Abstract
There is mounting evidence for a role of the growth factor granulocyte-macrophage colony-stimulating factor (GM-CSF) in inflammatory disease, including arthritis. In the present study, we examined the effectiveness of treatment of collagen-induced arthritis (CIA) with a neutralizing mAb to GM-CSF. DBA/1 mice were immunized for the development of CIA and treated at different times, and with different doses, with neutralizing mAb to GM-CSF or isotype control mAb. Anti-GM-CSF mAb treatment prior to the onset of arthritis, at the time of antigen challenge, was effective at ameliorating the ensuing disease. Modulation of arthritis was seen predominantly as a reduction in overall disease severity, both in terms of the number of limbs affected per mouse and the clinical score of affected limbs. Importantly, anti-GM-CSF mAb treatment ameliorated existing disease, seen both as a reduction in the number of initially affected limbs progressing and lower numbers of additional limbs becoming affected. By histology, both inflammation and cartilage destruction were reduced in anti-GM-CSF-treated mice, and the levels of tumor necrosis factor-a and IL-1? were also reduced in joint tissue washouts of these mice. Neither humoral nor cellular immunity to type II collagen, however, was affected by anti-GM-CSF mAb treatment. These results suggest that the major effect of GM-CSF in CIA is on mediating the effector phase of the inflammatory reaction to type II collagen. The results also highlight the essential role of GM-CSF in the ongoing development of inflammation and arthritis in CIA, with possible therapeutic implications for rheumatoid arthritis.
Keywords: antibodies, inflammatory mediators, in vivo animal models, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic, inflammatory disease characterized by the presence of numerous inflammatory mediators and the destruction of diarthrodial joints. GM-CSF was initially discovered for its role in the differentiation of hemopoietic precursor cells into mature granulocytes and macrophages [1]; however, it can also affect mature cell function and may be considered pro-inflammatory [2,3]. There is some evidence to suggest that GM-CSF may play a role in the pathogenesis of RA [4,5,6].
CIA is a commonly used model for RA, with which it has many immunologic and pathologic parallels [7]. In the present study, we examined the temporal requirement for GM-CSF in CIA by treating mice with a neutralizing mAb. The findings show that anti-GM-CSF mAb treatment can ameliorate arthritis not only when given prior to disease onset, but also post-onset.
Materials and methods
Collagen-induced arthritis
Male DBA/1 mice (ARC, Canning Vale, Western Australia, Australia), were immunized for CIA as previously described [8,9,10,11] (see Supplementary material). Mice were scored for arthritis using a scale of 0 (normal) to 3 (joint distortion and/or rigidity) for each limb. Antibodies to type II collagen (CII) were measured in serum by ELISA [9].
Paraffin-embedded sections of rear limbs were evaluated for infiltration of cells, cartilage damage and bone erosions (H & E stain), all scored separately from 0 (normal) to 3 (severe). Proteoglycan loss was also evaluated (safranin O, fast green stain), and scored from 0 (normal) to 3 (complete loss of staining) [12]. These scores were summed to give an overall histologic score out of 12.
mAb treatment
Neutralizing anti-mouse GM-CSF mAb (22E9.11 [13]; Dr J. Abrams, DNAX Research Institute, Palo Alto, CA, USA) and isotype control mAb (GL117.41; DNAX Research Institute) were purified on a protein G-sepharose column. Mice were treated intraperitoneally (i.p.) with 300 ?g of the appropriate mAb at different times (see Results and Supplementary material).
T-cell proliferation assay
Cells from inguinal lymph nodes were isolated from mice treated with mAb, and the proliferative response to CII was measured by [3H]thymidine incorporation [10] (see Supplementary material).
Cytokine ELISAs
Following sacrifice on day 35 after immunization, the tendons and synovium from the ankle joints of the hind limbs were dissected free from the surrounding tissue, cytokines were eluted for 1 hour at room temperature and supernatants were collected [14]. GM-CSF, tumor necrosis factor-a (TNFa) and IL-1? levels were measured in ankle joint tissue washouts and serum by ELISA (see Supplementary material).
Statistics
The Mann-Whitney two-sample rank test was used for differences in clinical and histologic scores, cytokine and anti-CII levels, and T-cell proliferation between treatment groups. The Spearman correlation coefficient was used for correlations, and the chi-squared test or two-way analysis of variance was used for differences in the number, clinical score and histologic features of individual limbs. P = 0.05 was considered significant.
Results
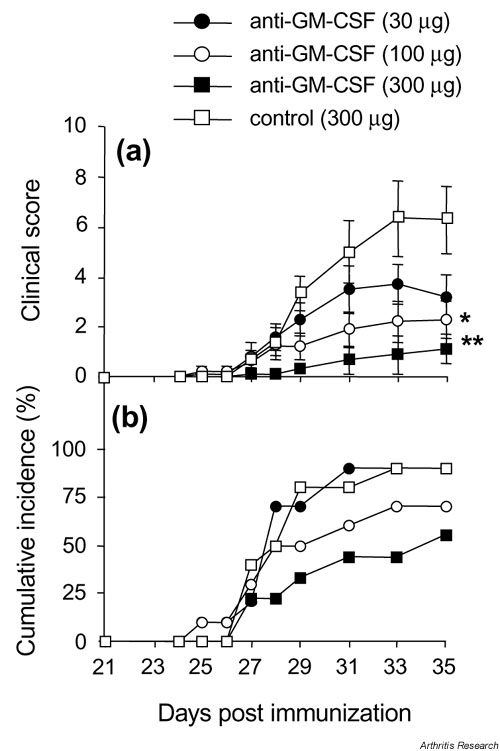
GM-CSF blockade prior to onset of arthritis results in less severe disease
Mice were treated i.p. with increasing doses of anti-GM-CSF (30, 100, and 300 ?g) beginning at day 21, and then every second day until day 31. Control mice received the highest dose (300 ?g) of control mAb. There was a dose-related suppression of the clinical score, with mice receiving 300 ?g anti-GM-CSF mAb showing a marked reduction of disease (Fig. 1a). This decrease in severity was seen both as a reduction in the number of affected limbs per mouse and as lower clinical scores for these affected limbs (see Supplementary Fig. 1). However, while there was a trend towards a lower incidence of arthritis with increasing doses of anti-GM-CSF mAb, this did not reach significance (Fig. 1b).
Figure 1.
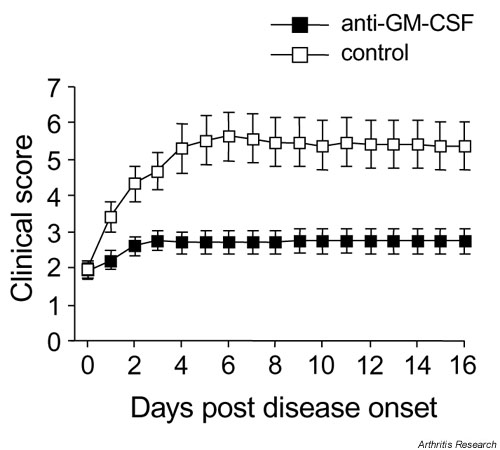
Effect of anti-GM-CSF treatment prior to the onset of arthritis. (a) Severity (mean clinical scores ? SEM). (b) Cumulative incidence. Mice were treated intraperitoneally every second day from days 21 to 31 with anti-GM-CSF mAb or control mAb. *P = 0.05 and **P = 0.01 compared with control mAb-treated. GM-CSF, Granulocyte macrophage-colony stimulating factor; mAb, monoclonal antibody.
Supplementary Figure 1.
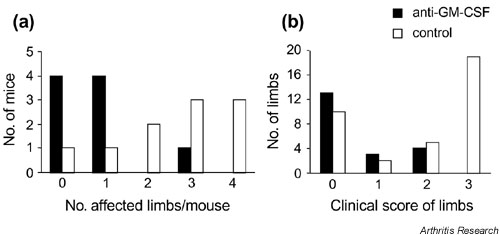
Effect of anti-GM-CSF treatment prior to the onset of arthritis on individual limb involvement. Data are from the experiment in Fig. 1 where mice were treated intraperitoneally with either 300 ?g anti-GM-CSF or 300 ?g isotype control every second day from days 21 to 31. (a) The number of mice that developed arthritis in a given number of limbs is presented. Anti-GM-CSF (n = 9) versus isotype control mice (n = 10) (P = 0.048; multiway chi-squared). There were no anti-GM-CSF-treated mice with all four limbs affected. (b) The number of individual limbs from arthritic mice only with a particular clinical score (severity) is presented. For the anti-GM-CSF-treated group, n = 20 limbs (5 mice); for the isotype control-treated group, n = 36 limbs (9 mice). There were no limbs from anti-GM-CSF-treated mice with a clinical score of 3. GM-CSF, Granulocyte macrophage-colony stimulating factor.
Decreasing the number of treatments resulted in a reduced effect on disease outcome (see Supplementary Fig. 2a).
Supplementary Figure 2.
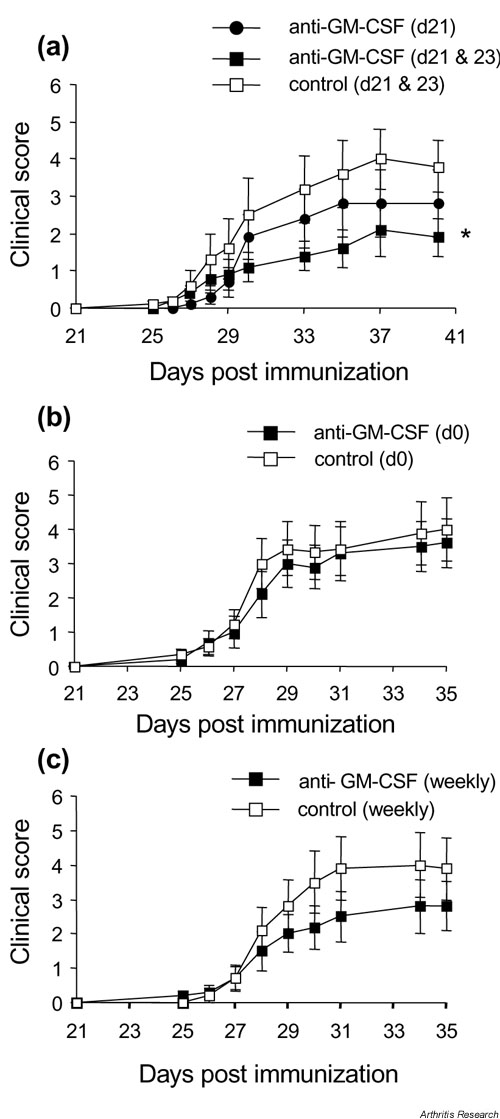
Effect of timing of anti-GM-CSF treatment on arthritis development. (a) Mice treated on day 21 (n = 17, anti-GM-CSF) or days 21 and 23 (n = 20, anti-GM-CSF; n = 20, isotype control). * Days 21 and 23 treatment, anti-GM-CSF treated versus isotype control treated mice (day 40) (P = 0.044; Mann-Whitney test). (b) Mice treated at the time of primary immunization (day 0) only (n = 10, anti-GM-CSF; n = 9, isotype control). (c) Mice treated at the time of primary immunization (day 0) then weekly until day 21 (n = 10, anti-GM-CSF; n = 10, isotype control). For all treatments, 300 ?g anti-GM-CSF or isotype control was used. Results are expressed as the mean ? SEM. GM-CSF, Granulocyte macrophage-colony stimulating factor.
Anti-GM-CSF mAb treatment before the booster injection (day 21), when the CII-specific immune response was developing, had no significant effect on disease (see Supplementary Fig. 2b,c).
GM-CSF blockade prevents the clinical progression of established arthritis
After observing that anti-GM-CSF could be beneficial when given appropriately prior to the onset of clinical arthritis, its therapeutic effect was sought by beginning treatment after the onset of clinical arthritis. Once mice had clinical signs of inflammation they were paired according to their arthritic score and assigned to either the anti-GM-CSF mAb or control mAb treatment group. Mice were treated i.p. daily (300 ?g) from the day of disease onset (day 0) to day 9 after onset (i.e. a total of 10 times).
Mice injected with anti-GM-CSF mAb showed no major progression of disease, with the mean clinical score not significantly different on day 10 compared with day 0 (disease onset) (2.8 ? 0.4 versus 1.9 ? 0.2, respectively; P > 0.05) (Fig. 2). Control mAb-treated mice, in contrast, showed typical disease progression with a significantly higher mean clinical score on day 10 compared with day 0 (5.4 ? 0.7 versus 1.9 ? 0.2, respectively; P = 0.0001) (Fig. 2). Anti-GM-CSF mAb-treated mice thus developed significantly milder disease than control mAb-treated mice (day 10, 2.8 ? 0.4 versus 5.4 ? 0.7; P = 0.007). There was no increase in the severity of arthritis following cessation of treatment with either mAb (day 10) (Fig. 2).
Figure 2.
Effect of anti-GM-CSF treatment on established arthritis. Mice were treated daily (days 0-9) with anti-GM-CSF mAb or control mAb. Results are expressed as the mean ? SEM (P = 0.007). GM-CSF, Granulocyte macrophage-colony stimulating factor; mAb, monoclonal antibody.
This absence of disease progression was seen as a relative lack of an increase in the clinical score of initially affected limbs following anti-GM-CSF treatment. Furthermore, anti-GM-CSF-treated mice showed a diminished recruitment of additional limbs that were normal at the commencement of treatment (see Supplementary Fig. 3).
Supplementary Figure 3.
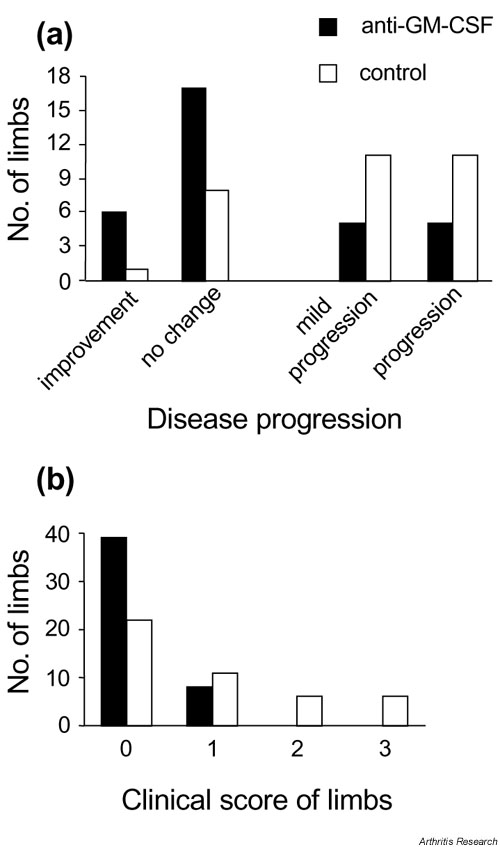
Effect of anti-GM-CSF treatment post-onset on individual limb involvement. Data are from the experiment in Fig. 2 where mice were treated intraperitoneally with 300 ?g anti-GM-CSF (n = 20 mice) or isotype control (n = 19 mice) daily from the day of disease onset (day 0) until day 9 (total, 10 times). (a) The progression of arthritis in limbs already affected prior to the commencement of treatment is presented: improvement, decrease in clinical score of individual limbs following treatment; no change, clinical score of individual limbs remained the same following treatment; mild progression, clinical score of individual limbs progressed from 1 initially to 2 at the end of treatment; progression, clinical score of individual limbs progressed from 1 or 2 initially to 3 at the end of treatment (n = 33 limbs [from 20 mice], anti-GM-CSF-treated group; and n = 31 limbs [from 19 mice], isotype control-treated group) (P = 0.01; multiway chi-squared). (b) The number of limbs, initially unaffected (clinical score 0, day 0), that developed a particular score at the end of the experiment is presented (n = 47 limbs [from 20 mice], anti-GM-CSF-treated group; and n = 45 limbs [from 19 mice], control-treated group) (P = 0.0007; multiway chi-squared). There were no limbs recruited in anti-GM-CSF-treated mice that developed a clinical score > 1. GM-CSF, Granulocyte macrophage-colony stimulating factor.
Reduced histopathology of joints following GM-CSF blockade
Both inflammation and joint destruction are aspects of arthritis, and while treatment may alleviate one aspect (e.g. inflammation) it may have no effect on another (e.g. joint destruction) [12]. The histologic features of infiltration, cartilage damage, proteoglycan depletion and bone erosions were lower in anti-GM-CSF mAb-treated mice compared with control mAb-treated mice (P < 0.0001, two-way analysis of variance; data not shown). The mean total score for the anti-GM-CSF mAb-treated group was significantly lower than for control mAb-treated mice when the histologic scores for each feature were combined for each treatment group (3.7 ? 0.5 versus 5.9 ? 0.8; P = 0.03). Figure 3 shows a normal joint and representative joints from arthritic mice following treatment with either control mAb or anti-GM-CSF mAb. There is less cellular infiltration and cartilage destruction in the anti-GM-CSF mAb-treated mouse compared with the control-treated mouse, in parallel with the reduction in clinical score.
Figure 3.
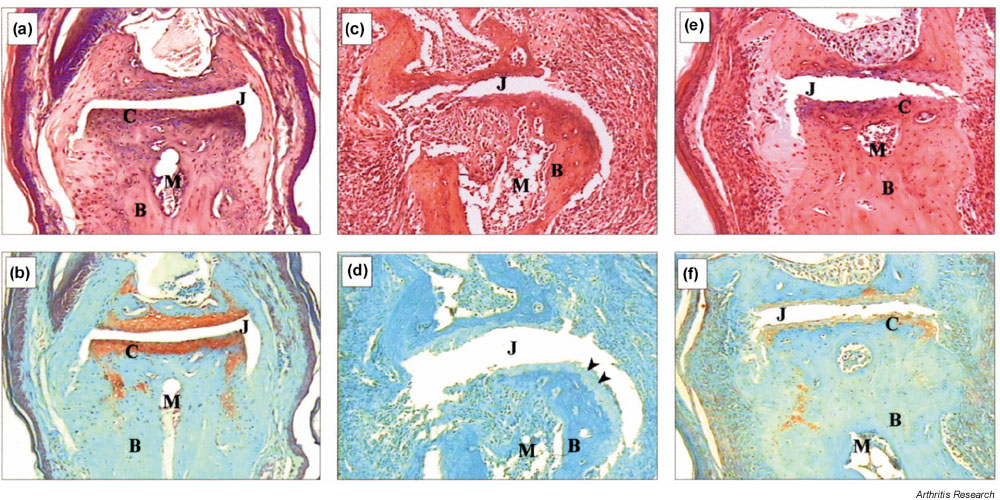
Effect of anti-GM-CSF treatment post-onset on joint histopathology of hind-limb distal interphalangeal joints with CIA. (a), (b) Normal joint; (c), (d) joint from a control mAb-treated mouse; and (e), (f) joint from an anti-GM-CSF mAb-treated mouse. For control and anti-GM-CSF mAb-treated mice, both limbs had a clinical score of 1 at onset. The control mAb-treated mouse limb progressed to a score of 3, whereas the anti-GM-CSF mAb-treated mouse limb showed no change over the course of treatment. These results are representative for each treatment group. (c) Severe inflammation and joint destruction is shown compared with (a). (e) Only mild infiltration and damage is evident with the architecture of the joint remaining intact. This is also reflected in (d), where there is loss of proteoglycan staining (arrowheads) compared with (b), while (f) shows intermediate staining. (a), (c) and (e) H & E staining; (b), (d) and (f) Safranin O, fast green staining. Magnification, x 125. B, Bone; C, cartilage; CIA, collagen-induced arthritis; GM-CSF, granulocyte macrophage-colony stimulating factor; J, joint space; M, bone marrow; mAb, monoclonal antibody.
Humoral and cellular response to CII following GM-CSF blockade in vivo
CIA development is dependent on a B-cell and T-cell response [10,15], and GM-CSF is implicated in the development of antigen presenting cells [16]. Treatment with anti-GM-CSF mAb had no effect on the anti-CII IgG response, nor the proliferative response of T cells to CII (see Supplementary Fig. 4).
Supplementary Figure 4.
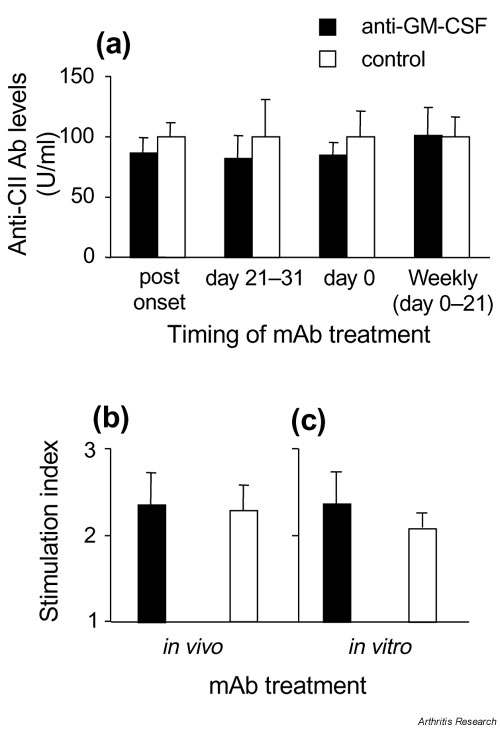
Effect of anti-GM-CSF treatment on CII-specific immune responses. (a) IgG Ab response to CII measured at the end of each experiment. Results are expressed as the mean ? SEM, where the mean level of anti-CII IgG for isotype control-treated mice was set at 100 U/ml for each experiment and compared with anti-GM-CSF-treated mice from the same experiment. (b) Proliferative response to CII measured in mice treated in vivo with 300 ?g of anti-GM-CSF or control mAb from every second day from days 21 to 31. (c) Proliferative response to CII measured in mice treated in vivo with control mAb, as in (b), except cells were cultured in the presence of 100 ?g/ml anti-GM-CSF or control mAb. (b), (c) Results are shown for cells cultured in the presence of 50 ?g/ml CII, and are expressed as a stimulation index (mean ? SEM) above background readings in the absence of CII (Stimulation Index = 1). CII, type II collagen; GM-CSF, Granulocyte macrophage-colony stimulating factor; mAb, monoclonal antibody.
Reduced cytokine levels in joint tissues following GM-CSF blockade in vivo
GM-CSF was only detectable in the joint washouts of mice with arthritis. Levels tended to be lower in anti-GM-CSF mAb-treated mice than control mAb-treated mice (Fig. 4).
Figure 4.
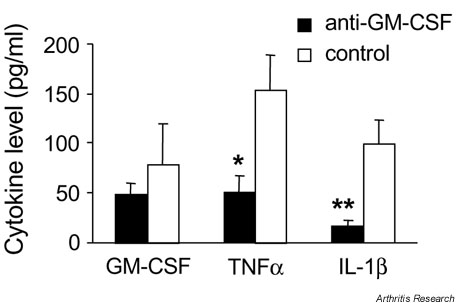
Effect of anti-GM-CSF treatment on cytokine levels in the joints. GM-CSF, TNFa and IL-1? levels were measured by enzyme-linked immunosorbent assay in washouts from ankle joints of arthritic mice for mice treated with anti-GM-CSF or control mAb every second day from days 21 to 31 post immunization. Results are expressed as the mean level ? SEM (pg/ml). *P = 0.02 and **P = 0.007 compared with control mAb-treated mice. GM-CSF, Granulocyte macrophage-colony stimulating factor; IL-1?, interleukin-1?; mAb, monoclonal antibody; TNFa, tumor necrosis factor-a.
There was also a significant reduction in the mean levels of both TNFa and IL-1? in the joint washouts for anti-GM-CSF mAb-treated mice compared with control mAb-treated mice (P = 0.02 and P = 0.007, respectively) (Fig. 4).
None of the cytokines could be detected in the serum of any of the mice at any time point measured for the various treatment regimes (data not shown).
Discussion
There is mounting evidence for a role of GM-CSF in inflammatory disease, including arthritis. In the present study, we confirm the essential role of GM-CSF in ongoing development of inflammation and arthritis in CIA using a neutralizing mAb that blocks GM-CSF. We show that it is not only possible to ameliorate arthritis when treated prior to onset of clinical disease at the time of antigen challenge but, importantly, arthritis is also reduced in mice with established disease. Treatment of established disease is what is required of a therapeutic agent for RA. We have previously shown that injection of GM-CSF exacerbates CIA [8], while GM-CSF gene-deficient mice are essentially resistant to CIA [9].
The apparent importance of timing for the effectiveness of anti-GM-CSF mAb treatment provides insight into the role that GM-CSF might play in the pathogenesis of arthritis. The fact that the antibody and cellular proliferative responses to CII were not reduced by anti-GM-CSF administration, and the lack of evidence so far for subsequent disease suppression if administered prior to antigen challenge, at least with the protocols tried, suggest that GM-CSF is involved in later events associated with the effector phase. Such events are subsequent to T-cell activation, and most probably effect mediators of inflammation (e.g. cytokines).
In keeping with the decrease in disease severity following anti-GM-CSF mAb treatment, we found the mean levels of TNFa and IL-1? were reduced in ankle joint washouts of anti-GM-CSF mAb-treated mice. These reduced levels correlated with disease severity and suggest that GM-CSF plays an important role in the local expression of the macrophage-derived TNFa and IL-1?, two key mediators in inflammatory joint disease. There is both in vitro and in vivo evidence for GM-CSF having a number of proinflammatory effects on both monocytes/macrophages and granulocytes [2,3,17,18,19,20,21,22,23,24], including priming of human monocytes for subsequent cytokine production.
GM-CSF may also be acting systemically because i.p. administration of anti-GM-CSF mAb largely prevented the spread of disease to new limbs. While it was not possible to measure serum GM-CSF levels, due to the short half-life of GM-CSF [25], this does not rule out a possible systemic role.
At sites of inflammation, including joints, GM-CSF has been proposed to form part of an inflammatory cytokine-driven feedback cascade termed the 'CSF network' [26,27,28,29,30]. Our predictions from this model are that exogenous GM-CSF will potentiate inflammatory disease and that it will be a therapeutic target ? there is now evidence for both these hypotheses. By blocking GM-CSF, fewer primed macrophages (and granulocytes) would thus be present at a site of inflammation, as evidenced by the current study, with the result that less mediators would be produced to promote inflammation and tissue destruction.
Conclusion
Experimental data indicating suppression of CIA by anti-TNFa antibody treatment preceded successful TNFa targeting in RA [12,31]. The present data show that anti-GM-CSF mAb treatment also suppresses CIA and, in particular, that existing disease can be ameliorated. These results suggest that the major effect of GM-CSF in CIA is in mediating the effector phase of the inflammatory reaction to CII. Both the inflammatory and erosive processes are affected, and the levels of key proinflammatory cytokines in RA, TNFa and IL-1?, are reduced, suggesting that consideration be likewise given to modulating GM-CSF clinically for inflammatory conditions.
Supplementary material
Materials and methods
Collagen-induced arthritis
Male DBA/1 mice, 8?12 weeks old (ARC, Canning Vale, WA, USA) were immunized intradermally in the base of the tail with 100 ?g chick CII (Sigma, St. Louis, MO, USA) emulsified in an equal volume of complete Freund's adjuvant containing 5 mg/ml heat-killed Mycobacterium tuberculosis (H37 Ra; Difco, Detroit, MI, USA). This procedure was repeated as a boost 21 days later, as previously published [8,9,10,11].
mAb treatment
Mice were treated i.p. with 300 ?g of the appropriate mAb in a volume of 100 ?l at various times. The first treatment was prior to the clinical onset of arthritis at the time of primary immunization (day 0), either once or at weekly intervals from days 0 to 21. Another treatment occurred at the time of antigen challenge (day 21), either once, twice (days 21 and 23), or every second day from days 21 to 31. For the latter regime, 30 and 100 ?g anti-GM-CSF mAb were also used. The final time for treatment was following clinical onset of arthritis. For treatment of existing arthritis, mAb was administered daily for a total of 10 days following onset of arthritis. Mice were paired according to their arthritic score and then assigned to either the anti-GM-CSF mAb treatment group or the isotype control mAb treatment group, such that both groups had approximately the same clinical score (mean ? SEM) on the first day of mAb administration (day 0).
T-cell proliferation assay
Cells from inguinal lymph nodes were isolated from mice treated every second day with 300 ?g of mAb from days 21 to 31. These cells were cultured (5 ? 105 cells/well, 2-3 mice/group), for 72 hours at 37?C (5% CO2), with 0?100 ?g/ml denatured CII (boiled for 10 min) in RPMI containing 50 ?M 2-ME and 5% (v/v) fetal calf serum (200 ?l/well) [10]. Either anti-GM-CSF mAb or isotype-control mAb were added to selected wells at concentrations of 100 ?g/ml. Cells were pulsed with 1 ?Ci [3H]TdR (Amersham Int., Amersham, UK) 16 hours prior to harvesting. Cells were harvested using an Inotech cell harvester (Inotech Biosystems International Inc, Rockville, MD, USA) and DNA synthesis measured by [3H]TdR incorporation using a Beckman ? scintillation counter (Beckman Instruments Inc., Irvine, CA, USA). Results are expressed as a Stimulation Index where cells cultured in media alone have an index of 1.
Cytokine ELISAs
The coating and capture antibodies, respectively, used in the cytokine ELISAs were anti-GM-CSF mAb (22E9.11; DNAX [13]) and a biotinylated anti-GM-CSF mAb (31G6.41; Pharmingen, San Diego, CA, USA) for GM-CSF; anti-TNFa mAb (G281-2626; Pharmingen) and a biotinylated anti-TNFa mAb (MP6-XT3; Pharmingen) for TNFa; and polyclonal anti-IL-1? Ab and a biotinylated anti-IL-1? mAb (Endogen, Woburn, MA, USA) for IL-1?. A streptavidin?horseradish peroxidase conjugate (Pharmingen) followed by TMB-peroxidase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD, USA) was used to detect the cytokines. A standard curve was constructed using serial dilutions of purified GM-CSF, TNFa, or IL-1? starting at a concentration of 2 ng/ml. Each ELISA was sensitive down to 15 pg/ml.
Results
GM-CSF blockade prior to onset of arthritis results in less severe disease
Mice treated i.p. with 300 ?g anti-GM-CSF beginning at day 21 and then every second day until day 31 showed a decrease in disease severity. The decrease in severity was seen both as a reduction in the number of affected limbs per mouse (Supplementary Fig. 1a) and lower clinical scores for these affected limbs (Supplementary Fig. 1b). For example, only 1 of 9 (11%) anti-GM-CSF-treated mice had more than one limb affected compared with 8 of 10 (80%) isotype control-treated mice (P = 0.003) (Supplementary Fig. 1a). Also, none of the affected limbs from anti-GM-CSF mAb-treated mice had a clinical score of 3 (maximum) compared with 19 individual limbs from isotype control-treated mice (Supplementary Fig. 1b).
Because continued treatment with anti-GM-CSF was effective in modifying the disease when initiated at antigen challenge (i.e. day 21 after primary immunization), the effect of the addition of anti-GM-CSF mAb at different times prior to disease onset was examined. Treatment with anti-GM-CSF mAb on days 21 and 23 resulted in a slight suppression of the clinical score that was significant on day 40 (P = 0.044) (Supplementary Fig. 2a). There was, however, no significant difference in the incidence, day of onset, or the number of limbs affected per mouse compared with isotype control-treated mice (data not shown). Treatment with anti-GM-CSF mAb on day 21 only did not result in any significant differences compared with isotype control-treated mice, although there was a trend towards slightly lower mean clinical scores (Supplementary Fig. 2a).
To assess the effectiveness of treatment with anti-GM-CSF mAb during the primary induction phase of disease, mice were treated i.p. with a single injection of 300 ?g anti-GM-CSF mAb at the time of immunization (day 0) (Supplementary Fig. 2b), or at weekly intervals from the time of immunization until day 21 (Supplementary Fig. 2c). There were no significant differences in the mean clinical scores (Supplementary Fig. 2b,c), the incidence or the day of onset (data not shown) between mice treated with anti-GM-CSF mAb and control mAb for either treatment regime. Mice treated weekly with anti-GM-CSF mAb up to and including day 21 showed a trend towards slightly lower mean clinical scores (Supplementary Fig. 2c); findings similar to mice treated on day 21 (Supplementary Fig. 2a).
GM-CSF blockade prevents the clinical progression of established arthritis
To further examine the suppression of disease seen in anti-GM-CSF mAb-treated mice with established disease, the progression of arthritis in initially affected limbs (Supplementary Fig. 3a) and the recruitment of additional limbs and their severity, analyzed individually (Supplementary Fig. 3b), were compared between the anti-GM-CSF mAb-treated and isotype control-treated groups. The absence of disease progression was seen as a relative lack of increase in the clinical score of initially affected limbs (Supplementary Fig. 3a). For example, the clinical score of 23 of the 33 (70%) initially affected limbs from the anti-GM-CSF mAb-treated group either did not change or improved (i.e. a lower score) following treatment, compared with only 9 of the 31 (29%) initially affected limbs from the control-treated group (P = 0.001). Furthermore, there was a diminished recruitment of additional limbs during treatment (Supplementary Fig. 3b). Of note, only 8 of 47 (17%) limbs that were normal at the commencement of treatment from the anti-GM-CSF-treated group developed arthritis compared with 23 of 45 (51%) limbs that were normal from the isotype control-treated group (P = 0.001). The arthritis that developed in limbs after the commencement of treatment with anti-GM-CSF mAb was very mild with a clinical score of only 1 in all cases. The arthritis that developed in limbs after the commencement of treatment with the control mAb, however, ranged from mild (clinical score of 1) to severe (clinical score of 3) (Supplementary Fig. 3b).
Reduced histopathology of joints following GM-CSF blockade
The cellular infiltration and degree of joint erosion were quantified from randomly selected mice from each treatment group; 124 joints from 18 hind limbs of anti-GM-CSF-treated mice and 142 joints from 16 hind limbs of control-treated mice were examined by microscopy. There was a significant correlation between the clinical score of each limb and each histologic feature for both treatment groups (data not shown).
Humoral and cellular response to CII following GM-CSF blockade in vivo
Antibodies to CII were measured in serum at the end of each experiment. Treatment with anti-GM-CSF mAb either post-onset or at various times prior to onset (days 21?31, day 0, or weekly from days 0 to 21) had no effect on the anti-CII IgG response (Supplementary Fig. 4a).
CII-specific T-cell expansion was measured in vitro from mice treated with mAb every second day from days 21 to 31. There was no difference in the proliferative response of T cells to CII from anti-GM-CSF mAb-treated mice compared with control mAb-treated mice (Supplementary Fig. 4b). Furthermore, in vitro treatment with anti-GM-CSF mAb also had no effect on the CII-specific proliferative response (Supplementary Fig. 4c).
Abbreviations
CIA = collagen-induced arthritis; CII = type II collagen; ELISA = enzyme-linked immunosorbent assay; GM-CSF = granulocyte-macrophage colony-stimulating factor; H & E = hematoxylin and eosin; IL = interleukin; mAb = monoclonal antibody; RA = rheumatoid arthritis; TNFa = tumor necrosis factor-a.
Acknowledgments
Acknowledgements
The authors thank Jennifer Davis for care of the mice, and Dr John Abrams (DNAX Research Institute, Palo Alto, CA, USA) for the hybridomas. This work was supported by the National Health and Medical Research Council of Australia.
References
- Metcalf D. The molecular control of cell division, differentiation commitment and maturation in haemopoietic cells. Nature. 1989;339:27–30. doi: 10.1038/339027a0. [DOI] [PubMed] [Google Scholar]
- Hamilton JA, Stanley ER, Burgess AW, Shadduck RK. Stimulation of macrophage plasminogen activator activity by colony-stimulating factors. J Cell Physiol. 1980;103:435–445. doi: 10.1002/jcp.1041030309. [DOI] [PubMed] [Google Scholar]
- Alvaro-Gracia JM, Zvaifler NJ, Firestein GS. Cytokines in chronic inflammatory arthritis. IV. Granulocyte/macrophage colony-stimulating factor-mediated induction of class II MHC antigen on human monocytes: a possible role in rheumatoid arthritis. J Exp Med. 1989;170:865–875. doi: 10.1084/jem.170.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu WD, Firestein GS, Taetle R, Kaushansky K, Zvaifler NJ. Cytokines in chronic inflammatory arthritis. II. Granulocyte-macrophage colony-stimulating factor in rheumatoid synovial effusions. J Clin Invest. 1989;83:876–882. doi: 10.1172/JCI113971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Clinton L. Expression of GM-CSF receptor in rheumatoid arthritis. Lancet. 1993;342:1244. doi: 10.1016/0140-6736(93)92229-m. [DOI] [PubMed] [Google Scholar]
- de Vries EG, Willemse PH, Biesma B, Stern AC, Limburg PC, Vellenga E. Flare-up of rheumatoid arthritis during GM-CSF treatment after chemotherapy. Lancet. 1991;338:517–518. doi: 10.1016/0140-6736(91)90594-f. [DOI] [PubMed] [Google Scholar]
- Myers LK, Rosloniec EF, Cremer MA, Kang AH. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997;61:1861–1878. doi: 10.1016/s0024-3205(97)00480-3. [DOI] [PubMed] [Google Scholar]
- Campbell IK, Bendele A, Smith DA, Hamilton JA. Granulocyte-macrophage colony stimulating factor exacerbates collagen-induced arthritis in mice. Ann Rheum Dis. 1997;56:364–368. doi: 10.1136/ard.56.6.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell IK, Rich MJ, Bischof RJ, Dunn AR, Grail D, Hamilton JA. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol. 1998;161:3639–3644. [PubMed] [Google Scholar]
- Campbell IK, Hamilton JA, Wicks IP. Collagen-induced arthritis in C57Bl/6 (H-2b) mice: new insights into an important disease model of rheumatoid arthritis. Eur J Immunol. 2000;30:1568–1575. doi: 10.1002/1521-4141(200006)30:6<1568::AID-IMMU1568>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Campbell IK, Rich MJ, Bischof RJ, Hamilton JA. The colony-stimulating factors and collagen-induced arthritis: exacerbation of disease by M-CSF and G-CSF and requirement for endogenous M-CSF. J Leuk Biol. 2000;68:144–150. [PubMed] [Google Scholar]
- Joosten LAB, Helsen MMA, van de Loo FAJ, Heinegard D, van den Berg WB. IL-1a ? blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF- a blockade only ameliorates joint inflammation. J Immunol. 1999;163:5049–5055. [PubMed] [Google Scholar]
- O'Garra A, Barbis D, Wu J, Hodgkin PD, Abrams J, Howard M. The BCL1 B lymphoma responds to IL-4, IL-5, and GM-CSF. Cell Immunol. 1989;123:189–200. doi: 10.1016/0008-8749(89)90279-7. [DOI] [PubMed] [Google Scholar]
- Kuiper S, Joosten LA, Bendele AM, Edwards CK, III, Arntz OJ, Helsen MM, Van de Loo FA, Van den Berg WB. Different roles of tumour necrosis factor alpha and interleukin 1 in murine streptococcal cell wall arthritis. Cytokine. 1988;10:690–702. doi: 10.1006/cyto.1998.0372. [DOI] [PubMed] [Google Scholar]
- Seki N, Sudo Y, Yoshioka T, Sugihara S, Fujitsu T, Sakuma S, Ogawa T, Hamaoka T, Senoh H, Fujiwara H. Type II collagen-induced murine arthritis. I. Induction and perpetuation of arthritis require synergy between humoral and cell-mediated immunity. J Immunol. 1988;140:1477–1484. [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmann J, Golde DW, Weisbart RH, Gasson JC. Granulocyte-macrophage colony-stimulating factor enhances phagocytosis of bacteria by human neutrophils. Blood. 1986;68:708–711. [PubMed] [Google Scholar]
- Weisbart RH, Kwan L, Golde DW, Gasson JC. Human GM-CSF primes neutrophils for enhanced oxidative metabolism in response to the major physiological chemoattractants. Blood. 1987;69:18–21. [PubMed] [Google Scholar]
- Morrissey PJ, Bressler L, Park LS, Alpert A, Gillis S. Granulocyte-macrophage colony-stimulating factor augments the primary antibody response by enhancing the function of antigen-presenting cells. J Immunol. 1987;139:1113–1119. [PubMed] [Google Scholar]
- Hart PH, Whitty GA, Piccoli DS, Hamilton JA. Synergistic activation of human monocytes by granulocyte-macrophage colony-stimulating factor and IFN-gamma. Increased TNF-alpha but not IL-1 activity. J Immunol. 1988;141:1516–1521. [PubMed] [Google Scholar]
- Sisson SD, Dinarello CA. Production of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor by human mononuclear cells stimulated with granulocyte-macrophage colony-stimulating factor. Blood. 1988;72:1368–1374. [PubMed] [Google Scholar]
- Hart PH, Vitti GF, Burgess DR, Whitty GA, Royston K, Hamilton JA. Activation of human monocytes by granulocyte-macrophage colony stimulating factor. Increased urokinase-type plasminogen activator activity. Blood. 1991;77:841–848. [PubMed] [Google Scholar]
- Tiegs G, Barsig J, Matiba B, Uhlig S, Wendel A. Potentiation by granulocyte macrophage colony-stimulating factor of lipopolysaccharide toxicity in mice. J Clin Invest. 1994;93:2616–2622. doi: 10.1172/JCI117274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette WH, Baker DA, Stam EJ, Umland JP, Griffiths RJ. GM-CSF rapidly primes mice for enhanced cytokine production in response to LPS and TNF. Cytokine. 1995;7:291–295. doi: 10.1006/cyto.1995.0035. [DOI] [PubMed] [Google Scholar]
- Metcalf D, Robb L, Dunn AR, Mifsud S, Di Rago L. Role of granulocyte-macrophage colony-stimulating factor and granulocyte colony-stimulating factor in the development of an acute neutrophil inflammatory response in mice. Blood. 1996;88:3755–3764. [PubMed] [Google Scholar]
- Zvaifler NJ, Firestein GS. Cytokines in chronic inflammatory synovitis. Scand J Rheumatol Suppl. 1988;76:203–210. doi: 10.3109/03009748809102970. [DOI] [PubMed] [Google Scholar]
- Leizer T, Cebon J, Layton JE, Hamilton JA. Cytokine regulation of colony-stimulating factor production in cultured human synovial fibroblasts: I. Induction of GM-CSF and G-CSF production by interleukin-1 and tumor necrosis factor. Blood. 1990;76:1989–1996. [PubMed] [Google Scholar]
- Hamilton JA. A colony-stimulating factor network involving mononuclear phagocytes and other cells. In Haematopoietic Growth Factors and Mononuclear Phagocytes Edited by van Furth R Basel: Karger. 1993. pp. 29–35.
- Hamilton JA. Colony stimulating factors, cytokines and monocyte-macrophages ? some controversies. Immunol Today. 1993;14:18–24. doi: 10.1016/0167-5699(93)90319-G. [DOI] [PubMed] [Google Scholar]
- Hamilton JA. Rheumatoid arthritis: opposing actions of haemopoietic growth factors and slow-acting anti-rheumatic drugs. Lancet. 1993;342:536–539. doi: 10.1016/0140-6736(93)91653-4. [DOI] [PubMed] [Google Scholar]
- Williams RO, Feldmann M, Maini RN. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 1992;89:9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]


