Abstract
BACKGROUND:
Mitragyna speciosa, also known as kratom, is obtained from the coffee plant family ‘Rubiaceae.’ Kratom is available in the form of capsules, whole, processed and powdered leaves, and as liquids. Secondary to its ‘natural herb’ status and opioid effects, it is misconceived to be a safe alternative for the treatment of chronic pain. The use of kratom has increased by tenfold in the United States since 2010.
METHODS AND RESULTS:
We report a term neonate who was born to a chronic kratom user and required treatment with opiates for neonatal drug withdrawal.
CONCLUSION:
Physicians should be aware of these herbal supplements and its potential withdrawal effects in newborn which cannot be picked up by the standard toxicology screen.
Keywords: Kratom, NAS – neonatal abstinence syndrome
1. Introduction
The incidence of illicit drug use during pregnancy is increasing in the United States (US) [1, 2]. The 2015 Substance Abuse and Mental Health Services Administration (SAMHSA) national survey on drug use and health estimated that 12.5% of pregnant women aged 15 – 44 years used illicit drugs, an increase from 4.5% in 2009 [2, 3]. Neonatal abstinence syndrome has become an epidemic throughout the US with increasing resource utilization over the last decade [4]. Newer herbal products such as ‘Kratom’ are gaining popularity and are being widely used in the United States [5]. Secondary to being touted as a natural herbal product, ‘Kratom’ is misconceived as a safe alternative for opioids by its users [5]. We present a case of withdrawal in a neonate who was born to a chronic kratom user.
2. Case
A full-term baby girl was born at an outlying facility to a 29-year-old single mother. This was her second pregnancy, which was complicated by chronic low back pain, fibromyalgia and anxiety. She was on prescribed medications throughout her pregnancy which included gabapentin (600 mg orally three times a day), clonazepam (0.5 mg orally 1–3 doses/day) and prenatal vitamins. She was also on over the counter oral herbal supplements in the form of tablets which she took (5 g each, 1–3 times a day). She was also a chronic smoker. All her prenatal labs were within normal limits, other than a screening for group B streptococcus (GBS) for which she received penicillin prophylaxis before delivery.
The baby was born by spontaneous vaginal delivery and had a normal transition at birth with Apgar scores of 9 at 1 and 5 minutes. She measured small for her gestational age which was attributed to maternal chronic tobacco use. She was started on formula feeding and after 24 hours of transition started exhibiting clinical signs which included reduced oral intake, jitteriness, hypertonia, sneezing and excessive crying. The urine toxicology sample was obtained 12 hours after birth. A complete blood count (CBC) with differential was obtained after 24 hours. The CBC was within normal limits and the urine toxicology panel was negative. The nursing assessment of signs and symptoms were consistent with neonatal withdrawal and the modified Finnegan scoring tool was used to assess severity [2, 6]. With rising Finnegan scores (10 and above) and unclear etiology, the baby was transferred to our regional perinatal center for further evaluation and management.
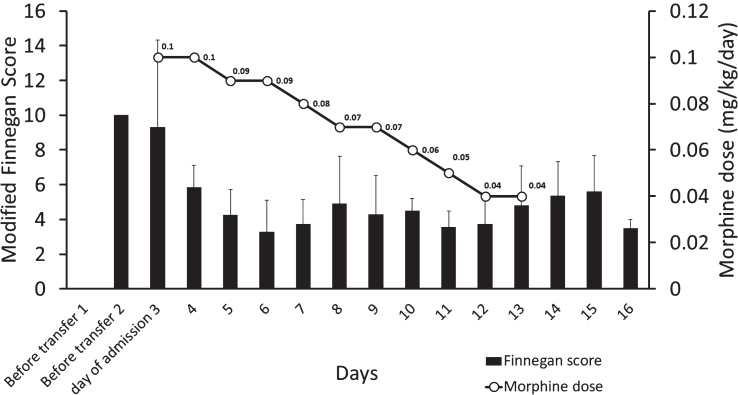
On admission to our neonatal intensive care unit (NICU), she was placed in an isolation room. Additional information obtained as part of history taking revealed that the mother’s herbal supplement was a pill sold over the counter known as kratom. Despite caring in a non-stimulant and isolated environment, the infant continued to sneeze, cry excessively, had increased muscle tone along with intermittent tachypnea and hyperthermia. She also had excessive suck, poor feeding associated with spit up. The Finnegan scores on admission were consistently >10 and warranted pharmacotherapy. These scores are repeated every 3–4 hours to decide on management and drug therapy. Kratom has an opioid effect and with the clinical picture consistent with neonatal withdrawal syndrome, oral morphine 0.1 mg/kg/day divided every 4 hours was started as part of the management (Fig. 1). During the NICU course, the baby was formula fed but bonded with the mother during the day. The infant responded to the morphine therapy and the weaning was initiated on day 3 of treatment and was gradually weaned off. She was observed for 48 hours after discontinuing pharmacotherapy and was discharged home. The infant stayed in our institution for a total of fourteen days.
Fig.1.
The figure shows the pharmacological management of the infant. X-axis shows the days, while y-axis shows the modified Finnegan scores (bar graph) and the secondary y-axis the dose of morphine (line graph).
3. Discussion
Mitragyna speciosa also known as kratom is obtained from the coffee plant family Rubiaceae [7]. They are indigenous to Southeast Asia where it was historically used for recreational and medicinal purposes. Kratom is available in the form of capsules, whole, processed and powdered leaves, and as liquids [5]. Secondary to its opioid effects and a ‘natural herb’ status, a general misconception of this drug is that it is a safe alternative for treatment of chronic pain. It can currently be purchased over the counter in some states in the United States and easily over the internet. Between 2010–2015, the United States poison center has noticed a 10-fold increase in the number of calls in regards to kratom exposure [8]. An online surveillance study reported an increase in the use of kratom to modulate opiate withdrawal [9]. Kratom dependence and unpleasant signs and symptoms of withdrawal have been previously reported in adults [10].
The drug enforcement administration (DEA) has listed kratom as a drug of concern [11]. At lower doses, the drug causes a stimulant effect, and at higher doses, has an opiate effect [5, 10, 12, 13]. These effects could lead to psychological and physiological dependence [11]. The two major components of Kratom are “Mitragynine” and “7 hydroxymitragynine”. These indole alkaloids are attributed to the opioid-like activity of Kratom [13]. These components mostly act on the μ and δ type opioid receptors [13–15]. Recently the food and drug administration (FDA) using their Public Health Assessment via Structural Evaluation (PHASE) methodology, provided stronger evidence for kratom’s opioid properties [7]. According to the FDA, ‘PHASE uses the molecular structure of a substance to predict its biological function in the body’. This three-dimensional technology identified that twenty-two of the most prevalent twenty-five compounds in kratom could bind to μ-opioid receptors [7]. These findings along with the available data confirm the opioid activity of kratom.
No published studies exist regarding the safety and efficacy of kratom for maternal use or its effect on the fetus during pregnancy. To our knowledge, this is the first report of neonatal withdrawal in the US secondary to maternal kratom use. Previously a prison case report from Thailand and recently a withdrawal in mother and baby in Canada have been reported [4, 16]. These reports and ours, raise some important questions. Is there evidence to support the claim that this drug crosses the placenta? A study in a murine model looking at the effect of in-utero exposure has shown that kratom could affect neural tube development suggesting transplacental transfer [17].
Could these withdrawal signs and symptoms in the neonate be secondary to gabapentin or clonazepam? A case series and a case report on neonatal withdrawal secondary to gabapentin have been reported [18, 19]. After six days of opiate or benzodiazepine therapy, the cases in these reports required gabapentin therapy which was not the case in our patient. Although there is literature to suggest that gabapentin abuse is usually associated with opioid use and may potentiate opioid effect, we do not know the synergistic effect of kratom and gabapentin [19, 20]. In regards to clonazepam, in a case series of thirty-eight women who were on chronic therapy, records obtained from twenty-seven babies did not show any adverse neonatal outcome/withdrawal [21].
Previous studies have suggested a possibility of neonatal nicotine withdrawal syndrome from excess maternal smoking [22–24]. Maternal smoking (>20 cigarettes/day) was associated with high urinary cotinine and hair nicotine [23] and was associated with low neonatal birth weight and increasing Finnegan scores. However, it is unclear if these symptoms were secondary to withdrawal or secondary to acute toxicity resulting in neurological injury associated with growth restriction [23]. Well-conducted studies are required to substantiate neonatal nicotine withdrawal further.
The signs and symptoms reported in our infant were consistent with opioid withdrawal. Conservative management and the response to morphine therapy and improvement in Finnegan score could be appreciated (Fig. 1). Our practice for pharmacologic therapy in neonatal withdrawal is to initiate morphine and if the symptoms are not controlled, use phenobarbital as a second agent. In our infant, we were able to wean morphine by ten percent every 24–48 hours until the medication was discontinued. The total duration of morphine therapy was eleven days. Breastfeeding and constant parental involvement could have impacted the neonatal course.
4. Conclusion
Kratom is an unregulated over-the-counter drug which has opioid-like activity. Physicians should be aware of the use of kratom and its potential withdrawal effects in newborn which cannot be picked up by the standard toxicology screening techniques. Conservative care and opiate therapy seems to be the current line of management for neonatal withdrawal secondary to kratom.
Funding source
None.
Financial disclosure
Canadian NRP grant – MR, American NRP grant – PC. Dr. Henry C. and Bertha H. Buswell Fellowship – MR, PC.
Conflict of interest
None.
Acknowledgments
We thank the mother who permitted us to submit this case report.
References
- [1]. Creanga AA, Sabel JC, Ko JY et al. Maternal drug use and its effect on neonates: A population-based study in Washington State. Obstet Gynecol. 2012;119(5):924–33. [DOI] [PubMed] [Google Scholar]
- [2]. Hudak ML, Tan RC, Committee On D, Committee On F, Newborn, American Academy of P. Neonatal drug withdrawal. Pediatrics. 2012;129(2):e540–60. [DOI] [PubMed] [Google Scholar]
- [3].Results from the 2015 National Survey On Drug UseAnd Health: Detailed Tables. https://wwwsamhsagov/data/sites/default/files/NSDUH-DetTabs-/NSDUH-DetTabs-/NSDUH-DetTabs-pdf.
- [4]. Trakulsrichai S, Tongpo A, Sriapha C et al. Kratom abuse in Ramathibodi Poison Center, Thailand: A five-year experience. J Psychoactive Drugs. 2013;45(5):404–8. [DOI] [PubMed] [Google Scholar]
- [5]. Voelker R.Kratom Products Seized. JAMA. 2016;316(11):1142. [DOI] [PubMed] [Google Scholar]
- [6]. D’Apolito KC.Assessing neonates for neonatal abstinence: Are you reliable? J Perinat Neonatal Nurs 2014;28(3):220–31. [DOI] [PubMed] [Google Scholar]
- [7].Statement from FDA Commissioner Scott Gottlieb, M.D. on FDAadvisory about deadly risks associatedwith kratom. https://wwwfdagov/NewsEvents/Newsroom/PressAnnouncements/ucm70htm.
- [8]. Anwar M, Law R, Schier J.Notes from the Field: Kratom (Mitragyna speciosa) Exposures Reported to Poison Centers - United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(29):748–9. [DOI] [PubMed] [Google Scholar]
- [9]. Boyer EW, Babu KM, Adkins JE, McCurdy CR, Halpern JH. Self-treatment of opioid withdrawal using kratom (Mitragynia speciosa korth). Addiction. 2008;103(6):1048–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Singh D, Muller CP, Vicknasingam BK.Kratom (Mitragyna speciosa) dependence, withdrawal symptoms and craving in regular users. Drug Alcohol Depend. 2014;139:132–7. [DOI] [PubMed] [Google Scholar]
- [11].Drugs of concern. A DEA Resource Guide: 2017 EDITION. 2017. https://www.dea.gov/pr/multimedia-library/publications/drug_of_abuse.pdf#page=84.
- [12]. Chang-Chien GC, Odonkor CA, Amorapanth P.Is Kratom the New ‘Legal High’ on the Block?: The Case of an Emerging Opioid Receptor Agonist with Substance Abuse Potential. Pain Physician. 2017;20(1):E195–E8. [PubMed] [Google Scholar]
- [13]. Prozialeck WC, Jivan JK, Andurkar SV.Pharmacology of kratom: An emerging botanical agent with stimulant, analgesic and opioid-like effects. J Am Osteopath Assoc. 2012;112(12):792–9. [PubMed] [Google Scholar]
- [14]. Matsumoto K, Hatori Y, Murayama T et al. Involvement of mu-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549(1-3):63–70. [DOI] [PubMed] [Google Scholar]
- [15]. Matsumoto K, Horie S, Takayama H et al. Antinociception, tolerance and withdrawal symptoms induced by 7-hydroxymitragynine, an alkaloid from the Thai medicinal herb Mitragyna speciosa. Life Sci. 2005;78(1):2–7. [DOI] [PubMed] [Google Scholar]
- [16]. Mackay L, Abrahams R. Novel case of maternal and neonatal kratom dependence and withdrawal. Can Fam Physician. 2018;64(2):121–2. [PMC free article] [PubMed] [Google Scholar]
- [17]. Bala Y. Muhammad ADA, Siti Nur Sharida Abd Kadir, Tariq Bn. Abdul Razak, Qamar U. Ahmed. In-Utero Effects of the Crude Ethanolic Extract of the Leaves of Mitragyna speciosa on Neural Tube Formation in Rats. Asian J Exp Biol Sci. 2010;1(2):404–8. [Google Scholar]
- [18]. Carrasco M, Rao SC, Bearer CF, Sundararajan S. Neonatal gabapentin withdrawal syndrome. Pediatr Neurol. 2015;53(5):445–7. [DOI] [PubMed] [Google Scholar]
- [19]. Loudin S, Murray S, Prunty L, Davies T, Evans J, Werthammer J. An atypical withdrawal syndrome in neonates prenatally exposed to gabapentin and opioids. J Pediatr. 2017;181:286–8. [DOI] [PubMed] [Google Scholar]
- [20]. Bastiaens L, Galus J, Mazur C. Abuse of gabapentin is associated with opioid addiction. Psychiatr Q. 2016;87(4):763–7. [DOI] [PubMed] [Google Scholar]
- [21]. Weinstock L, Cohen LS, Bailey JW, Blatman R, Rosenbaum JF. Obstetrical and neonatal outcome following clonazepam use during pregnancy: A case series. Psychother Psychosom. 2001;70(3):158–62. [DOI] [PubMed] [Google Scholar]
- [22]. Garcia-Algar O, Puig C, Vall O, Pacifici R, Pichini S. Effects of maternal smoking during pregnancy on newborn neurobehavior: Neonatal nicotine withdrawal syndrome. Pediatrics. 2004;113(3 Pt 1):623–4. [DOI] [PubMed] [Google Scholar]
- [23]. Law KL, Stroud LR, LaGasse LL, Niaura R, Liu J, Lester BM. Smoking during pregnancy and newborn neurobehavior. Pediatrics. 2003;111(6 Pt 1):1318–23. [DOI] [PubMed] [Google Scholar]
- [24]. Stroud LR, Paster RL, Papandonatos GD et al. Maternal smoking during pregnancy and newborn neurobehavior: Effects at 10 to 27 days. J Pediatr. 2009;154(1):10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]