Abstract
The environment is thought to affect outcomes in patients with cancer; however, this relationship has not been proven directly. Recently, an enriched environment, as a model of a positive environment, has been shown to suppress tumor growth by lowering leptin production through a pathway involving the hypothalamus/sympathetic nerve/leptin axis. We previously reported that a fragrant environment (FE) containing α-pinene suppressed tumor growth in mice; however, the underlying mechanism has not been elucidated. Accordingly, in this study, we investigated changes in the neuroendocrine and immune systems following exposure to an FE. Mice were exposed to α-pinene (5 h/day) for 4 weeks prior to tumor implantation with murine melanoma cells and 3 weeks after transplantation. In addition to the evaluation of tumor growth, the blood, spleen, and hypothalamus were collected 3 weeks after transplantation, and neuroendocrinological and immunological parameters were measured. Tumor size was ~40% smaller in mice exposed to FE. Moreover, plasma noradrenaline concentrations, which reflected sympathetic nervous activity, tended to increase, and leptin levels were significantly decreased in FE-exposed mice. Levels of stress hormones, such as plasma corticosterone and adrenaline, did not change in the 2 groups. In the hypothalamus, brain-derived neurotrophic factor protein levels and glucose-1-phosphate concentrations were decreased in the FE group. Additionally, numbers of B cells, CD4+ T cells, CD8+ T cells, and natural killer cells increased in the FE-exposed mice. These neurohormonal and immunological changes in the FE-exposed mice suggested that the FE may activate the hypothalamus/sympathetic nerve/leptin axis and immune system, thereby retarding tumor growth.
Keywords: α-pinene, fragrance, environment, cancer, hypothalamus, leptin, immune cell
Introduction
The influence of stressful factors on physiological and pathological processes has attracted much attention. Positive stress improves health, whereas negative stress is harmful to health and can promote cancer. Indeed, spiritual stress, depression, and social isolation stimulate cancer onset and progression. Conversely, individuals who have strong involvement with society tend to live longer.1-4
Housing in an enriched environment is a classic rodent model of positive stress. An enriched environment consisted of social interaction (14 mice in a large cage), stimulation of exploratory behavior with objects such as toys and a set of tunnels, and a running wheel for exercise. The complexity of the enriched environment influences brain structure and function5,6 and leads to changes in growth factor expression, enhancement of neurogenesis, and survival of cells within the central nervous system (CNS).7,8 In neuroscience, housing in an enriched environment has been reported to exhibit beneficial effects, including reduced anxiety levels, improved recovery from traumatic brain injury, and alleviation of various nerve disorders in animal models.5 In patients with breast cancer, social interactions, such as engagement in activities outside of the home, were associated with lower overall mortality after a breast cancer diagnosis.9,10 However, it has been difficult to demonstrate these changes experimentally without any possible mechanisms to explain the observed effects.
Recently, Cao et al11 reported that the growth of melanoma and colon cancer was suppressed in mice raised in an enriched environment. Subsequent studies have shown that this environment inhibits tumor growth in mouse models of breast cancer,12 glioma,13 and pancreatic cancer.14 These studies have provided solid evidence demonstrating that stimuli inducing positive stress from an enriched environment have inhibitory effects on tumor growth. Cao et al15 proposed that positive stress due to an enriched environment stimulates the hypothalamus, leading to activation of the sympathetic nervous system in the white adipose tissue, which in turn suppresses leptin release via β-adrenergic receptors. This marked drop in plasma leptin levels has antiproliferative effects in several types of cancers.
In addition to the hypothalamus/leptin pathway, an enriched environment also has beneficial effects on the immune system. Exposure to an enriched environment improves the activities of lymphocytes and natural killer (NK) cells.16 Both T lymphocytes and NK cells function in defense against cancer, and the important role of NK cells in the eradication of tumors has been demonstrated in many clinical and experimental studies.17 These findings suggested that immunological activation in an enriched environment may also explain tumor inhibition.
Fragrances can be provided as essential oils, which many people use in their houses and enjoy in daily life. The effects of fragrances on the body and mind have been studied extensively in recent years; indeed, fragrances can have various biological and psychological properties, resulting in reduced stress and enrichment of people’s lives.18,19 Thus, an FE may result in reduced stress and inhibition of tumor growth. We previously demonstrated that inhalation of α-pinene, a major volatile organic compound found in the forest, inhibits tumor growth in mice.20 This effect was not observed in cultured cells, suggesting that the inhibitory effects of α-pinene were not via a direct effect. However, the underlying mechanisms remain unclear.
Thus, in this study, we investigated whether neuroendocrinological and immunological changes mediated the observed inhibitory effects of the FE on the growth of cancer. Our findings provided important insights into the roles of FE with α-pinene in the hypothalamus/sympathetic nerve/leptin system and in the immune system.
Materials and Methods
FE Protocol
Four-week-old male C57BL/6 mice were purchased from CLEA Japan (Tokyo, Japan). All mice were housed and used for experiments in accordance with the standard ethical guidelines for the care and use of laboratory animals (Science Council of Japan; Guidelines for Proper Conduct of Animal Experiments, 2006), and this study was approved by the Animal Experiment Ethics Committee of Shizuoka Cancer Center.
The exposure protocol was described previously.20 Briefly, a 90-L polyethylene bag (Shimojima, Tokyo, Japan) was filled with high-purity air (O2 21 ± 0.5%; Toatsu Yamazaki, Tokyo, Japan) with or without (1R)-(+)-α-pinene (Tokyo Chemical Industry Co, Ltd, Tokyo, Japan). The estimated final concentration of α-pinene in the bag air was 180 ng/L. Mouse cages were transferred to an anesthesia chamber (60 L; Sanplatec Corporation, Osaka, Japan). Air in the anesthesia chamber was changed by attaching a bag to one outlet and removing all the air from the bag by suction with a DCI-NA flexible pump (Tech-jam, Osaka, Japan) via the outlet on the other side. After suctioning, we exchanged the empty bag for a new filled bag and then ventilated it by suction with a pump for 10 min/h. After 5 hours, the cages were removed from the anesthesia chamber and returned to standard laboratory conditions.
C57BL/6 mice were randomized into 2 groups: one was exposed to an environment containing α-pinene for 5 h/day (n = 8), and the other was exposed to highly purified air (n = 8), beginning from 4 weeks before melanoma implantation until 21 days after implantation. After 4 weeks of treatment with inhalation of α-pinene or air, mice underwent subcutaneous implantation of a syngeneic melanoma cell line (B16F10 cells) into the flank (1 × 105 cells/mouse) and were then returned to their respective cages. Tumor dimensions were measured with calipers at 10, 14, 17, and 21 days after inoculation, and tumor volume was calculated by the formula used for an ellipsoid (volume = 1/2 × long diameter × [short diameter]2).
Harvesting of Plasma and Measurement of Hormone Levels
The mice in each group were anesthetized with isoflurane, and blood was then collected from the carotid artery. All blood harvesting started at 10:00 am.
Plasma (n = 6 in each group) was assayed using the following commercially available assay kits: Leptin Assay Kit (Morinaga Institute of Biological Science, Inc, Yokohama, Japan), YK240 Corticosterone EIA (Yanaihara Institute Inc, Fujinomiya, Japan), and 2-CAT (A-N) Research ELISA (Labor Diagnostika Nord, Nordhorn, Germany).
Hypothalamic Brain-Derived Neurotrophic Factor (BDNF) Concentrations
Hypothalamic tissues were dissected on the day of sacrifice. Tissues were immediately frozen with liquid nitrogen and stored at −80°C before use. We used one hypothalamic tissue for BDNF assays and the other for metabolomic analysis (n = 6 in each group).
For BDNF assays, hypothalamic tissues were homogenized in lysis buffer (20 mM Tris-Cl, 137 mM NaCl, 15 NP40, 10% glycerol, 0.5 mM Na3VO4, and protease inhibitor cocktail). The extracts were acidified by dilution at 1:5 in Dulbecco’s phosphate-buffered saline to approximately pH 2.6 and then neutralized to pH 7.6. BDNF concentrations in the extracts were determined using a BDNF Emax ImmunoAssay System (Promega, Madison, WI) according to the methods described in the manufacturer’s manual.
Metabolomic Analysis of Hypothalamus Tissue
Tissue extractions and metabolomic analysis were performed as described previously.21,22 Briefly, tissues were completely disrupted with a mortar at 4°C. After the addition of 750 µL methanol containing internal standards (10 µM each of methionine sulfone and 2-[N-morpholino]-ethanesulfonic acid), the disrupted samples were sonicated for 30 seconds, mixed with Milli-Q water and chloroform at a volume ratio of 5:2:5, and centrifuged at 5000 × g for 5 minutes at 4°C The aqueous solutions were then centrifugally filtered through a 5-kDa cutoff filter (Millipore, Billerica, MA) to remove proteins. The filtrate was centrifugally concentrated, dissolved in 25 µL Milli-Q water, and immediately subjected to capillary electrophoresis-time-of-flight mass spectrometry (CE-TOFMS) analysis. All CE-TOFMS experiments were performed using an Agilent CE Capillary Electrophoresis System equipped with an Agilent 6224 TOFMS, an Agilent 1200 isocratic HPLC pump, an Agilent G1603 CE-MS adapter kit, and an Agilent G1607 CE-electrospray ionization-MS sprayer kit (Agilent Technologies, Santa Clara, CA). For system control and data acquisition, we used Agilent G2201AA ChemStation software for CE and MassHunter for TOFMS. Cationic and anionic metabolite analyses were performed with the HMT Metabolomics Solution Package (Human Metabolome Technologies, Inc, Yamagata, Japan) according to published methods. Briefly, the standard compounds were analyzed for confirmation of m/z values and migration time and then subjected to quantification. Raw data, which contained thousands of peaks, were processed using MasterHands software (Institute for Advanced Biosciences, Keio University, Yamagata, Japan) for the quantification of metabolites. The data processing flow consisted of the following steps: migration time alignment, peak detection, background subtraction, and integration of peak area from a 0.02 m/z-wide slice of the electropherogram. All target metabolites were identified by matching their m/z values and migration times with those of the standard compounds. In total, 113 compounds were annotated and quantified. For each sample, the measured metabolite concentrations were normalized to the sample weight.
Phenotypic Analysis of Immune Cells
Phenotypic analysis of immune cells (n = 7-8 in each group) was performed by flow cytometry using a previously described method.23 Briefly, splenocyte suspensions were prepared from spleens of C57/BL6 mice subjected to the previously mentioned tumor growth test. Splenocytes were pre-incubated with Mouse BD Fc Block (BD Biosciences, San Jose, CA) for 5 minutes to reduce Fc receptor-mediated binding by antibodies of interest. Cells were then incubated with fluorescent monoclonal antibodies or isotype control antibodies for 15 minutes at 4°C, washed with phosphate-buffered saline containing 0.5% bovine serum albumin and 0.01% sodium azide, and analyzed using a BD Accuri C6 flow cytometer (BD Biosciences). Fluorescently labeled antibodies against mouse molecules were purchased from BD Biosciences. For NK cell analysis, fluorescein isothiocyanate-conjugated anti-CD49b (DX5) and phycoerythrin (PE)-conjugated anti-CD3 (17A2) antibodies were used. To detect T cells, FITC-conjugated anti-CD3 (17A2) and PE-conjugated anti-CD4 (RM4-5) or -CD8 (53-6.7) antibodies were used.
Statistical Analysis
The data in the figures are presented as mean ± standard deviations. The significance of differences between groups was compared by Tukey tests or analysis of variance. The differences were considered statistically significant when the P values were less than .05.
Results
An FE With α-Pinene Reduced Tumor Growth
To investigate neuroendocrinological and immunological changes involved in FE-induced tumor inhibition, we first reconfirmed the reduction in B16 melanoma growth. Briefly, C57BL/6 mice were randomized to spend 5 h/day either in an FE with α-pinene or a pure air environment. After 4 weeks in FE with α-pinene or in pure air, both groups of mice received a subcutaneous injection of B16 melanoma cells (105 cells/mouse), and the tumor size in each mouse was then measured at specified intervals. The growth curves showed that the rate of tumor growth was significantly delayed in FE-housed mice compared with that in control mice. At 21 days after inoculation, the mean tumor volume for the FE-housed mice was about 40% lower than that of control mice (2734 ± 1094 vs 4450 ± 971 mm3, respectively; P < .05; Figure 1). However, body weight was not different between the 2 groups (FE = 30.68 ± 2.39 g vs control = 30.86 ± 2.53 g). These results were similar to those in previous reports.20
Figure 1.
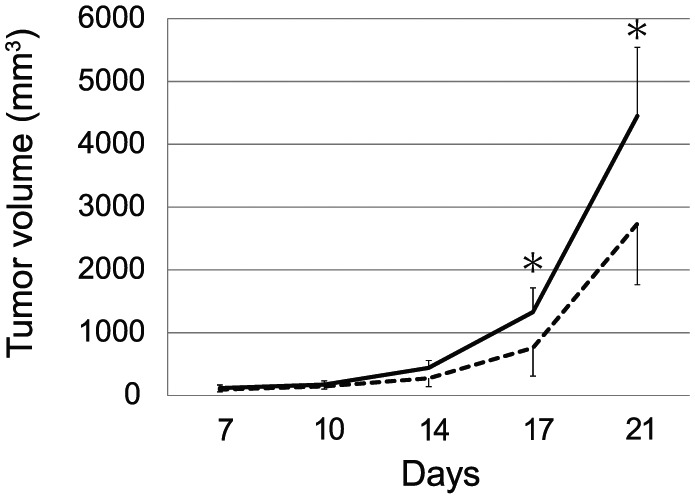
A fragrant environment (FE) reduced tumor growth. C57BL/6 mice were randomized into 2 groups: one was exposed to an environment containing α-pinene (180 ng/L in air) for 5 h/day (FE), and the other was exposed to a room containing pure air from 4 weeks prior to until 21 days after melanoma implantation. The tumor volume was measured at 7, 10, 14, 17, and 21 days after inoculation (n = 8 in each group, *P < .05).
Neuroendocrinological Examination
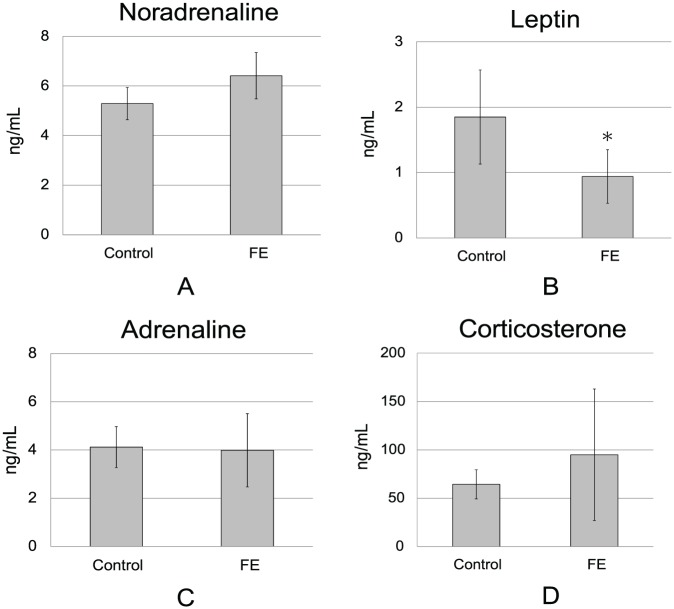
In an enriched environment, the hypothalamus/sympathetic nerve/leptin pathway is thought to be important for mediating tumor-suppressive effects.11 The plasma concentrations of noradrenaline, reflecting the activity of the sympathetic nervous system, tended to increase, although this increase was not significant (Figure 2A). Plasma leptin levels have also been shown to decrease in an enriched environment with body weight loss.11 In the present study, plasma leptin levels in the FE group were markedly reduced to about 50% that of controls (P < .05; Figure 2B), although body weights did not differ between the 2 groups.
Figure 2.
Effects of the FE on plasma neurohormonal levels in control and FE-exposed mice. Plasma was collected at 21 days after inoculation of melanoma cells. Plasma hormones were determined by ELISA (n = 6 in each group). Data are expressed as means ± standard deviations. *P < .05. (A) Plasma noradrenaline concentrations. (B) Plasma leptin concentrations. (C) Plasma adrenaline concentrations. (D) Plasma corticosterone concentrations.
In contrast, neither corticosterone nor adrenaline, which are both secreted from the adrenal glands in the presence of strong stress, differed between the 2 groups (Figure 2C and D). These results showed that the neuroendocrinological changes in the FE were similar to those previously reported for an enriched environment.
BDNF Measurement and Metabolomic Analysis of Hypothalamus Tissue
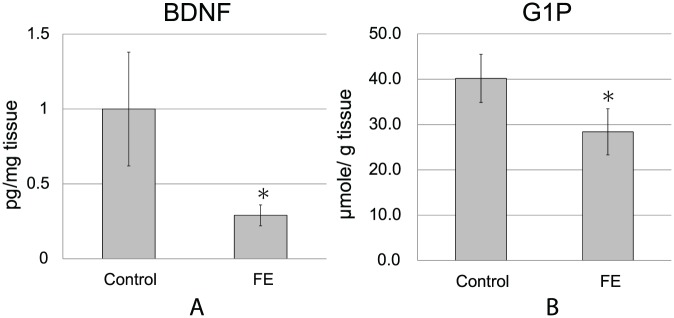
The hypothalamus plays an important role in the various effects of an enriched environment, and expression of BDNF mRNA in the hypothalamus is elevated in an enriched environment. In this study, we measured BDNF protein levels in the hypothalamus and found a marked reduction in expression in FE-exposed mice to about 30% that of control mice (FE = 0.29 ± 0.07 vs control = 1.00 ± 0.38 pg/mg tissue; P < .05; Figure 3A).
Figure 3.
FE decreased BDNF protein and glucose-1-phosphate concentrations in the hypothalamus. Hypothalamus tissues were collected at 21 days after inoculation of melanoma cells. (A) Hypothalamic BDNF protein concentrations. Hypothalamic BDNF levels were determined by ELISA (n = 6 in each group, *P < .05). (B) Hypothalamic glucose-1-phosphate concentrations. Hypothalamic metabolite levels were determined by CE-TOFMS (n = 6 in each group, *P < .05).
In the metabolomic analysis of the hypothalamus by CE-TOFMS, 91 metabolites were detected, and the concentrations were determined. Of these, glucose-1-phosphate (G1P) was significantly changed between the 2 groups (P < .05; Figure 3B, Supplemental Material Table 1, available online). The decrease in G1P concentration in the hypothalamus from FE mice indicated a decrease in stored sugar in the tissue, suggesting that the activity of the hypothalamus was higher in FE-exposed mice than in control mice.
Immunological Examinations
Another mechanism of resistance to tumor growth from an enriched environment is the effect of the immune system. An enriched environment has been reported to elevate the activity of NK cells and CD8+ T cells, which have important roles in tumor immunity.16,17 In addition, walking in a forest, which is an environment in which we are fully exposed to α-pinene, increases the activity of NK cells in humans.24,25 Therefore, we analyzed the immunological parameters of spleen cells at 21 days by flow cytometry.
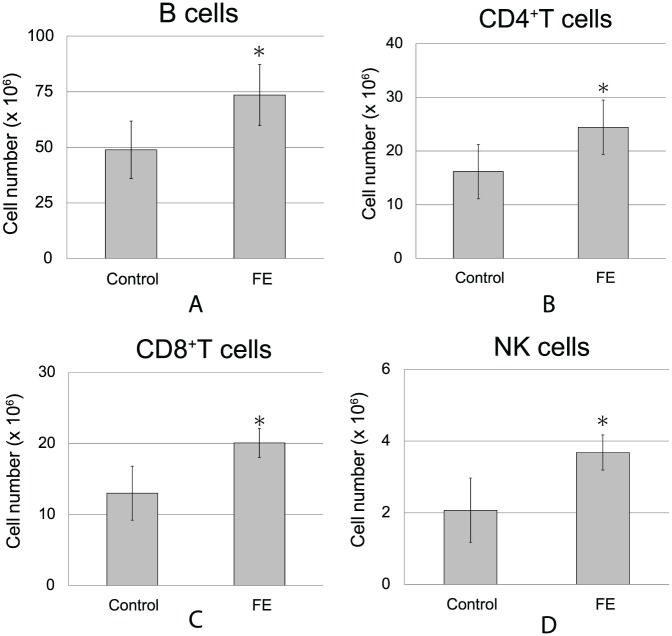
Spleen weights, total numbers of splenocytes, and the proportions of each cell subtype did not change under FE conditions (Supplemental Material Tables 2 and 3). However, the absolute numbers of various types of immune cells were elevated. For example, counts of B cells (Figure 4A), CD4+ T cells (Figure 4B), CD8+ T cells (Figure 4C), and NK cells (Figure 4D) were significantly increased in FE-housed mice compared with those in control mice (P < .05). These results suggested that an FE had beneficial effects on immunological responses to cancer cells consistent with cancer regression.
Figure 4.
Effects of the FE on immune cell numbers in the spleen. Flow cytometry analysis of splenocytes was performed 21 days after the inoculation of melanoma cells (n = 7-8 in each group, *P < .05). (A) B cells (CD19+CD45R/B220+). (B) CD4+ T cells (CD3+/CD4+). (C) CD8+ T cells (CD3+/CD8+). (D) NK cells (CD3−NK1.1+).
Discussion
Positive stress is known to be beneficial to human health, including not only the psychological state but also physical condition. Recently, several studies have reported that animals housed under enriched environment conditions, an animal model of positive stress, show a tumor-resistant phenotype.11-14,16 We previously reported the inhibitory effects of an FE with α-pinene on tumor growth.20 In this study, we examined neuroendocrinological and immunological changes in mice under an FE in order to evaluate the mechanisms through which the FE inhibits tumor growth. We found that the FE decreased plasma leptin levels and increased the numbers of B cells, CD4+ T cells, CD8+ T cells, and NK cells, accompanying a significant reduction in cancer burden. Moreover, we found that these changes were mediated by activation of the hypothalamus/sympathetic nervous system. These results indicated that the effects of the FE were similar to those of an enriched environment, expanding on the work of Cao et al and suggesting that positive stress tends to produce similar effects, regardless of the type of environment.
Cao et al11,15 reported that exposure to an enriched environment induces increased expression of BDNF in the hypothalamus, which activates the sympathetic nervous system, resulting in the production of noradrenaline and increased β-adrenergic receptors in white adipose tissue, thereby suppressing leptin expression. Here, we demonstrated that exposure to FE also induced activation of the hypothalamus/sympathetic nervous system and suppressed plasma leptin levels similar to that reported in mice housed in enriched environments, although we detected a decrease rather than an increase in BDNF. However, despite the observed decreases in leptin levels, body weights were not changed in our experiments. Cao et al also reported that running induces body weight loss, but does not decrease leptin levels and has no inhibitory effects on tumor growth. Thus, these findings suggested that weight loss and reduced leptin levels were not directly related. In our FE model, no weight loss was observed because the physical exercise level was low, despite stimulation of the hypothalamus.
The hypothalamus is an important CNS organ related to various sensations caused by odor. Odorant substances are low-molecular-weight organic compounds having molecular weights of about 300 Da or less. After entry into the nasal mucosa, odorant substances bind to olfactory receptors expressed in the olfactory nerve and electrically excite olfactory nerve cells. The electric signals generated in olfactory nerve cells following odor stimulation are transmitted to the olfactory bulb, which is the primary olfactory center. Thereafter, a signal is delivered to the secondary nerve and transmitted to brain regions, such as the anterior olfactory nucleus, olfactory tubercle, entorhinal cortex, piriform cortex, and amygdala. Signal inputs to the amygdala are then transmitted to the hypothalamus and are involved in emotion by odor through the neuroendocrine and autonomic nervous systems.26,27 Therefore, it is reasonable to speculate that the FE with α-pinene activated the hypothalamus in our model. This was also supported by our metabolomic data from the hypothalamus, in which the concentrations of G1P in the hypothalamus were decreased in FE-housed mice compared with those in control mice. G1P is an important carbohydrate intermediate in glucose metabolism and storage. Increased neuronal activity induced by sensory stimulation is associated with a decrease in glycogen levels in activated areas, demonstrating tight coupling between neuronal activity and glycogen mobilization.28,29 Decreased G1P levels in the hypothalamus in FE-exposed mice suggested hypothalamic activation and glycogen consumption. Leptin may be involved in reduction of G1P levels. Leptin also has major effects on glucose metabolism at both the systemic and cellular levels.30 At the cellular level, leptin enhances glucose uptake through GLUT1.31,32 In this study, a reduction in plasma leptin levels was observed. The reduction in leptin levels may have inhibited glucose uptake into cells and increased glycogenolysis.
BDNF is a member of the neurotrophin family of secreted proteins and plays an important role in neuronal growth and differentiation, thereby facilitating synaptic plasticity in the CNS33 and autonomic nervous system.34 Because BDGF gene expression is tightly regulated by epigenetic control in an activity-dependent and cell type-specific manner, BDGF is expected to be a key molecule in gene-environment interaction research.33 An enriched environment induces BDNF mRNA expression in the hypothalamus. Additionally, overexpression of BDNF in the hypothalamus mimics the tumor-suppressing effects of an enriched environment.11 However, BDNF protein levels were reduced in our model. Tissue content of protein is determined by a balance between production and release. Indeed, previous studies have reported that the expression patterns of BDNF mRNA and protein differ in glioblastoma cells, depending on the type and duration of stimulation.35 The discrepancy between BDNF mRNA and protein levels may be due to different environments used for enrichment and fragrance. Future studies will be necessary to elucidate the neurobiology of BDNF in the hypothalamus.
Recent studies have recognized the importance of cancer immunity due to the clinical introduction of immune checkpoint inhibitors.17,36 In particular, NK cells37 and CD8+ T cells38 are believed to play central roles in tumor immunity. However, the involvement of immunity in the induction of tumor resistance by an enriched environment is unclear. Cao et al argued that the involvement of immunity in the antitumor effects of an enriched environment is minimal because the activities of NK cells and CD8+ T cells are enhanced, even by simple exercise, and are unrelated to tumor resistance.11 However, in other reports, immunity has been reported to have a higher impact on tumor resistance than leptin.16 In our model of the FE, the numbers of NK cells and CD8+ T cells were increased as the concentration of leptin decreased. We only measured the numbers of immune cells in the spleen and did not assess intratumoral immune cell function or infiltration. From our experiments, we could not determine whether leptin or immunity was most important. In addition, the number of B cells was also increased under FE conditions. Although knowledge of the function of B cells in infection defense and autoimmune diseases has accumulated, the roles of B cells in tumor immunity are still unknown.39 There is no clear evidence demonstrating that antitumor antibodies naturally produced in vivo against tumors act as immune surveillance mechanisms. Drugs based on antibodies secreted by B cells can be used for the treatment of various tumors and have demonstrated remarkable results.40 In contrast, B cells are often harmful to the human body and act by suppressing tumor immunity41 and activating cancer cell proliferation.42 Further experiments are needed to clarify the importance of tumor immunity in the FE.
The possibility of a systemic effect of α-pinene should be considered. In our previous study, we showed that α-pinene had no direct inhibitory effect on tumor growth in vitro.20 Alternatively, the fragrance may have a direct effect on immune cells. Olfactory receptors are expressed by immune cells, such as polynuclear cells, T cells, and B cells.43 Only class I olfactory receptors, and not class II receptors, are expressed on immune cells. Class I olfactory receptors mainly bind to water-soluble ligands, whereas class II receptors bind to volatile ligands. The volatile compound α-pinene is thought to bind to class II receptors. Therefore, it appears unlikely that α-pinene would directly act on and affect immune cells.
Pleasant odors are positive stresses; however, unpleasant scents can become negative stresses. α-Pinene is a major component of wood scent, which is generally detected in the air of coniferous forests.44 It is unknown whether the scent of α-pinene is attractive or aversive to mice. However, α-pinene has been reported to have sedative effects in rats, suggesting that α-pinene is at least not an unpleasant odor to rats.45,46 In humans, direct exposure to α-pinene or fragrances that contained α-pinene decreased the early component of contingent negative variation, an index of sedative state,47 and increased the numbers of CD8+ cells and NK cells.48 However, no reports have described the effects of α-pinene on leptin.
Several limitations of the study should be discussed. First, we evaluated the effects of α-pinene in only one murine cancer model. To generalize our findings, it will be necessary to test other types of cancer models or spontaneous tumorigenesis models. The model we used in this experiment was exactly the same mouse strain and cell line as the model used by Cao et al11 in the enriched environment experiment. Additionally, the neuroendocrinological and immunological changes induced by the FE were similar to those in an enriched environment, suggesting that the effects of the FE on tumor suppression may also be applicable to other models. Second, we have examined only α-pinene, one type of fragrance, and we did not assess the possible effects of other fragrances on tumor growth. There are many types of scents derived from wood and non-wood sources, such as flowers. Therefore, other compounds should also be tested. However, preferences for or aversion to fragrances may differ between humans and mice, and the effects of fragrance on mice should be carefully assessed when considering their application to humans. Recently, Lasarte-Cia et al49 reported that some odors had immunostimulatory effects, whereas others had immunosuppressive effects. Furthermore, these immunomodulatory effects of odors varied between mouse strains. The authors reported that carvone, another terpene similar to α-pinene, was immunostimulatory in BALB/c female mice, but immunosuppressive in C57BL/6J female mice. Future studies will be necessary to elucidate the differences in the immunomodulatory effects of odors, mouse strains, and sex.
Conclusion
In this study, we demonstrated for the first time that exposure to an FE decreased plasma leptin concentrations and increased immune cells with significant inhibition of cancer growth in mice. The effects of FE involved a different mechanism but may activate pathways similar to those observed for enriched environments, thereby inhibiting the progression of cancer. Our results provide an expanded mechanistic view of how fragrance may exert beneficial effects against cancer and establish new potential avenues for cancer prevention and treatment.
Supplemental Material
Supplemental material, Supplemental_Material_fragrant_envieonment_tumor_growth_Kusuhara__ICT-2018-210.R1 for A Fragrant Environment Containing α-Pinene Suppresses Tumor Growth in Mice by Modulating the Hypothalamus/Sympathetic Nerve/Leptin Axis and Immune System by Masatoshi Kusuhara, Koji Maruyama, Hidee Ishii, Yoko Masuda, Kazutoshi Sakurai, Eiko Tamai and Kenichi Urakami in Integrative Cancer Therapies
Acknowledgments
We thank Ms Hasegawa and Ms Ichikawa for technical assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008;5:466-475. [DOI] [PubMed] [Google Scholar]
- 2. Hamer M, Chida Y, Molloy GJ. Psychological distress and cancer mortality. J Psychosom Res. 2009;66:255-258. [DOI] [PubMed] [Google Scholar]
- 3. Lin Y, Wang C, Zhong Y, et al. Striking life events associated with primary breast cancer susceptibility in women: a meta-analysis study. J Exp Clin Cancer Res. 2013;32:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lissoni P, Cangemi P, Pirato D, et al. A review on cancer—psychospiritual status interactions. Neuro Endocrinol Lett. 2001;22:175-180. [PubMed] [Google Scholar]
- 5. van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191-198. [DOI] [PubMed] [Google Scholar]
- 6. Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. 2006;7:697-709. [DOI] [PubMed] [Google Scholar]
- 7. Young D, Lawlor PA, Leone P, Dragunow M, During MJ. Environmental enrichment inhibits spontaneous apoptosis, prevents seizures and is neuroprotective. Nat Med. 1999;5:448-453. [DOI] [PubMed] [Google Scholar]
- 8. Cao L, Jiao X, Zuzga DS, et al. VEGF links hippocampal activity with neurogenesis, learning and memory. Nat Genet. 2004;36:827-835. [DOI] [PubMed] [Google Scholar]
- 9. Beasley JM, Newcomb PA, Trentham-Dietz A, et al. Social networks and survival after breast cancer diagnosis. J Cancer Surviv. 2010;4:372-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hinzey A, Gaudier-Diaz MM, Lustberg MB, DeVries AC. Breast cancer and social environment: getting by with a little help from our friends. Breast Cancer Res. 2016;18:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cao L, Liu X, Lin EJ, et al. Environmental and genetic activation of a brain-adipocyte BDNF/leptin axis causes cancer remission and inhibition. Cell. 2010;142:52-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nachat-Kappes R, Pinel A, Combe K, et al. Effects of enriched environment on COX-2, leptin and eicosanoids in a mouse model of breast cancer. PLoS One. 2012;7:e51525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garofalo S, D’Alessandro G, Chece G, et al. Enriched environment reduces glioma growth through immune and non-immune mechanisms in mice. Nat Commun. 2015;6:6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li G, Gan Y, Fan Y, et al. Enriched environment inhibits mouse pancreatic cancer growth and down-regulates the expression of mitochondria-related genes in cancer cells. Sci Rep. 2015;5:7856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cao L, During MJ. What is the brain-cancer connection? Annu Rev Neurosci. 2012;35:331-345. [DOI] [PubMed] [Google Scholar]
- 16. Song Y, Gan Y, Wang Q, et al. Enriching the housing environment for mice enhances their NK cell antitumor immunity via sympathetic nerve-dependent regulation of NKG2D and CCR5. Cancer Res. 2017;77:1611-1622. [DOI] [PubMed] [Google Scholar]
- 17. Lesterhuis WJ, Haanen JB, Punt CJ. Cancer immunotherapy—revisited. Nat Rev Drug Discov. 2011;10:591-600. [DOI] [PubMed] [Google Scholar]
- 18. Hur MH, Song JA, Lee J, Lee MS. Aromatherapy for stress reduction in healthy adults: a systematic review and meta-analysis of randomized clinical trials. Maturitas. 2014;79:362-369. [DOI] [PubMed] [Google Scholar]
- 19. Lakhan SE, Sheafer H, Tepper D. The effectiveness of aromatherapy in reducing pain: a systematic review and meta-analysis. Pain Res Treat. 2016;2016:8158693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kusuhara M, Urakami K, Masuda Y, et al. Fragrant environment with alpha-pinene decreases tumor growth in mice. Biomed Res. 2012;33:57-61. [DOI] [PubMed] [Google Scholar]
- 21. Urakami K, Zangiacomi V, Yamaguchi K, Kusuhara M. Quantitative metabolome profiling of Illicium anisatum by capillary electrophoresis time-of-flight mass spectrometry. Biomed Res. 2010;31:161-163. [DOI] [PubMed] [Google Scholar]
- 22. Yamakawa Y, Kusuhara M, Terashima M, et al. CD44 variant 9 expression as a predictor for gastric cancer recurrence: immunohistochemical and metabolomic analysis of surgically resected tissues. Biomed Res. 2017;38:41-52. [DOI] [PubMed] [Google Scholar]
- 23. Tai S, Cheng JY, Ishii H, et al. Effects of beta-tricalcium phosphate particles on primary cultured murine dendritic cells and macrophages. Int Immunopharmacol. 2016;40:419-427. [DOI] [PubMed] [Google Scholar]
- 24. Li Q. Effect of forest bathing trips on human immune function. Environ Health Prev Med. 2010;15:9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Li Q, Kobayashi M, Inagaki H, et al. A day trip to a forest park increases human natural killer activity and the expression of anti-cancer proteins in male subjects. J Biol Regul Homeost Agents. 2010;24:157-165. [PubMed] [Google Scholar]
- 26. Mori K, Sakano H. How is the olfactory map formed and interpreted in the mammalian brain? Annu Rev Neurosci. 2011;34:467-499. [DOI] [PubMed] [Google Scholar]
- 27. Zelano C, Sobel N. Humans as an animal model for systems-level organization of olfaction. Neuron. 2005;48:431-454. [DOI] [PubMed] [Google Scholar]
- 28. Belanger M, Allaman I, Magistretti PJ. Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011;14:724-738. [DOI] [PubMed] [Google Scholar]
- 29. Falkowska A, Gutowska I, Goschorska M, Nowacki P, Chlubek D, Baranowska-Bosiacka I. Energy metabolism of the brain, including the cooperation between astrocytes and neurons, especially in the context of glycogen metabolism. Int J Mol Sci. 2015;16:25959-25981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. D’Souza AM, Neumann UH, Glavas MM, Kieffer TJ. The glucoregulatory actions of leptin. Mol Metab. 2017;6:1052-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chowen JA, Frago LM, Fernandez-Alfonso MS. Physiological and pathophysiological roles of hypothalamic astrocytes in metabolism. J Neuroendocrinol. 2018:e12671. doi: 10.1111/jne.12671 [DOI] [PubMed] [Google Scholar]
- 32. Park HK, Ahima RS. Leptin signaling. F1000Prime Rep. 2014;6:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sasi M, Vignoli B, Canossa M, Blum R. Neurobiology of local and intercellular BDNF signaling. Pflugers Arch. 2017;469:593-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mattson MP, Wan R. Neurotrophic factors in autonomic nervous system plasticity and dysfunction. Neuromolecular Med. 2008;10:157-168. [DOI] [PubMed] [Google Scholar]
- 35. Jozwiak-Bebenista M, Jasinska-Stroschein M, Kowalczyk E. The differential effects of neuroleptic drugs and PACAP on the expression of BDNF mRNA and protein in a human glioblastoma cell line. Acta Neurobiol Exp (Wars). 2017;77:205-213. [PubMed] [Google Scholar]
- 36. Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Li Y, Sun R. Tumor immunotherapy: new aspects of natural killer cells. Chin J Cancer Res. 2018;30:173-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reading JL, Galvez-Cancino F, Swanton C, Lladser A, Peggs KS, Quezada SA. The function and dysfunction of memory CD8+ T cells in tumor immunity. Immunol Rev. 2018;283:194-212. [DOI] [PubMed] [Google Scholar]
- 39. Shen M, Wang J, Ren X. New insights into tumor-infiltrating B lymphocytes in breast cancer: clinical impacts and regulatory mechanisms. Front Immunol. 2018;9:470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sliwkowski MX, Mellman I. Antibody therapeutics in cancer. Science. 2013;341:1192-1198. [DOI] [PubMed] [Google Scholar]
- 41. Barbera-Guillem E, Nelson MB, Barr B, et al. B lymphocyte pathology in human colorectal cancer. Experimental and clinical therapeutic effects of partial B cell depletion. Cancer Immunol Immunother. 2000;48:541-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ammirante M, Luo JL, Grivennikov S, Nedospasov S, Karin M. B-cell-derived lymphotoxin promotes castration-resistant prostate cancer. Nature. 2010;464:302-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malki A, Fiedler J, Fricke K, Ballweg I, Pfaffl MW, Krautwurst D. Class I odorant receptors, TAS1R and TAS2R taste receptors, are markers for subpopulations of circulating leukocytes. J Leukoc Biol. 2015;97:533-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tsunetsugu Y, Park BJ, Miyazaki Y. Trends in research related to “Shinrin-yoku” (taking in the forest atmosphere or forest bathing) in Japan. Environ Health Prev Med. 2010;15:27-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Faturi CB, Leite JR, Alves PB, Canton AC, Teixeira-Silva F. Anxiolytic-like effect of sweet orange aroma in Wistar rats. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34:605-609. [DOI] [PubMed] [Google Scholar]
- 46. Yamaoka S, Tomita T, Imaizumi Y, Watanabe K, Hatanaka A. Effects of plant-derived odors on sleep-wakefulness and circadian rhythmicity in rats. Chem Senses. 2005;30(suppl 1):i264-i265. [DOI] [PubMed] [Google Scholar]
- 47. Sawada K, Ko MR, Yamashita Y, Suzuki Y. Fragrance of forest and its physiological effects. Aroma Res. 2000;1:67-71. [Google Scholar]
- 48. Kuriyama H, Watanabe S, Nakaya T, et al. Immunological and psychological benefits of aromatherapy massage. Evid Based Complement Alternat Med. 2005;2:179-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lasarte-Cia A, Lozano T, Perez-Gonzalez M, et al. Immunomodulatory properties of carvone inhalation and its effects on contextual fear memory in mice. Front Immunol. 2018;9:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplemental_Material_fragrant_envieonment_tumor_growth_Kusuhara__ICT-2018-210.R1 for A Fragrant Environment Containing α-Pinene Suppresses Tumor Growth in Mice by Modulating the Hypothalamus/Sympathetic Nerve/Leptin Axis and Immune System by Masatoshi Kusuhara, Koji Maruyama, Hidee Ishii, Yoko Masuda, Kazutoshi Sakurai, Eiko Tamai and Kenichi Urakami in Integrative Cancer Therapies