Abstract
Medicinal plants with antimicrobial action have been investigated for uses against biofilms, among which, Cymbopogon nardus, citronella, stands out as a promising species. The present study aims to evaluate the antimicrobial and antibiofilm action of the essential oil of C. nardus (EOCN) and geraniol on Gram-negative and positive bacteria from the determination of minimum inhibitory concentration (MIC) and minimum bactericidal concentration and inhibition of biofilms. In the results, the EOCN produced a 41 mm halo on S. aureus, which was susceptible with MIC values of 0.5 and 0.25 mg/mL for the EOCN and geraniol respectively, both with bactericidal effect. The antibiofilm action was confirmed, the EOCN and geraniol reduced the biofilm biomass of S. aureus up to 100% between 0.5 and 4 mg/mL concentrations. The reduction of cell viability was 0.25 and 1 mg/mL, of EOCN and geraniol, respectively. EOCN and geraniol were shown to be promising antibiotic against S. aureus.
Keywords: Antibiofilm, Cymbopogon nardus, Essential oil, Food, Geraniol
Introduction
Among the main causes of public health problems in the world are foodborne diseases (FDs). Foodborne diseases that are spread through the ingestion of contaminated water and/or food (Barreto et al., 2012). FDs are responsible for high rates of morbidity and mortality and for high costs of medical care (Fratamico et al., 2005).
The incidence of foodborne diseases has been underestimated. The World Health Organization estimates that 31 foodborne hazards have resulted in more than 600 million illnesses and 420,000 deaths worldwide in 2010. In Brazil, according to the Ministry of Health, there were 118 thousand registered cases of FDs from 2007 to 2016, and in 109 of these cases, the individuals died (Portal Saúde, 2016). Faced with this reality, it is necessary to find out the routes of exposure for key hazards for the prevention of FDs (Havelaar et al., 2015).
Contamination factors that contribute to the transmission of FDs are the following: direct transmission from animals to humans, vectors (insects), microbial adaptation, food handlers and food contaminated by processing surfaces (Hoffmann et al., 2017). Many pathogenic or corrosive microorganisms may be attached to food surfaces or may be present in the environments where food is prepared. One of the factors that exacerbates this issue is the lack of appropriate handling conditions and hygiene, which leads to surface and food contamination. S. aureus and E. coli are the predominant bacteria that form surface biofilms in the food industry and are responsible for numerous cases of food poisoning (Baptista, 2013, Di Conza et al., 2014; Freeman et al., 2014; Guimarães et al., 2017).
Biofilms are complex bacterial communities formed by the adhesion of microorganisms to a surface (biotic or abiotic); biofilms are embedded in an exopolymeric matrix composed of polysaccharides, nucleic acids, and lipids, among other compounds (Bersan et al., 2014). One way to combat biofilms is to use sanitizers. Countless studies are being carried out to develop new sanitizing products that are more effective and are less toxic to humans.
Essential oils and their major components stand out as new strategies to combat biofilms, with potential applicability in the food industry (Bordi and Bentzmann, 2011). Essential oils are volatile aromatic products composed of secondary metabolites of plants. Essential oils are found in a concentrated form in various parts of the plants, such as the leaves, fruits or bark, and their therapeutic properties are being studied in the search for new antimicrobial drugs and antibiofilm strategies. In this context, the use of medicinal plants with antimicrobial activities constitutes an important strategy to combat this problem (Aguiar et al., 2014; Oliveira et al., 2011).
The literature highlights the essential oils from the leaves of Cymbopogon nardus for its biological activity against Rhipicephalus microplus (Agnolin et al., 2010); antibacterial activity against Staphylococcus aureus, Listeria monocytogenes, E. coli, Salmonella choleraesuis and Pseudomonas aeruginosa (Andrade et al., 2012); and antifungal activity against Aspergillus spp. (Aguiar et al., 2014). Geraniol, a major component of this essential oil, has shown antibacterial activity against S. aureus strain ATCC 6538 (Henrique et al., 2015).
In view of the above observations, the present work aims to verify if the essential oil from the species C. nardus and its major component, geraniol, have antimicrobial activity and the potential to inhibit the formation of in vitro biofilms of strains of S. aureus and E. coli. The results of this study may guide future tests to validate the use of this essential oil as an antimicrobial.
Materials and methods
Collection and identification of botanical material
The leaves of C. nardus were collected in April 2014 in the municipality of Cariré, Ceará, Brazil. The plant material was identified by Dr. Elnatan Bezerra de Souza and deposited under accession number 20807 to the Dr. Francisco José de Abreu Matos Herbarium of the Vale do Acaraú State University, Sobral, CE, Brazil.
Extraction and chemical characterization of the essential oil of Cymbopogon nardus
The essential oil of C. nardus was obtained by extraction using the Cleavenger hydrodistillation method. For the extraction, 320 g of fresh leaves was used; the leaves were crushed, placed in a 5-L round-bottom flask with 2 L of distilled water, and boiled for 2 h. During this time, the water/oil mixture present in the dispenser was separated based on the density difference between the two. The obtained essential oil was weighed and stored under refrigeration in a labelled amber bottle. The yield of the essential oil was expressed as the percentage obtained from the ratio of the mass (g) of the oil extracted to the mass (g) of the leaves in the flask multiplied by 100.
The chemical constituents of the essential oil of C. nardus were analyzed in laboratory of Chemistry Natural Products at Federal University Rural of Rio de Janeiro, Seropédica, Rio de Janeiro. The major component of the essential oil, namely, geraniol, was commercially obtained from Sigma Aldrich®, Sigma, St. Louis, MO, USA.
Analyses of constituents of the essential oil
The qualitative analysis of the chemical composition of the essential oil was performed by GC-MS in a Shimadzu QP-2010 instrument (Shimadzu, Kyoto, Japan). The compounds were identified by analysis of the fragments patterns displayed in the mass spectra, and their identities were confirmed by comparing their mass spectra with those present in the database (NIST, 2006) and comparing their retention rates (Kovat) to of known compounds which were obtained by injection of a mixture of standards containing a homologous series of C8–C30 alkanes as described by Van Den Dol and Kratz (1963).
Substances and preparation of solutions
The analyzed substances were the essential oil of the C. nardus and the major component of this essential oil, geraniol; chlorhexidine and imipenem were used as positive controls.
Bacterial strains and culture conditions
The following strains were used in this study: S. aureus ATCC 6538, Staphylococcus epidermidis ATCC 12228, Enterococcus faecalis ATCC 4083, Streptococcus pyogenes ATCC 19615, E. coli ATCC 11303 and Pseudomonas aeruginosa ATCC 115442. The strains were purchased from the Institute Oswaldo Cruz-Fiocruz and are part of the American Type Culture Collection (ATCC). All strains were stored under refrigeration at − 18 °C in skim milk medium enriched with glycerol.
In the experiments, a 100 μL aliquot of the stock solution was inoculated in TSA medium (trypticase soy agar) and grown in a greenhouse at 37 °C for 24 h. After this initial activation, the culture was revived with the addition of 100 μL of inoculum to 10 mL of TSB (trypticase soy broth) followed by incubation for 18 h under the same conditions described above. In the antimicrobial activity assays, the cultures were washed with Mili-Rios water, and their concentrations were adjusted to 107–108 CFU/mL with the use of a microplate reader (SpectraMax i3 Multi-Mode Microplate Reader) at 620 nm.
Antimicrobial activity
The antimicrobial activities of the compounds were tested by performing antimicrobial susceptibility tests on the bacteria by the agar-diffusion method and by microdilution tests in broth; these tests were used to determine the minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC).
Antimicrobial susceptibility test by disc-diffusion in agar
For the antimicrobial susceptibility test by agar diffusion, modifications were made to the standard M2-A8 method (Clinical and Laboratory Standards Institute-CLSI) translated and distributed in Brazil by the National Agency of Sanitary Surveillance. The strains S. aureus, S. epidermidis, E. faecalis, S. pyogenes, E. coli and P. aeruginosa were activated in TSB, and two biological replicates were tested for each strain.
The cell densities of the bacterial cultures were adjusted with 0.85% saline solution to 106–108 CFU/mL or the equivalent of 0.5 on the McFarland scale and then inoculated on Müeller-Hinton agar medium. Sterile, 6-mm diameter white disks were soaked with 20 μL of essential oil Cymbopogon nardus (EOCN) (18 mg/mL), geraniol (19 mg/mL), imipenem positive control (10 mg/mL) or chlorhexidine positive control (10 mg/mL); after drying, the discs were placed in triplicate on the plates in which the bacteria were inoculated.
Minimum inhibitory concentration (MIC)
To determine the MICs by means of the microdilution test in broth, the compounds EOCN, geraniol and chlorhexidine were tested against the strains S. aureus and E. coli. The MIC test was standardized according to the Methods for Dilution Antimicrobial Susceptibility Test for Bacteria That Grow Aerobically; Approved Standard-Tenth Edition, CSLI document M07-A10 (2015) with modifications, as described below.
The minimum inhibitory concentration was determined by the microdilution technique in 96-well polystyrene plates. The calculations for the preparation of the solutions containing EOCN and geraniol were performed based on the densities of the substances. To facilitate the solubilization, 2% Tween 20 was used. For the assembly of the plates, EOCN, geraniol and chlorhexidine were prepared at an initial concentration of 16 mg/mL and serially diluted in culture medium so that the concentrations varied ranged from 0.125 to 8 mg/mL. Then 100 μl of culture medium containing the microorganisms were adjusted to the concentration of 107–108 CFU / ml and added. The negative control consisted of growing the microorganisms in the TSB culture medium with 2% Tween 20. The lowest concentration of each substance at which no microbial growth was detected was considered the MIC, through a visual reading the lower attention.
Minimum bactericidal concentration (MBC)
The minimum bactericidal concentration was determined by removing a 10-μL aliquot from the solutions considered to contain each substance at its MIC. The aliquot, in triplicate, was inoculated in Petri dishes containing TSA, and the dishes were placed in an incubator at 37 °C. Bacterial growth was observed after 24 h (Sá et al., 2012).
Antibiofilm activity
The ability to inhibit biofilm formation was determined by the indirect biomass-quantification technique using crystal violet (CV) and by the colony-forming unit (CFU)—counting technique, which provides data on the viability of the cells contained in the biofilm. The plaques were assembled in a process similar to the MIC test, and after 24 h, the antibiofilm activity was verified; the bacterium used was S. aureus.
Quantification of biofilm biomass
After the 24-hour incubation, the plates were washed with sterile water (200 μL/well) to remove the loosely adhered and air-dried cells. The adhered cells of the biofilm were fixed in the wells of the plate by the addition of 200 μL of methanol for 15 min. After 15 min, the methanol was removed, and 200 μL of 1% crystal violet was added for 15 min. Then, the plates were washed again and air dried. Next, 200 μL of ethanol (96%) was added to the wells, and the plates were left shaking for 5 min and then read on a microplate reader at 595 nm (Sá et al., 2012).
Counting of colony-forming units (CFU)
After the incubation period, the culture medium was removed, and the plates were subjected to three washes with distilled water. Then, 200 μL of 0.9% saline was added to the wells, and the plate was sonicated in an ultrasonic bath (LK-D32 Ultrasonic Bath) operating at 50 kHz for 10 min. The liquid from the wells was pooled to a total volume of 1 mL, from which a 20 μL aliquot was withdrawn and serially diluted in a volume of 180 μL of saline (10−1–10−6). In a Petri dish containing TSA, three aliquots of 10 μL were cultured, constituting a triplicate for each concentration. These plates were placed in a greenhouse for 24 h at 37 °C, the counting of the colony forming units was performed by manually counting the visible wells after incubation (Sá et al., 2012).
Statistical analysis
The statistical evaluation of the data was performed with the program Prism, GraphPad®, San Diego, California, USA, version 5.0. The statistical test used for multiple comparisons was ANOVA, followed by the Bonferroni test. Values of p < 0.01 were considered statistically significant and are indicated by an asterisk. The MBC and CFU tests were performed with three replicates. All tests were performed in three independent experiments.
Results and discussion
Yield and chemical composition of the essential oil of Cymbopogon nardus (Table 1)
Table 1.
Chemical composition, retention index of the literature (RILit), percentage of the identified components (%) from the essential oil of Cymbopogon nardus (EOCN) aerial parts
| No. | Compounds | RILit | % |
|---|---|---|---|
| 1 | Limonene | 1029 | 1.20 |
| 2 | γ-Terpinene | 1060 | 0.42 |
| 3 | Linalool | 1097 | 0.82 |
| 4 | Citronellal | 1153 | 27.55 |
| 5 | Citronellol | 1226 | 14.40 |
| 6 | Neral | 1238 | 0.90 |
| 7 | Carvone | 1243 | 10.06 |
| 8 | Geraniol | 1253 | 33.88 |
| 9 | Geranial | 1267 | 1.26 |
| 10 | Citronellyl acetate | 1352 | 0.51 |
| 11 | Neril acetate | 1361 | 0.66 |
| 12 | E-Muurola-3,5-diene | 1453 | 0.54 |
| 13 | δ-Cadinene | 1523 | 0.58 |
| 14 | Elemol | 1550 | 3.38 |
| 15 | Germacrene D-4-ol | 1575 | 1.75 |
| 16 | Z-Bisabolol-11-ol | 1619 | 0.80 |
| 17 | Eremoligenol | 1631 | 1.09 |
| Total | 99.80 |
The yield of the essential oil of C. nardus obtained by hydrodistillation was 1.33%. The extraction of the essential oils of aromatic plants depends on several factors, such as the extraction method, cultivation method, environmental conditions, period of collection, and altitude (Simões et al., 2010).
In the chemical analysis of the C. nardus essential oil, seventeen constituents were found (Table 1). The components found in higher amounts were the monoterpenes geraniol (33.88%), citronellal (27.55%) and citronellol (14.40%). The results found are in agreement with those of Hazwan et al. (2014), who found that geraniol constitutes 42.38% of the essential oil extracted from the leaves of the same plant.
The activity of geraniol has been described in other studies and seems to play a primary role in the antimicrobial activity of EOCN (Aguiar et al., 2014; Hazwan et al., 2014; Millezi et al., 2014).
Antimicrobial activity
Disc-diffusion in agar
The data for inhibition by EOCN and geraniol are shown in Table 2.
Table 2.
Values (mm) of the inhibition halos obtained by the use of E EOCN and geraniol against gram-positive and gram-negative bacteria
| Microorganisms | EOCN | Geraniol | IMP | CLR |
|---|---|---|---|---|
| Staphylococcus aureus | 41 ± 0.94 | 19 ± 0.47 | 59 ± 1.70 | 25 ± 0.47 |
| Staphylococcus epidermidis | 16 ± 2.05 | 19 ± 1.25 | 52 ± 2.05 | 18 ± 0.82 |
| Enterococcus faecalis | 13 ± 0.94 | 11 ± 0.94 | 35 ± 2.36 | 14 ± 0.47 |
| Streptococcus pyogenes | 12 ± 0.82 | 10 ± 1.25 | 38 ± 1.70 | 17 ± 3.09 |
| Pseudomonas aeruginosa | 0 | 10 ± 0.47 | 32 ± 0.94 | 17 ± 1.89 |
| Escherichia coli | 0 | 15 ± 1.63 | 19 ± 2.05 | 18 ± 2.16 |
EOCN Essential oil C. nardus, IMP Imipenem, CLR Chlorhexidine
Treatment with EOCN and geraniol led to the formation of inhibition halos 41 and 19 mm in diameter, respectively, when tested with S. aureus. Comparing these data with the values presented by commercial antibiotics, it can be seen that the inhibition halo formed in the EOCN treatment is 16 mm larger than the inhibition halo that formed in the chlorhexidine treatment.
The inhibition halos when S. epidermidis bacteria were tested with EOCN and geraniol were 16 and 19 mm in size, respectively. The sizes of the inhibition haloes of geraniol and chlorhexidine were close, i.e., 19 and 18 mm, and S. epidermidis were similar in the presence of geraniol and chlorhexidine.
Inhibition halos were observed for the bacteria E. faecalis and S. pyogenes when using EOCN, and the results were similar to the inhibition halos induced by chlorhexidine. EOCN did not induce the formation of inhibition halos for P. aeruginosa and E. coli bacteria. When treated with geraniol, E. coli presented a halo of 15 mm, and this value was close to the size of the halo formed upon chlorhexidine treatment, i.e., 18 mm.
Second, Carvalho (2012) attributed the antibacterial effect of C. nardus to the presence of the geraniol and citronellal constituents in its composition. Monoterpenes have the ability to cross the cell wall and the ability to permeabilize and depolarize the plasma membrane (Tong et al., 2013).
MIC and MBC values of EOCN and geraniol for S. aureus and E. coli
The results of the antimicrobial activities of EOCN and geraniol are shown in Table 3.
Table 3.
MIC and MBC values (mm/ml) of EOCN and geraniol for S. aureus and E. coli
| Microorganisms | MIC | MBC | ||
|---|---|---|---|---|
| EOCN (mg/ml) | Geraniol (mg/ml) | EOCN (mg/ml) | Geraniol (mg/ml) | |
| S. aureus | 0.5 | 0.25 | 4 | 2 |
| E. coli | > 8 | > 8 | > 8 | > 8 |
MIC Minimal inhibitory concentration, MBC Minimal bactericidal concentration, EOCN Essential oil C. nardus
The MICs of EOCN and geraniol for S. aureus were 0.5 mg/mL and 0.25 mg/mL, respectively. The data show that both EOCN and geraniol exhibit bacteriostatic activities at the concentrations stated above. The EOCN MIC values for S. aureus found in this study are in agreement with a study by Silveira et al. (2012) that evaluated the antimicrobial activity of EOCN from leaves and found geraniol to be the major component; that study found the EOCN MIC values to be 0.600 mg/mL. With respect to geraniol, the results of our study corroborate those found by Coutinho et al. (2015), who determined the MIC to be 0.24 mg/mL for S. aureus.
The MBC values for S. aureus bacteria were as follows: 4 mg/mL for EOCN and 2 mg/mL for geraniol. In one study, the MBC for S. aureus was found to be 2.5 mg/mL for geraniol. On the other hand, the MBC of EOCN and geraniol for E. coli was not detected (Coutinho et al., 2015).
Inhibition of bacterial biofilm formation by Staphylococcus aureus
The biomass reduction of the S. aureus biofilm occurred in both EOCN and geraniol treatments, as shown in Table 4.
Table 4.
Percentage reduction of S. aureus biofilm biomass by EOCN, geraniol and chlorhexidine (mg/mL)
| 4 mg/ml | 2 mg/ml | 1 mg/ml | 0.5 mg/ml | 0.25 mg/ml | 0.125 mg/ml | |
|---|---|---|---|---|---|---|
| EOCN | 100.0 ± 0.0Aa* | 98.9 ± 1.0Aa | 98.3 ± 1.6Aa | 95.0 ± 2.7Aa | 72.7 ± 7.1Ab | 61.0 ± 6.6Ab |
| GER | 98.5 ± 0.6Aa | 99.9 ± 0.0Aa | 99.0 ± 0.3Aa | 98.1 ± 0.6Aa | 95.0 ± 2.5Ba | 84.2 ± 3.5Ba |
| CLR | 95.7 ± 1.4Aa | 93.3 ± 2.2Aa | 100.0 ± 0.0Aa* | 99.7 ± 0.2Aa | 97.6 ± 1.1Ba | 99.8 ± 0.1Ba |
EOCN Essential oil Cymbopogon nardus, GER Geraniol, CLR Chlorhexidine
*Averages ± standard error followed by the same letter do not differ statistically from one another, upper-case letters correspond to the columns, and lower-case letters correspond to the rows. One-way ANOVA test (p < 0.01) with the Bonferroni post-test
According to the table, it can be seen that EOCN reduced the biomass between 95.0 and 100.0% when used in a concentration range of 0.5–4 mg/mL. Geraniol showed data similar to chlorhexidine at all concentrations; it is worth noting that at a concentration of 0.125 mg/mL there was a reduction of 84.2% in the biofilm biomass of S. aureus. It is observed that from 0.5 to 4 mg/mL the reduction in biomass ranged from 93.3 to 100%, and there was no statistically significant difference between these data at different concentrations or when using different substances. Thus, it can be concluded that the reduction in biomass promoted by the substances in this study was equivalent to the positive control at the measured concentrations.
The use of essential oils and their byproducts has become a major strategy to combat the formation and development of biofilms. Second, Chaieb et al. (2011) reported the inhibition of bacterial biofilm formation based on the bactericidal effects and antiadhesion potentials of many oils or isolated compounds. The tested substances, namely, EOCN and geraniol, show promise as inhibitors of biofilm formation by S. aureus, a bacterium that has caused many outbreaks of food poisoning (Guimarães et al., 2017). The cellular viability data are shown in Fig. 1.
Fig. 1.
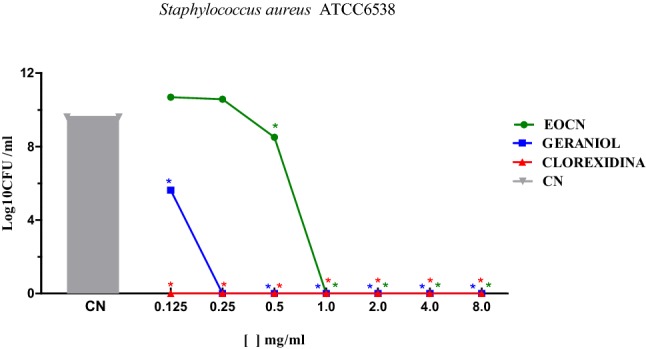
Cell viability in S. aureus biofilms after the action of EOCN and geraniol. *p < 0.01 compared to the negative control (CN). EOCN—Essential Oil C. nardus
The EOCN induced reduction in cell viability in the S. aureus biofilm occurred at EOCN concentrations up to 0.5 mg/mL; however, at concentrations from 1 to 8 mg/mL, the inhibition was 9.62 log CFU/mL. Geraniol reduced cell viability at the concentrations tested, with a reduction of 4 log CFU/mL compared to the negative control at 0.125 mg/mL. It is noteworthy that between 0.25 and 8 mg/mL of geraniol, the results are similar to those seen with chlorhexidine.
Geraniol is an acyclic monoterpene present in a large number of plant species. Second, Trombetta et al. (2005) showed that monoterpenes have antimicrobial activity against gram-positive bacteria. This antimicrobial activity occurs because the action of monoterpenes causes perturbation in the lipid fraction of the plasma membrane of the microorganism, which results in alterations of the permeability of the membrane and consequently in cellular death by plasmolysis.
For the food industry, the presence of biofilms entails serious economic losses. The use of essential oils is a new alternative for the disinfection of industrial surfaces. Oliveira et al. (2010) used the oil from the leaves of C. nardus alone or in combination and were able to reduce the number of cells in the biofilms of L. monocytogenes adherent to the surface by 100% (5.64 Log CFU/mL) after 60 min of contact.
The tested substances, namely, geraniol and the essential oil of C. nardus, presented antimicrobial activity; gram-positive bacteria were more susceptible in the planktonic form, and the data revealed values similar to those of the positive control. In addition, both EOCN and geraniol are bactericidal against S. aureus. The antibiofilm activity was evidenced by the inhibition of the formation of S. aureus biofilms and verified by the reduction in the formed biofilm biomass and the reduction in cell viability when compared to the negative control.
Acknowledgements
We would like to thank the Coordination of Improvement of Higher Education Personnel -CAPES for financial support.
References
- Agnolin CA, Olivo CJ, Leal MLR, Meinerz GR, Parra CLC, Machado PR, Foletto V, Bem CM, Nicolodi PRSJ. Efficacy of citronella oil Cymbopogon nardus (L.) Rendle in the control of bovine ectoparasites. Rev Bras Pl Med. 2010;12:482–487. doi: 10.1590/S1516-05722010000400012. [DOI] [Google Scholar]
- Aguiar RWS, Ootani MA, Ascencio SD, Ferreira TPS, Santos MM, Santos GR. Fumigant antifungal activity of Corymbia citriodora and Cymbopogon nardus essential oils and citronellal against three fungal species. Sci World J. 2014;492138:1–8. doi: 10.1155/2014/492138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade MA, Cardoso MG, Batista LR, Mallet ACT, Machado SMF. Essential oils of Cymbopogon nardus, Cinnamomum zeylanicum and Zingiber officinale: composition, antioxidant and antibacterial activities. Rev Cienc Agron. 2012;43:399–408. doi: 10.1590/S1806-66902012000200025. [DOI] [Google Scholar]
- Baptista MGFM. Mechanisms of resistance and selection of antibiotics. Masters dissertation. Universidade Lusófona de Humanidades e Tecnologia, Lisboa, Portugal (2013)
- Barreto NSE, Moura FCM, Teixeira JA, Assim DA, Miranda PC. Evaluation of hygienic-sanitary conditions of fish commercialized in the municipality of Cruz das Almas. Bahia. Rev Caatinga. 2012;25:86–95. [Google Scholar]
- Bersan SMF, Galvão LCC, Goes VFF, Sartoratto A, Figueira GM, Rehder VLG, Alencar SM, Duarte RMT, Rosalen PL, Duarte MCT. Action of essential oils from Brazilian native and exotic medicinal species on oral biofilms. J Evid Based Complementary Altern Med. 2014;14:451–463. doi: 10.1186/1472-6882-14-451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordi C, Bentzmann S. Hacking into bacterial biofilms: a new therapeutic challenge. Ann Intensive Care. 2011;1:1–8. doi: 10.1186/2110-5820-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho KIM. Effect of geraniol on experimental peptic ulcer disease. Masters dissertation. Institute of Biosciences of Botucatu, São Paulo, Brasil (2012)
- Chaieb K, Kouidhi B, Jrah H, Mahdouani K, Bakhrouf A. Antibacterial activity of thymoquinone, active principle of nigella sativa and its potency to prevent bacterial biofilm formation. BMC Complement Altern Med. 2011;11:11–29. doi: 10.1186/1472-6882-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; Fifteenth Informational Supplement. Document M100-S15 (ISBN 1- 56238-556-9). Pennsylvania, USA (2015)
- Coutinho HDM, Freitas MA, Gondim CNF Leite, Albuquerque RS, Ferreira JVA, Andrade JC. In vitro antimicrobial activity of geraniol and caryophyllene on Staphylococcus aureus. Rev Cubana Plant Med. 20: 98–105 (2015)
- Di Conza JA, Badaracco A, Ayala J, Rodríguez C, Famiglietti A, Gutkind GO. β-lactamases produced by amoxicillin clavulanate resistant enterobacteria isolated in Buenos Aires, Argentina: a new blatem gene. Rev Argent Microbiol. 2014;46:210–217. doi: 10.1016/S0325-7541(14)70075-6. [DOI] [PubMed] [Google Scholar]
- Fratamico PM, Bhunia A, Smith JL. Foodborne pathogens: microbiology and molecular biology. Emerg Infect Dis. 2005;12:453–454. [Google Scholar]
- Freeman JT, Nimmo J, Gregory E, Tiong A, Almeida M, McAuliffe GN, Roberts AS. Predictors of hospital surface contamination with extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae: patient and organism factors. Antimicrob Resist In. 2014;3:1–7. doi: 10.1186/2047-2994-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães CC, Ferreira TC, Oliveira RCF, Simioni PU, Ugrinovich LA. In vitro antimicrobial activity of aqueous extract and essential oil of rosemary (Rosmarinus officinalis L.) and clove (Caryophyllus aromaticus L.) against strains of Staphylococcus aureus and Escherichia coli. Rev Bras Biocienc. 15: 83–89 (2017)
- Havelaar AH, Kirk MD, Torgerson PR, Gibb HJ, Hald T, Lake JR, Praet N, Bellinger DC, Silva NR, Gargouri N, Speybroeck N, Cawthorne A, Mathers C. Organization global estimates and regional comparisons of the burden of foodborne disease in 2010. Plos Med. 2015;12:1–23. doi: 10.1371/journal.pmed.1001923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazwan HM, Man HC, Abidin ZZ, Jamaludin H. Comparison of Citronella oil extraction methods from Cymbopogon nardus grass by ohmic-heated hydro-distillation, hydro-distillation, and steam distillation. Bioresources. 2014;9:256–272. [Google Scholar]
- Henrique HC, Freitas MA, Gondim CNFL, Albuquerque RS, Ferreira JVA, Andrade JC. In vitro antimicrobial activity of geraniol and caryophyllene on Staphylococcus aureus. Rev Cubana Plant Med. 2015;20:98–105. [Google Scholar]
- Hoffmann S, Devleesschauwer B, Aspinall W, Cooke R, Corrigan T, Havelaar A, Angulo F, Gibb H, Kirk M, Speybroeck RLN, Torgerson P, Hald T. Attribution of global foodborne disease to specific foods: Findings from a World Health Organization structured expert elicitation. Plos One. 2017;14:1–26. doi: 10.1371/journal.pone.0183641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millezi AF, Baptista NN, Caixeta DS, Rossoni DF, Cardoso MG, Piccoli RH. Chemical characterization and antibacterial activity of essential oils of condiment and medicinal plants against Staphylococcus aureus and Escherichia coli. Rev Bras Pl Med. 2014;16:18–24. doi: 10.1590/S1516-05722014000100003. [DOI] [Google Scholar]
- NIST. National Institute of Standards and Technology. 2,6- Octadienal, 3,7-dimethyl. Disponível: http://webbook.nist.gov/cgi/cbook.cgi?ID=C5392405&Mask=200. Accessed on: 03/03/2016
- Oliveira MMM, Brugnera DF, Cardoso MG, Alves E, Piccoli RH. Disinfectant action of Cymbopogon sp. essential oils in different phases of biofilm formation by Listeria monocytogenes on stainless steel surface. Food Control. 21: 549–553 (2010)
- Oliveira MMM, Brugnera DF, Cardoso MG, Guimarães LGL, Piccoli RH. Yield, chemical composition and antilisterial activity of essential oils of Cymbopogon species. Rev Bras Pl Med. 2011;13:8–16. doi: 10.1590/S1516-05722011000100002. [DOI] [Google Scholar]
- Portal Saúde. Outbreaks of Foodborne Diseases in Brazil. Available in: http://portalsaude.gov.br. Accessed on: September 28, 2016
- Sá CSN, Cavalcante TT, Araújo AX, Santos HS, Albuquerque MR, Bandeira PN, Cunha RM, Cavada BS, Teixeira EH. Antimicrobial and antibiofilm action of casbane diterpene from Croton nepetaefolius against oral bacteria. Arch Oral Biol. 2012;5:550–555. doi: 10.1016/j.archoralbio.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Silveira SM, Júnior AC, Scheuermann GN, SecchiI FL, Verruk S, Krohn M, VieiraI CRW. Chemical composition and antibacterial activity of the essential oils of Cymbopogon winterianus (citronella), Eucalyptus paniculata (eucalypt) and Lavandula angustifolia (lavender) Rev Inst Adolfo Lutz. 2012;71:471–480. [Google Scholar]
- Simões CMO, Schenkel EP, Gosmann G, Mello JCP, Mentz LA, Petrovick PR. Pharmacognosy of the plant to the drug. 6. Florianópolis: UFRGS; 2010. [Google Scholar]
- Tong F, Gross AD, Dolan MC, Joel R. The phenolic monoterpenoid carvacrol inhibits the binding of nicotine to the housefly nicotinic acetylcholine receptor. Pest Manag Sci. 2013;69:775–780. doi: 10.1002/ps.3443. [DOI] [PubMed] [Google Scholar]
- Trombetta D, Castelli F, Sarpietro MG, Venuti V, Cristani M, Daniele C, Mazzanti SAG, Bisignano G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob Agents Ch. 2005;49:2474–2478. doi: 10.1128/AAC.49.6.2474-2478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Den Dol H, Kratz PD. A generalization of the retention index system incluinding linear temperature programmed gas liquid partition chromatography. J Chromatogr. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]


