Abstract
The vacuolar H+-ATPase (V-ATPase; V1Vo-ATPase) is an ATP-dependent proton pump that acidifies subcellular compartments in all eukaryotic organisms. V-ATPase activity is regulated by reversible disassembly into autoinhibited V1-ATPase and Vo proton channel subcomplexes, a process that is poorly understood on the molecular level. V-ATPase is a rotary motor, and recent structural analyses have revealed different rotary states for disassembled V1 and Vo, a mismatch that is likely responsible for their inability to reconstitute into holo V-ATPase in vitro. Here, using the model organism Saccharomyces cerevisiae, we show that a key impediment for binding of V1 to Vo is the conformation of the inhibitory C-terminal domain of subunit H (HCT). Using biolayer interferometry and biochemical analyses of purified mutant V1-ATPase and Vo proton channel reconstituted into vacuolar lipid-containing nanodiscs, we further demonstrate that disruption of HCT's V1-binding site facilitates assembly of a functionally coupled and stable V1Vo-ATPase. Unlike WT, this mutant enzyme was resistant to MgATP hydrolysis-induced dissociation, further highlighting HCT's role in the mechanism of V-ATPase regulation. Our findings provide key insight into the molecular events underlying regulation of V-ATPase activity by reversible disassembly.
Keywords: vacuolar ATPase, protein-protein interaction, biophysics, membrane protein, molecular motor, biolayer interferometry, lipid nanodisc, reversible disassembly
Introduction
The vacuolar H+-ATPase (V-ATPase, V1Vo-ATPase)3 is an ATP-dependent proton pump found on the endomembrane system of all eukaryotic organisms. This multisubunit nano-motor acidifies subcellular compartments and, in certain specialized tissues, the extracellular space. V-ATPase is essential for vital cellular processes such as pH homeostasis, protein sorting, autophagy, endocytosis, mTOR, and Notch signaling, as well as bone remodeling, urine acidification, hormone secretion, and neurotransmitter release (1). Although complete loss of V-ATPase function is embryonic lethal in mammals, aberrant activity has been associated with widespread human diseases including renal tubular acidosis (2), osteoporosis (3), neurodegeneration (4), diabetes (5), male infertility (6), and cancer (7), making V-ATPase a potential drug target (8, 9). However, because of its essential nature, global inhibition of V-ATPase is not a therapeutic option. Instead, there is a need for targeted modulation of the enzyme's activity, a goal that requires a detailed understanding of V-ATPase's catalytic and regulatory mechanisms.
V-ATPase is composed of two subcomplexes, a cytosolic ATPase called V1, and a membrane integral proton channel termed Vo (Fig. 1A). In yeast, the subunit compositions for the V1 and Vo are A3B3(C)DE3FG3H and ac8c′c″def, respectively (10). The A and B subunits of V1 are arranged in a hexamer (A3B3), with three catalytic sites at alternating AB interfaces. Located within the hexamer and extending from it in the direction of the membrane is subunit D that, together with F, provides the functional link between V1 and Vo. The Vo is constituted by subunit a that can be divided into cytosolic N-terminal and membrane-integral C-terminal domains (aNT and aCT), the c, c′ and c″ subunits (“proteolipids”) that form a ring (c-ring), and subunit d that connects the c-ring with V1 subunits D and F. V1 and Vo are held together by three heterodimers of subunits E and G (peripheral stalks EG1–3) that link the catalytic hexamer and the single copy C and H subunits to the membrane integral a subunit by binding to aNT.
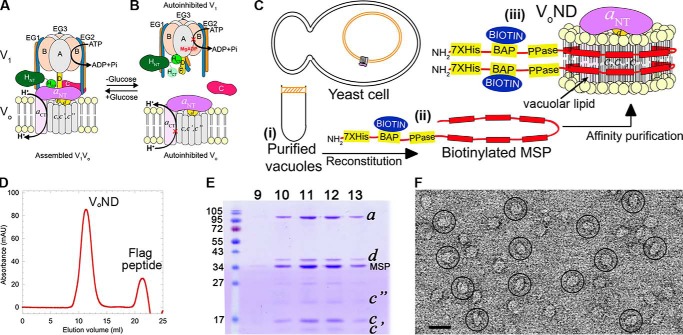
Figure 1.
Purification and characterization of VoND. A and B, schematic of V-ATPase regulation by reversible disassembly. C, purification strategy. Yeast vacuoles are isolated by flotation on a Ficoll gradient (panel i). Detergent-solubilized vacuolar proteins are mixed with biotinylated MSP (panel ii) and reconstituted into lipid nanodiscs followed by α-FLAG affinity capture of VoND (panel iii). D, size-exclusion chromatography of VoND. E, peak fractions were resolved using SDS-PAGE. F, negative stain EM of purified VoND. Bar, 20 nm.
V-ATPase is a rotary motor enzyme and employs a catalytic mechanism that is shared with the F-, A-, and A/V-type ATPases (11). In V-ATPase, ATP hydrolysis–driven rotation of the DFdc-ring central rotor is coupled to proton translocation at the interface of aCT and the c-ring. During catalysis, the three peripheral stalks, in conjunction with C, H, and aNT, resist the rotary torque to keep the A3B3 hexamer static against aCT for efficient energy coupling. However, unlike F-, A-, and A/V-type enzymes, eukaryotic V-ATPase is regulated by a unique mechanism referred to as “reversible disassembly,” wherein V1 detaches from Vo in response to e.g. nutrient shortage (12–14) (Fig. 1B), with concomitant silencing of V1's MgATPase (15, 16), and Vo's proton transport activities (17, 18). First described in yeast and insect, reversible disassembly is now emerging as an important and conserved regulatory mechanism, having been observed in mammalian systems as well (19–21). However, although we have some understanding of the process at the cellular level (22), less is known about the mechanism of reversible disassembly at the molecular scale. Biochemical studies in yeast have shown that the single copy C and H subunits, which are unique to eukaryotic V-ATPase, play key roles in enzyme regulation. Both C and H are two-domain proteins, with C composed of “foot” (Cfoot) and “head” (Chead), and H of N- (HNT) and C-terminal (HCT) domains. H has a dual role because it is required for both coupling MgATPase to proton-pumping activities and for stabilizing the autoinhibited state of membrane detached V1; C functions to stabilize the V1–Vo interface in the holo enzyme but dissociates from the complex upon regulated disassembly. Recent structural studies have revealed that in assembled V1Vo, HCT is bound to aNT (23), an interaction required for energy coupling (24) (Fig. 1A). Upon disassembly of V1 from Vo, HCT undergoes a 150° rotation to wedge an inhibitory loop between the B subunit of an open catalytic site and subunits DF of the central rotor (16) (Fig. S1, A–D, red spheres). At the same time, aNT moves from its peripheral position near Cfoot and EG in V1Vo (Fig. 1A) toward a more central position in free Vo to bind subunit d (18, 25–27) (Fig. S1, E–G). Moreover, cryoEM models of three distinct rotary states of holo V-ATPase (states 1–3) (23), along with the structures of autoinhibited V1 (16) and Vo (25, 27), revealed that although V1 is halted in state 2, Vo adopted state 3 (10) (Fig. S1). We hypothesized that the mismatch of rotational states observed in autoinhibited V1 and Vo, together with the large conformational changes of HCT and aNT that accompany enzyme dissociation, explain why V1 does not readily rebind free Vo under physiological conditions in vitro (10, 28), a safety mechanism that likely evolved to prevent spontaneous reassembly in vivo when the disassembled, inactive state is required.
We recently introduced biolayer interferometry (BLI) of purified V-ATPase in biotinylated and native lipid containing nanodiscs to analyze MgATP-dependent enzyme dissociation kinetics (29). Here, we have expanded on this approach to probe the interaction of V1 and Vo. Vo sector was reconstituted into vacuolar lipid containing nanodiscs (VoND) and immobilized on BLI sensors to screen V1 mutants for their ability to bind Vo. In line with available literature (28), WT V1 did not bind to VoND, presumably because of the “state mismatch” observed in the structures of free V1 and Vo (10). Previously, we generated a chimeric H subunit containing the yeast N-terminal and human C-terminal domains (Hchim) that does not inhibit free V1 because human HCT lacks an inhibitory loop that links an open catalytic site and the central rotor (16), and consequently, V1 containing Hchim is not restrained in any particular rotational state. We show that replacement of endogenous H in yeast V1 with Hchim (V1Hchim) (16) permits binding to VoND, and formation of a coupled holo V-ATPase (V1HchimVoND) with catalytic properties similar to the ones of the recently characterized WT V1VoND (29). However, V1HchimVoND was more resistant to ATP hydrolysis-induced disassembly compared with WT, highlighting the importance of HCT's conformational switch in driving V-ATPase disassembly. The in vitro data presented here thus provide key insight into the molecular steps that accompany V-ATPase regulation by reversible disassembly.
Results
Purification and characterization of native lipid nanodisc reconstituted Vo (VoND) and V1 mutants
Vo was extracted from yeast vacuoles using the “reconstitution before purification” strategy as described for V1Vo (29) (Fig. 1C, panels i–iii). The resultant VoND complex consisted of Vo embedded in endogenous vacuolar lipid containing nanodiscs encircled by biotinylated membrane scaffold protein (MSP; Fig. 1C, panel iii). The purified complex was monodisperse and contained all Vo subunits plus MSP based on gel filtration and SDS-PAGE (Fig. 1, D and E). Examination of purified VoND using negative stain EM showed single particles of VoND with the typical size and appearance as described previously (26) (Fig. 1F).
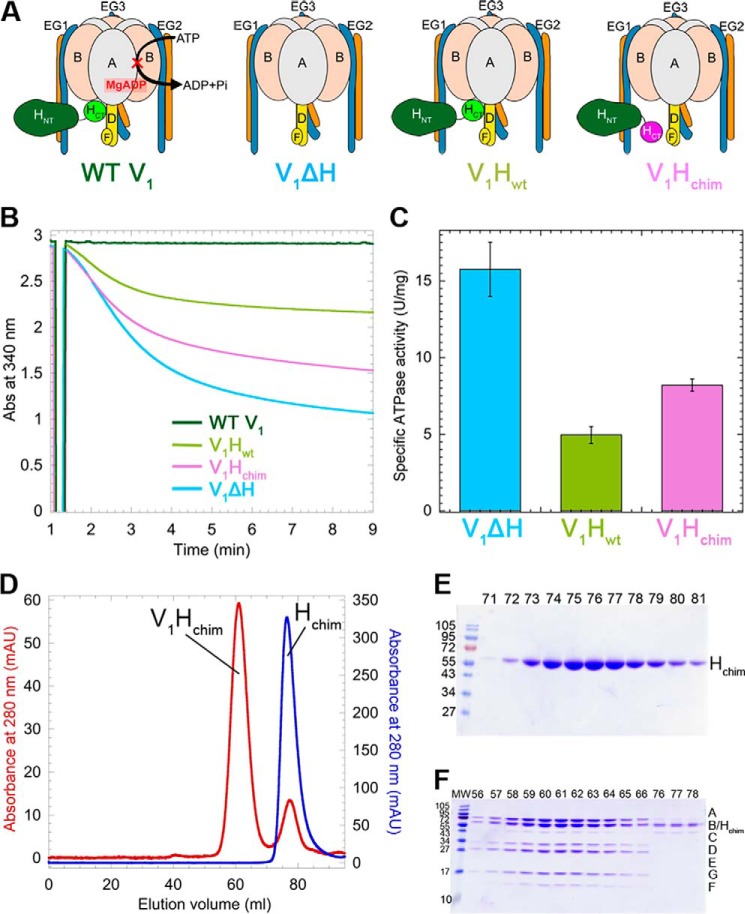
Four different V1 mutants were tested for their ability to bind VoND and form coupled holo V-ATPase (Fig. 2A): WT V1 (containing subunits A3B3DE3FG3H), V1 purified from a yeast strain deleted for subunit H (V1ΔH), V1ΔH reconstituted with recombinantly expressed WT H (V1Hwt), and V1ΔH reconstituted with chimeric H (V1Hchim) (16). Although WT V1 has no measurable MgATPase activity (Fig. 2B, dark green trace), V1ΔH had an initial specific activity of 15.7 ± 1.7 units/mg, consistent with previous reports (16, 30) (Fig. 2, B and C, blue trace and bar). Although MgATPase activity is measured in an ATP-regenerating system, the activity of V1ΔH decreases over time as MgADP gets trapped in a closed catalytic site, leading to the MgADP-inhibited state (15). Although V1Hwt has identical subunit composition to WT V1, V1Hwt distinguishes itself from WT by having only ∼0.4 instead of 1.3 mol/mol ADP in catalytic sites (16), and the complex therefore exhibits an initial MgATPase activity of ∼4.95 ± 0.55 units/mg before becoming MgADP-inhibited (Fig. 2, B and C, light green). V1ΔH reconstituted with Hchim to yield V1Hchim (Fig. 2, D–F) showed an initial MgATPase activity of 8.2 ± 0.4 units/mg that, as for the other V1 mutants, declined over time because of MgADP inhibition (Fig. 2, B and C, pink).
Figure 2.
Purification and characterization of V1 mutants. A, schematic representation of the V1 mutants. B, time-dependent MgATPase activities of the V1 mutants measured in an ATP regenerating assay. C, specific activities of the V1 mutants ± S.E. from at least two independent purifications per mutant. D, size-exclusion chromatography of Hchim (blue trace) and V1ΔH reconstituted with Hchim (V1Hchim, red trace). E and F, SDS-PAGE of column fractions of Hchim (E) and V1Hchim (F).
Binding of C subunit to V1
Experiments in yeast have shown that upon deletion of the gene encoding the C subunit, V1 does not stably/functionally associate with Vo. Moreover, biochemical analysis revealed that Chead binds isolated EG heterodimer with moderate affinity, whereas both Cfoot and EG bind aNT weakly (31, 32). From these data we concluded that V1 and Vo are held together by multiple weak interactions, resulting in an overall high-avidity interface, and that destabilization of one of these interactions by a cellular response to starvation would result in enzyme dissociation (10, 32). More recently we showed that although HNT binds isolated EG with a Kd of ∼0.2 μm, the affinity of the interaction is increased 40-fold when EG is part of V1 (30). Moreover, when we analyzed binding of H (and HNT) to V1ΔH, we found that MgATP hydrolysis destabilized the V1–H interaction, an effect likely caused by the cyclic conformational changes at the catalytic AB interfaces to which the EG heterodimers are bound (30). We therefore wished to determine whether C binding to EG on V1 is also enhanced compared with isolated EG, and if so, (i) what the affinity of the interaction is, and (ii) whether the interaction is also destabilized during ATP hydrolysis. Because V1 isolated from starving yeast has varying levels of substoichiometric amounts of C bound (15, 16, 33, 34), we purified V1 from a yeast strain in which C was deleted (V1ΔC) (16) to test for C binding. Using a BLI setup similar to the one we recently employed to analyze binding of H (and HNT) to V1ΔH (30), we found that V1ΔC binds C with a Kd of ∼0.7 nm (Fig. S4), indicating that one of the EG heterodimers bound to V1 is in a conformation that is more favorable for C binding compared with the isolated heterodimer. However, to test whether C binding is also destabilized as a result of ATP hydrolysis, we could not use V1ΔC because it contains the H subunit and so has no MgATPase activity, and we therefore used the catalytically active V1Hchim instead. As seen for the V1–H interaction, dissociation of V1Hchim from MBP-C loaded sensors was greatly accelerated only when the sensors were dipped into wells containing MgATP. Fitting the subunit C release in MgATP to two exponentials revealed a fast off rate of ∼0.012 ± 2.3 × 10−5 s−1 and a slower off rate of 5.1 × 10−4 ± 2 × 10−6 s−1, values similar to those observed for MgATP hydrolysis induced subunit H release as reported earlier (30) (Fig. S5). This suggests that the in vivo dissociation of the C subunit from the vacuolar membrane that occurs as a result of starvation is a direct result of MgATP hydrolysis–induced conformational changes of the EG heterodimer that is bound to Chead in the assembled enzyme (EG3) (23, 35). In summary, as for subunit H, binding of C to EG is significantly enhanced when EG is part of V1, and the V1–C interaction is greatly destabilized upon ATP hydrolysis.
V1Hchim and C subunit associate with VoND to form coupled V-ATPase in vitro
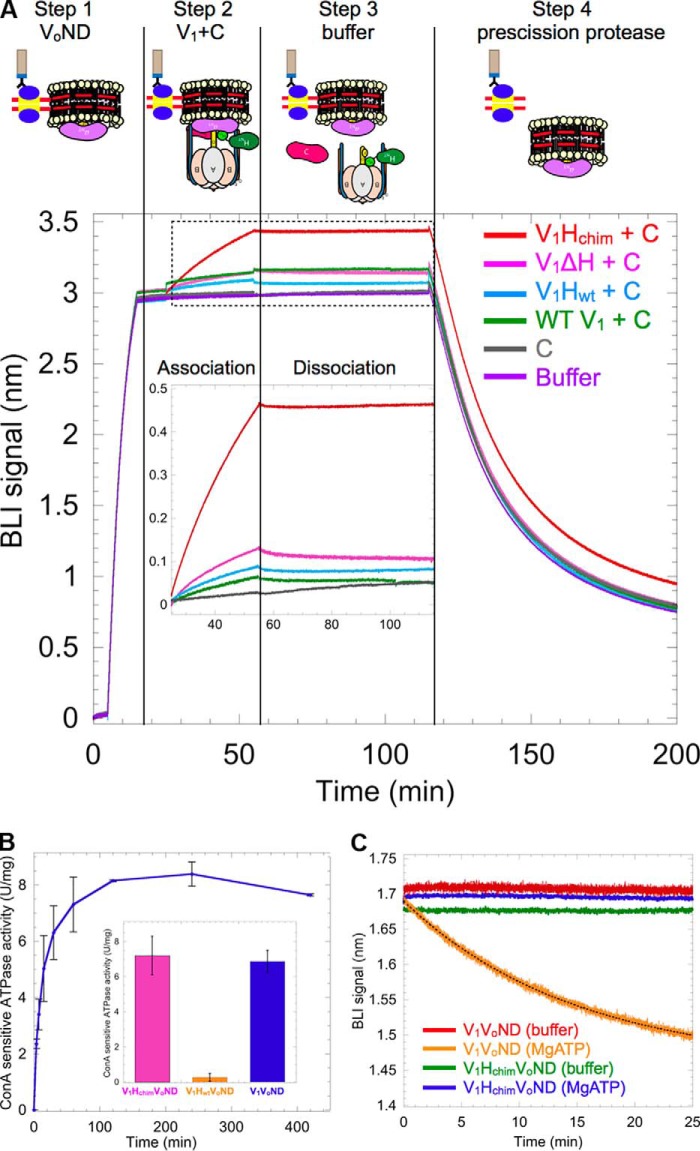
The ability of the V1 mutants depicted in Fig. 2A to interact with Vo was tested using BLI. VoND reconstituted with biotinylated MSP was immobilized on streptavidin-coated BLI sensors (Fig. 3A, step 1), which were then dipped in wells containing V1 mutants and subunit C (Fig. 3A, step 2). We found that of the four V1 mutants, only V1Hchim showed significant association with VoND (Fig. 3A, red trace). The sensors were then dipped in buffer to measure dissociation rates (Fig. 3A, step 3). However, no significant dissociation was observed, indicating stable assembly of V1Hchim with Vo. Without C, none of the V1 mutants showed significant binding (Fig. S3), consistent with studies in yeast that showed that deletion of C prevents assembly of V1Vo (36). As a control, the sensors were then dipped in PreScission protease to cleave and release any remaining complex (Fig. 3A, step 4). Further, we conducted BLI experiments in which VoND-coated sensors were first dipped into wells containing a mAb (10D7) against aNT, which recognizes a cryptic epitope only available for binding in free Vo (12), before dipping the sensors into V1Hchim plus C containing wells. Under these conditions, the observed on-rate (kobs) of V1Hchim was significantly (∼60-fold) reduced, indicating that the observed BLI signal upon dipping the sensors into V1Hchim plus C was indeed due to binding of V1Hchim to immobilized VoND (Fig. S2).
Figure 3.
V1Hchim and C associate with VoND to form coupled V1Vo-ATPase. A, VoND was immobilized on streptavidin-coated BLI sensors via biotinylated MSP (step 1). Sensors were then dipped into 0.4 μm of V1 mutants in presence of 1 μm C (association; step 2) followed by buffer (dissociation; step 3). Association with VoND was most efficient with V1Hchim (red trace). Sensors were then dipped in PreScission protease to verify that the BLI signal was not due to nonspecific binding (step 4). Inset shows an enlarged view of the association and dissociation steps. B, equimolar amounts of V1Hchim and VoND, and a 2-fold molar excess of C subunit were incubated at 22 °C, and the ConA-sensitive MgATPase activity was measured as a function of time. Each point represents the mean ± S.E. of two separate reconstitutions from two individual purifications. Inset, specific MgATPase activities of reconstituted V1HchimVoND and V1HwtVoND (± S.E. from two independent purifications) compared with purified V1VoND (29). C, following association of the V1HchimVoND complex, sensors were dipped in wells containing buffer (green) or buffer + 1 mm MgATP (blue) for dissociation rate measurement. The dissociation phase of WT V1VoND in buffer (red) and buffer +1 mm MgATP (orange) is included for comparison (data from Ref. 29).
Whereas the BLI experiment showed slow, but stable, association of V1Hchim and C with VoND, it was not clear whether functional V-ATPase was formed under these conditions. To address this question, we monitored MgATPase activity of a 1:1:2 mixture of V1Hchim, VoND, and C that is sensitive to the V-ATPase specific inhibitor, concanamycin A (ConA), as a function of time (Fig. 3B). Although V1Hchim has MgATPase activity on its own, ConA binds to the Vo complex and prevents c-ring rotation, so ATPase activity that is abolished by treatment with ConA is evidence of a functionally coupled V-ATPase complex. The experiment demonstrated that binding of V1Hchim and C to VoND resulted in the formation of a coupled V1HchimVoND complex and that the reconstitution under these conditions was complete in ∼2 h, with a final specific activity of 7.2 ± 1.09 units/mg, similar to what is reported for purified WT V1VoND (6.9 ± 0.6 units/mg) (29) (Fig. 3B, inset, pink and blue bars, respectively). The ability of V1Hchim to form a functional complex with VoND is consistent with the previous observation that Hchim can complement deletion of the native H subunit in yeast cells (16). ConA-sensitive ATPase activity was also measured with VoND, C, and V1Hwt and produced only 0.26 ± 0.2 units/mg of coupled activity (Fig. 3B, inset, orange bar). Therefore, reconstitution of V1Hwt with Vo and C is highly inefficient under these conditions, consistent with earlier in vitro studies (28) and the real-time BLI experiments presented here (Fig. 3A).
V1HchimVoND is more stable in presence of MgATP compared with V1VoND
Using BLI, we have previously demonstrated that V1's dissociation from Vo is negligible under non-ATP hydrolyzing conditions but that in presence of MgATP, the complex undergoes spontaneous dissociation with an off-rate of 1 × 10−3 ± 3.3 × 10−6 s−1 (29). We conducted a similar experiment using V1HchimVoND, wherein after association of V1Hchim and C with VoND on BLI sensors, we dipped the sensors in wells containing buffer or 1 mm MgATP. Significantly, unlike WT V1VoND, V1HchimVoND showed very little to no dissociation in the presence of MgATP, indicating that the assembled V1HchimVoND complex is inherently more stable than the WT complex (Fig. 3C). The experiment thus highlights the importance of HCT's conformational switch (from energy coupling in the holo enzyme to autoinhibition of membrane detached V1) in driving V-ATPase disassembly.
Structural and functional characterization of V1HchimVoND
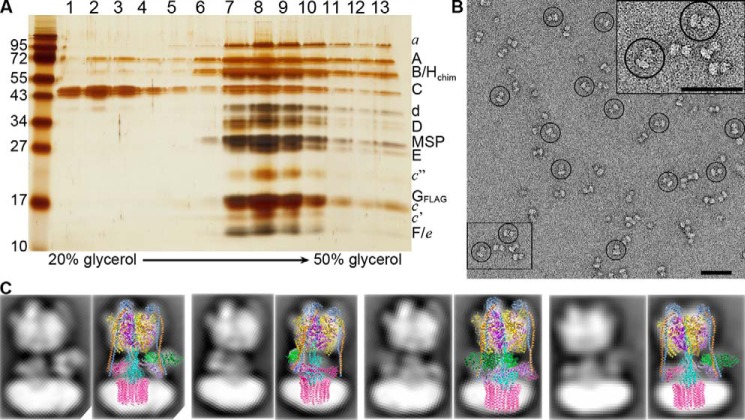
To determine the efficiency of V1HchimVoND complex formation, equimolar amounts of V1Hchim and VoND with a 2-fold molar excess of C were incubated for up to 16 h at 22 °C, and the reconstitution mixture was resolved by glycerol density gradient centrifugation. SDS-PAGE of the gradient fractions showed that the majority of V1 and Vo subunits co-migrated to fractions 7–10, similar to what was observed for purified WT V1VoND (29), with the excess C subunit remaining in lighter fractions (Fig. 4A). Negative stain EM of the peak fractions showed single particles of V1HchimVoND, with the typical dumbbell-shaped appearance of holo V-ATPase reported previously (29, 37, 38) (Fig. 4B). A more detailed analysis indicated a good match between averages obtained by reference free alignment and classification of a small data set of V1HchimVoND and corresponding projections of a cryoEM map of yeast V1Vo (23) (Fig. 4C). Taken together, these data show that reconstitution of V1Hchim with VoND and C result in a stable and coupled holo V-ATPase that is structurally similar to the WT enzyme.
Figure 4.
Structural and functional characterization of the V1HchimVoND complex. A, reconstituted V1HchimVoND was subjected to glycerol gradient centrifugation, and the gradient fractions were analyzed by silver-stained SDS-PAGE. B, negative stain EM of V1HchimVoND showing homogeneous and monodisperse dumbbell-shaped molecules. Inset in the top right shows 2× zoomed area highlighted in the bottom left. C, a data set of ∼5800 particle projections was subjected to reference-free alignment and classification, and selected class averages were overlaid with projections of the cryoEM model of yeast V1Vo (Protein Data Bank code 3J9U). Bars in B, 50 nm.
Discussion
In our experiments, only V1Hchim (in presence of C) shows significant binding to Vo. As mentioned earlier, human HCT lacks the inhibitory loop found in yeast HCT (16) (Fig. S1, A–D, red spheres), consistent with Hchim's inability to inhibit V1ΔH's MgATPase activity (Fig. 2, B and C). This lack of inhibition by Hchim is likely due to reduced binding of human HCT to the open catalytic site at the bottom of the A3B3 hexamer, with the remaining binding interaction between Hchim and V1 being mediated by HNT's interaction with one of the EG peripheral stalks. Therefore, we conclude that for V1 to reconstitute with Vo, HCT must be released from its inhibitory position on V1, so that HCT is available for binding to Vo's aNT. It is known that upon disassembly of V1 from Vo, aNT moves from a peripheral position in V1Vo to a more central position in autoinhibited Vo, where it binds subunit d (18, 25–27) (Fig. S1, E–G). The observation that V1Hchim, C, and autoinhibited VoND are sufficient to form a structurally and functionally coupled V-ATPase suggests that the release of HCT from its autoinhibitory position on V1 is necessary and sufficient for efficient reassembly of V1 and Vo. Our finding is consistent with the fact that reassembly of V1 with Vo on vacuoles is not inhibited by ConA, an inhibitor of c-ring rotation in the Vo sector (14).
HNT and HCT occupy specific binding sites on free V1, with HNT bound to EG1 and HCT bound to the bottom of the A3B3 hexamer, with its inhibitory loop wedged between the B subunit of an open catalytic site and the central stalk (Fig. S1, A–D) (16). The specific interaction of HCT with an open catalytic site maintains inhibitory MgADP in the adjacent closed catalytic site, locking autoinhibited V1 in rotational state 2. We have recently observed that transient MgATP hydrolysis on V1Hwt, which, unlike WT V1, is not in the MgADP-inhibited state (Fig. 2B), lowers H's affinity for V1, and we reasoned that this destabilization of the V1–H interaction is caused by MgATP hydrolysis driven conformational changes at the catalytic sites and the central (DF) and peripheral stalks (EG1–3) (30). We propose that an allosteric structural change at the open catalytic site that is driven by release of inhibitory MgADP from the closed catalytic site in autoinhibited, WT V1 by a yet unknown mechanism leads to the detachment of HCT from its inhibitory position so that it can bind aNT on Vo.
From studies in yeast it was shown that although C is required for binding of V1 to Vo on yeast vacuolar membranes (36), deletion of H allows assembly of a labile but inactive complex (39). The requirement of C for association of V1 with Vo is supported by our in vitro BLI experiments, wherein none of the V1 mutants reconstituted with Vo in absence of C (Fig. S3). However, unlike in vivo, the presence of C along with V1ΔH, WT V1, or V1Hwt is not sufficient for reconstituting V1Vo in vitro. This discrepancy is not due to a reduced affinity of C for V1, because we obtained a Kd of ∼0.7 nm for the interaction between C and V1ΔC (Fig. S4). A high-affinity interaction between C and V1ΔC is consistent with substoichiometric amounts of C remaining associated with purified V1ΔH and V1 (16), as well as the reported Kd of ∼42 nm for the EG–C interaction (31). However, tight and stable binding of C to V1ΔC would be inconsistent with the observed release of C into the cytosol upon disassembly of V1 from Vo (14), but, as shown here, ATP hydrolysis by V1 leads to the rapid release of the V1–C interaction, which likely explains why catalytically inactive enzyme does not disassemble upon glucose withdrawal (14). Although it has been reported that in the presence of the microtubule depolymerizing drug benomyl, C does not dissociate from V1Vo upon glucose removal (40), it is possible that C under these conditions quickly rebinds EG3 once V1 is in the autoinhibited state. In addition, a direct interaction between C and tubulin has been observed (40, 41), suggesting the possibility that, upon disassembly, C is sequestered by microtubules, preventing its reassociation with V1 and/or Vo.
Although reincorporation of C upon glucose addition does not require the microtubule network (40), efficient (re)assembly of holo V-ATPase requires a heterotrimeric chaperone complex referred to as RAVE (regulator of H+-ATPase of vacuolar and endosomal membranes). It has been proposed that upon receiving the signal for reassembly, RAVE recruits C and V1 to Vo on vacuolar membranes by directly interacting with C, EG (as part of V1), and aNT (as part of Vo) (42). Under the in vitro conditions employed here, it takes ∼2 h for a 1:1:2 mixture of V1Hchim, VoND, and C to complete reconstitution of V1HchimVoND, a relatively slow process compared with the kinetics of reassembly observed in vivo (∼5 min) (43). It is possible that the RAVE complex, by increasing the proximity of V1, C, and Vo, facilitates the otherwise low-affinity interactions at the V1–Vo interface (32), thereby accelerating reassembly.
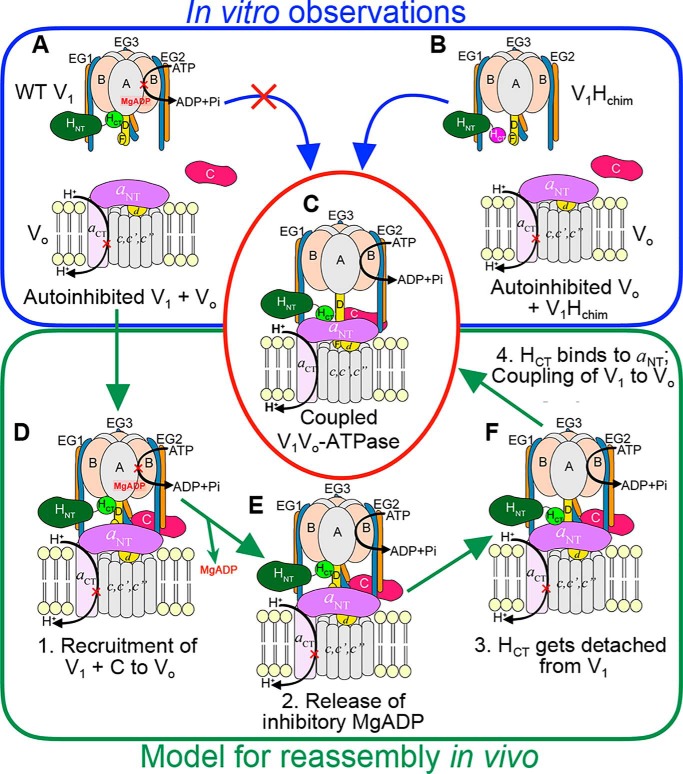
From the here presented data, we conclude that the detachment of HCT from V1 and the presence of C subunit are required for the reassembly of V1 with Vo (Fig. 5, A–C). In our in vitro reconstitutions, the association between V1Hchim and VoND is driven by the HCT–aNT interaction (Fig. 5B), but in vivo, the chain of events that leads to reassembly of autoinhibited V1 and Vo are probably different, because HCT is in its inhibitory conformation on V1 (Fig. 5A). We propose that in yeast, upon receiving cellular signals, autoinhibited V1 and C are first recruited to Vo (Fig. 5D), a process that is likely the rate-limiting step for reassembly. Our reason for this hypothesis is that even with the requirements for reassembly being met in our in vitro reconstitution of V1Hchim and C with Vo, the rate of reassembly was slow. In vivo, recruitment of V1 and C to Vo is facilitated and probably accelerated by the RAVE complex (42), but efficient (re)assembly in vivo requires additional factors such as the glycolytic enzymes aldolase (44) and phosphofructokinase (43), whose function in the process is currently not known. Once V1 and C are recruited to Vo at the vacuolar membrane, inhibitory MgADP is released upon opening of the closed catalytic site by a yet unknown mechanism (Fig. 5E). The release of inhibitory MgADP allows MgATP hydrolysis to resume, with concomitant conformational changes at the catalytic sites and rotation of the central stalk (DF), structural changes that result in detachment of HCT from V1 (30) (Fig. 5F). The proximity of aNT to V1-detached HCT facilitates the HCT–aNT interaction, a requirement for coupling of V1 to Vo in the holo-enzyme (24). The HCT–aNT interaction stabilizes the peripheral conformation of aNT such that the Cfoot–EG2–aNT ternary complex can be formed, thus completing functional (re)assembly (Fig. 5C).
Figure 5.
Model for reassembly of autoinhibited V1 and Vo. A–C, our in vitro experiments have shown that although WT H containing V1 does not readily bind VoND (A), V1Hchim spontaneously associates with VoND (B) to form a structurally and functionally coupled V-ATPase, albeit at a slow rate. C, in vivo, however, V1 exists in the autoinhibited conformation (A), and the rate of assembly with Vo is significantly faster (within 5 min). D–F, for in vivo (re)assembly, we propose that the following steps occur: step 1, recruitment of V1 and subunit C to the vacuolar membrane (D); step 2, release of inhibitory MgADP (E); step 3, detachment of HCT from its inhibitory position on V1; and step 4, HCT binding to aNT (F). For further details, see text.
V-ATPase regulation by reversible disassembly, originally discovered in lower eukaryotes, has been confirmed to be conserved in higher animals, including humans (19–21). Reconstitution of V1 with Vo has been investigated for mammalian V-ATPase from bovine brain clathrin-coated vesicles and in one study, chaotropically removed V1 reassembled with Vo on coated vesicle membranes upon dialysis, thereby regenerating ∼80% of the initial MgATPase activity (45). In another study, in vitro reconstitution of coated vesicle V-ATPase from V1 and Vo was shown to require the mammalian H subunit homolog SFD (sub-fifty-eight dimer) (46). However, in both cases, resulting V-ATPase complexes were not further characterized for subunit composition and structural integrity. Curiously, unlike yeast V1, removal of SFD from bovine coated vesicle V1 did not create a MgATP hydrolyzing V1-ATPase, suggesting the presence of other regulatory mechanisms in mammalian V1 (46). One of the likely reasons that few biochemical studies have focused upon the molecular mechanism of reversible disassembly in higher organisms is because mammalian V-ATPase is extraordinarily heterogeneous, with most subunits expressed as multiple isoforms or splice variants (including subunits H and a) (47), and to our knowledge, no in vitro system comparable to the one described here for the yeast V-ATPase has been reported for the mammalian enzyme. Yeast contains only one subunit with multiple isoforms (subunit a), and the two V-ATPase populations resulting from this single subunit difference appear to have different propensities to undergo dissociation, and only one of them requires the RAVE complex for (re)assembly (48, 49). It is likely that different isoform-containing enzymes in mammalian systems are subjected to differential regulatory mechanisms, resulting in variable propensities to dissociate. Because the human HCT does not silence yeast V1 but does facilitate efficient functional coupling in V1Vo, it will be of interest to explore the mechanism of regulation by reversible disassembly as a function of subunit isoform composition of the mammalian system in greater detail by using the tools developed and presented here for the yeast enzyme.
Experimental procedures
Strains
The yeast strain SF838–5Aα deleted for the vma2 gene (B subunit) vma2Δ::Nat was a kind gift from Dr. Patricia Kane, SUNY Upstate Medical University. A plasmid containing the FLAG tag with a KanMX6 marker, pFA6a-6xGly-FLAG-kanMX6 was a gift from Dr. Mark Hochstrasser (50) (Addgene plasmid no. 20751). The primers vph1CTFlagFWD (gct gtt gct agt gca agc tct tcc gct tca agc GGG GGA GGC GGG GGT GGAA) and vph1CTFlagREV (cct gga tgt gga ttt cga ttc taa cgt tac ccc aag gca aat gat ggt cac tgg GAA TTC GAG CTC GTT TAA AC) were used to amplify the FLAG tag and KanMX marker from pFA6a-6xGly-FLAG-kanMX6. The ∼1.8-kb product was gel purified and used for homologous recombination to insert the FLAG-KanMX cassette in the C terminus of vph1 in the yeast strain SF838–5Aα vma2Δ::Nat using the same primers as above. Colonies were selected for growth on YPD G418 plates, and the insertion of the FLAG tag at the C terminus of vph1 was confirmed by sequencing. The construction of chimeric H subunit (Hchim) encoding the N-terminal domain from Saccharomyces cerevisiae (residues 1–352) and the C-terminal domain (349–483) of the human H subunit into the yeast pRS316 vector has been discussed in Ref. 16. From the pRS316 vector, using the primers MalChimF (TTA GCC GGT ACC GGG AGC AAC GAA GAT ATT AAT GGA C) and MalChimR (TTA CCA AAG CTT TTA GCT TCG GGC GGC AG), Hchim was amplified. The primers additionally introduced the restriction sites 5′ KpnI and 3′ HindIII, which were used to insert the amplicon into a pMal vector. The resultant vector encoded MBP-tagged Hchim separated by a PreScission protease cleavage site, as confirmed by sequencing.
Purification of Vo and its reconstitution into endogenous vacuolar lipid
Vo was purified from yeast vacuoles and reconstituted into endogenous vacuolar lipid containing nanodiscs as described for V1VoND in Ref. 29. The steps are briefly described as follows.
Purification of biotinylated MSP
Biotinylated MSP was purified as described in Ref. 29. Briefly, BL21 (DE3) cells were co-transformed with the plasmids pHBPMSP1E3D1 and pBirAcm (encoding the BirA gene). The cells were grown in rich broth supplemented with 0.1 mm d-biotin, 34 μg/ml chloramphenicol, and 30 μg/ml kanamycin to an A595 of ∼0.5 at 37 °C followed by induction using 0.5 mm isopropyl β-d-thiogalactopyranoside for 3–4 h. Harvested cells were purified as described in Ref. 26. Briefly, the cells were lysed by sonicating three times for 30 s. The lysate was cleared by centrifugation at 13,000 × g and passed over a nickel–nitrilotriacetic acid affinity column. The column was washed with 10 column volumes of the each of the three buffers: 40 mm Tris-HCl, 300 mm NaCl, and 1% Triton X-100, pH 8; 40 mm Tris-HCl, 300 mm NaCl, 50 mm sodium cholate, and 5 mm immidazole, pH 8; and 40 mm Tris-HCl, 300 mm NaCl, and 10 mm immidazole, pH 8. MSP was eluted with a 10–column volume gradient of the elution buffer (40 mm Tris-HCl, 300 mm NaCl, and 100 mm immidazole, pH 8). Purified biotinylated MSP was dialyzed into 25 mm Tris, 150 mm NaCl, 0.5 mm EDTA, pH 7.2; concentrated to ∼5 mg/ml; snap frozen in liquid nitrogen; and stored at −80 °C until use.
Isolation of yeast vacuoles
Yeast vacuoles were isolated by flotation on a Ficoll density gradient as described in Ref. 51. Briefly, SF838–5Aɑ vma2Δ::Nat with a Flag tag on the C terminus of vph1 (a subunit) was grown to an A595 of ∼1.0 in YPD pH 5. 12 liters of cells were harvested by centrifugation at 5000 × g for 30 min. The pellet was washed and resuspended in 100 ml of 1.2 m sorbitol with ∼15 mg of zymolyase to form spheroplasts. The spheroplasts were recovered in 100 ml each of 2.4 m sorbitol and 2× YPD and then resuspended in buffer containing 12% Ficoll 400. The suspension was homogenized in a Dounce homogenizer and centrifuged at 71,000 × g for 40 min. Vacuole wafers from the top of the gradient were extracted, homogenized in buffer containing 8% Ficoll, and centrifuged at 71,000 × g for 40 min. The final vacuole wafers were resuspended in 1.5 mm Mes-Tris, pH 7.0, 5% glycerol, and 1 mm β-mercaptoethanol. Vacuolar protein concentration was measured using a modified BCA assay (18), and the vacuoles were frozen in liquid nitrogen until further use.
Extraction of Vo and reconstitution into lipid nanodiscs
Three batches of purified vacuoles (12 liters each) were typically used for one extraction as described in Ref. 29. Briefly, thawed vacuoles were combined, supplemented with protease inhibitors, and solubilized using 1.2 mg of n-dodecyl β-d-maltopyranoside/1 mg of vacuolar protein. To the detergent-solubilized sample, purified biotinylated MSP was added in a molar ratio of 1:50 (vacuolar protein:MSP). The mixture containing vacuolar protein, vacuolar lipids, and MSP was incubated at 4 °C for 1 h followed by detergent removal using bio-beads. Reconstituted vacuolar membrane proteins in biotinylated and endogenous vacuolar lipid containing nanodiscs were subjected to anti-FLAG affinity chromatography to purify Vo-containing nanodiscs. The eluate from the FLAG column was then subjected to size-exclusion chromatography using a Superdex 200 1 × 30-cm column. Peak fractions from gel filtration were combined and concentrated using a Vivaspin 100,000 molecular weight cutoff concentrator.
Purification of the chimeric H subunit (Hchim)
Escherichia coli Rosetta2 (Novagen) cells expressing N-terminal MBP-tagged Hchim were grown to an A600 of ∼0.5 (in LB, 0.2% glucose, 50 μg/ml carbenicillin, and 34 μg/ml chloramphenicol), and expression was induced with 0.5 mm isopropyl β-d-thiogalactopyranoside at 30 °C for 4 h. Protein was purified using amylose affinity chromatography, and the MBP tag was cleaved with PreScission protease as previously described (31). The pH of the cleavage product was adjusted to 7 by overnight dialysis in 25 mm sodium phosphate, pH 7, 0.5 mm EDTA, and 5 mm β-mercaptoethanol. At pH 7, Hchim has a predicted charge of +3.5, whereas the predicted charge of MBP is −9 (Protein Calculator v3.4), allowing separation of the two proteins using cation exchange (carboxymethyl) chromatography. The cleaved MBP came off the carboxymethyl column in the flow through and wash steps, whereas pure Hchim was eluted in dialysis buffer supplemented with 100 mm NaCl. The preparation was subjected to a final step using a Superdex 200 1.6 × 50-cm size-exclusion chromatography column.
Purification of V1 mutants and subunit C
WT V1, V1ΔH and V1ΔC were purified from vma10Δ::KanMX (29), vma13Δ::KanMX, vma10Δ::Nat (34), and vma5Δ::Nat, vma10Δ::URA3 (16), respectively, as described in Ref. 16. In all cases, yeast strains were transformed with a pRS315 vector containing N-terminally FLAG tagged vma10 (G subunit) for affinity purification on αFLAG agarose (33). Cells were grown to an A595 of ∼4 in synthetic dropout medium without leucine (SD −Leu) and harvested by centrifugation at 4000 × g for 15 min. The cells were lysed by ∼15 passes through a microfluidizer (Microfluidics M-110L). Unbroken cells were pelleted by centrifugation at 4000 × g for 30 min, and the resultant supernatant was cleared by centrifugation at 13,000 × g for 40 min. Cleared lysate was subjected to affinity chromatography using αFLAG resin. The eluate from the αFLAG column was concentrated and subjected to size-exclusion chromatography using a Superdex 200 1.6 × 50 cm column.
For preparation of V1Hchim and V1Hwt, V1ΔH eluted from the αFLAG column was incubated for 1 h at 4 °C with a ∼5-fold molar excess of either Hchim or Hwt (purified as in Ref. 30) to form the V1Hchim and V1Hwt complexes, respectively. V1 bound to Hchim or Hwt was then separated from the excess of Hchim or Hwt by size-exclusion chromatography using a Superdex 200 1.6 × 50-cm column. Subunit C was purified as previously described (31).
Biolayer interferometry
Interaction of Vo with the purified V1 mutants was screened using BLI, a light interference–based technique, similar to surface plasmon resonance. An Octet-RED system with streptavidin coated biosensors (FortéBio, SA biosensors, catalog no. 18-5019) were used for the experiments. All BLI experiments were conducted using 25 mm Tris-HCl, pH 7.2, 150 mm NaCl, 0.5 mm EDTA, 1 mm β-mercaptoethanol, 0.5 mg/ml BSA, except for experiments analyzing V1Hchim release from immobilized MBP-C, which required 10 mg/ml BSA because of an increased propensity of chimeric H containing V1 to bind nonspecificically to the BLI sensors. The temperature was maintained at 22 °C, with each biosensor stirred in 0.2 ml of sample at 1000 rpm and a standard measurement rate of 5 s−1. Streptavidin-coated biosensors were prewetted in BLI buffer and then dipped in wells containing 3 μg/ml of biotinylated VoND. A buffer control was included to show that none of the buffer components interacted with the sensors. Details of individual experiments have been described in the respective figure legends. The affinity of interaction between V1ΔC and MBP-C was measured using anti-mouse IgG Fc capturing biosensors (FortéBio, AMC biosensors catalog no. 18-5088) as described (30).
ATPase activity assay
MgATPase activity of purified V1 mutants and reconstitution mixtures (V1 mutants + VoND + subunit C) was measured using a coupled enzyme assay as described in Ref. 16. Briefly, 10 μg of the V1 mutant was added to an assay mixture containing 1 mm MgCl2, 5 mm ATP, 30 units/ml each of lactate dehydrogenase and pyruvate kinase, 0.5 mm NADH, 2 mm phosphoenolpyruvate, and 50 mm HEPES, pH 7.5, at 37 °C. The decrease of absorbance at 340 nm corresponding to the decline of NADH in the system was measured in the kinetics mode on a Varian Cary Bio100 spectrophotometer. In case of reconstitution mixtures, 20 μg of V1 mutant with equimolar amounts of VoND, and a 2× molar excess of C subunit was added to an assay containing 4 mm MgCl2.
Author contributions
S. S., R. A. O., and S. W. conceptualization; S. S., R. A. O., and M. M. K. investigation; S. S. writing-original draft; R. A. O. and S. W. writing-review and editing; S. W. funding acquisition.
Supplementary Material
Acknowledgments
We thank Dr. Patricia Kane for reagents and many helpful discussions and Dr. Thomas Duncan for assistance with BLI data collection and analysis.
This work was supported by National Institutes of Health Grant GM058600 (to S. W.). The authors declare that they have no conflicts of interest with the contents of this article. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This article contains Figs. S1–S5.
- V-ATPase
- vacuolar H+-ATPase
- V1
- ATPase sector of the V-ATPase
- Vo
- membrane sector of the V-ATPase
- aNT
- N-terminal cytoplasmic domain of the a subunit
- aCT
- C-terminal transmembrane domain of the a subunit
- HCT
- C-terminal domain of the H subunit
- HNT
- N-terminal domain of the H subunit
- MBP
- maltose-binding protein
- BLI
- biolayer interferometry
- ConA
- concanamycin A
- MSP
- membrane scaffold protein.
References
- 1. Forgac M. (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat. Rev. Mol. Cell Biol. 8, 917–929 10.1038/nrm2272 [DOI] [PubMed] [Google Scholar]
- 2. Karet F. E., Finberg K. E., Nelson R. D., Nayir A., Mocan H., Sanjad S. A., Rodriguez-Soriano J., Santos F., Cremers C. W., Di Pietro A., Hoffbrand B. I., Winiarski J., Bakkaloglu A., Ozen S., Dusunsel R., et al. (1999) Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat. Genet. 21, 84–90 10.1038/5022 [DOI] [PubMed] [Google Scholar]
- 3. Thudium C. S., Jensen V. K., Karsdal M. A., and Henriksen K. (2012) Disruption of the V-ATPase functionality as a way to uncouple bone formation and resorption: a novel target for treatment of osteoporosis. Curr. Protein Pept. Sci. 13, 141–151 10.2174/138920312800493133 [DOI] [PubMed] [Google Scholar]
- 4. Williamson W. R., and Hiesinger P. R. (2010) On the role of v-ATPase V0a1-dependent degradation in Alzheimer disease. Commun. Integr. Biol. 3, 604–607 10.4161/cib.3.6.13364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun-Wada G. H., Toyomura T., Murata Y., Yamamoto A., Futai M., and Wada Y. (2006) The a3 isoform of V-ATPase regulates insulin secretion from pancreatic β-cells. J. Cell Sci. 119, 4531–4540 10.1242/jcs.03234 [DOI] [PubMed] [Google Scholar]
- 6. Brown D., Smith P. J., and Breton S. (1997) Role of V-ATPase-rich cells in acidification of the male reproductive tract. J. Exp. Biol. 200, 257–262 [DOI] [PubMed] [Google Scholar]
- 7. Sennoune S. R., Bakunts K., Martínez G. M., Chua-Tuan J. L., Kebir Y., Attaya M. N., and Martínez-Zaguilan R. (2004) Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am. J. Physiol. Cell Physiol. 286, C1443–C1452 10.1152/ajpcell.00407.2003 [DOI] [PubMed] [Google Scholar]
- 8. Fais S., De Milito A., You H., and Qin W. (2007) Targeting vacuolar H+-ATPases as a new strategy against cancer. Cancer Res. 67, 10627–10630 10.1158/0008-5472.CAN-07-1805 [DOI] [PubMed] [Google Scholar]
- 9. Kane P. M. (2012) Targeting reversible disassembly as a mechanism of controlling V-ATPase activity. Curr. Protein Pept. Sci. 13, 117–123 10.2174/138920312800493142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Oot R. A., Couoh-Cardel S., Sharma S., Stam N. J., and Wilkens S. (2017) Breaking up and making up: the secret life of the vacuolar H+-ATPase. Protein Sci. 26, 896–909 10.1002/pro.3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muench S. P., Trinick J., and Harrison M. A. (2011) Structural divergence of the rotary ATPases. Q. Rev. Biophys. 44, 311–356 10.1017/S0033583510000338 [DOI] [PubMed] [Google Scholar]
- 12. Kane P. M. (1995) Disassembly and reassembly of the yeast vacuolar H+-ATPase in vivo. J. Biol. Chem. 270, 17025–17032 [PubMed] [Google Scholar]
- 13. Sumner J. P., Dow J. A., Earley F. G., Klein U., Jäger D., and Wieczorek H. (1995) Regulation of plasma membrane V-ATPase activity by dissociation of peripheral subunits. J. Biol. Chem. 270, 5649–5653 10.1074/jbc.270.10.5649 [DOI] [PubMed] [Google Scholar]
- 14. Parra K. J., and Kane P. M. (1998) Reversible association between the V1 and V0 domains of yeast vacuolar H+-ATPase is an unconventional glucose-induced effect. Mol. Cell Biol. 18, 7064–7074 10.1128/MCB.18.12.7064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parra K. J., Keenan K. L., and Kane P. M. (2000) The H subunit (Vma13p) of the yeast V-ATPase inhibits the ATPase activity of cytosolic V1 complexes. J. Biol. Chem. 275, 21761–21767 10.1074/jbc.M002305200 [DOI] [PubMed] [Google Scholar]
- 16. Oot R. A., Kane P. M., Berry E. A., and Wilkens S. (2016) Crystal structure of yeast V1-ATPase in the autoinhibited state. EMBO J. 35, 1694–1706 10.15252/embj.201593447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang J., Myers M., and Forgac M. (1992) Characterization of the V0 domain of the coated vesicle (H+)-ATPase. J. Biol. Chem. 267, 9773–9778 [PubMed] [Google Scholar]
- 18. Couoh-Cardel S., Milgrom E., and Wilkens S. (2015) Affinity purification and structural features of the yeast vacuolar ATPase Vo membrane sector. J. Biol. Chem. 290, 27959–27971 10.1074/jbc.M115.662494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Trombetta E. S., Ebersold M., Garrett W., Pypaert M., and Mellman I. (2003) Activation of lysosomal function during dendritic cell maturation. Science 299, 1400–1403 10.1126/science.1080106 [DOI] [PubMed] [Google Scholar]
- 20. Stransky L. A., and Forgac M. (2015) Amino acid availability modulates vacuolar H+-ATPase assembly. J. Biol. Chem. 290, 27360–27369 10.1074/jbc.M115.659128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bodzeta A., Kahms M., and Klingauf J. (2017) The presynaptic v-ATPase reversibly disassembles and thereby modulates exocytosis but is not part of the fusion machinery. Cell Rep. 20, 1348–1359 10.1016/j.celrep.2017.07.040 [DOI] [PubMed] [Google Scholar]
- 22. Parra K. J., Chan C. Y., and Chen J. (2014) Saccharomyces cerevisiae vacuolar H+-ATPase regulation by disassembly and reassembly: one structure and multiple signals. Eukaryot. Cell 13, 706–714 10.1128/EC.00050-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhao J., Benlekbir S., and Rubinstein J. L. (2015) Electron cryomicroscopy observation of rotational states in a eukaryotic V-ATPase. Nature 521, 241–245 10.1038/nature14365 [DOI] [PubMed] [Google Scholar]
- 24. Liu M., Tarsio M., Charsky C. M., and Kane P. M. (2005) Structural and functional separation of the N- and C-terminal domains of the yeast V-ATPase subunit H. J. Biol. Chem. 280, 36978–36985 10.1074/jbc.M505296200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mazhab-Jafari M. T., Rohou A., Schmidt C., Bueler S. A., Benlekbir S., Robinson C. V., and Rubinstein J. L. (2016) Atomic model for the membrane-embedded VO motor of a eukaryotic V-ATPase. Nature 539, 118–122 10.1038/nature19828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Stam N. J., and Wilkens S. (2017) Structure of the lipid nanodisc-reconstituted vacuolar ATPase proton channel: definition of the interaction of rotor and stator and implications for enzyme regulation by reversible dissociation. J. Biol. Chem. 292, 1749–1761 10.1074/jbc.M116.766790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roh S. H., Stam N. J., Hryc C. F., Couoh-Cardel S., Pintilie G., Chiu W., and Wilkens S. (2018) The 3.5-A cryoEM structure of nanodisc-reconstituted yeast vacuolar ATPase Vo proton channel. Mol. Cell 69, 993–1004.e3 10.1016/j.molcel.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Parra K. J., and Kane P. M. (1996) Wild-type and mutant vacuolar membranes support pH-dependent reassembly of the yeast vacuolar H+-ATPase in vitro. J. Biol. Chem. 271, 19592–19598 10.1074/jbc.271.32.19592 [DOI] [PubMed] [Google Scholar]
- 29. Sharma S., and Wilkens S. (2017) Biolayer interferometry of lipid nanodisc-reconstituted yeast vacuolar H+-ATPase. Protein Sci. 26, 1070–1079 10.1002/pro.3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sharma S., Oot R. A., and Wilkens S. (2018) MgATP hydrolysis destabilizes the interaction between subunit H and yeast V1-ATPase, highlighting H's role in V-ATPase regulation by reversible disassembly. J. Biol. Chem. 293, 10718–10730 10.1074/jbc.RA118.002951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Oot R. A., and Wilkens S. (2010) Domain characterization and interaction of the yeast vacuolar ATPase subunit C with the peripheral stator stalk subunits E and G. J. Biol. Chem. 285, 24654–24664 10.1074/jbc.M110.136960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Oot R. A., and Wilkens S. (2012) Subunit interactions at the V1–Vo interface in yeast vacuolar ATPase. J. Biol. Chem. 287, 13396–13406 10.1074/jbc.M112.343962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Z., Charsky C., Kane P. M., and Wilkens S. (2003) Yeast V1-ATPase: affinity purification and structural features by electron microscopy. J. Biol. Chem. 278, 47299–47306 10.1074/jbc.M309445200 [DOI] [PubMed] [Google Scholar]
- 34. Diab H., Ohira M., Liu M., Cobb E., and Kane P. M. (2009) Subunit interactions and requirements for inhibition of the yeast V1-ATPase. J. Biol. Chem. 284, 13316–13325 10.1074/jbc.M900475200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oot R. A., Huang L. S., Berry E. A., and Wilkens S. (2012) Crystal structure of the yeast vacuolar ATPase heterotrimeric EGC(head) peripheral stalk complex. Structure 20, 1881–1892 10.1016/j.str.2012.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Curtis K. K., Francis S. A., Oluwatosin Y., and Kane P. M. (2002) Mutational analysis of the subunit C (Vma5p) of the yeast vacuolar H+-ATPase. J. Biol. Chem. 277, 8979–8988 10.1074/jbc.M111708200 [DOI] [PubMed] [Google Scholar]
- 37. Wilkens S., Vasilyeva E., and Forgac M. (1999) Structure of the vacuolar ATPase by electron microscopy. J. Biol. Chem. 274, 31804–31810 10.1074/jbc.274.45.31804 [DOI] [PubMed] [Google Scholar]
- 38. Zhang Z., Zheng Y., Mazon H., Milgrom E., Kitagawa N., Kish-Trier E., Heck A. J., Kane P. M., and Wilkens S. (2008) Structure of the yeast vacuolar ATPase. J. Biol. Chem. 283, 35983–35995 10.1074/jbc.M805345200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ho M. N., Hirata R., Umemoto N., Ohya Y., Takatsuki A., Stevens T. H., and Anraku Y. (1993) VMA13 encodes a 54-kDa vacuolar H+-ATPase subunit required for activity but not assembly of the enzyme complex in Saccharomyces cerevisiae. J. Biol. Chem. 268, 18286–18292 [PubMed] [Google Scholar]
- 40. Tabke K., Albertmelcher A., Vitavska O., Huss M., Schmitz H. P., and Wieczorek H. (2014) Reversible disassembly of the yeast V-ATPase revisited under in vivo conditions. Biochem. J. 462, 185–197 10.1042/BJ20131293 [DOI] [PubMed] [Google Scholar]
- 41. Xu T., and Forgac M. (2001) Microtubules are involved in glucose-dependent dissociation of the yeast vacuolar [H+]-ATPase in vivo. J. Biol. Chem. 276, 24855–24861 10.1074/jbc.M100637200 [DOI] [PubMed] [Google Scholar]
- 42. Smardon A. M., Nasab N. D., Tarsio M., Diakov T. T., and Kane P. M. (2015) Molecular interactions and cellular itinerary of the yeast RAVE (regulator of the H+-ATPase of vacuolar and endosomal membranes) complex. J. Biol. Chem. 290, 27511–27523 10.1074/jbc.M115.667634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chan C. Y., and Parra K. J. (2014) Yeast phosphofructokinase-1 subunit Pfk2p is necessary for pH homeostasis and glucose-dependent vacuolar ATPase reassembly. J. Biol. Chem. 289, 19448–19457 10.1074/jbc.M114.569855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lu M., Ammar D., Ives H., Albrecht F., and Gluck S. L. (2007) Physical interaction between aldolase and vacuolar H+-ATPase is essential for the assembly and activity of the proton pump. J. Biol. Chem. 282, 24495–24503 10.1074/jbc.M702598200 [DOI] [PubMed] [Google Scholar]
- 45. Puopolo K., and Forgac M. (1990) Functional reassembly of the coated vesicle proton pump. J. Biol. Chem. 265, 14836–14841 [PubMed] [Google Scholar]
- 46. Xie X. S., Crider B. P., Ma Y. M., and Stone D. K. (1994) Role of a 50–57-kDa polypeptide heterodimer in the function of the clathrin-coated vesicle proton pump. J. Biol. Chem. 269, 25809–25815 [PubMed] [Google Scholar]
- 47. Toei M., Saum R., and Forgac M. (2010) Regulation and isoform function of the V-ATPases. Biochemistry 49, 4715–4723 10.1021/bi100397s [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kawasaki-Nishi S., Nishi T., and Forgac M. (2001) Yeast V-ATPase complexes containing different isoforms of the 100-kDa a-subunit differ in coupling efficiency and in vivo dissociation. J. Biol. Chem. 276, 17941–17948 10.1074/jbc.M010790200 [DOI] [PubMed] [Google Scholar]
- 49. Smardon A. M., Diab H. I., Tarsio M., Diakov T. T., Nasab N. D., West R. W., and Kane P. M. (2014) The RAVE complex is an isoform-specific V-ATPase assembly factor in yeast. Mol. Biol. Cell 25, 356–367 10.1091/mbc.e13-05-0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Funakoshi M., and Hochstrasser M. (2009) Small epitope-linker modules for PCR-based C-terminal tagging in Saccharomyces cerevisiae. Yeast 26, 185–192 10.1002/yea.1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Uchida E., Ohsumi Y., and Anraku Y. (1985) Purification and properties of H+-translocating, Mg2+-adenosine triphosphatase from vacuolar membranes of Saccharomyces cerevisiae. J. Biol. Chem. 260, 1090–1095 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.