Abstract
Type II diabetes mellitus (T2DM), characterized by abnormal blood glucose level, is a metabolic disease caused by pancreatic β-cell dysfunction and insulin resistance. Previous studies have reported that whole grain intake alleviated various metabolic syndromes. Here, the hypoglycemic effect of whole grain diet (WGD) on type II diabetes was investigated in C57BL/KsJ-db/db mice. WGD improved the regulation of fasting and postprandial blood glucose levels and reduced weight gain and lipid accumulation. On the molecular level, WGD up-regulated the glucose transporter type 4 and stimulated the insulin receptor substrate 1/phosphoinositide 3-kinase ((PI3K)/Akt) pathway. WGD stimulated the AMP-activated protein kinase (AMPK)/p38/Acetyl-CoA carboxylate pathway related to lipid metabolism and glucose uptake, and down-regulated the pro-inflammatory cytokines, C-reactive protein, interleukin (IL)-6, IL-1β, and tumor necrosis factor-alpha. Taken together, whole grains can be employed as functional food ingredients to alleviate T2DM by enhancing the PI3K/Akt and AMPK pathways.
Keywords: AMPK, Diabetes, Glucose uptake, PI3K/Akt pathway, Whole grains
Introduction
The prevalence of type II diabetes mellitus (T2DM) rapidly increases and has it become a global health threat (Domingueti et al., 2016; Kozuka et al., 2013). T2DM, which is characterized by abnormal blood glucose levels, is caused by insulin resistance in insulin target organs and impaired secretion of insulin from pancreatic β-cells (Gastaldelli, 2011). Under diabetic conditions, cells fail to properly respond to insulin or a key regulator of glucose metabolism, and glucose uptake and metabolism are impaired. Thus, blood glucose levels are maintained at continuously high levels, which leads severe complications (Domingueti et al., 2016). In response to this insulin-resistant state, pancreatic β-cells produce and secrete more insulin to lower the concentrations of circulating glucose in the blood (Gastaldelli, 2011). However, chronic insulin resistance leads to pancreatic β-cell dysfunction and the development of T2DM (Keane et al., 2015). Thus, increasing the sensitivity of insulin responses and/or reducing insulin resistance are effective ways to control blood glucose levels and improve the quality of life of patients with T2DM.
Insulin resistance is defined as the reduced ability of insulin to regulate glucose transport from the blood and the glucose metabolism in the muscle, liver, and adipose tissue. When insulin is bound to insulin receptor (IR) in muscle cells and adipocytes, the insulin receptor substrate 1 (IRS-1)/phosphatidylinositol 3 kinase (PI3K)/Akt signaling pathway is activated, subsequently stimulating the movement of glucose transporter type 4 (GLUT4), a protein involved in glucose uptake, from the cytoplasm to the cell membrane (Schultze et al., 2012). AMP-activated protein kinase (AMPK) is a major protein involved in regulation of energy homeostasis in cells (Hardie et al., 2012). Activation of AMPK not only recruits GLUT4 to the cell membrane through phosphorylation of p38 mitogen-activated protein kinase (MAPK) but also reduces synthesis of malonyl-CoA or a precursor of free fatty acids by inactivating acetyl-CoA carboxylase (ACC) (Day et al., 2017; Leem et al., 2016; Smith et al., 2016).
The insulin-resistant state in T2DM is closely related with chronic low-grade inflammation, which is featured by overproduction of pro-inflammatory cytokines including C-reactive protein (CRP), interleukin-1beta (IL-1β), IL-6, and tumor necrosis factor-alpha (TNF-α) (Kim et al., 2014). Increased production of these pro-inflammatory cytokines, which circulate throughout the body via blood vessels, confers cytotoxic effects on pancreatic β-cells, consequently resulting in impaired insulin secretion (Keane et al., 2015). Pro-inflammatory cytokines induce systemic insulin resistance in insulin target tissues by blunting the insulin-induced phosphorylation of IR and disturbing the IRS-1/PI3K/Akt pathway (Kim et al., 2014).
Global interest in functional foods with health benefits that are effective against metabolic diseases increases (Chung et al., 2014). Whole grains, which consist of starchy endosperm, germ, and bran, are an abundant source of minerals, vitamins, phytochemicals, and macronutrients which offer health advantages (Cho et al., 2013; Wu et al., 2015). Previous studies have demonstrated that whole grains possessed a broad range of biological activities, such as anti-obesity, hypolipidemic, anti-hypertensive, and anti-cancer effects (Gong et al., 2018; Lee et al., 2018). Scientific and clinical evidences have shown that whole grains had hypoglycemic effects and reduced the risk of developing of T2DM; however, its exact molecular mechanisms have not been fully clarified (Borneo and León, 2012; Xi and Liu, 2016). The present study investigated the inhibitory effect of whole grain diet (WGD) on T2DM, with particular emphasis on the underlying molecular mechanisms, including the PI3K/Akt pathway and AMPK activation, in C57BL/KsJ-db/db mice.
Materials and methods
Diet preparation
For the animal experiment, two types of experimental diets were formulated: a 30% WGD and a 60% WGD. The compositions of the whole grains used in this study were brown rice (15.19%), low glycemic index brown rice (15.19%), buckwheat (12.66%), barley (12.66%), black rice (10.13%), sorghum (7.6%), germinated brown rice (7.6%), roasting brown rice (7.6%), roasting barley (7.6%), and whey protein concentrate (7.6%). The whole grains consisted of starch (67.63%), protein (11.37%), fat (6.06%), dietary fiber (8.72%), ash (2.65%), and moisture (3.57%). The normal diet was AIN-93G diet (Unifaith, Inc., Seoul, Korea), and WGDs were formulated based on the normal diet. Whole grains replaced 30% and 60% of the normal diet in the 30% WGD and 60% WGD, respectively. All the experiment diets were adjusted to resolve any nutrient imbalance and had equal energy. The composition of each diet is shown in Table 1.
Table 1.
Composition of the experimental diets (%)
| Ingredients | Normal diet | 30% WGD | 60% WGD |
|---|---|---|---|
| Casein | 19.50 | 16.09 | 12.68 |
| l-Cystine | 0.3 | 0.3 | 0.3 |
| Corn starch | 47.89 | 27.60 | 7.31 |
| Maltodextrin 10 | 6.1 | 6.1 | 6.1 |
| Sucrose | 5 | 5 | 5 |
| Cellulose | 8.96 | 4.48 | 0 |
| Soybean oil | 7.50 | 5.68 | 3.86 |
| Mineral mixture | 3.5 | 3.5 | 3.5 |
| Vitamin mixture | 1 | 1 | 1 |
| Choline bitartrate | 0.25 | 0.25 | 0.5 |
| t-Butylhydroquinone | 0.0014 | 0.0014 | 0.0014 |
| Whole grain sample | 0 | 30 | 60 |
| Total | 100 | 100 | 100 |
| Total energy (kcal/g) | 3.87 | 3.87 | 3.87 |
Animal experiment
Five-week-old male C57BL/KsJ-db/db mice (Central Lab. Animal Inc., Seoul, Korea) were housed under environmentally controlled conditions (25 ± 2 °C, 55 ± 5% humidity, and a 12 h light/dark cycle) at the Yonsei Laboratory Animal Research Center (Seoul, Korea). Water and diets were provided ad libitum, except during the fasting period. After 2 weeks of circulation, the mice were randomly assigned into three experimental groups as follows (n = 7): normal diet-fed mice (CON), 30% WGD-fed mice (WG 30), and 60% WGD-fed mice (WG 60). For 5 weeks, body weight and blood glucose levels were measured twice a week after 8 h of fasting. Blood glucose was measured by using a GM505OA glucose meter (Handok, Inc., Seoul, Korea). At the end of the experiment, the mice were fasted for 10 h and then sacrificed. Epididymal, subcutaneous, and perirenal fat, gastrocnemius muscle, and liver were isolated from the mice. The masses of the liver and fat were measured. All the tissues were stored at − 70 °C and fixed in 10% formalin buffer for analysis. All the experimental procedures were carried out in accordance with the guidelines of Korea Food and Drug Administration (KFDA) for the care and use of laboratory animals and approved by the Institutional Animal Care and Use Committee (IACUC) of Yonsei University (Permit No. IACUC-A-201705-201-04).
Oral glucose tolerance test
One day before sacrifice, glucose (Sigma-Aldrich, St. Louis, MO, USA) was orally administered after 12 h of fasting. Blood glucose was measured every 30 min for 2 h by using a AGM-4000 glucometer (All Medicus Co., Ltd.., Gyeonggi, Korea).
Reverse transcription-polymerase chain reaction
Total RNA was isolated from the liver and gastrocnemius muscle tissues by using TRIzol solution (Takara, Shiga, Japan). For epididymal adipose tissue, RNA was isolated by using the RNeasy lipid tissue mini kit (Qiagen, Hilden, Germany). Reverse transcription-polymerase chain reaction (RT-PCR) was performed as described in a previous study (Lee et al., 2018). The following primer pairs (Bioneer, Daejeon, Korea) were used: TNF-α forward 5′-GCG GAG TCC GGG CAG GTC TA-3′ and TNF-α reverse 5′-TCC ACG ATT TCC CAG AGA AC-3′; IL-6 forward 5′-CAC GGC CTT CCT ACT TCA C-3′ and IL-6 reverse 5′-GGT CTT GGT CCT TAG CCA CT-3′; IL-1β forward 5′-CCT CAG TGC GGG CTA TGA CC-3′ and IL-1β reverse 5′-TCG GCC AAG ACA GGT CGC TC-3′; C-reactive protein (CRP) forward 5′-GAA CTG GCG GGC ACT GAA CTA-3′ and CRP reverse 5′-GGA GGT GCT TCA GGG TTC ACA-3′; and β-actin forward 5′-TAA CCA ACT GGG ACG ATA TG-3′ and β-actin reverse 5′-ATA CAG GGA CAG CAC AGC CT-3′. PCR products were stained with Loading STAR (Dynbio, Gyonggi, Korea), visualized with the G; BOX EF imaging system (Syngene, Cambridge, UK), and analysed with the GeneSys program.
Western blot assay
Total proteins were extracted from homogenised gastrocnemius muscle and epididymal fat tissue using NP-40 lysis buffer (Elpis Biotech, Daejeon, Korea). Western blot assay was conducted according to previously described method (Lee et al., 2018). The membrane was incubated with primary antibodies against GLUT4, p-IRS-1, IRS-1 p-PI3K, PI3K, p-Akt, Akt, p-AMPK, AMPK, p-p38, p38, p-ACC, ACC, and α-tubulin (Cell Signaling Technology, Beverly, MA, USA) for 18–24 h at 4 °C, and then by exposed to secondary horseradish peroxidase (HRP)-conjugated antibody (Bethyl Laboratories, Inc., Montgomery, TX, USA) for 2 h at 4 °C. The signals were developed using an enhanced chemiluminescence solution (Amersham Biosciences, Little Chalfont, UK) and detected using the G;BOX EF imaging system (Syngene) and the GeneSys program.
Serum biochemical measurements
Blood was collected from the mice by heart puncture, incubated for 10 min at room temperature, and centrifuged for 15 min at 4000 rpm. Sera were stored at − 70 °C until analysis. The levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), and insulin in serum were measured at Green Cross Labs (Gyeonggi, Korea).
Histological analysis
Epididymal fat and liver tissues were fixed in 10% formalin and embedded in paraffin to produce paraffin block slides. The tissues on the paraffin block slides were stained with hematoxylin and eosin. Stained tissues were analyzed using CK40 Inverted Microscope (Olympus, Tokyo, Japan) equipped with a T500 camera (magnification, 100 ×; eXcope, Daejeon, Korea). The size of the adipocytes was quantified from captured images using ImageJ software (version 1.47; National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Results are expressed as the mean ± standard deviation. All statistical analyses were conducted using SPSS version 23.0 (SPSS Inc., Chicago, IL, USA). Group differences were evaluated by one-way analysis of variance (ANOVA), followed by Duncan multiple range test. *p < 0.05 and **p < 0.01 were considered statistically significant.
Results and discussion
WGD improves blood glucose regulation
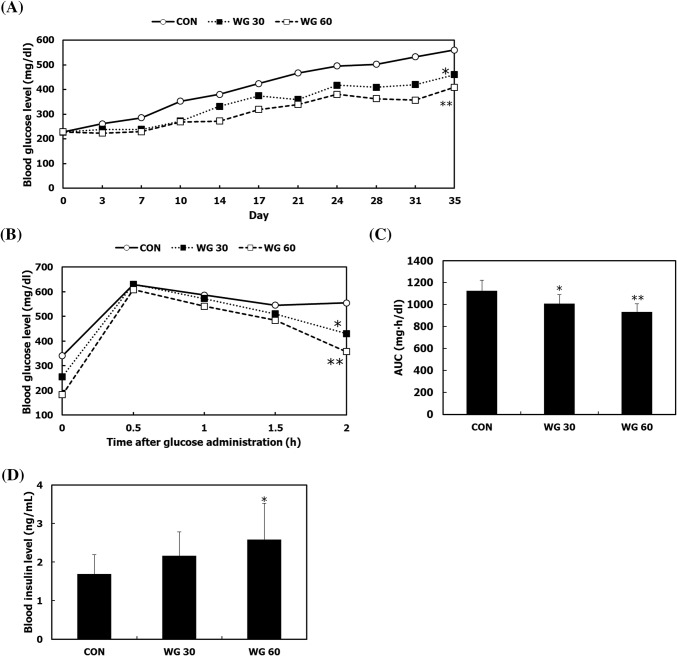
In this study, the beneficial effect of WGD on T2DM was evaluated by employing C57BL/KsJ-db/db mice which is a well-known animal model of T2DM (Lee et al., 2012). Dietary Guidelines for Americans recommend that at least half of the daily servings of grain consist of whole grains to reduce the prevalence of T2MD and obesity (Cho et al., 2013). Previous study presented whole grain diets containing 65% of wheat, barley, or oats improved plasma glucose and insulin resistance in an animal model of T2DM (Youn et al., 2012). Thus, we formulated 30% WGD and 60% WGD containing 30% and 60% whole grains, respectively. During the experimental period, the fasting blood glucose levels in all the groups were continuously increased; however, compared to the CON group, glucose levels were significantly decreased in the WG 30 and WG 60 groups (Fig. 1A). To assess the hypoglycemic effect of WGD, an oral glucose tolerance test was conducted. After oral administration of glucose, blood glucose was measured every 30 min for 2 h. Blood glucose levels were increased for the first 30 min. After that, in the CON group, a proper hypoglycemic response did not occur, and glucose levels remained the same, whereas in the WG groups, blood glucose levels were gradually decreased (Fig. 1B). WGD significantly reduced the total incremental area under the curve (AUC), compared to that in the CON group (Fig. 1C). The serum insulin levels in the WG 30 and WG 60 groups were higher than that in the CON group (Fig. 1D). Following the progression of type II diabetes, insulin resistance occurs and pancreatic β-cells are required to produce more insulin to maintain blood glucose levels (Keane et al., 2015). Whole grains not only reduce insulin resistance and triglycerides, but also improve glucose kinetics in individuals who have metabolic syndrome (Giacco et al., 2014; Malin et al., 2018). Whole grains consist of starchy endosperm, germ, and bran, and among these components, the bran or the outer layer of the grain is rich in beneficial nutrients, including various dietary fibers, vitamins, and phenolic compounds (Cho et al., 2013). Previous studies have recommended intake of bran-enriched foods or whole grains instead of refined grains to reduce the risk of T2DM (Aune et al., 2013). Several active compounds, such as γ-oryzanol, β-sitosterol, and ferulic acid, in whole grains can contribute to anti-diabetic effects. γ-Oryzanol improves glucose intolerance by reducing endoplasmic reticulum stress (Kozuka et al., 2012). Oral administration of β-sitosterol in rice bran reduces serum glucose and increases serum insulin level by preventing oxidative damage in diabetic rats (Gupta et al., 2011). Ferulic acid possesses therapeutic effects on diabetes and diabetic nephropathy (Choi et al., 2011). These results suggest that whole grains are helpful for lowering the high concentration of blood glucose in T2DM.
Fig. 1.
The effect of WGD on blood glucose regulation. Mice in the CON, WG 30, and WG 60 groups were fed a normal diet, 30% WGD, and 60% WGD, respectively, for 5 weeks. (A) Fasting blood glucose level was measured twice a week after 8 h of fasting, (B) postprandial blood glucose level was measured every 30 min for 2 h after orally administering glucose, (C) the area under the curve (AUC) for glucose level was quantified, (D) the serum insulin level was measured. Data are presented as the mean ± SD. *p < 0.05; **p < 0.01 compared with the CON group
WGD reduces lipid accumulation
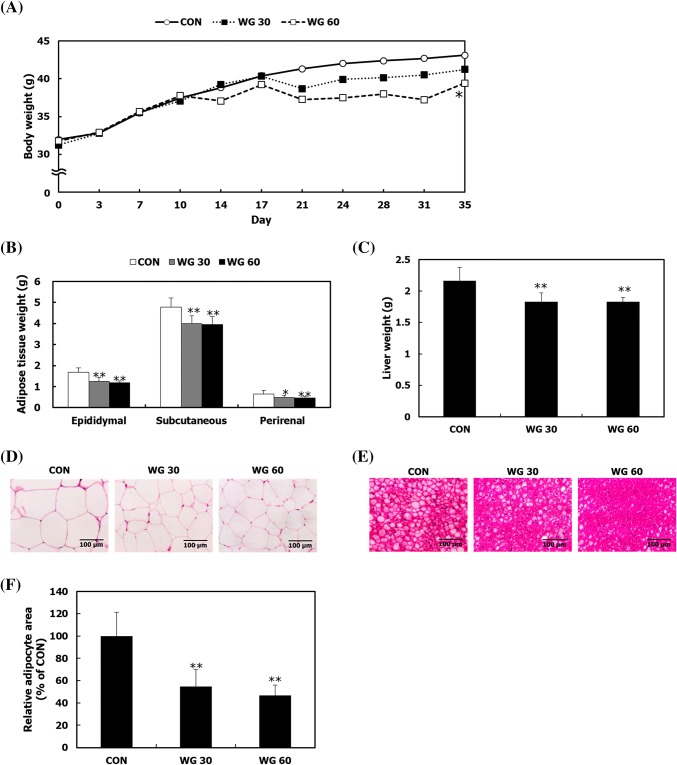
During the experimental period, the body weight of mice was gradually increased; however, mice fed with WGD showed a reduction in weight gain, compared to mice in the CON group (Fig. 2A). Fat and liver weights were also decreased in WG groups (Fig. 2B, C). To assess lipid accumulation in the fat tissues and liver, a histological analysis was performed on each tissue. WGD reduced lipid accumulation in the fat tissues and liver (Fig. 2D, E). In epididymal adipose tissue, the sizes of adipocyte in the WG groups were significantly lower than those in the CON group (Fig. 2F). Excessive lipid accumulation in adipose tissue causes insulin resistance and inflammation and up-regulates lipolysis, followed by secretion of free fatty acids into the blood (Jung and Choi, 2014). The free fatty acids in the blood enter the liver and accumulates as triglycerides or diacylglycerol, stimulating inflammation and insulin resistance (Asrih and Jornayvaz, 2015; Jung and Choi, 2014). A previous study presented that whole grain cereals containing various phytochemicals and dietary fibers attenuated the symptoms of obesity, such as fat accumulation and hyperlipidemia in obese mice (Lee et al., 2018). For example, brown rice and its major compound γ-oryzanol ameliorate lipid dysmetabolism in mice (Kozuka et al., 2013). The phenolic compounds including ferulic acid, caffeic acid, and coumaric acid found in whole grains possess anti-obesity effect by increasing fat metabolism, lowering lipid profile, and preventing fat deposition (Alam et al., 2016; Liu, 2007). Arabinoxylan, one of dietary fiber in whole grains, decreases body weight, fat mass development, and adipocyte size (Neyrinck et al., 2011). β-Glucan, which is found in the cell walls of whole grains, improves nonalcoholic fatty liver disease (Choi et al., 2010). These results suggest that the complex of phytochemicals and dietary fibers in whole grains collectively alleviates T2DM and obesity-related symptoms by decreasing lipid accumulation and improving lipid metabolism.
Fig. 2.
The effect of WGD on lipid accumulation in the liver and fat. Mice in the CON, WG 30, and WG 60 groups were fed a normal diet, 30% WGD, and 60% WGD, respectively, for 5 weeks. (A) Body weight was measured twice in a week, (B) the masses of the epididymal, subcutaneous, and perirenal fat were measured, (C) liver mass was measured. For the histological analysis, (D) epididymal fat tissue and (E) liver tissue were stained with hematoxylin and eosin (magnification, 100 ×), (F) the size of the epididymal adipocytes was quantified. Data are presented as the mean ± SD. *p < 0.05; **p < 0.01 compared with the CON group
WGD activates the IRS-1/PI3K/Akt pathway in skeletal muscle and fat
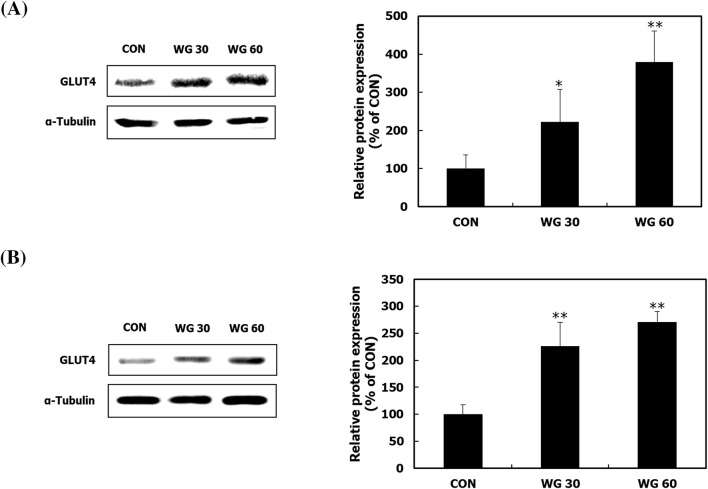
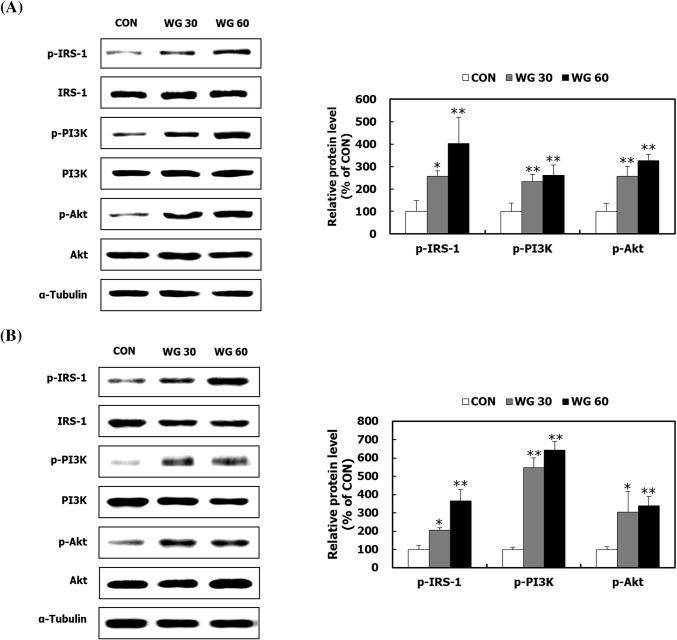
GLUT4 or a glucose transporter in skeletal muscle and adipose tissue is mainly responsible for glucose uptake (Fu et al., 2013; Keane et al., 2015). The protein expression of GLUT4 in skeletal muscle and adipose tissues was up-regulated in mice fed WGD, compared with that in mice in the CON group (Fig. 3A, B). GLUT4, which is embedded in transport vesicles and stored in the cytoplasm, is translocated to the cell membrane when the IRS-1/PI3K/Akt signalling pathway is activated in response to insulin (Schultze et al., 2012). The levels of p-IRS-1, p-PI3K, and p-Akt were significantly increased by WGD (Fig. 4A, B). Consistent with the current result, WGD was previously reported to activate the PI3K/Akt pathway in the skeletal muscle of HFD-induced obese mice (Lee et al., 2018). These results indicate that whole grains stimulate glucose uptake by activating an insulin-dependent cell signaling pathway.
Fig. 3.
The effect of WGD on the GLUT4 expression in skeletal muscle and fat. After the 5 week animal experiment, the gastrocnemius muscle tissue and epididymal adipose tissue were isolated. GLUT4 was measured in the (A) gastrocnemius muscle tissue and (B) epididymal adipose tissue by Western blot analysis. α-Tubulin was used as a loading control. Data are presented as the mean ± SD. *p < 0.05; **p < 0.01 compared with the CON group
Fig. 4.
The effect of WGD on the IRS-1/PI3K/Akt pathway in skeletal muscle and fat. After the 5 week animal experiment, the gastrocnemius muscle tissue and epididymal adipose tissue were isolated. The IRS-1/PI3K/Akt pathway was measured in (A) gastrocnemius muscle tissue and (B) epididymal adipose tissue by Western blot analysis. α-Tubulin was used as a loading control. Data are presented as the mean ± SD. *p < 0.05; **p < 0.01 compared with the CON group
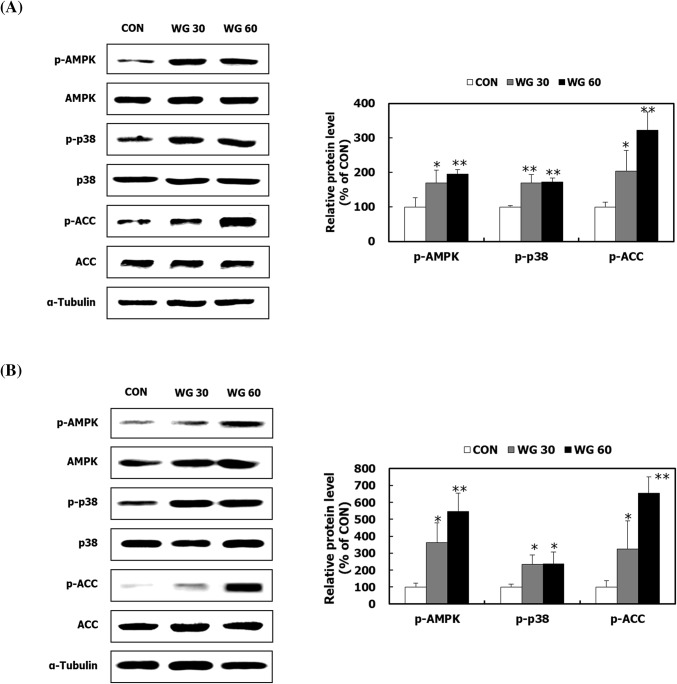
WGD activates the AMPK signaling pathway in skeletal muscle and fat
AMPK is a potential target for attenuating insulin resistance and improving lipid metabolism (Hardie et al., 2012). In this study, WGD up-regulated the protein expression of p-AMPK, p-p38 MAPK, and p-ACC in skeletal muscle and adipose tissues (Fig. 5A, B). AMPK induces GLUT4 recruitment to the plasma membrane by activating its downstream effector, p38 MAPK, which is an insulin-independent mechanism for GLUT4 translocation (Leem et al., 2016). Increased free fatty acids in the blood are another element that induces insulin resistance in target cells. ACC is a rate-limiting enzyme for free fatty acid synthesis that acts by converting acetyl-CoA into malonyl-CoA which is a metabolite for free fatty acid biosynthesis (Day et al., 2017; Smith et al., 2016). AMPK induces phosphorylation of ACC, which is an inactive form, consequently preventing lipid anabolism. Another function of AMPK is to stimulate lipid oxidation by activating genes (Kim et al., 2012). These results suggest that whole grains reduce blood glucose levels and lipid accumulation through AMPK activation.
Fig. 5.
The effect of WGD on AMPK signalling pathway in skeletal muscle and fat. After the 5 week animal experiment, the gastrocnemius muscle tissue and epididymal adipose tissue were isolated. The AMPK/p38/ACC pathway was measured in (A) gastrocnemius muscle tissue and (B) epididymal adipose tissue by Western blot analysis. α-Tubulin was used as a loading control. Data are presented as the mean ± SD. *p < 0.05; **p < 0.01 compared with the CON group
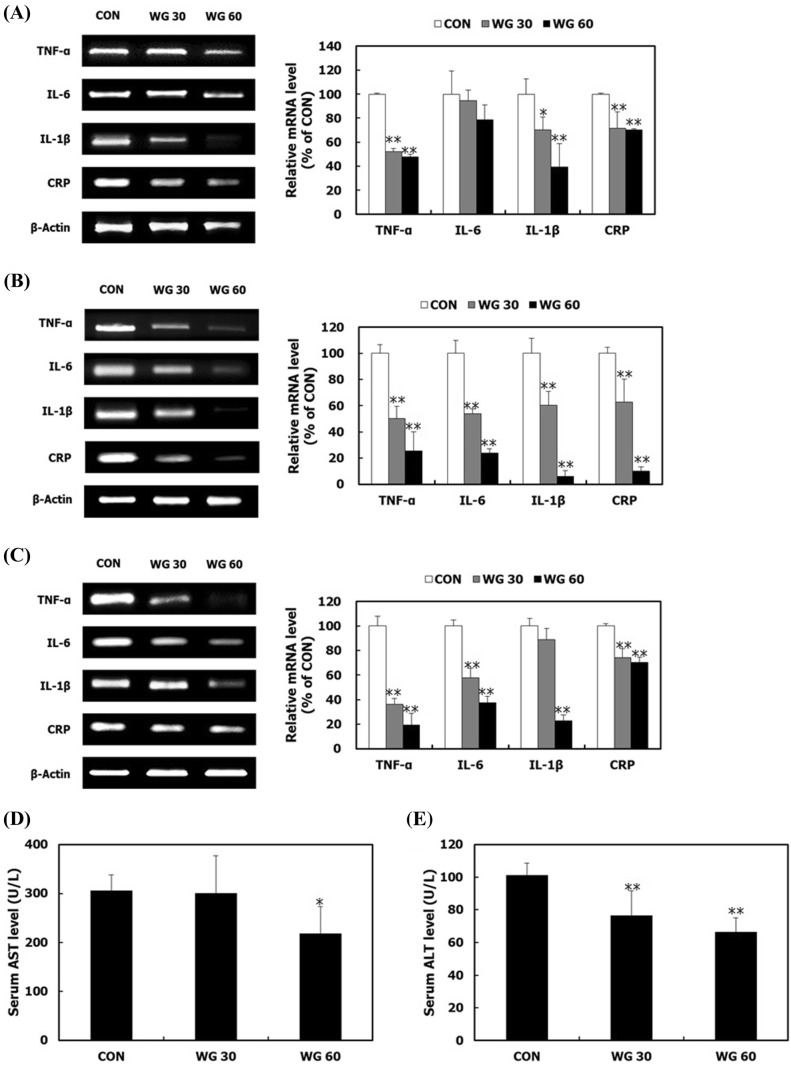
WGD decreases the expression of inflammatory cytokines
The overexpression and production of pro-inflammatory cytokines are risk factors that cause insulin resistance (Kim et al., 2014). WGD lowered the mRNA expression of these cytokines in gastrocnemius muscle, fat, and liver tissues (Fig. 6A–C). TNF-α and IL-6 interfere with the IRS-1/PI3K/Akt pathway and causes cytotoxic effects on pancreatic β-cells by inducing the overexpression of iNOS and the overproduction of NO (Fu et al., 2013; Tilg and Moschen, 2008). Hyperglycemia induces the production of IL-1β, which interferes with insulin signaling and causes β-cells dysfunction, leading to impaired insulin secretion (Kim et al., 2014). High levels of CRP lead to an increase in the nuclear translocation of NF-κB, which is a major protein responsible for the transcription of inflammatory cytokines (Kim et al., 2014). Excessive lipid accumulation and inflammation cause liver damage and increase blood level of AST and ALT (Dixon et al., 2001). In this study, WGD decreased blood levels of AST and ALT (Fig. 6D, E). These results suggest that whole grains attenuate inflammation-induced insulin resistance and protects the liver against inflammation.
Fig. 6.
The effect of WGD on inflammatory factor expression. After the 5 week animal experiment, gastrocnemius muscle tissue, epididymal adipose tissue, and liver were isolated. The mRNA expression levels of TNF-α, IL-6, IL-1β, and CRP in (A) epididymal adipose tissue, (B) gastrocnemius muscle tissue, and (C) liver tissue were measured by RT-PCR. β-Actin was used as a loading control. Blood was collected via cardiac puncture. The serum levels of (D) AST and (E) ALT were measured. Data are presented as the mean ± SD. *p < 0.05; **p < 0.01 compared with the CON group
In this study, we verified the hypoglycemic activity of a WGD and clarified the molecular mechanisms underlying WGD-mediated alleviation of T2DM in db/db mice. Phenotypically, WGD decreased fasting blood glucose levels and improved the hypoglycemic response. On the molecular level, WGD increased the recruitment of GLUT4 to the cellular membrane by stimulating the PI3K/Akt and AMPK pathways in skeletal muscle and fat. Also, WGD down-regulated the pro-inflammatory cytokines in insulin target cells. Taken together, these results show that whole grains can be useful as functional food ingredients for patients with T2DM to stimulate insulin sensitivity, enhance glucose metabolism, and reduce the inflammatory response by stimulating the PI3K/Akt and AMPK pathways. Further clinical research on the hypoglycemic activity of WGD is required for its usage as functional food ingredients.
Acknowledgements
We thank Prof. Jae-Kwon Lee (Kyunggi University, Suwon, Korea) for kindly supplying the mixture of whole grains. This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry, and Fisheries (IPET) through High Value-added Food Technology Development Program, funded by Ministry of Agriculture, Food, and Rural Affairs (MAFRA) (315071-03).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alam MA, Subhan N, Hossain H, Hossain M, Reza HM, Rahman MM, Ullah MO. Hydroxycinnamic acid derivatives: a potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016;13:27. doi: 10.1186/s12986-016-0080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asrih M, Jornayvaz FR. Metabolic syndrome and nonalcoholic fatty liver disease: is insulin resistance the link? Mol. Cell. Endocrinol. 2015;418:55–65. doi: 10.1016/j.mce.2015.02.018. [DOI] [PubMed] [Google Scholar]
- Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis of cohort studies. Eur J Epidemiol. 2013;28:845–858. doi: 10.1007/s10654-013-9852-5. [DOI] [PubMed] [Google Scholar]
- Borneo R, León AE. Whole grain cereals: functional components and health benefits. Food Funct. 2012;3:110–119. doi: 10.1039/C1FO10165J. [DOI] [PubMed] [Google Scholar]
- Cho SS, Qi L, Fahey GC, Jr, Klurfeld DM. Consumption of cereal fiber, mixtures of whole grains and bran, and whole grains and risk reduction in type 2 diabetes, obesity, and cardiovascular disease. AM. J. Clin. Nutr. 2013;98:594–619. doi: 10.3945/ajcn.113.067629. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim H, Jung MH, Hong S, Song J. Consumption of barley β-glucan ameliorates fatty liver and insulin resistance in mice fed a high-fat diet. Mol. Nutr. Food Res. 2010;54:1004–1013. doi: 10.1002/mnfr.200900127. [DOI] [PubMed] [Google Scholar]
- Choi R, Kim BH, Naowaboot J, Lee MY, Hyun MR, Cho EJ, Lee ES, Lee EY, Yang YC, Chung CH. Effects of ferulic acid on diabetic nephropathy in a rat model of type 2 diabetes. Exp. Mol. Med. 2011;43:676–683. doi: 10.3858/emm.2011.43.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SI, Kim TH, Rico CW, Kang MY. Effect of instant cooked giant embryonic rice on body fat weight and plasma lipid profile in high fat-fed mice. Nutrients. 2014;6:2266–2278. doi: 10.3390/nu6062266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day EA, Ford RJ, Steinberg GR. AMPK as a therapeutic target for treating metabolic diseases. Trends Endocrinol. Metab. 2017;28:545–560. doi: 10.1016/j.tem.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Dixon JB, Bhathal PS, O’brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- Domingueti CP, Dusse LMSA, das Graças Carvalho M, de Sousa LP, Gomes KB, Fernandes AP. Diabetes mellitus: the linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. J. Diabetes Complications. 2016;30:738–745. doi: 10.1016/j.jdiacomp.2015.12.018. [DOI] [PubMed] [Google Scholar]
- Fu Z, Gilbert E, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr. Diabetes Rev. 2013;9:25–53. doi: 10.2174/157339913804143225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaldelli A. Role of beta-cell dysfunction, ectopic fat accumulation and insulin resistance in the pathogenesis of type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2011;93:S60–S65. doi: 10.1016/S0168-8227(11)70015-8. [DOI] [PubMed] [Google Scholar]
- Giacco R, Costabile G, Della Pepa G, Anniballi G, Griffo E, Mangione A, Cipriano P, Viscovo D, Clemente G, Landberg R. A whole-grain cereal-based diet lowers postprandial plasma insulin and triglyceride levels in individuals with metabolic syndrome. Nutr. Metab. Cardiovasc. Dis. 2014;24:837–844. doi: 10.1016/j.numecd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Gong L, Cao W, Chi H, Wang J, Zhang H, Liu J, Sun B. Whole cereal grains and potential health effects: involvement of the gut microbiota. Food Res. Int. 2018;103:84–102. doi: 10.1016/j.foodres.2017.10.025. [DOI] [PubMed] [Google Scholar]
- Gupta R, Sharma AK, Dobhal M, Sharma M, Gupta R. Antidiabetic and antioxidant potential of β-sitosterol in streptozotocin-induced experimental hyperglycemia. J. Diabetes. 2011;3:29–37. doi: 10.1111/j.1753-0407.2010.00107.x. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014;15:6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keane KN, Cruzat VF, Carlessi R, de Bittencourt PIH, Newsholme P. Molecular events linking oxidative stress and inflammation to insulin resistance and β-cell dysfunction. Oxid. Med. Cell. Longev. 2015;2015:181643. doi: 10.1155/2015/181643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DY, Kim MS, Sa BK, Kim MB, Hwang JK. Boesenbergia pandurata attenuates diet-induced obesity by activating AMP-activated protein kinase and regulating lipid metabolism. Int. J. Mol. Sci. 2012;13:994–1005. doi: 10.3390/ijms13010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MB, Kim C, Song Y, Hwang JK. Antihyperglycemic and anti-Inflammatory effects of standardized Curcuma xanthorrhiza Roxb. extract and its active compound xanthorrhizol in high-fat diet-induced obese mice. Evid. Based Complement. Alternat. Med. 2014;2014:205915. doi: 10.1155/2014/205915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka C, Yabiku K, Sunagawa S, Ueda R, Taira S, Ohshiro H, Ikema T, Yamakawa K, Higa M, Tanaka H, Takayama C, Matsushita M, Oyadomari S, Shimabukuro M, Masuzaki H. Brown rice and its component, γ-oryzanol, attenuate the preference for high-fat diet by decreasing hypothalamic endoplasmic reticulum stress in mice. Diabetes. 2012;61:3084–3093. doi: 10.2337/db11-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka C, Yabiku K, Takayama C, Matsushita M, Shimabukuro M, Masuzaki H. Natural food science based novel approach toward prevention and treatment of obesity and type 2 diabetes: recent studies on brown rice and γ-oryzanol. Obes. Res. Clin. Pract. 2013;7:e165–e172. doi: 10.1016/j.orcp.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Lee SH, Min KH, Han JS, Lee DH, Park DB, Jung WK, Park PJ, Jeon BT, Kim SK, Jeon YJ. Effects of brown alga, Ecklonia cava on glucose and lipid metabolism in C57BL/KsJ-db/db mice, a model of type 2 diabetes mellitus. Food Chem. Toxicol. 2012;50:575–582. doi: 10.1016/j.fct.2011.12.032. [DOI] [PubMed] [Google Scholar]
- Lee S, Kim MB, Kim C, Hwang JK. Whole grain cereal attenuates obesity-induced muscle atrophy by activating the PI3K/Akt pathway in obese C57BL/6 N mice. Food Sci. Biotechnol. 2018;27:159–168. doi: 10.1007/s10068-017-0277-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leem KH, Kim MG, Hahm YT, Kim HK. Hypoglycemic effect of Opuntia ficus-indica var saboten is due to enhanced peripheral glucose uptake through activation of AMPK/p38 MAPK pathway. Nutrients. 2016;8:800. doi: 10.3390/nu8120800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RH. Whole grain phytochemicals and health. J. Cereal Sci. 2007;46:207–219. doi: 10.1016/j.jcs.2007.06.010. [DOI] [Google Scholar]
- Malin SK, Kullman EL, Scelsi AR, Haus JM, Filion J, Pagadala MR, Godin J-P, Kochhar S, Ross AB, Kirwan JP. A whole-grain diet reduces peripheral insulin resistance and improves glucose kinetics in obese adults: a randomized-controlled trial. Metabolism. 2018;82:111–117. doi: 10.1016/j.metabol.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyrinck AM, Possemiers S, Druart C, Van de Wiele T, De Backer F, Cani PD, Larondelle Y, Delzenne NM. Prebiotic effects of wheat arabinoxylan related to the increase in bifidobacteria, Roseburia and Bacteroides/Prevotella in diet-induced obese mice. PloS ONE. 2011;6:e20944. doi: 10.1371/journal.pone.0020944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultze SM, Hemmings BA, Niessen M, Tschopp O. PI3K/AKT, MAPK and AMPK signalling: protein kinases in glucose homeostasis. Expert Rev. Mol. Med. 2012;14:e1. doi: 10.1017/S1462399411002109. [DOI] [PubMed] [Google Scholar]
- Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, Steinberg GR. Treatment of nonalcoholic fatty liver disease: role of AMPK. Am. J. Physiol. Endocrinol. Metab. 2016;311:E730–E740. doi: 10.1152/ajpendo.00225.2016. [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 2008;14:222–231. doi: 10.2119/2007-00119.Tilg. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Flint AJ, Qi Q, Van Dam RM, Sampson LA, Rimm EB, Holmes MD, Willett WC, Hu FB, Sun Q. Association between dietary whole grain intake and risk of mortality: two large prospective studies in US men and women. JAMA Intern. Med. 2015;175:373–384. doi: 10.1001/jamainternmed.2014.6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi P, Liu RH. Whole food approach for type 2 diabetes prevention. Mol. Nutr. Food. Res. 2016;60:1819–1836. doi: 10.1002/mnfr.201500963. [DOI] [PubMed] [Google Scholar]
- Youn M, Csallany AS, Gallaher DD. Whole grain consumption has a modest effect on the development of diabetes in the Goto-Kakisaki rat. Br. J. Nutr. 2012;107:192–201. doi: 10.1017/S0007114511002741. [DOI] [PubMed] [Google Scholar]