Abstract
Functional neurological (conversion) disorder (FND) is a condition at the interface of neurology and psychiatry. A “software” vs. “hardware” analogy describes abnormal neurobiological mechanisms occurring in the context of intact macroscopic brain structure. While useful for explanatory and treatment models, this framework may require more nuanced considerations in the context of quantitative structural neuroimaging findings in FND. Moreover, high co-occurrence of FND and somatic symptom disorders (SSD) as defined in DSM-IV (somatization disorder, somatoform pain disorder, and undifferentiated somatoform disorder; referred to as SSD for brevity in this article) raises the possibility of a partially overlapping pathophysiology. In this systematic review, we use a transdiagnostic approach to review and appraise the structural neuroimaging literature in FND and SSD. While larger sample size studies are needed for definitive characterization, this article highlights that individuals with FND and SSD may exhibit sensorimotor, prefrontal, striatal-thalamic, paralimbic, and limbic structural alterations. The structural neuroimaging literature is contextualized within the neurobiology of stress-related neuroplasticity, gender differences, psychiatric comorbidities, and the greater spectrum of functional somatic disorders. Future directions that could accelerate the characterization of the pathophysiology of FND and DSM-5 SSD are outlined, including “disease staging” discussions to contextualize subgroups with or without structural changes. Emerging neuroimaging evidence suggests that some individuals with FND and SSD may have a “software” and “hardware” problem, although if structural alterations are present the neural mechanisms of functional disorders remain distinct from lesional neurological conditions. Furthermore, it remains unclear whether structural alterations relate to predisposing vulnerabilities or consequences of the disorder.
Keywords: Conversion disorder, Psychogenic, Neuroimaging, MRI, Functional neurological disorder, Somatic symptom disorder
Highlights
-
•
Transdiagnostic systematic review of structural MRI studies in FND and SSD
-
•
Sensorimotor-striatothalamic-limbic-paralimbic circuits implicated in both conditions.
-
•
Some small sample size FND studies did not show group-level structural alterations.
-
•
MRI alterations may relate to risk factors, compensatory changes or disease mechanisms.
-
•
Early-phase discussion on disease-staging algorithms outlined as a future direction.
1. Introduction
Functional neurological (conversion) disorder (FND) is a complex condition at the interface of neurology and psychiatry (Trimble and Reynolds, 2016). Prior to the Diagnostic and Statistical Manual of Mental Disorders 5th Edition (DSM-5) revised criteria (American Psychiatric Association, 2013; Stone et al., 2010a), FND for neurologists was largely a diagnosis for individuals with “medically unexplained” sensorimotor neurologic symptoms. As such, patients with FND were marginalized for much of the 20th century, with limited clinical and neuroscientific interest (Keynejad et al., 2017; Stone et al., 2008).
By contrast, founders of modern neurology and psychiatry were immensely intrigued by FND. Charcot theorized that functional motor symptoms were due to a “dynamic lesion” adversely impacting motor pathways (Bogousslavsky, 2014). Freud shifted the focus to the unconscious mind and theorized that psychological conflicts were “converted” to bodily symptoms to relieve distress (Breuer and Freud, 1956). The French psychologist Janet proposed a role for dissociation framed as a “retraction of the field of personal consciousness” (Janet, 1907). Recently, there is renewed interest in FND, catalyzed by the DSM-5 diagnostic criteria and pathophysiology-based research (Carson et al., 2012). Neurologically, emphasis is now given to identifying examination signs and semiologic features specific for FND (Avbersek and Sisodiya, 2010; Daum et al., 2014). Modern biopsychosocial formulations for FND incorporate the spectrum of predisposing vulnerabilities, acute precipitants, and perpetuating factors (Keynejad et al., 2018; Pick et al., 2018; Reuber, 2009).
The “software” vs. “hardware” analogy is a useful concept when discussing the diagnosis of FND with patients (Carson et al., 2016). According to this framing, the hardware (i.e. brain) lacks relevant structural abnormalities, however, the software (i.e. how the brain works) has a glitch that manifests in functional neurological symptoms. This framework is supported by the preservation of brain structure on clinical inspection of magnetic resonance imaging (MRI) scans at the macroscopic level. In parallel, there has been considerable advance using task and resting-state functional neuroimaging to delineate the emerging neurobiology of FND, summarized in several reviews and meta-analyses (Boeckle et al., 2016a; McSweeney et al., 2017; Perez et al., 2015b; Voon et al., 2016). Major themes across functional neuroimaging studies include: (1) heightened amygdalar reactivity to affectively valenced stimuli (Aybek et al., 2015; Aybek et al., 2014b; Hassa et al., 2017; Kanaan et al., 2007; Morris et al., 2017; Voon et al., 2010a); (2) increased limbic/paralimbic-sensorimotor connectivity (Espay et al., 2018b; Li et al., 2015a; Li et al., 2015b; Szaflarski et al., 2018; van der Kruijs et al., 2012, 2014; Voon et al., 2010a); (3) right temporoparietal junction/inferior parietal lobule hypoactivation and altered connectivity with sensorimotor cortices (Baek et al., 2017; Maurer et al., 2016; Voon et al., 2010b); (4) attentional dysregulation (Ghaffar et al., 2006; Mailis-Gagnon et al., 2003; Vuilleumier et al., 2001); and (5) deficits in motor planning (Voon et al., 2011), intention (de Lange et al., 2010; Spence et al., 2000), execution (Schrag et al., 2013; Stone et al., 2007) or inhibition (Cojan et al., 2009; Marshall et al., 1997; Tiihonen et al., 1995). Other abnormalities include implicit attentional biases (Pick et al., 2018), perceptual-cognitive inferences (Edwards et al., 2012), and mnemonic contributions to metacognitive processes disrupting subjective experience (Bègue et al., 2018b).
While the framing of FND as a “software” problem is well-received, this conceptualization may require more nuanced considerations. Notably, the shift from “psychogenic” to “functional” neurological disorders was proposed to eliminate false mind versus brain dichotomies (Edwards et al., 2014); similarly, structure-function relationships are well-recognized to be closely intertwined. Emerging structural neuroimaging findings point towards a parallel “hardware” related neurobiology in some FND populations, further bridging the divide between neurologic and psychiatric conceptualizations (Perez et al., 2018a). In addition, FND frequently co-exists clinically with the somatic symptom disorders (SSD) as largely defined in DSM-IV (somatization disorder, somatoform pain disorder, and undifferentiated somatoform disorder) (Kozlowska et al., 2018; Sar et al., 2004; Stone et al., 2010b); a few FND studies reported comorbidities rates with somatoform disorders above 50% (Bowman and Markand, 1996; Sar et al., 2004). The SSD category in the DSM-5 was designed to consolidate the DSM-IV diagnostic categories of somatization disorder, somatoform pain disorder, and undifferentiated somatoform disorder, although this reconceptualization has markedly different criteria based on cognitive-affective and behavioral aspects and is more explicit about including patients with defined medical conditions (Dimsdale et al., 2013). No studies have explicitly examined the overlap between FND and DSM-5 SSD, and we are unaware of any structural neuroimaging studies of DSM-5 SSD. Therefore, for the purposes of this article we use the term SSD to describe the somatic symptom disorders in DSM-IV. We acknowledge that this is not a one to one translation (see limitation section).
The explicit co-occurrence of FND and SSD was previously codified in part by the DSM-IV somatization disorder diagnostic category that encompassed individuals with functional neurological symptoms and other prominent somatic symptoms. FND and SSD share predisposing vulnerabilities (e.g. female predominance, high rates of depression-anxiety and adverse life event burden (Guz et al., 2004; Paras et al., 2009; Taylor, 2003)) further raising the possibility of a partially overlapping biology. Comorbid somatic symptoms in patients with FND also negatively impact healthcare utilization and prognosis (Ettinger et al., 1999; Glass et al., 2018; Ibrahim et al., 2009; Salinsky et al., 2016).
Reviews and meta-analyses have summarized the functional neurobiology of SSD compared to healthy controls (Boeckle et al., 2016b; Landa et al., 2012; Perez et al., 2015a), which includes: (1) increased limbic, paralimbic (insula, parahippocampal), and striato-thalamic activity during noxious tactile stimuli (Gundel et al., 2008; Luo et al., 2016; Stoeter et al., 2007); (2) decreased engagement of regulatory prefrontal regions during sensory and affective processing (Gundel et al., 2008; Noll-Hussong et al., 2013); (3) and sensorimotor, salience and default mode network resting-state alterations (Hakala et al., 2002; Karibe et al., 2010; Li et al., 2016; Zhao et al., 2017).
To aid the early-phase incorporation of structural neuroimaging findings in the development of biological models for FND and other functional somatic disorders, we used a transdiagnostic approach to conduct a systematic review and critically appraise the structural MRI literature in FND and SSD. We contextualized the structural neuroimaging literature within the neurobiology of stress-related neuroplasticity, gender differences, psychiatric comorbidities, and the greater spectrum of functional somatic disorders. Lastly, future directions were outlined that may help accelerate the characterization of the pathophysiology of these enigmatic conditions.
2. Methods
We followed PRISMA guidelines for systematic reviews (Moher et al., 2009).
Inclusion criteria were: (1) Articles written in English and including human subjects of any age; (2) Data published between January 1, 1980 and October 31, 2018; (3) Studies in somatosensory and/or motor FND using DSM-IV or DSM-5 criteria including functional movement disorders (e.g. functional tremor, gait, dystonia, tics/jerks, myoclonus), functional limb weakness, psychogenic nonepileptic seizures (PNES, also known as dissociative seizures), and nondermatomal sensory deficit disorders; (4) Studies in SSD using DSM-IV criteria including somatization disorder, somatoform pain disorder, undifferentiated somatoform disorder, and DSM-5 SSD; and (5) Quantitative structural brain imaging studies (manual tracings, volume-based (e.g. voxel-based morphometry (VBM)), surface-based (e.g. cortical thickness) and diffusion weighted imaging) comparing patients to healthy controls or employing within-group analyses.
Exclusion criteria were: (1) Studies without quantitative analyses of imaging data; (2) Investigations of body dysmorphic disorder, hypochondriasis and illness anxiety disorder; (3) Studies of other functional somatic disorders such as fibromyalgia and irritable bowel syndrome; (4) Mixed cohorts that included individuals with major neurological comorbidities (e.g. epileptic seizures); and (5) Functional neuroimaging and neurochemical studies (e.g. magnetic resonance spectroscopy).
3. Literature search strategy
Potential studies were identified through PubMed, PsycINFO and Embase. All available peer-reviewed records were searched using the following terms: “functional neurological disorder(s),” “conversion disorder,” “functional neurological symptom disorder,” “functional movement disorder(s),” “psychogenic movement disorder(s),” “functional gait,” “psychogenic gait,” “astasia-abasia,” “astasia abasia,” “functional tremor,” “psychogenic tremor,” “functional dystonia,” “psychogenic dystonia,” “fixed dystonia,” “psychogenic tic,” “psychogenic jerk,” “psychogenic myoclonus,” “functional limb weakness,” “functional weakness,” “psychogenic limb weakness,” “psychogenic weakness,” “psychogenic paralysis,” “functional paralysis,” “hysterical weakness,” “hysterical tremor,” “hysterical gait,” “hysterical dystonia,” “hysterical jerk,” “hysterical tic,” “hysterical myoclonus,” “nondermatomal sensory,” “psychogenic numbness,” “hysterical numbness,” “psychogenic anesthesia,” “hysterical anesthesia,” “somatoform disorder,” “somatization,” “undifferentiated somatoform,” OR “somatic symptom disorder” AND “magnetic resonance imaging,” “MRI,” “neuroimaging,” “voxel-based morphometry,” “VBM,” “cortical thickness,” “diffusion-weighted imaging”, OR “DTI.”
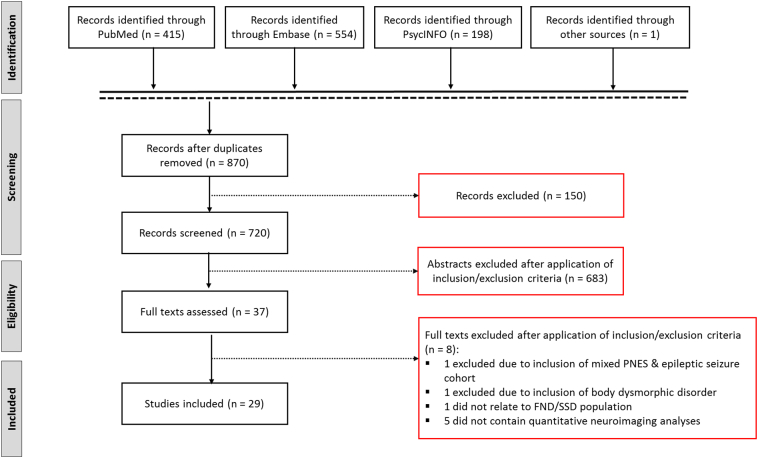
References of selected articles were also reviewed to ensure the search was comprehensive. This yielded 1124 items for a total of 870 articles after removing duplicates (see Fig. 1). Records were subsequently screened to ensure that they met the following preliminary criteria: (a) contained original data from a FND or SSD cohort; (b) structural neuroimaging was employed to quantify gray and/or white matter profiles; (c) written in English. This resulted in 720 records. To identify articles for final inclusion, two raters (I.B. and D.L.P.) independently reviewed all abstracts, selecting articles based on inclusion/exclusion criteria. This step resulted in 37 potentially eligible studies. In step 2, the same raters reviewed the full articles to determine if the article met inclusion criteria and any differences were reconciled. Of 37 original selections, 29 were included. Table 1 and Table 2 summarize the results in FND and SSD, respectively. Fig. 2 depicts commonly identified structural neuroimaging group-differences across FND and SSD.
Fig. 1.
Schematic overview of the systematic review process.
Table 1.
Structural MRI studies in functional neurological disorder.
| Study | Participants | Methods | Results | Limitations |
|---|---|---|---|---|
|
Atmaca et al. (2006) |
Unilateral motor FND (n = 12), all F, age 28.1 ± 5.1 | 1.5T | ↓ GMV in bilateral caudate and lentiform nucleus vs. HC | Small sample size |
| HC (n = 12) age & gender matched | Whole-brain manual tracings | ↓ GMV in R thalamus vs. HC | No apparent correction for multiple comparisons | |
|
FND Cohort: No comorbid psychiatric or neurologic disorders |
Between group comparisons |
(+) correlation between left caudate volumes and age of onset |
No FND phenotype details | |
| Findings not related to illness duration | ||||
|
Atmaca et al. (2016) |
Mixed FND (n = 20, 19 had PNES), all F, age 38.2 ± 8.3 | 1.5T | ↓ Pituitary volume vs. HC | Small sample size |
| Manual tracings of pituitary glands | (-) correlation between pituitary gland volumes and illness duration |
No neuroendocrine and autonomic data | ||
| HC (n = 20) age & gender matched | Between group comparisons | No information on adverse life events |
||
|
FND Cohort: No comorbid psychiatric or neurologic disorders No endocrine disorder or prior corticosteroid use |
Self-report measures: HDRS |
|||
|
Labate et al. (2011) |
PNES (n = 20), 55% F, age 36.7 ± 13.5 | 1.5T | ↓ GMV in bilateral cerebellum, R precentral gyrus, MFG, ACC, SMA vs. HC | Small sample size |
| HC (n = 40) age & gender matched | VBM and FreeSurfer CTH | |||
|
FND Cohort: No comorbid neurologic disorders |
Between and within group analyses | (-) correlation between depression scores with R dorsal premotor cortex GMV | Between-group differences not adjusted for depression and anxiety scores | |
| Psychiatric comorbidities: 75% with a mood disorder (lifetime); 95% with an anxiety disorder (lifetime) | Whole-brain and ROI based corrections | ↓ CTH in the R precentral gyrus, SFG, paracentral gyrus, and precuneus vs. HC | No information on adverse life events in patients | |
| No antipsychotic medication use |
Self-report measures: SDQ-20, BDI, STAI; DES-II Neuropsychiatric assessment: BIT, MCST; TMT-A; TMT-B |
(-) correlation between depression scores with R SFG, paracentral gyrus and orbitofrontal sulcus CTH | No psychiatric controls |
|
| (-) correlation between SDQ scores with L IFG and central sulcus CTH | ||||
|
Aybek et al. (2014a) |
FND-FW (n = 15), 73% F, age 37 ± 11.4 | 3T | No GMV or CTH differences across all FND vs. HC | Small sample size for FW subtypes (hemiparesis vs. paraparesis) |
| hemiparetic (n = 9), paraparetic (n = 6) | Dual VBM and VBCT | |||
| HC (n = 25) age & gender matched | Between and within-group comparisons | ↑ CTH bilateral premotor cortices in hemiparetic FND subgroup vs. HC | No whole-brain corrected analyses | |
|
FND Cohort: No major mental health or major neurologic comorbidities |
Secondary analyses stratified by motor subtype | No correlation between structural profiles and illness duration/severity |
No control group with limb immobility |
|
| ROI based corrections | ||||
| ROIs: primary motor, SMA, premotor areas | ||||
| Correlation with clinical data | ||||
|
Nicholson et al. (2014) |
FND-FW (n = 15), 67% F, mean age 37 | 3T | ↓ GMV L thalamus and lentiform nucleus vs. HC | Small sample size |
| HC (n = 31) age, gender, handedness, & IQ matched | FreeSurfer subcortical volumetric analysis | No correlation between GMV and laterality, illness duration or symptom severity |
No patient control group with impaired limb mobility | |
|
FND Cohort: No psychosis, bipolar disorder or major depression |
Between and within group comparisons | No contextualization of group-level findings with comorbidities (pain, anxiety) |
||
| ROI based corrections | ||||
| No major neurologic disorder |
ROIs: caudate, lentiform nuclei, thalamus and amygdala | |||
| Correlation between structural and clinical data | ||||
|
Ristic et al. (2015) |
PNES (n = 37), 84% F, age 37.3 ± 13.8 | 1.5T | No between-group difference in CSA and curvature | Did not account for medication effects |
| HC (n = 37) age & gender matched | FreeSurfer CTH, CSA, and cortical folding | ↑ CTH in bilateral medial-orbitofrontal, L insula and lateral-orbitofrontal vs. HC | No relationship between findings and clinical scores | |
|
FND Cohort: No comorbid epilepsy or major MRI abnormalities |
Between and within group analyses | ↓ CTH in bilateral precentral, R entorhinal and lateral-occipital region vs. HC | No psychometric measures of mood or anxiety |
|
| Presence of psychological abuse 11%; physical abuse 14%; sexual abuse 5% |
Whole-brain corrections | ↑ SD in bilateral insula, R rostral ACC, R posterior cingulate, L cuneus vs. HC | ||
| Neuropsychological assessment: Full-Scale IQ, Verbal IQ, Performance IQ; TMT-B; MCST |
↓ SD in bilateral medial-orbitofrontal sulci vs. HC |
|||
|
Riederer et al. (2017) |
NDSD with chronic pain (n = 25), 68% F, age 42.1 ± 9.9 | 3T | ↑ GM in R primary sensory cortex, thalamus vs. HC | Did not account for medication effects |
| VBM | ||||
| “Pain only” control (n = 23), 68% F; age 43.1 ± 10.5 | Between group analyses | ↑ GM bilaterally in lateral temporal regions vs. HC | No relation with pain-related clinical parameters (onset, duration, severity) |
|
| HC (n = 29), age 42.4 ± 9.8 | Whole-brain and ROI based corrections | ↑ GM bilaterally in hippocampus/fusiform gyrus vs. HC | ||
|
NDSD Cohort: No neurologic or severe psychiatric disorders |
ROIs: postcentral gyri, thalamus, insula, ACC | ↑ GM in L insula (ROI-based analyses) vs. HC | ||
| Self-report measures: HADS-A, HADS-D |
“Pain only” controls showed ↑ GM bilaterally in posterior insula and modest increases in sensory cortex GM vs. HC |
|||
|
Kozlowska et al. (2017) |
Pediatric FND-mixed, FMD, FW, PNES, NDSD (n = 25), 80% F, age 14.6 ± 2.0 | 3T | ↑ GMV in L SMA vs. HC | Modest sample size in mixed FND cohort |
| ↑ GMV in R STG and DMPFC vs. HC | ||||
| VBM | ||||
| HC (n = 24) age, gender & handedness matched | Between and within-groups analyses | (+) correlation between faster RT in EIT and SMA GMV | Between-group differences not adjusted for depression and anxiety scores | |
|
FND Cohort: No major neurologic disorder |
Whole-brain and ROI based corrections | No significant associations between GMVs and clinical scores |
No trauma or pain-related psychiatric controls |
|
| Presence of psychiatric disorders: 28% anxiety disorder, 24% depressive disorder, 84% with pain | ROIs: BG, thalamus, motor cortex, SMA, cerebellum | |||
| 36% with prior maltreatment Majority free of psychotropic medications |
Experimental measures: RT in emotion identification task (EIT) | |||
| Neuropsychiatric assessment: general functioning, IQ, attachment style, anxiety, depression, adverse life events | ||||
|
Perez et al. (2017a) |
FND-mixed including FMD, FW, PNES, NDSD (n = 23), 78% F, age 41.6 ± 11.6 | 3T | (-) correlation between L anterior insula GMV and FND symptom severity in women with FND | No control group |
| VBM | Mixed symptomatology and modest sample size | |||
|
FND Cohort: No major neurologic disorder |
Within group analyses | (-) correlation between L anterior insula GMV and childhood abuse burden in women with FND. | Low number of male subjects | |
| ROI-correction | ||||
| Current psychiatric disorders included: 35% MDD; 43% PD; 43% other somatic symptom disorders | ROIs: bilateral insula, ACC, amygdala, hippocampus | (-) between ACC GMV and PTSD severity across all patients | Did not account for possible medication effects |
|
| Majority on psychotropic medications |
Self-report measures: PHQ-15, SOMS:CD, CTQ, LEC-5, PCL-5, STAI, BDI |
(-) correlation of hippocampal GMV and lifetime adverse event burden across all patients |
||
|
Perez et al. (2017b) |
FND-mixed, FMD, FW, PNES, NDSD (n = 26), 81% F, age 40.3 ± 11.5 | 3T | No GMV differences across all FND vs. HC | Modest sample size in mixed FND cohort |
| VBM | ||||
| HC (n = 27) age & gender matched | Between and within-group analyses; stratified post-hoc analysis | ↓ L anterior insula GMV in FND with most impaired physical health vs. HC | ||
|
FND Cohort: No major neurologic disorders 24 of 26 patients with current psychiatric co-morbidities: 31% MDD; 38% GAD; 46% PD; 46% with other somatic symptom disorders |
Whole-brain and ROI based corrections | ↑ R postero-lateral cerebellum GMV in FND with most impaired mental health vs. HC | Did not account for possible medication effects | |
| No objective health status measure | ||||
| ROIs: bilateral insula, ACC, amygdala, periaqueductal gray | ↑ R amygdala GMV in FND correlated with impaired mental health and elevated trait anxiety | No psychiatric controls |
||
|
↑ Periaqueductal gray matter correlated with role limitations due to emotional problems | ||||
| Majority on psychotropic medications |
Self-report measures: SF-36; STAI-Trait; BDI |
|||
|
Perez et al. (2018c) |
FND-mixed, FMD, FW, PNES, NDSD (n = 22), 86% F, age 41.7 ± 11.0 | 3T | ↑ L anterior hippocampus GMV at baseline positively correlated with mental health improvement | Modest sample size in mixed FND |
| VBM | ||||
| HC (n = 27) age & gender matched | Within-group and stratified between group analyses | Above result not significant after controlling for baseline anxiety or trauma burden | Patients received individualized treatments | |
|
FND Cohort: No major neurologic disorder |
ROI-correction | ↓ R anterior hippocampus GMV at baseline in the 11 FND patients with the worst mental health outcomes vs. HC | Use of self-report outcome measures only |
|
| Presence of psychiatric comorbidities in the FND sample: MDD; GAD; PTSD; PD; SSD | ROIs: ACC, insula, amygdala, hippocampus | |||
| Majority on psychotropic medications |
Primary self-report measures: SF-36 at baseline and 6-month follow-up |
No baseline GMV correlations with physical health outcomes |
||
|
Maurer et al. (2018) |
FMD (n = 48; 17 also had FW), 79% F, age 44.6 ± 11.6 | 3T | ↑ L amygdala, striatum, fusiform, cerebellum and bilateral thalamus GMV vs. HC | Between-group differences not adjusted for depression and anxiety scores |
| VBM | ||||
| HC (n = 55), age, gender & education matched | Between and within group analyses | ↓ L sensorimotor cortex (precentral & postcentral gyri) GMV vs. HC | No psychiatric controls | |
| FND Cohort: | Whole-brain correction | (+) correlation between childhood trauma burden and L caudate and cerebellum GMV | No relationship with symptom severity or illness duration |
|
| No major neurologic disorder | ||||
| No psychosis, bipolar disorder or current substance abuse | Neuropsychiatric assessment: SCID-IV-TR, BDI, HAM-A, HAM-D STAI-S/T; CTQ, self-rated symptom severity |
(-) correlation between anxiety and L fusiform gyrus GMV | ||
| Presence of higher depression and anxiety scores in the FND sample | (+) correlation between depression and L cerebellar tonsil GMV |
|||
| 54% on psychotropic medications | ||||
|
Espay et al. (2018b) |
FT (n = 27), 85% F, age 50.9 ± 12.0 | 4T | ↓ L caudate volume vs. HC | Small sample size |
| Matched to HC (n = 25), 84% F, age 48.6 ± 11.4 | FSL-VBM | ↓ R post-central gyrus volume vs. HC |
Between-group differences not adjusted for depression and anxiety scores |
|
| ET (n = 16), 31% F, age 61.7 ± 9.3 | Between group analyses | |||
| FND Cohort: | Whole-brain correction | |||
| No neurologic disorder or history of severe psychiatric disorder (HDRS>24, HARS>25) | Neuropsychiatric assessment: MINI, HDRS, HARS | |||
| No benzodiazepine use |
||||
|
Perez et al. (2018b) |
FND-mixed, FMD, FW, PNES, NDSD (n = 26), 81% F, age 40.3 ± 11.5 | 3T | ↓L caudal ACC CTH in FND with high somatoform dissociation vs. HC | Small sample size for stratified between-group analyses |
| FreeSurfer CTH | ||||
| HC (n = 27) age & gender matched | Stratified between-group and within-group analyses | (-) correlation between SDQ scores and left caudal ACC CTH | No trauma and other dissociative disorder control group | |
|
FND Cohort: See Perez, Williams et al. 2017 |
Whole-brain correction | (+) correlation between depersonalization/derealization severity and right lateral occipital CTH | Only one surface measure used | |
| Self-report measures: SDQ; DES | Findings remained significant adjusting for mood/anxiety, PTSD/BPD, trauma burden, motor subtype and SSRI/SNRI use |
Same sample as Perez, Williams et al. 2017 |
||
| Posthoc analyses adjusted for: (1) lifetime PTSD and BPD; (2) STAI-T and BDI scores; (3) trauma burden; (4) motor FND subtypes; (5) SSRI/SNRI use | ||||
|
Williams et al. (2018) |
FND-mixed, FMD, FW, PNES, NDSD (n = 26), 81% F, age 40.3 ± 11.5 | 3T | (-) correlation between L parahippocampal CTH in women with FND and dismissing attachment style | Modest sample size |
| FreeSurfer CTH | Self-report measures only | |||
| HC (n = 27) age & gender matched | Between and within group analyses | (-) correlation between R hippocampal volume in women with FND and confrontive coping | Low number of male patients | |
|
FND Cohort: See Perez, Williams et al. 2017 |
Whole-brain correction | (+) correlation between R precentral gyrus CTH in women with FND and coping by accepting responsibility |
No attachment or coping style data in HC group | |
| Self report measures: CD-RISC, RSQ, WoC-R |
Same sample as Perez, Williams et al. 2017 |
|||
|
McSweeney et al. (2018) |
PNES (n = 20), 70% F, age 41.1 (range: 19-62) | 3T | ↑ CTH in bilateral cuneus, L paracentral and lingual regions vs. HC | Small sample size |
|
FND Cohort: No neurologic disorder or clinically significant MRI abnormalities. |
FreeSurfer Between and within group analyses |
↓ CTH in bilateral inferior frontal gyri, R superior temporal region and medial orbitofrontal cortices vs. HC | Between-group differences not adjusted for depression and anxiety scores | |
| 81% with comorbid depression; 56% with anxiety | Whole-brain correction | No association between CTH and trauma burden (based on retrospective chart review) | No psychiatric controls | |
| Did not account for medication effects | ||||
| 45% on antidepressants, 5% on antipsychotics |
No self-report measures |
|||
|
Ding et al. (2013) |
PNES (n = 17), 60% F, age 19.7 ± 7.6 | 3T | Altered (more lattice-like) small-worldness in structural and functional connectivity networks vs. HC | Small sample size |
| HC (n = 20) age & gender matched | DTI-20 directions | No psychiatric controls | ||
|
FND Cohort: No neurologic or psychiatric disorders |
Graph-theoretical analyses on structural (and functional) networks | PNES: alterations in regional characteristics in structural connectivity involved attentional, sensorimotor, subcortical and default-mode networks | No correlation with clinical measures |
|
| Whole-brain correction | ↓ in coupling strength of structural-functional connectivity vs. HC |
|||
| No self-report measures | ||||
|
Vasta et al. (2018) |
PNES (n = 23), 87% F, age 26.2 ± 12.4 | 3T | ↓ bilateral IFC pars triangularis, medial OFC, L caudal middle frontal, insula and R precentral gyrus surface area vs. HC | Small sample size for machine learning |
| HC (n = 21) age & gender matched | FreeSurfer surface area | Between-group differences not adjusted for depression and anxiety scores | ||
|
FND Cohort: No major neurologic disorder, severe depression and personality disorders |
Multivariate pattern analyses | ↑ L posterior cingulate surface area vs. HC | No psychiatric controls | |
| Whole-brain correction | Machine learning: differentiating profiles in R pars triangularis area, L posterior cingulate, R medial orbitofrontal area |
No correlation with clinical measures or phenotypes |
||
| Neuropsychiatric assessment | ||||
| Self-report measures: TAS-20, BDI-II, HAM-A, TEC, DES, SDQ-20 | ||||
|
Hernando et al. (2015) |
PNES (n = 8), 88% F, age 42.4 ± 16.4 | 3T (3 scanners) | Rightward asymmetry in number of UF streamlines in PNES vs. HC. | Very small sample size |
| HC (n = 7) age & gender matched | DTI 20-directions | (-) correlation between age of onset and UF rightward asymmetry |
Data acquired on multiple scanners | |
| Between and within group analyses | ||||
|
FND Cohort No neurologic or psychiatric disorders |
White Matter Tract of Interest: UF | Did not account for medication effects |
||
| Correlation between structural and clinical data | ||||
|
Lee et al., 2015 |
PNES (n = 16), 94% F, age 40.3 ± 13.8 | 3T (3 scanners) | ↑ FA in L internal and external capsules, corona radiata, UF, and STG vs. HC | Small sample size |
| HC (n = 16) age & gender matched | TBSS: DTI 32-directions | No within-group associations with event frequency and illness duration |
Data acquired on multiple scanners | |
|
FND Cohort No major neurologic disorder |
Between and within-group analyses | Between-group differences not adjusted for depression and anxiety scores |
||
| Psychiatric diagnoses: 44% with a history of depression, 31% with history of anxiety |
Whole-brain correction | |||
| Clinical data correlations | ||||
| Tomic et al. (2018) | “Fixed” functional dystonia (FixFD) (n = 13) | 1.5T | No differences in CTH or GMV in both FD patient groups vs. HC | Small sample size for FixFD subgroup |
| “Mobile” functional dystonia (MobFD) (n = 31) | DTI 65-directions FSL, TBSS, FreeSurfer CTH |
All FD subjects vs. HCs ↓ FA ↑ MD, RD in splenium of corpus callosum, corticospinal tract, anterior thalamic radiation, brainstem and R > L SLF, ILF, IFOF, UF, CB |
No contextualization of findings with clinical data | |
| Between-group differences not adjusted for depression and anxiety scores | ||||
| HC (n = 43) age & gender matched | Between and within-group analyses | |||
| FixFD cohort: fixed limb postures; comorbidity with CRPS; No botulinum toxin treatment response | Whole-brain correction |
MobFD vs. FixFD ↓ CTH in L orbitofrontal cortex, and medial and lateral parietal and posterior cingulate regions bilaterally |
Heterogeneity of dystonia distribution | |
| MobFD cohort: cranial, cervical or truncal localization; good botulinum toxin treatment response and potential presence of additional FMD/FND | Neuropsychiatric assessment: UDRS, BFMS, PMD, MMSE, HDRS, HARS, Apathy scale, SDQ, DES-II |
MobFD vs. HC ↓ GMV in L nucleus accumbens, putamen, thalamus, and bilateral caudate nuclei |
||
|
MobFD vs. FixFD ↓ GMV R hippocampus and globus pallidus |
Abbreviations: Anterior cingulate cortex (ACC); Beck Depression Inventory (BDI); Borderline personality disorder (BPD); Brief Intelligence Test (BIT); Computational Anatomy Toolbox for SPM (CAT12); Childhood Trauma Questionnaire (CTQ); Cingulum Bundle (CB); Connor-Davidson Resilience Scale (CD-RISC); Conversion Disorder subscale of the Screening for Somatoform Symptoms-7 scale (SOMS:CD); Cortical thickness (CTH); Complex regional pain syndrome (CRPS); Dissociative Experiences Scale (DES); Dorsomedial prefrontal cortex (DMPFC); Essential Tremor (ET); Epileptic seizures (ES); Female (F); Fractional anisotropy (FA); Functional movement disorder (FMD); FMRIB software library (FSL); Functional weakness (FW); General anxiety disorder (GAD); Gray matter volume (GMV); Hospital Anxiety and Depression Scale (HADS); Hospital Anxiety and Depression Scale Anxiety (HADS-A); Hospital Anxiety and Depression Scale Depression (HADS-D); Hamilton Depression Rating Scale (HDRS); Hamilton Anxiety Rating Scale (HARS); Healthy controls (HC); Inferior frontal gyrus (IFG); Inferior fronto-occipital fasciculus (IFOF); Inferior longitudinal fasciculus (ILF); Intelligence quotient (IQ); Left (L); Life Events Checklist-5 (LEC-5) ‘happened to me’; Major Depressive Disorder (MDD); Major Depressive Episode (MDE); Mean diffusivity (MD); Middle frontal gyrus (MFG); Mini International Neuropsychiatric Interview (MINI); Modified Card Sorting Test (MCST); Magnetic Resonance Imaging (MRI); Non-dermatomal sensory deficits (NDSD); Neuropsychiatry Unit Cognitive Assessment Tool (NUCOG); Pain Disorder (PD); Patient Health Questionnaire-15 (PHQ-15); Psychogenic non-epileptic seizures (PNES); PTSD Checklist for DSM-5 (PCL-5); Pain Perception Scale (PPS); Quality of life in epilepsy inventory 98 (QOLIE-98); Radial Diffusivity (RD); Right (R); Reaction times (RT); Region of interest (ROI); Relationship Scales Questionnaire (RSQ); Post-traumatic stress disorder (PTSD); Short Form Health Survey-36 (SF-36); Somatoform Dissociation Questionnaire-20 (SDQ); Somatization Subscale of Symptoms Checklist 90 (SCL-90); Somatic Symptom Disorder (SSD); Spielberger Trait Anxiety Inventory (STAI-T); Sulcal depth (SD); Supplementary motor area (SMA); Superior frontal gyrus (SFG); Superior longitudinal fasciculus (SLF); Superior temporal gyrus (STG); Symptom Checklist-90-R (SCL-90-R); Statistical Parametric Mapping (SPM); Tract-Based Spatial Statistics (TBSS); Trail Making Test (TMT) Part A (TMT-A) and Part B (TMT-B); Traumatic Experience Checklist (TEC); Uncinate fasciculus (UF); Unified Dystonia Rating Scale (UDRS); Burke-Fahn-Marsden Dystonia Rating Scale (BFMS); Psychogenic Movement Disorders Scale (PMD); Voxel-based Morphometry (VBM); Voxel-based cortical thickness (VBCT); Ways of Coping Scale-Revised (WoC); White matter volume (WMV). Healthy controls in all studies have no neurologic, psychiatric, or general medical conditions, unless mentioned otherwise. Ages provided in years. Legend: ↑, increase ; ↓, decrease ; (+), positive ; (-), negative.
Table 2.
Structural MRI studies in somatization disorder, somatoform pain disorder, and undifferentiated somatoform disorder.
| Study | Participants | Methods | Results | Limitations |
|---|---|---|---|---|
|
Hakala et al. (2004) |
SZD (n = 10), all F, age 46.8 ± 9.1 | 1.5T | ↑ bilateral caudate vs. HC |
Small sample size |
| HC (n = 16), all F, age 49.9 ± 6.1 | Manual tracings of caudate, putamen, hippocampus | No structured clinical interview | ||
|
SZD Cohort: No neurologic disorder or active Axis I diagnosis 10% on antipsychotics 4 patients with comorbid fibromyalgia |
Between group analyses | No apparent correction for multiple comparisons | ||
| Self-report measures: SCL-90 |
Did not account for possible medication effects |
|||
|
Atmaca et al. (2011) |
SZDa (n = 20), all F, age 43.6 ± 8.3 | 1.5T | ↓ bilateral amygdalar volume vs. HC | Small sample size |
| HC (n = 20), all F, age 40.0 ± 3.9 y. | GE Volume Viewer voxtool 4.2 program | No between-group hippocampal or whole-brain differences |
Relatively large slice thickness | |
|
SZD Cohort: No neurologic disorder No history of mania or psychosis 45% history of antidepressants, 30% history of antipsychotics |
Hippocampal and amygdala tracings | No apparent correction for multiple comparisons | ||
| Between group analyses | Presence of functional neurological symptom(s) not described |
|||
| Self-report measures: HDRS, HARS | ||||
|
Yildirim et al. (2012) |
SZD (n = 18), all F, age 42.3 ± 7.4 | 1.5T | ↓ pituitary volume vs. HC | Small sample size |
| HC (n = 18), all F, age 40.6 ± 3.6 | GE Volume Viewer voxtool 4.2 program | No correlations between pituitary volumes, duration of illness and depression |
No men included | |
| Pituitary tracings | ||||
| No psychiatric controls to account for psychiatric comorbidities | ||||
|
SZD Cohort: No neurologic disorder or psychiatric comorbidities (except for depression) |
Between and within group analyses | |||
| Presence of functional neurological symptom(s) not described | ||||
| No psychotropics in 2 weeks prior to study participation 44% with prior antidepressant use, 28% history of antipsychotic use |
Self-report measures: HDRS |
|||
|
Valet et al. (2009) |
PD (n = 14), all F, age 51.1 ± 11.1 diffuse headache, n = 4 low back pain, n = 7 temporomandibular (facial pain, n = 1 pelvic region, n = 1 lower limbs, n = 1 |
1.5T | ↓ GM in VMPFC, OFC, ACC, insular, parahippocampal and prefrontal cortices vs. HC (adjusted for depression) | Small sample size |
| VBM | ||||
| Between and within group analyses | No significant difference in global GM volumes between groups | No psychiatric or neurological controls with predominant pain | ||
| Whole-brain correction | (+) correlations between illness duration and R thalamic GMV | No attempt to adjust for anxiety |
||
| HC (n = 25), age & gender matched | Self-report measures: BDI, PPS |
(-) correlations between illness duration and L parahippocampal GMV |
||
|
PD Cohort: No neurologic disorder No fibromyalgia 50% had current MDE | ||||
|
Magon et al. (2018) |
PD (n = 23), 74% F, age 51 ± 9.9 | 3T | ↓ CTH in L precentral and postcentral gyri for PD vs. HC | Small sample size |
| HC (n = 23) age & gender matched | FreeSurfer 5.1 CTH | ↓ CTH in L inferior temporal sulcus vs. HC | No psychiatric controls | |
|
PD Cohort: No severe chronic medical disorders, unambiguous nociceptive pain (e.g., post-surgery pain) or phantom limb pain No hypochondriasis No comorbid major psychiatric disorders (i.e. schizophrenia, PTSD) |
Between group analyses | Between-group findings adjusted for depression | No contextualization of findings with PD-related clinical data | |
| Whole-brain correction | Findings did not hold adjusting for trait anxiety |
|||
| Neuropsychiatric assessment: SCID-IV-TR, BDI, TAS-20, STAI-S/T | ||||
|
Zhang et al. (2015) |
SZD (n = 25), 84% F, age 41.0 ± 10.8 | 3T | No group difference for FA values, MD, axial diffusivity and radial diffusivity (corrected p<0.05) | Interpretability to clinical populations with psychiatric comorbidities unclear |
| TBSS | ||||
| HC (n = 28), 79% F, age 38.7 ± 9.6 | Between and within group analyses | (+) correlation between right inferior fronto-occipital fasciculus and right cingulum FA with somatization scores | Presence of functional neurological symptom(s) not described | |
|
SZD Cohort: No neurologic or psychiatric comorbidities |
Whole-brain correction | Between-group differences not adjusted for depression and anxiety scores |
||
| Self-report measures: HDRS, HAMA, SCL-90 | ||||
|
Zhao et al. (2018) |
SZD (n = 25), 84% F, age 41.0 ± 10.8 | 3T | ↑ WMV in R IFG in SZD vs. HC | No use of DTI for WM integrity measurements |
| HC (n = 28), age, gender and education matched | CAT12 | ↓ WMV in L inferior longitudinal fasciculus in SZD vs. HC | Lack of clinical contextualization of WMV changes | |
|
SZD Cohort: No major neurologic or medical disorders Majority of patients had depressive disorders |
Between and within group analyses | Between-group findings adjusted for depression | ||
| Whole-brain correction | No correlation between abnormal WMV and clinical variables in SZD |
No psychiatric controls | ||
| Presence of functional neurological symptom(s) not described | ||||
| Self-report measures: HAMA, HAMD, somatization subscale of SCL-90, EPQ, WCST, digit symbol coding of WAIS | ||||
| Li et al. (2018) | SZD (n = 25), 84% F, age 41.0 ± 10.8 | 3T | ↓ R Cerebellum Crus I GMV vs. HC | No psychiatric controls |
| VBM | ↑ L ACC, angular gyrus, R MFG GMV vs. HC | Between-group differences not adjusted for depression and anxiety scores | ||
| HC (n = 28), age, gender and education matched | Between and within group analyses | (-) correlation of R MFG GMV with somatization subscale of the symptom checklist-90 (adjusted for depression and anxiety) | Presence of functional neurological symptom(s) not described | |
|
SZD Cohort: No major neurologic or medical disorders Majority of patients had depressive disorders (unspecified) |
Whole-brain correction | |||
| Self-report measures: HAMA, HAMD, somatization subscale of SCL-90, EPQ, WCST, digit symbol coding of WAIS |
Abbreviations: Anterior cingulate cortex (ACC); Beck Depression Inventory (BDI); Computational Anatomy Toolbox for SPM (CAT12); Cortical thickness (CTH); Female (F); Fractional anisotropy (FA); Gray matter volume (GMV); Hamilton Depression Rating Scale (HDRS); Hamilton Anxiety Rating Scale (HARS); Healthy controls (HC); Inferior frontal gyrus (IFG); Left (L); Major depressive episode (MDE); Mean diffusivity (MD); Middle frontal gyrus (MFG); Magnetic Resonance Imaging (MRI); Somatoform pain disorder (PD); Pain Perception Scale (PPS); Radial Diffusivity (RD); Right (R); Region of interest (ROI); Post-traumatic stress disorder (PTSD); Somatization Subscale of Symptoms Checklist 90 (SCL-90); Spielberger Trait Anxiety Inventory (STAI-T); Sulcal depth (SD); Supplementary motor area (SMA); Superior frontal gyrus (SFG); Superior longitudinal fasciculus (SLF); Superior temporal gyrus (STG); Symptom Checklist-90-R (SCL-90-R); Statistical Parametric Mapping (SPM); Somatization disorder (SZD); Tract-Based Spatial Statistics (TBSS); Trail Making Test (TMT) Part A (TMT-A) and Part B (TMT-B); Uncinate fasciculus (UF); Unified Dystonia Rating Scale (UDRS); White matter volume (WMV). Healthy controls in all studies have no neurologic, psychiatric, or general medical conditions, unless mentioned otherwise. aCohort used DSM-IIR diagnostic criteria via SCID for DSM-IV and included for completeness. Legend: ↑, increase ; ↓, decrease ; (+), positive ; (-), negative.
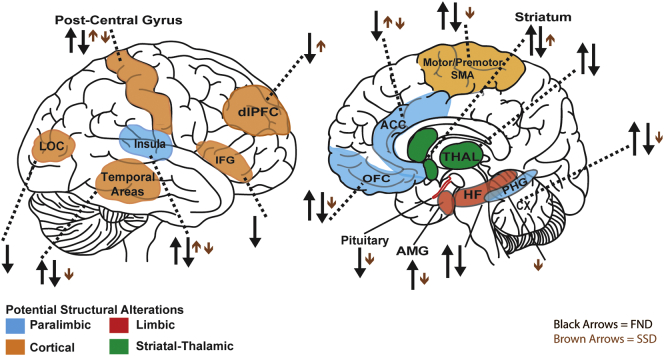
Fig. 2.
Overview of structural neuroimaging findings in functional neurological disorder (FND) and somatic symptom disorders (SSD; DSM-IV somatization disorder, somatoform pain disorder, and undifferentiated somatoform disorder). Directions of arrows show increases or decreases in regional brain structural measures compared to healthy controls. Several studies found no between group differences. Abbreviations: Anterior cingulate cortex (ACC); Amygdala (AMG); Cerebellum (Cx); Dorsolateral prefrontal cortex (dlPFC); Hippocampal formation (HF); Inferior frontal gyrus (IFG); Lateral occipital complex (LOC); Orbitofrontal cortex (OFC); Parahippocampal gyrus (PHG); Supplementary motor area (SMA); Thalamus (THAL).
4. Results
4.1. Manual tracings
Several studies used manual tracings to investigate structural profiles in patients with FND and SSD (Atmaca et al., 2006; Atmaca et al., 2016; Atmaca et al., 2011; Hakala et al., 2004; Yildirim et al., 2012). Atmaca and colleagues reported that 12 women with unilateral functional motor symptoms compared to controls showed smaller bilateral caudate and right thalamic volumes (Atmaca et al., 2006). Age of symptom onset positively correlated with left caudate volumes. By contrast, a study in 10 patients (somatization disorder (n = 6), undifferentiated somatoform disorder (n = 4)) showed increased bilateral caudate volumes compared to controls (Hakala et al., 2004). In another study, 20 women with somatization disorder compared to controls showed smaller bilateral amygdalar volumes (Atmaca et al., 2011).
Reduced pituitary volumes have been characterized in FND and somatization disorder populations compared to controls (Atmaca et al., 2016; Yildirim et al., 2012), although smaller pituitary volumes only correlated with longer illness durations in those with FND (Atmaca et al., 2016). Overall, manual tracing MRI studies identified smaller pituitary volumes across individuals with FND and somatization disorder, suggesting disturbances in stress-related neuroendocrine systems.
4.2. Other volumetric and voxel-based analyses
Volumetric investigations performed in FND and SSD cohorts identified structural alterations across prefrontal-subcortical-limbic areas (Aybek et al., 2014a; Espay et al., 2018b; Kozlowska et al., 2017; Li et al., 2018; Maurer et al., 2018; Nicholson et al., 2014; Perez et al., 2017a; Perez et al., 2017b; Perez et al., 2018c; Riederer et al., 2017; Valet et al., 2009). An early VBM study in 14 women with chronic somatoform pain disorder and high emotional pain responses showed decreased paralimbic (cingulo-insular, parahippocampal, orbitofrontal), prefrontal, inferior temporal, and cerebellar gray matter (GM) volumes compared to controls (Valet et al., 2009). Illness duration negatively correlated with left parahippocampal volumes and positively correlated with right thalamic volumes.
A VBM study in 25 patients with somatization disorder compared to controls showed decreased GM volume in the right cerebellum Crus I, and increased GM volumes in the left anterior cingulate cortex (ACC), right middle frontal gyrus, and left angular gyrus (Li et al., 2018). Middle frontal gyrus GM volume inversely correlated with somatization subscale scores, while left ACC GM volume negatively correlated with set-shifting (executive function) errors. Collectively, these two studies indicate possible structural changes within cortico-limbic-cerebellar circuits in patients with SSD, though volumetric research in these populations is in its early stages.
By contrast, quantitative volumetric studies in FND populations have been performed by several groups. A dual VBM and voxel-based cortical thickness (CTH) study conducted in 15 patients with functional limb weakness showed no group-level differences compared to controls (Aybek et al., 2014a). In secondary analyses, however, the functional hemiparesis subgroup exhibited increased bilateral premotor CTH compared to controls. In another study, Nicholson and colleagues used region-of-interest (ROI) FreeSurfer volumetric analyses to show that 14 patients with functional limb weakness exhibited smaller left thalamic and lentiform nucleus GM volumes compared to controls (Nicholson et al., 2014).
A within-group VBM study investigated structural associations with indices of patient-reported symptom severity, post-traumatic stress disorder (PTSD), and adverse life event burden in 23 patients with mixed FND (Perez et al., 2017a). Across all patients, there were no associations with FND symptom severity. However, in the 18 women with FND, parallel decreases in left anterior insular volume correlated with patient-reported symptom severity in ROI analyses. In women, the magnitude of childhood abuse burden also inversely correlated with left anterior insular volume. Each of these relationships held adjusting for trait anxiety, but did not remain significant controlling for depression. Across all individuals, PTSD severity inversely correlated with perigenual/dorsal anterior cingulate cortex (ACC) GM volume, potentially suggesting involvement of distinct salience network regions mediating FND symptom severity (insula) and PTSD symptom severity (ACC). Lifetime adverse event burden correlated with reduced left hippocampal volume across the entire cohort.
In a related study, Perez and colleagues expanded their cohort to 26 FND patients and 27 controls to investigate between-group and within-group associations with health-related quality of life, trait anxiety, and depression (Perez et al., 2017b). There were no volumetric differences between the complete FND cohort and controls. However, the sub-group of 13 FND patients with the most impaired physical health showed reduced left anterior insular GM volume compared to controls in ROI analyses; the sub-group of 13 patients with the greatest mental health impairments showed whole-brain corrected posterior lateral cerebellar volumetric increases (implicated in aversive responses) compared to controls. Within-group analyses showed that individual-differences in right amygdalar volume correlated with elevated trait anxiety and impaired mental health. In a pilot follow-up study performed on 22 of the initial 26 FND subjects, individual differences in baseline anterior hippocampal GM volume positively correlated with 6-month mental health outcomes; there were no volumetric associations with physical health outcomes (Perez et al., 2018c).
In additional support for structural alterations in limbic areas, 48 patients with functional movement disorders (17 also with functional limb weakness) exhibited increased left amygdalar GM volumes compared to controls in whole-brain corrected analyses (Maurer et al., 2018). Furthermore, increased left striatal, fusiform gyrus, cerebellar and bilateral thalamic GM volumes, alongside reduced left sensorimotor cortical volumes were observed in patients with functional movement disorders. Female gender, symptom lateralization, psychotropic medication use, and individuals with functional limb weakness vs. those with only hyperkinetic movements did not show robust subgroup effects. In within-group analyses, childhood trauma burden positively correlated with left caudate and cerebellar tonsil volumes; patients with higher anxiety exhibited smaller left fusiform gyrus volumes and those with more depression displayed larger cerebellar tonsil volumes. In this study, volumetric profiles did not correlate with illness duration or symptom severity. By contrast, another recently published VBM study reported decreased left caudate and right postcentral GM volumes in 27 individuals with functional tremor compared to controls (Espay et al., 2018b). These distinct striatal findings across functional movement disorder cohorts highlight the need for additional replication.
Developmental trajectories are important in contextualizing volumetric profiles in patients with FND. Kozlowska and colleagues applied VBM in 25 children and adolescents (80% female) with mixed FND to show increased left supplementary motor area (SMA) and right superior temporal and dorsomedial prefrontal volumes compared to controls in whole-brain corrected analyses (Kozlowska et al., 2017). In addition, SMA volumes positively correlated with faster reaction times in an emotion recognition task, interpreted to reflect enhanced motor readiness and vigilance to others' emotional states.
In the only VBM study performed in individuals with functional sensory deficits, 25 patients with nondermatomal somatosensory deficits (NDSD) and chronic widespread myofascial pain compared to controls showed increased bilateral middle/inferior temporal, posterior hippocampal/fusiform and right thalamic, precuneus and posterior cingulate GM volumes (Riederer et al., 2017). ROI analyses also identified increased right postcentral gyrus and left insular volumes in patients with NDSD compared to controls. Notably, patients with chronic pain without NDSD compared to healthy controls also showed post-central gyrus volumetric increases in ROI analyses.
In summary, volumetric studies across FND and SSD reported insular and thalamic structural alterations (Maurer et al., 2018; Perez et al., 2017b; Riederer et al., 2017; Valet et al., 2009), although a lack of group-level differences has also been described (Aybek et al., 2014a; Perez et al., 2017b) and studies have been inconsistent in delineating striatal abnormalities in functional movement disorders (Espay et al., 2018b; Maurer et al., 2018). Increases in motor/premotor GM volume have been reported in some adult and pediatric FND populations (Aybek et al., 2014a; Kozlowska et al., 2017), while others identified reduced sensorimotor volume (Espay et al., 2018b; Maurer et al., 2018). Patients with FND also exhibited increased amygdalar volumes compared to controls (Maurer et al., 2018). Across three questionnaires collected at one time point, left anterior insular volumes negatively correlated with patient-reported FND symptom severity using ROI analyses in one cohort (Perez et al., 2017a; Perez et al., 2017b). These brain-symptom severity findings have not been independently replicated.
4.3. Surface-based analyses
Several studies have applied surface-based approaches to delineate precentral gyrus and paralimbic alterations among other findings in patients with PNES and other motor FND subtypes (Labate et al., 2011; Perez et al., 2018b; Ristic et al., 2015; Williams et al., 2018). Labate and colleagues used dual surface-based CTH and VBM techniques in 20 patients (55% women) with PNES compared to 40 controls (Labate et al., 2011). In whole-brain corrected VBM analyses, patients compared to controls showed reduced bilateral cerebellum, right precentral, middle frontal gyrus, ACC, and SMA volumes. In within-group analyses, depression scores negatively correlated with right dorsal premotor volumes. In CTH analyses, patients with PNES showed thinning in the right precentral, superior frontal, paracentral gyri and precuneus. Depression scores correlated with right superior frontal and paracentral cortical thinning; somatoform dissociation severity inversely correlated with left inferior frontal and central sulcus CTH.
Surface-based measures examined in 37 patients (84% women) with PNES showed increased CTH in the bilateral medial orbitofrontal and left insular and lateral orbitofrontal cortices compared to controls (Ristic et al., 2015). Individuals with PNES also exhibited bilateral precentral and right entorhinal and lateral occipital cortical thinning; between-group sulcal depth abnormalities are described in Table 1. Another CTH and gyrification study in 20 patients with PNES showed reduced bilateral inferior frontal and right superior temporal and medial orbitofrontal CTH compared to controls; this PNES cohort also showed increased CTH in bilateral cuneus and left paracentral and lingual regions (McSweeney et al., 2018). There were no between-group gyrification differences.
In a recently published study, 23 patients with PNES showed decreased bilateral inferior frontal, medial orbitofrontal, left caudal middle frontal, right precentral gyri and left insular surface area compared to controls; increased left posterior cingulate surface area was also observed in patients with PNES (Vasta et al., 2018). Using machine learning, their MRI classification algorithm showed 75% accuracy in discriminating PNES from healthy subjects, with the inferior frontal, medial orbitofrontal and posterior cingulate cortices exhibiting the most differentiating profiles.
In addition to surface-based studies in isolated PNES, a CTH study in 26 patients with mixed FND compared to controls (same cohort as (Perez et al., 2017b) showed no whole-brain corrected differences (Perez et al., 2018b). However, the sub-group with high somatoform dissociation showed reduced left caudal ACC CTH compared to controls. In within-group analyses, somatoform dissociation severity inversely correlated to the left caudal ACC CTH across the FND cohort. A positive within-group correlation was also observed between depersonalization/derealization severity and right lateral occipital CTH. Both within-group relationships remained significant controlling for trait anxiety/depression, borderline personality disorder and PTSD, trauma burden, FND subtypes, and antidepressant use in separate post-hoc analyses. Another study in the same cohort showed that individual differences in dismissing attachment style correlated with reduced left parahippocampal CTH in women with FND (Williams et al., 2018). In addition, individual differences in confrontative coping and adaptive coping through accepting responsibility were associated with decreased right hippocampal volumes and increased ventral precentral gyrus CTH in women with FND, respectively.
Fourteen patients with chronic somatoform pain disorder showed thinning of left sensorimotor cortices (pre and postcentral gyri) and the left inferior temporal sulcus compared to controls (Magon et al., 2018). These results were interpreted as identifying brain areas implicated in sensory and affective processing of pain.
Overall, while the directionality of effects requires clarification, early evidence suggests that individual differences in caudal ACC and inferior frontal gyrus CTH in FND may be linked to somatoform dissociation (Labate et al., 2011; McSweeney et al., 2018; Perez et al., 2018b; Vasta et al., 2018). Notably, the ACC and inferior frontal gyrus are implicated in attentional/cognitive control and self-monitoring, and the caudal ACC is an integrative zone for cognitive control, negative affect and nociception (Shackman et al., 2011). Furthermore, CTH studies have been inconsistent in delineating possible motor/premotor/SMA alterations, with studies reporting reduced (Labate et al., 2011; Ristic et al., 2015), increased (McSweeney et al., 2018) or no group-level CTH differences (Aybek et al., 2014a; Perez et al., 2018b); sensorimotor cortical thinning was also observed in somatoform pain disorder (Magon et al., 2018).
4.4. White matter analyses
Several studies have used diffusion tensor imaging (DTI) to investigate white matter integrity in FND and somatization disorder (Ding et al., 2013; Hernando et al., 2015; Lee et al., 2015; Zhang et al., 2015). Lee and colleagues used tract-based spatial statistics (TBSS) to characterize increased fractional anisotropy (FA) in the left internal and external capsules, corona radiata, uncinate fasciculus, and white matter tracts adjacent to the superior temporal gyrus in 16 PNES patients (15 women) compared to controls (Lee et al., 2015). In a tractography study, a rightward asymmetry of the uncinate fasciculus was observed in 8 patients with PNES compared to controls (Hernando et al., 2015). Age of PNES onset inversely correlated with the magnitude of uncinate fasciculus asymmetry. In both DTI studies, data was acquired on multiple scanners, introducing a potential confound.
A graph-theory study identified that 17 patients with PNES exhibited a more lattice-like (small world) white matter organization and decreased coupling strength of structural and functional connectivity profiles compared to controls (Ding et al., 2013). In this study, structural connectivity was also altered across sensorimotor, attentional, subcortical, and default mode networks in patients with PNES compared to controls.
Another study examined gray and white matter changes in a cohort of 13 “fixed” functional dystonia, 31 “mobile” functional dystonia and 43 controls (Tomic et al., 2018). Their results showed no differences in CTH or GM volumes in both dystonia groups vs. controls. However, individuals with mobile functional dystonia exhibited decreased left nucleus accumbens, putamen, thalamic and bilateral caudate GM volumes compared to controls. In voxel-based white matter analyses, patients with functional dystonia showed decreased FA (and increased mean and radial diffusivity) in the corpus callosum, corticospinal tract, anterior thalamic radiations, cingulum bundle, uncinate fasciculus and brainstem among other sites. This study highlights the utility of multimodal neuroimaging techniques in elucidating the pathophysiology of FND.
A TBSS study in 25 patients with somatization disorder compared to 28 controls showed no between-group differences correcting for multiple comparisons (Zhang et al., 2015). Somatization severity positively correlated with FA values of the cingulum bundle and inferior fronto-occipital fasciculus. Zhao and colleagues showed that 26 patients with somatization disorder exhibited reduced white matter volume in the right inferior frontal gyrus and left inferior longitudinal fasciculus compared to controls (Zhao et al., 2018). These findings, however, did not relate to clinical variables.
While white matter investigations in FND and somatization disorder are at a particularly early stage, initial findings point towards possible limbic fiber bundle alterations (Hernando et al., 2015; Lee et al., 2015; Tomic et al., 2018; Zhang et al., 2015), with potentially more widespread disturbances.
5. Discussion
The MRI literature to date, while in early stages, supports that some FND and SSD populations exhibit structural brain alterations. Several implicated areas potentially overlap across both disorders.
5.1. Sensorimotor-striatothalamic-limbic-paralimbic circuits in FND and SSD
Structural changes involving primary and associative motor cortices have been reported in patients with FND compared to controls. Increases in premotor CTH and SMA volume have been identified in hemi-paretic FND-limb weakness (Aybek et al., 2014a) and mixed-FND pediatric (Kozlowska et al., 2017) populations, respectively. However, both decreases and increases in motor-related GM have been observed in patients with PNES (Labate et al., 2011; McSweeney et al., 2018; Ristic et al., 2015). These observations suggest heterogeneity in the magnitude and direction of potential motor alterations, even within specific FND subtypes. Alternatively, these findings may indicate differential involvement of motor regions during distinct ‘stages’ of disease (see future directions section) or cohort differences in psychotropic medication use. Lack of motor-premotor-SMA volumetric and CTH alterations have also been described in patients with FND (Aybek et al., 2014a; Perez et al., 2018b; Perez et al., 2017b; Tomic et al., 2018), suggesting that volumetric alterations may not be universally present in all patients with FND. Overall, these seemingly contradictory findings may relate to a multiplicity of distinct sample characteristics, and may be disease-related or compensatory. Nonetheless, these emerging structural alterations complement neuroimaging evidence showing motor circuit abnormalities using resting-state (Kozlowska et al., 2018; Li et al., 2015a; Wegrzyk et al., 2018) and motor tasks (Burgmer et al., 2006; Cojan et al., 2009; Marshall et al., 1997; Spence et al., 2000; Stone et al., 2007; Voon et al., 2011) in FND.
By contrast, studies in some SSD and NDSD cohorts compared to controls implicate sensory regions, including postcentral and occipital-temporal cortices (Magon et al., 2018; Riederer et al., 2017). In addition, while not universally identified, striatal-thalamic volumetric increases have been reported in SSD and in functional movement disorder patients (Hakala et al., 2004; Maurer et al., 2018). Smaller basal ganglia and thalamic volumes have been found in other FND studies (Atmaca et al., 2006; Espay et al., 2018b; Nicholson et al., 2014; Tomic et al., 2018). These changes complement growing evidence supporting a role for the thalamus beyond basic sensory-motor gating (Briggs and Usrey, 2008); reciprocal corticothalamic feedback serves to “sharpen the receptive fields,” but also potentially amplify sensory transmission arising from the periphery (Briggs and Usrey, 2008). Changes within these pathways may relate to altered somatosensory perceptions in these populations. Taken together, evidence points towards differential involvement of structural sensorimotor-striatal-thalamic circuits in FND and SSD, with commonly identified motor-related alterations in motor FND and somatosensory involvement in SSD.
Cingulo-insular structural alterations have also been described in FND and SSD populations (Labate et al., 2011; Perez et al., 2017a; Perez et al., 2018b; Perez et al., 2017b; Riederer et al., 2017; Ristic et al., 2015; Valet et al., 2009; Vasta et al., 2018). Notably, cingulo-insular (salience) network structural changes have been reported in PNES (Labate et al., 2011; Ristic et al., 2015; Vasta et al., 2018), mixed FND (Perez et al., 2017a; Perez et al., 2018b; Perez et al., 2017b), NDSD (Riederer et al., 2017), and somatoform pain disorder (Valet et al., 2009), with cingulo-insular volumetric reductions possibly driven by sub-populations reporting more severe functional neurologic symptoms. Furthermore, neuroimaging studies and meta-analyses in FND (Aybek et al., 2015; Boeckle et al., 2016a; Espay et al., 2018a; Stone et al., 2007) and SSD (Boeckle et al., 2016b; Perez et al., 2015a) support cingulo-insular functional alterations. The salience network, which includes cingulo-insular, amygdala and periaqueductal gray areas as core components, is implicated in the convergent processing of viscerosomatic (interoceptive) and nociceptive inputs with affective, threat-related, and motivational value (Seeley et al., 2007). Anterior insula and dorsal ACC areas are also implicated in cognitive (executive) control, and the anterior insula plays a role in self and emotional awareness (Craig, 2002; Paulus and Stein, 2006; Sinanaj et al., 2015). Integrating the FND, SSD and cognitive-affective neuroscience literature, we propose that structural and functional cingulo-insular alterations may contribute to impaired multimodal integration of affective and bodily-related information, which could also help explain the multiplicity of sensorimotor, affective and cognitive symptoms in some patients with FND and SSD (Bègue, 2018; Perez et al., 2015b; Vuilleumier, 2014).
Studies have also found prefrontal and paralimbic (orbitofrontal, parahippocampal gyrus) structural alterations in FND and SSD (Kozlowska et al., 2017; Labate et al., 2011; McSweeney et al., 2018; Ristic et al., 2015; Valet et al., 2009; Williams et al., 2018). Patients with PNES show reduced inferior frontal gyrus (Labate et al., 2011; McSweeney et al., 2018) and altered orbitofrontal CTH (McSweeney et al., 2018; Ristic et al., 2015) compared to controls. Inferior frontal gyrus CTH alterations, linked to somatoform dissociation (Labate et al., 2011), may relate more broadly to impaired top-down cognitive-emotional regulation. A volumetric study in pediatric FND suggests that developmental trajectories are also important for interpretative considerations (Kozlowska et al., 2017). In addition, parahippocampal volumetric reductions have been characterized in somatoform pain disorder (Valet et al., 2009), and inversely correlated with insecure attachment in patients with mixed FND (CTH) (Williams et al., 2018). More research is needed to contextualize these parahippocampal findings in relation to social-emotional, memory, and metacognitive abilities (Bègue et al., 2018b; Ward et al., 2014).
Finally, structural MRI findings in FND and somatization disorder populations point to changes in the amygdala and pituitary gland (Atmaca et al., 2016; Atmaca et al., 2011; Maurer et al., 2018; Perez et al., 2017b; Yildirim et al., 2012). In parallel, studies in patients with FND or SSD have identified heightened amygdalar activity at rest and/or during performance of affectively-valenced, nociceptive or metacognitive tasks (Aybek et al., 2015; Aybek et al., 2014b; Bègue et al., 2018b; Gundel et al., 2008; Hassa et al., 2017; Kanaan et al., 2007; Voon et al., 2010a). In addition, heightened amygdalar-motor control network connectivity in the resting-state (Morris et al., 2017; Wegrzyk et al., 2018) or during emotion processing (Aybek et al., 2015; Aybek et al., 2014b; Voon et al., 2010a) suggests an important pathway through which limbic structures may influence behavior. Notably, patients with SSD show potentially divergent amygdalar activations for external (environmental) emotional processing and bodily-related stimuli (de Greck et al., 2012; Gundel et al., 2008; Perez et al., 2015a).
The evidence reviewed here employing a transdiagnostic approach supports partially overlapping endophenotypes that may be disease related, compensatory and/or the consequence of shared predisposing vulnerabilities. To contextualize the structural neurobiology of FND and SSD, the subsequent sections briefly review aberrant neuroplasticity following adverse life events, the role of gender in the development of psychopathology, and the structural neurobiology of common neuropsychiatric comorbidities.
5.2. Stress-related neuroplasticity and gender differences
Adverse life events, including childhood abuse, are predisposing risk factors for the development of FND (Keynejad et al., 2018; Ludwig et al., 2018) and SSD (Loeb et al., 2018; Paras et al., 2009), and traumatic experiences have enduring neurobiological effects (Dannlowski et al., 2011; Lim et al., 2014; Teicher and Samson, 2013). Several areas implicated in the pathophysiology of FND and SSD such as the ACC, insula, orbitofrontal cortex and amygdala are susceptible to aberrant stress-mediated neuroplasticity (Dannlowski et al., 2011; Lim et al., 2014). Furthermore, childhood maltreatment in non-clinical populations is associated with automatic, biased negative emotional processing (Dannlowski et al., 2013) and sensitized amygdalar responsiveness (Dannlowski et al., 2011), providing a potential bridge between emotional processing and aberrant amygdala activations in patients with FND (Bègue et al., 2018b; Pick et al., 2018). Moreover, oxytocin receptor genotypes are connected with heightened amygdalar activity during negative emotional processing (Dannlowski et al., 2016), which fits well with preliminary findings of increased oxytocin receptor gene methylation in patients with FND (Apazoglou et al., 2017) and hypotheses of oxytocin abnormalities in SSD (Landa et al., 2012). Additionally, it remains an unanswered question whether some trauma-related neuroplastic changes increase a general predilection for the development of psychopathology (i.e. hippocampal reductions), while other changes may potentially facilitate the specific development of FND and/or SSD.
Given links between adverse life events and aberrant neuroplasticity in brain areas also implicated in the pathophysiology of FND and SSD, it is noteworthy that parallel autonomic and neuroendocrine abnormalities have been described in FND (Apazoglou et al., 2017; Bakvis et al., 2010; Kozlowska et al., 2015; Reinsberger et al., 2012) and SSD (Janssens et al., 2012; Rief and Barsky, 2005). Altered skin conductance and startle eyeblink responses have also been reported in FND cohorts (Kozlowska et al., 2018; Pick et al., 2017; Seignourel et al., 2007). Similarly, patients with SSD compared to controls exhibit decreased parasympathetic and increased sympathetic tone at baseline and during emotional and nociceptive processing (Houtveen and van Doornen, 2007; Pollatos et al., 2011a; Pollatos et al., 2011b).
Gender is another relevant factor when contextualizing indirect, nuanced links between adversity and the development of FND and SSD. For example, women exhibit higher rates of depression and anxiety than men exposed to similar traumatic experiences (MacMillan et al., 2001). A recent review focused on the contribution of sex-based biological differences on adult psychopathology across three domains that warrant study in FND and SSD (Tiwari and Gonzalez, 2018): (1) the role of gonadal hormones on modulating interactions between HPA axis functioning and adverse events across the lifespan; (2) gender differences in corticolimbic activations; (3) sex-specific interactions between epigenetic modifications in candidate genes modulating the effects of stress exposure and the development of psychopathology.
5.3. Neurocircuitry of psychiatric and other functional somatic disorders
Although this article focuses on the structural neurobiology of FND and SSD, it is important to note the overlapping neurocircuitry with the extended spectrum of other psychiatric and functional somatic disorders. In a VBM meta-analysis, patients with major depression compared to controls showed reduced rostral ACC and dorsolateral/dorsomedial prefrontal volumes; amygdalar and parahippocampal GM volume reductions were also commonly appreciated in those with mixed depression-anxiety (Bora et al., 2012). Similar volumetric abnormalities are observed in PTSD, involving bilateral ACC, orbitofrontal and hippocampal reductions (Kuhn and Gallinat, 2013). Borderline personality disorder, a comorbidity in some with FND, is linked to reduced bilateral amygdalar volumes (Ruocco et al., 2012).
Given the frequent co-occurrence of FND with other functional somatic disorders (i.e. fibromyalgia, irritable bowel syndrome) and chronic pain disorders, as well as their negative impact on clinical outcomes (Glass et al., 2018), it is notable that individuals with chronic back pain, chronic tension headache, and fibromyalgia exhibit neurocircuit alterations within the central pain matrix which strikingly overlap with many of the regions identified in the FND and SSD literature (Denk et al., 2014; Kuchinad et al., 2007; Schmidt-Wilcke et al., 2006; Schmidt-Wilcke et al., 2005; Yuan et al., 2017). Furthermore, prefrontal-striatal-thalamic-limbic structural alterations have also been described in fibromyalgia, irritable bowel syndrome and/or chronic fatigue syndrome (Cagnie et al., 2014; Finkelmeyer et al., 2018; Labus et al., 2014; Seminowicz et al., 2010; Shi et al., 2016). This argues for the need for considerable more research at the intersection of FND and the full spectrum of functional somatic disorders (Grinsvall et al., 2018).
6. Limitations
Methodological limitations in the FND and SSD structural MRI literature to date can be grouped into confounding factors, sample size issues, varied diagnostic criteria, and neuroimaging methodological concerns. See Table 1, Table 2 for specific limitations suggested for individual studies.
One major concern is heterogeneous patient characterization. Individual symptoms (e.g. weakness, seizures, pain, etc.) are not consistently reported across studies. Additionally, the systematic characterization of relevant clinical parameters (symptom severity, duration, number of relapses, medication use histories, psychiatric/neurological comorbidities etc.) is lacking. Since correlation of clinical measures to structural neuroimaging profiles is not routinely performed, or found to not be significant, this raises questions regarding whether reported structural group-differences represent disease mechanisms, compensatory alterations or markers of predisposing vulnerabilities. It should also be highlighted that several studies have reported no structural differences in FND cohorts vs. controls (Aybek et al., 2014a; Perez et al., 2018b; Perez et al., 2017b; Tomic et al., 2018). One interpretation may be that these studies were underpowered to capture quantifiable differences; alternatively, structural profiles may not be uniform across patients and perhaps influenced by symptom severity, disease chronicity, neuropsychiatric comorbidities and so on. In our opinion, a fruitful approach that embraces the inherent heterogeneity present in FND and SSD populations is to complement between-group analyses with stratified sub-group and within-group analyses based on clinically relevant characteristics (i.e. symptom severity, psychiatric comorbidities etc.). It also remains unclear whether the neurobiology of isolated functional deficits (e.g. limb weakness) differs significantly from mixed presentations.
The lack of comprehensive characterization of predisposing vulnerabilities, routine correction for depression and anxiety, and consideration of treatment effects (medications, psychotherapy, physiotherapy, etc.) are additional confounds. In particular, there is frequently limited characterization of comorbid psychiatric conditions and other functional somatic syndromes. Notably, it is our opinion that comorbid mood/anxiety symptoms are not simply “nuisance” variables. Instead, it seems likely that some nodes in the neurobiology of FND and SSD may be at the intersection of sensorimotor, viscerosomatic and affective processing. In addition, the apparent exclusion of psychiatric comorbidities in some studies (Hakala et al., 2004; Yildirim et al., 2012; Zhang et al., 2015) raises the question of generalizability for clinical populations where depression and anxiety are common. Nonetheless, it is important to perform additional analyses adjusting for co-morbid depression, anxiety and psychotropic medication use to evaluate these nuanced relationships. To aid the identification of specific brain-symptom relationships in FND and SSD, we suggest the future use of psychiatric and neuropsychiatric controls (e.g. PTSD, mood-anxiety disorders, dissociative disorders, migraine, mild traumatic brain injury etc.). Longitudinal studies would also help disentangle the effects of trait vs. state factors.
Another limitation is that some SSD cohorts appear poorly characterized clinically. This issue is particularly relevant for the studies in somatization disorder where the presence of functional neurological symptoms is ill-defined, and it is unclear if neurological examination signs were used as part of the basis for this categorization. As noted previously, it also remains an unanswered question how the prior diagnostic categories of somatization disorder, somatoform pain disorder and undifferentiated somatoform disorder will explicitly relate to the DSM-5 SSD category, even though the authors of the DSM-5 expected these diagnostic categories to be connected (Dimsdale et al., 2013). Other concerns with the DSM-5 SSD category have also been raised, including its potential use for patients with established medical or neurological etiologies for the patient's somatic symptoms (Mayou, 2014). Future research efforts should integrate the larger spectrum of functional somatic disorders, such as irritable bowel syndrome and fibromyalgia with positive diagnostic criteria.
Neuroimaging methodological concerns include underpowered sample sizes, variable correction for multiple comparisons (whole-brain corrected vs. ROI vs. uncorrected findings) and limited use of multimodal measures (e.g. CTH and GM volume). Across FND and SSD research there has also been a limited number of independent cohorts, underscoring the need for larger and a greater number of independent samples that can address statistical power and patient heterogeneity concerns. For example, some groups used the same cohort to examine MRI profiles related to physical health and predisposing vulnerabilities (Perez et al., 2017b; Williams et al., 2018); others may have applied distinct neuroimaging techniques (white matter volume and tract-based analyses) in the same cohort (Zhang et al., 2015; Zhao et al., 2018). Caution should be taken to not over interpret the structural neuroimaging literature outlined here, particularly given that the neural mechanisms of FND and SSD remain distinct from lesional neurological conditions. In addition, given the identified study limitations, the brain structure – patient cohort relationships detailed in this systematic review should be interpreted with caution and require replication. Furthermore, studies employing multimodal methods that combine neuroimaging endophenotypes with behavioral, autonomic, neuroendocrine, and genetic/epigenetic data are needed.
7. Future directions
Regarding the path forward, we advocate for research in disease staging to tackle heterogeneity issues. Staging models for chronic disorders such as hypertension and congestive heart failure are frequently used in medicine to delineate disease progression and inform treatment, yet this approach has been minimally used in neuropsychiatry (McGorry et al., 2014). Mechanistically, the staging concept captures functional and structural alterations as the disease progresses and implies the existence of at-risk individuals. Such individuals, in the context of gene-environment interactions, may have variable risk for the development of FND and/or SSD, as well as distinct prognoses. A staging algorithm could prove useful by placing a given patient in a continuum starting from an at-risk phase (stage 0) through to a chronic, relapsing/persistent phase (stage 3). In addition, staging algorithms would begin to operationalize cohort characterization for research practices.
Staging algorithms should incorporate developmental trajectories, with potentially distinct criteria for pediatric and adult populations. Staging classification scores might include:
-
a)
demographic parameters including age and gender.
-
b)
clinical parameters such as number and severity of individual symptoms, sites of involvement, episode duration, number of relapses etc.
-
c)
presence and severity of psychiatric comorbidities, concurrent functional somatic syndromes, and prior treatment considerations.
-
d)
impact on functionality (e.g. patient-rated, clinician-rated (Hinson et al., 2005)).
As a work-in-progress example: stage 0 – no clinical manifestation; stage 1 – within 12-months of first clinically salient episode; stage 2a – recurrent disability with multiple episodes (or persistent symptom duration > 1 year) and moderate impact on functioning; stage 2b – stage 2a with presence of major psychiatric comorbidities or another functional somatic disorder; stage 3 – chronically ill or with multiple relapses severely impacting functionality. Notably, the staging approach fits well with stepped-care and multidisciplinary treatment approaches for FND and SSD. Furthermore, as the field advances, MRI biomarkers may also be introduced into the criteria to provide greater biological specificity to disease staging algorithms.
In conclusion, emerging data suggests that some FND and SSD cohorts show evidence of both a “software” and “hardware” problem. The intersection of FND and DSM-5 SSD diagnostic categories have not yet been explicitly studied. Furthermore, current data does not allow conclusions regarding if the structural neuroimaging findings outlined here are disorder specific, or more closely related to predisposing risk factors and/or compensatory changes.
Funding
D.L.P. was funded by the NIMH K23MH111983-02, Sidney R. Baer Jr. Foundation and the Massachusetts General Hospital Physician-Scientist Development Award.
Acknowledgments
Acknowledgements
The authors would like to thank Patrik Vuilleumier for helpful comments in previous versions of this manuscript.
Disclosures/conflicts of interest
J.S. has received honorarium for lectures from the Movement Disorder Society, royalties from UpToDate and engages in independent expert personal injury and medical negligence testimony work in relation to FND and SSD.
D.L.P. has received honorarium for lectures from the American Academy of Neurology, Movement Disorder Society and Harvard Medical School Continuing Education programs.
All other authors do not have any conflicts of interests/disclosures.
References
- American Psychiatric Association . 5th ed. American Psychiatric Pub; Washington, DC: 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5) [Google Scholar]
- Apazoglou K., Mazzola V., Wegrzyk J., Polara G.F., Aybek S. Biological and perceived stress in motor functional neurological disorders. Psychoneuroendocrinology. 2017;85:142–150. doi: 10.1016/j.psyneuen.2017.08.023. [DOI] [PubMed] [Google Scholar]
- Atmaca M., Aydin A., Tezcan E., Poyraz A.K., Kara B. Volumetric investigation of brain regions in patients with conversion disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2006;30:708–713. doi: 10.1016/j.pnpbp.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Atmaca M., Sirlier B., Yildirim H., Kayali A. Hippocampus and amygdalar volumes in patients with somatization disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2011;35:1699–1703. doi: 10.1016/j.pnpbp.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Atmaca M., Baykara S., Mermi O., Yildirim H., Akaslan U. Pituitary volumes are changed in patients with conversion disorder. Brain Imaging Behav. 2016;10:92–95. doi: 10.1007/s11682-015-9368-6. [DOI] [PubMed] [Google Scholar]
- Avbersek A., Sisodiya S. Does the primary literature provide support for clinical signs used to distinguish psychogenic nonepileptic seizures from epileptic seizures? J. Neurol. Neurosurg. Psychiatry. 2010;81:719–725. doi: 10.1136/jnnp.2009.197996. [DOI] [PubMed] [Google Scholar]
- Aybek S., Nicholson T.R., Draganski B., Daly E., Murphy D.G., David A.S., Kanaan R.A. Grey matter changes in motor conversion disorder. J. Neurol. Neurosurg. Psychiatry. 2014;85:236–238. doi: 10.1136/jnnp-2012-304158. [DOI] [PubMed] [Google Scholar]
- Aybek S., Nicholson T.R., Zelaya F., O'Daly O.G., Craig T.J., David A.S., Kanaan R.A. Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry. 2014;71:52–60. doi: 10.1001/jamapsychiatry.2013.2842. [DOI] [PubMed] [Google Scholar]
- Aybek S., Nicholson T.R., O'Daly O., Zelaya F., Kanaan R.A., David A.S. Emotion-motion interactions in conversion disorder: an FMRI study. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek K., Donamayor N., Morris L.S., Strelchuk D., Mitchell S., Mikheenko Y., Yeoh S.Y., Phillips W., Zandi M., Jenaway A., Walsh C., Voon V. Impaired awareness of motor intention in functional neurological disorder: implications for voluntary and functional movement. Psychol. Med. 2017;47:1624–1636. doi: 10.1017/S0033291717000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakvis P., Spinhoven P., Giltay E.J., Kuyk J., Edelbroek P.M., Zitman F.G., Roelofs K. Basal hypercortisolism and trauma in patients with psychogenic nonepileptic seizures. Epilepsia. 2010;51:752–759. doi: 10.1111/j.1528-1167.2009.02394.x. [DOI] [PubMed] [Google Scholar]
- Bègue I. From emotion processing to metacognition: mnemonic contributions in conversion/functional neurological symptoms. Swiss Arch Neurol. Psychiatr. Psychother. 2018;169:253–259. [Google Scholar]
- Bègue I., Blakemore R., Klug J., Cojan Y., Galli S., Berney A., Aybek S., Vuilleumier P. Metacognition of visuomotor decisions in conversion disorder. Neuropsychologia. 2018;114:251–265. doi: 10.1016/j.neuropsychologia.2018.04.018. [DOI] [PubMed] [Google Scholar]
- Boeckle M., Liegl G., Jank R., Pieh C. Neural correlates of conversion disorder: overview and meta-analysis of neuroimaging studies on motor conversion disorder. BMC Psychiatry. 2016;16:195. doi: 10.1186/s12888-016-0890-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeckle M., Schrimpf M., Liegl G., Pieh C. Neural correlates of somatoform disorders from a meta-analytic perspective on neuroimaging studies. Neurol. Clin. 2016;11:606–613. doi: 10.1016/j.nicl.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogousslavsky J. Jean-Martin Charcot and his legacy. Front Neurol. Neurosci. 2014;35:44–55. doi: 10.1159/000359991. [DOI] [PubMed] [Google Scholar]
- Bora E., Fornito A., Pantelis C., Yucel M. Gray matter abnormalities in major depressive disorder: a meta-analysis of voxel based morphometry studies. J. Affect. Disord. 2012;138:9–18. doi: 10.1016/j.jad.2011.03.049. [DOI] [PubMed] [Google Scholar]
- Bowman E.S., Markand O.N. Psychodynamics and psychiatric diagnoses of pseudoseizure subjects. Am. J. Psychiatry. 1996;153:57–63. doi: 10.1176/ajp.153.1.57. [DOI] [PubMed] [Google Scholar]
- Breuer J., Freud S. Hogarth Press; London: 1956. Studies on Hysteria. [Google Scholar]
- Briggs F., Usrey W.M. Emerging views of corticothalamic function. Curr. Opin. Neurobiol. 2008;18:403–407. doi: 10.1016/j.conb.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgmer M., Konrad C., Jansen A., Kugel H., Sommer J., Heindel W., Ringelstein E.B., Heuft G., Knecht S. Abnormal brain activation during movement observation in patients with conversion paralysis. Neuroimage. 2006;29:1336–1343. doi: 10.1016/j.neuroimage.2005.08.033. [DOI] [PubMed] [Google Scholar]
- Cagnie B., Coppieters I., Denecker S., Six J., Danneels L., Meeus M. Central sensitization in fibromyalgia? A systematic review on structural and functional brain MRI. Semin. Arthritis Rheum. 2014;44:68–75. doi: 10.1016/j.semarthrit.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Carson A.J., Brown R., David A.S., Duncan R., Edwards M.J., Goldstein L.H., Grunewald R., Howlett S., Kanaan R., Mellers J., Nicholson T.R., Reuber M., Schrag A.E., Stone J., Voon V., Uk F.N.S. Functional (conversion) neurological symptoms: research since the millennium. J. Neurol. Neurosurg. Psychiatry. 2012;83:842–850. doi: 10.1136/jnnp-2011-301860. [DOI] [PubMed] [Google Scholar]
- Carson A., Lehn A., Ludwig L., Stone J. Explaining functional disorders in the neurology clinic: a photo story. Pract. Neurol. 2016;16:56–61. doi: 10.1136/practneurol-2015-001242. [DOI] [PubMed] [Google Scholar]
- Cojan Y., Waber L., Carruzzo A., Vuilleumier P. Motor inhibition in hysterical conversion paralysis. Neuroimage. 2009;47:1026–1037. doi: 10.1016/j.neuroimage.2009.05.023. [DOI] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Dannlowski U., Stuhrmann A., Beutelmann V., Zwanzger P., Lenzen T., Grotegerd D., Domschke K., Hohoff C., Ohrmann P., Bauer J., Lindner C., Postert C., Konrad C., Arolt V., Heindel W., Suslow T., Kugel H. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry. 2011;71:286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Dannlowski U., Kugel H., Huber F., Stuhrmann A., Redlich R., Grotegerd D., Dohm K., Sehlmeyer C., Konrad C., Baune B.T., Arolt V., Heindel W., Zwitserlood P., Suslow T. Childhood maltreatment is associated with an automatic negative emotion processing bias in the amygdala. Hum. Brain Mapp. 2013;34:2899–2909. doi: 10.1002/hbm.22112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U., Kugel H., Grotegerd D., Redlich R., Opel N., Dohm K., Zaremba D., Grogler A., Schwieren J., Suslow T., Ohrmann P., Bauer J., Krug A., Kircher T., Jansen A., Domschke K., Hohoff C., Zwitserlood P., Heinrichs M., Arolt V., Heindel W., Baune B.T. Disadvantage of social sensitivity: interaction of oxytocin receptor genotype and child maltreatment on brain structure. Biol. Psychiatry. 2016;80:398–405. doi: 10.1016/j.biopsych.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Daum C., Hubschmid M., Aybek S. The value of 'positive' clinical signs for weakness, sensory and gait disorders in conversion disorder: a systematic and narrative review. J. Neurol. Neurosurg. Psychiatry. 2014;85:180–190. doi: 10.1136/jnnp-2012-304607. [DOI] [PubMed] [Google Scholar]
- de Greck M., Scheidt L., Bolter A.F., Frommer J., Ulrich C., Stockum E., Enzi B., Tempelmann C., Hoffmann T., Han S., Northoff G. Altered brain activity during emotional empathy in somatoform disorder. Hum. Brain Mapp. 2012;33:2666–2685. doi: 10.1002/hbm.21392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange F.P., Toni I., Roelofs K. Altered connectivity between prefrontal and sensorimotor cortex in conversion paralysis. Neuropsychologia. 2010;48:1782–1788. doi: 10.1016/j.neuropsychologia.2010.02.029. [DOI] [PubMed] [Google Scholar]
- Denk F., McMahon S.B., Tracey I. Pain vulnerability: a neurobiological perspective. Nat. Neurosci. 2014;17:192–200. doi: 10.1038/nn.3628. [DOI] [PubMed] [Google Scholar]
- Dimsdale J.E., Creed F., Escobar J., Sharpe M., Wulsin L., Barsky A., Lee S., Irwin M.R., Levenson J. Somatic symptom disorder: an important change in DSM. J. Psychosom. Res. 2013;75:223–228. doi: 10.1016/j.jpsychores.2013.06.033. [DOI] [PubMed] [Google Scholar]
- Ding J.R., An D., Liao W., Li J., Wu G.R., Xu Q., Long Z., Gong Q., Zhou D., Sporns O., Chen H. Altered functional and structural connectivity networks in psychogenic non-epileptic seizures. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M.J., Adams R.A., Brown H., Parees I., Friston K.J. A Bayesian account of 'hysteria'. Brain. 2012;135:3495–3512. doi: 10.1093/brain/aws129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards M.J., Stone J., Lang A.E. From psychogenic movement disorder to functional movement disorder: it's time to change the name. Mov. Disord. 2014;29:849–852. doi: 10.1002/mds.25562. [DOI] [PubMed] [Google Scholar]
- Espay A.J., Maloney T., Vannest J., Norris M.M., Eliassen J.C., Neefus E., Allendorfer J.B., Chen R., Szaflarski J.P. Dysfunction in emotion processing underlies functional (psychogenic) dystonia. Mov. Disord. 2018;33:136–145. doi: 10.1002/mds.27217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espay A.J., Maloney T., Vannest J., Norris M.M., Eliassen J.C., Neefus E., Allendorfer J.B., Lang A.E., Szaflarski J.P. Impaired emotion processing in functional (psychogenic) tremor: a functional magnetic resonance imaging study. NeuroImage: Clinical. 2018;17:179–187. doi: 10.1016/j.nicl.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger A.B., Devinsky O., Weisbrot D.M., Goyal A., Shashikumar S. Headaches and other pain symptoms among patients with psychogenic non-epileptic seizures. Seizure. 1999;8:424–426. doi: 10.1053/seiz.1999.0334. [DOI] [PubMed] [Google Scholar]
- Finkelmeyer A., He J., Maclachlan L., Watson S., Gallagher P., Newton J.L., Blamire A.M. Grey and white matter differences in chronic fatigue syndrome – a voxel-based morphometry study. Neurol. Clin. 2018;17:24–30. doi: 10.1016/j.nicl.2017.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghaffar O., Staines W.R., Feinstein A. Unexplained neurologic symptoms: an fMRI study of sensory conversion disorder. Neurology. 2006;67:2036–2038. doi: 10.1212/01.wnl.0000247275.68402.fc. [DOI] [PubMed] [Google Scholar]
- Glass S.P., Matin N., Williams B., Mello J., Stephen C.D., Young S.S., Callahan J., LaFrance W.C., Jr., Perez D.L. Neuropsychiatric factors linked to adherence and short-term outcome in a US functional neurological disorders clinic: a retrospective cohort study. J. Neuropsychiatr. Clin. Neurosci. 2018;30:152–159. doi: 10.1176/appi.neuropsych.17060117. [DOI] [PubMed] [Google Scholar]
- Grinsvall C., Tornblom H., Tack J., Van Oudenhove L., Simren M. Relationships between psychological state, abuse, somatization and visceral pain sensitivity in irritable bowel syndrome. United Eur. Gastroenterol. J. 2018;6:300–309. doi: 10.1177/2050640617715851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundel H., Valet M., Sorg C., Huber D., Zimmer C., Sprenger T., Tolle T.R. Altered cerebral response to noxious heat stimulation in patients with somatoform pain disorder. Pain. 2008;137:413–421. doi: 10.1016/j.pain.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Guz H., Doganay Z., Ozkan A., Colak E., Tomac A., Sarisoy G. Conversion and somatization disorders; dissociative symptoms and other characteristics. J. Psychosom. Res. 2004;56:287–291. doi: 10.1016/S0022-3999(03)00069-2. [DOI] [PubMed] [Google Scholar]
- Hakala M., Karlsson H., Ruotsalainen U., Koponen S., Bergman J., Stenman H., Kelavuori J.P., Aalto S., Kurki T., Niemi P. Severe somatization in women is associated with altered cerebral glucose metabolism. Psychol. Med. 2002;32:1379–1385. doi: 10.1017/s0033291702006578. [DOI] [PubMed] [Google Scholar]
- Hakala M., Karlsson H., Kurki T., Aalto S., Koponen S., Vahlberg T., Niemi P.M. Volumes of the caudate nuclei in women with somatization disorder and healthy women. Psychiatry Res. 2004;131:71–78. doi: 10.1016/j.pscychresns.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Hassa T., Sebastian A., Liepert J., Weiller C., Schmidt R., Tüscher O. Symptom-specific amygdala hyperactivity modulates motor control network in conversion disorder. NeuroImage Clin. 2017;15:143–150. doi: 10.1016/j.nicl.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernando K.A., Szaflarski J.P., Ver Hoef L.W., Lee S., Allendorfer J.B. Uncinate fasciculus connectivity in patients with psychogenic nonepileptic seizures: a preliminary diffusion tensor tractography study. Epilepsy Behav. 2015;45:68–73. doi: 10.1016/j.yebeh.2015.02.022. [DOI] [PubMed] [Google Scholar]
- Hinson V.K., Cubo E., Comella C.L., Goetz C.G., Leurgans S. Rating scale for psychogenic movement disorders: scale development and clinimetric testing. Mov. Disord. 2005;20:1592–1597. doi: 10.1002/mds.20650. [DOI] [PubMed] [Google Scholar]
- Houtveen J.H., van Doornen L.J. Medically unexplained symptoms and between-group differences in 24-h ambulatory recording of stress physiology. Biol. Psychol. 2007;76:239–249. doi: 10.1016/j.biopsycho.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Ibrahim N.M., Martino D., van de Warrenburg B.P., Quinn N.P., Bhatia K.P., Brown R.J., Trimble M., Schrag A. The prognosis of fixed dystonia: a follow-up study. Parkinsonism Relat. Disord. 2009;15:592–597. doi: 10.1016/j.parkreldis.2009.02.010. [DOI] [PubMed] [Google Scholar]
- Janet P. Macmillan; New York: 1907. The Major Symptoms of Hysteria; Fifteen Lectures Given in the Medical School of Harvard University. [Google Scholar]
- Janssens K.A., Oldehinkel A.J., Verhulst F.C., Hunfeld J.A., Ormel J., Rosmalen J.G. Symptom-specific associations between low cortisol responses and functional somatic symptoms: the TRAILS study. Psychoneuroendocrinology. 2012;37:332–340. doi: 10.1016/j.psyneuen.2011.06.016. [DOI] [PubMed] [Google Scholar]
- Kanaan R.A., Craig T.K., Wessely S.C., David A.S. Imaging repressed memories in motor conversion disorder. Psychosom. Med. 2007;69:202–205. doi: 10.1097/PSY.0b013e31802e4297. [DOI] [PubMed] [Google Scholar]
- Karibe H., Arakawa R., Tateno A., Mizumura S., Okada T., Ishii T., Oshima K., Ohtsu M., Hasegawa I., Okubo Y. Regional cerebral blood flow in patients with orally localized somatoform pain disorder: a single photon emission computed tomography study. Psychiatry Clin. Neurosci. 2010;64:476–482. doi: 10.1111/j.1440-1819.2010.02119.x. [DOI] [PubMed] [Google Scholar]
- Keynejad R.C., Carson A.J., David A.S., Nicholson T.R. Functional neurological disorder: psychiatry's blind spot. Lancet Psychiatry. 2017;4:e2–e3. doi: 10.1016/S2215-0366(17)30036-6. [DOI] [PubMed] [Google Scholar]
- Keynejad R.C., Frodl T., Kanaan R., Pariante C., Reuber M., Nicholson T.R. Stress and functional neurological disorders: mechanistic insights. J. Neurol. Neurosurg. Psychiatry. 2018 doi: 10.1136/jnnp-2018-318297. Epub Ahead of Print. [DOI] [PubMed] [Google Scholar]
- Kozlowska K., Palmer D.M., Brown K.J., McLean L., Scher S., Gevirtz R., Chudleigh C., Williams L.M. Reduction of autonomic regulation in children and adolescents with conversion disorders. Psychosom. Med. 2015;77:356–370. doi: 10.1097/PSY.0000000000000184. [DOI] [PubMed] [Google Scholar]
- Kozlowska K., Griffiths K.R., Foster S.L., Linton J., Williams L.M., Korgaonkar M.S. Grey matter abnormalities in children and adolescents with functional neurological symptom disorder. Neurol. Clin. 2017;15:306–314. doi: 10.1016/j.nicl.2017.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowska K., Spooner C.J., Palmer D.M., Harris A., Korgaonkar M.S., Scher S., Williams L.M. "Motoring in idle": the default mode and somatomotor networks are overactive in children and adolescents with functional neurological symptoms. Neurol. Clin. 2018;18:730–743. doi: 10.1016/j.nicl.2018.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinad A., Schweinhardt P., Seminowicz D.A., Wood P.B., Chizh B.A., Bushnell M.C. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J. Neurosci. 2007;27:4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn S., Gallinat J. Gray matter correlates of posttraumatic stress disorder: a quantitative meta-analysis. Biol. Psychiatry. 2013;73:70–74. doi: 10.1016/j.biopsych.2012.06.029. [DOI] [PubMed] [Google Scholar]
- Labate A., Cerasa A., Mula M., Mumoli L., Gioia M.C., Aguglia U., Quattrone A., Gambardella A. Neuroanatomic correlates of psychogenic nonepileptic seizures: a cortical thickness and VBM study. Epilepsia. 2011;53:377–385. doi: 10.1111/j.1528-1167.2011.03347.x. [DOI] [PubMed] [Google Scholar]
- Labus J.S., Dinov I.D., Jiang Z., Ashe-McNalley C., Zamanyan A., Shi Y., Hong J.Y., Gupta A., Tillisch K., Ebrat B., Hobel S., Gutman B.A., Joshi S., Thompson P.M., Toga A.W., Mayer E.A. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain. 2014;155:137–149. doi: 10.1016/j.pain.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa A., Peterson B.S., Fallon B.A. Somatoform pain: a developmental theory and translational research review. Psychosom. Med. 2012;74:717–727. doi: 10.1097/PSY.0b013e3182688e8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Allendorfer J.B., Gaston T.E., Griffis J.C., Hernando K.A., Knowlton R.C., Szaflarski J.P., Ver Hoef L.W. White matter diffusion abnormalities in patients with psychogenic non-epileptic seizures. Brain Res. 2015;1620:169–176. doi: 10.1016/j.brainres.2015.04.050. [DOI] [PubMed] [Google Scholar]
- Li R., Li Y., An D., Gong Q., Zhou D., Chen H. Altered regional activity and inter-regional functional connectivity in psychogenic non-epileptic seizures. Sci. Rep. 2015;5 doi: 10.1038/srep11635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Liu K., Ma X., Li Z., Duan X., An D., Gong Q., Zhou D., Chen H. Altered functional connectivity patterns of the insular subregions in psychogenic nonepileptic seizures. Brain Topogr. 2015;28:636–645. doi: 10.1007/s10548-014-0413-3. [DOI] [PubMed] [Google Scholar]
- Li Q., Xiao Y., Li Y., Li L., Lu N., Xu Z., Mou X., Mao S., Wang W., Yuan Y. Altered regional brain function in the treatment-naive patients with somatic symptom disorder: a resting-state fMRI study. Brain Behav. 2016;6 doi: 10.1002/brb3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Liu F., Su Q., Zhang Z., Zhao J., Wang Y., Wu R., Zhao J., Guo W. Bidirectional causal connectivity in the cortico-limbic-cerebellar circuit related to structural alterations in first-episode, drug-naive somatization disorder. Front. Psychol. 2018;9 doi: 10.3389/fpsyt.2018.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L., Radua J., Rubia K. Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. Am. J. Psychiatry. 2014;171:854–863. doi: 10.1176/appi.ajp.2014.13101427. [DOI] [PubMed] [Google Scholar]
- Loeb T.B., Joseph N.T., Wyatt G.E., Zhang M., Chin D., Thames A., Aswad Y. Predictors of somatic symptom severity: the role of cumulative history of trauma and adversity in a diverse community sample. Psychol. Trauma. 2018;10:491–498. doi: 10.1037/tra0000334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig L., Pasman J.A., Nicholson T., Aybek S., David A.S., Tuck S., Kanaan R.A., Roelofs K., Carson A., Stone J. Stressful life events and maltreatment in conversion (functional neurological) disorder: systematic review and meta-analysis of case-control studies. Lancet Psychiatry. 2018;5:307–320. doi: 10.1016/S2215-0366(18)30051-8. [DOI] [PubMed] [Google Scholar]
- Luo Y., Yan C., Huang T., Fan M., Liu L., Zhao Z., Ni K., Jiang H., Huang X., Lu Z., Wu W., Zhang M., Fan X. Altered neural correlates of emotion associated pain processing in persistent somatoform pain disorder: an fMRI study. Pain Pract. 2016;16:969–979. doi: 10.1111/papr.12358. [DOI] [PubMed] [Google Scholar]
- MacMillan H.L., Fleming J.E., Streiner D.L., Lin E., Boyle M.H., Jamieson E., Duku E.K., Walsh C.A., Wong M.Y., Beardslee W.R. Childhood abuse and lifetime psychopathology in a community sample. Am. J. Psychiatry. 2001;158:1878–1883. doi: 10.1176/appi.ajp.158.11.1878. [DOI] [PubMed] [Google Scholar]
- Magon S., Sprenger T., Otti A., Papadopoulou A., Gündel H., Noll-Hussong M. Cortical thickness alterations in chronic pain disorder: an exploratory MRI study. Psychosom. Med. 2018;80:592–598. doi: 10.1097/PSY.0000000000000605. [DOI] [PubMed] [Google Scholar]
- Mailis-Gagnon A., Giannoylis I., Downar J., Kwan C.L., Mikulis D.J., Crawley A.P., Nicholson K., Davis K.D. Altered central somatosensory processing in chronic pain patients with "hysterical" anesthesia. Neurology. 2003;60:1501–1507. doi: 10.1212/wnl.60.9.1501. [DOI] [PubMed] [Google Scholar]
- Marshall J.C., Halligan P.W., Fink G.R., Wade D.T., Frackowiak R.S. The functional anatomy of a hysterical paralysis. Cognition. 1997;64:B1–B8. doi: 10.1016/s0010-0277(97)00020-6. [DOI] [PubMed] [Google Scholar]
- Maurer C.W., LaFaver K., Ameli R., Epstein S.A., Hallett M., Horovitz S.G. Impaired self-agency in functional movement disorders: a resting-state fMRI study. Neurology. 2016;87:564–570. doi: 10.1212/WNL.0000000000002940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer C.W., LaFaver K., Limachia G.S., Capitan G., Ameli R., Sinclair S., Epstein S.A., Hallett M., Horovitz S.G. Gray matter differences in patients with functional movement disorders. Neurology. 2018;91:e1870–e1879. doi: 10.1212/WNL.0000000000006514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayou R. Is the DSM-5 chapter on somatic symptom disorder any better than DSM-IV somatoform disorder? Br. J. Psychiatry. 2014;204:418–419. doi: 10.1192/bjp.bp.113.134833. [DOI] [PubMed] [Google Scholar]
- McGorry P., Keshavan M., Goldstone S., Amminger P., Allott K., Berk M., Lavoie S., Pantelis C., Yung A., Wood S. Biomarkers and clinical staging in psychiatry. World Psychiatry. 2014;13:211–223. doi: 10.1002/wps.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney M., Reuber M., Levita L. Neuroimaging studies in patients with psychogenic non-epileptic seizures: a systematic meta-review. Neurol. Clin. 2017;16:210–221. doi: 10.1016/j.nicl.2017.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSweeney M., Reuber M., Hoggard N., Levita L. Cortical thickness and gyrification patterns in patients with psychogenic non-epileptic seizures. Neurosci. Lett. 2018;678:124–130. doi: 10.1016/j.neulet.2018.04.056. [DOI] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G., Group, P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- Morris L.S., To, B, Baek K., Chang-Webb Y.C., Mitchell S., Strelchuk D., Mikheenko Y., Phillips W., Zandi M., Jenaway A., Walsh C., Voon V. Disrupted avoidance learning in functional neurological disorder: implications for harm avoidance theories. Neurol. Clin. 2017;16:286–294. doi: 10.1016/j.nicl.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson T.R., Aybek S., Kempton M.J., Daly E.M., Murphy D.G., David A.S., Kanaan R.A. A structural MRI study of motor conversion disorder: evidence of reduction in thalamic volume. J. Neurol. Neurosurg. Psychiatry. 2014;85:227–229. doi: 10.1136/jnnp-2013-305012. [DOI] [PubMed] [Google Scholar]
- Noll-Hussong M., Otti A., Wohlschlaeger A.M., Zimmer C., Henningsen P., Lahmann C., Ronel J., Subic-Wrana C., Lane R.D., Decety J., Guendel H. Neural correlates of deficits in pain-related affective meaning construction in patients with chronic pain disorder. Psychosom. Med. 2013;75:124–136. doi: 10.1097/PSY.0b013e31827e60f3. [DOI] [PubMed] [Google Scholar]
- Paras M.L., Murad M.H., Chen L.P., Goranson E.N., Sattler A.L., Colbenson K.M., Elamin M.B., Seime R.J., Prokop L.J., Zirakzadeh A. Sexual abuse and lifetime diagnosis of somatic disorders: a systematic review and meta-analysis. JAMA. 2009;302:550–561. doi: 10.1001/jama.2009.1091. [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Stein M.B. An insular view of anxiety. Biol. Psychiatry. 2006;60:383–387. doi: 10.1016/j.biopsych.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Perez D.L., Barsky A.J., Vago D.R., Baslet G., Silbersweig D.A. A neural circuit framework for somatosensory amplification in somatoform disorders. J. Neuropsychiatr. Clin. Neurosci. 2015;27:e40–e50. doi: 10.1176/appi.neuropsych.13070170. [DOI] [PubMed] [Google Scholar]
- Perez D.L., Dworetzky B.A., Dickerson B.C., Leung L., Cohn R., Baslet G., Silbersweig D.A. An integrative neurocircuit perspective on psychogenic nonepileptic seizures and functional movement disorders: neural functional unawareness. Clin EEG Neurosci. 2015;46:4–15. doi: 10.1177/1550059414555905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez D.L., Matin N., Barsky A., Costumero-Ramos V., Makaretz S.J., Young S.S., Sepulcre J., LaFrance W.C., Jr., Keshavan M.S., Dickerson B.C. Cingulo-insular structural alterations associated with psychogenic symptoms, childhood abuse and PTSD in functional neurological disorders. J. Neurol. Neurosurg. Psychiatry. 2017;88:491–497. doi: 10.1136/jnnp-2016-314998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez D.L., Williams B., Matin N., LaFrance W.C., Jr., Costumero-Ramos V., Fricchione G.L., Sepulcre J., Keshavan M.S., Dickerson B.C. Corticolimbic structural alterations linked to health status and trait anxiety in functional neurological disorder. J. Neurol. Neurosurg. Psychiatry. 2017;88:1052–1059. doi: 10.1136/jnnp-2017-316359. [DOI] [PubMed] [Google Scholar]
- Perez D.L., Keshavan M.S., Scharf J.M., Boes A.D., Price B.H. Bridging the great divide: what can neurology learn from psychiatry? J. Neuropsychiatr. Clin. Neurosci. 2018;30:271–278. doi: 10.1176/appi.neuropsych.17100200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez D.L., Matin N., Williams B., Tanev K., Makris N., LaFrance W.C., Jr., Dickerson B.C. Cortical thickness alterations linked to somatoform and psychological dissociation in functional neurological disorders. Hum. Brain Mapp. 2018;39:428–439. doi: 10.1002/hbm.23853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez D.L., Williams B., Matin N., Mello J., Dickerson B.C., LaFrance W.C., Jr., Keshavan M.S. Anterior hippocampal grey matter predicts mental health outcome in functional neurological disorders: an exploratory pilot study. J. Neurol. Neurosurg. Psychiatry. 2018;89:1221–1224. doi: 10.1136/jnnp-2017-317305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick S., Mellers J.D.C., Goldstein L.H. Autonomic and subjective responsivity to emotional images in people with dissociative seizures. J. Neuropsychol. 2017;12:341–355. doi: 10.1111/jnp.12144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pick S., Goldstein L.H., Perez D.L., Nicholson T.R. Emotional processing in functional neurological disorder: a review, biopsychosocial model and research agenda. J. Neurol. Neurosurg. Psychiatry. 2018 doi: 10.1136/jnnp-2018-319201. Epub Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O., Dietel A., Herbert B.M., Wankner S., Wachsmuth C., Henningsen P., Sack M. Blunted autonomic reactivity and increased pain tolerance in somatoform patients. Pain. 2011;152:2157–2164. doi: 10.1016/j.pain.2011.05.024. [DOI] [PubMed] [Google Scholar]
- Pollatos O., Herbert B.M., Wankner S., Dietel A., Wachsmuth C., Henningsen P., Sack M. Autonomic imbalance is associated with reduced facial recognition in somatoform disorders. J. Psychosom. Res. 2011;71:232–239. doi: 10.1016/j.jpsychores.2011.03.012. [DOI] [PubMed] [Google Scholar]
- Reinsberger C., Perez D.L., Murphy M.M., Dworetzky B.A. Pre- and postictal, not ictal, heart rate distinguishes complex partial and psychogenic nonepileptic seizures. Epilepsy Behav. 2012;23:68–70. doi: 10.1016/j.yebeh.2011.10.008. [DOI] [PubMed] [Google Scholar]
- Reuber M. The etiology of psychogenic non-epileptic seizures: toward a biopsychosocial model. Neurol. Clin. 2009;27:909–924. doi: 10.1016/j.ncl.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Riederer F., Landmann G., Gantenbein A.R., Stockinger L., Egloff N., Sprott H., Schleinzer W., Pirrotta R., Dumat W., Luechinger R., Baumgartner C., Kollias S., Sandor P.S. Nondermatomal somatosensory deficits in chronic pain are associated with cerebral grey matter changes. World J. Biol. Psychiatry. 2017;18:227–238. doi: 10.3109/15622975.2015.1073356. [DOI] [PubMed] [Google Scholar]
- Rief W., Barsky A.J. Psychobiological perspectives on somatoform disorders. Psychoneuroendocrinology. 2005;30:996–1002. doi: 10.1016/j.psyneuen.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Ristic A.J., Dakovic M., Kerr M., Kovacevic M., Parojcic A., Sokic D. Cortical thickness, surface area and folding in patients with psychogenic nonepileptic seizures. Epilepsy Res. 2015;112:84–91. doi: 10.1016/j.eplepsyres.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Ruocco A.C., Amirthavasagam S., Zakzanis K.K. Amygdala and hippocampal volume reductions as candidate endophenotypes for borderline personality disorder: a meta-analysis of magnetic resonance imaging studies. Psychiatry Res. 2012;201:245–252. doi: 10.1016/j.pscychresns.2012.02.012. [DOI] [PubMed] [Google Scholar]
- Salinsky M., Storzbach D., Goy E., Kellogg M., Boudreau E. Health care utilization following diagnosis of psychogenic nonepileptic seizures. Epilepsy Behav. 2016;60:107–111. doi: 10.1016/j.yebeh.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Sar V., Akyuz G., Kundakci T., Kiziltan E., Dogan O. Childhood trauma, dissociation, and psychiatric comorbidity in patients with conversion disorder. Am. J. Psychiatry. 2004;161:2271–2276. doi: 10.1176/appi.ajp.161.12.2271. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T., Leinisch E., Straube A., Kampfe N., Draganski B., Diener H.C., Bogdahn U., May A. Gray matter decrease in patients with chronic tension type headache. Neurology. 2005;65:1483–1486. doi: 10.1212/01.wnl.0000183067.94400.80. [DOI] [PubMed] [Google Scholar]
- Schmidt-Wilcke T., Leinisch E., Ganssbauer S., Draganski B., Bogdahn U., Altmeppen J., May A. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125:89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Schrag A.E., Mehta A.R., Bhatia K.P., Brown R.J., Frackowiak R.S., Trimble M.R., Ward N.S., Rowe J.B. The functional neuroimaging correlates of psychogenic versus organic dystonia. Brain. 2013;136:770–781. doi: 10.1093/brain/awt008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seignourel P.J., Miller K., Kellison I., Rodriguez R., Fernandez H.H., Bauer R.M., Bowers D., Okun M.S. Abnormal affective startle modulation in individuals with psychogenic [corrected] movement disorder. Mov. Disord. 2007;22:1265–1271. doi: 10.1002/mds.21451. [DOI] [PubMed] [Google Scholar]
- Seminowicz D.A., Labus J.S., Bueller J.A., Tillisch K., Naliboff B.D., Bushnell M.C., Mayer E.A. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57 e42. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shackman A.J., Salomons T.V., Slagter H.A., Fox A.S., Winter J.J., Davidson R.J. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat. Rev. Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Yuan C., Dai Z., Ma H., Sheng L. Gray matter abnormalities associated with fibromyalgia: a meta-analysis of voxel-based morphometric studies. Semin. Arthritis Rheum. 2016;46:330–337. doi: 10.1016/j.semarthrit.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Sinanaj I., Cojan Y., Vuilleumier P. Inter-individual variability in metacognitive ability for visuomotor performance and underlying brain structures. Conscious. Cogn. 2015;36:327–337. doi: 10.1016/j.concog.2015.07.012. [DOI] [PubMed] [Google Scholar]
- Spence S.A., Crimlisk H.L., Cope H., Ron M.A., Grasby P.M. Discrete neurophysiological correlates in prefrontal cortex during hysterical and feigned disorder of movement. Lancet. 2000;355:1243–1244. doi: 10.1016/S0140-6736(00)02096-1. [DOI] [PubMed] [Google Scholar]
- Stoeter P., Bauermann T., Nickel R., Corluka L., Gawehn J., Vucurevic G., Vossel G., Egle U.T. Cerebral activation in patients with somatoform pain disorder exposed to pain and stress: an fMRI study. Neuroimage. 2007;36:418–430. doi: 10.1016/j.neuroimage.2007.01.052. [DOI] [PubMed] [Google Scholar]
- Stone J., Zeman A., Simonotto E., Meyer M., Azuma R., Flett S., Sharpe M. FMRI in patients with motor conversion symptoms and controls with simulated weakness. Psychosom. Med. 2007;69:961–969. doi: 10.1097/PSY.0b013e31815b6c14. [DOI] [PubMed] [Google Scholar]
- Stone J., Hewett R., Carson A., Warlow C., Sharpe M. The 'disappearance' of hysteria: historical mystery or illusion? J. R. Soc. Med. 2008;101:12–18. doi: 10.1258/jrsm.2007.070129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone J., LaFrance W.C., Jr., Levenson J.L., Sharpe M. Issues for DSM-5: conversion disorder. Am. J. Psychiatry. 2010;167:626–627. doi: 10.1176/appi.ajp.2010.09101440. [DOI] [PubMed] [Google Scholar]
- Stone J., Warlow C., Sharpe M. The symptom of functional weakness: a controlled study of 107 patients. Brain. 2010;133:1537–1551. doi: 10.1093/brain/awq068. [DOI] [PubMed] [Google Scholar]
- Szaflarski J.P., Allendorfer J.B., Nenert R., LaFrance W.C., Barkan H.I., DeWolfe J., Pati S., Thomas A.E., Ver Hoef L. Facial emotion processing in patients with seizure disorders. Epilepsy Behav. 2018;79:193–204. doi: 10.1016/j.yebeh.2017.12.004. [DOI] [PubMed] [Google Scholar]
- Taylor G.J. Somatization and conversion: distinct or overlapping constructs? J. Am. Acad. Psychoanal. Dyn. Psychiatry. 2003;31:487–508. doi: 10.1521/jaap.31.3.487.22136. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A. Childhood maltreatment and psychopathology: a case for ecophenotypic variants as clinically and neurobiologically distinct subtypes. Am. J. Psychiatry. 2013;170:1114–1133. doi: 10.1176/appi.ajp.2013.12070957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiihonen J., Kuikka J., Viinamaki H., Lehtonen J., Partanen J. Altered cerebral blood flow during hysterical paresthesia. Biol. Psychiatry. 1995;37:134–135. doi: 10.1016/0006-3223(94)00230-Z. [DOI] [PubMed] [Google Scholar]
- Tiwari A., Gonzalez A. Biological alterations affecting risk of adult psychopathology following childhood trauma: a review of sex differences. Clin. Psychol. Rev. 2018;66:69–79. doi: 10.1016/j.cpr.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Tomic A., Agosta F., Sarasso E., Petrovic I., Basaia S., Pesic D., Kostic M., Fontana A., Kostic V.S., Filippi M. Are there two different forms of functional dystonia? A multimodal brain structural MRI study. Mol. Psychiatry. 2018 doi: 10.1038/s41380-018-0222-2. Epub Ahead of Print. [DOI] [PubMed] [Google Scholar]
- Trimble M., Reynolds E. Handbook of Clinical Neurology. Elsevier; 2016. A brief history of hysteria: from the ancient to the modern; pp. 3–10. [DOI] [PubMed] [Google Scholar]
- Valet M., Gundel H., Sprenger T., Sorg C., Muhlau M., Zimmer C., Henningsen P., Tolle T.R. Patients with pain disorder show gray-matter loss in pain-processing structures: a voxel-based morphometric study. Psychosom. Med. 2009;71:49–56. doi: 10.1097/PSY.0b013e31818d1e02. [DOI] [PubMed] [Google Scholar]
- van der Kruijs S.J., Bodde N.M., Vaessen M.J., Lazeron R.H., Vonck K., Boon P., Hofman P.A., Backes W.H., Aldenkamp A.P., Jansen J.F. Functional connectivity of dissociation in patients with psychogenic non-epileptic seizures. J. Neurol. Neurosurg. Psychiatry. 2012;83:239–247. doi: 10.1136/jnnp-2011-300776. [DOI] [PubMed] [Google Scholar]
- van der Kruijs S.J., Jagannathan S.R., Bodde N.M., Besseling R.M., Lazeron R.H., Vonck K.E., Boon P.A., Cluitmans P.J., Hofman P.A., Backes W.H., Aldenkamp A.P., Jansen J.F. Resting-state networks and dissociation in psychogenic non-epileptic seizures. J. Psychiatr. Res. 2014;54:126–133. doi: 10.1016/j.jpsychires.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Vasta R., Cerasa A., Sarica A., Bartolini E., Martino I., Mari F., Metitieri T., Quattrone A., Gambardella A., Guerrini R., Labate A. The application of artificial intelligence to understand the pathophysiological basis of psychogenic nonepileptic seizures. Epilepsy Behav. 2018;87:167–172. doi: 10.1016/j.yebeh.2018.09.008. [DOI] [PubMed] [Google Scholar]
- Voon V., Brezing C., Gallea C., Ameli R., Roelofs K., LaFrance W.C., Jr., Hallett M. Emotional stimuli and motor conversion disorder. Brain. 2010;133:1526–1536. doi: 10.1093/brain/awq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V., Gallea C., Hattori N., Bruno M., Ekanayake V., Hallett M. The involuntary nature of conversion disorder. Neurology. 2010;74:223–228. doi: 10.1212/WNL.0b013e3181ca00e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V., Brezing C., Gallea C., Hallett M. Aberrant supplementary motor complex and limbic activity during motor preparation in motor conversion disorder. Mov. Disord. 2011;26:2396–2403. doi: 10.1002/mds.23890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V., Cavanna A.E., Coburn K., Sampson S., Reeve A., LaFrance W.C., Jr. Functional neuroanatomy and neurophysiology of functional neurological disorders (conversion disorder) J. Neuropsychiatr. Clin. Neurosci. 2016;28:168–190. doi: 10.1176/appi.neuropsych.14090217. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. Brain circuits implicated in psychogenic paralysis in conversion disorders and hypnosis. Neurophysiol. Clin. 2014;44:323–337. doi: 10.1016/j.neucli.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P., Chicherio C., Assal F., Schwartz S., Slosman D., Landis T. Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain. 2001;124:1077–1090. doi: 10.1093/brain/124.6.1077. [DOI] [PubMed] [Google Scholar]
- Ward A.M., Schultz A.P., Huijbers W., Van Dijk K.R., Hedden T., Sperling R.A. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum. Brain Mapp. 2014;35:1061–1073. doi: 10.1002/hbm.22234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegrzyk J., Kebets V., Richiardi J., Galli S., de Ville D.V., Aybek S. Identifying motor functional neurological disorder using resting-state functional connectivity. Neurol. Clin. 2018;17:163–168. doi: 10.1016/j.nicl.2017.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams B., Jalilianhasanpour R., Matin N., Fricchione G.L., Sepulcre J., Keshavan M.S., LaFrance W.C., Jr., Dickerson B.C., Perez D.L. Individual differences in corticolimbic structural profiles linked to insecure attachment and coping styles in motor functional neurological disorders. J. Psychiatr. Res. 2018;102:230–237. doi: 10.1016/j.jpsychires.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yildirim H., Atmaca M., Sirlier B., Kayali A. Pituitary volumes are reduced in patients with somatization disorder. Psychiatry Investig. 2012;9:278–282. doi: 10.4306/pi.2012.9.3.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan C., Shi H., Pan P., Dai Z., Zhong J., Ma H., Sheng L. Gray matter abnormalities associated with chronic Back pain: a meta-analysis of voxel-based morphometric studies. Clin. J. Pain. 2017;33:983–990. doi: 10.1097/AJP.0000000000000489. [DOI] [PubMed] [Google Scholar]
- Zhang J., Jiang M., Yao D., Dai Y., Long L., Yu M., Liu J., Zhang Z., Xiao C., Guo W. Alterations in white matter integrity in first-episode, treatment-naive patients with somatization disorder. Neurosci. Lett. 2015;599:102–108. doi: 10.1016/j.neulet.2015.05.037. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Huang T., Tang C., Ni K., Pan X., Yan C., Fan X., Xu D., Luo Y. Altered resting-state intra- and inter- network functional connectivity in patients with persistent somatoform pain disorder. PLoS One. 2017;12 doi: 10.1371/journal.pone.0176494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Su Q., Liu F., Zhang Z., Li R., Zhu F., Wu R., Zhao J., Guo W. Regional white matter volume abnormalities in first-episode somatization disorder. Int. J. Psychophysiol. 2018;133:12–16. doi: 10.1016/j.ijpsycho.2018.09.003. [DOI] [PubMed] [Google Scholar]