Abstract
This study aimed to assess the biofilm formation by Bacillus cereus on two novel surfaces namely: aluminum and cold steel in comparison study with stainless steel and polystyrene. Also, it aimed to study the inhibitory effect of a new strain Pediococcus acidilactici against biofilm formation by B. cereus grown on these surfaces. In this study, B. cereus M50 isolated from milky machine surface was selected as the highest biofilm producer. The number of M50 cells adhered to aluminum and stainless steel surfaces were more than that adhered to polystyrene and cold steel, respectively. The antimicrobial, anti-adhesive and SEM studies revealed that the P. acidilactici P12 culture and its cell free filtrate showed a significant potential inhibition of biofilm formation of M50 on all tested surfaces under different conditions. These results demonstrated that P. acidilactici strain are considered a new biotreatment for biofilm destruction of food borne pathogens, food biopreservation and food safety.
Electronic supplementary material
The online version of this article (10.1007/s10068-018-0518-7) contains supplementary material, which is available to authorized users.
Keywords: Anti-adhesion, Biofilms, Hydrophilic and hydrophobic surfaces, LAB
Introduction
Biofilm is the ability of microorganisms to attach and grow on foods and food-contact surfaces under favorable conditions (van der Voort and Abee, 2013). Biofilms are complex microbial communities that embedded in an extracellular polymeric matrix. Since, the biofilms are very difficult to eliminate, the ability of bacteria to form biofilms possess a major problem in various industries, being a constant sources of contamination (Donlan and Costerton, 2002). Further, the formation of biofilms on industrial equipment can lead to serious, hygiene problems and economic losses due to food spoilage and equipment deterioration (Gram et al., 2007). Biofilms in food production environments can be formed on virtually all surfaces in contact with food causing recontamination of food (Majed et al., 2016).
Bacillus cereus is a spore former Gram positive bacilli and food spoilage pathogen, able to form biofilms on different surface materials and in different environmental conditions (Karunakaran and Biggs, 2011). Biofilms formed by B. cereus are influenced by substratum composition, surface chemistry and topography, and continuous fluid flow (Houry et al., 2010).
The best strategy for the industry to fight against B. cereus biofilms is to prevent its formation either by reduction of the spore load in raw materials or by early detection and eradication of the newly developing biofilms (Simões et al., 2010). Hygienic design of the equipment and effective cleaning procedures with proper frequency will help to reduce the risk of biofilm formation. Therefore, application of bacteriocin-producing LAB strains for control, inhibition of biofilm formation and/or killing of cells embedded in biofilms is considered a recent field of research (Gómez et al., 2012). LAB produces bacteriocins which can act synergistically or have an additive effect in the antimicrobial activity when combined with other antimicrobials agents. LAB may secrete organic acids, bacteriocins and biosurfactants (Gómez et al., 2012).
Various studies report B. cereus biofilm formation on abiotic surfaces including stainless steel, polystyrene and glass but a direct comparison between different substrata has not been performed (Karunakaran and Biggs, 2011). Therefore, this study aims to investigate the biofilm formation by foodborne B. cereus on aluminum and cold steel surfaces compared to stainless steel and polystyrene. Up till now, there is no previous comparison studies on the biofilm formation of B. cereus grown aluminum and cold steel with the other surfaces. Also, evaluation the antimicrobial and anti-biofilm potential of a new strain Pediococcus acidilactici against B. cereus strain was firstly discussed.
Materials and methods
Food samples
Twenty samples of raw milk and 10 milky machine surfaces, collected from diary factories in Dakhlia, Governorate, Egypt, were used for isolation of B. cereus. LAB was isolated from Yoghurt, pickles and cheese samples collected from market in Dakhlia Governorate, Egypt.
Isolation of B. cereus and lactic acid bacteria
Ten mL of raw milk samples were dispersed in 90 mL sterile saline solution (0.85% NaCl) and shaked for 2 min. Decimal dilutions were prepared and 0.1 mL of them was spread on the surface of prepared Mannitol Yolk polymyxin (MYP) (Sigma-Aldrich, St. Louis, MO, USA) agar plates. Meanwhile, the collected milky machine surface swaps were streaked directly on surface of MYP agar plates. The growing red colonies were purified and maintained on nutrient agar (Difco-Bacto, Carolina, USA) slants at 4 °C. Also, LAB were isolated from different food samples using surface spread technique on Man Rogosa and Sharpe agar (MRS) plates according to Shukla et al. (2008).
Identification of bacteria
Colonies morphology, cell shape and biochemical reactions of the selected B. cereus and LAB were recognized according to Holt et al. (1994). Their identification were confirmed using 16S rRNA genes sequencing according to Cibik et al. (2000). The 16S rDNA sequences which have been estimated in this study were deposited at NCBI web server (www.ncbi.nlm.nih.gov) under accession no. MH512943. Sequence analysis and comparison to published sequences made using the Basic Local Alignment Search Tool (BLAST) program (sequence-matching) (http://www.ncbi.nlm.nih.gov/blast).
Production and quantification of biofilm formation by B. cereus
One hundred of B. cereus cultures were isolated and screened for their biofilm formation. 200 µL of overnight B. cereus cultures (106 CFU/mL) was inoculated into 96-well microtiter plate (Spl LifeScience Co., Ltd., Naechon-Myeon, Pocheon, Korea) filled with 2 mL of brain heart infusion broth medium (BHI) (Sigma-Aldrich). The plates were incubated for 48 h at 30 °C.
The biofilm formation by each B. cereus culture was measured and quantified using Crystal Violet (CV) assay as described by Castelijn et al. (2012). After incubation, the wells were gently washed three times with 2 mL of phosphate buffered saline (PBS, Sigma-Aldrich) pH 7.1 (10 mM Na2HPO4, 1 mM KH2PO4, 140 mM NaCl, 3 mM KCl). The attached bacteria were fixed with 0.1% (W/V) crystal violet for 30 min. The excess of CV stain was discarded and the wells were washed three times with PBS. Thereafter, 2 mL of ethanol (70% (V/V)) were added to each well and incubated for 30 min to dislodge bounded biofilm. The optical density of solubilized bounded biofilm in each well was measured at 595 nm using ELISA Microplate reader (Microplate reader, Molecular Devices, LLC, San Jose, CA, USA). The negative control was determined in uninoculated BHI broth under the same conditions.
Biofilm formation on different commercial surfaces
Biofilm formation was detected on four different materials, stainless steel (AISI 304), pure polystyrene (prepared in Physical Lab., Faculty of Science, Zagazig university, Egypt), cold steel (1005) and aluminum (1050A H14) sheets. These sheets except polystyrene were obtained from companies in AL-Abour City, Egypt.
Preparation of sheets
The tested slide sheets (2 cm for each side) were cleaned by alcohol, acetone (Sigma-Aldrich, USA), air dried, soaked in 2 N HCl for 2 h, then rinsed with deionized water and air dried. Thereafter, all surfaces except polystyrene were autoclaved for 15 min at 121 °C. Polystyrene was sterilized by ethyl alcohol only. One sterile slide from each surface was transferred to 250 mL flask for further studies.
Enumeration of cells attached on sheets
One mL (106 CFU/mL) of B. cereus M50 was grown separately in BHI broth medium, in presence of the four tested surfaces, for 48 h at 35 °C. After incubation, the slides were removed and washed individually with 1 mL PBS pH 7.1 for 10 s to remove non adhered cells. Attached biofilms were collected with two sterile swabs. Afterwards, the cells were resuspended by vortexing swabs vigorously for 1 min in 9 mL of saline solution. The obtained suspensions were serially diluted and aliquots of 0.1 mL of appropriate dilutions were spread on MYP plates, then incubated for 24 h at 35 °C.
Contact angles and surface free energy analysis
The total surface energy of each surface was determined basing on contact angle measurements with water and glycerol using a travelling microscope, according to Owens–Wendt Method (Hejda et al., 2010).
where the total surface energy, polar and dispersion components of the surface free energy of the liquid are γl, γpl and γdl, respectively. θ is the contact angle between the sample and the liquid. Similarly, γs, γps, γds are the values for the tested surfaces. The values of γl, γpl and γdl, required for solid surface energy calculation were obtained from EL-Sayed et al. (2017).
Surface hydrophobicity of selected strain
Surface hydrophobicity of B. cereus M50 strain was determined using the bacterial adhesion to hydrocarbon (BATH) test according to Duary et al. (2011) with slight modification. The bacterial suspension was inoculated in tryptone soya broth medium (TSB) (Sigma-Aldrich) and incubated for 24 and 48 h at 37 °C. The cells were harvested by centrifugation (6000×g) and washed with sterile saline solution. One mL of toluene was added to 5 mL of each cell suspension, then incubated for 1 h at 37 °C. After incubation, the two-phase system were thoroughly mixed by vortexing for 3 min. Afterward, the aqueous phase was removed after 1 h of incubation at room temperature and its optical density was measured at 600 nm using spectrophotometer (New Brunsweak Scientific CO.). The bacterial culture alone was considered as control. Cell surface hydrophobicity (H%) was expressed as: H% = (1 − A1/A0) × 100
Antibacterial assay of LAB against selected strain
The antibacterial activity of cell free filtrates (CFF) of all different isolated LAB was determined using agar well diffusion method. The cell-free filtrate of each overnight grown culture of LAB was prepared by centrifuging at 6000×g for 20 min at 4 °C. The cell free filtrate was sterilized by membrane filtration (0.22 µm Millipore filter). The B. cereus M50 (106 CFU/mL) was spread over BHI agar surface. Afterwhich, a well with diameter of 6 mm by sterile cork poorer was done and each well filled with CFF. The plates were incubated for 24 h at 37 °C and the inhibition zones (mm) were determined (Djadouni and Mebrouk, 2012).
Antibacterial and anti-adhesive activities of LAB12 culture
The inhibitory effects of LAB12 culture on the growth of B. cereus M50 and its biofilm formation on the four experimental surfaces were determined under different conditions. Each surface slide was suspended in a 250 mL flask containing 50 mL BHI broth. This flask was inoculated with 1 mL (106 CFU/mL) overnight culture of B. cereus M50 and LAB12. The mixed cultures were incubated for different incubation periods (24, 48 and 72 h) at wide range of temperatures (10, 35 and 45 °C). The number of attached B. cereus cells on each tested surface was determined as mentioned above.
Furthermore, the inhibitory effect of cell free filtrate of LAB12 strain was investigated. The BHI broth media were inoculated with different inoculums sizes (105, 106, 107 and 108 CFU/mL) of LAB12 strain and incubated at 35 °C for 24, 48 and 72 h. Thereafter, these cultures were filtrated and examined for their potential to inhibit biofilm formation of B. cereus on tested surfaces. One mL (106 CFU/mL) of B. cereus M50 were added to a 50 mL beaker contained each of the tested surfaces and 9 mL of obtained CFFs of LAB12. The inoculated filtrates were incubated for 24 h at 35 °C. After incubation, the number of attached B. cereus cells was determined and then calculate the percentages of microbial adhesion on the surfaces as the following:
where Ac represents the number of CFU/cm2 treated with CFFs of LAB12 (obtained after 24, 48 and 27 h) and A0 the number of CFU/cm2 of control (without CFF). This allows to determine the percentage of microbial adhesion in relation to the control, which were set at 100% indicating total cells adhesion in the absence of CFF.
Scanning electron microscopy analysis
Slides treated with CFFs obtained after 24, 48 and 72 h in addition to control, were prepared for scanning electron microscopy. The biofilm on the surfaces was fixed and coated with gold/palladium in an Edwards S-150 sputter coater and the biofilm surface imaged using a JEOL-5400 SEM.
Statistical analysis
The attained data were examined statistically using one way analysis of variance (ANOVA). All statistical analysis were carried out using the software SPSS, version 16. *Values superscripted with different letters within the same line in all tables are significantly different at p < 0.05; each value is the mean of three replicates ± standard deviation.
Results and discussion
Isolation and screening of biofilm formation by B. cereus isolates
Biofilm formation was detected in 100% and 87.5% of B. cereus cultures isolated from milky machine surfaces and raw milk samples, respectively (Table 1). Several previous studies reported that, B. cereus is an endospore forming bacterium that frequently found in dairy products and diary environments (Salustiano et al., 2010). They have the ability to form biofilms and adhere to food processing equipments (Ribeiro et al., 2017). Hussain and Oh (2018) reported that 56 strains of B. cereus isolated from human and farm foods had biofilm formation abilities.
Table 1.
Prevalence and biofilm formation of B. cereus in raw milk samples and milky machine surfaces
| Source of sample | No. of sample | No. of total isolates | No. of biofilm forming isolates | % of biofilm formation |
|---|---|---|---|---|
| Raw milk | 20 | 80 | 70 | 87.5 |
| Milky machine | 10 | 20 | 20 | 100 |
| Total | 30 | 100 | 90 | 90 |
The isolate code 50 M which isolated from milky machine surface was selected as the highest biofilm producer bacteria. This isolate was characterized and identified using 16S rRNA gene sequence. The partial nucleotide sequence of amplified gene was deposited to GenBank under accession no. MH512943. BLAST program www.ncbi.n/m.gov/blast was used to assess the DNA similarities of obtained 16S rRNA gene sequence with other sequences deposited in GenBank (Figure S1).
Biofilm formation by B. cereus on different commercial surfaces
The biofilm formation was determined after incubation of B. cereus on the different surfaces for 48 h at 35 °C. As observed in Table 2, the ascending increase in the free surface energy of PS (23.81 mJ/m2) followed by CS (39.0 mJ/m2), SS (44.98 mJ/m2) and AL (54.80 mJ/m2), was paralleled to the increase in the biofilm formation to 2.1 × 105, 2.7 × 105, 3.5 × 106 and 4.3 × 106 CFU/mL, respectively. This explained that the maximum biofilm formation and cell number adhered to AL and SS may be related to their high free energy and hydrophilicity. Also, the cell surface hydrophobicity of vegetative and spores of B. cereus M50 cells was tested. The results showed that B. cereus spores possess greater hydrophobicity (60%) than vegetative cell (48%). The increase of bacterial spore’s hydrophobicity may be due to the relative abundance of proteins in the outer spore coats and exosporium compared to peptidoglycan on Gram positive vegetative cells surfaces (Henriques and Moran, 2007). As a result, B. cereus M50 cell hydrophobicity seemed to have little or no influence on adhesion. These results in agreement with Bonsaglia et al. (2014). They observed a great capacity to form biofilms on both hydrophobic and hydrophilic surfaces, whatever, biofilm formation was faster on the hydrophilic material. Also, Cerca et al. (2005) reported that all tested Staphylococcus epidermidis strains formed biofilms on hydrophilic and hydrophobic surfaces and cell surface hydrophobicity not have any influenced on adhesion. Hayrapetyan (2017) showed that stainless steel, as a contact surface, provides more favorable conditions for biofilm formation by B. cereus and maturation compared to polystyrene. Ribeiro et al. (2017) revealed that the higher adhesion of B. cereus spores which isolated from dairy industry to stainless steel surface (4.93 log CFU/cm2) was evaluated. Furthermore, our results are in agreement with Hyde et al. (1997) who explained that the surfaces with high free surface energy such as stainless steel and glass are more hydrophilic. These surfaces generally allow greater bacterial attachment and biofilm formation than hydrophobic surfaces, such as Teflon, nylon and polystyrene.
Table 2.
Total surface energy of experimental surfaces and biofilm formation by B. cereus M50 strain on their surfaces
| Experimental metal surface | Contact angle (degree) | Surface energy components (mJ/m2) | Biofilm formation | |||
|---|---|---|---|---|---|---|
| W | G | ϒDS | ϒPS | ϒS | CFU/cm2 | |
| Polystyrene | 85.0 ± 2.25a | 77 ± 2.03a | 14.84 ± 0.39c | 8.98 ± 0.24d | 23.81 ± 0.63d | 2.1 × 104 ± 0.05c |
| Cold steel | 63.61 ± 1.68b | 56.95 ± 1.51b | 18.3 ± 0.48b | 20.7 ± 0.55c | 39.0 ± 1.03c | 2.7 × 104 ± 0.07c |
| Stainless steel | 56.55 ± 1.49c | 48.92 ± 1.29c | 20.95 ± 0.55a | 24.03 ± 0.64b | 44.98 ± 1.19b | 3.5 × 105 ± 0.09b |
| Aluminum | 45.26 ± 1.19d | 52.64 ± 1.39bc | 8.37 ± 0.22d | 46.44 ± 0.12a | 54.80 ± 1.45a | 4.3 × 105 ± 0.116a |
W, Water; G: glycerol; ϒS, the total surface energy of the solid surface; ϒpS, polar component of the surface free energy of the solid surface; ϒDS, dispersion component of the surface free energy of the solid surface
Antimicrobial activity of LAB
A recent trends for the control of biofilm formation is the use of probiotics to colonized hard surfaces in order to counteract the proliferation of other bacterial species, based on the competitive exclusion principle (Hibbing et al., 2010). Several previous studies reported that bacteriocin producing LAB improved the bactericidal effect of biocides on bacterial biofilms (Gómez et al., 2012). Therefore, the biocontrol of biofilm formation of B. cereus M50 using LAB was investigated. The antagonistic effect of twenty LAB isolates against B. cereus M50 was studied using agar well diffusion method (Data not shown). LAB12 isolated from pickles, exhibited the highest antibacterial activity against M50 strain. The identification of isolate no. 12 was confirmed by amplification of its 16S rRNA gene as Pediococcus acidilactici and it was deposited in GenBank under accession no. MH512904 (Figure S2). Similarly, Reda (2014) reported that L. plantarum F14 isolated from pickles inhibited the enterotoxigenic B. cereus F23. LAB are recognized as potential interfering bacteria by producing various antimicrobial agents as hydrogen peroxide organic acids, bacteriocins and adhesion inhibitors (Tesfaye, 2014).
Antibacterial and anti-biofilm activities of LAB12 culture
The biofilm formation in various food processing contact surfaces have a great threat to human life. Therefore, new strategies are required worldwide to inhibit biofilm producer pathogens. In the present study, inhibition of biofilms formed by B. cereus M50 on tested surfaces was investigated. LAB12 culture was used as antimicrobial agent for inhibition of biofilms under different conditions. As observed in Table 3, the number of B. cereus M50 cells (CFU/cm2) that adhered to PS, CS, SS and AL surfaces were significantly increased by increasing of incubation periods at all incubation temperatures. As well as, the biofilm formation on all tested surfaces was increased at 35 and 45 °C for 48 h compared with 10 °C. Also, the inhibition of B. cereus biofilm formation on the four surfaces when incubated with mixed culture of LAB12 at different temperatures and times are shown in Table 3. As a result, the CFU/cm2 of B. cereus M50 attached on PS, CS, SS and AL surfaces were reduced efficiently after 48 h of incubation at 35 °C by about 4.24, 4.26, 5.28 and 4.45 log cycles, respectively. The reduced biofilm may be due to the antagonistic effect of LAB that decrease the pH of the food, competition for nutrients and production of inhibitory metabolites as reported by Soleimani et al. (2010). Moreover, the reduced biofilm ability of LAB12 culture and its metabolites may be they interfered with cell–cell interaction and cell assembly and reduced quorum sensing signals needed for biofilm formation, as in accordance with study of Sharma et al. (2018). They reported that the bacteriocins alone or combined with exopolysaccharides produced by Lactobacillus fermentum reduced the biofilm formation of Pseudomonas aeruginosa PAO1 at 37 °C. Reda (2014) reported that the pure culture or metabolites of LAB were used to control the growth of foodborne B. cereus strains.
Table 3.
Inhibition of biofilm formation of B. cereus M50 on polystyrene (PS), cold steel (CS), stainless steel (SS) and aluminum (AL) by mixed culture of P. acidilactici P12 under different temperature and time
| Temperature (°C) | Time (h) | Log No. of cells CFU/cm2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hydrophilic surfaces | Hydrophobic surfaces | ||||||||
| PS | CS | SS | AL | ||||||
| Control | Treated | Control | Treated | Control | Treated | Control | Treated | ||
| 10 | 24 | 1.14 ± 0.03c | 1.0 ± 0.03b | 1.52 ± 0.04c | 1.23 ± 0.03b | 2.1 ± 0.06c | 1.11 ± 0.03c | 2.70 ± 0.07b | 1.85 ± 0.05b |
| 48 | 2.34 ± 0.06a | 1.2 ± 0.03a | 2.45 ± 0.06a | 1.40 ± 0.04a | 3.22 ± 0.08b | 1.86 ± 0.05a | 3.63 ± 0.1a | 2.34 ± 0.06a | |
| 72 | 2.00 ± 0.05b | 1.0 ± 0.03b | 2.11 ± 0.05b | 1.21 ± 0.03b | 3.85 ± 0.1a | 1.40 ± 0.04b | 3.80 ± 0.1a | 1.85 ± 0.05b | |
| 35 | 24 | 3.25 ± 0.09b | 2.00 ± 0.05a | 3.70 ± 0.1b | 2.34 ± 0.06a | 4.10 ± 0.1b | 2.45 ± 0.06a | 4.63 ± 0.12b | 3.4 ± 0.09a |
| 48 | 5.46 ± 0.14a | 1.22 ± 0.03b | 5.72 ± 0.15a | 1.46 ± 0.04b | 6.60 ± 0.17a | 1.32 ± 0.03b | 6.95 ± 0.1a | 2.5 ± 0.07b | |
| 72 | 5.40 ± 0.14a | 1.28 ± 0.03b | 5.58 ± 0.15a | 1.53 ± 0.04b | 6.30 ± 0.17a | 1.30 ± 0.03b | 7.10 ± 0.19a | 2.00 ± 0.05c | |
| 45 | 24 | 1.88 ± 0.05b | 1.73 ± 0.04a | 2.1 ± 0.06b | 1.41 ± 0.04c | 2.90 ± 0.08b | 1.68 ± 0.04b | 3.22 ± 0.08b | 2.16 ± 0.06c |
| 48 | 3.42 ± 0.09a | 1.76 ± 0.05a | 3.70 ± 0.1a | 2.00 ± 0.05a | 4.26 ± 0.11a | 2.50 ± 0.07a | 5.19 ± 0.14a | 3.80 ± 0.1a | |
| 72 | 3.65 ± 0.1a | 1.82 ± 0.05a | 3.81 ± 0.1a | 1.81 ± 0.05b | 4.12 ± 0.11a | 2.44 ± 0.06a | 4.87 ± 0.13a | 3.39 ± 0.09b | |
Control: Bacillus cereus M50 alone
Treated: Bacillus cereus M50 grown with LAB12 culture
The relationships between biofilm reduction or inhibition on different surfaces and different temperatures are shown in Table 4. The results showed that B. cereus M50 biofilm was inhibited by LAB12 culture in all tested surfaces at all temperatures but the highest reduction was detected at 35 °C. Moreover, an inhibitory effect of LAB12 against B. cereus M50 was greater on stainless steel than others surfaces at all tested conditions. It can be concluded that, stainless steel AISI 304 surface was considered a more favorable surfaces for manufacturing food-processing surface equipment. The inhibition of biofilm formation on surfaces may be due to signaling molecules that block the attachment of bacterial cells to substrates, preventing synthesis of polymers in extracellular matrix and substances that block communication between bacteria (Suga and Smith, 2003).
Table 4.
Log cycle reduction of P. acidilactici P12 on polystyrene, cold steel, stainless steel and aluminum incubated at 10, 35 and 45 °C
| Temperature (°C) | Time (h) | Reduction of biofilm (log10CFU/cm2) | |||
|---|---|---|---|---|---|
| PS | CS | SS | AL | ||
| 10 | 24 | 0.65 ± 0.017c | 0.69 ± 0.018c | 1.01 ± 0.027c | 0.85 ± 0.022c |
| 48 | 1.66 ± 0.044b | 1.7 ± 0.045b | 1.36 ± 0.036b | 1.29 ± 0.034b | |
| 72 | 1.83 ± 0.048a | 2.0 ± 0.053a | 2.45 ± 0.065a | 1.95 ± 0.052a | |
| 35 | 24 | 1.20 ± 0.031b | 1.36 ± 0.036b | 1.65 ± 0.044b | 1.23 ± 0.033c |
| 48 | 4.24 ± 0.11a | 4.26 ± 0.11a | 5.28 ± 0.14a | 4.45 ± 0.12a | |
| 72 | 4.12 ± 0.11a | 4.05 ± 0.11a | 5.00 ± 0.13a | 4.10 ± 0.11b | |
| 45 | 24 | 0.14 ± 0.004c | 0.29 ± 0.008b | 1.22 ± 0.032b | 1.06 ± 0.028b |
| 48 | 1.40 ± 0.037a | 1.05 ± 0.028a | 1.76 ± 0.047a | 1.39 ± 0.037a | |
| 72 | 1.00 ± 0.026b | 0.9 ± 0.024c | 1.68 ± 0.044a | 1.48 ± 0.066a | |
The bold values meant as the highest biofilm reduction values by all tested surfaces
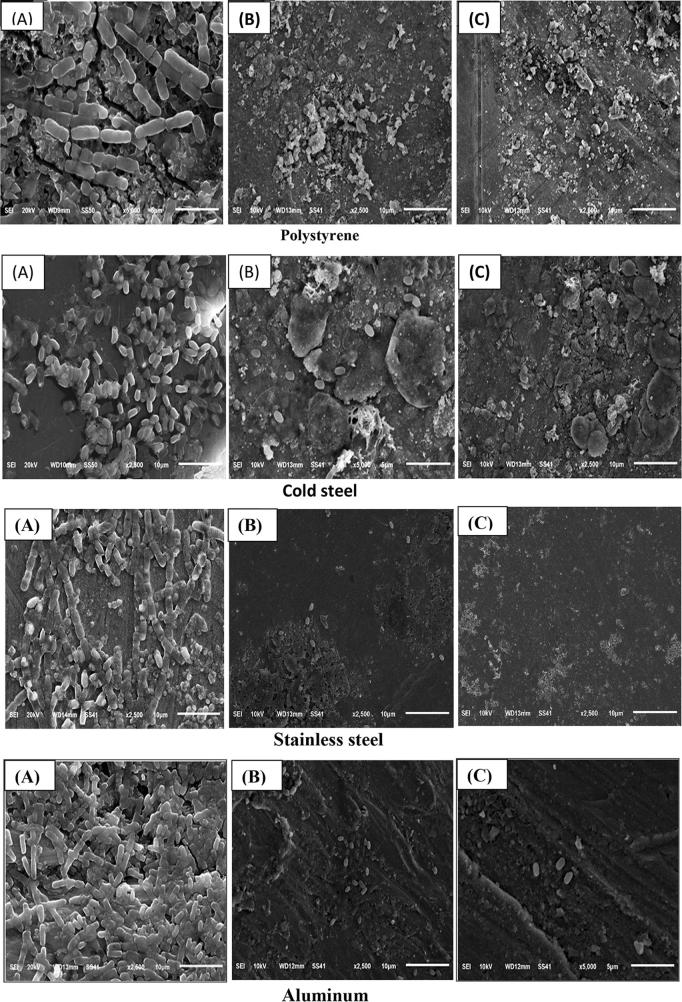
This study was extended for evolution of the effect of LAB filtrate, obtained from different incubation conditions, on adhesion of B. cereus M50. As observed in Table 5, the increasing of inoculum size of LAB12 leads to decreasing of microbial adhesion percentage till reach its maximum inhibition at 108 at all tested incubation conditions. Also, biofilm formation on all tested surfaces was completely inhibited in presence of 108 CFU/mL inoculums size of 48 h and 27 h LAB12 filtrates, as confirmed by Scanning electron micrographs (Fig. 1). The higher reduction in numbers of adherent cells on the surfaces may be due to the actions of antimicrobial compounds which present in LAB12 filtrate. The inhibitory effects of LAB12 strain may be due to its ability to produce antimicrobial agents such as organic acids, or lowering pH values, bacteriocin like substances, H2O2 and anti-biofilm substances (Li et al., 2014). The bactericidal and bacteriostatic effects of bacteriocin produced by LAB leads to pore formation in cell membrane, inhibition of peptidoglycan synthesis, degradation of cellular DNA and disruption through specific cleavage of 16SrRNA (Li et al., 2014). Bulgasem et al. (2015) observed the anti-adhesive efficiency of LAB supernatant against Candida species biofilms. Yong et al. (2015) showed that CFF of all LAB, isolated from meat, dairy and fermented products, exhibit a significant inhibition against Staphylococcus aureus. Denkova et al. (2013) revealed that probiotics have been shown to possess antimicrobial activity due to a variety of metabolites. Therefore, LAB is effective in food safety, developing characteristic new flavors, preserving food quality and control the growth of undesired microorganisms.
Table 5.
Effect of inoculums size of P. acidilactici P12 against B. cereus M50 biofilm formation on polystyrene, cold steel, stainless steel and aluminum at different incubation periods 24, 48 and 48 h
| Inoculum size (CFU/ml) | % Microbial adhesion | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PS | CS | SS | AL | |||||||||
| 24 | 48 | 72 (h) | 24 | 48 | 72 (h) | 24 | 48 | 72 (h) | 24 | 48 | 72 (h) | |
| 0 | 100 ± 2.65a | 100 ± 2.65a | 100 ± 2.65a | 100 ± 2.65a | 100 ± 2.65a | 100 ± 2.65a | 100 ± 2.65a | 100 ± 2.65a | 100 ± 2.65a | 100 ± 2.65a | 100 ± 2.64a | 100 ± 2.65a |
| 105 | 85 ± 2.25b | 50 ± 1.32b | 55 ± 1.45b | 87 ± 2.31b | 54 ± 1.43b | 57 ± 1.51b | 78 ± 2.06b | 46 ± 1.22b | 50 ± 1.32b | 90 ± 2.38b | 66 ± 1.75b | 58 ± 1.53b |
| 106 | 61 ± 1.61c | 22 ± 0.58c | 24 ± 0.63c | 63 ± 1.67c | 26 ± 0.69c | 27 ± 0.71c | 59 ± 1.56c | 21 ± 0.56c | 19 ± 0.51c | 73 ± 1.93c | 35 ± 0.93c | 31 ± 0.82c |
| 107 | 24 ± 0.63d | 10 ± 0.26d | 10 ± 0.26d | 28 ± 0.74d | 12 ± 0.32d | 14 ± 0.37d | 26 ± 0.69d | 12 ± 0.32d | 10 ± 0.26d | 35 ± 0.93d | 18 ± 0.47d | 16 ± 0.42d |
| 108 | 8 ± 0.21e | – | – | 10 ± 0.26e | – | – | 8 ± 0.21e | – | – | 15 ± 0.39e | – | – |
Fig. 1.
Scan electron micrographs showing effect of cell free filtrate of P. acidilactici P12 treatment on B. cereus M50 biofilm using four surfaces. (A) Bacillus cereus M50 only, (B) B. cereus M50 in presence of cell free filtrate after 24 h. (C) Bacillus cereus M50 in presence of cell free filtrate after 48 h
From these preliminary observations, it can be observed that B. cereus have the ability to form biofilms on different surfaces (hydrophilic and hydrophobic) independent to hydrophobicity of its cell surface, but formed greater in hydrophilic one. Also, it can be proposed that the CFF of P. acidilactici P12 (LAB12) and its cell culture exhibited significant antimicrobial and anti-adhesive activities against B. cereus M50. Therefore, LAB12 can be widely used in the food industry for biopreservation and as anti-adhesive agent in food processing equipments.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The author would like to thank Dr. Naglaa EL-Sayed for her guidance for studying hydrophobicity/hydrophylicity of surfaces.
Compliance with ethical standards
Conflict of interest
There is no conflict of interests regarding the publication of this paper.
References
- Bonsaglia ECR, Silva NCC, Junior AF, Junior JPA, Tsunemi MH, Rall VLM. Production of biofilm by Listeria monocytogenes in different materials and temperatures. Food Control. 2014;35:386–391. doi: 10.1016/j.foodcont.2013.07.023. [DOI] [Google Scholar]
- Bulgasem Y, Hassan Z, Abdalsadiq NKA, Yusoff WMM, et al. Anti-adhesion activity of lactic acid bacteria supernatant against human pathogenic Candida species biofilm. Health Sci. J. 2015;9:1–9. [Google Scholar]
- Castelijn GA, van der Veen S, Zwietering MH, Moezelaar R, Abee T. Diversity in biofilm formation and production of curlifimbriae and cellulose of Salmonella typhimurium strains of different origin in high and low nutrient medium. Biofouling. 2012;28:51–63. doi: 10.1080/08927014.2011.648927. [DOI] [PubMed] [Google Scholar]
- Cerca N, Pier GB, Vilanova M, Oliveira R, Azeredo J. Quantitative analysis of adhesion and biofilm formation on hydrophilic and hydrophobic surfaces of clinical isolates of Staphylococcus epidermidis. Res. Microbiol. 2005;156:506–514. doi: 10.1016/j.resmic.2005.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibik R, Lepage E, Tailliez P. Molecular diversity of Leuconostoc mesenteroides and Leuconostoc citreum isolated from traditional French cheeses as revealed by RAPD fingerprinting, 16S rDNA sequencing and 16S rDNA fragment amplification. Syst. Appl. Microbiol. 2000;23:267–278. doi: 10.1016/S0723-2020(00)80014-4. [DOI] [PubMed] [Google Scholar]
- Denkova R, Denkova Z, Yanakieva V, Blazheva D. Antimicrobial activity of probiotic lactobacilli, bifidobacteria and propionic acid bacteria, isolated from different sources. Microb. Path. Stratg. Comb. 857–864 (2013)
- Djadouni F, Mebrouk K. Antimicrobial activity of lactic acid bacteria and the spectrum of their biopeptides against spoiling germs in foods. Braz. Arch. Biol. Technol. 2012;3:435–443. doi: 10.1590/S1516-89132012000300015. [DOI] [Google Scholar]
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002;15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duary RK, Rajput YS, Batish VK, Grover S. Assessing the adhesion of putative indigenous probiotic lactobacilli to human colonic epithelial cells. Ind. J. Med. Res. 2011;134:664–671. doi: 10.4103/0971-5916.90992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EL-Sayed N, Reda FM, Farag O, Nasrallah D. Surface analysis of nitrogen plasma treated C60/PS nanocomposite films for antibacterial activity. J. Biol. Phys. 2017;43:211–224. doi: 10.1007/s10867-017-9447-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez NC, Abriouel H, Grande MJ, Pulido RP, Gálvez A. Effect of enterocin AS-48 in combination with biocides on planktonic and sessile Listeria monocytogenes. Food Microbiol. 2012;30:51–58. doi: 10.1016/j.fm.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Gram L, Bagge-Ravn D, Ng YY, Gymoese P, Vogel BF. Influence of food soiling matrix on cleaning and disinfection efficiency on surface attached Listeria monocytogenes. Food Control. 2007;18:1165–1171. doi: 10.1016/j.foodcont.2006.06.014. [DOI] [Google Scholar]
- Hayrapetyan H. Bacillus cereus Growth and Biofilm Formation: the Impact of Substratum, Iron Sources, and Transcriptional Regulator Sigma 54. Thesis Ph.D. at Wageningen University in the Aula (2017)
- Hejda F, Solar P, Kousal J. Surface free energy determination by contact angle measurements–a comparison of various approaches, WDS’10. In: Proceedings of Contributed Papers, Part III, 25 (2010)
- Henriques AO, Moran CP. Structure, assembly, and function of the spore surface layers. Ann. Rev. Microbiol. 2007;61:555–588. doi: 10.1146/annurev.micro.61.080706.093224. [DOI] [PubMed] [Google Scholar]
- Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010;8:15–25. doi: 10.1038/nrmicro2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt JG, Krieg NR, Sneath PHA, Staley JT, Williams ST. Bergey’s Manual of Determinative Bacteriology. 9. Baltimore: The Williams & Wilkins Co USA; 1994. [Google Scholar]
- Houry A, Briandet R, Aymerlch S, Gohar M. Involvement of motility and flagella in Bacillus cereus biofilm formation. Microbiology. 2010;156(4):1009–1018. doi: 10.1099/mic.0.034827-0. [DOI] [PubMed] [Google Scholar]
- Hussain MS, Oh DH. Impact of the isolation source on the biofilm formation characteristics of Bacillus cereus. J. Microbiol. Biotechnol. 2018;28:77–86. doi: 10.4014/jmb.1707.07023. [DOI] [PubMed] [Google Scholar]
- Hyde FW, Alberg M, Smith K. Comparison of fluorinated polymers against stain steel, glass and polypropylene in microbial biofilm adherence and removal. J. Ind. Microbiol. Biotechnol. 1997;19:142–149. doi: 10.1038/sj.jim.2900448. [DOI] [PubMed] [Google Scholar]
- Karunakaran E, Biggs CA. Mechanisms of Bacillus cereus biofilm formation: an investigation of the physicochemical characteristics of cell surfaces and extracellular proteins. Appl. Microbiol. Biotechol. 2011;89:1161–1175. doi: 10.1007/s00253-010-2919-2. [DOI] [PubMed] [Google Scholar]
- Li S, Huang R, Shah NP, Tao X, Xiong Y. Antioxidant and anti-bacterial activities of exopolysaccharides from Bifidobacterium bifidum WBIN03 and Lactobacillus plantarum R315. J. Dairy Sci. 2014;97:7334–7343. doi: 10.3168/jds.2014-7912. [DOI] [PubMed] [Google Scholar]
- Majed R, Faille C, Kallassy M, Gohar M. Bacillus cereus biofilms-same, only different. Front. Microbiol. 2016;7:1054. doi: 10.3389/fmicb.2016.01054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reda FM. Detoxification of enterotoxigenic Bacillus cereus (JX455159) isolated from meat by local strain of Lactobacillus plantarum (JX282192) Ann. Microbiol. 2014;64:287–296. doi: 10.1007/s13213-013-0662-5. [DOI] [Google Scholar]
- Ribeiro MC, Fernandes MS, Kuaye AY, Jimenez-Flores R, Gigante M. Preconditioning of the stainless steel surface affects the adhesion of Bacillus cereus spores. Int. Dairy J. 2017;66:108–114. doi: 10.1016/j.idairyj.2016.11.015. [DOI] [Google Scholar]
- Salustiano VC, Andrade NJ, Ribeiro Junior JI, Fernandes PE, Lopes JP, Bernardes PC, Portugal JG. Controlling Bacillus cereus adherence to stainless steel with different cleaning and sanitizing procedures used in dairy plants. Arq. Bras. Med. Vet. Zootec. 2010;62(6):1478–1483. doi: 10.1590/S0102-09352010000600026. [DOI] [Google Scholar]
- Sharma V, Harjai K, Shukla G. Effect of bacteriocin and exopolysaccharides isolated from probiotic on P. aeruginosa PAO1 biofilm. Folia Microbiol. 2018;63:181–190. doi: 10.1007/s12223-017-0545-4. [DOI] [PubMed] [Google Scholar]
- Shukla G, Devi P, Seghal R. Effect of Lactobacillus casei as a probiotic on modulation of giardiasis. Dig. Dis. Sci. 2008;53:2671–2679. doi: 10.1007/s10620-007-0197-3. [DOI] [PubMed] [Google Scholar]
- Simões M, Simões LC, Vieira MJ. A review of current and emergent biofilm control strategies. LWT Food Sci. Technol. 2010;43:573–583. doi: 10.1016/j.lwt.2009.12.008. [DOI] [Google Scholar]
- Soleimani NA, Kasra Kermanshahi R, Yakhchali B, Sattari TN. Antagonistic activity of probiotic lactobacilli against Staphylococcus aureus isolated from bovine mastitis. Afr. J. Microbiol. Res. 2010;420:2169–2173. [Google Scholar]
- Suga H, Smith K. Molecular mechanisms of bacterial quorum sensing as a new drug target. Curr. Opin. Chem. Biol. 2003;7:586–591. doi: 10.1016/j.cbpa.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Tesfaye A. Antagonism and primary in vitro probiotic evaluation of lactic acid bacteria recovered from Ergo. J. Agric. Biol. Sci. 2014;7:240–245. [Google Scholar]
- van der Voort M, Abee T. Sporulation environment of emetic toxin-producing Bacillus cereus strains determines spore size, heat resistance and germination capacity. J. Appl. Microbiol. 2013;114:1201–1210. doi: 10.1111/jam.12118. [DOI] [PubMed] [Google Scholar]
- Yong CC, Khoo BY, Sasidharan S. Activity of crude and fractionated extracts by lactic acid bacteria (LAB) isolated from local dairy, meat and fermented products against Staphylococcus aureus. Ann. Microbiol. 2015;65:1037–1047. doi: 10.1007/s13213-014-0949-1. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.