Abstract
The purpose of this study is to establish the best condition and microorganism for preparation of fermented ginseng including rich compound K. When raw ginseng parts were incubated with various microorganisms, there was an increase in compound K at 5 days in all samples fermented by Lactobacillus brevis (L. brevis) and Lactobacillus plantarum, isolated from kimchi. Especially, ginseng fine roots fermented with L. brevis (FR-B) included higher levels of compound K, total phenolic compounds, and antioxidant activities compared with other products. Conclusionally, these results indicate that the optimum condition for providing rich compound K product in fermented ginseng is ginseng fine roots are fermented with L. brevis for 5 days. Additionally, with FR-B there was greater improvement in physiochemical properties than with other products. Such information may be helpful for the manufacture of fermented ginseng including rich compound K as well as for understanding the biological features of fermented ginseng.
Electronic supplementary material
The online version of this article (10.1007/s10068-018-0504-0) contains supplementary material, which is available to authorized users.
Keywords: Antioxidant activity, Compound K, Ginseng fermentation, Lactobacillus brevis, Total phenolic compounds
Introduction
Ginseng (Panax ginseng) has been used as a component in traditional medicine in East Asia, and it has received worldwide attention as a constituent of functional food. Ginseng is known to possess various bioactive activities such as anti-cancer (Hofseth and Wargovich, 2007), anti-inflammation (Hofseth and Wargovich, 2007), and anti-obesity (Yin et al., 2008). Such effects of ginseng are closely associated with ginsenosides, active compounds of ginseng (Dai et al., 2017). Especially, red ginseng, which is a processed form of ginseng, shows more potent bioactive actions than ginseng because it is richer in ginsenosides (Shibata, 2001).
Fermentation for herbal medicines using microorganisms has been reported to improve the beneficial effects of the herbal medicines such as anti-allergy (Yoo et al., 2016), anti-cancer (Kim et al., 2015), anti-colitis (Kim et al., 2017), and neuroprotection (Park et al., 2016) because the microorganisms can convert glycosides to aglycones and/or produce their metabolites (Yoo et al., 2016). In addition, compound K, a bioactive ginsenoside, is converted from ginsenoside Rb1 and ginsenoside Rb2 by intestinal microflora (Akao et al., 1998); and it exerts various bioactivities such as anti-arteriosclerosis (Park et al., 2013), anti-cancer (Shibata, 2001), and neuroprotection (Oh and Kim, 2016). Recently, there have reports on fermentation of red ginseng and ginseng fruit by various microorganisms (Bae et al., 2004; Bai and Ganzle, 2015; Lee et al., 2015; Li et al., 2016; Park et al., 2017). Nonetheless, details of the process of raw ginseng fermentation and the best microorganism for the fermentation remain unknown.
In this study, to optimize a preparation method for fermented ginseng, we investigated the effects of various microorganisms such as Lactobacillus brevis [L. brevis, which is found in kimchi (Park and Oh, 2007)], Lactobacillus plantarum (L. plantarum), Saccharomyces cerevisiae (S. cerevisiae), Monascus purpureus (M. purpureus), Yoflex, and Bifidobacterium on ginseng fermentation in a time-dependent manner. Further, we evaluated the effects of L. brevis and L. plantarum, which were selected for preparation of fermented ginseng including rich compound K, on fine root, main root, and root bark of ginseng. To examine the change of physiochemical properties of ginseng, we determined total phenolic compound contents and antioxidant activities in products of non-fermented and fermented ginseng. In addition, to compare the ginsenoside contents in the products, we analyzed the contents of ginsenoside Rb1, ginsenoside Rg1, and compound K using a high-performance liquid chromatography (HPLC) system. Herein, we found that L. brevis was the best microorganism for the fermentation of ginseng including rich compound K and optimized the fermentation condition for the preparation of fermented ginseng from raw ginseng. Furthermore, we found that fine roots fermented with L. brevis (FR-B) showed high levels of compound K, total phenolic compounds, and antioxidant activities compared with the other products. These results may provide novel information for the manufacture of fermented ginseng and the application of FR-B as a functional food, and they may be helpful for understanding the biological features of fermented ginseng.
Materials and methods
Materials
Fine root, main root, root bark, and whole dried ginseng were purchased from Wooshin Co., Ltd (Geumsan-gun, Korea). L. brevis (KCTC 3498), L. plantarum (KCTC 3103), S. cerevisiae (KCTC 7904), and M. purpureus (KCTC 6121) were obtained from the Korean Collection for Type Cultures (Jeongeup, Korea). Yoflex and Bifidobacterium (BB-12) were procured from Chr. Hansen A/S (Hoersholm, Denmark). 2,2,-diphenyl-1-picrylhydrazyl (DPPH), 2,2′-azinobis (2-ethyl benzothiazoline-6-sulfonate (ABTS), ferric-reducing antioxidant power (FRAP), and all other chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA). All other chemicals were of analytical grade.
Sample preparation
Extraction of ginseng parts
Extracts of fine root, main root, root bark, and whole dried ginseng (100 g) were prepared by extraction with 70% ethanol (1 L) at 75 °C for 24 h, repeated 3 times, and then all the supernatant was collected. The supernatant was concentrated under vacuum at 40 °C using a rotary evaporator (MG-2100, Buchi, Flawil, Switzerland). The residues were collected when the weight of each residue reached about 25 g.
Fermentation for extracts of ginseng
The 2.5 g extracts were diluted with 50 mL of distilled water containing 7% α-herbzyme, and then the mixture was sterilized at 95 °C for 10 min. The sterilized solution was adjusted to pH 6.5 with 1 N NaOH, inoculated with microorganisms including L. brevis (1 × 105–107 CFU/mL), and then incubated at 37 °C for 3, 5, or 7 days. The fermented solution was centrifuged (1900×g, 4 °C) for 20 min, and then the supernatant was concentrated using a rotary evaporator.
Determination of total phenolic compounds
The amounts of total phenolic compounds in non-fermented and fermented ginseng extracts were determined in accordance with methods described previously (Yoo et al., 2014). Briefly, the solution (0.33 mL) was mixed with 2.5 mL of distilled water and then incubated with 0.16 mL of Folin–Ciocalteu reagent for 5 min. The above solution was further incubated for 30 min in darkness after treatment with 10% sodium bicarbonate solution (0.3 mL). Then absorbance at 760 nm was measured using a micro-plate reader (DU650, Beckman Coulter, Inc., Brea, CA, USA). A standard curve was prepared to express the results as caffeic acid equivalents.
Antioxidant activity assays
DPPH scavenging activity assay
DPPH radical scavenging activity was measured in accordance with the method reported previously (Birasuren et al., 2013). Briefly, various concentrations (0–10 mg/mL) of samples dissolved in methanol (200 μL) were mixed with 0.1 mM DPPH solution (4 mL) and then incubated for 30 min in the darkness. Absorbance at 517 nm was determined using a micro-plate reader.
Hydroxyl radical scavenging activity assay
Hydroxyl radical scavenging activity was determined in accordance with the method reported previously (Birasuren et al., 2013). Absorbance of the final mixture at 532 nm was measured using a micro-plate reader.
FRAP assay
FRAP assay was determined in accordance with the method reported previously (Birasuren et al., 2013). Absorbance of the final solution at 593 nm was measured using a micro-plate reader.
ABTS radical scavenging activity assay
ABTS radical scavenging activity assay was determined in accordance with the protocol reported previously (Birasuren et al., 2013). Absorbance of the mixture at 734 nm was measured using a micro-plate reader.
High-performance liquid chromatography analysis
To quantitate ginsenoside Rb1, ginsenoside Rg1, and compound K in all samples, HPLC analysis was performed using a HPLC system equipped with a 515 HPLC pump, 717 plus autosampler, and 2996 photodiode array detector (Waters Co., Milford, MA, USA) with a C18 column (4.6 × 250 mm, 5 μm; Sigma-Aldrich, St Louis, MO, USA). The ginsenosides were eluted in a gradient system composed of solvent A (deionized water) and solvent B (acetonitrile). The gradient was 20-20-32-50-65-90-90-20-20% of solvent B at the gradient time tG = 0-10-40-55-70-71-80-81-90 min, respectively. The column oven temperature was 40 °C, and the flow rate was 1.2 mL/min. An injection volume of 20 μL was applied. The UV/Vis detector was set at the wavelength range of 203 nm. The standards of ginsenoside Rb1, ginsenoside Rg1, and compound K as well as the samples (200 mg/mL) were dissolved and diluted in 50% methanol. All the sample solutions were used after filtering with a syringe filter (0.45 μm). Calibration curves constructed using linear least-squares regression were linear over the concentration range of the standards used. The relative standard deviation of the measured concentrations was used to assess the precision. A comparison of the mean measured concentration versus the corresponding nominal concentration was used to assess the accuracy. Data acquisition was performed using Empower 3 Chromatography Data Software (Waters Co., Milford, MA, USA).
Statistical analysis
The experimental results were listed as mean ± SD for all the experiments. One-way ANOVA was used for multiple comparisons (GraphPad Prism version 5.03 for Windows, San Diego, CA, USA). The Dunnett test was applied for significant variations between the non-fermented or fermented groups. Differences at the *p < 0.05 and **p < 0.01 levels were considered statistically significant.
Results and discussion
Effects of microorganisms on the fermentation of ginseng: comparison of ginsenoside contents
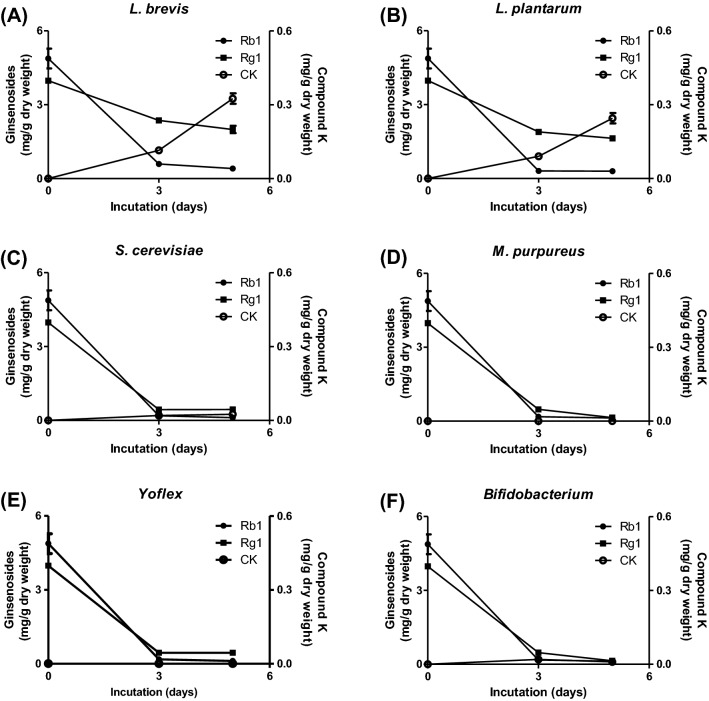
First, we investigated the effects of microorganisms such as L. brevis, L. plantarum, and S. cerevisiae on bioconversion of ginsenosides in whole ginseng extract because intestinal microflora are able to convert ginsenosides to compound K (Akao et al., 1998). When whole ginseng extract was fermented by L. brevis, L. plantarum, S. cerevisiae, M. purpureus, Yoflex, and Bifidobacterium for 3 days or 5 days, the level of compound K was rapidly elevated in the extracts of ginseng fermented by L. brevis [Fig. 1(A)] and L. plantarum [Fig. 1(B)] during the experimental period. In contrast, the levels of ginseonoside Rb1 and ginsenoside Rg1 were gently decreased in the period [Fig. 1(A, B)]. Meanwhile, the other microorganisms showed reduced levels of all the ginsenosides in ginseng extract during the experimental period [Fig. 1(C–F)]. These results suggest that the microorganisms can deglycosylate ginsenoside Rb1, ginsenoside Rg1, and compound K and that L. brevis and L. plantarum have lower abilities for compound K degradation than the other microorganisms. In support of this, it has been reported that the bioconversive activities of microorganisms are different when they convert ginsenosides (Park et al., 2017). In addition, microorganisms can deglycosylate and disintegrate ginsenosides (Odani et al., 1983). Collectively, L. brevis and L. plantarum are suitable microorganisms for preparation of fermented ginseng including rich compound K.
Fig. 1.
Effects of fermentation on ginsenoside contents in ginseng extracts. Ginseng extract was fermented by L. brevis, L. plantarum, S. cerevisiae, M. purpureus, Yoflex, or Bifidobacterium for 3 or 5 days. The ginsenoside contents in the samples were analyzed as described in “Materials and methods”. AL. brevis; BL. plantarum; CS. cerevisiae; DM. purpureus; E Yoflex; FBifidobacterium
Effects of L. brevis and L. plantarum on the fermentation of ginseng parts: comparison of ginsenoside contents
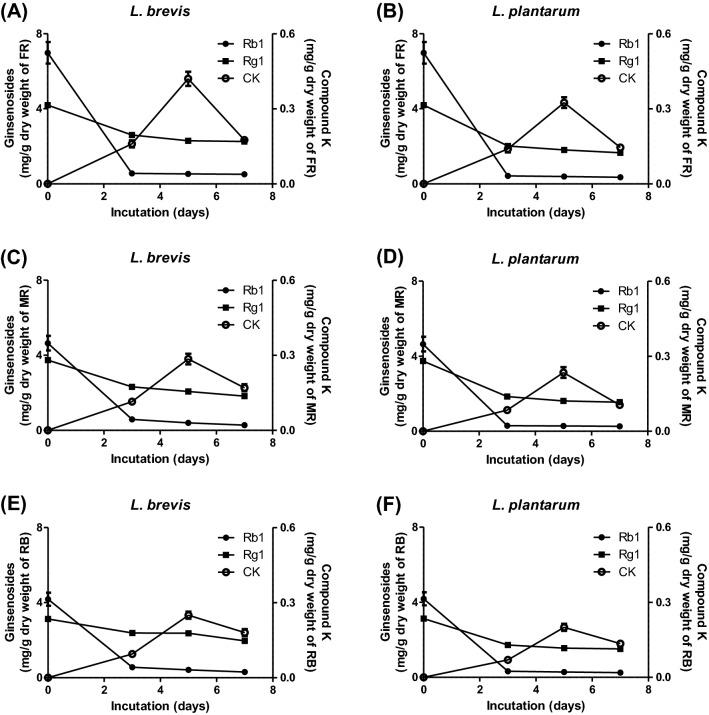
Because we found that L. brevis and L. plantarum could convert ginsenosides to compound K, we further examined the effects of them on fermentation for fine root, main root, and root bark of ginseng. Fine root is generally known to have a richer ginsenoside content than main root and root bark (Oh et al., 2014). Moreover, fine root is a by-product in the preparation of red ginseng because red ginseng is made from main root alone in Korea. When the amounts of ginsenosides in non-fermented fine root, main root, and root bark were analyzed using a HPLC system, fine root included higher levels of ginsenoside Rb1 and ginsenoside Rg1 than main root and root bark, but none of the samples contained compound K (Fig. 2). In contrast, when the extracts of fine root, main root, and root bark were fermented by L. brevis or L. plantarum in a time-dependent manner, the amounts of ginsenoside Rb1 and ginsenoside Rg1 in all the samples were decreased in proportion to fermentation time (Fig. 2). In addition, the amounts of compound K in fine root, main root, and root bark were decreased at 7 days after being elevated until 5 days (Fig. 2). The reason for the decrement of compound K at 7 days may be that the microorganisms can deglycosylate and disintegrate ginsenosides (Odani et al., 1983). The levels of compound K in all samples fermented by L. brevis were higher than those fermented by L. plantarum at the same fermentation time (Fig. 2). Especially, FR-B showed the highest level of compound K in comparison with main root fermented with L. brevis (MR-B) and root bark fermented with L. brevis (RB-B) [Fig. 2(A, C, E)]. These findings indicate that L. brevis is the best microorganism for preparation of fermented ginseng containing rich compound K, and the optimum condition for preparation of the best fermented ginseng is that using fine root fermented by L. brevis for 5 days.
Fig. 2.
Fermentation of fine root, main root, and root bark of ginseng by L. brevis and L. plantarum. The extracts of fine root, main root, and root bark were fermented by L. brevis or L. plantarum for 3, 5, or 7 days. The ginsenoside contents in the samples were analyzed as described in “Materials and methods”. A, B fine root; C, D main root; E, F root bark
Effects of fermentation by L. brevis and L. plantarum on physiochemical properties of ginseng parts: comparison of antioxidant activity and total phenol contents
Next, we were interested in the physiochemical properties of fermented ginseng by L. brevis and L. plantarum. Occasionally, the fermentation by microorganisms leads to improvement in the levels of total phenolic compounds and antioxidant activity (Liu et al., 2017). When fine root, main root, and root bark were fermented by L. brevis and L. plantarum, the antioxidant activities of all the fermented samples were significantly elevated (Table 1). In addition, the antioxidant activities of FR-B, MR-B, and RB-B were better than those of fine root fermented with L. plantarum (FR-P), main root fermented with L. plantarum (MR-P), and root bark fermented with L. plantarum (RB-P), respectively. Consistent with the compound K levels in FR-B, MR-B, and RB-B, the antioxidant activities of FR-B were significantly better than those of MR-B and RB-B. Furthermore, the amounts of total phenolic compounds in all the fermented samples were increased, and the ginseng fermentation by L. brevis also led to a greater increase in the total phenolic compounds than that by L. plantarum (Table 2). Moreover, the level of total phenolic compounds in FR-B was significantly higher than that in MR-B and RB-B (Table 2). These results suggest that the fermentation by microorganisms also improves the levels of antioxidant activities and total phenolic compounds in ginseng parts. Especially, FR-B showed higher levels of antioxidant activities and total phenolic compounds compared with the other fermented ginseng parts. Taken together, the results show that the FR fermentation by L. brevis elevates compound K as well as the physiochemical properties in ginseng. This information may be useful as an indicator for elevation of the quality of fermented ginseng. In addition, the regulation of fermentation time is very important for the preparation of high quality fermented ginseng product.
Table 1.
Antioxidant activities of ginseng parts fermented by L. brevis and L. plantarum
| Sample | Scavenging activity assays | |||
|---|---|---|---|---|
| DPPH (%) | Hydroxyl radical (%) | FRAP (mg/mL) | ABTS (%) | |
| FR | 28.86 ± 0.39** | 25.00 ± 0.44** | 0.130 ± 0.005** | 21.98 ± 0.18** |
| FR-B | 53.74 ± 0.19 | 46.80 ± 0.39 | 0.228 ± 0.003 | 52.49 ± 0.52 |
| FR-P | 51.68 ± 0.25** | 43.10 ± 0.58** | 0.215 ± 0.003* | 49.37 ± 0.12** |
| MR | 23.45 ± 0.01** | 21.04 ± 0.53** | 0.080 ± 0.003** | 14.60 ± 0.58** |
| MR-B | 44.89 ± 0.84## | 38.22 ± 0.73## | 0.198 ± 0.002## | 39.47 ± 0.65## |
| MR-P | 43.28 ± 0.53* | 36.95 ± 0.15* | 0.184 ± 0.004** | 37.26 ± 0.25** |
| RB | 22.75 ± 0.42** | 15.24 ± 0.54** | 0.071 ± 0.004** | 9.77 ± 0.76** |
| RB-B | 37.04 ± 0.92## | 30.81 ± 0.77## | 0.161 ± 0.005## | 26.55 ± 0.52## |
| RB-P | 29.83 ± 0.46** | 27.86 ± 0.60** | 0.154 ± 0.003 | 23.86 ± 0.16** |
The data are expressed as the mean ± SD values of three determinations
FR, fine root; MR, main root; RB root bark
*p < 0.05 and **p < 0.01 versus FR-B, MR-B, or RB-B group; ##p < 0.01 versus FR-B group
Table 2.
Composition of total phenolic compounds in ginseng parts fermented by L. brevis and L. plantarum
| Species | FR | MR | RB |
|---|---|---|---|
| 1.83 ± 0.01** | 1.60 ± 0.09** | 1.57 ± 0.06** | |
| L. brevis | 2.70 ± 0.01 | 2.32 ± 0.01## | 2.08 ± 0.05## |
| L. plantarum | 2.65 ± 0.01** | 2.26 ± 0.01 | 1.78 ± 0.04** |
The data are expressed as the mean ± SD values of three determinations
Unit: mg/g dry weight; FR, fine root; MR, main root; RB, root bark
**p < 0.01 versus FR-B, MR-B, or RB-B group; ##p < 0.01 versus FR-B group
In summary, this study established the optimum condition for preparation of fermented ginseng including rich compound K, and it demonstrated that L. brevis is the best microorganism for such preparation. In addition, it confirmed that the bioconversion of L. brevis is closely associated with changes of the physiochemical properties such as ginsenoside contents, antioxidant activities, and total phenolic compounds in ginseng parts. The improvements of compound K, antioxidant activities, and total phenolic compounds in FR-B may provide further information for the application of FR-B as a functional food for therapy and prevention of various diseases. Furthermore, the established fermentation condition can be applicable to the manufacture of FR-B possessing high levels of compound K, antioxidant activities, and total phenolic compounds.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was funded by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (Grant Number 2017R1D1A3B03027867).
Abbreviations
- FR-B
Fine root fermented with L. brevis
- FR-P
Fine root fermented with L. plantarum
- MR-B
Main root fermented with L. brevis
- MR-P
Main root fermented with L. plantarum
- RB-B
Root bark fermented with L. brevis
- RB-P
Root bark fermented with L. plantarum
Conflicts of interest
The authors declare no conflict of interest.
Human and animal rights
This article does not contain any studies performed by any of the authors with human participants or animals.
References
- Akao T, Kanaoka M, Kobashi K. Appearance of compound K, a major metabolite of ginsenoside Rb1 by intestinal bacteria, in rat plasma after oral administration–measurement of compound K by enzyme immunoassay. Biol. Pharm. Bull. 1998;21:245–249. doi: 10.1248/bpb.21.245. [DOI] [PubMed] [Google Scholar]
- Bae EA, Hyun YJ, Choo MK, Oh JK, Ryu JH, Kim DH. Protective effect of fermented red ginseng on a transient focal ischemic rats. Arch. Pharm. Res. 2004;27:1136–1140. doi: 10.1007/BF02975119. [DOI] [PubMed] [Google Scholar]
- Bai Y, Ganzle MG. Conversion of ginsenosides by Lactobacillus plantarum studied by liquid chromatography coupled to quadrupole trap mass spectrometry. Food Res. Int. 2015;76:709–718. doi: 10.1016/j.foodres.2015.07.040. [DOI] [PubMed] [Google Scholar]
- Birasuren B, Kim NY, Jeon HL, Kim MR. Evaluation of the Antioxidant Capacity and Phenolic Content of Agriophyllum pungens Seed Extracts from Mongolia. Prev. Nutr. Food Sci. 2013;18:188–195. doi: 10.3746/pnf.2013.18.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai D, Zhang CF, Williams S, Yuan CS, Wang CZ. Ginseng on Cancer: Potential Role in Modulating Inflammation-Mediated Angiogenesis. Am. J. Chin. Med. 2017;45:13–22. doi: 10.1142/S0192415X17500021. [DOI] [PubMed] [Google Scholar]
- Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. J. Nutr. 2007;137:183S–185S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- Kim A, Im M, Hwang YH, Yang HJ, Ma JY. Jaeumganghwa-Tang Induces Apoptosis via the Mitochondrial Pathway and Lactobacillus Fermentation Enhances Its Anti-Cancer Activity in HT1080 Human Fibrosarcoma Cells. PLoS One. 2015;10:e0127898. doi: 10.1371/journal.pone.0127898. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim DG, Lee MR, Yoo JM, Park KI, Ma JY. Fermented herbal formula KIOM-MA-128 protects against acute colitis induced by dextran sodium sulfate in mice. BMC Complement. Altern. Med. 2017;17:354. doi: 10.1186/s12906-017-1855-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Lee YC, Kim SS, Hong HD, Kim KT. Quality and antioxidant activity of ginseng seed processed by fermentation strains. J. Ginseng Res. 2015;39:178–182. doi: 10.1016/j.jgr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Ahn HJ, Kim NY, Lee YN, Ji GE. Korean Ginseng Berry Fermented by Mycotoxin Non-producing Aspergillus niger and Aspergillus oryzae: Ginsenoside Analyses and Anti-proliferative Activities. Biol. Pharm. Bull. 2016;39:1461–1467. doi: 10.1248/bpb.b16-00239. [DOI] [PubMed] [Google Scholar]
- Liu L, Zhang R, Deng Y, Zhang Y, Xiao J, Huang F, Wen W, Zhang M. Fermentation and complex enzyme hydrolysis enhance total phenolics and antioxidant activity of aqueous solution from rice bran pretreated by steaming with alpha-amylase. Food Chem. 2017;221:636–643. doi: 10.1016/j.foodchem.2016.11.126. [DOI] [PubMed] [Google Scholar]
- Odani T, Tanizawa H, Takino Y. Studies on the absorption, distribution, excretion and metabolism of ginseng saponins IV Decomposition of ginsenoside-Rg1 and -Rb1 in the digestive tract of rats. Chem. Pharm. Bull. 1983;31:3691–3697. doi: 10.1248/cpb.31.3691. [DOI] [PubMed] [Google Scholar]
- Oh J, Kim JS. Compound K derived from ginseng: neuroprotection and cognitive improvement. Food Funct. 2016;7:4506–4515. doi: 10.1039/C6FO01077F. [DOI] [PubMed] [Google Scholar]
- Oh JY, Kim YJ, Jang MG, Joo SC, Kwon WS, Kim SY, Jung SK, Yang DC. Investigation of ginsenosides in different tissues after elicitor treatment in Panax ginseng. J. Ginseng Res. 2014;38:270–277. doi: 10.1016/j.jgr.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park B, Hwang H, Lee J, Sohn SO, Lee SH, Jung MY, Lim HI, Park HW, Lee JH. Evaluation of ginsenoside bioconversion of lactic acid bacteria isolated from kimchi. J. Ginseng Res. 2017;41:524–530. doi: 10.1016/j.jgr.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park ES, Lee KP, Jung SH, Lee DY, Won KJ, Yun YP, Kim B. Compound K, an intestinal metabolite of ginsenosides, inhibits PDGF-BB-induced VSMC proliferation and migration through G1 arrest and attenuates neointimal hyperplasia after arterial injury. Atherosclerosis. 2013;228:53–60. doi: 10.1016/j.atherosclerosis.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Park HR, Lee H, Park H, Cho WK, Ma JY. Fermented Sipjeondaebo-tang Alleviates Memory Deficits and Loss of Hippocampal Neurogenesis in Scopolamine-induced Amnesia in Mice. Sci. Rep. 2016;6:22405. doi: 10.1038/srep22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KB, Oh SH. Cloning, sequencing and expression of a novel glutamate decarboxylase gene from a newly isolated lactic acid bacterium, Lactobacillus brevis OPK-3. Bioresour. Technol. 2007;98:312–319. doi: 10.1016/j.biortech.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Shibata S. Chemistry and cancer preventing activities of ginseng saponins and some related triterpenoid compounds. J. Korean Med. Sci. 2001;16(Suppl):S28–S37. doi: 10.3346/jkms.2001.16.S.S28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Zhang H, Ye J. Traditional chinese medicine in treatment of metabolic syndrome. Endocr. Metab. Immune Disord. Drug Targets. 2008;8:99–111. doi: 10.2174/187153008784534330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JM, Sok DE, Kim MR. Anti-allergic action of aged black garlic extract in RBL-2H3 cells and passive cutaneous anaphylaxis reaction in mice. J. Med. Food. 2014;17:92–102. doi: 10.1089/jmf.2013.2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo JM, Yang JH, Yang HJ, Cho WK, Ma JY. Inhibitory effect of fermented Arctium lappa fruit extract on the IgE-mediated allergic response in RBL2H3 cells. Int. J. Mol. Med. 2016;37:501–508. doi: 10.3892/ijmm.2015.2447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.