Short abstract
We explored the atypical functional connectivity between the anterior cingulate cortex and other brain areas in rats subjected to repeated meningeal nociception. The rat model was established by infusing an inflammatory soup through supradural catheters in conscious rats. Rats were subdivided according to the frequency of the inflammatory soup infusions. Functional connectivity analysis seeded on the anterior cingulate cortex was performed on rats 21 days after inflammatory soup infusion. Glyceryl trinitrate was injected following baseline scanning in the low-frequency inflammatory soup group and magnetic resonance imaging data were acquired 1 h after the injection. The rats exhibited nociceptive behavior after high-frequency inflammatory soup infusion. The anterior cingulate cortex showed increased functional connectivity with the cerebellum in the inflammatory soup groups. The medulla showed increased functional connectivity with the anterior cingulate cortex in the ictal period in the low-frequency inflammatory soup rats. Several areas showed increased functional connectivity with the anterior cingulate cortex in the high-frequency inflammatory soup group, including the pontine tegmentum, midbrain, thalamus, corpus callosum, hippocampus, and retrosplenial, visual, sensory, and motor cortices. This study indicated that the medulla participates in the early stage of a migraine attack and may be associated with the initiation of migraine. Sensitization of the trigeminal nociceptive pathway might contribute to the cutaneous allodynia seen in chronic migraine. Brain areas important for memory function may be related to the chronification of migraine. Electrophysiological studies should examine those migraine-related areas and provide new targets for migraine treatment and prevention.
Keywords: Migraine, cutaneous allodynia, anterior cingulate cortex, functional connectivity
Introduction
Migraine is the most common primary headache disorder and has high socioeconomic and personal costs.1 Migraine was ranked as the sixth leading cause of disability worldwide in the Global Burden of Disease Survey and the leading cause of disability in those under 50 years of age.2,3 Classified as a subset of migraine, chronic migraine (CM) is defined as headaches on ≥15 days/month. In approximately 2.5% of patients with episodic migraine (EM), the condition transitions to CM annually.4 CM imposes a greater burden, with disability scores nearly twice as high as those in EM.5
Except for the difference in headache-day frequency between EM and CM, there are two important indicators of progression to CM. Repeated migraine attacks may lead to central sensitization via the chronic stimulation of central pain pathways. As a symptomatic manifestation of central sensitization, cutaneous allodynia was defined as innocuous stimuli perceived as painful. The majority of migraineurs develop cutaneous allodynia during migraine attacks, and some have persistent sensitization even during the interictal phase.6,7 Cutaneous allodynia is more frequent and severe in CM than in EM; it is associated with female sex, headache frequency, increased body mass index, disability, and depression.8 Cutaneous allodynia may indicate migraine progression.4 Hyperalgesia during the interictal period is also regarded as an indicator of success in the preparation of animal models of CM.9 Major non-headache indicators associated with CM include anxiety, depression, and other affective disorders.10 Clinical and epidemiological studies have demonstrated that migraine has a bidirectional relationship with depression and anxiety.11,12 Migraineurs have a more than four-fold relative risk of developing depression compared with patients without migraine.13 Most CM rats show depression- and anxiety-like behaviors.14 However, functional brain changes related to the indicators of progression to CM remain unclear.
The anterior cingulate cortex (ACC) is a key structure involved in various higher brain functions, such as nociception, chronic pain, cognition, and emotions.15 Migraineurs had concordant decreases in the gray matter volume in the ACC, and the decreased gray matter volume was related to the estimated frequency of headache attacks in a meta-analysis.16 Activation in the ACC was also found in migraine patients.16 A CM group showed greater activation in the ACC on laser stimulus compared with both controls and migraine without aura (MWA) patients.17 Noxious trigeminal heat stimulation in migraine patients after external trigeminal neurostimulation induced a significantly reduced BOLD response in the ACC.18 A molecular-level study revealed altered N-acetyl aspartate/creatinine in the ACC in migraine patients.19 Taken together, these findings indicate that the ACC plays an important role in the pathogenesis of migraine, especially CM. The ACC is involved in chronic neuropathic pain and its associated anxiety.20 Nevertheless, the functional changes in the ACC that occur in migraine remain unknown.
Functional connectivity (FC) is a descriptive measure of the spatiotemporal correlations among distinct cerebral regions.21 Resting-state functional connectivity (rs-FC) shows co-activation of brain regions during the resting state and reveals the functional brain changes involved in the pathogenesis of diseases. Therefore, in this study, we used functional magnetic resonance imaging (fMRI) to investigate rs-FC based on the ACC in migraine model rats. We prepared animal models that mimic EM and CM via inflammatory soup (IS) infusions in the dura mater of rats at different frequencies.22 We hypothesized that there would be atypical FC of the ACC with (1) central pain pathways in the ictal stage in the low-frequency IS-stimulated group and (2) brain areas related to nociception, emotion processing, and pain modulation in the high-frequency IS-stimulated group, and (3) that cutaneous allodynia would be related to higher pain-modulating cortical areas.
Materials and methods
Ethical concerns and habituation
This study included 24 specific-pathogen-free Sprague Dawley adult male rats (180–220 g; six to seven weeks of age; Beijing Vital River Laboratory Animal Technology Co., Ltd.). The rats were housed separately in a temperature-controlled (22 ± 2°C) environment on a 12/12 h light/dark cycle and allowed food and water ad libitum. The experimental procedures were approved by the Laboratory Animal Center of the General Hospital of Chinese People's Liberation Army (Beijing, China) and were consistent with the ethical guidelines recommended by the International Association for the Study of Pain in experimental conscious animals.23 Efforts were made to minimize animal suffering.
Animal groups
The rats were divided randomly according to a sequence generated by a random-number table to avoid selection bias. According to the frequency of IS infusion, the rats were assigned to two experimental groups and corresponding control (Con) groups (n = 6/group), i.e., low-frequency infusion of IS (LF-IS; once every four days) and high-frequency infusion of IS (HF-IS; daily). The rats in the IS groups received infusions of IS (10 μL) for 5 min and the Con groups received sterile saline for 21 days. The IS (2 mM histamine, 2 mM 5-hydroxytryptamine, 2 mM bradykinin, and 0.2 mM prostaglandin E2 in saline, Sigma, USA) was prepared from stock solutions prior to use. The rats were placed in a plastic tube restraint for IS/saline infusion and tactile sensory testing. LF-IS group rats were infused with IS six times totally (less than eight times) to approximate the patients with EM. The HF-IS rats were infused with IS daily to mimic CM.23
Surgical procedures
The surgery was conducted according to the methods described in our previous research.24 Briefly, the rats were placed under general anesthesia (pentobarbital 50 mg/kg, intraperitoneal) and positioned in a stereotaxic apparatus (ZS-B/C, Beijing, China). An incision was made on the scalp to expose the skull. Next, two 8–10-mm long, 2-mm wide, and ∼0.5-mm deep troughs bilateral to the midsaggital suture (3–4 mm lateral to it) were drilled in the skull to orient and secure the PE10 tubing (62310, RWD Life Science Co., Ltd., Shenzhen, China). The catheters were then attached to the skull using 502 glue and dental cement. Finally, the incised skin was sutured. After surgery, rats recovered for about one week before used to proceed with the experiments. Tactile sensory thresholds were monitored during the recovery period to ensure that the thresholds returned back to their preoperative baseline.
Tactile sensory testing
Tactile sensory testing was carried out as described previously by Oshinsky et al.25 They were conducted every other day prior to the injection of IS or saline. Nociceptive thresholds were measured by perpendicularly applying a Von Frey monofilament (North Coast Medical Co., Ltd., Gilroy, CA, USA) to the periorbital region of the rats until a positive response was observed. The von Frey stimuli were presented in a sequential descending order to determine the threshold of response. The threshold is defined as a positive response to two of three, or in some cases three of five trials of a single von Frey monofilament. Rats that did not respond to the 10 g stimulus were assigned 10 g as their threshold for analysis. The evaluating experimenter was blind to the experimental group.
fMRI acquisition and FC analysis
The rats were imaged 24 h after the last infusion of IS/saline to mimic an interictal migraine state. The MRI data were acquired using a 7.0-T Bruker Pharma Scan system (Bruker BioSpin, Ettlingen, Germany) with a 38-mm diameter coil. Glyceryl trinitrate (0.1 mg/kg, i.p.) was injected following baseline scanning in LF-IS group.26 Cephalic mechanical allodynia were prominent in the rats at 1 h after glyceryl trinitrate infusion, so the MRI data were acquired 1 h after the injection to mimic an ictal migraine state.26 The MRI data collection were performed as described in our previous research.24 Briefly, the rats were anesthetized with isoflurane in a gas mixture of 40% O2 and 60% N2. Each rat was placed in the prone position on an MR-compatible stereotactic holder with a bite bar and a gas mask to exhaust the isoflurane. Respiration rate was monitored using a pressure sensor (SA Instruments, Stony Brook, NY, USA) throughout the scans.
High-resolution anatomical MRI data were collected using a T2-weighed RARE sequence. These T2-weighted images (T2WI) were obtained using a 2D-RARE sequence with the following parameters: repetition time = 6200 ms, effective echo time = 24 ms, flip angle =180 °, field of view = 35 × 35 mm2, matrix size = 256 × 256, slice thickness = 0.3 mm, slice gap = 0 mm, and total scan time = 20 min. Functional images were obtained using a gradient echo-planar imaging (EPI) sequence (repetition time = 2000 ms, echo time = 27.1 ms, number of segments = 2, flip angle = 90, slice thickness = 1 mm, slice gap = 0 mm, matrix = 128 × 128), and 150 continuous EPI functional volumes were acquired axially over 13 min 20 s.27
All functional image post-processing was performed by a single, experienced observer who was blinded to the treatment group. The preprocessing and data analysis were performed using the “spmratIHEP” toolbox within the SPM8 software (Welcome Department of Imaging Neuroscience; http://www.fil.ion.ucl.ac.uk/spm), which includes an fMRI rat brain template and the atlas of Paxinos and Watson.28,29
The functional data sets of all individual rats were pre-processed in spmratIHEP with the following major steps. (1) The first 10 volumes of each rat were discarded to allow for magnetization equilibration. (2) Slice timing: the differences in the slice acquisition times were corrected for using slice timing in each rat. (3) Realignment: the temporally processed volumes of each rat were realigned to the first volume to remove head motion, and a mean image was created over the 150 realigned volumes. All rats exhibited less than 1 mm of translation in the x, y, and z axes and 1° of rotation in each axis. (4) Spatial normalization: the realigned volumes were standardized spatially to the Paxinos and Watson space via normalization with the EPI template of a rat brain via their corresponding mean image. Then, all normalized images were resliced to 1.0 × 1.5 × 1.0 mm3 voxels. (5) Smoothing: the normalized functional series were smoothed with a Gaussian kernel of 2 × 4 × 2 mm3 full width at half-maximum.
Using DPARSF (http://rfmri.org/DPARSF), all smoothed images were then band-pass filtered at 0.01–0.08 Hz and further corrected for the effect of head movement by regressing out the translations and rotations of the head that were estimated during image realignment. FC was evaluated using the ACC as the seed region. One-sample T test on FC parameters has been performed for each group. Finally, to identify differences in FC between the IS group and the Con group, two-sample T tests were used. Significant FC was determined based on a voxel-level height threshold of P < 0.005 (uncorrected) and a cluster-extent threshold of 100 contiguous voxels.
Statistical analysis
The SPSS (ver. 20.0; IBM Corp., Armonk, NY, USA) for Windows, GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA) software packages and Adobe Photoshop CS6 (Adobe system Inc., San Diego, CA, USA) were used for data analyses and graph generation. All data are presented as the mean ± standard deviation. Levene’s test for homogeneity was conducted, and abnormally distributed data were analyzed using the Kruskal–Wallis test to determine differences among the groups. The data of facial tactile sensory threshold were abnormally distributed and analyzed using the Kruskal–Wallis test. P < 0.05 was considered to indicate statistical significance.
Results
Facial tactile sensory threshold
There was no significant difference in facial tactile threshold between LF-IS and low-frequency control (LF-Con; Kruskal–Wallis test, P > 0.05) (Figure 1). The HF-IS group showed a significantly greater decline in the periorbital tactile threshold compared with the high-frequency control (HF-Con) group from day 3 (Kruskal–Wallis test, χ2 = 6.036, P = 0.014) (Figure 1).
Figure 1.
Facial tactile thresholds during the 21 days experiment. The horizontal axis shows the day after measurement, and the vertical axis shows the facial tactile withdrawal thresholds of the rats. Mean ± standard deviation values are shown. No significant differences were shown between the LF-IS and LF-Con groups. The HF-IS group exhibited significant decreases in periorbital tactile thresholds compared with the HF-Con group (*P < 0.05, ***P < 0.001) from day 3 of the study. HF: high frequency; LF: low frequency; IS: inflammatory soup; Con: control.
FC analysis
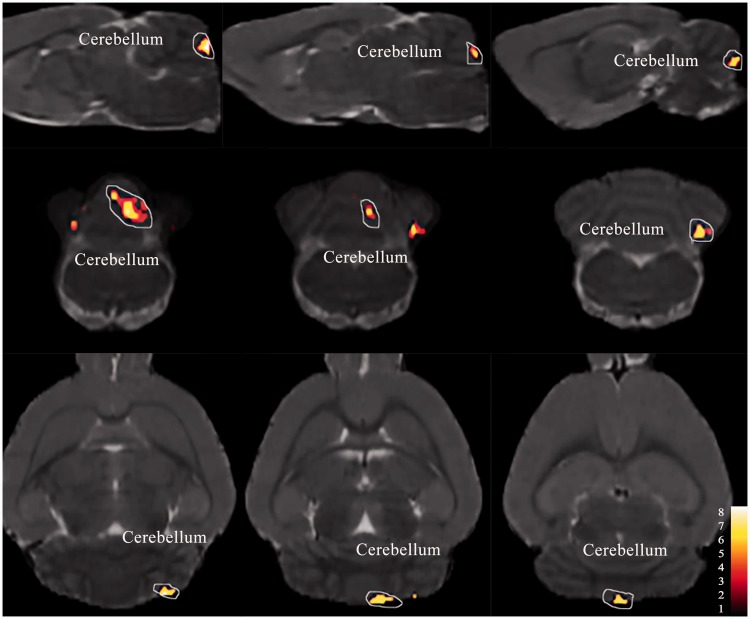
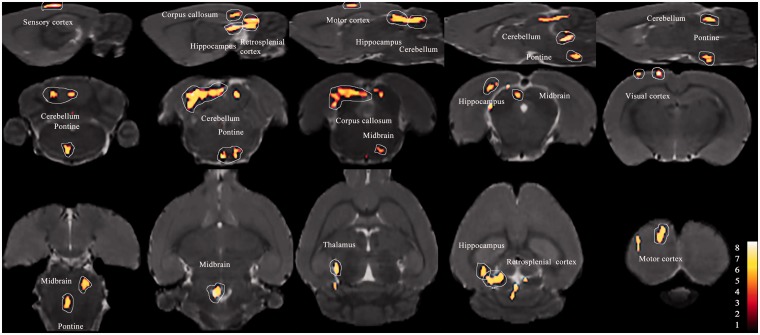
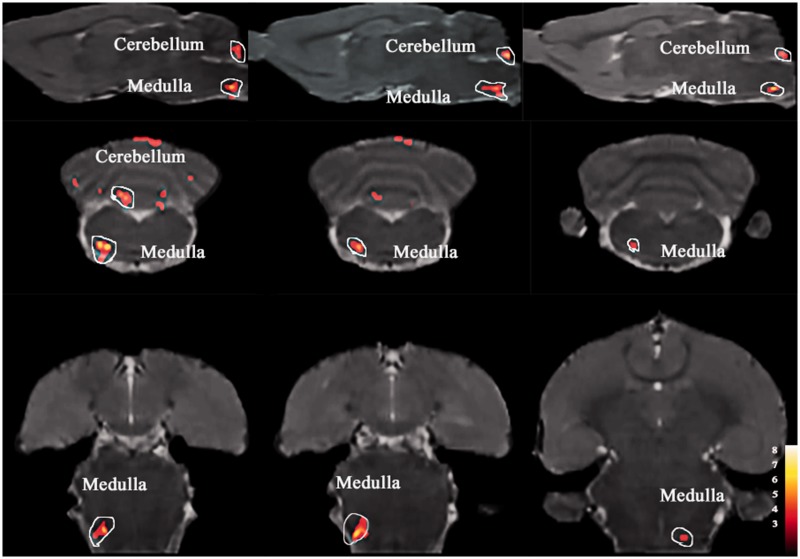
Figure 2 shows the anatomical boundaries of the ACC seeds. The results of one-sample t-test have shown that there was no significant difference in each group. The IS-infusion rats showed significantly increased functional correlations (P < 0.005, uncorrected, extent threshold k = 100 voxels) between the ACC and cerebellum compared with the saline-infusion rats (Figures 3 to 5 and Table 1). Except for the cerebellum, the medulla showed relatively increased FC with the ACC in the ictal period in the LF-IS rats compared with the LF-Con rats (P < 0.005, uncorrected, extent threshold k = 100 voxels) (Figure 4 and Table 1). Several areas in the HF-IS group showed relative increases in FC with the ACC compared with the HF-Con group. These areas included the pontine tegmentum, midbrain, thalamus, corpus callosum, hippocampus, and retrosplenial, visual, sensory, and motor cortices (Figure 5 and Table 1) (P < 0.005, uncorrected, extent threshold k = 100 voxels).
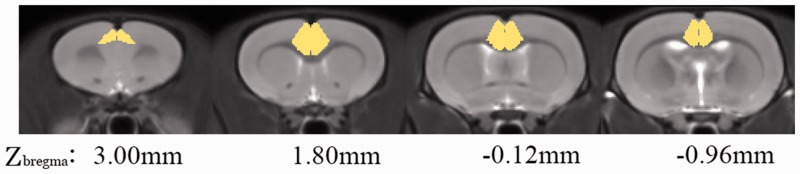
Figure 2.
Anterior cingulate cortex (ACC) seeds across the rats imposed on the T2-weighted MRI template and on the rat atlas structures. The anatomical boundaries for each rat were based on the atlas of Paxinos and Watson. The coronal slices of the ACC are shown.
Figure 3.
Cerebellum shown increased functional connectivity with the ACC (in red) in the LF-IS group compared with the LF-Con group (day 21) (P < 0.005, uncorrected, extent threshold k = 100 voxels) imposed on a T2-weighted magnetic resonance imaging template as well as on the rat atlas structures. Detail of the cluster shown is reported in Table 1. LF: low frequency; IS: inflammatory soup; Con: control; ACC: anterior cingulate cortex.
Figure 5.
Colored voxels represent clusters of significantly increased FC with the ACC in the HF-IS group compared with the HF-Con group (day 21). Details of the clusters shown are reported in Table 1. HF: high frequency; IS: inflammatory soup; Con: control; ACC: anterior cingulate cortex; FC: functional connectivity.
Table 1.
Brain regions with atypical functional connectivity with the anterior cingulate cortex in rats induced by dural inflammatory stimulation.
| Cluster or region of interest | Coordinates of peak(s) voxel (x, y, z) | Peak T value | Effect direction |
|---|---|---|---|
| Cerebellum | 0.7, 4.2, −14.5 | 5.44 | LF-IS>LF-Con (interictal stage) |
| −0.9, 4.1, −14.5 | 3.62 | HF-IS>HF-Con (interictal stage) | |
| Cerebellum | 1.5, 5.5, −14.3 | 6.50 | LF-IS>LF-Con (ictal stage) |
| −1.1, 5.2, −14.3 | 8.77 | ||
| Medulla | −2.4, 8.6, −13.8 | 5.21 | LF-IS>LF-Con (ictal stage) |
| Pontine (tegmentum of pons) | −0.0, 9.2, −9.0 | 5.95 | HF-IS>HF-Con (interictal stage) |
| Midbrain | −1.2, 2.5, −9.0 | 4.86 | HF-IS>HF-Con (interictal stage) |
| 1.2, 2.8, −9.2 | 4.95 | ||
| Thalamus | −1.6, 2.0, −7.8 | 4.55 | HF-IS>HF-Con (interictal stage) |
| Corpus callosum | −3.4, 1.8, −6.8 | 3.84 | HF-IS>HF-Con (interictal stage) |
| Hippocampus | −3.4, 3.7, −7.1 | 4.30 | HF-IS>HF-Con (interictal stage) |
| Retrosplenial cortex | −3.4, 2.6, −9.0 | 4.78 | HF-IS>HF-Con (interictal stage) |
| Visual cortex | −3.5, 1.6, −6.8 | 3.73 | HF-IS>HF-Con (interictal stage) |
| Sensory cortex | −4.0, −0.1, −2.5 | 3.79 | HF-IS>HF-Con (interictal stage) |
| Motor cortex | −2.0, −0.1, −2.3 | 6.21 | HF-IS>HF-Con (interictal stage) |
Note: Regions with changes in functional connectivity with the anterior cingulate were found in rats induced by subcranial (supradural) infusion of IS compared with saline-treated control rats. The coordinates according to Paxinos and Watson are given in mm: x (0 = center, left is negative), y (ventral to dorsal), and z (relative to bregma). Effect direction >: increased functional connectivity with the anterior cingulate cortex; interictal stage: the rats were imaged 24 h after the last infusion of IS/saline; ictal stage: the MRI data were acquired 1 h after the injection of glyceryl trinitrate in the LF-IS group. HF: high frequency; LF: low frequency; IS: inflammatory soup; Con: control.
Figure 4.
ACC based functional connectivity (FC) in LF-IS rats in ictal phase shown significantly increased FC with medulla and cerebellum compared with LF-Con group (day 21). Details of the clusters shown are reported in Table 1. LF: low frequency; IS: inflammatory soup; Con: control; ACC: anterior cingulate cortex.
Discussion
Migraine is believed to be a brain disorder influenced by the complex interaction of genetic and environment factors.30 The prevailing theory is that migraine is caused by abnormal sensory processing in the brain due to activation and sensitization of the trigeminovascular system innervating the intracranial vasculature and meninges.31 However, the mechanisms of the initiation and chronification of migraine are still unclear. In this study, we found that (1) the cerebellum was involved in all IS-infusion groups, in either the ictal or interictal stage, of the rat models of EM and CM; (2) atypical FC between ACC and medulla existed in the ictal stage of the LF-IS group (the rat model of EM), so the medulla may be involved in the initiation of migraine; and (3) central pain pathways (including the pontine, midbrain, thalamus, and sensory cortex) and modulation areas (such as the hippocampus, corpus callosum, visual cortex, and motor cortex) may be associated with the chronification of migraine and cutaneous allodynia.
The cerebellum is located solely and closely connected to the cerebrum, brainstem, and spinal cord. Traditionally, it is thought to play roles in motor control and coordination, and ataxia, dysarthria, and nystagmus arise from cerebellar dysfunction.32 Some recent studies have suggested that it also plays roles in cognition and pain processing.33,34 On nociceptive trigeminal input to the left nostril in a large number of volunteers, robust FC was found during nociception between the cerebellum and descending antinociceptive network (rostral part of the pons, periaqueductal gray, and thalamus), providing a solid basis for further research on cerebellar activity and connectivity in primary headache.35 Comparing the ictal and interictal scans in EM, activation was seen in the cerebellum.36 Increased cerebellar activity has also been found in response to trigeminal noxious stimuli in migraine patients and is highly interconnected with higher cognitive areas, implying that the cerebellum is involved in modulating the pain of migraine.37 A loss of macromolecules and/or micro-edema has been found in the cerebellum using T1 and magnetization transfer ratio contrasts in MWA patients, and the volume of the cerebellum was smaller in CM patients compared with healthy controls, although it is unclear whether the structural brain changes seen in migraine patients are the cause or result of headaches.38,39 Our study found increased FC between the cerebellum and ACC in the IS groups, further suggesting that the cerebellum is involved in the modulation of pain.
Other than the cerebellum, the only atypical FC we found was between the medulla (lateral portion) and ACC in the ictal phase of the LF-IS group (1 h after glyceryl trinitrate infusion, mimicking the ictal phase of EM). The headache phase of a migraine attack is thought to originate from the activation of nociceptors innervating the intracranial vasculature and meninges.31 Central processes of sensory afferents via the trigeminal tract enter the brainstem and pass caudally while giving off collaterals that terminate in the trigeminocervical complex, including the spinal trigeminal nucleus (SpVC, located in the lateral medulla) and upper cervical spinal cord (C1–3).40 During a migraine attack in a 42-year-old man with cutaneous allodynia after 1 h, fMRI showed activation of the SpVC, indicating that second-order neurons were activated by the impulses arising from peripheral nociceptors and initiated their sensitization.41 A positron emission tomography study found activation of the pons and other pain-related areas (right anterior cingulate, posterior cingulate, thalamus, insula, prefrontal cortex, and temporal lobes) in ictal stage scans in EM; the difference may be associated with the different scanning times after the migraine attack.36 Fos expression revealed that a single high-intensity IS stimulation on the dura mater of rats leads to widespread trigeminal and spinal central sensitization, concomitantly impairing the diffuse noxious inhibitory controls after repeated dural nociceptor activation.42 Combined with those results, our study further indicated that activation of the medulla (especially the SpVC) occurred in the early stage of a migraine attack and this might be associated with the initiation of migraine.
The interictal periorbital tactile threshold declined in the HF-IS group, mimicking CM. Cutaneous allodynia was present both during and between migraine attacks and is specific to migraine; it was present in the HF-IS group, indicating the establishment of a migraine animal model. Increased FC with the ACC was found in second-order neurons (pontine and midbrain), third-order neurons (thalamus), and higher centers (sensory cortex) in the trigeminal nociceptive pathway in the HF-IS group. Consistent with fMRI studies of migraineurs, trigemino-nociceptive activation was found in CM in the interictal phase, further suggesting that the development of sensitization contributes to the hypersensitivity seen in migraine (cutaneous allodynia in CM).43 Besides the trigemino-nociceptive system, activation of the hippocampus, corpus callosum, visual cortex, and motor cortex was also seen in the HF-IS group. The hippocampus and corpus callosum are important in memory, and damage to these structures leads to memory impairment.44 Increased FC between the ACC and the hippocampus and corpus callosum might play a role in the chronification of migraine. Cortical spreading depression in the visual cortex may be related to the aura of migraine and can cause sustained activation of meningeal nociceptors and thereby initiate the headache mechanisms; however, aura had not been observed in our study.45 Moreover, the visual and motor cortices are involved in modulating emotion and pain and atypical FC with the ACC in the HF-IS group may be involved in psychiatric comorbidities and pain relief of CM.46
Although there are important benefits to using animal models in brain imaging research, homology and consistency, and longitudinal studies are much more feasible, further histological and electrophysiological studies should be performed to identify the molecular mechanism in those migraine-related areas and provide new targets for migraine treatment and prevention.
Conclusion
This study found atypical FC of the ACC with brain regions primarily involved in the trigeminal nociceptive pathway, emotion processing, and pain modulation in rats following repeated stimulation of meningeal afferents (LF-IS, HF-IS, interictal, and ictal phases). This suggests that the cerebellum plays an important role in the modulation of pain. The medulla, especially the SpVC, participated in the early stage of a migraine attack and may be associated with the initiation of migraine. Sensitization of the trigeminal nociceptive pathway might contribute to the cutaneous allodynia in CM. Brain areas important in memory function may be related to the chronification of migraine.
Acknowledgment
The authors thanks Binbin Nie for the contribution made in the process of FC data analysis.
Author Contributions
Guarantor of integrity of entire study: YSY and ZHJ designed and supervised experiments; ZHJ, XYC, and DFZ contributed to data acquisition and analysis; WJT raised the mice; ZHJ contribute to manuscript drafting; YSY was involved in manuscript editing; All authors reviewed and approved the final manuscript.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Major National R&D Projects (grants Z161100002616013), National Science Foundation of China (grants 81771180), and Beijing Natural Science Foundation (grants 7162178).
References
- 1.Saylor D, Steiner TJ. The global burden of headache. Semin Neurol 2018; 38: 182–190. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2015 Disease and Injury Incidence and Prevalence Collaborators. Global, regional and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 1545–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steiner TJ, Stovner LJ, Vos T, Jensen R, Katsarava Z. Migraine is first cause of disability in under 50s: will health politicians now take notice? J Headache Pain 2018; 19: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aurora SK, Brin MF. Chronic migraine: an update on physiology, imaging, and the mechanism of action of two available pharmacologic therapies. Headache 2017; 57: 109–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology 2008; 71: 559–566. [DOI] [PubMed] [Google Scholar]
- 6.Mathew NT, Kailasam J, Seifert T. Clinical recognition of allodynia in migraine. Neurology 2004; 63: 848–852. [DOI] [PubMed] [Google Scholar]
- 7.Schwedt T J, Krauss M J, Frey K, Gereau R W. Episodic and chronic migraineurs are hypersensitive to thermal stimuli between migraine attacks. Cephalalgia 2011; 31: 6–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bigal ME, Ashina S, Burstein R, Reed ML, Buse D, Serrano D, Lipton RB. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology 2008; 70: 1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain 2014; 155: 269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cady RK, Schreiber CP, Farmer KU. Understanding the patient with migraine: the evolution from episodic headache to chronic neurologic disease. A proposed classification of patients with headache. Headache 2004; 44: 426–435. [DOI] [PubMed] [Google Scholar]
- 11.Beghi E, Bussone G, D'Amico D, Cortelli P, Cevoli S, Manzoni G C, Torelli P, Tonini M C, Allais G, De Simone R, D’Onofrio F, Genco S, Moschiano F, Beghi M, Salvi S. Headache, anxiety and depressive disorders: the HADAS study. J Headache Pain 2010; 11: 141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ratcliffe GE, Enns MW, Jacobi F, Belik SL, Sareen J. The relationship between migraine and mental disorders in a population-based sample. Gen Hosp Psychiatry 2009; 31: 14–19. [DOI] [PubMed] [Google Scholar]
- 13.Baskin SM, Lipchik GL, Smitherman TA. Mood and anxiety disorders in chronic headache. Headache 2006; 46: S76–S87. [DOI] [PubMed] [Google Scholar]
- 14.Zhang M, Liu Y, Zhao M, Tang W, Wang X, Dong Z, Yu S. Depression and anxiety behaviour in a rat model of chronic migraine. J Headache Pain 2017; 18: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsuda M, Koga K, Chen T, Zhuo M. Neuronal and microglial mechanisms for neuropathic pain in the spinal dorsal horn and anterior cingulate cortex. J Neurochem 2017; 141: 486–498. [DOI] [PubMed] [Google Scholar]
- 16.Jia Z, Yu S. Grey matter alterations in migraine: a systematic review and meta-analysis. NeuroImage Clin 2017; 14: 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Tommaso M, Losito L, Difruscolo O, Libro G, Guido M, Livrea P. Changes in cortical processing of pain in chronic migraine. Headache 2005; 45: 1208–1218. [DOI] [PubMed] [Google Scholar]
- 18.Russo A, Tessitore A, Esposito F. Functional changes of the perigenual part of the anterior cingulate cortex after external trigeminal neurostimulation in migraine patients. Front Neurol 2017; 8: 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becerra L, Veggeberg R, Prescot A, Jensen JE, Renshaw P, Scrivani S, Spierings ELH, Burstein R, Borsook D. A ‘complex’ of brain metabolites distinguish altered chemistry in the cingulate cortex of episodic migraine patients. NeuroImage Clin 2016; 11: 588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhuo M. Neural mechanisms underlying anxiety-chronic pain interactions. Trends Neurosci 2016; 39: 136–145. [DOI] [PubMed] [Google Scholar]
- 21.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 2007; 8: 700–711. [DOI] [PubMed] [Google Scholar]
- 22.Akerman S, Holland PR, Hoffmann J. Pearls and pitfalls in experimental in vivo models of migraine: dural trigeminovascular nociception. Cephalalgia 2013; 33: 577–592. [DOI] [PubMed] [Google Scholar]
- 23.Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain 1983; 16: 109–110. [DOI] [PubMed] [Google Scholar]
- 24.Jia Z, Tang W, Zhao D, Yu S. Disrupted functional connectivity between the periaqueductal gray and other brain regions in a rat model of recurrent headache. Sci Rep 2017; 7: 3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oshinsky ML, Sanghvi MM, Maxwell CR, Gonzalez D, Spangenberg RJ, Cooper M, Silberstein SD. Spontaneous trigeminal allodynia in rats: a model of primary headache. Headache 2012; 52: 1336–1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oshinsky ML, Gomonchareonsiri S. Episodic dural stimulation in awake rats: a model for recurrent headache. Headache 2007; 47: 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song T, Nie B, Ma E, Che J, Sun S, Wang Y, Shan B, Liu Y, Luo S, Ma G, Li K. Functional magnetic resonance imaging reveals abnormal brain connectivity in EGR3 gene transfected rat model of schizophrenia. Biochem Biophys Res Commun 2015; 460: 678–683. [DOI] [PubMed] [Google Scholar]
- 28.Nie B, Hui J, Wang L, Chai P, Gao J, Liu S, Zhang Z, Shan B, Zhao S. Automatic method for tracing regions of interest in rat brain magnetic resonance imaging studies. J Magn Reson Imaging 2010; 32: 830–835. [DOI] [PubMed] [Google Scholar]
- 29.Paxinos G, Watson C. The rat brain in stereotaxic coordinates – the new coronal set. 5th ed Cambridge, MA: Academic Press, 2004. [Google Scholar]
- 30.Ferrari MD, Klever RR, Terwindt GM, Ayata C, van den Maagdenberg A. Migraine pathophysiology: lessons from mouse models and human genetics. Lancet Neurol 2015; 14: 65–80. [DOI] [PubMed] [Google Scholar]
- 31.Noseda R, Burstein R. Migraine pathophysiology: anatomy of the trigeminovascular pathway and associated neurological symptoms, cortical spreading depression, sensitization, and modulation of pain. Pain 2013; 154: S44–S53. [DOI] [PubMed] [Google Scholar]
- 32.Apps R, Garwicz M. Anatomical and physiological foundations of cerebellar information processing. Nat Rev Neurosci 2005; 6: 297–311. [DOI] [PubMed] [Google Scholar]
- 33.Schmahmann JD. The cerebellum and cognition. Neurosci Lett 2018; 68: 62–75. [DOI] [PubMed] [Google Scholar]
- 34.Moulton EA, Elman I, Pendse G, Schmahmann J, Becerra L, Borsook D. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. J Neurosci 2011; 31: 3795–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mehnert J, Schulte L, Timmann D, May A. Activity and connectivity of the cerebellum in trigeminal nociception. NeuroImage 2017; 150: 112–118. [DOI] [PubMed] [Google Scholar]
- 36.Afridi SK, Giffin NJ, Kaube H, Friston KJ, Ward NS, Frackowiak RSJ, Goadsby PJ. A positron emission tomographic study in spontaneous migraine. Arch Neurol 2005; 62: 1270–1275. [DOI] [PubMed] [Google Scholar]
- 37.Mehnert J, May A. Functional and structural alterations in the migraine cerebellum. J Cereb Blood Flow Metab 2019; 39: 730–739. [DOI] [PMC free article] [PubMed]
- 38.Bilgic B, Kocaman G, Arslan AB, Noyan H, Sherifov R, Alkan A, Asil T, Parman Y, Baykan B. Volumetric differences suggest involvement of cerebellum and brainstem in chronic migraine. Cephalalgia 2016; 36: 301–308. [DOI] [PubMed] [Google Scholar]
- 39.Granziera C, Romascano D, Daducci A, Roche A, Vincent M, Krueger G, Hadjikhani N. Migraineurs without aura show microstructural abnormalities in the cerebellum and frontal lobe. Cerebellum 2013; 12: 812–818. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Broman J, Edvinsson L. Central projections of the sensory innervation of the rat middle meningeal artery. Brain Res 2008; 1208: 103–110. [DOI] [PubMed] [Google Scholar]
- 41.Burstein R, Cutrer MF, Yarnitsky D. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain 2000; 123: 1703–1709. [DOI] [PubMed] [Google Scholar]
- 42.Boyer N, Dallel R, Artola A, Monconduit L. General trigeminospinal central sensitization and impaired descending pain inhibitory controls contribute to migraine progression. Pain 2014; 155: 1196–1205. [DOI] [PubMed] [Google Scholar]
- 43.Schwedt TJ, Chiang C-C, Chong CD, Dodick DW. Functional MRI of migraine. Lancet Neurol 2015; 14: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaidel DW. The case for a relationship between human memory, hippocampus and corpus callosum. Biologic Res 1995; 28: 51–57. [PubMed] [Google Scholar]
- 45.Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol 2013; 75: 365–391. [DOI] [PubMed] [Google Scholar]
- 46.Peyron R, Faillenot I, Mertens P, Laurent B, Garcia-Larrea L. Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in the brain. A PET study. NeuroImage 2007; 34: 310–321. [DOI] [PubMed] [Google Scholar]