1% CaCO3 at 37 °C for 5 days and the succinic acid production was analyzed by shake-flasks analysis. The more productive strains were gained for the next round of genome shuffling. Three rounds of protoplast fusion were carried out and the pH values in screening plates were reduced after each rounds (3.9 and 3.5, respectively). Other unlabeled reagents were from Sigma Chemical(Co. St. Louis, MO, USA).
Shake-flasks and fed-batch fermentation analysis
As shake-flasks, strain was inoculated (10 mL) in 250 mL flasks containing 100 mL fermentation medium at 37 °C for 4 days. Fed-batch fermentation was reacted at 3.0 L fermentation medium in a 5 L bioreactor, the pH was maintained at 4.8 by adding 7.0 M NH4OH automatically, and agitated at 37 °C with the speed of 200 rpm (Biostat B2, Braun, Frankfurt, Germany). All tests were repeated three times. The supernatant was stored at −80 °C for further study.
Succinic acid and cell dry weight analysis
The yield of succinic acid was analyzed by using high-performance liquid chromatography (HPLC, Waters, Milford, MA, USA). The column was ZORBAX Eclipse XDB C18 column (150 mm × 4.6 mm, 5.0 µm), the mobile phase contained 93% 50 mmol/L H3PO4 and 7% acetonitrile while the detection wavelength was 210 nm, the flow rate was 0.5 mL/min and the injection volume was 20 µL.
Cell dry weight was measured after centrifuging, washing and drying the fermented liquid (50 mL) at 105 °C overnight. Each sample was analyzed for three times.
Statistical analysis
All experiments were performed in triplicate and the date is represented as the mean ± standard deviation. The significance of differences (p < 0.05) among the corresponding mean values were determined by Prism 7.0 for Windows (GraphPad Software, Inc.).
Results and discussion
Screening of starter strains
It is necessary to obtain the initial strains. Thus, UV and NTG treatments were used to establish the starting point. Cells were selected on screening plates with pH 4.2 and CaCO3, respectively, the succinic acid yield was analyzed in flasks. After that, four mutant strains were screened and named AS-UV1, AS-UV2, AS-NTG1 and AS-NTG2, respectively, which can grow at pH 4.5 (Table 1) with the production of succinic acid was slightly improved to 18.89, 18.61, 19.17, 17.88 g/L when fermented medium initial pH was 7.0 (Table 1), while the wild type strain AS-R2 only grow at pH 4.8 plate and yield of succinic acid was 17.07 g/L. Finally, four mutant strains were obtained for genome shuffling as starter strains.
Table 1.
Comparison of starter strains and mutant strains for acid-tolerance and the production of succinic acid at initial pH 7.0
| Strain | Acid-tolerance (pH) | Production (g/L) |
|---|---|---|
| AS-R2 | 4.8 | 17.07 |
| AS-UV1 | 4.5 | 18.89 |
| AS-UV2 | 4.5 | 18.61 |
| AS-NTG1 | 4.5 | 19.17 |
| AS-NTG2 | 4.5 | 17.88 |
Genome shuffling to generate acid tolerance strains
Genetic diversity is amplified by protoplast fusion as well as creates a new mutant combination and improves the performance of strains. Through the recombination processes, genome shuffling accelerates the evolution of strains(Biot-Pelletier and Martin, 2014). In this study, two populations of mutant strains, AS-NTG1, 2 and AS-UV1, 2 were suffered through protoplast formation, fusion and regeneration. After the first round, eight strains (AS-F11–AS-F18) were obtained. These strains were used for second round, and we selected four strains (AS-F21–AS-F24) for next round. Then, after the third round, four strains were obtained from the second shuffled library. After each round, the acid tolerant was increased gradually. Samples from round 1 strains (AS-F11–8) grew at pH 4.3, the round 2 strains (AS-F21–4) showed higher acid tolerance that could grow at pH 3.9 and the round 3 strains (AS-F31–4) grew under pH 3.5. Colonies were selected with the larger transparent circles on screening plates with 1% CaCO3 after low pH plate to each round. After three rounds of genome shuffling, these strains could grow steadily under pH 3.5 (Fig. 1), so the third round strains were used for further study.
Fig. 1.
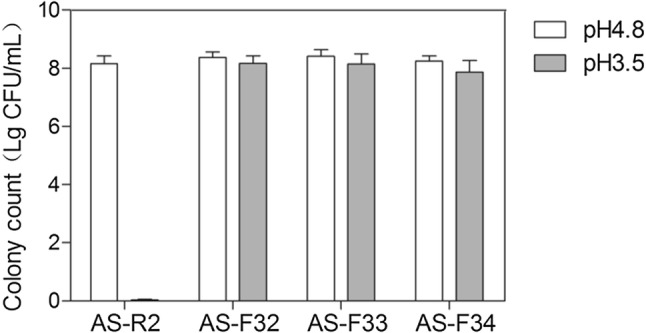
Starter strain and the third round of genome shuffling strains for acid-tolerance. The starter strains and the third round of genome shuffling strains were cultured in medium with pH 3.5 and 4.8. AS-R2: starter strains; AS-F32 and AS-F33: strains from the third round of genome shuffling
Cell dry weight and succinic acid production in pH 4.8 fermentation medium
To compare the cell dry weight and succinic acid yield between AS-R2 and other mutant strains, shake-flasks analysis was used and the data was recorded after 2d (Fig. 2). The results showed that the succinic acid yields of mutant strains by the mutation of NTG and UV treatments were slightly improved, and all shuffled strains were significantly improved compared to AS-R2 pH 4.8 fermentation medium (Fig. 1A) (p < 0.05). Moreover, cell dry weight of all shuffled strains were markedly raised compared to AS-R2 at the same condition (Fig. 1B) (p < 0.05). Among them, acid production and cell dry weight of the best performance of AS-F32 was 5.15 ± 0.05 and 4.82 ± 0.13 g/L in flask and improved 2.6 and 1.85 times over the start strain AS-R2. The fermentation broth’s end-point pH values of all shuffled strains were decreased to 3.34 ± 0.15 or lower, while AS-R2 only reduced pH to 4.6 ± 0.08. With the excellent performance of acid tolerance and succinic acid production, AS-F32 was selected and tested in fed batch fermentation.
Fig. 2.
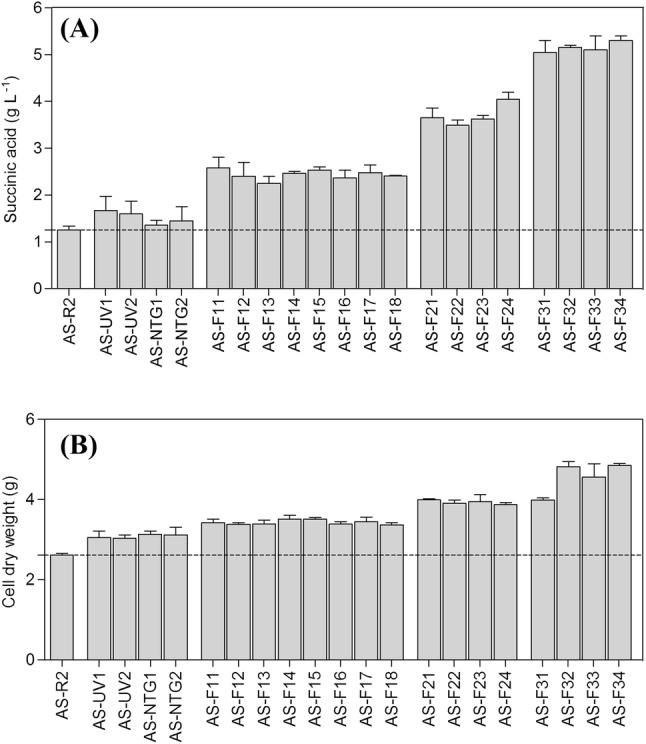
Cell dry weight and production of succinic acid by starter strains, mutant strains, and genome-shuffled strains in shake-flasks fermentation at pH 4.8. (A) Production of succinic acid. (B) Cell dry weight. AS-R2: starter strains; AS-UV1, AS-UV2: UV mutant strains; AS-NTG1, AS-NTG2: NTG mutant strains; AS-F11 to AS-F18: strains from the first round of genome shuffling; AS-F21 to AS-F24: strains from the second round of genome shuffling; AS-F31 to AS-F34: strains from the third round of genome shuffling
Fed-batch fermentation of AS-R2 and AS-F32 at low pH in 5 L containers
The selected strain AS-F32 and AS-R2 were fermented when maintained pH at 4.8 by adding 7.0 M NH4OH in 5 L containers, respectively. The variation of cell growth and succinic acid were investigated (Fig. 3). After 44 h, the acid yields and residual glucose concentrations of AS-F32 (Fig. 3A) and AS-R2 (Fig. 3B) were 31.20 g/L, 0.71 g/L and 15.10 g/L, 0.34 g/L, respectively. The results indicated that through genome shuffling, the productivity of AS-F32 was improved about 1.1 times and the dry cell weight was increased 44.4%, compared to AS-R2.
Fig. 3.
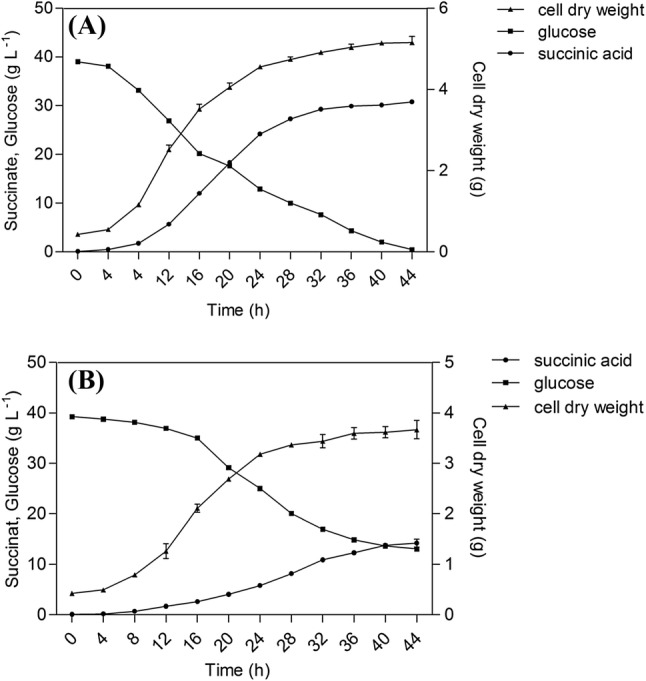
(A) Cell growth and fermentation results of AS-F32 in fed-batch fermentation at pH 4.5. (B) Cell growth and fermentation results of AS-R2 in fed-batch fermentation at pH 4.5. “circles” represent succinic acid; “squares” represent glucose; “triangles” represent cell dry weight
Succinic acid fermentation is a typical product-inhibited type of metabolism, namely, the growth of cells accompanied by the production of succinic acid. With the low pH value or the accumulation of other metabolites, cell growth and succinic acid production will be inhibited (Vuoristo et al., 2016). For industrial production, the expense and operations can be cut down if microorganisms can grow and work at lower pH value (Li et al., 2010). It also can be combined with high-performance in situ product removal when fermenting at lower pH value (Pleissner et al., 2017). Moreover, the responses of A. succinogenes to produce succinic acid are an intricate process as well as the effects of acid stress are complicated and it is still not known in detail. Therefore, it is difficult to enhance the acid resistance and improve the productivity of A. Succinogenes by directly using genetic manipulation or random mutations methods. Based on the above, in this research, the starter strains were obtained by using UV and NTG mutation methods. And then, both acid tolerance and acid production of A. Succinogenes were improved through genome shuffling. AS-F32 was obtained which the minimum growth pH is 3.5, the acid production was 5.01 g/L at initial pH 4.8 in shake-flasks and 31.2 g/L in fed-batch fermentation by maintaining pH at 4.8 with 7.0 M NH4OH, which were improved 2.6 and 1.1 times than AS-R2, respectively. Many researches were reported to enhance the production of succinic acid. Wu M. et al. enhanced succinic acid yield to 26.58 g/L at pH 5.8 by introduction of glutamate decarboxylase system in E. coli AFP111 (Wu et al., 2016; Ye et al., 2010). improved the succinic acid production A. succinogenes YZ25 by enhancement of ammonium-tolerant, which reached 32.68 g/L at pH 7.0 (Ye et al., 2010). In this study, both the acid-tolerance and succinic acid production of A. succinogenes are enhanced by using genome shuffling. In brief, the modified strain, AS-F32, has a profound meaning to produce succinic acid.
However, since the responses of A. succinogenes to produce succinic acid are an intricate process and the mechanism of improvement of succinic acid production in genome level is still not known in detail (Chen and Nielsen, 2016), researchers should try the best to elucidate the succinic acid metabolism completely. Genome shuffling is an effective method to reform the genes, but the reformed process is complicated and it is difficult to prove in the specific genes (Dai and Copley, 2004), although, the acid-tolerance and succinic acid production of A. succinogenes were improved, there still remain some questions to be illustrated. Further study should be warranted in molecular level by using transcriptomics and proteomics.
Acknowledgements
We are grateful to the Jilin Province Sci-tech Department, People’s Republic of China for providing financial support (Grant No. 20140519011JH).
Compliance with ethical standards
Conflict of interest
The authors whose names are listed above state that they have no conflict in terms of either financial interest or nonfinancial interest.
References
- Ahn JH, Jang YS, Sang YL. Production of succinic acid by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016;42:54–66. doi: 10.1016/j.copbio.2016.02.034. [DOI] [PubMed] [Google Scholar]
- Alonso S, Rendueles M, Díaz M. Microbial production of specialty organic acids from renewable and waste materials. Crit. Rev. Biotechnol. 2015;35:497–513. doi: 10.3109/07388551.2014.904269. [DOI] [PubMed] [Google Scholar]
- Biot-Pelletier D, Martin VJJ. Evolutionary engineering by genome shuffling. Appl. Microbiol. Biotechnol. 2014;98:3877. doi: 10.1007/s00253-014-5616-8. [DOI] [PubMed] [Google Scholar]
- Carlson A, Coggio B, Lau K, Mercogliano C, Millis J. Industrial Production of Succinic Acid. KGaA: Wiley-VCH Verlag GmbH & Co; 2016. [Google Scholar]
- Chen Y, Nielsen J. Biobased organic acids production by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016;37:165–172. doi: 10.1016/j.copbio.2015.11.004. [DOI] [PubMed] [Google Scholar]
- Dai MH, Copley SD. Genome Shuffling Improves Degradation of the Anthropogenic Pesticide Pentachlorophenol by Sphingobium chlorophenolicum ATCC 39723. Appl. Environ. Microbiol. 2004;70:2391–2397. doi: 10.1128/AEM.70.4.2391-2397.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Dai W, Xi Y, Wu M, Kong X, Ma J, Zhang M, Chen K, Wei P. Succinic acid production from sucrose by Actinobacillus succinogenes NJ113. Bioresour. Technol. 2014;153:327–332. doi: 10.1016/j.biortech.2013.11.062. [DOI] [PubMed] [Google Scholar]
- Lee PC, Lee SY, Hong SH, Chang HN, Park SC. Biological conversion of wood hydrolysate to succinic acid by Anaerobiospirillum succiniciproducens. Biotechnol. Lett. 2003;25:111. doi: 10.1023/A:1021907116361. [DOI] [PubMed] [Google Scholar]
- Li Q, Wang D, Wu Y, Li W, Zhang Y, Xing J, Su Z. One step recovery of succinic acid from fermentation broths by crystallization. Sep. Purif. Technol. 2010;72:294–300. doi: 10.1016/j.seppur.2010.02.021. [DOI] [Google Scholar]
- Liang LY, Liu RM, Ma JF, Chen KQ, Jiang M, Wei P. Increased production of succinic acid in Escherichia coli by overexpression of malate dehydrogenase. Biotechnol. Lett. 2011;33:2439–2444. doi: 10.1007/s10529-011-0707-4. [DOI] [PubMed] [Google Scholar]
- Liu R, Liang L, Li F, Wu M, Chen K, Ma J, Jiang M, Wei P, Ouyang P. Efficient succinic acid production from lignocellulosic biomass by simultaneous utilization of glucose and xylose in engineered Escherichia coli. Bioresour. Technol. 2013;149:84–91. doi: 10.1016/j.biortech.2013.09.052. [DOI] [PubMed] [Google Scholar]
- Luna-Flores CH, Palfreyman RW, Krömer JO, Nielsen LK, Marcellin E. Improved production of propionic acid using genome shuffling. Biotechnol. J. 12 (2016) [DOI] [PubMed]
- Oh IJ, Kim DH, Oh EK, Lee SY, Lee J. Optimization and scale-up of succinic acid production by Mannheimia succiniciproducens LPK7. J. Microbiol. Biotechnol. 2009;19:167. doi: 10.4014/jmb.0807.447. [DOI] [PubMed] [Google Scholar]
- Pinazo JM, Domine ME, Parvulescu V, Petru F. Sustainability metrics for succinic acid production: A comparison between biomass-based and petrochemical routes. Catal. Today. 2015;239:17–24. doi: 10.1016/j.cattod.2014.05.035. [DOI] [Google Scholar]
- Pleissner D, Dietz D, van Duuren JB, Wittmann C, Yang X, Lin CS, Venus J. Biotechnological production of organic acids from renewable resources. Adv. Biochem. Eng. Biotechnol. (2017) [DOI] [PubMed]
- Song H, Sang YL. Production of succinic acid by bacterial fermentation. Enzyme Microb. Technol. 2006;39:352–361. doi: 10.1016/j.enzmictec.2005.11.043. [DOI] [Google Scholar]
- Thuy NT, Kongkaew A, Flood A, Boontawan A. Fermentation and crystallization of succinic acid from Actinobacillus succinogenes ATCC55618 using fresh cassava root as the main substrate. Bioresour. Technol. 2017;233:342. doi: 10.1016/j.biortech.2017.02.114. [DOI] [PubMed] [Google Scholar]
- Vuoristo KS, Mars AE, Sanders JPM, Eggink G, Weusthuis RA. Metabolic Engineering of TCA Cycle for Production of Chemicals. Trends Biotechnol. 2016;34:191–197. doi: 10.1016/j.tibtech.2015.11.002. [DOI] [PubMed] [Google Scholar]
- Wang HK, Sun Y, Chen C, Sun Z, Zhou YC, Shen FD, Zhang HP, Dai YJ. Genome shuffling of Lactobacillus plantarum for improving antifungal activity. Food Control. 2013;32:341–347. doi: 10.1016/j.foodcont.2012.12.020. [DOI] [Google Scholar]
- Wang Y, Li Y, Pei X, Yu L, Feng Y. Genome-shuffling improved acid tolerance and L-lactic acid volumetric productivity in Lactobacillus rhamnosus. J. Biotechnol. 2007;129:510–515. doi: 10.1016/j.jbiotec.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Wittmann C, Liao JC, Ahn JH, Jang YS, Sang YL. Succinic Acid. KGaA: Wiley-VCH Verlag GmbH & Co; 2017. [Google Scholar]
- Wu M, Li X, Guo S, Lemma WD, Zhang W, Ma J, Jia H, Wu H, Jiang M, Ouyang P. Enhanced succinic acid production under acidic conditions by introduction of glutamate decarboxylase system in E. coli AFP111. Bioprocess Biosyst. Eng. 40: 1–9 (2016) [DOI] [PubMed]
- Ye G, Jiang M, Chen K, Li J, Xi Y, Huang X, Wei P. Breeding of ammonium-tolerant mutants of Actinobacillus succinogenes for succinic acid production and effect of ammonium. Chin. J. Agric. Biotechnol. 2010;26:183–188. [PubMed] [Google Scholar]
- Yu L, Pei X, Lei T, Wang Y, Feng Y. Genome shuffling enhanced L-lactic acid production by improving glucose tolerance of Lactobacillus rhamnosus. J. Biotechnol. 2008;134:154–159. doi: 10.1016/j.jbiotec.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Zhang YF, Liu SY, Du YH, Feng WJ, Liu JH, Qiao JJ. Genome shuffling of Lactococcus lactis subspecies lactis YF11 for improving nisin Z production and comparative analysis. J. Dairy Sci. 2014;97:2528–2541. doi: 10.3168/jds.2013-7238. [DOI] [PubMed] [Google Scholar]
- Zhang YX, Perry K, Vinci VA, Powell K, Stemmer WPC, Cardayré SBD. Genome shuffling leads to rapid phenotypic improvement in bacteria. Nature. 2002;415:644–646. doi: 10.1038/415644a. [DOI] [PubMed] [Google Scholar]
- Zhao J, Zhang C, Lu J, Lu Z. Enhancement of fengycin production in Bacillus amyloliquefaciens by ge. Can. J. Microbiol. 2015;62:431–436. doi: 10.1139/cjm-2015-0734. [DOI] [PubMed] [Google Scholar]


