Abstract
Chemical composition, antifungal and antioxidant properties of essential oil extracted from Cuminum cyminum from Iran was studied. GC–MS analysis revealed the presence of 18 components, with 3-caren-10-al and cuminal as the principal constituents. Hierarchical cluster analysis and antioxidant capacities showed that this essential oil made a single group at 64 unit distinct from other reported essential oils extracted from cumin in the literature and was with high antioxidant activity [150 µL exhibiting strong reducing power; 2200 (FRAP) μmol/L Fe+2 during 15 min and ~ 89 DPPH % at 60 min]. The antifungal effects of the essential oil against three postharvest fungal pathogens, Botrytis cinerea, Aspergillus niger and Penicillim expansum revealed that at concentrations of ≥ 750 µL/L, the mycelial growth of the tested fungi were completely inhibited. Overall, the essential oil derived from this new cumin chemovar could be a promising candidate for its utilization as a natural preservative.
Keywords: Chemovar, Essential oil, Antifungal, GC–MS
Introduction
Nowadays, contamination of the environment is becoming an alarming issue with growing use of different synthetic chemicals pesticides due to their gradual disintegration (Fenoll et al., 2013), development of resistance in different pests (Yu et al., 2014) and other potential hazards and risks to human health and other forms of life (Aktar et al., 2009). Based on these negative effects of the synthetic pesticides, there is a renewed interest in developing and using safer, more effective and eco-friendly alternative pest management strategies (Fenoll et al., 2013). Plants are genetically very diverse group and are used by people for food, either as edible products, or for culinary ingredients, for medicinal use (Cuce and Sokmen, 2017; Sengul et al., 2011; Vijayan et al., 2008). The use of natural plant derived compounds, due to their antimicrobial and insecticidal properties has gained popularity and scientific interest in recent years (Sonker et al., 2016). Aromatic and medicinal plants have a wide variety of bioactive compounds such as alkaloids, coumarins, flavonoids, phenols, saponins, tannins, terpenoids, quinones, glucosinolates and etc. which are contributed in bactericidal, virucidal, fungicidal, antihelminthic and insecticidal properties (Adorjan and Buchbauer, 2010). It has been reported that the concentration of bioactive compounds in each plant species is dependent on environmental conditions (Radušienė et al., 2012).
Postharvest diseases causes great losses on fruits and other plant products worldwide which affect quality, quantity and shelf life of products from harvest until actual use by consumers (Agrios, 2005). Although fungi, fungi-like protists and bacteria are considered as postharvest decaying agents, but fungi are the most important ones (Jahlegar et al., 2014). Aspergillus niger, Botrytis cinerea and Penicillium expansum are among the most important fungi causing postharvest rots in different plants (de Sousa et al., 2013; Tian et al., 2014).
Cumin (Cuminum cyminum L.), a member of Apiaceae family known as Zireh-e-sabz (= green cumin) in Iran, is widely used as an industrial and medicinal plant. This herb, which was originated from Egypt, Turkistan, and Eastern Mediterranean, nowadays is extensively cultivated in Iran (Moghaddam et al., 2015). Phenolic compounds and essential oils of cumin are used as natural antioxidants, in flavoring the foods and in the treatment of toothache, diarrhea, epilepsy, dyspepsia, and jaundice (Alinian et al., 2016; Nostro et al., 2015).
This study is aimed to: (1) extraction and assessing chemical composition of essential oil in a local seed lot of cumin in Iran. (2) evaluation the antifungal effects of essential oil under in vitro conditions against three important postharvest fungi, and (3) assessing antioxidant activity of essential oil with two different methods.
Materials and methods
Plant material
Ripe cumin seeds were taken from Ilkhchi region in East Azarbaijan province, Iran in 2015. Having classically semi-arid climate and at the altitude of 1500 m above the sea level, this region enjoys high precipitation throughout the autumn and winter months, whereas there is a little rainfall during the summer.
Essential oil extraction and analysis
Cumin seeds were dried at room temperature and darkness to minimize photo-oxidative changes. Essential oil extraction were done using 50 g of dried seeds by hydro-distillation for 4 h and a Clevenger type apparatus. Essential oil was maintained in darks crew capped vials at 4 °C for further analysis. The gas chromatography-mass spectrometry (GC–MS) analyses were performed on a Thermo Finnigan capillary gas chromatograph which was directly linked to the mass spectrometer system (model Trace GC/Trace MS Plus system; Finnigan, Hemel Hempstead, UK). A non-polar fused silica capillary column (HP-5 ms; 30 m × 0.25 mm i.d., 0.25 µm film thickness; Agilent Technologies, Palo Alto, CA, USA) was used. The GC Oven temperature was kept at 40 °C for two min and then was increased to 160 °C with the temperature rate of 3 °C/min and finally increased to 280 °C at a rate of 5 °C/min; and remain for two min. other injection information was, injector temperature 280 °C; ion source 200 °C and interface temperature 260 °C. The carrier gas was helium at a flow rate of 1 mL/min, and ionization energy was 70 eV. One µL of essential oil were solved in diethyl ether (2 µL of the oil in 2 mL solvent) was injected via a split injector (1:20). Also, n-alkenes (C6–C24) with the same injection conditions were used to calculate retention indices (RI). The calculated RI were compared with those of reported in the literature to identify of essential oil compounds (Sparkman, 2005). Mass spectra of the compounds were obtained using X-Calibur (2.07) with its available libraries. The compounds percentages were attained by the area normalization technique, without considering correction factors.
Fungal isolates
The tested fungi, Aspergillus niger (UUCC 1009), the causal agent of grapevine gray mold, Botrytis cinerea (UUCC 2012), the causal agent of grapevine black mold and Penicillium expansum (UUCC 1108), the causal agent of apple green molds, which were isolated and identified previously from infected grapes and apple fruits, were provided by the fungal culture collection at Plant Pathology laboratory, Urmia university. All the isolates were cultured on potato dextrose agar (PDA) medium at 25 °C for 7 days and were used in bioassays.
Antifungal bioassay
To determine the antifungal activity of essential oil against three postharvest fungi, food poisoning method was used. For this potato dextrose agar (PDA, Merck, Germany) medium was autoclaved at 121 °C for 20 min and allowed to cool to 45–50 °C. Different concentrations of essential oil (0, 250, 500, 750, 1000 and 1500 μL L−1) were incorporated into the medium and poured into petri plates (20 mL per each petri plate). Tween 80 was used at the rate of 0.01% as an oil disperser in all treatments. Mycelial discs (5 mm diameter) were cut from the edge of 5–7d old actively growing culture of the test fungi and placed in the center of solidified PDA plates upside down. Petri plates were incubated at 25 ± 1 °C. All the treatments were replicated four times. The mycelial growth was recorded daily until the growth of the fungi in controls covered the entire Petri plates and the percentage of mycelial growth inhibition (MGI %) in relation to the control treatments was calculated by the following formula:
where Dc and Dt represent colony diameter (mm) in control and treatment, respectively. In the cases that the mycelial growth was completely inhibited, the mycelial discs were taken and transferred into new PDA plates without any inhibitory substances and incubated again at 25 ± 1 °C for 72 h. While the growth of the fungi has occurred, the inhibitory effects of essential oil was considered fungistatic, otherwise, (no new growth), the effects of essential oil was considered fungicidal.
Antioxidant activity
FRAP assay Ferric reducing antioxidant power (FRAP) was used for assaying the antioxidant potential of essential oil (Benzie and Strain, 1996). According to the method, the capability of antioxidants to reduce the ferric ion (Fe3+) to the ferrous ion (Fe2+), generating the blue-colored Fe2+-tripyridyltriazine compound which rises absorbance at 593 nm. Essential oil was solved in distilled water and dimethyl sulfoxide (DMSO) (1% v/v) to obtain desired amounts (0–150 µL).
- DPPH Scavenging assay In this assay, free radical scavenging activity of essential oil was determined by an adapted version of Nakajima et al.’s principle (Nakajima et al., 2004) in line with (Chiou et al., 2007). Microliters ranging between 0 and 150 of essential oil with respect to the above-mentioned does were poured to 950 µL of 6 × 10−5 mol/L (free radical, 95%) in methanol. We shook the solution precisely and let it stay at room temperature at different time spans (X; 15, 30, 45, and 60 min). Next, the absorbance was gauged at 517 nm and the inhibition percentage of the DPPH radical was estimated through the following equation:
In which Abs control is the absorbance of DPPH solution mixtures without essential oil, while Abs sample is the absorbance of DPPH mixtures DPPH with essential oil sample presence on definite durations.
Statistical analysis
Statistical analyses of the data were performed using MSTAT-C statistical software and means were separated by DMRT at 0.01 probability level. Also, PAST software (ver. 2.17c) was used for determining the phytochemical distance via generating of hierarchical cluster analysis in ward’s method (Hammer et al., 2001).
Results and discussion
Essential oil yield and composition
The extracted essential oil in this study was found to be light yellow to cream at a yield of 1.1% (v/w) of dry weight. The chemical composition of cumin essential oil used in this study was depicted in Table 1. 3-caren-10-al (47.27%) and cuminal (25.92%) were the main compounds followed by 2-caren-10-al (8.05%), γ-terpinene (7.66%), (-)-β-pinene (5.11%), p -cymene (2.71%). Figure 1A gives detailed information about mass of dominant components from the chromatogram of GC–MS. Hydrocarbon (16.6%) and oxygenated monoterpenes (82.68%) were the major parts of essential oil. The synthesis of secondary metabolites in aromatic plants is mainly controlled by different internal and external factors. Genetic factors such as variety and chemo-type and many environmental factors such as location, altitude, temperature and light, agronomic characteristics and cultural management practices, extraction method and etc. affect yield and chemical composition of essential oils (Radušienė et al., 2012). The beneficial health effects of these plants are attributed to their essential oils. It is very important to searching for and identification of medicinal plants with higher essential oil content, better quality, novel components, and as a whole, a superior product. So in this study, the chemical composition, antifungal and antioxidant properties of essential oil from seeds of cumin collected from Ilkhchi, East Azarbaijan, Iran was evaluated.
Table 1.
Essential oil percentages of constituents of C. cyminumharvested from Ilkhchi–East Azarbaijan
| No. | Compounds | Empirical formula | RIa | RIb | Percentage* | Identification methods |
|---|---|---|---|---|---|---|
| 1 | α-pinene | C10H16 | 935 | 935 | 0.09 ± 0.00 | GC–MS, RI, Ref |
| 3 | Bicyclo[3.1.1]hept-2-ene, 3,6,6-trimethyl- | C10H16 | 938 | 938 | 0.18 ± 0.00 | GC–MS, RI |
| 4 | (-)-β-Pinene | C10H16 | 965 | 964 | 5.11 ± 0.10 | GC–MS, RI, Ref |
| 5 | β-Myrcene | C10H16 | 977 | 975 | 0.29 ± 0.01 | GC–MS, RI, Ref |
| 6 | α-Phellandrene | C10H16 | 983 | 986 | 0.18 ± 0.00 | GC–MS, RI, Ref |
| 7 | p -Cymene | C10H14 | 997 | 994 | 2.71 ± 0.05 | GC–MS, RI, Ref |
| 8 | d-Limonene | C10H16 | 999 | 995 | 0.40 ± 0.01 | GC–MS, RI, Ref |
| 9 | 1,8-Cineole | C10H18O | 1000 | 1002 | 0.32 ± 0.01 | GC–MS, RI, Ref |
| 10 | γ -Terpinene | C10H16 | 1044 | 1044 | 7.66 ± 0.19 | GC–MS, RI, Ref |
| 11 | cis Sabinene hydrate | C10H18O | 1091 | 1095 | 0.10 ± 0.00 | GC–MS, RI, Ref |
| 12 | cis-limonene oxide | C10H16O | 1167 | 1132 | 0.14 ± 0.00 | GC–MS, RI, Ref |
| 13 | Terpinene-4-ol | C10H18O | 1179 | 1179 | 0.14 ± 0.00 | GC–MS, RI, Ref |
| 14 | p-Mentha-1,8-dien-7-ol | C10H16O | 1197 | 1286 | 0.74 ± 0.01 | GC–MS, RI, Ref |
| 15 | Cuminal | C10H12O | 1253 | 1248 | 25.92 ± 0.49 | GC–MS, RI, Ref |
| 16 | 2-caren-10-al | C10H14O | 1297 | 1289 | 8.05 ± 0.16 | GC–MS, RI, Ref |
| 17 | 3-Caren-10-al | C10H14O | 1311 | 1311 | 47.27 ± 0.95 | GC–MS, RI, Ref |
| 18 | Bis (2-ethylhexyl) phthalate | C24H38O4 | 2092 | 2550 | 0.69 ± 0.01 | GC–MS, RI |
| Monoterpene hydrocarbons | 16.60 ± 0.37 | |||||
| Oxygenated monoterpenes | 82.68 ± 1.63 | |||||
| Others | 0.69 ± 0.01 | |||||
| Total percentage | 99.98 ± 2.01 | |||||
| Yield(% v/w) | 1.10 ± 0.10 |
GC gas chromatography, MS mass spectroscopy, RIa retention index determined on HP5-MS column, RIb retention index on HP5-MS column from the literature (Adams, 1995) and others (NIST); Ref references, those reported in literature
*Data obtained from three replication, mean ± Standard error
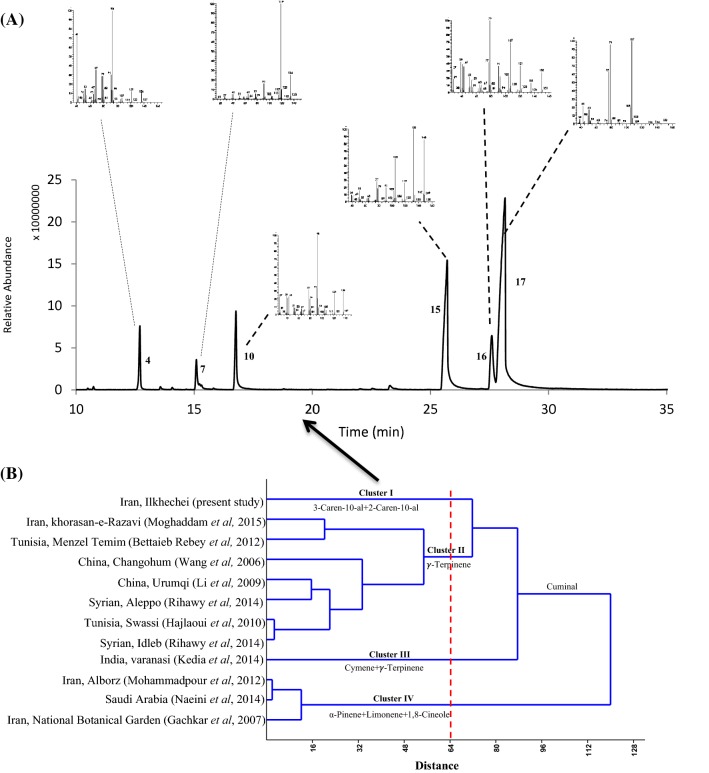
Fig. 1.
(A) GC-mass peaks and mass spectra spectrum of principal constitutes of Cuminum cyminum EO from the Ilkhchi–East Azarbaijan- Iran and (B) Dendrogram of hierarchical cluster analysis of C. cyminum EOs based on the chemical composition of our EO and those from the literature
Hierarchical cluster analysis (HCA)
HCA was performed based on 143 components for determining the novelty of the studied cumin chemovar (Fig. 1B). Dendrogram obtained based on the comparisons between the studied essential oil sample and those in the literature (Gachkar et al., 2007; Hajlaoui et al., 2010; Kedia et al., 2014; Li et al., 2009; Moghaddam et al., 2015; Mohammadpour et al., 2012; Naeini et al., 2014; Rebey et al., 2012; Rihawy et al., 2014; Wang et al., 2006) showed that the highest phytochemical distance obtained in ~ 120 units. Cumin essential oils studied by different researchers and from different locations were categorized into four groups in distance of 64 units. As it is evident in Fig. 1B, the studied essential oil made a separate group distinct from all the other reported essential oils in the literature. So, this is really a new chemovar and is differentiated by 3-caren-10-al/cuminal/2-caren-10-al (Cluster I) components. The second group (Cluster II), exemplified by seven samples, had cuminal/γ-terpinene/geranial as the major compounds (Gachkar et al., 2007; Hajlaoui et al., 2010; Li et al., 2009; Moghaddam et al., 2015; Rebey et al., 2012; Rihawy et al., 2014; Wang et al., 2006). Only one sample was in cluster III with cymene/γ-terpinene as its main compound (Kedia et al., 2014). Three samples grouped in Cluster IV have α-Pinene/Limonene/1, 8-cineole as their major compounds (Gachkar et al., 2007; Mohammadpour et al., 2012; Naeini et al., 2014). Major constituents of the reported cumin essential oils are shown in Table 2. Results of hierarchical cluster analysis of essential oil constituents showed that caren-10-al/cuminal are its dominant components, which has not been reported in previous studies (Gachkar et al., 2007; Hajlaoui et al., 2010; Kedia et al., 2014; Li et al., 2009; Moghaddam et al., 2015; Mohammadpour et al., 2012; Naeini et al., 2014; Rebey et al., 2012; Rihawy et al., 2014; Wang et al., 2006), so it is identified as a new chemovar. Doimo et al. (1999), based on the predominance of α-pinene and tasmanone in Eucalyptus cloeziana F. Muell. Essential oil, identified a new chemovar. Having high percentage of only a few components is one of the most important factors when bringing any product to market and industry.
Table 2.
Major constitute of essential oil of C. cyminum in literature
| Syrian-Idleb | Syrian-Aleppo | Tunisia-Swassi | China-Changohum | ||||
|---|---|---|---|---|---|---|---|
| Rihawy et al. (2014) | Rihawy et al. (2014) | Hajlaoui et al. (2010) | Wang et al. (2006) | ||||
| Cuminaldehyde | 38.5 | Cuminaldehyde | 32.8 | Cuminaldehyde | 39.48 | Cuminaldehyde | 45.75 |
| γ-Terpinene | 12.62 | γ-Terpinene | 16.3 | γ-Terpinene | 15.21 | 2-Ethylidene-6-methyl-3,5-heptadienal | 12.91 |
| β-Pinene | 11.5 | β-Pinene | 10.3 | O-Cymene | 11.82 | -Proyl-benzenemethanol | 12.76 |
| O-Cymene | 10.98 | O-Cymene | 9.74 | β-Pinene | 11.13 | 1-Methyl-4-(1-methylethyl)-1,4-cyclohexadiene | 7.54 |
| 2-Caren-10-al | 7.35 | 2-Caren-10-al | 6.24 | 2-Caren-10-al | 7.93 | Pulegone | 4.64 |
| Iran-National Botanical Garden | China-Urumqi | India-varanasi | Tunisia-Menzel Temim | ||||
| Gachkar et al. (2007) | Li et al. (2009) | Kedia et al. (2014) | Bettaieb Rebey et al. (2012) | ||||
| α-Pinene | 29.1 | Cuminaldehyde | 29.84 | Cymene | 47.08 | γ-Terpinene | 25.58 |
| Limonene | 21.5 | γ-Terpinene | 17.07 | γ-Terpinene | 19.36 | 1-Phenyl-1,2-ethanediol | 23.16 |
| Linalool | 17.9 | 2-Ethylidene-6-methyl-3,5-heptadienal | 8.2 | Cuminaldehyde | 14.92 | Cuminaldehyde | 15.31 |
| 1,8-Cyneole | 10.4 | -Proyl-benzenemethanol | 7.84 | Laevo-β-pinene | 11.5 | β-Pinene | 15.16 |
| Lynalyl acetate | 4.8 | 1-Methyl-4-(1-methylethyl)-1,4-cyclohexadiene | 5.87 | L-Limonene | 1.05 | P-Cymene | 9.05 |
| Iran-khorasan-e-Razavi | Saudi-Arabia | Iran-Ilkhchi | Iran-Alborz | ||||
| Moghaddam et al. (2015) | Naeini et al. (2014) | Mohammadpour et al. (2012) | |||||
| γ-Terpinene | 24.3 | α-Pinene | 30 | 3-Caren-10-al | 47.27 | α-Pinene | 29.2 |
| Cuminaldehyde | 21.07 | Limonene | 21 | Cuminaldehyde | 25.92 | Limonene | 21.7 |
| P-Cymene | 16.56 | 1,8-Cyneole | 18.5 | 2-Caren-10-al | 8.05 | 1,8-Cyneole | 18.1 |
| β-Pinene | 13.74 | Lynalool | 10 | γ-Terpinene | 7.66 | Linalool | 10.5 |
| Safranal | 12.59 | Linalyl acetate | 4 | β-Pinene | 5.11 | Linalyl acetate | 4.8 |
Antifungal activity of cumin essential oil
The results of antifungal bioassays showed that essential oil had a good inhibitory effect on mycelial growth of the tested fungi at p < 0.01 (Fig. 2A). Inhibitory effect of essential oil was concentration dependent. In the case of P. expansum, essential oil was completely inhibited mycelial growth at concentrations ≥ 1000 µL/L, and inhibitory effects were fungistatic, non-fungicidal, while in the case of B. cinereaand A. niger, it was happened at concentrations ≥ 750 µL/L and inhibitory effects were fungicidal.
Fig. 2.
Mycelial growth inhibition (%) (mean ± standard error) of the studied fungi as a base of EO concentration and antifungal activity of different concentration of EO. Penicillim expansum (a), Botrytis cinerea (b), Aspergillus niger (c)
The cumulative curves of mycelial growth diameter (MGD) of the tested fungi are shown in Fig. 3A–C. MGD was significantly affected by increasing in concentrations of essential oil at p < 0.01. Also, the reaction of different fungal species to essential oil was varied. Aspergillus niger was more susceptible and Pencillium expansum was less susceptible (Fig. 2).
Fig. 3.
Mycelial growth diameter (cm) and their means comparisons base of different concentration of essential oil (µL/L−1) and incubation time (Day). Penicillim expansum (A), Botrytis cinerea (B), Aspergillus niger (C)
Cumin seeds are used locally in Iran as a fragrant agent for medicinal and aromatic purposes. For example, recently the use of cumin seed as a natural preservative agent in processed cheese is expanded. In the present study, essential oil exhibited notable antifungal activity and can be used as the natural preservative agent. Antifungal property of essential oil is related with its constituents. Probably 3-caren-10-al, cuminal and 2-caren-10-al with high percentage have a major role in antifungal property. A high percentage of 3-Caren-10-al, cuminal and 2-caren-10-al with aldehyde (CH=O) groups were detected in cumin essential oil. Cell wall of pathogens is the main target of phenolic compounds. These compounds may disrupt the permeability barrier of cell membrane and prevent respiration. Hydrophobic property of essential oil components enables these compounds to penetrate into hydrophobic structure (lipid) of fungal cell membrane and mitochondria as a result disturbing their structure with accumulation of these compounds in the cell membrane of pathogen, energy deletion can be increase (Cox et al., 2001). In addition, metabolic pathways of microorganisms may be affected by essential oil. Phenolic compounds in low concentration disrupt proteins and in high concentrations damaged the enzymes outbreak in production of energy (Nychas, 1995). Farrag et al. (1989) mentioned that the antimicrobial activity of essential oils could be related to the presence of an aromatic nucleus and OH group that can affect hydrogen bonds of enzymes in microorganisms. The carbon atom in aldehyde groups could produce partial negative free electrons that plays a key role in forming covalent bonds with microorganisms, which prevent their growth (Asghari Marjanlo et al., 2009). Our in vitro antifungal assays demonstrated that cumin seed essential oil could consider as a candidate for natural antifungal agent in food preservation technology.
Antioxidant activity assessment (FRAP and DPPH scavenging)
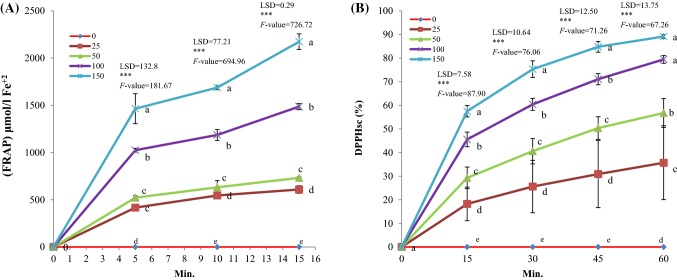
Figure 4A, B shows the antioxidant activity of Cumin essential oil (25–150 µL of EO) in comparison with control (p < 0.01). Based on FRAP method total antioxidant capacity (expressed as (FRAP) μmol/L Fe+2 of EO at three incubation times (5, 10 and 15 min) was increased based on concentration (Fig. 4A). In all of the incubation times, essential oil in the concentration of 150 µL exhibiting strong reducing power (1490–2200 (FRAP) μmol/L Fe+2). Also according to DPPH scavenging (DPPHsc %), the similar results was obtained. EO of C. cyminum had a significant scavenging effect at all the incubation times (15, 30 and 60 min) in comparison with control (p < 0.01). Essential oil in the concentration of 150 µL showed the highest antioxidant power (Fig. 4B). Antioxidant capacity at 150 µL of essential oil was higher (approximately threefold) than its capacity at 25 µL of essential oil. Antioxidants are substances that may protect the organism from damage caused by unstable molecules known as free radicals. There is a growing demand for natural sources of antioxidants. In the present study finding chemovar of cumin showed higher antioxidant power. This property can be made it as a good source of natural antioxidant. Essential oils possess different antioxidant capacities due to their diverse chemical structures (Dapkevicius et al., 2002). Normally monoterpene hydrocarbons are more active antioxidants than sesquiterpenes and non-isoprenoid components. Studies on antioxidant activity of different kinds of monoterpenes revealed that none of them are stronger than oxygenated monoterpenes (Zengin and Baysal, 2014). In this study, the major kind of terpenoid of identified components of essential oil was oxygen monoterpenes (82.68%). Among these monoterpenes, 3-caren-10-al, cuminal, γ-terpinene, p-cymene and β-pinene were noted, which previous studies have also identified that these monoterpenes and their antioxidant activity (Chen et al., 2014).
Fig. 4.
Antioxidant activity of different concentration of EO during studied time. (FRAP) µmol/L Fe+2 (A), DPPHsc (%) (B)
Acknowledgements
This research was supported by Urmia University, Urmia, Iran.
References
- Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. Allured Publ. Corp. Carol Stream IL. USA. Pp. 469 (1995)
- Adorjan B, Buchbauer G. Biological properties of essential oils: An updated review. Flavour Fragr. J. 2010;6:407–426. doi: 10.1002/ffj.2024. [DOI] [Google Scholar]
- Agrios GN. Plant Pathology. 5th ed. Elsevier Academic Press, USA. Pp. 952 (2005)
- Aktar W, Sengupta D, Chowdhury A. Impact of Pesticides Use in Agriculture: Their Benefits and Hazards. Interdiscip Toxicol. 2009;2:1–12. doi: 10.2478/v10102-009-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alinian S, Razmjoo J, Zeinali H. Flavonoids, anthocynins, phenolics and essential oil produced in cumin (Cuminum cyminum L.) accessions under different irrigation regimes. Ind Crops Prod. 81: 49–55 (2016)
- Asghari Marjanlo A, Mostofi Y, Shoeibi S, Fattahi M. Effect of Cumin Essential Oil on Postharvest Decay and Some Quality Factors of Strawberry. J. Med. Plants. 2009;3:25–43. [Google Scholar]
- Benzie IFF, Strain JJ. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- Chen S, Rotaru AE, Liu F, Philips J, Woodard TL, Nevin KP, Lovley DR. Carbon cloth stimulates direct interspecies electron transfer in syntrophic co-cultures. Bioresour Technol. 2014;173:82–86. doi: 10.1016/j.biortech.2014.09.009. [DOI] [PubMed] [Google Scholar]
- Chiou A, Karathanos VT, Mylona A, Salta FN, Preventi F, Andrikopoulos NK. Currants (Vitis vinifera L.) content of simple phenolics and antioxidant activity. Food Chem. 102: 516–522 (2007)
- Cox SD, Mann CM, Markham JL. Interactions between components of the essential oil of Melaleuca alternifolia. J Appl Microbial. 2001;91(3):492–497. doi: 10.1046/j.1365-2672.2001.01406.x. [DOI] [PubMed] [Google Scholar]
- Cuce M, Sokmen A. In vitro production protocol of Vaccinium uliginosum L. (bog bilberry) growing in the Turkish flora. Turk J Agric For. 41: 294–304 (2017).
- Dapkevicius A, Van Beek TA, Lelyveld GP, Van Veldhuizen A, De Groot A, Linssen JPH, Venskutonis R. Isolation and structure elucidation of radical scavengers from Thymus vulgaris leaves. J. Nat. Prod. 2002;65:892–896. doi: 10.1021/np010636j. [DOI] [PubMed] [Google Scholar]
- de Sousa LL, de Andrade SCA, Athayde AJAA, de Oliveira CEV, de Sales CV, Madruga MS, de Souza EL. Efficacy of Origanum vulgare L. and Rosmarinus officinalis L. essential oils in combination to control postharvest pathogenic Aspergilli and autochthonous mycoflora in Vitis labrusca L. (table grapes). Int J Food Microbiol. 165: 312–318 (2013) [DOI] [PubMed]
- Doimo L, Fletcher R J, D’Arcy BR, Southwell IA. A New Chemovar of Gympie Messmate (Eucalyptus cloeziana F. Muell.) Containing α-Pinene and Tasmanone. Oil Res. 11(1): 77–78 (1999)
- Farag RS, Daw ZY, Abo-Raya SH. Influence of some spice essential oils on Aspergillus parasiticus growth and production of aflatoxins in a synthetic medium. J Food Sci. 1989;54(1):74–76. doi: 10.1111/j.1365-2621.1989.tb08571.x. [DOI] [Google Scholar]
- Fenoll J, Sabater P, Navarro G, Vela N, Pérez-Lucas G, Navarro S. Abatement kinetics of 30 Sulfonylurea herbicide residues in water by photocatalytic treatment with semiconductor materials. J Environ Manage. 2013;130:361–368. doi: 10.1016/j.jenvman.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Gachkar L, Yadegari D, Rezaei MB, Taghizadeh M, Astaneh SA, Rasooli I. Chemical and biological characteristics of Cuminum cyminum and Rosmarinus officinalis essential oils. Food Chem. 2007;102:898–904. doi: 10.1016/j.foodchem.2006.06.035. [DOI] [Google Scholar]
- Hajlaoui H, Mighri H, Noumi E, Snoussi M, Trabelsi N, Ksouri R, Bakhrouf A. Chemical composition and biological activities of Tunisian Cuminum cyminum L. essential oil: A high effectiveness against Vibrio spp. strains. Food Chem Toxicol. 48: 2186–2192 (2010) [DOI] [PubMed]
- Hammer Ø, Harper DATaT, Ryan PD. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica. 4: 1–9 (2001)
- Jhalegar MJ, Sharma RR, Singh D. Antifungal efficacy of botanicals against major postharvest pathogens of Kinnow mandarin and their use to maintain postharvest quality. Fruits. 2014;69(3):223–237. doi: 10.1051/fruits/2014012. [DOI] [Google Scholar]
- Kedia A, Prakash B, Mishra PK, Chanotiya CS, Dubey NK. Antifungal, antiaflatoxigenic, and insecticidal efficacy of spearmint (Mentha spicata L.) essential oil. Int Biodeterior Biodegradation. 89: 29–36 (2014)
- Li XM, Tian SL, Pang ZC, Shi JY, Feng ZS, Zhang YM. Extraction of Cuminum cyminum essential oil by combination technology of organic solvent with low boiling point and steam distillation. Food Chem. 2009;115:1114–1119. doi: 10.1016/j.foodchem.2008.12.091. [DOI] [Google Scholar]
- Moghaddam M, Miran SNK, Pirbalouti AG, Mehdizadeh L, Ghaderi Y. Variation in essential oil composition and antioxidant activity of cumin (Cuminum cyminum L.) fruits during stages of maturity. Ind Crops Prod. 70: 163–169 (2015)
- Mohammadpour H, Moghimipour E, Rasooli I, Fakoor MH, Astaneh SA, Moosaie SS, Jalili Z. Chemical composition and antifungal activity of Cuminum cyminum L. essential oil from Alborz mountain against Aspergillus species. Jundishapur J Nat Pharm Prod. 7: 50–55 (2012) [PMC free article] [PubMed]
- Naeini A, Jalayer Naderi N, Shokri H. Analysis and in vitro anti-Candida antifungal activity of Cuminum cyminum and Salvadora persica herbs extracts against pathogenic Candida strains. J Mycol Med. 2014;24:13–18. doi: 10.1016/j.mycmed.2013.09.006. [DOI] [PubMed] [Google Scholar]
- Nakajima J, Tanaka I, Seo S, Yamazaki M, Saito K. LC/PDA/ESI-MS profiling and radical scavenging activity of anthocysnins in various berries. J Biomed Biotechnol. 2004;5:241–247. doi: 10.1155/S1110724304404045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nostro A, Cellini L, Di Bartolomeo S, Di Campli E, Grande R, Cannatelli MA, Alonzo V. Antibacterial effect of plant extracts against Helicobacter pylori. Phytother. Res. 2005;19:198–202. doi: 10.1002/ptr.1640. [DOI] [PubMed] [Google Scholar]
- Nychas GJE. Natural antimicrobials from plants. pp. 58–89. In: New Methods of Food Preservation. London, United Kingdom. Blackie Academic and Professional, London, United Kingdom (1995)
- Radušienė J, Karpavičienė B, Stanius Ž. Effect of external and internal factors on secondary metabolites accumulation in St. John’s worth. Bot Lith. 2012;18(2):101–108. [Google Scholar]
- Rebey I, Jabri-Karoui I, Hamrouni-Sellami I, Bourgou S, Limam FMB. Effect of drought on the biochemical composition and antioxidant activities of cumin (Cuminum cyminum L.)seeds. Ind Crops Prod. 36: 238–245 (2012)
- Rihawy MS, Bakraji EH, Odeh A. PIXE and GC-MS investigation for the determination of the chemical composition of Syrian Cuminum cyminum L. Appl. Radiat. Isot. 2014;86:118–125. doi: 10.1016/j.apradiso.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Sengul M, Ercisli S, Yildiz H, Gungor N, Kavaz A, Cetin B. Antioxidant, antimicrobial activity and total phenolic content with the aerial parts of Artemisia absinthum, Artemisia santonicum and Saponaria officinalis. Iran J Pharm Res. 2011;10(1):49–55. [PMC free article] [PubMed] [Google Scholar]
- Sonker N, Pandey AK, Singh P. Strategies to control post-harvest diseases of table grape: a review. J Wine Res. 2016;27(2):105–122. doi: 10.1080/09571264.2016.1151407. [DOI] [Google Scholar]
- Sparkman OD. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy Robert P. Adams. J Am Soc Mass Spectrom. 2005;16:1902–1903. doi: 10.1016/j.jasms.2005.07.008. [DOI] [Google Scholar]
- Tian J, Zeng X, Feng Z, Miao X, Peng X, Wang Y. Zanthoxylum molle Rehd. essential oil as a potential natural preservative in management of Aspergillus flavus. Ind Crops Prod. 60: 151–159 (2014)
- Vijayan K, Chakraborti SP, Ercisli S, Ghosh PD. NaCI induced morpho-biochemical and anatomical changes in mulberry (Morus spp.). Plant Growth Regul. 56 (1): 61–69 (2008).
- Wang Z, Ding L, Li TC, Zhou X, Wang L, Zhang H, He H. Improved solvent-free microwave extraction of essential oil from dried Cuminum cyminum L. and Zanthoxylum bungeanum Maxim. J. Chromatogr. A 1102: 11–17 (2006) [DOI] [PubMed]
- Yu C, Zeng L, Sheng K, Chen F, Zhou T, Zheng X, Yu T. Aminobutyric acid induces resistance against Penicillium expansum by priming of defence responses in pear fruit. Food Chem. 2014;159:29–37. doi: 10.1016/j.foodchem.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Zengin H, Baysal AH. Antibacterial and antioxidant activity of essential oil terpenes against pathogenic and spoilage-forming bacteria and cell structure-activity relationships evaluated by SEM microscopy. Molecules. 2014;19:17773–17798. doi: 10.3390/molecules191117773. [DOI] [PMC free article] [PubMed] [Google Scholar]