Abstract
The aim of this study was to produce ready to drink (RTD) iced teas from sage and linden. For this purpose, phenolics of sage and linden were extracted by pressurized hot water extraction (PHWE) and then the extracts obtained were spray dried by the addition of maltodextrin. The powders produced by spray drying were processed into RTD sage and linden iced teas by adding sucrose and citric acid. The optimum conditions of PHWE of sage and linden were at 160 °C and for 10 min and 5 min, respectively. The solubility and microencapsulation efficiency of spray dried powders were found to be greater than 97%. In addition, total phenolic content and antioxidant activity of RTD iced teas are parallel with those of microencapsulated sage and linden powders after pasteurization at 80 °C for 5 min. The sensory analysis of RTD iced teas showed that products were well-accepted by the panelists.
Keywords: Sage, Linden, Pressurized hot water extraction, Spray drying, Ready to drink iced tea
Introduction
Tea and tea-based products are popular beverages, consumed by people worldwide (Hakim et al., 2000). Tea is prepared from the leaves of Camellia sinensis and herbal substances such as leaves, flowers, seed, barks, and fruits of medicinal and aromatic plants (Malongane et al., 2017). Apart from C. sinensis, teas prepared from herbs such as thyme, peppermint, melissa, sage, and linden have been popular because of their fragrances and health benefits (Chan et al., 2010). Numerous studies have been published regarding antioxidant, anti-inflammatory, antimicrobial, anti-cancer and anti-aging activities of herbal teas (Atoui et al., 2005; Oh et al., 2013; Zhao et al., 2013).
Salvia officinalis L. (Sage) and Tilia cordata (Linden) have been used in folk medicine due to their medicinal properties since the ancient times. Previous studies indicated that water extracts and herbal teas of both plants possess potent antioxidant activity, thereby provide protection against oxidation-related disease (Atoui et al., 2005; Yıldırım et al., 2000).
Extraction is the key step in the acquisition of bioactive compounds such as phenolics from plant tissues. Since the polarity of phenolics range from polar to non-polar, the percent recovery and antioxidant activities of extracts depend on the type of solvent used in any extraction. However, organic solvents are regarded as environmentally non-friendly. Besides, when water’s polarity is manipulated by changing temperature and pressure by pressurized hot water extraction (PHWE), it can be used as an alternative to the organic solvents (Liu et al., 2013). Hence, PHWE have become a popular green extraction technique for the recovery of bioactive compounds from plants in recent years (Herrero et al., 2005).
While the industry has developed several tea-based beverages such as ready to drink (RTD) iced teas and flavored teas which are produced from C. sinensis, a limited number of beverages have been produced from herbal substances. Recently, there have been an increasing demand for the tea consumption in the form of RTD iced tea in the worldwide (Loizzo et al., 2012). Moreover, RTD iced tea is more popular than brewed tea, especially the spring and summer due to its refreshing nature (Del Rio et al., 2010). RTD iced tea is commonly prepared with cooling of brewed tea or instant tea powder (Kumar et al., 2013). Similarly, microencapsulated herbal tea powder produced by spray drying may be used for production of RTD herbal iced teas by reconstituting with cold water.
Thus, the objectives of this study were (i) to assess PHWE of sage leaves and linden flowers, (ii) to produce microencapsulated sage and linden powder by spray drying and (iii) to evaluate physicochemical properties, antioxidant activities and sensory properties of RTD herbal iced teas produced from microencapsulated powders.
Materials and methods
Herbs and chemicals
Dried sage leaves and linden flowers were purchased from a spice seller (Arifoğlu, Kayseri, Turkey). HPLC grade water was obtained from Millipore Simplicity 185 water purification system (Millipore, Darmstadt, Germany). All reagents and solvents were of analytical grade. Folin-Ciocalteu’s phenol reagent, sodium carbonate, citric acid, and methanol were purchased from Merck (Germany). DPPH (2, 2-diphenyl-1-picrylhydrazyl), ABTS+ [2,2-azinobis-(3-ethyl-benzothiazoline-6-sulphonic acid)], Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), gallic acid, and maltodextrin (DE 13-17) were purchased from Sigma Aldrich Co. (St. Louis, MI, USA). Sucrose was obtained from a local market (Beğendik, Kayseri, Turkey).
Pressurized hot water extraction (PHWE)
PHWE was performed by an Accelerated Solvent Extraction device (ASE® 350, Dionex, Sunnyvale, CA, USA). A disposable filter paper (30 mm, cellulose, Dionex) was inserted at the bottom of 100 mL stainless steel extraction cell to prevent the collection of suspended particles in the extracts. A 10 g of herbs (sage or linden) were filled into the extraction cell. After that water was pumped into the cell, pressure was elevated and kept at 10.34 MPa (fixed manufacturing setting) with the help of N2 gas. Single factor experiments were used to determine the optimum conditions for extracting phenolics from sage and linden. Five levels of extraction temperatures (40, 80, 120,160, and 200 °C) and four levels of extraction times (1, 5,10, and 30 min) were studied to determine the effect of extraction temperature and static time on recovery of phenolics. Before experiments, an extraction cell heat-up time was performed for a given time. The duration of the initial heat-up step depends on the temperature set point for the method (approximately 5 min at 40 and 80 °C, 6 min at 120 °C, 8 min at 160 °C, 9 min at 200 °C). Experiments were conducted by changing the level of a factor while keeping all other factors as fixed. Finally, the resulting extracts were collected in the bottles by purging the cell with N2. The extracts were stored at − 18 °C until analysis.
Feed preparation and spray drying
Spray drying of the extracts was performed according to Çam et al. (2018). After PHWE of the herbs, the total soluble solids (3-3.6°Brix) of the extracts were adjusted to 8°Brix by concentrating with a rotary evaporator (Hei-Vap Value G1, Heidolph, Schwabach, Germany). Maltodextrin was added to resulting extracts at core/coating material ratio of 1/1 (w/w) with constant mixing by a homogenizer (T-18 Ultra Turrax, IKA, Staufen, Germany). The feed solutions (200 mL) were then spray dried using a Mini Spray Dryer (Buchi-B290, Flawil, Switzerland). The spray dryer was operated at 160 °C inlet temperature, air flow rate (600 L/h), the feed flow rate (8 mL/min). The obtained microencapsulated sage and linden powders were stored at − 18 °C until analysis.
Production of RTD herbal iced teas from microencapsulated powders
RTD sage and linden iced teas were produced from microencapsulated sage and linden powders. The ingredients of RTD herbal iced teas were determined according to commercial iced teas in Turkish market. The formulations of RTD iced teas contained 0.5% (w/v) microencapsulated powder, 7% (w/v) sucrose and 0.15% (w/v) citric acid. After mixing dry ingredients (microencapsulated powder, sucrose, citric acid), water was added onto dry ingredients with constant mixing. The volume was adjusted to 250 mL, and then the prepared formulations were pasteurized at 80 °C for 5 min in a water bath (Julabo, Seelbach, Germany).
Analyses of the extracts, tea powders and RTD herbal iced teas
Color properties, dry matter content, pH and water activity
The color of microencapsulated powders and RTD herbal iced teas as L* (lightness), a* (red to green), and b* (yellow to blue) were measured by using a chromameter (Minolta CR103, Japan). The pH values of RTD herbal iced teas were determined by a pH meter (Hanna, Italy). The dry matter content of the microencapsulated powders was measured by hot air oven at 105 °C for 16 h. The water activity (Aw) values of the microencapsulated powders were determined using an automatic water activity device (Aqua-Lab Series 3, Decagon Devices Inc., Pullman, WA, USA) at 25 °C.
Total phenolics content (TPC) and surface phenolic content (SPC)
Total and surface phenolics of the microcapsules were extracted according to the procedure of Çam et al. (2014) with some modifications. For extraction of total phenolics from the microcapsules, 1 g of the microcapsules were dispersed in 10 mL of distilled water. The dispersion was agitated in a vortex for 5 min and then filtered through a micro filter. Similarly, in case of phenolics in the surface, 1 g of microcapsules were treated with 10 mL of ethanol. The mixture was mixed by vortex at room temperature for 5 min and then filtered. The extracts obtained by PHWE and RTD herbal iced teas were only filtered through a micro filter (0.45 μm).
TPC of the extracts, microcapsules and RTD herbal iced teas as well as SPC of the microcapsules were determined using the Folin-Ciocalteu assay (Singleton et al., 1999). Results were expressed in mg of gallic acid equivalents (GAE) per g or 100 mL sample.
Microencapsulation yield (MY)
MY of microencapsulated powders was calculated from the following formula (Çam et al., 2014):
Microencapsulation efficiency (MEE)
MEE of microencapsulated powders was calculated as described by Çam et al. (2014) according to the following formula:
Antioxidant activity
The free radical-scavenging activity of microencapsulated powders and RTD herbal iced teas was determined according to the method of Re et al. (1999), using ABTS radical. The results were reported as mg of Trolox equivalents antioxidant capacity (TEAC) per g or 100 mL of sample.
The antioxidant activity was also determined using DPPH as a free radical, according to Brand-Williams et al. (1995). The results were determined as the half maximal effective concentration (EC50). EC50 was expressed as g or mL of sample to scavenge a gram of DPPH.
Sensory analysis
A test panel was conducted on RTD herbal iced teas. Fifteen volunteer panelists (9 men and 6 women, aged 18–45 years) were selected from both undergraduate and graduate students of Department of Food Engineering of Erciyes University. The panelists were asked to assess the appearance, odor, aroma, taste, and overall acceptability of iced teas; using a 9-point hedonic scale (1 = unacceptable, 9 = acceptable) (Amofa-Diatuo et al., 2017).
Statistical analysis
The data analysis was carried out using SPSS 22 statistical package for Windows (SPSS Inc., Chicago, IL, USA) Analysis of variance (ANOVA) was performed to determine significant differences (p < 0.05) among the samples. Differences between means were detected using Tukey’s test.
Results and discussion
PHWE of sage and linden
The effects of temperature on the PHWE of sage and linden were investigated between 40 and 200 °C by applying fixed static time as 10 min. The results demonstrated that different extraction temperatures significantly (p < 0.05) affected the extraction of phenolics from sage and linden (Fig. 1A). TPC of sage extracts significantly increased (p < 0.05) as the temperature was changed from 40 to 200 °C with 40 °C increments. Every increments in temperature with 40 °C intervals resulted in significant increase compared to former temperature point. The exception was between 80 and 120 °C where TPC remained unchanged. Overall, almost 2.5-fold higher results were obtained in TPC for 200 °C (as 75.60 mg/GAE) than for 40 °C (as 30.30 mg/GAE). Similar results for PHWE of sage by-products was reported by Pavlić et al. (2016), who found TPC as 41.37–78.25 mg GAE/g by applying different temperatures (120–220 °C) and static times (10–30 min). Similarly, TPC of extracts of linden flower also showed an increasing trend with increasing temperature. Parallel results, indicating the effects of temperature on TPC, were reported by Vergara-Salinas et al. (2012), Herrero et al. (2005) and Kanmaz and Saral (2017), who extracted polyphenols from deodorized thyme, antioxidants from Spirulina platensis, phenolics from mandarin peel, respectively. Conversely, negative effect of increasing temperature on TPC was also reported by Monrad et al. (2009) who studied on procyanidins form red grape pomace. The TPC obtained by PHWE of linden flower was higher than the result of Akyuz et al. (2014) who reported as 17.37 mg GAE/g in methanolic extract. In addition, it should be noted that when extraction temperature of 200 °C was applied, TPC of sage and linden extracts increased but thermal degradation was observed in terms of color properties. Since this observation could affect color properties of the resulting products, an extraction temperature of 160 °C was selected as the optimum temperature for extracting phenolics from sage and linden. RTD iced teas which reflecting the original color of infusions obtained from plant materials are preferable by the consumers. In most cases, the desired color is certain tones of yellow. Group of phenolics, specifically flavonoids, are responsible from the yellow color of plant infusions.
Fig. 1.
Effect of temperature (A) and static time (B) on PHWE of sage and linden. (Values of different letters on the same line are significantly different (p < 0.05))
Applying the optimum temperature (160 °C), different static times significantly (p < 0.05) affected the extraction of phenolics from sage and linden. The results showed that TPC of sage extracts increased with the increase in static times up to 10 min whereas TPC of linden extracts remained unchanged between 5 and 10 min (Fig. 1B). However, applying static time of 30 min resulted in significant (p < 0.05) loss in TPC of the both extracts. This might be due to the degradation of phenolics because of elongated extraction time. These results are consistent with Lee et al. (2018), who reported decrease in TPCs of red ginseng extracts when applied temperature of 150–200 °C and extraction times of 25–30 min. According to results of our study, static times of 10 and 5 min were selected as the best static times for PHWE of sage and linden, respectively. Previously, optimum parameters for maximized TPC, total flavonoid and antioxidant activity were reported at 201.5 °C for 15.8 min in PHWE of sage by-products (Pavlić et al., 2016), however, the authors gave no information about the color properties of their extracts. Additionally, Ollanketo et al. (2002) evaluated the antioxidant activity of sage by PHWE and noted the highest recovery of analyte at 150 °C; however, highest antioxidant activity was observed at 100 °C for 60 min extraction. In another work, Hossain et al. (2010) reported that optimum extraction conditions of sage by pressurized liquid extraction were 129 °C, 5 min, and 58% aqueous methanol for temperature, static time and extraction solvent composition, respectively. No comparable results were found regarding the PHWE conditions of linden in literature.
Physicochemical properties and antioxidant activities of microencapsulated powders
Sage and linden extracts obtained by PHWE in optimum conditions were spray dried using maltodextrin as a coating material. Table 1 shows physicochemical properties of microencapsulated powders. MY is used for evaluating the economic and performance aspect of microencapsulation process and must be more than 50% for a successful process (Bhandari et al., 1997). The MY values for microencapsulated sage and linden were 61.24 and 63.48%, respectively. MEE of maltodextrin for phenolics of sage and linden was approximately 97%, indicating that it is a suitable coating material for microencapsulation of phenolics of sage and linden. Dry matter and water activity values of microencapsulated powders are consistent with the findings of several studies (Quek et al., 2007; Şahin-Nadeem et al., 2013). Higher dry matter and lower water activity values ensure microbial, physical and chemical stability of microencapsulated powders (Quek et al., 2007). Solubility of microencapsulated powders is the main parameter that determines their utility as ingredients in beverage formulation and is closely linked to the properties of coating material used in microencapsulation. Maltodextrin as a coating material is commonly used in production of water-soluble teas (Çam et al., 2018; Şahin-Nadeem et al., 2013) due to its higher water solubility compared to other coating materials. In this study, solubility of microencapsulated powders was found to be greater than 97%.
Table 1.
Physicochemical properties of microencapsulated sage and linden powders
| Analyses | MEE (%) | MY (%) | Dry matter (%) | Aw | Solubility (%) | Color | ||
|---|---|---|---|---|---|---|---|---|
| L* | a* | b* | ||||||
| Sage | 97.73 ± 0.02 | 61.24 ± 6.09 | 98.6 ± 1.14 | 0.18 ± 0.01 | 98.13 ± 0.18 | 74.58 ± 1.23 | 1.58 ± 0.24 | 17.78 ± 0.33 |
| Linden | 97.40 ± 0.01 | 63.48 ± 2.08 | 97.7 ± 0.69 | 0.20 ± 0.00 | 97.22 ± 0.27 | 71.25 ± 0.53 | 9.05 ± 0.28 | 22.34 ± 0.58 |
MEE Microencapsulation efficiency, MY Microencapsulation yield
The levels of TPC present in microencapsulated sage and linden were 81.16 and 61.95 mg GAE/g, respectively (Table 2). The values obtained for the TPC of microencapsulated sage and linden powder were higher than those of PHWE of sage and linden. Some researchers have reported a decrease in TPC of extracts during spray drying due to high temperature (Georgetti et al., 2008; Sablania et al., 2018). In this study, an increase in TPC of the extracts is due to the concentration process applied before the spray drying despite possible loss of phenolics during spray drying. The obtained EC50 and TEAC values for microencapsulated sage and linden powders are shown in Table 2. Antioxidant activities of both microencapsulated powders were quite similar. The EC50 was 1.33 and 1.38 g/g DPPH, respectively, for microencapsulated sage and linden powders. In addition, the TEAC values of microencapsulated sage and linden powders were 207.38 and 209.35 mg TEAC/g, respectively.
Table 2.
The phenolic contents and antioxidant activity of microencapsulated powders
| TPC (mg GAE/g) | SPC (mg GAE/g) | EC50 (g/g DPPH) | TEAC (mg/g) | |
|---|---|---|---|---|
| Sage | 81.16 ± 1.47 | 1.84 ± 0.01 | 1.33 ± 0.07 | 207.39 ± 21.77 |
| Linden | 61.95 ± 0.38 | 1.61 ± 0.01 | 1.38 ± 0.01 | 209.35 ± 29.73 |
TPC total phenolic contents; SPC surface phenolic contents, GAE gallic acid equivalent, EC50 the amount of sample to decrease the initial DPPH concentration by 50%, TEAC trolox equivalent antioxidant capacity
Properties of RTD herbal iced teas
To produce RTD iced teas from sage and linden, their microencapsulated powders produced by spray drying were reconstituted with cold water by adding citric acid and sucrose. The TPCs, antioxidant activities and physicochemical properties of RTD iced teas are shown in Table 3. The results showed that TPC and antioxidant activity of RTD iced teas were parallel to those of 0.5 g of microencapsulated powders used to produce 100 mL RTD iced tea. Furthermore, there was no the effect of pasteurization process on TPC and antioxidant activity of RTD iced teas. These observations were consistent with a previous work (Joubert et al., 2009) in which the effect of pasteurization and sterilization was studied on the phenolics of fermented rooibos iced teas. Similar results were also observed by Santos et al. (2018), who reported no significant difference in the TPC and antioxidant activity of a beverage made from a mixture of rooibos, mate and white tea after pasteurization. In this study, TPCs of RTD iced teas were higher than those of commercial RTD iced teas in Mexico (Flores-Martínez et al., 2018) and Brazil (Kodama et al., 2010).
Table 3.
The phenolic contents, antioxidant activity and physicochemical properties of RTD iced teas
| RTD iced teas | TPC (mg/100 mL) | EC50 (mL/g DPPH) | TEAC (mg/100 mL) | pH | Color | ||
|---|---|---|---|---|---|---|---|
| L* | a* | b* | |||||
| Sage | 39.47 ± 1.21 | 345.58 ± 8.27 | 103.51 ± 8.62 | 3.45 ± 0.01 | 25.16 ± 0.10 | 0.10 ± 0.01 | 2.47 ± 0.09 |
| Linden | 29.64 ± 1.42 | 358.21 ± 6.13 | 98.59 ± 11.76 | 3.05 ± 0.00 | 23.70 ± 0.05 | 4,83 ± 0.09 | 1.77 ± 0.06 |
TPC total phenolic contents, GAE gallic acid equivalent, EC50 the amount of sample to decrease the initial DPPH concentration by 50%, TEAC trolox equivalent antioxidant capacity
The lightness of RTD iced teas was found lower than that of microencapsulated powders. The probable reason for lower degree of lightness of RTD iced teas might be due to the presence of cold-water insoluble compounds in sage and linden extracts obtained at high temperature. Another explanation may be non-enzymatic browning reaction materializes as undesired during spray drying (Şahin-Nadeem et al., 2013). In addition, pH values of RTD iced teas were lower than the previous study of Flores-Martínez et al. (2018) evaluating pH values of RTD flavored-colored commercial teas in Mexico.
Sensory properties of RTD herbal iced teas
The RTD iced teas were evaluated for their sensory properties as shown in Fig. 2. RTD iced teas made from microencapsulated powders were accepted, with scores for appearance, odor, aroma, taste, and overall acceptability ranging from 6.6 to 8.07 on hedonic scale. The appearance, aroma, and taste for the two RTD iced teas were quite similar. Regarding overall acceptability and odor of RTD iced teas, it was determined that panelists preferred RTD sage iced tea. Despite the reduction in lightness during production of RTD iced teas from microencapsulated tea powders, the appearances of RTD iced teas were found desirable by panelist.
Fig. 2.
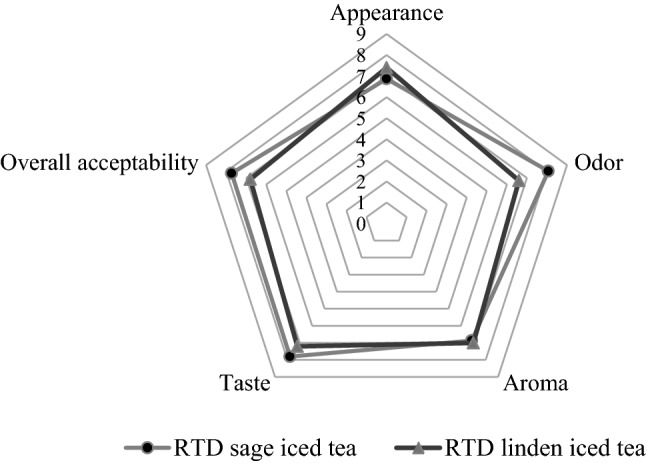
Sensory properties of RTD iced teas. (A 9- point hedonic scale (9 = Excellent, 1 = Extremely poor) was used for sensory evaluation)
In conclusion, this is the first study conducted on production of RTD sage and linden iced teas. In addition, there is no previous study regarding PHWE and microencapsulation of linden. TPC of sage and linden extracts increased with increasing temperature, extending static time after 10 min decreased TPC of the extracts. Microencapsulated powders obtained by using maltodextrin as a coating material had high MEE for phenolics. Pasteurization process was no affect TPC and antioxidant activity of RTD iced teas produced from microencapsulated powders. Future studies are necessary to evaluate the changes in the physicochemical properties and antioxidant activities of RTD iced teas produced after microcapsule powders are stored.
Acknowledgements
This research was financially supported by the Ministry of Science, Industry and Technology, Republic of Turkey under the Project Number of 1483.TGSD.2015.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hamza Alaşalvar, Email: hamza.alasalvar@ohu.edu.tr.
Mustafa Çam, Email: mcam@erciyes.edu.tr.
References
- Akyuz E, Şahin H, Islamoglu F, Kolayli S, Sandra P. Evaluation of phenolic compounds in Tilia rubra Subsp. caucasica by HPLC-UV and HPLC-UV-MS/MS. Int. J. Food Prop. 2014;17:331–343. [Google Scholar]
- Amofa-Diatuo T, Anang DM, Barba FJ, Tiwari BK. Development of new apple beverages rich in isothiocyanates by using extracts obtained from ultrasound-treated cauliflower by-products: Evaluation of physical properties and consumer acceptance. J. Food Compost. Anal. 2017;61:73–81. doi: 10.1016/j.jfca.2016.10.001. [DOI] [Google Scholar]
- Atoui AK, Mansouri A, Boskou G, Kefalas P. Tea and herbal infusions: Their antioxidant activity and phenolic profile. Food Chem. 2005;89:27–36. doi: 10.1016/j.foodchem.2004.01.075. [DOI] [Google Scholar]
- Bhandari BR, Datta N, Crooks R, Howes T, Rigby S. A semi-empirical approach to optimise the quantity of drying aids required to spray dry sugar-rich foods. Drying Technol. 1997;15:2509–2525. doi: 10.1080/07373939708917373. [DOI] [Google Scholar]
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- Chan EWC, Lim YY, Chong KL, Tan JBL, Wong SK. Antioxidant properties of tropical and temperate herbal teas. J. Food Compost. Anal. 2010;23:185–189. doi: 10.1016/j.jfca.2009.10.002. [DOI] [Google Scholar]
- Çam M, Dinç Işıklı M, Yüksel E, Alaşalvar H, Başyiğit B. Application of pressurized water extraction and spray drying techniques to produce soluble spearmint tea. J. Food Meas. Charact. 2018;12:1927–1934. doi: 10.1007/s11694-018-9808-2. [DOI] [Google Scholar]
- Çam M, İçyer NC, Erdoğan F. Pomegranate peel phenolics: Microencapsulation, storage stability and potential ingredient for functional food development. LWT - Food Sci. Technol. 2014;55:117–123. doi: 10.1016/j.lwt.2013.09.011. [DOI] [Google Scholar]
- Del Rio D, Calani L, Scazzina F, Jechiu L, Cordero C, Brighenti F. Bioavailability of catechins from ready-to-drink tea. Nutrition. 2010;26:528–533. doi: 10.1016/j.nut.2009.06.013. [DOI] [PubMed] [Google Scholar]
- Flores-Martínez D, Urías-Orona V, Hernández-García L, Rubio-Carrasco W, Silva-Gutiérrez K, Guevara-Zambrano M, Prieto-Cadena J, Serna-Méndez T, Muy-Rangel D, Niño-Medina G. Physicochemical parameters, mineral composition, and nutraceutical properties of ready-to-drink flavored-colored commercial teas. J. Chem. 2018 [Google Scholar]
- Georgetti SR, Casagrande R, Souza CRF, Oliveira WP, Fonseca MJV. Spray drying of the soybean extract: Effects on chemical properties and antioxidant activity. LWT - Food Sci. Technol. 2008;41:1521–1527. doi: 10.1016/j.lwt.2007.09.001. [DOI] [Google Scholar]
- Hakim I, Weisgerber U, Harris R, Balentine D, Van-Mierlo C, Paetau-Robinson I. Preparation, composition and consumption patterns of tea-based beverages in Arizona. Nutr. Res. 2000;20:1715–1724. doi: 10.1016/S0271-5317(00)00275-X. [DOI] [Google Scholar]
- Herrero M, Martín-Álvarez PJ, Senorans FJ, Cifuentes A, Ibáñez E. Optimization of accelerated solvent extraction of antioxidants from Spirulina platensis microalga. Food Chem. 2005;93:417–423. doi: 10.1016/j.foodchem.2004.09.037. [DOI] [Google Scholar]
- Hossain M, Brunton N, Martin-Diana A, Barry-Ryan C. Application of response surface methodology to optimize pressurized liquid extraction of antioxidant compounds from sage (Salvia officinalis L.), basil (Ocimum basilicum L.) and thyme (Thymus vulgaris L.). Food Funct. 1: 269–277 (2010) [DOI] [PubMed]
- Joubert E, Viljoen M, De Beer D, Manley M. Effect of heat on aspalathin, iso-orientin, and orientin contents and color of fermented rooibos (Aspalathus linearis) iced tea. J. Agric. Food Chem. 2009;57:4204–4211. doi: 10.1021/jf9005033. [DOI] [PubMed] [Google Scholar]
- Kanmaz EÖ, Saral Ö. The effect of extraction parameters on antioxidant activity of subcritical water extracts obtained from mandarin peel. Gida. 2017;42:405–412. doi: 10.15237/gida.GD16073. [DOI] [Google Scholar]
- Kodama DH, Gonçalves AEDSS, Lajolo FM, Genovese MI. Flavonoids, total phenolics and antioxidant capacity: comparison between commercial green tea preparations. Food Sci. Technol. 2010;30:1077–1082. doi: 10.1590/S0101-20612010000400037. [DOI] [Google Scholar]
- Kumar CS, Subramanian R, Rao LJ. Application of enzymes in the production of RTD black tea beverages: a review. Crit. Rev. Food Sci. Nutr. 2013;53:180–197. doi: 10.1080/10408398.2010.520098. [DOI] [PubMed] [Google Scholar]
- Lee J-H, Ko M-J, Chung M-S. Subcritical water extraction of bioactive components from red ginseng (Panax ginseng C.A. Meyer) J. Supercrit. Fluid. 2018;133:177–183. doi: 10.1016/j.supflu.2017.09.029. [DOI] [Google Scholar]
- Liu F, Ong ES, Li SFY. A green and effective approach for characterisation and quality control of Chrysanthemum by pressurized hot water extraction in combination with HPLC with UV absorbance detection. Food Chem. 2013;141:1807–1813. doi: 10.1016/j.foodchem.2013.04.083. [DOI] [PubMed] [Google Scholar]
- Loizzo MR, Pugliese A, Menichini F. Radical scavenging, antioxidant and ferric reducing activities of commercial mineral water enriched with fruit and ready to drink flavoured teas. Open Nutraceuticals J. 2012;5:160–168. [Google Scholar]
- Malongane F, McGaw LJ, Mudau FN. The synergistic potential of various teas, herbs and therapeutic drugs in health improvement: a review. J. Sci. Food Agric. 2017;97:4679–4689. doi: 10.1002/jsfa.8472. [DOI] [PubMed] [Google Scholar]
- Monrad JK, Howard LR, King JW, Srinivas K, Mauromoustakos A. Subcritical solvent extraction of procyanidins from dried red grape pomace. J. Agric. Food Chem. 2009;58:4014–4021. doi: 10.1021/jf9028283. [DOI] [PubMed] [Google Scholar]
- Oh J, Jo H, Cho AR, Kim S-J, Han J. Antioxidant and antimicrobial activities of various leafy herbal teas. Food Control. 2013;31:403–409. doi: 10.1016/j.foodcont.2012.10.021. [DOI] [Google Scholar]
- Ollanketo M, Peltoketo A, Hartonen K, Hiltunen R, Riekkola M-L. Extraction of sage (Salvia officinalis L.) by pressurized hot water and conventional methods: antioxidant activity of the extracts. Eur. Food Res. Technol. 2002;215:158–163. doi: 10.1007/s00217-002-0545-7. [DOI] [Google Scholar]
- Pavlić B, Vidović S, Radosavljević R, Cindrić M, Zeković Z. Subcritical water extraction of sage (Salvia officinalis L.) by-products—Process optimization by response surface methodology. J. Supercrit. Fluid. 2016;116:36–45. doi: 10.1016/j.supflu.2016.04.005. [DOI] [Google Scholar]
- Quek SY, Chok NK, Swedlund P. The physicochemical properties of spray-dried watermelon powders. Chem. Eng. Process. 2007;46:386–392. doi: 10.1016/j.cep.2006.06.020. [DOI] [Google Scholar]
- Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Bio. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- Sablania V, Bosco SJD, Rohilla S, Shah MA. Microencapsulation of Murraya koenigii L. leaf extract using spray drying. J. Food Meas. Charact. 2018;12:892–901. doi: 10.1007/s11694-017-9704-1. [DOI] [Google Scholar]
- Santos JS, Deolindo CTP, Hoffmann JF, Chaves FC, do Prado-Silva L, Sant’Ana AS, Azevedo L, do Carmo MAV, Granato D. Optimized Camellia sinensis var. sinensis, Ilex paraguariensis, and Aspalathus linearis blend presents high antioxidant and antiproliferative activities in a beverage model. Food Chem. 2018;254:348–358. doi: 10.1016/j.foodchem.2018.02.021. [DOI] [PubMed] [Google Scholar]
- Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- Şahin-Nadeem H, Dinçer C, Torun M, Topuz A, Özdemir F. Influence of inlet air temperature and carrier material on the production of instant soluble sage (Salvia fruticosa Miller) by spray drying. LWT-Food Sci. Technol. 2013;52:31–38. doi: 10.1016/j.lwt.2013.01.007. [DOI] [Google Scholar]
- Vergara-Salinas JR, Pérez-Jiménez J, Torres JL, Agosin E, Pérez-Correa JR. Effects of temperature and time on polyphenolic content and antioxidant activity in the pressurized hot water extraction of deodorized thyme (Thymus vulgaris) J. Agric. Food Chem. 2012;60:10920–10929. doi: 10.1021/jf3027759. [DOI] [PubMed] [Google Scholar]
- Yıldırım A, Mavi A, Oktay M, Kara AA, Algur ÖF, Bilaloǧlu V. Comparison of antioxidant and antimicrobial activities of Tilia (Tilia argentea Desf ex DC), sage (Salvia triloba L.), and Black tea (Camellia sinensis) extracts. J. Agric. Food Chem. 2000;48:5030–5034. doi: 10.1021/jf000590k. [DOI] [PubMed] [Google Scholar]
- Zhao J, Deng JW, Chen YW, Li SP. Advanced phytochemical analysis of herbal tea in China. J. Chromatogr. A. 2013;1313:2–23. doi: 10.1016/j.chroma.2013.07.039. [DOI] [PubMed] [Google Scholar]



