Abstract
α-Klotho, a known anti-aging protein, exerts diverse physiological effects including: maintenance of phosphate and calcium homeostasis, modulation of cell proliferation, and enhanced buffering of reactive oxygen species. However, the role of α-Klotho in the regulation of energy metabolism is complex and poorly understood. Here we investigated the effects of 5 weeks peripheral administration of α-Klotho in high fat diet induced obese mice. Food intake, blood glucose, and body weight were measured daily. Energy expenditure was determined with indirect calorimetry and body composition with magnetic resonance imaging. Liver and adipose tissue were collected for lipid content measurements and gene expression analysis. α-Klotho-treated mice experienced reduced adiposity, increased lean mass, and elevated energy expenditure, despite no changes in food intake, body weight, or fed blood glucose levels. Lipid accumulation in liver and adipose tissue was also reduced compared to controls. Furthermore, Real-time quantitative PCR showed reduced expression of key lipogenic genes in α-Klotho treated mice in these organs. Taken together, these data suggest encouraging therapeutic potential of α-Klotho and highlight a need for further research into the specific mechanisms explaining improved body composition, elevated energy expenditure, and reduced lipid content in both liver and adipose tissue in α-Klotho-treated mice.
Keywords: Molecular biology, Physiology
1. Introduction
Obesity is associated with dysregulated body-weight homeostasis, and is the primary risk factor for the development of type 2 diabetes, hypertension, and cardiovascular disease [1]. Obesity continues to reach epidemic proportions in North America, elevating morbidity and mortality rates, while providing a cumbersome economic burden. These trends highlight a need for further development in the field of metabolic physiology, an area that has been advanced by the identification of endogenous compounds regulating food intake and energy expenditure.
One such compound is α-Klotho, an anti-aging protein secreted by the kidney and the brain to exhibit multiple hormonal effects [2] including: maintenance of vitamin D and calcium homeostasis [3, 4], modulation of cell senescence [5], and production of reactive oxygen species buffering enzymes [6]. α-Klotho may also be associated with whole-body energy metabolism, evidenced by impaired circulating α-Klotho concentrations in obese and diabetic human patients [7, 8]. α-Klotho overexpression in db/db mice attenuates hyperglycemia and hyperphagia [6], and can even extend the lifespan in healthy mice [9]. Furthermore, genetic deletion of α-Klotho suppresses uncoupling protein 1 gene expression, decreases temperature in brown adipose tissue, and trends to increase food intake relative to body weight [10].
Despite this encouraging evidence, the specific role of α-Klotho in regulation of energy homeostasis is complicated and not completely understood. For example, similar to overexpression models, genetic knockdown of α-Klotho in mice also increases insulin sensitivity [11]. α-Klotho provides negative feedback to insulin and IGF-1 signaling to modulate cell proliferation and improve tolerance to oxidative stress [12, 13, 14, 15], but also facilitates insulin release via transient receptor protein channel modification and improved calcium flux [16]. Additionally, α-Klotho deletion promotes extreme leanness, but ultimately results in premature death in mouse models [9, 17]. These mice experience drastically impaired development, manifesting as osteoporosis, reduced adiposity, and atrophied organs [10, 17].
Overall, α-Klotho's exact involvement in the pathophysiology, or even treatment, of obesity is not understood completely. Models genetically altering α-Klotho levels provide encouraging evidence indicating a prominent role of α-Klotho in whole-body energy metabolism, however, these models have conflicting results and lack insight into α-Klotho's tissue-specific mechanisms of action. Consequently, this study aimed to investigate the effects of intraperitoneal α-Klotho administration on the physiology of metabolically active organs. Furthermore, this model provides a direct translational look into the therapeutic potential of α-Klotho to treat obesity and metabolic disease.
2. Materials and methods
2.1. Materials
2.1.1. Animals
All aspects of animal care and experimentation were conducted in accordance with the National Institutes Health “Guide for the Care and Use of Laboratory Animals” (NIH Publication No. 85-23, revised 1996) and approved by the Institutional Animal Care and Use Committees of East Carolina Diabetes and Obesity Institute. 24 C57BL6 male mice (JAX Stock No: 00664) from Jackson lab (The Jackson Laboratory, Bar Harbor, Maine) were fed a high-fat diet with a kilocalorie composition of 58%, 25%, and 17% fat, carbohydrate, and protein respectively for 8 weeks (D12331, Research Diets, New Brunswick, NJ). Mice were housed under controlled temperatures at 22–24 °C and a 12 h light/12 h dark cycle.
2.1.2. Reagent
The recombinant mouse α-Klotho protein (aa 35-982) was obtained from R & D Systems (Minneapolis, MN).
2.2. Methods
2.2.1. Treatment protocol
Six-week-old, male, C57BL/6 mice were fed a high-fat diet (HFD) for 8 weeks. Mice were then intraperitoneally treated with α-Klotho protein (0.02 mg/kg) or saline every 48 h. for 5 weeks. At week 18 (4 weeks into α-Klotho or saline treatment) indirect calorimetry was performed and at week 19 mice were euthanized for further analysis of metabolically active tissues.
2.2.2. Body composition and food intake
Body weight and food intake were recorded weekly. Total fat and lean mass were assessed using EchoMRI (Echo Medical Systems, Houston, TX). Epididymal white adipose tissue (eWAT) was harvested. Mice were individually housed for 1 week, followed by weekly measurement of food intake. Food intake data were combined, averaged, and analyzed by unpaired Student's t tests. To maximize accuracy of food intake measurements, a white bedding paper under the food bowl was used to collect residual food crumbs and all uneaten food was measured. This amount was subtracted from the total food given to calculate total amount of food intake.
2.2.3. Energy expenditure
Energy expenditure was measured by assessing oxygen consumption with indirect calorimetry. Individually housed male mice on a HFD and 18 weeks of age (4 weeks into α-Klotho or saline treatment) were studied, using the Comprehensive Lab Animal Monitoring System (CLAMS, Columbus Instruments, Columbus, OH). Mice were acclimated in the CLAMS chambers for 72 hours before data collection. Mice had free access to food and water for the duration of the studies. Values from individual time points were totaled and analyzed by unpaired Student's t tests comparing two groups. All data were normalized for body weight.
2.2.4. Histopathology
At the end of the experiment, livers were frozen in liquid hydrogen and then cryostat-sectioned at a thickness of 5 μm onto poly-l-lysine slides for lipid deposition analysis by Oil Red O staining. eWAT was frozen in liquid hydrogen and then cryostat-sectioned at a thickness of 20 μm onto poly-l-lysine slides for adipocyte size measurement. The red areas and adipocyte size were quantified by Image J [18].
2.2.5. Quantitative real-time PCR
Total RNA from liver or eWAT was extracted using TRIzol reagent (Invitrogen) and subjected to quantitative real-time PCR as described previously [19]. Gene-specific primer sequences are listed in Supplemental Table 1. Relative gene expressions were calculated with the delta-delta CT method with 18s ribosomal RNA normalization, using ViiA 7 system (Applied Biosystems).
2.2.6. Triglyceride content in blood, adipose tissue and liver
To quantify changes in triglyceride concentrations in blood, adipose tissue, and liver, a Triglyceride Colorimetric Assay Kit (Cat. # 10010303, Cayman Chemical, Ann Arbor MI) was used according to the manufacturer's instruction. Each biological replicate was analyzed in duplicate.
2.2.7. Statistical analysis
Data are expressed as the mean ± SEM. Unpaired Student's t tests were used throughout this study to compare two distinct groups using GraphPad Prism 5 software (San Diego, CA). P < 0.05 was considered statistically significant.
3. Results
3.1. α-Klotho improves body composition in obese mice despite no changes in food intake and body weight
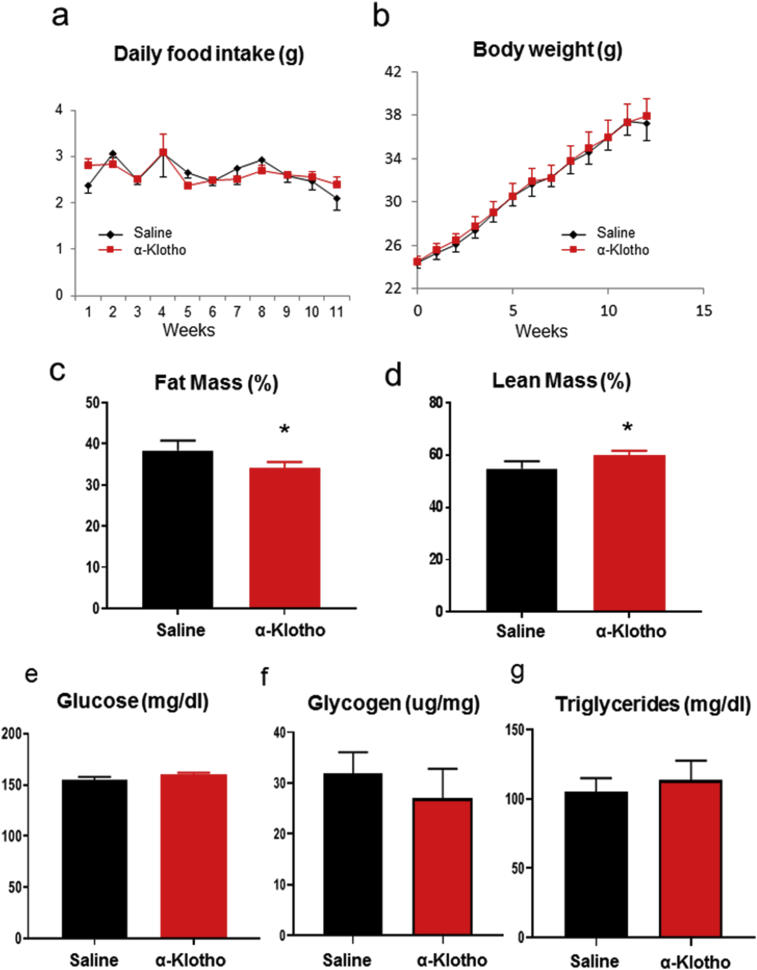
There were no differences in food intake and body weight between α-Klotho and saline groups throughout the experiment (Fig. 1a and b). Despite no changes in body weight, at the end of the experiment fat mass was decreased (Saline: 38.3% ± 1.0% versus α-Klotho: 34.0% ± 0.6%) (Fig. 1c) and lean mass was increased (Saline: 54.8% ± 1.2% versus α-Klotho: 60.0% ± 0.7%) (Fig. 1d) in α-Klotho treated mice. There were no differences in fed glucose levels, liver glycogen contents, and serum triglyceride concentrations between groups (Fig. 1e, f, g).
Fig. 1.
α-Klotho decreases fat mass and increases lean mass without changing body weight or food intake. (a) Food intake, (b) Body weight, (c) Percent fat mass, (d) Percent lean mass, (e) Fed glucose levels, (f) Liver glycogen content, and (g) Serum triglyceride concentrations in α-Klotho treated and control mice. Bar graphs show Mean + SEM. n = 6–7 per group; * indicates p < 0.05 vs saline group.
3.2. α-Klotho increases energy expenditure in obese mice
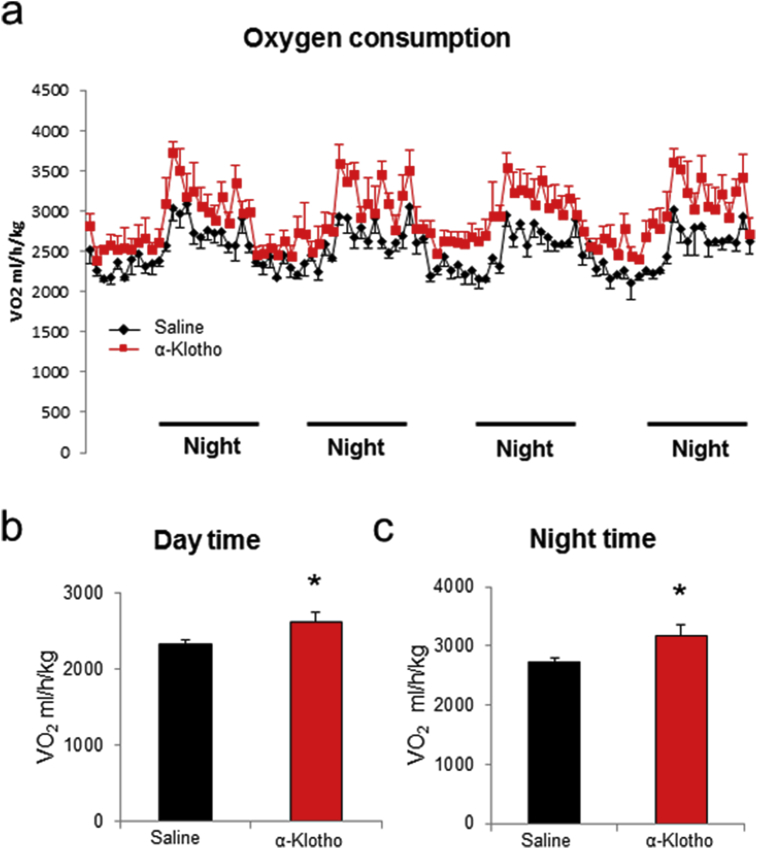
Energy expenditure was measured via indirect calorimetry using the Comprehensive Lab Animal Monitoring System over 7 days. Data were collected and analyzed from the last four days (Fig. 2a). α-Klotho-treated mice show significantly increased oxygen consumption during both day time (Saline: 2333 ± 113 ml/h/kg versus α-Klotho: 2628 ± 206 ml/h/kg) (Fig. 2b) and night time (Saline: 2726 ± 135 ml/h/kg versus α-Klotho: 3182 ± 263 ml/h/kg) (Fig. 2c).
Fig. 2.
α-Klotho increases energy expenditure. (a) Oxygen consumption normalized to body weight, (b) Oxygen consumption during the day time, (c) and night time in α-Klotho treated and control mice. Bar graphs show Mean + SEM. n = 6–7 per group. * indicates p < 0.05 vs saline group.
3.3. α-Klotho reduces lipid accumulation in liver and white adipose tissue of obese mice
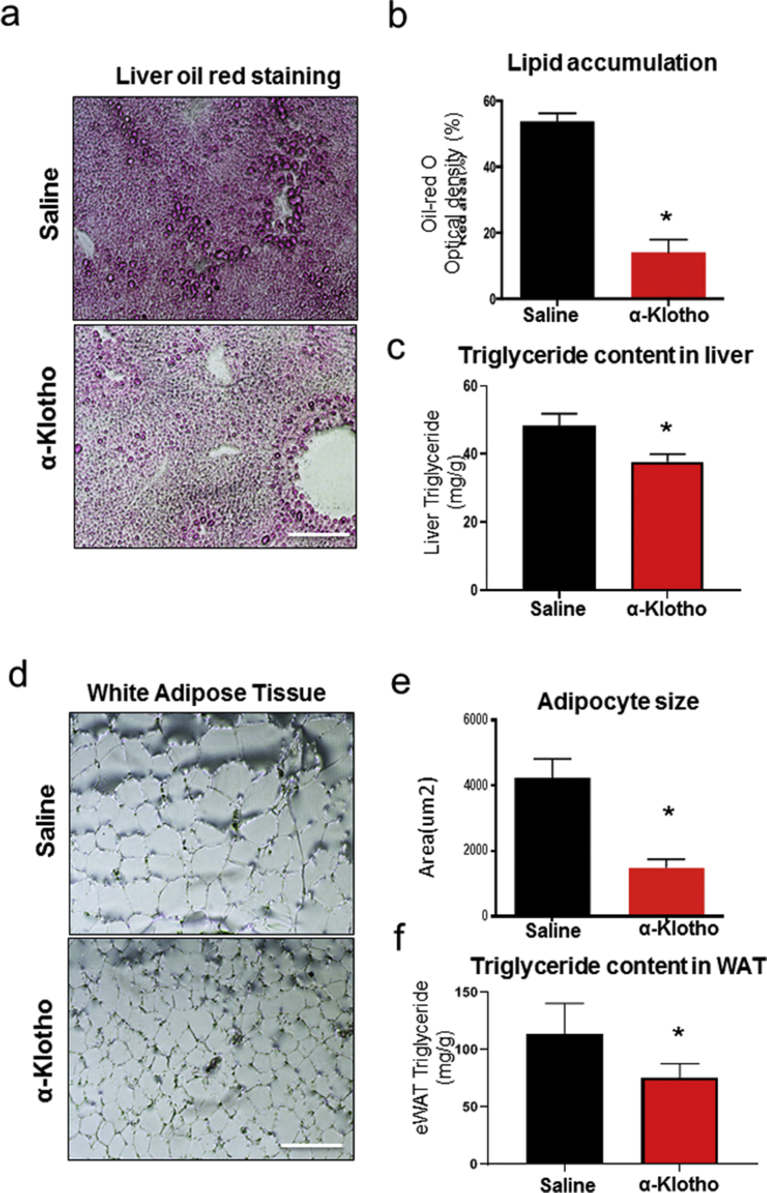
5-weeks administration of α-Klotho decreased lipid accumulation in the liver (Fig. 3a and b) (Frozen sections were stained by Oil Red O) and quantified by Image J (Fig. 3b). Triglyceride content was significantly decreased in α-Klotho treated mice compared with controls (Saline: 48.4 ± 3.3 mg/g versus α-Klotho: 37.7 ± 2.2 mg/g) (Fig. 3c). α-Klotho treatment also significantly decreased adipocyte size (Fig. 3d and e) and triglyceride content in epididymal white adipose tissue (Saline: 113.2 ± 25.2 mg/g versus α-Klotho: 75.7 ± 11.9 mg/g) (Fig. 3f).
Fig. 3.
α-Klotho reduces liver lipid content and decreases adipocyte size. (a) Oil red o staining of liver, (b) Quantification of lipid area, and (c) liver triglyceride content, (d) Representative images of eWAT sections, (e) Quantification of adipocyte size, and (f) Epididymal white adipose tissue triglyceride content in α-Klotho treated and control mice. Bar graphs show Mean + SEM. n = 6–7 per group; The scale bars represent 50 μm * indicates p < 0.05 vs saline group.
3.4. α-Klotho modulates key gene expression related to lipid metabolism in liver and white adipose tissue
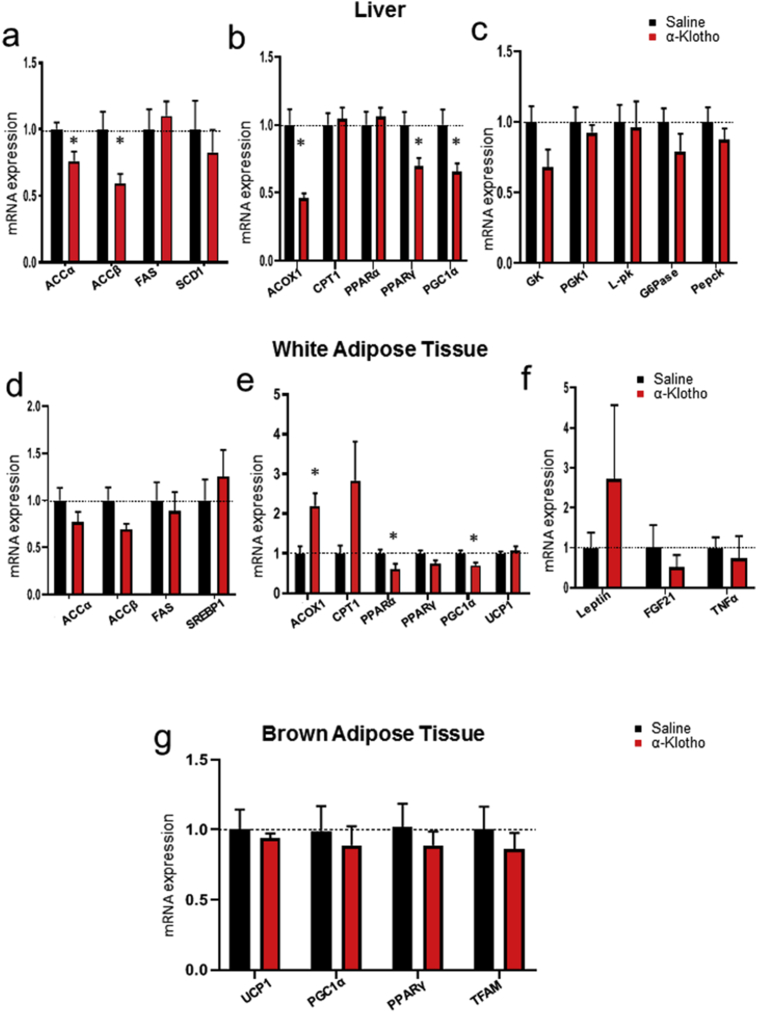
To elucidate the molecular mechanism of α-Klotho's lipid lowering effects, we investigated expression of key genes related to lipid metabolism in liver and adipose tissue. Compared to saline-treated controls, α-Klotho treated mice had changes in liver gene expression associated with decreased lipogenesis, including reductions in ACC-α (0.75 fold) and ACC-β (0.60 fold) (Fig. 4a). Interestingly, ACOX1 (0.48 fold), PGC1α (0.6 fold), and PPARγ (0.65 fold), genes associated with fat oxidation, were also decreased in the livers of the α-Klotho group compared to controls (Fig. 4b). There were no changes in key gene expressions involved in hepatic glucose metabolism (Fig. 4c). In white adipose tissue, α-Klotho-treated mice showed significantly increased ACOX1 (2.2 fold) gene expression, while PPARα (0.63 fold) and PGC1α (0.7 fold) were decreased compared with controls (Fig. 4e). We also found strong trends toward decreased PPARγ and ACC-β and strong trends toward increased CPT1 and leptin in the adipose tissue of α-Klotho treated mice compared with controls (Fig. 4d, e and f). In brown adipose tissue, there was no alteration in key gene expression involved in thermogenesis (Fig. 4g).
Fig. 4.
The effects of α-Klotho on gene expression in liver, eWAT, and BAT. (a) Hepatic levels of key gene expression involved in lipogenesis, (b) fat oxidation, (c) glucose metabolism. (d) eWAT levels of key gene expression involved in in lipogenesis, (e) fat oxidation, (f) secreting cytokines. (g) BAT levels of key gene expression involved in thermogenesis in α-Klotho treated and control mice. Bar graphs show Mean + SEM. n = 6–7 per group; * indicates p < 0.05 vs saline group.
4. Discussion
The discovery of the “anti-aging protein” α-Klotho has generated research interest that has significantly advanced our current understanding of the aging process. Although α-Klotho was identified nearly 20 years ago, its specific function involving energy metabolism and related disease-states remains incompletely understood. We investigated the effects of intraperitoneal α-Klotho injection in the high fat diet induced obese (DIO) mouse model. We revealed a previously uncovered beneficial role of α-Klotho in the regulation of lipid accumulation in liver and adipose tissue. Overall, α-Klotho treatment resulted in elevated energy expenditure, decreased fat mass, and increased lean mass, despite no change in food intake or body weight. Further gene expression analysis began to elucidate the tissue-specific mechanisms underlying α-Klotho regulation of liver and adipose tissue metabolism.
In the livers of DIO mice treated with α-Klotho for 5 weeks, gene expression of the two isoforms of Acetyl-CoA carboxylase (ACC) was significantly decreased, indicating reduced inhibition of CPT-1, and increased fat oxidation. In the adipose tissue of α-Klotho-treated mice, ACOX1 and CPT1 gene expression was elevated, also suggesting increased fat oxidation. Interestingly, the thermogenic gene expression (UCP1) was not altered in α-Klotho treated mice, suggesting that browning of white adipose tissue is unlikely. α-Klotho also does not increase key gene expression associated with thermogenesis in brown adipose tissue. Moreover, decreased PPARγ and PGC1α mRNA in both these tissues indicates reduced lipogenesis. Notably, there were no changes in gene expressions that related to hepatic glucose metabolism, such as Phosphoglycerate Kinase 1 (PGK1), Glucose 6-phosphatase (G6Pase), Glucokinase (GK), liver type pyruvate kinase (L-PK), and Phosphoenolpyruvate carboxykinase (PEPCK). Supporting this data, no change in fed blood glucose levels were observed. However, no assays were performed during the fasted status. Due to this limitation, we cannot rule out the possibility that α-Klotho alters glucose levels or gene expression related to glucose metabolism during fasting conditions. Nonetheless, our data suggest distinct responses in lipid upon α-Klotho administration. The general trends in α-Klotho-induced changes in liver and adipose gene expression illustrate a shift in lipid metabolism favoring oxidation rather than storage. This metabolic shift would explain the reduced lipid accumulation observed in α-Klotho-treated DIO mice.
Interestingly, α-Klotho knock-out mice experience atrophy in many metabolic organs, including adipose tissue and liver [9, 10]. One report even observed α-Klotho to induce C/EBPα- and PPARγ gene expression, which promotes adipocyte differentiation in cultured 3T3-L1 adipocytes [20]. While the source of discrepancy between the studies is not clear, it is evident that the nature of α-Klotho's role in regulation of adipogenesis and lipid metabolism is complex. Additional research is still needed and the signaling mechanisms responsible for α-Klotho-mediated changes in lipid-related gene expression remain unknown.
Overall the current study indicates improved energy expenditure and reduced adiposity in α-Klotho-treated DIO mice. This encouraging therapeutic potential is supported by past literature observing beta cell specific α-Klotho overexpression to attenuate the progression of diabetes via improved insulin release and ROS buffering [6, 16, 21]. Furthermore, in α-Klotho deficient mice, non-shivering thermogenesis and gene expression of uncoupling protein 1 are impaired, while gluconeogenic gene expression in the liver is elevated [10]. However, the role of α-Klotho in the pathophysiology of metabolic disease is complex. α-Klotho overexpressing mice live longer, but experience insulin resistance [9]. Interestingly, these mice do not experience hyperglycemia, adiposity, or hyperphagia associated with clinical insulin resistance [9]. Conversely, mice with impaired α-Klotho gene expression have increased insulin sensitivity, but experience many disease phenotypes associated with accelerated aging [10, 17]. These convoluted results may be explained by α-Klotho acting as a homeostatic modulator of insulin and IGF-1 signaling to prevent hypoglycemia, and enhance FOXO activity to reduce apoptosis and improve ROS buffering [9, 22, 23].
Currently, the major gap in knowledge regarding α-Klotho's functions continues to be uncertainty surrounding the direct targets of α-Klotho. For example, the receptor for α-Klotho has yet to be discovered. Emerging evidence suggests that α-Klotho forms constitutive scaffolding complexes with fibroblast growth factor receptors (FGFR's) 1c, 3c, and 4 to increase the affinity of these FGFRs to fibroblast growth factor (FGF23) [24]. Since FGFRs are ubiquitous, but α-Klotho expression is tissue-specific, it seems α-Klotho's presence governs whether a cell is an FGF23 target. Furthermore, FGFR's affinity to FGF23 in the absence of α-Klotho is very low, indicating α-Klotho may be required to activate FGFRs and their downstream signaling molecules [11]. It is possible that the α-Klotho-FGFR complex and subsequent signaling pathways may be responsible for α-Klotho's roles regulating energy metabolism. This hypothesis is supported by the previously described FGF21-FGFR signaling mechanisms that result in elevated energy expenditure, improved insulin signaling, and reversed hepatic steatosis in DIO mice [25, 26]. Specifically, deletion of FGFR1c in adipose tissue abolished the therapeutic ability of FGFs to lower plasma glucose, increase insulin and triglycerides, and alter serum levels of adipokines [26]. Furthermore, dominant-negative fibroblast growth factor receptor (FGFR) expression in hepatocytes of transgenic mice resulted in a fatty liver phenotype [27]. Taken together these studies suggest FGFR signaling in adipose tissue and liver is important to homeostatic regulation of fat metabolism. Future studies should investigate the hypothesis that α-Klotho may result in activation of FGFR1 and its downstream signaling pathway, leading to transcriptional changes related to lipid metabolism and reduced adiposity.
Among many signaling pathways activated by FGFRs, PI3Kinase (PI3K) signaling is involved in homeostatic regulation of energy metabolism [28, 29, 30, 31]. Activated PI3K signaling phosphorylates forkhead box proteins, FOXO1, FOXO3a, and FOXO4, preventing translocation into the nucleus, and resulting in a loss of their transcriptional activity [32]. One report suggests that FOXO1 positively regulates adipogenesis through its binding to the promoter region of PPARγ [33]. Therefore, FGFR-mediated PI3kinase signaling, and subsequent FOXO1 inhibition, could be a possible mechanism explaining reduced adiposity via decreased PPARγ activity.
Although α-Klotho is theoretically not permeable to the blood brain barrier (BBB) due to its molecular size [34], there is a recent study reporting that α-Klotho-F, a fragment of the α-Klotho protein described in this study, can acutely improve cognitive and motor functions following peripheral administration in young, aging, and hSYN transgenic mice [35]. One must consider that some of the lipid reducing effects by α-Klotho may also be associated with the central nervous system (CNS). However, how peripheral α-Klotho transmits a signal to the CNS remains to be answered. Possibilities include modification of other circulating factors that can cross the BBB or alteration of central α-Klotho levels, either directly or indirectly. Our study does not rule out that α-Klotho might also act with the CNS but, rather, poses the question of the possibility of such a phenomenon.
In conclusion, our study demonstrates that 5-weeks administration of α-Klotho improves adiposity in DIO mice, mainly via lowering the lipid accumulation in liver and adipose tissue. The observed shift in lipid metabolism favoring oxidation rather than storage in liver and adipose begins to shed light on the tissue specific mechanisms responsible for α-Klotho's role in energy metabolism. The encouraging therapeutic potential of α-Klotho shown in the current study highlights a need for further research into this phenomenon, including investigations into likely α-Klotho induced FGFR1 signaling in liver and adipose tissue.
Declarations
Author contribution statement
Zhijian Rao: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Taylor Landry: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Peixin Li: Performed the experiments.
Wyatt Bunner, Brenton Thomas Laing, Yuan Yuan: Contributed reagents, materials, analysis tools or data.
Hu Huang: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by start-up funds from East Carolina University to H. Huang.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Alec Brendan Chaves at East Carolina University for proofreading and discussion.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Kopelman P.G. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 2.Huang C.-L. Regulation of ion channels by secreted Klotho: mechanisms and implications. Kidney Int. 2010;77:855–860. doi: 10.1038/ki.2010.73. [DOI] [PubMed] [Google Scholar]
- 3.Huang C.-L., Moe O.W. Klotho: a novel regulator of calcium and phosphorus homeostasis. Pflügers Arch. Eur. J. Physiol. 2011;462:185–193. doi: 10.1007/s00424-011-0950-5. [DOI] [PubMed] [Google Scholar]
- 4.Tsujikawa H., Kurotaki Y., Fujimori T., Fukuda K., Nabeshima Y.-I. Klotho, a gene related to a syndrome resembling human premature aging, functions in a negative regulatory circuit of vitamin D endocrine system. Mol. Endocrinol. 2003;17:2393–2403. doi: 10.1210/me.2003-0048. [DOI] [PubMed] [Google Scholar]
- 5.Liu H., Fergusson M.M., Castilho R.M., Liu J., Cao L., Chen J., Malide D., Rovira I.I., Schimel D., Kuo C.J., Gutkind J.S., Hwang P.M., Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 6.Lin Y., Sun Z. In vivo pancreatic β-cell-specific expression of antiaging gene Klotho: a novel approach for preserving β-cells in type 2 diabetes. Diabetes. 2015;64:1444–1458. doi: 10.2337/db14-0632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wan Q., He Y., Yuan M. Klotho in diabetes and diabetic nephropathy: a brief update review. Int. J. Clin. Exp. Med. 2017;10:4342–4349. [Google Scholar]
- 8.Amitani M., Asakawa A., Amitani H., Kaimoto K., Sameshima N., Koyama K.I., Haruta I., Tsai M., Nakahara T., Ushikai M., Cheng K., Hamada S., Inui A. Plasma klotho levels decrease in both anorexia nervosa and obesity. Nutrition. 2013;29:1106–1109. doi: 10.1016/j.nut.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 9.Kurosu H., Yamamoto M., Clark J.D., V Pastor J., Nandi A., Gurnani P., McGuinness O.P., Chikuda H., Yamaguchi M., Kawaguchi H., Shimomura I., Takayama Y., Herz J., Kahn C.R., Rosenblatt K.P., Kuro-o M. Suppression of aging in mice by the hormone klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mori K., Yahata K., Mukoyama M., Suganami T., Makino H., Nagae T., Masuzaki H., Ogawa Y., Sugawara A., Nabeshima Y., Nakao K. Disruption of klotho gene causes an abnormal energy homeostasis in mice. Biochem. Biophys. Res. Commun. 2000;278:665–670. doi: 10.1006/bbrc.2000.3864. [DOI] [PubMed] [Google Scholar]
- 11.Kurosu H., Ogawa Y., Miyoshi M., Yamamoto M., Nandi A., Rosenblatt K.P., Baum M.G., Schiavi S., Hu M.-C., Moe O.W., Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen C.-D., Podvin S., Gillespie E., Leeman S.E., Abraham C.R. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc. Natl. Acad. Sci. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolf I., Levanon-Cohen S., Bose S., Ligumsky H., Sredni B., Kanety H., Kuro-o M., Karlan B., Kaufman B., Koeffler H.P., Rubinek T. Klotho: a tumor suppressor and a modulator of the IGF-1 and FGF pathways in human breast cancer. Oncogene. 2008;27:7094–7105. doi: 10.1038/onc.2008.292. [DOI] [PubMed] [Google Scholar]
- 14.van Heemst D. Insulin, IGF-1 and longevity. Aging Dis. 2010;1:147–157. [PMC free article] [PubMed] [Google Scholar]
- 15.Bluher M., Kahn B.B., Kahn C.R. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299:572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 16.Lin Y., Sun Z. Antiaging gene Klotho enhances glucose-induced insulin secretion by up-regulating plasma membrane levels of TRPV2 in MIN6 β-cells. Endocrinology. 2012;153:3029–3039. doi: 10.1210/en.2012-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuro-o M., Nabeshima Y., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Ohyama Y., Kurabayashi M., Kaname T., Kume E., Iwasaki H., Iida A., Shiraki-Iida T., Nishikawa S., Nagai R. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 18.Deutsch M.J., Schriever S.C., Roscher A.A., Ensenauer R. Digital image analysis approach for lipid droplet size quantitation of Oil Red O-stained cultured cells. Anal. Biochem. 2014;445:87–89. doi: 10.1016/j.ab.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 19.Do K., Laing B.T., Landry T., Bunner W., Mersaud N., Matsubara T., Li P., Yuan Y., Lu Q., Huang H. The effects of exercise on hypothalamic neurodegeneration of Alzheimer's disease mouse model. PLoS One. 2018;13 doi: 10.1371/journal.pone.0190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chihara Y., Rakugi H., Ishikawa K., Ikushima M., Maekawa Y., Ohta J., Kida I., Ogihara T. Klotho protein promotes adipocyte differentiation. Endocrinology. 2006;147:3835–3842. doi: 10.1210/en.2005-1529. [DOI] [PubMed] [Google Scholar]
- 21.Lin Y., Sun Z. Antiaging gene klotho attenuates pancreatic β-cell apoptosis in type 1 diabetes. Diabetes. 2015;64:4298–4311. doi: 10.2337/db15-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeldich E., Chen C.-D., Colvin T.A., Bove-Fenderson E.A., Liang J., Tucker Zhou T.B., Harris D.A., Abraham C.R. The neuroprotective effect of klotho is mediated via regulation of members of the redox system. J. Biol. Chem. 2014;289:24700–24715. doi: 10.1074/jbc.M114.567321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yamamoto M., Clark J.D., V Pastor J., Gurnani P., Nandi A., Kurosu H., Miyoshi M., Ogawa Y., Castrillon D.H., Rosenblatt K.P., Kuro-o M. Regulation of oxidative stress by the anti-aging hormone klotho. J. Biol. Chem. 2005;280:38029–38034. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen G., Liu Y., Goetz R., Fu L., Jayaraman S., Hu M.-C., Moe O.W., Liang G., Li X., Mohammadi M. α-Klotho is a non-enzymatic molecular scaffold for FGF23 hormone signaling. Nature. 2018;553:461–466. doi: 10.1038/nature25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu J., Lloyd D.J., Hale C., Stanislaus S., Chen M., Sivits G., Vonderfecht S., Hecht R., Li Y.-S., Lindberg R.A., Chen J.-L., Jung D.Y., Zhang Z., Ko H.-J., Kim J.K., Véniant M.M. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foltz I.N., Hu S., King C., Wu X., Yang C., Wang W., Weiszmann J., Stevens J., Chen J.S., Nuanmanee N., Gupte J., Komorowski R., Sekirov L., Hager T., Arora T., Ge H., Baribault H., Wang F., Sheng J., Karow M., Wang M., Luo Y., McKeehan W., Wang Z., Veniant M.M., Li Y. Treating diabetes and obesity with an FGF21-mimetic antibody activating the klotho/FGFR1c receptor complex. Sci. Transl. Med. 2012;4 doi: 10.1126/scitranslmed.3004690. 162ra153–162ra153. [DOI] [PubMed] [Google Scholar]
- 27.Steiling H., Wüstefeld T., Bugnon P., Brauchle M., Fässler R., Teupser D., Thiery J., Gordon J.I., Trautwein C., Werner S. Fibroblast growth factor receptor signaling is crucial for liver homeostasis and regeneration. Oncogene. 2003;22:4380–4388. doi: 10.1038/sj.onc.1206499. [DOI] [PubMed] [Google Scholar]
- 28.Chen G.J., Weylie B., Hu C., Zhu J., Forough R. FGFR1/PI3K/AKT signaling pathway is a novel target for antiangiogenic effects of the cancer drug Fumagillin (TNP-470) J. Cell. Biochem. 2007;101:1492–1504. doi: 10.1002/jcb.21265. [DOI] [PubMed] [Google Scholar]
- 29.Dey J.H., Bianchi F., Voshol J., Bonenfant D., Oakeley E.J., Hynes N.E. Targeting fibroblast growth factor receptors blocks PI3K/AKT signaling, induces apoptosis, and impairs mammary tumor outgrowth and metastasis. Cancer Res. 2010;70:4151–4162. doi: 10.1158/0008-5472.CAN-09-4479. [DOI] [PubMed] [Google Scholar]
- 30.Liu L., Xie Y., Lou L. PI3K is required for insulin-stimulated but not EGF-stimulated ERK1/2 activation. Eur. J. Cell Biol. 2006;85:367–374. doi: 10.1016/j.ejcb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Yu J.S.L., Cui W. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 2016;143:3050–3060. doi: 10.1242/dev.137075. [DOI] [PubMed] [Google Scholar]
- 32.Smith G.R., Shanley D.P. Modelling the response of FOXO transcription factors to multiple post-translational modifications made by ageing-related signaling pathways. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fan W., Imamura T., Sonoda N., Sears D.D., Patsouris D., Kim J.J., Olefsky J.M. FOXO1 transrepresses peroxisome proliferator-activated receptor gamma transactivation, coordinating an insulin-induced feed-forward response in adipocytes. J. Biol. Chem. 2009;284:12188–12197. doi: 10.1074/jbc.M808915200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pardridge W.M. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. doi: 10.1602/neurorx.2.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leon J., Moreno A.J., Garay B.I., Chalkley R.J., Burlingame A.L., Wang D., Dubal D.B. Peripheral elevation of a klotho fragment enhances brain function and resilience in young, aging, and α-synuclein transgenic mice. Cell Rep. 2017;20:1360–1371. doi: 10.1016/j.celrep.2017.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.